Abstract
Engineered nanoparticles could trigger inflammatory responses and potentiate a desired innate immune response for efficient immunotherapy. Here we report size-dependent activation of innate immune signaling pathways by gold (Au) nanoparticles. The ultrasmall-size (<10 nm) Au nanoparticles preferentially activate the NLRP3 inflammasome for Caspase-1 maturation and interleukin-1β production, while the larger-size Au nanoparticles (>10 nm) trigger the NF-κB signaling pathway. Ultrasmall (4.5 nm) Au nanoparticles (Au4.5) activate the NLRP3 inflammasome through directly penetrating into cell cytoplasm to promote robust ROS production and target autophagy protein-LC3 (microtubule-associated protein 1-light chain 3) for proteasomal degradation in an endocytic/phagocytic-independent manner. LC3-dependent autophagy is required for inhibiting NLRP3 inflammasome activation and plays a critical role in the negative control of inflammasome activation. Au4.5 nanoparticles promote the degradation of LC3, thus relieving the LC3-mediated inhibition of the NLRP3 inflammasome. Finally, we show that Au4.5 nanoparticles could function as vaccine adjuvants to markedly enhance ovalbumin (OVA)-specific antibody production in an NLRP3-dependent pattern. Our findings have provided molecular insights into size-dependent innate immune signaling activation by cell-penetrating nanoparticles and identified LC3 as a potential regulatory target for efficient immunotherapy.
Keywords: NLRP3 inflammasome, cell-penetrating ultrasmall-sized gold nanoparticles, autophagy, microtubule-associated protein 1-light chain 3 (LC3), adjuvant activity, antibody production
Graphical Abstract
A host's immune system can be harnessed to develop protective or therapeutic immunity against infectious disease, tissue injury, and cancer. Among them, innate immune response is the first line of defense against invading pathogens and transformed tumor cells, and it is critical for subsequent adaptive immune response.1,2 Nanoparticles, which are designed as synthetic building blocks at the nanometer scale (1 to 100 nm) for drug delivery and other medical applications, have been shown to interact with biological entities and trigger innate immune responses.3,4 Inflammatory responses induced by nanoparticles have been reported to cause toxicities, such as tissue fibrosis or allergy,5,6 but such a response could also promote or enhance humoral and cellular immune responses against infection or cancer, depending on nanoparticle physicochemical properties.7,8 However, which specific characteristic of nanoparticles is required for triggering innate immune response and what are the mechanisms underlying these innate immune responses and signaling pathways are poorly understood. As the first defense system, immune cells monitor foreign invaders and trigger innate immune signaling pathways, including NF-κB, type I interferon (IFN), or inflammasome activation through recognition of pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs) by several classes of pattern recognition receptors (PRRs).1 Different toll-like receptors (TLRs) or NOD-like receptors (NLRs) have distinct locations for specific recognition of pathogens or stress. Therefore, the intracellular location and the internalization pathways of nanoparticles are critical for dictating the activation of specific innate immune signaling pathways.
NLRP3 (NLR family, pyrin domain containing 3) is a key cytoplasmic innate immune receptor that detects or senses particles including silica, aluminum salts, monosodium urate (MSU), and calcium pyrophosphate dihydrate (CPPD), and protein aggregates caused by inappropriate oligomerization or misfolding.9-11 NLRP3 inflammasome activation has also been implicated for cancer immunotherapy and alum’s adjuvant activity for antibody production.12,13 Recent studies show that manufactured nanoparticles could also activate the NLRP3 inflammasome and enhance the antibody production depending on nanoaprticle size, surface charge, and surface modification.14-24 The currently proposed mechanisms of nanoparticle-induced NLRP3 inflammasome activation include enhanced uptake of inflammsome activators, excessive reactive oxygen species (ROS) production, suppressed degradation of the inflammasome due to compromised lysosome activity, and nanoparticle-induced lysosomal cathepsin or other danger signal release.9-11,14,24-31 However, the role of the physicochemical properties in controlling the pattern-specific immune response and the precise molecular targets of nanoparticles for activating the inflammasomes remain unclear. Further investigations on the regulatory mechanism of nanoparticle-induced inflammasome activation are needed. For example, many nanoparticles are capable of generating robust ROS production,32 but only a limited number of nanoparticles can activate inflammasomes,23,24,33,34 suggesting that other contributing factors might be involved in the regulation of inflammasome activation.
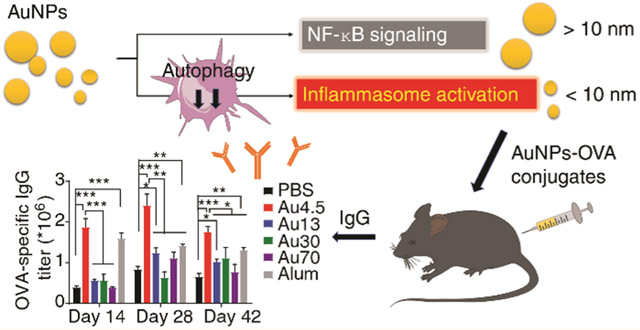
Autophagy is a homeostatic process that removes damaged cellular components or ubiquitinated proteins through autophagosomal degradation and is involved in the regulation of inflammasome activation.35-37 Of particular note, nanoparticles that are approximately the same size as self-cellular components and invading microorganisms have been found to induce autophagy through an oxidative stress- or mTOR-dependent pathway via endocytosis/phagocytosis-dependent mechanisms.38-41 On the contrary, nanoparticles could also suppress autophagic degradation due to the impairment of autophagosome fusion with lysosomes or impaired lysosome function.28-30,38,41-45 However, how autophagy regulates inflammasome activation in response to the stimulations by nanoparticles with different physicochemical properties remains unclear. In this study, we investigated differential inflammatory responses induced by PEGylated Au nanoparticles of different sizes. Our results showed that Au nanoparticles of ultrasmall size (<10 nm) could markedly activate the NLRP3 inflammasome, while large-size nanoparticles failed to do so. We further demonstrated that the ultrasmall-sized Au nanoparticles (Au4.5) could directly penetrate into the cytoplasm to trigger ROS production and target LC3 (Map1lc3b, microtubule-associated protein 1-light chain 3B, a central autophagic machinery protein) for degradation in an endocytic/phagocytic-independent manner. Cells that are deficient in LC3 could release their inhibitory effect on NLRP3 inflammasome activation through autophagy-mediated degradation. Finally, we showed that cell-penetrating Au-nanoparticle-mediated inflammasome activation could markedly enhance antibody production and induce a potent adjuvant effect compared to larger-size Au nanoparticles and alum adjuvant. Thus, our findings have identified a previously unrecognized role and mechanism for ultrasmall-sized Au nanoparticles in NLRP3 inflammasome activation for adjuvating strong antibody response. Further, these findings provide a scientific basis for the use of nanoparticles in developing efficient immunotherapeutics for infectious diseases.
RESULTS
Size-Dependent Innate Immune Responses Are Induced by Nanoparticles.
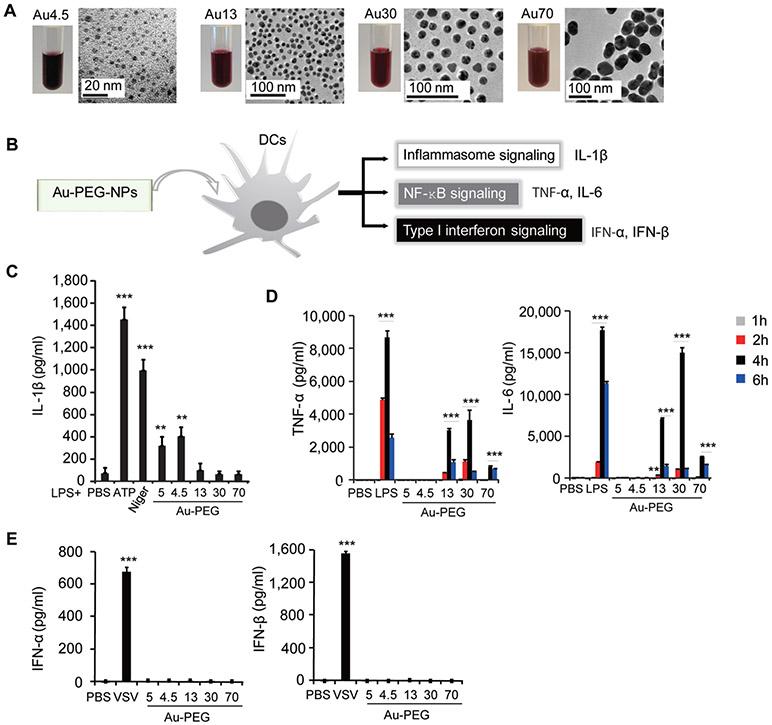
To gain insights into how engineered nanoparticles trigger a specific innate immune response, we first synthesized different sized polyethylene glycol-coated gold nanoparticles (Au-PEG-NPs) with mean diameters of 4.5, 13, 30, and 70 nm (determined by transmission electron microscopy, TEM) for mechanistic study (Figure 1A). The hydrodynamic size distribution and zeta potential of Au nanoparticles in H2O and biological solutions, including saline (0.9% NaCl) and RPMI1640 medium, were characterized (Figure S1A-F). We also evaluated the endotoxin contamination of all Au nanoparticle samples and showed that the endotoxin levels of Au nanoparticle samples were <0.01 EU/mL (Figure S1G). Mouse bone marrow-derived dendritic cells (BMDCs) were prepared and used to determine the innate immune response triggered by different sized Au nanoparticles (Figure 1B). BMDCs were >80% in purity, as determined by flow cytometry using two different DC markers (CD11c+ or MHC Class II+ population) (Figure S2A). We first primed BMDCs with lipopolysaccharide (LPS) in order to induce the expression of interleukin-1β precursor (pro-IL-1β); we then treated primed BMDCs with different Au nanoparticles we synthesized, commercialized 5 nm Au-PEG-NPs (Sigma), and positive controls (ATP or nigericin), respectively. We found significant amounts of IL-1β were produced by LPS-primed cells after treatment with ultrasmall-sized Au nanoparticles, including 4.5 nm Au-PEG-NPs (Au4.5) and commercialized 5 nm Au-PEG-NPs (Au5) (Figure 1C). By contrast, large-sized Au nanoparticles could not activate inflammasomes for IL-1β production but instead triggered the NF-κB signaling pathway (Figure 1D). Upon stimulation by 13, 30, and 70 nm Au-PEG-NPs (Au13, Au30, and Au70), BMDCs produced high levels of proinflammatory cytokines such as tumor necrosis factor (TNF-α) and interleukin-6 (IL-6) (Figure 1D). Consistently, we found that I kappa B kinase (IKK) was phosphorylated (p-IKK) after stimulation by larger-sized nanoparticles (Figure S2B). However, we did not observe any appreciable activation of type I interferon (IFN) signaling. There was no detectable IFN-α/β in all nanoparticle-treated BMDCs (Figure 1E). Consistently, we did not detect the phosphorylation of interferon regulatory factor 3 (IRF-3) after incubation of BMDCs with different sized Au nanoparticles (Figure S2C). Taken together, these results suggest that the size of nanoparticles is critical in dictating the activation of specific innate immune signaling pathways. We therefore selected ultrasmall-sized Au nanoparticles for further inflammasome studies.
Figure 1.
Size-dependent innate immune responses are induced by nanoparticles. (A) Optical images of Au nanoparticle suspension and transmission electron microscopy analysis of Au nanoparticles (top row). Scale bar presents as indicated. (B) Scheme for the screening strategy for innate immune responses to nanoparticles. (C) ELISA for the induction of IL-1β in supernatants of bone marrow derived dendritic cells (BMDCs) primed with LPS (100 ng/mL, 3 h) alone or followed by Au-PEG-NPs treatment at 200 μg/mL for 6 h. ATP was added at 5 mM for 1 h. Nigericin was added at 1 μM for 4–6 h. Mean diameter of each nanoparticle is indicated. (D) ELISA test for secretion of TNF-α and IL-6 in the supernatants of BMDCs stimulated with indicated Au nanoparticles (200 μ/mL) or LPS (100 ng/mL) for 1, 2, 4, and 6 h. (E) ELISA test for induction of IFN-α and IFN-β in supernatants of BMDCs stimulated with Au nanoparticles (200 μg/mL) or vesicular stomatitis virus (VSV) for 24 h. *p < 0.05, **p < 0.01, ***p < 0.001; NS, not significant. Data are representative of three experiments (mean ± SD).
NLRP3 Is Required for Inflammasome Activation by Ultrasmall Nanoparticles.
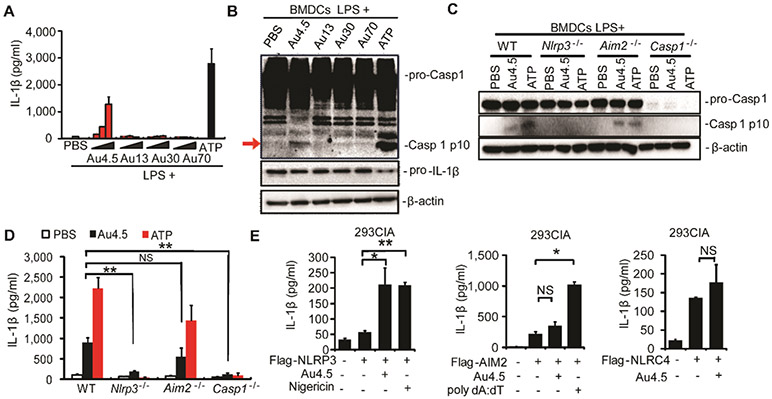
Next, we sought to determine how inflammasome signaling was activated by ultrasmall nanoparticles. We found that Au4.5 nanoparticles markedly activated the inflammasome and induced IL-1β secretion by mature Caspase-1 (p10) in a dose-dependent manner (Figure 2A,B). To test the possibility that the inflammsome pathway could be activated by residual molecules from the synthesis procedure and/or PEG coating, we first separated the Au4.5 nanoparticles from the suspending solution using Amicon Ultra centrifugal filter units (Ultra-15, MWCO 30 kDa) and found that only the Au4.5 nanoparticle fraction, but not the suspending solution, could activate the inflammasome for IL-1β production (Figure S3A), suggesting the irrelevance of potential residual molecules for inflammasome activation. Next, we compared 5 nm sized non-PEG-coated Au nanoparticles with the Au4.5 nanoparticles and found that the Au5 (non-PEG-coated) could activate the inflammasome to a similar extent as Au4.5 (Figure S3A). These data suggest that the Au4.5-induced inflammasome activation was dispensable of PEG coating and residuals. Because there are several inflammasome complexes composed of different NOD-like receptors, including NLRP3, Aim2 (absent in melanoma 2), and NLRC4 (NLR family, CARD domain containing 4), we isolated BMDCs from Nlrp3-, Aim2-, and Caspase-1-deficient mice and tested their ability to activate the inflammasome for IL-1β release after nanoparticle treatment. We found that the Caspase-1 and IL-1β production and activation were completely abolished in Nlrp3−/− and Caspase-1−/− BMDCs upon Au4.5 and ATP (a strong NLRP3-inflammasome inducer) stimulations (Figure 2C,D). By contrast, the cleaved Caspase-1 and IL-1β levels in Aim2−/− BMDCs remain markedly high upon ATP and Au4.5 stimulation (Figure 2C,D). Furthermore, we showed that inflammasome activation could be induced by Au4.5 nanoparticles in 293CIA cells46 (stably expressing Caspase-1, pro-IL-1β, and ASC) transfected with NLRP3, but not AIM2 or NLRC4 genes, based on the IL-1β production (Figure 2E). These results suggest that NLRP3 is specifically required for inflammsome activation in response to Au4.5 nanoparticle stimulation.
Figure 2.
NLRP3 is required for inflammasome activation by ultrasmall nanoparticles. (A,B) ELISA test for the induction of IL-1β production in supernatants (A) and immunoblot analysis of whole cell lysates (B) of bone marrow derived dendritic cells (BMDCs) primed with LPS (100 ng/mL, 3 h) alone or followed by 6 h of Au4.5, Au13, Au30, or Au70 (50, 100, 200 μg/mL) treatment or 1 h of ATP (5 mM) treatment. Whole cell lysates were immunoblotted with indicated antibodies (B). (C,D) BMDCs from wild-type, Nlrp3−/−, Aim2−/−, and Caspase-1−/− mice were primed with LPS (100 ng/mL, 3h) and then treated with Au4.5 nanoparticles at 200 μg/mL for 6 h or ATP for 1 h. Immunoblot analysis of cell lysates of pro-Caspase-1 and cleaved Caspase-1 p10 are shown in (C). Production of IL-1β in the supernatants was analyzed by ELISA (D). (E) ELISA for IL-1β production in cell supernatants of 293CIA cells transfected with plasmids encoding HA-NLRP3, Flag-Aim2, or Flag-NLRC4, respectively, followed by treatment with Au4.5 (200 μg/mL) or nigericin (1 μM) for 6 h or transfection with poly dA:dT for 6 h. *p < 0.05, **p < 0.01; NS, not significant. Data are representative of three experiments (mean ± SD).
Au4.5 Nanoparticles Activate NLRP3 Inflammasome Complex through ROS Production and Targeting LC3 for Degradation.
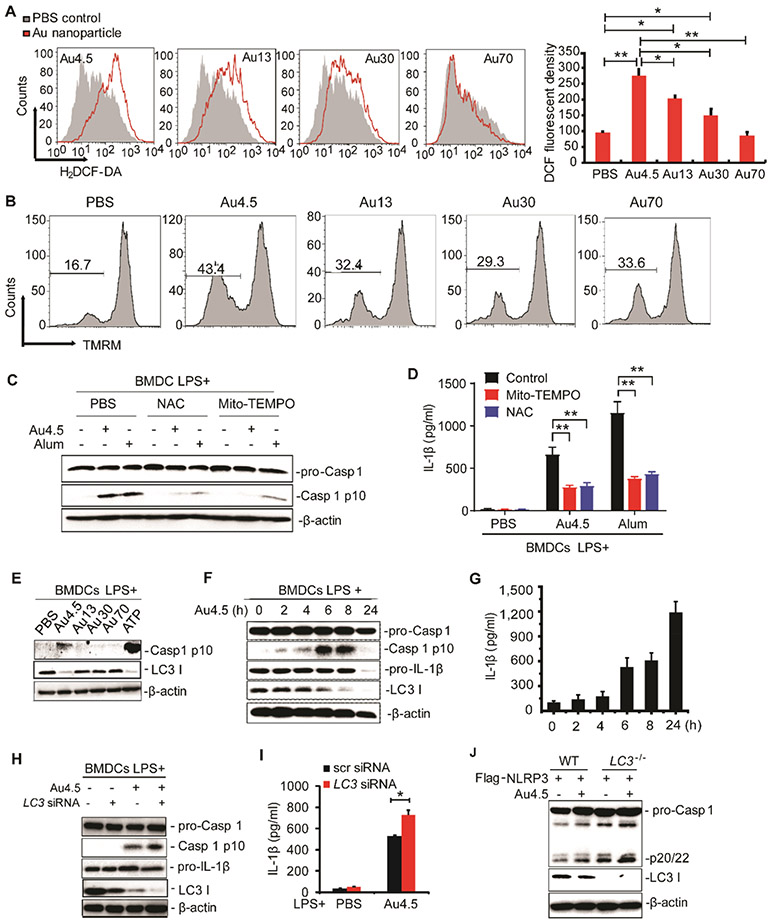
Previous studies have shown that particles or crystals trigger the NLRP3 inflammasome complex by providing danger signals such as lysosomal destabilization and ROS production upon uptake of these particles.11,33 Consistently, we found that Au4.5 nanoparticles generated the highest level of intracellular ROS among different groups of nanoparticles in sizes (Figure 3A). The presence of more depolarized mitochondria in Au4.5-treated cells was consistent with the ROS production (Figure 3B). To further determine the role of ROS in Au4.5-induced inflammasome activation, we applied two different ROS scavengers/inhibitors, MitoTEMPO and n-acetyl cysteine (NAC), to test their effect on inflammasome activity. Both MitoTEMPO and NAC could successfully reduce the ROS production in Au4.5-treated cells to a level similar to nontreated controls (Figure S3B). Our results show that both cleaved caspase 1 and IL-1β production induced by Au4.5 or alum (positive control) were significantly reduced after treatment of ROS scavengers, suggesting an important role of ROS in Au4.5-induced inflammasome activation (Figure 3C,D).
Figure 3.
Au4.5 nanoparticles activate the NLRP3 inflammasome through ROS production and targeting LC3 for degradation. (A) Flow cytometry analysis of BMDCs’ intracellular ROS. BMDCs were labeled with H2DCF-DA to trace the intracellular ROS levels after incubation with 200 μg/mL of Au4.5, 13, 30, and 70 nanoparticles for 6 h, respectively. Quantities of fluorescent density are normalized from two independent experiments. (B) Flow cytometry analysis of BMDCs’ mitochondria depolarization using fluorescent probe TMRM upon treatment with 200 μ/mL of Au4.5, 13, 30, and 70 nanoparticles for 6 h, respectively. (C,D) Immunoblot analysis of cleaved caspase 1 in cell lysates (C) and ELISA for IL-1β in supernatants (D) of LPS-primed BMDCs, which were pretreated with MitoTEMPO (1 μM) or NAC (2 mM) for 3 h, followed by incubation with 200 μg/mL of Au4.5 or 400 μg/mL of alum for 6 h along with the MitoTEMPO or NAC. (E) Immunoblot analysis of cell lysates of BMDCs primed with LPS (100 ng/mL, 3 h) and then left untreated or followed by 6 h of Au4.5, Au13, Au30, or Au70 (200 μg/mL) treatment or 1 h of ATP treatment. (F,G) Immunoblot analysis of cell lysates (F) and ELISA for IL-1β in supernatants (G) of BMDCs primed with LPS (100 ng/mL, 3 h), followed by treatment with 200 μg/mL of Au4.5 for a different time. (H,I) Immunoblot analysis of pro-Caspase-1, Caspase-1 p10, and LC3 in cell lysates (H) and ELISA for IL-1β production in cell supernatants (I) of BMDCs transfected with scramble siRNA or LC3 siRNA and then primed with LPS (100 ng/mL, 3 h) alone or followed by 6 h of Au4.5 (200 μg/mL) treatment. (J) Immunoblot analysis of pro-Caspase-1, Caspase-1 p20/22, and LC3 in lysates of wild-type (WT) 293CIA cells or LC3 knockout 293CIA cells treated with Au4.5 (200 μg/mL) for 6 h. Data are representative of three experiments (mean ± SD). *p < 0.05.
It has been known that autophagy negatively regulates inflammasome activation through the removal of inflammasome-activating endogenous signals (such as mitochondrial DNA) and the sequestration and degradation of inflammasome components.35-37,47 Cells defective in autophagy would elevate inflammasome activity and IL-1β production, resulting in the pathogenesis of inflammatory diseases.36,37 To further determine whether autophagy plays any role in Au4.5-induced NLRP3 inflammasome activation, we next tested the autophagy protein levels after nanoparticle treatment and found that Au4.5 nanoparticle treatment could specifically decrease the protein level of autophagic protein-LC3 (Figure 3E) but not other autophagy proteins tested such as Beclin 1, ATG13, or ATG14 (data not shown). Importantly, the increased production of mature Caspase-1 p10 and IL-1β was inversely correlated with the decrease of the LC3 protein level (Figure 3F,G). In order to determine whether the Au4.5 nanoparticle-induced inflammasome activation was directly regulated by LC3, we transfected BMDCs with LC3b-specific small interfering RNA (siRNA) to knock down endogenous LC3. We found that knockdown of LC3 in BMDCs could significantly enhance Caspase-1 activation and IL-1β secretion after Au4.5 stimulation (Figure 3H,I). Similar results were obtained in LC3-deficient 293CIA (293CIALC3−/−) cells when compared to wild-type 293CIA (Figure 3J). Caspase-1 cleaved product (p20/22) in 293CIALC3−/− cells was much higher than that in wild-type 293CIAWT (Figure 3J). These data suggest that NLRP3 inflammasome activity is negatively regulated by LC3 protein and that Au4.5 may contribute to the enhanced inflammasome activity by targeting LC3 for degradation. Interestingly, the commercialized Au5 was also found to reduce the LC3 protein level (Figure S3C), suggesting that ultrasmall Au nanoparticles might promote inflammasome activation through degradation of LC3 as a common pathway.
LC3-Dependent Macroautophagy Negatively Regulates the NLRP3 Inflammasome Activity.
To determine the molecular mechanisms by which LC3 regulates NLRP3 inflammasome activation, we first tested whether LC3 interacted with NLRP3 before and after Au4.5 stimulation. We found that LC3 interacted with endogenous NLRP3 even before Au4.5 stimulation (Figure 4A), but such an interaction became stronger after Au4.5 stimulation (Figure 4B). Using confocal microscopy, we further showed that NLRP3 was colocalized with GFP-LC3+ puncta (Figures 4C and S4A). LC3 is essential in autophagosome membrane elongation for autophagosome formation. To determine if LC3 reduction caused by Au4.5 compromises macroautophagy, we monitored the LC3+ puncta formation and calculated the LC3+ puncta numbers per cell to compare the autophagic flux before and after Au nanoparticle stimulation. We found that Au4.5 treatment markedly reduced the overall LC3 fluorescent levels as well as the number of LC3+ puncta (Figure S4B), which is consistent with our finding that the LC3 protein level is reduced in Au4.5-treated cells. Consistently, the p62 protein (macroautophagy substrate) was accumulated after Au4.5 or Au5 (purchased from Sigma) nanoparticle treatment, suggesting that the ultrasmall Au nanoparticles indeed suppress autophagy (Figure S4C). Furthermore, induction of autophagy using autophagy inducer (4-OH tamoxifen) or starvation could decrease IL-1β production and Caspase-1 p10 (Figure 4D,E), suggesting the negative regulatory role of autophagy in inflammasome activation. Moreover, elevated NLRP3 levels were observed in ultrasmall Au nanoparticle-treated BMDCs (Figure S4D), showing that NLRP3 was accumulated in Au4.5-treated cells due to compromised LC3-dependent macroautophagy.
Figure 4.
Au4.5 activates the NLRP3 inflammasome through its inhibitory effect on macroautophagy. (A) Immunoblot analysis of cell lysates from BMDCs primed with LPS (100 ng/mL, 3 h) and stimulated by Au4.5 (200 μg/mL) for 6 h, followed by immunoprecipitation with anti-NLRP3 and immunoblot analysis (antibodies, left margin). (B) Immunoblot of 293CIA cells transfected with plasmid for HA-NLRP3 and treated with Au4.5 or nigericin for 6 h. Cell lysates were immunoprecipitated with anti-LC3 and immunoblot for NLRP3. (C) Confocal microscopy analysis of colocalization of LC3 and NLRP3 in 293CIA cells transfected with plasmids encoding EGFP-LC3 and NLRP3, followed by Au4.5 stimulation for 6 h. Cells were fixed and stained with DAPI (blue) and anti-NLRP3 followed with RPE-conjugated anti-mouse IgG antibody (red). Scale bar, 10 μm. Arrows indicate colocalization. (D,E) ELISA analysis (D) and immunoblot (E) of BMDCs primed with LPS, followed by Au4.5, ATP, or nigericin stimulation in normal, starvation condition, or along with autophagy inducer 4-OH tamoxifen treatment (5–10 μM, 12 h prior to stimulation and also applied during stimulation). Immunoblot of pro-Caspase-1 and Caspase-1 p10 were performed. Niger represents nigericin (E). (F,G) 293CIA, 293CIALC3−/−, 293CIAATG16L−/−, and 293CIABECN1−/− cells were transfected with Flag-NLRP3 and then stimulated by Au4.5 or nigericin for 6 h. Relative quantity of Caspase-1 p20/22 bands are shown on the right side (F). Induction of IL-1β in cell supernatants was analyzed by ELISA (G). Data are representative of three experiments (mean ± SD). *p < 0.05, **p < 0.01, ***p < 0.001.
LC3 exists in a cytoplasmic form (LC3-I) and a membrane-associated form (LC3-II) after autophagy activation. During autophagy, the autophagosome engulfs cytoplasmic components; concomitantly, the cytosolic form of LC3 (LC3-I) is conjugated to phosphatidylethanolamine (PE) to form lipidated LC3 (LC3-II), a process called lipidation. LC3-II is recruited to autophagosomal membranes, and then, autophagosomes fuse with lysosomes to degrade the intra-autophagosomal components. The conversion of LC3-I to LC3-II is a crucial step for autophagosome maturation.48 Therefore, we next tested whether two other autophagy genes, ATG16L1 (required for LC3 lipidation) and BECN1 (upstream of LC3 lipidation process), are required for inhibiting NLRP3 activation. We used sgRNA-guided genome editing by CRISPR-Cas9 technology to generate both ATG16L1 knock-out and BECN1 knockout 293CIA cells and confirmed the successful KO of specific genes using Western blotting and the Surveyor assay (Figures 4F and S4E). In both ATG16L1 knockout cells and BECN1 knockout cells, we found that Caspase-1 p20/22 activity and IL-1β production were markedly enhanced, compared to WT cells (Figure 4F,G), which supports the critical role of LC3 lipidation in suppression of inflammasome activity. Collectively, we demonstrated that ultrasmall nanoparticles activated the NLRP3 inflammasome through suppression of LC3-dependent autophagic degradation of NLRP3, and manipulation of autophagy using nanoparticles could regulate the activity of NLRP3 inflammasome.
Au4.5 Nanoparticles Are Capable to Directly Penetrate into Cells to Promote LC3 K48-Linked Ubiquitination for Proteasomal Degradation.
To understand how Au4.5 nanoparticles inhibit the LC3 protein level, we first examined LC3 at the mRNA level after stimulation with different sized Au nanoparticles and found no significant difference after treatment (Figure S5A), suggesting that Au nanoparticles do not affect LC3 mRNA expression. Furthermore, we did not detect direct binding between Au4.5 nanoparticles and LC3 protein in an in vitro assay using Au4.5 nanoparticle mixing and culture with recombinant human LC3 protein in PBS (supplement with 1 mM EDTA) (Figure S5B). No appreciable difference was observed for Au4.5-induced LC3 degradation when ROS scavengers were applied (Figure S5C), suggesting that ROS are not required for Au4.5-induced LC3 degradation. However, using bafilomycin A1 for inhibiting autophagy and MG132 for inhibiting the proteasome pathway, we showed that the LC3 degradation by Au4.5 could be blocked by MG132 but not by bafilomycin A1 (Figure S5D), suggesting that Au4.5-induced LC3 degradation is mainly mediated through the proteasome pathway. To determine whether Au4.5 affects the ubiquitination of LC3, we immunoprecipitated EGFP-LC3 and found that LC3 was ubiquitinated by K48-linked ubiquitin chains after Au4.5 treatment (Figure S5E). We then used a site-directed mutagenesis approach to substitute Lys5 (K5) and Lys8 (K8) of LC3 with arginine (R) to create LC3K5R and LC3K8R single mutants and a LC3K5R,K8R double mutant. We found that the LC3K5R or LC3K8R single mutant partially reduced the K48-linked ubiquitination of LC3, while the LC3K5R,K8R double mutant almost completely abolished the Au4.5 nanoparticle-induced K48-linked ubiquitination of LC3 (Figure S5F). Although it remains unclear how ultrasmall Au nanoparticles affect the ubiquitination of LC3, a previous report shows that ubiquitin can bind to citrate-coated silver nanoparticles (AgNPs) to form protein coronas.49 Future studies are warranted to assess whether ubiquitin also binds to Au nanoparticles and affects the ubiquitination of LC3 using computational and experimental characterizations.
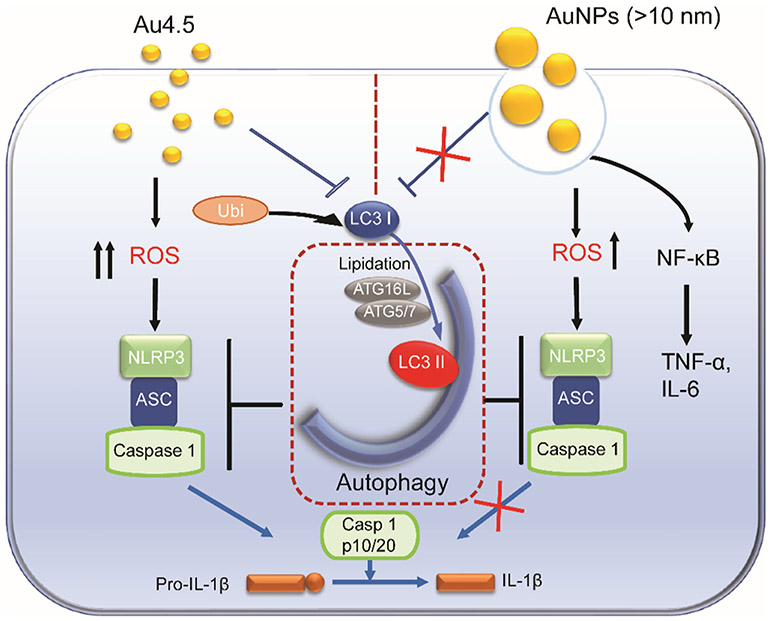
Physicochemical properties of nanoparticles largely determine their uptake, intracellular location, and biological feedback.4,50 Using transmission electron microscopy (TEM), we observed that Au4.5 nanoparticles were mainly distributed in the cytoplasm outside phagosomal or endolysosomal compartments, whereas larger Au nanoparticles were mainly localized within the endosomes/phagosomes (Figures 5A and S6). This observation is consistent with previous findings that ultrasmall sizes or cationic surface modifications can facilitate nanoparticles (such as metal nanoclusters, dendrimers, and carbon nanotubes) penetrating or trafficking into the cytoplasm.51,52 Furthermore, we used inductively coupled plasma-mass spectrometry (ICP-MS) to quantify the uptake of Au nanoparticles by BMDCs under the 4 °C condition (which inhibits energy-dependent cellular uptake) or upon internalization inhibitor treatment. We showed that only a small portion (32% of responsiveness) of Au4.5 nanoparticles were blocked from internalization under the 4 °C condition, suggesting that the majority of Au4.5 were taken up through an energy-independent pathway (Figure 5B). By contrast, Au13, 30, and 70 nanoparticles were mainly taken up through energy-dependent cellular uptake pathways (>80% inhibited from internalization under the 4 °C condition) (Figure 5B). We next determined the Au4.5 nanoparticle-induced inflammasome activity at 4 °C and found that the IL-1β could still be produced after Au4.5 stimulation although at a lower level (Figure 5C). The silica crystals (US Silica, MIN-U-SIL-15, median diameter 3.4 μm), which have been demonstrated to be taken up through energy-dependent cell phagocytosis and activate NLRP3 inflammasome through destabilizing the lysosomes, served as a control (Figure 5C).11 By using inhibitors to block clathrin-dependent endocytosis (chlorpromazine) or macropinocytosis/phagocytosis (cytochalasin D), we found that a small portion of Au4.5 could be taken up through a macropinocytosis or endocytosis pathway, which may be due to the aggregation or agglomeration of ultrasmall nanoparticles50 (Figure 5D). On the contrary, Au13 was mainly internalized through either a macropinocytosis (~45%) or endocytosis (~50%) pathway. The majority of Au30 nanoparticles were taken up through a macropinocytosis/ phagocytosis pathway (>75% responsive to cytochalasin D treatment), and a small portion was internalized through endocytosis (~20% responsive to chlorpromazine treatment). By contrast, Au70 was predominately taken up through a macropinocytosis/phagocytosis pathway (>80% responsive to cytochalasin D treatment) (Figure 5D). These results demonstrated that majority of Au4.5 nanoparticles could directly penetrate into the cell cytoplasm in an energy-independent manner, suggesting an endocytosis/phagocytosis-independent way for ultrasmall-nanoparticle-induced inflammasome activation (Figure 6). By contrast, large nanoparticles are mainly taken up through energy-dependent macropinocytosis/phagocytosis or endocytosis and are trapped in phagosomal or endolysosomal compartments for NF-κB signaling activation (Figure 6). These findings were supported by previous reports that nanoparticles of ultrasmall size (<10 nm) with a well-designed surface could facilitate their uptake through direct cell bilayer penetration51,52 or escape from the endosomes/phagosomes after internalization. Although previous studies show that phagocytosis of crystals such as silica and aluminum salts was required for NLRP3 inflammasome activation,11,23 our data show that inflammasome activation induced by ultrasmall nanoparticles was independent of phagocytosis. A recent study also shows that 20 nm TiO2 nanoparticles induce the activation of the inflammasome without phagocytosis.24 Most TiO2 nanoparticles are localized in the cytoplasm in monocytes; disruption of actin-mediated phagocytosis does not affect inflammasome activation and IL-1β production.24 Once inside the cytoplasm, ultrasmall nanoparticles have the ability to impair mitochondrial function and degrade LC3, which could both generate excessive ROS and relieve the NLRP3 suppression function of LC3 for NLRP3 inflammasome activation.53 Thus, our study shows that nanoparticle size and intracellular localization play critical roles in the selection and activation of specific innate immune signaling pathways.
Figure 5.
Cell-penetrating Au4.5 nanoparticles are responsible for activating the NLRP3 inflammasome. (A) TEM analysis for Au nanoparticle intracellular locations in BMDCs incubated with 200 μg/mL of Au nanoparticles for 6 h. (B) ICP-MS analysis of the amounts of Au nanoparticle uptake by BMDCs at 37 or 4 °C for 6 h (left). Percentage of Au nanoparticles responsive to 4 °C condition treatment compared to 37 °C condition treatment is shown on the right. (C) IL-1β production from LPS-primed BMDCs treated with Au nanoparticles at 37 or 4 °C for 6 h. (D) ICP-MS analysis of the amounts of Au nanoparticle uptake by BMDCs treated with PBS, chlorpromazine (an inhibitor for clathrin-mediated endocytosis, 20 μg/mL), or cytochalasin D (an inhibitor for phagocytosis/macropinocytosis, 40 μM) (left). Percentage of Au nanoparticles responsive to chlorpromazine (20 μg/mL) or cytochalasin D (40 μM) treatment is shown on the right.
Figure 6.
Scheme illustration for Au4.5-induced NLRP3 inflammasome activation. Au4.5-nanoparticle-induced inflammasome activation requires dual functions: (1) generating robust ROS production as an inflammasome-stimulating signal and (2) releasing the autophagy-mediated inhibitory effect on inflammasome activity through targeting LC3 for degradation. By contrast, Au13 failed to activate the NLRP3 inflammasome, since the autophagy-mediated inhibition of the inflammasome pathway is still active. Large Au-NPs (>10 nm) are taken up through endocytosis and trigger NF-κB signaling for TNF-α and IL-6 production. ROS, reactive oxygen species.
Au4.5 Nanoparticles Generate Strong Adjuvant Activity for Antibody Production.
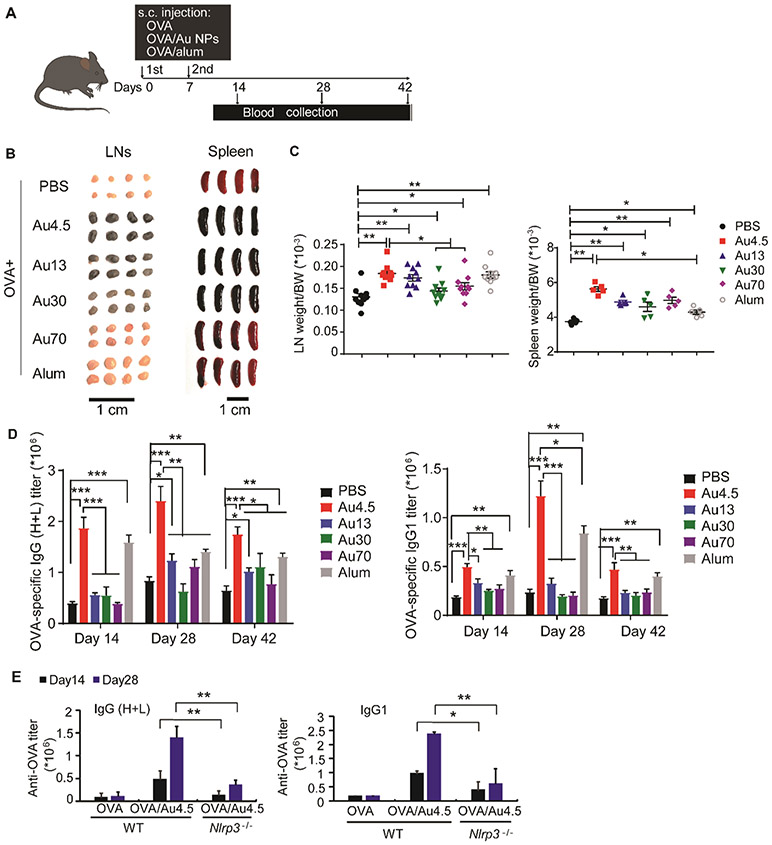
NLRP3 inflammasome activation has been implicated for cancer immunotherapy54 and antibody production.12,13 Mice deficient in Nlrp3 or Caspase-1 fail to elicit a significant antibody response to ovalbumin (OVA) complexed with alum adjuvants.54 For this reason, we next evaluated the effects of Au4.5-nanoparticle-mediated NLRP3 inflammasome activation on antibody production. After immunization of mice with OVA antigen alone or along with different sized Au nanoparticles or alum adjuvants (Figure 7A), we measured serum antibody production and isolated the secondary lymphoid organs from mice. The spleens and lymph nodes in all Au nanoparticle-treated mice were enlarged compared to the OVA-alone group, since the activation of either inflammasome or NF-κb signaling could induce inflammatory responses in mice (Figure 7B,C). Notably, significant amounts of Au4.5 and Au13 nanoparticles, and less amounts of Au30 nanoparticles, were found to target the lymph nodes, which is supported by recent studies showing that ultrasmall nanoparticles (3–25 nm in size) are favorable for lymphatic transport in comparison with larger nanoparticle counterparts, and generate stronger adaptive immunity for antibody production or antitumor T cell responses.5,40,55 The sizes and weights of lymph nodes in Au4.5-adjuvated mice were similar to those of the Au13 group but bigger than those from Au30 and Au70 groups, probably due to significant amounts of Au4.5 and Au13 nanoparticles transported to the lymph nodes for inflammatory responses. Although the sizes and weights of spleens in Au4.5-adjuvated mice were similar to larger-sized Au nanoparticle-treated mice, the serum antibody production from Au4.5 adjuvanted mice, including OVA-specific IgG1 and IgG (H+L), was much higher compared to larger-sized Au nanoparticle groups (Figure 7D). In addition, mice receiving Au4.5 and OVA vaccine produced potent OVA-specific IgG1 and IgG (H+L) at a similar level to alum-treated mice on days 14 and 42. On day 28, Au4.5 nanoparticles even induced a stronger antibody response than alum adjuvants (Figure 7D). Importantly, Au4.5-enhanced OVA-specific IgG1 and IgG (H+L) production was abolished in Nlrp3-deficient mice, suggesting the critical role of the NLRP3 inflammasome signaling in the Au4.5-adjuvanted antibody response (Figure 7E).
Figure 7.
Potent NLRP3-dependent adjuvant activity of Au4.5 nanoparticles enhances antibody production in vivo. (A) Scheme illustration of mice receiving ovalbumin (OVA) immunization and antibody titer analysis. Female wild-type or NLRP3-deficient mice (6–8 weeks old) were injected subcutaneously with PBS, OVA (50 μg) alone, OVA (50 μg) adjuvanted by Au4.5 (2 mg), or OVA (50 μg) adjuvanted by alum (2 mg) on days 0 and 7. Serum samples were collected on days 14, 28, and 42 for antibody titer analysis (n = 5). (B) Images of lymph nodes and spleens from mice immunized by OVA, OVA adjuvanted by alum, and OVA adjuvanted by Au nanoparticles (n = 5). (C) Lymph nodes and spleen weight/body weight (BW) ratio of mice immunized by OVA, OVA adjuvanted by alum, and OVA adjuvanted by Au nanoparticles (n = 5). (D) OVA-specific IgG1 and IgG (H+L) production in serum of mice immunized by PBS, OVA, OVA adjuvanted by Au nanoparticles, and OVA adjuvanted by alum (n = 5). (E) OVA-specific IgG1 and IgG (H+L) production in serum of wild-type or NLRP3-deficient mice immunized by OVA and OVA adjuvanted by Au4.5 nanoparticles (n = 5). *p < 0.05, **p < 0.01. Data are representative of three experiments (mean ± SEM).
CONCLUSIONS
In summary, our study shows that the size of a nanoparticle is critical in dictating the activation of specific innate immune signaling pathways. The Au nanoparticles of ultrasmall size, such as Au4.5 and Au5, preferentially trigger NLRP3 inflammasome activation, while the Au nanoparticles of large sizes (Au13, Au30, and Au70) trigger the NF-κB signaling pathway. Our TEM observations as well as pharmacological interference for nanoparticle internalization experiments further show that different sized Au nanoparticles had distinct intracellular locations—Au4.5 nanoparticles were mainly localized in cytoplasm, whereas larger-sized counterparts were trapped within phagosomal or endolysosomal compartments. This was consistent with a previous finding that showed the size of a nanoparticle could largely determine its internalizing pathway and intracellular location.50,52 Although ROS production serves as a stimulating signal for Au4.5-mediated inflammasome activation, our results show that cell-penetrating ultrasmall nanoparticles target the central autophagic protein LC3 for proteasomal degradation, which relieved the LC3-mediated inhibition of the NLRP3 inflammasome, to fully activate the NLRP3 inflammasome. A blockade of negative regulators of inflammasome signaling could induce a strong proinflammatory response, as desired for vaccination or immunotherapy. The NLRP3 inflammasome and the release of IL-1β have been implicated in improving the quality of the adaptive response in the protection against specific pathogens or malignancy.13,56 Our findings indicate that ultrasmall Au nanoparticles could serve as adjuvants for enhancing antigen-specific antibody production in an NLRP3-dependent manner. Compared to larger-sized Au nanoparticles and alum adjuvant, Au4.5 nanoparticles exhibit a higher potency for eliciting an OVA-specific antibody response. Our findings have not only provided insights into the molecular mechanisms of how ultrasmall-size nanoparticles activate NLRP3 inflammasome pathways but also demonstrated their immune-stimulating adjuvant activity for antibody responses. Identification of LC3 as a checkpoint inhibition for inflammasome activation may serve as a potential target for rational designs of therapeutic agents for efficient nanovaccine development against infectious diseases.
METHODS
Nanoparticle Preparation and Characterization.
PEGylated Au nanoparticles (13, 30, and 70 nm Au-PEG-NPs) were prepared by reacting citrate-coated Au nanoparticles with thiolated PEG.57,58 Briefly, to synthesize different sized citrate-coated Au nanoparticles (Au-PEG-NPs), a certain volume of 1.0 mM HAuCl4 (as listed below) (Sigma, 254169) was added into a 200 mL breaker containing 100 mL of sterile ultrapure water on a stirring hot plate and heated to 100 °C. Then, 1% trisodium citrate dihydrate (volume listed below) (Sigma, S4641) was quickly added to the rapidly stirred boiling solution. The gold sol gradually forms as the citrate reduces the gold(III). The heat was removed once the solution turned to deep red to form citrate-coated Au nanoparticles. To obtain the PEGylated gold nanoparticle, 600 μg of thiolated PEG (HS-PEG, JENKEM Technolgy Co. Ltd.) was dissolved in 1 mL of sterile ultrapure water and added to the citrate-coated gold nanoparticle suspensions (100 mL) and reacted for 3 h. After that, the PEGylated gold nanoparticle was purified by centrifugation (14 000 rpm) using sterile ultrapure water in order to remove the excessive trisodium citrate and HS-PEG.
The synthesis of 4.5 nm PEGylated gold nanoparticles was followed by protocol as previously described.59 Briefly, an aqueous solution of hydrogen tetrachloroautate (3.1 mL, 2%) (Sigma, 254169) was mixed with teraoctylammonium bromide (0.5 g) (Sigma, 294136) in toluene (14 mL). Then, 1.9 mL of sterile untrapure water was added. The two-phase mixture was vigorously stirred until all the tetrachloroaurate was transferred into the organic phase (turned to deep orange color). Aqueous sodium borohydride (60 mg) (Sigma, 71320) dissolved in 8 mL of sterile ultrapure water was rapidly added into the above mixture with continuous stirring. After the mixture was stirred for 2 h, the organic phase was extracted and washed three times with sterile ultrapure water. The organic phase was evaporated under nitrogen to obtain a crude product. The crude product was suspended in CH2Cl2 (8 mL), followed by adding HSPEG3OH (6 mg) and stirring under nitrogen gas for 1 day. After the solvent was removed, the slurry of the crude Au4.5-PEG was redissolved and purified by column chromatography using Sephadex LH-20 to remove byproducts, excess TOAB, and free ligand. The pure Au4.5-PEG nanoparticles were stored as dry powder at −20 °C until use.
The morphology and size of Au-PEG-NPs were evaluated using transmission electron microscopy (TEM, JEM-200CX, Jeol Ltd., Japan) (Figure 2A). The nanoparticle hydrodynamic size distribution and zeta potentials in H2O and biological solutions including saline (0.9% NaCl) and RPMI1640 medium were determined by a ZetaSizer Nanoseries Nano-ZS (Malvern Instruments Ltd., Malvern, UK). The endotoxin contamination was evaluated in all Au nanoparticle samples using Pierce LAL Chromogenic Endotoxin Quantitation Kit (Thermo, A39552S); the background of Au nanoparticle absorbance at 405–410 nm were subtracted using the same Au samples without adding substrate solution. The suspensions were sonicated for 5 min before use. The 5 nm sized PEG-coated (808628) and non-PEG-coated Au nanoparticles (752568) were both purchased from Sigma. Silica crystals were purchased from US Silica (MIN-U-SIL-15). Imject alum was obtained from Thermo Scientific (77161). The materials and cells all tested negative for Mycoplasma (MycoAlert ratio <0.9) using the MycoAlert Mycoplasma Detection Kit (Lonza).
Antibodies and Reagents.
Anti-GFP (FL; sc-8334), anti-mouse Caspase-1 (sc-514), and anti-IL-1β (sc-23460) were from Santa Cruz Biotechnology. Anti-human Caspase-1 (3688S), anti-phosphorylated-IKKα/β, anti-phosphorylated-IκBα, anti-IκBα, and anti-p-IRF3 (phosphorylated at Ser396) were from Cell Signaling Technology. Anti-LC3b and anti-Atg16L1 were from MBL. Anti-NLRP3 (AG-20B-0014) was from Adipogen. Horseradish peroxidase-anti-Flag (M2) and anti-β-actin (A1978) were from Sigma. LPS was from Sigma. ATP and nigericin were obtained from Invivogen. Ovalbumin and H2DCF-DA were purchased from Thermo Fisher. NAC (A7250–10G) and MitoTEMPO (SML0737–5MG) were purchased from Sigma.
Mice.
Wild-type C57BL/6 mice, NLRP3-, Aim2-, and Caspase-1-deficient mice were obtained from Jackson Laboratory. All mice were 6–12 weeks of age at use. Mice were housed under specific pathogen-free conditions. All animal experiments and protocols were approved by the Animal Care and Use Committee of the Houston Methodist Research Institute. Female wild-type or NLRP3-deficient mice (6–8 weeks old) were injected subcutaneously with PBS, ovalbumin (OVA) (50 μg) alone, OVA (50 μg) adjuvanted by Au4.5 (2 mg), or OVA (50 μg) adjuvanted by alum (2 mg) on days 0 and 7 for OVA-specific antibody generation. To prepare the alum or Au4.5 adjuvanted OVA formulation, alum or Au4.5 nanoparticles were added dropwise with constant mixing with the OVA solution at a volume ratio of adjuvant to OVA of 1:1, and then continually vortexed for 30 min and injected subcutaneously within 10 min. Serum samples were collected on days 14, 28, and 42.
Cell Isolation and Culture.
BMDCs were prepared as described.60 In general, bone marrow was flushed from mouse femurs and tibias. Bone marrow progenitor cells were cultured in complete RPMI-1640 medium containing mouse granulocyte/macrophage colony-stimulating factor (GM-CSF, 20 ng/mL), mouse interleukin-4 (IL-4, 10 ng/mL), and β-Me (55 μM). BMDCs were used at 6 to 7 days of culture. 293CIA cells were maintained in DMEM (Mediatech) containing 10% heat-inactivated FCS. For inflammasome activation studies, BMDCs were primed for 3 h with LPS (100 ng/mL), followed by treatment with ATP (5 mM, 1 h) or nigericin (Sigma-Aldrich, 1 μM, 4–6 h). Au nanoparticles were used at concentrations of 50, 100, and 200 μg/mL for 6–8 h. Alum and silica were used at 400 μg/mL for 6 h, respectively. 293CIA cells were transfected with HA-NLRP3, Flag-NLRP3, Flag-Aim2, or Flag-NLRC4 plasmid, respectively, and then, cells were treated with different ligands and particulates at 24 h post-transfection. Nigericin was applied at a concentration of 1 μM for 4–6 h.
ELISA.
Mouse IFN-α and IFN-β ELISA kits are obtained from PBL Biomedical Laboratories. Capture and detection antibodies for mouse TNF-α, IL-6, IL-1β (eBioscience), and human IL-1β (R&D) were applied according to the manufacturer’s protocols for detection of cytokines in cell supernatants. Serum levels of OVA-specific antibody production were measured using HRP-conjugated anti-mouse (C57BL/6) IgG (H+L) and IgG1 antibodies from Southern Biotech (5300–05B).
In Vitro LC3-Au4.5 Binding.
To check whether LC3 could directly bind to Au4.5 nanoparticles, 500 μg of Au4.5 nanoparticles were gently mixed with 50 μg of recombinant human LC3 protein (Abcam, ab103506) in PBS (supplement with 1 mM EDTA) and incubated at 4 °C for 18 h with gentle rotation. The Au4.5 nanoparticles were washed using PBS (supplement with 1 mM EDTA) and centrifuged at 4000 rpm for 30 min in Amicon Ultra centrifugal filter units (Ultra-15, MWCO 30 kDa) for a total of five times to remove the nonbinding or excess LC3 protein (18 kDa). The potential Au4.5-bound LC3 protein was disassociated by adding 2× SDS loading buffer (10% w/v SDS, 20% w/v glycerol, 10% v/v 2-mercaptoethanol, 125 mM Tris-HCl, pH 6.8, bromophenol blue) and separated by 15% SDS-PAGE.
Immunoblot Analysis and Immunoprecipitation.
Cell lysates were prepared in low-salt lysis buffer, separated by SDS-PAGE, and transferred to PVDF membrane (Bio-Rad) for immunoblot. For immunoprecipitation, whole cell lysates were obtained at 24 h posttransfection or after ligand stimulation using low-salt lysis buffer by shaking on ice for 30 min. The 293CIA or BMDC cell lysates (2–3 million cells for each sample) were immunoprecipitated with 2 μg of anti-LC3, 2 μg of anti-NLRP3, or 3 μg of anti-GFP antibody per sample and protein A/G-agarose beads (40 μL/sample) at 4 °C overnight. The beads were washed five times with low-salt lysis buffer, and immunoprecipitates were eluted with SDS loading buffer and subjected to SDS-PAGE gel electrophoresis, followed by immunoblotting. LumiGlo chemiluminescent substrate (KPL) was used for protein detection.
RNA-Mediated Interference.
Stealth small interfering RNA sets for mouse Map1lc3b were obtained from Life Technologies. Nucleofector transfection (Amaxa) was applied for the LC3 knockdown in BMDCs using the suggested protocol.
Transmission Electron Microscopy (TEM).
BMDCs were fixed in 2.5% (v/v) glutaraldehyde after stimulation by LPS and Au nanoparticles (200 μg/mL) for 6 h as described elsewhere in this paper. The ultrathin sections (70–100 nm) of cell pellets were stained with lead citrate and uranyl acetate. The samples were viewed under a JEOL JEM-1400 TEM.
CRISPR Technology for Gene Knockout in Cells.
We designed human LC3B, ATG16L, and BECN1 sgRNAs using an online CRISPR design tool (crispr.mit.edu) by inputting a targeted exon sequence. Designed sgRNAs were cloned into the BsmB1 site of pLenti-Crispr-Cas9 v2 vectors (Addgene) and verified by sequencing analysis. The puromycin selection marker of pLenti-Crispr-Cas9 v2 vector was replaced by a zeocin selection marker for gene knockout in 293CIA cells, since 293CIA cells are puromycin resistant.46 The sgRNA-containing plasmids were transfected into HEK293T cells with psPAX2 (12260, Addgene) and pMD2.G (12259, Addgene) plasmids. After 2 days, the virus-containing medium was subjected to ultracentrifugation (20 000g at 4 °C for 2 h) to concentrate the viruses. 293CIA cells were then transduced with LC3B, ATG16L, or BECN1-targeting sgRNA-containing lentiCRISPR viruses, followed by zeocin selection at 400 μg/mL for 6 days. The knockout efficiency was confirmed using Surveyor assay (purchased from IDT) and immunoblot analysis. The sgRNAs for human LC3B, ATG16L, and BECN1 gene knockout in 293CIA cells and the primers for Surveyor assay are listed as below.
Immunofluorescence.
For confocal microscope imaging, 293CIA cells were transfected with GFP-LC3 and Flag-NLRP3. After 24 h of transfection, cells were left untreated or treated with Au4.5 nanoparticles for 6 h. Cells were washed twice with PBS, fixed overnight at −20 °C with cold methanol, and washed twice with PBS. Nonspecific receptors on cells were blocked for 1 h with 5% bovine serum albumin (BSA, Sigma-Aldrich). Triton (0.1%) was applied to permealize the cells, followed by labeling with NLRP3 antibody and secondary RPE-conjugated anti-mouse IgG antibody (Life technology). Cells were labeled and mounted using proLong Gold Antifade Mountant with DAPI (P36941, Life Technology).
Molecular Cloning of Human NLRP3, LC3, and Related Mutated Genes.
A complete open reading frame of human NLRP3 and LC3 were obtained from the entry clone library (Thermo Fisher) and subsequently subcloned into pcDNA3.1 or pEGFP-C2 vectors. The mutations of human LC3 were generated by PCR using the following primers and subsequently subcloned into the pEGFP-C2 vector. Flag-NLRP3 was generated using PCR-based Gateway Technology (Life Technologies). The LC3 point mutation was created by PCR-based site-directed mutagenesis using primers listed below.
Inductively Coupled Plasma-Mass Spectrometry (ICP-MS).
BMDCs were incubated with Au nanoparticles for 6 h at 4 or 37 °C. Chlorpromazine (20 μg/mL) or cytochalasin D (40 μM) was applied 2 h prior to Au nanoparticle treatment and maintained in the culture during Au treatment. Cells were washed with PBS five times. Then, the cell samples were digested using aqua fortis (nitric acid/hydrochloric acid 3:1). After the solution volume was adjusted to 2 mL using 2% nitric acid and 1% hydrochloride acid (1:1), gold level measurements were performed using ICP-MS instrument (NexION 300X, PerkinElmer).
Real-Time PCR Analysis.
Total RNA was isolated from BMDCs primed with LPS (100 ng/mL, 3 h), followed by Au nanoparticle stimulation at 200 μg/mL for 6 h. First-strand cDNA was generated from total RNA using oligo-dT primers and reverse transcriptase II (Invitrogen). Real-time PCR was conducted with specific primers and SYBR GreenERq PCR Super Mix Universal (Invitrogen) using an ABI Prism 7000 analyzer (Applied Biosystems). The expression levels of the target gene expression were normalized to mouse GAPDH. The following primers were used for LC3 real-time PCR: forward primer (GATGTCCGACTTATTCGAGAGC) and reverse primer (TTGAGCTGTAAGCGCCTTCTA).
Statistical Analysis.
Statistical analysis was performed using GraphPad Prism v6.0 and Excel. A one-way ANOVA or two-tailed Student’s t-test was applied for comparisons. p-Values of less than 0.05 were considered statistically significant
Supplementary Material
Table 1.
HAuCl4 and Trisodium Citrate Dihydrate Volume Usage for the Synthesis of Citrate-Coated Au Nanoparticles
| size (nm) | HAuCl4 (mL) | 1% trisodium citrate dihydrate (mL) |
|---|---|---|
| 13 | 1.7 | 10 |
| 30 | 0.25 | 1.5 |
| 70 | 0.5 | 0.6 |
Table 2.
Sequences for sgRNAs Targeting Human LC3B, ATG16L, and BECN1 Genes and the Primers for the Surveyor Assay
| LC3B sgRNA | TTCAAGCAGCGCCGCACCTT |
| ATG16L1 sgRNA | CAATTTAGTCCCGGACATGA |
| BECN1 sgRNA | ATTTATTGAAACTCCTCGCC |
| LC3B KO Surveyor primer forward | CGCCAGAGTCGGATTCGCCG |
| LC3B KO Surveyor primer reverse | CGGGGTGATTCAGCAGGCCC |
| ATG16L KO Surveyor primer forward | GGCTTTGTGAACATGTTTCTG |
| ATG16L KO Surveyor primer reverse | GGGCCTCAATCTGCCTGGTC |
| BECN1 KO Surveyor primer forward | CCTGACTGCTAAGTGTTGAG |
| BECN1 KO Surveyor primer reverse | CATCTCTATCACCTGGCTC |
Table 3.
Primer Sequences for Generating Plasmids Encoding Point-Mutated LC3
| LC3 K5R f | ATGCCGTCGGAGAGGACCTTCAAGCAGCGC |
| LC3 K5R r | GCGCTGCTTGAAGGTCCTCTCCGACGGCAT |
| LC3 K8R f | TCGGAGAAGACCTTCAGGCAGCGCCGCACCTTC |
| LC3 K8R r | GAAGGTGCGGCGCTGCCTGAAGGTCTTCTCCGA |
| LC3 K5R,K8R f | ATGCCGTCGGAGAGGACCTTCAGGCAGCGC |
| LC3 K5R,K8R r | GCGCTGCCTGAAGGTCCTCTCCGACGGCAT |
ACKNOWLEDGMENTS
This work was supported by grants from the NCI and NIH (R01CA101795 and U54CA210181), Department of Defense (DoD) CDMRP BCRP (BC151081), and the startup funding from the Houston Methodist Research Institute and University of Southern California to R.W.
Footnotes
Supporting Information
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsnano.0c00962.
Additional data for the characterization of Au nanoparticles, different innate immune signaling pathways activated by Au nanoparticles, LC3 degradation by ultrasmall Au nanoparticles, macroautophagy in Au-nanoparticle-treated cells, polyubiquitination of LC3 induced by Au4.5 nanoparticles, and TEM analysis for Au4.5 nanoparticle intracellular locations in BMDCs (PDF)
Original Western blots for all main figures (PDF)
The authors declare no competing financial interest.
Contributor Information
Motao Zhu, Department of Medicine, and Norris Comprehensive Cancer Center, Keck School of Medicine, University of Southern California, Los Angeles, California 90033, United States; CAS Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety, CAS Center for Excellence in Nanoscience, National Center for Nanoscience and Technology of China, Beijing 100190, China; Center for Inflammation and Epigenetics, Houston Methodist Research Institute, Houston, Texas 77030, United States.
Libo Du, State Key Laboratory for Structural Chemistry of Unstable Species, Center for Molecular Sciences, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China.
Ruifang Zhao, CAS Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety, CAS Center for Excellence in Nanoscience, National Center for Nanoscience and Technology of China, Beijing 100190, China; Center for Inflammation and Epigenetics, Houston Methodist Research Institute, Houston, Texas 77030, United States.
Helen Y. Wang, Department of Medicine, and Norris Comprehensive Cancer Center, Keck School of Medicine, University of Southern California, Los Angeles, California 90033, United States; Center for Inflammation and Epigenetics, Houston Methodist Research Institute, Houston, Texas 77030, United States
Yuliang Zhao, CAS Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety, CAS Center for Excellence in Nanoscience, National Center for Nanoscience and Technology of China, Beijing 100190, China.
Guangjun Nie, CAS Key Laboratory for Biomedical Effects of Nanomaterials and Nanosafety, CAS Center for Excellence in Nanoscience, National Center for Nanoscience and Technology of China, Beijing 100190, China.
Rong-Fu Wang, Department of Medicine, and Norris Comprehensive Cancer Center, Keck School of Medicine and Department of Pediatrics, Children’s Hospital Los Angeles, Keck School of Medicine, University of Southern California, Los Angeles, California 90033, United States; Center for Inflammation and Epigenetics, Houston Methodist Research Institute, Houston, Texas 77030, United States.
REFERENCES
- (1).Iwasaki A; Medzhitov R Control of Adaptive Immunity by the Innate Immune System. Nat. Immunol 2015, 16, 343–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Coffman RL; Sher A; Seder RA Vaccine Adjuvants: Putting Innate Immunity to Work. Immunity 2010, 33, 492–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Hubbell JA; Thomas SN; Swartz MA Materials Engineering for Immunomodulation. Nature 2009, 462, 449–460. [DOI] [PubMed] [Google Scholar]
- (4).Valsami-Jones E; Lynch I NANOSAFETY. How Safe Are Nanomaterials? Science 2015, 350, 388–389. [DOI] [PubMed] [Google Scholar]
- (5).Hirai T; Yoshioka Y; Izumi N; Ichihashi K; Handa T; Nishijima N; Uemura E; Sagami K; Takahashi H; Yamaguchi M; Nagano K; Mukai Y; Kamada H; Tsunoda S; Ishii KJ; Higashisaka K; Tsutsumi Y Metal Nanoparticles in the Presence of Lipopolysaccharides Trigger the Onset of Metal Allergy in Mice. Nat. Nanotechnol 2016, 11, 808–816. [DOI] [PubMed] [Google Scholar]
- (6).Ma JY; Young SH; Mercer RR; Barger M; Schwegler-Berry D; Ma JK; Castranova V Interactive Effects of Cerium Oxide and Diesel Exhaust Nanoparticles on Inducing Pulmonary Fibrosis. Toxicol. Appl. Pharmacol 2014, 278, 135–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Goldberg MS Immunoengineering: How Nanotechnology Can Enhance Cancer Immunotherapy. Cell 2015, 161, 201–204. [DOI] [PubMed] [Google Scholar]
- (8).Kim J; Li WA; Choi Y; Lewin SA; Verbeke CS; Dranoff G; Mooney DJ Injectable, Spontaneously Assembling, Inorganic Scaffolds Modulate Immune Cells In Vivo and Increase Vaccine Efficacy. Nat. Biotechnol 2015, 33, 64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Guo H; Callaway JB; Ting JP Inflammasomes: Mechanism of Action, Role in Disease, and Therapeutics. Nat. Med 2015, 21, 677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Dostert C; Petrilli V; Van Bruggen R; Steele C; Mossman BT; Tschopp J Innate Immune Activation through Nalp3 Inflammasome Sensing of Asbestos and Silica. Science 2008, 320, 674–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Hornung V; Bauernfeind F; Halle A; Samstad EO; Kono H; Rock KL; Fitzgerald KA; Latz E Silica Crystals and Aluminum Salts Activate the NALP3 Inflammasome through Phagosomal Destabilization. Nat. Immunol 2008, 9, 847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Flach TL; Ng G; Hari A; Desrosiers MD; Zhang P; Ward SM; Seamone ME; Vilaysane A; Mucsi AD; Fong Y; Prenner E; Ling CC; Tschopp J; Muruve DA; Amrein MW; Shi Y Alum Interaction with Dendritic Cell Membrane Lipids Is Essential for Its Adjuvanticity. Nat. Med 2011, 17, 479–487. [DOI] [PubMed] [Google Scholar]
- (13).Eisenbarth SC; Colegio OR; O’Connor W; Sutterwala FS; Flavell RA Crucial Role for the Nalp3 Inflammasome in the Immunostimulatory Properties of Aluminium Adjuvants. Nature 2008, 453, 1122–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Assis NRG; Caires AJ; Figueiredo BC; Morais SB; Mambelli FS; Marinho FV; Ladeira LO; Oliveira SC The Use of Gold Nanorods as a New Vaccine Platform against Schistosomiasis. J. Controlled Release 2018, 275, 40–52. [DOI] [PubMed] [Google Scholar]
- (15).Wang YT; Lu XM; Zhu F; Huang P; Yu Y; Zeng L; Long ZY; Wu YM The Use of a Gold Nanoparticle-Based Adjuvant to Improve the Therapeutic Efficacy of hNgR-Fc Protein Immunization in Spinal Cord-Injured Rats. Biomaterials 2011, 32, 7988–7998. [DOI] [PubMed] [Google Scholar]
- (16).Sanchez-Guzman D; Le Guen P; Villeret B; Sola N; Le Borgne R; Guyard A; Kemmel A; Crestani B; Sallenave JM; Garcia-Verdugo I Silver Nanoparticle-Adjuvanted Vaccine Protects against Lethal Influenza Infection through Inducing BALT and IgA-Mediated Mucosal Immunity. Biomaterials 2019, 217, 119308. [DOI] [PubMed] [Google Scholar]
- (17).Franchi L; Nunez G The Nlrp3 Inflammasome Is Critical for Aluminium Hydroxide-Mediated IL-1beta Secretion But Dispensable for Adjuvant Activity. Eur. J. Immunol 2008, 38, 2085–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Oyewumi MO; Kumar A; Cui Z Nano-Microparticles as Immune Adjuvants: Correlating Particle Sizes and the Resultant Immune Responses. Expert Rev. Vaccines 2010, 9, 1095–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Maquieira A; Brun EM; Garces-Garcia M; Puchades R Aluminum Oxide Nanoparticles as Carriers and Adjuvants for Eliciting Antibodies from Non-Immunogenic Haptens. Anal. Chem 2012, 84, 9340–9348. [DOI] [PubMed] [Google Scholar]
- (20).Li X; Aldayel AM; Cui Z Aluminum Hydroxide Nanoparticles Show a Stronger Vaccine Adjuvant Activity than Traditional Aluminum Hydroxide Microparticles. J. Controlled Release 2014, 173, 148–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Hem SL; Hogenesch H Relationship between Physical and Chemical Properties of Aluminum-Containing Adjuvants and Immunopotentiation. Expert Rev. Vaccines 2007, 6, 685–798. [DOI] [PubMed] [Google Scholar]
- (22).Fifis T; Gamvrellis A; Crimeen-Irwin B; Pietersz GA; Li J; Mottram PL; McKenzie IF; Plebanski M Size-Dependent Immunogenicity: Therapeutic and Protective Properties of Nano-Vaccines against Tumors. J. Immunol 2004, 173, 3148–3154. [DOI] [PubMed] [Google Scholar]
- (23).Lunov O; Syrovets T; Loos C; Nienhaus GU; Mailander V; Landfester K; Rouis M; Simmet T Amino-Functionalized Polystyrene Nanoparticles Activate the NLRP3 Inflammasome in Human Macrophages. ACS Nano 2011, 5, 9648–9657. [DOI] [PubMed] [Google Scholar]
- (24).Yazdi AS; Guarda G; Riteau N; Drexler SK; Tardivel A; Couillin I; Tschopp J Nanoparticles Activate the NLR Pyrin Domain Containing 3 (Nlrp3) Inflammasome and Cause Pulmonary Inflammation through Release of IL-1alpha and IL-1beta. Proc. Natl. Acad. Sci. U. S. A 2010, 107, 19449–19454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Yang EJ; Kim S; Kim JS; Choi IH Inflammasome Formation and IL-1beta Release by Human Blood Monocytes in Response to Silver Nanoparticles. Biomaterials 2012, 33, 6858–6867. [DOI] [PubMed] [Google Scholar]
- (26).Sharp FA; Ruane D; Claass B; Creagh E; Harris J; Malyala P; Singh M; O’Hagan DT; Petrilli V; Tschopp J; O’Neill LA; Lavelle EC Uptake of Particulate Vaccine Adjuvants by Dendritic Cells Activates the NALP3 Inflammasome. Proc. Natl. Acad. Sci. U. S. A 2009, 106, 870–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Niikura K; Matsunaga T; Suzuki T; Kobayashi S; Yamaguchi H; Orba Y; Kawaguchi A; Hasegawa H; Kajino K; Ninomiya T; Ijiro K; Sawa H Gold Nanoparticles as a Vaccine Platform: Influence of Size and Shape on Immunological Responses In Vitro and In Vivo. ACS Nano 2013, 7, 3926–3938. [DOI] [PubMed] [Google Scholar]
- (28).Mishra AR; Zheng J; Tang X; Goering PL Silver Nanoparticle-Induced Autophagic-Lysosomal Disruption and NLRP3-Inflammasome Activation in HepG2 Cells Is Size-Dependent. Toxicol. Sci 2016, 150, 473–487. [DOI] [PubMed] [Google Scholar]
- (29).Mao BH; Tsai JC; Chen CW; Yan SJ; Wang YJ Mechanisms of Silver Nanoparticle-Induced Toxicity and Important Role of Autophagy. Nanotoxicology 2016, 10, 1021–1040. [DOI] [PubMed] [Google Scholar]
- (30).Jessop F; Hamilton RF; Rhoderick JF; Shaw PK; Holian A Autophagy Deficiency in Macrophages Enhances NLRP3 Inflammasome Activity and Chronic Lung Disease Following Silica Exposure. Toxicol. Appl. Pharmacol 2016, 309, 101–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Jessop F; Hamilton RF Jr.; Rhoderick JF; Fletcher P; Holian A Phagolysosome Acidification is Required for Silica and Engineered Nanoparticle-Induced Lysosome Membrane Permeabilization and Resultant NLRP3 Inflammasome Activity. Toxicol. Appl. Pharmacol 2017, 318, 58–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Nel A; Xia T; Madler L; Li N Toxic Potential of Materials at the Nanolevel. Science 2006, 311, 622–627. [DOI] [PubMed] [Google Scholar]
- (33).Tschopp J; Schroder K NLRP3 Inflammasome Activation: The Convergence of Multiple Signalling Pathways on ROS Production? Nat. Rev. Immunol 2010, 10, 210–215. [DOI] [PubMed] [Google Scholar]
- (34).Palomaki J; Valimaki E; Sund J; Vippola M; Clausen PA; Jensen KA; Savolainen K; Matikainen S; Alenius H. Long, Needle-Like Carbon Nanotubes and Asbestos Activate the NLRP3 Inflammasome through a Similar Mechanism. ACS Nano 2011, 5, 6861–6870. [DOI] [PubMed] [Google Scholar]
- (35).Shi CS; Shenderov K; Huang NN; Kabat J; Abu-Asab M; Fitzgerald KA; Sher A; Kehrl JH Activation of Autophagy by Inflammatory Signals Limits IL-1beta Production by Targeting Ubiquitinated Inflammasomes for Destruction. Nat. Immunol 2012, 13, 255–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Saitoh T; Fujita N; Jang MH; Uematsu S; Yang BG; Satoh T; Omori H; Noda T; Yamamoto N; Komatsu M; Tanaka K; Kawai T; Tsujimura T; Takeuchi O; Yoshimori T; Akira S Loss of the Autophagy Protein Atg16L1 Enhances Endotoxin-Induced IL-1beta Production. Nature 2008, 456, 264–268. [DOI] [PubMed] [Google Scholar]
- (37).Nakahira K; Haspel JA; Rathinam VA; Lee SJ; Dolinay T; Lam HC; Englert JA; Rabinovitch M; Cernadas M; Kim HP; Fitzgerald KA; Ryter SW; Choi AM Autophagy Proteins Regulate Innate Immune Responses by Inhibiting the Release of Mitochondrial DNA Mediated by the NALP3 Inflammasome. Nat. Immunol 2011, 12, 222–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Khan MI; Mohammad A; Patil G; Naqvi SA; Chauhan LK; Ahmad I Induction of ROS, Mitochondrial Damage and Autophagy in Lung Epithelial Cancer Cells by Iron Oxide Nanoparticles. Biomaterials 2012, 33, 1477–1488. [DOI] [PubMed] [Google Scholar]
- (39).Huang D; Zhou H; Gao J Nanoparticles Modulate Autophagic Effect in a Dispersity-Dependent Manner. Sci. Rep 2015, 5, 14361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Li H; Li Y; Jiao J; Hu HM Alpha-Alumina Nanoparticles Induce Efficient Autophagy-Dependent Cross-Presentation and Potent Antitumour Response. Nat. Nanotechnol 2011, 6, 645–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Peynshaert K; Manshian BB; Joris F; Braeckmans K; De Smedt SC; Demeester J; Soenen SJ Exploiting Intrinsic Nanoparticle Toxicity: The Pros and Cons of Nanoparticle-Induced Autophagy in Biomedical Research. Chem. Rev 2014, 114, 7581–7609. [DOI] [PubMed] [Google Scholar]
- (42).Zhang L; Wang X; Miao Y; Chen Z; Qiang P; Cui L; Jing H; Guo Y Magnetic Ferroferric Oxide Nanoparticles Induce Vascular Endothelial Cell Dysfunction and Inflammation by Disturbing Autophagy. J. Hazard. Mater 2016, 304, 186–195. [DOI] [PubMed] [Google Scholar]
- (43).Li R; Ji Z; Qin H; Kang X; Sun B; Wang M; Chang CH; Wang X; Zhang H; Zou H; Nel AE; Xia T Interference in Autophagosome Fusion by Rare Earth Nanoparticles Disrupts Autophagic Flux and Regulation of an Interleukin-1beta Producing Inflammasome. ACS Nano 2014, 8, 10280–10292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Zhang P; Chiu YC; Tostanoski LH; Jewell CM Polyelectrolyte Multilayers Assembled Entirely from Immune Signals on Gold Nanoparticle Templates Promote Antigen-Specific T Cell Response. ACS Nano 2015, 9, 6465–6477. [DOI] [PubMed] [Google Scholar]
- (45).Ma X; Wu Y; Jin S; Tian Y; Zhang X; Zhao Y; Yu L; Liang XJ Gold Nanoparticles Induce Autophagosome Accumulation through Size-Dependent Nanoparticle Uptake and Lysosome Impairment. ACS Nano 2011, 5, 8629–8639. [DOI] [PubMed] [Google Scholar]
- (46).Yu JW; Wu J; Zhang Z; Datta P; Ibrahimi I; Taniguchi S; Sagara J; Fernandes-Alnemri T; Alnemri ES Cryopyrin and Pyrin Activate Caspase-1, but Not NF-κB, via ASC Oligomerization. Cell Death Differ. 2006, 13, 236–249. [DOI] [PubMed] [Google Scholar]
- (47).Cadwell K Crosstalk between Autophagy and Inflammatory Signalling Pathways: Balancing Defence and Homeostasis. Nat. Rev. Immunol 2016, 16, 661–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (48).Nath S; Dancourt J; Shteyn V; Puente G; Fong WM; Nag S; Bewersdorf J; Yamamoto A; Antonny B; Melia TJ Lipidation of the LC3/GABARAP Family of Autophagy Proteins Relies on a Membrane-Curvature-Sensing Domain in Atg3. Nat. Cell Biol 2014, 16, 415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Ding F; Radic S; Chen R; Chen P; Geitner NK; Brown JM; Ke PC Direct Observation of a Single Nanoparticle-Ubiquitin Corona Formation. Nanoscale 2013, 5, 9162–9169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Zhu M; Nie G; Meng H; Xia T; Nel A; Zhao Y Physicochemical Properties Determine Nanomaterial Cellular Uptake, Transport, and Fate. Acc. Chem. Res 2013, 46, 622–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Verma A; Uzun O; Hu Y; Hu Y; Han H-S; Watson N; Chen S; Irvine DJ; Stellacci F Surface-Structure-Regulated Cell-Membrane Penetration by Monolayer-Protected Nanoparticles. Nat. Mater 2008, 7, 588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Guo Y; Terazzi E; Seemann R; Fleury JB; Baulin VA Direct Proof of Spontaneous Translocation of Lipid-Covered Hydrophobic Nanoparticles through a Phospholipid Bilayer. Sci. Adv 2016, 2, No. e1600261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Zhou R; Yazdi AS; Menu P; Tschopp J A Role for Mitochondria in NLRP3 Inflammasome Activation. Nature 2011, 469, 221–225. [DOI] [PubMed] [Google Scholar]
- (54).Ghiringhelli F; Apetoh L; Tesniere A; Aymeric L; Ma Y; Ortiz C; Vermaelen K; Panaretakis T; Mignot G; Ullrich E; Perfettini JL; Schlemmer F; Tasdemir E; Uhl M; Genin P; Civas A; Ryffel B; Kanellopoulos J; Tschopp J; Andre F; et al. et al. Activation of the NLRP3 Inflammasome in Dendritic Cells Induces IL-1beta-Dependent Adaptive Immunity against Tumors. Nat. Med 2009, 15, 1170–1178. [DOI] [PubMed] [Google Scholar]
- (55).Reddy ST; van der Vlies AJ; Simeoni E; Angeli V; Randolph GJ; O’Neil CP; Lee LK; Swartz MA; Hubbell JA Exploiting Lymphatic Transport and Complement Activation in Nanoparticle Vaccines. Nat. Biotechnol 2007, 25, 1159–1164. [DOI] [PubMed] [Google Scholar]
- (56).Kool M; Petrilli V; De Smedt T; Rolaz A; Hammad H; van Nimwegen M; Bergen IM; Castillo R; Lambrecht BN; Tschopp J Cutting Edge: Alum Adjuvant Stimulates Inflammatory Dendritic Cells through Activation of the NALP3 Inflammasome. J. Immunol 2008, 181, 3755–3759. [DOI] [PubMed] [Google Scholar]
- (57).Cho WS; Cho M; Jeong J; Choi M; Cho HY; Han BS; Kim SH; Kim HO; Lim YT; Chung BH; et al. Acute Toxicity and Pharmacokinetics of 13 nm-Sized PEG-Coated Gold Nanoparticles. Toxicol. Appl. Pharmacol 2009, 236, 16–24. [DOI] [PubMed] [Google Scholar]
- (58).Frens G Controlled Nucleation for the Regulation of the Particle Size in Monodisperse Gold Suspensions. Nature, Phys. Sci 1973, 241, 20–22. [Google Scholar]
- (59).Du L; Suo S; Wang G; Jia H; Liu KJ; Zhao B; Liu Y Mechanism and Cellular Kinetic Studies of the Enhancement of Antioxidant Activity by Using Surface-Functionalized Gold Nanoparticles. Chem. - Eur. J 2013, 19, 1281–1287. [DOI] [PubMed] [Google Scholar]
- (60).Inaba K; Inaba M; Romani N; Aya H; Deguchi M; Ikehara S; Muramatsu S; Steinman RM Generation of Large Numbers of Dendritic Cells from Mouse Bone Marrow Cultures Supplemented with Granulocyte/Macrophage Colony-Stimulating Factor. J. Exp. Med 1992, 176, 1693–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.