Abstract
Convalescent plasma therapy has been implemented in a few cases of severe coronavirus disease 2019. No report about convalescent plasma therapy in treating patients with prolonged positivity of SARS-CoV-2 RNA has been published. In this study, we conducted a retrospective observational study in 27 patients with prolonged positivity of SARS-CoV-2 RNA, the clinical benefit of convalescent plasma therapy were analyzed. qRT-PCR test of SARS-CoV-2 RNA turned negative (≤ 7 days) in a part of patients (early negative group, n = 15) after therapy, others (late negative group, n = 12) turned negative in more than 7 days. Pulmonary imaging improvement was confirmed in 7 patients in early negative group and 8 in late negative group after CP therapy. Viral load decreased in early negative group compared with late negative group at day 3, 5, 7 after implementing convalescent plasma therapy. Patients in early negative group had a shorter median length of hospital stay. In conclusion, convalescent plasma therapy might help eliminate virus and shorten length of hospital stay in patients with prolonged positivity of SARS-CoV-2 RNA.
Keywords: Coronavirus disease 2019 (COVID-19), SARS-CoV-2, Prolonged positivity, Convalescent plasma therapy
Introduction
The severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) emerged in late 2019 (Zhu et al. 2020), has been rapidly spreading and causing a worldwide pandemic (Kirby 2020; Saglietto et al. 2020). The pneumonia induced by SARS-CoV-2 is known as coronavirus disease 2019 (COVID-19) (Ivers and Walton 2020). To date, the virus has infected millions of people all over the world.
Recently, many studies about long-term viral duration in COVID-19 patients have been published (Liu et al. 2020; Wan et al. 2020; Zhou et al. 2020; Shi et al. 2020; Yang JR et al. 2020; Li et al. 2020). The longest duration observed was 83 days in one patient’s upper respiratory tract samples (Li et al. 2020). Although the association between viral duration and disease severity or older age was inconsistent in different studies, some studies reported longer viral duration correlated with severe disease or older age in COVID-19 patients (Yan et al. 2020; Zhang YC et al. 2020; Hu et al. 2020). Besides, there are limited evidences about the infectivity of SARS-CoV-2 for patients with prolonged positivity of SARS-CoV-2 RNA (Walsh et al. 2020). Thus, treating prolonged positive patients might be necessary during this COVID-19 pandemic.
By now, no anti-viral therapy has been proven effective in treating COVID-19. Convalescent plasma (CP) therapy is a classical passive antibody therapy used to treat viral pandemic historically, such as influenza A (HIN1) (Hung et al. 2011), Ebola virus disease (Sahr et al. 2017) and SARS (Cheng et al. 2005). Recently case reports showed that CP collected from recovered patients might be effective to treat critically ill patients with COVID-19 (Zhang B et al. 2020; Ahn et al. 2020; Duan et al. 2020; Shen et al. 2020). However, in these studies, critically ill patients at the early stages of illness were mainly aimed. There is not any report about implementing CP therapy in patients with prolonged positivity of SARS-CoV-2 RNA.
We noticed that convalescent plasma was sometimes given to these patients with prolonged positivity of SARS-CoV-2 RNA. Herein, we perform a retrospective study to analyze the clinical benefit of CP therapy in patients with prolonged positivity of SARS-CoV-2 RNA.
Materials and Methods
Design and Study Participants
This single-center retrospective observational study was performed in Jinyintan Hospital, which is one of the earliest designated hospitals for COVID-19 in Wuhan, China. Patients who confirmed COVID-19 admitted into Jinyintan Hospital from January 1 to April 20, 2020, were included for initial screen. We investigated all patients with COVID-19 who received CP therapy during hospitalization without enrolled in any other random control trial. Patients were excluded if their SARS-CoV-2 tests were negative before infusion of CP. The clinical outcomes (discharges, mortality, length of hospital stay) were monitored up to April 25, 2020. The discharged patients in Jinyintan Hospital must need to meet the following criteria: patients with two consecutive negative tests of respiratory specimens; patients’ symptom resolved; no hospitalization was required as assessed by clinicians. In our study, all discharged patients still need to transport to other isolation sites for medical observation for 2 weeks.
In our study, we defined patients with tests of SARS-CoV-2 turned negative ≤ 7 days after the first infusion of CP to be in early negative group (EN group), others were defined to be in late negative group (LN group).
Clinical Information
Clinical information of patients was collected from the electronic medical information system of Jinyintan Hospital, including the following factors: demographic data; date of symptom onset, admission, first CP infusion and discharge; laboratory data before and after infusion of CP, including white blood cell count, neutrophil count, lymphocyte count, liver and kidney function test, and inflammatory factors such as high sensitive C-reaction protein (HsCRP); results of SARS-CoV-2 test and cycle threshold value (Ct value) of quantitative reverse transcription-polymerase chain reaction; patients’ status and treatments before or after the CP therapy, including the vital signs, anti-virus therapy, oxygen therapy, and other treatments; total volume dose of CP; pulmonary imaging examination data; information on complications such as transfusion-related adverse reactions.
Quantitative Reverse Transcription-Polymerase Chain Reaction
Test of SARS-CoV-2 were performed in a laboratory in Jinyintan Hospital. Respiratory tract specimens (including nasopharyngeal, sputum, bronchial alveolar lavage fluid) collected from patient were transferred to the laboratory within 4 h. The quantitative reverse transcription-polymerase chain reaction (RT-PCR) of SARS-CoV-2 has already been described previously (Corman et al. 2020). Total nucleic acid extraction was performed on the specimens using the RNA Viral Kit (Life River). The E gene, N gene, and RdRP gene of SARS-CoV-2 (located in ORFlab reading frame) was detected using a specific kit (Life River), which was approved by the China Food and Drug Administration. Ct value is the number of cycles required for the fluorescent signal to cross the threshold for a positive test, and a higher Ct value is correlated with lower viral load. According to the instruction of the kit, Ct values of specimens with E gene, N gene, and RdRP gene < 43 were considered to be positive, and results were highly reliable.
Artificial Intelligence Analysis of Computed Tomography
The imaging artificial intelligence (AI)-assisted diagnostic system can quickly identify COVID-19, delineate and quantify lesions designed by the Chinese Academy of Sciences, National Biological Information Center, Tsinghua University, and Hospital of Zhongshan University. It is developed by applying advanced AI technologies, such as deep learning, transfer learning, and using the multiple neural network architecture training models. Besides AI, X-ray and computed tomography (CT) images of all patients have been manually reviewed by a group, consisting of three experienced imaging specialists.
CP of Donors
All CP were obtained from donors recovered from COVID-19, the interval between symptom onset and donation of donors were > 3 weeks; all donors must meet the discharge standard of the seventh Trial Version of the New Coronavirus Pneumonia Prevention and Control Program (Chinese National Health Commission 2020). All the donors were confirmed without transfusion-related infectious diseases before donation. Interval between discharge and donation must be > 10 days. The neutralizing antibody titer was evaluated before transfusion, convalescent plasma with titer of > 1:160 were used for patients in our study. After the clinician applying for a blood transfusion, convalescent plasma would be transferred from the blood center to the isolation ward on the same day.
Statistical Analysis
Data were expressed as categorical variable and continuous variable. To compare the EN group and LN group, Chi-square test was used to analyze the categorical variable. For the continuous variable, results of Data were shown as median and inter-quartile range (IQR). Mann–Whitney test was used to perform nonparametric test. A two-sided P value < 0.05 were considered statistically significantly different between the two groups. SPSS 22.0 is used for statistical analysis.
Results
Demographics and Baseline Characteristics of Patients with Prolonged Positivity of SARS-CoV-2 RNA before CP Therapy
As shown in Table 1, a total of 27 adult patients, with a median 44.0 (30.0–47.0) days between symptom onset and last positive test of SARS-CoV-2 RNA before CP therapy, were included. Their median age was 64.0 (57.0–72.0) years and 15 (55.5%) patients were male.
Table 1.
Demographic and clinical characteristics of patient before CP therapy.
| Characteristic | Total n = 27 |
EN group n = 15 |
LN group n = 12 |
P value |
|---|---|---|---|---|
| Age, median (IQR)—y | 64.0 (57.0–72.0) | 63.0 (57.0–72.0) | 65.0 (56.2–78.0) | 0.581 |
| Male sex—no. (%) | 15 (55.5) | 6 (40) | 9 (75) | 0.121 |
| Coexisting chronic disease—no. (%) | ||||
| Hypertension | 12 (44.4) | 6 (40) | 6 (50) | 0.707 |
| Coronary artery disease | 2 (7.4) | 1 (6.6) | 1 (8.3) | 1.000 |
| Diabetes | 2 (7.4) | 1 (6.6) | 1 (8.3) | 1.000 |
| Malignant tumor | 3 (11.1) | 2 (13.3) | 1 (8.3) | 1.000 |
| Neurological disorders | 6 (22.2) | 3 (20) | 3 (25) | 1.000 |
| Chronic kidney disease | 1 (3.7) | 0 | 1 (8.3) | |
| HIV/AIDS | 2 (7.4) | 0 | 2 (16.6) | |
| Chronic liver disease | 2 (7.4) | 0 | 1 (8.3) | |
| Laboratory tests before transfusion | ||||
| White-cell count, median (IQR)—(× 10−9/L) | 5.37 (4.81–7.99) | 6.49 (4.81–7.99) | 4.16(3.31–7.47) | 0.075 |
| Neutrophil count, median (IQR)—(× 10−9/L) | 3.57 (2.58–5.58) | 4.25 (2.94–5.70) | 2.83(1.91–4.26) | 0.083 |
| Lymphocyte count, median (IQR)—(× 10−9/L) | 1.24 (0.62–1.85) | 1.44 (0.46–1.85) | 1.07 (0.64–1.33) | 0.614 |
| Platelet count, median (IQR)—(× 10−9/L) | 175 (137–194) | 185 (163–219) | 148 (78–183) | 0.054 |
| Hematocrit, median (IQR)—(%) | 33.4 (28.4–38.4) | 33.4 (26.4–40.1) | 34.1 (28.7–38.3) | 0.943 |
| Serum creatinine, median (IQR)—(μmol/L) | 69.1 (57.4–75.0) | 66.0 (56.0–75.0) | 70.5 (59.0–112.0) | 0.683 |
| Total bilirubin, median (IQR)—(μmol/L) | 11.4 (8.6–18.0) | 11.9 (9.4–18.0) | 10.8(8.1–20.0) | 0.648 |
| Alanine aminotransferase, median (IQR)— (U/L) | 17.0 (10.0-28.0) | 24.0 (13.0–33.0) | 13.0 (8.5–26.0) | 0.103 |
| Aspartate aminotransferase, median (IQR)—(U/L) | 26.0 (20.0–42.0.0) | 27.0 (21.0–60.0) | 24.5 (15.5–34.5) | 0.516 |
| High-sensitivity C-reactive protein, median (IQR)—(mg/L) | 3.1 (0.8–37.8) | 3.1 (0.8–38.1) | 3.9 (0.7–42.8) | 0.733 |
We conducted a subgroup analysis between patients of EN group and LN group. Demographic data was shown as Table 1, median age, percentage of male patients, coexisting chronic diseases of patients in both groups were not significantly different (Table 1). Each patient of both groups underwent laboratory tests before CP therapy including white cell count, neutrophil count, lymphocyte count, platelet count, hematocrit, serum creatinine test, serum total bilirubin, serum alanine aminotransferase, serum aspartate aminotransferase, and hsCRP test, and the results were shown as Table 1.
As shown in Table 2, patients in both groups have a longer median interval between symptom onset and date of CP transfusion as compared to former reports [40.0 (26.0–47.0) days in EN group and 45.5 (41.2–57.0) days in LN group]. The median body temperature and oxygen therapy before CP transfusion were not significant different. The median fraction of inspiration O2 (FiO2), peripheral oxygen saturation and anti-viral therapies of both groups before CP therapy are shown in Table 2. Before transfusion, eight patients in EN group and seven in LN group received broad-spectrum antibiotic therapy, three patients in EN group and two in LN group received corticoid therapy after admission. Four patients in EN group and two in LN group received infusion of immunoglobulin after admission. As shown in Tables 1, 2, demographics and baseline characteristics of patients in EN group and LN group were not significant different before CP therapy.
Table 2.
Patients’ status and treatments received before CP therapy.
| Characteristic | Total n = 27 |
EN group n = 15 |
LN group n = 12 |
P value |
|---|---|---|---|---|
| Interval between symptom onset and transfusion, median (IQR)—d | 45.0 (35.0–49.0) | 40.0 (26.0–47.0) | 45.5 (41.2–57.0) | 0.075 |
| Interval between symptom onset and last positive test before CP therapy, median (IQR)—d | 44.0 (30.0–47.0) | 39.0 (24.0–45.0) | 44.5 (38.2–54.7) | 0.126 |
| Body temperature, median (IQR)—°C | 36.9 (36.6–37.0) | 36.8 (36.5–37.2) | 36.9 (36.7–37.0) | 0.516 |
| Fever—no. (%) | 6 (22.2) | 4 (26.6) | 2 (16.6) | 0.662 |
| Oxygen therapy—no. (%) | ||||
| No oxygen treatment | 19 (70.3) | 10 (66.6) | 9 (75.0) | 0.696 |
| Nasal catheter oxygen therapy | 3 (11.1) | 3 (20) | 0 (0) | |
| Mechanical ventilation | 5 (18.5) | 2 (13.3) | 3 (25.0) | 1.000 |
| Extracorporeal membrane oxygenation | 1 (3.7) | 1 (6.6) | 0 (0) | |
| Fraction of inspiration O2 (n = 26), median (IQR)—% | 21.0 (21.0–33.0) | 21.0 (21.0–33.0) | 21.0 (21.0–35.2) | 0.809 |
| Respiratory rate > 24 times/min—no. (%) | 5 (18.5) | 3 (20) | 2 (16.6) | 1.000 |
| Peripheral oxygen saturation, median (IQR)—% | 98.0 (97.0–99.0) | 97.0 (97.0–99.0) | 97.0 (97.0–98.5) | 0.905 |
| Vasopressors—no. (%) | 4 (14.8) | 1 (6.6) | 3 (25.0) | 0.294 |
| Anti-virus therapy—no. (%) | ||||
| Ribavirin | 4 (14.8) | 2 (13.3) | 2 (16.6) | 1.000 |
| Lopinavir | 8 (29.6) | 3 (20.0) | 5 (41.6) | 0.398 |
| Favipiravir | 2 (7.4) | 2 (13.3) | 0 (0) | |
| Definite or suspected coinfection—no. (%) | 6 (22.2) | 3 (20.0) | 3 (25.0) | 0.433 |
| Broad-spectrum antibiotic therapy—no. (%) | 15 (55.5) | 8 (53.3) | 7 (58.3) | 1.000 |
| Corticoid therapy—no. (%) | 5 (18.5) | 3 (20) | 2 (16.6) | 1.000 |
| Immunoglobulin therapy—no. (%) | 6 (22.2) | 4 (26.6) | 2 (16.6) | 0.662 |
Clinical Benefit and Outcome of Patients with Prolonged Positivity of SARS-CoV-2 RNA after CP Therapy
As shown in Table 3, the median and interquartile ranged total volume of CP transfusion was 400 (200–400) mL in EN group and 400 (400–800) mL in LN group. No adverse reactions related to blood transfusion were found during infusion in both groups. The median interval between transfusion and discharge was 7.0 (4.0–11.0) days in EN group and 24.0 (14.7–28.7) days in LN group. Most patients underwent X-ray or CT scan before and after transfusion (EN group = 8; LN group = 12), and pulmonary imaging improvement was confirmed in 7 patients in EN group and 8 in LN group after CP therapy.
Table 3.
Patients’ status after transfusion and outcome after CP therapy.
| Characteristic | Total n = 27 |
EN group n = 15 |
LN group n = 12 |
P value |
|---|---|---|---|---|
| Total volume dose of CP, median (IQR)—mL | 400 (200–600) | 400 (200–400) | 400 (400–800) | 0.861 |
| Transfusion-related adverse reactions—no. (%) | 0 (0) | 0 (0) | 0 (0) | |
| Interval between first transfusion and discharge, median (IQR)—d | 11.0 (6.0–25.0) | 7.0 (4.0–11.0) | 24.0 (14.7–28.7) | |
| Pulmonary imaging improvement | 15/20 | 7/8 | 8/12 | 0.603 |
| Length of hospital stay, median (IQR)—d | 43.0 (24.0–54.0) | 37.0 (19.0–50.0) | 52.0 (35.0–63.7) | |
| Mortality of 60 days—no. (%) | 3 (11.1) | 0 (0) | 3 (25) |
The median length of hospitalization in EN group was 37.0 days and 52.0 days in LN group, as shown in Table 3. Due to the definition of EN group and LN group, the median length of hospitalization in LN group was much longer than EN group, thus we didn’t make a comparative analysis. Three patients died in LN group within 60 days, two died from refractory hypoxemia and one in LN group died from severe septic shock. No patients died in EN group within 60 days.
Comparison of Lung Imaging before and after CP Therapy in Patients with Prolonged Positivity of SARS-CoV-2 RNA
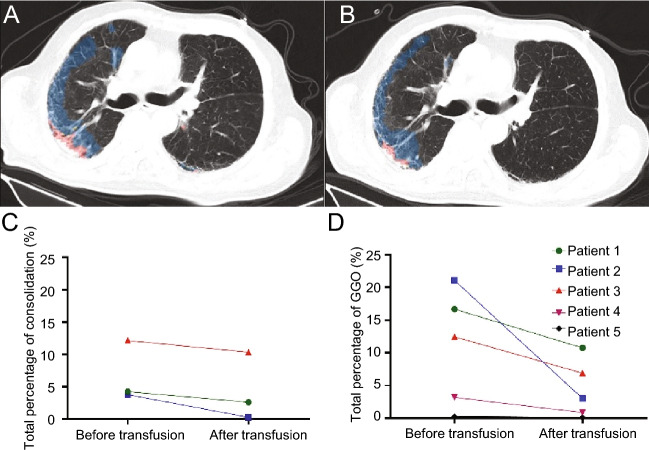
Five patients in our study underwent CT scan before (within 3 days) and reexamination after transfusion (within 5–8 days). For these patients, comparison and analysis of CT images were performed before and after transfusion by using the AI-assisted diagnostic system described above (Fig. 1A, 1B). Three (60%) patients showed as consolidation of CT images before CP therapy (Fig. 1C), five all showed as ground-glass opacity (GGO) (Fig. 1D) before CP therapy, which were similar to the former report about CT findings in COVID-19 patients (Adair and Ledermann 2020). After transfusion, the total consolidation percentage decreased after transfusion in three patients (Fig. 1C), and the total GGO percentage decreased in five all patients’ CT images (Fig. 1D).
Fig. 1.
CT images before and after CP therapy. A Results of AI-assisted diagnostic system in patient 2 before CPT, blue areas represent GGO in CT images, red areas represent consolidation in CT images. B Results of AI-assisted diagnostic system in patient 2 after CPT. C Consolidation of CT in patients 1, 2, and 3 decreased after the transfusion. D GGO of CT in patients 1, 2, 3, 4, and 5 decreased after CP therapy.
Variation Trend of Viral Load before and after CP Therapy in Patients with Prolonged Positivity of SARS-CoV-2 RNA
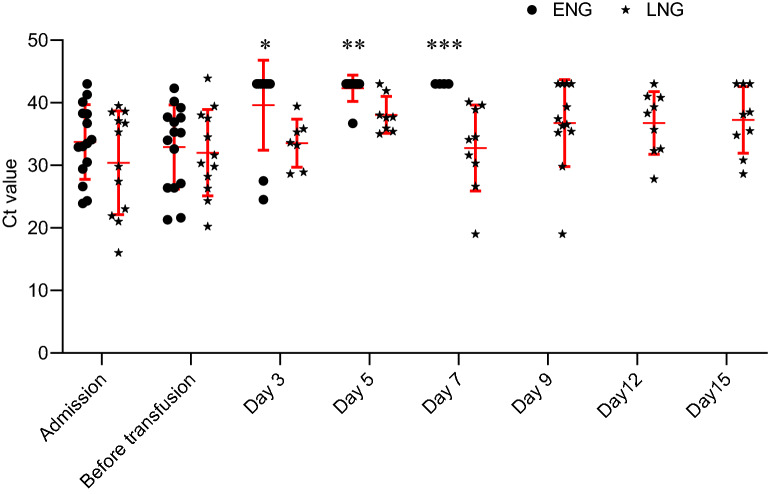
After admission, patients in both groups underwent SARS-CoV-2 tests by using RT-PCR as described above. The variation trend of Ct value in both groups are show as Fig. 2. The median Ct value on admission is 33.0 (28.7–38.2) in EN group and 32.5 (22.1–38.1) in LN group, without significant differences (P = 0.591, Fig. 2). Besides, the median Ct values was not significant between EN group and LN group before transfusion [34.0 (26.4–38.3) vs. 30.9 (26.7–35.7), P = 0.591, Fig. 2]. After transfusion, Ct values of patients in EN group increased and > 43 within 7 days gradually and most of them (n = 13) discharged within 10 days and were unable to detect at 9, 12, and 15 days. Conversely, the median Ct values of patients in LN group remained < 43 at 3, 5, 7, 9, and 12 days after transfusion, 6 patients in LN group still remained < 43 at 15 days after transfusion (Fig. 2).
Fig. 2.
Variation trend of viral load of patients before and after CP therapy: Ct value of < 43 is defined to be positive, and Ct value of > 47 would be undetectable. *The median Ct value in early negative group (ENG) was significantly greater than late negative group (LNG) on day 3 after the transfusion, P = 0.043. **: The median Ct value in early negative group was significantly greater than late negative group on day 5, P = 0.008. ***: The median Ct value in early negative group was significantly greater than late negative group on day 7, P = 0.003.
Discussion
Our study explored the efficiency of CP therapy in COVID-19 patients at a later stage of the illness. All patients with prolonged positivity of SARS-CoV-2 RNA in our study were implemented CP therapy including mild cases. We confirmed that the viral load rapidly decreased after CP therapy in some patients (EN group), whereas others remain positive 7 days after CP therapy (LN group). The difference in baseline information, viral load, and other interventions was not significant before transfusion. After CP therapy, more than half patients obtained a rapid decrease of viral load.
CP therapy is a classic therapy against pandemic that can be traced back to the early twentieth century and clinicians treated the 1918 Spanish influenza with convalescent sera (Luke et al. 2006), which was found to be effective in decreasing the mortality of 1918 Spanish influenza pandemic. In the 21st century researches have shown that CP therapy effectively and safely treats H1N1 and SARS at the early stage of illness. Besides SARS and H1N1, there is also some anecdotal information on the use of convalescent serum in seriously ill individuals. Two patients diagnosed with Ebola viral disease received CP therapy on the early stage of illness (day 8 and 3 after symptom onset) and recovered without serious long-term sequelae to date (Kraft et al. 2015). One patient diagnosed with H5N1 received CP therapy on day 8 after symptom onset recovered and successfully discharged (Zhou et al. 2007). Thus, CP therapy might be an effective therapy for certain viral diseases, especially in early stage of illness.
However, to our knowledge, no reports about convalescent plasma therapy in viral disease at later stage have been published. Recently one case report suggests efficiency of CP therapy in treating 5 patients with severe COVID-19 (Corman et al. 2020), the interval between the symptom onset and transfusion was < 20 days. However, the interval in our study is much longer than the previously mentioned report. In our study, empirical anti-viral therapies were already implemented in these patients, but Ct value of respiratory tract specimens collected from these patients were still < 43 before CP therapy, represented a high viral load. These patients with prolonged positivity of SARS-CoV-2 RNA [44.0 (30.0–47.0) days] still needed to hospitalize and separate, which may cause a huge cost during COVID-19 pandemic. In fact, individual human case studies reported long periods of viral shedding that in Middle East respiratory syndrome (MERS) pandemic (Kraaij-Dirkzwager et al. 2014; Spanakis et al. 2014). One report showed that 42-days positive test outcome in RT-PCR assay of MERS in a healthcare worker in Saudi Araba (Al-Gethamy et al. 2015). Recently, a report have shown long-term coexisting of SARS-CoV-2 in some patients, and one of them did not produce any SARS-CoV-2—specific IgG with a positive test of SARS-CoV-2 in sputum after 46 days of illness (Wang B et al. 2020). No specific therapies have proven to treat patients with prolonged positivity of SARS-CoV-2 RNA. In our study, more than half of the prolonged positive patients (EN group) met the discharge standard within 7 days and discharged rapidly after CP therapy. Thus, patients with prolonged positivity of SARS-CoV-2 RNA might benefit from CP therapy with shorter length of hospitalization and less cost. Besides, RT-PCR of SARS-CoV-2 turned negative within 15 days after first infusion in five patients in LN group, one turned negative at 21 days, and six were still positive until the deadline.
Our research showed that the viral load of the respiratory tract specimen in two groups differed after CP therapy. Viral load may correlate with transmission potential in COVID-19 (Little et al. 2020). Recently, a report have shown viral load that was detected in the asymptomatic patient was similar to that in the symptomatic patients, which suggested the transmission potential of asymptomatic or minimally symptomatic patients (Zou et al. 2020). Besides, CP treatment may discontinue SARS-CoV-2 shedding although didn’t reduce mortality in critically end-stage COVID-19 patients (Zeng et al. 2020). In our study most patients might still remain transmission potential even though symptom were mild before CP therapy. Thus, implementing CP therapy in patients with prolonged positivity of SARS-CoV-2 RNA might help to decrease viral road and potential of transmission in our study.
In addition, Reports suggests the mortality of severe COVID-19 patients was more than 60% at 28 days (Yang X et al. 2020a, 2020b; Wang Z et al. 2020). Whether CP therapy can reduce mortality of patient with COVID-19 is unclear in our study. Symptoms of most patients in our study were mild, so we didn’t do a mortality analysis. After CP therapy, three patients in LN group died in our study. We assumed that virus duration might be a vital reason of death in these three patients for their viral durations were quite long (59, 53, 53 days).
This study has several limitations. First, it is a retrospective observational study, a randomized double-blind trial would be more accurate to assess the efficacy of CP therapy in COVID-19. Second, symptoms of most patients in our study were mild, including more critically ill patients would be helpful in determining whether CP therapy could reduce mortality in COVID-19. Third, no studies have reported the appropriate time and dosage of CP implemented in patients with COVID-19; therefore, all decisions of CP therapy intervention were made by clinicians empirically. Forth, we did not monitor the neutralizing antibody in patients after CP therapy.
In conclusion, this retrospective observational study on CP therapy shows that patients with prolonged positivity of SARS-CoV-2 RNA can benefit from a rapid decrease of viral load and improvement in pulmonary images. The appropriate time to implement CP therapy and the optimal CP dosage are still to be explored in the future.
Acknowledgements
This study was supported by the Fundamental Research Funds for the Central Universities (2020kfyXGYJ092).
Author Contributions
YW, KH and LR collected the epidemiological and clinical data. XY, SP, JZ, JX, LC summarised all data. YW, KH and LR drafted the manuscript. YS and CH revised the final manuscript and approved the manuscript.
Compliance with Ethical Standards
Conflict of interest
The authors declare that they have no conflict of interest.
Animal and Human Rights Statement
This study was approved by the ethical committee from Jinyintan Hospital. Written informed consent was waived due to the rapid emergence of this infectious disease.
Footnotes
Yongran Wu, Ke Hong and Lianguo Ruan contributed equally to this work.
Contributor Information
Chaolin Huang, Email: chaolin2020@163.com.
You Shang, Email: you_shanghust@163.com.
References
- Adair LB, 2nd, Ledermann EJ. Chest CT findings of early and progressive phase COVID-19 infection from a US patient. Radiol Case Rep. 2020;15:819–824. doi: 10.1016/j.radcr.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn JY, Sohn Y, Lee SH, Cho Y, Hyun JH, Baek YJ, Jeong SJ, Kim JH, Ku NS, Yeom JS, Roh J, Ahn MY, Chin BS, Kim YS, Lee H, Yong D, Kim HO, Kim S, Choi JY. Use of convalescent plasma therapy in two COVID-19 patients with acute respiratory distress syndrome in Korea. J Korean Med Sci. 2020;35:e149. doi: 10.3346/jkms.2020.35.e149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Gethamy M, Corman VM, Hussain R, Al-Tawfiq JA, Drosten C, Memish ZA. A case of long-term excretion and subclinical infection with Middle East respiratory syndrome coronavirus in a healthcare worker. Clin Infect Dis. 2015;60:973–974. doi: 10.1093/cid/ciu1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Wong R, Soo YO, Wong WS, Lee CK, Ng MH, Chan P, Wong KC, Leung CB, Cheng G. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24:44–46. doi: 10.1007/s10096-004-1271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinese National Health Commission (2020) New coronavirus pneumonia prevention and control program. Accessed on http://www.nhc.gov.cn/yzygj/s7653pd/202003/056b2ce9e13142e6a70ec08ef970f1e8.shtml
- Corman VM, Landt O, Kaiser M, Molenkamp R, Meijer A, Chu DK, Bleicker T, Brünink S, Schneider J, Schmidt ML, Mulders DG, Haagmans BL, van der Veer B, van den Brink S, Wijsman L, Goderski G, Romette JL, Ellis J, Zambon M, Peiris M, Goossens H, Reusken C, Koopmans MP, Drosten C. Detection of 2019 novel coronavirus (2019-nCoV) by real-time RT-PCR. Euro Surveill. 2020;25:2000045. doi: 10.2807/1560-7917.ES.2020.25.3.2000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan K, Liu B, Li C, Zhang H, Yu T, Qu J, Zhou M, Chen L, Meng S, Hu Y, Peng C, Yuan M, Huang J, Wang Z, Yu J, Gao X, Wang D, Yu X, Li L, Zhang J, Wu X, Li B, Xu Y, Chen W, Peng Y, Hu Y, Lin L, Liu X, Huang S, Zhou Z, Zhang L, Wang Y, Zhang Z, Deng K, Xia Z, Gong Q, Zhang W, Zheng X, Liu Y, Yang H, Zhou D, Yu D, Hou J, Shi Z, Chen S, Chen Z, Zhang X, Yang X. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A. 2020;117:9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X, Xing Y, Jia J, Ni W, Liang J, Zhao D, Song X, Gao R, Jiang F. Factors associated with negative conversion of viral RNA in patients hospitalized with COVID-19. Sci Total Environ. 2020;728:138812. doi: 10.1016/j.scitotenv.2020.138812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung IF, To KK, Lee CK, Lee KL, Chan K, Yan WW, Liu R, Watt CL, Chan WM, Lai KY, Koo CK, Buckley T, Chow FL, Wong KK, Chan HS, Ching CK, Tang BS, Lau CC, Li IW, Liu SH, Chan KH, Lin CK, Yuen KY. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis. 2011;52:447–456. doi: 10.1093/cid/ciq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivers LC, Walton DA. Novel coronavirus disease (COVID-19): global health equity in pandemic response. Am J Trop Med Hyg. 2020;102:1149–1150. doi: 10.4269/ajtmh.20-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirby T. South America prepares for the impact of COVID-19. Lancet Respir Med. 2020;8:551–552. doi: 10.1016/S2213-2600(20)30218-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraaij-Dirkzwager M, Timen A, Dirksen K, Gelinck L, Leyten E, Groeneveld P, Jansen C, Jonges M, Raj S, Thurkow I, van Gageldonk-Lafeber R, van der Eijk A, Koopmans M, MERS-CoV Outbreak Investigation Team of the Netherlands (2014) Middle East respiratory syndrome coronavirus (MERS-CoV) infections in two returning travellers in the Netherlands, May 2014. Euro Surveill 19:20817 [DOI] [PubMed]
- Kraft CS, Hewlett AL, Koepsell S, Winkler AM, Kratochvil CJ, Larson L, Varkey JB, Mehta AK, Lyon GM 3rd, Friedman-Moraco RJ, Marconi VC, Hill CE, Sullivan JN, Johnson DW, Lisco SJ, Mulligan MJ, Uyeki TM, McElroy AK, Sealy T, Campbell S, Spiropoulou C, Ströher U, Crozier I, Sacra R, Connor MJ Jr, Sueblinvong V, Franch HA, Smith PW, Ribner BS; Nebraska Biocontainment Unit and The Emory Serious Communicable Diseases Unit (2015) The use of TKM-100802 and convalescent plasma in 2 patients with Ebola virus disease in the United States. Clin Infect Dis 61:496–502 [DOI] [PMC free article] [PubMed]
- Li N, Wang X, Lv T. Prolonged SARS-CoV-2 RNA shedding: not a rare phenomenon. J Med Virol. 2020 doi: 10.1002/jmv.25952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little P, Read RC, Amlôt R, Chadborn T, Rice C, Bostock J, Yardley L. Reducing risks from coronavirus transmission in the home-the role of viral load. BMJ. 2020;369:m1728. doi: 10.1136/bmj.m1728. [DOI] [PubMed] [Google Scholar]
- Liu WD, Chang SY, Wang JT, Tsai MJ, Hung CC, Hsu CL, Chang SC. Prolonged virus shedding even after seroconversion in a patient with COVID-19. J Infect. 2020;81:318–356. doi: 10.1016/j.jinf.2020.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luke TC, Kilbane EM, Jackson JL, Hoffman SL. Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment. Ann Intern Med. 2006;145:599–609. doi: 10.7326/0003-4819-145-8-200610170-00139. [DOI] [PubMed] [Google Scholar]
- Saglietto A, D’Ascenzo F, Zoccai GB, De Ferrari GM. COVID-19 in Europe: the Italian lesson. Lancet. 2020;395:1110–1111. doi: 10.1016/S0140-6736(20)30690-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahr F, Ansumana R, Massaquoi TA, Idriss BR, Sesay FR, Lamin JM, Baker S, Nicol S, Conton B, Johnson W, Abiri OT, Kargbo O, Kamara P, Goba A, Russell JB, Gevao SM. Evaluation of convalescent whole blood for treating Ebola Virus Disease in Freetown, Sierra Leone. J Infect. 2017;74:302–309. doi: 10.1016/j.jinf.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, Wang F, Li D, Yang M, Xing L, Wei J, Xiao H, Yang Y, Qu J, Qing L, Chen L, Xu Z, Peng L, Li Y, Zheng H, Chen F, Huang K, Jiang Y, Liu D, Zhang Z, Liu Y, Liu L. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323:1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi D, Wu W, Wang Q, Xu K, Xie J, Wu J, Lv L, Sheng J, Guo J, Wang K, Fang D, Li Y, Li L. Clinical characteristics and factors associated with long-term viral excretion in patients with SARS-CoV-2 infection: a single center 28-day study. J Infect Dis. 2020;222:910–918. doi: 10.1093/infdis/jiaa388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spanakis N, Tsiodras S, Haagmans BL, Raj VS, Pontikis K, Koutsoukou A, Koulouris NG, Osterhaus AD, Koopmans MP, Tsakris A. Virological and serological analysis of a recent Middle East respiratory syndrome coronavirus infection case on a triple combination antiviral regimen. Int J Antimicrob Agents. 2014;44:528–532. doi: 10.1016/j.ijantimicag.2014.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh KA, Jordan K, Clyne B, Rohde D, Drummond L, Byrne P, Ahern S, Carty PG, O’Brien KK, O’Murchu E, O’Neill M, Smith SM, Ryan M, Harrington P. SARS-CoV-2 detection, viral load and infectivity over the course of an infection: SARS-CoV-2 detection, viral load and infectivity. J Infect. 2020;81:357–371. doi: 10.1016/j.jinf.2020.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan R, Mao ZQ, He LY, Hu YC, Chen W. Evidence from two cases of asymptomatic infection with SARS-CoV-2: are 14 days of isolation sufficient. Int J Infect Dis. 2020;95:174–175. doi: 10.1016/j.ijid.2020.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Wang L, Kong X, Geng J, Xiao D, Ma C, Jiang XM, Wang PH. Long-term coexistence of SARS-CoV-2 with antibody response in COVID-19 patients. J Med Virol. 2020 doi: 10.1002/jmv.25946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Yang B, Li Q, Wen L, Zhang R. Clinical features of 69 cases with coronavirus disease 2019 in Wuhan, China. Clin Infect Dis. 2020;71:769–777. doi: 10.1093/cid/ciaa272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan D, Liu XY, Zhu YN, Huang L, Dan BT, Zhang GJ, Gao YH. Factors associated with prolonged viral shedding and impact of Lopinavir/Ritonavir treatment in hospitalised non-critically ill patients with SARS-CoV-2 infection. Eur Respir J. 2020;56:2000799. doi: 10.1183/13993003.00799-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JR, Deng DT, Wu N, Yang B, Li HJ, Pan XB. Persistent viral RNA positivity during the recovery period of a patient with SARS-CoV-2 infection. J Med Virol. 2020 doi: 10.1002/jmv.25940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Yu Y, Xu J, Shu H, Xia J, Liu H, Wu Y, Zhang L, Yu Z, Fang M, Yu T, Wang Y, Pan S, Zou X, Yuan S, Shang Y. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8:475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Yang Q, Wang Y, Wu Y, Xu J, Yu Y, Shang Y. Thrombocytopenia and its association with mortality in patients with COVID-19. J Thromb Haemost. 2020;18:1469–1472. doi: 10.1111/jth.14848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yongchen Z, Shen H, Wang X, Shi X, Li Y, Yan J, Chen Y, Gu B. Different longitudinal patterns of nucleic acid and serology testing results based on disease severity of COVID-19 patients. Emerg Microbes Infect. 2020;9:833–836. doi: 10.1080/22221751.2020.1756699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng QL, Yu ZJ, Gou JJ, Li GM, Ma SH, Zhang GF, Xu JH, Lin WB, Cui GL, Zhang MM, Li C, Wang ZS, Zhang ZH, Liu ZS. Effect of convalescent plasma therapy on viral shedding and survival in COVID-19 patients. J Infect Dis. 2020;222:38–43. doi: 10.1093/infdis/jiaa228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Liu S, Tan T, Huang W, Dong Y, Chen L, Chen Q, Zhang L, Zhong Q, Zhang X, Zou Y, Zhang S. Treatment with convalescent plasma for critically ill patients with SARS-CoV-2 infection. Chest. 2020;158:e9–e13. doi: 10.1016/j.chest.2020.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou B, Zhong N, Guan Y. Treatment with convalescent plasma for influenza A (H5N1) infection. N Engl J Med. 2007;357:1450–1451. doi: 10.1056/NEJMc070359. [DOI] [PubMed] [Google Scholar]
- Zhou B, She J, Wang Y, Ma X. The duration of viral shedding of discharged patients with severe COVID-19. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, Zhao X, Huang B, Shi W, Lu R, Niu P, Zhan F, Ma X, Wang D, Xu W, Wu G, Gao GF, Tan W, China Novel Coronavirus Investigating and Research Team (2020) China novel coronavirus investigating and research team. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med 382:727–733 [DOI] [PMC free article] [PubMed]
- Zou L, Ruan F, Huang M, Liang L, Huang H, Hong Z, Yu J, Kang M, Song Y, Xia J, Guo Q, Song T, He J, Yen HL, Peiris M, Wu J. SARS-CoV-2 viral load in upper respiratory specimens of infected patients. N Engl J Med. 2020;382:1177–1179. doi: 10.1056/NEJMc2001737. [DOI] [PMC free article] [PubMed] [Google Scholar]