SUMMARY
Redox cycling of extracellular electron shuttles can enable the metabolic activity of subpopulations within multicellular bacterial biofilms that lack direct access to electron acceptors or donors. How these shuttles catalyze extracellular electron transfer (EET) within biofilms without being lost to the environment has been a long-standing question. Here, we show that phenazines mediate efficient EET through interactions with extracellular DNA (eDNA) in Pseudomonas aeruginosa biofilms. Retention of pyocyanin (PYO) and phenazine carboxamide in the biofilm matrix is facilitated by eDNA binding. In vitro, different phenazines can exchange electrons in the presence or absence of DNA and can participate directly in redox reactions through DNA. In vivo, biofilm eDNA can also support rapid electron transfer between redox active intercalators. Together, these results establish that PYO:eDNA interactions support an efficient redox cycle with rapid EET that is faster than the rate of PYO loss from the biofilm.
Graphical Abstract
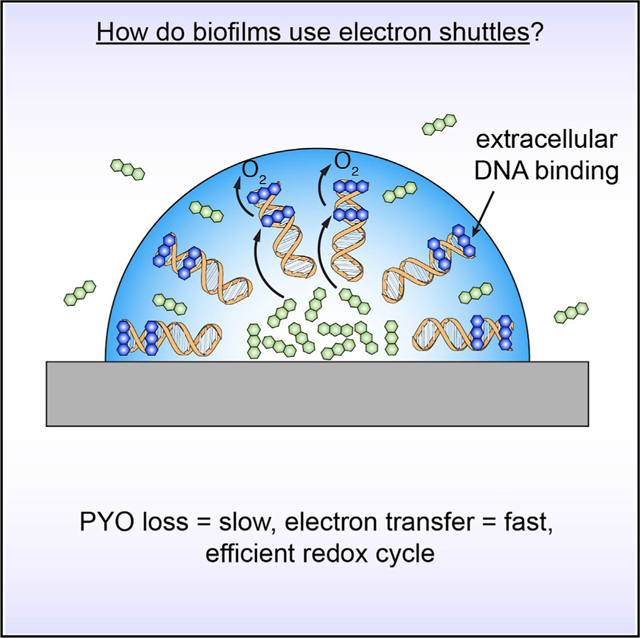
In Brief
Phenazines are retained in biofilms through binding to extracellular DNA, and together these biofilm components mediate efficient extracellular electron transfer to support bacterial metabolism
INTRODUCTION
Microbial biofilms are ubiquitous in natural and engineered contexts, spanning plant roots to chronic human infections to anaerobic digestors (Hall-Stoodley et al., 2004; Pandit et al., 2020). As biofilms develop, metabolic stratification occurs, driven by steep concentration gradients of substrates, such as oxygen, that are consumed by cells at the biofilm periphery faster than the substrates can diffuse into the biofilm interior (Stewart 2003; Stewart and Franklin, 2008; Liu et al., 2015). Indeed, oxidant limitation is a generic challenge for cells that inhabit biofilm microenvironments where electron donors are abundant, yet electron acceptors are not. One widespread strategy microbes employ to overcome this challenge is to channel electrons derived from intracellular metabolism to extracellular oxidants at a distance (Shi et al., 2016). Known as “extracellular electron transfer” (EET), this process requires electron carriers to bridge the gap, be they outer membrane-associated or extracellular cytochromes (Richter et al., 2009; Xu et al., 2018; Jiménez Otero et al., 2018; Nevin et al., 2009), various structures called “nanowires” (Steidl et al., 2016; Subramanian et al., 2018; Wang et al., 2019; Reguera et al., 2005; Malvankar et al., 2011), cable bacteria conductive filaments (Cornelissen et al., 2018), or redox-active small molecules (Glasser et al., 2017a). Although the putative molecular components underpinning different EET processes have been described in a variety of organisms, a detailed understanding of how these components achieve EET remains an important research goal across diverse systems.
In contrast to the rapid progress in understanding the molecular basis by which cytochromes and nanowires facilitate EET, less progress has been made on how soluble (physically diffusive) electron shuttles facilitate EET beyond interactions at the cell surface (Light et al., 2018; Xu et al., 2016; Marsili et al., 2008). In part, this is due to the challenges involved in identifying and studying small molecule metabolites within a complex extracellular matrix. Accordingly, to study extracellular electron shuttling, we have chosen to work with a model system that employs a relatively well studied and tractable set of shuttles called phenazines. Phenazines are colorful redox-active molecules produced by numerous microbial species including the bacterium, Pseudomonas aeruginosa (Turner and Messenger, 1986; Chincholkar and Thomashow, 2013). P. aeruginosa strains are ubiquitous yet perhaps most well-known for their roles in chronic infections where their growth as biofilms renders them antibiotic tolerant and contributes to patient morbidity and mortality (Costerton et al., 1999); importantly, phenazines support the development of anoxic, antibiotic tolerant biofilm regions. Two dimensional mapping of phenazine production in the agar underlying colony biofilms has revealed that different phenazines localize to distinct zones (Bellin et al., 2014, 2016). Here, we set out to better understand how phenazines facilitate EET within the P. aeruginosa biofilm matrix.
Intriguingly, although the P. aeruginosa biofilm matrix comprises a heterogeneous group of polymers (Colvin et al., 2012), extracellular DNA (eDNA) from dead cells is a significant contributor (Allesen-Holm et al., 2006; Jennings et al., 2015; Whitchurch et al., 2002), accounting for the majority of the matrix polymers in some cases (Steinberger and Holden, 2005; Matsukawa and Greenberg, 2004). Phenazines have long been known to intercalate into double stranded DNA in vitro (Hollstein and Van Gemert, 1971). Recently, it was suggested that the phenazine pyocyanin (PYO) can participate in DNA-mediated charge transfer in vitro (Das et al., 2015), and phenazine-eDNA interactions might facilitate biofilm EET (Das et al., 2015). Notably, the ability of PYO to stimulate cell lysis (Das and Manefield, 2012) changes according to the environment: when cells are oxidatively stressed (i.e., oxidant replete but reductant limited) and ATP limited, PYO is toxic; whereas when they are reductively stressed (i.e., reductant replete but oxidant limited), PYO promotes viability and biofilm aggregate expansion (Meirelles and Newman, 2018; Costa et al., 2017). This observation raises the intriguing possibility that cell lysis by a small percentage of the population early on might later promote EET once biofilms have developed anoxic zones where extracellular electron shuttles support metabolism. Although a variety of roles for eDNA in biofilms have been proposed, including serving as a structural support, nutrient, and/or genetic reservoir (Flemming and Wingender, 2010), to our knowledge, that biofilm eDNA may facilitate EET has not been demonstrated.
The current model of phenazine redox cycling in biofilms can be broadly defined (Figure 1A). In anoxic regions, oxidized phenazines are reduced intracellularly by metabolic reactions that support these cells (Jo et al., 2017; Glasser et al., 2014, 2017b; Wang et al., 2010). These reduced phenazines are thought to physically diffuse through the extracellular matrix toward the oxic region where they react abiotically with molecular oxygen. The oxidized phenazines then return to the anoxic region of the biofilm to complete the redox cycle. Although studies have begun to characterize the reactions on either side of the redox cycle, very little is known about how phenazines operate in the intervening extracellular matrix. Theoretical studies suggest that physical diffusion of oxidized phenazine toward the biofilm interior and reduced phenazine toward the biofilm periphery may be fast enough to support the metabolism of the oxidant limited cells (Glasser et al., 2017a; Kempes et al., 2014), but these models have assumed the biofilm is a closed system in which freely diffusing phenazines are not lost to the outer environment. It is well established, however, that phenazines can escape P. aeruginosa biofilms (Ramos et al., 2010), which, if left unchecked, would greatly reduce the efficiency of phenazine redox cycling. This is a key challenge these biofilms must solve.
Figure 1. Colony Biofilms Retain PYO and PCN.
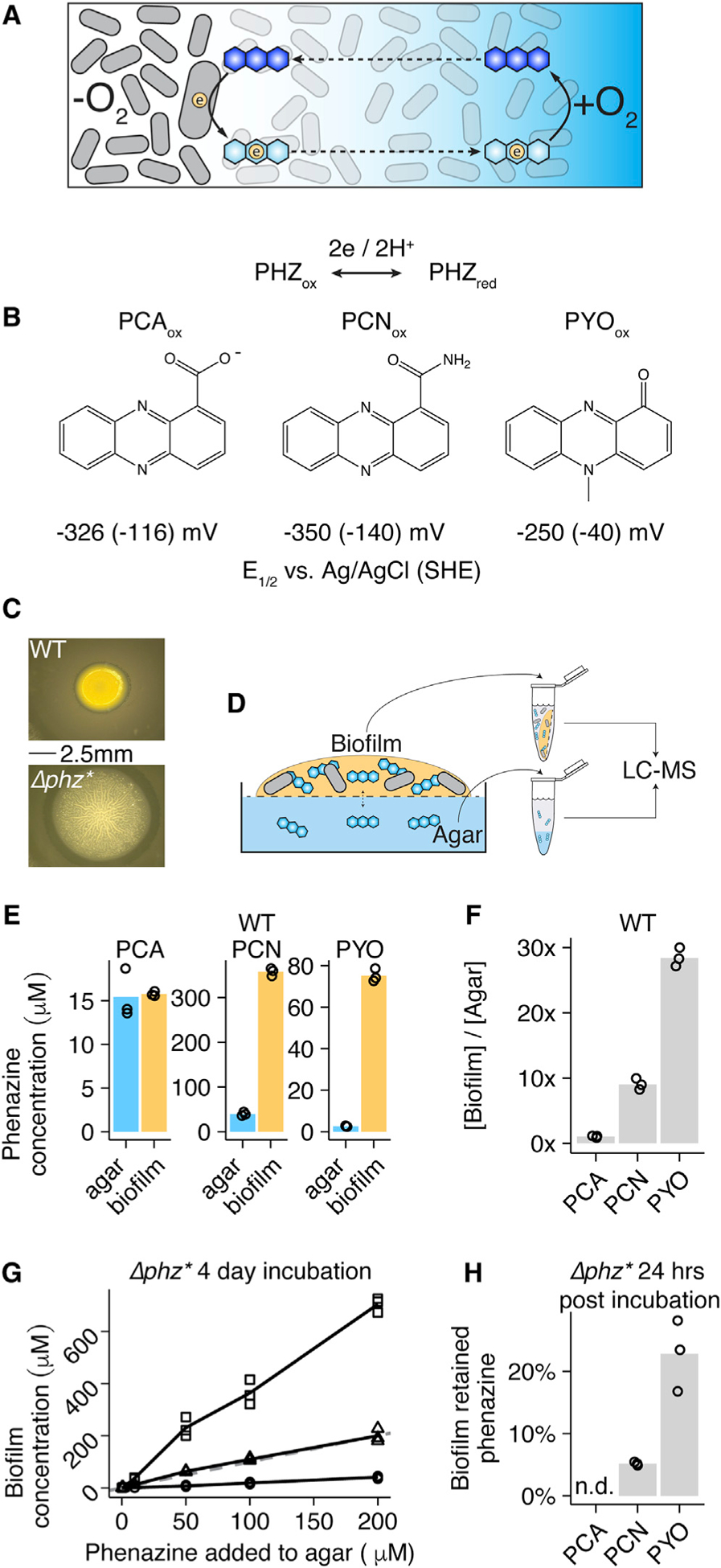
(A) Diagram of the phenazine redox cycle in a biofilm. Cells are shown as gray rods, phenazines are shown as blue hexagons, electrons are shown as circles, and the oxygen gradient is shown as the blue background.
(B) Structures of the three studied phenazines in their oxidized states produced by P. aeruginosa—phenazine carboxylate (PCA), phenazine carboxamide (PCN), and pyocyanin (PYO). All three phenazines (PHZ) undergo two proton two electron reductions and the midpoint potentials are shown for the reduction of each phenazine.
(C) Images of WT (top) and Δphz* (bottom) colony biofilms.
(D) Schematic of phenazine extractions from colony biofilms and agar. The 0.2 μm membrane is shown as the dashed line.
(E) Biofilm and agar concentrations for PCA, PCN, and PYO from three WT biofilms.
(F) The same data as (C), represented as retention ratios ([Biofilm]/[Agar]).
(G) Recovered phenazine concentrations from Δphz* colony biofilms grown with different levels of synthetic phenazine in the underlying agar for 4 days. The dashed gray line shows 1:1 (biofilm concentration:added phenazine).
(H) Accumulated phenazine from three Δphz* colony biofilms following 3 days of growth with synthetic phenazine (day 3), and 1 day later after transfer to fresh agar (day 4). Data are represented as the percentage day 4/day 3. PCA was not detected (n.d.) on day 4.
In (E)–(H), values for individual biofilms are shown by open symbols, and lines or bars represent the mean.
See also Figure S1.
This study investigates how phenazine electron transfer may be reconciled with phenazine retention. Specifically, we ask: are phenazines retained and if so to what extent? What mechanisms of electron transfer are compatible with phenazine retention? Our motivation to answer these questions arises not only from a desire to constrain the model of phenazine redox cycling within P. aeruginosa biofilms, but more broadly, to identify a potentially generalizable strategy for how diverse electron shuttles enable EET.
RESULTS
We studied three major phenazine derivatives made by P. aeruginosa strain UCBPP-PA14 (Schroth et al., 2018): phenazine carboxylate (PCA), phenazine carboxamide (PCN), and pyocyanin (PYO); at the pH relevant for our experiments, the dominant form of PCA is negatively charged whereas PCN and PYO are uncharged (Figure 1B). Beyond studying wild-type (WT)-produced phenazines, we also determine the effects of individual synthetic phenazines on a mutant that does not produce phenazines, Δphz (ΔphzA1-G1, ΔphzA2-G2), or on a mutant that is also incapable of modifying PCA to make PCN and PYO, Δphz* (Δphz, ΔphzMS, ΔphzH). Experiments were performed with two different types of biofilms: macroscopic colony biofilms grown on nutrient agar surfaces (Figures 1C and S1A) and microscopic biofilms attached to the surfaces of an interdigitated microelectrode array (IDA) suspended in liquid medium. Phenazine-dependent biofilm phenotypes operate similarly at both scales (Ramos et al., 2010), so we selected the biofilm cultivation method for any given experiment based on which was best suited to answering our specific research question.
Colony Biofilms Retain PCN and PYO, but Not PCA
First, we sought to quantify phenazine retention by colony biofilms (Figures 1C and 1D). We used liquid chromatographymass spectrometry (LC-MS) to quantify extracted endogenous phenazines from the biofilms and compared their concentrations to that in the underlying agar (Figures 1D–1F). Colony biofilms could be cleanly separated from the agar, because they were separated by a 0.2 μm membrane filter, which did not affect the results (Figures S1B and S1C). Overall, PCA, PCN, and PYO concentrations varied by more than 10-fold in the biofilms reaching concentrations of ~15 μM PCA, ~400 μM PCN, and ~80 μM PYO. Comparing biofilm to agar concentrations showed that PCN and PYO were enriched in the biofilm 10-fold and 30-fold, respectively, while PCA reached similar concentrations in the biofilm and the agar (Figures 1E and 1F). This suggested that PCN and PYO were strongly retained by the biofilm and PCA was not, as would be expected based on their charge. Lysing resuspended biofilm cells by sonication prior to phenazine quantification did not strongly affect the results (Figure S1D), indicating that the measured pools of phenazines were predominantly retained extracellularly rather than intracellularly.
To test if differential phenazine retention requires endogenous phenazines, we grew Δphz* colony biofilms with synthetic phenazines in the agar and quantified phenazines taken up by the biofilm. Incubation with ≥50 μM PYO resulted in >200 μM PYO accumulation in the biofilm (Figure 1G). PCN accumulated to a lesser extent, and PCA biofilm uptake was minimal (<50 μM) even with 200 μM added to the agar) (Figure 1G). Δphz* colonies transferred from phenazine agar to fresh agar after 3 days of growth retained phenazines in the same pattern as the WT over 24 h (Figure 1H), demonstrating that the observed phenazine retention does not depend on endogenous phenazine production. WT colony biofilms exhibit relatively thick and smooth morphologies that contain deep anoxic regions that are thought to be supported by phenazine EET. Δphz* colony biofilms exhibit different colony morphologies that are thin and highly wrinkled, which is thought to be a physiological adaptation to maximize surface area and oxygen penetration in the absence of phenazines as shown for Δphz (Dietrich et al., 2013). Notably, only incubation of Δphz* colonies with exogenous PYO appreciably complemented the colony wrinkling phenotype (Figure S1A), demonstrating that retained PYO is used by the colony biofilm for phenazine EET metabolism in vivo. P. aeruginosa colony biofilms thus appear able to take up and use significant amounts of exogenous PYO, and PCN to a lesser extent. These results predict that colony biofilms contain an extracellular component that binds and effectively retains PYO and PCN, but not PCA.
Phenazines Differentially Bind Extracellular DNA
Because we measured considerable biofilm-retained PYO and PCN that was insensitive to cell lysis by sonication, we hypothesized these phenazines might bind an abundant component of the biofilm extracellular matrix. The extracellular matrix in P. aeruginosa PA14 biofilms is known to be composed primarily of two polymers: DNA from dead cells (eDNA) and the polysaccharide, Pel (Colvin et al., 2011; Das and Manefield, 2012), and recent work suggested that PYO intercalates into DNA in vitro (Das et al., 2015). To test the hypothesis that eDNA in the biofilm matrix was responsible for binding phenazines, we quantified the binding affinity of oxidized PCA, PCN, and PYO for a 29 base pair double-stranded DNA (dsDNA) molecule in vitro using isothermal titration calorimetry (Figure 2A). As expected, oxidized PCA showed no detectable binding because it is negatively charged, as is the phosphate backbone of DNA at pH 7. In contrast, oxidized PCN (KD = 194 μM; 95% confidence interval [CI] = 148–305 μM) and PYO (KD = 13 μM; 95% CI = 6.5–49 μM) both bind dsDNA, and their dominant forms are neutral at pH 7 (Costa et al., 2017). These results are consistent with ethidium bromide displacement and microscale thermophoresis binding assays (Figures S2A and S2B) (Das et al., 2015). Notably, these in vitro phenazine-DNA binding affinities correlate with their in vivo retention ratio ([biofilm]/[agar]), where PYO is retained in the biofilm significantly more than PCN, and PCA is not retained. Reduced PYO showed no change in endogenous fluorescence upon addition of calf thymus DNA (Figure S2C), suggesting that the DNA binding affinity of PYO is redox dependent.
Figure 2. Phenazines Interact with DNA In Vitro and In Vivo.
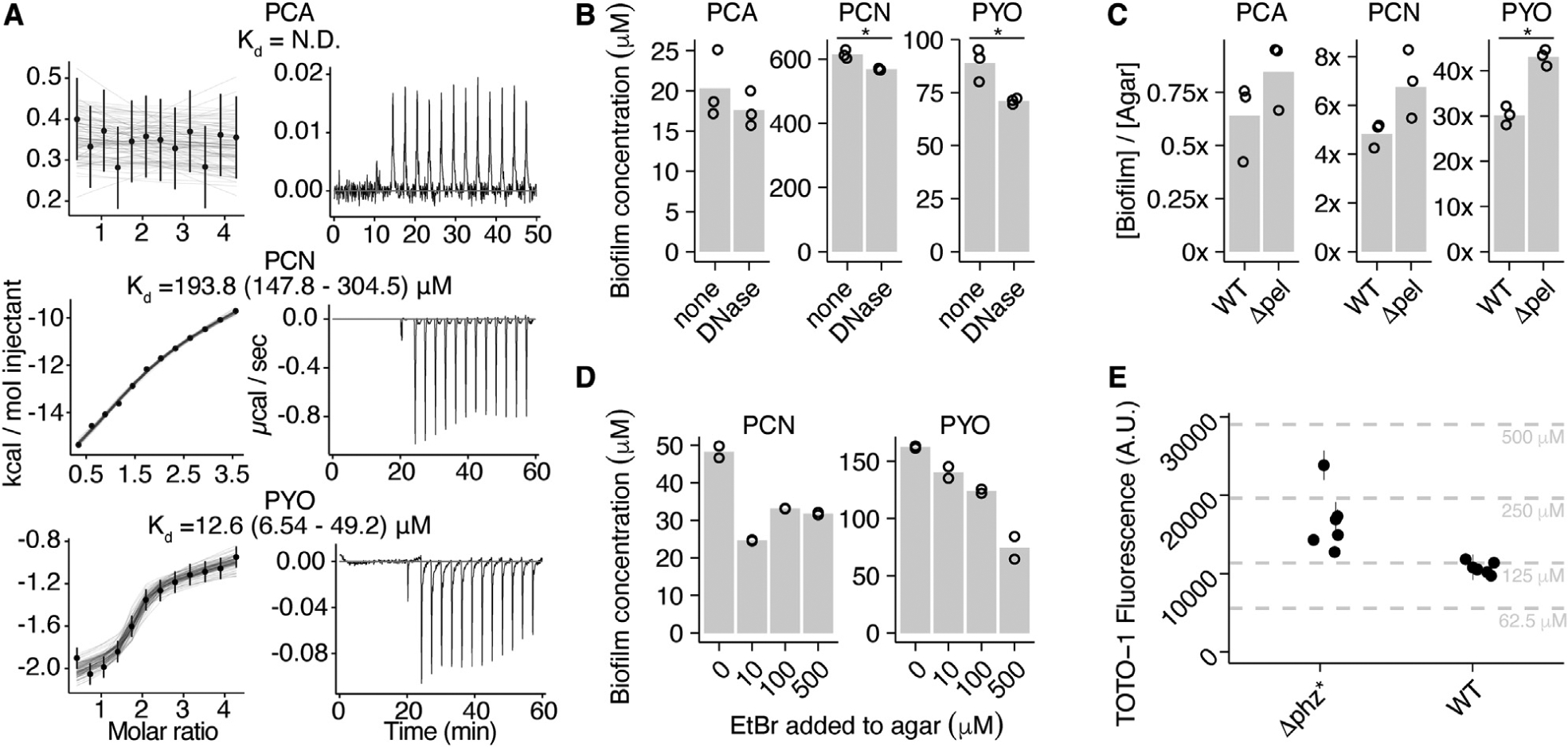
(A) Representative isothermal titration calorimetry (ITC) data for each phenazine injected into a solution of dsDNA (29 base pairs). Exothermic reactions are depicted as negative values. Integrated peak data was fit with a Bayesian model to calculate the Kd (in base pairs DNA) with 95% confidence intervals (Duvvuri et al., 2018).
(B) Biofilm phenazine concentrations for WT biofilms treated with or without DNase I in the underlying agar for 24 h (n = 3 per condition). Bars with asterisks denote measurements that differ significantly (p < 0.05) by a Welch’s single-tailed t test.
(C) Phenazine retention ratios for WT and Δpel colony biofilms (n = 3 per condition). Statistical test same as in (B).
(D) Accumulated phenazine concentrations for Δphz* biofilms incubated with 50 μM PCN or PYO and increasing concentrations of the competitive intercalator, ethidium bromide, in the underlying agar (n = 2 per condition).
(E) eDNA quantified in six WT and Δphz* colony biofilms with the dye TOTO-1. Error bars show standard deviation from two technical replicates. Dashed lines show calf thymus DNA standards, with concentrations back calculated for the biofilm volume.
In (B)–(D), values for individual biofilms are shown by point symbols, and bars represent the mean.
See also Figures S1, S2, and S3.
To determine whether phenazine-eDNA binding occurs in vivo, we treated 3-day-old WT biofilms with DNase I for 24 h. These experiments were performed with DNase I spotted on tryptone agar medium rather than its optimal buffer, as controls showed that buffer alone significantly disturbed the biofilm (Figures S3A–S3C). A previous study also observed that mature P. aeruginosa biofilms were minimally affected by DNase and suggested that extracellular proteases may inactivate DNase (Whitchurch et al., 2002). Despite a low activity for DNase under these conditions, DNase-treated biofilms retained significantly lower biofilm PCN and PYO concentrations than their untreated counterparts; moreover, PCA concentration was unchanged (Figure 2B). P. aeruginosa eDNA originates from the genomic DNA of dead cells, is high molecular weight and bound by other biomolecules (Kavanaugh et al., 2019); accordingly, complete elimination of phenazine binding sites with DNase is not expected. We also compared the phenazine retention in the WT to a Pel mutant (Δpel) and found that biofilms without Pel retained significantly more PYO (Figure 2C). Because Pel, the dominant exopolysaccharide in PA14 biofilms is known to bind eDNA (Jennings et al., 2015), these results suggest that Pel may partially block access to eDNA by PYO, although this remains to be tested in vitro. Because eDNA and Pel are the primary matrix constituents of PA14 biofilms (Colvin et al., 2011; Das and Manefield, 2012), that PYO was well retained in Δpel biofilms indicates that eDNA is the dominant matrix component responsible for its retention.
To further test if phenazine-eDNA binding occurs in vivo, w e competed phenazines against ethidium bromide, a well-known DNA intercalator. Because PCN and PYO compete for DNA binding sites with ethidium in vitro (Figure S2A), and ethidium is largely excluded from cells (Jernaes and Steen, 1994), we reasoned that these intercalators could compete for binding sites in the biofilm eDNA. We grew Δphz* biofilms with 50 μM oxidized PCN or oxidized PYO and increasing amounts of ethidium in the underlying agar. Figure 2D shows that increasing concentrations of ethidium resulted in successively less PYO accumulating in the biofilms, while PCN accumulated to a similar lower level in the presence of any amount of added ethidium. These results demonstrate that more than 50% of biofilm-accumulated PYO resides in binding sites that can be competitively inhibited by ethidium, whereas more than 30% of the more weakly bound PCN resides in binding sites that are saturated at ethidium concentrations ≥10 μM. Although these results reveal eDNA binding sites for PYO and PCN, they also show fractions that are ethidium insensitive. This suggests there may be other binding sites for phenazines such as Pel-eDNA complexes, cell surfaces, filamentous phage or extracellular proteins. Regardless, the ability of ethidium to displace significant portions of PCN and PYO indicates that these phenazines intercalate into biofilm eDNA.
Confocal microscopy of WT and Δphz* colony biofilms with a cell-impermeable dsDNA dye, TOTO-1 (Okshevsky and Meyer, 2014), showed abundant eDNA localized in dead cells and in between cells (Figure S3D). We quantified the bulk concentration of eDNA in colony biofilms by incubating biofilm suspensions with TOTO-1 and measuring dye fluorescence. Both WT and Δphz* biofilm suspensions yielded large fluorescence values when incubated with TOTO-1. These values fall within the range of 60–500 μM base pairs dsDNA in the colony biofilms, when calibrated against standards of calf thymus DNA (Figure 2E). However, adding calf thymus DNA to the biofilm suspensions did not yield the expected increase in dye fluorescence (Figure S3E), which suggests that the dye may be partially inhibited by biofilm components. Therefore, this order of magnitude estimate of biofilm eDNA should be interpreted as a lower bound on the true value. Given this estimate, the biofilm eDNA (>100 μM base pairs) is in excess of PYO (~80 μM), but it may not be in excess of PCN (>300 μM). Due to its poor aqueous solubility, it is probable that PCN crystallizes extracellularly at the observed biofilm concentrations, which could lead to its measured retention (Hernandez et al., 2004). Together, our in vivo and in vitro results are consistent with eDNA providing binding sites for oxidized PCN and oxidized PYO in the biofilm extracellular matrix.
Constraints on Phenazine Electron Transfer Mechanisms In Vitro and In Vivo
Given that phenazines are differentially bound and retained in biofilm eDNA, we next sought to constrain how electron transfer might be achieved in this context. Previous research has shown distinct localization patterns for different phenazines within biofilms, with the lowest potential phenazines (e.g., PCA) in the interior, and the highest potential phenazine (e.g., PYO) at the oxic periphery (Bellin et al., 2014, 2016). To test whether electron transfer could occur between these molecules in solution, we mixed different oxidized and reduced phenazines under anoxic conditions and monitored the absorbance spectra before and after mixing (Figures 3A and 3B). Because PYO exhibited the largest changes in absorbance upon reduction, we monitored different mixtures of PYO with PCA or PCN at 690 nm (unique PYO absorbance maximum) starting 1 min after mixing, at which point equilibrium had been achieved. Reactions proceeded as expected from the redox potentials of the phenazines, where PYO was almost completely reduced by the lower potential PCA and PCN, and reduction of PCA and PCN by the higher potential PYO was minimal (Figures 3B and S4A). In addition to establishing that electron transfer can occur between different phenazines, given their similar structures, these results suggest that electron transfer between unbound (physically diffusing) like phenazines (e.g., PYO-PYO electron self-exchange) may also contribute to EET. Moreover, reactions between reduced PCA or PCN and oxidized PYO proceeded faster than oxidation of any of these phenazines by molecular oxygen (Figure 3C), because PYOred appeared in these reactions immediately upon the addition of PCAred or PCNred in the presence of O2, with subsequent oxidation of PYOred by O2 proceeding more slowly. We next wondered whether the presence of DNA would affect the extent of PYO reduction. PCA or PCN fully reduce PYO in the presence of DNA (Figure 3B). Because PCA does not bind DNA, this result suggests electron transfer is occurring in solution between PCA in solution and unbound PYO. For PCN and PYO that both bind DNA, it is also possible that electron transfer is achieved by their unbound counterparts in solution. However, it has long been known that DNA can facilitate electron transfer between bound redox molecules (Genereux and Barton, 2010), motivating us to test whether such a process could also occur within our P. aeruginosa biofilms.
Figure 3. Inter-Phenazine Electron Transfer and DNA Charge Transfer.
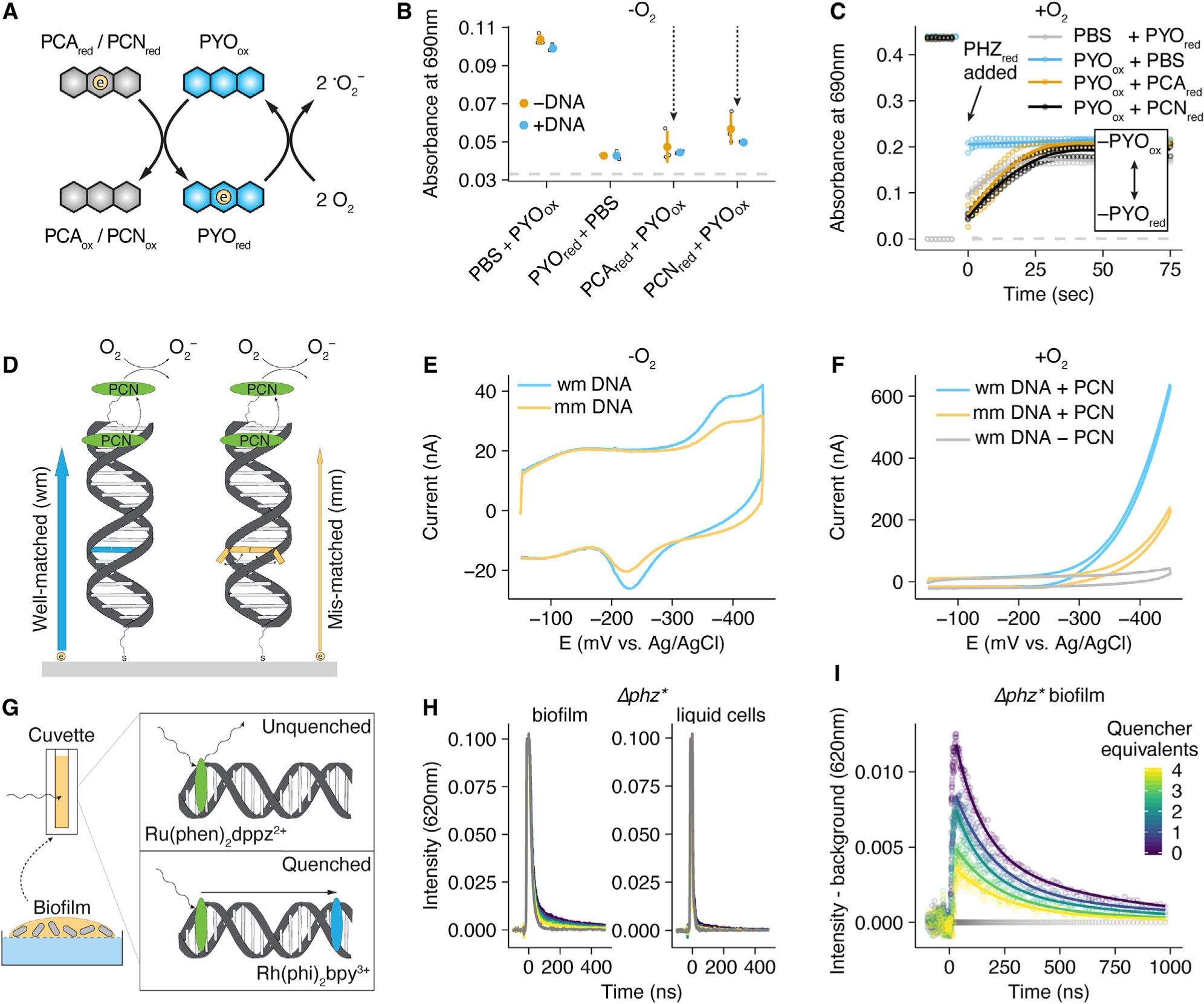
(A) Diagram showing an electron transfer reaction in solution between a reduced phenazine and oxidized PYO and between reduced PYO and molecular oxygen.
(B) Reaction progress after 1 min measured at 690 nm for mixtures of phenazines shown in (A), compared to oxidized and reduced PYO alone. Each reaction was performed in the presence and absence of calf thymus DNA. For each condition n = 3 and error bars are one standard deviation.
(C) PYO oxidation state measured at 690 nm over time (diagnostic for oxidized PYO) for different reactions in the presence of oxygen. Points are individual measurements, lines are loess smoothed for each set of triplicate measurements.
(D) Schematic showing a DNA modified electrode with tethered PCN (green oval) and the expected electron transfer for well-matched duplexes (blue arrow and base pair) and duplexes containing a mismatch (orange arrow and base pair). Mismatched bases are less likely to be in a well stacked position, which is necessary for electron transfer through the DNA π-stack. PCN is shown both intercalated into and outside of DNA to convey its reversible DNA binding.
(E) Representative cyclic voltammetry of the well-matched (wmDNA) and mismatched (mmDNA) constructs shown in (D) under anoxic conditions, acquired at 100 mV/s.
(F) Representative cyclic voltammetry of the well matched, mismatched, or no phenazine constructs under the aerobic conditions described in (D), acquired at 100 mV/s.
(G) Diagram of time resolved spectroscopy of the photoexcited electron donor Ru(phen)2dppz2+ quenched by Rh(phi)2bpy3+ with biofilm eDNA.
(H) Comparison of Ru(phen)2dppz2+ fluorescence in the presence of a concentrated liquid P. aeruginosa culture and a resuspended biofilm containing eDNA. Gray lines show background biological fluorescence before Ru(phen)2dppz2+ was added. The color map is the same as (I).
(I) The background subtracted data from the biofilm panel of (H). The amount of Rh(phi)2bpy3+ is color coded as quencher equivalents relative to Ru(phen)2dppz2+.
Dots are raw data, lines are fit bi-exponential decays.
See also Figure S4 and STAR Methods.
DNA facilitates charge transfer through the π-stacked base pairs (Genereux and Barton, 2010), and recent studies have shown that DNA charge transfer can occur over kilobase distances (Tse et al., 2019). Using single stranded DNA (ssDNA) modified electrodes and hybridizing complementary DNA, a previous study suggested that PYO might be able to participate in electron transfer via DNA (Das et al., 2015). However, this specific method likely produces heterogeneous ssDNA/dsDNA monolayers, and the use of PYO in solution makes possible direct electron transfer between PYO and the electrode, confounding DNA charge transfer signals. To avoid background phenazine-electrode reactions, we revisited these experiments with a carefully controlled dsDNA modified electrode and a covalently tethered phenazine. Because it was not possible to directly synthesize a tethered PYO derivative, we tethered PCN, the other DNA binding phenazine, to a DNA strand (17 base pairs) through a flexible alkane linker (see STAR Methods). We chose to work with the PCN-like tethered phenazine because it was the simplest synthetic route and nearest possible representation of a PA14 phenazine. To form dsDNA, the complementary strand containing a thiol linker (17 base pairs) was annealed to the PCN strand. The gold electrode was modified with the thiolated dsDNA according to standard protocols (Kelley et al., 1997; Slinker et al., 2010, 2011), yielding a packed DNA monolayer where the distal end of each dsDNA molecule contained a tethered PCN that can be either bound (intercalated within dsDNA) or unbound while covalently attached (Figure 3D).
The efficiency of DNA charge transfer depends upon the integrity of the π-stacking of base pairs within the dsDNA (Genereux and Barton, 2010). Mismatched DNA base pairs stack less efficiently, but otherwise do not affect DNA structure, thus providing a convenient perturbation of DNA CT. Therefore, we used cyclic voltammetry to compare electron transfer between PCN and the electrode through well-matched dsDNA monolayers to dsDNAs containing a single base mismatch (Figure 3D). We utilized multiplexed DNA chips to facilitate replicate comparisons between well-matched and mismatched DNA monolayers (Figures S4B–S4D); measurements with a non-intercalating control probe showed that these different monolayers had very similar surface coverages (Figures S4E and S4F) (Slinker et al., 2010). Figure 3E shows that the mismatched construct yielded diminished current in the phenazine redox peak, consistent with the charge transfer being DNA-mediated; the presence of the intervening mismatch inhibits DNA charge transfer. This mismatch effect was consistent across replicate low density (32%–54% decrease) and high density (36%–69% decrease) DNA monolayers (Figure S4C; STAR Methods, “DNA modified electrode controls”). These results displayed mismatch attenuation similar to that observed for well-characterized small molecules shown to stack with the DNA duplex and carry out DNA-mediated charge transfer (Slinker et al., 2011). Strikingly, in the presence of oxygen, a classic voltammetric signal characteristic of electrocatalysis was obtained (Figure 3F) centered on the phenazine redox peak. Hence, the DNA-tethered phenazine is able to accept electrons from the electrode through the DNA π-stack and then reduce oxygen in a catalytic fashion. Together, these results demonstrate that a P. aeruginosa phenazine can participate in DNA charge transfer in vitro.
To test if biofilm eDNA could support DNA charge transfer, we incubated Δphz* colony biofilms suspended in PBS with well-characterized intercalators that can perform DNA charge transfer reactions, which can be monitored by time-resolved spectroscopy (Arkin et al., 1996b). The quenching of photoexcited Ru(phen)2dppz2+ by Rh(phi)2bpy3+ is well-characterized and known to occur by a redox mechanism (Stemp et al., 1995) and not energy transfer (e.g., fluorescence resonance energy transfer [FRET]), with both the forward and back electron transfers occurring predominantly on the picosecond timescale (Figures 3G and S4G) (Arkin et al., 1996a). Both of these intercalators bind to dsDNA more than an order of magnitude more tightly than do phenazines. Moreover, Ru(phen)2dppz2+ is luminescent in aqueous solution when intercalated in dsDNA (or otherwise protected from water), precluding a 2nd order reaction between the complexes in solution (Friedman et al., 1990). Thus, in time-resolved emission experiments on the nanosecond timescale, static quenching, where quenching is fast and occurs without a change in Ru(phen)2dppz2+ lifetime, is consistent with DNA-mediated charge transfer (Arkin et al., 1996b), while slower dynamic quenching, which leads to a change in emission lifetime, is consistent with a slower diffusive process. We first compared the Ru(phen)2dppz2+ signals of liquid grown and biofilm suspensions (of the same optical density) to determine if the signal was specific to eDNA; the ruthenium complex is not expected to be taken up by the cells and bind to genomic DNA on the timescale of this experiment (Figure 3H). We only observed ruthenium luminescence in the presence of the biofilm suspension, consistent with ruthenium luminescence being associated with binding to eDNA. We then examined the pattern of quenching of Ru(phen)2dppz2+ by Rh(phi)2bpy3+. Figure 3I shows static quenching where the intensity of the Ru(phen)2 dppz2+ signal decreases, while the observed decay kinetics are unchanged (Figure S4H). Therefore, we conclude that biofilm eDNA can support rapid DNA charge transfer between these two metal complexes, faster than the timescale for diffusion.
Electrode-Grown Biofilms Retain PYO Capable of Extracellular Electron Transfer
Having investigated phenazine retention and electron transfer separately, we next wanted to establish a system in which we could monitor both of these processes simultaneously in vivo. We took an electrochemical approach to achieve this, growing P. aeruginosa biofilms on interdigitated microelectrode electrode arrays (IDA) (Figure 4A). Biofilms were grown by incubating IDAs in bioelectrochemical reactors with planktonic cultures under oxic conditions and stirring (Figure 4B). After 4 days, with medium replaced daily, mature biofilms were transferred to anoxic reactors with fresh medium for electrochemical measurements. Confocal and SEM imaging revealed that the IDA biofilms were heterogeneous in composition, but consistently contained multicellular structures of live cells and abundant eDNA (Figures 4C–4E, S5A, and S5B). Under these conditions, the cells predominately produced PYO (and some PCA), as measured by LC-MS (Figure S5C). Because PYO was the most tightly binding phenazine in colony biofilms and in vitro, we focused on this phenazine for the remainder of these experiments.
Figure 4. P. aeruginosa Forms Biofilms on Electrodes and Shows PYO-Dependent Conductivity.
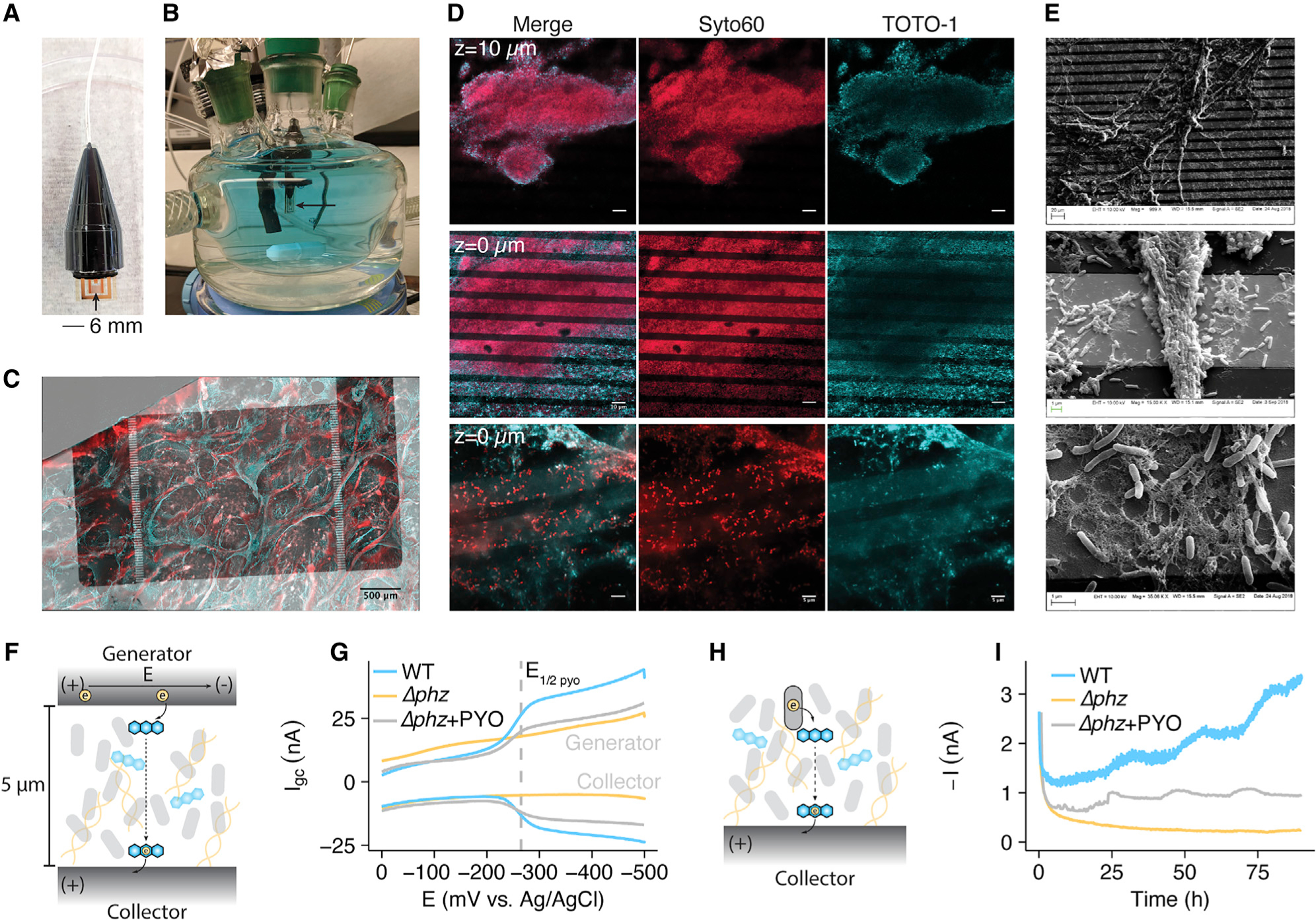
(A) Photograph of a sterile IDA.
(B) Photograph of the growth/electrochemical reactor with a submerged IDA in a fresh medium + PYO solution.
(C) A max intensity projection of a WT IDA biofilm imaged using Syto60 (cell permeable – all DNA), shown as red, and TOTO-1 (cell impermeable – eDNA), shown as cyan. Fluorescence channels are overlaid on a transmitted light channel showing the opaque gold regions of the electrode in gray scale.
(D) Fluorescence microscopy of IDA biofilms with the same dyes as in (C). Top: a 63× confocal image of a Δphz* IDA biofilm from a z stack 10 μm above the electrode surface (scale bar = 10 μm). Middle: a confocal image from the same z stack as top, but at the electrode surface (scale bar = 10 μm). Bottom: a confocal slice of a different Δphz* biofilm from a 63× zoom with Airyscan, showing single live cells and eDNA on the electrode surface (scale bar = 5 μm).
(E) SEM images at increasing magnification showing cells and extracellular matrix on the IDA electrode bands.
(F) Diagram showing how a generator-collector (GC) two electrode system can generate current through PYO reduction.
(G) GC data is displayed as the current at each electrode versus the generator potential, (E). Representative measurements are shown for WT, Δphz, and Δphz + PYO biofilms.
(H) Diagram showing how cells generate metabolism dependent current through phenazine reduction.
(I) Metabolic current described in (H) is measured by chronoamperometry for Δphz and WT biofilms over several days. Representative data shown. See also Figure S5.
Originally used to measure conductivity of abiotic materials, the IDA has a 2-working electrode geometry and recently was adapted to study EET through microbial biofilms (Xu et al., 2018; Yates et al., 2015; Boyd et al., 2015; Snider et al., 2012). Measurements are made by driving electron transfer between the two electrode bands across a 5-mm gap (Figure 4F), resulting in EET that is decoupled from the cells’ metabolic activity. Specifically, we used a generator-collector (GC) strategy to measure EET through the biofilm, where the “generator” electrode is swept from an oxidizing potential (E = 0 mV versus Ag/AgCl) to a reducing potential (E = −500 mV), while the “collector” electrode maintains a fixed oxidizing potential (E = 0 mV). In this GC arrangement, electron transfer into the biofilm from the generator occurs as the potential of the generator is swept negatively, reducing PYO (E1/2 = −250 mV) at the biofilm/generator interface. Electrons are conducted across the gap through the biofilm due to EET, either by physical diffusion of PYO or other mechanisms. The electron transfer at both the generator and collector is measured as current (Igc) and plotted against the potential of the generator electrode. Implicit in generating a sustained Igc is recycling of the redox molecules supporting current. Figure 4G shows that WT and Δphz + PYO biofilms supported current (above background) across the 5-μm gap over a ~3 min scan as the generator potential approached PYO’s redox potential (−250 mV versus Ag/AgCl), consistent with PYO-mediated EET; significantly, Δphz alone did not. Dependency of current on the generator potential observed here is consistent with PYO-mediated EET being a diffusive process. Reduction of PYO at the generator and oxidation of PYO at the collector generates a redox gradient that drives EET through the intervening biofilm, resulting in current centered on the PYO midpoint potential that saturates at strongly reducing generator potentials (Snider et al., 2012). Here, the diffusive nature of EET would reflect either physical diffusion of PYO through the biofilm (reduced PYO from the generator to collector, oxidized PYO from the collector to the generator) or the effective diffusion of electrons through bound PYO (Strycharz-Glaven et al., 2011).
To test whether these short-term GC measurements of biofilm EET indicate long-term ability to support metabolic activity, we poised the IDA electrodes at an oxidizing potential (+100 mV versus Ag/AgCl) and monitored the current produced by the biofilm over 4 days in the presence of 40 mM succinate as the electron donor for cellular metabolism under anoxic conditions. In this configuration, the observed current would be due to cellular oxidation of succinate coupled with PYO-catalyzed EET to the poised electrode, where the electrode acts as the terminal electron acceptor instead of oxygen (Figure 4H). Figure 4I shows that both WT biofilms relying solely on endogenous PYO and Δphz biofilms + exogenous PYO generate robust current over many days, whereas Δphz alone does not. The daily periodic rise in current likely reflects the impact of slight temperature or light fluctuations in the room on the cells’ metabolic activity over the course of the experiment (Kahl et al., 2016, 2019). Differences in current magnitude between the WT and Δphz + PYO runs are likely due to stochastic differences in PYO abundance in the biofilms and/or efficiency of cellular PYO reduction. Together, these results demonstrate that the IDA biofilms can use retained PYO for EET to support metabolic activity. Although we focus on phenazine-mediated EET, the small current observed for Δphz biofilms could indicate that there are also phenazine-independent mechanisms of EET perhaps mediated by conductive pili, flagella, or other metabolites (Maruthupandy et al., 2015; Liu et al., 2019).
To determine whether our IDA biofilms retained phenazines in the same manner as colony biofilms, Δphz* IDA biofilms were soaked in PYO in one reactor and then transferred to another reactor with fresh medium lacking PYO (Figure 5A). Comparing GC scans of a biofilm in the soak and transfer reactors showed that PYO was clearly retained on this timescale and supported biofilm conductivity (Figure 5B). In contrast, GC scans of a Δphz* biofilm soaked in PCA showed a signal that became immediately undetectable in the transfer reactor (Figure 5C). Therefore, PCA rapidly diffused out of the biofilm, as expected because it does not bind DNA. The equilibration of PYO (from the IDA biofilm) with the fresh medium was monitored in more detail by square wave voltammetry (SWV) (Figure 5D), for which peak current (Iswv) is proportional to the concentration of the PYO remaining in the biofilm at each time interval (Bard et al., 1980). Thus, the rate of decay of Iswv provides a means to assess the loss rate of PYO from the biofilm, a measure of how long PYO is retained. Upon transfer, the biofilm PYO peak current (Iswv) decays in 30–45 min, whereas for a blank IDA (no biofilm) dipped in PYO, Iswv decays in 2–3 min (Figure 5G). Thus, like colony biofilms, IDA biofilms appear to retain PYO but not PCA, and use retained PYO for phenazine EET metabolism in vivo.
Figure 5. PYO-Mediated Electron Transfer Is Faster Than PYO Loss.
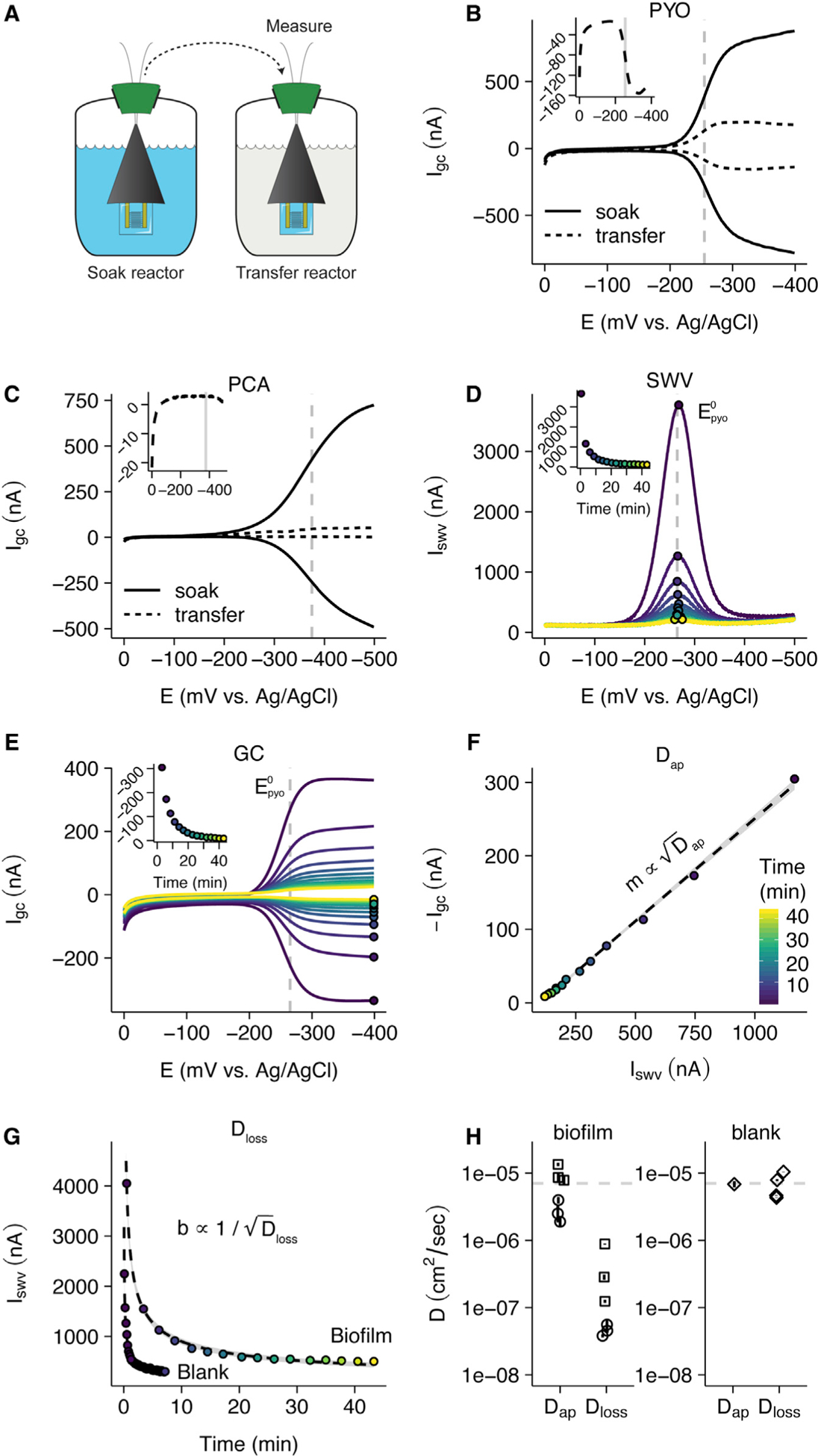
(A) Schematic showing how measurements are made to maximize biofilm-specific PYO signals by transferring IDAs to fresh medium. PYO is shown in blue.
(B) Representative generator collector measurements of Δphz* biofilms soaked with PYO. Measurements were acquired during the soak and immediately following transfer to a reactor with fresh medium. Inset shows a zoomed in view of transfer data.
(C) Same measurements as (B), except the Δphz* biofilm was soaked in PCA. The inset figure shows the collector current with more detail.
(D) Repeated SWV measurements were taken over time as the Δphz* + PYO biofilm equilibrates with the solution in the transfer reactor. Points show peak current within each scan and inset shows the quantified peak currents versus time. The color map is the same for (D)–(G).
(E) Repeated GC measurements were taken concurrently with SWV measurements over time as the same biofilm equilibrates. Points and inset are same as in (D).
(F) Plot of the peak GC versus peak SWV currents fit with a line (gray area is 95% confidence interval).
(G) Comparison of SWV signal over time in the transfer reactor from IDAs with or without a biofilm. Data are fit with a 1D diffusion model, as discussed in the STAR Methods section Dloss measurement theory. Dashed lines are best fit models and gray regions show 95% confidence intervals.
(H) Measurements of Dap and Dloss for two Δphz* biofilm IDAs soaked in 75 μM PYO (shown as sets of open circles and squares) and a blank IDA soaked in different concentrations of PYO (open diamonds). A parameter for calculating Dloss (scan time ts) was solved by assuming that Dap = Dloss for the blank IDA (see STAR Methods section Dloss model assumptions). Error bars for Dap are 95% confidence intervals from the linear fit. Error bars for Dloss assume I0 was the peak current in the soak reactor and 95% confidence interval intervals for each technical replicate were determined from the nonlinear fit for Dloss. The dotted line is at 7 × 10−6 cm2/s, the measured D for the similarly sized small molecule, caffeine (Niesner and Heintz, 2000). See also Figures S5, S6, and S7 and STAR Methods.
Electron Transfer through Biofilms Is Faster Than PYO Loss
Next, we sought to understand the efficiency of PYO-mediated EET in the biofilm. We reasoned that a determination of “efficiency” would compare the rate of electron transfer (that supports the metabolism of the O2 limited cells) to the loss rate of PYO from the biofilm (that limits the utility of each PYO molecule). These two processes can both be described by diffusion coefficients, so in a single electrochemical experiment, we measured the apparent diffusion coefficient for the PYO-mediated EET, Dap, which characterizes all of the redox processes with the electrode, and the diffusion coefficient for PYO as it is lost from the biofilm, Dloss.
We determined Dap for EET by PYO in an IDA Δphz* biofilm to avoid confounding PYO retention with production, although WT biofilms with endogenous PYO yielded similar results (Figures S6A and S6B). Each Δphz* biofilm was soaked with PYO in one reactor then transferred to a second reactor lacking PYO (Figure 5A), and the equilibration of the biofilm PYO into the fresh medium was monitored by paired SWV and GC measurements over time (Figures 5D and 5E). Noting that Iswv is proportional to , where C is the effective PYO concentration (Bard et al., 1980), whereas Igc is proportional to C * Dap (Strycharz-Glaven et al., 2011), plotting Igc versus Iswv for each time point is expected to yield a linear dependency with a slope (m) proportional to (Figure 5F) when Dap is independent of the concentration of PYO in the biofilm (Akhoury et al., 2013; White et al., 1982). In this manner, it is possible to measure Dap for PYO remaining in the biofilm at any given instance when its concentration is unknown.
Applying this approach, for two biological replicates of Δphz* biofilms (three technical replicates each), we found that the mean Dap for PYO is 6.4 × 10−6 cm2/s over a wide range of PYO biofilm concentrations (Figures 5H and S6C). To validate our approach, we measured Dap using a blank IDA with no biofilm, only solution PYO for which we expect Dap ≈ Dloss. Using known PYO concentrations with a blank IDA (no biofilm) we obtained nearly identical estimates (Dap ≈ 6.8 × 10−6 cm2/s) using our Igc versus Iswv method (concentration unknown) or established methods (Igc versus [PYO] and Iswv versus [PYO]) that depend on known concentrations (Figures 5H and S6D–S6G; STAR Methods section Dap measurement theory) (Bard et al., 1980). We further validated our measurement scheme by comparing it to an established chronocoulometry technique with two other redox molecules (hexammineruthenium(III) and ferrocenemethanol) in the presence and absence of the polymer Nafion. In all cases, estimates of Dap by the two methods were within ~2× (Figure S6H).
To estimate the upper limit for Dloss of PYO lost from P. aeruginosa biofilms, we applied a semi-infinite one-dimensional diffusion model (STAR Methods, Dloss measurement theory) to fit the decay of the same Iswv measurements used above (Figures 5D and 5G). Although each SWV scan depends on Dap, the decay process of Iswv results from loss of PYO out of the biofilm (Dloss). This calculation requires a scan time constant, whose value we constrain by using the blank control where we assume Dap = Dloss, allowing us to solve for this constant (Figure S7A; STAR Methods, section Dloss model assumptions). For Δphz* biofilms, this diffusion model yields a mean Dloss of 2.0 × 10−7 cm2/s (Figure S7B). Hence, Dap for PYO-mediated biofilm EET is more than 25-fold higher than Dloss (Figure 5H). Although this model simplifies the actual physical system, it provides a means to estimate an upper limit for the rate of PYO loss from the biofilm. Such a low Dloss is consistent with the relatively long time it takes for Iswv to decay when transferred to fresh medium for PYO in a biofilm (~45 min) compared to PYO for a blank IDA (<2 min) (Figure 5G) or to PCA in a biofilm (<1 min) (Figure 5C). Moreover, the conservative assumptions of our model make it likely that we have underestimated the true difference between Dloss and Dap in the biofilm. Collectively, these observations support the idea that PYO EET occurs rapidly compared to the loss of PYO diffusing out of the IDA biofilms.
DISCUSSION
The redox activity of phenazine metabolites produced by P. aeruginosa has intrigued researchers since the 1930s (Friedheim, 1934), and over the last 20 years a model has emerged that links phenazine electron transfer to biofilm metabolism (Hernandez and Newman, 2001; Jo et al., 2017; Dietrich et al., 2013). Using the well-characterized P. aeruginosa-phenazine system as a model for studying EET mediated by extracellular electron shuttles, here we addressed the conundrum of how phenazines complete their redox cycle within the biofilm matrix without being lost to the environment. Our results point to eDNA as being a critical component of the matrix that facilitates phenazine retention and redox cycling for EET.
Quantifying phenazine retention in a simple biofilm system was our first goal. Although past work has measured phenazines in culture supernatants and in agar underlying colony biofilms (Bellin et al., 2014, 2016; Dietrich et al., 2006), phenazine concentrations within any type of biofilm system were unknown. We found that the degree of retention varied dramatically between the three studied phenazines in colony biofilms, with PCN and PYO being strongly enriched in the biofilm, in contrast to PCA, which readily diffuses away (Figure 1). Further, only PYO complemented the wrinkled morphology of the Δphz* mutant, showing that this retained PYO is used for phenazine EET to support anoxic biofilm metabolism in vivo (Figure S1A). We observed a similar trend for biofilms grown in liquid medium attaching to IDAs, where PCA was not retained, but PYO was retained and supported biofilm conductivity and metabolism in vivo (Figures 4 and 5).
Notably, oxidized PCN and PYO bind ds DNA in vitro, and perturbing extracellular DNA binding sites disrupted PYO retention (and PCN to a lesser extent) in vivo (Figure 2). Previous studies have shown PYO actually promotes eDNA release via cell lysis (Das and Manefield, 2012; Meirelles and Newman, 2018), so PYO retention by eDNA suggests a connection between eDNA production and utilization. Although certain enzymes and cytochromes are known to bind extracellular polysaccharides (Tielen et al., 2013; Rollefson et al., 2011), to our knowledge, PYO retention in eDNA is the first example of an electron shuttling small molecule being bound by the extracellular matrix of a biofilm. Moreover, that such binding underpins PYO-mediated biofilm EET is an important new result. Because eDNA is found in biofilms made by diverse species and many small molecules exhibit DNA binding capacity, our results may be broadly relevant to diverse biofilm functions involving extracellular metabolites.
Recognizing that a primary function for phenazines is extracellular electron transfer, we characterized this process in vivo. Our IDA experiments suggest that PYO simultaneously can be retained (low Dloss) and facilitate fast EET (high Dap), establishing the efficiency of this process (Figure 5). To understand the mechanism that underpins this efficient EET, we can interpret our results in the context of electron transfer theory from the study of redox polymers (Dalton and Murray, 1990). This theory holds that the measured Dap is the sum of the effective diffusion coef-ficient of electrons due to self-exchange reactions among bound shuttles (De) and the physical diffusion coefficient (Dphys) of any unbound shuttles (Dap = D e + D phys). In this context, we can explain our electrochemical results that biofilm PYO Dap (~6.4 × 10−6 cm2/s) is higher than biofilm PYO Dloss (~2.0 × 10−7 cm2/s), and similar to the blank IDA (solution PYO) D values (~6.8 × 10−6 cm2/s) in two ways (Figure 5H). First, if we assume our measured Dloss is the same as PYO Dphys within the biofilm, the difference between Dap and Dphys can be explained by De. Our in vitro data suggest that such self-exchange reactions (De) could be DNA-mediated. In the case of very rapid electron transfer among eDNA-bound PYO, Dap would still be limited by counter ion diffusion, consistent with a measured Dap similar to that of a small molecule in solution (~7 × 10−6 cm2/s) (White et al., 1982). Alternatively, the measured loss of PYO from the biofilm, Dloss, may not accurately reflect physical diffusion of PYO within the biofilm, Dphys. Because the IDA biofilms do not completely cover the electrodes, PYO may be able to physically diffuse in solution adjacent to them. In this case, diffusion in solution would be consistent with the measured Dap, and the low Dloss measurement would reflect the slow release of PYO from the IDA biofilm due to its retention by eDNA. Regardless, the striking measured difference between Dap and Dloss indicates that PYO electron transfer promotes efficient biofilm EET metabolism because electron transfer occurs rapidly, while loss of PYO to the environment is slow due to its retention by eDNA.
Together, our results allow us to refine our model for how phenazine EET may operate within biofilms (Figure 6). Intriguingly, PYO biosynthesis requires O2, whereas PCN and PCA do not, and electrochemical imaging underneath colony biofilms has shown lower potential phenazines in the anoxic interior (PCA and PCN) and the higher potential phenazine (PYO) near the oxic periphery (Bellin et al., 2014, 2016). Reduced PYO is also known to react with oxygen significantly faster than PCA and PCN (Wang and Newman, 2008). As such, it is tempting to speculate that phenazines are ordered in the biofilm matrix in a sequence akin to a large extracellular electron transport chain—from reduced PCA/PCN in the anoxic interior, to eDNA bound PYO at the oxic periphery, and ultimately to molecular oxygen (Figure 6A). How might phenazines exchange electrons within this framework? Noting that the heterogeneity of the biofilm matrix makes it possible that different electron transfer pathways could occur in these complex systems, we favor two mechanisms mediated by eDNA for how phenazine EET may operate in the matrix (Figures 6B and 6C). Both mechanisms assume that reduced PCA and PCN will diffuse from the anoxic zone to the oxic zone (i), where PYOox is bound to eDNA. In one model (Figure 6B), PYO’s binding equilibrium will result in some PYOox un-binding from the eDNA, allowing it to react with PCAred or PCNred (ii). PYO red then reacts with oxygen generating PYOox (iii), which rebinds DNA. In the other model (Figure 6C), reduced phenazines (likely PCN) intercalate into eDNA and reduce PYOox via DNA charge transfer (ii). PYO red unbinds DNA, reacts with oxygen (iii), and PYOox rebinds DNA. Given that PCAred and PCNred react more quickly with PYOox than O2, then both models would facilitate the reoxidation of the interior phenazines and promote diffusion back toward the anoxic interior (iv). These models integrate what is known about phenazine O2 reactivities, redox potentials and biosynthesis zones, and help explain how PYO and eDNA interactions enhance EET in P. aeruginosa biofilms by facilitating retention and electron transfer. Testing these models in physiochemically well-defined synthetic matrixes in addition to complex biofilms represents an exciting challenge for future research.
Figure 6. Proposed Models of Phenazine Electron Transfer and Retention.
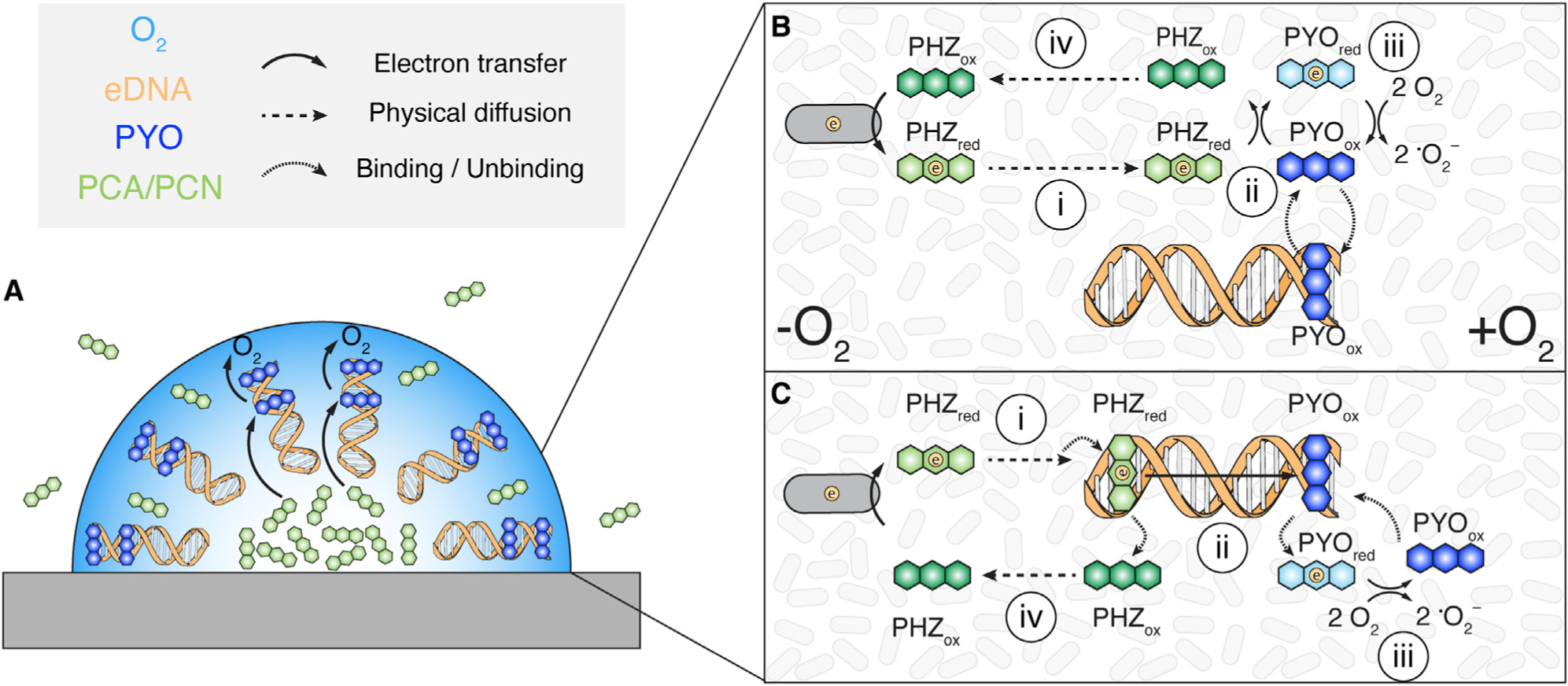
(A) Overview of phenazine electron transport to oxygen in the biofilm. Note the DNA molecules are drawn radially for explanatory purposes only. (B) and (C) show proposed models for how the scheme shown in (A) occurs.
(B and C) The top model shows electron transfer in solution (B), and the bottom model shows electron transfer via DNA charge transfer (C). In both models oxidized PYO is mostly bound and retained in the oxic region of the biofilm. PCN and PCA (PHZ) are reduced in the anoxic region of the biofilm and diffuse outward (i). The reduced phenazine reduces oxidized PYO (ii). Reduced PYO then reacts with molecular oxygen (iii), and the oxidized PYO re-equilibrates with the DNA binding sites. This facilitates the re-oxidation of PCA and PCN, allowing some diffusion back toward the interior (iv).
In conclusion, our findings illustrate how eDNA may provide a mechanism to resolve how otherwise diffusible extracellular electron shuttles can catalyze efficient EET in real world, open systems. Beyond serving as a structural support, carbon source, or genetic reservoir, our studies reveal that interactions between extracellular electron shuttles and eDNA in biofilms underpin their metabolic vitality. It is noteworthy that eDNA is abundant in many biofilms (Flemming and Wingender, 2010) and diverse biofilm-forming bacteria have the potential to produce extracellular electron shuttles (Glasser et al., 2017a). Accordingly, eDNA retention of these electron shuttles—and perhaps of other biologically useful molecules—may represent a widespread strategy whereby a reactive extracellular matrix supports bacterial biofilms in unexpected and physiologically significant ways.
STAR★METHODS
RESOURCE AVAILABILITY
Lead Contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the Lead Contact, Dianne Newman (dkn@caltech.edu).
Materials Availability
All bacterial strains are available upon request.
Data and Code Availability
All code used for data processing and analysis is available in a GitHub repository (https://github.com/DKN-lab/phz_eDNA_2019). Html versions of the Rmarkdown notebooks are also available as a website (https://DKN-lab.github.io/phz_eDNA_2019). Any raw data not included in the repository, such as files in proprietary formats or large imaging files, are available upon request.
EXPERIMENTAL MODEL AND SUBJECT DETAILS
All Pseudomonas aeruginosa UCBPP-PA14 strains were plated on LB agar from −80°C glycerol stocks and grown overnight at 37°C. Plates were stored at −4°C for up to a week and were used to inoculate liquid cultures. Unless otherwise noted, liquid cultures were grown in 3 mL of LB medium in glass culture tubes (VWR #47729–583) in an orbital shaker (New Brunswick, Innova 44) at 37°C shaking at 250 rpm. Colony biofilms were grown on 1% tryptone (BD #211705), 1% agar (BD #214010) in 6-well plates (Fisher Scientific #08-772-49) at room temperature (~22°C) in the dark for 3 to 4 days. IDA biofilms were grown in a minimal medium (MM) with succinate as the carbon source (14.15 mM KH2PO4, 38.85 mM K2HPO4, 42.8 mM NaCl, 9.3 mM NH4Cl, 40 mM Na-succinate, adjusted to pH 7.2 and autoclaved then 1x SL-10 trace element solution (Atlas, 2004) and 1 mM MgSO4). From a stationary phase 3 mL MM liquid culture, the bioelectrochemical reactors with 180 mL MM were inoculated to an OD500 of 0.005. The medium was exchanged every 24 hours, and biofilms were grown on the IDA for 3 or 4 days in this fashion. During growth, constant temperature was maintained at ~31°C, reactors were stirred at 250 rpm (VWR #58948–138) and air was bubbled into two reactors at a time with a small aquarium bubbler (Tetra #77851).
METHOD DETAILS
General
Chemicals were obtained from commercial sources (Sigma Aldrich, Fisher Scientific, VWR, and New England Biolabs) and used without further purification unless otherwise specified. All solutions were made with Milli-Q water (> 18 MΩ cm). Phosphate buffered saline (PBS) used for resuspending cells or contacting biofilms (137mM NaCl, 10mM PO4 (1.44 g / L Na2HPO4, 0.24 g / L KH2PO4), 2.7mM KCl, pH 7.2) was different than PBS used for chemical stocks and in vitro assays (10mM PO4 (0.952 g/L Na2HPO4, 0.592 g/L NaH2PO4), 50mM NaCl, pH 7.0).
PCA was synthesized by Dr. Stuart Conway’s Lab and was a gift. PCN was obtained from Princeton Biochem (#PBMR030086). PYO was synthesized by shining light on phenazine methosulfate (VWR #AAH56718–06) and purified with repeated organic (dichloromethane) and acid (HCl) extractions as described previously (Costa et al., 2017). PYO was further purified by reverse phase HPLC or repeated hexane precipitations. Stocks of PYO were either made in PBS (pH 7.0) to 1 mM or in 20mM HCl to 10 mM and quantified by absorbance at 690 nm with the extinction coefficient ε690 = 4306 M−1 cm−1. Stocks of PCA were made in PBS to 1–2 mM or in 10 mM NaOH to 10 mM. Stocks of PCN were made in DMSO to 20 mM.
Colony Biofilms
Growth
Colony biofilms were grown on 1% tryptone (BD #211705), 1% agar (BD #214010) in 6-well plates (Fisher Scientific #08-772-49) at room temperature (~22°C) in the dark for 3 to 4 days. The medium was autoclaved and cooled to 60°C, then in a biosafety cabinet (The Baker Company #SG603A-HE) 5mL of molten agar was transferred into each well of the 6-well plate and left to solidify for 60min with no lid and constant airflow. When phenazines were added to the medium, concentrated stocks were added first, then the medium, and then the wells were stirred with sterile pipettes until uniform. Membrane filters (Sigma-Aldrich # WHA110606) were gently placed shiny side down onto the center of each agar well, and colonies were inoculated by pipetting 10μL of stationary phase culture (from LB liquid) onto the center of the membranes.
For DNase treated biofilms, after 3 days of growth membranes/biofilms were transferred to fresh agar wells containing 20uL of DNase 1 (2 Kunitz units / μL) (Sigma-Aldrich #D4263) spotted directly underneath the biofilm. Biofilms then grew for another 24 hr. DNase was prepared in NEB Buffer 4 or water.
Imaging
All colony biofilms were imaged in the 6 well plates at 20x zoom with a Keyence digital microscope (VHX-600) before extraction. Colonies were stained for confocal imaging by growing them with 1 μM TOTO-1 and 10 μM Syto60 in the underlying agar. Biofilms on their membranes were transferred to imaging dishes. High magnification images were taken by gently dropping coverslips (#1.5) onto the top surface of the colony and imaging through the coverslip with an upright confocal microscope, as described below (IDA biofilms – Fluorescence imaging). Colony biofilms on membranes were fixed for scanning electron microscopy (SEM) by floating the membrane on a paraformaldehyde solution and then submerging. Upon submersion, the colony came off of the membrane mostly intact, leaving only a thin layer of cells fixed to the membrane. See SEM imaging section below for further details.
Extraction
Membranes, with the colony biofilms stably attached, were lifted off the agar with fine tweezers and carefully placed in microfuge tubes containing 800μL or 1mL of PBS. For colonies that were smaller in diameter than the microfuge tube, the membrane was placed upside down directly on top of the open tube, so that the colony sat above the PBS hanging from the membrane. Then, the colony was gently pushed down into the tube, by pushing with tweezers from the center. If the colony diameter was greater than the tube the membrane/biofilm was carefully held with the tweezers and allowed to flop over. While the membrane/biofilm was curved over it was gently threaded into the tube. If any part of the colony touched the rim of the tube, the tweezers were used to scrape as much as possible into the inside.
Once the membrane/biofilm was in the tube, each tube was vortexed (Fisher Scientific #12–812) at maximum speed for 1 min, after which the vast majority of the biofilm was resuspended in the liquid and no longer associated with the membrane. The membrane was removed from each tube with tweezers. The biofilm suspension was then centrifuged (Eppendorf #5418) at 6000 rcf. for 5min. The supernatant was removed and immediately prepared for LC-MS or frozen at −20°C. This process was done for many biofilms at once, so biofilms typically sat for 30–60min in PBS from the time the membrane was first added to the time the samples were centrifuged.
Biofilm volumes were estimated by comparing biofilm suspension volumes to controls that only had bare membranes added and removed. Volumes were measured with a p200 pipette. Significant variation was observed in volume measurements of replicate biofilms that looked identical, so the mean WT colony volume of 60μL was used to normalize all colony measurements, although there are likely subtle volume differences between the differently treated colonies/strains.
The 6-well agar plates were extracted, by adding 2 or 3mL of PBS to the 5mL of agar. The agar was scarred with a pipette tip to facilitate agar/liquid exchange. The 6 well plates were left on an orbital shaker at 250rpm (Ika #KS-260) for 6 hours, which was determined to be optimal. Then, 1mL of the liquid from each well was transferred to a microfuge tube and processed for LC-MS or frozen at −20°C.
For the sonication experiment, resuspended biofilms were treated for 1 min on ice (Fisher Scientific, Sonic Dismembrator 550), then processed normally. CFU counts showed that > 65% of cells died. For the no-membrane experiment, 3mL of PBS were added directly to the wells containing the biofilm. The biofilm was quickly resuspended in the liquid using a cell scraper and 1mL of liquid was saved, while the rest was removed. Then the agar was extracted as normal.
LC-MS
Samples were filtered with 0.2 mm spin filters (Corning #8160) and transferred to sample vials (Waters #600000668CV) and loaded into the autosampler at 10°C. Samples were run on a Waters LC-MS system (Waters e2695 Separations Module, 2998 PDA Detector, QDA Detector). 10μL of each sample was injected onto a reverse phase C-18 column (XBridge #186006035) running a gradient of 100% H2O + 0.04% NH4OH to 70% acetonitrile + 0.04% NH4OH over 11 min (run times were 20min total). UV-Vis and positive MS scans were acquired for each run. PCA, PCN, and PYO were distinguished by retention time (~3 min, 6 min and 8.85 min respectively), detected at 364 nm (PCA and PCN) or 313 nm (PYO), and manually verified by examining masses 225.2 (PCA), 224.2 (PCN) and 210.24 (PYO). Peaks were automatically identified in the UV-Vis channels by retention time, quantified using the apex track algorithm, converted to concentrations using standard curves (> 6 points from 100 nM to 100 μM), and then exported as text files from the Empower software.
eDNA measurements
Resuspended colony biofilms were assayed for eDNA by measuring TOTO-1 fluorescence on a Tecan Spark 10M plate reader in black 96 well plates. Wells were prepared with 65 μL PBS and 10 μL of TOTO-1 (10 μM stock, 1 μM final) and 25 μL of biofilm suspension or calf thymus DNA was added to each well and gently mixed by pipetting. TOTO-1 fluorescence was monitored with 480 nm excitation and 535 nm emission after ~20 minutes of incubation at room temperature. Colony biofilms were measured with six biological replicates and technical triplicates. A standard curve was obtained by diluting a stock of calf thymus DNA twofold serially, yielding measurements from 500 μg / mL to 7 ng / mL.
DNA binding assays
Phenazine stocks were made in PBS (pH 7.0 10mM PO4, 50mM NaCl) to 1mM (PCA, PYO) or 500 μM (PCN with 5% DMSO). All assays were performed in the same PBS buffer unless otherwise noted. Complementary 29 base pair oligos from IDT were annealed (cooled incrementally over 1 hour following denaturation) to form the ds DNA for ITC and the ethidium displacement assays (see Table S2 for sequences). Ds DNA for MST was prepared by PCR amplifying an 80 base pair region from P. aeruginosa genomic DNA using the primers listed in Table S2. One of the primers contained a Cy3 fluorophore for the MST readout.
Isothermal titration calorimetry (ITC)
ITC was performed with a MicroCal ITC200 (Malvern). Phenazine stocks were loaded directly into the ITC syringe. The cell was loaded with 10 to 50 μM ds DNA in PBS (or PBS + 5% DMSO for PCN). Results shown are representative of replicates taken at various ds DNA concentrations. Thermograms were recorded at 21°C with stirring at 300rpm and reference power 2. There were 13 injections of 3.2μL volume spaced by 240 s with 600 s of settle time.
Thermograms were integrated and baseline corrected in the Origin software. Peak integrations were then loaded into the GUI version of pytc (Duvvuri et al., 2018) with the default 0.1 units of uncertainty added to each measurement. Binding curves were plotted with the molar ratio phenazine / oligomer. The data were fit with the Bayesian model and parameter estimates with confidence intervals were generated. The K value was converted to a dissociation constant by taking the inverse and converted from the oligomer concentration to base-pair concentration by multiplying by 29 base pair / fraction competent (f_x = 1.6 for PYO and 1.3 for PCN).
Ethidium bromide displacement
In black 96 well plates (Nunc #237105) 5 μM ethidium bromide (EtBr) was prepared in wells with increasing concentrations of PCA, PCN, or PYO. Fluorescence readings were taken on a Tecan Spark 10M plate reader with excitation 480 nm and emission 600 nm. Then a small volume of 29 base pair ds DNA (prepared the same as for ITC) was added to a final concentration of 1 μM. The plates were mixed with rotary shaking and incubated at room temperature for at least 5 min protected from light. Then fluorescence was read again, and the bound ethidium signal was calculated by subtracting the pre-DNA reading from the post-DNA reading. IC50 was calculated by fitting the data to the hill equation. IC50 was converted into Ki with the equation Ki = IC50 / (1 + [EtBr] / Kd), using an empirical Kd for ethidium (under the same conditions) of 1 μM.
Microscale thermophoresis (MST)
Microscale thermophoresis was performed with a NanoTemper Monolith instrument following the manufacturer’s protocol (capillaries - NanoTemper #MO-K022). Briefly, capillary solutions were prepared with 50 nM ds DNA and two-fold dilutions of phenazines starting at concentrations greater than or equal to 1mM. Thermophoresis was performed at an ambient temperature of 22.5°C, with the thermorphoresis laser power 40% and the fluorescence excitation laser at 20% power. Thermophoresis was recorded for 30 s and evaluated using the T-jump strategy. Fluorescence peak shapes along the x axis of each capillary were very uniform and peak intensity did not vary meaningfully between phenazine concentrations. Kd was calculated by fitting the quantified data to a hill equation. Results shown are representative of multiple MST runs with varied settings.
Endogenous fluorescence of reduced phenazines
The endogenous fluorescence of reduced phenazines was measured with different concentrations of calf thymus DNA with a BioTek Synergy 4 plate reader placed in an anaerobic chamber. Reduced phenazines (100 μM solutions) were prepared by bulk electrolysis of oxidized phenazine solutions in electrochemical chambers described previously (Wang et al., 2010). The solutions were transferred into stoppered serum bottles and moved to the anaerobic chamber containing the plate reader. 135μL reduced phenazine was incubated with 15μL PBS containing different amounts of calf thymus DNA, thus each well contained 90 μM phenazine. Fluorescence was monitored in a black 96 well plate with filter cubes to control excitation and emission wavelengths. PYO was excited using a 360 nm light and emission was monitored at 460 nm. For PCA and PCN excitation was at 485 nm and emission was at 528 nm.
Phenazine electron transfer in vitro
Electron transfer reactions between phenazines under anoxic conditions were monitored in the anaerobic plate reader described above. Reduced phenazines (described above) were mixed with oxidized phenazines (67.5 μL each) and 15 μL PBS with or without DNA (2 mg / mL) to yield mixtures containing 45 μM of each phenazine. Reactions in clear bottom, black walled 96 well plates were measured at 690 nm absorbance approximately one minute following mixing. For each well, an absorbance scan and fluorescence measurements (described above) were also taken, and these data matched the results obtained at 690 nm. Time series (2.5 min) of absorbance and fluorescence measurements were taken for each well, but no changes were observed, indicating the reactions had reached equilibrium.
Phenazine redox reactions were monitored over time under oxic conditions with an aerobic Beckman Coulter DU 800 spectrophotometer. The instrument was blanked with PBS. Plastic cuvettes were filled with 500 μL PBS (oxic) or PYOox and placed inside the instrument and the lid was shut. Reduced phenazine or anoxic PBS was drawn (~500 μL) from stoppered serum bottles (anoxic) into needled 1 mL syringes. Absorbance measurements at 690 nm (1.5 s intervals) were started on the cuvette and proceeded for 10 s to acquire baseline values. Then the lid was opened, and the syringe was quickly emptied into the cuvette and the lid was closed again. The measurement proceeded until 90 s had elapsed. Reactions were repeated in triplicate.
DNA modified electrodes
Preparation of thiol-modified DNA strand
A single-stranded DNA sequence with the 5 end modified with a C6 S-S phosphoramidite was purchased from Integrated DNA Technologies (see Table S1 for DNA sequences). The oligonucleotide was reduced using dithiothreitol (DTT, Sigma Aldrich, 100 mM) in a buffer solution (50 mM Tris-HCl, pH 8.4, 50 mM NaCl) for 2 h. The reduced thiol-modified DNA was then purified by size exclusion chromatography (Nap5 Sephadex, G-25, GE Healthcare) with phosphate buffer (pH 7.0, 5 mM NaH2PO4, 50 mM NaCl) as the eluent. Subsequently, high pressure liquid chromatography (HPLC, HP 1100, Agilent) was performed using a reverse-phase PLRP-S column (Agilent) using a gradient of acetonitrile and 50 mM ammonium acetate (5%–15% ammonium acetate over 35 minutes). After HPLC purification, the thiol-modified ss DNA was characterized using matrix-assisted laser desorption ionization (MALDI) characterization using a Autoflex MALDI TOF/TOF (Bruker), and quantified using a 100 Bio UV-visible spectrophotometer (Cary, Agilent). Another ss DNA strand for installing a CC mismatch near the interface between DNA duplexes and self-assembled monolayer (SAM) linker was prepared in an analogous manner as detailed above.
Preparation of amine-DNA strand
A single-stranded DNA sequence with the 5 end modified with a C6 NH2 phosphoramidite was purchased from Integrated DNA Technologies. HPLC was performed using a reverse-phase PLRP-S column using a gradient of acetonitrile and 50 mM ammonium acetate (5%–15% ammonium acetate over 35 minutes). After HPLC purification, the amine-modified oligonucleotide was characterized using MADLI-TOF-MS and quantified using UV-visible spectrophotometry.
Preparation of phenazine-modified DNA strand
Phenazine-1-carboxylic acid (PCA, 4.9 mg, 22 μmol) was added to dicyclohexylcarbodiimide (DCC, 9.3 mg, 45 μmol) and N-hydroxysuccinimide (NHS, 5.2 mg, 45 μmol) in degassed, anhydrous dimethylformamide (DMF, 500 μL) at RT in a scintillation vial wrapped in aluminum foil. The barely soluble PCA turned from green to yellow upon stirring overnight in the dark under Ar. The NHS-activated PCA solution was reduced to low volume (100 μL) and was then added to a solution containing amine-modified DNA (0.42 μmol) in the presence of hydroxybenzotriazole (HOBT, 1 mg, 7.4 μmol) and N,N,N,N-tetramethyl-O-(1H-benzotriazol-1-yl)uronium hexafluoro-phosphate (HBTU, 1 mg, 2.6 μmol) in sodium bicarbonate solution (200 μL, pH 8, 0.1 M) overnight in the dark with the 1.5 mL Eppendorf tube wrapped in aluminum foil and mixed thoroughly using the shake function of a benchtop vortexer. The 1.5 mL tube secured using a clip to avoid spilling. The crude product was then buffer exchanged using a NAP-5 size-exclusion column into phosphate buffer (pH 7.0, 5 mM NaH2PO4, 50 mM NaCl). The treated product was subsequently purified using HPLC on a reverse-phase PLRP-S column with a gradient of acetonitrile and 50 mM ammonium acetate (5%–15% ammonium acetate over 35 minutes) while monitoring 252, 260, 280, 354, and 365 nm simultaneously. Pale yellow liquid fractions were collected and freeze-dried on a lyophilizer (Labconco). The resulting yellow dried smear was resuspended in minimal MQ H2O and was subsequently analyzed by MALDI-TOF-MS.
Formation of double-stranded DNA duplexes
The two strands of a duplex are synthesized separately, purified, desalted, EtOH precipitated, and stored frozen at −20°C. Prior to electrochemical experiments, the two strands of a duplex were mixed in equimolar (50 μM) in 200 μL phosphate buffer (pH 7.0, 5 mM NaH2PO4, 50 mM NaCl). The DNA solution was deoxygenated by bubbling Ar for at least 5 minutes per mL, and then annealed on a thermocycler (Beckman Instruments) by initial heating to 90°C followed by slow cooling over a span of 90 min.
Preparation of DNA-modified electrodes
Multiplexed chips are gently cleaned by sonicating with acetone once then isopropanol three times before drying with Ar. They are then cleaned with UV/Ozone using a UVO cleaner for 20 minutes. Immediately after cleaning the surface, a plastic clamp and rubber gasket (Buna-N) were affixed to the Au surface to create a well for liquid and 50 μM duplex DNA in phosphate buffer (5 mM phosphate, pH 7, 50 mM NaCl) to make ds DNA-modified surfaces. The ds DNA was incubated on the surface for 18–24 h in the absence of light. Once the ds DNA is affixed to the surface, it cannot be dried without compromising the structure and subsequently the measured properties of the ds DNA-modified surfaces. The solution was then exchanged 3 × with phosphate buffer (pH 7, 5 mM phosphate, 50 mM NaCl, 5% glycerol) and incubated with 1 mM mercaptohexanol in phosphate buffer (pH 7, 5 mM phosphate, 50 mM NaCl, 5% glycerol) for 45 minutes. Lastly the surface was rinsed at least 5 × with phosphate buffer (pH 7, 5 mM phosphate, 50 mM NaCl) that was degassed by leaving open in an anaerobic chamber (Coy Lab Products) for at least 3 days.
Multiplexed electrochemical measurements
Experiments performed were replicated at least three times using different samples, and data presented are from representative trials. Cyclic voltammetry (CV), square wave voltammetry (SWV), and differential pulse voltammetry (DPV) were carried out using a 620D Electrochemical Workstation (CH Instruments) at room temperature inside an anaerobic chamber. The atmosphere of the anaerobic chamber (< 1 ppm O2, ca. 3.4% H2) was monitored using a CAM-12 O2 and H2 sensor (Coy Lab Products). The chamber was maintained O2-free by using two ventilated Pd catalyst packs (Coy Lab Products).
Electrochemical experiments were carried out in a three-electrode set-up under an anaerobic atmosphere. CV was conducted at a scan rate of 100 mV/s unless otherwise specified. The central well around the multiplexed electrode surfaces created by the plastic clamp was filled with aqueous buffer containing degassed phosphate buffer (pH 7, 5 mM phosphate, 50 mM NaCl). An Ag/AgCl reference electrode (BASi) was coated with a solidified mixture of 1% agarose and NaCl (3 M) in water inside a long, thin pipette tip. The tip was cut so that the salt bridge could connect the electrode to the buffer from the top of the well. A freshly-polished Pt wire used as an auxiliary electrode was also submerged in the buffer from the top of the well. The working electrode contacted a dry part of unmodified gold surface. Scan rate dependence studies (10–5000 mV/s) were carried out to determine whether the phenazine moiety was covalently attached to the DNA-modified electrodes. A linear relationship between scan rates and measured peak currents signified that phenazine was covalently attached. For O2 electrocatalysis studies, CV was conducted in open air in O2-saturated phosphate buffer (pH 7, 5 mM phosphate, 50 mM NaCl). Hexaammineruthenium(III) chloride (RuHex, 1–500 μM in pH 7, 5 mM phosphate, 50 mM NaCl) was used in control experiments to probe whether electron transfer occurred between the Au electrode and the covalently attached PCN through DNA.
DNA modified electrode controls
The purpose of carrying out the control experiments shown in Figure S4 using electrodes modified using self-assembled monolayers (SAMs) of double-stranded DNA (ds DNA) was to probe the electron transfer mechanism between the redox probe and the electrode surface. Four sets of control experiments were conducted to probe whether DNA-mediated charge transport (DNA charge transfer) occurs between PCN and the electrode via the base stack of ds DNA: (i) WM-versus MM-DNA, (ii) high- versus low-density monolayers, (iii) PCN versus RuHex, and (iv) scan rate dependence studies.
WM- versus MM-DNA: Electrodes modified using SAMs of ds DNA that are well-matched (WM) or contain a single base pair mismatch (MM) were prepared under identical conditions. WM-DNA electrodes and MM-DNA electrodes have similar physical properties but exhibit different charge transfer properties that occur through the aromatic base stacks of DNA. If the electron transfer process between the redox probe and the electrode occurs via DNA charge transfer, then a decrease in the yield of DNA charge transfer is expected due to the perturbation to the base stack introduced by the MM lesion. If the electron transfer process occurs via other modes such as physical diffusion of a charge carrier or physical contact between the redox probe and the electrode surface, then no difference in the current measured would be expected.
High- versus low-density monolayers: Electrodes modified using high- and low-density SAMs of ds DNA were used to probe the effect of the number of ds DNA on the charge transport mechanism. A low-density ds DNA monolayer promotes electron transport within one ds DNA, but ds DNA may adopt various conformations because the individual ds DNA are more distantly spaced. A high-density ds DNA monolayer encourages ds DNA to align with each other in a more orderly fashion due to more ds DNA present in a confined space. Regardless of a high- or low-density monolayer, for the case of PCN-modified DNA-SAM, MM discrimination was observed, suggesting that DNA charge transfer is the major mode of electron transport between the electrode and the redox probe.
PCN versus RuHex: RuHex is a positively-charged species that binds electrostatically to the negatively-charged phosphate backbone. RuHex also associates to the OH groups at the terminus of the SAM surface passivated by backfilling using mercaptohexanol. Electrons tunnel through the SAM from the electrode to the RuHex situated at the SAM terminus. Electrons can then be transferred and be distributed among the Ruhex bound to the SAM surface and on the DNA phosphate backbone. Residual RuHex present in solution could help with ET through hopping and diffusion in solution, and the contribution of solution-based ET to the current measured depends on the concentration of RuHex used. Therefore a number of RuHex concentrations were screened in order to optimize the conditions for this measurement. For species that do not participate in DNA charge transfer, no MM discrimination will be observed. The RuHex experiments showthat the number of ds DNA on the SAM-modified electrodes is similar for both the WM and MM cases. The observation of a sizable MM discrimination demonstrates that PCN bound on DNA participates in DNA charge transfer.
Scan rate dependence studies (10–5000 mV/s) were carried out to determine whether the phenazine moiety was covalently attached to the DNA-modified electrodes. A linear relationship between scan rates and measured peak currents signified that phenazine was covalently attached.
Time resolved spectroscopy
Colony biofilms (Δphz*) were grown and suspensions were prepared in 500 μL PBS, as described above for the LC-MS and eDNA measurements. 400 μL of the suspension was transferred to a clean quartz cuvette. Time-resolved emission measurements utilized a YAG laser (λexc = 532 nm), with laser powers of ~2 mJ per pulse. A colored-glass longpass filter (λ > 600 nm) was used to minimize scattered laser light and the emission of the Ru(phen)2dppz2+ complex was monitored at 620 nm. An excitation pulse (532 nm) was delivered and emission was recorded at 620 nm for 1.9 ms and each time point was 0.2 ns. A background scan was acquired with only the biofilm suspension. Then 5 μM Ru(phen)2dppz2+ was added and a scan was recorded. Subsequently, 5 μM Rh(phi)2bpy3+ was added after each scan to acquire the 1, 2, 3 and 4 quencher equivalent datasets. This process was repeated for multiple biofilms and a liquid Δphz* culture that was concentrated to the same optical density (500 nm) as the biofilm suspensions. Datasets were background subtracted from the biofilm only background scan and were fit with biexponential decay models.
IDA biofilms
Electrode and reactor preparation
Gold IDA electrodes were prepared as previously described (Boyd et al., 2015). IDAs fabricated on glass substrate were ordered from CH Instruments (#012125). A passivation membrane covered the working electrodes except for the overlapping regions that form the 5 mm gap and where the leads were attached. Insulated wires (Digi-Key #W7-ND) were attached to the two working electrodes of the IDA with a conductive epoxy (Electron Microscopy Sciences #12670-EE) that was cured at 80°C for 1 hour. A shell for the IDA construct was constructed from 15mL conical vials that were sawed off at the 2.5 mL mark. Rough edges were smoothed with a razor blade, and two holes were poked in the bottom of the vial. The IDA with attached wires was placed inside the conical vial and the wires were threaded through the holes at the bottom. A nonconductive epoxy (Amron International #2131-B) was carefully pipetted into the vial with the IDA until it was full to the rim. The epoxy was allowed to set at room temperature in a fume hood for 24–48 hours. IDA constructs were then used immediately or stored in Petri dishes and covered to protect from light and dust.
Reactors (Pine Instruments, 265 mL water-jacketed electrochemical reactor) used to grow biofilms on the IDA were cleaned thoroughly and autoclaved between uses. For gas control, the ports were stoppered or sealed with o-rings and screw clamps. Gas inflow and outflow lines were attached via 6 inch gassing needles and 16 g 1.5 in needles, respectively, pierced through separate stoppers. Gas outflow was attached to a liquid overflow vessel, a 500 mL Erlenmeyer flask that also had 16 g needles as inflow and outflows. The water jacket of each reactor was connected in series to a water chiller (Lytron) to heat the vessels to ~31°C. Each reactor was filled with 180mL of sterile medium and the IDA construct was suspended from a stopper (size 4) by threading the wires through 16 g needles and then removing the needles, so that the electrode was fully submerged in the liquid. IDAs were sterilized in 10% bleach for 30 s, then rinsed in sterile medium before submerging in the reactor.
Biofilm growth
Biofilms were grown in a minimal medium (MM) with succinate as the carbon source (14.15 mM KH2PO4, 38.85 mM K2HPO4, 42.8 mM NaCl, 9.3 mM NH4Cl, 40 mM Na-succinate, adjusted to pH 7.2 and autoclaved then 1x SL-10 trace element solution [Atlas, 2004] and 1 mM MgSO4). From a stationary phase MM culture, the reactors with 180 mL MM were inoculated to an OD500 of 0.005. The medium was exchanged every 24 hours, and biofilms were grown on the IDA for 3 or 4 days in this fashion. During growth, constant temperature was maintained at ~31°C, reactors were stirred at 250 rpm (VWR #58948–138) and air was bubbled into two reactors at a time with a small aquarium bubbler (Tetra #77851). Depending on the experiment, biofilms were grown with or without the full electrochemical setup described below. At a minimum, biofilms were grown with the IDA working electrode (disconnected from potentiostat).
Electrochemical setup
Electrochemistry was performed using a three-electrode setup and CHI 760b or CHI 760e bipotentiostats. The working electrode(s) were on the IDA and connected to wires as described above. The counter electrode was a separate graphite rod (Alfa Aesar #14738) and the reference electrode was a separate Ag/AgCl electrode (BASi #MW-2030 or #MF-2079) assembled as described in the above DNA charge transfer section. Before measurements were made in a reactor, the medium was sparged with N2 for at least 10 min. Reactors were gently bubbled with N2 throughout the measurements. Unless otherwise noted Generator-Collector scans were acquired at 3 mV/s, with the collector held at 0 mV. Square wave voltammetry was performed at 300 hz with an amplitude of 25 mV and an increment of 1 mV. Chronoamperometry of metabolic current was acquired at +100 mV.
Measurement scheme
For experiments where biofilms were soaked in synthetic PYO, biofilms were soaked for at least 10 min. To transfer the IDA biofilm, the potentiostat leads were removed and the reference and counter leads were attached to the transfer reactor electrodes. The IDA biofilm was dipped in fresh medium to remove solution PYO and then submerged in the transfer reactor. The working electrodes were attached, and the open circuit potential was measured to ensure proper connections – this took roughly 30 s. For time sensitive experiments, SWVs were immediately recorded. For biofilm Dap and Dloss measurements SWV and GC scans were taken consecutively (manually or with a macro) until 15 of each scan had been acquired. For blank Dloss measurements, rapid consecutive SWV scans were taken in an automated fashion using the “repeated runs” option in the CHI software. For blank Dap measurements, the blank IDA was incubated with increasing concentrations of PYO in the soak reactor.
Fluorescence imaging
IDA biofilms were prepared for imaging by removing the biofilm coated region of the IDA from the epoxy encased shell. The exposed region of the glass substrate near the epoxy interface was scored with a diamond tipped scribe and then it was snapped off from the rest of the vial/epoxy assembly. With tweezers the IDA fragment was transferred to a slide with a multi-well silicone spacer and dye mixture was added with a pipette directly to the edge of the IDA fragment until it was fully coated. The dye mixture was 10 μM Syto60 (Invitrogen #S11342) and 1 or 2 μM TOTO-1 (Invitrogen T3600) in PBS. Coverslips (#1.5) were added directly on top of the mixture and sealed with clear nail polish to the spacer.
Slides were then imaged on a confocal microscope. Most imaging was done using an upright Zeiss LSM 880 with Fast Airyscan. Syto60 was excited with a 633 nm laser and emission was typically recorded with a bandpass filter from 650 to 750 nm. TOTO-1 was excited with a 488 or 514 nm laser and emission was typically recorded with a bandpass filter from 530 to 630 nm. Tilescans were taken using a 10x objective and high magnification images were taken with a 63x objective. Several images were taken at 63x magnification using the Airyscan module with superresolution settings. For Airyscan images the appropriate filter cubes were used.
Other imaging was done on an inverted confocal Leica model TCS SPE with a 10x objective for tile scanning. Syto60 was excited with a 633 nm laser, TOTO-1 was excited with a 488 nm laser, and emissions were set by band pass filters as above.
Dap measurement theory
To measure Dap, we compared two electrochemical measurements that depend on Dap in different ways. By performing this comparison at multiple concentrations, we could fit the data points to a line whose slope can be defined by known parameters, yielding Dap. Δphz* biofilms were soaked in 75 μM of PYO and then transferred to fresh medium lacking PYO. The biofilm PYO concentration dropped over the course of 45 min as equilibration with the medium occurred. Approximately 15 sets of scans were taken during this time period.
The first measurement was square wave voltammetry (SWV) (Figure 5B). The SWV peak current (Iswv) is defined in terms of concentration of redox molecules (C) reacting directly (by physical diffusion) or indirectly (through electron self-exchange reactions) with the electrode (Dap), the area of the electrode (A), the number of electrons per redox reaction (n), the Faraday constant (F), the pulse width (tp) and a normalization constant (φ). Note that Iswv depends on the square root of Dap.
| (Equation 1) |
The second measurement was a generator-collector (GC) measurement (Figure 5C). The maximum GC current (Igc) is also defined in terms of C, Dap, n, and F, but depends on a geometric factor (S) (Boyd et al., 2015) as opposed to the electrode area. Note that Igc depends directly on Dap.
| (Equation 2) |
A plot of experimentally determined Igc versus Iswv values yields linear relationships for PYO in biofilms and in solution (i.e., blank IDA) (Figures 5D and S6), with slope (m) that can be defined in terms Igc and Iswv:
| (Equation 3) |
| (Equation 4) |
This relationship enables determination of Dap from the experimentally determined dependency of Igc to Iswv (i.e., m) in terms of known experimental parameters (Figure 5F). Importantly, it provides a means of determining Dap that is not dependent on knowing C, which for our system is unknown and changes over time as PYO diffuses out of the biofilm.
| (Equation 5) |
Note that because the slope of these plots is linear, it suggests that Dap is constant for a biofilm despite how the concentration of PYO changes as it diffuses. We assume a constant Dap by fitting the data with a linear model.
Dloss measurement theory
In order to measure the physical diffusion coefficient of PYO molecules lost from the IDA-grown biofilms, we monitored the loss of biofilm PYO over time as it equilibrated with the fresh medium similar to an approach taken with polymer films (White et al., 1982). To quantify this process, we used successive SWV scans acquired over 45min following transfer to fresh medium (the SWV subset of the Dap data). A 1-dimensional diffusion model was then applied to fit the decay of Iswv yielding an estimate of Dloss.
Considering 1-dimensional diffusion of an initial mass (M0) of a substance from a point source, the solution of Fick’s second law describing the time-dependent concentration gradient is given by Equation 6 where x is distance normal from the source, A is the cross-sectional area in 3D space, and Dloss is the physical diffusion coefficient.
| (Equation 6) |
When the source is located at no-flux boundary such that the mass diffuses only to one side,
| (Equation 7) |
where C(x = 0, t) is the time dependent concentration at the surface of the no-flux boundary (e.g., the IDA surface).
To connect this model to the data, the concentration and initial mass can be defined in terms of the measured SWV current (Iswv).
For SWV, the concentration, C, is given by:
| (Equation 8) |
Concentration can be defined as the mass per volume, so the initial mass, M0, can be expressed in terms of the effective volume probed by the electrode (V):
| (Equation 9) |
The initial current is defined as Iswv (t = 0) = I0, which is experimentally estimated from the last Iswv in the PYO soak, before transfer to PYO-free medium. This is a conservative overestimate, because some of the soak signal comes from the solution PYO. Substituting the values for C (Equation 8) and M0 (Equation 9) into Equation 7 yields:
| (Equation 10) |
The term V refers to the biofilm volume from which the mass of PYO was detected by the electrode. For a 1D electrode process, the concentration gradient extends from the electrode-solution interface (C = 0) to the edge of the diffusion layer, d (C = Cbulk) in a near linear fashion (Bard et al., 1980). There is no region where all of the mass is detected, but the electrode has detected one half of the mass in the volume A × δ, therefore the effective V can be defined:
| (Equation 11) |
The diffusion layer, δ, for a single potential step can be estimated by:
| (Equation 12) |
where ts is the amount of time that the driving potential is held. SWV is a series of forward and reverse potential steps for which we could not unequivocally define an equivalent ts value, however, we discuss reasonable bounds in the assumptions section below. Substituting into Equation 11 yields , therefore Equation 10 can be written as:
| (Equation 13) |
And with that expression we can fit the decay of Iswv(t) to a model of the form:
| (Equation 14) |
where the coefficient, b, is described in known variables except for Dloss and where a accounts for any constant background signal.
| (Equation 15) |
Therefore, Dloss can be calculated from the fit data as (Figures 5F and S7):
| (Equation 16) |
Dloss model assumptions
The Dloss analysis described above is based on a number of assumptions. It assumes, for example, that the biofilms are homogeneous. In reality, they are heterogeneous, containing many voids and obstacles (e.g., cells and exopolysaccharides) through which diffusion would not occur. We contend heterogeneity would affect Dloss and Dap in a similar manner, as it reduces the biofilm volume in which PYO resides. As such, we don’t expect biofilm heterogeneity to greatly affect the determination of Dloss from Dap. Importantly, our analysis assumes a single infinitely thick phase described by a single Dloss. In reality, there are at least two phases, a thin biofilm adjoining an infinitely thick solution. If Dloss in solution is greater than in the biofilm, then at any instance the concentration gradient of PYO across the biofilm will be steeper than predicted by the model. Since the flux of PYO out of the biofilm at any instance is proportional to the product of the gradient and Dloss, as the model fits the rate of change of PYO in the biofilm the assumption of a shallower gradient than the actual gradient is expected to result in a calculated Dloss that is higher than the actual Dloss.
To estimate the scan time parameter, ts, we assumed that for the blank IDA Dap = Dloss and solved for ts that best fit the Iswv decay for the blank IDA. We then used this value, ts = 21ms, to calculate Dloss for the biofilm IDAs. The scan time parameter is intended to estimate the thickness of the diffusion layer that is formed during a single potential step that drives the flux of the electrode reactant resulting in the observed current. SWV is, however, a series of forward and reverse potential steps superimposed on a series of forward potential steps. Therefore, determining the effective scan time that describes the change in thickness of the diffusion layer that occurs during the forward pulse contributing to Iswv is nontrivial (we only consider the forward pulse as the reverse scan partially replenishes the electrode reactant that is depleted during the forward scan). One approach to estimate ts is setting the SWV expression equal to twice the Cottrell equation (Equations 17 & 18), since Iswv is the net current from the forward and reverse pulses. For a pulse amplitude of 0.025 V and step increment of 0.001 V as used here, each potential step is effectively 0.05 V. The potential at which the Iswv occurs is the formal potential of the electrode reactant and applying the Nernst equation, the fraction of electrode reactant in the oxidized state at the electrode surface at the start of the forward potential step generating Iswv (E = Eo’+ 0.025 V) is 82.6% and at the end (E = Eo 0.025 V) is 17.4%. The Cottrell equation assumes the potential step drives a redox reaction in which 100% of the electrode accessible redox molecules at the electrode go from oxidized to reduced (or vice versa). Replacing C in the Cottrell equation with 0.655 × C to reflect the fraction of PYO at the electrode surface that changes oxidation state during the forward potential step of the SWV yields an estimate ts z6ms.
| (Equation 17) |
| (Equation 18) |
As such, our ts estimate (21 ms) based on the blank Dap = Dloss used to estimate Dloss for the biofilm may be an overestimate. Noting that Dloss scales linearly with ts (Equation 16), this would conservatively underestimate the difference between biofilm Dap and Dloss.
Parameters for electrochemical calculations
The surface area of the electrode for SWV, A = 0.025 cm2, and the geometric factor for GC, S = 18.4 cm, were calculated for a blank IDA using the known concentration and Dap for ferrocene methanol (Boyd et al., 2015). All quantified SWVs were acquired with a pulse amplitude of 25 mV at a frequency of 300 Hz and an increment of 1 mV. The SWV pulse time, tp, is one half the square wave period (½ * 1/300 = 1/600 s). Peak separation from CV of PYO in solution indicated that it did not undergo the full 2 electron reduction, but on average underwent electron transfer with n ≈ 1.8. From these acquisition parameters, φ = 0.7 was inferred from a table of existing values (Lovríc, 2010). For the Dloss estimate, it was assumed that I0 was the soak SWV peak current. The equivalent scan time, ts, for the Dloss calculation is discussed above.
Abiotic IDA measurements with nafion
Electrochemical experiments were recorded with a bi-potentiostat (CHI, Model #760) in a Teflon cell designed in-house to mount gold interdigitated array (IDA) electrodes from CHI. The counter and reference electrodes were a Pt coiled wire and an Ag/AgCl reference electrode. All measurements were recorded at room temperature in 0.2 M Na2SO4 as the electrolyte purged with Argon. The IDA electrodes were modified with Nafion film by pipetting 20 μL of a 5% wt Nafion solution (Sigma Aldrich) onto the IDA electrodes and allowing a film to form as the solution dried at room temperature. After 2 hours, the modified IDAs were rinsed with ethanol and DI water and then mounted into the base of the Teflon cell.
The Nafion films on the IDAs mounted in the Teflon cell were loaded with various amounts of Ru(NH3)63+ by either exposing the films to a 10 mM solution of Ru(NH3)6Cl3 (Sigma Aldrich) in 0.2 M Na2SO4 at time points from several minutes to several hours or by allowing Ru(NH3)63+ in the films to diffuse out into bulk electrolyte for several hours. The Nafion films were loaded with Ferrocenemethanol (Fc-OH) by exposing the films to a 250 μM solution of Fc-OH in 0.2 M Na2SO4 for several hours. To decrease the amount of Fc-OH in loaded films, the films were allowed to have the Fc-OH in the films to diffuse out into bulk electrolyte for several hours. For each electrochemical measurement after loading or depleting the film, the cell was rinsed with DI water and 2 mL of fresh electrolyte was added. The counter and reference electrodes were position above the IDA electrodes in a Teflon lid, and the electrolyte were purged with Argon for 15 minutes with a blanket of Argon maintained in the head space of the Teflon cell. Three conditions were measured with the Fc-OH - (i) Fc-OH in solution with naked IDAs, (ii) Fc-OH in solution at IDAs modified with Nafion and (iii) Fc-OH loaded into the Nafion modified IDAs.
For the SWV versus GC method, SWV and GC scans were acquired as described above, and Equation 5 was used to calculate Dap with the following parameters: A = 0.0213 cm2, Ψ = 0.505, S = 18.4 cm, tp = 1/600 s. For chronocoulometry, voltage was stepped from 0 mV to −500 mV versus Ag/AgCl. From the slope of plots of charge versus (time)1/2, Dap was calculated from the following equation:
where S is the chronocoulometric slope (C cm−2 s−1/2), Φ is the film thickness (cm), Γ is the total quantity of Ferrocenemethanol in the film (mol cm2), and F is the Faraday constant and C is concentration in moles/cm3 (C = 2.5 × 10−7 moles/cm3). A film thickness of 10 μm was estimated from the reported density of casted Nafion films by knowing the amount of Nafion deposited and the area covered by the film.
Scanning electron microscopy (SEM)
IDA and colony biofilms were prepared for SEM using the following protocol. Delicate samples were gently transferred between solutions using metal spatulas or plastic grids as supports. Material was fixed in 4% paraformaldehyde in PBS for 2 hours or overnight, then washed twice with PBS. Material was then fixed in 1% OsO4 in water for 1 hour, then washed twice with PBS. Next, the samples were dehydrated by sequentially immersing in 50, 70, 90, 95 and 100% ethanol (EtOH) solutions for 10 min, and then again in 100% EtOH for 1 hour. Samples were then transferred to hexamethyldisilazane (HMDS) solutions of 1:2 then 2:1 HMDS:EtOH for 20 min each, followed by two incubations in 100% HMDS for 20 min each. Samples were then removed from the solution and air-dried before attaching to imaging stubs with conductive tape. Imaging stubs were then sputter coated with 10 nm of palladium before being loaded into the SEM. Imaging was done with a Zeiss 1550VP field emission SEM using the SE2 detector.
QUANTIFICATION AND STATISTICAL ANALYSIS
Nearly all data processing and analysis was done in R (R Development Core Team, 2018) using tidyverse packages including ggplot2, dplyr, readr (Wickham, 2017), broom (Robinson and Hayes, 2018) and hms (Müller, 2018). The nonlinear least-squares (‘nls’) function in base R was used for Dloss fits and the linear model (‘lm’) function was used for Dap fits. Electrochemical data was exported from CHI software as text files with raw data, acquisition parameters and timestamps and then imported into R. Confocal images were stitched (for tilescans) and processed into z-slices or maximum intensity projections using the Zeiss Zen Black software and Fiji (Schindelin et al., 2012).
Throughout the manuscript sample sizes are reported as biological or technical replicates, as appropriate. Typically, center points are means and error bars are 95% confidence intervals or two standard deviations, although it is noted when error bars represent other measures. Welch’s t test (unequal variance – one-sided) was used to compare means. Statistically significant difference in means was determined for p values < 0.05. All code for each figure, including statistical tests, is available in a GitHub repository (https://github.com/DKN-lab/phz_eDNA_2019) and html versions of the Rmarkdown notebooks are also available as a website (https://DKN-lab.github.io/phz_eDNA_2019).
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Bacterial and Virus Strains | ||
| Pseudomonas aeruginosa UCBPP-PA14 (strain DKN_WT) | Schroth et al., 2018 | BEI resources: NR-50573 |
| P. aeruginosa UCBPP-PA14 ΔphzA1-G1 ΔphzA2-G2 (derivative of DKN_WT); Δphz | Dietrich et al., 2006 | N/A |
| P. aeruginosa UCBPP-PA14 ΔphzA1-G1 ΔphzA2-G2 ΔphzMS ΔphzH (derivative of DKN_WT); Δphz* | This paper. | N/A |
| P. aeruginosa UCBPP-PA14 (strain MRP_WT) | Schroth et al., 2018 | BEI resources: NR-50573 |
| P. aeruginosa UCBPP-PA14 ΔpelB (derivative of MRP_WT); Δpel | Colvin et al., 2011 | N/A |
| Chemicals, Peptides, and Recombinant Proteins | ||
| Pyocyanin (PYO) | This study | CAS: 85-66-5 |
| Phenazine methosulfate | VWR | Cat#AAH56718–06; CAS: 299-11-6 |
| Phenazine-1-carboxylic acid (PCA) | Dr. Stuart Conway, Department of Chemistry, University of Oxford | CAS: 2538-68-3 |
| Phenazine-1-carboxamide (PCN) | Princeton BioMolecular Research | Cat#PBMR030086; CAS: 550-89-0 |
| TOTO-1 iodide | ThermoFisher Scientific | Cat#T3600 |
| SYTO 60 | ThermoFisher Scientific | Cat#S11342 |
| Deposited Data | ||
| Code notebooks for data processing and analysis to generate figures. | This study | https://github.com/dkn-lab/phz_eDNA_2019 |
| Oligonucleotides (5’−3’) | ||
| ITC binding assay - top strand (29 basepairs): GTGGCAGGTCAGTCAAGTATACTGCACTA | This study | N/A |
| ITC binding assay - bottom strand (29 basepairs): TAGTGCAGTATACTTGACTGACCTGCCAC | This study | N/A |
| MST binding assay - forward primer (80 basepair product): Cy3/AGAGCAGTTGGTCAAGGC | Bergkessel et al., 2019 | N/A |
| MST binding assay - reverse primer (80 basepair product): GAAAATAACGCTTGACGGAA | Bergkessel et al., 2019 | N/A |
| DNA modified electrode - top strand before PCN modification (17 basepairs): (NH2)-C6/GCTCAGTACGACGTCGA | This study | N/A |
| DNA modified electrode - well-matched bottom strand (17 basepairs): (SH)-C6/TCGACGTCGTACTGAGC | This study | N/A |
| DNA modified electrode - mis-matched bottom strand (17 basepairs): (SH)-C6/TCGACCTCGTACTGAGC | This study | N/A |
| Software and Algorithms | ||
| R, including functions lm(), nls(), and t.test() as well as varied tidyverse tools. | R Development Core Team 2018; Wickham, 2017; Robinson and Hayes, 2018; Muller, 2018 | https://www.r-project.org/ |
| Fiji | Schindelin et al., 2012 | https://imagej.net/Fiji |
| Pytc (ITC data) | Duvvuri etal., 2018 | https://pytc.readthedocs.io/en/latest/ |
| Other | ||
| Whatman nuclepore membranes (filters); 0.2 μm pores; polycarbonate | Sigma-Aldrich | Cat#WHA110606 |
| IDA gold electrode | CH Instruments | Cat#012125 |
| Bipotentiostat | CH Instruments | Model: 760b, 760e |
Highlights.
PYO and PCN bind extracellular DNA, which facilitates their retention in biofilms
Electrode biofilms support fast PYO electron transfer and slow PYO loss
Phenazines rapidly exchange electrons and are capable of DNA charge transfer in vitro
ACKNOWLEDGMENTS
We thank Jeanyoung Jo, Lars Dietrich, and Matthew Parsek for providing strains and the Biological Imaging Center, GPS Division Analytical Facility, and Beckman Institute Laser Resource Center at Caltech for supporting confocal imaging, SEM imaging, and time resolved spectroscopy, respectively. We also thank three anonymous reviewers for their constructive comments. This work was supported by NIH (1R01AI127850-01A1 to D.K.N. and GM126904 to J.K.B.); ARO (W911NF-17-1-0024 to J.K.B.); ONR (N0001418WX00436 to M.D.Y., S.A.T., and L.M.T.); and Rosen Bioen-gineering Center at Caltech (to S.H.S., D.K.N., and J.K.B.). E.C.M.T. was supported by a Croucher Foundation Research Fellowship.
Footnotes
DECLARATION OF INTERESTS
The authors declare no competing interests.
REFERENCES
- Akhoury A, Bromberg L, and Hatton TA (2013). Interplay of electron hopping and bounded diffusion during charge transport in redox polymer electrodes. J. Phys. Chem. B 117, 333–342. [DOI] [PubMed] [Google Scholar]
- Allesen-Holm M, Barken KB, Yang L, Klausen M, Webb JS, Kjelleberg S, Molin S, Givskov M, and Tolker-Nielsen T (2006). A characterization of DNA release in Pseudomonas aeruginosa cultures and biofilms. Mol. Microbiol 59, 1114–1128. [DOI] [PubMed] [Google Scholar]
- Arkin MR, Stemp ED, Holmlin RE, Barton JK, Hörmann A, Olson EJ, and Barbara PF (1996a). Rates of DNA-mediated electron transfer between metallointercalators. Science 273, 475–480. [DOI] [PubMed] [Google Scholar]
- Arkin MR, Stemp EDA, Turro C, Turro NJ, and Barton JK (1996b). Luminescence Quenching in Supramolecular Systems: A Comparison of DNA- and SDS Micelle-Mediated Photoinduced Electron Transfer between Metal Complexes. J. Am. Chem. Soc 118, 2267–2274. [Google Scholar]
- Atlas R (2004). Handbook of Microbiological Media, Third Edition (CRC Press; ). [Google Scholar]
- Bard AJ, Faulkner LR, Leddy J, and Zoski CG (1980). Electrochemical Methods: Fundamentals and Applications (Wiley; ). [Google Scholar]
- Bellin DL, Sakhtah H, Rosenstein JK, Levine PM, Thimot J, Emmett K, Dietrich LE, and Shepard KL (2014). Integrated circuit-based electrochemical sensor for spatially resolved detection of redox-active metabolites in biofilms. Nat. Commun 5, 3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellin DL, Sakhtah H, Zhang Y, Price-Whelan A, Dietrich LEP, and Shepard KL (2016). Electrochemical camera chip for simultaneous imaging of multiple metabolites in biofilms. Nat. Commun 7, 10535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd DA, Snider RM, Erickson JS, Roy JN, Strycharz-Glaven SM, and Tender LM (2015). Theory of redox conduction and the measurement of electron transport rates through electrochemically active biofilms In Biofilms in Bioelectrochemical Systems, Beyenal H and Babauta J, eds. (John Wiley & Sons, Inc.). [Google Scholar]
- Chincholkar S, and Thomashow L (2013). Microbial Phenazines: Biosynthesis, Agriculture and Health (Springer Science & Business Media; ). [Google Scholar]
- Colvin KM, Gordon VD, Murakami K, Borlee BR, Wozniak DJ, Wong GC, and Parsek MR (2011). The pel polysaccharide can serve a structural and protective role in the biofilm matrix of Pseudomonas aeruginosa. PLoS Pathog 7, e1001264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colvin KM, Irie Y, Tart CS, Urbano R, Whitney JC, Ryder C, Howell PL, Wozniak DJ, and Parsek MR (2012). The Pel and Psl polysaccharides provide Pseudomonas aeruginosa structural redundancy within the biofilm matrix. Environ. Microbiol 14, 1913–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelissen R, Bøggild A, Thiruvallur Eachambadi R, Koning RI, Kremer A, Hidalgo-Martinez S, Zetsche EM, Damgaard LR, Bonné R, and Drijkoningen J (2018). The cell envelope structure of cable bacteria. Front. Microbiol 9, 3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa KC, Glasser NR, Conway SJ, and Newman DK (2017). Pyocyanin degradation by a tautomerizing demethylase inhibits Pseudomonas aeruginosa biofilms. Science 355, 170–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costerton JW, Stewart PS, and Greenberg EP (1999). Bacterial biofilms: a common cause of persistent infections. Science 284, 1318–1322. [DOI] [PubMed] [Google Scholar]
- Das T, and Manefield M (2012). Pyocyanin promotes extracellular DNA release in Pseudomonas aeruginosa. PLoS ONE 7, e46718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das T, Kutty SK, Tavallaie R, Ibugo AI, Panchompoo J, Sehar S, Aldous L, Yeung AW, Thomas SR, and Kumar N (2015). Phenazine virulence factor binding to extracellular DNA is important for Pseudomonas aeruginosa biofilm formation. Sci. Rep 5, 8398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich LEP, Price-Whelan A, Petersen A, Whiteley M, and Newman DK (2006). The phenazine pyocyanin is a terminal signalling factor in the quorum sensing network of Pseudomonas aeruginosa. Mol. Microbiol 61, 1308–1321. [DOI] [PubMed] [Google Scholar]
- Dietrich LEP, Okegbe C, Price-Whelan A, Sakhtah H, Hunter RC, and Newman DK (2013). Bacterial community morphogenesis is intimately linked to the intracellular redox state. J. Bacteriol 195, 1371–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvvuri H, Wheeler LC, and Harms MJ (2018). pytc: Open-Source Python Software for Global Analyses of Isothermal Titration Calorimetry Data. Biochemistry 57, 2578–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flemming H-C, and Wingender J (2010). The biofilm matrix. Nat. Rev. Microbiol 8, 623–633. [DOI] [PubMed] [Google Scholar]
- Friedheim EA (1934). The effect of pyocyanine on the respiration of some normal tissues and tumours. Biochem. J 28, 173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman AE, Chambron JC, Sauvage JP, Turro NJ, and Barton JK (1990). A molecular light switch for DNA: Ru(bpy)2(dppz)2+. J. Am. Chem. Soc 112, 4960–4962. [Google Scholar]
- Genereux JC, and Barton JK (2010). Mechanisms for DNA charge transport. Chem. Rev 110, 1642–1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser NR, Kern SE, and Newman DK (2014). Phenazine redox cycling enhances anaerobic survival in Pseudomonas aeruginosa by facilitating generation of ATP and a proton-motive force. Mol. Microbiol 92, 399–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser NR, Saunders SH, and Newman DK (2017a). The colorful world of extracellular electron shuttles. Annu. Rev. Microbiol 71, 731–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser NR, Wang BX, Hoy JA, and Newman DK (2017b). The Pyruvate and a-Ketoglutarate Dehydrogenase Complexes of Pseudomonas aeruginosa Catalyze Pyocyanin and Phenazine-1-carboxylic Acid Reduction via the Subunit Dihydrolipoamide Dehydrogenase. J. Biol. Chem 292, 5593–5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall-Stoodley L, Costerton JW, and Stoodley P (2004). Bacterial biofilms: from the natural environment to infectious diseases. Nat. Rev. Microbiol 2, 95–108. [DOI] [PubMed] [Google Scholar]
- Hernandez ME, and Newman DK (2001). Extracellular electron transfer. Cell. Mol. Life Sci 58, 1562–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez ME, Kappler A, and Newman DK (2004). Phenazines and other redox-active antibiotics promote microbial mineral reduction. Appl. Environ. Microbiol 70, 921–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollstein U, and Van Gemert RJ Jr. (1971). Interaction of phenazines with polydeoxyribonucleotides. Biochemistry 10, 497–504. [DOI] [PubMed] [Google Scholar]
- Jennings LK, Storek KM, Ledvina HE, Coulon C, Marmont LS, Sadovskaya I, Secor PR, Tseng BS, Scian M, and Filloux A (2015). Pel is a cationic exopolysaccharide that cross-links extracellular DNA in the Pseudomonas aeruginosa biofilm matrix. Proc. Natl. Acad. Sci. USA 112, 11353–11358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jernaes MW, and Steen HB (1994). Staining of Escherichia coli for flow cytometry: influx and efflux of ethidium bromide. Cytometry 17, 302–309. [DOI] [PubMed] [Google Scholar]
- Jiménez Otero F, Chan CH, and Bond DR (2018). Identification of Different Putative Outer Membrane Electron Conduits Necessary for Fe(III) Citrate, Fe(III) Oxide, Mn(IV) Oxide, or Electrode Reduction by Geobacter sulfurreducens. J. Bacteriol 200, e00347–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo J, Cortez KL, Cornell WC, Price-Whelan A, and Dietrich LE (2017). An orphan cbb3-type cytochrome oxidase subunit supports Pseudomonas aeruginosa biofilm growth and virulence. eLife 6, e30205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahl LJ, Morales D, and Dietrich L (2016). Light- and Temperature-Controlled Redox Cycling in Pseudomonas Aeruginosa Biofilms. Free Radic. Biol. Med 100, S185. [Google Scholar]
- Kahl LJ, Price-Whelan A, and Dietrich LEP (2019). Light-mediated decreases in cyclic di-GMP levels are potentiated by pyocyanin and inhibit structure formation in Pseudomonas aeruginosa biofilms. bioRxiv 10.1101/797357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanaugh JS, Flack CE, Lister J, Ricker EB, Ibberson CB, Jenul C, Moormeier DE, Delmain EA, Bayles KW, and Horswill AR (2019). Identification of Extracellular DNA-Binding Proteins in the Biofilm Matrix. MBio 10, e01137–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley SO, Barton JK, Jackson NM, and Hill MG (1997). Electrochemistry of methylene blue bound to a DNA-modified electrode. Bioconjug. Chem 8, 31–37. [DOI] [PubMed] [Google Scholar]
- Kempes CP, Okegbe C, Mears-Clarke Z, Follows MJ, and Dietrich LEP (2014). Morphological optimization for access to dual oxidants in biofilms. Proc. Natl. Acad. Sci. USA 111, 208–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light SH, Su L, Rivera-Lugo R, Cornejo JA, Louie A, Iavarone AT, Ajo-Franklin CM, and Portnoy DA (2018). A flavin-based extracellular electron transfer mechanism in diverse Gram-positive bacteria. Nature 562, 140–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Prindle A, Humphries J, Gabalda-Sagarra M, Asally M, Lee DY, Ly S, Garcia-Ojalvo J, and Süel GM (2015). Metabolic co-dependence gives rise to collective oscillations within biofilms. Nature 523, 550–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Wang S, Xu A, Zhang L, Liu H, and Ma LZ (2019). Biological synthesis of high-conductive pili in aerobic bacterium Pseudomonas aeruginosa. Appl. Microbiol. Biotechnol 103, 1535–1544. [DOI] [PubMed] [Google Scholar]
- Malvankar NS, Vargas M, Nevin KP, Franks AE, Leang C, Kim BC, Inoue K, Mester T, Covalla SF, and Johnson JP (2011). Tunable metallic-like conductivity in microbial nanowire networks. Nat. Nanotechnol 6, 573–579. [DOI] [PubMed] [Google Scholar]
- Marsili E, Baron DB, Shikhare ID, Coursolle D, Gralnick JA, and Bond DR (2008). Shewanella secretes flavins that mediate extracellular electron transfer. Proc. Natl. Acad. Sci. USA 105, 3968–3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruthupandy M, Anand M, Maduraiveeran G, Beevi ASH, and Priya RJ (2015). Electrical conductivity measurements of bacterial nanowires fromPseudomonas aeruginosa. Adv. Nat. Sci.-Nanosci 6, 045007. [Google Scholar]
- Matsukawa M, and Greenberg EP (2004). Putative exopolysaccharide synthesis genes influence Pseudomonas aeruginosa biofilm development. J. Bacteriol 186, 4449–4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meirelles LA, and Newman DK (2018). Both toxic and beneficial effects of pyocyanin contribute to the lifecycle of Pseudomonas aeruginosa. Mol. Microbiol 110, 995–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller K (2018). hms: Pretty Time of Day (CRAN; ). [Google Scholar]
- Nevin KP, Kim B-C, Glaven RH, Johnson JP, Woodard TL, Methé BA, Didonato RJ, Covalla SF, Franks AE, Liu A, and Lovley DR (2009). Anode biofilm transcriptomics reveals outer surface components essential for high density current production in Geobacter sulfurreducens fuel cells. PLoS ONE 4, e5628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niesner R, and Heintz A (2000). Diffusion coefficients of aromatics in aqueous solution. J. Chem. Eng. Data 45, 1121–1124. [Google Scholar]
- Okshevsky M, and Meyer RL (2014). Evaluation of fluorescent stains for visualizing extracellular DNA in biofilms. J. Microbiol. Methods 105, 102–104. [DOI] [PubMed] [Google Scholar]
- Pandit A, Adholeya A, Cahill D, Brau L, and Kochar M (2020). Microbial biofilms in nature: unlocking their potential for agricultural applications. J. Appl. Microbiol 129, 199–211. [DOI] [PubMed] [Google Scholar]
- R Development Core Team (2018). R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing; ). [Google Scholar]
- Ramos I, Dietrich LE, Price-Whelan A, and Newman DK (2010). Phenazines affect biofilm formation by Pseudomonas aeruginosa in similar ways at various scales. Res. Microbiol 161, 187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reguera G, McCarthy KD, Mehta T, Nicoll JS, Tuominen MT, and Lovley DR (2005). Extracellular electron transfer via microbial nanowires. Nature 435, 1098–1101. [DOI] [PubMed] [Google Scholar]
- Richter H, Nevin KP, Jia H, Lowy DA, Lovley DR, and Tender LM (2009). Cyclic voltammetry of biofilms of wild type and mutant Geobacter sulfurreducens on fuel cell anodes indicates possible roles of OmcB, OmcZ, type IV pili, and protons in extracellular electron transfer. Energy Environ. Sci 2, 506. [Google Scholar]
- Robinson D, and Hayes A (2018). broom: Convert Statistical Analysis Objects into Tidy Tibbles (CRAN; ). [Google Scholar]
- Rollefson JB, Stephen CS, Tien M, and Bond DR (2011). Identification of an extracellular polysaccharide network essential for cytochrome anchoring and biofilm formation in Geobacter sulfurreducens. J. Bacteriol 193, 1023–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, and Schmid B (2012). Fiji: an open-source platform for biological-image analysis. Nat. Methods 9, 676–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroth MN, Cho JJ, Green SK, and Kominos SD; Microbiology Society Publishing (2018). Epidemiology of Pseudomonas aeruginosa in agricultural areas. J. Med. Microbiol 67, 1191–1201. [DOI] [PubMed] [Google Scholar]
- Shi L, Dong H, Reguera G, Beyenal H, Lu A, Liu J, Yu HQ, and Fredrickson JK (2016). Extracellular electron transfer mechanisms between microorganisms and minerals. Nat. Rev. Microbiol 14, 651–662. [DOI] [PubMed] [Google Scholar]
- Slinker JD, Muren NB, Gorodetsky AA, and Barton JK (2010). Multiplexed DNA-modified electrodes. J. Am. Chem. Soc 132, 2769–2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slinker JD, Muren NB, Renfrew SE, and Barton JK (2011). DNA charge transport over 34 nm. Nat. Chem 3, 228–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider RM, Strycharz-Glaven SM, Tsoi SD, Erickson JS, and Tender LM (2012). Long-range electron transport in Geobacter sulfurreducens biofilms is redox gradient-driven. Proc. Natl. Acad. Sci. USA 109, 15467–15472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steidl RJ, Lampa-Pastirk S, and Reguera G (2016). Mechanistic stratification in electroactive biofilms of Geobacter sulfurreducens mediated by pilus nanowires. Nat. Commun 7, 12217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberger RE, and Holden PA (2005). Extracellular DNA in single- and multiple-species unsaturated biofilms. Appl. Environ. Microbiol 71, 5404–5410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemp EDA, Arkin MR, and Barton JK (1995). Electron transfer between metallointercalators bound to DNA: Spectral identification of the transient intermediate. J. Am. Chem. Soc 117, 2375–2376. [Google Scholar]
- Stewart PS (2003). Diffusion in biofilms. J. Bacteriol 185, 1485–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart PS, and Franklin MJ (2008). Physiological heterogeneity in biofilms. Nat. Rev. Microbiol 6, 199–210. [DOI] [PubMed] [Google Scholar]
- Strycharz-Glaven SM, Snider RM, Guiseppi-Elie A, and Tender LM (2011). On the electrical conductivity of microbial nanowires and biofilms. Energy Environ. Sci 4, 4366. [Google Scholar]
- Subramanian P, Pirbadian S, El-Naggar MY, and Jensen GJ (2018). Ultrastructure of Shewanella oneidensis MR-1 nanowires revealed by electron cryotomography. Proc. Natl. Acad. Sci. USA 115, E3246–E3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tielen P, Kuhn H, Rosenau F, Jaeger K-E, Flemming H-C, and Wingender J (2013). Interaction between extracellular lipase LipA and the polysaccharide alginate of Pseudomonas aeruginosa. BMC Microbiol 13, 159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse ECM, Zwang TJ, Bedoya S, and Barton JK (2019). Effective Distance for DNA-Mediated Charge Transport between Repair Proteins. ACS Cent. Sci 5, 65–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner JM, and Messenger AJ (1986). Occurrence, biochemistry and physiology of phenazine pigment production. Adv. Microb. Physiol 27, 211–275. [DOI] [PubMed] [Google Scholar]
- Wang Y, and Newman DK (2008). Redox reactions of phenazine antibiotics with ferric (hydr)oxides and molecular oxygen. Environ. Sci. Technol 42, 2380–2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Kern SE, and Newman DK (2010). Endogenous phenazine antibiotics promote anaerobic survival of Pseudomonas aeruginosa via extracellular electron transfer. J. Bacteriol 192, 365–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Gu Y, O’Brien JP, Yi SM, Yalcin SE, Srikanth V, Shen C, Vu D, Ing NL, and Hochbaum AI (2019). Structure of Microbial Nanowires Reveals Stacked Hemes that Transport Electrons over Micrometers. Cell 177, 361–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitchurch CB, Tolker-Nielsen T, Ragas PC, and Mattick JS (2002). Extracellular DNA required for bacterial biofilm formation. Science 295, 1487. [DOI] [PubMed] [Google Scholar]
- White HS, Leddy J, and Bard AJ (1982). Polymer films on electrodes. 8. Investigation of charge-transport mechanisms in Nafion polymer modified electrodes. J. Am. Chem. Soc 104, 4811–4817. [Google Scholar]
- Wickham H (2017). tidyverse: Easily Install and Load the “Tidyverse.” (CRAN; ). [Google Scholar]
- Xu S, Jangir Y, and El-Naggar MY (2016). Disentangling the roles of free and cytochrome-bound flavins in extracellular electron transport from Shewanella oneidensis MR-1. Electrochim. Acta 198, 49–55. [Google Scholar]
- Xu S, Barrozo A, Tender LM, Krylov AI, and El-Naggar MY (2018). Multiheme Cytochrome Mediated Redox Conduction through Shewanella oneidensis MR-1 Cells. J. Am. Chem. Soc 140, 10085–10089. [DOI] [PubMed] [Google Scholar]
- Yates MD, Golden JP, Roy J, Strycharz-Glaven SM, Tsoi S, Erickson JS, El-Naggar MY, Calabrese Barton S, and Tender LM (2015). Thermally activated long range electron transport in living biofilms. Phys. Chem. Chem. Phys 17, 32564–32570. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All code used for data processing and analysis is available in a GitHub repository (https://github.com/DKN-lab/phz_eDNA_2019). Html versions of the Rmarkdown notebooks are also available as a website (https://DKN-lab.github.io/phz_eDNA_2019). Any raw data not included in the repository, such as files in proprietary formats or large imaging files, are available upon request.


