Abstract
The hyperactive RAS and inflammation are closely associated. The angiotensin-II/AT1R axis of the RAS has been explored extensively for its role in inflammation and a plethora of pathological conditions. Understanding the role of AT2R in inflammation is an emerging area of research. The AT2R is expressed on a variety of immune and non-immune cells, which upon activation triggers the release of a host of cytokines and has multiple effects that coalesce to anti-inflammation and prevents maladaptive repair. The anti-inflammatory outcomes of AT2R activation are linked to its well-established signaling pathways involving formation of nitric oxide and activation of phosphatases. Collectively, these effects promote cell survival and tissue function. The consideration of AT2R as a therapeutic target requires further investigations.
Keywords: Angiotensin-II type 2 receptor, inflammation, nitric oxide, pathological conditions, maladaptive repair, immune and non-immune cells
1. INTRODUCTION
The RAS plays a pivotal role in the pathogenesis of hypertension and various renal and cardiovascular diseases. Ang-II is a major hormone of the RAS and acts via AT1R and AT2R. There is well-documented evidence suggesting the role of AT1R in various diseases, including its proinflammatory role and its involvement in hypertension and tissue injury. AT2R later surfaced, owing to the anti-inflammatory effects in immune [1, 2] and non-immune cells [3, 4]. During pathological conditions, a variety of immune cells infiltrate to the effector organs and modulate the organ microenvironment by releasing an array of cytokines, chemokines and effector molecules. These infiltrated cells (mainly granulocytes, lymphocytes and monocytes) interact with the resident cells via chemokines and cytokines and modulate the site of injury initially as a pro-inflammatory deleterious phase followed by an anti-inflammatory repair phase. The RAS has considerable influence on immune cells (adaptive as well as innate), injury and repair.
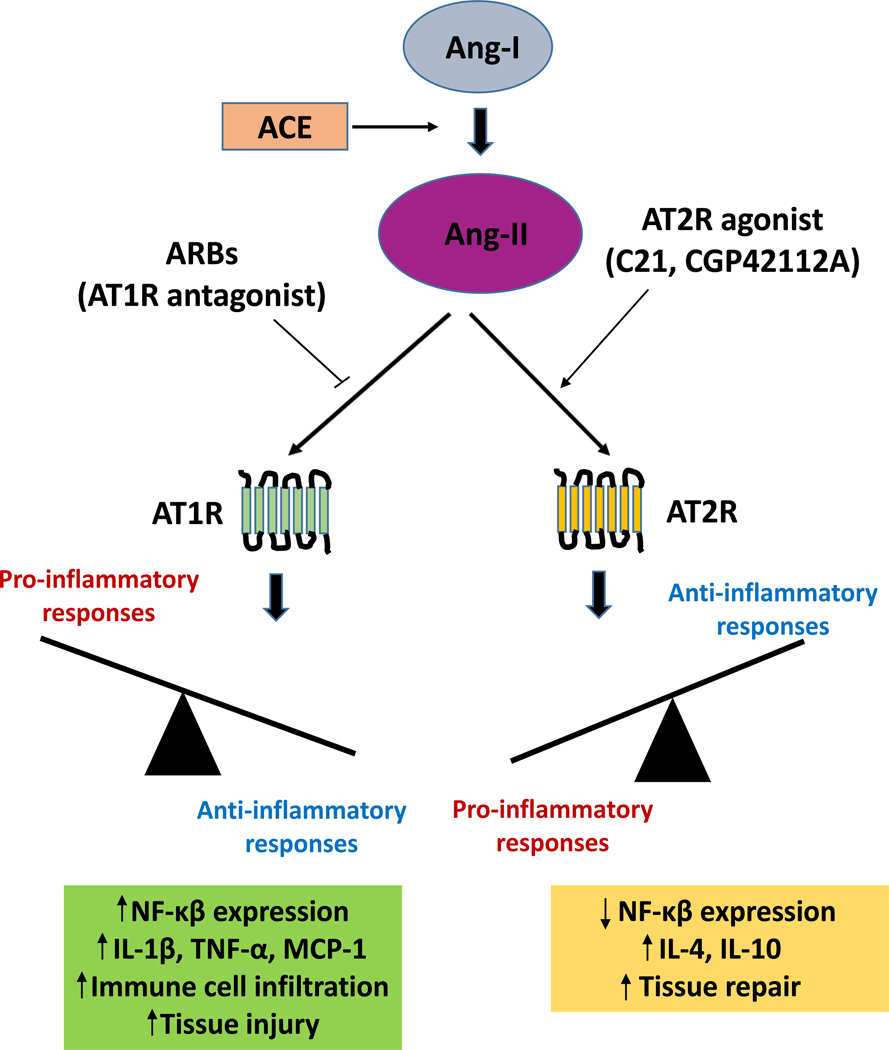
In general, AT2R expression is lower as compared to its counterpart, AT1R [5], but strongly upregulated following tissue injury [6]. Since the discovery of orally bioavailable selective AT2R agonist C21, the anti-inflammatory role of AT2R has become increasingly clear [7]. The goal of this article is to review the expression and function of AT2R in response to pharmacological activation in various immune and non-immune cells and their relationship with organ-specific effects and BP regulation. Fig. (1) summarizes the balance between pro- and anti-inflammatory responses which are differentially regulated by Ang II.
Fig. (1).
Balance between pro- and anti-inflammatory responses regulated by Ang-II.
1.1. Adaptive Immune System
T-cells are the central players of an adaptive immune system. Various T-cells (CD4/CD8) play a pivotal role in pathogen/tumor clearance and maintenance of immune homeostasis. AT2R expressing CD4+ T-cells were elevated in heart failure in human and rodent model. These AT2R+CD4+ T-cells expressed FoxP3 (regulatory T-cells), secreted IL-10 (a major anti-inflammatory cytokine) and other anti-inflammatory cytokines [1]. In vivo AT2R stimulation also leads to an increase in AT2R+ cell population in the infarcted myocardium and reduced apoptosis of cardiomyocytes in rats with acute myocardial infarction [6]. Additionally, AT2R expression was also detected in a population of CD8 T-cells infiltrating in the periinfarct myocardium. A subset of CD8+AT2R+ was detected seven days after myocardial infarction in rats. These cells showed upregulated anti-inflammatory IL-10 and downregulated pro-inflammatory IL-2 and IFN-γ [2], which was thought to be a part of the protective response to counter AT1R mediated aggravation of cardiomyocytes.
Recently, a population of CD4+AT2R+ cells was identified in thoracic aortic aneurysm, a progressive fatal aortic pathological dilation. These cells displayed an inhibitory effect on proliferation and MMP2 expression in endothelial cells as opposed to the CD4+AT2R− cells which promoted proliferation and MMP2 expression in endothelial cells [8]. AT1R through the activation of immune cells induces arterial inward remodeling and thus reduced blood flow in cardiovascular disorders. On the contrary, AT2R expressing T lymphocytes, by secreting IL-17, have been involved in flow (shear stress) mediated outward remodeling, collateral arteries growth in ischemic diseases and revascularization. Mice lacking AT2R or athymic mice (lacking T-cells) did not observe flow (shear stress) mediated outward remodeling [9].
The role of other immune cells like B cells, NK cells and NKT (natural killer T) cells has rarely been studied in renal or cardiac injury model. However, the AT1R and AT2R expression on uterine NK cells and their role in BP management during pregnancy in mice have been reported [10]. This further supports the notion that T-cells also have a role to play in renal, cardiac and neuronal pathologies resulting from stroke or ischemic attack. This may pave the ways to further explore the possibilities of studying AT2R expression on immune cells in pathological conditions such as in chronic and acute renal injuries. Also, we have substantial evidence that AT2R signaling can be modulated using receptor-ligand interactions as studied by our lab and many others [3, 11–13]. Another study provides evidence that T-cells and NK cells possess a fully functional RAS, and the ability to produce and deliver Ang-II to inflammatory sites. It revealed the presence of renin, its receptor, angiotensinogen and ACE in these cells by mRNA analysis. Both the AT1R and AT2R are expressed, and serve their functions in chemotaxis, cell proliferation and calcium signaling. However, antagonists specific to these receptors could not completely abolish the Ang-II-mediated effects, which indicates that another functional angiotensin receptor might be present in T-cells and NK cells and involved in these effects [14].
1.2. Innate Immune System
The innate immune response acts as the first line of defense against an array of pathogens. It is non-specific, and acts rapidly, beginning within a few hours of infection [15]. The major components of the innate immune system include physical barriers, such as epidermis of the skin, tears, saliva, mucus membranes and their secretions, and cells such as macrophages, DCs and neutrophils, which are the major phagocytic cells of the immune system. Besides, leukocytes including basophils and eosinophils are also important for pathogen clearance and immunoregulation [16]. Research establishing a link between innate immune system and hypertension has flourished in the recent years. While the role of AT1R present on the immune cells has been sufficiently discussed in the past, there is only a limited discussion on the role of AT2R in this context [17–18].
1.2.1. Monocytes/macrophages
Almost two decades ago, it was reported that macrophages express all the components of RAS, except chymase. A 6-fold increase in AT2R expression was found during the differentiation of monocytes to macrophages which may be involved in the development of atherosclerosis [19]. AT2R was upregulated in the glomerular cells and macrophages, thereby attenuating acute glomerular lesions in glomerulonephritis [20]. Another study on adjuvant-induced arthritis rat model showed that direct AT2R stimulation with agonist CGP42112 in vitro inhibited the activity of IL-1β-stimulated monocytes, along with AT1R downregulation and AT2R upregulation in the stimulated monocytes [21]. The anti-inflammatory role of AT2R activation by its agonist C21, in TLR-4 mediated inflammation indicated a critical role for IL-10. This study also provides evidence regarding the involvement of ERK1/2 pathway in the release of IL-10 following AT2R stimulation in macrophages [22]. In a similar study, the direct activation of AT2R on THP-1 and U937 cells by agonist C21 was anti-inflammatory since it attenuated the early inflammatory responses mediated by TLR-4, through the regulation of pro- and anti-inflammatory cytokines at both the gene and protein level [23]. In an experimental mice model of AE, direct activation of AT2R by agonist C21 inhibited the activation of resting microglia, and infiltration of the activated microglia (a subpopulation of macrophages in the brain) in the lumbar spinal cord [24]. It was confirmed by this study that AT2R was expressed in microglia, T-cells, spinal cord and brain aggregates.
In contrast to the anti-inflammatory role of AT2R, peripheral macrophages expressing AT2R predominantly infiltrate the site of nerve injury and are critical for triggering pain sensitization. Selective AT2R antagonism by the depletion of peripheral macrophages chemo-genetically and transplantation of AT2R-null hematopoietic cells was found beneficial in inhibiting the neuropathic pain hypersensitivity [25]. In another study by the same group of researchers, it was observed that the activation of AT2R on peripheral/skin macrophages that infiltrate the injury site, triggers the production of ROS and RNS. This mediated the trans-activation of cell damage sensing ion channel TRPA1, resulting in the excitation of mouse and human sensory dorsal root ganglion which represents a peripheral mechanism for the induction of chronic neuropathic pain [26]. This ROS and RNS production was scavenged by AT2R antagonist PD123319 and N-acetyl cysteine, which indicates a mechanism for the attenuation of nociceptor excitation to provide relief from pain.
Considerable evidence suggests that AT2R has antihypertensive, anti-inflammatory and anti-proliferative effect, but some of the above mentioned recent studies highlighting the role of macrophage AT2R in promoting chronic neuropathic pain hypersensitivity would impose a challenge on the existing knowledge about AT2R, and necessitates elucidating other properties of this receptor, which may have not been addressed yet.
1.2.2. Dendritic Cells
For the first time, the differential expression of RAS components was identified in immature and mature DCs by using human cDNA microarray (27). The blockade of AT2R resulted in the development of DCs expressing significantly higher levels of CD1a, a well-characterized marker for DCs differentiation, with high endocytic capacity and allostimulatory activities, as compared to control DCs (28). It was also reported that DCs sufficiently express the components of RAS including the two axis: ACE-Ang II-ATjR and the ACE2-Ang-(1–7)-MasR. Phosphorylation of ERK1/2 induced by Ang-II was markedly enhanced by Ang-(1–7) co-treatment, but this effect was significantly inhibited by AT2R antagonist PD123319 [29]. In another study, the importance of Ang-II in the regulation of DCs was highlighted as being pro-inflammatory and immunomodulatory in function. The proliferation and phagocytic activity of DCs was markedly inhibited by Ang-II treatment, but the maturation of DCs and their migratory activity were significantly enhanced. However, it did not indicate any specific role of AT2R in the maturation or proliferation of DCs [30].
1.2.3. Neutrophils
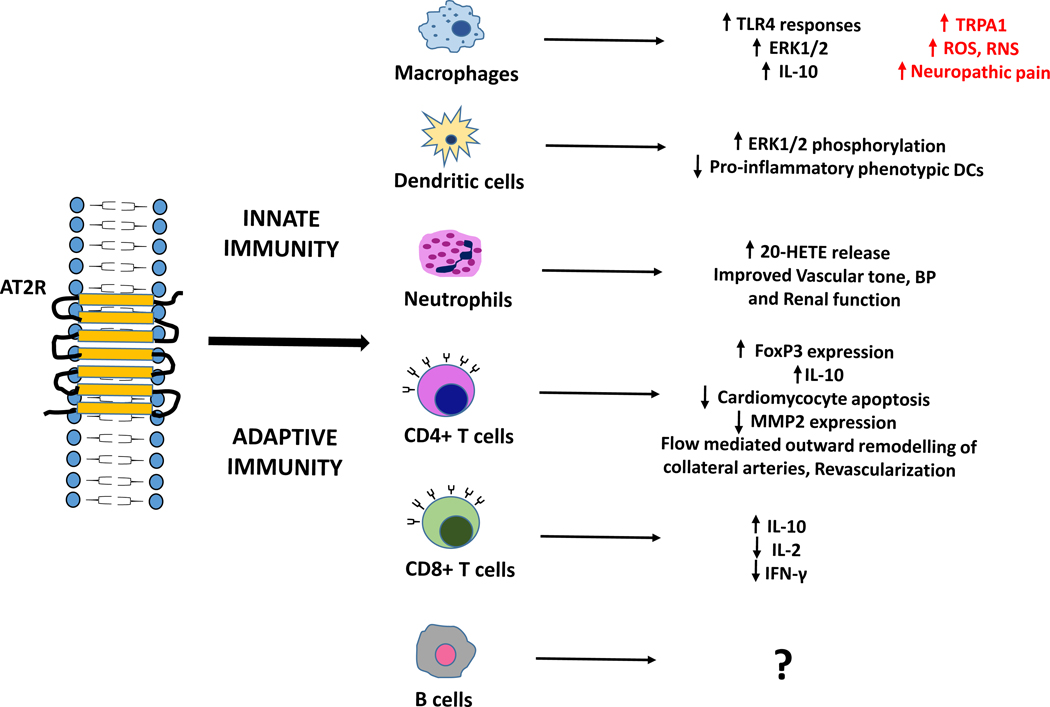
Neutrophils overexpressing ACE were more efficient in killing methicillin-resistant Staphylococcus aureus during infection, probably due to increased production of ROS, especially the superoxide radical generation by neutrophils. This indicates that neutrophils overexpressing ACE could boost the immune response to bacterial infections which are antibiotic-resistant [31]. However, the specific role of AT2R in these studies was not reported. Since AT2R is important for Ang-II induced mechanical hypersensitivity, Ang-II-induced infiltration of macrophages and neutrophils in the hind paw of the mouse was investigated for AT2R expression. However, no amplification for either AT1R or AT2R could be seen in peritoneal PMN [26]. In another study, human neutrophil and platelet content, and release of 20-HETE, which is synthesized in response to Ang-II and essential for regulating vascular tone, BP and renal function, was significantly enhanced by Ang-II treatment predominantly via AT2R. The receptor blockade by its antagonist PD123319 markedly reduced both the content and release of 20-HETE from neutrophils and platelets [32]. These findings related to the role of AT2R in innate and adaptive immunity have been summarized in Fig. (2).
Fig. (2).
Role of AT2R in innate and adaptive immunity.
1.3. Non-immune Cells
1.3.1. Vascular Endothelial Cells
Endothelial cells harbor and release a number of factors involved in (patho)physiology of hypertension. NO is one of these factors critically involved in vasodilation. Endothelial dysfunction, regardless of the type of injury leading to decreased bioavailability of NO, contributes to change in cell surface electrostatic charge and increases permeability to proteins and inflammatory cells [33]. AT2R is strongly linked to NO formation [34, 35] which is corroborated by studies showing exacerbation of oxidative stress and injury in AT2R/ApoE-double KO mice [36] and reduced expression of inflammatory mediators in atherosclerotic plaque during overexpression of AT2R [37, 38]. C21 also reduces aortic infiltration of immune cells and vascular stiffness by reducing ECM components (fibronectin, collagen) [39]. Additionally, anti-inflammatory effects of AT2R may encompass suppressed expression and signaling of lectin-like oxidized low-density lipoprotein scavenger receptor-1, a scavenger receptor of ox-LDL [38, 40] which requires further elucidation.
Obesity is characterized by chronic low-grade inflammation and is associated with impairment in sodium excretion. Accumulation of sodium is also linked to endothelial glycocalyx damage and impairment in vascular permeability [41]. We have observed infiltration of CD68+ monocyte/macrophage in obese kidney which was reduced considerably with AT2R agonist C21 treatment [42]. As AT2R function is linked to NO formation and sodium excretion [43, 44], the AT2R-mediated reduction in immune cell infiltration may partly involve reduction in sodium accumulation and preservation of vascular permeability.
1.3.2. Vascular Smooth Muscle Cells
The Ang-II is a potent stimulator of cytokine release and expression of MCP-1 and other mediators (ICAM-1, VCAM-1, MMP, plasminogen activation inhibitor) involved in vascular inflammation through NFκB activation. The AT2R function is associated with activation of phosphatases. The expression of AT2R is transcriptionally upregulated during vascular injury. This suggests that pharmacological activation can be targeted to contain inflammation. Additionally, anti-inflammatory effects of AT2R may also partly involve relaxation of vasculature that involves NO formation, inhibition of ROS and RhoA-Rho kinase-dependent myosin light chain phosphorylation and modulation of calcium sensitivity [45].
Tyrosine and serine phosphorylation of STATs is critical for its activation and IFN-γ signaling. IFN-γ and many other signaling molecules (IL-1β, Ang-II, glucose and insulin) upregulate AT2R expression through interferon regulatory factor-1 [46]. Hence, AT2R is speculated to have immunomodulatory function in VSMCs. This finding has been supported by another study performed in PC12W cells that expresses AT2R but not ATiR. AT2R stimulation by CGP42112 reduced STAT phosphorylation and TNF-α production [47].
1.3.3. Renal Epithelial Cells
RPTCs are vulnerable to a number of inflammatory stimuli including hypertension, proteinuria, endotoxemia (lipopolysaccharide), ischemia, glucose, fatty acids, etc.
1.3.3.1. Hypertension
Sodium may accumulate with or without fluid accumulation in the renal interstitium [48]. Intake of HSD [43] and experimental hypertensive simulations (e.g. 2-kidney-1-clip) have been associated with angiotensinemia. Ang-II is reported to facilitate sodium reabsorption which may further accelerate hypertensive phenotype [49]. Ang-II also may directly induce expression of cytokines (IL-1β, IL-6, IL-8, TNF-α) in epithelial cells [69]. Particularly in 2-kidney-1-clip hypertensive model, the AT2R agonist C21 treatment reduced mRNA and protein expression of cytokines (TNF-α, IL-6 and TGF-β1) in renal interstitial fluid [50]. In stroke-prone SHR rats, C21 reduced ED1+ monocytes/macrophages infiltration, expression of collagen and tubular damage [51].
1.3.3.2. Proteinuria
Diet low [52] or rich [53] in sodium can modify immune response and kidney disease progression. Proteinuria is frequently associated with HSD intake and is an indicator of several comorbid conditions including obesity, diabetes and hypertension. The exposure of RPTCs to luminal protein (albumin) load per se has been reported to activate PKC-NOX-NFκB pathway [54] and aid in release of potent pro-inflammatory mediators from epithelial cells and influx of leukocytes causing injury and apoptosis [55]. AT2R is located in RPTCs and has been reported to be upregulated during obesity [56]. Pharmacological stimulation of AT2R by CGP42112a has been reported to reduce inflammatory mediators in obese rat kidney [57]. Likewise, another selective AT2R agonist C21 has been reported to reduce proteinuria [51, 58] and several other inflammatory stimuli including Ang-II [59,60], hypertension [60, 61] and oxidative stress [57, 58]. In another study, anti-proteinuric and anti-oxidative benefits upon chronic treatment with C21 were correlated with anti-inflammatory changes in STZ-induced diabetic kidney [62].
1.3.3.3. Endotoxemia
In HK-2 cells, we have reported that AT2R stimulation via NO formation stimulates the release of anti-inflammatory IL-10, which may have reduced the release of pro-inflammatory cytokines (TNF-α, IL-6) upon endotoxemic challenge with LPS [42]. This change is paralleled in obese Zucker rat (a model of low-grade endotoxemia), showing reductions in circulating and renal TNF-α and IL-6 upon C21 treatment [42]. Moreover, acute administration of C21 has been reported to increase renal IL-10, which is hypothesized to reduce renal content of MCP-1 and IL-6 and limit infiltration of CD11b+ leukocytes in mice kidney challenged with LPS [3].
1.3.3.4. Ischemia
The anti-inflammatory role of AT2R in animal models of ischemic renal injury has been recognized based on early findings showing aggravation of renal interstitial infiltration and activation of macrophages and fibrocytes upon deletion of AT2R [63]. Along the same line, pharmacological activation of AT2R by C21 demonstrated reduced myeloperoxidase activity (marker of neutrophil influx) and circulating MCP-1 in rats subjected to myocardial ischemia [64].
Ischemia also causes cell cycle arrest in RPTCs. Such cells are prone to produce and release growth factors (i.e. TGF-β1, MCP-1) which through paracrine signaling, can activate and transform adjacent pericytes and fibroblast to myofibroblast with marked upregulation of collagen [65]. RPTCs may also acquire mesenchymal characteristics (secretory, motility and plasticity), undergo remodeling and transform into fibroblasts via multiple pathways in response to numerous stimuli [65–67]. In HK-2 cells, AT2R is co-localized with TGF-βRII and CGP stimulation strengthens AT2R-TGFβRII interaction, reduces TGFβRII expression and prevents epithelial-to-mesenchymal transition in NO-dependent manner [68]. Renal tubules may directly release these stimuli and participate in propagation of oxidative stress, activation of inflammasome, recruitment of immune cells and formation of ECM. The latter involves either synthesis or inhibition of breakdown. The breakdown process is dependent on a balance between MMP and tissue inhibitor of MMP [67]. However, the mechanism through which AT2R reduces ECM is not known and needs to be explored.
1.4. Pulmonary Epithelial Cells
Anti-inflammatory effects of AT2R were estimated to extend to lung parenchyma based on reports showing aggravated acid-induced or sepsis-induced acute lung injury [69]. This finding has been corroborated by a report showing reduced monocytic, neutrophilic and eosinophilic infiltration and lung injury in neonatal rat pups exposed to hyperoxia upon administration of novel and highly specific AT2R ligand MOR107 [70]. Hyperoxia is a stimulus associated with inflammation and oxidative stress in respiratory diseases [71]. However, there were some inconsistencies as the anti-inflammatory effects of AT2R agonist MOR107 did not correlate with mRNA expression of potent chemoattractants (MCP-1, chemokine-induced neutrophilic chemoattractant-1) and other indices of lung injury such as alveolarization, vascularization and capillary alveolar leakage [70].
1.5. Neuronal Cells
1.5.1. Ischemic Stroke
The potential of AT2R as an anti-inflammatory target emerged from a study showing greater ischemic injury and neurological deficits in AT2R-KO mice as compared to wild-type mice and ATiR blockade was less effective in containing injury in AT2R-KO mice as compared to wild-type mice [72]. Pharmacological activation of AT2R reduced the activation of microglia (local antigen presenting cell), apoptosis, infarct volume and motor deficits, and increased neuronal survival in a BP and CBF-independent manner in a focal reperfusion model of stroke-induced in conscious SHR by administering endothelin-1 (inflammatory vasoconstrictor) to the middle cerebral artery through a surgically implanted cannula [73–75]. However, one study showed improvement in CBF with C21 treatment after stroke induction by middle cerebral artery occlusion [76]. AT2R-coupled vasodilatory NO can modulate neuronal bioavailability of free radicals [77]. Hence, anti-inflammatory effect of AT2R in microglia can be ascribed to cerebral vasorelaxation [75], phosphatase-mediated direct inhibition of PKC activation and p47phox phosphorylation [78] and superoxide formation [74, 76]. The neuronal survival exerted by AT2R in animals subjected to cerebral ischemia is partly dependent on hypoxia-inducible factor-α [79], brain-derived neurotrophic factor [24, 79], and vascular endothelial growth factor, which is involved in the regenerative processes [80]. These effects are AT2R-specific as PD123319 totally [75, 78, 80] or partially reversed [73, 74]. In another study, peripheral administration of C21 after stroke induction reduced the inflammatory markers in cerebral cortex 24-hr post-ischemic stroke [81]. Moreover, the role of AT2R in revascularization [82], in improving regenerative efficacy and migration of bone marrow mononuclear cells [83, 84] and mesenchymal stem cells [85, 86], and in cell survival [6, 83, 84] has been established.
The anti-inflammatory attributes of AT2R in non-immune cells are summarized in Table 1.
Table 1.
Role of AT2R as anti-inflammatory in non-immune cells.
| Non-immune Cell Types | AT2R Anti-Inflammatory Functions | References | |
|---|---|---|---|
| Vascular endotdelial cells | Reduced aortic immune cell infiltration Decreased fibronectin and collagen Reduced vascular stiffness Reduced CD68+ monocyte/macrophage in obese kidney | [39, 42] | |
| Vascular smooth muscle cells | Decreased ROS, enhanced NO bioavailability, myosin light chain phosphorylation, modulation of Ca2+ sensitivity, Reduced STAT phosphorylation | [45, 47] | |
| Renal epithelial cells | Hypertension | Reduced IL-6, TNF-α and ED1+ monocyte/macrophage infiltration Reduced tubular damage | [50, 51, 69] |
| Endotoxemia | Increased NO formation and IL-10, reduced CD11b+ leukocytes infiltration in AKI | [3, 42] | |
| Ischemia | Decreased neutrophilic influx, MCP-1 and inflammasome activation | [64, 67] | |
| Pulmonary epithelial cells | Reduced monocytic, eosinophilic and neutrophilic infiltration during lung injury | [70] | |
| Neuronal cells | Decreased PKC activation and enhanced NO bioavailability Reduced superoxide formation Cerebral vasorelaxation during ischemic stroke | [74, 75, 78] | |
CONCLUSION
The anti-inflammatory function of AT2R has been sufficiently evidenced by its involvement in modulating numerous key mediators (Ang-II, cytokines, chemokines, and oxidative stress) that establishes communicative pathways amid non-immune cells (neuronal, epithelial, endothelial and smooth cells) and cells of innate and adaptive immunity. The anti-inflammatory effects of AT2R seem to be BP-independent [39, 50] and involve NO formation and activation of phosphatases as an underlying mechanism. The benefits of AT2R activation are not only limited to hypertension, CKD, obesity, diabetes, pulmonary arterial hypertension and atherosclerosis [53] but extend to retinal diseases [87] and autoimmune diseases such as encephalomyelitis [24, 88, 89] and rheumatoid arthritis [90]. Anti-inflammation mediated by AT2R activation is promising in myocardial infarction and cardiac repair [64, 84]. However, further studies are needed to investigate the therapeutic utility of AT2R since macrophage AT2R promotes chronic neuropathic pain hypersensitivity.
Acknowledgments
FUNDING
Financial support from the National Institutes of Health NIDDK R01 DK117495 and R01 DK61578, USA is acknowledged.
LIST OF ABBREVIATIONS
- ACE
Angiotensin converting enzyme
- AE
Autoimmune encephalomyelitis
- AKI
Acute kidney injury
- Ang-I
Angiotensin-I
- Ang-II
Angiotensin-II
- ARBs
Angiotensin receptor blockers
- AT1R
Angiotensin-II type 1 receptor
- AT2R
Angiotensin-II type 2 receptor
- AT2R-KO
Angiotensin-II type 2 receptor knock out
- BP
Blood pressure
- CBF
Cerebral blood flow
- CKD
Chronic kidney diseases
- DCs
Dendritic cells
- ECM
Extracellular matrix
- ERK
Extracellular signal-regulated kinase
- 20-HETE
20-hydroxyeicosatetraenoic acid
- HK-2
Human kidney-2
- ICAM-1
Intracellular cell adhesion molecule-1
- IL-1β
Interleukin-1β
- IL-10
Interleukin-10
- IL-6
Interleukin-6
- MCP-1
Monocyte chemoattractant protein-1
- MMP
Matrix metalloproteinase
- NFκB
Nuclear factor kappa B
- NK
Natural killer
- NKT
Natural killer T lymphocytes
- NO
Nitric oxide
- NOX
NADPH oxidase
- ox-LDL
Oxidized LDL
- PMN
Polymorphonuclear
- RAS
Renin-angiotensin system
- ROS
Reactive oxygen species
- RPTCs
Renal proximal tubular epithelial cells
- RNS
Reactive nitrogen species
- SHR
Spontaneously hypertensive rat
- STAT
Signal transducers and activators of transcription
- TGF-β1
Transforming growth factor-β1
- TGFβRII
TGF-β1 receptor II
- TNF-α
Tumor necrosis factor-α
- VCAM-1
Vascular cell adhesion molecule-1
- VSMCs
Vascular smooth muscle cells
Footnotes
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- [1].Skorska A, von Haehling S, Ludwig M, et al. The CD4(+) AT2R(+) T cell subpopulation improves post-infarction remodelling and restores cardiac function. J Cell Mol Med 2015; 19(8): 1975–85. 10.1111/jcmm.12574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Curato C, Slavic S, Dong J, et al. Identification of noncytotoxic and IL-10-producing CD8+AT2R+ T cell population in response to ischemic heart injury. J Immunol 2010; 185(10): 6286–93. 10.4049/jimmunol.0903681 [DOI] [PubMed] [Google Scholar]
- [3].Patel S, Dhande I, Gray EA, Ali Q, Hussain T. Prevention of lipopolysaccharide-induced CD11b+ immune cell infiltration in the kidney: role of AT2 receptors. Biosci Rep 2019; 39(5): BSR20190429. 10.1042/BSR20190429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rompe F, Artuc M, Hallberg A, et al. Direct angiotensin II type 2 receptor stimulation acts anti-inflammatory through epoxyeicosatrienoic acid and inhibition of nuclear factor kappaB. Hypertension 2010; 55(4): 924–31. 10.1161/HYPERTENSIONAHA.109.147843 [DOI] [PubMed] [Google Scholar]
- [5].Steckelings UM, Rompe F, Kaschina E, et al. The past, present and future of angiotensin II type 2 receptor stimulation. J Renin Angiotensin Aldosterone Syst 2010; 11(1): 67–73. 10.1177/1470320309347791 [DOI] [PubMed] [Google Scholar]
- [6].Altarche-Xifró W, Curato C, Kaschina E, et al. Cardiac c-kit+AT2+ cell population is increased in response to ischemic injury and supports cardiomyocyte performance. Stem Cells 2009; 27(10): 2488–97. 10.1002/stem.171 [DOI] [PubMed] [Google Scholar]
- [7].Wan Y, Wallinder C, Plouffe B, et al. Design, synthesis, and biological evaluation of the first selective nonpeptide AT2 receptor agonist. J Med Chem 2004; 47(24): 5995–6008. 10.1021/jm049715t [DOI] [PubMed] [Google Scholar]
- [8].Wang C, Wu T, Hu X, et al. Identification and characterization of CD4(+)AT2(+) T lymphocyte population in human thoracic aortic aneurysm. Am J Transl Res 2015; 7(2): 232–41. [PMC free article] [PubMed] [Google Scholar]
- [9].Caillon A, Grenier C, Grimaud L, et al. The angiotensin II type 2 receptor activates flow-mediated outward remodelling through T cells-dependent interleukin-17 production. Cardiovasc Res 2016; 112(1): 515–25. 10.1093/cvr/cvw172 [DOI] [PubMed] [Google Scholar]
- [10].Hatta K, Carter AL, Chen Z, et al. Expression of the vasoactive proteins AT1, AT2, and ANP by pregnancy-induced mouse uterine natural killer cells. Reprod Sci 2011; 18(4): 383–90. 10.1177/1933719110385136 [DOI] [PubMed] [Google Scholar]
- [11].Ahmed HA, Ishrat T, Pillai B, et al. Angiotensin receptor (AT2R) agonist C21 prevents cognitive decline after permanent stroke in aged animals-A randomized double-blind pre-clinical study. Behav Brain Res 2019; 359: 560–9. 10.1016/j.bbr.2018.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Castoldi G, di Gioia CRT, Roma F, et al. Activation of angiotensin type 2 (AT2) receptors prevents myocardial hypertrophy in Zucker diabetic fatty rats. Acta Diabetol 2019; 56(1): 97–104. 10.1007/s00592-018-1220-1 [DOI] [PubMed] [Google Scholar]
- [13].Lange C, Sommerfeld M, Namsolleck P, Kintscher U, Unger T, Kaschina E. AT2R (angiotensin AT2 receptor) agonist, compound 21, prevents abdominal aortic aneurysm progression in the rat. Hypertension 2018; 72(3): e20–9. 10.1161/HYPERTENSIONAHA.118.11168 [DOI] [PubMed] [Google Scholar]
- [14].Jurewicz M, McDermott DH, Sechler JM, et al. Human T and natural killer cells possess a functional renin-angiotensin system: further mechanisms of angiotensin II-induced inflammation. J Am Soc Nephrol 2007; 18(4): 1093–102. 10.1681/ASN.2006070707 [DOI] [PubMed] [Google Scholar]
- [15].Chaplin DD. Overview of the immune response. J Allergy Clin Immunol 2010; 125(2)(Suppl. 2): S3–23. 10.1016/j.jaci.2009.12.980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Geering B, Stoeckle C, Conus S, Simon HU. Living and dying for inflammation: neutrophils, eosinophils, basophils. Trends Immunol 2013; 34(8): 398–409. 10.1016/j.it.2013.04.002 [DOI] [PubMed] [Google Scholar]
- [17].Yang T, Xu C. Physiology and pathophysiology of the intrarenal renin-angiotensin system: an update. J Am Soc Nephrol 2017; 28(4): 1040–9. 10.1681/ASN.2016070734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Ribeiro-Oliveira A Jr, Nogueira AI, Pereira RM, Boas WW, Dos Santos RA, Simões e Silva AC. The renin-angiotensin system and diabetes: an update. Vasc Health Risk Manag 2008; 4(4): 787–803. [PMC free article] [PubMed] [Google Scholar]
- [19].Okamura A, Rakugi H, Ohishi M, et al. Upregulation of renin-angiotensin system during differentiation of monocytes to macrophages. J Hypertens 1999; 17(4): 537–45. 10.1097/00004872-199917040-00012 [DOI] [PubMed] [Google Scholar]
- [20].Mii A, Shimizu A, Masuda Y, et al. Angiotensin II receptor blockade inhibits acute glomerular injuries with the alteration of receptor expression. Lab Invest 2009; 89(2): 164–77. 10.1038/labinvest.2008.128 [DOI] [PubMed] [Google Scholar]
- [21].Wang D, Hu S, Zhu J, et al. Angiotensin II type 2 receptor correlates with therapeutic effects of losartan in rats with adjuvant-induced arthritis. J Cell Mol Med 2013; 17(12): 1577–87. 10.1111/jcmm.12128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Dhande I, Ma W, Hussain T. Angiotensin AT2 receptor stimulation is anti-inflammatory in lipopolysaccharide-activated THP-1 macrophages via increased interleukin-10 production. Hypertens Res 2015; 38(1): 21–9. 10.1038/hr.2014.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Menk M, Graw JA, von Haefen C, et al. Stimulation of the angiotensin II AT2 receptor is anti-inflammatory in human lipopolysaccharide-activated monocytic cells. Inflammation 2015; 38(4): 1690–9. 10.1007/s10753-015-0146-9 [DOI] [PubMed] [Google Scholar]
- [24].Valero-Esquitino V, Lucht K, Namsolleck P, et al. Direct angiotensin type 2 receptor (AT2R) stimulation attenuates T-cell and microglia activation and prevents demyelination in experimental autoimmune encephalomyelitis in mice. Clin Sci (Lond) 2015; 128(2): 95–109. 10.1042/CS20130601 [DOI] [PubMed] [Google Scholar]
- [25].Shepherd AJ, Mickle AD, Golden JP, et al. Macrophage angiotensin II type 2 receptor triggers neuropathic pain. Proc Natl Acad Sci USA 2018; 115(34): E8057–66. 10.1073/pnas.1721815115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Shepherd AJ, Copits BA, Mickle AD, et al. Angiotensin II triggers peripheral macrophage-to-sensory neuron redox crosstalk to elicit pain. J Neurosci 2018; 38(32): 7032–57. 10.1523/JNEUROSCI.3542-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lapteva N, Nieda M, Ando Y, et al. Expression of renin-angiotensin system genes in immature and mature dendritic cells identified using human cDNA microarray. Biochem Biophys Res Commun 2001; 285(4): 1059–65. 10.1006/bbrc.2001.5215 [DOI] [PubMed] [Google Scholar]
- [28].Nahmod KA, Vermeulen ME, Raiden S, et al. Control of dendritic cell differentiation by angiotensin II. FASEB J 2003; 17(3): 491–3. 10.1096/fj.02-0755fje [DOI] [PubMed] [Google Scholar]
- [29].Nie W, Yan H, Li S, et al. Angiotensin-(1–7) enhances angiotensin II induced phosphorylation of ERK1/2 in mouse bone marrow-derived dendritic cells. Mol Immunol 2009; 46(3): 355–61. 10.1016/j.molimm.2008.10.022 [DOI] [PubMed] [Google Scholar]
- [30].Meng Y, Chen C, Liu Y, Tian C, Li HH. Angiotensin II regulates dendritic cells through activation of NF-κB/p65, ERK1/2 and STAT1 pathways. Cell Physiol Biochem 2017; 42(4): 1550–8. 10.1159/000479272 [DOI] [PubMed] [Google Scholar]
- [31].Khan Z, Shen XZ, Bernstein EA, et al. Angiotensin-converting enzyme enhances the oxidative response and bactericidal activity of neutrophils. Blood 2017; 130(3): 328–39. 10.1182/blood-2016-11-752006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Tsai IJ, Croft KD, Puddey IB, Beilin LJ, Barden A. 20-Hydroxyeicosatetraenoic acid synthesis is increased in human neutrophils and platelets by angiotensin II and endothelin-1. Am J Physiol Heart Circ Physiol 2011; 300(4): H1194–200. 10.1152/ajpheart.00733.2010 [DOI] [PubMed] [Google Scholar]
- [33].Ruggenenti P, Remuzzi G. Time to abandon microalbuminuria?. Kidney Int 2006; 70(7): 1214–22. 10.1038/sj.ki.5001729 [DOI] [PubMed] [Google Scholar]
- [34].Toedebusch R, Belenchia A, Pulakat L. Cell-specific protective signaling induced by the novel AT2R-agonist NP-6A4 on human endothelial and smooth muscle cells. Front Pharmacol 2018; 9: 928 10.3389/fphar.2018.00928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Taguchi K, Matsumoto T, Kamata K, Kobayashi T. Angiotensin II type 2 receptor-dependent increase in nitric oxide synthase activity in the endothelium of db/db mice is mediated via a MEK pathway. Pharmacol Res 2012; 66(1): 41–50. 10.1016/j.phrs.2012.02.010 [DOI] [PubMed] [Google Scholar]
- [36].Iwai M, Chen R, Li Z, et al. Deletion of angiotensin II type 2 receptor exaggerated atherosclerosis in apolipoprotein E-null mice. Circulation 2005; 112(11): 1636–43. 10.1161/CIRCULATIONAHA.104.525550 [DOI] [PubMed] [Google Scholar]
- [37].Dandapat A, Hu CP, Chen J, et al. Over-expression of angiotensin II type 2 receptor (agtr2) decreases collagen accumulation in atherosclerotic plaque. Biochem Biophys Res Commun 2008; 366(4): 871–7. 10.1016/j.bbrc.2007.11.061 [DOI] [PubMed] [Google Scholar]
- [38].Hu C, Dandapat A, Chen J, et al. Over-expression of angiotensin II type 2 receptor (agtr2) reduces atherogenesis and modulates LOX-1, endothelial nitric oxide synthase and heme-oxygenase-1 expression. Atherosclerosis 2008; 199(2): 288–94. 10.1016/j.atherosclerosis.2007.11.006 [DOI] [PubMed] [Google Scholar]
- [39].Rehman A, Leibowitz A, Yamamoto N, Rautureau Y, Paradis P, Schiffrin EL. Angiotensin type 2 receptor agonist compound 21 reduces vascular injury and myocardial fibrosis in stroke-prone spontaneously hypertensive rats. Hypertension 2012; 59(2): 291–9. 10.1161/HYPERTENSIONAHA.111.180158 [DOI] [PubMed] [Google Scholar]
- [40].Wang X, Phillips MI, Mehta JL. LOX-1 and angiotensin receptors, and their interplay. Cardiovasc Drugs Ther 2011; 25(5): 401–17. 10.1007/s10557-011-6331-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Oberleithner H, Peters W, Kusche-Vihrog K, et al. Salt overload damages the glycocalyx sodium barrier of vascular endothelium. Pflugers Arch 2011; 462(4): 519–28. 10.1007/s00424-011-0999-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Dhande I, Ali Q, Hussain T. Proximal tubule angiotensin AT2 receptors mediate an anti-inflammatory response via interleukin-10: role in renoprotection in obese rats. Hypertension 2013; 61(6): 1218–26. 10.1161/HYPERTENSIONAHA.111.00422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Ali Q, Hussain T. AT2 receptor non-peptide agonist C21 promotes natriuresis in obese Zucker rats. Hypertens Res 2012; 35(6): 654–60. 10.1038/hr.2012.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Carey RM, Padia SH. Angiotensin AT2 receptors: control of renal sodium excretion and blood pressure. Trends Endocrinol Metab 2008; 19(3): 84–7. 10.1016/j.tem.2008.01.003 [DOI] [PubMed] [Google Scholar]
- [45].Savoia C, Tabet F, Yao G, Schiffrin EL. M. R, Touyz RM. Negative regulation of RhoA-Rho kinase by angiotensin II type 2 receptor in vascular smooth muscle cells - role in angiotensin II-induced vasodilation in stroke-prone SHR. J Hypertens 2005; 23: 1037–45. 10.1097/01.hjh.0000166845.49850.39 [DOI] [PubMed] [Google Scholar]
- [46].Ali Q, Sabuhi R, Hussain T. High glucose up-regulates angiotensin II subtype 2 receptors via interferon regulatory factor-1 in proximal tubule epithelial cells. Mol Cell Biochem 2010; 344(1–2): 65–71. 10.1007/s11010-010-0529-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Abadir PM, Walston JD, Carey RM, Siragy HM. Angiotensin II Type-2 receptors modulate inflammation through signal transducer and activator of transcription proteins 3 phosphorylation and TNFα production. J Interferon Cytokine Res 2011; 31(6): 471–4. 10.1089/jir.2010.0043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Titze J, Machnik A. Sodium sensing in the interstitium and relationship to hypertension. Curr Opin Nephrol Hypertens 2010; 19(4): 385–92. 10.1097/MNH.0b013e32833aeb3b [DOI] [PubMed] [Google Scholar]
- [49].Xie P, Joladarashi D, Dudeja P, Sun L, Kanwar YS. Modulation of angiotensin II-induced inflammatory cytokines by the Epac1-Rap1A-NHE3 pathway: implications in renal tubular pathobiology. Am J Physiol Renal Physiol 2014; 306(11): F1260–74. 10.1152/ajprenal.00069.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Matavelli LC, Huang J, Siragy HM. Angiotensin AT2 receptor stimulation inhibits early renal inflammation in renovascular hypertension. Hypertension 2011; 57(2): 308–13. 10.1161/HYPERTENSIONAHA.110.164202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Gelosa P, Pignieri A, Fändriks L, et al. Stimulation of AT2 receptor exerts beneficial effects in stroke-prone rats: focus on renal damage. J Hypertens 2009; 27(12): 2444–51. 10.1097/HJH.0b013e3283311ba1 [DOI] [PubMed] [Google Scholar]
- [52].Braam B, Huang X, Cupples WA, Hamza SM. Understanding the two faces of low-salt intake. Curr Hypertens Rep 2017; 19(6): 49 10.1007/s11906-017-0744-z [DOI] [PubMed] [Google Scholar]
- [53].Cao W, Li A, Wang L, et al. A salt-induced reno-cerebral reflex activates renin-angiotensin systems and promotes CKD progression. J Am Soc Nephrol 2015; 26(7): 1619–33. 10.1681/ASN.2014050518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Cao W, Zhou QG, Nie J, et al. Albumin overload activates intrarenal renin-angiotensin system through protein kinase C and NADPH oxidase-dependent pathway. J Hypertens 2011; 29(7): 1411–21. 10.1097/HJH.0b013e32834786f0 [DOI] [PubMed] [Google Scholar]
- [55].Macconi D, Remuzzi G, Benigni A. Key fibrogenic mediators: old players. Renin-angiotensin system. Kidney Int Suppl (2011) 2014; 4(1): 58–64. 10.1038/kisup.2014.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Hakam AC, Hussain T. Renal angiotensin II type-2 receptors are upregulated and mediate the candesartan-induced natriuresis/diuresis in obese zucker rats. Hypertension 2005; 45(2): 270–5. [DOI] [PubMed] [Google Scholar]
- [57].Sabuhi R, Ali Q, Asghar M, Al-Zamily NR, Hussain T. Role of the angiotensin II AT2 receptor in inflammation and oxidative stress: opposing effects in lean and obese zucker rats. Am J Physiol Renal Physiol 2011; 300(3): F700–6. 10.1152/ajprenal.00616.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Patel SN, Ali Q, Hussain T. Angiotensin II type 2-receptor agonist C21 reduces proteinuria and oxidative stress in kidney of high-salt-fed obese zucker rats. Hypertension 2016; 67(5): 906–15. 10.1161/HYPERTENSIONAHA.115.06881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Siragy HM, Xue C, Abadir P, Carey RM. Angiotensin subtype-2 receptors inhibit renin biosynthesis and angiotensin II formation. Hypertension 2005; 45(1): 133–7. 10.1161/01.HYP.0000149105.75125.2a [DOI] [PubMed] [Google Scholar]
- [60].Kemp BA, Howell NL, Gildea JJ, Keller SR, Padia SH, Carey RM. AT receptor activation induces natriuresis and lowers blood pressure. Circ Res 2014; 115(3): 388–99. 10.1161/CIRCRESAHA.115.304110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Ali Q, Patel S, Hussain T. Angiotensin AT2 receptor agonist prevents salt-sensitive hypertension in obese zucker rats. Am J Physiol Renal Physiol 2015; 308(12): F1379–85. 10.1152/ajprenal.00002.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Matavelli LC, Zatz R, Siragy HM. A nonpeptide angiotensin II type 2 receptor agonist prevents renal inflammation in early diabetes. J Cardiovasc Pharmacol 2015; 65(4): 371–6. 10.1097/FJC.0000000000000207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Sakai N, Wada T, Matsushima K, et al. The renin-angiotensin system contributes to renal fibrosis through regulation of fibrocytes. J Hypertens 2008; 26(4): 780–90. 10.1097/HJH.0b013e3282f3e9e6 [DOI] [PubMed] [Google Scholar]
- [64].Kaschina E, Grzesiak A, Li J, et al. Angiotensin II type 2 receptor stimulation: a novel option of therapeutic interference with the renin-angiotensin system in myocardial infarction?. Circulation 2008; 118(24): 2523–32. 10.1161/CIRCULATIONAHA.108.784868 [DOI] [PubMed] [Google Scholar]
- [65].Liu BC, Tang TT, Lv LL. How tubular epithelial cell injury contributes to renal fibrosis. Adv Exp Med Biol 2019; 1165: 233–52. 10.1007/978-981-13-8871-2_11 [DOI] [PubMed] [Google Scholar]
- [66].Buckley ST, Medina C, Ehrhardt C. Differential susceptibility to epithelial-mesenchymal transition (EMT) of alveolar, bronchial and intestinal epithelial cells in vitro and the effect of angiotensin II receptor inhibition. Cell Tissue Res 2010; 342(1): 39–51. 10.1007/s00441-010-1029-x [DOI] [PubMed] [Google Scholar]
- [67].Rüster C, Wolf G. Angiotensin II as a morphogenic cytokine stimulating renal fibrogenesis. J Am Soc Nephrol 2011; 22(7): 1189–99. 10.1681/ASN.2010040384 [DOI] [PubMed] [Google Scholar]
- [68].Guo HL, Liao XH, Liu Q, Zhang L. Angiotensin II type 2 receptor decreases transforming growth factor-β type II receptor expression and function in human renal proximal tubule cells. PLoS One 2016; 11(2): e0148696 10.1371/journal.pone.0148696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Imai Y, Kuba K, Rao S, et al. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature 2005; 436(7047): 112–6. 10.1038/nature03712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Wagenaar GT, Laghmani H, Fidder M, et al. Agonists of MAS oncogene and angiotensin II type 2 receptors attenuate cardiopulmonary disease in rats with neonatal hyperoxia-induced lung injury. Am J Physiol Lung Cell Mol Physiol 2013; 305(5): L341–51. 10.1152/ajplung.00360.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Baraldi E, Filippone M. Chronic lung disease after premature birth. N Engl J Med 2007; 357(19): 1946–55. 10.1056/NEJMra067279 [DOI] [PubMed] [Google Scholar]
- [72].Iwai M, Liu HW, Chen R, et al. Possible inhibition of focal cerebral ischemia by angiotensin II type 2 receptor stimulation. Circulation 2004; 110(7): 843–8. 10.1161/01.CIR.0000138848.58269.80 [DOI] [PubMed] [Google Scholar]
- [73].McCarthy CA, Vinh A, Broughton BR, Sobey CG, Callaway JK, Widdop RE. Angiotensin II type 2 receptor stimulation initiated after stroke causes neuroprotection in conscious rats. Hypertension 2012; 60(6): 1531–7. 10.1161/HYPERTENSIONAHA.112.199646 [DOI] [PubMed] [Google Scholar]
- [74].McCarthy CA, Vinh A, Callaway JK, Widdop RE. Angiotensin AT2 receptor stimulation causes neuroprotection in a conscious rat model of stroke. Stroke 2009; 40(4): 1482–9. 10.1161/STROKEAHA.108.531509 [DOI] [PubMed] [Google Scholar]
- [75].McCarthy CA, Vinh A, Miller AA, et al. Direct angiotensin AT2 receptor stimulation using a novel AT2 receptor agonist, compound 21, evokes neuroprotection in conscious hypertensive rats. PLoS One 2014; 9(4): e95762 10.1371/journal.pone.0095762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Min LJ, Mogi M, Tsukuda K, et al. Direct stimulation of angiotensin II type 2 receptor initiated after stroke ameliorates ischemic brain damage. Am J Hypertens 2014; 27(8): 1036–44. 10.1093/ajh/hpu015 [DOI] [PubMed] [Google Scholar]
- [77].Wang G, Coleman CG, Glass MJ, et al. Angiotensin II type 2 receptor-coupled nitric oxide production modulates free radical availability and voltage-gated Ca2+ currents in NTS neurons. Am J Physiol Regul Integr Comp Physiol 2012; 302(9): R1076–83. 10.1152/ajpregu.00571.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Bhat SA, Sood A, Shukla R, Hanif K. AT2R activation prevents microglia pro-inflammatory activation in a NOX-dependent manner: inhibition of PKC activation and p47(phox) phosphorylation by PP2A. Mol Neurobiol 2019; 56(4): 3005–23. 10.1007/s12035-018-1272-9 [DOI] [PubMed] [Google Scholar]
- [79].Umschweif G, Shabashov D, Alexandrovich AG, Trembovler V, Horowitz M, Shohami E. Neuroprotection after traumatic brain injury in heat-acclimated mice involves induced neurogenesis and activation of angiotensin receptor type 2 signaling. J Cereb Blood Flow Metab 2014; 34(8): 1381–90. 10.1038/jcbfm.2014.93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Mateos L, Perez-Alvarez MJ, Wandosell F. Angiotensin II type-2 receptor stimulation induces neuronal VEGF synthesis after cerebral ischemia. Biochim Biophys Acta 2016; 1862(7): 1297–308. 10.1016/j.bbadis.2016.03.013 [DOI] [PubMed] [Google Scholar]
- [81].Joseph JP, Mecca AP, Regenhardt RW, et al. The angiotensin type 2 receptor agonist Compound 21 elicits cerebroprotection in endothelin-1 induced ischemic stroke. Neuropharmacology 2014; 81: 134–41. 10.1016/j.neuropharm.2014.01.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Ludwig M, Skorska A, Tölk A, et al. Characterization of ion currents of murine CD117(pos) stem cells in vitro and their modulation under AT2 R stimulation. Acta Physiol (Oxf) 2013; 208(3): 274–87. 10.1111/apha.12115 [DOI] [PubMed] [Google Scholar]
- [83].Du M, Schmull S, Zhang W, et al. c-kit(+)AT2R(+) bone marrow mononuclear cell subset is a superior subset for cardiac protection after myocardial infarction. Stem Cells Int 2016; 2016: 4913515 10.1155/2016/4913515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Xu Y, Hu X, Wang L, et al. Preconditioning via angiotensin type 2 receptor activation improves therapeutic efficacy of bone marrow mononuclear cells for cardiac repair. PLoS One 2013; 8(12): e82997 10.1371/journal.pone.0082997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Xu XP, Huang LL, Hu SL, et al. Genetic modification of mesenchymal stem cells overexpressing angiotensin II Type 2 receptor increases cell migration to injured lung in lps-induced acute lung injury mice. Stem Cells Transl Med 2018; 7(10): 721–30. 10.1002/sctm.17-0279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Xu XP, He HL, Hu SL, et al. Ang II-AT2R increases mesenchymal stem cell migration by signaling through the FAK and RhoA/Cdc42 pathways in vitro. Stem Cell Res Ther 2017; 8(1): 164 10.1186/s13287-017-0617-z [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- [87].Verma A, Zhu P, de Kloet A, Krause E, Sumners C, Li Q. Angiotensin receptor expression revealed by reporter mice and beneficial effects of AT2R agonist in retinal cells. Exp Eye Res 2019; 187: 107770 10.1016/j.exer.2019.107770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Stegbauer J, Lee DH, Seubert S, et al. Role of the renin-angiotensin system in autoimmune inflammation of the central nervous system. Proc Natl Acad Sci USA 2009; 106(35): 14942–7. 10.1073/pnas.0903602106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Lanz TV, Ding Z, Ho PP, et al. Angiotensin II sustains brain inflammation in mice via TGF-beta. J Clin Invest 2010; 120(8): 2782–94. 10.1172/JCI41709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Terenzi R, Manetti M, Rosa I, et al. Angiotensin II type 2 receptor (AT2R) as a novel modulator of inflammation in rheumatoid arthritis synovium. Sci Rep 2017; 7(1): 13293 10.1038/s41598-017-13746-w [DOI] [PMC free article] [PubMed] [Google Scholar]