Abstract
Background:
In primary aldosteronism (PA), kidney function impairment could be concealed by relative hyperfiltration and emerge after adrenalectomy. We hypothesized transtubular gradient potassium gradient (TTKG), a kidney aldosterone bioactivity indicator, could correlate to end organ damage and forecast kidney function impairment after adrenalectomy.
Methods:
In the present prospective study, we enrolled lateralized PA patients who underwent adrenalectomy and were followed up 12 months after operation in the Taiwan Primary Aldosteronism Investigation (TAIPAI) registry from 2010 to 2018. The clinical outcome was kidney function impairment, defined as estimated glomerular filtration rate (eGFR) <60 ml/min/1.73 m2 at 12 months after adrenalectomy. End organ damage is determined by microalbuminuria and left ventricular mass.
Results:
In total, 323 patients [mean, 50.8 ± 10.9 years old; female 178 (55.1%)] were enrolled. Comparing pre-operation and post-operation data, systolic blood pressure, serum aldosterone, urinary albumin to creatinine ratio and eGFR decreased. TTKG ⩾ 4.9 correlated with pre-operative urinary albumin to creatinine ratio >50 mg/g [odds ratio (OR) = 2.42; p = 0.034] and left ventricular mass (B = 20.10; p = 0.018). Multivariate logistic regression analysis demonstrated that TTKG ⩾ 4.9 could predict concealed chronic kidney disease (OR = 5.42; p = 0.011) and clinical success (OR = 2.90, p = 0.017) at 12 months after adrenalectomy.
Conclusions:
TTKG could predict concealed kidney function impairment and cure of hypertension in PA patients after adrenalectomy. TTKG more than 4.9 as an adverse surrogate of aldosterone and hypokalaemia correlated with pre-operative end organ damage in terms of high proteinuria and cardiac hypertrophy.
Keywords: adrenalectomy, kidney function impairment, primary aldosteronism, transtubular potassium gradient
Introduction
Primary aldosteronism (PA) is the most common cause of secondary hypertension.1 Related to persistent hypertension and volume retention,2 PA patients have high incidence of end organ damage3,4 and cardiovascular events5 when compared with essential hypertension patients. In aldosterone producing adenoma (APA), refractory hypertension could be correctable when patients receive adrenalectomy.6
In addition to high blood pressure that damages kidney structure,7,8 aldosterone directly contributes to injury to the kidneys via the genomic and non-genomic pathway.9 In rodent studies, aldosterone infusion induced glomerular, kidney vascular injury10 and interstitial fibrosis.11 Furthermore, longstanding aldosterone excess accelerates hyperfiltration in the kidneys.12 Kidney hyperfiltration contributes to early renal damage in pre-diabetes and pre-hypertension patients.13 Related to enhanced sodium reabsorption by excess aldosterone, kidney hyperfiltration in PA12,14 is more significant and will augment kidney damage together with the presence of abnormal urinary albumin excretion.4,15 However, relative kidney hyperfiltration masks deteriorating kidney function12,16 and makes kidney function impairment difficult to isolate. Therefore, kidney function impairment may be concealed in PA patients and emerge after adrenalectomy.
Low serum potassium was reported to predict declined estimated glomerular filtration rate after adrenalectomy in PA patients.17 Potassium secretion in the kidney cortical collecting duct is mainly modulated by aldosterone.18 PA patients may have refractory hypokalaemia related to persistent aldosterone stimulation in kidney. Transtubular potassium gradient (TTKG), a formula to gauge renal potassium secretion by the cortical collecting duct,19,20 had been used as bioactivity marker of aldosterone on kidney.20,21 The aim of the present study was to investigate whether TTKG could predict kidney function impairment after surgical treatment in PA patients and its relationship with end organ injury.
Methods
Ethics statement
The study complied with the Declaration of Helsinki and was approved by the National Taiwan University Hospital Research Ethics Committee (No. 200611031R). All participants received comprehensive written information and signed a consent form before inclusion in the study.
Patients’ selection
The present study is a cohort study enrolling PA patients from 2010 to 2018 who received adrenalectomy and were followed up to 12 months with serum creatinine data. The individuals were registered in the Taiwan Primary Aldosteronism Investigation (TAIPAI) database.22–31 The study group included two medical centres (National Taiwan University Hospital (NTUH), Taipei, Taiwan; Taipei University Hospital, Taipei, Taiwan) and five regional hospitals (Cardinal Tien Hospital, New Taipei City, Taiwan; Taipei Tzu Chi Hospital, New Taipei City, Taiwan; Yun- Lin Branch of NTUH, Douliou City, Taiwan; Hsin-Chu Branch of NTUH, Hsin-Chu City, Taiwan; Zhongxing Branch of Taipei City Hospital, Taipei, Taiwan).32
All antihypertensive medications were discontinued for at least 21 days before confirmation tests. Doxazosin and/or diltiazem were administered to control markedly high blood pressure when required.25
The diagnosis of PA in hypertensive patients was based on the following criteria:
Confirmation
Fulfilment of the following three conditions confirmed a diagnosis of PA:28,31
(a) Autonomous excess aldosterone production evidenced with a 24-h urinary aldosterone level (Uald-24 h) more than 20.3 µg; (b) a TAIPAI score larger than 60%;26 (c) Seated post-saline loading plasma aldosterone concentration (PAC) >16 ng/dl or PAC/plasma renin activity (PRA > 35 (ng/dl)/(ng/ml/h) shown in a post-captopril test.
The probability of PA (TAIPAI score)26 was equal to: = 1/1 + e−β, where β = [PAC (ng/dl) × (0.063)] + [PRA (ng/ml/h) × (–0.205)] + [(ARR × 0.001) + BMI(kg/m2) x (0.067)]+[Male × (–0.738) +Serum potassium(mmol/L) × (–1.512)]+ [eGFR(ml/min/1.73 m2)× (0.017)] + [(propensity score) ×(−0.539) +(1.851)], where ARR represents aldosterone-renin ratio, BMI represents body mass index and eGFR represents estimated glomerular filtration rate.
The propensity score was described as our previous report.26
Functional survey
The aldosterone concentration was measured by radioimmunoassay using a commercial kit (Aldosterone Maia Kit, Adaltis Italia S.P.A., Bologna, Italy)33 and PRA was measured by the generation of angiotensin I in vitro using a commercially available radioimmunoassay kit (DiaSorin, Stillwater, MN, USA).34
Lateralization and subtype identification
APA is identified on the basis of PA patients following:31 (a) lateralization of aldosterone secretion at adrenal venous sampling (AVS) or during dexamethasone suppressing NP-59 SPECT/CT35 and confirm adrenal adenoma in hematoxylin and eosin (HE) stain; or (b) pathologically proven adenoma after an adrenalectomy and stained with positive CYP11B2 stain.28
Clinical parameters and assessment of outcome
General information about age, sex, body weight, BMI, systolic blood pressure (SBP) and diastolic blood pressure (DBP) were recorded. We collected biochemistry data about serum creatinine, potassium and osmolality, and urine creatinine, potassium, albumin and osmolality. The estimated glomerular filtration rate was calculated via the Chronic Kidney Disease Epidemiology Collaboration formula. Transtubular potassium gradient was calculated by using the formula: urine K/plasma K ÷ urine osmolality/plasma osmolality. Cardiac echo was conducted before surgical treatment. All echocardiography36 was performed using a Hewlett-Packard 5500 ultrasound system with an S3 transducer (1.0–3.0 MHz). Two-dimensional, M-mode, Doppler and tissue Doppler ultrasonography were performed in each patient, and the dimensions of the chamber, wall thickness and left ventricular ejection fraction (M-mode) were measured according to the guidelines of the American Society of Echocardiography.37
An eGFR of less than 60 ml/min/1.73 m2 was defined as kidney function impairment.7,38,39 Since declining eGFR became steady 6–12 months after adrenalectomy was reported,7,16,40 we assessed post-operative eGFR at 12 months after adrenalectomy as the primary endpoint.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation (SD) and categorical variables were expressed as frequency and percentage. Paired t-test and Chi-squared test were used to distinguish the differences prior to and after surgical treatment. An independent t-test was applied to distinguish variables between different groups. Pearson correlation test and multivariate linear regression analysis were carried out to evaluate the relationship between interested variables. Covariates were age, sex, body weight, BMI, SBP, DBP, log aldosterone values, PRA, ARR, pre-operation eGFR, serum potassium levels and TTKG value. In order to depict the implications of TTKG for individual patients, a generalized additive model (GAM) incorporating the subject-specific (longitudinal) random effects were plotted and adjusted with other parameters to predict the possibility of outcome.30,41 The optimal cut-off value was defined as the log odd equalling zero.31 After finding the optimal cut-off value of TTKG, univariate and multivariate logistic regression analyses were applied to evaluate TTKG and other covariates relative to the clinical outcome and end organ damage. Binary logistic regression analysis with a stepwise variable selection procedure was adopted using available variables to identify the important factors associated with post-operative complete clinical success. The significance levels for entry and for stay were conservatively set at 0.15.
Statistical significance was defined as two-sided p value < 0.05. Statistical analyses were performed with IBM SPSS statistics version 17 (Armonk, NY: IBM Corp) software and R software. All analyses were performed with R software, version 3.2.2 (Free Software Foundation, Inc., Boston, MA).42
Results
Clinical characteristic prior to and after 12 months of adrenalectomy
The present study enrolled 341 patients of unilateral PA patients who underwent adrenalectomy and were followed up to 12 months after the operation. Although eGFR was supposed to decline due to relief of hyperfiltration after adrenalectomy, we could not exclude the possibility that improved blood pressure had beneficial effects on renal function recovery. We observed that some individuals who had pre-operative eGFR less than 60 ml/min/1.73 m2, which we defined as renal function impairment, could recover after adrenalectomy. But none of the individuals who had pre-operative eGFR less than 45 ml/min/1.73 m2 recovered to above eGFR 60ml/min/1.73 m2. Thus, we excluded the 18 individuals with an eGFR less than 45 ml/min/1.73 m2 at the baseline.43 Finally, 323 enrolees were included for analysis. Included participants possessed a mean age of 50.8 ± 10.9 years and were 44.9% male. Comparing values before and 12 months after the operation, there were significant improvements in SBP, DBP, plasma aldosterone levels, PRA, ARR, serum potassium levels and urine albumin to creatinine ratio (ACR); however, eGFR was decreased (Table 1).
Table 1.
Characteristics of study cohort in primary aldosteronism patients before and 12 months of adrenalectomy.
| Variables | Before adrenalectomy | 12 months after adrenalectomy | p value |
|---|---|---|---|
| No of participants | 323 | 323 | |
| Age (years) | 50.8 ± 10.9 | NA | |
| Sex, Female (%) | 178 (55.1%) | NA | |
| Body weight (kg) | 68.5 ± 14.3 | NA | |
| Body mass index (kg/m2) | 25.6 ± 4.0 | NA | |
| ACEI or ARB | 143 (44.1%) | 62 (19.1%) | <0.001 |
| α-blocker | 77 (23.8%) | 12 (3.7%) | 0.014 |
| β-blocker | 139 (42.9%) | 50 (15.4%) | <0.001 |
| CCB | 225 (69.4%) | 87 (26.9%) | 0.022 |
| Vasodilator | 18 (5.6%) | 9 (2.8%) | 0.027 |
| Diuretics | 36 (11.1%) | 8 (2.5%) | <0.001 |
| Hypertension duration (years) | 7.6 ± 7.0 | NA | |
| SBP (mm Hg) | 154.6 ± 21.3 | 135.0 ± 17.7 | <0.001 |
| DBP (mm Hg) | 92.8 ± 13.9 | 83.9 ± 11.5 | <0.001 |
| Plasma aldosterone level (ng/dl) | 60.4 ± 40.9 | 31.0 ± 19.6 | <0.001 |
| Plasma renin activity (ng/ml/hr) | 0.72 ± 2.80 | 3.34 ± 6.73 | <0.001 |
| Aldosterone renin ratio | 1358 ± 2807 | 122 ± 596 | <0.001 |
| Serum creatinine level (mg/dl) | 0.89 ± 0.25 | 1.06 ± 0.84 | <0.001 |
| eGFR (EPI-Cr, ml/min/1.73 m2) | 88.8 ± 19.7 | 78.9 ± 22.3 | <0.001 |
| Kidney function impairment | 37 (11.5%) | 70 (21.7%) | <0.001 |
| Serum potassium level (mEq/L) | 3.5 ± 0.7 | 4.3 ± 0.4 | <0.001 |
| Urine albumin over creatinine ratio (mg/g) | 86 ± 22 | 32 ± 96 | 0.001 |
ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker; CCB, calcium channel blocker; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; EPI-Cr, Epidemiology Collaboration creatinine equation; NA, not available; SBP, systolic blood pressure; TTKG, transtubular potassium gradient.
TTKG predicted kidney function impairment 1 year after adrenalectomy in PA patients
To find the adequate cut-point value of pre-operative TTKG that predicted kidney function impairment at 12 months after adrenalectomy, a GAM plot was plotted. At the cut point of 4.9, higher TTKG forecasted an eGFR less than 60 ml/min/1.73 m2 at 1 year after surgical treatment (Figure 1). In full adjusted multivariate logistic regression analysis, systolic blood pressure [odds ratio (OR) = 1.06; 95% confidence interval (CI), 1.02–1.11; p = 0.002], pre-operative eGFR (OR = 0.91; 95% CI, 0.87–0.95; p < 0.001) and TTKG ⩾ 4.9 (OR = 5.42; 95% CI, 1.48–19.85; p = 0.011) were independent predictors of kidney function impairment (Table 2). Further assessment was conducted to evaluate confounding of anti-hypertension drugs (Supplemental Table S1).
Figure 1.
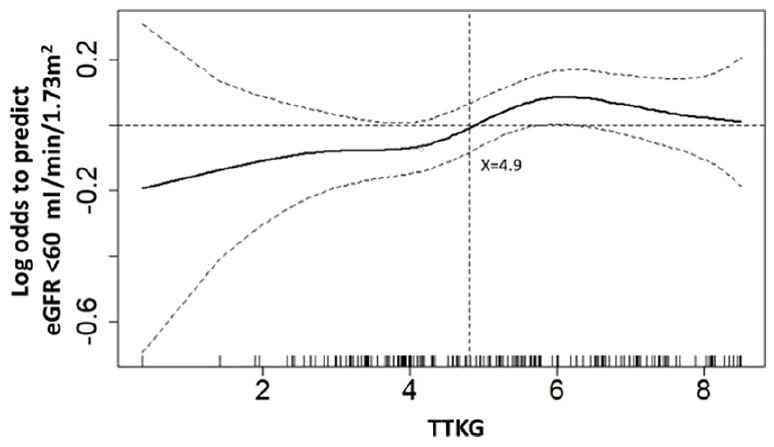
GAM plot for TTKG and eGFR < 60 ml/min/1.73 m2. GAM plot for the probability of TTKG and eGFR < 60 ml/min/1.73 m2 after 12 months of adrenalectomy against TTKG of APA patients incorporating the subject-specific (longitudinal) random effects expressed as the logarithm of the odds (logit). The probability of outcome events was constructed with hypertensive duration have an average of zero over the range of the data, that is, TTKG = 4.9. The dashed lines indicate approximated pointwise 95% CI. Dotted curves indicate 95% CIs for the smoothed hazard.
APA, aldosterone producing adenoma; ARR, aldosterone to renin ratio; BMI, body mass index; BW, body weight; CI, confidence interval; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; GAM, generalized additive model; PRA, plasma renin activity; SBP, systolic blood pressure; TTKG, transtubular potassium gradient.
Table 2.
Baseline characteristics predicting eGFR < 60 ml/min/1.73 m2 after 12 months of adrenalectomy by logistic regression analysis.
| Univariable |
Multivariable |
|||
|---|---|---|---|---|
| Odds ratio (95% CI) | p value | Odds ratio (95% CI) | p value | |
| Age (year) | 1.06 (1.03–1.09) | <0.001 | ||
| Gender (Male) | 0.34 (0.19–0.58) | <0.001 | ||
| Body weight (kg) | 1.02 (1.01–1.04) | 0.012 | ||
| BMI (kg/m2) | 1.07 (1.01–1.15) | 0.032 | ||
| SBP (mmHg) | 1.04 (1.02–1.05) | <0.001 | 1.06 (1.02–1.11) | 0.002 |
| DBP (mmHg) | 1.02 (1.00–1.04) | 0.014 | ||
| eGFR (EPI-Cr, ml/min/1.73m2) | 0.93 (0.91–0.94) | <0.001 | 0.91 (0.87–0.95) | <0.001 |
| Log aldosterone | 4.27 (1.55–11.81) | 0.005 | ||
| Plasma renin activity (ng/ml/hr) | 1.26 (1.02–1.55) | 0.031 | ||
| Log aldosterone renin ratio | 0.52 (0.32–0.84) | 0.007 | ||
| Potassium (mEq/L) | 0.66 (0.44–1.00) | 0.049 | ||
| TTKG ⩾ 4.9 | 2.10 (0.97–4.53) | 0.059 | 5.42 (1.48–19.85) | 0.011 |
BMI, body mass index; CI, confidence interval; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; EPI-Cr, Epidemiology Collaboration creatinine equation; SBP, systolic blood pressure; TTKG, transtubular potassium gradient.
Baseline factors associated with TTKG
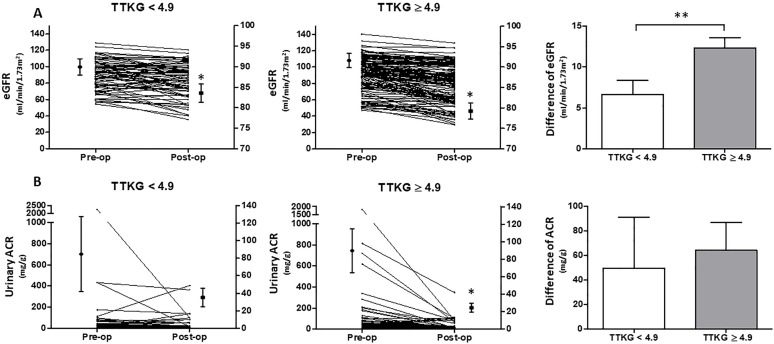
Pearson correlation test demonstrated that TTKG correlated with pre-operative log aldosterone values (r = 0.30; p < 0.001) and serum potassium levels (r = –0.39; p < 0.001). Pre-operative log aldosterone values (OR = 5.93; 95% CI, 1.56–22.50; p = 0.009) and serum potassium levels (OR = 0.33; 95% CI, 0.19–0.59, p < 0.001) were associated with TTKG ⩾ 4.9 by logistic regression analysis (Supplemental Table S2). When comparing between TTKG ⩾ 4.9 and TTKG < 4.9 individuals (Supplemental Table S3), pre-operative aldosterone values (64.83 ± 42.74 versus 45.56 ± 24.27; p < 0.001) and serum potassium (3.39 ± 0.64 versus 3.77 ± 0.56; p < 0.001) were significantly different. In comparison with TTKG < 4.9 individuals (Supplemental Table S3), TTKG ⩾ 4.9 individuals were more influenced by adrenalectomy. TTKG ⩾ 4.9 individuals had a higher difference of eGFR12 before and after adrenalectomy and a significant decline of urinary ACR12 by the time of operation (Figure 2).
Figure 2.
The eGFR and urinary ACR before and after adrenalectomy in TTKG < 4.9 or TTKG ⩾ 4.9 groups. The patients were categorized into TTKG < 4.9 or TTKG ⩾ 4.9 groups. The eGFR and urinary ACR were more influenced by adrenalectomy in TTKG ⩾ 4.9 group. (A) After operation, the eGFR in both groups decreased significantly (*). However, the difference of eGFR before and after adrenalectomy was more significant in TTKG ⩾ 4.9 group versus TTKG < 4.9 group (**). (B) The urinary ACR dropped more remarkably in patients with TTKG ⩾ 4.9 after adrenalectomy, whereas TTKG < 4.9 patients did not have significant decline of urinary ACR. Left Y axis indicates the values of variables. Right Y axis indicates the values of mean ± SEM.
*p represented < 0.05 and statistical analysis was conducted by compare t-test.
**p represented < 0.05 and statistical analysis was conducted by independent t-test.
eGFR, estimated glomerular filtration; TTKG, transtubular potassium gradient; Urinary ACR, urinary albumin to creatinine ratio.
In addition to the binominal definition of kidney function impairment, we further tested the percentage change of eGFR before and after adrenalectomy. TTKG ⩾ 4.9 also was associated with percentage decrease of eGFR (Supplemental Table S4) and predicted a 20% decrease of eGFR after adrenalectomy (OR = 2.55; 95% CI, 1.11–5.88, p = 0.028) (Supplemental Table S5).
Sensitivity analysis
TTKG was originally to be applied when urine osmolality is more than serum osmolality.44 We further excluded patients who had urine osmolality less than serum osmolality (n = 167). In light of our main result, TTKG could constantly predict kidney function impairment (OR = 5.57; 95% CI, 1.22–25.35; p = 0.026) by full adjustment of variables.
Furthermore, we excluded individuals with a pre-operative eGFR of less than 60 ml/min/1.73 m2 (n = 183).16,40,45 TTKG ⩾ 4.9 could constantly forecast kidney function impairment at 1 year of adrenalectomy (OR = 5.05; 95% CI, 1.31–19.48; p = 0.019).
Diabetic mellitus (DM) interferes with endothelium dysfunction and might affect the prediction of TTKG. Therefore, we attempted to exclude diabetic patients for analysis. In non-DM patients (n = 182), TTKG ⩾ 4.9 correlates with kidney impairment after adrenalectomy (OR = 3.43; 95% CI, 1.17–10.07; p = 0.025).
Since hypertension could promote kidney impairment,46 while increased body mass index would be a risk factor for impaired kidney function,47 we further applied subgroup analysis by categorizing patients into SBP > 140 or ⩽140 mmHg and BMI ⩾ 25 or <25 (kg/m2). In patients with pre-operative hypertension (OR = 3.51; 95% CI, 1.36–9.01; p = 0.009), and in patients with increased BMI (OR = 3.39; 95% CI, 1.12–10.33; p = 0.031), TTKG ⩾ 4.9 could predict kidney function impairment after adrenalectomy.
TTKG correlated with end organ injury
With linear regression analysis, females, increased body weight, SBP, log aldosterone level and TTKG ⩾ 4.9 correlated with increased pre-operative left ventricular mass (Table 3). Furthermore, we assessed the relationship of TTKG to proteinuria. TTKG ⩾ 4.9 predicted pre-operative ACR >50 mg/g (OR = 2.42; 95% CI, 1.07–5.47; p = 0.034) (Table 4).
Table 3.
The association between pre-operation variables predicting LV mass by linear regression analysis.
| Univariable |
Multivariable |
|||
|---|---|---|---|---|
| B Coefficient | p value | B Coefficient | p value | |
| Age (year) | −0.593 | 0.137 | ||
| Gender (Male) | −65.93 | <0.001 | −29.21 | 0.018 |
| Body weight (kg) | 2.46 | <0.001 | 1.79 | 0.029 |
| BMI (kg/m2) | 6.12 | <0.001 | ||
| SBP (mmHg) | 1.11 | <0.001 | 0.78 | 0.005 |
| DBP (mmHg) | 1.47 | <0.001 | ||
| eGFR (EPI-Cr, ml/min/1.73 m2) | −0.54 | 0.016 | ||
| Log aldosterone | 38.97 | 0.026 | 33.58 | 0.033 |
| Plasma renin activity (ng/ml/hr) | 3.17 | 0.251 | ||
| Log aldosterone renin ratio | −7.45 | 0.311 | ||
| Potassium (mEq/L) | −17.49 | 0.010 | ||
| TTKG ⩾ 4.9 | 9.46 | 0.381 | 20.10 | 0.018 |
The multivariate regression analysis was conducted by full adjustment of variables.
BMI, body mass index; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; EPI-Cr, Epidemiology Collaboration creatinine equation; LV, left ventricle; SBP, systolic blood pressure; TTKG, transtubular potassium gradient.
Table 4.
Pre-operation characteristics predicting urinary ACR > 50 mg/g by logistic regression analysis.
| Univariable |
Multivariable (Backward
conditional) |
|||
|---|---|---|---|---|
| Odds ratio (95% CI) | p value | Odds ratio (95% CI) | p value | |
| Age (year) | 1.00 (0.98–1.02) | 0.838 | ||
| Gender (Male) | 0.90 (0.54–1.51) | 0.699 | ||
| Body weight (kg) | 1.01 (0.99–1.03) | 0.258 | ||
| BMI (kg/m2) | 1.08 (1.01–1.15) | 0.016 | 1.11 (1.01–1.21) | 0.027 |
| SBP (mmHg) | 1.02 (1.01–1.04) | <0.001 | 1.02 (1.00–1.04) | 0.045 |
| DBP (mmHg) | 1.04 (1.02–1.06) | <0.001 | ||
| eGFR (EPI-Cr, ml/min/1.73 m2) | 0.99 (0.98–1.01) | 0.243 | ||
| Log aldosterone | 1.21 (0.45–3.23) | 0.706 | ||
| Plasma renin activity (ng/ml/hr) | 1.01 (0.92–1.10) | 0.900 | ||
| Log aldosterone renin ratio | 0.96 (0.63–1.45) | 0.832 | ||
| Potassium (mEq/L) | 0.82 (0.56–1.21) | 0.326 | ||
| TTKG ⩾ 4.9 | 2.01 (0.95–4.28) | 0.070 | 2.42 (1.07–5.47) | 0.034 |
The multivariate regression analysis was conducted by full adjustment of variables.
ACR, albumin to creatinine ratio; BMI, body mass index; CI, confidence interval; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; EPI-Cr, Epidemiology Collaboration creatinine equation; SBP, systolic blood pressure; TTKG, transtubular potassium gradient.
Cure of hypertension after adrenalectomy
We assessed the variables to cure hypertension after adrenalectomy according to the Primary Aldosteronism Surgery Outcome (PASO) consensus. We categorized patients into clinical success groups, in terms of complete success, partial success and absent success groups. TTKG ⩾ 4.9 could predict clinical success after adrenalectomy (OR = 2.90; 95% CI, 1.21–6.92; p = 0.017) (Supplemental Table S6).
Discussion
Our findings suggest that TTKG, a marker to gauge renal potassium secretion by the cortical collecting duct in terms of hyperaldosteronism and hypokalaemia, could predict kidney function impairment after adrenalectomy even with a high possibility to cure hypertension. TTKG could correlate to end organ damage such as increased left ventricle (LV) mass, microalbuminuria. The demonstration of enhanced potassium excretion in patients with PA opens new avenues for better understanding the mechanisms of aldosterone-induced kidney dysfunction.
TTKG in PA
PA patients with TTKG ⩾ 4.9 had high aldosterone values and low serum potassium levels. TTKG ⩾ 4.9 individuals were more affected by adrenalectomy since they had higher difference of eGFR and significant urinary ACR decline. The results indicated that TTKG ⩾ 4.9 individuals had higher pre-operative kidney hyperfiltration compared with TTKG < 4.9 individuals. In multiple variate logistic regression analysis, TTKG was independent of serum aldosterone and potassium levels in predicting kidney function impairment after adrenalectomy. This implies that high TTKG patients had higher aldosterone production and mineralocorticoid activity than their counterpart. Potassium excretion in principle cells rely on epithelium sodium channel (ENac) to reabsorb sodium and subsequently trigger potassium efflux from apical (potassium) K+ channel.48 Moreover, hyperfiltration relative to sodium retention increases urinary flow rate in the cortical collecting duct and further stimulates potassium secretion.48 Taken together, inappropriately high TTKG was associated with abnormal ENac activation by hyperaldosterone and low serum potassium levels49 in our data.
TTKG associate with kidney fibrosis
In patients with hyperaldosteronism, high TTKG reflects adverse activity of aldosterone on kidney fibrosis. In PA patients, the pre-treatment plasma potassium level was reported as an independent risk factor for eGFR decline after treatment.17
Long-term stimulation of ENac by aldosterone could increase intracellular sodium concentration in kidney tubular cells. Intracellular overload of sodium subsequently induces intracellular calcium overload50 and stimulates calcium calmodulin kinase II (CaMKII)50,51 which is found in human renal tubule cells52 and modulates ENac activity.53 CaMKII contributes to aldosterone associated fibrosis in kidney collecting duct cells.54 Furthermore, colocalization of signals for urinary potassium/urinary creatinine and CaMKIIG was found in genome-wide association studies (GWAS).55 Sustained high TTKG might be accompanied by persistent high intracellular sodium concentration, urinary potassium excretion and CaMKII activation, and further associated with kidney fibrosis.
TTKG correlates with organ damage
In our analysis, high TTKG was associated with end organ damage with the manifestation of increased LV mass. Inappropriate high TTKG in PA is accompanied with volume expansion and hyperfiltration. High intraglomerular pressure results in podocyte injury and subsequently proteinuria. Inflammation caused by aldosterone15 contributes to glomerulus damage. In regards to the heart, in addition to direct aldosterone effect, increased renal ENac subunits play a pivotal role in the pathogenesis of chronic heart failure in rodent models.56 Hypokalaemia in PA also could stimulate CaMKII50 and further provoke cardiac inflammation57 and hypertrophy.58,59
Furthermore, decreased cardiac pump function eliminates blood supply to the severely oxygen-dependent kidney.60 Despite whole kidney hyperfiltration in PA and increased GFR, damage to endothelium cells and microstructure in the kidney and accompanied heart dysfunction ultimately results in kidney impairment.
High urinary potassium excretion has been reported to increase the risk of kidney function impairment progression.61 Hyperfiltration related to sustained ENac activation could induce proteinuria.62,63 Moreover, genomic colocalization of urinary ACR and urinary potassium/urinary creatinine in glycine amidinotransferase locus was found in GWAS analysis55 and provided evidence that urinary potassium excretion correlated to albuminuria. This could explain why high TTKG was associated with microalbuminuria in our study, as microalbuminuria reflects systemic vascular endothelium dysfunction.64 In the long-term follow-up of primary hypertension without DM and other major diseases, baseline microalbuminuria increased the risk of declined renal function.65 Taken together, we showed that high urine potassium excretion was associated with an increased risk of developing kidney function impairment in PA patients after adrenalectomy at least partially via increased proteinuria.55
TTKG predicts clinical cure after adrenalectomy
Adrenalectomy decreased blood pressure and urinary albuminuria in our PA patients7 that could be beneficial to organ injury. However, in the PASO study,66 the plasma aldosterone levels did not have significant differences between the groups of complete clinical success versus absent clinical success, or between partial clinical success versus absent clinical success. In our analysis, high TTKG is found to be a predictive factor to clinical success after adrenalectomy according to the PASO criteria.66 It is possible that TTKG reflects mineralocorticoid bioactivity together with hypokalaemia on the target organ and predicts more accurately the clinical outcome after the correction of aldosterone excess by adrenalectomy.
Study limitation
TTKG is suggested to be operated when urine osmolality is more than serum osmolality.44 In light of this, we excluded individuals whose urine osmolality was less than serum osmolality, and high TTKG could predict post-operative kidney function impairment.
Baseline kidney function is associated with impaired kidney function after adrenalectomy and could interfere with the evaluation of TTKG. We have found that some patients who have pre-operative eGFR less than 60 ml/min/1.73m2 were improved after adrenalectomy.43 Nonetheless, even if we excluded patients who had pre-operative eGFR less than 60 ml/min/1.73m2, defined as our primary endpoint, TTKG could be associated with post-operative kidney impairment.
Conclusions and implication
In summary, our study strengthens the body of observational literature suggesting that kidney potassium handling and hyperaldosterone, in terms of TTKG, could predict kidney impairment and cure hypertension at 12 months after adrenalectomy in unilateral PA patients. High TTKG was associated with end organ damage; more specifically, it was correlated with LV mass and urinary albumin excretion, even with a high possibility to cure hypertension.
Supplemental Material
Supplemental material, Supplementary_Materials_TAJ-20-02-OA-032.R1_1 for Transtubular potassium gradient predicts kidney function impairment after adrenalectomy in primary aldosteronism by Hung-Wei Liao, Shuo-Meng Wang, Chieh-Kai Chan, Yen-Hung Lin, Po-Chih Lin, Chen-Hsun Ho, Yu-Chun Liu, Jeff S Chueh and Vin-Cent Wu in Therapeutic Advances in Chronic Disease
Acknowledgments
We thank the Membership of the Taiwan Primary Aldosteronism Investigation (TAIPAI) Study Group: Tai-Shuan Lai; Vin-Cent Wu; Shao-Yu Yang; Kao-Lang Liu; Chin-Chen Chang; Bo-Chiag Lee; Shuo-Meng Wang; Kuo-How Huang; Po-Chih Lin; Yen-Hung Lin; Lian-Yu Lin; Shih-Cheng Liao; Ruoh-Fang Yen; Ching-Chu Lu (National Taiwan University Hospital, Taipei, Taiwan); Chieh-Kai Chan (NTUH Hsin-Chu branch); Leay-Kiaw Er; Ya-Hui Hu; Chia-Hui Chang; Che-Hsiung Wu; Yao-Chou Tsai (Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, Taipei, Taiwan); Shih-Chieh Jeff Chueh (Cleveland Clinic Institute of Urology and Kidneys); Chen-Hsun Ho (Taipei Medical University-Shuang Ho Hospital, Ministry of Health and Welfare); Wei-Chieh Huang (New Taipei City Hospital); Ying-Ying Chen (MacKay Memorial Hospital); Kwan-Dun Wu (National Taiwan University Hospital, Taipei, Taiwan NTUH, Director of Coordinating Center).
The manuscript has been revised again by a native English speaker (Eric Chueh, B.A, MBA program [in progress]; Case Western Reserve University, Cleveland, OH, USA).
Footnotes
Author contributions: HWL: acquisition of data, analysis and interpretation of data and wrote the first draft. SMW: interpreted the revised result and provided the methods for statistics. CKC: conceived the review topic, provided additional supplemental results and drafted the revised article. YHL: conceived the review topic, interpreted the response letter and responded with further statistics. PCL: revised and approved the final version of the manuscript and drafted the revised article. CHH: revised and approved the final version of the manuscript and acquired the results. YCL: revised and approved the final version of the manuscript, responded with further statistics and revised it critically for important intellectual content. JSC: critical revision of manuscript for intellectual content. VCW: study concept and design, interpretation of data, critical revision of manuscript for intellectual content and final approval of the version to be published.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Data availability statement: The data that support the findings of this study are available on request from the corresponding author, V.C.W. The data are not publicly available due to their containing information that could compromise the privacy of research participants.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by Taiwan National Science Council [104-2314-B-002-125-MY3, 106-2314-B-002 -166 -MY3,107-2314-B-002-026-MY3], National Health Research Institutes [PH-102-SP-09)], National Taiwan University Hospital [106-FTN20, 106-P02, UN106-014, 106-S3582, NTUH 107-A141, 107-S3809, 107-T02,PC1246,VN109-09,109-S4634,UN109-041 ] and Ministry of Science and Technology (MOST) of the Republic of China (Taiwan) [grant number, MOST 106-2321-B-182-002]
ORCID iD: Hung-Wei Liao  https://orcid.org/0000-0003-3090-1937
https://orcid.org/0000-0003-3090-1937
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Hung-Wei Liao, Chinru Clinic, Taipei.
Shuo-Meng Wang, Department of Urology, National Taiwan University Hospital, Taipei.
Chieh-Kai Chan, Department of Internal Medicine, National Taiwan University Hospital, Hsin-Chu branch, Hsin-Chu.
Yen-Hung Lin, Department of Internal Medicine, National Taiwan University Hospital, Taipei.
Po-Chih Lin, Department of Internal Medicine, National Taiwan University Hospital, Taipei.
Chen-Hsun Ho, Division of Urology, Department of Surgery, Shin Kong Wu Ho-Su Memorial Hospital, Taipei, Taiwan.
Yu-Chun Liu, Far Eastern Polyclinic, Taipei City.
Jeff S Chueh, Glickman Urological and Kidney Institute, and Cleveland Clinic Lerner College of Medicine, Cleveland Clinic, Cleveland, OH, USA.
Vin-Cent Wu, Department of Internal Medicine, National Taiwan University Hospital, Room 1555, Clinical Research Building, 7 Chung-Shan South Road, Taipei 100.
References
- 1. Douma S, Petidis K, Doumas M, et al. Prevalence of primary hyperaldosteronism in resistant hypertension: a retrospective observational study. Lancet 2008; 371: 1921–1926. [DOI] [PubMed] [Google Scholar]
- 2. Satoh F, Morimoto R, Iwakura Y, et al. Primary aldosteronism: a Japanese perspective. Rev Endocr Metab Disord 2011; 12: 11–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Monticone S, Burrello J, Tizzani D, et al. Prevalence and clinical manifestations of primary aldosteronism encountered in primary care practice. J Am Coll Cardiol 2017; 69: 1811–1820. [DOI] [PubMed] [Google Scholar]
- 4. Rossi GP, Bernini G, Desideri G, et al. Renal damage in primary aldosteronism: results of the PAPY study. Hypertension 2006; 48: 232–238. [DOI] [PubMed] [Google Scholar]
- 5. Milliez P, Girerd X, Plouin PF, et al. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol 2005; 45: 1243–1248. [DOI] [PubMed] [Google Scholar]
- 6. Carter Y, Roy M, Sippel RS, et al. Persistent hypertension after adrenalectomy for an aldosterone-producing adenoma: weight as a critical prognostic factor for aldosterone’s lasting effect on the cardiac and vascular systems. J Surg Res 2012; 177: 241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Catena C, Colussi G, Nadalini E, et al. Relationships of plasma renin levels with renal function in patients with primary aldosteronism. Clin J Am Soc Nephrol 2007; 2: 722–731. [DOI] [PubMed] [Google Scholar]
- 8. Sechi LA, Novello M, Lapenna R, et al. Long-term renal outcomes in patients with primary aldosteronism. JAMA 2006; 295: 2638–2645. [DOI] [PubMed] [Google Scholar]
- 9. Marney AM, Brown NJ. Aldosterone and end-organ damage. Clin Sci (Lond) 2007; 113: 267–278. [DOI] [PubMed] [Google Scholar]
- 10. Rocha R, Chander PN, Zuckerman A, et al. Role of aldosterone in renal vascular injury in stroke-prone hypertensive rats. Hypertension 1999; 33: 232–237. [DOI] [PubMed] [Google Scholar]
- 11. Brem AS, Morris DJ, Ge Y, et al. Direct fibrogenic effects of aldosterone on normotensive kidney: an effect modified by 11β-HSD activity. Am J Physiol Renal Physiol 2010; 298: F1178–F1187. [DOI] [PubMed] [Google Scholar]
- 12. Ribstein J, Du Cailar G, Fesler P, et al. Relative glomerular hyperfiltration in primary aldosteronism. J Am Soc Nephrol 2005; 16: 1320–1325. [DOI] [PubMed] [Google Scholar]
- 13. Palatini P. Glomerular hyperfiltration: a marker of early renal damage in pre-diabetes and pre-hypertension. Nephrol Dial Transplant 2012; 27: 1708–1714. [DOI] [PubMed] [Google Scholar]
- 14. Kuo CC, Wu VC, Tsai CW, et al. Relative kidney hyperfiltration in primary aldosteronism: a meta-analysis. J Renin Angiotensin Aldosterone Syst 2011; 12: 113–122. [DOI] [PubMed] [Google Scholar]
- 15. Bernardi S, Toffoli B, Zennaro C, et al. Aldosterone effects on glomerular structure and function. J Renin Angiotensin Aldosterone Syst 2015; 16: 730–738. [DOI] [PubMed] [Google Scholar]
- 16. Utsumi T, Kawamura K, Imamoto T, et al. Preoperative masked renal damage in Japanese patients with primary aldosteronism: identification of predictors for chronic kidney disease manifested after adrenalectomy. Int J Urol 2013; 20: 685–691. [DOI] [PubMed] [Google Scholar]
- 17. Kramers BJ, Kramers C, Lenders JW, et al. Effects of treating primary aldosteronism on renal function. J Clin Hypertens (Greenwich) 2017; 19: 290–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Palmer LG, Frindt G. Aldosterone and potassium secretion by the cortical collecting duct. Kidney Int 2000; 57: 1324–1328. [DOI] [PubMed] [Google Scholar]
- 19. Joo KW, Chang SH, Lee JG, et al. Transtubular potassium concentration gradient (TTKG) and urine ammonium in differential diagnosis of hypokalemia. J Nephrol 2000; 13: 120–125. [PubMed] [Google Scholar]
- 20. Sakaguchi T, Hirata A, Kashiwase K, et al. Transtubular potassium concentration gradient as a surrogate measure of arterial underfilling in acute decompensated heart failure. Circ J 2016; 80: 1965–1970. [DOI] [PubMed] [Google Scholar]
- 21. Rodríguez-Soriano J, Ubetagoyena M, Vallo A. Transtubular potassium concentration gradient: a useful test to estimate renal aldosterone bio-activity in infants and children. Pediatr Nephrol 1990; 4: 105–110. [DOI] [PubMed] [Google Scholar]
- 22. Wu VC, Kuo CC, Wang SM, et al. Primary aldosteronism: changes in cystatin C-based kidney filtration, proteinuria, and renal duplex indices with treatment. J Hypertens 2011; 29: 1778–1786. [DOI] [PubMed] [Google Scholar]
- 23. Wu VC, Lo SC, Chen YL, et al. Endothelial progenitor cells in primary aldosteronism: a biomarker of severity for aldosterone vasculopathy and prognosis. J Clin Endocrinol Metab 2011; 96: 3175–3183. [DOI] [PubMed] [Google Scholar]
- 24. Wu VC, Huang KH, Peng KY, et al. Prevalence and clinical correlates of somatic mutation in aldosterone producing adenoma-Taiwanese population. Sci Rep 2015; 5: 11396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. TAIPAI Study Group, Wu VC, Chueh SC, et al. Association of kidney function with residual hypertension after treatment of aldosterone-producing adenoma. Am J Kidney Dis 2009; 54: 665–673. [DOI] [PubMed] [Google Scholar]
- 26. Wu VC, Yang SY, Lin JW, et al. Kidney impairment in primary aldosteronism. Clin Chim Acta 2011; 412: 1319–1325. [DOI] [PubMed] [Google Scholar]
- 27. Wu VC, Chang HW, Liu KL, et al. Primary aldosteronism: diagnostic accuracy of the losartan and captopril tests. Am J Hypertens 2009; 22: 821–827. [DOI] [PubMed] [Google Scholar]
- 28. Wu VC, Hu YH, Er LK, et al. Case detection and diagnosis of primary aldosteronism - the consensus of Taiwan society of aldosteronism. J Formos Med Assoc 2017; 116: 993–1005. [DOI] [PubMed] [Google Scholar]
- 29. Peng KY, Chang HM, Lin YF, et al. miRNA-203 modulates aldosterone levels and cell proliferation by targeting Wnt5a in aldosterone-producing adenomas. J Clin Endocrinol Metab 2018; 103: 3737–3747. [DOI] [PubMed] [Google Scholar]
- 30. Wu VC, Wang SM, Chang CH, et al. Long term outcome of aldosteronism after target treatments. Sci Rep 2016; 6: 32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chan CK, Kim JH, Chueh E, et al. Aldosterone level after saline infusion test could predict clinical outcome in primary aldosteronism after adrenalectomy. Surgery 2019; 166: 362–368. [DOI] [PubMed] [Google Scholar]
- 32. Wu VC, Hu YH, Wu CH, et al. Administrative data on diagnosis and mineralocorticoid receptor antagonist prescription identified patients with primary aldosteronism in Taiwan. J Clin Epidemiol 2014; 67: 1139–1149. [DOI] [PubMed] [Google Scholar]
- 33. Wu VC, Kuo CC, Chang HW, et al. Diagnosis of primary aldosteronism: comparison of post-captopril active renin concentration and plasma renin activity. Clin Chim Acta 2010; 411: 657–663. [DOI] [PubMed] [Google Scholar]
- 34. Wu CH, Yang YW, Hu YH, et al. Comparison of 24-h urinary aldosterone level and random urinary aldosterone-to-creatinine ratio in the diagnosis of primary aldosteronism. PLoS One 2013; 8: e67417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yen RF, Wu VC, Liu KL, et al. 131I-6beta-iodomethyl-19-norcholesterol SPECT/CT for primary aldosteronism patients with inconclusive adrenal venous sampling and CT results. J Nucl Med 2009; 50: 1631–1637. [DOI] [PubMed] [Google Scholar]
- 36. Hung CS, Wu XM, Chen CW, et al. The relationship among cardiac structure, dietary salt and aldosterone in patients with primary aldosteronism. Oncotarget 2017; 8: 73187–73197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American society of echocardiography’s guidelines and standards committee and the chamber quantification writing group, developed in conjunction with the European association of echocardiography, a branch of the European society of cardiology. J Am Soc Echocardiogr 2005; 18: 1440–1463. [DOI] [PubMed] [Google Scholar]
- 38. Arima S, Kohagura K, Xu HL, et al. Nongenomic vascular action of aldosterone in the glomerular microcirculation. J Am Soc Nephrol 2003; 14: 2255–2263. [DOI] [PubMed] [Google Scholar]
- 39. Wu CH, Yang YW, Hung SC, et al. Effect of treatment on body fluid in patients with unilateral aldosterone producing adenoma: adrenalectomy versus spironolactone. Sci Rep 2015; 5: 15297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Tanase-Nakao K, Naruse M, Nanba K, et al. Chronic kidney disease score for predicting postoperative masked renal insufficiency in patients with primary aldosteronism. Clin Endocrinol (Oxf) 2014; 81: 665–670. [DOI] [PubMed] [Google Scholar]
- 41. Shu KH, Wang CH, Wu CH, et al. Urinary π-glutathione S-transferase predicts advanced acute kidney injury following cardiovascular surgery. Sci Rep 2016; 6: 26335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Wang JJ, Chi NH, Huang TM, et al. Urinary biomarkers predict advanced acute kidney injury after cardiovascular surgery. Crit Care 2018; 22: 108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. James MT, Pannu N, Hemmelgarn BR, et al. Derivation and external validation of prediction models for advanced chronic kidney disease following acute kidney injury. JAMA 2017; 318: 1787–1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ethier JH, Kamel KS, Magner PO, et al. The transtubular potassium concentration in patients with hypokalemia and hyperkalemia. Am J Kidney Dis 1990; 15: 309–315. [DOI] [PubMed] [Google Scholar]
- 45. Musso C, Liakopoulos V, Stefanidis I, et al. Correlation between creatinine clearance and transtubular potassium concentration gradient in old people and chronic renal disease patients. Saudi J Kidney Dis Transpl 2007; 18: 551–555. [PubMed] [Google Scholar]
- 46. Griffin KA. Hypertensive kidney injury and the progression of chronic kidney disease. Hypertension 2017; 70: 687–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Garland JS. Elevated body mass index as a risk factor for chronic kidney disease: current perspectives. Diabetes Metab Syndr Obes 2014; 7: 347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Unwin RJ, Luft FC, Shirley DG. Pathophysiology and management of hypokalemia: a clinical perspective. Nat Rev Nephrol 2011; 7: 75–84. [DOI] [PubMed] [Google Scholar]
- 49. Perucca J, Bichet DG, Bardoux P, et al. Sodium excretion in response to vasopressin and selective vasopressin receptor antagonists. J Am Soc Nephrol 2008; 19: 1721–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Weiss JN, Qu Z, Shivkumar K. Electrophysiology of hypokalemia and hyperkalemia. Circ Arrhythm Electrophysiol 2017; 10: e004667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yao L, Fan P, Jiang Z, et al. Nav1.5-dependent persistent Na+ influx activates CaMKII in rat ventricular myocytes and N1325S mice. Am J Physiol Cell Physiol 2011; 301: C577–C586. [DOI] [PubMed] [Google Scholar]
- 52. Kubokawa M, Nakamura K, Komagiri Y. Interaction between calcineurin and Ca/Calmodulin kinase-II in modulating cellular functions. Enzyme Res 2011; 2011: 587359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Alli AA, Bao HF, Liu BC, et al. Calmodulin and CaMKII modulate ENaC activity by regulating the association of MARCKS and the cytoskeleton with the apical membrane. Am J Physiol Renal Physiol 2015; 309: F456–F463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Park EJ, Jung HJ, Choi HJ, et al. miR-34c-5p and CaMKII are involved in aldosterone-induced fibrosis in kidney collecting duct cells. Am J Physiol Renal Physiol 2018; 314: F329–F342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Zanetti D, Rao A, Gustafsson S, et al. Identification of 22 novel loci associated with urinary biomarkers of albumin, sodium, and potassium excretion. Kidney Int 2019; 95: 1197–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zheng H, Liu X, Rao US, et al. Increased renal ENaC subunits and sodium retention in rats with chronic heart failure. Am J Physiol Renal Physiol 2011; 300: F641–F649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Beckendorf J, van den Hoogenhof MMG, Backs J. Physiological and unappreciated roles of CaMKII in the heart. Basic Res Cardiol 2018; 113: 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Singh MV, Anderson ME. Is CaMKII a link between inflammation and hypertrophy in heart? J Mol Med (Berl) 2011; 89: 537–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Anderson ME, Brown JH, Bers DM. CaMKII in myocardial hypertrophy and heart failure. J Mol Cell Cardiol 2011; 51: 468–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Shiba N, Shimokawa H. Chronic kidney disease and heart failure–bidirectional close link and common therapeutic goal. J Cardiol 2011; 57: 8–17. [DOI] [PubMed] [Google Scholar]
- 61. He J, Mills KT, Appel LJ, et al. Urinary sodium and potassium excretion and CKD progression. J Am Soc Nephrol 2016; 27: 1202–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Lee SM, Park JY, Park MS, et al. Association of renal hyperfiltration with incident proteinuria - a nationwide registry study. PLoS One 2018; 13: e0195784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Aviv A, Hollenberg NK, Weder AB. Sodium glomerulopathy: tubuloglomerular feedback and renal injury in African Americans. Kidney Int 2004; 65: 361–368. [DOI] [PubMed] [Google Scholar]
- 64. Pedrinelli R, Giampietro O, Carmassi F, et al. Microalbuminuria and endothelial dysfunction in essential hypertension. Lancet 1994; 344: 14–18. [DOI] [PubMed] [Google Scholar]
- 65. Viazzi F, Leoncini G, Conti N, et al. Microalbuminuria is a predictor of chronic renal insufficiency in patients without diabetes and with hypertension: the MAGIC study. Clin J Am Soc Nephrol 2010; 5: 1099–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Williams TA, Lenders JWM, Mulatero P, et al. Outcomes after adrenalectomy for unilateral primary aldosteronism: an international consensus on outcome measures and analysis of remission rates in an international cohort. Lancet Diabetes Endocrinol 2017; 5: 689–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_Materials_TAJ-20-02-OA-032.R1_1 for Transtubular potassium gradient predicts kidney function impairment after adrenalectomy in primary aldosteronism by Hung-Wei Liao, Shuo-Meng Wang, Chieh-Kai Chan, Yen-Hung Lin, Po-Chih Lin, Chen-Hsun Ho, Yu-Chun Liu, Jeff S Chueh and Vin-Cent Wu in Therapeutic Advances in Chronic Disease