Abstract
The pathogenesis of pulmonary arterial hypertension is closely associated with dysregulated inflammation. Recently, abnormal alterations in gut microbiome composition and function were reported in a pulmonary arterial hypertension experimental animal model. However, it remains unclear whether these alterations are a result or the cause of pulmonary arterial hypertension. The purpose of this study was to investigate whether alterations in the gut microbiome affected the hemodynamics in SU5416/hypoxia rats. We used the SU5416/hypoxia rat model in our study. SU5416/hypoxia rats were treated with a single SU5416 injection (30 mg/kg) and a three-week hypoxia exposure (10% O2). Three SU5416/hypoxia rats were treated with a combination of four antibiotics (SU5416/hypoxia + ABx group) for four weeks. Another group was exposed to hypoxia (10% O2) without the SU5416 treatment, and control rats received no treatment. Fecal samples were collected from each animal, and the gut microbiota composition was analyzed by 16S rRNA sequencing. The antibiotic treatment significantly suppressed the vascular remodeling, right ventricular hypertrophy, and increase in the right ventricular systolic pressure in SU5416/hypoxia rats. 16S rRNA sequencing analysis revealed gut microbiota modification in SU5416/hypoxia + ABx group. The Firmicutes-to-Bacteroidetes ratio in SU5416/hypoxia rats was significantly higher than that in control and hypoxia rats. Compared with the control microbiota, 14 bacterial genera, including Bacteroides and Akkermansia, increased, whereas seven bacteria, including Rothia and Prevotellaceae, decreased in abundance in SU5416/hypoxia rats. Antibiotic-induced modification of the gut microbiota suppresses the development of pulmonary arterial hypertension. Dysbiosis may play a causal role in the development and progression of pulmonary arterial hypertension.
Keywords: vascular remodeling, dysbiosis, pulmonary hypertension experimental, pathogenesis, inflammation
Introduction
Pulmonary arterial hypertension (PAH) is a progressive cardiovascular disease characterized by elevated pulmonary arterial pressure, leading to right heart failure.1 PAH prognosis is unfavorable, with a median survival of <3 years during the natural clinical disease course.2 Although advanced diagnostic strategies and selective pulmonary vasodilators have contributed to improved PAH prognosis,3,4 PAH is still incurable and demands life-long treatments.5,6 Additionally, patients who have severe PAH and do not respond to maximal medical therapy require lung transplantation.5,6
Pulmonary vascular remodeling is a pathological feature observed in the pulmonary arteries in humans and experimental PAH models.1,7,8 This remodeling causes obstruction of the pulmonary arteries and formation of complex vascular lesions, including plexiform-like, dilatation, or onion-skin lesions.1,7 Furthermore, smooth muscle cells (SMCs) and endothelial cells (ECs) abnormally accumulate in remodeled pulmonary arteries.1,9,10 The accumulation of SMCs and ECs involves mutated or dysfunctional transforming growth factor-β or bone morphogenic protein, and abnormal signal transduction.1,9 Abnormal inflammation also plays a role in the progression of the vascular remodeling.1,11 Inflammatory cells, including macrophages, T- and B-lymphocytes, dendritic cells, and natural killer cells are observed around remodeled vascular PAH lesions.11 Elevated serum levels of inflammatory cytokines, such as interleukin-6 (IL-6) and tissue necrosis factor-α (TNF-α), are associated with poor PAH prognosis.12,13
The role of inflammation in the development of PAH has been investigated in experimental animal models. In the SU5416/hypoxia (Su/Hx) model, PAH is induced by chronic hypoxia exposure and SU5416, which is an inhibitor of the vascular endothelial growth factor receptor. This is a widely used representative animal model for PAH.8,14 Chronically elevated pulmonary arterial pressure15 and vascular remodeling are observed in Su/Hx rats,8 similar to the observations in humans.8 In a Su/Hx model of athymic rats lacking T cells, more severe pulmonary hypertension and vascular remodeling have been observed than in rats with normal immunity.16 Therefore, abnormal inflammation and regulatory T cell dysfunction16 are associated with the development of PAH.1,11
Microbiota includes the entire microbial community that populates a specific organ,17 and the collective microbiota genome is called the “microbiome”.17,18 Recent technological advances have contributed to the understanding of microbiota and microbiome19 and have revealed the relationship between “dysbiosis,” which is abnormal microbiome composition and function,17,18 and the development of cardiovascular diseases, such as atherosclerosis, systemic hypertension, and heart failure.17,20,21 However, the involvement of dysbiosis in PAH development remains unclear.22 Callejo and colleagues have recently reported alterations in gut microbiota composition and function in the Su/Hx rat model.23 However, whether these alterations are a cause or the result of PAH is still unknown.
In this study, we investigated whether alterations in the gut microbiome affected the hemodynamics in Su/Hx rats. Accordingly, we modified the gut microbiota of Su/Hx rats through an antibiotic treatment and analyzed the differential microbiota pattern between the treated and untreated animals. Our results demonstrate that modifying the gut microbiota in Su/Hx rats suppresses the vascular remodeling and the deterioration of pulmonary hemodynamics.
Methods and materials
Animals
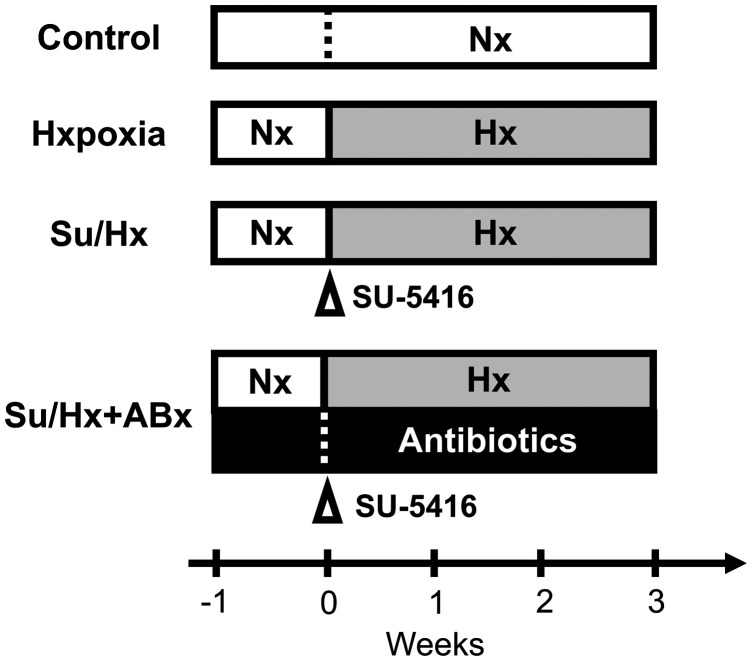
Five-week-old specific pathogen-free male Sprague Dawley rats were purchased from Japan CLEA Co. Ltd (Tokyo, Japan). The animals were kept in autoclaved cages (22.5 × 33.8 × 14.0 cm) with bedding at 24 ℃ under a 12-h light/dark cycle in the animal experimental facility of Chiba University. All rats had free access to food and sterilized water. General animal food (type MF) for rats was purchased from Oriental Yeast. Co., Ltd. Cages, bedding, drinking water, and food were replaced three times a week. Animals were randomly divided into four groups as follows: untreated rats (control group); rats exposed to only hypoxia for three weeks without SU5416 injection (Hx group); rats treated with SU5416 and exposed to hypoxia (Su/Hx group); and Su/Hx rats treated with antibiotics (Su/Hx + ABx group; Fig. 1).
Fig. 1.
Study protocol. One week before the beginning of the study, all rats were acclimatized in normoxic (Nx) conditions. The control group remained in Nx conditions. A second group of rats was exposed to hypoxia (10% O2) for three weeks but did not receive SU5416 injection. A third group of rats received a subcutaneous injection of SU5416 (30 mg/kg) followed by exposure to hypoxia (10% O2) for three weeks (Su/Hx group). A fourth group of rats received antibiotic treatment one week before and four weeks after receiving the SU5416 injection and exposure to hypoxia (Su/Hx + Abx group).
Experimental PAH model
All rats were kept in normoxia for a week before the experiments for acclimation to the experimental room. Su/Hx rats were treated as previously reported.8,24 Briefly, rats received a single subcutaneous injection of 30 mg/kg SU5416 (R&D Systems, Minneapolis, MN) and then kept in ventilated hypoxic chambers (10% O2, normobaric) for three weeks (Fig. 1).
Antibiotic treatment of Su/Hx rats
To modify intestinal bacteria composition, Su/Hx rats were treated with a cocktail of antibiotics (Fig. 1). The antibiotic cocktail contained 1 g/L ampicillin (Sigma), 500 mg/L vancomycin (Wako, Osaka, Japan), 1 g/L neomycin (Wako), and 1 g/L metronidazole (Wako), according to a previous report.25 Antibiotics were dissolved in sterilized drinking water. The drinking water and food were replaced with fresh water and food three times a week. Antibiotic administration (pretreatment) was started one week before SU5416 treatment and continued until the end of the study period.
Hemodynamics measurements
Hemodynamics were evaluated by right-sided heart catheterization (RHC), as described in our previous report.24 Briefly, rats were intraperitoneally anesthetized with 0.3 mg/kg medetomidine, 4 mg/kg midazolam, and 5 mg/kg butorphanol tartrate before RHC. A polyethylene catheter (internal diameter: 0.5 mm) was inserted through the right jugular vein and into the right ventricle (RV) to measure the right ventricular systolic pressure (RVSP). The catheter was connected to a transducer (LT0380/D; AD Instruments, Sydney, Australia). The signals were amplified with a Bio Amp (FE132; AD Instruments) and recorded using a PowerLab 8/35 computer system (AD Instruments). Wave data were analyzed using the Chart software (AD instrument). Rats were sacrificed by blood removal under anesthesia. The lungs and hearts were subsequently resected for pathological evaluations. The RV and left ventricle plus septum (LV + S) of each animal were weighed, and the RV/LV + S ratio, which is an indicator of RV hypertrophy,26 was calculated.
Bacterial DNA isolation from fecal samples
Fecal samples were collected from each animal <1 h before starting hypoxia exposure at 0 weeks and the RHC at three weeks. Feces were directly collected from the anuses while being discharged and transferred to sterilized tubes by using sterilized needles. Each sample was frozen within 30 min of collection and stored at –80 ℃ until DNA isolation. Bacterial genomic DNA was extracted using a NuculeoSpin Tissue DNA kit (Macherey-Nagel GmbH & Co. KG, Düren, Germany) according to the manufacturer's data sheet.
16S rRNA analysis
A two-step PCR was performed for preparing the libraries of 16S rRNA gene sequences. During the first step, the V3-V4 region of 16S RNA gene was amplified using a 16S (V3-V4) Metagenomic Library Construction kit for NGS (Takara Bio) and primers 341F (5'-TCGTCGGCAGCGTCAGATGTGTATAAGAGACAG-3') and 806R (5'-GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAG-3') according to the product datasheet. During the second step, the samples were indexed using a Nextera XT Index Kit (Illumina, San Diego, CA, USA) according to the datasheet. The prepared libraries were analyzed on a MiSeq instrument (Illumina) with 250-bp paired-end sequencing. Genome sequencing was performed at the Takara Bio Biochemical Center (Kusatsu, Shiga, Japan). Subsequent data processing was performed at the National Institutes of Biomedical Innovation, Health, and Nutrition. Clustering, assembly, chimeric evaluation, and operational taxonomic unit definition were performed using the QIIME pipeline (v 1.9.1), as previously described.27 Taxonomy assignment was performed using the USEARCH algorithm at 97% similarity with SILVA 128 reference database. Principal coordinate analysis (PCoA) was performed using R package vegan, ade4 based on Bray-Curtis distance matrix at the genus level, and clustering analysis was performed using the R package vegan, stats based on Bray-Curtis distance matrix at the genus level and by using the ward.D2 method (nboost = 10,000).
Quantification of bacterial abundance in fecal samples
The abundance of each bacterium in the fecal samples was estimated by quantitative PCR. The ZymoBIOMICS Spike-in Control I (Zymo Research, Irvine, CA, USA) was used as a bacterial standard. Twenty microliters of the standard sample contained 2 × 107 of Imtechella halotolerans and Allobacillus halotolerans cells. The standard samples were serially 10-fold diluted and used to generate a standard curve. The bacterial genomic DNA from the standard and fecal samples was extracted using a NuculeoSpin Tissue DNA kit (Macherey-Nagel GmbH & Co. KG) according to the manufacturer's data sheet. Then, 2 µL of DNA samples, 14 µL of RT2 SYBR Green Mastermix (Qiagen, Hilden, Germany), 1-µL forward primer (5′-ACT CCT ACG GGA GGC AGC AGT-3′), 1-µL reverse primer (5′-ATT ACC GCG GCT GCT GGC-3′), and 10 µL of DNase-free water were combined into a reaction mixture. PCR was performed using an ABI 7300 system (Applied Biosystems, Foster City, CA, USA). The PCR protocol was as follows: 10 min at 95 ℃; 40 cycles of 95 ℃ for 15 s, 60 ℃ for 30 s, and 72 ℃ for 32 s; followed by 15 s at 95 ℃, 30 s at 60 ℃, and the final ramp to 95 ℃. Cycle thresholds were measured, and the abundance of each bacterium was calculated according to the standard curve.
Pathological examination
Resected lung tissues were fixed in 10% buffered formalin (Wako) for ≥ 48 h. The samples were embedded in paraffin and sliced into 3-µm thick sections. Lung and heart tissues were stained with Elastica van Gieson staining. The pathological quantification of the pulmonary vessels was based on a previous report.24 We identified all the pulmonary vessels in at least two slides per animal. The pulmonary vessels were scored by intimal occlusion severity as follows: 0, no neointima formation; 1, mild neointima formation and luminal narrowing ( <50%); and 2, severe neointima formation and luminal occlusion ( >50%). The histological findings were assessed by two investigators (T.J.S and H.S.) in a blinded manner, and final evaluations were based on their consensus.
Statistical analysis
Data were analyzed using GraphPad Prism (ver. 8.2.1, GraphPad Software Inc. San Diego, CA, USA). Continuous variables are described as means ± standard deviation unless otherwise stated. Differences between two groups were evaluated using the Student's t-test, and one-way ANOVA was used for assessing differences among multiple groups. The p-values were adjusted using the Bonferroni correction for multiple comparisons. p < 0.05 was considered significant.
Results
Modification of gut microbiota suppresses Su/Hx-induced RV hypertrophy and aggravation of hemodynamics
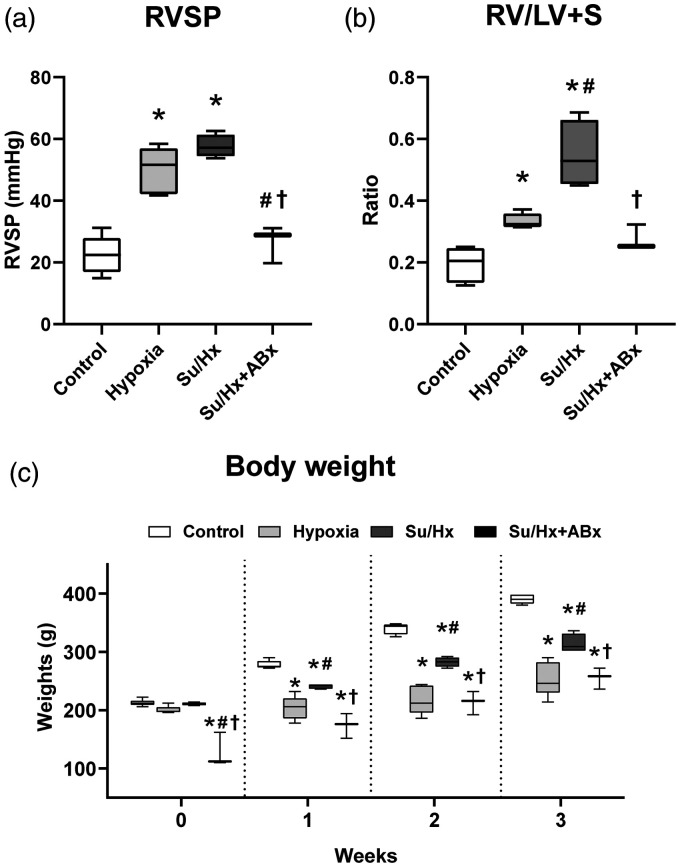
The RVSP values of control, Hx, Su/Hx, and Su/Hx + ABx groups were 22.6 ± 5.9, 50.0 ± 7.5, 57.7 ± 3.7, and 26.6 ±6.0 mmHg, respectively (Fig. 2a). Su/Hx and Hx groups had significantly higher RVSP values than did the control group (control vs. Hx: p < 0.0001; control vs. Su/Hx: p < 0.0001). There was no significant difference between the RVSP values of Su/Hx + ABx and control groups (p = 1.0). The RVSP of Su/Hx + ABx group was significantly lower than those of Hx and Su/Hx groups (vs. Hx: p = 0.0007; vs. Su/Hx: p < 0.0001). The RV/LV + S ratios of control, Hx, Su/Hx, and Su/Hx + ABx groups were 0.20 ±0.05, 0.33 ± 0.02, 0.55 ± 0.11, and 0.28 ± 0.04, respectively (Fig. 2b). Hx and Su/Hx rats had significantly higher RV/LV + S values than did the control group (control vs. Hx: p = 0.02, control vs. Su/Hx: p < 0.0001). The RVSP value of Su/Hx + ABx was significantly lower than that of the Su/Hx group (p = 0.0004), but did not differ from that of the control or Hx group (vs. control: p = 0.6, vs. Hx: p = 1.0).
Fig. 2.
Comparison of the hemodynamics and body weights among control, Su/Hx, and Su/Hx + Abx rats. (a) Right ventricular systolic pressure (RVSP). (b) The ratio of the right ventricle weight to left ventricle and septum weights (RV/LV + S). (c) Body weights of the four groups.
Su/Hx: SU5416/hypoxia; Su/Hx + ABx: SU5416/hypoxia rats treated with antibiotics. *p < 0.05, vs. control; #p < 0.05, vs. Hx rats; †p < 0.05, vs. Su/Hx rats.
Body weight
Fig. 2c shows the bodyweight data from each group. At 0 weeks, the body weights were as follows: control, 212.7 ± 5.3; Hx, 200.3 ± 5.9; Su/Hx, 210.5 ± 2.5; and Su/Hx + ABx, 128.0 ± 29.5 g. Su/Hx + ABx rats, which received one-week antibiotic pretreatment (Fig. 1) weighed significantly less than did the rats in the other groups (vs. control: p < 0.0001; vs. Hx: p < 0.0001; vs. Su/Hx: p < 0.0001). At three weeks, the body weights of the four groups were 390.0 ± 8.2, 254.0 ± 29.3, 314.0 ± 16.1, and 255.3 ± 18.1 g, respectively (Fig. 2c). The weights of Hx, Su/Hx, and Su/Hx + ABx rats were significantly lower than those of control rats (vs. Hx: p < 0.0001; vs. Su/Hx: p = 0.0002; vs. Su/Hx + ABx: p < 0.0001).
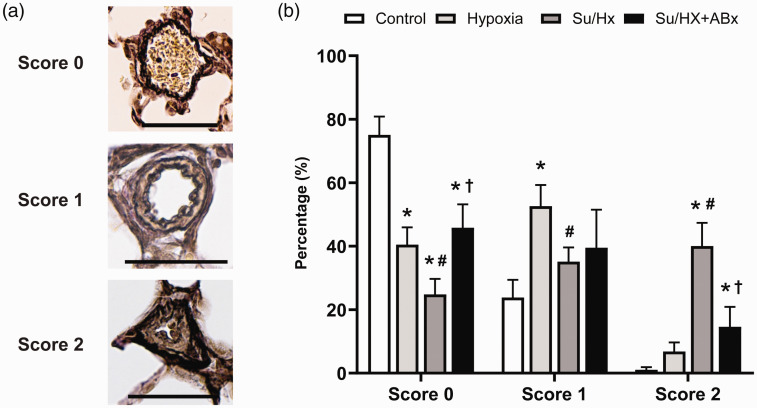
Gut microbiota modification suppresses Su/Hx-induced progression of vascular remodeling
The percent of obstructive vasculopathy (score 2) in control, Hx, Su/Hx, and Su/Hx + ABx groups were 1.1 ± 0.8, 6.8 ± 2.9, 40.1 ± 7.3, and 14.6 ± 6.3%, respectively. Su/Hx group had significantly more vessels with score 2 than the control or Hx group (vs. control: p < 0.0001; vs. Hx: p < 0.0001, Fig. 3). This difference was more pronounced than that observed between the Su/Hx + ABx group and the control or Hx group, indicating that the antibiotic pre-treatment suppressed the development of obstructive vasculopathy (p < 0.0001) (Fig. 3).
Fig. 3.
Vascular remodeling in control, Su/Hx, and Su/Hx + ABx rats. (a) Representative images showing each grade of vascular remodeling. Scale bars show 50 µm. (b) The proportion of each grade of vascular remodeling among the three groups.
Su/Hx: SU5416/hypoxia; Su/Hx + ABx: SU5416/hypoxia rats treated with antibiotics.
*p < 0.05, vs. control; #p < 0.05, vs. Hx rats; †p < 0.05, vs. Su/Hx rats.
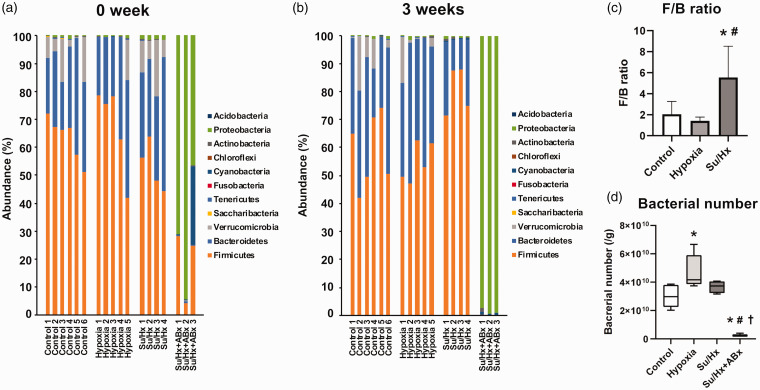
Gut microbiota compositions
Fig. 4a and b show the gut microbiota compositions at the beginning and end of the study. The ratio of Firmicutes-to-Bacteroidetes (F/B) ratio, which is a biomarker of dysbiosis,28 was significantly higher in Su/Hx rats than in the control and Hx groups (vs. control: p = 0.02, vs. Hx: p = 0.01, Fig. 4c) at three weeks, although there was no difference among the three groups at 0 weeks (p = 0.3). The abundance of Bacteroides was 0% in all the Su/Hx + ABx rats, and therefore, the F/B ratios in these rats were incalculable.
Fig. 4.
Gut microbiota compositions. (a) The gut microbiota compositions at the beginning of the study. (b) The gut microbiota compositions after three weeks of hypoxic exposure. (c) The Firmicutes-to-Bacteroidetes (F/B) ratios in control, hypoxia, and Su/Hx groups at three weeks. (d) The bacterial numbers in fecal samples at three weeks.
Su/Hx: SU5416/hypoxia; Su/Hx + ABx: SU5416/hypoxia rats treated with the antibiotic cocktail.
*p < 0.05, vs. control; #p < 0.05, vs. Hx rats; †p < 0.05, vs. Su/Hx rats.
Antibiotic treatments modify gut microbiota composition in rats
Fig. 4d shows the total number of bacteria in the fecal samples from each group. The bacterial number in the Su/Hx + ABx group was significantly lower than those in the other groups (vs. control: p = 0.002; vs. Hx: p < 0.0001; Su/Hx: p < 0.0005). Moreover, the gut microbiota composition in Su/Hx + ABx rats was different from those of the other groups (Fig. 4a and b). At the phylum level, the abundance of Proteobacteria in the Su/Hx + ABx group was 98.6 ± 1.1% at three weeks and was significantly higher than those in other groups (control: 0.5 ± 0.5%, p < 0.0001; Hx: 0.5 ± 0.5%, p < 0.0001; Su/Hx: 0.8 ± 0.3%, p < 0.0001). At the genus level, Shigella accounted for 95.8 ± 3.1% of the gut microbiota in Su/Hx + ABx rats (Supplemental Fig.).
Gut microbiota composition differ between PAH and control rats
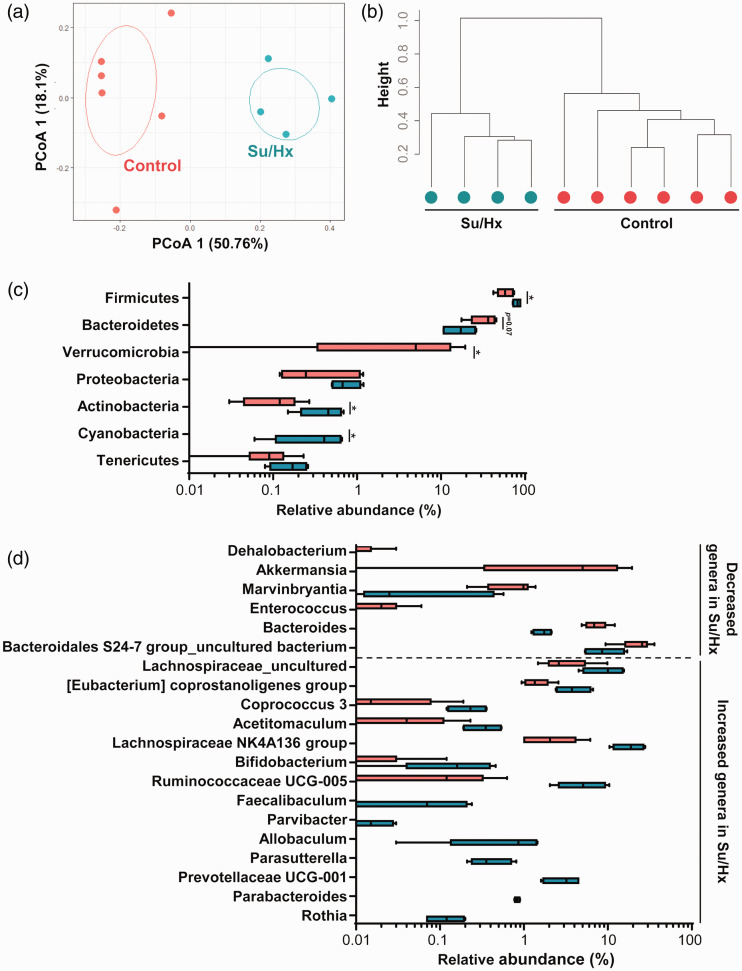
16S rRNA analysis revealed that Su/Hx rats had a microbiota composition distinct from that of control rats at three weeks (Fig. 5). PCoA, which is a metric analysis for visualizing the similarities and differences among communities,19 revealed that control and Su/Hx groups formed different clusters (Fig. 5a). The results of dendrogram analysis also showed differences in gut microbiota composition between control and Su/Hx groups (Fig. 5b). Fig. 5c shows the microbiota compositions of the two groups at the phylum level. Firmicutes, Actinobacteria, and Cyanobacteria were significantly less abundant in the Su/Hx group than in the control group (Firmicutes: p = 0.03; Actinobacteria: p = 0.03; and Cyanobacteria: p = 0.009; Fig. 5c). The abundance of Bacteroidetes was also lower, although the difference was not significant (p = 0.07; Fig. 5c). Fig. 5d shows the differential bacterial composition pattern between control and Su/Hx groups at the genus level. The abundances of six bacteria were significantly decreased in the Su/Hx group when compared with the control (Dehalobacterium: p =0.025; Akkermansia: p = 0.048; Marvinbryantia: p = 0.043; Enterococcus: p = 0.034; Bacteroides: p = 0.014; and Bacteroidetes S24-7 group uncultured bacterium: p = 0.043). Conversely, the abundances of 14 bacteria were significantly increased in the Su/Hx group (Lachnospiraceae uncultured: p = 0.043; Eubacterium coprostanoligenes group: p = 0.025; Coprococcus 3: p = 0.040; Acetitomaculum: p = 0.042; Lachnospiraceae NK4A136 group: p = 0.014; Bifidobacterium: p = 0.017; Ruminococcaceae UCG-005: p = 0.014; Faecalibaculum: p = 0.026; Parvibacter: p =0.026; Allobaculum: p = 0.0057; Parasutterella: p = 0.0057; Prevotellaceae UCG-001: p = 0.0057; Parabacteroides: p = 0.0057; and Rothia: p = 0.0056).
Fig. 5.
Differences in gut microbiota between control and Su/Hx rats at three weeks. (a) Principal coordinates analysis (PCoA). (b) Dendrogram of the two groups. (c) The compositions of gut microbiota at the phylum level. *p < 0.05, vs. control. (d) Significantly increased and decreased genera in Su/Hx rats compared with those in the control group.
Su/Hx: SU5416/hypoxia.
Discussion
In this study, we first explored the effects of altered gut microbiota in a PAH rat model. The antibiotic treatment modified the gut microbiota of Su/Hx rats, and the Su/Hx rats treated with antibiotics developed less vascular remodeling and PAH. The results suggested that gut microbiota is possibly related to the pathogenesis of PAH.
Recently, Callejo et al. have reported the altered composition of gut microbiota in Su/Hx rats as compared with control rats.23 They also described that the F/B ratio was higher in Su/Hx rats than in control rats, consistent with our results. Several other studies have described the relationship between cardiovascular diseases and dysbiosis.20,21,23,28 For example, Yang et al. have demonstrated that F/B ratios are higher in two different animal models of systemic hypertension than in control rats.28 They have also shown that patients with systemic hypertension have distinct gut microbiota compositions from those of healthy control subjects.28 Moreover, Emoto et al. have reported that alterations in gut microbiota can be related to the severity of coronary artery disease.20
The underlying cause of dysbiosis in PAH remains unclear. Hypoxia exposure reduces the oxygen pressure within the small intestine, which can induce alterations in gut microbiota.29 In the current study, there was no difference in the F/B ratios between the control and Hx rats. The discrepancy suggests that other factors can be involved with dysbiosis in Su/Hx rats. There is no report describing that the effects of SU5416 on gut microbiota, although the possibility that SU5416 treatments may play a potential role in the dysbiosis cannot be excluded. Sympathetic nervous system is activated in Su/Hx rats.30 Activated sympathetic nervous system can increase the permeability of the gut,31 which may be related to dysbiosis in Su/Hx rats.23 Heart failure patients have increased gut permeability and gut dysbiosis,32,33 although it is still unclear whether dysbiosis is a cause of heart failure. Thus, the dysbiosis in Su/Hx rats may depend on several different systems. Our results suggest the causative role of dysbiosis in Su/Hx rats, but whether the dysbiosis is a cause or the result of PAH is open to discussion. The precise mechanisms of dysbiosis in PAH should be investigated in future studies.
We observed an imbalance in the gut microbiota composition of Su/Hx rats relative to that of the control rats both at the phylum and genus levels. Bacteroides and Akkermansia, which are related to the suppression of inflammation, were decreased in Su/Hx rats. Bacteroides are known to be responsible for the induction of regulatory T cells (Tregs) via polysaccharide A production.34,35 Tregs suppress the excessive inflammatory response after vascular injuries, and their reduction may induce the development of PAH.16,36 Akkermansia suppresses vascular inflammation and the development of atherosclerosis via suppression of the expressed inflammatory cytokines, such as TNF-α and IL-1β.37,38 Kim et al. have recently reported that the abundances of Bacteroides and Akkermansia are decreased in PAH patients compared with those in control subjects.39 These decreases might be related to the development of PAH both in humans and experimental animal models. Increased bacteria in Su/Hx rats, such as Rothia and Prevotellaceae, might be associated with the induction of inflammation. Rothia dentocariosa is commonly isolated from the oral cavity and is associated with TNF-α production and chronic inflammation in periodontal diseases.40 Furthermore, Rothia is related to increased IL-8 levels and disease severity in patients with obstructive sleep apnea syndrome.41 Increased Prevotellaceae levels are commonly observed in patients with new-onset untreated rheumatoid arthritis (RA),42,43 and plays a causative role in the development of RA.43 Thus, we think that imbalanced abundances of several different bacteria might be associated with the induction or suppression of inflammation, which might be related to the development of PAH.
Modification of the gut microbiota using antibiotics suppressed the progression of PAH in our study. Indeed, antibiotic treatments dramatically altered the gut microbiota composition in Su/Hx + ABx rats. Shigella accounted for > 95% of the gut microbiota in Su/Hx + ABx rats. It has been acknowledged that Shigella is not pathogenic to rats, mice, rabbits, or guinea pigs.44,45 This bacterium can acquire resistance to antibiotics, including β-lactams, aminoglycosides, and fluoroquinolones, as a result of abused antibiotic treatments.45,46 Thus, we think that microbial substitution occurred in Su/Hx + ABx rats.
Antibiotic treatments can suppress the development of cardiovascular diseases. Yang et al. have described that minocycline improves angiotensin II-induced systemic hypertension via restoring the gut microbiota.28 Koeth et al. have reported that broad-spectrum antibiotic treatments suppress the progression of atherosclerosis induced in Apoe knockout mice.47 Other gut microbiota disturbances may also affect cardiovascular diseases. Marques et al. have demonstrated that high-fiber diet changes the gut microbiota and suppresses the progression of systemic hypertension and heart failure in mice with mineralocorticoid-induced hypertension.48 These data support the notion that dysbiosis plays causal roles in the development of PAH and restoring the gut microbiota might offer an alternative strategy for the prevention of PAH.
In the current study, the body weights of Su/Hx + ABx rats were lower than those of the rats in the other groups. Gut microbiota play important roles in energy biogenesis and vitamin synthesis.17 The lack or modification of gut microbiota can affect body weight. For example, mice that are housed under germ-free conditions and thus have not been exposed to any living bacterium weigh 40% less than those housed under regular conditions.49 Treatments using multiple antibiotics can also induce weight losses in rats.50 Finally, chronic hypoxia exposure can be a cause of weight loss in rats.51 Thus, it seems that the weight loss in Su/Hx + ABx rats in our study might have resulted from the combined effects of antibiotic and hypoxia treatments.
The current study had some limitations. First, the body weights of rats in the three groups were different, which might have affected the observed changes in gut microbiota and in the progression of PAH. Second, the sample size of Su/Hx + ABx rats was small. Third, we did not analyze the function of the gut microbiota by metagenome analysis. Fourth, the therapeutic effect of antibiotic treatments for PAH was unclear under the design of the current study. The reason for this was the fact that the antibiotic treatment in this study was preventive and not therapeutic. The therapeutic effects of altering gut microbiota on PAH should be investigated in the future studies. Finally, our results were obtained only from animal experiments. Therefore, it is unclear whether our results are applicable to human patients with PAH. At this moment, we do not recommend long-term antibiotic treatment for these patients with PAH and the risk factors because such it would be too invasive. In future clinical studies, other interventions, such as probiotic treatments, should be considered. Despite these limitations, we believe that the results of this study indicate the important relationship between the pathogenesis of PAH and gut microbiota.
In conclusion, modification of gut microbiota with antibiotics suppresses the development of PAH. Dysbiosis may play a causal role in the development and progression of PAH, and modification of gut microbiota might be a new option for the prevention of PAH.
Supplemental Material
Supplemental material, sj-pdf-1-pul-10.1177_2045894020929147 for Gut microbiota modification suppresses the development of pulmonary arterial hypertension in an SU5416/hypoxia rat model by Takayuki J. Sanada, Koji Hosomi, Hiroki Shoji, Jonguk Park, Akira Naito, Yumiko Ikubo, Asako Yanagisawa, Takayuki Kobayashi, Hideki Miwa, Rika Suda, Seiichiro Sakao, Kenji Mizuguchi, Jun Kunisawa, Nobuhiro Tanabe and Koichiro Tatsumi in Pulmonary Circulation
Acknowledgments
We thank Ms. Tomoko Misawa of the Department of Respirology, Graduate School of Medicine, Chiba University for her technical support. We also would like to thank Editage (www.editage.com) for English-language editing.
Conflict of interest
T.J.S was a member of an endowed department sponsored by Actelion Pharmaceuticals Ltd and received a Grant-in-Aid for Young Scientists from the Japan Society for the Promotion of Science (JSPS) (No. JP 16K19444) and a scholarship from MSD Life Science Foundation, Public Interest Incorporated Foundation. K.H. received a Grant-in-Aid for Young Scientists from the Japan Society for the Promotion of Science (JSPS) (No. JP18K17997) and a grant from the Ministry of Health, Labour, and Welfare of Japan (No. JP19KA3001). J.P. was supported by the Ministry of Education, Culture, Sports, Science, and Technology of Japan; the Japan Society for the Promotion of Science (Nos. JP18H02150, JP18H02674, and JP17K09604); the AMED (Nos. JP17fk0108223h0002, JP17ek0410032s0102, JP17fk0108207h0002, JP17ek0210078h0002, JP17ak0101068h0001, JP17gm1010006s0101, JP18ck0106243h0003, and JP19ek0410062h0001); the Science and Technology Research Promotion Program for Agriculture, Forestry, Fisheries, and Food Industry; the Terumo Foundation for Life Sciences and Arts; the ONO Medical Research Foundation; the Canon Foundation; the Cross-ministerial Strategic Innovation Promotion Program; and the Joint Research Project between the Institute of Medical Science, the University of Tokyo, and Public/Private R&D Investment Strategic Expansion PrograM (PRISM). H.M. received a Grant-in-Aid for Young Scientists from the Japan Society for the Promotion of Science (No. JP19K17664). R.S. received a Grant-in-Aid for Young Scientists from the Japan Society for the Promotion of Science (No. 18K15944). S.S. was supported by grants endowed to the Intractable Respiratory Diseases and Pulmonary Hypertension Research Group by the Ministry of Health, Labour, and Welfare of Japan (No. 27280401) and a Grant-in-Aid for Scientific Research B from the Japan Society for the Promotion of Science (No. JP18H03664) and received honoraria for lectures from Nippon Shinyaku Co., Ltd, Bayer, Actelion Pharmaceuticals, and Pfizer. K.M. received a Grant-in-Aid for Scientific Research C from the Japan Society for the Promotion of Science (No. 17K07268). J.K. received a Grant-in-Aid for Scientific Research B from the Japan Society for the Promotion of Science (No. JP18H03664) and funding from the AMED (CREST, 19gm1010006s0103). N.T. was supported by grants from Japan Society for the Promotion of Science and by grants endowed to the Intractable Respiratory Diseases and Pulmonary Hypertension Research Group by the Ministry of Health, Labour, and Welfare of Japan; and received personal fees from Actelion Pharmaceuticals Ltd, from the Bayer AG, the Daiichi-Sankyo Company, and from the Nippon Shinyaku Co., Ltd. K.T. was supported by a Grant-in-Aid for Scientific Research (No. 17H04181) and grants endowed to the Intractable Respiratory Diseases and Pulmonary Hypertension Research Group by the Ministry of Health, Labour, and Welfare of Japan and those endowed to the same group by the AMED (No. 16ek0109127h0002) and received lecture honoraria from Actelion Pharmaceuticals and Nippon Boehringer Ingelheim. The other authors had no potential conflicts of interest.
Ethical statements
All the experimental procedures involving animals were approved by Chiba University Instrumental Animal and Use Committee (approval numbers 29-231 and 30-446).
Funding
This study was supported by a Grant-in-Aid for Scientific Research C from the Japan Society for the Promotion of Science (JSPS) (No. 17K09604) and by grants endowed to the Intractable Respiratory Diseases and Pulmonary Hypertension Research Group by the Ministry of Health, Labour, and Welfare of Japan, and by those endowed to the same group by Japan Agency for Medical Research and Development (AMED) (No. 16ek0109127h0002). The funders had no role in the study design, data collection or analysis, decision to publish, or preparation of the manuscript.
ORCID iDs
Takayuki J. Sanada https://orcid.org/0000-0001-5725-1810
Seiichiro Sakao https://orcid.org/0000-0001-6719-1022
Supplemental Material
Supplemental material for this article is available online.
References
- 1.Humbert M, Guignabert C, Bonnet S, et al. Pathology and pathobiology of pulmonary hypertension: state of the art and research perspectives. Eur Respir J 2019; 53: pii:1801887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fuster V, Steele PM, Edwards WD, et al. Primary pulmonary hypertension: natural history and the importance of thrombosis. Circulation 1984; 70: 580–587. [DOI] [PubMed] [Google Scholar]
- 3.Humbert M, Sitbon O, Yaici A, et al. Survival in incident and prevalent cohorts of patients with pulmonary arterial hypertension. Eur Respir J 2010; 36: 549–555. [DOI] [PubMed] [Google Scholar]
- 4.Farber HW, Miller DP, Poms AD, et al. Five-year outcomes of patients enrolled in the REVEAL Registry. Chest 2015; 148: 1043–1054. [DOI] [PubMed] [Google Scholar]
- 5.Galiè N, Humbert M, Vachiery J-L, et al. 2015 ESC/ERS guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS) endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2015; 37: 67–119. [DOI] [PubMed] [Google Scholar]
- 6.Galie N, Channick RN, Frantz RP, et al. Risk stratification and medical therapy of pulmonary arterial hypertension. Eur Respir J 2019; 53: pii:1801889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heath D, Edwards JE. The pathology of hypertensive pulmonary vascular disease: a description of six grades of structural changes in the pulmonary arteries with special reference to congenital cardiac septal defects. Circulation 1958; 18: 533–547. [DOI] [PubMed] [Google Scholar]
- 8.Toba M, Alzoubi A, O'Neill KD, et al. Temporal hemodynamic and histological progression in Sugen5416/hypoxia/normoxia-exposed pulmonary arterial hypertensive rats. Am J Physiol Heart Circ Physiol 2014; 306: H243–H250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rol N, Kurakula KB, Happe C, et al. TGF-beta and BMPR2 signaling in PAH: two black sheep in one family. Int J Mol Sci 2018; 19: 2585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jonigk D, Golpon H, Bockmeyer CL, et al. Plexiform lesions in pulmonary arterial hypertension composition, architecture, and microenvironment. Am J Pathol 2011; 179: 167–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rabinovitch M, Guignabert C, Humbert M, et al. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circ Res 2014; 115: 165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soon E, Holmes AM, Treacy CM, et al. Elevated levels of inflammatory cytokines predict survival in idiopathic and familial pulmonary arterial hypertension. Circulation 2010; 122: 920–927. [DOI] [PubMed] [Google Scholar]
- 13.Cracowski JL, Chabot F, Labarere J, et al. Proinflammatory cytokine levels are linked to death in pulmonary arterial hypertension. Eur Respir J 2014; 43: 915–917. [DOI] [PubMed] [Google Scholar]
- 14.Taraseviciene-Stewart L, Kasahara Y, Alger L, et al. Inhibition of the VEGF receptor 2 combined with chronic hypoxia causes cell death-dependent pulmonary endothelial cell proliferation and severe pulmonary hypertension. Faseb J 2001; 15: 427–438. [DOI] [PubMed] [Google Scholar]
- 15.de Raaf MA, Schalij I, Gomez-Arroyo J, et al. SuHx rat model: partly reversible pulmonary hypertension and progressive intima obstruction. Eur Respir J 2014; 44: 160–168. [DOI] [PubMed] [Google Scholar]
- 16.Taraseviciene-Stewart L, Nicolls MR, Kraskauskas D, et al. Absence of T cells confers increased pulmonary arterial hypertension and vascular remodeling. Am J Respir Crit Care Med 2007; 175: 1280–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lynch SV, Pedersen O. The human intestinal microbiome in health and disease. N Engl J Med 2016; 375: 2369–2379. [DOI] [PubMed] [Google Scholar]
- 18.Cho I, Blaser MJ. The human microbiome: at the interface of health and disease. Nat Rev Genet 2012; 13: 260–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuczynski J, Lauber CL, Walters WA, et al. Experimental and analytical tools for studying the human microbiome. Nat Rev Genet 2011; 13: 47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Emoto T, Yamashita T, Sasaki N, et al. Analysis of gut microbiota in coronary artery disease patients: a possible link between gut microbiota and coronary artery disease. J Atheroscler Thromb 2016; 23: 908–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang WH, Kitai T, Hazen SL. Gut microbiota in cardiovascular health and disease. Circ Res 2017; 120: 1183–1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thenappan T, Khoruts A, Chen Y, et al. Can intestinal microbiota and circulating microbial products contribute to pulmonary arterial hypertension?. Am J Physiol Heart Circ Physiol 2019; 317: H1093–H1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Callejo M, Mondejar-Parreno G, Barreira B, et al. Pulmonary arterial hypertension affects the rat gut microbiome. Sci Rep 2018; 8: 9681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takeuchi T, Sakao S, Kato F, et al. Pulmonary haemodynamics are correlated with intimal lesions in a rat model of severe PAH: attenuation of pulmonary vascular remodelling with ambrisentan. Histol Histopathol 2016; 31: 1357–1365. [DOI] [PubMed] [Google Scholar]
- 25.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, et al. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell 2004; 118: 229–241. [DOI] [PubMed] [Google Scholar]
- 26.Fulton RM, Hutchinson EC, Jones AM. Ventricular weight in cardiac hypertrophy. Br Heart J 1952; 14: 413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mohsen A, Park J, Chen YA, et al. Impact of quality trimming on the efficiency of reads joining and diversity analysis of Illumina paired-end reads in the context of QIIME1 and QIIME2 microbiome analysis frameworks. BMC Bioinformatics 2019; 20: 581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang T, Santisteban MM, Rodriguez V, et al. Gut dysbiosis is linked to hypertension. Hypertension 2015; 65: 1331–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moreno-Indias I, Torres M, Montserrat JM, et al. Intermittent hypoxia alters gut microbiota diversity in a mouse model of sleep apnoea. Eur Respir J 2015; 45: 1055–1065. [DOI] [PubMed] [Google Scholar]
- 30.Yoshida K, Saku K, Kamada K, et al. Electrical vagal nerve stimulation ameliorates pulmonary vascular remodeling and improves survival in rats with severe pulmonary arterial hypertension. JACC Basic Transl Sci 2018; 3: 657–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun Y, Fihn BM, Sjovall H, et al. Enteric neurones modulate the colonic permeability response to luminal bile acids in rat colon in vivo. Gut 2004; 53: 362–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pasini E, Aquilani R, Testa C, et al. Pathogenic gut flora in patients with chronic heart failure. JACC Heart Fail 2016; 4: 220–227. [DOI] [PubMed] [Google Scholar]
- 33.Hayashi T, Yamashita T, Watanabe H, et al. Gut microbiome and plasma microbiome-related metabolites in patients with decompensated and compensated heart failure. Circ J 2018; 83: 182–192. [DOI] [PubMed] [Google Scholar]
- 34.Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature 2008; 453: 620–625. [DOI] [PubMed] [Google Scholar]
- 35.Round JL, Mazmanian SK. Inducible Foxp3 + regulatory T-cell development by a commensal bacterium of the intestinal microbiota. Proc Natl Acad Sci USA 2010; 107: 12204–12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tamosiuniene R, Tian W, Dhillon G, et al. Regulatory T cells limit vascular endothelial injury and prevent pulmonary hypertension. Circ Res 2011; 109: 867–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang L, Qin Q, Liu M, et al. Akkermansia muciniphila can reduce the damage of gluco/lipotoxicity, oxidative stress and inflammation, and normalize intestine microbiota in streptozotocin-induced diabetic rats. Pathog Dis 2018, pp. 76–DOI:10.1093/femspd/fty028. [DOI] [PubMed] [Google Scholar]
- 38.Li J, Lin S, Vanhoutte PM, et al. Akkermansia muciniphila protects against atherosclerosis by preventing metabolic endotoxemia-induced inflammation in Apoe –/– mice. Circulation 2016; 133: 2434–2446. [DOI] [PubMed] [Google Scholar]
- 39.Kim S, Rigatto K, Gazzana MB, et al. Altered gut microbiome profile in patients with pulmonary arterial hypertension. Hypertension 2020; 75: 1063–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kataoka H, Taniguchi M, Fukamachi H, et al. Rothia dentocariosa induces TNF-alpha production in a TLR2-dependent manner. Pathog Dis 2014; 71: 65–68. [DOI] [PubMed] [Google Scholar]
- 41.Wu BG, Sulaiman I, Wang J, et al. Severe obstructive sleep apnea is associated with alterations in the nasal microbiome and an increase in inflammation. Am J Respir Crit Care Med 2019; 199: 99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scher JU, Sczesnak A, Longman RS, et al. Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. Elife 2013; 2: e01202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maeda Y, Kurakawa T, Umemoto E, et al. Dysbiosis contributes to arthritis development via activation of autoreactive T cells in the intestine. Arthritis Rheumatol 2016; 68: 2646–2661. [DOI] [PubMed] [Google Scholar]
- 44.Kim YJ, Yeo SG, Park JH, et al. Shigella vaccine development: prospective animal models and current status. Curr Pharm Biotechnol 2013; 14: 903–912. [DOI] [PubMed] [Google Scholar]
- 45.Lampel KA, Formal SB, Maurelli AT. A brief history of Shigella. EcoSal Plus 2018, pp. 8–DOI: 10.1128/ecosalplus.ESP-0006-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ranjbar R, Farahani A. Shigella: antibiotic-resistance mechanisms and new horizons for treatment. Infect Drug Resist 2019; 12: 3137–3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koeth RA, Wang Z, Levison BS, et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat Med 2013; 19: 576–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Marques FZ, Nelson E, Chu PY, et al. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation 2017; 135: 964–977. [DOI] [PubMed] [Google Scholar]
- 49.Lee WJ, Hase K. Gut microbiota-generated metabolites in animal health and disease. Nat Chem Biol 2014; 10: 416–424. [DOI] [PubMed] [Google Scholar]
- 50.Hoban AE, Moloney RD, Golubeva AV, et al. Behavioural and neurochemical consequences of chronic gut microbiota depletion during adulthood in the rat. Neuroscience 2016; 339: 463–477. [DOI] [PubMed] [Google Scholar]
- 51.Hislop A, Reid L. New findings in pulmonary arteries of rats with hypoxia-induced pulmonary hypertension. Br J Exp Pathol 1976; 57: 542–554. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-pul-10.1177_2045894020929147 for Gut microbiota modification suppresses the development of pulmonary arterial hypertension in an SU5416/hypoxia rat model by Takayuki J. Sanada, Koji Hosomi, Hiroki Shoji, Jonguk Park, Akira Naito, Yumiko Ikubo, Asako Yanagisawa, Takayuki Kobayashi, Hideki Miwa, Rika Suda, Seiichiro Sakao, Kenji Mizuguchi, Jun Kunisawa, Nobuhiro Tanabe and Koichiro Tatsumi in Pulmonary Circulation