Abstract
Amyloid-β (Aβ) has long been shown to be critical in Alzheimer’s disease pathophysiology. Microglia contributes to the earliest responses to Aβ buildup, by direct interaction through multiple receptors. Microglial cells operate Aβ clearance and trigger inflammatory/regenerative processes that take place in the long years of silent disease progression that precede symptomatic appearance. But in time and with aging, the fine balance between pro- and anti-inflammatory activity of microglia deranges, negatively impacting its Aβ-clearing ability. Furthermore, in recent years, microglial activation has proven to be much more complex than the mere dichotomic pro/anti-inflammatory polarization previously accepted. Microglia can display a wide spectrum of phenotypes, which can even be mixed. On these bases, it is evident that while pharmacological intervention aiding microglia to prolong its ability to cope with Aβ buildup could be extremely relevant, its feasibility is hampered by such high complexity, which still needs to be completely understood.
Keywords: Alzheimer’s disease, β-amyloid receptors, TREM2, CD33, neuroinflammation, microglial activation
1. Introduction
Alzheimer’s Disease (AD) is a slow-developing neurodegenerative pathology representing the dominant worldwide cause of dementia in the elderly [1, 2]. Sporadic forms of AD become symptomatic by the age of 65 and over, but its molecular triggers affect selective areas of the brain decades earlier [1, 3]. Endogenous protective responses have been described, attempting to compensate for the pathological loss of function during the initial stages of AD [4, 5]. These events could account for the extremely slow progression of neurodegeneration. Compensation has also been proposed to explain why some individuals display a great degree of resilience and preserve cognitive functions even in the presence of pathological hallmarks [4, 6]. Accumulation of extracellular β-amyloid protein (Aβ) to generate plaques, and abnormal phosphorylation of intracellular tau protein causing disruption of neuronal functions, are still considered the key elements in AD [7]. Accordingly, the “amyloid cascade hypothesis” that involves both events, initially proposed about 20 years ago, then revised and updated, is still the paramount paradigm [8, 9]. Unfortunately, up to now, targeting solely Aβ and/or tau has not proven to be a winning therapeutic strategy [2], as no drug in development, acting as anti-Aβ or anti-p-tau, has yet successfully completed its program to the clinics. Understanding the sequence of events that occur in the slow course of AD development appears mandatory to identify targets for an early, preventive intervention. In this sense, it is worthwhile to revisit the role played by microglia in the early molecular steps that pave the way to AD onset. Microglial activation has traditionally been linked to neuroinflammation and thus interpreted as a fundamentally negative event [10]. However, in recent years it has become more evident that microglia-sustained inflammatory responses can initially be beneficial and can remain such if timely converted into reparative and inflammation-resolving ones [11]. In particular, in AD, early microglial activation to phagocyte accumulating Aβ is anti-inflammatory in nature and precedes the appearance of a pro-inflammatory and potentially harmful phenotype observed at later stages, when amyloid load increases. We will here review the role played by microglia in AD, taking into account the dynamics of its activation, its ability to interact with Aβ and the real potential of targeting microglia for pharmacological intervention.
2. Redefining microglia in AD: from the M1/M2 dichotomy to the phenotypic activation continuum
Emerging evidence indicates that the involvement of non-neuronal cells cannot merely be considered a corollary to the progression of neuronal damage, but appears crucial in the sequence of neuropathological events [6, 12-15]. It is worth noting that the central role of microglia has been corroborated by genome-wide association studies and, accordingly, several of the mutations recognized as risk factors for sporadic AD are predominantly expressed in microglia [16, 17]. In addition, the above mentioned compensatory mechanisms, that may significantly intervene on disease development and progression, primarily rely on non-neuronal cells, mainly the microglia population. Microglial cells, physiologically exert a dual role. They are in fact considered the resident immune cells of the central nervous system (CNS) aimed at maintaining CNS homeostasis, but, at the same time, they behave as sensors, able to become reactive to any perturbing signal to the system [18]. A general simplified classification in two distinct phenotypes, M1 and M2 is largely accepted within the scientific community [19]. The M1 is a pro-inflammatory phenotype able to release several molecules, including cytokines, chemokines and growth factors, all endowed with pro-inflammatory properties, and thus actively involved in neuroinflammatory processes [20, 21]. M2 polarization is also referred to as an “alternative activation”, characterized by the release of anti-inflammatory cytokines and neurotrophins and mainly involved in reparative and restorative processes that take place during the resolution of acute inflammatory events [21]. Despite this consolidated classification, the current trend acknowledges microglia in a myriad of different activation states that can exhibit opposite properties, according to what largely described, but also intermediate features to represent a continuum between the two extreme phenotypes [22].
The hypothesis that microglia activation state can impact AD is now well supported and, certainly, admission of neuroinflammation as a central event in the neurodegenerative process has also contributed to revise the initial “amyloid cascade hypothesis” [23]. According to the more recent view, inflammation and Aβ deposition are strictly interconnected so that, reciprocally, one facilitates the other to generate a vicious cycle that characterizes disease progression [23]. Microglia uptake and degrade Aβ [24, 25], and this property has been previously associated with the M2-like phenotype, whereas it has been shown to be markedly impaired in M1-like activated cells [19, 24, 26]. More interestingly, the transition from one state of activation to another has been shown to occur even in a stage-dependent manner during the progression of disease in the APP/PS1 AD animal model [27]. The switch from M2 towards M1 polarization was, in this case, evident as pathology worsened. Strikingly, however, a number of in vivo studies on AD models have reported that even anti-inflammatory cytokines such as IL-10, IL-4 or TGF-β can negatively impact plaque pathology [28-31]. IL-10 signaling, in particular, has been linked to a reduced ability to clear Aβ, which can in turn further affect microglial activation and promote toxicity [28, 30]. The lesson learned from this evidence, which further confirms microglial complexity, is that the general idea that just promoting an anti-inflammatory phenotype will automatically have a beneficial outcome is not quite correct. Instead, we need to keep in mind that polarization of microglia in AD is not exclusively harmful or beneficial, but has to be finely tuned to prevent dysfunction. Any potentially viable therapeutic strategy meant to promote the transition from M1 to M2, or for the “continuum concept” described above, from a M1-like to a more M2-prone phenotype, will then require a deep knowledge of the context-dependent responses of microglia in order to avoid perturbing a very delicate equilibrium (Fig. 1).
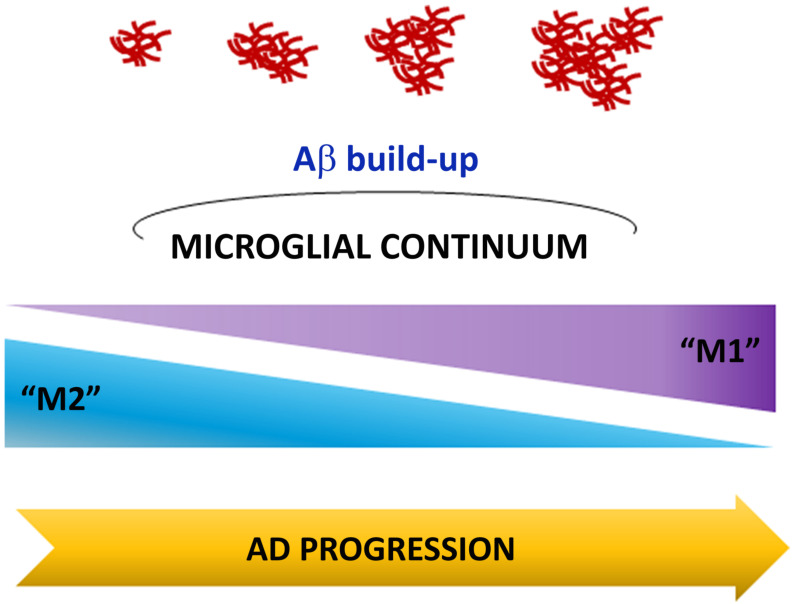
Fig. (1).
The microglial continuum. During Alzheimer’s disease progression, microglia dynamically change in a continuum from two different, but potentially overlapping states of activation (M2 and M1 phenotypes), that eventually converge towards a prevalent chronic inflammatory condition. This event leads to neurotoxic accumulation of Aβ peptide. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
3. AΒ interactions at the cell surface: where it all starts
The direct interaction of Aβ oligomers with cellular targets is the key event that triggers the very beginning of the complex signaling that gives life to the “amyloid cascade”. Aβ has not been found to have selective cognate receptors but is able to bind to all cell types in the CNS through a variety of binding partners at the cell surface. Interest has been mostly focused on neurons, which synthesize Aβ and are the main sufferers of its toxicity, so that interactions of Aβ with neuronal surface molecules have been more deeply analyzed and described. At this level, Aβ has been reported to interact, among others, with NMDA glutamate receptors, Na/K ATPases, insulin receptors, prion protein receptors and integrins [32-36]. Particularly relevant is the interaction with α1-integrins, which was shown to prompt aberrant neuronal cell cycle re-entry, dependent on DNA polymerase β (polβ) [37-39]. Polβ-mediated cell cycle activation leads to neuronal death and, consistently, polβ inhibitors were shown to be neuroprotective [40, 41]. In addition, Aβ can directly modify the membrane lipid bilayer, altering permeability and excitability [42-45]. Finally, Aβ is actively internalized by different receptors such as insulin receptors, the receptor for advanced glycation end-products (RAGE), low-density lipoprotein receptor-related protein 1 and nicotinic acetylcholine receptors [46]. Once inside, Aβ oligomers contribute to the generation of reactive oxidant species [47-49], alter mitochondrial function [50, 51], and more generally disrupt neuronal homeostasis determining cell damage [52].
Whereas Aβ interaction with neurons is responsible for direct cell damage, interactions at the microglial level comprise a multifaceted response. Aβ-microglia contacts are mediated by a variety of receptors and represent the first, necessary step for the clearance of the peptide [53]. At the same time, such interactions lead to activation of signaling cascades that are determinants of the destiny of microglia polarization and thus, indirectly, for the state of neurons. The acute response to the buildup of Aβ includes microglia activation aimed at eliminating the peptide. Much of what happens next depends on the ability of microglia to keep up the rate of Aβ clearance to balance its overproduction to prevent its accumulation. The imbalance in these processes is a primary cause for a microglial switch towards a phenotype unable to control Aβ pathology and lacking protective functions [12, 54]. In agreement, aging has been related to impaired microglial phagocytic ability and is considered a key risk factor in AD as well as other age-associated neurological disorders [55, 56].
Many of the receptors that mediate binding to Aβ on microglia are shared with neurons; among these are complement receptors [53, 57], Fc receptors [58], Formyl Peptide Receptors [59], scavenger receptors A, Cluster of differentiation 36 (CD36) [60], Toll-Like Receptors [61] and RAGE [62]. A peculiar binding molecule for Aβ is High Mobility Group Box 1 (HMGB1), a non-histone chromosomal protein, whose function is not limited to the control of transcription. It, in fact, behaves as an inflammatory mediator in several pathological conditions, being released by injured and inflammatory cells [63-65]. Accordingly, it is present in high concentrations in neuroinflammatory processes in the AD brain where, within the plaque, it associates with Aβ contrasting its phagocytosis by microglial cells [66, 67]. Interestingly in cultured microglia, HMGB1 directly binds to and stabilizes Aβ1-42 oligomers, thereby reducing microglial uptake [67]. Hence HMGB1 behaves as an extracellular binding site for Aβ, impairing its phagocytosis and clearance by microglia. Moreover, HMGB1-bound Aβ1-40 is internalized in microglia and increases in the cytoplasm, but its degradation is inhibited [67]. The high levels of HMGB1 in AD and its co-localization with microglia in senile plaques make this molecule a risk factor for neurodegeneration, that can participate in AD pathophysiology by impairing the early “M2-like” functions of microglia [67]. The involvement of HMGB1 in AD pathophysiology and, in particular, in microglia clearing function, appears more intriguing since HMGB1, in other cellular systems, has been shown to favor cell migration [68, 69], a property that is peculiar to activated microglia and also to induce proliferation and differentiation of neural precursors towards the neuronal lineage [70].
A number of receptors with binding affinity for Aβ are selectively or predominantly expressed in microglia, and among these, perhaps the most relevant are the triggering receptor expressed on myeloid cells 2 (TREM2) and CD33 (Fig. 2). Large-scale genetic studies have contributed to the identification of these receptors as risk factors for AD [71], which represent ideal candidate targets in the strategy to steer microglia towards compensatory/restorative activation. We will here discuss current knowledge on the roles of these two microglial receptors, whose functions have recently been shown to be intertwined.
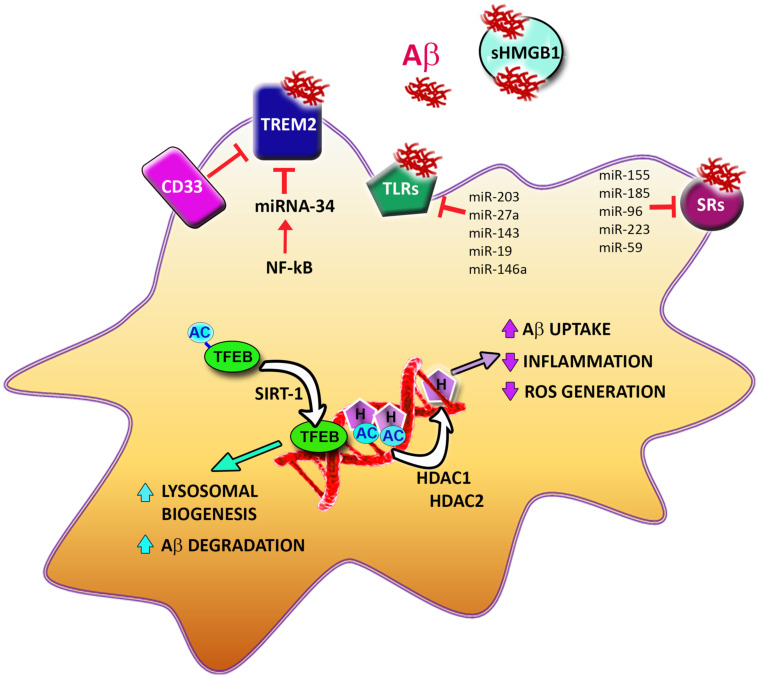
Fig. (2).
Microglial interactions with Aβ and epigenetic mechanisms controlling Aβ removal. At the microglial cell surface, aggregated Aβ interacts with the triggering receptor expressed on myeloid cells 2 (TREM2), Toll Like Receptors (TLRs) and Scavenger Receptors (SRs), which all mediate its uptake. CD33 negatively affects TREM2’s ability to bind Aβ. Soluble High Mobility Group Box 1 (sHMGB1) binds to Aβ in the extracellular compartment, preventing its uptake. Epigenetic mechanisms affect multiple steps involved in Aβ removal. NF-kB increases transcription of miRNA-34, which represses TREM2 production. A number of other miRNAs regulate the expression of TLRs and SRs. The transcription factor EB (TFEB) is activated by SIRT1-mediated deacetylation and upregulates genes involved in lysosome biogenesis and Aβ degradation. Histone (H) deacetylation by histone deacetylases (HDAC) 1 and 2 leads to upregulation of genes involved in Aβ uptake and downregulation of genes involved in inflammation and radical oxygen species (ROS) generation. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
TREM2 belongs to the TREM family of receptors that are cell surface glycoproteins with immunoglobulin-like extracellular domains and short cytoplasmic tails. Aβ has been shown to bind TREM2 with nanomolar affinity [72, 73] and gene mutations that compromise this affinity are evidenced as risk factors linked to AD [74, 75]. This observation, together with the bulk of the work on TREM2 in AD, both in vitro and in vivo, have mainly related Aβ-TREM2 interaction to the mediation of Aβ clearance and containment of plaque formation [72, 76, 77]. In particular, Aβ binding was shown to favor interaction between TREM2 and adaptor protein DAP12, required for downstream signaling leading to Aβ clearance [72]. Lack of TREM2 affected nascent plaques, which appeared less compacted, with a negative impact on neurites [78, 79]. Similarly, the induction of TREM2 expression in AD mice, with a transgenic approach, revealed a dose-dependent beneficial effect. In this case, activation of genes
involved in phagocytosis and resolution of inflammation preceded activation of genes associated with neurodegenerative events [77]. This is perfectly in line with the idea of a compensatory response set up by microglia being associated with the initial appearance of Aβ aggregates. Apparently, contradictory results came from another study, linking the amelioration of AD pathology to TREM2 deficiency in AD mice [80]. Such discrepancy could be accounted by the different time points tested in different studies, and therefore by the existence of stage-dependent effects [81, 82]. In agreement, using animals of different ages, it was recently shown that Aβ deposition was lower at early time points but exacerbated at later stages [83]. This observation still awaits to be completely understood, but current hypotheses suggest both a differential role for TREM2 in resident microglia vs. infiltrated macrophages, and a differential involvement of these two cell types during disease development [81, 83]. In particular, TREM2 expression would have a beneficial role in microglia and a negative one in macrophages [81]. In addition to its regulatory role in phagocytic functions, TREM2 was shown to promote microglial survival through downstream signaling that involves the Wnt/β-catenin pathway [84]. To further complicate the picture, ADAM proteases were shown to release a soluble ectodomain of TREM2 (sTREM2), the real role of which is still a matter of debate. Results from animal models showed sTREM2 to enhance microglial pro-inflammatory cytokine expression, thus suggesting an action opposite to that of its transmembrane counterpart [85]. On the other hand, evidence from clinical studies pointed to a decrease of sTREM2 in the CSF from patients in early but asymptomatic phases of AD [86], and an increase of sTREM2 in CSF from patients in early symptomatic stages [87, 88]. Interestingly, sTREM2 decrease coincided with abnormal Aβ, but not tau, pathology and correlated with the absence of neurodegeneration [86]. Its increase appeared modulated by membrane-spanning 4-domains subfamily A (MS4A) proteins [88] and was linked to later age of onset and slower disease progression [87]. Using a model for ectopic expression of TREM2, a recent study provided insight into the possible distinct roles for TREM2 and its soluble domain: the former appears to be associated with phagocytosis promotion and the latter required for pro-inflammatory signaling [89].
CD33 belongs to the sialic acid-binding immunoglobulin-type lectin family and is expressed on the cell surface where it takes part in cell adhesion and proliferative processes as well as modulation of endocytosis and cytokine release [90]. In monocytes, CD33 has been suggested to modulate the activation state and regulate the release of inflammatory cytokines by sensing levels of sialic acid in the microenvironment [91]. Great interest has revolved around this protein since its identification as a genetic risk factor for AD in genome-wide association studies [92]. CD33 interacts with Aβ selectively through its extracellular sialic-binding domain [93]. The deletion of the specific CD33 mRNA exon coding for the sialic-binding domain of the protein was in fact associated with a decreased risk for AD [94]. Following Aβ binding, an intracellular tyrosine-inhibitory domain becomes phosphorylated and cross-activates Src homology 2 domain-containing phosphatases, that inhibits downstream targets involved in phagocytosis [93]. So, overall, CD33 dampens the microglial ability to clear Aβ by inhibiting phagocytosis. Accordingly, studies on CD33-/- AD mice have shown increased microglial uptake and clearance of Aβ and have revealed that CD33 acts upstream of TREM2, leading to increased microglial pathology [95, 96]. This is in agreement with the evidence that CD33 and TREM2 exert opposing effects on microglia. Furthermore, the tight relationship between the two receptors was confirmed by the observation that CD33 knockout in AD mice was only beneficial if functional TREM2 was preserved [95]. Consistent with preclinical evidence, CD33 was also found to be increased in microglial cells from AD patients [96].
4. Epigenetic mechanisms as potential targets to aid microglial AΒ clearance
As described so far, the ability of microglia to effectively remove Aβ is central in neuroprotection against the harmful consequences of peptide buildup. In this regard, epigenetic mechanisms, such as histone deacetylation and translational repression by microRNAs, could play an important role by modulating genes coding for proteins involved in microglial clearance functions. Notably, epigenetic modulation of microglia-operated clearance is already known to occur during development, when synaptic pruning and removal of apoptotic neuronal debris take place [97, 98]. Accordingly, in the absence of dying neurons, microglial genes coding for proteins involved in clearance functions are downregulated [99]. Epigenetic mechanisms shown to impact Aβ clearance comprise histone deacetylation and translational repression by microRNAs. In 5x FAD mice, selective genetic ablation of the transcriptional repressors histone deacetylase (HDAC) 1 and 2 in microglia, was shown to significantly increase Aβ phagocytosis and improve cognitive impairment [100]. These effects were mediated by the upregulation of genes involved in microglial clearance functions and downregulation of pro-inflammatory and ROS-generating genes [100]. On the opposite, HDAC3 was shown to negatively regulate spatial memory in APP/PS1, where its overexpression increased amyloid deposition while its inhibition led to decreased Aβ levels and plaque burden [101]. Consistent results were observed from a study on 3x Tg mice, where inhibition of HDAC3 correlated with decreased Aβ1-42 protein level, as well as improved spatial learning and memory [102]. Aβ degradation was shown to be regulated by epigenetic modulation of microglial lysosomal pathways, mediated by transcription factor EB (TFEB). In particular, TFEB acetylation by SIRT1, a deacetylase endowed with several neuroprotective properties, appeared to enhance transcriptional upregulation of downstream genes involved in lysosomal biogenesis. This, in turn, facilitated Aβ degradation [103]. Among miRNAs, particularly relevant appears to be the role of miRNA-34a, shown to significantly down-regulate TREM2 in a murine microglial cell line [104] and in the hippocampus from sporadic AD patients, compared to age-matched controls [105]. Furthermore, miRNA-34a transcription was shown to be positively regulated by inflammatory transcription factor NF-kB [104, 105].
Interestingly, the use of a selective antagomir was able to prevent TREM2 downregulation in a functional assay in murine microglial cells [105]. In addition, a number of other micro-RNAs have been shown to affect the expression of cell surface molecules necessary for Aβ phagocytosis, such as toll-like receptors (TLR) and scavenger receptors (SR) [106]. Among these are miR-203, miR-27a, miR-143, miR-19, and miR-146a, involved in TLR modulation and miR-155, miR-185, miR-96, miR-223, and miR-59, involved in SR modulation [106]. The mentioned mechanisms of epigenetic control of microglial function, impacting Aβ clearance, are illustrated in Fig. 2.
5. Challenges and perspectives
Almost 30 years have passed since the role of Aβ has found a definition in the amyloid cascade hypothesis, but the cure for AD still appears to be far out of our reach. What has clearly emerged in these years is the complexity of Aβ-cellular interactions, given its ability to bind to several different receptors, both transmembrane and soluble, on different cell types. This exponentially amplifies the signaling pathways involved in AD pathogenesis, i.e. the potential therapeutical targets implicated in disease onset. To further complicate this picture, the strict stage-dependence of the actual effects of Aβ buildup makes it particularly hard to identify the right opportunity for intervention in a disease that develops over decades. By the time symptoms become even slightly evident, neuronal damage is so advanced that it appears unlikely that neuronal function could be rescued. This would in part explain the persistent failure of direct Aβ-targeting therapies [107]. Microglial cells deserve particular attention now that it is ascertained that they are not mere spectators but the main characters in AD pathogenesis. Microglia are early responders to Aβ and the way they are inclined to react in a particular context, in a particular time and a particular individual, will affect susceptibility to disease progression. The debate on the real function of microglia in the development of Aβ toxicity is not certainly new and, in this context, the contraposing concept of M1-M2 phenotypes, as mentioned above, needs to be overcome. We should move to a broader view of much more numerous and complex microglia activation states that occur, probably, but not necessarily, in time sequence. Different microglia types can in fact even coexist at the same time [108], as they can proliferate, phagocyte cellular debris, express markers of activation such as cytokines, but also release neuroprotective and neurotrophic factors [98, 109, 110]. Microglia can also progressively evolve toward disease-associated microglia (DAM) preferentially localized in the proximity of Aβ plaques with a definite protective function [111]. Accordingly, both in AD human brains and animal models, the absence of microglia worsens the pathological events [112]. However, synaptic pruning and consequent cognitive impairments have been also described in AD animal models and related to microglia function [113].
The question should then be what is the real druggability of microglia as a target in AD and whether those identified specific microglia markers can represent valuable targets. Certainly, identification of microglial TREM2 and the vast knowledge gathered on its function in the last several years, make this molecule one of the most promising targets. However, the literature on TREM2 so far appears still hazy and confirms the existence of a time-course of differential expression and function even for this crucial receptor. This, once again, highlights the undeferrable need to identify the right time window in order for any therapeutic intervention to be successful. Finally, controversies still exist on whether TREM2 should be considered only a promising potential biomarker of disease onset and progression or whether it can be viewed as a non-neuronal pharmacologically or genetically modifiable target. Even if attempts to modify TREM2 expression have been carried out and related to disease severity in AD animal models [77], the possibility to translate this into clinical research is still full of challenges. Even more complicated is the possibility to hit CD33, due to its specific molecular nature and the consequent paucity of identified ligands [17] and also to the species-specific contrasting roles ascribed to this membrane molecule. Hence, microglial specific targets appear promising but much has to be done to identify the appropriate tools to move in the direction to selectively hit them.
Acknowledgements
This work was supported by PRIN 2017 to MAS.
Consent for Publication
Not applicable.
Funding
None.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
References
- 1.Frozza R.L., Lourenco M.V., De Felice F.G. Challenges for Alzheimer’s disease therapy: Insights from novel mechanisms beyond memory defects. Front. Neurosci. 2018;12:37. doi: 10.3389/fnins.2018.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cummings J., Lee G., Ritter A., Zhong K. Alzheimer’s disease drug development pipeline: 2018. Alzheimers Dement. (N. Y.) 2018;4:195–214. doi: 10.1016/j.trci.2018.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sperling R., Mormino E., Johnson K. The evolution of preclinical Alzheimer’s disease: Implications for prevention trials. Neuron. 2014;84(3):608–622. doi: 10.1016/j.neuron.2014.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Merlo S., Spampinato S.F., Sortino M.A. Early compensatory responses against neuronal injury: A new therapeutic window of opportunity for Alzheimer’s Disease? CNS Neurosci. Ther. 2019;25(1):5–13. doi: 10.1111/cns.13050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merlo S., Spampinato S.F., Beneventano M., Sortino M.A. The contribution of microglia to early synaptic compensatory responses that precede β-amyloid-induced neuronal death. Sci. Rep. 2018;8(1):7297. doi: 10.1038/s41598-018-25453-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Strooper B., Karran E. The cellular phase of Alzheimer’s disease. Cell. 2016;164(4):603–615. doi: 10.1016/j.cell.2015.12.056. [DOI] [PubMed] [Google Scholar]
- 7.Chong F.P., Ng K.Y., Koh R.Y., Chye S.M. Tau Proteins and Tauopathies in Alzheimer’s Disease. Cell. Mol. Neurobiol. 2018;38(5):965–980. doi: 10.1007/s10571-017-0574-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hardy J., Selkoe D.J. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297(5580):353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 9.Cline E.N., Bicca M.A., Viola K.L., Klein W.L. The amyloid-β oligomer hypothesis: beginning of the third decade. J. Alzheimers Dis. 2018;64(s1):S567–S610. doi: 10.3233/JAD-179941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kinney J.W., Bemiller S.M., Murtishaw A.S., Leisgang A.M., Salazar A.M., Lamb B.T. Inflammation as a central mechanism in Alzheimer’s disease. Alzheimers Dement. (N. Y.) 2018;4:575–590. doi: 10.1016/j.trci.2018.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caraci F., Merlo S., Drago F., Caruso G., Parenti C., Sortino M.A. Rescue of noradrenergic system as a novel pharmacological strategy in the treatment of chronic pain: focus on microglia activation. Front. Pharmacol. 2019;10:1024. doi: 10.3389/fphar.2019.01024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai Z., Hussain M.D., Yan L.J. Microglia, neuroinflammation, and beta-amyloid protein in Alzheimer’s disease. Int. J. Neurosci. 2014;124(5):307–321. doi: 10.3109/00207454.2013.833510. [DOI] [PubMed] [Google Scholar]
- 13.Webers A., Heneka M.T., Gleeson P.A. The role of innate immune responses and neuroinflammation in amyloid accumulation and progression of Alzheimer’s disease. Immunol. Cell Biol. 2020;98(1):28–41. doi: 10.1111/imcb.12301. [DOI] [PubMed] [Google Scholar]
- 14.Minter M.R., Taylor J.M., Crack P.J. The contribution of neuroinflammation to amyloid toxicity in Alzheimer’s disease. J. Neurochem. 2016;136(3):457–474. doi: 10.1111/jnc.13411. [DOI] [PubMed] [Google Scholar]
- 15.Beneventano M., Spampinato S.F., Merlo S., Chisari M., Platania P., Ragusa M., Purrello M., Nicoletti F., Sortino M.A. Shedding of microvesicles from microglia contributes to the effects induced by metabotropic glutamate receptor 5 activation on neuronal death. Front. Pharmacol. 2017;8:812. doi: 10.3389/fphar.2017.00812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Takatori S., Wang W., Iguchi A., Tomita T. Genetic risk factors for alzheimer disease: emerging roles of microglia in disease pathomechanisms. Adv. Exp. Med. Biol. 2019;1118:83–116. doi: 10.1007/978-3-030-05542-4_5. [DOI] [PubMed] [Google Scholar]
- 17.Biber K., Bhattacharya A., Campbell B.M., Piro J.R., Rohe M., Staal R.G.W., Talanian R.V., Möller T. Microglial drug targets in ad: opportunities and challenges in drug discovery and development. Front. Pharmacol. 2019;10:840. doi: 10.3389/fphar.2019.00840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wolf S.A., Boddeke H.W., Kettenmann H. Microglia in physiology and disease. Annu. Rev. Physiol. 2017;79:619–643. doi: 10.1146/annurev-physiol-022516-034406. [DOI] [PubMed] [Google Scholar]
- 19.Cherry J.D., Olschowka J.A., O’Banion M.K. Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J. Neuroinflammation. 2014;11:98. doi: 10.1186/1742-2094-11-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Du L., Zhang Y., Chen Y., Zhu J., Yang Y., Zhang H.L. Role of microglia in neurological disorders and their potentials as a therapeutic target. Mol. Neurobiol. 2017;54(10):7567–7584. doi: 10.1007/s12035-016-0245-0. [DOI] [PubMed] [Google Scholar]
- 21.Kabba J.A., Xu Y., Christian H., Ruan W., Chenai K., Xiang Y., Zhang L., Saavedra J.M., Pang T. Microglia: Housekeeper of the central nervous system. Cell. Mol. Neurobiol. 2018;38(1):53–71. doi: 10.1007/s10571-017-0504-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ransohoff R.M. A polarizing question: do M1 and M2 microglia exist? Nat. Neurosci. 2016;19(8):987–991. doi: 10.1038/nn.4338. [DOI] [PubMed] [Google Scholar]
- 23.Karran E., Mercken M., De Strooper B. The amyloid cascade hypothesis for Alzheimer’s disease: an appraisal for the development of therapeutics. Nat. Rev. Drug Discov. 2011;10(9):698–712. doi: 10.1038/nrd3505. [DOI] [PubMed] [Google Scholar]
- 24.Koenigsknecht-Talboo J., Landreth G.E. Microglial phagocytosis induced by fibrillar beta-amyloid and IgGs are differentially regulated by proinflammatory cytokines. J. Neurosci. 2005;25(36):8240–8249. doi: 10.1523/JNEUROSCI.1808-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weldon D.T., Rogers S.D., Ghilardi J.R., Finke M.P., Cleary J.P., O’Hare E., Esler W.P., Maggio J.E., Mantyh P.W. Fibrillar beta-amyloid induces microglial phagocytosis, expression of inducible nitric oxide synthase, and loss of a select population of neurons in the rat CNS in vivo. J. Neurosci. 1998;18(6):2161–2173. doi: 10.1523/JNEUROSCI.18-06-02161.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cherry J.D., Olschowka J.A., O’Banion M.K. Are “resting” microglia more “m2”? Front. Immunol. 2014;5:594. doi: 10.3389/fimmu.2014.00594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jimenez S., Baglietto-Vargas D., Caballero C., Moreno-Gonzalez I., Torres M., Sanchez-Varo R., Ruano D., Vizuete M., Gutierrez A., Vitorica J. Inflammatory response in the hippocampus of PS1M146L/APP751SL mouse model of Alzheimer’s disease: age-dependent switch in the microglial phenotype from alternative to classic. J. Neurosci. 2008;28(45):11650–11661. doi: 10.1523/JNEUROSCI.3024-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chakrabarty P., Li A., Ceballos-Diaz C., Eddy J.A., Funk C.C., Moore B., DiNunno N., Rosario A.M., Cruz P.E., Verbeeck C., Sacino A., Nix S., Janus C., Price N.D., Das P., Golde T.E. IL-10 alters immunoproteostasis in APP mice, increasing plaque burden and worsening cognitive behavior. Neuron. 2015;85(3):519–533. doi: 10.1016/j.neuron.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chakrabarty P., Tianbai L., Herring A., Ceballos-Diaz C., Das P., Golde T.E. Hippocampal expression of murine IL-4 results in exacerbation of amyloid deposition. Mol. Neurodegener. 2012;7:36. doi: 10.1186/1750-1326-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guillot-Sestier M.V., Doty K.R., Gate D., Rodriguez J., Jr Leung, B.P.; Rezai-Zadeh, K.; Town, T. Il10 deficiency rebalances innate immunity to mitigate Alzheimer-like pathology. Neuron. 2015;85(3):534–548. doi: 10.1016/j.neuron.2014.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Town T., Laouar Y., Pittenger C., Mori T., Szekely C.A., Tan J., Duman R.S., Flavell R.A. Blocking TGF-beta-Smad2/3 innate immune signaling mitigates Alzheimer-like pathology. Nat. Med. 2008;14(6):681–687. doi: 10.1038/nm1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DiChiara T., DiNunno N., Clark J., Bu R.L., Cline E.N., Rollins M.G., Gong Y., Brody D.L., Sligar S.G., Velasco P.T., Viola K.L., Klein W.L. Alzheimer’s toxic amyloid beta oligomers: unwelcome visitors to the na/k atpase alpha3 docking station. Yale J. Biol. Med. 2017;90(1):45–61. [PMC free article] [PubMed] [Google Scholar]
- 33.De Felice F.G., Vieira M.N., Bomfim T.R., Decker H., Velasco P.T., Lambert M.P., Viola K.L., Zhao W.Q., Ferreira S.T., Klein W.L. Protection of synapses against Alzheimer’s-linked toxins: insulin signaling prevents the pathogenic binding of Abeta oligomers. Proc. Natl. Acad. Sci. USA. 2009;106(6):1971–1976. doi: 10.1073/pnas.0809158106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao W.Q., De Felice F.G., Fernandez S., Chen H., Lambert M.P., Quon M.J., Krafft G.A., Klein W.L. Amyloid beta oligomers induce impairment of neuronal insulin receptors. FASEB J. 2008;22(1):246–260. doi: 10.1096/fj.06-7703com. [DOI] [PubMed] [Google Scholar]
- 35.Texidó L., Martín-Satué M., Alberdi E., Solsona C., Matute C. Amyloid β peptide oligomers directly activate NMDA receptors. Cell Calcium. 2011;49(3):184–190. doi: 10.1016/j.ceca.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y., Zhao Y., Zhang L., Yu W., Wang Y., Chang W. Cellular prion protein as a receptor of toxic amyloid-β42 oligomers is important for Alzheimer’s disease. Front. Cell. Neurosci. 2019;13:339. doi: 10.3389/fncel.2019.00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Copani A., Caraci F., Hoozemans J.J., Calafiore M., Sortino M.A., Nicoletti F. The nature of the cell cycle in neurons: focus on a “non-canonical” pathway of DNA replication causally related to death. Biochim. Biophys. Acta. 2007;1772(4):409–412. doi: 10.1016/j.bbadis.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 38.Frasca G., Carbonaro V., Merlo S., Copani A., Sortino M.A. Integrins mediate beta-amyloid-induced cell-cycle activation and neuronal death. J. Neurosci. Res. 2008;86(2):350–355. doi: 10.1002/jnr.21487. [DOI] [PubMed] [Google Scholar]
- 39.Herrup K., Neve R., Ackerman S.L., Copani A. Divide and die: cell cycle events as triggers of nerve cell death. J. Neurosci. 2004;24(42):9232–9239. doi: 10.1523/JNEUROSCI.3347-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Merlo S., Basile L., Giuffrida M.L., Sortino M.A., Guccione S., Copani A. Identification of 5-Methoxyflavone as a novel dna polymerase-beta inhibitor and neuroprotective agent against beta-amyloid toxicity. J. Nat. Prod. 2015;78(11):2704–2711. doi: 10.1021/acs.jnatprod.5b00621. [DOI] [PubMed] [Google Scholar]
- 41.Copani A., Sortino M.A., Caricasole A., Chiechio S., Chisari M., Battaglia G., Giuffrida-Stella A.M., Vancheri C., Nicoletti F. Erratic expression of DNA polymerases by beta-amyloid causes neuronal death. FASEB J. 2002;16(14):2006–2008. doi: 10.1096/fj.02-0422fje. [DOI] [PubMed] [Google Scholar]
- 42.Canale C., Seghezza S., Vilasi S., Carrotta R., Bulone D., Diaspro A., San Biagio P.L., Dante S. Different effects of Alzheimer’s peptide Aβ(1-40) oligomers and fibrils on supported lipid membranes. Biophys. Chem. 2013;182:23–29. doi: 10.1016/j.bpc.2013.07.010. [DOI] [PubMed] [Google Scholar]
- 43.Kagan B.L., Hirakura Y., Azimov R., Azimova R., Lin M.C. The channel hypothesis of Alzheimer’s disease: Current status. Peptides. 2002;23(7):1311–1315. doi: 10.1016/S0196-9781(02)00067-0. [DOI] [PubMed] [Google Scholar]
- 44.Bode D.C., Baker M.D., Viles J.H. Ion channel formation by amyloid-β42 oligomers but not amyloid-β40 in cellular membranes. J. Biol. Chem. 2017;292(4):1404–1413. doi: 10.1074/jbc.M116.762526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Drolle E., Hane F., Lee B., Leonenko Z. Atomic force microscopy to study molecular mechanisms of amyloid fibril formation and toxicity in Alzheimer’s disease. Drug Metab. Rev. 2014;46(2):207–223. doi: 10.3109/03602532.2014.882354. [DOI] [PubMed] [Google Scholar]
- 46.Mohamed A., Posse de Chaves E. Aβ internalization by neurons and glia. Int. J. Alzheimers Dis. 2011;2011:127984. doi: 10.4061/2011/127984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheignon C., Tomas M., Bonnefont-Rousselot D., Faller P., Hureau C., Collin F. Oxidative stress and the amyloid beta peptide in Alzheimer’s disease. Redox Biol. 2018;14:450–464. doi: 10.1016/j.redox.2017.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Caruso G., Fresta C.G., Musso N., Giambirtone M., Grasso M., Spampinato S.F., Merlo S., Drago F., Lazzarino G., Sortino M.A., Lunte S.M., Caraci F. Carnosine prevents aβ-induced oxidative stress and inflammation in microglial cells: a key role of TGF-β1. Cells. 2019;8(1):E64. doi: 10.3390/cells8010064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Caruso G., Spampinato S.F., Cardaci V., Caraci F., Sortino M.A., Merlo S. beta-amyloid and oxidative stress: perspectives in drug development. Curr. Pharm. Des. 2019;25(45):4771–4781. doi: 10.2174/1381612825666191209115431. [DOI] [PubMed] [Google Scholar]
- 50.De Felice F.G., Velasco P.T., Lambert M.P., Viola K., Fernandez S.J., Ferreira S.T., Klein W.L. Abeta oligomers induce neuronal oxidative stress through an N-methyl-D-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J. Biol. Chem. 2007;282(15):11590–11601. doi: 10.1074/jbc.M607483200. [DOI] [PubMed] [Google Scholar]
- 51.Reddy P.H. Amyloid beta, mitochondrial structural and functional dynamics in Alzheimer’s disease. Exp. Neurol. 2009;218(2):286–292. doi: 10.1016/j.expneurol.2009.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mattson M.P., Cheng B., Davis D., Bryant K., Lieberburg I., Rydel R.E. beta-Amyloid peptides destabilize calcium homeostasis and render human cortical neurons vulnerable to excitotoxicity. J. Neurosci. 1992;12(2):376–389. doi: 10.1523/JNEUROSCI.12-02-00376.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Doens D., Fernández P.L. Microglia receptors and their implications in the response to amyloid β for Alzheimer’s disease pathogenesis. J. Neuroinflammation. 2014;11:48. doi: 10.1186/1742-2094-11-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dionisio-Santos D.A., Olschowka J.A., O’Banion M.K. Exploiting microglial and peripheral immune cell crosstalk to treat Alzheimer’s disease. J. Neuroinflammation. 2019;16(1):74. doi: 10.1186/s12974-019-1453-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Gabande-Rodriguez E., Keane L., Capasso M. Microglial phagocytosis in aging and Alzheimer’s disease. J. Neurosci. Res. 2019;••• doi: 10.1002/jnr.24419. [DOI] [PubMed] [Google Scholar]
- 56.Clayton K.A., Van Enoo A.A., Ikezu T. Alzheimer’s disease: the role of microglia in brain homeostasis and proteopathy. Front. Neurosci. 2017;11:680. doi: 10.3389/fnins.2017.00680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yanamadala V., Friedlander R.M. Complement in neuroprotection and neurodegeneration. Trends Mol. Med. 2010;16(2):69–76. doi: 10.1016/j.molmed.2009.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van der Kleij H., Charles N., Karimi K., Mao Y.K., Foster J., Janssen L., Yang C. P.; Kunze, W.; Rivera, J.; Bienenstock, J. Evidence for neuronal expression of functional Fc (epsilon and gamma) receptors. J. Allergy Clin. Immunol. 2010;125(3):757–760. doi: 10.1016/j.jaci.2009.10.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cattaneo F., Guerra G., Ammendola R. Expression and signaling of formyl-peptide receptors in the brain. Neurochem. Res. 2010;35(12):2018–2026. doi: 10.1007/s11064-010-0301-5. [DOI] [PubMed] [Google Scholar]
- 60.Husemann J., Loike J.D., Anankov R., Febbraio M., Silverstein S.C. Scavenger receptors in neurobiology and neuropathology: their role on microglia and other cells of the nervous system. Glia. 2002;40(2):195–205. doi: 10.1002/glia.10148. [DOI] [PubMed] [Google Scholar]
- 61.Okun E., Griffioen K.J., Mattson M.P. Toll-like receptor signaling in neural plasticity and disease. Trends Neurosci. 2011;34(5):269–281. doi: 10.1016/j.tins.2011.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yan S.D., Chen X., Fu J., Chen M., Zhu H., Roher A., Slattery T., Zhao L., Nagashima M., Morser J., Migheli A., Nawroth P., Stern D., Schmidt A.M. RAGE and amyloid-beta peptide neurotoxicity in Alzheimer’s disease. Nature. 1996;382(6593):685–691. doi: 10.1038/382685a0. [DOI] [PubMed] [Google Scholar]
- 63.Takata K., Kitamura Y. Molecular approaches to the treatment, prophylaxis, and diagnosis of Alzheimer’s disease: tangle formation, amyloid-β, and microglia in Alzheimer’s disease. J. Pharmacol. Sci. 2012;118(3):331–337. doi: 10.1254/jphs.11R10FM. [DOI] [PubMed] [Google Scholar]
- 64.Scaffidi P., Misteli T., Bianchi M.E. Release of chromatin protein HMGB1 by necrotic cells triggers inflammation. Nature. 2002;418(6894):191–195. doi: 10.1038/nature00858. [DOI] [PubMed] [Google Scholar]
- 65.Vijayakumar E.C., Bhatt L.K., Prabhavalkar K.S. High mobility group box-1 (hmgb1): a potential target in therapeutics. Curr. Drug Targets. 2019;20(14):1474–1485. doi: 10.2174/1389450120666190618125100. [DOI] [PubMed] [Google Scholar]
- 66.Takata K., Kitamura Y., Kakimura J., Shibagaki K., Tsuchiya D., Taniguchi T., Smith M.A., Perry G., Shimohama S. Role of high mobility group protein-1 (HMG1) in amyloid-beta homeostasis. Biochem. Biophys. Res. Commun. 2003;301(3):699–703. doi: 10.1016/S0006-291X(03)00024-X. [DOI] [PubMed] [Google Scholar]
- 67.Takata K., Takada T., Ito A., Asai M., Tawa M., Saito Y., Ashihara E., Tomimoto H., Kitamura Y., Shimohama S. Microglial amyloid-β1-40 phagocytosis dysfunction is caused by high-mobility group box protein-1: implications for the pathological progression of Alzheimer’s disease. Int. J. Alzheimers Dis. 2012;2012:685739. doi: 10.1155/2012/685739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sinagra T., Merlo S., Spampinato S.F., Pasquale R.D., Sortino M.A. High mobility group box 1 contributes to wound healing induced by inhibition of dipeptidylpeptidase 4 in cultured keratinocytes. Front. Pharmacol. 2015;6:126. doi: 10.3389/fphar.2015.00126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Straino S., Di Carlo A., Mangoni A., De Mori R., Guerra L., Maurelli R., Panacchia L., Di Giacomo F., Palumbo R., Di Campli C., Uccioli L., Biglioli P., Bianchi M.E., Capogrossi M.C., Germani A. High-mobility group box 1 protein in human and murine skin: involvement in wound healing. J. Invest. Dermatol. 2008;128(6):1545–1553. doi: 10.1038/sj.jid.5701212. [DOI] [PubMed] [Google Scholar]
- 70.Bortolotto V., Grilli M. Every cloud has a silver lining: proneurogenic effects of aβ oligomers and hmgb-1 via activation of the rage-nf-κb axis. CNS Neurol. Disord. Drug Targets. 2017;16(10):1066–1079. doi: 10.2174/1871527315666160803153459. [DOI] [PubMed] [Google Scholar]
- 71.Malik M., Parikh I., Vasquez J.B., Smith C., Tai L., Bu G., LaDu M.J., Fardo D.W., Rebeck G.W., Estus S. Genetics ignite focus on microglial inflammation in Alzheimer’s disease. Mol. Neurodegener. 2015;10:52. doi: 10.1186/s13024-015-0048-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhao Y., Wu X., Li X., Jiang L.L., Gui X., Liu Y., Sun Y., Zhu B., Pina-Crespo J.C., Zhang M., Zhang N., Chen X., Bu G., An Z., Huang T.Y., Xu H. 2018.
- 73.Zhong L., Wang Z., Wang D., Wang Z., Martens Y.A., Wu L., Xu Y., Wang K., Li J., Huang R., Can D., Xu H., Bu G., Chen X.F. Amyloid-beta modulates microglial responses by binding to the triggering receptor expressed on myeloid cells 2 (TREM2). Mol. Neurodegener. 2018;13(1):15. doi: 10.1186/s13024-018-0247-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jin S.C., Benitez B.A., Karch C.M., Cooper B., Skorupa T., Carrell D., Norton J.B., Hsu S., Harari O., Cai Y., Bertelsen S., Goate A.M., Cruchaga C. Coding variants in TREM2 increase risk for Alzheimer’s disease. Hum. Mol. Genet. 2014;23(21):5838–5846. doi: 10.1093/hmg/ddu277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jonsson T., Stefansson H., Steinberg S., Jonsdottir I., Jonsson P.V., Snaedal J., Bjornsson S., Huttenlocher J., Levey A.I., Lah J.J., Rujescu D., Hampel H., Giegling I., Andreassen O.A., Engedal K., Ulstein I., Djurovic S., Ibrahim-Verbaas C., Hofman A., Ikram M.A., van Duijn C.M., Thorsteinsdottir U., Kong A., Stefansson K. Variant of TREM2 associated with the risk of Alzheimer’s disease. N. Engl. J. Med. 2013;368(2):107–116. doi: 10.1056/NEJMoa1211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yeh F.L., Hansen D.V., Sheng M. TREM2, Microglia, and Neurodegenerative Diseases. Trends Mol. Med. 2017;23(6):512–533. doi: 10.1016/j.molmed.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 77.Lee C.Y.D., Daggett A., Gu X., Jiang L.L., Langfelder P., Li X., Wang N., Zhao Y., Park C.S., Cooper Y., Ferando I., Mody I., Coppola G., Xu H., Yang X.W. 2018.
- 78.Wang Y., Ulland T.K., Ulrich J.D., Song W., Tzaferis J.A., Hole J.T., Yuan P., Mahan T.E., Shi Y., Gilfillan S., Cella M., Grutzendler J., DeMattos R.B., Cirrito J.R., Holtzman D.M., Colonna M. TREM2-mediated early microglial response limits diffusion and toxicity of amyloid plaques. J. Exp. Med. 2016;213(5):667–675. doi: 10.1084/jem.20151948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yuan P., Condello C., Keene C.D., Wang Y., Bird T.D., Paul S.M., Luo W., Colonna M., Baddeley D., Grutzendler J. TREM2 Haplodeficiency in Mice and Humans Impairs the Microglia Barrier Function Leading to Decreased Amyloid Compaction and Severe Axonal Dystrophy. Neuron. 2016;90(4):724–739. doi: 10.1016/j.neuron.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jay T.R., Miller C.M., Cheng P.J., Graham L.C., Bemiller S., Broihier M.L., Xu G., Margevicius D., Karlo J.C., Sousa G.L., Cotleur A.C., Butovsky O., Bekris L., Staugaitis S.M., Leverenz J.B., Pimplikar S.W., Landreth G.E., Howell G.R., Ransohoff R.M., Lamb B.T. TREM2 deficiency eliminates TREM2+ inflammatory macrophages and ameliorates pathology in Alzheimer’s disease mouse models. J. Exp. Med. 2015;212(3):287–295. doi: 10.1084/jem.20142322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tanzi R.E. TREM2 and Risk of Alzheimer’s Disease--Friend or Foe? N. Engl. J. Med. 2015;372(26):2564–2565. doi: 10.1056/NEJMcibr1503954. [DOI] [PubMed] [Google Scholar]
- 82.Udeochu J., Sayed F.A., Gan L. TREM2 and Amyloid Beta: A Love-Hate Relationship. Neuron. 2018;97(5):991–993. doi: 10.1016/j.neuron.2018.02.018. [DOI] [PubMed] [Google Scholar]
- 83.Jay T.R., Hirsch A.M., Broihier M.L., Miller C.M., Neilson L.E., Ransohoff R.M., Lamb B.T., Landreth G.E. Disease Progression-Dependent Effects of TREM2 Deficiency in a Mouse Model of Alzheimer’s Disease. J. Neurosci. 2017;37(3):637–647. doi: 10.1523/JNEUROSCI.2110-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zheng H., Jia L., Liu C.C., Rong Z., Zhong L., Yang L., Chen X.F., Fryer J.D., Wang X., Zhang Y.W., Xu H., Bu G. TREM2 Promotes Microglial Survival by Activating Wnt/β-Catenin Pathway. J. Neurosci. 2017;37(7):1772–1784. doi: 10.1523/JNEUROSCI.2459-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhong L., Chen X.F., Wang T., Wang Z., Liao C., Wang Z., Huang R., Wang D., Li X., Wu L., Jia L., Zheng H., Painter M., Atagi Y., Liu C.C., Zhang Y.W., Fryer J.D., Xu H., Bu G. Soluble TREM2 induces inflammatory responses and enhances microglial survival. J. Exp. Med. 2017;214(3):597–607. doi: 10.1084/jem.20160844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Suárez-Calvet M., Morenas-Rodríguez E., Kleinberger G., Schlepckow K., Araque Caballero M.A., Franzmeier N., Capell A., Fellerer K., Nuscher B., Eren E., Levin J., Deming Y., Piccio L., Karch C.M., Cruchaga C., Shaw L.M., Trojanowski J.Q., Weiner M., Ewers M., Haass C. Alzheimer’s Disease Neuroimaging Initiative. Early increase of CSF sTREM2 in Alzheimer’s disease is associated with tau related-neurodegeneration but not with amyloid-β pathology. Mol. Neurodegener. 2019;14(1):1. doi: 10.1186/s13024-018-0301-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ewers M., Franzmeier N., Suárez-Calvet M., Morenas-Rodriguez E., Caballero M.A.A., Kleinberger G., Piccio L., Cruchaga C., Deming Y., Dichgans M., Trojanowski J.Q., Shaw L.M., Weiner M.W., Haass C. Alzheimer’s Disease Neuroimaging Initiative. Increased soluble TREM2 in cerebrospinal fluid is associated with reduced cognitive and clinical decline in Alzheimer’s disease. Sci. Transl. Med. 2019;11(507):eaav6221. doi: 10.1126/scitranslmed.aav6221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Deming Y., Filipello F., Cignarella F., Cantoni C., Hsu S., Mikesell R., Li Z., Del-Aguila J.L., Dube U., Farias F.G., Bradley J., Budde J., Ibanez L., Fernandez M.V., Blennow K., Zetterberg H., Heslegrave A., Johansson P.M., Svensson J., Nellgård B., Lleo A., Alcolea D., Clarimon J., Rami L., Molinuevo J.L., Suárez-Calvet M., Morenas-Rodríguez E., Kleinberger G., Ewers M., Harari O., Haass C., Brett T.J., Benitez B.A., Karch C.M., Piccio L., Cruchaga C. The MS4A gene cluster is a key modulator of soluble TREM2 and Alzheimer’s disease risk. Sci. Transl. Med. 2019;11(505):eaau2291. doi: 10.1126/scitranslmed.aau2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yao H., Coppola K., Schweig J.E., Crawford F., Mullan M., Paris D. Distinct signaling pathways regulate TREM2 phagocytic and NFκB antagonistic activities. Front. Cell. Neurosci. 2019;13:457. doi: 10.3389/fncel.2019.00457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Crocker P.R., Paulson J.C., Varki A. Siglecs and their roles in the immune system. Nat. Rev. Immunol. 2007;7(4):255–266. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 91.Lajaunias F., Dayer J.M., Chizzolini C. Constitutive repressor activity of CD33 on human monocytes requires sialic acid recognition and phosphoinositide 3-kinase-mediated intracellular signaling. Eur. J. Immunol. 2005;35(1):243–251. doi: 10.1002/eji.200425273. [DOI] [PubMed] [Google Scholar]
- 92.Naj A.C., Jun G., Beecham G.W., Wang L.S., Vardarajan B.N., Buros J., Gallins P.J., Buxbaum J.D., Jarvik G.P., Crane P.K., Larson E.B., Bird T.D., Boeve B.F., Graff-Radford N.R., De Jager P.L., Evans D., Schneider J.A., Carrasquillo M.M., Ertekin-Taner N., Younkin S.G., Cruchaga C., Kauwe J.S., Nowotny P., Kramer P., Hardy J., Huentelman M.J., Myers A.J., Barmada M.M., Demirci F.Y., Baldwin C.T., Green R.C., Rogaeva E., St George-Hyslop P., Arnold S.E., Barber R., Beach T., Bigio E.H., Bowen J.D., Boxer A., Burke J.R., Cairns N.J., Carlson C.S., Carney R.M., Carroll S.L., Chui H.C., Clark D.G., Corneveaux J., Cotman C.W., Cummings J.L., DeCarli C., DeKosky S.T., Diaz-Arrastia R., Dick M., Dickson D.W., Ellis W.G., Faber K.M., Fallon K.B., Farlow M.R., Ferris S., Frosch M.P., Galasko D.R., Ganguli M., Gearing M., Geschwind D.H., Ghetti B., Gilbert J.R., Gilman S., Giordani B., Glass J.D., Growdon J.H., Hamilton R.L., Harrell L.E., Head E., Honig L.S., Hulette C.M., Hyman B.T., Jicha G.A., Jin L.W., Johnson N., Karlawish J., Karydas A., Kaye J.A., Kim R., Koo E.H., Kowall N.W., Lah J.J., Levey A.I., Lieberman A.P., Lopez O.L., Mack W.J., Marson D.C., Martiniuk F., Mash D.C., Masliah E., McCormick W.C., McCurry S.M., McDavid A.N., McKee A.C., Mesulam M., Miller B.L., Miller C.A., Miller J.W., Parisi J.E., Perl D.P., Peskind E., Petersen R.C., Poon W.W., Quinn J.F., Rajbhandary R.A., Raskind M., Reisberg B., Ringman J.M., Roberson E.D., Rosenberg R.N., Sano M., Schneider L.S., Seeley W., Shelanski M.L., Slifer M.A., Smith C.D., Sonnen J.A., Spina S., Stern R.A., Tanzi R.E., Trojanowski J.Q., Troncoso J.C., Van Deerlin V.M., Vinters H.V., Vonsattel J.P., Weintraub S., Welsh-Bohmer K.A., Williamson J., Woltjer R.L., Cantwell L.B., Dombroski B.A., Beekly D., Lunetta K.L., Martin E.R., Kamboh M.I., Saykin A.J., Reiman E.M., Bennett D.A., Morris J.C., Montine T.J., Goate A.M., Blacker D., Tsuang D.W., Hakonarson H., Kukull W.A., Foroud T.M., Haines J.L., Mayeux R., Pericak-Vance M.A., Farrer L.A., Schellenberg G.D. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer’s disease. Nat. Genet. 2011;43(5):436–441. doi: 10.1038/ng.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Zhao L. CD33 in Alzheimer’s Disease - biology, pathogenesis, and therapeutics: A mini-review. Gerontology. 2019;65(4):323–331. doi: 10.1159/000492596. [DOI] [PubMed] [Google Scholar]
- 94.Estus S., Shaw B.C., Devanney N., Katsumata Y., Press E.E., Fardo D.W. Evaluation of CD33 as a genetic risk factor for Alzheimer’s disease. Acta Neuropathol. 2019;138(2):187–199. doi: 10.1007/s00401-019-02000-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Griciuc A., Patel S., Federico A.N., Choi S.H., Innes B.J., Oram M.K., Cereghetti G., McGinty D., Anselmo A., Sadreyev R.I., Hickman S.E., El Khoury J., Colonna M., Tanzi R.E. 2019. [DOI] [PMC free article] [PubMed]
- 96.Griciuc A., Serrano-Pozo A., Parrado A.R., Lesinski A.N., Asselin C.N., Mullin K., Hooli B., Choi S.H., Hyman B.T., Tanzi R.E. Alzheimer’s disease risk gene CD33 inhibits microglial uptake of amyloid beta. Neuron. 2013;78(4):631–643. doi: 10.1016/j.neuron.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Paolicelli R.C., Bolasco G., Pagani F., Maggi L., Scianni M., Panzanelli P., Giustetto M., Ferreira T.A., Guiducci E., Dumas L., Ragozzino D., Gross C.T. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333(6048):1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- 98.Neumann H., Kotter M.R., Franklin R.J. Debris clearance by microglia: an essential link between degeneration and regeneration. Brain. 2009;132(Pt 2):288–295. doi: 10.1093/brain/awn109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ayata P., Badimon A., Strasburger H.J., Duff M.K., Montgomery S.E., Loh Y.E., Ebert A., Pimenova A.A., Ramirez B.R., Chan A.T., Sullivan J.M., Purushothaman I., Scarpa J.R., Goate A.M., Busslinger M., Shen L., Losic B., Schaefer A. Epigenetic regulation of brain region-specific microglia clearance activity. Nat. Neurosci. 2018;21(8):1049–1060. doi: 10.1038/s41593-018-0192-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Datta M., Staszewski O., Raschi E., Frosch M., Hagemeyer N., Tay T.L., Blank T., Kreutzfeldt M., Merkler D., Ziegler-Waldkirch S., Matthias P., Meyer-Luehmann M., Prinz M. 2018. [DOI] [PubMed]
- 101.Zhu X., Wang S., Yu L., Jin J., Ye X., Liu Y., Xu Y. HDAC3 negatively regulates spatial memory in a mouse model of Alzheimer’s disease. Aging Cell. 2017;16(5):1073–1082. doi: 10.1111/acel.12642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Janczura K.J., Volmar C.H., Sartor G.C., Rao S.J., Ricciardi N.R., Lambert G., Brothers S.P., Wahlestedt C. Inhibition of HDAC3 reverses Alzheimer’s disease-related pathologies in vitro and in the 3xTg-AD mouse model. Proc. Natl. Acad. Sci. USA. 2018;115(47):E11148–E11157. doi: 10.1073/pnas.1805436115. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 103.Bao J., Zheng L., Zhang Q., Li X., Zhang X., Li Z., Bai X., Zhang Z., Huo W., Zhao X., Shang S., Wang Q., Zhang C., Ji J. Deacetylation of TFEB promotes fibrillar Aβ degradation by upregulating lysosomal biogenesis in microglia. Protein Cell. 2016;7(6):417–433. doi: 10.1007/s13238-016-0269-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Alexandrov P.N., Zhao Y., Jones B.M., Bhattacharjee S., Lukiw W.J. Expression of the phagocytosis-essential protein TREM2 is down-regulated by an aluminum-induced miRNA-34a in a murine microglial cell line. J. Inorg. Biochem. 2013;128:267–269. doi: 10.1016/j.jinorgbio.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhao Y., Bhattacharjee S., Jones B.M., Dua P., Alexandrov P.N., Hill J.M., Lukiw W.J. Regulation of TREM2 expression by an NF-кB-sensitive miRNA-34a. Neuroreport. 2013;24(6):318–323. doi: 10.1097/WNR.0b013e32835fb6b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Madadi S., Schwarzenbach H., Saidijam M., Mahjub R., Soleimani M. Potential microRNA-related targets in clearance pathways of amyloid-β: novel therapeutic approach for the treatment of Alzheimer’s disease. Cell Biosci. 2019;9:91. doi: 10.1186/s13578-019-0354-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cummings J., Lee G., Ritter A., Sabbagh M., Zhong K. Alzheimer’s disease drug development pipeline: 2019. Alzheimers Dement. (N. Y.) 2019;5:272–293. doi: 10.1016/j.trci.2019.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ulland T.K., Colonna M. TREM2 - a key player in microglial biology and Alzheimer disease. Nat. Rev. Neurol. 2018;14(11):667–675. doi: 10.1038/s41582-018-0072-1. [DOI] [PubMed] [Google Scholar]
- 109.Lue L.F., Kuo Y.M., Beach T., Walker D.G. Microglia activation and anti-inflammatory regulation in Alzheimer’s disease. Mol. Neurobiol. 2010;41(2-3):115–128. doi: 10.1007/s12035-010-8106-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Parkhurst C.N., Yang G., Ninan I., Savas J.N., Yates J.R., III, Lafaille J.J., Hempstead B.L., Littman D.R., Gan W.B. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 2013;155(7):1596–1609. doi: 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Keren-Shaul H., Spinrad A., Weiner A., Matcovitch-Natan O., Dvir-Szternfeld R., Ulland T.K., David E., Baruch K., Lara-Astaiso D., Toth B., Itzkovitz S., Colonna M., Schwartz M., Amit I. A Unique microglia type associated with restricting development of Alzheimer's disease. 2017. [DOI] [PubMed]
- 112.Ulland T.K., Song W.M., Huang S.C., Ulrich J.D., Sergushichev A., Beatty W.L., Loboda A.A., Zhou Y., Cairns N.J., Kambal A., Loginicheva E., Gilfillan S., Cella M., Virgin H.W., Unanue E.R., Wang Y., Artyomov M.N., Holtzman D.M., Colonna M. 2017.
- 113.Hong S., Beja-Glasser V.F., Nfonoyim B.M., Frouin A., Li S., Ramakrishnan S., Merry K.M., Shi Q., Rosenthal A., Barres B.A., Lemere C.A., Selkoe D.J., Stevens B. Complement and microglia mediate early synapse loss in Alzheimer mouse models. Science. 2016;352(6286):712–716. doi: 10.1126/science.aad8373. [DOI] [PMC free article] [PubMed] [Google Scholar]