Abstract
OCTA imaging in optic neuropathies.
Keywords: Alzheimer’s disease, dominant optic atrophy, erythrocyte mediated angiography, glaucoma, Leber’s hereditary optic neuropathy, optic neuropathy, optical coherence tomography angiography, Parkinson’s disease, retinal blood flow, schizophrenia, vasomotion, Wolfram Syndrome
Introduction
Optical coherence tomography angiography (OCTA) is an emerging technique for non-invasively imaging the ocular vasculature. OCTA enables en-face visualization of the retinal circulation into anatomic slabs of the superficial as well as deep vascular networks supplying the various retinal layers (Figure 1). In addition, OCTA provides quantitative measurements of retinal vascular anatomy, permitting an objective approach for evaluating vascular pathology in disease.1 Vessel density and perfusion density are two OCTA metrics frequently reported in the literature. In particular, vessel density represents the percentage of blood vessels occupying a given area following image binarization, whereas perfusion density reflects the percentage of blood vessels occupying a given area following image skeletonization.1
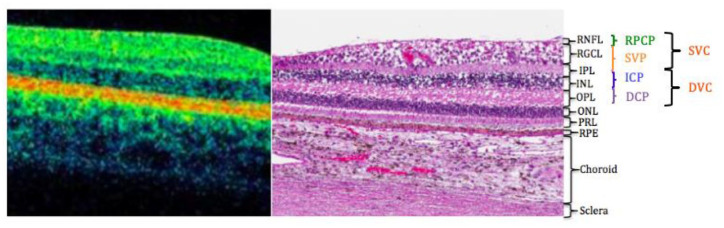
Figure 1.
Retinal vascular plexuses corresponding with a histological section of the human retina showing anatomic layers from spectral domain optical coherence tomography.
The four vascular plexuses can be grouped into superficial and deep vascular complexes (SVC and DVC), reflecting the anatomic location of the ICP at the IPL/INL interface.
Abbreviations: DCP, deep capillary plexus; ICP, intermediate capillary plexus; INL, inner nuclear layer; IPL, inner plexiform layer; ONL, outer nuclear layer; OPL, outer plexiform layer; PRL, photoreceptor layer; RGCL, retinal ganglion cell layer; RNFL, retinal nerve fiber layer; RPCP, radial peripapillary capillary plexus; RPE, retinal pigment epithelium; SVP, superficial vascular plexus.
The eye, and more particularly, the optic nerve and the retina share many vascular characteristics with the central nervous system (CNS). Measuring the structural and functional changes within the ocular vasculature may be useful as an objective, quantitative means of non-invasively evaluating the cerebrovascular architecture.2,3 The current article reviews the most recent applications of OCTA imaging at the eye–brain interface and highlights the emerging vascular biomarkers for neurodegenerative optic neuropathies.
Alzheimer’s disease
Alzheimer’s disease (AD) is the leading cause of dementia, affecting more than 5.8 million Americans, and is the most expensive disease in the United States at a total cost of $290 billion in 2019.4 As the field of medicine prolongs life, the number of people suffering from AD increases together with aging and is estimated to quadruple by 2050.4 Ophthalmologic impairments including loss of visual acuity, color recognition, and motion perception have all been reported in AD. Intriguingly, these visual changes have been documented not only in the early stages of disease, but also in the presymptomatic stages.5–10 In addition, visual abnormalities in AD have been associated with degeneration of the anterior visual pathways, as first demonstrated histopathologically using postmortem human tissues.11–14
Vascular abnormalities in AD include impaired β-amyloid (Aß) clearance and subsequent breakdown of the blood–brain barrier followed by cerebrovascular dysfunction.15–18 Similar mechanisms of cortical neurodegeneration involving amyloid deposition have also been observed in the optic nerve and retina.19–21 Given this resemblance between the cerebral and the intraocular vasculature, investigators have increasingly sought to identify potential oculovascular biomarkers for AD.
Retinal vascular pathology in AD
Histological studies in AD human retina have provided evidence of vascular pathology resembling that of the cerebral vasculature including narrowed vessel caliber, reduced blood flow, and increased blood oxygen saturation as well as tortuosity.20,22–30 Schultz and colleagues reported significant differences in islet amyloid polypeptide levels, pericyte number, and vessel length between retinal fractions derived from AD and non-demented patient cases. In addition, these retinal parameters significantly correlated with corresponding variables in respective hippocampal fractions.31 Initial live-patient studies conducted by Berisha and colleagues hypothesized that retinal atrophy in AD may be associated with abnormal retinal blood flow. In particular, Berisha and colleagues measured retinal blood flow column diameter and flow rates in early AD using laser Doppler imaging. AD subjects featured significant retinal nerve fiber layer (RNFL) loss superiorly accompanied by decreased flow rate and narrowing of the superior temporal retinal vein relative to controls.20 Using fundus photography and automated vessel segmentation software, Cheung and colleagues23 demonstrated narrowing of retinal vessel caliber, decreased fractal dimension, and pronounced vessel tortuosity in AD patients relative to controls.
Bulut and colleagues were the first to evaluate the vascular density and foveal avascular zone (FAZ) of the retina in AD using OCTA. Relative to controls, they found significant enlargement of the FAZ and decreased vascular density for all retinal zones of the superficial vascular complex in AD.15 These vascular changes may be associated with reduced angiogenesis from the binding of vascular endothelial growth factor (VEGF) to Aβ.19,21 Aβ accumulation and subsequent plaque formation in vessel walls may also occlude and thus impair blood flow.20,32 Studies conducted by Jiang and colleagues similarly showed loss of retinal vessel density in AD. In particular, the superficial capillary plexus (SCP) and deep capillary plexus (DCP) were both markedly attenuated in AD, whereas only the DCP was significantly affected in mild cognitive impairment (MCI).33 In addition, a progressive pattern of vessel loss from MCI to AD was observed, suggesting OCTA’s potential role in detecting the conversion from MCI to AD.33 Given the non-uniform distribution of retinal ganglion cells and their corresponding vascular supply, Jiang and colleagues33 also reported sectoral differences with respect to vascular pathology, whereby vessel density was highest between 0.5 to 1.25 mm from the fovea. Notably, however, Mini–Mental State Examination (MMSE) scores did not correlate with retinal vascular changes in AD relative to controls. This demonstrates that there is relatively low sensitivity of the MMSE to differentiate MCI from AD.34,35 Further studies by Yoon and colleagues36 have confirmed retinal vascular changes in AD and MCI. In this relatively large cross-sectional study, AD participants showed significantly reduced macular vessel density and perfusion density of the SCP relative to MCI and control subjects. In contrast to previous studies, the FAZ did not significantly differ among the three cohorts. These variable findings may be attributed to confounding factors including glaucoma and retinal disease, segmentation algorithms, and the diagnostic criteria for AD and MCI.37–39 Additional diagnostics using positron emission tomography (PET) imaging or lumbar puncture may be necessary for homogenizing cohorts in future studies. This is especially important since MCI is a complex, heterogeneous group that includes patients who may not progress to AD, may revert back to being cognitively normal, or may demonstrate non-AD dementia.40
Querques and colleagues41 evaluated the functional and morphological characteristics of the retinal vasculature in AD and MCI. Using both dynamic vessel analyzer (DVA) and OCTA, the study demonstrated significant impairment of retinal neurovascular coupling not only in AD, but also in MCI. In addition, vascular impairment was inversely correlated with the level of Aβ in the cerebrospinal fluid (CSF). During DVA testing, the group observed an overall reduction of vessel response, mostly involving arterial vasodilation. DVA analysis also demonstrated an altered vascular response to flicker stimulus in MCI.41 Therefore, functional alterations in the retinal vasculature may occur earlier in disease pathogenesis and precede structural changes. These findings further support those by Jiang and colleagues,33 whereby vascular parameters may aid in predicting the transition from MCI to AD. Notably, CSF Aβ levels directly correlated with arterial dilation level after flicker, suggesting a potential relationship between Aβ load and neurovascular coupling. This is consistent with the known inverse correlation between Aβ levels in the brain with CSF Aβ levels, and the direct correlation with CSF phosphorylated-tau levels.42
However, the clinical utility of the retinal vasculature as a purported surrogate marker for AD remains inconclusive, given the inconsistent findings between groups and the various limitations of OCTA associated with live-patient studies. Problems include reduced cooperation in patients who are cognitively compromised and the persistent challenge of unequivocally diagnosing AD clinically.42,43 Results may be confounded by artifacts or poor scan quality, as may be caused by poor subject compliance and fixation deficits.15 Therefore, data derived from subjects with advanced disease may not always reliably reflect AD.
Notably, more recent OCTA studies have also evaluated the retinal vasculature in cognitively healthy, preclinical AD. Reported findings include increased FAZ area along with decreased vessel density and flow in these presymptomatic stages.44,45 In contrast, den Haan and colleagues showed no significant decrease in vessel density or increase in FAZ in asymptomatic participants with AD pathology, after adjusting for age and sex. In addition, there were no associations between retinal vascular parameters and CSF biomarkers or MMSE scores.46 Taken together, these findings suggest that retinal vascular pathology may possibly precede cognitive impairment. Nevertheless, results have varied across groups and additional studies involving larger cohorts are necessary for validation.
Correlations with primary open-angle glaucoma
Primary open-angle glaucoma (POAG) is a progressive neurodegenerative optic neuropathy characterized by retinal ganglion cell atrophy and associated visual field loss. Aside from elevated intraocular pressure, vascular pathology, among other etiologies, has long been implicated in glaucoma pathogenesis.47 Early fluorescein angiography (FA) studies have shown reduced optic nerve head fluorescence, delayed arterial-venous transit times, and diffuse hypoperfusion of glaucomatous discs.48–50 Since the development of OCTA, numerous studies have provided evidence of vascular pathology in glaucoma and the diagnostic potential of the peripapillary and macular vasculature in different stages of disease (see Bekkers and colleagues51 for comprehensive review). More recently, OCTA has also been used to evaluate the potential relationship between dementia and POAG. AD and POAG are neurodegenerative diseases associated with apoptosis of nerve cells and impairment of microvasculature.52 Both AD and POAG affect similar populations of older people and are commonly referred to as diseases of accelerated aging.53 Studies on the pathogenesis of glaucoma have shown a significant decrease in Aβ as well as an increase in tau protein levels in the vitreous body.54 These results resemble CSF protein levels in AD.55 Retinal vascular changes have also been compared between glaucoma and AD. Notably, the superficial vessels may be more impaired in POAG, whereas the vessels supplying the deeper layers of the retina are more affected in AD.56 Zabel and colleagues showed decreased vascular density in deep vascular plexus (DVP) and FAZ enlargement in AD relative to glaucoma and healthy control subjects. For the POAG group, significant changes occurred in the radial peripapillary capillary plexus (RPCP) and in the SCP, which correlated with peripapillary RNFL thickness reduction.56 These OCTA findings suggest notable patterns of vessel attenuation in the retina that may be used to discern between AD and POAG. Nevertheless, additional studies confirming and elaborating on these findings are necessary, especially given the potentially confounding effects of anti-glaucoma medication on ocular hemodynamics.56
Parkinson’s disease
Parkinson’s disease (PD) is a leading neurodegenerative disorder characterized by abnormal deposition of cytoplasmic α-synuclein (α-syn) protein inclusions leading to progressive loss of dopaminergic neurons.57 In particular, dopamine depletion following neuronal loss in the nigrostriatal pathway leads to characteristic motor symptoms in PD including bradykinesia, resting tremor, rigidity, and postural instability.58,59 However, non-motor symptoms have similarly been described in this disease including behavioral and autonomic nervous system disturbances, dementia, and ophthalmologic impairments.58–62 In fact, studies have provided significant histopathological evidence of retinal α-syn aggregation in PD.63 These retinal findings may possibly explain the visual impairments frequently observed in PD including visual acuity, color discrimination, motion perception, and contrast sensitivity.60
Several studies have provided evidence of vascular pathology in PD retina. Miri and colleagues64 quantified the perifoveal capillary network in 23 PD patients and 13 healthy controls using FA. PD patients exhibited significant reduction in FAZ area, which correlated inversely with foveal thinning. In addition, foveal thinning was highly associated with disease duration and motor impairment in PD patients.64 More recently, OCTA retinal imaging has also been applied in PD. Studies conducted by Kwapong and colleagues first described vascular pathology in 38 early PD and 28 control subjects using OCTA. Microvascular density was markedly reduced in the total 2.5-mm annular zone (TAZ) of the macula, most pronounced in the SCP, whereas the DCP was overall spared in PD patients relative to healthy controls. In addition, correlation analysis revealed a significant relationship between SCP vessel attrition and ganglion cell-inner plexiform layer (GC-IPL) thickness, suggesting a vascular contribution to retinal degeneration in PD.65
Schizophrenia
Schizophrenia, a severe mental illness characterized by psychotic symptoms and behavioral disturbances, is a leading cause of disease-related disability in the world.66 Notably, however, its pathogenesis remains unclear largely due to the difficulty of directly assessing its neuronal-level dysfunctions. Aside from psychiatric abnormalities, visual and ophthalmologic impairments from color recognition to motion perception have also been reported in schizophrenia spectrum disorders (SSDs).67 Recent studies have shown neurological features in SSD similar to AD and PD including cognitive impairment and dopamine dysregulation, respectively.68 Since the advent of OCT, there has been a renewed interest in ophthalmologic studies of schizophrenia. Intriguingly, recent reports have provided evidence of retinal ganglion cell and nerve fiber atrophy, suggesting potential ocular biomarkers for schizophrenia as a neurodegenerative disease process.68
Neuroimaging studies have illustrated impaired circulation in schizophrenic patients, suggesting vascular involvement in this disease.69–71 More recently, researchers have similarly sought to evaluate the retinal vasculature in SSD retina. Initial retinal imaging studies conducted by Meier and colleagues showed significant widening of retinal venule caliber in schizophrenia, which notably correlated with psychotic symptoms reported in adulthood as well as in childhood. The authors proposed that these vascular findings may reflect a limited brain oxygen supply, since subjects with wider venules developed schizophrenia.72,73 Several studies have also evaluated the retinal vasculature in SSD using OCTA. Kasetty and colleagues performed OCT and OCTA imaging in 19 SSD patients and eight healthy controls. While retinal thickness did not significantly differ between groups, vessel and perfusion densities of the parafoveal superficial vascular plexus (SVP) were markedly reduced in SSD patients relative to controls. Intriguingly, however, DCP perfusion density was notably greater in patients as compared to controls.74 In a prospective study of 39 SSD patients and 27 healthy controls, Asanad and colleagues75 reported a significant reduction in peripapillary SVP perfusion density temporally and increased central macular SVP vessel density in patients relative to controls. Lizano and colleagues analyzed the macular SVP in 24 SSD patients and 16 healthy controls using Swept Source OCTA. Relative to controls, Lizano and colleagues found no significant differences in vessel density in SSD patients. Notably, however, SVP vessel diameter was markedly increased in SSD. In addition, increased vessel diameter was associated with worse negative symptoms, whereas decreased vessel tortuosity was associated with reduced cognitive function.76 Additional studies in schizophrenic patients may provide important insight into disease pathogenesis and diagnosis.
Mitochondrial optic neuropathies
Mitochondrial optic neuropathies are another class of neuro-ophthalmic disease where OCTA has recently been applied in identifying vascular biomarkers. Among these include Leber’s hereditary optic neuropathy (LHON), dominant optic atrophy (DOA), and Wolfram syndrome (WS).
LHON
LHON is a rare, mitochondrial neurodegenerative disease of the optic nerve, characteristically involving bilateral vision loss and preferentially occuring in males as compared to females, having a 9:1 gender distribution ratio, respectively.77 Various studies have advanced our understanding of LHON pathogenesis in relation to the microvascular blood supply. Nikoskelainen and colleagues first provided evidence of vascular pathology in LHON. In asymptomatic patients, FA showed peripapillary microangiopathy with arteriovenous shunting in telangiectatic vessels.78–80 In acute LHON, Nikoskelainen and colleagues reported maximal dilation and rapid flow of arteries and telangiectatic vessels with reduced filling of the papillomacular capillaries. In chronic stages, FA showed diminished optic nerve head vascularity and delayed arteriovenous circulation.80 Recent applications of OCTA have expanded upon these early qualitative findings by providing more objective and precise quantitative measures of vascular pathology. Balducci and colleagues used OCTA to detect significant peripapillary microvascular changes over the disease course of LHON. Intriguingly, decreased RPCP vessel density corresponded with loss of the RGC-IPL and preceded thinning of the RNFL.81
In addition to the peripapillary region, OCTA studies have also provided evidence of pathology associated with the macular vasculature in different disease stages of LHON.82–84 OCTA in acute LHON showed increased vascular perfusion along with microangiopathic vascular telangiectasias (Figure 2), whereas subacute LHON featured vascular attrition of the FAZ, which was markedly enlarged.84 Borrelli and colleagues showed quantitative differences in the macular retinal and choroidal circulation in chronic LHON. Similar to acute and subacute LHON, vascular attenuation in chronic disease stages preferentially involved the macular region comprising the papillomacular bundle (PMB), which contains the smallest caliber retinal ganglion cell fibers that are the most vulnerable to mitochondrial dysfunction.84–86 OCTA has improved upon previous imaging technologies through enhanced visualization of the optic disc and the retinal microvasculature, non-invasively. Relative to previous subjective findings by conventional angiography, these OCTA studies provide new insights into the evolution of mitochondrial optic neuropathies and introduce the clinical utility of vascular metrics as objective, quantitative biomarkers for LHON.
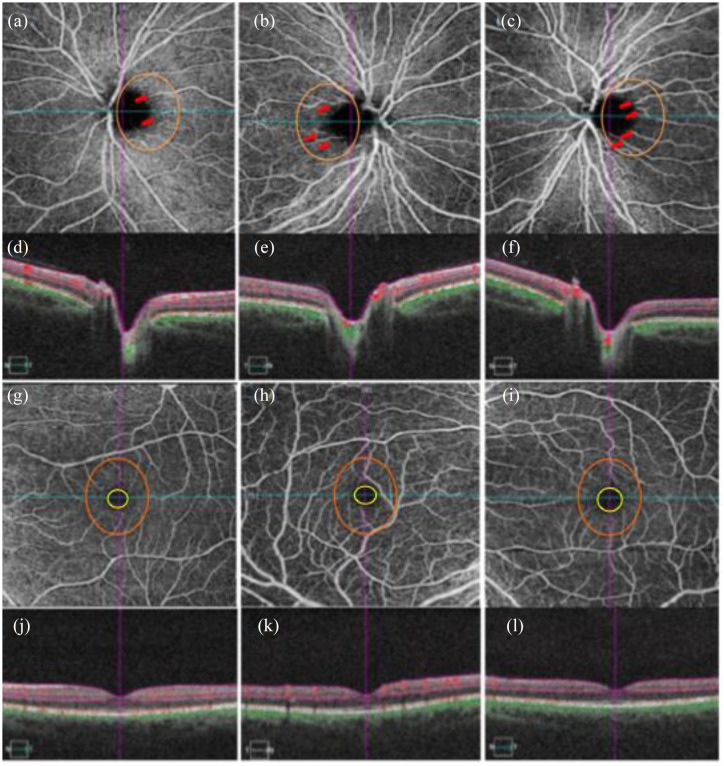
Figure 2.
Optical coherence tomography angiography (OCTA) images of the peripapillary superficial vascular plexus (SVP) (a–c) in an asymptomatic LHON mitochondrial DNA mutation 3460 (mt3460) carrier (a), and affected LHON mtDNA 3460 patient right (b) and left (c) eyes. OCT cross-sections (d–f) show overlaying retinal flow (red) on OCT reflectance (gray scale). Corresponding OCTA images (g–i) of the macular SVP in the carrier (g), and affected patient (h,i) show increasing size of the foveal avascular zone (FAZ) (yellow circle) (FAZ increase from g to i). Qualitative assessment of the parafoveal vasculature (orange circle) revealed telangiectatic blood vessels and vascular tortuosity more prominent in the patient’s more recently affected eye (h) relative to the primary affected eye (i). Such parafoveal vascular features are less apparent in the unaffected mother (a). OCT cross-sections overlaying retinal flow (red) on OCT reflectance (gray scale) (j–l) show perfusion defects for both temporal and nasal regions of the primary affected left eye (l). In contrast, the patient’s right eye revealed increased perfusion for both temporal and nasal regions relative to both the patient’s left eye (l) and the unaffected eye (j) from Asanad and colleagues.82,83
DOA
DOA is another inherited mitochondrial optic neuropathy affecting the retinal ganglion cells with similarities to LHON. DOA is most commonly associated with a mutation in OPA1, a nuclear gene on chromosome 3q28-q29, which regulates normal mitochondrial morphology.87 Among its roles, OPA1 maintains mitochondrial membrane integrity for modulating apoptosis and is also important for mitochondrial dynamics, oxidative phosphorylation, and the maintenance of mitochondrial DNA (mtDNA).88
In contrast to LHON, which typically manifests in the second and third decades of life, DOA is most often diagnosed at latency age (7–10 years old) and presents as slowly progressive and symmetric loss of vision that stops progressing at about age 20 years old. DOA patients are frequently asymptomatic in early disease stages, and children may not always complain of symptoms. Consequently, diagnostic work-up commonly follows incidental visual field findings, pertinent family history, or optic atrophy on funduscopic examination.89 DOA pathophysiology has been suggested to involve a neurodegenerative etiology. OCT in DOA characteristically shows significant reduction of the peripapillary RNFL thickness in all quadrants, although most pronounced in the temporal and inferotemporal quadrants.88,90,91 Vascular pathology in DOA had not previously been reported by conventional FA. Using OCTA, Balducci and colleagues found that the temporal networks of the peripapillary and optic nerve head microvasculature were reduced in DOA patients, which is consistent with the preferential involvement of the PMB that similarly is a hallmark feature in LHON.92,93 Intriguingly, Balducci and colleagues also showed that radial peripapillary capillary vessel density strongly correlated with both functional and structural parameters including best-corrected visual acuity, visual field testing, and RNFL thickness. These findings suggest that perfusion indices may potentially be useful for staging and monitoring the natural history of DOA.92
Further studies conducted by Martins and colleagues evaluated the macular microvascular blood supply and the link between structural and vascular changes in DOA using OCTA.94 Their analysis of the ratio between parafoveal SCP vessel density and macular ganglion cell complex thickness showed more severe relative vascular pathology. Specifically, vascular dysfunction was more pronounced in the macular region, whereas neuronal and vascular dysfunctions were equally prominent in the peripapillary region. This study also found a significant correlation between global circumpapillary vessel density and temporal RNFL thickness in DOA eyes, which further suggests a neurovascular degenerative process in disease pathogenesis.94
WS
WS, also known by the acronym DIDMOAD (diabetes insipidus, diabetes mellitus, optic atrophy, and deafness), is an autosomal recessive, progressive neurodegenerative disease. Patients with WS characteristically present with early onset diabetes mellitus and optic atrophy in the first decade of life, diabetes insipidus and deafness in the second decade, and urinary tract with neurological complications in the third decade.95 WS has been associated with mutations in the WFS1 or CISD2 (WFS2) gene, which likely lead to impaired calcium homeostasis followed by widespread cellular apoptosis. Nevertheless, there remains a limited fund of knowledge of disease pathophysiology.92 Studies have postulated that WS pathophysiology may not be solely restricted to the endoplasmic reticulum, as previously suggested, but may also include a mitochondrial-related etiology. Recent studies have provided evidence for a mitochondrial-associated etiology by demonstrating a striking similarity in the histopathological pattern of optic nerve atrophy between WS and LHON, an established mitochondrial optic neuropathy.96 Al-Till and colleagues97 previously noted mild window defects but otherwise normal fovea in WS individuals using FA. Until recently, however, vascular pathology in WS had not been characterized. Asanad and colleagues98 first described OCTA findings in WS, which showed appreciable vessel dropout in the SCP of the temporal optic nerve head and peripapillary microvasculature (Figure 3). This pattern of vascular attenuation is consistent with the preferential involvement of the small axons comprising the PMB, a hallmark feature associated with well-established mitochondrial optic neuropathies such as LHON.92 The unmyelinated retinal ganglion cell axons of the PMB are long and narrow in caliber, thus exhibiting a high surface area to volume ratio. This anatomical design with high energy demands for axonal transport make the PMB fibers most vulnerable in mitochondrial optic neuropathy.84–86 The temporal involvement in WS was also consistent with the vascular attenuation patterns seen in OCTA and laser speckle flowgraphy (LSFG) studies in DOA.99,100 In addition, OCTA showed remarkable similarities between WS and LHON with respect to peripapillary telangiectatic blood vessels and vascular tortuosity (Figure 3 (b) and (e)), which are pathognomonic findings for LHON.98,101
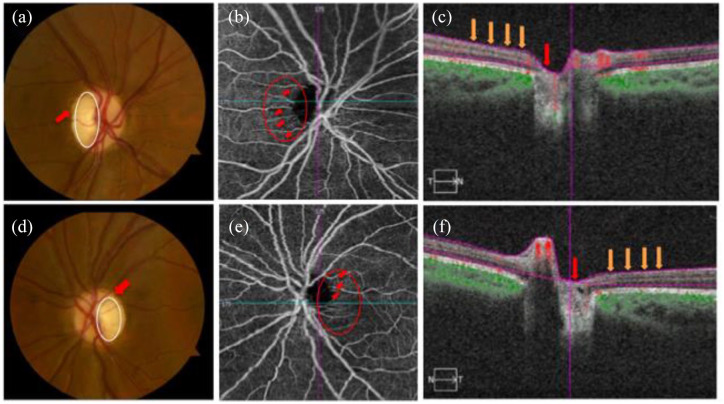
Figure 3.
Shows images of the right and left eyes in a patient with Wolfram syndrome (WS). Fundus photographs (a, d) show temporal pallor (white circle) and disc edema (red arrow) in the right (a) and left eyes (d). OCTA images of the superficial capillary plexus (b, e) demonstrate visible attenuation of the temporal microvascular networks of the optic nerve head (ONH) and peripapillary area (red circle) along with peripapillary telangiectatic blood vessels and vascular tortuosity (red arrows) for the right (b) and left eyes (e). OCT cross-sections (c, f) overlaying retinal flow (red) on OCT reflectance (gray scale) show a perfusion defect associated with ONH (red arrow) and temporal peripapillary nerve fiber layer (orange arrows) for the right (c) and left (f) eyes from Asanad and colleagues.98
Future directions
OCTA has served as a transformative tool for mapping vascular perfusion at the structural and qualitative functional levels.17,102,103 In addition to structural indices of the retinal vasculature, retinal blood flow has also been recognized as a potentially valuable biomarker for neurodegenerative optic neuropathies.40,104,105 Various methods of measuring retinal blood flow have been developed including color Doppler imaging, laser speckle imaging, Doppler OCT, and more recently OCTA and its derivatives. These imaging modalities determine retinal blood flow indirectly from surrogates of blood flow, as opposed to absolute measures of retinal blood flow.106–110 Indirect measurements of blood flow may be more prone to artifact, which may decrease the signal-to-noise ratio of blood flow measurements, possibly contributing to disagreement in vascular findings in optic neuropathies. In addition, OCTA is relatively limited by its spatial resolution and imaging speed.111,112 In turn, quantitative assessment of absolute retinal blood flow in the human retina has yet to be elucidated.112,113 Therefore, there is a critical need for an accurate and precise gold standard for quantifying human retinal blood flow to validate these current devices and to establish the use of ocular microvascular blood flow as a robust biomarker for neurodegenerative optic neuropathies.112,114 Direct observation and quantification of absolute retinal blood flow may help improve our understanding of these optic nerve diseases.
Erythrocyte-mediated angiography
Erythrocyte-mediated angiography (EMA) is a novel imaging technique which directly measures erythrocyte velocity in vivo.114,115 Following cell preparation, using a protocol previously described by Flower and colleagues,116,117 autologous indocyanine green (ICG)-labeled erythrocyte ghosts are injected into the bloodstream, providing a wide-field view of retinal blood flow in the peripapillary and macular regions (Figure 4) and individual erythrocytes are tracked over time to determine retinal blood flow (Figure 5). By measuring vessel diameter using an automated approach, flow rate can further be determined.114,115 A recent study conducted by Tracey and colleagues114 showed EMA as having an intrasession variability of about 4% and intersession variability of approximately 5%. These findings support the premise that EMA may reliably determine retinal blood flow.
Figure 4.
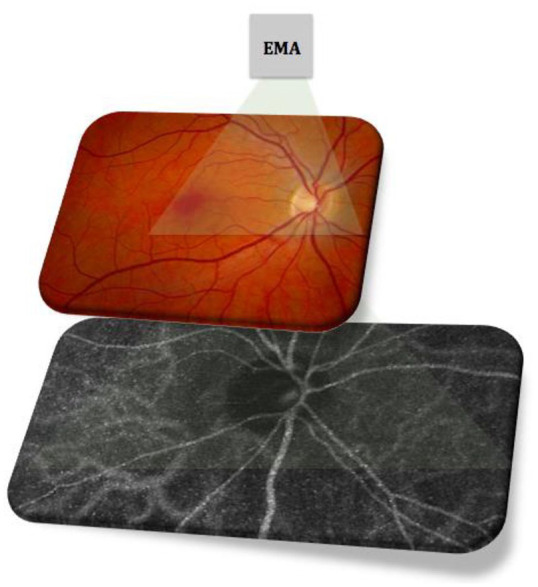
Erythrocyte-mediated angiography (EMA) providing a wide-field view of retinal blood flow in the peripapillary and macular regions using autologous ICG-labeled erythrocyte ghost cells.
Figure 5.
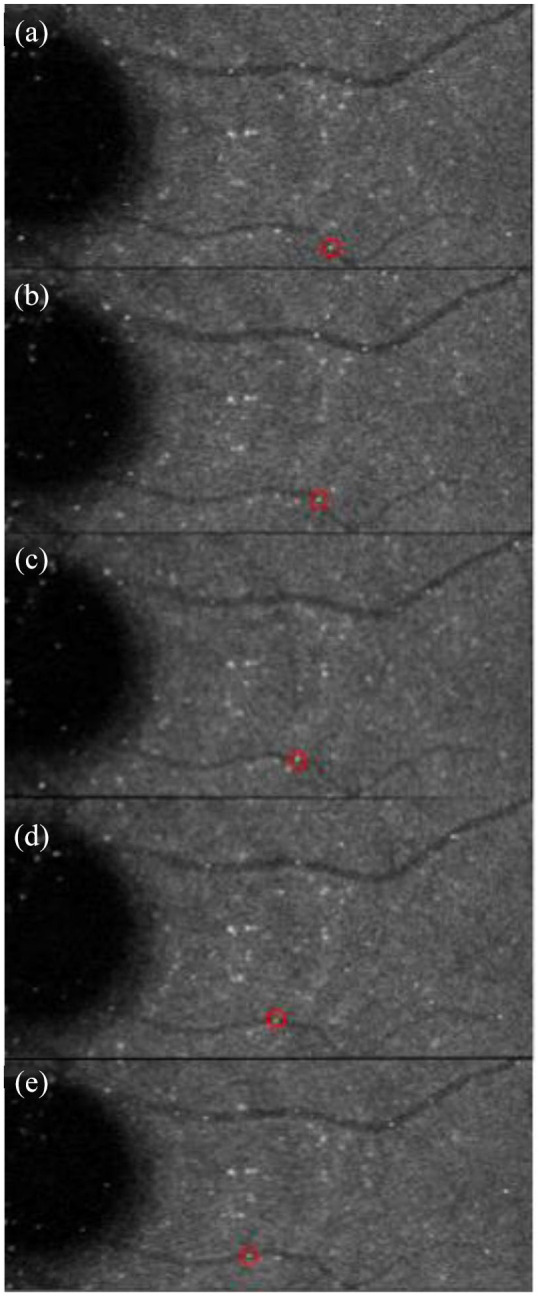
Representative image of erythrocyte tracking using erythrocyte-mediated angiography. Depicts the same erythrocyte (red circle) flowing frame-by-frame along a retinal vein (a–e). From Tracey and colleagues114 (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6934793/)
Retinal neurovascular units may underlie the regulation of retinal blood flow through neurovascular coupling (neuron-associated changes in vascular flow). Specifically, the metabolic demands of local retinal tissue are coupled with the corresponding blood supply through spontaneous rhythmic changes in vessel tonicity, also known as vasomotion. Impaired neurovascular coupling, manifesting as impaired vasomotion, reduces circulatory efficiency and may play a role in early glaucoma pathogenesis and AD.117–119 The early detection of vasomotion impairment by EMA may not only enhance our understanding of retinal blood flow dysregulation, but also may be useful as a complementary technology to OCTA. Taken together, these synergistic imaging modalities may provide new insights into the vascular pathology associated with optic neuropathies, providing more sensitive as well as objective biomarkers for predicting capillary structural changes and diagnosing disease.
Conclusions and final remarks
Recent applications of OCTA have provided valuable new insights into the pathogenesis of diseases of the optic nerve. These findings have introduced a new era of biomarkers that allows for better structure/function understanding in neuro-ophthalmology. OCTA, and the creative platforms building upon it, may together provide a foundation for evaluating the efficacy of purported therapies for neuro-ophthalmic diseases, allowing for early interventions and ultimately monitoring of disease.
Footnotes
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Samuel Asanad  https://orcid.org/0000-0002-7556-0155
https://orcid.org/0000-0002-7556-0155
Contributor Information
Samuel Asanad, Department of Ophthalmology and Visual Sciences, University of Maryland Eye Associates, University of Maryland Medical Center and University of Maryland School of Medicine, 419 W. Redwood St., Baltimore, MD 21201, USA.
Isa Mohammed, Department of Ophthalmology and Visual Sciences, University of Maryland School of Medicine, Baltimore, MD, USA.
Alfredo A. Sadun, Doheny Eye Center, Los Angeles, CA, USA; Department of Ophthalmology, David Geffen School of Medicine, University of California, Los Angeles, Los Angeles, CA, USA
Osamah J. Saeedi, Department of Ophthalmology and Visual Sciences, University of Maryland School of Medicine, Baltimore, MD, USA
References
- 1. Rabiolo A, Gelormini F, Sacconi R, et al. Comparison of methods to quantify macular and peripapillary vessel density in optical coherence tomography angiography. PLoS ONE 2018; 13: e0205773. DOI: 10.1371/journal.pone.0205773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wylegala A. Principles of OCTA and applications in clinical neurology. Curr Neurol Neurosci Rep 2018; 18: 96. DOI: 10.1007/s11910-018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nelis P, Kleffner I, Burg MC, et al. OCT-angiography reveals reduced vessel density in the deep retinal plexus of CADASIL patients. Sci Rep 2018; 8: 8148. DOI: 10.1038/s41598-018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Alzheimer’s Association. 2019 Alzheimer’s disease facts and figures. Alzheimers Dement 2019; 15: 321–87. [Google Scholar]
- 5. Pillai JA, Cummings JL. Clinical trials in predementia stages of Alzheimer disease. Med Clin North Am 2013; 97: 439–457. DOI: 10.1016/j.mcna.2013.01.002. [DOI] [PubMed] [Google Scholar]
- 6. Katz B, Rimmer S. Ophthalmologic manifestations of Alzheimer’s disease. Surv Ophthalmol 1989; 34: 31–43. DOI: 10.1016/0039-6257(89)90127-6. [DOI] [PubMed] [Google Scholar]
- 7. Javaid FZ, Brenton J, Guo L, et al. Visual and ocular manifestations of Alzheimer’s disease and their use as biomarkers for diagnosis and progression. Front Neurol 2016; 7: 55. DOI: 10.3389/fneur.2016.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Salamone G, Di Lorenzo C, Mosti S, et al. Color discrimination performance in patients with Alzheimer’s disease. Dement Geriatr Cogn Disord 2009; 27: 501–507. DOI: 10.1159/000218366. [DOI] [PubMed] [Google Scholar]
- 9. Tzekov R, Mullan M. Vision function abnormalities in Alzheimer disease. Surv Ophthalmol 2014; 59: 414–433. DOI: 10.1016/j.survophthal.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 10. Uhlmann RF, Larson EB, Koepsell TD, et al. Visual impairment and cognitive dysfunction in Alzheimer’s disease. J Gen Intern Med 1991; 6: 126–132. DOI: 10.1007/BF02598307. [DOI] [PubMed] [Google Scholar]
- 11. Blanks JC, Hinton DR, Sadun AA, et al. Retinal ganglion cell degeneration in Alzheimer’s disease. Brain Res 1989; 501: 364–372. DOI: 10.1016/0006-8993(89)90653. [DOI] [PubMed] [Google Scholar]
- 12. Blanks JC, Torigoe Y, Hinton DR, et al. Retinal pathology in Alzheimer’s disease. I. Ganglion cell loss in foveal/parafoveal retina. Neurobiol Aging 1996; 17: 377–384. DOI: 10.1016/0197-4580(96)00010-3. [DOI] [PubMed] [Google Scholar]
- 13. Hinton DR, Sadun AA, Blanks JC, et al. Optic-nerve degeneration in Alzheimer’s disease. N Engl J Med 1986; 315: 485–487. DOI: 10.1056/NEJM198608213150804. [DOI] [PubMed] [Google Scholar]
- 14. Sadun AA, Borchert M, DeVita E, et al. Assessment of visual impairment in patients with Alzheimer’s disease. Am J Ophthalmol 1987; 104: 113–120. DOI: 10.1016/0002-9394(87)90001. [DOI] [PubMed] [Google Scholar]
- 15. Bulut M, Kurtulusş; F, Gözkaya O, et al. Evaluation of optical coherence tomography angiographic findings in Alzheimer’s type dementia. Br J Ophthalmol 2018; 102: 233–237. [DOI] [PubMed] [Google Scholar]
- 16. Koronyo-Hamaoui M, Koronyo Y, Ljubimov AV, et al. Identification of amyloid plaques in retinas from Alzheimer’s patients and noninvasive in vivo optical imaging of retinal plaques in a mouse model. Neuroimage 2011; 54(Suppl. 1): S204–S217. DOI: 10.1016/j.neuroimage.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Koustenis A, Jr, Harris A, Gross J, et al. Optical coherence tomography angiography: an overview of the technology and an assessment of applications for clinical research. Br J Ophthalmol 2017; 101: 16–20. DOI: 10.1136/bjophthalmol-2016-309389. [DOI] [PubMed] [Google Scholar]
- 18. La Morgia C, Ross-Cisneros FN, Koronyo Y, et al. Melanopsin retinal ganglion cell loss in Alzheimer disease. Ann Neurol 2016; 79: 90–109. DOI: 10.1002/ana.24548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brown WR, Thore CR. Review: cerebral microvascular pathology in ageing and neurodegeneration. Neuropathol Appl Neurobiol 2011; 37: 56–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Berisha F, Feke GT, Trempe CL, et al. Retinal abnormalities in early Alzheimer’s disease. Invest Ophthalmol Vis Sci 2007; 48: 2285–2289. DOI: 10.1167/iovs.06-1029. [DOI] [PubMed] [Google Scholar]
- 21. Hunter JM, Kwan J, Malek-Ahmadi M, et al. Morphological and pathological evolution of the brain microcirculation in aging and Alzheimer’s disease. PLoS ONE 2012; 7: e36893. DOI: 10.1371/journal.pone.0036893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Armstrong R, Kergoat H. Oculo-visual changes and clinical considerations affecting older patients with dementia. Ophthalmic Physiol Opt 2015; 35: 352–376. DOI: 10.1111/opo.12220. [DOI] [PubMed] [Google Scholar]
- 23. Cheung CY, Ong YT, Ikram MK, et al. Microvascular network alterations in the retina of patients with Alzheimer’s disease. Alzheimers Dement 2014; 10: 135–142. DOI: 10.1016/j.jalz.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 24. Einarsdottir AB, Hardarson SH, Kristjansdottir JV, et al. Retinal oximetry imaging in Alzheimer’s disease. J Alzheimers Dis 2016; 49: 79–83. DOI: 10.3233/JAD-150457. [DOI] [PubMed] [Google Scholar]
- 25. Frost S, Martins RN, Kanagasingam Y. Ocular biomarkers for early detection of Alzheimer’s disease. J Alzheimers Dis 2010; 22: 1–16. DOI: 10.3233/JAD-2010-100819. [DOI] [PubMed] [Google Scholar]
- 26. Hart NJ, Koronyo Y, Black KL, et al. Ocular indicators of Alzheimer’s: exploring disease in the retina. Acta Neuropathol 2016; 132: 767–787. DOI: 10.1007/s00401-016-1613-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stefansson E, Olafsdottir OB, Einarsdottir AB, et al. Retinal oximetry discovers novel biomarkers in retinal and brain diseases. Invest Ophthalmol Vis Sci 2017; 58: BIO227–BIO233. DOI: 10.1167/iovs.17-21776. [DOI] [PubMed] [Google Scholar]
- 28. Tsai CS, Ritch R, Schwartz B, et al. Optic nerve head and nerve fiber layer in Alzheimer’s disease. Arch Ophthalmol 1991; 109: 199–204. DOI: 10.1001/archopht.1991.01080020045040. [DOI] [PubMed] [Google Scholar]
- 29. Williams MA, McGowan AJ, Cardwell CR, et al. Retinal microvascular network attenuation in Alzheimer’s disease. Alzheimers Dement (Amst) 2015; 1: 229–235. DOI: 10.1016/j.dadm.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Feke GT, Hyman BT, Stern RA, et al. Retinal blood flow in mild cognitive impairment and Alzheimer’s disease. Alzheimers Dement (Amst) 2015; 1: 144–151. DOI: 10.1016/j.dadm.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Schultz N, Byman E. The Netherlands Brain Bank, et al. Levels of retinal IAPP are altered in Alzheimer’s disease patients and correlate with vascular changes and hippocampal IAPP levels. Neurobiol Aging 2018; 69: 94–101. DOI: 10.1016/j.neurobiolaging.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 32. Dorr A, Sahota B, Chinta LV, et al. Amyloid-beta-dependent compromise of microvascular structure and function in a model of Alzheimer’s disease. Brain 2012; 135: 3039–3050. DOI: 10.1093/brain/aws243. [DOI] [PubMed] [Google Scholar]
- 33. Jiang H, Wei Y, Shi Y, et al. Altered macular microvasculature in mild cognitive impairment and Alzheimer disease. J Neuroophthalmol 2018; 38: 292–298. DOI: 10.1097/WNO.0000000000000580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Arevalo-Rodriguez I, Smailagic N, Roque IFM, et al. Mini-Mental State Examination (MMSE) for the detection of Alzheimer’s disease and other dementias in people with mild cognitive impairment (MCI). Cochrane Database Syst Rev 2015; 2015: CD010783. DOI: 10.1002/14651858.CD010783.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tsoi KK, Chan JY, Hirai HW, et al. Cognitive tests to detect dementia: a systematic review and meta-analysis. JAMA Intern Med 2015; 175: 1450–1458. DOI: 10.1001/jamainternmed.2015.2152. [DOI] [PubMed] [Google Scholar]
- 36. Yoon SP, Grewal DS, Thompson AC, et al. Retinal microvascular and neurodegenerative changes in Alzheimer’s disease and mild cognitive impairment compared with control participants. Ophthalmol Retina 2019; 3: 489–499. DOI: 10.1016/j.oret.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chan VTT, Sun Z, Tang S, et al. Spectral-domain OCT measurements in Alzheimer’s disease: a systematic review and meta-analysis. Ophthalmology 2019; 126: 497–510. DOI: 10.1016/j.ophtha.2018.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lad EM, Mukherjee D, Stinnett SS, et al. Evaluation of inner retinal layers as biomarkers in mild cognitive impairment to moderate Alzheimer’s disease. PLoS ONE 2018; 13: e0192646. DOI: 10.1371/journal.pone.0192646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Leite MT, Rao HL, Weinreb RN, et al. Agreement among spectral-domain optical coherence tomography instruments for assessing retinal nerve fiber layer thickness. Am J Ophthalmol 2011; 151: 85–92. DOI: 10.1016/j.ajo.2010.06.041. [DOI] [PubMed] [Google Scholar]
- 40. Larrieu S, Letenneur L, Orgogozo JM, et al. Incidence and outcome of mild cognitive impairment in a population-based prospective cohort. Neurology 2002; 59: 1594–1599. DOI: 10.1212/01.wnl.0000034176.07159.f8. [DOI] [PubMed] [Google Scholar]
- 41. Querques G, Borrelli E, Sacconi R, et al. Functional and morphological changes of the retinal vessels in Alzheimer’s disease and mild cognitive impairment. Sci Rep 2019; 9: 63. DOI: 10.1038/s41598-018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tapiola T, Alafuzoff I, Herukka SK, et al. Cerebrospinal fluid {beta}-amyloid 42 and tau proteins as biomarkers of Alzheimer-type pathologic changes in the brain. Arch Neurol 2009; 66: 382–389. DOI: 10.1001/archneurol.2008.596. [DOI] [PubMed] [Google Scholar]
- 43. Dubois B, Padovani A, Scheltens P, et al. Timely diagnosis for Alzheimer’s disease: a literature review on benefits and challenges. J Alzheimers Dis 2016; 49: 617–631. DOI: 10.3233/JAD-150692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Amieva H, Le Goff M, Millet X, et al. Prodromal Alzheimer’s disease: successive emergence of the clinical symptoms. Ann Neurol 2008; 64: 492–498. DOI: 10.1002/ana.21509. [DOI] [PubMed] [Google Scholar]
- 45. O’Bryhim BE, Apte RS, Kung N, et al. Association of preclinical Alzheimer disease with optical coherence tomographic angiography findings. JAMA Ophthalmol 2018; 136: 1242–1248. DOI: 10.1001/jamaophthalmol.2018.3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. den Haan J, van de Kreeke JA, van Berckel BN, et al. Is retinal vasculature a biomarker in amyloid proven Alzheimer’s disease? Alzheimers Dement (Amst) 2019; 11: 383–391. DOI: 10.1016/j.dadm.2019.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Flaxman SR, Bourne RRA, Resnikoff S, et al. Global causes of blindness and distance vision impairment 1990-2020: a systematic review and meta-analysis. Lancet Glob Health 2017; 5: e1221–e1234. DOI: 10.1016/S2214-109X(17)30393-5. [DOI] [PubMed] [Google Scholar]
- 48. Hitchings RA, Spaeth GL. Fluorescein angiography in chronic simple and low-tension glaucoma. Br J Ophthalmol 1977; 61: 126–132. DOI: 10.1136/bjo.61.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. O’Brart DP, de Souza Lima M, Bartsch DU, et al. Indocyanine green angiography of the peripapillary region in glaucomatous eyes by confocal scanning laser ophthalmoscopy. Am J Ophthalmol 1997; 123: 657–666. DOI: 10.1016/s0002-9394(14)71078-5. [DOI] [PubMed] [Google Scholar]
- 50. Schwartz B, Rieser JC, Fishbein SL. Fluorescein angiographic defects of the optic disc in glaucoma. Arch Ophthalmol 1977; 95: 1961–1974. DOI: 10.1001/archopht.1977.04450110055002. [DOI] [PubMed] [Google Scholar]
- 51. Bekkers A, Borren N, Ederveen V, et al. Microvascular damage assessed by optical coherence tomography angiography for glaucoma diagnosis: a systematic review of the most discriminative regions. Acta Ophthalmol. Epub ahead of print 16 March 2020. DOI: 10.1111/aos.14392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bayer AU, Keller ON, Ferrari F, et al. Association of glaucoma with neurodegenerative diseases with apoptotic cell death: Alzheimer’s disease and Parkinson’s disease. Am J Ophthalmol 2002; 133: 135–137. DOI: 10.1016/s0002-9394(01)01196-5. [DOI] [PubMed] [Google Scholar]
- 53. Sadun AA, Asanad S. The eye in Alzheimer’s disease. Ophthalmology 2019; 126: 511–512. DOI: 10.1016/j.ophtha.2018.10.001. [DOI] [PubMed] [Google Scholar]
- 54. Yoneda S, Hara H, Hirata A, et al. Vitreous fluid levels of beta-amyloid((1-42)) and tau in patients with retinal diseases. Jpn J Ophthalmol 2005; 49: 106–108. DOI: 10.1007/s10384-004-0156-x. [DOI] [PubMed] [Google Scholar]
- 55. Engelborghs S, De Vreese K, Van de Casteele T, et al. Diagnostic performance of a CSF-biomarker panel in autopsy-confirmed dementia. Neurobiol Aging 2008; 29: 1143–1159. DOI: 10.1016/j.neurobiolaging.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 56. Zabel P, Kaluzny JJ, Wilkosc-Debczynska M, et al. Comparison of retinal microvasculature in patients with Alzheimer’s disease and primary open-angle glaucoma by optical coherence tomography angiography. Invest Ophthalmol Vis Sci 2019; 60: 3447–3455. DOI: 10.1167/iovs.19-27028. [DOI] [PubMed] [Google Scholar]
- 57. Nussbaum RL, Ellis CE. Alzheimer’s disease and Parkinson’s disease. N Engl J Med 2003; 348: 1356–1364. DOI: 10.1056/NEJM2003ra020003. [DOI] [PubMed] [Google Scholar]
- 58. Fahn S. Description of Parkinson’s disease as a clinical syndrome. Ann N Y Acad Sci 2003; 991: 1–14. DOI: 10.1111/j.1749-6632.2003.tb07458.x. [DOI] [PubMed] [Google Scholar]
- 59. Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 2015; 30: 1591–1601. DOI: 10.1002/mds.26424. [DOI] [PubMed] [Google Scholar]
- 60. Archibald NK, Clarke MP, Mosimann UP, et al. The retina in Parkinson’s disease. Brain 2009; 132: 1128–1145. DOI: 10.1093/brain/awp068. [DOI] [PubMed] [Google Scholar]
- 61. Archibald NK, Clarke MP, Mosimann UP, et al. Retinal thickness in Parkinson’s disease. Parkinsonism Relat Disord 2011; 17: 431–436. DOI: 10.1016/j.parkreldis.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 62. Nowacka B, Lubinski W, Honczarenko K, et al. Ophthalmological features of Parkinson disease. Med Sci Monit 2014; 20: 2243–2249. DOI: 10.12659/MSM.890861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bodis-Wollner I, Kozlowski PB, Glazman S, et al. Alpha-synuclein in the inner retina in Parkinson disease. Ann Neurol 2014; 75: 964–966. DOI: 10.1002/ana.24182. [DOI] [PubMed] [Google Scholar]
- 64. Miri S, Glazman S, Mylin L, et al. A combination of retinal morphology and visual electrophysiology testing increases diagnostic yield in Parkinson’s disease. Parkinsonism Relat Disord 2016; 22(Suppl. 1): S134–S137. DOI: 10.1016/j.parkreldis.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 65. Kwapong WR, Ye H, Peng C, et al. Retinal microvascular impairment in the early stages of Parkinson’s disease. Invest Ophthalmol Vis Sci 2018; 59: 4115–4122. DOI: 10.1167/iovs.17-23230. [DOI] [PubMed] [Google Scholar]
- 66. World Health Organization. Mental health report 2001—mental health: new understanding, new hope, https://apps.who.int/iris/handle/10665/42390
- 67. Gracitelli CP, Abe RY, Diniz-Filho A, et al. Ophthalmology issues in schizophrenia. Curr Psychiatry Rep 2015; 17: 28. DOI: 10.1007/s11920-015-0569-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Silverstein SM, Rosen R. Schizophrenia and the eye. Schizophr Res Cogn 2015; 2: 46–55. DOI: 10.1016/j.scog.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Andreasen NC, Calarge CA, O’Leary DS. Theory of mind and schizophrenia: a positron emission tomography study of medication-free patients. Schizophr Bull 2008; 34: 708–719. DOI: 10.1093/schbul/sbn034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Curtis CE, Iacono WG, Beiser M. Relationship between nailfold plexus visibility and clinical, neuropsychological, and brain structural measures in schizophrenia. Biol Psychiatry 1999; 46: 102–109. DOI: 10.1016/s0006-3223(98)00363. [DOI] [PubMed] [Google Scholar]
- 71. Uranova NA, Zimina IS, Vikhreva OV, et al. Ultrastructural damage of capillaries in the neocortex in schizophrenia. World J Biol Psychiatry 2010; 11: 567–578. DOI: 10.3109/15622970903414188. [DOI] [PubMed] [Google Scholar]
- 72. Meier MH, Gillespie NA, Hansell NK, et al. Retinal microvessels reflect familial vulnerability to psychotic symptoms: a comparison of twins discordant for psychotic symptoms and controls. Schizophr Res 2015; 164: 47–52. DOI: 10.1016/j.schres.2015.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Meier MH, Shalev I, Moffitt TE, et al. Microvascular abnormality in schizophrenia as shown by retinal imaging. Am J Psychiatry 2013; 170: 1451–1459. DOI: 10.1176/appi.ajp.2013.13020234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Kasetty M, Lizano P, Zeng R, et al. A pilot study of chorioretinal structural and vascular abnormalities in psychosis, 2019, https://www.issoct.com/a-pilot-study-of-chorioretinal-structural-and-vascular-abnormalities-in-psychosis-895/
- 75. Asanad S, Addis H, Chen S, et al. Retinal thickness and vascular pathology as ocular biomarkers for schizophrenia: morphometric analysis of the peripapillary and macular regions using OCT and OCTA in vivo. Invest Ophthalmol Vis Sci 2020; 61: 5105. [Google Scholar]
- 76. Lizano P, Bannai D, Adhan I, et al. Superficial retinal vascular abnormalities in schizophrenia as shown by Swept Source OCT-angiography: a preliminary study. Biol Psychiatry 2020; 87: S445. [Google Scholar]
- 77. Leber T. About hereditary and congenital optic nerve disorders. Albrect Von Graefes Arch Clin Exp Ophthalmol 1871; 17: 249–291. [Google Scholar]
- 78. Nikoskelainen E, Hoyt WF, Nummelin K. Ophthalmoscopic findings in Leber’s hereditary optic neuropathy. I. Fundus findings in asymptomatic family members. Arch Ophthalmol 1982; 100: 1597–1602. DOI: 10.1001/archopht.1982.01030040575003. [DOI] [PubMed] [Google Scholar]
- 79. Nikoskelainen E, Hoyt WF, Nummelin K. Ophthalmoscopic findings in Leber’s hereditary optic neuropathy. II. The fundus findings in the affected family members. Arch Ophthalmol 1983; 101: 1059–1068. DOI: 10.1001/archopht.1983.01040020061011. [DOI] [PubMed] [Google Scholar]
- 80. Nikoskelainen E, Hoyt WF, Nummelin K, et al. Fundus findings in Leber’s hereditary optic neuroretinopathy. III. Fluorescein angiographic studies. Arch Ophthalmol 1984; 102: 981–989. DOI: 10.1001/archopht.1984.01040030783017. [DOI] [PubMed] [Google Scholar]
- 81. Balducci N, Cascavilla ML, Ciardella A, et al. Peripapillary vessel density changes in Leber’s hereditary optic neuropathy: a new biomarker. Clin Exp Ophthalmol 2018; 46: 1055–1062. DOI: 10.1111/ceo.13326. [DOI] [PubMed] [Google Scholar]
- 82. Asanad S, Meer E, Fantini M, et al. Leber’s hereditary optic neuropathy: shifting our attention to the macula. Am J Ophthalmol Case Rep 2019; 13: 13–15. DOI: 10.1016/j.ajoc.2018.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Asanad S, Meer E, Tian JJ, et al. Leber’s hereditary optic neuropathy: severe vascular pathology in a severe primary mutation. Intractable Rare Dis Res 2019; 8: 52–55. DOI: 10.5582/irdr.2018.01126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Borrelli E, Balasubramanian S, Triolo G, et al. Topographic macular microvascular changes and correlation with visual loss in chronic Leber’s hereditary optic neuropathy. Am J Ophthalmol 2018; 192: 217–228. DOI: 10.1016/j.ajo.2018.05.029. [DOI] [PubMed] [Google Scholar]
- 85. Pan BX, Ross-Cisneros FN, Carelli V, et al. Mathematically modeling the involvement of axons in Leber’s hereditary optic neuropathy. Invest Ophthalmol Vis Sci 2012; 53: 7608–7617. DOI: 10.1167/iovs.12-10452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Sadun AA, La Morgia C, Carelli V. Mitochondrial optic neuropathies: additional facts and concepts—response. Clin Exp Ophthalmol 2014; 42: 207–208. DOI: 10.1111/ceo.12147. [DOI] [PubMed] [Google Scholar]
- 87. Cornille K, Milea D, Amati-Bonneau P, et al. Reversible optic neuropathy with OPA1 exon 5b mutation. Ann Neurol 2008; 63: 667–671. DOI: 10.1002/ana.21376. [DOI] [PubMed] [Google Scholar]
- 88. Barboni P, Savini G, Cascavilla ML, et al. Early macular retinal ganglion cell loss in dominant optic atrophy: genotype-phenotype correlation. Am J Ophthalmol 2014; 158: 628–636. DOI: 10.1016/j.ajo.2014.05.034. [DOI] [PubMed] [Google Scholar]
- 89. Asanad S, Tian JJ, Frousiakis S, et al. Optical coherence tomography of the retinal ganglion cell complex in Leber’s hereditary optic neuropathy and dominant optic atrophy. Curr Eye Res 2019; 44: 638–644. DOI: 10.1080/02713683.2019.1567792. [DOI] [PubMed] [Google Scholar]
- 90. Corajevic N, Larsen M, Ronnback C. Thickness mapping of individual retinal layers and sectors by Spectralis SD-OCT in Autosomal Dominant Optic Atrophy. Acta Ophthalmol 2018; 96: 251–256. DOI: 10.1111/aos.13588. [DOI] [PubMed] [Google Scholar]
- 91. Kim TW, Hwang JM. Stratus OCT in dominant optic atrophy: features differentiating it from glaucoma. J Glaucoma 2007; 16: 655–658. DOI: 10.1097/IJG.0b013e31804d23aa. [DOI] [PubMed] [Google Scholar]
- 92. Balducci N, Ciardella A, Gattegna R, et al. Optical coherence tomography angiography of the peripapillary retina and optic nerve head in dominant optic atrophy. Mitochondrion 2017; 36: 60–65. DOI: 10.1016/j.mito.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 93. Carelli V, Ross-Cisneros FN, Sadun AA. Mitochondrial dysfunction as a cause of optic neuropathies. Prog Retin Eye Res 2004; 23: 53–89. DOI: 10.1016/j.preteyeres.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 94. Martins A, Rodrigues TM, Soares M, et al. Peripapillary and macular morpho-vascular changes in patients with genetic or clinical diagnosis of autosomal dominant optic atrophy: a case-control study. Graefes Arch Clin Exp Ophthalmol 2019; 257: 1019–1027. DOI: 10.1007/s00417-019-04267-5. [DOI] [PubMed] [Google Scholar]
- 95. Gunn T, Bortolussi R, Little JM, et al. Juvenile diabetes mellitus, optic atrophy, sensory nerve deafness, and diabetes insipidus: a syndrome. J Pediatr 1976; 89: 565–570. DOI: 10.1016/s0022-3476(76)80387. [DOI] [PubMed] [Google Scholar]
- 96. Ross-Cisneros FN, Pan BX, Silva RA, et al. Optic nerve histopathology in a case of Wolfram syndrome: a mitochondrial pattern of axonal loss. Mitochondrion 2013; 13: 841–845. DOI: 10.1016/j.mito.2013.05.013. [DOI] [PubMed] [Google Scholar]
- 97. Al-Till M, Jarrah NS, Ajlouni KM. Ophthalmologic findings in fifteen patients with Wolfram syndrome. Eur J Ophthalmol 2002; 12: 84–88. DOI: 10.1177/112067210201200202. [DOI] [PubMed] [Google Scholar]
- 98. Asanad S, Wu J, Nassisi M, et al. Optical coherence tomography-angiography in Wolfram syndrome: a mitochondrial etiology in disease pathophysiology. Can J Ophthalmol 2019; 54: e27–e30. DOI: 10.1016/j.jcjo.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 99. Granse L, Bergstrand I, Thiselton D, et al. Electrophysiology and ocular blood flow in a family with dominant optic nerve atrophy and a mutation in the OPA1 gene. Ophthalmic Genet 2003; 24: 233–245. DOI: 10.1076/opge.24.4.233.17230. [DOI] [PubMed] [Google Scholar]
- 100. Inoue M, Himori N, Kunikata H, et al. The reduction of temporal optic nerve head microcirculation in autosomal dominant optic atrophy. Acta Ophthalmol 2016; 94: e580–e585. DOI: 10.1111/aos.12999. [DOI] [PubMed] [Google Scholar]
- 101. Meyerson C, Van Stavern G, McClelland C. Leber hereditary optic neuropathy: current perspectives. Clin Ophthalmol 2015; 9: 1165–1176. DOI: 10.2147/OPTH.S62021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Ang M, Tan ACS, Cheung CMG, et al. Optical coherence tomography angiography: a review of current and future clinical applications. Graefes Arch Clin Exp Ophthalmol 2018; 256: 237–245. DOI: 10.1007/s00417-017-3896-2. [DOI] [PubMed] [Google Scholar]
- 103. Gu B, Wang X, Twa MD, et al. Noninvasive in vivo characterization of erythrocyte motion in human retinal capillaries using high-speed adaptive optics near-confocal imaging. Biomed Opt Express 2018; 9: 3653–3677. DOI: 10.1364/BOE.9.003653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Wei X, Balne PK, Meissner KE, et al. Assessment of flow dynamics in retinal and choroidal microcirculation. Surv Ophthalmol 2018; 63: 646–664. DOI: 10.1016/j.survophthal.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 105. Nicolela MT, Walman BE, Buckley AR, et al. Ocular hypertension and primary open-angle glaucoma: a comparative study of their retrobulbar blood flow velocity. J Glaucoma 1996; 5: 308–310. [PubMed] [Google Scholar]
- 106. de Carlo TE, Romano A, Waheed NK, et al. A review of optical coherence tomography angiography (OCTA). Int J Retina Vitreous 2015; 1: 5. DOI: 10.1186/s40942-015-0005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Guan K, Hudson C, Flanagan JG. Variability and repeatability of retinal blood flow measurements using the Canon Laser Blood Flowmeter. Microvasc Res 2003; 65: 145–151. DOI: 10.1016/s0026-2862(03)00007-4. [DOI] [PubMed] [Google Scholar]
- 108. Mohindroo C, Ichhpujani P, Kumar S. Current imaging modalities for assessing ocular blood flow in glaucoma. J Curr Glaucoma Pract 2016; 10: 104–112. DOI: 10.5005/jp-journals-10008-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Rege A, Cunningham SI, Liu Y, et al. Noninvasive assessment of retinal blood flow using a novel handheld laser speckle contrast imager. Transl Vis Sci Technol 2018; 7: 7. DOI: 10.1167/tvst.7.6.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Spaide RF, Fujimoto JG, Waheed NK, et al. Optical coherence tomography angiography. Prog Retin Eye Res 2018; 64: 1–55. DOI: 10.1016/j.preteyeres.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Lee B, Novais EA, Waheed NK, et al. En face Doppler optical coherence tomography measurement of total retinal blood flow in diabetic retinopathy and diabetic macular edema. JAMA Ophthalmol 2017; 135: 244–251. DOI: 10.1001/jamaophthalmol.2016.5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Zhu J, Merkle CW, Bernucci MT, et al. Can OCT angiography be made a quantitative blood measurement tool? Appl Sci (Basel) 2017; 7: 687. DOI: 10.3390/app7070687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Borrelli E, Sadda SR, Uji A, et al. Pearls and pitfalls of optical coherence tomography angiography imaging: a review. Ophthalmol Ther 2019; 8: 215–226. DOI: 10.1007/s40123-019-0178-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Tracey BM, Mayo LN, Le CT, et al. Measurement of retinal microvascular blood velocity using erythrocyte mediated velocimetry. Sci Rep 2019; 9: 20178. DOI: 10.1038/s41598-019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Wang D, Haytham A, Mayo L, et al. Automated retinal microvascular velocimetry based on erythrocyte mediated angiography. Biomed Opt Express 2019; 10: 3681–3697. DOI: 10.1364/BOE.10.003681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Flower R, Peiretti E, Magnani M, et al. Observation of erythrocyte dynamics in the retinal capillaries and choriocapillaris using ICG-loaded erythrocyte ghost cells. Invest Ophthalmol Vis Sci 2008; 49: 5510–5516. DOI: 10.1167/iovs.07-1504. [DOI] [PubMed] [Google Scholar]
- 117. Flower RW, Kling R. Observation and characterization of microvascular vasomotion using erythrocyte mediated ICG angiography (EM-ICG-A). Microvasc Res 2017; 113: 78–87. DOI: 10.1016/j.mvr.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 118. O’Brien JT. Vascular cognitive impairment. Am J Geriatr Psychiatry 2006; 14: 724–733. DOI: 10.1097/01.JGP.0000231780.44684.7e. [DOI] [PubMed] [Google Scholar]
- 119. Li Y, Xie L, Huang T, et al. Aging neurovascular unit and potential role of DNA damage and repair in combating vascular and neurodegenerative disorders. Front Neurosci 2019; 13: 778. DOI: 10.3389/fnins.2019.00778. [DOI] [PMC free article] [PubMed] [Google Scholar]