Abstract
Purpose
To investigate the clinicopathological and prognostic factors related to early gastric cancer recurrence after curative resection.
Patients and Methods
Between October 2006 and August 2018, a total of 149 patients with recurrence of gastric cancer/adenocarcinoma of the esophagogastric junction after curative resection were enrolled from our treatment group. A retrospective clinical analysis was performed on these patients with gastric cancer recurrence after curative resection.
Results
Among the 149 patients, 99 (66.4%) had only one recurrence pattern, and 50 (33.6%) had multiple recurrence patterns. The median recurrence-free survival (RFS) was 18.2 months (95% CI 15.0–21.4). Ninety-four patients (63.1%) experienced early recurrence (recurrence within 24 months after curative resection), and 55 patients (36.9%) experienced late recurrence (recurrence beyond 24 months after curative resection). The univariate analysis showed that perineural invasion (P=0.002), depth of invasion (P=0.026), postoperative chemotherapy (P=0.036) and postoperative complications (P=0.004) were significant factors associated with early recurrence after curative resection for gastric cancer. Perineural invasion (P=0.003), postoperative chemotherapy (P=0.036) and postoperative complications (P=0.042) were independent factors associated with early recurrence after curative resection in the multivariate analysis. The survival analysis showed that perineural invasion (P=0.011) and postoperative complications (P=0.007) were independent prognostic factors. The median survival time of early recurrence patients was significantly shorter than that of late recurrence patients (25.4 vs 62.9 months, P<0.001).
Conclusion
Perineural invasion, postoperative chemotherapy and postoperative complications were independent factors associated with early recurrence after curative resection. Patients with early recurrence after curative resection had poorer survival.
Keywords: stomach neoplasms, gastrectomy, neoplasm recurrence and metastasis, prognosis
Introduction
Gastric cancer is the fifth most common malignancy and the third leading cause of cancer death worldwide.1 It is also one of the most common malignant tumors in China. Statistics released in 2019 showed that the morbidity and mortality of gastric cancer rank second and third in the country, respectively.2 Treatments for gastric cancer include surgery, chemotherapy, radiotherapy, targeted therapy, and immunotherapy. To date, curative resection has been considered the only way to cure gastric cancer. Approximately 60.8% of patients with gastric cancer in China will experience recurrence, and 42.5% of patients experience recurrence within two years after curative resection. The recurrence of tumors has become the main cause of death for patients with gastric cancer.3 This study retrospectively analyzed the clinicopathological factors of 149 patients with recurrence after curative resection. The parameters that determined the timing of recurrence and their association with prognosis were investigated by univariate and multivariate analyses. The findings of this study are helpful for predicting whether patients will experience early recurrence after surgery. Taking effective measures accordingly may prevent early recurrence and improve survival.
Patients and Methods
Patients
Between October 2006 and August 2018, a total of 149 patients with recurrence of gastric cancer/adenocarcinoma of the esophagogastric junction after curative resection (R0 resection) were enrolled from our treatment group (Table 1). All of these patients underwent lymphadenectomy higher than D2 (complete removal of group 1 and 2 lymph nodes). All patients’ clinicopathological characteristics, including gender, age at surgery, histological type, perineural invasion, lymphovascular invasion, depth of invasion (T stage), lymph node metastasis (N stage), TNM stage (according to the 8th Edition of the American Joint Committee on Cancer staging manual),4 postoperative chemotherapy, recurrence patterns, tumor location, postoperative complications, and survival data, were retrospectively reviewed based on operative notes, medical records and telephone follow-ups. Among 123 patients who received postoperative adjuvant chemotherapy, 26 patients (21.1%) received chemotherapy containing S-1 and oxaliplatin (SOX), 22 patients (17.9%) received chemotherapy containing fluorouracil and oxaliplatin (FOLFOX), 21 patients (17.1%) received chemotherapy containing fluorouracil and paclitaxel, 10 patients (8.1%) received chemotherapy containing capecitabine and oxaliplatin (XELOX), and 44 patients (35.8%) received other regimens.
Table 1.
Clinicopathological Characteristics of 149 Patients
| Clinicopathological Characteristics | n (%) |
|---|---|
| Gender | |
| Male | 106 (71.1) |
| Female | 43 (28.9) |
| Age at surgery, years, median (range) | 54.8 (20–78) |
| Histological type | |
| Well-differentiated tumors | 36 (24.2) |
| Poorly differentiated tumors | 113 (75.8) |
| Perineural invasion | |
| Negative | 70 (47.0) |
| Positive | 79 (53.0) |
| Lymphovascular invasion | |
| Negative | 68 (45.6) |
| Positive | 81 (54.4) |
| Depth of invasion (T stage) | |
| T1 | 5 (3.4) |
| T2 | 11 (7.4) |
| T3 | 38 (25.5) |
| T4 | 95 (63.7) |
| Lymph node metastasis (N stage) | |
| N0 | 25 (16.8) |
| N1 | 13 (8.7) |
| N2 | 43 (28.9) |
| N3 | 68 (45.6) |
| TNM stage | |
| I | 10 (6.7) |
| II | 19 (12.8) |
| III | 120 (80.5) |
| Postoperative chemotherapy | |
| 0 | 26 (17.4) |
| 1–3 cycles | 32 (21.5) |
| ≥4 cycles | 91 (61.1) |
| Postoperative complications | |
| Negative | 141 (94.6) |
| Positive | 8 (5.4) |
| Tumor location | |
| Upper | 53 (35.6) |
| Middle | 55 (36.9) |
| Lower | 37 (24.8) |
| Entire | 4 (2.7) |
| Recurrence patterns | |
| Locoregional recurrence | 33 (22.2) |
| Hematogenous metastasis | 32 (21.5) |
| Distant lymphatic recurrence | 0 |
| Peritoneal implantation | 34 (22.7) |
| Multiple recurrence patterns | 50 (33.6) |
Abbreviation: TNM, tumor node metastasis.
Clinicopathological Factor Identification
The patients were divided into 2 groups: the early recurrence group, which included patients who had recurrence within 2 years after curative resection, and the late recurrence group, which included patients who had recurrence more than 2 years after curative resection. Well-differentiated tumors included papillary carcinoma and highly or moderately differentiated tubular adenocarcinoma. Poorly differentiated tumors included low differentiated tubular adenocarcinoma, mucinous adenocarcinoma, and signet ring cell carcinoma. According to the postoperative chemotherapy strategy, the patients were divided into three groups: no chemotherapy, 1–3 cycles of chemotherapy and ≥ 4 cycles of chemotherapy. Postoperative complications included infection, anastomotic leakage, postoperative hemorrhage and intestinal obstruction occurring within 60 days after radical gastrectomy. The main patterns of recurrence were recorded as the first site of detectable failure at the time of diagnosis. The recurrence patterns included locoregional recurrence (gastric or nodal), hematogenous metastasis (such as liver, lung, and bone), distant lymphatic recurrence, peritoneal implantation (peritoneal nodules, ascites, and Krukenberg tumors), and multiple recurrence patterns.
Postoperative Follow-Up
The patients were followed closely until September 2019; the average length of follow-up was 46.9 (range 4.1–194.9) months. Follow-up assessments were performed every 3 months for the first 2 years after surgery, every 6 months for 3–5 years, and yearly thereafter. Routine follow-up consisted of physical examination, laboratory tests, chest radiography, abdominopelvic ultrasonography, computed tomography (CT) and magnetic resonance imaging (MRI). Recurrence-free survival (RFS) was defined as the time from surgery to recurrence. Overall survival (OS) was defined as the time from curative resection of gastric cancer to death or the last follow-up time.
Statistical Analysis
All data were analyzed with SPSS 21.0 (SPSS Inc., Chicago, IL, USA) software. Categorical variables were analyzed with a chi-squared test and Fisher’s exact test. Univariate analyses were performed with the Kaplan–Meier method. In the multivariate analysis, a Cox regression analysis was applied to identify independent clinicopathological factors that were associated with early recurrence and survival. The survival analyses and curves were established with the Kaplan–Meier method and compared with the Log rank test. P < 0.05 was considered statistically significant.
Results
Length of Time to Recurrence and Recurrence Patterns
The median RFS was 18.2 months (95% CI 15.0–21.4). The early recurrence group included 94 (63.1%) patients, and the late recurrence group included 55 (36.9%) patients. The median RFS of the early recurrence group was 12.2 months (95% CI 10.2–14.1). The median RFS of the late recurrence group was 37.5 months (95% CI 28.1–46.9).
Among the 149 patients, 99 (66.4%) had only one recurrence pattern, and 50 (33.6%) had multiple recurrence patterns. As a single pattern, peritoneal implantation (22.7%) was observed most frequently, followed by locoregional recurrence (22.2%) and hematogenous metastasis (21.5%). The most common site of hematogenous metastasis was the liver, occurring in 18 patients (12.1%) in total and as the only site of recurrence in 16 patients. No patient had solitary distant lymphatic recurrence. The recurrence pattern of distant lymphatic recurrence was accompanied by other recurrence patterns.
Clinicopathological Factors According to the Recurrence Time
The clinicopathological factors of the early recurrence group and the late recurrence group were compared in Table 2. In the early recurrence group, perineural invasion occurred frequently (63.8% vs 36.2% of patients; χ2=11.946, P = 0.001). In addition, gender, age at surgery, histological type, lymphovascular invasion, depth of invasion, lymph node metastasis, TNM stage, postoperative chemotherapy, postoperative complications, tumor location and recurrence patterns were not significantly different between the two groups (P> 0.05).
Table 2.
Comparison of Clinicopathological Factors Between Early and Late Recurrence of Gastric Cancer After Curative Resection n (%)
| Clinicopathologic Factors | Early Recurrence | Late Recurrence | χ2 | P-value |
|---|---|---|---|---|
| (n = 94) | (n = 55) | |||
| Gender | 0.178 | 0.710 | ||
| Male | 68 (72.3) | 38 (69.1) | ||
| Female | 26 (27.7) | 17 (30.9) | ||
| Age at surgery | 0.016 | 1.000 | ||
| ≤60 years | 64 (68.1) | 38 (69.1) | ||
| >60 years | 30 (31.9) | 17 (30.9) | ||
| Histological type | 0.461 | 0.554 | ||
| Well-differentiated tumors | 21 (22.3) | 15 (27.3) | ||
| Poorly differentiated tumors | 73 (77.7) | 40 (72.7) | ||
| Perineural invasion | 11.946 | 0.001 | ||
| Negative | 34 (36.2) | 36 (65.5) | ||
| Positive | 60 (63.8) | 19 (34.5) | ||
| Lymphovascular invasion | 0.976 | 0.395 | ||
| Negative | 40 (42.6) | 28 (50.9) | ||
| Positive | 54 (57.4) | 27 (49.1) | ||
| Depth of invasion (T stage) | 4.733 | 0.202 | ||
| T1 | 2 (2.1) | 3 (5.5) | ||
| T2 | 6 (6.4) | 5 (9.1) | ||
| T3 | 29 (30.9) | 9 (16.4) | ||
| T4 | 57 (60.6) | 38 (69.0) | ||
| Lymph node metastasis (N stage) | 3.263 | 0.354 | ||
| N0 | 12 (12.8) | 13 (23.6) | ||
| N1 | 9 (9.6) | 4 (7.3) | ||
| N2 | 27 (28.7) | 16 (29.1) | ||
| N3 | 46 (48.9) | 22 (40.0) | ||
| TNM stage | 2.897 | 0.230 | ||
| I | 4 (4.3) | 6 (10.9) | ||
| II | 11 (11.7) | 8 (14.5) | ||
| III | 79 (84.0) | 41(74.6) | ||
| Postoperative chemotherapy | 2.525 | 0.302 | ||
| 0 | 16 (17.0) | 10 (18.2) | ||
| 1–3 cycles | 24 (25.5) | 8 (14.5) | ||
| ≥4 cycles | 54 (57.5) | 37 (67.3) | ||
| Postoperative complications | 2.164 | 0.259 | ||
| Negative | 87 (92.6) | 54 (98.2) | ||
| Positive | 7 (7.4) | 1 (1.8) | ||
| Tumor location | 6.710 | 0.081 | ||
| Upper | 31 (33.0) | 22 (40.0) | ||
| Middle | 41 (43.6) | 14 (25.5) | ||
| Lower | 21 (22.3) | 16 (29.1) | ||
| Entire | 1 (1.1) | 3 (5.4) | ||
| Recurrence patterns | 1.827 | 0.614 | ||
| Locoregional recurrence | 21 (22.3) | 12 (21.8) | ||
| Hematogenous metastasis | 22 (23.4) | 10 (18.2) | ||
| Peritoneal implantation | 23 (24.5) | 11(20.0) | ||
| Multiple recurrence patterns | 28 (29.8) | 22 (40.0) |
Abbreviation: TNM, tumor node metastasis.
Univariate and Multivariate Analyses of Early Recurrence
Univariate analyses were performed with the Kaplan–Meier method. They indicated that perineural invasion (P= 0.002), depth of invasion (P= 0.026), postoperative chemotherapy (P= 0.036), and postoperative complications (P= 0.004) were significant factors associated with early recurrence after curative resection. Gender, age at surgery, histological type, lymphovascular invasion, lymph node metastasis, TNM stage, tumor location and recurrence patterns did not show significant associations with early recurrence (Table 3).
Table 3.
Results of the Univariate Analysis of Factors for Early Recurrence of Gastric Cancer After Curative Resection
| Clinicopathological Factors | Median RFS Months (95% CI) | χ2 | P-value |
|---|---|---|---|
| Gender | 0.194 | 0.66 | |
| Male | 16.3(13.7–18.9) | ||
| Female | 19.3(12.8–25.8) | ||
| Age at surgery | 0 | 0.996 | |
| ≤60 years | 18.3(14.9–21.6) | ||
| >60 years | 16.2(12.2–20.3) | ||
| Histological type | 0.298 | 0.585 | |
| Well-differentiated tumors | 18.3(12.3–24.2) | ||
| Poorly differentiated tumors | 16.3(12.3–20.4) | ||
| Perineural invasion | 9.154 | 0.002 | |
| Negative | NR | ||
| Positive | 14.2(11.6–16.9) | ||
| Lymphovascular invasion | 1.326 | 0.25 | |
| Negative | 19.3(13.2–25.4) | ||
| Positive | 16.2(11.8–207) | ||
| Depth of invasion (T stage) | 9.256 | 0.026 | |
| T1 | NR | ||
| T2 | 14.3 | ||
| T3 | 11.1(6.6–15.7) | ||
| T4 | 19.3(16.1–22.6) | ||
| Lymph node metastasis (N stage) | 1.804 | 0.614 | |
| N0 | NR | ||
| N1 | 15.2(10.5–19.9) | ||
| N2 | 18.2(13.0–23.4) | ||
| N3 | 17.2(13.1–21.4) | ||
| TNM stage | 1.659 | 0.428 | |
| I | NR | ||
| II | 15.2(4.9–25.5) | ||
| III | 17.3(14.8–19.8) | ||
| Postoperative chemotherapy | 6.653 | 0.036 | |
| 0 | 14.2(5.3–23.1) | ||
| 1–3 cycles | 13.2(3.3–23.0) | ||
| ≥4 cycles | 19.3(15.9–22.8) | ||
| Postoperative complications | 8.453 | 0.004 | |
| Negative | 18.3(15.4–21.1) | ||
| Positive | 3.1(2.2–4.0) | ||
| Tumor location | 5.456 | 0.141 | |
| Upper | 19.3(13.2–25.4) | ||
| Middle | 15.2(11.4–19.0) | ||
| Lower | 17.2(10.0–24.5) | ||
| Entire | NR | ||
| Recurrence patterns | 1.654 | 0.647 | |
| Locoregional recurrence | 17.2(11.5–22.9) | ||
| Hematogenous metastasis | 12.2(6.5–17.9) | ||
| Peritoneal implantation | 19.2(13.5–24.9) | ||
| Multiple recurrence patterns | 18.3(13.6–23.0) |
Abbreviations: TNM, tumor node metastasis; RFS, recurrence-free survival; CI, confidence interval; NR, not reached.
A multivariate analysis was performed using a Cox regression. The independent risk factors associated with the early recurrence of gastric cancer after curative resection were perineural invasion (P= 0.003), postoperative chemotherapy (P =0.036) and postoperative complications (P = 0.042) (Table 4).
Table 4.
Results of the Multivariate Analysis of Factors for Early Recurrence of Gastric Cancer After Curative Resection
| Factors | B | SE | Wald | df | P | Exp(B) | 95.0% CI |
|---|---|---|---|---|---|---|---|
| Perineural invasion | 0.667 | 0.222 | 9.02 | 1 | 0.003 | 1.948 | 1.261~3.011 |
| Postoperative chemotherapy | −0.278 | 0.133 | 4.391 | 1 | 0.036 | 0.757 | 0.584~0.982 |
| Postoperative complications | −0.813 | 0.401 | 4.123 | 1 | 0.042 | 0.443 | 0.202~0.972 |
Abbreviations: B, regression coefficient; SE, standard error; df, degree of freedom; Exp(B), odds ratio; CI, confidence interval.
Survival Analysis
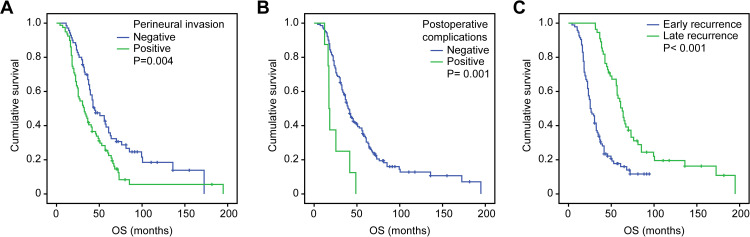
The univariate analysis indicated that perineural invasion (P= 0.004) and postoperative complications (P= 0.001) were significant factors associated with OS. Gender, age at surgery, histological type, lymphovascular invasion, lymph node metastasis, depth of invasion, TNM stage, postoperative chemotherapy, tumor location and recurrence patterns did not show significant associations with OS (Table 5, Figure 1A and B). The multivariate analysis indicated that OS was independently influenced by perineural invasion (P= 0.011) and postoperative complications (P = 0.007) (Table 6). The median survival time was 25.4 months (95% CI 19.7~31.1) in early recurrence patients and 62.9 months (95% CI 55.8~70.1) in late recurrence patients. The median OS of early recurrence patients was significantly shorter than that of late recurrence patients (P<0.001, Figure 1C).
Table 5.
Results of the Univariate Analysis of Prognostic Factors for Recurrence of Gastric Cancer After Curative Radical Resection
| Clinicopathological Factors | Median OS Months (95% CI) | χ2 | P-value |
|---|---|---|---|
| Gender | 0.007 | 0.931 | |
| Male | 38.6(34.0–43.1) | ||
| Female | 40.6(29.1–52.1) | ||
| Age at surgery | 0.627 | 0.429 | |
| ≤60 years | 39.5(32.5–46.6) | ||
| >60 years | 38.6(31.9–45.2) | ||
| Histological type | 0.762 | 0.383 | |
| Well-differentiated tumors | 41.6(36.9–46.3) | ||
| Poorly differentiated tumors | 36.5(28.5–44.6) | ||
| Perineural invasion | 8.21 | 0.004 | |
| Negative | 44.6(29.1–60.2) | ||
| Positive | 32.4(23.6–41.2) | ||
| Lymphovascular invasion | 0.962 | 0.327 | |
| Negative | 41.6(32.6–50.6) | ||
| Positive | 35.5(26.7–44.3) | ||
| Depth of invasion (T stage) | 1.525 | 0.677 | |
| T1 | 45.7(30.4–60.9) | ||
| T2 | 40.6(9.3–71.9) | ||
| T3 | 32.4(21.9–43.0) | ||
| T4 | 39.6(33.6–45.6) | ||
| Lymph node metastasis (N stage) | 6.263 | 0.099 | |
| N0 | 64.9(27.2–102.6) | ||
| N1 | 37.6(22.1–53.0) | ||
| N2 | 41.6(34.0–49.2) | ||
| N3 | 33.4(26.4–40.4) | ||
| TNM stage | 4.319 | 0.115 | |
| I | 60.9(0–131.5) | ||
| II | 39.5(20.7–58.4) | ||
| III | 37.5(30.1–44.1) | ||
| Postoperative chemotherapy | 5.844 | 0.054 | |
| 0 | 39.6(36.3–42.9) | ||
| 1–3 cycles | 20.2(3.5–37.1) | ||
| ≥4 cycles | 42.6(30.8–54.3) | ||
| Postoperative complications | 10.892 | 0.001 | |
| Negative | 40.6(35.7–45.5) | ||
| Positive | 17.3(15.8–18.7) | ||
| Tumor location | 1.651 | 0.648 | |
| Upper | 38.6(32.4–44.8) | ||
| Middle | 37.5(29.5–45.5) | ||
| Lower | 46.6(33.6–59.6) | ||
| Entire | 33.4(8.6–58.2) | ||
| Recurrence patterns | 2.740 | 0.433 | |
| Locoregional recurrence | 45.7(36.9–54.4) | ||
| Hematogenous metastasis | 33.5(22.3–44.7) | ||
| Peritoneal implantation | 36.5(32.2–40.9) | ||
| Multiple recurrence patterns | 38.6(28.3–48.8) |
Abbreviations: TNM, tumor node metastasis; OS, overall survival; CI, confidence interval.
Figure 1.
Kaplan–Meier curves for overall survival were well separated according to (A) perineural invasion, (B) postoperative complications and (C) the time of recurrence.
Abbreviation: OS, overall survival.
Table 6.
Results of the Multivariate Analysis of Factors for the Length of the Survival Time of Gastric Cancer After Curative Resection
| Factors | B | SE | Wald | df | P | Exp(B) | 95.0% CI |
|---|---|---|---|---|---|---|---|
| Perineural invasion | 0.476 | 0.187 | 6.459 | 1 | 0.011 | 1.61 | 1.115~2.325 |
| Postoperative complications | −1.013 | 0.376 | 7.26 | 1 | 0.007 | 0.363 | 0.174~0.759 |
Abbreviations: B, regression coefficient; SE, standard error; df, degree of freedom; Exp(B), odds ratio; CI, confidence interval.
Discussion
The morbidity and mortality of gastric cancer are very high in China. At present, surgery is still the only way to cure gastric cancer, but recurrence after radical gastrectomy is an important factor affecting the survival of patients. No standard exists for determining the time of early recurrence after radical surgery for gastric cancer. Studies show that the postoperative recurrence of gastric cancer mainly occurs 2 years after surgery.5 Most domestic and foreign scholars define recurrence times ≤ 24 months as early recurrence and > 24 months as late recurrence.6–8 Therefore, this study divided early and late recurrence by 24 months. The median RFS was 18.2 months (95% CI 15.0–21.4), and recurrence within 24 months accounted for 63.1%, which is similar to the results of previous studies.5–8
Although numerous studies on the clinicopathological factors related to the recurrence time of gastric cancer after curative resection have been conducted, their conclusions are different. Xu et al8 showed that tumor size, lymph node metastasis and adjuvant chemotherapy were independent factors of the early recurrence of gastric cancer after curative resection. Tumor size and TNM stage are both important prognostic factors.8 Shiraishi et al9 reported that tumor size, lymphatic invasion, the level of lymph node metastasis, the stage of disease, and the extent of lymph node dissection are the factors most significantly associated with early recurrence (within 2 years after gastrectomy). Choi et al10 reported that TNM stage and perineural invasion are independent factors of early recurrence and metastasis after radical gastrectomy. In our study, the clinicopathological factors of the early recurrence group and the late recurrence group were compared. In the early recurrence group, perineural invasion occurred frequently. The univariate analysis showed that gender and age at surgery were not related to the early recurrence of gastric cancer after surgery. Perineural invasion, depth of invasion, postoperative chemotherapy, and postoperative complications were significant factors associated with early recurrence after curative resection, which is consistent with most research results.8,11 However, the multivariate analysis showed that only perineural invasion, postoperative chemotherapy and postoperative complications were independent risk factors involved in the early recurrence of gastric cancer after curative resection. The results showed that depth of invasion was the only synergistic factor of early recurrence after radical gastrectomy, which may have some influence on the time interval of recurrence. However, it was not an independent risk factor involved in early recurrence. Perineural invasion (PNI) is considered a key pathological feature of many other malignancies, including those of the pancreas, colon and rectum, prostate, biliary tract, and stomach.12–16 Some authors agree that perineural invasion is the most significant route of extracapsular spread in prostate cancer.17 The mechanism of PNI is still unclear. Studies have shown that PNI is related to the interaction among tumor cells, the cell matrix and nerves. On the one hand, nerve tissue promotes tumor proliferation and invasion through specific signals; on the other hand, tumor cells can lead to the formation and extension of nerve cell axons and make nerve fibers thicker, which is conducive to the contact, adhesion and migration of tumor cells and nerves.18–20 A meta-analysis including 30,590 gastric cancer patients who had undergone curative gastrectomy from 24 studies showed that the median rate of positive perineural invasion was 40.9% (6.8–75.6%).21 The positivity rate of PNI increased when tumors were undifferentiated and the depth of invasion, the size, and clinical stage of tumor increased. It is associated with the recurrence and prognosis of gastric cancer after curative resection.16 In our study, the positive rate of PNI was 53% (79/149). PNI was also an independent factor associated with early recurrence and independent prognostic factor. Therefore, for cases with perineural invasion, even if radical resection is performed, the possibility of recurrence is high. Follow-up visits should be regularly performed, which may help to detect early recurrence. Our study suggests that postoperative adjuvant chemotherapy is also an independent risk factor for early recurrence, which is consistent with most research results. Histological type, lymphovascular invasion, lymph node metastasis, TNM stage, tumor location and recurrence patterns did not show significant associations with early recurrence. This finding is not consistent with the findings of the above studies. In the future, we need to expand the sample size of retrospective studies or conduct prospective studies to obtain more accurate results.
To investigate the prognostic value of postoperative complications, the incidence and severity of the complications must be properly evaluated. Clavien and Dindo proposed the CD classification, which is a treatment-oriented and objective standard for postoperative complications.22,23 In this study, we used the CD classification to assess the severity of complications. We defined the postoperative complications of gastric cancer, including infection, anastomotic leakage, postoperative hemorrhage and intestinal obstruction occurring within 60 days post-surgery, as grade II or more severe complications. Of the 149 patients, 8 cases of postoperative complications occurred, including 1 case of pneumonia, 2 cases of celiac infection, 3 cases of celiac hemorrhage, 1 case of anastomotic leakage and 1 case of intestinal obstruction. Seven cases were in the early recurrence group, and 1 case was in the late recurrence group. In our study, the univariate analysis showed a strong correlation between postoperative complications and early recurrence after curative resection (P = 0.004). The multivariate analysis also showed that it was an independent risk factor involved in early recurrence. Multiple clinical studies have also shown that postoperative complications are independent risk factors involved in the early recurrence of gastric cancer after curative resection.24,25 Generally, postoperative complications cause a systemic inflammatory response syndrome. Proinflammatory cytokine cascades, including tumor necrosis factor-alpha and interleukins 1, 6 and 8, can affect the function and regulation of cytotoxic T-lymphocytes, natural killer cells, and antigen-presenting cells.26–28 IL-6 was shown to increase vascular endothelial growth factor (VEGF) expression in various cancer cell lines, and VEGF was reported to induce tumor angiogenesis, leading to increased tumor recurrence in mice.29–31 In addition to its role in promoting angiogenesis, VEGF is also an important immunoregulatory factor in the tumor microenvironment. VEGF can inhibit tumor immunity through the accumulation of suppressive immune cell subtypes, including myeloid-derived suppressor cells and T regulatory cells.32,33 Residual tumor cells can grow during this postoperative period with host immunosuppression. In addition, patients with postoperative complications were less likely to receive adjuvant therapy or delay the initiation of adjuvant therapy. Thus, the mechanism above are possible mechanisms of early recurrence in patients with postoperative complications.
Conclusions
The survival analysis in this study showed that perineural invasion and postoperative complications were independent risk factors for the survival time of gastric cancer patients after curative resection. The median survival time of early recurrence patients was significantly shorter than that of late recurrence patients, which indicated that patients with early recurrence after curative resection have poorer survival. Based on the results above, our study suggested that patients with perineural invasion should be followed up regularly, which could help to detect early recurrence. Measures should be considered to prevent and decrease postoperative complications to improve long-term outcomes.
Acknowledgments
This work was supported by Science and Technology Program of Fujian Province, China (2018Y2003); Natural Science Foundation of Fujian Province, China (2018J01267); and Joint Funds for the Innovation of Science and Technology, Fujian province (2017Y9077). We thank Jessica W. from American Journal Experts for providing professional language editing service on this manuscript.
Funding Statement
This work was supported by Science and Technology Program of Fujian Province, China (2018Y2003), Natural Science Foundation of Fujian Province, China (2018J01267), Joint Funds for the Innovation of Science and Technology, Fujian Province (2017Y9077).
Abbreviations
TNM, tumor node metastasis; CT, computed tomography; MRI, magnetic resonance imaging; RFS, recurrence-free survival; OS, overall survival; B, regression coefficient; SE, standard error; df, degree of freedom; Exp(B), odds ratio; CI, confidence interval; NR, not reached.
Data Sharing Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Informed Consent
The study was approved by the responsible ethics committee (ethics committee of Fujian Cancer Hospital & Fujian Medical University Cancer Hospital, Fuzhou, People’s Republic of China) for the retrospective analysis of clinical data (permit number YKT2019-019-01). Additional examinations were not performed. Patient records and information were anonymized and de-identified prior to analysis. Informed consent was obtained from all individual participants included in the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
All authors declare that they have no conflict of interest.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Zheng RS, Sun KX, Zhang SW, et al. Report of cancer epidemiology in China, 2015. Zhonghua Zhong Liu Za Zhi. 2019;41(1):19–28. doi: 10.3760/cma.j.issn.0253-3766.2019.01.005 [DOI] [PubMed] [Google Scholar]
- 3.Liu D, Lu M, Li J, et al. The patterns and timing of recurrence after curative resection for gastric cancer in China. World J Surg Oncol. 2016;14(1):305. doi: 10.1186/s12957-016-1042-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amin MB, Edge S, Greene F, et al. American Joint Committee on Cancer Staging Manual. New York, NY: Springer; 2017. [Google Scholar]
- 5.Lee JH, Kim HI, Kim MG, Ha TK, Jung MS, Kwon SJ. Recurrence of gastric cancer in patients who are disease-free for more than 5 years after primary resection. Surgery. 2016;159(4):1090–1098. doi: 10.1016/j.surg.2015.11.002 [DOI] [PubMed] [Google Scholar]
- 6.Yoo CH, Noh SH, Shin DW, Choi SH, Min JS. Recurrence following curative resection for gastric carcinoma. Br J Surg. 2000;87(2):236–242. doi: 10.1046/j.1365-2168.2000.01360.x [DOI] [PubMed] [Google Scholar]
- 7.Sakar B, Karagol H, Gumus M, et al. Timing of death from tumor recurrence after curative gastrectomy for gastric cancer. Am J Clin Oncol. 2004;27(2):205–209. doi: 10.1097/01.coc.0000092703.12189.a2 [DOI] [PubMed] [Google Scholar]
- 8.Xu X, Fang L, Ji Y, Lu M, Huang P. Clinicopathologic and prognostic factors related to early recurrence of gastric cancer after curative radical resection. J Mod Oncol. 2018;26(5):728–733. [Google Scholar]
- 9.Shiraishi N, Inomata M, Osawa N, Yasuda K, Adachi Y, Kitano S. Early and late recurrence after gastrectomy for gastric carcinoma. Univariate and multivariate analyses. Cancer. 2000;89(2):255–261. doi: [DOI] [PubMed] [Google Scholar]
- 10.Choi JY, Ha TK, Kwon SJ. Clinicopathologic characteristics of gastric cancer patients according to the timing of the recurrence after curative surgery. J Gastric Cancer. 2011;11(1):46–54. doi: 10.5230/jgc.2011.11.1.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li FX, Zhang RP, Liu H, Quan JC, Liang H. Risk factors for early recurrence after radical resection of proximal gastric cancer. Zhonghua Wei Chang Wai Ke Za Zhi. 2012;15(2):129–132. [PubMed] [Google Scholar]
- 12.Su CH, Tsay SH, Wu CC, et al. Factors influencing postoperative morbidity, mortality, and survival after resection for hilar cholangiocarcinoma. Ann Surg. 1996;223(4):384–394. doi: 10.1097/00000658-199604000-00007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ozaki H, Hiraoka T, Mizumoto R, et al. The prognostic significance of lymph node metastasis and intrapancreatic perineural invasion in pancreatic cancer after curative resection. Surg Today. 1999;29(1):16–22. doi: 10.1007/BF02482964 [DOI] [PubMed] [Google Scholar]
- 14.Beard CJ, Chen MH, Cote K, et al. Perineural invasion is associated with increased relapse after external beam radiotherapy for men with low-risk prostate cancer and may be a marker for occult, high-grade cancer. Int J Radiat Oncol Biol Phys. 2004;58(1):19–24. doi: 10.1016/S0360-3016(03)01433-0 [DOI] [PubMed] [Google Scholar]
- 15.Law WL, Chu KW. Anterior resection for rectal cancer with mesorectal excision: a prospective evaluation of 622 patients. Ann Surg. 2004;240(2):260–268. doi: 10.1097/01.sla.0000133185.23514.32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bilici A, Seker M, Ustaalioglu BB, et al. Prognostic significance of perineural invasion in patients with gastric cancer who underwent curative resection. Ann Surg Oncol. 2010;17(8):2037–2044. doi: 10.1245/s10434-010-1027-y [DOI] [PubMed] [Google Scholar]
- 17.Ayala GE, Dai H, Ittmann M, et al. Growth and survival mechanisms associated with perineural invasion in prostate cancer. Cancer Res. 2004;64(17):6082–6090. doi: 10.1158/0008-5472.CAN-04-0838 [DOI] [PubMed] [Google Scholar]
- 18.Feng Y, Hu X, Liu G, et al. M3 muscarinic acetylcholine receptors regulate epithelial-mesenchymal transition, perineural invasion, and migration/metastasis in cholangiocarcinoma through the AKT pathway. Cancer Cell Int. 2018;18:173. doi: 10.1186/s12935-018-0667-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Entschladen F, Palm D, Niggemann B, Zaenker KS. The cancer’s nervous tooth: considering the neuronal crosstalk within tumors. Seminars Cancer Biol. 2008;18(3):171–175. doi: 10.1016/j.semcancer.2007.12.004 [DOI] [PubMed] [Google Scholar]
- 20.Xia Q, Chen L. Cellular and molecular mechanisms of perineural invasion in gastric cancer. Zhonghua Wei Chang Wai Ke Za Zhi. 2015;18(2):198–200. [PubMed] [Google Scholar]
- 21.Deng J, You Q, Gao Y, et al. Prognostic value of perineural invasion in gastric cancer: a systematic review and meta-analysis. PLoS One. 2014;9(2):e88907. doi: 10.1371/journal.pone.0088907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi: 10.1097/01.sla.0000133083.54934.ae [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–196. doi: 10.1097/SLA.0b013e3181b13ca2 [DOI] [PubMed] [Google Scholar]
- 24.Li QG, Li P, Tang D, Chen J, Wang DR. Impact of postoperative complications on long-term survival after radical resection for gastric cancer. World J Gastroenterol. 2013;19(25):4060–4065. doi: 10.3748/wjg.v19.i25.4060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lui S-A, Tan WB, Tai BC, et al. Predictors of survival outcome following radical gastrectomy for gastric cancer. ANZ J Surg. 2019;89(1–2):84–89. doi: 10.1111/ans.15011 [DOI] [PubMed] [Google Scholar]
- 26.Menetrier-Caux C, Montmain G, Dieu MC, et al. Inhibition of the differentiation of dendritic cells from CD34(+) progenitors by tumor cells: role of interleukin-6 and macrophage colony-stimulating factor. Blood. 1998;92(12):4778–4791. doi: 10.1182/blood.V92.12.4778 [DOI] [PubMed] [Google Scholar]
- 27.Horn F, Henze C, Heidrich K. Interleukin-6 signal transduction and lymphocyte function. Immunobiology. 2000;202(2):151–167. doi: 10.1016/S0171-2985(00)80061-3 [DOI] [PubMed] [Google Scholar]
- 28.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–545. doi: 10.1016/S0140-6736(00)04046-0 [DOI] [PubMed] [Google Scholar]
- 29.Cohen T, Nahari D, Cerem LW, Neufeld G, Levi BZ. Interleukin 6 induces the expression of vascular endothelial growth factor. J Biol Chem. 1996;271(2):736–741. doi: 10.1074/jbc.271.2.736 [DOI] [PubMed] [Google Scholar]
- 30.Salgado R, Vermeulen PB, Benoy I, et al. Platelet number and interleukin-6 correlate with VEGF but not with bFGF serum levels of advanced cancer patients. Br J Cancer. 1999;80(5–6):892–897. doi: 10.1038/sj.bjc.6690437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bohle B, Pera M, Pascual M, et al. Postoperative intra-abdominal infection increases angiogenesis and tumor recurrence after surgical excision of colon cancer in mice. Surgery. 2010;147(1):120–126. doi: 10.1016/j.surg.2009.06.035 [DOI] [PubMed] [Google Scholar]
- 32.Terme M, Pernot S, Marcheteau E, et al. VEGFA-VEGFR pathway blockade inhibits tumor-induced regulatory T-cell proliferation in colorectal cancer. Cancer Res. 2013;73(2):539–549. doi: 10.1158/0008-5472.CAN-12-2325 [DOI] [PubMed] [Google Scholar]
- 33.Horikawa N, Abiko K, Matsumura N, et al. Expression of vascular endothelial growth factor in ovarian cancer inhibits tumor immunity through the accumulation of myeloid-derived suppressor cells. Clin Cancer Res. 2017;23(2):587–599. doi: 10.1158/1078-0432.CCR-16-0387 [DOI] [PubMed] [Google Scholar]