Abstract
In stroke (cerebral ischemia), despite continuous efforts both at the experimental and clinical level, the only approved pharmacological treatment has been restricted to tissue plasminogen activator (tPA). Stroke is the leading cause of functional disability and mortality throughout worldwide. Its pathophysiology starts with energy pump failure, followed by complex signaling cascade that ultimately ends in neuronal cell death. Ischemic cascade involves excessive glutamate release followed by raised intracellular sodium and calcium influx along with free radicals’ generation, activation of inflammatory cytokines, NO synthases, lipases, endonucleases and other apoptotic pathways leading to cell edema and death. At the pre-clinical stage, several agents have been tried and proven as an effective neuroprotectant in animal models of ischemia. However, these agents failed to show convincing results in terms of efficacy and safety when the trials were conducted in humans following stroke. This article highlights the various agents which have been tried in the past but failed to translate into stroke therapy along with key points that are responsible for the lagging of experimental success to translational failure in stroke treatment.
Keywords: Stroke, cerebral ischemia, neuroprotection, pathophysiology, pharmacotherapy, pre-clinical studies, clinical trials, STAIR criteria
1. INTRODUCTION
Stroke or cerebral ischemia or cerebrovascular accident (CVA), a devastating neurological emergency, is a paramount cause of mortality and neurological impairment in developed countries [1-3]. It ranks fifth for causing functional disability in the United States [4]. In stroke, there is a reduced supply of blood to the brain that occurs either cause of occlusion of blood vessels supplying blood to the brain (ischemic stroke; 87%) or cause of hemorrhagic bleed (hemorrhagic stroke; 13%) [4]. An abrupt decrease in oxygen, as well as glucose supply to the brain, triggers an ischemic cascade that results in the spread of death of neuronal cells within minutes of stroke onset [5]. Restoring blood flow to the ischemic area, reperfusion is the only way that helps in limiting neuronal injury post ischemia [2]. Currently, tissue plasminogen activator (tPA), is the only US-FDA approved thrombolytic therapy for the management of acute ischemic stroke (AIS) [6]. However, reperfusion therapy itself is associated with further damage to the neurons and brain microcirculation by activating inflammatory response, thus causing tissue injury [2].
The involvement of complex pathophysiology in cerebral ischemia is the key culprit; responsible for failure in translating preclinical agents into successful clinical therapy in stroke management [7]. It is the need of the hour to explore agents which are able to interrupt pathways at multiple steps or to explore the combinations that can act in a sequential manner so as to halt ischemic damage especially in penumbra (region surrounding core infarct), called ‘neuroprotection’. Many agents have been tested at the experimental level in various animal models of stroke, where they reduced infarct size and improved neurological outcome in these animal models and thus found to be neuroprotective in stroke. However, among these, not even a single agent has successfully developed as a therapy in the management of stroke when tried clinically. The purpose of this review is (i) to provide an outline of stroke or cerebral ischemia, its pathophysiology and understanding of the underlying mechanisms at the cellular and molecular level; (ii) to provide a brief idea in relation to the various experimental agents that have been investigated for neuroprotective effects in stroke; (iii) key points for the translational failure of these neuroprotective agents to clinical therapy in stroke along with STAIR criteria; and (iv) the current status of various agents in clinical trials of stroke.
2. PATHOPHYSIOLOGY OF STROKE
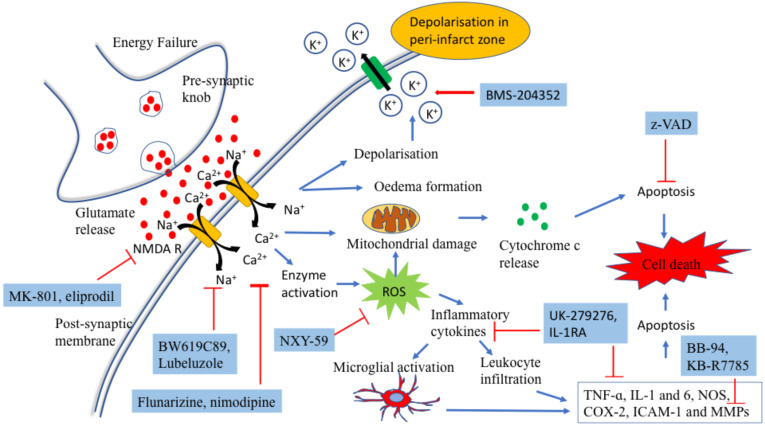
In stroke, degree and duration of reduced blood supply are two important factors that account for the extent of neuronal injury. The ischemic area is composed of two zones involving the core and infarct border zone (IBZ). Core is the area which is critically damaged by reduced cerebral blood flow (CBF). During cerebral ischemia, cell death occurs because of activation of complex downstream signaling cascade starting from energy failure to the imbalance in ionic homeostasis. Under anaerobic environment, the cell fails to generate enough adenosine triphosphate (ATP), which is required to maintain cellular integrity. Under hypoxic conditions, there is a plethora of glutamate release into the extracellular space; a key regulator of inducing ischemic cascade [8]. Immediately following its release, it causes activation of AMPA, NMDA and kainate receptors present on neuronal cell membrane, which in turn causes an increase in calcium (Ca2+) and sodium (Na+) influx, which subsequently activated a series of adverse events including the activation of NO synthases, lipases, endonucleases, generation of reactive oxygen (ROS) nitrogen species (RNS), lipid peroxidation, mitochondrial dysfunction, activation of NF-kB (upregulating the expression of TNF-ɑ, IL-1 and 6, NOS, COX-2, ICAM-1 and MMPs), recruitment of proinflammatory cytokines (IL-1, IL-6, IL-10, TNF-ɑ, TGF-1β and HMG-1) and edema formation; the key contributors in neuronal death via necrosis and activating apoptotic phenomenon [2, 8-11] (Fig. 1). Also the increased intracellular calcium concentrations are responsible for activating calpains, with proteases responsible for inhibiting protein synthesis and leading to neuronal death [10, 12]. In contrast, there is another area that surrounds the core infarct and havs moderate CBF because it tends to have perfusion from surrounding collateral vascular structures, making it functionally less active but metabolically active. This region is called penumbra or infarct border zone (IBZ) [2, 9, 13, 14]. Genes in this region activate gradually and take hours to days to undergo the process of apoptosis in the absence of adequate reperfusion [2]. Thus, it provides potential space that can be salvaged and targeted for halting ischemic cascade during post-ischemic phase neuronal death, called ‘ neuroprotection’ [2, 9].
Fig. (1).
Pathophysiology of stroke. (Blue arrows depict downstream signalling cascade following stroke; red arrows depict inhibition in the signalling pathway). (A higher resolution / colour version of this figure is available in the electronic copy of the article).
3. EPIGENETIC MODIFICATIONS AND their ROLE IN CEREBRAL ISCHEMIA
Epigenetic changes like DNA modification, histone deacetylases (HDACs) and microRNAs (miRNAs) are important modulators for regulating gene function and play a vital role in brain vascular remodeling and synaptic plasticity following stroke [15, 16].
DNA methylation: DNA methylation is responsible for brain tissue damage via suppressing the expression of neuroprotective genes. DNA methylation is catalyzed by DNA methyltransferase enzymes (DNMTs), which causes an increase in CpG island methylation. Following ischemia, DNMTs levels get enhanced acutely responsible for secondary brain injury by causing neuroprotective gene repression [15]. Treatment with 5-aza-2′-deoxycytidine (DNMT inhibitor) improved functional outcomes in rodents following stroke revealing the role of DNA methylation in stroke [17].
Histone deacetylases (HDACs): Stroke induces a marked decrease in histone acetylation levels (H3 and H4) that starts within hours of stroke induction and remains in that state for a period of weeks. Decreased histone acetylation occurs due to an imbalance in the combined activity of HDACs and HATs [16]. Following stroke, HDACs levels get significantly increased in IBZ, which are treated as important mediators to induce neuronal tissue damage. This enhanced HDACs expression is considered to increase oxidative stress in the brain tissue and can be targeted to induce tissue repair in cerebral ischemia via its suppression. Ischemic injury causes a reduction in phosphorylation of HDAC2 at Ser 394, inhibiting HDAC2-FOXO3a interaction responsible for oxidative stress-induced tissue death [18]. HDAC inhibitors such as VPA and sodium butyrate were found to be neuroprotective in stroke [19, 20].
MicroRNAs (miRNAs): These are the non-coding RNAs (usually 20-25 nucleotides) responsible for the inhibition of translating into proteins. miRNAs are widely expressed in the central nervous system and play an important role in brain vascular repair processes such as angiogenesis and neuronal outgrowth. The miRNA profiling of rat brain has identified a number of miRNAs (miR-139, miR-335, miR-15a, miR-155 and miR-107 in angiogenesis, miR-124 and miR-17-92 cluster in neurogenesis and miR-9; miR-200b and miR-146a in oligodendrogenesis), which play a vital role in regulating neuronal vascular repair following cerebral ischemia [15, 16].
4. CURRENT TREATMENT AND OTHER INVESTIGATIONAL THERAPIES IN STROKE
Till date, intravenous tissue plasminogen activator (tPA) (thrombolytic therapy, the only FDA approved pharmacological treatment (Table 1)) [21] or mechanical thrombectomy are the only treatment options available for recanalization of occluded blood vessels in the primary management of AIS, which is effective when given within restricted time period (3-4.5 hours). Owing to the narrow time window of its administration, only 5% or less percentage of stroke patients are eligible to undergo rt-PA therapy. Secondly, the therapy itself is responsible for increasing the risk of hemorrhagic bleed and reperfusion injury [5, 9, 22, 23]. Halting downstream ischemic cascade at the level of various biochemical, metabolic and molecular in the infarct border zone (IBZ) using a pharmacological or non-pharmacological neuroprotective agent may be proven as another therapeutic approach in salvaging the neurons following stroke [9, 24]. Following are the various strategies that have been tried in the past experimental studies as neuroprotective agents in the treatment of acute ischemic stroke (Tables 2 and 3).
Table 1.
Thrombolytic agents for treatment of stroke.
| 1. First generation thrombolytics 1. Streptokinase 2. Urokinase 2. Second generation thrombolytics 1. Alteplase 2. Pro-urokinase 3. Third generation thrombolytics 1. Reteplase 2. Anoteplase 3. Tenecteplase |
Table 2.
Neuroprotective strategies in stroke.
| 1. Glutamatergic activity inhibitors 1. Inhibition of presynaptic glutamate release 2. Postsynaptic inhibition of glutamate receptors 2. Ion channel modulators 1. Calcium-channel blockers 2. γ-Aminobutyric acid (GABA) modulators 3. Potassium channel activator 3. Antioxidants: Free radical scavengers 4. Anti-apoptotic agents 5. Anti-inflammatory agents 6. Other investigational therapies in cerebral ischemia 1. GPIIb/IIIa platelet inhibitor 2. Serotonin Agonists 3. Opioid receptor modulators 4. Matrix metalloproteinases (MMPs) inhibitors 5. Citicoline 6. Lubeluzole 7. Gangliosides 8. Growth factors 9. Albumin 10. Metal ion chelator 11. Ancrod 12. CEPO |
Table 3.
Brief summary of important agents that have been investigated for treatment of stroke and their outcome.
| Drugs Investigated | Outcome | |||
|---|---|---|---|---|
| Pre Clinical | Clinical | |||
| Glutamatergic activity inhibitors A. Sodium channel blocker a. BW619C89 b. Lubeluzole B. NMDA receptor antagonist a. Dizocilpine (MK-801) b. Cerestat c. Eliprodil d. Magnesium sulfate C. AMPA receptor antagonist a. YM-872 |
Effective [132, 133] Effective [134] Effective [135] Effective [36] Effective [38] Effective [136] Effective [39, 40] |
Side effects [26] Ineffective [28, 119] Ineffective [137] Ineffective [37] Effective [43] Unknown [41] |
||
| Ion channel modulators A. Calcium-channel blockers a. Nimodipine b. PY 108- 068 c. Flunarizine B. GABA modulators a. Clomethiazole C. Potassium channel activators a. BMS-204352 |
Effective [138] Effective [139] Effective [140] Effective [141] Effective [61] |
Ineffective [52] Ineffective [53] Ineffective [54] Ineffective [59] Ineffective [62] |
||
| Free radical scavengers a. NXY-059 b. Edaravone c. Ebselen d. Tirilazad |
Effective [64, 65] Effective [66, 67] Effective [69] Effective [71] |
Ineffective [142] Effective [72, 143] Effective [73] Worsens the outcome [74, 75] |
||
| Anti-inflammatory agents A. Anti-ICAM-1 a. Enlimomab B. Anti-CD11b/CD18 a. UK-279276 C. IL-1RA D. MAPK inhibitor a. SB 239063 |
Effective [144] Effective [85] Effective [87] Effective [89, 90] |
Ineffective [84] Ineffective [86] Safe but worsens the outcome [88] No data in stroke patients |
||
| GPIIb/IIIa platelet inhibitor a. Abciximab b. SM-20302 |
Effective [91, 92] Effective [93, 94] |
Withdrawn because of safety concern [96] No data |
||
| Serotonin agonist a. BAYX3702 |
Effective [100] | Not known [102] | ||
| Opioid receptor modulators a. Nalmefene b. Naloxone c. BRL52537 d. U-50,488E e. U-50,488H |
Effective [145] Effective [146] Effective [103] Effective [104] Effective [105] |
Safe but not effective [107] Ineffective [106] No data No data No data |
||
| Drugs Investigated | Outcome | |||
| Pre Clinical | Clinical | |||
| Matrix metalloproteinases inhibitors a. BB-94 b. KB-R7785 |
Effective [110] Effective [111] |
No data No data |
||
| Citicoline | Effective [114] | Ineffective [118] | ||
| Albumin | Effective [126] | Ineffective [127] | ||
| Ancrod | Effective [147] | Ineffective [129] | ||
4.1. Glutamergic Activity Inhibitors
Glutamate, an excitatory amino acid, plays an important role in neuronal damage following ischemia. Inhibiting the activity was thought to be beneficial in preventing ischemia related neuronal damage and associated functional disability. Inhibition of glutamatergic associated excitatory activity can be achieved at different levels of glutamate release and its action [25].
4.1.1. Inhibition of Presynaptic Glutamate Release
Sodium channel blockers: These agents prevent the release of glutamate into synaptic space. Therefore, they may be beneficial in preventing neuronal damage post-ischemia.
A clinical trial using pyrimidine derivative BW619C89 was withdrawn because of the safety concerns associated with the trial drug [26]. In the US study conducted by Grotta et al. [27] involving lubeluzole (Na+ channel inhibitor), no significant differences were obtained in mortality between the treatment and control group. However, significant improvement was observed in neurological outcome and disability between both the groups, which was contrary to the European study where the use of lubeluzole following ischemia showed no significant improvement in neurological impairment between the treatment and control groups [28]. Similarly, other clinical trials with sodium channel blockers involving the use of Lub-Int-13, fosphenytoin and lifaricine, were found to be non-beneficial following ischemia and thus their studies and further clinical development were terminated [29, 30].
4.1.2. Postsynaptic Inhibition of Glutamate Receptor
N-methyl-D-aspartate (NMDA) and alpha-amino-3-hydroxy-5-methyl-4- isoxazole propionic acid (AMPA) are two subtypes of postsynaptic glutamate receptors that are targeted for preventing post-ischemia neuronal cell death. At present, instead, more focus has been given towards finding an agent that can target not only single receptor but the NMDA complex having a receptor-regulated calcium channel with an affinity for modulators, which provide loci of glycine as well as polyamine [31]. Various noncompetitive NMDA receptor antagonists (dextromethorphan [32], dizocilpine (MK-801) [33, 34] and cerestat [35, 36]), a competitive NMDA antagonist (eliprodil (an ‘atypical’ antagonist) [37, 38]) and an AMPA antagonist (zonampanel (YM-872) [39-41]) have been tested and showed neuroprotection in experimental models of stroke. However, none of these agents were able to reach the clinical development stage as trials were terminated on the basis of ineffectiveness and increased adverse effect to benefit ratio in stroke [42]. A clinical trial with magnesium sulphate, anNMDA antagonist in acute stroke, has been found to decrease mortality with an improved neurological impairment in the treatment group as compared to that of placebo-controlled group [43].
4.2. Ion Channel Modulators
4.2.1. Calcium Channel Blockers
Ischemic cascade involves elevated calcium influx via the activation of glutamate receptor (NMDA or AMPA), voltage-dependent calcium channels (VDCC) and inhibition of Ca2+-ATPases activity, which is responsible for mediating neuronal damage following ischemia. Inhibiting this increased intracellular calcium influx may help in preventing neuronal death and reducing stroke associated neurological impairment and disability. Different calcium channel inhibitors including nicardipine, amlodipine, nimodipine or flunarizine, have been tested experimentally to block the activity of these receptors or channels and are found to be neuroprotective in stroke [44-48]. Leyden et al. in 1988 have conducted an experimental study of focal ischemia in rabbits using cerebro-selective calcium channel antagonists (lidoflazine, nimodipine, or nicardipine), where they observed poor neurological functional outcome and thus concluded that calcium channel antagonists are of no benefit in stroke [49]. When the clinical trials using calcium channel blockers were conducted, none of the agents were found to be significantly beneficial in cerebral ischemia [50, 51]. Clinical trials involving the use of nimodipine, nicardipine and darodipine (PY 108- 068) were terminated, as the results of these studies showeed no efficacy in ischemic stroke [50, 52-54]. Also it has been seen that the use of flunarizine was associated with worsening of stroke outcome [54].
4.2.2. γ-Aminobutyric Acid (GABA) Modulators
GABA, an inhibitory neurotransmitter, seems to have an important role in stroke recovery as it antagonizes glutamatergic excitation in neurons following ischemia [55-57]. Based on this concept, many scientists have demonstrated that enhancing GABAergic transmission in neurons during post-ischemia in rodents provides neuroprotection [57, 58]. Thus, various clinical trials to find GABAergic modulators as a potential therapy for stroke treatment come into play. The multicenter clinical trials involving the use of clomethiazole or diazepam have been found to be ineffective as results have shown no significant improvement in neurological outcome in the treatment group when compared to placebo-controlled group [59, 60].
4.2.3. Potassium Channel Activator
Activation of potassium channels is responsible for causing neuronal hyperpolarization and thereby reduces excitatory activity in neurons. BMS-204352; potassium channel activator had shown neuroprotection in animal models of ischemia by reducing the infarct size. However, the agent failed to show its efficacy when the trial was conducted in humans following stroke [61, 62].
4.3. Antioxidants: Free Radical Scavengers
Following cerebral ischemia, tissue damage mainly occurs because of the production of free radicals, including hydroxyl, hydrogen peroxide or superoxide. Thus, the agents which can either block its production or inhibit its activation might be proven beneficial in stroke. Improved neurological outcome was obtained in experimental studies of stroke when the animals were treated with free radical scavengers including NXY-59 [63-65], ɑ-phenyl-N-tert-butyl-nitrone, edaravone [66-68], ebselen [69, 70] and tirilazad [71]. Trials of edaravone have provided evidence for its use in combination with endovascular therapy in stroke [72]. In a randomized placebo-controlled double-blinded clinical trial, ebselen was given in a dose of 150 mg b.i.d orally as ebselen granules suspended in water and found to improve outcome in stroke patients at the end of 1 month [73]. A clinical trial using tirilazad was associated with worsening of outcome and resulted in increased death and disability in one-fifth of stroke patients [74, 75].
4.4. Anti-apoptotic Agents
Inhibiting the caspases and proapoptotic genes expression or enhancing anti-apoptotic genes expression, provides a potential target for treatment in ischemic stroke [76]. z-VAD (in two forms fluoro-methyl-ketone (fmk) or dichloro-benzoyl-oxo-pentanoic acid (dcb)), z-DVED-fmk and Ac-YVAD-cmk are important inhibitors of caspases that have been demonstrated to improve the neurological outcome in animals following stroke and thus provide neuroprotection [77-80].
4.5. Anti-inflammatory Agents
Selectins, integrins and ICAMs, play an important role in mediating inflammatory response in ischemia. Studies have revealed that agents like anti-selectin and anti-ICAM-1 are found to be effective by decreasing infarct size in an experimental model of stroke. In one of the clinical trials involving enlimomab, murine anti-ICAM-1 antibody has proven to worsen neurological outcomes in stroke patients along with increased mortality [81-84]. However, another study involving UK-279276, anti-CD11b/CD18 agent has shown to improve neurological deficit in the experimental model of stroke but failed to show efficacy when tried in stroke patients [85, 86]. Interleukin-1 (IL-1) receptor antagonist (IL-1RA) was found to be effective when studied in animal models of stroke [87] but failed to show efficacy when the trial was conducted in patients after acute stroke [88]. SB 239063, p38 mitogen-activated protein kinase inhibitor (MAPK), is another inflammatory agent, which has been found to be effective neuroprotective when tested in animals following cerebral ischemia [89, 90].
4.6. GPIIb/IIIa Platelet Inhibitor
Trials have been conducted using abciximab and SM-20302 either as combination therapy with alteplase or alone in stroke patients within 5-6 hours of symptom onset [91-94]. The Abciximab Emergent Stroke Treatment Trial (AbESTT) was done to evaluate the safety and efficacy of abciximab in patients following acute stroke. Phase II trials showed better outcomes at the end of 3 months in patients following stroke [95]. However, phase III trial was terminated prematurely because its use was associated with bleeding
complications and thrombocytopenia [96]. Despite such complications, these agents may be treated as potential targets as an adjuvant therapy in stroke.
4.7. Serotonin Agonists
These agents activate the serotonin receptor (5-HT1A) post-synaptically. The activation of these receptors causes neuronal hyperpolarization via inducing potassium efflux, thereby inhibiting excitatory activity in the neurons. BAYX3702 (Repinotan HCl); 5-HT1A receptor agonist tends to inhibit glutamate release following ischemia and has shown neuroprotection by reducing infarct size in an experimental model of focal cerebral ischemia [97-101]. Phase II randomized double-blinded study was done to evaluate the safety and efficacy of BAYX3702, which was infused at a rate of 1.25 mg/day in patients following acute stroke. But no results are known till date [102].
4.8. Opioid Receptor Modulators
κ-opioid receptor agonists [BRL52537 [103], U-50,488E [104] and U-50,488H [105]] have shown to provide neuroprotection when tested in animals following cerebral ischemia. Clinical trials using nalmefene, an μ-opiate receptor antagonist were done, which revealed that the drug is safe to be used in stroke patients, however, no improvement in clinical outcome has been observed in these patients following stroke [106-108]. In another trial, nalmefene was found to improve prognosis in patients following large cerebral infarctions caused by middle cerebral artery occlusion [109].
4.9. Matrix Metalloproteinases (MMPs) Inhibitors
BB-94 [110] and KB-R7785 [111] are two promising inhibitors of matrix metalloproteinases (MMPs), which have shown good results and improved neurological deficit score when tested in animals following temporary and permanent stroke [112].
4.10. Citocoline
The agent has improved neurological deficit score and reduced infarct size in animals following focal ischemia. A large number of experimental studies have been conducted to explore the efficacy of citicoline in ischemic stroke [113-115]. Its efficacy at the pre-clinical level was concluded in a meta-analysis by Bustamante et al. [116]. Also, a systematic review done by Secades et al. [117] concluded its efficacy in stroke patients. However, citicoline was not found to be effective in another trial (ICTUS trial) involving a larger population and was terminated [118].
4.11. Lubeluzole
It is a sodium and calcium channel blocker along with an additional inhibitory effect on glutamate release and nitric oxide synthesis [119]. It is a benzothiazole derivative and was shown to be neuroprotective in animal models of cerebral ischemic tissue injury [120, 121]. However, it was not found to have a promising neuroprotective effect when tried in patients with acute ischemic stroke [119].
4.12. Gangliosides
These are large-molecular-weight glycosphingolipids, mediating neuroprotection via increasing the expression of brain-derived neurotrophic factor (BDNF), blocking neuronal excitatory amino acid activity, normalizing altered phosphorylation of proteins and stabilizing the plasma membrane structure [122]. Based on preclinical evidence, clinical trial (Italian Acute Stroke Study) was conducted, enrolling 502 subjects where the agent failed to show its efficacy in patients following stroke [123].
4.13. Growth Factors
Neurotrophic factors are important for maintaining as well as survival of neurons. Haemopoietic growth factors (granulocyte-CSFs, erythropoietin, granulocyte-macrophage colony-stimulating factor, SCF, VEGF, stromal cell-derived factor-1α), BDNF, FGF-2 and IGF-1, PGRN, HB-EGF, HGF, EGF and GDNF have been tried in animal models of stroke, which successfully improved neurological outcome, reduced infarct size and decreased mortality [124, 125].
4.14. Albumin
In an experimental model of stroke, human albumin was found to improve neurological deficit and reduced infarct size in MCAO rats when given in doses of 1.25 g/kg i.v at 4hr post MCAO [126]. In ALIAS (albumin in acute ischemic stroke) trial, albumin was administered at 2g/kg intravenously to patients who were presented to hospital within 5 hours following stroke and evaluated efficacy outcome both as neurological and functional improvements at 90 days. The study revealed that albumin administration did not improve clinical outcome but caused an increase in intracerebral bleeding and pulmonary edema in these patients [127].
4.15. Metal Ion Chelator
In a small-scale multicenter placebo-controlled trial, DP-b99, a membrane-activated metal zinc and calcium ion chelator was evaluated in stroke patients. At 90 days, improved clinical outcome was observed in DP-b99 treated group with no differences in mortality rate and safety outcomes [128].
4.16. Ancrod
It is a serine protease obtained from pit viper venom that acts as a defibrogenating agent. Ancrod in Stroke Program (ASP)-I and -II trials were conducted where ancrod was administered via i/v infusion at 0.167 IU/kg per hour starting from 6 hours after the onset of stroke symptoms. The results were compared with the placebo group, which demonstrated that ancrod was not efficacious in improving the clinical outcome and also its use is associated with an increased risk of intracerebral bleeding in stroke patients [129].
4.17. CEPO
Carbamylated form of erythropoietin (EPO), which is a granulocyte colony-stimulating factor, has shown to improve neurological deficit in an animal model of ischemia [130]. As it does not compete with erythropoietin for EPO receptor, thereby it does not affect the hematocrit levels [130]. Currently, CEPO has completed with phase-1 clinical trials where the drug was assessed for its safety and pharmacokinetic profile in patients with AIS [131].
5. FAILURE FROM BENCH TO BEDSIDE TRANSLATION; REASONS BEHIND AND FUTURE DIRECTIONS
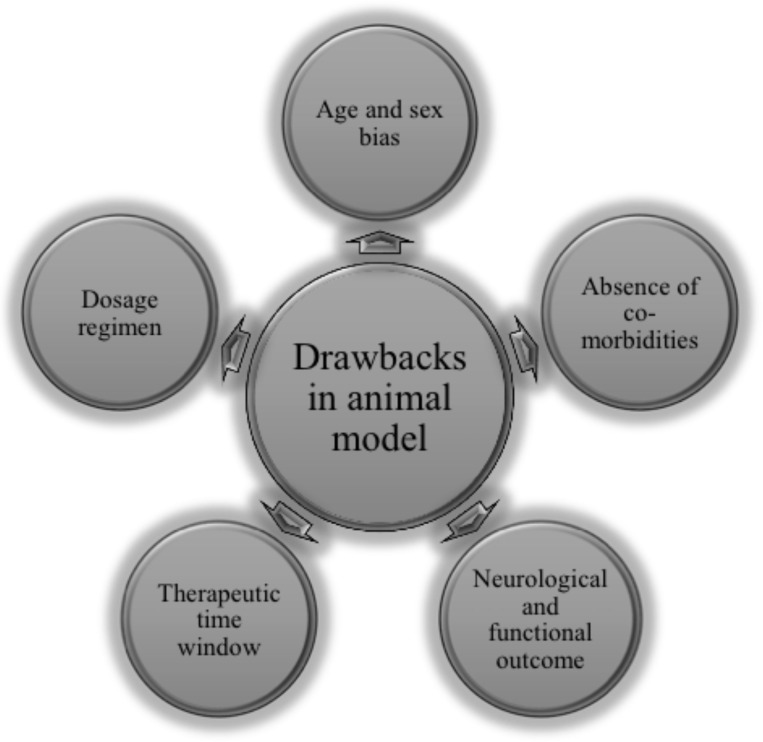
Despite numerous experimental studies that have been conducted successfully in animals for the treatment in stroke, none of the agents have been translated to clinical setting yet. Following are the key points for lagging behind the translation failure of neuroprotective strategies from animals to humans in stroke management (Fig. 2) [7, 148].
Fig. (2).
Translational failure: drawbacks in animal model of stroke. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Model selection and success criteria: Different models are being used to induce cerebral ischemia: in vivo models (middle cerebral artery occlusion, photo thrombosis, endothelin-1 and thromboembolic stroke model) and in vitro (glucose deprivation (GD) and Combined oxygen-glucose deprivation (OGD)). Are these models successful enough to mimic the exact clinical situation? Is infarct volume comparable in all these models? No criteria are there to evaluate the successful induction of stroke in these models. The success rate and standardization of these models vary from one laboratory to another.
Outcome measures and functional assessment: In all experimental studies of stroke, the success of the treatment arm is done based on infarct size and functional assessment like that of morris water maze (MWM) and novel object recognition (NOR). However, in a clinical setting, neurological functions are assessed by using ranking or scoring systems like the NIH Stroke Scale and Barthel Index.
Therapeutic time window: In most of the experimental studies, agents are administered either before induction of stroke or immediately following stroke. This is not in accordance with clinical trials and not mimicking the exact situation. As in a clinical scenario, a long duration is required to diagnose a patient clinically and obtain MRI scans. No evidence is available that suggests the effectiveness of these experimental agents when administered beyond 6 hours following stroke onset.
Drug-dosing regimens: In experimental studies, drugs are administered in low doses and for short duration of time, in order to avoid toxicity. However, in humans, drugs are administered in a highly variable manner that ranges from single intravenous injection/infusion to multiple oral dosing, which may extend to months duration.
Age and pre-morbid conditions: Another lag between experimental and clinical set up is the presence of pre-morbid conditions like arteriosclerosis, hypertension, diabetes mellitus, and hyperlipidemia. These factors are generally ignored, while conducting preclinical studies. In an experimental setup, researchers generally employ fresh and young animals, while stroke occurs mostly in the aged population that too presented with multiple diseases.
Sex bias: Female sex hormones are neuroprotective and to prevent the hormonal cycle interference in elucidating the neuroprotective active action of newer agents, preclinical studies on stroke are preferably conducted using male animals. There are studies which showed differences in responses obtained when a pathway is targeted among both sexes. The AIF-knockout Harlequin male rats have been found to be more tolerable against ischemic injury when compared to female rats. Similarly, the neuroprotective effect of dextromethorphan was more evident in male rats than in female rats.
5.1. Challenges in Conducting Clinical Trials in Stroke [149-151]
Incompatible preclinical models, poor planning and analysis of experimental data are usually not correlated using neuroimaging modalities.
Poor design, blinding and randomization protocols for conducting clinical trials.
Inadequate sample size: limitation of enrolling a considerable number of subjects in the clinical study with comparable characteristics, which represents preliminary assessment only.
No control arm: Some of the clinical trials are conducted without having any control arm.
Scoring system and outcome measures: For outcome measures in stroke studies, different scoring systems are adopted including NIHSS, mRS, and BI evaluation that do not correlate with infarct volume and brain tissue damage. Also, for adequate comparison, each arm must have similar scoring that can be easily compared without any bias.
Epidemiological factors and sex differences: A systematic review conducted in East Asian countries by Venketasubramanian et al revealed the lowest incidence rate in Malaysia (67/100,000 person-years), while the highest rates were observed in Japan (422/100,000 person-years among men and 212/100,000 person-years among women) and Taiwan (330/100,000 person-years) [152]. Western European epidemiological studies have revealed that males had 30% higher stroke incidence as compared to females. However, it tended to be more severe in females than in males [153].
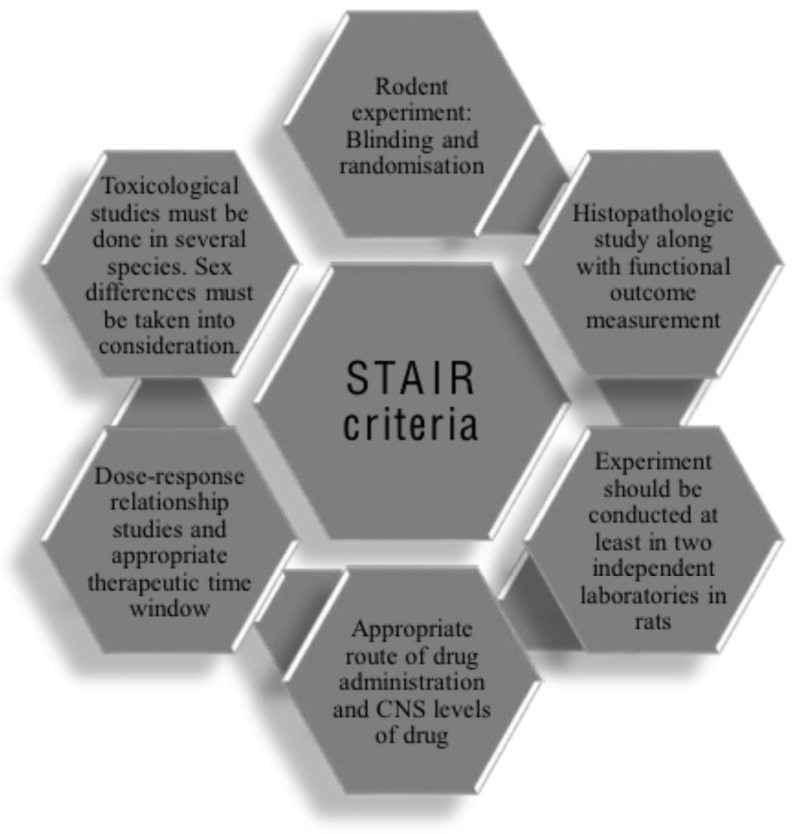
As a result of continuous negative results and failures in clinical trials, in the year 1999, a task force called Stroke
Therapy and Academic Industry Roundtable (STAIR) developed guidelines as STAIR criteria for facilitating preclinical studies into clinical translation in stroke (Fig. 3) [148, 154].
Fig. (3).
STAIR criteria for conducting pre-clinical studies in stroke. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
5.2. On-going Clinical Trials in Stroke
Despite past failures, continuous efforts are being made to find newer drugs in the treatment of stroke and many agents are there in the development phase of clinical trials in stroke (Table 4).
Table 4.
Brief summary of on-going clinical trials in stroke.
| Intervention | Condition | Allocation & Blinding | Phase of Trial | Outcome | |||||
|---|---|---|---|---|---|---|---|---|---|
| Butylphthalide (NBP) | Large-artery Atherosclerosis Acute ischemic stroke |
Randomized, triple blind | Phase 4 [155] | On-going | |||||
| Recombinant human urokinase (rhPro-UK) (PROST) | Acute ischemic stroke | Randomized, open label | Phase 3 [156] | On-going | |||||
| JPI-289 | Acute ischemic stroke | Randomized, quadruple blind | Phase 2 [157] | On-going | |||||
| Recombinant human tissue kallikrein (DM199) | Acute ischemic stroke | Randomized, double blind | Phase 2 [158] | On-going | |||||
| DLBS1033 | Acute ischemic stroke | Randomized, quadruple blind | Phase 2 and 3 [159] | On-going | |||||
| Fingolimod (FTY720) | Acute ischemic stroke | Non randomized, open label | Completed phase 2 [160] | Effective and well tolerated [161] | |||||
| Cilostazol (CAIST) | Cerebral Infarction | Randomized, double blind | Completed phase 4 [162] | Safe and effective [163] | |||||
| Atorvastatin | Acute ischemic stroke | Randomized, open label | Completed phase 4 [164] | Better functional outcome [165] | |||||
| Edaravone (EDO) | Acute ischemic stroke | Randomized, open label | Completed phase 4 [166, 167] |
Effective in acute noncardioembolic ischemic stroke [167] | |||||
| Intervention | Condition | Allocation & Blinding | Phase of Trial | Outcome | |||||
| Histamine- glutamate antagonist |
Acute Cerebrovascular Accident Cerebral Edema |
Randomized, open label | Completed phase 2 [168] | No data | |||||
| Uric Acid (URICO-ICTUS) | Acute ischemic stroke | Randomized, quadruple blind | Completed phase 2 and 3 [169-171] | Safe and improved stroke outcomes | |||||
| Bone Marrow-derived Stem Cells Therapy (InVeST) | Acute ischemic stroke | Randomized, open label | Completed phase 2 [172] | Safe but ineffective [173] | |||||
| Recombinant human erythropoietin alfa | Acute ischemic stroke | Randomized, quadruple blind | Completed phase 2 & 3 [174] |
Safety concern | |||||
| Ginsenoside-Rd | Acute ischemic stroke | Randomized, double blind | Completed phase 2 [175] | Effective | |||||
| HT047 | Acute ischemic stroke | Randomized, triple blind | Completed phase 2 [176] | No results provided | |||||
| Cerebrolysin | Acute ischemic stroke | Randomized, quadruple blind | Completed phase 4 [177] | Favorable outcome | |||||
| Mild hypothermia | Acute ischemic stroke | Single-arm, open-label | Completed phase 1 [178] | Safe | |||||
| 3K3A-APC (RHAPSODY) |
Acute ischemic stroke | Randomized, quadruple blind | Completed phase 2 [179] | Low intracranial hemorrhage rate | |||||
| Lu AA24493 (CEPO) | Acute ischemic stroke | Randomised, double blind | Completed phase 1 [180] | No results were provided | |||||
| Natalizumab (ACTION and ACTION2) |
Acute ischemic stroke | Randomized, quadruple blind | Completed phase 2 [181, 182] |
No improvement in functional outcome [183] | |||||
| Human plasmin | Acute ischemic stroke | Non randomised, open label | Completed phase 1 and 2 [184] | Safe and well tolerated | |||||
| Human Immune Globulin Intravenous (IVIg) | Acute ischemic stroke | Randomized, quadruple blind | Withdrawn from phase 1 [185] | Difficult recruitment and associated black box warning | |||||
CONCLUSION
In the past, many agents have been shown to be promising agents for providing neuroprotection in animal models of stroke. So far, none of these agents have been successfully translated into stroke therapy. Various important factors including animal age, sex, comorbid conditions, optimal dosing regimen, therapeutic time window, route/dosage form/time of drug administration and an appropriate neurological scoring system need to be elucidated for successful translation of pre-clinical studies into an effective clinical therapy in the treatment and management of stroke.
Ischemic cascade being a series of complex downstream signaling pathways, targeting a single pathway will not provide an effective way to manage stroke. Thus finding such an agent or a combination of agents (called “drug cocktail” in stroke) which act at multiple pathways may provide a promising future in the management of stroke.
Acknowledgements
Declared none.
list of ABBREVIATIONS
- AIS
Acute ischemic stroke
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolone proprionic acid
- ATP
Adenosine triphosphate
- BDNF
Brain–derived neurotrophic factor
- CBF
Cerebral blood flow
- COX
Cyclooxygenase
- CSFs
Colony-stimulating factors
- CVA
Cerebrovascular accident
- EGF
Epidermal growth factor
- FGF-2
Fibroblast growth factor-2
- GDNF
Glial cell line-derived neurotrophic factor
- HB-EGF
Heparin-binding epidermal growth factor-like growth factor
- HGF
Hepatocyte growth factor
- HMG-1
High mobility group protein-1
- IBZ
Infarct border zone
- IGF-1
Insulin-like Growth Factor-1
- IL-1
Interleukin-1
- IL-10
Interleukin-10
- IL-6
Interleukin-6
- MAPK
Mitogen-activated protein kinase inhibitor
- MMPs
Matrix metalloproteinases inhibitors
- NMDA
N-methyl-D-aspartate
- NOS
Nitric oxide synthase
- PGRN
Progranulin
- rt-TPA
Recombinant tissue plasminogen activator
- SCF
Stem cell factor
- TNFα
Tumor necrosis factor α
Consent for Publication
Not applicable.
Funding
None.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Musuka T.D., Wilton S.B., Traboulsi M., Hill M.D. Diagnosis and management of acute ischemic stroke: speed is critical. CMAJ. 2015;187(12):887–893. doi: 10.1503/cmaj.140355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woodruff T.M., Thundyil J., Tang S.C., Sobey C.G., Taylor S.M., Arumugam T.V. Pathophysiology, treatment, and animal and cellular models of human ischemic stroke. Mol. Neurodegener. 2011;6(1):11. doi: 10.1186/1750-1326-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deb P., Sharma S., Hassan K.M. Pathophysiologic mechanisms of acute ischemic stroke: An overview with emphasis on therapeutic significance beyond thrombolysis. Pathophysiology. 2010;17(3):197–218. doi: 10.1016/j.pathophys.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Seifert H.A., Offner H. The splenic response to stroke: from rodents to stroke subjects. J. Neuroinflammation. 2018;15(1):195. doi: 10.1186/s12974-018-1239-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shuaib A., Hussain M.S. The past and future of neuroprotection in cerebral ischaemic stroke. Eur. Neurol. 2008;59(1-2):4–14. doi: 10.1159/000109254. [DOI] [PubMed] [Google Scholar]
- 6.National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. Tissue plasminogen activator for acute ischemic stroke. N. Engl. J. Med. 1995;333(24):1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 7.Cheng Y.D., Al-Khoury L., Zivin J.A. Neuroprotection for ischemic stroke: two decades of success and failure. NeuroRx. 2004;1(1):36–45. doi: 10.1602/neurorx.1.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prentice H., Modi J.P., Wu J-Y. Mechanisms of neuronal protection against excitotoxicity, endoplasmic reticulum stress, and mitochondrial dysfunction in stroke and neurodegenerative diseases. Oxid. Med. Cell. Longev. 2015;2015:964518. doi: 10.1155/2015/964518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weinberger J.M. Evolving therapeutic approaches to treating acute ischemic stroke. J. Neurol. Sci. 2006;249(2):101–109. doi: 10.1016/j.jns.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 10.Froehler M.T., Ovbiagele B. Therapeutic hypothermia for acute ischemic stroke. Expert Rev. Cardiovasc. Ther. 2010;8(4):593–603. doi: 10.1586/erc.09.129. [DOI] [PubMed] [Google Scholar]
- 11.Tănăsescu R., Nicolau A., Ticmeanu M., Luca D., Caraiola S., Cojocaru I.M., Frăsineanu A., Ionescu R., Hristea A., Ene A., Tănăsescu R., Baicuş C. An immunological approach to cerebral ischemia (I). Immune cells and adhesion molecules. Rom. J. Intern. Med. 2008;46(1):3–8. [PubMed] [Google Scholar]
- 12.Neumar R.W., Hagle S.M., DeGracia D.J., Krause G.S., White B.C. Brain μ-calpain autolysis during global cerebral ischemia. J. Neurochem. 1996;66(1):421–424. doi: 10.1046/j.1471-4159.1996.66010421.x. [DOI] [PubMed] [Google Scholar]
- 13.Astrup J., Siesjö B.K., Symon L. Thresholds in cerebral ischemia - the ischemic penumbra. Stroke. 1981;12(6):723–725. doi: 10.1161/01.STR.12.6.723. [DOI] [PubMed] [Google Scholar]
- 14.Ginsberg M.D., Belayev L., Zhao W., Huh P.W., Busto R. The acute ischemic penumbra: topography, life span, and therapeutic response. Acta Neurochir. Suppl. (Wien) 1999;73:45–50. doi: 10.1007/978-3-7091-6391-7_7. [DOI] [PubMed] [Google Scholar]
- 15.Jhelum P., Karisetty B.C., Kumar A., Chakravarty S. Implications of epigenetic mechanisms and their targets in cerebral ischemia models. Curr. Neuropharmacol. 2017;15(6):815–830. doi: 10.2174/1570159X14666161213143907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kassis H., Shehadah A., Chopp M., Zhang Z.G. Epigenetics in stroke recovery. Genes (Basel) 2017;8(3):E89. doi: 10.3390/genes8030089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Endres M., Meisel A., Biniszkiewicz D., Namura S., Prass K., Ruscher K., Lipski A., Jaenisch R., Moskowitz M.A., Dirnagl U. DNA methyltransferase contributes to delayed ischemic brain injury. J. Neurosci. 2000;20(9):3175–3181. doi: 10.1523/JNEUROSCI.20-09-03175.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao S., Yan F., Cheng J., Huang L., Chen H. HDAC2 Selectively regulates foxo3a-mediated gene transcription during oxidative stress-induced neuronal cell death. J. Neurosci. 2015;35(3):1250–1259. doi: 10.1523/JNEUROSCI.2444-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim H.J., Leeds P., Chuang D.M. The HDAC inhibitor, sodium butyrate, stimulates neurogenesis in the ischemic brain. J. Neurochem. 2009;110(4):1226–1240. doi: 10.1111/j.1471-4159.2009.06212.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kassis H., Shehadah A., Li C., Zhang Y., Cui Y., Roberts C., Sadry N., Liu X., Chopp M., Zhang Z.G. Class IIa histone deacetylases affect neuronal remodeling and functional outcome after stroke. Neurochem. Int. 2016;96:24–31. doi: 10.1016/j.neuint.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lapchak P.A., Araujo D.M. Advances in ischemic stroke treatment: neuroprotective and combination therapies. Expert Opin. Emerg. Drugs. 2007;12(1):97–112. doi: 10.1517/14728214.12.1.97. [DOI] [PubMed] [Google Scholar]
- 22.George P.M., Steinberg G.K. Novel stroke therapeutics: unraveling stroke pathophysiology and its impact on clinical treatments. Neuron. 2015;87(2):297–309. doi: 10.1016/j.neuron.2015.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaur H., Prakash A., Medhi B. Drug therapy in stroke: from preclinical to clinical studies. Pharmacology. 2013;92(5-6):324–334. doi: 10.1159/000356320. [DOI] [PubMed] [Google Scholar]
- 24.Lees K.R., Barer D., Ford G.A., Hacke W., Kostulas V., Sharma A.K., Odergren T. SA-NXY-0004 Investigators. Tolerability of NXY-059 at higher target concentrations in patients with acute stroke. Stroke. 2003;34(2):482–487. doi: 10.1161/01.STR.0000053032.14223.81. [DOI] [PubMed] [Google Scholar]
- 25.Martínez-Vila E., Sieira P.I. Current status and perspectives of neuroprotection in ischemic stroke treatment. Cerebrovasc. Dis. 2001;11(Suppl. 1):60–70. doi: 10.1159/000049127. [DOI] [PubMed] [Google Scholar]
- 26.Muir K.W., Lees K.R., Hamilton S.J.C.A., George C.F., Hobbiger S.F., Lunnon M.W. A randomized, double-blind, placebo-controlled ascending dose tolerance study of 619C89 in acute stroke. Ann. N. Y. Acad. Sci. 1995;765(1):328–329. doi: 10.1111/j.1749-6632.1995.tb16605.x. [DOI] [PubMed] [Google Scholar]
- 27.Grotta J., The US and Canadian Lubeluzole Ischemic Stroke Study Group Lubeluzole treatment of acute ischemic stroke. Stroke. 1997;28(12):2338–2346. doi: 10.1161/01.STR.28.12.2338. [DOI] [PubMed] [Google Scholar]
- 28.Diener H.C., European and Australian Lubeluzole Ischaemic Stroke Study Group Multinational randomised controlled trial of lubeluzole in acute ischaemic stroke. Cerebrovasc. Dis. 1998;8(3):172–181. doi: 10.1159/000015847. [DOI] [PubMed] [Google Scholar]
- 29.Pulsinelli W., Mann M., Welch K., Zivin J., Biller J. Fosphenytoin in acute ischemic stroke: efficacy results. Neurology. 1999;52(Suppl. 2):A384. [Google Scholar]
- 30.Squire I.B., Lees K.R., Pryse-Phillips W., Kertesz A., Bamford J., Lifarizine Study Group Efficacy and tolerability of lifarizine in acute ischemic stroke. A pilot study. Ann. N. Y. Acad. Sci. 1995;765(1):317–318. doi: 10.1111/j.1749-6632.1995.tb16599.x. [DOI] [PubMed] [Google Scholar]
- 31.Scatton B., Carter C., Benavides J., Giroux C. N-Methyl-D-Aspartate receptor antagonists: a novel therapeutic perspective for the treatment of ischemic brain injury. Cerebrovasc. Dis. 1991;1(3):121–135. doi: 10.1159/000108829. [DOI] [Google Scholar]
- 32.Block F., Schwarz M. Dextromethorphan reduces functional deficits and neuronal damage after global ischemia in rats. Brain Res. 1996;741(1-2):153–159. doi: 10.1016/S0006-8993(96)00916-X. [DOI] [PubMed] [Google Scholar]
- 33.Olney J.W., Labruyere J., Price M.T. Pathological changes induced in cerebrocortical neurons by phencyclidine and related drugs. Science. 1989;244(4910):1360–1362. doi: 10.1126/science.2660263. [DOI] [PubMed] [Google Scholar]
- 34.Buchan A.M., Slivka A., Xue D. The effect of the NMDA receptor antagonist MK-801 on cerebral blood flow and infarct volume in experimental focal stroke. Brain Res. 1992;574(1-2):171–177. doi: 10.1016/0006-8993(92)90814-P. [DOI] [PubMed] [Google Scholar]
- 35.Lees K.R. Cerestat and other NMDA antagonists in ischemic stroke. Neurology. 1997;49(5) Suppl. 4:S66–S69. doi: 10.1212/WNL.49.5_Suppl_4.S66. [DOI] [PubMed] [Google Scholar]
- 36.Pitsikas N., Brambilla A., Besozzi C., Bonali P., Fodritto F., Grippa N., Scandroglio A., Borsini F. Effects of cerestat and NBQX on functional and morphological outcomes in rat focal cerebral ischemia. Pharmacol. Biochem. Behav. 2001;68(3):443–447. doi: 10.1016/S0091-3057(00)00469-X. [DOI] [PubMed] [Google Scholar]
- 37.Giroux C., Rosen P., Scatton B. 1994. [Google Scholar]
- 38.Ibarrola D., Seegers H., Jaillard A., Hommel M., Décorps M., Massarelli R. The effect of eliprodil on the evolution of a focal cerebral ischaemia in vivo. Eur. J. Pharmacol. 1998;352(1):29–35. doi: 10.1016/S0014-2999(98)00330-6. [DOI] [PubMed] [Google Scholar]
- 39.Takahashi M., Ni J.W., Kawasaki-Yatsugi S., Toya T., Ichiki C., Yatsugi S.I., Koshiya K., Shimizu-Sasamata M., Yamaguchi T. Neuroprotective efficacy of YM872, an α-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor antagonist, after permanent middle cerebral artery occlusion in rats. J. Pharmacol. Exp. Ther. 1998;287(2):559–566. [PubMed] [Google Scholar]
- 40.Takahashi M., Kohara A., Shishikura J., Kawasaki-Yatsugi S., Ni J.W., Yatsugi S., Sakamoto S., Okada M., Shimizu-Sasamata M., Yamaguchi T. YM872: a selective, potent and highly water-soluble alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptor antagonist. CNS Drug Rev. 2002;8(4):337–352. doi: 10.1111/j.1527-3458.2002.tb00232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. https://clinicaltrials.gov/ct2/show/NCT00044070
- 42.Akins P.T., Atkinson R.P. Glutamate AMPA receptor antagonist treatment for ischaemic stroke. Curr. Med. Res. Opin. 2002;18(Suppl. 2):s9–s13. doi: 10.1185/030079902125000660. [DOI] [PubMed] [Google Scholar]
- 43.Muir K.W., Lees K.R.A. A randomized, double-blind, placebo-controlled pilot trial of intravenous magnesium sulfate in acute stroke. Ann. N. Y. Acad. Sci. 1995;765:315–316. doi: 10.1111/j.1749-6632.1995.tb16598.x. [DOI] [PubMed] [Google Scholar]
- 44.Grotta J.C., Pettigrew L.C., Rosenbaum D., Reid C., Rhoades H., McCandless D. Efficacy and mechanism of action of a calcium channel blocker after global cerebral ischemia in rats. Stroke. 1988;19(4):447–454. doi: 10.1161/01.STR.19.4.447. [DOI] [PubMed] [Google Scholar]
- 45.Lukic-Panin V., Kamiya T., Zhang H., Hayashi T., Tsuchiya A., Sehara Y., Deguchi K., Yamashita T., Abe K. Prevention of neuronal damage by calcium channel blockers with antioxidative effects after transient focal ischemia in rats. Brain Res. 2007;1176:143–150. doi: 10.1016/j.brainres.2007.07.038. [DOI] [PubMed] [Google Scholar]
- 46.Gelmers H.J. Calcium-channel blockers: effects on cerebral blood flow and potential uses for acute stroke. Am. J. Cardiol. 1985;55(3):144B–148B. doi: 10.1016/0002-9149(85)90623-X. [DOI] [PubMed] [Google Scholar]
- 47.Abe K., Kogure K., Watanabe T. Prevention of ischemic and postischemic brain edema by a novel calcium antagonist (PN200-110). J. Cereb. Blood Flow Metab. 1988;8(3):436–439. doi: 10.1038/jcbfm.1988.81. [DOI] [PubMed] [Google Scholar]
- 48.Meyer J.S., Takashima S., Terayama Y. Cerebral Ischemia and Basic Mechanisms. Berlin, Heidelberg: Springer; 1994. Calcium channel blockers prevent delayed cerebral ischemia after intracranial aneurysmal subarachnoid hemorrhage. pp. 113–124. [Google Scholar]
- 49.Lyden P.D., Zivin J.A., Kochhar A., Mazzarella V. Effects of calcium channel blockers on neurologic outcome after focal ischemia in rabbits. Stroke. 1988;19(8):1020–1026. doi: 10.1161/01.STR.19.8.1020. [DOI] [PubMed] [Google Scholar]
- 50.Horn J., Limburg M. Calcium antagonists for ischemic stroke: a systematic review. Stroke. 2001;32(2):570–576. doi: 10.1161/01.STR.32.2.570. [DOI] [PubMed] [Google Scholar]
- 51.Zhang J., Yang J., Zhang C., Jiang X., Zhou H., Liu M. Calcium antagonists for acute ischemic stroke. Cochrane Database Syst. Rev. 2012;(5):CD001928. doi: 10.1002/14651858.cd001928.pub2. [DOI] [PubMed] [Google Scholar]
- 52.Wahlgren N.G., MacMahon D.G., De Keyser J. Intravenous nimodipine west european stroke trial (inwest) of nimodipine in the treatment of acute ischaemic stroke. Cerebrovasc. Dis. 1994;4(3):204–210. doi: 10.1159/000108483. [DOI] [Google Scholar]
- 53.Oczkowski W.J., Hachinski V.C., Bogousslavsky J., Barnett H.J., Carruthers S.G.A. A double-blind, randomized trial of PY108-068 in acute ischemic cerebral infarction. Stroke. 1989;20(5):604–608. doi: 10.1161/01.STR.20.5.604. [DOI] [PubMed] [Google Scholar]
- 54.Franke C.L., Palm R., Dalby M., Schoonderwaldt H.C., Hantson L., Eriksson B., Lang-Jenssen L., Smakman J. Flunarizine in stroke treatment (FIST): a double-blind, placebo-controlled trial in Scandinavia and the Netherlands. Acta Neurol. Scand. 1996;93(1):56–60. doi: 10.1111/j.1600-0404.1996.tb00171.x. [DOI] [PubMed] [Google Scholar]
- 55.Paik N-J., Yang E. Role of GABA plasticity in stroke recovery. Neural Regen. Res. 2014;9(23):2026–2028. doi: 10.4103/1673-5374.147920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chi O.Z., Hunter C., Liu X., Chi Y., Weiss H.R. Effects of GABA(A) receptor blockade on regional cerebral blood flow and blood-brain barrier disruption in focal cerebral ischemia. J. Neurol. Sci. 2011;301(1-2):66–70. doi: 10.1016/j.jns.2010.10.024. [DOI] [PubMed] [Google Scholar]
- 57.Schwartz-Bloom R.D., Sah R. γ-Aminobutyric acid(A) neurotransmission and cerebral ischemia. J. Neurochem. 2001;77(2):353–371. doi: 10.1046/j.1471-4159.2001.00274.x. [DOI] [PubMed] [Google Scholar]
- 58.He W-M., Ying-Fu L., Wang H., Peng Y-P. Delayed treatment of α5 GABAA receptor inverse agonist improves functional recovery by enhancing neurogenesis after cerebral ischemia-reperfusion injury in rat MCAO model. Sci. Rep. 2019;9(1):2287. doi: 10.1038/s41598-019-38750-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wahlgren N.G., The Clomethiazole Acute Stroke Study Collaborative Group The Clomethiazole Acute Stroke Study (CLASS): Results of a Randomised Controlled Study of Clomethiazole versus Placebo in 1360 Acute Stroke Patients. Cerebrovasc. Dis. 1997;7(Suppl. 4):24–30. doi: 10.1159/000108249. [DOI] [PubMed] [Google Scholar]
- 60.Liu J., Wang L-N., Ma X., Ji X. Gamma aminobutyric acid (GABA) receptor agonists for acute stroke. Cochrane Database Syst. Rev. 2016;10:CD009622. doi: 10.1002/14651858.CD009622.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gribkoff V.K., Starrett J.E., Jr, Dworetzky S.I., Hewawasam P., Boissard C.G., Cook D.A., Frantz S.W., Heman K., Hibbard J.R., Huston K., Johnson G., Krishnan B.S., Kinney G.G., Lombardo L.A., Meanwell N.A., Molinoff P.B., Myers R.A., Moon S.L., Ortiz A., Pajor L., Pieschl R.L., Post-Munson D.J., Signor L.J., Srinivas N., Taber M.T., Thalody G., Trojnacki J.T., Wiener H., Yeleswaram K., Yeola S.W. Targeting acute ischemic stroke with a calcium-sensitive opener of maxi-K potassium channels. Nat. Med. 2001;7(4):471–477. doi: 10.1038/86546. [DOI] [PubMed] [Google Scholar]
- 62.Jensen B.S. BMS-204352: a potassium channel opener developed for the treatment of stroke. CNS Drug Rev. 2002;8(4):353–360. doi: 10.1111/j.1527-3458.2002.tb00233.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lapchak P.A., Araujo D.M. Development of the nitrone-based spin trap agent NXY-059 to treat acute ischemic stroke. CNS Drug Rev. 2003;9(3):253–262. doi: 10.1111/j.1527-3458.2003.tb00252.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sydserff S.G., Borelli A.R., Green A.R., Cross A.J. Effect of NXY-059 on infarct volume after transient or permanent middle cerebral artery occlusion in the rat; studies on dose, plasma concentration and therapeutic time window. Br. J. Pharmacol. 2002;135(1):103–112. doi: 10.1038/sj.bjp.0704449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhao Z., Cheng M., Maples K.R., Ma J.Y., Buchan A.M. NXY-059, a novel free radical trapping compound, reduces cortical infarction after permanent focal cerebral ischemia in the rat. Brain Res. 2001;909(1-2):46–50. doi: 10.1016/S0006-8993(01)02618-X. [DOI] [PubMed] [Google Scholar]
- 66.Okamura K., Tsubokawa T., Johshita H., Miyazaki H., Shiokawa Y. Edaravone, a free radical scavenger, attenuates cerebral infarction and hemorrhagic infarction in rats with hyperglycemia. Neurol. Res. 2014;36(1):65–69. doi: 10.1179/1743132813Y.0000000259. [DOI] [PubMed] [Google Scholar]
- 67.Fujiwara N., Som A.T., Pham L-D.D., Lee B.J., Mandeville E.T., Lo E.H., Arai K. A free radical scavenger edaravone suppresses systemic inflammatory responses in a rat transient focal ischemia model. Neurosci. Lett. 2016;633:7–13. doi: 10.1016/j.neulet.2016.08.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Yagi K., Kitazato K.T., Uno M., Tada Y., Kinouchi T., Shimada K., Nagahiro S. Edaravone, a free radical scavenger, inhibits MMP-9-related brain hemorrhage in rats treated with tissue plasminogen activator. Stroke. 2009;40(2):626–631. doi: 10.1161/STROKEAHA.108.520262. [DOI] [PubMed] [Google Scholar]
- 69.He M., Xing S., Yang B., Zhao L., Hua H., Liang Z., Zhou W., Zeng J., Pei Z. Ebselen attenuates oxidative DNA damage and enhances its repair activity in the thalamus after focal cortical infarction in hypertensive rats. Brain Res. 2007;1181:83–92. doi: 10.1016/j.brainres.2007.08.072. [DOI] [PubMed] [Google Scholar]
- 70.Namura S., Nagata I., Takami S., Masayasu H., Kikuchi H. Ebselen reduces cytochrome c release from mitochondria and subsequent DNA fragmentation after transient focal cerebral ischemia in mice. Stroke. 2001;32(8):1906–1911. doi: 10.1161/01.STR.32.8.1906. [DOI] [PubMed] [Google Scholar]
- 71.Sena E., Wheble P., Sandercock P., Macleod M. Systematic review and meta-analysis of the efficacy of tirilazad in experimental stroke. Stroke. 2007;38(2):388–394. doi: 10.1161/01.STR.0000254462.75851.22. [DOI] [PubMed] [Google Scholar]
- 72.Enomoto M., Endo A., Yatsushige H., Fushimi K., Otomo Y. Clinical effects of early edaravone use in acute ischemic stroke patients treated by endovascular reperfusion therapy. Stroke. 2019;50(3):652–658. doi: 10.1161/STROKEAHA.118.023815. [DOI] [PubMed] [Google Scholar]
- 73.Yamaguchi T., Sano K., Takakura K., Saito I., Shinohara Y., Asano T., Yasuhara H., Ebselen Study Group Ebselen in acute ischemic stroke: a placebo-controlled, double-blind clinical trial. Stroke. 1998;29(1):12–17. doi: 10.1161/01.STR.29.1.12. [DOI] [PubMed] [Google Scholar]
- 74.International Steering 2001.
- 75.Tirilazad International Steering Committee Tirilazad mesylate in acute ischemic stroke: A systematic review. Stroke. 2000;31(9):2257–2265. doi: 10.1161/01.STR.31.9.2257. [DOI] [PubMed] [Google Scholar]
- 76.Kuschinsky W., Gillardon F. Apoptosis and cerebral ischemia. Cerebrovasc. Dis. 2000;10(3):165–169. doi: 10.1159/000016052. [DOI] [PubMed] [Google Scholar]
- 77.Prunell G.F., Arboleda V.A., Troy C.M. Caspase function in neuronal death: delineation of the role of caspases in ischemia. Curr. Drug Targets CNS Neurol. Disord. 2005;4(1):51–61. doi: 10.2174/1568007053005082. [DOI] [PubMed] [Google Scholar]
- 78.Hara H., Friedlander R.M., Gagliardini V., Ayata C., Fink K., Huang Z., Shimizu-Sasamata M., Yuan J., Moskowitz M.A. Inhibition of interleukin 1β converting enzyme family proteases reduces ischemic and excitotoxic neuronal damage. Proc. Natl. Acad. Sci. USA. 1997;94(5):2007–2012. doi: 10.1073/pnas.94.5.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lin X., Ye H., Siaw-Debrah F., Pan S., He Z., Ni H., Xu Z., Jin K., Zhuge Q., Huang L. AC-YVAD-CMK inhibits pyroptosis and improves functional outcome after intracerebral hemorrhage. BioMed Res. Int. 2018;2018:3706047. doi: 10.1155/2018/3706047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sun Y., Xu Y., Geng L. Caspase-3 inhibitor prevents the apoptosis of brain tissue in rats with acute cerebral infarction. Exp. Ther. Med. 2015;10(1):133–138. doi: 10.3892/etm.2015.2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang R.L., Chopp M., Jiang N., Tang W.X., Prostak J., Manning A.M., Anderson D.C. Anti-intercellular adhesion molecule-1 antibody reduces ischemic cell damage after transient but not permanent middle cerebral artery occlusion in the Wistar rat. Stroke. 1995;26(8):1438–1442. doi: 10.1161/01.STR.26.8.1438. [DOI] [PubMed] [Google Scholar]
- 82.Schneider D., Berrouschot J., Brandt T., Hacke W., Ferbert A., Norris S.H., Polmar S.H., Schäfer E. Safety, pharmacokinetics and biological activity of enlimomab (anti-ICAM-1 antibody): an open-label, dose escalation study in patients hospitalized for acute stroke. Eur. Neurol. 1998;40(2):78–83. doi: 10.1159/000007962. [DOI] [PubMed] [Google Scholar]
- 83.Furuya K., Takeda H., Azhar S., McCarron R.M., Chen Y., Ruetzler C.A., Wolcott K.M., DeGraba T.J., Rothlein R., Hugli T.E., del Zoppo G.J., Hallenbeck J.M. Examination of several potential mechanisms for the negative outcome in a clinical stroke trial of enlimomab, a murine anti-human intercellular adhesion molecule-1 antibody: a bedside-to-bench study. Stroke. 2001;32(11):2665–2674. doi: 10.1161/hs3211.098535. [DOI] [PubMed] [Google Scholar]
- 84.Enlimomab Acute Stroke Trial Investigators Use of anti-ICAM-1 therapy in ischemic stroke: results of the Enlimomab Acute Stroke Trial. Neurology. 2001;57(8):1428–1434. doi: 10.1212/WNL.57.8.1428. [DOI] [PubMed] [Google Scholar]
- 85.Zhang L., Zhang Z.G., Zhang R.L., Lu M., Krams M., Chopp M. Effects of a selective CD11b/CD18 antagonist and recombinant human tissue plasminogen activator treatment alone and in combination in a rat embolic model of stroke. Stroke. 2003;34(7):1790–1795. doi: 10.1161/01.STR.0000077016.55891.2E. [DOI] [PubMed] [Google Scholar]
- 86.Krams M., Lees K.R., Hacke W., Grieve A.P., Orgogozo J-M., Ford G.A., ASTIN Study Investigators Acute stroke therapy by inhibition of neutrophils (ASTIN): an adaptive dose-response study of UK-279,276 in acute ischemic stroke. Stroke. 2003;34(11):2543–2548. doi: 10.1161/01.STR.0000092527.33910.89. [DOI] [PubMed] [Google Scholar]
- 87.Lee J.H., Kam E.H., Kim J.M., Kim S.Y., Kim E.J., Cheon S.Y., Koo B-N. Intranasal administration of interleukin-1 receptor antagonist in a transient focal cerebral ischemia rat model. Biomol. Ther. (Seoul) 2017;25(2):149–157. doi: 10.4062/biomolther.2016.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Smith C.J., Hulme S., Vail A., Heal C., Parry-Jones A.R., Scarth S., Hopkins K., Hoadley M., Allan S.M., Rothwell N.J., Hopkins S.J., Tyrrell P.J. SCIL-STROKE (Subcutaneous interleukin-1 receptor antagonist in ischemic stroke): A randomized controlled phase 2 trial. Stroke. 2018;49(5):1210–1216. doi: 10.1161/STROKEAHA.118.020750. [DOI] [PubMed] [Google Scholar]
- 89.Barone F.C., Irving E.A., Ray A.M., Lee J.C., Kassis S., Kumar S., Badger A.M., White R.F., McVey M.J., Legos J.J., Erhardt J.A., Nelson A.H., Ohlstein E.H., Hunter A.J., Ward K., Smith B.R., Adams J.L., Parsons A.A. SB 239063, a second-generation p38 mitogen-activated protein kinase inhibitor, reduces brain injury and neurological deficits in cerebral focal ischemia. J. Pharmacol. Exp. Ther. 2001;296(2):312–321. [PubMed] [Google Scholar]
- 90.Legos J.J., Erhardt J.A., White R.F., Lenhard S.C., Chandra S., Parsons A.A., Tuma R.F., Barone F.C. SB 239063, a novel p38 inhibitor, attenuates early neuronal injury following ischemia. Brain Res. 2001;892(1):70–77. doi: 10.1016/S0006-8993(00)03228-5. [DOI] [PubMed] [Google Scholar]
- 91.Eckert B., Koch C., Thomalla G., Roether J., Zeumer H. Acute basilar artery occlusion treated with combined intravenous Abciximab and intra-arterial tissue plasminogen activator: report of 3 cases. Stroke. 2002;33(5):1424–1427. doi: 10.1161/01.STR.0000014247.70674.7F. [DOI] [PubMed] [Google Scholar]
- 92.Kleinschnitz C., Pozgajova M., Pham M., Bendszus M., Nieswandt B., Stoll G. Targeting platelets in acute experimental stroke: impact of glycoprotein Ib, VI, and IIb/IIIa blockade on infarct size, functional outcome, and intracranial bleeding. Circulation. 2007;115(17):2323–2330. doi: 10.1161/CIRCULATIONAHA.107.691279. [DOI] [PubMed] [Google Scholar]
- 93.Lapchak P.A., Araujo D.M., Song D., Zivin J.A. The nonpeptide glycoprotein IIb/IIIa platelet receptor antagonist SM-20302 reduces tissue plasminogen activator-induced intracerebral hemorrhage after thromboembolic stroke. Stroke. 2002;33(1):147–152. doi: 10.1161/hs0102.100530. [DOI] [PubMed] [Google Scholar]
- 94.Horisawa S., Kaneko M., Ikeda Y., Ueki Y., Sakurama T. Antithrombotic effect of SM-20302, a nonpeptide GPIIb/IIIa antagonist, in a photochemically induced thrombosis model in guinea pigs. Thromb. Res. 1999;94(4):227–234. doi: 10.1016/S0049-3848(98)00215-1. [DOI] [PubMed] [Google Scholar]
- 95.Abciximab Emergent Stroke Treatment Trial (AbESTT) Investigators Emergency administration of abciximab for treatment of patients with acute ischemic stroke: results of a randomized phase 2 trial. Stroke. 2005;36(4):880–890. doi: 10.1161/01.STR.0000157668.39374.56. [DOI] [PubMed] [Google Scholar]
- 96.Adams H.P., Jr, Effron M.B., Torner J., Dávalos A., Frayne J., Teal P., Leclerc J., Oemar B., Padgett L., Barnathan E.S., Hacke W. AbESTT-II Investigators. Emergency administration of abciximab for treatment of patients with acute ischemic stroke: results of an international phase III trial: Abciximab in Emergency Treatment of Stroke Trial (AbESTT-II). Stroke. 2008;39(1):87–99. doi: 10.1161/STROKEAHA.106.476648. [DOI] [PubMed] [Google Scholar]
- 97.Mauler F., Fahrig T., Horváth E., Jork R. Inhibition of evoked glutamate release by the neuroprotective 5-HT(1A) receptor agonist BAY x 3702 in vitro and in vivo. Brain Res. 2001;888(1):150–157. doi: 10.1016/S0006-8993(00)03074-2. [DOI] [PubMed] [Google Scholar]
- 98.Schaper C., Zhu Y., Kouklei M., Culmsee C., Krieglstein J. Stimulation of 5-HT(1A) receptors reduces apoptosis after transient forebrain ischemia in the rat. Brain Res. 2000;883(1):41–50. doi: 10.1016/S0006-8993(00)02876-6. [DOI] [PubMed] [Google Scholar]
- 99.Mauler F., Horváth E. Neuroprotective efficacy of repinotan HCl, a 5-HT1A receptor agonist, in animal models of stroke and traumatic brain injury. J. Cereb. Blood Flow Metab. 2005;25(4):451–459. doi: 10.1038/sj.jcbfm.9600038. [DOI] [PubMed] [Google Scholar]
- 100.Lutsep H.L. Repinotan, A 5-HT1A agonist, in the treatment of acute ischemic stroke. Curr. Drug Targets CNS Neurol. Disord. 2005;4(2):119–120. doi: 10.2174/1568007053544165. [DOI] [PubMed] [Google Scholar]
- 101.Berends A.C., Luiten P.G.M., Nyakas C. A review of the neuroprotective properties of the 5-HT1A receptor agonist repinotan HCl (BAYx3702) in ischemic stroke. CNS Drug Rev. 2005;11(4):379–402. doi: 10.1111/j.1527-3458.2005.tb00055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. https://clinicaltrials.gov/ct2/show/record/NCT00044915
- 103.Goyagi T., Toung T.J.K., Kirsch J.R., Traystman R.J., Koehler R.C., Hurn P.D., Bhardwaj A. Neuroprotective κ-opioid receptor agonist BRL 52537 attenuates ischemia-evoked nitric oxide production in vivo in rats. Stroke. 2003;34(6):1533–1538. doi: 10.1161/01.STR.0000072512.30658.E7. [DOI] [PubMed] [Google Scholar]
- 104.Tang A.H. Protection from cerebral ischemia by U-50,488E, a specific kappa opioid analgesic agent. Life Sci. 1985;37(16):1475–1482. doi: 10.1016/0024-3205(85)90178-X. [DOI] [PubMed] [Google Scholar]
- 105.Gannon R.L., Terrian D.M. U-50,488H inhibits dynorphin and glutamate release from guinea pig hippocampal mossy fiber terminals. Brain Res. 1991;548(1-2):242–247. doi: 10.1016/0006-8993(91)91127-M. [DOI] [PubMed] [Google Scholar]
- 106.Olinger C.P., Adams H.P., Jr, Brott T.G., Biller J., Barsan W.G., Toffol G.J., Eberle R.W., Marler J.R. High-dose intravenous naloxone for the treatment of acute ischemic stroke. Stroke. 1990;21(5):721–725. doi: 10.1161/01.STR.21.5.721. [DOI] [PubMed] [Google Scholar]
- 107.Clark W.M., Raps E.C., Tong D.C., Kelly R.E., The Cervene Stroke Study Investigators Cervene (Nalmefene) in acute ischemic stroke: final results of a phase III efficacy study. Stroke. 2000;31(6):1234–1239. doi: 10.1161/01.STR.31.6.1234. [DOI] [PubMed] [Google Scholar]
- 108.Zheng J., Li H., Guo R., Chen R., Lin S., Liu M., You C. Neuroprotection of nalmefene for postoperative patients with spontaneous intracerebral hemorrhage. Int. J. Neurosci. 2015;125(12):918–923. doi: 10.3109/00207454.2014.985294. [DOI] [PubMed] [Google Scholar]
- 109.Li X-P., Hou W-C., Song L. nalmefene improves prognosis in patients with a large cerebral infarction: study protocol and preliminary results of a randomized, controlled, prospective trial. Clin. Trials Degener. Dis. 2017;2(4):101. doi: 10.4103/2542-3975.222182. [DOI] [Google Scholar]
- 110.Sumii T., Lo E.H. Involvement of matrix metalloproteinase in thrombolysis-associated hemorrhagic transformation after embolic focal ischemia in rats. Stroke. 2002;33(3):831–836. doi: 10.1161/hs0302.104542. [DOI] [PubMed] [Google Scholar]
- 111.Jiang X., Namura S., Nagata I. Matrix metalloproteinase inhibitor KB-R7785 attenuates brain damage resulting from permanent focal cerebral ischemia in mice. Neurosci. Lett. 2001;305(1):41–44. doi: 10.1016/S0304-3940(01)01800-6. [DOI] [PubMed] [Google Scholar]
- 112.Romanic A.M., White R.F., Arleth A.J., Ohlstein E.H., Barone F.C. Matrix metalloproteinase expression increases after cerebral focal ischemia in rats: inhibition of matrix metalloproteinase-9 reduces infarct size. Stroke. 1998;29(5):1020–1030. doi: 10.1161/01.STR.29.5.1020. [DOI] [PubMed] [Google Scholar]
- 113.Alvarez-Sabín J., Román G.C. The role of citicoline in neuroprotection and neurorepair in ischemic stroke. Brain Sci. 2013;3(3):1395–1414. doi: 10.3390/brainsci3031395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shuaib A., Yang Y., Li Q. Evaluating the efficacy of citicoline in embolic ischemic stroke in rats: neuroprotective effects when used alone or in combination with urokinase. Exp. Neurol. 2000;161(2):733–739. doi: 10.1006/exnr.1999.7314. [DOI] [PubMed] [Google Scholar]
- 115.Onal M.Z., Li F., Tatlisumak T., Locke K.W., Sandage B.W., Jr, Fisher M. Synergistic effects of citicoline and MK-801 in temporary experimental focal ischemia in rats. Stroke. 1997;28(5):1060–1065. doi: 10.1161/01.STR.28.5.1060. [DOI] [PubMed] [Google Scholar]
- 116.Bustamante A., Giralt D., Garcia-Bonilla L., Campos M., Rosell A., Montaner J. Citicoline in pre-clinical animal models of stroke: a meta-analysis shows the optimal neuroprotective profile and the missing steps for jumping into a stroke clinical trial. J. Neurochem. 2012;123(2):217–225. doi: 10.1111/j.1471-4159.2012.07891.x. [DOI] [PubMed] [Google Scholar]
- 117.Secades J.J., Alvarez-Sabín J., Castillo J., Díez-Tejedor E., Martínez-Vila E., Ríos J., Oudovenko N. Citicoline for acute ischemic stroke: a systematic review and formal meta-analysis of randomized, double-blind, and placebo-controlled trials. J. Stroke Cerebrovasc. Dis. 2016;25(8):1984–1996. doi: 10.1016/j.jstrokecerebrovasdis.2016.04.010. [DOI] [PubMed] [Google Scholar]
- 118.Dávalos A., Alvarez-Sabín J., Castillo J., Díez-Tejedor E., Ferro J., Martínez-Vila E., Serena J., Segura T., Cruz V.T., Masjuan J., Cobo E., Secades J.J. International Citicoline Trial on acUte Stroke (ICTUS) trial investigators. Citicoline in the treatment of acute ischaemic stroke: an international, randomised, multicentre, placebo-controlled study (ICTUS trial). Lancet. 2012;380(9839):349–357. doi: 10.1016/S0140-6736(12)60813-7. [DOI] [PubMed] [Google Scholar]
- 119.Gandolfo C., Sandercock P., Conti M. Lubeluzole for acute ischaemic stroke. 2002. [DOI] [PubMed]
- 120.Haseldonckx M., Van Reempts J., Van de Ven M., Wouters L., Borgers M. Protection with lubeluzole against delayed ischemic brain damage in rats. A quantitative histopathologic study. Stroke. 1997;28(2):428–432. doi: 10.1161/01.STR.28.2.428. [DOI] [PubMed] [Google Scholar]
- 121.De Ryck M., Keersmaekers R., Duytschaever H., Claes C., Clincke G., Janssen M., Van Reet G. Lubeluzole protects sensorimotor function and reduces infarct size in a photochemical stroke model in rats. J. Pharmacol. Exp. Ther. 1996;279(2):748–758. [PubMed] [Google Scholar]
- 122.Svennerholm L. Gangliosides--a new therapeutic agent against stroke and Alzheimer’s disease. Life Sci. 1994;55(25-26):2125–2134. doi: 10.1016/0024-3205(94)00393-9. [DOI] [PubMed] [Google Scholar]
- 123.Argentino C., Sacchetti M.L., Toni D., Savoini G., D’Arcangelo E., Erminio F., Federico F., Milone F.F., Gallai V., Gambi D. GM1 ganglioside therapy in acute ischemic stroke. Italian Acute Stroke Study--Hemodilution + Drug. Stroke. 1989;20(9):1143–1149. doi: 10.1161/01.STR.20.9.1143. [DOI] [PubMed] [Google Scholar]
- 124.Lanfranconi S., Locatelli F., Corti S., Candelise L., Comi G.P., Baron P.L., Strazzer S., Bresolin N., Bersano A. Growth factors in ischemic stroke. J. Cell. Mol. Med. 2011;15(8):1645–1687. doi: 10.1111/j.1582-4934.2009.00987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Larpthaveesarp A., Ferriero D.M., Gonzalez F.F. Growth factors for the treatment of ischemic brain injury (growth factor treatment). Brain Sci. 2015;5(2):165–177. doi: 10.3390/brainsci5020165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Belayev L., Liu Y., Zhao W., Busto R., Ginsberg M.D. Human albumin therapy of acute ischemic stroke: marked neuroprotective efficacy at moderate doses and with a broad therapeutic window. Stroke. 2001;32(2):553–560. doi: 10.1161/01.STR.32.2.553. [DOI] [PubMed] [Google Scholar]
- 127.Martin R.H., Yeatts S.D., Hill M.D., Moy C.S., Ginsberg M.D., Palesch Y.Y. ALIAS Parts 1 and 2 and NETT Investigators. ALIAS (Albumin in Acute Ischemic Stroke) Trials: Analysis of the Combined Data From Parts 1 and 2. Stroke. 2016;47(9):2355–2359. doi: 10.1161/STROKEAHA.116.012825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Diener H-C., Schneider D., Lampl Y., Bornstein N.M., Kozak A., Rosenberg G. DP-b99, a membrane-activated metal ion chelator, as neuroprotective therapy in ischemic stroke. Stroke. 2008;39(6):1774–1778. doi: 10.1161/STROKEAHA.107.506378. [DOI] [PubMed] [Google Scholar]
- 129.Levy D.E., del Zoppo G.J., Demaerschalk B.M., Demchuk A.M., Diener H-C., Howard G., Kaste M., Pancioli A.M., Ringelstein E.B., Spatareanu C., Wasiewski W.W. Ancrod in acute ischemic stroke: results of 500 subjects beginning treatment within 6 hours of stroke onset in the ancrod stroke program. Stroke. 2009;40(12):3796–3803. doi: 10.1161/STROKEAHA.109.565119. [DOI] [PubMed] [Google Scholar]
- 130.Wang Y., Zhang Z.G., Rhodes K., Renzi M., Zhang R.L., Kapke A., Lu M., Pool C., Heavner G., Chopp M. Post-ischemic treatment with erythropoietin or carbamylated erythropoietin reduces infarction and improves neurological outcome in a rat model of focal cerebral ischemia. Br. J. Pharmacol. 2007;151(8):1377–1384. doi: 10.1038/sj.bjp.0707285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. https://clinicaltrials.gov/ct2/show/NCT00870844
- 132.Smith S.E., Lekieffre D., Sowinski P., Meldrum B.S. Cerebroprotective effect of BW619C89 after focal or global cerebral ischaemia in the rat. Neuroreport. 1993;4(12):1339–1342. doi: 10.1097/00001756-199309150-00013. [DOI] [PubMed] [Google Scholar]
- 133.Smith S.E., Hodges H., Sowinski P., Man C.M., Leach M.J., Sinden J.D., Gray J.A., Meldrum B.S. Long-term beneficial effects of BW619C89 on neurological deficit, cognitive deficit and brain damage after middle cerebral artery occlusion in the rat. Neuroscience. 1997;77(4):1123–1135. doi: 10.1016/S0306-4522(96)00530-1. [DOI] [PubMed] [Google Scholar]
- 134.Aronowski J., Strong R., Grotta J.C. Treatment of experimental focal ischemia in rats with lubeluzole. Neuropharmacology. 1996;35(6):689–693. doi: 10.1016/0028-3908(96)84640-5. [DOI] [PubMed] [Google Scholar]
- 135.Lin B., Dietrich W.D., Ginsberg M.D., Globus M.Y., Busto R. MK-801 (dizocilpine) protects the brain from repeated normothermic global ischemic insults in the rat. J. Cereb. Blood Flow Metab. 1993;13(6):925–932. doi: 10.1038/jcbfm.1993.115. [DOI] [PubMed] [Google Scholar]
- 136.Westermaier T., Stetter C., Kunze E., Willner N., Raslan F., Vince G.H., Ernestus R-I. Magnesium treatment for neuroprotection in ischemic diseases of the brain. Exp. Transl. Stroke Med. 2013;5(1):6. doi: 10.1186/2040-7378-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Albers G.W., Goldstein L.B., Hall D., Lesko L.M., Aptiganel Acute Stroke Investigators Aptiganel hydrochloride in acute ischemic stroke: a randomized controlled trial. JAMA. 2001;286(21):2673–2682. doi: 10.1001/jama.286.21.2673. [DOI] [PubMed] [Google Scholar]
- 138.Horn J., de Haan R.J., Vermeulen M., Luiten P.G., Limburg M. Nimodipine in animal model experiments of focal cerebral ischemia: a systematic review. Stroke. 2001;32(10):2433–2438. doi: 10.1161/hs1001.096009. [DOI] [PubMed] [Google Scholar]
- 139.Wiernsperger N., Gygax P., Hofmann A. Calcium antagonist PY 108-068: demonstration of its efficacy in various types of experimental brain ischemia. Stroke. 1984;15(4):679–685. doi: 10.1161/01.STR.15.4.679. [DOI] [PubMed] [Google Scholar]
- 140.De Ryck M., Van Reempts J., Borgers M., Wauquier A., Janssen P.A. Photochemical stroke model: flunarizine prevents sensorimotor deficits after neocortical infarcts in rats. Stroke. 1989;20(10):1383–1390. doi: 10.1161/01.STR.20.10.1383. [DOI] [PubMed] [Google Scholar]
- 141.Sydserff S.G., Cross A.J., Murray T.K., Jones J.A., Green A.R. Clomethiazole is neuroprotective in models of global and focal cerebral ischemia when infused at doses producing clinically relevant plasma concentrations. Brain Res. 2000;862(1-2):59–62. doi: 10.1016/S0006-8993(00)02071-0. [DOI] [PubMed] [Google Scholar]
- 142.Shuaib A., Lees K.R., Lyden P., Grotta J., Davalos A., Davis S.M., Diener H-C., Ashwood T., Wasiewski W.W., Emeribe U. SAINT II Trial Investigators. NXY-059 for the treatment of acute ischemic stroke. N. Engl. J. Med. 2007;357(6):562–571. doi: 10.1056/NEJMoa070240. [DOI] [PubMed] [Google Scholar]
- 143.Yang J., Cui X., Li J., Zhang C., Zhang J., Liu M. Edaravone for acute stroke: Meta-analyses of data from randomized controlled trials. Dev. Neurorehabil. 2015;18(5):330–335. doi: 10.3109/17518423.2013.830153. [DOI] [PubMed] [Google Scholar]
- 144.Vemuganti R., Dempsey R.J., Bowen K.K. Inhibition of intercellular adhesion molecule-1 protein expression by antisense oligonucleotides is neuroprotective after transient middle cerebral artery occlusion in rat. Stroke. 2004;35(1):179–184. doi: 10.1161/01.STR.0000106479.53235.3E. [DOI] [PubMed] [Google Scholar]
- 145.Graham S.H., Shimizu H., Newman A., Weinstein P., Faden A.I. Opioid receptor antagonist nalmefene stereospecifically inhibits glutamate release during global cerebral ischemia. Brain Res. 1993;632(1-2):346–350. doi: 10.1016/0006-8993(93)91175-R. [DOI] [PubMed] [Google Scholar]
- 146.Anttila J.E., Albert K., Wires E.S., Mätlik K., Loram L.C., Watkins L.R., Rice K.C., Wang Y., Harvey B.K., Airavaara M. 2018. [DOI] [PMC free article] [PubMed]
- 147.Elger B., Hornberger W., Schwarz M., Seega J. MRI study on delayed ancrod therapy of focal cerebral ischaemia in rats. Eur. J. Pharmacol. 1997;336(1):7–14. doi: 10.1016/S0014-2999(97)01217-X. [DOI] [PubMed] [Google Scholar]
- 148.Xu S-Y., Pan S-Y. The failure of animal models of neuroprotection in acute ischemic stroke to translate to clinical efficacy. Med. Sci. Monit. Basic Res. 2013;19:37–45. doi: 10.12659/MSMBR.883750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Reis C., Akyol O., Ho W.M., Araujo C., Huang L., Applegate R.I.I., Zhang J.H., Phase I., Phase I.I. Therapies for acute ischemic stroke: an update on currently studied drugs in clinical research. BioMed Res. Int. 2017;2017:4863079. doi: 10.1155/2017/4863079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kikuchi K., Tanaka E., Murai Y., Tancharoen S. Clinical trials in acute ischemic stroke. CNS Drugs. 2014;28(10):929–938. doi: 10.1007/s40263-014-0199-6. [DOI] [PubMed] [Google Scholar]
- 151.Furlan A.J. Challenges in acute ischemic stroke clinical trials. Curr. Cardiol. Rep. 2012;14(6):761–766. doi: 10.1007/s11886-012-0311-9. [DOI] [PubMed] [Google Scholar]
- 152.Venketasubramanian N., Yoon B.W., Pandian J. Stroke Epidemiology in South, East, and South-East Asia: A Review. J. Stroke Cerebrovasc. Dis. 2017;19(3):286–294. doi: 10.5853/jos.2017.00234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Appelros P., Stegmayr B., Terént A. Sex differences in stroke epidemiology: a systematic review. Stroke. 2009;40(4):1082–1090. doi: 10.1161/STROKEAHA.108.540781. [DOI] [PubMed] [Google Scholar]
- 154.Liu S., Zhen G., Meloni B.P., Campbell K., Winn H.R. Rodent stroke model guidelines for preclinical stroke trials in. J. Exp. Stroke Transl. Med. 2009;2(2):2–27. doi: 10.6030/1939-067x-2.2.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. https://clinicaltrials.gov/ct2/show/NCT03413202
- 156. https://clinicaltrials.gov/ct2/show/NCT03541668
- 157.Clinical Trial to Evaluate the Efficacy and Safety of JPI-289 in Patients With Acute Ischemic Stroke - Full Text View - ClinicalTrials https://clinicaltrials.gov/ct2/show/NCT03062397
- 158.Evaluation to Assess Safety and Tolerability of DM199 in Subjects With Acute Ischemic Stroke - Full Text View https://clinicaltrials.gov/ct2/show/NCT03290560
- 159.DLBS1033 for Acute Ischemic Stroke Patients - Full Text View https://clinicaltrials.gov/ct2/show/NCT02133521
- 160.Efficacy and Safety of FTY720 for Acute Stroke - Full Text View https://clinicaltrials.gov/ct2/show/NCT02002390
- 161.Zhu Z., Fu Y., Tian D., Sun N., Han W., Chang G., Dong Y., Xu X., Liu Q., Huang D., Shi F.D. Combination of the immune modulator fingolimod with alteplase in acute ischemic stroke: a pilot trial. Circulation. 2015;132(12):1104–1112. doi: 10.1161/CIRCULATIONAHA.115.016371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. https://clinicaltrials.gov/ct2/show/NCT00272454
- 163.Lee Y-S., Bae H-J., Kang D-W., Lee S-H., Yu K., Park J-M., Cho Y-J., Hong K-S., Kim D-E., Kwon S.U., Lee K.B., Rha J.H., Koo J., Han M.G., Lee S.J., Lee J.H., Jung S.W., Lee B.C., Kim J.S. Cilostazol in Acute Ischemic Stroke Treatment (CAIST Trial): a randomized double-blind non-inferiority trial. Cerebrovasc. Dis. 2011;32(1):65–71. doi: 10.1159/000327036. [DOI] [PubMed] [Google Scholar]
- 164. https://clinicaltrials.gov/ct2/show/NCT02225834
- 165.Tuttolomondo A., Di Raimondo D., Pecoraro R., Maida C., Arnao V., Della Corte V., Simonetta I., Corpora F., Di Bona D., Maugeri R., Iacopino D.G., Pinto A. Early High-dosage atorvastatin treatment improved serum immune-inflammatory markers and functional outcome in acute ischemic strokes classified as large artery atherosclerotic stroke: A Randomized Trial. Medicine (Baltimore) 2016;95(13):e3186. doi: 10.1097/MD.0000000000003186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Edaravone-Sodium Ozagrel Comparative Post-Marketing Study on Acute Ischemic Stroke - Study Results - ClinicalTrials https://clinicaltrials.gov/ct2/show/results/NCT00200356
- 167.Shinohara Y., Saito I., Kobayashi S., Uchiyama S. Edaravone (radical scavenger) versus sodium ozagrel (antiplatelet agent) in acute noncardioembolic ischemic stroke (EDO trial). Cerebrovasc. Dis. 2009;27(5):485–492. doi: 10.1159/000210190. [DOI] [PubMed] [Google Scholar]
- 168.Histamine Glutamate Antagonism in Stroke - Full Text View https://clinicaltrials.gov/ct2/show/NCT02142712
- 169.Chamorro Á., Amaro S., Castellanos M., Gomis M., Urra X., Blasco J., Arenillas J.F., Román L.S., Muñoz R., Macho J., Cánovas D., Marti-Fabregas J., Leira E.C., Planas A.M., URICO-ICTUS Investigators Uric acid therapy improves the outcomes of stroke patients treated with intravenous tissue plasminogen activator and mechanical thrombectomy. Int. J. Stroke. 2017;12(4):377–382. doi: 10.1177/1747493016684354. [DOI] [PubMed] [Google Scholar]
- 170.Amaro S., Laredo C., Renú A., Llull L., Rudilosso S., Obach V., Urra X., Planas A.M., Chamorro Á., URICO-ICTUS investigators uric acid therapy prevents early ischemic stroke progression: a tertiary analysis of the urico-ictus trial (efficacy study of combined treatment with uric acid and r-tpa in acute ischemic stroke). Stroke. 2016;47(11):2874–2876. doi: 10.1161/STROKEAHA.116.014672. [DOI] [PubMed] [Google Scholar]
- 171. https://clinicaltrials.gov/ct2/show/NCT00860366
- 172.Stem Cell Therapy For Acute Ischemic Stroke Patients - Full Text View https://clinicaltrials.gov/ct2/show/NCT02425670
- 173.Prasad K., Sharma A., Garg A., Mohanty S., Bhatnagar S., Johri S., Singh K.K., Nair V., Sarkar R.S., Gorthi S.P., Hassan K.M., Prabhakar S., Marwaha N., Khandelwal N., Misra U.K., Kalita J., Nityanand S., Inve S.T. Study Group. Intravenous autologous bone marrow mononuclear stem cell therapy for ischemic stroke: a multicentric, randomized trial. Stroke. 2014;45(12):3618–3624. doi: 10.1161/STROKEAHA.114.007028. [DOI] [PubMed] [Google Scholar]
- 174.Ehrenreich H., Weissenborn K., Prange H., Schneider D., Weimar C., Wartenberg K., Schellinger P.D., Bohn M., Becker H., Wegrzyn M., Jähnig P., Herrmann M., Knauth M., Bähr M., Heide W., Wagner A., Schwab S., Reichmann H., Schwendemann G., Dengler R., Kastrup A., Bartels C., EPO Stroke Trial Group Recombinant human erythropoietin in the treatment of acute ischemic stroke. Stroke. 2009;40(12):e647–e656. doi: 10.1161/STROKEAHA.109.564872. [DOI] [PubMed] [Google Scholar]
- 175.Liu X., Xia J., Wang L., Song Y., Yang J., Yan Y., Ren H., Zhao G. Efficacy and safety of ginsenoside-Rd for acute ischaemic stroke: a randomized, double-blind, placebo-controlled, phase II multicenter trial. Eur. J. Neurol. 2009;16(5):569–575. doi: 10.1111/j.1468-1331.2009.02534.x. [DOI] [PubMed] [Google Scholar]
- 176.Clinical Trial to Assess the Efficacy and to Evaluate Safety of HT047 in Patients With Acute Ischemic Stroke - Full Text View - ClinicalTrials https://clinicaltrials.gov/ct2/show/NCT02828540
- 177.Heiss W-D., Brainin M., Bornstein N.M., Tuomilehto J., Hong Z., Cerebrolysin Acute Stroke Treatment in Asia (CASTA) Investigators Cerebrolysin in patients with acute ischemic stroke in Asia: results of a double-blind, placebo-controlled randomized trial. Stroke. 2012;43(3):630–636. doi: 10.1161/STROKEAHA.111.628537. [DOI] [PubMed] [Google Scholar]
- 178.Horn C.M., Sun C-H.J., Nogueira R.G., Patel V.N., Krishnan A., Glenn B.A., Belagaje S.R., Thomas T.T., Anderson A.M., Frankel M.R., Schindler K.M., Gupta R. Endovascular reperfusion and cooling in cerebral acute ischemia (ReCCLAIM I). J. Neurointerv. Surg. 2014;6(2):91–95. doi: 10.1136/neurintsurg-2013-010656. [DOI] [PubMed] [Google Scholar]
- 179.Lyden P., Pryor K.E., Coffey C.S., Cudkowicz M., Conwit R., Jadhav A., Sawyer R.N., Jr, Claassen J., Adeoye O., Song S., Hannon P., Rost N.S., Hinduja A., Torbey M., Lee J.M., Benesch C., Rippee M., Rymer M., Froehler M.T., Clarke Haley E., Johnson M., Yankey J., Magee K., Qidwai J., Levy H., Mark Haacke E., Fawaz M., Davis T.P., Toga A.W., Griffin J.H., Zlokovic B.V. NeuroNEXT Clinical Trials Network NN104 Investigators. Final results of the rhapsody trial: a multi-center, phase 2 trial using a continual reassessment method to determine the safety and tolerability of 3k3a-apc, a recombinant variant of human activated protein c, in combination with tissue plasminogen activator, mechanical thrombectomy or both in moderate to severe acute ischemic stroke. Ann. Neurol. 2019;85(1):125–136. doi: 10.1002/ana.25383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Safety Study of Carbamylated Erythropoietin (CEPO) to Treat Patients With Acute Ischemic Stroke - Full Text View - ClinicalTrials https://clinicaltrials.gov/ct2/show/NCT00756249
- 181.Safety and Efficacy of Intravenous Natalizumab in Acute Ischemic Stroke - Full Text View https://clinicaltrials.gov/ct2/show/NCT02730455
- 182.Effect of Natalizumab on Infarct Volume in Acute Ischemic Stroke - Full Text View https://clinicaltrials.gov/ct2/show/NCT01955707
- 183.Elkins J., Veltkamp R., Montaner J., Johnston S.C., Singhal A.B., Becker K., Lansberg M.G., Tang W., Chang I., Muralidharan K., Gheuens S., Mehta L., Elkind M.S.V. Safety and efficacy of natalizumab in patients with acute ischaemic stroke (ACTION): a randomised, placebo-controlled, double-blind phase 2 trial. Lancet Neurol. 2017;16(3):217–226. doi: 10.1016/S1474-4422(16)30357-X. [DOI] [PubMed] [Google Scholar]
- 184. https://clinicaltrials.gov/ct2/show/NCT01014975
- 185.IVIG in Acute Ischemic Stroke https://clinicaltrials.gov/ct2/show/NCT01628055