Abstract
Dopamine-receptor blocking agent-associated akathisia (DRBA-A) is an adverse effect that can significantly limit the use of these important medications for the treatment of a variety of psychiatric diseases, yet there is no unifying theory regarding its pathophysiology. This knowledge gap limits clinicians’ ability to effectively manage DRBA-A and mitigate negative outcomes in an already vulnerable patient population. Based on a review of the current literature on the subject, it is hypothesized that dopaminergic and noradrenergic signaling is perturbed in DRBA-A. Accordingly, it is proposed that the optimal agent to manage this extrapyramidal symptom should increase dopamine signaling in the affected areas of the brain and counteract compensatory noradrenergic signaling via antagonism of adrenergic or serotonergic receptors.
Keywords: akathisia, antipsychotic, dopamine receptor blocking agent, extrapyramidal symptom, mechanism, treatment
Introduction
In their 1977 publication, psychiatrists Robert Belmaker and David Wald described their experience after receiving a single dose of 5 mg intravenous haloperidol. They reported experiencing a myriad of debilitating symptoms, including anxiety and “profound inner restlessness” that rendered them unable to work for more than 36 hours.1 This anecdote clearly demonstrates the consequences of the common yet often underappreciated side effect of dopamine receptor blocking agents (DRBAs) known as akathisia.
Akathisia is a psychomotor syndrome involving subjective feelings of agitation and dysphoria, accompanied by repetitive and/or purposeless movement and symptoms of cognitive dysfunction such as selective attention deficit, perceptual disorder, and impaired coping responses.2–4 Akathisia may develop at different time points during treatment with DRBAs. Acute akathisia has a relatively rapid onset and short duration, and is associated with intense dysphoria.5 Chronic akathisia persists beyond 6 months and is generally associated with less severe feelings of restlessness than during the acute phase.5 This discussion will focus on acute and chronic akathisia as opposed to tardive akathisia, which is more closely related to tardive dyskinesia and may develop via a different mechanism.5
Akathisia can be detected using the Barnes Akathisia-Rating Scale (BARS),6 yet it is still frequently overlooked or misdiagnosed.5,7–9 Symptoms can develop soon after DRBA administration, and may continue despite intervention. Patients often experience the effects of DRBA-associated akathisia (DRBA-A) within days to weeks of taking the medication, and lingering symptoms can persist even after a dose reduction.10 The effects of DRBA-A can greatly impact daily functioning and wellbeing. Akathisia severity was found to be negatively correlated with quality of life in patients with schizophrenia,11 and those with DRBA-A demonstrated significantly lower quality of life than those who regularly took DRBAs without experiencing akathisia.12 Other significant consequences of DRBA-A include treatment non-adherence,13 worsening psychiatric symptoms,14–16 and suicidality.17–20 Though the rates of DRBA-A are high,21 data on morbidity and mortality is scarce.
Discussion
Current understanding of pathophysiology
DRBAs block dopamine signaling via dopamine D2 receptors. In specific areas of the brain, this dopamine receptor antagonism confers a therapeutic effect, but in the striatum it can trigger extrapyramidal symptoms (EPS) such as akathisia.22 D2 receptor occupancy is believed to be paramount in understanding the development of DRBA-related EPS, which also includes dystonia, parkinsonism, and tardive dyskinesia.23–26 It has been observed that when > 80% of these receptors are occupied by DRBAs, this risk of EPS substantially increases.27–29 Along these lines, genotyping results among patients with schizophrenia have revealed an increased rate of DRBA-A with the use of second generation agents in those possessing a genetic variation associated with reduced striatal dopamine D2 receptor density,30 which would allow for this occupancy threshold to be surpassed more readily. Other patient-specific risk factors for DRBA-A include young age, male gender, and concomitant substance use, while medication-related factors such as the use of high potency DRBAs, rapid DRBA dose escalation, and DRBA polytherapy can also increase the risk of akathisia (Table 1).
Table 1.
Description of different types of EPS and other movement disorders that can be associated with the use of DRBA.
| Akathisia8,23,31–40 | Dystonia29,33–36,41–43 | Parkinsonism23,29,33–36,44 | Tardive Dyskinesia29,33–36,41,45–49 | |
|---|---|---|---|---|
| Description | Feeling of internal restlessness, pacing | Sustained abnormal postures and muscle spasm | Bradykinesia, tremor, rigidity, postural instability | Choreic or stereotypic repetitive movements (often orofacial) |
| Prevalence range (first-generation) |
5–75% | 2–90% | 30% | 20–32% |
| Prevalence range (second-generation) | 1–27% | 1–14% | 2–33% | 2–13% |
| Onset | Hours to days | Hours to days | Weeks | Months to years |
| Known risk factors | • Young age • Male gender • Parkinsonism • Bipolar depression • Substance use • Brain damage • Palliative care • High potency DRBAs • Naïve to DRBAs • Rapid DRBA dose increase • DRBA polytherapy • Parenteral route of administration • Abrupt DRBA discontinuation |
• Young age • Male gender • Dystonia (personal or family history) • Substance use |
• Old age • Female gender • Acute/early EPS • Cognitive deficit |
• Old age • Female gender • Non-Caucasian race • Acute/early EPS • Negative symptoms • Affective disorder • Substance use • Brain damage • Diabetes mellitus • Past exposure to first-generation DRBAs • Past exposure to anticholinergic medications • Longer DRBA treatment duration |
| Common Pharmacological Treatment | • Propranolol 10–40 mg BID • Benztropine 1–2 mg BID |
• Benztropine 1–2 mg PO or IM • Diphenhydramine 25–50 mg PO or IM • Trihexyphenidyl 5–15 mg divided 3–4 times per day |
• Benztropine 1–2 mg BID • Trihexyphenidyl 5–15 mg divided 3–4 times per day • Amantadine 100 mg 2–3 times per day |
• Valbenazine 40–80 mg daily • Deutetrabenazine 6 mg BID |
This table was compiled using information from resources referenced throughout the manuscript. These references are cited in the column header. Prevalence ranges are based on the lowest and highest observed prevalence reported in the literature.
BID, twice daily; DRBAs, dopamine receptor blocking agents; EPS, extrapyramidal symptoms; IM, intramuscular injection; PO, by mouth.
It is generally believed that the first generation of DRBA medications developed in this class (e.g., chlorpromazine, haloperidol, perphenazine) confer a greater risk of EPS than second-generation agents.23,26,31,50 First-generation DRBAs are high potency striatal D2 receptor antagonists, whereas second-generation agents bind more loosely and can be rapidly displaced from D2 by intrinsic dopamine, thereby ameliorating movement symptoms.51 Nigrostriatal dopamine D2 receptor affinity and occupancy, as well as upregulation of the D2 receptor, have been correlated with the severity of EPS.52 In vivo neuroimaging studies support the association between EPS and dopamine D2/3 receptor binding in the substantia nigra.53
Second-generation DRBAs also block serotonin 5-HT2A receptors, which normally inhibit dopaminergic neurotransmission, thereby resulting in dopaminergic upregulation in the nigrostriatal region.54 The ratio of 5-HT2A:D2 antagonism is believed to be another dynamic influencing a DRBA’s tendency to produce EPS,36 as greater 5-HT2A receptor antagonism can mitigate the effects of EPS caused by dopamine blockade in the ventral striatum.55–58 The relative “EPS advantage” of second-generation agents is touted as producing multiple clinical benefits,59,60 but movement side effects can still occur in second-generation agents despite lower D2 receptor occupancy.5,24,34,61,62
The risk of akathisia, for instance, may be comparable or even greater in second-generation agents.52 Akathisia does not correspond well with other types of EPS in terms of symptom severity and treatments, suggesting that there are other important mechanisms underlying its development.63 For example, DRBA-A may result from an intrinsic homeostatic response to the depletion of dopaminergic activity in the ventral striatum portion of the brain.64 The nucleus accumbens is an area specifically implicated in the pathophysiology of akathisia due to its importance for reward and movement. It is theorized that compensatory overstimulation of this region may result in “senseless” behaviors and feelings of dysphoria observed with akathisia.65
Incidence rates of DRBA-A vary among different agents in the second generation class66 (Table 2). For example, quetiapine is often considered to be “akathisia-sparing”, while aripiprazole has been associated with higher risks of DRBA-A development.10,67 Despite being structurally related to aripiprazole68 and demonstrating a similar magnitude of D2 receptor antagonism,69 brexpiprazole is believed to have lower rates of DRBA-A.70,71 This may be due to brexpiprazole possessing greater binding affinity for the 5-HT2A receptor than aripiprazole.69
Table 2.
Rates of akathisia for second-generation DRBAs.
| Second generation DRBAs | Incidence rate of akathisia in adults when taking oral dosage forms |
|---|---|
| Aripiprazole (Abilify®) | 2–25%72 |
| Asenapine (Saphris®) | 4–15%73 |
| Brexpiprazole (Rexulti®) | 4–14%74 |
| Cariprazine (Vraylar®) | Schizophrenia = 9%75
Bipolar = 20%75 |
| Clozapine (Clozaril®) | 3%76 |
| Iloperidone (Fanapt®) | None reported for akathisia77
Extrapyramidal disease = 4–5%77 |
| Lurasidone (Latuda®) | 5.6–22%78 |
| Olanzapine (Zyprexa®) | 5–27%79 |
| Paliperidone (Invega®) | 3–17%80 |
| Quetiapine (Seroquel®) | 1–4.8%81 |
| Risperidone (Risperdal®) | Up to 11%82 |
| Ziprasidone (Geodon®) | 8–10%83 |
DRBAs, dopamine receptor blocking agents.
Indeed, greater 5-HT2A compared with D2 receptor antagonism appears to negatively correlate with DRBA-A risk.84 Aripiprazole has serotonin 5-HT2A blocking effects that are less potent than D2, while quetiapine, which is associated with lower rates of DRBA-A, binds 5-HT2A to a greater extent than D2.54 This preferential binding to 5-HT2A over D2 is most striking with clozapine (Table 3). Although DRBA-A from clozapine appears to be a rare clinical occurrence, there have been case reports of patients who both developed DRBA-A from clozapine and experienced a reduction of DRBA-A symptoms from clozapine. 85–87 Serotonin receptor activity has also been linked to risk of DRBA-A caused by first-generation agents. Patients with schizophrenia and reduced 5-HT1B receptor density were found to develop more frequent and severe akathisia when taking haloperidol compared with those with normal 5-HT1B receptor density.88
Table 3.
Comparison of dopamine D2 receptor binding affinity to serotonin 5-HT2A receptor binding affinity for selected DRBAs.
| DRBA | D2 Ki89 | 5-HT2A Ki89 | D2 Ki/5-HT2A Ki | DRBA-A risk |
|---|---|---|---|---|
| Aripiprazole | 0.7 | 8.7 | 0.08 | High |
| Brexpiprazole | 0.3 | 0.47 | 0.64 | High |
| Clozapine | 210 | 2.59 | 81.08 | Very low |
| Haloperidol | 2.6 | 61 | 0.04 | High |
| Quetiapine | 770 | 31 | 24.84 | Low |
Lower Ki connotes higher binding affinity.
D2 Ki/5-HT2A Ki > 1 indicates relatively greater 5-HT2A receptor antagonism compared to D2 receptor antagonism.
DRBAs, dopamine receptor blocking agents.
Treatment mechanisms
Strategies for managing DRBA-A include psychosocial and pharmacological interventions.5 Expert consensus guidelines recommend lowering the dosage of the DRBA, switching to an antipsychotic carrying lower akathisia risk (such as quetiapine), or initiating a short-term adjunctive medication as initial treatment options for DRBA-A.90–92 If it is safe to do so, decreasing the dose by 50% or completely discontinuing the DRBA may be required to relieve symptoms of akathisia.31 If medications are used to treat DRBA-A, response rates for the commonly used agents propranolol and mirtazapine have been found to be 30% and 43.3%, respectively.93
In general, support for the use of many DRBA-A treatments is based on anecdotal and empirical findings, often using data derived from studies with small sample sizes. Evidence for the use of the beta-blocking medication propranolol in treating DRBA-A dates back to the 1980s, making it one of the most well-studied treatments. Doses of 60–120 mg/day have been demonstrated to be effective and well-tolerated for reducing DRBA-A symptoms.94–97 The hypothetical mechanism of action, shared by the alpha-2 agonist clonidine,98 is suppression of compensatory noradrenergic signaling that may trigger psychomotor activation associated with akathisia. D2 stimulation in the locus ceruleus normally inhibits norepinephrine outflow,99 so it follows that reduced dopamine signaling caused by DRBAs subsequently increases norepinephrine signaling in the midbrain as part of a feedback response.65 Noradrenergic antagonists such as propranolol and clonidine likely act by suppressing this excessive sympathetic response. Pramipexole, a dopamine agonist, is currently being studied to treat akathisia and other types of extrapyramidal symptoms related to DRBA therapy.100 It acts not only to directly restore dopamine signaling in the ventral striatal region, but also to suppress noradrenergic overstimulation. These agents should be used with caution, however, as dopamine agonism carries with it the risk of exacerbating psychotic symptoms.32
Recent literature has called into question the predominance of propranolol in the treatment of DRBA-A.101 Comparative studies have shown cyproheptadine, zolmitriptan, and vitamin B6 produce similar levels of DRBA-A symptom reduction when compared with a range of doses (40–120 mg/day) of propranolol.102–104 Serotonin 5-HT2A receptor antagonists, such as mirtazapine,105–110 trazodone,111 fluvoxamine,112 zolmitriptan,103 mianserin,113 and cyproheptadine,102 represent another class of treatments for DRBA-A.114 Blocking this receptor likely plays a role in reducing symptoms by increasing downstream dopamine signaling in areas of the brain affected by DRBAs.65 Though selective serotonin reuptake inhibitors (SSRIs) generally increase serotonergic neurotransmission and can precipitate akathisia, fluvoxamine possesses weak dopamine reuptake inhibition properties that have been shown to offset the resultant dopaminergic decrease.112,115 Mirtazapine, mianserin, and cyproheptadine also share antihistamine effects (Table 4), which may produce sedation and thereby ameliorate symptoms of akathisia.
Table 4.
Net neurotransmitter effects of compounds that have demonstrated efficacy in the treatment of dopamine-receptor blocking agent-associated akathisia (DRBA-A).
| Net neurotransmitter effects* | |||||||
|---|---|---|---|---|---|---|---|
| Compound | Acetylcholine | Dopamine | GABA | Glutamate | Histamine | Norepinephrine | Serotonin |
| Amantadine | NA | + | NA | – | NA | NA | NA |
| Benztropine | – | + | NA | NA | – | NA | NA |
| Clonazepam116 | NA | + | + | NA | NA | NA | NA |
| Clonidine | NA | NA | NA | NA | NA | − | NA |
| Cyproheptadine | − | NA | NA | NA | − | NA | − |
| Diazepam116,117 | NA | + | + | NA | NA | NA | NA |
| Diphenhydramine | − | NA | NA | NA | − | NA | NA |
| Fluvoxamine | NA | + | NA | NA | NA | NA | + |
| Gabapentin118 | NA | NA | + | − | NA | NA | NA |
| Lorazepam116 | NA | + | + | NA | NA | NA | NA |
| Mianserin119 | NA | + | NA | NA | − | + | + |
| Mirtazapine120 | NA | + | NA | NA | − | + | + |
| Pramipexole99 | NA | + | NA | NA | NA | − | + |
| Propranolol | NA | NA | NA | NA | NA | − | NA |
| Trazodone121 | NA | NA | NA | NA | − | − | + |
| Trihexyphenidyl122 | − | + | NA | NA | NA | NA | NA |
| Zolmitriptan | NA | NA | NA | NA | NA | NA | + |
Assume doses used to treat akathisia.
DRBAs, dopamine receptor blocking agents.
| Legend: | |

|
No significant activity |
| Stimulation | |
| Inhibition | |
Sources: PubChem, Micromedex, Lundbeck Institute, Stahl’s Prescriber Guides, & StatPearls.
Anticholinergic medications are commonly used to treat other forms of extrapyramidal symptoms related to DRBA therapy, such as dystonia and parkinsonism, and may be useful when akathisia is present in combination with these types of EPS.123 The purported mechanism of anticholinergics in the treatment of DRBA-A is restoration of dopamine signaling in areas of the brain where it is depleted by DRBAs.65 Specifically, D2 receptors located on cholinergic interneurons in the basal ganglia, which normally inhibit acetylcholine release,124 can activate the extrapyramidal pathway when blocked. This pathophysiologic theory is supported by the reduced rates of EPS seen with clozapine, which has greater intrinsic anti-muscarinic anticholinergic activity than other DRBAs.24,51,125 Excessive cholinergic outflow can be counteracted with the administration of agents such as benztropine,126 diphenhydramine,127 and trihexyphenidyl.128 Unfortunately, the use of anticholinergics is limited by adverse effects92 such as those related to cardiovascular, gastrointestinal, and cognitive dysfunction.
Combination therapy with propranolol and a benzodiazepine such as diazepam can also be effective in reducing DRBA-A symptoms.129 Benzodiazepines are believed to counteract gamma-aminobutyric acid (GABA) inhibition caused by DRBAs.101 One proposed mechanism of DRBA-A is via downregulation of GABA signaling in the pallidus through the blockade of D2 receptors.123 5-HT2A receptor antagonism may also decrease GABA signaling in the prefrontal cortex,130 which would offer an explanation as to why akathisia is still observed at high rates with second-generation DRBAs. In addition to correcting GABA hypofunctioning, benzodiazepines may also act via GABAergic depression of the central nervous system to reduce physical and psychological symptoms of agitation associated with DRBA-A.
Short (1–2 week) courses of clonazepam131 and lorazepam132 have been associated with a reduction in DRBA-A symptoms. Intravenous diazepam can rapidly relieve DRBA-A symptoms,133 making it an acceptable option in the acute setting. However, the adverse effect profile, risk of overdose, and abuse potential of benzodiazepines make them far less attractive options for routine chronic and/or preventative treatment of DRBA-A.134 Gabapentin enacarbil is another pharmacotherapy option that acts by potentiating GABA signaling and is associated with relatively fewer risks compared with benzodiazepines. Treatment with gabapentin enacarbil has been shown to significantly decrease the severity of DRBA-A within 2 weeks at doses similar to those recommended for restless leg syndrome.135 Amantadine is an antagonist of the N-methyl-d-aspartate (NMDA) receptor that opposes glutamatergic signaling similarly to GABA, and has also been found to be effective in treating DRBA-A.21
Other treatments for DRBA-A have various mechanisms of action. Preladenant works by antagonism the adenosine receptor and has been studied to treat DRBA-A.136 Reduced iron levels were found to correlate with DRBA-A development in patients with schizophrenia,137 and the administration of IV iron produces a reduction in DRBA-A symptoms.138 Heavy smoking has been associated with fewer instances of DRBA-A among patients with schizophrenia when compared with light smokers.139 Accordingly, the administration of nicotine patches has been found to significantly reduce DRBA-A symptoms in non-smoking inpatients.140 Vitamin B6 significantly reduces subjective symptoms of restlessness, distress, and global symptoms of DRBA-A compared with placebo.141 Vitamin B6 is important for the synthesis of dopamine, serotonin, and GABA.101 This treatment may also act outside of the neurotransmitter system to reduce symptoms via free radical scavenging.142 Another antioxidant that may be useful for the treatment of DRBA-A is N-acetyl cysteine, which significantly decreases akathisia when used adjunctively in patients with schizophrenia taking DRBAs.143
Conclusion
DRBA-A is a prevalent and potentially serious adverse effect of medications that are used widely in the field of psychiatry. It is crucial that DRBA-A is screened for in all patients prescribed these agents throughout all time points in their treatment. An accurate description of possible symptoms, including both common and uncommon presentations, must be provided to patients at the initiation of therapy so that they are aware of what to possibly expect. Any new motor or psychiatric symptoms resembling anxiety should include DRBA-A as a differential diagnosis.
The prevailing theory holds that dopamine hypofunctioning in the ventral striatal region of the brain is implicated in the pathophysiology of EPS such as akathisia, though this is likely not the only significant mechanism as rates of DRBA-A remain high in second generation DRBAs despite their comparatively lower dopamine D2 blocking potency than first generation agents. Figure 1 attempts to visualize neural circuits implicated in DRBA-A and illustrate their interactions in the pathophysiology of this drug-induced phenomenon.
Figure 1.
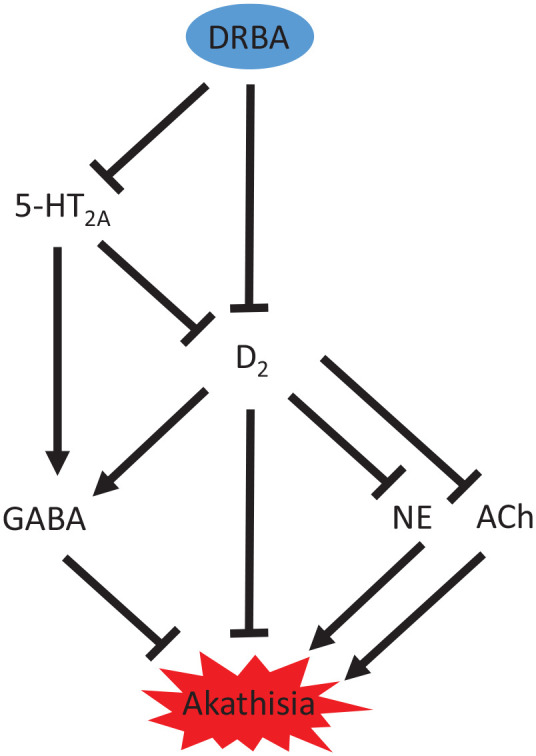
Proposed neural circuitry underlying second generation DRBA-A.
5-HT2A, serotonin receptor subtype; Ach, acetylcholine; D2, dopamine receptor subtype; DRBA, dopamine receptor blocking agent; DRBA-A, dopamine-receptor blocking agent-associated akathisia; GABA, gamma-aminobutyric acid; NE, norepinephrine.
Stimulatory pathways end with an arrow (▼) and inhibitory pathways end with a line (▬).
While dopamine neurotransmission via the nigrostriatal pathway is believed to be important for other types of EPS, akathisia may more heavily involve alterations in the mesolimbic pathway. A deficiency in dopamine signaling via the former results in movement dysfunction, while effects on the latter can be linked to goal-directed behavior. Interruption in dopamine outflow through the ventral striatum may underlie agitation and obsessive-compulsive types of thoughts and behaviors unique to akathisia, such as purposeless and/or repetitive movements.
Noradrenergic signaling is likely also important in the pathogenesis of DRBA-A, and is already central to the treatment paradigm. It has been established that dopamine blockade by DRBAs can result in norepinephrine overactivity,144 and thus it is reasonable to correlate this sympathetic response with the physical and psychological manifestation of akathisia. Consequently, it is key that any treatment for DRBA-A involve suppression of this system, by way of either alpha-1 receptor antagonism, alpha-2 agonism, or beta antagonism.
The development of new treatments for DRBA-A is relevant as traditional treatments are not always effective.92 Based on the mechanisms of currently available therapies for DRBA-A, an effective agent should increase dopamine signaling in the affected areas of the brain (either via anticholinergic, serotonin receptor antagonism, or direct dopamine receptor stimulatory activity) and counteract compensatory noradrenergic signaling via one of the pathways mentioned previously. When balancing the two effects, a higher priority should be placed on noradrenergic inhibition than dopamine stimulation in order to avoid exacerbating psychotic symptoms. Therapies with significant GABAergic, anticholinergic, and/or antihistamine effects may have less impact on psychiatric symptoms (both beneficial and detrimental) than those with dopaminergic effects, but should generally be avoided due side effect burden. Ultimately, the management of DRBA-A should be a collaborative effort between patient and provider, and the benefit versus risk should be carefully weighed for any medications used.
Footnotes
Conflict of interest statement: The authors declare that there is no conflict of interest.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Shaina Musco  https://orcid.org/0000-0002-6040-8425
https://orcid.org/0000-0002-6040-8425
Contributor Information
Shaina Musco, Department of Clinical Sciences, High Point University Fred Wilson School of Pharmacy, One University Parkway, High Point, NC 27262, USA.
Vivian McAllister, High Point University David R. Hayworth College of Arts and Sciences, One University Parkway, High Point, NC, USA.
Ian Caudle, High Point University Fred Wilson School of Pharmacy, One University Parkway, High Point, NC, USA.
References
- 1. Belmaker RH, Wald D. Haloperidol in normals. Br J Psychiatry 1977; 131: 222–223. [DOI] [PubMed] [Google Scholar]
- 2. Lohr JB, Eidt CA, Abdulrazzaq Alfaraj A, et al. The clinical challenges of akathisia. CNS Spectr 2015; 20(Suppl. 1): 1–14; quiz 5–6. [DOI] [PubMed] [Google Scholar]
- 3. Kim JH, Byun HJ. Association of subjective cognitive dysfunction with akathisia in patients receiving stable doses of risperidone or haloperidol. J Clin Pharm Ther 2007; 32: 461–467. [DOI] [PubMed] [Google Scholar]
- 4. Sachdev PS, Brune M. Animal models of acute drug-induced akathisia - a review. Neurosci Biobehav Rev 2000; 24: 269–277. [DOI] [PubMed] [Google Scholar]
- 5. Kane JM, Fleischhacker WW, Hansen L, et al. Akathisia: an updated review focusing on second-generation antipsychotics. J Clin Psychiatry 2009; 70: 627–643. [DOI] [PubMed] [Google Scholar]
- 6. Barnes TR. A rating scale for drug-induced akathisia. Br J Psychiatry 1989; 154: 672–676. [DOI] [PubMed] [Google Scholar]
- 7. Hirose S. The causes of underdiagnosing akathisia. Schizophr Bull 2003; 29: 547–558. [DOI] [PubMed] [Google Scholar]
- 8. Forcen FE. Akathisia: is restlessness a primary condition or an adverse drug effect? Curr Psychiatry 2015; 14: 14–18. [Google Scholar]
- 9. Walker L. Sertraline-induced akathisia and dystonia misinterpreted as a panic attack. Psychiatr Serv 2002; 53: 1477–1478. [DOI] [PubMed] [Google Scholar]
- 10. Juncal-Ruiz M, Ramirez-Bonilla M, Gomez-Arnau J, et al. Incidence and risk factors of acute akathisia in 493 individuals with first episode non-affective psychosis: a 6-week randomised study of antipsychotic treatment. Psychopharmacology (Berl) 2017; 234: 2563–2570. [DOI] [PubMed] [Google Scholar]
- 11. Awad AG, Voruganti L, Heslegrave R. A conceptual model of quality of life in schizophrenia: description and preliminary clinical validation. Qual Life Res 1997; 6: 21–26. [DOI] [PubMed] [Google Scholar]
- 12. Browne S, Clarke M, Gervin M, et al. Quality of life after treatment for a first presentation of schizophrenia. Schizophr Res 2000; 1: 301. [Google Scholar]
- 13. Velligan DI, Weiden PJ, Sajatovic M, et al. The expert consensus guideline series: adherence problems in patients with serious and persistent mental illness. J Clin Psychiatry 2009; 70(Suppl. 4): 1–46; quiz 7–8. [PubMed] [Google Scholar]
- 14. Jouini L, Ouali U, Ouanes S, et al. What about the hidden face of Akathisia? Eur Psychiatry 2017; 41(Suppl): S688. [Google Scholar]
- 15. Baynes D, Mulholland C, Cooper SJ, et al. Depressive symptoms in stable chronic schizophrenia: prevalence and relationship to psychopathology and treatment. Schizophr Res 2000; 45: 47–56. [DOI] [PubMed] [Google Scholar]
- 16. Majadas S, Olivares J, Galan J, et al. Prevalence of depression and its relationship with other clinical characteristics in a sample of patients with stable schizophrenia. Compr Psychiatry 2012; 53: 145–151. [DOI] [PubMed] [Google Scholar]
- 17. Cheng HM, Park JH, Hernstadt D. Akathisia: a life-threatening side effect of a common medication. BMJ Case Rep 2013; 2013: bcr2012007713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Cem Atbasoglu E, Schultz SK, Andreasen NC. The relationship of akathisia with suicidality and depersonalization among patients with schizophrenia. J Neuropsychiatry Clin Neurosci 2001; 13: 336–341. [DOI] [PubMed] [Google Scholar]
- 19. Seemuller F, Lewitzka U, Bauer M, et al. The relationship of Akathisia with treatment emergent suicidality among patients with first-episode schizophrenia treated with haloperidol or risperidone. Pharmacopsychiatry 2012; 45: 292–296. [DOI] [PubMed] [Google Scholar]
- 20. Reutfors J, Clapham E, Bahmanyar S, et al. Suicide risk and antipsychotic side effects in schizophrenia: nested case-control study. Hum Psychopharmacol 2016; 31: 341–345. [DOI] [PubMed] [Google Scholar]
- 21. Miller CH, Fleischhacker WW. Managing antipsychotic-induced acute and chronic akathisia. Drug Saf 2000; 22: 73–81. [DOI] [PubMed] [Google Scholar]
- 22. Snyder S, Greenberg D, Yamamura HI. Antischizophrenic drugs and brain cholinergic receptors: affinity for muscarinic sites predicts extrapyramidal effects. Arch Gen Psychiatry 1974; 31: 58–61. [DOI] [PubMed] [Google Scholar]
- 23. Barnes TR, McPhillips MA. Novel antipsychotics, extrapyramidal side effects and tardive dyskinesia. Int Clin Psychopharmacol 1998; 13(Suppl. 3): 49–57. [DOI] [PubMed] [Google Scholar]
- 24. Schillevoort I, Herings RMC, Hugenholtz G, et al. Antipsychotic-induced extrapyramidal syndromes in psychiatric practice: a case-control study. Pharm World Sci 2005; 27: 285–289. [DOI] [PubMed] [Google Scholar]
- 25. de Greef R, Maloney A, Olsson-Gisleskog P, et al. Dopamine D2 occupancy as a biomarker for antipsychotics: quantifying the relationship with efficacy and extrapyramidal symptoms. The AAPS Journal 2010; 13: 121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Leucht S, Wahlbeck K, Hamann J, et al. New generation antipsychotics versus low-potency conventional antipsychotics: a systematic review and meta-analysis. Lancet 2003; 361: 1581–1589. [DOI] [PubMed] [Google Scholar]
- 27. Nordström A-L, Farde L, Wiesel FA, et al. Central D2-dopamine receptor occupancy in relation to antipsychotic drug effects: a double-blind PET study of schizophrenic patients. Biol Psychiatry 1993; 33: 227–235. [DOI] [PubMed] [Google Scholar]
- 28. Remington G, Kapur S. D2 and 5-HT2 receptor effects of antipsychotics: bridging basic and clinical findings using PET. J Clin Psychiatry 1999; 60(Suppl 10): 15–19. [PubMed] [Google Scholar]
- 29. Casey DE. Pathophysiology of antipsychotic drug-induced movement disorders. J Clin Psychiatry 2004; 65(Suppl 9): 25–28. [PubMed] [Google Scholar]
- 30. Lawford B, Barnes M, Swagell C, et al. DRD2/ANKK1 Taq1A (rs 1800497 C> T) genotypes are associated with susceptibility to second generation antipsychotic-induced akathisia. J Psychopharmacol 2013; 27: 343–348. [DOI] [PubMed] [Google Scholar]
- 31. Keller DM, Myhre KE, Dowben JS, et al. Biological perspectives: akathisia: ants in your pants. Perspect Psychiatr Care 2013; 49: 149–151. [DOI] [PubMed] [Google Scholar]
- 32. Naguy A. Akathisia-A psychopharmacologic treatment “menu”. Asia Pac Psychiatry 2017; 9. [DOI] [PubMed] [Google Scholar]
- 33. Pierre JM. Extrapyramidal symptoms with atypical antipsychotics: incidence, prevention and management. Drug Saf 2005; 28: 191–208. [DOI] [PubMed] [Google Scholar]
- 34. Divac N, Prostran M, Jakovcevski I, et al. Second-generation antipsychotics and extrapyramidal adverse effects. Biomed Res Int 2014; 2014: 656370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Blair DT, Dauner A. Extrapyramidal symptoms are serious side-effects of antipsychotic and other drugs. Nurse Pract 1992; 17: 56, 62–64, 67. [DOI] [PubMed] [Google Scholar]
- 36. Dayalu P, Chou KL. Antipsychotic-induced extrapyramidal symptoms and their management. Expert Opin Pharmacother 2008; 9: 1451–1462. [DOI] [PubMed] [Google Scholar]
- 37. Berna F, Misdrahi D, Boyer L, et al. Akathisia: prevalence and risk factors in a community-dwelling sample of patients with schizophrenia. Results from the FACE-SZ dataset. Schizophr Res 2015; 169: 255–261. [DOI] [PubMed] [Google Scholar]
- 38. Kurlawala Z, Vatsalya V. Heavy alcohol drinking associated akathisia and management with quetiapine XR in alcohol dependent patients. J Addict 2016; 2016: 6028971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wielenga-Boiten JE, Ribbers GM. Akathisia–rare cause of psychomotor agitation in patients with traumatic brain injury: case report and review of literature. J Rehabil Res Dev 2012; 49: 1349–1354. [DOI] [PubMed] [Google Scholar]
- 40. Musco S, Ruekert L, Myers J, et al. Characteristics of patients experiencing extrapyramidal symptoms or other movement disorders related to dopamine receptor blocking agent therapy. J Clin Psychopharmacol 2019; 39: 336–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Shirzadi AA, Ghaemi SN. Side effects of atypical antipsychotics: extrapyramidal symptoms and the metabolic syndrome. Harv Rev Psychiatry 2006; 14: 152–164. [DOI] [PubMed] [Google Scholar]
- 42. Spina E, Sturiale V, Valvo S, et al. Prevalence of acute dystonic reactions associated with neuroleptic treatment with and without anticholinergic prophylaxis. Int Clin Psychopharmacol 1993; 8: 21–24. [DOI] [PubMed] [Google Scholar]
- 43. van Harten PN, Hoek HW, Kahn RS. Acute dystonia induced by drug treatment. BMJ 1999; 319: 623–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gershanik OS. Drug-induced parkinsonism in the aged. Recognition and prevention. Drugs Aging 1994; 5: 127–132. [DOI] [PubMed] [Google Scholar]
- 45. Kulkarni SK, Naidu PS. Pathophysiology and drug therapy of tardive dyskinesia: current concepts and future perspectives. Drugs Today (Barc) 2003; 39: 19–49. [DOI] [PubMed] [Google Scholar]
- 46. Coplan J, Gugger JJ, Tasleem H. Tardive dyskinesia from atypical antipsychotic agents in patients with mood disorders in a clinical setting. J Affect Disord 2013; 150: 868–871. [DOI] [PubMed] [Google Scholar]
- 47. Correll CU, Kane JM, Citrome LL. Epidemiology, prevention, and assessment of tardive dyskinesia and advances in treatment. J Clin Psychiatry 2017; 78: 1136–1147. [DOI] [PubMed] [Google Scholar]
- 48. Casey DE. Tardive dyskinesia and atypical antipsychotic drugs. Schizophr Res 1999; 35(Suppl): S61–S66. [DOI] [PubMed] [Google Scholar]
- 49. Correll CU, Schenk EM. Tardive dyskinesia and new antipsychotics. Curr Opin Psychiatry 2008; 21: 151–156. [DOI] [PubMed] [Google Scholar]
- 50. Jeste DV, Lacro JP, Bailey A, et al. Lower incidence of tardive dyskinesia with risperidone compared with haloperidol in older patients. J Am Geriatr Soc 1999; 47: 716–719. [DOI] [PubMed] [Google Scholar]
- 51. Kapur S, Seeman P. Does fast dissociation from the dopamine D2 receptor explain the action of atypical antipsychotics?: A new hypothesis. Am J Psychiatry 2001; 158: 360–369. [DOI] [PubMed] [Google Scholar]
- 52. Tarsy, Baldessarini, Tarazi Effects of newer antipsychotics on extrapyramidal function. CNS Drugs 2002; 16: 23–45. [DOI] [PubMed] [Google Scholar]
- 53. Tuppurainen H, Kuikka JT, Viinamaki H, et al. Extrapyramidal side-effects and dopamine D(2/3) receptor binding in substantia nigra. Nord J Psychiatry 2010; 64: 233–238. [DOI] [PubMed] [Google Scholar]
- 54. Stahl SM. Stahl’s essential psychopharmacology: neuroscientific basis and practical applications. 4th ed. Cambridge University Press, New York, 2013, p. 626. [Google Scholar]
- 55. Suzuki H, Gen K. The relationship between the plasma concentration of blonanserin, and its plasma anti-serotonin 5-HT2A activity/anti-dopamine D2 activity ratio and drug-induced extrapyramidal symptoms. Psychiatry Clin Neurosci 2012; 66: 146–152. [DOI] [PubMed] [Google Scholar]
- 56. Ennis C, Kemp JD, Cox B. Characterisation of inhibitory 5-hydroxytryptamine receptors that modulate dopamine release in the striatum. J Neurochem 1981; 36: 1515–1520. [DOI] [PubMed] [Google Scholar]
- 57. Huttunen M. The Evolution of the serotonin-dopamine antagonist concept. J Clin Psychopharmacol 1995; 15(Suppl. 1): 4S–10S. [DOI] [PubMed] [Google Scholar]
- 58. Matsui-Sakata A, Ohtani H, Sawada Y. Pharmacokinetic-pharmacodynamic analysis of antipsychotics-induced extrapyramidal symptoms based on receptor occupancy theory incorporating endogenous dopamine release. Drug Metab Pharmacokinet 2005; 20: 187–199. [DOI] [PubMed] [Google Scholar]
- 59. Tandon R, Jibson MD. Extrapyramidal side effects of antipsychotic treatment: scope of problem and impact on outcome. Ann Clin Psychiatry 2002; 14: 123–129. [DOI] [PubMed] [Google Scholar]
- 60. Tandon R. Safety and tolerability: how do newer generation “atypical” antipsychotics compare? Psychiatr Q 2002; 73: 297–311. [DOI] [PubMed] [Google Scholar]
- 61. Demyttenaere K, Detraux J, Racagni G, et al. Medication-induced akathisia with newly approved antipsychotics in patients with a severe mental illness: a systematic review and meta-analysis. CNS Drugs 2019; 33: 549–566. [DOI] [PubMed] [Google Scholar]
- 62. Chow CL, Kadouh NK, Bostwick JR, et al. Akathisia and newer second-generation antipsychotic drugs: a review of current evidence. Pharmacotherapy 2020; 40: 565–574. [DOI] [PubMed] [Google Scholar]
- 63. Bratti IM, Kane JM, Marder SR. Chronic restlessness with antipsychotics. Am J Psychiatry 2007; 164: 1648–1654. [DOI] [PubMed] [Google Scholar]
- 64. Metin B, Metin SZ, Gunduz A, et al. Brainstem reflexes are hyperactive in patients with drug-induced akathisia. Neurol Sci 2017; 38: 1683–1689. [DOI] [PubMed] [Google Scholar]
- 65. Stahl SM, Lonnen AJM. The mechanism of drug-induced Akathsia. CNS Spectr 2011; 16: 7–10. [PubMed] [Google Scholar]
- 66. Poyurovsky M, Weizman A. Treatment of antipsychotic-related akathisia revisited: the role of serotonin 2A receptor antagonists. J Clin Psychopharmacol 2015; 35: 711–714. [DOI] [PubMed] [Google Scholar]
- 67. Yoshimura B, Sato K, Sakamoto S, et al. Incidence and predictors of acute akathisia in severely ill patients with first-episode schizophrenia treated with aripiprazole or risperidone: secondary analysis of an observational study. Psychopharmacology (Berl) 2019; 236: 723–730. [DOI] [PubMed] [Google Scholar]
- 68. Stahl SM. Mechanism of action of brexpiprazole: comparison with aripiprazole. CNS Spectr 2016; 21: 1–6. [DOI] [PubMed] [Google Scholar]
- 69. Solmi M, Murru A, Pacchiarotti I, et al. Safety, tolerability, and risks associated with first- and second-generation antipsychotics: a state-of-the-art clinical review. Ther Clin Risk Manag 2017; 13: 757–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Citrome L, Ota A, Nagamizu K, et al. The effect of brexpiprazole (OPC-34712) and aripiprazole in adult patients with acute schizophrenia: results from a randomized, exploratory study. Int Clin Psychopharmacol 2016; 31: 192–201. [DOI] [PubMed] [Google Scholar]
- 71. Ishigooka J, Iwashita S, Tadori Y. Efficacy and safety of brexpiprazole for the treatment of acute schizophrenia in Japan: a 6-week, randomized, double-blind, placebo-controlled study Brexpiprazole for schizophrenia. Psychiatry Clin Neurosci 2018; 72: 692–700. [DOI] [PubMed] [Google Scholar]
- 72. U.S. Food and Drug Administration. Abilify (aripiprazole) [package insert]. Otsuka Pharmaceutical Co., Ltd.: Tokyo, https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/021436s038,021713s030,021729s022,021866s023lbl.pdf (2014, accessed October 14, 2019). [Google Scholar]
- 73. U.S. Food and Drug Administration. Saphris (asenapine) [package insert]. Allergan USA, Inc.: Irvine, https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/022117s020s021lbl.pdf (2017, accessed October 14, 2019). [Google Scholar]
- 74. U.S. Food and Drug Administration. Rexulti (brexpiprazole) [package insert]. Otsuka Pharmaceutical Co., Ltd.: Tokyo, https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/205422s002lbl.pdf (2015, accessed October 14, 2019). [Google Scholar]
- 75. U.S. Food and Drug Administration. Vraylar (cariprazine) [package insert]. Actavis Pharma, Inc.: NJ, https://www.accessdata.fda.gov/drugsatfda_docs/label/2015/204370lbl.pdf (2015, accessed October 14, 2019). [Google Scholar]
- 76. U.S. Food and Drug Administration. Clozaril (clozapine) [package insert]. HLS Therapeutics (USA), Inc.: PA, https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/019758s084lbl.pdf (2017, accessed October 14, 2019). [Google Scholar]
- 77. U.S. Food and Drug Administration. Fanapt (iloperidone) [package insert]. Vanda Pharmaceuticals, Inc.: Rockville, MD, https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/022192lbl.pdf (2009, accessed October 14, 2019). [Google Scholar]
- 78. U.S. Food and Drug Administration. Latuda (lurasidone) [package insert]. Sunovion Pharmaceuticals, Inc.: MA, https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/200603lbls10s11.pdf (2013, accessed October 14, 2019). [Google Scholar]
- 79. U.S. Food and Drug Administration. Zyprexa (olanzapine) [package insert]. Eli Lilly and Company, IN, https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/020592s057,021086s036,021253s045lbl.pdf (2010, accessed October 14, 2019).
- 80. U.S. Food and Drug Administration. Invega (paliperidone) [package insert]. Ortho-McNeil-Janssen Pharmaceuticals, Inc., NJ, https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021999s018lbl.pdf (2010, accessed October 14, 2019).
- 81. U.S. Food and Drug Administration. Seroquel (quetiapine) [package insert]. AstraZeneca Pharmaceuticals LP., DE, https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/020639s064lbl.pdf (2013, accessed October 14, 2019).
- 82. U.S. Food and Drug Administration. Risperdal (risperidone) [package insert]. Ortho-McNeil-Janssen Pharmaceuticals, Inc., NJ, https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020272s056,020588s044,021346s033,021444s03lbl.pdf (2009, accessed October 14, 2019).
- 83. U.S. Food and Drug Administration. Geodon (ziprasidone) [package insert]. Pfizer, Inc., NY, https://www.accessdata.fda.gov/drugsatfda_docs/label/2009/020825s035,020919s023lbl.pdf (2008, accessed October 14, 2019).
- 84. Poyurovsky M, Weizman A. Serotonin-based pharmacotherapy for acute neuroleptic-induced akathisia: a new approach to an old problem. Br J Psychiatry 2001; 179: 4–8. [DOI] [PubMed] [Google Scholar]
- 85. Grover S, Sahoo S. Clozapine induced akathisia: a case report and review of the evidence. Indian J Pharmacol 2015; 47: 234–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Pondé MP, Freire ACC. Increased anxiety, akathisia, and suicidal thoughts in patients with mood disorder on aripiprazole and lamotrigine. Case Rep Psychiatry 2015; 2015: 1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Patchan KM, Richardson C, Gopal V, et al. The risk of suicide after clozapine discontinuation: cause for concern. Ann Clin Psychiatry 2015; 27: 253–256. [PMC free article] [PubMed] [Google Scholar]
- 88. Grubor M, Zivkovic M, Sagud M, et al. HTR1A, HTR1B, HTR2A, HTR2C and HTR6 gene polymorphisms and extrapyramidal side effects in haloperidol-treated patients with schizophrenia. Int J Mol Sci 2020; 21: 2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Correll CU. From receptor pharmacology to improved outcomes: individualising the selection, dosing, and switching of antipsychotics. Eur Psychiatry 2010; 25(Suppl. 2): S12–S21. [DOI] [PubMed] [Google Scholar]
- 90. Javed A, Arthur H, Curtis L, et al. Practical guidance on the use of lurasidone for the treatment of adults with schizophrenia. Neurol Ther 2019; 8: 215–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Taylor D, Barnes TRE, Young AH. The maudsley prescribing guidelines in psychiatry. 13th ed. Hoboken, NJ: Wiley, 2018. [Google Scholar]
- 92. Poyurovsky M. Acute antipsychotic-induced akathisia revisited. Br J Psychiatry 2010; 196: 89–91. [DOI] [PubMed] [Google Scholar]
- 93. Hieber R, Dellenbaugh T, Nelson LA. Role of mirtazapine in the treatment of antipsychotic-induced akathisia. Ann Pharmacother 2008; 42: 841–846. [DOI] [PubMed] [Google Scholar]
- 94. Adler L, Angrist B, Peselow E, et al. A controlled assessment of propranolol in the treatment of neuroleptic- induced akathisia. Br J Psychiatry 1986; 149: 42–45. [DOI] [PubMed] [Google Scholar]
- 95. Lipinski JF, Jr, Zubenko GS, Cohen BM, et al. Propranolol in the treatment of neuroleptic-induced akathisia. Am J Psychiatry 1984; 141: 412–415. [DOI] [PubMed] [Google Scholar]
- 96. Kramer MS, Gorkin R, DiJohnson C. Treatment of neuroleptic-induced akathisia with propranolol: a controlled replication study. Hillside J Clin Psychiatry 1989; 11: 107–119. [PubMed] [Google Scholar]
- 97. Irwin M, Sullivan G, Van Putten T. Propranolol as a primary treatment of neuroleptic-induced akathisia. Hillside J Clin Psychiatry 1988; 10: 244–250. [PubMed] [Google Scholar]
- 98. Zubenko GS, Cohen BM, Lipinski JF, Jr, et al. Use of clonidine in treating neuroleptic-induced akathisia. Psychiatry Res 1984; 13: 253–259. [DOI] [PubMed] [Google Scholar]
- 99. Chernoloz O, El Mansari M, Blier P. Sustained administration of pramipexole modifies the spontaneous firing of dopamine, norepinephrine, and serotonin neurons in the rat brain. Neuropsychopharmacology 2009; 34: 651–661. [DOI] [PubMed] [Google Scholar]
- 100. ClinicalTrials.gov. Li H, Yu W. A pilot study of pramipexole to treat extrapyramidal symptoms induced by antipsychotics NCT03430596. 2018. https://clinicaltrials.gov/ct2/show/NCT03430596 (accessed 10 March 2019).
- 101. Sethuram K, Gedzior J. Akathisia: case presentation and review of newer treatment agents. Psychiatr Ann 2014; 44: 391–396. [Google Scholar]
- 102. Fischel T, Hermesh H, Aizenberg D, et al. Cyproheptadine versus propranolol for the treatment of acute neuroleptic-induced akathisia: a comparative double-blind study. J Clin Psychopharmacol 2001; 21: 612–615. [DOI] [PubMed] [Google Scholar]
- 103. Avital A, Gross-Isseroff R, Stryjer R, et al. Zolmitriptan compared to propranolol in the treatment of acute neuroleptic-induced akathisia: a comparative double-blind study. Eur Neuropsychopharmacol 2009; 19: 476–482. [DOI] [PubMed] [Google Scholar]
- 104. Shams-Alizadeh N, Bakhshayesh H, Rezaei F, et al. Effect of vitamin B6 versus propranolol on antipsychotic-induced akathisia: a pilot comparative double-blind study. Iran J Pharm Res 2018; 17(Suppl): 130–135. [PMC free article] [PubMed] [Google Scholar]
- 105. Wilson MS., II Mirtazapine for akathisia in bipolar disorder. J Clin Psychopharmacol 2005; 25: 394–395. [DOI] [PubMed] [Google Scholar]
- 106. Ranjan S, Chandra PS, Chaturvedi SK, et al. Atypical antipsychotic-induced akathisia with depression: therapeutic role of mirtazapine. Ann Pharmacother 2006; 40: 771–774. [DOI] [PubMed] [Google Scholar]
- 107. Poyurovsky M, Weizman A. Very low-dose mirtazapine (7.5 mg) in treatment of acute antipsychotic-associated akathisia. J Clin Psychopharmacol 2018; 38: 609–611. [DOI] [PubMed] [Google Scholar]
- 108. Poyurovsky M, Bergman J, Pashinian A, et al. Beneficial effect of low-dose mirtazapine in acute aripiprazole-induced akathisia. Int Clin Psychopharmacol 2014; 29: 296–298. [DOI] [PubMed] [Google Scholar]
- 109. Praharaj SK, Kongasseri S, Behere RV, et al. Mirtazapine for antipsychotic-induced acute akathisia: a systematic review and meta-analysis of randomized placebo-controlled trials. Ther Adv Psychopharmacol 2015; 5: 307–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Poyurovsky M, Pashinian A, Weizman R, et al. Low-dose mirtazapine: a new option in the treatment of antipsychotic-induced akathisia. A randomized, double-blind, placebo- and propranolol-controlled trial. Biol Psychiatry 2006; 59: 1071–1077. [DOI] [PubMed] [Google Scholar]
- 111. Stryjer R, Rosenzcwaig S, Bar F, et al. Trazodone for the treatment of neuroleptic-induced acute akathisia: a placebo-controlled, double-blind, crossover study. Clin Neuropharmacol 2010; 33: 219–222. [DOI] [PubMed] [Google Scholar]
- 112. Furuse T, Hashimoto K. Fluvoxamine for aripiprazole-associated akathisia in patients with schizophrenia: a potential role of sigma-1 receptors. Ann Gen Psychiatry. 2010; 9: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Miodownik C, Lerner V, Statsenko N, et al. Vitamin B6 versus mianserin and placebo in acute neuroleptic-induced akathisia: a randomized, double-blind, controlled study. Clin Neuropharmacol 2006; 29: 68–72. [DOI] [PubMed] [Google Scholar]
- 114. Pringsheim T, Gardner D, Addington D, et al. The assessment and treatment of antipsychotic-induced akathisia. Can J Psychiatry 2018; 63: 719–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Nepal H, Black E, Bhattarai M. Self-harm in sertraline-induced akathisia. Prim Care Companion CNS Disord 2016; 18. [DOI] [PubMed] [Google Scholar]
- 116. Riegel AC, Kalivas PW. Neuroscience: lack of inhibition leads to abuse. Nature 2010; 463: 743–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Rincon-Cortes M, Gagnon KG, Dollish HK, et al. Diazepam reverses increased anxiety-like behavior, social behavior deficit, and dopamine dysregulation following withdrawal from acute amphetamine. Neuropsychopharmacology 2018; 43: 2418–2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Hara K, Sata T. Inhibitory effect of gabapentin on N-methyl-D-aspartate receptors expressed in Xenopus oocytes. Acta Anaesthesiol Scand 2007; 51: 122–128. [DOI] [PubMed] [Google Scholar]
- 119. Tanda G, Bassareo V, Di Chiara G. Mianserin markedly and selectively increases extracellular dopamine in the prefrontal cortex as compared to the nucleus accumbens of the rat. Psychopharmacology (Berl) 1996; 123: 127–130. [DOI] [PubMed] [Google Scholar]
- 120. Nakayama K, Sakurai T, Katsu H. Mirtazapine increases dopamine release in prefrontal cortex by 5-HT1A receptor activation. Brain Res Bull 2004; 63: 237–241. [DOI] [PubMed] [Google Scholar]
- 121. Maj J, Palider W, Rawlow Trazodone, a central serotonin antagonist and agonist. J Neural Transm 1979; 44: 237–248. [DOI] [PubMed] [Google Scholar]
- 122. Downs AM, Fan X, Donsante C, et al. Trihexyphenidyl rescues the deficit in dopamine neurotransmission in a mouse model of DYT1 dystonia. Neurobiol Dis 2019; 125: 115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Iqbal N, Lambert T, Masand P. Akathisia: problem of history or concern of today. CNS Spectr 2007; 12(9 Suppl. 14): 1–13. [DOI] [PubMed] [Google Scholar]
- 124. Lim SA, Kang UJ, McGehee DS. Striatal cholinergic interneuron regulation and circuit effects. Front Synaptic Neurosci 2014; 6: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Strange PG. Antipsychotic drugs: importance of dopamine receptors for mechanisms of therapeutic actions and side effects. Pharmacol Rev 2001; 53: 119–133. [PubMed] [Google Scholar]
- 126. Adler LA, Peselow E, Rosenthal M, et al. A controlled comparison of the effects of propranolol, benztropine, and placebo on akathisia: an interim analysis. Psychopharmacol Bull 1993; 29: 283–286. [PubMed] [Google Scholar]
- 127. Vinson DR, Drotts DL. Diphenhydramine for the prevention of akathisia induced by prochlorperazine: a randomized, controlled trial. Ann Emerg Med 2001; 37: 125–131. [DOI] [PubMed] [Google Scholar]
- 128. Salem H, Nagpal C, Pigott T, et al. Revisiting antipsychotic-induced akathisia: current issues and prospective challenges. Curr Neuropharmacol 2017; 15: 789–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Oflaz S, Bakay H, Cekic E, et al. Atypical antipsychotics induced chronic akathisia: a case report. Journal of Mood Disorders 2014; 4: 175. [Google Scholar]
- 130. Abi-Saab WM, Bubser M, Roth RH, et al. 5-HT2 receptor regulation of extracellular GABA levels in the prefrontal cortex. Neuropsychopharmacology 1999; 20: 92–96. [DOI] [PubMed] [Google Scholar]
- 131. Lima AR, Soares-Weiser K, Bacaltchuk J, et al. Benzodiazepines for neuroleptic-induced acute akathisia. Cochrane Database Syst Rev 2002; 1: Cd001950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Bartels M, Heide K, Mann K, et al. Treatment of akathisia with lorazepam. An open clinical trial. Pharmacopsychiatry 1987; 20: 51–53. [DOI] [PubMed] [Google Scholar]
- 133. Hirose S, Ashby CR. Immediate effect of intravenous diazepam in neuroleptic-induced acute akathisia: an open-label study. J Clin Psychiatry 2002; 63: 524–527. [DOI] [PubMed] [Google Scholar]
- 134. Fontanella CA, Campo JV, Phillips GS, et al. Benzodiazepine use and risk of mortality among patients with schizophrenia: a retrospective longitudinal study. J Clin Psychiatry 2016; 77: 661–667. [DOI] [PubMed] [Google Scholar]
- 135. Takeshima M, Ishikawa H, Kanbayashi T, et al. Gabapentin enacarbil for antipsychotic induced akathisia in schizophrenia patients: a pilot open-labeled study. Neuropsychiatr Dis Treat 2018; 14: 3179–3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. ClinicalTrials.gov. Merck Sharp & Dohme Corp. Study of preladenant for the treatment of neuroleptic induced akathisia. NCT00693472. 2007. (accessed 10 March 2019).
- 137. Kuloglu M, Atmaca M, Üstündag B, et al. Serum iron levels in schizophrenic patients with or without akathisia. Eur Neuropsychopharmacol 2003; 13: 67–71. [DOI] [PubMed] [Google Scholar]
- 138. Cotter PE, O’Keeffe ST. Improvement in neuroleptic-induced akathisia with intravenous iron treatment in a patient with iron deficiency. J Neurol Neurosurg Psychiatry 2007; 78: 548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Barnes M, Lawford BR, Burton SC, et al. Smoking and schizophrenia: is symptom profile related to smoking and which antipsychotic medication is of benefit in reducing cigarette use? Aust N Z J Psychiatry 2006; 40: 575–580. [DOI] [PubMed] [Google Scholar]
- 140. Anfang MK, Pope HG., Jr. Treatment of neuroleptic-induced akathisia with nicotine patches. Psychopharmacology 1997; 134: 153–156. [DOI] [PubMed] [Google Scholar]
- 141. Lerner V, Bergman J, Statsenko N, et al. Vitamin B6 treatment in acute neuroleptic-induced akathisia: a randomized, double-blind, placebo-controlled study. J Clin Psychiatry 2004; 65: 1550–1554. [DOI] [PubMed] [Google Scholar]
- 142. Cadet JL, Lohr JB. Possible involvement of free radicals in neuroleptic-induced movement disorders. Evidence from treatment of tardive dyskinesia with vitamin E. Ann N Y Acad Sci 1989; 570: 176–185. [DOI] [PubMed] [Google Scholar]
- 143. Berk M, Copolov D, Dean O, et al. N-acetyl cysteine as a glutathione precursor for schizophrenia–a double-blind, randomized, placebo-controlled trial. Biol Psychiatry 2008; 64: 361–368. [DOI] [PubMed] [Google Scholar]
- 144. Piercey MF, Smith MW, Lum-Ragan JT. Excitation of noradrenergic cell firing by 5-hydroxytryptamine1A agonists correlates with dopamine antagonist properties. J Pharmacol Exp Ther 1994; 268: 1297–1303. [PubMed] [Google Scholar]


