Abstract
Traumatic brain injury (TBI) is the principal cause of invalidity and death in the population under 45 years of age worldwide.
This mini-review aims to systematize the forensic approach in neuropathological studies, highlighting the proper elements to be noted during external, radiological, autoptical, and histological examinations with particular attention paid to immunohistochemistry and molecular biology.
In the light of the results of this mini-review, an accurate forensic approach can be considered mandatory in the examination of suspected TBI with medico-legal importance, in order to gather all the possible evidence to corroborate the diagnosis of a lesion that may have caused, or contributed to, death. From this point of view, only the use of an evidence-based protocol can reach a suitable diagnosis, especially in those cases in which there are other neuropathological conditions (ischemia, neurodegeneration, neuro-inflammation, dementia) that may have played a role in death.
This is even more relevant when corpses, in an advanced state of decomposition, are studied, where the radiological, macroscopic and histological analyses fail to give meaningful answers. In these cases, immune-histochemical and molecular biology diagnostics are of fundamental importance and a forensic neuropathologist has to know them. Particularly, MiRNAs are promising biomarkers for TBI both for brain damage identification and for medico-legal aspects, even if further investigations are required to validate the first experimental studies. In the same way, the genetic substrate should be examined during any forensic examination, considering its importance in the outcome of TBI.
Keywords: Traumatic brain injury, neuropathology, forensic radiology, autoptic approach, histopathology, miRNA, molecular biology
1. INTRODUCTION
Traumatic brain injury (TBI) is the principal cause of invalidity and death in the population under 45 years of age worldwide [1]. The Brain Injury Association of America (2011) defines TBI as “an alteration in brain function, or other evidence of brain pathology, caused by an external force”. In 2014, there were 56,800 TBI-related deaths in the US, including 2,529 who were children. Intentional self-harm, unintentional falls, and motor vehicle crashes were reported to be the most common mechanisms of TBI-related death injuries. These three principal mechanisms of injury accounted for 32.5%, 28.1%, 18.7%, of the reported TBI-related deaths, respectively. Rates of TBI-related deaths per 100,000 population were highest among adults over the age of 75 (78.5), those aged from 65 to 74 (24.7), and individuals aged 55 to 64 (19.1) [2]. An aggregate hospitalized plus fatal TBI incidence rate of about 235 per 100,000 was derived from a European retrospective study of twenty-three European national reports. An average mortality rate of about 15 per 100,000 and case fatality rate of about 11 per 100 were derived [3].
Open head injuries account for 30% of TBIs, which occur when an object penetrates the skull and induces damage to brain tissue, resulting in neurological impairment. The remaining 70% of TBIs are closed head injuries following a rapid acceleration and/or deceleration of the head, or a blow to the head or impact of the head against an object [4]. Motor vehicle accidents, falls, assaults, bicycle accidents, and sports injuries are the most common causes. The highest incidence of TBIs is related to young children, older adolescents and the elderly, while men are 1.5 to 3 times more likely to sustain a TBI than women [5].
Head injuries can be classified into two broad categories, according to the mechanism by which the injury is produced: impact injuries and acceleration/deceleration injuries. Impact injuries are described as the result of the impact of an object on the head, and they are related to the local effects of contact between the head and the object. Consistently, these injuries include soft tissue injuries (lacerations, abrasions, and contusions of the scalp), fracture of the skull, contusions of the brain, epidural hematomas, and intracerebral hemorrhages [6, 7].
Acceleration/deceleration injuries are the result of an abrupt movement of the head after the moment of the injury, leading to the variation of intracranial pressure gradients and to the brain experiencing both shearing and tensile forces. The two types of injuries typically produced are subdural hematomas (following the tearing of the subdural bridging veins) and diffuse axonal injuries (the consequence of axonal injury) [8-10].
From a neuro-pathological point of view, the development of TBI has been divided into two main stages as regards brain damage after a head injury: primary damage occurring at the moment of the lesion, such as lacerations of the scalp, fractures of the skull, surface contusions and lacerations of the brain, the diffusion of the axonal injury and intracranial hemorrhage; and secondary damage caused by complicated processes triggered at the moment of the injury but clinically not evident, such as brain damage due to raised intracranial pressure, ischemia, swelling, and infection [11, 12]. In the past few years, specific techniques of neuroradiology have contributed to the classification of brain injury after a head injury [13]. These imaging techniques [14] provide functional correlations of the structural damage, subsequently confirmed by autopsy. As a result, the classification of focal damage - which includes laceration of the scalp, fracture of the skull, surface contusions and lacerations, intracranial hematoma, and raised intracranial pressure, and diffuse damage which includes ischaemic brain damage, diffuse axonal injury, and diffuse brain swelling - is now largely recognized.
Considering the aforementioned reasons, the aim of this review is to establish a single systematic evidence-based post mortem protocol for a better objectification of TBI damage.
2. FORENSIC IMAGING
As the study of traumas in clinical settings cannot be made without the use of radiology, forensic pathology can benefit from the use of imaging techniques to plan the subsequent autoptical approach in cases of cranium-encephalic injuries [15]. Among the different imaging techniques, post-mortem computed tomography (PM-CT) certainly plays the main role, guaranteeing the highlighting of typical lesions. First of all, this technique can detect the presence of fractures involving neurocranium, and viscerocranium, up to the cervical vertebrae. Furthermore, the presence of cranial open-fractures can be associated with another characteristic, seen with the same method, that is the presence of air, such as: gas embolism in the cerebral and pulmonary circulation (both venous and arterial), or pneumoencephalon and pneumorachis. The interpretation of this finding, however, deserves two considerations: on the one hand, the presence of bubbles is difficult to detect during autopsy, thus it is possible to plan particular autoptical approaches, on the other hand, it enters into the differential diagnosis with putrefactive gases [16, 17]. Furthermore, PM-CT is useful in detecting intracranial hematomas with epidural, subdural, and subarachnoid localization or intraventricular hemorrhages; as well as intraocular hemorrhages associated with fractures of the base of the orbit or skull or in cases of shaken baby syndrome (SBS) [18]. In addition, in the study of bleeding, compared to PM-CT, post-mortem magnetic resonance imaging (PM-MRI) shows a high sensitivity to highlight subarachnoid hemorrhages and subgaleal hematomas, as well as detecting lesions in cases of trauma with typical blow and kickback dynamics [19]. On the other hand, the absence of the use of ionizing radiation in MRI would legitimize its use on living people. In fact, in cases of SBS, MRI can demonstrate the presence of intracranial bleedings in different stages of evolution, providing information on the time of production, although a precise dating is unlikely. Furthermore, clinical forensic medicine can benefit from the use of MRI in attempted homicide in cases of strangulation, allowing the visualization of bleeding or edema of the soft and muscular tissues of the neck, as well as the suffering of the lymph nodes of this anatomical district. Moreover, always in a clinical field, the possibility of this diagnostic technique for the evaluation of bone age is discussed through the study of cartilage and their modifications with growth, always without the use of radiation [20].
Aghayev et al., also demonstrated the ability of PM-MRI to identify herniation of cerebellar tonsils through the foramen magnum as a sign of elevated intracranial cerebral pressure [21]. Finally, PM-MRI can count on diffusion tensor imaging (DTI) to assess traumatic brain damage: tractography can highlight dislocation and rupture of the fibers in cases of post-traumatic cerebral hemorrhage, or the interruption of the fibers following the passage of a bullet [22, 23]. However, for the detection of suspected foreign bodies, the gold standard is PM-CT, which allows them to be identified in terms of number, shape, size, integrity, and localization. This technique also allows the identification of a penetrating tract, both from a firearm or a sharp weapon, usually conical with the base of the entrance wound [24]. The limitation of this technique is, obviously, the fact that the presence of metallic elements leaves artifacts that cover further injuries, for example hemorrhages.
Post-traumatic subarachnoid hemorrhage can be showed by post-mortem computed tomography angiography (PM-CTA), with evidence of the origin of the vascular lesion, frequently breaking the vertebrobasilar artery. Furthermore, PM-CTA can highlight the presence of post-traumatic aneurisms of the intracranial vessels, which are difficult to detect on macroscopic autopsy or on the investigation after formalin fixation of the brain, according to the site [25]. Therefore, this technique is particularly effective in traffic accidents [26].
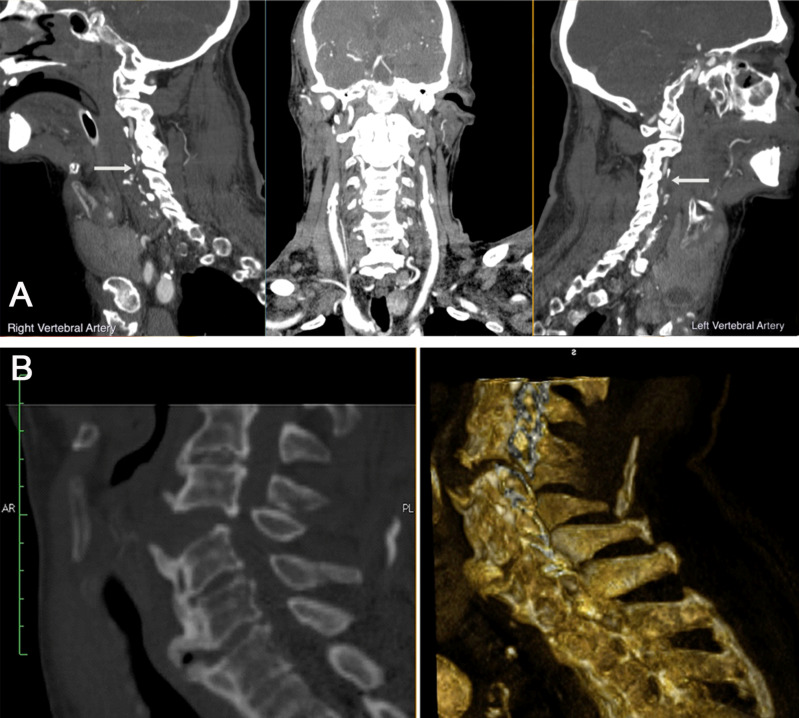
As far as this kind of death is concerned, a case is reported dealing with a 79-year-old man, involved in a frontal-impact vehicle crash. He was taken to the Emergency Department by ambulance where he arrived comatose (GCS 3). The patient immediately underwent brain CT scan and angio-CT scan, directed, in particular, to study epiaortic vessels. The results were extensive hypodensity in the subcortical areas of the left frontal lobe and extensive hypodensity of the cortico-subcortical regions of the parietal, occipital and cerebellar lobes of probable ischemic origin, bilaterally. The angio-CT study documented a complete occlusion of both vertebral arteries from their origin, where they appear threadlike, up to the C3 vertebral body. From this level, they appeared reperfused, even if the right one seemed to be reduced in size and less opacified than the left one (Fig. 1A). The man died about 4 days after his admittance to the hospital. The external examination of the body was not remarkable for any signs of trauma. However, a PM-CT conducted the day after detected a large hypodense area in the parietal and frontal lobe, bilaterally, of probable infarct origin; parietal calcifications of the carotid axes at the level of bifurcation and of the siphons; parietal calcifications of the right vertebral artery. Dislocation of C5 from C4 with large dehiscence of the disc space and associated thickening of paravertebral tissues (Fig. 1B).
Fig. (1).
Radiological approach. A. Angio CT-Premortem: extensive hypodensity in the subcortical areas of the left frontal lobe. Extensive hypodensity affects the cortico-subcortical regions of the parietal, occipital and cerebellar lobes, of probable ischemic meaning, bilaterally. The angio-CT study documented a complete occlusion of both vertebral arteries from their origin, where they appear threadlike, up to the C3 vertebral body. From this level, they appeared reperfused, even if the right one seems to be reduced in size and less opacified than the left one. No modifications were detected on the basilar trunk. B. CT-Postmortem: a large hypodense area in the parietal and frontal lobes, bilaterally, of probable infarct origin; parietal calcifications of the carotid axes at the level of bifurcation and of the siphons; parietal calcifications of the right vertebral artery. Dislocation of C5 from C4 with large dehiscence of the disc space and associated thickening of paravertebral tissues. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
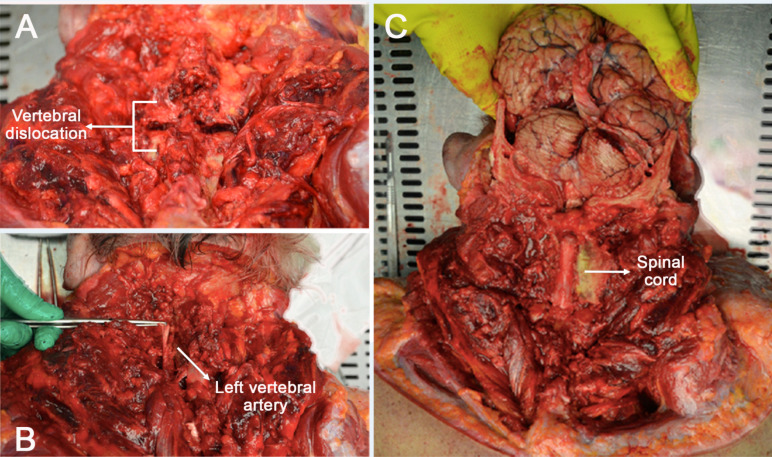
Thus, a posterior approach during autoptical examination was preferred. The splenic muscles of the head were uncovered, the semi-spinal muscle was exposed and appeared hemorrhagic, bilaterally. The nuchal ligament was removed, the spinous process of the C4-vertebra was detected and this vertebra was dislocated from the C5-vertebra with posterior exposure of the spinal cord and hemorrhagic dural sac (Fig. 2A). Through the use of a rongeur, the transverse processes were sectioned to visualize the course of the vertebral arteries into the transverse foramen; which presented a regular course (Fig. 2B). Thereafter, the brain was removed with the spinal cord up to the upper border of the C5-vertebra (Fig. 2C).
Fig. (2).
Posterior approach during autopsy. A. C4-vertebra dislocated from the C5-vertebra with posterior exposure of the spinal cord and hemorrhagic dural sac. B. After the transverse processes had been sectioned, the course of the vertebral arteries into the transverse foramen was visualized. C. The anatomical preparation composed of the brain and the spinal cord up to the upper border of the C5-vertebra. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
The right vertebral artery showed a lumen reduction. Microscopic examination with Hematoxylin and Eosin (H&E) showed extended intraparenchymal erythrocyte collections that substituted large tracts of nervous tissue; eccentric atherosclerotic plaque, causing a lumen reduction of about 35-40%. Above all, there was an interruption of the dura mater and the arachnoid layer at the C4-C5 spinal specimen with contextual erythrocyte presence below and in the context of the sheets themselves. The cause of death was then attributed to a progressive multi-organ failure resulting from trauma that occurred after the road accident, determined by: vertebro-basilar insufficiency, causing a massive cerebral ischemia with a state of coma; respiratory failure with the need for mechanical ventilation; acute renal failure; pre-existing comorbidities
This case shows the importance of a post-mortem radiological approach to plan subsequent autopsy.
3. AUTOPSY
Autopsy has the aim of identifying both primary and secondary brain damage. The scalp incision takes place with the well-known bimastoid resection [27]. If necessary, in order to visualize the orbital cavities, for example in Shaken Baby Syndrome (SBS), the sectioning of the skin and subcutaneous tissues of the splanchnocranium can be performed, according to the Rutty technique [28]. SBS combines subdural hemorrhage, acute encephalopathy, retinal hemorrhage, optic nerve sheath hemorrhage, and sparse or absent signs of external injury [29]. Searching for these signs, the removal of the eyeballs can be useful for subsequent histological studies. Moreover, considering that asymmetry between the eyes of an individual can occur, both eyes should be collected and studied [30]. After the overturning of the scalp strips, the examination and the description of the scalp are carried out before dissection. In cases of hemorrhagic infiltration or gunshot wounds, the fragments of bone and skin are removed for microscopic examination. In traumatic skull injuries, the evaluation and description of various fracture types are performed before skull removal [31-33].
Once the skull is removed, the brain has to be observed and described. At the macroscopic level, the visualization of the brain can evidence lacerations, contusions and hematomas [34, 35].
Subdural hematomas can be classified as acute (symptoms occurring within 72 h), subacute (3 days to 3 weeks) or chronic (more than 3 weeks after the injury). This acceleration/deceleration injury is the result of a shearing force acting upon the parasagittal bridging veins [10] and can be located either on the ipsilateral or contralateral side of the impact area or bilaterally, but is not associated with skull fractures (even though some studies suggest a dural origin for the subdural bleeding seen in young infants) [35].
Hemorrhages are streak-like and can be both solitary and multiple, while the amount of bleeding that continues until death depends on the type of vessel injured and on the presence of necrosis. In cases of profuse bleeding, this area may expand into the white matter and the subarachnoid space (intracerebral hemorrhage) [36, 37].
Subarachnoid hemorrhage is the most common consequence of traumatic head injury. Lacerations of the internal carotid, vertebral or basilar arteries have been proved to cause traumatic subarachnoid hemorrhage over the base of the brain, therefore being immediately fatal [38, 39].
Subarachnoid hemorrhage can be produced postmortem due to the lysis of blood cells, loss of vascular integrity with consequent blood leakage in the subarachnoid space. Furthermore, during the evisceration of the brain, minimal subarachnoid hemorrhage may be produced. While removing the skullcap, cerebral veins and the arachnoid membrane are torn, with subsequent diffusion of blood into the subarachnoid space in the posterior aspect (dependent portion) of the cerebral hemispheres and cerebellum. Despite the fact that this hemorrhage is usually minor, if the brain is not removed from the cranial cavity immediately but rather left to sit for a while, a considerable quantity of subarachnoid hemorrhage may accumulate [10].
Brain swelling can occur following significant head injury [39, 40], due to the development of a severe state of brain swelling for a certain time, herniation of the brain or secondary brain stem hemorrhage. A rapid progression of this process can result in tonsillar and/or transtentorial herniation of the brain, with consequent necrosis, secondary infarction, and Duret hemorrhages [41]. Violent hyperextension of the head and neck can cause lacerations at the junction of the pons and medulla [42-46].
After the evisceration of the brain, which in this case must be in toto, it is formalin-fixed for further studies, and it is possible to observe the basilar skull fractures, which are very common because of the construction and irregular shape of the base of the skull: hinge fractures (consisting in basilar fractures that completely bisect the base of the skull), ring fractures (circular fractures of the base of the skull that surrounds the foramen magnum, may be due to impacts on the top of the head that drive the skull downward onto the vertebral column and impacts the tip of the chin), contrecoup fractures of the anterior cranial fossae (isolated fractures of the anterior cranial fossae associated with contrecoup injuries of the brain, with the impact point on the opposite side of the skull) [47-49].
Severe injury to the vertebral arteries is caused by blunt traumas to the neck. The upper third of the cervical region is the area where the vertebral artery is most susceptible to traumas of two types: a traumatically induced dissection in the vessel wall with rupture into the subarachnoid space at the base of the brain; a similar type of dissection characterized, however, by the presence of thrombosis of the lumen with infarction of brain tissue, instead of the rupture of the vessel wall. Injury of the vertebral artery should be suspected when an individual collapses and dies almost immediately after receiving a blow to the neck. The most common causes of vertebral artery trauma are blows to the neck, motor vehicle accidents, falls, and cervical spine manipulation [50, 51].
The autoptic method in cases of access to the dorsal spine is a posterior approach consisting in a semicircular bisacromial incision or a median perpendicular/sagittal incision (Fig. 2), for the inspection and isolation of the posterior neck muscles, paravertebral muscles, ligaments, vertebrae (spinal and transverse processes as well as vertebral bodies), and vertebral arteries. This approach gives easy access to the cervical trunk, consenting the immediate visualization of the cranial–cervical joint, and, of course, allows for complete resection and isolation of the cord. This method is preferable in cases when death happens as a result of surgery [26]. In cases where visualization of the vertebral arteries is necessary, as in the case already mentioned in the radiology section, the posterior approach is certainly the gold standard technique; furthermore, in that specific case, to limit the possibility of damaging the vessels, a Kerrison rongeur was used to access the transverse holes of the cervical vertebrae and visualize the arteries in situ.
After evisceration it is possible to proceed with the observation of the brain and its dissection with Virchow or Ludwig procedure [27]: the occurrence of macroscopic damage will be observed, e.g. intra-parenchymal bleedings and contusions [38, 52].
There are six types of contusions: coup contusions, which occur in the site of impact, inflicting tensile force injuries to the brain; contrecoup contusions, which occur in the brain at locations directly opposite to the point of impact; fracture contusions, associated with fractures of the skull; intermediary coup contusions, which consist in hemorrhagic contusions in the deep structures of the brain (the white matter, basal ganglia, and corpus callosum, typically observed in falls); gliding contusions, focal hemorrhages located in the cortex and underlying white matter of the dorsal surfaces of the cerebral hemispheres, principally in the frontal region (observed in falls and vehicle accidents); Herniation contusions, typically caused by impaction of the medial portion of the temporal lobes against the edge of the tentorium, or the cerebellar tonsils against the foramen magnum [36, 42].
4. TOXICOLOGY
TBI is frequently associated with substance abuse, in a two-way relationship [53]: on the one hand, the use of substances represents a risk factor in the genesis of TBI, also influencing outcomes; on the other hand, subjects with TBI are at greater risk of developing a substance abuse disorder. Among the different substances abused, alcohol is certainly the most studied and investigated in cases of TBI [54] followed by drugs, including marijuana and cocaine [55]. Moreover, in the last few decades, the use of anabolic androgenic steroids (AAS) has been constantly increasing in the general population, not only in athletes, particularly for aesthetic purposes. In this regard, a recent review described the correlation between AAS use/abuse and anxiety or aggression, analyzing the two pathways that could be involved in AAS-induced behavioral disorders [56]. On this theme, as suggested in the paragraph on Molecular Biology, the use of new molecular biomarkers, such as miRNAs, could become very important for forensic purposes. For example, in a pilot study, in drug abuser tissue, the expression levels of miR-132 and miR-34 were higher than control groups, suggesting a specific pathway in consumption-induced neurodegeneration [57].
Furthermore, a study also linked the pattern of injury and the severity of the lesions according to the different concentrations of alcohol in the blood by detecting how concentrations higher than 2.5 g/l were statistically related to head injuries and more serious injuries [58]. Recent studies, however, present contrasting results between the severity of TBI and substance use, noting how high blood alcohol [59] or methamphetamine [60] values can constitute protective factors with respect to mortality in head traumas. Although further studies are needed in order to better understand these phenomena, it clearly emerges as a complete understanding of the toxicological examination.
However, when questioning whether a substance has caused or simply contributed to death, the reflection cannot focus only on illegal substances. Even drugs legally prescribed for therapeutic purposes can influence the evolution or extent of head trauma. The chronic use of anticoagulants at the time of the traumatic event may cause, indeed, a hemorrhage greater than in free-from-drug individuals or may be associated with a rare but sufficiently dangerous complication, such as delayed traumatic subarachnoid hemorrhages [61]. In addition, the activity of warfarin can be influenced by the genetic component, which should be explored in cases of autopsies. The most important pharmacogenetic association has been identified in the polymorphisms in the gene encoding the epoxide reductase of vitamin K and in the cytochrome P450 CYP2C9 gene [62]. In these cases, the finding of a high dose of warfarin in pre-mortem blood samples could be therapeutic and not a sign of overdose or over-prescription, excluding any medical liability.
On the other hand, it has been shown that the same head trauma alters the pharmacokinetics of some substances, thus a higher dose is necessary to reach therapeutic plasma concentrations, such as paracetamol [63], cyclosporine A [64] and phenytoin [65].
However, as with all substances, post-mortem concentrations cannot be easily interpreted to ascertain the ante-mortem concentrations. Therefore, further studies should be performed to define a direct relationship between the ante- and post-mortem concentrations of these substances.
5. HISTOLOGICAL AND IMMUNOHISTOCHEMICAL STUDIES
Histological studies are usually performed with H&E staining. At the microscopic level, the organization of a subdural hematoma can be divided into different stages, allowing the determination of its timing and evolution, according to Lindenberg [36], and McCormick [66] (easier to appreciate with the use of Masson’s trichrome staining [37]): i) after 24 h: intact erythrocytes; fibrin among the dura mater and the hemorrhage; ii) from 24 to 48 h: fibrin deposition; neutrophils in the hemorrhage; proliferation of fibroblasts among dura mater and the hemorrhage; iii) from 48 to 72 h: endothelial proliferation; iv) from 3 to 5 days: appearance of macrophages; early erythrocyte breakdown; increasing of neomembrane; v) from 5 to 10 days: revascularization of the hemorrhage; lack of erythrocytes; increasing of neomembrane; vi) up to 14 days: hemosiderin macrophages (being hemosiderin deposits highlight with Perls’s staining); neomembrane thickness up to twice that of the dura mater; dilated capillaries; vii) up to 21 days: hemorrhage is absorbed; increased vascular proliferation; neomembrane constituted by fibrovascular tissue; viii) up to 1 month: the neomembrane thickness is similar to the thickness of the dura madre; collagen deposits; formation of new arteries; ix) up to 6 months: rare hemosiderin macrophages; fusion between neomembrane and the dura mater; x) up to 1 year: neomembrane is undistinguishable from the dura mater; hemosiderin macrophage presence.
Subarachnoid hemorrhages yield the same microscopic picture as described for subdural hematomas. However, the demonstration of antibodies against Beta-Amyloid Precursor Protein (β-APP) can be used [66].
Diffuse axonal injury (DAI), despite being undetected, is included among the results of traumatic changes in the CNS [67-76]. Sudden cerebral swelling and death due to craniocerebral trauma have also been observed in children and young adults. Although the etiology remains unknown, vasodilation and initial hyperemia with redistribution of blood are included in the basic pathophysiology [77]. In cases with a short survival time, it is difficult to detect DAI using conventional techniques, such as hematoxylin-eosin staining, which can identify the axon injury after about 24 h, but an immunohistochemical technique that uses β-APP allows identification of damaged axons a 2–3 h after injury [78-81]. In cases of TBIs the use of routine histology as well as immunohistochemical techniques during the earliest appearance and observation period are useful to investigate brain tissue modification [82-85].
The most commonly accepted histological and immunohistochemical parameters for approximate age determination of brain tissue lesions, according to DiMaio et al. [10] and Dettmeyer et al. [36] are: immediately: edematous swelling (up to 6 days) [86], neuronal degeneration, shrinkage, neuronal vacuolization; after 45-125 min: apoptosis [87, 88]; after 2 h: neutrophils, red neurons [41], neuron-specific enolase (NSE) in the peri-contusion zone [89]; after 3-4 h: apolipoprotein E (ipsilateral hemisphere) [90], Glial fibrillary acidic protein (GFAP) [91]; after 10 h: axonal swelling (up to 20 h) [92], erythrophages (from 4 days) [93]; after 24 h: nuclear swelling [92], vascular proliferation [94], leukocyte common antigen, lipophages (between 24-72 h) [93], CD68+ macrophages [95-96], erythrocytes (up to 5 days) [41], CD15 neutrophils [96]; after 2 days: CD3+ T lymphocytes, [84], siderophages [93]; after 6-7 days: hematoidin [97], tenascin [98].
For a correct formulation of the cause of death, the morphological demonstration of hypoxic brain injury is of considerable interest in forensic pathology [36, 95]. The histological changes are typically related to chromatin agglutination in nerve cells (after a few minutes), the loosening of Nissl bodies (after approximately 20 min) and their decay (after approximately 2 h), the homogenization of karyoplasm with shrinkage of nucleus and cytoplasmic eosinophilia (after approximately 7 h, visible after 12-18 h), swelling of endothelial cells, pericytes, and astrocytic appendages (visible after 12 h), axonal swelling at the margins (after approximately 24 h). First occurrence of macrophages at the border of the source (after 30 h) and their increase, along with the visible presence of micro-hemorrhages, siderophages (after approximately 48 h), the presence of lipophages (Sudan III staining, after approximately 48 h) [36], increasing of ionized calcium-binding adapter molecule-1 (IBA-1) (after approximately 3 days and progressively increasing in the next 15 to 20 days), aquaporin-4 (AQP4) (after 7 to 30 days), and Hypoxia-Inducible Factor 1-alpha (HIF-1α), capillary sprouts beginning at the periphery (2–3 weeks) [95]. Another aspect to be investigated, concerning immunohistochemical studies for the diagnosis of TBI, is oxidative stress. In fact, according to the hypothesis of Schiavone et al., TBI would cause an increase in NOX2 expression in PV-positive GABAergic interneurons resulting in increased ROS production and neuronal death [99]. Although there are no randomized studies that identify these parameters as TBI markers, an increased NOX2 expression combined with the increase of 8-hydroxy-2′-deoxyguanosine (8OHdG) could be positively related to the immunoreactivity showed in similar cases. Immunoreactivity in GABAergic neurons and, in particular, in PV-positive interneurons associated with a depletion of the same neurons at the cerebral cortical level, could be sought. Further studies are mandatory for the general acceptance of these findings.
6. MOLECULAR BIOLOGY
The role of genetics concerning traumatic brain injury can be linked to two important aspects: genetic predisposition, which is able to determine the outcome of the patients, and identification of new molecular biomarkers, both for the identification of the damaged anatomic region and for the forensic examiner during autopsy, with the aim of identifying the exact cause of the death.
6.1. Genetic Substrate
In the last few decades, the identification of a common genetic substrate underlying the outcome of TBI has been considered an important research field by the scientific community. The nonspecific characteristics of TBI symptomatology, compounded by the variable presentation of TBI, have made the genetic approach challenging. By collecting family history, it has been possible to identify that genetic patterns may play a pivotal role in a patient's biological response to TBI. To date, it is well known that genetic factors, through different pathological pathways, can be considered determinant both for short-term survival and long-term neurological and functional outcomes after TBI.
Several genetic variants are strictly related to short-term survival, influencing inflammation, the severity of axonal injury, and blood-brain disruption. In a similar manner, genetic factors may influence long-term outcome, regulating plasticity and neuronal regeneration. One of the genes that has been most investigated and that can be very important for short-term and long-term prognosis after TBI is the Apolipoprotein E (APOE) gene. This gene encodes the main apolipoprotein in the central nervous system and is involved in cholesterol and lipid metabolism. Based on both in vitro and in vivo studies, during regeneration processes after a brain injury (traumatic and non-traumatic), apolipoprotein plays an important role in the recycling of plasma lipoproteins to build new neuronal cell membranes, neurites, and synapses [100, 101]. APOE has been characterized by three alleles (APOE ε2, ε3 and ε4): the ε4 allele is strictly linked to Alzheimer’s disease, as demonstrated in studies on twins [102], even if it can also influence the prognosis of other brain disorders, such as brain hemorrhage, although the mechanisms underlying these associations are unclear [103]. Moreover, this allele can be related to the severity of axonal injury, with a poor prognosis in severe TBI patients [104]. Several studies have described an association between TBI and several polymorphisms in genes such as BDNF (Brain-derived neurotrophic factor), which acts on certain neurons of the central nervous system and the peripheral nervous system, helping to support the survival of existing neurons, and encouraging the growth and differentiation of new neurons and synapses [105]. Moreover, the family of interleukin (IL) genes can be considered very important, regulating the inflammatory response [106]. Several papers have described an important role for cerebrospinal fluid (CSF) that enters the brain via the periarterial space and interchanges with interstitial fluid (ISF). Ilif et al. demonstrated an animal model that this exchange system can be called the glymphatic system because it shares many similarities with the lymphatic system in peripheral tissue, and the presence of glial aquaporin-4 (AQP4) water channels facilitates its activity [107].
Other important proteins that play an important role in TBI is the Tau protein that stabilizes microtubules. Indeed, the main function of the Tau protein is to modulate the stability of axonal microtubules.
The genetic substrate is very important for Tau protein expression: in fact, the microtubule-associated protein tau (MAPT) gene is located on chromosome 17q21, containing 16 exons [108]. The particular characteristics of this gene are linked with the transcript: Exons 2, 3 and 10 are alternatively spliced and lead to the formation of six tau isoforms with a range of 352-441 amino acids [109]. Even if TBI can be divided into acute brain injury (comprising mild TBI, including its short-term sequelae) and catastrophic brain injury (it may lead to death), the genetic substrate of Tau protein is very important in so-called chronic brain injury. This kind of event is frequently underestimated, it is linked with several different sport activities characterized by repeated head trauma such as professional boxers, American football players, hockey players, and professional kickboxing. The cellular and molecular changes linked to the different tau isoforms (for example tau phosphorylation) are strictly connected with the severity of the trauma, improving or reducing neuronal functions [110].
The AQP4 gene encodes a protein that is the predominant aquaporin found in the brain, playing an important role in brain-water homeostasis. Secondary edema and brain damage may correlate with the expression of AQP-4 mRNA in the peri-hematoma brain edema area. For these reasons, several gene variations could be very important in TBI outcome: for example, one non-synonymous single-nucleotide polymorphism (nsSNP) (rs3906956, M278T) has been described to be linked to increasing water permeability [95, 111]. In light of these findings, genetic investigations could be considered an important tool in the evaluation of the prognosis for patients with TBI. Moreover, it could be very important to identify the variants on the genetic substrate because the same traumatic event may have different consequences. To consolidate this important concept, several association studies were performed with the aim of linking TBI with different genetic variations. For example, in a study conducted in aneurysmal subarachnoid hemorrhage (SAH) patients, the polymorphism –786 T/C (rs2070744) on the endothelial nitric oxide synthase (NOS3) gene was associated with cerebral vasospasm [112], and the C-alleles were linked to a reduction of cerebral blood flow [113]. Another interesting study reported an association between a polymorphism located on the tumor protein 53 gene (TP53) and a bad outcome in TBI: analyzing the Argp53Pro polymorphism, Martinez-Lucas et al. [114] described a bad outcome in patients with the Arg/Arg genotype. These considerations could be relevant in sports traumas: for example, soccer is undoubtedly considered a contact sport with a relevant number of craniocerebral injuries, which are statistically comparable to American football and hockey. There are two principal ways to cause a TBI in soccer: head injuries could be generated from unintentional collisions with the head of another player (head to head) or collisions between the head and different body parts (elbow/legs to head) [115]. TBI gravity could be linked to the genotype of the players.
In conclusion, TBI can be defined as a multifactorial event with subjective symptoms that are strictly linked to the genotype of the subject. These considerations opened new scenarios analyzing the medico-legal aspects, both from penal and insurance points of view. Indeed, in organ damage evaluation, the genetic substrate could be relevant: for these reasons, in the near future, genetic tests could be mandatory before life insurance or penal evaluation of damages.
6.2. TBI: New Molecular Biomarkers
The knowledge about TBI could be very important to identify new molecular biomarkers using them both for diagnosis and therapy and for the identification of the exact cause of death in forensic examinations. A myriad of attempts by researchers to find TBI biomarkers have been made: nevertheless, to date, none of them have shown results definitive enough to warrant use in clinical settings.
The starting point of this kind of study is undoubtedly related to the aberrant expression of intra- and extra-cellular proteins linked to the injured brain [116, 117]. An alteration of the expression levels of these proteins could be the key to the identification processes of damage. Biomarkers, such as metabolites, proteins, and neuronal imaging, have been investigated in TBI patients. In this way, extracellular vesicles (EVs), which are membranous nanoparticles subdivided into exosomes (originating in the endosomal/multivesicular body system) and micro-vesicles (larger EVs produced through budding of the plasma membrane), are generated by all cells and are secreted into the extracellular environment. After a TBI, EVs can be collected from peripheral blood samples and their contents quantitatively examined to identify potential changes occurring after brain damage [118]. Particularly, different studies on protein biomarkers have been performed with the aim of identifying the perfect marker for diagnosing, monitoring, and predicting the course of concussions. Several markers, such as GFAP, S-100, ubiquitin carboxy-terminal hydrolase L1, and tau, have been investigated in CSF and blood with the aim of assessing oxidative stress, inflammation, excitotoxicity and other pathological mechanisms linked to TBI [119, 120]. Obviously, the main aims of the recent systematic reviews are directed towards clinical applications. To date, S100 is the only molecular serum biomarker of BI that has been supported by scientific evidence [116], while CFS seems to be the most reliable biological fluid [121]. As suggested in the previous paragraph, the phosphorylated form of Tau can be considered a valuable CSF biomarker in patients suffering from TBI. Indeed, this biomarker was found to be elevated from 24 up to 48 h following hypoxic brain injury. For this reason, ultra-sensitive immunoassays are currently available to measure less than 10pg of P-Tau protein [122].
In the last few decades, the role of miRNAs has been studied by the scientific community in several diseases, particularly in cancer. miRNAs composed of 20–24 nucleotides are often located within introns and are known as short noncoding regulatory molecules, playing a key role in regulating gene/protein expression [107, 123]. Moreover, their pivotal role as molecular cellular modulators suggested numerous studies in different fields of medicine with the aim of identifying new molecular biomarkers. Particularly, based on their important roles in the regulation of various cellular functions in the brain, their activity on TBI has been focused on by several experimental studies [124]. This particular kind of biomarker could be used both for identification of TBI in the patient outcome and in autopsy with the aim of identifying the anatomical region of brain damage, and, consequently, the exact cause of death. Different plasma miRNA profiles were investigated in TBI patients, these showed under-expression levels (miR-16 and miR-92a) or over-expression levels (miR-765, miR-93, miR-191, and miR-499) [125, 126].
A recent paper described an experimental study that aimed to identify serum miRNA biomarkers able to discriminate mild and severe TBI. miR-425-5p and miR-502 seem to be downregulated early after a mild TBI, while miR-21 and miR-335 were described upregulated in severe TBI patients [127]. The main miRNAs investigated related to TBI are miR-21 and miR-16, frequently investigated in both human and animal TBI studies: indeed, both miRNAs have been found upregulated in brain damage. On the other hand, miR-107 and mir-27a are down-regulated after injury: in fact, low levels of miR-107 are critical for inflammatory processes, whereas a down-regulation of miR-27a facilitates programmed cell death [128]. Finally, it is very interesting to report the research idea suggested in a recent study to use miRNA biomarkers as easily available tools in medico-legal investigations to ascertain the exact cause of death in suspected brain injury cases [57].
In conclusion, miRNAs are promising biomarkers for TBI both for brain damage identification and for medico-legal aspects, even if further investigations are required to validate the first experimental studies.
DISCUSSION AND CONCLUSION
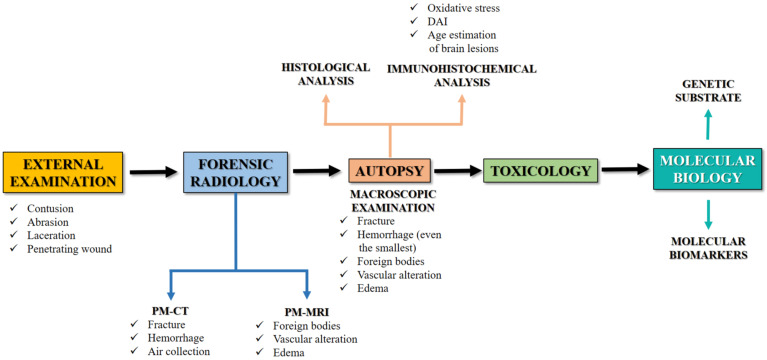
An accurate forensic approach can be considered mandatory in the examination of a suspected TBI with medico-legal importance, in order to gather all the possible evidence to corroborate the diagnosis of a lesion that may have caused or contributed to death. From this point of view, only the use of an evidence-based protocol can reach a suitable diagnosis, especially in those cases in which there are other neuropathological conditions (ischemia, neurodegeneration, neuroinflammation, dementia) that may have played a role in death. This mini-review has, in fact, the objective of systematizing the forensic approach in neuropathological studies, highlighting the proper elements to be noted during external, radiological, autoptical, histological examinations with particular attention to immunohistochemistry and molecular biology (Fig. 3).
Fig. (3).
This flow chart summarizes the multidisciplinary forensic approach in TBI. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
These two methods, in fact, allow a critical approach also to those cases that up until now have always been considered axioms in normal forensic practice, such as, for example, hemorrhage. Hypostatic bleeding can occur in the subarachnoid space and within the cerebral parenchyma after death in the absence of trauma, which can be difficult to differentiate from traumatic hemorrhages [129, 130]. Moreover, this is even more important when corpses in an advanced state of decomposition are studied, where the radiological, macroscopic and histological analyses fail to give meaningful answers. In these cases, immunohistochemical and molecular biology diagnostics, which can count on the relative stability of miRNAs compared to the post-mortem time interval [131] are of fundamental importance and a forensic neuropathologist has to know them. In the same way, the genetic substrate should be focused on during the forensic examination, considering its importance in the outcome of TBI.
In other words, it is essential to highlight the pivotal role of molecular biology in the case of TBI. Notably, on the one hand, it could be considered the genetic predisposition to envelop brain damage with a different degree of gravity: as previously discussed, the genetic substrate is an important factor because the same brain trauma could have a different prognosis. On the other hand, molecular investigation also offers the possibility of discovering new biomarkers to use in a clinical setting, identifying pathways activated in TBI or associated with disease conditions.
Finally, the efforts made in TBI studies should be sustained by the scientific community because to date, the different aspects of TBI have not been completely clarified, revealing the extreme complexity of the central nervous system and how it responds to injury.
Acknowledgements
We wish to thank the Scientific Bureau of the University of Catania for language support.
Consent for Publication
Not applicable.
Funding
None.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Werner C., Engelhard K. Pathophysiology of traumatic brain injury. Br. J. Anaesth. 2007;99(1):4–9. doi: 10.1093/bja/aem131. [DOI] [PubMed] [Google Scholar]
- 2.Peterson A.B., Xu L., Daugherty J., Breiding M.J. 2019 https://www.cdc.gov/
- 3.Tagliaferri F., Compagnone C., Korsic M., Servadei F., Kraus J. A systematic review of brain injury epidemiology in Europe. Acta Neurochir. (Wien) 2006;148(3):255–268. doi: 10.1007/s00701-005-0651-y. [DOI] [PubMed] [Google Scholar]
- 4.Ommaya A.K., Goldsmith W., Thibault L. Biomechanics and neuropathology of adult and paediatric head injury. Br. J. Neurosurg. 2002;16(3):220–242. doi: 10.1080/02688690220148824. [DOI] [PubMed] [Google Scholar]
- 5.Wilson B.A., Winegardner J., van Heugten C.M., Ownsworth T. Neuropsychological Rehabilitation: The International Handbook. Vol. 6. London: Routledge; 2017. p. 6. [Google Scholar]
- 6.Reilly P., Bullock R., Anderson R., McLean J. 2013. Biomechanics of Closed Head Injury. [Google Scholar]
- 7.Murie-Fernandez M., Burneo J.G., Teasell R.W. In: The Causes of Epilepsy: Common and Uncommon Causes in Adults and Children. Shorvon S.D., Andermann F., Guerrini R., editors. Vol. 58. Cambridge: Cambridge University Press; 2011. pp. 400–406. [Google Scholar]
- 8.Corrigan J.D., Selassie A.W., Orman J.A. The epidemiology of traumatic brain injury. J. Head Trauma Rehabil. 2010;25(2):72–80. doi: 10.1097/HTR.0b013e3181ccc8b4. [DOI] [PubMed] [Google Scholar]
- 9.Langlois J.A., Rutland-Brown W., Wald M.M. The epidemiology and impact of traumatic brain injury: a brief overview. J. Head Trauma Rehabil. 2006;21(5):375–378. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- 10.DiMaio D., DiMaio V.J.M. Forensic Pathology. 2nd ed. Vol. 6. CRC Press, LLC; 2001. pp. 147–183. [Google Scholar]
- 11.Romner B., Grände P.O. Traumatic brain injury: Intracranial pressure monitoring in traumatic brain injury. Nat. Rev. Neurol. 2013;9(4):185–186. doi: 10.1038/nrneurol.2013.37. [DOI] [PubMed] [Google Scholar]
- 12.Graham D.I., Adams J.H., Nicoll J.A.R., Maxwell W.L., Gennarelli T.A. The nature, distribution and causes of traumatic brain injury. Brain Pathol. 1995;5(4):397–406. doi: 10.1111/j.1750-3639.1995.tb00618.x. [DOI] [PubMed] [Google Scholar]
- 13.Marshall L.F., Marshall S.B., Klauber M.R., Clark M. van B., Eisenberg H.M., Jane J.A., Luerssen T.G., Marmarou A., Foulkes M.A. A new classification of head injury based on computerized tomography. J. Neurosurg. 1991;75(Suppl.):S14–S20. doi: 10.3171/sup.1991.75.1s.0s14. [DOI] [Google Scholar]
- 14.Teasdale E., Cardoso E., Galbraith S., Teasdale G. CT scan in severe diffuse head injury: physiological and clinical correlations. J. Neurol. Neurosurg. Psychiatry. 1984;47(6):600–603. doi: 10.1136/jnnp.47.6.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cafarelli F. P., Grilli G., Zizzo G., Bertozzi G., Giuliani N., Mahakkanukrauh P., Pinto A., Guglielmi G. 2018. [DOI] [PubMed]
- 16.Egger C., Bize P., Vaucher P., Mosimann P., Schneider B., Dominguez A., Meuli R., Mangin P., Grabherr S. Distribution of artifactual gas on post-mortem multidetector computed tomography (MDCT). Int. J. Legal Med. 2012;126(1):3–12. doi: 10.1007/s00414-010-0542-5. [DOI] [PubMed] [Google Scholar]
- 17.Egger C., Vaucher P., Doenz F., Palmiere C., Mangin P., Grabherr S. Development and validation of a postmortem radiological alteration index: the RA-Index. Int. J. Legal Med. 2012;126(4):559–566. doi: 10.1007/s00414-012-0686-6. [DOI] [PubMed] [Google Scholar]
- 18.Panda A., Kumar A., Gamanagatti S., Mishra B. Virtopsy computed tomography in trauma: normal postmortem changes and pathologic spectrum of findings. Curr. Probl. Diagn. Radiol. 2015;44(5):391–406. doi: 10.1067/j.cpradiol.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 19.Bešenski N. Traumatic injuries: imaging of head injuries. Eur. Radiol. 2002;12(6):1237–1252. doi: 10.1007/s00330-002-1355-9. [DOI] [PubMed] [Google Scholar]
- 20.Grabherr S., Egger C., Vilarino R., Campana L., Jotterand M., Dedouit F. Modern post-mortem imaging: an update on recent developments. Forensic Sci. Rev. 2017;4(3):211–222. doi: 10.1080/20961790.2017.1330738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aghayev E., Yen K., Sonnenschein M., Ozdoba C., Thali M., Jackowski C., Dirnhofer R. Virtopsy post-mortem multi-slice computed tomography (MSCT) and magnetic resonance imaging (MRI) demonstrating descending tonsillar herniation: comparison to clinical studies. Neuroradiology. 2004;46(7):559–564. doi: 10.1007/s00234-004-1212-4. [DOI] [PubMed] [Google Scholar]
- 22.Yen K., Weis J., Kreis R., Aghayev E., Jackowski C., Thali M., Boesch C., Maier S.E., Dirnhofer R., Lövblad K.O. Line-scan diffusion tensor imaging of the posttraumatic brain stem: changes with neuropathologic correlation. AJNR Am. J. Neuroradiol. 2006;27(1):70–73. [PMC free article] [PubMed] [Google Scholar]
- 23.Ruder T.D., Thali M.J., Hatch G.M. Essentials of forensic post-mortem MR imaging in adults. Br. J. Radiol. 2014;87:1036. doi: 10.1259/bjr.20130567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fais P., Cecchetto G., Boscolo-Berto R., Toniolo M., Viel G., Miotto D., Montisci M., Tagliaro F., Giraudo C. Morphometric analysis of stab wounds by MSCT and MRI after the instillation of contrast medium. Radiol. Med. (Torino) 2016;121(6):494–501. doi: 10.1007/s11547-015-0612-3. [DOI] [PubMed] [Google Scholar]
- 25.Chiba F., Inokuchi G., Makino Y., Torimitsu S., Motomura A., Yamaguchi R., Hashimoto M., Hoshioka Y., Nasgasawa S., Sakuma A., Yajima D., Saito H., Iwase H. Postmortem angiography revealing traumatic rupture of the intracranial internal carotid artery. Int. J. Legal Med. 2018;132(2):589–592. doi: 10.1007/s00414-017-1752-x. [DOI] [PubMed] [Google Scholar]
- 26.Chatzaraki V., Thali M.J., Ampanozi G., Schweitzer W. Fatal Road traffic vehicle collisions with pedestrian victims: forensic postmortem computed tomography and autopsy correlation. Am. J. Forensic Med. Pathol. 2018;39(2):130–140. doi: 10.1097/PAF.0000000000000382. [DOI] [PubMed] [Google Scholar]
- 27.Pomara C., Karch S.B., Fineschi V. Forensic Autopsy: A Handbook and Atlas. 1st ed. CRC Press; 2010. [Google Scholar]
- 28.Rutty G. Reports, Documentation and Statements: In the Hospital Autopsy: A Manual of Fundamental Autopsy Practice. 3rd ed. Vol. 10. London: CRC Press; 2012. pp. 324–336. [Google Scholar]
- 29.Duhaime A.C., Alario A.J., Lewander W.J., Schut L., Sutton L.N., Seidl T.S., Nudelman S., Budenz D., Hertle R., Tsiaras W. Head injury in very young children: mechanisms, injury types, and ophthalmologic findings in 100 hospitalized patients younger than 2 years of age. Pediatrics. 1992;90(2 Pt 1):179–185. [PubMed] [Google Scholar]
- 30.Gilliland M.G.F., Levin A.V., Enzenauer R.W., Smith C., Parsons M.A., Rorke-Adams L.B., Lauridson J.R., La Roche G.R., Christmann L.M., Mian M., Jentzen J., Simons K.B., Morad Y., Alexander R., Jenny C., Wygnanski-Jaffe T. Guidelines for postmortem protocol for ocular investigation of sudden unexplained infant death and suspected physical child abuse. Am. J. Forensic Med. Pathol. 2007;28(4):323–329. doi: 10.1097/PAF.0b013e31815b4c00. [DOI] [PubMed] [Google Scholar]
- 31.Gurdjian E.S., Webster J.E., Lissner H.R. The Mechanism of Skull Fracture. J. Neurosurg. 2009;7(2):106–114. doi: 10.3171/jns.1950.7.2.0106. [DOI] [PubMed] [Google Scholar]
- 32.Kimpara H., Iwamoto M. Mild traumatic brain injury predictors based on angular accelerations during impacts. Ann. Biomed. Eng. 2012;40(1):114–126. doi: 10.1007/s10439-011-0414-2. [DOI] [PubMed] [Google Scholar]
- 33.Zbinden B., Kaiser G. Specific Aspects of Depressed Skull Fractures in Childhood. Eur. J. Pediatr. Surg. 2008;44(1):3–7. doi: 10.1055/s-2008-1042634. [DOI] [PubMed] [Google Scholar]
- 34.Fisher R.S., Petty C.S. Forensic Pathology, A handbook for pathologist. Am. J. Forensic Med. Pathol. 2006;1(3):286. doi: 10.1097/00000433-198009000-00022. [DOI] [Google Scholar]
- 35.Squier W., Mack J. The neuropathology of infant subdural haemorrhage. Forensic Sci. Int. 2009;187(1-3):6–13. doi: 10.1016/j.forsciint.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 36.Lindenberg R. Trauma of meninges and brain. Pathology of the nervous system. Vol. 2 McGraw-Hill; 1971. [Google Scholar]
- 37.Dettmeyer R.B., Verhoff M.A., Schütz H.F. Forensic Medicine. Fundamentals and Perspectives. 2014;9:135–153. [Google Scholar]
- 38.Haines D.E. 5th ed. Vol. 7. The Meninges. In Fundamental Neuroscience for Basic and Clinical Applications; 2017. pp. 107–121. [Google Scholar]
- 39.Martin G.T. Acute brain trauma. Ann. R. Coll. Surg. Engl. 2016;98(1):6–10. doi: 10.1308/rcsann.2016.0003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kobrine A.I., Timmins E., Rajjoub R.K., Rizzoli H.V., Davis D.O. Demonstration of massive traumatic brain swelling within 20 minutes after injury. Case report. J. Neurosurg. 1977;46(2):256–258. doi: 10.3171/jns.1977.46.2.0256. [DOI] [PubMed] [Google Scholar]
- 41.Shahlaie K., Zwienenberg-Lee M., Muizelaar J.P. Clinical pathophysiology of traumatic brain injury. In: Hr W., editor. Youmans Neurological Surgery. 5th ed. Philadelphia: Saunders; 2004. pp. 5039–5064. [Google Scholar]
- 42.Finnie J.W. Forensic pathology of traumatic brain injury. Vet. Pathol. 2016;53(5):962–978. doi: 10.1177/0300985815612155. [DOI] [PubMed] [Google Scholar]
- 43.Hashimoto T., Nakamura N., Richard K.E., Frowein R.A. Primary brain stem lesions caused by closed head injuries. Neurosurg. Rev. 1993;16(4):291–298. doi: 10.1007/BF00383839. [DOI] [PubMed] [Google Scholar]
- 44.Britt R.H., Herrick M.K., Mason R.T., Dorfman L.J. Traumatic lesions of pontomedullary junction. Neurosurgery. 1980;6(6):623–631. doi: 10.1227/00006123-198006000-00002. [DOI] [PubMed] [Google Scholar]
- 45.Gunji H., Mizusawa I., Hiraiwa K. The mechanism underlying the occurrence of traumatic brainstem lesions in victims of traffic accidents. Leg. Med. (Tokyo) 2002;4(2):84–89. doi: 10.1016/S1344-6223(02)00012-3. [DOI] [PubMed] [Google Scholar]
- 46.Stan A.C., Guenther D., Fieguth A., Hori A. Traumatic ponto-medullary tear: a case report. Forensic Sci. Int. 1996;77(1-2):37–43. doi: 10.1016/0379-0738(95)01842-5. [DOI] [PubMed] [Google Scholar]
- 47.Adams V.I. Neck Injuries: I. Occipitoatlantal dislocation—a pathologic study of twelve traffic fatalities. J. Forensic Sci. 2015;37:556–564. doi: 10.1520/jfs13262j. [DOI] [PubMed] [Google Scholar]
- 48.Hopper R.H., McElhaney J.H., Myers B.S. 1994. Mandibular and basilar skull fracture tolerance. [DOI] [PubMed] [Google Scholar]
- 49.Hein P.M., Schulz E. Contrecoup fractures of the anterior cranial fossae as a consequence of blunt force caused by a fall. Acta Neurochir. (Wien) 1990;105(1-2):24–29. doi: 10.1007/BF01664853. [DOI] [PubMed] [Google Scholar]
- 50.Harrigan M.R., Hadley M.N., Dhall S.S., Walters B.C., Aarabi B., Gelb D.E., Hurlbert R.J., Rozzelle C.J., Ryken T.C., Theodore N. Management of vertebral artery injuries following non-penetrating cervical trauma. Neurosurgery. 2013;72(Suppl. 2):234–243. doi: 10.1227/NEU.0b013e31827765f5. [DOI] [PubMed] [Google Scholar]
- 51.Desouza R.M., Crocker M.J., Haliasos N., Rennie A., Saxena A. Blunt traumatic vertebral artery injury: a clinical review. Eur. Spine J. 2011;20(9):1405–1416. doi: 10.1007/s00586-011-1862-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Adams J.H., Doyle D., Graham D.I., Lawrence A.E., McLellan D.R. Deep intracerebral (basal ganglia) haematomas in fatal non-missile head injury in man. J. Neurol. Neurosurg. Psychiatry. 1986;49(9):1039–1043. doi: 10.1136/jnnp.49.9.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bjork J.M., Grant S.J. Does traumatic brain injury increase risk for substance abuse? J. Neurotrauma. 2009;26(7):1077–1082. doi: 10.1089/neu.2008.0849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Taylor B., Irving H.M., Kanteres F., Room R., Borges G., Cherpitel C., Greenfield T., Rehm J. The more you drink, the harder you fall: a systematic review and meta-analysis of how acute alcohol consumption and injury or collision risk increase together. Drug Alcohol Depend. 2010;110(1-2):108–116. doi: 10.1016/j.drugalcdep.2010.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kelly M.P., Johnson C.T., Knoller N., Drubach D.A., Winslow M.M. Substance abuse, traumatic brain injury and neuropsychological outcome. Brain Inj. 1997;11(6):391–402. doi: 10.1080/026990597123386. [DOI] [PubMed] [Google Scholar]
- 56.Bertozzi G., Sessa F., Albano G.D., Sani G., Maglietta F., Roshan M.H.K., Volti G.L., Bernardini R., Avola R., Pomara C. The role of anabolic androgenic steroids in disruption of the physiological function in discrete areas of the central nervous system. Mol. Neurobiol. 2017;55:5548–5556. doi: 10.1007/s12035-017-0774-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sessa F., Maglietta F., Bertozzi G., Salerno M., Di Mizio G., Messina G., Montana A., Ricci P., Pomara C. Human brain injury and mirnas: an experimental study. Int. J. Mol. Sci. 2019;20(7):E1546. doi: 10.3390/ijms20071546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Johnston J.J.E., McGovern S.J. Alcohol related falls: An interesting pattern of injuries. Emerg. Med. J. 2004;21(2):185–188. doi: 10.1136/emj.2003.006130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yaghoubian A., Kaji A., Putnam B., De Virgilio N., De Virgilio C. Elevated blood alcohol level may be protective of trauma patient mortality. Am. Surg. 2009;75(10):950–953. [PubMed] [Google Scholar]
- 60.Dockree P.M., Robertson I.H. Electrophysiological markers of cognitive deficits in traumatic brain injury: A review. Int. J. Psychophysiol. 2011;82(1):53–60. doi: 10.1016/j.ijpsycho.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 61.Arai N., Tabuse M., Nakamura A., Miyazaki H. Delayed traumatic subarachnoid hemorrhage related to anticoagulant use: A case report. J. Spine Neurosurg. 2016;5:5. [Google Scholar]
- 62.Krynetskiy E., McDonnell P. Building individualized medicine: prevention of adverse reactions to warfarin therapy. J. Pharmacol. Exp. Ther. 2007;322(2):427–434. doi: 10.1124/jpet.106.117952. [DOI] [PubMed] [Google Scholar]
- 63.Parker S.L., Saxena M., Gowardman J., Lipman J., Myburgh J., Roberts J.A. Population pharmacokinetics of intravenous paracetamol in critically ill patients with traumatic brain injury. J. Crit. Care. 2018;47:15–20. doi: 10.1016/j.jcrc.2018.05.016. [DOI] [PubMed] [Google Scholar]
- 64.Empey P.E., McNamara P.J., Young B., Rosbolt M.B., Hatton J. Cyclosporin A disposition following acute traumatic brain injury. J. Neurotrauma. 2006;23(1):109–116. doi: 10.1089/neu.2006.23.109. [DOI] [PubMed] [Google Scholar]
- 65.Empey P.E., Velez de Mendizabal N., Bell M.J., Bies R.R., Anderson K.B., Kochanek P.M., Adelson P.D., Poloyac S.M. Therapeutic hypothermia decreases phenytoin elimination in children with traumatic brain injury. Crit. Care Med. 2013;41(10):2379–2387. doi: 10.1097/CCM.0b013e318292316c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McCormick W.F. Pathology of closed head injury. In: Wilkins R.H., editor. Neurosurgery. New York: McGraw-Hill; 1985. pp. 1544–1570. [Google Scholar]
- 67.Squier W., Scheimberg I., Smith C. Spinal nerve root β-APP staining in infants is not a reliable indicator of trauma. Forensic Sci. Int. 2011;212(1-3):e31–e35. doi: 10.1016/j.forsciint.2011.04.027. [DOI] [PubMed] [Google Scholar]
- 68.Orihara Y., Tsuda R., Ikematsu K., Nakasono I., Ogata M. 2003. Immunohistochemical Study on the Induction of Heme Oxygenase-1 by Traumatic Brain Injury. [DOI] [PubMed] [Google Scholar]
- 69.Geddes J.F., Whitwell H.L., Graham D.I. Traumatic axonal injury: practical issues for diagnosis in medicolegal cases. Neuropathol. Appl. Neurobiol. 2000;26(2):105–116. doi: 10.1046/j.1365-2990.2000.026002105.x. [DOI] [PubMed] [Google Scholar]
- 70.Hausmann R., Kaiser A., Lang C., Bohnert M., Betz P. A quantitative immunohistochemical study on the time-dependent course of acute inflammatory cellular response to human brain injury. Int. J. Legal Med. 1999;112(4):227–232. doi: 10.1007/s004140050241. [DOI] [PubMed] [Google Scholar]
- 71.Nogami M., Takatsu A., Endo N., Ishiyama I. IgG immunohistochemistry for the assessment of brain injuries in forensic autopsies. Leg. Med. (Tokyo) 1999;1(2):76–79. doi: 10.1016/S1344-6223(99)80016-9. [DOI] [PubMed] [Google Scholar]
- 72.Nogami M., Takatsu A., Endo N., Ishiyama I. Immunohistochemical localization of heat shock protein 70 in the human medulla oblongata in forensic autopsies. Leg. Med. (Tokyo) 1999;1(4):198–203. doi: 10.1016/S1344-6223(99)80038-8. [DOI] [PubMed] [Google Scholar]
- 73.Ogata M., Tsuganezawa O. Neuron-specific enolase as an effective immunohistochemical marker for injured axons after fatal brain injury. Int. J. Legal Med. 1999;113(1):19–25. doi: 10.1007/s004140050273. [DOI] [PubMed] [Google Scholar]
- 74.Herczeg L., Gorombey S., Vaszily M. Morphological damage to the central nervous system (CNS) following open heart surgery. Forensic Sci. Int. 1996;79(2):103–111. doi: 10.1016/0379-0738(96)01896-8. [DOI] [PubMed] [Google Scholar]
- 75.Kitamura O. Immunohistochemical investigation of hypoxic/ischemic brain damage in forensic autopsy cases. Int. J. Legal Med. 1994;107(2):69–76. doi: 10.1007/BF01225492. [DOI] [PubMed] [Google Scholar]
- 76.Vowles G.H., Scholtz C.L., Cameron J.M. Diffuse axonal injury in early infancy. J. Clin. Pathol. 1987;40(2):185–189. doi: 10.1136/jcp.40.2.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McQuillen J.B., McQuillen E.N., Morrow P. Trauma, sport, and malignant cerebral edema. Am. J. Forensic Med. Pathol. 1988;9(1):12–15. doi: 10.1097/00000433-198803000-00004. [DOI] [PubMed] [Google Scholar]
- 78.Reichard R.R., Smith C., Graham D.I. The significance of β-APP immunoreactivity in forensic practice. Neuropathol. Appl. Neurobiol. 2005;31(3):304–313. doi: 10.1111/j.1365-2990.2005.00645.x. [DOI] [PubMed] [Google Scholar]
- 79.Kaur B., Rutty G.N., Timperley W.R. The possible role of hypoxia in the formation of axonal bulbs. J. Clin. Pathol. 1999;52(3):203–209. doi: 10.1136/jcp.52.3.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Harrington D., Rutty G.N., Timperley W.R. β -amyloid precursor protein positive axonal bulbs may form in non-head-injured patients. J. Clin. Forensic Med. 2000;7(1):19–25. doi: 10.1054/jcfm.2000.0359. [DOI] [PubMed] [Google Scholar]
- 81.Frati A., Cerretani D., Fiaschi A.I., Frati P., Gatto V., La Russa R., Pesce A., Pinchi E., Santurro A., Fraschetti F., Fineschi V. Diffuse axonal injury and oxidative stress: a comprehensive review. Int. J. Mol. Sci. 2017;18(12):E2600. doi: 10.3390/ijms18122600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wang Q., Ishikawa T., Michiue T., Zhu B.L., Guan D.W., Maeda H. Quantitative immunohistochemical analysis of human brain basic fibroblast growth factor, glial fibrillary acidic protein and single-stranded DNA expressions following traumatic brain injury. Forensic Sci. Int. 2012;221(1-3):142–151. doi: 10.1016/j.forsciint.2012.04.025. [DOI] [PubMed] [Google Scholar]
- 83.Tao L., Chen X., Qin Z., Bian S. Could NF-kappaB and caspase-3 be markers for estimation of post-interval of human traumatic brain injury? Forensic Sci. Int. 2006;162(1-3):174–177. doi: 10.1016/j.forsciint.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 84.Cervós-Navarro J., Lafuente J.V. Traumatic brain injuries: Structural changes. J. Neurol. Sci. 1991;103(Suppl.):S3–S14. doi: 10.1016/0022-510X(91)90002-O. [DOI] [PubMed] [Google Scholar]
- 85.Oehmichen M., Raff G. Timing of cortical contusion. Correlation between histomorphologic alterations and post-traumatic interval. Z. Rechtsmed. 1980;84(2):79–94. doi: 10.1007/BF02114577. [DOI] [PubMed] [Google Scholar]
- 86.Hausmann R., Vogel C., Seidl S., Betz P. Value of morphological parameters for grading of brain swelling. Int. J. Legal Med. 2006;120(4):219–225. doi: 10.1007/s00414-005-0021-6. [DOI] [PubMed] [Google Scholar]
- 87.Hausmann R., Biermann T., Wiest I., Tübel J., Betz P. Neuronal apoptosis following human brain injury. Int. J. Legal Med. 2004;118(1):32–36. doi: 10.1007/s00414-003-0413-4. [DOI] [PubMed] [Google Scholar]
- 88.Ng I., Yeo T.T., Tang W.Y., Soong R., Ng P.Y., Smith D.R. Apoptosis occurs after cerebral contusions in humans. Neurosurgery. 2000;46(4):949–956. doi: 10.1097/00006123-200004000-00034. [DOI] [PubMed] [Google Scholar]
- 89.Finch C.E. Kessler; Pfaff; Heuser; Visser; Smith; Thijssen; Swaab. Neurons, glia, and plasticity in normal brain aging. Neurobiol. Aging. 2003;24(S1):S123–S127. doi: 10.1016/S0197-4580(03)00051-4. [DOI] [PubMed] [Google Scholar]
- 90.Orihara Y., Nakasono I. Induction of apolipoprotein E after traumatic brain injury in forensic autopsy cases. Int. J. Legal Med. 2002;116(2):92–98. doi: 10.1007/s00414-001-0265-8. [DOI] [PubMed] [Google Scholar]
- 91.Hausmann R., Riess R., Fieguth A., Betz P. Immunohistochemical investigations on the course of astroglial GFAP expression following human brain injury. Int. J. Legal Med. 2000;113(2):70–75. doi: 10.1007/PL00007711. [DOI] [PubMed] [Google Scholar]
- 92.Lundesgaard E.J.M., Opdal S.H., Rognum T.O., Stray-Pedersen A. Postmortem evaluation of brain edema: an attempt with measurements of water content and brain-weight-to-inner-skull-circumference ratio. J. Forensic Leg. Med. 2019;64:1–6. doi: 10.1016/j.jflm.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 93.Oehmichen M., Eisenmenger W., Raff G., Berghaus G. Brain macrophages in human cortical contusions as indicator of survival period. Forensic Sci. Int. 1986;30(4):281–301. doi: 10.1016/0379-0738(86)90136-2. [DOI] [PubMed] [Google Scholar]
- 94.Ragaisis V. Brain contusion: morphology, pathogenesis and treatment. Medicina (Kaunas) 2002;38(3):243–249. [PubMed] [Google Scholar]
- 95.Neri M., Frati A., Turillazzi E., Cantatore S., Cipolloni L., Di Paolo M., Frati P., La Russa R., Maiese A., Scopetti M., Santurro A., Sessa F., Zamparese R., Fineschi V. Immunohistochemical evaluation of aquaporin-4 and its correlation with cd68, iba-1, hif-1α, gfap, and cd15 expressions in fatal traumatic brain injury. Int. J. Mol. Sci. 2018;19(11):E3544. doi: 10.3390/ijms19113544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dressler J., Hanisch U., Kuhlisch E., Geiger K.D. Neuronal and glial apoptosis in human traumatic brain injury. Int. J. Legal Med. 2007;121(5):365–375. doi: 10.1007/s00414-006-0126-6. [DOI] [PubMed] [Google Scholar]
- 97.van Gijn J., Rinkel G.J.E. Subarachnoid haemorrhage: diagnosis, causes and management. Brain. 2001;124(Pt 2):249–278. doi: 10.1093/brain/124.2.249. [DOI] [PubMed] [Google Scholar]
- 98.Hausmann R., Betz P. The time course of the vascular response to human brain injury--an immunohistochemical study. Int. J. Legal Med. 2000;113(5):288–292. doi: 10.1007/s004149900126. [DOI] [PubMed] [Google Scholar]
- 99.Schiavone S., Neri M., Trabace L., Turillazzi E. The NADPH oxidase NOX2 mediates loss of parvalbumin interneurons in traumatic brain injury: human autoptic immunohistochemical evidence. Sci. Rep. 2017;7(1):8752. doi: 10.1038/s41598-017-09202-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Huang Y., Mahley R.W., Apolipoprotein E. Apolipoprotein E: structure and function in lipid metabolism, neurobiology, and Alzheimer’s diseases. 2014. [DOI] [PMC free article] [PubMed]
- 101.Poirier J. Apolipoprotein E in animal models of CNS injury and in Alzheimer’s disease. Trends Neurosci. 1994;17(12):525–530. doi: 10.1016/0166-2236(94)90156-2. [DOI] [PubMed] [Google Scholar]
- 102.Rao A.T., Degnan A.J., Levy L.M. Genetics of Alzheimer disease. AJNR Am. J. Neuroradiol. 2014;35(3):457–458. doi: 10.3174/ajnr.A3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Verghese P.B., Castellano J.M., Holtzman D.M. Apolipoprotein E in Alzheimer’s disease and other neurological disorders. Lancet Neurol. 2011;10(3):241–252. doi: 10.1016/S1474-4422(10)70325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zeng S., Jiang J-X., Xu M-H., Xu L-S., Shen G-J., Zhang A-Q., Wang X-H. Prognostic value of apolipoprotein E epsilon4 allele in patients with traumatic brain injury: a meta-analysis and meta-regression. Genet. Test. Mol. Biomarkers. 2014;18(3):202–210. doi: 10.1089/gtmb.2013.0421. [DOI] [PubMed] [Google Scholar]
- 105.Blennow K., Brody D.L., Kochanek P.M., Levin H., McKee A., Ribbers G.M., Yaffe K., Zetterberg H. Traumatic brain injuries. Nat. Rev. Dis. Primers. 2016;2:16084. doi: 10.1038/nrdp.2016.84. [DOI] [PubMed] [Google Scholar]
- 106.Davidson J., Cusimano M.D., Bendena W.G. Post-Traumatic brain injury: genetic susceptibility to outcome. Neuroscientist. 2015;21(4):424–441. doi: 10.1177/1073858414543150. [DOI] [PubMed] [Google Scholar]
- 107.Iliff J.J., Wang M., Liao Y., Plogg B.A., Peng W., Gundersen G.A., Benveniste H., Vates G.E., Deane R., Goldman S.A., Nagelhus E.A., Nedergaard M. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci. Transl. Med. 2012;4(147):147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Neve R.L., Harris P., Kosik K.S., Kurnit D.M., Donlon T.A. Identification of cDNA clones for the human microtubule-associated protein tau and chromosomal localization of the genes for tau and microtubule-associated protein 2. Brain Res. 1986;387(3):271–280. doi: 10.1016/0169-328X(86)90033-1. [DOI] [PubMed] [Google Scholar]
- 109.Sergeant N., Delacourte A., Buée L. Tau protein as a differential biomarker of tauopathies. Biochim. Biophys. Acta. 2005;1739(2-3):179–197. doi: 10.1016/j.bbadis.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 110.Blennow K., Hardy J., Zetterberg H. The neuropathology and neurobiology of traumatic brain injury. Neuron. 2012;76(5):886–899. doi: 10.1016/j.neuron.2012.11.021. [DOI] [PubMed] [Google Scholar]
- 111.Sorani M.D., Manley G.T., Giacomini K.M. Genetic variation in human aquaporins and effects on phenotypes of water homeostasis. Hum. Mutat. 2008;29(9):1108–1117. doi: 10.1002/humu.20762. [DOI] [PubMed] [Google Scholar]
- 112.Ko N.U., Rajendran P., Kim H., Rutkowski M., Pawlikowska L., Kwok P.Y., Higashida R.T., Lawton M.T., Smith W.S., Zaroff J.G., Young W.L. Endothelial nitric oxide synthase polymorphism (-786T->C) and increased risk of angiographic vasospasm after aneurysmal subarachnoid hemorrhage. Stroke. 2008;39(4):1103–1108. doi: 10.1161/STROKEAHA.107.496596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Garry P.S., Ezra M., Rowland M.J., Westbrook J., Pattinson K.T.S. The role of the nitric oxide pathway in brain injury and its treatment--from bench to bedside. Exp. Neurol. 2015;263:235–243. doi: 10.1016/j.expneurol.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 114.Martínez-Lucas P., Moreno-Cuesta J., García-Olmo D.C., Sánchez-Sánchez F., Escribano-Martínez J., del Pozo A.C., Lizán-García M., García-Olmo D. Relationship between the Arg72Pro polymorphism of p53 and outcome for patients with traumatic brain injury. Intensive Care Med. 2005;31(9):1168–1173. doi: 10.1007/s00134-005-2715-0. [DOI] [PubMed] [Google Scholar]
- 115.Bunc G., Ravnik J., Velnar T. May heading in soccer result in traumatic brain injury? a review of literature. Med. Arh. 2017;71(5):356–359. doi: 10.5455/medarh.2017.71.356-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Najem D., Rennie K., Ribecco-Lutkiewicz M., Ly D., Haukenfrers J., Liu Q., Nzau M., Fraser D.D., Bani-Yaghoub M. Traumatic brain injury: classification, models, and markers. Biochem. Cell Biol. 2018;96(4):391–406. doi: 10.1139/bcb-2016-0160. [DOI] [PubMed] [Google Scholar]
- 117.Sessa F., Salerno M., Di Mizio G., Bertozzi G., Messina G., Tomaiuolo B., Pisanelli D., Maglietta F., Ricci P., Pomara C. Anabolic androgenic steroids: searching new molecular biomarkers. Front. Pharmacol. 2018;9:1321. doi: 10.3389/fphar.2018.01321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Karnati H.K., Garcia J.H., Tweedie D., Becker R.E., Kapogiannis D., Greig N.H. Neuronal enriched extracellular vesicle proteins as biomarkers for traumatic brain injury. J. Neurotrauma. 2018;36(7):975–987. doi: 10.1089/neu.2018.5898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Reis C., Wang Y., Akyol O., Ho W.M., Ii R.A., Stier G., Martin R., Zhang J.H. What’s new in traumatic brain injury: update on tracking, monitoring and treatment. Int. J. Mol. Sci. 2015;16(6):11903–11965. doi: 10.3390/ijms160611903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mondello S., Sorinola A., Czeiter E., Vámos Z., Amrein K., Synnot A., Donoghue E., Sándor J., Wang K.K.W., Diaz-Arrastia R., Steyerberg E.W., Menon D.K., Maas A.I.R., Buki A. Blood-Based protein biomarkers for the management of traumatic brain injuries in adults presenting to emergency departments with mild brain injury: a living systematic review and meta-analysis. 2018. [DOI] [PMC free article] [PubMed]
- 121.Agoston D.V., Shutes-David A., Peskind E.R. Biofluid biomarkers of traumatic brain injury. Brain Inj. 2017;31(9):1195–1203. doi: 10.1080/02699052.2017.1357836. [DOI] [PubMed] [Google Scholar]
- 122.Randall J., Mörtberg E., Provuncher G.K., Fournier D.R., Duffy D.C., Rubertsson S., Blennow K., Zetterberg H., Wilson D.H. Tau proteins in serum predict neurological outcome after hypoxic brain injury from cardiac arrest: results of a pilot study. Resuscitation. 2013;84(3):351–356. doi: 10.1016/j.resuscitation.2012.07.027. [DOI] [PubMed] [Google Scholar]
- 123.Bafunno V., Santacroce R., Chetta M., Peyvandi F., Sessa F., Chinni E., Longo V., Margaglione M. Polymorphic miRNA-mediated gene contribution to inhibitor development in haemophilia A. Haemophilia. 2012;18(6):1003–1007. doi: 10.1111/j.1365-2516.2012.02882.x. [DOI] [PubMed] [Google Scholar]
- 124.Meissner L., Gallozzi M., Balbi M., Schwarzmaier S., Tiedt S., Terpolilli N.A., Plesnila N. Temporal profile of microrna expression in contused cortex after traumatic brain injury in mice. J. Neurotrauma. 2016;33(8):713–720. doi: 10.1089/neu.2015.4077. [DOI] [PubMed] [Google Scholar]
- 125.Yang T., Song J., Bu X., Wang C., Wu J., Cai J., Wan S., Fan C., Zhang C., Wang J. Elevated serum miR-93, miR-191, and miR-499 are noninvasive biomarkers for the presence and progression of traumatic brain injury. J. Neurochem. 2016;137(1):122–129. doi: 10.1111/jnc.13534. [DOI] [PubMed] [Google Scholar]
- 126.Redell J.B., Moore A.N., Ward N.H., III, Hergenroeder G.W., Dash P.K. Human traumatic brain injury alters plasma microRNA levels. J. Neurotrauma. 2010;27(12):2147–2156. doi: 10.1089/neu.2010.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Di Pietro V., Ragusa M., Davies D., Su Z., Hazeldine J., Lazzarino G., Hill L.J., Crombie N., Foster M., Purrello M., Logan A., Belli A. MicroRNAs as novel biomarkers for the diagnosis and prognosis of mild and severe traumatic brain injury. J. Neurotrauma. 2017;34(11):1948–1956. doi: 10.1089/neu.2016.4857. [DOI] [PubMed] [Google Scholar]
- 128.Atif H., Hicks S.D. A review of MicroRNA biomarkers in traumatic brain injury. J. Exp. Neurosci. 2019;131179069519832286 doi: 10.1177/1179069519832286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Xiang L., Zhou G., Su P., Xia S., Han B., Wang Y., Zhang T. Could postmortem hemorrhage occur in the brain? A preliminary study on the establishment and investigation of postmortem hypostatic hemorrhage using rabbit models. Am. J. Forensic Med. Pathol. 2013;34(2):147–149. doi: 10.1097/PAF.0b013e31828877f0. [DOI] [PubMed] [Google Scholar]
- 130.Bockholdt B., Maxeiner H., Hegenbarth W. Factors and circumstances influencing the development of hemorrhages in livor mortis. Forensic Sci. Int. 2005;149(2-3):133–137. doi: 10.1016/j.forsciint.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 131.MacKenzie J.M. Examining the decomposed brain. Am. J. Forensic Med. Pathol. 2014;35(4):265–270. doi: 10.1097/PAF.0000000000000111. [DOI] [PubMed] [Google Scholar]