Abstract
Background
Pharmacological treatment for alcohol dependence has only three approved drugs: disulfiram, naltrexone and acamprosate. The effects of these drugs are, however, limited, presenting several side effects and a modestly higher efficacy compared to placebo. The administration of omega-3 might bring new perspectives to relapse prevention.
Methods
This systematic review aimed to analyze the available literature, compiling the studies that used omega-3 to prevent relapse in alcohol dependents.
Results
The databases used were PubMed and Web of Science. We identified 2,231 studies and only five articles addressed the administration of omega-3 and alcoholism. Preclinical studies evaluating the effects of PUFAs related to chronic alcohol administration showed improvements in behavioral, cellular and molecular levels. The clinical trial yielded inconclusive results.
Conclusion
Despite the reduced number of studies, omega-3 interventions seem to be promising for controlling issues related to alcohol dependence.
Keywords: PUFAs, omega-3, ethanol, relapse, treatment, craving
1. INTRODUCTION
Pharmacological treatment for alcohol dependence has only three approved drugs: disulfiram, naltrexone and acamprosate. Disulfiram acts as an aversion therapy (acetaldehyde buildup) and the latter treatments, naltrexone and acamprosate, target craving. The effects of these drugs are, however, limited, presenting several side effects and a modestly higher efficacy compared to placebo [1]. Challenges in the treatment of alcohol use disorders include recurrent relapses, with rates ranging from 40% to 60% within one year [2]. Meta-analyses of available treatments show modest efficacy in maintaining abstinence even in the short term, thus the need for new pharmacological alternatives is compelling [3].
The administration of omega-3 might bring new perspectives to relapse prevention. Several pre-clinical studies suggest that omega-3 might have protective effects related to the chronic exposure to alcohol. Shi et al. (2019) [4] reported that dendritic alterations of neurons in the nucleus accumbens resulting from chronic exposure to alcohol decreased significantly with omega-3 administration. Moreover, low plasma levels of docosahexaenoic acid (DHA) seem to be associated with an increased relapse vulnerability in substance abusers [5]. Nevertheless, the chronic administration of alcohol reduces the DHA concentration in the blood and the nervous system [6].
Ethanol inhibits the activity of delta-6-desaturase and delta-5-desaturase [7-9]. These enzymes participate in the metabolic pathways by which linoleic and alpha-linolenic acids become polyunsaturated fatty acids: omega-6 and omega-3, respectively. The metabolites of linoleic acid (LA) or omega-6 are gammalinolenic acids (GLA), dihomogamalinolenic acids (DGLA) and arachidonic acids (AA). Alpha-linolenic acid (ALA) or omega-3 metabolites are eicosapentaenoic acids (EPA) and docosahexanoic acids (DHA). The long carbon chain and unsaturation number give these fatty acids the classification of polyunsaturated fatty acids (PUFAs).
These two classes of PUFAs are metabolically and functionally distinct. They have opposite physiological functions: while omega-3 suppresses the synthesis of IL-1 and IL-6 cytokines by increasing IL-2, Omega-6 increases cytokine production via AA due to increased release of corticotropin-releasing hormone (CRH) [10]. Moreover, they are also constituents of cell membranes, determining their biological properties and cellular response to many stimuli [11]. Omega-6 series PUFAs are related to homeostasis, pain, inflammation and tumor genesis, conditions closely related to prostaglandins. The omega-3 series PUFAs suppresses the synthesis of IL-1 and IL-6 cytokines, have anti-inflammatory action and are related to the functioning of some neurotransmitters [10, 11]. These neurotransmitters are closely related to alcohol dependence. Hence, omega-3 deficiency leads to hypofunction of the mesocortical and mesolimbic pathways (well-known reinforcement pathways of abusive substance use).
On the other hand, the administration of omega-3 increases the central activity of 5HT (prefrontal cortex area), decreasing aggressiveness and impulsivity. Omega-3 has a neuroprotective effect on the glutamatergic system, especially against the exocitoxicity induced by NMDA. It is known that this system is hyperfunctioning due to chronic exposure to alcohol, as well as in its sudden suppression [12, 13], which seems to influence the relapse [14, 15].
Some studies evaluated the role of PUFAs in alcoholism, relating them to liver damage caused by alcohol [16-18]; effects on tolerance [19], and attenuation of negative effects of chronic alcohol use [6, 20, 21]. Glen et al. (1984) [22] showed that alcohol dependents who received PUFAs presented a fast decrease in the level of gamma glutamyl transferase, required less benzodiazepines for detoxification, and presented a faster cognitive improvement when compared to the placebo group.
The pioneering work of Goldstein (1987) [23] showed that alcohol alters the cell membrane environment, mainly by modifying the permeability of the lipid fraction, altering their fluidity and protein function. PUFAs, the group to which omega-3 belongs have, among their functions, cell signaling, enzymatic regulation, eicosanoid synthesis, regulation of neuronal migration, determination of synaptic plasticity and modulation of cytokines that have neuromodulatory and neurotransmitter activity [24-26].
Our hypothesis is that the administration of omega-3 could minimize the damage caused by chronic alcohol use by normalizing the functioning of the mesolimbic pathway (reinforcement pathway), providing a neuroprotective effect on the glutamatergic system, and stabilizing the neuronal membranes. Therefore, regularizing these functions could have benefits over decreasing relapses in alcohol dependents. This systematic review aimed to analyze the literature regarding the role of omega-3 in preventing relapse in alcohol dependents and related outcomes, considering both clinical and preclinical studies.
2. METHODS
2.1. Bibliographic Search
In order to identify the studies related to the potential effect of omega-3 in the prevention of alcohol relapse, a systematic bibliographic search was conducted in two databases: PubMed/Medline and Web of Science. Primary search strategy was the following:
(Drinking behavior[mesh] OR Alcohol-related disorders[mesh] OR Alcoholic beverages[mesh] OR (Alcohol*[tiab] AND (drink* OR binge OR beer OR wine OR spirits OR beverage*)) OR (Drink*[tiab] AND (alcohol* OR binge OR beer OR wine OR spirits OR beverage*)) OR (Ethanol*[tiab] AND (drink* OR binge OR beer OR wine OR spirits OR beverage*)) OR Binge OR Beer OR Wine OR Spirits) AND (“Fish oils”[mesh] OR Fatty Acids, Omega-3[mesh] OR Fatty Acids, Omega-6[mesh] OR “dietary fats, unsaturated”[MeSH Terms] OR “cod liver oil” OR “Docosahexaenoic Acid*” OR “Eicosapentaenoic Acid*” OR “n-3 fatty” OR “n3 fatty” OR “n-3 polyunsaturated” OR “n3 polyunsaturated” OR “Omega 3” OR PUFA OR PUFAS OR “n-3 oil*” OR “n3 oil*” OR “n-6 fatty” OR “n6 fatty” OR “n-6 polyunsaturated” OR “n6 polyunsaturated” OR “Omega 6” OR “n-6 oil*” OR “n6 oil*” OR alpha-Linolenic OR gamma-Linolenic OR Linoleic OR “polyunsaturated fatty” OR (DHA[Tiab] AND (fatty OR oil OR omega)) OR (EPA[Tiab] AND (fatty OR oil OR omega)) OR (ALA[Tiab] AND (fatty OR oil OR omega))).
We used this strategy for PubMed search and an adapted version for Web of Science. Articles were selected in a two-step process: titles and abstracts were assessed to evaluate if they were in accordance with the topic of this review. The second step included full text evaluations and data extractions. Two authors (JCFG and AGB) applied inclusion and exclusion criteria in both steps concurrently. In case of discrepancy between these authors, a third one (GNP) evaluated the material.
2.2. Inclusion and Exclusion Criteria
Only original and peer-reviewed articles assessing the effects of omega-3 on alcohol dependence-related outcomes were included. For this purpose, we considered all clinical trials and experimental studies in which omega-3 were the intervention (independent variable) and any kind of alcohol dependence-related measure was the outcome (dependent variable). We excluded study designs not complying with these parameters, as well as reviews and other theoretical articles, and articles for which we could not find full texts. There were no restrictions regarding population (i.e., both clinical and preclinical studies were considered eligible), as long as it was related to alcohol dependence or relapse reduction. There was no restriction regarding publication date and language.
2.3. Risk of Bias
Information about possible biases affecting the quality of selected articles was obtained using the Cochrane Collaboration Risk of Bias tool - for clinical trials [27], and SYRCLE´s Risk of Bias Tool, a specific instrument for animal studies [28].
3. RESULTS
3.1. Selected Studies and Sample Description
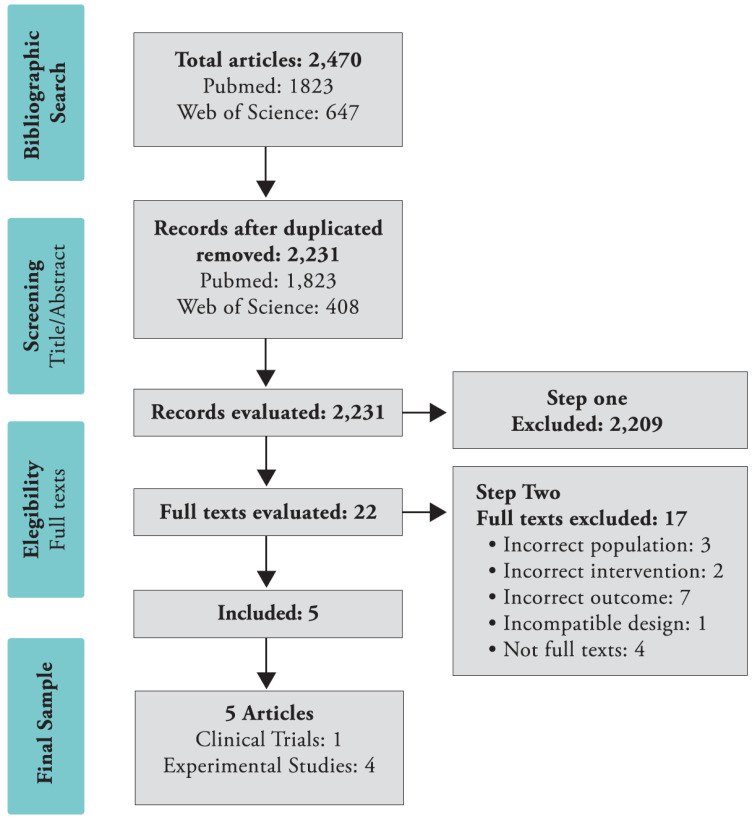
We retrieved 2,470 articles from our literature search and after removing duplicate articles, we elected 2,231 articles for the composition of our initial sample. Among them, 1,823 were original from PubMed/Medline and the remaining 408 were found exclusively in Web of Science. After screening of title and abstracts, 2,209 articles were excluded and 22 articles remained eligible to step two. Among these, 17 articles were excluded from our second step. Only five articles composed our final sample. The selection process appears in Fig. 1.
Fig. (1).
PRISMA systematic review search flowchart. (A higher resolution / colour version of this figure is available in the electronic copy of the article).
Only one article is a clinical trial addressing the main objective of this review [29]. The other articles are experimental studies and were included in order to evaluate potential translational effects of this intervention in alcohol dependence. A full description of these pre-clinical articles and their main features is available in Table 1. In the following section, we described and compared qualitatively the selected articles.
Table 1.
List and description of selected preclinical studies and clinical trial.
| Article | Alcoholic Model | Species | Strain | Gender | Age | Sample Size | Intervention Composition |
Dose,
Frequency and Duration |
Effects Evaluated | Outcome Tests | Conclusion |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Balaszczuk et al. (2019) [30] | Subcutaneous injection of ethanol | Rat | Wistar | Male and Female | PND 7 - PND 14, PND 20, PND 30 |
Up to 67 in different experiments | Omega 3 | 720mg/kg/single dose | Binge alcohol neurotoxicity | OFT, EPM | Benefits on locomotion behavior |
| Shi et al. (2019) [4] | Diet enriched with alcohol (28 days) | Mice | C57BL/6 J | Male | 8-10 weeks | 24 | Fish Oil (5mg EPA and 30mg DHA) | 50uL in corn oil, 14 days before and during alcohol treatment | Physical distress during acute ethanol withdrawal | Locomotion, CPP | Hyperlocomotion reduction and less preference towards the alcohol paired chamber in CPP test. Fish oil prevented ethanol-induced dendritic morphological changes in the nucleus accumbens |
| He et al. (2018) [31] | Intragastric; 4.8g/kg/day; 5 weeks | Rat | F344 | Male | 7 weeks | 48 | DHA | 20mg/kg/day; 5 weeks | A binge ethanol model | OFT | DHA was not able to reverse the locomotor pattern |
| Raabe et al. (2014) [32] |
Diluted alcohol | C. elegans | Wild-type N2 (var Bristol), BX24 fat-1 (wa9), BX17 fat-4 (wa14), BX30 fat-3 (wa22) |
Adult | No description | 6 | EPA and AA | 160uM | A model to evaluate acute functional tolerance to ethanol |
Locomotion, Speed | Recovery from acute functional tolerance defect |
| Fogaça et al. (2011) [29] |
Clinical trail (double-blind, placebo-controlled) |
Human | -- | Male | 30-50 years old | 80 | 1 g Borago officinalis oil 1 g of fish oil | 120 mg of GLA and 400 mg EPA + DHA/ daily (90 days) | Serum level of PUFAS, Dependency level, Compulsion |
HPLC, SADD, OCDS Scale |
There were no differences compared to placebo |
Abbreviations: OFT: Open Field Test; EPM: Elevated Plus Maze; CPP: Conditioned Place Preference; HPLC: High Performance Liquid Chromatography technique; SADD: Short Alcohol Dependence Data; OCDS Scale: Obsessive-Compulsive Drinking Scale.
3.2. Descriptive Results
3.2.1. Clinical Trial-Description and Main Results
This was a placebo-controlled double-blind clinical trial [29]. The inclusion criteria in this clinical trial were alcohol dependence by DSM IV criteria, male gender, 30 to 50 years old, without other drugs dependence. Patients were randomly allocated into four groups, with 20 participants in each group: placebo; naltrexone; PUFAs; and Naltrexone + PUFAs. The PUFAs administered were composed of Borago officinalis oil (rich in omega 6) and fish oil (rich in omega 3) capsules. The number of patients who completed the study in each group was, placebo (n = 11); naltrexone (n = 11); PUFAS (n = 12); and naltrexone + PUFAS (n = 9). The tools used to evaluate the alcohol dependence in this trial were the Short Alcohol Dependence Data Questionnaire (SADD) and the Obsessive Compulsive Disorder and Addiction (OCDS). All patients who completed the study decreased the amount of alcohol consumed, the compulsion and the degree of severity of dependence at the end of three months of treatment when compared to their initial scores, regardless the group to which they belonged. In addition, omega-3 (EPA and DHA) and omega-6 (AA and LA) levels remained identical throughout the study even among those patients who received PUFA supplements. The study had several limitations: small sample size, high dropout rate, simultaneous administration of omega-6 and omega-3, the prescribed doses (160 mg of EPA, 240 mg of DHA and 120 mg of GLA) and no change in PUFAs dosages before and after administration.
3.2.2. Pre-clinical Studies-Description and Main Results
Among the final sample, four articles reported experiments in animal’s models. Of this sample, two of them reported data using rats, one using mice and one using C. elegans. The tests used to observe alcohol dependence and related behaviors were Open Field Test (OFT), Elevated Plus Maze (EPM), Conditioned Place Preference (CPP), Locomotion and Speed of Locomotion.
Balaszczuk et al. (2019) [30] evaluated a binge alcohol neurotoxic model during postnatal development. In this study, newborn rats were administered with ethanol (2.5g/kg) at the seventh postpartum day. A single dose of omega-3 (720mg/kg) reduced hyperlocomotion and anxiety-like behaviors induced by ethanol after one week (postpartum day 14). The benefits over locomotion remained until the 30th postpartum day, but no long-term effects on anxiety-like behaviors were observed.
He et al. (2018) [31] evaluated the effects of DHA administration on a binge ethanol model, both in control and in HIV-infected rats. Contrarily to Balaszczuk et al. (2019) [31], DHA was not able to reverse the locomotor pattern induced by ethanol administration.
Shi et al. (2019) [4] employed a mice model of alcohol dependence induction to demonstrate that fish oil treatment relieved the physical distress during acute ethanol withdrawal at 6, 12 and 24 hours. The authors described hyperlocomotion reduction after 7-day abstinence in the treated group, as well as less preference towards the alcohol pared chamber in CPP test. In addition, fish oil prevented ethanol-induced dendritic morphological changes in the nucleus accumbens.
Raabe et al. (2014) [32] used C. elegans as a model to evaluate acute functional tolerance to ethanol. They assessed locomotion behavior in different mutants after exposition to ethanol. These mutants presented altered fatty acids metabolism and the authors associated these mutations to different responses in acute functional tolerance to ethanol. Later, they described that a dietary supplementation of EPA could rescue the acute functional tolerance defect in these mutants.
3.3. Risk of Bias Analysis
Regarding the single clinical trial included in this review, the risk of bias showed no high risk on any of the items evaluated, using the Cochrane Risk of Bias Took. Several item were marked as unclear though, due to a lack of descriptive information. Regarding the preclinical studies, the majority of items evaluated by SYRCLE’s Risk of Bias Tool demonstrated an unclear risk of bias. This is a common feature in the preclinical risk of bias assessment and reflects the lack of methodological guidance in the report of preclinical results. All results are related to the risk of bias evaluation, both from the clinical trial and from the preclinical studies (Table 2).
Table 2.
Risk of bias evaluation.
| - | Random Sequence Generation | Baseline Characteristics | Allocation Concealment | Random Housing | Blinding (Performance) | Random Outcome Assessment | Blinding (Outcome Detection) | Incomplete Outcome Data | Selective Reporting | Other Biases |
|---|---|---|---|---|---|---|---|---|---|---|
| Clinical trials | - | - | - | - | - | - | - | - | - | - |
| Fogaça et al. (2011) [29] | ? | NA | ? | NA | ? | NA | ? | + | + | + |
| Preclinical trials | - | - | - | - | - | - | - | - | - | - |
| Balanszczuk et al. (2019) [30] | ? | + | ? | ? | ? | ? | + | ? | ? | + |
| Shi et al. (2019) [4] | ? | + | ? | ? | ? | ? | + | ? | ? | + |
| He et al. (2018) [31] | ? | + | ? | ? | ? | ? | ? | ? | ? | + |
| Raabe et al. (2014) [32] | ? | + | ? | ? | ? | ? | ? | ? | ? | + |
Abbreviations: Risk of bias was evaluated using either Cochrane's or Syrcle's tool. Green cells represent low risk of bias, yellow cells represent unclear risk of bias, red cells represent high risk of bias. NA: not applicable (items evaluated only by SYRCLE's but not by Cochrane's tool).
4. DISCUSSION
Although, it seems promising to test the effects of omega-3 on relapse prevention in alcohol addicts, we found very few studies addressing this issue. The closest study to test this hypothesis was conducted by Fogaça et al. (2011) [29]. The highlight of their study is the simultaneous administration of omega-6 and omega-3. The current Western diet contains high levels of omega-6 and low levels of omega-3 leading to an unbalance between omega-6 / omega-3, sometimes reaching a ratio of 20:1, very different from the Paleolithic era diet, which contained the 1:1 ratio [33]. The ideal ratio between omega-6 and omega-3 is still unknown, but some studies claim it should be around 4:1 respectively. Therefore, the administration of omega-6 may have masked the possible benefits of omega-3. Another outstanding aspect in the study by Fogaça et al. (2011) [29] refers to the blood dosages of PUFAs, which remained unchanged. Regarding this result, we can think of two hypotheses: either patients did not follow the prescription or there were problems in the absorption/ metabolization of these acids. In addition, the observation that all patients who completed the study decreased or stopped alcohol consumption, regardless of the group they were allocated to, may reflect only their initial motivation to change behavior [34].
Studies on tobacco dependence have shown that smokers have decreased levels of blood DHA [35, 36] and clinical trials demonstrated that daily administration of fish oil capsules decreased the dependence and craving levels of smokers [36, 37]. Among cocaine-dependent individuals, studies have shown that low levels of fatty acids are related to relapse vulnerability and aggression [38, 39]. There is also evidence of association between supplementation of PUFAs and decreased anxiety and anger among substance users in general [40]. In abstinent alcoholics, the administration of omega-3 reduced perceived stress and basal secretion of cortisol [41]. Some studies have suggested that psychiatric conditions are associated with PUFAs depletion, and maybe a risk factor of suicide [42]. Whilst there are favorable indications of the application of omega-3 in the treatment of addiction or other mood disorders, to date the main evidence of effectiveness is restricted to the treatment of unipolar and bipolar depression [43].
5. Limitations
Although the number of preclinical studies is higher than the clinical ones, they are also scanty. Additionally, as they are methodologically heterogeneous (i.e. different species, methods and omega-related interventions), it is hard to summarize findings and generate robust conclusions. This does not hamper the importance of such preclinical studies, since they allow researchers to study conditions that would hardly be present in human beings.
Conclusion
Despite the reduced number of studies, omega-3 interventions seem promising for alcohol-related outcomes, especially relapse prevention. The four studies included showed that preclinical models might be able to assess the effects of PUFAs on alcohol-related outcomes in behavioral, cellular and molecular levels. Once the actual effects of PUFAs in alcohol dependence in humans are established, these models would be important to determine their mechanisms of action. Therefore, robust randomized, double blind, placebo-controlled clinical trials are needed to elucidate the possible therapeutic effects in preventing relapse into alcoholism.
Acknowledgements
All authors have read and approved the final manuscript.
Consent for Publication
Not applicable.
Funding
The Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP) [grant # 2015/19472-5], Associação Fundo de Incentivo à Pesquisa (AFIP) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) supported this work.
Conflict of Interest
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- 1.Zindel L.R., Kranzler H.R. Pharmacotherapy of alcohol use disorders: seventy-five years of progress. J. Stud. Alcohol Drugs Suppl. 2014;75(Suppl. 17):79–88. doi: 10.15288/jsads.2014.75.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McLellan A.T., Lewis D.C., O’Brien C.P., Kleber H.D. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 2000;284(13):1689–1695. doi: 10.1001/jama.284.13.1689. [DOI] [PubMed] [Google Scholar]
- 3.Maisel N.C., Blodgett J.C., Wilbourne P.L., Humphreys K., Finney J.W. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: When are these medications most helpful? Addiction. 2013;108(2):275–293. doi: 10.1111/j.1360-0443.2012.04054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shi Z., Xie Y., Ren H., He B., Wang M., Wan J.B., Yuan T.F., Yao X., Su H. Fish oil treatment reduces chronic alcohol exposure induced synaptic changes. Addict. Biol. 2019;24(4):577–589. doi: 10.1111/adb.12623. [DOI] [PubMed] [Google Scholar]
- 5.Buydens-Branchey L., Branchey M., Hibbeln J.R. Low plasma levels of docosahexaenoic acid are associated with an increased relapse vulnerability in substance abusers. Am. J. Addict. 2009;18(1):73–80. doi: 10.1080/10550490802544003. [DOI] [PubMed] [Google Scholar]
- 6.Umhau J.C., Zhou W., Thada S., Demar J., Hussein N., Bhattacharjee A.K., Ma K., Majchrzak-Hong S., Herscovitch P., Salem N., Jr, Urish A., Hibbeln J.R., Cunnane S.C., Rapoport S.I., Hirvonen J. Brain docosahexaenoic acid [DHA] incorporation and blood flow are increased in chronic alcoholics: A positron emission tomography study corrected for cerebral atrophy. PLoS One. 2013;8(10):e75333. doi: 10.1371/journal.pone.0075333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nervi A.M., Peluffo R.O., Brenner R.R., Leikin A.I. Effect of ethanol administration on fatty acid desaturation. Lipids. 1980;15(4):263–268. doi: 10.1007/BF02535837. [DOI] [PubMed] [Google Scholar]
- 8.Reitz R.C. Relationship of the ocyl-CoA desaturase to certain membrane fatty acid changes induced by ethanol consumption. Proc. West. Pharmacol. Soc. 1984;27:247–249. [Google Scholar]
- 9.Nakamura M.T., Tang A.B., Villanueva J., Halsted C.H., Phinney S.D. Selective reduction of delta 6 and delta 5 desaturase activities but not delta 9 desaturase in micropigs chronically fed ethanol. J. Clin. Invest. 1994;93(1):450–454. doi: 10.1172/JCI116981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yehuda S. Omega-6/omega-3 ratio and brain-related functions. World Rev. Nutr. Diet. 2003;92:37–56. doi: 10.1159/000073791. [DOI] [PubMed] [Google Scholar]
- 11.Das U.N. Fams, Long-chain polyunsaturated fatty acids in the growth and development of the brain and memory. Nutrition. 2003;19(1):62–65. doi: 10.1016/S0899-9007(02)00852-3. [DOI] [PubMed] [Google Scholar]
- 12.Hermann D., Weber-Fahr W., Sartorius A., Hoerst M., Frischknecht U., Tunc-Skarka N., Perreau-Lenz S., Hansson A.C., Krumm B., Kiefer F., Spanagel R., Mann K., Ende G., Sommer W.H. Translational magnetic resonance spectroscopy reveals excessive central glutamate levels during alcohol withdrawal in humans and rats. Biol. Psychiatry. 2012;71(11):1015–1021. doi: 10.1016/j.biopsych.2011.07.034. [DOI] [PubMed] [Google Scholar]
- 13.Holmes A., Spanagel R., Krystal J.H. Glutamatergic targets for new alcohol medications. Psychopharmacology (Berl.) 2013;229(3):539–554. doi: 10.1007/s00213-013-3226-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalivas P.W. The glutamate homeostasis hypothesis of addiction. Nat. Rev. Neurosci. 2009;10(8):561–572. doi: 10.1038/nrn2515. [DOI] [PubMed] [Google Scholar]
- 15.Spanagel R. Alcoholism: a systems approach from molecular physiology to addictive behavior. Physiol. Rev. 2009;89(2):649–705. doi: 10.1152/physrev.00013.2008. [DOI] [PubMed] [Google Scholar]
- 16.Shiraishi K., Matsuzaki S., Itakura M., Ishida H. Abnormality in membrane fatty acid compositions of cells measured on erythrocyte in alcoholic liver disease. Alcohol. Clin. Exp. Res. 1996;20(1) Suppl.:56A–59A. doi: 10.1111/j.1530-0277.1996.tb01730.x. [DOI] [PubMed] [Google Scholar]
- 17.Pita M.L., Rubio J.M., Murillo M.L., Carreras O., Delgado M.J. Chronic alcoholism decreases polyunsaturated fatty acid levels in human plasma, erythrocytes, and platelets--influence of chronic liver disease. Thromb. Haemost. 1997;78(2):808–812. doi: 10.1055/s-0038-1657633. [DOI] [PubMed] [Google Scholar]
- 18.Varatharajalu R., Garige M., Leckey L.C., Reyes-Gordillo K., Shah R., Lakshman M.J. Protective role of dietary curcumin in the prevention of the oxidative stress induced by chronic alcohol with respect to hepatic injury and antiatherogenic markers. Oxid. Med. Cell. Longev. 2016;••• doi: 10.1155/2016/5017460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meehan E., Beaugé F., Choquart D., Leonard B.E. Influence of an n-6 polyunsaturated fatty acid-enriched diet on the development of tolerance during chronic ethanol administration in rats. Alcohol. Clin. Exp. Res. 1995;19(6):1441–1446. doi: 10.1111/j.1530-0277.1995.tb01005.x. [DOI] [PubMed] [Google Scholar]
- 20.Duffy O., Ménez J.F., Leonard B.E. Attenuation of the effects of chronic ethanol administration in the brain lipid content of the developing rat by an oil enriched in gamma linolenic acid. Drug Alcohol Depend. 1992;31(1):85–89. doi: 10.1016/0376-8716(92)90012-2. [DOI] [PubMed] [Google Scholar]
- 21.Le-Niculescu H., Case N.J., Hulvershorn L., Patel S.D., Bowker D., Gupta J., Bell R., Edenberg H.J., Tsuang M.T., Kuczenski R., Geyer M.A., Rodd Z.A., Niculescu A.B. Convergent functional genomic studies of ω-3 fatty acids in stress reactivity, bipolar disorder and alcoholism. Transl. Psychiatry. 2011;1e:4. doi: 10.1038/tp.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glen I., Glen E., MacDonnell L. Possible pharmacologic approaches to the prevention and treatment of alcohol-related CNS impairment: results of a double blind trial of essential fatty acids. In: Littleton J., editor. Pharmacological Treatments for Alcoholism. London: Croon-Helm; 1984. [Google Scholar]
- 23.Goldstein D.B. Ethanol-induced adaptation in biological membranes. Ann. N. Y. Acad. Sci. 1987;492:103–111. doi: 10.1111/j.1749-6632.1987.tb48658.x. [DOI] [PubMed] [Google Scholar]
- 24.Dyall S.C. 2011.
- 25.Tian C., Fan C., Liu X., Xu F., Qi K. Brain histological changes in young mice submitted to diets with different ratios of n-6/n-3 polyunsaturated fatty acids during maternal pregnancy and lactation. Clin. Nutr. 2011;30(5):659–667. doi: 10.1016/j.clnu.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Tanaka K., Farooqui A.A., Siddiqi N.J., Alhomida A.S., Ong W.Y. Effects of docosahexaenoic acid on neurotransmission. Biomol. Ther. (Seoul) 2012;20(2):152–157. doi: 10.4062/biomolther.2012.20.2.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higgins J.P., Altman D.G., Gøtzsche P.C., Jüni P., Moher D., Oxman A.D., Savovic J., Schulz K.F., Weeks L., Sterne J.A., Cochrane Statistical Methods Group The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. 2011. [DOI] [PMC free article] [PubMed]
- 28.Hooijmans C.R., Rovers M.M., de Vries R.B., Leenaars M., Ritskes-Hoitinga M., Langendam M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014;14:43. doi: 10.1186/1471-2288-14-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fogaça M.N., Santos-Galduróz R.F., Eserian J.K., Galduróz J.C.F. The effects of polyunsaturated fatty acids in alcohol dependence treatment--a double-blind, placebo-controlled pilot study. BMC Clin. Pharmacol. 2011;11:10. doi: 10.1186/1472-6904-11-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Balaszczuk V., Salguero J.A., Villarreal R.N., Scaramuzza R.G., Mendez S., Abate P. 2019. [DOI] [PubMed]
- 31.He J., Huang W., Zheng S., Vigorito M., Chang S.L. Effects of docosahexaenoic acid on locomotor activity in ethanol-treated HIV-1 transgenic rats. J. Neurovirol. 2018;24(1):88–97. doi: 10.1007/s13365-017-0597-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raabe R.C., Mathies L.D., Davies A.G., Bettinger J.C. 2014.
- 33.Simopoulos A.P. An Increase in the omega-6/omega-3 fatty acid ratio increases the risk for obesity. Nutrients. 2016;8(3):128. doi: 10.3390/nu8030128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Witkiewitz K., Litten R.Z., Leggio L. Advances in the science and treatment of alcohol use disorder. Sci. Adv. 2019;5(9):eaax4043. doi: 10.1126/sciadv.aax4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scaglia N., Chatkin J., Chapman K.R., Ferreira I., Wagner M., Selby P., Allard J., Zamel N. The relationship between omega-3 and smoking habit: A cross-sectional study. Lipids Health Dis. 2016;15:61. doi: 10.1186/s12944-016-0220-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zaparoli J.X., Sugawara E.K., de Souza A.A., Tufik S., Galduróz J.C. Omega-3 levels and nicotine dependence: a cross-sectional study and clinical trial. Eur. Addict. Res. 2016;22(3):153–162. doi: 10.1159/000439525. [DOI] [PubMed] [Google Scholar]
- 37.Rabinovitz S. Effects of omega-3 fatty acids on tobacco craving in cigarette smokers: A double-blind, randomized, placebo-controlled pilot study. J. Psychopharmacol. (Oxford) 2014;28(8):804–809. doi: 10.1177/0269881114536477. [DOI] [PubMed] [Google Scholar]
- 38.Buydens-Branchey L., Branchey M., McMakin D.L., Hibbeln J.R. Polyunsaturated fatty acid status and relapse vulnerability in cocaine addicts. Psychiatry Res. 2003;120(1):29–35. doi: 10.1016/S0165-1781(03)00168-9. [DOI] [PubMed] [Google Scholar]
- 39.Buydens-Branchey L., Branchey M., McMakin D.L., Hibbeln J.R. Polyunsaturated fatty acid status and aggression in cocaine addicts. Drug Alcohol Depend. 2003;71(3):319–323. doi: 10.1016/S0376-8716(03)00168-6. [DOI] [PubMed] [Google Scholar]
- 40.Buydens-Branchey L., Branchey M., Hibbeln J.R. Associations between increases in plasma n-3 polyunsaturated fatty acids following supplementation and decreases in anger and anxiety in substance abusers. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2008;32(2):568–575. doi: 10.1016/j.pnpbp.2007.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barbadoro P., Annino I., Ponzio E., Romanelli R.M., D’Errico M.M., Prospero E., Minelli A. Fish oil supplementation reduces cortisol basal levels and perceived stress: a randomized, placebo-controlled trial in abstinent alcoholics. Mol. Nutr. Food Res. 2013;57(6):1110–1114. doi: 10.1002/mnfr.201200676. [DOI] [PubMed] [Google Scholar]
- 42.Pompili M., Longo L., Dominici G., Serafini G., Lamis D.A., Sarris J., Amore M., Girardi P. Polyunsaturated fatty acids and suicide risk in mood disorders: A systematic review. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2017;74(74):43–56. doi: 10.1016/j.pnpbp.2016.11.007. [DOI] [PubMed] [Google Scholar]
- 43.Bozzatello P., Rocca P., Mantelli E., Bellino S. Polyunsaturated fatty acids: What is their role in treatment of psychiatric disorders? Int. J. Mol. Sci. 2019;20(21):E5257. doi: 10.3390/ijms20215257. [DOI] [PMC free article] [PubMed] [Google Scholar]