Abstract
Introduction
A disintegrin and metallopeptidase with thrombospondin motifs (ADAMTSs), whose expression is dysregulated in various cancers, is implicated in cancer development. Herein, we aimed to investigate the functional role of ADAMTS8 in breast cancer (BC) and explore the underlying mechanisms.
Methods
The protein expression of ADAMTS8 in BC cell lines and tumor tissues from BC patients was quantified by Western blot. ADAMTS8 overexpression was induced by transfection with pEZ-M90-ADAMTS8 plasmid using lipofectamine 2000. To generate ADAMTS8 stable knockdown cells, MDA-MB-231 cells were transfected with psi-H1-ADAMTS8siRNA plasmids. Cell counting kit-8 (CCK-8) assay, wound-healing assay, transwell assay and flow cytometry assay were employed to analyze the effects of ADAMTS8 on the proliferation, migration, invasion and apoptosis of BC cells. Chemosensitivity also was assessed using CCK-8 assay. The expressions of β-catenin, MMP-7 and c-Myc were measured by Western blot.
Results
Our results showed that ADAMTS8 expression was significantly lower in BC tissues than that in adjacent non-tumor tissues. Overexpression of ADAMTS8 in MDA-MB-453 cells could inhibit the cell proliferation, migration and invasion and promote apoptosis. ADAMTS8 knockdown displayed the reverse effect in MDA-MB-231 cells. Consistently, in vivo data showed that ADAMTS8 overexpression led to a reduction in tumor growth. In addition, chemosensitivity testing in MDA-MB-453 cells transfected with pEZ-M90-ADAMTS8 plasmid indicated that cisplatin inhibited cell growth dramatically. Furthermore, attenuated β-catenin, MMP-7 and c-Myc level was detected after ADAMTS8 overexpression.
Conclusion
These results indicate that increased ADAMTS8 expression could modify the progression of BC by inhibiting cell proliferation and invasion while promoting the apoptosis of BC cells. Thus, ADAMTS8 represents a potential therapeutic target for BC therapy.
Keywords: ADAMTS8, breast cancer, BC, proliferation, invasion, apoptosis
Introduction
Breast cancer (BC) is the most common cancer and the sixth cause of cancer-related deaths in Chinese females.1 A large number of hormonal and reproductive factors are contributed to the increased risk of BC, including early menarche, late menopause, limited breast-feeding, nulliparity, abortion.2–5 Moreover, accumulating evidence has suggested that genetic and epigenetic alterations play crucial roles in initiation and progression of BC.6–9 Therefore, there is an urgent need for molecular investigations that can direct the development of effective biomarkers and therapeutic targets in the treatment of BC.
A disintegrin and metalloprotease with thrombospondin motifs (ADAMTSs), a family of extracellular matrix metalloproteinases comprising 19 members in humans, are similar to matrix metalloproteinases (MMPs) and ADAMs in terms of both structure and function.10,11 Members of the ADAMTS family are distinguished by one or more thrombospondin type 1 repeat domains in their ancillary regions.12 The ADAMTS genes participate in a wide range of biological processes, including extracellular matrix assembly and degradation, hemostasis, hemostasis, organogenesis, and angiogenesis.13 They also play an important role in tumorigenesis and the progression of a variety of cancers, such as esophageal, nasopharyngeal, gastric, colorectal, pancreatic, lung, hepatic and breast cancers.14–20
ADAMTS8, also known as METH-2, is a member of the ADAMTS family and has antiangiogenic properties.21 ADAMTS8 can inhibit vascular endothelial growth factor (VEGF)-mediated angiogenesis in endothelial cells in vitro.22 Increasing evidence has indicated that ADAMTS8 expression was decreased in some cancers.21,23,24 Another study demonstrated that ADAMTS8 has inhibitory effects on the proliferation and invasion of hepatocellular carcinoma.19 However, as far as we know, no specific reports on the clinical significance and unique roles of ADAMTS8 in BC have been published. Therefore, in the current study, we aimed to investigate the functional role of ADAMTS8 in BC and explore the underlying mechanisms.
Materials and Methods
Tissue Specimens of the Patients with BC
Tissue specimens were collected at the Fourth Hospital of Hebei University from 12 BC patients who underwent tumor resection in the Department of Surgery in May 2020. All procedures were supervised and approved by the hospital’s Human Tissue Research Committee. Inform consent was provided to all patients enrolled in this study and the methods were carried out in accordance with the approved guidelines.
Cell Lines and Cell Culture
Five human BC cell lines (MDA-MB-231, MDA-MB-468, MDA-MB-453, MCF-7 and T47D) were purchased from the Shanghai Institute of Biological Sciences, Chinese Academy of Sciences (Shanghai, China) and cultured in RPMI 1640 medium (Gibco® BRL; Thermo Fisher Scientific) supplemented with 10% FBS (Gibco® BRL; Thermo Fisher Scientific), 100 U/mL of penicillin, 100 U/mL of streptomycin at 37°C, and 5% CO2 in a humidified atmosphere incubator.
Western Blot Analysis
The total protein from each BC cell and tissue specimens of the patients with BC was extracted using RIPA lysis buffer (Roche, Basel, Switzerland) containing phenylmethylsulphonyl fluoride (PMSF). The total protein concentration was determined using a bicinchoninic acid (BCA) kit (Sigma-Aldrich Co., St Louis, MO, USA). Then, 50 μg of protein was separated by 10% sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto polyvinylidene difluoride (PVDF) membranes (Roche). The membranes were blocked with 5% skim milk for 2 hours at room temperature, and then they were incubated first with mouse monoclonal antibody against human ADAMTS8 (1:2000; Origene Technologies, Rockville, MD, USA), β-catenin (1:2000; Santa Cruz, CA, USA), MMP-7 (1:2000; Santa Cruz, CA, USA), c-Myc (1:2000; Santa Cruz, CA, USA), or β-actin (1:10,000; Abcam, Cambridge, MA, USA) at 4°C overnight, followed by incubation with a secondary antibody HRP-conjugated anti-mouse immunoglobulin (Ig)G (1:5000; Thermo Fisher Scientific, Waltham, MA, USA). The enhanced chemiluminescence (ECL) reagent (Thermo Fisher Scientific) was dripped onto the membranes and exposed to a FluorChem® HD2 protein imprinting imaging system (Alpha InnoTec, San Leandro, CA, USA).
Cell Transfection
The human BC cell line MDA-MB-453 in the logarithmic growth phase was seeded onto six-well plates. MDA-MB-453 cells were transfected instantaneously with pEZ-M90-ADAMTS8 and pEZ-M90 (vector) plasmids labeled with green fluorescent protein (GFP) (GeneCopoeia, Rockville, MD, USA) using Lipofectamine 2000 (Thermo Fisher Scientific) according to the manufacturer’s instructions (GeneCopoeia, Rockville, MD, USA). MDA-MB-231 cells were transfected with psi-H1-ADAMTS8siRNA and psi-H1 plasmids. Successful overexpression and knockdown of ADAMTS8 were confirmed by Western blot analysis using an anti-ADAMTS8 antibody (Origene, Rockville, MD, USA). Following transfection for 72 hours, 0.6 µg/mL of puromycin (Sigma-Aldrich Co., St. Louis, MO, USA) was used for clone selection. Stable cells were harvested 4 weeks later for subsequent experiments.
Cell Proliferation Assay
Cell proliferation was examined using Cell Counting Kit (CCK)-8 (Dojindo Lab, Kumamoto, Japan). Briefly, stably transfected cells were harvested and seeded onto 96-well microplates (Corning Incorporated, Corning, NY, USA) at a density of 1×103 cells per well. Six duplicate wells were set up for each group. At each time point (0 hours, 12 hours, 24 hours, 48 hours, and 72 hours), 10 µL of CCK-8 was added to each well, according to the manufacturer’s protocol, followed by incubation at 37°C with 5% humidified CO2 for 2 hours. The absorbance of the wells was measured at 450 nm using a Microplate Autoreader (Bio-Tek Instruments, Winooski, VT, USA). All assays were carried out independently in triplicate.
Wound-Healing Assay
Stably transfected cells were seeded onto six-well plates and cultured to approximately 90% confluence. Then, a straight wound was drawn with a 200 μL pipette tip on the cell monolayer. Subsequently, the cells continued to be cultured with serum-free medium for 24 hours. The wound closure was monitored and photographed under a microscope at 0 hours, 12 hours, and 24 hours. At least five fields were analyzed for each scratch, and the migration rates were calculated as the width of a scratch divided by the initial width of the same scratch, as described previously.25
Cell Migration and Invasion Assay
The migratory and invasive potential of the cells was evaluated using Transwell chambers (8 μm pores; Corning). Then, 1×104 cells suspended in 200 μL of serum-free medium were added to the upper chamber (a Matrigel-uncoated chamber for the migration assay and a Matrigel-coated chamber for the invasion assay), and 500 μL of medium with 10% FBS was added into the lower chamber. Following incubation for 24 hours at 37°C, the cells that did not migrate or invade through the pores were removed using a cotton swab, and the cells on the lower surface of the membrane were fixed with methanol, stained with 0.1% crystal violet, and counted.
Flow Cytometry Assay
Cell apoptosis was detected by flow cytometry analysis. Each group of cells was harvested, washed twice with cold PBS, and resuspended in binding buffer; then, PE Annexin V and 7-AAD (BD Biosciences, Pharmingen, San Diego, CA, USA) were added to the cells in darkness for 15 minutes at 25°C. Cell apoptosis was analyzed using a fluorescence-activated cell sorting (FACS) Aria II flow cytometer (BD Biosciences). The experiment was repeated at least three times.
In vivo Tumorigenesis Experiments
The animal experiments were approved by the Institutional Animal Care and Ethics Committee of The Fourth Hospital of Hebei Medical University (approval number: 2,019,062). Thirty 4-week-old male BALB/c nude mice were purchased from Charles River Laboratories (Beijing, China; permission no. SCXK [Jing] 2016–0006). For the xenograft tumor growth assay, MDA-MB-453 cells (2×106 cells per mouse) were stably transfected with a negative control or an ADAMTS8 overexpressing plasmid was injected subcutaneously into the left scapular region of each mouse. Tumor size was measured every 3 days with a caliper, and tumor volume (v) was calculated with the following formula: v = (length×width2)/2. After 4 weeks, the mice were euthanized, and the tumors were removed and weighed. All animal experiments were performed according to the National Institutes of Health Animal Use Guidelines on the Use of Experimental Animals.
Cell Chemosensitivity Assay
The cell chemosensitivity was assessed by CCK-8 assay as described above. The chemotherapeutic reagents cisplatin, doxorubicin, and cyclophosphamide (TCI chemicals, Shanghai, China) were used in this assay. Cells were treated with chemotherapy reagents for 72 hours prior to CCK-8 assay with the following: cisplatin, 0, 0.2, 0.4, 0.6, 0.8, 1.0, 1.2 mg/mL; doxorubicin, 0, 0.2, 0.4, 0.6, 0.8, 1.2 μg/mL; cyclophosphamide, 0, 2, 4, 6, 8, 10, 12 μg/mL.
Statistical Analysis
Data are expressed as means ± standard deviation. Statistical analysis between two groups was performed with Student’s t-test, and the comparison between three or more groups was performed with One-way ANOVA. P<0.05 was considered to indicate a statistically significant difference. Statistical analyses were performed using the SPSS version 19.0 software (IBM Corporation, Armonk, NY, USA).
Results
ADAMTS8 Expression Levels are Decreased in BC Tissues
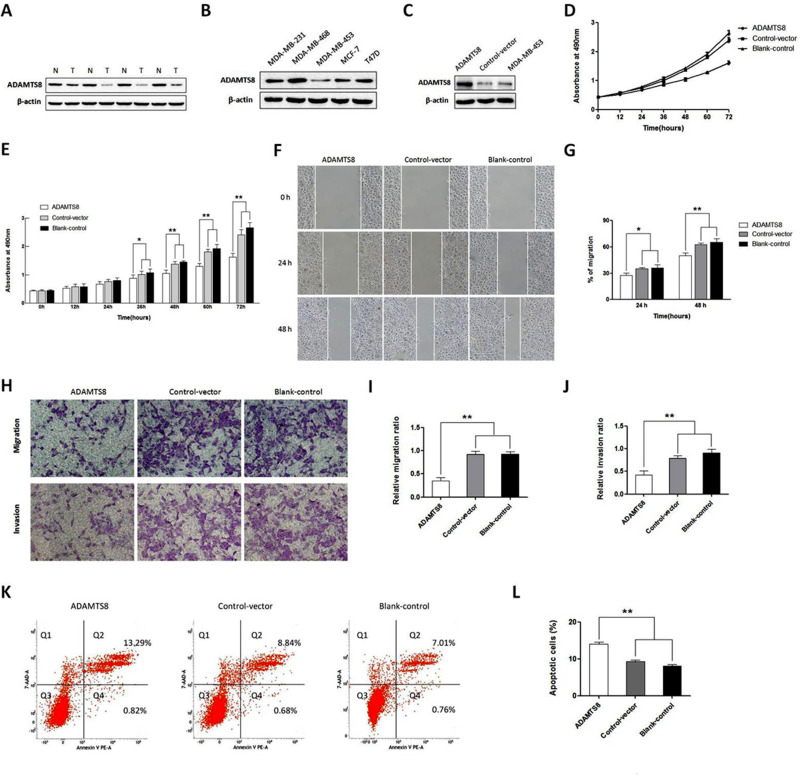
Twelve BC patients were enrolled to evaluate the expression levels of ADAMTS8 by Western blot. The result showed that ADAMTS8 expression was significantly decreased in the BC tissue specimens compared with the adjacent non-tumor tissue specimens (Figure 1A).
Figure 1.
Overexpression of ADAMTS8 inhibits proliferation, migration, invasion and induces apoptosis of BC cells. (A) Western blotting assays of ADAMTS8 protein expression in patients with BC tissues. T: BC tumor tissues; N: corresponding non-tumor tissues. (B) Expression of ADAMTS8 protein levels was examined in different BC cell lines with Western blotting. (C) Expression of ADAMTS8 protein was examined with Western blotting in MDA-MB-453 cells stably transfected with pEZ-M90-ADAMTS8 or pEZ-M90 plasmids. (D) CCK-8 assay showing that overexpression of ADAMTS8 inhibits proliferation of MDA-MB-453 cells. (E) Quantification of results from D. (F) Wound-healing assay showing that overexpression of ADAMTS8 suppresses migration of MDA-MB-453 cells. (G) Quantification of results from F. (H) Transwell assay showing that overexpression of ADAMTS8 suppresses invasion of MDA-MB-453 cells. (I, J) Quantification of results from H. (K) Flow cytometry showing that overexpression of ADAMTS8 induces apoptosis of MDA-MB-453 cells. (L) Quantification of results from (K). *P < 0.05, **P < 0.01.
Abbreviations: ADAMTS8, a disintegrin and metallopeptidase with thrombospondin motif 8; BC, breast cancer; CCK-8, cell counting kit-8.
Overexpression of ADAMTS8 Inhibits Proliferation and Induces Apoptosis Among BC Cells
To explore the functional roles of ADAMTS8 in BC, the ADAMTS8 protein level was first evaluated in five BC cell lines by Western blot (Figure 1B). Since MDA-MB-453 cells exhibited low ADAMTS8 expression, whereas MDA-MB-231 cells displayed high ADAMTS8 expression, which have a high rate of migration and invasion, we use these two cell lines for subsequent overexpression and knockdown assays. We then transfected pEZ-M90-ADAMTS8 or pEZ-M90 (Vector) plasmids into the MDA-MB-453 cells. As shown in Figure 1C, ADAMTS8 protein expression had significantly increased in MDA-MB-453 cells transfected with pEZ-M90-ADAMTS8 when compared with that observed in MDA-MB-453 cells and MDA-MB-453 cells transfected with pEZ-M90. Therefore, these MDA-MB-453 cells were used for further experiments.
CCK-8 assays were performed to determine the proliferation capacity of MDA-MB-453 cells. The CCK-8 assay showed that the overexpression of ADAMTS8 could significantly inhibit the proliferative ability at 36 hours and 72 hours in MDA-MB-453 cells, as compared with the control vector and blank control groups (Figure 1D and E).
To explore the function of ADAMTS8 in inducing apoptosis of BC cells, flow-cytometry was performed. As presented in Figure 1K and L, the percentage of apoptotic cells in the pEZ-M90-ADAMTS8 group was significantly higher than that of the control vector and blank control groups.
In summary, these results suggest that the overexpression of ADAMTS8 could inhibit the proliferation and induce apoptosis of MDA-MB-453 cells.
Overexpression of ADAMTS8 Attenuates the in vitro Migration and Invasion of BC Cells
The effects of ADAMTS8 on the migration and invasion ability of BC cells were further investigated by wound-healing and Transwell assays. In the wound-healing assay and transwell migration assay, the migration ability was markedly decreased in the MDA-MB-453 cells that overexpressed ADAMTS8 when compared with that of the control cells (Figure 1F–I). In the Matrigel invasion assay, overexpressing ADAMTS8 attenuated the invasiveness of MDA-MB-453 (Figure 1H and J). Taken together, these results indicated that the overexpression of ADAMTS8 remarkably attenuated the migration and invasion of BC cells.
Downregulation of ADAMTS8 Promotes Proliferation, Migration, Invasion and Inhibits Apoptosis of BC Cells
As shown in Figure 2A, ADAMTS8 protein expression had decreased in MDA-MB-231 cells transfected with psi-H1-ADAMTS8siRNA when compared with that observed in MDA-MB-231 cells and MDA-MB-231 cells transfected with psi-H1. The effect of ADAMTS8 knockdown on BC progression in terms of proliferation, migration and invasion was evaluated in MDA-MB-231 cells. Cells transfected with psi-H1-ADAMTS8siRNA promoted proliferation from 48 to 72 h (P < 0.05, Figure 2B and C), migration (P < 0.05, Figure 2D–G), and invasion (P < 0.01, Figure 2F and H) compared with cells transfected with psi-H1 or blank control cells. Apoptosis was also measured by flow cytometry. Apoptotic cells were decreased in psi-H1- ADAMTS8siRNA group compared with that of the blank control groups (P < 0.05, Figure 2I and J). These data demonstrated that downregulation of ADAMTS8 promotes proliferation, migration, invasion and inhibits apoptosis of BC cells.
Figure 2.
ADAMTS8 knockdown promotes proliferation, migration, invasion and inhibits apoptosis of BC cells. (A) Expression of ADAMTS8 protein was examined with Western blotting in MDA-MB-231 cells stably transfected with psi-H1-ADAMTS8siRNA or psi-H1 plasmids. (B) CCK-8 assay showing that ADAMTS8 knockdown promotes proliferation of MDA-MB-231 cells. (C) Quantification of results from B. (D) Wound-healing assay showing that ADAMTS8 knockdown promotes migration of MDA-MB-231 cells. (E) Quantification of results from D. (F) Transwell assay showing that ADAMTS8 knockdown promotes invasion of MDA-MB-231 cells. (G, H) Quantification of results from F. (I) Flow cytometry showing that ADAMTS8 knockdown inhibits apoptosis of MDA-MB-231 cells. (J) Quantification of results from (I). *P < 0.05, **P < 0.01.
Abbreviations: ADAMTS8, a disintegrin and metallopeptidase with thrombospondin motif 8; BC, breast cancer; CCK-8, cell counting kit-8.
Overexpression of ADAMTS8 Inhibits the Tumor Growth of BC Cells in vivo
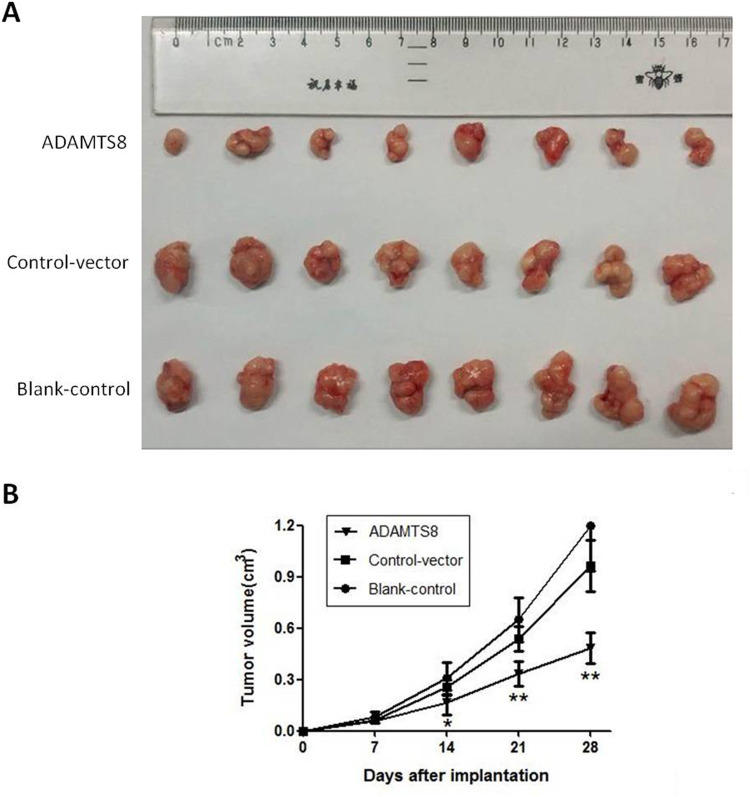
To confirm the effects of ADAMTS8 on BC cell growth in vivo, a xenograft tumor assay was performed in nude mice. As shown in Figure 3A, tumors formed from MDA-MB-453 cells stably transfected with pEZ-M90-ADAMTS8 were much smaller than those from the control vector and blank control groups. The volume of the tumors that stemmed from MDA-MB-453 cells stably transfected with pEZ-M90-ADAMTS8 were markedly reduced compared to that of the control vector and blank control groups 21 days after implantation (Figure 3B). These data demonstrated that the overexpression of ADAMTS8 could inhibit BC cell growth in vivo.
Figure 3.
Overexpression of ADAMTS8 inhibits the growth of BC cell in vivo. (A) Photographs of mice bearing human MDA-MB-453 xenograft. (B) Growth curves of xenograft tumors. *P < 0.05, **P < 0.01.
Abbreviations: ADAMTS8, a disintegrin and metallopeptidase with thrombospondin motif 8; BC, breast cancer.
Overexpression of ADAMTS8 Enhanced the Chemosensitivity of BC Cells to Cisplatin
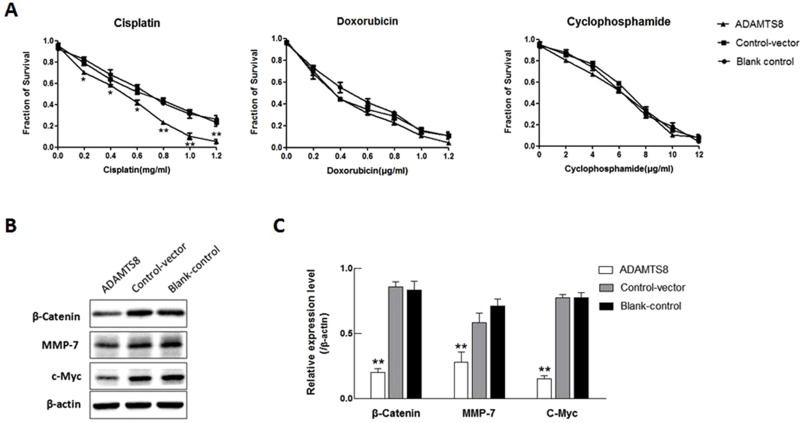
To explore the role of ADAMTS8 in chemotherapy, we treated MDA-MB-453 cells with different concentrations of cisplatin, doxorubicin, and cyclophosphamide. CCK-8 assay revealed that the proliferation of MDA-MB-453 cells was significantly inhibited in the ADAMTS8 group after treatment with cisplatin in all doses used (Figure 4A). These results indicated that overexpression of ADAMTS8 increased chemotherapy sensitivity of MDA-MB-453 cells to cisplatin.
Figure 4.
Overexpression of ADAMTS8 increases the chemotherapy sensitivity and attenuates β-catenin, MMP-7 and c-Myc protein levels in BC cell. (A) MDA-MB-453 cells stably transfected with pEZ-M90-ADAMTS8 or pEZ-M90 plasmids were treated with increasing concentrations of chemotherapy drug cisplatin, doxorubicin, or cyclophosphamide. CCK-8 assay analyzed cell survival. (B) Expression of β-catenin, MMP-7 and c-Myc protein was examined with Western blotting in MDA-MB-453 cells stably transfected with pEZ-M90-ADAMTS8 or pEZ-M90 plasmids. (C) Quantification of results from (B). *P < 0.05, **P < 0.01.
Abbreviations: ADAMTS8, a disintegrin and metallopeptidase with thrombospondin motif 8; MMP-7, matrix metalloproteinase-7; BC, breast cancer; CCK-8, cell counting kit-8.
Influence on the Wnt/β-Catenin Signalling Pathway May Be Involved in ADAMTS8-Induced Inhibition of MDA-MB-453 Cells
To determine the signalling pathway that is influenced by ADAMTS8, the effect of ADAMTS8 on the activity of Wnt/β-Catenin signalling was evaluated, which is involved in the inhibition of MDA-MB-453 cells. As shown in Figure 4B and C, ADAMTS8 significantly suppressed the activities of target genes of the Wnt/β-catenin pathway, including β-catenin, MMP7, c-MYC. These data indicate that ADAMTS8 affects the Wnt/β-catenin signaling pathway, which regulates MDA-MB-453 proliferation, migration, invasion and apoptosis of MDA-MB-453 cells.
Discussion
It is well known that carcinogenesis is a complex, multi-step process involving various environmental and genetic factors.26 In addition, many abnormal events in the tumor microenvironment contribute to cancer occurrence and development, such as the loss of cell-cycle control, changes in apoptotic and angiogenic functions, and destruction of the extracellular matrix (ECM).27 As a secreted proteinase family binding to ECM, the dysregulated expression of ADAMTSs in diverse types of cancer has been reported.15,16,20,22,28 However, there are limited data on the role of ADAMTSs in BC.
In our current work, the subsequent functional analysis illustrated that overexpression of ADAMTS8 could inhibit proliferation, migration, and invasion, as well as promote the apoptosis of BC cells, which is in line with the findings in hepatocellular carcinoma.19 In addition, ADAMTS8 overexpression elevated chemosensitivity of BC cells to cisplatin. Moreover, ADAMTS8 also exhibits inhibitory effects on BC cell growth in vivo. These results suggest that ADAMTS8 display antitumorigenic activity and might modify the outcomes of BC.
ADAMTS8 was shown to be an antiangiogenic factor;22 anti-angiogenic activity is thought to be mediated through its thrombospondin-1 motifs, which block capillary-like tube formation and endothelial cell proliferation,29 or directly influence it through VEGF binding.22 These findings suggest that ADAMTS8 holds potential as a tumor suppressor. As a metalloproteinase, ADAMTS8 plays a pivotal role in tumor growth and the multi-step processes of invasion and metastasis, including proteolytic degradation of ECM, alteration of the cell-cell and cell-ECM interactions, regulation of growth factor activities and integrin functions, and angiogenesis.30,31 Emerging evidence suggests that ADAMTS8 can inhibit tumor cell growth and induce apoptosis by modulating the EGFR-MEK-ERK signaling pathway.19,24 Furthermore, ADAMTS8 frequently shows promoter methylation in some cancers, suggesting that the epigenetic silencing of ADAMTS8 may be related to tumorigenesis.18,21,32 The Wnt/β-catenin pathway plays a crucial role in the differentiation, proliferation, migration, invasion, and cellular death processes.33,34 β-Catenin, a core component of Wnt/β-catenin signaling, exerts an important role in cancers. Some studies showed that Wnt/β-catenin pathway is abnormally activated in breast cancer, and high β-catenin activity has been reported to correlate with poor prognosis and tumor progression in breast cancer.35,36 Li et al also demonstrate that ADAMTS8 functions as a tumor suppressor in colorectal cancer through Wnt/β-catenin signaling pathway.37 The present study demonstrated that overexpression of ADAMTS8 inhibited proliferation and invasion by mediating the expression of the Wnt/β-catenin target genes c-Myc and MMP-7 in BC cells. Taken together, these mechanisms are potentially involved in the progression of BC induced by ADAMTS8. The full mechanism of ADAMTS8 in BC process and its associated signaling pathways need further explored.
Conclusion
These results indicate that ADAMTS8 may modify BC development and progression expression by influencing cell proliferation, migration, invasion and apoptosis of BC cells potentially via Wnt/β-catenin signaling. Therefore, targeting ADAMTS8 might represent a promising therapeutic strategy for BC patients.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
All authors declare that they have no competing interests.
References
- 1.Fan L, Strasser-Weippl K, Li JJ, et al. Breast cancer in China. Lancet Oncol. 2014;15(7):e279–e289. doi: 10.1016/S1470-2045(13)70567-9 [DOI] [PubMed] [Google Scholar]
- 2.Gao YT, Shu XO, Dai Q, et al. Association of menstrual and reproductive factors with breast cancer risk: results from the Shanghai Breast Cancer Study. Int J Cancer. 2000;87:295–300. doi: [DOI] [PubMed] [Google Scholar]
- 3.Zhang Q, Liu LY, Wang F, Mu K, Yu ZG. The changes in female physical and childbearing characteristics in China and potential association with risk of breast cancer. BMC Public Health. 2012;12:368. doi: 10.1186/1471-2458-12-368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Key TJ, Verkasalo PK, Banks E. Epidemiology of breast cancer. Lancet Oncol. 2001;2(3):133–140. doi: 10.1016/S1470-2045(00)00254-0 [DOI] [PubMed] [Google Scholar]
- 5.Yan R, Wang K, Peng R, et al. Genetic variants in lncRNA SRA and risk of breast cancer. Oncotarget. 2016;7(16):22486–22496. doi: 10.18632/oncotarget.7995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mavaddat N, Michailidou K, Dennis J, et al. Polygenic risk scores for prediction of breast cancer and breast cancer subtypes. Am J Hum Genet. 2019;104(1):21–34. doi: 10.1016/j.ajhg.2018.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Decker B, Allen J, Luccarini C, et al. Rare, protein-truncating variants in ATM, CHEK2 and PALB2, but not XRCC2, are associated with increased breast cancer risks. J Med Genet. 2017;54(11):732–741. doi: 10.1136/jmedgenet-2017-104588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang F, Zhang Y, Deng Z, et al. Genetic variants in the acylphosphatase 2 gene and the risk of breast cancer in a Han Chinese population. Oncotarget. 2016;7(52):86704–86712. doi: 10.18632/oncotarget.13495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perreault AA, Sprunger DM, Venters BJ. Epigenetic and transcriptional profiling of triple negative breast cancer. Sci Data. 2019;6:190033. doi: 10.1038/sdata.2019.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang BL. ADAMTS: a novel family of extracellular matrix proteases. Int J Biochem Cell Biol. 2001;33(1):33–44. doi: 10.1016/s1357-2725(00)00061-3 [DOI] [PubMed] [Google Scholar]
- 11.Kelwick R, Desanlis I, Wheeler GN, Edwards DR. The ADAMTS (a disintegrin and metalloproteinase with thrombospondin motifs) family. Genome Biol. 2015;16(1):113. doi: 10.1186/s13059-015-0676-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Porter S, Clark IM, Kevorkian L, Edwards DR. The ADAMTS metalloproteinases. Biochem J. 2005;386(Pt 1):15–27. doi: 10.1042/BJ20040424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wagstaff L, Kelwick R, Decock J, Edwards DR. The roles of ADAMTS metalloproteinases in tumorigenesis and metastasis. Front Biosci. 2011;16:1861–1872. doi: 10.2741/3827 [DOI] [PubMed] [Google Scholar]
- 14.Jin H, Wang X, Ying J, et al. Epigenetic identification of ADAMTS18 as a novel 16q23.1 tumor suppressor frequently silenced in esophageal, nasopharyngeal and multiple other carcinomas. Oncogene. 2007;26(53):7490–7498. doi: 10.1038/sj.onc.1210559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kilic MO, Aynekin B, Kara A, Icen D, Demircan K. Differentially regulated ADAMTS1, 8, and 18 in gastric adenocarcinoma. Bratisl Lek Listy. 2017;118(2):71–76. doi: 10.4149/BLL_2017_014 [DOI] [PubMed] [Google Scholar]
- 16.Filou S, Korpetinou A, Kyriakopoulou D, et al. ADAMTS expression in colorectal cancer. PLoS One. 2015;10(3):e0121209. doi: 10.1371/journal.pone.0121209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yi JM, Guzzetta AA, Bailey VJ, et al. Novel methylation biomarker panel for the early detection of pancreatic cancer. Clin Cancer Res. 2013;19(23):6544–6555. doi: 10.1158/1078-0432.CCR-12-3224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dunn JR, Panutsopulos D, Shaw MW, et al. METH-2 silencing and promoter hyper- methylation in NSCLC. Br J Cancer. 2004;91(6):1149–1154. doi: 10.1038/sj.bjc.6602107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao X, Yang C, Wu J, Nan Y. ADAMTS8 targets ERK to suppress cell proliferation, invasion, and metastasis of hepatocellular carcinoma. Onco Targets Ther. 2018;11:7569–7578. doi: 10.2147/OTT.S173360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Porter S, Span PN, Sweep FCGJ, et al. ADAMTS8 and ADAMTS15 expression predicts survival in human breast carcinoma. Int J Cancer. 2006;118(5):1241–1247. doi: 10.1002/ijc.21476 [DOI] [PubMed] [Google Scholar]
- 21.Dunn JR, Reed JE, Du Plessis DG, et al. Expression of ADAMTS-8, a secreted protease with antiangiogenic properties, is downregulated in brain tumours. Br J Cancer. 2006;94(8):1186–1193. doi: 10.1038/sj.bjc.6603006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vázquez F, Hastings G, Ortega MA, et al. METH-1, a human ortholog of ADAMTS-1, and METH-2 are members of a new family of proteins with angio-inhibitory activity. J Biol Chem. 1999;274(33):23349–23357. doi: 10.1074/jbc.274.33.23349 [DOI] [PubMed] [Google Scholar]
- 23.Porter S, Scott SD, Sassoon EM, et al. Dysregulated expression of adamalysin-thrombospon- din genes in human breast carcinoma. Clin Cancer Res. 2004;10(7):2429–2440. doi: 10.1158/1078-0432.ccr-0398-3 [DOI] [PubMed] [Google Scholar]
- 24.Choi GC, Li J, Wang Y, et al. The metalloprotease ADAMTS8 displays antitumor properties through antagonizing EGFR-MEK-ERK signaling and is silenced in carcinomas by CpG methylation. Mol Cancer Res. 2014;12(2):228–238. doi: 10.1158/1541-7786.MCR-13-0195 [DOI] [PubMed] [Google Scholar]
- 25.Guan D, Factor D, Liu Y, Wang Z, Kao HY. The epigenetic regulator UHRF1 promotes ubiquitination-mediated degradation of the tumor-suppressor protein promyelocytic leukemia protein. Oncogene. 2013;32(33):3819–3828. doi: 10.1038/onc.2012.406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Migliore L, Coppedè F. Genetic and environmental factors in cancer and neurodegenerative diseases. Mutat Res. 2002;512(2–3):135–153. doi: 10.1016/s1383-5742(02)00046-7 [DOI] [PubMed] [Google Scholar]
- 27.Soysal SD, Tzankov A, Muenst SE. Role of the tumor microenvironment in breast cancer. Pathobiology. 2015;82(3–4):142–152. doi: 10.1159/000430499 [DOI] [PubMed] [Google Scholar]
- 28.Li S, Li T, Li X, et al. MicroRNA-32 regulates development and progression of hepatocellular carcinoma by targeting ADAMTS9 and affects its prognosis. Med Sci Monit Basic Res. 2018;24:177–187. doi: 10.12659/MSMBR.910522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lawler J. The functions of thrombospondin-1 and-2. Curr Opin Cell Biol. 2000;12(5):634–640. doi: 10.1016/s0955-0674(00)00143-5 [DOI] [PubMed] [Google Scholar]
- 30.Yoon SO, Park SJ, Yun CH, Chung AS. Roles of matrix metalloproteinases in tumor metastasis and angiogenesis. J Biochem Mol Biol. 2003;36(1):128–137. doi: 10.5483/bmbrep.2003.36.1.128 [DOI] [PubMed] [Google Scholar]
- 31.Mochizuki S, Okada Y. ADAMs in cancer cell proliferation and progression. Cancer Sci. 2007;98(5):621–628. doi: 10.1111/j.1349-7006.2007.00434.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rodríguez-Rodero S, Fernández AF, Fernández-Morera JL, et al. DNA methylation signatures identify biologically distinct thyroid cancer subtypes. J Clin Endocrinol Metab. 2013;98(7):2811–2821. doi: 10.1210/jc.2012-3566 [DOI] [PubMed] [Google Scholar]
- 33.Clevers H, Nusse R. Wnt/β-catenin signaling and disease. Cell. 2012;149(6):1192–1205. doi: 10.1016/j.cell.2012.05.012 [DOI] [PubMed] [Google Scholar]
- 34.Ren L, Chen H, Song J, et al. MiR-454-3p-mediated Wnt/β-catenin signaling antagonists suppression promotes breast cancer metastasis. Theranostics. 2019;9(2):449–465. doi: 10.7150/thno.29055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khramtsov AI, Khramtsova GF, Tretiakova M, et al. Wnt/beta-catenin pathway activation is enriched in basal-like breast cancers and predicts poor outcome. Am J Pathol. 2010;176(6):2911–2920. doi: 10.2353/ajpath.2010.091125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lin SY, Xia W, Wang JC, et al. β-catenin, a novel prognostic marker for breast cancer: its roles in cyclin D1 expression and cancer progression. Proc Natl Acad Sci U S A. 2000;97(8):4262–4266. doi: 10.1073/pnas.060025397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li L, Yuan S, Zhao X, et al. ADAMTS8 is frequently down-regulated in colorectal cancer and functions as a tumor suppressor. Biochem Biophys Res Commun. 2020;524(3):663–671. doi: 10.1016/j.bbrc.2020.01.020 [DOI] [PubMed] [Google Scholar]