Abstract
Background
Dual pathway inhibition with 2.5 mg rivaroxaban twice daily plus 100 mg aspirin once daily may be a promising alternative to 100 mg aspirin antiplatelet therapy for the prevention of cardiovascular events in patients with coronary artery disease and/or peripheral arterial disease. However, treatment costs and bleeding risks are higher, and there is another treatment option for peripheral arterial disease, 75 mg clopidogrel. A comprehensive assessment of benefits, risks and costs of dual pathway inhibition versus standard of care is needed.
Methods
We used a state transition model including cardiovascular, ischaemic limb and bleeding events to compare dual pathway inhibition to aspirin antiplatelet therapy in coronary artery disease, and additionally to clopidogrel antiplatelet therapy in peripheral arterial disease patients. We calculated the incremental cost-effectiveness ratio from costs and quality-adjusted life-years of lifelong treatment, and the cost-effectiveness probability at a €50,000/quality-adjusted life-year threshold.
Results
Quality-adjusted life-years and costs of dual pathway inhibition were highest, the incremental cost-effectiveness ratios versus aspirin were €32,109 in coronary artery disease and €26,381 in peripheral arterial disease patients, with 92% and 56% cost-effectiveness probability, respectively (clopidogrel was extendedly dominated). Incremental cost-effectiveness ratios were below €20,000 in comorbid peripheral arterial disease patients and coronary artery disease patients younger than 65 years, incremental cost-effectiveness ratios were above €50,000 in carotid artery disease patients and coronary artery disease patients older than 75 years.
Conclusion
Lifelong preventive treatment of coronary artery disease and peripheral arterial disease patients at risk of cardiovascular events with dual pathway inhibition improves health outcomes and seems overall cost-effective relative to aspirin antiplatelet therapy and also to clopidogrel antiplatelet therapy for peripheral arterial disease, particularly in comorbid patients, but not in older patients and in carotid artery disease patients. These findings may warrant a targeted approach.
Keywords: Peripheral arterial disease, coronary artery disease, rivaroxaban, aspirin, clopidogrel, cost-benefit analysis
Introduction
Rivaroxaban is a selective oral factor Xa inhibitor that can be taken in combination with aspirin for cardiovascular risk reduction in patients with a history of cardiovascular disease.1 The COMPASS trial assessed the effectiveness of dual pathway inhibition (DPI) with rivaroxaban 2.5 mg twice daily plus 100 mg aspirin once daily (European Medicines Agency-recommended dose for atherosclerotic event prevention) compared with single antiplatelet therapy (ATP) with 100 mg aspirin in coronary artery disease (CAD) and peripheral arterial disease (PAD) patients.1,2 A cost-effectiveness analysis quantifying costs and health effects (quality of life (QoL) and survival) of DPI and relevant comparators, including clopidogrel 75 mg for PAD, is needed to support decision-making regarding the treatment of CAD and PAD patients with DPI.
The main outcome of the COMPASS trial was major adverse cardiovascular events (MACE), the composite endpoint of non-fatal myocardial infarction (MI), ischaemic and haemorrhagic stroke (IS and HS) and cardiovascular death, and in PAD patients also, major adverse limb events (MALE), the composite endpoint of acute and chronic limb ischaemia and major vascular amputation. The trial showed a reduction of MACE and MALE with DPI versus aspirin ATP (CAD: hazard ratio (HR) MACE 0.74, 95% confidence interval (CI) 0.65–0.86;2 PAD: HR MACE 0.72, 95% CI 0.57–0.90,1 HR MALE 0.54, 95% CI 0.35–0.82).1 However, the number of major bleedings was also higher (HR 1.66, 95% CI 1.37–2.03 in CAD,2 HR 1.61, 95% CI 1.12–2.31 in PAD).1
Risk reduction with anticoagulant and antiplatelet therapies are cornerstones in preventing cardiovascular and ischaemic limb events, which are responsible for the substantial morbidity and mortality associated with cardiovascular disease.3,4 New anticoagulant drugs such as rivaroxaban may reduce this disease burden. However, adding costly new drugs to the treatment regimen may put considerable health and financial burden on patients and society. Recently, health technology assessment bodies in England and The Netherlands recommended DPI for CAD and PAD treatment based on analyses provided by the manufacturer.5,6 A thorough assessment of health and financial effects by an independent party is needed.
Based on results of the COMPASS trial and other literature, we estimated the cost-effectiveness of DPI with rivaroxaban plus aspirin versus aspirin ATP in CAD and PAD patients. Using a decision analytical model allowed us to consider a lifetime time horizon and to include clopidogrel ATP for PAD, which was not included in the COMPASS trial. The analysis reflects the costs and health consequences of cardiovascular events, limb events and bleeding events, and thereby addresses the trade-off between benefits and risks, and health outcomes and costs. The aim of this study is to provide a comprehensive assessment of the value of DPI in CAD and PAD patients.
Methods
Cost-effectiveness analyses compare health outcomes and costs of two or more interventions. Relevant health states (e.g. ‘stable disease’, ‘active disease’ and ‘dead’) are defined with specified costs, QoL and probabilities for events which are likely to be treatment dependent. Often several sources inform these parameters. By simulating the progression of a patient population through the health states over time, the costs and health outcomes, in this case from the Dutch societal perspective, are estimated. Health outcomes are expressed in life years and in quality-adjusted life years (QALYs, number of life years multiplied with the QoL during those years). The incremental cost-effectiveness ratio (ICER) summarises the results as the additional costs per additional QALY of one intervention compared with another.
This study follows the recommendations of the Consolidated Health Economic Evaluation Reporting Standards (CHEERS).7 Future health outcomes are discounted at 1.5%, costs at 4% according to Dutch guidelines.8
Intervention, comparator and patient population
The cost-effectiveness of 2.5 mg rivaroxaban twice daily plus 100 mg aspirin once daily (DPI), was compared with 100 mg aspirin once daily and to 75 mg clopidogrel once daily (through an indirect comparison) for PAD patients, as recommend in the guidelines.3,4
The patient populations were defined as in the COMPASS trial,1,2 and were similar to Dutch CAD and PAD populations according to expert opinion. CAD patients had a history of MI, multi-vessel CAD, angina, or coronary revascularisation and a risk factor. PAD patients had been diagnosed via ankle-brachial index or a history of cerebral or peripheral revascularisation, or greater than 50% stenosis shown via angiography.
Model structure
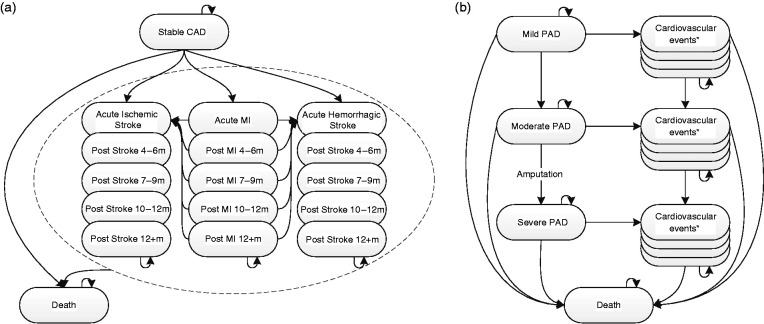
A health state transition model with a lifetime time horizon and a cycle length of 3 months was developed in Microsoft Excel, reflecting the effects of primary and recurrent cardiovascular and bleeding events on costs and health outcomes in CAD patients. The model was extended also to model ischaemic limb events in PAD patients (see Figure 1).
Figure 1.
Coronary artery disease (CAD) and peripheral arterial disease (PAD) model structure. M: months; MI: myocardial infarction.
*PAD cardiovascular event tunnel states as in the CAD model structure.
CAD patients started in the ‘stable CAD’ health state and could transition into MI and stroke tunnel states or to death. Tunnel states are a set of health states through which patients migrate in a predefined order reflecting consecutive consequences triggered by a health event. Recurrent cardiovascular events with permanent effects on utility, costs or probability of further events were explicitly modelled and otherwise reflected through temporary costs and disutilities, lasting one cycle. Patients alive were at risk of background mortality9 (i.e. for death from non-cardiovascular causes) and for major and minor bleeds, reflected through temporary costs and disutilities lasting one cycle.
PAD patients started in the ‘mild PAD’ health state, those with a history of revascularisation started in the ‘moderate PAD’ health state. ‘Mild PAD’ corresponded to Rutherford stages 0 to 3, ‘moderate PAD’ was defined by a history of unsuccessful revascularisation and corresponded to stages 1 to 4. Progression to ‘moderate PAD’ occurred through acute or chronic limb ischaemia, which is irreversible in a proportion of patients. Progression to ‘severe PAD’ occurred on major vascular amputation. Tunnel states for MI and stroke were defined by PAD severity. PAD progression also occurred in tunnel states.
Effectiveness
Primary events
Treatment effectiveness was modelled by digitising cumulative hazard curves of the COMPASS publications1,2 using the algorithm developed by Guyot et al.10 The curves described MACE-free survival in CAD and PAD, and MALE-free survival in PAD patients. Parametric time-to-event (TTE) curves were fitted for extrapolation of effectiveness. Based on statistical fit, visual inspection and expert opinion, Weibull curves and exponential curves were selected for MACE and MALE, respectively (see Supplementary Appendix). Treatment effectiveness with clopidogrel APT was modelled through the MACE HR of clopidogrel APT versus aspirin APT from the CAPRIE trial.11 CAPRIE and COMPASS trial populations, treatments and outcomes were deemed sufficiently similar despite the higher aspirin dose (325 mg), expert opinion considered an effect on the effectiveness of aspirin or the HR of clopidogrel unlikely (see Supplementary Appendix).
The probabilities of MI, IS, HS and cardiovascular death in ‘stable CAD’, ‘mild’ and ‘moderate PAD’ health states were obtained by multiplying the probability of MACE with the proportions of individual events in COMPASS (CAPRIE for clopidogrel). The probabilities in ‘severe PAD’ were informed by patients post MALE in COMPASS.12 It was assumed that by age 85 years all cardiovascular events would be fatal,13,14 a scenario analysis explored alternative assumptions.
The transition probability to ‘moderate PAD’ was calculated from the probability of MALE, multiplied with the proportion of acute and chronic limb ischaemia from COMPASS; 22% of these patients transitioned to ‘moderate PAD.15 The transition probability to ‘severe PAD’ was calculated from the number of major vascular amputations and the population at risk in COMPASS, assumed to be patients with a revascularisation history (Tables 1 and 2 in Anand et al.).1 No data were available to model PAD progression on clopidogrel, therefore probabilities were assumed equal to aspirin.
Table 1.
Utilities and costs of health states and events.
| Health states | Utility, annual | (SE) | Related healthcare costs | Patient and family costs | Intersectoral costsa | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Stable CAD | 0.671 | (0.046) | 20 | €134 | 25 | €0 | €0 | |||
| Mild PAD, | 0.681 | NA | €508 | 34 | €423 | 34 | €268 | 35 | ||
| Moderate PAD | 0.607 | NA | €863 | 34 | €358 | 34 | €268 | 35 | ||
| Severe PAD | 0.430 | (0.108) | 23 | €3551 | 24 | €358 | 34 | €268 | 35 | |
| CV events | CAD and mild PADb | CAD and mild PADc | CAD and PAD | |||||||
| Acute MI: 0–3, 4–6 months | 0.603 | (0.022) | 20 | €6630 | 28 | €154 | 36,d | €1255 | 36 | |
| Sub-acute MI 7–9, 10–12 months | 0.671 | (0.046) | 20 | €2841 | 28 | €33 | 36,d | €0 | ||
| Post MI 12+ months | 0.671 | (0.046) | 20 | €287 | 28 | €0 | €0 | |||
| Acute stroke, 0–3 months | 0.523 | (0.019) | 20 | €7440 | 28 | €4742 | 30 | €1212 | 36 | |
| Sub-acute stroke, 4–6 months | 0.542 | (0.044) | 20 | €7440 | 28 | €4742 | 30 | €1212 | 36 | |
| Sub-acute stroke 7–9, 10–12 months | 0.542 | (0.044) | 20 | €3189 | 28 | €1666 | 30 | €0 | ||
| Post stroke 12+ months | 0.542 | (0.044) | 20 | €2776 | 28 | €0 | €0 | |||
|
Events |
Decrement (SE) |
|
Per event |
|
||||||
| Recurrent MI | 0.063 | (0.013) | 21 | €6630 | 28 | |||||
| Recurrent stroke | 0.117 | (0.035) | 21 | €7440 | 28 | |||||
| Major bleeding | 0.047 | (0.001) | 16 | €7819 | 32 | |||||
| Minor bleedinge | 0.039 | (0.003) | 16 | €200 | 33 | |||||
| Peripheral revascularisation | 0.133 | (0.002) | 24 | €3454 | 35 | |||||
| Amputation | 0.161 | (0.032) | 24 | €15,127 | 23 | |||||
| CV death | €6630 | |||||||||
| Non-CV death | €1227 | 31 | ||||||||
|
Components PAD utilities |
Utility |
(SE) |
|
|||||||
| General population aged 65+ years (mild) | 0.870 | (0.001) | 21 | |||||||
| Rutherford 1–3 (mild, moderate) | 0.620 | (0.155) | 22 | |||||||
| Rutherford 4 (moderate) | 0.490 | (0.123) | 22 | |||||||
| CV event with PAD | Decrement (SE) | |||||||||
| Acute MI, 0–3, 4–6 months | 0.063 | (0.013) | 20 | |||||||
| Sub-acute, post MI, 7+ months | 0 | assumed | ||||||||
| Acute stroke, 0–3 months | 0.117 | (0.041) | 20 | |||||||
| Sub-acute, post stroke, 4+ months | 0.067 | (0.025) | 20 | |||||||
CAD: coronary artery disease; CV: cardiovascular; HS: haemorrhagic stroke; IS: ischaemic stroke; MI: myocardial infarction; PAD: peripheral arterial disease; SE: standard error.
aCost of missed work, assumed to be unpaid for this retired population. A day of missed work is assumed to contain 3 hours of unpaid work, as seen in patients with rheumatoid arthritis.38
bFor PAD utilities with CV events: apply ‘CV event with PAD’ decrement to mild/moderate/severe PAD utility.
cFor PAD-related healthcare costs with CV events: add moderate/severe PAD costs to the costs of CV event with CAD/mild PAD.
dAssuming the number of healthcare visits (GP, specialist, etc.) is equal to patients post stroke.
eDisutility of minor bleed assumed to last one month.
Table 2.
Probabilistic cost-effectiveness results.
| Incremental | |||||
|---|---|---|---|---|---|
| CAD | Aspirin | DPI | DPI vs. aspirin | ||
| Costs | €99,807 | €109,941 | €10,134 | ||
| Life years | 11.697 | 12.131 | 0.434 | ||
| QALYs | 7.773 | 8.089 | 0.316 | ||
| ICER | €32,035 | ||||
|
PAD |
Aspirin |
DPI |
Clopidogrel |
Incremental |
Incremental |
|
DPI vs. aspirin |
Clopidogrel vs. aspirin |
||||
| Costs | €156,905 | €166,945 | €165,843 | €10,040 | €8938 |
| Life years | 11.667 | 12.127 | 12.114 | 0.460 | 0.446 |
| QALYs | 7.489 | 7.868 | 7.761 | 0.379 | 0.272 |
| ICER | €26,463 | *€32,913 |
CAD: coronary artery disease; DPI: dual pathway inhibition; ICER: incremental cost-effectiveness ratio; PAD: peripheral arterial disease; QALY: quality-adjusted-life-year.
*Clopidogrel is extendedly dominated, i.e. the ICER of DPI vs. aspirin is lower than the ICER of clopidogrel vs. aspirin.
Recurrent cardiovascular events
No data on recurrent cardiovascular events in the COMPASS trial were published. Reconstructed TTE models of the PEGASUS trial treating patients with a MI history with ticagrelor DPI or aspirin were therefore used for CAD and ‘mild PAD’ (see Supplementary Appendix).16 Data stemmed from National Institute for Health and Care Excellence (NICE) committee papers.17 Probabilities in ‘moderate’ and ‘severe PAD’ health states were informed by patients post MALE in COMPASS.12 All probabilities were treatment independent.
Treatment discontinuation
A cyclic treatment discontinuation of 2%, up to 16% total was modelled based on the COMPASS trial. Scenario analyses explore assumptions of no discontinuation and of continuous discontinuation up to 41%, in line with previous findings.18 Patients post HS were assumed to discontinue rivaroxaban and clopidogrel and receive aspirin APT, in line with clinical practice.
Adverse events
Probabilities for major and minor bleeds on DPI and aspirin were taken from the COMPASS trial.19 Probabilities on clopidogrel treatment could not be obtained from the CAPRIE trial but were informed relative to those of ticagrelor DPI.16,20 Scenarios explore alternative bleeding risks.21
Utilities
QALYs were calculated based on EQ-5D utility estimates of health states and disutilities of events. Utility data collected in the COMPASS trial were not published; therefore, values were obtained from the literature (see Table 1, alternative utilities in Supplementary Appendix).
Health state utilities of CAD patients were informed by UK EQ-5D scores for multiple health conditions.22 The ‘stable CAD’ utility was assumed equal to patients with an old MI. Utilities in MI and stroke states (IS and HS assumed equal) were taken from patients with acute MI or cerebrovascular disease, old MI and late effects of cerebrovascular disease. The PEGASUS trial informed disutilities of major and minor bleeds.17
PAD health state utilities were not available from previously mentioned sources. The ‘mild PAD’ utility was a weighted average of asymptomatic patients (Dutch general population)23 and patients with mild to severe claudication (Dutch patients with Rutherford 1–3),24 using proportions calculated from COMPASS (27% and 73%).1 The ‘moderate PAD’ utility was a weighted average of mild to severe claudication and rest pain utilities (Dutch patients with Rutherford 1–3 and Rutherford 4,24 proportions estimated by two clinical experts (90% and 10%)). The utility of ‘severe PAD’ and disutilities of revascularisation and amputation were obtained from matching Dutch populations.25,26
Utilities in PAD tunnel states were computed from the PAD health state utility (mild, moderate, severe) and a disutility attributed to the cardiovascular event (acute MI or cerebrovascular disease, late effects of cerebrovascular disease, from CAD health state utilities source).22
Resource use and costs
Each health state was associated with cyclic treatment, healthcare, patient and family (travel and informal care), and inter-sectorial costs (productivity losses); additionally, costs applied to acute events (see Table 1). Age-dependent unrelated healthcare costs were applied in accordance with Dutch guidelines (see Supplementary Appendix).27 Treatment costs per cycle were €232.33, €17.35 and €31 for DPI, aspirin and clopidogrel, considering prices per dose of €1.18 for 2.5 mg rivaroxaban, €0.19 for 100 mg aspirin and €0.34 for 75 mg clopidogrel.28 All prices were inflated to 2018,29 prices in pounds were converted to Euros (€1 is £0.87, February 2019). Sources used in dossiers of the Dutch healthcare institute were screened, the latest costs were selected.
Healthcare costs of ‘stable CAD’ were based on costs in Dutch CAD patients.27 Costs in ‘stable CAD’ and ‘mild PAD’ tunnel states were informed by first year costs of MI and stroke30 (IS and HS assumed equal, 51% and 49% major and minor strokes).31 Based on previous studies, 70% of first-year MI and stroke costs occurred within the first 6 months.32 The costs of cardiovascular death were averaged costs of acute MI and stroke (proportions from the COMPASS trial); the costs of non-cardiovascular death were taken from van Hout and Simoons.33 Costs of bleeding and perforated peptic ulcers informed the costs of major bleeding.34 Minor bleeds were assumed to be GP treated.5
Healthcare costs in ‘mild PAD’ were based on a walking advice strategy, and costs in ‘moderate PAD’ on a supervised exercise strategy.35 One source informed the costs in ‘severe PAD’ and the cost of undergoing amputation.25 The cost of revascularisation was informed by Spronk et al.36 Costs in ‘moderate’ and ‘severe PAD’ tunnel states were obtained by inflating the costs of ‘moderate’ and ‘severe PAD’ treatment with costs in CAD tunnel states.
Sources of healthcare costs were used for patient and family and intersectoral costs when possible. Additional sources reporting patient and caregiver days lost after MI and stroke, and home care post stroke were used in CAD and PAD tunnel states.32,37
Analyses
Fully incremental deterministic and probabilistic results are presented. Probabilistic numbers needed to treat/harm were calculated from the incremental number of ischaemic and bleeding events for different time horizons.
In the deterministic sensitivity analyses, utilities, costs and effectiveness parameters were varied one-by-one within their 95% CI or plausible ranges to determine the most influential parameters. The results are presented in tornado diagrams. In the probabilistic sensitivity analysis, these parameters were varied simultaneously according to assigned probability distributions to explore the effect of joint uncertainty (details in the Supplementary Appendix). The results are presented in incremental cost-effectiveness planes (iCE planes) displaying the incremental QALY plotted against the incremental cost of each iteration, and in cost-effectiveness acceptability curves (CEACs) showing the probability of cost-effectiveness at different willingness-to-pay (WTP) thresholds. Corresponding to the disease burden of CAD and PAD (0.42 and 0.44, respectively) in The Netherlands, the relevant WTP is €50,000/QALY.
Scenario analyses explored alternative assumptions, results of the most influential scenarios are presented (details and complete results in the Supplementary Appendix). Subgroup analyses used subgroup-specific hazards for the aspirin arm and HRs for the relative effectiveness of DPI.1,2
Based on drug and healthcare costs, the average annual incremental healthcare expenditure with DPI versus aspirin was estimated, considering 26% of 730,000 Dutch CAD, and 40% of 569,000 PAD patients eligible.5,38,39
Validation
In line with the AdViSHE tool,40 the conceptual model and model inputs were validated against clinical expert opinion and the literature. Experts agreed the model reflected the clinical disease, disease stages and their consequences, and deemed the model results in line with expectations. The computerised model was reviewed and tested, issues were resolved. A cross-validation against COMPASS trial results found numbers of events observed and modelled differing by less than 1% (see Supplementary Appendix).
Results
In both populations, DPI costs and QALYs were highest (Table 2). The incremental costs were mainly driven by higher drug and unrelated healthcare costs of DPI (see Supplementary Appendix). In CAD patients, the probabilistic incremental QALYs and costs were 0.315 and €10,111, the ICER versus aspirin was €32,109/QALY gained, the deterministic results were similar (see Supplementary Appendix). In PAD patients, the ICER of clopidogrel versus aspirin was €30,118, clopidogrel was extendedly dominated; the ICER of DPI versus aspirin was €26,381. In both populations, 10% of the incremental QALYs were generated within 5 years of treatment, 31% within 10 years (see Supplementary Appendix). The average annual incremental healthcare expenditure with DPI was €38.7 million and €29.0 million in CAD and PAD patients, respectively.
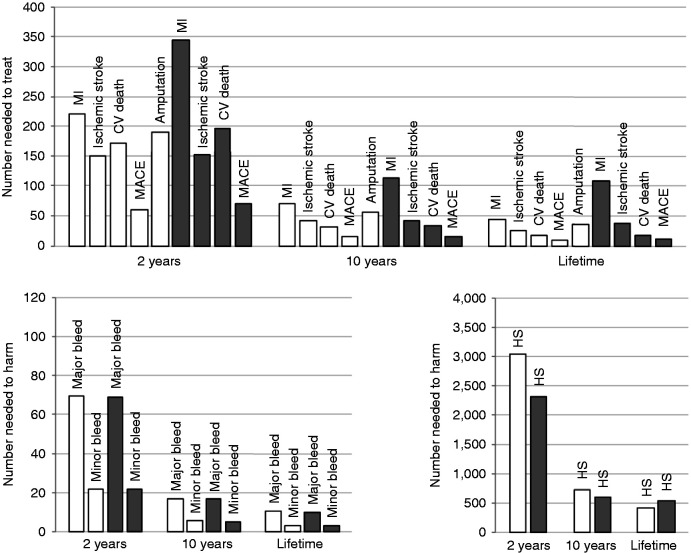
Over a lifetime horizon, 12 CAD or nine PAD patients need to be treated with DPI versus aspirin to avoid one additional incidence of MACE; the number needed to harm was three for an additional minor bleed and 10 for a major bleed in both populations (see Figure 2).
Figure 2.
Numbers needed to treat to avoid an event and number needed to harm (with an additional event) of dual pathway inhibition (DPI) versus aspirin at three time points; coronary artery disease (CAD) (grey), peripheral arterial disease (PAD) (white). CV: cardiovascular; HS: haemorrhagic stroke; MACE: major adverse cardiovascular event; MI: myocardial infarction. Numbers needed to treat/harm decrease with longer timeframes.
Deterministic sensitivity analysis
The most influential parameter in both populations was the relative effectiveness of DPI versus aspirin on MACE. Moreover, influential parameters were the ‘stable CAD’ utility and the scale parameter of the CAD MACE TTE curve, and the proportion of cardiovascular death with aspirin for PAD and the ‘mild PAD’ utility (see Supplementary Appendix).
Probabilistic sensitivity analysis
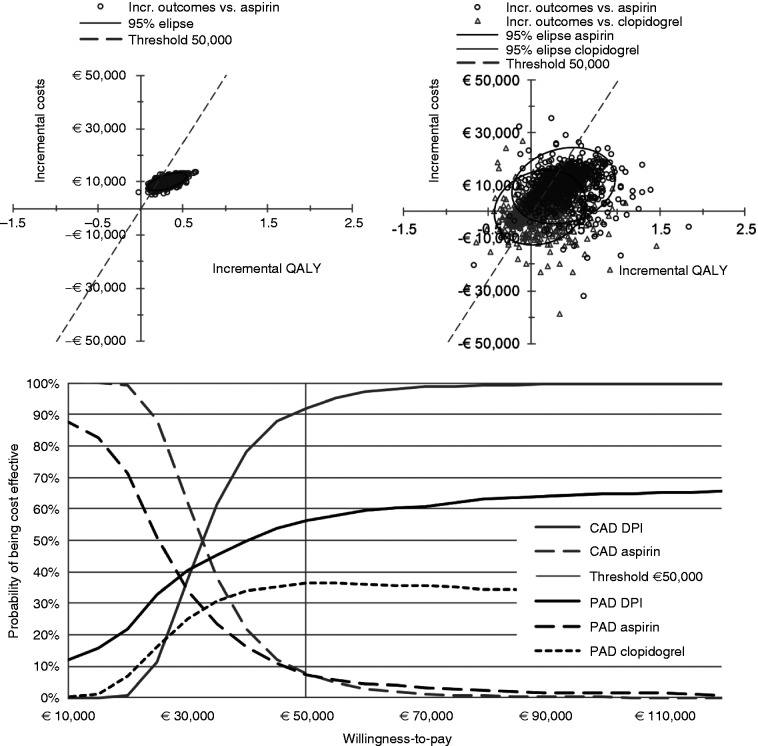
The iCE plane showed higher costs and larger effects of DPI compared with aspirin in most iterations, and mixed results for the comparison with clopidogrel for PAD. The probabilities of DPI being cost-effective at a WTP threshold of €50,000 were 92% and 56% in CAD and PAD patients, these decreased at lower WTP thresholds (Figure 3).
Figure 3.
iCE planes of the CAD population (top left) and of the PAD population (top right), and CEACs (bottom). CAD: coronary artery disease; CEAC: cost-effectiveness acceptability curve; DPI: dual pathway inhibition; iCE plane: incremental cost-effectiveness plane; PAD: peripheral arterial disease; QALY: quality-adjusted life year; WTP: willingness to pay. iCE planes present the incremental QALY and incremental cost per iteration of the probabilistic sensitivity analysis, to express joint uncertainty; the CEACs show the probability of cost-effectiveness of each treatment option at different WTP thresholds.
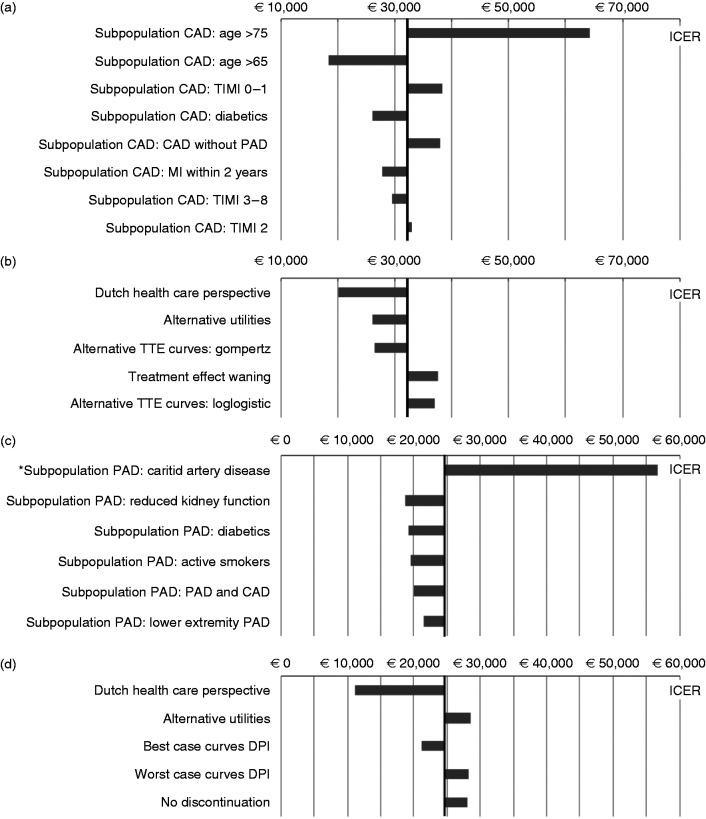
Subgroup and scenario analyses
The CAD ICER was highest in patients older than 75 years and lowest in patients younger than 65 years. The PAD ICER was highest in patients with carotid artery disease (vs. clopidogrel) and lowest in patients with reduced kidney function. The most influential scenarios used the Dutch healthcare perspective, alternative utility values or alternative TTE curves for MACE (and MALE for PAD, see Supplementary Appendix). The ICER of DPI from a UK payer perspective was £23,605 in CAD patients and £40,889 in PAD patients (vs. clopidogrel). Clopidogrel was (extendedly) dominated in all other scenarios (Figure 4).
Figure 4.
(a) ICERs in subgroup analyses CAD; (b) ICERs in scenario analyses CAD; (c) ICERs in subgroup analyses PAD; (d) ICERs in scenario analyses PAD. Black vertical line represents the base-case ICER as reference, *ICER DPI versus clopidogrel. CAD: coronary artery disease; DPI: dual pathway inhibition; ICER: incremental cost-effectiveness ratio; PAD: peripheral arterial disease; TIMI: trials in myocardial infarction secondary prevention risk score (high score = higher risk of major vascular events); TTE: time-to-event.
Discussion
In this cost-effectiveness analysis QALYs and costs of DPI with rivaroxaban plus aspirin were higher than with aspirin alone, with an ICER of €32,109 in CAD and €26,381 in PAD patients. DPI extendedly dominated clopidogrel for PAD. At a €50,000/QALY threshold, the cost-effectiveness probability of DPI was 92% and 56% for CAD and PAD, respectively. DPI was more cost-effective in CAD patients younger than 65 years and PAD patients with comorbidities, and not cost-effective in patients with carotid artery disease and CAD patients older than 75 years. Over a lifetime horizon, the numbers needed to treat to avoid one additional MACE were 12 for CAD and nine for PAD; the numbers needed to harm were three and 10 for a minor and major bleed in both populations.
The strength of these analyses is the high level of granularity at which they were conducted, reflecting acute and long-term effects of primary and recurrent cardiovascular and limb events, and providing ample information about comparators and subgroups. The methods used were reported transparently so the reader can interpret the results in light of the underlying assumptions. The analyses have a number of limitations relating to the unavailability of data. We attempted to overcome these by requesting individual time-to-event curves and utility data from the researchers of the COMPASS trial, but this request was not granted (personal communication between Joore and Elkeboom, 02.12.2019). Firstly, the COMPASS trial did not prove superiority of DPI over all standard of care treatments, as clopidogrel ATP was not tested. Through an indirect comparison we enabled a crude comparison of DPI versus clopidogrel. However, assumptions were necessary regarding bleeding risks and the effectiveness for MALE, these were explored in scenario analyses and found to be non-influential. It cannot be ruled out that changes in cardiovascular prevention that occurred since the CAPRIE trial (e.g. use of statins) impair the comparability of the CAPRIE and the COMPASS trial. Secondly, the COMPASS publications reported the main outcomes as composite endpoints (MACE and MALE) instead of individual Kaplan–Meier curves of MI, IS, HS and cardiovascular death. Assumptions about the proportions of events within MACE, about the relationship between age and cardiovascular death, and about the populations at risk of limb ischaemia and major amputation were necessary. These were described and explored in scenario analyses when possible, which showed no substantial impact on the results. It is the uncertainty not captured, i.e. the possibility that the effectiveness data of 23 months follow-up were immature and not sufficiently reflective of long-term (relative) effectiveness, which remains a limitation of the study. Thirdly, primary but not recurrent major bleeds were reported in the COMPASS trial, this may underestimate the number of major bleeds in favour of DPI, as shown in a scenario analysis. Likewise, scarce reporting of recurrent cardiovascular events and no reporting of costs and utilities from the COMPASS trial necessitated the use of other sources, causing issues of indirectness. Especially for patients with both cardiovascular and MALE events, cardiovascular risk may be underestimated and disutilities of cardiovascular and MALE events may be overestimated as these were assumed to be additive. As explored in scenario analyses, this may favour DPI.
The Scottish Medicines Consortium compared DPI with aspirin APT in patients with CAD or PAD and reported an ICER of £16,311/QALY.41 The Markov model differed and COMPASS trial data were used that were unobtainable to us (effectiveness, utilities, costs). Comparison with a weighted average of CAD and PAD outcomes of our UK payer perspective scenario showed Scottish Medicines Consortium QALYs were larger and costs were lower, but incremental QALYs, costs and the ICER of £20,629 were comparable (see Supplementary Appendix). The model submitted to the Dutch authorities also differed in model structure and input parameters, utilities and disutilities were larger and unrelated healthcare costs were not considered. The resulting ICER was €6,954/QALY.5
The CAD subgroup results showed an ICER exceeding €60,000 and a cost-effectiveness probability of 20% in patients above 75 years of age, while in patients 65 years and younger the ICER was below €20,000 and 100% cost-effective. Considering the estimated incremental healthcare expenditure of €38.7 million on DPI treatment in CAD patients, a targeted approach may be warranted to reduce the financial burden on the healthcare system. The results of PAD patients are based on strong assumptions regarding the effectiveness of clopidogrel. Considering the cost-effectiveness of DPI for PAD remained uncertain overall (56% cost-effectiveness probability DPI, 36% clopidogrel), additional evidence on clopidogrel APT could reduce this uncertainty. Future research should also overcome the transparency shortcomings in the reporting of trial results to enable independent researchers to conduct cost-effectiveness analyses based on best available evidence.
Conclusion
Lifelong preventive treatment of CAD and PAD patients at risk of cardiovascular events with rivaroxaban plus aspirin DPI improves health outcomes and seems overall to be cost-effective compared with aspirin ATP, and clopidogrel ATP for PAD, particularly in comorbid patients; however, not in patients older than 75 years and in carotid artery patients. The considerable economic impact of DPI may warrant a targeted approach. Further research is needed to address the long-term effectiveness of DPI and the effectiveness of clopidogrel for PAD.
Supplemental Material
Supplemental material, CPR913380 Supplementary material for Rivaroxaban plus aspirin for the prevention of ischaemic events in patients with cardiovascular disease: a cost-effectiveness study by Svenja Petersohn, Xavier Pouwels, Bram Ramaekers, Arina ten Cate-Hoek and Manuela Joore in European Journal of Preventive Cardiology
Acknowledgements
The author(s) would like to thank Barend Mees and Hugo ten Cate for their support developing the PAD model structure and validating model input and results with their expert opinion; the contents of this publication are solely the responsibility of the authors.
Author contribution
SP, XP, BR, AtC and MJ contributed to the conception or design of the work, contributed to the acquisition, analysis and interpretation and critically revised the manuscript. XP and SP drafted the manuscript. All author(s) gave final approval and agree to be accountable for all aspects of the work ensuring integrity and accuracy.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Anand SS, Bosch J, Eikelboom JW, et al. Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet 2018; 391: 219–229. [DOI] [PubMed] [Google Scholar]
- 2.Connolly SJ, Eikelboom JW, Bosch J, et al. Rivaroxaban with or without aspirin in patients with stable coronary artery disease: an international, randomised, double-blind, placebo-controlled trial. Lancet 2018; 391: 205–218. [DOI] [PubMed] [Google Scholar]
- 3.Montalescot G, Sechtem U, Achenbach S, et al. 2013 ESC Guidelines on the management of stable coronary artery disease. The Task Force on the Management of Stable Coronary Artery Disease of the European Society of Cardiology. Eur Heart J 2013; 34: 2949–3003. [DOI] [PubMed] [Google Scholar]
- 4.Aboyans V, Ricco JB, Bartelink MEL, et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries. Endorsed by: the European Stroke Organization (ESO), the Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J 2018; 39: 763–816. [DOI] [PubMed] [Google Scholar]
- 5.Knies S. Budget impact analyse van rivaroxaban (Xarelto®) voor de preventie van atherotrombotische complicaties bij volwassen patiënten met coronaire hartziekte (CHZ) of symptomatische perifeer arterieel vaatlijden (PAV) met een hoog risico op ischemische voorvallen. 2019. Diemen: Zorginstituut Nederland.
- 6.National Institute for Health and Care Excellence. Final appraisal document: Rivaroxaban for preventing atherothrombotic events in people with coronary or peripheral artery disease. 2019. London: National Institute for Health and Care Excellence. [Google Scholar]
- 7.Husereau D, Drummond M, Petrou S, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) – explanation and elaboration: a report of the ISPOR Health Economic Evaluation Publication Guidelines Good Reporting Practices Task Force. Value Health 2013; 16: 231–250. [DOI] [PubMed] [Google Scholar]
- 8.Zorginstituut Nederland. Guideline for economic evaluations in healthcare. 2016. Diemen: Zorginstituut Nederland. [Google Scholar]
- 9.Centraal Bureau voor de Statistiek. Age- and gender-specific general mortality 1950–2014 http://statline.cbs.nl/StatWeb/publication/?DM=SLNL&PA=37530NED&D1=1,3,5&D2=101-120&D3=0&D4=l&HDR=T,G3&STB=G2,G1&VW=T (2015, accessed 12 February 2019).
- 10.Guyot P, Ades A, Ouwens MJ, et al. Enhanced secondary analysis of survival data: reconstructing the data from published Kaplan–Meier survival curves. BMC Med Res Methodol 2012; 12: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. The CAPRIE steering committee. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 1996; 348: 1329–1339. [DOI] [PubMed] [Google Scholar]
- 12.Anand SS, Caron F, Eikelboom JW, et al. Major adverse limb events and mortality in patients with peripheral artery disease: the COMPASS Trial. J Am Coll Cardiol 2018; 71: 2306–2315. [DOI] [PubMed] [Google Scholar]
- 13.Saposnik G, Cote R, Phillips S, et al. Stroke outcome in those over 80. Stroke 2008; 39: 2310–2317. [DOI] [PubMed] [Google Scholar]
- 14.Smilowitz NR, Mahajan AM, Roe MT, et al. Mortality of myocardial infarction by sex, age, and obstructive coronary artery disease status in the ACTION Registry – GWTG (Acute Coronary Treatment and Intervention Outcomes Network Registry – Get With the Guidelines). Circ Cardiovasc Qual Outcomes 2017; 10: e003443. [DOI] [PubMed]
- 15.Je HG, Kim BH, Cho KI, et al. Correlation between patient-reported symptoms and ankle-brachial index after revascularization for peripheral arterial disease. Int J Mol Sci 2015; 16: 11355–11368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonaca MP, Bhatt DL, Cohen M, et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med 2015; 372: 1791–1800. [DOI] [PubMed] [Google Scholar]
- 17.National Institute for Health and Care Excellence. Ticagrelor for preventing atherothrombotic events after myocardial infarction [ID813]: committee papers. NICE technology appraisal guidance 420 [Internet] 2016.
- 18.Bowry ADK, Shrank WH, Lee JL, et al. A systematic review of adherence to cardiovascular medications in resource-limited settings. J Gen Intern Med 2011; 26: 1479–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eikelboom JW, Connolly SJ, Bosch J, et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med 2017; 377: 1319–1330. [DOI] [PubMed] [Google Scholar]
- 20.Arora S, Shemisa K, Vaduganathan M, et al. Premature ticagrelor discontinuation in secondary prevention of atherosclerotic CVD: JACC Review Topic of the Week. J Am Coll Cardiol 2019; 73: 2454–2464. [DOI] [PubMed] [Google Scholar]
- 21.van Rein N, le Cessie S, van Vliet IP, et al. Increased risk of major bleeding after a minor bleed during treatment with vitamin K antagonists is determined by fixed common risk factors. J Thromb Haemost 2016; 14: 948–952. [DOI] [PubMed] [Google Scholar]
- 22.Sullivan PW, Slejko JF, Sculpher MJ, et al. Catalogue of EQ-5D Scores for the United Kingdom. Med Decis Making 2011; 31: 800–804. [DOI] [PubMed] [Google Scholar]
- 23.Bernert S, Fernández A, Haro JM, et al. Comparison of different valuation methods for population health status measured by the EQ-5D in three European countries. Value in Health 2009; 12: 750–758. [DOI] [PubMed] [Google Scholar]
- 24.de Vries M, Ouwendijk R, Kessels AG, et al. Comparison of generic and disease-specific questionnaires for the assessment of quality of life in patients with peripheral arterial disease. J Vasc Surg 2005; 41: 261–268. [DOI] [PubMed] [Google Scholar]
- 25.Oostenbrink JB, Tangelder MJD, Busschbach JJV, et al. Cost-effectiveness of oral anticoagulants versus aspirin in patients after infrainguinal bypass grafting surgery. J Vasc Surg 2001; 34: 254–262. [DOI] [PubMed] [Google Scholar]
- 26.van Stel HF, Busschbach JJV, Hunink MGM, et al. Impact of secondary cardiovascular events on health status. Value in Health 2012; 15: 175–182. [DOI] [PubMed] [Google Scholar]
- 27.van Baal PHM, Wong A, Slobbe LCJ, et al. Standardizing the inclusion of indirect medical costs in economic evaluations. PharmacoEconomics 2011; 29: 175–187. [DOI] [PubMed] [Google Scholar]
- 28.Zorginstituut Nederland. Medicijnkosten. Medicijnkosten.nl (2019, accessed 18 February 2019).
- 29.Centraal Bureau voor de Statistiek. Harmonized consumer price index. https://statline.cbs.nl/Statweb/publication/?DM=SLEN&PA=83133ENG&D1=0-1,4-5&D2=0&D3=286-302&HDR=T&STB=G1,G2&VW=T (2019, accessed 18 February 2019).
- 30.Greving J, Visseren F, de Wit G, et al. Statin treatment for primary prevention of vascular disease: whom to treat? Cost-effectiveness analysis. BMJ 2011; 342. [DOI] [PubMed] [Google Scholar]
- 31.Berkhemer OA, Fransen PSS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015; 372: 11–20. [DOI] [PubMed] [Google Scholar]
- 32.van Eeden M, van Heugten C, van Mastrigt GA, et al. The burden of stroke in the Netherlands: estimating quality of life and costs for 1 year poststroke. BMJ Open 2015; 5: e008220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Hout BA, Simoons ML. Cost-effectiveness of HMG coenzyme reductase inhibitors. Whom to treat? Eur Heart J 2001; 22: 751–761. [DOI] [PubMed] [Google Scholar]
- 34.de Leest H, van Dieten H, van Tulder M, et al. Costs of treating bleeding and perforated peptic ulcers in The Netherlands. J Rheumatol 2004; 31: 788–791. [PubMed] [Google Scholar]
- 35.van Asselt AD, Nicolai SP, Joore MA, et al. Cost-effectiveness of exercise therapy in patients with intermittent claudication: supervised exercise therapy versus a ‘go home and walk’ advice. Eur J Vasc Endovasc Surg 2011; 41: 97–103. [DOI] [PubMed] [Google Scholar]
- 36.Spronk S, Bosch JL, den Hoed PT, et al. Cost-effectiveness of endovascular revascularization compared to supervised hospital-based exercise training in patients with intermittent claudication: a randomized controlled trial. J Vasc Surg 2008; 48: 1472–1480. [DOI] [PubMed] [Google Scholar]
- 37.Kotseva K, Gerlier L, Sidelnikov E, et al. Patient and caregiver productivity loss and indirect costs associated with cardiovascular events in Europe. Eur J Prev Cardiol 2019; 26: 1150–1157. [DOI] [PubMed]
- 38.Leening MJG, Siregar S, Vaartjes I, et al. Heart disease in the Netherlands: a quantitative update. Netherlands heart journal: monthly journal of the Netherlands Society of Cardiology and the Netherlands Heart Foundation 2014; 22: 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bartelink MEL, Elsman BHP, Oostindjer A, et al. NHG-Standaard Perifeer arterieel vaatlijden (tweede herziening). Huisarts Wet 2014; 57: 81. [Google Scholar]
- 40.Vemer P, Corro Ramos I, van Voorn GAK, et al. AdViSHE: a validation-assessment tool of health-economic models for decision makers and model users. Pharmacoeconomics 2016; 34: 349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scottish Medicines Consortium. Rivaroxaban (Xarelto) in patients with stable coronary artery disease that does not require dual antiplatelet therapy. https://www.scottishmedicines.org.uk/medicines-advice/rivaroxaban-xarelto-fullsubmission-smc2128/ (2019, accessed March 2019).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, CPR913380 Supplementary material for Rivaroxaban plus aspirin for the prevention of ischaemic events in patients with cardiovascular disease: a cost-effectiveness study by Svenja Petersohn, Xavier Pouwels, Bram Ramaekers, Arina ten Cate-Hoek and Manuela Joore in European Journal of Preventive Cardiology