Abstract
Background and Purpose
The role of aspirin plus clopidogrel (A+C) therapy compared with aspirin monotherapy in patients presenting with acute ischemic stroke (IS) or transient ischemic attack remains uncertain. We conducted this study to determine the optimal period of efficacy and safety of A+C compared with aspirin monotherapy.
Methods
Ten randomized controlled trials (15434 patients) were selected using MEDLINE, EMBASE, and the Cochrane Central Register of Controlled Trials (CENTRAL) (inception June 2018) comparing A+C with aspirin monotherapy in patients with transient ischemic attack or IS. The primary efficacy outcome was recurrent IS, and the primary safety outcome was major bleeding. The secondary outcomes were major adverse cardiovascular events (composite of stroke, myocardial infarction, and cardiovascular mortality) and all-cause mortality. We stratified analysis based on the short- (≤1 month), intermediate- (≤3 month), and long-term (>3 month) A+C therapy. Effects were estimated as relative risk (RR) with 95% CI.
Results
A+C significantly reduced the risk of recurrent IS at short-term (RR, 0.53; 95% CI, 0.37–0.78) and intermediate-term (RR, 0.72; 95% CI, 0.58–0.90) durations. Similarly, major adverse cardiovascular event was significantly reduced by short-term (RR, 0.68; 95% CI, 0.60–0.78) and intermediate-term (RR, 0.76; 95% CI, 0.61–0.94) A+C therapy. However, long-term A+C did not yield beneficial effect in terms of recurrent IS (RR, 0.81; 95% CI, 0.63–1.04) and major adverse cardiovascular events (RR, 0.87; 95% CI, 0.71–1.07). Intermediate-term (RR, 2.58; 95% CI, 1.19–5.60) and long-term (RR, 1.87; 95% CI, 1.36–2.56) A+C regimens significantly increased the risk of major bleeding as opposed to short-term A+C (RR, 1.82; 95% CI, 0.91–3.62). Excessive all-cause mortality was limited to long-term A+C (RR, 1.45; 95% CI, 1.10–1.93).
Conclusions
Short-term A+C is more effective and equally safe in comparison to aspirin alone in patients with acute IS or transient ischemic attack.
Keywords: aspirin, clopidogrel, stroke, transient ischemic attack
The role of antiplatelets for the secondary prevention of ischemic stroke (IS) or transient ischemic attack (TIA) is well established1-3; still the antiplatelet regimen with optimal efficacy and safety is to be determined. Aspirin has been known for years to reduce recurrent strokes after the initial episode.1-3 Dual antiplatelet therapy with aspirin and clopidogrel (A+C) is recommended in acute coronary syndromes,4 but the data in acute IS or TIA has been inconsistent.5-7 European guidelines do not recommend A+C combination in patients with recent occurrence of stroke.8 However, current American Heart Association/American Stroke Association5 guidelines recommend 21-day treatment with A+C to be started within 24 hours of symptom onset with minor stroke (Class IIa; Level of Evidence B) based on the CHANCE trial (Clopidogrel in High-Risk Patients With Acute Nondisabling Cerebrovascular Events) in Chinese population which demonstrated 33% reduction of recurrent IS with no increase in major bleeding.9 Recently, the multinational POINT trial (Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke) revealed 28% reduction in subsequent IS with A+C in comparison to aspirin monotherapy; however, major bleeding events were doubled.10 However, certain trials11-13 failed to demonstrate any reduction in recurrent IS, accompanied by increased risk of major bleeding. Furthermore, prior meta-analyses have shown inconsistent results and were not able to propose a definite strategy regarding the optimal duration of A+C treatment.6,7,14-17 Because of the lack of consensus on the optimal A+C duration, we performed a systematic review and metaanalysis to assess the efficacy and safety of subgroups based on the duration of dual antiplatelet therapy (DAPT).
Methods
We performed and reported the meta-analysis as stated by the Cochrane collaboration guidelines,18 AHA Journal’s Transparency and Openness Promotion guidelines,19 and Preferred Reporting Items for Systematic Reviews and Meta-Analyses report.20 Authors declare that all supporting data are available within the article and in the online-only Data Supplement.
Search Strategy
Two authors (Drs Rahman and Khan) conducted the search by using online databases MEDLINE (PubMed), EMBASE, and the Cochrane Central Register of Controlled Trials (CENTRAL) from inception of databases to June 31, 2018. We used the following words and MeSH terms in combination: ischemic stroke, cerebral infarction, cerebrovascular disease, transient ischemic attacks, aspirin, clopidogrel, aspirin and clopidogrel combination, antiplatelets, randomized controlled trials. The search was limited to studies only on human subjects published as full text articles. The search strategy is reported in the online-only Data Supplement. To supplement the electronic database search, bibliographies of the relevant articles were reviewed. All citations were uploaded into EndNote X7 (Thompson ISI Research Soft, Philadelphia, PA). Duplicates were removed manually and by using EndNote X7.
Selection Criteria
Studies were evaluated at title and abstract level followed by full text scrutiny by 2 authors (Drs Nasir and Hammad) based on the following inclusion standard. The whole process was supervised by a third person (Dr Rahman), and disagreements were resolved by consensus. Studies that met the following criteria were included: (1) randomized controlled trials; (2) comparing A+C with aspirin alone in patients with noncardioembolic IS or TIA; (3) not suitable for thrombolysis; (4) adults ≥18 years of age; and (5) reported at least recurrent IS and major bleeding as end points. The above criterion was not restricted to language, sample size, comorbidities and follow-up duration.
Data Extraction and Quality Assessment
Data extraction was performed (Drs Khan and Nasir) using 3 different collection forms comprising baseline characteristics of participants (sample size and comorbidities), study characteristics (study design, demographics, intervention doses, and follow-up duration), and outcomes (events, sample size, event rate, and crude point estimates). Quality assessment of randomized controlled trials was provided based on the Cochrane bias risk assessment21 (Table I in the online-only Data Supplement).
Outcome Measures
Since after TIA or IS, the early 3-month risk of recurrent stroke ranges from 5% to 20% and is highest within the first 1 month,22-25 we aimed to assess the efficacy and safety of interventions based on the duration of DAPT. Therefore, analyses were stratified according to short- (≤1 month), intermediate- (≤3 month), and long-term (>3 month) durations. We determined the primary efficacy outcome as recurrent IS. It was defined as rapid onset of a new or worsening of existing focal neurologic deficit, with clinical or imaging evidence of infarction that was not attributable to a nonischemic etiology. The primary safety end point was major bleeding. There was slight variation in the definition of major bleeding among different trials as mentioned in Table II in the online-only Data Supplement. The secondary outcomes were all-cause mortality and major adverse cardiovascular events (MACE), which was defined as composite of recurrent stroke, cardiovascular mortality, and myocardial infarction.
Statistical Analysis
The current meta-analysis was conducted using generic invariance weighted random effects model.26 Random effects were used to account for heterogeneity in patient population of the included studies. Estimates were reported as risk ratio (RR) with 95% CI, supplemented by risk difference. Relative risk was used to report outcomes in the forest plots, and risk difference estimates are reported in Table III in the online-only Data Supplement. A P of ≤0.05 was considered significant. Heterogeneity was evaluated using the Q statistics and quantified with the I2 index with values ≥50% consistent with a high degree of heterogeneity.27
We conducted stratified analysis based on the duration of A+C therapy. Moment of methods meta regression analysis was conducted to assess the impact of various study and baseline patient characteristics on the primary outcomes. Publication bias was assessed using Egger’s regression test.28 Comprehensive meta-analysis software version 3.0 (Biostat, Englewood, NJ) was used for conducting all analyses.
Results
A total of 10 randomized controlled trials9-12,29-34 comprising 15434 patients were eventually selected comparing A+C therapy with aspirin alone in patients with IS or TIA. The initial database search recovered 14 560 articles; 9248 were duplicates, and 5113 records were removed at title and abstract level. Additionally, on full-text review, 189 studies were removed when desired outcomes were not reported or when A+C combination was not compared with aspirin alone or when studies were systematic reviews and meta-analyses (Figure 1). The MATCH trial (the Management of Atherothrombosis With Clopidogrel in High-Risk Patients)13 was excluded because it compared A+C with clopidogrel alone.
Figure 1.
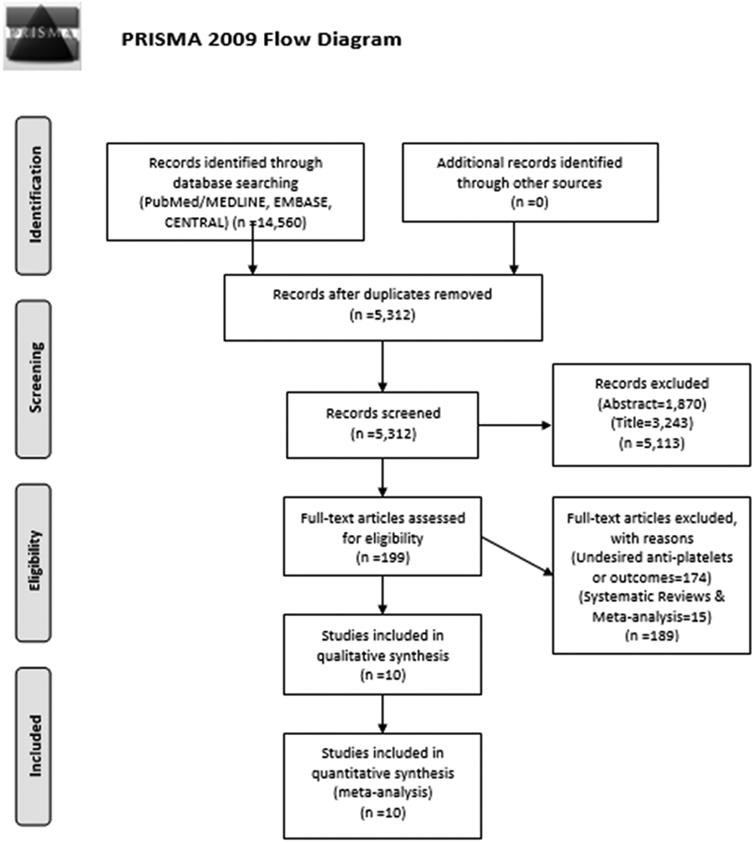
PRISMA flow chart showing study selection process. PRISMA indicates Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Among the included trials (Table), 7 studies were double blinded9-12,29,33,34; 3 were conducted worldwide10,11,33; 5 were conducted in Asian population9,29-32 and 1 trial each in North America12 and Europe.34 Three studies enrolled solely patients with IS,11,29,31 and the rest of the studies included both IS and TIA patients. Onset to treatment after an IS or TIA was within 72 hours in majority of studies. The mean (SD) age of the participants was 64.0±3.4 years, and 61.1% of the participants were males (Table IV in the online-only Data Supplement). As our analysis was based on the duration of DAPT, 6 studies met the criteria for short-term analysis and 2 studies each were included in intermediate- and long-term analysis. The POINT trial10 measured outcomes at 1 week, 1 month, and 3 month for MACE and major bleeding, so we included both 1-month and 3-month data in our short- and intermediate-term analysis, respectively. Subgroup analysis of measured outcomes separately for IS or TIA, type of strokes, and timing of DAPT initiation could not be performed because of inaccessible patient-level data.
Table.
Study Characteristics
| Studies | Setting | Design | Arms | n | Type of Patients |
Onset to Treatment |
Duration of Dual Therapy |
Stroke Severity (NIHSS) |
Follow- Up mo |
|---|---|---|---|---|---|---|---|---|---|
| POINT 201810 | Worldwide, 269 centers | Double-blind | C (LD 600 mg, then 75 mg QD)+A (50–325 mg QD) | 2432 | Minor stroke or high-risk TIA | ≤12 h | 3 mo | 2 | 3 |
| A (50–325 mg QD) | 2449 | 2 | |||||||
| COMPRESS 201629 | Korea, 20 centers | Double-blind | C (75 mg QD without LD)+A (LD 300 mg, then 100 mg QD) | 174 | IS | ≤48 h | 1 mo | 3 | 1 |
| A (LD 300 mg, then 100 mg QD) | 175 | 3 | |||||||
| He et al 201430 | China, single center | Open-label | C (LD 300 mg, then 75 mg QD)+A (100 mg QD) | 321 | Minor stroke or TIA | ≤72 h | 14 days | 3.7 | 14 days |
| A (300 mg QD) | 326 | 3.3 | |||||||
| Yi et al 201431 | China, 2 centers | Open-label, blinded outcomes | C (75 mg QD)+A (200 mg QD) for 30 days, then C alone (75 mg QD) | 284 | IS | ≤48 h | 1 mo | 11.2 | 1 |
| A (200 mg QD for 30 days, then 100 mg QD) | 286 | 11.5 | |||||||
| CHANCE 20139 | China, 114 centers | Double-blind | C (LD 300 mg, then 75 mg QD)+A (75 mg QD) for 21 days then C alone 75 mg | 2584 | Minor Stroke or high-risk TIA | ≤24 h | 21 days | ≤3 | 3 |
| A (75 mg QD) | 2586 | ≤3 | |||||||
| SPS3 201211 | America & Spain, 82 centers | Double-blind | C (75 mg QD without LD)+A (325 mg QD) | 1517 | Lacunar infarcts | <180 days | 3.4 y | … | 41 |
| A (325 mg QD) | 1503 | … | |||||||
| CLAIR 201032 | Asia, multicenter | Open-label, blinded outcomes | C (LD 300 mg QD, then 75 mg QD)+A (75–160 mg QD) | 46 | IS or TIA | <7 days | 7 days | <8 | 7 days |
| A (75–160 mg QD) | 52 | <8 | |||||||
| FASTER 200712 | North America, 18 centers | Double-blind | C (LD 300 mg, then 75 mg QD)+A (LD 162 mg, then 81 mg QD) | 198 | Minor Stroke or TIA | ≤24 h | 3 mo | 0.75 | 3 |
| A (LD 162 mg, then 81 mg QD) | 194 | 1 | |||||||
| CHARISMA 200633 | Worldwide, 768 centers | Double-blind | C (75 mg QD without LD)+A (75–162 mg QD) | 98 | IS or TIA | ≤24 h | 28 mo | … | 28 |
| A (75–162 mg QD) | 118 | … | |||||||
| CARESS 200534 | Europe, 11 centers | Double-blind | C (LD 300 mg, then 75 mg QD)+A (75 mg QD) | 51 | IS or TIA with ≥50% CS | ≤3 mo | 7 days | ≤22 | 7 days |
| A (75 mg QD) | 56 |
A indicates aspirin; C, clopidogrel; CARESS, Clopidogrel and Aspirin for Reduction of Emboli in Symptomatic Carotid Stenosis; CHANCE, Clopidogrel in High-Risk Patients With Acute Nondisabling Cerebrovascular Events; COMPRESS, Combination of Clopidogrel and Aspirin for Prevention of Early Recurrence in Acute Atherothrombotic Stroke; CS, carotid stenosis; FASTER, Fast Assessment of Stroke and TIA to Prevent Early Recurrence; IS, ischemic stroke; LD, loading dose; n, number of patients; NIHSS, National Institutes of Health Stroke Scale; POINT, Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke; QD, daily; SPS3, Secondary Prevention of Small Subcortical Strokes; and TIA, transient ischemic attack.
Primary Efficacy Outcome
A+C therapy significantly reduced the risk of recurrent IS at both short-term (6.4% versus 10.0%; RR, 0.53; 95% CI, 0.37–0.78; I2=21%) and intermediate-term (4.8% versus 6.7%; RR, 0.72; 95% CI, 0.58–0.90; I2=0%) durations compared with aspirin monotherapy. Conversely, there was no significant difference between both the groups at long-term duration (6.3% versus 7.7%; RR, 0.81; 95% CI, 0.63–1.04; I2=0%; Figure 2).
Figure 2.
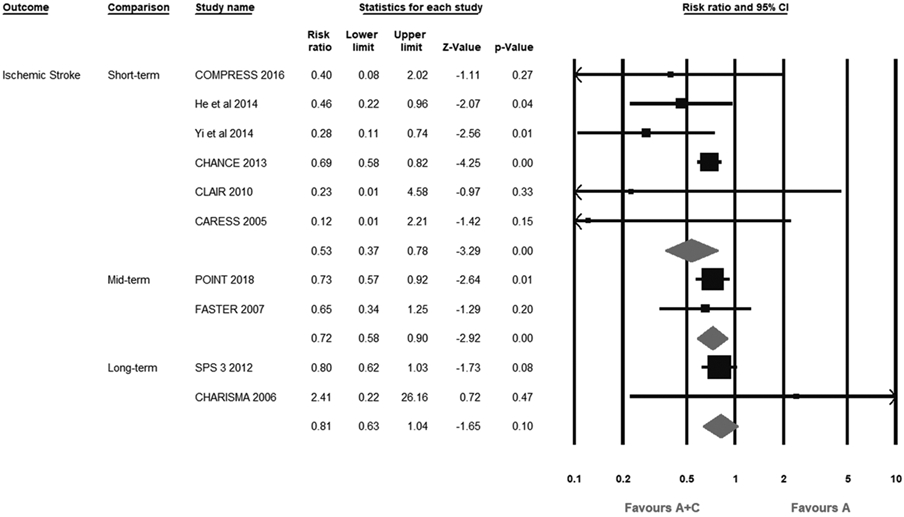
Forest plot comparing aspirin plus clopidogrel (A+C) vs aspirin alone (A) for recurrent ischemic stroke. CARESS indicates Clopidogrel and Aspirin for Reduction of Emboli in Symptomatic Carotid Stenosis; CHANCE, Clopidogrel in High-Risk Patients With Acute Nondisabling Cerebrovascular Events; COMPRESS, Combination of Clopidogrel and Aspirin for Prevention of Early Recurrence in Acute Atherothrombotic Stroke; FASTER, Fast Assessment of Stroke and TIA to Prevent Early Recurrence; POINT, Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke; and SPS 3, Secondary Prevention of Small Subcortical Strokes.
Primary Safety Outcome
At short-term duration, A+C therapy was comparable to aspirin monotherapy (0.4% versus 0.2%; RR, 1.82; 95% CI, 0.91–3.62; I2=0%). On the contrary, both intermediate-term (1.1% versus 0.4%; RR, 2.58; 95% CI, 1.19–5.60; I2=2.1%) and long-term (6.6% versus 3.4%; RR: 1.87; 95% CI, 1.36–2.56; I2=0%) strategies significantly increased the risk of major bleeding (Figure 3). Interestingly, no events of major bleeding occurred in 7-day and 14-day follow-up trials, although they were small,30,32,34 so the result of short-term major bleeding outcome was based on 4 trials9,10,29,31 of around 1-month A+C therapy, which indicates that the safety of 1-month dual therapy is comparable to that of aspirin alone.
Figure 3.
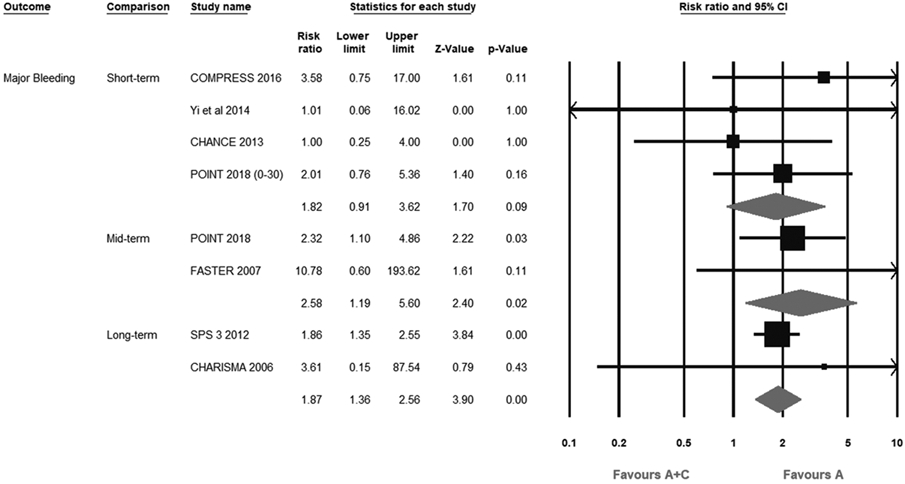
Forest plot comparing aspirin plus clopidogrel (A+C) vs aspirin alone (A) for major bleeding. CHANCE indicates Clopidogrel in High-Risk Patients With Acute Nondisabling Cerebrovascular Events; CHARISMA, Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance; COMPRESS, Combination of Clopidogrel and Aspirin for Prevention of Early Recurrence in Acute Atherothrombotic Stroke; FASTER, Fast Assessment of Stroke and TIA to Prevent Early Recurrence; POINT, Platelet-Oriented Inhibition in New TIA and Minor Ischemic Stroke; and SPS 3, Secondary Prevention of Small Subcortical Strokes.
Secondary Outcomes
In terms of MACE, A+C therapy was superior to aspirin monotherapy at both short-term (5.9% versus 8.7%; RR, 0.68; 95% CI, 0.60–0.78; I2=0%) and intermediate-term (5.2% versus 6.9%; RR, 0.76; 95% CI, 0.61–0.94; I2=0%) durations. Long-term AC strategy did not provide significant efficacy benefit (10.1% versus 11.6%; RR, 0.87; 95% CI, 0.71–1.07; I2=0%; Figure I in the online-only Data Supplement). Finally, in comparison to aspirin monotherapy, all-cause mortality was significantly increased by long-term A+C therapy (7.4% versus 5.1%; RR, 1.45; 95% CI, 1.10–1.93; I2=0%) as opposed to short-term (0.5% versus 0.4%; RR, 1.15; 95% CI, 0.53–2.48; I2=0%) and intermediate-term (0.7% versus 0.5%; RR, 1.56; 95% CI, 0.77–3.18; I2=0%) treatments (Figure II in the online-only Data Supplement).
Meta Regression Analysis
Moment of meta regression analysis did not reveal any significant association of the primary efficacy and safety outcomes with various covariates (Table V in the online-only Data Supplement).
Discussion
Our analysis comprising 10 randomized controlled trials was stratified based on the duration of A+C therapy. The short-term A+C strategy demonstrated maximal benefit of 47% relative risk reduction in recurrent IS and 32% relative risk reduction in MACE without significant increase in major bleeding. Intermediate A+C strategy led to 28% and 24% relative risk reduction in recurrent IS and MACE, respectively. This strategy, however, also led to >2-fold increase in major bleeding. To identify any net advantage of mid-term aspirin and clopidogrel regimen, the number needed to treat to prevent one MACE was 59 and number needed to harm to cause one more major bleeding event was 139 patients. Long-term A+C failed to reduce recurrent IS or MACE, instead it significantly enhanced the risk of major bleeding and all-cause mortality when compared with aspirin alone.
The risk reduction of recurrent IS by A+C therapy seems to be maximum during the short-term duration, which most likely corresponds to the greater risk of recurrent IS within few days to first month after IS or TIA.22-25 Of the total 3-month recurrent IS in the POINT10 and the CHANCE9,35 trials, around 64% and 74% occurred within the first week and nearly 84% and 87% occurred within the first month after TIA or minor IS, respectively. In the POINT trial, the rate of major ischemic vascular events during the period of 31 to 90 days was not significant among the treatment arms. It is possible that the significant reduction of recurrent IS in our mid-term analysis was mainly because of substantial beneficial effect achieved within the first week or first month.
The timing of DAPT initiation after an IS or TIA is the key determinant of the efficacy of the therapy. In the POINT10 trial, DAPT was introduced within 12 hours and in the CHANCE trial9 within 24 hours of a minor IS or TIA. The time-to-event hazard ratio curves for recurrent IS in both studies were divergent in favor of DAPT within the first 1 to 2 days, indicating early onset of benefit of DAPT. In contrast, in the ESPRIT (European/Australasian Stroke Prevention in Reversible Ischaemia Trial)36 trial, which randomized patients to DAPT and aspirin monotherapy within 6 months of a minor IS or TIA, the slope of time-to-event hazard ratio curve for ischemic events was steady and nondivergent during the initial course of therapy. This suggests that DAPT should be initiated within 12 to 24 hours after a minor IS or TIA to achieve maximal efficacy.
With respect to the long-term DAPT, our analysis was predominantly based on the SPS3 trial (Secondary Prevention of Small Subcortical Strokes)11 which enrolled patients within 180 days of subcortical lacunar IS and did not demonstrate significant reduction of recurrent IS with A+C, rather than there was higher risk of major bleeding and all-cause mortality. These findings might be related to very late A+C initiation, consequently falling outside the desired therapeutic efficacy time frame and rendering the therapy ineffective. This study enrolled subjects with small cerebral artery disease in which the pathophysiology is less likely related to the atherothrombosis, which is the primary mechanism for large cerebral artery disease.37 The results of the SPS3 are particularly at odds with randomized trials comparing long-term use of aspirin and extended-release dipyridamole, which demonstrated significant reduction in major vascular events in patients with minor stroke or TIA.36,38 It would be intriguing to directly compare regimens like aspirin and extended-release dipyridamole with A+C during long-term exposure specifically in patients with minor IS or high-risk TIA with low-risk bleeding profile.
The reluctance to use DAPT after an IS or TIA is primarily because of the fear of bleeding and especially intracerebral hemorrhage. Unfortunately, the natural history of bleeding after an IS has not been clearly established as opposed to recurrent IS. For instance, one recent post hoc analysis of 6 randomized trials indicated about 2-fold greater risk of major bleeding in the initial 30 days of DAPT (A+C or aspirin+dipyridamole) as compared with 31 to 90 days.39 Also, the major bleedings within 30 days of DAPT were mainly caused by gastrointestinal bleeds rather than the intracerebral hemorrhage events. In contrast, the MATCH trial13 did not show any early increase in life-threatening bleeding and intracerebral hemorrhage with A+C treatment, and the bleeding complications were steady over the span of 18 months of dual therapy, pointing toward a time margin at which risks exceed the benefits as evident in our analysis.
In the contemporary POINT trial,10 the major bleeding events were slightly higher in the first 30 days as compared with those in the 31 to 90 days, regardless of DAPT or single antiplatelet therapy. This high early bleeding risk could be explained by several possible mechanisms like increased sensitivity to antiplatelets initially, adjunctive anticoagulation and procedures, and unrecognized vulnerability causing predisposed patients bleed earlier, leaving behind less bleeding susceptible population.39-41 In the analysis of the EXPRESS (Early Use of Existing Preventive Strategies for Stroke) and the FASTER (Fast Assessment of Stroke and TIA to Prevent Early Recurrence) trials, there was higher risk of major bleeding with A+C therapy in aspirin-naive patients as compared with the patients previously on aspirin.42 Therefore, the patients not on any antiplatelet therapy before an IS or TIA should be carefully screened for other bleeding risks (unexplained microcytic or iron deficiency anemia, uncontrolled hypertension, advance age, renal and liver disease, history of peptic ulcer disease or other bleeding diathesis, large-territory ISs, thrombocytopenia, and anticoagulation) that might argue against DAPT. Better long-term blood pressure control after an IS has pivotal role in preventing major bleeding and recurrent stroke.43
The data regarding intermediate duration A+C therapy should be interpreted with caution as oftentimes major bleedings can be more incapacitating than ischemic events. Moreover, this analysis did not include minor bleedings which may generate additional negative impact on patients’ well-being and use of medical resources. Among the events categorized as major bleeding, gastrointestinal bleeding, the most common, is less likely to result in permanent impairment as opposed to intracerebral hemorrhage or recurrent IS.11,39 The delicate risk benefit balance with intermediate duration A+C therapy should be further investigated among various IS and TIA subpopulations. Few ongoing trials will provide more insight about various other antiplatelet strategies (Table VI in the online-only Data Supplement).
The current analysis has provided better evidence and decisive approach towards optimal duration of A+C therapy after an IS or TIA. Previous meta-analyses did not analyze efficacy and safety of A+C therapy separately at ≤1-month and ≤3-month durations. Zhang et al6 and Tan et al7 analyzed A+C therapy at ≤3-month and >3-month durations. Their results were contrasting in terms of both major bleeding at ≤3-month duration and recurrent IS at >3-month duration. Zhang et al6 also included the MATCH trial which compared A+C with clopidogrel rather than aspirin as monotherapy. Few studies15,16 did not stratify analysis based on the duration of A+C, whereas others14,17 included patients without recent IS or TIA.
Our study has several limitations: (1) there were significant variations in the patient characteristics, timing of DAPT initiation after IS or TIA,11,34 severity and mechanism of strokes and the brain territory at risk, duration of DAPT, follow-up period, and dose of antiplatelet and adjunctive therapy. These could not be elucidated because of unavailability of patient-level data. (2) Prevalence of CYP2C19 genetic variants in the clopidogrel group and their association of genotypes with clinical outcomes could not be assessed. (3) The potential impact of preexisting use of antiplatelet agents or concurrent medical therapy could not be investigated because of lack of access to patient-level data. (4) The subgroup analyses to further investigate the 1-week, 2-week, or 2- to 4-week outcomes could not be performed because of unavailability of data and variation in the onset to treatment. (5) Certain estimates might lack statistical power to show clinical differences. For instance, despite the lack of statistical significance, the use of long-term A+C might still be beneficial in terms of recurrent IS and MACE because the overall point estimates were still in favor of A+C therapy.
In conclusion, A+C therapy is most effective and adequately safe in reducing recurrent stroke and MACE when administered in the initial weeks after the reference IS or TIA. Use of midterm (up to 3 month) combination strategy could only be considered in carefully selected IS or TIA subjects who are at high risk for recurrent IS and carry low bleeding propensity. Finally, long-term (≥3 month) A+C treatment did not show significant benefit rather increased the bleeding risk compared with aspirin monotherapy. However. the long-term efficacy of A+C therapy might be affected by limited statistical power and late initiation of A+C therapy. Hence, long-term A+C therapy should be further investigated in well-powered clinical trials.
Supplementary Material
Footnotes
The online-only Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.118.023978.
Disclosures
None.
Contributor Information
Hammad Rahman, Department of Medicine, Guthrie Health System/Robert Packer Hospital, Sayre, PA.
Safi U. Khan, Department of Medicine, West Virginia University, Morgantown.
Fahad Nasir, Department of Medicine, Guthrie Health System/Robert Packer Hospital, Sayre, PA.
Tehseen Hammad, Department of Medicine, Services Hospital, Lahore, Pakistan.
Michael A. Meyer, Division of Neurology, Guthrie Health System/Robert Packer Hospital, Sayre, PA.
Edo Kaluski, Department of Medicine, Guthrie Health System/Robert Packer Hospital, Sayre, PA; Division of Cardiology, Rutgers New Jersey Medical School, Newark; Division of Cardiology, The Geisinger Commonwealth Medical College, Scranton, PA.
References
- 1.Rothwell PM, Algra A, Chen Z, Diener HC, Norrving B, Mehta Z. Effects of aspirin on risk and severity of early recurrent stroke after transient ischaemic attack and ischaemic stroke: time-course analysis of randomised trials. Lancet. 2016;388:365–375. doi: 10.1016/S0140-6736(16)30468-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baigent C, Blackwell L, Collins R, Emberson J, Godwin J, Peto R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet (London, England). 2009;373:1849–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antithrombotic Trialists' Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324:71–86. doi: 10.1136/bmj.324.7329.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, et al. 2016 acc/aha guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease. A report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. 2016;68:1082–1115. [DOI] [PubMed] [Google Scholar]
- 5.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. ; American Heart Association Stroke Council. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46–e110. doi: 10.1161/STR.0000000000000158 [DOI] [PubMed] [Google Scholar]
- 6.Zhang Q, Wang C, Zheng M, Li Y, Li J, Zhang L, et al. Aspirin plus clopidogrel as secondary prevention after stroke or transient ischemic attack: a systematic review and meta-analysis. Cerebrovasc Dis. 2015;39:13–22. doi: 10.1159/000369778 [DOI] [PubMed] [Google Scholar]
- 7.Tan S, Xiao X, Ma H, Zhang Z, Chen J, Ding L, et al. Clopidogrel and aspirin versus aspirin alone for stroke prevention: a meta-analysis. PLoS One. 2015;10:e0135372. doi: 10.1371/journal.pone.0135372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.European Stroke Organisation (ESO) Executive Committee; ESO Writing Committee. Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis. 2008;25:457–507. doi: 10.1159/000131083 [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, Wang Y, Zhao X, Liu L, Wang D, Wang C, et al. ; CHANCE Investigators. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369:11–19. doi: 10.1056/NEJMoa1215340 [DOI] [PubMed] [Google Scholar]
- 10.Johnston SC, Easton JD, Farrant M, Barsan W, Conwit RA, Elm JJ, et al. Clinical Research Collaboration, Neurological Emergencies Treatment Trials Network, and the POINT Investigators. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med. 2018;379:215–225. doi: 10.1056/NEJMoa1800410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benavente OR, Hart RG, McClure LA, Szychowski JM, Coffey CS, Pearce LA; SPS3 Investigators. Effects of clopidogrel added to aspirin in patients with recent lacunar stroke. N Engl J Med. 2012;367:817–825. doi: 10.1056/NEJMoa1204133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kennedy J, Hill MD, Ryckborst KJ, Eliasziw M, Demchuk AM, Buchan AM; FASTER Investigators. Fast assessment of stroke and transient ischaemic attack to prevent early recurrence (FASTER): a randomised controlled pilot trial. Lancet Neurol. 2007;6:961–969. doi: 10.1016/S1474-4422(07)70250-8 [DOI] [PubMed] [Google Scholar]
- 13.Diener HC, Bogousslavsky J, Brass LM, Cimminiello C, Csiba L, Kaste M, et al. ; MATCH Investigators. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:331–337. doi: 10.1016/S0140-6736(04)16721-4 [DOI] [PubMed] [Google Scholar]
- 14.Chen S, Shen Q, Tang Y, He L, Li Y, Li H, et al. Efficacy and safety of adding clopidogrel to aspirin on stroke prevention among high vascular risk patients: a meta-analysis of randomized controlled trials. PLoS One. 2014;9:e104402. doi: 10.1371/journal.pone.0104402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou X, Tian J, Zhu MZ, He CK. A systematic review and meta-analysis of published randomized controlled trials of combination of clopidogrel and aspirin in transient ischemic attack or minor stroke. Exp Ther Med. 2017;14:324–332. doi: 10.3892/etm.2017.4459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang Y, Zhou M, Zhong X, Wang Y, Zhao X, Liu L, et al. Dual versus mono antiplatelet therapy for acute non-cardioembolic ischaemic stroke or transient ischaemic attack: a systematic review and meta-analysis. Stroke Vasc Neurol. 2018;3:107–116. doi: 10.1136/svn-2018-000168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gouya G, Arrich J, Wolzt M, Huber K, Verheugt FW, Gurbel PA, et al. Antiplatelet treatment for prevention of cerebrovascular events in patients with vascular diseases: a systematic review and meta-analysis. Stroke. 2014;45:492–503. doi: 10.1161/STROKEAHA.113.002590 [DOI] [PubMed] [Google Scholar]
- 18.Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0: Updated March 2011 http://handbook-5-1.cochrane.org/. Assessed October 19, 2018.
- 19.AHA/ASA Journals. Transparency and Openness Promotion (TOP) Guidelines for Authors Publishing in an American Heart Association Journal. http://www.ahajournals.org/TOP-guidelines. Assessed October 20, 2018.
- 20.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. ; Cochrane Bias Methods Group; Cochrane Statistical Methods Group. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kleindorfer D, Panagos P, Pancioli A, Khoury J, Kissela B, Woo D, et al. Incidence and short-term prognosis of transient ischemic attack in a population-based study. Stroke. 2005;36:720–723. doi: 10.1161/01.STR.0000158917.59233.b7 [DOI] [PubMed] [Google Scholar]
- 23.Coull AJ, Lovett JK, Rothwell PM; Oxford Vascular Study. Population based study of early risk of stroke after transient ischaemic attack or minor stroke: implications for public education and organisation of services. BMJ. 2004;328:326. doi: 10.1136/bmj.37991.635266.44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnston SC, Gress DR, Browner WS, Sidney S. Short-term prognosis after emergency department diagnosis of TIA. JAMA. 2000;284:2901–2906. [DOI] [PubMed] [Google Scholar]
- 25.Rosamond W, Flegal K, Furie K, Go A, Greenlund K, Haase N, et al. ; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–146. doi: 10.1161/CIRCULATIONAHA.107.187998 [DOI] [PubMed] [Google Scholar]
- 26.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. [DOI] [PubMed] [Google Scholar]
- 27.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hong KS, Lee SH, Kim EG, Cho KH, Chang DI, Rha JH, et al. ; COMPRESS Investigators. Recurrent ischemic lesions after acute atherothrombotic stroke: clopidogrel plus aspirin versus aspirin alone. Stroke. 2016;47:2323–2330. doi: 10.1161/STROKEAHA.115.012293 [DOI] [PubMed] [Google Scholar]
- 30.He F, Xia C, Zhang JH, Li XQ, Zhou ZH, Li FP, et al. Clopidogrel plus aspirin versus aspirin alone for preventing early neurological deterioration in patients with acute ischemic stroke. J Clin Neurosci. 2015;22:83–86. doi: 10.1016/j.jocn.2014.05.038 [DOI] [PubMed] [Google Scholar]
- 31.Yi X, Lin J, Wang C, Zhang B, Chi W. A comparative study of dual versus monoantiplatelet therapy in patients with acute large-artery atherosclerosis stroke. J Stroke Cerebrovasc Dis. 2014;23:1975–1981. doi: 10.1016/j.jstrokecerebrovasdis.2014.01.022 [DOI] [PubMed] [Google Scholar]
- 32.Wong KS, Chen C, Fu J, Chang HM, Suwanwela NC, Huang YN, et al. ; CLAIR Study Investigators. Clopidogrel plus aspirin versus aspirin alone for reducing embolisation in patients with acute symptomatic cerebral or carotid artery stenosis (CLAIR study): a randomised, open-label, blinded-endpoint trial. Lancet Neurol. 2010;9:489–497. doi: 10.1016/S1474-4422(10)70060-0 [DOI] [PubMed] [Google Scholar]
- 33.Bhatt DL, Fox KA, Hacke W, Berger PB, Black HR, Boden WE, et al. ; CHARISMA Investigators. Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med. 2006;354:1706–1717. doi: 10.1056/NEJMoa060989 [DOI] [PubMed] [Google Scholar]
- 34.Markus HS, Droste DW, Kaps M, Larrue V, Lees KR, Siebler M, et al. Dual antiplatelet therapy with clopidogrel and aspirin in symptomatic carotid stenosis evaluated using doppler embolic signal detection: the Clopidogrel and Aspirin for Reduction of Emboli in Symptomatic Carotid Stenosis (CARESS) trial. Circulation. 2005;111:2233–2240. doi: 10.1161/01.CIR.0000163561.90680.1C [DOI] [PubMed] [Google Scholar]
- 35.Pan Y, Jing J, Chen W, Meng X, Li H, Zhao X, et al. ; CHANCE Investigators. Risks and benefits of clopidogrel-aspirin in minor stroke or TIA: time course analysis of CHANCE. Neurology. 2017;88:1906–1911. doi: 10.1212/WNL.0000000000003941 [DOI] [PubMed] [Google Scholar]
- 36.Halkes PH, van Gijn J, Kappelle LJ, Koudstaal PJ, Algra A; ESPRIT Study Group. Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial. Lancet. 2006;367:1665–1673. doi: 10.1016/S0140-6736(06)68734-5 [DOI] [PubMed] [Google Scholar]
- 37.Wardlaw JM. What causes lacunar stroke? J Neurol Neurosurg Psychiatry. 2005;76:617–619. doi: 10.1136/jnnp.2004.039982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Diener HC, Cunha L, Forbes C, Sivenius J, Smets P, Lowenthal A. European Stroke Prevention Study. 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci. 1996;143:1–13. [DOI] [PubMed] [Google Scholar]
- 39.Hilkens NA, Algra A, Kappelle LJ, Bath PM, Csiba L, Rothwell PM, et al. ; CAT Collaboration. Early time course of major bleeding on antiplatelet therapy after TIA or ischemic stroke. Neurology. 2018;90:e683–e689. doi: 10.1212/WNL.0000000000004997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Helgason CM, Bolin KM, Hoff JA, Winkler SR, Mangat A, Tortorice KL, et al. Development of aspirin resistance in persons with previous ischemic stroke. Stroke. 1994;25:2331–2336. [DOI] [PubMed] [Google Scholar]
- 41.Pulcinelli FM, Pignatelli P, Celestini A, Riondino S, Gazzaniga PP, Violi F. Inhibition of platelet aggregation by aspirin progressively decreases in long-term treated patients. J Am Coll Cardiol. 2004;43:979–984. doi: 10.1016/j.jacc.2003.08.062 [DOI] [PubMed] [Google Scholar]
- 42.Geraghty OC, Kennedy J, Chandratheva A, Marquardt L, Buchan AM, Rothwell PM. Preliminary evidence of a high risk of bleeding on aspirin plus clopidogrel in aspirin-naive patients in the acute phase after TIA or minor ischaemic stroke. Cerebrovasc Dis. 2010;29:460–467. doi: 10.1159/000297961 [DOI] [PubMed] [Google Scholar]
- 43.Benavente OR, Coffey CS, Conwit R, Hart RG, McClure LA, Pearce LA, et al. ; SPS3 Study Group. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet. 2013;382:507–515. doi: 10.1016/S0140-6736(13)60852-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


