Abstract
Background:
Recent data suggest a beneficial effect of add-on treatment with Viscum album L (VA) on the survival in cancer patients. The objective of this study was to compare the impact of standard oncological therapy plus add-on VA treatment (S+VA) versus standard oncological therapy alone (S) on the overall survival (OS) of patients with nonmetastasized non–small cell lung carcinoma (NSCLC).
Methods:
The multicenter real-world data study was conducted using data from the Network Oncology Clinical Registry. The primary end point was OS. OS and impact on hazard in both treatment groups were compared.
Results:
A total of 275 patients with stages I to IIIA NSCLC were enrolled (mean age = 67.6 years, 57.2% male patients). No significant difference of OS was observed between both groups. Even though not significant, for a subgroup of unresected patients with stage I NSCLC, adenocarcinoma or squamous cell carcinoma, a medium effect size OS improvement was observed for S+VA compared to S.
Conclusions:
Our findings support the importance of surgery as the most effective intervention in nonmetastasized NSCLC patients. Add-on VA therapy shows here no additional effect in resected patients. However, a small subgroup analysis suggests a possible role of add-on VA for nonresected subgroups. Our results complement existing knowledge on the clinical impact of add-on VA therapy in NSCLC patients and may serve as hypothesis-generating data for further examinations in this cohort. Further research could be directed towards the role of combined therapy for nonresected early-stage NSCLC.
Keywords: NSCLC, advanced, nonmetastasized, lung cancer, Viscum album L, overall survival
Introduction
Lung cancer is the most common cancer worldwide and the most common cause of death from cancer worldwide.1-3 Five-year lung cancer survival rate has been increasing in several countries due to eliminating smoking initiations, increasing smoking cessations, lung screening programs in high-risk patients, and, last but not least, by new targeted therapies and immune-oncological treatments having been approved and applied during the last years.4-7 Non–small cell lung carcinoma (NSCLC) accounts for 85% of lung cancer.8 ESMO (European Society for Medical Oncology) Clinical Practice guidelines suggest that surgery is the gold standard for stages I and II NSCLC with anatomical resection being preferred over wedge resection.9 Furthermore, adjuvant chemotherapy (CTx) should be offered to patients with resected stages II and III NSCLC with a 2-drug combination with cisplatin. While targeted agents should not be used in the adjuvant setting, (neo-)adjuvant anti-PD(L)-1 checkpoint inhibitors are currently evaluated in addition to current standard of care. For locally advanced stage III NSCLC, surgery should be followed by adjuvant CTx (if N2 disease is only documented intraoperatively). For curative treatment, patients should be able to undergo platinum-based CTx. For unresectable stages IIIA and IIIB concurrent CTx is the gold standard treatment, and if not possible, sequential CTx followed by definitive radiotherapy is an effective alternative.9 Viscum album L (VA) is applied in integrative oncology concepts as an adjuvant to standard oncological CTx therapy to improve health-related quality of life.10-15 Add-on VA makes CTx more tolerable by alleviating disease-related and treatment-related symptoms and can thus support and augment its effect.16,17 Even though its potential beneficial effect on cancer survival has been described in a plethora of studies,11,15,16,18-21 further systematic research is needed to evaluate add-on VA’s impact on survival,15,22,23 which is discussed controversially between stakeholders. For stage IV NSCLC patients, we recently observed a clinical significant survival advantage for patients treated with combined CTx and add-on VA compared with CTx alone.24 The objective of the present multicenter real-world data (RWD) study was to evaluate the effect of additional VA treatment on the survival of stages I to IIIA NSCLC patients treated with standard oncological treatment.
Materials and Methods
Study Design and Patients
A noncontrolled, nonrandomized multicenter observational cohort study was conducted revealing RWD25 by analyzing patient registry data (Network Oncology [NO]). The NO is a conjoint clinical register of hospitals, practitioners, and outpatient centers26 of which 3 study centers participated. Patients were included who were 18 years or older, who gave written consent, with a histologically proven primary diagnosis of stages I to IIIA NSCLC seen between February 2012 and October 2017, and receiving standard oncological treatment surviving more than 28 days. Patients receiving monoclonal antibodies, tyrosine kinase inhibitors, or immuno-oncological therapy as standard oncological treatment were not excluded. Patients were not included if they did not give written consent, or when death date or last contact date was not available. Follow-up was performed routinely 6 months after first diagnosis and annually during the next years. Loss to follow-up was defined as no follow-up visits.
Ethics Approval and Consent to Participate
This study is a RWD study of the NO that has been approved by the Ethical Committee of the Medical Association Berlin (Berlin—Ethik-Kommission der Ärztekammer Berlin). The reference number is Eth-27/10. This study has been retrospectively registered at the World Health Organization–approved registry German Register for Clinical Trials (Deutsches Register Klinischer Studien, DRKS), trial registration number DRKS00013335 (http://www.drks.de/drks_web/setLocale_EN.do). Written informed consent has been obtained from all the patients prior study enrolment. The study complies with the principles laid down in the Declaration of Helsinki.
Data Collection
Structured Query Language inquiries on records of patients were run for lung cancer patients (International Classification of Diseases code: C34) using the clinical database NO. For queried patients, demographic data and hospital-related data (diagnosis, histology, pretreatment, and treatment) were retrieved from the NO. In addition, recorded TNM (tumor, node, metastasis) stages or documented metastases were queried with their according date and translated into Union for International Cancer Control (UICC) stages according to the seventh edition of TNM Classification of Malignant Tumours. UICC stage at first diagnosis was defined as the earliest recorded stage within a month of the diagnosis date. Furthermore, chemotherapeutic applications were queried with their according date. Surgical interventions were coded according to the German procedure classification 2013 (DIMDI; http://www.dimdi.de/static/en/klassi/ops/index.htm). Application of VA extracts in the context of an integrative oncological setting was retrieved with start and end dates, application type, and the pharmaceutical used. VA therapy was defined as lasting more than 4 weeks.
Classification of Groups
Included NSCLC patients were classified into the histological subgroups non–squamous cell carcinoma, squamous cell carcinoma, or large cell carcinoma. We then classified patients to 1 of 2 groups: (a) control (ctrl) group—patients received only standard oncological treatment and no VA therapy and (b) combined group—patients who received concomitant VA therapy ≥4 weeks. Ctrl or combined were applied as per routine clinical care. Nonrandomized allocation to the treatment groups was performed by the physician after elaborate information and patient’s decision on treatment options. VA therapy was applied subcutaneously according to SmPC.27-29 Off-label intravenous application was performed in individual cases.
Determination of Sample Size
The study was designed as a study with independent cases and controls (allocation scheme of 1:7 case–control). It was assumed that at least 3 explanatory variables were required to yield good results for binary outcome prediction. According to Harrell et al,30 10 cases per variable at minimum for logistic regression modelling would yield a stable model. Thus, 30 events in the case cohort and 210 events in the control cohort were needed leading to a total sample size of at least 240 patients for adjusted multivariate regression analysis.
Endpoints
The objective of this study was to evaluate the effect of VA in addition to standard oncological treatment on survival in stages I to IIIA NSCLC patients. The primary outcome of the study was the evaluation of overall survival (OS) and to test the hypothesis that stages I to IIIA NSCLC patients receiving additional VA to CTx have a longer OS than patients receiving CTx only. The secondary outcome was the assessment of factors for their association with the hazard of dying.
Statistical Analysis
The start date for survival analysis was the first date of available histology (index date), which was ±28 days of date of first diagnosis of stages I to IIIA lung cancer. Patient survival was calculated from index date until the patient’s last record, which was either the date of death, or the last documentation of personal contact, interdisciplinary tumor board, or follow-up (for follow-up measures please see study design and patients). A year lasted 365.25 days, and a month was 365.25/12 days. Kaplan-Meier survival was calculated for both groups. We employed the parametric and accelerated Weibull model in the analysis of survival data to estimate both relative event rates and relative extension in survival time. The Weibull distribution model as a parametric failure-time analysis allows a broad set of inferences to be made. Among other models, it is uniquely proportional and accelerated at the same time so that both relative event rates and relative extension in survival time can be analyzed.31,32
To analyze how different factors influence the hazard on patient survival and to reduce potential confounding bias, potential confounders (age, gender, and oncological treatment) were addressed. Verification analyses were performed, whether or not proportional hazard assumptions were met. All analyses were conducted using the software R, version 3.3.0—2016-05-03, R-Studio version 0.99.896, a language and environment for statistical computing.33 Continuous variables were described as median with interquartile range; categorical variables were summarized as absolute and relative frequencies. Data distributions were inspected graphically using box plots and histograms and were arithmetically examined for skewness. Patients with missing data were not included. For both groups, baseline characteristics and treatment regimens were compared using the unpaired Student’s t test for independent samples. For comparison of categorical variables, χ2 test analysis was performed. All tests were performed 2-sided. P < .05 were considered significant.
For survival analysis including Kaplan-Meier curves and right-censored time-to-event analyses the R-package34 “survival” was applied, version 2.41-3, published by Terry M. Therneau and Thomas Lumley on April 4, 2017 (https://CRAN.R-project.org/package=survival). To draw survival curves the package “survminer” was used, version 0.4.0, by Alboukadel Kassambara, Marcin Kosinski, and Przemyslaw Biecek published on June 7, 2017 (https://CRAN.R-project.org/package=survminer). For the Weibull model, the package “SurvRegCensCov,” version 1.4, published by Stanislas Hubeaux and Kaspar Rufibach October 8, 2015 (https://cran.r-project.org/web/packages/SurvRegCensCov/index.html) was applied. For the implementation of nonparametric estimators for censored event history (survival) analysis, the package “prodlim” was applied, version 1.6.1, published by Thomas A. Gerds on March 6, 2017 (https://CRAN.R-project.org/package=prodlim).
Results
Patients
A total of 275, stages I to IIIA, NSCLC cancer patients that were diagnosed between February 2012 and October 2017 (follow-up total: 2070 days; average 285.38 days) in the NO revealed complete histological data and showed survival of greater than 28 days after index date rendering eligibility of these patients for subsequent survival analysis (see study flow chart, Figure 1).
Figure 1.
Flow chart of the study population. NSCLC, non-small cell lung carcinoma; VA, Viscum album L, mistletoe.
Table 1 shows the main characteristics of 275 analyzed patients at baseline. No significant differences between groups with regard to demographic characteristics, tumor histology subtypes, smoker status, cancer-directed surgery, and radiation were seen. Two hundred thirty-seven (86.2%) patients received standard oncological therapy, while 38 patients (13.8%) received additional VA treatment. Mean age of the total cohort was 67.6 years with no significant differences between both groups. The sex ratio (male/female) was 1.33 in the total cohort. Of the total analyzed cohort, 135 patients were diagnosed with non-squamous (48.9%), 123 with squamous (44.6%), and 18 with large cell carcinoma (6.5%). The proportion of histology classes was well distributed between both groups.
Table 1.
| All patients (n = 275) |
Ctrl (n = 237) |
aoVA (n = 38) |
P | ||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Total number of patients | 275 | 100.0 | 237 | 86.2 | 38 | 13.8 | — |
| Age at diagnosis, mean (SD), years | 67.6 | 12.3 | 68.0 | 12.7 | 65.3 | 9.4 | .115 |
| Gender | |||||||
| Female | 118 | 42.8 | 96 | 40.5 | 22 | 56.4 | .092 |
| Male | 158 | 57.2 | 141 | 59.5 | 17 | 43.6 | |
| Body mass index | |||||||
| <25 | 102 | 45.9 | 84 | 45.2 | 18 | 50.0 | .639 |
| 25-29.9 | 91 | 41.0 | 76 | 40.9 | 15 | 41.7 | |
| 30+ (obese) | 29 | 13.1 | 26 | 14.0 | 3 | 8.30 | |
| Histology | |||||||
| ADC | 135 | 48.9 | 114 | 48.1 | 21 | 53.8 | .953 |
| SQC | 123 | 44.6 | 107 | 45.1 | 16 | 41.0 | |
| LCC | 18 | 6.5 | 16 | 6.8 | 2 | 5.1 | |
| Smoker | |||||||
| Current/past | 216 | 94.3 | 183 | 94.8 | 33 | 91.7 | .720 |
| Never | 13 | 5.70 | 10 | 5.2 | 3 | 8.30 | |
| Cancer-directed surgeryc | |||||||
| No | 110 | 39.9 | 93 | 39.2 | 17 | 43.6 | .736 |
| Yes | 166 | 60.1 | 144 | 60.8 | 22 | 56.4 | |
| Chemotherapy | |||||||
| No | 185 | 67.0 | 170 | 71.7 | 15 | 38.5 | 9.172e-05 |
| Yes | 91 | 33.0 | 67 | 28.3 | 24 | 61.5 | |
| Radiation therapy (lung) | |||||||
| No | 250 | 90.6 | 215 | 90.7 | 35 | 89.7 | 1.0 |
| Yes | 26 | 9.4 | 22 | 9.3 | 4 | 10.3 | |
| Partner status | |||||||
| Married/life partnership | 114 | 41.3 | 93 | 39.1 | 12 | 30.8 | .211 |
| Living alone | 62 | 22.5 | 56 | 23.6 | 6 | 15.4 | |
| NA | 100 | 36.2 | 88 | 37.2 | 12 | 30.8 | |
Abbreviations: Ctrl, control group; aoVA, add-on Viscum album L, SD, standard deviation; ADC, adenocarcinoma; SQC, squamous cell carcinoma; LCC, large cell carcinoma; NA, not specified.
Characteristics of patients with stages I to IIIA non–small cell lung cancer; percentages of sub-characteristics may not add up to 100% due to rounding procedures.
Chi-square analysis for categorical variables (or Fisher’s exact test in case frequencies were ≤5); Student’s t test for age distribution.
In one patient, cancer-directed surgery was not specified.
The majority of patients were current or past smokers (n = 216, 94.3%), with the proportions of smokers being equivalent between both groups. CTx was significantly different between both groups with a higher percentage of patients receiving CTx in the combinational arm (n = 26, 66.7%) versus the control arm (n = 67, 28.3%). One hundred fourteen patients (41.3%) of the total cohort lived in a marriage or life partnership, around one third (n = 62, 22.5%) lived alone, and for around one third (n = 100, 36.2%), the partner status was not known. Slightly but not significantly more patients in the control group lived in a marriage or life partnership. The years of diagnosis (2012-2017) were well distributed (data not shown).
Oncological Treatment
As to first-line CTx treatment, significant differences were seen between both groups (Table 1). As to first-line CTx treatment, platinum-compounds were received by 86 (94.5%) of all patients, mostly in combination with vinorelbine (n = 74, 81.3%) and pemetrexed (n = 7, 7.7%; see Table 2).
Table 2.
Composition of First-Line and (Neo)Adjuvant CTx Regimena.
| N (%) | Ctrl | Combined | |
|---|---|---|---|
| CTx | 91(100) | 66 (100) | 25 (100) |
| Platinum compounds | 86 (94.5) | 62 (93.9) | 24 (96) |
| + Vinorelbine | 74 (81.3) | 54 (81.8) | 20 (80) |
| + Pemetrexed | 7 (7.7) | 5 (7.58) | 2 (8.0) |
| + Paclitaxel/docetaxel | 2 (2.2) | 1 (1.5) | 1 (4.0) |
| + Etoposide | 1 (1.1) | 1 (1.5) | 0 (0) |
| + Gemcitabine | 1 (1.1) | 0 (0) | 1 (4.0) |
| Gemcitabine alone | 2 (2.2) | 2(3.0) | 0 (0) |
| Pemetrexed alone | 1 (1.1) | 1 (1.5) | 0 (0) |
| Epirubicine alone | 1 (1.1) | 1 (1.5) | 0 (0) |
| Vinorelbine alone | 1 (1.1) | 0 (0) | 1 (4.0) |
Abbreviations: CTx, chemotherapy; Ctrl, control group; NSCLC, non–small cell lung carcinoma.
First-line chemotherapy applied to patients with stages I to IIIA NSCLC. n = 91; numbers in rows and columns do not necessarily add to 100% as patients may have received various combinations of preparations.
Radiation was applied in 10 of 111 patients (9%) with stage I tumors, in 7 of 68 patients (10.3%) with stage II, and in 9 of 97 patients (9.3%) with stage IIIA. Ctx was applied in 12 of 111 patients (10.8%) with stage I cancer, in 28 of 68 patients (41.2%) with stage II, and in 51 of 97 patients (52.6%) with stage IIIA (data not shown).
As to second-line treatment (n = 25), the most often applied Ctx were platinum compounds (n = 12, 48.0%) in combination with vinorelbine (n = 6, 24.0%) or pemetrexed (n = 4, 16.0%), followed by nivolumab alone (n = 3, 12.0%) and docetaxel alone (n = 3, 12.0%), data not shown.
In addition to standard oncological treatment, 38 patients (13.8%) received extracts of VA (see Table 3). The most frequent type of application for mistletoe agents was off-label intravenous injection in 20 patients (52.6% of all VA patients), followed closely by subcutaneous injections in 15 (39.5%) patients, respectively. In general, abnobaviscum extracts (n = 12, 31.6%), mainly used for subcutaneous application (n = 8), and helixor extracts (n = 20, 52.6%), mainly used for intravenous application (n = 18), were the mistletoe remedies most often prescribed followed by subcutaneous Iscador preparations (n = 6, 15.8%).
Table 3.
Add-On VA Application in the Combined Groupa.
| Total | Abnobaviscum preparations | Iscador preparations | Helixor preparations | |
|---|---|---|---|---|
| Total number of patients, n (%) | 38 (100) | 12 (100) | 6 (100) | 20 (100) |
| Subcutaneous application, n (%) | 15 (39.5) | 8 (66.7) | 6 (100) | 1 (5.0) |
| Intravenous application, n (%) | 20 (52.6) | 2 (16.7) | — | 18 (90.0) |
| NA, n (%) | 3 (7.9) | 2 (16.7) | — | 1 (5.0) |
Abbreviations: VA, Viscum album L (mistletoe); NA, not specified.
Characteristics of VA therapy and application type applied additionally to standard oncological treatment (n = 16). Numbers in rows and columns do not necessarily add to 100% as patients may have received various combinations of preparations.
Outcomes
Two hundred seventy-five patients were included in the OS analysis. A survival benefit was seen for patients having received surgery (Surgery group) compared with patients having received no surgery (No Surgery group; see Figure 2 and Table 4). The median OS was 59.4 months in the surgery group (95% CI [confidence interval]: NA-NA) and 21.3 months (95% CI: NA-NA) in the group without surgery with 0 out of 14 events (see Table 4). This difference was statistically highly significant (χ2 = 26.1, P = 3e-07). One-year OS rates were 94.2% for the surgery group and 76.5% for patients who received no surgery; 3-year OS rates were 83.2% and 28.2%, respectively. Comparing surgery patients receiving further standard oncology therapy and surgery patients receiving standard oncology therapy plus VA no significant differences could be detected (χ2 = 1.5, P = .2).
Figure 2.
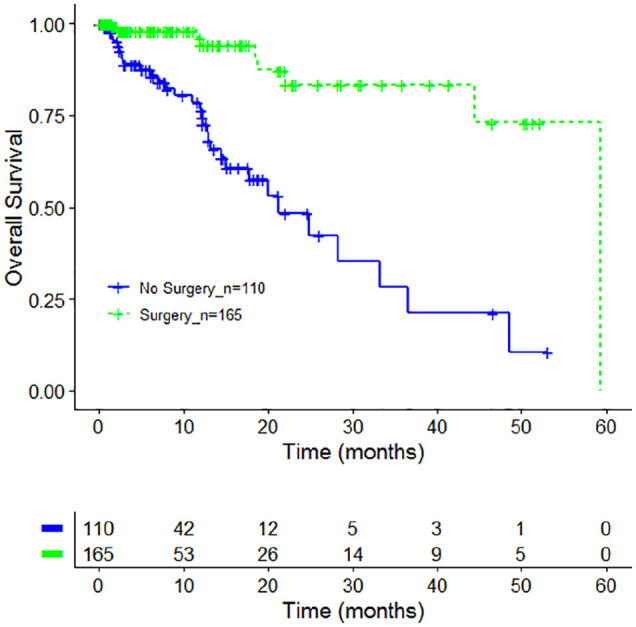
Overall survival by cancer-related surgery. Kaplan-Meier survival curves displaying overall survival in stages I to IIIA NSCLC (non–small cell lung carcinoma) patients in accordance to cancer-related surgery (green line) or no cancer-related surgery (blue line), n = 275.
Table 4.
Median Overall Survival According to Treatment and Cancer-Related Surgerya.
| N | Events | Median (months) | CI (months) | |
|---|---|---|---|---|
| All_no surgery | 110 | 31 | 21.3 | 15.1-NA |
| All_surgery | 165 | 9 | 59.4 | NA-NA |
| Log rank test χ² = 26.1 on 1 degree of freedom, P = 3e-07 | ||||
Abbreviations: CI, confidence interval; NA, not available; Ctrl, control group; VA, Viscum album L.
Median overall survival in accordance to treatment and cancer-related surgery, n = 311. Ctrl, oncological standard treatment; combined, oncological standard treatment plus add-on VA.
Factors Associated With Prolonged Survival
Cancer-directed surgery compared with no cancer-directed surgery significantly decreased hazard of death by 78% (hazard ratio: 0.22, 95% CI: 0.10-0.53, P = .0009) as shown by multivariate Weibull analysis adjusting for age, gender, tumor stage, cancer-directed surgery, radiation, CTx, and add-on VA therapy (see Table 5). While the effect for other covariates were not significant, the direction of impact on hazard was positive for male gender, UICC stage IIIA and radiation, and negative for CTx. Age and addition of VA therapy showed no direction of association with hazard of death and were not significant. Even though a significantly higher proportion of patients from the combined group received CTx, the survival hazard of CTx in the cohort was not significant.
Table 5.
| HR | Estimate (SE) | Value (error) | P | |
|---|---|---|---|---|
| Total number of patients n = 275 | ||||
| Age, median (IQR), years | 1.02 | 0.03 (0.02) | −0.02 (0.02) | .27 |
| Gender | ||||
| Female | Ref | |||
| Male | 1.46 | 0.37 (0.34) | −0.30 (0.28) | .28 |
| UICC stage | ||||
| I | Ref | |||
| II | 1.07 | 0.07(0.47) | −0.05 (0.37) | .88 |
| IIIA | 1.53 | 0.42 (0.46) | −0.34 (0.37) | .36 |
| Add-on VA therapy | ||||
| No | Ref | |||
| Yes | 1.00 | 0.007 (0.38) | −0.005 (0.30) | .99 |
| Cancer-directed surgery | ||||
| No | Ref | |||
| Yes | 0.22 | −1.49 (0.43) | 1.20 (0.30) | <.001*** |
| Radiation lung | ||||
| No | Ref | |||
| Yes | 1.17 | 0.15 (0.40) | −0.12 (0.32) | .70 |
| CTx first | ||||
| No | Ref | |||
| Yes | 0.83 | −0.19 (0.37) | 0.15 (0.30) | .61 |
Abbreviations: NSCLC, non–small cell lung cancer; HR, hazard ratio; SE, standard error; IQR, interquartile range; Ref, reference; UICC, Union for International Cancer Control; VA, Viscum album L; CTx, chemotherapy.
Multivariate regression analysis, HR based on Weibull model; model for each group adjusted for demographic variables and treatment regimens.
Except age being a continuous variable all other explanatory variables were of categorical nature.
P ≤ .001.
Subgroup Analysis 1: Association of OS With Treatment Group
A subgroup analysis was performed for patients not having undergone surgery (see flowchart in Figure 1). One hundred ten patients were included in the subgroup OS analysis, and of them 92 (83.6%) receiving standard oncological therapy (ctrl group) and 17 (15.5%) standard oncological plus VA therapy (combined group). As to OS, no significant differences were seen between combined and ctrl group (χ2 = 1.7, P = 0.2; see Figure 3 and Table 6). The median OS was 33.2 months (95% CI: 14.4-NA) in the combined group and 21.3 months in the ctrl group (95% CI: 13.2-NA).
Figure 3.
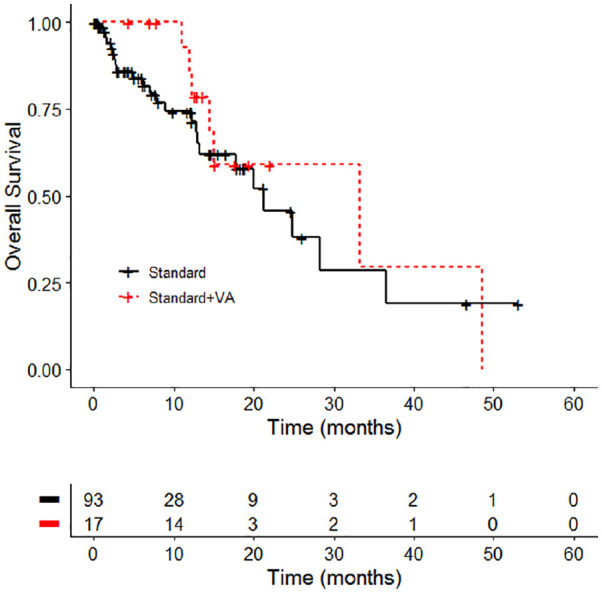
Overall survival by treatment group standard versus standard + Viscum album L (VA). Kaplan-Meier survival curves displaying overall survival in stages I to IIIA non–small cell lung carcinoma patients in accordance to the treatment, n = 110; standard, oncological standard treatment, standard + VA, oncological standard treatment plus add-on VA.
Table 6.
Median Overall Survival According to Treatment Groupsa.
| N | Events | Median (months) | CI (months) | |
|---|---|---|---|---|
| Ctrl | 93 | 24 | 21.3 | 13.2-NA |
| Combined | 17 | 7 | 33.2 | 14.4-NA |
| Log rank test χ2 = 1.7 on 1 degrees of freedom, P = .2 | ||||
Abbreviations: CI, confidence interval; Ctrl, control group; NA, not available; VA, Viscum album L.
Median overall survival, n = 110. Standard, oncological standard treatment, Standard + VA, oncological standard treatment plus add-on VA.
Subgroup Analysis 2: Association of OS With Histology and Treatment Group
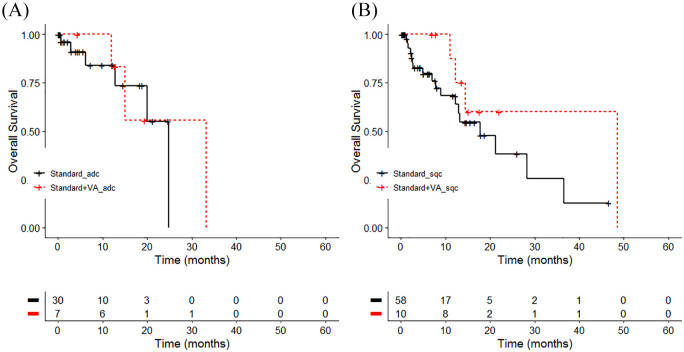
A second subgroup analysis was performed in the nonresected cohort investigating the effect of treatment on survival within the different histology group. No significant differences in survival were detected between the 3 histology groups as to treatment (χ2 = 1.7, P = .2; see Figure 4a and b and Table 7). For adenocarcinoma, patients’ OS was 8.4 months longer in the combined treatment group (median = 33.2 months) compared with the ctrl group with 24.8 months (χ2 = 1.7, P = .2). The same tendency was observed for patients with squamous cell carcinoma with a 30.8 months longer OS for patients receiving the combined treatment (median = 48.4 months) compared with patients receiving standard oncological treatment only (median = 17.7 months; χ2 = 1.6, P = .2). For large cell carcinoma, a comparison between both treatment groups was not possible due to absence of LCC patients in the combined group. Cohen’s d calculation revealed a small to medium effect size difference for patients with unresected stages I to IIIA adenocarcinoma (d = 0.4) and with unresected stages I to IIIA squamous cell carcinoma (d = 0.3) that were treated with the combined therapy compared with ctrl group.
Figure 4.
Overall survival by treatment and histology group. Kaplan-Meier survival curves displaying overall survival in stages I to IIIA non–small cell lung carcinoma (NSCLC) patients in accordance to the NSCLC histology group (A) adenocarcinoma (n = 37), χ2 = 1.7, P = .2, d = 0.44, or (B) squamous cell carcinoma (n = 68), χ2 = 1.6, P = .2, d = 0.31; Standard, oncological standard treatment; Standard + Viscum album L (VA), oncological standard treatment plus add-on VA; adc, adenocarcinoma; lcc, large cell carcinoma; sqc, squamous cell carcinoma.
Table 7.
Median Overall Survival According to Treatment and Histologya.
| N | Events | Median (months) | CI (months) | |
|---|---|---|---|---|
| Standard strata = adc | 30 | 6 | 24.8 | 20.0-NA |
| Standard + VA_strata = adc | 7 | 3 | 33.2 | 15.1-NA |
| Standard_strata = sqc | 58 | 18 | 17.7 | 12.3-NA |
| Standard +VA_strata = sqc | 10 | 4 | 48.5 | 14.4-NA |
| Standard_strata = lcc | 5 | 0 | NA | NA-NA |
| Log rank test χ2 = 1.7 on 1 degrees of freedom, P = .2 | ||||
Abbreviations: CI, confidence interval; NA, not available; VA, Viscum album L.
Median overall survival in accordance to treatment and histology, n = 110. Standard, oncological standard treatment; standard + VA, oncological standard treatment plus add-on VA.
Subgroup Analysis 3: Association of OS With Tumor stage and Treatment Group
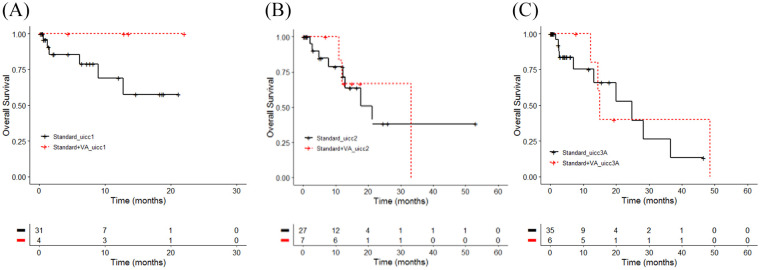
For the subgroup of patients who did not undergo surgery a third analysis was performed to detect potential survival differences between various tumor stage groups in accordance to treatment. UICC stage I NSCLC patients treated with additional VA showed a better but not significant survival, with both groups not reaching the median OS (χ2 = 1.7, P= .2; see Figure 5A-C and Table 8).
Figure 5.
Overall survival by treatment and Union for International Cancer Control (UICC) tumor stage. Kaplan-Meier survival curves displaying overall survival in stages I to IIIA non–small cell lung carcinoma patients in accordance to the UICC stages: (A) I (n = 35), χ2 =1.7, P = 0.2, d = 0.44; (B) stage II (n = 34), χ2 = 0, P = 0.9, d = 0; or (C) stages IIIA (n = 41), χ2 = 0.1, P = 0.7, d = 0.09; Standard, oncological standard treatment; standard + VA, oncological standard treatment plus add-on VA; UICC, UICC tumor stage.
Table 8.
Median Overall Survival of Patients With Stages I to IIIA NSCLC According to Treatment and UICC Stagea.
| N | Events | Median (months) | CI (months) | |
|---|---|---|---|---|
| Standard_uicc I | 31 | 6 | NA | 8.93-NA |
| Standard + VA_uicc I | 4 | 0 | NA | NA-NA |
| Standard_uicc II | 27 | 8 | 21.3 | 12.87-NA |
| Standard + VA_uicc II | 7 | 3 | 33.2 | 12.03-NA |
| Standard_uicc IIIA | 35 | 10 | 24.8 | 13.20-NA |
| Standard+VA_UICC IIIA | 6 | 4 | 15.1 | 14.43-NA |
| Log rank test Χ²= 2.2 on 5 degrees of freedom, p= 0.8 | ||||
Abbreviations: NSCLC, non–small cell lung carcinoma; UICC, Union for International Cancer Control; CI, confidence interval; NA, not available; VA, Viscum album L.
Median overall survival in accordance to treatment and UICC stage, n = 110. Standard, oncological standard treatment; Standard + VA, oncological standard treatment plus add-on VA.
A prolonged but not significant median OS was observed for UICC stage II NSCLC patients with 33.2 (combined group) versus 21.3 months OS (ctrl group; χ2 = 0, P = .9). A shorter but not significant median OS was calculated for UICC stages IIIA NSCLC patients with 15.1 (combined group) versus 24.8 months OS (ctrl group; χ2 = 0.1, P = .7). A medium effect size difference (d = 0.4) for patients with unresected stage I NSCLC who were treated with the combined therapy compared with ctrl group was observed (see Figure 5a-c and Table 8).
Discussion
The results of the present study on stages I to IIIA NSCLC patients reveal that add-on VA does not change OS of stages I to IIIA NSCLC patients treated with standard oncological therapy. Cancer-directed surgery showed here a highly significant association with improved OS outcome and the impact of surgery in patients with NSCLC remains indisputably the most important cure option for resectable stages I through IIIA NSCLC.8 Thus, we further investigated whether the addition of add-on VA may play an effective role in a subgroup of NSCLC patients who did not undergo surgery. Overall, the findings revealed that no significant OS differences were detected between unresected stages I to IIIA NSCLC patients with and without add-on VA treatment. Nevertheless, associations with OS prolongations of small to medium effect sizes were observed in unresected stage I NSCLC, unresected stages I to IIIA adenocarcinoma, and unresected stages I to IIIA squamous cell carcinoma patients when VA was added to standard oncological therapy. However, these differences were not significant and further research needs to be directed toward the role of add-on VA for nonresected early-stage NSCLC. Mistletoe preparations are total extracts from the whole plant. Subcutaneous VA application supplemented by off-label intravenous applications can stabilize the physical and mental condition of the oncological patient. Clinical studies on add-on VA therapy, among them various randomized controlled trial (RCT) studies being summarized in systematic reviews with meta-analyses, acknowledge a 41% to 51% reduction of hazard of death in oncological patients.19,21,35 In line with that, our group could confirm these results in 2 real-world studies for metastasized pancreatic (60% hazard reduction, P < .001) and stage IV NSCLC patients (56% hazard reduction, P < .005), respectively.24,36 In the latter published work, we could show as well a survival benefit for stage IV adenocarcinoma NSCLC patients compared with stage IV NSCLC with other histology types,24 underlining the clinical effect of add-on VA for this special histology type. Our results are difficult to compare with existing literature: From 7 RCTs published so far, where VA’s impact in lung cancer patients was investigated, 6 reported results on survival of lung cancer patients. From these only 3 RCTs included all stages of NSCLC,36-38 of which 2 were considered eligible for Cochrane analysis published in 2009.36,37 One of these 3 RCTs38 dealing with NSCLC patients was a randomized matched-pair study nested within a cohort study. As only 6 pairs of stages I and IV NSCLC patients were included in the randomized matched-pair part of the trial, no results according to RCT criteria were available for this entity. However, the nonrandomized prospective matched-pair part of this study38 revealed 52 pairs of stage- and standard oncological treatment-matched NSCLC patients showing 3.08 years of mean survival time in the add-on VA (Iscador) group versus 2.60 years in the control group (no add-on VA; log-rank test: P = .05), showing a tendency toward significance for this outcome and this entity. Thus, the outcome of this RCT is not fully comparable with the outcome of the present study as stage IV patients were not included in our study. The other 2 “Cochrane” studies showed no significant improved survival in the mistletoe arm versus ctrol groups. Nevertheless, it has to be remarked that in the other of the 3 RCTs,36 87 patients with surgery and add-on VA were compared with 96 surgery patients. As surgery has a great impact on the survival of lung cancer including NSCLC patients (as shown in our present study as well), survival differences as to add-on VA impact might have been masked.
Our study revealed that not more than 16% of the patients with unresectable NSCLC received combinational treatment and thus our results have to be interpreted conservatively. This may be due to the fact that stage IV rather than stages I to IIIA NSCLC patients comply with long-term (>4 weeks) VA treatments: in a recently published real-world study, 31.6% of stage IV NSCLC patients applied long-term add-on VA therapy.24 The latter number is in line with a study revealing that between 23% and 66% of lung cancer patients seek and continuously apply integrative oncology treatment options.39 Even up to 77% of lung cancer patients are applying add-on complementary therapies including VA therapy, but the respective study indicated that these patients are mainly late-stage (IIIB and IV) lung cancer patients.40
Limitations of the study may be its observational nature implying that our findings and conclusions have to be handled with caution and should be interpreted in light of existing randomized, controlled trials. Furthermore, unwanted biases (eg, preference bias) may have been introduced into the analysis, for example, the assignment of treatment with add-on VA was performed in a nonrandomized, noncontrolled, and unblinded fashion and physicians could have unintentionally selected patients with better prognoses for VA therapy. In addition, subgroup analyses included nonresected patients who mainly perform poorer than resected lung cancer patients; however, due to the nature of data analysis, it is not possible to evaluate whether some of the nonresected patients refused resection and could have performed better. Further risk of bias could have been included as patients in the combined group received add-on VA ≥4 weeks. Small sample size in the subgroup analyses implies that the results have to be treated conservatively. Immortal time bias was not controlled for which may affect estimates of therapeutic effectiveness. The strengths of our study are the integration of multicenter and real-world daily care data under typical hospital conditions and the external validity of our results as the characteristics of the patients and relevant factors (eg, surgery) being associated with OS of lung cancer patients are comparable with published data. Thus, our findings may complement the recently reported clinical impact of add-on VA therapy in NSCLC patients.
Conclusions
Our findings support the importance of surgery as the most effective intervention in nonmetastasized NSCLC patients. Furthermore, they indicate that add-on VA therapy has no additional effect in resected nonmetastasized NSCLC. However, a small subgroup analysis suggests a possible role of add-on VA for nonresected subgroups. These findings have to be reevaluated in further prospective randomized studies. The results of our study complement existing knowledge on the clinical impact of add-on VA therapy in NSCLC patients and may serve as hypothesis-generating data for further examinations in this cohort. Further research could be directed toward the role of combined therapy for nonresected early-stage NSCLC.
Acknowledgments
We would like to thank all staff members at the GKH and the FIH involved in the present work.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: BM received fees for lectures or advisory boards from AstraZeneca, Boehringer Ingelheim, Helixor, Kyowa-Kirin, Leo, Lilly, Roche, Teva, outside the submitted work. BM received grants for travelling from AstraZeneca, BMS, Boehringer Ingelheim, Celgene, Helixor, Iscador, Janssen, Kyowa-Kirin, Leo, Lilly, Novartis, MSD, Pfizer, Roche, Teva. FS reports grants from Helixor Heilmittel GmbH (travel costs and honoraria for speaking), grants from AstraZeneca (travel costs and honoraria for speaking), grants from Abnoba GmbH, grants from Iscador AG, outside the submitted work. CG reports grants from Iscador AG, outside the submitted work. The other authors have declared that no competing interests exist. No payment was received for any other aspects of the submitted work. There are no patents, products in development or marketed products to declare. There are no other relationships, conditions, or circumstances that present a potential conflict of interest.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The Network Oncology was funded by unrestricted research grants from Iscador AG Arlesheim, Switzerland; ABNOBA GmbH Pforzheim, Germany; and Helixor GmbH Rosenfeld, Germany. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. By contract, researchers were independent from the funder.
ORCID iD: Friedemann Schad  https://orcid.org/0000-0002-6928-6209
https://orcid.org/0000-0002-6928-6209
References
- 1. Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359-E386. [DOI] [PubMed] [Google Scholar]
- 2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7-30. [DOI] [PubMed] [Google Scholar]
- 3. EMA. Keytruda, INN-pembrolizumab. Annex I: summary of product characteristics. Accessed November 27, 2017 http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/003820/WC500190990.pdf
- 4. Gettinger S, Horn L, Jackman D, et al. Five-year follow-up of nivolumab in previously treated advanced non-small-cell lung cancer: results from the CA209-003 study. J Clin Oncol. 2018;36:1675-1684. [DOI] [PubMed] [Google Scholar]
- 5. Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [DOI] [PubMed] [Google Scholar]
- 6. National Lung Screening Trial Research Team, Aberle DR, Adams AM, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365:395-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391:1023-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83:584-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Postmus PE, Kerr KM, Oudkerk M, et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2017;28(suppl 4):iv1-iv21. [DOI] [PubMed] [Google Scholar]
- 10. Lange-Lindberg AM, Garrido MV, Busse R. Mistletoe treatments for minimising side effects of anticancer chemotherapy. GMS Health Technol Assess. 2006;2:Doc18. [PMC free article] [PubMed] [Google Scholar]
- 11. Ostermann T, Raak C, Bussing A. Survival of cancer patients treated with mistletoe extract (Iscador): a systematic literature review. BMC Cancer. 2009;9:451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bussing A, Raak C, Ostermann T. Quality of life and related dimensions in cancer patients treated with mistletoe extract (iscador): a meta-analysis. Evid Based Complement Alternat Med. 2012;2012:219402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Greenlee H, Balneaves LG, Carlson LE, et al. Clinical practice guidelines on the use of integrative therapies as supportive care in patients treated for breast cancer. J Natl Cancer Inst Monogr. 2014;2014:346-358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Greenlee H, DuPont-Reyes MJ, Balneaves LG, et al. Clinical practice guidelines on the evidence-based use of integrative therapies during and after breast cancer treatment. CA Cancer J Clin. 2017;67:194-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Horneber MA, Bueschel G, Huber R, Linde K, Rostock M. Mistletoe therapy in oncology. Cochrane Database Syst Rev. 2008;2008(2):CD003297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bock PR, Friedel WE, Hanisch J, Karasmann M, Schneider B. Efficacy and safety of long-term complementary treatment with standardized European mistletoe extract (Viscum album L.) in addition to the conventional adjuvant oncologic therapy in patients with primary non-metastasized mammary carcinoma. Results of a multi-center, comparative, epidemiological cohort study in Germany and Switzerland [in German]. Arzneimittelforschung. 2004;54:456-466. [DOI] [PubMed] [Google Scholar]
- 17. Troger W, Zdrale Z, Tisma N, Matijašević M. Additional therapy with a mistletoe product during adjuvant chemotherapy of breast cancer patients improves quality of life: an open randomized clinical pilot trial. Evid Based Complement Alternat Med. 2014;2014:430518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kienle GS, Kiene H. Complementary cancer therapy: a systematic review of prospective clinical trials on anthroposophic mistletoe extracts. Eur J Med Res. 2007;12:103-119. [PubMed] [Google Scholar]
- 19. Ostermann T, Bussing A. Retrolective studies on the survival of cancer patients treated with mistletoe extracts: a meta-analysis. Explore (NY). 2012;8:277-281. [DOI] [PubMed] [Google Scholar]
- 20. Schad F, Atxner J, Buchwald D, et al. Intratumoral mistletoe (Viscum album L) therapy in patients with unresectable pancreas carcinoma: a retrospective analysis. Integr Cancer Ther. 2014;13:332-340. [DOI] [PubMed] [Google Scholar]
- 21. Troger W, Galun D, Reif M, Schumann A, Stanković N, Milićević N. Viscum album [L.] extract therapy in patients with locally advanced or metastatic pancreatic cancer: a randomised clinical trial on overall survival. Eur J Cancer. 2013;49:3788-3797. [DOI] [PubMed] [Google Scholar]
- 22. Kienle GS, Berrino F, Bussing A, Portalupi E, Rosenzweig S, Kiene H. Mistletoe in cancer—a systematic review on controlled clinical trials. Eur J Med Res. 2003;8:109-119. [PubMed] [Google Scholar]
- 23. Bar-Sela G, Wollner M, Hammer L, Agbarya A, Dudnik E, Haim N. Mistletoe as complementary treatment in patients with advanced non-small-cell lung cancer treated with carboplatin-based combinations: a randomised phase II study. Eur J Cancer. 2013;49:1058-1064. [DOI] [PubMed] [Google Scholar]
- 24. Schad F, Thronicke A, Steele ML, et al. Overall survival of stage IV non-small cell lung cancer (NSCLC) patients treated with Viscum album L. in addition to chemotherapy, a real-world observational multicenter analysis. PLoS One. 2018;13:e0203058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Khozin S, Blumenthal GM, Pazdur R. Real-world data for clinical evidence generation in oncology. J Natl Cancer Inst. 2017;109. doi: 10.1093/jnci/djx187 [DOI] [PubMed] [Google Scholar]
- 26. Schad F, Axtner J, Happe A, et al. Network Oncology (NO)—a clinical cancer register for health services research and the evaluation of integrative therapeutic interventions in anthroposophic medicine. Forsch Komplementmed. 2013;20:353-360. [DOI] [PubMed] [Google Scholar]
- 27. Fachinformation Helixor. Published 2017. Accessed December 18, 2017 http://misteltherapie.at/die-misteltherapie/gebrauchs-fachinformation
- 28. Arzneimittelinformation AbnobaVISCUM. Published 2017. Accessed December 18, 2017 http://www.abnoba.de
- 29. Arzneimittelinformation Iscador. Published 2017. Accessed December 18, 2017 https://www.iscador.com/de/
- 30. Harrell FE, Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;15:361-387. [DOI] [PubMed] [Google Scholar]
- 31. Carroll KJ. On the use and utility of the Weibull model in the analysis of survival data. Control Clin Trials. 2003;24:682-701. [DOI] [PubMed] [Google Scholar]
- 32. Weibull W. A statistical distribution of wide applicability. J Appl Mechanics. 1951;18:293-297. [Google Scholar]
- 33. R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Published 2016. Accessed November 27, 2017 https://www.R-project.org/
- 34. Ostermann T, Appelbaum S, Poier D, Boehm K, Raak C, Büssing A. A systematic review and meta-analysis on the survival of cancer patients treated with a fermented Viscum album L. extract (iscador): an update of findings. Complement Med Res. Published online January 10, 2020. doi: 10.1159/000505202 [DOI] [PubMed] [Google Scholar]
- 35. Axtner J, Steele M, Kroz M, Spahn G, Matthes H, Schad F. Health services research of integrative oncology in palliative care of patients with advanced pancreatic cancer. BMC Cancer. 2016;16:579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Salzer G, Danmayr E, Wutzlhofer F, et al. Adjuvante Iscador Behandlung operierter nicht kleinzelliger Bronchuskarzinom—Ergebnisse einer randomisierten Studie. Deutsche Zeitschrift für Onkologie. 1991;23:93-98. [Google Scholar]
- 37. Dold U, Edler L, Mäurer HC, et al. Krebszusatztherapie beim fortgeschrittenen nicht-kleinzelligen Bronchialkarzinom. Thieme; 1991. [Google Scholar]
- 38. Grossarth-Maticek R, Kiene H, Baumgartner SM, Ziegler R. Use of Iscador, an extract of European mistletoe (Viscum album), in cancer treatment: prospective nonrandomized and randomized matched-pair studies nested within a cohort study. Altern Ther Health Med. 2001;7:57-66, 68-72, 74-56. [PubMed] [Google Scholar]
- 39. Frenkel M, Slater R, Sapire K, et al. Complementary and integrative medicine in lung cancer: questions and challenges. J Altern Complement Med. 2018;24:862-871. [DOI] [PubMed] [Google Scholar]
- 40. Thronicke A, Oei SL, Merkle A, Sierpina V. Integrative cancer care in a certified cancer centre of a German anthroposophic hospital. Complement Ther Med. 2018;40:151-157. [DOI] [PubMed] [Google Scholar]