Abstract
Purpose
The primary objective was to evaluate the maximum tolerated dose (within 10 weeks after treatment) associated with increasing hypofractionation to the prostate fossa (PF). We hypothesized that escalating the dose per fraction (fx) to the PF would have acceptable toxicity.
Materials and Methods
Tested dose levels (DLs) were 3.6 Gy × 15 fx (DL1); 4.7 Gy × 10 fx (DL2); and 7.1 Gy × 5 fx (DL3). Escalation followed a 6 + 6 rules-based design with 12 patients required at the maximum tolerated dose. Doselimiting toxicity was defined as grade (G) ≥3, gastrointestinal (GI) or genitourinary (GU) toxicity by National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.03). Patients completed quality-of-life questionnaires.
Results
Twenty-four patients with indications for adjuvant or salvage radiation therapy (RT) enrolled (6 at DL1 and 2; 12 at DL3). All patients had at least 6 months of follow-up (median follow-up, 14.1 months). Four patients received concurrent androgen deprivation therapy. No G ≥ 3 GI or GU toxicity was seen at any DL; 2 of 6 patients in the DL1 group, 3 of 6 in DL2, and 7 of 12 in DL3 experienced G2 GI toxicity during RT. Except in 1 patient, all acute G2 GI toxicity resolved by 10 weeks. Three of 12 patients reported an increase to G1 and G2 GU toxicity in the 2 weeks after RT in groups DL1 and DL2 and 1 of 12 patients in DL3. At week 2 after RT, decline in the 26-item Expanded Prostate Cancer Index Composite bowel domain met criteria for a minimally important difference in 71% of patients. At week 10, 1 of 6, 2 of 6, and 7 of 11 patients at DLs 1, 2, and 3, respectively, still met minimally important difference criteria. International Prostate Symptom Scores worsened 2 weeks after treatment but improved by 6 to 10 weeks.
Conclusions
Dose escalation up to 7.1 Gy × 5 fx to the PF was completed without acute G ≥ 3 toxicity. There was transient G2 rectal toxicity at all DLs during and immediately after RT. We must perform long-term follow-up and assessment of late toxicity of SBRT to the PF.
Introduction
Over the past decade, the proportion of men with newly diagnosed prostate cancer (CaP) undergoing radical prostatectomy (RP) has increased.1 Recurrent disease will develop in between 30% and 60% of patients who undergo RP.2 Three randomized controlled trials support the use of post-RP radiation therapy (RT) and demonstrated improved biochemical recurrence-free survival (BCRFS) for patients with stage pT3 disease, positive surgical margins, and/or persistently elevated prostate-specific antigen (PSA).3–5 These studies delivered conventionally fractionated RT with 2 Gy per fraction, which has become the standard fractionation regimen for postoperative RT.
Tissues with low α/β ratios are particularly sensitive to fraction size, with higher doses resulting in disproportionate increases in cell death. Although most tumors have high α/β values,6 CaP has an estimated α/β value of 1.5 Gy.7 With a lower α/β ratio, CaP should have an improved therapeutic ratio with hypofractionation. Furthermore, if the α/β formalism and assumed values for the CaP area correct and one maintains a constant biologically effective dose for normal tissues, there is the potential for increased tumor control with hypofractionation in this setting.8 Additionally, it has been shown in the definitive management of CaP that SBRT is more cost-effective than fractionated IMRT.9–11 Moderate post-RP hypofractionation has been explored in prospective single-institution trials using doses of approximately 2.5 to 3.1 Gy/fraction.12–17 The acute toxicity reports are similar to toxicity from standard fractionation after RP.13,15–19 Three studies using moderate hypofractionation with more than 15 months of follow-up report no increase in late toxicity.15–17 However, there have been reports of higher than expected late grade (G) 3 genitourinary (GU) toxicity (18.1%−28% vs. <5%).3,5,20–23 Our study evaluates 3 dose levels (DLs) (Table E1; available online at https://doi.org/10.1016/j.ijrobp.2018.12.047) based on the same biologically effective dose as that reported by Kruser et al because they reported no acute or late G ≥ 3 gastrointestinal (GI) toxicities and acceptable acute/late G2 toxicities (7% G2 and 15% G3 GU; 14% G2 and 4% G3 GI).15 To our knowledge, no published reports of stereotactic body RT (SBRT) or extreme hypofractionation regimens in the postoperative setting exist in the literature.
This single-institution phase 1 study evaluated physician-scored toxicity and patient- reported (PR) quality of life (QOL) of moderate to extreme post-RP hypofractionation. To evaluate tolerance of increasingly hypofractionated regimens, the dose per fraction increased over decreasing number of treatments. Doses evaluated in this trial were higher than in any previously reported studies, and the final DL used only 5 fractions to treat the prostate fossa (PF). All fractionation schedules were designed to yield a similar predicted toxicity profile based on an equivalent dose at 2 Gy fractions (EQD2) of 71 Gy. Because of the similar EQD2, we hypothesized that the toxicity of prostate fossa SBRT (PF-SBRT) would be well tolerated and comparable to standard fractionation.
Methods and Materials
This was an institutional review board–approved (clinicaltrials.gov NCT02446366) single-institution, phase 1 prospective clinical trial of moderate to extreme hypofractionation to the PF for patients at risk of microscopic residual disease after surgery. The primary objective was to determine the maximum tolerated dose (MTD) per fraction based on acute urinary and rectal toxicity at 10 weeks.
Patient eligibility
Eligible men were >18 years of age with prostate adenocarcinoma who had undergone RP of any kind, with a postoperative Eastern Cooperative Oncology Group performance status of 0 or 1. Patients with T2 pathology were eligible if they had positive surgical margins or a rising post-RP PSA level, whereas patients with pT3a/pT3b disease were eligible regardless of margin status. Patients with pathologic and radiographic nodal involvement were excluded. Neoadjuvant or concurrent hormonal therapy was allowed per physician discretion. Patients were excluded if they had prior radiation to the pelvis, gross residual disease on post-RP imaging, neoadjuvant or postoperative chemotherapy, or a history of inflammatory bowel disease.
Immobilization and treatment planning
Regardless of DL, the following strategy was used for immobilization and treatment planning. A urologist placed 3 PF fiducial markers under transrectal ultrasound guidance 1 to 2 weeks before computed tomography (CT) simulation.24,25 The fiducial markers were placed in the PF to correspond to what would have been the right mid gland, left base, and left apex of an intact prostate gland. A bowel regimen of either 30 mL of milk of magnesia the night before simulation and before each treatment or a Fleet enema before simulation and each treatment was required. A 60- to 100-cm3 rectal balloon was inserted for simulation and each treatment to push the lateral rectal walls out of the high-dose area26,27 (Fig. E1; available online at https://doi.org/10.1016/j.ijrobp.2018.12.047). All patients were asked to obtain a comfortably full bladder within the range of 150 to 250 mL and had an ultrasound scan before CT simulation (2 patients at DL1 were unable to receive presimulation and pretreatment ultrasound and were treated with a comfortably full bladder, alignment of fiducial markers, and rectal balloon). For daily treatment, the patient would have an ultrasound scan that verified the bladder volume to be within 10% of the calculated bladder volume based on CT simulation. Clinical target volume (CTV) was similar to the European Organisation for Research and Treatment of Cancer guidelines for target volume in postoperative RT.28 The CTV covered the vesicourethral anastomosis and bladder neck because of high rates of local recurrence but was carved out of the bladder superior to that (Fig. E2; available online at https://doi.org/10.1016/j.ijrobp.2018.12.047).28,29 The CTV was uniformly expanded 3 mm to create the planning target volume (PTV).
RT was delivered using volumetric arc RT or step-and-shoot intensity modulated RT (IMRT) on a linear accelerator (Trilogy, 21iX, or TrueBeam STX; Varian Medical Systems, Palo Alto, CA) using 6-MV photons. The dose was prescribed to cover ≥95% of the PTV.
Organs at risk included the rectum (divided into anterior, lateral, and posterior walls in the region of the PTV); bladder; bladder wall (outer 5 mm of the bladder contour); bowel space; and femoral heads. Organ at risk constraints were based on SBRT constraints for definitive prostate treatment and postoperative hypofractionated constraints.18,19,26,30,31 The following constraints were applied to all DLs: The anterior rectal wall was limited to 105% of prescription. No more than 3 cm3 of the lateral walls was allowed to receive 90% or more of the prescription dose. The posterior rectal wall maximum dose was limited to ≤45% of prescription. Less than 50% of the rectal wall circumference received 24 Gy.30 A rectal volume of <3 cm3 should receive 50 Gy, and <35% of the rectal circumference should receive >39 Gy. The bladder wall was limited to 105% of prescription.26 If this constraint could not be met, the bladder V40 Gy was limited to <22.9 cm3 and the V50 was limited to <13.2 cm3.19 The bowel maximum dose (to 0.5 cm3) was 30 Gy.31
Treatment
Daily image guidance localized the PF fiducials using megavoltage or kilovoltage x-rays. On the first day of therapy in the DL1 and 2 groups, patients had a cone beam CT (CBCT) to evaluate rectal volume and rectal balloon position. If the CBCT matched the fiducial alignment, no further CBCT scans were obtained. For patients on DL 3, CBCT scans were performed daily. Patients were treated on consecutive days regardless of fractionation. DL1 consisted of 15 fractions of 3.6 Gy, DL2 consisted of 10 fractions of 4.7 Gy, and DL3 consisted of 5 fractions of 7.1 Gy. Patients on DL3 used Anusol-HC (Salix Pharmaceuticals, Inc.) each night during treatment and twice a day for 2 weeks after treatment.
Study endpoints and statistics
The primary objective was to determine the MTD per fraction based on acute urinary and rectal toxicity at 10 weeks after treatment completion. The MTD was defined as the highest DL tested in which none or only 1 patient experienced dose-limiting toxicity (DLT), with 12 patients enrolled at the MTD. Dose escalation overview was undertaken by the USC Norris Cancer Center phase 1 Monitoring Committee. For decisions regarding dose escalation/de-escalation, DLT was defined as symptomatic G3 to G5 GI or GU toxicity based on the Common Terminology Criteria of Adverse Events (CTCAE) (version 4.03) within 10 weeks of RT19 completion and considered to be at least possibly attributed to RT. Dose escalations proceeded according to a 6 + 6 scheme,32 intending to select a dose with a low likelihood of DLT in which 5% to 10% was considered desirable and 20% undesirable. Six patients were enrolled at each new DL; if none of the 6 patients experienced DLT, the dose was escalated; if 1 of the 6 experienced DLT, 6 more patients would be treated at that DL; if 2 or more patients experienced DLT, dose escalation was stopped.
After completion of the SBRT, patients were assessed for toxicity and PR-QOL at 2, 6, and 10 weeks and at 6, 12, 18, 24, 30, and 36 months. The PSA level was measured at 10 weeks and then every 3 months for the first year and every 6 months thereafter.
The secondary objective was to assess the short-term toxicities and adverse events. The instruments used to assess PR-QOL were the Expanded Prostate Cancer Index Composite (EPIC)-26 and the International Prostate Symptom Score (IPSS). In evaluating the EPIC-26, we defined the minimally important difference (MID), or threshold beyond which changes in PR-QOL are considered clinically relevant, based on the mean calculated by Skolarus et al.33
Adverse events were listed and summarized by DL and time from RT completion. Associations between acute GI/GU toxicities and dosimetric parameters were evaluated by Cochran–Mantel–Haenszel tests and radar plots. For PR-QOL surveys, difference scores from baseline were calculated. Medians, ranges, and first and second quartiles were reported for each DL. PSA values were plotted.
Results
Twenty-four patients were enrolled and were evaluable between June 2015 and January 2018. Median follow-up was 30.1, 18.2, and 9.1 months for DL1, DL2, and DL3, respectively. Median patient age was 66 (54–79) years. The median PSA for all 3 DLs was <0.2 ng/mL before initiation of RT (Table 1).
Table 1.
Baseline patient characteristics
| Radiation dose |
||||
|---|---|---|---|---|
| Characteristics | 3.6 Gy × 15 fractions (n = 6) | 4.7 Gy × 10 fractions (n = 6) | 7.1 Gy × 5 fractions (n = 12) | Total (n = 24) |
| Age (y), median (range) | 64 (63–68) | 66 (54–71) | 67 (57–79) | 66 (54–79) |
| Mo from prostatectomy to radiation | 7.7 (3.7, 8.3) | 5.0 (3.7, 25.0) | 12.1 (8.9, 31.3) | 8.9 (4.6, 18.7) |
| therapy, median (Q1, Q3) | (3.6, 9.1) | (3.4, 26.6) | (3.1, 72.2) | (3.1, 72.2) |
| Range (minimum, maximum) | ||||
| Follow-up time (mo), median | 30.1 (27.1, 30.3) | 18.2 (17.0, 18.2) | 9.1 (6.3, 12.0) | 14.1 (9.1, 24.1) |
| (Q1, Q3) | (24.0–30.6) | (16.1–24.2) | (6.0–12.1) | (6.0–30.6) |
| Range (minimum, maximum) | ||||
| Baseline PSA, median (range) | 0.14 (0.03, 0.36) | 0.05 (0.03, 0.16) | 0.05 (0.03, 0.15) | 0.06 (0.03, 0.36) |
| Race | ||||
| Asian | 0 (0%) | 0 (0%) | 2 (17%) | 2 (8%) |
| Black or African American | 1 (17%) | 0 (0%) | 1 (8%) | 2 (8%) |
| Hispanic | 1 (17%) | 0 (0%) | 0 (0%) | 1 (4%) |
| Non-Hispanic White | 4 (67%) | 6 (100%) | 9 (75%) | 19 (79%) |
| Concurrent ADT | ||||
| No | 6 (100%) | 6 (100%) | 8 (67%) | 20 (83%) |
| Yes | 0 (0%) | 0 (0%) | 4 (33%) | 4 (17%) |
| Gleason score | ||||
| 3 + 4 | 3 (50%) | 4 (67%) | 7 (58%) | 14 (58%) |
| 4 + 3 | 2 (33%) | 2 (33%) | 2 (17%) | 6 (25%) |
| 4 + 4 | 0 (0%) | 0 (0%) | 1 (8%) | 1 (4%) |
| 4 + 5 | 1 (17%) | 0 (0%) | 2 (17%) | 3 (13%) |
| Pathologic T stage | ||||
| T2c | 0 (0%) | 0 (0%) | 3 (25%) | 3 (13%) |
| T3a | 3 (50%) | 6 (100%) | 8 (67%) | 17 (71%) |
| T3b | 3 (50%) | 0 (0%) | 1 (8%) | 4 (17%) |
| Surgical margins | ||||
| Negative | 3 (50%) | 1 (17%) | 5 (42%) | 9 (38%) |
| Positive | 3 (50%) | 5 (83%) | 7 (58%) | 15 (63%) |
| Perineural invasion | ||||
| No | 0 (0%) | 1 (17%) | 2 (17%) | 3 (13%) |
| Yes | 6 (100%) | 5 (83%) | 10 (83%) | 21 (88%) |
Abbreviations: ADT = androgen deprivation therapy; PSA = prostate-specific antigen; Q = quartile.
No DLT was seen within 10 weeks of RT in DL1/2; 12 patients were enrolled at DL3. Patients experiencing G1 and G2 physician-scored toxicity by DL during different points of treatment and recovery are listed in Table 2. Because many men had GU toxicity related to surgery, we documented the maximum increase in toxicity over their postsurgical/pre-RT baseline. The only general disorder patients reported on CTCAE (version 4.03) was G1 and G2 fatigue; this resolved by 6 months after treatment in all but 1 patient from DL1 and 1 patient from DL3. One patient from DL2 experienced G2 fatigue that resolved by 6 weeks after treatment. The remainder of the toxicity observed was in the GI or GU axis.
Table 2.
Change of maximum adverse event grade from preradiation baseline
| 3.6 Gy × 15 fractions (n = 6) |
|||||||||
| System organ class | Change of grade from baseline | During TX | 2 wk Post TX | 6 wk Post TX | 10 wk Post TX | 6 mo Post TX | |||
| General disorders and administration site conditions* | 0–1 | 2 (33%) | 3 (50%) | 2 (33%) | 2 (33%) | 1 (17%) | |||
| 0–2 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | ||||
| 1–2 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | ||||
| Gastrointestinal disorders | 0–1 | 1 (17%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |||
| 0–2 | 1 (17%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | ||||
| 1–2 | 1 (17%) | 1 (17%) | 1 (17%) | 0 (0%) | 0 (0%) | ||||
| Renal and urinary disorders | 0–1 | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | |||
| 1–2 | 1 (17%) | 2 (33%) | 1 (17%) | 0 (0%) | 0 (0%) | ||||
| 4.7 Gy × 10 fractions (n = 6) |
7.1 Gy × 5 fractions (n = 12) |
||||||||
| During TX | 2 wk Post TX | 6 wk Post TX | 10 wk Post TX | 6 mo Post TX | During TX | 2 wk Post TX | 6 wk Post TX | 10 wk Post TX | 6 mo Post TX |
| 1 (17%) | 1 (17%) | 1 (17%) | 0 (0%) | 0 (0%) | 4 (33%) | 3 (25%) | 2 (17%) | 1 (8%) | 1 (8%) |
| 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| 1 (17%) | 1 (17%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| 2 (33%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 2 (17%) | 4 (33%) | 2 (17%) | 2 (17%) | 3 (25%) |
| 2 (33%) | 2 (33%) | 0 (0%) | 0 (0%) | 0 (0%) | 6 (50%) | 1 (8%) | 1 (8%) | 0 (0%) | 1 (8%) |
| 1 (17%) | 1 (17%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (8%) | 0 (0%) | 0 (0%) | 1 (8%) | 0 (0%) |
| 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) |
| 0 (0%) | 1 (17%) | 0 (0%) | 0 (0%) | 0 (0%) | 0 (0%) | 1 (8%) | 0 (0%) | 0 (0%) | 0 (0%) |
Abbreviation: TX = treatment.
Note: Toxicity with possible, probable, or definite attribution reported.
Only 1 type of toxicity (fatigue) was reported by patients in general disorders and administration site conditions.
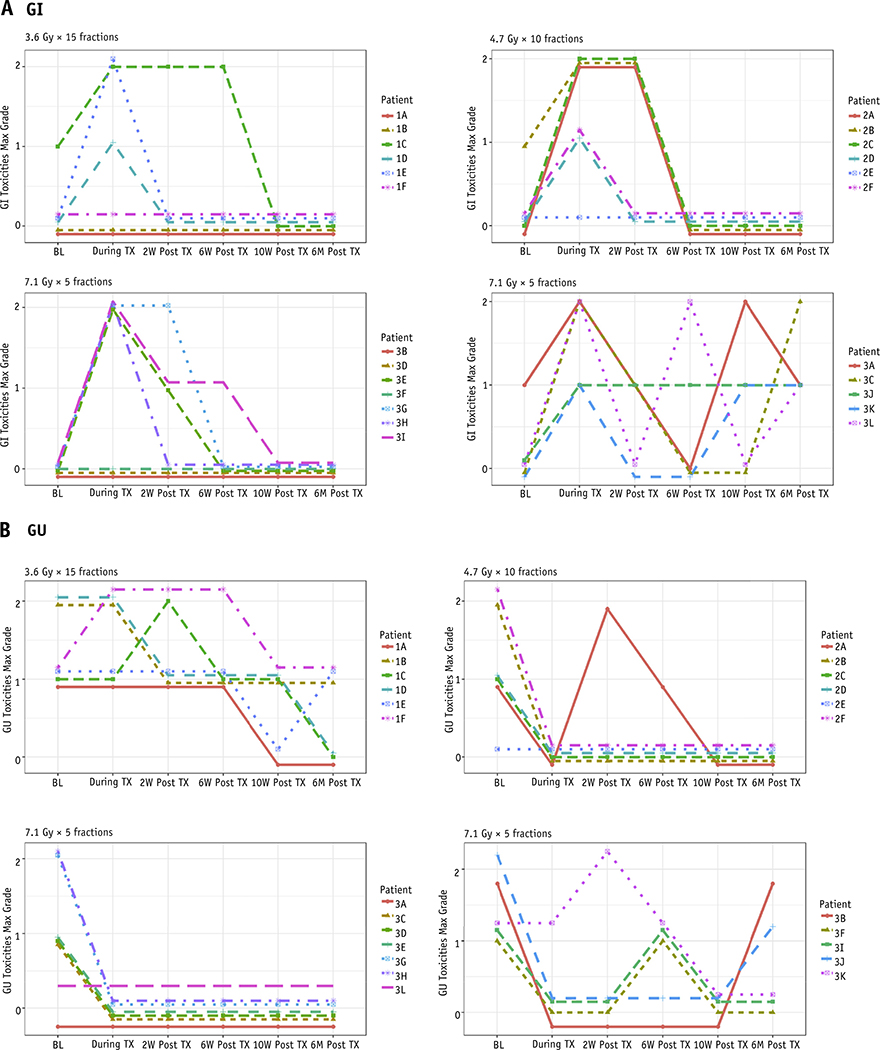
No men experienced acute G3+ GI or GU toxicity. The most common acute GI toxicities were proctitis and blood seen on toilet tissue after a bowel movement. Although 3 of 6 and 7 of 12 of men on DL2 and DL3, respectively, had G2 GI toxicity during treatment, these resolved to G0 by 10 weeks after treatment in all patients in DL2. On DL3, 4 of 7 toxicities resolved to G0, 1 of 7 decreased to G1, and 2 and 7 remained in G2 by 10 weeks after treatment. Figure 1A shows physician-reported GI toxicity per patient at each DL. For patients in DL1 and DL2, all GI toxicities resolved by 10 weeks after treatment. For 10 of the 12 patients in DL3 symptoms resolved by 10 weeks after treatment; the other 2 patients had an up-and-down pattern to their GI toxicity, with 1 patient reporting G1 toxicity at 6 months after RT that was not present before RT. The most common GU side effects reported were urgency and frequency of urination, and these resolved by 10 weeks after radiation in all 3 DLs. Of the 4 patients (2 of 6, 1 of 6, and 1 of 12 in DL1, DL2, and DL3, respectively) who experienced an increase in GU toxicity, all experienced this increase at 2 weeks after treatment with return to baseline in all by week 10 (Table 2, Fig. 1B). Overall, no meaningful scientific cutoff for volumetric dose was correlated to acute G2 GI/GU toxicity.
Fig. 1.
A, Gastrointestinal (GI) and (B) genitourinary (GU) adverse events based on National Institutes of Health Common Terminology Criteria of Adverse Events (version 4.03) per patient at each dose level. Abbreviations: BL = baseline; max = maximum; TX = treatment.
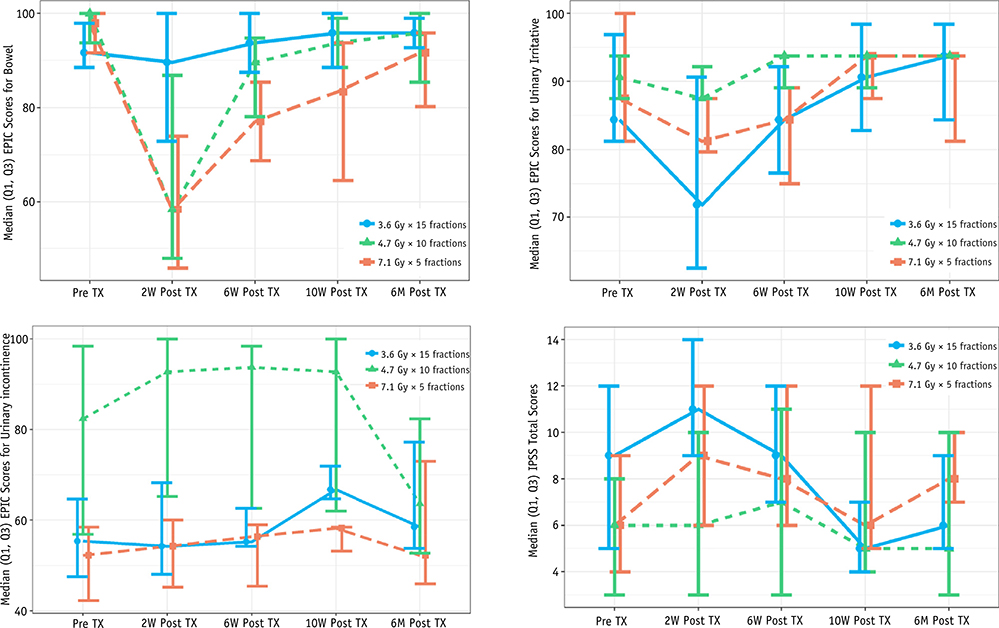
Patient compliance with PR-QOL was 100% with surveys up to 6 months for all 3 DLs except for 1 patient on DL3 who did not comply at 10 weeks. Figure 2 and Table 3 summarize the differences over PR baseline for each DL for the EPIC-26 bowel domain and the EPIC-26 urinary irritative and incontinence scores along with the IPSS score. At 2 weeks after RT, patients reported a maximal decline in the EPIC-26 bowel domain: 3 of 6, 4 of 6, and 10 of 12 patients in DL1, DL2, and DL3, respectively, experienced decreases that were clinically relevant based on the MID (defined as a decline of 6+ points on the EPIC-26).33 This decreased GI QOL at week 2 improved across all DLs at 10 weeks after RT with only 1 of 6, 2 of 6, and 7 of 11 patients (1 patient on DL3 refused to complete QOL questionnaires at week 10) in DLs 1, 2, and 3, respectively, having residual EPIC score changes greater than the MID (Table E2; available online at https://doi.org/10.1016/j.ijrobp.2018.12.047). Despite these EPIC results, no patients on DLs 1 or 2 reported “moderate” or “big” problems with their bowel habits, and only 1 patient on DL3 reported a “moderate” problem at 10 weeks after RT (Table E3; available online at https://doi.org/10.1016/j.ijrobp.2018.12.047).
Fig. 2.
Medians and lower (quartile 1 [Q1]) and upper (quartile 3 [Q3]) quartiles for 26-item Expanded Prostate Cancer Index (EPIC-26) bowel and International Prostate Symptom Score (IPSS).
Table 3.
Patient-reported quality-of-life assessments
| 2-wk posttreatment difference from baseline |
|||||
| Assessment tool | Dose level | No. | Median (Min, Q1, Q3, Max) | ||
| EPIC-26: Bowel* | 3.6 Gy × 15 fractions | 6 | −4.2 (−50.0, −20.8, 0, 8.3) | ||
| 4.7 Gy × 10 fractions | 6 | −33.3 (−87,5, −54.2, 0, 3.3) | |||
| 7.1 Gy × 5 fractions | 12 | −31.3 (−75.0, −54.2, −14.6, 8.3) | |||
| EPIC-26: Urinary irritative* | 3.6 Gy × 15 fractions | 6 | −6.3 (−25, −18.8, 0, 6.3) | ||
| 4.7 Gy × 10 fractions | 6 | 0 (−6.3, −6.3, 0, 12.5) | |||
| 7.1 Gy × 5 fractions | 12 | −6.3 (−56.3, −18.8, −3.1, 12.5) | |||
| EPIC-26: Urinary incontinence* | 3.6 Gy × 15 fractions | 6 | 4.1 (−12.5, −8.3, 8.3, 8.3) | ||
| 4.7 Gy × 10 fractions | 6 | 3.1 (0, 0, 6.3, 14.5) | |||
| 7.1 Gy × 5 fractions | 12 | 3.1 (−31.3, −11.5, 6.3, 62.8) | |||
| IPSS total score† | 3.6 Gy × 15 fractions | 6 | 2 (−3, −3, 7, 9) | ||
| 4.7 Gy × 10 fractions | 6 | 1 (−1, −1, 3, 4) | |||
| 7.1 Gy × 5 fractions | 12 | 2 (−3, 0, 3, 14) | |||
| 6-wk posttreatment difference from baseline |
10-wk posttreatment difference from baseline |
6-mo posttreatment difference from baseline |
|||
| No. | Median (Min, Q1, Q3, Max) | No. | Median (Min, Q1, Q3, Max) | No. | Median (Min, Q1, Q3, Max) |
| 6 | 0 (−25.0, 0, 4.2, 8.3) | 6 | 0 (−16.7, 0, 8.3, 8.3) | 6 | −2.1 (−4.2, −4.2, 8.3, 12.5) |
| 6 | −6.3 (−37.5, −25, 0, 12.5) | 6 | −2.1 (−25.0, −12.5, 0, 16.7) | 6 | −4.2 (−8.3, −8.3, 0, 0) |
| 12 | −16.7 (−50.0, −31.3, −2.1, 8.3) | 11 | −16.7 (−54,2, −20.8, 0, 8.3) | 12 | −6.3 (−41.7, −12.5, 0, 11.7) |
| 6 | −6.3 (−12.5, −6.3, −6.3, 31.3) | 6 | 0 (−12.5, 0, 6.3, 37.5) | 6 | 0 (−6.3, 0, 12.5, 37.5) |
| 6 | 0 (−6.3, 0, 6.3, 18.8) | 6 | 0 (−6.3, −6.3, 6.3, 25) | 6 | 3.1 (0, 0, 6.3, 6.3) |
| 12 | −6.3 (−43.8, −12.5, 0, 6.3) | 11 | 0 (−6.3, −6.3, 6.3, 25) | 12 | 0 (−50, −6.3, 6.3, 25) |
| 6 | 1 (−27, −12.5, 8.3, 33.3) | 6 | 6.3 (0, 6.3, 22.8, 41.8) | 6 | 0 (−33.3, −14.5, 45.8, 60.5) |
| 6 | 0 (−8.3, −6.3, 6.3, 22.8) | 6 | 1 (−14.5, 0, 6.3, 29) | 6 | −7.3 (−20.8, −16.8, 0, 0) |
| 12 | 2 (−21, 0, 9.4, 35.8) | 11 | 0 (−12.5, −6.3, 6.3, 27) | 12 | −3.1 (−21, −10.4, 21.9, 69) |
| 6 | 2 (−7, −3, 5, 7) | 6 | −1.5 (−9, −7, 0, 2) | 6 | 0.5 (−9, −6, 3, 4) |
| 6 | 2 (−2, 0, 3, 3) | 6 | 2 (−14, 0, 3, 4) | 6 | 1 (−3, −1, 3, 3) |
| 12 | 1 (−9, −2.5, 7.5, 14) | 11 | 0 (−6, 0, 4, 9) | 12 | 2 (−7, −2.5, 4.5, 18) |
Abbreviations: EPIC = Expanded Prostate Cancer Index; IPSS = International Prostate Symptom Score; max = maximum; Min = minimum.
EPIC-26 (range 0–100). The higher the score, the better the patient is doing.
IPSS (range 0–35). Scores 1 to 7 = mild, 8 to 19 = moderate, 20 to 35 = severe prostate symptoms.
GU QOL was measured by IPSS absolute number along with EPIC-26 urinary irritative and incontinence scores. The IPSS increased from baseline at 2 weeks in 3 of 6, 3 of 6, and 8 of 12 patients on DL1, DL2, and DL3, respectively. At 10 weeks, the IPSS returned to baseline or lower category (mild, moderate, severe) in all but 3 patients (all 3 in DL 3; Table 3, Fig. 2). Similar to the IPSS, the GU QOL as measured by the EPIC-26 urinary irritative score worsened at 2 weeks but improved by 10 weeks. At 2 weeks, 2 of 6, 0 of, and 5 of 12 patients in DL1, DL2, and DL3, respectively, experienced MID as defined as a decline in 7 points; at 10 weeks, 1 of 6, 0 of 6, and 0 of 11 patients in DL1, DL2, and DL3 experienced MID (Table E2; available online at https://doi.org/10.1016/j.ijrobp.2018.12.047).
GU QOL as measured by the EPIC-26 urinary incontinence score remained the same or improved slightly until 10 weeks after RT for the majority of patients in all DLs. Incontinence worsened from 10 weeks to 6 months, especially in the DL2 group (Table 3 and Fig. 2). At 2 weeks, 1 of 6, 0 of 6, and 3 of 12 patients in DL1, DL2, and DL3, respectively, experienced MID as defined as a decline in 9 points. At 10 weeks, 0 of 6, 1 of 6, and 1 of 11 patients in DL1, DL2, and 3, respectively, experienced MID. At 6 months, 2 of 6, 3 of 6, and 3 of 12 patients in DL1, DL2, and DL3, respectively, experienced MID. Only 1 patient across all DLs reported a “big” problem with his overall urinary function at 6 months (Table E3; available online at https://doi.org/10.1016/j.ijrobp.2018.12.047). Despite a decline in the EPIC-26 urinary incontinence score at 6 months, no patients reported an increase in CTCAE GU grade from their pre-RT baseline at 6 months across all DLs (Table 2).
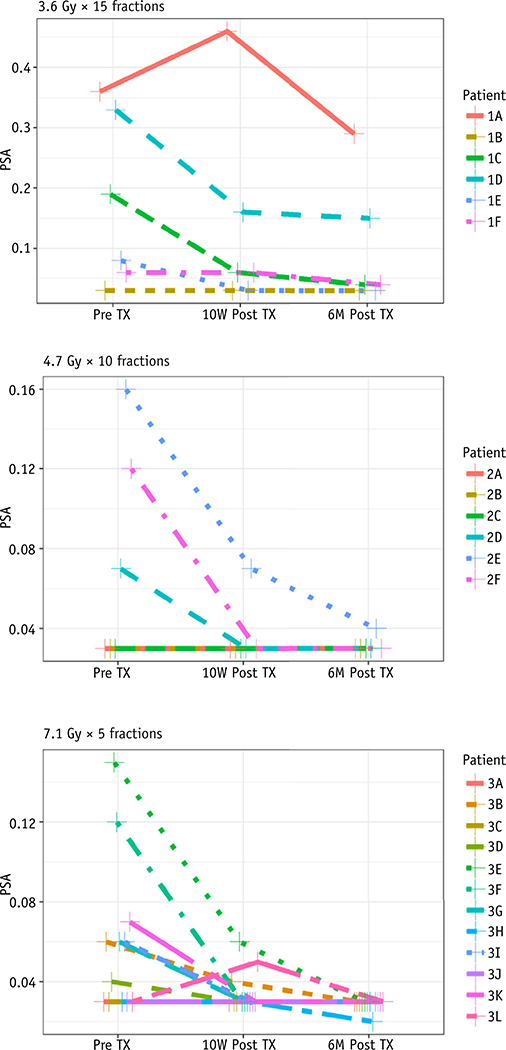
Although BCRFS was not the primary outcome of this study, PSA levels did decline for all but 1 patient at 10 weeks after treatment. The 1 patient who had a PSA increase at week 10 had a decline in PSA level to below the pre-RT baseline at 6 months after treatment (Fig. 3). It is far too early to ascribe any PSA-related benefit to this treatment, and this will be monitored closely over time.
Fig. 3.
Prostate-specific antigen (PSA) level over time per patient at each dose level.
Discussion
Three trials show a benefit to adjuvant RT for BCRFS in the setting of high-risk pathologic features,3–5 and salvage RT in the setting of a rising PSA level is the only curative intervention in this setting. Unfortunately, the pooled, BCRFS outcomes after either intervention are approximately 50%.5,34 Ways to improve BCRFS and survival include the addition of ADT postoperatively35 and dose escalation.12,34,36–38 Dose escalation with standard fractionation has been well tolerated in the post-RP setting.38,39
Given the low α/β ratio of CaP, another potential way to improve BCRFS is by hypofractionation. This approach has been studied in several prospective post-RP trials12–15 and is the subject of an ongoing randomized trial (https://clinicaltrials.gov/ct2/show/NCT03274687). The acute toxicity reported from these postoperative studies has been similar to toxicity from standard fractionation with acute G2 GU toxicity between 6% and 16% and acute G2 GI toxicity between 4% and 33%.13,15,18,19,21 The late toxicity profile of hypofractionation to the PF has varied; 3 studies with >15 months’ follow-up report no increase in late effects using moderate hypofractionation and advanced delivery techniques.15–17 There have, however, been reports of higher than expected late G3 GU toxicity (18.1% and 28%).20,21
This single-institution phase 1 study prospectively evaluated acute toxicity and PR-QOL to increasingly hypofractionated PF treatment. To our knowledge, it is the first study to evaluate these doses of postoperative hypofractionation and SBRT treatment to the PF.
Toxicity reporting
Acute toxicity reporting in definitive SBRT CaP is typically done at 1 or 3 months after RT.40,41 The maximal acute toxicity is thus often underreported because it is typically seen in the first 1 to 2 weeks after RT. In a study that evaluated proctitis in the 1 week after prostate SBRT, Paydar et al showed that, similar to our findings, GI toxicity peaks early and recovers by approximately 3 months after RT.42 Their G2 proctitis rate at 1 week was 23%; our G2 GI toxicity at 2 weeks after treatment was 17% in DL1 patients, 50% in DL2 patients, and 8% in DL3 patients. Because we captured toxicity during treatment and 2 weeks after RT, our PR-QOL outcomes indicate large adverse effects on QOL (34% with “moderate or big” bowel problems on DL1, 40% on DL2, and 50% on DL3). As Paydar et al contend, despite the recall period for the EPIC-26 being 4 weeks, it appears that patients underreport early side effects if queried only at 1 month after RT.42
GI/GU toxicity and QOL
No G3 acute GU/GI toxicity was seen at any DL. At 10 weeks after RT, none of the 24 patients had an increase in their physician-scored pre-RT GU toxicity (0%; 95% exact one-sided binomial confidence interval [0, 12]). This result appears to be better than studies that used 70 to 75 Gy in standard fractionation to the PF (48.6%39 G1, 28%22,39 G2) and similar to studies that evaluated 2.5 to 3.1 Gy postoperatively (4%−6% G2).15,18–20,43 Ten-week PR-QOL median IPSS and EPIC-26 urinary irritative and incontinence scores were at baseline or slightly improved from the start of treatment (Table 3 and Fig. 2). An increase in the EPIC-26–reported urinary incontinence score was seen at 6 months; 8 patients had an increase in incontinence that met MID. This may be the start of late toxicity that we will continue to closely monitor in these patients and report once data mature further. This finding also highlights the importance of PR-QOL in the postoperative patient population. The CTCAE (version 4.03) grading for incontinence revolves around a patient’s need for a pad (G1: occasional incontinence, pads not indicated; G2: spontaneous incontinence, pads indicated), which many postoperative patients need at baseline.
Acute G2 GI toxicity for hypofractionated treatment has ranged from 14%15 to 29.7%.43 Sampath et al presented their PF-SBRT results (for use of up to 9 Gy per fraction) in abstract form and noted a G2 GI toxicity rate of 35.7% (5 of 14) within 3 months of RT.44 Our 10-week G2 GI toxicity was 8% (exact two-sided 95% confidence interval [<1, 38]) on DL3, in which only 1 patient reported a “moderate problem.”
Daily treatment
Because of the dose-per-fraction escalation, all patients regardless of DL were treated daily. It is likely we would have had lower acute GI toxicity if we had treated patients every other day. In a phase 2 SBRT trial for localized CaP, King et al reported that late rectal toxicity (deemed to be “moderate” or “big” problems) decreased from 24% to 0% when treatment was altered from daily to every other day. Radiation Therapy Oncology Group G2 GI toxicity decreased from 6% for daily treatment to 0% for every-other-day treatment, which was not a statistically significant difference. Late Radiation Therapy Oncology Group G3 GU toxicity also decreased from 6% to 2%,45,46 which was not statistically significant.
Quon et al evaluated once-weekly versus every-other-day prostate SBRT and found that patients treated once weekly had superior acute bowel QOL. Acute urinary QOL also improved in the once-weekly arm (78% vs. 94%). There were no differences in 2-year urinary or bowel QOL.47 The effect of every-week treatment on PSA was not reported.
One limitation of our study was the small sample size. Because this was one of the first SBRT trials in postoperative CaP patients and because no DLT had ever been defined, we purposefully restricted the number of study patients to limit the number of patients at risk. Another limitation is the short follow-up. Although data on acute toxicity were mature for all patients, longer follow-up is needed to assess late toxicity and will be reported when data further mature. The lack of acute G3 toxicity does not exclude the possibility of significant late toxicities and must be closely monitored. This study also demonstrates the importance of evaluating patients at 2 weeks after RT to assess peak GU/GI side effects.
Conclusion
Moderate to extreme PF hypofractionation causes transient G2 GI/GU toxicity in the first 2 weeks after RT. The toxicity decreased by week 10 in almost all cases, which is comparable to other acute post-RP hypofractionation results. All 3 tested DLs were well tolerated based on acute toxicity. Because of these favorable acute results, further study is warranted to evaluate long-term toxicity and BCFRS.
Supplementary Material
Summary.
This phase 1 trial evaluated the tolerability of moderate to extreme hypofractionation to the prostate fossa, as measured by physician-scored toxicity and patient-reported outcomes. Given the similar equivalent dose in 2-Gy fractions of all dose levels, we hypothesized that prostate fossa stereotactic body radiation therapy would be well tolerated, with toxicity comparable to that of standard fractionation. There was transient grade 2 rectal toxicity at all dose levels during and immediately after radiation therapy. We must await long-term follow-up to assess late toxicity.
Footnotes
Conflicts of interest: none.
This protocol is registered with ClinicalTrials.gov, NCT number: NCT02446366.
Supplementary material for this article can be found at https://doi.org/10.1016/j.ijrobp.2018.12.047.
References
- 1.Cooperberg MR, Carroll PR. Trends in management for patients with localized prostate cancer, 1990–2013. JAMA 2015;314:80–82. [DOI] [PubMed] [Google Scholar]
- 2.Pfister D, Bolla M, Briganti A, et al. Early salvage radiotherapy following radical prostatectomy. Eur Urol 2014;65:1034–1043. [DOI] [PubMed] [Google Scholar]
- 3.Bolla M, van Poppel H, Tombal B, et al. Postoperative radiotherapy after radical prostatectomy for high-risk prostate cancer: Long-term results of a randomised controlled trial (EORTC trial 22911). Lancet 2012;380:2018–2027. [DOI] [PubMed] [Google Scholar]
- 4.Thompson IM, Tangen CM, Paradelo J, et al. Adjuvant radiotherapy for pathological T3N0M0 prostate cancer significantly reduces risk of metastases and improves survival: Long-term followup of a randomized clinical trial. J Urol 2009;181:956–962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wiegel T, Bartkowiak D, Bottke D, et al. Adjuvant radiotherapy versus wait-and-see after radical prostatectomy: 10-year follow-up of the ARO 96–02/AUO AP 09/95 trial. Eur Urol 2014;66:243–250. [DOI] [PubMed] [Google Scholar]
- 6.Williams MV, Denekamp J, Fowler JF. A review of alpha/beta ratios for experimental tumors: Implications for clinical studies of altered fractionation. Int J Radiat Oncol Biol Phys 1985;11:87–96. [DOI] [PubMed] [Google Scholar]
- 7.Brenner DJ, Martinez AA, Edmundson GK, et al. Direct evidence that prostate tumors show high sensitivity to fractionation (low alpha/beta ratio), similar to late-responding normal tissue. Int J Radiat Oncol Biol Phys 2002;52:6–13. [DOI] [PubMed] [Google Scholar]
- 8.Ritter M Rationale, conduct, and outcome using hypofractionated radiotherapy in prostate cancer. Semin Radiat Oncol 2008;18:249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halpern JA, Sedrakyan A, Dinerman B, et al. Indications, utilization and complications following prostate biopsy: New York State analysis. J Urol 2017;197:1020–1025. [DOI] [PubMed] [Google Scholar]
- 10.Sher DJ, Parikh RB, Mays-Jackson S, et al. Cost-effectiveness analysis of SBRT versus IMRT for low-risk prostate cancer. Am J Clin Oncol 2014;37:215–221. [DOI] [PubMed] [Google Scholar]
- 11.Muralidhar V, Nguyen PL. Maximizing resources in the local treatment of prostate cancer: A summary of cost-effectiveness studies. Urol Oncol 2017;35:76–85. [DOI] [PubMed] [Google Scholar]
- 12.Cozzarini C, Fiorino C, Di Muzio N, et al. Hypofractionated adjuvant radiotherapy with helical tomotherapy after radical prostatectomy: Planning data and toxicity results of a phase I-II study. Radiother Oncol 2008;88:26–33. [DOI] [PubMed] [Google Scholar]
- 13.Ippolito E, Cellini N, Digesu C, et al. Postoperative intensity-modulated radiotherapy with simultaneous integrated boost in prostate cancer: A dose-escalation trial. Urol Oncol 2013;31:87–92. [DOI] [PubMed] [Google Scholar]
- 14.Krause S, Sterzing F, Neuhof D, et al. Hypofractionated helical intensity-modulated radiotherapy of the prostate bed after prostatectomy with or without the pelvic lymph nodesdthe PRIAMOS trial. BMC Cancer 2012;12:504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kruser TJ, Jarrard DF, Graf AK, et al. Early hypofractionated salvage radiotherapy for postprostatectomy biochemical recurrence. Cancer 2011;117:2629–2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fersino S, Tebano U, Mazzola R, et al. Moderate hypofractionated postprostatectomy volumetric modulated arc therapy with daily image guidance (VMAT-IGRT): A mono-institutional report on feasibility and acute toxicity. Clin Genitourin Cancer 2017;15:e667–e673. [DOI] [PubMed] [Google Scholar]
- 17.Barra S, Belgioia L, Marcenaro M, et al. Moderate hypofractionated radiotherapy after prostatectomy for cancer patients: Toxicity and clinical outcome. Cancer Manag Res 2018;10:473–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wong GW, Palazzi-Churas KL, Jarrard DF, et al. Salvage hypofractionated radiotherapy for biochemically recurrent prostate cancer after radical prostatectomy. Int J Radiat Oncol Biol Phys 2008;70:449–455. [DOI] [PubMed] [Google Scholar]
- 19.Katayama S, Striecker T, Kessel K, et al. Hypofractionated IMRT of the prostate bed after radical prostatectomy: Acute toxicity in the PRIAMOS-1 trial. Int J Radiat Oncol Biol Phys 2014;90:926–933. [DOI] [PubMed] [Google Scholar]
- 20.Cozzarini C, Fiorino C, Deantoni C, et al. Higher-than-expected severe (grade 3–4) late urinary toxicity after postprostatectomy hypofractionated radiotherapy: A single-institution analysis of 1176 patients. Eur Urol 2014;66:1024–1030. [DOI] [PubMed] [Google Scholar]
- 21.Lewis SL, Patel P, Song H, et al. Image guided hypofractionated postprostatectomy intensity modulated radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys 2016;94:605–611. [DOI] [PubMed] [Google Scholar]
- 22.De Meerleer G, Fonteyne V, Meersschout S, et al. Salvage intensity-modulated radiotherapy for rising PSA after radical prostatectomy. Radiother Oncol 2008;89:205–213. [DOI] [PubMed] [Google Scholar]
- 23.Mak RH, Hunt D, Efstathiou JA, et al. Acute and late urinary toxicity following radiation in men with an intact prostate gland or after a radical prostatectomy: A secondary analysis of RTOG 94–08 and 96–01. Urol Oncol 2016;34:430.e1–430.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klayton T, Price R, Buyyounouski MK, et al. Prostate bed motion during intensity-modulated radiotherapy treatment. Int J Radiat Oncol Biol Phys 2012;84:130–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schiffner DC, Gottschalk AR, Lometti M, et al. Daily electronic portal imaging of implanted gold seed fiducials in patients undergoing radiotherapy after radical prostatectomy. Int J Radiat Oncol Biol Phys 2007;67:610–619. [DOI] [PubMed] [Google Scholar]
- 26.Boike TP, Lotan Y, Cho LC, et al. Phase I dose-escalation study of stereotactic body radiation therapy for low- and intermediate-risk prostate cancer. J Clin Oncol 2011;29:2020–2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smeenk RJ, van Lin EN, van Kollenburg P, et al. Endorectal balloon reduces anorectal doses in post-prostatectomy intensity-modulated radiotherapy. Radiother Oncol 2011;101:465–470. [DOI] [PubMed] [Google Scholar]
- 28.Poortmans P, Bossi A, Vandeputte K, et al. Guidelines for target volume definition in post-operative radiotherapy for prostate cancer, on behalf of the EORTC Radiation Oncology Group. Radiother Oncol 2007;84:121–127. [DOI] [PubMed] [Google Scholar]
- 29.Connolly JA, Shinohara K, Presti JC, et al. Local recurrence after radical prostatectomy: Characteristics in size, location, and relationship to prostate-specific antigen and surgical margins. Urology 1996;47:225–231. [DOI] [PubMed] [Google Scholar]
- 30.Kim DW, Cho LC, Straka C, et al. Predictors of rectal tolerance observed in a dose-escalated phase 1–2 trial of stereotactic body radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys 2014; 89:509–517. [DOI] [PubMed] [Google Scholar]
- 31.Kavanagh BD, Pan CC, Dawson LA, et al. Radiation dose-volume effects in the stomach and small bowel. Int J Radiat Oncol Biol Phys 2010;76(3 suppl):S101–S107. [DOI] [PubMed] [Google Scholar]
- 32.Lin Y, Shih WJ. Statistical properties of the traditional algorithmbased designs for phase I cancer clinical trials. Biostatistics 2001;2: 203–215. [DOI] [PubMed] [Google Scholar]
- 33.Skolarus TA, Dunn RL, Sanda MG, et al. Minimally important difference for the Expanded Prostate Cancer Index Composite Short Form. Urology 2015;85:101–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tendulkar RD, Agrawal S, Gao T, et al. Contemporary update of a multi-institutional predictive nomogram for salvage radiotherapy after radical prostatectomy. J Clin Oncol 2016;34:3648–3654. [DOI] [PubMed] [Google Scholar]
- 35.Shipley WU, Seiferheld W, Lukka HR, et al. Radiation with or without antiandrogen therapy in recurrent prostate cancer. N Engl J Med 2017; 376:417–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.King CR. The dose-response of salvage radiotherapy following radical prostatectomy: A systematic review and meta-analysis. Radiother Oncol 2016;121:199–203. [DOI] [PubMed] [Google Scholar]
- 37.Pisansky TM, Agrawal S, Hamstra DA, et al. Salvage radiation therapy dose response for biochemical failure of prostate cancer after prostatectomy–A multi-institutional observational study. Int J Radiat Oncol Biol Phys 2016;96:1046–1053. [DOI] [PubMed] [Google Scholar]
- 38.Cozzarini C, Montorsi F, Fiorino C, et al. Need for high radiation dose (>or=70 gy) in early postoperative irradiation after radical prostatectomy: A single-institution analysis of 334 high-risk, node-negative patients. Int J Radiat Oncol Biol Phys 2009;75:966–974. [DOI] [PubMed] [Google Scholar]
- 39.Ghadjar P, Hayoz S, Bernhard J, et al. Acute toxicity and quality of life after dose-intensified salvage radiation therapy for biochemically recurrent prostate cancer after prostatectomy: First results of the randomized Trial SAKK 09/10. J Clin Oncol 2015;33:4158–4166. [DOI] [PubMed] [Google Scholar]
- 40.King CR, Collins S, Fuller D, et al. Health-related quality of life after stereotactic body radiation therapy for localized prostate cancer: Results from a multi-institutional consortium of prospective trials. Int J Radiat Oncol Biol Phys 2013;87:939–945. [DOI] [PubMed] [Google Scholar]
- 41.King CR, Freeman D, Kaplan I, et al. Stereotactic body radiotherapy for localized prostate cancer: Pooled analysis from a multi-institutional consortium of prospective phase II trials. Radiother Oncol 2013;109:217–221. [DOI] [PubMed] [Google Scholar]
- 42.Paydar I, Cyr RA, Yung TM, et al. Proctitis 1 week after stereotactic body radiation therapy for prostate cancer: Implications for clinical trial design. Front Oncol 2016;6:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Massaccesi M, Cilla S, Deodato F, et al. Hypofractionated intensity-modulated radiotherapy with simultaneous integrated boost after radical prostatectomy: Preliminary results of a phase II trial. Anticancer Res 2013;33:2785–2789. [PubMed] [Google Scholar]
- 44.Sampath S, Yuh P, Frankel P, et al. Prostate bed stereotactic body radiotherapy (PB-SBRT) for postprostatectomy biochemical recurrence: First toxicity results of a phase 1 dose-escalation trial. Int J Radiat Oncol Biol Phys 2016;96(2 suppl):e227–e228. [Google Scholar]
- 45.King CR, Brooks JD, Gill H, et al. Stereotactic body radiotherapy for localized prostate cancer: Interim results of a prospective phase II clinical trial. Int J Radiat Oncol Biol Phys 2009;73:1043–1048. [DOI] [PubMed] [Google Scholar]
- 46.King CR, Brooks JD, Gill H, et al. Long-term outcomes from a prospective trial of stereotactic body radiotherapy for low-risk prostate cancer. Int J Radiat Oncol Biol Phys 2012;82:877–882. [DOI] [PubMed] [Google Scholar]
- 47.Quon HC, Ong A, Cheung P, et al. Once-weekly versus every-other-day stereotactic body radiotherapy in patients with prostate cancer (PATRIOT): A phase 2 randomized trial. Radiother Oncol 2018;127: 206–212. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.