Abstract
Epileptogenesis refers to the process in which a normal brain becomes epileptic, and is characterized by hypersynchronous spontaneous recurrent seizures involving a complex epileptogenic network. Current available pharmacological treatment of epilepsy is generally symptomatic in controlling seizures but is not disease-modifying in epileptogenesis. Cumulative evidence suggests that adult neurogenesis, specifically in the subgranular zone of the hippocampal dentate gyrus, is crucial in epileptogenesis. In this review, we describe the pathological changes that occur in adult neurogenesis in the epileptic brain and how adult neurogenesis is involved in epileptogenesis through different interventions. This is followed by a discussion of some of the molecular signaling pathways involved in regulating adult neurogenesis, which could be potential druggable targets for epileptogenesis. Finally, we provide perspectives on some possible research directions for future studies.
Keywords: Epileptogenesis, adult neurogenesis, drug target, optogenetic, chemogenetic, neural circuit
1. Introduction
Epilepsy is a multifarious and deliberating disease, characterized by excessive or hypersynchronous spontaneous recurrent seizures (SRS) affecting ~1% of the population [1, 2]. The term “Epileptogenesis” refers to the process by which a normal brain becomes epileptic and involves a complex epileptogenic network. Anti-epileptic drugs (AEDs) are the first-choice treatment for epilepsy in clinical practice. Although there are currently ~30 AEDs with diverse molecular targets, these AEDs are generally symptomatic in controlling seizures (also called anti-seizure drugs) but are not disease-modifying in epileptogenesis [3]; no effective pharmacological prevention or cure has been identified for epilepsy. Thus, progress towards deeper and alternative insights into the mechanisms of epileptogenesis is critical for developing better therapies to prevent epilepsy in order to ease the significant burden on patients with epilepsy.
Neurogenesis, the process of generating functionally integrated neurons, is traditionally believed to occur only during embryonic stages in the mammalian Central Nervous System (CNS), but its persistence throughout adulthood in mammals (adult neurogenesis) [4-9] has recently become generally accepted. Adult neurogenesis occurs specifically in the Subgranular Zone (SGZ) of the Hippocampal Dentate Gyrus (DG) and the Subventricular Zone (SVZ) of the forebrain lateral ventricles. Specifically in the SGZ, adult-born neurons still make up about 6% of the Granular Cell Layer (GCL) in adult rats [10]. Accumulative evidence suggests that adult neurogenesis is extremely crucial for learning and memory, including encoding of temporal information into memories, spatial memory, context-dependent memory, and pattern separation, as well as cognitive flexibility [11-19]. Meanwhile, adult neurogenesis is also implicated in a variety of pathological CNS diseases, including psychiatric disorders (schizophrenia, major depression, addiction and anxiety) [20-25], neurodegenerative diseases (Parkinson’s disease, Alzheimer’s disease and Huntington’s disease) [26], and epilepsy.
Emerging evidence from both animal models and human data suggests a critical role of neurogenesis in epilepsy, especially in epileptogenesis. Recently, the field of “adult neurogenesis in epilepsy” has been propelled by technical advances, including genetic marking with retroviruses, transgenic animal models that allow visualization and specific manipulation of newborn neurons, and virus-mediated tracing methods, as well as optogenetic/chemogenetic intervention methods. In this review, we mainly concentrate on the role of adult neurogenesis in epileptogenesis. We review pathological changes that occur in adult neurogenesis in the epileptic brain and the functional relevance of neurogenesis in epileptogenesis through different updated interventions. Furthermore, we discuss some molecular signaling pathways regulating adult neurogenesis, which can be potential druggable targets for epileptogenesis, and propose some perspectives for future studies.
2. Adult Neurogenesis in the Epileptic Brain
2.1. Seizure-induced Cell Proliferation
Epileptogenesis is usually triggered by initial epileptogenic insults, such as status epilepticus (SE), childhood febrile seizures, head trauma, and stroke, followed by a latent period between initial insults and later chronic spontaneous recurrent seizures [27]. Different studies have been performed to identify the dramatic disturbances in adult neurogenesis in SGZ and SVZ based on various types of epileptogenic insults (Table 1), including pilocarpine and kainic acid (KA)-induced SE, as well as kindling stimulation [28-34]. Parent et al. (1997) used exogenous nucleotide bromodeoxyuridine (BrdU) mitotic labeling to assess cell proliferation and found a transient upregulation of neurogenesis after pilocarpine-induced SE [28]. A subsequent study using a KA-induced SE model provided further evidence that on both the ipsilateral and contralateral sides of KA injection, neurogenesis increased compared with controls [29]. Additionally, in kindling models, cell proliferation increased in animals that experienced more than 9 fully amygdala kindled seizures [31]. Examination of the hippocampus from temporal lobe epilepsy (TLE) patients also suggested increased cell proliferation [35, 36], which was consistent with studies in animal models.
Table 1.
Impact of seizures on neurogenesis, survival, morphology, and integration of adult-born GCs in different epilepsy models.
| Types of Epilepsy | Species | Region | Findings | Refs. | ||||
|---|---|---|---|---|---|---|---|---|
| Pilocarpine-induced epilepsy model | Rat | SVZ | Increased neuroblasts at 7 days after SE, returned after 21days; Increased neuroblast migration to the olfactory bulb |
Parent, J. M. et al. (2002) | ||||
| Rat | SVZ | No increase in cSVZ neurogenesis; Increased cSVZ gliogenesis |
Parent, J. M. et al. (2006) | |||||
| Rat | SGZ | Increase in neurogenesis 3, 6, and 13d after SE, recovered by 27 d; Ectopic location | Parent, J. M. et al. (1997) | |||||
| Rat | SGZ | induced HBDs which are postsynaptic to mossy fibers | Ribak, C. E. et al. (2000) | |||||
| Rat | SGZ | Adult-born DGCs located at the hilar/CA3 border several weeks after SE and they were synchronized with CA3 pyramidal cells | Scharfman, H. E et al. (2000) | |||||
| Rat | SGZ | Perforant path activation led to robust activation of newborn hilar GCs | Scharfman, H.E et al. (2003) | |||||
| Rat | SGZ | HBDs were longer than control; 20% HBDs of newborn neurons in epileptic rat, <2% in control; HBDs were adjacent to astrocytic process in the hilus |
Shapiro, L. A. et al. (2005) | |||||
| Rat | SGZ | More ectopic hilar GCs, more frequent seizures | McCloskey, D. P. et al. (2006) | |||||
| Rat | SGZ | Progenitors migrated aberrantly to the hilus and ML in rat DG; Ectopic DGCs were found in the hilus and ML |
Parent, J. M. et al. (2006) | |||||
| Mouse | SGZ | 2 weeks or 1 month after seizures, the length and complexity of dendrites of immature (~12d) GCs were both increased; Dendrite outgrowth correlated with the severity of initial seizures; 5-16 days after seizure, PP stimulation evoked glutamatergic input to newborn GCs |
Overstreet-Wadiche, L. S. et al. (2006) | |||||
| Mouse | SGZ | ~50% of immature GCs exhibited aberrant HBDs compared with only ~9% of immature GCs; newborn cells were even more severely impacted than immature cells | Danzer, S. et al. (2007) | |||||
| Mouse | SGZ | Positive correlations were found between seizure frequency and the percentage of hilar ectopic GCs; the amount of MFS; the extent of mossy cell death |
Hester, M. S et al. (2013) | |||||
| Rat | SGZ | Low incidence of severe seizures enhanced neurogenesis and the generation of ectopic hGCs; High incidence of severe seizures impaired GCL and reduced newly-generated cells |
Uemori, T. et al. (2017) | |||||
| KA-induced epilepsy model | Rat | SGZ | KA-induced seizures led to neurogenesis | Bengzon, J. et al. (1997) | ||||
| Rat | SGZ | 1 week after KA, neurogenesis was increased bilaterally; > 6-fold ipsilateral, 3-fold contralateral | Gray, W. P. et al. (1998) | |||||
| Rat | SGZ | Neurogenesis began to increase at day 3, peaked at day 5 and returned to baseline at day 10 | Nakagawa, E. et al. (2000) | |||||
| Rat | SGZ | Induced HBDs which are postsynaptic to mossy fibers | Ribak, C. E. et al. (2000) | |||||
| Rat | SGZ | Adult-born DGCs located at the hilar/CA3 border several weeks after SE and they were synchronized with CA3 pyramidal cells | Scharfman, H. E et al. (2000) | |||||
| Rat | SGZ | DCX-expressing cells were increased 16 days after ICV or IP KA; Conversely, neurogenesis declined after 5 months | Hattiangady, B. et al. (2004) | |||||
| Mouse | SGZ | Increased cell proliferation and new neurons persisted for months; Stimulus stimulated the division of late type-3 progenitor cells (expressing DCX) |
Jessberger, S. et al. (2005) | |||||
| Types of Epilepsy | Species | Region | Findings | Refs. | ||||
| KA-induced epilepsy model | Mouse | SGZ | GCL dispersion within the lesion is negatively associated with ipsi neurogenesis, the contra exhibits neurogenesis without local neuronal loss; similar survival rate in KA as in control |
Kralic, J. E. et al. (2005) | ||||
| Mouse | SGZ | Early, transient increase in neurogenesis bilaterally, becoming microglial cells and astrocytes instead of neurons; later, neurogenesis stops in ipsi but not in contra | Heinrich, C. et al. (2006) | |||||
| Rat | SGZ | Granule cells born after SE extended abnormal HBDs and became morphologically and functionally integrated; GCs born before SE didn't show aberrant dendritic growth |
Jessberger, S. et al. (2007) | |||||
| Perforant path stimulation | Rat | SGZ | Prolonged, focal seizure discharges increased mitotic activity | Parent, J. M. et al. (1997) | ||||
| Rat | SGZ | Neurogenesis began to increase after 5 consecutive stageIseizures | Nakagawa, E. et al. (2000) | |||||
| Hippocampal kindling model | Rat | SGZ | 1 and 40 kindling stimulations induced neurogenesis | Bengzon, J. et al. (1997) | ||||
| Rat | SGZ | SE of varying severity triggered similar short-term (1 week) proliferation of neural progenitors; More new neurons survived at 4 weeks after partially convulsive SE compared with fully convulsive |
Mohapel, P. et al. (2004) | |||||
| Amygdala kindling model | Rat | SGZ | 9 or more class 4/5 kindled seizures increased cell proliferation in DG | Parent, J. M. et al. (1998) | ||||
| Rat | SGZ | No significant change during focal seizures; Neurogenesis was enhanced by 75-140% after generalized seizures |
Scott, B. W. et al. (1998) | |||||
| Electrovonculsive seizure | Rat | SGZ | A single seizure increased the number of newborn cells, surviving for > 3 months; dose-dependent mechanism |
Madsen, T. M. et al. (2000) | ||||
| Flurothyl kindling model (forebrain seizures) |
Mouse | SGZ | Significant increases in either 1 or 8 fluorothyl-induced seizures; Increases were observed at 1 and 3 days after 1 seizures, and at 0, 1, 3 and 7 days after 8 seizures |
Ferland, R. J. et al. (2002) | ||||
| Electrically induced, self-sustained SE | Rat | SGZ | The degree of survival of newly generated neurons was determined primarily by the initial SE instead of additional seizures |
Ekdahl, C. T. et al. (2003) | ||||
| Rat | SGZ | A substantial proportion of the mature GCs at 6 months are generated during the first 2 weeks after SE and survive | Bonde, S. et al. (2006) | |||||
| TLE | Human | SGZ | Progenitors migrated aberrantly to the hilus and ML in rat DG; Ectopic DGCs were found in the hilus and ML |
Parent, J. M. et al. (2006) | ||||
The response in adult neurogenesis is also intriguing at different stages after initial SE. Increased neurogenesis after acute seizures returns to baseline or even below baseline level about 1 month after the initial seizure episode in rats [28, 37, 38]. Hattiangady et al. (2004) employed doublecortin (DCX) as a marker of newly born neurons and reported that neurogenesis declined at 5 months post-KA administration [37]. Accordingly, another study by Heinrich and colleagues reported a gradual decrease in neurogenesis by 1 week and below baseline by 4-6 weeks after the initial seizure episode [38]. One possible explanation is that the potential for adult neurogenesis might be reduced due to the “exhaustion” of the neural stem cell (NSC) pool or alterations in the neurogenic niche providing support for NSCs [37, 39, 40]. Moreover, the degree of abnormal adult neurogenesis is also associated with the severity of the initial SE, which is also a key determinant factor for later chronic SRS. It was demonstrated that SE severity influenced the long-term outcome instead of short-term neurogenesis in a hippocampal kindling model; animals exhibiting less severe SE states (partially convulsive) had more newborn Dentate Granule Cells (DGCs) survive after 4 weeks (long-term) compared with the fully convulsive group, while SE of varying severity triggered similar neurogenesis after 1 week (short-term) [41, 42]. On the contrary, Uemori et al. (2017) found in a pilocarpine-induced SE model that seizure-induced damage and aberrant adult neurogenesis observed 10 days after seizure induction were dependent on the frequency of severe seizures (falling and jumping); fewer severe seizures were associated with enhanced neurogenesis and the generation of ectopic hilar DGCs, while the opposite effects were exerted by more severe seizures [43].
Interestingly, conflicting results were observed when evaluating adult neurogenesis in neonatal rats. In contrast to the increased cell proliferation observed after acute epileptogenic insults in adult animals, neurogenesis decreased after acute SE in young pups, and a modest increase in neurogenesis even at 2 months after SE was found [44-47]. Thus, it seems that the change of adult neurogenesis depends on the developmental state of the brain at the time of the initial seizure induction.
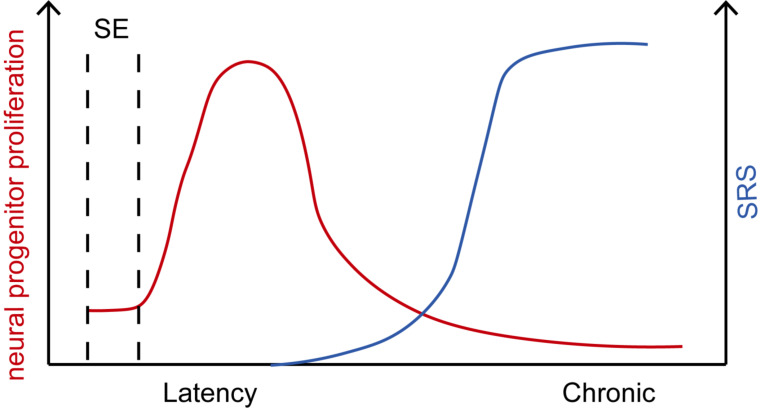
Generally, adult neurogenesis is transiently upregulated by initial epileptogenic insults, and then returns to baseline or even below baseline during the SRS period (Fig. 1). The change of adult neurogenesis is influenced by many important factors, including the severity of initial epileptogenic insult, the stage of epileptogenesis, and the age of brain. Notably, as epileptogenesis is involved in a multifarious process, whether other various epileptogenic insults lead to similar change of adult neurogenesis still needs to be widely verified.
Fig. (1).
Changes of neural progenitor proliferation and SRS in epileptogenesis over time. Epileptogenesis is usually triggered by initial epileptogenic insults, such as status epilepticus (SE), followed by a latent quiescent period between initial insults and later chronic spontaneous recurrent seizures (SRS). The quiescent latency lasts ranging from several days to several weeks and ends at the time of the appearance of the first spontaneous seizure. Frequency of SRS increases with time and gradually becomes stable. On the other hand, seizure activity leads to a dramatic increase in neural progenitor proliferation judged by Ki67 expression or short-pulse bromodeoxyuridine (BrdU) labeling in the DG after SE. Neural progenitor proliferation returns to baseline levels approximately 3 to 4 weeks following the initial SE episode. At later stages following SE, the potential for adult neurogenesis might even be reduced, probably due to an “exhaustion” of the NSC pool. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
2.2. Abnormal Maturation and Integration of Adult-generated GCs
In addition to the quantitative changes in neurogenesis after acute SE, major qualitative alterations have also been reported [48-50]. Seizure-induced changes in the local environment were found to accelerate the functional integration of adult-generated DGCs, which affected not only the newborn DGCs generated immediately after seizure, but also impacted subsequent generations [51]. Some populations of seizure-generated DGCs show severe morphological abnormalities that may critically affect dentate connectivity. In striking contrast to cells born under normal conditions, which typically have a single dendrite arising from the apical portion of the cell body branching in the outer GCL or inner molecular layer, seizure-generated DGCs tend to extend additional basal dendrites toward the hilus [52, 53]. In the rat pilocarpine model, about a third of adult-born DGCs that were 2 weeks old at the time of SE or born 4 days after SE developed Hilar Basal Dendrites (HBDs) [54]. This leads to a dramatic shift in the percentage of granule cells with HBDs: from 1% in controls to ≈20% in epileptic animals [55]. Moreover, newly generated DGCs display a significantly greater percentage of HBDs as compared to mature DGCs following SE [56, 57]. Meanwhile, granule cells with HBDs received significant recurrent inputs from neighboring granule cells, as evidenced by both anatomical and electrophysiological studies [52, 58, 59].
In addition to aberrant dendritic growth, SE also alters the migration of adult-born neurons. In the adult rat SVZ, prolonged seizures increased neuroblast migration to the olfactory bulb and induced a portion of neuroblasts to exit the migratory stream prematurely and enter non-olfactory forebrain regions [34]. Although the vast majority of newborn DGCs during adult life migrated into the GCL, DGCs were found in rodent models of epilepsy to migrate instead into the hilus or through the GCL into the inner molecular
layer after SE [28, 37]. Similar ectopically located DGCs were also reported to appear in the epileptic human hippocampus [60]. Accompanying their abnormal localization, the electrophysiological features of ectopic granule cells are different from cells born under normal conditions. Bursting is not typical of normal DGCs, while many of these ectopic DGCs burst in synchrony with CA3 pyramidal cells, and this property has been suggested to be pro-epileptogenic [61]. Meanwhile, it was also shown that ectopic DGCs exhibited higher ratios of excitatory to inhibitory inputs than normal DGCs [62]. Combining retroviral birth dating with rabies virus-mediated retrograde trans-synaptic tracing, Du et al. (2017) found substantial hippocampal circuit remodeling after an epileptogenic insult. Prominent excitatory monosynaptic inputs (from other DGCs, CA3, and CA1 pyramidal cells) were all increased for early-born and adult-born DGCs [63]. Using the same retrograde tracing technology, a more recent study by Zhu et al. (2019) also revealed that newborn DGCs formed excessive de novo excitatory connections, as well as recurrent excitatory loops [64]. Conversely, seizure-generated DGCs had less excitatory but increased inhibitory input that resulted in overall decreased excitability compared with newborn DGCs induced by running [65]. These findings suggest that seizure-induced adult neurogenesis may play a compensatory role in epileptogenesis.
In general, although controversy exists, there are abundant pathologies identified among DGCs, including changed cell proliferation, abnormal maturation, and functional integration in the epileptic brain, which are thought to contribute to epileptogenesis through the establishment of hyper-excitatory networks. However, whether these abnormal newborn DGCs are causal or resultant factors for epileptogenesis remains to be determined.
3. Functional significance of adult neurogenesis in epileptogenesis
While intriguing, morphological and electrophysiological studies are only correlation studies. They are not adequate to definitively establish that the integration of adult-born DGCs contributes to epileptogenesis. Thus, subsequent studies have used different intervention approaches to block or reduce adult neurogenesis (loss of function) to assess its real role in epileptogenesis (Table 2).
Table 2.
Functional relevance of adult neurogenesis in epilepsy with different interventions.
| Interventions |
Timepoints of
Intervention |
Species | Animal Models | Findings | Refs. |
|---|---|---|---|---|---|
| Inhibition of neurogenesis by antimitotic Ara-C | from 1 day before SE for 14 days | Rat | Pilo-induced epilepsy | Reduced frequency and duration of seizures; No obvious difference of neuronal damage |
Jung K. H. et al. (2004) |
| Inhibition of neurogenesis by COX-2 inhibitor | from 1 day after SE to 14 or 28 days after SE | Rat | Pilo-induced epilepsy | Reduced frequency and duration of seizures; Neuroprotective effect |
Jung K. H. et al. (2006) |
| Inhibition of neurogenesis by radiation | 1 day before starting kindling | Rat | Hippo kindling model | Decreased ADT and developed more severe seizure more rapidly | Raedt, R. et al. (2007) |
| Inhibition of neurogenesis by Levetiracetam | from 1 day after SE for 25 days | Mouse | KA-induced epilepsy | Decreased the mean duration of seizures 58 days later | Sugaya, Y. et al. (2010) |
| Inhibition of neurogenesis by radiation | 1 day before starting kindling | Rat | Amygdala kindling model | No effects on kindling acquisition and kindled seizures | Pekcec, A. et al. (2011) |
| Genetic ablation of neurogenesis (Nestin-TK) |
GCV for 4 weeks until injection of Pilo | Mouse | Pilo-induced epilepsy | Reduced frequency of SRS; Restored cognitive function |
Cho, K. O. et al. (2015) |
| Reduction of neurogenesis by X-irradiation or genetic ablation (GFAP-TK) |
from 6 weeks of age (3 doses of X-irradiation, 3 days between doses) 7 weeks later, KA; from 6 weeks of age (VGCV for 6 weeks) 2 weeks later, KA |
Mouse | KA-induced epilepsy | Increased the acute effects of KA (decrease in the latency to the first convulsive seizure, increased number, duration and mortality) |
Iyengar, S. S. et al. (2015) |
| Genetic ablation of neurogenesis (NestinCreERT2:: DTr) |
from 3 weeks of age for 4 weeks (tamoxifen, weekly), 1 week later, Pilo; DT began the 3rd day after SE daily for 5d | Mouse | Pilo-induced epilepsy | Reduced seizure frequency; Increased seizure duration |
Hosford, B. E. et al. (2016) |
| Inhibition of neurogenesis by MAM | both 4 weeks ahead of and after SE; with intervals of 48 hours for 4 weeks | Mouse | Pilo-induced epilepsy | eHGCs, MFS and HBDs were eliminated; No alterations in frequency, duration, or severity |
Zhu, K. et al. (2017) |
| Inhibition of neurogenesis by ephrin-B3 | from 7 days after SE for 7 days | Rat | Pilo-induced epilepsy | Reduced seizure frequency; Reduced amplitudes and mean duration of EEG seizures |
Liu, T. et al. (2018) |
| Chemogenetic excitation/inhibition of newborn neurons (RV-hM3Dq/RV-hM4Di) |
3 days after SE, RV-hM4Dq/hM3Di injected; 2.5 months later, EEG recording, 1-3 d baseline, 4-6 d CNO |
Mouse | Pilo-induced epilepsy | Inhibition reduced epileptic spikes and SRS; Activation increased epileptic spikes and SRS |
Zhou, Q. G. et al. (2019) |
| Genetic ablation of neurogenesis (Nestin-TK) | GCV for 4 weeks post-SE, EEG recording from 5 to 7 weeks post-SE; GCV for 8 weeks post-SE, EEG recording from 5 to 7 weeks post-SE or EEG recording from 18 weeks to 20 weeks post-SE |
Mouse | Pilo-induced epilepsy | 4 weeks of ablation, no effect on SRS frequency or duration; 8 weeks of ablation, 65% reduction of SRS frequency, last for 10 days |
Varma, P. et al. (2019) |
Using a variety of antimitotic agents or radiation to limit adult neurogenesis before an epileptogenic brain injury, early studies have produced conflicting results. Raedt and colleagues used low-dose radiation to suppress hippocampal adult neurogenesis in a kindling model of TLE and found that radiated rats developed severe seizures more rapidly, while the final establishment of the permanent fully kindled state was not influenced [66]. Meanwhile, other studies showed no detrimental or protective effects of newborn DGCs on hippocampal network function [65-68], suggesting that hyper-excitability within the epileptic hippocampal circuitry might be compensated for by more inhibited newborn DGCs. By contrast, studies demonstrated that decreasing DGC neurogenesis by using antimitotic agents, including cytosine-b-D-arabinofuranoside (Ara-C) or a selective Cyclooxygenase-2 (COX-2) inhibitor, reduced the frequency of SRS and suppressed epileptogenesis [69, 70]. Nonetheless, all approaches discussed above (anti-mitotic agents, radiation, etc.) have other non-specific effects apart from reducing newborn DGCs; meanwhile, the role of adult neurogenesis may vary since different epilepsy models are applied. Thus, the effect (or lack of effect) of these approaches is not adequate to define the precise role of adult neurogenesis in epileptogenesis.
A conditional and inducible transgenic cell ablation strategy was used to ablate adult neurogenesis more selectively and at specified time points [71-73]. In a pilocarpine-induced SE model, Nestin-δ-HSV-thymidine kinase-EGFP (Nestin-TK) transgenic mice were used to achieve selective ablation of dividing neural stem/progenitors by ganciclovir (GCV) administration. Remarkably, in this way, ablation of adult neurogenesis for 4 weeks before acute seizures led to a reduction in the frequency of SRS, which lasted for nearly 1 year [72]. A subsequent study extended these findings by beginning ablation from 5 weeks before SE until 3d after the epileptogenic insult, using a conditional and inducible diphtheria-toxin receptor (DTr) expression strategy. This approach produced a 50% reduction in the frequency of SRS and a 20% increase in the duration of SRS [73]. More recently, more than 4 weeks of continuous and concurrent ablation of seizure-induced neurogenesis in the Nestin-TK mice after SE was reported to reduce the formation of SRS by 65% [74], while the therapeutic effective time was very limited. Whether adult neurogenesis in later phases of epileptogenesis may consistently contribute to epileptogenic circuits and lower the therapeutic efficacy of targeting early adult neurogenesis still remains to be studied.
In addition, although these above studies indicate that abnormal hippocampal newborn DGCs contribute to epileptogenic circuits, it remains to be determined how these abnormal hippocampal DGCs contribute to the onset of SRS. Recently, chemogenetic approaches have been developed as a valuable platform for manipulating neuronal and non-neuronal signal transduction in a cell-type-specific manner in freely moving animals [75]. Compared with ablation, the chemogenetic method only controls the activity of newborn DGCs in an inducible and reversible manner without interrupting neural circuit formation during epileptogenesis. Using this method to inhibit newborn DGCs, Zhou et al. (2019) found that epileptic spikes and SRS were both reduced, revealing an essential role for newborn DGCs in the production of epileptic spikes and SRS. Conversely, specific activation of
newborn DGCs dramatically increased the frequency of epileptic spikes and SRS, indicating that activation of newborn DGCs was sufficient to activate epileptic neural circuits [64]. This is the first research published that focused on attempting to activate newborn DGCs (gain of function) rather than merely ablating them, which indicates that newborn DGCs are necessary and sufficient for remodeling hippocampal neural circuits underling induction of SRS during epileptogenesis.
However, uncertainty still remains as to the exact contribution of newborn DGCs to epileptogenesis. In 2015, Iyengar and colleagues demonstrated that ablating newborn neurons before a challenge with (KA) increased the severity of the resulting seizures, suggesting that the newborn DGCs before SE may serve a protective role during acute seizures [76]. On the other hand, another study by Cho et al. (2015) reported that there was no observed effect from ablation on acute SE induced by pilocarpine [72]. Meanwhile, in this work, investigators found that pre-SE ablation was effective at reducing later SRS, whereas pre- plus post-SE ablation, which provided near-complete ablation, turned out to be not effective. Using a transgenic mouse model to reduce neurogenesis by deleting the transcription factor NeuroD1 from progenitors, Brulet and colleagues found that although this approach produced a partial reduction in neurogenesis, SRS was similar between control and knockout mice treated by pilocarpine [77]. Considering these contradictory findings, there is a possibility that functionally heterogeneous populations of adult-born neurons exist. In other words, adult-born neurons are a mixture of hyper- and hypo-excitable cells. To address this hypothesis, there remains a need to develop specific methods to target or modulate subpopulations of adult-born neurons that are structurally or functionally abnormal. Besides, differences among mouse strains, timepoints, seizure models, or other technical aspects all contribute to these conflicting findings. Future studies will need to use improved tools to precisely distinguish detrimental and protective adult-generated neurons.
4. Signaling mechanism regulating adult neurogenesis: Looking for new potential druggable targets for epileptogenesis
4.1. The Challenge for Current AEDs: Control of Seizure but not Epileptogenesis
Although the pathophysiological mechanism underlying epilepsy is multifactorial, it is commonly recognized that epileptic seizures are caused by generalized hyperexcitability and excessive or hypersynchronous activity with enhanced neuronal excitability [1, 78]. There is no doubt that this concept has assisted our discovery of many AEDs with different targets. Currently available AEDs are mostly based on the following main mechanisms: (1) modulation of voltage-gated ion channels, including the voltage-gated sodium, potassium, and calcium channels, which are essential to the intrinsic excitability of neuron, and play a key role in epilepsy [79-86]; (2) inhibition of excitatory synaptic transmission, such as glutamate, one of the predominant excitatory neurotransmitters in the adult mammalian brain, which is closely involved with epilepsy [87-94]; (3) enhancement of inhibitory synaptic transmission, such as GABA, one of the most important inhibitory neurotransmitters in the brain and one that provides a key functional mechanism for AED [95-101].
However, epilepsy is still associated with considerable therapeutic pharmacological treatment challenges. One foremost challenge is drug-resistance, with 30% of epilepsy patients unable to be efficiently controlled through current therapies [102, 103]. Meanwhile, most available AEDs are not without side effects (including ataxia, dizziness, cognitive impairment, and depression) [104, 105]. Another primary challenge is that no one AED is able to inhibit or prevent epileptogenesis. All current AEDs actually work as symptomatic drugs alleviating seizure activity. Apart from the seizure itself, the burden of comorbidities (including depression, anxiety, and cognitive deficits), which are significant in people with epilepsy [106], are not well controlled by AEDs. Without a doubt, investigations of novel therapeutics for epileptogenesis should extend beyond directly manipulating the excitatory and inhibitory imbalance. From this point of view, new concepts and novel targets should be introduced based on a deeper and more comprehensive understanding of the precise mechanism of epileptogenesis, such as abnormal adult neurogenesis.
4.2. Druggable Target of Adult Neurogenesis for Epileptogenesis
Fortunately, recent progress in adult neurogenesis represents a promising target for the intervention of epileptogenesis. The balance between progenitor maintenance, proliferation, and neuronal differentiation is regulated by signals provided by the microenvironment that respond to both physiological and pathological stimuli. Thus, a better understanding of these signaling pathways can lead to a more defined regulation of neurogenesis for treatment in the future. Various signaling pathways and neurochemicals, such as Wnt [107-113], Notch [114-118], sonic hedgehog (Shh) [119-121], Bone Morphogenetic Proteins (BMPs) [122, 123], Brain-Derived Neurotrophic Factor (BDNF) [124-128], vascular Endothelial Growth Factor (VEGF) [129], and neurotransmitters like glutamate and GABA [130-132], as well as inflammatory pathways [133-135], have all been found to be important regulators of neurogenesis (Table 3). Meanwhile, some of the above have been reported to produce notable changes in epilepsy, suggesting that these signaling pathways take part in certain phases of epileptogenesis. In the following section, we describe potential pathways that may be used to prevent epileptogenesis through modulation of adult neurogenesis (Fig. 2).
Table 3.
Overview of signaling pathways regulating neurogenesis.
| Molecules | Effect on Neurogenesis | Species | Region | Refs. | ||||
|---|---|---|---|---|---|---|---|---|
| Morphogens | ||||||||
| Wnt | Increases neurogenesis; Involved in the control of neuronal fate commitment |
Rat | SGZ | Lie et al. (2005) | ||||
| Required for progenitor proliferation | Mouse | SVZ | Adachi, K. et al. (2007) | |||||
| Mouse | SGZ | Mao, Y. et al. (2009) | ||||||
| Mouse | SGZ/SVZ | Qu, Q. et al. (2010) | ||||||
| Required for dendritic development | Mouse | SGZ | Gao, X. et al. (2007) | |||||
| Required for neuronal differentiation | Mouse | SGZ | Kuwabara, T. et al. (2009); Gao Z. et al. (2009) | |||||
| Required for the survival | Mouse | SGZ/ SVZ | Gao Z. et al. (2009) | |||||
| Shh | Required for progenitor proliferation | Rat | SGZ | Lai, K. et al. (2003); Banerjee, S.B. et al. (2005) | ||||
| Mouse | SGZ/SVZ | Machold R. et al. (2003); Ahn, S. et al. (2005) | ||||||
| Required for formation of adult NSCs | Mouse | SGZ | Han, Y. G. et al. (2008) | |||||
| Notch | Required for NSC self-renewal, maintenance | Mouse | SVZ | Chojanacki, A. et al. (2003); Imayoshi, I. et al. (2010) | ||||
| Mouse | SGZ | Breuning, J. J. et al. (2007); Ables, J. L. et al. (2010); Ehm, O. et al. (2010) | ||||||
| Required for dendritic arborization | Mouse | SGZ | Breuning, J. J. et al. (2007) | |||||
| Increases SVZ neurogenesis | Rat | SVZ | Wang X. et al. (2009) | |||||
| Required for maturation of neurons | Mouse | SVZ | Fujimoto, M. et al. (2009) | |||||
| BMP | Decreases neurogenesis; Promotes glia differentiation; Promotes neuroblasts survival |
Mouse | SVZ | Lim, D. A. et al. (2000) | ||||
| Blocking BMP via Noggin is required for NSC self-renewal | Mouse | SGZ | Bonaguidi, M. A. et al. (2008) | |||||
| Blocking BMP via Noggin increases neurogenesis | Mouse | SGZ | Guo, W. et al. (2011) | |||||
| Neurotrophins | ||||||||
| BDNF | Required for survival of newborn neurons | Mouse | SGZ/SVZ | Linnarsson, S. et al. (2000); Bergami, M. et al. (2008) | ||||
| Increases neurogenesis | Rat | neurogenic and non-neurogenic niches | Benraiss et al. (2001); Pencea et al. (2001) | |||||
| Rat | SGZ | Katoh-Semba, R. et al. (2002); Scharfman, H. et al. (2005) |
||||||
| No effect in neurogenesis in mice; Decreases neurogenesis in rats |
Rat/Mouse | SVZ | Galvao, R. P. et al. (2008) | |||||
| Required for progenitor proliferation | Mouse | SGZ | Li, Y. et al. (2008) | |||||
| Required for dendritic arborization and functional integration of newborn neurons | Mouse | SGZ | Bergami, M. et al. (2008) | |||||
| NT-3 | Required for neuronal differentiation | Mouse | SGZ | Shimazu, K. et al. (2006) | ||||
| NGF | Promotes survival of neurons | Rat | SGZ | Frielingsdorf, H. et al. (2007) | ||||
| Molecules | Effect on Neurogenesis | Species | Region | Refs. | ||||
| Growth factors | ||||||||
| FGF-2 | Increases neurogenesis; Enhances dendritic growth |
Rat | SGZ | Rai, K. S. et al. (2007) | ||||
| Required for progenitor proliferation | Mouse | SGZ | Zhao, M. et al. (2007) | |||||
| IGF-1 | Increases neurogenesis | Rat | SGZ | Aberg M. A. et al. (2000); Lichtenwalner R. J. et al. (2001) |
||||
| Instructs multipotent progenitors to become oligodendrocytes | Rat | SGZ | Heish, J. et al. (2004) | |||||
| Required for migration from SVZ to OB | Mouse | SVZ | Hurtado-Chong, A. et al. (2009) | |||||
| VEGF | Increases neurogenesis | Rat | SGZ/SVZ | Jin, K. et al. (2002) | ||||
| Required for progenitor proliferation | Rat | SGZ | Warner-Schmidt, J. L. (2007) | |||||
| Neurotransmitters | ||||||||
| Glutamate | Reduces neurogenesis | Rat | SGZ | Cameron, H. A. (1995) | ||||
| Increases neurogenesis | Rat | SGZ | Bai, F. et al. (2003) | |||||
| Required for survival of new neurons | Mouse | SGZ | Tashiro, A. et al. (2006) | |||||
| Required for migrating neuroblasts survival | Mouse | SVZ | Platel, J. C. et al. (2010) | |||||
| GABA | Reduces the speed of migrating neuroblasts | Mouse | SVZ | Bolteus A. J. et al. (2004) | ||||
| Reduces neurogenesis | Mouse | SVZ | Liu, X. et al. (2005) | |||||
| Required for synaptic integration and dendritic development of new neurons | Mouse | SGZ | Ge, S. et al. (2006) | |||||
| Required for NSC maintenance | Mouse | SGZ | Song J, et al. (2012) | |||||
| Promotes survival of newborn progenitors | Mouse | SGZ | Song J, et al. (2013) | |||||
| Dopamine | Required for progenitor proliferation | Mouse | SGZ/SVZ | Hoglinger, G. U. et al. (2004); Baker, S. A. et al. (2004) |
||||
| Increases neurogenesis | Rat | SVZ | Van Kampen, J. M. et al (2004) | |||||
| Required for progenitor proliferation in GCL; Deafferentation increases neurogenesis in the glomerular layer |
Rat | SVZ | Winner, B. et al. (2006) | |||||
| Inflammatory cytokines | Decreases neurogenesis | Rat | SGZ | Ekdahl C.T. et al. (2003) | ||||
| Decreases neurogenesis | Rat | SGZ | Monje, M. L. et al. (2003) | |||||
| Decreases neurogenesis | Mouse | SGZ | Iosif, R. E. et al. (2006) | |||||
| Decreases neurogenesis; Promotes astroglial differentiation |
Mouse | SGZ | Zonis, S. et al. (2013) | |||||
Fig. (2).
Hippocampal circuitry remodeling in the epileptic brain and signaling pathways regulating adult hippocampal neurogenesis. Upper panel: Schematic of the hippocampus in normal physiological conditions, sparse DGC-DGC connections are shown. Newborn neurons in the SGZ pass through several consecutive developmental stages. Different signaling pathways exert stage- and cell-specific effects. Lower panel: Schematic of the epileptic brain demonstrating increased recurrent DGC-DGC connections and preferential inputs from hilar ectopic DGCs to adult-born DGCs; back projections from CA3 pyramidal cells preferentially onto adult-born DGCs. Inhibitory inputs (blue dashed line) onto adult-born DGCs from interneurons are maintained, while those onto early-born DGCs are diminished. Increased excitatory inputs (red dashed line) and outputs (black dashed line) indicate their contributions to epileptogenesis. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
4.3. Wnt/β-catenin
Several studies have addressed the role of Wnt signaling in adult neurogenesis. It was reported that overexpression of Wnt3 increased neurogenesis; in contrast, blocking Wnt signaling reduced neurogenesis significantly, suggesting a critical role of Wnt signaling in progenitor proliferation [113]. Apart from the convincing evidence for the role of this pathway in promoting proliferation, Wnt-signaling was also reported to be required in neuronal differentiation based on the evidence that adult NSCs were not able to transit to
immature and mature granule neurons in β-catenin cKO mice [110].
Recent studies on epileptic animal models and human data highlight the importance of the Wnt/β-catenin signaling pathway in epilepsy. Elevated expression of Wnt signaling was relevant to the increased neurogenesis and neuronal death commonly observed after various seizures [125, 136-139]. In a study by Qu and colleagues, knocking down β-catenin was found to attenuate aberrant neurogenesis induced by KA injection via Wnt signaling [138]. Unfortunately, the effect on seizures or epileptogenesis was not reported. Other studies have been conducted to investigate the role of the Wnt signaling pathway in epilepsy. After the conditional deletion of β-catenin in the dorsal telencephalon using the cre-loxP system, β-catenin knockout mice exhibited increased seizure susceptibility and an increased number of seizures compared with wildtype (WT) mice [140]. Instead, Yang and colleagues found confusing results that both deletion and overexpression of β-catenin had a notable impact on seizure susceptibility after pentylenetetrazole (PTZ) seizures [141]. However, whether the role of the Wnt signaling pathway in epilepsy specifically depended on adult neurogenesis is still unknown and requires further investigation.
4.4. BDNF
BDNF has been shown to play an active role in regulating neurogenesis. For example, Linnarsson and colleagues demonstrated that BDNF was required for the survival of the continuously regenerating populations of neurons in the adult DG and SVZ based on the discovery that the number of new cells was decreased in heterozygote BDNF knockout mice [142]. On the other hand, the use of riluzole to increase BDNF in the hippocampus or chronic BDNF infusion directly into the adult DG both increased proliferation of granule precursor cells [126, 128]. Conditional loss of TrkB in BDNF/TrkB signaling in newly-generated neurons was also reported to be required for neurogenesis in the adult DG [143]. Another study, however, indicated that survival, dendritic arborization, and functional integration of newborn neurons depended on the BDNF/TrkB signaling pathway [144].
In the chronic phase of epilepsy, BDNF decreased coinciding with the decrease of neurogenesis [127]. In addition, emerging evidence suggests that activation of BDNF and its tropomyosin receptor kinase B (TrkB) occurred in both animal models and patients with epilepsy [145, 146]. Moreover, augmentation of BDNF-TrkB signaling by direct infusion or transgenic overexpression of BDNF resulted in increased seizure susceptibility [147, 148], while conditional knockout or chemical-genetic inhibition of TrkB protected against epileptogenesis [149, 150]. However, whether there is a causal relationship between the anti-epileptogenesis effect of BDNF and its broad regulation of neurogenesis is still an unanswered question.
4.5. Neurotransmitters
Neurotransmitters are small diffusible molecules that act as the basis of chemical communication between neurons. Glutamate is the predominant excitatory neurotransmitter in the adult mammalian brain and plays important role in numerous complex processes. Both electrophysiological and immunohistochemical evidence suggests glutamate receptors are expressed on neural progenitor cells in adult neurogenic niches. In the adult DG, NMDA receptors were shown to regulate the survival of new neurons during a short, critical period soon after birth [151]. Single cell knock-out of the NMDA receptor resulted in apoptosis of migrating neuroblasts, indicating that glutamate mediated the survival and functional integration of neuroblasts via NMDA signaling [152]. In addition, AMPA receptors also take part in regulation of adult neurogenesis. Chronic administration of an AMPA receptor potentiator significantly increased cell proliferation in the hippocampus, which was the first in vivo study investigating the role of AMPA receptors in neurogenesis [153].
GABA is the key inhibitory neurotransmitter in the adult brain. Intriguingly, similar to its double-edged role in epilepsy [154, 155], it exerts a dual role on immature GCs: initially depolarizing and subsequently hyperpolarizing, depending on the level of intracellular chloride [156]. In the SVZ, nonsynaptic GABA released from neuroblasts reduced the proliferation of GFAP-expressing progenitors, which provides a negative feedback mechanism for controlling the proliferation of progenitors [132]. Furthermore, the speed of neuroblast migration in RMS was studied in acute sagittal brain slices, and the results suggested GABA reduced the speed of cell migration through GABAA receptor activation [131]. GABA was also shown to be required for synaptic integration and dendritic development of newborn neurons [156]. In addition, Ge and colleagues demonstrated that parvalbumin (PV+) interneuron released GABA acted through the gamma2 subunit containing GABAA receptors to maintain adult NSC quiescence and inhibit symmetric self-renewal and astrocyte fate choice [157]. Their subsequent study indicated that activation of PV+ interneurons promoted survival and development of newborn progenitors [158].
Although these above-mentioned neurotransmitter pathways are commonly accepted to be involved in seizure susceptibility and epileptogenesis, whether they act through modulation of adult neurogenesis remains to be further investigated. Neurotransmitters like GABA can exert diverse functions based on the level of subtypes of GABAergic neurons and microcircuits or circuits, such as feed-back circuits, feed-forward circuits, and disinhibition circuits [159]. Thus, further studies are needed to dissect the roles of neurotransmitters and their specific effects on adult neurogenesis at the microcircuit and circuit level, which may be of great benefit for anti-epileptogenic therapeutics.
4.6. Inflammatory cytokines
A great deal of work has been devoted to dissecting the role of cytokines in the direct or indirect regulation of neurogenesis. First of all, neural progenitors constitutively express receptors for pro-inflammatory cytokines [160]. Despite controversies resulting from the use of different experimental models, most in vitro experiments showed that pro-inflammatory cytokines generally suppressed proliferation both through direct and indirect mechanisms [133, 161]. For example, interleukin (IL)-6 markedly induced expression of p21 in hippocampus-derived neuronal progenitor cells and arrested proliferation of progenitors of neuronal lineage, while astroglial cells continued to proliferate [161]. Using animal models with loss of tumor necrosis factor receptor (TNF-R1 and TNF-R2), it was also revealed that both under basal conditions and after SE, cell proliferation in the DG was elevated in TNF-R1(-/-) and TNF-R1/R2(-/-) mice [162]. In addition to experiments with single cytokine exposure, administration of lipopolysaccharide (LPS), a method commonly used to stimulate the innate inflammatory response, was reported to disrupt progenitor proliferation, decrease differentiation into neurons, and reduce survival of neuroblasts [134, 135, 163]. Experiments with intraventricular LPS injections demonstrated that cytokines and activated microglia were, to a large extent, responsible for the decrease in neurogenesis [163, 164].
Inflammatory pathways have also been commonly accepted to be involved in seizure susceptibility and epileptogenesis [165-169]. Transient increases in IL-1β after prolonged febrile seizures promoted adult epileptogenesis [170, 171]. Roseti et al. (2015) found that pathophysiological concentrations of IL-1β decreased the GABA amplitude by up to 30% in specimens from patients with TLE and led to seizure generation due to neuronal hyper-excitability, suggesting an epileptogenesis role for IL-1β [172]. Besides, toll-like receptors (TLRs) were also associated with epileptogenesis. In a pilocarpine model of SE, deletion of TLR3 decreased epileptogenesis while reducing levels of cytokines IL-1β and microglial activity [173]. Antagonists or knockout of high mobility group box 1 (HMGB1)-TLR4 signaling in an animal model also decreased acute and chronic seizure recurrence [174-177]. However, whether these inflammatory pathways act through modulation of adult neurogenesis remains to be investigated.
Several signaling molecules associated with adult neurogenesis have been found to produce notable changes in epilepsy, suggesting that these signaling pathways take part in certain phases of epileptogenesis by mediating neurogenesis to a certain degree. Considering the close relationship among epilepsy, neurogenesis, and its regulation pathways, it is natural for us to take advantage of these pathways to target neurogenesis as one of the future directions of pharmacological intervention for epileptogenesis. However, small molecules targeting these pathways and epileptogenesis have yet to be clearly identified. Meanwhile, whether small molecule modulation of these pathways to mediate aberrant neurogenesis will ultimately be therapeutically beneficial for epileptogenesis still remains to be determined. Furthermore, it is important to consider the broader effect of small molecules on other functions, since the signaling pathways we mentioned above are also critical for many normal processes.
5. Perspectives
Despite a relatively comprehensive understanding of changes to adult neurogenesis and preliminary evidence for its role in epileptogenesis, there are still a number of research directions that require significant investigation. Further studies in these directions with more advanced technologies may help adult-born GCs to be better applied to future epilepsy therapy.
5.1. Direction 1: Adult Neurogenesis and its Underlying Neural Circuit Mechanisms in Different Types of Epilepsies
Epileptogenesis is known to be associated with an extensive set of brain changes that play either causal or resultant roles, including cell loss, inflammation, neurogenesis, and mossy fiber sprouting. Although neurogenesis may play important causal roles in epilepsy, its role may vary from essential in some models to irrelevant in others. The mechanisms underlying seizures among different animals may also differ. Determining which models require neurogenesis and whether and how these models are related to human epilepsy is of great importance.
Meanwhile, newborn DGCs may be heterogeneous in different types of epilepsies or even one epilepsy with different stages. Consistent with this idea, various roles for adult-born GCs were presented in different experimental paradigms [66, 67, 70, 72, 76]. Also, as was described by Cho et al. (2015), although the single round of GCV treatment before acute SE led to a reduction of SRS, no significant reduction was observed after more complete ablation of neurogenesis was achieved by two rounds of GCV [72]. One possible explanation is that populations of newborn neurons with the ability to suppress chronic seizures were ablated by two rounds of GCV treatment. If the epileptic DG contains both pathological and protective newborn DGCs, which integrate into different circuits, the net effect of early ablation techniques that act on the entire population may vary depending on the relative ratio of pathological and protective newborn DGCs. However, more suitable tools are urgently required to dissect the sole contribution of different DGCs and provide direct evidence for the existence of both pathological and protective DGCs. Advances may come from providing direct evidence for the role played by hilar ectopic DGCs and HBDs. Their morphological and electrophysiological properties generally suggest that they are pro-epileptic because they may integrate into the existing circuit with more excitatory projections received and sent compared with normotopic DGCs [178-181]. Existing contradictory studies used various approaches to ablate neurogenesis effectively. The approaches used to ablate neurogenesis may exert different effects on hilar ectopic DGCs and HBDs, which may also help to clarify the existing contradictory findings.
Furthermore, the contribution of newborn neurons to epileptogenesis may follow complex temporal dynamics. Rabies virus-mediated mapping studies revealed that DGCs born 7 days before or 3 days after SE showed significant increases in the connectivity ratio of excitatory-to-inhibitory connections, while the connectivity ratio of DGCs born 21 days before or 14 days after SE was not altered [64]. However, studies to determine the functional contributions of DGCs born at different timepoints relative to SE induction are still lacking. Newborn DGCs generated prior to, immediately after SE, or at chronic stages of epilepsy may play different roles based on different morphology and connection properties. Meanwhile, the morphological and physiological phenotypes of adult-born cells change markedly while they mature and young adult-born DGCs (~ 4-weeks-old) were reported to influence hippocampal function and behavior in a different manner compared with mature ones (>8 weeks-old) [182-185]. For example, Gu and colleagues [184] found that reversibly silencing ~4-weeks-old cells after training significantly disrupted retrieval of hippocampal memory, as compared to other ages. These functional distinctions may be attributed to the different integration patterns of mature DGCs from young adult-born DGCs. In summary, understanding the specific roles of heterogeneous adult-born DGCs and their integration patterns may be critical for optimizing the intervention timepoints and providing precise pharmacological candidates for preventing epileptogenesis.
5.2. Direction 2
Adult neurogenesis as a potential target for comorbidities of epilepsies including cognitive deficit and depression.
The patients with epilepsy are often at an increased risk for depression, anxiety, sleep disturbances, and cognitive impairment [186-189]. Recently, it has been increasingly recognized that the expression of comorbid conditions may precede seizures and that these conditions are not uniformly resolved even if seizures are fully controlled. In addition, AEDs may also cause cognitive and psychiatric disturbances. Thus, to improve the quality of life for many patients with epilepsy, a desirable “cure” must involve more than stopping or preventing seizures, but also must ameliorate the accompanying comorbidities [190, 191].
Newborn DGCs have long been considered to be critical in several hippocampus-dependent functions. The first evidence comes from correlational studies linking the levels of neurogenesis with performance in classical behavioral tasks probing the function of the hippocampal formation, such as the Morris water maze [192]. Environmental conditions and strategies that target cell death of adult-born neurons or other approaches that can increase neurogenesis were shown to enhance hippocampus-dependent learning and memory, indicating a possible functional link between neurogenesis and learning memory performance [11, 12, 193, 194]. Likewise, a number of negative factors, including stress and aging, showed an association between decreased levels of neurogenesis with reduced hippocampus-dependent memory performance [20, 192].
At present, no single AED seems to treat accompanying cognitive deficits. However, as is the case for adult neurogenesis under normal conditions, adult neurogenesis may be promising in treating epilepsy while improving epilepsy-associated cognitive decline. Consistent with this idea, existing studies have already made some progress: genetic ablation of neurogenesis prior to SE was shown to reduce chronic seizure frequency and normalize epilepsy-associated cognitive deficits [72]. Additionally, a widely prescribed drug for epilepsy, valproic acid, was found to potently suppress seizure induced neurogenesis through modulation of Wnt signaling and restored hippocampal-dependent memory function [195]. Inflammatory cytokines such as IL-1 might affect cognitive function by neurogenesis and LTP [196], while they were also shown to be associated with epilepsy both in animal models and clinical patients [170, 171, 197-199].
In addition, a substantial number of psychiatric diseases (e.g. depression and anxiety) were also found to be closely related to neurogenesis. Newborn neurons may be critically involved in the disease process of depression and represent a potential treatment target owing to the fact that a number of clinically used antidepressants, such as fluoxetine, strongly enhance neurogenesis [21, 200]. Furthermore, direct evidence for the role played by neurogenesis in depression was provided by taking advantage of transgenic and radiation methods to specifically alter adult neurogenesis [24, 201]. Hippocampal IL-1β may be a factor contributing to depression in TLE. Induction of IL-1β expression in rats with chronic TLE may ultimately lead to epilepsy-associated depressive-like behavior. Pharmacological blockade of hippocampal IL-1R exerts an antidepressant effect in the post-SE model, suggesting its potential use for treating depression and epilepsy. In addition, using chemical-genetic approaches to selectively inhibit activation of TrkB, a receptor for BDNF, can prevent the development of TLE and ameliorates comorbid anxiety-like behavior and destruction of hippocampal neurons [149].
Taken together, further studies are urgently needed to clarify the functional characteristics of neurogenesis in different comorbidities of epilepsies and their underlying mechanisms.
5.3. Direction 3: Druggable Signaling that Drives Abnormal Adult Neurogenesis and its Underlying Neural Circuit in Epileptogenesis
Although numerous signaling pathways are critically involved in various aspects of physiological neurogenesis, studies addressing whether and how components of these pathways may directly or indirectly mediate different aspects of epileptogenesis are relatively limited; a focus on seizure-induced neurogenesis, seizure susceptibility, and the development of epilepsy with respect to these pathways is urgently needed. In addition, several studies examined neurogenesis in both the acute and chronic periods following seizures, but many studies investigating protein levels did not correlate with these time periods. Therefore, it is difficult to decide what role a certain signaling pathway may have in regulating neurogenesis at different time periods following seizures. Based on the dissection of contributions of adult-born DGCs born at different timepoints and different ages, investigations for optimization of when modulation of this pathway may be most beneficial and appropriate as a therapeutic target is of great importance. In addition, when manipulating signaling pathways, it is critical to assess the effects of manipulation on other downstream functions. With recent advances in single-cell sequencing technology, the possibility exists of distinguishing aberrant and normal neurogenesis. This would provide a more explicit understanding of the heterogeneity of adult neurogenesis. In this way, we may find more specific and more efficient targets for epileptogenesis.
In addition, based on the classic view that epilepsy is caused by an imbalance of “excitatory-inhibition”, searching for methods to instruct adult-born DGCs to preferentially differentiate into GABAergic interneurons (fate change) may be of great potential in preventing epileptogenesis. Results from research taking advantage of an induced pluripotent stem cell (iPSCs) model suggest this possibility for dysregulation of GABA/Glutamate differentiation [202]. Encouragingly, some in vitro studies also show promising results by taking advantage of a cocktail of small molecules (e.g. lineage-specific transcription factors) that can change the cellular environment and achieve specific fate decisions [203-205]. The fate choice of neural progenitors in vivo can be dictated by the environment of the neurogenic niche; however, with regard to searching for appropriate molecules to control the fate of aberrant newborn DGCs during epilepsy, significant research is still required to fully understand this process. Meanwhile, whether it is possible to reduce ectopic hilar DGCs by urging DGCs to integrate into normal rather than pro-epileptic circuits may also be of great value. Future studies using new technologies like optogenetics and single-cell sequencing are expected to alter fate commitment, as well as the integration patterns of adult DGCs.
Conclusion
In this review, we outlined the connection between epilepsy and various aspects of neurogenesis (proliferation, survival, migration and integration) from the perspective of the neurogenesis playing a role in epileptogenesis. Besides, interventions of neurogenesis with pharmacological and genetic methods and their consequences were summarized. However, the complex association between neurogenesis and epileptogenesis presents a great obstacle for researchers and hinders the outcome of potential therapies. The functional implications of neurogenesis haven’t yet been fully elucidated. In addition, numerous signaling pathways that can regulate neurogenesis and are critically involved in epileptogenesis were also presented. Future investigations may revolutionize the therapies of epilepsy by preventing or controlling epilepsy, as well as ameliorating comorbidities.
Acknowledgements
Declared none.
Consent for Publication
Not applicable.
Funding
This project was supported by grants from the National Natural Science Foundation of China (81630098, 81603084) and the Young Elite Scientist Sponsorship Program by CAST (2018QNRC001).
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Devinsky O., Vezzani A., O’Brien T.J., Jette N., Scheffer I.E., de Curtis M., Perucca P. Epilepsy. Nat. Rev. Dis. Primers. 2018;4:18024. doi: 10.1038/nrdp.2018.24. [DOI] [PubMed] [Google Scholar]
- 2.Thijs R.D., Surges R., O’Brien T.J., Sander J.W. Epilepsy in adults. Lancet. 2019;393(10172):689–701. doi: 10.1016/S0140-6736(18)32596-0. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y., Chen Z. An update for epilepsy research and antiepileptic drug development: Toward precise circuit therapy. Pharmacol. Ther. 2019;201:77–93. doi: 10.1016/j.pharmthera.2019.05.010. [DOI] [PubMed] [Google Scholar]
- 4.Altman J. Are new neurons formed in the brains of adult mammals? Science. 1962;135(3509):1127–1128. doi: 10.1126/science.135.3509.1127. [DOI] [PubMed] [Google Scholar]
- 5.Eriksson P.S., Perfilieva E., Björk-Eriksson T., Alborn A.M., Nordborg C., Peterson D.A., Gage F.H. Neurogenesis in the adult human hippocampus. Nat. Med. 1998;4(11):1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- 6.Gould E., Tanapat P., McEwen B.S., Flügge G., Fuchs E. Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc. Natl. Acad. Sci. USA. 1998;95(6):3168–3171. doi: 10.1073/pnas.95.6.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gross C.G. Neurogenesis in the adult brain: death of a dogma. Nat. Rev. Neurosci. 2000;1(1):67–73. doi: 10.1038/35036235. [DOI] [PubMed] [Google Scholar]
- 8.Bergmann O., Spalding K.L., Frisén J. Adult neurogenesis in humans. Cold Spring Harb. Perspect. Biol. 2015;7(7):a018994. doi: 10.1101/cshperspect.a018994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ming G.L., Song H. Adult neurogenesis in the mammalian central nervous system. Annu. Rev. Neurosci. 2005;28:223–250. doi: 10.1146/annurev.neuro.28.051804.101459. [DOI] [PubMed] [Google Scholar]
- 10.Cameron H.A., McKay R.D. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J. Comp. Neurol. 2001;435(4):406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- 11.Chen L., Gong S., Shan L.D., Xu W.P., Zhang Y.J., Guo S.Y., Hisamitsu T., Yin Q.Z., Jiang X.H. Effects of exercise on neurogenesis in the dentate gyrus and ability of learning and memory after hippocampus lesion in adult rats. Neurosci. Bull. 2006;22(1):1–6. [PubMed] [Google Scholar]
- 12.Sahay A., Scobie K.N., Hill A.S., O’Carroll C.M., Kheirbek M.A., Burghardt N.S., Fenton A.A., Dranovsky A., Hen R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011;472(7344):466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huckleberry K.A., Shue F., Copeland T., Chitwood R.A., Yin W., Drew M.R. Dorsal and ventral hippocampal adult-born neurons contribute to context fear memory. Neuropsychopharmacology. 2018;43(12):2487–2496. doi: 10.1038/s41386-018-0109-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aimone J.B., Wiles J., Gage F.H. Potential role for adult neurogenesis in the encoding of time in new memories. Nat. Neurosci. 2006;9(6):723–727. doi: 10.1038/nn1707. [DOI] [PubMed] [Google Scholar]
- 15.Becker S., Wojtowicz J.M. 2007. [Google Scholar]
- 16.Chambers R.A., Potenza M.N., Hoffman R.E., Miranker W. Simulated apoptosis/neurogenesis regulates learning and memory capabilities of adaptive neural networks. Neuropsychopharmacology. 2004;29(4):747–758. doi: 10.1038/sj.npp.1300358. [DOI] [PubMed] [Google Scholar]
- 17.Ko H.G., Jang D.J., Son J., Kwak C., Choi J.H., Ji Y.H., Lee Y.S., Son H., Kaang B.K. Effect of ablated hippocampal neurogenesis on the formation and extinction of contextual fear memory. Mol. Brain. 2009;2:1. doi: 10.1186/1756-6606-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anacker C., Hen R. Adult hippocampal neurogenesis and cognitive flexibility - linking memory and mood. Nat. Rev. Neurosci. 2017;18(6):335–346. doi: 10.1038/nrn.2017.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deng W., Aimone J.B., Gage F.H. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat. Rev. Neurosci. 2010;11(5):339–350. doi: 10.1038/nrn2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gould E., Tanapat P. Stress and hippocampal neurogenesis. Biol. Psychiatry. 1999;46(11):1472–1479. doi: 10.1016/S0006-3223(99)00247-4. [DOI] [PubMed] [Google Scholar]
- 21.Malberg J.E., Eisch A.J., Nestler E.J., Duman R.S. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J. Neurosci. 2000;20(24):9104–9110. doi: 10.1523/JNEUROSCI.20-24-09104.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Santarelli L., Saxe M., Gross C., Surget A., Battaglia F., Dulawa S., Weisstaub N., Lee J., Duman R., Arancio O., Belzung C., Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301(5634):805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 23.Duan X., Chang J.H., Ge S., Faulkner R.L., Kim J.Y., Kitabatake Y., Liu X.B., Yang C.H., Jordan J.D., Ma D.K., Liu C.Y., Ganesan S., Cheng H.J., Ming G.L., Lu B., Song H. Disrupted-In-Schizophrenia 1 regulates integration of newly generated neurons in the adult brain. Cell. 2007;130(6):1146–1158. doi: 10.1016/j.cell.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Snyder J.S., Soumier A., Brewer M., Pickel J., Cameron H.A. Adult hippocampal neurogenesis buffers stress responses and depressive behaviour. Nature. 2011;476(7361):458–461. doi: 10.1038/nature10287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller B.R., Hen R. The current state of the neurogenic theory of depression and anxiety. Curr. Opin. Neurobiol. 2015;30:51–58. doi: 10.1016/j.conb.2014.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Winner B., Winkler J. Adult neurogenesis in neurodegenerative diseases. Cold Spring Harb. Perspect. Biol. 2015;7(4):a021287. doi: 10.1101/cshperspect.a021287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pitkänen A., Sutula T.P. Is epilepsy a progressive disorder? Prospects for new therapeutic approaches in temporal-lobe epilepsy. Lancet Neurol. 2002;1(3):173–181. doi: 10.1016/S1474-4422(02)00073-X. [DOI] [PubMed] [Google Scholar]
- 28.Parent J.M., Yu T.W., Leibowitz R.T., Geschwind D.H., Sloviter R.S., Lowenstein D.H. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J. Neurosci. 1997;17(10):3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gray W.P., Sundstrom L.E. Kainic acid increases the proliferation of granule cell progenitors in the dentate gyrus of the adult rat. Brain Res. 1998;790(1-2):52–59. doi: 10.1016/S0006-8993(98)00030-4. [DOI] [PubMed] [Google Scholar]
- 30.Jessberger S., Römer B., Babu H., Kempermann G. Seizures induce proliferation and dispersion of doublecortin-positive hippocampal progenitor cells. Exp. Neurol. 2005;196(2):342–351. doi: 10.1016/j.expneurol.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 31.Parent J.M., Janumpalli S., McNamara J.O., Lowenstein D.H. Increased dentate granule cell neurogenesis following amygdala kindling in the adult rat. Neurosci. Lett. 1998;247(1):9–12. doi: 10.1016/S0304-3940(98)00269-9. [DOI] [PubMed] [Google Scholar]
- 32.Scott B.W., Wang S., Burnham W.M., De Boni U., Wojtowicz J.M. Kindling-induced neurogenesis in the dentate gyrus of the rat. Neurosci. Lett. 1998;248(2):73–76. doi: 10.1016/S0304-3940(98)00355-3. [DOI] [PubMed] [Google Scholar]
- 33.Madsen T.M., Treschow A., Bengzon J., Bolwig T.G., Lindvall O., Tingström A. Increased neurogenesis in a model of electroconvulsive therapy. Biol. Psychiatry. 2000;47(12):1043–1049. doi: 10.1016/S0006-3223(00)00228-6. [DOI] [PubMed] [Google Scholar]
- 34.Parent J.M., Valentin V.V., Lowenstein D.H. Prolonged seizures increase proliferating neuroblasts in the adult rat subventricular zone-olfactory bulb pathway. J. Neurosci. 2002;22(8):3174–3188. doi: 10.1523/JNEUROSCI.22-08-03174.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blümcke I., Schewe J.C., Normann S., Brüstle O., Schramm J., Elger C.E., Wiestler O.D. Increase of nestin-immunoreactive neural precursor cells in the dentate gyrus of pediatric patients with early-onset temporal lobe epilepsy. Hippocampus. 2001;11(3):311–321. doi: 10.1002/hipo.1045. [DOI] [PubMed] [Google Scholar]
- 36.Thom M., Martinian L., Williams G., Stoeber K., Sisodiya S.M. Cell proliferation and granule cell dispersion in human hippocampal sclerosis. J. Neuropathol. Exp. Neurol. 2005;64(3):194–201. doi: 10.1093/jnen/64.3.194. [DOI] [PubMed] [Google Scholar]
- 37.Hattiangady B., Rao M.S., Shetty A.K. Chronic temporal lobe epilepsy is dentate neurogenesis in the adult associated with severely declined hippocampus. Neurobiol. Dis. 2004;17:473–490. doi: 10.1016/j.nbd.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 38.Heinrich C., Nitta N., Flubacher A., Müller M., Fahrner A., Kirsch M., Freiman T., Suzuki F., Depaulis A., Frotscher M., Haas C.A. Reelin deficiency and displacement of mature neurons, but not neurogenesis, underlie the formation of granule cell dispersion in the epileptic hippocampus. J. Neurosci. 2006;26(17):4701–4713. doi: 10.1523/JNEUROSCI.5516-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sierra A., Martín-Suárez S., Valcárcel-Martín R., Pascual-Brazo J., Aelvoet S.A., Abiega O., Deudero J.J., Brewster A.L., Bernales I., Anderson A.E., Baekelandt V., Maletić-Savatić M., Encinas J.M. Neuronal hyperactivity accelerates depletion of neural stem cells and impairs hippocampal neurogenesis. Cell Stem Cell. 2015;16(5):488–503. doi: 10.1016/j.stem.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kralic J.E., Ledergerber D.A., Fritschy J.M. Disruption of the neurogenic potential of the dentate gyrus in a mouse model of temporal lobe epilepsy with focal seizures. Eur. J. Neurosci. 2005;22(8):1916–1927. doi: 10.1111/j.1460-9568.2005.04386.x. [DOI] [PubMed] [Google Scholar]
- 41.Mohapel P., Ekdahl C.T., Lindvall O. Status epilepticus severity influences the long-term outcome of neurogenesis in the adult dentate gyrus. Neurobiol. Dis. 2004;15(2):196–205. doi: 10.1016/j.nbd.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 42.Ekdahl C.T., Zhu C., Bonde S., Bahr B.A., Blomgren K., Lindvall O. Death mechanisms in status epilepticus-generated neurons and effects of additional seizures on their survival. Neurobiol. Dis. 2003;14(3):513–523. doi: 10.1016/j.nbd.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 43.Uemori T., Toda K., Seki T. Seizure severity-dependent selective vulnerability of the granule cell layer and aberrant neurogenesis in the rat hippocampus. Hippocampus. 2017;27(10):1054–1068. doi: 10.1002/hipo.22752. [DOI] [PubMed] [Google Scholar]
- 44.Wasterlain C.G. Developmental brain damage after chemically induced epileptic seizures. Eur. Neurol. 1975;13(6):495–498. doi: 10.1159/000114705. [DOI] [PubMed] [Google Scholar]
- 45.Xiu-Yu S., Ruo-Peng S., Ji-Wen W. Consequences of pilocarpine-induced recurrent seizures in neonatal rats. Brain Dev. 2007;29(3):157–163. doi: 10.1016/j.braindev.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 46.Liu H., Kaur J., Dashtipour K., Kinyamu R., Ribak C.E., Friedman L.K. Suppression of hippocampal neurogenesis is associated with developmental stage, number of perinatal seizure episodes, and glucocorticosteroid level. Exp. Neurol. 2003;184(1):196–213. doi: 10.1016/S0014-4886(03)00207-3. [DOI] [PubMed] [Google Scholar]
- 47.Shi X.Y., Wang J.W., Lei G.F., Sun R.P. Morphological and behavioral consequences of recurrent seizures in neonatal rats are associated with glucocorticoid levels. Neurosci. Bull. 2007;23(2):83–91. doi: 10.1007/s12264-007-0012-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shapiro L.A., Ribak C.E., Jessberger S. Structural changes for adult-born dentate granule cells after status epilepticus. Epilepsia. 2008;49(Suppl. 5):13–18. doi: 10.1111/j.1528-1167.2008.01633.x. [DOI] [PubMed] [Google Scholar]
- 49.Kokaia M. Seizure-induced neurogenesis in the adult brain. Eur. J. Neurosci. 2011;33(6):1133–1138. doi: 10.1111/j.1460-9568.2011.07612.x. [DOI] [PubMed] [Google Scholar]
- 50.Jessberger S., Parent J.M. Epilepsy and adult neurogenesis. Cold Spring Harb. Perspect. Biol. 2015;7(12):7. doi: 10.1101/cshperspect.a020677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Overstreet-Wadiche L.S., Bromberg D.A., Bensen A.L., Westbrook G.L. Seizures accelerate functional integration of adult-generated granule cells. J. Neurosci. 2006;26(15):4095–4103. doi: 10.1523/JNEUROSCI.5508-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ribak C.E., Tran P.H., Spigelman I., Okazaki M.M., Nadler J.V. Status epilepticus-induced hilar basal dendrites on rodent granule cells contribute to recurrent excitatory circuitry. J. Comp. Neurol. 2000;428(2):240–253. doi: 10.1002/1096-9861(20001211)428:2<240:AID-CNE4>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 53.Shapiro L.A., Korn M.J., Ribak C.E. Newly generated dentate granule cells from epileptic rats exhibit elongated hilar basal dendrites that align along GFAP-immunolabeled processes. Neuroscience. 2005;136(3):823–831. doi: 10.1016/j.neuroscience.2005.03.059. [DOI] [PubMed] [Google Scholar]
- 54.Kron M.M., Zhang H., Parent J.M. The developmental stage of dentate granule cells dictates their contribution to seizure-induced plasticity. J. Neurosci. 2010;30(6):2051–2059. doi: 10.1523/JNEUROSCI.5655-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hirtz M., Fuchs H., Chi L. Influence of substrate treatment on self-organized pattern formation by langmuir-blodgett transfer. J. Phys. Chem. B. 2008;112(3):824–827. doi: 10.1021/jp0767664. [DOI] [PubMed] [Google Scholar]
- 56.Walter C., Murphy B.L., Pun R.Y., Spieles-Engemann A.L., Danzer S.C. Pilocarpine-induced seizures cause selective time-dependent changes to adult-generated hippocampal dentate granule cells. J. Neurosci. 2007;27(28):7541–7552. doi: 10.1523/JNEUROSCI.0431-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jessberger S., Zhao C., Toni N., Clemenson G.D., Jr, Li Y., Gage F.H. Seizure-associated, aberrant neurogenesis in adult rats characterized with retrovirus-mediated cell labeling. J. Neurosci. 2007;27(35):9400–9407. doi: 10.1523/JNEUROSCI.2002-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Austin J.E., Buckmaster P.S. Recurrent excitation of granule cells with basal dendrites and low interneuron density and inhibitory postsynaptic current frequency in the dentate gyrus of macaque monkeys. J. Comp. Neurol. 2004;476(3):205–218. doi: 10.1002/cne.20182. [DOI] [PubMed] [Google Scholar]
- 59.Shapiro L.A., Ribak C.E. Newly born dentate granule neurons after pilocarpine-induced epilepsy have hilar basal dendrites with immature synapses. Epilepsy Res. 2006;69(1):53–66. doi: 10.1016/j.eplepsyres.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 60.Parent J.M., Elliott R.C., Pleasure S.J., Barbaro N.M., Lowenstein D.H. Aberrant seizure-induced neurogenesis in experimental temporal lobe epilepsy. Ann. Neurol. 2006;59(1):81–91. doi: 10.1002/ana.20699. [DOI] [PubMed] [Google Scholar]
- 61.Scharfman H.E., Goodman J.H., Sollas A.L. Granule-like neurons at the hilar/CA3 border after status epilepticus and their synchrony with area CA3 pyramidal cells: functional implications of seizure-induced neurogenesis. J. Neurosci. 2000;20(16):6144–6158. doi: 10.1523/JNEUROSCI.20-16-06144.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhan R.Z., Timofeeva O., Nadler J.V. High ratio of synaptic excitation to synaptic inhibition in hilar ectopic granule cells of pilocarpine-treated rats. J. Neurophysiol. 2010;104(6):3293–3304. doi: 10.1152/jn.00663.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Du X., Zhang H., Parent J.M. Rabies tracing of birthdated dentate granule cells in rat temporal lobe epilepsy. Ann. Neurol. 2017;81(6):790–803. doi: 10.1002/ana.24946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhou Q.G., Nemes A.D., Lee D., Ro E.J., Zhang J., Nowacki A.S., Dymecki S.M., Najm I.M., Suh H. Chemogenetic silencing of hippocampal neurons suppresses epileptic neural circuits. J. Clin. Invest. 2019;129(1):310–323. doi: 10.1172/JCI95731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jakubs K., Nanobashvili A., Bonde S., Ekdahl C.T., Kokaia Z., Kokaia M., Lindvall O. Environment matters: synaptic properties of neurons born in the epileptic adult brain develop to reduce excitability. Neuron. 2006;52(6):1047–1059. doi: 10.1016/j.neuron.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 66.Raedt R., Boon P., Persson A., Alborn A.M., Boterberg T., Van Dycke A., Linder B., De Smedt T., Wadman W.J., Ben-Menachem E., Eriksson P.S. Radiation of the rat brain suppresses seizure-induced neurogenesis and transiently enhances excitability during kindling acquisition. Epilepsia. 2007;48(10):1952–1963. doi: 10.1111/j.1528-1167.2007.01146.x. [DOI] [PubMed] [Google Scholar]
- 67.Pekcec A., Lüpke M., Baumann R., Seifert H., Potschka H. Modulation of neurogenesis by targeted hippocampal irradiation fails to affect kindling progression. Hippocampus. 2011;21(8):866–876. doi: 10.1002/hipo.20802. [DOI] [PubMed] [Google Scholar]
- 68.Zhu K., Yuan B., Hu M., Feng G.F., Liu Y., Liu J.X. Reduced abnormal integration of adult-generated granule cells does not attenuate spontaneous recurrent seizures in mice. Epilepsy Res. 2017;133:58–66. doi: 10.1016/j.eplepsyres.2017.04.004. [DOI] [PubMed] [Google Scholar]
- 69.Jung K.H., Chu K., Kim M., Jeong S.W., Song Y.M., Lee S.T., Kim J.Y., Lee S.K., Roh J.K. Continuous cytosine-b-D-arabinofuranoside infusion reduces ectopic granule cells in adult rat hippocampus with attenuation of spontaneous recurrent seizures following pilocarpine-induced status epilepticus. Eur. J. Neurosci. 2004;19(12):3219–3226. doi: 10.1111/j.0953-816X.2004.03412.x. [DOI] [PubMed] [Google Scholar]
- 70.Jung K.H., Chu K., Lee S.T., Kim J., Sinn D.I., Kim J.M., Park D.K., Lee J.J., Kim S.U., Kim M., Lee S.K., Roh J.K. Cyclooxygenase-2 inhibitor, celecoxib, inhibits the altered hippocampal neurogenesis with attenuation of spontaneous recurrent seizures following pilocarpine-induced status epilepticus. Neurobiol. Dis. 2006;23(2):237–246. doi: 10.1016/j.nbd.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 71.Pun R.Y., Rolle I.J., Lasarge C.L., Hosford B.E., Rosen J.M., Uhl J.D., Schmeltzer S.N., Faulkner C., Bronson S.L., Murphy B.L., Richards D.A., Holland K.D., Danzer S.C. Excessive activation of mTOR in postnatally generated granule cells is sufficient to cause epilepsy. Neuron. 2012;75(6):1022–1034. doi: 10.1016/j.neuron.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cho K.O., Lybrand Z.R., Ito N., Brulet R., Tafacory F., Zhang L., Good L., Ure K., Kernie S.G., Birnbaum S.G., Scharfman H.E., Eisch A.J., Hsieh J. Aberrant hippocampal neurogenesis contributes to epilepsy and associated cognitive decline. Nat. Commun. 2015;6:6606. doi: 10.1038/ncomms7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hosford B.E., Liska J.P., Danzer S.C. Ablation of newly generated hippocampal granule cells has disease-modifying effects in epilepsy. J. Neurosci. 2016;36(43):11013–11023. doi: 10.1523/JNEUROSCI.1371-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Varma P., Brulet R., Zhang L., Hsieh J. Targeting seizure-induced neurogenesis in a clinically-relevant time-period leads to transient but not persistent seizure reduction. J. Neurosci. 2019;39(35):7019–7028. doi: 10.1523/JNEUROSCI.0920-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Roth B.L. DREADDs for neuroscientists. Neuron. 2016;89(4):683–694. doi: 10.1016/j.neuron.2016.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Iyengar S.S., LaFrancois J.J., Friedman D., Drew L.J., Denny C.A., Burghardt N.S., Wu M.V., Hsieh J., Hen R., Scharfman H.E. Suppression of adult neurogenesis increases the acute effects of kainic acid. Exp. Neurol. 2015;264:135–149. doi: 10.1016/j.expneurol.2014.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brulet R., Zhu J., Aktar M., Hsieh J., Cho K.O. Mice with conditional NeuroD1 knockout display reduced aberrant hippocampal neurogenesis but no change in epileptic seizures. Exp. Neurol. 2017;293:190–198. doi: 10.1016/j.expneurol.2017.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vossler D.G., Weingarten M., Gidal B.E. American epilepsy society treatments committee. Summary of antiepileptic drugs available in the United States of America: working toward a world without epilepsy. Epilepsy Curr. 2018;18(4) Suppl. 1:1–26. doi: 10.5698/1535-7597.18.4s1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bartolomei F., Gastaldi M., Massacrier A., Planells R., Nicolas S., Cau P. Changes in the mRNAs encoding subtypes I, II and III sodium channel alpha subunits following kainate-induced seizures in rat brain. J. Neurocytol. 1997;26(10):667–678. doi: 10.1023/A:1018549928277. [DOI] [PubMed] [Google Scholar]
- 80.Vreugdenhil M., Faas G.C., Wadman W.J. Sodium currents in isolated rat CA1 neurons after kindling epileptogenesis. Neuroscience. 1998;86(1):99–107. doi: 10.1016/S0306-4522(98)00041-4. [DOI] [PubMed] [Google Scholar]
- 81.Gastaldi M., Robaglia-Schlupp A., Massacrier A., Planells R., Cau P. mRNA coding for voltage-gated sodium channel beta2 subunit in rat central nervous system: cellular distribution and changes following kainate-induced seizures. Neurosci. Lett. 1998;249(1):53–56. doi: 10.1016/S0304-3940(98)00394-2. [DOI] [PubMed] [Google Scholar]
- 82.Cooper E.C. Potassium Channels (including KCNQ) and Epilepsy. Jasper's Basic Mechanisms of the Epilepsies; 2012. [PubMed] [Google Scholar]
- 83.Köhling R., Wolfart J. Potassium Channels in Epilepsy. Cold Spring Harb. Perspect. Med. 2016;6(5):6. doi: 10.1101/cshperspect.a022871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Coulter D.A., Huguenard J.R., Prince D.A. Characterization of ethosuximide reduction of low-threshold calcium current in thalamic neurons. Ann. Neurol. 1989;25(6):582–593. doi: 10.1002/ana.410250610. [DOI] [PubMed] [Google Scholar]
- 85.Wei F., Yan L.M., Su T., He N., Lin Z.J., Wang J., Shi Y.W., Yi Y.H., Liao W.P. ion channel genes and epilepsy: functional alteration, pathogenic potential, and mechanism of epilepsy. Neurosci. Bull. 2017;33(4):455–477. doi: 10.1007/s12264-017-0134-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhang S., Zhang Z., Shen Y., Zhu Y., Du K., Guo J., Ji Y., Tao J. scn9a epileptic encephalopathy mutations display a gain-of-function phenotype and distinct sensitivity to oxcarbazepine. Neurosci. Bull. 2019;36(1):11–24. doi: 10.1007/s12264-019-00413-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kanda T., Kurokawa M., Tamura S., Nakamura J., Ishii A., Kuwana Y., Serikawa T., Yamada J., Ishihara K., Sasa M. Topiramate reduces abnormally high extracellular levels of glutamate and aspartate in the hippocampus of spontaneously epileptic rats (SER). Life Sci. 1996;59(19):1607–1616. doi: 10.1016/0024-3205(96)00492-4. [DOI] [PubMed] [Google Scholar]
- 88.Shank R.P., Gardocki J.F., Streeter A.J., Maryanoff B.E. An overview of the preclinical aspects of topiramate: pharmacology, pharmacokinetics, and mechanism of action. Epilepsia. 2000;41(Suppl. 1):S3–S9. doi: 10.1111/j.1528-1157.2000.tb02163.x. [DOI] [PubMed] [Google Scholar]
- 89.Gryder D.S., Rogawski M.A. Selective antagonism of GluR5 kainate-receptor-mediated synaptic currents by topiramate in rat basolateral amygdala neurons. J. Neurosci. 2003;23(18):7069–7074. doi: 10.1523/JNEUROSCI.23-18-07069.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kim H.J., Kim I.K., Song W., Lee J., Park S. The synergic effect of regular exercise and resveratrol on kainate-induced oxidative stress and seizure activity in mice. Neurochem. Res. 2013;38(1):117–122. doi: 10.1007/s11064-012-0897-8. [DOI] [PubMed] [Google Scholar]
- 91.Hanada T., Hashizume Y., Tokuhara N., Takenaka O., Kohmura N., Ogasawara A., Hatakeyama S., Ohgoh M., Ueno M., Nishizawa Y. Perampanel: a novel, orally active, noncompetitive AMPA-receptor antagonist that reduces seizure activity in rodent models of epilepsy. Epilepsia. 2011;52(7):1331–1340. doi: 10.1111/j.1528-1167.2011.03109.x. [DOI] [PubMed] [Google Scholar]
- 92.Li Z., You Z., Li M., Pang L., Cheng J., Wang L. protective effect of resveratrol on the brain in a rat model of epilepsy. Neurosci. Bull. 2017;33(3):273–280. doi: 10.1007/s12264-017-0097-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xu X.X., Luo J.H. Mutations of N-Methyl-D-Aspartate receptor subunits in epilepsy. Neurosci. Bull. 2018;34(3):549–565. doi: 10.1007/s12264-017-0191-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xu X.X., Liu X.R., Fan C.Y., Lai J.X., Shi Y.W., Yang W., Su T., Xu J.Y., Luo J.H., Liao W.P. functional investigation of a grin2a variant associated with rolandic epilepsy. Neurosci. Bull. 2018;34(2):237–246. doi: 10.1007/s12264-017-0182-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gale K. GABA in epilepsy: the pharmacologic basis. Epilepsia. 1989;30(Suppl. 3):S1–S11. doi: 10.1111/j.1528-1157.1989.tb05825.x. [DOI] [PubMed] [Google Scholar]
- 96.Stringer J.L., Lothman E.W. Pharmacological evidence indicating a role of GABAergic systems in termination of limbic seizures. Epilepsy Res. 1990;7(3):197–204. doi: 10.1016/0920-1211(90)90015-N. [DOI] [PubMed] [Google Scholar]
- 97.De Biase D., Barra D., Bossa F., Pucci P., John R.A. Chemistry of the inactivation of 4-aminobutyrate aminotransferase by the antiepileptic drug vigabatrin. J. Biol. Chem. 1991;266(30):20056–20061. [PubMed] [Google Scholar]
- 98.Rudolph U., Crestani F., Benke D., Brünig I., Benson J.A., Fritschy J.M., Martin J.R., Bluethmann H., Möhler H. Benzodiazepine actions mediated by specific gamma-aminobutyric acid(A) receptor subtypes. Nature. 1999;401(6755):796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- 99.Cossart R., Bernard C., Ben-Ari Y. Multiple facets of GABAergic neurons and synapses: multiple fates of GABA signalling in epilepsies. Trends Neurosci. 2005;28(2):108–115. doi: 10.1016/j.tins.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 100.Chang Y.Y., Gong X.W., Gong H.Q., Liang P.J., Zhang P.M., Lu Q.C. gabaa receptor activity suppresses the transition from inter-ictal to ictal epileptiform discharges in juvenile mouse hippocampus. Neurosci. Bull. 2018;34(6):1007–1016. doi: 10.1007/s12264-018-0273-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Yu X., Yang L., Li J., Li W., Li D., Wang R., Wu K., Chen W., Zhang Y., Qiu Z., Zhou W. De Novo and inherited setd1a variants in early-onset epilepsy. Neurosci. Bull. 2019;35(6):1045–1057. doi: 10.1007/s12264-019-00400-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kwan P., Brodie M.J. Early identification of refractory epilepsy. N. Engl. J. Med. 2000;342(5):314–319. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- 103.Löscher W., Klitgaard H., Twyman R.E., Schmidt D. New avenues for anti-epileptic drug discovery and development. Nat. Rev. Drug Discov. 2013;12(10):757–776. doi: 10.1038/nrd4126. [DOI] [PubMed] [Google Scholar]
- 104.Stephen L.J., Wishart A., Brodie M.J. 2017.
- 105.Perucca P., Gilliam F.G. Adverse effects of antiepileptic drugs. Lancet Neurol. 2012;11(9):792–802. doi: 10.1016/S1474-4422(12)70153-9. [DOI] [PubMed] [Google Scholar]
- 106.Keezer M.R., Sisodiya S.M., Sander J.W. Comorbidities of epilepsy: Current concepts and future perspectives. Lancet Neurol. 2016;15(1):106–115. doi: 10.1016/S1474-4422(15)00225-2. [DOI] [PubMed] [Google Scholar]
- 107.Adachi K., Mirzadeh Z., Sakaguchi M., Yamashita T., Nikolcheva T., Gotoh Y., Peltz G., Gong L., Kawase T., Alvarez-Buylla A., Okano H., Sawamoto K. Beta-catenin signaling promotes proliferation of progenitor cells in the adult mouse subventricular zone. Stem Cells. 2007;25(11):2827–2836. doi: 10.1634/stemcells.2007-0177. [DOI] [PubMed] [Google Scholar]
- 108.Gao X., Arlotta P., Macklis J.D., Chen J. Conditional knock-out of beta-catenin in postnatal-born dentate gyrus granule neurons results in dendritic malformation. J. Neurosci. 2007;27(52):14317–14325. doi: 10.1523/JNEUROSCI.3206-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gao Z., Ure K., Ables J.L., Lagace D.C., Nave K.A., Goebbels S., Eisch A.J., Hsieh J. Neurod1 is essential for the survival and maturation of adult-born neurons. Nat. Neurosci. 2009;12(9):1090–1092. doi: 10.1038/nn.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kuwabara T., Hsieh J., Muotri A., Yeo G., Warashina M., Lie D.C., Moore L., Nakashima K., Asashima M., Gage F.H. Wnt-mediated activation of NeuroD1 and retro-elements during adult neurogenesis. Nat. Neurosci. 2009;12(9):1097–1105. doi: 10.1038/nn.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mao Y., Ge X., Frank C.L., Madison J.M., Koehler A.N., Doud M.K., Tassa C., Berry E.M., Soda T., Singh K.K., Biechele T., Petryshen T.L., Moon R.T., Haggarty S.J., Tsai L.H. Disrupted in schizophrenia 1 regulates neuronal progenitor proliferation via modulation of GSK3beta/beta-catenin signaling. Cell. 2009;136(6):1017–1031. doi: 10.1016/j.cell.2008.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Qu Q., Sun G., Li W., Yang S., Ye P., Zhao C. Orphan nuclear receptor TLX activates Wnt/beta-catenin signalling to stimulate neural stem cell proliferation and self-renewal. Nat. Cell Biol. 2010;12(1):31–40. doi: 10.1038/ncb2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lie D.C., Colamarino S.A., Song H.J., Désiré L., Mira H., Consiglio A., Lein E.S., Jessberger S., Lansford H., Dearie A.R., Gage F.H. Wnt signalling regulates adult hippocampal neurogenesis. Nature. 2005;437(7063):1370–1375. doi: 10.1038/nature04108. [DOI] [PubMed] [Google Scholar]
- 114.Hitoshi S., Alexson T., Tropepe V., Donoviel D., Elia A.J., Nye J.S., Conlon R.A., Mak T.W., Bernstein A., van der Kooy D. Notch pathway molecules are essential for the maintenance, but not the generation, of mammalian neural stem cells. Genes Dev. 2002;16(7):846–858. doi: 10.1101/gad.975202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Chojnacki A., Shimazaki T., Gregg C., Weinmaster G., Weiss S. Glycoprotein 130 signaling regulates Notch1 expression and activation in the self-renewal of mammalian forebrain neural stem cells. J. Neurosci. 2003;23(5):1730–1741. doi: 10.1523/JNEUROSCI.23-05-01730.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Breunig J.J., Silbereis J., Vaccarino F.M., Sestan N., Rakic P. Notch regulates cell fate and dendrite morphology of newborn neurons in the postnatal dentate gyrus. Proc. Natl. Acad. Sci. USA. 2007;104(51):20558–20563. doi: 10.1073/pnas.0710156104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Wang X., Mao X., Xie L., Greenberg D.A., Jin K. Involvement of Notch1 signaling in neurogenesis in the subventricular zone of normal and ischemic rat brain in vivo. J. Cereb. Blood Flow Metab. 2009;29(10):1644–1654. doi: 10.1038/jcbfm.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Imayoshi I., Sakamoto M., Yamaguchi M., Mori K., Kageyama R. Essential roles of Notch signaling in maintenance of neural stem cells in developing and adult brains. J. Neurosci. 2010;30(9):3489–3498. doi: 10.1523/JNEUROSCI.4987-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Lai K., Kaspar B.K., Gage F.H., Schaffer D.V. Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat. Neurosci. 2003;6(1):21–27. doi: 10.1038/nn983. [DOI] [PubMed] [Google Scholar]
- 120.Machold R., Hayashi S., Rutlin M., Muzumdar M.D., Nery S., Corbin J.G., Gritli-Linde A., Dellovade T., Porter J.A., Rubin L.L., Dudek H., McMahon A.P., Fishell G. Sonic hedgehog is required for progenitor cell maintenance in telencephalic stem cell niches. Neuron. 2003;39(6):937–950. doi: 10.1016/S0896-6273(03)00561-0. [DOI] [PubMed] [Google Scholar]
- 121.Han Y.G., Spassky N., Romaguera-Ros M., Garcia-Verdugo J.M., Aguilar A., Schneider-Maunoury S., Alvarez-Buylla A. Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat. Neurosci. 2008;11(3):277–284. doi: 10.1038/nn2059. [DOI] [PubMed] [Google Scholar]
- 122.Lim D.A., Tramontin A.D., Trevejo J.M., Herrera D.G., García-Verdugo J.M., Alvarez-Buylla A. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron. 2000;28(3):713–726. doi: 10.1016/S0896-6273(00)00148-3. [DOI] [PubMed] [Google Scholar]
- 123.Bonaguidi M.A., Peng C.Y., McGuire T., Falciglia G., Gobeske K.T., Czeisler C., Kessler J.A. Noggin expands neural stem cells in the adult hippocampus. J. Neurosci. 2008;28(37):9194–9204. doi: 10.1523/JNEUROSCI.3314-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pencea V., Bingaman K.D., Wiegand S.J., Luskin M.B. Infusion of brain-derived neurotrophic factor into the lateral ventricle of the adult rat leads to new neurons in the parenchyma of the striatum, septum, thalamus, and hypothalamus. J. Neurosci. 2001;21(17):6706–6717. doi: 10.1523/JNEUROSCI.21-17-06706.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Fasen K., Beck H., Elger C.E., Lie A.A. Differential regulation of cadherins and catenins during axonal reorganization in the adult rat CNS. J. Neuropathol. Exp. Neurol. 2002;61(10):903–913. doi: 10.1093/jnen/61.10.903. [DOI] [PubMed] [Google Scholar]
- 126.Katoh-Semba R., Asano T., Ueda H., Morishita R., Takeuchi I.K., Inaguma Y., Kato K. Riluzole enhances expression of brain-derived neurotrophic factor with consequent proliferation of granule precursor cells in the rat hippocampus. FASEB J. 2002;16(10):1328–1330. doi: 10.1096/fj.02-0143fje. [DOI] [PubMed] [Google Scholar]
- 127.Shetty A.K., Zaman V., Shetty G.A. Hippocampal neurotrophin levels in a kainate model of temporal lobe epilepsy: a lack of correlation between brain-derived neurotrophic factor content and progression of aberrant dentate mossy fiber sprouting. J. Neurochem. 2003;87(1):147–159. doi: 10.1046/j.1471-4159.2003.01979.x. [DOI] [PubMed] [Google Scholar]
- 128.Scharfman H., Goodman J., Macleod A., Phani S., Antonelli C., Croll S. Increased neurogenesis and the ectopic granule cells after intrahippocampal BDNF infusion in adult rats. Exp. Neurol. 2005;192(2):348–356. doi: 10.1016/j.expneurol.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 129.Jin K., Zhu Y., Sun Y., Mao X.O., Xie L., Greenberg D.A. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc. Natl. Acad. Sci. USA. 2002;99(18):11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Cameron H.A., McEwen B.S., Gould E. Regulation of adult neurogenesis by excitatory input and NMDA receptor activation in the dentate gyrus. J. Neurosci. 1995;15(6):4687–4692. doi: 10.1523/JNEUROSCI.15-06-04687.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bolteus A.J., Bordey A. GABA release and uptake regulate neuronal precursor migration in the postnatal subventricular zone. J. Neurosci. 2004;24(35):7623–7631. doi: 10.1523/JNEUROSCI.1999-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liu X., Wang Q., Haydar T.F., Bordey A. Nonsynaptic GABA signaling in postnatal subventricular zone controls proliferation of GFAP-expressing progenitors. Nat. Neurosci. 2005;8(9):1179–1187. doi: 10.1038/nn1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ben-Hur T., Ben-Menachem O., Furer V., Einstein O., Mizrachi-Kol R., Grigoriadis N. Effects of proinflammatory cytokines on the growth, fate, and motility of multipotential neural precursor cells. Mol. Cell. Neurosci. 2003;24(3):623–631. doi: 10.1016/S1044-7431(03)00218-5. [DOI] [PubMed] [Google Scholar]
- 134.Ekdahl C.T., Claasen J.H., Bonde S., Kokaia Z., Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc. Natl. Acad. Sci. USA. 2003;100(23):13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Monje M.L., Toda H., Palmer T.D. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302(5651):1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- 136.Madsen T.M., Newton S.S., Eaton M.E., Russell D.S., Duman R.S. Chronic electroconvulsive seizure up-regulates beta-catenin expression in rat hippocampus: role in adult neurogenesis. Biol. Psychiatry. 2003;54(10):1006–1014. doi: 10.1016/S0006-3223(03)00700-5. [DOI] [PubMed] [Google Scholar]
- 137.Rubio C., Rosiles-Abonce A., Trejo-Solis C., Rubio-Osornio M., Mendoza C., Custodio V., Martinez-Lazcano J.C., Gonzalez E., Paz C. Increase signaling of Wnt/β-catenin pathway and presence of apoptosis in cerebellum of kindled rats. CNS Neurol. Disord. Drug Targets. 2017;16(7):772–780. doi: 10.2174/1871527316666170117114513. [DOI] [PubMed] [Google Scholar]
- 138.Qu Z., Su F., Qi X., Sun J., Wang H., Qiao Z., Zhao H., Zhu Y. Wnt/β-catenin signalling pathway mediated aberrant hippocampal neurogenesis in kainic acid-induced epilepsy. Cell Biochem. Funct. 2017;35(7):472–476. doi: 10.1002/cbf.3306. [DOI] [PubMed] [Google Scholar]
- 139.Huang C., Fu X.H., Zhou D., Li J.M. the role of wnt/β-catenin signaling pathway in disrupted hippocampal neurogenesis of temporal lobe epilepsy: a potential therapeutic target? Neurochem. Res. 2015;40(7):1319–1332. doi: 10.1007/s11064-015-1614-1. [DOI] [PubMed] [Google Scholar]
- 140.Campos V.E., Du M., Li Y. Increased seizure susceptibility and cortical malformation in beta-catenin mutant mice. Biochem. Biophys. Res. Commun. 2004;320(2):606–614. doi: 10.1016/j.bbrc.2004.05.204. [DOI] [PubMed] [Google Scholar]
- 141.Yang J., Zhang X., Wu Y., Zhao B., Liu X., Pan Y., Liu Y., Ding Y., Qiu M., Wang Y.Z., Zhao G. Wnt/β-catenin signaling mediates the seizure-facilitating effect of postischemic reactive astrocytes after pentylenetetrazole-kindling. Glia. 2016;64(6):1083–1091. doi: 10.1002/glia.22984. [DOI] [PubMed] [Google Scholar]
- 142.Linnarsson S., Willson C.A., Ernfors P. Cell death in regenerating populations of neurons in BDNF mutant mice. Brain Res. Mol. Brain Res. 2000;75(1):61–69. doi: 10.1016/S0169-328X(99)00295-8. [DOI] [PubMed] [Google Scholar]
- 143.Li Y., Luikart B.W., Birnbaum S., Chen J., Kwon C.H., Kernie S.G., Bassel-Duby R., Parada L.F. TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron. 2008;59(3):399–412. doi: 10.1016/j.neuron.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bergami M., Rimondini R., Santi S., Blum R., Götz M., Canossa M. Deletion of TrkB in adult progenitors alters newborn neuron integration into hippocampal circuits and increases anxiety-like behavior. Proc. Natl. Acad. Sci. USA. 2008;105(40):15570–15575. doi: 10.1073/pnas.0803702105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Binder D.K., Croll S.D., Gall C.M., Scharfman H.E. BDNF and epilepsy: too much of a good thing? Trends Neurosci. 2001;24(1):47–53. doi: 10.1016/S0166-2236(00)01682-9. [DOI] [PubMed] [Google Scholar]
- 146.McNamara J.O., Scharfman H.E. Temporal Lobe Epilepsy and the BDNF Receptor, TrkB. Jasper's Basic Mechanisms of the Epilepsies; 2012. [PubMed] [Google Scholar]
- 147.Scharfman H.E., Goodman J.H., Sollas A.L., Croll S.D. Spontaneous limbic seizures after intrahippocampal infusion of brain-derived neurotrophic factor. Exp. Neurol. 2002;174(2):201–214. doi: 10.1006/exnr.2002.7869. [DOI] [PubMed] [Google Scholar]
- 148.Heinrich C., Lähteinen S., Suzuki F., Anne-Marie L., Huber S., Häussler U., Haas C., Larmet Y., Castren E., Depaulis A. Increase in BDNF-mediated TrkB signaling promotes epileptogenesis in a mouse model of mesial temporal lobe epilepsy. Neurobiol. Dis. 2011;42(1):35–47. doi: 10.1016/j.nbd.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 149.Liu G., Gu B., He X.P., Joshi R.B., Wackerle H.D., Rodriguiz R.M., Wetsel W.C., McNamara J.O. Transient inhibition of TrkB kinase after status epilepticus prevents development of temporal lobe epilepsy. Neuron. 2013;79(1):31–38. doi: 10.1016/j.neuron.2013.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.He X.P., Kotloski R., Nef S., Luikart B.W., Parada L.F., McNamara J.O. Conditional deletion of TrkB but not BDNF prevents epileptogenesis in the kindling model. Neuron. 2004;43(1):31–42. doi: 10.1016/j.neuron.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 151.Tashiro A., Sandler V.M., Toni N., Zhao C., Gage F.H. NMDA-receptor-mediated, cell-specific integration of new neurons in adult dentate gyrus. Nature. 2006;442(7105):929–933. doi: 10.1038/nature05028. [DOI] [PubMed] [Google Scholar]
- 152.Platel J.C., Dave K.A., Gordon V., Lacar B., Rubio M.E., Bordey A. NMDA receptors activated by subventricular zone astrocytic glutamate are critical for neuroblast survival prior to entering a synaptic network. Neuron. 2010;65(6):859–872. doi: 10.1016/j.neuron.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bai F., Bergeron M., Nelson D.L. Chronic AMPA receptor potentiator (LY451646) treatment increases cell proliferation in adult rat hippocampus. Neuropharmacology. 2003;44(8):1013–1021. doi: 10.1016/S0028-3908(03)00104-7. [DOI] [PubMed] [Google Scholar]
- 154.Wang Y., Wang Y., Chen Z. Double-edged GABAergic synaptic transmission in seizures: The importance of chloride plasticity. Brain Res. 2018;1701:126–136. doi: 10.1016/j.brainres.2018.09.008. [DOI] [PubMed] [Google Scholar]
- 155.Wang Y., Xu C., Xu Z., Ji C., Liang J., Wang Y., Chen B., Wu X., Gao F., Wang S., Guo Y., Li X., Luo J., Duan S., Chen Z. Depolarized GABAergic signaling in subicular microcircuits mediates generalized seizure in temporal lobe epilepsy. Neuron. 2017;95(5):1221. doi: 10.1016/j.neuron.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 156.Ge S., Goh E.L., Sailor K.A., Kitabatake Y., Ming G.L., Song H. GABA regulates synaptic integration of newly generated neurons in the adult brain. Nature. 2006;439(7076):589–593. doi: 10.1038/nature04404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Song J., Zhong C., Bonaguidi M.A., Sun G.J., Hsu D., Gu Y., Meletis K., Huang Z.J., Ge S., Enikolopov G., Deisseroth K., Luscher B., Christian K.M., Ming G.L., Song H. Neuronal circuitry mechanism regulating adult quiescent neural stem-cell fate decision. Nature. 2012;489(7414):150–154. doi: 10.1038/nature11306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Song J., Sun J., Moss J., Wen Z., Sun G.J., Hsu D., Zhong C., Davoudi H., Christian K.M., Toni N., Ming G.L., Song H. Parvalbumin interneurons mediate neuronal circuitry-neurogenesis coupling in the adult hippocampus. Nat. Neurosci. 2013;16(12):1728–1730. doi: 10.1038/nn.3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Paz J.T., Huguenard J.R. Microcircuits and their interactions in epilepsy: is the focus out of focus? Nat. Neurosci. 2015;18(3):351–359. doi: 10.1038/nn.3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Green H.F., Treacy E., Keohane A.K., Sullivan A.M., O’Keeffe G.W., Nolan Y.M. A role for interleukin-1β in determining the lineage fate of embryonic rat hippocampal neural precursor cells. Mol. Cell. Neurosci. 2012;49(3):311–321. doi: 10.1016/j.mcn.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 161.Zonis S., Ljubimov V.A., Mahgerefteh M., Pechnick R.N., Wawrowsky K., Chesnokova V. p21Cip restrains hippocampal neurogenesis and protects neuronal progenitors from apoptosis during acute systemic inflammation. Hippocampus. 2013;23(12):1383–1394. doi: 10.1002/hipo.22192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Iosif R.E., Ekdahl C.T., Ahlenius H., Pronk C.J., Bonde S., Kokaia Z., Jacobsen S.E., Lindvall O. Tumor necrosis factor receptor 1 is a negative regulator of progenitor proliferation in adult hippocampal neurogenesis. J. Neurosci. 2006;26(38):9703–9712. doi: 10.1523/JNEUROSCI.2723-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kohman R.A., Rhodes J.S. Neurogenesis, inflammation and behavior. Brain Behav. Immun. 2013;27(1):22–32. doi: 10.1016/j.bbi.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Borsini A., Zunszain P.A., Thuret S., Pariante C.M. The role of inflammatory cytokines as key modulators of neurogenesis. Trends Neurosci. 2015;38(3):145–157. doi: 10.1016/j.tins.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 165.Dey A., Kang X., Qiu J., Du Y., Jiang J. Anti-Inflammatory Small Molecules To Treat Seizures and Epilepsy: From Bench to Bedside. Trends Pharmacol. Sci. 2016;37(6):463–484. doi: 10.1016/j.tips.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Vezzani A. Anti-inflammatory drugs in epilepsy: does it impact epileptogenesis? Expert Opin. Drug Saf. 2015;14(4):583–592. doi: 10.1517/14740338.2015.1010508. [DOI] [PubMed] [Google Scholar]
- 167.Barker-Haliski M.L., Löscher W., White H.S., Galanopoulou A.S. Neuroinflammation in epileptogenesis: Insights and translational perspectives from new models of epilepsy. Epilepsia. 2017;58(Suppl. 3):39–47. doi: 10.1111/epi.13785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Rana A., Musto A.E. The role of inflammation in the development of epilepsy. J. Neuroinflammation. 2018;15(1):144. doi: 10.1186/s12974-018-1192-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Vezzani A., French J., Bartfai T., Baram T.Z. The role of inflammation in epilepsy. Nat. Rev. Neurol. 2011;7(1):31–40. doi: 10.1038/nrneurol.2010.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Feng B., Tang Y., Chen B., Xu C., Wang Y., Dai Y., Wu D., Zhu J., Wang S., Zhou Y., Shi L., Hu W., Zhang X., Chen Z. Transient increase of interleukin-1β after prolonged febrile seizures promotes adult epileptogenesis through long-lasting upregulating endocannabinoid signaling. Sci. Rep. 2016;6:21931. doi: 10.1038/srep21931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Chen B., Feng B., Tang Y., You Y., Wang Y., Hou W., Hu W., Chen Z. Blocking GluN2B subunits reverses the enhanced seizure susceptibility after prolonged febrile seizures with a wide therapeutic time-window. 2016. [DOI] [PubMed]
- 172.Roseti C., van Vliet E.A., Cifelli P., Ruffolo G., Baayen J.C., Di Castro M.A., Bertollini C., Limatola C., Aronica E., Vezzani A., Palma E. GABAA currents are decreased by IL-1β in epileptogenic tissue of patients with temporal lobe epilepsy: implications for ictogenesis. Neurobiol. Dis. 2015;82:311–320. doi: 10.1016/j.nbd.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 173.Gross A., Benninger F., Madar R., Illouz T., Griffioen K., Steiner I., Offen D., Okun E. Toll-like receptor 3 deficiency decreases epileptogenesis in a pilocarpine model of SE-induced epilepsy in mice. Epilepsia. 2017;58(4):586–596. doi: 10.1111/epi.13688. [DOI] [PubMed] [Google Scholar]
- 174.Iori V., Maroso M., Rizzi M., Iyer A.M., Vertemara R., Carli M., Agresti A., Antonelli A., Bianchi M.E., Aronica E., Ravizza T., Vezzani A. Receptor for Advanced Glycation Endproducts is upregulated in temporal lobe epilepsy and contributes to experimental seizures. Neurobiol. Dis. 2013;58:102–114. doi: 10.1016/j.nbd.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 175.Zhao J., Wang Y., Xu C., Liu K., Wang Y., Chen L., Wu X., Gao F., Guo Y., Zhu J., Wang S., Nishibori M., Chen Z. Therapeutic potential of an anti-high mobility group box-1 monoclonal antibody in epilepsy. Brain Behav. Immun. 2017;64:308–319. doi: 10.1016/j.bbi.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 176.Maroso M., Balosso S., Ravizza T., Liu J., Aronica E., Iyer A.M., Rossetti C., Molteni M., Casalgrandi M., Manfredi A.A., Bianchi M.E., Vezzani A. Toll-like receptor 4 and high-mobility group box-1 are involved in ictogenesis and can be targeted to reduce seizures. Nat. Med. 2010;16(4):413–419. doi: 10.1038/nm.2127. [DOI] [PubMed] [Google Scholar]
- 177.Zurolo E., Iyer A., Maroso M., Carbonell C., Anink J.J., Ravizza T., Fluiter K., Spliet W.G., van Rijen P.C., Vezzani A., Aronica E. Activation of Toll-like receptor, RAGE and HMGB1 signalling in malformations of cortical development. Brain. 2011;134(Pt 4):1015–1032. doi: 10.1093/brain/awr032. [DOI] [PubMed] [Google Scholar]
- 178.Cameron M.C., Zhan R.Z., Nadler J.V. Morphologic integration of hilar ectopic granule cells into dentate gyrus circuitry in the pilocarpine model of temporal lobe epilepsy. J. Comp. Neurol. 2011;519(11):2175–2192. doi: 10.1002/cne.22623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Kelly T., Beck H. Functional properties of granule cells with hilar basal dendrites in the epileptic dentate gyrus. Epilepsia. 2017;58(1):160–171. doi: 10.1111/epi.13605. [DOI] [PubMed] [Google Scholar]
- 180.Scharfman H.E., Sollas A.E., Berger R.E., Goodman J.H., Pierce J.P. Perforant path activation of ectopic granule cells that are born after pilocarpine-induced seizures. Neuroscience. 2003;121(4):1017–1029. doi: 10.1016/S0306-4522(03)00481-0. [DOI] [PubMed] [Google Scholar]
- 181.Hester M.S., Danzer S.C. Accumulation of abnormal adult-generated hippocampal granule cells predicts seizure frequency and severity. J. Neurosci. 2013;33(21):8926–8936. doi: 10.1523/JNEUROSCI.5161-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Clelland C.D., Choi M., Romberg C., Clemenson G.D., Jr, Fragniere A., Tyers P., Jessberger S., Saksida L.M., Barker R.A., Gage F.H., Bussey T.J. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325(5937):210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Aimone J.B., Deng W., Gage F.H. 2010. [Google Scholar]
- 184.Gu Y., Arruda-Carvalho M., Wang J., Janoschka S.R., Josselyn S.A., Frankland P.W., Ge S. Optical controlling reveals time-dependent roles for adult-born dentate granule cells. Nat. Neurosci. 2012;15(12):1700–1706. doi: 10.1038/nn.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Nakashiba T., Cushman J.D., Pelkey K.A., Renaudineau S., Buhl D.L., McHugh T.J., Rodriguez Barrera V., Chittajallu R., Iwamoto K.S., McBain C.J., Fanselow M.S., Tonegawa S. Young dentate granule cells mediate pattern separation, whereas old granule cells facilitate pattern completion. Cell. 2012;149(1):188–201. doi: 10.1016/j.cell.2012.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Elger C.E., Helmstaedter C., Kurthen M. Chronic epilepsy and cognition. Lancet Neurol. 2004;3(11):663–672. doi: 10.1016/S1474-4422(04)00906-8. [DOI] [PubMed] [Google Scholar]
- 187.Tellez-Zenteno J.F., Patten S.B., Jetté N., Williams J., Wiebe S. Psychiatric comorbidity in epilepsy: a population-based analysis. Epilepsia. 2007;48(12):2336–2344. doi: 10.1111/j.1528-1167.2007.01222.x. [DOI] [PubMed] [Google Scholar]
- 188.Jacobs M.P., Leblanc G.G., Brooks-Kayal A., Jensen F.E., Lowenstein D.H., Noebels J.L., Spencer D.D., Swann J.W. Curing epilepsy: progress and future directions. Epilepsy Behav. 2009;14(3):438–445. doi: 10.1016/j.yebeh.2009.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ji C., Zhu L., Chen C., Wang S., Zheng L., Li H. volumetric changes in hippocampal subregions and memory performance in mesial temporal lobe epilepsy with hippocampal sclerosis. Neurosci. Bull. 2018;34(2):389–396. doi: 10.1007/s12264-017-0186-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Meador K.J. Cognitive and memory effects of the new antiepileptic drugs. Epilepsy Res. 2006;68(1):63–67. doi: 10.1016/j.eplepsyres.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 191.Gomer B., Wagner K., Frings L., Saar J., Carius A., Härle M., Steinhoff B.J., Schulze-Bonhage A. The influence of antiepileptic drugs on cognition: a comparison of levetiracetam with topiramate. Epilepsy Behav. 2007;10(3):486–494. doi: 10.1016/j.yebeh.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 192.Drapeau E., Mayo W., Aurousseau C., Le Moal M., Piazza P.V., Abrous D.N. Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proc. Natl. Acad. Sci. USA. 2003;100(24):14385–14390. doi: 10.1073/pnas.2334169100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kempermann G., Kuhn H.G., Gage F.H. More hippocampal neurons in adult mice living in an enriched environment. Nature. 1997;386(6624):493–495. doi: 10.1038/386493a0. [DOI] [PubMed] [Google Scholar]
- 194.van Praag H., Kempermann G., Gage F.H. Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus. Nat. Neurosci. 1999;2(3):266–270. doi: 10.1038/6368. [DOI] [PubMed] [Google Scholar]
- 195.Jessberger S., Nakashima K., Clemenson G.D., Jr, Mejia E., Mathews E., Ure K., Ogawa S., Sinton C.M., Gage F.H., Hsieh J. Epigenetic modulation of seizure-induced neurogenesis and cognitive decline. J. Neurosci. 2007;27(22):5967–5975. doi: 10.1523/JNEUROSCI.0110-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.McAfoose J., Baune B.T. Evidence for a cytokine model of cognitive function. Neurosci. Biobehav. Rev. 2009;33(3):355–366. doi: 10.1016/j.neubiorev.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 197.Tao A.F., Xu Z.H., Chen B., Wang Y., Wu X.H., Zhang J., Tang Y.S., Xu C.L., Zhao H.W., Hu W.W., Shi L.Y., Zhang S.H., Chen Z. The Pro-inflammatory cytokine interleukin-1β is a key regulatory factor for the postictal suppression in mice. CNS Neurosci. Ther. 2015;21(8):642–650. doi: 10.1111/cns.12416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ravizza T., Noé F., Zardoni D., Vaghi V., Sifringer M., Vezzani A. Interleukin Converting Enzyme inhibition impairs kindling epileptogenesis in rats by blocking astrocytic IL-1beta production. Neurobiol. Dis. 2008;31(3):327–333. doi: 10.1016/j.nbd.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 199.DeSena A.D., Do T., Schulert G.S. Systemic autoinflammation with intractable epilepsy managed with interleukin-1 blockade. J. Neuroinflammation. 2018;15(1):38. doi: 10.1186/s12974-018-1063-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Sahay A., Hen R. Adult hippocampal neurogenesis in depression. Nat. Neurosci. 2007;10(9):1110–1115. doi: 10.1038/nn1969. [DOI] [PubMed] [Google Scholar]
- 201.Anacker C., Luna V.M., Stevens G.S., Millette A., Shores R., Jimenez J.C., Chen B., Hen R. Hippocampal neurogenesis confers stress resilience by inhibiting the ventral dentate gyrus. Nature. 2018;559(7712):98–102. doi: 10.1038/s41586-018-0262-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Mariani J., Coppola G., Zhang P., Abyzov A., Provini L., Tomasini L., Amenduni M., Szekely A., Palejev D., Wilson M., Gerstein M., Grigorenko E.L., Chawarska K., Pelphrey K.A., Howe J.R., Vaccarino F.M. FOXG1-Dependent dysregulation of GABA/Glutamate neuron differentiation in autism spectrum disorders. Cell. 2015;162(2):375–390. doi: 10.1016/j.cell.2015.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Sypecka J., Sarnowska A., Domanska-Janik K. Crucial role of the local micro-environment in fate decision of neonatal rat NG2 progenitors. Cell Prolif. 2009;42(5):661–671. doi: 10.1111/j.1365-2184.2009.00618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Caiazzo M., Giannelli S., Valente P., Lignani G., Carissimo A., Sessa A., Colasante G., Bartolomeo R., Massimino L., Ferroni S., Settembre C., Benfenati F., Broccoli V. Direct conversion of fibroblasts into functional astrocytes by defined transcription factors. Stem Cell Reports. 2015;4(1):25–36. doi: 10.1016/j.stemcr.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Colasante G., Lignani G., Rubio A., Medrihan L., Yekhlef L., Sessa A., Massimino L., Giannelli S.G., Sacchetti S., Caiazzo M., Leo D., Alexopoulou D., Dell’Anno M.T., Ciabatti E., Orlando M., Studer M., Dahl A., Gainetdinov R.R., Taverna S., Benfenati F., Broccoli V. Rapid conversion of fibroblasts into functional forebrain gabaergic interneurons by direct genetic reprogramming. Cell Stem Cell. 2015;17(6):719–734. doi: 10.1016/j.stem.2015.09.002. [DOI] [PubMed] [Google Scholar]