Abstract
Background and aims:
Concentrated fish oils, containing a mixture of long-chain monounsaturated fatty acids (LCMUFA) with aliphatic chains longer than 18 C atoms (i.e., C20:1 and C22:1), have been shown to attenuate atherosclerosis development in mouse models. It is not clear, however, how individual LCMUFA isomers may act on atherosclerosis.
Methods:
In the present study, we used saury fish oil-derived concentrates enriched in either C20:1 or C22:1 isomer fractions to investigate their individual effect on atherosclerosis and lipoprotein metabolism. LDLR-deficient (LDLr−/−) mice were fed a Western diet supplemented with 5% (w/w) of either C20:1 or C22:1 concentrate for 12 wk.
Results:
Compared to the control Western diet with no supplement, both LCMUFA isomers increased hepatic levels of LCMUFA by 2~3-fold (p < 0.05), and decreased atherosclerotic lesion areas by more than 40% (p < 0.05), although there were no major differences in plasma lipoproteins or hepatic lipid content. Both LCMUFA isomers significantly decreased plasma CRP levels, improved Abca1-dependent cholesterol efflux capacity of apoB-depleted plasma, and enhanced Ppar transcriptional activities in HepG2 cells. LC-MS/MS proteomic analysis of lipoproteins (HDL, LDL and VLDL) revealed that both LCMUFA isomer diets resulted in similar potentially beneficial alterations in proteins involved in complement activation, blood coagulation, and lipid metabolism. Several lipoprotein proteome changes were significantly correlated with atherosclerotic plaque reduction.
Conclusions:
Dietary supplementation with the LCMUFA isomers C20:1 or C22:1 was equally effective in reducing atherosclerosis in LDLr−/−mice and this may partly occur through activation of the Ppar signaling pathways and favorable alterations in the proteome of lipoproteins.
Keywords: long-chain monounsaturated fatty acids, atherosclerosis, lipoprotein proteome, peroxisome proliferator-activated receptors, inflammation, cholesterol efflux
1. Introduction
Dietary modifications are typically recommended as the first-line approach for reducing cardiovascular disease (CVD) risk. Numerous studies have examined the effect of different types of dietary lipids on atherosclerosis [1]. Compared with saturated fatty acid, the consumption of diets rich in monounsaturated fatty acid (MUFA) has been linked to lower cardiovascular events and mortality [2]. Most MUFA studies related to CVD risk have exclusively focused on oleic acid (C18:1), which is abundant in the Mediterranean diet. Limited information, however, is available regarding the effect of other dietary MUFA, such as long-chain MUFA (LCMUFA) with carbon chains longer than 18 (i.e., C20:1 and C22:1 isomers). Although not found in most dietary sources, LCMUFA are abundantly found in some types of fish, such as saury, pollock, and herring [3]. Our recent animal studies showed that unlike oleic acid-rich olive oil, supplementation with a saury fish oil-derived LCMUFA concentrate, containing a mixture of C20:1 and C22:1, suppressed atherosclerosis development in LDLR-knockout (LDLr−/−) mice [4]. In addition, two human cohort-based studies have shown a positive correlation between red blood cell LCMUFA isomer C22:1 levels or dietary consumption of LCMUFA isomer C20:1 with reduced CVD risk even after adjustment for EPA (C20:5) and DHA (C22:6) levels [5,6]. However, no study has yet investigated LCMUFA isomer-specific effects on atherosclerosis. Although the ratio of C20:1 to C22:1 tends to be similar in most fish [3], this is still a potentially important issue. If only one LCMUA isomer is effective in reducing atherosclerosis and the other has no effect or is even pro-atherogenic, a fish oil specifically enriched in the beneficial LCMUFA isomers may better reduce CVD risk.
Our previous LCMUFA supplementation study, with both isomers, showed that despite a significant reduction in aortic plaque area, total plasma lipids or cholesterol in individual lipoprotein fractions were not altered in LDLr−/− mice by a LCMUFA-rich diet [4]. Therefore, lipoprotein lipid levels alone did not provide sufficient mechanistic insight into the beneficial actions of LCMUFA. Increasing evidence has demonstrated that a wide variety of proteins involved in complement regulation, blood coagulation, and proteolysis are physically associated with lipoproteins, and changes in the lipoprotein proteome can modulate their function [7]. Previous studies have also shown that diets enriched in omega-3 fatty acids can alter the proteome of lipoproteins [8]. Therefore, a detailed characterization of lipoprotein proteomics after consumption of LCMUFAs is needed to gain additional insights into their role in modifying the progression of atherosclerosis.
In the present study, we investigated the individual effect of dietary supplementation of fish oil-derived C20:1 and C22:1 isomer fractions on the proteomes of lipoprotein subclasses and the development of atherosclerosis in LDLr−/−mice. The results show that both LCMUFA isomers behaved similarly in modulating the lipoprotein proteome and reducing atherosclerotic progression.
2. Materials and methods
Animals study design
The C20:1 and C22:1 fatty acids were in the form of ethyl esters in purified saury oil (Nippon Suisan Kaisha, Tokyo, Japan) and were separated through an octadecylsilyl silica gel column. The fatty acid composition is shown in Supplemental Table 1, and omega-11 LCMUFA isomers are the major components (77% C20:1 n-11 in C20:1 fraction and 96% C22:1 n-11 in C22:1 fraction). Eight-week old female LDLr−/− mice (the Jackson Laboratory) maintained at 12 h/12 h day/night cycles were treated according to the NIH guidelines and with the approval of the Institutional Animal Care Committee. Animals were randomly assigned to one of three groups (n = 12): Western Adjusted Calories Diet (TD.88137; Teklad, Harlan Laboratories Inc.) supplemented with a final concentration of 5% (w/w) of C20:1 or C22:1 or the control diet (TD.88137) with no fatty acid supplement. The three diets were formulated to be nearly identical in their overall composition (Supplemental Table 2). At the end of the 12-week feeding period, non-fasting blood, aortas and livers were collected at sacrifice. An aliquot of fresh plasma was used for separation of lipoproteins by fast protein liquid chromatography (FPLC) and was analyzed by proteomic analysis as described below. Residual plasma and liver tissue was stored at −80°C for subsequent analysis.
Evaluation of atherosclerotic lesions
The whole aorta in each mouse was isolated, mounted en face and stained for lipid with Sudan IV, as described previously [4]. The atherosclerotic lesion severity was expressed as the area percentage of positive Sudan IV staining.
Hepatic lipid and fatty acid composition analyses
To measure hepatic lipid content, total lipids were extracted by the method of Folch, and the gas chromatographic determination of fatty acid composition in liver was performed as described previously [4]. Plasma and liver levels of total cholesterol (TC), free cholesterol (FC), phospholipids (PL), and triglycerides (TG) were measured enzymatically (Wako Chemicals).
Plasma lipid analysis and FPLC analysis of pooled plasma samples
Plasma lipoproteins VLDL, LDL, and HDL were separated by FPLC from 100 μl of pooled plasma (n = 6). TC, FC, PL and TG concentrations in plasma and each lipoprotein fraction were enzymatically quantified as described above.
In vitro assessment of cholesterol efflux capacity of plasma
Pooled apoB-depleted plasma from each group (n = 6) were used for the cholesterol efflux assessment in BHK cells stably transfected with ABCA1, ABCG1, or not (MOCK) as described previously [4]. In brief, [3H]cholesterol-labeled BHK cells were incubated with 1% of apoB-depleted plasma at 37°C for 4 hr before radioactivity was counted in media and cell lysate. The cholesterol efflux rate from cells to apoB-depleted plasma was expressed as the % of [3H]cholesterol transferred from cells to medium.
Measurement of plasma C-reactive protein (CRP) levels
Plasma inflammatory cytokine CRP levels was analyzed, using a commercially available ELISA kit (Abcam, Cambridge, MA).
Transfection and luciferase assay
Peroxisome proliferator-activated receptor (PPAR) transcriptional activities in HepG2 cells were estimated as described previously [9]. In brief, HepG2 cells were co-transfected with the PPRETK-luciferase reporter plasmid and internal control pRL-TK, followed by 24-hour treatment of 0.1% of LCMUFA isomers or not (control) in medium containing 1% BSA. The Luciferase activity was normalized to internal Renilla luciferase activity.
Proteomic analysis
Proteomic profiling of HDL, LDL, and VLDL fractions isolated by FPLC in each group (n = 6) was performed by LC-MS/MS on an Orbitrap Elite mass spectrometer (Thermo Scientific) as previously described [10]. The abundance of lipoprotein-associated proteins was estimated using a semi-quantitative spectrum counting method [11]. A gene ontology (GO) enrichment analysis with Gene Ontology for Functional Analysis (GOFFA) was performed to provide a functional annotation of the altered genes derived from the proteomics study [12].
Statistical analysis
Unless otherwise indicated, all the data were analyzed using GraphPad Prism statistical software (GraphPad Software Inc. version 6.00, La Jolla, CA). Statistical analysis between three groups was completed using one-way analysis of variance (ANOVA) followed by the Tukey’s post hoc test where appropriate. All data were expressed as mean ± SEM unless otherwise stated, and differences were considered significant when p ≤ 0.05. Evaluation of relationships between the lipoprotein proteomes and lesion area was performed by partial least squares regression analysis using JMP Statistical Discovery software (SAS, version 12.2.0). Using total spectrum counts as the relative abundance measure for each protein and total lesion area as the response variable, analysis was performed using the NIPALS method and leave-one-out validation. A variable importance for projection (VIP) value greater than 1.25 was considered statistically significant.
3. Results
3.1. Atherosclerosis lesion development
To induce atherosclerosis, LDLr−/− mice were placed for 12 weeks on a Western high-fat diet (42% energy from fat) supplemented with either C20:1 or C22:1 (5%; w/w) or with no fat supplements (control diet). Although the total energy content from protein, carbohydrate, and fat in the three diets was nearly identical (Supplemental Table 2), both the C20:1 and C22:1 supplemented diets inhibited to a similar degree (> 40%, p < 0.05) the development of atherosclerotic lesions in mice compared to mice on the control diet (Fig. 1A).
Fig. 1.
Progression of atherosclerosis and changes in plasma and hepatic lipids.
(A) Representative images of plaque on aorta surfaces obtained from en face Sudan IV staining (upper panel), and quantitative analysis of atherosclerotic area (lower panel); (B) hepatic proportion (%) of LCMUFA measured by gas chromatography; (C) plasma lipid levels; (D) hepatic lipid levels; (E and F) plasma FPLC analysis of plasma TC and TG. Data are expressed as mean ± SEM (n = 12; FPLC data: n = 6). ***p < 0.001.
3.2. Changes in hepatic fatty acid composition
Dietary C20:1 and C22:1 supplementation significantly (p < 0.05) increased hepatic corresponding LCMUFA isomers, and accordingly increased total LCMUFA levels significantly (p < 0.05) by 2~3-fold (Fig. 1B). No noticeable differences were found in the hepatic levels of shorter-chain MUFA, such as C16:1 and C18:1, saturated FA and polyunsaturated FA (PUFA) among the three diet groups. The full fatty acid composition of the liver from the 3 diet groups is shown in Supplemental Table 3.
3.3. Hepatic and plasma lipid levels
C20:1- or C22:1-rich diet did not change the liver and plasma levels of TC, FC, PL and TG (Fig. 1C and D). Analysis of lipoproteins by FPLC analysis also showed no difference in the major lipid levels in VLDL, LDL, and HDL fractions among the 3 diet groups (Fig. 1E and F, and Supplemental Table 4).
3.4. Cholesterol efflux capacity
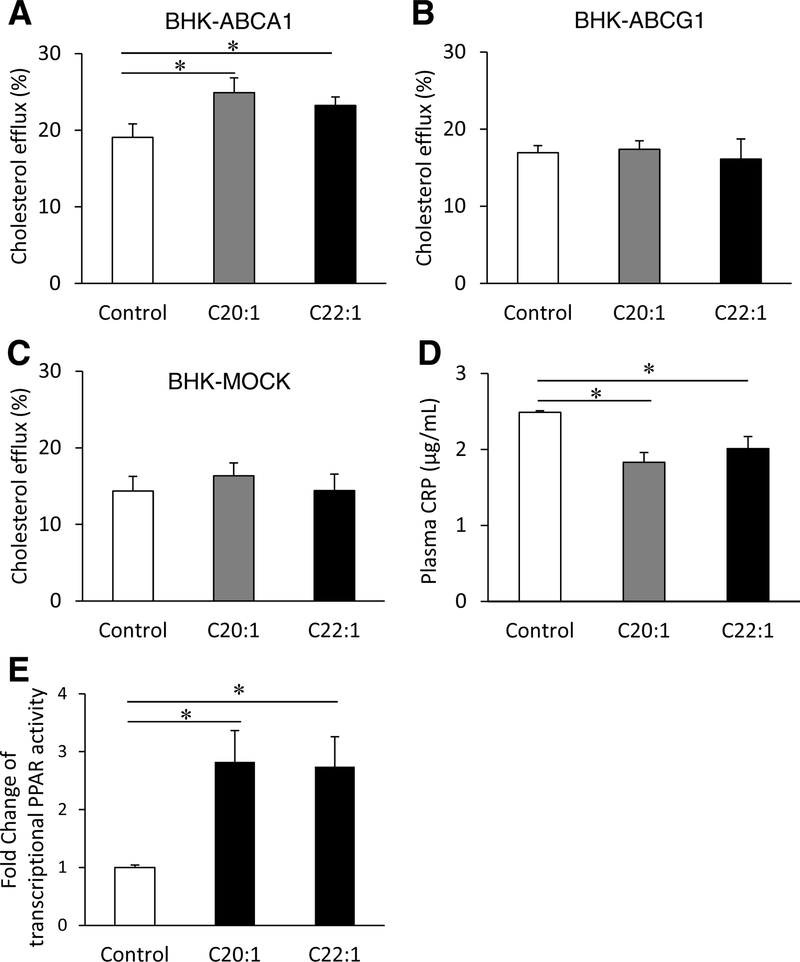
To investigate the mechanism for decreased atherosclerosis in mice supplemented with LCMUFA isomers despite the lack of significant changes in lipoprotein levels, we examined the effect of LCMUFA on plasma cholesterol efflux capacity. Incubation of BHK-ABCA1 cells with apoB-depleted plasma from LCMUFA diet groups resulted in significantly (p < 0.05) higher cholesterol efflux rates compared to control plasma (Fig. 2A). There were no differences in cholesterol efflux capacity from BHK-ABCG1 cells or BHK-MOCK cells among the three diet groups (Fig. 2B and C).
Fig. 2.
Dietary LCMUFA isomers improve cholesterol efflux capacity, inflammation, and PPAR reporter gene activity.
(A-C) Cholesterol efflux rate from BHK-ABCA1, BHK-ABCG1, and BHK-MOCK cells, respectively, to apoB-depleted plasma (n = 6); (D) plasma C-creative protein (CRP) levels (n = 12) and (E) fold changes in PPAR transcriptional activity in HepG2 cells treated with 0.1% of LCMUFA ethyl ester isomers following 24-hr transfection of PPRE-TK-luciferase reporter plasmid (n = 3). Data are expressed as mean ± SEM. *p < 0.05.
3.5. Plasma CRP levels
To further explore other potential mechanisms for the beneficial effect of LCMUFA, we measured plasma CRP as an indicator of systemic inflammation, which we previously showed decreased in mice after a diet enriched in both LCMUFA isomers [4]. Dietary supplementation with LCMUFA isomer fractions significantly decreased (p < 0.05) plasma concentration of CRP compared with control (Fig. 2D).
3.6. PPAR transcriptional activities
We previously showed that dietary LCMUFA favorably regulated the hepatic PPAR target genes in different atherosclerosis mouse models [4]. To clarify the effect of each individual LCMUFA isomer on PPAR transcriptional activity, we used a PPAR reporter gene assay in HepG2 cells. Consistent with previous observations, C20:1 and C22:1 both produced ~1.8-fold increases (p < 0.05) in PPAR transcriptional activity compared to control (Fig. 2E).
3.7. Modulation of the lipoprotein proteome
Lipoprotein proteome composition was analyzed by LC-MS/MS analysis. 91 HDL-associated, 49 LDL-associated, and 39 VLDL-associated proteins were detected in at least 50% of plasma samples from each diet group (Supplemental Table 5). In Fig. 3, We examined the effect of LCMUFA supplementation on the lipoprotein proteome and identified 14 HDL-associated, 9 LDL-associated, and 3 VLDL-associated proteins that were significantly (p < 0.05) altered. Both LCMUFA isomer diets increased the abundance of the following proteins: HDL-associated Ig lambda-1 chain C region (Lac1), coagulation factor X (Fa10), carboxypeptidase N catalytic chain (Cbpn), serum albumin (Albu), histidine-rich glycoprotein (Hrg), and protein Z-dependent protease inhibitor (Zpi), LDL-associated cluster of Pregnancy zone protein (Pzp), clusterin (Clus), and fibronectin (Finc), and VLDL-associated fibrinogen alpha chain (Fiba). In contrast, both dietary LCMUFA isomers decreased the abundance of the following proteins: HDL-associated complement factor B (Cfab), LDL-associated Ig mu chain C region (Ighm), Ig kappa chain C region (Igkc), and apolipoprotein A-IV (Apoa4). Overall, the two LCMUFA isomer fractions acted similarly in altering the lipoprotein proteome. Significantly altered proteins were further grouped into functional categories using Gene Ontology (GO) analysis (Table 1). This analysis revealed that most altered proteins (21%) were related to transport, followed by complement activation (17%) and blood coagulation-related proteins (12%). Several lipid/cholesterol metabolism-related proteins were also significantly altered by LCMUFA isomer diets.
Fig. 3.
Dietary LCMUFA isomers alter lipoprotein proteome.
Fold changes in HDL, LDL, and VLDL proteomes in LCMUFA isomer diet groups compared with control (n = 6). *p < 0.05 compared with control, #p < 0.05 compared with C20:1 group.
Table 1.
Pearson correlation (r-value) for lipoprotein-associated proteins that significantly (p < 0.05) altered by LCMUFA diets and atherosclerotic plaque area (%). Gene Ontology (GO) annotations for the altered proteins were indicated.
| Compltement activation | Blood coagulation | Antigen binding | Cholesterol metabolism | Lipid metabolism | Response to glucocorticoid stimulus | Activation of JNK activity | Vascular endothelial growth factor Receptor signaling | Transport | Lipid binding | Cellular matrix | Stem cell differentiation | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lipoproteln | Protein ID | UniProt ID | r (p value) | Gene Ontology (GO) term | |||||||||||
| HDL | |||||||||||||||
| Alub | P07724 | −0.58 (0.01) | ● | ● | ● | ||||||||||
| Apoc1 | P34928 | −0.34 (0.2) | ● | ● | ● | ||||||||||
| Cbpn | Q9JJN5 | −0.66 (<0.01) | ● | ||||||||||||
| Cfab | P04186 | 0.33 (0.2) | ● | ||||||||||||
| Cs1a | Q8CG14 | 0.1 (0.7) | ● | ||||||||||||
| Cs1b | Q8CFG8 | 0.27 (0.3) | ● | ||||||||||||
| Fa10 | 088947 | −0.28 (0.3) | ● | ● | |||||||||||
| Gcab | P01864 | 0.32 (0.2) | ● | ||||||||||||
| Hrg | Q9ESB3 | −0.69 (<0.01) | ● | ● | |||||||||||
| Lac1 | P01843 | −0.42 (0.08) | ● | ||||||||||||
| Trfe | Q921I1 | 0.45 (0.06) | ● | ● | ● | ||||||||||
| Vgfr3 | P35917 | 0.27 (0.3) | ● | ||||||||||||
| Zpi | Q8R121 | 0.52 (0.02) | ● | ||||||||||||
| LDL | |||||||||||||||
| Apoa4 | P06728 | 0.7 (<0.01) | ● | ● | ● | ● | |||||||||
| Clus | Q06890 | −0.71 (<0.01) | ● | ● | ● | ||||||||||
| Co3 | P01027 | 0.34 (0.2) | ● | ● | ● | ● | ● | ||||||||
| Finc | P11276 | −0.63 (<0.01) | ● | ||||||||||||
| Ighm | P01872 | 0.49 (0.04) | ● | ● | ● | ||||||||||
| Igkc | P01837 | 0.51 (0.03) | ● | ● | ● | ||||||||||
| Masp2 | Q91WP0 | −0.17 (0.5) | ● | ||||||||||||
| Mbl2 | P41317 | −0.61 (<0.01) | ● | ● | ● | ||||||||||
| Pzp | Q61838 | −0.64 (<0.01) | ● | ||||||||||||
| VLDL | |||||||||||||||
| Fiba | E9PV24 | −0.53 (0.02) | ● | ||||||||||||
| Finc | P11276 | −0.36 (0.1) | ● | ||||||||||||
| Tspl1 | P35441 | −0.17 (0.5) | ● | ● | ● | ● | |||||||||
3.8. Association between altered lipoprotein composition and atherosclerosis
Partial least squares (PLS) regression analysis was used to evaluate the relationship between lipoprotein-bound proteins and atherosclerotic lesion area and to identify LCMUFA-altered proteins that may contribute to the observed atheroprotection. PLS is a statistical approach used to generate models for high-dimension data sets, such as in proteomics and genomics studies, where the number of variables often exceed the number of observations [13]. Several components of the HDL proteome were strongly associated with atherosclerosis burden, among these were five of the LCMUFA modified proteins: Hrg, Cbpn, Albu, Zpi, and Trfe (Fig. 4A; the green spots above the dotted line). For LDL, 7 of the 9 proteins influenced by LCMUFA diets were correlated with lesion area (Fig. 4B; the green spots above the dotted line), and, for VLDL, 1 of 3 (Fig. 4C; the green spot above the dotted line). The correlation between the LCMUFA-derived altered lipoprotein-associated proteins and atherosclerosis were also confirmed by using Pearson correlation analysis (Table 1). For every LCMUFA altered protein, the directionality of change in protein abundance was consistent with atheroprotection. For example, proteins that were negatively associated with lesion area were increased by LCMUFA diet, and vice versa.
Fig. 4.
Association between lipoprotein proteome and atherosclerosis.
Partial least squares (PLS) regression analysis was used to evaluate associations between lipoprotein bound proteins and atherosclerosis lesion area for HDL (A), LDL (B), and VLDL (C). Volcano-style plots of coefficient vs. variable importance projection (VIP) display the strength of association and significance, respectively. VIP greater than 1.25 is considered statistically significant. Proteins of interest are labeled with protein ID and green points identify proteins that were affected by LCMUFA diet.
4. Discussion
The present study showed for the first time that dietary supplementation with the different LCMUFA isomer fractions (C20:1 and C22:1) individually suppresses atherosclerosis development in a mouse model. Dietary supplementation also increased the corresponding hepatic levels of the individual LCMUFA isomers in the liver without changes in the concentration of the major hepatic lipid classes. We cannot exclude from this data the possibility that LCMUFA could be differentially enriched in certain classes of lipids or in certain other tissues, such as the aorta, and is an important topic for future investigation.
In line with our previous mixed LCMUFA study [4], the beneficial effects of each LCMUFA isomer include decreased inflammation, as measured by CRP, and increased plasma capacity for cholesterol efflux by the ABCA1 transporter. Similar to EPA and DHA [14], our results also show that each LCMUFA isomer also enhanced PPAR transcriptional activity. Several other naturally occurring fatty acids, such as medium-chain saturated and monounsaturated fatty acids, have also been reported to bind and activate PPAR isomers [14]. PPARs are members of the nuclear-hormone-receptor superfamily and are known to regulate the inflammatory response through their transactivation and transrepression capacities [15] and thus could account for the reduced CRP observed in this study and in the reduction of other pro-inflammatory cytokines that we previously observed with LCMUFA supplementation [4]. Furthermore, stimulation of PPAR pathways also leads to enhanced cholesterol efflux by the induction of the ABCA1 transporter [16], and thus may reduce atherosclerosis by enhancing the reverse-cholesterol-transport pathway. Besides ABCA1, we previously observed that supplementation with mixed LCMUFA isomers [4], alters the hepatic expression in mice of several other PPAR target genes relevant to atherosclerosis, such as Cyp7a1, Adipor2, Rxr and Lxralpha. Thus, changes in gene expression in the liver and or in other tissues, such as the vessel wall, in response to altered PPAR signaling likely contributes to the beneficial effects of LCMUFA dietary supplementation, but identifying the exact mechanism will require additional studies.
Most notably, each LCMUFA isomer decreased atherosclerosis in LDLr−/−mice to a similar degree and showed an equal effect on the other measured parameters. LCMUFA supplementation in type II diabetic mice has also been shown to reduce plasma lipids and improve insulin sensitivity [17]. Based on these positive findings, we are now starting a human clinical trial of saury oil containing a mixture of LCMUFA (ClinicalTrials.gov Identifier: NCT03043365). If this trial shows some benefit in metabolic parameters, further improvements are not likely based on this study from the use of purified LCMUFA isomers, which is costly and difficult to do on a large scale. It is important to note that the LCMUFA dose used in this animal study is relatively high but is similar to other animal studies of other dietary fatty acids [18], and in the human clinical trial, the daily dose of saury oil is 4g, which is the usual maximum dose of fish oils.
Interestingly, neither LCMUFA isomer affected total plasma lipid levels or the distribution of the major lipoprotein subclasses in LDLr−/− mice unlike what we observed in diabetic mice on a mixed LCMUFA rich diet [17]. There is growing evidence, however, that simple measurement of cholesterol concentration on lipoproteins has limited predictive value for CVD in human studies. It is well known that approximately half of individuals with myocardial infarction do not appear to be at great risk based on LDL-C [19]. Similarly, studies have shown that measures of HDL function related to cholesterol efflux are superior to LDL-C in predicting CVD risk [20]. Recent proteomic studies have also shown that besides the major apolipoprotein structural proteins, as many as 200 individual proteins can be found in lipoprotein fractions, and that the lipoprotein proteome may be predictive of CVD risk [21]. Therefore, we performed proteomic profiling of the major lipoprotein fractions in mice on the different diets.
LCMUFA supplementation had the greatest effect on the lipoprotein proteome related to complement activation and blood coagulation, both of which are known to contribute to the pathogenesis of atherosclerosis [22]. In addition, more than half of the LDL- and VLDL-associated proteins altered by dietary LCMUFA were also favorably correlated with atherosclerosis suppression. Most notably, Clus (Apoj) in LDL had the strongest correlation with decreased atherosclerosis. Studies have demonstrated that plasma glycoprotein Clus plays a protective role in atherosclerosis development through several mechanisms, including anti-inflammatory and cytoprotective actions, regulation of the immune system and LDL aggregation [23]. Future studies, however, will be needed to investigate whether these changes in the lipoprotein proteome in response to the LCMUFA-rich diets are mechanistically linked to atherosclerosis.
In summary, our results demonstrate that LCMUFA rich diets caused enrichment of LCMUFA in the liver and both LCMUFA isomers individually attenuated the progression of atherosclerotic lesions in LDLr−/− mice, possibly through stimulation of PPAR pathways. Finally, protein compositional changes in lipoproteins in response to LCMUFA were also observed and found to be associated with decreased atherosclerosis.
Supplementary Material
Highlights.
Some fish oils are enriched in long-chain monounsaturated fatty acids (LCMUFA)
LCMUFA isomers (C20:1, C22:1) fractions inhibited atherosclerosis in LDLR-KO mice
There were no major differences in plasma lipoproteins or hepatic lipid content
Proteomic analysis revealed lipoprotein composition changes by LCMUFA isomers
Several lipoprotein proteome changes significantly correlated with atherosclerotic reduction
Acknowledgements
We thank NHLBI Proteomics Core Facility for helpful advice and access to the mass spectrometer.
Financial support
This research was supported by the Intramural Research Program of the National Heart, Lung and Blood Institute (NHLBI) and the Office of Dietary Supplements (ODS) Research Scholars Program at National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
The authors declared they do not have anything to disclose regarding conflict of interest with respect to this manuscript.
References
- 1.Hooper L, Summerbell CD, Thompson R, Sills D, Roberts FG, et al. Reduced or modified dietary fat for preventing cardiovascular disease. Cochrane Database Syst Rev. 2011;7:CD002137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schwingshackl L, Hoffmann G. Monounsaturated fatty acids, olive oil and health status: a systematic review and meta-analysis of cohort studies. Lipids Health Dis. 2014; 13:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang ZH, Emma-Okon B, Remaley AT. Dietary marine-derived long-chain monounsaturated fatty acids and cardiovascular disease risk: a mini review. Lipids Health Dis. 2016;15:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang ZH, Bando M, Sakurai T, Chen Y, Emma-Okon B, et al. Long-chain monounsaturated fatty acid-rich fish oil attenuates the development of atherosclerosis in mouse models. Mol Nutr Food Res. 2016, 60:2208–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsumoto C, Matthan NR, Lichtenstein AH, Gaziano JM, Djoussé L. Red blood cell MUFAs and risk of coronary artery disease in the Physicians’ Health Study. Am J Clin Nutr. 2013;98:749–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim DS, Maden SK, Burt AA, Ranchalis JE, Furlong CE, et al. Dietary fatty acid intake is associated with paraoxonase 1 activity in a cohort-based analysis of 1,548 subjects. Lipids Health Dis. 2013;12:183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gordon SM, Remaley AT. High density lipoproteins are modulators of protease activity: Implications in inflammation, complement activation, and atherothrombosis. Atherosclerosis. 2017;259:104–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burillo E, Martín-Fuentes P, Mateo-Gallego R, Baila-Rueda L, Cenarro A, et al. , Omega-3 fatty acids and HDL. How do they work in the prevention of cardiovascular disease? Curr Vasc Pharmacol. 2012;10,432–441. [DOI] [PubMed] [Google Scholar]
- 9.Wang S, Awad KS, Elinoff JM, Dougherty EJ, Ferreyra GA, et al. , G Protein-coupled Receptor 40 (GPR40) and Peroxisome Proliferator-activated Receptor γ (PPARγ): AN INTEGRATED TWO-RECEPTOR SIGNALING PATHWAY, J Biol Chem. 2015;290:19544–19557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gordon SM, McKenzie B, Kemeh G, Sampson M, Perl S, et al. Rosuvastatin Alters the Proteome of High Density Lipoproteins: Generation of alpha-1-antitrypsin Enriched Particles with Anti-inflammatory Properties. Mol Cell Proteomics. 2015;14:3247–3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gordon SM, Deng J, Lu LJ, Davidson WS. Proteomic characterization of human plasma high density lipoprotein fractionated by gel filtration chromatography. J Proteome Res. 2010;9:5239–5249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun H, Fang H, Chen T, Perkings R, Tong W. GOFFA: Gene Ontology For Functional Analysis - Software for gene ontology-based functional analysis of genomic and proteomic data. BMC Bioinformatics. 2006;7(Suppl 2):S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boulesteix AL, Strimmer K. Partial least squares: a versatile tool for the analysis of high-dimensional genomic data. Brief Bioinform. 2007;8:32–44. [DOI] [PubMed] [Google Scholar]
- 14.Grygiel-Górniak B Peroxisome proliferator-activated receptors and their ligands: nutritional and clinical implications--a review. Nutr J. 2014;13:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Daynes RA, Jones DC. Emerging roles of PPARs in inflammation and immunity. Nat Rev Immunol. 2002;2:748–759. [DOI] [PubMed] [Google Scholar]
- 16.Chinetti G, Lestavel S, Bocher V, Remaley AT, Neve B, et al. , PPAR-alpha and PPAR-gamma activators induce cholesterol removal from human macrophage foam cells through stimulation of the ABCA1 pathway. Nat Med. 2001;7:53–58. [DOI] [PubMed] [Google Scholar]
- 17.Yang ZH, Miyahara H, Iwasaki Y, Takeo J, Katayama M. Dietary supplementation with long-chain monounsaturated fatty acids attenuates obesity-related metabolic dysfunction and increases expression of PPAR gamma in adipose tissue in type 2 diabetic KK-Ay mice. Nutr Metab (Lond). 2013;10:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mori T, Kondo H, Hase T, Tokimitsu I, Murase T. Dietary fish oil upregulates intestinal lipid metabolism and reduces body weight gain in C57BL/6J mice. J Nutr. 2007;137:2629–2634. [DOI] [PubMed] [Google Scholar]
- 19.Davidson MH, Ballantyne CM, Jacobson TA, Bittner VA, Braun LT, et al. , Clinical utility of inflammatory markers and advanced lipoprotein testing: advice from an expert panel of lipid specialists. J Clin Lipidol. 2011;5:338–367. [DOI] [PubMed] [Google Scholar]
- 20.Keene D, Price C, Shun-Shin MJ, Francis DP. Effect on cardiovascular risk of high density lipoprotein targeted drug treatments niacin, fibrates, and CETP inhibitors: meta-analysis of randomised controlled trials including 117,411 patients. BMJ. 2014;349:g4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shah AS, Tan L, Long JL, Davidson WS. Proteomic diversity of high density lipoproteins: our emerging understanding of its importance in lipid transport and beyond. J Lipid Res. 2013; 54:2575–2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carter AM. Complement activation: an emerging player in the pathogenesis of cardiovascular disease. Scientifica (Cairo). 2012;2012:402783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park S, Mathis KW, Lee IK. The physiological roles of apolipoprotein J/clusterin in metabolic and cardiovascular diseases. Rev Endocr Metab Disord. 2014;15:45–53. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.