Temporal metabolic profiles in sepsis are altered by the hormonal changes related to reduced food intake before admission and fasting after mechanical ventilation (MV), followed by refeeding [1]. Animal models suggest that preterminal rats were hypometabolic, compared to surviving animals [2]. This was further corroborated among septic patients, where higher metabolic rates and lower respiratory quotients (RQ) were observed [3]. However, longitudinal data using indirect calorimetry (IC) remain sparse. We aimed to determine the temporal trends of energy expenditure (EE) and RQ among septic patients receiving MV.
All MV septic adults in the intensive care unit of a tertiary hospital were screened between September 2018 and December 2019 with ethics approval (DSRB 2017/01001). Patients were excluded if they received MV < 3 days, dialysis, fraction of inspired oxygen > 0.6, and chest drain.
Patients demographics, anthropometric indices, outcomes, energy (target 25 kcal/kg/day) and protein (target 1.5 g/kg/day) delivery, propofol dose, and glucose infusions were collected. We initiated enteral feeding within 24 h of MV commencement. IC data collected (RQ, oxygen consumption, carbon dioxide production, and total EE) using Carescape B650 (GE Healthcare, USA), standardized for temperature, barometric pressure, and humidity, were obtained post-intubation (baseline), within 2 h of feeding initiation and thereafter, daily up to 5 days or till extubation, whichever was earlier. A steady state of 30 min was mandated to ensure IC data validity.
Categorical and continuous variables were reported as proportion and mean (SD) or median (IQR), respectively. Comparisons of medians were performed using Mann–Whitney U test. All statistical tests were two-tailed. P < 0.05 was considered significant. To determine metabolism status, we followed the methods by Giovannini and Fried [2, 3]. Hyper- and hypometabolism were determined by positive and negative EE when compared to the resting state by Harris-Benedict equation. To understand substrate utilization, we followed the RQ trends. A balanced diet’s RQ is approximately 0.8. We divided the cohort into RQ ≤ 0.8 (lipid as predominant substrate) and > 0.8 (carbohydrate as predominant substrate). STATA 14 (Stata Corp, College Station, TX, USA) was used.
Table 1 displays the baseline characteristics between survivors (n = 20) and non-survivors (n = 14). Median age and BMI were 63.5 (24–73) years and 22.5 (20.4–22.7) kg/m2, respectively. Mean APACHE II 27.7 (7.8), SOFA 14.3 (2.7), and mNUTRIC 6.2 (1.5) scores were noted.
Table 1.
Comparison between survivors and non-survivors
| Survivors (n = 20) | Non-survivors (n = 14) | |||||||||||
| Demographics | ||||||||||||
| Age, median (IQR) (years) | 63.5 (24–72.5) | 63.5 (24–74) | ||||||||||
| Male (%) | 12 (60%) | 12 (85.7%) | ||||||||||
| BMI, median (IQR) Kg/m2 | 22.23 (15.4–22.8) | 22.74 (18.61–22.07) | ||||||||||
| Clinical severity | ||||||||||||
| APACHE II, mean (SD) | 27.5 ± 6.1 | 27.9 ± 9.3 | ||||||||||
| SOFA, mean (SD) | 13.8 ± 2.8 | 15.1 ± 2.5 | ||||||||||
| mNUTRIC, mean (SD) | 6.25 ± 1.61 | 6.21 ± 1.25 | ||||||||||
| SGA, median (IQR) | 6 (3–6) | 5 (1–5) | ||||||||||
| Vasopressor use (%) | 12 (60%) | 11 (78.6%) | ||||||||||
| Time to initiate feeding, median (range), hours | 8.16 (3–45.1) | 6.62 (3–23.25) | ||||||||||
| Comorbidities | ||||||||||||
| Diabetes mellitus (%) | 10 (50%) | 4 (28.6%) | ||||||||||
| Ischemic heart disease (%) | 5 (25%) | 4 (28.6%) | ||||||||||
| Cerebrovascular accident (%) | 5 (25%) | 1 (7.1%) | ||||||||||
| Chronic liver disease (%) | 3 (15%) | 1 (7.1%) | ||||||||||
| Chronic kidney disease (%) | 7 (35%) | 3 (21.4%) | ||||||||||
| Malignancy (%) | 2 (10%) | 3 (21.4%) | ||||||||||
| Connective tissue disease (%) | 5 (25%) | 4 (28.6%) | ||||||||||
| Diagnosis | ||||||||||||
| Pneumonia | 8 (40%) | 8 (57.1%) | ||||||||||
| Line sepsis | 2 (10%) | 1 (7.1%) | ||||||||||
| Other sites of sepsis | 10 (50%) | 5 (35.7%) | ||||||||||
| Details of calories and macronutrient delivered | ||||||||||||
| Total | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Total | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | |
| Calories delivered, median, IQR (kcal) | 4655.45 (3439.96–5716.79) | 821.01 (384.25–999.65) | 845.40 (669.03–1102.08) | 1072.22 (815.25–1329.05) | 953.37 (817.38–1281.80) | 1139.75 (1028.50–1421.00) | 4600.76 (3754.91–6067.63) | 590.71 (330.82–896.00) | 1038.65 (637.48–1584.00) | 1143.54 (808.75–1620.66) | 941.25 (753.12–1419.75) | 1063.03 (76.25–1490.29) |
| Carbohydrates delivered, median, IQR (g) | 498.50 (379.50–646.00) | 51.58 (34.07–98.77) | 106.87 (69.52–117.94) | 117.93 (96.86–145.8) | 117 (109.33–129.6) | 126.22 (98.28–159.69) | 482.00 (400.00–581.00) | 35.21 (24.95–83.02) | 101.83 (62.24–129.6) | 103.53 (86.65–131.53) | 107.56 (86.4–125.95) | 107.56 (93.79–132.53) |
| Lipid delivered, median, IQR (g) | 190.50 (158.00–294.00) | 23.43 (10.59–52.17) | 55.61 (19.02–69.36) | 43.2 (34.38–69.33) | 43.2 (31.20–67.00) | 57.29* (47.52–69.33) | 154.50 (109.00–282.00) | 19.38 (7.60–28.62) | 36.47 (26.13–51.84) | 35.91 (23.10–53.72) | 34.62 (24.66–71.02) | 29.95* (25.01–55.95) |
| Protein delivered, median, IQR (g) | 323.74 (261.78–372.05) | 44.92 (25.49–66.47) | 61.17 (47.01–79.98) | 74.88 (55.2–86.84) | 66.36 (30.33–77.80) | 70.25 (62.59–91.15) | 279.33 (110.16–398.17) | 34.32 (34.32–17.41) | 69 (30.78–76.48) | 66.8 (47.66–86.14) | 61.91 (42.80–90.78) | 77.68 (43.22–88.32) |
Abbreviations: IQR interquartile range, BMI body mass index, APACHE Acute Physiology And Chronic Health Evaluation, SOFA sequential organ failure assessment, mNUTRIC modified Nutrition Risk in Critically ill, SGA Subjective Global Assessment, IC indirect calorimetry
*p = 0.02
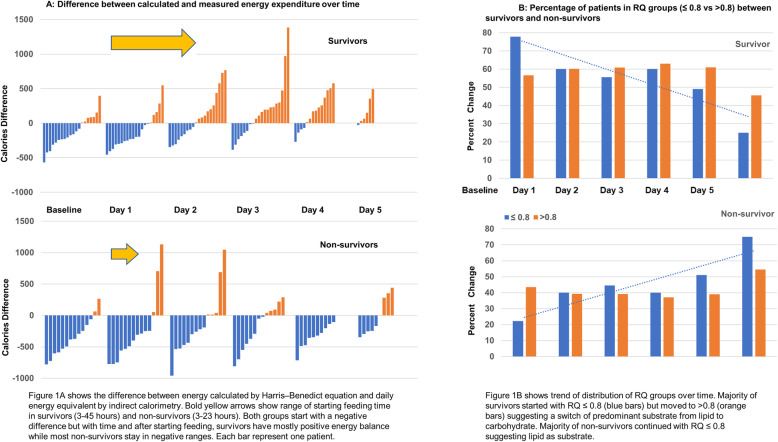
The metabolic profiles differed between survivors and non-survivors (Fig. 1a). Both groups had negative energy balance during fasting state. Survivors transitioned to a hypermetabolic state following feeding initiation, achieving positive energy balance. Non-survivors remained hypometabolic despite feeding.
Fig. 1.
a Difference between calculated and measured energy expenditure over time. b Percentage of patients in RQ groups (≤ 0.8 vs > 0.8) between survivors and non-survivors
Most patients’ initial RQ were ≤ 0.8. Survivors transitioned into > 0.8 following feeding initiation, suggesting a change to carbohydrate metabolism. Most non-survivors RQ remained ≤ 0.8 (Fig. 1b). There was no difference in the daily energy and macronutrients delivered between survivors and non-survivors (Table 1).
Our study advances the understanding of energy balance and substrate utilization in sepsis. During fasting, low insulin with elevated counter-regulatory hormones promotes lipolysis; muscle glycogen is depleted at an exponential rate greater than athletes running marathons [4]. The predominant energy substrate switches from carbohydrates to lipids—the hallmark of fasting physiology. This explains the low RQ in early sepsis, when patients are preferentially utilizing lipids (RQ ≤ 0.8) during permissive underfeeding [5]. The hypermetabolic state and inability for non-survivors to transit to carbohydrate utilization suggest ongoing debilitating mitochondrial dysfunction, consistent with associated multi-organ failure [6]. However, whether adjusting the feeding types and regimen to alter these patterns and improve outcomes remain unknown.
In conclusion, EE and substrate utilization patterns in early sepsis differ between survivors and non-survivors. Survivors assume higher EE and used carbohydrate as the main substrate while non-survivors have lower EE and predominantly utilized lipid.
Acknowledgements
The authors would like to acknowledge Ms. Sandy Lim Hong Hong for her invaluable logistic support and the ICU nurses for obtaining the readings of the indirect calorimetry.
Abbreviations
- MV
Mechanical ventilation
- RQ
Respiratory quotient
- EE
Energy expenditure
- IC
Indirect calorimetry
Authors’ contributions
Andrew Li and Amartya Mukhopadhyay were involved in the conceptualization, material preparation, writing, data analysis, and conduct of the study. The author(s) read and approved the final manuscript.
Funding
Financial support was provided by the masters of clinical investigation program.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The National Healthcare Group Domain-Specific Review Board approved the study with a waiver of informed consent due to the non-interventional study design (DSRB 2017/01001).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Heyland DK, Dhaliwal R, Jiang X, Day AG. Identifying critically ill patients who benefit the most from nutrition therapy: the development and initial validation of a novel risk assessment tool. Crit Care. 2011;15:R268. doi: 10.1186/cc10546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fried RC, Bailey PM, Mullen JL, Stein TP, Crosby LO, Buzby GP. Alterations in exogenous substrate metabolism in sepsis. Arch Surg. 1986;121:173–178. doi: 10.1001/archsurg.1986.01400020059007. [DOI] [PubMed] [Google Scholar]
- 3.Giovannini I, Boldrini G, Castagneto M, Sganga G, Nanni G, Pittiruti M, et al. Respiratory quotient and patterns of substrate utilization in human sepsis and trauma. JPEN J Parenter Enteral Nutr. 1983;7:226–230. doi: 10.1177/0148607183007003226. [DOI] [PubMed] [Google Scholar]
- 4.Wischmeyer PE, San-Millan I. Winning the war against ICU-acquired weakness: new innovations in nutrition and exercise physiology. Crit Care. 2015;19(Suppl 3):S6. doi: 10.1186/cc14724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arabi YM, Aldawood AS, Haddad SH, Al-Dorzi HM, Tamim HM, Jones G, et al. Permissive underfeeding or standard enteral feeding in critically ill adults. N Engl J Med. 2015;372:2398–2408. doi: 10.1056/NEJMoa1502826. [DOI] [PubMed] [Google Scholar]
- 6.Leverve XM. Mitochondrial function and substrate availability. Crit Care Med. 2007;35(9 Suppl):S454–S460. doi: 10.1097/01.CCM.0000278044.19217.73. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.