Abstract
It is well-established that cardiovascular disease continues to represent a growing health problem and significant effort has been made to elucidate the underlying mechanisms. In this review, we report on past and recent high impact publications in the field of intracrine network signaling, focusing specifically on opioids and their interrelation with key modulators of the cardiovascular system and the onset of related disease. We present an overview of studies outlining the scope of cardiovascular and cerebrovascular processes that are affected by opioids, including heart function, ischemia, reperfusion, and blood flow. Specific emphasis is placed on the importance of dynorphin molecules in cerebrovascular and cardiovascular regulation. Evidence suggests that excessive or insufficient dynorphin could make an important contribution to cardiovascular physiology, yet numerous paradoxical observations frequently impede a clear understanding of the role of dynorphin. Thus, we argue that dynorphin-mediated signaling events for which an immediate regulatory effect is disputed should not be dismissed as unimportant, as they may play a role in cross-talk with other signaling networks. Finally, we consider the most recent evidence on the role of dynorphin during cardiovascular-related inflammation and on the potential value of endogenous and exogenous inhibitors of kappa-opioid receptor, a major dynorphin A receptor, to limit or prevent cardiovascular disease and its related sequelae.
Keywords: Dynorphins, signaling, cardiovascular, I/R injury
1. INTRODUCTION
The components of the cardiovascular (CV) system are tightly-controlled and adapt quite rapidly to chemical fluctuations within circulation to ensure cellular survival. This malleability is mediated through four signaling networks, namely autocrine, paracrine, intracrine, and neurotransmitter signaling. This intricate network of signal transduction exchange occurs between the cells of four different tissues, namely the nervous system, renal system, vascular endothelial cells, and cardiac muscle. Among these tissues, neurotransmitters along with intracrine signaling factors, are involved in many mechanical and chemical processes such as muscle contraction, heart rate (HR), as well as water and electrolyte regulation. Irreversible interruption of this signaling network promotes CV tissue damage and disease progression.
CV disease is an inflammatory disorder, with secondary renal dysfunction, vascular compromise, and central nervous system compensation for the vascular and cardiac aberrations, or a combination of any of these disorders. CV disease is characterized by pro- and anti-oxidant mechanisms [1], pro- and antiangiogenic factors [2], fluid imbalance [3, 4], disorders of acid-base balance [5], and sympathetic/-parasympathetic tone imbalance [6, 7]. Although much is known regarding the interactions of these aforementioned tissues with various physiological systems, such as immune components and the nervous system during normal CV responses, their function in CV diseases is not well-understood. High blood pressure (BP) promotes the development of coronary artery disease, stroke, and heart failure, but mechanistic intricacies have yet to be documented. Numerous neuropeptides are implicated in the CV system [8, 9], including dynorphin-dependent signaling, which is the focus of this review. Understanding the mechanisms by which neuropeptides, such as dynorphins, influence CV function is critical for understanding the molecular origin of heart failure and for developing novel treatment strategies. This review focuses on whether our current understanding of dynorphins supports the existence of a causal relationship between altered dynorphins and changes in cardiovascular function. We explore the specific interrelation between dynorphin with (1) the cerebrovascular system (cerebral blood flow), (2) blood pressure effects (hypertension, heart rate, endothelial, and plasma catecholamines), (3) heart function, (4) heart failure (coronary artery disease and myocardial infarction), (5) arrhythmia, (6) inflammation, and (7) stroke. We, therefore, thoroughly review the most seminal works conducted some twenty years ago and most recent literature pertaining to the role of dynorphin going from one extreme of the CV spectrum, BP regulation, to the other end of spectrum, cardiomyogenic differentiation.
2. SYNTHESIS AND METABOLISM OF DYNORPHIN
Dynorphin was described by Goldstein and colleagues who reported the opioid nature of the peptide [10]. In humans, the prodynorphin (pDyn) gene is composed of four exons that can exist up to 10 kb from each other [11–13]. The 5’ end of exon 3 and 3’ end of exon 4 encode the entire preprodynorphin sequence [12]. The 254 amino acid preprodynorphin, a biologically inactive precursor, is converted to pDyn by signal peptidases, which remove the 20 amino acid signal peptide from its N-terminus. Dynorphin is synthesized as a component of pDyn [12], while the remaining opioid peptides are produced as components of two distinct precursors known as proopiomelanocortin and proenkephalin. The full details of the synthesis and secretion of dynorphins are beyond the scope of this review and the reader is directed towards a more comprehensive review of this process [12].
pDyn, a polypeptide 234 amino acids in length, undergoes proteolytic cleavages to yield several opioid peptides among which are two extended forms of dynorphin-B, known as big dynorphin (consisting of both dynorphin A and dynorphin-B) comprised of 32 amino acids, and a leumorphin, comprised of 31 amino acids [12]. Big dynorphin is proteolytically-processed to yield dynorphin A of 17 amino acid residues, a definitive hormone product (Fig. 1). In peripheral circulation, both dynorphin A and dynorphin-B undergo further proteolytic cleavage to yield N- and C-terminal fragments [14], such as dynorphin A (1–13) (Dyn-A1–13), dynorphin A (1–8) (Dyn-A1–8) and dynorphin-B (1–13) (Dyn-B1–13, Rimorphin), which retain biological activity (Figure 1). There is extensive sequence homology at the N-termini of these two hormones.
Fig. (1). Metabolism of dynorphin peptides.
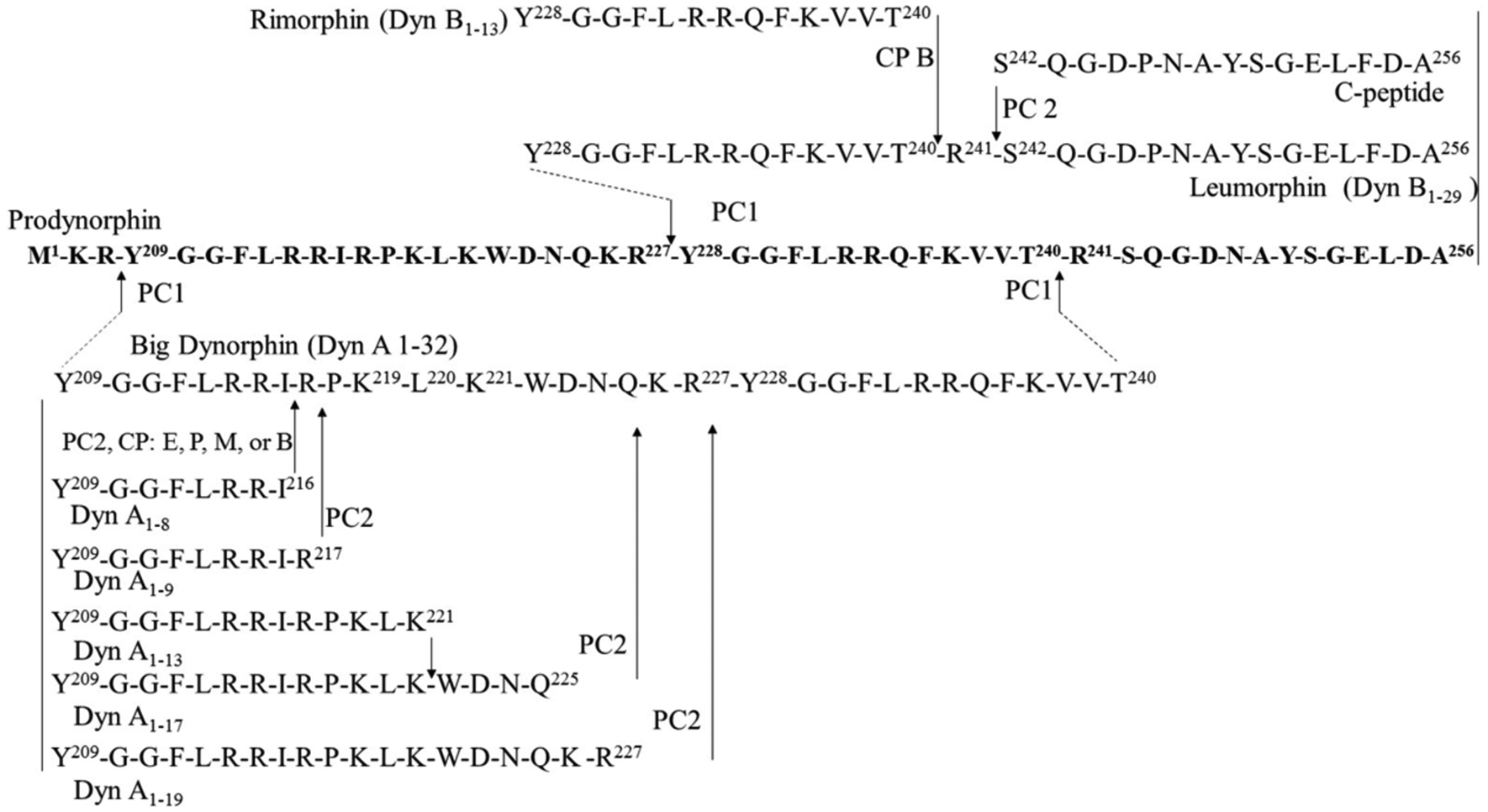
The enzymes known to convert the dynorphin peptides have been curated from primary literatures and shown within the arrows. All Dyn molecules contain the Leucine-enkephalin sequence (Y-G-G-F-L), but diverge on remaining residues. CPB; carboxypeptidase B, Dyn; dynorphin, PC1; prohormone convertase 1; PC2; prohormone convertase 2.
2.1. Pharmacokinetics.
Recent evidence shows that endogenous dynorphins affect the CV system (Table 1). Dynorphin is present in blood at physiological conditions ranging from 0.1–1.5 nM [15–17]. The degradation rate of dynorphin A in the peripheral blood is 40 pmol/min/μl [18]. Dynorphin half-life in plasma is reported to be less than 1 min [19] or to range between 0.5–4 minutes [20].
Table 1.
Direct and indirect effects of endogenous Dynorphins on the cardiovascular system.
| Dynorphin Fragment | Tissue | Effect | IC50/ EC50 | Implication | Ref. |
|---|---|---|---|---|---|
| Big Dyn | Hela cells | Ability to cross plasma membrane | 1 μM | Dyn molecules affect intracellular processes in absence of KOP | [140] |
| Dyn B 1–13 | Rat cardiac sarcolemma membrane | Inhibit oubain binding site on Na+-K+ TPase | 16.0 μM | Putative Mechanism for Na+-K+ ATPase inhibition | [39] |
| Embryonic stem cells | Bind to KOP receptors on nuclei and upregulate GATA-4, Nkx-2.5 transcription | Intracellular actions of Dynorphins and involvement in cardiac differentiation | [53] | ||
| Rat fetal Brain aggregates | Inhibited DNA synthesis 7 day old fetal brain aggregates, but stimulated DNA synthesis in 21 day old fetal brain aggregates | nM*, 1 μM | Potential to modulate fetal glial cell proliferation in time-dependent manner | [54] | |
| Dyn A 1–13 | Hela cells | Ability to cross plasma membrane | 10 μM | Demonstrates ability of Dyn molecules to affect intracellular processes in absence of KOP | [140] |
| Cardiac | Inhibit Na+-K+ ATPase | 30 μM | Increase intracellular [Na+] | [141] | |
| Cardiac | Indirect inhibitory effect on cardiac Dopamine Receptor 2-induced NE release | 10 μM | Demonstrates direct, inhibitory role of sympathetic nervous system by dynorphins on cardiovascular tissue | [30] | |
| Rat cardiac sarcolemma membrane | Inhibit Oubain binding site on Na+-K+ ATPase | 2.9 μM | Putative Mechanism for Na+-K+ ATPase inhibition | [39] |
represents the IC50 value for 7 day old fetal brain aggregates.
3. PHARMACOLOGY OF DYNORPHIN
Dynorphin A, dynorphin-B, and big dynorphin act on the human kappa opioid receptor (KOP) [12]. Albeit, dynorphin-B has a lower affinity for human KOP compared to dynorphin A and big dynorphin. In large part due to the basic residues found in the C-terminus, dynorphin A binds the KOP with high-selectivity in physiological concentration [21], but at a high concentration, dynorphin A also exhibits affinity for non-opioid receptors such as bradykinin B2 (B2) receptors [22], inducing pain. However, the blockade of BK-induced, B2-mediated receptor activation by dynorphin has significant implications for the role of dynorphin in the regulation of hypertension and inflammation. The reader is directed to recent reviews for more detailed information on opioid binding receptor function [23, 24].
4. PHYSIOLOGY OF DYNORPHINS IN THE CV SYSTEM
4.1. Mechanisms of Dynorphin Control of CV Response
In the past four decades, various regulatory mechanisms of dynorphin-mediated signaling have been elucidated in human disease states, including stress, obesity, pain, and addiction. It is only in recent years, that dynorphins have emerged as potential key players in CV disease. Ventura and colleagues [25, 26] performed numerous studies detailing the effects of pDyn localization, and pathways mediating pDyn upregulation in normal and cardiomyopathic myocytes [25, 26], and rat ventricular cardiomyocytes [27]. In support, increased levels of dynorphin in plasma and ventricular tissue are observed following heart transplantation [28]. This observation suggests that functionally pDyn is enhanced within the heart under physiological and pathophysiological conditions. It is presently unclear how pDn contributes to this disease. KOP is shown to be localized to the nuclei and sarcoplasmic reticulum of cardiomyocytes [25]. Dynorphin-B-induced activation of the isolated nuclei results in elevated opioid peptide gene transcription [25]. Cardiomyogenic differentiation has been described for numerous progenitor cell types. A recent study finds that KOP and delta-opioid receptors (DOP) expression are elevated during mouse embryonic stem cell differentiation [29]. Furthermore, dynorphin-mediated KOP activation causes an increase in the contractility of cardiomyocytes. Dynorphin is reportedly expressed within the atria and ventricles of the heart [15]. Notably, its content is not influenced by unilateral vagotomy [15], supporting its potential physiological role in cardiac tissues. Thus, a local dynorphin-mediated KOP pathway signaling may have an impact on cardiac remodeling and tissues. However, physiological roles being mediated via the CNS and PNS cannot be yet ruled out given evidence for indirect modulation of dynorphin A via pre-synaptic dopaminergic receptors on cardiac tissues [30]. The complexity of dynorphin-mediated signaling has been associated with numerous neuromediators identified as being co-expressed with dynorphin and involved in the control of cardiac and vascular function [31, 32]. Thus, collective experimental evidence shows an intriguing relationship among the cardiac tissues, the microenvironment of the cardiomyogenic lineage, and dynorphin as it pertains to CV function.
4.2. Physiology and Function
During the last two decades, an increasing number of research groups have focused on the expression and function of dynorphin signaling processes in the heart in order to understand how dynorphin physiology was intertwined with other known major biological processes. Studies of KOP using rat ventricular cardiomyocytes have revealed its presence in the T-tubule with Cav1.2 channels, plasma membrane, sarcoplasmic reticulum with ryanodine receptors, and mitochondria [33]. The T-tubule is part of the cell membrane containing numerous ion channels that play a critical role in excitation-contraction coupling in cardiomyocytes [34]. Evidence revealed that the synthesis of the pDyn gene involved protein kinase C-α following the activation of KOP by Dyn-B1–13 [35]. Bian and colleagues [36] began to suggest that KOP-opioid stimulation of PKC also decreases cAMP levels in isolated ventricular myocytes. Increased dynorphin was associated with abnormal heart rhythm. There is limited information on the effect of dynorphin on abnormal heart rhythm and currently, only two studies on dynorphin-induced abnormal heart rhythm have been reported. The primary cause of KOP-induced abnormal heart rhythm is found to be due to an increase in PKC-dependent, Na+/H+ antiporter function coupled to ATPase activity, which is argued to be congruent with secondary elevations in Na+ and Ca2+ [37, 38]; (Fig. 2). Dumont and colleagues [39] demonstrated that fragments of Dyn-A1–13 can inhibit the ouabain binding site on Na+/K+ ATPase and inhibit Na+ uptake in cardiomyocytes affecting the membrane voltage of the cell. Thus, the dynorphin-induced cardiac abnormal heart rhythm is a KOP-independent mechanism. While these studies show that the dynorphin-mediated signaling pathways are important for the heart function, the impact that a change in cardiac dynorphin expression and function may have on the cardiac contractility in humans, is well worth revisiting.
Fig. (2).
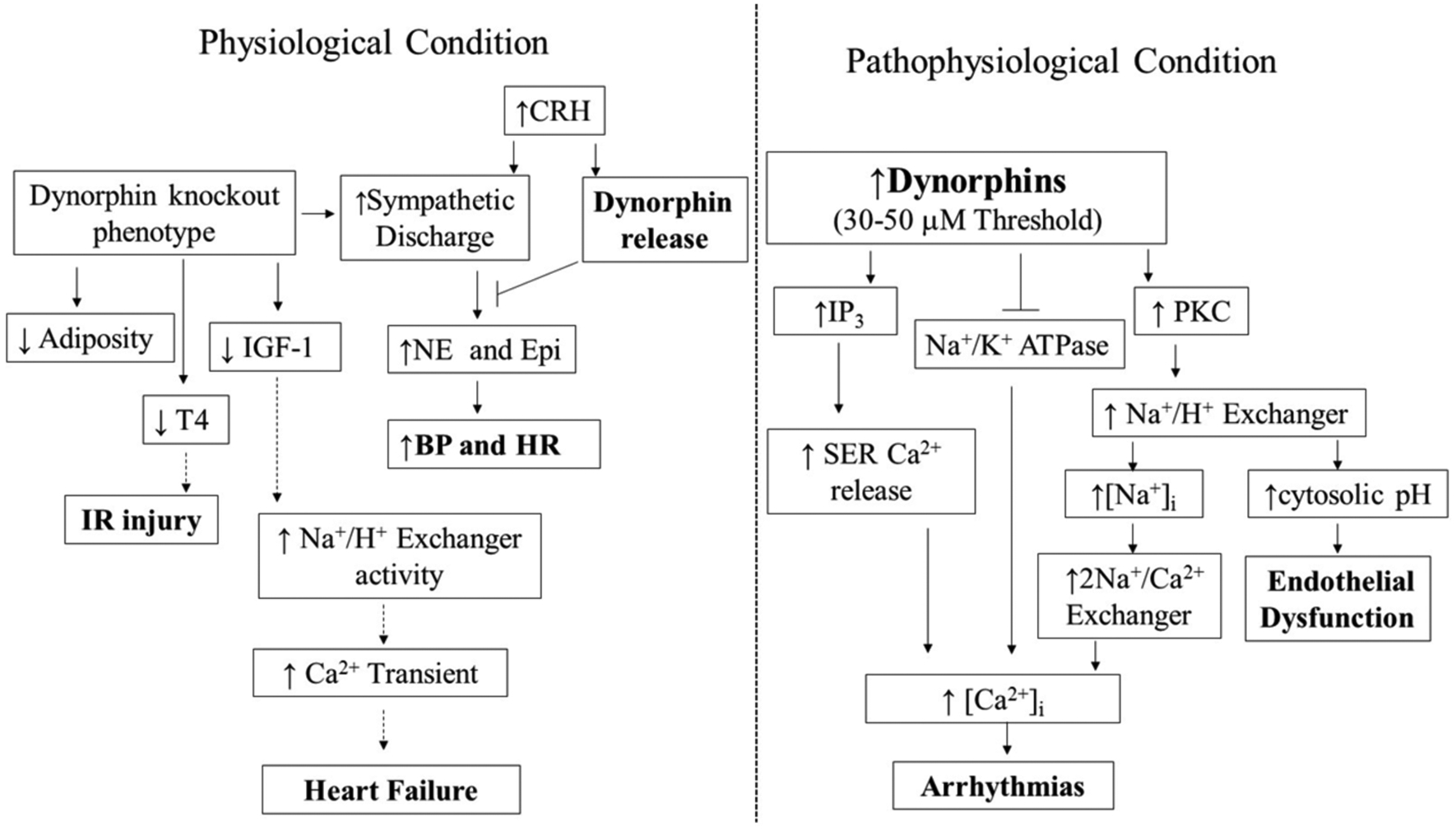
Schematic interpretation of the importance of dynorphins under physiological and pathophysiological processes in the cardiovascular system. Dynorphin fragments (Dyn A 1–17, Dyn A 1–13, and Dyn B 1–13) are involved in the regulation of tissues within the CV system. Panel A. Dynorphin A is cardioprotective. Dyn A 1–17 and Dyn A 1–13 dampen the sympathetic-induced increases in blood pressure and heart rate. Dyn deficient mice exhibit increased sympathetic activation. Lack of Dyn causes reduced IGF-1, T4, and free fatty acid levels. IGF-1 is capable of inhibiting Na+/H+ antiporter. Thus, increased IGF-1 may increase the development of heart failure via uncontrolled Na+/H+ antiporter hyperactivity-mediated increased calcium transient. Increased T4 protects cardiac tissue following I/R injury. Panel B. Arrhythmogenic signaling of dynorphins. Once a threshold is reached (30–50 μM), dynorphins induce arrhythmias through PKC- and IP3 - dependent mechanisms. Lastly, inhibition of Na+/K+ ATPase by Dyn leads to arrhythmias via intracellular calcium mobilization and activation of PKC -dependent Ca2+ signaling pathway. The flow of physiological changes that require further investigations are shown in dashed arrows. BP; blood pressure, CRH; corticotropic-releasing hormone, Epi; epinephrine, FFAs; free fatty acids, HR; heart rate, IGF-1; insulin-like growth factor 1, IP3; NE; norepinephrine, PKC; protein kinase C, SER; sarco/endoplasmic reticulum-associated calcium storage organelle, T4; thyroxine.
4.3. Pharmacology
The relationship between isoprenaline treatment and increased lipid peroxidation and antioxidant enzymes (GSH, SOD, and catalase) has been established in rat tissues [40]. Dynorphin treatment caused a reduction in GSH levels in isolated rat hearts [41]. Evidence also demonstrated that U50488 reduced the isoprenaline-increased Ca2+ current flow through voltage-gated Ca2+ channels in the sarcoplasmic reticulum [42]. All effects of U50488 were abolished by nor-Binaltorphimine (nor-BNI) [42]. Investigations were therefore performed to determine isoprenaline-induced lipid peroxidation influences on antioxidant enzyme levels and to provide mechanistic evidence that linked increased catecholamines to a dynorphin-dependent pathway in cardiomyocytes [43]. U50488 was capable of reducing lipid peroxidation and depletion of antioxidant enzymes in isoprenaline-mediated cardiac hypertrophy (Fig. 2A); [43]. Moreover, isoprenaline treatment caused an increase in myocardial fibrosis beneath the endocardium, as evidenced by increases in Type I and Type III collagen and fibronectin in the left ventricles, which could be reduced by U50488 treatment [42]. The U50488 reduction in fibrosis following isoprenaline treatment was later confirmed by a second study [43]. These studies indicate experimental evidence of the dynorphin-mediated signaling are crucially involved in numerous physiological processes in the CV system including tissue remodeling and protection against sympathetic over-activation (Fig. 2A).
4.4. Dynorphin and Fetal Development
It is now widely accepted that there is a critical window of vulnerability in which fetal development is heavily influenced by environmental stress [44] and disease risk [45], but the fetal heart has limited ability to respond to stress via sympathetic stimulation in gestation [46]. Dynorphin and KOP are both proclaimed to have a role in gestational vascularization in the brain (Fig. 3) [47]. Given that Leu-enkephalin from the adrenal gland concentration increases with each passing month of fetal development [48], it was suggested that the adrenal gland could contribute to CV development during the first two months of fetal development [48] and that adrenal enkephalins might play a modulatory role for circulating catecholamines in fetal development [48]. This implicates dynorphin playing a significant role because Leu-enkepalin and pDyn are formed from the same precursor molecule, ACTH [11]. Evidence shows that norepinephrine and epinephrine can increase HR and BP in fetal sheep [49], which are necessary for fetal survival [50], and KOP agonism is cardioprotective in neonatal hearts [42]. Dynorphin decreases the expression of the VEGF receptors, FLK1 and NRP [47]. The need to confirm the influence of dynorphin on VEGF receptor expression cannot be understated given that NRP1 influences the development of the sympathetic nervous system throughout gestation [51]. Together with other reports demonstrating dynorphin may have a role in cardiomyocyte differentiation [52, 53] and glial cell proliferation [54], Dynorphin has a potential physiological role in fetal development that could be modulated by maternal stressors.
Fig. (3). Schematic depiction of the importance of dynorphins during cardiovascular development.
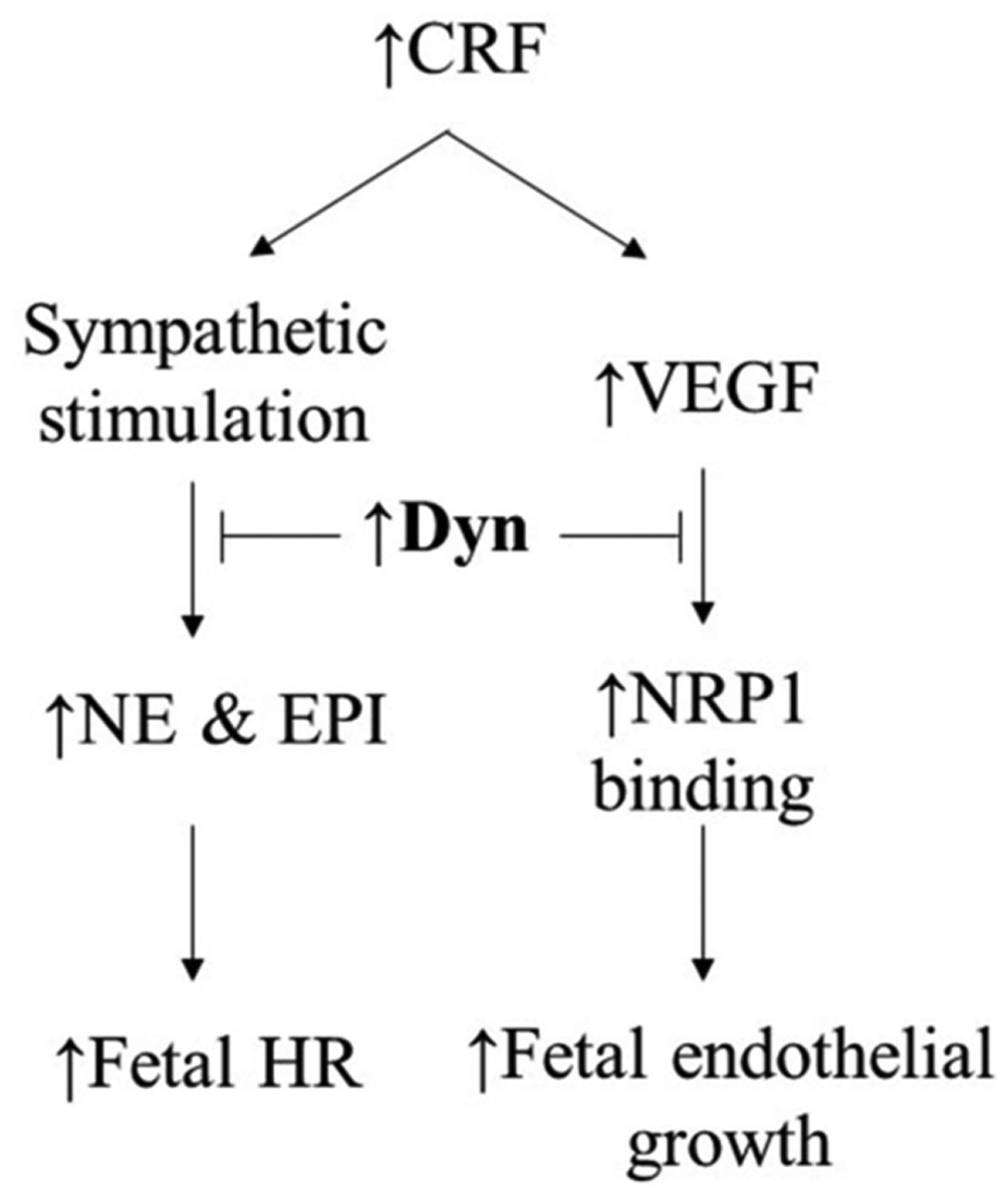
CRF, master stress hormone, is capable of elevating MAP and heart rate via noradrenergic sympathetic nervous outflow as well as serving as angiogenic stimulator in fetal endothelial cells through production of vascular endothelial growth factor (VEGF). Dyn B appears to be involved in embryonic cardiogenesis and catecholamines have been shown to maintain fetal heart rate survival Dynorphin A appears to be a modulator of both noradrenergic sympathetic nervous outflow and VEGF signaling.
5. GENETIC INFLUENCES
5.1. pDyn Mutation is not an Independent Predictor of CV Disease
Although physiological complexity exists, genetic defects in dynorphin [55] that are observed in mice can inform its role in human disease states, beyond its well-recognized involvement in pain and addiction. These genetic defects include elevated expression due to alterations in carboxypeptidase [56] and prohormone convertase activities, a lack of the transcriptional repressor, Downstream Regulatory Element Antagonistic Modulator (DREAM; [57], or epigenetic modifications.
Mice that carry the mutation, R212W, in pDyn (pDyn R212W) show increased levels of mutant dynorphin A expression without any change in dynorphin-B. The pDyn R212W mouse exhibits many clinical features of spinocerebellar ataxia type 23 (SCA23) [58, 59]. Although pDyn is expressed in myocardial tissues [27], patients with SCA23 [60] or pDyn R212W mice do not present with abnormalities in autonomic function or cardiomyocyte function. However, SCA 23 is a very rare form of the SCA1 autosomal dominant and is diagnosed in a small number of cases worldwide. Further investigations are required to address whether the pDyn R212W mice exhibit heart defects. Needless to say, the focus of research has been on pDyn and its neuropeptides that are expressed in the brain, particularly those neurons involved in motor neuron disease. But, it remains unclear as to how the mutation of pDyn, which is expressed in the brain and the peripheral tissues, affects only selected tissues. Although the experimental study of pDyn R212W mice provides significant insight into the role of dynorphin in the CNS pathways regulating glutamatergic signaling, it should not discourage the careful use of pDyn R212W mice which can serve as an important genetic tool to study the role of pDyn and its neuropeptides in the heart and other tissues. For instance, dynorphin A regulates alpha-cell glucagon secretion [61], which plays a critical role in the regulation of glycaemia and ensuing glucose homeostasis. Further careful characterization of pDyn R212W mice might contribute toward the understanding of various pathways associated with dynorphin-mediated signaling.
KOP is encoded by the opioid receptor kappa 1 (OPRK1) gene, which is located on chromosome 8q11.2. Several single nucleotide polymorphism (SNP) and insertion/deletion of nucleotides upstream of the translational start site in OPRK1 of KOP have been reported for the human [62]. Although the SNPs are associated with alcohol dependence, the phenotypes, CV or otherwise, of these SNPs and insertion/deletion have not been investigated.
5.2. Other Biological Processes
In knockout studies, pDyn knockout mice had increased sympathetic activation, decreased IGF-1, T4, and free fatty acids (Fig. 2A), which can have a negative effect on the body’s function. Dynorphin-knockout mice exhibited increased fatty acid oxidation and corresponding reductions in adiposity. Evidence indicates that Dyn-A1–17 administration causes an increase in total free fatty acids and nor-BNI reduces Dyn-A1–17-induced motor dysfunction [63]. Importantly, recent studies suggest that IGF-1 is cardioprotective by inhibiting Na+/H+ exchanger activity via phosphorylation at Ser648 of the exchanger [64] and T3 aids in early-phase CV angiogenic sprouting after ischemia/reperfusion (I/R) through angiopoietin-1 and VEGF upregulation [65]; (Fig. 2A). Previous research has shown that thyroxin (T4, a precursor of T3) induces a decrease in liver tissue glutathione (GSH) leading to increased liver I/R-induced damage [66]. Of interest, propylthiouracil (an antithyroid agent) reverses severe skin scald injury-induced GSH reduction [67], while increasing GSH concentration at the end of reperfusion [66]. Hypothyroid hearts are less vulnerable to I/R injury [68]. It is tempting to speculate that dynorphin-knockout mice, exhibiting reduced thyroid hormone, would be protected from I/R injury (Fig. 2). Most recently, T3 is found to stimulate the expression of AT2R/MAS1-ACE2 in an in vivo myocardial I/R model using rats [69]. The outcome of the aforementioned studies is that dynorphin signaling is important in the CV system. Since the removal of pDyn is not lethal, one may argue that pDyn is not critical to the CV system. As opposed to studies in genetically altered mice, the roles of dynorphins on CV function is mainly extrapolated from the studies of the effects of pharmacological inhibition of KOP. The findings of the above studies provide an opportunity to explore the complex and intriguing relationship among IGF-1, T4, free fatty acids and dynorphin as it relates to many CV-related organs including brain, heart, kidney, and liver.
6. DYNORPHIN IN CV SYSTEMIC DISEASE
6.1. Dynorphins and Blood Pressure Regulation
Herein, we divide our discussion into two parts: the first sub-section being devoted to the role of dynorphin in the blood vessels of the brain and its cardiovascular consequences, and the second part dedicated to the blood vessels residing outside of the brain.
6.1.1. Modulatory Role for Dynorphins at the Level of Central Nervous System
The coordinated interactions between the autonomic nervous system (ANS) and the sensory system appear to modulate cerebrovascular tone under pathological condition [70, 71]. Moreover, many endogenous paracrine and intracrine peptide hormones can also specifically modify this coordinated interactive state. Notable examples are peptides of the renin-angiotensin system (RAS), kallikrein-kinin system (KKS), endothelin, and corticotropin-releasing factor (CRF).
Attempts to determine the mechanisms of action for dynorphin on cerebral vasculature are complicated due to its interactions with several different proteins, the heterogeneity of neurovascular unit cell population, species dependency of its receptor [72], and the individual patterns of hemodynamic alteration under BP regulation [73]. Over the last three decades, the possibility that dynorphin is involved in ANS/sensory system/paracrine and intracrine peptide hormones axes has been examined [74].
Evidence suggests dynorphin can suppress one of the vasoconstriction pathways [75] depending on the particular blood vessel or vascular bed studied [76]. Dynorphin plays a role as an endogenous hypotensive peptide in healthy rats [77] and those that undergo subsequent bradycardia [78]. Notably, dynorphin modulates sympathetic activity via stimulation of atrial natriuretic factor [79], which can reduce BP in hypertensive subjects. In addition, dynorphin appears to control urine flow (i.e. diuresis) by acting at KOP [80]. A dramatic and sustained increase in dynorphin level might interfere with non-reabsorbed solute used to treat polyuria associated with diabetes mellitus.
To date, although angiotensin 2 converting enzyme, a major protease of the RAS, is capable of hydrolyzing Dyn-A1–13 [81], the interaction between endogenous opioids and the RAS is unclear. Positive and negative relationships have been reported, and some reports argue for no relationship at all [82]. But the question remains as to whether dynorphin-mediated signaling possesses some degree of physiologically important CV activity that is simply too low to quantify by the methods used so far. Ang II and aldosterone can act in the brain to increase sympathetic outflow and ensuing hypertension. Ang II has been reported to increase the expression of the pDyn gene in porcine [82, 83] and Ang II treated cells decrease the release of aldosterone in the presence of a KOP receptor agonist [83], which may be due to non-specific binding of the KOP receptor agonist to the Ang II receptor, angiotensin type 1 receptor (AT1R). KOP antagonists surprisingly reduce BP elevation by interfering with Ang II-induced vasoconstriction in the brain [75]. Studies reported that KOP receptor antagonism by MR 2266 had a partial inhibitory effect on Ang II’s actions on HR and diastolic BP [75, 82]. Of particular interest is how dynorphin signaling/RAS interactions may affect the development or progression of hypertension and its potential complications.
Dynorphin may play a pivotal role in the development of hypertension and is known to exert a critical role in patients with chronic hypertension. Patients with severe hypertension demonstrate both plasma opioid and catecholamines (norepinephrine, epinephrine) elevations [84]. Interestingly, an earlier study on the role of dynorphin in hypertensive patients with pheochromocytoma indicates that opioids [85], including Dyn-A1–13, can suppress the secretion of catecholamines [86]. It is the inhibition of the opioid receptors that induce hypertension [85]. Under a stressful situation, the body releases epinephrine, which causes an increase in HR and raises BP. Although the association between stress and hypertension is not well-established, dynorphin A-mediated pathways may be a key player in the hypertension-stress relationship [87].
Dynorphins exhibit a high level of control by acting in the central nervous system (CNS) to offset the increases in CRF-induced cardiac function. The intracerebroventricular administration of Dyn-A1–17, but not Dyn-A1–13, is capable of attenuating CRF-induced elevations of mean arterial pressure (MAP), HR, and plasma catecholamine levels in conscious unrestrained rats [88]. It is shown that if Dyn1–8 is administered into the hippocampal formation, it results in a decrease in BP with no effect on HR in conscious hypertensive and normotensive rats [89]. The combination of Dyn1–17 and CRF did not result in a surge of norepinephrine or epinephrine levels as was observed in groups treated with CRF alone [88]. Moreover, there is a reciprocal release action between CRF and dynorphin A [90, 91] within the hypothalamus. CRF-induced corticotropin-releasing factor 2b (CRF2β) activation causes cerebrovascular vasodilation leading to increased cerebral blood flow in a dose-dependent manner [92]. Urocortins are members of the CRF peptide family, which contribute to cerebrovasculature and vasohumoral function in cardiac-normative and - diseased states (in addition to their well-recognized role in the hypothalamopituitary-adrenal stress axis; [93]. Urocortin 2 is found to be a vasoactive and cardioprotective peptide. It induces vasorelaxation by activating CRF2β receptors located in the intracerebral arterioles [92]. Since CRF is an endogenous trigger of dynorphin A, the balance between overproduction or reduced degradation of endogenous CRF and dynorphin A in favor of vasodilation could be a major factor in their vasodilatory action and the extent of vasodilation during sever prolonged ischemia in animals.
Dynorphin is present in human cerebral perivascular nerves [94], which carry out similar roles to cerebrovascular functions. Dynorphin B, in particular, is expressed in human blood vessels [95]. Although dynorphin A does not alter vessel tone in vitro, it inhibits the release of neurotransmitters from afferent and sympathetic axons which could influence the autonomic functions of the vasculature [95]. Studies have been carried out to establish whether dynorphin has a protective role after BP increases. Dynorphin appears to exert its vasodilatory effect during the normotensive phase, though it induces vasoconstriction during hypotension in the cerebrovasculature [96]. This intriguing observation has been the subject of little additional research.
Studies support a modulatory role for dynorphins at the level of CNS, which subsequently affect vascular reactivity. Dyn1–13 induces vasodilation via acting on the presynaptic neuronal NF-κB/p65 KOP [97]. Dynorphin A-mediated KOP activation sustains the contraction of rat cerebral arteries [94]. The KOP antagonist, nor-BNI [89], and Gi/o antagonist, pertussis toxin, fail to completely abolish dynorphin-dependent vasoconstriction [94]. Des-Tyr1 dynorphin (2–13), a non-KOP agonist, also exerts vasoconstrictive actions; however, nor-BNI fails to prevent des-Tyr1 dynorphin (2–13)-induced vasoconstriction. These data imply the importance of the Tyr residue at the amino terminus of the Dyn1–13 peptide as a determinant of the capability of dynorphin A to activate KOP and regulate cerebral arteries [94]. Dynorphin A has also been reported to be localized in the adventitia layer of the middle cerebral artery and the basilar artery [94]. The basilar artery and middle cerebral artery have both been reported to demonstrate dose-dependent increases in contraction after administration of Dyn1–13 and Dyn1–17, with the former having larger effects at 10 μM in the basilar artery and the latter having larger effects in the middle cerebral artery [94]. Dynorphin-B exerted negligible effects on contraction in both the basilar and middle cerebral artery [94]. Local interaction of dynorphin with the noncerebrovasculture may also be an important variable that influences cerebrovascular reactivity [94]. Taken together, dynorphin-mediated signaling plays an important role in the cerebral circulation and contraction function.
6.1.2. Modulatory Role for Dynorphins at Vessels Residing Outside the Brain
The extant literature reveals a complex picture of dynorphin’s role in systemic BP regulation. The importance of dynorphins in the control of CV function emerged from the 1983 empirical study examining the effects of dynorphins on BP [98]. Dynorphin1–13 caused a 60 mmHg drop in MAP [98]. The drop in pressure only occurred upon intracisternal administration, with no changes reported upon intravenous administration. The modulation of dynorphin-mediated reduction in blood flow by vascular aminopeptidase (s) cannot be ignored in animals with intravenous injection of dynorphin with absolute certainty. Further investigation is required to confirm the potential acute pressor action of dynorphin via intravenous administration. However, the results of the above study suggested that the hemodynamic consequences may occur as a result of interference with the ANS, the master circuit in the regulation of systemic BP, by the intracisternal administration of dynorphin.
The physiological function of dynorphin in the peripheral vasculature has not been thoroughly investigated. Dynorphin appears to decrease vascular reactivity through endothelial mechanisms. KOP receptor mRNA is expressed in the endothelial cells. Studies have documented the effects of KOP receptor agonism in the endothelium [47]. Tian and collaborators [99] demonstrated that KOP agonism via U50488 rescued pathological symptoms of hyperlipidemia associated with aortic endothelial cell morphology. In a well-designed experiment, dynorphin-KOP signaling was shown to be involved in vascular development, in particular in endothelial differentiation and proper vascular pathfinding processes [47].
Despite over a decade of research into the mystery of dynorphin interactions with CV function, a key question remains unresolved: how does dynorphin contribute to hypertension? U50488H decreases the left ventricular systolic pressure in hamsters subjected to untreated chronic hypertension [100]. Herein, we review the recent findings describing the potential cross-talk between dynorphins and major local autocrine/paracrine factors known to control smooth muscle contraction. The intimate interaction between RAS (a system well-known for its regulation of BP and fluid homeostasis), endothelin-1 (ET-1, a paracrine regulator), and dynorphin for protection against Ang II-induced constriction may provide possible explanations to the above question. ET-1 is involved in the development of fibrosis [101]. Like dynorphin, the ET-1 level is upregulated in CSF following fluid percussion injury [102]. ET-1 receptor antagonists partially restore dynorphin-induced vasodilation after such injury. Naloxone, a non-selective and competitive opioid receptor antagonist, is found to be ineffective in blocking metorphamide-induced ET-1 release suggesting that these effects occur via opioid receptor-independent mechanisms. Metorphamide, an opioid with a high affinity for MOP [~50% less affinity to KOP and negligible activity at DOP; [103]], promotes the release of ET-1 in aortic endothelial cell culture [104]. Examining the dynorphin-ET-1 relationship in vivo may provide important clues as to how these interactions occur and whether dynorphin-ET-1 signaling in the peripheral vasculature influences systemic hemodynamics. It is presently unclear whether dynorphins function influences peripheral vasculature.
6.1.3. Dynorphin and Heart Failure
Patients with advanced heart failure are generally treated with opioids to alleviate breathlessness and unload the heart [100]. Evidence suggests that pDyn gene expression may be involved in controlling heart function via autocrine- or paracrine-dependent mechanisms [105]. The presence of dynorphin in postganglionic sympathetic nerve fibers innervating coronary blood vessels and cardiomyocytes have been reported [106].
Dynorphin appears to aid in an ensuing fall in BP and maintaining cardiovascular activities both centrally and locally under pathological conditions such as heart failure, myocardial infarction (MI) and acute respiratory failure [107]. In a model of congestive heart failure, KOP and pDyn expression in the left ventricle were increased compared with the control [33]. All congestive heart failure rats presented with dilated hearts and severe systolic and diastolic dysfunction [33]. While, KOP mRNA expression is low in the heart [108], a rat model of renovascular hypertension (2K1C rats) exhibits increased KOP, along with heterodimerization of apelin receptors and KOP, leading to altered cardiac inotropic effects [109].
The functional behavior of dynorphin changes with the severity of heart failure. [110]. Recent evidence suggests that the KOP agonist is an anti-infarct agent and its effect is mediated via ERK1/2 [111]. Dynorphin stimulates the release of an atrial natriuretic factor in patients with acute heart failure, but it attenuates this factor in patients with severe heart failure [79]. This observation indicates that dynorphin not only has the ability to switch biological activities in physiological conditions versus pathological conditions, as in fluid percussion injury, but also that the biological roles of dynorphin can switch in a disease state manner (acute vs severe). These data suggest new avenues for research targeting the primary mechanism through which dynorphin signaling influences heart and the development of heart failure. For instance, the physiological significance of dynorphin-mediated signaling in the animal model of coronary artery occlusion that compromises coronary blood flow, promotes vascular endothelial dysfunction, and thrombus formation must be investigated in order to determine whether altered dynorphin is indicator or causal in the genesis of the heart failure.
6.1.4. Dynorphin and Cardiac Arrhythmia
Ischemia influences the level of dynorphin in the non-preconditioned heart [112]. This observation is in line with the notion that the dynorphin-mediated KOP signaling pathway is involved in I/R arrhythmia (Fig. 2B). In support, MR2266, a KOP antagonist, reduced arrhythmia [112]. U50488 has been reported to act on ventricular myocytes by two separate pathways; a cAMP-dependent pathway and a phospholipase Cdiacylglycerol pathway. At 1 μmol/L, U50488 decreased noradrenaline-cAMP stimulation [113]. Exposure of the heart to U50488 (50 μmol/L) caused IP3-dependent arrhythmias [113]. This observation is in accord with evidence that dynorphin-induced cardiac arrhythmias are concentration-dependent [114]. In the same study, the arrhythmia surprisingly appears to be triggered by myocardial cAMP. The above studies suggest that caution should be exercised when IP3 formation alone is elevated, which can have deleterious effects on heart. Dynorphin is also capable of potentiating epinephrine-induced arrhythmias through CNS mechanisms [115], which might be due to Ca2+ overload (Fig. 3). A previous study also demonstrated that a combination of dynorphin and epinephrine can markedly influence the severity of arrhythmia [116]. In contrast, U-62066 (a selective agonist of KOP) protected rats from epinephrine-induced arrhythmias (Table 2; [117]). In addition, dynorphin potentiated digitoxin-induced arrhythmia in guinea pigs [118]. However, KOP knockout mice exhibited a decrease in triglyceride synthesis in the liver and reduced malonyl-CoA [119]. Although the exact mechanism(s) by which the malonyl-CoA is altered in the KOP knockout mice are not clear, the control of malonyl-CoA levels, a major inhibitor of mitochondrial fatty acid uptake, is considered important in heart disease [120, 121]. Chronic elevation of fatty acid oxidation is a major contributor to the severity of ischemic heart disease. Evidence suggests that improved fatty acid suppression or reduction in myocardial fatty acid uptake may improve myocardial ischemia tolerance in human type 2 diabetes mellitus [122]. The reduction in malonyl-CoA is linked to an increase in fatty acid oxidation rates during reperfusion of ischemic hearts [123].
Table 2.
KOP blockers and activator.
| Synthetic molecules | KOP | Tissue | Effect | Ref. |
|---|---|---|---|---|
| Agonists U50488H | Selective | Heart | Activate Na+-H+ exchanger via PKC activation Reduces oxidative stress |
[36] [43] |
| Spiradoline (U-62066) | Highly selective | Cerebral ventricle | Protects from epinephrine-induced arrhythmias | [117] |
| Antagonists MR2266 | Non-selective | Myocardium | Reduced severity of I/R induced arrhythmia Reduced infarct size Enhanced coronary blood |
[112] |
| Nor-BNI | Selective | Intrathecal administration Lateral cerebral ventricle Heart | Reduces the pressor actions of Dyn A administration Inhibitory effect on Ang II induced increases in HR and BP via CNS-mediated mechanisms Reduces isoprenaline-induced cardiac hypertrophy and fibrosis |
[142] [75] [42] |
Ang II; angiotensin II, I/R; ischemia/reperfusion, Nor-BNI; Norbinaltorphimine (nor-BNI; 17,17′-Bis (cyclopropylethyl)-6,6′,7,7′-tetradehydro-4,5:4′,5′- diepoxy-6,6′- (imino)[7,7′-bimorphinan]-3,3′,14,14′-tetrol), PKC; protein kinase C
Although the literature suggests that dynorphin-mediated KOP signaling may have a role in treating arrhythmia, this conclusion is based on agonist and antagonist activity at KOP. It would be of interest to determine whether such function can be detected in dynorphin overexpression models and/or address the tissue-specific functions of KOP by creating conditional KOP null alleles.
6.1.5. Dynorphin-mediated Effects to Inflammation-induced Cardiac Injury
Ischemic cardiac injury initiates the activation of toll-like receptor (TLR)-mediated innate immune response and increases the chemokine levels as well as cytokine synthesis in the infarcted heart [124]. Inflammation and the recruitment and infiltration of inflammatory cells are intertwined with MI in order to bring about infarct healing, angiogenesis, and ventricular remodeling [124].
The role, if any, that dynorphins play in inflammation has not been well-documented in the pathophysiology of heart disease. White blood cell (WBC) count is altered during infection-, toxin-, or drug-induced inflammation (Fig. 4). Increased WBC count is a strong predictor of coronary risk in patients with and without coronary heart disease (CHD; [125] independent of the traditional risk factors that include diabetes, hypertension, dyslipidemia, and obesity.
Fig. (4). Dynorphins and cardiac inflammation.
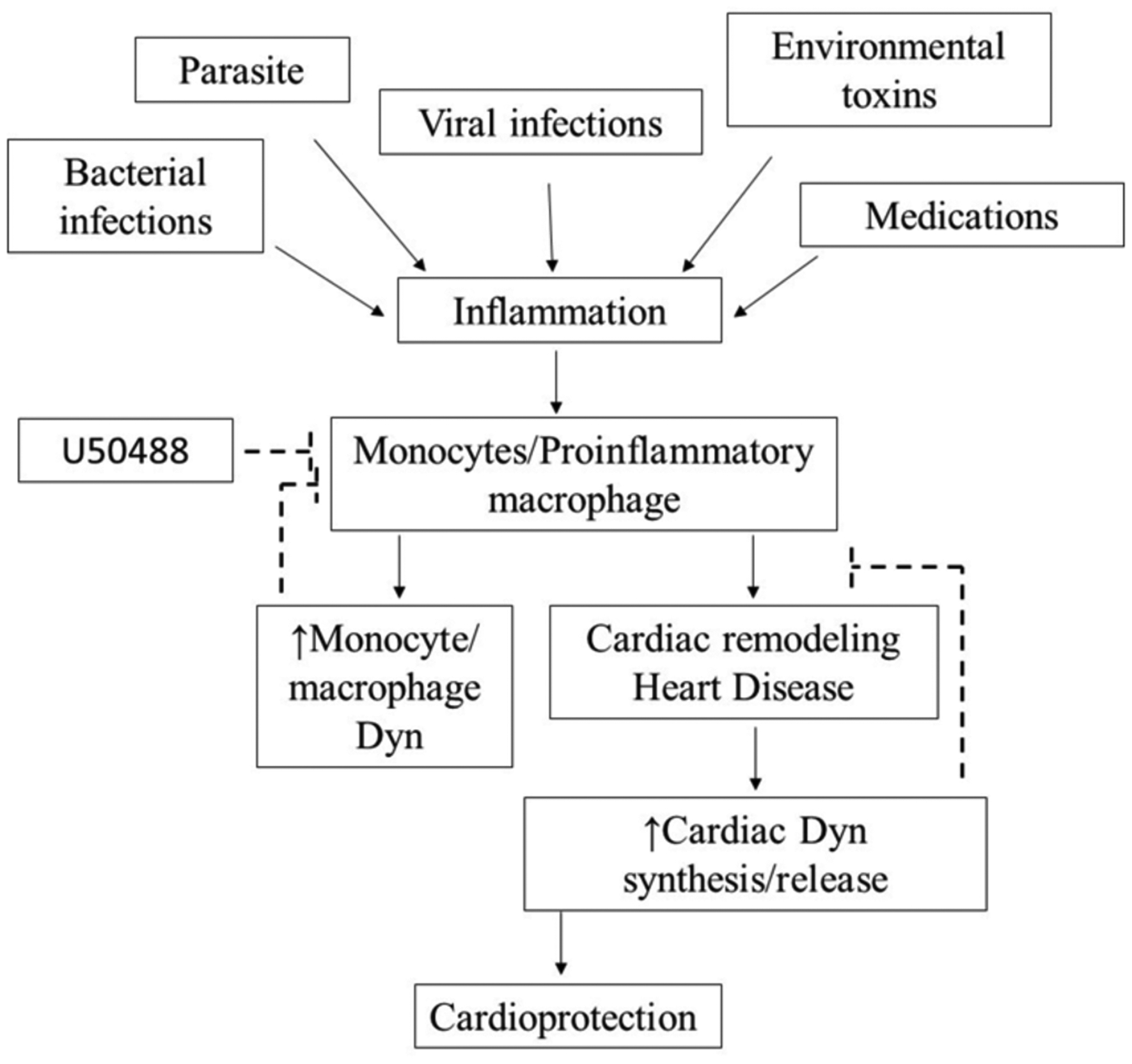
Inflammation is controlled by multiple factors that could include environmental toxins, medications, parasite, bacteria or viral infection, Inflammation-induced cytokine production can cause cardiac remodeling and heart disease through increased monocyte and pro-macrophage activation. During inflammation, there is an increase in the synthesis and release of Dyn. Released Dyn modulates macrophage-mediated cytotoxicity avoiding arrhythmogenesis and eliciting cardioprotective effects.
KOP expression increases in LPS-stimulated monocytes [126]. Dynorphins exert anti-inflammatory effects partly by inhibiting NF-κB/p65 nuclear translocation in LPS-stimulated THP1 monocytes [126]. Dyn1–17, Dyn1–6, Dyn1–7, and Dyn1–9, or U50488, decrease NF-κB/p65 nuclear expression [126]. Further, selective Dyn metabolites reduce the expression of proinflammatory cytokines, including IL-1β and TNF-α [126]. However, others are reported to increase TNF-α release [127].
Myocardial opioid peptides appear to exert key roles in the cascade of signaling pathways that regulate the heart [109]. Evidence indicates that myocardial opioid-mediated receptor activation inhibits cardiac excitation-contraction coupling and protects the heart against hypoxia and ischemic injury, independent of enkephalin release [128]. Nor-BNI pretreatment increases dynorphin levels following myocardial stunning (transient ischemia; [129]). The detrimental effects and beneficial role of opioid-mediated KOP activation may be attributable to the concentration of endogenous dynorphin, stimulating exogenous opioids, or functional diversity in the response of inflammatory cells.
The immune responses of dynorphin are not limited to monocyte-derived immune cells. Dynorphin effects in the immune system have been reported in many types of immune cells [refer to [18]]. Importantly, U50488 suppresses IL-6, IL-1β and TNF-α production in macrophages and decreases monocyte chemotaxis. Under pathological conditions, such as HIV, dynorphin A can stimulate TNF-α and IL-6, which were blocked by nor-BNI [18]. Interruption of dynorphin-dependent signalling may be beneficial for the treatment of inflammation, which is common for heart disease as well as stroke patients.
U50488 has been shown to be cardio-protective against reactive oxygen species (ROS)-mediated damage (106); supporting the notion that dynorphin can confer protection against ROS in some tissues, but not others. Alternatively, at lower concentrations than those investigated previously (106), dynorphin may not be cardioprotective and may contribute to ROS-induced cardiac damage. These findings are relevant to a number of CV-related pathologies given that ROS plays a role in hypertension, cardiomyopathy, cardiac hypertrophy [130] and promotes inflammation by activation of NF-κB and advanced glycation end products that stimulate cytokine monocytes to release TNF-α in prediabetic patients [131]. The anti-inflammatory and/or pro-inflammatory properties of dynorphins need to be elucidated and currently represent an understudied therapeutic target.
6.1.6. Dynorphin and Stroke
The endogenous dynorphin peptides are implicated in the pathogenesis of stroke [132, 133]. Dynorphin A reduces cerebral ischemic injury [134]. Evidence supports a neuroprotective role for dynorphin in stroke [135]. Dynorphin is increased transiently in the ipsilateral central nucleus of the amygdala following a stroke that may mediate the CV complications [136]. Dynorphin A decreases infarct volume following a local brain I/R injury, which can be attenuated by a KOP antagonist [134]. Most patients with ischemic stroke experience blockage of the middle cerebral artery. The mouse model of middle cerebral artery occlusion exhibits a decrease in Purkinje cell number [137]. As mentioned earlier, similar neuron defects are observed in mice with targeted pDyn mutation, pDyn R212W. A better understanding of the mechanisms of dynorphin using pDyn R212W mice would not only highlight its importance in cerebrovascular physiology and cerebral circulation, but it would enable us to realize its therapeutic potential in the treatment of ischemic stroke. Further studies will be necessary to unveil whether the mutated pDyn may lead to a loss of protection from stroke in the context of SCA23. Additional studies of the post-ischemic pathophysiology of each endogenous dynorphin peptide using current models of stroke (for instance using endovascular trapping techniques) may provide a rational basis for establishing the role of dynorphins in stroke.
CONCLUSION
Understanding the biology of the dynorphin-mediated signaling in the CV system is a challenging scientific problem with numerous clinical relevance. We are now aware of the presence of a dynorphin-mediated signaling network in the various tissues within the CV system, which raises the question as to the function of dynorphins in regulating/modulating the response of the cell or tissue in which it is found. Under physiological condition, dynorphin functions include control of cardiac regulation, modulation of the flow of blood through the cerebral arteries, and modulation of inflammatory mediators in inflammatory cells. The peripheral dynorphin-mediated signaling may have a protective function against CV risk. Under pathological conditions, there is a strong association between elevated dynorphin function in cardiac tissues and heart failure. KOP expression changes in the model of congestive heart failure and that of renovascular hypertension. KOP agonists have recently been implicated in renal protection against ischemic injury. Collectively, dynorphins have physiological functions in the cerebrovasculature and the CV tissues. We should no longer consider dynorphins to be only for neurons.
During the last three decades, a large number of studies have focused on dynorphins and their role in the CV system of small animals. The use of small-animal models in the field of dynorphins has been historically important in helping investigators understand the biological and physiological roles of dynorphins in CV regulation. This approach has laid a strong foundation for our conceptual understanding of the expression and function of dynorphins within the CV system. Clinical issues related to dynorphin excess or dynorphin deficiency in humans have been under-investigated. Given the experimental limitations inherent to clinical work, a thorough understanding of the physiology and pathology of dynorphins in appropriate large-animal models [138] could bridge the gap between basic science findings and clinical observation. Large animals more resemble the human CV system with a high percentage of genetic conservation which is necessary to translate data to human children, adults, or the elderly. For instance, the pig heart resembles the young human heart which lacks anastomosis; a feature not present in small-animal models. Likewise, elderly or diabetic patients often experience myocardial infarction and myocardial cells generally recover if the infarct zone is adequately perfused by collateral circulation. Like the aged human heart, the canine heart has the innate ability to promote collateral growth. The canine heart further resembles the contractility of the adult human heart, supporting its utility in the assessment of cardiomyopathy. The dynorphin-mediated signaling research in large animals will provide opportunities to learn a great deal about dynorphins. The use of higher-order animal models can further translate these findings to human disease states. Despite the limitations of working with large animals (horse, cow, dog, pig), their suitability in expanding our knowledge of CV function, including interactions with conserved genes that contribute to multifactorial disease states, cannot be understated [139]. As such, translation to large-animal models presents as the next step to study dynorphins in a “human-like” CV organ to further elucidate their role in normative CV function and CV disease.
ACKNOWLEDGEMENTS
This work was supported by the University of Mississippi overhead account (Z.S.) and the National Institutes on Drug Abuse: R00 DA039791 (J.J.P.)
LIST OF ABBREVIATIONS
- ANS
Autonomic nervous system
- CV
Cardiovascular
- CRF
corticotropin-releasing factor
- Dyn-A1–13
Dynorphin A (1–13)
- Dyn-A1–13
Dynorphin A (1–8)
- Dyn-B1–13
Dynorphin B (1–13), Rimorphin
- Dyn-A1–17
Dynorphin B (1–17)
- IGF-1
Insulin-like growth factor-1
- I/R
ischemia/reperfusion
- KOP
Kappa opioid receptor
- Nor-BNI
nor-Binaltorphimine
- OPRK1
opioid receptor kappa 1
- pDyn
Prodynorphin
- SNP
Single nucleotide polymorphism
- SCA1
spinocerebellar ataxia type 1
- SCA23
spinocerebellar ataxia type 23
- T3
Triiodothyronine
- T4
Thyroid hormone thyroxine
Footnotes
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- [1].Billingsley HE, Carbone S. The antioxidant potential of the Mediterranean diet in patients at high cardiovascular risk: an in-depth review of the PREDIMED. Nutr Diabetes 2018; 8(1): 13 [ 10.1038/s41387-018-0025-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Marek-Trzonkowska N, Kwieczyńska A, Reiwer-Gostomska M, Koliński T, Molisz A, Siebert J. Arterial Hypertension Is Characterized by Imbalance of Pro-Angiogenic versus Anti-Angiogenic Factors. PLoS One 2015; 10(5)e0126190 [ 10.1371/journal.pone.0126190] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Ok E, Levin NW, Asci G, Chazot C, Toz H, Ozkahya M. Interplay of volume, blood pressure, organ ischemia, residual renal function, and diet: certainties and uncertainties with dialytic management. Semin Dial 2017; 30(5): 420–9. [ 10.1111/sdi.12612] [DOI] [PubMed] [Google Scholar]
- [4].Lim LM, Tsai NC, Lin MY, et al. Hyponatremia is Associated with Fluid Imbalance and Adverse Renal Outcome in Chronic Kidney Disease Patients Treated with Diuretics. Sci Rep 2016; 6: 36817 [ 10.1038/srep36817] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kraut JA, Madias NE. Adverse Effects of the Metabolic Acidosis of Chronic Kidney Disease. Adv Chronic Kidney Dis 2017; 24(5): 289–97. [ 10.1053/j.ackd.2017.06.005] [DOI] [PubMed] [Google Scholar]
- [6].Shen MJ, Zipes DP. Role of the autonomic nervous system in modulating cardiac arrhythmias. Circ Res 2014; 114(6): 1004–21. [ 10.1161/CIRCRESAHA.113.302549] [DOI] [PubMed] [Google Scholar]
- [7].Silvani A, Calandra-Buonaura G, Dampney RA, Cortelli P. Brain-heart interactions: physiology and clinical implications. Philos Trans- Royal Soc, Math Phys Eng Sci 2016; 374(2067)20150181 [ 10.1098/rsta.2015.0181] [DOI] [PubMed] [Google Scholar]
- [8].Tanaka K, Kersten JR, Riess ML. Opioid-induced cardioprotection. Curr Pharm Des 2014; 20(36): 5696–705. [ 10.2174/1381612820666140204120311] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Feng Y, He X, Yang Y, Chao D, Lazarus LH, Xia Y. Current research on opioid receptor function. Curr Drug Targets 2012; 13(2): 230–46. [ 10.2174/138945012799201612] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Goldstein A, Tachibana S, Lowney LI, Hunkapiller M, Hood L. Dynorphin-(1–13), an extraordinarily potent opioid peptide. Proc Natl Acad Sci USA 1979; 76(12): 6666–70. [ 10.1073/pnas.76.12.6666] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Civelli O, Douglass J, Goldstein A, Herbert E. Sequence and expression of the rat prodynorphin gene. Proc Natl Acad Sci USA 1985; 82(12): 4291–5. [ 10.1073/pnas.82.12.4291] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Schwarzer C 30 years of dynorphins--new insights on their functions in neuropsychiatric diseases. Pharmacol Ther 2009; 123(3): 353–70. [ 10.1016/j.pharmthera.2009.05.006] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Horikawa S, Takai T, Toyosato M, et al. Isolation and structural organization of the human preproenkephalin B gene. Nature 1983; 306(5943): 611–4. [ 10.1038/306611a0] [DOI] [PubMed] [Google Scholar]
- [14].Day R, Lazure C, Basak A, et al. Prodynorphin processing by proprotein convertase 2. Cleavage at single basic residues and enhanced processing in the presence of carboxypeptidase activity. J Biol Chem 1998; 273(2): 829–36. [ 10.1074/jbc.273.2.829] [DOI] [PubMed] [Google Scholar]
- [15].Spampinato S, Goldstein A. Immunoreactive dynorphin in rat tissues and plasma. Neuropeptides 1983; 3(3): 193–212. [ 10.1016/0143-4179(83)90016-1] [DOI] [PubMed] [Google Scholar]
- [16].Spampinato S, Paradisi R, Canossa M, et al. Immunoreactive dynorphin A-like material in extracted human hypothalamic-hypophysial plasma. Life Sci 1993; 52(2): 223–30. [ 10.1016/0024-3205(93)90143-Q] [DOI] [PubMed] [Google Scholar]
- [17].Müller S, Ho B, Gambus P, Millard W, Hochhaus G. An HPLC/RIA method for dynorphin A1–13 and its main metabolites in human blood. J Pharm Biomed Anal 1997; 16(1): 101–9. [ 10.1016/S0731-7085(97)00010-1] [DOI] [PubMed] [Google Scholar]
- [18].Gein SV. Dynorphins in regulation of immune system functions. Biochemistry (Mosc) 2014; 79(5): 397–405. [ 10.1134/S0006297914050034] [DOI] [PubMed] [Google Scholar]
- [19].Brugos B, Hochhaus G. Metabolism of dynorphin A(1–13). Pharmazie 2004; 59(5): 339–43. [PubMed] [Google Scholar]
- [20].Müller S, Hochhaus G. Metabolism of dynorphin A 1–13 in human blood and plasma. Pharm Res 1995; 12(8): 1165–70. [ 10.1023/A:1016211910107] [DOI] [PubMed] [Google Scholar]
- [21].Chavkin C, Goldstein A. Specific receptor for the opioid peptide dynorphin: structure--activity relationships. Proc Natl Acad Sci USA 1981; 78(10): 6543–7. [ 10.1073/pnas.78.10.6543] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Luiz AP, Schroeder SD, Rae GA, Calixto JB, Chichorro JG. Contribution and interaction of kinin receptors and dynorphin A in a model of trigeminal neuropathic pain in mice. Neuroscience 2015; 300: 189–200. [ 10.1016/j.neuroscience.2015.05.015] [DOI] [PubMed] [Google Scholar]
- [23].Pathan H, Williams J. Basic opioid pharmacology: an update. Br J Pain 2012; 6(1): 11–6. [ 10.1177/2049463712438493] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Sobczak M, Sałaga M, Storr MA, Fichna J. Physiology, signaling, and pharmacology of opioid receptors and their ligands in the gastrointestinal tract: current concepts and future perspectives. J Gastroenterol 2014; 49(1): 24–45. [ 10.1007/s00535-013-0753-x] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ventura C, Maioli M, Pintus G, Posadino AM, Tadolini B. Nuclear opioid receptors activate opioid peptide gene transcription in isolated myocardial nuclei. J Biol Chem 1998; 273(22): 13383–6. [ 10.1074/jbc.273.22.13383] [DOI] [PubMed] [Google Scholar]
- [26].Ventura C, Guarnieri C, Vaona I, Campana G, Pintus G, Spampinato S. Dynorphin gene expression and release in the myocardial cell. J Biol Chem 1994; 269(7): 5384–6. [PubMed] [Google Scholar]
- [27].Ventura C, Canossa M, Vaona I, et al. Prodynorphin mRNA is synthesized in adult cultured rat ventricular cardiomyocytes. Cardioscience 1993; 4(1): 21–4. [PubMed] [Google Scholar]
- [28].Ationu A, Sorensen K, Whitehead B, Singer D, Carter N. Ventricular expression and circulating levels of immunoreactive dynorphin in heart transplant recipients. Clin Sci (Lond) 1993; 85(1): 1–4. [ 10.1042/cs0850001] [DOI] [PubMed] [Google Scholar]
- [29].Šínová R, Kudová J, Nešporová K, et al. Opioid receptors and opioid peptides in the cardiomyogenesis of mouse embryonic stem cells. J Cell Physiol 2019; 234(8): 13209–19. [ 10.1002/jcp.27992] [DOI] [PubMed] [Google Scholar]
- [30].Dumont M, Lemaire S. Interactions of dynorphin A-(1–13) and nociceptin with cardiac D2 binding sites: inhibition of ischemia-evoked release of noradrenaline from synaptosomal-mitochondrial fractions. J Mol Cell Cardiol 2000; 32(8): 1567–74. [ 10.1006/jmcc.2000.1192] [DOI] [PubMed] [Google Scholar]
- [31].Andrews BT, McIntosh TK, Gonzales MF, Weinstein PR, Faden AI. Levels of endogenous opioids and effects of an opiate antagonist during regional cerebral ischemia in rats. J Pharmacol Exp Ther 1988; 247(3): 1248–54. [PubMed] [Google Scholar]
- [32].Lu X, Hong X, Wang C. [Effect of dynorphin A1–13 on hypoxia-ischemic brain injury in neonatal rats]. Zhonghua Fu Chan Ke Za Zhi 1997; 32(4): 198–201. [Effect of dynorphin A1–13 on hypoxia-ischemic brain injury in neonatal rats]. [PubMed] [Google Scholar]
- [33].Treskatsch S, Shaqura M, Dehe L, et al. Upregulation of the kappa opioidergic system in left ventricular rat myocardium in response to volume overload: Adaptive changes of the cardiac kappa opioid system in heart failure. Pharmacol Res 2015; 102: 33–41. [ 10.1016/j.phrs.2015.09.005] [DOI] [PubMed] [Google Scholar]
- [34].Ibrahim M, Gorelik J, Yacoub MH, Terracciano CM. The structure and function of cardiac t-tubules in health and disease. Proc Biol Sci 2011; 278(1719): 2714–23. [ 10.1098/rspb.2011.0624] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ventura C, Pintus G. Opioid peptide gene expression in the primary hereditary cardiomyopathy of the Syrian hamster. III. Autocrine stimulation of prodynorphin gene expression by dynorphin B. J Biol Chem 1997; 272(10): 6699–705. [ 10.1074/jbc.272.10.6699] [DOI] [PubMed] [Google Scholar]
- [36].Bian JS, Wang HX, Zhang WM, Wong TM. Effects of kappa-opioid receptor stimulation in the heart and the involvement of protein kinase C. Br J Pharmacol 1998; 124(3): 600–6. [ 10.1038/sj.bjp.0701857] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Barra de la Tremblaye P, Plamondon H. Alterations in the corticotropin-releasing hormone (CRH) neurocircuitry: Insights into post stroke functional impairments. Front Neuroendocrinol 2016; 42: 53–75. [ 10.1016/j.yfrne.2016.07.001] [DOI] [PubMed] [Google Scholar]
- [38].Ventura C, Pintus G, Tadolini B. Opioid Peptide gene expression in the myocardial cell. Trends Cardiovasc Med 1998; 8(3): 102–10. [ 10.1016/S1050-1738(97)00140-0] [DOI] [PubMed] [Google Scholar]
- [39].Dumont M, Lemaire S. Interactions of dynorphin A and related peptides with cardiac ouabain binding sites. J Mol Cell Cardiol 1996; 28(3): 615–21. [ 10.1006/jmcc.1996.0057] [DOI] [PubMed] [Google Scholar]
- [40].Rathore N, John S, Kale M, Bhatnagar D. Lipid peroxidation and antioxidant enzymes in isoproterenol induced oxidative stress in rat tissues. Pharmacol Res 1998; 38(4): 297–303. [ 10.1006/phrs.1998.0365] [DOI] [PubMed] [Google Scholar]
- [41].Yang CS, Tsai PJ, Chou ST, Niu YL, Lai JS, Kuo JS. The roles of reactive oxygen species and endogenous opioid peptides in ischemia-induced arrhythmia of isolated rat hearts. Free Radic Biol Med 1995; 18(3): 593–8. [ 10.1016/0891-5849(94)00153-B] [DOI] [PubMed] [Google Scholar]
- [42].Yin W, Zhang P, Huang JH, et al. Stimulation of kappa-opioid receptor reduces isoprenaline-induced cardiac hypertrophy and fibrosis. Eur J Pharmacol 2009; 607(1–3): 135–42. [ 10.1016/j.ejphar.2009.01.050] [DOI] [PubMed] [Google Scholar]
- [43].Jaiswal A, Kumar S, Seth S, Dinda AK, Maulik SK. Effect of U50,488H, a κ-opioid receptor agonist on myocardial α-and β-myosin heavy chain expression and oxidative stress associated with isoproterenol-induced cardiac hypertrophy in rat. Mol Cell Biochem 2010; 345(1–2): 231–40. [ 10.1007/s11010-010-0577-4] [DOI] [PubMed] [Google Scholar]
- [44].Sominsky L, Spencer SJ. Eating behavior and stress: a pathway to obesity. Front Psychol 2014; 5(MAY): 434 [ 10.3389/fpsyg.2014.00434] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Stout SA, Espel EV, Sandman CA, Glynn LM, Davis EP. Fetal programming of children’s obesity risk. Psychoneuroendocrinology 2015; 53: 29–39. [ 10.1016/j.psyneuen.2014.12.009] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Lebowitz EA, Novick JS, Rudolph AM. Development of myocardial sympathetic innervation in the fetal lamb. Pediatr Res 1972; 6(12): 887–93. [ 10.1203/00006450-197212000-00006] [DOI] [PubMed] [Google Scholar]
- [47].Yamamizu K, Furuta S, Katayama S, et al. The κ opioid system regulates endothelial cell differentiation and pathfinding in vascular development. Blood 2011; 118(3): 775–85. [ 10.1182/blood-2010-09-306001] [DOI] [PubMed] [Google Scholar]
- [48].Dunlap CE III, Sundberg DK, Rose JC. Characterization of opioid peptides from maternal and fetal sheep adrenal glands. Peptides 1985; 6(3): 483–9. [ 10.1016/0196-9781(85)90114-7] [DOI] [PubMed] [Google Scholar]
- [49].Jones CT, Ritchie JW. The cardiovascular effects of circulating catecholamines in fetal sheep. J Physiol 1978; 285: 381–93. [ 10.1113/jphysiol.1978.sp012577] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Portbury AL, Chandra R, Groelle M, et al. Catecholamines act via a beta-adrenergic receptor to maintain fetal heart rate and survival. Am J Physiol Heart Circ Physiol 2003; 284(6): H2069–77. [ 10.1152/ajpheart.00588.2002] [DOI] [PubMed] [Google Scholar]
- [51].Maden CH, Gomes J, Schwarz Q, Davidson K, Tinker A, Ruhrberg C. NRP1 and NRP2 cooperate to regulate gangliogenesis, axon guidance and target innervation in the sympathetic nervous system. Dev Biol 2012; 369(2): 277–85. [ 10.1016/j.ydbio.2012.06.026] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Schaub MC, Hefti MA, Harder BA, Eppenberger HM. Various hypertrophic stimuli induce distinct phenotypes in cardiomyocytes. J Mol Med (Berl) 1997; 75(11–12): 901–20. [ 10.1007/s001090050182] [DOI] [PubMed] [Google Scholar]
- [53].Ventura C, Zinellu E, Maninchedda E, Maioli M. Dynorphin B is an agonist of nuclear opioid receptors coupling nuclear protein kinase C activation to the transcription of cardiogenic genes in GTR1 embryonic stem cells. Circ Res 2003; 92(6): 623–9. [ 10.1161/01.RES.0000065169.23780.0E] [DOI] [PubMed] [Google Scholar]
- [54].Gorodinsky A, Barg J, Belcheva MM, et al. Dynorphins modulate DNA synthesis in fetal brain cell aggregates. J Neurochem 1995; 65(4): 1481–6. [ 10.1046/j.1471-4159.1995.65041481.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Smeets CJ, Zmorzyńska J, Melo MN, et al. Altered secondary structure of Dynorphin A associates with loss of opioid signalling and NMDA-mediated excitotoxicity in SCA23. Hum Mol Genet 2016; 25(13): 2728–37. [ 10.1093/hmg/ddw130] [DOI] [PubMed] [Google Scholar]
- [56].Berman Y, Mzhavia N, Polonskaia A, Devi LA. Impaired prohormone convertases in Cpe(fat)/Cpe(fat) mice. J Biol Chem 2001; 276(2): 1466–73. [ 10.1074/jbc.M008499200] [DOI] [PubMed] [Google Scholar]
- [57].Cheng HY, Pitcher GM, Laviolette SR, et al. DREAM is a critical transcriptional repressor for pain modulation. Cell 2002; 108(1): 31–43. [ 10.1016/S0092-8674(01)00629-8] [DOI] [PubMed] [Google Scholar]
- [58].Verbeek DS. Spinocerebellar ataxia type 23: a genetic update. Cerebellum 2009; 8(2): 104–7. [ 10.1007/s12311-008-0085-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Smeets CJ, Jezierska J, Watanabe H, et al. Elevated mutant dynorphin A causes Purkinje cell loss and motor dysfunction in spinocerebellar ataxia type 23. Brain 2015; 138(Pt 9): 2537–52. [ 10.1093/brain/awv195] [DOI] [PubMed] [Google Scholar]
- [60].Smeets CJ, Verbeek DS. Reply: SCA23 and prodynorphin: is it time for gene retraction? Brain 2016; 139(Pt 8)e43 [ 10.1093/brain/aww094] [DOI] [PubMed] [Google Scholar]
- [61].Jacobson DA, Cho J, Landa LR Jr, et al. Downstream regulatory element antagonistic modulator regulates islet prodynorphin expression. Am J Physiol Endocrinol Metab 2006; 291(3): E587–95. [ 10.1152/ajpendo.00612.2005] [DOI] [PubMed] [Google Scholar]
- [62].Edenberg HJ, Wang J, Tian H, et al. A regulatory variation in OPRK1, the gene encoding the kappa-opioid receptor, is associated with alcohol dependence. Hum Mol Genet 2008; 17(12): 1783–9. [ 10.1093/hmg/ddn068] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Bakshi R, Newman AH, Faden AI. Dynorphin A-(1–17) induces alterations in free fatty acids, excitatory amino acids, and motor function through an opiate-receptor-mediated mechanism. J Neurosci 1990; 10(12): 3793–800. [ 10.1523/JNEUROSCI.10-12-03793.1990] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Yeves AM, Burgos JI, Medina AJ, Villa-Abrille MC, Ennis IL. Cardioprotective role of IGF-1 in the hypertrophied myocardium of the spontaneously hypertensive rats: A key effect on NHE-1 activity. Acta Physiol (Oxf) 2018; 224(2)e13092 [ 10.1111/apha.13092] [DOI] [PubMed] [Google Scholar]
- [65].Sabatino L, Kusmic C, Nicolini G, et al. T3 enhances Ang2 in rat aorta in myocardial I/R: comparison with left ventricle. J Mol Endocrinol 2016; 57(3): 139–49. [ 10.1530/JME-16-0118] [DOI] [PubMed] [Google Scholar]
- [66].Vairetti M, Ferrigno A, Rizzo V, Richelmi P, Cillo U, Imberti R. Liver damage during ischemia/reperfusion and glutathione: implications for potential organ donors. Transplant Proc 2007; 39(6): 1768–70. [ 10.1016/j.transproceed.2007.06.001] [DOI] [PubMed] [Google Scholar]
- [67].Sener G, Sehirli O, Velioğlu-Oğünç A, et al. Propylthiouracil (PTU)-induced hypothyroidism alleviates burn-induced multiple organ injury. Burns 2006; 32(6): 728–36. [ 10.1016/j.burns.2006.01.002] [DOI] [PubMed] [Google Scholar]
- [68].Seara FAC, Maciel L, Barbosa RAQ, et al. Cardiac ischemia/reperfusion injury is inversely affected by thyroid hormones excess or deficiency in male Wistar rats. PLoS One 2018; 13(1)e0190355 [ 10.1371/journal.pone.0190355] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Sabatino L, Balzan S, Kusmic C, Iervasi G. Modification of gene expression profiling related to renin-angiotensin system in an ischemia/reperfusion rat model after T3 infusion. Mol Cell Biochem 2018; 449(1–2): 277–83. [ 10.1007/s11010-018-3364-2] [DOI] [PubMed] [Google Scholar]
- [70].Uddman R, Edvinsson L. Neuropeptides in the cerebral circulation. Cerebrovasc Brain Metab Rev 1989; 1(3): 230–52. [PubMed] [Google Scholar]
- [71].Armstead WM, Mirro R, Zuckerman SL, Leffler CW. Vasopressin modulates cerebrovascular responses to opioids in newborn pigs. J Pharmacol Exp Ther 1992; 260(3): 1107–12. [PubMed] [Google Scholar]
- [72].Broad J, Maurel D, Kung VW, et al. Human native kappa opioid receptor functions not predicted by recombinant receptors: Implications for drug design. Sci Rep 2016; 6: 30797 [ 10.1038/srep30797] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Fontana F, Bernardi P, Pich EM, et al. Opioid peptide modulation of circulatory and endocrine response to mental stress in humans. Peptides 1997; 18(2): 169–75. [ 10.1016/S0196-9781(96)00319-1] [DOI] [PubMed] [Google Scholar]
- [74].Moskowitz SI, Basu SB, Bergold PJ. Chronic and cyclical neuronal loss in hippocampal slice cultures following transient inhibition of the type 1 isoform of superoxide dismutase. Brain Res 2001; 913(2): 207–19. [ 10.1016/S0006-8993(01)02756-1] [DOI] [PubMed] [Google Scholar]
- [75].Rabkin SW. Endogenous kappa opioids mediate the action of brain angiotensin II to increase blood pressure. Neuropeptides 2007; 41(6): 411–9. [ 10.1016/j.npep.2007.09.003] [DOI] [PubMed] [Google Scholar]
- [76].Champion HC, Pierce RL, Kadowitz PJ. Nociceptin, a novel endogenous ligand for the ORL1 receptor, dilates isolated resistance arteries from the rat. Regul Pept 1998; 78(1–3): 69–74. [ 10.1016/S0167-0115(98)00117-7] [DOI] [PubMed] [Google Scholar]
- [77].Barnes MJ, Jen KL, Dunbar JC. The effect of CNS opioid on autonomic nervous and cardiovascular responses in dietinduced obese rats. Peptides 2004; 25(1): 71–9. [ 10.1016/j.peptides.2003.11.009] [DOI] [PubMed] [Google Scholar]
- [78].Feuerstein G, Faden AI. Differential cardiovascular effects of mu, delta and kappa opiate agonists at discrete hypothalamic sites in the anesthetized rat. Life Sci 1982; 31(20–21): 2197–200. [ 10.1016/0024-3205(82)90117-5] [DOI] [PubMed] [Google Scholar]
- [79].Fontana F, Bernardi P, Pich EM, et al. Relationship between plasma atrial natriuretic factor and opioid peptide levels in healthy subjects and in patients with acute congestive heart failure. Eur Heart J 1993; 14(2): 219–25. [ 10.1093/eurheartj/14.2.219] [DOI] [PubMed] [Google Scholar]
- [80].Kapusta DR, Sezen SF, Chang JK, Lippton H, Kenigs VA. Diuretic and antinatriuretic responses produced by the endogenous opioid-like peptide, nociceptin (orphanin FQ). Life Sci 1997; 60(1): PL15–21. [DOI] [PubMed] [Google Scholar]
- [81].Warner FJ, Smith AI, Hooper NM, Turner AJ. Angiotensinconverting enzyme-2: a molecular and cellular perspective. Cell Mol Life Sci 2004; 61(21): 2704–13. [ 10.1007/s00018-004-4240-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Bali A, Randhawa PK, Jaggi AS. Interplay between RAS and opioids: opening the Pandora of complexities. Neuropeptides 2014; 48(4): 249–56. [ 10.1016/j.npep.2014.05.002] [DOI] [PubMed] [Google Scholar]
- [83].Krazinski BE, Koziorowski M, Brzuzan P, Okrasa S. The expression of genes encoding opioid precursors and the influence of opioid receptor agonists on steroidogenesis in porcine adrenocortical cells in vitro. J Physiol Pharmacol 2011; 62(4): 461–8. [PubMed] [Google Scholar]
- [84].Fontana F, Bernardi P, Spampinato S, Boschi S, De Iasio R, Grossi G. Pressor effects of endogenous opioid system during acute episodes of blood pressure increases in hypertensive patients. Hypertension 1997; 29(1 Pt 1): 105–10. [ 10.1161/01.HYP.29.1.105] [DOI] [PubMed] [Google Scholar]
- [85].Mannelli M, Maggi M, DeFeo ML, et al. Opioid modulation of normal and pathological human chromaffin tissue. J Clin Endocrinol Metab 1986; 62(3): 577–82. [ 10.1210/jcem-62-3-577] [DOI] [PubMed] [Google Scholar]
- [86].Yanase T, Nawata H, Kato K, Ibayashi H. Catecholamines and opioid peptides in human phaeochromocytomas. Acta Endocrinol (Copenh) 1986; 113(3): 378–84. [ 10.1530/acta.0.1130378] [DOI] [PubMed] [Google Scholar]
- [87].Anderson RI, Becker HC. Role of the Dynorphin/Kappa Opioid Receptor System in the Motivational Effects of Ethanol. Alcohol Clin Exp Res 2017; 41(8): 1402–18. [ 10.1111/acer.13406] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Overton JM, Fisher LA. Modulation of central nervous system actions of corticotropin-releasing factor by dynorphin-related peptides. Brain Res 1989; 488(1–2): 233–40. [ 10.1016/0006-8993(89)90713-0] [DOI] [PubMed] [Google Scholar]
- [89].Wang JQ, Ingenito AJ. Cardiovascular effects of microinjection of dynorphin-A(1–8) into the hippocampus in conscious, spontaneously hypertensive and normotensive Wistar-Kyoto rats. Clin Exp Hypertens 1994; 16(2): 229–43. [ 10.3109/10641969409067951] [DOI] [PubMed] [Google Scholar]
- [90].Fisher LA, Brown MR. Central regulation of stress responses: regulation of the autonomic nervous system and visceral function by corticotrophin releasing factor-41. Baillieres Clin Endocrinol Metab 1991; 5(1): 35–50. [ 10.1016/S0950-351X(05)80095-3] [DOI] [PubMed] [Google Scholar]
- [91].Nikolarakis KE, Almeida OF, Herz A. Stimulation of hypothalamic beta-endorphin and dynorphin release by corticotropin-releasing factor (in vitro). Brain Res 1986; 399(1): 152–5. [ 10.1016/0006-8993(86)90610-4] [DOI] [PubMed] [Google Scholar]
- [92].De Michele M, Touzani O, Foster AC, Fieschi C, Sette G, McCulloch J. Corticotropin-releasing factor: effect on cerebral blood flow in physiologic and ischaemic conditions. Exp Brain Res 2005; 165(3): 375–82. [ 10.1007/s00221-005-2303-0] [DOI] [PubMed] [Google Scholar]
- [93].Davis ME, Pemberton CJ, Yandle TG, et al. Urocortin 2 infusion in healthy humans: hemodynamic, neurohormonal, and renal responses. J Am Coll Cardiol 2007; 49(4): 461–71. [ 10.1016/j.jacc.2006.09.035] [DOI] [PubMed] [Google Scholar]
- [94].Ruisanchez É, Cselenyák A, Papp RS, et al. Perivascular expression and potent vasoconstrictor effect of dynorphin A in cerebral arteries. PLoS One 2012; 7(5)e37798 [ 10.1371/journal.pone.0037798] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Moskowitz MA, Saito K, Brezina L, Dickson J. Nerve fibers surrounding intracranial and extracranial vessels from human and other species contain dynorphin-like immunoreactivity. Neuroscience 1987; 23(2): 731–7. [ 10.1016/0306-4522(87)90090-X] [DOI] [PubMed] [Google Scholar]
- [96].Armstead WM, Mirro R, Busija DW, Leffler CW. Prostanoids modulate opioid cerebrovascular responses in newborn pigs. J Pharmacol Exp Ther 1990; 255(3): 1083–9. [PubMed] [Google Scholar]
- [97].Sun FY, Zhang AZ. Dynorphin receptor in the blood vessel. Neuropeptides 1985; 5(4–6): 595–8. [ 10.1016/0143-4179(85)90088-5] [DOI] [PubMed] [Google Scholar]
- [98].Laurent S, Schmitt H. Central cardiovascular effects of kappa agonists dynorphin-(1–13) and ethylketocyclazocine in the anaesthetized rat. Eur J Pharmacol 1983; 96(1–2): 165–9. [ 10.1016/0014-2999(83)90547-2] [DOI] [PubMed] [Google Scholar]
- [99].Tian F, Zheng XY, Li J, et al. κ-Opioid Receptor Stimulation Improves Endothelial Function via Akt-stimulated NO Production in Hyperlipidemic Rats. Sci Rep 2016; 6: 26807 [ 10.1038/srep26807] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Bolte C, Newman G, Schultz JelJ. Hypertensive state, independent of hypertrophy, exhibits an attenuated decrease in systolic function on cardiac kappa-opioid receptor stimulation. Am J Physiol Heart Circ Physiol 2009; 296(4): H967–75. [ 10.1152/ajpheart.00909.2008] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Seccia TM, Maniero C, Belloni AS, et al. Role of angiotensin II, endothelin-1 and L-type calcium channel in the development of glomerular, tubulointerstitial and perivascular fibrosis. J Hypertens 2008; 26(10): 2022–9. [ 10.1097/HJH.0b013e328309f00a] [DOI] [PubMed] [Google Scholar]
- [102].Kasemsri T, Armstead WM. Endothelin production links superoxide generation to altered opioid-induced pial artery vasodilation after brain injury in pigs. Stroke 1997; 28(1): 190–6. [ 10.1161/01.STR.28.1.190] [DOI] [PubMed] [Google Scholar]
- [103].Weber E, Esch FS, Böhlen P, et al. Metorphamide: isolation, structure, and biologic activity of an amidated opioid octapeptide from bovine brain. Proc Natl Acad Sci USA 1983; 80(23): 7362–6. [ 10.1073/pnas.80.23.7362] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Arendt RM, Schmoeckel M, Wilbert-Lampen U, Plasse A, Heucke L, Werdan K. Bidirectional effects of endogenous opioid peptides on endothelin release rates in porcine aortic endothelial cell culture: mediation by delta opioid receptor and opioid receptor antagonist-insensitive mechanisms. J Pharmacol Exp Ther 1995; 272(1): 1–7. [PubMed] [Google Scholar]
- [105].Ventura C, Pintus G, Vaona I, Bennardini F, Pinna G, Tadolini B. Phorbol ester regulation of opioid peptide gene expression in myocardial cells. Role of nuclear protein kinase. J Biol Chem 1995; 270(50): 30115–20. [ 10.1074/jbc.270.50.30115] [DOI] [PubMed] [Google Scholar]
- [106].Wegener K, Kummer W. Sympathetic noradrenergic fibers as the source of immunoreactive alpha-neoendorphin and dynorphin in the guinea pig heart. Acta Anat (Basel) 1994; 151(2): 112–9. [ 10.1159/000147651] [DOI] [PubMed] [Google Scholar]
- [107].Fontana F, Bernardi P, Tartuferi L, Boschi S, Di Toro R, Spampinato S. Opioid peptides attenuate blood pressure increase in acute respiratory failure. Peptides 2001; 22(4): 631–7. [ 10.1016/S0196-9781(01)00373-4] [DOI] [PubMed] [Google Scholar]
- [108].Peng J, Sarkar S, Chang SL. Opioid receptor expression in human brain and peripheral tissues using absolute quantitative real-time RT-PCR. Drug Alcohol Depend 2012; 124(3): 223–8. [ 10.1016/j.drugalcdep.2012.01.013] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Rostamzadeh F, Najafipour H, Yeganeh-Hajahmadi M, Esmaeili-Mahani S, Joukar S, Iranpour M. Heterodimerization of apelin and opioid receptors and cardiac inotropic and lusitropic effects of apelin in 2K1C hypertension: Role of pERK1/2 and PKC. Life Sci 2017; 191: 24–33. [ 10.1016/j.lfs.2017.09.044] [DOI] [PubMed] [Google Scholar]
- [110].Wu JP, Chen YT, Lee AY. Opioids in myocardial ischaemia: potentiating effects of dynorphin on ischaemic arrhythmia, bradycardia and cardiogenic shock following coronary artery occlusion in the rat. Eur Heart J 1993; 14(9): 1273–7. [ 10.1093/eurheartj/14.9.1273] [DOI] [PubMed] [Google Scholar]
- [111].Kim JH, Jang YH, Chun KJ, et al. Kappa-opioid receptor activation during reperfusion limits myocardial infarction via ERK1/2 activation in isolated rat hearts. Korean J Anesthesiol 2011; 60(5): 351–6. [ 10.4097/kjae.2011.60.5.351] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Fu LL, Xia Q, Shen YL, Wong TM. [Involvement of endogenous opioids in cardioprotective effects of ischemic preconditioning in the isolated rat heart]. Sheng Li Xue Bao 1998; 50(6): 603–10. [Involvement of endogenous opioids in cardioprotective effects of ischemic preconditioning in the isolated rat heart]. [PubMed] [Google Scholar]
- [113].Yu X, Zhang W, Bian J, Wong TM. Pro- and anti-arrhythmic effects of a kappa opioid receptor agonist: a model for the biphasic action of a local hormone in the heart. Clin Exp Pharmacol Physiol 1999; 26(10): 842–4. [ 10.1046/j.1440-1681.1999.03143.x] [DOI] [PubMed] [Google Scholar]
- [114].Lee AY, Wong TM. Effects of dynorphin1–13 on cardiac rhythm and cyclic adenosine monophosphate (cAMP) levels in the isolated perfused rat heart. Neurosci Lett 1987; 80(3): 289–92. [ 10.1016/0304-3940(87)90469-1] [DOI] [PubMed] [Google Scholar]
- [115].Lishmanov Iu B, Maslov LN, Ugdyzhekova DS. Eksp Klin Farmakol 1995; 58(4): 26–8. [An experimental study of the pharmacological activity of opiate receptor ligands on models of adrenaline-induced arrhythmias]. [PubMed] [Google Scholar]
- [116].Rabkin SW. Dynorphin A (1–13) in the brain suppresses epinephrine-induced ventricular premature complexes and ventricular tachyarrhythmias. Regul Pept 1992; 41(2): 95–107. [ 10.1016/0167-0115(92)90039-W] [DOI] [PubMed] [Google Scholar]
- [117].Maslov LN, Lishmanov IuB, Krylatov AV, Ugdyzhekova DS. [The participation of the central and peripheral kappa-opiate receptors in the mechanism of the anti-arrhythmia action of benzeneacetamide derivatives]. Eksp Klin Farmakol 1996; 59(6): 20–2. [The participation of the central and peripheral kappa-opiate receptors in the mechanism of the anti-arrhythmia action of benzeneacetamide derivatives]. [PubMed] [Google Scholar]
- [118].Rabkin SW. Morphine and the endogenous opioid dynorphin in the brain attenuate digoxin-induced arrhythmias in guinea pigs. Pharmacol Toxicol 1992; 71(5): 353–60. [ 10.1111/j.1600-0773.1992.tb00561.x] [DOI] [PubMed] [Google Scholar]
- [119].Czyzyk TA, Nogueiras R, Lockwood JF, et al. kappa-Opioid receptors control the metabolic response to a high-energy diet in mice. FASEB J 2010; 24(4): 1151–9. [ 10.1096/fj.09-143610] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Dyck JR, Cheng JF, Stanley WC, et al. Malonyl coenzyme a decarboxylase inhibition protects the ischemic heart by inhibiting fatty acid oxidation and stimulating glucose oxidation. Circ Res 2004; 94(9): e78–84. [ 10.1161/01.RES.0000129255.19569.8f] [DOI] [PubMed] [Google Scholar]
- [121].Folmes CD, Lopaschuk GD. Role of malonyl-CoA in heart disease and the hypothalamic control of obesity. Cardiovasc Res 2007; 73(2): 278–87. [ 10.1016/j.cardiores.2006.10.008] [DOI] [PubMed] [Google Scholar]
- [122].Mather KJ, Hutchins GD, Perry K, et al. Assessment of myocardial metabolic flexibility and work efficiency in human type 2 diabetes using 16-[18F]fluoro-4-thiapalmitate, a novel PET fatty acid tracer. Am J Physiol Endocrinol Metab 2016; 310(6): E452–60. [ 10.1152/ajpendo.00437.2015] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Kudo N, Barr AJ, Barr RL, Desai S, Lopaschuk GD. High rates of fatty acid oxidation during reperfusion of ischemic hearts are associated with a decrease in malonyl-CoA levels due to an increase in 5′-AMP-activated protein kinase inhibition of acetyl-CoA carboxylase. J Biol Chem 1995; 270(29): 17513–20. [ 10.1074/jbc.270.29.17513] [DOI] [PubMed] [Google Scholar]
- [124].Liu J, Wang H, Li J. Inflammation and Inflammatory Cells in Myocardial Infarction and Reperfusion Injury: A Double- Edged Sword. Clin Med Insights Cardiol 2016; 10: 79–84. [ 10.4137/CMC.S33164] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Madjid M, Fatemi O. Components of the complete blood count as risk predictors for coronary heart disease: in-depth review and update. Tex Heart Inst J 2013; 40(1): 17–29. [PMC free article] [PubMed] [Google Scholar]
- [126].Fazalul Rahiman SS, Morgan M, Gray P, Shaw PN, Cabot PJ. Dynorphin 1–17 and Its N-Terminal Biotransformation Fragments Modulate Lipopolysaccharide-Stimulated Nuclear Factor-kappa B Nuclear Translocation, Interleukin-1beta and Tumor Necrosis Factor-alpha in Differentiated THP-1 Cells. PLoS One 2016; 11(4)e0153005 [ 10.1371/journal.pone.0153005] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Rahiman SSF, Morgan M, Gray P, Shaw PN, Cabot PJ. Inhibitory effects of dynorphin 3–14 on the lipopolysaccharide-induced toll-like receptor 4 signalling pathway. Peptides 2017; 90: 48–54. [ 10.1016/j.peptides.2017.02.004] [DOI] [PubMed] [Google Scholar]
- [128].Pepe S, van den Brink OW, Lakatta EG, Xiao RP. Cross-talk of opioid peptide receptor and beta-adrenergic receptor signalling in the heart. Cardiovasc Res 2004; 63(3): 414–22. [ 10.1016/j.cardiores.2004.04.022] [DOI] [PubMed] [Google Scholar]
- [129].Grosse Hartlage MA, Theisen MM, Monteiro de Oliveira NP, Van Aken H, Fobker M, Weber TP. Kappa-opioid receptor antagonism improves recovery from myocardial stunning in chronically instrumented dogs. Anesth Analg 2006; 103(4): 822–32. [ 10.1213/01.ane.0000237246.40665.34] [DOI] [PubMed] [Google Scholar]
- [130].Pham-Huy LA, He H, Pham-Huy C. Free radicals, antioxidants in disease and health. Int J Biomed Sci 2008; 4(2): 89–96. [PMC free article] [PubMed] [Google Scholar]
- [131].Wasserman DH, Wang TJ, Brown NJ. The Vasculature in Prediabetes. Circ Res 2018; 122(8): 1135–50. [ 10.1161/CIRCRESAHA.118.311912] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Scavini C, Rozza A, Bo P, et al. Kappa-opioid receptor changes and neurophysiological alterations during cerebral ischemia in rabbits. Stroke 1990; 21(6): 943–7. [ 10.1161/01.STR.21.6.943] [DOI] [PubMed] [Google Scholar]
- [133].Vuong C, Van Uum SH, O’Dell LE, Lutfy K, Friedman TC. The effects of opioids and opioid analogs on animal and human endocrine systems. Endocr Rev 2010; 31(1): 98–132. [ 10.1210/er.2009-0009] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Kao TK, Ou YC, Liao SL, et al. Opioids modulate post-ischemic progression in a rat model of stroke. Neurochem Int 2008; 52(6): 1256–65. [ 10.1016/j.neuint.2008.01.007] [DOI] [PubMed] [Google Scholar]
- [135].Kuroda H, Baskin DS, Matsui T, Loh HH, Hosobuchi Y, Lee NM. Effects of dynorphin1–13 on opiate binding and dopamine and GABA uptake in stroked cat brain. Brain Res 1986; 379(1): 68–74. [ 10.1016/0006-8993(86)90256-8] [DOI] [PubMed] [Google Scholar]
- [136].Cechetto DF, Hachinski V. Cardiovascular consequence of experimental stroke. Baillieres Clin Neurol 1997; 6(2): 297–308. [PubMed] [Google Scholar]
- [137].Baek H, Sariev A, Kim MJ, Lee H, Kim J, Kim H. A neuroprotective brain stimulation for vulnerable cerebellar Purkinje cell after ischemic stroke: a study with low-intensity focused ultrasound. Conf Proc IEEE Eng Med Biol Soc 2018; 2018: 4744–7. [ 10.1109/EMBC.2018.8513138] [DOI] [PubMed] [Google Scholar]
- [138].Tsang HG, Rashdan NA, Whitelaw CB, Corcoran BM, Summers KM, MacRae VE. Large animal models of cardiovascular disease. Cell Biochem Funct 2016; 34(3): 113–32. [ 10.1002/cbf.3173] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Camacho P, Fan H, Liu Z, He JQ. Large Mammalian Animal Models of Heart Disease. J Cardiovasc Dev Dis 2016; 3(4)E30 [ 10.3390/jcdd3040030] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Marinova Z, Vukojevic V, Surcheva S, et al. Translocation of dynorphin neuropeptides across the plasma membrane. A putative mechanism of signal transmission. J Biol Chem 2005; 280(28): 26360–70. [ 10.1074/jbc.M412494200] [DOI] [PubMed] [Google Scholar]
- [141].Maeda S, Nakamae J, Inoki R. Inhibition of cardiac Na+, K+− ATPase activity by dynorphin-A and ethylketocyclazocine. Life Sci 1988; 42(4): 461–8. [ 10.1016/0024-3205(88)90085-9] [DOI] [PubMed] [Google Scholar]
- [142].Thornhill JA, Pittman QJ. Hemodynamic responses of conscious rats following intrathecal injections of prodynorphin-derived opioids: independence of action of intrathecal arginine vasopressin. Can J Physiol Pharmacol 1990; 68(2): 174–82. [ 10.1139/y90-028] [DOI] [PubMed] [Google Scholar]


