Abstract
Introduction
In chronic obstructive pulmonary disease (COPD), endothelial dysfunction and stiffness of systemic arteries may contribute to increased cardiovascular risk. Pulmonary vascular disease (PVD) is frequent in COPD. The association between PVD and systemic vascular dysfunction has not been thoroughly evaluated in COPD.
Methods
A total of 108 subjects were allocated into four groups (non-smoking controls, smoking controls, COPD without PVD and COPD with PVD). In systemic arteries, endothelial dysfunction was assessed by flow-mediated dilation (FMD) and arterial stiffness by pulse wave analysis (PWA) and pulse wave velocity (PWV). PVD was defined by a mean pulmonary artery pressure (PAP) ≥25 mmHg at right heart catheterization or by a tricuspid regurgitation velocity >2.8 m/s at doppler echocardiography. Biomarkers of inflammation and endothelial damage were assessed in peripheral blood.
Results
FMD was lower in COPD patients, with or without PVD, compared to non-smoking controls; and in patients with COPD and PVD compared to smoking controls. PWV was higher in COPD with PVD patients compared to both non-smoking and smoking controls in a model adjusted by age and the Framingham score; PWV was also higher in patients with COPD and PVD compared to COPD without PVD patients in the non-adjusted analysis. FMD and PWV correlated significantly with forced expiratory volume in the first second (FEV1), diffusing capacity for carbon monoxide (DLCO) and systolic PAP. FMD and PWV were correlated in all subjects.
Discussion
We conclude that endothelial dysfunction of systemic arteries is common in COPD, irrespective if they have PVD or not. COPD patients with PVD show increased stiffness and greater impairment of endothelial function in systemic arteries. These findings suggest the association of vascular impairment in both pulmonary and systemic territories in a subset of COPD patients.
Keywords: COPD, pulmonary circulation and pulmonary hypertension, emphysema, cardiovascular diseases
Plain Language Summary
Patients who suffer from an obstruction to the flow of air in their airways (a condition that is called chronic obstructive pulmonary disease, or COPD) present with stiffness of the peripheral blood vessels and an impairment of normal dilation of the arteries in response to changes in systemic arterial pressure, as compared to smokers as well as to healthy subjects. Furthermore, patients who suffer from disease of the pulmonary vessels, leading to a higher pressure in the pulmonary circulation, also present the increased stiffness and reduced dilation of peripheral arteries to a greater degree: this finding may suggest that the dysfunction in pulmonary and systemic vascular territories might be associated within this patient subgroup. For this reason, the non-invasive evaluation of dilation and stiffness in the peripheral arteries could potentially lead to a suspicion of elevated pressure in the pulmonary circulation of patients with COPD, a condition that usually requires an invasive procedure for its diagnosis.
Introduction
Chronic obstructive pulmonary disease (COPD) is a chronic inflammatory lung disorder with systemic effects that has been associated with increased cardiovascular risk.1 Indeed, cardiovascular disease is a major cause of death in COPD patients, accounting for 25% of the overall mortality.2 Previous studies have shown that COPD is associated with changes in systemic vascular function3,4 and circulating biomarkers of vascular competence5 thereby suggesting the presence of associated peripheral artery disease.6 The severity of endothelial dysfunction in systemic arteries, as assessed by flow-mediated dilation (FMD),7 correlates with the severity of airflow obstruction and the extent of emphysema in COPD.3 Furthermore, COPD patients exhibit vascular stiffness in systemic arteries,8,9 which is correlated with the severity of emphysema.10
Pulmonary vascular disease (PVD), a term11 that encompasses abnormal pulmonary hemodynamics and changes suggestive of pulmonary hypertension12 at echocardiography, is a frequent complication of COPD, especially in advanced disease stages.13 Pulmonary hypertension is a strong and independent prognostic factor for mortality in COPD patients14 and the presence of PVD has been associated with more frequent exacerbation episodes and greater use of healthcare resources.15,16
The relationship between PVD and systemic peripheral disease has not been well established. Conceivably, given that cigarette smoke exposure is a common risk factor for alterations in both vascular territories, patients with PVD might be more prone to develop peripheral vascular disease. In this respect, in a previous study, we showed that COPD patients with PVD had reduced FMD compared with patients without PVD.17
The association of COPD with markers of vascular function in systemic vessels, namely stiffness and endothelial function, has been assessed in separated cohorts. Furthermore, the association of PVD and systemic vascular function in COPD has not been thoroughly evaluated. Accordingly, the current study aimed to assess systemic endothelial function and arterial compliance in the same cohort of patients with COPD, as well as to analyze their potential relationship with PVD.
Materials and Methods
Study Population
Sixty-one patients with COPD, with and without PVD, and 47 control subjects with normal pulmonary function (20 of them current smokers) were prospectively evaluated. COPD was defined by smoking habit, a compatible clinical history, and evidence of chronic airflow obstruction on forced spirometry (post-bronchodilator forced expiratory volume in the first second/forced vital capacity ratio, FEV1/FVC, <70%).18 Patients were clinically stable at the time of the study without exacerbation episodes or oral steroid treatment during the previous 4 months. All COPD patients were on regular bronchodilator treatment and most were also receiving inhaled corticosteroids; PVD was considered to be present when at right heart catheterization mean pulmonary artery pressure (mPAP) was ≥25mmHg, fulfilling the definition of pulmonary hypertension when the study was initiated,12 or when tricuspid regurgitation velocity was >2.8 m/s at Doppler echocardiography. Patients with left ventricle ejection fraction <50% at echocardiography were excluded. Healthy subjects were allocated into two groups according to their smoking status (non-smokers and active smokers).
A complete clinical history, physical examination, laboratory tests, electrocardiogram, pulmonary function tests and echocardiogram were performed in all subjects. In patients with COPD, arterial blood gas analysis was additionally performed. The following inflammatory and endothelial biomarkers were also analyzed: brain natriuretic peptide (BNP), high-sensitivity C reactive protein (hs-CRP), fibrinogen, vascular endothelial growth factor (VEGF), transforming growth factor-beta (TGF-β), interleukin-6 (IL-6), hepatocyte growth factor (HGF), angiopoietin-2 (ANG-2), cyclic guanosine monophosphate (cGMP), soluble tumor necrosis factor receptor type I (sTNF-αRI), soluble intercellular adhesion molecule-1 (sICAM-1), leptin, adiponectin, and soluble tyrosine kinase receptor Axl (sAXL).
The study was approved by the Ethics Committee of Hospital Clínic of Barcelona (20095026) and conducted in accordance with the Declaration of Helsinki on ethical principles for medical research involving human subjects. All subjects gave written informed consent before being included in the study.
All the measurements of vascular distensibility and endothelial function were performed in a quiet, temperature-controlled room (22±2ºC). Subjects fasted and avoided physical exercise, caffeine, alcohol, drugs and stimulants for at least 6 hours and rested supine for 15 minutes before starting the measurements, maintaining this position throughout the measurements. All studies were performed at the same time of day (afternoon) with the same ultrasound machine and by the same operator. FMD testing was performed 15–30 minutes after the completion of the arterial stiffness tests.
Vascular Stiffness
Arterial stiffness was assessed according to current guidelines.19 Both pulse wave analysis (PWA) and pulse wave velocity (PWV) measurements were performed by applanation tonometry (SphygmoCor System, AtCor Medical, Sydney, Australia). Blood pressure was measured twice using an automated, non-invasive oscillometric sphygmomanometer.
Pulse wave analysis was obtained on the radial artery of the right wrist and measured by specifically trained staff. The machine derived an aortic pulse pressure waveform from the radial artery wave and calculated the component of pulse pressure related to the reflection wave due to the increased stiffness of the arterial wall by means of the pressure difference between the reflection wave peak and the incident wave peak, expressed as percentage of the central pulse pressure (augmentation index, AI).20 Since the timing of the reflection wave is obviously affected by heart rate, the AI was also normalized at a standard heart rate of 75 beats per minute (AI75),21 in order to avoid the potential bias of different heart rates across the study groups.
Pulse wave velocity was measured by specifically trained staff. We used the R-wave of a simultaneously recorded ECG to identify the onset of the pressure wave, and applanation tonometry at the carotid and femoral arteries to record the pressure waveform, establishing the time delay between the R-wave and the beginning of the pulse pressure for each of the two central pulses. This distance (in meters) divided by the time delay of the pressure waveform (seconds) equaled carotid-femoral PWV.22 A measurement was accepted when it was reproducible at least twice with minimal variation. The final result was the mean of the measurements performed.
Endothelial Function
Endothelial function was assessed by FMD on the brachial artery, according to current guidelines23,24 as previously described.17 In brief, a blood pressure cuff was placed around the forearm. Brachial artery diameter was measured longitudinally 5–10 cm above the elbow by ultrasound, using a linear vascular transducer 7.5/5.5 MHz connected to an echocardiogram (Sonos 5500, Philips Medical Systems), and a three-lead electrocardiography was connected to the ultrasound machine. A 60-second baseline period was recorded prior to the cuff inflation. After this, the blood pressure cuff was inflated to 250 mmHg for 5 minutes to achieve total brachial arterial occlusion, and then rapidly deflated. Recording recommenced immediately after deflation and continued for 2 minutes. The brachial artery recovered for 15 minutes, after which another baseline scan was recorded for 15 seconds.
All the recorded images were transferred to a computer for measurement by the automated edge detection software (Brachial artery analyzer, MIA-LLC, IA, USA). The FMD response was calculated as the percentage of change from baseline to peak diameter of the brachial artery after cuff deflation.
Statistical Analysis
Data are expressed as mean ±SD when variable distribution was normal and as median and percentile 25–75 for non-normal distributions. The four groups were compared with a non-adjusted analysis using ANOVA with post-hoc tests (Tukey for continuous variables and Bonferroni for categorical variables). Since comorbidities were not equally represented within the four groups, and in order to correct for their possible confounding effect over endothelial dysfunction and arterial stiffness, a linear regression model was created comprising all the conditions and discarding them one by one using Akaike information criterion (AICc), in order to find out which variables (such as age, systemic arterial hypertension, diabetes or Framingham score) had an effect on the overall differences of FMD, PWA and PWV between groups and should thus be included in the final model for each study variable, while maintaining the group category as the variable of interest. With the linear model thus created, we performed Sidak and Tukey multiple comparison tests in order to carry out pairwise comparisons. Correlations among variables were analyzed using Pearson’s correlation tests. P<0.05 was considered significant in all cases.
Results
Anthropometric, clinical and functional characteristics of the subjects are shown in Table 1. In the COPD without PVD group, 3 patients were in GOLD 1 stage, 16 patients in GOLD 2, 13 patients in GOLD 3 and 14 patients in GOLD 4; in the COPD with PVD group no patient was in stage 1, one patient was in GOLD 2, 5 patients in GOLD 3 and 9 patients in GOLD 4. Fifteen patients overall (one patient in the smoking group, 4 patients in the COPD without PVD group and 10 in the COPD with PVD group) underwent right heart catheterization, while the other patients were evaluated by means of echocardiography. Fifteen patients with COPD fulfilled the criteria for PVD. Age, male gender proportion and the number of packs-year were higher in COPD patients. COPD patients with PVD had greater airflow obstruction, lower DLCO and lower PaO2 than COPD patients without PVD. Systemic arterial hypertension and diabetes mellitus were more prevalent in COPD patients with PVD.
Table 1.
Characteristics of the Study Subjects
| Non-Smoking Controls (n=27) | Smoking Controls (n=20) | COPD without PVD (n=46) | COPD with PVD (n=15) | |
|---|---|---|---|---|
| Age, years | 56 ± 8 | 54 ± 8 | 62 ± 7 ab | 64 ± 6 ab |
| Male gender, n (%) | 12 (44%) | 9 (45%) | 38 (83%) ab | 13 (87%) ab |
| Smoking status | ||||
| Current smokers, n (%) | 0 (0%) | 20 (100%) | 14 (30%) | 2 (13%) |
| Ex-smokers, n (%) | 9 (33%) | 0 (0%) | 32 (70%) | 13 (87%) |
| Pack-years smoking | 4 ± 8 | 30 ± 24 | 62 ± 29 ab | 69 ± 29 ab |
| GOLD stage 1-2-3-4, n | NA | NA | 3–16-13-14 | 0-1-5-9 |
| FEV1, % ref | 107 ± 12 | 103 ± 10 | 48 ± 20 ab | 30 ± 10 abc |
| FVC, % ref | 106 ± 11 | 104 ± 12 | 84 ± 19 ab | 65 ± 13 abc |
| FEV1/FVC, % | 79 ± 5 | 77 ± 5 | 43 ± 14 | 34 ± 9 |
| TLC, % ref | 106 ± 8 | 106 ± 9 | 116 ± 19 a | 113 ± 21 |
| RV, % ref | 108 ± 18 | 110 ± 23 | 181 ± 57 ab | 197 ± 62 ab |
| DLCO, % ref | 92 ± 15 | 85 ± 9 | 57 ± 20 ab | 39 ± 12 abc |
| PaO2, mmHg | NA | NA | 73 ± 9 | 63 ± 10c |
| Systemic arterial hypertension, n (%) | 5 (19%) | 3 (15%) | 21 (46%) ab | 10 (67%) abc |
| Dyslipidemia, n (%) | 7 (26%) | 7 (35%) | 14 (30%) | 6 (40%) |
| Diabetes mellitus, n (%) | 1 (4%) | 0 (0%) | 2 (4%) | 5 (33%) abc |
| Framingham score, points | 4.9 ± 5.1 | 7.00 ± 6.0 | 10.2 ± 5.8 a | 12.1 ± 6.8 ab |
| Systolic pulmonary artery pressure, mmHg | 27 ± 4 | 27 ± 3 | 31 ± 3 | 43 ± 10 abc |
| Mean pulmonary artery pressure, mmHg * | NA | NA | 21.2 ± 1.9 | 29.9 ± 5.9 abc |
| Cardiac index, L/min/m2 * | NA | NA | 2.24 ± 0.89 | 2.77 ± 0.47 abc |
| Pulmonary artery wedge pressure, mmHg * | NA | NA | 8.3 ± 3.1 | 9.30 ± 4.1 |
| Pulmonary vascular resistance, dyn·s·cm−5 * | NA | NA | 247 ± 40 | 328 ± 113 |
| Left ventricular ejection fraction, % | 64 ± 3 | 64 ± 3 | 62 ± 5 | 60 ± 6 |
| Systolic blood pressure, mmHg | 124 ± 18 | 123 ± 16 | 130 ± 20 | 135 ± 19 |
Notes: Results are expressed in mean±standard deviation. a p<0.05 compared with non-smokers; b p<0.05 compared with smokers; c p<0.05 compared with COPD without PVD; * Right heart catheterization was performed in 5 patients of the COPD without PVD group and in 10 patients of the COPD with PVD group.
Abbreviations: COPD, chronic obstructive pulmonary disease; PVD, pulmonary vascular disease; GOLD, Global Initiative for Chronic Obstructive Lung Disease; FEV1, forced expiratory volume in 1st second; FVC, forced vital capacity; TLC, total lung capacity; RV, residual volume; DLCO, diffusing capacity for carbon monoxide; PaO2, partial pressure of arterial oxygen; NA, not available.
Endothelial Function of Systemic Arteries
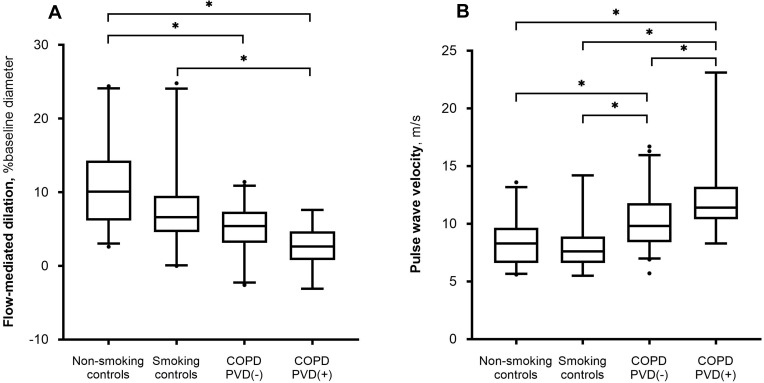
In the non-adjusted analysis, FMD was lower in both COPD groups, with and without PVD, as compared with non-smoking controls; and lower in the COPD with PVD group compared with smoking controls (Table 2, Figure 1A). In consideration of the different baseline vessel diameters, we formulated the ratio between flow-mediated dilation and baseline brachial artery diameter, which is an index of endothelial dysfunction independent of vessel diameter; this parameter yielded the same results. None of the individual conditions (age, gender, BMI) and comorbidities (systemic arterial hypertension, diabetes, hypercholesterolemia, renal disease, chronic ischemic disease, cardiac failure, Framingham score) included in the linear regression model had an effect on the differences between groups, so the adjusted analysis was not performed.
Table 2.
Vascular Function in Systemic Arteries
| Non-Smoking Controls (n = 26) | Smoking Controls (n = 20) | COPD without PVD (n = 45) |
COPD with PVD (n = 15) |
|
|---|---|---|---|---|
| Flow-mediated dilation, % change from baseline diameter | 10.10 (6.15–14.30) | 6.60 (4.58–9.50) | 5.40 (3.13–7.30) a | 2.70 (0.78–4.70) ab |
| Flow-mediated dilation/Baseline brachial artery diameter, %/mm | 2.50 (1.48–3.82) | 1.34 (0.80–2.04) | 1.16 (0.49–1.65) a | 0.49 (0.18–1.02) ab |
| Augmentation index, % | 25.0 (19.3–35.3) | 33.0 (20.0–43.0) | 25.0 (17.3–30.0) | 27.0 (17.3–34.0) |
| Augmentation index 75, % | 23.5 (17.0–29.3) | 26.0 (20.0–40.0) | 27.0 (16.8–31.0) | 26.0 (19.5–31.8) |
| Pulse wave velocity, m/s | 8.3 (6.6–9.7) | 7.6 (6.6–8.9) | 9.8 (8.4–11.8) ab | 11.4 (10.4–13.2) abc |
Notes: Results are expressed as median (percentile 25-percentile 75). Non-adjusted comparisons: ap<0.05 compared with non-smokers; b p<0.05 compared with smokers; c p<0.05 compared with COPD without PVD.
Abbreviations: COPD, chronic obstructive pulmonary disease; PVD, pulmonary vascular disease.
Figure 1.
Results of (A) endothelial function, assessed with flow-mediated dilation, and (B) arterial stiffness, assessed with pulse wave velocity, in non-smoking controls, smoking controls, chronic obstructive pulmonary disease (COPD) without pulmonary vascular disease (PVD) [COPD PVD(-)] and COPD with PVD [COPD PVD(+)]. Boxplots show median and 25–75 percentiles, whiskers show 5 and 95 percentiles, points show values out of this range. Between-group differences were analyzed with unadjusted ANOVA using Tukey as post-hoc test; *p<0.05.
Vascular Stiffness of Systemic Arteries
In the non-adjusted analysis, PWV was higher in both COPD groups compared with control subjects (smokers and non-smokers). It was also higher in the COPD with PVD group compared with the COPD without PVD group (p =0.037). In the linear model adjusted for age and the Framingham score (selected by the Akaike information criterion method), PWV was higher in the COPD with PVD group compared to non-smoking and smoking control subjects (Table 2, Figure 1B). There was also a trend towards higher PWV in the COPD with PVD group compared to the COPD without PVD group (p=0.06) in the adjusted model. There were no differences in PWV between non-smokers and smokers control groups. Measurements of PWA, expressed as AI and AI75, did not differ between groups, neither in the non-adjusted nor in the adjusted models (Table 2).
Relationship Between Systemic Vascular Function and Pulmonary Function
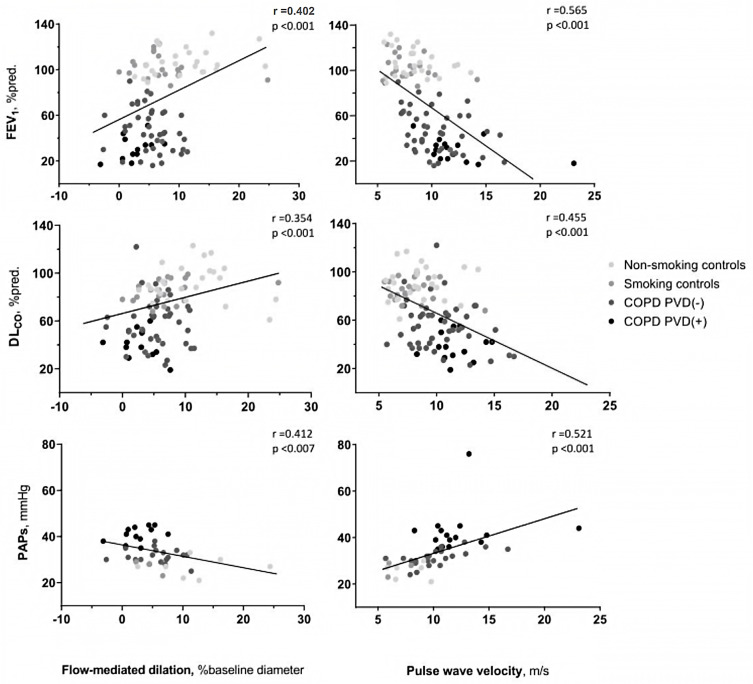
Both FMD and PWV correlated with FEV1, DLCO and systolic PAP values (Figure 2); also, the number of pack/years correlated with FMD (r =0.393, p <0.001) and PWV (r =0.357, p <0.001). FMD and PWV were inversely correlated (r=−0.3; p=0.04). Since the group of non-smoking healthy subjects might lead to a correlation bias, we repeated the analysis excluding this group. Correlations between lung function and sPAP and PWV persisted statistically significant, whereas correlations with FMD did not. We tested the performance of FMD and PWV in predicting the presence of PVD using ROC analysis. Both tests had good diagnostic performance in identifying PVD, with areas under the curve (AUC) of 0.815 and 0.803, FMD and PWV, respectively (Figure S1, supplementary material). A FMD <4.5% had a sensitivity of 77% and a specificity of 75% in identifying PVD. A PWV >10.4 m/s had a sensitivity of 87%, and specificity of 72% in identifying PVD.
Figure 2.
Correlation between flow-mediated dilation (left column) and pulse wave velocity (right column) with forced expiratory volume during the first second (FEV1), diffusing capacity for carbon monoxide (DLCO) and systolic pulmonary artery pressure (PAPs; estimated by doppler echocardiography in patients with tricuspid regurgitation and thus a measurable PAPs or measured at right heart catheterization). Patients are grouped as non-smoking controls, smoking controls, chronic obstructive pulmonary disease (COPD) without pulmonary vascular disease (PVD) [COPD PVD(-)] and COPD with PVD [COPD PVD(+)].
Inflammatory and Vascular Markers
The plasma levels of the soluble receptor of tumor necrosis factor-alpha (sTNF-αRI) were higher in COPD patients with PVD, compared with both non-smoking and smoking control groups (Table 3). Fibrinogen was increased in COPD patients without PVD, compared with both the non-smoking and smoking control groups.
Table 3.
Vascular and Systemic Inflammatory Markers
| Non-Smoking Controls (n=27) | Smoking Controls (n=20) | COPD without PVD (n=46) | COPD with PVD (n=15) | |
|---|---|---|---|---|
| hsCRP, mg/dL | 0.15 (0.05–0.24) | 0.09 (0.05–0.33) | 0.40 (0.12–0.87) | 0.27 (0.20–0.80) |
| BNP, pg/mL | 13.7 (4.1–23.4) | 12.0 (7.6–28.1) | 13.5 (7.7–24.9) | 21.2 (8.4–40.5) |
| Fibrinogen, g/L | 3.40 (3.00–3.70) | 3.30 (2.90–3.78) | 3.80 (3.10–5.05)ab | 4.00 (3.60–4.50) |
| Glycemia, mg/dL | 92 (85–99) | 92 (83–99) | 96 (87–109) | 112 (94–138) |
| VEGF, detectable % | 12 (44.4) | 12 (60) | 22 (47.8) | 7 (46.7) |
| VEGF, pg/mL | 47.0 (20.8–87.3) | 13.3 (8.9–40.1) | 9.5 (4.6–39.5) | 20.7 (16.8–30.0) |
| TGF-β, ng/mL | 2.31 (1.42–3.43) | 2.08 (1.39–5.70) | 3.00 (2.06–3.65) | 2.18 (1.83–3.29) |
| IL-6, pg/mL | 1.74 (0.96–5.23) | 2.55 (0.88–8.00) | 1.84 (0.87–3.26) | 1.12 (0.54–2.07) |
| HGF, pg/mL | 274 (220–437) | 257 (218–365) | 336 (309–407) | 338 (309–448) |
| Leptin. ng/mL | 11.0 (8.8–16.9) | 13.9 (6.6–17.4) | 11.5 (8.2–22.5) | 16.6 (9.5–20.5) |
| Angiopoietin-2, pg/mL | 776 (593–939) | 942 (599–1319) | 923 (746–1172) | 1244 (844–1551) |
| cGMP, nmol/mL | 2.52 (2.02–3.18) | 2.19 (1.82–3.00) | 2.23 (1.81–3.10) | 2.58 (2.20–3.09) |
| sTNF-αRI, pg/mL | 1039 (706–1348) | 897 (691–1159) | 1050 (878–1359) | 1303 (1207–1659) ab |
| sICAM-1, ng/mL | 77.1 (59.0–94.5) | 85.8 (67.0–144.8) | 96.0 (80.7–190.5) | 92.6 (82.8–212.4) |
| Adiponectin, ng/mL | 1046 (795–1494) | 1118 (859–1628) | 927 (691–1223) | 815 (717–996) |
| sAxl, pg/mL | 38.3 (15.8–70.9) | 42.4 (20.8–69.3) | 38.8 (23.0–57.7) | 31.9 (20.3–72.7) |
Notes: Results are expressed in median (percentile 25-percentile 75). a p<0.05 compared with non-smokers; b p<0.05 compared with smokers.
Abbreviations: hsCRP, high sensitivity C-reactive protein; BNP, brain natriuretic peptide; VEGF, vascular endothelial growth factor; TGF-β, transforming growth factor; IL-6, Interleukin-6; HGF, hepatocyte growth factor; cGMP, cyclic guanosine monophosphate; sTNF-αRI, soluble receptor of tumor necrosis factor-alpha; sICAM-1, soluble intercellular adhesion molecule-1; sAxl, adiponectin, soluble tyrosine kinase receptor Axl. COPD, chronic obstructive pulmonary disease; PVD, pulmonary vascular disease.
Discussion
The results of the present study show that in patients with COPD, the presence of PVD is associated with systemic arterial dysfunction, characterized by increased vascular stiffness and worse endothelial function in systemic arteries. This association is irrespective of the presence of cardiovascular risk factors, which are more prevalent in COPD patients with PVD.
Systemic arterial stiffness, as assessed by PWV, was increased in COPD patients compared with control subjects, being patients with COPD and PVD those who presented the highest PWV values, in the non-adjusted analysis. Nevertheless, since in our cohort COPD patients presented more frequently cardiovascular risk factors, we analyzed the potential effect of associated comorbidities in an adjusted linear regression model. In the adjusted model, differences between COPD patients with PVD and both non-smoking and smoking controls persisted after adjusting for potential confounding factors. Furthermore, there was a trend to greater PWV in COPD patients with PVD compared with those without PVD. Accordingly, greater systemic arterial stiffness in the COPD with PVD group cannot be attributed to the effect of existing conditions or comorbidities.
Higher PWV in COPD patients has been previously shown,10 but to our knowledge, this is the first time that this observation is adjusted for potential confounding comorbidities, namely cardiovascular risk factors, and related to the presence of PVD. Taken together our current findings and previous observations suggest that greater systemic arterial stiffness in COPD might be the result of increased cardiovascular risk factors in this population. However, a subgroup of COPD patients with greater stiffness in systemic arteries might be more prone to develop PVD and eventually pulmonary hypertension, potentially as a result of greater stiffness also in pulmonary vessels.25
Several studies have previously reported endothelial dysfunction of systemic arteries in COPD patients,3,26 and our own group17 has already demonstrated the presence of reduced FMD in COPD patients with PVD compared to patients without pulmonary vascular impairment. In the current study, we extend these previous observations and demonstrate that endothelial dysfunction of systemic arteries in COPD is independent of the presence of cardiovascular risk factors and that both endothelial dysfunction and arterial stiffness are present in the same cohort of patients, being inversely related among them. Furthermore, COPD patients with PVD consistently show the greatest impairment in systemic vascular function, as shown in both unadjusted and adjusted linear models.
The reasons why COPD patients with PVD show greater systemic vascular disease are not clear. Airway inflammation has been previously linked with reduced vascular nitric oxide production,27 which could possibly explain a mechanism for endothelial dysfunction. However, the hypothesis of a “spill-over” of inflammatory mediators from the lung to the systemic circulation is not supported by the current findings, since there were no consistent differences among groups in the inflammatory mediators that were tested. Cigarette smoking is a common etiologic factor for both systemic28 and pulmonary vascular disease.29 Endothelial dysfunction might predispose to greater vascular damage and pulmonary vascular remodeling which in turn may lead to pulmonary artery stiffness. In fact, the number of pack/years correlated with both FMD and PWV in our cohort. Pulmonary artery stiffness has been extensively reported in COPD patients,25,30 and this finding, as measured by magnetic resonance pulse wave velocity, is able to identify pulmonary hypertension in COPD and is predictive of major cardiovascular events.31 In our study, arterial stiffness was not associated with smoking habit in patients without COPD, as no differences were observed between control smokers and non-smokers. Conceivably, vessel stiffness might be a contributing factor for the development of PVD. Similar to what has been shown in other forms of pulmonary hypertension,32 factors involved in vessel stiffening, such as metalloproteases,33 collagen and extra-cellular matrix gene expression and transcription,34,35 which have been shown in pulmonary arteries of COPD patients,36 might contribute to the development of PVD in COPD. Arterial stiffness could also be induced as a result of greater sympathetic nervous activity, through an increased vascular tone enhanced by associated sleep apnea37 or elevated carotid chemoreceptor activity.38 However, this seems unlikely in our patients since all of them were clinically screened to rule out sleep apnea.
Interestingly, sTNF-αRI was significantly elevated in COPD patients with PVD. sTNF-αRI has been shown to be elevated in patients with COPD and systemic hypertension,39 or coronary artery disease.40 TNFα was also elevated in COPD patients with pulmonary hypertension.41
Taken together, the interplay between pulmonary and systemic vascular disease in COPD patients could be more complex than previously considered, although they share physiopathological mechanisms42,43 and similar therapeutic strategies.44 It is therefore conceivable that COPD patients, through the effect of cigarette smoking, develop endothelial dysfunction and that, as the disease progresses, this leads to remodeling of both pulmonary and systemic vascular beds producing stiffness of the vessel wall. Indeed, endothelial damage has recently been suggested as a possible pathogenetic mechanism in the development of COPD, alongside other more traditionally studied pathophysiological pathways.45,46 Our observations could support this hypothesis, although further studies are needed to better define the role of endothelial dysfunction in this disease.
Results of the present study indicate that pulmonary and systemic vascular impairments are interrelated in COPD. These findings concur with the previous observations of altered markers of vascular competence (defined as an imbalance between injury and repair capacity of the endothelium) in COPD patients with PVD,5 suggesting common pathways in the pathobiology of alterations in both vascular territories.
Our study has some limitations. First, even though the overall cohort comprises more than one hundred patients, when broken down into groups, the sample size might not be large enough to discern some differences between groups. Second, right heart catheterization was not available in all subjects. Therefore, we cannot ascertain that patients with echocardiographic findings suggestive of pulmonary hypertension certainly had it. For this reason, we used the term PVD to define this subgroup of subjects. The proceedings from the 6th World Symposium on Pulmonary Hypertension (Nice 2018) suggested to lower the mPAP threshold for the diagnosis of pulmonary hypertension;47 however, we chose to follow the European guidelines12 that were in effect when the study was started. Nevertheless, the recent proposal to lower the cut-off mPAP value to define pulmonary hypertension47,48 and the observation in COPD that mPAP values lower than 25mmHg are associated with adverse clinical outcomes15 are in favor that patients we classified as having PVD certainly had it. Third, we cannot rule out the possibility that some of the patients in the PVD group on the basis of echocardiographic findings had left ventricular dysfunction. We excluded patients with reduced ejection fraction at echocardiography and those with intermediate or high probability of heart failure with preserved ejection fraction based on clinical criteria.49 Nevertheless, we cannot completely exclude the latter possibility.
Conclusion
The results of the present study show an association between systemic and pulmonary vascular impairment that suggests common pathophysiological mechanisms. The concurrence of endothelial dysfunction, which might be triggered by cigarette smoke products, and vascular stiffness appears as a potential common mechanism, not fully explained by conventional cardiovascular risk factors. Our results suggest that exploring factors associated with systemic vascular impairment in COPD may also shed light on the understanding of the development of pulmonary hypertension and contribute to reduce the impact of vascular comorbidities in this disease.
Acknowledgments
The authors are deeply indebted to Prof. Lluis de Jover (Biostatistics Unit, Department of Public Health, School of Medicine, University of Barcelona; Barcelona, Spain), deceased, for his invaluable assistance in the statistical analysis.
Funding Statement
The present study was supported by grants PS090536, PI12/00510 and PI13/00836 from the Instituto de Salud Carlos III (ISCiii), 197/2015 from the Spanish Society of Respiratory Medicine (SEPAR), Catalan Society of Pulmonology (SOCAP) and Fundación Contra la Hipertensión Pulmonar (FCHP).
Abbreviations
AICc, Akaike information criterion; AI, augmentation index; AI75, augmentation index normalized at 75 bpm; ANG-2, angiopoietin-2; BNP, brain natriuretic peptide; cGMP, cyclic guanosine monophosphate; COPD, chronic obstructive pulmonary disease; hs-CRP, high-sensitivity C reactive protein; FEV1, post-bronchodilator forced expiratory volume in the first second; FMD, flow-mediated dilation; FVC, forced vital capacity; HGF, hepatocyte growth factor; IL-6, interleukin-6; PH, pulmonary hypertension; PVD, pulmonary vascular disease; PWA, pulse wave analysis; PWV, pulse wave velocity; sAXL, soluble tyrosine kinase receptor; sICAM-1, soluble intercellular adhesion molecule-1; sTNF-αRI, soluble tumor necrosis factor receptor type I; TNFα, tumor necrosis factor α; VEGF, vascular endothelial growth factor.
Ethical Approvement Statement
The study was approved by the Ethics Committee of Hospital Clínic of Barcelona (20095026) and all subjects gave written informed consent before being included in the study.
Disclosure
Dr García-Lucio reports grants from Instituto de Salud Carlos III, outside of the current study. The authors report no other potential conflicts of interest for this work.
References
- 1.Chen W, Thomas J, Sadatsafavi M, FitzGerald JM. Risk of cardiovascular comorbidity in patients with chronic obstructive pulmonary disease: a systematic review and meta-analysis. Lancet Respir Med. 2015;3(8):631–639. doi: 10.1016/S2213-2600(15)00241-6 [DOI] [PubMed] [Google Scholar]
- 2.McGarvey LP, John M, Anderson JA, Zvarich M, Wise RA; TORCH Clinical Endpoint Committee. Ascertainment of cause-specific mortality in COPD: operations of the TORCH Clinical Endpoint Committee. Thorax. 2007;62(5):411–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barr RG, Mesia-Vela S, Austin JH, et al. Impaired flow-mediated dilation is associated with low pulmonary function and emphysema in ex-smokers: the Emphysema and Cancer Action Project (EMCAP) study. Am J Respir Crit Care Med. 2007;176(12):1200–1207. doi: 10.1164/rccm.200707-980OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chandra D, Gupta A, Strollo PJ Jr, et al. Airflow limitation and endothelial dysfunction: unrelated and independent predictors of atherosclerosis. Am J Respir Crit Care Med. 2016;194(1):38–47. doi: 10.1164/rccm.201510-2093OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.García-Lucio J, Peinado VI, de Jover L, et al. Imbalance between endothelial damage and repair capacity in chronic obstructive pulmonary disease. PLoS One. 2018;13(4):e0195724. doi: 10.1371/journal.pone.0195724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Houben-Wilke S, Jörres RA, Bals R, et al. Peripheral artery disease and its clinical relevance in patients with chronic obstructive pulmonary disease in the COPD and systemic consequences–comorbidities network study. Am J Respir Crit Care Med. 2017;195(2):189–197. doi: 10.1164/rccm.201602-0354OC [DOI] [PubMed] [Google Scholar]
- 7.Celermajer DS, Sorensen KE, Gooch VM, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340(8828):1111–1115. doi: 10.1016/0140-6736(92)93147-F [DOI] [PubMed] [Google Scholar]
- 8.Mills NL, Miller JJ, Anand A, et al. Increased arterial stiffness in patients with chronic obstructive pulmonary disease: a mechanism for increased cardiovascular risk. Thorax. 2008;63(4):306–311. doi: 10.1136/thx.2007.083493 [DOI] [PubMed] [Google Scholar]
- 9.Maclay JD, McAllister DA, Mills NL, et al. Vascular dysfunction in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2009;180(6):513–520. doi: 10.1164/rccm.200903-0414OC [DOI] [PubMed] [Google Scholar]
- 10.McAllister DA, Maclay JD, Mills NL, et al. Arterial stiffness is independently associated with emphysema severity in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2007;176(12):1208–1214. doi: 10.1164/rccm.200707-1080OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petrosillo N. Pulmonary vascular disease and infection: a tale of two diseases. Clin Microbiol Infect. 2011;17(1):5–6. doi: 10.1111/j.1469-0691.2010.03291.x [DOI] [PubMed] [Google Scholar]
- 12.Galiè N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: the Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J. 2015;46(4):903–975. doi: 10.1183/13993003.01032-2015 [DOI] [PubMed] [Google Scholar]
- 13.Portillo K, Torralba Y, Blanco I, et al. Pulmonary hemodynamic profile in chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2015;14(10):1313–1320. doi: 10.2147/COPD.S78180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Andersen KH, Iversen M, Kjaergaard J, et al. Prevalence, predictors, and survival in pulmonary hypertension related to end-stage chronic obstructive pulmonary disease. J Heart Lung Transplant. 2012;31(4):373–380. doi: 10.1016/j.healun.2011.11.020 [DOI] [PubMed] [Google Scholar]
- 15.Kessler R, Faller M, Fourgaut G, Mennecier B, Weitzenblum E. Predictive factors of hospitalization for acute exacerbation in a series of 64 patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;159(1):158–164. doi: 10.1164/ajrccm.159.1.9803117 [DOI] [PubMed] [Google Scholar]
- 16.Wells JM, Washko GR, Han MK, et al. Pulmonary arterial enlargement and acute exacerbations of COPD. N Engl J Med. 2012;367(10):913–921. doi: 10.1056/NEJMoa1203830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pizarro S, García-Lucio J, Peinado VI, et al. Circulating progenitor cells and vascular dysfunction in chronic obstructive pulmonary disease. PLoS One. 2014;9(8):e106163. doi: 10.1371/journal.pone.0106163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Global Initiative for Chronic Obstructive Lung Disease. [homepage on the Internet]. Available from: https://goldcopd.org/gold-reports. Accessed June21, 2020..
- 19.Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J. 2006;27(21):2588–2605. doi: 10.1093/eurheartj/ehl254 [DOI] [PubMed] [Google Scholar]
- 20.Butlin M, Qasem A. Large artery stiffness assessment using sphygmocor technology. Pulse (Basel). 2017;4(4):180–192. doi: 10.1159/000452448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilkinson IB, MacCallum H, Flint L, Cockcroft JR, Newby DE, Webb DJ. The influence of heart rate on augmentation index and central arterial pressure in humans. J Physiol. 2000;525(Pt 1):263–270. doi: 10.1111/j.1469-7793.2000.t01-1-00263.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harvey RE, Barnes JN, Hart EC, Nicholson WT, Joyner MJ, Casey DP. Influence of sympathetic nerve activity on aortic hemodynamics and pulse wave velocity in women. Am J Physiol Heart Circ Physiol. 2017;312(2):H340‐H346. doi: 10.1152/ajpheart.00447.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thijssen DH, Black MA, Pyke KE, et al. Assessment of flow-mediated dilation in humans: a methodological and physiological guideline. Am J Physiol Heart Circ Physiol. 2011;300(1):H2–12. doi: 10.1152/ajpheart.00471.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. J Am Coll Cardiol. 2002;39(2):257–265. doi: 10.1016/S0735-1097(01)01746-6 [DOI] [PubMed] [Google Scholar]
- 25.Liu CY, Parikh M, Bluemke DA, et al. Pulmonary artery stiffness in chronic obstructive pulmonary disease (COPD) and emphysema: the Multi-Ethnic Study of Atherosclerosis (MESA) COPD Study. J Magn Reson Imaging. 2018;47(1):262–271. doi: 10.1002/jmri.25753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vaes AW, Spruit MA, Theunis J, et al. Endothelial function in patients with chronic obstructive pulmonary disease: a systematic review of studies using flow mediated dilatation. Expert Rev Respir Med. 2017;11(12):1021–1031. doi: 10.1080/17476348.2017.1389277 [DOI] [PubMed] [Google Scholar]
- 27.Csoma B, Bikov A, Nagy L, et al. Dysregulation of the endothelial nitric oxide pathway is associated with airway inflammation in COPD. Respir Res. 2019;20(1):156. doi: 10.1186/s12931-019-1133-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Celermajer DS, Sorensen KE, Georgakopoulos D, et al. Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation. 1993;88:2149–2155. doi: 10.1161/01.CIR.88.5.2149 [DOI] [PubMed] [Google Scholar]
- 29.Peinado VI, Barbera JA, Ramirez J, et al. Endothelial dysfunction in pulmonary arteries of patients with mild COPD. Am J Physiol. 1998;274(6):L908–13. doi: 10.1152/ajplung.1998.274.6.L908 [DOI] [PubMed] [Google Scholar]
- 30.Domingo E, Grignola JC, Aguilar R, Messeguer ML, Roman A. Pulmonary arterial wall disease in COPD and interstitial lung diseases candidates for lung transplantation. Respir Res. 2017;18(1):85. doi: 10.1186/s12931-017-0568-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agoston-Coldea L, Lupu S, Mocan T. Pulmonary artery stiffness by cardiac magnetic resonance imaging predicts major adverse cardiovascular events in patients with chronic obstructive pulmonary disease. Sci Rep. 2018;8(1):14447. doi: 10.1038/s41598-018-32784-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Farrero M, Blanco I, Batlle M, et al. Pulmonary hypertension is related to peripheral endothelial dysfunction in heart failure with preserved ejection fraction. Circ Heart Fail. 2014;7(5):791–798. doi: 10.1161/CIRCHEARTFAILURE.113.000942 [DOI] [PubMed] [Google Scholar]
- 33.Wright JL, Tai H, Wang R, Wang X, Churg A. Cigarette smoke upregulates pulmonary vascular matrix metalloproteinases via TNF-alpha signaling. Am J Physiol Lung Cell Mol Physiol. 2007;292(1):L125–33. doi: 10.1152/ajplung.00539.2005 [DOI] [PubMed] [Google Scholar]
- 34.Hoffmann J, Wilhelm J, Marsh LM, et al. Distinct differences in gene expression patterns in pulmonary arteries of patients with chronic obstructive pulmonary disease and idiopathic pulmonary fibrosis with pulmonary hypertension. Am J Respir Crit Care Med. 2014;190(1):98–111. doi: 10.1164/rccm.201401-0037OC [DOI] [PubMed] [Google Scholar]
- 35.Karmouty-Quintana H, Weng T, Garcia-Morales LJ, et al. Adenosine A2B receptor and hyaluronan modulate pulmonary hypertension associated with chronic obstructive pulmonary disease. Am J Respir Cell Mol Biol. 2013;49(6):1038–1047. doi: 10.1165/rcmb.2013-0089OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Llinàs L, Peinado VI, Ramon Goñi J, et al. Similar gene expression profiles in smokers and patients with moderate COPD. Pulm Pharmacol Ther. 2011;24(1):32–41. doi: 10.1016/j.pupt.2010.10.010 [DOI] [PubMed] [Google Scholar]
- 37.Seravalle G, Mancia G, Grassi G. Sympathetic nervous system, sleep, and hypertension. Curr Hypertens Rep. 2018;20(9):74. doi: 10.1007/s11906-018-0874-y [DOI] [PubMed] [Google Scholar]
- 38.Phillips DB, Steinback CD, Collins SÉ, et al. The carotid chemoreceptor contributes to the elevated arterial stiffness and vasoconstrictor outflow in chronic obstructive pulmonary disease. J Physiol. 2018;596(15):3233‐3244. doi: 10.1113/JP275762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eagan TML, Ueland T, Wagner PD, et al. Systemic inflammatory markers in COPD: results from the Bergen COPD Cohort Study. Eur Respir J. 2010;35(3):540–548. doi: 10.1183/09031936.00088209 [DOI] [PubMed] [Google Scholar]
- 40.Safranow K, Dziedziejko V, Rzeuski R, et al. Plasma concentrations of TNF-α and its soluble receptors sTNFR1 and sTNFR2 in patients with coronary artery disease. Tissue Antigens. 2009;74(5):386–392. doi: 10.1111/j.1399-0039.2009.01332.x [DOI] [PubMed] [Google Scholar]
- 41.Joppa P, Petrasova D, Stancak B, Tkacova R. Systemic inflammation in patients with COPD and pulmonary hypertension. Chest. 2006;130(2):326–333. doi: 10.1378/chest.130.2.326 [DOI] [PubMed] [Google Scholar]
- 42.Vriz O, Motoji Y, Ferrara F, Bossone E, Naeije R. The right heart-pulmonary circulation unit in systemic hypertension. Heart Fail Clin. 2018;14(3):247–253. doi: 10.1016/j.hfc.2018.02.002 [DOI] [PubMed] [Google Scholar]
- 43.Zuo L, Rose BA, Roberts WJ, He F, Banes-Berceli AK. Molecular characterization of reactive oxygen species in systemic and pulmonary hypertension. Am J Hypertens. 2014;27(5):643–650. doi: 10.1093/ajh/hpt292 [DOI] [PubMed] [Google Scholar]
- 44.Leggio M, Fusco A, Limongelli G, Sgorbini L. Exercise training in patients with pulmonary and systemic hypertension: a unique therapy for two different diseases. Eur J Intern Med. 2018;47:17–24. doi: 10.1016/j.ejim.2017.09.010 [DOI] [PubMed] [Google Scholar]
- 45.Wouters EFM, Franssen FM. Chronic obstructive pulmonary disease: shifting the paradigm to the vasculature. Am J Respir Crit Care Med. 2019;199(3):258–259. doi: 10.1164/rccm.201808-1542ED [DOI] [PubMed] [Google Scholar]
- 46.Oelsner EC, Balte PP, Grams ME, et al. Albuminuria, lung function decline, and risk of incident chronic obstructive pulmonary disease. The NHLBI pooled cohorts study. Am J Respir Crit Care Med. 2019;199(3):321–332. doi: 10.1164/rccm.201803-0402OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nathan SD, Barberà JA, Gaine SP, et al. Pulmonary hypertension in chronic lung disease and hypoxia. Eur Respir J. 2019;53(1):1801914. doi: 10.1183/13993003.01914-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53(1):1801913. doi: 10.1183/13993003.01913-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vachiéry JL, Tedford RJ, Rosenkranz S, et al. Pulmonary hypertension due to left heart disease. Eur Respir J. 2019;53(1):1801897. doi: 10.1183/13993003.01897-2018 [DOI] [PMC free article] [PubMed] [Google Scholar]