Abstract
Background
Post-stroke depression (PSD) is one of the most common complications in stroke survivors. But, there are still no objective methods to diagnose PSD. This study aims to identify potential biomarkers for diagnosing PSD in middle-aged stroke survivors.
Methods
Middle-aged subjects aged 30 to 59 years (92 PSD patients and 89 stroke survivors without depression) were included in this study. Urinary metabolites were detected by gas chromatography-mass spectrometry (GC-MS). Differential urinary metabolites and potential biomarkers were screened by applying statistical analysis.
Results
The different urinary metabolic phenotypes between PSD patients and stroke survivors without depression were identified. A total of 12 differential urinary metabolites were accurately identified by using orthogonal partial least-squares-discriminant analysis. After analyzing those 12 differential urinary metabolites by step-wise logistic regression analysis, only seven metabolites (palmitic acid, hydroxylamine, myristic acid, glyceric acid, lactic acid, tyrosine and azelaic acid) were finally selected as potential biomarkers for diagnosing PSD in middle-aged stroke survivors. A panel consisting of these potential biomarkers could effectively diagnose middle-aged PSD patients.
Conclusion
Urinary metabolic profiles were different between middle-aged PSD patients and stroke survivors without depression. Our results would be helpful in future for developing an objective method to diagnose PSD in middle-aged stroke survivors.
Keywords: post-stroke depression, biomarkers, metabolites
Introduction
Cerebrovascular diseases are often coupled with a very high incidence of neuropsychiatric disorders. Post-stroke depression (PSD), as one of the most common complications in stroke survivors, has been widely investigated in recent decades.1 It is estimated that one third of stroke survivors experience significant depressive symptoms during the first year after stroke.2 A study reported that PSD was diagnosed in 16.4% of patients in Hong Kong at the first three months after stroke.3 Another study found that there were 15.5% of patients with PSD at the first one month after stroke.4 Moreover, PSD could increase the morbidity and mortality rates among stroke survivors.5
PSD harms the rehabilitation of stroke survivors. It may reduce the patients’ compliance with rehabilitation programs, and at last result in a prolonged hospital stay.6 A previous study reported that patients with PSD were associated with poorer functional and cognitive recovery compared to stroke survivors without depression.7 The depression severity is found to be an independent predictor of severity of impairment in activities of daily living (ADL). Consistent with these findings, a longitudinal study reported that PSD patients receiving nortriptyline or fluoxetine showed a decreased disability than PSD patients receiving placebo.8 Similarly, PSD patients who responded to treatment with nortriptyline or fluoxetine had significantly better improvement in ADL than non-responders.9 Therefore, it is vital to treat PSD during the management of stroke survivors.
However, PSD appears to remain frequently unrecognized and untreated. The pathophysiology of PSD is still poorly understood, and no objective methods for diagnosing PSD exist. The clinicians mainly use the subjective evaluation scales, such as DSM-5 criteria, to diagnose PSD. However, this method often causes misdiagnosis or under-diagnosis.10 To solve this issue, many works have been done. Liu et al suggested that malondialdehyde had the potential ability to be a biomarker for PSD.11 Serum IL-18 was found to be a potential predictor of PSD at six months after stroke.12 Currently, metabolomics has been widely used to identify potential biomarkers for diseases and provide valuable insights into disease mechanisms.13–15 Using metabolomics, we have identified some potential biomarkers for diagnosing bipolar disorder patients during depressive episode and hepatitis B virus-infected patients with depression.16,17 In this study, we planned to identify some potential urine-accessible metabolite biomarkers for objectively diagnosing PSD in middle-aged (30–59 years) stroke survivors.
Patients and Methods
Recruiting Qualified Subjects
In this study, the middle-aged subjects (92 PSD patients and 89 stroke survivors without depression) were included. All the included patients provided the written informed consent. This study was approved by the Ethical Committee of Chongqing Emergency Medical Center (CEMC20200107) and conducted following the Declaration of Helsinki. The stroke was diagnosed using the criteria that were confirmed by the fourth National Conference on Cerebrovascular Diseases. The depressive symptoms were assessed using Hamilton Depression Rating Scale (HDRS).4 The HDRS-rating was conducted by an experienced psychiatrist. The included stroke survivors were without other mental disorders before stroke, and were also without alcohol abuse, illicit drug use, somatic comorbidities and systemic medical illness. The detailed information is presented in Table 1.
Table 1.
Clinical Characteristics of Middle-Aged Subjects in Different Groups
| Variables | Training Set | Testing Set | ||||
|---|---|---|---|---|---|---|
| PSD | Non-Depressed Stroke Survivors |
p-value | PSD | Non-Depressed Stroke Survivors |
p-value | |
| Samples | 58 | 59 | – | 34 | 30 | – |
| Age | 54.27(4.19) | 52.27(5.25) | 0.14 | 54.14(4.01) | 51.77(4.49) | 0.13 |
| Education (years) | 9.21(5.54) | 9.33(5.48) | 0.89 | 9.47(6.29) | 9.17(5.66) | 0.84 |
| Married (Yes/No) | 51/7 | 50/9 | 0.62 | 28/6 | 27/3 | 0.57 |
| Sex(F/M) | 25/23 | 28/21 | 0.85 | 19/15 | 16/14 | 0.84 |
| BMI | 24.02(3.34) | 23.74(3.17) | 0.73 | 22.43(2.87) | 23(2.23) | 0.62 |
| HDRS | 20.26(5.65) | 1.32(1.68) | <0.00001 | 20.29(0.23) | 1.07(1.46) | <0.00001 |
Abbreviations: PSD, post-stroke depression; F, female; M, male; BMI, body mass index; HDRS, Hamilton Depression Rating Scale.
Sample Preparation and Metabolites Acquisition
Samples, sample preparation procedures, and metabolites data acquisition were extracted from the previous studies.18,19 Briefly, after thawing in an ice-water bath, the urine samples were vortexed. A 15µL aliquot of each urine sample was mixed with 10µL aliquot of each internal standard solution (L-leucine-13C6, 0.02 mg/mL). To degrade the urea, 15µlurease was added into the mixture solution. One hour later (under 37°C), the 240μL and 80μL ice-cold methanol were successively used to extract the mixture solution. After vortexing for 30 s and centrifugation at 14000rpm for 5 min (under 4°C), 224 µL of supernatant was vacuum-dried at room temperature. Then, 30µlmethoxyamine (20 mg/mL) was used to derivate the dried metabolic extract for 90 min at 37°C. To obtain trimethylsilyl derivatives, 30µlBSTFA with 1% TCMS was added into the mixture. After heating the mixture for 1 h at 70°C, 1.0µL cooled derivatives were added into the GC-MS system (Agilent 7980 GC system) to obtain metabolic data.
Statistical Data Analysis
Multivariate data analysis was performed to visualize metabolic alterations between PSD patients and stroke survivors without depression. We used the creatinine to normalize the metabolite data, and then the data were unit variance scaled. Orthogonal partial least-squares-discriminant analysis (OPLS-DA) was conducted using metabolic data. The variable importance in the projection (VIP) value from the OPLS-DA model was used to select urinary metabolite responsible for the group discrimination. Those variables with VIP>1.0 (equivalent to a p-value of less than 0.05) were identified. Additionally, the nonparametric Mann–Whitney U-test was applied to measure the significance of each metabolite with VIP>1.0 in discriminating the different groups. Here, we used the Benjamini and Hochberg False Discovery Rate to correct the p-values from multiple testing. Differential urinary metabolites were selected by consideration of both VIP (>1.0) and adjusted p-value (<0.05). To obtain potential biomarkers for diagnosing PSD in middle-aged stroke survivors, the step-wise logistic regression analysis based on Akaike’s information criterion (AIC) rule was used to analyze these differential urinary metabolites further. The AIC value was used to assess the quality of each built logistic regression model. It could simultaneously take the goodness of fit and simplicity of the model into account. The model with minimum AIC value was identified as the preferred model. Finally, we used the receiver operating characteristic (ROC) curve analysis to evaluate the clinical application ability of the preferred model. The SIMCA-P 14.0 and SPSS 19.0 were used to do statistical analysis in this study.
Results
Regrouping the Included Subjects
It was essential to use independent samples to validate potential biomarkers. Therefore, the included subjects in this study were randomly assigned into training set (58 PSD patients, 59 stroke survivors without depression) and testing set (34 PSD patients, 30 stroke survivors without depression). There were no significant differences on age, sex, body mass index (BMI) and HDRS scores between two groups (see Table 1). The training set was used to find the differential urinary metabolites, and the testing set was used to validate these metabolites.
Metabolic Alterations in Middle-Aged PSD Patients
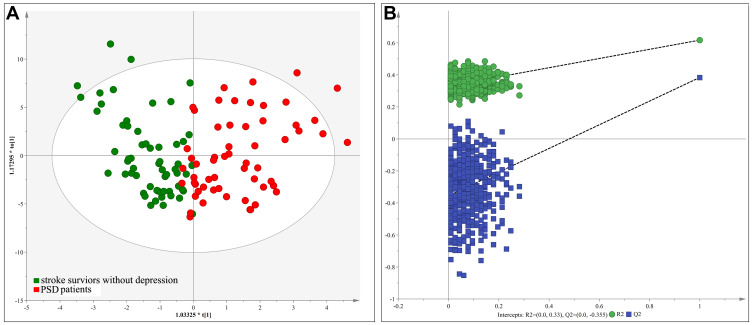
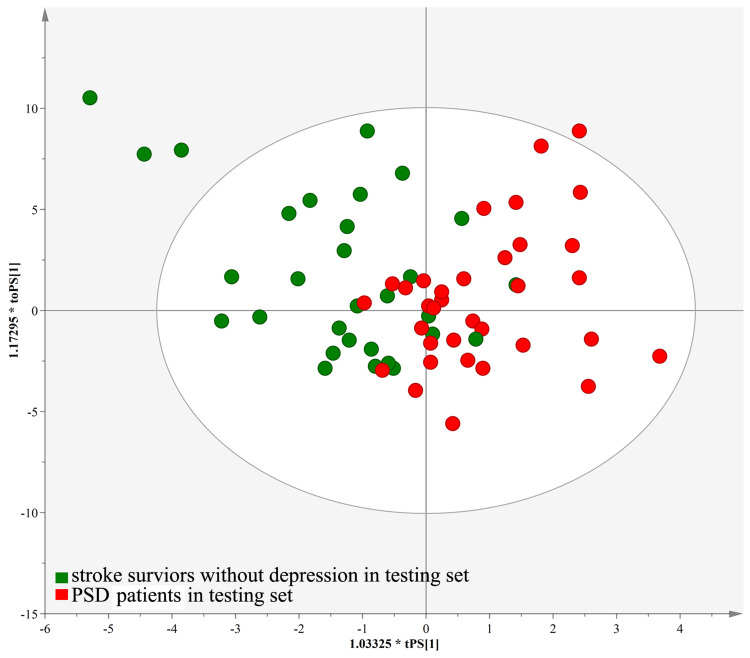
An OPLS-DA model was constructed by using the training set, which showed the satisfactory group classification ability. As represented in the score scatter plot (Figure 1A), PSD patients and stroke survivors without depression were correctly separated with little overlap, representing the strong explanatory power of the data. The results of 599-items permutation test (R2=(0.0; 0.33), Q2=(0.0; −0.355)) further demonstrated the valid and stable of the built OPLS-DA model (Figure 1B). Meanwhile, the T-predicted scatter plot revealed a clear separation between PSD patients and stroke survivors without depression from the testing set, indicating a good predictive ability of the built OPLS-DA model (Figure 2). These results suggested the robust metabolic alterations in middle-aged PSD patients. Also, we found that high body mass index (BMI) could not significantly affect the urine metabolomic signature (see Supplementary file 1). The information of all analyzed metabolites and representative extracted ion chromatograms were also provided in Supplementary file 1.
Figure 1.
The scores plot of the OPLS-DA model: (A) OPLS-DA model was constructed using middle-aged PSD patients (red circle) and stroke survivor without depression (green circle); (B) 599-items permutation test showed that the model was valid and stable.
Abbreviation: PSD, post-stroke depression.
Figure 2.
T-predicted scatter plot of the OPLS-DA model. The model could correctly predict the middle-aged PSD patients (red circle) and stroke survivor without depression (green circle) in the testing set.
Abbreviation: PSD, post-stroke depression.
Differential Urinary Metabolites Identification
Urinary metabolites with VIP>1 in the OPLS-DA model as well as the adjusted p-value<0.05 were selected as differential urinary metabolites responsible for subjects classification. The corresponding OPLS-DA loading plot identified 18 significantly changed urinary metabolites with VIP>1. But only 12 of these18 metabolites were with an adjusted p-value less than 0.05. Thus, 12 differential urinary metabolites were identified in this study. These differential urinary metabolites between PSD patients and stroke survivors without depression were palmitic acid, hydroxylamine, myristic acid, glucose, fructose, glyceric acid, lactic acid, tyrosine, azelaic acid, α-aminobutyric acid, pyroglutamic acid and uric acid. Those 12 differential urinary metabolites are summarized in Table 2.
Table 2.
Differential Urinary Metabolites Responsible for Subjects Classification
| Metabolites | VIP | P-valuea | Adjust P-valueb | Fold Changec |
|---|---|---|---|---|
| Palmitic acid | 1.92 | 7.74E-05 | 6.97E-04 | 0.52 |
| Hydroxylamine | 1.91 | 1.03E-05 | 1.86E-04 | 0.51 |
| Myristic acid | 1.77 | 1.21E-04 | 5.46E-04 | 0.56 |
| Glucose | 1.55 | 5.08E-03 | 1.02E-02 | 0.68 |
| Fructose | 1.51 | 1.96E-02 | 3.21E-02 | 0.64 |
| Glyceric acid | 1.32 | 7.74E-05 | 4.64E-04 | 0.45 |
| Lactic acid | 1.31 | 1.33E-04 | 4.78E-04 | 0.60 |
| Tyrosine | 1.22 | 4.28E-03 | 1.10E-02 | 1.85 |
| Azelaic acid | 1.14 | 7.70E-04 | 2.31E-03 | 0.40 |
| Α-aminobutyric acid | 1.08 | 1.88E-02 | 3.38E-02 | 0.71 |
| Pyroglutamic acid | 1.06 | 3.30E-02 | 4.96E-02 | 0.86 |
| Uric acid | 1.01 | 4.43E-03 | 9.97E-03 | 0.78 |
Notes: aP-value was from the nonparametric Mann–Whitney U-test the Benjamini and Hochberg False. bAdjust P-value was from the Benjamini and Hochberg False Discovery Rate. c>1.0 and <1.0 indicated lower and higher level, respectively, in middle-aged PSD patients.
Potential Assessment of Metabolites Markers for Middle-Aged PSD
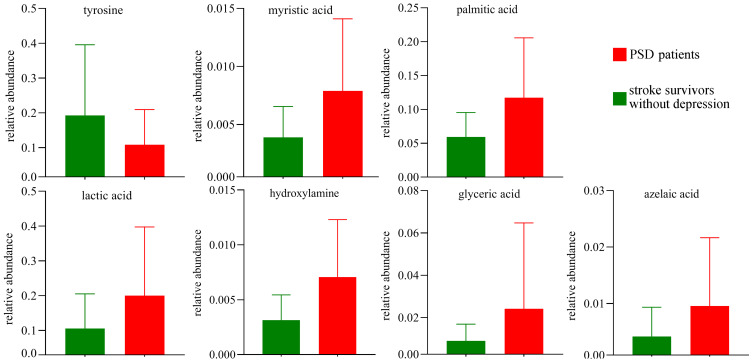
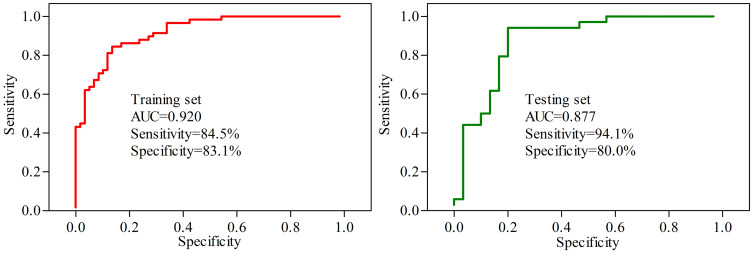
Based on the characteristic analysis of urinary metabolic profiles of middle-aged PSD patients shown above, we tried to explore the potential diagnostic biomarkers further. The results of step-wise logistic regression analysis showed that the panel consisting of seven differential urinary metabolites could effectively separate PSD patients from stroke survivors without depression. These seven differential urinary metabolites were palmitic acid, hydroxylamine, myristic acid, glyceric acid, lactic acid, tyrosine and azelaic acid (Figure 3). The clinical application potential of this panel was evaluated by ROC curve analysis. As shown in Figure 4, this panel demonstrated good potential for the discrimination of PSD patients with an area under curve value (AUC)=0.920 in the training set and an AUC=0.877 in the testing set.
Figure 3.
Column bar graphs of seven potential biomarkers for PSD in middle-aged stroke survivors.
Abbreviation: PSD, post-stroke depression.
Figure 4.
ROC curve analysis of the panel consisting of seven potential biomarkers.
Abbreviation: AUC, area under the curve.
Pathway Analysis of Differential Metabolites
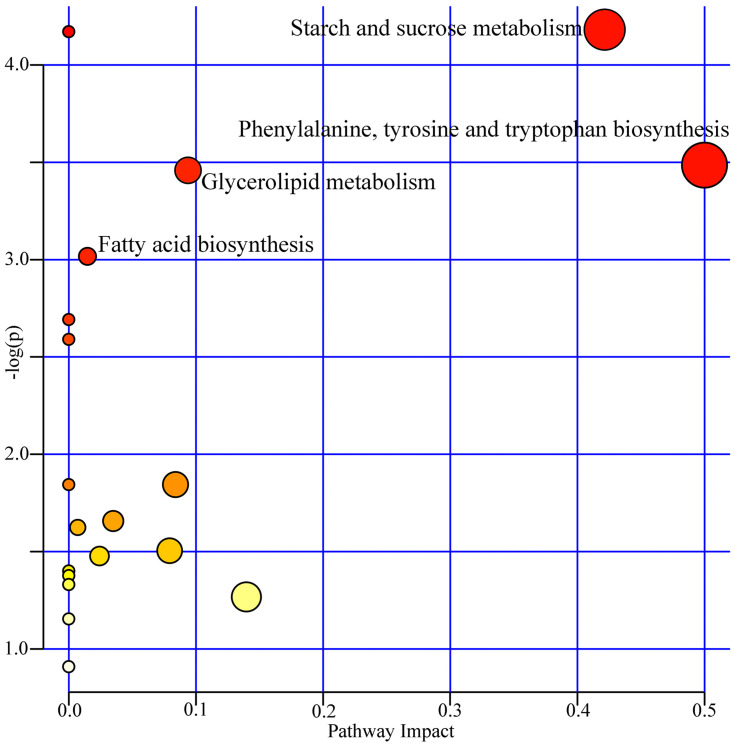
To further analyze the biological functions of these identified differential urinary metabolites, we used the online software MetaboAnalyst4.0 to analyze the related pathways for differential metabolites. According to the results of pathway analysis and enrichment analysis, four metabolic pathways were screened out in this study. These pathways were: phenylalanine, tyrosine and tryptophan biosynthesis (p=0.031, impact=0.50); fatty acid biosynthesis (p=0.048, impact=0.015); Glycerolipid metabolism (p=0.032, impact=0.093); and starch and sucrose metabolism (p=0.008, impact=0.42) (Figure 5). These pathways were related to biosynthesis, oxidative stress response and energy balance, such as Fatty acid biosynthesis and Starch and sucrose metabolism. These results might reveal the potential contributors to the metabolic changes in depression after stroke.
Figure 5.
Metabolic pathway analyses of the differential urinary metabolites.
Discussion
In recent years, many studies have employed metabolomics to identify disease-related markers.13–17 To the best of our knowledge, this is the first study to explore the differences of urinary metabolite markers in middle-aged stroke survivors with depression. In the present study, the built OPLS-DA model demonstrated that there were significant differences of urinary metabolic profiles between PSD patients and stroke survivors without depression. In total, 12 differential urinary metabolites were identified, and a panel consisting of seven potential metabolic biomarkers could effectively diagnose PSD in middle-aged stroke survivors. These results suggested that detecting differential urinary metabolites could be a valid way of diagnosing PSD in middle-aged stroke survivors.
The optimal method for PSD diagnosis remains unclear. A meta-analysis reported that the Center of Epidemiological Studies-Depression Scale (CES-D), HDRS, and 9-item Patient Health Questionnaire (PHQ-9) appeared to be the optimal measures for diagnosing PSD.20 However, PHQ-9 may be more pragmatic in a busy clinical practice when taking into account the feasibility of screening tools, although HDRS and CES-D may have high sensitivity.20 However, this kind of method using scales to detect depressive symptoms has its limitations, as these scales are not capable of adequately characterizing the highly heterogeneous of depressive symptoms in clinical presentation.21 Here, seven differential urinary metabolites were selected as potential biomarkers for diagnosing PSD in middle-aged stroke survivors. Our results could provide valuable information to facilitate searching PSD-specific biomarkers.
A previous study reported that the level of tyrosine was significantly decreased in depressed patients compared to healthy controls, and it could be a potential biomarker for depression.22 In this study, tyrosine with the significantly decreased level in PSD patients compared to stroke survivors without depression was identified as a potential biomarker for PSD. Meanwhile, the disturbed phenylalanine, tyrosine and tryptophan biosynthesis pathway was found here. Interestingly, an animal study reported that depressive behavior was associated with significantly lower levels of metabolites in the tyrosine-phenylalanine pathway.23 These findings suggested that the inhibited tyrosine-phenylalanine pathway might be related to the pathogenesis of PSD.
As the final product of purinergic catabolism, uric acid has an important role in antioxidant defense.24 In purine catabolism, the purine is preferentially metabolized to uric acid to respond to the increased oxidative stress. Here, the uric acid level was significantly increased in PSD patients relative to stroke survivors without depression. Meanwhile, the previous study also reported a significantly higher level of uric acid in depressed patients compared to healthy controls.22 These results indicated the increased oxidative stress in PSD patients. Also, a significantly higher level of azelaic acid was found in PSD patients, and it was also identified as a potential biomarker for PSD in this study. Jones found that azelaic (nonanedioic) acid could inhibit reactive oxygen species (ROS) generation through nicotinamide adenine dinucleotide phosphate (NADPH) oxidase inhibition.25 These findings indicated that the increased oxidative stress might be related to the pathogenesis of PSD.
Increasing numbers of studies have shown that gut microbiota could play an important role in the development of psychiatric disorders.26,27 Our previous study found that there were age-specific differences on gut microbiota in patients with depression.28 Meanwhile, the dysbiosis of gut microbiota has been found in stroke survivors.29,30 In this study, tyrosine, a metabolic byproduct of gut microbiota, was identified as a potential biomarker for middle-aged PSD. Also, using germ-free mice, we found that the gut microbiota could affect the host behaviors and lipid metabolism in the prefrontal cortex of mice.31 Interestingly, the Fatty acid biosynthesis was found here to be significantly affected in middle-aged PSD patients. Combining our results with related reports, we assumed that the metabolism disorder resulted from the disturbed gut microbiota might promote the occurrence of depression in middle-aged stroke survivors.
There are several limitations in this study. First, all the included patients received medications to treat stroke, as medications could directly affect metabolomics profiles, our results needed further validation and support. Secondly, all the included patients were of the same ethnicity. Thus, the ethno-specific biases could not be ruled out here. Thirdly, we did not assess the effects of sociodemographic variables on urinary metabolites, such as education and marital status; these variable were also important factors in depression. Fourthly, due to the relatively small sample size, we did not analyze the sex-specific metabolic profiles; although the metabolic profiles likely also differ to sex. Finally, we only analyzed the urine metabolites; future studies should further analyze the metabolites in serum samples or cerebrospinal fluid to ensure these potential urinary biomarkers physiologically relate to PSD.
In conclusion, using both univariate and multivariate statistical analysis, we found the divergent urinary metabolic phenotypes between middle-aged PSD patients and middle-aged stroke survivors without depression. The 12 significantly changed metabolites were identified, and seven metabolites were selected as potential biomarkers for PSD in middle-aged stroke survivors. A panel consisting of these potential biomarkers showed excellent diagnostic performance in both training set and testing set. Limited by the relatively small sample size, we will further validate these potential biomarkers in a large-scale study.
Disclosure
The authors declare no financial or other conflicts of interest for this work.
References
- 1.Egorova N, Cumming T, Shirbin C, Veldsman M, Werden E, Brodtmann A. Lower cognitive control network connectivity in stroke participants with depressive features. Transl Psychiatry. 2018;7(11):4. doi: 10.1038/s41398-017-0038-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Towfighi A, Ovbiagele B, El Husseini N, et al. Poststroke depression: a scientific statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2017;48(2):e30–e43. doi: 10.1161/STR.0000000000000113 [DOI] [PubMed] [Google Scholar]
- 3.Tang WK, Chan SS, Chiu HF, et al. Poststroke depression in Chinese patients: frequency, psychosocial, and radiological determinants. J Geriatr Psychiatry Neurol. 2005;18:45–51. doi: 10.1177/0891988704271764 [DOI] [PubMed] [Google Scholar]
- 4.Fang J, Yan W, Jiang G, et al. Serotonin transporter gene polymorphism in Chinese patients with poststroke depression: a case-control study. Stroke. 2011;42(5):1461–1463. doi: 10.1161/STROKEAHA.110.598672 [DOI] [PubMed] [Google Scholar]
- 5.Bartoli F, Lillia N, Lax A, et al. Depression after stroke and risk of mortality: a systematic review and meta-analysis. Stroke Res Treat. 2013;2013:862978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sugawara N, Metoki N, Hagii J, et al. Effect of depressive symptoms on the length of hospital stay among patients hospitalized for acute stroke in Japan. Neuropsychiatr Dis Treat. 2015;11:2551–2556. doi: 10.2147/NDT.S91303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang WN, Pan YH, Wang XY, Zhao Y. A prospective study of the incidence and correlated factors of post-stroke depression in China. PLoS One. 2013;8(11):e78981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mikami K, Jorge RE, Adams HP Jr, et al. Effect of antidepressants on the course of disability following stroke. Am J Geriatr Psychiatry. 2011;19:1007–1015. doi: 10.1097/JGP.0b013e31821181b0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chemerinski E, Robinson RG, Arndt S, et al. The effect of remission of poststroke depression on activities of daily living in a double-blind randomized treatment study. J Nerv Ment Dis. 2001;189:421–425. [DOI] [PubMed] [Google Scholar]
- 10.Pan JX, Xia JJ, Deng FL, et al. Diagnosis of major depressive disorder based on changes in multiple plasma neurotransmitters: a targeted metabolomics study. Transl Psychiatry. 2018;8(1):130. doi: 10.1038/s41398-018-0183-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu Z, Zhu Z, Zhao J, et al. Malondialdehyde: a novel predictive biomarker for post-stroke depression. J Affect Disord. 2017;220:95–101. doi: 10.1016/j.jad.2017.05.023 [DOI] [PubMed] [Google Scholar]
- 12.Yang L, Zhang Z, Sun D, et al. The serum interleukin-18 is a potential marker for development of post-stroke depression. Neurol Res. 2010;32(4):340–346. doi: 10.1179/016164110X12656393665080 [DOI] [PubMed] [Google Scholar]
- 13.Sellgren CM, Gracias J, Jungholm O, et al. Peripheral and central levels of kynurenic acid in bipolar disorder subjects and healthy controls. Transl Psychiatry. 2019;9(1):37. doi: 10.1038/s41398-019-0378-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen JJ, Bai SJ, Li WW, et al. Urinary biomarker panel for diagnosing patients with depression and anxiety disorders. Transl Psychiatry. 2018;8(1):192. doi: 10.1038/s41398-018-0245-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen JJ, Xie J, Li WW, et al. Age-specific urinary metabolite signatures and functions in patients with major depressive disorder. Aging (Albany NY). 2019;11(17):6626–6637. doi: 10.18632/aging.102133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie J, Chen C, Hou LJ, Zhou CJ, Fang L, Chen JJ. Dual metabolomic platforms identified a novel urinary metabolite signature for Hepatitis B virus-infected patients with depression. Diabetes Metab Syndr Obes. 2020;13:1677–1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen JJ, Xie J, Zeng L, Zhou CJ, Zheng P, Xie P. Urinary metabolite signature in bipolar disorder patients during depressive episode. Aging (Albany NY). 2019;11(3):1008–1018. doi: 10.18632/aging.101805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang W, Zhang XA. A novel urinary metabolite signature for non-invasive post-stroke depression diagnosis. Cell Biochem Biophys. 2015;72(3):661–667. doi: 10.1007/s12013-014-0472-9 [DOI] [PubMed] [Google Scholar]
- 19.Liang ZH, Jia YB, Li ZR, et al. Urinary biomarkers for diagnosing poststroke depression in patients with type 2 diabetes mellitus. Diabetes Metab Syndr Obes. 2019;12:1379–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meader N, Moe-Byrne T, Llewellyn A, Mitchell AJ. Screening for post-stroke major depression: a meta-analysis of diagnostic validity studies. J Neurol Neurosurg Psychiatry. 2014;85:198–206. doi: 10.1136/jnnp-2012-304194 [DOI] [PubMed] [Google Scholar]
- 21.Chen L, Eaton WW, Gallo JJ, Nestadt G. Understanding the heterogeneity of depression through the triad of symptoms, course and risk factors: a longitudinal, population-based study. J Affect Disord. 2000;59(1):1–11. doi: 10.1016/S0165-0327(99)00132-9 [DOI] [PubMed] [Google Scholar]
- 22.Zheng P, Chen JJ, Huang T, et al. A novel urinary metabolite signature for diagnosing major depressive disorder. J Proteome Res. 2013;12(12):5904–5911. doi: 10.1021/pr400939q [DOI] [PubMed] [Google Scholar]
- 23.Zheng S, Yu M, Lu X, et al. Urinary metabonomic study on biochemical changes in chronic unpredictable mild stress model of depression. Clin Chim Acta. 2010;411(3–4):204–209. doi: 10.1016/j.cca.2009.11.003 [DOI] [PubMed] [Google Scholar]
- 24.Yao JK, Dougherty GG Jr, Reddy RD, et al. Homeostatic imbalance of purine catabolism in first-episode neuroleptic-naïve patients with schizophrenia. PLoS One. 2010;5(3):e9508. doi: 10.1371/journal.pone.0009508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jones DA. Rosacea, reactive oxygen species, and azelaic Acid. J Clin Aesthet Dermatol. 2009;2(1):26–30. [PMC free article] [PubMed] [Google Scholar]
- 26.Lukić I, Getselter D, Ziv O, et al. Antidepressants affect gut microbiota and Ruminococcusflavefaciens is able to abolish their effects on depressive-like behavior. Transl Psychiatry. 2019;9(1):133. doi: 10.1038/s41398-019-0466-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmidtner AK, Slattery DA, Gläsner J, et al. Minocycline alters behavior, microglia and the gut microbiome in a trait-anxiety-dependent manner. Transl Psychiatry. 2019;9(1):223. doi: 10.1038/s41398-019-0556-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen JJ, He S, Fang L, et al. Age-specific differential changes on gut microbiota composition in patients with major depressive disorder. Aging (Albany NY). 2020;12(3):2764–2776. doi: 10.18632/aging.102775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan BYQ, Paliwal PR, Sharma VK. Gut microbiota and stroke. Ann Indian Acad Neurol. 2020;23(2):155–158. doi: 10.4103/aian.AIAN_483_19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen R, Wu P, Cai Z, et al. PuerariaeLobatae Radix with chuanxiong Rhizoma for treatment of cerebral ischemic stroke by remodeling gut microbiota to regulate the brain-gut barriers. J Nutr Biochem. 2019;65:101–114. [DOI] [PubMed] [Google Scholar]
- 31.Chen JJ, Xie J, Zeng BH, et al. Absence of gut microbiota affects lipid metabolism in the prefrontal cortex of mice. Neurol Res. 2019;41(12):1104–1112. doi: 10.1080/01616412.2019.1675021 [DOI] [PubMed] [Google Scholar]