Abstract
Objectives:
The objective of this study was to evaluate the costs and consequences of introducing "self-administration of medication" (SAM) during hospitalization as compared with nurse-led dispensing and administration of medication.
Methods:
This pragmatic randomized controlled trial was performed in a Danish Cardiology Unit. Patients ⩾18 years old capable of self-administering medication were eligible. In the intervention group, patients self-administered their medication. In the control group, medication was dispensed and administered by nurses. The implementation of SAM was used to evaluate the cost–consequences. The micro-costing analysis used the hospital perspective and a short-term incremental costing approach. The costs for medication, materials, and nursing time were included. Consequences included the dispensing error proportion, patients’ perceptions regarding medication, satisfaction, and deviations in the medication list at follow-up. In addition, the number of readmissions and general practitioner (GP) contacts within 30 days after discharge was included.
Results:
The total cost (TC) per patient in the intervention group was 49.9€ (95% CI: 46.6–53.2) compared with 52.6€ (95% CI: 46.6–58.6) in the control group. The difference between the groups was not statistically significant (p = 0.09). Sensitivity analysis consistently showed TCs favoring the intervention. The dispensing error proportion was 9.7% (95% CI: 7.9–11.6) in the intervention group compared with 12.8% (95% CI: 10.9–15.6) in the control group. The difference was statistically significant (p = 0.02). The analysis also found changes in the perceptions regarding medication (indicating higher medication adherence), increased satisfaction, and fewer patients with deviations in the medication list at follow-up. No statistically significant differences between the groups in relation to readmissions and GP contacts within 30 days were observed.
Conclusions:
SAM seems to cost less although the cost difference was small and not statistically significant. As SAM had positive effects on patient outcomes, the results indicate that SAM may be cost-effective.
Plain language summary
Self-administration of medication: a research study of the costs and consequences
Objectives
To evaluate the costs and consequences of introducing “self-administration of medication” (SAM) during hospitalization compared to medication dispensed by nurses.
Methods
This research study included patients ≥18 years capable of self-administering medication and was performed in a Danish cardiology unit. Patients self-administered their own medication during hospitalization in the intervention group, whereas nurses dispensed and administered the medication in the control group. Patients were allocated between groups by randomization. The costs of SAM were analyzed from a hospital perspective and included costs for medication, materials, and nursing time. The consequences included the proportion of dispensing errors, patients’ perceptions regarding medication, patient satisfaction, deviations in the medication list at follow-up, the number of readmissions and general practitioner (GP) contacts within 30 days after discharge.
Results
The total cost per patient was 49.9€ in the intervention group compared to 52.6€ in the control group (p = 0.09). The cost difference between groups was not significant. The proportion of dispensing errors was significantly lower in the intervention group compared to the control group. In addition the research study found changes in the perceptions regarding medication, increased satisfaction, and fewer patients with deviations in the medication list at follow-up. For readmissions and GP contacts within 30 days no significant differences between groups were found.
Conclusion
SAM cost less or equal to medication dispensing and administration by nurse. SAM had positive impacts on patient outcomes. Therefore, SAM may be cost-effective.
Keywords: health economic evaluation, cost analysis, cost–consequence, self-administration, self-management, dispensing error, beliefs about medicines, satisfaction
Introduction
When patients are admitted to Danish hospitals, healthcare professionals mostly take over responsibility for their medication. However, healthcare systems are moving towards incorporating more patient involvement since patients increasingly expect to be able to influence the course of their treatment.1,2
Patient involvement means that patients and healthcare professionals work in collaboration.3 Self-management support is a central component of patient involvement, aiming to improve patients’ knowledge, skills, and confidence in managing their health conditions.1 To support self-management, healthcare professionals must encourage patients to actively participate in their treatment as much as they want and can.1
Patient information and “self-administration of medication” (SAM) during hospitalization are key elements in self-management support. Previous research on SAM has shown patient advantages such as independence, increased knowledge, empowerment, and a sense of control and higher medication safety.4–9
Increased medication safety and adherence to prescribed treatment can potentially lead to better health and, consequently, to a reduced need for healthcare. On the other hand, the risk of errors, such as a too high/low medication dose and nonadherence, could negatively affect health.5 Such medication errors may increase hospitalization length, healthcare costs, and mortality.10
Apart from safety issues and the possible effects on health and derived healthcare use, concerns about resource requirements for the intervention (e.g. additional time and staffing) is a barrier to the successful implementation of patient involvement.3
In a busy working day, healthcare professionals have many different, and maybe competing, tasks to perform. Hence, supporting patients in self-management may not be their first priority. However, healthcare professionals may also be relieved when patients are self-managing during hospitalization.11
As healthcare resources are scarce, health economic evaluation is important in choosing the best, safest, and most economically advantageous way to manage medication at a hospital. Hence, economic evaluations of patient involvement initiatives may help inform current concerns about the scarcity of healthcare resources and the need to maximize health improvements.1,12
SAM is a complex intervention, involving changes in working procedures, procedure coordination, and concerted effort of multiple parties (e.g. patients, nurses, and doctors). Complex interventions often have a variety of outcomes that cannot be easily converted to monetary terms or reported in one health measure. Therefore, a cost–consequence analysis (CCA) is recommended when evaluating such an intervention’s health economic impact.13 A CCA shows the total cost (TC) of implementing an intervention as well as its consequences and allows the reader to form their own opinion of the intervention’s relevance and importance in the context of their decision-making.13
To the best of the authors’ knowledge, no previous studies have made a full health economic evaluation of the costs and consequences of introducing SAM during hospitalization.
Therefore, the objective of this study was to evaluate the costs and consequences of introducing SAM during hospitalization as compared with nurse-led medication dispensing and administration from a hospital perspective.
Methods
The study is reported according to the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement.14
Trial design and study setting
The economic evaluation used data from a pragmatic randomized controlled trial (RCT) of the consequences of introducing SAM compared with nurse-led medication dispensing and administration.15 The RCT was performed at the Medical Department (Cardiology Unit, 28 beds) of Randers Regional Hospital in Denmark from August 2017 to September 2018.
In Denmark, every citizen has free, tax-funded access to the healthcare system16 and medication is usually provided by the hospital during hospitalization. It is, however, permitted to ask patients to bring and use their own medication during hospitalization. Prescribed medication in the primary healthcare sector is largely also paid for by public funds (regional authorities and municipalities). Co-payment varies with type of medicine, annual medicine consumption and social conditions with a maximum co-payment of 560€ per year.17
Resource use and cost data for a CCA were collected alongside the RCT.
Participants
Patients were eligible for inclusion if they were capable of SAM, if they self-administered their medication at home, if they received at least one medication suitable for self-administration, if they spoke Danish, and if they were ⩾18 years old. SAM capability was assessed by nurses.15
Patients were consecutively recruited from Monday to Thursday. Written informed consent was obtained on inclusion.
Intervention
The medication brought by patients to the hospital was assessed by a nurse regarding the quantity (amount) and quality (e.g. expiry data)18 and was compared with prescriptions in the electronic Medication Administration Record (eMAR), which also links to prescriptions in the primary sector. A doctor was consulted in case of any uncertainties. Any missing prescribed medication or any newly prescribed medication was provided by the hospital. Medication was provided as a whole package if available as small blister packages (⩽20 pieces), as a blister card if only available in large blister packages, or as loose tablets in a container. Medication to be used at the hospital was placed in a green bag, whereas medication not in use was placed in a red bag. Medication was stored in the patient’s wardrobe. The key was kept by the patient. Self-administration was documented in the eMAR.
A nurse instructed the patient in the use of the medication and provided written information about new medication as well as an updated medication list. The patient was informed about any medication changes, and the patient’s SAM capability was reviewed by a nurse at least once daily.
Patients self-administered their medication during hospitalization [except medication not suitable for self-administration (once-only prescriptions; medication stored in the refrigerator, except insulin; injections and infusions, such as diuretics or antibiotics; and variable high-dose digoxin and inhalations taken through nebulization)]. At discharge, the patients were provided with an updated medication list; however, they were already informed and instructed about their medication through SAM.
Control group
Hospital-provided medication during hospitalization was dispensed and administered by a nurse at each prescribed time point. First, the medication was dispensed in the medicine room, with every dispensing of a medication being documented in the eMAR. Second, the nurse walked from the medicine room to the patient’s room to deliver the filled medicine cup to the patients (administration). Patients could self-administer inhalation and nonstocked medication brought to the hospital. At discharge, the patients were provided with an updated medication list and were primarily informed and instructed about their medication at that time point. Medication for 1–3 days was dispensed by a nurse if the patient was unable to go to a community pharmacy right after discharge.
Sampling
Patients for the RCT were recruited from August 2017 to September 2018.
The sample size for the RCT was determined based on the primary outcome (error proportion) and was calculated to be the observation of 1020 opportunities for error (OEs) in each group (based on pilot measurements), corresponding to approximately 150 patients in each of the study groups. The randomization was performed by the Hospital Pharmacy’s Department of Quality Assurance.15
A time study was, due to resource constraints, performed in a subsample of patients from April 2018 to June 2018. At that point, the intervention had been running for some time and was expected to be routine work reflecting resource use if SAM was to be implemented and scaled up.
The calculation of the time study’s sample size was based on the means and standard deviations (SD) of the pilot time measurements of the “SAM start-up” and “Administration” processes, with a statistical significance level of 5% and a 25% difference considered appropriate. The sample size was calculated to be at least 16 time measurements for each time cost category in each group.
CCA
The TC of implementing the intervention in relation to its consequences was presented in the CCA.
Costs
A cost analysis was performed from a hospital perspective using a short-term incremental costing approach. The analysis of the processes within the intervention (unpublished) found that the costs related to medication, materials, and nursing time spent on different tasks would change. The intervention was assumed to have no influence on the overhead costs, including hospital administration, cleaning, and rent. The costs of planning, developing, and implementing the intervention were not part of the cost analysis.
Micro-costing level was used. The measurement of the use of medication and materials was performed throughout the patients’ hospitalization by reviewing the eMAR prescriptions and by observing the nurses. Only medication suitable for self-administration was included. The nursing time spent dispensing medication was measured using stopwatches when the dispensing process was observed in the control group. The nursing time spent on medication administration in the control group, the SAM start-up in the intervention group, and the discharge preparation in both groups were measured in the time study. The measurements of the time spent on administration and the SAM start-up were performed by the principal investigator using stopwatches, whereas the time used for discharge preparation was self-reported by the nurses. The number of dispensed doses, administrations (medication rounds), and self-administered medications were registered by reviewing the eMAR prescriptions.
The valuation of the identified cost items was performed by multiplying the quantity and the unit cost. The unit costs for each medication and material were registered once under the assumption that it would not change throughout the study period.
The mean hourly labor costs for the Cardiology Unit nurses were obtained from the hospital personnel system and were in line with a similar Danish study24 multiplied by 1.3 to adjust for breaks, meetings, and days off.
Table 1 describes how the identified costs were valued and calculated from the measurements.
Table 1.
Cost items.
| Identified costs | Measurements | Valuation |
|---|---|---|
| CSAMstart-up | ||
| Costs for nursing time used on starting SAM |
One SAM start-up per patient in the intervention group | Nursing time estimated in the time study* was multiplied with the mean nurse labor cost for each patient in the intervention group |
| Cnewmedication | ||
| Costs for providing new medication | The number of doses was obtained from the eMAR | Hospital Pharmacy prices 2018 |
| Cusualmedication | ||
| Costs for providing patient’s usual medication | The number of doses was obtained from the eMAR | Hospital Pharmacy prices 2018 |
| Cmaterials | ||
| Costs for materials (plastic bags, medicine cups, dosage boxes) | The number of pieces provided was obtained by observing the start of the intervention and from the eMAR | Central Denmark Region prices 2018 |
| Cdispensing | ||
| Costs for nursing time used on dispensing of medication (only medication suitable for self-administration) | The number of dispensed doses was obtained from the eMAR | Nursing time on dispensing* was multiplied with the mean nurse labor cost for each patient |
| Cadministration | ||
| Costs for nursing time used on administration of medication (delivering the filled medicine cup) | The number of administrations was obtained from the eMAR | Nursing time estimated in the time study* was multiplied with the mean nurse labor cost for each patient |
| Cdischarge | ||
| Costs for nursing time used on discharge preparation | One discharge per patient | Nursing time estimated in the time study* was multiplied with the mean nurse labor cost for every patient in the respective groups |
eMAR, electronic Medication Administration Record; SAM, self-administration of medication.
Nursing time used on dispensing was measured when the dispensing process was observed in the control group. Nursing time on SAM start-up, medication administration, and preparation of discharge was measured in a time study from April 2018 to June 2018. See Appendix 1.
The TC was calculated for each alternative from the following equations:
The incremental costs were calculated as the difference between the TCintervention group and the TCControl group.
Consequences
Patient outcomes were explored in the RCT.15 The applied methods are presented in brief terms here.
In addition, information on the patients’ derived consumption of healthcare resources [general practitioner (GP) contacts and readmissions within 30 days after discharge] was obtained.
Patient outcomes
Dispensing errors
The primary outcome was the dispensing error proportion observed through modified disguised observation19,21–23 of the patients in the intervention group and the nurses in the control group.
Dispensing errors were defined as “the dispensing of a dose of medication that deviates from the prescription, from hospital guidelines, or written procedures”15 and were categorized into clinical and procedural errors. A clinical error happened when the patient did not receive the medication as prescribed in the eMAR.20,24 A procedural error happened when the nurse deviated from the written procedures or guidelines.20,24,25 An OE was defined as any dose dispensed plus any dose prescribed but omitted.21,24 The error proportion was calculated by dividing the number of dispensing errors by the number of OEs observed and multiplying with 100%.15
Perceptions regarding medication
The patients’ perceptions regarding medication were explored upon their study inclusion and through a telephone call two weeks after discharge. A Danish version26 of the original Beliefs about Medicines Questionnaire (BMQ)27–29 was used. The BMQ consists of 18 items divided into 4 factors (Necessity, Concerns, Overuse, Harm) scored on a 5-point Likert scale (1 = Strongly disagree to 5 = Strongly agree). The total factor scores range from 5 to 25 for “necessity” and “concerns” and from 4 to 20 for “overuse” and “harm.”27 Scores were calculated for each patient and as mean scores for the groups at each time point and as the change over time.15
Patient satisfaction
The patients were contacted by telephone 2 weeks after discharge and were asked about their satisfaction with the way they received medication during hospitalization; a 5-point Likert scale was used (1 = Very unsatisfactory to 5 = Very satisfactory).15
Deviations in medication list at follow-up
During the telephone interviews, patients were asked to report their medication list at the day of the interview. Deviations from the medication list in the discharge letter that were not confirmed by the patient’s GP or the hospital were registered.15 The number of patients with deviations and the mean number of deviations per patient were calculated for each study group.
Healthcare sector outcomes
Readmissions and GP contacts
Information on the number of readmissions and GP contacts within 30 days from discharge was obtained from the Central Denmark Region registries for each patient. It was not possible to gain information on the reason for contacting the GP or the readmissions; thus, the total numbers were obtained. Transfer to another hospital on the day of discharge from the Cardiology Unit was not considered a readmission. Contacts with more than one hospital on the same date were considered one readmission.
Data analysis
The continuous outcomes were compared using Student’s t-test or Wilcoxon–Mann–Whitney test. The binary outcomes were compared using a chi-squared test or Fisher’s exact test.
The time measurements were analyzed using linear regression analyses to explore the associations between the number of medications, the dispensings, and the administrations (Appendix 1). As time use was not measured for all the patients, dispensings, and administrations, the estimated associations were used to predict total use of nurse time for each patient, based on the number of medications, dispensings, and administrations.
Confidence intervals (CIs) of the cost items except Cdischarge were calculated by bootstrapping with 1000 replications. The CIs for Cdischarge were calculated from the CIs of the time measurements.
Finally, we translated our findings into estimated annual costs and consequences to provide the findings in a format that would better match the types of decisions and considerations undertaken by decision-makers. The number of patients capable of self-administration per year was estimated from the patient flow in the RCT.15 A total of 632 of the 1666 assessed patients were capable of self-administration. A total of 19% (68/354) of the invited patients declined to self-administer. We assume that 19% of the remaining 278 patients would also decline to participate (53 patients). Of the 1666 assessed patients, this yields 511 SAM candidates (31%). The Cardiology Unit has an annual intake of approximately 2000 patients; we therefore reasonably assume that 620 patients annually are SAM candidates (31%).
We used this number to estimate the annual costs and consequences if the intervention were implemented on a routine basis in the Cardiology Unit.
Cost-effectiveness planes for the annual incremental costs and effects were generated by bootstrapping the datasets with 1000 replications and extrapolating to annual numbers.
Sensitivity analyses of the TCs were performed as one-way (univariate) analyses using bootstrapped CIs as the minimum and maximum values of each cost item, holding others constant.12,30
In addition, sensitivity was assessed in scenario analyses with changes in basic intervention assumptions.12,30 The first scenario analysis assumed that every patient in the intervention group brought and used their usual medication during hospitalization, which removed the cost for providing typical medication. This assumption was chosen as SAM originally builds upon the idea that patients bring and use their usual medication at a hospital. The second scenario analysis assumed that only nursing time cost items were relevant to include in the TC. This assumption attempted to shift the analysis from a hospital to a healthcare perspective, where medication and material costs are assumed to compensate across healthcare subsectors. The third scenario analysis assumed nurse’s mean hourly labor cost on a national level31 instead of the local level. This assumption was chosen as many nurses in the Cardiology Unit (Randers) are young and newly educated.
The incremental cost calculated in each sensitivity analysis was compared with the incremental cost in the base case (the RCT). A tornado diagram was used to demonstrate the results.
Stata® v.15 (StataCorp, 4905 Lakeway Drive, TX, USA) was used for the analysis of statistics.
Ethics
The intervention included no biomedical intervention; according to Danish legislation, the Central Denmark Committee of Health Research Ethics waived the need for approval. The study procedures followed the ethical standards of the Helsinki Declaration.32
The study was registered in ClinicalTrials.gov [ClinicalTrials.gov identifier: NCT03541421] and the Danish Data Protection Agency (1-16-02-106-17).
Results
A total of n = 250 patients were recruited, with 125 in each study group. Three patients from the intervention group withdrew due to a decline in their SAM capability. One patient withdrew due to refusal to be in the control group. A total of n = 7 patients were discharged earlier than expected and were not observed. For the analysis, n = 119 patients (1033 OEs) remained in the intervention group, and n = 120 patients (1028 OEs) remained in the control group.
Baseline data
The baseline characteristics of the patients were similar with no statistically significant differences between groups.15 In the intervention group, 71% were men, with a mean age of 62.8 years (95% CI: 60.6–65.0) and a median length of stay of 3.1 days (IQR: 2.4). They received a mean of 4.5 medications per patient at admission (95% CI: 3.7–5.2), and 18% brought all their usual medication to the hospital. In the control group, 63% were men, with a mean age of 65.5 years (95% CI: 63.4–67.7) and a median length of stay of 3.0 days (IQR: 2.0). They received a mean of 5.1 medications per patient at admission (95% CI: 4.4–5.8), and 22% brought all their usual medication to the hospital.
There was no statistically significant difference in the BMQ scores between the groups at the time of inclusion.15
Costs
The unit costs and nursing time spent on the SAM start-up, dispensing, administration, and discharge preparation processes are presented in Table 2.
Table 2.
Time measurements and unit cost estimates.
| Alternatives |
||||
|---|---|---|---|---|
| Intervention group |
Control group |
|||
| Time (minutes) | Unit costs (2018€) | Time (minutes) | Unit costs (2018€) | |
| SAM start-up by nurse | ||||
| Fixed component (start-up cost per patient) | 6.40 | 3.95 | Not relevant | |
| Variable component (cost per self-administered medication) | 2.60 | 1.60 | ||
| Medication | ||||
| Differs from medication to medication Unit costs for specific medications were obtained from hospital pharmacy prices | ||||
| Materials | ||||
| Plastic bag (green/red) per piece | 0.13 | Not relevant | ||
| Medicine cup per piece | 0.01 | 0.01 | ||
| Dosage box per piece | 0.57 | 0.57 | ||
| Dispensing by nurse | ||||
| Cost per medication dispensed | 0.55 | 0.34 | 0.55 | 0.34 |
| Administration by nurse | ||||
| Cost per administration | 1.30 | 0.80 | 1.30 | 0.80 |
| Discharge preparation | ||||
| Cost per discharge | 27.55 | 16.99 | 37.03 | 22.83 |
| Nurses involved (including 30% to cover days off work, meetings, breaks) | ||||
| Labor cost per hour | 36.98 | 36.98 | ||
SAM, self-administration of medication.
Nursing time in relation to SAM start-up consists of a fixed time and a variable time depending on the number of self-administered medications. Other time measurements are presented and used as means. See Appendix 1.
The time measurements analysis for the SAM start-up revealed that the time spent was determined by a fixed component that would apply to every patient (start-up costs) and a variable component depending on the number of medications. Thus, irrespective of the number of medications, the nurse would spend an average of 6.4 min on the start-up process and, in addition, they would spend an average of 2.6 min per medication (providing medication, printing medication information, and instructing the patient; Appendix 1). The time spent on medication dispensing depended on the number of dispensed medications, whereas the time spent on medication administration depended on the number of administrations (medication rounds) during the day (Appendix 1). The time spent on preparation for discharge did not depend on the number of medications; thus, a mean time per patient was used (one discharge per patient); see Appendix 1.
The costs per patient and per year for the intervention and control groups are presented in Table 3.
Table 3.
Costs per patient and annual incremental cost of SAM (Intervention) and usual practice (Control) (2018€).
| Costs per patient (2018€) |
Estimated annual change (n = 620) |
|||
|---|---|---|---|---|
| Intervention group (I) |
Control group (C) |
Incremental cost (I–C) | Incremental cost (I–C) | |
|
Cnewmedication
Mean, 95% CI |
12.6 10.0; 15.2 |
10.6 7.4; 13.8 |
+2.0 −2.1; +6.2 |
+1247 −1330; +3825 |
|
Cusualmedication
Mean, 95% CI |
3.7 2.4; 5.0 |
3.5 1.4; 5.6 |
+0.2 −2.3; +2.7 |
+144 −1400; +1689 |
|
Cmaterials
Mean, 95% CI |
0.7 0.6; 0.7 |
0.2 0.2; 0.3 |
+0.4 +0.4; +0.5 |
+267 +223; +312 |
|
Cdispensing
Mean, 95%CI |
0.9 0.6; 1.1 |
9.2 7.4; 11.0 |
−8.3 −10.2; −6.5 |
−5165 −6311; −4018 |
|
Cadministration
Mean, 95%CI |
1.4 0.9; 1.8 |
6.2 5.2; 7.3 |
−4.9 −6.0; −3.74 |
−3018 −3727; −2308 |
|
CSAMstart-up
Mean, 95%CI |
13.6 12.7; 14.5 |
0.0 0.0; 0.0 |
+13.6 +12.8; +14.5 |
+8458 +7913; +9003 |
|
Cdischarge
Mean, 95%CI |
17.0 12.3; 21.7 |
22.8 15.9; 29.7 |
−5.8 −14.4; 2.7 |
−3621 −8898; +1656 |
|
Total Cost
Mean, 95%CI |
49.9
46.6; 53.2 |
52.6
46.6; 58.6 |
−2.7
−9.5; +4.1 |
−1686
−5893; +2521 |
Notes: Confidence intervals (CIs) of the cost items except Cdischarge were calculated by bootstrapping with 1000 replications. The CIs for Cdischarge were calculated from bootstrapped CIs of the time-measurements (1000 replications). The costs for medication only included medicine suitable for self-administration. The calculations were based on exact values and presented as rounded numbers.
A “+” means a difference in favor of the control group (dispensing and administration by nurse).
A “–“ means a difference in favor of the intervention (self-administration).
In the intervention group, we found an average TC per patient of 49.9€ as compared with 52.6€ in the control group. The incremental cost was −2.7€ in favor of the intervention. The difference between the alternatives was not statistically significant (p = 0.09).
In the intervention group, new medication was provided by the hospital as whole packages/blister packages, resulting in a larger cost (Cnewmedication=12.6€) than for the control group (Cnewmedication=10.6€) (Table 3). When looking at the costs related to nursing time, we found that it was time-consuming to start SAM in the intervention group (CSAMstart-up=13.6€); however, the nurses saved time on dispensing, administration, and also on discharge preparation since the patients were already informed about their medication (Table 3). In fact, nursing time accounted for the largest part of the TCs in both groups.
When looking at the estimated annual TC, we found a TC of 30,923€ in the intervention group and 32,609€ in the control group. Therefore, the incremental annual cost was −1686€ in favor of the SAM-intervention (i.e. a saving, as the TC for SAM is slightly lower than the TC for usual practice).
Consequences
The consequences of the alternatives are presented in Table 4 as patient and healthcare sector outcomes.
Table 4.
Consequences per alternative.
| Consequences per group or per patient |
Estimated annual change (n = 620) |
|||
|---|---|---|---|---|
| Intervention group (I) | Control group (C) | Incremental effect (I–C) | Incremental effect (I–C) | |
| Consequences: Patient outcomes | ||||
| Dispensing errors, total numbers per group | ||||
| Dispensing errors, | −32.6 | −498* | ||
| n/OEs | 100/1033 | 132/1028 | ||
| (%) | (9.7) | (12.8) | ||
| 95% CI | 7.9–11.6 | 10.9–15.0 | ||
| Clinical errors, | −21.2 | −324* | ||
| n/OEs | 25/1033 | 46/1028 | ||
| (%) | (2.4) | (4.5) | ||
| 95% CI | 1.6–3.6 | 3.3–5.9 | ||
| Procedural errors, | −11.4 | −174* | ||
| n/OEs | 75/1033 | 86/1028 | ||
| (%) | (7.3) | (8.4) | ||
| 95% CI | 5.8–9.0 | 6.7–10.2 | ||
| Patients with dispensing errors | −26.7 | −138 | ||
| n/Patients | 41/119 | 68/120 | ||
| (%) | 34.5 | 56.7 | ||
| Patient satisfaction, total numbers per group | ||||
| Highly satisfied | ||||
| n/Patients | 99/114 | 55/110 | +42.0 | +229 |
| (%) | 86.8 | 50.0 | ||
| Satisfied | ||||
| n/Patients | 13/114 | 38/110 | −26.9 | −149 |
| (%) | 11.4 | 34.5 | ||
| Neutral or unsatisfied | ||||
| n/Patients | 2/114 | 17/110 | −15.1 | −81 |
| (%) | 1.8 | 15.5 | ||
| Perception regarding medication (change in patient mean factor scores over time) | ||||
| Necessity | +0.30 | +0.40 | −0.10 | Not relevant |
| Concern | −0.67 | +0.28 | −0.95 | |
| Overuse | −0.38 | −0.29 | −0.09 | |
| Harm | −0.60 | +0.17 | −0.77 | |
| Deviations in medication list at follow-up per group and per patient | ||||
| Patients with deviations | ||||
| n/Patients | 10/114 | 22/110 | −12.8 | −70 |
| (%) | 8.8 | 20.0 | ||
| Mean number | 0.13 | 0.29 | −0.16 | −99 |
| 95% CI | 0.05–0.22 | 0.16–0.42 | ||
| Consequences - Healthcare sector outcomes | ||||
| Readmissions within 30 days after discharge per patient | ||||
| Mean number | 0.21 | 0.23 | −0.02 | −12 |
| 95% CI | 0.11–0.31 | 0.12–0.33 | ||
| GP contacts within 30 days after discharge per patient | ||||
| Mean number | 2.04 | 2.53 | −0.49 | −304 |
| 95% CI | 1.69–2.39 | 2.14–2.91 | ||
Source: The effectiveness data stem from the RCT15 and Central Denmark Region registries.
Notes: *In the RCT a mean of 25.44 OEs per patient was registered during study inclusion. This yields 15,773 OEs in total per year (n = 620). The expected number of dispensing errors per year was calculated from error proportions and this annual number of OEs; that is, dispensing errors in total = 15,773 OEs × 0.0968 = 1527 errors in the intervention group compared with 15,773 OEs × 0.1284 = 2025 errors in the control group.
The difference between alternatives is calculated per year by multiplying the incremental effect per patient by n = 620 patients.
A “–” means a difference in favor of the intervention (i.e. self-administration); although, for patient satisfaction a “+” means a difference in favor of the intervention.
Patient outcomes
A statistically significant difference in the total number of dispensing errors was observed (p = 0.02), with 100 errors out of the 1033 OEs in the intervention group and 132 errors out of 1028 OEs in the control group (Table 4). When dividing these into clinical and procedural errors, a statistically significant difference was observed in the number of clinical errors (p = 0.01) but not in the number of procedural errors (p = 0.35).
The patients in the intervention group were more satisfied (p = 0.00) with the way they received medication during hospitalization (Table 4).
When comparing the BMQ change over time between the groups, we observed a statistically significant difference in relation to the factors “concerns” (p = 0.05) and “harm” (p < 0.01), with lower values in the intervention group (Table 4).
There were statistically significantly fewer patients (p = 0.02) with deviations in the medication list at follow-up in the intervention group as compared with the control group. In addition, the mean number of deviations per patient was statistically significantly different between the groups (p = 0.02) (Table 4).
Healthcare sector outcomes
In the intervention group, there were 25 readmissions within 30 days after discharge (0.21 readmissions per patient) as compared to 27 readmissions in the control group (0.23 readmissions per patient, p = 0.84; Table 4).
In the intervention group, there were 243 GP contacts within 30 days after discharge (2.04 per patient) as compared with 303 GP contacts in the control group (2.53 per patient). The difference between the groups was not statistically significant at the 5% level, though it was close (p = 0.07) (Table 4).
Comparing costs and consequences
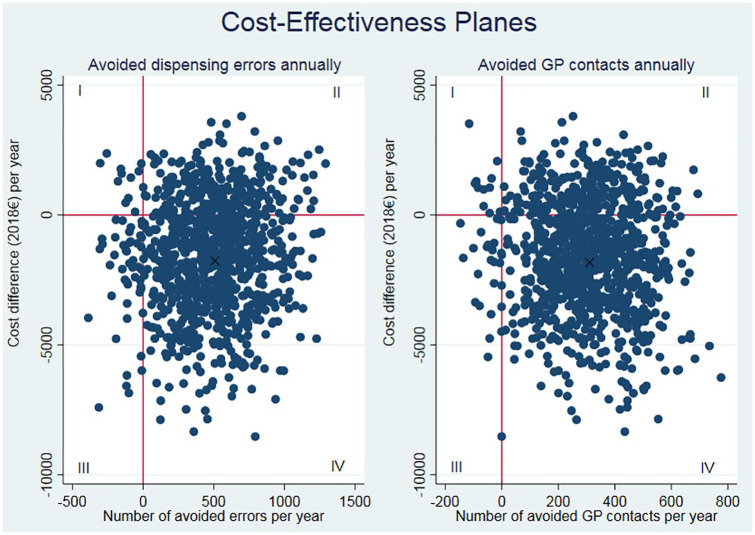
To convey to decision-makers the implications of introducing SAM as a routine practice corresponding to an estimated annual number of 620 patients, we extrapolated the costs and consequences. The overall positive effect in annual terms can be seen in Table 4. To further illustrate the relationship between the incremental costs and consequences and the uncertainty, we generated cost-effectiveness planes. Figure 1 presents the cost-effectiveness planes for avoided dispensing errors and avoided GP contacts.
Figure 1.
Cost-effectiveness planes for avoided dispensing errors and avoided GP contacts.
Note: The datasets were bootstrapped with 1000 replications. These were extrapolated to annual numbers and the incremental costs and effects were plotted in two-way scatter plots. The “X” illustrates the base case with the annual numbers from Table 4.
In both cost-effectiveness planes, the dots are widespread; however, the majority of cases are in quadrant IV, where the intervention is cost-effective with lower costs and better outcomes as compared with the control group.
Sensitivity analysis
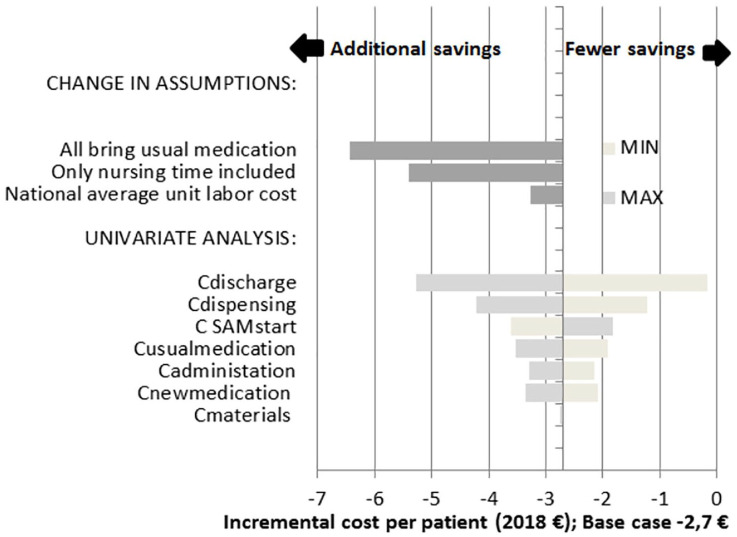
A total of 17 sensitivity analyses were performed, of which 14 were one-way (univariate) analyses of the cost items, and three were analyses with changes in assumptions.
The incremental cost for each analysis was in favor of the intervention with more or less savings; however, the numbers were small, and only a few euros apart from one another (see Figure 2 and Appendix 2). For example, using the minimum and maximum values of the SAM start-up CIs made the TC vary by only ±0.9€ compared with the base case.
Figure 2.
Tornado-diagram of results from the sensitivity analyses.
Note: Change in assumptions: three analyses with changes in the assumptions:
(i) assuming that every SAM patient brings and uses their own medication during hospitalization;
(ii) assuming that only nursing time was relevant; and
(iii) assuming an average hourly labor cost on the national level (31).
Univariate analysis: bootstrapped CIs were used as minimum and maximum values of each cost item holding others constant.
Values to the left show additional savings when compared with the base case whereas values to the right show cases with lower savings.
The largest saving (−6.4€) was seen when assuming that all patients bring and use their own medication during hospitalization.
By only looking at the nursing time, the incremental savings increased as well (from −2.7€ to −5.4€). Further, the variation in the nursing time used in discharge preparation resulted in large variation in the incremental cost.
Discussion
In the cost analysis, on average, a lower TC was found in the intervention than in the control group; however, the difference between the alternatives were not statistically significant (p = 0.09). In the intervention group, the costs for providing new medication and materials were higher than in the control group. Although it was time-consuming to start the self-administration process in the intervention group, the nurses saved time on dispensing, administration, and discharge preparation since the patients were already informed about their medication. The sensitivity analyses showed either no difference in the TCs between the groups or more or less savings in favor of the intervention; however, the numbers were small, with only a few euros difference between the groups. From a hospital perspective, the largest saving is possible when the SAM patients bring and use their usual medication during hospitalization. This is not surprising because costs are shifted to the primary healthcare sector and to patients. The increased financial burden on patients depends on the extent of co-payment applying to the particular medication and patient, but at the individual patient level it is small (Table 3). Disregarding the question of who pays for the medication it is relevant to note that overall, nurses save time when introducing SAM and supporting patients in self-management. This saved time can be used on other tasks in the ward. Even if patients do not bring their own medication this would be an advantage. This is, of course, also worth considering from the hospital perspective.
With regard to the consequences, fewer dispensing errors were found in the intervention group than in the control group. Patients instructed in medication self-administration had fewer concerns about their medication at follow-up and found medication to be less harmful in general. Patients from the intervention group were more satisfied with the medication management at the hospital, and fewer had deviations in the medication list at follow-up than the control group. There were no differences between the groups in relation to readmissions within 30 days after discharge. Fewer GP contacts within 30 days after discharge were found in the intervention group; however, the difference between the alternatives fell short of reaching statistical significance at the 5% level (p = 0.07).
To the best of the authors’ knowledge, no previous studies have made a full health economic evaluation of the costs and consequences of introducing SAM at hospitals, which makes it impossible to compare our results with those of others.
A recent non-randomized study from Denmark presents the results from a cost analysis of introducing one-stop dispensing, where patients self-administer their own medication when assessed as ready to do so. The study found no statistically significant difference in medication costs. Nursing time was studied in relation to dispensing, administration, and self-administration start-up. Time consumption was reduced by 12 min per patient per hospitalization in the self-administering group.11 In the present study, a cost difference (nursing time items) of 5.3€ was found, which corresponds to a reduction of 9 min per patient in the self-administering group.
Few studies have compared incremental costs with incremental effects such as avoided medication errors. Decision-makers may have to consider and choose between different medication approaches that are primarily intended to reduce medication errors (and thereby improve quality of care). In such cases, it is considered relevant to use avoided medication errors as a denominator.
Vermeulen et al. found an incremental cost-effectiveness ratio of +3.54€ per avoided medication error when comparing computerized physician order entry with a paper-based prescription system.33 Risør et al. found an incremental cost-effectiveness ratio of +2.01€ per avoided administration error when comparing an automated medication system with nurse-led medication dispensing and administration.34 In the present study, the intervention was not more costly than usual practice, and the intervention group had better effects compared to those in the control group (e.g. fewer errors, changed perceptions about medication indicating better medication adherence, higher satisfaction, fewer deviations in the medication list at discharge).
The errors’ clinical consequences were not investigated. The patients did not receive the medication as prescribed when a clinical error happened; thus, these were the most important errors to avoid.20,24 Medication errors may be costly to the healthcare system, as some increase medication costs and length of stay in the hospital and result in additional hospitalizations.35 Hence, SAM may introduce additional healthcare savings due to the reduced number of dispensing errors. Further studies on the errors’ clinical consequences and economic impacts are recommended (e.g. a model-based analysis could be considered).
Strengths and limitations
The study has some strengths and limitations that merit further discussion.
In this RCT, we used micro-costing for the cost analysis to obtain as precise results as possible. The unit costs for the medication and materials were measured once under the assumption that they would not change throughout the study period and to avoid disturbance of the results unrelated to the intervention. We acknowledge the risk of information bias as prices may change over time; however, the bias was nondifferential.
The time spent on discharge preparation was self-reported by the nurses, whereas the time spent on dispensing, administration, and SAM start-up was measured by the principal investigator using stopwatches. This difference in the data-collection method may have introduced an inaccuracy; however, this was similar for both groups. More attention to this is recommended in future studies.
In the time study (administration, SAM start-up, discharge), we used a sample size of 16 measurements per group for each nursing task. The sample size calculation was based on the feasibility and pilot study, with only a few measurements of time used on dispensing, administration, and SAM start-up but with no pilot measurements on the time used on discharge preparation. It is possible that the number of measurements was too small to display a significant difference.
When a patient in the intervention group had not brought a medication, or when a new medication was prescribed, the hospital provided the medication. In most cases, the medication was provided as blister cards or as loose tablets in a container. However, in n = 16 patients, the medication was provided as a large blister package, making the cost larger than necessary. The mean cost for providing medication in the intervention group was therefore slightly larger than it should have ideally been.
The concept of SAM builds upon the assumption that patients bring and use their own medication while in the hospital. Far from every patient brought and used own medication and the cost of providing the usual medication was larger than it should have ideally been. The sensitivity analysis confirmed additional savings when the SAM patients brought and used their usual medication.
During the study period, several nurses were replaced; hence, only few nurses became highly experienced with SAM, and inexperienced nurses may have missed some of the intervention tasks. This may be a limitation; however, it reflects the circumstances in a typical hospital ward.
Patients were recruited from Monday to Thursday at a cardiological unit. There may be more or fewer patients eligible for SAM in other types of wards and on weekends. In addition, the patients had low comorbidity levels and we cannot generalize our results to patients with more comorbidity. SAM capability must be assessed from time to time.
Conclusion
SAM seems to cost less although the cost difference was small and not statistically significant. As SAM had a positive effect on patient outcomes, the results indicate that SAM may be cost-effective.
Supplemental Material
Supplemental material, Appendix_TAW for Cost–consequence analysis of self-administration of medication during hospitalization: a pragmatic randomized controlled trial in a Danish hospital setting by Charlotte Arp Sørensen, Annette de Thurah, Marianne Lisby, Charlotte Olesen, Signe Bredsgaard Sørensen and Ulrika Enemark in Therapeutic Advances in Drug Safety
Supplemental material, CHEERS-Checklist_CCA_revised for Cost–consequence analysis of self-administration of medication during hospitalization: a pragmatic randomized controlled trial in a Danish hospital setting by Charlotte Arp Sørensen, Annette de Thurah, Marianne Lisby, Charlotte Olesen, Signe Bredsgaard Sørensen and Ulrika Enemark in Therapeutic Advances in Drug Safety
Acknowledgments
This study could not have been performed without the engaged work performed by the nurses in the Cardiology unit when they delivered the intervention. Thanks also to the engaged staff at the Danish Centre for Healthcare Improvements at Aalborg University, Denmark, who provided professional sparring about the analysis.
Footnotes
Author contributions: CAS, AT, CO, ML and UE were responsible for the planning and design of the study. CAS was responsible for data collection and analysis. CAS wrote the first draft. AT, CO, ML, SBS and UE provided critical revision. All authors read and approved the final manuscript.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was funded by the Hospital Pharmacy, Central Denmark Region, the Hospital Pharmacies, and Amgros’ Research and Development Foundation and Randers Regional Hospital. The funders paid the salary for the principal investigator and had no other role in the study.
Conflict of interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Data availability statement: Data are available from the corresponding author upon reasonable request.
ORCID iD: Charlotte Arp Sørensen  https://orcid.org/0000-0003-0417-1379
https://orcid.org/0000-0003-0417-1379
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Charlotte Arp Sørensen, Randers Regional Hospital, Dronningborg Boulevard 16D, Randers NØ, 8930, Denmark; Hospital Pharmacy, Central Denmark Region, Medical Department, Department of Clinical Medicine, Aarhus University, Denmark.
Annette de Thurah, Department of Clinical Medicine, Aarhus University, Health, Denmark; Department of Rheumatology, Aarhus University Hospital, Denmark.
Marianne Lisby, Department of Clinical Medicine, Aarhus University, Health, Denmark; Research Center for Emergency Medicine, Aarhus University Hospital, Denmark.
Charlotte Olesen, Hospital Pharmacy Central Denmark Region, Clinical Pharmacy, Aarhus University Hospital, Denmark.
Signe Bredsgaard Sørensen, Medical Department, Cardiology unit, Randers Regional Hospital, Denmark.
Ulrika Enemark, Department of Public Health, Aarhus University, Denmark.
References
- 1. Ahmad N, Ellins J, Krelle H, et al. Person-centred care: from ideas to action bringing together the evidence on shared decision making and self-management support. London: The Health Foundation, 2014. [Google Scholar]
- 2. Lomborg K, Bregnballe V, Rodkjær L, et al. Patientinvolvering - et begreb med praktisk potentiale. Sygeplejersken 2015; 12: 70–73. [Google Scholar]
- 3. European Commission. Eurobarometer qualitative study: patient involvement -aggregate report. 2012. [Google Scholar]
- 4. Richardson SJ, Brooks HL, Bramley G, et al. Evaluating the effectiveness of self-administration of medication (SAM) schemes in the hospital setting: a systematic review of the literature. PLoS One 2014; 9: e113912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Collingsworth S, Gould D, Wainwright SP. Patient self-administration of medication: a review of the literature. Int J Nurs Stud 1997; 34: 256–269. [DOI] [PubMed] [Google Scholar]
- 6. Manias E, Beanland C, Riley R, et al. Self-administration of medication in hospital: patients’ perspectives. J Adv Nurs 2004; 46: 194–203. [DOI] [PubMed] [Google Scholar]
- 7. Vanwesemael T, Boussery K, Manias E, et al. Self-management of medication during hospitalisation: Healthcare providers’ and patients’ perspectives. J Clin Nurs 2018; 27: 753–768. [DOI] [PubMed] [Google Scholar]
- 8. Wright J, Emerson A, Stephens M, et al. Hospital inpatient self-administration of medicine programmes: a critical literature review. Pharm World Sci 2006; 28: 140–151. [DOI] [PubMed] [Google Scholar]
- 9. Mahler TE, Sørensen CA, Hansen TG. Patients’ perspective on self-administration during hospitalisation - a qualitative pilot study. Eur J Pers Cent Healthc 2019; 7: 358–366. [Google Scholar]
- 10. Classen DC, Pestotnik SL, Evans RS, et al. Adverse drug events in hospitalized patients: excess length of stay, extra costs, and attributable mortality. JAMA 1997; 277: 301–306. [PubMed] [Google Scholar]
- 11. Houlind MB, McNulty HBO, Treldal C, et al. One-stop dispensing: hospital costs and patient perspectives on self-management of medication. Pharmacy (Basel) 2018; 6: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Drummond MF, Sculpher MJ, Claxton K, et al. Methods for the Economic Evaluation of Health Care Programmes, 4th ed. Oxford: Oxford University Press; , 2015. [Google Scholar]
- 13. Hartfiel N, Edwards RT. Cost–consequence analysis of public health interventions. In: Edwards RT, McIntosh E. (eds) Applied Health Economics for Public Health Practice and Research, 1st ed. Oxford: Oxford University Press, 2019, pp. 233–245. [Google Scholar]
- 14. Husereau D, Drummond M, Petrou S, et al. Consolidated health economic evaluation reporting standards (CHEERS) statement. Value Health 2013; 16: e1–e5. [DOI] [PubMed] [Google Scholar]
- 15. Sørensen CA, Lisby M, Olesen C, et al. Self-administration of medication: a pragmatic randomized controlled trial of the impact on dispensing errors, perceptions, and satisfaction. Ther Adv Drug Saf 2020; 11: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. The Ministry of Health. Healthcare in Denmark: an overview. Denmark: The Ministry of Health, 2017. [Google Scholar]
- 17. Danish Medicines Agency. Medicintilskudsgrænser. 2019. [Google Scholar]
- 18. Nielsen T, Joergensen M, Honoré SP. The quantity and quality of patients’ own medicines brought to hospital during admission Eur J Hosp Pharm Sci Pract 2012; 19: 164. [Google Scholar]
- 19. Dean B, Barber N. Validity and reliability of observational methods for studying medication administration errors. Am J Health Syst Pharm 2001; 58: 54–59. [DOI] [PubMed] [Google Scholar]
- 20. Westbrook JI, Li L, Lehnbom EC, et al. What are incident reports telling us? A comparative study at two Australian hospitals of medication errors identified at audit, detected by staff and reported to an incident system. Int J Qual Health Care 2015; 27: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Allan EL, Barker KN. Fundamentals of medication error research. Am J Hosp Pharm 1990; 47: 555–571. [PubMed] [Google Scholar]
- 22. Barker KN. Data collection techniques: observation. Am J Hosp Pharm 1980; 37: 1235–1243. [PubMed] [Google Scholar]
- 23. Barker KN, Flynn EA, Pepper GA. Observation method of detecting medication errors. Am J Health Syst Pharm 2002; 59: 2314–2316. [DOI] [PubMed] [Google Scholar]
- 24. Risør BW, Lisby M, Sørensen J. An automated medication system reduces errors in the medication administration process: results from a Danish hospital study. Eur J Hosp Pharm 2016; 23: 189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Central Denmark Region. Medication dispensing and administration, regional guideline. [In Danish: Lægemiddeldispensering og -administration, regional retningslinje]. 2019. [Google Scholar]
- 26. de Thurah A, Nørgaard M, Harder I, et al. Compliance with methotrexate treatment in patients with rheumatoid arthritis: influence of patients? Beliefs about the medicine. A prospective cohort study. Rheumatol Int 2010; 30: 1441–1448. [DOI] [PubMed] [Google Scholar]
- 27. Horne R, Weinman J, Hankins M. The beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychol Health 1999; 14: 1–24. [Google Scholar]
- 28. Horne R, Weinman J. Patients’ beliefs about prescribed medicines and their role in adherence to treatment in chronic physical illness. J Psychosom Res 1999; 47: 555–567. [DOI] [PubMed] [Google Scholar]
- 29. Horne R, Chapman SCE, Parham R, et al. Understanding patients’ adherence-related beliefs about medicines prescribed for long-term conditions: a meta-analytic review of the Necessity-Concerns Framework. PLoS One 2013; 8: e80633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fox-Rushby J, Cairns J. Efficiency and economic evaluation, 1st ed. Berkshire: Open University Press, 2005, pp. 7–18. [Google Scholar]
- 31. Dansk Sygepleje R. Lønstatistik for sygeplejersker ansat i kommuner og regioner. 2019. [Google Scholar]
- 32. The World Medical Association. WMA declaration of Helsinki – ethical principles for medical research involving human subjects. 2018. [Google Scholar]
- 33. Vermeulen KM, van Doormaal JE, Zaal RJ, et al. Cost-effectiveness of an electronic medication ordering system (CPOE/CDSS) in hospitalized patients. Int J Med Inform 2014; 83: 572–580. [DOI] [PubMed] [Google Scholar]
- 34. Risør BW, Lisby M, Sørensen J. Cost-Effectiveness analysis of an automated medication system implemented in a Danish hospital setting. Value Health 2016; 19: A626. [DOI] [PubMed] [Google Scholar]
- 35. Walsh EK, Hansen CR, Sahm LJ, et al. Economic impact of medication error: a systematic review. Pharmacoepidemiol Drug Saf 2017; 26: 481–497. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Appendix_TAW for Cost–consequence analysis of self-administration of medication during hospitalization: a pragmatic randomized controlled trial in a Danish hospital setting by Charlotte Arp Sørensen, Annette de Thurah, Marianne Lisby, Charlotte Olesen, Signe Bredsgaard Sørensen and Ulrika Enemark in Therapeutic Advances in Drug Safety
Supplemental material, CHEERS-Checklist_CCA_revised for Cost–consequence analysis of self-administration of medication during hospitalization: a pragmatic randomized controlled trial in a Danish hospital setting by Charlotte Arp Sørensen, Annette de Thurah, Marianne Lisby, Charlotte Olesen, Signe Bredsgaard Sørensen and Ulrika Enemark in Therapeutic Advances in Drug Safety