Abstract
Purpose
To report the surgical outcomes of combined gonioscopy-assisted transluminal trabeculotomy (GATT) with ab interno canaloplasty (ABiC) in conjunction with phacoemulsification for primary open-angle glaucoma (POAG).
Patients and Methods
This prospective, interventional, non-comparative case series included POAG patients who underwent combined GATT and ABiC in conjunction with phacoemulsification performed between January 2018 and August 2018. Main outcome measures include surgical success rate, changes in intraocular pressure (IOP), number of antiglaucoma medications, corrected distance visual acuity (CDVA), and complications.
Results
We enrolled twenty eyes of 19 patients in our study. The mean age was 61.2 ± 6 years, and all the patients completed a 12-month follow-up. The overall success rate was 100%. The mean baseline IOP was 19.75 ± 4.68 mmHg, and at 12 months, the mean IOP was 13.30 ± 1.30 mmHg (IOP reduction of 32.7%). The baseline number of antiglaucoma medications was 3.4 ± 0.6 (range: 2 to 4 medications), and after 12 months follow-up, the number was reduced to 1.1 ± 1.0 (range: 0 to 2 medications). The CDVA for all 20 eyes was 0.85±0.58 LogMAR at baseline, and 0.16±0.30 LogMAR at 12-month follow-up. Only six eyes developed hyphaema, which had cleared by the first postoperative month, and three eyes needed medical treatment for postoperative IOP spikes.
Conclusion
The 12-month results of our study suggest that combined GATT with ABiC in conjunction with phacoemulsification is a safe and effective alternative in decreasing the IOP and number of antiglaucoma medications in POAG patients.
Keywords: primary open-angle glaucoma, Minimally Invasive Glaucoma Surgery, Gonioscopy-Assisted Transluminal Trabeculotomy, Ab Interno Canaloplasty, Schlemm's Canal, glaucoma medication
Introduction
Trabeculectomy and aqueous shunts are the most widely performed procedures for the management of primary open-angle glaucoma (POAG).1 Although effective, these procedures carry the risk of severe postoperative complications, including bleb-related complications, hypotony, shallow anterior chambers, and choroidal effusion.2 Furthermore, when performed in the same setting of cataract surgery, the outcomes of these procedures can be affected due to the increased postoperative inflammation and subsequent bleb fibrosis.3,4
In recent years, glaucoma management has expanded after the development of various types of minimally invasive glaucoma surgery (MIGS).5 MIGS procedures have the advantages of rapid recovery for the patients and high safety profile because of their ab-interno method, which preserves the conjunctiva and does not require scleral dissection.6
The gonioscopy-assisted transluminal trabeculotomy (GATT) consists of a modified technique of trabeculotomy, which was newly developed as a MIGS procedure, with an ab interno approach that eliminated most of the downsides of ab externo trabeculotomy.7 This technique reduces the intraocular pressure (IOP) by enhancing the conventional pathway of aqueous outflow without creating a scleral flap or violating the conjunctiva.8 On the other hand, Ab interno canaloplasty (ABiC) is another MIGS procedure that addresses all the aspects of the aqueous outflow system.9 Similar to ab externo canaloplasty, ABiC acts in the same way, but without the need for tensioning suture, which makes the procedure an angioplasty but without a stent.10
Various retrospective studies have reported the outcomes of GATT and ABiC as stand-alone procedures or in combination with cataract surgery for the treatment of different types of glaucoma.7,8,10-17 Herein, we report the outcomes of combined GATT with ABiC in conjunction with phacoemulsification for POAG management.
Patients and Methods
This study was a prospective, non-randomized, non-comparative, interventional case series of patients who underwent combined GATT with ABiC in conjunction with phacoemulsification for the management of POAG. All the procedures were performed by a single surgeon (A.H.) between January 2018 and August 2018 at King Fahad Hospital of the University Khobar, Saudi Arabia. The inclusion criteria of the study included POAG patients with an IOP above the target on maximum tolerated antiglaucoma medications or when the IOP was stable, but there was a desire to decrease the antiglaucoma medications. The exclusion criteria were other forms of glaucoma, cloudy cornea, peripheral anterior synechiae, and active uveitis. The main outcome measures included surgical success rate, changes in IOP, number of antiglaucoma medications, corrected distance visual acuity (CDVA), and complications. The study was approved by the affiliated institutional review board of the hospitals, and adhered to the Declaration of Helsinki and its amendments. All the patients were informed about the other surgical alternatives and signed a written consent before the surgery.
Pre-operative data included gender, age, diagnosis, number of antiglaucoma medications, corrected distance visual acuity (CDVA), slit-lamp and gonioscopic examination, IOP measurement, and fundoscopy. Biometry measurements were obtained using the IOL Master 500 (Carl Zeiss Meditec, Germany) machine, and the formula was chosen depending on the axial length of each eye. All the patients were examined postoperatively at day one, two weeks, and one, three, six, and 12 months. Postoperative data included CDVA, IOP, number of antiglaucoma medications, and complications.
Surgical Technique
Initially, all the patients underwent a standard temporal clear corneal phacoemulsification with intraocular lens implantation under peribulbar anesthesia consisting of bupivacaine and lidocaine. The GATT and ABIC part of the procedure started with creating a port-side incision (either superiorly or inferiorly) directed toward the nasal angle. After injecting an intracameral miotic agent (acetylcholine chloride 1%), the anterior chamber was filled with a viscoelastic agent (sodium hyaluronate). The nasal angle landmarks were identified using a Volk Transcend Vold Gonio (TVG) surgical lens after adjusting the patient’s head and the surgical microscope. A small localized goniotomy of a two mm width was performed horizontally in the nasal angle to gain access to the Schlemm canal. After that, a flexible illuminated microcatheter (iTrack, Ellex, California, USA) was passed circumferentially 360° through the goniotomy site using microsurgical forceps with the assistance of Healon/Healon GV injection (2 Clicks for each clock hour while sliding the illuminated tip of the microcatheter). After canulating the entire canal, the microcatheter’s distal end was removed through the main corneal incision, and the proximal part was pulled into the anterior chamber making a 360° GATT. The viscoelastic material was aspirated partially using the irrigation/aspiration probe, and a small amount was left to prevent bleeding from the canal. The corneal wounds were then hydrated to ensure a watertight seal. Finally, a subconjunctival injection of gentamycin and dexamethasone was given. After the surgery, topical moxifloxacin was prescribed four times a day for one week, and all eyes received a tapering course of topical prednisolone acetate 1.0% over four weeks.
Statistical Analysis
The effectiveness and safety parameters, including mean IOP, number of antiglaucoma medications, CDVA, and postoperative complication rates, were analyzed for all eyes at each visit. The CDVA measures were converted into a logarithm of the minimum angle of resolution (logMAR) notation for statistical analysis. The surgery was considered successful if the postoperative IOP reduction was more than 20% from baseline or if the IOP was between 5 and 21 mm Hg at or after 12 months of follow-up. Eyes that did not achieve the intended IOP reduction or required further glaucoma surgery for IOP control during the follow-up were considered a failure. Normally distributed data were summarized with mean and standard deviation, while non-normally distributed data were summarized with mean and range. Categorical data were summarized in count and percentage.
Baseline and follow-up parameters were compared by repeated-measure analysis of variances (ANOVA) with Bonferroni correction. Categorical data were compared with the Wilcoxon signed-rank test. A P value <0.05 was considered statistically significant.
Results
Twenty eyes of 19 patients (11 males and 8 females) were enrolled in this study. The mean age was 61.2 ± 6 years (range 52 to 80). All the 20 eyes had a diagnosis of POAG and underwent Combined GATT and ABiC with phacoemulsification. The follow-up duration in this study was 12 months for all patients. Demographics and ocular characteristics of the study subjects are summarized in (Table 1).
Table 1.
Demographic Data and Clinical Characteristics of the Study Patients
| Parameters | Value |
|---|---|
| Patients, no | 19 |
| Eyes, no | 20 |
| Mean age ± SD | 61.2 ± 6 years (range 52 to 80 years) |
| Gender (male/female), no (%) | 11(57.9%)/8 (42.1%) |
| Cupping of the optic disc ± SD | 0.59 ± 0.15 (range 0.30 to 0.80) |
Abbreviations: no, number; SD, standard deviation.
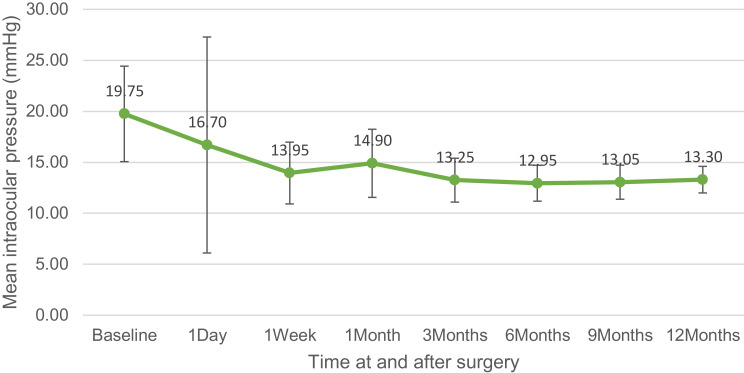
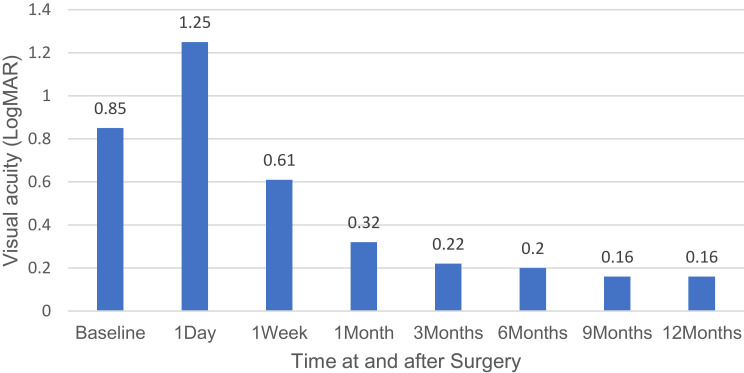
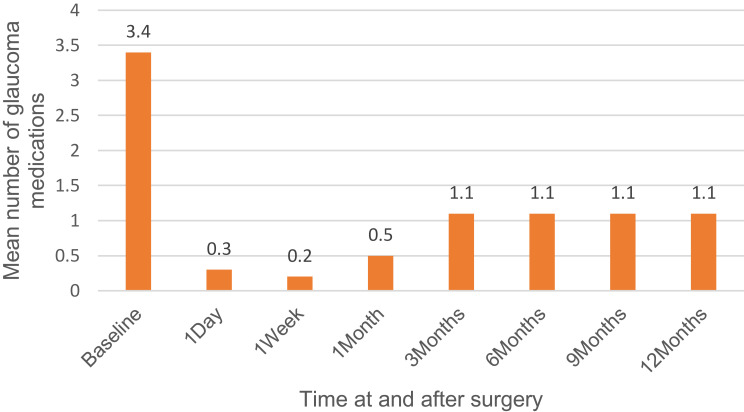
The overall success rate was 100%. The mean baseline IOP for all 20 eyes was 19.75 ± 4.68 mmHg, and at 12 months, the mean IOP was 13.30 ± 1.30 mmHg (p<0.001; IOP reduction of 32.7%). The baseline number of antiglaucoma medications for all 20 eyes was 3.4 ± 0.6 medications (range: 2 to 4 medications), and after 12 months follow-up, the number of antiglaucoma medications was 1.1 ± 1.0 medications (range: 0 to 2 medications) (p<0.001). The CDVA for all 20 eyes was 0.85 ± 0.58 LogMAR at baseline, and 0.16 ± 0.30 LogMAR at 12-month follow-up (p<0.001). Surgical efficacy and safety data are represented in Table 2. Changes in CDVA, IOP, and the number of antiglaucoma medications over the course of the study are shown in (Figures 1–3).
Table 2.
Efficacy of Surgery for All Patients in the Study
| Parameters | Time | Value | P-value |
|---|---|---|---|
| CDVA in LogMAR ± SD (Snellen) | Preoperatively | 0.85 ± 0.58 (20/142) | |
| Day 1 | 1.25 ± 0.87 (20/356) | 1.0 | |
| Week 1 | 0.61 ± 0.46 (20/81) | 1.0 | |
| Month 1 | 0.32 ± 0.44 (20/42) | 0.017 | |
| Month 3 | 0.22 ± 0.44 (20/33) | 0.005 | |
| Month 6 | 0.20 ± 0.45 (20/32) | 0.003 | |
| Month 9 | 0.16 ± 0.30 (20/29) | <0.001 | |
| Month 12 | 0.16 ± 0.30 (20/29) | <0.001 | |
| IOP (mmHg) ± SD | Preoperatively | 19.75 ± 4.68 (range 12 to 30) | |
| Day 1 | 16.70 ± 10.59 (range 8 o 53) | 1.0 | |
| Week 1 | 13.95 ± 3.03 (range 10 to 20) | 0.002 | |
| Month 1 | 14.90 ± 3.34 (range 10 to 20) | 0.016 | |
| Month 3 | 13.25 ± 2.15 (range 11 to 19) | 0.001 | |
| Month 6 | 12.95 ± 1.76 (range 11 to 18) | <0.001 | |
| Month 9 | 13.05 ± 1.67 (range 11 to 17) | <0.001 | |
| Month 12 | 13.30 ± 1.30 (range 12 to 17) | <0.001 | |
| Glaucoma medications number | Preoperatively | 3.4 ± 0.6 (range 2 to 4) | |
| Day 1 | 0.3 ± 0.8 (range 0 to 3) | <0.001 | |
| Week 1 | 0.2 ± 0.6 (range 0 to 2) | <0.001 | |
| Month 1 | 0.5 ± 0.9 (range 0 to 2) | <0.001 | |
| Month 3 | 1.1 ± 1.0 (range 0 to 2} | <0.001 | |
| Month 6 | 1.1 ± 1.0 (range 0 to 2} | <0.001 | |
| Month 9 | 1.1 ± 1.0 (range 0 to 2} | <0.001 | |
| Month 12 | 1.1 ± 1.0 (range 0 to 2} | <0.001 |
Abbreviations: CDVA, corrected distance visual acuity; logMAR, logarithm of the minimum angle of resolution; SD, standard deviation.
Figure 2.
Intraocular pressure over the time of study.
Figure 1.
Visual acuity over the time of study.
Figure 3.
Number of antiglaucoma medications over the time of study.
None of the operated eyes required further glaucoma procedure. Only three eyes had IOP spikes on the first postoperative day and were managed with topical antiglaucoma medications. Additionally, hyphaema of less than one-third of the anterior chamber was observed in 6 eyes within the first postoperative week, which had cleared by the first postoperative month. No other complications occurred during or after the surgery.
Discussion
In the era of MIGS, the focus had moved to the conjunctival sparing surgical approaches. These approaches aim for a safer, less invasive way to decrease IOP than traditional surgeries and improve the patients’ quality of life by lowering the number of topical antiglaucoma medications, which ultimately reduces side effects.11
The effectiveness of GATT was first described by Grover et al in 2014. In their 12 months follow-up retrospective study, they reported an IOP reduction of 11.1 ± 6.1 mmHg (40%) on an average of 1.1 ± 1.8 fewer antiglaucoma medications in 57 patients who underwent GATT with or without cataract surgery for POAG. Furthermore, they reported an overall success rate of 91% in patients who underwent GATT with or without cataract surgery.7 Similarly, Rahmatnejad et al reported an IOP reduction of 9.3 ± 10.8 mmHg (38%) after 12 months follow-up in 66 POAG patients who underwent GATT. The overall success rate of GATT in their study was 63%.11 On the other hand, Gallardo et al reported promising results on 75 eyes treated with ABiC with and without cataract surgery for POAG. In their study, the mean IOP decreased by 32.3% (from 20.4 ± 4.7 to 13.3 ± 1.9 mmHg), and the mean postoperative number of antiglaucoma medications decreased by 60% (from 2.8 ± 0.9 to 1.1 ± 1.1 medications) after 12 months follow-up.13 In another study of 36 eyes of 28 POAG patients treated by ABiC with and without cataract surgery, Davids et al reported an IOP decrease from 19.8 ± 4.1 to 13.8 ± 3 mmHg at 12 months follow-up. Furthermore, the number of antiglaucoma medications was stabilized at 2.1 ± 1.6 medications postoperatively.14
Some investigators have evaluated the additive effect of ab interno 360-degree suture trabeculotomy in patients previously treated by ab externo canaloplasty.18,19 Seuthe et al reported at 12 months a mean IOP reduction of 41.2% with a decrease of antiglaucoma medications from 2.7 to 1.6 in their 88-patient cohort.18 Additionally, the results of Voykov et al study showed a 41.4% decrease in the mean IOP and reduction of antiglaucoma medications to 1.2 at 12 months in 31 patients with POAG.19 In the present study, we report the 12-month outcomes of combined GATT with ABiC in conjunction with phacoemulsification for the management of POAG. Combining the two different surgical procedures in the same setting addresses multiple points of outflow resistance, and could, therefore, serve as an indirect indicator of surgical success.20 The overall success rate in our study was 100%. The IOP reduction was 6.45 ± 3.38 mmHg (IOP reduction of 32.7%) after 12 months, which is relatively lower than previously reported because these eyes had a low baseline IOP. Furthermore, the number of antiglaucoma medications decreased to 1.1 ± 1.0 at 12 months post-operatively. Our results showed comparable IOP lowering and antiglaucoma medication reduction to previous studies that evaluated the effectiveness of GATT and ABiC as stand-alone procedures or in combination with phacoemulsification and other MIGS options. However, the success rate in our study was higher than those in previous studies.
The most commonly reported complications after GATT and ABiC are hyphaema, and transient IOP spikes.8 Hyphaema is deemed as a sign of surgical success and usually resolves spontaneously in the early postoperative period.12,21 In our study, six eyes developed hyphaema, which had cleared by the first postoperative month, and three eyes needed medical treatment for postoperative IOP spikes. No other complications occurred during or after the surgery, and none of the operated eyes required further glaucoma procedure.
Similar to the procedure in our study, the OMNI Surgical Device (Sight Sciences), provides the ability to perform a circumferential viscodilation and ab interno trabeculotomy. This newly introduced device showed promising preliminary results for the management of moderate and advanced glaucoma. Nevertheless, limited data are currently available about its safety and efficacy, because of the short time this device has been marketed.20
Limitations to our study include the limited number of patients and the lack of long follow-up duration. Furthermore, it was a nonrandomized study with no control group. Despite these limitations, we believe that combined GATT with ABiC in conjunction with phacoemulsification has the potential to be a great alternative option for the management of POAG, given the favorable efficacy outcomes and high safety profile in all eyes in our study.
This prospective study, to our knowledge, is the first to report on the outcomes of combined GATT with ABiC in conjunction with phacoemulsification for POAG. According to the 12-month results of our research, the combined procedure appears to be safe and effective in decreasing the IOP and the number of antiglaucoma medications in POAG patients. We recommend that more extensive randomized controlled studies be performed with a longer follow-up in order to assess the long-term outcomes of this procedure in such patient population.
Disclosure
The study has not been presented at any meeting. The authors declare that they do not have competing interests for this work.
References
- 1.Gedde SJ, Schiffman JC, Feuer WJ, et al. Treatment outcomes in the Tube Versus Trabeculectomy (TVT) study after five years of follow-up. Am J Ophthalmol. 2012;153(5):789–803.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zahid S, Musch DC, Niziol LM, et al. Risk of endophthalmitis and other long-term complications of trabeculectomy in the Collaborative Initial Glaucoma Treatment Study (CIGTS). Am J Ophthalmol. 2013;155(4):674–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cillino S, Di Pace F, Casuccio A, et al. Deep sclerectomy versus punch trabeculectomy with or without phacoemulsification; a randomized clinical trial. J Glaucoma. 2004;24:500–506. [DOI] [PubMed] [Google Scholar]
- 4.Murthy SK, Damji KF, Pan Y, Hodge WG. Trabeculectomy and phacotrabeculectomy, with mitomycin-C, show similar two-year target IOP outcomes. Can J Ophthalmol. 2006;41:51–59. [DOI] [PubMed] [Google Scholar]
- 5.Francis BA, Singh K, Lin SC, et al. Novel glaucoma procedures: a report by the American Academy of Ophthalmology. Ophthalmology. 2011;118(7):1466±1480. [DOI] [PubMed] [Google Scholar]
- 6.Lavia C, Dallorto L, Maule M, et al. Minimally-invasive glaucoma surgeries (MIGS) for open-angle glaucoma: a systematic review and meta-analysis. PLoS One. 2017;12(8):e0183142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grover DS, Godfrey DG, Smith O, et al. Gonioscopy-assisted transluminal trabeculotomy, ab interno trabeculotomy: technique report and preliminary results. Ophthalmology. 2014;121(4):855–861. [DOI] [PubMed] [Google Scholar]
- 8.Baykara M, Poroy C, Erseven C. Surgical outcomes of combined gonioscopy‑assisted transluminal trabeculotomy and cataract surgery. Indian J Ophthalmol. 2019;67(4):505–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Byszewska A, Konopińska J, Kicińska AK, Mariak Z, Rękas M. Canaloplasty in the treatment of primary open-angle glaucoma: patient selection and perspectives. Clin Ophthalmol. 2019;13:2617–2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Körber N. Ab interno canaloplasty for the treatment of glaucoma: a case series study. Spektrum Augenheilkd. 2018;32(6):223–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rahmatnejad K, Pruzan NL, Amanullah S, et al. Surgical outcomes of gonioscopy‑assisted transluminal trabeculotomy (GATT) in patients with open‑angle glaucoma. J Glaucoma. 2017;26:1137‑43. [DOI] [PubMed] [Google Scholar]
- 12.Grover DS, Smith O, Fellman RL, et al. Gonioscopy assisted transluminal trabeculotomy: an ab interno circumferential trabeculotomy 24-month follow-up. J Glaucoma. 2018;27(5):393–401. [DOI] [PubMed] [Google Scholar]
- 13.Gallardo MJ, Supnet RA, Ahmed IIK. Viscodilation of Schlemm’s canal for the reduction of IOP via an ab-interno approach. Clin Ophthalmol. 2018;23(12):2149–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davids AM, Pahlitzsch M, Boeker A, et al. Ab interno canaloplasty (ABiC)-12-month results of a new minimally invasive glaucoma surgery (MIGS). Graefes Arch Clin Exp Ophthalmol. 2019;257(9):1947–1953. [DOI] [PubMed] [Google Scholar]
- 15.Aktas Z, Ucgul AY, Bektas C, Sahin Karamert S. Surgical outcomes of prolene gonioscopy-assisted transluminal trabeculotomy in patients with moderate to advanced open-angle glaucoma. J Glaucoma. 2019;28(10):884–888. [DOI] [PubMed] [Google Scholar]
- 16.Cubuk MO, Ucgul AY, Unsal E. Gonioscopy-assisted transluminal trabeculotomy as an option after failed trabeculectomy. Int Ophthalmol. 2020;40(8):1923–1930. [DOI] [PubMed] [Google Scholar]
- 17.Hirabayashi MT, Lee D, King JT, Thomsen S, An JA. Comparison of surgical outcomes of 360° circumferential trabeculotomy versus sectoral excisional goniotomy with the kahook dual blade at 6 months. Clin Ophthalmol. 2019;13:2017–2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Seuthe AM, Januschowski K, Szurman P. Micro-invasive 360-degree suture trabeculotomy after successful canaloplasty – one-year results. Graefes Arch Clin Exp Ophthalmol. 2016;254:155–159. [DOI] [PubMed] [Google Scholar]
- 19.Voykov B, Szurman P, Dimopoulos S, et al. Micro-invasive suture trabeculotomy after canaloplasty: preliminary results. Clin Exp Ophthalmol. 2015;43:409–414. [DOI] [PubMed] [Google Scholar]
- 20.Dickerson JE Jr, Brown RH. Circumferential canal surgery: a brief history. Curr Opin Ophthalmol. 2020;31(2):139–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yalinbas D, Aktas Z, Hepsen İ, Dilekmen N. An unusual complication of combined gonioscopy-assisted transluminal trabeculotomy and phacoemulsification: vision loss due to intracapsular hematoma. Int Ophthalmol. 2018;38(5):2223–2226. [DOI] [PubMed] [Google Scholar]