Abstract
Study objectives
Women in the luteal phase of the menstrual cycle exhibit better cognitive performance overnight than women in the follicular phase, although the mechanism is unknown. Given the link between core body temperature (CBT) and performance, one potential mechanism is the thermoregulatory role of progesterone (P4), estradiol (E2), and their ratio (P4/E2), which change across the menstrual cycle. We examined the role of P4/E2 in modulating performance during extended wake in premenopausal women. Additionally, we compared the acute effects of nighttime light exposure on performance, CBT, and hormones between the menstrual phases.
Methods
Participants were studied during a 50 h constant routine and a 6.5 h monochromatic nighttime light exposure. Participants were 16 healthy, naturally cycling women (eight follicular; eight luteal). Outcome measures included reaction time, attentional failures, self-reported sleepiness, CBT, melatonin, P4, and E2.
Results
As compared to women in the luteal phase, women in the follicular phase exhibited worse performance overnight. CBT was significantly associated with performance, P4, and P4/E2 but not with other sex hormones. Sex hormones were not directly related to performance. Light exposure that suppressed melatonin improved performance in the follicular phase (n = 4 per group) to levels observed during the luteal phase and increased CBT but without concomitant changes in P4/E2.
Conclusions
Our results underscore the importance of considering menstrual phase when assessing cognitive performance during sleep loss in women and indicate that these changes are driven predominantly by CBT. Furthermore, this study shows that vulnerability to sleep loss during the follicular phase may be resolved by exposure to light.
Keywords: alertness, sleep, performance, menstrual cycle, core body temperature, reproductive hormones
Statement of Significance.
We examined a possible endocrine mechanism underlying the elevated performance impairment observed in women during the follicular phase of the menstrual cycle as compared to the luteal phase. We show that differences in performance may be driven by sex steroid-mediated changes in core body temperature across the menstrual cycle. Furthermore, we provide evidence that ocular light exposure that suppresses melatonin can restore overnight performance in women in the follicular phase to levels equivalent to those observed in the luteal phase. These data have important implications for understanding the occupational risks of shiftwork for women and provide a possible countermeasure for mitigating the performance impairments due to menstrual phase.
Introduction
Daily fluctuations in alertness and cognitive performance are associated with the 24 h rhythm in core body temperature (CBT), which displays a daily variation of approximately 1°C [1, 2]. On average, a 0.17°C increase in nocturnal CBT is associated with improvement in working memory, cognitive throughput, self-reported alertness, and reaction times [3]. In naturally menstruating women, the circadian rhythm in CBT is also affected by the phase of the menstrual cycle. As compared to the follicular phase, the circadian decrease in CBT during the night is attenuated in the luteal phase, resulting in a decreased amplitude of the 24 h CBT rhythm [4–7] and a ~0.4°C higher CBT overnight [4, 6, 8, 9]. Therefore, the ~0.4°C nocturnal increase in CBT associated with the luteal phase of the menstrual cycle would be expected to increase alertness and improve cognitive performance relative to the follicular phase during extended wakefulness. Two studies investigating the effects of menstrual phase on cognitive performance during sleep loss have confirmed this relationship and shown that women in the luteal phase of the menstrual cycle perform better than women in the follicular phase, particularly during the night [10, 11], but neither of these studies examined the role of reproductive hormones in mediating the differences in cognitive performance between menstrual phases.
The changes in CBT during the menstrual cycle are attributed to changes in progesterone (P4), which has a hyperthermic effect [12–15]. Circulating P4 concentrations are low throughout the follicular phase but increase during the luteal phase, which coincides with the elevation in CBT during the luteal phase [4, 8]. Moreover, estradiol (E2), through its hypothermic actions, has been shown to modify the hyperthermic effects of P4 such that combined administration of P4 and E2 leads to lower body temperatures than adminstration of P4 alone [12]. Accordingly, the P4/E2 ratio has been shown to be associated with CBT in naturally cycling women; specifically, an increase in the P4/E2 ratio across the menstrual cycle was associated with an increase in the 24 h average body temperature and a decrease in the amplitude of the CBT rhythm [16, 17]. The modulatory role of P4 and E2 on body temperature is, therefore, a potential mechanism through which menstrual phase-dependent differences in these hormones affect neurobehavioral performance. We, therefore, examined the relationship between P4, P4/E2 ratio, CBT, and neurobehavioral performance across the menstrual cycle.
Furthermore, light exposure also affects both neurobehavioral performance and CBT [18–20], but whether the beneficial effects of light on neurobehavioral performance depend on menstrual phase has not been examined. Additionally, therefore, we compared the effects of light exposure on neurobehavioral performance and concomitant changes in P4, P4/E2 ratio, and CBT between the follicular and luteal phases of the menstrual cycle.
Methods
Participants
Participants were 16 healthy women aged 19–29 years (mean age 22.94 ± 2.57 years), with 8 studied in the follicular phase and 8 in the luteal phase of their menstrual cycle. All participants underwent comprehensive physical, psychiatric, and medical screening including an ophthalmologic examination and Ishihara color blindness test. All participants self-reported a regular menstrual cycle lasting 26–35 days and were not using oral contraception (OC) for at least 3 months prior to the start of the study. Participant demographic information is shown in Table 1.
Table 1.
Participant demographics and menstrual phase allocation on constant routine and light exposure study days†
| Participant ID | Age | Bed time | DLMO | CBTmin | Menst cycle length | Constant routine menst. phase | Light exposure menst. phase |
|---|---|---|---|---|---|---|---|
| 22K7V | 19 | 23.97 | 23.12 | 5.70 | 30 | F | F |
| 26H6V* | 19 | 23.07 | 23.57 | 7.87 | 31.5 | F | L |
| 2614V | 22 | 24.00 | 23.49 | 5.63 | 28 | F | F |
| 26F2V | 22 | 24.07 | 24.12 | 5.10 | 33 | F | F |
| 25N6V | 23 | 22.67 | 20.87 | 2.10 | 28 | F | F |
| 2692V | 23 | 23.55 | 22.97 | 5.30 | 28 | F | F |
| 26G6V | 23 | 21.00 | 21.77 | 3.33 | 29 | F | F |
| 26G3V | 24 | 23.05 | 22.61 | 5.03 | 29 | F | F |
| 26R1V | 20 | 21.93 | 19.18 | 1.13 | 32.5 | L | L |
| 2622V | 22 | 22.68 | 21.10 | 4.13 | 27 | L | L |
| 2251V | 23 | 25.53 | 24.95 | 5.73 | 28 | L | L |
| 25Q2V | 23 | 23.00 | 23.23 | 3.53 | 27 | L | L |
| 22A1V | 24 | 22.02 | 21.93 | 4.60 | 29 | L | L |
| 22K3V* | 24 | 22.05 | — | 3.00 | 27 | L | F |
| 21B8V | 27 | 23.20 | 21.04 | 4.05 | 28 | L | L |
| 26P1V | 29 | 22.85 | 21.54 | 2.58 | 28 | L | L |
| Fol M (SD) | 21.88 (1.89) | 23.17 (1.02) | 22.81 (1.05) | 5.01 (1.71) | 29.56 (1.84) | n = 8 | n = 8 |
| Lut M (SD) | 24.00 (2.83) | 22.91 (1.17) | 21.85 (1.82) | 3.60 (1.39) | 28.31 (1.83) | n = 8 | n = 8 |
†CBTmin = core body temperature minimum; DLMO = dim light melatonin onset; menst. = menstrual; F = follicular phase; L = luteal phase. The mean ± SD of age, bedtime, DLMO, CBTmin, and menstrual cycle length for each menstrual phase is based on the menstrual phase classifications during CR. Allocation to the luteal phase was based on a cutoff of P4 > 3 ng/mL. The total number of participants (n) in each menstrual phase, follicular (Fol), and luteal (Lut) on different study days is presented in the bottom two rows. The participant IDs of women who changed menstrual phase during the study are marked with an asterisk (*).
To determine menstrual phase, daily average P4 levels were calculated for each participant for the constant routine (CR) and light exposure days, and women with P4 concentrations >3 ng/mL were considered to have ovulated [21, 22] and were assigned to the luteal phase. Based on these criteria, two women changed menstrual phase status between the CR and light exposure, one from follicular to luteal (with concomitant changes in follicle-stimulating hormone [FSH] and luteinizing hormone [LH] indicating ovulation) and one from luteal to follicular (Table 1). As the effect of light exposure on P4 is not well understood, the calculation of P4 concentrations for determining menstrual phase status on study days 6 and 7, when scheduled light exposure occurred, excluded all time points after the beginning of the light exposure. Individual and group-average hormone values are shown in Supplementary Figure S1.
For 3 weeks prior to the laboratory study, participants maintained a self-selected 8:16 h sleep/wake schedule that was confirmed with time-stamped call-ins at bed and wake times and with actigraphy (Actiwatch-L, Philips Respironics, Bend, OR) and sleep diaries for at least 7 days prior to entering the laboratory. Participants were asked to refrain from using any prescription and nonprescription medications, supplements, recreational drugs, caffeine, alcohol, or nicotine. Compliance was confirmed with a urine toxicology test during screening and upon admission to the laboratory. The study was approved by the Partners Human Research Committee (2007P000566) and the Monash University Human Research Ethics Committee (2018–13325) and written informed consent was given by participants before commencing the study.
Study protocol
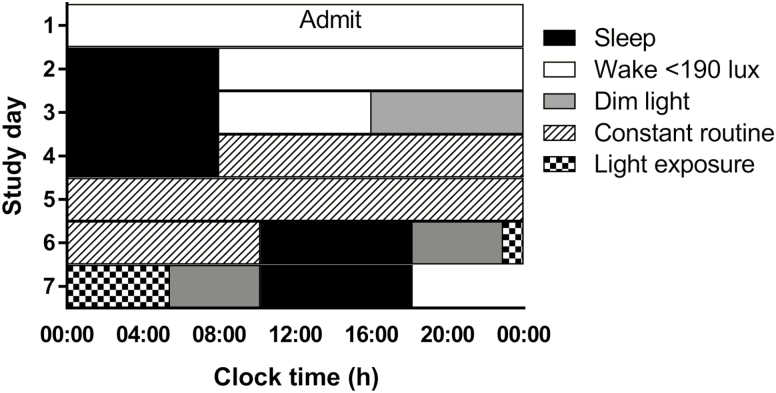
Participants were studied individually for 9 days in an environment free of time cues (no access to windows, clocks, live TV, radio, and internet and continually supervised by staff trained not to reveal the time). The study schedule consisted of (1) three baseline nights with 8:16 h sleep/wake schedule, (2) a 49 h 40 min CR, followed by an 8 h sleep opportunity, (3) a 6.5 h nighttime light exposure starting 4.75 h post-wake, followed by an 8 h sleep opportunity, and, finally, (4) a second 29 h 40 min CR followed by an 8 h sleep opportunity and then discharge. During the CRs, participants remained awake in a semirecumbent posture in dim light (<3 lux) and were fed hourly isocaloric snacks (150 mEq Na+/100mEq K+ (±20%); 1.3 × basal energy expenditure; 2000 mL fluids/24 h day). All study events were timed relative to the habitual wake time of each participant calculated from time-stamped call-ins and confirmed with actigraphy and sleep diaries prior to admitting. The current analyses are limited to data collected from the start of the first CR and until the end of the 6.5 h light exposure (Figure 1).
Figure 1.
Participants completed a 9 day laboratory protocol. The protocol is depicted in relative clock time with a relative bedtime of midnight. White bars represent wake episodes in <190 lux, black bars represent scheduled sleep episodes with lights off (0 lux), gray bars represent wake episodes in dim light (<3 lux) not under CR conditions, and bars with a diagonal pattern represent the CR in dim light (<3 lux). The checkered bar represents the 6.5 h light exposure occurring 4.75 h postwake on day 6. The wake episode for the CR includes an additional 30 min not under CR conditions, during which time participants prepared for bed. The study schedule consisted of (1) three baseline nights with 8:16 h sleep/wake schedule, (2) a 49 h 40 min CR, followed by an 8 h sleep opportunity, (3) a 6.5 h nighttime light exposure starting 4.75 h postwake, followed by an 8 h sleep opportunity, and, finally, (4) a second 29 h 40 min CR followed by an 8 h sleep opportunity and then discharge (study days not shown).
Lighting
Study lighting conditions have been described in detail previously [18, 23, 24]. During baseline days, maximum ambient light (ceiling mounted 4100 K fluorescent lamps F96T12/41U/HO/EW, 95 W; F32T8/ADV841/A, 32 W; F25T8/TL841, 25 W; Philips Lighting, Eindhoven, The Netherlands) during scheduled wake episodes was ~48 µW/cm2 (~190 lux) when measured vertically and ~23 µW/cm2 (~88 lux) when measured horizontally at a height of 187 and 137 cm, respectively. Midway through day 3, maximum ambient light was reduced to <0.4 µW/cm2 (<3 lux) when measured vertically and ~0.6 lux when measured horizontally. This level of light was maintained for the remainder of the study except during scheduled sleep episodes that occurred in darkness and during the light exposure on days 6 and 7 when ambient lighting was switched off.
The 6.5 h monochromatic light exposure (half peak bandwidth = 10–15 nm) began on day 6 of the protocol (Figure 1) starting 4.75 h after wake (or 9.25 h prior to respective wake time during each participants’ baseline days). Light wavelength and irradiance were selected as part of different studies examining the role of light on melatonin suppression and circadian phase resetting unrelated to menstrual phase [23, 25, 26]. In the current analysis, we compared neurobehavioral performance, CBT, and hormone levels in participants in whom light either did or did not induce melatonin suppression (≥33% or <33%, respectively) independent of the wavelength and irradiance. Participants were exposed to 420 nm (0.2–13.25 µW/cm2; follicular n = 3; luteal n = 1), 460 nm (2.37–55 µW/cm2; follicular n = 3; luteal n = 2), 507 nm (0.7–10.95 µW/cm2; follicular n = 0; luteal n = 2), or 555 nm (0.4–12.1 µW/cm2; follicular n = 2; luteal n = 3) light. Participants wore black-out goggles for the 15 min prior to the light exposure following administration of a pupil dilator (ophthalmologic preparation of 0.5% cyclopentolate hydrochloride, Cyclogel, Alcon, TX). During the light exposure, participants remained seated under continuous supervision by study staff and were asked to maintain a fixed gaze for 90 min in the Ganzfeld dome followed by a 10 min free gaze, repeated throughout the light exposure. Further details of the light exposure procedure and light exposure system can be found in Rahman et al., Gooley et al., and Brainard et al. [18, 23, 27].
Neurobehavioral Performance, Hormone, and Body Temperature Assessments
Participants completed alertness and neurobehavioral performance assessments every hour starting 2.5 h post-wake during the first CR and every hour starting 1 h postwake on the light exposure day. Self-reported sleepiness was assessed using the Karolinska Sleepiness Scale (KSS; [28]), a nine-point scale from 1 (“very alert”) to 9 (“very sleepy, fighting sleep”). KSS scores were collected by pressing the appropriate number on a keyboard when prompted. During the monochromatic light exposure, participants completed the KSS by responding verbally after the identical instructions and options presented during the CR were read to them. Objective neurobehavioral performance was measured using the 10 min auditory psychomotor vigilance task (aPVT), where a tone was presented at random intervals between 1 and 9 s and participants were asked to respond by pressing a button as quickly as possible after hearing the sound. Mean reaction time and attentional failures (reaction times >500 ms) were calculated for each 10 min aPVT session.
Plasma was collected from an indwelling intravenous cannula inserted into a forearm vein and kept patent with a heparinized saline infusion (5 IU heparin/mL 0.45% NaCl infused at 40–42 mL/h). Blood samples were transferred to ethylenediaminetetraacetic acid (EDTA) tubes and kept on ice before centrifugation. The plasma fraction was transferred into plastic tubes and stored at −20°C. During the first CR, blood samples were collected every 30–60 min until the beginning of the light exposure where samples were collected every 20 min. Two-hourly samples from the CR were assayed and all available samples on the light exposure day were assayed. Plasma melatonin was assayed using radioimmunoassay (ALPCO Diagnostics, Salem NH). Plasma intra-assay and interassay coefficients of variation (CVs) were <9% and <11%, respectively, at 1.94 and 16.59 pg/ml. Plasma E2, FSH, LH, P4, and sex-hormone binding globulin (SHBG) were assayed using Access Chemiluminescent Immunoassay (Beckman Coulter, Fullerton, CA). Intra-assay and interassay CVs were 12%–20% for E2, 3.1%–5.6% for FSH, 4.3%–6.4% for LH, and 6.11%–11.19% for P4. The P4/E2 ratio was calculated as . Given that data for other reproductive hormones involved in the menstrual cycle (FSH, LH, and SHBG) were available, these hormones were also included in the analyses to provide validation of the menstrual phase classification.
Core body temperature was measured every minute via a rectal thermistor (Yellow Springs Instruments Inc., Yellow Springs, OH) throughout the study protocol. CBT data were averaged in 1 h bins prior to analysis following inspection and removal of artifact.
Data analysis
The first 5 h of neurobehavioral performance, hormone, and body temperature data were excluded from the analysis of CR data to remove masking effects from the prior sleep episode and changes in posture [29]. For analyses of the light exposure data, only time points collected between experimental lights on (4.75 h postwake) and experimental lights off (11.25 h postwake) were analyzed. The figures, however, include data prior to and after light exposure to illustrate temporal changes in neurobehavioral performance, hormone, and CBT levels before, during, and after the light exposure. LH was below the assay’s limit of detection for one participant (26F2V) and for another participant (22A1V), FSH data were excluded from analysis due to abnormally high FSH levels (mean ± SD, 39.9 ± 18.6 mUI/mL; Supplementary Figure S1) without abnormal values for any of the other hormones. To account for differences in sample frequencies between individuals due to missing samples, values were binned in 2 and 1 h bins for CR and light exposure data, respectively, prior to analysis. Missing data during the CR was 2% for the aPVT and CBT and between 6% and 13% for the sex hormones, and during light exposure was 11% for the aPVT, 4% for CBT, and between 15% and 20% for the sex hormones.
Linear mixed model analyses were performed to compare neurobehavioral performance, hormones, and body temperature between (1) menstrual phases during the CR and (2) menstrual phases and melatonin suppression and nonsuppression groups during the light exposure. Time and group were modeled as fixed effects, with participant modeled as a random effect. Because the irradiance and wavelength of the light exposures were not consistent between participants, in order to analyze the effect of light, participants were dichotomized to melatonin suppression and nonsuppression groups based on whether they exhibited a clear biological response to the light exposure (≥33% melatonin suppression [30]). To determine the relationship between body temperature, hormones, and neurobehavioral performance during the CR, regression analyses were conducted on the 42 h (6–48 h awake) area under the curve (AUC) data for each variable. AUCs were used for this analysis to minimize effects due to the pulsatility of the hormones. Data were first analyzed with a linear regression model; however, given that previous research has shown a nonlinear quadratic (y = ax2 + bx + c) model best describes the relationship between the P4/E2 ratio and CBT [16, 17], we also fit this model to the data. Path analysis was conducted on the AUC data to examine the direct and indirect relationships between neurobehavioral performance, CBT, and hormones. All statistical analyses were conducted in SAS 9.4. (Cary, NC).
Results
Effect of menstrual phase on neurobehavioral performance, hormones, and body temperature
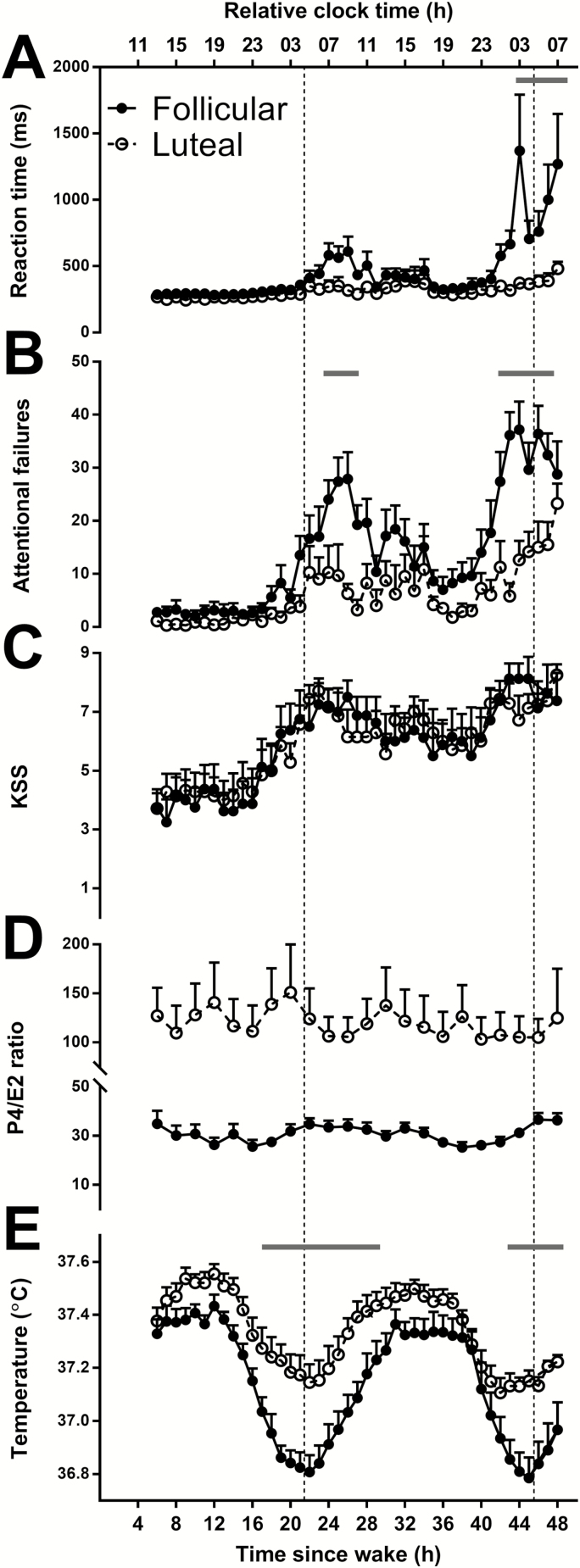
Self-reported sleepiness (KSS scores: F42, 497 = 5.01, p < .0001) and neurobehavioral performance impairment (aPVT attentional failures: F42, 490 = 7.28, p < .0001; mean reaction time: F42, 486 = 4.8, p < .0001) increased with increasing time awake (Figure 2, A–C). Women in the follicular phase had significantly slower mean reaction times (F1, 13 = 10.19, p < .01) and more attentional failures (F1, 13= 6.52, p < .03) than women in the luteal phase, but there was no difference in self-reported sleepiness between menstrual phases. Additionally, a significant time by menstrual phase interaction for both mean reaction time (F42, 486 = 2.47, p < .0001) and attentional failures (F42, 490 = 1.77, p < .003) showed that differences in neurobehavioral performance between the groups were most pronounced within ~4 h after CBT minimum during the first night and within ~3 h before and after CBT minimum on the second night (Figure 2, A, B, and E).
Figure 2.
The mean ± SEM of mean reaction time (A) and attentional failures (B) on the aPVT, KSS (C), P4/E2 ratio (D), and core body temperature (E) for women in the follicular (closed circles) and luteal phase (open circles) of the menstrual cycle during the CR. Corresponding clock times are reported relative to scheduled wake. Time = 0 relative to scheduled wake was defined as 0700 h based on the group mean (mean ± SD: 0704 ± 0108 h) for illustrative purposes. Vertical dotted lines represent the CBT minimum at 0430 h based on the group mean (mean ± SD: 0418 ± 0140 h). Significant false discovery rate (FDR) corrected post hoc t-tests are denoted by gray bars above time points that were significant. Unadjusted data are plotted.
As expected, menstrual phase was associated with significant changes in overall levels of P4 (F1, 14 = 8.62, p < .02), P4/E2 ratio (F1, 14 = 10.84, p < .01), LH (F1, 13 = 5.54, p < .04), FSH (F1, 13 = 19.27, p < .001), and CBT (F1, 14 = 10.41, p < .01). P4 (data not shown), P4/E2 ratio (Figure 2, D), and CBT (Figure 2, E) were higher, and FSH and LH (data not shown) were lower in the luteal phase of the menstrual cycle. In contrast, melatonin, E2, and SHBG levels were not different between menstrual phases (data not shown). Moreover, there was a significant interaction effect of time and menstrual phase on CBT (F42, 533= 1.72, p < .003), showing greatest differences ~5 h before and after CBT minimum (Figure 2, E).
Relationship between body temperature, neurobehavioral performance, and P4, E2, and the P4/E2 ratio
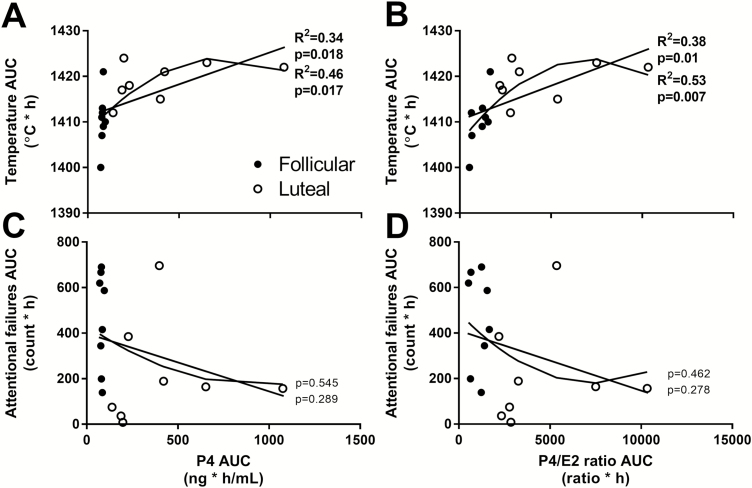
Regression analyses of the AUC from 6 to 48 h during the CR showed that CBT was inversely associated with attentional failures (R2 = .27, p = .04) but not mean reaction time (Supplementary Figure S2). P4 (R2 = .34, p = .02) and the P4/E2 ratio (R2 = .38, p = .01) were significantly associated with CBT (Figure 3, A and B), but neither P4 nor the P4/E2 ratio predicted attentional failures (Figure 3, C and D). CBT, mean reaction time, or attentional failures were not linearly associated with E2, however (Supplementary Figure S3). Using the quadratic regression model, both P4 (R2 = .46, p = .02) and the P4/E2 ratio (R2 = .53, p = .007) were significantly associated with CBT (Figure 3, A and B). Similar to the linear model, however, neither P4 nor the P4/E2 ratio were significantly associated with attentional failures (Figure 3, C and D) or mean reaction time.
Figure 3.
Linear and nonlinear quadratic regression analyses of the association of P4 (left panel) and the P4/E2 ratio (right panel) with temperature (A, B) and attentional failures (C, D). Closed circles represent women in the follicular phase and open circles represent women in the luteal phase of the menstrual cycle. R2 values are shown for significant associations only.
As the P4/E2 ratio and P4 were significantly associated with CBT and CBT was associated with attentional failures, we performed path analysis to determine whether the P4/E2 ratio or P4 were indirectly associated with attentional failures via a relationship with CBT. There was a trend (p = .05) toward an indirect effect of the P4/E2 ratio on lapses but there was no significant direct effect (Supplementary Figure S4). There were no significant direct or indirect effects of P4 or E2 on attentional failures.
Effect of light exposure on neurobehavioral performance, hormones, and body temperature
To examine the effects of light exposure on neurobehavioral performance, hormones, and body temperature, we compared these outcomes between four groups (n = 4/group): (1) luteal, ≥33% melatonin suppression; (2) follicular, ≥33% melatonin suppression; (3) luteal, no suppression (<33%); and (4) follicular, no suppression (<33%).
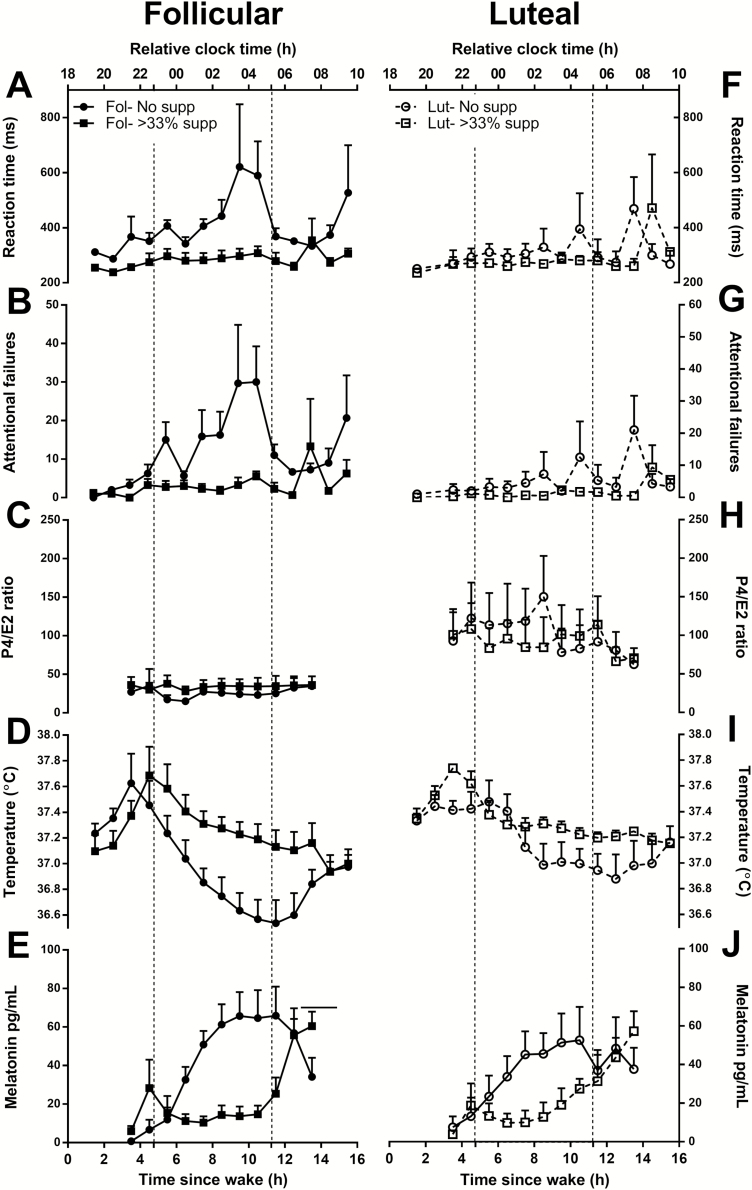
Shown in Figure 4, there was a significant effect of group for mean reaction time (F3, 12 = 5.76, p < .02), attentional failures (F3, 12 = 5.38 p < .02), CBT (F3, 12 = 3.58, p < .05), and melatonin (F3, 12 = 3.72, p < .05). Post hoc analysis showed significantly fewer attentional failures (t12 = 3.23, p < .01) and faster mean reaction times (t12 = 3.39, p < .01) during the follicular phase with concomitant melatonin suppression than during the follicular phase without melatonin suppression (Figure 4, A and B). In contrast, melatonin suppression status was not associated with attentional failures or mean reaction time during the luteal phase (Figure 4, F and G). When melatonin suppression did not occur, women demonstrated worse neurobehavioral performance during the follicular phase compared to the luteal phase as assessed by mean reaction time (t12 = 2.89, p < .02) and attentional failures (t12 = 2.73, p < .02), consistent with the CR results. When melatonin levels were suppressed by light exposure, mean reaction time (t12 = 0.34, p = .740) and attentional failures (t12 = 0.42, p = .682) were not different between the luteal and follicular phases.
Figure 4.
The mean ± SEM of mean reaction time (A, F) and attentional failures (B, G) on the aPVT, the P4/E2 ratio (C, H), core body temperature (D, I), and melatonin (E, J) for women in the follicular (left panel) and luteal phases (right panel) who showed suppression (≥33% melatonin suppression; squares) versus no suppression (<33% suppression; circles) during the 6.5 h light exposure. Corresponding clock times are reported relative to scheduled wake. Time = 0 relative to scheduled wake was defined as 0700 h based on the group mean (mean ± SD: 0704 ± 0108 h) for illustrative purposes. The dotted lines mark the start and the end of the light exposure. Unadjusted data are plotted.
Consistent with the CR condition, CBT trended toward being higher in the luteal phase compared to the follicular phase when comparing women without melatonin suppression (Figure 4, D and I), but this difference did not reach statistical significance (t12 = 1.86, p = .087). When light exposure induced melatonin suppression, however, body temperature in the follicular phase was similar to that in the luteal phase (t12 = 0.09, p = .930; Figure 4, D and I). None of the four groups differed significantly in the P4/E2 ratio (Figure 4, C and H) nor in any of the other reproductive hormones.
Conclusion/Discussion
In the current study, we found that neurobehavioral performance impairment was associated with menstrual phase, specifically that women in the follicular phase showed greater attentional failures and slower mean reaction times as compared to women in the luteal phase when exposed to acute overnight sleep loss. Moreover, we provided evidence that ocular light exposure might be an effective countermeasure for mitigating the neurobehavioral performance vulnerability to sleep loss due to menstrual phase. Additionally, changes in neurobehavioral performance were associated with changes in CBT, which also trended toward being modulated by the P4/E2 ratio. This endocrine association identifies a possible mechanistic role of these hormones in modulating neurobehavioral performance vulnerability to sleep loss based on menstrual phase.
While a previous study has shown greater impairment in the follicular phase during the night on tasks measuring cognitive throughput, there were no significant differences in reaction time between the menstrual phases following a single night of sleep deprivation (~24 h awake [10]). In the current study, the differences in mean reaction time between the menstrual phases appeared only following significant sleep loss (post hoc comparisons significant only after 44 h awake), consistent with Wright and Badia [10]. Vidafar et al. [11], however, did show differences in PVT performance between menstrual phases during a single night of sleep deprivation (30 h awake) in a larger cohort, which included the current study participants. We expand their findings by showing that the differences in PVT performance persist during a second night of sleep deprivation such that women in the luteal phase remain more resilient even when prolonged wakefulness is extended past 30 h. While such extended acute sleep loss is less common than chronic sleep restriction, the medical and firefighter professions routinely schedule 24 h or longer shifts without guarantee of sleep [31] and, therefore, our findings have important implications for women experiencing acute sleep loss. Furthermore, chronic sleep loss [32, 33], particularly chronic variable sleep loss [34], can induce the same degree of impairment as 24 or more hours awake in only a few weeks. It will be important to examine whether menstrual phase differences in neurobehavioral performance are observed in response to chronic, as well as acute, sleep loss.
Light exposure is an effective countermeasure for improving alertness and neurobehavioral impairment during adverse circadian phases as occurs during shift work [18–20, 24, 35]. Therefore, we tested whether light could mitigate the greater cognitive impairment to sleep loss observed during the follicular phase compared to the luteal phase. We found that ocular light exposure that suppressed circulating melatonin levels, confirming a robust physiological response to light exposure, also reduced the risk of attentional failures and mean reaction time in women in the follicular phase to levels observed in women in the luteal phase. Importantly, light exposure too weak to suppress melatonin levels did not improve neurobehavioral performance, demonstrating that the positive effects were not placebo responses. Comparing neurobehavioral performance between women with and without melatonin suppression in the luteal phase showed a trend for performance improvement with light exposure that also suppressed melatonin levels, but the difference was not statistically significant (p = .09) likely due to the limited sample size in each group (n = 4). The current study was designed to induce a range of melatonin suppression responses by changing the spectral and irradiance characteristics of the light exposure. This approach, however, precluded analysis of the spectral and irradiance responses of neurobehavioral performance at each menstrual phase due to a limited sample size in each light-exposure condition. Future research is warranted to further explore these relationships to design optimal lighting interventions as a countermeasure for differences in overnight neurobehavioral performance between the follicular and luteal phase as observed in this study.
Prior work, mostly in men [1, 3, 36–39], has shown that cognitive performance paralells the 24 h rhythm in CBT, and directly manipulating body temperature can alter cognitive performance [40–42]. We observed a similar relationship between body temperature and neurobehavioral performance in premenopausal women such that the time course of performance was similar to that of CBT during both phases of the menstrual cycle (Figure 2, A, B, and E). Furthermore, the light exposure experiments in our study support a modulatory role for CBT in explaining the difference in neurobehavioral performance between the menstrual phases. The improvement in neurobehavioral performance observed in the follicular phase in response to light was accompanied by an increase in CBT. Furthermore, the order of groups from best to worst performers was identical to the order of groups from highest to lowest body temperature (Figure 4, D and I). Taken together, these results suggest that differences in CBT between the menstrual phases may account for the differences in neurobehavioral performance.
We also examined whether E2 and P4 can modulate CBT and neurobehavioral performance. Both the linear and nonlinear quadratic regression models showed that P4 and the P4/E2 ratio were associated with CBT and, consistent with previous findings [16, 17], the latter explained more variance in CBT (45% and 54%, respectively; Figure 3). Our preliminary analysis in a limited sample suggests that these changes in P4/E2 ratio may have an indirect effect on neurobehavioral performance (attentional failures) through its influence on CBT but not directly (Supplementary Figure S4). These analyses, therefore, provide a potential mechanism through which menstrual phase can affect neurobehavioral vulnerability to sleep loss. The lower P4/E2 ratio during the follicular phase causes a greater reduction and lower absolute body temperature when awake overnight, which in turn leads to poorer neurobehavioral performance.
Although our study provides novel insight on the association between neurobehavioral performance, CBT, and female reproductive hormones, it has limitations. First, the small sample size means that the positive effect of light on neurobehavioral performance in women in the follicular phase, while evident (Figure 4, A and B), should be interpreted with caution. These findings need to be replicated in a larger sample. Second, the effect of the changes in the hormonal milieu within a menstrual cycle phase (i.e., early vs. late follicular or luteal) was not investigated in the current study. The use of a within-participants-crossover design may help to determine how neurobehavioral performance changes directly in response to hormonal changes that occur throughout the menstrual cycle. Third, the participants were young, healthy naturally cycling women. These results are, therefore, not readily generalizable to women with varying hormonal profiles due to hormonal contraception, hormone replacement therapy, or during perimenopause or postmenopause. Consequently, it will be important to determine the effects of hormonal contraception on CBT and neurobehavioral performance given that ~30% of US women of reproductive age currently use one form of hormonal contraception [43]. Several studies have shown that OC use increases CBT to levels higher than the follicular phase [4, 8, 44, 45], which would be expected to improve neurobehavioral performance, although recent evidence suggests that there is also a slight variation of CBT across the quasifollicular and quasiluteal phases [46]. Consistent with the hypothesis that CBT increases associated with OC may improve performance, however, one study has shown that cognitive throughput in OC users is greater than women in the follicular phase [10].
Consideration of our findings in the context of menopause and aging is also important. Increasing age is associated with a decrease in the amplitude of the circadian CBT rhythm [47–49] and higher absolute body temperatures overnight [50]. The CBT rhythm in older women, like women in the luteal phase, has been shown to have a smaller amplitude and higher absolute minimum overnight compared to both young women and young men [48, 50]. This body temperature change would be expected to improve neurobehavioral performance overnight and, consistent with this hypothesis, older adults show less neurobehavioral impairment than do young individuals in response to sleep loss [51–53]. While neurobehavioral performance in premenopausal and postmenopausal women has not been compared directly, based on our results, we may expect that menopause, like the luteal phase, could be protective against neurobehavioral performance impairment resulting from sleep loss.
In summary, we have shown that the greater vulnerability to neurobehavioral performance impairment overnight in women in the follicular phase may be mediated by changes in core body temperature induced by changes in the P4/E2 ratio with menstrual phase. This neurobehavioral performance impairment appears to be countered by light exposure. These findings have important implications for safety and development of countermeasures for women working extended shifts and overnight during the follicular phase, and more broadly that menstrual phase should be monitored and reported when investigating the effects of sleep loss and light on neurobehavioral performance in women.
Supplementary Material
Supplementary material is available at SLEEP online.
Figure S1. Individual and group-average E2 (A-B), FSH (C-D), LH (E-F), P4 (G-H), SHBG (I-J) during the constant routine (left panel) and light exposure (right panel) for women in the follicular and luteal phases of the menstrual cycle. Individual values are calculated as the average for each participant during the constant routine and prior to the light exposure (LE). LE averages include only samples collected before experimental lights on. The dotted line in G and H indicates the 3ng/mL cutoff for P4 used to allocate participants to each menstrual phase. The red data point in C and D represents the participant (22A1V) excluded from the FSH analysis due to abnormally high FSH values. The LH and FSH data points indicating ovulation for the participant (26H6V) that transitioned from the follicular to luteal phase of the menstrual cycle during the course of the inpatient study are annotated in D and F.
Figure S2. Linear regression analyses of the relationship between core body temperature (CBT) and neurobehavioral performance on aPVT attentional failures (A) and mean reaction time (B). Temperature was a significant predictor of attentional failures, but not mean reaction time. Closed circles represent women in the follicular phase (●) and open circles represent women in the luteal phase (○) of the menstrual cycle.
Figure S3. Linear regression analyses examining the relationship between E2 and core body temperature (A), attentional failures (B) and mean reaction time (C). There was not a relationship between E2 and temperature or neurobehavioral performance. Closed circles represent women in the follicular phase (●) and open circles represent women in the luteal phase (○) of the menstrual cycle.
Figure S4. Path diagram showing the direct effects of the P4/E2 ratio and CBT on attentional failures. Arrows are labeled with the standardized effect values. Solid lines indicate significant associations and dashed lines indicate non-significant associations. The gray curved line represents the indirect relationship when trended toward significance (p=0.05). Lines are weighted to reflect the strength of the association. Significance is denoted by * p≤0.05, ** p<0.01.
Acknowledgments
We thank the technical, dietary, and laboratory staff, nurses and physicians, participant recruiters, and the study participants at the Center for Clinical Investigation and Division of Sleep and Circadian Disorders, Brigham and Women’s Hospital; Ralph Todesco (Brigham and Women’s Hospital), John Hanifin, PhD (Thomas Jefferson University), Ron Kovak, and Jon Cooke (Photon Technology Inc., Lawrenceville, NJ) for technical support of the monochromatic light equipment; and Eric Chua, PhD (Singapore Institute of Technology) for assistance with performance data management. This work was conducted at Brigham and Women’s Hospital.
Funding
This work was supported by the National Institute of Neurological Disorders and Stroke (R01 NS36590 to G.C.B.), the National Institute of Mental Health (2R01 MH45130-11A1 to C.A.C. and S.W.L.), the National Center for Complementary and Alternative Medicine (R01 AT002129 to C.A.C. and S.W.L.), and the National Institute of Environmental Health Sciences (R21 ES017112-01A1 to S.W.L.). G.C.B, C.A.C., and S.W.L. were supported in part by the National Space Biomedical Research Institute through NASA NCC 9–58. The project was supported by the National Center for Research Resources through grants to Brigham and Women’s Hospital General Clinical Research Center (NCRR M01 RR02635) and the Harvard Clinical and Translational Science Center (NCRR UL1 RR025758). L.K.G. was supported by an Australian Government Research Training Program (RTP) Scholarship (Australian Government, Department of Education).
Disclosure Statement
L.K.G. and J.J.G. have nothing to declare. M.S.H. has provided limited consulting to The MathWorks, Inc. S.M.W.R. is a Program Leader and serves as a consultant to the CRC for Alertness, Safety and Productivity, Australia. S.M.W.R. reports receiving research grants from the CRC for Alertness, Safety and Productivity, Philips Respironics, Rio Tinto, Shell, Linfox Australia, and Teva Pharma Australia, and has received equipment support and consultancy fees through his institution from Vanda Pharmaceuticals, Optalert, Tyco Healthcare, Compumedics, BHP, and Teva Pharmaceuticals, which are not related to this paper. G.C.B. has no conflicts of interest relative to the scientific content of this manuscript. In the spirit of open disclosure, however, he reports that he and his research program have received financial, material, and travel support from a range of federal, industrial, and philanthropic organizations in the past 2 years through present time. C.A.C. reports grants from Cephalon Inc., Jazz Pharmaceuticals Plc., Inc., National Football League Charities, Optum, Philips Respironics, Inc., Regeneron Pharmaceuticals, ResMed Foundation, San Francisco Bar Pilots, Sanofi S.A., Sanofi-Aventis, Inc, Schneider Inc., Sepracor, Inc, Mary Ann & Stanley Snider via Combined Jewish Philanthropies, Sysco, Takeda Pharmaceuticals, Teva Pharmaceuticals Industries, Ltd., and Wake Up Narcolepsy; and personal fees from Bose Corporation, Boston Celtics, Boston Red Sox, Cephalon, Inc., Columbia River Bar Pilots, Ganésco Inc., Institute of Digital Media and Child Development, Klarman Family Foundation, Samsung Electronics, Quest Diagnostics, Inc., Teva Pharma Australia, Vanda Pharmaceuticals, Washington State Board of Pilotage Commissioners, Zurich Insurance Company, Ltd. In addition, C.A.C. holds a number of process patents in the field of sleep/circadian rhythms (e.g., photic resetting of the human circadian pacemaker) and holds an equity interest in Vanda Pharmaceuticals, Inc. Since 1985, C.A.C. has also served as an expert on various legal and technical cases related to sleep and/or circadian rhythms, including those involving the following commercial entities: Casper Sleep Inc., Comair/Delta Airlines, Complete General Construction Company, FedEx, Greyhound, HG Energy LLC, Purdue Pharma, LP, South Carolina Central Railroad Co., Steel Warehouse Inc., Stric-Lan Companies LLC, Texas Premier Resource LLC, and United Parcel Service (UPS). C.A.C. receives royalties from the New England Journal of Medicine; McGraw Hill; Houghton Mifflin Harcourt/Penguin; and Philips Respironics, Inc. for the Actiwatch-2 and Actiwatch-Spectrum devices. C.A.C.’s interests were reviewed and managed by Brigham and Women’s Hospital and Partners HealthCare in accordance with their conflict of interest policies. S.W.L. has had a number of commercial interests in the last 24 months (2017–2019). No other interests are directly related to the research or topic reported in this paper but, in the interests of full disclosure, are outlined below. S.W.L. has received consulting fees from the BHP Billiton, EyeJust Inc., Noble Insights, and Team C Racing; honoraria and/or paid travel from BHP Billiton, DIN, IES, Ineos, SLTBR, and Teague; has current consulting contracts with Akili Interactive; Apex 2100 Ltd.; Consumer Sleep Solutions; Headwaters Inc.; Hintsa Performance AG; Light Cognitive; Lighting Science Group Corporation; Mental Workout; PlanLED; Six Senses; Stantec; and Wyle Integrated Science and Engineering; has received unrestricted equipment gifts from Bionetics Corporation and F. Lux Software LLC; royalties from Oxford University Press; and has served as a paid expert in legal proceedings related to light, sleep, and health. S.A.R. holds patents for prevention of circadian rhythm disruption by using optical filters and improving sleep performance in participants exposed to light at night. S.A.R. owns equity in Melcort Inc. S.A.R. has provided paid consulting services to Sultan & Knight Ltd and Bambu Vault LLC. S.A.R. has received honoraria as an invited speaker and travel funds from Starry Skies Lake Superior, University of Minnesota Medical School, PennWell Corp., and Seoul Semiconductor Co. Ltd.
References
- 1. Dijk DJ, et al. . Circadian and sleep/wake dependent aspects of subjective alertness and cognitive performance. J Sleep Res. 1992;1(2):112–117. [DOI] [PubMed] [Google Scholar]
- 2. Kräuchi K, et al. . Circadian rhythm of heat production, heart rate, and skin and core temperature under unmasking conditions in men. Am J Physiol. 1994;267(3 Pt 2):R819–R829. [DOI] [PubMed] [Google Scholar]
- 3. Wright KP Jr, et al. . Relationship between alertness, performance, and body temperature in humans. Am J Physiol Regul Integr Comp Physiol. 2002;283(6):R1370–R1377. [DOI] [PubMed] [Google Scholar]
- 4. Kattapong KR, et al. . Effect of sex, menstrual cycle phase, and oral contraceptive use on circadian temperature rhythms. Chronobiol Int.. 1995;12:257–266 [Google Scholar]
- 5. Shechter A, et al. . Circadian variation of sleep during the follicular and luteal phases of the menstrual cycle. Sleep. 2010;33(5):647–656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cagnacci A, et al. . Modification of circadian body temperature rhythm during the luteal menstrual phase: role of melatonin. J Appl Physiol (1985). 1996;80(1):25–29. [DOI] [PubMed] [Google Scholar]
- 7. Lee KA. Circadian temperature rhythms in relation to menstrual cycle phase. J Biol Rhythms.. 1988;3:255–263 [Google Scholar]
- 8. Baker FC, et al. . Sleep and 24 hour body temperatures: a comparison in young men, naturally cycling women and women taking hormonal contraceptives. J Physiol. 2001;530(Pt 3):565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Driver HS, et al. . Sleep and the sleep electroencephalogram across the menstrual cycle in young healthy women. J Clin Endocrinol Metab. 1996;81(2):728–735. [DOI] [PubMed] [Google Scholar]
- 10. Wright KP Jr, et al. . Effects of menstrual cycle phase and oral contraceptives on alertness, cognitive performance, and circadian rhythms during sleep deprivation. Behav Brain Res. 1999;103(2):185–194. [DOI] [PubMed] [Google Scholar]
- 11. Vidafar P, et al. . Increased vulnerability to attentional failure during acute sleep deprivation in women depends on menstrual phase. Sleep. 2018;41(8). doi:10.1093/sleep/zsy098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Stachenfeld NS, et al. . Estrogen modifies the temperature effects of progesterone. J Appl Physiol (1985). 2000;88(5):1643–1649. [DOI] [PubMed] [Google Scholar]
- 13. Israel SL, et al. . The thermogenic property of progesterone. Obstet Gynecol Surv. 1950;5:532–533 [Google Scholar]
- 14. Rothchild I, et al. . The effects of dosage, and of estrogen, androgen or salicylate administration on the degree of body temperature elevation induced by progesterone. Endocrinology. 1952;50(4):485–496. [DOI] [PubMed] [Google Scholar]
- 15. Buxton CL, et al. . Hormonal factors involved in the regulation of basal body temperature during the menstrual cycle and pregnancy. J Clin Endocrinol Metab. 1948;8(7):544–549. [DOI] [PubMed] [Google Scholar]
- 16. Cagnacci A, et al. . Regulation of the 24h body temperature rhythm of women in luteal phase: role of gonadal steroids and prostaglandins. Chronobiol Int. 2002;19(4):721–730. [DOI] [PubMed] [Google Scholar]
- 17. Cagnacci A, et al. . Regulation of the 24-hour rhythm of body temperature in menstrual cycles with spontaneous and gonadotropin-induced ovulation. Fertil Steril. 1997;68(3):421–425. [DOI] [PubMed] [Google Scholar]
- 18. Rahman SA, et al. . Diurnal spectral sensitivity of the acute alerting effects of light. Sleep. 2014;37(2):271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cajochen C, et al. . High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light. J Clin Endocrinol Metab. 2005;90(3):1311–1316. [DOI] [PubMed] [Google Scholar]
- 20. Wright KP Jr, et al. . Caffeine and light effects on nighttime melatonin and temperature levels in sleep-deprived humans. Brain Res. 1997;747(1):78–84. [DOI] [PubMed] [Google Scholar]
- 21. Stricker R, et al. . Establishment of detailed reference values for luteinizing hormone, follicle stimulating hormone, estradiol, and progesterone during different phases of the menstrual cycle on the Abbott ARCHITECT analyzer. Clin Chem Lab Med. 2006;44(7):883–887. [DOI] [PubMed] [Google Scholar]
- 22. Israel R, et al. . Single luteal phase serum progesterone assay as an indicator of ovulation. Am J Obstet Gynecol. 1972;112(8):1043–1046. [DOI] [PubMed] [Google Scholar]
- 23. Gooley JJ, et al. . Spectral responses of the human circadian system depend on the irradiance and duration of exposure to light. Sci Transl Med. 2010;2:31ra33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lockley SW, et al. . Short-wavelength sensitivity for the direct effects of light on alertness, vigilance, and the waking electroencephalogram in humans. Sleep. 2006;29(2):161–168. [PubMed] [Google Scholar]
- 25. Lockley SW, et al. . High sensitivity of the human circadian melatonin rhythm to resetting by short wavelength light. J Clin Endocrinol Metab. 2003;88(9):4502–4505. [DOI] [PubMed] [Google Scholar]
- 26. Amundadottir ML. Light-Driven Model for Identifying Indicators of Non-Visual Health Potential in the Built Environment [PhD thesis]. Lausanne, Switzerland: École Polytechnique Fédérale de Lausanne; 2016. [Google Scholar]
- 27. Brainard GC, et al. . Human melatonin regulation is not mediated by the three cone photopic visual system. J Clin Endocrinol Metab. 2001;86(1):433–436. [DOI] [PubMed] [Google Scholar]
- 28. Akerstedt T, et al. . Subjective and objective sleepiness in the active individual. Int J Neurosci. 1990;52(1–2):29–37. [DOI] [PubMed] [Google Scholar]
- 29. Brown EN, et al. . The statistical analysis of circadian phase and amplitude in constant-routine core-temperature data. J Biol Rhythms. 1992;7(3):177–202. [DOI] [PubMed] [Google Scholar]
- 30. Hull JT, et al. . Suppression of melatonin secretion in totally visually blind people by ocular exposure to white light: clinical characteristics. Ophthalmology. 2018;125(8):1160–1171. [DOI] [PubMed] [Google Scholar]
- 31. Barger LK, et al. . Neurobehavioral, health, and safety consequences associated with shift work in safety-sensitive professions. Curr Neurol Neurosci Rep. 2009;9(2):155–164. [DOI] [PubMed] [Google Scholar]
- 32. Van Dongen HP, et al. . The cumulative cost of additional wakefulness: dose-response effects on neurobehavioral functions and sleep physiology from chronic sleep restriction and total sleep deprivation. Sleep. 2003;26(2):117–126. [DOI] [PubMed] [Google Scholar]
- 33. Belenky G, et al. . Patterns of performance degradation and restoration during sleep restriction and subsequent recovery: a sleep dose-response study. J Sleep Res. 2003;12(1):1–12. [DOI] [PubMed] [Google Scholar]
- 34. St Hilaire MA, et al. . Modeling neurocognitive decline and recovery during repeated cycles of extended sleep and chronic sleep deficiency. Sleep. 2017;40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cajochen C, et al. . Dose-response relationship for light intensity and ocular and electroencephalographic correlates of human alertness. Behav Brain Res. 2000;115(1):75–83. [DOI] [PubMed] [Google Scholar]
- 36. Johnson MP, et al. . Short-term memory, alertness and performance: a reappraisal of their relationship to body temperature. J Sleep Res. 1992;1(1):24–29. [DOI] [PubMed] [Google Scholar]
- 37. Monk TH, et al. . Task variables determine which biological clock controls circadian rhythms in human performance. Nature. 1983;304(5926):543–545. [DOI] [PubMed] [Google Scholar]
- 38. Wyatt JK, et al. . Circadian temperature and melatonin rhythms, sleep, and neurobehavioral function in humans living on a 20-h day. Am J Physiol. 1999;277(4 Pt 2):R1152–R1163. [DOI] [PubMed] [Google Scholar]
- 39. Cajochen C, et al. . EEG and ocular correlates of circadian melatonin phase and human performance decrements during sleep loss. Am J Physiol. 1999;277(3 Pt 2):R640–R649. [DOI] [PubMed] [Google Scholar]
- 40. Fort A, et al. . Psychometric performance: circadian rhythms and effect of raising body temperature. J Physiol. 1973;231(2):114P–115P. [PubMed] [Google Scholar]
- 41. Fort A, et al. . The relationship between deep body temperature and performance on psychometric tests. J Physiol. 1971;219(2):17P–18P. [PMC free article] [PubMed] [Google Scholar]
- 42. Raymann RJ, et al. . Time-on-task impairment of psychomotor vigilance is affected by mild skin warming and changes with aging and insomnia. Sleep. 2007;30(1):96–103. [DOI] [PubMed] [Google Scholar]
- 43. Daniels K, et al. . Current contraceptive status among women aged 15–44: United States, 2011–2013. NCHS Data Brief. 2014;173:1–8. [PubMed] [Google Scholar]
- 44. Baker FC, et al. . Oral contraceptives alter sleep and raise body temperature in young women. Pflugers Arch. 2001;442(5):729–737. [DOI] [PubMed] [Google Scholar]
- 45. Tenaglia SA, et al. . Influence of menstrual cycle and oral contraceptives on tolerance to uncompensable heat stress. Eur J Appl Physiol Occup Physiol. 1999;80(2):76–83. [DOI] [PubMed] [Google Scholar]
- 46. Lei TH, et al. . On exercise thermoregulation in females: interaction of endogenous and exogenous ovarian hormones. J Physiol. 2019;597(1):71–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Campbell SS, et al. . Relationships between sleep and body temperature in middle-aged and older subjects. J Am Geriatr Soc. 1998;46(4):458–462. [DOI] [PubMed] [Google Scholar]
- 48. Czeisler CA, et al. . Association of sleep-wake habits in older people with changes in output of circadian pacemaker. Lancet. 1992;340(8825):933–936. [DOI] [PubMed] [Google Scholar]
- 49. Dijk DJ, et al. . Ageing and the circadian and homeostatic regulation of human sleep during forced desynchrony of rest, melatonin and temperature rhythms. J Physiol. 1999;516(Pt 2):611–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Cagnacci A, et al. . Hypothermic effect of melatonin and nocturnal core body temperature decline are reduced in aged women. J Appl Physiol (1985). 1995;78(1):314–317. [DOI] [PubMed] [Google Scholar]
- 51. Blatter K, et al. . Gender and age differences in psychomotor vigilance performance under differential sleep pressure conditions. Behav Brain Res. 2006;168(2):312–317. [DOI] [PubMed] [Google Scholar]
- 52. Duffy JF, et al. . Healthy older adults better tolerate sleep deprivation than young adults. J Am Geriatr Soc. 2009;57(7):1245–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Silva EJ, et al. . Circadian and wake-dependent influences on subjective sleepiness, cognitive throughput, and reaction time performance in older and young adults. Sleep. 2010;33(4):481–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.