Abstract
Background and Aims
CD39/ENTPD1 scavenges pro-inflammatory nucleotides, to ultimately generate immunosuppressive adenosine, which has a central role in immune homeostasis. Global deletion of Cd39 increases susceptibility to experimental colitis while single nucleotide polymorphisms within the human CD39 promoter, and aberrant patterns of expression during experimental hypoxia, predispose to Crohn’s disease. We aimed to define the impact of transgenic human CD39 [hTG] overexpression in experimental colitis and to model therapeutic effects using the recombinant apyrase APT102 in vivo. We also determined the in vitro effects of APT102 on phenotypic and functional properties of regulatory T-lymphocytes derived from patients with Crohn’s disease.
Methods
Colitis was induced by administration of dextran sulfate sodium in wild-type [WT] or hTG mice, and, in another model, by adoptive transfer of CD45RBhigh cells with or without WT or hTG regulatory T cells [Treg]. In additional experiments, mice were treated with APT102. The effects of APT102 on phenotype and function of Treg and type-1 regulatory T [Tr1] cells were also evaluated, after purification from peripheral blood and lamina propria of Crohn’s disease patients [n = 38].
Results
Overexpression of human CD39 attenuated experimental colitis and protected from the deleterious effects of systemic hypoxia, pharmacologically induced by deferoxamine. Administration of APT102 in vivo enhanced the beneficial effects of endogenous Cd39 boosted by the administration of the aryl hydrocarbon receptor [AhR] ligand unconjugated bilirubin [UCB]. Importantly, supplemental APT102 restored responsiveness to AhR stimulation by UCB in Treg and Tr1 cells, obtained from Crohn’s disease patients.
Conclusions
hCD39 overexpression ameliorated experimental colitis and prevented hypoxia-related damage in vivo. Exogenous administration of APT102 boosted AhR-mediated regulatory effects in vivo while enhancing Treg functions in Crohn’s disease in vitro.
Keywords: ATPase/ADPase, ectonucleotidase, regulatory cells, Crohn’s disease
1. Introduction
Defective regulatory cell functions1–3 contribute to disordered immune homeostasis in inflammatory bowel disease [IBD]. Impaired regulatory cell function in IBD results, at least in part, from low levels of CD39, an ectonucleotidase with a pivotal role in immunity as it hydrolyses pro-inflammatory ATP and ADP into AMP, which is subsequently converted into immunosuppressive adenosine by the 5′-ectoenzyme CD73.4,5 CD39 is mainly expressed by endothelial cells in the vasculature and by a variety of immune cells such as B lymphocytes, myeloid cells, T-cells including Th17, regulatory T cells [Treg] and type-1 regulatory T [Tr1] cells.5 Adoptive transfer of Treg, isolated from Entpd1−/− mice does not protect from development of colitis.6 Furthermore, in humans, previous studies have shown impairment of CD39 expression in Treg obtained from the peripheral blood and lamina propria of Crohn’s disease patients. Low CD39 levels may result from polymorphisms in the ENTPD1/CD39 promoter7 or from the associated impaired responses to aryl hydrocarbon receptor [AhR] activation by immunometabolites such as unconjugated bilirubin [UCB].8,9 AhR is a modulator of toxin responses10,11 and adaptive immunity and is, in turn, inhibited by hypoxia, resulting from protracted inflammation.12,13
Transgenic expression of human CD39 [hCD39] in mice results in impaired platelet aggregation, resistance to thromboembolism14 and in longer survival in a cardiac transplant model of vascular rejection.14 Such hCD39 transgenic overexpression confers protection in a model of warm ischaemia reperfusion injury resulting in reduced kidney damage and in preserved levels of serum creatinine and urea.15 Beneficial effects of CD39 in vivo have been further supported by pre-clinical evidence that administration of the human soluble domain of CD3916 or APT102, the extracellular domain with improved ADPase activity of human nucleoside triphosphate diphosphohydrolase-3 [CD39L3], a member of the CD39 family, confers protection in models of acute myocardial infarction,16 ischaemia reperfusion injury,17–19 vein graft bypass surgery20 and coronary artery occlusion.17
Here we investigated the impact of hCD39 transgenic overexpression on experimental colitis models in mice; we also tested the therapeutic effects of APT102 administration in vivo and, in vitro, on the phenotypic and functional properties of Treg and Tr1-cells, derived from Crohn’s disease patients.
We show that hCD39 overexpression ameliorates experimental colitis while protecting from pharmacological systemic hypoxia-associated injury. Furthermore, administration of APT102 has therapeutic effects in experimental colitis in vivo and corrects Crohn’s disease-derived regulatory cell functionality in vitro, by synergizing with AhR ligation/activation to boost endogenous purinergic cytoprotective pathways.
2. Methods
2.1. Mice
hCD39 transgenic [hTG] mice were originally generated on a C57BL/6-CBA background using a construct containing the murine H-2kb promoter to drive hCD39 expression.14 They were backcrossed more than six times onto C57BL/6. The derivation and characterization of Entpd1−/− mice on the C57BL/6 background has been previously described.21 C57BL/6 wild type [WT] mice, originally purchased from Taconic, were bred in our animal facility for four to six generations before being used and subjected to experimental colitis.
Rag2−/− immunodeficient mice were from Taconic. In each group, 8- to 10-week-old male and female mice were studied in accordance with the National Institutes of Health guide for the care and use of Laboratory animals. Protocols were approved by the Animal Care and Use Committee at Beth Israel Deaconess Medical Center [BIDMC], Boston, MA.
2.2. Subjects
Peripheral blood mononuclear cells [PBMCs] and lamina propria mononuclear cells [LPMCs] were isolated from 38 patients with Crohn’s disease [median Harvey–Bradshaw index 2, range 0–8], recruited from the Gastroenterology Division, BIDMC. Sixteen patients were studied during active disease, while the remaining were on clinical remission. At the time of study, 17 patients were on infliximab, six were on steroids and five were receiving azathioprine/6-mercaptopurine. PBMCs were also obtained from 15 healthy blood donors [Blood Donor Center at Children’s Hospital, Boston]. Human studies received IRB approval at BIDMC. Written consent was obtained from all study participants prior to inclusion in the study.
2.3. APT102
APT102 was produced and purified from a stably transfected Chinese hamster ovary cell line as described previously.22 The protein was stable for several years with purity of >99% and endotoxin level of <1 EU/mg. Pharmacokinetic/pharmacodynamic analysis revealed that the elimination-phase half-life of a single bolus intraperitoneal injection is ~35 h in mice [Supplementary Figure 1A,B].
2.4. Cell isolation, polarization and culture
2.4.1. Mouse
Mononuclear cells for in vitro experiments were obtained from the spleen of WT, hTG and Entpd1−/− mice and subjected to CD4 T-cell isolation according to the manufacturer’s instructions [Miltenyi Biotec]. The purity of the sorted CD4 cell population exceeded 92%. CD4 cells were then cultured in RPMI 1640 medium, supplemented with 2 mM l-glutamine, 100 IU/mL penicillin, 100 mg/mL streptomycin, 1% non-essential amino acids and 10% FBS and exposed for 5 days to Treg or Tr1 cell polarizing conditions. Treg polarizing conditions consisted of interleukin 2 [IL2; 100 ng/mL], transforming growth factor β [TGF β; 10 ng/mL] and Dynabeads Mouse T activator CD3/CD28 for T-cell expansion [bead/cell ratio: 1/2, ThermoFisher Scientific]; Tr1 polarizing conditions consisted of IL27 [30 ng/mL]. All cytokines were from R&D Systems. In some of the cultures, cells were exposed to APT102 at 0.5 mg/mL for the last 12 h of culture and/or UCB at 20 μM for the last 6 h of culture.8,13 Mononuclear cells were also isolated from the spleen, mesenteric lymph nodes [MLNs], intra-epithelial [IELs] and lamina propria [LPs] lymphocytes, obtained from dextran sulfate sodium [DSS] colitic mice at harvest.8,13 Mononuclear cell phenotype was then assessed by flow cytometry [see below].
2.4.2. Human
PBMCs were obtained by density gradient centrifugation on Ficoll-Paque [GE Healthcare Life Sciences].23 LPMCs were obtained from freshly biopsied colonic tissue in 13 patients with Crohn’s disease. In these patients, tissue was biopsied from both inflamed and non-inflamed areas [three or four samples per bioptic area]. LPMCs were isolated as previously described8,23 and results from the sites compared. PBMC and LPMC viability always exceeded 98%. CD4 cells were purified from both PBMC and LPMC preparations according to the manufacturer’s recommendations [Miltenyi Biotec]. The purity of the sorted CD4+ cells exceeded 92%. Cells were resuspended in RPMI 1640 supplemented with 10% fetal bovine serum [FBS] and exposed to Treg and Tr1 polarizing conditions. Cells were polarized under the same culture conditions and upon exposure to the same cytokine concentrations as described above in the mouse section. All cytokines were from R&D Systems. In parallel cultures, cells were exposed to APT102 in the absence or presence of UCB, as indicated above.
2.5. Induction and assessment of colitis
2.5.1. DSS-induced colitis
C57BL/6 WT and hTG mice were treated with 3% DSS in standard drinking water, provided ad libitum for 6 days. In additional experiments, mice were concomitantly injected with either: UCB [20 μmol/kg/day intraperitoneally, i.p.] alone or in combination with deferoxamine [100 mg/kg/day i.p.] to induce systemic hypoxia; or APT102 [1 mg/kg/day i.p.]. UCB resuspension was conducted as described previously.8 Deferoxamine and APT102 were both resuspended in 1×PBS. UCB, deferoxamine and/or APT102 administration was continued after day 7, when DSS was replaced with standard drinking water. In the experiments where the effects of deferoxamine were tested, mice were killed on day 10, after 4 days of recovery. When therapeutic effects of APT102 were evaluated in longer-term experiments, mice were exposed to 2% instead of 3% DSS and killed on day 30, after 24 days of recovery. In additional experiments, colitis was induced upon injection of CD4+CD45RBhigh cells, obtained by immunomagnetic isolation from WT splenocytes. CD45RBhigh cells were injected i.p. [4 × 105] into 8-week-old Rag2−/− mice, alone or in combination with CD4+CD25high Treg [1 × 105] from WT or hTG mice.24
In both models, disease activity index [range 0–8] was calculated on the basis of body weight loss [‘0’: 0–1%; ‘1’: >1–5%; ‘2’: >5–10%; ‘3’: >10–15%; ‘4’: >15–20%], presence of gross blood [‘0’: absence of blood; ‘1’: presence of blood; ‘2’: overt bleeding] and stool consistency [‘0’: firm; ‘1’: loose; ‘2’: diarrhoea] and assessed on a weekly basis in the T-cell transfer model6,24 and on a daily basis in the DSS model.8 On the day of harvest, colons were dissected and colon length was measured from the ileocecal junction to the anal verge. Colitis histology score was calculated after haematoxylin and eosin staining.8
2.6. Flow cytometry staining
Flow cytometry was performed as previously described23 following mouse or human mononuclear cell staining with fluorochrome-conjugated monoclonal antibodies [Supplementary Table 1]. Cells were acquired on a BD LSRII [BD Biosciences] and analysed using FlowJo 2 software [version 10, TreeStar]. Positively stained cell populations were gated based on unstained and single stained controls. The fluorescence-minus-one method was used to adjust compensation.
2.7. Cell proliferation assay
Proliferation of murine Treg and Tr1 cells was performed following exposure to Dynabeads Mouse T activator CD3/CD28 for T-cell expansion [bead/cell: 1/2] and IL2 at 30 ng/mL for 3 days. Proliferation was measured based on 3H-thymidine incorporation.25
2.8. Thin layer chromatography
Thin layer chromatography [TLC] was performed upon cell incubation with 2 mCi/mL [14C] ADP.23
2.9. Suppressive function
The suppressive function of murine and human Treg and Tr1 cells was assessed in co-culture experiments, in which polarized Treg and Tr1 cells were initially sorted as CD25highCD127− and CD49b+LAG-3+ cells and then added at a ratio of 1/8 to CD4+CD25− target cells.8 Cultures of CD4+CD25− targets without Treg or Tr1 cells were performed in parallel, under identical conditions. Target cells were initially stained with Cell Trace Violet [1/4000, Invitrogen], activated using IL2 [30 ng/mL] and Dynabeads Mouse or Human T activator CD3/CD28 for T-cell expansion [bead/cell ratio: 1/2] and tested for IL17 and IFNγ production after 4 days of co-culture.
2.10. qPCR
Expression of A1, A2A, A2B and A3 adenosine receptors, Cd49b, Lag-3 and Foxp3 was determined by quantitative PCR [PCR], following total RNA extraction by TRIzol [Thermo Fisher Scientific] and mRNA reverse-transcription using iScript cDNA synthesis kit [Bio-Rad Laboratories]. Primers for adenosine receptors, Cd49b, Lag-3 and Foxp3 were as previously described.26–28 Relative gene expression was determined after normalization to mouse β-actin.
2.11. Immunohistochemistry
Frozen tissue sections [6 μm] of spleen and colon were incubated overnight at 4°C with rabbit anti-mouse CD39 [a gift from Dr Jean Sévigny, Quebec, Canada] or with biotin-labelled mouse anti-human CD39 antibody [clone # BU61, Ancell]. Immunohistochemistry was carried out as previously reported.29
2.12. Statistics
Results are expressed as mean ± SEM. Normality of variable distribution was assessed by Kolmogorov–Smirnov goodness-of-fit tests. Comparisons were performed using parametric [paired or unpaired Student’s t test] or non-parametric [Wilcoxon signed-rank or Mann–Whitney test] tests according to data distribution. One-way ANOVA or Kruskal–Wallis tests, followed by Tukey’s or Dunn’s multiple comparison tests, were used when comparing more than two sets of data. p < 0.05 was considered significant; p ≤ 0.1 was considered indicating a trend to significance. Statistical analysis was performed using SPSS version 22 and GraphPad Prism version 7.
3. Results
3.1. hCD39 overexpression enhances Treg function
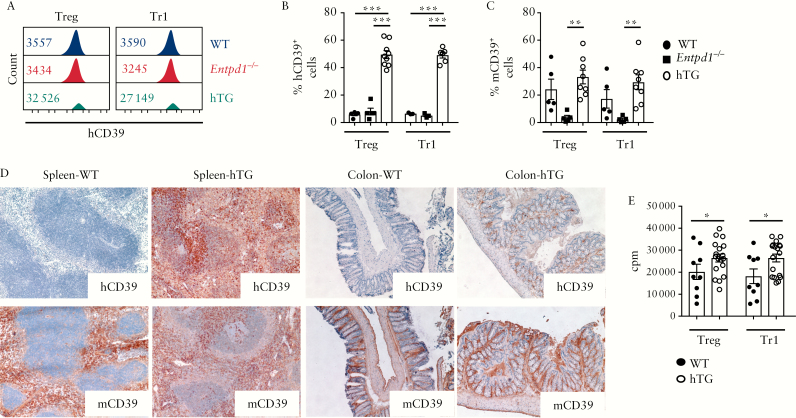
We tested the effects of hCD39 overexpression on the phenotypic and functional properties of Treg and Tr1 cells obtained from mice that express human ENTPD1 under control of the H-2kb promoter. We noted elevated levels of hCD39 and high frequencies of hCD39+ cells in Treg and Tr1 subsets [of hTG mice], in contrast to WT and Entpd1−/− mice [Figure 1A,B]. Notably, Treg and Tr1 subsets from hTG mice also exhibited increases in cells positive for mouse CD39 [mCD39], when compared with Entpd1−/− mice, although this was a trend in the WT mice [Figure 1C].
Figure 1.
Treg and Tr1 cells from hTG mice display higher frequencies of mCD39+ cells and show increased proliferative capacity. [A] Histogram plots of human CD39 [hCD39] MFI of Treg and Tr1 cells obtained from a representative WT, Entpd1−/− and hTG mouse. Numerical values of hCD39 MFI are indicated within the histogram plots. [B] Mean ± SEM frequency of hCD39+ cells within Treg and Tr1 cells of WT [n = 3–5], Entpd1−/− [n = 3–5] and hTG mice [n = 6–9]. [C] Mean ± SEM frequency of mCD39+ cells within Treg and Tr1 cells of WT [n = 5], Entpd1−/− [n = 5] and hTG mice [n = 8]. [D] Representative hCD39 and mCD39 staining of frozen sections from the spleen and colon of WT and hTG mice. Staining from one of three independent experiments is shown [original magnification, ×20]. [E] Proliferation of Treg and Tr1 cells obtained from WT [n = 9] and hTG [n = 18] mice was measured by 3H-thymidine incorporation. Mean ± SEM counts (cpm) are shown. *p ≤ 0.05;**p ≤ 0.01;***p ≤ 0.001.
hCD39 expression did not impact the differentiation of CD4 cells into Treg and Tr1 cells, as reflected by similar levels of CD25 and FOXP3 in Treg, and of CD49b, LAG-3 and IL10 expression in Tr1 cells [Supplementary Figure 2A]. hCD39 expression in hTG mice was noted both in the spleen white and red pulp as well as in colonic tissue sections where it was present in the lamina propria and muscularis mucosae [Figure 1D]. In hTG mice, hCD39 staining within the colon was comparable with that of mCD39 [Figure 1D].
Treg and Tr1 cells obtained from hTG mice displayed higher proliferative ability [Figure 1E] and comparable frequencies of TIM-3+ [Supplementary Figure 2B] and annexin V+ cells [Supplementary Figure 2C], in contrast to WT mice.
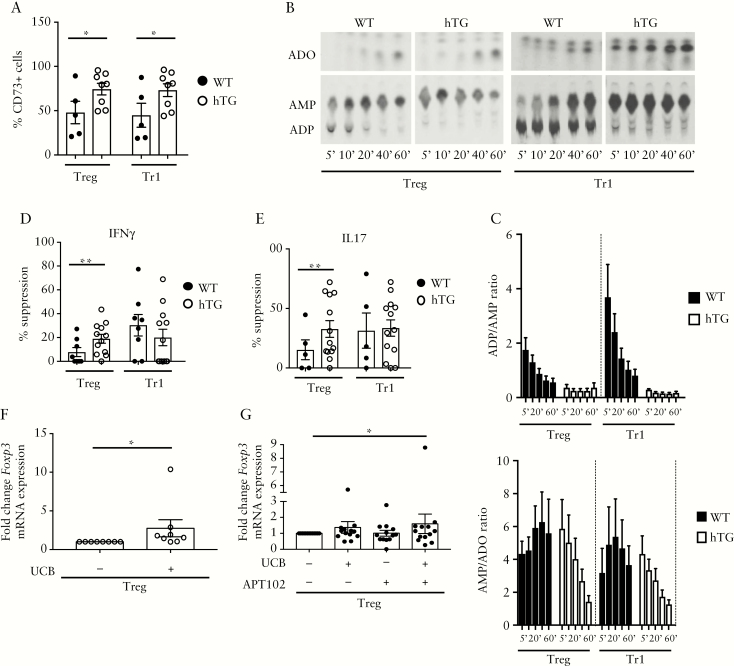
When tested for CD73, the ectoenzyme that works in tandem with CD39 and hydrolyses AMP to adenosine, hTG-derived Treg and Tr1 cells displayed heightened proportions of CD73+ lymphocytes [Figure 2A]. hTG Treg and Tr1 cells showed elevated ADPase activity, as manifested by a higher ADP/AMP ratio, and higher AMPase activity, as represented by the AMP/adenosine ratio, compared to WT counterparts [Figure 2B,C]. This indicates that the increased CD39 and CD73 expression was mirrored by heightened ectoenzymatic activity.
Figure 2.
Treg and Tr1 cells from hTG mice display heightened ectoenzymatic activity. [A] Mean ± SEM frequency of CD73+ cells within Treg and Tr1 cells from WT [n = 5] and hTG [n = 8] mice. [B] Cell ADPase ectoenzymatic activity was determined upon cell incubation with14C-labelled ADP by TLC. One representative of four independent experiments is shown. [C] Mean + SEM ADP/AMP and AMP/adenosine [ADO] ratio of Treg and Tr1 cells [WT n = 4; hTG n = 4]. Mean ± SEM percentage suppression of Treg and Tr1 cells over IFNγ [D] and IL17 [E] production by CD4+CD25− cells [WT n = 5–8; hTG n = 12–13]. Mean ± SEM fold change in Foxp3 mRNA expression in untreated and UCB-treated hTG Treg [n = 8] [F], or in untreated, UCB-, APT102- or UCB plus APT102-treated WT Treg [n = 13]. *p ≤ 0.05;**p ≤ 0.01.
Treg and Tr1 cells differentiated from hTG mice expressed higher A2B and similar A1, A2A and A3 receptor levels when compared with WT mice [Supplementary Figure 3A–D].
As CD39 expression has been associated with Treg and Tr1 suppressive function,4,12 we tested the ability of these cells to suppress IFNγ and IL17 production by CD4+CD25− responder cells. Treg from hTG mice displayed greater suppression over IFNγ and IL17 production, as compared to WT Treg [Figure 2D,E]. No differences in the ability to suppress were noted between hTG and WT Tr1 cells [Figure 2D,E].
Collectively, these data show that overexpression of hCD39 is accompanied by increased ADPase and AMPase enzymatic activity by Treg and Tr1 cells and by heightened suppressive function in Treg.
3.2. APT102 increases WT Treg response to AhR ligation
Here we evaluated the response of WT and hTG Treg and Tr1 cells to AhR activation in the presence of UCB, an immunosuppressive metabolite with beneficial properties in boosting CD39 and other protective factors in experimental colitis and human IBD.8,9 Analysis of Foxp3, Lag-3, Cd49b and mCd39 mRNA levels was carried out using WT or hTG cells as an internal control to assess variations in the response to UCB as compared to baseline levels.
Exposure of hTG, but not WT Treg, to UCB resulted in significant augmentation of Foxp3 mRNA levels [Figure 2F]. In WT Treg, addition of APT102, which provides exogenous ADPase activity, boosted Foxp3 in Treg concomitantly exposed to UCB [Figure 2G]. No changes in Lag-3, Cd49b and mCd39 mRNA levels were noted in hTG and WT Treg and Tr1 cells upon exposure to UCB, APT102, or a combination of UCB and APT102 [Supplementary Figure 4A–F]. mRNA data were confirmed by flow cytometry analysis, showing significant increases in FOXP3 mean fluorescence intensity [MFI] following hTG Treg exposure to UCB and after WT Treg treatment with UCB and APT102 [Supplementary Figure 5A], and unchanged LAG-3, CD49b and CD39 MFI in WT and hTG Treg and Tr1 cells under the same culture conditions [Supplementary Figure 5B–D]. Overall, these data indicate that addition of APT102 to WT Treg enhances the response to UCB, as reflected by augmented Foxp3 mRNA expression and MFI, mimicking the effects noted in hTG Treg in the presence of UCB only.
3.3. Overexpression of hCD39 ameliorates experimental colitis
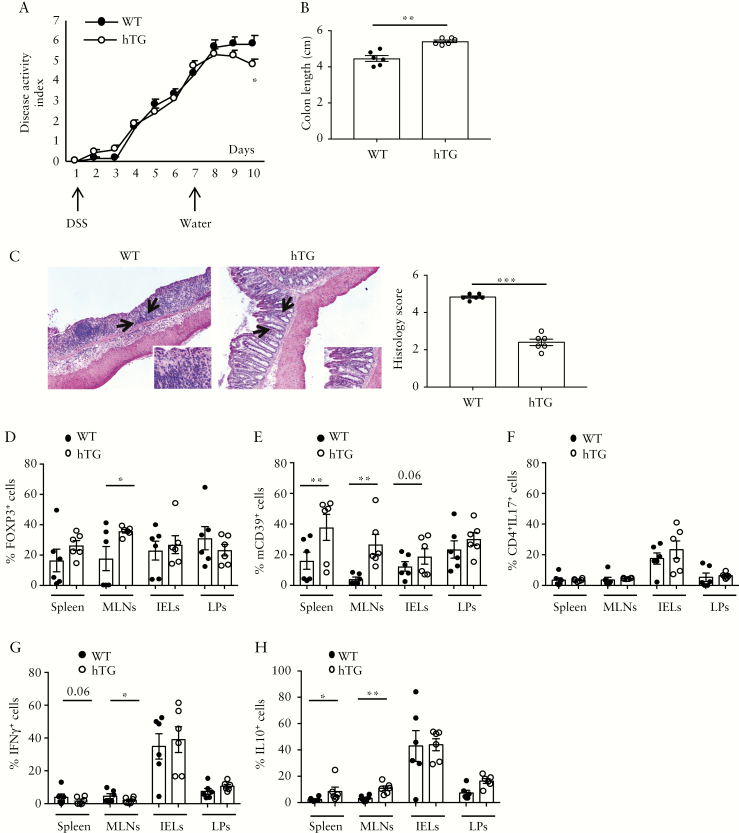
The effects of hCD39 overexpression were also tested in vivo in experimental models of colitis, induced either by DSS or by adoptive transfer of CD45RBhigh cells into Rag2−/− mice. In the DSS model, DSS was provided for six consecutive days [induction phase] and then replaced with normal drinking water for four additional days [recovery phase]. Compared to WT, hTG mice had a lower disease activity index [DAI] during recovery [Figure 3A], greater colon length [Figure 3B] and lower histology score [Figure 3C]. Furthermore, hTG animals displayed higher proportions of FOXP3+ cells within MLNs [Figure 3D and Supplementary Figure 6A] and higher frequencies of mCD39+ cells within spleen, MLNs and IELs [Figure 3E and Supplementary Figure 6B]. Compared to WT, hTG mice had also lower frequencies of IFNγ + [Figure 3G and Supplementary Figure 6D] and higher proportions of IL10+ lymphocytes [Figure 3H and Supplementary Figure 6E] in the spleen and MLNs. No significant differences between hTG and WT mice were noted in the proportion of IL17-producing CD4 cells in all compartments studied [Figure 3F and Supplementary Figure 6C].
Figure 3.
hCD39 overexpression in mice ameliorates DSS colitis in vivo. WT [n = 6] and hTG [n = 6] mice were treated with 3% DSS for 6 days. DSS treatment was then replaced with normal water for an additional 4 days. [A] Mean+SEM disease activity index in WT and hTG mice. [B] Mean ± SEM colon length [cm] at the time of harvesting. [C] Haematoxylin and eosin staining of colon sections [original magnification, ×10]; arrows indicate the area magnified in the insets [×20]; mean ± SEM histology score at the time of harvesting is also shown. Mean ± SEM frequency of [D] CD4+FOXP3+, [E] CD4+mCD39+, [F] CD4+IL17+, [G] CD4+IFNγ + and [H] CD4+IL10+ lymphocytes among spleen, MLN, IEL and LP mononuclear cells. *p ≤ 0.05;**p ≤ 0.01;***p ≤ 0.001.
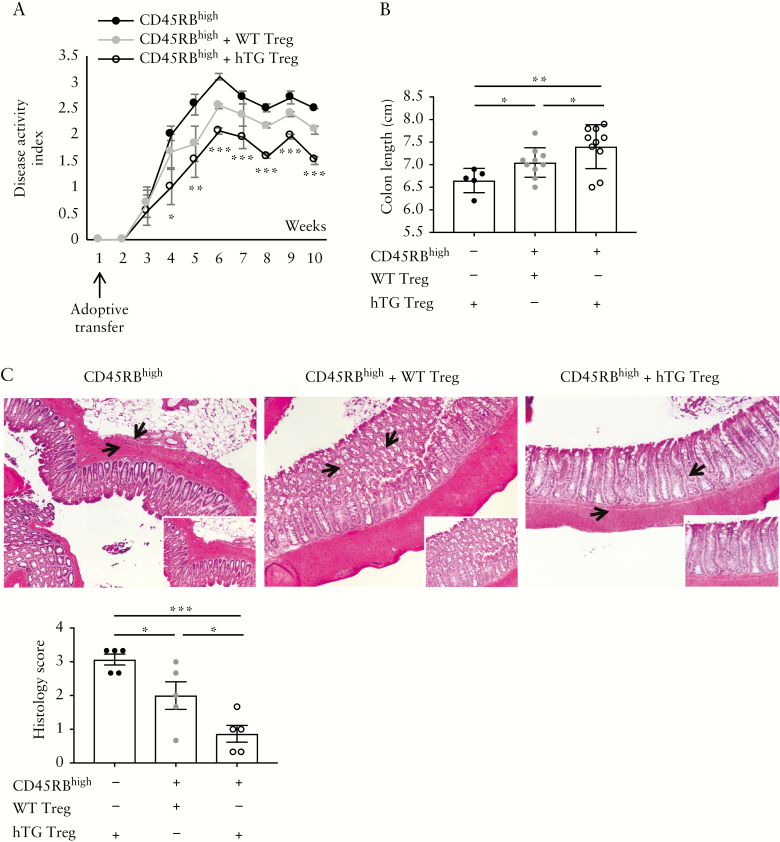
In the adoptive transfer model, injection of hTG Treg ameliorated the course of colitis induced by CD45RBhigh cells more effectively than in the setting with WT Treg [Figure 4A]. Recipients of hTG Treg had also greater colon length and lower histology score than mice injected with CD45RBhigh alone or in association with WT Treg [Figure 4B,C].
Figure 4.
hCD39 overexpressing Treg ameliorate experimental colitis, induced by adoptive transfer. CD45RBhigh lymphocytes, obtained from the spleen of WT mice, were injected into Rag-2−/− recipients, alone or in association with WT or hTG Treg. Mice were kept for up to 10 weeks and disease activity index was monitored throughout. [A] Mean+SEM disease activity index in Rag-2−/− mice injected with CD45RBhigh [n = 5], CD45RBhigh plus WT Treg [n = 10] or CD45RBhigh plus hTG Treg [n = 10]. [B] Mean ± SEM colon length [cm] at the time of harvesting. [C] Haematoxylin and eosin staining of colon sections [original magnification, ×10]; arrows indicate the area magnified in the insets [×20]; mean ± SEM histology score at the time of harvesting is also shown. *p ≤ 0.05;**p ≤ 0.01;***p ≤ 0.001.
Overall, these data show that overexpression of hCD39 has beneficial effects on T-cell transfer as well as the recovery phase of DSS colitis.
3.4. Overexpression of hCD39 protects from deleterious effects of hypoxia
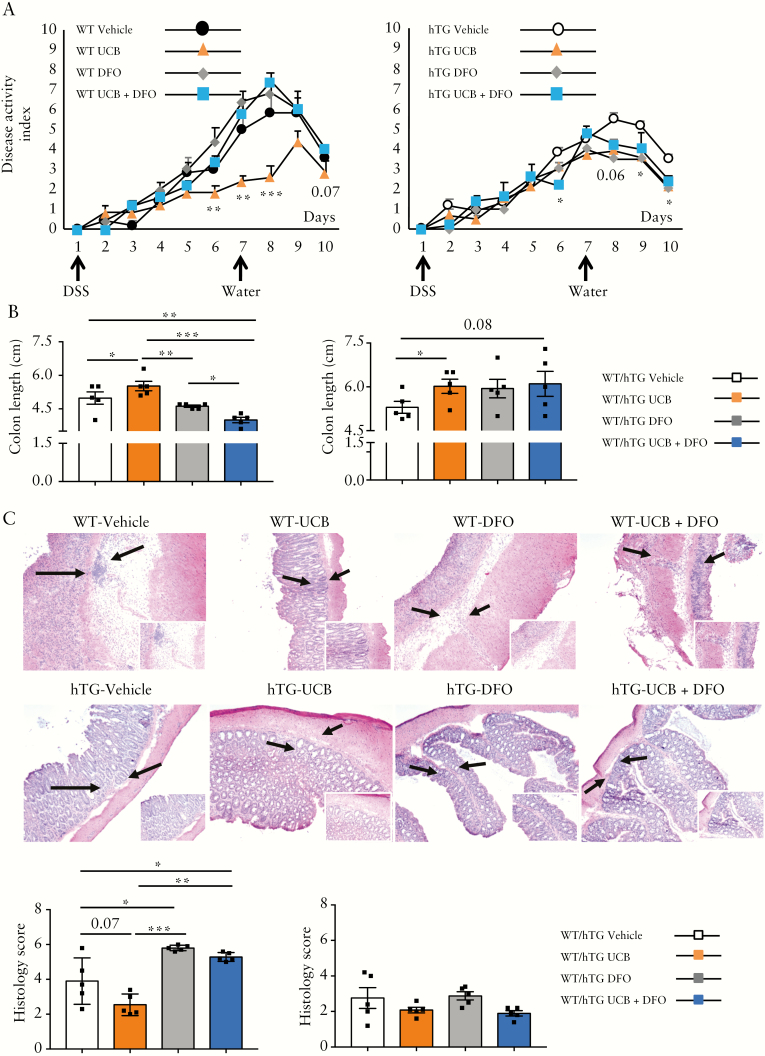
In the context of protracted inflammation, hypoxia has deleterious effects by limiting UCB-induced AhR activation, with consequent impairment in the ability of T cells to upregulate CD39.13 Here we tested whether overexpression of hCD39 could antagonize the inhibitory effects of hypoxia, systemically induced upon administration of deferoxamine, an iron chelator. Deferoxamine was administered alone or in combination with UCB to hTG and WT mice during the induction and recovery phase of DSS colitis.
hTG mice treated with UCB had lower DAI compared to vehicle-treated mice on days 9 and 10, and this beneficial effect remained unchanged in the presence of deferoxamine [Figure 5A]. In contrast, deferoxamine- or deferoxamine plus UCB-treated WT animals displayed a worsened disease course [Figure 5A]. DAI in the presence of deferoxamine and UCB was lower in hTG than WT mice on days 8, 9 and 10 [p < 0.05 for all]. Similar deleterious effects of deferoxamine were noted in WT but not hTG mice, when analysing colon length and histology score [Figure 5B,C]. Compared to vehicle and/or UCB-treated animals, WT mice administered deferoxamine alone displayed: a decreased frequency of CD4 cells positive for FOXP3 in the spleen, MLNs, IELs and LPs [Supplementary Figure 7A]; reduced proportions of CD4 cells positive for mCD39 in the spleen, MLNs and IELs [Supplementary Figure 7B]; decreased frequencies of CD4+IL17+ cells in the spleen and MLNs [Supplementary Figure 7C]; and reduced proportions of CD4+IL10+ cells in the MLNs and IELs [Supplementary Figure 7E]. When deferoxamine was combined with UCB, some of these effects were reverted, as indicated by increased frequencies of CD4+mCD39+ cells in MLNs and LPs [Supplementary Figure 7B] and of CD4+IL10+ lymphocytes in the IEL and LP compartments; while others were worsened as indicated by heightened proportions of CD4+IL17+ and CD4+IFNγ + lymphocytes in LPs [Supplementary Figure 7C,D].
Figure 5.
hCD39 overexpression protects against deleterious systemic effects of deferoxamine-induced hypoxia. WT [n = 24] and hTG [n = 24] mice were exposed to 3% DSS for 6 days and then to normal water for four additional days. For the whole duration of the experiment, mice were administered vehicle [n = 6], UCB [n = 6], deferoxamine [DFO, n = 6], or UCB plus DFO [n = 6]. [A] Mean+SEM disease activity index in vehicle, UCB-, DFO- and UCB plus DFO-treated WT and hTG mice. [B] Mean ± SEM colon length [cm] at the time of harvest. [C] Haematoxylin and eosin staining of colon sections [original magnification, ×10]; arrows indicate the area magnified in the insets [×20]; mean ± SEM histology score at the time of harvesting is also shown. *p ≤ 0.05;**p ≤ 0.01;***p ≤ 0.001.
In hTG mice, no substantial changes were noted when deferoxamine was administered alone, apart from an increase in the proportion of CD4+IL10+ cells in LPs [Supplementary Figure 7E]. In the same compartment, when deferoxamine was associated with UCB, we noted increased proportions of CD4+FOXP3+ and CD4+IL10+ cells [Supplementary Figure 7A,E] and decreased frequencies of CD4+IFNγ + lymphocytes [Supplementary Figure 7D].
Collectively, these data show that hCD39 overexpression is protective in the setting of systemic hypoxia and this favours maintenance of responses to AhR stimulation, as with UCB.
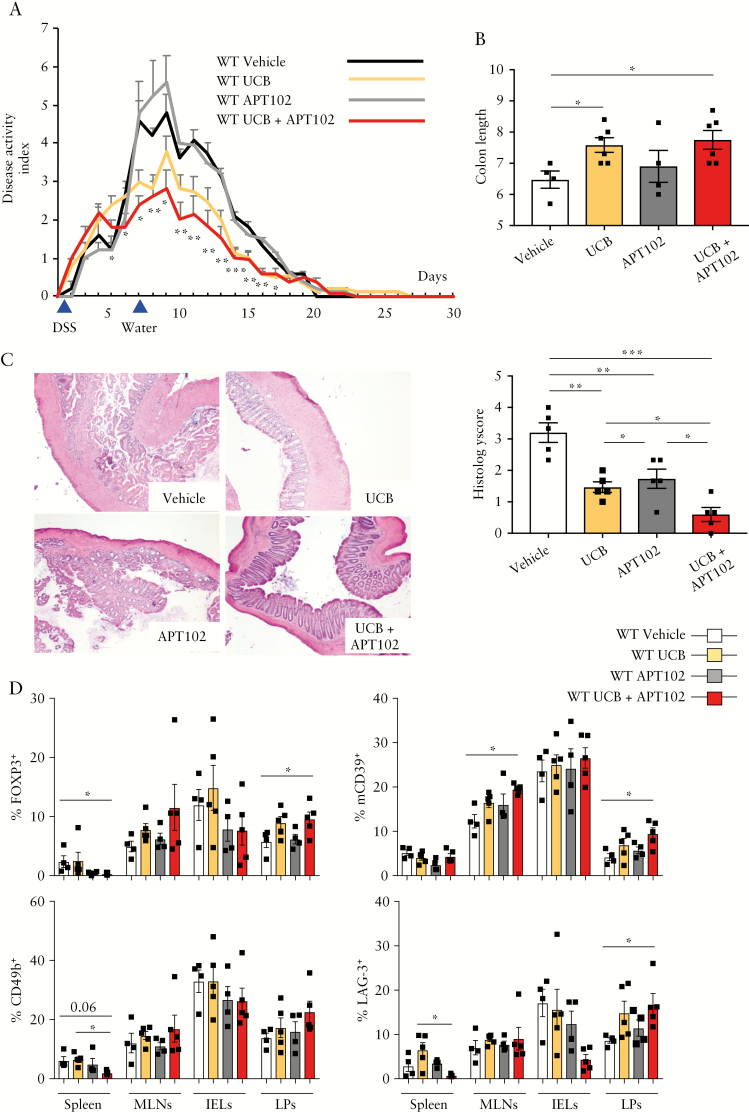
3.5. APT102 augments the benefit of UCB in experimental colitis
As we noted increased response to UCB in those WT Treg exposed to APT102 [Figure 2G], we then tested the effects of APT102 administration, alone or in combination with UCB, in the DSS colitis model. APT102 was well tolerated and no toxicity was noted throughout the experiment. Combination treatments with APT102 and UCB resulted in amelioration of DAI during the recovery phase of colitis, compared to animals treated with vehicle or APT102 [days 7–17] or UCB [days 5–7] alone [Figure 6A], and also in greater colon length and lower histology score, when compared to vehicle or APT102-treated mice [Figure 6B,C]. Colon length was similar and histology score was lower compared with UCB-treated animals [Figure 6B,C]. Compared to vehicle, combinations of APT102 and UCB decreased the frequency of CD4+FOXP3+ cells in the spleen while increasing the frequency within LPs [Figure 6D]; this combination augmented the proportion of CD4+mCD39+ lymphocytes within MLNs and LPs [Figure 6D] and that of CD4+LAG-3+ cells within LPs. Compared to UCB only, combinations of APT102 and UCB decreased the proportion of CD4+CD49b+ and CD4+LAG-3+ cells in the spleen [Figure 6D]. Furthermore, exposure to UCB and APT102 resulted in lower frequencies of CD4+IL17+ cells in the spleen but a higher proportion of these cells in MLNs [Supplementary Figure 8A]; no changes were noted in the frequency of CD4+IFNγ + and CD4+IL10+ cells [Supplementary Figure 8B,C].
Figure 6.
APT102 boosts UCB immunoregulatory properties in DSS colitis in vivo. WT mice were treated with 2% DSS for 6 days. DSS treatment was then replaced with normal water for an additional 24 days. For the whole duration of the experiment, mice were exposed to vehicle [n = 4], UCB [n = 6], APT102 [n = 4] or UCB plus APT102 [n = 6]. [A] Mean+SEM disease activity index. Asterisks indicate statistical significance as determined by ANOVA. [B] Mean ± SEM colon length [cm]. [C] Haematoxylin and eosin staining of colon sections [original magnification, ×10]; mean ± SEM histology score at the time of harvesting is also shown. [D] Mean ± SEM frequency of CD4+FOXP3+, CD4+mCD39+, CD4+CD49b and CD4+LAG-3+ lymphocytes within spleen, MLN, IEL and LP mononuclear cells. *p ≤ 0.05;**p ≤ 0.01;***p ≤ 0.001.
3.6. Addition of APT102 enhances AhR stimulation in Crohn’s-derived Treg and Tr1 cells
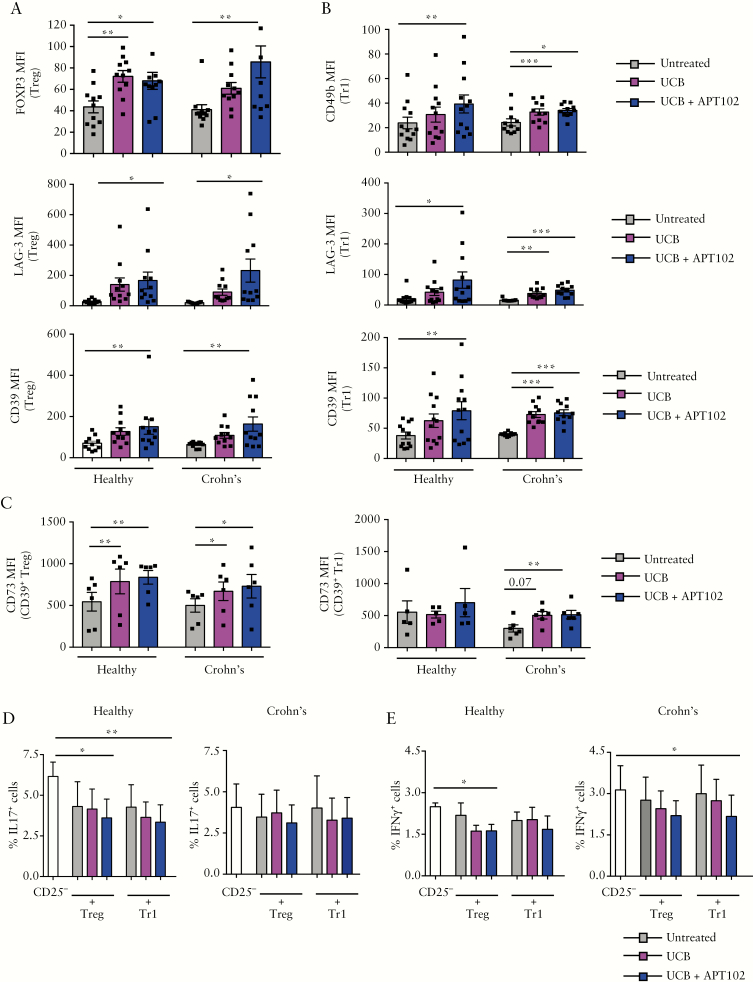
APT102 boosted the response of WT Treg to UCB-induced AhR activation in vitro [Figure 2G]. Hence, we tested the effects of APT102 on the response to UCB of Treg and Tr1 cells, derived from the peripheral blood and lamina propria of Crohn’s disease patients.
Regarding peripheral blood-derived cells, we noted that exposure of Treg to a combination of UCB and APT102 boosted FOXP3, LAG-3 and CD39 MFI compared to baseline levels in both healthy subjects and Crohn’s disease samples [Figure 7A and Supplementary Figure 9A]. Treg from healthy subjects upregulated FOXP3 levels also upon addition of UCB only [Figure 7A]. Similar to Treg, addition of UCB and APT102 to Tr1 cells increased CD49b, LAG-3 and CD39 levels in healthy subjects and in Crohn’s disease samples [Figure 7B and Supplementary Figure 9B]. The combination of UCB and APT102 boosted CD73 MFI in CD39+ Treg of healthy subjects and Crohn’s patients as well as in CD39+ Tr1-cells from Crohn’s disease patients [Figure 7C].
Figure 7.
APT102 promotes additional AhR activation in Treg and Tr1 cells from Crohn’s disease patients. Treg and Tr1 cells were obtained from peripheral blood-derived CD4+ lymphocytes of healthy subjects and Crohn’s disease patients. Mean ± SEM [A] FOXP3, LAG-3 and CD39 MFI in untreated, UCB- or UCB plus APT102-treated Treg; [B] CD49b, LAG-3 and CD39 MFI in untreated, UCB- or UCB plus APT102-treated Tr1 cells [healthy subjects, n = 11 for Treg, n = 12 for Tr1 cells; Crohn’s patients, n = 11]. [C] Mean ± SEM CD73 MFI of untreated, UCB- and UCB plus APT102-treated CD39+ Treg and CD39+ Tr1 cells [healthy subjects, n = 6 for Treg and n = 5 for Tr1 cells; Crohn’s patients, n = 6]. Mean ± SEM frequency of [D] IL17 and [E] IFNγ-producing cells within CD4+CD25− cells in the absence or presence of untreated, UCB- or UCB plus APT102-treated Treg and Tr1 cells [healthy subjects n = 5; Crohn’s patients n = 5]. *p ≤ 0.05;**p ≤ 0.01;***p ≤ 0.001.
No differences were noted in CD39, FOXP3, CD49b and LAG-3 in Treg and Tr1 cells exposed to APT102 only [data not shown]. APT102 treatment tended to increase CD73 MFI in CD39+ Tr1 cells from Crohn’s disease patients [299 ± 57 vs 444 ± 71, p = 0.07].
There was higher FOXP3 MFI in Treg from patients treated with azathioprine/6-mercaptopurine and higher CD49b MFI in Tr1 cells from anti-tumour necrosis factor α-treated patients compared to patients whose drug regimens did not include these two drugs [FOXP3 MFI: 72.8 ± 9.8 vs 51.6 ± 5.4, p = 0.09; CD49b MFI: 30.1 ± 2.3 vs 22.8 ± 3.7; p = 0.007]. Furthermore, CD39 and CD49b MFI in Tr1 cells tended to be positively correlated with the duration of infliximab therapy [p = 0.1 in both cases].
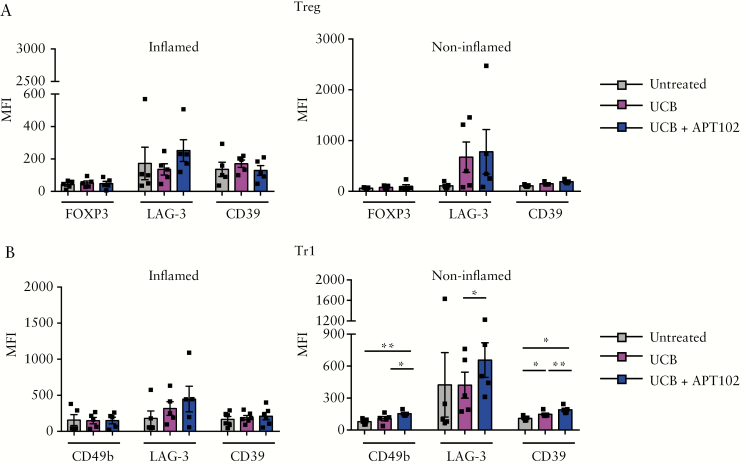
In healthy subjects, addition of APT102 improved the ability of UCB to enhance Treg and Tr1 cell suppression of IL17 and, in Treg, also IFNγ production by CD4+CD25− responder cells [Figure 7D,E]. In Crohn’s disease, treatment with APT102 and UCB boosted the ability of Tr1 cells to suppress IFNγ production by responders [Figure 7E]. In lamina propria-derived Treg and Tr1 cells obtained from ‘inflamed’ biopsied areas, no changes were noted in the expression of T-cell lineage-defining markers and CD39 after cell exposure to UCB alone or in combination with APT102 [Figure 8A,B]. In contrast, when lamina propria-derived cells were obtained from ‘non-inflamed’ biopsied areas [used as internal controls] we noted that addition of APT102 significantly increased the expression of CD49b, LAG-3 and CD39 in Tr1 cells concomitantly exposed to UCB [Figure 8B].
Figure 8.
Exposure to APT102 and UCB boosts the regulatory phenotype of lamina propria-derived Tr1 cells in Crohn’s disease. Treg and Tr1 cells were derived from lamina propria CD4 lymphocytes of Crohn’s disease patients [n = 6] and exposed to APT102 for 12 h in combination with UCB, added for the last 6 h of culture. Treg and Tr1 cells were obtained from both inflamed and non-inflamed biopsied areas. Mean ± SEM [A] FOXP3, LAG-3 and CD39 MFI in Treg; and [B] CD49b, LAG-3 and CD39 MFI in Tr1 cells. Addition of APT102 to UCB boosts CD49b, LAG-3 and CD39 MFI in Tr1 cells obtained from non-inflamed biopsied areas. *p ≤ 0.05;**p ≤ 0.01.
4. Discussion
We show here that overexpression of hCD39 boosts Treg function and bolsters responses to UCB in vitro, ameliorates the course of experimental colitis, while protecting against the deleterious effects of systemic hypoxia in vivo. These effects are mirrored by the ATPase/ADPase APT102 that synergizes with UCB to improve colitis in vivo and confer augmented suppressive properties to Crohn’s-derived regulatory cell subsets in vitro.
Our findings support an important role of CD39 in conferring benefit in experimental colitis in mice, as reflected by ameliorated histopathological features and experimental outcomes in both DSS and adoptive transfer models. hCD39 overexpression also results in greater suppressive abilities of adoptively transferred Treg, as well as in increased percentages of CD4+mCD39+ cells preferentially in the systemic compartment of DSS mice. This pattern was felt to be associated with heightened local levels of adenosine that might further induce native CD39 expression in a cAMP-dependent manner.23,30 These results mirror previous studies, in which hCD39 overexpression resulted in improved survival after transplant and myocardial ischaemia reperfusion and after warm renal ischaemia reperfusion with preservation of kidney function in both murine and swine models.15,31
Beneficial effects of hCD39 overexpression were supported also by in vitro data showing enhanced ectoenzymatic properties of hTG Tr1 cells and Treg, when compared to the WT counterpart. These effects were further enhanced in Treg, where heightened suppressor ability was noted possibly as the result of increased A2B receptor levels that were previously associated with enhanced Treg differentiation and expansion.4,32
hCD39 overexpression confers an advantage also in the response to UCB, a prototypic metabolite that serves as an AhR endogenous ligand.8,9 This effect is mimicked by APT102, an optimized form of human apyrase with preferential ADPase activity.17 Addition of APT102 boosted UCB immunoregulatory properties of WT Treg, mirroring the findings observed in hTG Treg. In Crohn’s-derived samples, treatment with APT102 enhanced the regulatory properties of UCB-AhR signalling in peripheral blood-derived Treg and Tr1 cells and in Tr1 cells from the lamina propria. The predominant effect of APT102 on Tr1 cells in Crohn’s-derived samples might be beneficial given that Tr1 cells are the main regulatory subset during protracted inflammation and are involved in the healing process of tissue injury. As we previously reported for Th17 cells,8 treatment with UCB alone did not result in major changes in the phenotypes of regulatory cell subsets obtained from the inflamed biopsied areas. These data indicate the need for initial core immunosuppression to contain the effects of pro-inflammatory mediators and to enable immunomodulatory strategies such as APT102 to succeed. This is supported by the evidence of higher Treg FOXP3 MFI and increased Tr1 CD49b MFI in patients treated with azathioprine/6-mercaptopurine and infliximab respectively, and by a trend towards positive correlation between the duration of infliximab therapy and Tr1 CD39 and CD49b MFI. Whether the inability to upregulate CD39 by Treg or Tr1 cells obtained from inflamed biopsied areas results from aberrant upregulation of gut homing receptors cannot be ruled out and awaits further investigation; in this regard we have previously reported that a genetic deficiency of CD39 is associated with an aberrant increase in α4β7 in murine hepatic CD8 cells.33 Should future data indicate heightened expression of gut homing receptors in lamina propria-derived regulatory cell subsets from Crohn’s disease patients, combinatorial treatment with antibodies targeting these molecules would be adequate.
In vivo, treatment with APT102 augmented the benefits of UCB, resulting in significant amelioration of histopathological scores and boosting of immunoregulatory markers in the LP compartments of WT colitic mice, further supporting the salutary immunoregulatory effects of this exogenous ADPase.
Another important finding of this study derives from the observation that hCD39 overexpression protects animals from hypoxic exacerbation of colitis triggered by deferoxamine, without abrogating the salutary effects of UCB. In contrast, exposure of WT mice to deferoxamine worsens colitis, also in the presence of UCB, and results in downregulation of FOXP3, mCD39 and IL10 in CD4 cells of both systemic and local colonic compartments. The effects of deferoxamine, alone or in combination with UCB, on the phenotype of CD4 cells from hTG mice appear to mainly impact the colonic compartment, as reflected by the paradoxical decrease in IFNγ and increases in FOXP3 and IL10 levels. Enhancement of FOXP3+ Treg under hypoxic conditions is known to occur,34 suggesting differential effects of hypoxia on select cell subtypes.13,35,36
In conclusion, we provide evidence for protective roles of hCD39 overexpression during intestinal inflammation by abrogating the deleterious effects of hypoxia while preserving beneficial responses to AhR ligation, resulting in further expression of native CD39. We also note that increased ATPase/ADPase activity administered in the form of APT102 boosts Treg and Tr1 cells in Crohn’s disease. These data support the use of APT102 and related compounds together with modulated AhR-signalling to both preserve and enable T-lymphocyte regulatory properties in IBD. Such combined approaches might thereby maintain immune-tolerance and quiescence of disease activity in this important chronic inflammatory condition.
Funding
This work was supported by the National Institutes of Health [R01 DK108894 to M.S.L.; P01 HL107152 and R21 CA164970 to S.C.R.]; American Association for the Study of Liver Diseases Pilot Research Award [to M.S.L.]; the Helmsley Charitable Trust [grant 281574.5069091.0010 to A.C.M. and S.C.R.]; and by the Department of Defense Award W81XWH-16–0464 [to S.C.R.].
Conflict of Interest
R.J.R., S.M., M.V., A.X., R.H., P.C., E.C., Y.W., A.C.M., S.C.R. & M.S.L.: nothing to disclose; R.C.: a shareholder of APT Therapeutics.
Author Contributions
R.J.R., S.M., M.V.: acquisition of data; analysis and interpretation of data; drafting of the manuscript; A.X., R.H., E.C.: acquisition of data; P.C., Y.W., R.C., A.C.M., S.C.R., M.S.L.: critical revision of the manuscript for important intellectual content.
Supplementary Material
Acknowledgments
We thank Barbora Gromova and Dr Luiza Abrahão Frank for technical assistance with some of the experiments.
References
- 1. Josefowicz SZ, Niec RE, Kim HY, et al.. Extrathymically generated regulatory T cells control mucosal TH2 inflammation. Nature 2012;482:395–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Maillard MH, Cotta-de-Almeida V, Takeshima F, et al.. The Wiskott-Aldrich syndrome protein is required for the function of CD4(+)CD25(+)Foxp3(+) regulatory T cells. J Exp Med 2007;204:381–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol 2005;6:1142–51. [DOI] [PubMed] [Google Scholar]
- 4. Deaglio S, Dwyer KM, Gao W, et al.. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med 2007;204:1257–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Allard B, Longhi MS, Robson SC, Stagg J. The ectonucleotidases CD39 and CD73: novel checkpoint inhibitor targets. Immunol Rev 2017;276:121–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gibson DJ, Elliott L, McDermott E, et al.. Heightened expression of CD39 by regulatory T lymphocytes is associated with therapeutic remission in inflammatory bowel disease. Inflamm Bowel Dis 2015;21:2806–14. [DOI] [PubMed] [Google Scholar]
- 7. Friedman DJ, Künzli BM, A-Rahim YI, et al.. From the Cover: CD39 deletion exacerbates experimental murine colitis and human polymorphisms increase susceptibility to inflammatory bowel disease. Proc Natl Acad Sci U S A 2009;106:16788–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Longhi MS, Vuerich M, Kalbasi A, et al.. Bilirubin suppresses Th17 immunity in colitis by upregulating CD39. JCI Insight 2017;2: pii: 92791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Phelan D, Winter GM, Rogers WJ, Lam JC, Denison MS. Activation of the Ah receptor signal transduction pathway by bilirubin and biliverdin. Arch Biochem Biophys 1998;357:155–63. [DOI] [PubMed] [Google Scholar]
- 10. Quintana FJ, Basso AS, Iglesias AH, et al.. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature 2008;453: 65–71. [DOI] [PubMed] [Google Scholar]
- 11. Veldhoen M, Hirota K, Westendorf AM, et al.. The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature 2008;453:106–9. [DOI] [PubMed] [Google Scholar]
- 12. Mascanfroni ID, Takenaka MC, Yeste A, et al.. Metabolic control of type 1 regulatory T cell differentiation by AHR and HIF1-α. Nat Med 2015;21:638–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xie A, Robles RJ, Mukherjee S, et al.. HIF-1α-induced xenobiotic transporters promote Th17 responses in Crohn’s disease. J Autoimmun 2018;94:122–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dwyer KM, Robson SC, Nandurkar HH, et al.. Thromboregulatory manifestations in human CD39 transgenic mice and the implications for thrombotic disease and transplantation. J Clin Invest 2004;113:1440–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Crikis S, Lu B, Murray-Segal LM, et al.. Transgenic overexpression of CD39 protects against renal ischemia-reperfusion and transplant vascular injury. Am J Transplant 2010;10:2586–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Köhler D, Eckle T, Faigle M, et al.. CD39/ectonucleoside triphosphate diphosphohydrolase 1 provides myocardial protection during cardiac ischemia/reperfusion injury. Circulation 2007;116:1784–94. [DOI] [PubMed] [Google Scholar]
- 17. Moeckel D, Jeong SS, Sun X, et al.. Optimizing human apyrase to treat arterial thrombosis and limit reperfusion injury without increasing bleeding risk. Sci Transl Med 2014;6:248ra105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ibrahim M, Wang X, Puyo CA, et al.. Human recombinant apyrase therapy protects against canine pulmonary ischemia-reperfusion injury. J Heart Lung Transplant 2015;34:247–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sugimoto S, Lin X, Lai J, et al.. Apyrase treatment prevents ischemia-reperfusion injury in rat lung isografts. J Thorac Cardiovasc Surg 2009;138:752–9. [DOI] [PubMed] [Google Scholar]
- 20. Ji Y, Adeola O, Strawn TL, Jeong SS, Chen R, Fay WP. Recombinant soluble apyrase APT102 inhibits thrombosis and intimal hyperplasia in vein grafts without adversely affecting hemostasis or re-endothelialization. J Thromb Haemost 2017;15:814–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mizumoto N, Kumamoto T, Robson SC, et al.. CD39 is the dominant Langerhans cell-associated ecto-NTPDase: modulatory roles in inflammation and immune responsiveness. Nat Med 2002;8:358–65. [DOI] [PubMed] [Google Scholar]
- 22. Uluçkan O, Eagleton MC, Floyd DH, et al.. APT102, a novel adpase, cooperates with aspirin to disrupt bone metastasis in mice. J Cell Biochem 2008;104:1311–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Longhi MS, Moss A, Bai A, et al.. Characterization of human CD39+ Th17 cells with suppressor activity and modulation in inflammatory bowel disease. PLoS One 2014;9:e87956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J Immunol 2003;170:3939–43. [DOI] [PubMed] [Google Scholar]
- 25. Liberal R, Grant CR, Yuksel M, et al.. Regulatory T-cell conditioning endows activated effector T cells with suppressor function in autoimmune hepatitis/autoimmune sclerosing cholangitis. Hepatology 2017;66:1570–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Clemente-Casares X, Blanco J, Ambalavanan P, et al.. Expanding antigen-specific regulatory networks to treat autoimmunity. Nature 2016;530:434–40. [DOI] [PubMed] [Google Scholar]
- 27. Zhang Y, Morris KL, Sparrow SK, et al.. Defective renal water handling in transgenic mice over-expressing human CD39/NTPDase1. Am J Physiol Renal Physiol 2012;303:F420–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Vaeth M, Müller G, Stauss D, et al.. Follicular regulatory T cells control humoral autoimmunity via NFAT2-regulated CXCR5 expression. J Exp Med 2014;211:545–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Künzli BM, Bernlochner MI, Rath S, et al.. Impact of CD39 and purinergic signalling on the growth and metastasis of colorectal cancer. Purinergic Signal 2011;7:231–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liao H, Hyman MC, Baek AE, Fukase K, Pinsky DJ. cAMP/CREB-mediated transcriptional regulation of ectonucleoside triphosphate diphosphohydrolase 1 (CD39) expression. J Biol Chem 2010;285:14791–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wheeler DG, Joseph ME, Mahamud SD, et al.. Transgenic swine: expression of human CD39 protects against myocardial injury. J Mol Cell Cardiol 2012;52:958–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ehrentraut H, Westrich JA, Eltzschig HK, Clambey ET. Adora2b adenosine receptor engagement enhances regulatory T cell abundance during endotoxin-induced pulmonary inflammation. PLoS One 2012;7: e32416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Peng ZW, Rothweiler S, Wei G, et al.. The ectonucleotidase ENTPD1/CD39 limits biliary injury and fibrosis in mouse models of sclerosing cholangitis. Hepatol Commun 2017;1:957–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Clambey ET, McNamee EN, Westrich JA, et al.. Hypoxia-inducible factor-1 alpha-dependent induction of FoxP3 drives regulatory T-cell abundance and function during inflammatory hypoxia of the mucosa. Proc Natl Acad Sci U S A 2012;109:E2784–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Caldwell CC, Kojima H, Lukashev D, et al.. Differential effects of physiologically relevant hypoxic conditions on T lymphocyte development and effector functions. J Immunol 2001;167:6140–9. [DOI] [PubMed] [Google Scholar]
- 36. Lukashev D, Klebanov B, Kojima H, et al.. Cutting edge: hypoxia-inducible factor 1alpha and its activation-inducible short isoform I.1 negatively regulate functions of CD4+ and CD8+ T lymphocytes. J Immunol 2006;177:4962–5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.