Abstract
Background:
Topical diclofenac, a nonsteroidal anti-inflammatory drug, has proven efficacy and safety in the management of osteoarthritis pain. We investigated penetration of topical diclofenac into knee synovial tissue and fluid (primary objective) and evaluated relative exposure in the knee versus plasma (secondary objective).
Methods:
In this phase I, double-blind, multicenter study, patients scheduled for arthroplasty for end-stage knee osteoarthritis were randomly assigned 2:1 to 4 g diclofenac diethylamine 2.32% w/w gel (92.8 mg diclofenac diethylamine, equivalent to 74.4 mg diclofenac, per application) or placebo gel, applied to the affected knee by a trained nurse/designee every 12 h for 7 days before surgery. Diclofenac concentrations were measured in synovial tissue, synovial fluid and plasma from samples obtained during surgery ⩾12 h after last application. Treatment-emergent adverse events (TEAEs) were evaluated.
Results:
Evaluable synovial tissue or fluid samples were obtained from 45 (diclofenac n = 29; placebo n = 16) of 47 patients. All diclofenac-treated participants had measurable diclofenac concentrations in synovial tissue [geometric mean 1.57 (95% confidence interval (CI) 1.12, 2.20) ng/g] and fluid [geometric mean 2.27 (95% CI 1.87, 2.76) ng/ml] ⩾12 h after the last dose. Geometric mean (95% CI) ratio of diclofenac in synovial tissue:plasma was 0.32 (0.23, 0.45) and in synovial fluid:plasma was 0.46 (0.40, 0.54). TEAE rates were similar for diclofenac (55.2%) and placebo (58.8%); none were treatment related.
Conclusions:
Topical diclofenac diethylamine 2.32% w/w gel penetrated into the osteoarthritic knee after repeated application and remained detectable in synovial tissue and fluid at the end of the final 12 h dosing cycle.
Keywords: diclofenac, nonsteroidal anti-inflammatory agents, osteoarthritis, pharmacokinetics; tissue distribution
Introduction
Topical nonsteroidal anti-inflammatory drugs (NSAIDs) are a generally well-tolerated and effective treatment for pain related to osteoarthritis (OA).1–3 Clinical guidelines support a role for topical NSAIDs for symptom management in patients with knee and/or hand OA.4–9 Topical diclofenac, one of the most-studied topical NSAIDs, has a well-established efficacy and safety profile.1–3 In head-to-head trials, efficacy was at least equivalent to that of some oral NSAIDs.10–12 Adverse events (AEs) of topical diclofenac are primarily local skin and subcutaneous tissue disorders,13 with minimal systemic AEs due to low systemic concentrations (3–5% of total systemic absorption for oral diclofenac).14
Topical NSAIDs deliver active drug directly to the site of pain and inflammation, avoid first-pass metabolism, and minimize systemic AEs.15 Therapeutic efficacy is presumably dependent on skin penetration and the ability to deliver pharmacodynamically active concentrations to the underlying site of pain and inflammation in the affected joint.15,16 However, the disposition of topical diclofenac is not fully characterized, and no such studies have been performed using topical diclofenac diethylamine 2.32% w/w gel.
The current study investigated topical diclofenac diethylamine 2.32% w/w gel penetration into subdermal tissues and plasma. The primary objective was to determine whether diclofenac penetrates into the treated knee joint after repeated topical application. A post hoc analysis on the primary endpoint was done to determine whether diclofenac’s penetration of the knee joint was impacted by body mass index (BMI). The secondary objective was to evaluate relative exposure of diclofenac in the knee joint versus plasma. Exploratory objectives were to evaluate treatment effects on cyclooxygenase-2 (COX-2) inhibition and inflammatory cytokines in the knee joint.
Methods
Study design and procedures
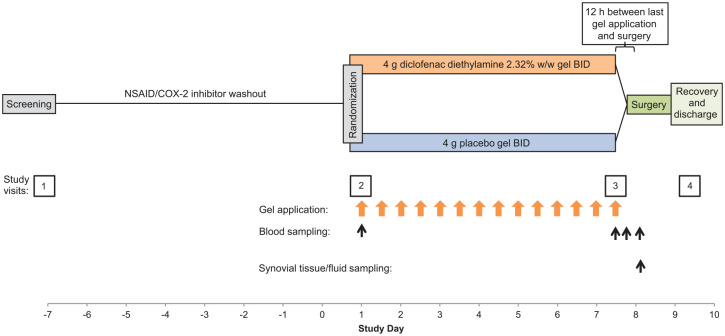
This phase I, randomized, double-blind, placebo-controlled steady-state pharmacokinetic study [ClinicalTrials.gov identifier: NCT03497039] was conducted at five European sites (four in the UK, one in Germany) from 19 July 2018 through 1 March 2019. Participants were randomly assigned 2:1 to receive 4 g diclofenac diethylamine 2.32% w/w gel (92.8 mg diclofenac diethylamine, equivalent to 74.4 mg diclofenac, per application) or placebo gel, applied to the target knee twice daily at 12 h intervals during the 7 days before scheduled knee arthroplasty for symptomatic end-stage OA (Figure 1).
Figure 1.
Study design.
BID, twice daily; COX-2, cyclo-oxygenase-2; NSAID, nonsteroidal anti-inflammatory drug.
There were four study-site assessment visits: a screening visit at study day −7, a baseline visit on study day 1 (7 days before surgery), a third assessment period lasting from hospital admittance the evening of day 7 through day 8 (day of surgery), and a final assessment before discharge, between days 8 and 10.
A trained nurse or designee applied study medication using a standardized method either at the study site (first/last dose) or at the participant’s home or other convenient location (all intervening doses). Each 4 g dose was measured using a dosing card, applied to a 400 cm2 surface of the anterior aspect of the knee, centred over the knee joint line, that was first marked with a surgical site marking pen and stencil, and rubbed into the skin for about 1 min. This dose represents the registered posology for this approved product in the countries where the study sites were located. The final dose was administered approximately 12 (−1 to +3) h before arthroplasty; if surgery was postponed, dosing continued for up to an additional week, after which the participant was withdrawn from the study if there were continued delays.
Paracetamol (maximum 4 g daily) was provided as rescue medication between screening and study day 7 to be used not only for knee pain but any pain (e.g. headache) or fever. Codeine or tramadol could be prescribed at the investigator’s discretion if additional relief was needed.
During surgery, a tourniquet was used to provide a bloodless surgical field. Synovial fluid was collected by aspiration of the joint before arthrotomy and partitioned into four aliquots of 2.5–3 ml. Two synovial tissue samples, approximately 2–3 cm3 each were obtained by sharp dissection. The synovium was resected from the supra-patellar pouch and from medial and lateral gutters; samples were not differentiated by specific site of collection. Samples were immediately frozen (−80°C) before being shipped to the lab for analysis. Diclofenac concentrations were measured in two aliquots each of synovial tissue, synovial fluid and plasma.
Blood was drawn between anaesthesia and surgery completion. Blood samples were also drawn within 1 h before the first treatment dose at baseline, within 1 h before the last treatment dose, and between the last dose and time of surgery.
Safety assessments included physical examinations and vital signs at all study visits; 12-lead electrocardiogram (ECG) and standard laboratory assessments at screening, before surgery and at the final visit; and assessment of AEs at all study visits and nurses’ home visits.
Randomization and blinding
Randomization was stratified by study centre. Participants were assigned numbers via an interactive response technology system according to a schedule generated by the statistics department at the contract research organization (PPD). Participants, investigators, study site staff, the statistician, the sponsor and any vendors who could influence study outcomes were blinded to treatment assignment. Active and placebo gels had identical odour, packaging, labelling and administration schedule, and were as identical as possible in appearance.
Selection of study population
Men and women ⩾50 years of age who had scheduled unilateral arthroplasty for treatment of OA with a radiographically confirmed Kellgren–Lawrence grade ⩾2 within the past 6 months were eligible to participate. Participants had to have a BMI of 17.5 to <40 kg/m2 and total body weight >50 kg, had to be fit for surgery with no clinically relevant abnormalities and had to be able and willing to comply with scheduled study procedures. Key exclusion criteria included damaged, open, or diseased skin around the knee, and acute or chronic medical or psychiatric condition or laboratory abnormality that could increase the participant’s risk, affect interpretation of study results, or interfere with drug absorption.
Participants could not use NSAIDs, COX-2 inhibitors, or dietary supplements within 7 days or five half-lives (whichever was longer) prior to the first dose of study medication and during the study (see washout period, Figure 1). Other prohibited medications included intra-articular or periarticular procedures or injections in either knee within 3 months of study entry, systemic corticosteroids within 6 weeks, and any anticoagulants (warfarin, heparin, etc.) within the preceding week or anti-aggregants (clopidogrel, ticagrelor, dipyridamole, abciximab, vorapaxar, etc.) within the past month. Permitted exceptions included stable low doses of aspirin started ⩾1 month before randomization and anticoagulant therapy for surgery. Any chondroprotectant or disease-modifying OA drugs (e.g. glucosamine or chondroitin sulfate) had to be stable for ⩾1 month prior to study entry and maintained throughout the study.
Complete inclusion and exclusion criteria are provided in online Supplemental Table S1.
Ethical considerations
The study protocol was reviewed and approved by an Ethics Committee in each country (Ethics Committee of the University of Würzburg, Versbacher Str. 9, Würzburg, Germany, D-97078; NHS Health Research Authority South Central: Hampshire A Research Ethics Committee, Level 3, Block B, Whitefriars, Lewins Mead, Bristol, BS1 2NT, UK). The study was conducted in accordance with the International Council for Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use Good Clinical Practice guidelines and the principles outlined in the Declaration of Helsinki. Participants provided written informed consent prior to performance of any study procedure.
Study outcomes
Primary pharmacokinetic endpoints consisted of diclofenac concentrations in synovial tissue and synovial fluid of the treated knee 12 h after the last diclofenac application in the 7-day treatment period. Secondary pharmacokinetic endpoints included the ratio of diclofenac concentrations in the synovial tissue of the treated knee and plasma concentration and the ratio of diclofenac concentrations in the synovial fluid of the treated knee and plasma concentration at time of surgery. Exploratory pharmacodynamic (PD) endpoints included prostaglandin E2 (PGE2), interleukin-6 (IL-6), and tumour necrosis factor alpha (TNFα) levels in synovial tissue and fluid of the treated knee. Safety outcomes consisted of incidence, severity, and relation to treatment of treatment-emergent AEs (TEAEs) and serious AEs; laboratory, vital sign, or ECG abnormalities; TEAEs leading to treatment or study discontinuation; and deaths.
Bioanalytical methodology
A detailed description of the bioanalytical methodology is provided in the online supplemental material.
Diclofenac concentrations in synovial tissue, synovial fluid, and plasma were assayed by high-performance liquid chromatography tandem mass spectrometry, using diclofenac-d4 as the internal standard. The lower limit of quantitation was 0.23 ng/g in synovial tissue, 0.10 ng/ml in synovial fluid, and 0.098 ng/ml in plasma. Across the range of quantitation, the percent coefficient of variation (% CV) ranged from 0.1% to 2.9% in synovial tissue, 0.9% to 5.5% in synovial fluid, and 0.9% to 2.9% in plasma; the percent bias was −0.9% to 1%, −1.7% to 3.0% and −9.3% to 4.8% in synovial tissue, synovial fluid and plasma, respectively.
PGE2 concentrations in synovial tissue and fluid were measured using an enzyme-linked immunosorbent assay. The lower limit of quantification was 2.62 ng/ml in synovial tissue and 0.013 ng/ml in synovial fluid. Percent relative error (%RE) for PGE2 detection was −3.2% to 3.2%; % CV was 2.9–11.2%.
IL-6 and TNFα were measured with an electrochemiluminescence assay with a Meso Scale Discovery (MSD) Sector Imager 6000 (Meso Scale Diagnostics, Rockville, Maryland, USA) electrochemiluminescence reader. Diluted samples were analyzed using an MSD V-PLEX human cytokine multiplex pro-inflammatory panel (excluding pro-inflammatory markers other than IL-6 and TNFα) (Meso Scale Diagnostics). Analysis was done using MSD’s Discovery Workbench version V4.0.12.1 software. The range of quantification was 3.16–976.00 pg/ml for IL-6 and 1.38–496.00 pg/ml for TNFα in both synovial tissue and fluid. Intra-assay accuracy (%bias) and precision (%CV) for IL-6 and TNFα were both 20% (25% upper and lower limit of quantification).
Statistical analyses
No formal estimation of sample size was conducted. Enrolment of 50 participants was planned to ensure evaluable data from at least 45 (30 assigned to active treatment and 15 to placebo), which was considered adequate to characterize diclofenac levels in synovial tissue and synovial fluid based on similar studies of oral or topical NSAIDs or joint inflammation.17–22 No power calculations were made for exploratory PD endpoints.
The safety population comprised all participants who were randomized and received at least one dose of study treatment. The analysis population comprised those from the safety population who completed surgery and had evaluable synovial tissue or fluid samples.
Diclofenac concentrations in synovial tissue and fluid were summarized descriptively. Geometric means with their two-sided 95% confidence interval (CI) were calculated, assuming data on the log scale were normally distributed (subsequently confirmed on the data using q–q plots). Geometric means provide a robust summary measure accounting for the specific nature of concentration data, which are bounded by zero and positively skewed, as illustrated by a median lower than the arithmetic mean. While no formal hypothesis testing was performed, the criterion for success was that diclofenac would be detectable within synovial tissue or fluid. Post hoc analyses were performed to calculate Spearman’s rank correlation coefficient (r) between BMI and diclofenac concentration in synovial tissue and synovial fluid.
The same statistical approach as for the primary outcomes was used to summarize the ratios between diclofenac concentrations in synovial tissue or fluid and plasma and the concentrations of PGE2, IL-6 and TNFα. For each exploratory endpoint, a two-sided t-test at an alpha level of 0.05 was conducted to compare the log-transformed mean levels. The geometric mean ratio between the treatment groups was calculated as a measure of the contrast between the groups, also known as the effect size. The geometric mean ratio gives an estimate of the relative effect of diclofenac versus placebo on the corresponding PD biomarker. The associated 95% CI was calculated to give a range of plausible values (i.e. compatible with the observed data) for this effect.
All analyses were conducted using SAS version 9.4 software (SAS Institute, New York, USA).
Results
Subject disposition, baseline characteristics, and compliance
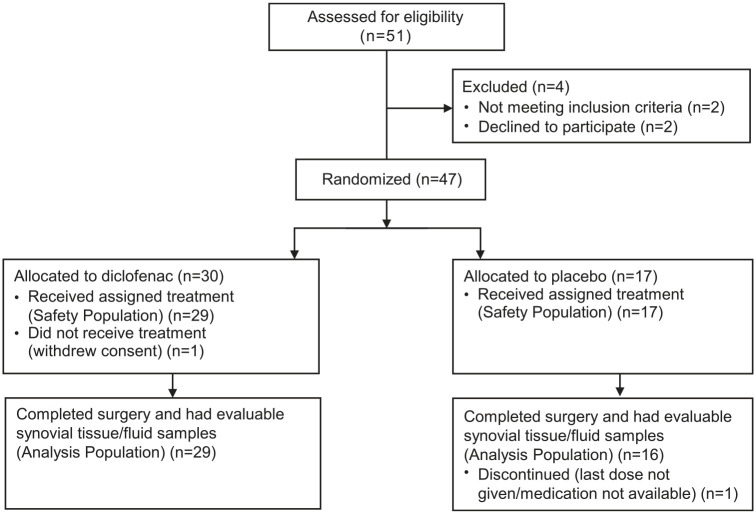
A total of 47 participants were enrolled: 30 in the diclofenac group and 17 in the placebo group. A total of 45 (95.7%; diclofenac n = 29; placebo n = 16) completed the study and had evaluable synovial tissue or fluid (Figure 2). Surgery was postponed past day 7 in 5/45 participants (2 diclofenac, 3 placebo). Average number of days from baseline to surgery was 7.27 [standard deviation (SD) 1.01]. All participants had protocol deviations (Supplemental Table S2), which most commonly concerned collection and handling of samples and storage of study treatment. The bioanalytical laboratory determined that none of these protocol deviations prevented proper analysis of the samples.
Figure 2.
Participant flow.
Mean (SD) age was 71.2 (7.9) years, 52.2% were women, and mean (SD) BMI was 30.7 (4.8) kg/m2 (Table 1). All subjects had 100% of scheduled treatment applications, and mean exposure was 59.17 g of gel in the diclofenac group and 61.3 g in the placebo group. Rescue medication (paracetamol) was used by 79.3% of the diclofenac group and 75.0% of the placebo group; median (range) number of 500 mg tablets used was 8 (1–54) and 13 (6–42), respectively. In addition, 16.7% of the diclofenac group and 17.6% of the placebo group used NSAIDs or corticosteroids between screening and collection of synovial samples, although these medications were prohibited during the trial.
Table 1.
Demographics, safety population.
| Diclofenac diethylamine 2.32% w/w gel (n = 29) | Placebo gel (n = 17) | |
|---|---|---|
| Age, mean (SD), years | 70.9 (7.6) | 71.7 (8.6) |
| Sex, n (%) | ||
| Female | 15 (51.7) | 9 (52.9) |
| Male | 14 (48.3) | 8 (47.1) |
| Height, mean (SD), cm | 168.0 (7.8) | 166.3 (8.9) |
| Weight, mean (SD), kg | 88.0 (15.9) | 82.8 (10.8) |
| BMI, mean (SD), kg/m2 | 31.2 (5.3) | 30.0 (4.0) |
BMI, body mass index; SD, standard deviation.
Synovial tissue and fluid concentrations
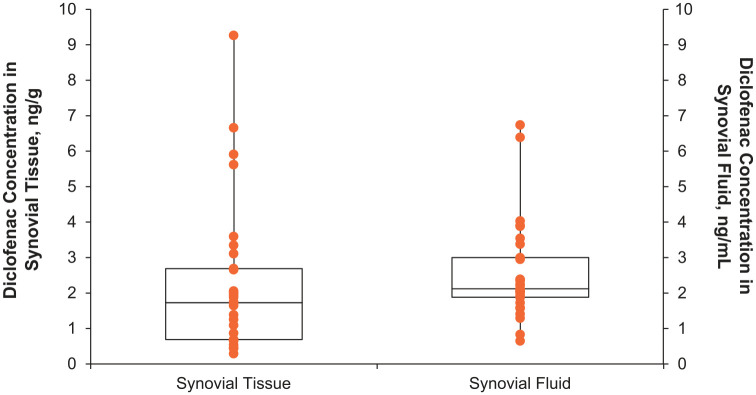
All participants treated with topical diclofenac had measurable concentrations of diclofenac in synovial tissue and synovial fluid at 12–15 h after the last application (Figure 3). Geometric mean (95% CI) diclofenac concentrations were 1.57 (1.12, 2.20) ng/g in synovial tissue and 2.27 (1.87, 2.76) ng/ml in synovial fluid (Table 2), which were well above the limits of detection for the assay (0.23 ng/g and 0.10 ng/ml, respectively). Diclofenac concentrations ranged from 0.29 to 9.27 ng/g in synovial tissue and 0.65–6.74 ng/ml in synovial fluid. Thus, although there was a degree of variability in individual synovial tissue and fluid concentrations, diclofenac penetrated into the affected joint in all participants. No correlation (r = −0.003) between BMI and synovial fluid diclofenac concentration, and weak positive correlation (r = 0.315) between BMI and synovial tissue diclofenac concentration were observed. These results suggest that BMI has no impact on diclofenac’s penetration into the knee.
Figure 3.
Diclofenac concentration in synovial tissue and synovial fluid 12 h after last diclofenac dose in a 7-day topical treatment regimen, analysis population.
Table 2.
Diclofenac concentrations in synovial tissue, synovial fluid, and plasma 12 h after last administration of topical diclofenac diethylamine 2.32% w/w gel 4 g BID for 7 days (n = 29), analysis population.
| Synovial tissue concentration, ng/ga | Synovial fluid concentration, ng/mla | Plasma concentration, ng/ml | |
|---|---|---|---|
| Median | 1.73 | 2.12 | 4.76 |
| Range | 0.29–9.27 | 0.65–6.74 | 0.92–16.72 |
| Geometric meanb | 1.57 | 2.27 | ND |
| 95% CI | 1.12, 2.20 | 1.87, 2.76 | ND |
Primary endpoint.
The geometric mean (95% CI) is calculated by back-transforming the mean (95% CI) of the log-transformed data.
BID, twice daily; CI, confidence interval; ND, not determined.
Ratio of synovial tissue and fluid concentrations to plasma concentrations
Plasma concentrations are reported descriptively in Table 2. The geometric mean (95% CI) ratio of diclofenac concentration in synovial tissue:plasma was 0.32 (0.23, 0.45) and the ratio of synovial fluid:plasma was 0.46 (0.40, 0.54), indicating greater diclofenac concentrations in plasma than in the joint 12 h after the last dose (i.e. trough levels; Table 3).
Table 3.
Ratios of diclofenac concentration in synovial tissue:plasma and synovial fluid:plasma 12 h after last administration of topical diclofenac diethylamine 2.32% w/w gel 4 g BID for 7 days (diclofenac group, n = 29), analysis population.
| Ratio of synovial tissue concentration to plasma concentration, (ng/g)/(ng/ml) | Ratio of synovial fluid concentration to plasma concentration, (ng/ml)/(ng/ml) | |
|---|---|---|
| Median | 0.242 | 0.448 |
| Range | 0.10–10.04 | 0.20–2.19 |
| Geometric meana | 0.320 | 0.463 |
| 95% CI | 0.228, 0.450 | 0.397, 0.539 |
The geometric mean (95% CI) is calculated by back-transforming the mean (95% CI) of the log-transformed data.
BID, twice daily; CI, confidence interval.
Exploratory endpoints: inflammatory markers
Concentrations of PGE2, IL-6, and TNFα in synovial tissue and fluid at about 12 h after the last dose are presented in Supplemental Table S3. Results of this exploratory analysis were inconclusive. TNFα and IL-6 could not be quantified in synovial tissue, and TNFα was quantifiable in synovial fluid in <35% of the diclofenac group and 25% of the placebo group. Even for parameters that were satisfactorily quantified (PGE2 in synovial tissue and fluid and IL-6 in synovial fluid), the observed variability was too large to draw conclusions regarding diclofenac’s effect. The 95% CIs for the geometric mean ratios (which give a range of plausible values for the relative effect of diclofenac versus placebo on the corresponding biomarkers) were wide.
Safety
Overall, 16 (55.2%) participants treated with topical diclofenac and 10 (58.8%) in the placebo group experienced TEAEs, most commonly nausea (17.2% versus 11.8%), vomiting (13.8% versus 5.9%, all in the postoperative setting) and falls (10.3% versus 0; Table 4). No TEAEs were considered by the investigator to be related to treatment.
Table 4.
Summary of safety outcomes, safety population.
| Diclofenac diethylamine 2.32% w/w gel (n = 29), n (%) | Placebo gel (n = 17), n (%) | |
|---|---|---|
| Any AE | 18 (62.1) | 11 (64.7) |
| Any TEAE | 16 (55.2) | 10 (58.8) |
| Serious TEAEs | 1 (3.4) | 0 |
| Treatment-related TEAEs | 0 | 0 |
| TEAEs leading to treatment discontinuation | 0 | 0 |
| TEAEs leading to study discontinuation | 0 | 0 |
| Deaths | 0 | 0 |
| TEAEs occurring in ⩾2 participants in either arm | ||
| Nausea | 5 (17.2) | 2 (11.8) |
| Vomiting | 4 (13.8) | 1 (5.9) |
| Fall | 3 (10.3) | 0 |
| Bursal fluid accumulation | 2 (6.9) | 1 (5.9) |
| C-reactive protein increased | 2 (6.9) | 1 (5.9) |
| Dizziness | 0 | 2 (11.8) |
AE, adverse event; TEAE, treatment-emergent adverse event.
One participant in the diclofenac group experienced two serious TEAEs consisting of moderate grade 2 Escherichia coli urinary tract infection and moderate grade 2 hypotension. The only severe TEAE was a postprocedural complication (vasovagal syncope on first mobilization, not considered diclofenac related) in a participant who had received diclofenac. There were no deaths; no TEAEs leading to study drug or study discontinuation, or dose reduction or interruption; and no clinically notable changes in laboratory parameters or vital signs.
Discussion
Local concentrations of NSAIDs in the joint are thought to be important to their therapeutic effect in management of OA-related pain because inflammation in the joint is a key component of the pathogenesis, and synovitis in particular is associated with joint pain.17,23–26 Like all NSAIDs, diclofenac relieves pain by preferentially blocking COX-2, thereby inhibiting production of pro-inflammatory PGE227–29 and limiting prostaglandin-induced inflammation and pain.28,30 The study met its primary objective of demonstrating that diclofenac diethylamine 2.32% w/w gel penetrates into underlying target tissues after repeated topical application to the knee in patients with OA. Diclofenac was detected in the synovial tissue and synovial fluid at 12–15 h after the last application in all subjects treated with diclofenac diethylamine 2.32% w/w gel for 7 days. Thus, local exposure at the site of action persisted through the 12 h dosing interval, supporting the current twice-daily dosing posology.
Mean BMI in the diclofenac group was 31.2 kg/m2. The high prevalence of obesity in our study participants is not unexpected because obesity is a risk factor for knee OA.31 In all participants, diclofenac was detectable after 12 h, including in those who were overweight or obese and presumably had a thicker fatty tissue layer to penetrate compared with normal-weight individuals. In the post hoc analysis, no correlation between BMI and synovial fluid diclofenac concentration, and a weak positive correlation between BMI and synovial tissue diclofenac concentration were observed. Taken together, these results suggest that BMI does not impact diclofenac penetration into the knee. The mechanisms by which highly protein-bound topical agents such as diclofenac penetrate into deep tissue largely involve convective blood, lymphatic and interstitial flow.32 These mechanisms likely apply to adipose tissue as well, because adipose tissue is highly vascularized,33 which may explain the lack of a negative correlation between BMI and diclofenac penetration.
Our overall findings on the primary endpoint support those of previous studies showing that topical diclofenac permeates underlying tissues and enters the synovium.22,34–36 For example, in patients undergoing arthroplasty for knee joint effusions, after 3 days of topical diclofenac sodium 4% spray gel (80 mg/d or 120 mg/d) application, the median (range) diclofenac concentration in synovial tissue was 36.2 (1.2–1232.0) ng/g with the 80 mg dose and 42.8 (0.8–594.0) ng/g with 120 mg. The median (range) concentration in synovial fluid was 2.6 (0.4–408.5) ng/ml and 2.8 (0.2–47.1) ng/ml, respectively, and in plasma was 3.9 (1.3–302.2) ng/ml and 4.1 (1.1–23.0) ng/ml, respectively.22 In patients undergoing arthroplasty for knee OA, a single dose of diclofenac sodium tape (15 mg) applied in two strips to the medial and lateral aspects of the knee 12 h before surgery resulted in mean (SD) diclofenac concentrations of 4.99 (3.84) ng/ml in synovial membrane, 1.96 (0.68) ng/ml in synovial fluid, and 4.70 (1.95) ng/ml in plasma.35 In another study, 23 patients scheduled for knee arthroplasty for OA (91%), trauma (4.5%) or polyarthritis (4.5%) were treated with 80 mg diclofenac diethylammonium emulsion gel three times daily for 2–5 days through the morning of surgery, applied medially and laterally (40 mg each) to either the presurgical or contralateral knee.36 When the gel was applied to the affected knee, mean (range) diclofenac concentrations were 25.4 (5.7–81.2) ng/mg in the synovial membrane, 19.2 (4.5–87.1) ng/ml in the synovia, and 18.0 (4.8–44.9) ng/ml in the plasma.36 Variability in diclofenac concentrations across studies may relate to differences in study parameters such as dosing regimens, dosing frequency, sampling time, administration site, diclofenac formulation, thickness of participants’ stratum corneum layer, and body mass and constitution.
Geometric means and their 95% CIs of diclofenac concentration in synovial tissue:plasma and synovial fluid:plasma ratios were all below 1, indicating greater concentrations in plasma than in the joint at ⩾12 h after the final dose. This differs from prior studies of topical diclofenac described previously, which typically showed plasma levels that were lower than synovial tissue levels and higher than or similar to synovial fluid levels.22,35,36
Speculating on potential reasons why our results do not conform to this pattern, one may suspect inferior and/or slower direct penetration to the joint. However, it is also conceivable that tissue penetration is particularly fast, along with facilitated systemic redistribution and low retention within the joint. The latter scenario would suggest that diclofenac diethylamine 2.32% w/w gel might be a rapidly effective topical formulation, and the maximum concentration in synovial tissue and fluid along with a high fluid/tissue-to-plasma ratio occur early during the anticipated 12 h duration of action. Accordingly, our results would confirm that even after the end of the 12 h dosing interval, significant concentrations of the active compound are still detectable in the synovial fluid and tissue.
This hypothesis of sufficient tissue penetration to yield an appropriate analgesic effect across the 12 h dosing interval is supported by the finding that a smaller proportion of participants in the diclofenac group required paracetamol rescue medication compared with the placebo group, even though the minimum effective diclofenac concentrations in synovial tissue, synovial fluid and plasma are still being defined. Furthermore, topical diclofenac has established efficacy in a variety of conditions, including OA.1–3,37–41
IL-1β and TNFα are predominant pro-inflammatory cytokines that regulate production of various other pro-inflammatory cytokines, such as IL-6 and IL-8.42–44 Inflammatory cytokines downstream of PGE2 in the signal transduction pathway (e.g. TNFα, IL-6) are reduced when PGE2 production is inhibited by NSAIDs.29,45 Therefore, this study sought to characterize these inflammatory biomarkers to further explore whether they were reduced in the presence of diclofenac in the joint. It is important to note that the study was not statistically powered to detect a difference between treatment groups for these PD biomarkers, as they were exploratory endpoints. At the time of study design, no in vivo data were available on the effect of diclofenac on these markers. As such, the potential size of the effect, variability of the data and probability of detecting a difference between the groups were unknown. In the end, the precision around the estimates derived in the study was poor, which can be put into perspective with several considerations. First, the variability of the PD biomarkers data was found to be high compared with the relatively small number of participants included in the study. Secondly, PD markers were only measured once, during surgery, and not at baseline, which avoided the burden of an additional invasive procedure but did not allow for within-subject comparisons, which would have reduced variability. Finally, a 2:1 randomization ratio was chosen to put emphasis on the primary and secondary objectives by enrolling more subjects in the diclofenac arm, but was suboptimal for exploratory comparisons; a 1:1 randomization ratio would have increased the precision around the observed effect size.
Levels of PGE2, IL-6, and TNFα in synovial tissue and fluid of patients with OA are highly variable,17,46,47 and published data regarding the minimal effective therapeutic concentrations of diclofenac in target tissues as assessed by the diclofenac IC50 (i.e. the concentration that produces 50% of the maximum inhibition of prostaglandin synthesis) for PGE2 are inconsistent.15 Accordingly, the minimum concentration of diclofenac in synovial tissue and fluid required to produce meaningful reductions in these biomarkers remains to be characterized. Even though synovial tissue and fluid concentrations attained in this study may appear comparatively low, we cannot draw any conclusions as to whether these were sufficient to yield a clinically meaningful effect in terms of pain reduction.
Our results were in line with those of Rosengren et al.48 who found no detectable TNFα in synovial tissue from 15 patients undergoing hip or knee replacement for OA. The low or undetectable levels of TNFα and IL-6 may be due to limited inflammatory activity in the knee joints of our participants, resulting from limited loading of the joint in advance of surgery or longstanding, advanced-stage OA (which is associated with less inflammation compared with early-stage OA17). Low/undetectable levels of inflammatory markers even in the placebo group confirm minimal inflammatory activity in these joints, limiting the potential for further reductions in those parameters. Research is ongoing to determine what other mediators of local pain and inflammation may play a role in patient-experienced discomfort; it is unknown to what extent those mediators are affected when exposed to diclofenac in the joint.
Topical diclofenac diethylamine 2.32% w/w gel had an overall rate of TEAEs similar to that of placebo, and there were no treatment-related TEAEs. It has been reported that AEs with topical diclofenac are largely local application-site reactions13,49 with fewer systemic effects, especially gastrointestinal events and liver-enzyme elevations, compared with oral NSAIDs.49 Here, although the incidence of gastrointestinal TEAEs was numerically higher in the diclofenac arm than in the placebo arm, none were considered treatment related. Local reactions at the application site were not more common with diclofenac than placebo.
This study has a number of strengths and limitations. Concentrations of diclofenac and inflammatory biomarkers were objectively measured using validated procedures. This study used a newer assay with an improved ability to detect low concentrations of diclofenac (lower limit of quantitation: 0.233 ng/g in synovial tissue, 0.100 ng/ml in synovial fluid, and 0.0977 ng/ml in plasma) compared with previous studies (synovial tissue: 0.24 to 1.50 ng/g; synovial fluid: ⩽0.15–8.00 ng/ml; plasma: ⩽0.15–8.00 ng/ml).22,35,36 Treatment was applied by a trained nurse or designee using a standardized procedure to ensure consistent dosing and compliance. One limitation is that the synovial tissue and fluid were collected only at 12 h after last dose (the end of the dosing cycle), after 7 days of diclofenac application. Thus, diclofenac concentrations and cytokine levels earlier in the dosing cycle were not assessed. In addition, the study population consisted of those undergoing knee arthroplasty for OA and is not necessarily generalizable to those with earlier-stage OA. This phase II study was designed to assess pharmacokinetics only and did not include efficacy outcomes; accordingly, evaluating the relationship between diclofenac concentrations in the joint and therapeutic efficacy should be subject to further clinical studies, building on these findings.
Conclusion
Topical diclofenac diethylamine 2.32% w/w gel 4 g applied twice daily for 7 days was absorbed by the skin and successfully penetrated into the joint, with detectable levels found in both synovial tissue and fluid at the end of the final 12 h dosing interval. Additional studies are needed to identify the minimum concentration in synovial tissue, synovial fluid, and/or plasma needed to obtain pain relief, and to clarify the effects of topical diclofenac on inflammatory biomarkers. Topical diclofenac diethylamine 2.32% w/w gel had a similar incidence of TEAEs compared with placebo and no TEAEs were considered by the investigator to be treatment related.
Supplemental Material
Supplemental material, Seefried_et_al_SUPPLEMENTAL_INFO_FOR_RESUBMISSION_6-8-20_1 for Penetration of topical diclofenac into synovial tissue and fluid of osteoarthritic knees: a multicenter, randomized, placebo-controlled, pharmacokinetic study by Lothar Seefried, Mark Blyth, Rohit Maheshwari, Stephen M. McDonnell, Guillaume Frappin, Martina Hagen, Nadine Maybaum, Sebastian Moreira and Hemant Pandit in Therapeutic Advances in Musculoskeletal Disease
Acknowledgments
The authors thank all of the patients and the clinical and research staff for their contributions to this study. The authors would also like to acknowledge Kamal Deep, MD, from Golden Jubilee National Hospital, Clydebank, United Kingdom, who was a trial investigator. Medical writing assistance was provided to the authors by Peloton Advantage, an OPEN Health company, Parsippany, NJ, and funded by GSK Consumer Healthcare S.A., Nyon, Switzerland.
Footnotes
Conflict of interest statement: Lothar Seefried’s institution (University of Würzburg) received financial support from GSK for participating in this study.
Mark Blyth reports no conflicts of interest.
Rohit Maheshwari reports no conflicts of interest.
Stephen M. McDonnell reports no conflicts of interest.
Guillaume Frappin, Martina Hagen, Nadine Maybaum, and Sebastian Moreira are employees of GSK Consumer Healthcare.
Hemant Pandit’s institution (University of Leeds) received financial support from GSK for participating in this study.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: the sponsor, GSK Consumer Healthcare S.A., Nyon, Switzerland, participated in the study design, as well as data collection, analysis and interpretation. The sponsor provided funding for medical writing support and participated in the decision to submit the manuscript for publication.
ORCID iD: Lothar Seefried  https://orcid.org/0000-0003-1154-3388
https://orcid.org/0000-0003-1154-3388
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Lothar Seefried, Clinical Trial Unit, Orthopaedic Department, University Würzburg, Brettreichtstr 11, Würzburg, Bavaria 97074, Germany.
Mark Blyth, Department of Orthopaedic Surgery, Glasgow Royal Infirmary, Glasgow, UK.
Rohit Maheshwari, Department of Orthopaedic Surgery, Golden Jubilee National Hospital, Clydebank, UK.
Stephen M. McDonnell, Addenbrooke’s Hospital, Cambridge University Hospitals NHS Foundation Trust, Cambridge, UK
Guillaume Frappin, GlaxoSmithKline Consumer Healthcare S.A., Nyon, Switzerland.
Martina Hagen, GlaxoSmithKline Consumer Healthcare S.A., Nyon, Switzerland.
Nadine Maybaum, GSK Consumer Healthcare, Warren, NJ, USA.
Sebastian Moreira, GSK Consumer Healthcare, Warren, NJ, USA.
Hemant Pandit, Leeds Institute of Rheumatic and Musculoskeletal Medicine, University of Leeds, Chapel Allerton Hospital, Leeds, UK.
References
- 1. Zeng C, Wei J, Persson MSM, et al. Relative efficacy and safety of topical non-steroidal anti-inflammatory drugs for osteoarthritis: a systematic review and network meta-analysis of randomised controlled trials and observational studies. Br J Sports Med 2018; 52: 642–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Stewart M, Cibere J, Sayre EC, et al. Efficacy of commonly prescribed analgesics in the management of osteoarthritis: a systematic review and meta-analysis. Rheumatol Int 2018; 38: 1985–1997. [DOI] [PubMed] [Google Scholar]
- 3. Derry S, Conaghan P, Da Silva JA, et al. Topical NSAIDs for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev 2016; 4: CD007400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Treatment of osteoarthritis of the knee. Evidence-based guideline. 2nd ed. Rosemont, IL: American Academy of Orthopaedic Surgeons, 2013. [DOI] [PubMed] [Google Scholar]
- 5. Kolasinski SL, Neogi T, Hochberg MC, et al. 2019 American College of Rheumatology/Arthritis Foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Care Res (Hoboken) 2020; 72: 149–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bruyere O, Cooper C, Pelletier JP, et al. A consensus statement on the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) algorithm for the management of knee osteoarthritis-from evidence-based medicine to the real-life setting. Semin Arthritis Rheum 2016; 45: S3–S11. [DOI] [PubMed] [Google Scholar]
- 7. Kloppenburg M, Kroon FP, Blanco FJ, et al. 2018 update of the EULAR recommendations for the management of hand osteoarthritis. Ann Rheum Dis 2019; 78: 16–24. [DOI] [PubMed] [Google Scholar]
- 8. Bannuru RR, Osani MC, Vaysbrot EE, et al. OARSI guidelines for the non-surgical management of knee, hip, and polyarticular osteoarthritis. Osteoarthritis Cartilage 2019; 27: 1578–1589. [DOI] [PubMed] [Google Scholar]
- 9. Rillo O, Riera H, Acosta C, et al. PANLAR consensus recommendations for the management in osteoarthritis of hand, hip, and knee. J Clin Rheumatol 2016; 22: 345–354. [DOI] [PubMed] [Google Scholar]
- 10. Tugwell PS, Wells GA, Shainhouse JZ. Equivalence study of a topical diclofenac solution (Pennsaid®) compared with oral diclofenac in symptomatic treatment of osteoarthritis of the knee: a randomized controlled trial. J Rheumatol 2004; 31: 2002–2012. [PubMed] [Google Scholar]
- 11. Simon LS, Grierson LM, Naseer Z, et al. Efficacy and safety of topical diclofenac containing dimethyl sulfoxide (DMSO) compared with those of topical placebo, DMSO vehicle and oral diclofenac for knee osteoarthritis. Pain 2009; 143: 238–245. [DOI] [PubMed] [Google Scholar]
- 12. Zacher J, Burger KJ, Farber L, et al. Topical diclofenac emulgel versus oral ibuprofen in the treatment of active osteoarthritis of the finger joints (Heberden’s and/or Bouchard’s nodes): a double-blind, controlled, randomized study. Postgrad Med 2011; 123: 1–7. [Google Scholar]
- 13. Honvo G, Leclercq V, Geerinck A, et al. Safety of topical non-steroidal anti-inflammatory drugs in osteoarthritis: outcomes of a systematic review and meta-analysis. Drugs Aging 2019; 36: 45–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Altman R, Bosch B, Brune K, et al. Advances in NSAID development: evolution of diclofenac products using pharmaceutical technology. Drugs 2015; 75: 859–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hagen M, Baker M. Skin penetration and tissue permeation after topical administration of diclofenac. Curr Med Res Opin 2017; 33: 1623–1634. [DOI] [PubMed] [Google Scholar]
- 16. Singh P, Roberts MS. Skin permeability and local tissue concentrations of nonsteroidal anti-inflammatory drugs after topical application. J Pharmacol Exp Ther 1994; 268: 144–151. [PubMed] [Google Scholar]
- 17. Benito MJ, Veale DJ, FitzGerald O, et al. Synovial tissue inflammation in early and late osteoarthritis. Ann Rheum Dis 2005; 64: 1263–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fowler PD, Shadforth MF, Crook PR, et al. Plasma and synovial fluid concentrations of diclofenac sodium and its major hydroxylated metabolites during long-term treatment of rheumatoid arthritis. Eur J Clin Pharmacol 1983; 25: 389–394. [DOI] [PubMed] [Google Scholar]
- 19. Alvarez-Soria MA, Largo R, Santillana J, et al. Long term NSAID treatment inhibits COX-2 synthesis in the knee synovial membrane of patients with osteoarthritis: differential proinflammatory cytokine profile between celecoxib and aceclofenac. Ann Rheum Dis 2006; 65: 998–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gallelli L, Galasso O, Urzino A, et al. Characteristics and clinical implications of the pharmacokinetic profile of ibuprofen in patients with knee osteoarthritis. Clin Drug Investig 2012; 32: 827–833. [DOI] [PubMed] [Google Scholar]
- 21. Gallelli L, Galasso O, Falcone D, et al. The effects of nonsteroidal anti-inflammatory drugs on clinical outcomes, synovial fluid cytokine concentration and signal transduction pathways in knee osteoarthritis. A randomized open label trial. Osteoarthritis Cartilage 2013; 21: 1400–1408. [DOI] [PubMed] [Google Scholar]
- 22. Efe T, Sagnak E, Roessler PP, et al. Penetration of topical diclofenac sodium 4% spray gel into the synovial tissue and synovial fluid of the knee: a randomised clinical trial. Knee Surg Sports Traumatol Arthrosc 2014; 22: 345–350. [DOI] [PubMed] [Google Scholar]
- 23. Fu K, Robbins SR, McDougall JJ. Osteoarthritis: the genesis of pain. Rheumatology (Oxford) 2018; 57: iv43–iv50. [DOI] [PubMed] [Google Scholar]
- 24. Hunter DJ, Bierma-Zeinstra S. Osteoarthritis. Lancet 2019; 393: 1745–1759. [DOI] [PubMed] [Google Scholar]
- 25. Baker K, Grainger A, Niu J, et al. Relation of synovitis to knee pain using contrast-enhanced MRIs. Ann Rheum Dis 2010; 69: 1779–1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Leung YY, Huebner JL, Haaland B, et al. Synovial fluid pro-inflammatory profile differs according to the characteristics of knee pain. Osteoarthritis Cartilage 2017; 25: 1420–1427. [DOI] [PubMed] [Google Scholar]
- 27. Brune K. Persistence of NSAIDs at effect sites and rapid disappearance from side-effect compartments contributes to tolerability. Curr Med Res Opin 2007; 23: 2985–2995. [DOI] [PubMed] [Google Scholar]
- 28. Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol 2011; 31: 986–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Van Erk MJ, Wopereis S, Rubingh C, et al. Insight in modulation of inflammation in response to diclofenac intervention: a human intervention study. BMC Med Genomics 2010; 3: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Minami T, Nakano H, Kobayashi T, et al. Characterization of EP receptor subtypes responsible for prostaglandin E2-induced pain responses by use of EP1 and EP3 receptor knockout mice. Br J Pharmacol 2001; 133: 438–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Jiang L, Tian W, Wang Y, et al. Body mass index and susceptibility to knee osteoarthritis: a systematic review and meta-analysis. Joint Bone Spine 2012; 79: 291–297. [DOI] [PubMed] [Google Scholar]
- 32. Dancik Y, Anissimov YG, Jepps OG, et al. Convective transport of highly plasma protein bound drugs facilitates direct penetration into deep tissues after topical application. Br J Clin Pharmacol 2012; 73: 564–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gu P, Xu A. Interplay between adipose tissue and blood vessels in obesity and vascular dysfunction. Rev Endocr Metab Disord 2013; 14: 49–58. [DOI] [PubMed] [Google Scholar]
- 34. Brunner M, Dehghanyar P, Seigfried B, et al. Favourable dermal penetration of diclofenac after administration to the skin using a novel spray gel formulation. Br J Clin Pharmacol 2005; 60: 573–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Miyatake S, Ichiyama H, Kondo E, et al. Randomized clinical comparisons of diclofenac concentration in the soft tissues and blood plasma between topical and oral applications. Br J Clin Pharmacol 2009; 67: 125–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gondolph-Zink B, Gronwald U. [Active substance concentrations in articular and periarticular tissues of the knee joint after the cutaneous application of diclofenac diethelammonium Emulgel]. Akt Rheumatol 1996; 21: 298–304. [Google Scholar]
- 37. Niethard FU, Gold MS, Solomon GS, et al. Efficacy of topical diclofenac diethylamine gel in osteoarthritis of the knee. J Rheumatol 2005; 32: 2384–2392. [PubMed] [Google Scholar]
- 38. Altman RD, Dreiser RL, Fisher CL, et al. Diclofenac sodium gel in patients with primary hand osteoarthritis: a randomized, double-blind, placebo-controlled trial. J Rheumatol 2009; 36: 1991–1999. [DOI] [PubMed] [Google Scholar]
- 39. Predel HG, Koll R, Pabst H, et al. Diclofenac patch for topical treatment of acute impact injuries: a randomised, double blind, placebo controlled, multicentre study. Br J Sports Med 2004; 38: 318–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Predel HG, Hamelsky S, Gold M, et al. Efficacy and safety of diclofenac diethylamine 2.32% gel in acute ankle sprain. Med Sci Sports Exerc 2012; 44: 1629–1636. [DOI] [PubMed] [Google Scholar]
- 41. Predel HG, Giannetti B, Pabst H, et al. Efficacy and safety of diclofenac diethylamine 1.16% gel in acute neck pain: a randomized, double-blind, placebo-controlled study. BMC Musculoskelet Disord 2013; 14: 250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Inoue H, Takamori M, Shimoyama Y, et al. Regulation by PGE2 of the production of interleukin-6, macrophage colony stimulating factor, and vascular endothelial growth factor in human synovial fibroblasts. Br J Pharmacol 2002; 136: 287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Guerne PA, Carson DA, Lotz M. IL-6 production by human articular chondrocytes. Modulation of its synthesis by cytokines, growth factors, and hormones in vitro. J Immunol 1990; 144: 499–505. [PubMed] [Google Scholar]
- 44. Lotz M, Terkeltaub R, Villiger PM. Cartilage and joint inflammation. Regulation of IL-8 expression by human articular chondrocytes. J Immunol 1992; 148: 466–473. [PubMed] [Google Scholar]
- 45. Mahdy AM, Galley HF, Abdel-Wahed MA, et al. Differential modulation of interleukin-6 and interleukin-10 by diclofenac in patients undergoing major surgery. Br J Anaesth 2002; 88: 797–802. [DOI] [PubMed] [Google Scholar]
- 46. Pearson MJ, Herndler-Brandstetter D, Tariq MA, et al. IL-6 secretion in osteoarthritis patients is mediated by chondrocyte-synovial fibroblast cross-talk and is enhanced by obesity. Sci Rep 2017; 7: 3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Orita S, Koshi T, Mitsuka T, et al. Associations between proinflammatory cytokines in the synovial fluid and radiographic grading and pain-related scores in 47 consecutive patients with osteoarthritis of the knee. BMC Musculoskelet Disord 2011; 12: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rosengren S, Firestein GS, Boyle DL. Measurement of inflammatory biomarkers in synovial tissue extracts by enzyme-linked immunosorbent assay. Clin Diagn Lab Immunol 2003; 10: 1002–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Roth SH, Fuller P. Diclofenac topical solution compared with oral diclofenac: a pooled safety analysis. J Pain Res 2011; 4: 159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Seefried_et_al_SUPPLEMENTAL_INFO_FOR_RESUBMISSION_6-8-20_1 for Penetration of topical diclofenac into synovial tissue and fluid of osteoarthritic knees: a multicenter, randomized, placebo-controlled, pharmacokinetic study by Lothar Seefried, Mark Blyth, Rohit Maheshwari, Stephen M. McDonnell, Guillaume Frappin, Martina Hagen, Nadine Maybaum, Sebastian Moreira and Hemant Pandit in Therapeutic Advances in Musculoskeletal Disease