Abstract
Aim
To develop and validate a nomogram for predicting the overall survival (OS) in patients with recurrent hepatocellular carcinoma (HCC) after hepatectomy who underwent microwave ablation (MWA).
Methods
The training cohort included 299 patients with recurrent HCCs after hepatectomy who met the Milan criteria and received MWA from April 2007 to December 2017. Baseline characteristics were collected to identify risk factors for the determination of death after MWA. A multivariate Cox proportional hazards model based on significant risk factors was used to develop the nomogram, which was then assessed for its predictive accuracy using Harrell’s C-index and the area under the curve (AUC). The nomogram was validated by internal (n = 240) and external cohorts (n = 205) from another hospital.
Results
After a median follow-up of 32.3 months, 38.8% (116/299) of patients had died. Multivariate Cox proportional hazards analyses showed that comorbid disease, early recurrence, and albumin-bilirubin (ALBI) grades 2–3 were independent prognostic factors for poor OS. This nomogram accurately stratified patients into subgroups with low or high risk. The 1-, 3- and 5-year OS rates in the low-risk subgroup were 99.4%, 97.2%, and 86.1%, respectively, and they were 92.8%, 70.3%, and 45.8% in the high-risk subgroup (P < 0.001). The nomogram predicted OS in the training cohort with a C-index score of 0.801 (95% CI 0.761–0.841). The nomogram was validated by internal and external cohorts, with C-index scores of 0.792 (95% CI 0.738–0.846) and 0.744 (95% CI 0.703–0.785), respectively.
Conclusion
The nomogram provides individualized risk estimates for long-term OS for patients with recurrent HCC after hepatectomy who underwent MWA.
Keywords: recurrent hepatocellular carcinoma, microwave ablation, overall survival, nomogram, hepatectomy
Introduction
Hepatocellular carcinoma (HCC) is the sixth most common type of cancer and causes the third highest number of cancer-related deaths worldwide; the morbidity and mortality rates of HCC are still increasing.1–3 Clinical guidelines have recommended liver transplantation (LT), hepatectomy and local radical ablation therapy (LRAT) as first-line treatment options for HCC patients with Barcelona Clinic Liver Cancer (BCLC) stage 0 or A.4,5 Among these options, hepatectomy is the mainstay treatment for early-stage HCC. However, recurrence following hepatectomy remains an important factor for prognosis, as the risk can be as high as 70% within 5 years.6,7 For these recurrent HCCs, salvage LT has been found to be beneficial only for a limited number of patients due to donor shortages. Moreover, repeat hepatectomy and LRAT have been more commonly used to treat recurrent HCCs.8
Most studies have reported that minimizing invasiveness can be used to maintain a good hepatic function reserve, and this is a feasible and effective treatment strategy for recurrent HCC. As a minimally invasive surgery, microwave ablation (MWA) has many advantages compared with radiofrequency ablation (RFA),9–11 including higher intra-tumoural temperature, shorter operation duration and lower electrical conductivity dependence. Furthermore, a larger ablation range, which originates from the internally cooled microwave generator, can extend the suitability of MWA for lesions smaller than 3 cm up to those that are 5 cm. Most studies have reported that the therapeutic effect of MWA on HCC is comparable to that of RFA, surgical resection, and even liver transplantation.12–14 The benefits of MWA treatment for recurrent HCC after hepatectomy have been confirmed and accepted by interventional radiologists. However, the risk factors that impact the overall survival (OS) associated with recurrent HCC among patients who have received MWA after hepatectomy remain unclear, and no relevant studies have been conducted until now.
Nomograms are pictorial representations of complex mathematical formulas and are commonly used to determine prognosis in medicine and oncology.15–17 Nomograms can be used to identify an individual’s probability of experiencing a clinical event through the integration of a variety of prognostic and determinant variables, resulting in biologically and clinically integrated models for the development of personalized medicine. Nomogram-derived prognosis evaluation has been incorporated into clinical decision making, since it offers several advantages compared with conventional staging, including the rapid calculation on graphical user-friendly interfaces, higher level of accuracy and ease of comprehension.
Here, to investigate risk factors of post-surgical survival, we developed and validated a nomogram to predict the OS of patients with recurrent HCC after hepatectomy who underwent MWA.
Patients and Methods
Patients and Study Design
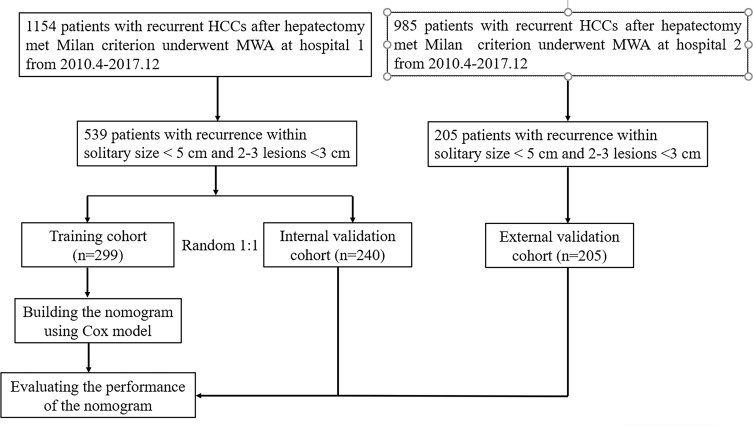
All the patients who were enrolled provided written informed consent. The Institutional Ethics Committees of Linyi City Central Hospital approved the study protocol, which complied with the Declaration of Helsinki. Figure 1 shows a flow chart of the patient selection process. This was a retrospective study on consecutive recurrent HCC patients who met the Milan criteria and received MWA after hepatectomy at Linyi City Central Hospital between April 2007 and December 2017. HCC was diagnosed by either imaging or histological evaluation based on the European Association for the Study of Liver (EASL) and the American Association for the Study of Liver Disease (AASLD) guidelines. The inclusion criteria for the recurrent HCC patients were as follows: (1) Eastern Cooperative Oncology Group performance score of ≤2; (2) Child-Pugh Class A or B; (3) complete movement of the primary HCC by hepatectomy; (4) maximum recurrent tumour diameter of ≤5 cm if only a single tumour was present or ≤3 recurrent tumours with a maximum diameter of ≤3 cm each; (5) absence of vascular invasion or extrahepatic metastases; and (6) no other previous malignancies reported. The following exclusion criteria were applied: (1) incomplete clinical data; (2) cause of death was not HCC progression; (3) loss to follow up within 3 months after ablation; and (4) serious heart, lung and renal dysfunction and active severe infection. After screening, 539 patients with recurrent HCCs were enrolled and assigned to either the training cohort (n = 299) or the internal validation cohort (n = 240) through computer-generated randomization based on a proportion of 1:1. The external validation cohort consisted of 116 recurrent HCC patients who received MWA after hepatectomy who were enrolled at another hospital during the same period.
Figure 1.
Flow diagram shows study patient accrual process.
The following clinical data were collected: (1) patient characteristics (sex, age, comorbid disease [hypertension, diabetes, heart disease, renal disease and oesophageal gastric varices], pathological differentiation, aetiology, cirrhosis, time to recurrence, BCLC stage and performance status at recurrence); (2) tumour characteristics (size, number and location); and (3) laboratory indices (Child-Turcotte-Pugh [CTP] grade, albumin-bilirubin [ALBI] grade, a-fetoprotein, serum albumin, total bilirubin, aspartic aminotransferase [AST] and alanine aminotransferase [ALT] levels). The ALBI grade does not use variables, such as ascites and encephalopathy, which are subjective but are used for the CTP grade.18 The following formula was utilized for the calculation of the ALBI score before treatment: (log 10 bilirubin [BI] [μ mol/L] × 0.66) + (albumin [AL] [g/L] ×-0.085). Values of ≤ −2.60, > −2.60 to −1.39, and > −1.39 were used to classify the scores as grades 1, 2, and 3, respectively.
Microwave Ablation Treatment
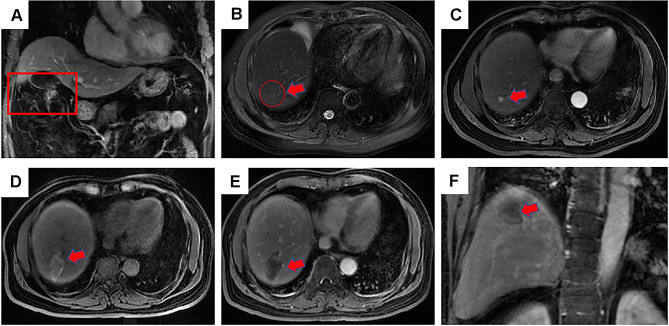
Three interventional radiologists, L.Z., C.H.Q. and W.W.B., with 25, 10 and 10 years of experience with MWA, respectively, administered the ablations. All the MWA procedures were performed under CT guidance. The microwave needle was inserted into the tumour, and the deployment degree scale was determined based on the tumour size and shape. The patients were kept on a scanning bed in either the supine or the prone position, depending on the location of the lesions. Local and intravenous anaesthesia were administered before each MWA procedure was conducted. After anaesthesia, at a predetermined angle, a 15-gauge, 18-cm MWA antenna (MTC-3C, Nanjing Qinghai Research Institute of Microwave Electric, China) was inserted into the tumour. The position of the ablation electrode was confirmed as accurate using CT image scanning, which was performed again before ablation. The power and duration of ablation were determined based on standard recommendations provided by the manufacturer of the equipment. Each MWA session used an overlapping technique to ensure that the entire tumour was eradicated. Figure 2 shows the post-SR diagnosis of HCC, treatment, and post-ablation assessment of one patient who received MWA.
Figure 2.
Contrast-enhanced magnetic resonance imaging (MRI) revealed a 54-years-old man with HCC of 3.7 cm in maximum diameter in segment 7 who underwent microwave ablation (MWA). (A) MRI axial scan showed a residual liver in portal phase after 1 month underwent hepatectomy, the red frame shows the cut into parts; (B) a slightly higher signal nodule in red circle was shown in segment 7 in MRI T2WI (red arrow), which was defined as a recurrence lesion; (C) a high signal nodule is shown in arterial phase axial MRI image after MWA (red arrow); (D) high-density MWA zone is shown in T1WI axial MRI image after 3 months (red arrow); (E) low-density MWA zone is shown in delay phase axial MRI image after 3 months (red arrow); (F) low-density MWA zone is shown in delay phase coronal MRI image after 3 months (red arrow).
Treatment and Follow-Up
Follow-up was conducted 1–3 months after the treatment, within 1 year and then at 3- to 6-month intervals. Further follow-up visits included a standard physical examination; determination of tumour marker levels; prothrombin time; total bilirubin and serum albumin using laboratory tests; and US, CT or magnetic resonance imaging (MRI) to obtain contrast-enhanced images. Treatment modalities for recurrence were identified by the multidisciplinary team and the consent of the patients, who were randomly assigned to receive surgical, MWA, RFA, TACE and supportive treatments.
Statistical Analysis
SPSS 21.0 software (SPSS, USA) and R 3.0.2 software were used to conduct the statistical analyses. Categorical variables were compared using Pearson chi-squared analysis or Fisher exact tests, whereas continuous variables were compared using the independent sample t-test and Mann–Whitney U-test. The Log rank test was used to compare survival curves, and the Kaplan-Meier method was used for their construction. Independent prognostic factors were identified by entering variables that were significant in the univariate analysis into the multivariable Cox proportional hazard regression analysis. The Akaike information criterion and the backward process were used to select significant independent variables, which were then used to construct the nomogram. The Schoenfeld residual test and plots were used to verify the proportional hazards assumption, whereas the variation inflation factor was used to evaluate multicollinearity. Harrell’s concordance index (C-index) was used to determine the discriminatory ability and calibration of the model to evaluate its performance. The tertiles of the predictions and calibration curves of the internal and external validation cohorts were used to create the Kaplan-Meier curves. Bootstrapping involving 1,000 resampled datasets was performed for these activities. The Cox proportional hazards model was used along with the Akaike information criterion, which identifies all statistical models that can be used to predict recurrent HCC patient OS. The model was more representative of the lower Akaike information criterion. A parametric survival analysis was used to determine homogeneity after multiplying the likelihood ratio by 2. A p value of less than 0.05 indicated statistical significance.
Results
Patient Characteristics
In our study, 539 patients with recurrent HCC (79 females and 460 males; average age of 56.2 ± 11.4 years [standard deviation]) with a total of 782 HCC nodules who were followed for more than 5 years were included. Within the median follow-up period of 32.3 months (range of 7.8–113.3 months), 38.8% (116/299) of patients died and 11.2% (33/299) patients relapsed again in the training cohort. The baseline characteristics of the training and validation datasets are described in Table 1. The clinical characteristics and follow-up data did not vary significantly among the three groups (P = 0.527–0.679). At medical centre 1, 539 patients with 782 tumours received a total of 944 treatment sessions. At medical centre 2, 116 patients with 138 tumours received a total of 162 treatment sessions. The complete ablation rates were 96.2% (752/782) at medical centre 1 and 98.5% (136/138) at medical centre 2, respectively. In the training cohort, the 1-year, 3-year and 5-year OS rates were 96.2%, 85.1% and 68.9%, respectively. In the internal validation cohort, the 1-year, 3-year and 5-year OS rates were 97.4%, 83.0% and 66.6%, respectively. In the external validation cohort, the cumulative 1-, 3- and 5-year OS rates were 96.2%, 85.1% and 68.9%, respectively. The 1-year, 3-year and 5-year OS rates of the training, internal validation and external validation cohorts did not reveal any significant differences (P = 0.826).
Table 1.
Clinical Characteristics of Patients with Recurrent HCC Underwent MWA
| Parameters | Training Cohort | Internal Validation | External Validation | P value |
|---|---|---|---|---|
| (n=299) | Cohort (n=240) | Cohort (n=205) | ||
| Age (y) | 0.527 | |||
| Mean±SD | 57.8±10.8 | 55.8±11.5 | 54.3±11.2 | |
| Range | 31–81 | 36–85 | 27–82 | |
| Sex | 0.103 | |||
| Male | 254 (84.9) | 206 (85.8) | 174 (84.9) | |
| Female | 45 (15.1) | 34 (14.2) | 31 (15.1) | |
| Comorbid disease | 0.065 | |||
| No | 129 (43.1) | 99 (41.3) | 56 (27.3) | |
| Yes | 170 (56.9) | 141 (58.8) | 149 (72.7) | |
| Pathological differentiation | 0.521 | |||
| Well/moderately | 201 (67.2) | 197 (82.1) | 163 (79.5) | |
| Poorly | 98 (32.8) | 43 (17.9) | 42 (20.5) | |
| Etiology | 0.322 | |||
| HBV | 247 (82.6) | 200 (83.3) | 188 (91.7) | |
| HCV | 38 (12.7) | 28 (11.7) | 13 (6.3) | |
| Alcohol-induced | 14 (4.7) | 12 (6.0) | 4 (2.0) | |
| Cirrhosis | 0.589 | |||
| Yes | 278 (93.0) | 215 (89.6) | 182 (88.8) | |
| No | 21(7.0) | 25 (10.4) | 23 (11.2) | |
| Size of recurrence (cm)* | ||||
| Mean±SD Range <3.0 3.0–5.0 |
2.3±0.9 0.8–5.0 223 (75.6) 76 (24.4) |
2.5±0.9 0.8–5.0 186 (77.5) 54 (22.5) |
2.1±0.9 0.8–5.0 179 (87.3) 26 (12.7) |
0.206 |
| Recurrence no. | 0.462 | |||
| 1 | 163 (54.5) | 143 (59.6) | 137 (66.8) | |
| 2–3 | 136 (45.5) | 97 (40.4) | 68 (33.2) | |
| Adjacent to organ | 0.212 | |||
| No | 66 (22.1) | 44 (18.3) | 32 (15.6) | |
| Major vessels | 77 (25.8) | 40 (16.7) | 37 (18.0) | |
| Diaphragm | 89 (29.8) | 57 (23.8) | 63 (30.7) | |
| Gastrointestinal tract | 67 (22.4) | 102 (42.2) | 73 (35,6) | |
| Time to recurrence (year) | 0.394 | |||
| >1 | 195 (65.2) | 162 (67.5) | 104 (50.7) | |
| ≤1 | 104 (34.8) | 78 (32.5) | 101 (49.3) | |
| CTP grade | 0.895 | |||
| A | 295 (98.7) | 234 (97.5) | 195 (95.1) | |
| B | 4 (1.3) | 6 (2.5) | 10 (4.9) | |
| ALBI grade | 0.745 | |||
| 1 | 279 (93.3) | 230 (92.9) | 184 (89.8) | |
| 2–3 | 20 (6.7) | 10 (7.1) | 21 (10.2) | |
| α-fetoprotein level (ng/mL)† | 14.8 (1.4–223.1) | 17.8 (2.2–223.1) | 22.4 (2.4–266.9) | 0.105 |
| Albumin level (μmol/L)† | 37.1 (12.5–54.2) | 38.9 (12.6–47.8) | 33.5 (13.9–57.7) | 0.864 |
| Total bilirubin level (μmol/L)† | 16.2 (4.3–51.6) | 15.2 (4.3–44.9) | 17.9 (5.1–56.6) | 0.215 |
| ALT level (U/L)† | 54.2 (7.6–274.6) | 43.7 (8.7–277.4) | 65.1 (9.9–234.6) | 0.346 |
| AST level (U/L)† | 60.1(17.2–313.0) | 41.2 (14.7–221.3) | 48.2 (14.2–387.0) | 0.679 |
Notes: Unless otherwise indicated, data are number of patients, with percentage in parentheses; *Data are means ± standard deviation; †Data are medians, with interquartile range in parentheses.
Abbreviations: ALBI, albumin-bilirubin; CTP, Child-Turcotte-Pugh; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Independent Prognostic Factors of the Training Cohort
Sixteen possible risk factors for OS were evaluated using a Cox regression model. Univariate and multivariate analyses were conducted on the data from 299 patients with recurrent HCC who had undergone MWA. Table 2 shows the results of these analyses. Significant differences between the OS rates that were affected by comorbid disease (hazard ratio [HR] = 1.989; 95% confidence interval [CI]: 1.347, 2.935; P = 0.001), recurrence time (HR = 0.407; 95% CI: 0.207, 0.672; P = 0.001), CTP grade (HR = 10.626; 95% CI: 6.737, 23.466; P = 0.032), and ALBI grade (HR = 12.573; 95% CI: 6.737, 23.466; P < 0.001) were found through the univariate analyses. The multivariate analysis demonstrated that the OS rate was significantly altered by comorbid disease (HR = 1.840; 95% CI: 1.237, 2.735; P = 0.003), recurrence time (HR = 0.460; 95% CI: 0.301, 0.704; P < 0.001) and ALBI grade (HR = 11.525; 95% CI: 5.661, 23.463; P < 0.001). However, CTP grade was not a significant risk factor for OS.
Table 2.
Univariate and Multivariable Analysis of Risk Factors for OS
| Parameters | Univariate Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age (year) | 1.143 (0.716–1.824) | 0.577 | … | … |
| <65 | ||||
| ≥65 | ||||
| Sex | 0.941 (0.561–1.579) | 0.819 | … | … |
| Male | ||||
| Female | ||||
| Comorbid disease | 1.989 (1.347–2.935) | 0.001* | 1.840 (1.237–2.735) | 0.003* |
| No | ||||
| Yes | ||||
|
Etiology HBV |
ref | … | … | … |
| HCV | 1.201 (0.486–2.967) | 0.691 | … | … |
| Alcohol-induced | 1.691 (0.643–4.447) | 0.287 | … | … |
| Pathological differentiation | 1.523 (0.682–1.921) | 0.372 | … | … |
| Well/moderately | ||||
| Poorly | ||||
| Cirrhosis | 0.927 (0.431–1.994) | … | … | |
| No | ||||
| Yes | ||||
| Recurrence time (years) | 0.407 (0.272–0.607) | 0.001* | 0.460 (0.301–0.704) | <0.001* |
| <1 | ||||
| ≥1 | ||||
| Tumor size (cm) | 0.940 (0.624–1.417) | 0.768 | … | … |
| <3 | ||||
| 3–5 | ||||
| Tumor number | 0.767 (0.534–1.101) | 0.150 | … | … |
| 1 | ||||
| 2–3 | ||||
| Adjacent to organ | ||||
| No | Ref | … | ||
| Major vessels | 1.623 (0.517–2.982) | 0.382 | … | … |
| Diaphragm | 1.787 (0.612–2.981) | 0.562 | … | … |
| Gastrointestinal tract | 1.112 (0.311–1.872) | 0.781 | … | … |
| CTP grade | 10.626 (2.487–45.410) | 0.032* | … | … |
| A | ||||
| B | ||||
| ALBI grade | 12.573 (6.737–23.466) | <0.001* | 11.525(5.661–23.463) | <0.001* |
| 1 | ||||
| 2–3 | ||||
| α-fetoprotein level (ng/mL) | 1.418 (0.966–2.082) | 0.074 | … | … |
| ≤20 | ||||
| >20 | ||||
Note: * shows statistically significant result.
Abbreviations: ALBI, albumin-bilirubin; CTP, Child-Turcotte-Pugh; BCLC, Barcelona Clinic Liver cancer; TACE, transarterial chemoembolization; RFA, radiofrequency ablation; MWA, microwave ablation.
Construction and Validation of the OS Prediction Nomogram
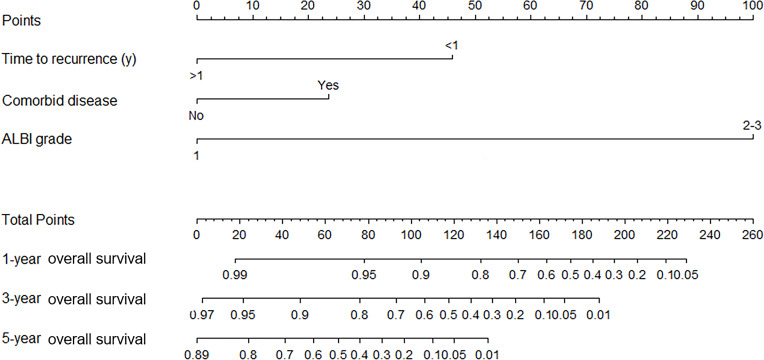
The β-coefficients were used for the construction of a nomogram that could assess the contribution of the independent prognostic factors that were found to be significant towards prognosis and their association with OS in the multivariate analysis. The sum of the points for each prognostic variable was used to calculate an individualized grade for each patient included in the study. The total point (range of 0–260) projections are shown on scales in Figure 3, which shows the predicted 1-year, 3-year and 5-year OS.
Figure 3.
The nomogram was developed in the validation data set, with comorbid disease, recurrence time, and ALBI grade.
Discrimination Ability of the Prognostic Nomogram
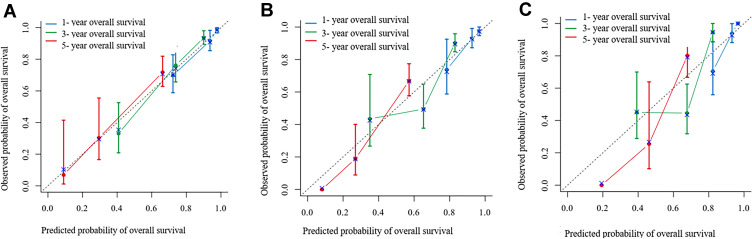
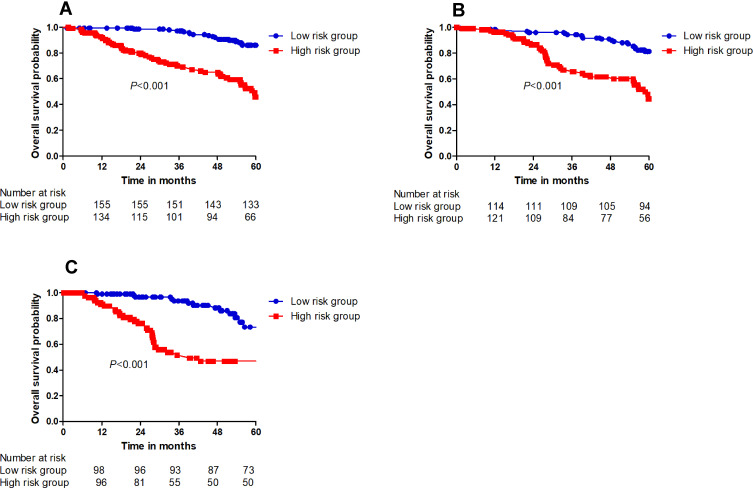
The OS determination model constructed using the training cohort yielded a C-index score of 0.801 (95% CI: 0.761–0.841). The calibration curves plotted for 1-year, 3-year and 5-year OS using the training cohort corresponded well with the idealized 45° line (Figure 4A). The C-index score of the OS determination model using the internal cohort was 0.792 (95% CI: 0.738–0.846), whereas that using the external cohort was 0.744 (95% CI: 0.703–0.785). The 1-year, 3-year and 5-year OS calibration curves also corresponded well with those of the internal and external validation cohorts (Figure 4B and C). The OS probabilities were divided into low-risk and high-risk subgroups and were used to plot Kaplan-Meier curves to analyse the discrimination ability of the nomogram. The 1-year, 3-year and 5-year OS rates in the low-risk subgroup were 99.4%, 97.2% and 86.1%, respectively. These rates were 92.8%, 70.3% and 45.8%, respectively, in the high-risk group of the training cohort (P < 0.001). In the internal validation cohort, the 1-year, 3-year and 5-year OS rates in the low-risk subgroup were 98.4%, 94.3%, and 86.1%, respectively. These rates were 96.3%, 67.0% and 45.8%, respectively, in the high-risk group (P < 0.001). In the external validation cohort, the 1-year, 3-year and 5-year OS rates in the low-risk subgroup were 99.0%, 93.6%, and 73.3%, respectively. These rates were 91.0%, 51.6%, and 46.9%, respectively, in the high-risk group (P < 0.001). Significant differences were detected between the low-risk and high-risk subgroups of the training and the internal and external validation cohorts (P < 0.001) (Figure 5A–C).
Figure 4.
Calibration curve for predicting OS after MWA at (A) 1-, 3-, and 5- years in the training data set, at (B) 1-, 3-, and 5- years in the internal validation data set and at (C) 1-, 3-, and 5- years in the external validation data set. Nomogram-predicted probability of OS is plotted on the x-axis; actual OS is plotted on the y-axis.
Figure 5.
The cumulative OS rate stratified by risk score of the nomogram was then used to plot Kaplan-Meier curves. (A) The cumulative OS rate in high-risk group was higher than that in low-risk group in training sets. (B) The cumulative OS rate in high-risk group was higher than that in low-risk group in internal validation sets. (C) The cumulative OS rate in high-risk group was higher than that in low-risk group in external validation sets.
Prognostic Performance of Serum Marker Associations with OS
Homogeneity and the Akaike information criterion were used to evaluate the prognostic performance of ALBI grade, CTP grade, BCLC grade, albumin level, bilirubin level, a-fetoprotein level, ALT level and AST level. Table 3 shows that, among these markers, homogeneity was the highest (likelihood ratio X2) and the Akaike information criterion value was the lowest for ALBI grade. The predictive ability of ALBI grade was superior to that of CTP grade, BCLC grade and a-fetoprotein level for the prediction the OS of recurrent HCC patients who received MWA.
Table 3.
Assessment Accuracy of Serum Markers for OS After MWA for Patients with Recurrent HCC
| Serum Marker | Discriminatory Ability (Linear Trend χ2) | Homogeneity (Likelihood Ratio χ2) | Akaike Information Criterion |
|---|---|---|---|
| ALBI grade | 26.781 | 61.293 | 2001.895 |
| CTP grade | 10.238 | 17.293 | 2103.672 |
| BCLC grade | 2.289 | 32.234 | 2282.342 |
| Albumin level | 13.211 | 33.921 | 2045.238 |
| Bilirubin level | 9.982 | 42.912 | 2127.872 |
| a-fetoprotein level | 5.823 | 9.723 | 2189.273 |
| ALT level | 11.234 | 8.294 | 2089.343 |
| AST level | 10.829 | 9.990 | 2078.118 |
Abbreviations: ALBI, albumin-bilirubin; CTP, Child-Turcotte-Pugh; BCLC stage, Barcelona Clinic Liver Cancer stage; ALT, alanine aminotransferase; AST, aspartate aminotransferase.
Discussion
The recurrence rate after hepatectomy is very high and seriously affects the prognosis of HCC patients.19 Treatment for recurrent lesions is an important issue that urgently needs to be resolved. The optimal treatment method for recurrent HCC after hepatectomy has not been established. The EASL guidelines for the management of HCC have suggested that repeat hepatectomy or percutaneous thermal ablation may be attempted for intrahepatic recurrence.4 In our study, a cohort of patients with recurrent HCC who received MWA after hepatectomy was observed. In addition, risk factors related to OS were analysed. The three major findings of our study are as follows. First, the OS rates of patients with recurrent HCC who received MWA were acceptable, with a 5-year rate of 68.9% after MWA treatment of recurrent lesions. Second, a nomogram was constructed based on clinicopathological factors that may be utilized for the prediction of individual OS of recurrent HCC patients post-MWA. Third, ALBI grade was confirmed to be superior to CTP grade, BCLC grade and a-fetoprotein level in predicting the OS of recurrent HCC patients after MWA using the homogeneity analysis and Akaike information criterion methods.20
The results generated from the nomogram indicated that the condition of the patient, characteristics of recurrence tumours, hepatic function, and treatment for recurrence were important factors that impacted the OS of patients with recurrent HCC after MWA. Many studies have reported that comorbid diseases, including hypertension, diabetes, heart disease, renal disease and oesophageal gastric varices, are independent risk factors for the poor survival of primary and recurrent HCC patients.21–23 Furthermore, the mechanism has been explained. Regarding the characteristics of recurring tumours, it has been reported that tumour size, number and recurrence time are significantly related to OS.24–26 In our study, an early recurrence time after MWA was found to be an independent prognostic factor for poor OS, which was similar to that presented in previous reports. The mechanism of early recurrence should be further explored because the effect of ablation on tumour progression was not fully elucidated. Early recurrence may influence the tumour and body immune microenvironment after ablation.27,28 Moreover, hepatic reserve function not only affected treatment choice but also impacted prognosis after treatment, especially for recurrent HCC patients. Traditionally, the CTP system has been used for cirrhosis patients. Five parameters, including coagulation function, degree of hepatic encephalopathy, extent of ascites, bilirubin level and serum albumin level, are used to determine the CTP score. Ascites and hepatic encephalopathy determination are partially subjective processes. Therefore, compared with CTP grade, ALBI grade incorporates both serum albumin and bilirubin levels,18,29 which allows it to offer an objective evidence-based assessment of liver reserve function. ALBI grade showed a high degree of accuracy for OS compared to other prognostic factors and was found to be a promising alternative for the assessment of liver reserve function, given its simple, objective, evidence-based advantages.
Nomograms can more accurately predict the prognosis of certain cancers compared to conventional staging systems. The results showed that the nomogram developed in our study showed a good C-index of 0.801, and the result was verified using both internal and external validation. The BCLC staging system was created to assess the association between staging and treatment indications for primary HCC rather than recurrent HCC. However, the BCLC staging system has also been reported to be suitable for the prediction of OS in patients with HCC after treatment. Therefore, the nomogram created in our study was verified to be an efficient method for predicting the OS of recurrent HCC patients who received MWA. The results were further verified using stratification analysis. Membership in the low-risk or high-risk subgroup of recurrent HCC patients stratified using the nomogram was predictive of OS with better performance.
In the OS-predicting nomograms, death rates should ideally be over 10 times the number of variables to decrease the expected error to <10% in the probability predictions.30 Nomogram validation methods, resampling with bootstrapping and data splitting were used to generate unbiased predictions of model performance without affecting the sample size. A disadvantage of data splitting is the inevitable loss of accuracy, since the final model cannot be validated. Overall, 84 patients died in our study, making the death rate more than 28 times higher than the total number of risk-related variables. The OS-predicting nomograms were also subjected to external validation using a resampling method, with 1000 bootstrapped replications for the entire cohort, resulting in good C-indexes of 0.792 and 0.744. These results indicate that the nomograms possess a high degree of reliability and precision.
However, our study has several limitations. First, as a retrospective study, it contains inherent biases. Second, the MWA was guided using ultrasound for the training and internal validation cohorts, whereas CT guidance was utilized for the external validation cohort. The different guidance methods may have led to an artificial discrepancy. Third, patients who received liver transplantation, radiotherapy, or molecular targeting treatment were not included; therefore, it was not possible to determine the effect of MWA for recurrence following different procedures. Finally, a larger sample with participation from multiple centres is needed to further validate the nomogram.
In conclusion, a nomogram was developed to predict the OS of patients with recurrent HCC who received MWA. This nomogram showed a high level of performance in internal and external validation. The nomogram had a significant ability to predict 1-year, 3-year and 5-year OS. Serum markers of ALBI grade were found to be important for predicting the OS of recurrent HCC patients after MWA. This model may help clinicians to create personalized therapeutic strategies that improve the long-term prognosis of recurrent HCC patients.
Data Sharing Statement
Please contact the corresponding author for all data requests.
Ethics Approval
The ethics committee board of the Linyi City Central Hospital (B2020-100-06) approved the use of patients with recurrent HCCs after MWA for this study.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest for this work.
References
- 1.Feng RM, Zong YN, Cao SM, Xu RH. Current cancer situation in China: good or bad news from the 2018 global cancer statistics. Cancer Commun. 2019;39(1):22. doi: 10.1186/s40880-019-0368-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 3.Cai Z, Liu Q. Understanding the global cancer statistics 2018: implications for cancer control. Sci China Life Sci. 2019. doi: 10.1007/s11427-019-9816-1 [DOI] [PubMed] [Google Scholar]
- 4.Galle PR, Forner A, Llovet JM, et al. Electronic address: easloffice@easloffice.eu, European association for the study of the liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018;69(1):182–236. doi: 10.1016/j.jhep.2018.03.019 [DOI] [PubMed] [Google Scholar]
- 5.Heimbach JK, Kulik LM, Finn RS, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018;67(1):358–380. doi: 10.1002/hep.29086 [DOI] [PubMed] [Google Scholar]
- 6.Zou H, Zhu CZ, Wang C, et al. Recurrence of Barcelona clinic liver cancer stage A hepatocellular carcinoma after hepatectomy. Am J Med Sci. 2017;354(3):262–267. doi: 10.1016/j.amjms.2017.05.014 [DOI] [PubMed] [Google Scholar]
- 7.Lee KF, Chong C, Fong A, et al. Pattern of disease recurrence and its implications for postoperative surveillance after curative hepatectomy for hepatocellular carcinoma: experience from a single center. Hepatobiliary Surg Nutr. 2018;7(5):320–330. doi: 10.21037/hbsn.2018.03.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xing H, Sun LY, Yan WT, et al. Repeat hepatectomy for patients with early and late recurrence of hepatocellular carcinoma: a multicenter propensity score matching analysis. Surgery. 2019. doi: 10.1016/j.surg.2019.11.005 [DOI] [PubMed] [Google Scholar]
- 9.Liang P, Yu J, Yu XL, et al. Percutaneous cooled-tip microwave ablation under ultrasound guidance for primary liver cancer: a multicentre analysis of 1363 treatment-naive lesions in 1007 patients in China. Gut. 2012;61(7):1100–1101. doi: 10.1136/gutjnl-2011-300975 [DOI] [PubMed] [Google Scholar]
- 10.Glassberg MB, Ghosh S, Clymer JW, et al. Microwave ablation compared with radiofrequency ablation for treatment of hepatocellular carcinoma and liver metastases: a systematic review and meta-analysis. Onco Targets Ther. 2019;12:6407–6438. doi: 10.2147/OTT.S204340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Izzo F, Granata V, Grassi R, et al. Radiofrequency ablation and microwave ablation in liver tumors: an update. Oncologist. 2019;24(10):e990. doi: 10.1634/theoncologist.2018-0337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Du S, Yang JZ, Chen J, Zhou WG, Sun YY. Comparisons of recurrence-free survival and overall survival between microwave versus radiofrequency ablation treatment for hepatocellular carcinoma: a multiple centers retrospective cohort study with propensity score matching. PLoS One. 2020;15(1):e0227242. doi: 10.1371/journal.pone.0227242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu W, Zou R, Wang C, et al. Microwave ablation versus resection for hepatocellular carcinoma within the Milan criteria: a propensity-score analysis. Ther Adv Med Oncol. 2019;11:1758835919874652. doi: 10.1177/1758835919874652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sayon-Orea C, Moreno-Iribas C, Delfrade J, et al. Inverse-probability weighting and multiple imputation for evaluating selection bias in the estimation of childhood obesity prevalence using data from electronic health records. BMC Med Inform Decis Mak. 2020;20(1):9. doi: 10.1186/s12911-020-1020-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huo RR, Liu X, Cui J, et al. Development and validation a nomogram for assessing survival in patients with hepatocellular carcinoma after hepatectomy. Biosci Rep. 2020. doi: 10.1042/BSR20192690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ge D, Luo Z, Mao R, et al. Development and validation of a nomogram-based prognostic evaluation model for sarcomatoid hepatocellular carcinoma. Adv Ther. 2020;37(7):3185–3205. doi: 10.1007/s12325-020-01357-3 [DOI] [PubMed] [Google Scholar]
- 17.Liu J, Lu J, Ma Z, Li W. A nomogram based on a three-gene signature derived from AATF coexpressed genes predicts overall survival of hepatocellular carcinoma patients. Biomed Res Int. 2020;2020:7310768. doi: 10.1155/2020/3920284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee IC, Hung YW, Liu CA, et al. A new ALBI-based model to predict survival after transarterial chemoembolization for BCLC stage B hepatocellular carcinoma. Liver Int. 2019;39(9):1704–1712. doi: 10.1111/liv.14194 [DOI] [PubMed] [Google Scholar]
- 19.Wang K, Liu G, Li J, et al. Early intrahepatic recurrence of hepatocellular carcinoma after hepatectomy treated with re-hepatectomy, ablation or chemoembolization: a prospective cohort study. Eur J Surg Oncol. 2015;41(2):236–242. doi: 10.1016/j.ejso.2014.11.002 [DOI] [PubMed] [Google Scholar]
- 20.Kao WY, Su CW, Chiou YY, et al. Hepatocellular carcinoma: nomograms based on the albumin-bilirubin grade to assess the outcomes of radiofrequency ablation. Radiology. 2017;285(2):670–680. doi: 10.1148/radiol.2017162382 [DOI] [PubMed] [Google Scholar]
- 21.Tian YL, Ji JJ, Chen LN, et al. Risk factors for long-term prognosis of hepatocellular carcinoma patients after anatomic hepatectomy. World J Clin Cases. 2020;8(4):713–722. doi: 10.12998/wjcc.v8.i4.713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang YY, Xiang BD, Ma L, et al. Development and validation of a nomogram to preoperatively estimate post-hepatectomy liver dysfunction risk and long-term survival in patients with hepatocellular carcinoma. Ann Surg. 2020. doi: 10.1097/SLA.0000000000003803 [DOI] [PubMed] [Google Scholar]
- 23.Hong YM, Cho M, Yoon KT, et al. Risk factors of early recurrence after curative hepatectomy in hepatocellular carcinoma. Tumour Biol. 2017;39(10):1010428317720863. doi: 10.1177/1010428317720863 [DOI] [PubMed] [Google Scholar]
- 24.Xu X, Chen J, Wang F, Ni Q, Naimat U, Chen Z. Recurrence of hepatocellular carcinoma after laparoscopic hepatectomy: risk factors and treatment strategies. J Laparoendosc Adv Surg Tech A. 2017;27(7):676–684. doi: 10.1089/lap.2016.0541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamaoka M, Kobayashi T, Ishiyama K, et al. Evaluation of the risk factors and prognostic factors of hepatectomy for hepatocellular carcinoma in patients aged 80 years or more. J Hepatobiliary Pancreat Sci. 2017;24(1):58–64. doi: 10.1002/jhbp.413 [DOI] [PubMed] [Google Scholar]
- 26.Kwon SK, Yun SS, Kim HJ, Lee DS. The risk factors of early recurrence after hepatectomy in hepatocellular carcinoma. Ann Surg Treat Res. 2014;86(6):283–288. doi: 10.4174/astr.2014.86.6.283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang XP, Chen ZH, Zhou TF, et al. Chinese national research cooperative group for diagnosis and treatment of hepatocellular carcinoma with tumour thrombus. A nomogram to predict early postoperative recurrence of hepatocellular carcinoma with portal vein tumour thrombus after R0 liver resection: a large-scale, multicenter study. Eur J Surg Oncol. 2019;45(9):1644–1651. doi: 10.1016/j.ejso.2019.03.043 [DOI] [PubMed] [Google Scholar]
- 28.He W, Peng B, Tang Y, et al. Nomogram to predict survival of patients with recurrence of hepatocellular carcinoma after surgery. Clin Gastroenterol Hepatol. 2018;16(5):756–764.e10. doi: 10.1016/j.cgh.2017.12.002 [DOI] [PubMed] [Google Scholar]
- 29.Ho SY, Liu PH, Hsu CY, et al. An Albumin-Bilirubin (ALBI) grade-based prognostic model for patients with hepatocellular carcinoma within milan criteria. Am J Clin Oncol. 2019;42(9):698–704. doi: 10.1097/COC.0000000000000581 [DOI] [PubMed] [Google Scholar]
- 30.Iasonos A, Schrag D, Raj GV, Panageas KS. How to build and interpret a nomogram for cancer prognosis. J Clin Oncol. 2008;26(8):1364–1370. doi: 10.1200/JCO.2007.12.9791 [DOI] [PubMed] [Google Scholar]