Herbivores have the highest representation of at-risk species in the present day, the recent past, and the late Pleistocene.
Abstract
As a result of their extensive home ranges and slow population growth rates, predators have often been perceived to suffer higher risks of extinction than other trophic groups. Our study challenges this extinction-risk paradigm by quantitatively comparing patterns of extinction risk across different trophic groups of mammals, birds, and reptiles. We found that trophic level and body size were significant factors that influenced extinction risk in all taxa. At multiple spatial and temporal scales, herbivores, especially herbivorous reptiles and large-bodied herbivores, consistently have the highest proportions of threatened species. This observed elevated extinction risk for herbivores is ecologically consequential, given the important roles that herbivores are known to play in controlling ecosystem function.
INTRODUCTION
Over the past 500 years, at least 368 vertebrate species have gone extinct (1), most as a result of direct (e.g., hunting) and indirect (e.g., habitat loss) human influences (2–4). Furthermore, 18% of extant vertebrates have been identified as threatened with extinction (1), and the expansion of anthropogenic disturbances combined with climate change is expected to exacerbate species declines (5). Because species drive ecosystem functions, this species loss, like the historical mass extinction events before it, is likely to profoundly influence the ecology of our planet (6, 7). Historical extinctions have taught us that the disproportionate loss of large-bodied species and species within a specific trophic group (predators, herbivores, or omnivores) has substantial ecological and evolutionary impacts that extend well beyond just the loss of taxonomic diversity (7–9). For example, megaherbivore extinctions in the Late Quaternary altered plant-herbivore interactions in a way that led to marked shifts in plant communities, which, in turn, altered fire regimes and biogeochemical cycling (9, 10). Herbivores also produce a substantial amount of the greenhouse gas methane, and studies have suggested that the resulting methane reduction from megaherbivore extinctions in the Late Quaternary could have been responsible for a 0.08° to 0.20°C decrease in Earth’s climate, leading into the Younger Dryas (8). Although there is strong evidence that current species loss continues to affect large vertebrates disproportionately (11, 12), our understanding of which trophic group is at the greatest risk of extinction is primarily driven by anecdotal evidence and correlations with species traits linked to extinction risk.
Trophic group, an organism’s position in a food chain, is hypothesized to be associated with modern-day extinction risk (13–15). Specifically, higher trophic groups (e.g., predators) are thought to be more at risk than lower ones (e.g., herbivores) (13–16). Extinction risk is predicted to correlate with trophic position for several reasons, including the greater energetic demands (14) and dependence of predators on lower trophic levels for food, which themselves may be in decline (15, 17). Carbone and Gittleman (18) estimated that 10,000 kg of prey is needed to support just 90 kg of a given carnivore species, and prey depletion has been linked to declines of many large carnivores, including tigers, dholes, and several species of leopard (17). Declining populations of natural prey combined with retracting home ranges also increase human-predator conflicts as predators expand into human-occupied areas (19). The tenuous relationship between humans and predators is exemplified by the fact that hunting and trapping has, at least in part, been implicated in the decline of 80% of threatened species in the order Carnivora (1).
Understanding how risk varies among different trophic groups is important because, regardless of the traits driving extinction risk, nonrandom patterns of extinction across trophic groups can result in changes to trophic structure that may influence many aspects of an ecosystem (13). For example, declines in scavenging vultures in India have led to increased risk of the spread of diseases such as rabies and anthrax (13). In another example, declines in large-bodied frugivores in tropical forests are reshaping tree communities, which, in turn, is predicted to lead to an overall reduction in forest carbon storage (20).
Extinction risk is shaped by historical and contemporary socio-ecological factors that have affected a region (e.g., time since human colonization, gross national product, and industrial development) (2, 16). The spatial variability in the human footprint (21) may ultimately affect trophic groups differently, leading to geographic- or habitat-level differences in extinction risk among predators, omnivores, and herbivores. Furthermore, humans have played a role in species extinctions since at least the late Pleistocene (4), and patterns of threatened species across trophic groups may be derived from past extinctions. These processes can cause hysteresis in extinction threat: If humans heavily targeted predators in the past, then the predatory species that survived are likely to be the most resistant species to anthropogenic impacts and thus may be less likely to be threatened by contemporary drivers.
We examined possible differences in extinction risk across trophic groups using three different approaches. First, we examined contemporary patterns in extinction risk among herbivores, omnivores, and predators. To do this, we focused on comparing threat patterns in trophic groups and diets across major taxonomic groups (mammals, birds, and reptiles) globally, across different terrestrial and oceanic regions, and across different habitat types. Second, we examined the role that past extinctions may have played in the trajectory of contemporary patterns by examining the proportions of recently extinct mammal, bird, and reptile species and late Pleistocene extinct mammals in each trophic group. Last, we determined whether trophic group was an important factor driving extinction risk by examining how body size and trophic group interact to affect a species’ threat status. To examine our objectives, we assembled a database categorizing the trophic group (predator, herbivore, and omnivore) and diet of all non–data-deficient mammals, birds, and reptiles (22,166 species) assessed by the International Union for Conservation of Nature (IUCN) (1), the global authority on species extinction and extinction risk.
RESULTS
Patterns of extinction risk across trophic groups and diets
The background fraction of all IUCN threatened mammal, bird, and reptile species that we examined, calculated by dividing the total number of threatened species by the total number of all species, was estimated to be 18%. Within these species, we found that herbivores were the most at-risk trophic group globally, with ~25% [95% confidence interval (CI), 24 to 27%] of all herbivore species listed as threatened (Fig. 1A). This amounts to ~300 more herbivores listed as threatened than would be expected given the background threatened fraction of 18%. The proportion of herbivores at risk of extinction was higher than omnivores and predators (table S1), with 8% (±0.57%) more herbivores listed as threatened compared to omnivores and 10% (±0.52%) more than predators. The pattern of higher extinction risk in herbivores relative to other trophic groups was consistent even when the three taxonomic classes were analyzed separately (table S1 and Fig. 1A).
Fig. 1. Patterns of extinction risk by trophic group and diet.
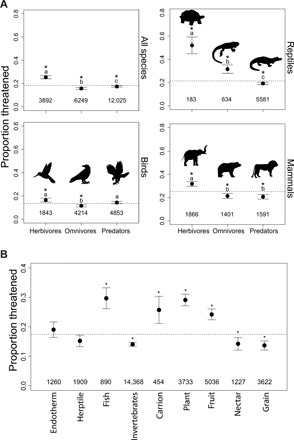
(A) Mean proportions of threatened vertebrate species (±95% CI) within herbivores, omnivores, and predators summarized for all species combined and within mammals, birds, and reptiles. Letters indicate the results for comparisons across trophic group using Tukey’s post hoc tests. Differing letters indicate significant differences among trophic groups (P < 0.05). (B) Mean proportions of threatened species (±95% CI) within each diet group across all species combined. Some species can be classified in multiple diet groups. Asterisks indicate where the proportions of threatened species are significantly different from the background fraction (dashed horizontal line). Numbers indicate sample size.
Overall, herbivorous reptiles (e.g., tortoises) had the highest proportion of at-risk species, with 52% (45 to 59%) listed as threatened. It should be noted that as of 2019, ~70% of described reptile species have been evaluated by the IUCN, whereas complete assessments have been made for mammals and birds. If this subsampling of reptiles was, in any way, trophically biased, then it could have introduced bias into our results for reptiles.
To investigate the potential ecosystem-level effects of species loss across trophic groups, we reran our analyses using more detailed diet groups. In terms of herbivorous diets, we found that consumers of fruit and general plant parts (e.g., leaves, roots, and stems) all had higher proportions of threatened species compared to the background fraction of 18% (Fig. 1B). Predatory diets that exhibited elevated risk of extinction included piscivores and scavengers (Fig. 1B).
Patterns of extinction risk in trophic groups across geographic regions
To identify region-specific patterns in the extinction risk of different trophic groups, we examined the proportions of threatened species in herbivores, omnivores, and predators for the five major marine regions and 13 land regions classified by the IUCN. The most consistent pattern shared across geographic regions for all species combined and for individual taxonomic classes was again an elevated vulnerability of herbivores to extinction risk (table S2 and Fig. 2). We identified elevated risk (proportion higher than the background fraction) for herbivores in at least one taxonomic class in 80% of marine regions and 85% of land regions (table S2 and Fig. 2). Herbivorous reptiles, followed by herbivorous mammals, contributed the majority of this risk. The second most common at-risk trophic group–taxonomic class pairing occurred in omnivorous reptiles, which had elevated risk in 80% of marine regions and 46% of land regions (table S2 and Fig. 2). Last, predators had elevated levels of risk in only 23% of land regions and 40% of the marine regions. Predatory reptiles and predatory birds (e.g., seabirds) drove the geographic patterns observed in at-risk predators. Overall, sub-Saharan Africa and the Atlantic Ocean had the highest numbers of taxonomic classes and trophic groups classified as threatened (Fig. 2).
Fig. 2. Trophic group patterns in extinction risk across land and marine regions.
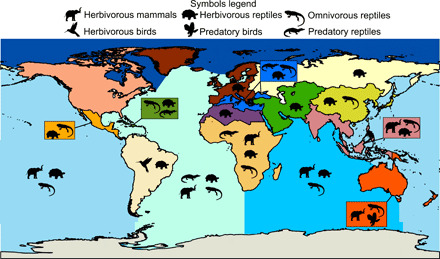
Silhouettes indicate trophic groups and taxonomic classes with higher proportions of threatened species in a given region compared to the background fraction. Regions without symbols had either similar or lower proportions of threatened species compared to the background fraction. Colors illustrate land and marine regional boundaries. Summary statistics can be found in table S1.
Patterns of extinction risk in trophic groups across habitats
We also examined trophic group patterns in extinction risk across five aquatic (marine and inland wetland habitats) and seven terrestrial habitats. Consistent with our previous findings, herbivores, followed by omnivores, had the greatest representation of at-risk species across habitat types. Herbivores had elevated risk in 100% of aquatic habitats and 57% of terrestrial habitats (table S3 and Fig. 3). Herbivorous reptiles inhabiting aquatic ecosystems were highly threatened, with 100% of herbivorous reptiles in marine oceanic, marine intertidal, and marine neritic habitats listed as threatened (Fig. 3). In terrestrial systems, forests had the highest risk of extinction in herbivores, with all three taxonomic classes expressing elevated risk of extinction (Fig. 3). Similar to herbivores, omnivores also showed high levels of risk in all aquatic habitats. Patterns of extinction risk across habitats for omnivores were driven almost entirely by reptiles, except in marine neritic habitats where both omnivorous reptiles and mammals showed elevated risks of extinction (table S3 and Fig. 3). Higher proportions of threatened predators occurred in 60% of aquatic habitats and 14% of terrestrial habitats (table S3 and Fig. 4). Predatory birds, especially those in marine habitats (e.g., seabirds), drove our observed patterns in extinction risk in predators (table S3 and Fig. 3).
Fig. 3. Trophic group patterns in extinction risk across habitat types.
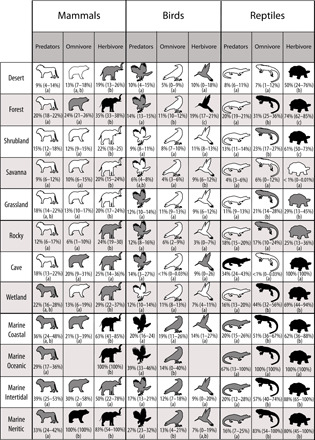
Icons indicate trophic groups with lower proportions (white), similar proportions (gray), or higher proportions (black) of threatened species compared to the background fraction. Numbers indicate the mean proportion (±95% CI) listed as threatened for each trophic group–habitat combination. Letters indicate the results for comparisons across trophic group using Tukey’s post hoc tests. Differing letters indicate significant differences among trophic groups (P < 0.05). Summary statistics can be found in table S2.
Fig. 4. Historical patterns in extinctions across trophic groups.

The mean proportions (±95% CI) of extinct species for recently extinct birds, mammals, and reptiles combined (all species) and separately, and Pleistocene extinct mammals. Asterisks indicate where the proportions of extinct species are significantly different from the background fraction (dashed horizontal line). Numbers indicate total number of species within each group. Letters indicate the results for comparisons across trophic group using Tukey’s post hoc tests. Differing letters indicate significant differences among trophic groups (P < 0.05).
Historical patterns in trophic group extinctions
To examine whether and how past extinctions have influenced current patterns in threatened species, we compared the proportions of recently extinct mammal, bird, and reptile species (i.e., species classified as extinct or extinct in the wild in the last 500 years by the IUCN) and late Pleistocene extinct mammals (beginning 11,000 years ago for Africa, North America, and South America and 50,000 years ago for Australia) in each of our three trophic groups (22). In both recently extinct species and late Pleistocene extinct mammals, we found herbivores to have the greatest proportion of extinct species (table S4 and Fig. 4). In contrast, predators had lower proportions of extinctions compared to herbivores and, except for recently extinct birds, had lower levels of extinction compared to the background proportion. Omnivores had similar levels of extinction compared to the background proportion, with the exception of Pleistocene extinct mammals, which had a significantly lower proportion (table S4 and Fig. 4).
Potential drivers of trophic group patterns in extinction risk
Our above analyses show trophic group patterns in extinction risk across multiple spatial and temporal scales, but it does not determine whether trophic group itself is an important factor driving extinction risk. To answer this question, we ran phylogenetic generalized linear models (GLMs) to test associations between extinction risk, body size, and trophic group. We found evidence that trophic group and body size are important factors driving the extinction risk patterns observed in our study. However, the overall importance and relationship between these two factors varied for mammals, birds, and reptiles (Fig. 5 and table S5).
Fig. 5. Relationships between body mass, trophic group, and threat status.
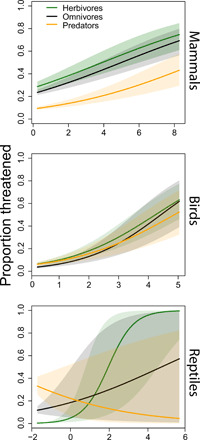
The relationship between body size, trophic group, and the proportion of mammals, birds, and reptiles listed as threatened by the IUCN. Models account for phylogenetic relatedness. Solid lines represent means, while shaded areas represent 95% CI. See table S5 for summary statistics.
For mammals, we found that body mass and trophic group were both important factors that independently affected threat status, with large-bodied organisms and herbivores experiencing the greatest risk of extinction (table S5 and Fig. 5). Neither individual anthropogenic threat drivers (e.g., biological resource use and habitat alteration) nor the total number of anthropogenic drivers experienced by a trophic group helped explain the overrepresentation of threatened herbivores in mammals (table S6 and Fig. 6). In many cases, herbivorous mammals were the least affected or similarly affected by a driver compared to predatory and omnivorous mammals (table S6 and Fig. 6).
Fig. 6. Impacts of anthropogenic drivers of global change on threatened species.
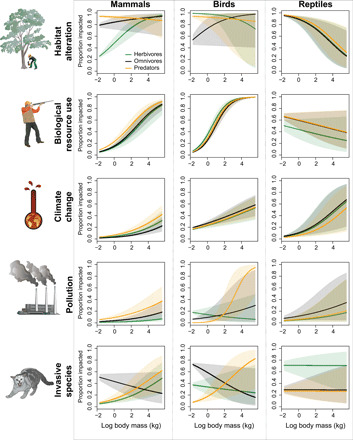
The relationship between body size, trophic group, and the proportion of species affected by habitat alteration, biological resource use, climate change, pollution, and invasive species. Models account for phylogenetic relatedness. Solid lines represent means, while shaded areas represent 95% CI. See tables S6 to S8 for summary statistics.
In birds, we found that body mass was positively correlated with threat status. However, trophic group only marginally affected the threat status of birds, with herbivores having a slightly higher proportion of threatened species compared to predators (table S5 and Fig. 5). In birds, small-bodied herbivores were more affected by habitat alteration and pollution compared to omnivores (Fig. 6). Small herbivorous birds were also more affected by invasive species and pollution compared to small predatory birds; however, this pattern was the reverse in large-bodied birds (table S7 and Fig. 6).
In reptiles, trophic group mediated the association between body mass and threat status. Here, we found that the probability of being threatened was positively related to body mass in herbivores and omnivores, but that the inverse was true for predatory reptiles (table S5 and Fig. 5). For reptiles, invasive species was the only anthropogenic pressure to significantly affect herbivores more than other trophic groups (table S8 and Fig. 6).
DISCUSSION
Numerous studies have helped to identify species’ traits that correlate with extinction risk to shed light on current patterns in the Anthropocene extinction crisis (12, 14, 15). Findings from some of these past studies, alongside a host of single species research focused on extinction threat in charismatic predators, have led to the general assumption that predators are, for a variety of reasons, at a higher risk of extinction than other trophic groups (14–16, 19). By systematically examining the patterns of at-risk species across different trophic groups, our study identified that the threat of extinction is trophically skewed. However, contrary to many of these previous expectations, we found herbivores to be the most at-risk trophic group among mammals, birds, and reptiles. Although geography and habitat influenced taxonomic class-specific results, herbivores consistently had the highest representation of at-risk species in the present day, the recent past, and the late Pleistocene. In many cases, reptiles were the primary group driving our observed patterns in extinction risk of modern herbivores. This result is noteworthy, given that much has yet to be determined regarding their contemporary functional roles in ecosystems (23).
We identified a few instances where predators show elevated risk of extinction. When more detailed diet categories were analyzed, both piscivores and scavengers had elevated risk compared to background levels. Consistent with the higher extinction risk in piscivores, we found that elevated risk of extinction in predators occurred almost exclusively in marine habitats, which suggests that extinction pressures threatening predators may be greater in the ocean than on land. Past research has proposed that, in many regions, humans are preferentially and unsustainably exploiting marine organisms at the top of the food chain (24). However, this research has focused mainly on fish (24), which we did not include in our study. A likely productive future research endeavor would be to determine how the inclusion of bony and cartilaginous fishes and other taxonomic classes not in this study (e.g., invertebrates) would influence interpretations about how trophic group shapes extinction risk overall, as well as how it influences our observations about the relative risk for different trophic groups in terrestrial and aquatic environments.
Although we were unable to identify a single anthropogenic driver for the global decline in herbivores, we did find that certain drivers disproportionately affect some groups of herbivores more relative to other trophic groups. For example, we found that invasive species affect herbivorous reptiles disproportionately compared to omnivores and predators. Invasive vertebrates (e.g., rats), insects (e.g., fire ants), and plants (e.g., Hottentot fig) have all been implicated in the decline and even extinction of several reptiles (25). Furthermore, we found that invasive species, pollution, and habitat alteration affect small herbivorous birds disproportionately. However, for both of these cases, it is unclear why these anthropogenic drivers would target herbivores more than other trophic groups. A new challenge for conservation biology will be to identify clear mechanisms responsible for this apparent association between trophic group and extinction risk. These studies are likely to include investigations into the interactive effects of multiple anthropogenic drivers and their impact upon intrinsic species traits that are associated with herbivory.
Our study determined that trophic group is an important factor driving extinction risk in reptiles, mammals, and birds. Past studies examining the effect of trophic group or diet on extinction risk have found either no effect of trophic group (26) or that higher-order predators are at the highest risk of extinction (14, 16). These past studies, however, focused on select groups of organisms like animals in the order Carnivora (16), or squamate reptiles (26), suggesting that these more focused studies mask the more general role of trophic group as a significant variable that influences extinction patterns in mammals, birds, and reptiles collectively. Critically, our results show that any future endeavors to explain patterns of extinction risk in mammals, birds, and reptiles need to account for trophic group.
In addition to trophic group, our study found that body size is an important trait that independently drives extinction risk in mammals and birds and interacts with trophic group to influence extinction risk in reptiles. Since the late Pleistocene, the average body mass of organisms has been declining because of size-selective threats that are robustly linked to human activities (27). Our results agree with findings from past studies that the selection against large-bodied organisms is likely to continue under business as usual management scenarios (11, 12). Our study adds to this body of literature by showing that, except for predatory reptiles, large-bodied organisms across all three trophic groups are at a high risk of extinction, but herbivores are disproportionately the most at-risk trophic group within these large-bodied species. The ecological effects of the loss of large-bodied herbivores will depend, to some degree, on the capacity of smaller-bodied species to compensate numerically and/or functionally (28). Many body size–specific functions (e.g., dispersal of large-seeded fruits by sloths) cannot, however, be replicated by simply increasing abundances of smaller-bodied species (28).
Prehistoric extinctions of megaherbivores drastically changed the structure and functioning of Earth’s ecosystems by altering vegetation dynamics, fire regimes, carbon cycling, and biogeochemical cycling (7–9). Our results highlight that Earth is once again experiencing declines and extinctions that are disproportionately affecting large herbivores. However, how these declines and extinctions of herbivores are likely to affect the trajectory of life on Earth is not yet known, but studies have linked modern herbivores with ecosystem processes as diverse as the evolution of plant and predator traits, ecosystem resilience/resistance, nutrient cycling, fire regimes, greenhouse gas dynamics, plant regeneration, and primary production (29).
MATERIALS AND METHODS
We created a database containing biological, ecological, and geographical data on all extant mammal (4858), bird (10,910), and reptile (6398) species assessed on the 2019-2 IUCN Red List of Threatened Species (1) as well as recently extinct (extinct in the wild and extinct on the IUCN Red List of Threatened Species) mammals, birds, and reptiles and late Pleistocene extinct mammals derived from McCauley et al. (2) and Smith et al. (27). First, we assigned binary threat assessments to each species. Second, we characterized the diets of each species based on available information. Third, we classified the trophic groups of each species based on our diet characterization. We then combined these data with species-specific geographic location, habitat, and threat information from the IUCN Red List of Threatened Species. We used the completed database to quantify the observed proportions of threatened or extinct species in specific trophic groups (i.e., predator, omnivore, and herbivore) in relation to each other and the background fractions of threatened or extinct species.
Binary threat classification
For each extant species assessed by the IUCN, we assigned a binary threat classification of “threatened” or “nonthreatened.” Species with IUCN Red List assessments of “critically endangered,” “endangered,” and “vulnerable” were considered threatened with extinction, and species with “near threatened,” “least concern,” and “lower risk” assessments were considered nonthreatened. This binary classification system represents the two potential outcomes in an extinction event (i.e., extinction or survival) and is consistent with analytical approaches previously used for examining extinction risk (11). Species assessed as “extinct” or “extinct in the wild” were not included in threatened status analyses. Instead, they were classified as recently extinct in analyses looking at historical trophic group patterns. Species categorized as data deficient by IUCN were not used in our analyses. Mammals from the extinct species lists from McCauley et al. (2) and Smith et al. (27) were classified as extinct in the late Pleistocene and were only used in analyses of historical trophic group patterns.
Diet categories
We classified the diet of all mammal, bird, and reptile species assessed by the IUCN using published literature, reference texts, databases, or extrapolation from related taxonomic class. Species’ diets were determined from various data types (i.e., percent, ranked presence-absence, and unranked presence-absence of specific food items; table S9). We classified diet categories using a binary response of present or absent. Only adult diet information from wild populations was used. The primary diet categories (with subcategories in parentheses) were endotherm (mammal and bird), herptile (amphibian and reptile), fish, invertebrate (insect), carrion, plant (foliage, root, and wood), fruit, nectar, and grain (30, 31). Carrion eaters (i.e., scavengers) were defined as species that consume animal-based carrion. To be classified in the plant category, a species must consume algae, fungus, leaves, shoots, roots, wood, flowers, pollen, sepals, or other miscellaneous plant material (e.g., vegetable matter) (30).
For diets described in the literature as percentages, we used specific percentage cutoffs to classify diet categories as either present or absent. Percentage cutoffs were adjusted on the basis of the number of unique diet items included in a species’ diet, because as the number of diet items increases, the weight of a diet category increases. For example, if a species only consumed two diet items, and one diet item comprised ≤20% of the diet, then that item was classified as absent. However, if the species’ diet had more than two diet items, then any item with a ≥20% contribution was classified as present.
For diets described as presence-absence data, diet categories were classified according to keywords. We included diet items associated with the following keywords: “primary,” “secondary,” “mostly,” “also,” “frequently,” “regularly,” “usually,” and “fair amount.” We excluded diet items associated with the keywords “tertiary,” “sometimes,” “occasionally,” “rarely,” “small quantities,” “and even,” “opportunistically,” and “at times supplemented with.” If a diet item was associated with uncertainty statements of “probably,” “possibly,” “may,” or “presumably,” then it was excluded unless no other diet information was available. For diets described using unranked presence-absence data (e.g., “eats fruit, insects, and seeds”), all listed diet items were classified as present.
Trophic group
Using dietary information, we classified species into three trophic groups: predator, omnivore, and herbivore. For ranked presence-absence data, predators were defined as animals that primarily consumed animal-based diet items and could consume plant-based diet items in small amounts; herbivores primarily consumed plant-based diet items and could consume animal-based diet items in small amounts, and omnivores primarily or secondarily consumed a mixture of both types of diet items. For unranked presence-absence data, predators consumed only animal-based diet items, herbivores consumed only plant-based diet items, and omnivore diets included items from both categories. To explore the sensitivity of results to our classification of trophic groups, trophic groups were classified in three different ways where the contributions of the herbivore or predator diet shifted: 70% cutoff, 80% cutoff, and 90% cutoff. Results described in the main text represent the most conservative view of predators and herbivores, with predators defined as species that consume a ≥90% animal-based diet, herbivores as species that consume a ≥90% plant-based diet, and omnivores as species that consume an 11 to 89% animal or plant-based diet. The 80% diet defines predators as species that consume a ≥80% animal-based diet, herbivores as species that consume a ≥80% plant-based diet, and omnivores as species that consume a 21 to 79% animal or plant-based diet. The 70% diet defines predators as species that consume a ≥70% animal-based diet, herbivores as species that consume a ≥70% plant-based diet, and omnivores as species that consume a 31 to 69% animal or plant-based diet. In general, our conclusions about how trophic group shaped extinction risk were insensitive to our methods for defining trophic groups (fig. S1).
When diet information could not be found for a species, we extrapolated trophic group from the sister taxonomic class. If a species was formerly classified as another species or a subspecies, then the trophic group from the former classification was used. If the species was not formally classified as another species or a subspecies, then trophic group was extrapolated from the closest living congener or confamiliar using previously published phylogenies (32–34). To ground-truth this approach, we first removed all extrapolated species from our dataset and then split the remaining data into training data (75% of the data) and test data (25%). We then assigned trophic group to species in our test data set using the trophic group of the closest living relative in our training data. We repeated this process across 1000 bootstrapped phylogenies to provide a mean accuracy (±SE) and compared our extrapolated trophic group results with the known trophic groups in the original data set. We found that our accuracies for predicting the trophic group of mammals, birds, and reptiles were 94% (±0.02), 82% (±0.03), and 88% (±0.05), respectively.
For late Pleistocene extinct mammals as listed by Smith et al. (27), only trophic group was classified and not diet due to a paucity of specific diet information. Trophic group was categorized on the basis information collated from the primary literature, databases, and closest extant congeners using similar protocols to the dietary categorization described previously.
Regions, habitats, and anthropogenic global change drives
To explore anthropogenic effects on trophic group patterns and the variability in threat classifications, we downloaded all species-specific extrinsic variables (i.e., habitats, regions, and associated anthropogenic threats) from the IUCN Red List of Threatened Species. Major geographic (land and marine) regions, habitats, and anthropogenic threats were classified as either present or absent for each species. The major anthropogenic global change drives classified by the IUCN were simplified into five major anthropogenic threat categories (3): habitat alteration (residential and commercial development, agriculture and aquaculture, energy production and mining, transportation and service corridors, human intrusions and disturbance, or natural system modifications), biological resource use (e.g., overexploitation), climate change, pollution, and invasive species.
Body size
We used adult body size for classification. When male and female body sizes were given, we used the geometric mean of the two values. When a range of body sizes was given, we used the geometric mean of the smallest and largest value (30). Body mass was most readily available for birds and mammals. However, body size measurements for reptiles were not standardized across taxonomic orders. Body size for Squamata was commonly reported as total length or snout-vent length, while body size for Testudines was reported as standard carapace length. To overcome nonstandardization methods for measuring body size, we converted all length measurements to body mass using previously published family-level length-mass regressions. References for length-mass regressions are provided in the Supplementary Materials. If previously published regressions were not available, then we developed regressions from species in our data that contained both body mass and length data. For species with missing body size data, we extrapolated body size using the average of all congeners (excluding congeners that had been previously extrapolated this way).
Analyses
The IUCN Red List of Threatened Species consists of imperfect data as many species are classified as data deficient, and others have not been described at all. Because of the imperfect nature of the IUCN data, we used the available data to generate mean estimates and 95% confidence limits of the proportions of threatened species for different trophic groups. Therefore, although we may not know what the exact true proportion of threatened species is for each trophic group, our CIs indicate boundaries within which the true number likely lies. We investigated patterns in the proportion of threatened species for each trophic group (herbivore, omnivore, and predator) at the global scale for extant vertebrate species (mammals, birds, and reptiles), recently extinct vertebrate species (mammals, birds, and reptiles), and Pleistocene extinct vertebrate species (mammals only). Differences in proportions of threatened species among trophic groups were analyzed using GLMs, with threat status as the response variable and trophic group as the predictor variable. We used a binomial error structure (threatened or not threatened) and a logit link function. Each species was treated as a single replicate and recorded as either threatened or not, or when historical patterns were examined, extinct or not. Following the GLM, post hoc Tukey’s tests were used to identify differences among trophic groups in the proportions of threatened or extinct species. For each trophic group, we produced mean estimates and 95% CIs of the proportion of threatened species using the “predict” function in R. These 95% CIs were compared to the proportion of threatened or extinct species across background levels, which were calculated by taking the number of threatened species and dividing it by the total number of species in that analysis. If the 95% CI did not intersect the background fraction of threatened species (i.e., background level), then the trophic group was designated as having a higher or lower proportion of threatened species than the background fraction. After running the analysis across all taxa in the IUCN Red List of Threatened Species, we split the data on the basis of taxonomic class and ran individual models for each class separately.
To understand the relationships between specific diet types and the proportion of threatened species, we ran subsequent analyses where each diet type was treated as a binary descriptive variable. This technique meant that each species could be classified in multiple diet categories (i.e., an herbivore could be classed as consuming nectar and grain), meaning that the diet categories were not mutually exclusive. We used a GLM to understand how different diet categories are compared to the background fraction of threatened species, and again used the predict function to generate means and 95% CIs for each diet type. These 95% CIs for each specific diet were then compared to the background fraction of threatened species.
Following the global analyses, we split our data on the basis of habitat type and geographic region and repeated the analyses to understand how patterns in threat status of different trophic groups varied across geographic regions and habitat types. For each habitat or geographic region, we repeated the techniques described above for the global analyses. First, we used a GLM and post hoc Tukey’s test to assess differences in the proportion of threatened species among trophic groups for each habitat or region. We then used the predict function to generate mean proportions and 95% CIs for each taxonomic class and compared them to the proportion of threatened species within that taxonomic class (either all species, mammals, birds, or reptiles) across the globe. All analyses were carried out in R.
To determine potential drivers of extinction risk, we ran phylogenetic GLMs with both trophic group and body size as predictor variables. To determine whether specific anthropogenic drivers or the total number of anthropogenic drivers disproportionately affected herbivores, we examined the proportion of threatened species in each trophic group affected by resource use, climate change, habitat alteration, invasive species, and pollution while accounting for both body mass and phylogeny. Phylogenetic signal is well documented in IUCN threat status data (33, 35), and to ensure that this would not affect our models assessing support for potential mechanisms (36), we used a phylogenetic comparative approach. Our use of phylogenetic GLMs (37), as implemented in phylolm (38), ensures that our model estimates are robust to species shared evolutionary history. Further, to account for uncertainty about phylogenetic topology or divergence dates, we repeated our analyses across Bayesian posterior distributions of phylogenetic trees [reviewed in (39)]. We took 1000 phylogenies for each taxonomic group—Faurby and Svenning (32) for mammals, Jetz et al. (34) for birds, and Tonini et al. (33) for reptiles—and repeated all analyses across them, reporting mean parameter estimates across all these models. To ensure that our trait and phylogenetic data were compatible, we matched all phylogenetic data to the taxonomy used by IUCN (that is, the basis of our trait data; see above) using taxize (40). We used Akaike information criterion to select the most parsimonious model. R codes to repeat our analyses are given in the Supplementary Materials.
Supplementary Material
Acknowledgments
Symbols in Figs. 1 to 5 were provided by Freepik, and images in Fig. 6 are courtesy of the Integration and Application Network, University of Maryland Center for Environmental Science (ian.umces.edu/symbols/). Funding: Funding was provided by a USU Ecology Center and Graduate Enhancement Award to S.A.V.; an Early Career Research Fellowship from the Gulf Research Program of the National Academies of Sciences, Engineering, and Medicine to T.B.A.; a gift to UC Santa Barbara from Marc and Lynne Benioff in the form of support for the Benioff Ocean Initiative to D.J.M.; Utah State University–Utah Agricultural Experiment Station (journal paper number 9282); and the NSF (ABI-1759965 and EF-1802605) and the U.S. Forest Service (18-CS-11046000-041) grants to W.D.P. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Gulf Research Program of the National Academies of Sciences, Engineering, and Medicine. Author contributions: T.B.A. and S.A.V. contributed equally to the work. T.B.A., E.H., E.M.P.M., D.J.M., and S.A.V. designed the study; S.A.V. and K.H.B. collected data; E.H. and W.D.P. analyzed data; and S.A.V. and T.B.A. wrote the manuscript. All authors contributed to editing of the manuscript. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/32/eabb8458/DC1
REFERENCES AND NOTES
- 1.IUCN, The IUCN Red List of Threatened Species. Version 2019–2 (IUCN, 2019); http://www.iucnredlist.org.
- 2.McCauley D. J., Pinsky M. L., Palumbi S. R., Estes J. A., Joyce F. H., Warner R. R., Marine defaunation: Animal loss in the global ocean. Science 347, 1255641 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Young H. S., Mccauley D. J., Galetti M., Dirzo R., Patterns, causes, and consequences of Anthropocene defaunation. Annu. Rev. Ecol. Evol. Syst. 47, 333–358 (2016). [Google Scholar]
- 4.Barnosky A. D., Koch P. L., Feranec R. S., Wing S. L., Shabel A. B., Assessing the causes of late Pleistocene extinctions on the continents. Science 306, 70–75 (2004). [DOI] [PubMed] [Google Scholar]
- 5.Urban M., Accelerating extinction risk from climate change. Science 348, 571–573 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Hooper D. U., Adair E. C., Cardinale B. J., Byrnes J. E. K., Hungate B. A., Matulich K. L., Gonzalez A., Duffy J. E., Gamfeldt L., O’Connor M. I., A global synthesis reveals biodiversity loss as a major driver of ecosystem change. Nature 486, 105–108 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Barnosky A. D., Lindsey E. L., Villavicencio N. A., Bostelmann E., Hadly E. A., Wanket J., Marshall C. R., Variable impact of late-Quaternary megafaunal extinction in causing ecological state shifts in North and South America. Proc. Natl. Acad. Sci. U.S.A. 113, 856–861 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith F. A., Hammond J. I., Balk M. A., Elliott S. M., Lyons S. K., Pardi M. I., Tomé C. P., Wagner P. J., Westover M. L., Exploring the influence of ancient and historic megaherbivore extirpations on the global methane budget. Proc. Natl. Acad. Sci. U.S.A. 113, 874–879 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gill J. L., Ecological impacts of the late Quaternary megaherbivore extinctions. New Phytol. 201, 1163–1169 (2014). [DOI] [PubMed] [Google Scholar]
- 10.Gill J. L., Williams J. W., Jackson S. T., Lininger K. B., Robinson G. S., Pleistocene megafaunal collapse, novel plant communities, and enhanced fire regimes in North America. Science 326, 1100–1104 (2009). [DOI] [PubMed] [Google Scholar]
- 11.Ripple W. J., Wolf C., Newsome T. M., Hoffmann M., Wirsing A. J., McCauley D. J., Extinction risk is most acute for the world’s largest and smallest vertebrates. Proc. Natl. Acad. Sci. U.S.A. 114, 10678–10683 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cardillo M., Mace G. M., Jones K. E., Bielby J., Bininda-Emonds O. R. P., Sechrest W., Orme C. D. L., Purvis A., Multiple causes of high extinction risk in large mammal species. Science 309, 1239–1241 (2005). [DOI] [PubMed] [Google Scholar]
- 13.Estes J. A., Terborgh J., Brashares J. S., Power M. E., Berger J., Bond W. J., Carpenter S. R., Essington T. E., Holt R. D., Jackson J. B. C., Marquis R. J., Oksanen L., Oksanen T., Paine R. T., Pikitch E. K., Ripple W. J., Sandin S. A., Scheffer M., Schoener T. W., Shurin J. B., Sinclair A. R. E., Soulé M. E., Virtanen R., Wardle D. A., Trophic downgrading of planet Earth. Science 333, 301–306 (2011). [DOI] [PubMed] [Google Scholar]
- 14.Purvis A., Gittleman J. L., Cowlishaw G., Mace G. M., Predicting extinction risk in declining species. Proc. Biol. Sci. 267, 1947–1952 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holt R. D., Lawton J. H., Polis G. A., Martinez N. D., Trophic rank and the species-area relationship. Ecology 80, 1495–1504 (1999). [Google Scholar]
- 16.Cardillo M., Purvis A., Sechrest W., Gittleman J. L., Bielby J., Mace G. M., Human population density and extinction risk in the world’s carnivores. PLOS Biol. 2, e197 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wolf C., Ripple W. J., Prey depletion as a threat to the world’s large carnivores. R. Soc. Open Sci. 3, 160252 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carbone C., Gittleman J. L., A common rule for the scaling of carnivore density. Science 295, 2273–2276 (2002). [DOI] [PubMed] [Google Scholar]
- 19.Ripple W. J., Estes J. A., Beschta R. L., Wilmers C. C., Ritchie E. G., Hebblewhite M., Berger J., Elmhagen B., Letnic M., Nelson M. P., Schmitz O. J., Smith D. W., Wallach A. D., Wirsing A. J., Status and ecological effects of the world’s largest carnivores. Science 343, 1241484 (2014). [DOI] [PubMed] [Google Scholar]
- 20.Bello C., Galetti M., Pizo M. A., Magnago L. F. S., Rocha M. F., Lima R. A. F., Peres C. A., Ovaskainen O., Jordano P., Defaunation affects carbon storage in tropical forests. Sci. Adv. 1, e1501105 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Venter O., Sanderson E. W., Magrach A., Allan J. R., Beher J., Jones K. R., Possingham H. P., Laurance W. F., Wood P., Fekete B. M., Levy M. A., Watson J. E. M., Sixteen years of change in the global terrestrial human footprint and implications for biodiversity conservation. Nat. Commun. 7, 12558 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smith F. A., Lyons S. K., Ernest S. K. M., Jones K. E., Kaufman D. M., Dayan T., Marquet P. A., Brown J. H., Haskell J. P., Body mass of late Quaternary mammals. Ecology 84, 3403 (2003). [Google Scholar]
- 23.de Miranda E. B. P., The plight of reptiles as ecological actors in the tropics. Front. Ecol. Evol. 5, 159 (2017). [Google Scholar]
- 24.Myers R. A., Worm B., Rapid worldwide depletion of predatory fish communities. Nature 423, 280–283 (2003). [DOI] [PubMed] [Google Scholar]
- 25.Gibbons J. W., Scott D. E., Ryan T. J., Buhlmann K. A., Tuberville T. D., Metts B. S., Greene J. L., Mills T., Leiden Y., Poppy S., Winne C. T., The global decline of reptiles, Déjà Vu Amphibians: Reptile species are declining on a global scale. Six significant threats to reptile populations are habitat loss and degradation, introduced invasive species, environmental pollution, disease, unsustainable use, and global climate change. BioScience 50, 653–666 (2000). [Google Scholar]
- 26.Böhm M., Williams R., Bramhall H. R., Mcmillan K. M., Davidson A. D., Garcia A., Bland L. M., Bielby J., Collen B., Correlates of extinction risk in squamate reptiles: The relative importance of biology, geography, threat and range size. Glob. Ecol. Biogeogr. 25, 391–405 (2016). [Google Scholar]
- 27.Smith F. A., Smith R. E. E., Lyons S. K., Payne J. L., Body size downgrading of mammals over the late Quaternary. Science 313, 310–313 (2018). [DOI] [PubMed] [Google Scholar]
- 28.Sekar N., Lee C.-L., Sukumar R., Functional nonredundancy of elephants in a disturbed tropical forest. Conserv. Biol. 31, 1152–1162 (2017). [DOI] [PubMed] [Google Scholar]
- 29.Forbes E. S., Cushman J. H., Burkepile D. E., Young T. P., Klope M., Young H. S., Synthesizing the effects of large, wild herbivore exclusion on ecosystem function. Funct. Ecol. 33, 1597–1610 (2019). [Google Scholar]
- 30.Wilman H., Belmaker J., Jennifer S., de la Rosa C., Rivadeneira M. M., Jetz W., EltonTraits 1.0: Species-level foraging attributes of the world’s birds and mammals. Ecology 95, 2027 (2014). [Google Scholar]
- 31.Kissling W. D., Dalby L., Fløjgaard C., Lenoir J., Sandel B., Sandom C., Trøjelsgaard K., Svenning J.-C., Establishing macroecological trait datasets: Digitalization, extrapolation, and validation of diet preferences in terrestrial mammals worldwide. Ecol. Evol. 4, 2913–2930 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Faurby S., Svenning J.-C., A species-level phylogeny of all extant and late Quaternary extinct mammals using a novel heuristic-hierarchical Bayesian approach. Mol. Phylogenet. Evol. 84, 14–26 (2015). [DOI] [PubMed] [Google Scholar]
- 33.Tonini J. F. R., Beard K. H., Ferreira R. B., Jetz W., Pyron R. A., Fully-sampled phylogenies of squamates reveal evolutionary patterns in threat status. Biol. Conserv. 204, 23–31 (2016). [Google Scholar]
- 34.Jetz W., Thomas G. H., Joy J. B., Hartmann K., Mooers A. O., The global diversity of birds in space and time. Nature 491, 444–448 (2012). [DOI] [PubMed] [Google Scholar]
- 35.Cannicci S., Burrows D., Fratini S., Smith T. J. III, Offenberg J., Dahdouh-Guebas F., Faunal impact on vegetation structure and ecosystem function in mangrove forests: A review. Aquat. Bot. 89, 186–200 (2008). [Google Scholar]
- 36.Cooper N., Jetz W., Freckleton R. P., Phylogenetic comparative approaches for studying niche conservatism. J. Evol. Biol. 23, 2529–2539 (2010). [DOI] [PubMed] [Google Scholar]
- 37.Ives A. R., Garland T. Jr., Phylogenetic logistic regression for binary dependent variables. Syst. Biol. 59, 9–26 (2010). [DOI] [PubMed] [Google Scholar]
- 38.Tung Ho L. S., Ané C., A linear-time algorithm for Gaussian and non-Gaussian trait evolution models. Syst. Biol. 63, 397–408 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Huelsenbeck J. P., Ronquist F., Nielsen R., Bollback J. P., Bayesian inference of phylogeny and its impact on evolutionary biology. Science 294, 2310–2314 (2001). [DOI] [PubMed] [Google Scholar]
- 40.Chamberlain S. A., Szöcs E., Taxize: Taxonomic search and retrieval in R. F1000Res. 2, 191 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/32/eabb8458/DC1


