Abstract
Pain is a complex physiological process that includes many components. Growing evidence supports the idea that oxidative stress and Ca2+ signaling pathways participate in pain detection by neurons. The main source of endogenous reactive oxygen species (ROS) is mitochondrial dysfunction induced by membrane depolarization, which is in turn caused by Ca2+ influx into the cytosol of neurons. ROS are controlled by antioxidants, including selenium. Selenium plays an important role in the nervous system, including the brain, where it acts as a cofactor for glutathione peroxidase and is incorporated into selenoproteins involved in antioxidant defenses. It has neuroprotective effects through modulation of excessive ROS production, inflammation, and Ca2+ overload in several diseases, including inflammatory pain, hypersensitivity, allodynia, diabetic neuropathic pain, and nociceptive pain. Ca2+ entry across membranes is mediated by different channels, including transient receptor potential (TRP) channels, some of which (e.g., TRPA1, TRPM2, TRPV1, and TRPV4) can be activated by oxidative stress and have a role in the induction of peripheral pain. The results of recent studies indicate the modulator roles of selenium in peripheral pain through inhibition of TRP channels in the dorsal root ganglia of experimental animals. This review summarizes the protective role of selenium in TRP channel regulation, Ca2+ signaling, apoptosis, and mitochondrial oxidative stress in peripheral pain induction.
Keywords: Calcium ion, neurological diseases, oxidative stress, peripheral pain, TRP channels, selenium
1. Introduction
Pain can be induced by the excessive production of ROS or RNS species [1], and several kinds of cation channels act as sensors of oxidative stress and join the pain perception and transduction pathway [2, 3]. Yabe-Nishimura et al. [4] suggested that NADPH oxidase-generated ROS increases TRPV1 channel activity and enhances the translocation of protein kinase C (PKC), causing inflammatory pain in mice DRG neurons. An experimentally thermal hyperalgesia model can be induced by peroxynitrite, which occurs by directly combining subplantar superoxide radical injection and endogenous nitric oxide (NO). In a study, a powerful antioxidant, melatonin, has been found to be responsible for hyperalgesia healing because of its anti-inflammatory activity [5]. Khattab showed that TEMPOL is a superoxide dismutase mimetic radical scavenger that promotes carrageenan-induced hyperalgesia via its analgesic and anti-inflammatory activity [6]. In another study, researchers evaluated the role of a strong protein antioxidant, sestrin 2, in neuropathic pain after peripheral nerve injury induction. They found that sestrin 2 protein expression decreases ROS levels and that it may help in achieving oxidant/antioxidant balance after injury and in suppressing ROS-dependent neuropathic pain in mice [7].
Different stages of the afferent pain pathway, including the detection of environmental stimuli, generation of action potentials, and propagation of signal transduction through the release of neuropeptides, are related to calcium-permeable cation channels [2]. Calcium channel blockers can be useful for reducing nociception [8]. However, there is a positive feedback mechanism among intracellular calcium concentrations, and mitochondrial membrane depolarization results in ROS production that is linked to calcium ion-permeable transient receptor potential (TRP) channels. Deciding where the feedback loop can be cut is therefore important in the development of new strategies for the treatment of painful sensation.
Pain is as old as human history, and everyone experiences it in one or more stages of life [9]. Although it can be unpleasant, pain is nonetheless an essential part of human survival and adaptation mechanisms [10]. It is often classified into two main categories—nociceptive and neuropathic pain. Nociceptive pain is a subjective experience that leads to tissue injury, and is often described as a sharp, aching, and pulsating pain. Nociceptors are activated by extreme temperature, high pressure, tissue damage-induced inflammation, and chemicals, such as substance P, serotonin, acetylcholine, low-pH solutions, ATP, and lactic acid [11], and they can be induced by alcoholism, chemotherapy, and diabetes [12]. Anticonvulsant and antidepressant drugs have been used for the treatment of neuropathic pain with limited success. Although the etiology of neuropathic pain is not fully understood, recent data suggest the important roles of excessive oxidative stress and calcium ion (Ca2+) overload in neurons [13, 14].
Oxidative stress results from an imbalance between the increased production of reactive oxygen species (ROS) and oxidative stress regulation by antioxidant systems. Lipid peroxidation-mediated oxidative tissue damage occurs during neurological disease and peripheral pain. In addition to the exogenous overproduction of ROS, in general, ROS accumulation results from mitochondrial respiratory chain pathways [13]. Enzymatic and non-enzymatic antioxidants have important roles in scavenging the ROS produced by the mitochondria [15]. For this reason, glutathione peroxidase (GPx) plays a critical role in protecting neurons from the harmful effects of ROS. Selenium is a co-factor for GPx enzymatic activity, and mitochondrial respiratory chain reactions are also modulated by selenium [16]. Excessive ROS production and increased Ca2+ influx are implicated in several diseases, including inflammatory pain, hypersensitivity, allodynia, diabetic neuropathic pain, and nociceptive pain [14, 17, 18]. Selenium may exert neuroprotective effects through modulation of ROS overproduction and Ca2+ entry in several neuronal diseases by supporting GPx activity and inhibiting cationic movements into the cytosol [19].
Increases in Ca2+ entry in peripheral pain and neuropathy occur through the activation of Ca2+ channels, such as voltage-gated calcium channels (VGCCs), and other channels, such as TRP channels. The mammalian TRP superfamily consists of 28 channels with 6 different subgroups. Some TRP channels are expressed in peripheral neurons and also in the central nervous system (CNS) and dorsal root ganglion (DRG), with the members of TRP sub-families (TRPA1, TRPM8, TRPV1, and TRPV2) being grouped as nociceptive TRP channels [20]. Neuronal TRP channels are activated by nociceptive stimuli, resulting in the neuronal depolarization and generation of action potentials [11]. After perception of the peripheral stimuli, nociceptive neurons initiate a series of action potentials in primary afferent fibers of the DRG neurons to stimulate postsynaptic neurons via pain mediators, including the calcitonin gene-related peptide (CGRP), substance P, and other excitatory neurotransmitters, such as glutamate. Peripheral pain signals are directed by nociceptive neurons through Aδ and C fibers to pain-related locations of the brain and the cerebellum, so the peripheral and CNS are connected to each other through pain mediators in the transmission of painful stimuli [21]. Moreover, previous studies have shown that distinct TRP channels are functionally expressed in primary nociceptors and in Aδ and C fibers [22-24], although the molecular mechanisms involved in peripheral pain have not yet been clarified.
Some clues on neuropathic pain have been found in recent data, and they show the roles of TRP channels in the etiology of neuropathic pain in experimental animal research. For example, Klein et al. [25] investigated the role of TRPA1, TRPC5, TRPM3, TRPM8, TRPV1, TRPV2, TRPV3, and TRPV4 in somatosensory function by using the von Frey test, and suggested the role of the TRPV3 channel in response to temperature, touch, and chemicals as a part of avoidance behavior. Other data from Salat et al. [26] reported the anti-nociceptive activities of TRPA1, TRPM8, and TRPV1 antagonists in neurogenic and neuropathic pain in a mouse model. Collectively, these behavioral and pharmacological studies show that TRP channels are directly involved in pain pathways. In some experiments, essential peripheral pain mediator neuropeptide release in sensory nerves has been attributed to TRP channels. Kirkwood et al. [27] investigated the role of TRPV1 channels in CGRP and substance P release in acute pancreatic pain. They suggest that the activation of TRPV1 promotes substance P release from pancreatic sensory nerves. The effects of TRPA1, CGRP, and substance P on colitis in a mice model were investigated, and TRPA1 channel activation by the induction of colitis was increased via Ca2+ signaling and substance P release [28]. In an experimental animal study, the involvement of TRPA1, but not TRPV1 channels has been reported to be related to CGRP release [29]. The report was confirmed by De Col et al. [30], who demonstrated that TRPA1 agonists induce CGRP release through the dura mater of rodents. Several studies report that antioxidant treatments, including those with selenium, can regulate the harmful effects of oxidative stress and irregular Ca2+ accumulation in neuronal cells. Several ROS-sensitive TRP channels may be responsible for cytosolic Ca2+ overload and cause neurological diseases and neuropathic pain; these are discussed below.
Inflammation occurs as a result of a pathological condition that produces pain, and it is sensed by the peripheral nervous system and CNS. Inflammatory cytokines, such as TNF-α, IL-6, and IL-1β are released from activated macrophages when neural or other tissues are damaged in pathological pain [31]. Some studies suggest that inflammation is related to the numbers and types of ion channels at nerve endings [32, 33].
TRP channels constitute the largest group of activator chemicals and are involved in the generation of pain sensation in mammals [34]. The expression level of pain-related TRP channels, such as TRPA1 and TRPV1, has been reported to increase the risk of pain induction in animal models [5, 6, 14]. Recently, interest in the role of TRP channels in neurological diseases, including neurodegenerative diseases (Table 1), and peripheral pain (Table 2) has increased. Although the results of previous data on TRPA1, TRPC5, and TRPV1 in neurons indicated that the oxidation of cysteine groups is very important for the activation of these channels [35, 36], novel studies point to modulator role of selenium treatments in neuronal channels [37]. Selenoproteins are selenocysteine-containing proteins, and they are mainly expressed in the human brain and DRG; they are most likely involved in antioxidant processes, which are the key factors in preventing the onset and progression of peripheral pain [38]. In addition, some newly synthesized selenoproteins and selenium products have been found to possess remarkable physiological properties [39, 40]. Despite accumulating evidence to implicate the involvement of several TRP channels in a wide range of neurological diseases, including peripheral pain, only 4 of the 28 mammalian TRP channel subunits, namely TRPA1, TRPM8, TRPV1, and TRPV3, have been exploited thus far to reach the clinical stage of drug development [34]. Because of the high potency of TRP channel inhibitors, selenium might serve as an alternative to conventional therapeutic drugs through inhibition of the TRP channel for the treatment of neurological diseases, including peripheral pain.
Table 1.
Effects of antioxidants and selenium treatments on possible therapeutic targets in different experimental neurological disease models of rodents (TBI, traumatic brain injury; SCI, spinal cord injury).
| Channel | Agent | Material | Value/Effect | Refs. |
|---|---|---|---|---|
| TRPA1 | NAC | Mice | Ischemia and oxidative stress induced peripheral postischemic dysesthesia treatment | [24, 87] |
| TRPA1 | Resveratrol | Rat DRG and HEK-293 cells | TRPA1 inhibition | [83] |
| TRPA1-TRPV1 | NAC | Rats | Cigarette smoke induced superior laryngeal irritation treatment | [192] |
| TRPA1-TRPV1 | 17-β estradiol, tamoxifen and raloxifene | Rat DRG | Inhibition of peripheral pain, TRPA1 and TRPV1 | [88] |
| TRPM3 | Flavones | Rats | Inhibition of TRPM3 mediated thermal hyperalgesia | [193] |
| TRPV1 | Hypericum perforatum extract | Rat DRG | SCI-induced pain and neuronal death reduction | [194] |
| TRPV1 | NAC and GSH | Mice DRG | TRPV1 Inhibition | [72] |
| TRPV1 | Selenium and NAC | Rat hippocampus | Treatment of TBI induced rats | [118] |
| TRPV1 | Dexmedetomidine | Rat hippocampus and DRG | Neuroprotective effects on cerebral ischemia induced-oxidative stress | [195] |
| TRPV1 | Vitamin C, E and NAC | Rats | Treatment of cortical neuronal death via TRPV1 | [196] |
| TRPV1 | Curcumin | Rats | Inhibits TRPV1 mediated pain hypersensitivity | [197] |
| TRPV1 | Selenium | Rats | Inhibits memory impairments | [117] |
| TRPV1 | Hypericum perforatum | Rats | Inhibiting SCI | [198] |
| TRPV1 | Melatonin and selenium | Rat hippocampus and DRG | Neuroprotection in diabetic rats | [19] |
| TRPV1 | Melatonin and selenium | MCF-7 cells | Inhibiting TRPV1 and enhanced chemotherapeutic action | [115, 116] |
| TRPV4 | Trolox and MitoE | Rats | Oxidative stress-induced neuronal and astrocytic damage attenuated in hippocampal slices | [186] |
| TRPV4 | Trolox | Rat hippocampal slices | Amyloid β induced neuronal and astrocytic damage attenuated | [143] |
Table 2.
Effects of different forms of selenium on possible therapeutic targets in different animal and human experimental pain model studies.
| Model | Form of Se | Effect | Pathway | Refs. |
|---|---|---|---|---|
| Human | Selenium | Antioxidant | Inflammatory state | [160] |
| Human | Selenium | Antioxidant | Reduce pain in patients suffering from chronic pancreatitis. | [162] |
| Mice | 1,2-bis-(4-methoxyphenylselanyl) styrene | Anti-nociceptive and anti-inflammatory | Regulation of serotoninergic system | [154] |
| Mice | 3-(4-chlorophenylselanyl)-1-methyl-1H-indole | Anti-nociceptive and anti-inflammatory | Involvement of neurotransmitters systems | [155] |
| Mice | (OMePhSe)2 | Anti-nociceptive action and incorporation | Restored the changes in inflammatory and apoptotic protein contents | [156] |
| Mice | (OMePhSe)2 | Anti-nociceptive action | Thermal stability and the anti-nociceptive action | [157] |
| Mice | (m-CF3-PhSe)2 | Anti-nociceptive and antidepressant-like actions | Inflammation-induced depression and chronic pain | [161] |
| Rat | Selenium nanoparticles | Anti-inflammatory | Radioprotective effect by increasing antioxidant activity | [153] |
| Rat | Selenium | Anti-nociceptive and anti-inflammatory | Pharmacological targets in the treatment of FM-induced apoptosis and peripheral pain | [37] |
| Rat | (OMePhSe)2 | Antioxidant | Supplemented diet for antidepressants and analgesics | [158] |
This review summarizes two main topics: (1) the role of TRP channels expressed by primary sensory neurons in the development of pain associated with peripheral pathologies and the possible approaches to translate preclinical data to the development of effective new analgesics and (2) the potential role of selenium as a novel inhibitor of TRP cation channels and its potential for treating neurological diseases and peripheral neuropathic pain.
2. Role of calcium and oxidative stress in pain perception
Anions or cations intervene in various cellular metabolic processes either directly or indirectly as co-factors of enzymes. The electrical activity of excitable cells leads to cationic mobilization, including that of Ca2+. The cellular microenvironment of neurons is also well organized for cationic mobilization because the difference in Ca2+ concentrations in the extracellular ([Ca2+]e = ~1.2–3.0 mM) and intracellular milieu ([Ca2+]c = ~100 nM) is more than 10,000 fold. Transient increases in [Ca2+]c from 100 nM to µM initiate the physiological pathways of neuronal response. The main sources of Ca2+ are extracellular fluid and Ca2+ storage organelle, such as the endoplasmic reticulum and the mitochondria [41]. There are many types of calcium-permeable channels/pumps in the plasma membrane and intracellular organelle receptors that regulate intracellular Ca2+ signals within microseconds to mediate physiological reactions. Ca2+ entry from the extracellular space is mediated by several kinds of channels, such as VGCCs, store-operated calcium entry channels, ionotropic N-methyl-D-aspartate (NMDA) receptors, ligand-gated calcium channels, and TRP channels. Ca2+ release from intracellular stores mainly occurs by stimulation of ryanodine receptors (RyR) and inositol triphosphate receptors (IP3R). However, prolonged high levels of [Ca2+]c threaten cellular integrity by activation of cysteine-dependent aspartate proteases (caspases) [42-44]. Thus, Ca2+ store organelle also sequesters Ca2+ to restore cytosolic Ca2+ levels to the normal cytosolic values. However, Ca2+ overloading in the mitochondria disturbs their normal function by depolarizing the intermembrane space and inducing apoptotic cascades via the release of cytochrome c and by generating ROS from the respiratory chain [45, 46].
Neuronal cells are sensitive to abnormal ROS production because of the nervous system’s lipid-rich environment. ROS and reactive nitrogen species (RNS) indirectly trigger IP3R activation via phospholipase C (PLC)-mediated IP3 production, causing Ca2+ release [47]. ROS also directly induce Ca2+ influx into the cytosol by activation of ROS-sensitive Ca2+ permeable channels in the cell membrane. This positive feedback mechanism, therefore, creates a cycle that gradually increases Ca2+ concentration, leading to cell death [48]. Moreover, aged neurons have much concentrated resting Ca2+ levels [49].
Intracellular Ca2+ levels are regulated by several Ca2+ channels in the cell membrane and by Ca2+ release from the intracellular organelle. Two major intracellular stores of Ca2+ are present, the endoplasmic reticulum and the mitochondria. VGCCs are localized in the outer membrane of the mitochondria, allowing the diffusion of Ca2+ from the cytosol to the intermembrane space. The mitochondrial Ca2+ uniporter is an essential channel for the transport of Ca2+ through the inner membrane into the matrix by using an electrochemical gradient. The sodium-calcium exchanger transports one Ca2+ and three Na+ from the matrix to the intermembrane space. To accumulate large amounts of Ca2+ for prolonged durations, calcium phosphate precipitations form near the cristae and then migrate to free zones in the matrix of the mitochondria. However, excessive deposition of Ca2+ causes mitochondrial depolarization, an increase in ROS production, mitochondrial cytochrome c and nucleic acid release, cellular swelling, and, finally, apoptotic cell death. The mitochondria not only have a role in Ca2+ homeostasis, but they also need Ca2+ for intrinsic functions, including ATP synthesis, tricarboxylate cycle function, and generation of reducing agents, such as NADH and FADH2 [50, 51].
The mitochondria generate endogenous ROS following mitochondrial depolarization that is triggered by Ca2+ overload. The ROS-related activation of PLC pathways can lead to IP3R-mediated Ca2+ release from the endoplasmic reticulum. Therefore, the mitochondria and the endoplasmic reticulum modulate intracellular calcium signaling [52].
Interestingly, mitochondrial functions and Ca2+ signaling are closely related processes, as Ca2+ in one store acts as a messenger between the cytosol and its mitochondrial units to regulate the energy requirements of neurons.
Ca2+ has also been presented as a key regulator of cell survival, but it can cause apoptosis in response to a number of pathological conditions. In addition, the mitochondria act as Ca2+ buffers by sequestering excess Ca2+ from the cytosol [53]. Blockade of Ca2+ influx into the intracellular organelle, such as the endoplasmic reticulum, the mitochondria, and the cytosol, can effectively produce a rapid, simultaneous, and reversible cessation of movements. Ca2+ overloading in the mitochondria induces an apoptotic program by stimulating the release of apoptosis-promoting factors, such as cytochrome c, and by generating ROS as a result of respiratory chain damage. Mitochondrial function is also essential for neuronal survival because neurons critically depend on ATP synthesis generated by mitochondrial oxidative phosphorylation [53]. In fact, the release of Ca2+ from endoplasmic reticulum stores by IP3 receptors and the entry of Ca2+ through TRP channels in neurons have been implicated in multiple models of apoptosis as being directly responsible for mitochondrial Ca2+ overload [33]. An increase in apoptotic death through an increase in mitochondrial membrane potential, as well as a decrease in ATP synthesis and mitochondrial Bcl-2 protein production through activation of the TRPV1 channel, was reported in pancreatic neuroendocrine tumor cells by capsaicin [54]. However, a decrease in mitochondrial membrane depolarization through inhibition of Ca2+ entry was reported in SH-SY5Y neuroblastoma cells by antioxidant (curcumin) treatment [55].
3. Selenium
An essential trace element for vertebrates, selenium was first reported in 1817 by Berzelius [56]. It exists in two natural forms in nature either inorganic (selenite, selenide, and selenate) or organic (selenocysteine, selenomethionine, and Se-methylselenocristeine) [57]. The molecules of selenium act as co-factors for different enzymes, such as GPx, thioredoxin reductase (TrxRs), and iodothyronine deiodinases [38, 58]. It attracted much attention because of its role in preventing various diseases [59]. The useful properties of selenium are attributed to its ability to be incorporated into various proteins, with 25 selenoproteins formed by selenocysteines. Although high concentrations of selenium have cytotoxic effects, low-dose selenium can scavenge ROS and reduce pain [60, 61].
Selenium forms several allotropes (red, black, and gray) that interconvert with temperature changes. It has two opposite physiological features. Concentration-dependent selenium can exert therapeutic or toxic effects. High doses of selenium promote the proliferation of cancer cells and have neurotoxic effects, although low and intermediate doses inhibit cancer cell proliferation and have therapeutic effects on neurological diseases [60, 61]. There are several antioxidant nanoparticles, such as selenium nanoparticles. The metabolism of selenium nanoparticles through the up-regulation of antioxidant enzymes but down-regulation of ROS products in cells and neurons has been summarized in recent papers [60, 61].
4. TRP channels
As mentioned above, intracellular Ca2+ signals are controlled by ion pumps and cation channels, including the superfamily members of TRP channels that are responsible for non-specific cationic influx into the cytosol. The trp gene was first identified in the eye cells of Drosophila and has since been also found in vertebrates. There are six subfamilies (Ankyrin: TRPA, Canonical: TRPC, Melastatin: TRPM, Mucolipin: TRPML, Polycystin: TRPP, and Vanilloid: TRPV) and 28 subtypes of these channels in mammals, with 27 of these being functionally expressed in humans, except for the TRPC2 pseudogene. The TRP channels have six transmembrane segments with a binding loop S5 to S6 and have a tetrameric structure. The majority of TRP channel subtypes, excluding TRPM4 and TRPM5, are permeable to Ca2+ and are widely expressed in the brain and sensorial tissues, such as those in the hippocampus, cerebral cortex, DRG, and trigeminal ganglion (TG) neurons [62-64]. Several reports have proposed that TRP channel-mediated Ca2+ signaling mediates pain sensation. For example, Kahya et al. [19] suggested the role of TRP channels in diabetic neuropathic pain via overloading of cytosolic Ca2+ levels, in which hyperglycemia and diabetes stimulate Ca2+ influx into the cytosol through TRP channels by elevation of ROS levels. Because of the disruption of intracellular Ca2+ homeostasis, depolarization of mitochondrial membranes and enhanced ROS production result in the activation of oxidative stress-sensitive TRP channels and play an important role in the pathophysiology of diabetic neuropathy. We also emphasized the role of TRP channels in the transduction of diabetic pain via sensory neurons that may be mediated by the TRPC, TRPV, and TRPM subfamilies [34].
TRP channel expression levels were increased in DRG and TG neurons by chemotherapeutic agents, such as cisplatin, oxaliplatin, and paclitaxel [65]. Moreover, the antagonists of these TRP channels attenuated chemotherapeutic-induced mitochondrial oxidative stress, inflammation, cold allodynia, and hyperalgesia. There are high expression levels of seven TRP superfamily members in pain-related neurons (e.g., DRG and TG), and these are TRPA1, TRPM3, TRPM8, TRPV1, TRPV2, TRPV3, and TRPV4. Moreover, Mori et al. [35] reported that most of these channels are activated by oxidative stress. The neuroprotective effects of several antioxidants, including selenium, on different neurological diseases are well understood because of their ROS scavenging activity and antioxidant capacity [66-69]. Furthermore, oxidative stress-sensitive TRP channels can be inhibited by antioxidant treatment, and this contributes to cell survival; mitochondrial functions likewise reduce apoptotic cascades [70-76]. To understand the role of these seven types of TRP channels in the neurobiology of pain, understanding first their structural and functional differences, as well as their activation and inactivation mechanisms, is necessary.
5. Antioxidants and TRP channels in pain
5.1. TRPA1
The TRPA1 channel is a member of the ankyrin subfamily and is localized mainly in DRG and TG neurons, nodose ganglia, cerebral cortex, hippocampus, and non-excitable tissues. A unique feature of the ankyrin subfamily is that repeats of cysteine-rich ankyrin regions are located close to the N-terminal domain of TRPA1 channels, suggesting the sensor role of environmental irritants and pungent stimuli [77, 78]. TRPA1 channels are gated by cool temperatures (<18°C) and some environmental irritants, such as cinnamaldehyde, mustard oil, allicin, icilin, carvacrol, eugenol, and gingerol [79]. Oxidative stressors can also activate TRPA1 channels [80], and the inhibition of TRPA1 channels can be by antagonists (for example, by high concentrations of camphor, mecamylamine, AP-18, HC-030031, and compound 31 from Novartis), as well as by antioxidants (such as resveratrol) [81].
TRPA1 channels are activated by neuropathic pain, nociception, allodynia, and cold hyperalgesia [82, 83]. The role of TRPA1 in the induction of pain is well known, for example, in diabetic neuropathic pain [83], inflammatory pain [84], mechanical allodynia [85], and chemotherapeutic agent-induced pain [86]. However, reports on the role of antioxidants in the inhibition or activation of TRPA1 channels in pain are limited, with no reports on selenium and TRPA1 activity. A recent study by Stenger et al. [87] found that N-acetyl-cysteine (NAC, but not GSH) inhibits TRPA1-dependent calcium influx in transfected HEK-293 cells, whereas Yu et al. [83] demonstrated that the antioxidant resveratrol (and other stilbenoids) inhibits TRPA1-related currents and reduces pain-related behaviors in rats [83]. In addition, Yazgan and Nazıroğlu [88] reported that 17-β estradiol, tamoxifen, and raloxifene also cause the inhibition of TRPA1 and pain [88].
5.2. TRPM8
The TRPM8 channel is another member of the melastatin subfamily and is widely expressed in primary nociceptive fiber (Aδ and C) sensory neurons in the skin and mucosa, urogenital system, lung epithelial cells, and myocytes [89]. TRPM8 cationic currents can be induced by low temperatures (T<22°C –26°C) and by agonists, such as menthol, eucalyptol, geraniol, icilin, and linalool [90]. Non-selective TRPM8 cationic currents can be inhibited by capsazepine, clotrimazole, econazole, AMG2850, and N-(4-tertiarybutylphenyl)-4-(3-chloropyridin-2-yl) tetrahydropyrazine-1(2H)-carbox-amide (BCTC). It is important to note that capsazepine and BCTC were first described as antagonists of TRPV1 channels and later as inhibitors of TRPM8 [91]. TRPM8 channels respond to cold temperatures, and inhibition of these channel currents may provide cold hyperalgesia and allodynia [92, 93], with reports of cold activation of TRPM8 channels in DRG neurons [94]. The role of TRPM8 in the transduction of pain has been known for a long time, for example, in cold hyperalgesia [95, 96], chemotherapeutic-induced hypersensitivity [97, 98], inflammatory pain [99], migraine [100], and mechanical allodynia [101]. However, studies on the role of antioxidants in the inhibition or activation of TRPM8 channels in pain and on the effects of selenium on TRPM8 activity are limited.
5.3. TRPV1
TRPV1 cation channels are abundantly expressed in the TG, DRG, hypothalamus, cerebellum, cerebral cortex, hippocampus, thalamus, substantia nigra, and peptidergic neurons which are responsible for painful sensation and neurogenic pain [63]. Similar to TRPA1, TRPV1 channels also contain short ankyrin repeats in their structures [102]. TRPV1 channels are activated by higher temperatures (T>43°C), chemicals, such as capsaicin and resiniferatoxin (a vanilloid), fatty acid derives, including anandamide, N-acylethanolamines, and N-arachidonoyl dopamine, the environmental irritants camphor and allicin, and oxidative stress [103, 104]. TRPV1 channels can be gated by an intracellular acidic pH (<6.5) and some lipid derivatives [105]. TRPV1 cationic currents are inhibited by antagonists, such as capsazepine, 5′-iodoresiniferatoxin, ruthenium red, and antioxidant administration [19, 106]. The relationship between TRPV1 channels and nociception or neuropathic pain was confirmed in knock-out animal studies [107]. Another study on pain concluded that eriodictyol reduces nociception by inhibiting TRPV1 channels and protecting cells from oxidative stress [108].
The role of TRPV1 channels in pain and heat sensation has been known for some time, with studies on diabetic neuropathic pain [19, 109, 110], inflammatory pain [111, 112], hyperalgesia [113], and cancer-induced neuropathy [114]. However, research on the role of antioxidants in the inhibition or activation of TRPV1 channels in pain induction remains limited. In a cancer cell line (MCF-7), both melatonin and selenium inhibit cell proliferation caused by the anticancer drugs doxorubicin and cisplatin by modulating to TRPV1 channels [115, 116]. Balaban et al. [117] suggested that selenium modulates TRPV1-mediated calcium signaling, inhibits ROS production by enhancing GPx activity, and reduces mitochondrial depolarization in the neurons of dementia-induced rat [117].
We have previously reported that NAC and selenium in traumatic brain injury have protective effects on apoptosis, oxidative stress, and Ca2+ influx via inhibition of TRPV1 channel activation in hippocampal neurons. The effects of NAC are greater than those of selenium [118]. Studies by Yüksel et al. [37] investigated the effects of selenium on apoptosis, oxidative stress, and Ca2+ influx mediated by TRPM2 and TRPV1 cation channels in DRG and sciatic nerve neurons in a rat model of fibromyalgia. They reported that the pain associated with fibromyalgia is related to the activation of TRPM2 and TRPV1 channels induced by mitochondrial ROS production and apoptosis in the DRG and sciatic neurons. They also observed that the pain, oxidative damage, and apoptotic effects of fibromyalgia are minimized by the blockage of TRPM2 and TRPV1 in the neurons by selenium [37].
5.4. TRPV2
TRPV2 cation channels are members of the vanilloid subfamily. Although the molecular structure of these channels has the features of the vanilloid type, they are insensitive to vanilloids, such as capsaicin. TRPV2 channels are also different from TRPV1 channels in their ankyrin repeats and responses to temperature. Mammalian TRPV2 channels can be activated by temperatures higher than 52°C and by chemicals, such as aminoethoxydiphenyl borate (2-APB) and carvacrol [119]. Inhibition of TRPV2 channels can be achieved by antagonists, such as ruthenium red, transilat, amiloride, lanthanide ions (La3+), and SKF96365 [62]. The expression pattern of TRPV2 channels is mainly in the brain, vascular smooth muscle cells, intestines, macrophages, neurons, neuroendocrine cells, key cells of innate immunity, and several types of cancers [119, 120]. Axelsson et al. [121] investigated the expression of TRPV2 channels in primary afferent nociceptors and keratinocytes, and they reported the colocalization of TRPV2 channels, CGRP, and substance P sensory neuropeptides in C-fiber primary afferents [121]. Mihara et al. [122] demonstrated that TRPV2 causes NO production through Ca2+ influx in myenteric neurons, and they suggested that inhibition of the TRPV2 channel could be a therapeutic target in NO-related pathologies [122]. Another study [123] found that probenecid, a uricosuric drug, activates TRPV2 channels in sensory neurons, suggesting that this drug could be a useful agent for TRPV2-mediated pain. Experiments by Park et al. [124] investigated TRPV2 and TRPV3 polymorphisms in Korean patients with fibromyalgia and indicated that functionally expressed TRPV2 channels are not directly related to fibromyalgia but rather to the polymorphisms of TRPV2 channels [124]. More studies on the effects of antioxidants and oxidative stress on TRPV2 channels are needed.
5.5. TRPV3
TRPV3 cation channels are expressed in neuronal and non-neuronal tissues, including the keratinocytes, brain, testis [125], and skin and dermatological tissues [126]. TRPV3 channels are activated by 2-APB, carvacarol (from oregano), camphor, thyme, and eugenol. The activation threshold of these channels is between 30°C and 39°C, suggesting the role of TRPV3 channels at physiological temperatures [127]. Park et al. [124] reported that although TRPV3 channels are not directly related to fibromyalgia, the polymorphisms of these channels are associated with the severity of fibromyalgia. In a related study, Huang et al. [128] proposed that TRPV3 channels in keratinocytes mediate heat-induced prostaglandin release and trigger acute nociception and hyperalgesia in keratinocyte sensory function. However, a recent finding from this laboratory proposed that C57BL6 mice lacking TRPV3 channels have a similar heat sensitivity as the control mice [129]. Carreño et al. [130] investigated the single nucleotide polymorphisms of the TRPV1 and TRPV3 channels in the migraine susceptibility of a Spanish population and found that the TRPV1 and TRPV3 channels are important in genetic susceptibility to migraine [130]. Brederson et al. [131] reviewed the specific antagonists of TRPV3 channels used to reduce pain sensation, thermal hyperalgesia, and burn or inflammation in animal studies [131].
5.6. TRPV4
TRPV4 non-selective cation channels are also members of the vanilloid subfamily; they have at least three subunits of ankyrin repeats in the N-terminal region of the channel structure. TRPV4 channels are widely expressed in the brain, TG and DRG neurons, salivary glands, and in non-excitable tissues, including those in the liver, lungs, and trachea and in the basolateral membranes of the kidney epithelium [132]. TRPV4 channels are gated by temperature (25°C to 34°C), extracellular osmotic changes and alterations of pH (<6), and mechanical/chemical stimuli [132-134]. In addition, the activation of TRPV4 in the hippocampus, astrocytes, and DRG by extracellular H2O2 has been reported, although the channel is inhibited in the neurons by antioxidant treatments (Trolox, MitoE, and reduced glutathione, GSH). The prolonged activation of TRPV4 channels in neurons, such as those in the hippocampus and astrocytes, leads to Ca2+ overload, causing oxidative stress and cell injury [109]. Increased Ca2+ causes oxidative stress through multiple mechanisms, and it has been shown to be linked to cell injury induced by different insults [135]. There are a limited number of antagonists for TRPV4 cationic currents, but similar to other members of the vanilloid subfamily, ruthenium red is used to inactivate these channels. Trivalent cations, such as Gd3+ and La3+, also inhibit TRPV4 channels [136]. The functional importance of TRPV4 channels is directly related to protease activated receptor 2 (PAR2), which mediates inflammatory and neurogenic pain. Inhibition of TRPV4 activation reduces PAR2-induced neurogenic inflammation in rat primary nociceptive neurons [136-138]. Alessandri-Haber et al. [139] assessed the role of TRPV4 in chemotherapeutic-induced neuropathic pain in the peripheral endings of the saphenous nerve and showed that taxol induces peripheral neuropathy and mechanical hyperalgesia, and that nociception is decreased by antisense treatment to TRPV4 channels. Materazzi et al. [140] reported that TRPV4 activation causes ROS-related neuropeptide release in sensory nerve endings in paclitaxel-induced neuropathy. Liu et al. [141] investigated the inhibition of TRPV4 channel expression by RNAi in DRG neurons and observed that substance P is co-expressed with TRPV4 channels in rat DRG neurons, as is the case also for TRPV1. Recent findings show that TRPV4-mediated Ca2+ influx can also be triggered by ROS and that TEMPOL or a mitochondrial-specific antioxidant (MitoQ) reverses excessive ROS production and Ca2+ influx [142]. Bai and Lipski investigated the potential role of TRPM2 and TRPV4 channels in oxidative stress-induced neuronal death in hippocampal tissue cultures and confirmed that amyloid β-induced neuronal damage is associated with oxidative stress and partly involved in the stress-sensitive TRPM2 and TRPV4 channels, as treatment with an antioxidant (Trolox) increased cell survival [143].
Inhibition of TRPV4 channels by RNAi or channel blockers eliminated acrolein-induced oxidative and cell injury in mouse cultured urothelial cells, whereas treatment with the antioxidant NAC had similar effects as TRPV4 antagonists in reducing Ca2+ influx [144]. Some reports also have contrasting findings, as in the studies by Ma et al., who found that the plant flavone and antioxidant apigenin trigger TRPV4-mediated Ca2+ signaling in transfected HEK-293 cells [145].
6. Selenium, calcium signaling, and TRP channels
The dynamic and narrow concentration range of Ca2+ intracellular signaling requires well-coordinated regulatory mechanisms (Fig. 1). Mitochondrial function is essential for neuronal survival because neurons critically depend on ATP synthesis generated by mitochondrial oxidative phosphorylation [13, 50, 146, 147]. Zeng et al. [148] reported that extracellular selenium application can inhibit TRPM2 cation channel currents induced by intracellular ADPR. This shows the dual role of selenium in TRP channels—Se acts as a potential inhibitor in TRPM2 channels via the extracellular site of the channel, and it also demonstrates its antioxidant ability via GPx; it helps reduce oxidative stress from the cytosol and decreases ROS-sensitive TRP channel gating induced by an intracellular oxidative status.
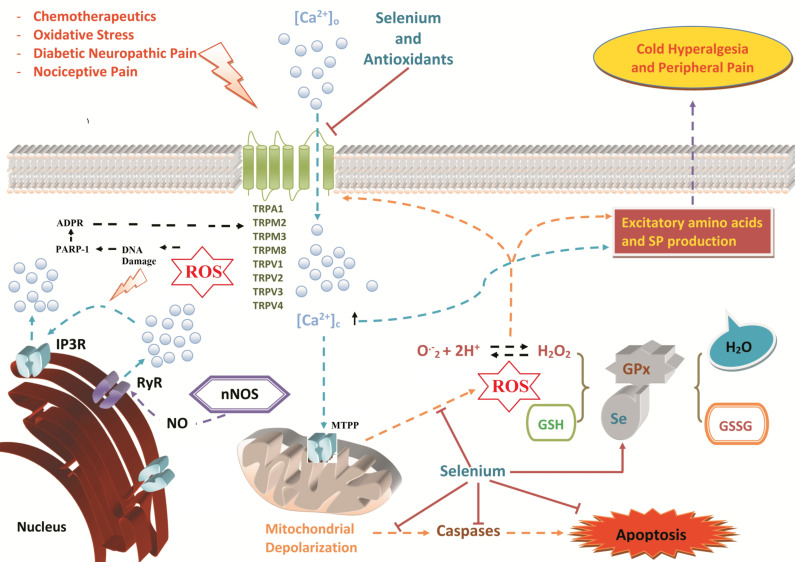
Fig. (1).
Role of selenium supplementation in TRP channel-mediated pathological mechanisms in neurons. Neural tissues are very vulnerable to oxidative stress-dependent cell death because of their lipid-rich structures. Many TRP channels (TRPA1, TRPC5, TRPM2, and TRPV1) are expressed in neuronal tissues and have unique functional and structural properties [3, 199]. Other neural TRP channels (TRPM3, TRPM8, TRPV2, TRPV3, and TRPV4) act as thermal sensors to hot or cold temperature. Chemotherapeutic agents, oxidative stress, diabetic neuropathic pain, and inflammatory and nociceptive pain may stimulate TRP channels to rapidly increase cytosolic Ca2+ concentrations and trigger calcium-activated calcium channel (CACC) entry. Overloading of cytosolic Ca2+ concentrations results in depolarization of the mitochondria via the opening of mitochondria transition permeability pores (MTPP). As a result of mitochondrial dysfunction, either caspase activity may result in apoptosis, or ROS overproduction activates oxidative stress-sensitive TRP channels. Excessive Ca2+ entry and ROS stimulate the release of pain mediators, such as substance P and other excitatory amino acids, causing PKC activation, NO synthesis, RyR activation and IP3 receptor activation (IP3R) [13]. TRPM2 channel is activated in the peripheral neurons by ROS-induced DNA damage and ADP-ribose (ADPR) production through PARP-1 enzyme activation. Several reports have concluded that selenium (Se) inhibits the activation of TRP channels and Ca2+ signaling, as well as decreases mitochondrial depolarization and ROS overproduction. Supplementation with selenium prevents apoptotic cell death by reducing caspase 3 and 9 activities. Selenium is also a cofactor for the antioxidant GPx in reducing excessive ROS production in cytosolic compartments. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Mitochondrial depolarization activity depends on Ca2+ and is fueled by Ca2+ entry from the extracellular space via TRP channels when triggered by neuronal activity [13]. Overloaded Ca2+ in the cytosol induces apoptosis if the accumulation will not be buffered by the mitochondria [13, 44, 51]. Growing evidence indicates that the production of excessive ROS and the stimulation of apoptotic pathways, including caspase activities, such as caspase 3 and 9, are increased in the hippocampus and DRG by increased mitochondrial membrane depolarization [63, 88] because the neurons have high oxygen consumption and polyunsaturated fatty acid content. Caspase 3 is well known to be an important indicator of the extrinsic and intrinsic pathways of apoptosis. Yousuf et al. [149] examined the neuroprotective actions of selenium in cerebral ischemia-induced damage in rat hippocampus and reported that a low dose of intraperitoneal sodium selenite (0.1 mg/kg each day for a week) reduces intracellular Ca2+ concentrations, caspase 3 activity, and mitochondrial dysfunction. Uğuz and Nazıroğlu [150] reported that selenium reduces oxidative stress-induced activation of DRG neurons by decreasing oxidative stress-induced apoptotic pathways and cytosolic calcium levels. Similarly, Kahya et al. [150] reported the protective effects of selenium on caspase 3 and caspase 9 activity in diabetes-induced rats.
Reeves et al. [151] investigated the overexpression of selenium-containing protein M (Selenoprotein M) in murine HT22 hippocampal neurons, primary cultured neuronal cells, and cerebellar C8-D1A astrocytes. The increased expression of selenoprotein M reduces ROS and apoptotic parameters, although shRNA-silenced selenoprotein M increases H2O2-induced apoptosis and ROS production. Selenoprotein M overexpression also attenuates the Ca2+ influx evoked by H2O2. Disturbances in Ca2+ mobilization have an important role in the etiology of pain. Ca2+ overload induces pain through substance P (and CGRP) production in neurons through direct channel activation or excessive ROS production and induction of apoptosis through depolarization of the mitochondria [65].
7. Selenium and pain
Immune cell activation occurs in neurodegenerative diseases in the CNS. Inflammation in the CNS causes microglial and macrophage SN. Dominiak et al. [152] investigated the protective effect of Selol against inflammation in rat brain and found that Selol prevents LPS-mediated (100 μg/kg) inflammation in the CNS by regulating antioxidant levels. The authors suggested that Selol could be used to decrease inflammation in neurodegenerative diseases. Uğuz et al. [150] investigated H2O2-induced oxidative stress in the DRG sensory neurons of rats by monitoring lipid peroxidation, cytosolic Ca2+ release, GPx, glutathione (GSH), apoptosis, and cell viability (MTT assay). They proposed that selenium acts as a ROS scavenger in DRG neurons in oxidative stress-induced neurological diseases [150]. El-Ghazaly et al. [153] examined the limitations of selenium because of bioavailability and toxicity in a study in which rats were exposed to radiation and administered nano-Se orally (2.55 mg/kg). They demonstrated the positive effects of nano-Se on inflammation induced in irradiated rats. Anversa et al. [154] suggested the anti-nociceptive and anti-inflammatory effects of selenium-containing compound [1,2-bis-(4-methoxyphenylselanyl), an organic Se] styrene, through regulation of the serotoninergic system in mice. Similarly, Birmann et al. [155] suggested a selenium containing compound (3-(4-chlorophenylselanyl)-1-methyl-1H-indole) in the treatment of pain and inflammation. p,p’-methoxyl-diphenyl diselenide (OMePhSe2) contains a selenium molecule, and its anti-nociceptive action in the model of neuropathic pain was reported by Marcondes et al. [156]. Similarly, Sari et al. [157] observed its modulator role on anti-nociception in Swiss mice [157]. Oliveira et al. [158] reported the protective effects of a MeOPhSe2-supplemented diet on depression and pain comorbidity in rats [158]. Mansel et al. [159] examined the protective effects of selenium on pain in women with fibrocystic changes in the breast. Yüksel et al. [37] evaluated the protective effects of selenium on ROS and of Ca2+ influx on fibromyalgia in the DRG and sciatic nerve induced rat fibromyalgia model, as well as examined selenium mediation by inhibition of TRP channels (TRPM2 and TRPV1). Barros-Neto et al. [160] investigated the relationship between chronic musculoskeletal pain and malnutrition and reported that myofascial pain is associated with changes in intracellular zinc and selenium stores caused by inadequate food intake.
Chronic pain and depression are common in many patients, leading to increased use of antidepressants and pain relievers. A study conducted by Brüning et al. [161] examined the effects of organoselenium (m-CF3-PhSe)2 on the immune system and the relationship between chronic pain and depression. They reported that m-CF3-PhSe2 decreases the levels of pro-inflammatory mediators and the damage caused by partial nerve ligation [161]. Patients with chronic pancreatitis have abdominal pain that is usually resistant to analgesic strategies, with some studies suggesting that the pain of chronic pancreatitis could be attributed to ROS-induced pancreatic damage, opening the possibility for the use of selenium as an antioxidant trace element to improve the quality of life and reduce pain in patients with chronic pancreatitis [162].
8. Selenium and TRP channels in diabetes-induced neuropathic pain
Diabetes is a metabolic disease affecting about 100 million people worldwide. Peripheral diabetic neuropathy occurs in about 60% of these patients, and diabetic neuropathy emerges as a complication of the disease. Oxidative stress and inflammation have a main role in diabetic neuropathic pain generation [13, 163-165]. Inhibition of ROS generation may contribute to the reduction of diabetic neuropathy [166]. Antioxidant applications can also be useful for the attenuation of diabetes and hyperglycemia-induced neuropathy. Different studies have evaluated the effects of alpha lipoic acid administration on diabetic neuropathy, and researchers have found significant results; a correlation exists between alpha lipoic acid administration and the reduction of diabetic neuropathy in patients with diabetes [167, 168]. There are several reports about the use of antioxidant treatments, such as those involving vitamin E [169], coenzyme Q10 [170], and the selenoprotein Selol [171], in limiting diabetic neuropathy both in patients and in experimental studies.
Selenium deficiency and excessive Ca2+ entry through activation of TRP channels have important roles in the etiology of diabetic neuropathic pain. There are several studies of TRP channel function in diabetic neuropathic pain, with a focus on the TRPC, TRPM, and TRPV channels. In a post-mortem study, researchers found that TRP channel expression levels may change in diabetic neuropathic patients compared with normal human tissues [172]. The regulation of TRP channels is gaining prominence as a therapeutic target for neuropathic pain in diabetic patients [165]. TRPV1 channel activation without changes in expression levels occurs in native rat sensory neurons and HEK293 cells expressing TRPV1 (but not in TRPA1-deficient mice) stimulated by glucose and hypoxic conditions. Alkaline pH causes pain and TRPA1 activation [173]. Wei et al. [174] investigated the endogenous compounds affecting TRPA1 ion channel activation by mechanical hypersensitivity, a symptom of diabetes mellitus. They noted that the progress of hypersensitivity is prevented in streptozotocin-induced diabetic rats treated with a TRPA1-antagonist [174].
A limited number of studies have investigated neuropathic pain and TRP channel regulation by selenium and other antioxidant applications. Sözbir and Naziroglu [75] found that N-acetyl cysteine treatment has the regulatory effects of oxidative stress-induced TRPM2 channel activation and increased antioxidant parameters in rat brain. They also suggested that modulation of TRPM2 via the ROS-dependent pathway can be a therapeutic target for the inhibition of diabetic neuropathy. In another study, Kahya et al. [19] proposed that the TRPM2 and TRPV1 channels are involved in the ROS-dependent mechanisms of diabetic neuropathic pain induction and that melatonin and selenium treatments can be a therapeutic approach to decrease peripheral pain.
9. Selenium and chemotherapeutic agent-induced neuropathic pain
Anti-cancer drugs induce the activation of the cell membrane-embedded cation channels of DRG and dorsal horn neurons, including the Ca2+, Na+, and K+ channels, and NMDA receptors, and they change cytosolic ion concentration, especially [Ca2+]c, which initiates other alterations to induce neuropathic pain [175]. It is well known that a poor antioxidant defense system and elevated oxidative stress arise after drug treatment, contributing to painful neuropathy [176]. Neuropathic pain occurs when pressure is applied to the nerve cells of cancer patients, who describe the symptoms as “a burning or heavy sensation, or numbness along the path of the affected nerve” [177]. The molecular basis of this pain shows the contributory role of TRP channels, especially of the TRPA1, TRPM8, and TRPV1 subtypes [178, 179]. Chiba et al. [180] investigated the role of TRPV1 in peripheral neuropathy after anticancer drug therapy and suggested the role of TRPV1 expression in vincristine-induced pain in peripheral neuropathy. Similarly, Chukyo et al. [181] reported that increases in TRPA1, TRPM8, and TRPV1 expression in DRG neurons may contribute to the development of oxaliplatin-induced neuropathic pain in the somatosensory system. Cisplatin and selenium synergistically interact in the TRPV1 channel function in a breast cancer cell line (MCF-7), suggesting that a combination of these drugs could have a greater anticancer effect by modulation of TRPV1 [115]. Li et al. [182] showed that the TRPA1 and oxidative stress signaling pathway has an important role in oxaliplatin-induced pain, and they also suggested that blocking miRNA-155 increases NADPH oxidase, resulting in oxidative stress and TRPA1 expression in rat dorsal horn neurons. Melatonin has powerful antioxidant properties, and several reports also found that it changes TRP channel gating. Torsney et al. [183] developed a rat model of paclitaxel-induced peripheral neuropathy and showed that melatonin can contribute to restricting mitochondrial dysfunction; because of antioxidant capacity, the development of neuropathy is limited. Zhang et al. [184] demonstrated that using an electroacupuncture antioxidant system could be activated and that paclitaxel-induced neuropathic pain decreases in the DRG neurons of rats. Collectively, in terms of the regulatory effect on TRP channels, selenium treatment may be useful in enhancing the antioxidative system and reducing neuropathic pain induced by chemotherapeutic agents. Further studies are needed to understand the effects of selenium supplementation on chemotherapeutic-induced neuropathic pain models.
Conclusion and future research directions
Neuropathic pain is different from general pain and is described as a shooting or burning pain. The molecular pathways of DRG neurons have a major role in the induction of neuropathic pain. The neurons in the DRG and CNS are affected by complications of alcoholism, chemotherapy treatment, and diabetes. The treatment of neuropathic pain includes the use of anticonvulsant and antidepressant drugs, but their success rate in pain treatment is poor. However, accumulating evidence indicates the important roles of oxidative stress and overload Ca2+ in neurons in human and rodents with neuropathic pain.
ROS production occurs during physiological functions, such as phagocytosis and mitochondrial functions. The ROS in neurons and the brain are controlled by enzymatic and non-enzymatic antioxidants. A co-factor of GPx, selenium is believed to play a critical role in protecting neurons from hazardous mitochondrial and inflammation-induced ROS production. Increasing evidence implicates the protective role of selenium or selenium-containing molecules, such as OMePhSe2 and 1,2-bis-(4-methoxyphenylselanyl), in the inhibition of excessive ROS production and overload Ca2+ entry in the etiology of several neuronal diseases, including inflammatory pain, hypersensitivity, allodynia, diabetic neuropathic pain, and nociceptive pain.
The expression level of TRP channels varies in tissues according to their functions in these tissues. For example, TRPM2 channels are mainly responsible for the phagocytic activity and are highly expressed in phagocytic cells. The high expression levels of seven TRP channels (TRPA1, TRPM3, TRPM8, TRPV1, TRPV2, TRPV3, and TRPV4) in the DRG and TG are mainly responsible for mediating neuropathic pain. Some TRP channels, such as TRPA1 and TRPV1, are activated by oxidative stress. Low selenium levels can be found in the plasma of patients with neuropathic pain and neurological diseases. A limited number of studies indicate the protective roles of selenium and its derivatives via regulation of TRP channels in humans and rodents with neurological diseases and pain. However, there are no reports of selenium activity in oxidative stress-dependent activated TRP channels, such as TRPM2 and TRPM7, suggesting the need to further investigate the effects of selenium on other TRP channels involved in peripheral pain models.
High levels of ROS and low levels of antioxidants are known to play a pivotal role in the pathobiology of peripheral pain [185]. As already mentioned, the TRPA1, TRPV1, and TRPV4 channels are activated by several stimuli, including oxidative stress [13, 186]. The involvement of cysteine residues and the antioxidant dithiothreitol in the N domain of TRPA1 has been established in a mass spectrometry study [187]. TRPA1 activation through oxidative modifications of the cysteine residues in the DRG of wild and TRPA1 knockout mice has also been reported [80, 188]. The activation of TRPV1 channels in different cells and neurons by oxidative alterations of multiple extracellular [189] and intracellular sources [190]. Cysteine groups as a source of the thiol redox system act as the main source of different antioxidants, such as GSH and Se–GPx [16, 191]. Therefore, TRPV1 is activated in the DRG [72] of rats by depletion of intracellular GSH, although the channel is inhibited in cells following treatment with thiol redox cycle members, such as GSH, selenium, and NAC [19, 72, 118]. Our recent results indicate the protective role of selenium through inhibition of TRPA1 and TRPV1 in different pain models [19, 37]. Therefore, this corroborates the growing evidence on the potential role of selenium as a modulator of TRP channel activation-induced neuropathic pain in the clinic. The relative contributions of the activation of TRP channels or the inhibition of oxidative stress by selenium and selenoproteins remain unclear, though. This topic should be clarified in future studies.
Acknowledgements
There is no financial disclosure of the current study. Compliance with Ethical Standards.
List of Abbreviations
- [Ca2+]c
Cytosolic free calcium ion
- CACC
Calcium activated calcium channel
- CNS
Central nervous system
- DRG
Dorsal root ganglion neuron
- GPx
Glutathione peroxidase
- GSH
Reduced glutathione
- MTP
Mitochondria transition permeability
- NAC
N-acetyl-cysteine
- ROS
Reactive oxygen species
- SCI
Spinal cord injury
- Se
Selenium
- TBI
Traumatic brain injury
- TRP
Transient receptor potential
- TRPA1
Transient receptor potential ankyrin 1
- TRPM3
Transient receptor potential melastatin 3
- TRPM8
Transient receptor potential melastatin 8
- TRPV1
Transient receptor potential vanilloid 1
- TRPV2
Transient receptor potential vanilloid 2
- TRPV3
Transient receptor potential vanilloid 3
- TRPV4
Transient receptor potential vanilloid 4
- VGCC
Voltage-gated calcium channels
Authors’ contributions
Mustafa Nazıroğlu formulated the present hypothesis. Ahmi Öz and Kenan Yıldızhan were responsible for writing the report. Mustafa Nazıroğlu made critical revisions of the manuscript.
Consent for Publication
Not applicable.
Funding
The study was supported by BSN Health, Analysis and Innovation Ltd. Inc. Teknokent, Isparta, Turkey (Project No: 2018-15).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- 1.Little J.W., Doyle T., Salvemini D. Reactive nitroxidative species and nociceptive processing: determining the roles for nitric oxide, superoxide, and peroxynitrite in pain. Amino Acids. 2012;42(1):75–94. doi: 10.1007/s00726-010-0633-0. [DOI] [PubMed] [Google Scholar]
- 2.Bourinet E., Altier C., Hildebrand M.E., Trang T., Salter M.W., Zamponi G.W. Calcium-permeable ion channels in pain signaling. Physiol. Rev. 2014;94(1):81–140. doi: 10.1152/physrev.00023.2013. [DOI] [PubMed] [Google Scholar]
- 3.Shimizu S., Takahashi N., Mori Y. TRPs as chemosensors (ROS, RNS, RCS, gasotransmitters). Handb. Exp. Pharmacol. 2014;223:767–794. doi: 10.1007/978-3-319-05161-1_3. [DOI] [PubMed] [Google Scholar]
- 4.Ibi M., Matsuno K., Shiba D., Katsuyama M., Iwata K., Kakehi T., Nakagawa T., Sango K., Shirai Y., Yokoyama T., Kaneko S., Saito N., Yabe-Nishimura C. Reactive oxygen species derived from NOX1/NADPH oxidase enhance inflammatory pain. J. Neurosci. 2008;28(38):9486–9494. doi: 10.1523/JNEUROSCI.1857-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Esposito E., Paterniti I., Mazzon E., Bramanti P., Cuzzocrea S. Melatonin reduces hyperalgesia associated with inflammation. J. Pineal Res. 2010;49(4):321–331. doi: 10.1111/j.1600-079X.2010.00796.x. [DOI] [PubMed] [Google Scholar]
- 6.Khattab M.M. TEMPOL, a membrane-permeable radical scavenger, attenuates peroxynitrite- and superoxide anion-enhanced carrageenan-induced paw edema and hyperalgesia: a key role for superoxide anion. Eur. J. Pharmacol. 2006;548(1-3):167–173. doi: 10.1016/j.ejphar.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 7.Kallenborn-Gerhardt W., Lu R., Syhr K.M., Heidler J., von Melchner H., Geisslinger G., Bangsow T., Schmidtko A. Antioxidant activity of sestrin 2 controls neuropathic pain after peripheral nerve injury. Antioxid. Redox Signal. 2013;19(17):2013–2023. doi: 10.1089/ars.2012.4958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diniz D.M., de Souza A.H., Pereira E.M., da Silva J.F., Rigo F.K., Romano-Silva M.A., Binda N., Castro C.J., Jr, Cordeiro M.N., Ferreira J., Gomez M.V. Effects of the calcium channel blockers Phα1β and ω-conotoxin MVIIA on capsaicin and acetic acid-induced visceral nociception in mice. Pharmacol. Biochem. Behav. 2014;126:97–102. doi: 10.1016/j.pbb.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 9.Reuler J.B., Girard D.E., Nardone D.A. The chronic pain syndrome: misconceptions and management. Ann. Intern. Med. 1980;93(4):588–596. doi: 10.7326/0003-4819-93-4-588. [DOI] [PubMed] [Google Scholar]
- 10.Falk S., Dickenson A.H. Pain and nociception: mechanisms of cancer-induced bone pain. J. Clin. Oncol. 2014;32(16):1647–1654. doi: 10.1200/JCO.2013.51.7219. [DOI] [PubMed] [Google Scholar]
- 11.Dubin A.E., Patapoutian A. Nociceptors: the sensors of the pain pathway. J. Clin. Invest. 2010;120(11):3760–3772. doi: 10.1172/JCI42843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baron R. Neuropathic pain: a clinical perspective. Handb. Exp. Pharmacol. 2009;(194):3–30. doi: 10.1007/978-3-540-79090-7_1. [DOI] [PubMed] [Google Scholar]
- 13.Carrasco C., Naziroǧlu M., Rodríguez A.B., Pariente J.A. Neuropathic pain: delving into the oxidative origin and the possible implication of transient receptor potential channels. Front. Physiol. 2018;9:95. doi: 10.3389/fphys.2018.00095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jang Y., Cho P.S., Yang Y.D., Hwang S.W. Nociceptive Roles of TRPM2 Ion Channel in Pathologic Pain. Mol. Neurobiol. 2018;55(8):6589–6600. doi: 10.1007/s12035-017-0862-2. [DOI] [PubMed] [Google Scholar]
- 15.Naziroğlu M. New molecular mechanisms on the activation of TRPM2 channels by oxidative stress and ADP-ribose. Neurochem. Res. 2007;32(11):1990–2001. doi: 10.1007/s11064-007-9386-x. [DOI] [PubMed] [Google Scholar]
- 16.Nazıroglu M. Role of selenium on calcium signaling and oxidative stress-induced molecular pathways in epilepsy. Neurochem. Res. 2009;34(12):2181–2191. doi: 10.1007/s11064-009-0015-8. [DOI] [PubMed] [Google Scholar]
- 17.Pickering G., Morel V. Memantine for the treatment of general neuropathic pain: a narrative review. Fundam. Clin. Pharmacol. 2018;32(1):4–13. doi: 10.1111/fcp.12316. [DOI] [PubMed] [Google Scholar]
- 18.Brefel-Courbon C., Ory-Magne F., Thalamas C., Payoux P., Rascol O. Nociceptive brain activation in patients with neuropathic pain related to Parkinson’s disease. Parkinsonism Relat. Disord. 2013;19(5):548–552. doi: 10.1016/j.parkreldis.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 19.Kahya M.C., Nazıroğlu M., Övey I.S. Modulation of diabetes-induced oxidative stress, apoptosis, and ca2+ entry through trpm2 and trpv1 channels in dorsal root ganglion and hippocampus of diabetic rats by melatonin and selenium. Mol. Neurobiol. 2017;54(3):2345–2360. doi: 10.1007/s12035-016-9727-3. [DOI] [PubMed] [Google Scholar]
- 20.Mickle A.D., Shepherd A.J., Mohapatra D.P. Nociceptive TRP Channels: Sensory Detectors and Transducers in Multiple Pain Pathologies. Pharmaceuticals (Basel) 2016;9(4):72. doi: 10.3390/ph9040072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzalez-Ramirez R., Chen Y., Liedtke W.B., Morales-Lazaro S.L. TRP channels and pain. In: Emir T.L.R., editor. Neurobiology of trp channels, nd. Boca Raton, FL: 2017. pp. 125–147. [PubMed] [Google Scholar]
- 22.Kobayashi K., Fukuoka T., Obata K., Yamanaka H., Dai Y., Tokunaga A., Noguchi K. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J. Comp. Neurol. 2005;493(4):596–606. doi: 10.1002/cne.20794. [DOI] [PubMed] [Google Scholar]
- 23.Ji G., Zhou S., Carlton S.M. Intact Adelta-fibers up-regulate transient receptor potential A1 and contribute to cold hypersensitivity in neuropathic rats. Neuroscience. 2008;154(3):1054–1066. doi: 10.1016/j.neuroscience.2008.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sasaki A., Mizoguchi S., Kagaya K., Shiro M., Sakai A., Andoh T., Kino Y., Taniguchi H., Saito Y., Takahata H., Kuraishi Y. A mouse model of peripheral postischemic dysesthesia: involvement of reperfusion-induced oxidative stress and TRPA1 channel. J. Pharmacol. Exp. Ther. 2014;351(3):568–575. doi: 10.1124/jpet.114.217570. [DOI] [PubMed] [Google Scholar]
- 25.Klein A.H., Trannyguen M., Joe C.L., Iodi C.M., Carstens E. Thermosensitive transient receptor potential (TRP) channel agonists and their role in mechanical, thermal and nociceptive sensations as assessed using animal models. Chemosens. Percept. 2015;8(2):96–108. doi: 10.1007/s12078-015-9176-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sałat K., Filipek B. Antinociceptive activity of transient receptor potential channel TRPV1, TRPA1, and TRPM8 antagonists in neurogenic and neuropathic pain models in mice. J. Zhejiang Univ. Sci. B. 2015;16(3):167–178. doi: 10.1631/jzus.B1400189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wick E.C., Hoge S.G., Grahn S.W., Kim E., Divino L.A., Grady E.F., Bunnett N.W., Kirkwood K.S. Transient receptor potential vanilloid 1, calcitonin gene-related peptide, and substance P mediate nociception in acute pancreatitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2006;290(5):G959–G969. doi: 10.1152/ajpgi.00154.2005. [DOI] [PubMed] [Google Scholar]
- 28.Engel M.A., Leffler A., Niedermirtl F., Babes A., Zimmermann K., Filipović M.R., Izydorczyk I., Eberhardt M., Kichko T.I., Mueller-Tribbensee S.M., Khalil M., Siklosi N., Nau C., Ivanović-Burmazović I., Neuhuber W.L., Becker C., Neurath M.F., Reeh P.W. TRPA1 and substance P mediate colitis in mice. Gastroenterology. 2011;141(4):1346–1358. doi: 10.1053/j.gastro.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 29.Pozsgai G., Hajna Z., Bagoly T., Boros M., Kemény Á., Materazzi S., Nassini R., Helyes Z., Szolcsányi J., Pintér E. The role of transient receptor potential ankyrin 1 (TRPA1) receptor activation in hydrogen-sulphide-induced CGRP-release and vasodilation. Eur. J. Pharmacol. 2012;689(1-3):56–64. doi: 10.1016/j.ejphar.2012.05.053. [DOI] [PubMed] [Google Scholar]
- 30.Denner A.C., Vogler B., Messlinger K., De Col R. Role of transient receptor potential ankyrin 1 receptors in rodent models of meningeal nociception - Experiments in vitro. Eur. J. Pain. 2017;21(5):843–854. doi: 10.1002/ejp.986. [DOI] [PubMed] [Google Scholar]
- 31.Zhang J.M., An J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007;45(2):27–37. doi: 10.1097/AIA.0b013e318034194e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rahman W., Dickenson A.H. Voltage gated sodium and calcium channel blockers for the treatment of chronic inflammatory pain. 2013. [DOI] [PubMed]
- 33.Gwanyanya A., Macianskiene R., Mubagwa K. Insights into the effects of diclofenac and other non-steroidal anti-inflammatory agents on ion channels. J. Pharm. Pharmacol. 2012;64(10):1359–1375. doi: 10.1111/j.2042-7158.2012.01479.x. [DOI] [PubMed] [Google Scholar]
- 34.Holzer P., Izzo A.A. The pharmacology of TRP channels. Br. J. Pharmacol. 2014;171(10):2469–2473. doi: 10.1111/bph.12723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mori Y., Takahashi N., Polat O.K., Kurokawa T., Takeda N., Inoue M. Redox-sensitive transient receptor potential channels in oxygen sensing and adaptation. Pflugers Arch. 2016;468(1):85–97. doi: 10.1007/s00424-015-1716-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takahashi N., Mori Y. TRP Channels as Sensors and Signal Integrators of Redox Status Changes. Front. Pharmacol. 2011;2:58. doi: 10.3389/fphar.2011.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yüksel E., Nazıroğlu M., Şahin M., Çiğ B. Involvement of TRPM2 and TRPV1 channels on hyperalgesia, apoptosis and oxidative stress in rat fibromyalgia model: Protective role of selenium. Sci. Rep. 2017;7(1):17543. doi: 10.1038/s41598-017-17715-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pillai R., Uyehara-Lock J.H., Bellinger F.P. Selenium and selenoprotein function in brain disorders. IUBMB Life. 2014;66(4):229–239. doi: 10.1002/iub.1262. [DOI] [PubMed] [Google Scholar]
- 39.Kim S., Park S.E., Sapkota K., Kim M.K., Kim S.J. Leaf extract of Rhus verniciflua Stokes protects dopaminergic neuronal cells in a rotenone model of Parkinson’s disease. J. Pharm. Pharmacol. 2011;63(10):1358–1367. doi: 10.1111/j.2042-7158.2011.01342.x. [DOI] [PubMed] [Google Scholar]
- 40.Nazıroğlu M., Muhamad S., Pecze L. Nanoparticles as potential clinical therapeutic agents in Alzheimer’s disease: focus on selenium nanoparticles. Expert Rev. Clin. Pharmacol. 2017;10(7):773–782. doi: 10.1080/17512433.2017.1324781. [DOI] [PubMed] [Google Scholar]
- 41.Verkhratsky A.J., Petersen O.H. Neuronal calcium stores. Cell Calcium. 1998;24(5-6):333–343. doi: 10.1016/S0143-4160(98)90057-4. [DOI] [PubMed] [Google Scholar]
- 42.Mata A., Marques D., Martínez-Burgos M.A., Silveira J., Marques J., Mesquita M.F., Pariente J.A., Salido G.M., Singh J. Effect of hydrogen peroxide on secretory response, calcium mobilisation and caspase-3 activity in the isolated rat parotid gland. Mol. Cell. Biochem. 2008;319(1-2):23–31. doi: 10.1007/s11010-008-9873-7. [DOI] [PubMed] [Google Scholar]
- 43.González D., Espino J., Bejarano I., López J.J., Rodríguez A.B., Pariente J.A. Caspase-3 and -9 are activated in human myeloid HL-60 cells by calcium signal. Mol. Cell. Biochem. 2010;333(1-2):151–157. doi: 10.1007/s11010-009-0215-1. [DOI] [PubMed] [Google Scholar]
- 44.Bejarano I., Espino J., González-Flores D., Casado J.G., Redondo P.C., Rosado J.A., Barriga C., Pariente J.A., Rodríguez A.B. Role of Calcium Signals on Hydrogen Peroxide-Induced Apoptosis in Human Myeloid HL-60 Cells. Int. J. Biomed. Sci. 2009;5(3):246–256. [PMC free article] [PubMed] [Google Scholar]
- 45.Ureshino R.P., Hsu Y.T., do Carmo L.G., Yokomizo C.H., Nantes I.L., Smaili S.S. Inhibition of cytoplasmic p53 differentially modulates Ca(2+) signaling and cellular viability in young and aged striata. Exp. Gerontol. 2014;58:120–127. doi: 10.1016/j.exger.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 46.Berridge M.J. The Inositol trisphosphate/calcium signaling pathway in health and disease. Physiol. Rev. 2016;96(4):1261–1296. doi: 10.1152/physrev.00006.2016. [DOI] [PubMed] [Google Scholar]
- 47.Di Meo S., Reed T.T., Venditti P., Victor V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxid. Med. Cell. Longev. 2016;2016:1245049. doi: 10.1155/2016/1245049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hecquet C.M., Malik A.B. Role of H(2)O(2)-activated TRPM2 calcium channel in oxidant-induced endothelial injury. Thromb. Haemost. 2009;101(4):619–625. doi: 10.1160/TH08-10-0641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gómez-Gonzalo M., Martin-Fernandez M., Martínez-Murillo R., Mederos S., Hernández-Vivanco A., Jamison S., Fernandez A.P., Serrano J., Calero P., Futch H.S., Corpas R., Sanfeliu C., Perea G., Araque A. Neuron-astrocyte signaling is preserved in the aging brain. Glia. 2017;65(4):569–580. doi: 10.1002/glia.23112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bianchi K., Rimessi A., Prandini A., Szabadkai G., Rizzuto R. Calcium and mitochondria: mechanisms and functions of a troubled relationship. Biochim. Biophys. Acta. 2004;1742(1-3):119–131. doi: 10.1016/j.bbamcr.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 51.Arruda A.P., Hotamisligil G.S. Calcium Homeostasis and organelle function in the pathogenesis of obesity and diabetes. Cell Metab. 2015;22(3):381–397. doi: 10.1016/j.cmet.2015.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Díaz-Vegas A.R., Cordova A., Valladares D., Llanos P., Hidalgo C., Gherardi G., De Stefani D., Mammucari C., Rizzuto R., Contreras-Ferrat A., Jaimovich E. Mitochondrial calcium increase induced by ryr1 and ip3r channel activation after membrane depolarization regulates skeletal muscle metabolism. Front. Physiol. 2018;9:791. doi: 10.3389/fphys.2018.00791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hajnóczky G., Csordás G., Das S., Garcia-Perez C., Saotome M., Sinha Roy S., Yi M. Mitochondrial calcium signalling and cell death: approaches for assessing the role of mitochondrial Ca2+ uptake in apoptosis. Cell Calcium. 2006;40(5-6):553–560. doi: 10.1016/j.ceca.2006.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Skrzypski M., Sassek M., Abdelmessih S., Mergler S., Grötzinger C., Metzke D., Wojciechowicz T., Nowak K.W., Strowski M.Z. Capsaicin induces cytotoxicity in pancreatic neuroendocrine tumor cells via mitochondrial action. Cell. Signal. 2014;26(1):41–48. doi: 10.1016/j.cellsig.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 55.Uğuz A.C., Öz A., Nazıroğlu M. Curcumin inhibits apoptosis by regulating intracellular calcium release, reactive oxygen species and mitochondrial depolarization levels in SH-SY5Y neuronal cells. J. Recept. Signal Transduct. Res. 2016;36(4):395–401. doi: 10.3109/10799893.2015.1108337. [DOI] [PubMed] [Google Scholar]
- 56.Rayman M.P. Selenium and human health. Lancet. 2012;379(9822):1256–1268. doi: 10.1016/S0140-6736(11)61452-9. [DOI] [PubMed] [Google Scholar]
- 57.Yakubov E., Buchfelder M., Eyüpoglu I.Y., Savaskan N.E. Selenium action in neuro-oncology. Biol. Trace Elem. Res. 2014;161(3):246–254. doi: 10.1007/s12011-014-0111-8. [DOI] [PubMed] [Google Scholar]
- 58.Nazıroğlu M., Yıldız K., Tamtürk B., Erturan İ., Flores-Arce M. Selenium and psoriasis. Biol. Trace Elem. Res. 2012;150(1-3):3–9. doi: 10.1007/s12011-012-9479-5. [DOI] [PubMed] [Google Scholar]
- 59.Bai K., Hong B., Hong Z., Sun J., Wang C. Selenium nanoparticles-loaded chitosan/citrate complex and its protection against oxidative stress in D-galactose-induced aging mice. J. Nanobiotechnology. 2017;15(1):92. doi: 10.1186/s12951-017-0324-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Huang B., Zhang J., Hou J., Chen C. Free radical scavenging efficiency of Nano-Se in vitro. Free Radic. Biol. Med. 2003;35(7):805–813. doi: 10.1016/S0891-5849(03)00428-3. [DOI] [PubMed] [Google Scholar]
- 61.Navarro-Alarcon M., Cabrera-Vique C. Selenium in food and the human body: a review. Sci. Total Environ. 2008;400(1-3):115–141. doi: 10.1016/j.scitotenv.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 62.Flockerzi V. An introduction on TRP channels. Handb. Exp. Pharmacol. 2007;179:1–19. doi: 10.1007/978-3-540-34891-7_1. [DOI] [PubMed] [Google Scholar]
- 63.Pingle S.C., Matta J.A., Ahern G.P. Capsaicin receptor: TRPV1 a promiscuous TRP channel. Handb. Exp. Pharmacol. 2007;179:155–171. doi: 10.1007/978-3-540-34891-7_9. [DOI] [PubMed] [Google Scholar]
- 64.Demirdaş A., Nazıroğlu M., Övey I.S. Duloxetine reduces oxidative stress, apoptosis, and ca2+ entry through modulation of trpm2 and trpv1 channels in the hippocampus and dorsal root ganglion of rats. Mol. Neurobiol. 2017;54(6):4683–4695. doi: 10.1007/s12035-016-9992-1. [DOI] [PubMed] [Google Scholar]
- 65.Nazıroğlu M., Braidy N. Thermo-sensitive trp channels: novel targets for treating chemotherapy-induced peripheral pain. Front. Physiol. 2017;8:1040. doi: 10.3389/fphys.2017.01040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bräuer A.U., Savaskan N.E. Molecular actions of selenium in the brain: neuroprotective mechanisms of an essential trace element. Rev. Neurosci. 2004;15(1):19–32. doi: 10.1515/REVNEURO.2004.15.1.19. [DOI] [PubMed] [Google Scholar]
- 67.Nazıroğlu M., Çelik Ö., Uğuz A.C., Bütün A. Protective effects of riboflavin and selenium on brain microsomal Ca2+-ATPase and oxidative damage caused by glyceryl trinitrate in a rat headache model. Biol. Trace Elem. Res. 2015;164(1):72–79. doi: 10.1007/s12011-014-0199-x. [DOI] [PubMed] [Google Scholar]
- 68.Wilhelm E.A., Ferreira A.T., Pinz M.P., Reis A.S.D., Vogt A.G., Stein A.L., Zeni G., Luchese C. Antioxidant effect of quinoline derivatives containing or not selenium: Relationship with antinociceptive action quinolines are antioxidant and antinociceptive. An. Acad. Bras. Cienc. 2017;89(1) Suppl.:457–467. doi: 10.1590/0001-3765201720160668. [DOI] [PubMed] [Google Scholar]
- 69.Solovyev N.D. Importance of selenium and selenoprotein for brain function: From antioxidant protection to neuronal signalling. J. Inorg. Biochem. 2015;153:1–12. doi: 10.1016/j.jinorgbio.2015.09.003. [DOI] [PubMed] [Google Scholar]
- 70.Naziroğlu M., Lückhoff A. Effects of antioxidants on calcium influx through TRPM2 channels in transfected cells activated by hydrogen peroxide. J. Neurol. Sci. 2008;270(1-2):152–158. doi: 10.1016/j.jns.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 71.Crouzin N., Ferreira M.C., Cohen-Solal C., Barbanel G., Guiramand J., Vignes M. Neuroprotection induced by vitamin E against oxidative stress in hippocampal neurons: involvement of TRPV1 channels. Mol. Nutr. Food Res. 2010;54(4):496–505. doi: 10.1002/mnfr.200900188. [DOI] [PubMed] [Google Scholar]
- 72.Nazıroğlu M., Ciğ B., Ozgül C. Neuroprotection induced by N-acetylcysteine against cytosolic glutathione depletion-induced Ca2+ influx in dorsal root ganglion neurons of mice: role of TRPV1 channels. Neuroscience. 2013;242:151–160. doi: 10.1016/j.neuroscience.2013.03.032. [DOI] [PubMed] [Google Scholar]
- 73.Celik O., Nazıroğlu M. Melatonin modulates apoptosis and TRPM2 channels in transfected cells activated by oxidative stress. Physiol. Behav. 2012;107(3):458–465. doi: 10.1016/j.physbeh.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 74.Nazıroğlu M., Özgül C., Küçükayaz M., Çiğ B., Hebeisen S., Bal R. Selenium modulates oxidative stress-induced TRPM2 cation channel currents in transfected Chinese hamster ovary cells. Basic Clin. Pharmacol. Toxicol. 2013;112(2):96–102. doi: 10.1111/j.1742-7843.2012.00934.x. [DOI] [PubMed] [Google Scholar]
- 75.Sözbir E., Nazıroğlu M. Diabetes enhances oxidative stress-induced TRPM2 channel activity and its control by N-acetylcysteine in rat dorsal root ganglion and brain. Metab. Brain Dis. 2016;31(2):385–393. doi: 10.1007/s11011-015-9769-7. [DOI] [PubMed] [Google Scholar]
- 76.Öz A., Çelik Ö. Curcumin inhibits oxidative stress-induced TRPM2 channel activation, calcium ion entry and apoptosis values in SH-SY5Y neuroblastoma cells: Involvement of transfection procedure. Mol. Membr. Biol. 2016;33(3-5):76–88. doi: 10.1080/09687688.2017.1318224. [DOI] [PubMed] [Google Scholar]
- 77.Zayats V., Samad A., Minofar B., Roelofs K.E., Stockner T., Ettrich R. Regulation of the transient receptor potential channel TRPA1 by its N-terminal ankyrin repeat domain. J. Mol. Model. 2013;19(11):4689–4700. doi: 10.1007/s00894-012-1505-1. [DOI] [PubMed] [Google Scholar]
- 78.Cordero-Morales J.F., Gracheva E.O., Julius D. Cytoplasmic ankyrin repeats of transient receptor potential A1 (TRPA1) dictate sensitivity to thermal and chemical stimuli. Proc. Natl. Acad. Sci. USA. 2011;108(46):E1184–E1191. doi: 10.1073/pnas.1114124108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Clapham D.E. SnapShot: mammalian TRP channels. Cell. 2007;129(1):220. doi: 10.1016/j.cell.2007.03.034. [DOI] [PubMed] [Google Scholar]
- 80.Andersson D.A., Gentry C., Moss S., Bevan S. Transient receptor potential A1 is a sensory receptor for multiple products of oxidative stress. J. Neurosci. 2008;28(10):2485–2494. doi: 10.1523/JNEUROSCI.5369-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Baraldi P.G., Preti D., Materazzi S., Geppetti P. Transient receptor potential ankyrin 1 (TRPA1) channel as emerging target for novel analgesics and anti-inflammatory agents. J. Med. Chem. 2010;53(14):5085–5107. doi: 10.1021/jm100062h. [DOI] [PubMed] [Google Scholar]
- 82.Marwaha L., Bansal Y., Singh R., Saroj P., Bhandari R., Kuhad A. TRP channels: potential drug target for neuropathic pain. Inflammopharmacology. 2016;24(6):305–317. doi: 10.1007/s10787-016-0288-x. [DOI] [PubMed] [Google Scholar]
- 83.Yu L., Wang S., Kogure Y., Yamamoto S., Noguchi K., Dai Y. Modulation of TRP channels by resveratrol and other stilbenoids. Mol. Pain. 2013;9:3. doi: 10.1186/1744-8069-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nassini R., Materazzi S., Benemei S., Geppetti P. The TRPA1 channel in inflammatory and neuropathic pain and migraine. Rev. Physiol. Biochem. Pharmacol. 2014;167:1–43. doi: 10.1007/112_2014_18. [DOI] [PubMed] [Google Scholar]
- 85.Kwan K.Y., Allchorne A.J., Vollrath M.A., Christensen A.P., Zhang D.S., Woolf C.J., Corey D.P. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50(2):277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 86.Trevisan G., Materazzi S., Fusi C., Altomare A., Aldini G., Lodovici M., Patacchini R., Geppetti P., Nassini R. Novel therapeutic strategy to prevent chemotherapy-induced persistent sensory neuropathy by TRPA1 blockade. Cancer Res. 2013;73(10):3120–3131. doi: 10.1158/0008-5472.CAN-12-4370. [DOI] [PubMed] [Google Scholar]
- 87.Stenger B., Popp T., John H., Siegert M., Tsoutsoulopoulos A., Schmidt A., Mückter H., Gudermann T., Thiermann H., Steinritz D. N-Acetyl-L-cysteine inhibits sulfur mustard-induced and TRPA1-dependent calcium influx. Arch. Toxicol. 2017;91(5):2179–2189. doi: 10.1007/s00204-016-1873-x. [DOI] [PubMed] [Google Scholar]
- 88.Yazğan Y., Nazıroğlu M. Ovariectomy-induced mitochondrial oxidative stress, apoptosis, and calcium ion influx through trpa1, trpm2, and trpv1 are prevented by 17β-estradiol, tamoxifen, and raloxifene in the hippocampus and dorsal root ganglion of rats. Mol. Neurobiol. 2017;54(10):7620–7638. doi: 10.1007/s12035-016-0232-5. [DOI] [PubMed] [Google Scholar]
- 89.Pérez de Vega M.J., Gómez-Monterrey I., Ferrer-Montiel A., González-Muñiz R. Transient receptor potential melastatin 8 channel (trpm8) modulation: cool entryway for treating pain and cancer. J. Med. Chem. 2016;59(22):10006–10029. doi: 10.1021/acs.jmedchem.6b00305. [DOI] [PubMed] [Google Scholar]
- 90.DeFalco J., Steiger D., Dourado M., Emerling D., Duncton M.A. 5-benzyloxytryptamine as an antagonist of TRPM8. Bioorg. Med. Chem. Lett. 2010;20(23):7076–7079. doi: 10.1016/j.bmcl.2010.09.099. [DOI] [PubMed] [Google Scholar]
- 91.Block C.H., Hoffman G.E. Neuropeptide and monoamine components of the parabrachial pontine complex. Peptides. 1987;8(2):267–283. doi: 10.1016/0196-9781(87)90102-1. [DOI] [PubMed] [Google Scholar]
- 92.Dhaka A., Murray A.N., Mathur J., Earley T.J., Petrus M.J., Patapoutian A. TRPM8 is required for cold sensation in mice. Neuron. 2007;54(3):371–378. doi: 10.1016/j.neuron.2007.02.024. [DOI] [PubMed] [Google Scholar]
- 93.Broad L.M., Mogg A.J., Beattie R.E., Ogden A.M., Blanco M.J., Bleakman D. TRP channels as emerging targets for pain therapeutics. Expert Opin. Ther. Targets. 2009;13(1):69–81. doi: 10.1517/14728220802616620. [DOI] [PubMed] [Google Scholar]
- 94.Naziroğlu M., Ozgül C. Effects of antagonists and heat on TRPM8 channel currents in dorsal root ganglion neuron activated by nociceptive cold stress and menthol. Neurochem. Res. 2012;37(2):314–320. doi: 10.1007/s11064-011-0614-z. [DOI] [PubMed] [Google Scholar]
- 95.Su L., Shu R., Song C., Yu Y., Wang G., Li Y., Liu C. Downregulations of TRPM8 expression and membrane trafficking in dorsal root ganglion mediate the attenuation of cold hyperalgesia in CCI rats induced by GFRα3 knockdown. Brain Res. Bull. 2017;135:8–24. doi: 10.1016/j.brainresbull.2017.08.002. [DOI] [PubMed] [Google Scholar]
- 96.Gong K., Jasmin L. Sustained Morphine Administration Induces TRPM8-Dependent Cold Hyperalgesia. J. Pain. 2017;18(2):212–221. doi: 10.1016/j.jpain.2016.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Pan Y., Chen F., Huang S., Cai Z., Lan H., Tong Y., Yu X., Zhao G. TRPA1 and TRPM8 receptors may promote local vasodilation that aggravates oxaliplatin-induced peripheral neuropathy amenable to 17β-estradiol treatment. Curr. Neurovasc. Res. 2016;13(4):309–317. doi: 10.2174/1567202613666160601144254. [DOI] [PubMed] [Google Scholar]
- 98.Kato Y., Tateai Y., Ohkubo M., Saito Y., Amagai S.Y., Kimura Y.S., Iimura N., Okada M., Matsumoto A., Mano Y., Hirosawa I., Ohuchi K., Tajima M., Asahi M., Kotaki H., Yamada H. CKato. Gosha-jinki-gan reduced oxaliplatin-induced hypersensitivity to cold sensation and its effect would be related to suppression of the expression of TRPM8 and TRPA1 in rats. Anticancer Drugs. 2014;25(1):39–43. doi: 10.1097/CAD.0000000000000022. [DOI] [PubMed] [Google Scholar]
- 99.Caceres A.I., Liu B., Jabba S.V., Achanta S., Morris J.B., Jordt S.E. Transient receptor potential cation channel subfamily m member 8 channels mediate the anti-inflammatory effects of eucalyptol. Br. J. Pharmacol. 2017;174(9):867–879. doi: 10.1111/bph.13760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dussor G., Cao Y.Q. TRPM8 and Migraine. Headache. 2016;56(9):1406–1417. doi: 10.1111/head.12948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hsieh Y.L., Chen H.Y., Yang C.H., Yang C.C. Analgesic effects of transcutaneous ultrasound nerve stimulation in a rat model of oxaliplatin-induced mechanical hyperalgesia and cold allodynia. Ultrasound Med. Biol. 2017;43(7):1466–1475. doi: 10.1016/j.ultrasmedbio.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 102.Lishko P.V., Procko E., Jin X., Phelps C.B., Gaudet R. The ankyrin repeats of TRPV1 bind multiple ligands and modulate channel sensitivity. Neuron. 2007;54(6):905–918. doi: 10.1016/j.neuron.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 103.Nazıroğlu M. Molecular role of catalase on oxidative stress-induced Ca(2+) signaling and TRP cation channel activation in nervous system. J. Recept. Signal Transduct. Res. 2012;32(3):134–141. doi: 10.3109/10799893.2012.672994. [DOI] [PubMed] [Google Scholar]
- 104.Kurogi M., Kawai Y., Nagatomo K., Tateyama M., Kubo Y., Saitoh O. Auto-oxidation products of epigallocatechin gallate activate TRPA1 and TRPV1 in sensory neurons. Chem. Senses. 2015;40(1):27–46. doi: 10.1093/chemse/bju057. [DOI] [PubMed] [Google Scholar]
- 105.Bevan S., Quallo T., Andersson D.A. Trpv1. Handb. Exp. Pharmacol. 2014;222:207–245. doi: 10.1007/978-3-642-54215-2_9. [DOI] [PubMed] [Google Scholar]
- 106.Edwards J.G. TRPV1 in the central nervous system: synaptic plasticity, function, and pharmacological implications. Prog. Drug Res. 2014;68:77–104. doi: 10.1007/978-3-0348-0828-6_3. [DOI] [PubMed] [Google Scholar]
- 107.Danigo A., Magy L., Demiot C. TRPV1 in neuropathic pain: from animal models to therapeutical prospects. 2013. [DOI] [PubMed]
- 108.Rossato M.F., Trevisan G., Walker C.I., Klafke J.Z., de Oliveira A.P., Villarinho J.G., Zanon R.B., Royes L.F., Athayde M.L., Gomez M.V., Ferreira J. Eriodictyol: a flavonoid antagonist of the TRPV1 receptor with antioxidant activity. Biochem. Pharmacol. 2011;81(4):544–551. doi: 10.1016/j.bcp.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 109.Pabbidi R.M., Yu S.Q., Peng S., Khardori R., Pauza M.E., Premkumar L.S. Influence of TRPV1 on diabetes-induced alterations in thermal pain sensitivity. Mol. Pain. 2008;4:9. doi: 10.1186/1744-8069-4-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wilder-Smith E.P., Ong W.Y., Guo Y., Chow A.W. Epidermal transient receptor potential vanilloid 1 in idiopathic small nerve fibre disease, diabetic neuropathy and healthy human subjects. Histopathology. 2007;51(5):674–680. doi: 10.1111/j.1365-2559.2007.02851.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Gouin O., L’Herondelle K., Lebonvallet N., Le Gall-Ianotto C., Sakka M., Buhé V., Plée-Gautier E., Carré J.L., Lefeuvre L., Misery L., Le Garrec R. TRPV1 and TRPA1 in cutaneous neurogenic and chronic inflammation: pro-inflammatory response induced by their activation and their sensitization. Protein Cell. 2017;8(9):644–661. doi: 10.1007/s13238-017-0395-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang Y., Gao Y., Tian Q., Deng Q., Wang Y., Zhou T., Liu Q., Mei K., Wang Y., Liu H., Ma R., Ding Y., Rong W., Cheng J., Yao J., Xu T.L., Zhu M.X., Li Y. TRPV1 sumoylation regulates nociceptive signaling in models of inflammatory pain. Nat. Commun. 2018;9(1):1529. doi: 10.1038/s41467-018-03974-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ji R.R., Samad T.A., Jin S.X., Schmoll R., Woolf C.J. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron. 2002;36(1):57–68. doi: 10.1016/S0896-6273(02)00908-X. [DOI] [PubMed] [Google Scholar]
- 114.Maqboul A., Elsadek B. Expression profiles of TRPV1, TRPV4, TLR4 and ERK1/2 in the dorsal root ganglionic neurons of a cancer-induced neuropathy rat model. PeerJ. 2018;6:e4622. doi: 10.7717/peerj.4622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sakallı Çetin E., Nazıroğlu M., Çiğ B., Övey I.S., Aslan Koşar P. Selenium potentiates the anticancer effect of cisplatin against oxidative stress and calcium ion signaling-induced intracellular toxicity in MCF-7 breast cancer cells: involvement of the TRPV1 channel. J. Recept. Signal Transduct. Res. 2017;37(1):84–93. doi: 10.3109/10799893.2016.1160931. [DOI] [PubMed] [Google Scholar]
- 116.Koşar P.A., Nazıroğlu M., Övey I.S., Çiğ B. Synergic effects of doxorubicin and melatonin on apoptosis and mitochondrial oxidative stress in mcf-7 breast cancer cells: involvement of trpv1 channels. J. Membr. Biol. 2016;249(1-2):129–140. doi: 10.1007/s00232-015-9855-0. [DOI] [PubMed] [Google Scholar]
- 117.Balaban H., Nazıroğlu M., Demirci K., Övey I.S. The protective role of selenium on scopolamine-induced memory impairment, oxidative stress, and apoptosis in aged rats: the involvement of trpm2 and trpv1 channels. Mol. Neurobiol. 2017;54(4):2852–2868. doi: 10.1007/s12035-016-9835-0. [DOI] [PubMed] [Google Scholar]
- 118.Nazıroğlu M., Senol N., Ghazizadeh V., Yürüker V. Neuroprotection induced by N-acetylcysteine and selenium against traumatic brain injury-induced apoptosis and calcium entry in hippocampus of rat. Cell. Mol. Neurobiol. 2014;34(6):895–903. doi: 10.1007/s10571-014-0069-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shibasaki K. Physiological significance of TRPV2 as a mechanosensor, thermosensor and lipid sensor. J. Physiol. Sci. 2016;66(5):359–365. doi: 10.1007/s12576-016-0434-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kojima I., Nagasawa M. Trpv2. Handb. Exp. Pharmacol. 2014;222:247–272. doi: 10.1007/978-3-642-54215-2_10. [DOI] [PubMed] [Google Scholar]
- 121.Axelsson H.E., Minde J.K., Sonesson A., Toolanen G., Högestätt E.D., Zygmunt P.M. Transient receptor potential vanilloid 1, vanilloid 2 and melastatin 8 immunoreactive nerve fibers in human skin from individuals with and without Norrbottnian congenital insensitivity to pain. Neuroscience. 2009;162(4):1322–1332. doi: 10.1016/j.neuroscience.2009.05.052. [DOI] [PubMed] [Google Scholar]
- 122.Mihara H., Boudaka A., Shibasaki K., Yamanaka A., Sugiyama T., Tominaga M. Involvement of TRPV2 activation in intestinal movement through nitric oxide production in mice. J. Neurosci. 2010;30(49):16536–16544. doi: 10.1523/JNEUROSCI.4426-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bang S., Kim K.Y., Yoo S., Lee S.H., Hwang S.W. Transient receptor potential V2 expressed in sensory neurons is activated by probenecid. Neurosci. Lett. 2007;425(2):120–125. doi: 10.1016/j.neulet.2007.08.035. [DOI] [PubMed] [Google Scholar]
- 124.Park D.J., Kim S.H., Nah S.S., Lee J.H., Kim S.K., Lee Y.A., Hong S.J., Kim H.S., Lee H.S., Kim H.A., Joung C.I., Kim S.H., Lee S.S. Polymorphisms of the TRPV2 and TRPV3 genes associated with fibromyalgia in a Korean population. Rheumatology (Oxford) 2016;55(8):1518–1527. doi: 10.1093/rheumatology/kew180. [DOI] [PubMed] [Google Scholar]
- 125.Colton C.K., Zhu M.X. 2-Aminoethoxydiphenyl borate as a common activator of TRPV1, TRPV2, and TRPV3 channels. Handb. Exp. Pharmacol. 2007;179:173–187. doi: 10.1007/978-3-540-34891-7_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Nilius B., Bíró T. TRPV3: a ‘more than skinny’ channel. Exp. Dermatol. 2013;22(7):447–452. doi: 10.1111/exd.12163. [DOI] [PubMed] [Google Scholar]
- 127.Guatteo E., Chung K.K., Bowala T.K., Bernardi G., Mercuri N.B., Lipski J. Temperature sensitivity of dopaminergic neurons of the substantia nigra pars compacta: involvement of transient receptor potential channels. J. Neurophysiol. 2005;94(5):3069–3080. doi: 10.1152/jn.00066.2005. [DOI] [PubMed] [Google Scholar]
- 128.Huang S.M., Lee H., Chung M.K., Park U., Yu Y.Y., Bradshaw H.B., Coulombe P.A., Walker J.M., Caterina M.J. Overexpressed transient receptor potential vanilloid 3 ion channels in skin keratinocytes modulate pain sensitivity via prostaglandin E2. J. Neurosci. 2008;28(51):13727–13737. doi: 10.1523/JNEUROSCI.5741-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Huang S.M., Li X., Yu Y., Wang J., Caterina M.J. TRPV3 and TRPV4 ion channels are not major contributors to mouse heat sensation. Mol. Pain. 2011;7:37. doi: 10.1186/1744-8069-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Carreño O., Corominas R., Fernández-Morales J., Camiña M., Sobrido M.J., Fernández-Fernández J.M., Pozo-Rosich P., Cormand B., Macaya A. SNP variants within the vanilloid TRPV1 and TRPV3 receptor genes are associated with migraine in the Spanish population. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 2012;159B(1):94–103. doi: 10.1002/ajmg.b.32007. [DOI] [PubMed] [Google Scholar]
- 131.Luo J., Hu H. Thermally activated TRPV3 channels. Curr. Top. Membr. 2014;74:325–364. doi: 10.1016/B978-0-12-800181-3.00012-9. [DOI] [PubMed] [Google Scholar]
- 132.Plant T.D., Strotmann R. In: TRPV4: A Multifunctional Nonselective Cation Channel with Complex Regulation.TRP Ion Channel Function in Sensory Transduction and Cellular Signaling Cascades; Liedtke, W.B. Heller S., editor. Boca Raton, FL: 2007. [PubMed] [Google Scholar]
- 133.Alessandri-Haber N., Yeh J.J., Boyd A.E., Parada C.A., Chen X., Reichling D.B., Levine J.D. Hypotonicity induces TRPV4-mediated nociception in rat. Neuron. 2003;39(3):497–511. doi: 10.1016/S0896-6273(03)00462-8. [DOI] [PubMed] [Google Scholar]
- 134.Kumagami H., Terakado M., Sainoo Y., Baba A., Fujiyama D., Fukuda T., Takasaki K., Takahashi H. Expression of the osmotically responsive cationic channel TRPV4 in the endolymphatic sac. Audiol. Neurotol. 2009;14(3):190–197. doi: 10.1159/000180290. [DOI] [PubMed] [Google Scholar]
- 135.Zhang L.P., Kline R.H., IV, Deevska G., Ma F., Nikolova-Karakashian M., Westlund K.N. Alcohol and high fat induced chronic pancreatitis: TRPV4 antagonist reduces hypersensitivity. Neuroscience. 2015;311:166–179. doi: 10.1016/j.neuroscience.2015.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vincent F., Acevedo A., Nguyen M.T., Dourado M., DeFalco J., Gustafson A., Spiro P., Emerling D.E., Kelly M.G., Duncton M.A. Identification and characterization of novel TRPV4 modulators. Biochem. Biophys. Res. Commun. 2009;389(3):490–494. doi: 10.1016/j.bbrc.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 137.Poole D.P., Amadesi S., Veldhuis N.A., Abogadie F.C., Lieu T., Darby W., Liedtke W., Lew M.J., McIntyre P., Bunnett N.W. Protease-activated receptor 2 (PAR2) protein and transient receptor potential vanilloid 4 (TRPV4) protein coupling is required for sustained inflammatory signaling. J. Biol. Chem. 2013;288(8):5790–5802. doi: 10.1074/jbc.M112.438184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Grace M.S., Lieu T., Darby B., Abogadie F.C., Veldhuis N., Bunnett N.W., McIntyre P. The tyrosine kinase inhibitor bafetinib inhibits PAR2-induced activation of TRPV4 channels in vitro and pain in vivo. Br. J. Pharmacol. 2014;171(16):3881–3894. doi: 10.1111/bph.12750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Alessandri-Haber N., Dina O.A., Yeh J.J., Parada C.A., Reichling D.B., Levine J.D. Transient receptor potential vanilloid 4 is essential in chemotherapy-induced neuropathic pain in the rat. J. Neurosci. 2004;24(18):4444–4452. doi: 10.1523/JNEUROSCI.0242-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Materazzi S., Fusi C., Benemei S., Pedretti P., Patacchini R., Nilius B., Prenen J., Creminon C., Geppetti P., Nassini R. TRPA1 and TRPV4 mediate paclitaxel-induced peripheral neuropathy in mice via a glutathione-sensitive mechanism. Pflugers Arch. 2012;463(4):561–569. doi: 10.1007/s00424-011-1071-x. [DOI] [PubMed] [Google Scholar]
- 141.Liu T.T., Bi H.S., Lv S.Y., Wang X.R., Yue S.W. Inhibition of the expression and function of TRPV4 by RNA interference in dorsal root ganglion. Neurol. Res. 2010;32(5):466–471. doi: 10.1179/174313209X408945. [DOI] [PubMed] [Google Scholar]
- 142.Suresh K., Servinsky L., Jiang H., Bigham Z., Yun X., Kliment C., Huetsch J., Damarla M., Shimoda L.A. Reactive oxygen species induced Ca2+ influx via TRPV4 and microvascular endothelial dysfunction in the SU5416/hypoxia model of pulmonary arterial hypertension. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018;314(5):L893–L907. doi: 10.1152/ajplung.00430.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Bai J.Z., Lipski J. Involvement of TRPV4 channels in Aβ(40)-induced hippocampal cell death and astrocytic Ca(2+) signalling. Neurotoxicology. 2014;41:64–72. doi: 10.1016/j.neuro.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 144.Zhang X., Gao S., Tanaka M., Zhang Z., Huang Y., Mitsui T., Kamiyama M., Koizumi S., Fan J., Takeda M., Yao J. Carbenoxolone inhibits TRPV4 channel-initiated oxidative urothelial injury and ameliorates cyclophosphamide-induced bladder dysfunction. J. Cell. Mol. Med. 2017;21(9):1791–1802. doi: 10.1111/jcmm.13100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ma X., He D., Ru X., Chen Y., Cai Y., Bruce I.C., Xia Q., Yao X., Jin J. Apigenin, a plant-derived flavone, activates transient receptor potential vanilloid 4 cation channel. Br. J. Pharmacol. 2012;166(1):349–358. doi: 10.1111/j.1476-5381.2011.01767.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Santo-Domingo J., Demaurex N. Calcium uptake mechanisms of mitochondria. Biochim. Biophys. Acta. 2010;1797(6-7):907–912. doi: 10.1016/j.bbabio.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 147.Hongpaisan J., Winters C.A., Andrews S.B. Strong calcium entry activates mitochondrial superoxide generation, upregulating kinase signaling in hippocampal neurons. J. Neurosci. 2004;24(48):10878–10887. doi: 10.1523/JNEUROSCI.3278-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zeng B., Chen G.L., Xu S.Z. Divalent copper is a potent extracellular blocker for TRPM2 channel. Biochem. Biophys. Res. Commun. 2012;424(2):279–284. doi: 10.1016/j.bbrc.2012.06.107. [DOI] [PubMed] [Google Scholar]
- 149.Yousuf S., Atif F., Ahmad M., Hoda M.N., Khan M.B., Ishrat T., Islam F. Selenium plays a modulatory role against cerebral ischemia-induced neuronal damage in rat hippocampus. Brain Res. 2007;1147:218–225. doi: 10.1016/j.brainres.2007.01.143. [DOI] [PubMed] [Google Scholar]
- 150.Uğuz A.C., Nazıroğlu M. Effects of selenium on calcium signaling and apoptosis in rat dorsal root ganglion neurons induced by oxidative stress. Neurochem. Res. 2012;37(8):1631–1638. doi: 10.1007/s11064-012-0758-5. [DOI] [PubMed] [Google Scholar]
- 151.Reeves M.A., Bellinger F.P., Berry M.J. The neuroprotective functions of selenoprotein M and its role in cytosolic calcium regulation. Antioxid. Redox Signal. 2010;12(7):809–818. doi: 10.1089/ars.2009.2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Dominiak A., Wilkaniec A., Jęśko H., Czapski G.A., Lenkiewicz A.M., Kurek E., Wroczyński P., Adamczyk A. Selol, an organic selenium donor, prevents lipopolysaccharide-induced oxidative stress and inflammatory reaction in the rat brain. Neurochem. Int. 2017;108:66–77. doi: 10.1016/j.neuint.2017.02.014. [DOI] [PubMed] [Google Scholar]
- 153.El-Ghazaly M.A., Fadel N., Rashed E., El-Batal A., Kenawy S.A. Anti-inflammatory effect of selenium nanoparticles on the inflammation induced in irradiated rats. Can. J. Physiol. Pharmacol. 2017;95(2):101–110. doi: 10.1139/cjpp-2016-0183. [DOI] [PubMed] [Google Scholar]
- 154.Anversa R.G., Sousa F.S.S., Birmann P.T., Lima D.B. ; Lenardao E.J., Bruning C.A., Savegnago L. Antinociceptive and anti-inflammatory effects of 1,2-bis-(4 methoxyphenylselanyl) styrene in mice: involvement of the serotonergic system. J. Pharm. Pharmacol. 2018;70(7):901–909. doi: 10.1111/jphp.12907. [DOI] [PubMed] [Google Scholar]
- 155.Birmann P.T., Sousa F.S.S., de Oliveira D.H., Domingues M., Vieira B.M., Lenardão E.J., Savegnago L. 3-(4-Chlorophenylselanyl)-1-methyl-1H-indole, a new selenium compound elicits an antinociceptive and anti-inflammatory effect in mice. Eur. J. Pharmacol. 2018;827:71–79. doi: 10.1016/j.ejphar.2018.03.005. [DOI] [PubMed] [Google Scholar]
- 156.Marcondes Sari M.H., Zborowski V.A., Ferreira L.M., Jardim N.D.S., Araujo P.C.O., Brüning C.A., Cruz L., Nogueira C.W. Enhanced pharmacological actions of p,p′-methoxyl-diphenyl diselenide-loaded polymeric nanocapsules in a mouse model of neuropathic pain: Behavioral and molecular insights. J. Trace Elem. Med. Biol. 2018;46:17–25. doi: 10.1016/j.jtemb.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 157.Sari M.H.M., Ferreira L.M. AngonesiZborowski, V.; Araujo, P.C.O.; Nadal, J.M.; Farago, P.V.; Cruz, L.; Nogueira, C.W. p,p′-Methoxyl-diphenyl diselenideincorporation into polymeric nanocapsules improves its antinociceptive action: Physicochemical and behavioral studies. Colloids Surf. B Biointerfaces. 2017;157:464–472. doi: 10.1016/j.colsurfb.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 158.Oliveira C.E.S., Marcondes Sari M.H.M., Zborowski V.A., Prado V.C., Nogueira C.W., Zeni G. Pain-depression dyad induced by reserpine is relieved by p,p′-methoxyl-diphenyl diselenide in rats. Eur. J. Pharmacol. 2016;791:794–802. doi: 10.1016/j.ejphar.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 159.Mansel R.E., Das T., Baggs G.E., Noss M.J., Jennings W.P., Cohen J., Portman D., Cohen M., Voss A.C. A Randomized controlled multicenter trial of an investigational liquid nutritional formula in women with cyclic breast pain associated with fibrocystic breast changes. J. Womens Health (Larchmt.) 2018;27(3):333–340. doi: 10.1089/jwh.2017.6406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Barros-Neto J.A., Souza-Machado A., Kraychete D.C., Jesus R.P., Cortes M.L., Lima M.D., Freitas M.C., Santos T.M., Viana G.F., Menezes-Filho J.A. Selenium and. PLoS One. 2016;11(10):e0164302. doi: 10.1371/journal.pone.0164302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Brüning C.A., Martini F., Soares S.M., Sampaio T.B., Gai B.M., Duarte M.M., Nogueira C.W. m-Trifluoromethyl-diphenyl diselenide, a multi-target selenium compound, prevented mechanical allodynia and depressive-like behavior in a mouse comorbid pain and depression model. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2015;63:35–46. doi: 10.1016/j.pnpbp.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 162.Kirk G.R., White J.S., McKie L., Stevenson M., Young I., Clements W.D., Rowlands B.J. Combined antioxidant therapy reduces pain and improves quality of life in chronic pancreatitis. J. Gastrointest. Surg. 2006;10(4):499–503. doi: 10.1016/j.gassur.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 163.Ozkul A., Ayhan M., Yenisey C., Akyol A., Guney E., Ergin F.A. The role of oxidative stress and endothelial injury in diabetic neuropathy and neuropathic pain. Neuroendocrinol. Lett. 2010;31(2):261–264. [PubMed] [Google Scholar]
- 164.Kiasalari Z., Rahmani T., Mahmoudi N., Baluchnejadmojarad T., Roghani M. Diosgenin ameliorates development of neuropathic pain in diabetic rats: Involvement of oxidative stress and inflammation. Biomed. Pharmacother. 2017;86:654–661. doi: 10.1016/j.biopha.2016.12.068. [DOI] [PubMed] [Google Scholar]
- 165.Nazıroğlu M., Dikici D.M., Dursun S. Role of oxidative stress and Ca2+ signaling on molecular pathways of neuropathic pain in diabetes: focus on TRP channels. Neurochem. Res. 2012;37(10):2065–2075. doi: 10.1007/s11064-012-0850-x. [DOI] [PubMed] [Google Scholar]
- 166.Olukman M., Önal A., Celenk F.G., Uyanıkgil Y., Cavuşoğlu T., Düzenli N., Ülker S. Treatment with NADPH oxidase inhibitor apocynin alleviates diabetic neuropathic pain in rats. Neural Regen. Res. 2018;13(9):1657–1664. doi: 10.4103/1673-5374.232530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Ziegler D., Ametov A., Barinov A., Dyck P.J., Gurieva I., Low P.A., Munzel U., Yakhno N., Raz I., Novosadova M., Maus J., Samigullin R. Oral treatment with alpha-lipoic acid improves symptomatic diabetic polyneuropathy: the SYDNEY 2 trial. Diabetes Care. 2006;29(11):2365–2370. doi: 10.2337/dc06-1216. [DOI] [PubMed] [Google Scholar]
- 168.Agathos E., Tentolouris A., Eleftheriadou I., Katsaouni P., Nemtzas I., Petrou A., Papanikolaou C., Tentolouris N. Effect of α-lipoic acid on symptoms and quality of life in patients with painful diabetic neuropathy. J. Int. Med. Res. 2018;46(5):1779–1790. doi: 10.1177/0300060518756540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rajanandh M.G., Kosey S., Prathiksha G. Assessment of antioxidant supplementation on the neuropathic pain score and quality of life in diabetic neuropathy patients - a randomized controlled study. Pharmacol. Rep. 2014;66(1):44–48. doi: 10.1016/j.pharep.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 170.Zhang Y.P., Song C.Y., Yuan Y., Eber A., Rodriguez Y., Levitt R.C., Takacs P., Yang Z., Goldberg R., Candiotti K.A. Diabetic neuropathic pain development in type 2 diabetic mouse model and the prophylactic and therapeutic effects of coenzyme Q10. Neurobiol. Dis. 2013;58:169–178. doi: 10.1016/j.nbd.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 171.Bujalska M., Winecka R., Gumułka S.W. Effect of selol on the opioids activity in streptozotocin induced hyperalgesia. Acta Pol. Pharm. 2008;65(6):691–696. [PubMed] [Google Scholar]
- 172.Facer P., Casula M.A., Smith G.D., Benham C.D., Chessell I.P., Bountra C., Sinisi M., Birch R., Anand P. Differential expression of the capsaicin receptor TRPV1 and related novel receptors TRPV3, TRPV4 and TRPM8 in normal human tissues and changes in traumatic and diabetic neuropathy. BMC Neurol. 2007;7:11. doi: 10.1186/1471-2377-7-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Tominaga M. 2010.
- 174.Wei H., Hämäläinen M.M., Saarnilehto M., Koivisto A., Pertovaara A. Attenuation of mechanical hypersensitivity by an antagonist of the TRPA1 ion channel in diabetic animals. Anesthesiology. 2009;111(1):147–154. doi: 10.1097/ALN.0b013e3181a1642b. [DOI] [PubMed] [Google Scholar]
- 175.Jaggi A.S., Singh N. Mechanisms in cancer-chemotherapeutic drugs-induced peripheral neuropathy. Toxicology. 2012;291(1-3):1–9. doi: 10.1016/j.tox.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 176.Duggett N.A., Griffiths L.A., McKenna O.E., de Santis V., Yongsanguanchai N., Mokori E.B., Flatters S.J. Oxidative stress in the development, maintenance and resolution of paclitaxel-induced painful neuropathy. Neuroscience. 2016;333:13–26. doi: 10.1016/j.neuroscience.2016.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Colloca L., Ludman T., Bouhassira D., Baron R., Dickenson A.H., Yarnitsky D., Freeman R., Truini A., Attal N., Finnerup N.B., Eccleston C., Kalso E., Bennett D.L., Dworkin R.H., Raja S.N. Neuropathic pain. Nat. Rev. Dis. Primers. 2017;3:17002. doi: 10.1038/nrdp.2017.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Binder A., May D., Baron R., Maier C., Tölle T.R., Treede R.D., Berthele A., Faltraco F., Flor H., Gierthmühlen J., Haenisch S., Huge V., Magerl W., Maihöfner C., Richter H., Rolke R., Scherens A., Uçeyler N., Ufer M., Wasner G., Zhu J., Cascorbi I. Transient receptor potential channel polymorphisms are associated with the somatosensory function in neuropathic pain patients. PLoS One. 2011;6(3):e17387. doi: 10.1371/journal.pone.0017387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Tominaga M. Nociception and TRP channels. Handb. Exp. Pharmacol. 2007;179:489–505. doi: 10.1007/978-3-540-34891-7_29. [DOI] [PubMed] [Google Scholar]
- 180.Chiba T., Oka Y., Sashida H., Kanbe T., Abe K., Utsunomiya I., Taguchi K. Vincristine-induced peripheral neuropathic pain and expression of transient receptor potential vanilloid 1 in rat. J. Pharmacol. Sci. 2017;133(4):254–260. doi: 10.1016/j.jphs.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 181.Chukyo A., Chiba T., Kambe T., Yamamoto K., Kawakami K., Taguchi K., Abe K. Oxaliplatin-induced changes in expression of transient receptor potential channels in the dorsal root ganglion as a neuropathic mechanism for cold hypersensitivity. 2017. [DOI] [PubMed]
- 182.Miao F., Wang R., Cui G., Li X., Wang T., Li X. Engagement of MicroRNA-155 in Exaggerated Oxidative Stress Signal and TRPA1 in the Dorsal Horn of the Spinal Cord and Neuropathic Pain During Chemotherapeutic Oxaliplatin. Neurotox. Res. 2019;36(4):712–723. doi: 10.1007/s12640-019-00039-5. [DOI] [PubMed] [Google Scholar]
- 183.Galley H.F., McCormick B., Wilson K.L., Lowes D.A., Colvin L., Torsney C. Melatonin limits paclitaxel-induced mitochondrial dysfunction in vitro and protects against paclitaxel-induced neuropathic pain in the rat. J. Pineal Res. 2017;63(4):e12444. doi: 10.1111/jpi.12444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Zhao X., Liu L., Wang Y., Wang G., Zhao Y., Zhang Y. Electroacupuncture enhances antioxidative signal pathway and attenuates neuropathic pain induced by chemotherapeutic paclitaxel. Physiol. Res. 2019;68(3):501–510. doi: 10.33549/physiolres.934084. [DOI] [PubMed] [Google Scholar]
- 185.Waseem M., Kaushik P., Tabassum H., Parvez S. Role of Mitochondrial mechanism in chemotherapy-induced peripheral neuropathy. Curr. Drug Metab. 2018;19(1):47–54. doi: 10.2174/1389200219666171207121313. [DOI] [PubMed] [Google Scholar]
- 186.Bai J.Z., Lipski J. Differential expression of TRPM2 and TRPV4 channels and their potential role in oxidative stress-induced cell death in organotypic hippocampal culture. Neurotoxicology. 2010;31(2):204–214. doi: 10.1016/j.neuro.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 187.Macpherson L.J., Dubin A.E., Evans M.J., Marr F., Schultz P.G., Cravatt B.F., Patapoutian A. Noxious compounds activate TRPA1 ion channels through covalent modification of cysteines. Nature. 2007;445(7127):541–545. doi: 10.1038/nature05544. [DOI] [PubMed] [Google Scholar]
- 188.Salazar H., Llorente I., Jara-Oseguera A., García-Villegas R., Munari M., Gordon S.E., Islas L.D., Rosenbaum T. A single N-terminal cysteine in TRPV1 determines activation by pungent compounds from onion and garlic. Nat. Neurosci. 2008;11(3):255–261. doi: 10.1038/nn2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Yoshida T., Inoue R., Morii T., Takahashi N., Yamamoto S., Hara Y., Tominaga M., Shimizu S., Sato Y., Mori Y. Nitric oxide activates TRP channels by cysteine S-nitrosylation. Nat. Chem. Biol. 2006;2(11):596–607. doi: 10.1038/nchembio821. [DOI] [PubMed] [Google Scholar]
- 190.Chung Y.W., Jeong D., Noh O.J., Park Y.H., Kang S.I., Lee M.G., Lee T.H., Yim M.B., Kim I.Y. Antioxidative role of selenoprotein W in oxidant-induced mouse embryonic neuronal cell death. Mol. Cells. 2009;27(5):609–613. doi: 10.1007/s10059-009-0074-3. [DOI] [PubMed] [Google Scholar]
- 191.Sen C.K., Packer L. Thiol homeostasis and supplements in physical exercise. Am. J. Clin. Nutr. 2000;72(2) Suppl.:653S–669S. doi: 10.1093/ajcn/72.2.653S. [DOI] [PubMed] [Google Scholar]
- 192.Liu B.Y., Tsai T.L., Ho C.Y., Lu S.H., Lai C.J., Kou Y.R. Role of TRPA1 and TRPV1 in the ROS-dependent sensory irritation of superior laryngeal capsaicin-sensitive afferents by cigarette smoke in anesthetized rats. Pulm. Pharmacol. Ther. 2013;26(3):364–372. doi: 10.1016/j.pupt.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 193.Straub I., Krügel U., Mohr F., Teichert J., Rizun O., Konrad M., Oberwinkler J., Schaefer M. Flavanones that selectively inhibit TRPM3 attenuate thermal nociception in vivo. Mol. Pharmacol. 2013;84(5):736–750. doi: 10.1124/mol.113.086843. [DOI] [PubMed] [Google Scholar]
- 194.Özdemir U.S., Nazıroğlu M., Şenol N., Ghazizadeh V. Hypericum perforatum attenuates spinal cord injury-induced oxidative stress and apoptosis in the dorsal root ganglion of rats: involvement of TRPM2 and TRPV1 channels. Mol. Neurobiol. 2016;53(6):3540–3551. doi: 10.1007/s12035-015-9292-1. [DOI] [PubMed] [Google Scholar]
- 195.Akpınar H., Nazıroğlu M., Övey I.S., Çiğ B., Akpınar O. The neuroprotective action of dexmedetomidine on apoptosis, calcium entry and oxidative stress in cerebral ischemia-induced rats: Contribution of TRPM2 and TRPV1 channels. Sci. Rep. 2016;6:37196. doi: 10.1038/srep37196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Shirakawa H., Yamaoka T., Sanpei K., Sasaoka H., Nakagawa T., Kaneko S. TRPV1 stimulation triggers apoptotic cell death of rat cortical neurons. Biochem. Biophys. Res. Commun. 2008;377(4):1211–1215. doi: 10.1016/j.bbrc.2008.10.152. [DOI] [PubMed] [Google Scholar]
- 197.Yeon K.Y., Kim S.A., Kim Y.H., Lee M.K., Ahn D.K., Kim H.J., Kim J.S., Jung S.J., Oh S.B. Curcumin produces an antihyperalgesic effect via antagonism of TRPV1. J. Dent. Res. 2010;89(2):170–174. doi: 10.1177/0022034509356169. [DOI] [PubMed] [Google Scholar]
- 198.Uslusoy F., Nazıroğlu M., Çiğ B. Inhibition of the TRPM2 and TRPV1 Channels through Hypericum perforatum in sciatic nerve injury-induced rats demonstrates their key role in apoptosis and mitochondrial oxidative stress of sciatic nerve and dorsal root ganglion. Front. Physiol. 2017;8:335. doi: 10.3389/fphys.2017.00335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Ogawa N., Kurokawa T., Mori Y. Sensing of redox status by TRP channels. Cell Calcium. 2016;60(2):115–122. doi: 10.1016/j.ceca.2016.02.009. [DOI] [PubMed] [Google Scholar]