Abstract
Purpose
The hypertriglyceridemic waist (HTGW) phenotype can predict cardiovascular disease (CVD) risk. Additionally, strong evidence indicates that elevated urinary albumin–creatinine ratio (UACR) is associated with increased prevalence of CVD. However, few studies have explored the association between the HTGW phenotype and UACR.
Patients and Methods
In this cross-sectional descriptive study, a total of 40,674 subjects (28,562 women and 12,112 men older than 40 years) were recruited from seven different geographic regional centres. The HTGW phenotype was defined as increased triglyceride levels (triglyceride ≥ 1.5 mmol/L for female and ≥2.0 mmol/L for male) and waist circumference (WC; WC ≥ 85 for female and WC ≥ 90 cm for male). Logistic regression analyses were performed to assess the relationship between UACR and the HTGW phenotype.
Results
Subjects with the HTGW phenotype showed a more significant trend towards increased excretion of UACR [among all subjects, odds ratio (OR) = 1.303, 95% CI: 1.132–1.499, P < 0.001; among men, OR = 1.406, 95% CI: 1.057–1.870, P = 0.019; among women, OR = 1.268, 95% CI: 1.074–1.496, P = 0.005]. Furthermore, the stratified analysis showed that the OR for high-risk significantly increased in individuals in the HTGW group aged below 65 years, with 5.6 ≤ fasting blood glucose < 7.0 or 7.8 ≤ post-load blood glucose <11.1 mmol/L, 120 ≤ systolic blood pressure < 140 or 80 ≤ diastolic blood pressure < 90, 24 ≤ body mass index < 28 kg/m2, and estimated globular filtration rate > 90 mL/min per 1.73 m2.
Conclusion
This study has advanced the understanding of visceral obesity and our results supported the fact that the HTGW phenotype is associated with elevated UACR excretion among general Chinese adults.
Keywords: waist circumference, hypertriglyceridemia, chronic kidney disease, urinary albumin-creatinine ratio
Introduction
Recently, the prevalence of visceral obesity has continued to notably rise. Visceral obesity plays an essential role in the occurrence of insulin resistance (IR), metabolic syndrome (MetS), and cardiovascular disease (CVD).1 Compelling evidence had been detailed in specific prospective studies on the regional adipose tissue distribution,2 while others have indicated that visceral obesity is closely associated with CVD risk, especially in women.3,4
Moreover, the hypertriglyceridemic waist (HTGW) phenotype is defined as having at-risk triglyceride (TG) values and at-risk waist circumference (WC).5 Visceral obesity is more closely related to the HTGW phenotype than it is to WC.6 Increasing evidence has confirmed that the HTGW phenotype is also associated with the onset and prevalence of specific diseases. Specifically, studies have reported that the HTGW phenotype may serve as a strong indicator of atherogenic trends among postmenopausal women.4 Further, a study in the Chinese population suggests that the HTGW phenotype increases the prevalence of prediabetes;7 meanwhile, a STROBE compliant study showed that the HTGW phenotype indicates endothelial dysfunction, which can result in hypertension.8 Furthermore, the HTGW phenotype rather than body mass index (BMI) was recommended as a useful screening tool to predict the risk of CVD and MetS.9 The HTGW phenotype has also been reported to contribute to a greater risk of coronary artery disease.10 In fact, the HTGW phenotype can be used as a clinical indicator of atherosclerotic metabolic characteristics in males, with its value in assessing the 7.5-year CVD risk having been confirmed.11
The urinary albumin excretion rate can be generally described in terms of the urinary albumin–creatinine ratio (UACR). Elevated UACRs have been described as a symbol for impaired function of kidney and is a crucial risk factor for CVD.12 Early renal dysfunction is closely associated with a higher incidence of CVD.13 Furthermore, compared with normal UACR values, diabetic patients with micro- and macro-albuminuria have 1.5- and 3.7-times higher mortality rates associated with CVD, respectively.14 Hence, considering that visceral obesity is a common risk factor for both CVD and chronic renal disease development,15 the aim of the present study was to establish if an association exists between the HTGW phenotype and increased UACR.
Materials and Methods
Study Population
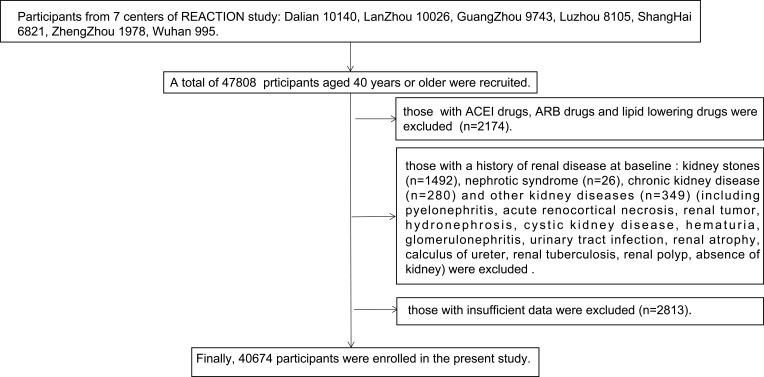
This study was drawn from a longitudinal REACTION study, designed to examine the relationship between type 2 diabetes mellitus (T2DM) and cancer risk in a Chinese population. The REACTION study used the multistage random sampling method and was conducted among adults aged over 40 years old from both urban and rural areas with various states of economic development and urbanisation, across mainland China from 2011 to 2012.16 A total of 47,808 participants, which were representative of China, were recruited from seven different geographic regional centres (Dalian, Guangzhou, Lanzhou, Luzhou, Shanghai, Zhengzhou, and Wuhan). Participants who used lipid lowering or ACEI/ARB drugs, those who had been diagnosed with primary kidney disease at baseline, and those with incomplete demographic or clinical characteristic data were excluded. A total of 40,674 participants (12,112 men and 28,562 women) were enrolled in the present analysis. The flow chart describing the enrolment of the subjects in this study is presented in Figure 1.
Figure 1.
The flow chart describing the enrolment of the subjects in this study.
Abbreviations: ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker.
Ethics Approval and Informed Consent
Before data collection, informed consent was acquired from all participants, and the protocols of this study were approved by the Committee on Human Research of Rui-Jin Hospital affiliated with the School of Medicine, Shanghai Jiao Tong University.
Data Collection
According to the standardised procedures, the subjects underwent anthropometric assessments, self-administered questionnaires, blood collection, and a 75 g-oral glucose tolerance test (OGTT). Detailed information of the participants regarding demographic characteristics, clinical medical record, and lifestyle were collected via standard examination questionnaires. The clinical medical record included history of nephritic diseases, hyperlipidaemia, hypertension, T2DM, CVD [including stroke, myocardial infarction (MI), and coronary heart disease (CHD)], and the use of drugs. Smoking practices were also determined based on whether the participant smoked more than once per day. Drinking habits were determined based on whether the frequency of consumption was more than once per week.
The body height, weight, and WC were measured according to the standard protocol. Before measurements were taken, the subjects were asked to remove their hats, coats, and shoes. WC was measured on the ligature’s midpoint level between the twelfth rib’s inferior margin with anterior superior spine. BMI was calculated as body weight/height2 (kg/m2). Blood pressure (BP) was calculated based on three sequential measurements with 1-min intervals each. The three values of systolic BP (SBP) and diastolic BP (DBP) were averaged for analysis. All subjects had specific biochemical parameters assessed, including aspartate transaminase (AST), alanine transaminase (ALT), serum creatinine (Scr), Haemoglobin A1c (HbA1c), fasting blood glucose (FBG), 2 h post-load blood glucose (PBG), low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), total cholesterol (TC), and TG. Blood samples were collected by venipuncture, and the blood parameters were assessed by quality control procedures. Fasting blood samples were obtained from the subjects following an overnight fast (≥10 hours). The 75 g-OGTT or the 100 g-steamed-bread meal test was performed on all subjects regardless of their history of diabetes, and the venepuncture blood specimen was collected to measure PBG after 2 hours. The estimated glomerular filtration rate (eGFR, mL/min per 1.73 m2) was calculated according to the following formula:
eGFR = 186 × [serum creatinine × 0.011]− 1.154 × [age]− 0.203 × [0.742 if female] × 1.233, where serum creatinine is presented as μmol/L. These values were calculated using the Modification of Diet in Renal Disease (MDRD), which was recalibrated for the population of China.17
Definition of Variables
According to the questionnaires provided to each participant, self-reported history of diabetes diagnosed by physicians was regarded as history of diabetes. Self-reported history of hypertension by physicians was regarded as history of hypertension. Urinary albumin and creatinine concentrations were tested by the first-void sterile urine samples in the early morning. The UACR was defined as the ratio of the urine albumin concentration to the urinary creatinine concentration. The UACR data were defined as categorical variables, which were separated into two groups: UACR ≥ 30 mg/g or UACR < 30 mg/g. Normal WC was defined as <85 cm for women and <90 cm for men; the normal TG was defined as <1.5 mmol/L for women and <2.0 mmol/L for men. Based on these cut-off values, subjects were separated into the following four groups: normal waist-normal triglycerides (NWNT), normal waist-elevated triglycerides (HTG), enlarged waist-normal triglycerides (EW), and HTGW with elevated triglycerides-enlarged waist.5
Statistical Analyses
All statistical analyses were conducted with SPSS version 24.0 (IBM, Chicago, IL, USA). One-way analysis of variance was used to compare the differences among the continuous variables of the four phenotypes. The least significant difference was used as the multiple comparison test, and continuous variables were expressed as means ± standard deviations (SD). The continuous variables that were not normally distributed were expressed as medians (Inter-Quartile Range, IQR). Categorical variables were described as percentages (%). Logistic regression analyses were carried out to estimate the odds ratios (ORs) and 95% confidence intervals (CIs) to explore the associations of the HTGW phenotype with UACR. All analyses were performed by sex. Model 1 was the unadjusted model. Model 2 was adjusted for age, centres, and sex. Model 3 was further adjusted for educational status, smoking habits, drinking habits, and CVD history based on Model 2. Model 4 was further adjusted for diabetes history, hypertension history, and diabetes or hypertension drug usage status. Model 5 was further adjusted for BMI, eGFR, HDL-C, LDL-C, TC, AST, ALT, FBG, PBG, SBP, and DBP. After controlling potential confounders, the relationship between UACR and the HTGW phenotype was also explored within subgroups, which were stratified based on blood pressure (Normal BP: SBP < 120 and DBP < 80 mmHg; Prehypertension: DBP ≥ 80 to < 90 or SBP ≥ 120 to < 140 mmHg; Hypertension: DBP ≥ 90 or SBP ≥ 140 mmHg); blood glucose (Diabetes: FBG ≥ 7.0 or 2-h PBG ≥ 11.1 mmol/L, Prediabetes: FBG ≥ 5.6 to < 7.0 or PBG ≥ 7.8 to < 11.1, mmol/L; Normal: FBG < 5.6 and PBG < 7.8, mmol/L); BMI (Obese: BMI ≥ 28 kg/m2; General overweight: BMI ≥ 24 to < 28, kg/m2; Normal weight BMI ≥ 18.5 to <24, kg/m2; Underweight: BMI < 18.5 kg/m2); eGFR (eGFR < 60, mL/min per 1.73 m2; eGFR ≥ 60 to < 90, mL/min per 1.73 m2; eGFR ≥ 90 mL/min per 1.73 m2); and age (age < 55 years; age ≥ 55 to ≤ 64; age ≥ 65 years). Analyses of the interactions between the HTGW phenotype and the stratified variables were examined among populations with increased risk of UACR. Two-tailed P < 0.05 were considered to be statistically significant.
Results
Clinical Characteristics of the Study Participants
Ultimately, 40,674 participants (28,562 women and 12,112 men) were recruited. Table 1 shows the clinical and biochemical characteristics of the respective four subject groups. The mean age of the HTGW group was 60.08 ± 9.17 years. Compared with the other three groups, individuals in the HTGW group had higher prevalence of diabetes (NWNT: 6.9%; HTG: 9.3%; EW: 11.6%; HTGW: 15.2%, P < 0.001), hypertension (NWNT: 10.6%; HTG: 17.7%; EW: 21.4%; HTGW: 29.9%, P < 0.001), MI (NWNT: 0.2%; HTG: 0.2%; EW: 0.5%; HTGW: 0.5%, P < 0.001), CHD (NWNT: 2.4%; HTG: 2.8%; EW: 4.4%; HTGW: 5.5%, P < 0.001), and stroke (NWNT: 0.7%; HTG: 0.9%; EW: 1.4%; HTGW: 1.6%, P < 0.001). Additionally, the HTGW group was characterised by higher BMI, LDL-C, AST, ALT, FBG, PBG, SBP, and DBP values, while their eGFR values were lower (P < 0.001).
Table 1.
Baseline Characteristics Based on the Hypertriglyceridemic Waist Phenotype
| Variable | NWNT (n=15,250) |
HTG (n=6077) |
EW (n=10,488) |
HTGW (n=8859) |
P value |
|---|---|---|---|---|---|
| Age, years Male sex, no. (%) Female sex, no. (%) |
56.53±9.13 5147(42.5) 10,103(35.4) |
57.08±8.16 1169(9.7) 4908(17.2) |
59.63±9.60 3825(31.6) 6663(23.3) |
60.08±9.17 1971(16.3) 6888(24.1) |
<0.001a,b,c,d,e,f <0.001a,b,c,d,e,f <0.001a,b,c,d,e,f |
| Waist circumference, cm | 77.65±6.36 | 79.50±5.17 | 93.82±6.61 | 94.02±6.95 | <0.001a,b,c,d,e |
| Triglycerides, mmol/L | 1.00(0.11–1.99) | 2.14(1.50–15.93) | 1.13(0.23–1.99) | 2.26(1.50–15.90) | <0.001a,b,c,d,e,f |
| Body mass index, kg/m2 | 22.25±2.89 | 23.20±2.75 | 26.32±3.25 | 27.01±3.35 | <0.001a,b,c,d,e,f |
| Total cholesterol, mmol/L | 4.76±1.12 | 5.44±1.14 | 4.87±1.10 | 5.41±1.10 | <0.001a,b,c,d,f |
| LDL-C, mmol/L | 2.80±0.88 | 3.09±0.94 | 2.98±0.88 | 3.10±0.91 | <0.001a,b,c,d,f |
| HDL-C, mmol/L | 1.41±0.37 | 1.25±0.30 | 1.32±0.34 | 1.20±0.26 | <0.001a,b,c,d,e,f |
| UACR, mg/g ALT, U/L AST, U/L SBP, mmHg DBP, mmHg Resting heart rate, (beat/min) |
8.89(5.44–16.64) 15.48±0.11 20.67±0.10 124.29±0.16 74.26±0.09 78.36±0.10 |
11.20(6.53–21.29) 18.61±0.18 22.86±0.19 128.63±0.26 76.79±0.14 80.33±0.16 |
9.47(5.52–18.68) 18.47±0.15 21.84±0.12 135.65±0.21 78.54±0.11 77.99±0.12 |
12.26(6.73–24.53) 21.94±0.19 24.09±0.13 138.37±0.22 80.23±0.12 80.10±0.13 |
<0.001c,e,f <0.001a,b,c,e,f <0.001a,b,c,d,e,f <0.001a,b,c,d,e,f <0.001a,b,c,d,e,f <0.001a,b,c,d,f |
| FBG, mmol/L | 5.64±1.43 | 5.97±1.70 | 6.01±1.62 | 6.43±2.10 | <0.001a,b,c,e,f |
| PBG, mmol/L | 7.63±3.44 | 8.83±3.82 | 8.56±3.95 | 9.89±4.38 | <0.001a,b,c,d,e,f |
| eGFR, mL/min per 1.73m2 | 124.31±27.12 | 115.37±20.41 | 122.46±26.41 | 113.71±21.76 | <0.001a,c,d,e,f |
| Married, % | 88.8% | 87.4% | 88.7% | 85.7% | <0.001 |
| High-school education, % | 53.3% | 51.1% | 45.6% | 40.6% | <0.001 |
| Current smoker, % | 13.0% | 9.2% | 12.8% | 10.1% | <0.001 |
| Past smoker, % | 11.5% | 7.1% | 13.2% | 8.7% | <0.001 |
| Current alcohol drinker, % | 6.4% | 4.3% | 8.4% | 5.7% | <0.001 |
| Past alcohol drinker, % | 4.8% | 3.0% | 6.3% | 4.2% | <0.001 |
| History of hypertension, % | 10.6% | 17.7% | 21.4% | 29.9% | <0.001 |
| History of diabetes, % | 6.9% | 9.3% | 11.6% | 15.2% | <0.001 |
| Previous MI, % | 0.2% | 0.2% | 0.5% | 0.5% | <0.001 |
| Previous stroke, % | 0.7% | 0.9% | 1.4% | 1.6% | <0.001 |
| Previous CHD, % Taking diabetic drugs, % Taking hypertension drugs, % |
2.4% 5.8% 1.4% |
2.8% 8.0% 2.2% |
4.4% 10.1% 2.1% |
5.5% 13.0% 2.8% |
<0.001 <0.001 <0.001 |
Notes: Data were mean ± SD or median (IQR) for skewed variables or numbers (proportions) for categorical variables: a, b, c, d, e, fP < 0.05 for G2 vs G1, G3 vs G1, G4 vs G1, G3 vs G2, G4 vs G2, and G4 vs G3, respectively. G1: NWNT; G2: HTG; G3: EW; G4: HTGW.
Abbreviations: NWNT, normal waist and normal triglyceride; HTG, normal waist with hypertriglyceridemia; EW, enlarged waist circumference; HTGW, hyper-triglyceridemic waist; LDL-C, low-density lipoprotein cholesterol; HDL-C, high-density lipoprotein cholesterol; eGFR: estimated glomerular filtration rate; MI, myocardial infarction; CHD, coronary heart disease; ALT, alanine aminotransferase; AST, aspartate aminotransferase; SBP, systolic blood pressure; DBP, diastolic blood pressure; FBG, fasting blood glucose; PBG, 2 h post-load blood glucose; UACR, urinary albumin-creatinine ratio.
Association Between UACR and the HTGW Phenotype
Logistic regression analyses were applied to explore the relationship between the HTGW phenotype and UACR after adjusting for confounders. As shown in Table 2, the HTGW and HTG groups showed significant associations with UACR using models 1–5; Table 3 shows an association of the HTGW phenotype with UACR in the respective genders after adjusting for confounders. Furthermore, compared with the other three groups, only the HTGW group was significantly associated with UACR in both sexes after adjusting for confounding factors (OR: 1.303, 95% CI: 1.132–1.499, P < 0.001 in all subjects; OR: 1.268, 95% CI: 1.074–1.496, P = 0.005 in women; OR: 1.406, 95% CI: 1.057–1.870, P = 0.019 in men) (Tables 2 and 3). The association between the HTG phenotype and UACR was significant (OR: 1.182, 95% CI: 1.016–1.376, P = 0.030; Table 3); however, the association between HTG and UACR disappeared in men.
Table 2.
Odds Ratios (95% Confidence Interval) for UACR by HTGW Phenotype Among All Subjects
| NWNT (n=15,250) |
HTG (n=6077) |
EW (n=10,488) |
HTGW (n=8859) |
|
|---|---|---|---|---|
| Model 1 OR(95% CI) P value |
Reference |
1.550(1.420–1.693)* <0.001 |
1.344(1.244–1.452)* <0.001 |
2.068(1.920–2.229)* <0.001 |
| Model 2 OR(95% CI) P value |
Reference |
1.417(1.308–1.534)* <0.001 |
1.259(1.150–1.378)* <0.001 |
1.830(1.694–1.978)* <0.001 |
| Model 3 OR(95% CI) P value |
Reference |
1.395(1.287–1.512)* <0.001 |
1.142(1.133–1.362)* <0.001 |
1.787(1.652–1.933)* <0.001 |
| Model 4 OR(95% CI) P value |
Reference |
1.277(1.145–1.424)* <0.001 |
1.110(0.983–1.252) 0.137 |
1.498(1.349–1.665)* <0.001 |
| Model 5 OR(95% CI) P value |
Reference |
1.243(1.096–1.410)* <0.001 |
1.026(0.898–1.172) 0.704 |
1.303(1.132–1.499)* <0.001 |
Notes: *Significant data (P<0.05): Model 1: unadjusted; Model 2: adjusted for age, sex, centres; Model 3: further adjusted for education status, smoking habits, drinking habits, CVD status; Model 4: further adjusted for diabetes history, hypertension history, use of diabetes or hypertension drugs status; Model 5: further adjusted for BMI, eGFR, HDL-C, LDL-C, TC, AST, ALT, FBG, PBG, SBP, DBP.
Abbreviations: NWNT, normal waist and normal triglyceride; HTG, normal waist with hypertriglyceridemia; EW, enlarged waist circumference; HTGW, hypertriglyceridemic waist; UACR, urinary albumin-creatinine ratio.
Table 3.
Odds Ratios (95% Confidence Interval) for UACR by HTGW Phenotype Among Men and Women
| Phenotype Model 1 Model 2 Model 3 Model 4 Model 5 |
|---|
| groups OR(95% CI) P value OR(95% CI) P value OR(95% CI) P value OR(95% CI) P value OR(95% CI) P value |
| Men |
| NWNT(5147) Reference Reference Reference Reference Reference |
| HTG(1169) 1.537(1.235–1.913)* 0.047 1.524(1.217–1.909)* <0.001 1.371(1.114–1.688)* 0.044 1.338(1.083–1.652)* 0.007 1.272(0.986–1.639) 0.064 |
| EW(3825) 1.499(1.284–1.750)* <0.001 1.412(1.204–1.655)* <0.001 1.342(1.007–1.786)* <0.001 1.291(0.964–1.730) 0.087 1.292(0.945–1.767) 0.108 |
| HTGW(1971) 2.111(1.810–2.463)* <0.001 2.011(1.714–2.359)* <0.001 1.663(1.352–2.045)* <0.001 1.576(1.274–1.948)* <0.001 1.406 (1.057–1.870)* 0.019 |
| Women |
| NWNT(10,103) Reference Reference Reference Reference Reference |
| HTG(4908) 1.587(1.437–1.753)* <0.001 1.410(1.283–1.548)* <0.001 1.395(1.268–1.533)* <0.001 1.276(1.120–1.453)* <0.001 1.182(1.016–1.376)* 0.030 |
| EW(6663) 1.315(1.196–1.445)* <0.001 1.209(1.094–1.336)* <0.001 1.195(1.079–1.322)* 0.001 1.076(0.941–1.231) 0.284 1.020(0.878–1.186) 0.793 |
| HTGW(6888) 2.073(1.901–2.262)* <0.001 1.724(1.574–1.888)* <0.001 1.676(1.529–1.838)* <0.001 1.459(1.289–1.653)* <0.001 1.268(1.074–1.496)* 0.005 |
Notes: *Significant data (P<0.05): Model 1: unadjusted; Model 2: adjusted for age, centres; Model 3: further adjusted for education status, smoking habits, drinking habits, CVD status; Model 4: further adjusted for diabetes history, hypertension history, use diabetes or hypertension drugs status; Model 5: further adjusted for BMI, eGFR, HDL-C, LDL-C, TC, AST, ALT, FBG, PBG, SBP, DBP.
Abbreviations: NWNT, normal waist and normal triglyceride; HTG, normal waist with hypertriglyceridemia; EW, enlarged waist circumference; HTGW, hyper-triglyceridemic waist; UACR, urinary albumin-creatinine ratio.
Association Between the HTGW Phenotype and UACR Among Subgroups
To further verify whether the association between UACR and the HTGW was robust, stratified analyses of blood glucose and BP were conducted. As shown in Table 4, among participants aged below 65 years, the HTGW group had a remarkably significant relationship with UACR (age < 55: OR = 1.407, 95% CI = 1.094–1.809, P = 0.008; 55 ≤ age ≤ 64: OR = 1.338, 95% CI = 1.061–1.686, P = 0.014). As for the blood glucose level, a significant association between HTGW and UACR was observed in subjects with prediabetes (5.6 ≤ FBG < 7.0 or 7.8 ≤ PBG < 11.1 mmol/L, OR: 1.425, 95% CI: 1.124–1.808, P = 0.004). A significant result was also found in the HTG group with prediabetes (OR: 1.243, 95% CI: 1.001–1.543, P= 0.049). Additionally, for blood pressure and BMI, similar and significant results were observed in the HTGW group with high-normal BP (120 ≤ SBP < 140 and/or 80 ≤ DBP < 90 mmHg, OR: 1.268, 95% CI: 1.038–1.549, P = 0.020) and overweight (24 ≤ BMI < 28 kg/m2, OR: 1.268, 95% CI: 1.008–1.595, P = 0.043). Meanwhile, a significant relationship was observed between the HTGW and UACR groups when eGFR ≥ 90 mL/min per 1.73 m2 (OR: 1.199, 95% CI: 1.047–1.484, P=0.009). A significant interaction between blood glucose and the HTGW phenotype can be seen in Table 4 (P =0.002). To analyse the effect of FBG and PBG on the interaction, the prediabetic subjects were divided into the impaired fasting glucose group (IFG) (FBG [5.6, 7.0], PBG [0, 7.8], mmol/L) and the impaired glucose tolerance group (IGT) (FBG [0, 7.0], FBG [7.8, 11.1], mmol/L), as seen in Table 5. The association between UACR and HTGW was also significant in the IFG group (OR: 1.407, 95% CI: 1.020–1.941, P = 0.037).
Table 4.
The Association of the HTGW Phenotype with UACR in Different Subgroups of Age, Blood Glucose and Blood Pressure
| Variable | NWNT HTG EW HTGW | P-values | |||
|---|---|---|---|---|---|
| Reference | OR(95% CI) P value | OR(95% CI) P value | OR(95% CI) P value | For Interaction | |
| Age, year a | 0.127 | ||||
| <55 (n=15,704) | 1 | 1.260(0.974–1.629) 0.079 | 1.007(0.790–1.284) 0.954 | 1.407(1.094–1.809)* 0.008 | |
| 55–64 (n=15,264) | 1 | 1.197(0.970–1.476) 0.093 | 0.938(0.759–1.158) 0.552 | 1.338(1.061–1.686)* 0.014 | |
| ≥65 (n=9706) | 1 | 0.972(0.781–1.210) 0.800 | 1.208(0.927–1.574) 0.163 | 1.086(0.836–1.411) 0.536 | |
| Blood glucose, mmol/L b | 0.002 | ||||
| FBG<5.6 and PBG<7.8 (n=16,035) | 1 | 1.145(0.943–1.390) 0.173 | 1.038(0.846–1.273) 0.722 | 1.217 (0.992–1.492) 0.059 | |
| 5.6≤FBG<7.0 or 7.8≤PBG<11.1 (n=16,107) | 1 | 1.243(1.001–1.543)* 0.049 | 1.153(0.923–1.441) 0.209 | 1.425(1.124–1.808)* 0.004 | |
| FBG≥7.0 or PBG≥11.1 (n=8532) | 1 | 1.017(0.858–1.206) 0.845 | 1.193(0.988–1.440) 0.067 | 1.146(0.922–1.407) 0.193 | |
| BP, mmHg c | 0.234 | ||||
| SBP<120 and DBP<80 (n=13,190) | 1 | 1.236(0.896–1.705) 0.197 | 0.954(0.714–1.276) 0.751 | 1.299(0.960–1.756) 0.090 | |
| 120≤SBP<140 and/or 80≤DBP<90 (n=15,384) | 1 | 1.128(0.956–1.331) 0.152 | 1.153(0.960–1.385) 0.127 | 1.268(1.038–1.549)* 0.020 | |
| SBP≥140 or DBP≥90 (n=12,100) | 1 | 1.048(0.875–1.255) 0.613 | 1.097(0.890–1.352) 0.388 | 1.170(0.941–1.456) 0.158 | |
| eGFR, mL/min per 1.73m2 d eGFR≥90 (n=37,617) |
1 |
1.108(0.952–1.290) 0.186 |
1.079(0.932–1.249) 0.311 |
1.199(1.047–1.484)* 0.009 |
0.183 |
| 60≤eGFR<90 (n=2808) | 1 | 1.253(0.796–1.974) 0.330 | 1.242(0.831–1.858) 0.291 | 1.337(0.910–1.946) 0.139 | |
| eGFR<60 (249) | 1 | 0.988(0.617–1.583) 0.961 | 1.110(0.735–1.678) 0.619 | 1.131(0.759–1.685) 0.546 | |
| BMI, kg/m2 e BMI<18.5 (n=1117) |
1 |
1.305(0.961–1.772) 0.088 |
0.952(0.747–1.215) 0.695 |
1.247(0.965–1.610) 0.091 |
0.136 |
| 18.5≤BMI<24 (n=18,097) | 1 | 1.107(0.806–1.519) 0.530 | 1.007(0.783–1.296) 0.956 | 1.074(0.842–1.399) 0.598 | |
| 24≤BMI<28 (n=15,669) | 1 | 1.002(0.833–1.206) 0.981 | 1.170(0.948–1.444) 0.143 | 1.268(1.008–1.595)* 0.043 | |
| BMI≥28 (n=5791) | 1 | 1.019(0.779–1.335) 0.889 | 0.964(0.746–1.246) 0.779 | 1.086(0.787–1.499) 0.615 | |
Notes: *Significant data (P<0.05): aFor age subgroup: adjusted for sex, centres, ALT, AST, LDL-C, HDL-C, TC, PBG, PBG, SBP, DBP, eGFR, BMI, smoking habits, drinking habits, CVD status, hypertension history, diabetes history, use diabetes drugs and hypertension drugs status: bFor blood glucose subgroup: adjusted for age, sex, centres, ALT, AST, LDL-C, HDL-C, TC, SBP, DBP, eGFR, BMI, smoking habits, drinking habits, CVD status, hypertension history, use hypertension drugs status: cFor blood pressure subgroup: adjusted for age, sex, centres, ALT, AST, LDL-C, HDL-C, TC, FBG, PBG, eGFR, BMI, smoking habits, drinking habits, CVD status, diabetes history, use diabetes drugs status: dFor eGFR subgroup: adjusted for age, sex, centres, ALT, AST, LDL-C, HDL-C, TC, FBG, PBG, SBP, DBP, BMI, smoking habits, drinking habits, CVD status, hypertension history, diabetes history, use diabetes drugs and hypertension drugs status: eFor BMI subgroup: adjusted for age, sex, centres, ALT, AST, LDL-C, HDL-C, TC, FBG, PBG, eGFR, SBP, DBP, smoking habits, drinking habits, CVD status, hypertension history, diabetes history, use diabetes drugs and hypertension drugs status.
Abbreviations: NWNT, normal waist and normal triglyceride; HTG, normal waist with hypertriglyceridemia; EW, enlarged waist circumference; HTGW, hypertriglyceridemic waist; UACR, urinary albumin-creatinine ratio; FPG, fasting plasma glucose; PPG, postprandial plasma glucose; BP, blood pressure; SBP, systolic blood pressure; DBP, diastolic blood pressure.
Table 5.
The Association of the HTGW Phenotype with UACR in Different Subgroups of Prediabetes
| Variable NWNT HTG EW HTGW |
|---|
| Reference OR(95%CI) P value OR(95%CI) P value OR(95%CI) P value |
| IFG (n=6133) |
| 5.6≤FBG<7.0 and 1 1.099(0.830–1.456) 0.511 1.012(0.742–1.380) 0.941 1.407(1.020–1.941)* 0.037 |
| PBG<7.8 |
| IGT (n=9974) |
| FBG<7.0 and 1 0.909(0.750–1.101) 0.329 1.019(0.833–1.247) 0.854 0.878(0.707–1.091) 0.241 |
| 7.8≤PBG<11.1 |
Notes: *Significant data (P<0.05): Adjusted for age, sex, centres, ALT, AST, LDL-C, HDL-C, TC, SBP, DBP, eGFR, BMI, smoking habits, drinking habits, CVD status, hypertension history, use hypertension drugs status.
Abbreviations: NWNT, normal waist and normal triglyceride; HTG, normal waist with hypertriglyceridemia; EW, enlarged waist circumference; HTGW, hypertriglyceridemic waist; UACR, urinary albumin-creatinine ratio.
Discussion
Herein, we found that the HTGW phenotype exhibited the most significant association with increased UACR risk, which was nearly 1.92- to 2.23-fold in all participants. In fact, only the association between UACR and the HTGW phenotype retained significance after controlling for confounding factors in males. For females, increased UACR was observed in both the HTGW and the HTG groups; however, the association with UACR was more significant for the HTGW group. Furthermore, the risk of UACR was significantly increased in the HTGW group aged less than 65 years, with eGFR ≥ 90 mL/min per 1.73 m2, prediabetes, prehypertension, or borderline values of BMI. Therefore, these results may possibly be proved useful and significative for monitoring populations more likely to benefit from the avoidance and amelioration of the associated factors such as TG and WC. Here, we also first verified a noteworthy association between UACR and the HTGW phenotype.
The association between HTGW phenotype and UACR may be attributable to visceral obesity; however, the underlying mechanism is unclear. In previous studies, the HTGW phenotype was reported to be remarkably associated with visceral obesity, and it was suggested that the combined presence of TG and WC may serve as the optimal indicator for CVD risk among menopausal women.4 Moreover, it is well established that abdominal adipose tissue serves as an endocrine organ that produces various bioactive substances, affecting the body’s metabolic status.18 The accumulation of fat is likely to be an essential risk factor for kidney injury, and increases in WC and TG indicate accumulation of fat in abdominal adipose tissue. There were other visceral adiposity indices also exploiting WC and TG, such as visceral adiposity index (VAI) and the lipid accumulation product index (LAPI), and it was reported that VAI level is associated with elevated urine albumin excretion and renal dysfunction.19 The results of this study were consistent with our present findings. In addition, some previous studies showed that prevalence of CKD is significantly associated with both VAI and LAPI.20,21 Excess fat can indirectly induce kidney damage by increasing the prevalence of atherosclerosis, hypertension, or type 2 diabetes.22,23 Furthermore, excess fat can directly exacerbate kidney injury via the inflammatory response, which produces adipokines such as TNF-α and IL-6 leading to change in renal hemodynamics. These changes include an increased demand for renal metabolism and dilation of mesangium, leading to the development of glomerular hypertrophy, excessive filtration, and hypertension. Increased glomerular filtration rates eventually cause proteinuria and glomerulosclerosis.24–26 Besides, the production of adipokine leptin is also increased, which can subsequently increase the activity of the sympathetic nervous system and the degree of oxidative stress, ultimately leading to glomerulosclerosis, renal fibrosis, and proteinuria.27
As seen in Tables 2 and 3, we also determined that the association with UACR was significant in women in Model 5 when the TG level increased, as well as with the HTGW phenotype, a combination of increased WC and TG, had the most significant association with UACR. In men, a significant association was only found between UACR and the HTGW phenotype, which is consistent with previous studies. We showed that especially in postmenopausal women, excessive lipid accumulation, such as hypertriglyceridemia, increased the risk of CVD.28–30 Increased WC and elevated TG also had a significant cumulative effect on cardiovascular risk.31,32 Further, a study among Asian adults showed that CVD was independently associated with high level of albuminuria.33 These differing results of gender may be partially explained by the difference in the sample ratio of gender. Nevertheless, in the present study, we were unable to identify the actual mechanisms of gender difference. Therefore, possible mechanisms with regard to gender-specific associations of the HTGW phenotype with UACR need to be further explored.
It is interesting to find that the association of HTGW phenotype with UACR in borderline subgroups compared with the two other subgroups was significant, as shown in Table 4. The possible reason, at least in part, might be due to the populations of old age, hypertension, obese and diabetes possibly had better compliance with medical orders, had more time to exercise, and dietary habits may also improve. Some studies showed that physical activities reduced inflammation which played a key role in the loss of renal function34,35 and the relationship between HTGW and UACR may attenuate in the abnormal subgroups. Moreover, interaction between HTGW and potential confounders among the UACR group was analysed in the present study. The interaction between the HTGW phenotype and blood glucose was significant. The present result indicated that among the prediabetic population, FBG is more closely related to HTGW and UACR. A Mexican study, whose results were consistent with the findings of our study, suggested that kidney dysfunction was worsened among people with high TG levels, in those with increased WC, and in those who smoke, as well as, most notably, in people with diabetic diseases.22 Moreover, evidence showed that the glucose abnormality and the functional impairment of β-cell were both caused by the overloaded islets TG content.36 Furthermore, another study indicated that visceral adiposity was significantly associated with IFG, and isolated IFG had been shown to lead to hepatic IR.37 Because patients may have undiagnosed IFG for an extended period of time, asymptomatic complications such as diabetic nephropathy can eventually develop.38 Therefore, the findings of the present study may offer proof for prevention of early diabetic nephropathy via the improvement of the HTGW phenotype.
Our study provides additional evidence for the association between HTGW and UACR in a different population. However, several limitations of this study need to be considered. First, since this research population only included populations over the age of 40 years, our findings may not be applicable to younger individuals. Furthermore, the gender ratio is skewed; thus, the associations obtained in this study should be considered with caution. Second, the selected population excluded those who used lipid lowering and ACEI/ARB drugs but did not exclude the population using other drugs that may affect the association. Therefore, we cannot be certain how the use of other drugs may have affected the results of this study. Third, a limitation to the HTGW phenotype is its dichotomous nature of representation. With cut-off points originated from western populations and not validated in China, using the same cut-off points for HTGW in a Chinese population is a limitation. Finally, due to the cross-sectional nature of the current study, the causal association of UACR and the HTGW phenotype was undetermined, and prospective studies are surely needed to prove whether the HTGW phenotype could predict increased UACR.
Conclusion
In conclusion, we found a noticeable association between the HTGW phenotype and UACR. When the population belonged to the HTGW group aged less than 65 years with eGFR ≥ 90 mL/min per 1.73 m2, prediabetes, prehypertension, and a borderline BMI value, the risk for increase of UACR was more likely to be augmented. The present study may supply proofs to suggests that awareness of the HTGW phenotype should be increased, and the HTGW phenotype was associated with increased early renal dysfunction or chronic diseases such as CKD and CVD. Further prospective epidemiological studies are needed to support the findings of our study.
Acknowledgments
We are indebted to the participants and their families for participating in this study. Kang Chen, Wenhua Yan, Anping Wang, Weiqing Wang, Zhengnan Gao, Xuelei Tang, Li Yan, Qin Wan, Zuojie Luo, Guijun Qin, Lulu Chen, Guang Ning. For these people’s invaluable help in collecting data.
Funding Statement
This study was supported by the Chinese Society of Endocrinology, the Key Laboratory for Endocrine and Metabolic Diseases of Ministry of Health (1994DP131044); the National Key New Drug Creation and Manufacturing Program of Ministry of Science and Technology (2012ZX09303006-001); the National High Technology Research and Development Program of China (863 Program, 2011AA020107); the National Science and Technology Major Project 288 (2011ZX09307-001-8); the Beijing Municipal Science and Technology Commission (No. D141107005314004); and the Scientific and Technological Innovation Program of Sanya (2016YW31).
Data Sharing Statement
All data used to support the findings of this study are available from the corresponding author upon request.
Author Contributions
Wanlu Su and Jie Wang contributed equally to this article. Wanlu Su performed the statistical analysis and interpreted the data, drafted and revised the manuscript. Jie Wang contributed to design the conception of the manuscript, interpret the data, draft and revise the manuscript. Yiming Mu offered many help for data collection and acquisition and revised the manuscript. Every author contributed in the final approval of the version to be published and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Disclosure
The authors report no conflicts of interest in this work. This study was conducted in accordance with the Declaration of Helsinki.
References
- 1.Despres JP, Lemieux I, Bergeron J, et al. Abdominal obesity and the metabolic syndrome: contribution to global cardiometabolic risk. Arterioscler Thromb Vasc Biol. 2008;28(6):1039–1049. doi: 10.1161/ATVBAHA.107.159228 [DOI] [PubMed] [Google Scholar]
- 2.Fujimoto WY, Bergstrom RW, Boyko EJ, et al. Visceral adiposity and incident coronary heart disease in Japanese-American men: the 10-year follow-up results of the seattle Japanese-American community diabetes study. Diab Care. 1999;22(11):1808–1812. doi: 10.2337/diacare.22.11.1808 [DOI] [PubMed] [Google Scholar]
- 3.Nicklas BJ, Penninx BW, Ryan AS, et al. Visceral adipose tissue cutoffs associated with metabolic risk factors for coronary heart disease in women. Diab Care. 2003;26(5):1413–1420. doi: 10.2337/diacare.26.5.1413 [DOI] [PubMed] [Google Scholar]
- 4.Tankó LB, Bagger YZ, Qin G, et al. Enlarged waist combined with elevated triglycerides is a strong predictor of accelerated atherogenesis and related cardiovascular mortality in postmenopausal women. Circulation. 2005;111(15):1883–1890. doi: 10.1161/01.CIR.0000161801.65408.8D [DOI] [PubMed] [Google Scholar]
- 5.Lemieux I, Pascot A, Couillard C, et al. Hypertriglyceridemic waist: a marker of the atherogenic metabolic triad (hyperinsulinemia; hyperapolipoprotein B; small, dense LDL) in men? Circulation. 2000;102(2):179–184. doi: 10.1161/01.CIR.102.2.179 [DOI] [PubMed] [Google Scholar]
- 6.Sam S, Feinstein S, Haffner S, et al. Hypertriglyceridemic waist phenotype predicts increased visceral fat in subjects with type 2 diabetes. Diab Care. 2009;32(10):1916e20. doi: 10.2337/dc09-0412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao K, Yang SS, Wang HB, et al. Association between the hypertriglyceridemic waist phenotype and prediabetes in Chinese adults aged 40 years and older. J Diab Res. 2018;2018:1031939. doi: 10.1155/2018/1031939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen S, Guo X, Yu S, et al. Hypertriglyceridemic waist phenotype and metabolic abnormalities in hypertensive adults: A STROBE compliant study. Medicine. 2016;95:49. doi: 10.1097/MD.0000000000005613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang A, Li Z, Zhou Y, et al. Hypertriglyceridemic waist phenotype and risk of cardiovascular diseases in China: results from the Kailuan Study. Int J Cardiol. 2014;174(1):106–109. doi: 10.1016/j.ijcard.2014.03.177 [DOI] [PubMed] [Google Scholar]
- 10.Arsenault BJ, Lemieux I, Despres JP, et al. The hypertriglyceridemic-waist phenotype and the risk of coronary artery disease: results from the EPIC-Norfolk prospective population study. CMAJ. 2010;182(13):1427–1432. doi: 10.1503/cmaj.091276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Czernichow S, Bruckert E, Bertrais S, et al. Hypertriglyceridemic waist and 7.5-year prospective risk of cardiovascular disease in asymptomatic middle-aged men. Int J Obes. 2007;31(5):791–796. doi: 10.1038/sj.ijo.0803477 [DOI] [PubMed] [Google Scholar]
- 12.Amadi CE, Mbakwem AC, Kushimo OA, et al. Prevalence of positive chronic kidney Disease screening in professional male long haul drivers at risk of cardiovascular Disease in Lagos, Nigeria: a cross-section study. BMC Public Health. 2019;19(1):1032. doi: 10.1186/s12889-019-7328-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.J KW X, Devereux RB, Yeh J, et al. Albuminuria within the “normal” range and risk of cardiovascular disease and death in American Indians: the strong heart study. Am J Kidney Dis. 2007;49(2):208–216. doi: 10.1053/j.ajkd.2006.10.017 [DOI] [PubMed] [Google Scholar]
- 14.Resnick HE, Lindsay RS, McDermott MM, et al. Relationship of high and low ankle brachial index to all-cause and cardiovascular disease mortality: the strong heart study. Circulation. 2004;109(6):733–739. doi: 10.1161/01.CIR.0000112642.63927.54 [DOI] [PubMed] [Google Scholar]
- 15.Teixeira da Cunha França AK, Dos Santos AM, Salgado JV, et al. Usefulness of visceral adipose tissue estimation in the prevention of chronic kidney disease in hypertensive patients in primary health care. Nutr Hosp. 2018;35(4):948–956. doi: 10.20960/nh.1534 [DOI] [PubMed] [Google Scholar]
- 16.Ning G. Risk Evaluation of cAncers in Chinese diabeTic Individuals: a lONgitudinal (REACTION) study. J Diab. 2012;4(2):172–173. doi: 10.1111/j.1753-0407.2012.00182.x [DOI] [PubMed] [Google Scholar]
- 17.Ma YC, Zuo L, Chen JH, et al. Modified glomerular filtration rate estimating equation for Chinese patients with chronic kidney disease. J Am Soc Nephrol. 2006;17(10):2937–2944. doi: 10.1681/ASN.2006040368 [DOI] [PubMed] [Google Scholar]
- 18.Fortuño ARA, Gómez-Ambrosi J, Frühbeck G, et al. Adipose tissue as an endocrine organ: role of leptin and adiponectin in the pathogenesis of cardiovascular diseases. J Physiol Biochem. 2003;59(1):51–60. doi: 10.1007/BF03179868 [DOI] [PubMed] [Google Scholar]
- 19.Sun K, Lin D, Feng L, et al. Visceral adiposity index is associated with increased urinary albumin excretion: a population-based study. Clin Nutr. 2019;38(3):1332–1338. doi: 10.1016/j.clnu.2018.05.025 [DOI] [PubMed] [Google Scholar]
- 20.Xiao H, Xiong C, Xiaofei S, et al. Visceral adiposity index and Chronic kidney disease in a non-diabetic population: a cross-sectional study. Diab Metab Syndr Obes. 2020;13:257–265. doi: 10.2147/DMSO.S231656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dai D, Chang Y, Yintao C, et al. Visceral adiposity index and lipid accumulation product index: two alternate body indices to identify chronic kidney disease among the rural population in Northeast China. Int J Environ Res Public Health. 2016;13(12):1231. doi: 10.3390/ijerph13121231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stenvinkel PZC, Ikizler TA. Obesity in CKD: what should nephrologists know? J Am Soc Nephrol. 2013;24:1727–1736. doi: 10.1681/ASN.2013040330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wanner CJK. Kidneys do not like excess body fat. Lancet Diab Endocrinol. 2015;3:669–671. doi: 10.1016/S2213-8587(15)00235-1 [DOI] [PubMed] [Google Scholar]
- 24.Mallamaci F, Tripepi G. Obesity and CKD progression: hard facts on fat CKD patients. Nephrol Dial Transplant. 2013;28:iv105–108. doi: 10.1093/ndt/gft391 [DOI] [PubMed] [Google Scholar]
- 25.Spoto B, Zoccali C. Spleen IL-10, a key player in obesity-driven renal risk. Nephrol Dial Transplant. 2013;28(5):1061–1064. doi: 10.1093/ndt/gft094 [DOI] [PubMed] [Google Scholar]
- 26.Stenvinkel P, Ketteler M, Johnson RJ, et al. IL-10, IL-6, and TNF-alpha: central factors in the altered cytokine network of uremia–the good, the bad, and the ugly. Kidney Int. 2005;67(4):1216–1233. doi: 10.1111/j.1523-1755.2005.00200.x [DOI] [PubMed] [Google Scholar]
- 27.Wolf G, Ziyadeh FN. Leptin and renal fibrosis. Contrib Nephrol. 2006;151:175–183. [DOI] [PubMed] [Google Scholar]
- 28.Tanko LB, Bagger YZ, Alexandersen P, et al. Peripheral adiposity exhibits an independent dominant antiatherogenic effect in elderly women. Circulation. 2003;107(12):1626–1631. doi: 10.1161/01.CIR.0000057974.74060.68 [DOI] [PubMed] [Google Scholar]
- 29.Howard BV, Criqui MH, Curb JD, et al. Risk factor clustering in the insulin resistance syndrome and its relationship to cardiovascular disease in postmenopausal white, black, hispanic, and Asian/Pacific Islander women. Metabolism. 2003;52(3):362–371. doi: 10.1053/meta.2003.50057 [DOI] [PubMed] [Google Scholar]
- 30.Bass KMNC, Klag MJ, Bush TL. Plasma lipoprotein levels as predictors of cardiovascular death in women. Arch Intern Med. 1993;153:2209–2216. doi: 10.1001/archinte.1993.00410190045006 [DOI] [PubMed] [Google Scholar]
- 31.Kahn HS, Valdez R. Metabolic risks identified by the combination of enlarged waist and elevated triacylglycerol concentration. Am J Clin Nutr. 2003;78(5):928–934. doi: 10.1093/ajcn/78.5.928 [DOI] [PubMed] [Google Scholar]
- 32.Cheal KL, Abbasi F, Lamendola C, et al. Relationship to insulin resistance of the adult treatment panel III diagnostic criteria for identification of the metabolic syndrome. Diabetes. 2004;53(5):1195–1200. doi: 10.2337/diabetes.53.5.1195 [DOI] [PubMed] [Google Scholar]
- 33.Lim CC, Teo BW, Ong PG, et al. Chronic kidney disease, cardiovascular disease and mortality: A prospective cohort study in a multi-ethnic Asian population. Eur J Prev Cardiol. 2015;22(8):1018–1026. doi: 10.1177/2047487314536873 [DOI] [PubMed] [Google Scholar]
- 34.King DE, Carek P, Mainous III AG, et al. Inflammatory markers and exercise: differences related to exercise type. Med Sci Sports Exerc. 2003;35(4):575–581. doi: 10.1249/01.MSS.0000058440.28108.CC [DOI] [PubMed] [Google Scholar]
- 35.AbramsonandV.Vaccarino JL. Relationship between physical activity and inflammation among apparently healthy middle-aged and older US adults. JAMA Intern Cine. 2002;162(11):1286–1292. doi: 10.1001/archinte.162.11.1286 [DOI] [PubMed] [Google Scholar]
- 36.Unger RH. Lipotoxicity in the pathogenesis of obesity‐dependent NIDDM. Genetic and clinical implications. Diabetes. 1995;44(8):780863. doi: 10.2337/diab.44.8.863 [DOI] [PubMed] [Google Scholar]
- 37.Elizalde-Barrera CI, Rubio-Guerra AF, Lozano-Nuevo JJ, et al. Triglycerides and waist to height ratio are more accurate than visceral adiposity and body adiposity index to predict impaired fasting glucose. Diab Res Clin Pract. 2019;153:49–54. doi: 10.1016/j.diabres.2019.05.019 [DOI] [PubMed] [Google Scholar]
- 38.de Simone G, Wang W, Best LG, et al. Target organ damage and incident type 2 diabetes mellitus: the strong heart study. Cardiovasc Diabetol. 2017;16(1):64. doi: 10.1186/s12933-017-0542-6 [DOI] [PMC free article] [PubMed] [Google Scholar]