Abstract
Purpose:
The primary objective of this study was to determine the rate of pathological response after preoperative celecoxib and concurrent taxane-based chemotherapy in patients with cancer of the esophagus and gastroesophageal junction.
Methods:
Thirty-nine patients were enrolled in this single-arm, phase II clinical trial. Patients were administered daily celecoxib in combination with two to three cycles of carboplatin and paclitaxel with preoperative intent. Levels of cyclooxygenase (COX)-2 expression in resected tumors were analyzed by immunohistochemistry and correlated with clinical outcome measures. Postoperatively, patients were administered daily celecoxib for 1 year or until documented tumor recurrence.
Results:
All patients received two to three cycles of chemotherapy plus celecoxib 800 mg/d. Toxicities were as expected. A major clinical response (complete response + partial response) was noted in 22 patients (56%); six patients (15%) had a complete clinical response. Thirty-seven patients underwent esophagectomy. Five patients had a major pathological response (12.8%). Four-year overall and disease-free survivals were 40.9% and 30.3%, respectively. Patients with tumors expressing COX-2 demonstrated a higher likelihood of a major clinical response response (62% versus 50%) and an improved overall survival, compared with patients with COX-2-negative tumors.
Conclusions:
Preoperative celecoxib with concurrent chemotherapy demonstrated sufficient effect on pathologic response to warrant further study. Patients with tumors expressing COX-2 demonstrated trends toward improved response to preoperative therapy and improved overall survival compared with nonexpressors.
Keywords: COX-2, Celecoxib, Esophageal cancer
Carcinoma of the esophagus is associated with a poor prognosis.1 Five-year survival rarely exceeds 15 to 20% after surgery alone and is only modestly improved if surgery is preceded by chemotherapy or chemoradiotherapy.2–11 Therefore, there is a clear need for the development of novel treatment strategies to improve outcomes for patients with esophageal cancer. Considerable data indicate that cyclooxygenase (COX)-2, an early response gene induced by various mitogenic and inflammatory stimuli, is a potential therapeutic target in a number of solid tumors including esophageal cancer.12–26 For example, increased expression of COX-2 is observed in 70 to 80% of esophageal adeno- and squamous cell carcinomas of the esophagus, and COX-2 overexpression is independently associated with significantly diminished survival of patients with esophageal cancer.21,27–31 The negative impact of COX-2 expression on survival may be due, in part, to its key role in intratumoral prostaglandin synthesis. Prostaglandins are known tumor promoters that enhance cancer growth by inhibition of apoptosis, and stimulation of angiogenesis, and metastasis.17,20–22
Increased expression of COX-2 has also been noted to occur in response to chemotherapy and radiotherapy, a phenomenon that may potentially reduce the efficacy of cytotoxic therapy.32–34 There is evidence from laboratory studies and some clinical trials that inhibition of COX-2 activity may enhance the cytotoxicity of chemotherapy agents and radiation therapy.35–40 This study was designed to test the hypothesis that celecoxib, administered at doses known to inhibit COX-2 function in vivo, would improve the pathological response rate mediated by preoperative taxane-based chemotherapy in patients with potentially resectable cancer of the esophagus and cardia. Additionally, we attempted to explore the potential efficacy of adjuvant celecoxib in reducing or delaying disease recurrence.
METHODS
Patients and Methods
From March 2003 to November 2005, 39 patients with histologically confirmed carcinoma of the esophagus and cardia were enrolled in a phase II, open-labeled, single-arm trial. Patients were considered eligible if their clinical stage was T2-T3/N0, T1-T3/ N1, or T1-T3/M1a-M1b (lymph). Patients with clinical T4 tumors or metastases to distant organ sites were not eligible. In all patients, pretreatment evaluation included a complete history and physical examination, computed tomography of the chest and upper abdomen, 18 fluoro-deoxyglucose positron emission tomography, and upper gastrointestinal endoscopy. Additionally, every patient underwent esophageal endoscopic ultrasonography to determine malignant mural penetration and nodal involvement. Lymph node metastases were identified on the basis of standard echogenic criteria and/or fine needle aspiration cytology of suspicious nodes. Additional eligibility criteria included Karnofsky performance status ≥80%, acceptable hepatic, renal, and bone marrow function, absence of significant cardiovascular disease, and willingness to abstain from the use of steroidal or nonsteroidal antiinflammatory drugs (other than low-dose acetylsalicylic acid or inhaled steroids) for the duration of the study. The trial was approved by the institutional review board, and an informed consent document clearly describing the investigational nature of the treatment plan and the ability to withdraw from the study at anytime was signed by all patients. A separate institutional review board approval was obtained to enable immunohistochemical analysis of COX-2 expression in the resected tumors. All clinical and pathological staging was based on the sixth edition of the American Joint Committee on Cancer.41
Treatment Plan
Two to three cycles of paclitaxel and carboplatin were administered 21 days apart. Paclitaxel was administered as a 3-hour infusion at a dose of 200 mg/m2, followed by a 1-hour infusion of carboplatin dosed to an area under the curve of 6 by the Calvert formula. All patients were premedicated by dexamethasone, diphenhydramine, and H2-receptor antagonist.
Preoperative Celecoxib
Celecoxib was administered orally at a dose of 400 mg twice daily starting 3 to 7 days before the first day of chemotherapy and continued daily until the morning of surgery. Compliance was determined by pill counts at each follow-up visit, and patients were considered compliant if at least 80% of the drug was taken.
Surgical Resection
Esophagectomy with reconstruction of the gastrointestinal tract was planned to occur approximately 3 to 4 weeks after the last cycle of chemotherapy provided that restaging positron emission tomography and computed tomography scanning showed no evidence of disease progression.
Adjuvant Celecoxib
Celecoxib was administered orally at 400 mg twice daily starting no later than 3 months postoperatively provided there was adequate wound healing. Celecoxib was to be continued for 1 year or until documented tumor recurrence. Compliance with adjuvant celecoxib was determined by pill counts at each follow-up visit, and patients were considered compliant if at least 80% of the drug was taken. Following the publication of information regarding the frequency of cardiovascular adverse events associated with long-term use of celecoxib, all patients were required to sign a revised consent form that clearly incorporated all new information regarding adverse events possibly related to celecoxib, before starting the adjuvant part of the trial.42
Criteria for Response
A major clinical response was defined as either a complete or partial response, criteria for which are listed in Table 1. Major pathological response was defined as either a complete pathological response and/or minimal microscopic residual disease in the resected specimen. Absence of any tumor in the resected esophagus and lymph nodes on histological examination was considered a complete pathologic response. Minimal microscopic residual disease was defined as the presence of either microscopic foci of carcinoma in the absence of a mass or scattered foci of microscopic carcinoma, each not exceeding 2 mm in size, and in aggregate comprising no more than 10% of the tumor mass.
TABLE 1.
Criteria for Clinical Response
| Complete response | Disappearance of all clinical evidence of tumor on endoscopic examination and absence of activity on restaging PET scanning |
| Partial response | Fifty-percent reduction in the length of the primary tumor as determined by endoscopy |
| Stable disease | Less than 50% reduction in endoscopic tumor length |
| Disease progression | A 25% or greater increase in endoscopic tumor length |
PET, positron emission tomography.
Immunohistochemistry
Resected tissues were fixed in formalin, routinely processed, and embedded in paraffin. Sections were cut at 4 μm and dehydrated through alcohol gradients. Antigen retrieval was performed in a laboratory-grade microwave by boiling and incubating for 20 minutes in Target Retrieval Solution pH 9.0 (S2367; DAKO, Carpinteria, CA.) Endogenous peroxidase activity was inhibited by incubation in Dual Endogenous Enzyme Block (S2003; DAKO) for 10 minutes. Sections were then rinsed in a Tris-based wash buffer (S3006; DAKO) and incubated at room temperature for 1 hour with a Cox-2-specific murine monoclonal antibody (clone CX-294, M3617; DAKO) diluted 1:75. After washing in Tris buffer, samples were incubated with horseradish peroxidase-conjugated secondary antibody (EnvisionFlex/HRP; DAKO) for 1 hour at room temperature, rinsed in Tris buffer, and incubated in 3,3′-diaminobenzidine substrate buffer (DAKO) for 5 minutes at room temperature. Specimens were rinsed in H2O and counterstained with hematoxylin, dehydrated, and mounted. Colon carcinoma sections were used as positive controls. To confirm the specificity of results, additional sections were processed for staining without incubation with the primary anti-COX-2 antibody.
COX-2 expression in tumor tissues was scored using a 0 to 4+ scale, with no tumor cells stained = 0, and 1 to 25%, 26 to 50%, 51 to 75%, and more than 75% of positive cells being scored as 1+, 2+, 3+, and 4+, respectively. Results of preliminary histopathologic analysis were independently verified in a blinded manner by a second anatomic pathologist.
Statistical Considerations
Sample Size
The primary objective of this phase II trial was to determine the rate of major pathologic response, as previously defined, in the resected specimens of patients receiving preoperative paclitaxel/carboplatin/celecoxib. A Simon two-stage minimax design was applied to test the efficacy of the combination regimen.43 Thirty patients were to be enrolled in the first stage, and if one or no major pathologic responses were observed, the trial would be terminated early and declared to have a negative result. If two or more major pathologic responses were observed, then an additional nine patients (total 39) were to be enrolled. If on completion of the trial, five or more major pathologic responses were observed (≥13%) out of the 39 enrolled patients, the treatment would be declared effective and worthy of further study. This design yielded a 0.95 probability of a positive result (beta error = 0.05) if the true major pathologic response rate was ≥22% and a 0.95 probability of a negative result (alpha error = 0.05) if the true major pathologic response rate was ≤5%. With a total sample size of 39 patients, a 95% confidence interval (CI) could be constructed to be within ±11% of the true major pathologic response rate.
Statistical Analysis
Descriptive statistics were calculated for baseline demographic and clinical/prognostic characteristics. The χ2 test or Fisher’s exact test were used to assess the univariate association between categorical predictor variables and categorical outcome variables of interest (i.e., depending on the expected cell frequencies in the cross-tabulation analyses). The two-sample t test or the nonparametric Wilcoxon-rank sum test were used, as appropriate, to assess the univariate association between categorical predictor variables and continuous outcome variables of interest. Kaplan-Meier survival analysis was performed to evaluate disease-free survival (DFS) and overall survival (OS) for all study patients, and the log-rank test was used to compare DFS/OS between levels of clinical/prognostic factors of interest. DFS was defined as the time from first treatment day (i.e., date of first treatment with celecoxib) until tumor recurrence or death from any cause (or until date of last follow-up if no recurrence/death). OS was defined as the time from first treatment day (i.e., date of first treatment with celecoxib) until death from any cause (or until date of last follow-up if no death). All DFS and OS analyses were based on an intention-to-treat analysis (i.e., all patients were analyzed regardless of full compliance with celecoxib treatment). Median follow-up time for the study group was computed based on the surviving patients. Given the small sample size in the study, no multivariate analysis for DFS or OS could be performed because the number of recurrences/deaths in the cohort would not allow for multivariate models with stable estimates for the model coefficients. All p values were two sided with statistical significance evaluated at the 0.05 alpha level. Ninety-five percent CIs were calculated to assess the precision of the obtained estimates. All analyses were performed in SAS Version 9.2 (SAS Institute, Inc., Cary, NC) and STATA Version 11.0 (StataCorp, College Station, TX).
RESULTS
Thirty-nine patients were enrolled in this single-arm phase II trial. Patient’s characteristics are listed in Table 2. Median age was 63 years, and the majority had adenocarcinoma. Approximately two thirds of patients had either stage III or IV disease. Approximately 87% were men; and 72% of all patients in the study were smokers.
TABLE 2.
Patient Characteristics (n = 39)
| Patients | |
| Median age | 63 (38–76) |
| Males | 34 |
| Females | 5 |
| Smoking status | |
| Current | 9 |
| Former | 19 |
| Never | 11 |
| Clinical stage | |
| IIA (T2N0/T3N0) | 9 (3/6) |
| IIB (T2N1) | 4 |
| III (T3N1) | 20 |
| IVA (T3N0M1a/T4N1M1a) | 2 |
| IVB (T1N1M1ba/T3N1M1ba) | 4 (1/3) |
| Cell type | |
| Adenocarcinoma | 33 |
| Squamous cell carcinoma | 6 |
| Postsurgical staging (n = 37) | |
| yp 0 | 1 |
| yp I | 1 |
| yp IIA (T2N0M0/T3N0M0) | 9 (3/6) |
| yp IIB (T0N1M0/T2N1M0) | 6 (1/5) |
| yp III (T3N1M0/T4NxM0) | 16 (15/1) |
| yp IVA (T3N1M1a) | 1 |
| yp IVB (T1N0M1b/T3N1M1b/T4N1M1b) | 5 (1/3/1) |
The clinical M1b classification referred to nonregional lymph node metastases.
Preoperative Therapy
All patients received the per-protocol planned courses of chemotherapy. Twenty-eight patients received two cycles, and after a protocol amendment, 11 patients received three cycles. All patients completed the prescribed preoperative celecoxib treatment without incident.
Response to Induction Therapy
A major clinical response, as previously defined, was noted in 22 patients (56%). Six patients (15%) had a complete clinical response, with no clinical or histological evidence of carcinoma seen at endoscopy.
Surgical Treatment
Two patients were explored but not resected due to previously unsuspected airway invasion. An esophagectomy with mediastinal and abdominal lymph node dissection was performed in all remaining patients. Twenty patients had additional dissection of the cervical and superior mediastinal lymph nodes. The median number of resected lymph nodes was 30. Postsurgical staging is presented in Table 2. There was no operative or hospital mortality.
Pathological Response
Five patients had a major pathological response as previously defined (12.8%; 95% CI: 4.8–28.2%), which resulted in the primary end point of the study being met. A complete pathological response was noted in one patient, and four patients had minimal residual disease. Thirty-two patients had no discernable treatment effect on pathological examination, whereas two patients had disease progression precluding resection.
Treatment-Related Toxicity
There were no treatment-related deaths. Toxicities attributable to preoperative treatment are listed in Table 3. Five patients (15%) had pulmonary emboli, and 13 (33%) had atrial fibrillation in the perioperative period.
TABLE 3.
Neoadjuvant Grade 3 and 4 Events (n = 39)
| Adverse Events | Grade 3 | Grade 4 |
|---|---|---|
| Musculoskeletal | ||
| Myalgia | 2 | 0 |
| Constitutional | ||
| Fatigue | 1 | 0 |
| Cardiovascular | ||
| Hypotension | 1 | 0 |
| Atrial fibrillation | 1 | 0 |
| Syncope | 2 | 0 |
| Preoperative hematological | ||
| Neutropenia | 7 | 7 |
| Neutropenic fever | 3 | 2 |
| Anemia | 2 | 0 |
| Thrombocytopenia | 2 | 0 |
Adjuvant Celecoxib
Nine patients received adjuvant celecoxib treatment for less than 6 months, and 19 patients received adjuvant celecoxib for 6 months or more. Two patients who had been on celecoxib for 1 month and 8 months developed deep vein thrombosis and shortly thereafter exhibited recurrent disease. Eleven patients did not receive adjuvant celecoxib, of whom two patients developed deep vein thrombosis and subsequent disease recurrence.
Survival
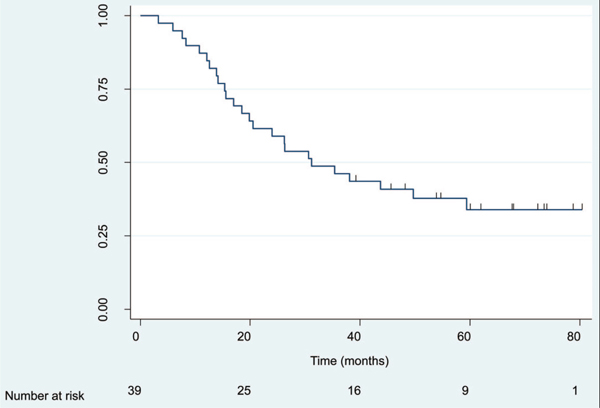
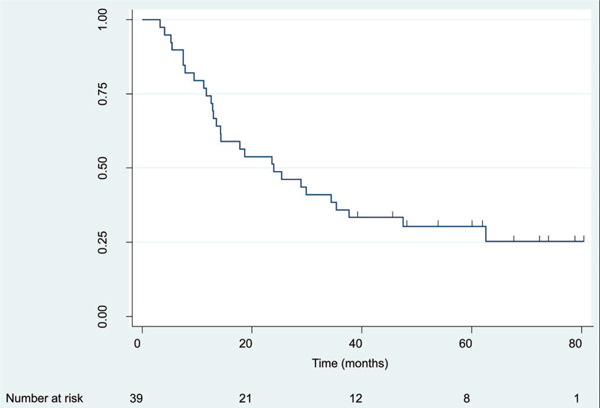
Median follow-up for surviving patients was 64 months (range: 39.0–67.2 months). Four-year OS and DFS were 40.9% (95% CI = 25.5–55.7%) and 30.3% (95% CI = 16.7–45.0%), respectively (Figures 1 and 2). Corresponding median OS and DFS were 31.1 months (95% CI = 18.5–59.4 months) and 24.0 months (95% CI = 13.0–37.7 months), respectively. There was a nonsignificant trend for improved OS in patients who had a major clinical response (CR + PR; N = 22) compared with patients who had minimal response or stable disease (median OS = 35.4 months versus 30.7 months, respectively, p = 0.20). Similar results were observed for improvement in DFS among patients with a major clinical response versus all others (median DFS = 25.4 months versus 18.7 months, respectively, p = 0.04). There was no difference in either OS or DFS between patients who received adjuvant celecoxib for less than 6 months versus 6 months or more (p = 0.75 for OS and p = 0.78 for DFS).
FIGURE 1.
Overall survival (OS) for the entire cohort. N = 39 patients, 25 deaths. Median OS time 31.1 months (95% confidence interval [CI] = 18.5–59.4). Four-year OS = 40.9% (95% CI = 25.5–55.7%).
FIGURE 2.
Disease-free survival (DFS) for the entire cohort. N = 39 patients, 28 recurrences or deaths. Median DFS = 24.0 months (95% confidence interval [CI] = 13.0–37.7 months). Four-year DFS = 30.3% (95% CI = 16.7–45.0%).
COX-2 Immunohistochemistry
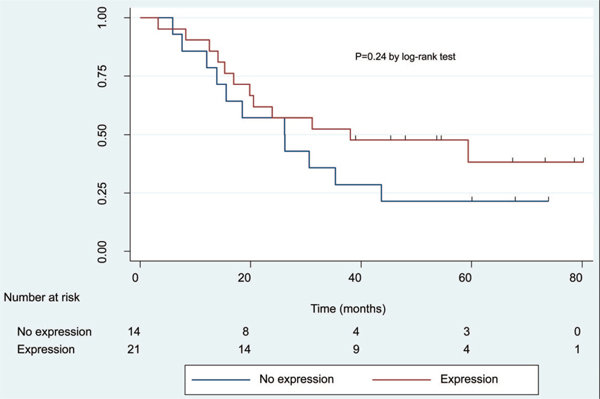
Tumor specimens were available from 35 patients for immunohistochemistry analysis of intratumoral COX-2 expression. Fourteen (40%) had no detectable COX-2 protein expression, whereas 21 (60%) had at least 10% of tumor cells showing cytoplasmic COX-2 staining. Patients who had COX-2 expressing tumors were more likely to have a major clinical response (13/21, 62%) than those with COX-2-negative tumors (7/14; 50%); however, the difference was not statistically significant Interestingly, tissue was available for COX-2 immunohistochemistry in three of the four patients who had microscopic residual disease on pathological examination; all three patients had COX-2 protein expression in their residual tumors. Furthermore, there was a nonsignificant trend favoring improved OS among patients whose tumors expressed COX-2 compared with nonexpressors. Specifically, median and 4-year OS survival were 38.1 months and 47.6%, respectively, in patients whose tumors expressed COX-2, versus 26.2 months and 21.4% for the nonexpressors (p = 0.24) (Figure 3). Median and 4-year DFS were also slightly higher for COX-2 expressors (23.7 months and 33.3%, respectively) versus nonexpressors (17.8 months and 21.4%, respectively, p = 0.42).
FIGURE 3.
Overall survival (OS) comparison between expressors and nonexpressors of cyclooxygenase (COX)-2. No expression: N = 14 patients, 11 deaths. Median OS time = 26.2 months (95% confidence interval [CI] = 12.1–43.7). Four-year OS = 21.4% (95% CI = 5.2–44.8%). Expression: N = 21 patients, 12 deaths. Median OS time = 38.1 months (95% CI = 17.0, not estimated). Four-year OS = 47.6% (95% CI = 25.7–66.7%).
DISCUSSION
Despite evidence that chemotherapeutic agents including taxanes are potent inducers of COX-2 and that abrogation of COX-2 activity may potentially enhance their cytotoxicity,32–34,40 trials examining the efficacy of COX-2 inhibitors administered in conjunction with chemotherapy or chemoradiotherapy have yielded mixed and mostly disappointing results.35,38,44–46 For example, in several phase II trials, the addition of celecoxib at 800 mg/d to 5-flourouracil and either irinotecan or oxaliplatin resulted in no improvements in response or survival in patients with metastatic colorectal cancer.44–46 In contrast, celecoxib enhanced the effects of preoperative taxane-based chemotherapy in patients with early-stage lung cancer.38 These conflicting results may be due to multiple factors including potentially complex pharmacodynamic interactions between celecoxib and different chemotherapeutic agents, the magnitude of intratumoral COX-2 expression, and ABC transporter expression.47–50 The significance of COX-2 expression in tumors treated by celecoxib was elegantly shown in a randomized phase II trial reported by Edelman et al.51 Patients with advanced lung cancer treated with gemcitabine and carboplatin were randomly assigned to receive celecoxib, the lipoxygenase inhibitor zeluton, or both of these drugs. In a prospectively planned subset analysis, patients with increased intratumoral COX-2 expression who were treated with celecoxib had significantly improved survivals compared with patients with COX-2-positive tumors not receiving celecoxib. Importantly, patients with tumors lacking COX-2 expression who received celecoxib had significantly worse survivals than those with COX-2-negative tumors who were not treated with celecoxib.
Despite the relatively small sample size in the current trial, it is interesting to note that celecoxib seemed to have a beneficial effect in terms of response and survival only in patients whose tumors expressed COX-2. Although the results fell short of statistical significance, patients with COX-2-positive tumors were more likely to experience a major clinical response and improvement in overall and DFS than those with COX-2-negative tumors. It is also of interest that all three patients with microscopic residual disease from whom tissue was available for IHC had elevated COX-2 expression in their tumors.
Although the trial met its primary objective of achieving complete pathological response and/or minimal residual disease in 13% of patients (i.e., ≥5 major pathologic responses out of 39 patients), several cautionary notes are warranted before further evaluation of celecoxib therapy in esophageal cancer. First, the data regarding the predictive benefit of COX-2 expression were derived from an unplanned posthoc analysis and was not a prespecified end point in the trial design. Furthermore, by necessity, COX-2 analysis was performed on posttreatment resected tissues rather than pretreatment biopsies; hence this observation will need to be verified in a larger trial with pre/posttreatment COX-2 expression as specified end points. Although our COX-2 expression may have been induced by chemotherapy, the hypothesis of the study examining the effect of abrogation of COX-2 activity on response would likely be unaffected by such induction. Second, the preoperative administration of celecoxib in this trial was associated with a higher than expected incidence of venous thromboembolic adverse events despite standard perioperative prophylactic measures.52 We did not observe a similar adverse events profile in a previous trial of preoperative celecoxib in patients with early stage lung cancer.38 Further trials evaluating the role of celecoxib in patients undergoing esophagectomy or other major abdominal surgery should include intensification of prophylactic measures to decrease the propensity for venous thromboembolic events and careful perioperative monitoring of cardiac arrhythmias. In this regard, it is important to point out that in the current trial, patients continued to take celecoxib up to the day of surgery. It is conceivable that stopping treatment at least a week before any planned surgical intervention might have blunted some of the prothrombotic effects of COX-2 inhibition. Finally, although this trial was not designed to address the benefit of adjuvant celecoxib, we were unable to detect a signal suggesting efficacy of the COX-2 inhibitor in either preventing or delaying cancer recurrence.
ACKNOWLEDGMENTS
Supported by a grant from Pfizer. Dr. Paul Christos was partially supported by the following grant: Clinical Translational Science Center (CTSC) (UL1-RR024996).
The authors thank Martha Quezado, MD, (National Institutes of Health) for independent confirmation of COX-2 immunohistochemistry results.
Footnotes
Disclosure: Dr. Brendon Stiles reports that after completion of the trial his spouse was subsequently hired by Pfizer, and Dr. Stiles has therefore made disclosures with regard to immediate family employment and stock ownership in this organization.
REFERENCES
- 1.Stewart BW, Kleihues P. World Cancer Report, Lyon, France: IARC Press, 2008. [Google Scholar]
- 2.Posner MC, Minsky BD, Ilson DH. Cancer of the esophagus, Ch. 39, Section 2 In T Vincent Devita J, Lawrence TS, et al. (Eds.), Devita, Hellman, & Rosenberg’s Cancer: Principles and Practice of Oncology. Philadelphia, PA: Lippincott Williams & Wilkins, 2008, pp. 993–1043. [Google Scholar]
- 3.Bates BA, Detterbeck FC, Bernard SA, et al. Concurrent radiation therapy and chemotherapy followed by esophagectomy for localized esophageal carcinoma. J Clin Oncol 1996;14:156–163. [DOI] [PubMed] [Google Scholar]
- 4.Bidoli P, Bajetta E, Stani SC, et al. Ten-year survival with chemotherapy and radiotherapy in patients with squamous cell carcinoma of the esophagus. Cancer 2002;94:352–361. [DOI] [PubMed] [Google Scholar]
- 5.Bosset JF, Gignoux M, Triboulet JP, et al. Chemoradiotherapy followedby surgery compared with surgery alone in squamous-cell cancer of the esophagus. N Engl J Med 1997;337:161–167. [DOI] [PubMed] [Google Scholar]
- 6.Kelsen DP, Ginsberg R, Pajak TF, et al. Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N Engl J Med 1998;339:1979–1984. [DOI] [PubMed] [Google Scholar]
- 7.Kelsen DP, Minsky B, Smith M, et al. Preoperative therapy for esophageal cancer: a randomized comparison of chemotherapy versus radiation therapy. J Clin Oncol 1990;8:1352–1361. [DOI] [PubMed] [Google Scholar]
- 8.Medical Research Council Oesophageal Cancer Working Group. Surgical resection with or without preoperative chemotherapy in oesophageal cancer: a randomised controlled trial. Lancet 2002;359: 1727–1733. [DOI] [PubMed] [Google Scholar]
- 9.Gebski V, Burmeister B, Smithers BM, et al. Survival benefits from neoadjuvant chemoradiotherapy or chemotherapy in oesophageal carcinoma: a meta-analysis. Lancet Oncol 2007;8:226–234. [DOI] [PubMed] [Google Scholar]
- 10.Law S, Fok M, Chow S, et al. Preoperative chemotherapy versus surgical therapy alone for squamous cell carcinoma of the esophagus: a prospective randomized trial. J Thorac Cardiovasc Surg 1997;114:210–217. [DOI] [PubMed] [Google Scholar]
- 11.Ancona E, Ruol A, Santi S, et al. Only pathologic complete response to neoadjuvant chemotherapy improves significantly the long term survival of patients with resectable esophageal squamous cell carcinoma: final report of a randomized, controlled trial of preoperative chemotherapy versus surgery alone. Cancer 2001;91:2165–2174. [PubMed] [Google Scholar]
- 12.Sharma S, Stolina M, Yang SC, et al. Tumor cyclooxygenase 2-dependent suppression of dendritic cell function. Clin Cancer Res 2003;9: 961–968. [PubMed] [Google Scholar]
- 13.Riedl K, Krysan K, Pold M, et al. Multifaceted roles of cyclooxygenase-2 in lung cancer. Drug Resist Updat 2004;7:169–184. [DOI] [PubMed] [Google Scholar]
- 14.Reddy BS, Rao CV, Seibert K. Evaluation of cyclooxygenase-2 inhibitor for potential chemopreventive properties in colon carcinogenesis. Cancer Res 1996;56:4566–4569. [PubMed] [Google Scholar]
- 15.Kawamori T, Rao CV, Seibert K, et al. Chemopreventive activity ofc elecoxib, a specific cyclooxygenase-2 inhibitor, against colon carcinogenesis. Cancer Res 1998;58:409–412. [PubMed] [Google Scholar]
- 16.Harris RE, Alshafie GA, Abou-Issa H, et al. Chemoprevention of breast cancer in rats by celecoxib, a cyclooxygenase 2 inhibitor. Cancer Res 2000;60:2101–2103. [PubMed] [Google Scholar]
- 17.Masferrer JL, Leahy KM, Koki AT, et al. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res 2000;60:1306–1311. [PubMed] [Google Scholar]
- 18.Alshafie GA, Abou-Issa HM, Seibert K, et al. Chemotherapeutic evaluation of celecoxib, a cyclooxygenase-2 inhibitor, in a rat mammary tumor model. Oncol Rep 2000;7:1377–1381. [DOI] [PubMed] [Google Scholar]
- 19.Steinbach G, Lynch PM, Phillips RK, et al. The effect of celecoxib, a cyclooxygenase-2 inhibitor, in familial adenomatous polyposis. N Engl J Med 2000;342:1946–1952. [DOI] [PubMed] [Google Scholar]
- 20.Dannenberg AJ, Subbaramaiah K. Targeting cyclooxygenase-2 in human neoplasia: rationale and promise. Cancer Cell 2003;4:431–436. [DOI] [PubMed] [Google Scholar]
- 21.Zimmermann KC, Sarbia M, Weber AA, et al. Cyclooxygenase-2 expression in human esophageal carcinoma. Cancer Res 1999;59:198–204. [PubMed] [Google Scholar]
- 22.Wilson KT, Fu S, Ramanujam KS, et al. Increased expression of inducible nitric oxide synthase and cyclooxygenase-2 in Barrett’s esophagus and associated adenocarcinomas. Cancer Res 1998;58:2929–2934. [PubMed] [Google Scholar]
- 23.Li Z, Shimada Y, Kawabe A, et al. Suppression of N-nitrosomethylbenzylamine (NMBA)-induced esophageal tumorigenesis in F344 rats by JTE-522, a selective COX-2 inhibitor. Carcinogenesis 2001;22:547–551. [DOI] [PubMed] [Google Scholar]
- 24.Sawaoka H, Kawano S, Tsuji S, et al. Cyclooxygenase-2 inhibitors suppress the growth of gastric cancer xenografts via induction of apoptosis in nude mice. Am J Physiol 1998;274:G1061–G1067. [DOI] [PubMed] [Google Scholar]
- 25.Oshima M, Dinchuk JE, Kargman SL, et al. Suppression of intestinal polyposis in Apc delta716 knockout mice by inhibition of cyclooxygenase 2 (COX-2). Cell 1996;87:803–809. [DOI] [PubMed] [Google Scholar]
- 26.Sandler AB, Dubinett SM. COX-2 inhibition and lung cancer. Semin Oncol 2004;31:45–52. [DOI] [PubMed] [Google Scholar]
- 27.Buskens CJ, Van Rees BP, Sivula A, et al. Prognostic significance of elevated cyclooxygenase 2 expression in patients with adenocarcinoma of the esophagus. Gastroenterology 2002;122:1800–1807. [DOI] [PubMed] [Google Scholar]
- 28.Takatori H, Natsugoe S, Okumura H, et al. Cyclooxygenase-2 expression is related to prognosis in patients with esophageal squamous cell carcinoma. Eur J Surg Oncol 2008;34:397–402. [DOI] [PubMed] [Google Scholar]
- 29.Altorki N. COX-2: a target for prevention and treatment of esophageal cancer. J Surg Res 2004;117:114–120. [DOI] [PubMed] [Google Scholar]
- 30.Altorki NK, Subbaramaiah K, Dannenberg AJ. COX-2 inhibition in upper aerodigestive tract tumors. Semin Oncol 2004;31:30–36. [DOI] [PubMed] [Google Scholar]
- 31.Xi H, Baldus SE, Warnecke-Eberz U, et al. High cyclooxygenase-2 expression following neoadjuvant radiochemotherapy is associated with minor histopathologic response and poor prognosis in esophageal cancer. Clin Cancer Res 2005;11:8341–8347. [DOI] [PubMed] [Google Scholar]
- 32.Altorki NK, Port JL, Zhang F, et al. Chemotherapy induces the expression of cyclooxygenase-2 in non-small cell lung cancer. Clin Cancer Res 2005;11:4191–4197. [DOI] [PubMed] [Google Scholar]
- 33.Subbaramaiah K, Hart JC, Norton L, et al. Microtubule-interfering agents stimulate the transcription of cyclooxygenase-2. Evidence for involvement of ERK1/2 AND p38 mitogen-activated protein kinase pathways. J Biol Chem 2000;275:14838–14845. [DOI] [PubMed] [Google Scholar]
- 34.Huang WZ, Fu JH, Wang DK, et al. Overexpression of cyclooxygenase-2 is associated with chemoradiotherapy resistance and prognosis in esophageal squamous cell carcinoma patients. Dis Esophagus 2008;21: 679–684. [DOI] [PubMed] [Google Scholar]
- 35.Schneider BJ, Kalemkerian GP, Kraut MJ, et al. Phase II study of celecoxib and docetaxel in non-small cell lung cancer (NSCLC) patients with progression after platinum-based therapy. J Thorac Oncol 2008;3: 1454–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mutter R, Lu B, Carbone DP, et al. A phase II study of celecoxib in combination with paclitaxel, carboplatin, and radiotherapy for patients with inoperable stage IIIA/B non-small cell lung cancer. Clin Cancer Res 2009;15:2158–2165. [DOI] [PubMed] [Google Scholar]
- 37.Tuynman JB, Buskens CJ, Kemper K, et al. Neoadjuvant selective COX-2 inhibition down-regulates important oncogenic pathways in patients with esophageal adenocarcinoma. Ann Surg 2005;242:840–849, discussion 849–850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Altorki NK, Keresztes RS, Port JL, et al. Celecoxib, a selective cyclooxygenase-2 inhibitor, enhances the response to preoperative paclitaxel and carboplatin in early-stage non-small-cell lung cancer. J Clin Oncol 2003;21:2645–2650. [DOI] [PubMed] [Google Scholar]
- 39.Milas L, Kishi K, Hunter N, et al. Enhancement of tumor response to gamma-radiation by an inhibitor of cyclooxygenase-2 enzyme. J Natl Cancer Inst 1999;91:1501–1504. [DOI] [PubMed] [Google Scholar]
- 40.Hida T, Kozaki K, Muramatsu H, et al. Cyclooxygenase-2 inhibitor induces apoptosis and enhances cytotoxicity of various anticancer agents in non-small cell lung cancer cell lines. Clin Cancer Res 2000;6:2006–2011. [PubMed] [Google Scholar]
- 41.Greene FL, Compton CC, Fritz AG, et al. AJCC Cancer Staging Atlas, 6th ed., New York, NY: Springer, 2006. [Google Scholar]
- 42.Solomon SD, McMurray JJ, Pfeffer MA, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med 2005;352:1071–1080. [DOI] [PubMed] [Google Scholar]
- 43.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials 1989;10:1–10. [DOI] [PubMed] [Google Scholar]
- 44.Kohne CH, De Greve J, Hartmann JT, et al. Irinotecan combined with infusional 5-fluorouracil/folinic acid or capecitabine plus celecoxib or placebo in the first-line treatment of patients with metastatic colorectal cancer. EORTC study 40015. Ann Oncol 2008;19:920–926. [DOI] [PubMed] [Google Scholar]
- 45.Maiello E, Giuliani F, Gebbia V, et al. FOLFIRI with or without celecoxib in advanced colorectal cancer: a randomized phase II study of the Gruppo Oncologico dell’Italia Meridionale (GOIM). Ann Oncol 2006;17(Suppl 7):vii55–vii59. [DOI] [PubMed] [Google Scholar]
- 46.Fuchs CS, Marshall J, Mitchell E, et al. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first-line treatment of metastatic colorectal cancer: results from the BICC-C Study. J Clin Oncol 2007;25:4779–4786. [DOI] [PubMed] [Google Scholar]
- 47.Robey RW, To KK, Polgar O, et al. ABCG2: a perspective. Adv Drug Deliv Rev 2009;61:3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Szczuraszek K, Materna V, Halon A, et al. Positive correlation between cyclooxygenase-2 and ABC-transporter expression in non-Hodgkin’s lymphomas. Oncol Rep 2009;22:1315–1323. [DOI] [PubMed] [Google Scholar]
- 49.An Y, Ongkeko WM. ABCG2: the key to chemoresistance in cancer stem cells? Expert Opin Drug Metab Toxicol 2009;5:1529–1542. [DOI] [PubMed] [Google Scholar]
- 50.Huang D, Gao Q, Guo L, et al. Isolation and identification of cancer stem-like cells in esophageal carcinoma cell lines. Stem Cells Dev 2009;18:465–473. [DOI] [PubMed] [Google Scholar]
- 51.Edelman MJ, Watson D, Wang X, et al. Eicosanoid modulation in advanced lung cancer: cyclooxygenase-2 expression is a positive predictive factor for celecoxib + chemotherapy—Cancer and Leukemia Group B Trial 30203. J Clin Oncol 2008;26:848–855. [DOI] [PubMed] [Google Scholar]
- 52.Altorki N, Kent M, Ferrara C, et al. Three-field lymph node dissection for squamous cell and adenocarcinoma of the esophagus. Ann Surg 2002;236:177–183. [DOI] [PMC free article] [PubMed] [Google Scholar]