Abstract
Metabotropic glutamate (mGlu) receptors represent the largest family of glutamate receptors in mammals and act as fine tuners of the chemical transmission in central nervous system (CNS).
In the last decade, results concerning the expression and the subcellular localization of mGlu receptors further clarified their role in physio-pathological conditions. Concomitantly, their pharmacological characterization largely improved thanks to the identification of new compounds (chemical ligands and antibodies recognizing epitopic sequences of the receptor proteins) that allowed to decipher the protein compositions of the naive receptors.
mGlu receptors are expressed at the presynaptic site of chemical synapses. Here, they modulate intraterminal enzymatic pathways controlling the migration and the fusion of vesicles to synaptic membranes as well as the phosphorylation of colocalized receptors. Both the control of transmitter exocytosis and the phosphorylation of colocalized receptors elicited by mGlu receptors are relevant events that dictate the plasticity of nerve terminals, and account for the main role of presynaptic mGlu receptors as modulators of neuronal signalling.
The role of the presynaptic mGlu receptors in the CNS has been the matter of several studies and this review aims at briefly summarizing the recent observations obtained with isolated nerve endings (we refer to as synaptosomes). We focus on the pharmacological characterization of these receptors and on their receptor-receptor interaction / oligo-dimerization in nerve endings that could be relevant to the development of new therapeutic approaches for the cure of central pathologies.
Keywords: Synaptosomes, transmitter release, presynaptic receptors, oligomerization, receptor-receptor interaction, mGlu1/5, mGlu2/3, mGlu7
1. INTRODUCTION
Metabotropic glutamate (mGlu) receptors have a widespread distribution in the organism including the Central Nervous System (CNS), where they act as fine tuners of the chemical transmission [1-6].
The mGlu receptor family consists of eight different subtypes that are subdivided in three main groups depending on the homology of the protein sequences, the coupled G proteins and the second messenger pathways that transduce their signals in cells.
The group I of the mGlu receptors includes the mGlu1 and mGlu5 receptor subtypes. These receptors positively couple Gq/11 proteins and activate phospholipase C (PLC) and adenylyl cyclase (AC)-dependent intraterminal pathway, increasing the dynamics of cytosolic Ca2+ ions and of proteins phosphorylation [4, 5, 7-9].
The group II includes the mGlu2 and mGlu3 receptors. The huge homology of the receptor protein sequences made it very difficult to distinguish them by a pharmacological point of view. Nonetheless, the synthesis of new compounds [10-16] and the proposal of a novel approach based on antibodies recognizing the epitopic outer sequence of the receptor protein [17, 18] largely overcame the problem.
Finally, the III group consists of the mGlu4, mGlu6, mGlu7 and mGlu8 receptors. Several papers in the literature are dedicated to the mGlu4, 7 and 8 subtypes, while the mGlu6 receptor still represents an almost so far unexplored entity (the cDNA encoding the human mGlu6 was isolated from a human retinal cDNA library, [19, 20]).
The mGlu receptors belonging to the second and the third groups couple Gi/o proteins and negatively control AC activities and Voltage-Sensitive Ca2+ Channels (VSCCs), but activate the mitogen activated protein kinase (MAPK) dependent pathways [21]. Activation of these receptors inhibits the influx of positive charges within the cells and activate the K+ rectifying channels [6, 22-24].
The mGlu receptors are widely distributed in all the CNS regions and expressed in neurons, in astrocytes and glial cells, as well as in oligodendrocytes [25-28]. This diffuse distribution accounts for the interest into this receptor family, since modulators of mGlu receptors could represent therapeutic approaches to recover functional unbalance within the quad-partite synapse, the functional unit which consists of the pre and the post synaptic component of the chemical synapses as well as of the surrounding astrocytes and the microglial cells [29]. Ligands modulating these receptors can restore physiological transmission between neurons and astrocytes, but also counteract pathological microglia activation, defining the fate and the maturation of oligodendrocytes and myelin [28, 30].
In the last decade several reviews have been dedicated to the new results concerning the expression and localization of mGlu receptors as well as their role in pathological conditions [10, 11, 31, 32]. The impact of mGlu receptor ligands/modulators in neurodegenerative disorders, however, still represents matter of discussion [33].
Presynaptic mGlu receptors control intraterminal enzymatic pathways dictating the migration and the fusion of vesicles to the synaptic membranes, then tuning transmitters outflow [1, 2, 34, 35]. Meanwhile, the modulation of the intraterminal phosphorylative pathways also influences the phosphorylation – dephosphorylation of by-side receptor proteins (i.e. ionotropic receptors such as NMDA receptors, as well as other metabotropic receptors including GABAB and 5-HT2A receptors [36-42]), indirectly tuning their insertion in plasma membranes and their functions. Both events, i.e. the control of transmitter exocytosis and the phosphorylation of colocalized receptors, promote the plasticity of nerve terminals and account for the main role that presynaptic mGlu receptors play as modulators of neuronal signalling in CNS.
The cross-talk of mGlu receptors with by-standing receptors implies either receptors oligomerization or receptor-receptor interaction [43-45]. The term oligomerization refers to the physical association of colocalized receptors to form receptor assembly with a high level of complexity, where one receptor protein allosterically controls the function of the other receptor(s). The phenomenon occurs throughout the CNS, including in nerve endings, and it affects the efficiency of chemical transmission either presynaptically or postsynaptically. As far as the mGlu receptors are concerned, they can either homo or heterodimerize [7, 45]. The heteromeric association was first proposed to involve only receptors belonging to the same group (i.e. mGlu1 and mGlu5 receptors [46, 47] or mGlu receptors belonging to groups sharing a common transducing pathway (i.e. mGlu2 / mGlu4 receptor heterodimers [48, 49]). Recently, however, evidence was provided showing that mGlu receptors also can heterodimerize with metabotropic receptors transducing opposite intraterminal signalling (i.e. the mGlu5 / mGlu3 receptor-receptor interaction [50]) or with non-glutamatergic receptors (the 5-HT2A / mGlu2 [18, 40] and the mGlu1 / GABAB receptors complexes [42]). These new findings dramatically increase the complexity of the scenario. Some examples of this heterodimeric association occurring presynaptically in synaptosomes will be discussed in this review [41].
The term receptor-receptor interaction refers to the colocalization of two or more receptors (mGlu 1/5 / NMDA receptors [36, 37], mGlu2/3/ NMDA receptors [51], mGlu7 / A1 / GABAB receptors [52], mGlu7 / β receptors [53]), but it does not imply the physical association of the receptor proteins involved (that on the contrary represents a prerequisite for an efficient receptor homo/heterodimerization).
Both the receptor-receptor interaction and the receptor oligomerization trigger and sustain the mechanism of “metamodulation” [54, 55]. The term “metamodulation” refers to the cooperation linking different neurotransmitters to modulate synaptic transmission in the CNS. Neurotransmitters are often analysed individually owing to decipher their impact on chemical transmission, under-estimating their ability to cooperate and reciprocally interact to control neuronal activity. The complexity that originates from the converging activity of transmitters acting at colocalized receptors is impressive and might be taken into consideration when predicting the final outcome of therapeutic interventions with drugs or when considering the onset and the progression of neuropathological diseases [40].
The role of the presynaptic mGlu receptors in the CNS has been a matter of several investigations and studies have been already dedicated to resume the knowledge of the functional and pharmacological characteristics of these receptors [1, 2, 35, 56]. This review aims at updating the observations from studies with isolated nerve endings (we refer to as synaptosomes) that recently improved our knowledge of the pharmacological profile and of the receptor-receptor interaction / dimerization of presynaptic release-regulating mGlu receptors.
2. SYNAPTOSOMES IN SUPERFUSION: AN IN VITRO MODEL FOR INVESTIGATING THE RECEPTOR-RECEPTOR INTERACTION AND THE RECEPTOR ASSOCIATION AT THE PRESYNAPTIC LEVEL
Synaptosomes are pinched-off nerve terminals that retain all the functional features of the structures they originate from. During homogenization, nerve endings are detached from the axonal process and resealed to form these structures (see for recent reviews, [35, 58]. Synaptosomes possess the naïve receptors and the proteomic repertoire that assures their functional activities, including the control of transmitter release [57, 58]. In 1974 Raiteri and colleagues described for the first time the technique of the “up-down superfusion of a thin layer of synaptosomes” [59]. Briefly, monolayers of synaptosomes are plated on microporous filters in parallel chambers and continuously down superfused. In these conditions, the solution continuously removes the transmitters released by the superfused particles before they can activate targets on by-standing particles or retrogradely act at the nerve endings they are released from. Auto and heteroreceptor-mediated feed-back mechanisms of regulation of transmitter release are therefore minimized and do not affect the outcome (the release of transmitters, see [57] for technical details).
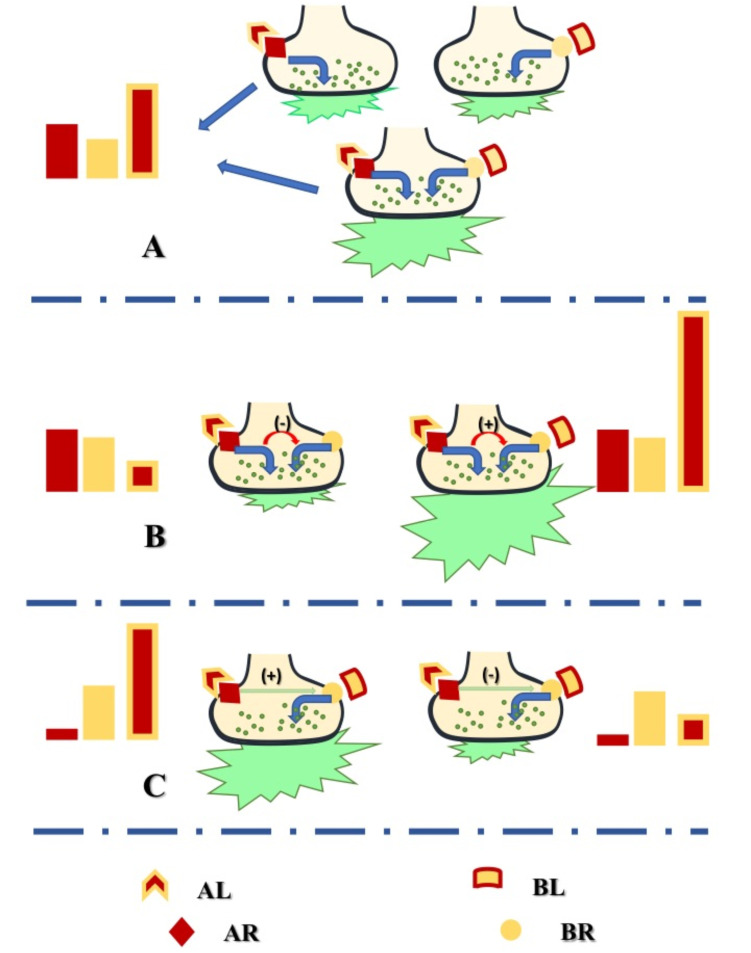
During superfusion, synaptosomes are exposed to agonists /antagonists of selected receptors in the absence or presence of depolarizing stimuli. Superfusate fractions are collected and quantified for their content in transmitters. The changes in transmitter overflow detected in superfusate fractions correlate to the exposure of synaptosomes to receptor ligands and confirm (by a functional point of view) the existence of the receptor in a synaptosomal subfamily. Actually, in superfused synaptosomes, the finding that a certain agonist AL dissolved in the superfusion medium causes significant changes in the basal or in the depolarization-evoked transmitter exocytosis from synaptosomes implies that the superfused synaptosomes possess the receptor targeted by the agonist (AR) and that the binding of AL to AR mediates functional responses that emerge as modification of transmitter release (Fig. 1A).
Fig. (1).
Correlation between agonist-receptor interaction and release efficiency in superfused synaptosomes. Synaptosomes are endowed with several receptor subtypes (i.e. AR and BR) that may colocalize in the same particles and that, once activated by selected ligands (i.e. AL and BL), might control the transmitter release. A) AR and BR exist on different synaptosomes or coexist in the same particles where they control transmitter exocytosis when activated by the respective agonists AL and BL independently one from each other. The concomitant activation of the two receptors would lead to a releasing activity corresponding to the sum of the releasing activity elicited by each receptor. B) AR and BR are functionally coupled and control transmitter exocytosis. The binding of AL to AR controls the release of the transmitter but also activates transducing pathways that reverberate on the colocalized BR influencing the releasing activity elicited by BL, either potentiating it (B, right) or decreasing it (B, left). C) AL acting at AR does not modify per se the transmitter release but affects the releasing activity elicited by BL acting at BR, either potentiating (C, left) or reducing (C, right) it. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
This is, however, a simplified picture of what can occur in superfused synaptosomes, since usually, these particles possess several receptors that, once activated, might either control the transmitter release independently one to each other or, alternatively, cooperate in an allosteric manner to modulate the exocytosis (Fig. 1A and 1B). In the former case, (Fig. 1A) the concomitant activation of the colocalized receptors would lead to a releasing activity that amounts to the sum of each single releasing effect. In the latter case (Fig. 1B), the concomitant activation of the colocalized receptors would elicit a releasing activity lower or higher than the sum of the releasing effects caused by the receptors. The possibility also exists that the exposure of synaptosomes to AL acting at the AR receptor does not cause per se changes in the transmitter release but influences the releasing activity elicited by another agonist (i.e. BL) acting at a colocalized B receptor (BR, Fig. 1C). In this case, the AR receptor is “silent”, but functionally cross-talks with the colocalized receptor to influence its outcomes [60, 61]. Whatever the picture, it might be concluded that the technique of the up-down superfusion of a thin monolayer of synaptosomes represents a method of choice to highlight by a functional point of view the co-existence of the two receptors on the same terminals and their allosteric interactions (Fig. 1B and 1C).
The functional results from release studies are, however, insufficient to demonstrate the physical interaction of colocalized receptors. To definitively address this point, parallel immunochemical studies must be also performed to unveil the physical association of the receptor proteins [18]. Furthermore, the results stating the physical receptor-receptor interaction can be further implemented by confocal microscopy confirming the presence of the receptor proteins in the selected synaptosomal subpopulations under study [18, 42, 46, 47, 62, 63].
Last but not least, the “up-down superfusion of a thin layer of synaptosomes” permits to evaluate the threshold of the activation of the receptors, as it allows to discriminate between the receptors that modify the spontaneous release from those whose release-modulating activity emerges in depolarized conditions. This information is fundamental to predict the relevance and the role of selected receptor subtypes in dictating the efficacy of synaptic connection in CNS.
3. PRESYNAPTIC RELEASE-REGULATING mGlu RECEPTORS BELONGING TO THE FIRST GROUP
3.1. The Group I mGlu Receptors: Pharmacological Profile and Intra-group Homo/heterodimerization
In general, the lack of selective ligands slowed the pharmacological characterization of the mGlu receptors, with the exception of the mGlu5 and the mGlu1 receptors, for which agonist / antagonists and allosteric modulators were available starting from the late ‘90 [64-67].
Group I mGlu receptors exist as auto and heteroreceptors.
The first evidence proving the existence of presynaptic release regulating group I mGlu autoreceptors was provided by Sanchez-Prieto and colleagues starting from the early ’90. The authors demonstrated the existence of presynaptic mGlu5 autoreceptors in the hippocampus and the cortex of rodents [34, 68-73]. Despite these findings and the general consensus on the existence of presynaptic release-regulating mGlu5 autoreceptors, almost concomitantly data emerged in the literature supporting the existence also of presynaptic mGlu1 autoreceptors [74-78]. This hypothesis was proven in 2008 by Pittaluga and collaborators [46] that demonstrated the presence of high affinity presynaptic release-regulating mGlu5 autoreceptors and of low affinity presynaptic release-regulating mGlu1 autoreceptors in isolated cortical nerve endings controlling the exocytosis of glutamate elicited by a mild depolarizing stimulus (Table 1, Fig. 2). The existence of both presynaptic release-regulating mGlu1 and mGlu5 autoreceptors in cortical synaptosomes was further supported by results obtained with animals bearing both a spontaneous and an engineered genetic mutation of the GRM1 and GRM5 genes, respectively (the Grm1crv4 and the Grm5 knock-out, k.o. mice [46, 79, 80]).
Table 1.
Pharmacological profile of the group I mGlu autoreceptors in isolated nerve endings (synaptosomes) from selected regions of the central nervous system.
| CNS Region | mGlu5 | mGlu1 | 3,5-DHPG (µM) | Basal Release | Depolarization-Evoked Release |
Glutamate Release in the
Presence of 3,5-DHPG |
Refs. | ||
|---|---|---|---|---|---|---|---|---|---|
|
Effect of
3,5-DHPG |
Effect of MPEP |
Effect of
CPCCOEt |
Effect of
LY367385 |
||||||
| Rat hippocampus | + | a | 0.3 µM | n.d. | 50 μM 4-AP + arachidonic acid, ⇑ |
1 µM, ⇓ | n.e. | 1 µM, ⇓ | [68] |
| Mouse cortex | + | 0.3 µM | n.d. | 12 mM KCl, ⇑ | 1 µM, ⇓ | 5 µM, n.e. | 1 µM, ⇓ | [46, 47] | |
| + | 30 µM | n.d. | 12 mM KCl, ⇑ | 1 µM, n.e. | 5 µM, ⇓ | 1 µM, ⇓ | |||
| Mouse cerebellum | + | 10 µM | n.d. | 12 mM KCl, ⇑ | 1 µM, n.e. | 5 µM, ⇓ | n.d. | [79] | |
| Mouse spinal cord | + | + | 30 µM | ⇑ | n.e. | 1 µM, ⇓ | 5 µM, ⇓ | 1 µM, ⇓ | [81] |
a = no data available in synaptosomes; n.e.= no effect; n.d.= not determined; ⇑ = increase glutamate release; ⇓ = decrease glutamate release.
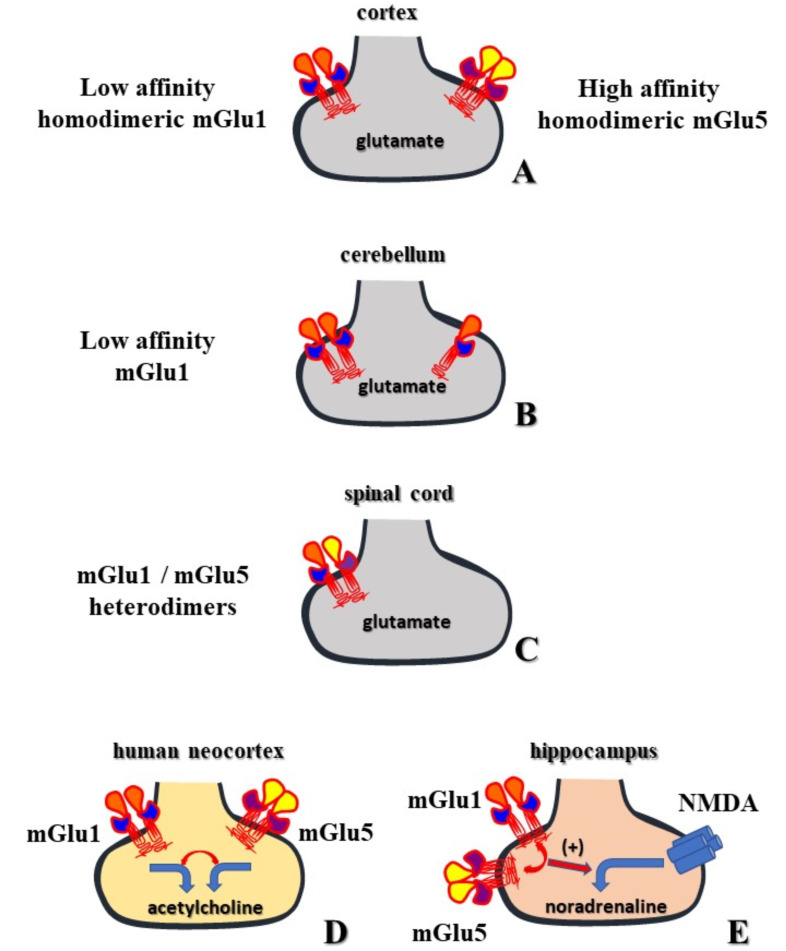
Fig. (2).
The contribution of the group I mGlu receptors to the presynaptic control of transmitter release from synaptosomes. A. mGlu1 and mGlu5 homodimeric receptors colocalize in mouse cortical glutamatergic nerve endings, but respond differently to the orthosteric agonists, representing respectively the low and the high affinity receptors. The two receptors do not modify the spontaneous release of glutamate but potentiate the depolarization-evoked glutamate exocytosis. B. mGlu1 receptors are present in cerebellar glutamatergic nerve endings, but the data so far available does not allow to predict whether the receptors exist in monomeric or dimeric assembly. The mGlu1 receptor does not affect the spontaneous release of glutamate but reinforces the depolarization-evoked glutamate exocytosis. C. mGlu1 and mGlu5 autoreceptors exist and colocalize in spinal cord synaptosomes, where they functionally associate to release glutamate. The two receptors increase the spontaneous release of glutamate. D. mGlu1/ mGlu5 containing receptors exist in human cortical cholinergic terminals where their activation elicits the release of acetylcholine. The two receptors compensate one each other, since the blockade of the 3,5-DHPG-evoked releasing activity is achieved only when the mGlu1 and the mGlu5 receptor antagonists are concomitantly added. The very low percentage of the cholinergic synaptosomes did not allow to perform immunocytochemical analysis to investigate the receptor protein assembly but the functional results seems best interpreted by assuming the colocalization of mGlu1 and mGlu5 homodimeric receptors. E. mGlu1 and mGlu5 receptors also exist in hippocampal noradrenergic terminals and cooperate in an exclusive manner. The receptors do not modify on their own the spontaneous release of the amine but potentiate the NMDA-mediated releasing activity. We propose that homodimeric mGlu1 and mGlu5 heteroreceptors colocalize in these terminals to modulate the releasing activity elicited by the activation of colocalized NMDA receptors. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Concomitantly, results from immunochemical analysis with confocal microscopy and western blot techniques were also provided well in line with the functional observations.
Specifically, cortical synaptosomal lysates and their presynaptic components showed immunopositivity for both the mGlu5 and the mGlu1 receptor proteins, that were detected either in the monomeric and the dimeric forms and confocal microscopy confirmed the presence of both mGlu5 and mGlu1 staining in vesicular glutamate transporter-1 (VGLUT-1) positive, sintaxin-1A containing glutamatergic particles [46].
These results were however insufficient to answer the question of whether the two autoreceptors colocalized on the same glutamatergic terminals or if they were expressed on different subpopulations of nerve endings. The following observations, however, filled the gap, suggesting that mGlu1 and mGlu5 receptors homodimers colocalize and physically associate in the same particle: i) mGlu1 immunoprecipitates from the cortical synaptosomes were also immunopositive for the mGlu5 receptor proteins, ii) the genetic deletion of the mGlu1 receptor also abolished the mGlu5-mediated component of the 3,5-Dihydroxyphenylglycine (3,5-DHPG)-induced releasing activity, iii) the mGlu1 orthosteric antagonist LY367385 also reduced the mGlu5-mediated potentiation of glutamate exocytosis [46, 47].
Group I mGlu receptors exist also in other CNS regions, including the cerebellum and the spinal cord. Cerebellar synaptosomes were found to be endowed with mGlu1 autoreceptors (Table 1, Fig. 2), whose activation failed to affect the basal release of glutamate but potentiated in a concentration-dependent manner the depolarization-evoked glutamate exocytosis [79]. Immunochemical studies confirmed the presence of the mGlu1 receptor proteins in synaptosomal lysates but did not give information on whether the receptor adopts a mono or a dimeric form (Fig. 2).
Group I mGlu autoreceptors were also detected in spinal cord synaptosomes. In this case, the activation of the presynaptic mGlu autoreceptors potentiated the basal release of glutamate. The 3,5-DHPG-evoked amplification of the glutamate basal outflow was prevented to a comparable extent by the mGlu5 or the mGlu1 receptor antagonists when added alone. The functional results were predictive of the presence of mGlu1 / mGlu5 heterodimeric assemblies, where the two receptors cooperate to control glutamate release ([81], Table 1). Western blot analysis of the spinal cord synaptosomal lysates unveiled the presence of both the mGlu1 and the mGlu5 receptor proteins in the monomeric form, but definitive results supporting their physical association in a heterodimeric assembly were not provided.
As far as the group I mGlu heteroreceptors are concerned, evidence exist proving the existence of these receptors in the hippocampal noradrenergic and in the human cortical cholinergic synaptosomes [37, 38, 82, 83].
The main characteristics of these receptors complex have been already reviewed in a previous work [35] and are compatible with the coexistence of both mGlu1 and mGlu5 receptors in both the synaptosomal subpopulations.
The most relevant difference between the two systems relies on the functional interaction linking the two mGlu receptors. In human cholinergic terminals both the mGlu1 and the mGlu5 receptors participate in the 3,5-DHPG-induced release of acetylcholine being reciprocally exclusive, since when added alone, antagonists acting at both mGlu receptor null the releasing activity elicited by the receptor agonist [82]. Differently, in hippocampal noradrenergic nerve endings, the 3,5-DHPG-induced facilitation of the NMDA-evoked [3H]noradrenaline release was impeded only if mGlu1 and mGlu5 antagonists are added concomitantly [37, 38]. In this case, the results were best interpreted by assuming that the mGlu1 and the mGlu5 heteroreceptors colocalize and compensate one for each other. Unfortunately, the low percentage of both the cortical cholinergic and the hippocampal noradrenergic synaptosomal subpopulations with respect to the entire synaptosomal population(less than the 1% of the total hippocampal synaptosomes) did not allow to verify the colocalization and the physical interaction of the mGlu1 and the mGlu5 receptor proteins, leaving so far unproven this hypothesis.
The relative contribution of the group I mGlu receptors to the presynaptic control of transmitter release from synaptosomes is resumed in Fig. 2.
3.2. The Group I mGlu Receptors: Inter-group Heterodimerization and Receptor-receptor Interaction with Non-glutamatergic Receptors
Evidence in the last decade clearly demonstrated that mGlu receptors can heterodimerize with mGlu receptors belonging to other groups (inter-dimerization, mGlu2 / mGlu4 receptors [48], mGlu5 / mGlu3 receptors [50]) as well as associate to G protein coupled receptors (GPCRs) belonging to other non-glutamatergic receptors [40].
It was recently described a functional interaction linking the mGlu5 receptor and the mGlu3 receptor [50]. In particular, mGlu3 receptor was shown to support the mGlu5 receptor signalling in neurons, amplifying the poly-phosphoinositide hydrolysis elicited by mGlu5 agonist, while mGlu5 activation subserved the mGlu3-dependent long-term depression in the prefrontal cortex. The novelty of these findings is that the receptor-receptor interaction involves receptors that physiologically transduce opposite signalling (i.e. the activation of PLC-mediated poly-phosphoinositide breakdown and the inhibition of the AC-mediated signalling, respectively). Whether such a kind of receptor-receptor interaction also can occur at nerve endings has not been so far investigated, but surely deserves attention.
Differently, it was recently demonstrated that mGlu1 receptors colocalize and functionally interact with other non-glutamatergic metabotropic receptors. It is the case of the presynaptic release-regulating GABAB receptors in both GABAergic and glutamatergic nerve endings [42].
The existence of presynaptic release-regulating mGlu1 receptors in GABAergic terminals has been a matter of discussion during the time. Selective mGlu1 receptor antagonists were shown to be neuroprotective and the beneficial effect was ascribed to the reinforcement of the GABAergic transmission in the hippocampus [84-86]. This effect was proposed not to involve the activation of mGlu1 receptors located on GABAergic nerve terminals, but rather to rely on an indirect cascade of events leading to the reduction of the inhibitory inputs of CB1 receptors on GABA exocytosis [87]. In line with the proposed indirect mGlu1 receptor-mediated control of GABA exocytosis, it was shown that neither the group I agonists nor the antagonists were able to modify the depolarization-evoked release of GABA from cortical and hippocampal synaptosomes [47, 88], although, more recently, 3,5-DHPG was reported to increase the spontaneous release of GABA from rat parietal-cortical synaptosomes [89].
To reconciliate these observations, it was speculated that mGlu1 receptors on GABAergic terminals can trigger a cytosolic cascade of events insufficient to elicit evident changes in transmitter exocytosis, but sufficient to modulate colocalized receptors, as already observed in noradrenergic terminals. Accordingly, mGlu1 receptor was found to tune colocalized GABAB receptors through protein kinase C (PKC)-mediated events. It is known that PKC-mediated processes phosphorylate the carboxy terminus of the GABAB1 subunit and assure its dissociation from the N-ethylmaleimide-sensitive fusion protein, favouring the desensitization of the receptor [90]. By hampering the PKC phosphorylative processes in synaptosomes, mGlu1 antagonists could reinforce the GABAB receptor-mediated control of GABA exocytosis, stabilizing the GABAB receptors in synaptosomal plasma membranes and slowing their internalization [42]. This cascade of events was proposed to underline the mGlu1-GABAB receptor-receptor interaction in these terminals. This hypothesis appears well in line with the previous evidence in the literature. In particular, evidence supporting the mGlu1 / GABAB receptor-receptor association and functional cross-talk were already reported in the literature starting from the early 2000 [91-95] and data were provided demonstrating that activation of GABAB receptors tunes the calcium responses generated by mGlu1 receptors [92, 96].
It is interesting to note that a comparable antagonist-like, receptor-receptor interaction also bridges mGlu1 autoreceptors and GABAB heteroreceptors in glutamatergic nerve endings, suggesting that the receptor-receptor cross-talk represents a general mechanism of mGlu1-mediated metamodulation of the GABAB receptor, at least at the cortical level.
Finally, it is worth reminding that, beside the mGlu5 / mGlu3 and the mGlu1 / GABAB receptor-receptor interaction, group I mGlu receptors also tune the NMDA-evoked release of noradrenaline from hippocampal synaptosomes. Again, the low percentage of the noradrenergic synaptosomal subpopulation in the hippocampal synaptosomal preparation did not allow to support the functional results with immunological results. Nonetheless, the results from release studies in isolated synaptosomes are well in line with the hypothesis of the group I mGlu /NMDA receptor-receptor interaction at this level [37, 38].
3.3. Molecular Mechanism for the Regulation of Transmitter Release Elicited by Presynaptic mGlu Receptors Belonging to the First Group
Group I mGlu receptors positively couple Gs proteins and trigger PLC-dependent intraterminal pathways leading to the activation of PKC and the mobilization of cytosolic calcium ions from intraterminal stores [4]. This cascade of events would favour the recruitment and the fusion of vesicles with synaptosomal plasma membranes, causing transmitter exocytosis.
Contrary to the expectation, however, it emerged that, depending on the mGlu receptor subtype involved and on the CNS region under study, the functional consequences elicited by the presynaptic mGlu1/5 receptors in term of transmitter release are heterogenous and impact this functional parameter differently (Table 1).
The impact of the group I mGlu receptors on transmitter release could be roughly resumed in three different models as follows:
mGlu 1/5 receptors can trigger transmitter release by mobilizing Ca2+ ions in the cytosol to a level high enough to elicit the docking and the fusion of vesicles and the consequent exocytosis of transmitters. It is the case of the presynaptic mGlu1/ mGlu5 autoreceptors in spinal cord glutamatergic nerve endings, that causes a significant production of inositol-triphosphates and a marked increase of [Ca2+] in the cytosol and triggers glutamate outflow also in basal, non-depolarized conditions [81] (Fig. 2C and D).
mGlu1/5 receptors can reinforce the release of transmitters elicited by a mild depolarizing stimulus by further increasing the Ca2+ ions level within the terminal. The amount of Ca2+ ions mobilized by the group I mGlu receptors is insufficient to trigger exocytosis but sufficient to amplify the releasing effect elicited by a mild depolarizing stimulus. It is the case of the presynaptic release-regulating mGlu1 and mGlu5 autoreceptors in cortical synaptosomes [46] (Fig. 2A and B).
mGlu1/5 receptors can indirectly modulate transmitter release by modifying the phosphorylation of aminoacid residues in the carboxi-terminus of colocalized receptors influencing their functions, including the control of exocytosis. It is the case of the mGlu1 and mGlu5 receptors colocalized with NMDA receptors in noradrenergic nerve endings [38] (Fig. 2E).
The dissection of the molecular events through which group I mGlu receptors control transmitter release is important to predict their role in the mechanisms of synaptic plasticity in the CNS.
4. PRESYNAPTIC RELEASE-REGULATING mGlu RECEPTORS BELONGING TO THE SECOND GROUP
4.1. The Group II mGlu Receptors: New Insights on the Pharmacological Characterization and the Intra-group Homo/Heterodimerization
Starting from their discovery, data confirmed the presynaptic localization of mGlu2/3 receptors in glutamatergic nerve endings from several CNS regions, including the cortex, the striatum and the spinal cord [1, 97-99]. In these regions, the pre-terminal portion of the axonal processes as well as the synaptic boutons were particularly enriched in mGlu2/3 receptor proteins, that, if activated by glutamate released by by-standing neurons and neighbouring astrocytes, negatively control transmitter exocytosis [17, 18, 41, 100-102]. mGlu2/3 receptors are therefore proposed as suitable targets for “neuroprotection” in central disorders typified by hyper-glutamatergicity.
The pharmacological characterization of the presynaptic release-regulating mGlu2/3 receptors and the description of the relative role of the mGlu2 and the mGlu3 receptor proteins was hindered by the lack of selective ligands able to discriminate between the two subunits. Furthermore, the genetic deletion of the mGlu2 or the mGlu3 receptor protein caused adaptative modification in the glutamate exocytosis, that might alter the results in studies aimed at deciphering the mGlu composition of the receptors [100].
The first selective ligands for mGlu2/3 receptors were the orthosteric agonists (2R,4R)-4-Aminopyrrolidine-2,4-dicarboxylate [(2R,4R)-APDC] [103] and (2S,2'R,3'R,)-2-(2',3'-Dicarboxycyclopropyl)glycine (DGC-IV) [104]. Both the compounds were typified by a micromolar affinity for the group II mGlu receptors but they were not selective for these receptors, since (2R,4R)-APDC also binds with lower affinity with other mGlu receptors while DGC-IV interacts, although less efficiently, with NMDA receptors and cannot be used in “in vivo” studies because of its limited distribution. Both compounds were reported to inhibit efficiently the exocytosis of glutamate from cortical synaptosomes [1, 77, 105].
A significant improvement came from the “second generation” of agonists, that were more potent and selective for the group II mGlu receptors. This class of orthosteric mGlu2/3 compounds includes bicyclic analogues of glutamate. The prototypes of these drugs are LY354740 and LY379268 [10, 13, 106, 107]. A large body of evidence supports their selectivity and efficacy in preclinical “in vitro” studies and clinical studies have been also developed to verify the efficacy of some of them for the treatment of central disorders, including generalized anxiety disorders, panic attack and schizophrenia [4, 10, 108, 109].
Beside these orthosteric agonists, the non-selective competitive group II antagonist LY341495 [110] also helped to identify the presence and the role of mGlu2/3 receptors. Unfortunately, however, also these compounds lack the selectivity towards the mGlu2 and the mGlu3 receptors and did not allow to discriminate the respective roles of the two proteins in naïve receptors. More recently, the synthesis of the mixed ligand LY541850, which selectively activates mGlu2 receptors but antagonizes mGlu3 receptors [111], and of the selective positive allosteric modulators (PAMs) and negative allosteric modulators (NAMs) for the two receptor subtypes, (i.e. the mGlu2 PAMs LY566332 and BINA and the mGlu3 NAMs LY2389575 and ML337 [98, 112-116], respectively) made the prediction of the protein composition of mGlu proteins assembly possible. Table 2 summarizes the pharmacological profile of the mGlu2/3 ligands reported in the text.
Table 2.
Ligands of the group II mGlu cited in the text.
| Ligands | Pharmacological Profile | Refs. |
|---|---|---|
| (2R,4R)-APDC | Broad spectrum group II mGlu agonist, also active at other mGlu receptors | [1, 103] |
| DGC-IV | Broad spectrum group II mGlu agonist | [1, 104] |
| NAAG | mGlu3 agonist | [123, 124] |
| LY354740 | mGlu2/3 agonist | [13, 106, 107] |
| LY379268 | mGlu2/3 agonist | [13, 106, 107] |
| LY341495 | Broad spectrum group II mGlu antagonist | [110] |
| LY541850 | Mixed ligand (mGlu2 agonist / mGlu3 antagonist) | [1] |
| LY566332 | mGlu2 PAM | [99, 113] |
| LY2389575 | mGlu3 NAM | [114] |
| BINA | mGlu2 PAM | [112] |
| ML 337 | mGlu3 NAM | [116] |
| DN10 | Nanobody, mGlu2 PAM, partial agonist at mGlu2 | [17] |
| DN13 | Nanobody, mGlu2 PAM | [17] |
Beside the classic ligand-receptor approach to characterize receptors, a new method, which we refer to as “the immunopharmacological approach”, was proposed for discriminating between the two receptors subtypes. It consists in the use of antibodies recognizing the outer side of the receptor protein as selective ligands able to interfere with the receptor-mediated functions [17, 18, 117-121]. The impact of the antibody / receptor complex on the releasing efficiency from synaptosomes is strictly dependent on the targeted receptors, as indicated by the results in the literature showing that the presence of the antibody-receptor complex at the outer side of the synaptosomal plasmamembranes can favour receptor internalization [121], modulating the receptor-mediated effects [17, 117-119] but also activate complement-mediated responses at nerve terminals [122].
As far as the group II mGlu receptors are concerned, Jean Philippe Pin and collaborators firstly described nanobodies (DN10 and DN13) that selectively bind the active form of the mGlu2 receptor protein acting as PAM in transfected cells and brain slices [17]. These nanobodies specifically bind the homodimeric association of the mGlu2 receptors, but not their heterodimeric association with mGlu3 and mGlu4 receptors. In acute hippocampal slices, the nanobodies potentiated the mGlu2 receptors controlling calcium transients in the presynaptic mossy fibers terminals also slowing the off-rate kinetic of inhibition produced by the group II agonist DCG-IV. These effects were prevented by the mGlu2/3 antagonist LY341495, definitively supporting the selective activity of the nanobodies at the mGlu2 receptors. Moreover, the results also improved the pharmacological characterization of the group II receptors in mossy fibers terminals [17].
The first review dealing with presynaptic mGlu2/3 receptors dates back to 2000 and describes the information available at that time about the presynaptic release-regulating mGlu receptors, reporting also the data available from synaptosomes [1]. The data suggesting the existence of the presynaptic release-regulating group II mGlu autoreceptors were obtained by studying the effects of DCG-IV and of 2R,4R-APDC by using synaptosomes isolated from several CNS regions. The pharmacological characterization of the presynaptic group II mGlu receptors in cortical and spinal cord synaptosomes was improved in 2016 [101] by taking advantage of selective receptors ligands, including LY379268, LY341495, LY541850 and the dipeptide N-acetyl-aspartyl-glutamate (NAAG, [123, 124]). The functional results seemed compatible with the conclusion that cortical synaptosomes possess mGlu2-preferring, mGlu3-sensitive autoreceptors, while spinal cord synaptosomes are endowed with almost pure mGlu3-preferring autoreceptors. In this work the use of an anti-mGlu2/3 antibody also confirmed the presence of mGlu2/3 receptor proteins in both the synaptosomal preparations. Interestingly, the western blot analysis unveiled that the receptor proteins preferentially adopt the dimeric association in both cortical and spinal cord particles (i.e. the monomeric form of the receptor protein was almost undetectable). Confocal microscopy confirmed the colocalization of mGlu2/3 immunostaining with VGLUT-1 and syntaxin-1A signal.
These observations were however insufficient to propose the respective role of the mGlu2 and the mGlu3 receptor proteins in the expression of the receptor complexes. This aspect was clarified by using mGlu2 PAMs (LY566332 and BINA) and mGlu3 NAMs (LY2389575 and ML337) as well as selective anti-mGlu2 and anti-mGlu3 antibodies recognizing the outer side of the mGlu2 and the mGlu3 proteins [18]. The results from these studies are briefly summarized in Table 3. The observations from this set of experiments were largely confirmatory of the presence of mGlu3-preferring autoreceptors in the spinal cord and of mGlu2-preferring, mGlu3-sensitive receptors in the cortex.
Table 3.
Pharmacological profile of the mGlu2/3 autoreceptors controlling glutamate exocytosis from cortical and spinal cord nerve endings.
| Ligands | Applied Stimulus | Cortex | Spinal Cord | Refs. |
|---|---|---|---|---|
| (2R,4R)-APDC | 50 µM veratridine | ⇓ | n.d. | [105] |
| DGC-IV | 50 µM veratridine, 200 mM 4-AP |
⇓ | n.d. | [77, 105] |
| LY379268 | 12 / 15 mM KCl-enriched solution | ⇓; EC50: 1.50 ± 1.15 nM | ⇓; EC50: 0.15 ± 0.37 pM | [101] |
| NAAG | “ | n.e. | ⇓; EC50: 0.054 ± 0.003 pM | [101] |
| LY541850 | “ | ⇓; EC50: 1-100 nM | 1000 nM, inactive on its own, ⇑ LY379268-mediated inhibition (10-100 nM) |
[101] |
| LY341495 | “ | 100 nM inactive on its own, ⇑ LY379268-mediated inhibition (10-100 nM), ⇑ LY541850-mediated inhibition (10-100 nM) |
100 nM inactive on its own, ⇑ LY379268-mediated inhibition (10-100 nM) ⇑ NAAG-mediated inhibition, (100 nM) |
[101] |
| LY566332 | “ | 1 µM, inactive on its own ⇓⇓ 3 nM LY379268-mediated inhibition |
1 µM, inactive on its own inactive on the 30 pM LY379268-mediated inhibition |
[18] |
| LY2389575 | “ | 1 µM, inactive on its own ⇑ 10 nM LY379268-mediated inhibition |
1 µM, inactive on its own ⇑ 30 pM LY379268-mediated inhibition |
[18] |
| BINA | “ | 1 µM, inactive on its own ⇓⇓ 3 nM LY379268-mediated inhibition |
1 µM, inactive on its own inactive on the 10 pM LY379268-mediated inhibition |
[18] |
| ML-337 | “ | 1 µM, inactive on its own ⇑ 10 nM LY379268-mediated inhibition |
1 µM, inactive on its own ⇑ 30 pM LY379268-mediated inhibition |
[18] |
| Anti mGlu2 | “ | inactive on its own ⇑ 3 nM LY379268-mediated inhibition |
inactive on its own inactive on the 30 pM LY379268-mediated inhibition |
[18] |
| Anti mGlu3 | inactive on its own partial ⇑ 3 nM LY379268-mediated inhibition |
inactive on its own ⇑ on the 30 pM LY379268-mediated inhibition |
[18] |
⇓ = inhibition of glutamate release; ⇓⇓ = increased inhibition of glutamate release n.e = no effect; ⇑ = block of the inhibition of glutamate release.
In particular, as far as the results of the two anti-mGlu antibodies are concerned, the two antibodies confirmed the presence of the mGlu2 and the mGlu3 receptor proteins in both synaptosomal preparations with either western blot analysis and confocal microscopy, but they also supported the proposed subclassification of the autoreceptors when used in the “immunopharmacological approach” (Table 3). Differently from what has been observed by Scholler and colleagues in 2017, however, the antibodies did not potentiate but prevented the agonist-induced activity. In particular, the incubation of synaptosomes with the antibodies reduced, although to a different extent and in region-specific manner, the presynaptic release-regulating activity of LY379268 in spinal cord and cortical synaptosomes. The incubation of spinal cord synaptosomes with the anti-mGlu3 antibody efficiently impeded the LY3792768-induced inhibition of glutamate release, while the anti-mGlu2 antibody was almost inactive. Differently, in cortical synaptosomes, incubation of the isolated nerve endings with the anti-mGlu2 antibody nulled the inhibitory effect of LY379268, while the anti-mGlu3 antibody affected it to a lower extent. Concomitantly, Western blot analysis unveiled a very low expression of the mGlu2 receptor protein in spinal cord synaptosomes when compared to mGlu3 receptor protein (both proteins were detected in the dimeric association). On the contrary, the mGlu2 dimeric immunopositivity prevailed in cortical synaptosomal lysates where also the mGlu3 dimeric staining was observed, although less pronounced. In a whole, these observations confirm that the mGlu2/3 autoreceptors in spinal cord glutamatergic synaptosomes consists of mGlu3 dimeric assembly that is typified by a very low picomolar affinity for LY379268. The high affinity of the agonists in this region is compatible with previous observations concerning the presynaptic release-regulating mGlu2/3 receptors in another subpopulation of spinal cord synaptosomes, namely the glycinergic one [102]. Also these terminals possess NAAG-sensitive mGlu2/3 heteroreceptors typified by an high affinity for the agonist (NAAG provoked about 40% inhibition of the KCl-evoked glycine exocytosis when added at 0.001 nM). Differently, in cortical glutamatergic nerve endings both the mGlu2 and the mGlu3 proteins participate to the expression of the mGlu2/3 autoreceptors (although to a different level) that are characterized by a nanomolar affinity for LY379268.
Presynaptic release-regulating mGlu2/3 autoreceptors have a widespread distribution in CNS and data concerning the characterization of these receptors also in other regions are available in the literature. mGlu3-preferring autoreceptors were proposed to exist in mice striatal nerve endings [100]. The conclusion was proposed on the basis of the results obtained when studying the effect of LY379268 on the release of endogenous glutamate elicited by veratridine in striatal synaptosomes from wild type mice as well as from animals knocked out for the mGlu2 (mGlu2 -/- k.o. mice) and for the mGlu3 (mGlu3 -/- k.o. mice). Interestingly, LY379268 failed to modify significantly the release of endogenous GABA in striatal synaptosomes in both control and mGlu3-/- mice, but significantly reduced it in striatal synaptosomes from mGlu2-/- animals. The functional adaptations due to the genetic deletion of the receptor proteins that emerged from this study in nerve endings suggest caution in the use of the mGlu k.o. mice to define the role of mGlu proteins in receptor expression [100].
4.2. The Group II mGlu Receptors: Inter-group Hetero-dimerization and Receptor-receptor Interaction with Non-glutamatergic Receptors
While studying the dimeric association of mGlu receptors, it was demonstrated that group II and III mGlu receptors (i.e. the mGlu2 and the mGlu4 receptor proteins) can associate in a dimeric intergroup association [48]. The impact and the role of this heterodimeric complex is far to be elucidated and evidence so far available concerning their distribution in synapses are lacking. Nonetheless, evidence was consistent with the presence of both mGlu2 and mGlu4 receptors at presynaptic level in corticostriatal projections, where their activation reduces excitatory transmission [125]. Similarly, data in literature suggest that both mGlu2 and mGlu4 receptors exist in medial prefrontal cortex axon terminals as well as at the presynaptic level in thalamocortical synapses [114, 126-128]. Data definitively proving the mGlu2 and mGlu4 receptor proteins heterodimerization at the presynaptic level and the functional relevance of this receptor-receptor association are however lacking.
As already discussed before, the mGlu3 receptors were recently reported to colocalize and heterodimerize with mGlu5 receptors. Again, the existence of this receptor complex at the presynaptic level in nerve terminals remains so far unexplored [50].
A striking finding of recent years, however, is that, beside the existence of heterodimers consisting of mGlu receptors belonging to the II and the III groups (i.e. the mGlu2/4 heterodimers [128]), mGlu2/3 receptors also heterodimerize with non-glutamatergic metabotropic receptors. In particular, mGlu2/3 receptors were found to functionally couple to the serotonergic metabotropic receptor 5-HT2A. This receptor-receptor cross-talk was firstly shown to occur in the prefrontal cortex of mammals [129], where it was proposed as a functional mechanism through which glutamate modulates the efficiency of serotonin receptors [126, 130-132]. Briefly, data were provided showing that the two receptors colocalize and functionally cross-talk to modulate glutamate signalling The striking unexpected finding was that the two receptors interact in an antagonist-like manner, since activation of one receptor reduces the functional outcome of the other receptor. This antagonist-like functional cross-talk would permit to use the mGlu2/3 selective ligands to modulate the functions of 5-HT2A receptors, giving a rationale for the antipsychotic activity of mGlu2/3 receptors modulators [133]. Furthermore, data from autoptic brain of psychotic patients suggested that mGlu2/3/5-HT2A receptors heterodimerization would be dysregulated in patients suffering from schizophrenia, also giving a rationale for the different sensitivity of patients to antipsychotic therapy [129, 132]. More recently, the mGlu2/3 /5-HT2A receptors heterodimerization was also reported to exist presynaptically in spinal cord glutamatergic nerve endings [41]. At this level the two receptors modulate one another in an antagonist-like fashion. The heterodimeric association of the two receptors in these terminals was proven with immunoprecipitation studies since anti-mGlu2/3 immunoprecipitates from spinal cord synaptosomal lysates were also immunopositive for the 5-HT2A protein, consistent with the physical association of the two receptor proteins
As already discussed in the introduction, the colocalization of two receptors in synaptosomes does not necessarily implies the physical association of the two proteins in a receptor complex. For instance, evidence exists showing that mGlu2/3 receptors control presynaptically the NMDA-mediated facilitation of acetylcholine exocytosis in synaptosomes [51]. Immunochemical analysis investigating the association of the NMDA receptor subunits and the mGlu2/3 receptor proteins are lacking.
5. PRESYNAPTIC RELEASE-REGULATING mGlu RECEPTORS BELONGING TO THE THIRD GROUP
5.1. The Group III mGlu Receptors: New Insights on the Pharmacological Characterization and the Intra-group Homo/Heterodimerization
The results concerning the existence and the functional role of presynaptic group III mGlu receptors in CNS and in particular those studies where synaptosomes were used to define these aspects mainly concern the mGlu7 receptor subtypes. The existence of mGlu4, mGlu7 and mGlu8 receptor subtypes in nerve endings isolated from cerebral cortices was demonstrated by immunohistochemistry, with confocal microscopy [134]. Concomitant calcium imaging unveiled that cortical synaptosomes were endowed with mGlu receptors with high and low affinity for (l)-2-amino-4-phosphonobutyrate (l-AP4). These receptors were then identified by immunocytochemistry as mGlu4 and mGlu7 receptors respectively. Their activation caused the reduction of glutamate release from cortical nerve endings by reducing the Ca2+mediated responses. The two receptors were proposed to have a different localization, since mGlu4 autoreceptors were largely expressed in nerve terminals endowed with both N- and P/Q-type Ca2+ channels, while mGlu7 autoreceptors were preferentially located in N-type Ca2+ channel-containing synaptosomes. Almost concomitantly, the authors demonstrated that the inhibitory effect elicited by l-AP4 on glutamate release mainly relied on the inhibition of AC-mediated pathway [135]. In all these studies, the involvement of mGlu7 receptors was deduced from immunocytochemistry analysis in synaptosomes, since selective ligands for this receptor subtype were not available at that time. Interestingly, Sanchez-Prieto and his collaborators found that the prolonged exposure of synaptosomes to l-AP4 caused a shift from inhibition to potentiation of glutamate release. Facilitation of glutamate exocytosis involved an intraterminal pathway that relies on PLC and consequently increased phosphatidylinositol (4,5)-bisphosphate hydrolysis that favour translocation to the active zone of munc 13-1, a protein essential for synaptic vesicle priming, [136]. The shift from facilitation to inhibition or vice versa of GPCRs exposed to agonists during time was already reported for other GPCRs including the group I mGlu receptors [46, 69, 137] and well support the dynamic profile of these receptors.
The definitive pharmacological characterization of mGlu7 autoreceptors in cortical nerve endings was recently provided by Wang et al. [138] by using N,N’-dibenzyhydryl-ethane-1,2-diamine dihydrochloride (AMN082, [139]) and 6-(4-Methoxyphenyl)-5-methyl-3-(4-pyridinyl)-isoxazolo[4,5-c]pyridin-4(5H)-one hydrochloride (MMPIP, [140]), respectively PAM and NAM of the mGlu7 receptor subtype. The authors demonstrated that AMN082 efficiently inhibited the release of glutamate elicited by the transient exposure of synaptosomes to 4-aminopyridine and that the inhibitory effect was reversed by MMPIP. They also described the intraterminal pathway involved in the inhibitory effect that, well consistent with previous observation, was found to rely on a significant reduction of the AC-induced, cAMP-mediated cascade of events.
The mGlu7 heteroreceptors were also reported to exist in hippocampal GABAergic synaptosomes. The exposure of cortical synaptosomes to AMN802 (in the nanomolar range) caused a drastic reduction of the GABA exocytosis elicited by a mild depolarizing stimulus (high KCl containing medium) in a MMPIP-sensitive manner. The receptor protein was present in the synaptosomal lysate in the monomeric form and confocal microscopy confirmed its presence in sintaxin-1A positive, vesicular GABA transporter-containing synaptosomes [141]. Table 4 summarizes the pharmacological profile of the mGlu7 ligands reported in the text.
Table 4.
Group III mGlu receptor ligand and their impact on transmitter exocytosis.
| Ligands | Pharmacological Profile | Impact on Glutamate Release | Impact on GABA Release | Refs. |
|---|---|---|---|---|
| l-AP4 | Broad spectrum agonist of group III mGlu | ⇓ 1 mM 4-AP-induced exocytosis | ⇓ 12 mM KCl-evoked exocytosis | [134-137, 141] |
| AMN082 | mGlu7 positive allosteric modulator | ⇓, 1 mM 4-AP-induced exocytosis | ⇓ 12 mM KCl-evoked exocytosis | [138, 141] |
| MPPIP | mGlu7 negative allosteric modulator | ⇑ AMN082-mediated inhibition of 1 mM 4-AP-induced exocytosis | ⇑ AMN082-mediated inhibition of 1 mM 4-AP-induced exocytosis | [138, 141] |
⇓ = inhibition of transmitter release; ⇑ = reversal of the inhibition of transmitter release.
5.2. The Group III mGlu Receptors: Inter-group Heterodimerization and Receptor-receptor Interaction with Non-glutamatergic Receptors
In 2010, Martin and colleagues [136] provided evidence that the mGlu7 receptor proteins in cerebro-cortical nerve terminals are present in a dimeric association, consistent with the conclusion that at this level the receptors adopt this assembly.
The presynaptic release-regulating mGlu7 receptors were also reported to colocalize and functionally interact with other non-mGlu receptors. Functional results concerning glutamate exocytosis and calcium movements in synaptosomes demonstrated that mGlu7 autoreceptors colocalize with A1 adenosine receptors and GABAB receptors in hippocampal and cerebrocortical synaptosomes to modulate the efficiency of transmitter release. The concomitant activation of the three receptors occludes their inhibitory effects on glutamate exocytosis [52].
Release studies were also performed suggesting that the presynaptic mGlu7 heteroreceptors and the GABAB autoreceptors located on GABAergic nerve endings are not functionally coupled. This conclusion relied on the findings that i) the inhibition of GABA exocytosis and cAMP production elicited by AMN082 and (-)Baclofen were additive, ii) the GABAB receptor antagonist CGP52432 failed to affect the mGlu7-mediated inhibition of GABA exocytosis and iii) the (-)baclofen-induced control of GABA overflow was insensitive to the concomitant presence of MMPIP. Differently, these observations were best interpreted by assuming that mGlu7 and GABAB receptors are located on different subpopulation of mouse hippocampal GABAergic nerve terminals or, if colocalized, that they are not functionally coupled. Well in line with the first hypothesis, Somogyi et al. [142] demonstrated that mGlu7 immunoreactivity is restricted to the GABAergic interneurons that receive innervation from mGlu7 receptor-enriched glutamatergic terminals.
Finally, evidence was also provided demonstrating the colocalization and the functional cross-talk of mGlu7 and β receptor in cerebrocortical synaptosomes with confocal microscopy and release experiments. The interaction linking the two receptors was dependent on pertussis-toxin-sensitive pathway and mediated the presynaptic control of glutamate exocytosis [53].
CONCLUSION
The main aspect highlighted in the review concerns the complexity of presynaptic release-regulating mGlu receptors in the CNS. The availability of new ligands disclosed the huge heterogeneity of mGlu receptors association in nerve endings. This heterogeneity might account for the complexity of the effects elicited by these receptors [5, 7, 10], but it also could give the rationale for the difference in the potency and efficacy of selected mGlu receptor ligands in different CNS regions [18, 101].
The results reported in the review demonstrate that at the presynaptic level mGlu receptors participate to the expression of oligomeric structures having heterogeneous receptor composition that differ in term of efficiency of signal transduction and of affinity for the available agonists, antagonists and allosteric modulators.
Notably, a relevant aspect is the observation that the composition of the presynaptic mGlu-containing receptor oligomers does not consist only of intra-group mGlu receptors combinations (i.e. the mGlu1/mGlu5 receptor complex [46, 81]) but also of association of mGlu receptors with other non-glutamatergic receptors [41, 42]. In the latter case, the counterpart of the mGlu receptor in the presynaptic release-regulating oligomer might be a receptor sharing a common intraterminal pathway with the glutamatergic receptor (i.e. it is the case of the 5-HT2A receptor that functionally couple to the mGlu2/3 receptors, [41]) or a receptor transducing a different intraterminal signal (i.e. the GABAB receptors coupled to the mGlu1 receptors, [42]). As already highlighted by Nicoletti and collaborators [50], the partnership among metabotropic receptor subtypes coupled to different G proteins may stimulate reconsideration of the role of mGlu receptors, including those located presynaptically, in CNS.
These findings also shed new light on the concept of metamodulation. The fact that receptors belonging to different groups and/or families could participate to oligomeric structures at the presynaptic level, then dictating the efficiency of transmitter release and phosphorylative pathways, suggests reconsidering their role in synaptic plasticity and in neurological disorders. Beside its intrinsic dynamism, the impact of metamodulation in CNS can vary depending on the physio-pathological conditions of individuals and the related changes in the expression and / or functions of the receptors involved in the functional association (i.e the 5-HT2A receptors in the patients affected by schizophrenia [129, 132]). All these aspects surely deserve attention and synaptosomes as well as their up-down superfusion to monitor transmitter release represent appropriate techniques to study the presynaptic release- regulating receptors in nerve terminals and their interaction with other by-standing receptors.
ACKNOWLEDGEMENTS
Declared none
LIST OF ABBREVIATIONS
- (2R,4R)-APDC
(2R,4R)-4-Aminopyrrolidine-2,4-dicarboxylate
- 3,5-DHPG
(S)-3,5-Dihydroxyphenylglycine
- AC
adenylyl cyclase
- AMN082
N,N’-dibenzyhydryl-ethane-1,2-diamine dihydrochloride
- CNS
central nervous system
- DGV-IV
(2S,2'R,3'R,)-2-(2',3'-Dicarboxycyclopropyl)glycine
- GPCR
G protein coupled receptor
- l-AP4
(l)-2-amino-4-phosphonobutyrate
- MAPK
mitogen activated protein kinase
- mGlu receptor
metabotropic glutamate receptor
- MMPIP
6-(4-Methoxyphenyl)-5-methyl-3-(4-pyridinyl)-isoxazolo[4,5-c]pyridin-4(5H)-one hydrochloride
- NAAG
N-acetyl-aspartyl-glutamate
- NAM
negative allosteric modulators
- PAM
positive allosteric modulator
- PKC
protein kinase C
- PLC
phospholipase C
- VGLUT-1
vesicular glutamate transporter-1
- VSCC
voltage-sensitive Ca2+ channel
CONSENT FOR PUBLICATION
Not applicable
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise
REFERENCES
- 1.Cartmell J., Schoepp D.D. Regulation of neurotransmitter release by metabotropic glutamate receptors. J. Neurochem. 2000;75(3):889–907. doi: 10.1046/j.1471-4159.2000.0750889.x. [DOI] [PubMed] [Google Scholar]
- 2.Schoepp D.D. Unveiling the functions of presynaptic metabotropic glutamate receptors in the central nervous system. J. Pharmacol. Exp. Ther. 2001;299(1):12–20. [PubMed] [Google Scholar]
- 3.Nicoletti F., Battaglia G., Storto M., Ngomba R.T., Iacovelli L., Arcella A., Gradini R., Sale P., Rampello L., De Vita T., Di Marco R., Melchiorri D., Bruno V. Metabotropic glutamate receptors: beyond the regulation of synaptic transmission. Psychoneuroendocrinology. 2007;32(Suppl. 1):S40–S45. doi: 10.1016/j.psyneuen.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 4.Nicoletti F., Bockaert J., Collingridge G.L., Conn P.J., Ferraguti F., Schoepp D.D., Wroblewski J.T., Pin J.P. Metabotropic glutamate receptors: from the workbench to the bedside. Neuropharmacology. 2011;60(7-8):1017–1041. doi: 10.1016/j.neuropharm.2010.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicoletti F., Bruno V., Ngomba R.T., Gradini R., Battaglia G. Metabotropic glutamate receptors as drug targets: what’s new? Curr. Opin. Pharmacol. 2015;20:89–94. doi: 10.1016/j.coph.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 6.Mercier M.S., Lodge D. Group III metabotropic glutamate receptors: pharmacology, physiology and therapeutic potential. Neurochem. Res. 2014;39(10):1876–1894. doi: 10.1007/s11064-014-1415-y. [DOI] [PubMed] [Google Scholar]
- 7.De Blasi A., Conn P.J., Pin J., Nicoletti F. Molecular determinants of metabotropic glutamate receptor signaling. Trends Pharmacol. Sci. 2001;22(3):114–120. doi: 10.1016/s0165-6147(00)01635-7. [DOI] [PubMed] [Google Scholar]
- 8.Pin J.P., Acher F. The metabotropic glutamate receptors: structure, activation mechanism and pharmacology. Curr. Drug Targets CNS Neurol. Disord. 2002;1(3):297–317. doi: 10.2174/1568007023339328. [DOI] [PubMed] [Google Scholar]
- 9.Iacovelli L., Nicoletti F., De Blasi A. Molecular mechanisms that desensitize metabotropic glutamate receptor signaling: an overview. Neuropharmacology. 2013;66:24–30. doi: 10.1016/j.neuropharm.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Bruno V., Battaglia G., Copani A., D’Onofrio M., Di Iorio P., De Blasi A., Melchiorri D., Flor P.J., Nicoletti F. Metabotropic glutamate receptor subtypes as targets for neuroprotective drugs. J. Cereb. Blood Flow Metab. 2001;21(9):1013–1033. doi: 10.1097/00004647-200109000-00001. [DOI] [PubMed] [Google Scholar]
- 11.Moldrich R.X., Chapman A.G., De Sarro G., Meldrum B.S. Glutamate metabotropic receptors as targets for drug therapy in epilepsy. Eur. J. Pharmacol. 2003;476(1-2):3–16. doi: 10.1016/s0014-2999(03)02149-6. [DOI] [PubMed] [Google Scholar]
- 12.Chiechio S., Copani A., Melchiorri D., Canudas A.M., Storto M., Calvani M., Nicolai R., Nicoletti F. Metabotropic receptors as targets for drugs of potential use in the treatment of neuropathic pain. J. Endocrinol. Invest. 2004;27(6) Suppl.:171–176. [PubMed] [Google Scholar]
- 13.Imre G. The preclinical properties of a novel group II metabotropic glutamate receptor agonist LY379268. CNS Drug Rev. 2007;13(4):444–464. doi: 10.1111/j.1527-3458.2007.00024.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marek G.J. Metabotropic glutamate2/3 (mGlu2/3) receptors, schizophrenia and cognition. Eur. J. Pharmacol. 2010;639(1-3):81–90. doi: 10.1016/j.ejphar.2010.02.058. [DOI] [PubMed] [Google Scholar]
- 15.Fell M.J., McKinzie D.L., Monn J.A., Svensson K.A. Group II metabotropic glutamate receptor agonists and positive allosteric modulators as novel treatments for schizophrenia. Neuropharmacology. 2012;62(3):1473–1483. doi: 10.1016/j.neuropharm.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 16.Chaki S., Fukumoto K. mGlu receptors as potential targets for novel antidepressants. Curr. Opin. Pharmacol. 2018;38:24–30. doi: 10.1016/j.coph.2018.02.00. [DOI] [PubMed] [Google Scholar]
- 17.Scholler P., Nevoltris D., de Bundel D., Bossi S., Moreno-Delgado D., Rovira X., Møller T.C., El Moustaine D., Mathieu M., Blanc E., McLean H., Dupuis E., Mathis G., Trinquet E., Daniel H., Valjent E., Baty D., Chames P., Rondard P., Pin J.P. Allosteric nanobodies uncover a role of hippocampal mGlu2 receptor homodimers in contextual fear consolidation. Nat. Commun. 2017;8(1):1967. doi: 10.1038/s41467-017-01489-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olivero G., Bonfiglio T., Vergassola M., Usai C., Riozzi B., Battaglia G., Nicoletti F., Pittaluga A. Immuno-pharmacological characterization of group II metabotropic glutamate receptors controlling glutamate exocytosis in mouse cortex and spinal cord. Br. J. Pharmacol. 2017;174(24):4785–4796. doi: 10.1111/bph.14061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laurie D.J., Schoeffter P., Wiederhold K.H., Sommer B. Cloning, distribution and functional expression of the human mGlu6 metabotropic glutamate receptor. Neuropharmacology. 1997;36(2):145–152. doi: 10.1016/s0028-3908(96)00172-4. [DOI] [PubMed] [Google Scholar]
- 20.Valerio A., Zoppi N., Ferraboli S., Paterlini M., Ferrario M., Barlati S., Spano P. Alternative splicing of mGlu6 gene generates a truncated glutamate receptor in rat retina. Neuroreport. 2001;12(12):2711–2715. doi: 10.1097/00001756-200108280-00024. [DOI] [PubMed] [Google Scholar]
- 21.Pin J.P., Duvoisin R. The metabotropic glutamate receptors: structure and functions. Neuropharmacology. 1995;34(1):1–26. doi: 10.1016/0028-3908(94)00129-G. [DOI] [PubMed] [Google Scholar]
- 22.Lavreysen H., Dautzenberg F.M. Therapeutic potential of group III metabotropic glutamate receptors. Curr. Med. Chem. 2008;15(7):671–684. doi: 10.2174/092986708783885246. [DOI] [PubMed] [Google Scholar]
- 23.Amalric M., Lopez S., Goudet C., Fisone G., Battaglia G., Nicoletti F., Pin J.P., Acher F.C. Group III and subtype 4 metabotropic glutamate receptor agonists: discovery and pathophysiological applications in Parkinson’s disease. Neuropharmacology. 2013;66:53–64. doi: 10.1016/j.neuropharm.2012.05.026. [DOI] [PubMed] [Google Scholar]
- 24.Williams C.J., Dexter D.T. Neuroprotective and symptomatic effects of targeting group III mGlu receptors in neurodegenerative disease. J. Neurochem. 2014;129(1):4–20. doi: 10.2174/092986708783885246. [DOI] [PubMed] [Google Scholar]
- 25.D’Antoni S., Berretta A., Bonaccorso C.M., Bruno V., Aronica E., Nicoletti F., Catania M.V. Metabotropic glutamate receptors in glial cells. Neurochem. Res. 2008;33(12):2436–2443. doi: 10.1007/s11064-008-9694-9. [DOI] [PubMed] [Google Scholar]
- 26.Wierońska J.M., Pilc A. Metabotropic glutamate receptors in the tripartite synapse as a target for new psychotropic drugs. Neurochem. Int. 2009;55(1-3):85–97. doi: 10.1016/j.neuint.2009.02.019. [DOI] [PubMed] [Google Scholar]
- 27.Bradley S.J., Challiss R.A. G protein-coupled receptor signalling in astrocytes in health and disease: a focus on metabotropic glutamate receptors. Biochem. Pharmacol. 2012;84(3):249–259. doi: 10.1016/j.bcp.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 28.Spampinato S.F., Copani A., Nicoletti F., Sortino M.A., Caraci F. Metabotropic glutamate receptors in glial cells: a new potential target for neuroprotection? Front. Mol. Neurosci. 2018;11:414. doi: 10.3389/fnmol.2018.00414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schafer D.P., Lehrman E.K., Stevens B. The “quad-partite” synapse: microglia-synapse interactions in the developing and mature CNS. Glia. 2013;61(1):24–36. doi: 10.1002/glia.22389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fazio F., Zappulla C., Notartomaso S., Busceti C., Bessede A., Scarselli P., Vacca C., Gargaro M., Volpi C., Allegrucci M., Lionetto L., Simmaco M., Belladonna M.L., Nicoletti F., Fallarino F. Cinnabarinic acid, an endogenous agonist of type-4 metabotropic glutamate receptor, suppresses experimental autoimmune encephalomyelitis in mice. Neuropharmacology. 2014;81:237–243. doi: 10.1016/j.neuropharm.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 31.Palucha A., Pilc A. Metabotropic glutamate receptor ligands as possible anxiolytic and antidepressant drugs. Pharmacol. Ther. 2007;115(1):116–147. doi: 10.1016/j.pharmthera.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 32.Nickols H.H., Conn P.J. Development of allosteric modulators of GPCRs for treatment of CNS disorders. Neurobiol. Dis. 2014;61:55–71. doi: 10.1016/j.nbd.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bruno V., Caraci F., Copani A., Matrisciano F., Nicoletti F., Battaglia G. The impact of metabotropic glutamate receptors into active neurodegenerative processes: A “dark side” in the development of new symptomatic treatments for neurologic and psychiatric disorders. Neuropharmacology. 2017;115:180–192. doi: 10.1016/j.neuropharm.2016.04.044. [DOI] [PubMed] [Google Scholar]
- 34.Raiteri M. Presynaptic metabotropic glutamate and GABAB receptors. Handb. Exp. Pharmacol. 2008;(184):373–407. doi: 10.1007/978-3-540-74805-2_12. [DOI] [PubMed] [Google Scholar]
- 35.Pittaluga A. Presynaptic release-regulating mglu1 receptors in central nervous system. 2016. [DOI] [PMC free article] [PubMed]
- 36.Benquet P., Gee C.E., Gerber U. Two distinct signaling pathways upregulate NMDA receptor responses via two distinct metabotropic glutamate receptor subtypes. J. Neurosci. 2002;22(22):9679–9686. doi: 10.1523/JNEUROSCI.22-22-09679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Longordo F., Feligioni M., Chiaramonte G., Sbaffi P.F., Raiteri M., Pittaluga A. The human immunodeficiency virus-1 protein transactivator of transcription up-regulates N-methyl-D-aspartate receptor function by acting at metabotropic glutamate receptor 1 receptors coexisting on human and rat brain noradrenergic neurones. J. Pharmacol. Exp. Ther. 2006;317(3):1097–1105. doi: 10.1124/jpet.105.099630. [DOI] [PubMed] [Google Scholar]
- 38.Luccini E., Musante V., Neri E., Brambilla Bas M., Severi P., Raiteri M., Pittaluga A. Functional interactions between presynaptic NMDA receptors and metabotropic glutamate receptors co-expressed on rat and human noradrenergic terminals. Br. J. Pharmacol. 2007;151(7):1087–1094. doi: 10.1038/sj.bjp.0707280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Baki L., Fribourg M., Younkin J., Eltit J.M., Moreno J.L., Park G., Vysotskaya Z., Narahari A., Sealfon S.C., Gonzalez-Maeso J., Logothetis D.E. Cross-signaling in metabotropic glutamate 2 and serotonin 2A receptor heteromers in mammalian cells. Pflugers Arch. 2016;468(5):775–793. doi: 10.1007/s00424-015-1780-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Delille H.K., Becker J.M., Burkhardt S., Bleher B., Terstappen G.C., Schmidt M., Meyer A.H., Unger L., Marek G.J., Mezler M. Heterocomplex formation of 5-HT2A-mGlu2 and its relevance for cellular signaling cascades. Neuropharmacology. 2012;62(7):2184–2191. doi: 10.1016/j.neuropharm.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 41.Olivero G., Grilli M., Vergassola M., Bonfiglio T., Padolecchia C., Garrone B., Di Giorgio F.P., Tongiani S., Usai C., Marchi M., Pittaluga A. 5-HT2A-mGlu2/3 receptor complex in rat spinal cord glutamatergic nerve endings: A 5-HT2A to mGlu2/3 signalling to amplify presynaptic mechanism of auto-control of glutamate exocytosis. Neuropharmacology. 2018;133:429–439. doi: 10.1016/j.neuropharm.2018.02.030. [DOI] [PubMed] [Google Scholar]
- 42.Vergassola M., Olivero G., Cisani F., Usai C., Bossi S., Puliti A., Pittaluga A. Presynaptic mGlu1 receptors control gabab receptors in an antagonist-like manner in mouse cortical gabaergic and glutamatergic nerve endings. Front. Mol. Neurosci. 2018;11:324. doi: 10.3389/fnmol.2018.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.G-protein-coupled receptors. Trends Pharmacol. Sci. 2007;28(12):615–620. doi: 10.1016/j.tips.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 44.Prezeau L., Rives M.L., Comps-Agrar L., Maurel D., Kniazeff J., Pin J.P. Functional crosstalk between GPCRs: with or without oligomerization. Curr. Opin. Pharmacol. 2010;10(1):6–13. doi: 10.1016/j.coph.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 45.Pin J.P., Bettler B. Organization and functions of mGlu and GABAB receptor complexes. Nature. 2016;540(7631):60–68. doi: 10.1038/nature20566. [DOI] [PubMed] [Google Scholar]
- 46.Musante V., Neri E., Feligioni M., Puliti A., Pedrazzi M., Conti V., Usai C., Diaspro A., Ravazzolo R., Henley J.M., Battaglia G., Pittaluga A. Presynaptic mGlu1 and mGlu5 autoreceptors facilitate glutamate exocytosis from mouse cortical nerve endings. Neuropharmacology. 2008;55(4):474–482. doi: 10.1016/j.neuropharm.2008.06.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Musante V., Summa M., Neri E., Puliti A., Godowicz T.T., Severi P., Battaglia G., Raiteri M., Pittaluga A. The HIV-1 viral protein Tat increases glutamate and decreases GABA exocytosis from human and mouse neocortical nerve endings. Cereb. Cortex. 2010;20(8):1974–1984. doi: 10.1093/cercor/bhp274. [DOI] [PubMed] [Google Scholar]
- 48.Doumazane E., Scholler P., Zwier J.M., Trinquet E., Rondard P., Pin J.P. A new approach to analyze cell surface protein complexes reveals specific heterodimeric metabotropic glutamate receptors. FASEB J. 2011;25(1):66–77. doi: 10.1096/fj.10-163147. [DOI] [PubMed] [Google Scholar]
- 49.Liu J., Zhang Z., Moreno-Delgado D., Dalton J.A., Rovira X., Trapero A., Goudet C., Llebaria A., Giraldo J., Yuan Q., Rondard P., Huang S., Liu J., Pin J.P. Allosteric control of an asymmetric transduction in a G protein-coupled receptor heterodimer. 2017. [DOI] [PMC free article] [PubMed]
- 50.Di Menna L., Joffe M.E., Iacovelli L., Orlando R., Lindsley C.W., Mairesse J., Gressèns P., Cannella M., Caraci F., Copani A., Bruno V., Battaglia G., Conn P.J., Nicoletti F. Functional partnership between mGlu3 and mGlu5 metabotropic glutamate receptors in the central nervous system. Neuropharmacology. 2018;128:301–313. doi: 10.1016/j.neuropharm.2017.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mela F., Marti M., Fiorentini C., Missale C., Morari M. Group-II metabotropic glutamate receptors negatively modulate NMDA transmission at striatal cholinergic terminals: role of P/Q-type high voltage activated Ca++ channels and endogenous dopamine. Mol. Cell. Neurosci. 2006;31(2):284–292. doi: 10.1016/j.mcn.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 52.Martín R., Ladera C., Bartolomé-Martín D., Torres M., Sánchez-Prieto J. The inhibition of release by mGlu7 receptors is independent of the Ca2+ channel type but associated to GABAB and adenosine A1 receptors. Neuropharmacology. 2008;55(4):464–473. doi: 10.1016/j.neuropharm.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 53.Ferrero J.J., Ramírez-Franco J., Martín R., Bartolomé-Martín D., Torres M., Sánchez-Prieto J. Cross-talk between metabotropic glutamate receptor 7 and beta adrenergic receptor signaling at cerebrocortical nerve terminals. Neuropharmacology. 2016;101:412–425. doi: 10.1016/j.neuropharm.2015.07.025. [DOI] [PubMed] [Google Scholar]
- 54.Katz P.S., Edwards D.H. Metamodulation: the control and modulation of neuromodulation. 1999.
- 55.Ribeiro J.A., Sebastião A.M. Modulation and metamodulation of synapses by adenosine. Acta Physiol. (Oxf.) 2010;199(2):161–169. doi: 10.1111/j.1748-1716.2010.02115.x. [DOI] [PubMed] [Google Scholar]
- 56.Pinheiro P.S., Mulle C. Presynaptic glutamate receptors: physiological functions and mechanisms of action. 2008. [DOI] [PubMed]
- 57.Raiteri L., Raiteri M. Synaptosomes still viable after 25 years of superfusion. Neurochem. Res. 2000;25(9-10):1265–1274. doi: 10.1023/A:1007648229795. [DOI] [PubMed] [Google Scholar]
- 58.Pittaluga A. Acute functional adaptations in isolated presynaptic terminals unveil synaptosomal learning and memory. Int. J. Mol. Sci. 2019;20(15):3641. doi: 10.3390/ijms20153641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Raiteri M., Angelini F., Levi G. A simple apparatus for studying the release of neurotransmitters from synaptosomes. Eur. J. Pharmacol. 1974;25(3):411–414. doi: 10.1016/0014-2999(74)90272-6. [DOI] [PubMed] [Google Scholar]
- 60.Pittaluga A., Bonfanti A., Raiteri M. Somatostatin potentiates NMDA receptor function via activation of InsP(3) receptors and PKC leading to removal of the Mg(2+) block without depolarization. Br. J. Pharmacol. 2000;130(3):557–566. doi: 10.1038/sj.bjp.0703346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Summa M., Di Prisco S., Grilli M., Marchi M., Pittaluga A. Hippocampal AMPA autoreceptors positively coupled to NMDA autoreceptors traffic in a constitutive manner and undergo adaptative changes following enriched environment training. Neuropharmacology. 2011;61(8):1282–1290. doi: 10.1016/j.neuropharm.2011.07.032. [DOI] [PubMed] [Google Scholar]
- 62.Hervás C., Pérez-Sen R., Miras-Portugal M.T. Presence of diverse functional P2X receptors in rat cerebellar synaptic terminals. Biochem. Pharmacol. 2005;70(5):770–785. doi: 10.1016/j.bcp.2005.05.033. [DOI] [PubMed] [Google Scholar]
- 63.Rodrigues R.J., Almeida T., Díaz-Hernández M., Marques J.M., Franco R., Solsona C., Miras-Portugal M.T., Ciruela F., Cunha R.A. Presynaptic P2X1-3 and α3-containing nicotinic receptors assemble into functionally interacting ion channels in the rat hippocampus. Neuropharmacology. 2016;105:241–257. doi: 10.1016/j.neuropharm.2016.01.022. [DOI] [PubMed] [Google Scholar]
- 64.Schoepp D.D., Goldsworthy J., Johnson B.G., Salhoff C.R., Baker S.R. 3,5-dihydroxyphenylglycine is a highly selective agonist for phosphoinositide-linked metabotropic glutamate receptors in the rat hippocampus. J. Neurochem. 1994;63(2):769–772. doi: 10.1046/j.1471-4159.1994.63020769.x. [DOI] [PubMed] [Google Scholar]
- 65.Salt T.E., Binns K.E., Turner J.P., Gasparini F., Kuhn R. Antagonism of the mGlu5 agonist 2-chloro-5-hydroxyphenylglycine by the novel selective mGlu5 antagonist 6-methyl-2-(phenylethynyl)-pyridine (MPEP) in the thalamus. Br. J. Pharmacol. 1999;127(5):1057–1059. doi: 10.1038/sj.bjp.0702677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hermans E., Nahorski S.R., Challiss R.A. Reversible and non-competitive antagonist profile of CPCCOEt at the human type 1alpha metabotropic glutamate receptor. Neuropharmacology. 1998;37(12):1645–1647. doi: 10.1016/S0028-3908(98)00132-4. [DOI] [PubMed] [Google Scholar]
- 67.Litschig S., Gasparini F., Rueegg D., Stoehr N., Flor P.J., Vranesic I., Prézeau L., Pin J.P., Thomsen C., Kuhn R. CPCCOEt, a noncompetitive metabotropic glutamate receptor 1 antagonist, inhibits receptor signaling without affecting glutamate binding. Mol. Pharmacol. 1999;55(3):453–461. [PubMed] [Google Scholar]
- 68.Herrero I., Miras-Portugal M.T., Sánchez-Prieto J. Positive feedback of glutamate exocytosis by metabotropic presynaptic receptor stimulation. 1992. [DOI] [PubMed]
- 69.Herrero I., Miras-Portugal M.T., Sánchez-Prieto J. Rapid desensitization of the metabotropic glutamate receptor that facilitates glutamate release in rat cerebrocortical nerve terminals. 1994. [DOI] [PubMed]
- 70.Shigemoto R., Nomura S., Ohishi H., Sugihara H., Nakanishi S., Mizuno N. Immunohistochemical localization of a metabotropic glutamate receptor, mGluR5, in the rat brain. 1993. [DOI] [PubMed]
- 71.Vázquez E., Herrero I., Miras-Portugal M.T., Sánchez-Prieto J. Developmental change from inhibition to facilitation in the presynaptic control of glutamate exocytosis by metabotropic glutamate receptors. Neuroscience. 1995;68(1):117–124. doi: 10.1016/0306-4522(95)00121-X. [DOI] [PubMed] [Google Scholar]
- 72.Rodríguez-Moreno A., Sistiaga A., Lerma J., Sánchez-Prieto J. Switch from facilitation to inhibition of excitatory synaptic transmission by group I mGluR desensitization. Neuron. 1998;21(6):1477–1486. doi: 10.1016/S0896-6273(00)80665-0. [DOI] [PubMed] [Google Scholar]
- 73.Sistiaga A., Herrero I., Conquet F., Sánchez-Prieto J. The metabotropic glutamate receptor 1 is not involved in the facilitation of glutamate release in cerebrocortical nerve terminals. 1998. [DOI] [PubMed]
- 74.Fotuhi M., Sharp A.H., Glatt C.E., Hwang P.M., von Krosigk M., Snyder S.H., Dawson T.M. Differential localization of phosphoinositide-linked metabotropic glutamate receptor (mGluR1) and the inositol 1,4,5-trisphosphate receptor in rat brain. J. Neurosci. 1993;13(5):2001–2012. doi: 10.1523/JNEUROSCI.13-05-02001.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lujan R., Nusser Z., Roberts J.D., Shigemoto R., Somogyi P. Perisynaptic location of metabotropic glutamate receptors mGluR1 and mGluR5 on dendrites and dendritic spines in the rat hippocampus. 1996. [DOI] [PubMed]
- 76.Moroni F., Cozzi A., Lombardi G., Sourtcheva S., Leonardi P., Carfì M., Pellicciari R. Presynaptic mGlu1 type receptors potentiate transmitter output in the rat cortex. Eur. J. Pharmacol. 1998;347(2-3):189–195. doi: 10.1016/S0014-2999(98)00124-1. [DOI] [PubMed] [Google Scholar]
- 77.Reid M.E., Toms N.J., Bedingfield J.S., Roberts P.J. Group I mGlu receptors potentiate synaptosomal [3H]glutamate release independently of exogenously applied arachidonic acid. Neuropharmacology. 1999;38(4):477–485. doi: 10.1016/S0028-3908(98)00217-2. [DOI] [PubMed] [Google Scholar]
- 78.Fazal A., Parker F., Palmer A.M., Croucher M.J. Characterisation of the actions of group I metabotropic glutamate receptor subtype selective ligands on excitatory amino acid release and sodium-dependent re-uptake in rat cerebrocortical minislices. 2003. [DOI] [PubMed]
- 79.Rossi P.I., Musante I., Summa M., Pittaluga A., Emionite L., Ikehata M., Rastaldi M.P., Ravazzolo R., Puliti A. Compensatory Molecular and Functional Mechanisms in Nervous System of the Grm1(crv4) Mouse Lacking the mGlu1 Receptor: A Model for Motor Coordination Deficits. Cereb. Cortex. 2013;23(9):2179–2189. doi: 10.1093/cercor/bhs200. [DOI] [PubMed] [Google Scholar]
- 80.Bossi S., Musante I., Bonfiglio T., Bonifacino T., Emionite L., Cerminara M., Cervetto C., Marcoli M., Bonanno G., Ravazzolo R., Pittaluga A., Puliti A. Genetic inactivation of mGlu5 receptor improves motor coordination in the Grm1crv4 mouse model of SCAR13 ataxia. 2018. [DOI] [PubMed]
- 81.Giribaldi F., Milanese M., Bonifacino T., Anna Rossi P.I., Di Prisco S., Pittaluga A., Tacchetti C., Puliti A., Usai C., Bonanno G. Group I metabotropic glutamate autoreceptors induce abnormal glutamate exocytosis in a mouse model of amyotrophic lateral sclerosis. Neuropharmacology. 2013;66:253–263. doi: 10.1016/j.neuropharm.2012.05.018. [DOI] [PubMed] [Google Scholar]
- 82.Feligioni M., Raiteri L., Pattarini R., Grilli M., Bruzzone S., Cavazzani P., Raiteri M., Pittaluga A. The human immunodeficiency virus-1 protein Tat and its discrete fragments evoke selective release of acetylcholine from human and rat cerebrocortical terminals through species-specific mechanisms. J. Neurosci. 2003;23(17):6810–6818. doi: 10.1523/JNEUROSCI.23-17-06810.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Parodi M., Patti L., Grilli M., Raiteri M., Marchi M. Nicotine has a permissive role on the activation of metabotropic glutamate 5 receptors coexisting with nicotinic receptors on rat hippocampal noradrenergic nerve terminals. Neurochem. Int. 2006;48(2):138–143. doi: 10.1016/j.neuint.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 84.Pellegrini-Giampietro D.E. The distinct role of mGlu1 receptors in post-ischemic neuronal death. Trends Pharmacol. Sci. 2003;24(9):461–470. doi: 10.1016/S0165-6147(03)00231-1. [DOI] [PubMed] [Google Scholar]
- 85.Battaglia G., Bruno V., Pisani A., Centonze D., Catania M.V., Calabresi P., Nicoletti F. Selective blockade of type-1 metabotropic glutamate receptors induces neuroprotection by enhancing gabaergic transmission. 2001. [DOI] [PubMed]
- 86.Ferraguti F., Crepaldi L., Nicoletti F. Metabotropic glutamate 1 receptor: current concepts and perspectives. 2008. [DOI] [PubMed]
- 87.Landucci E., Boscia F., Gerace E., Scartabelli T., Cozzi A., Moroni F., Mannaioni G., Pellegrini-Giampietro D.E. Involvement of endocannabinoid signaling in the neuroprotective effects of subtype 1 metabotropic glutamate receptor antagonists in models of cerebral ischemia. Int. Rev. Neurobiol. 2009;85:337–350. doi: 10.1016/S0074-7742(09)85023-X. [DOI] [PubMed] [Google Scholar]
- 88.Zucchini S., Pittaluga A., Brocca-Cofano E., Summa M., Fabris M., De Michele R., Bonaccorsi A., Busatto G., Barbanti-Brodano G., Altavilla G., Verlengia G., Cifelli P., Corallini A., Caputo A., Simonato M. Increased excitability in tat-transgenic mice: role of tat in HIV-related neurological disorders. Neurobiol. Dis. 2013;55:110–119. doi: 10.1016/j.nbd.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 89.Bragina L., Bonifacino T., Bassi S., Milanese M., Bonanno G., Conti F. Differential expression of metabotropic glutamate and GABA receptors at neocortical glutamatergic and GABAergic axon terminals. Front. Cell. Neurosci. 2015;9:345. doi: 10.3389/fncel.2015.00345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Terunuma M., Pangalos M.N., Moss S.J. Functional modulation of GABAB receptors by protein kinases and receptor trafficking. Adv. Pharmacol. 2010;58:113–122. doi: 10.1016/S1054-3589(10)58005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ige A.O., Bolam J.P., Billinton A., White J.H., Marshall F.H., Emson P.C. Cellular and sub-cellular localisation of GABA(B1) and GABA(B2) receptor proteins in the rat cerebellum. Brain Res. Mol. Brain Res. 2000;83(1-2):72–80. doi: 10.1016/S0169-328X(00)00199-6. [DOI] [PubMed] [Google Scholar]
- 92.Tabata T., Araishi K., Hashimoto K., Hashimotodani Y., van der Putten H., Bettler B., Kano M. Ca2+ activity at GABAB receptors constitutively promotes metabotropic glutamate signaling in the absence of GABA. Proc. Natl. Acad. Sci. USA. 2004;101(48):16952–16957. doi: 10.1073/pnas.0405387101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Luján R., Shigemoto R. Localization of metabotropic GABA receptor subunits GABAB1 and GABAB2 relative to synaptic sites in the rat developing cerebellum. Eur. J. Neurosci. 2006;23(6):1479–1490. doi: 10.1111/j.1460-9568.2006.04669.x. [DOI] [PubMed] [Google Scholar]
- 94.Rives M.L., Vol C., Fukazawa Y., Tinel N., Trinquet E., Ayoub M.A., Shigemoto R., Pin J.P., Prézeau L. Crosstalk between GABAB and mGlu1a receptors reveals new insight into GPCR signal integration. EMBO J. 2009;28(15):2195–2208. doi: 10.1038/emboj.2009.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tadavarty R., Rajput P.S., Wong J.M., Kumar U., Sastry B.R. Sleep-deprivation induces changes in GABA(B) and mGlu receptor expression and has consequences for synaptic long-term depression. PLoS One. 2011;6(9):e24933. doi: 10.1371/journal.pone.0024933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hirono M., Yoshioka T., Konishi S. GABA(B) receptor activation enhances mGluR-mediated responses at cerebellar excitatory synapses. Nat. Neurosci. 2001;4(12):1207–1216. doi: 10.1038/nn764. [DOI] [PubMed] [Google Scholar]
- 97.Luján R., Roberts J.D., Shigemoto R., Ohishi H., Somogyi P. Differential plasma membrane distribution of metabotropic glutamate receptors mGluR1 alpha, mGluR2 and mGluR5, relative to neurotransmitter release sites. J. Chem. Neuroanat. 1997;13(4):219–241. doi: 10.1016/S0891-0618(97)00051-3. [DOI] [PubMed] [Google Scholar]
- 98.Tamaru Y., Nomura S., Mizuno N., Shigemoto R. Distribution of metabotropic glutamate receptor mGluR3 in the mouse CNS: differential location relative to pre- and postsynaptic sites. Neuroscience. 2001;106(3):481–503. doi: 10.1016/S0306-4522(01)00305-0. [DOI] [PubMed] [Google Scholar]
- 99.Hemstapat K., Da Costa H., Nong Y., Brady A.E., Luo Q., Niswender C.M., Tamagnan G.D., Conn P.J. A novel family of potent negative allosteric modulators of group II metabotropic glutamate receptors. J. Pharmacol. Exp. Ther. 2007;322(1):254–264. doi: 10.1124/jpet.106.117093. [DOI] [PubMed] [Google Scholar]
- 100.Corti C., Battaglia G., Molinaro G., Riozzi B., Pittaluga A., Corsi M., Mugnaini M., Nicoletti F., Bruno V. The use of knock-out mice unravels distinct roles for mGlu2 and mGlu3 metabotropic glutamate receptors in mechanisms of neurodegeneration/neuroprotection. J. Neurosci. 2007;27(31):8297–8308. doi: 10.1523/JNEUROSCI.1889-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Di Prisco S., Merega E., Bonfiglio T., Olivero G., Cervetto C., Grilli M., Usai C., Marchi M., Pittaluga A. Presynaptic, release-regulating mGlu2 -preferring and mGlu3 -preferring autoreceptors in CNS: pharmacological profiles and functional roles in demyelinating disease. Br. J. Pharmacol. 2016;173(9):1465–1477. doi: 10.1111/bph.13442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Romei C., Raiteri M., Raiteri L. Glycine release is regulated by metabotropic glutamate receptors sensitive to mGluR2/3 ligands and activated by N-acetylaspartylglutamate (NAAG). Neuropharmacology. 2013;66:311–316. doi: 10.1016/j.neuropharm.2012.05.030. [DOI] [PubMed] [Google Scholar]
- 103.Monn J.A., Valli M.J., Johnson B.G., Salhoff C.R., Wright R.A., Howe T., Bond A., Lodge D., Spangle L.A., Paschal J.W., Campbell J.B., Griffey K., Tizzano J.P., Schoepp D.D. Synthesis of the four isomers of 4-aminopyrrolidine-2,4-dicarboxylate: identification of a potent, highly selective, and systemically-active agonist for metabotropic glutamate receptors negatively coupled to adenylate cyclase. J. Med. Chem. 1996;39(15):2990–3000. doi: 10.1021/jm9601765. [DOI] [PubMed] [Google Scholar]
- 104.Cartmell J., Adam G., Chaboz S., Henningsen R., Kemp J.A., Klingelschmidt A., Metzler V., Monsma F., Schaffhauser H., Wichmann J., Mutel V. Characterization of [3H]-(2S,2‘R,3’R)-2-(2′,3′-dicarboxy-cyclopropyl)glycine ([3H]-DCG IV) binding to metabotropic mGlu2 receptor-transfected cell membranes. Br. J. Pharmacol. 1998;123(3):497–504. doi: 10.1038/sj.bjp.0701647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Attwell P.J., Singh Kent N., Jane D.E., Croucher M.J., Bradford H.F. Anticonvulsant and glutamate releaseinhibiting properties of the highly potent metabotropic glutamate receptor agonist (2S,29R,39R)-2-(29,39-dicarboxycyclopropyl)glycine (DCG-IV). Brain Res. 1998;805(1-2):138–143. doi: 10.1016/S0006-8993(98)00698-2. [DOI] [PubMed] [Google Scholar]
- 106.Monn J.A., Valli M.J., Massey S.M., Wright R.A., Salhoff C.R., Johnson B.G., Howe T., Alt C.A., Rhodes G.A., Robey R.L., Griffey K.R., Tizzano J.P., Kallman M.J., Helton D.R., Schoepp D.D. Design, synthesis, and pharmacological characterization of (+)-2-aminobicyclo[3.1.0]hexane-2,6-dicarboxylic acid (LY354740): a potent, selective, and orally active group 2 metabotropic glutamate receptor agonist possessing anticonvulsant and anxiolytic properties. J. Med. Chem. 1997;40(4):528–537. doi: 10.1021/jm9606756. [DOI] [PubMed] [Google Scholar]
- 107.Monn J.A., Valli M.J., Massey S.M., Hansen M.M., Kress T.J., Wepsiec J.P., Harkness A.R., Grutsch J.L., Jr, Wright R.A., Jr, Johnson B.G., Andis S.L., Kingston A., Tomlinson R., Lewis R., Griffey K.R., Tizzano J.P., Schoepp D.D. Synthesis, pharmacological characterization, and molecular modeling of heterobicyclic amino acids related to (+)-2-aminobicyclo[3.1.0] hexane-2,6-dicarboxylic acid (LY354740): identification of two new potent, selective, and systemically active agonists for group II metabotropic glutamate receptors. J. Med. Chem. 1999;42(6):1027–1040. doi: 10.1021/jm980616n. [DOI] [PubMed] [Google Scholar]
- 108.Moghaddam B., Adams B.W. Reversal of phencyclidine effects by a group II metabotropic glutamate receptor agonist in rats. Science. 1998;281(5381):1349–1352. doi: 10.1126/science.281.5381.1349. [DOI] [PubMed] [Google Scholar]
- 109.Flor P.J., Battaglia G., Nicoletti F., Gasparini F., Bruno V. Neuroprotective activity of metabotropic glutamate receptor ligands. Adv. Exp. Med. Biol. 2012;513:197–223. doi: 10.1007/978-1-4615-0123-7_7. [DOI] [PubMed] [Google Scholar]
- 110.Kingston A.E., Ornstein P.L., Wright R.A., Johnson B.G., Mayne N.G., Burnett J.P., Belagaje R., Wu S., Schoepp D.D. LY341495 is a nanomolar potent and selective antagonist of group II metabotropic glutamate receptors. Neuropharmacology. 1998;37(1):1–12. doi: 10.1016/S0028-3908(97)00191-3. [DOI] [PubMed] [Google Scholar]
- 111.Hanna L., Ceolin L., Lucas S., Monn J., Johnson B., Collingridge G., Bortolotto Z., Lodge D. Differentiating the roles of mGlu2 and mGlu3 receptors using LY541850, an mGlu2 agonist/mGlu3 antagonist. Neuropharmacology. 2013;66:114–121. doi: 10.1016/j.neuropharm.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 112.Jin X., Semenova S., Yang L., Ardecky R., Sheffler D.J., Dahl R., Conn P.J., Cosford N.D., Markou A. The mGluR2 positive allosteric modulator BINA decreases cocaine self-administration and cue-induced cocaine-seeking and counteracts cocaine-induced enhancement of brain reward function in rats. Neuropsychopharmacology. 2010;35(10):2021–2036. doi: 10.1038/npp.2010.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Molinaro G., Traficante A., Riozzi B., Di Menna L., Curto M., Pallottino S., Nicoletti F., Bruno V., Battaglia G. Activation of mGlu2/3 metabotropic glutamate receptors negatively regulates the stimulation of inositol phospholipid hydrolysis mediated by 5-hydroxytryptamine2A serotonin receptors in the frontal cortex of living mice. Mol. Pharmacol. 2009;76(2):379–387. doi: 10.1124/mol.109.056580. [DOI] [PubMed] [Google Scholar]
- 114.Benneyworth M.A., Xiang Z., Smith R.L., Garcia E.E., Conn P.J., Sanders-Bush E. A selective positive allosteric modulator of metabotropic glutamate receptor subtype 2 blocks a hallucinogenic drug model of psychosis. Mol. Pharmacol. 2007;72(2):477–484. doi: 10.1124/mol.107.035170. [DOI] [PubMed] [Google Scholar]
- 115.Caraci F., Molinaro G., Battaglia G., Giuffrida M.L., Riozzi B., Traficante A., Bruno V., Cannella M., Merlo S., Wang X., Heinz B.A., Nisenbaum E.S., Britton T.C., Drago F., Sortino M.A., Copani A., Nicoletti F. Targeting group II metabotropic glutamate (mGlu) receptors for the treatment of psychosis associated with Alzheimer’s disease: selective activation of mGlu2 receptors amplifies beta-amyloid toxicity in cultured neurons, whereas dual activation of mGlu2 and mGlu3 receptors is neuroprotective. Mol. Pharmacol. 2011;79(3):618–626. doi: 10.1124/mol.110.067488. [DOI] [PubMed] [Google Scholar]
- 116.Wenthur C.J., Morrison R., Felts A.S., Smith K.A., Engers J.L., Byers F.W., Daniels J.S., Emmitte K.A., Conn P.J., Lindsley C.W. Discovery of (R)-(2-fluoro-4-((-4-methoxyphenyl)ethynyl)phenyl) (3-hydroxypiperidin-1-yl)methanone (ML337), an mGlu3 selective and CNS penetrant negative allosteric modulator (NAM). J. Med. Chem. 2013;56(12):5208–5212. doi: 10.1021/jm400439t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Musante V., Longordo F., Neri E., Pedrazzi M., Kalfas F., Severi P., Raiteri M., Pittaluga A. RANTES modulates the release of glutamate in human neocortex. J. Neurosci. 2008;28(47):12231–12240. doi: 10.1523/JNEUROSCI.3212-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Di Prisco S., Olivero G., Merega E., Bonfiglio T., Marchi M., Pittaluga A. CXCR4 and NMDA receptors are functionally coupled in rat hippocampal noradrenergic and glutamatergic nerve endings. J. Neuroimmune Pharmacol. 2016;11(4):645–656. doi: 10.1007/s11481-016-9677-6. [DOI] [PubMed] [Google Scholar]
- 119.Di Prisco S., Summa M., Chellakudam V., Rossi P.I., Pittaluga A. RANTES-mediated control of excitatory amino acid release in mouse spinal cord. J. Neurochem. 2012;121(3):428–437. doi: 10.1111/j.1471-4159.2012.07720.x. [DOI] [PubMed] [Google Scholar]
- 120.Ayoub M.A., Crépieux P., Koglin M., Parmentier M., Pin J.P., Poupon A., Reiter E., Smit M., Steyaert J., Watier H., Wilkinson T. Antibodies targeting G protein-coupled receptors: Recent advances and therapeutic challenges. MAbs. 2017;9(5):735–741. doi: 10.1080/19420862.2017.1325052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Olivero G., Vergassola M., Cisani F., Usai C., Pittaluga A. Immuno-pharmacological characterization of presynaptic glun3a-containing nmda autoreceptors: relevance to anti-nmda receptor autoimmune diseases. Mol. Neurobiol. 2019;56(9):6142–6155. doi: 10.1007/s12035-019-1511-8. [DOI] [PubMed] [Google Scholar]
- 122.Merega E., Prisco S.D., Severi P., Kalfas F., Pittaluga A. Antibody/receptor protein immunocomplex in human and mouse cortical nerve endings amplifies complement-induced glutamate release. Neurosci. Lett. 2015;600:50–55. doi: 10.1016/j.neulet.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 123.Neale J.H. N-acetylaspartylglutamate is an agonist at mGluR3 in vivo and in vitro. J. Neurochem. 2011;119(5):891–895. doi: 10.1111/j.1471-4159.2011.07380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wroblewska B., Santi M.R., Neale J.H. N-acetylaspartylglutamate activates cyclic AMP-coupled metabotropic glutamate receptors in cerebellar astrocytes. Glia. 1998;24(2):172–179. doi: 10.1002/(SICI)1098-1136(199810)24:2<172:AID-GLIA2>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 125.Yin S., Noetzel M.J., Johnson K.A., Zamorano R., Jalan-Sakrikar N., Gregory K.J., Conn P.J., Niswender C.M. Selective actions of novel allosteric modulators reveal functional heteromers of metabotropic glutamate receptors in the CNS. J. Neurosci. 2014;34(1):79–94. doi: 10.1523/JNEUROSCI.1129-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Marek G.J., Wright R.A., Schoepp D.D., Monn J.A., Aghajanian G.K. Physiological antagonism between 5-hydroxytryptamine(2A) and group II metabotropic glutamate receptors in prefrontal cortex. J. Pharmacol. Exp. Ther. 2000;292(1):76–87. [PubMed] [Google Scholar]
- 127.Zhang C., Marek G.J. Group III metabotropic glutamate receptor agonists selectively suppress excitatory synaptic currents in the rat prefrontal cortex induced by 5-hydroxytryptamine2A receptor activation. J. Pharmacol. Exp. Ther. 2007;320(1):437–447. doi: 10.1124/jpet.106.107490. [DOI] [PubMed] [Google Scholar]
- 128.Kammermeier P.J. Functional and pharmacological characteristics of metabotropic glutamate receptors 2/4 heterodimers. Mol. Pharmacol. 2012;82(3):438–447. doi: 10.1124/mol.112.078501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wischhof L., Koch M. 5-HT2A and mGlu2/3 receptor interactions: on their relevance to cognitive function and psychosis. Behav. Pharmacol. 2016;27(1):1–11. doi: 10.1097/FBP.0000000000000183. [DOI] [PubMed] [Google Scholar]
- 130.Aghajanian G.K., Marek G.J. Serotonin, via 5-HT2A receptors, increases EPSCs in layer V pyramidal cells of prefrontal cortex by an asynchronous mode of glutamate release. Brain Res. 1999;825(1-2):161–171. doi: 10.1016/S0006-8993(99)01224-X. [DOI] [PubMed] [Google Scholar]
- 131.González-Maeso J., Ang R.L., Yuen T., Chan P., Weisstaub N.V., López-Giménez J.F., Zhou M., Okawa Y., Callado L.F., Milligan G., Gingrich J.A., Filizola M., Meana J.J., Sealfon S.C. Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature. 2008;452(7183):93–97. doi: 10.1038/nature06612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Delille H.K., Mezler M., Marek G.J. The two faces of the pharmacological interaction of mGlu2 and 5-HT2A - relevance of receptor heterocomplexes and interaction through functional brain pathways. Neuropharmacology. 2013;70:296–305. doi: 10.1016/j.neuropharm.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 133.Chaki S. Group II metabotropic glutamate receptor agonists as a potential drug for schizophrenia. Eur. J. Pharmacol. 2010;639(1-3):59–66. doi: 10.1016/j.ejphar.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 134.Millán C., Luján R., Shigemoto R., Sánchez-Prieto J. 2002.
- 135.Millán C., Luján R., Shigemoto R., Sánchez-Prieto J. Subtype-specific expression of group III metabotropic glutamate receptors and Ca2+ channels in single nerve terminals. J. Biol. Chem. 2002;277(49):47796–47803. doi: 10.1074/jbc.M207531200. [DOI] [PubMed] [Google Scholar]
- 136.Martín R., Durroux T., Ciruela F., Torres M., Pin J.P., Sánchez-Prieto J. The metabotropic glutamate receptor mGlu7 activates phospholipase C, translocates munc-13-1 protein, and potentiates glutamate release at cerebrocortical nerve terminals. J. Biol. Chem. 2010;285(23):17907–17917. doi: 10.1074/jbc.M109.080838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Olivero G., Cisani F., Vergassola M., Pittaluga A. Prolonged activation of CXCR4 hampers the release-regulating activity of presynaptic NMDA receptors in rat hippocampal synaptosomes. Neurochem. Int. 2019;126:59–63. doi: 10.1016/j.neuint.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 138.Wang C.C., Kuo J.R., Huang S.K., Wang S.J. Metabotropic glutamate 7 receptor agonist AMN082 inhibits glutamate release in rat cerebral cortex nerve terminal. Eur. J. Pharmacol. 2018;823:11–18. doi: 10.1016/j.ejphar.2018.01.038. [DOI] [PubMed] [Google Scholar]
- 139.Mitsukawa K., Yamamoto R., Ofner S., Nozulak J., Pescott O., Lukic S., Stoehr N., Mombereau C., Kuhn R., McAllister K.H., van der Putten H., Cryan J.F., Flor P.J. A selective metabotropic glutamate receptor 7 agonist: activation of receptor signaling via an allosteric site modulates stress parameters in vivo. Proc. Natl. Acad. Sci. USA. 2005;102(51):18712–18717. doi: 10.1073/pnas.0508063102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Suzuki G., Tsukamoto N., Fushiki H., Kawagishi A., Nakamura M., Kurihara H., Mitsuya M., Ohkubo M., Ohta H. In vitro pharmacological characterization of novel isoxazolopyridone derivatives as allosteric metabotropic glutamate receptor 7 antagonists. J. Pharmacol. Exp. Ther. 2007;323(1):147–156. doi: 10.1124/jpet.107.124701. [DOI] [PubMed] [Google Scholar]
- 141.Summa M., Di Prisco S., Grilli M., Usai C., Marchi M., Pittaluga A. Presynaptic mGlu7 receptors control GABA release in mouse hippocampus. Neuropharmacology. 2013;66:215–224. doi: 10.1016/j.neuropharm.2012.04.020. [DOI] [PubMed] [Google Scholar]
- 142.Somogyi P., Dalezios Y., Luján R., Roberts J.D., Watanabe M., Shigemoto R. High level of mGluR7 in the presynaptic active zones of select populations of GABAergic terminals innervating interneurons in the rat hippocampus. Eur. J. Neurosci. 2003;17(12):2503–2520. doi: 10.1046/j.1460-9568.2003.02697.x. [DOI] [PubMed] [Google Scholar]