Abstract
Cartilage injuries are typically caused by trauma, chronic overload, and autoimmune diseases. Owing to the avascular structure and low metabolic activities of chondrocytes, cartilage generally does not self-repair following an injury. Currently, clinical interventions for cartilage injuries include chondrocyte implantation, microfracture, and osteochondral transplantation. However, rather than restoring cartilage integrity, these methods only postpone further cartilage deterioration. Stem cell therapies, especially mesenchymal stem cell (MSCs) therapies, were found to be a feasible strategy in the treatment of cartilage injuries. MSCs can easily be isolated from mesenchymal tissue and be differentiated into chondrocytes with the support of chondrogenic factors or scaffolds to repair damaged cartilage tissue. In this review, we highlighted the full success of cartilage repair using MSCs, or MSCs in combination with chondrogenic factors and scaffolds, and predicted their pros and cons for prospective translation to clinical practice.
Keywords: Mesenchymal stem cell, chondrogenic factor, cartilage injury, cartilage tissue engineering, cartilage regeneration
Introduction
Cartilage has limited self-repair ability due to its complex structure and the relatively low metabolic activities of chondrocytes. There are many causes of cartilage injuries, including trauma, chronic overload, and autoimmune disease. Injury frequently leads to gradual cartilage tissue impairment, resulting in pain, functional damage, and degenerative diseases.1 However, the treatment of cartilage injury is still a perplexing problem for orthopedic surgeons, owing to unsatisfactory therapeutic outcomes. Early clinical intervention for symptomatic cartilage lesions focuses on non-surgical management techniques, such as cell therapy, hormone therapy, and cytokine therapy.2 However, these interventions also have their limitations, including the leakage of drugs, a relatively low concentration at the injured area, and severe systemic side effects. The surgical management of cartilage injuries includes microfracture, autologous osteochondral transplantation, and total joint replacement. Autograft is an effective treatment for cartilage injuries, but transplantation is also associated with the risk of various complications, such as limited cartilage mass, donor site complications, and infections.3
The limitations of effective clinical treatment for cartilage injury have prompted the development of regenerative medical therapies. Stem cells are extensively used in a variety of regenerative medicine field due to its capacity to proliferate and directionally differentiate.4 The outstanding chondrogenic potential of mesenchymal stem cells (MSCs) has made MSC therapy a potential alternative strategy for cartilage repair (Scheme 1). MSCs can be isolated from tissue easily, while extensive pre-clinical trials confirmed MSCs could differentiate into cartilage tissue under chondrogenic factors, facilitating their use for the repair of injured cartilage.5 Moreover, in the process of differentiation, MSCs could produce various extracellular matrices (ECMs), which are essential for the recovery of cartilage functions.6 At the targeted repair areas, MSCs could release various cytokines, growth factors, and chemokines, driving endogenous MSCs to enter lesion areas and creating an appropriate regenerative microenvironment while aiding the regeneration of cartilage tissue simultaneously.4 The combination of MSCs with exogenous biochemical or biomechanical stimuli, as well as the engineered scaffolds in MSC-based therapies, has demonstrated significant advances in cartilage regeneration (Scheme 2).7
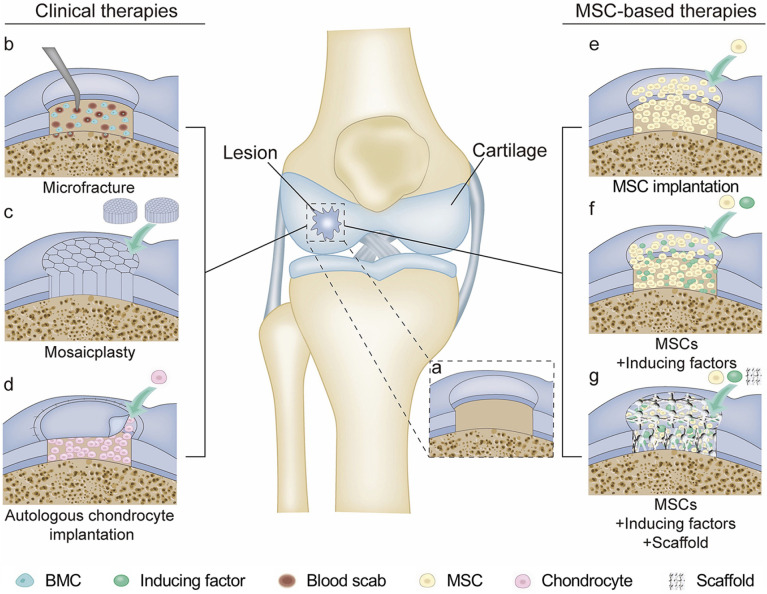
Scheme 1.
Cartilage repair modalities. (a) Full-thickness cartilage injury. (b) Microfracture. Channels were produced 3−4 mm deep to penetrate the subchondral bone to allow BM-MSCs to migrate from the bone marrow to the defect area. (c) Mosaicplasty. Healthy osteochondral plugs were implanted from the little weight-bearing area into the defect area. (d) Autologous chondrocyte implantation. The defect area was filled with autologous chondrocytes and covered with a periosteal slice. (e) MSC implantation. The defect area was filled with MSCs. (f) The combination of MSCs and inducing factors was loaded into the defect. (g) The biocompatible scaffold contained MSCs and inducing factors were implanted into the defect area to repair the lesion.
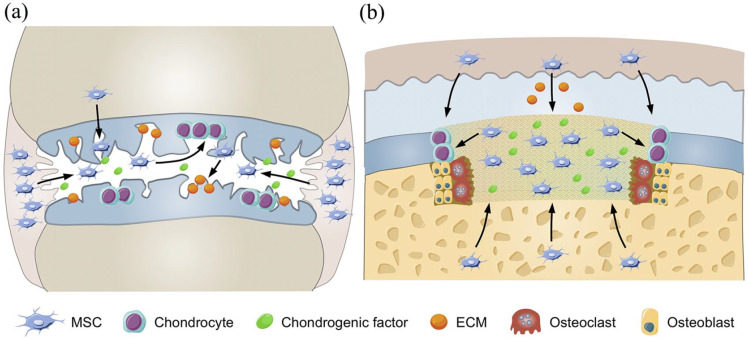
Scheme 2.
Cartilage repair process. (a) The chondrogenic factors recruit endogenous MSCs to repair cartilage injury. The arrow indicates the migration of MSCs from the joint synovium, synovial fluid, and bone marrow to the injured cartilage. Moreover, chondrogenic factors induce the differentiation of MSCs to chondrocytes and the production of cartilage-related ECMs in the process of cartilage repair. (b) Engineering scaffold repairs osteochondral defect. The MSCs and chondrogenic factors were seeded into the scaffold, and the scaffold provides a 3D micro environment for proliferation and differentiation of MSCs. The arrow indicates the migration of MSCs to the defect area and MSC differentiation into chondrocytes in the presence of chondrogenic factors. When the scaffold is degraded by osteoclasts gradually, the chondrocytes and osteoblasts are filled into the vacancy to repair the osteochondral tissue.
This review provides a detailed presentation of the outcomes of different MSC-based strategies for cartilage regeneration and discusses their prospective translation to clinical practice. We believe this is a comprehensive overview that promotes the theoretical basis for MSC-based strategies for cartilage regeneration.
MSCs for cartilage regeneration
MSCs are derived from various tissues and have the potential to differentiate into chondrocytes.8 Moreover, MSCs produce a variety of ECM molecules that are critical for cartilage function, including collagens (Cols), fibronectin, proteoglycans, and glycosaminoglycans (GAGs), as well as a variety of cytokines.9 Generally, the source of MSCs for cartilage regeneration can be acquired endogenously or exogenously.
Endogenous MSCs
Microfracture surgery is a commonly used technique for early-phase cartilage injury. In microfracture surgery, the surgeon drills several holes in the subchondral bone to discharge bone marrow marrow-derived mesenchymal stem cells (BM-MSCs), cytokines, and platelets from the marrow, which can stimulate the regeneration of cartilage.10 Microfracture surgery has been preferred by the majority of orthopedic surgeons for its simple single-stage technology and confined invasiveness. Moreover, microfracture was 90% successful in relieving pain postoperatively in cartilage lesions.11 After applying microfracture surgery in full-thickness cartilage defect, the histological evaluation of the early changes of cartilage units showed that the repair was caused by endochondral ossification in depths of microfracture punctures.12 Moreover, endochondral ossification could activate osteoclast and induce the reconstruction of cartilage, which regenerates earlier than the subchondral bone. The Food and Drug Administration (FDA) considered microfracture a splendid prognosis in the treatment of small size cartilage injuries. Many types of research showed microfracture could postpone cartilage degeneration regardless of the lesion size.13,14 However, some studies illustrated that the post-surgical microenvironment of microfracture failed to induce the differentiation of BM-MSCs appropriately, leading to the formation of relatively unstable fibrous tissue rather than cartilage tissue.15
Exogenous MSCs
The exogenous way of acquiring MSCs is obtaining it through other tissues within the same host. Currently, BM-MSCs, adipose tissue–derived mesenchymal stem cells (AT-MSCs), and peripheral blood-derived mesenchymal stem cells (PB-MSCs) have been widely investigated both in surgical and in injective measures.16 These MSCs could be implanted into the joint by surgical incision or intra-articular injection for small size cartilage repair. The selection of treatment measures mainly depends on the specific cartilage pathology.
Wakitani et al.17 reported in a clinical trial that BM-MSC implantation was used for the treatment of patients with osteoarthritis (OA), and hyaline cartilage–like tissue was observed at the defect areas after 42 weeks of implantation. A post-surgical arthroscopic assessment after AT-MSC implantation in human OA knee was carried out to explore its clinical prognosis.18 The prognosis was assessed based on the International Knee Documentation Committee (IKDC) score and the Tegner activity level. A remarkable improvement in the IKDC score and the Tegner activity level was observed after the operation.18 However, high body mass index (BMI) and large lesion size were regarded as a potential predictor of poor clinical outcomes in OA knee with the treatment of AT-MSC implantation. According to the International Cartilage Repair Society (ICRS) standards, 76% of patients were reported to have abnormal cartilage repair by a post-surgical arthroscopic study, indicating the unsatisfied outcomes of OA with the treatment of MSCs alone. For the treatment of large cartilage lesions, it may be necessary to combine the surgical approaches with tissue engineering technology.
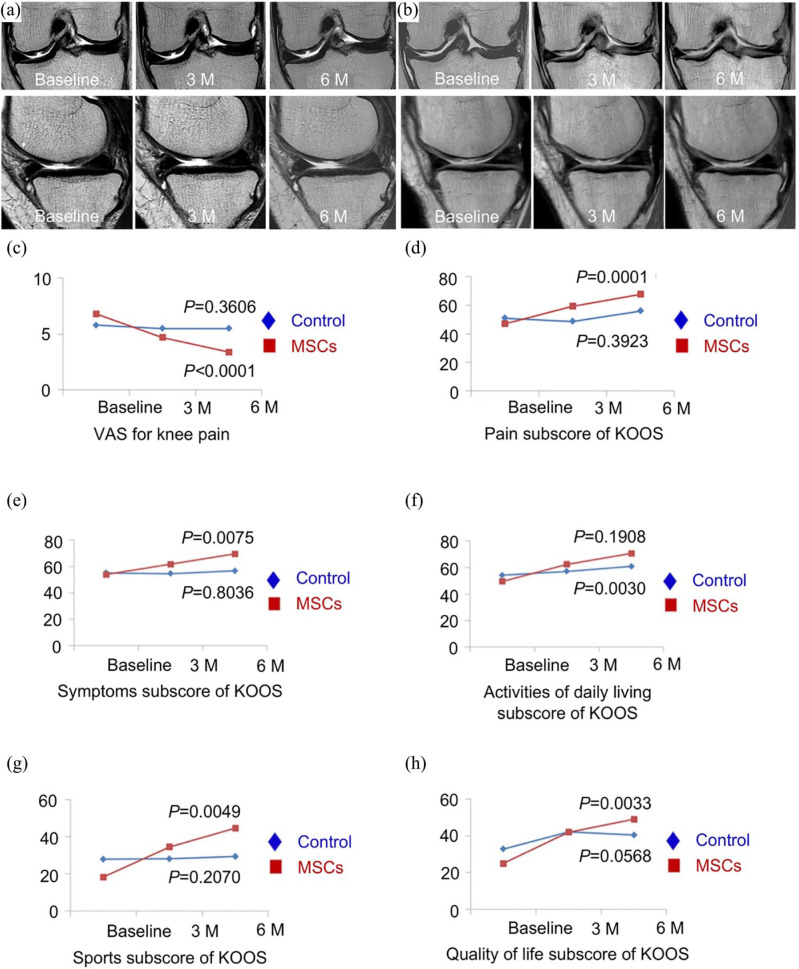
Compared with MSC implantation, intra-articular injection of MSCs increased the risk of MSCs migrating to non-target tissue.16 Thus, this approach seems to be only suitable for cartilage degeneration in OA patients. A patient with severe knee OA showed positive result after a single-dose injection of BM-MSCs, without the use of supplemental drugs.19 Magnetic resonance imaging (MRI) showed that cartilage thickness enhanced and subchondral edema decreased in patients at six months’ follow-up.20 In a phase IIb, stochastic clinical trial, Lee et al. assessed the therapeutic effect of the intra-articular injection of autologous AT-MSCs into joint OA (Figure 1).21 After six months of AT-MSC injection, the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scores were improved. In addition, MRI showed no significant variations in cartilage defect with AT-MSC injection at six months, while the defect in the control group was found to be deteriorated.
Figure 1.
Safety and efficacy of intra-articular injection of AT-MSCs for knee osteoarthritis. (a) MRI images of tibial condyles and medial femoral of the knee before or three or six months after the intra-articular injection of AT-MSCs showed a change in cartilage defect within six months. (b) At different time points, MRI images of femoral and tibial condyles showed a significant increase in cartilage defect at six months’ follow-up after injection of normal saline. (c−h) Visual analog scale (VAS) changes in knee pain and knee injury, and osteoarthritis outcome score (KOOS) score changes in AT-MSC intra-articular injection group and control group during six months. Adopted from Lee et al. and reprinted with permission of John Wiley and Sons.21
There are several issues related to the isolation and manipulation of MSCs in the process of cartilage repair, including the selection of the most appropriate tissue source and proper delivery route to the cartilage lesion. There are several challenges to be overcome before MSC implantation becomes a practical cartilage repair approach in the clinic.
Combination therapy of MSCs and chondrogenic factors
Small molecule drugs
Small molecule drugs have crucial roles in MSC-based cartilage regeneration and show unique advantages over traditional growth factors in cartilage repair. First, small molecule drugs are too small to induce immune response compared with macromolecular substances, such as proteins.22 In addition, manufacturing cost and cross-species contamination risk can be significantly decreased with the application of small molecule drugs compared to protein-based growth factors.23 Numerous small molecule drugs had been developed for cartilage regeneration, including kartogenin (KGN) and various natural bioactive compounds.24
KGN is one of the most commonly used small molecule chondrogenic factors, which could induce MSC chondrogenesis in a dose-dependent pattern, and could also improve the production of chondrogenesis-related protein of MSCs, including Col II and aggrecan.24 Moreover, KGN is very stable at room temperature, easing storage and transportation. KGN-pretreated BM-MSCs were shown to be more effective in the formation of chondral matrix and postponement of cartilage deterioration than the normal BM-MSCs.22 In addition, Spakova et al.25 found that KGN was a chondrogenic promoter of BM-MSCs in the process of cartilage regeneration. They collected osteochondral cylinders and BM-MSCs from OA patients, and then KGN was added into the co-culture of osteochondral cylinders and BM-MSCs. The BM-MSCs without KGN were treated as a control group. After 21 days of co-culture, histological and scanning electron microscopic (SEM) analysis revealed that BM-MSCs had colonized on the surface of OA cartilage in the presence of KGN, and secretion of GAGs and proteoglycans was also detected, while the chondrogenic markers were barely detectable in the control group, indicating the effectiveness of KGN in the process of cartilage regeneration.
In addition to KGN, the extracts curcumin and resveratrol also demonstrated great potential in inducing MSC chondrogenic differentiation. Curcumin belongs to the polyphenol ingredient segregated from Curcuma longa, which has powerful antioxidant, anti-carcinogenic, anti-angiogenesis, and anti-inflammatory effects.26 Curcumin demonstrated a high capacity to inhibit the secretion of inflammatory cytokines in arthritis, assisting MSCs in cartilage regeneration. It is well known that OA and rheumatoid arthritis (RA) are characterized as cartilage degeneration in the inflammatory microenvironment. Traditional MSC implantation therapies for OA and RA usually do not have satisfying outcomes as pro-inflammatory cytokines could hinder the differentiation of MSCs, which reside in the cartilage or surrounding tissue. Curcumin impedes the secretion of pro-inflammatory cytokines and builds a microenvironment to antagonize the pro-inflammatory condition, thus facilitating chondrogenesis of AT-MSCs.27 Buhrmann et al.27 demonstrated that curcumin restrained transcription factor nuclear factor-κB (NF-κB) transcription as well as the activation of caspase-3 induced by interleukin-1β (IL-1β) in AT-MSCs. In addition, curcumin treatment improved Col II production and proteoglycan synthesis, indicating that curcumin treatment may support the repair of cartilage by modifying the inflammatory microenvironment for AT-MSCs in joint.
Similar to curcumin, resveratrol was found to have immunomodulatory, antioxidative, and anti-inflammatory roles.28 Resveratrol could protect MSCs and ensure the process of chondrogenic differentiation in the inflammatory microenvironment by inhibiting the activation of apoptosis signaling and reversing the catabolic effect. In addition, an exciting insight showed resveratrol may be a promising anti-aging drug for the treatment of age-related cartilage illness.28 Csaki et al. found that resveratrol could inhibit IL-1β-induced caspase-3 activation, reactive oxygen species (ROS) generation, and p53-related apoptosis in time- and dose-dependent patterns.29,30 Moreover, resveratrol was reported to inhibit IL-1β and protect BM-MSC-derived chondrocytes from inflammatory factors.31 When BM-MSCs were stimulated with IL-1β, Col II and aggrecan production was inhibited, while matrix metalloprotein-13 (MMP-13) production was increased. In addition, resveratrol was reported to reverse catabolism by decreasing the translocation of NF-κB, indicating that resveratrol could protect BM-MSC-derived chondrocytes from IL-1β by inhibiting NF-κB.
Cartilage-inducing factors
Growth factors are considered to be one of the most essential substances in the development and homeostasis of cartilage. However, growth factors are always limited by relatively short storage periods and heterotopic ossification. The emergence of MSC-based therapies has provided excellent prospects for growth factors to be used in therapies for cartilage injuries. Growth factors activate MSC differentiation, and this process recruits endogenous MSCs and induces the formation of cartilage-related extracellular matrices, providing promising therapeutic efficacy for cartilage injuries.
In the various clinical trials exploring the chondrogenic potential of growth factors, bone morphogenetic proteins (BMPs) demonstrated fascinating roles in MSC-based therapies for cartilage regeneration. BMPs not only enhance the chondrogenic differentiation of MSCs but also recruit endogenous MSCs to the injured area to stimulate the repair process of cartilage. BMP2 and BMP7 have proven to have the ability to induce MSCs into chondrogenic differentiation.32 Moreover, the combination of MSCs and BMPs activated endogenous cell migration, which then promotes cartilage healing. Dorman et al. evaluated the effects of BMP treatment on undifferentiated MSCs. As early as 48 h after BMP treatment, MSCs showed morphological changes, and the number of cells increased, indicating BMP could induce the proliferation and differentiation of MSCs effectively.33 Grande et al.34 isolated periosteum MSCs, transfected BMP-7 gene into cells, and implanted them to full-thickness osteochondral defects of the middle trochlear nerve. The results demonstrated that the transfected MSCs dramatically improved the quality of repaired tissue compared with the normal MSC group, indicating that the combination of BMPs and MSCs is a potential therapy for cartilage injuries.
As another member of the transforming growth factor (TGF) superfamily, TGF-β is also essential for the regulation of MSC differentiation in the process of cartilage repair.35 TGF-β is commonly reported as an effective activator for the synthesis of proteoglycans and Col II.36 In addition, TGF-β could stimulate chondrogenesis of MSCs.37 The application of TGF-β3 was reported to further improve the functional chondrogenesis differentiation of BM-MSCs under dynamic compression.38 Furthermore, compared with the continuous exposure to TGF-β, transient exposure to TGF-β could also improve the functional chondrogenesis of BM-MSCs while reducing chondrocyte hypertrophy.39 The study investigated the differentiation and maturation of BM-MSCs in transient exposure to TGF-β3 at various doses and durations. The results demonstrated that brief exposure to a high dose of 100.0 ng mL−1 was sufficient to stimulate cartilage regeneration. Moreover, the mechanical and biochemical characteristics of the regeneration tissue in brief exposure to a high dose of TGF-β3 exceeded the sustaining exposure to low dose of 10.0 ng mL−1.39
Insulin-like growth factor (IGF) is a critical cartilage-inducing factor that regulates the chondrogenic behavior of MSCs, and the existence of IGF enhances the production of chondrocyte-related matrices.40 The IGF family consists of two ligands, with IGF-1 being widely used in cartilage repair. IGF-1 is considered a fundamental mediator of cartilage homeostasis because it stimulates proteoglycan synthesis and promotes chondrocyte proliferation.40 In addition, IGF-1 induces BM-MSCs to differentiate into the chondrocyte phenotype by improving the production of GAG.41 The transfection of the IGF gene into MSCs promoted the chondrogenic differentiation potential without the induction of hypertrophic phenotype.42 The content of GAG and the expression of Col IIA1 were improved in the IGF-MSC group, and IGF-induced tissue showed a similar prosperity with the chondrocyte phenotype.
Fibroblast growth factor (FGF) has various functions in regulating MSCs to repair cartilage injuries.43 FGF promotes the proliferation of undifferentiated MSCs in vitro. Moreover, FGF improved the chondrogenic ability of MSCs during the differentiation process. During the in vitro development of MSCs, FGF ligands could promote the proliferation of MSCs while maintaining the undifferentiated state of cells.44 FGF-2 could synergistically improve MSC proliferation and subsequent chondrogenic differentiation with Wingless (Wnt) signal. The treatment of BM-MSCs with FGF-2 enhanced the production of cartilage-related components during the process of chondrogenesis.45 However, while the differences in chondrogenic gene expression between the FGF-2 and control groups were gradually decreased, an increasing GAG content distinction was maintained. The result indicated that FGF could induce early differentiation and enhance the chondrogenesis of BM-MSCs in vitro. In addition, treatment with FGF-2 in equine BM-MSCs during monolayer expansion was reported to improve subsequent chondrogenesis in the three-dimensional (3D) culture system, which was essential for cartilage repair dependent on BM-MSC treatment.46
Other chondrogenic proteins
In addition to small molecule drugs and growth factors, platelet-rich plasma protein (PRP) is also used in MSC-based therapies for cartilage regeneration. PRP could induce MSC proliferation and increase ECM production to repair the damaged cartilage.47 PRP can be acquired by centrifuging blood samples, which releases factors for the repair of injured tissues.48 Chen et al. analyzed the PRP therapeutic effect with and without MSCs for OA models.47 The PRP + MSC treatment demonstrated superior repair outcomes in morphology and GAG production than the other groups, and the Osteoarthritis Research Society International (OASRI) scores were significantly lower than those of cartilage treated with other therapies. The combination of PRP and l-ascorbic acid promoted the chondrogenesis of AT-MSCs.49 With the addition of 10% PRP, the proliferation and chondrogenic differentiation ability of AT-MSCs increased. Moreover, proteoglycan production also increased.49
Parathyroid hormone-related protein (PTHrP) has been reported to perform fundamental roles in cartilage homeostasis, maintaining the chondrocyte phenotype. The effect of PTHrP on MSCs mainly depends on the pattern of application. Constant application of PTHrP could inhibit chondrogenic differentiation of MSCs, while intermittent supplements enhanced chondrogenic differentiation of MSCs and inhibited chondrocyte hypertrophy at the same time.50 Constant supplementation of PTHrP during BM-MSC chondrogenic differentiation inhibited chondrocyte hypertrophy and suppressed the chondrogenesis of BM-MSC to its articular phenotype.50 However, an intermittent pulsed PTHrP for 6 h promoted BM-MSC chondrogenic differentiation and restrained the hypertrophy.51 Similarly, although the continuous exposure to PTHrP inhibited BM-MSC chondrogenic differentiation, the pulsed exposure to PTHrP dramatically enhanced the deoxyribonucleic acid (DNA) content of Col II and proteoglycan after 6 weeks. Intermittent exposure to PTHrP increased MSC chondrogenic differentiation and decreased endochondral ossification, which indicated that periodic application of PTHrP might be an effective method for increasing the chondrogenesis of BM-MSCs.52 Furthermore, during the process of BM-MSC chondrogenic differentiation, PTHrP was found to regulate chondrocyte hypertrophy in the development of embryonic cartilage in a hypertrophy model of chondrogenic BM-MSCs in vitro.50
Biomechanical factors
Cartilage is one of the load-bearing tissues that have significant roles in diarthrodial joints. The importance of physical loading in chondrocyte maturation and phenotype maintenance is well known. The biomechanical characteristics of the cartilage depend on the composition and arrangement of ECMs.53 Reduced biomechanical loading usually leads to atrophy, while mechanical overloading can lead to irreversible injuries.53 In MSC-based therapies, pre-stimulating MSCs with physical biomechanical stimuli could improve cartilage regeneration. The biomechanical characteristics of articular joints are hydrostatic pressure, shear, and compression. Therefore, the commonly used mechanical stimuli in cartilage repair are compression, hydrostatic pressure, and shear stress. The comprehension of the importance of physical and biomechanical factors on MSC behavior might offer a unique insight into stimuli factors for cartilage regeneration.
Static compression could increase the chondrogenesis of embryonic limb bud by upregulating expression of Col II, Sox9, and aggrecan.54 Moreover, the invention of bioreactors could achieve the application of various complicated physical loading in MSCs, including hydrostatic pressure and compression.55 The application of intermittent hydrostatic pressure stimulated the expression of genes Col II, Sox9, and aggrecan.56 Moreover, the effect of dynamic compression on MSC chondrogenesis has been extensively investigated. Huang et al.57 observed the expression of Col II, Sox9, and aggrecan following dynamic compression on rabbit BM-MSCs. Given that, multiple modal bioreactors were used to mimic these biomechanical properties in vivo, and the application of multi-modal bioreactors would further improve the chondrogenesis of MSCs.58 Compared with the usage of compression or shear alone, the combination of cyclic compression with shear not only increased the chondrogenic differentiation of BM-MSCs but also enhanced GAG and Col II deposition without exogenous growth factor stimulation.59 Further study has shown that physical stimuli could induce the potential secretion activity of BM-MSCs. Gardner et al. observed that shear and compression promoted the endogenous production and secretion of TGF-β1.60
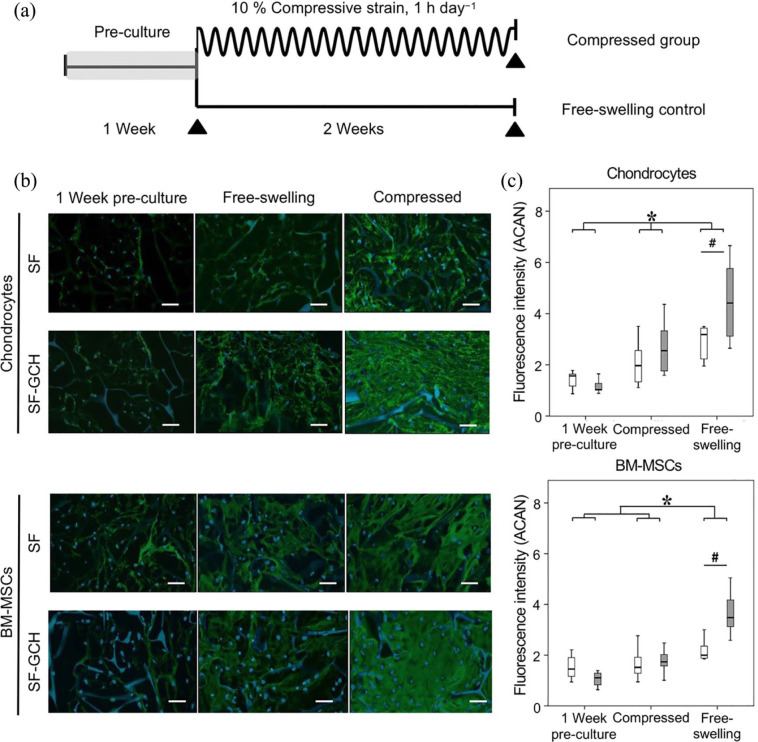
Moreover, the combination of the scaffold with mechanical stimuli that mimic the native cartilage microenvironment has been found to enhance chondrogenesis for cartilage repair (Figure 2).61 Porous scaffolds based on silk fibroin (SF) and SF with gelatin/chondroitin sulfate/hyaluronate (SF-GCH) were developed, and then BM-MSCs or chondrocytes were seeded into the scaffold with or without dynamic compression. The dynamic compression significantly increased the chondrogenesis of MSCs and chondrocyte biosynthesis in both scaffolds.61 The microenvironment provided by scaffolds and compression improved the production of chondrogenic matrices, aggrecan, and Col IIA1 in BM-MSCs and chondrocytes.61 By spatially controlled local biochemical and mechanical characteristics of the scaffold, the outside dynamic compression induced cell deformation into the scaffold.62 Neven et al. developed a hydrogel with layers of varying stiffness, and then dynamic compression was applied to the hydrogels to produce different strains. The results demonstrated that although the same dynamic mechanical stimulation was applied, high strains stimulated Col II production, while low strains simulated Col I production.62 This finding showed that appropriate mechanical stimulations are potent regulators of MSC differentiation and may act as a potential stimulus factor for the treatment of cartilage injuries.
Figure 2.
Efficacy of dynamic compression on BM-MSC and chondrocyte behavior. (a) Overview of study design. The constructs were motivated for 2 weeks with 1 h compression per day at 10% compressive strain (1 Hz, 23 h of rest period per day). (b) Immunofluorescence staining of aggrecan in chondrocyte and BM-MSC constructs under different conditions. Immunofluorescence staining of aggrecan (green) with nucleus (blue) in chondrocyte construct or BM-MSC construct. Scale bars = 50 μm. (c) Integrated fluorescence intensities of aggrecan in chondrocyte and BM-MSC constructs. The chondrocyte- and BM-MSC-seeded scaffolds under dynamic compression showed higher aggrecan staining than other conditions. Moreover, the aggrecan staining was found in SF-GCH constructs than in SF constructs under dynamic compression. Adopted from Sawatjui et al. and reprinted with permission of John Wiley and Sons.61
MSCs with engineered scaffolds for cartilage regeneration
Scaffolds play an essential role in successful cartilage repair. The appropriate scaffold for cartilage repair should be made from biodegradable and biocompatible materials, which support chondrogenesis. The degradation rate of the scaffold may match the cartilage formation speed, and the mechanical properties of the scaffold should satisfy the physical loading to afford enough space for tissue regeneration.7 Moreover, the engineered scaffold should process appropriately porous structure to permit nutrients and waste products produced by cells to migrate to the synovial fluid.63 Various polymers were investigated to find satisfactory scaffolds for cartilage regeneration. Based on the characteristics of engineered scaffolds, we could divide them into natural and synthetic scaffolds. To examine and summarize some of the various options within the natural and synthetic polymers, we expect to provide an optimizing choice of MSC-based therapies for cartilage regeneration.
Natural polymer scaffolds
Natural polymers for cartilage regeneration can be mainly classified into proteins and polysaccharides. The composition of natural polymers is similar to the cartilage ECM components. Natural polymers can be degraded hydrolytically and enzymatically, and the degradation products are usually not harmful to the surrounding tissue.7 Besides cartilage ECM-related proteins, non-cartilaginous tissue-associated proteins, including fibrin and silk, are also utilized to manufacture scaffolds with different physical and chemical properties.64
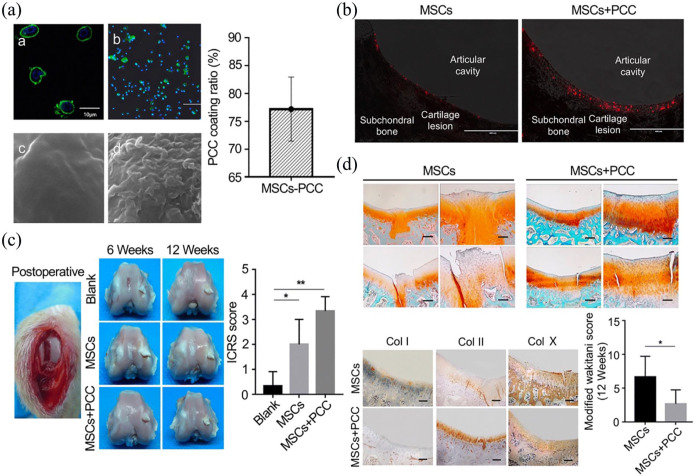
Col is the main component of cartilage ECM and makes an optimized material for scaffold fabrication. Cartilage ECM has the capacity to promote BM-MSC proliferation, improve cartilaginous matrix formation, and inhibit hypertrophic differentiation of BM-MSC-derived chondrocytes.65 It has been reported that pericellular Col I coating enhanced BM-MSC homing and chondrogenic differentiation during intra-articular injection (Figure 3).66 Xia et al.66 developed a pericellular Col I coating (PCC) for BM-MSCs, and the capability of BM-MSC-PCC homing and chondrogenic differentiation was evaluated in rabbit cartilage defect models. After the injection of BM-MSC-PCC for 12 weeks, PCC-coated BM-MSCs were found to enhance the quality of cartilage regeneration.66 In addition, the implantation of Col I and Col III gels seeded with BM-MSCs was also found to induce cartilage regeneration and the repair of subchondral bone in osteochondral defects.67 The Col II-coated surface accelerated the deposition of calcium, and Col II modulated early osteogenic differentiation of BM-MSCs by activating runt-related transcription factor (RUNX).68 According to the digestion of cartilage ECMs, Cols had been found to have the ability to promote chondrogenic differentiation. Screening of multiple Col types demonstrated Col IX had the ability to induce chondrogenesis and promote cell proliferation and matrix production, and inhibit matrix degradation at the same time.65 After evaluating the chondrogenic effects of MSCs in different ECM types, Col II was found to produce favorable conditions for the chondrogenic phenotype expression of BM-MSCs.69 The application of Col in clinical cartilage repair was also actively investigated, and the autologous BM-MSCs/Col-scaffolds were used in the restoration of torn meniscal cartilage.70 Autologous BM-MSCs were segregated from iliac crest in patients, and expanded and seeded into Col scaffold, and then the BM-MSC-Col scaffold was implanted into the area of meniscal tear prior to the vertical mattress suture operation. During two years of postoperative follow-up, five patients showed significant clinical improvement, indicating the combination of MSCs with Col scaffold could improve meniscal repair outcomes in some patients.70
Figure 3.
Col I coating on BM-MSCs after intra-articular injection for cartilage defect. (A) Pericellular Col I coating. (a) Laser confocal microscopy observation of BM-MSCs coated with PCC. Scale bar = 10 μm. (b) Observation of PCC-coated BM-MSCs by fluorescence microscope. Scale bar = 500 μm. (c) SEM image of BM-MSC at 10,000× magnification. (d) SEM image of Col I–coated BM-MSCs at 10,000× magnification. (B) Homing and retention of BM-MSCs in vivo. Red particles represented BM-MSCs. (C) Macroscopic observation and histological scoring of the repaired cartilage. (D) Safranin O/fast green staining of regenerated cartilage and the modified Wakitani score of the regeneration of cartilage and immunohistochemical staining of Col I, Col II, and Col X in cartilage defect of trochlear groove after intra-articular injection of BM-MSCs and BM-MSC-PCC for 12 weeks. Scale bar = 100 μm. Adopted from Xia et al.66 and reprinted with permission of Springer Nature.
Fibrin is another protein-based scaffold prepared by fibrinogen polymerization.71 It can be stimulated and polymerized under vascular pathological conditions.72 Fibrin glue was commonly the cell delivery system during the process of cartilage repair for generating new cartilage matrices.64 In addition, fibrin glue promotes the proliferation of MSCs.73 Kim et al.74 found that the fibrin glue scaffold maintains the functional survival and paracrine ability of MSCs within the scaffold. Haleem et al.75 seeded autologous BM-MSCs into a fibrin glue scaffold and then transplanted it into the osteochondral defect in femoral condyles to evaluate the clinical treatment in human patients, and the clinical symptoms of all patients were improved over the 12 months’ follow-up. Fibrin had been developed as a feasible scaffold for AT-MSC implantation. Compared with the implantation with only AT-MSCs, better ICRS grades were presented to the group of AT-MSCs loaded in fibrin glue.76 Fibrin-based hydrogel-encapsulated BM-MSCs could induce BM-MSC chondrogenic differentiation, supporting the use of fibrin as the encapsulating matrix for the cartilage phase in an osteochondral construct.77
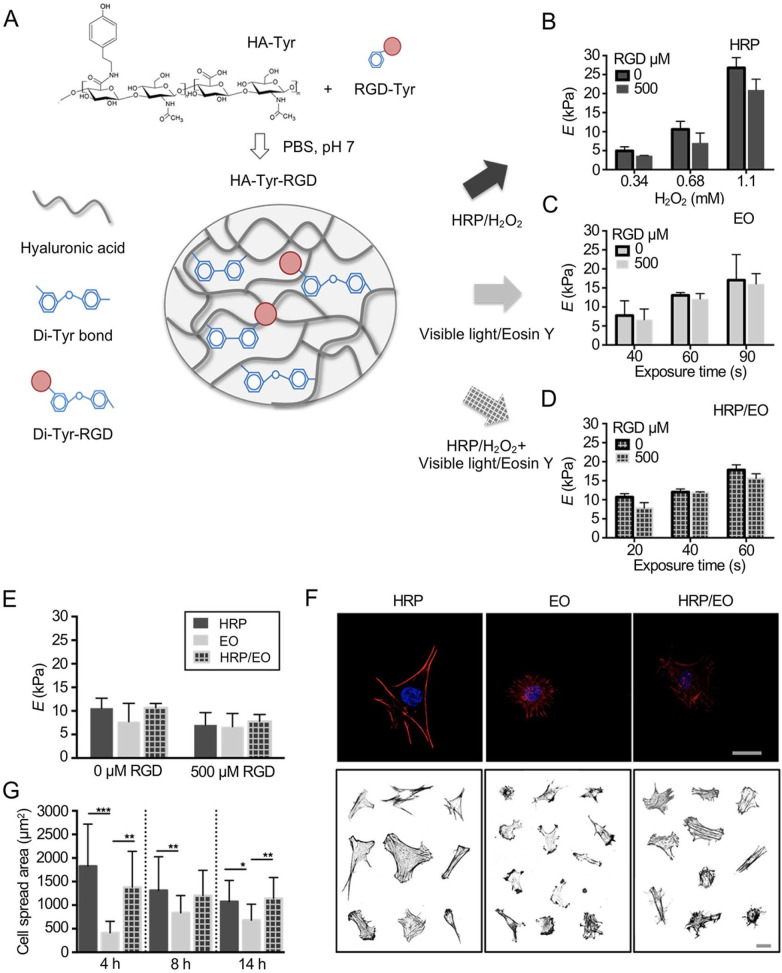
Hyaluronic acid (HA) is an essential material for the hydration and organization of the proteoglycan framework. It is also a critical component of the cartilaginous ECM, which can be degraded by hyaluronidases naturally in vivo.2 However, HA is not suitable for cartilage repair under unmodified conditions due to its relatively low mechanical property. Therefore, it usually needs to be cross-linked with other molecules to enhance its mechanical characteristics in cartilage engineering. The cross-linking density not only impacts the physical features of hydrogels but also affects MSC chondrogenesis and hypertrophy.78 Increased cross-linking by changing the HA macromer concentration or extended light exposure time resulted in decreased matrix production and deposition.78 This process also induced MSC hypertrophy, leading to matrix calcification.78 Given the importance of HA hydrogels in tissue engineering, it was necessary to investigate how the differences between chemical and physical characteristics of the scaffold direct cell fate. To that end, a hyaluronan-tyramine (HA-Tyr) hydrogel was fabricated by enzymatic cross-linking using horseradish peroxidase (HRP) or by photo-cross-linking to investigate the effect of different hydrogel cross-linking mechanisms on the behavior of BM-MSCs (Figure 4).79 The results illustrated that when hydrogels are fabricated with equal Young’s moduli, BM-MSCs cultivated on enzymatically formed HA-Tyr hydrogels showed improved cell diffusion and longer length of focal adhesion than cells grown on light-triggered gelation matrices. Similarly, Kim et al. found that the change of mechanics and adhesiveness of HA fibers affected MSC interactions and gene expressions. The proliferation of MSCs was decided by the adhesiveness of hydrogels, and the expression of chondrogenic markers relied on mechanics and adhesiveness properties. The softer fiber increased the chondrogenesis of MSCs.80
Figure 4.
Impact of different cross-linking of HA-Tyr hydrogels on behaviors of BM-MSCs. (a) The overview of fabricating HA-Tyr substrates. HA was reacted with tyramine to generate HA-Tyr conjugates, and hydrogels were formed enzymatically either with HRP/H2O2 (HRP) by adding a photosensitizer and light illumination of an enzymatically pre-cross-linked matrix (HRP/EO) or without H2O2 (EO). (b−d) The atomic force microscopy measured initial Young’s modulus of HA-Tyr hydrogels, unmodified or modified with 500 μm RGD formed (b) enzymatically (HRP), (c) with light (EO), or (d) combination of both (HRP/EO). (e) Initial Young’s modulus of the HA-Tyr substrates modified with 500 μm RGD. (f) Actin cytoskeletal organization of representative BM-MSCs cultured on HRP, EO, and HRO/EO cross-linked hydrogels (7 kPa) for 8 h and stained for F-actin (red) and cell nuclei (blue). Scale bars = 25 μm. (g) Quantification of cell spread area. Adopted from Loebel et al.79 and reprinted with permission of American Chemical Society.
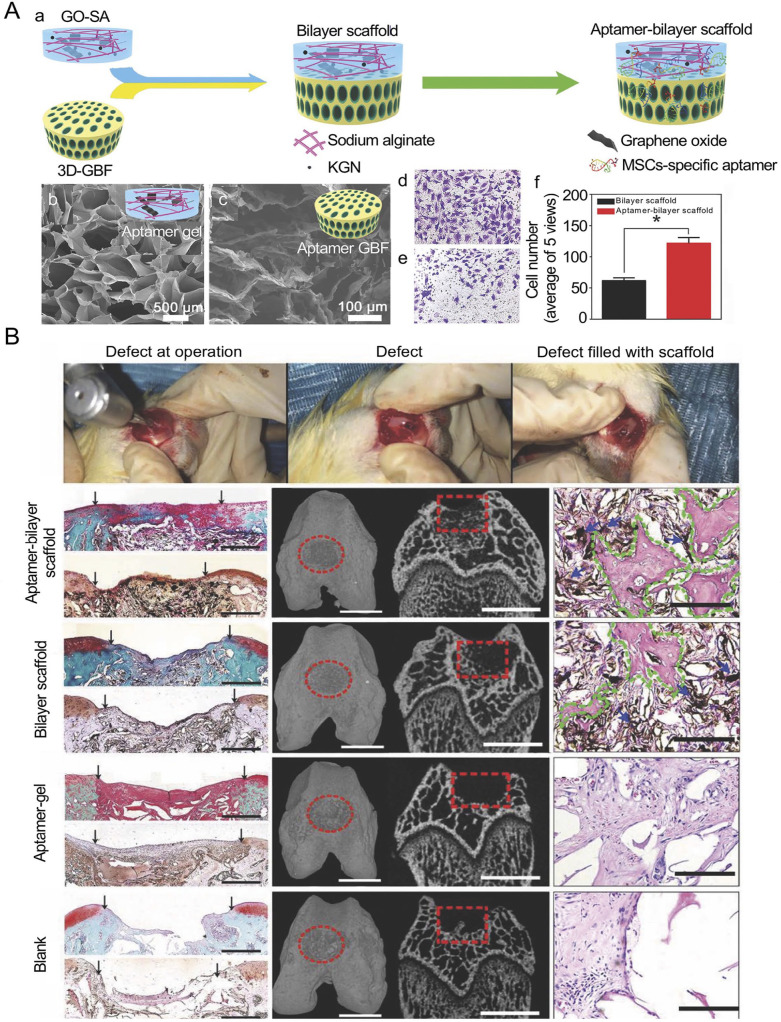
Alginate and agarose are the commonly used matrices of cartilage scaffolds. They are easily gelated and have excellent biocompatibility to encapsulate cells. Alginate derived from algae undergoes reversible gelation by cross-linking with various cations. Alginate had been utilized as a matrix for cultivating MSCs and chondrocytes for its capacity to maintain the chondrocyte phenotype. Moreover, alginate could be used in the repair of osteochondral structure in bilayer scaffolds. The scaffold based on sodium alginate (SA) and graphene oxide (GO) was fabricated for autologous BM-MSC recruitment. Thus, the repair of osteochondral tissue can be achieved simultaneously (Figure 5).81 GO was cross-linked to an SA meshwork with the addition of KGN. A 3D GO-based biomineral framework (3D-GBF) was used for the repair of subchondral bone defect. Then, BM-MSC-specific aptamers were attached to the scaffold for capturing BM-MSCs. The scaffold recruited endogenous BM-MSCs from bone marrow, and KGN promoted chondrogenic differentiation of BM-MSCs, while GBF accelerated osteoblastic differentiation of BM-MSCs. As alginate and fibrin hydrogels had been widely used in cartilage repair, a fibrin/alginate blended hydrogel was fabricated to harness the beneficial characteristics of both materials. The blended hydrogels not only preserved gel extensibility and promoted BM-MSC proliferation but also facilitated the synthesis of GAG and Col II, resulting in chondrogenic differentiation.82 Agarose is also a category of polysaccharide containing galactose residues, which segregated from algae. It can be used for the cultivation of cells and induces AT-MSC chondrogenic differentiation.83 The implantation of agarose-coating chondrocytes and BM-MSCs resulted in the production of Cols and proteoglycans in the area of osteochondral defects.84 BM-MSCs were filled into agarose and cast in specific molds to manufacture various scaffold constructs, and then the different scaffold architectures were combined with dynamic culture conditions to investigate the optimal cultivation methods for BM-MSCs.85 To investigate the fate of BM-MSCs in long-term maturation in a 3D scaffold, bovine BM-MSCs were cultivated in agarose to form cartilage tissue. The results showed that chondrogenesis occurred in BM-MSC-seeded hydrogels, while the mechanical properties of these neo-tissues were similar to the native chondrocyte matrix.
Figure 5.
Bilayer scaffold for osteochondral defect in knee joint. (A-a) Preparation of aptamer-bilayer scaffold. (b) SEM image of aptamer-gel. Scale bars = 500 μm. (c) SEM image of aptamer-GBF. Scale bars = 100 μm. (d) Light microscopy image of transwell assay of BM-MSCs on aptamer-bilayer scaffold and (e) bilayer scaffold. (f) Statistical data of transwell assay. (B) Scaffold implantation process. Histomorphology analysis of the neo-cartilage tissue in different scaffolds. Scale bars = 0.5 mm. Micro-CT reconstruction of the osteochondral defect in scaffolds. Scale bars = 2 mm. Histomorphology analysis of newly formed bone tissue after treatment with scaffolds. Scale bars = 100 μm. The black arrow points to the interface between the repair tissue and the host cartilage, the red elliptical ring and red box represent the osteochondral defect, and the blue arrow and green shape represent the scaffold and newly formed bone tissue, respectively. Adopted from Hu et al. and reprinted with permission of John Wiley and Sons.81
Chitosan belongs to the deacetylated derivate of chitin, lysosomes could degrade chitosan in vivo, and chitosan-containing matrices have been widely used in the region of cellular encapsulation, drug release, and cell culture.86,87 A novel polyvinyl alcohol–chitosan composite hydrogel seeded with MSCs provided comparable treatment outcomes to the traditional alginate-MSC construct implantation in cartilage defects at the medial condyle of femora, supporting the efficacy of chitosan in cartilage repair.88
As a denatured form of Col, gelatin also exhibited good cell-adhesion ability and thus became a promising scaffold in cartilage regeneration. A gelatin absorbable sponge was developed as a feasible carrier for the regeneration of cartilage during BM-MSC-based therapies.89 After culture for 21 days in vitro, the gelatin sponge with BM-MSCs generated a cartilage-like ECM. Gelatin was also reported to develop into a microsphere, which could release TGF-β1 to induce chondrogenesis of MSCs as a selective method to supplement growth factors in the culture medium.90 However, gelatin had also shown some disadvantages in the process of cartilage repair, including the weakness to resist mechanical stresses.91 Modifying gelatin by cross-linking could solve this problem. As seen, it enhanced the mechanical characteristics of the scaffold in the implantation of BM-MSCs seeded with cross-linked gelatin. The scaffold demonstrated excellent biocompatibility and repairing effect after implantation into the osteochondral defect.91
Although silk is not a component of cartilage, silk and its derivatives belong to a kind of naturally degradable fibrin with distinct mechanical properties and prominent biocompatibility that could support the proliferation of MSCs in the presence of chondrogenic growth factors.92 The application of autologous BM-MSCs and 3D porous silk scaffolds had the possibility of successful cartilage repair.93 After three weeks of cultivation of MSCs in the silk scaffold, cell alignment and Col deposition in scaffold resembled natural cartilage, indicating the potential of the novel 3D silk scaffold in MSC-based cartilage repair. Moreover, the hybridization of dense Col (DC) with silk fibrin (SF) produced multi-layered DC-SF-DC scaffolds.94 The multi-layered scaffolds promoted BM-MSC differentiation by upregulating Col IIA1 and aggrecan expression. In addition, compared to the pure DC and SF scaffolds, the multi-layered scaffold promoted the production of Col II and improved the sustained chondrogenic and osteoblastic differentiation of BM-MSCs.
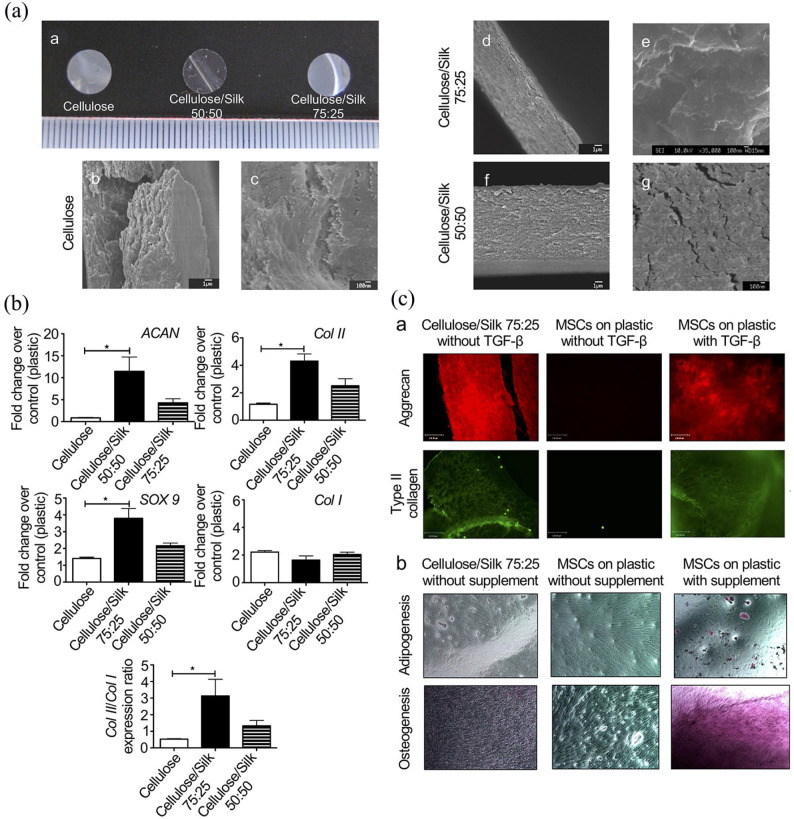
Other polysaccharides, including different forms of cellulose, also attracted the attention of researchers to develop MSC-based, engineered scaffolds for cartilage regeneration. Cellulose is a semi-crystalline glucose polymer, and cellulose polymers satisfy the chondrocyte proliferation and reveal excellent biocompatibility in vitro.95 Carboxymethylcellulose (CMC) is one of the derivatives of cellulose. BM-MSCs encapsulated in photo-cross-linked CMC scaffolds supplemented with TGF-β3 were reported to stimulate cartilage matrix deposition and were able to elaborate functional ECMs consistent with the cartilaginous tissue phenotype.96 Huang et al.97 found that chondrogenesis was increased in the lowest concentrations of sodium cellulose sulfate (NaCS) scaffold, as seen with the highest Col II production and expression of cartilage-specific genes. In addition, the cellulose/silk blended scaffold was reported to be used as a transplantable strategy to stimulate BM-MSCs for cartilage repair (Figure 6).98 The scaffold upregulated Col II, Sox9, and aggrecan expression without growth factor induction. No lipogenic and osteogenic differentiation was found, indicating the blended scaffold induced specific MSC differentiation into the chondrogenic lineage.98
Figure 6.
Composite scaffold-directed chondrogenesis of BM-MSCs. (A) Macroscopic and microscopic presentation of polymer membranes. (b, d, f) Low-resolution images. (c, e, g) High-resolution images. (B) Chondrogenic gene expression of BM-MSCs on polymer membranes. The expression of BM-MSCs on different polymers was collected and transcribed into cDNA for quantitative PCR analysis of the chondrogenic markers. The BM-MSCs cultured on the specific blend combination of cellulose and silk in 75:25 ratio upregulated the chondrogenic marker genes Col II, Sox9, and aggrecan in the absence of TGF-β. (C-a) Chondrogenic commitment of BM-MSCs on the polymer membranes. BM-MSCs were cultured in different polymers or plastics for 21 days in the presence or absence of TGF-β. (b) BM-MSCs were cultured with or without adipogenic and osteogenic supplements. Chondrogenic differentiation of BM-MSCs was assessed by staining for aggrecan (red fluorescence) and Col II (green fluorescence). Fat vacuoles were stained with Oil Red O to evaluate adipogenesis and differentiation. Osteogenic differentiation was assessed by staining calcium with Alizarin Red. No adipogenic or osteogenic differentiation was found on the cellulose/silk composite scaffold in 75:25 ratio. Adopted from Singh et al.98 and reprinted with permission of American Chemical Society.
In summary, engineered scaffolds based on natural polymers lead to satisfying outcomes of MSC therapies in cartilage regeneration in terms of the relatively excellent biocompatibility and chondrogenesis support. However, the disadvantages of natural polymers are apparent, including the difficulty of processing the scaffold into desired structures and the difficulty of functionalizing the scaffold. The application of synthetic polymer scaffolds can overcome most of the restrictions of natural polymer scaffolds.
Synthetic polymer scaffolds
Various synthetic polymers are fabricated for biomedical applications. Compared with natural polymers, the advantages of the application of synthetic polymers in cartilage regeneration mainly focus on their stability and diversity. We can manipulate their biochemical and mechanical properties to meet the repair requirements by changing their components and structures. The most common synthetic polymers in cartilage regeneration are poly(α-esters), such as poly(lactic-co-glycolic acid) (PLGA), poly(lactic acid) (PLA), poly(glycolic acid) (PGA), and poly(ethylene glycol) (PEG).
As the copolymer of PGA/PLA, PLGA has also demonstrated the capacity to assist MSCs in the repair of damaged cartilage.99 PLGA was reported as a hydrolyzable scaffold containing BM-MSCs for the fabrication of trachea cartilage.100 After 4 weeks of incubation, BM-MSCs obtained the chondrogenic ability with the induction of TGF-β. Xin et al. fabricated a PLGA-based nanofiber to improve adhesive ability, growth, and differentiation of BM-MSCs.101 The majority of BM-MSCs were proliferated in PLGA scaffolds for 14 days, and BM-MSCs in the PLGA scaffold were successfully differentiated into chondrocytes.
PLA is a biodegradable polymer that was approved by the FDA in various medical applications. The application of gene-modified MSCs with tissue engineering scaffolds could significantly improve the repair of the subchondral bone.102 TGF-β1 gene-modified MSCs seeded on poly-l-lysine-coated PLA bionic scaffold for the treatment of osteochondral defects.103 After 24 weeks of implantation, the cartilage tissue filled in the chondral zone and the trabecular bone filled in the subchondral region. Similarly, Yan and Yu104 seeded four types of chondrogenic cells, including chondrocytes, BM-MSCs, fibroblasts, and umbilical cord blood stem cells, into PLAs separately, and cell/PLA composites were transplanted into femoral trochlear groove cartilage defects. The application of BM-MSCs and PLA scaffold showed better neo-chondrocyte arrangement in the chondral region, and the integration with surrounding cartilage was successfully repaired.
PGA is a polymer with high tensile strength. Due to its appropriate degradation and excellent mechanical characteristics, it had been widely used in cartilage engineering. In addition, BM-MSCs combined with PGA were reported to induce chondrogenic differentiation, and the content of Col II and aggrecan was dramatically increased.105
PEG and polycaprolactone (PCL) represented the most commonly used synthetic polymer hydrogel in cartilage repair. Hydrogels with a 3D-aggregated structure could maintain a large amount of water.106 Hydrogel matrices are able to obtain the desired shape and proper mechanical characteristics after implantation.107 The specific combinations of PEG hydrogels with chondroitin sulfate (CS) and matrix metalloproteinase–sensitive peptides (MMP-pep) in a layer-by-layer organization were reported to induce the BM-MSCs to differentiate into chondrocytes and different phenotypes in the surface, transitional, and deep layers of cartilage tissue.108 The shallow region consisted of PEG, CS, and MMP-pep, the middle layer consisted of CS and PEG, and the bottom layer consisted of HA and PEG. The results demonstrated that the Col II content decreased from the superficial to deep region step by step. In contrast, the increase in Col X and proteoglycan resulted in the difference in compressive modulus in different cartilage layers. Moreover, the PEG/Col mimetic peptide (CMP) composite hydrogels were developed for the encapsulation of MSCs into neo-cartilage.109 Compared to PEG hydrogels without CMP, the hybrid scaffolds provided a microenvironment, which increased the chondrogenic differentiation of MSCs and promoted the production of ECMs.109 Furthermore, matrix elasticity was found to have an impact on the chondrogenesis of MSCs, the soft matrix leading to a higher extent of chondrogenesis and the stiff matrix with the opposite effect.109
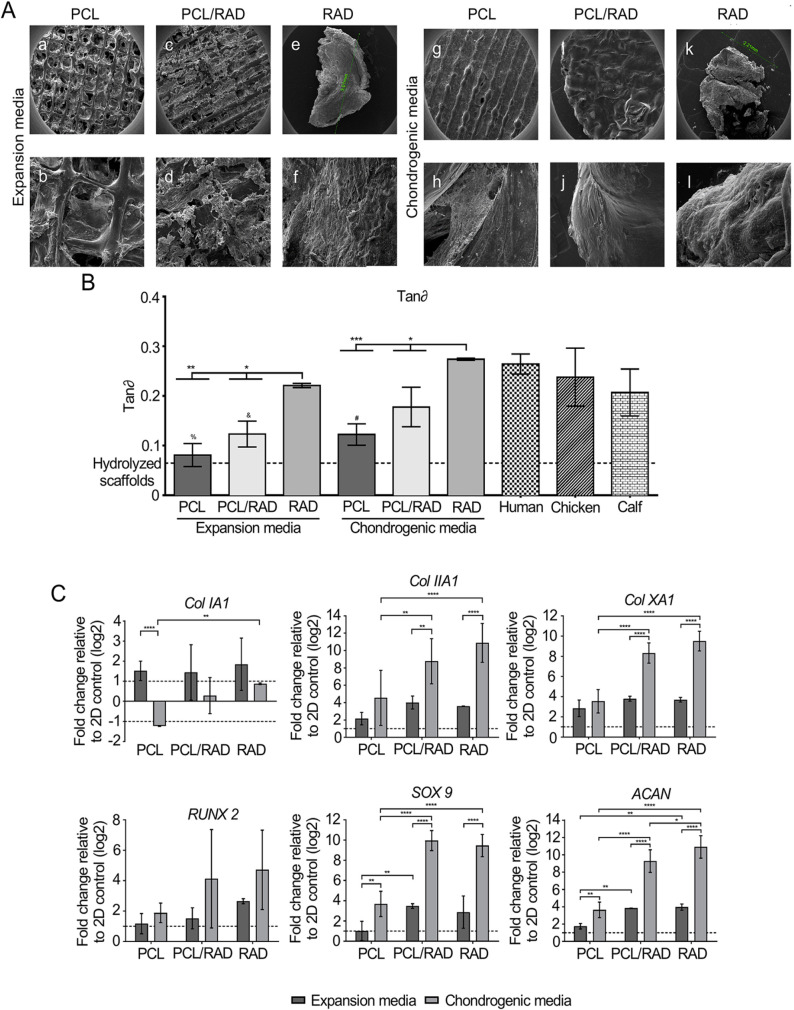
PCL is the commonly used polyester approved by the FDA for biomedical applications.110 Furthermore, PCL could induce cell attachment, proliferation, and matrix generation.111 Dahlin et al. found the capacity of the co-culture of chondrocytes and MSCs in the electrospinning PCL scaffolds to repair osteochondral defects in the trochlear groove in rats. The co-culture in the scaffold resulted in repaired tissue composed of more cartilage-like tissue compared to the empty treatment.112 Similarly, the effect of direct cell–cell contact in BM-MSC and chondrocyte co-cultures on chondroid ECM deposition in non-woven fibrous PCL scaffolds was assessed. Chondrocytes and BM-MSCs were co-cultured in the PCL scaffolds directly or indirectly, and the results demonstrated that chondrocytes affected the chondrogenesis of BM-MSCs by increasing the cartilaginous ECM synthetic capacity.113 PCL scaffold with nanofibrous texture allowed BM-MSCs to express aggrecans, and the augmentation with hyaluronan and TGF-β1 in the scaffold was helpful for the chondrogenic differentiation of BM-MSCs.114 Moreover, PCL could be used in 3D tissue engineering for cartilage repair by combining the PCL macrostructure with the self-assembly peptide RAD16-I (Figure 7).115 The scaffold provided a microenvironment for BM-MSC proliferation and differentiation into chondrocytes and had a mechanical property similar to native cartilage, providing a potential treatment for cartilage regeneration.
Figure 7.
Bioengineered scaffold for cartilage regeneration. (A) SEM images of composite PCL, PCL/RAD, and RAD cultured in expansion medium or chondrogenic medium. Scale bar images a, c, e, g, i, and k = 1 mm, images b and d = 300 μm, image j = 100 μm, and images f, h, and l = 50 μm. (B) Mechanical characteristics of PCL, PCL/RAD, and RAD constructs in expansion or chondrogenic media. Tan delta (tan∂) represented the statistical differences compared with human, chicken, and calf. tan∂ related to the ratio of loss and storage modulus. (C) Chondrogenic and hypertrophic gene expression of PCL, PCL/RAD, and RAD constructs in expansion or chondrogenic media after 30 days of culture. 2D monolayer cultured cells in expansion medium were used as a control sample.
Natural and synthetic polymer composite scaffolds
Considering the advantages and limitations of natural and synthetic polymers, most recent studies pursued the combination of natural and artificial materials to exploit the optimal scaffold for cartilage regeneration.116 While synthetic polymers could customize the blended scaffold to possess necessary mechanical characteristics and structures, the addition of natural polymers could afford bioactive molecules, which are also important for the proliferation and differentiation of MSCs.
To investigate different ECM contents in regulating differentiation of BM-MSCs, Hwang et al. encapsulated BM-MSCs in PEG-based hydrogels containing either Col or HA and cultivated them in chondrogenic medium.117 In Col-based PEG hydrogel, chondrogenic differentiation of BM-MSCs was increased, while osteogenic differentiation of BM-MSCs was induced by PEG hydrogel containing HA. In addition, Liao et al. fabricated the PCL microfiber coated with decellularized cartilage ECM. The composite scaffold supported the chondrogenic differentiation of BM-MSCs in vitro. Moreover, a higher GAG synthetic activitiy of BM-MSCs was observed in composite scaffold.118
The effect of hydroxyapatite on cartilage repair had been widely reported. To establish an appropriate environment for the synthesis of cartilage ECM, nanoscale hydroxyapatite was loaded on poly(l-lactic acid) (PLLA) fibers, and MSCs were seeded on the surface of the composite scaffold.119 After 14 days of culture, cartilage-specific proteoglycan immunostaining confirmed the presence of chondrogenic ECM in the scaffold. Similarly, Zhou et al.120 developed a hybrid scaffold consisting of PGA-hydroxyapatite and autologous BM-MSCs, which facilitated the repair of osteochondral defect in femoral intercondylar fossa. At 16 weeks after implantation, the study found that the neo-tissue in the repaired area was integrated with the surrounding tissue.
Considering the physical properties of cartilage, the scaffold for cartilage repair should demonstrate proper mechanical characteristics. In a variety of cartilage repairing studies, chitosan has been compounded with other synthetic polymers to increase the mechanical properties and cell attachment. Ploy(l-lactide-co-ε-caprolactone) (PLCL) scaffold was cross-linked with chitosan to enhance wettability, while the chitosan surface increased homogeneous distribution of BM-MSCs within the scaffold.121 BM-MSCs attached to the chitosan-modified composite scaffold quickly and formed F-actin fiber to constitute clusters. This phenomenon was delayed in an unmodified scaffold. Moreover, the mechanical properties of neo-tissue were improved on a modified composite scaffold.
Conclusion
Current clinical treatment does not usually provide satisfying repair outcomes because of limited sources of transplanted cartilage and the failure of integration among regenerative tissue and surrounding tissue.
Emerging MSC-based therapies represent a satisfying approach for cartilage repair, to circumvent the limitations of current clinical treatments, in terms of their accessibility, minimization of donor morbidity, and splendid capacity of chondrogenic differentiation (Table 1).
Table 1.
Comparison of clinical strategies with MSC-based therapies for cartilage damage treatment.
| Strategy | Advantage | Disadvantage | Reference |
|---|---|---|---|
| Distraction | Increase joint space and reduce pressure in joint Significant improvements in pain and mobility of joint Technical simplicity Low cost |
Pin tract infection Neuropraxia Thrombosis |
Mastbergen et al.6 |
| Autologous osteochondral implantation | Generally biocompatible in vivo
Improved the prognosis of large size osteochondral defect |
Largely invasive Graft separation |
Daher et al.2 |
| Allograft osteochondral implantation | Maintain the original structure for repaired tissue | Immune reaction Display variability among different samples Limited donor source |
Daher et al.2 |
| Autologous chondrocyte implantation | Appropriate phenotype required for cartilage repair Limited invasiveness Avoid potential immune complications |
Dedifferentiation to fibroblast-like cells in monolayer culture Limited mitotic activity Two-stage operation |
Djouad et al.4 |
| Joint replacement | Metal prostheses provide excellent fracture resistance and mechanical strength Ceramic prostheses showed great osteoconduction ability |
Metal toxic degradation products and immunogenicity Do not provide suitable microenvironment for tissue growth |
Smith and Grande7 |
| MSC-based therapies | Regeneration of a relative complete, functional cartilage tissue Can be modified and processed to desired specifications with consistent quality Relatively good biocompatibility and similar biomechanical properties with the target tissue |
Increased complexity for fabricating Difficult to process in clinical practice |
Zhu et al.63 |
MSC: mesenchymal stem cell.
Using MSCs alone, the progression of cartilage degeneration could be delayed. This strategy has succeeded in relieving pain and improving joint function in OA and RA patients. Moreover, it was also demonstrated the MSCs have the potential to prevent chondrocyte apoptosis through a paracrine effect. Implantation of MSCs has been used in various pre-clinical studies for its low operation trauma. However, this method just postpones cartilage deterioration and fails to regenerate the injured cartilage. The application of chondrogenic factors could regulate the differentiation, proliferation, and metabolic activity of MSCs. The usage of MSCs with chondrogenic factors was reported in the treatment of early-stage and small size cartilage injuries. Chondrogenic factors had been proven to increase the therapeutic efficacies of MSCs.
For the treatment of cartilage defect, the regeneration of neo-tissue is relatively unstable in mechanical properties compared with the native cartilage tissue. The 3D environment provided by the scaffold has a crucial role in maintaining the chondrocyte phenotype of MSCs. Scaffold not only enables the homogeneous distribution of MSCs but also provides the appropriate substrate for cell growth and mechanical integrity for post-surgical implantation. The strategy could induce the regeneration of a relatively complete, functional cartilage tissue, which is significant for the repair of full-thickness cartilage defect. The combination of MSCs with chondrogenic factors achieved better-repaired outcomes compared with MSCs alone. In addition, the addition of engineered scaffolds enhanced ECM synthesis and significantly improved the therapeutic results of severe or full-thickness cartilage defects.
The purpose of MSC-based therapies is to create bionic tissues, which could mimic the physical characteristics of native cartilage. However, the approaches should meet the elementary requirement of integrated cartilage regeneration and much still need to be investigated. In the process of cartilage regeneration, we need to maintain the chondrocyte phenotype of existing chondrocytes and improve complete MSC chondrogenic differentiation. In the process of applying MSC-based therapies to the clinical setting, we need to determine what constitutes a healthy, cartilage-like regeneration tissue. We believe the integration of MSC-based therapies into current clinical approaches will overcome the existing challenges and enable us to develop a genuinely biomimetic cartilage regenerative therapy.
Footnotes
Author contributions: J.D, F.C., Y.W., and X.Z. conceived the article; H.L. wrote the article with support from W.X.; all authors revised the article and approved the final version.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was financially supported by the National Natural Science Foundation of Chain (Grant Nos. 51973216, 51873207, and 81671804), the Program of the Development and Reform Commission of Jilin Province (Grant No. 2018C052-4), the Science and Technology Development Program of Jilin Province (Grant Nos. 20200404182YY and 20190304121YY), and the Scientific Research Planning Project of the Education Department of Jilin Province (Grant No. JJKH20201075KJ).
ORCID iD: Jianxun Ding  https://orcid.org/0000-0002-5232-8863
https://orcid.org/0000-0002-5232-8863
References
- 1. Lories RJ, Luyten FP. The bone-cartilage unit in osteoarthritis. Nat Rev Rheumatol 2011; 7: 43–49. [DOI] [PubMed] [Google Scholar]
- 2. Daher RJ, Chahine NO, Greenberg AS, et al. New methods to diagnose and treat cartilage degeneration. Nat Rev Rheumatol 2009; 5(11): 599–607. [DOI] [PubMed] [Google Scholar]
- 3. Wang C, Feng N, Chang F, et al. Injectable cholesterol-enhanced stereocomplex polylactide thermogel loading chondrocytes for optimized cartilage regeneration. Adv Healthcare Mater 2019; 8(14): 1900312. [DOI] [PubMed] [Google Scholar]
- 4. Djouad F, Bouffi C, Ghannam S, et al. Mesenchymal stem cells: Innovative therapeutic tools for rheumatic diseases. Nat Rev Rheumatol 2009; 5(7): 392–399. [DOI] [PubMed] [Google Scholar]
- 5. Ringe J, Burmester GR, Sittinger M. Regenerative medicine in rheumatic disease-progress in tissue engineering. Nat Rev Rheumatol 2012; 8(8): 493–498. [DOI] [PubMed] [Google Scholar]
- 6. Mastbergen SC, Saris DBF, Lafeber FPJG. Functional articular cartilage repair: Here, near, or is the best approach not yet clear? Nature Rev Rheumatol 2013; 9: 277–290. [DOI] [PubMed] [Google Scholar]
- 7. Smith BD, Grande DA. The current state of scaffolds for musculoskeletal regenerative applications. Nat Rev Rheumatol 2015; 11(4): 213–222. [DOI] [PubMed] [Google Scholar]
- 8. Han Y, Li X, Zhang Y, et al. Mesenchymal stem cells for regenerative medicine. Cells 2019; 8: 886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Somoza RA, Welter JF, Correa D, et al. Chondrogenic differentiation of mesenchymal stem cells: Challenges and unfulfilled expectations. Tissue Eng, Part B 2014; 20(6): 596–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hurst JM, Steadman JR, O’Brien L, et al. Rehabilitation following microfracture for chondral injury in the knee. Clin Sports Med 2010; 29(2): 257–265. [DOI] [PubMed] [Google Scholar]
- 11. Mithoefer K, McAdams T, Williams RJ, et al. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee. Am J Sports Med 2009; 37(10): 2053–2063. [DOI] [PubMed] [Google Scholar]
- 12. Hayashi S, Nakasa T, Ishikawa M, et al. Histological evaluation of early-phase changes in the osteochondral unit after microfracture in a full-thickness cartilage defect rat model. Am J Sports Med 2018; 46(12): 3032–3039. [DOI] [PubMed] [Google Scholar]
- 13. Gudas R, Gudaitė A, Mickevičius T, et al. Comparison of osteochondral autologous transplantation, microfracture, or debridement techniques in articular cartilage lesions associated with anterior cruciate ligament injury: A prospective study with a 3-year follow-up. Arthroscopy 2013; 29(1): 89–97. [DOI] [PubMed] [Google Scholar]
- 14. Goyal D, Keyhani S, Lee EH, et al. Evidence-based status of microfracture technique: A systematic review of level I and II studies. Arthroscopy 2013; 29(9): 1579–1588. [DOI] [PubMed] [Google Scholar]
- 15. Steadman JR, Briggs KK, Rodrigo JJ, et al. Outcomes of microfracture for traumatic chondral defects of the knee: Average 11-year follow-up. Arthroscopy 2003; 19(5): 477–484. [DOI] [PubMed] [Google Scholar]
- 16. Reissis D, Tang QO, Cooper NC, et al. Current clinical evidence for the use of mesenchymal stem cells in articular cartilage repair. Expert Opin Biol Ther 2016; 16(4): 535–557. [DOI] [PubMed] [Google Scholar]
- 17. Wakitani S, Imoto K, Yamamoto T, et al. Human autologous culture expanded bone marrow mesenchymal cell transplantation for repair of cartilage defects in osteoarthritic knees. Osteoarthritis Cartilage 2002; 10(3): 199–206. [DOI] [PubMed] [Google Scholar]
- 18. Koh YG, Choi YJ, Kwon OR, et al. Second-look arthroscopic evaluation of cartilage lesions after mesenchymal stem cell implantation in osteoarthritic knees. Am J Sports Med 2014; 42(7): 1628–1637. [DOI] [PubMed] [Google Scholar]
- 19. Centeno CJ, Busse D, Kisiday J, et al. Increased knee cartilage volume in degenerative joint disease using percutaneously implanted, autologous mesenchymal stem cells. Pain Physician 2008; 11(3): 343–353. [PubMed] [Google Scholar]
- 20. Davatchi F, Abdollahi BS, Mohyeddin M, et al. Mesenchymal stem cell therapy for knee osteoarthritis. Preliminary report of four patients. Int J Rheum Dis 2016; 19(3): 219–225. [DOI] [PubMed] [Google Scholar]
- 21. Lee WS, Kim HJ, Kim KI, et al. Intra-Articular injection of autologous adipose tissue-derived mesenchymal stem cells for the treatment of knee osteoarthritis: A phase IIb, randomized, placebo-controlled clinical trial. Stem Cells Transl Med 2019; 8(6): 504–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu C, Li Y, Yang Z, et al. Kartogenin enhances the therapeutic effect of bone marrow mesenchymal stem cells derived exosomes in cartilage repair. Nanomedicine (Lond) 2020; 15(3): 273–288. [DOI] [PubMed] [Google Scholar]
- 23. Feng X, Li J, Zhang X, et al. Electrospun polymer micro/nanofibers as pharmaceutical repositories for healthcare. J Controlled Release 2019; 302: 19–41. [DOI] [PubMed] [Google Scholar]
- 24. Johnson K, Zhu S, Tremblay MS, et al. A stem cell-based approach to cartilage repair. Science 2012; 336: 717–721. [DOI] [PubMed] [Google Scholar]
- 25. Spakova T, Plsikova J, Harvanova D, et al. Influence of kartogenin on chondrogenic differentiation of human bone marrow-derived MSCs in 2D culture and in co-cultivation with OA osteochondral explant. Molecules 2018; 23: 181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chandran B, Goel A. A randomized, pilot study to assess the efficacy and safety of curcumin in patients with active rheumatoid arthritis. Phytother Res 2012; 26(11): 1719–1725. [DOI] [PubMed] [Google Scholar]
- 27. Buhrmann C, Mobasheri A, Matis U, et al. Curcumin mediated suppression of nuclear factor-κB promotes chondrogenic differentiation of mesenchymal stem cells in a high-density co-culture microenvironment. Arthritis Res Ther 2010; 12(4): R127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Saiko P, Szakmary A, Jaeger W, et al. Resveratrol and its analogs: Defense against cancer, coronary disease and neurodegenerative maladies or just a fad? Mutat Res 2008; 658: 68–94. [DOI] [PubMed] [Google Scholar]
- 29. Csaki C, Keshishzadeh N, Fischer K, et al. Regulation of inflammation signalling by resveratrol in human chondrocytes in vitro. Biochem Pharmacol 2008; 75: 677–687. [DOI] [PubMed] [Google Scholar]
- 30. Shakibaei M, John T, Seifarth C, et al. Resveratrol inhibits IL-1β-induced stimulation of caspase-3 and cleavage of PARP in human articular chondrocytes in vitro. Ann N Y Acad Sci 2007; 1095: 554–563. [DOI] [PubMed] [Google Scholar]
- 31. Lei M, Liu SQ, Liu YL. Resveratrol protects bone marrow mesenchymal stem cell derived chondrocytes cultured on chitosan-gelatin scaffolds from the inhibitory effect of interleukin-1β. Acta Pharmacol Sin 2008; 29(11): 1350–1356. [DOI] [PubMed] [Google Scholar]
- 32. Scarfì S. Use of bone morphogenetic proteins in mesenchymal stem cell stimulation of cartilage and bone repair. World J Stem Cells 2016; 26: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dorman LJ, Tucci M, Benghuzzi H. In vitro effects of BMP-2, BMP-7, and BMP-13 on proliferation and differentiation of mouse mesenchymal stem cells. Biomed Sci Instrum 2012; 48: 8181–8177. [PubMed] [Google Scholar]
- 34. Grande DA, Mason J, Light E, et al. Stem cells as platforms for delivery of genes to enhance cartilage repair. J Bone Joint Surg Am 2003; 85(Suppl. 2): 111–116. [DOI] [PubMed] [Google Scholar]
- 35. Ding J, Zhang J, Li J, et al. Electrospun polymer biomaterials. Prog Polym Sci 2019; 90: 1–34. [Google Scholar]
- 36. Goldring MB. Update on the biology of the chondrocyte and new approaches to treating cartilage diseases. Best Pract Res Clin Rheumatol 2006; 20(5): 1003–1025. [DOI] [PubMed] [Google Scholar]
- 37. Facchini A, Lisignoli G, Cristino S, et al. Human chondrocytes and mesenchymal stem cells grown onto engineered scaffold. Biorheology 2006; 43(34): 471–480. [PubMed] [Google Scholar]
- 38. Thorpe SD, Buckley CT, Vinardell T, et al. The response of bone marrow-derived mesenchymal stem cells to dynamic compression following TGF-β3 induced chondrogenic differentiation. Ann Biomed Eng 2010; 38: 2896–2909. [DOI] [PubMed] [Google Scholar]
- 39. Kim M, Erickson IE, Choudhury M, et al. Transient exposure to TGF-β3 improves the functional chondrogenesis of MSC-laden hyaluronic acid hydrogels. J Mech Behav Biomed Mater 2012; 11: 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yoon DM, Fisher JP. Effects of exogenous IGF-1 delivery on the early expression of IGF-1 signaling molecules by alginate embedded chondrocytes. Tissue Eng, Part A 2008; 14(7): 1263–1273. [DOI] [PubMed] [Google Scholar]
- 41. Uebersax L, Merkle HP, Meinel L. Insulin-like growth factor I releasing silk fibroin scaffolds induce chondrogenic differentiation of human mesenchymal stem cells. J Controlled Release 2008; 127: 12–21. [DOI] [PubMed] [Google Scholar]
- 42. Ikeda Y, Sakaue M, Chijimatsu R, et al. IGF-1 gene transfer to human synovial MSCs promotes their chondrogenic differentiation potential without induction of the hypertrophic phenotype. Stem Cells Int 2017; 2017: 5804147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Liu Z, Xu J, Colvin JS, et al. Coordination of chondrogenesis and osteogenesis by fibroblast growth factor 18. Genes Dev 2002; 16: 859–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Cleary MA, van Osch GJ, Brama PA, et al. FGF, TGFβ and Wnt crosstalk: Embryonic to in vitro cartilage development from mesenchymal stem cells. J Tissue Eng Regen Med 2015; 9(4): 332–342. [DOI] [PubMed] [Google Scholar]
- 45. Cheng T, Yang C, Weber N, et al. Fibroblast growth factor 2 enhances the kinetics of mesenchymal stem cell chondrogenesis. Biochem Biophys Res Commun 2012; 426: 544–550. [DOI] [PubMed] [Google Scholar]
- 46. Stewart AA, Byron CR, Pondenis H. Effect of fibroblast growth factor-2 on equine mesenchymal stem cell monolayer expansion and chondrogenesis. Am J Vet Res 2007; 68(9): 941–945. [DOI] [PubMed] [Google Scholar]
- 47. Chen YC, Hsu YM, Tan KP, et al. Intraarticular injection for rabbit knee osteoarthritis: Effectiveness among hyaluronic acid, platelet-rich plasma, and mesenchymal stem cells. J Taiwan Inst Chem Eng 2018; 91: 138–145. [Google Scholar]
- 48. Eppley BL, Pietrzak WS, Blanton M. Platelet-rich plasma: A review of biology and applications in plastic surgery. Plast Reconstr Surg 2006; 118(6): 147e–159e. [DOI] [PubMed] [Google Scholar]
- 49. Barlian A, Judawisastra H, Alfarafisa NM, et al. Chondrogenic differentiation of adipose-derived mesenchymal stem cells induced by L-ascorbic acid and platelet rich plasma on silk fibroin scaffold. PeerJ 2018; 6: e5809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mueller MB, Fischer M, Zellner J, et al. Effect of parathyroid hormone-related protein in an in vitro hypertrophy model for mesenchymal stem cell chondrogenesis. Int Orthop 2013; 37(5): 945–951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fischer J, Ortel M, Hagmann S, et al. Role of PTHrP(1-34) pulse frequency versus pulse duration to enhance mesenchymal stromal cell chondrogenesis. J Cell Physiol 2016; 231(12): 2673–2681. [DOI] [PubMed] [Google Scholar]
- 52. Fischer J, Aulmann A, Dexheimer V, et al. Intermittent PTHrP(1-34) exposure augments chondrogenesis and reduces hypertrophy of mesenchymal stromal cells. Stem Cells Dev 2014; 23: 2513–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Zhuo Q, Yang W, Chen J, et al. Metabolic syndrome meets osteoarthritis. Nat Rev Rheumatol 2012; 8(12): 729–737. [DOI] [PubMed] [Google Scholar]
- 54. Takahashi T, Nuckolls GH, Takahashi K, et al. Compressive force promotes sox9, type II collagen and aggrecan and inhibits IL-1β expression resulting in chondrogenesis in mouse embryonic limb bud mesenchymal cells. J Cell Sci 1998; 111: 2067–2076. [DOI] [PubMed] [Google Scholar]
- 55. O’Conor CJ, Case N, Guilak F. Mechanical regulation of chondrogenesis. Stem Cell Res Ther 2013; 4: 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Miyanishi K, Trindade M, Lindsey DP, et al. Effects of hydrostatic pressure and transforming growth factor-β3 on adult human mesenchymal stem cell chondrogenesis in vitro. Tissue Eng 2006; 12(6): 1419–1428. [DOI] [PubMed] [Google Scholar]
- 57. Huang CYC, Reuben PM, Cheung HS. Temporal expression patterns and corresponding protein inductions of early responsive genes in rabbit bone marrow-derived mesenchymal stem cells under cyclic compressive loading. Stem Cells 2005; 23(8): 1113–1121. [DOI] [PubMed] [Google Scholar]
- 58. Grad S, Eglin D, Alini M, et al. Physical stimulation of chondrogenic cells in vitro: A review. Clin Orthop Relat Res 2011; 469(10): 2764–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Schätti O, Grad S, Goldhahn J, et al. A combination of shear and dynamic compression leads to mechanically induced chondrogenesis of human mesenchymal stem cells. Eur Cell Mater 2011; 22: 214–225. [DOI] [PubMed] [Google Scholar]
- 60. Gardner OFW, Fahy N, Alini M, et al. Joint mimicking mechanical load activates TGF-β1 in fibrin-poly(ester-urethane) scaffolds seeded with mesenchymal stem cells. J Tissue Eng Regen Med 2017; 11: 2663–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sawatjui N, Limpaiboon T, Schrobback K, et al. Biomimetic scaffolds and dynamic compression enhance the properties of chondrocyte- and MSC-based tissue-engineered cartilage. J Tissue Eng Regen Med 2018; 12(5): 1220–1229. [DOI] [PubMed] [Google Scholar]
- 62. Steinmetz NJ, Aisenbrey EA, Westbrook KK, et al. Mechanical loading regulates human MSC differentiation in a multi-layer hydrogel for osteochondral tissue engineering. Acta Biomater 2015; 21: 142–153. [DOI] [PubMed] [Google Scholar]
- 63. Zhu T, Cui Y, Zhang M, et al. Engineered three-dimensional scaffolds for enhanced bone regeneration in osteonecrosis. Bioact Mater 2020; 5(3): 584–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Ahmed TA, Giulivi A, Griffith M, et al. Fibrin glues in combination with mesenchymal stem cells to develop a tissue-engineered cartilage substitute. Tissue Eng, Part A 2011; 17(3–4): 323–335. [DOI] [PubMed] [Google Scholar]
- 65. Li A, Wei Y, Hung C, et al. Chondrogenic properties of collagen type XI, a component of cartilage extracellular matrix. Biomaterials 2018; 173: 47–57. [DOI] [PubMed] [Google Scholar]
- 66. Xia H, Liang C, Luo P, et al. Pericellular collagen I coating for enhanced homing and chondrogenic differentiation of mesenchymal stem cells in direct intra-articular injection. Stem Cell Res Ther 2018; 9: 174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wakitani S, Goto T, Pineda SJ, et al. Mesenchymal cell-based repair of large, full-thickness defects of articular cartilage. J Bone Joint Surg Am 1994; 76(4): 579–592. [DOI] [PubMed] [Google Scholar]
- 68. Chiu LH, Lai WF, Chang SF, et al. The effect of type II collagen on MSC osteogenic differentiation and bone defect repair. Biomaterials 2014; 35(9): 2680–2691. [DOI] [PubMed] [Google Scholar]
- 69. Bosnakovski D, Mizuno M, Kim G, et al. Chondrogenic differentiation of bovine bone marrow mesenchymal stem cells (MSCs) in different hydrogels: Influence of collagen type II extracellular matrix on MSC chondrogenesis. Biotechnol Bioeng 2006; 93: 1152–1163. [DOI] [PubMed] [Google Scholar]
- 70. Whitehouse MR, Howells NR, Parry MC, et al. Repair of torn avascular meniscal cartilage using undifferentiated autologous mesenchymal stem cells: From in vitro optimization to a first-in-human study. Stem Cells Transl Med 2017; 6(4): 1237–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sims CD, Butler PE, Cao YL, et al. Tissue engineered neocartilage using plasma derived polymer substrates and chondrocytes. Plast Reconstr Surg 1998; 101(6): 1580–1585. [DOI] [PubMed] [Google Scholar]
- 72. Hunziker EB. Articular cartilage repair: Basic science and clinical progress. A review of the current status and prospects. Osteoarthr Cartil 2002; 10: 432–463. [DOI] [PubMed] [Google Scholar]
- 73. Liu H, Cheng Y, Chen J, et al. Component effect of stem cell-loaded thermosensitive polypeptide hydrogels on cartilage repair. Acta Biomater 2018; 73: 103–111. [DOI] [PubMed] [Google Scholar]
- 74. Kim I, Lee SK, Yoon JI, et al. Fibrin glue improves the therapeutic effect of MSCs by sustaining survival and paracrine function. Tissue Eng, Part A 2013; 19(21–22): 2373–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Haleem AM, Singergy AAE, Sabry D, et al. The clinical use of human culture-expanded autologous bone marrow mesenchymal stem cells transplanted on platelet-rich fibrin glue in the treatment of articular cartilage defects. Cartilage 2010; 1(4): 253–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Kim YS, Choi YJ, Suh DS, et al. Mesenchymal stem cell implantation in osteoarthritic knees: Is fibrin glue effective as a scaffold. Am J Sports Med 2015; 43(1): 176–185. [DOI] [PubMed] [Google Scholar]
- 77. Ho ST, Cool SM, Hui JH, et al. The influence of fibrin based hydrogels on the chondrogenic differentiation of human bone marrow stromal cells. Biomaterials 2010; 31(1): 38–47. [DOI] [PubMed] [Google Scholar]
- 78. Bian L, Hou C, Tous E, et al. The influence of hyaluronic acid hydrogel cross-linking density and macromolecular diffusivity on human MSC chondrogenesis and hypertrophy. Biomaterials 2013; 34: 413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Loebel C, Szczesny SE, Cosgrove BD, et al. Cross-linking chemistry of tyramine-modified hyaluronan hydrogels alters mesenchymal stem cell early attachment and behavior. Biomacromolecules 2017; 18: 855–864. [DOI] [PubMed] [Google Scholar]
- 80. Kim IL, Khetan S, Baker BM, et al. Fibrous hyaluronic acid hydrogels that direct MSC chondrogenesis through mechanical and adhesive cues. Biomaterials 2013; 34(22): 5571–5580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Hu X, Wang Y, Tan Y, et al. A difunctional regeneration scaffold for knee repair based on aptamer-directed cell recruitment. Adv Mater 2017; 29(15): 1605235. [DOI] [PubMed] [Google Scholar]
- 82. Ma K, Titan AL, Stafford M, et al. Variations in chondrogenesis of human bone marrow-derived mesenchymal stem cells in fibrin/alginate blended hydrogels. Acta Biomater 2012; 8(10): 3754–3764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Awad HA, Wickham MQ, Leddy HA, et al. Chondrogenic differentiation of adipose-derived adult stem cells in agarose, alginate, and gelatin scaffolds. Biomaterials 2004; 25(16): 3211–3222. [DOI] [PubMed] [Google Scholar]
- 84. Diduch DR, Jordan LC, Mierisch CM, et al. Marrow stromal cells embedded in alginate for repair of osteochondral defects. Arthroscopy 2000; 16(6): 571–577. [DOI] [PubMed] [Google Scholar]
- 85. Sheehy EJ, Buckley CT, Kelly DJ. Chondrocytes and bone marrow-derived mesenchymal stem cells undergoing chondrogenesis in agarose hydrogels of solid and channelled architectures respond differentially to dynamic culture conditions. J Tissue Eng Regen Med 2011; 5(9): 747–758. [DOI] [PubMed] [Google Scholar]
- 86. Montembault A, Tahiri K, Korwin-Zmijowska C, et al. A material decoy of biological media based on chitosan physical hydrogels: Application to cartilage tissue engineering. Biochimie 2006; 88(5): 551–564. [DOI] [PubMed] [Google Scholar]
- 87. Li S, Dong S, Xu W, et al. Antibacterial hydrogels. Adv Sci 2018; 5: 1700527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Dashtdar H, Murali MR, Abbas AA, et al. PVA-chitosan composite hydrogel versus alginate beads as a potential mesenchymal stem cell carrier for the treatment of focal cartilage defects. Knee Surg Sports Traumatol Arthrosc 2013; 23: 1368–1377. [DOI] [PubMed] [Google Scholar]
- 89. Ponticiello MS, Schinagl RM, Kadiyala S, et al. Gelatin-based resorbable sponge as a carrier matrix for human mesenchymal stem cells in cartilage regeneration therapy. J Biomed Mater Res 2000; 52(2): 246–255. [DOI] [PubMed] [Google Scholar]
- 90. Kudva AK, Dikina AD, Luyten FP, et al. Gelatin microspheres releasing transforming growth factor drive in vitro chondrogenesis of human periosteum derived cells in micromass culture. Acta Biomater 2019; 90: 287–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Mazaki T, Shiozaki Y, Yamane K, et al. A novel, visible light-induced, rapidly cross-linkable gelatin scaffold for osteochondral tissue engineering. Sci Rep 2014; 4: 4457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Bhardwaj N, Nguyen QT, Chen AC, et al. Potential of 3-D tissue constructs engineered from bovine chondrocytes/silk fibroin-chitosan for in vitro cartilage tissue engineering. Biomaterials 2011; 32(25): 5773–5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Wang Y, Kim U-J, Blasioli DJ, et al. In vitro cartilage tissue engineering with 3D porous aqueous-derived silk scaffolds and mesenchymal stem cells. Biomaterials 2005; 26: 7082–7094. [DOI] [PubMed] [Google Scholar]
- 94. Ghezzi CE, Marelli B, Donelli I, et al. Multilayered dense collagen-silk fibroin hybrid: A platform for mesenchymal stem cell differentiation towards chondrogenic and osteogenic lineages. J Tissue Eng Regen Med 2017; 11(7): 2046–2059. [DOI] [PubMed] [Google Scholar]
- 95. Müller FA, Müller L, Hofmann I, et al. Cellulose-based scaffold materials for cartilage tissue engineering. Biomaterials 2006; 27(21): 3955–3963. [DOI] [PubMed] [Google Scholar]
- 96. Gupta MS, Cooper ES, Nicoll SB. Transforming growth factor-β3 stimulates cartilage matrix elaboration by human marrow-derived stromal cells encapsulated in photocrosslinked carboxymethylcellulose hydrogels: Potential for nucleus pulposus replacement. Tissue Eng, Part A 2011; 17: 2903–2910. [DOI] [PubMed] [Google Scholar]
- 97. Huang GP, Molina A, Tran N, et al. Investigating cellulose derived glycosaminoglycan mimetic scaffolds for cartilage tissue engineering applications. J Tissue Eng Regen Med 2018; 12(1): e592–e603. [DOI] [PubMed] [Google Scholar]
- 98. Singh N, Rahatekar SS, Koziol KKK, et al. Directing chondrogenesis of stem cells with specific blends of cellulose and silk. Biomacromolecules 2013; 14: 1287–1298. [DOI] [PubMed] [Google Scholar]
- 99. Zhang Y, Liu X, Zeng L, et al. Polymer fiber scaffolds for bone and cartilage tissue engineering. Adv Funct Mater 2019; 29: 1903279. [Google Scholar]
- 100. Liu L, Wu W, Tuo X, et al. Novel strategy to engineer trachea cartilage graft with marrow mesenchymal stem cell macroaggregate and hydrolyzable scaffold. Artif Organs 2010; 34(5): 426–433. [DOI] [PubMed] [Google Scholar]
- 101. Xin X, Hussain M, Mao JJ. Continuing differentiation of human mesenchymal stem cells and induced chondrogenic and osteogenic lineages in electrospun PLGA nanofiber scaffold. Biomaterials 2007; 28(2): 316–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Cui L, Zhang J, Zou J, et al. Electroactive composite scaffold with locally expressed osteoinductive factor for synergistic bone repair upon electrical stimulation. Biomaterials 2020; 230: 119617. [DOI] [PubMed] [Google Scholar]
- 103. Guo X, Zheng Q, Yang S, et al. Repair of full-thickness articular cartilage defects by cultured mesenchymal stem cells transfected with the transforming growth factor β1 gene. Biomed Mater 2006; 1: 206–215. [DOI] [PubMed] [Google Scholar]
- 104. Yan H, Yu C. Repair of full-thickness cartilage defects with cells of different origin in a rabbit model. Arthroscopy 2007; 23(2): 178–187. [DOI] [PubMed] [Google Scholar]
- 105. Wu X, Li S, Lou L, et al. The effect of the microgravity rotating culture system on the chondrogenic differentiation of bone marrow mesenchymal stem cells. Mol Biotechnol 2012; 54: 331–336. [DOI] [PubMed] [Google Scholar]
- 106. Qiu H, Guo H, Li D, et al. Intravesical hydrogels as drug reservoirs. Trends Biotechnol 2020; 38(6): 579–583. [DOI] [PubMed] [Google Scholar]
- 107. Nesic D, Whiteside R, Brittberg M, et al. Cartilage tissue engineering for degenerative joint disease. Adv Drug Deliv Rev 2006; 58: 300–322. [DOI] [PubMed] [Google Scholar]
- 108. Nguyen LH, Kudva AK, Saxena NS, et al. Engineering articular cartilage with spatially-varying matrix composition and mechanical properties from a single stem cell population using a multi-layered hydrogel. Biomaterials 2011; 32(29): 6946–6952. [DOI] [PubMed] [Google Scholar]
- 109. Liu SQ, Tian Q, Hedrick JL, et al. Biomimetic hydrogels for chondrogenic differentiation of human mesenchymal stem cells to neocartilage. Biomaterials 2010; 31(28): 7298–7307. [DOI] [PubMed] [Google Scholar]
- 110. Jeong CG, Hollister SJ. A comparison of the influence of material on in vitro cartilage tissue engineering with PCL, PGS, and POC 3D scaffold architecture seeded with chondrocytes. Biomaterials 2010; 31(15): 4304–4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Cui Y, Zhu T, Li D, et al. Bisphosphonate-functionalized scaffolds for enhanced bone regeneration. Adv Healthcare Mater 2019; 8: 1901073. [DOI] [PubMed] [Google Scholar]
- 112. Dahlin RL, Kinard LA, Lam J, et al. Articular chondrocytes and mesenchymal stem cells seeded on biodegradable scaffolds for the repair of cartilage in a rat osteochondral defect model. Biomaterials 2014; 35(26): 7460–7469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Levorson EJ, Santoro M, Kasper FK, et al. Direct and indirect co-culture of chondrocytes and mesenchymal stem cells for the generation of polymer/extracellular matrix hybrid constructs. Acta Biomater 2014; 10(5): 1824–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Schagemann JC, Paul S, Casper ME, et al. Chondrogenic differentiation of bone marrow-derived mesenchymal stromal cells via biomimetic and bioactive poly-ε-caprolactone scaffolds. J Biomed Mater Res A 2013; 101(6): 1620–1628. [DOI] [PubMed] [Google Scholar]
- 115. Rubí-Sans G, Recha-Sancho L, Pérez-Amodio S, et al. Development of a three-dimensional bioengineered platform for articular cartilage regeneration. Biomolecules 2019; 10: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Zhang Y, Yu J, Ren K, et al. Thermosensitive hydrogels as scaffolds for cartilage tissue engineering. Biomacromolecules 2019; 20: 1478–1492. [DOI] [PubMed] [Google Scholar]
- 117. Hwang NS, Varghese S, Li H, et al. Regulation of osteogenic and chondrogenic differentiation of mesenchymal stem cells in PEG-ECM hydrogels. Cell Tissue Res 2011; 344(3): 499–509. [DOI] [PubMed] [Google Scholar]
- 118. Liao J, Guo X, Grande-Allen KJ, et al. Bioactive polymer/extracellular matrix scaffolds fabricated with a flow perfusion bioreactor for cartilage tissue engineering. Biomaterials 2010; 31(34): 8911–8920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Spadaccio C, Rainer A, Trombetta M, et al. Poly-L-lactic acid/hydroxyapatite electrospun nanocomposites induce chondrogenic differentiation of human MSC. Ann Biomed Eng 2009; 37(7): 1376–1389. [DOI] [PubMed] [Google Scholar]
- 120. Zhou XZ, Leung VY, Dong QR, et al. Mesenchymal stem cell-based repair of articular cartilage with polyglycolic acid-hydroxyapatite biphasic scaffold. Int J Artif Organs 2008; 31(6): 480–489. [DOI] [PubMed] [Google Scholar]
- 121. Yang Z, Wu Y, Li C, et al. Improved mesenchymal stem cells attachment and in vitro cartilage tissue formation on chitosan-modified poly(L-lactide-co-ε-caprolactone) scaffold. Tissue Eng, Part A 2012; 18: 242–251. [DOI] [PMC free article] [PubMed] [Google Scholar]