Abstract
Objective
This study was set out to explore the expression and related mechanism of XIST and miR-200a-3p in retinoblastoma (Rb).
Patients and Methods
Fifty-four children with Rb who came to our hospital for surgery from January 2018 to September 2019 were collected. In addition, Rb cells and human retinal epithelial cells were purchased. XIST-siRNA (si-XIST), XIST-shRNA (sh-XIST), empty vector plasmid (siRNA-NC), miR-200a-3p-mimics and miR −200a-3p-inhibition were transfected into Y79 cells. The expression of XIST and miR-200a-3p in the samples were determined by qRT-PCR. β-catenin, cyclin B1, cyclin D1, Bax, Caspase-3, N-cadherin, vimentin, Snail, E-Cadherin and ZO-1 protein levels were measured by WB. MTT, Transwell and flow cytometry were utilized to detect cell proliferation, invasion, and apoptosis, respectively.
Results
XIST was highly expressed while miR-200a-3p was lowly expressed in patients’ tissues, and the AUC of both was over 0.8. XIST and miR-200a-3p was related to differentiation degree in Rb patients. Y79 cells were selected for transfection. Compared with the siRNA-NC group, XIST was significantly reduced in the siRNA-XIST group, and it was significantly increased in the shRNA-XIST group (P<0.01). The proliferation capacity of siRNA-XIST group was decreased, while that of shRNA-XIST group was up-regulated. The apoptosis rate of siRNA-XIST group was significantly up-regulated, while that of shRNA-XIST group was decreased (P<0.001). The invasive capacity of siRNA-XIST group was decreased, while that of shRNA-XIST group was up-regulated (P<0.001). Silencing XIST and over-expressed miR-200a-3p could inhibit cell epithelial–mesenchymal transition (EMT), proliferation, invasion, and promote apoptosis. WB detection showed that Carboplatin + LncRNA XIST intervention group could more significantly inhibit β-catenin, cyclin B1, cyclin D1, N-cadherin, vimentin, Snail protein, and promote the up-regulation of Bax, Caspase-3, E-Cadherin and ZO-1 expression.
Conclusion
Inhibition of XIST expression can up-regulate miR-200a-3p-mediated PI3K-Akt/MAPK-ERK signaling pathway and affect cell EMT, proliferation, invasion, and apoptosis, which is expected to be a potential therapeutic target for Rb.
Keywords: lncRNA-XIST, miR-200a-3p, NRP1, epithelial–mesenchymal transition, retinoblastoma, biological mechanism
Introduction
Retinoblastoma (Rb) is a family hereditary intraocular malignant tumor derived from photoreceptor precursor cells, which is common in infants and young children.1,3 Retinoblastoma is a serious threat to blindness of infants and young children. If the tumor volume or metastatic area of Rb exceeds half of the eyeball, the last resort would be the enucleation of the eyeball. Therefore, the detection, diagnosis and treatment of the disease at the early stage are critical to improve the cure rate and reduce the mortality rate.4,6 Carboplatin, as a first-line tumor chemotherapy drug, has been widely used in clinical practice. However, its therapeutic efficiency is not ideal, and its application is limited by its potential serious toxic side effects and drug resistance.7,8 In recent years, molecular targeting has shown its vital role in the diagnosis, staging and comprehensive treatment of Rb. Some studies have revealed that the occurrence and development of Rb are related to long non-coding RNA (lncRNA), and that both carboplatin and lncRNA affect the EMT of Rb cells and the biological function of cancer cells, which suggests that studies on the targeted treatment of Rb by carboplatin and lncRNA have shown broad development prospects.9,10
Evidence has shown that the location of LncRNA is closely associated with protein-coding genes, and is involved in Rb metastasis, which affects cell proliferation, apoptosis and differentiation.11 A recent study has proposed that the abnormal expression of LncRNA XIST and lncRNA in cancers has important clinical value for cancer diagnosis and outcomes in patients.12 LncRNA XIST is up-regulated in various tumors such as colorectal cancer, lung cancer, and Rb.13,14 However, at present, little research has been conducted on the expression and predictive significance of carboplatin and lncRNA targeted therapy in Rb. Therefore, in the respective of molecular biology, this paper aims to offer a theoretical basis for the diagnosis and treatment of carboplatin and lncRNA targeted therapy.
Here, by detecting the expression of lncRNA in Rb, we explored the inhibitory effect of carboplatin on the progression of Rb through the lncRNA XIST/miR-200a-3p/NRP1 axis, so as to find reliable tumor markers and potential drug targets for clinical diagnosis and prognosis of Rb.
Patients and Methods
Materials
A total of 54 children with Rb who came to the First Affiliated Hospital of Zhengzhou University from January 2018 to September 2019 were collected. Exclusion and inclusion criteria: Inclusion criteria: Children with Rb were confirmed by pathology, cytology and imaging,15 and they were not treated with anti-tumor treatment such as preoperative chemotherapy, immunotherapy, or radiation. Exclusion criteria: Children complicated with liver cirrhosis or coagulation dysfunction, those with incomplete clinical data and an estimated survival time of less than 1 month, or those who did not cooperate or lost to follow up. This study was carried out under the approval of the Medical Ethics Committee of the First Affiliated Hospital of Zhengzhou University, and was in accordance with Helsinki Declaration, the legal guardian of the participant had signed the informed consent in advance.
Main Instruments and Reagents
Y79 cells of human Rb cell line and ARPE-19 cells of human retinal epithelial cells (BNCC341293, BNCC337713) were purchased from BeNa Culture Collection. Lipofectamine™ 2000 Transfection Kit, ABI Stepone Plus Real-Time PCR System, Annexin V/PI Apoptosis Detection Kit, and Trizol Extraction Kit (Invitrogen, Carlsbed, CA, USA). SYBR Green PCR Master Mix (Applied Biosystems, Waltham, MA, USA). MTT kit (Beyotime Biotechnology Co., Ltd., Shanghai, China, product number: C0009). Transwell kit, 10% fetal bovine serum (FBS), FACSCanto flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA). DR5000 UV-visible spectrophotometer (BioRad, Hercules, USA). The design and synthesis of all the primer sequences were carried out by Sangong Bioengineering Co., Ltd., Shanghai, China.
Detection Methods
Cell Culture and Transfection Experiments
Cell experiment: Y79 cells were transfected into DMEM containing 10% FBS and penicillin-streptomycin mixed solution, and cultured in a cell incubator at 37°C with 5% CO2 and saturated humidity. According to the instructions of LipofectamineTM 2000 transfection kit, the cells were transfected with XIST-siRNA (si-XIST), XIST-shRNA (sh-XIST) and empty vector plasmid (siRNA-NC) respectively. Then, the primers were transfected into the cells with the greatest difference in XIST expression, and then they resumed culture in a medium containing 10% FBS 6hrs after transfection. The transfection efficiency was verified by qRT-PCR.
Drug test: Y79 cells were cultured in DMEM containing 100 mg·L-1 double antibody and 10% FBS under the culture conditions of 5% CO2, 37°C and saturated humidity. Then, the cultured Y79 cells were randomly divided into Carboplatin + LncRNA XIST intervention group, Carboplatin intervention group, normal saline group, and siRNA-LncRNA XIST intervention group.
QRT-PCR Detection
QRT-PCR was employed to detect mRNA expression in tissues and cells. According to the instructions of Trizol reagent, the total tissue RNA was extracted and dissolved in 20 μL DEPC, and then reversely transcribed using the reverse transcription kit. Reaction conditions: 95°C, 15 min; 95°C, 15 s; 58°C, 30 s, totaling 35 cycles; at last, extension was performed at 72°C for 15 mins. Three duplicate holes were set for each sample and the experiment was repeated three times. Finishing reaction, the amplification and fusion curves of real-time PCR were determined, and the relative quantities of target genes were calculated based on the result parameters. The relative quantification of target genes was worked out by 2−ΔCt Table 1.
Table 1.
MiR-200a-3p, XIST and Their Internal Reference Primer Sequences
| Gene | Forward Primer | Reverse Primer |
|---|---|---|
| miR-200a-3p | 5ʹ-TAACACTGTCTGGTAACGATGT-3’ | 5ʹ-CATCTTACCGGACAGTGCTGGA-3’ |
| NRP1 | 5′-GCTCTAGAGAATGCTTCTAGAAACTTCCAGC-3′ | 5ʹ-GCTCTAGATACAGTTCAGTTCTATGTGGTTTTTATATG-3’ |
| XIST | 5ʹ-CTCTCCATTGGGTTCAC-3’ | 5ʹ-GCGGCAGGTCTTAAGAGATGAG-3’ |
| U6 | 5ʹ-CTCGCTTCGGCAGCACA-3’ | 5ʹ-AACGCTTCACGAATTTGCGT-3’ |
| GAPDH | 5ʹ-CAAAGGTGGATCAGATTCAAG-3’ | 5ʹ-GGTGAGCATTATCACCCAGAA-3’ |
Western Blot Detection
After lysis, the cells were collected and transferred to a centrifuge tube, where they were centrifuged at 12,000×g at 4°C for 10mins, and the obtained supernatant was collected as a protein sample. The protein concentration was determined by BCA assay, and protein samples were diluted with Lysis buffer to prepare a protein of 20mg/mL. After that, development was carried out in a dark room, the excess liquid on the membrane was firstly blotted, and then illuminated by ECL to develop. The protein bands were scanned and the grayscale values were analyzed by Quantity One software (Molecular Devices Corp, The Bay Area, CA, USA).
Cell Proliferation Experiment
The viability of cells was detected by MTT. Twenty-four hours after transfection, the cells were harvested and adjusted to 5×103 cells per well. Dimethyl sulfoxide (200 μL) was added to each well, and the OD value of each group of cells was measured using a spectrophotometer at a wavelength of 570 mm.
Transwell Invasion Experiment
The Transwell chamber was painted with Matrigel and stood at 37°C for 30 min. Cells were resuspended in a tissue-free DMEM with a cell density of 4×105/mL. Then, 200 μL cell suspension was added to the upper chamber, while 800 μL DMEM (containing 10% FBS) was put into the lower chamber. The cells passing through the basement membrane of the compartment, which indicated the cell invasion ability was finally counted under an optical microscope with 10 high-power fields randomly selected.
Apoptosis Experiment
After 48 hrs of transfection, cells were digested with 0.25% trypsin and washed twice with PBS. Then, the cells were resuspended with 100 μL AnnexinV binding buffer, configured to a suspension of 1×106 cells/mL, added with 5 μL Annexin-V/FITC solution and cultured at 4°C for 15 mins. Followed by the addition of 5 μL PI staining solution, and then the culture was resumed for 5 mins at 4°C. The cell apoptosis was tested by Flow cytometry. The experiment was repeated for 3 times and averaged.
Statistical Methods
SPSS 20.0 (SPSS, Inc, Chicago, IL, USA) was responsible for statistical analysis. Normally distributed data were described as mean ± standard deviation (mean ± SD), and inter-group comparison of measurement data were conducted using the independent sample t-test. Multi-time data were compared by repeated-measures analysis of variance, and Bonferroni method was used for post hoc test. One-way ANOVA was employed for the comparison of Mean among multiple groups, and LSD-t method was applied for post hoc test. ROC curve was utilized to evaluate the diagnostic value, and Pearson test for correlation analysis. P<0.05 indicated a statistically significant difference.
Results
Expression of lncRNA XIST in Rb
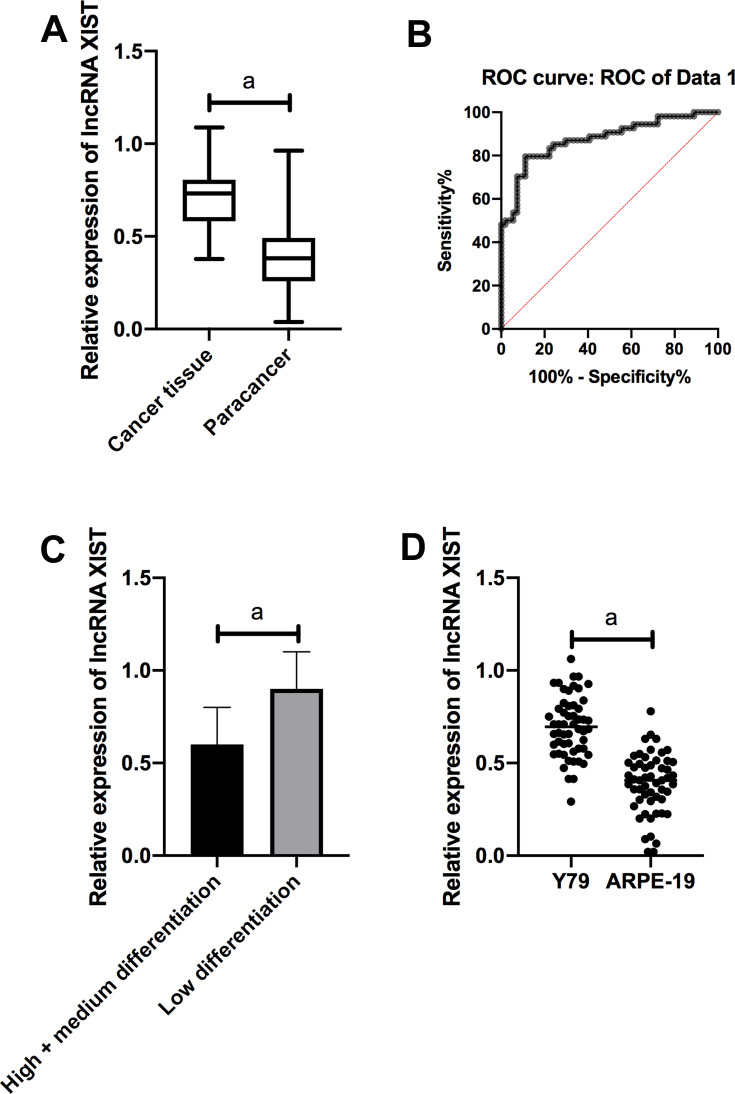
QRT-PCR quantitatively detected the expression of XIST in Rb tissues and cell lines. The results showed that XIST was markedly up-regulated in Rb tissues compared with normal tissues adjacent to the cancer. ROC analysis revealed that the AUC of XIST was > 0.8. Further, according to the median expression of XIST, the patients were divided into high- and low-expression groups. The results revealed that XIST was related to the differentiation degree of Rb patients. Compared with human normal retinal epithelial cells, XIST was dramatically increased in Y79 cells (Figure 1).
Figure 1.
Expression of lncRNA XIST in Rb. (A) XIST was remarkably up-regulated in Rb tissues compared with normal tissues adjacent to the cancer. (B) ROC analysis showed that the AUC of XIST was over 0.8. (C) XIST was notably lower in high/medium differentiated tissues than in low differentiated tissues. (D) Compared with human normal retinal epithelial cells ARPE-19, XIST was noticeably increased in Rb cell line Y79.
Note: aIndicates P<0.001.
Effects of lncRNA XIST on Biological Function of Rb Cells
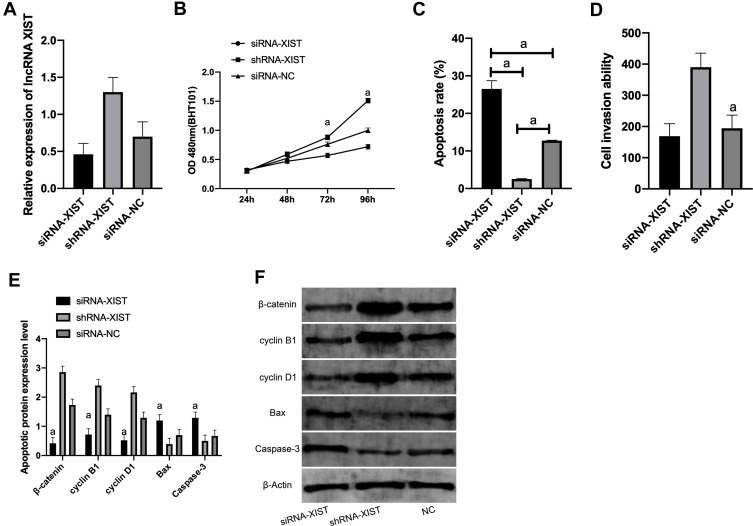
Y79 cell line, which showed the largest expression difference, was selected for transfection. XIST was notably lower in the siRNA-XIST group than in the siRNA-NC group (P<0.01), and that in the shRNA-XIST group was obviously up-regulated compared with the siRNA-NC group (P<0.01). MTT results showed that, compared with the siRNA-NC group, the proliferation ability in the siRNA-XIST group was markedly lower, and that in the shRNA-XIST group was higher (P<0.05). The comparison of apoptosis rate detected by Flow cytometry revealed that the apoptosis rate in the siRNA-XIST group and shRNA-XIST group was greatly lower than the siRNA-NC group (both P<0.001). Transwell results exhibited that the cell invasion ability of the siRNA-XIST group was remarkably lower and that of the shRNA-XIST group was remarkably higher compared with the siRNA-NC group (both P<0.001). (Figure 2)
Figure 2.
Expression of XIST in cells and its effect on cell biological function. (A) Expression of XIST after transfection with Y79. (B) Proliferation of Y79 after transfection. (C) Apoptosis of Y79 after transfection. (D) Invasion of Y79 after transfection. (E) Expression of apoptosis-related proteins in Y79 after transfection. (F) WB diagram.
Note: aIndicates P<0.001.
Targeted Binding of LncRNA XIST to miR-200a-3p
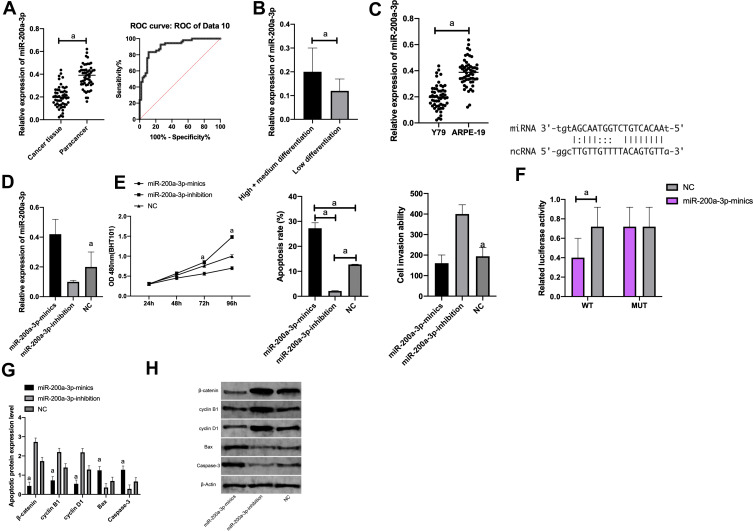
The miR-200a-3p expression in each cell line was tested by qRT-PCR. It showed that, compared with ARPE-19 normal retinal epithelial cells, miR-200a-3p was markedly down-regulated in Y79 cells (P<0.05). Therefore, we selected Y79 cells for transfection, and found that the miR-200a-3p level in the miR-200a-3p-mimics group was significantly higher than that in the NC group, while that in the miR-200a-3p-inhibition group was notably lower than the NC group. Regarding cell proliferation capacity, CCK-8 demonstrated that the proliferation capacity in the miR-200a-3p-mimics group was noticeably lower while that in the miR-200a-3p-inhibition group was dramatically higher compared with the NC group (P<0.001). As to apoptosis, flow cytometry revealed that the apoptosis rate in the miR-200a-3p-mimics group was remarkably higher while that in the miR-200a-3p-inhibition group was statistically lower compared with the NC group (both P<0.001). Concerning cell invasive ability, Transwell assay indicated that the cell invasion in the miR-200a-3p-mimics group was dramatically lower while that in the miR-200a-3p-inhibition group was greatly higher compared with the NC group (both P<0.001). (Figure 3).
The luciferase activity of pmirGLO-XIST WT/MUT and miR-200a-3p mimics after transfection in cells was detected using DLR gene assay. It was observed that transfection of pmirGLO XIST WT and miR-200a-3p mimics brought notably inhibited Y79 luciferase activity, while transfection with pmirGLO-XIST MUT and miR-200a-3p mimics had nothing to do with Y79 luciferase activity (Figure 3).
Figure 3.
Targeted binding of LncRNA XIST to miR-200a-3p. (A) MiR-200a-3p expression in cancer tissues and adjacent cancer tissues; The diagnostic AUC of miR-200a-3p was >0.8. (B) miR-200a-3p was noticeably higher in high/medium differentiation tissues than in low differentiation tissues. (C) Compared with ARPE-19, miR-200a-3p was notably down-regulated in Y79. (D) MiR-200a-3p expression in Y79 after transfection. (E) Proliferation, apoptosis, and invasion of Y79 after transfection. (F) Luciferase activity of Y79. (G) Expression of apoptosis-related proteins in Y79 after transfection. (H) WB diagram.
Note: aIndicates P<0.001.
IncRNA XIST/miR-200a-3p/NRP1 Molecular Axis
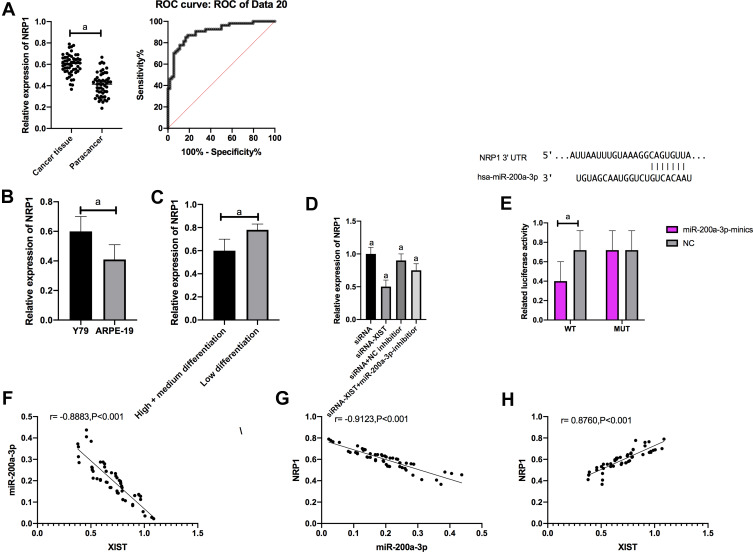
QRT-PCR was employed to measure the NRP1 level in each cell line. Compared with ARPE-19 normal human retinal epithelial cells, NRP1 was dramatically increased in Y79 cells (P<0.001), so we selected Y79 for transfection. The luciferase activity of pmirGLO-NRP1 WT/MUT and miR-200a-3p mimics after transfection in cells was detected by DLR gene assay. Y79 luciferase activity was noticed to be notably suppressed after transfecting pmirGLO NRP1 WT and miR-200a-3p mimics, while it changed little after transfecting pmirGLO-NRP1 MUT and miR-200a-3p mimics.
QPCR results showed that IncRNA XIST knockdown markedly inhibited NRP1 expression, while transfection with miR-200a-3p-inhibitor reversed the inhibition of NRP1 expression.
Correlation analysis results revealed a negative link between XIST and miR-200a-3p (r= −0.8883, P<0.001), a negative link between miR-200a-3p and NRP1 (r= −0.9123, P<0.001), and a positive link between XIST and NRP1 expression (r= 0.8760, P<0.001).(Figure 4)
Figure 4.
IncRNA XIST targeted miR-200a-3p to up-regulate NRP1 expression. (A) NRP1 expression in cancer tissues and adjacent tissues. The diagnostic AUC of NRP1 was > 0.8. (B) Compared with ARPE-19, NRP1 was significantly up-regulated in Y79. (C) NRP1 was significantly down-regulated in high/medium differentiation tissues than that in low differentiation tissues. (D) NRP1 expression after transfection with Y79. (E) Luciferase activity of Y79; (F) XIST was negatively correlated with miR-200a-3p (r= −0.8883, P<0.001). (G) MiR-200a-3p was negatively correlated with NRP1 (r= −0.9123, P<0.001). (H) XIST was positively correlated with NRP1 expression (r= 0.8760, P<0.001).
Note: aIndicates P<0.001.
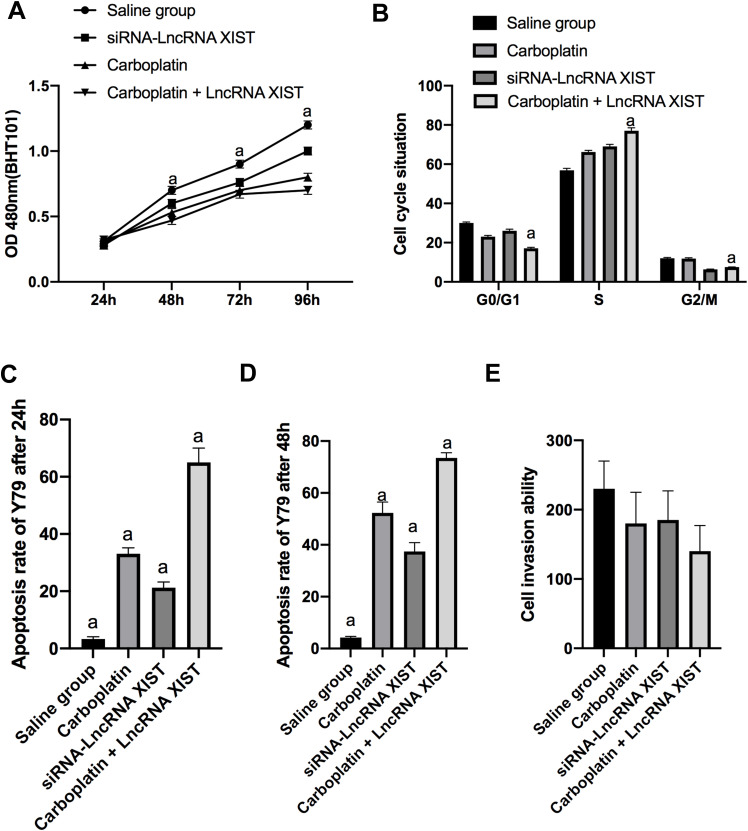
Effects of Carboplatin Combined with lncRNA XIST on Biological Function of Rb Cells
MTT detection results showed that the viability of Y79 in Carboplatin + LncRNA XIST intervention group was markedly lower, while the apoptosis rate was remarkably higher than that in other interference groups from 48 h onward, and the survival rate of cells at 48hrs was noticeably higher than that at 24 h (all P<0.001).
Flow cytometry results exhibited that cells in the carboplatin intervention group decreased in G0/G1 phase and increased in S phase, with a statistically significant difference compared with the normal saline group (P<0.01), while the number of cells in M phase changed little (P>0.05). In the siRNA-LncRNA XIST intervention group, the number of cells in G2/M phase decreased, while the number of cells in S phase increased, which was significantly different from those in the normal saline group (P<0.01), and there was little change in G0/G1 phase cells (P>0.05). The Carboplatin + LncRNA XIST intervention group showed decreased G0/G1 cells, increased S cells, and reduced G2/M cells (all P<0.001).(Figure 5)
Figure 5.
Effects of carboplatin combined with lncRNA XIST on biological function of Rb cells. (A) Cell proliferation in each group. (B) Cell cycle in each group. (C) Apoptosis at 24 hrs. (D) Apoptosis at 48 hrs. (E) Cell invasion.
Note: aIndicates P<0.001.
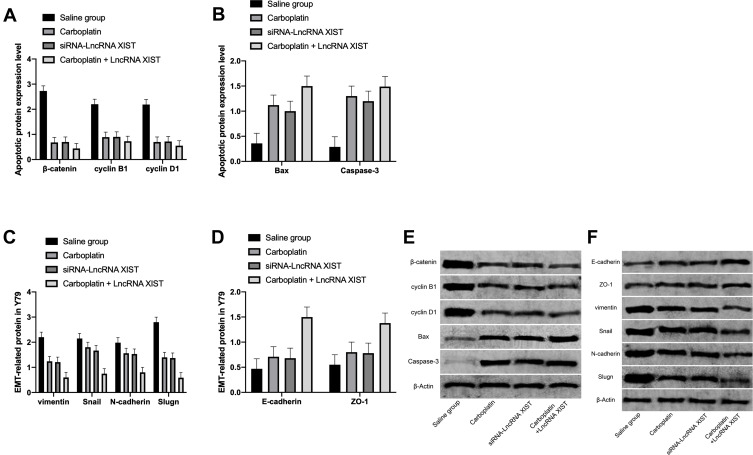
Effects of Carboplatin Combined with lncRNA XIST on on Apoptosis Proteins and ETM of Rb Cells
WB assay exhibited that the levels of apoptosis-related proteins β-catenin, cyclin B1 and cyclin D1 in Rb cells in the Carboplatin + LncRNA XIST intervention group were remarkably down-regulated compared with the normal saline group, while Bax and Caspase-3 levels were dramatically up-regulated.
Western Blot assay exhibited that compared with the normal saline group, the levels of ETM-related proteins N-cadherin, vimentin and Snail in the Carboplatin + LncRNA XIST intervention group were notably down-regulated, while E-cadherin and ZO-1 levels were significantly up-regulated.(Figure 6)
Figure 6.
Effects of carboplatin combined with lncRNA XIST on on apoptosis proteins and ETM of Rb cells. (A) Compared with the normal saline group, the expression levels of β-catenin, cyclin B1 and cyclin D1 proteins of cells in the Carboplatin + LncRNA XIST group were markedly down-regulated. (B) Compared with the normal saline group, the expression levels of Bax and Caspase-3 of cells in the Carboplatin + LncRNA XIST groups were remarkably up-regulated. (C) Compared with the normal saline group, the expression levels of N-cadherin, vimentin and Snail protein in the Carboplatin + LncRNA XIST group were notably down-regulated. (D) Compared with the normal saline group, the expression levels of E-Cadherin and ZO-1 in the Carboplatin + LncRNA XIST intervention group were obviously up-regulated. (E) WB diagram. (F) WB diagram.
Discussion
At present, chemotherapy is a mainstream treatment for Rb. However, the dose of chemotherapy drugs is more risky for infants whose body functions are not yet mature.2 Studies in recent years have found a close relationship between LncRNA and multiple tumors, and it is reported that LncRNA plays a critical role in carcinogenesis and tumor progression.16,17 LncRNA XIST, an oncogene in various malignant tumors, has attracted extensive attention of scholars.18 Some tumor treatment studies have revealed that the regulation of lncRNA XIST can promote the progression of osteosarcoma by miR-195-5p targeted YAP, and interfering with lncRNA exerts a favorable inhibitory effect on tumor cells in vitro, but the effect in vivo is unsatisfactory.19 This study is to explore the inhibitory effect of carboplatin on the progression and biological function of Rb through lncRNA XIST/miR-200a-3p/NRP1 axis, in order to provide a new theoretical basis for the diagnosis and treatment of Rb in molecular biology.
In the present study, we applied qRT-PCR to detect the expression of XIST in Rb. We noticed that XIST was abnormally up-regulated in Rb patients’ tissues, and compared with human normal retinal epithelial cells, the expression of XIST in Rb cell line Y79 was noticeably increased. By further analyzing the clinicopathological features of the patients, we found that the high XIST expression was correlated with the differentiation of Rb patients, and ROC curve identified that the AUC of XIST was more than 0.8. Latest reports have verified that lncRNA XIST inhibits the growth, migration and invasion of liver cancer cells by sponging miR-155-5p, and lncRNA XIST can regulate cell biological functions by targeting downstream genes.20,21 The targeting relationships between lncRNA XIST and miR-200a-3p, and between miR-200a-3p and NRP1 were further discovered through TargetScan database analysis. In addition, miR-200a-3p and NRP1 were found to be correlated with the differentiation of Rb, and that their AUCs were more than 0.8. MiR-200a-3p showed low expression in gastric cancer, cervical cancer and retina-related diseases, while NRP1, on the contrary, affected the clinicopathological characteristics of cancer.22 In the study of Sun et al, lncRNA MALAT1 was found to regulate the apoptosis of cardiomyocytes by regulating the miR-200a-3p/PDCD4 axis.23 However, whether lncRNA XIST can act as a ceRNA to regulate the gene of Rb cells through the miR-200a-3p/NRP1 axis and affect the cell cycle of Rb cells remains to be explored.
In the cell experiment, we compared human retinoblastoma cell line Y79 with normal retinal epithelial cells of ARPE-19, and found that XIST and NRP1 were highly expressed in Rb cell line, while miR-200a-3p was low. Correlation analysis results showed a negative correlation between XIST and miR-200a-3p, a negative correlation between miR-200a-3p and NRP1, and a positive correlation between XIST and NRP1. Then, we up- and down-regulated the XIST, miR-200a-3p, and NRP1 levels in Y79. The results showed that the knockdown of IncRNA XIST dramatically inhibited NRP1 expression, while the simultaneous transfection of miR-200a-3p-inhibitor reversed the inhibition of NRP1 expression. Observation of cell biological function revealed that inhibited XIST and overexpressed miR-200a-3p greatly prevented cell proliferation and invasion. In addition, β-catenin, cyclin B1, and cyclin D1 of apoptosis-related proteins showed obviously reduced expression, while Bax and Caspase-3 presented remarkably elevated levels. All these suggest that lncRNA XIST/miR-200a-3p/NRP1 axis inhibits Rb, which is a potential target for the treatment of Rb, but the inhibition of chemotherapy drug carboplatin combined with lncRNA XIST/miR-200a-3p/NRP1 axis on Rb remains poorly understood.
Reports on the EMT process of Rb cells showed that carboplatin had a great inhibitory effect on Rb cells.24 In recent years, related studies have revealed that EMT is indispensable for tumor cell growth and survival.25 In this study, we investigated the effects of carboplatin on the cytotoxicity of Rb cells through the lncRNA XIST/miR-200a-3p/NRP1 axis and found that carboplatin and micro-RNA-34a could inhibit EMT of Rb cells. The proliferation, invasion and ETM inhibition of Rb cells in carboplatin combined with siRNA-LncRNA XIST intervention group were stronger than those in carboplatin or siRNA-LncRNA XIST intervention group alone. SiRNA-LncRNA XIST could promote carboplatin induced transformation of Y79, which was consistent with the results of tumor cell proliferation inhibition, while the carboplatin intervention group and the combined intervention group showed stronger ability to induce cell resistance than the siRNA-lncRNA XIST intervention group to induce tumor cell correction alone, indicating that gene intervention has a weak therapeutic effect on tumors and needs to be combined with other cytotoxic chemotherapy drugs. siRNA-lncRNA XIST intervention resulted in increased cell arrest in S phase, and decreased cells in G0/G1 phase and G2/M phase, suggesting that all drug intervention groups could inhibit the growth of transplanted Rb tumors, and the strongest inhibitory effect on tumor growth was found in the carboplatin combined with siRNA-lncRNA XIST intervention group. Reports on the treatment of tumors with chemotherapeutic drugs combined with LncRNA also indicate the inhibitory effect of carboplatin combined with LncRNA UCA1 in inhibiting cell proliferation and tumor growth in Rb, leading to cell cycle stagnation and apoptosis in Rb.26
We still have certain limitations in this study. The regulation network of carboplatin on XIST is not clear, and further studies are needed to determine whether carboplatin can influence the development of tumor through other pathways. Therefore, in future studies, we hope to explore the regulatory network of chemical drugs combined with XIST through bioinformatics analysis, so as to provide more evidence for our experiments.
Conclusion
Carboplatin inhibits the proliferation and EMT transformation of Rb cells via lncRNA XIST/miR-200a-3p/NRP1 axis. Carboplatin combined with lncRNA XIST/miR-200a-3p/NRP1 axis is a potential therapy and is expected to become a potential clinical treatment for Rb.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Xiao J, Ma M, Liang Ming, et al. [Site-directed mutagenesis of long gene by partial amplification combining with double fragments ligation]. Sheng Wu Gong Cheng Xue Bao 2020;36:1232–1240. [DOI] [PubMed] [Google Scholar]
- 2.Golabchi K, Soleimani-Jelodar R, Aghadoost N, et al. MicroRNAs in retinoblastoma: potential diagnostic and therapeutic biomarkers. J Cell Physiol. 2018;233(4):3016–3023. doi: 10.1002/jcp.26070 [DOI] [PubMed] [Google Scholar]
- 3.Singh L, Singh MK, Rizvi MA, et al. Clinical relevance of the comparative expression of immune checkpoint markers with the clinicopathological findings in patients with primary and chemoreduced retinoblastoma. Cancer Immunol. Immunother 2020;69:1087–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiménez I, Laé M, Tanguy M, et al. Craniofacial second primary tumors in patients with germline retinoblastoma previously treated with external beam radiotherapy: A retrospective institutional analysis. Pediatr Blood Cancer. 2020;67:e28158. [DOI] [PubMed] [Google Scholar]
- 5.Damato B, Afshar AR, Everett L, et al. The University of California, San Francisco documentation system for retinoblastoma: preparing to improve staging methods for this disease. Ocul Oncol Pathol. 2019;5(1):36–45. doi: 10.1159/000488147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas NA, Abraham RG, Dedi B, et al. Targeting retinoblastoma protein phosphorylation in combination with EGFR inhibition in pancreatic cancer cells. Int J Oncol. 2019;54(2):527–536. doi: 10.3892/ijo.2018.4658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cruz-Galvez CC, Ortiz-Lazareno PC, Pedraza-Brindis EJ, et al. Pentoxifylline enhances the apoptotic effect of carboplatin in Y79 Retinoblastoma Cells. In Vivo. 2019;33(2):401–412. doi: 10.21873/invivo.11487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Soliman SE, D'Silva CrN, Dimaras H, et al. Clinical and genetic associations for carboplatin-related ototoxicity in children treated for retinoblastoma: A retrospective noncomparative single-institute experience. Pediatr Blood Cancer 2018;65:e26931. [DOI] [PubMed] [Google Scholar]
- 9.Nikpayam E, Tasharrofi B, Sarrafzadeh S, et al. The role of long non-coding RNAs in ovarian cancer. Iran Biomed J. 2017;21(1):3. doi: 10.18869/acadpub.ibj.21.1.3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang D, Sun X, Zhang Y. Downregulation of LncRNA PMS2L2 in endometrial adenocarcinoma upon carboplatin treatment. Cancer Manag Res. 2019;11:8905. doi: 10.2147/CMAR.S221274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng Y, Chang Q, Zheng B, et al. LncRNA XIST promotes the epithelial to mesenchymal transition of retinoblastoma via sponging miR-101. Eur J Pharmacol. 2019;843:210–216. doi: 10.1016/j.ejphar.2018.11.028 [DOI] [PubMed] [Google Scholar]
- 12.Wei W, Liu Y, Lu Y, et al. LncRNA XIST promotes pancreatic cancer proliferation through miR-133a/EGFR. J Cell Biochem. 2017;118(10):3349–3358. doi: 10.1002/jcb.25988 [DOI] [PubMed] [Google Scholar]
- 13.Tang Y, He R, An J, et al. lncRNA XIST interacts with miR-140 to modulate lung cancer growth by targeting iASPP. Oncol Rep. 2017;38(2):941–948. doi: 10.3892/or.2017.5751 [DOI] [PubMed] [Google Scholar]
- 14.Chen D, Chen L, Lu Y, et al. Long noncoding RNA XIST expedites metastasis and modulates epithelial–mesenchymal transition in colorectal cancer. Cell Death Dis. 2017;8(8):e3011. doi: 10.1038/cddis.2017.421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou S, Wang L, Yang Q, et al. Systematical analysis of lncRNA–mRNA competing endogenous RNA network in breast cancer subtypes. Breast Cancer Res Treat. 2018;169(2):267–275. doi: 10.1007/s10549-018-4678-1 [DOI] [PubMed] [Google Scholar]
- 16.Königsrainer A, Rau B. Cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC): don’t throw the baby out with the bathwater. Pleura Peritoneum. 2018;3(4). doi: 10.1515/pp-2018-0131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Biasini A, Smith AAT, Abdulkarim B, et al. The Contribution of lincRNAs at the Interface between Cell Cycle Regulation and Cell State Maintenance. iScience 2020;23:101291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lv L, Wei M, Lin P, et al. Integrated mRNA and lncRNA expression profiling for exploring metastatic biomarkers of human intrahepatic cholangiocarcinoma. Am J Cancer Res. 2017;7(3):688. [PMC free article] [PubMed] [Google Scholar]
- 19.Yang C, Wu K, Wang S, et al. Long non‐coding RNA XIST promotes osteosarcoma progression by targeting YAP via miR‐195‐5p. J Cell Biochem. 2018;119(7):5646–5656. doi: 10.1002/jcb.26743 [DOI] [PubMed] [Google Scholar]
- 20.Lin X, Huang Z, Chen X, et al. XIST induced by JPX suppresses hepatocellular carcinoma by sponging miR-155-5p. Yonsei Med J. 2018;59(7):816–826. doi: 10.3349/ymj.2018.59.7.816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shi J, Tan S, Song L, et al. LncRNA XIST knockdown suppresses the malignancy of human nasopharyngeal carcinoma through XIST/miRNA-148a-3p/ADAM17 pathway in vitro and in vivo. Biomed Pharmacother. 2020;121:109620. doi: 10.1016/j.biopha.2019.109620 [DOI] [PubMed] [Google Scholar]
- 22.Li SP, Xu HX, Yu Y, et al. LncRNA HULC enhances epithelial-mesenchymal transition to promote tumorigenesis and metastasis of hepatocellular carcinoma via the miR-200a-3p/ZEB1 signaling pathway. Oncotarget. 2016;7(27):42431. doi: 10.18632/oncotarget.9883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun R, Zhang L. Long non-coding RNA MALAT1 regulates cardiomyocytes apoptosis after hypoxia/reperfusion injury via modulating miR-200a-3p/PDCD4 axis. Biomed Pharmacother. 2019;111:1036–1045. doi: 10.1016/j.biopha.2018.12.122 [DOI] [PubMed] [Google Scholar]
- 24.Avior Y, Lezmi E, Yanuka D, et al. Modeling developmental and tumorigenic aspects of trilateral retinoblastoma via human embryonic stem cells. Stem Cell Rep. 2017;8(5):1354–1365. doi: 10.1016/j.stemcr.2017.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Egger JV, Lane MV, Antonucci LA, et al. Dephosphorylation of the retinoblastoma protein (Rb) inhibits cancer cell EMT via Zeb. Cancer Biol Ther. 2016;17(11):1197–1205. doi: 10.1080/15384047.2016.1235668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu X, Xue L, Yao Y, et al. The FoxM1-ABCC4 axis mediates carboplatin resistance in human retinoblastoma Y-79 cells. Acta Biochim Biophys Sin (Shanghai). 2018;50(9):914–920. doi: 10.1093/abbs/gmy080 [DOI] [PubMed] [Google Scholar]