Abstract
Purpose
The aim of the present study was to evaluate the potential wound healing activity of the hydroalcoholic extract of Salvia haenkei on in vitro and in vivo experimental models.
Materials and Methods
Preliminary analytical characterization of the hydroalcoholic extract of Salvia haenkei was made by reversed-phase high performance liquid chromatography (RP-HPLC) that permitted identification of a qualitative fingerprint of the extract of aerial parts. The wound healing activity of the hydroalcoholic extract of Salvia haenkei was evaluated in vitro by the scratch assay on human dermal fibroblasts and human epidermal keratinocytes and in vivo by standardized mouse excisional splinting model. Real-time PCR (RT-PCR) experiments were performed to analyze gene expression levels of inflammatory markers.
Results
The scratch assay tests showed that the treatment with the hydroalcoholic extract of Salvia haenkei did not induce an increase in the fibroblasts migration rate with respect to the positive control. Instead, the hydroalcoholic extract of Salvia haenkei was effective in improving the wound closure rate on keratinocyte cell cultures with an almost total invasion of the scratch after 48 h of treatment; whereas the positive control, at the same time point, showed only a 67% reduction of the wound size. In vivo experiments showed that the groups treated with the extract of Salvia haenkei completely re-epithelized the wound in 2.7 days, a timing that was comparable with the action of the positive control that took only 2.1 days. Gene expression analysis showed that Salvia haenkei positively regulated the signaling pathway of the nuclear factor-κB (NF-κB) transcription factor.
Conclusion
The results suggested that the hydroalcoholic extract of Salvia haenkei induced a clear wound healing effect.
Keywords: wound healing, cosmetics, animal model, scratch assay
Introduction
Wound healing is a properly timed, iterative, and dynamic process, mainly characterized by three fundamental physiological stages: inflammation, proliferation and a final phase of remodeling, which determines the strength and appearance of the healed tissue.1
The reported stages require a tethering between several molecular and biological events such as cellular proliferation and migration, cytokines and growth factor production that are principally orchestrated by the cooperation of dermal fibroblasts and epidermal keratinocytes.2 Furthermore, the mutual cutaneous processes of repairing and regeneration are promoted by a cross-talk of a double paracrine way, where the cellular migration plays a fundamental role in the physiological repair processes.3
The wound healing process is promoted efficiently by the use of traditional remedies, which are mainly based on plant sources. These remedies have been shown to affect one or more stages of the healing process. In this context, traditional medicine provides a vast source for the discovery of original bioactive compounds and for the development of new pharmaceutical applications.
Salvia haenkei, a plant species of the genus Salvia (family Lamiaceae),4 popularly known as Pampa Salvia, is mainly distributed in Bolivia and Peru.5 Salvia haenkei occupies an interesting role in traditional Bolivian medicine .
Apillapampa, a community of Quechua-speaking people, located in the Bolivian Andes, uses Salvia haenkei as constituent of lejia, a water-based mixture of the ashes of 20 plant species commonly associated with practice of chewing coca. The alkaline substances present in the lejia promote the transformation of stimulating coca alkaloids to free bases, enhancing their physiological and psychoactive effects.6
The traditional Bolivian medicine also includes the use of Salvia haenkei as a digestive or antiemetic agent, to reduce colds and bronchitis symptoms, and against tuberculosis.7
Thanks to the preservation and dissemination activities of Kallawaya knowledge of herbal cures by the Sociedad Boliviana de Medicna Tradicional Medicine (SOBOMETRA), Abdel-Malek et al8 demonstrated an anti-HIV activity of this plant.
Recently, a new medical use was attributed to Salvia haenkei, thanks to the research of Alimonti et al, that demonstrated an antisenescence activity of Salvia haenkei, suggesting a potential use of Salvia haenkei based products for antiaging skin treatments.9,10
The traditional uses and the recent research focused on skin application inspired our studies to investigate the wound healing capacity of Salvia haenkei.
The hydroalcoholic extract of the aerial parts of Salvia haenkei was tested in vitro, by the scratch assay, which is a widely consolidated tool for the screening of potential wound healing agents11 on human dermal fibroblasts and human epidermal keratinocytes, and in vivo, by the standardized mouse excisional splinting model to observe the re-epithelialization of wounds in a more complex and realistic system.12
Our data demonstrated for the first time that hydroalcoholic extract of Salvia haenkei promoted wound healing activity, indicating its use as a potential ingredient in developing new pharmaceutical formulations to treat wounds, cuts, and other skin injuries.
Materials and Methods
Standard and Reagents for HPLC Analysis
Ethanol, methanol, distilled water, acetonitrile, and formic acid, were purchased from VWR (Milan, Italy). The European Pharmacopoeia Reference Standard of Rosmarinic Acid (RA) was from EDQM (Strasbourg, France, code Y0000786). All chemicals used in experimental procedure were of analytical grade purity.
Plant Material
The aerial parts of Salvia haenkei, also known as Pampa Salvia, were collected from a private cultivation situated in Piemonte (Italy). The plant specimen was identified by botanists of Martin Bauer S.p.A, Italy and a plant sample was deposited at their herbarium.
The plant material was harvested and the aerial parts were put in a ventilated stove at 45°C for 24 h and then ground into a fine powder using a hammer mill. A quantity of 100 g of powdered dried plant material was placed in a percolator and covered by 200 mL of water/ethanol (30–70% v/v) mixture. The obtained suspension was incubated for two hours at room temperature and subsequently the extract was collected in a new flask (170 mL approximately). The remained humid plant material was covered for the second time by 200 mL of water/ethanol (30–70% v/v) mixture for two hours. After the second collection process, the extraction procedure was repeated, reaching an amount of six extractions. All the collected extracts were pooled and the obtained hydroalcoholic total extract was filtered through a filter paper and concentrated under vacuum (approximately 11 mmHg) up to 30–35 mL by rotary evaporator, using a water bath at low temperature (30°C), obtaining a viscous solution. The filtrate was left open for 12 h in a well-ventilated hood at 30°C until complete evaporation of the last traces of solvent. The plant/dry extract ratio was 7:1. Finally, 10 g of maltodextrin (DE10) were added to the dry extract in order to improve the consistency and the mixture was ground in the mill and sifted, obtaining 23.7 g of dry extract.
HPLC Analysis
The RA standard was dissolved in methanol at the theoretical final concentration of 0.04 mg/mL. The plant dry extract (50 mg) was accurately weighed into a 100 mL volumetric flask and dissolved in methanol. Both final solutions were filtered through PTFE 0.22 µm filters prior to injection into HPLC system.
The HPLC analysis of hydroalcoholic extract of Salvia haenkei were performed using the Agilent 1260 Series (Santa Clara, CA, USA) equipped with DAD (diode array detector). The separation was carried out by a gradient system, using a reverse-phase Phenomenex (Torrance, CA, USA) Luna C18 (100×4.6 mm, 3 µm) column maintained at 40°C. Injection volume was 3 µL. UV spectra were collected across the range of 200–600 nm. The wavelength of 329 nm, with a sampling frequency of 20 Hz, was selected as the detection wavelength for the identification of the peak of the RA. The mobile phase was composed of solvent (A), acetonitrile and solvent (B), 0.25% (v/v) formic acid solution in water. A linear gradient elution (Table 1), with a flow rate of 1.2 mL/min was used. The presence of RA in the extract was identified by comparing its retention time and spectrum with retention time and spectrum of the RA standard. The estimation of the RA content in the extract was performed using linear regression analysis.
Table 1.
HPLC Linear Gradient
| Time (min) | % A | % B |
|---|---|---|
| 0 | 2 | 98 |
| 5 | 14 | 86 |
| 10 | 20 | 80 |
| 16 | 95 | 5 |
| 18 | 95 | 5 |
| 20 | 2 | 98 |
| 25 | 2 | 98 |
Cell Culture
“Human adult primary dermal fibroblasts” (ATCC PCS-201-012) and “Human skin keratinocyte-human papillomavirus 16 (HPV-16) E6/E7 transformed” (ATCC CRL-2404), were grown in the appropriate minimal medium, fibroblast basal medium supplemented with “fibroblast growth kit-low serum” (ATCC, American Type Culture Collection, Manassas, VA, USA) and 0.2% FBS, and Keratinocyte SFM (Thermo Fisher Scientific, Monza, Italy)supplemented with EGF (5 ng/mL), respectively. The culture conditions were 37°C in a moist atmosphere containing 5% CO2.
The Scratch Wound Healing Assay
The hydroalcoholic dry extract of Salvia haenkei was dissolved in 10% ethanol and then diluted to the final concentration (0.031%) in the appropriate cell culture medium in order to obtain a 1% ethanol working solution. The final solution was sterilized by filtration with 0.22 µm sterile membrane filter. Working concentration of Salvia haenkei was determined because of previous cellular proliferation test and solubilization limits of the plant extract (data not shown).
Dermal fibroblasts (5×105 cells/well) and epidermal keratinocytes (2×105 cells/well) were seeded in 12-well tissue culture plates, in triplicate for each treatment. Seeding conditions were assessed previously based on the growth curves (data not shown), in order to obtain a confluence of 80–90% after 24 h of culture. Twenty-four hours postseeding, the cell monolayers were scraped mechanically with a sterile 1000 μL pipette tip in a straight line to create the “scratches”. Detached cells and debris were washed away with PBS.13 The culture media containing the hydroalcoholic extract of Salvia haenkei were added to fibroblasts and keratinocytes. The appropriate minimal media were added as negative control; complete media supplemented with 2% FBS or 10 ng/mL EGF were added to cell cultures as positive control for fibroblasts and keratinocyte, respectively.
Immediately after wound induction (T0), and at 3, 6, 24, 48 h after treatments, the dishes were placed under a phase-contrast microscope, equipped with a digital camera, for the acquisition of the images of the wounds. At least two fields/well were evaluated for a total amount of 10 images/treatment/time point. In order to maintain fixed the fields during the image acquisition, markings with an ultrafine tip marker were drawn close to the scratch as reference points.
Distance covered by cells moving into the scratched area was measured using the Tscratch software (Gebäck, Zurich, Switzerland).14 Images were analyzed per condition, per time point, and means and standard deviations were calculated. Student’s t-test was used for statistical evaluation. The results were considered significant when P-value was lower than 0.05.
Gene Expression Assays
Dermal fibroblasts (5×105 cells/well) and epidermal keratinocytes (3×105 cells/well) were seeded in six-well tissue culture plates, in triplicate for each treatment. Seeding conditions were assessed previously based on the growth curves (data not shown), in order to obtain a confluence of approximately 50–60% of confluence at the day of the treatment. Twenty-four hours after seeding, the appropriate culture media containing the hydroalcoholic extract of Salvia haenkei, prepared as previously described, were added to fibroblasts and keratinocytes. As negative control, appropriate fresh medium was added to three wells for each cell line. Cells were scraped at the established time points (1.5, 3, 6, 18, and 24 h after the treatment) and collected in RLT buffer. Total RNA was isolated from cells using RNeasy Mini Kit. The reverse transcriptase reaction was performed using 1.5 μg of total RNA in a 10 μL reaction. Ten nanograms of the resulting cDNA was used in the subsequent amplification step along with 1 μL of each 20X TaqMan® probe (Assay ID: Hs00174128_m1, TNFα; Hs01042014_m1, NF-κB; Hs01075529_m1, NOS2; Hs00153133_m1, PTGS2; Hs00174131_m1, IL-6). The expression levels of the eukaryotic 18S rRNA (Assay ID Hs03003631_g1) was used as normalization factor. Real-time PCR reactions were performed in duplicate by using the TaqMan® Master Mix in a 7900HT system. All the reagents for RNA extraction and retrotranscription were from Qiagen NV (Milan, Italy), whereas reagents and instruments for real-time PCR were from Thermo Fisher Scientific (Monza, Italy). The relative transcription level was calculated by using the ΔΔCt method. Statistical analysis were performed through a one-way ANOVA followed by the Dunnett’s test with control. The results were considered significant when P-value was lower than 0.05.
Animals
The Italian “Ministero della Salute” approved the animal protocol (protocol no. 4561/16). All experimental procedures involving animal and their care were carried out in accordance with Italian approved guidelines. Eighteen females, not pregnant BALB/c mice (eight weeks old) were purchased from Charles River Laboratories (Calco, Italy). Animals were housed under controlled room temperature (22±2°C) and humidity (55±10%) with a 12:12-h light–dark cycle. Mice were randomly divided in three experimental groups.
In vivo Wound Healing Experiments
A standardized mouse excisional splinting model12 was used. Briefly, after shaving the back area of the mice, the wound healing model was prepared by pulling with fingers the dorsal skin of the back and punching the folded skin with a 3 mm diameter biopsy punch in order to create a full-thickness wound of Ø 3 mm in diameter at left and right side of the midline. Then, a silicone splinting ring was placed and fastened around the wound perimeter to prevent wound closure caused by skin contraction, in order to obtain a healing process very similar to that in humans.
The wounded area of six mice was treated topically, once a day for 18 days, with a water based cream (glycerine, sorbitol 70%, carbomer, sodium hydroxide and water) containing the hydroalcoholic dry extract of Salvia haenkei (0.5% w/w) whereas that of the mice of the positive control group was treated with a commercially available unguent composed by collagenase and chloramphenicol (Iruxol®, Smith & Nephew GmbH, Monza, Italy). An untreated group was used as negative control group. Wounded areas were photographed immediately after wound induction and then at days 2, 4, 6, 8, 11, 14, and 18. To assure the same distance between the lens of the camera and the back of the mice, we used a standardized and fixed system applied to the stereomicroscope. In addition, we put a measuring tape near the wound as length reference for each collected image. After each image acquisition, a removable sterile dressing was placed on each wound to protect them from the external environment. Photographs were processed with the software ImageJ (Rasband, Bethesda, MD, USA)15 to measure the closure of the wounds, normalized taking into account the absolute error of measurement. The percentage of healing, expressed as re-epithelialized area with respect to wounded area at day 0 (assumed as 100%), was calculated as follows: 100- (wounded area at day x (mm2)/wounded area at day 0 (mm2))×100. Statistical analysis was performed using the software GraphPad Prism® 6.07 (GraphPad Software Inc., La Jolla, CA, USA). Correlation and statistical significance were calculated through Student’s t-test and Pearson’s correlation (P-value <0.01).
Results
Chemical Analyses of Salvia haenkei Extract
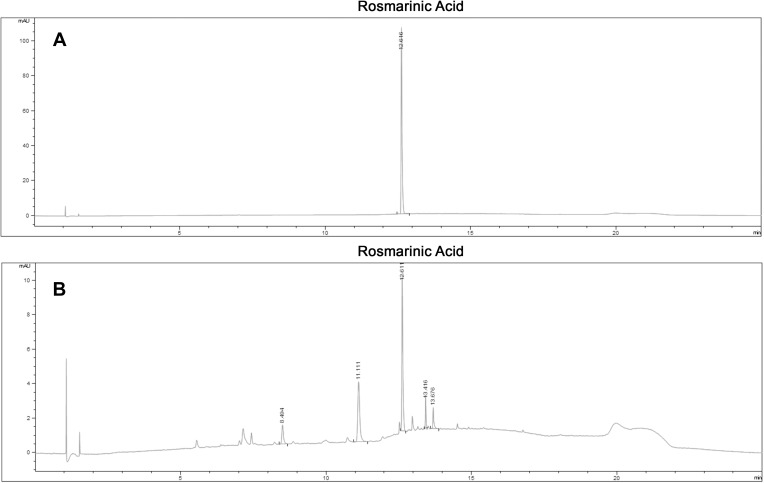
The chromatographic profile, or fingerprint, of Salvia haenkei hydroalcoholic extract, obtained by HPLC (Figure 1), confirmed the RA as the characteristics constituent. The calibration curve of RA standard solution was found to be linear (y=6051.2x + 1.0085; R2=0.9994) in the concentration range 0.61–39.00 µg/mL. The content of RA in the hydroalcoholic extract was roughly 0.45% w/w.
Figure 1.
The chromatographic profile of Salvia haenkei extract obtained by HPLC. (A) Chromatogram of RA standard. (B) Chromatogram of hydroalcoholic Salvia haenkei extract. The average retention time of RA was 12.61 min.
Effect of Salvia haenkei Extract on Cell Migration
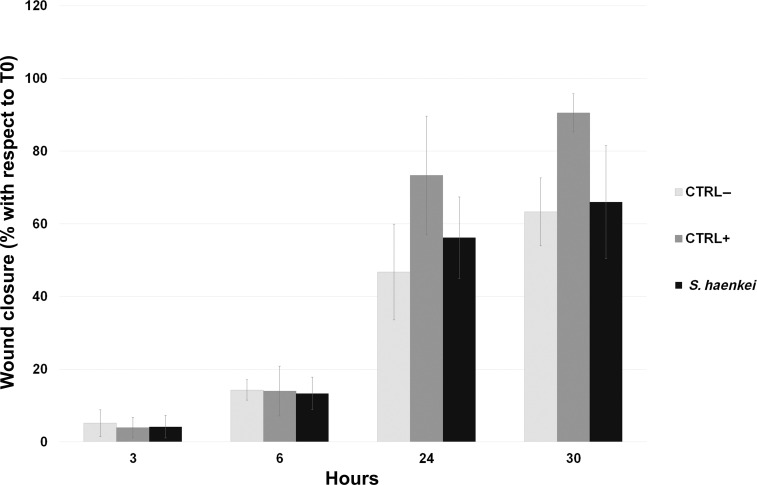
In order to investigate the potential healing activity of the hydroalcoholic extract of Salvia haenkei, we used the in vitro model of wound healing assay, the scratch assay. Specifically, we investigated the healing potential of Salvia haenkei by evaluating the ability of the extract of Salvia haenkei to induce cell migration on human dermal fibroblasts and human epidermal keratinocytes, in comparison to that of the positive control. To this aim, we created scratches in the cell monolayers by scraping cells with a pipette tip. The migration rate, ie, the time used by cells to invade the scratch, of both cell lines was evaluated by monitoring the size of the wound at time 0, immediately after scratching, and at four different time points after the treatment with Salvia haenkei or the positive control. Our results showed that the extract of Salvia haenkei did not induce a statistically significant increase in the fibroblasts migration rate (Figure 2).
Figure 2.
“Scratch assay” on human dermal fibroblasts. Graphical representation of the percentage of the wound closure respect to T0. Statistical significance calculated respect to negative control.
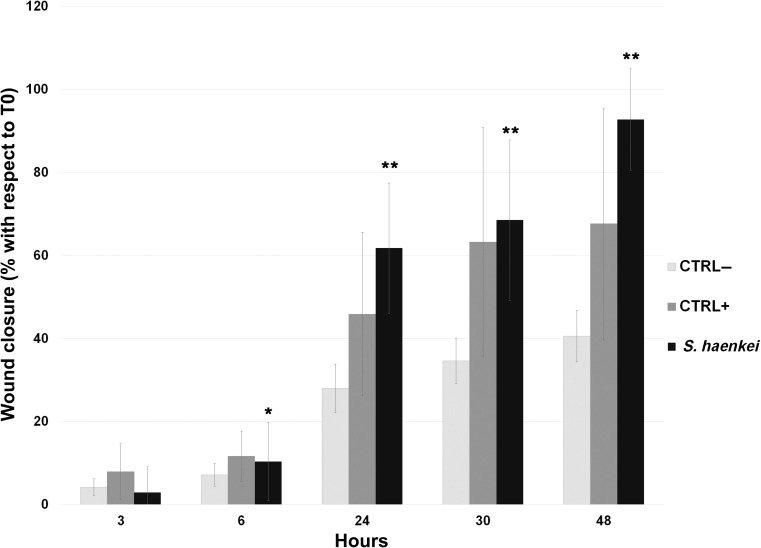
Conversely, the extract of Salvia haenkei was effective in improving the cell migration rate on keratinocytes cell cultures, showing an increase of the wound closure with respect to the negative control even higher than that of the positive control. Indeed, 24 h after treatment, the extract of Salvia haenkei induced a wound closure of about 60%, with a nearly total invasion of the scratch in 48 h of treatment whereas the positive control showed at the same time point only a 67% reduction of the size of the wound (Figure 3).
Figure 3.
“Scratch assay” on human epidermal keratinocytes. Graphical representation of the percentage of the wound closure respect to T0. Statistical significance calculated with respect to negative control. P-value: *<0.05 and **<0.005.
Effect of Salvia haenkei Extract on Pro-inflammatory Genes Transcription
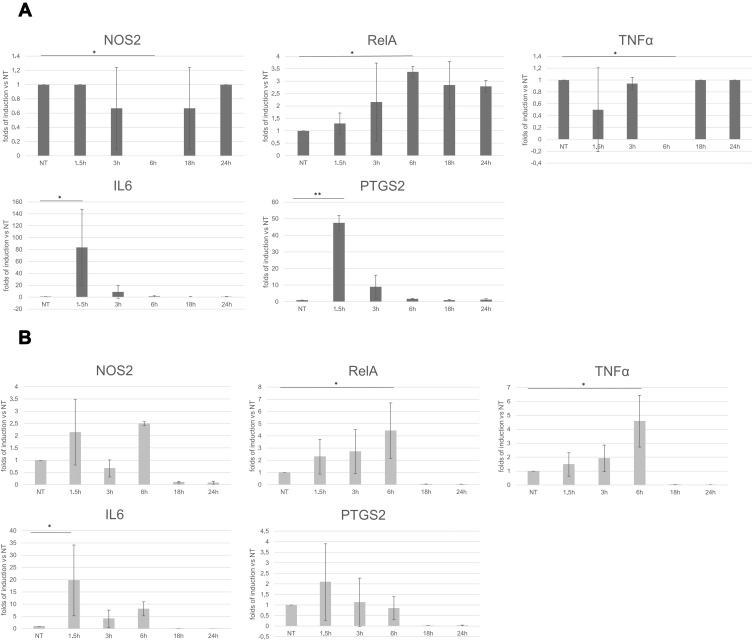
Subsequently, we tried to elucidate if Salvia haenkei could exert an effect also at the molecular levels on fibroblasts and keratinocytes. In fact, during wound healing, the classical nuclear factor-κB (NF-κB) pathway is activated as an innate immune reaction and it promotes the transcription of many cytokines, chemokines, adhesion molecules, enzymes which produce secondary inflammatory mediators necessary for the correct sequence of the next healing steps.16 Thus, we verified if the gene transcription pattern of the two cellular types could be affected by the treatment, at defined time points, with the hydroalcoholic extract of Salvia haenkei. Specifically, we analyzed the mRNA levels of some of the genes involved in the early inflammatory phase of skin repair: the transcriptionally active subunit of the transcription factor NF-κB (RelA), the inflammatory cytokines interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNFα), the inducible nitric oxide synthase (iNOS or NOS2) and the inducible prostaglandin synthesizing enzyme, cyclooxygenase-2 (COX-2) (Figure 4).
Figure 4.
RT-PCR on (A) human dermal fibroblasts and (B) human epidermal keratinocytes. Graphical representation of the gene expression levels, expressed as folds of induction respect to the not-treated control. Statistical significance calculated respect to not-treated control. P-value: *<0.05 and **<0.005.
RelA transcription levels mildly increased in keratinocytes in the early phase of stimulation, from 1.5 to 6 h, with the highest value at 6 h (4.4 folds of induction). However, mRNA levels of RelA in the human dermal fibroblasts remained slightly increased (about 2–3 folds of induction) for all the time when the extract of Salvia haenkei was in the culture medium.
It is known that IL-6 modulates immune response and is essential for timely wound healing,17,18 whereas regulated and balanced TNFα signaling is important to assure the activation of the correct signal transduction pathways leading to the expression of some growth factors promoting re-epithelialization (for example, FGF-7).19 Our results showed that IL-6 expression had a huge increase after 1.5 h of stimulation with Salvia haenkei both in fibroblasts and in keratinocytes (83.6 and 19.7 folds of induction, respectively), whereas TNFα levels had just a mild increasing trend in the early times of treatment, with the highest value at six hours (4.6 folds of induction), only in epidermal keratinocytes.
Data obtained from iNOS-deficient mice clearly indicated an important role for iNOS-derived NO in wound healing. The average time of wound closure was significantly delayed in the knockout animals in a model of full-thickness excisional wounding.20,21 Regarding iNOS transcription levels, the Figure 4 shows that, except for the complete silencing of the gene at six hours, they were not affected by the treatment with Salvia haenkei in fibroblast cell line. A slight increase of the NOS mRNA could be appreciated, instead, in keratinocytes at 1.5 and six hours (2.1 and 2.5 folds of induction, respectively). Though the role of cyclooxygenases in wound repair remains still not completely clarified,22 some evidences exists that COX-2 induction may be important in cutaneous wound healing.23
The treatment with the extract of Salvia haenkei induced the transcription of COX-2 gene at 1.5 h time point in both cell lines, with a major effect on fibroblasts (47.5 folds of induction).
In vivo Wound Healing Potential of Salvia haenkei
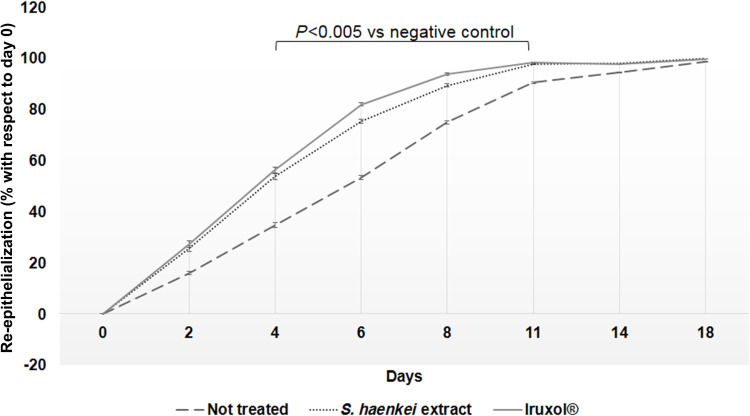
To better investigate the wound healing potential of the extract of Salvia haenkei, we generated a mouse model of wound, the standardized mouse excisional splinting model.12 Therefore, we performed two full thickness round wounds with a biopsy punch on the skin of the back of the mice and then we treated the wounded areas topically once a day with a formulation containing the extract of Salvia haenkei (0.5%). Similarly, we treated another group of mice with an unguent composed by collagenase and chloramphenicol as positive control. Finally, we generated a third group of wounded, not treated mice as negative control for re-epithelialization. Immediately after injuring and then at days 2, 4, 6, 8, 11, 14, and 18, we photographed the back of the mice and the images were used to measure the size of the wounded area. The healing potential of the tested products was expressed in terms of wound closure, ie as percentage of re-epithelialized skin at day x with respect to the wounded area at day 0. As shown in Figures 5 and 6, the topical administration of the extract of Salvia haenkei resulted in a wound-healing rate higher than that of the negative control (P-value <0.05) and very similar to that of the positive control, with a re-epithelialization of about 60% at day four.
Figure 5.
Graphical representation of the wound healing process, expressed as percentage of re-epithelialized area with respect to wounded area at day 0 (P-value <0.05).
Figure 6.
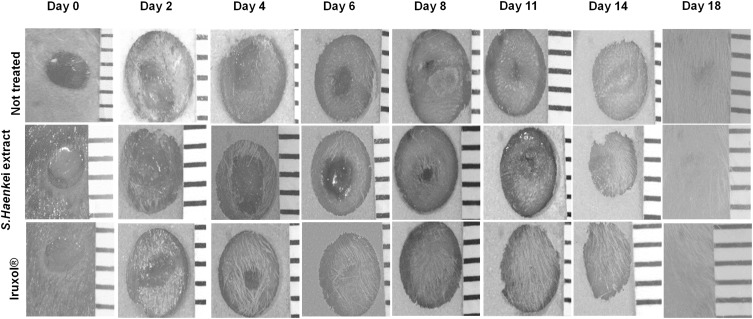
Representative images of the effect of Salvia haenkei on wound closure in mice skin. The scale bar was reported on the right side of each picture. The distance between each horizontal line of the scale bar is 1 mm.
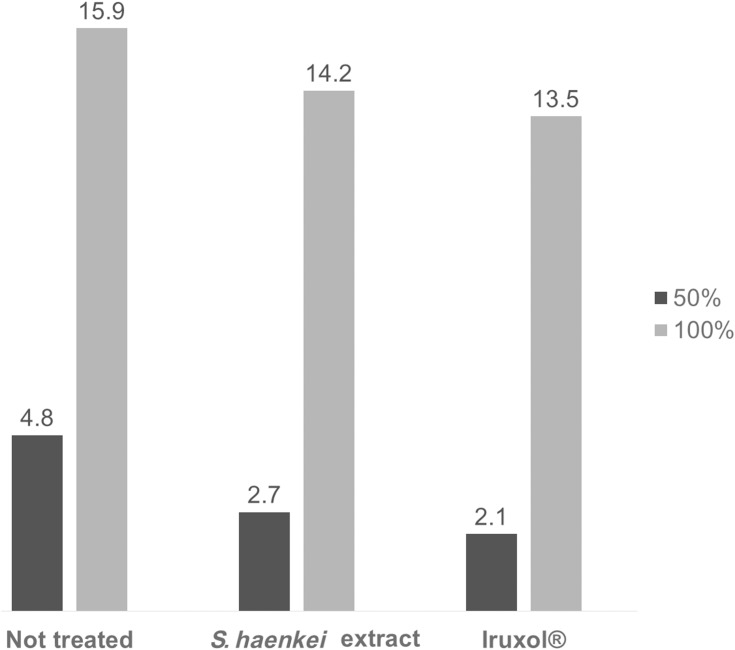
In addition, although at day 18 we observed the complete wound closure for all the three groups, is noteworthy that the treatment with the Salvia haenkei based formulation, like the positive control, was particularly effective in the early stage of the healing process. Through a statistical extrapolation, we were able to calculate the time needed to reach the 50% and the 100% of wound closure for all the three experimental group. As shown in Figure 7, we observed that, while the not treated group needed 4.8 days to reach the 50% of wound closure, the groups treated with the extract of Salvia haenkei and the positive control took only 2.7 and 2.1 days, respectively.
Figure 7.
Graphical representation of time needed to reach the 50% and the 100% of the wound closure, expressed as days.
Discussion and Conclusion
Several traditional remedies, mainly based on plant sources, are commonly involved in the treatment of wounds. These remedies have been shown to affect one or more stages of the healing process.
The genus Salvia presents several species directly involved in the wound healing mechanism.24–26
In this study, an investigation on the involvement of Salvia heankei hydroalcoholic extract in the wound healing process was made for the first time, thanks to a combined approach of in vitro and in vivo experimental techniques.
A preliminary quali-quantitative analysis of the hydroalcoholic extract of Salvia haenkei was carried out by RP-HPLC to characterize the aerial parts extract used for in vitro and in vivo tests. The obtained fingerprint of Salvia haenkei (Figure 1) at the reported analytical conditions (see Materials and Methods), showed a well characteristic spectrum with a predominant presence of rosmarinic acid, a metabolite widely used as preservative agent in pharmaceutical and food industry,27 with a pronounced antiviral, antibacterial, anti-inflammatory, anti-allergic, and anti-oxidant properties.25,28 The content of rosmarinic acid in the hydroalcoholic extract was approximately 0.45% w/w.
Thanks to the scratch assay, one of the major methods used as in vitro model of wound healing assay, we monitored the wound healing process promoted by the extract of Salvia haenkei at different physiological levels. The scrath assay was carried out on dermal fibroblasts and epidermal keratinocytes, two cell lines that are synergistically implicated in wound healing mechanisms,
Indeed, fibroblasts play a fundamental role in the production and remodeling of the extracellular matrix; in addition, the proliferation and migration of these cells play a key role in the formation of granulation tissue and in the further steps of wound repair.29 Keratinocytes, however, migrate from the basal layers of the epidermis to a more superficial one, triggering a series of biochemical events that lead to the re-epithelialization of the skin, a fundamental parameter of successful wound closure.11
Interestingly, our in vitro investigations revealed that, though the hydroalcoholic extract of Salvia haenkei did not induce a pronounced fibroblast migration (Figure 2), it showed, in epithelial keratinocyte cultures, statistical significance related to wound closure efficacy (Figure 3). In detail, the hydroalcoholic extract of Salvia haenkei induced, after 48 h of treatment, a 25.1% higher coverage of the wound with respect to the positive control.
In addition, at the molecular level, we observed that extract of Salvia haenkei was able to modulate positively the transcription of some of the genes mainly involved in the early inflammatory phase of skin repair.
The first phase of healing, in fact, is characterized by reactions mediated by cytokines, chemokines and growth factors produced by both fibroblasts, keratinocytes and inflammatory cells that migrate to the wounded area. All these factors are essential in the early protective response against pathogens and then become necessary for the migration of phagocytic and inflammatory cells to the injured tissues. Many of them are target genes of the transcription factor NF-κB.16,19,30
In our experimental conditions, we observed a positive regulation of the transcription factor NF-κB and, consequently, a modulation of the expression levels of its target genes. Noteworthy is the ability of Salvia haenkei to induce IL-6 expression at high levels. In fact, IL-6 has been reported as one of the key molecules promoting the wound healing process through multiple mechanisms including leukocyte recruitment, angiogenesis, and collagen production.18 In addition, Gallucci et al31 showed that IL-6 could influence wound healing by inducing keratinocyte migration, paving the way to explain, at least in part, the molecular mechanisms through which Salvia haenkei promotes wound closure in the in vitro assay.
The wound healing effects of Salvia haenkei were also observed in the in vivo experiment performed by the excisional wound model in mice. The water-based cream containing extract of Salvia haenkei permitted 50% of wound closure in 2.7 days with respect to 2.1 days of the positive control, an approved drug for sores and wound healing (Figure 7).
The obtained data reinforced the in vitro results, and all together revealed more evidence that Salvia haenkei is involved in the wound healing mechanism.
However, more evidence is needed to completely elucidate at the molecular level the physiological re-epithelialization process.
The literature suggests that terpenoids and flavonoids, widely present in the genus Salvia32–35 and probably contained in the Salvia haenkei phytocomplex,36 could be the major promoters of the observed healing mechanism, even if, as in this case, further investigations on qualitative and quantitative Salvia haenkei phytocomplex composition are needed to confirm the presented hypothesis.
In conclusion, this study offers, for the first time, scientific value in support of our considerations inspired by the recent research focused on skin application of Salvia haenkei, promoting a reliable use of the extract of Salvia haenkei as an ingredient in new pharmaceutical forms for the treatment of skin lesions.
Acknowledgment
The authors acknowledge Dr Immacolata Polvere for her important role in the planning and execution of gene expression experiments.
Disclosure
All authors are employees of IBSA Farmaceutici Italia, an Italian pharmaceutical company, and report personal fees from IBSA Farmaceutici Italia, during the conduct of the study and outside the submitted work. In addition, Andrea Maria Giori has a patent pending: IT102017000146620. The authors report no other conflicts of interest in this work.
References
- 1.Martin P. Wound healing–aiming for perfect skin regeneration. Science. 1997;276(5309):75–81. doi: 10.1126/science.276.5309.75 [DOI] [PubMed] [Google Scholar]
- 2.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341:738–746. doi: 10.1056/NEJM199909023411006 [DOI] [PubMed] [Google Scholar]
- 3.Wang Z, Wang Y, Farhangfar F, Zimmer M, Zhang Y. Enhanced keratinocyte proliferation and migration in co-culture with fibroblasts. PLoS One. 2012;7:e40951. doi: 10.1371/journal.pone.0040951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bentham G. Labiatarum Genera Et Species: Or, a Description of the Genera and Species of Plants of the Order Labiatæ; with Their General History, Characters, Affinities, and Geographical Distribution. London: Ridgway and Sons; 1836. [Google Scholar]
- 5.Epling C. A revision of Salvia, subgenus Calosphace. Feddes Reper Beih. 1939;110:1–383. [Google Scholar]
- 6.Thomas E. Quantitative Ethnobotanical Research on Knowledge and Use of Plants for Livelihood Among Quechua, Yuracaré and Trinitario Communities in the Andes and Amazon Regions of Bolivia. Ghent: Ghent University; 2008. [Google Scholar]
- 7.Chavez Canaviri AM. Medicina Tradicional y la medicina Occidental ed el Manejo de la Tubercolosis. La Paz: Universidad Mayor de San Andrés; 2007. [Google Scholar]
- 8.Abdel-Malek S, Bastien JW, Mahler WF, et al. Drug leads from the Kallawaya herbalists of Bolivia. 1. Background, rationale, protocol and anti-HIV activity. J Ethnopharm. 1996;50:157–166. doi: 10.1016/0378-8741(96)01380-3 [DOI] [PubMed] [Google Scholar]
- 9.Alimonti A, Matic I Screening method and substances for contrasting aging. US20160106661A1, 2014.
- 10.Matic I, Revandkar A, Chen J, et al. Identification of Salvia haenkei as gerosuppressant agent by using an integrated senescence-screening assay. Aging. 2016;8:3223. doi: 10.18632/aging.101076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/S0092-8674(00)81280-5 [DOI] [PubMed] [Google Scholar]
- 12.Xusheng Wang JG, Tredget EE, Yaojiong W. The mouse excisional wound splinting model, including applications for stem cell transplantation. Nat Prot. 2013;8:302–309. doi: 10.1038/nprot.2013.002 [DOI] [PubMed] [Google Scholar]
- 13.Chun-Chi Liang AYP, Guan J-L. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Prot. 2007;2:329–333. doi: 10.1038/nprot.2007.30 [DOI] [PubMed] [Google Scholar]
- 14.Gebäck SM, Koumoutsakos P, Detmar M. T Scratch: a novel and simple software tool for automated analysis of monolayer wound healing assays. Biotech. 2009;4:265–274. doi: 10.2144/000113083 [DOI] [PubMed] [Google Scholar]
- 15.Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Meth. 2012;9:671. doi: 10.1038/nmeth.2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ambrozova N, Ulrichova J, Galandakova AJ. Models for the study of skin wound healing. The role of Nrf2 and NF-κB. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2017;161:1. doi: 10.5507/bp.2016.063 [DOI] [PubMed] [Google Scholar]
- 17.McFarland-Mancini MM, Funk HM, Paluch AM, et al. Differences in wound healing in mice with deficiency of IL-6 versus IL-6 receptor. J Immunol. 2010;184(12):7219–7228. doi: 10.4049/jimmunol.0901929 [DOI] [PubMed] [Google Scholar]
- 18.Lin ZQ, Kondo T, Ishida Y, Takayasu T, Mukaida NJ. Essential involvement of IL‐6 in the skin wound‐healing process as evidenced by delayed wound healing in IL‐6‐deficient mice. J Leuk Biol. 2003;73:713–721. doi: 10.1189/jlb.0802397 [DOI] [PubMed] [Google Scholar]
- 19.Barrientos S, Stojadinovic O, Golinko MS, Brem H. Tomic‐Canic. Growth factors and cytokines in wound healing. Wound Rep Reg. 2008;16(5):585–601. doi: 10.1111/j.1524-475X.2008.00410.x [DOI] [PubMed] [Google Scholar]
- 20.Frank S, Kämpfer H, Wetzler C, Pfeilschifter JJ. Nitric oxide drives skin repair: novel functions of an established mediator. Kid Int. 2002;61(3):882–888. doi: 10.1046/j.1523-1755.2002.00237.x [DOI] [PubMed] [Google Scholar]
- 21.Kitano T, Yamada H, Kida M, et al. Impaired healing of a cutaneous wound in an inducible nitric oxide synthase-knockout mouse. Derm Res Pract. 2017;2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. J Inv Derm. 2007;127:514–525. doi: 10.1038/sj.jid.5700701 [DOI] [PubMed] [Google Scholar]
- 23.Futagami A, Ishizaki M, Fukuda Y, Kawana S, Yamanaka NJ. Wound healing involves induction of cyclooxygenase-2 expression in rat skin. Lab Inv. 2002;82:1503–1513. doi: 10.1097/01.LAB.0000035024.75914.39 [DOI] [PubMed] [Google Scholar]
- 24.Güzel S, Özay Y, Kumaş M, et al. Wound healing properties, antimicrobial and antioxidant activities of Salvia kronenburgii Rech. f. and Salvia euphratica Montbret, Aucher & Rech. f. var. euphratica on excision and incision wound models in diabetic rats. Biomed Pharmacother. 2019;111:1260–1276. doi: 10.1016/j.biopha.2019.01.038 [DOI] [PubMed] [Google Scholar]
- 25.Süntar İ, Akkol EK, Şenol FS, Keles H, Orhan IE. Investigating wound healing, tyrosinase inhibitory and antioxidant activities of the ethanol extracts of Salvia cryptantha and Salvia cyanescens using in vivo and in vitro experimental models. J Ethnopharm. 2011;135:71–77. doi: 10.1016/j.jep.2011.02.022 [DOI] [PubMed] [Google Scholar]
- 26.Salimikia I, Aryanpour M, Bahramsoltani R, et al. Phytochemical and wound healing effects of methanolic extract of Salvia multicaulis Vahl. in rat. J Med Pla. 2016;1:38–46. [Google Scholar]
- 27.Savona M, Barberini S, Bassolino L, et al. Strategies for optimization of the production of rosmarinic acid in Salvia officinalis L. and Salvia dolomitica Codd biomass with several biotechnological approaches Salvia Biotechnology. Springer, Cham; 2017:209–239. [Google Scholar]
- 28.Kamatou G, Chen W, Viljoen A. Quantification of rosmarinic acid in Salvia species indigenous to South Africa by HPTLC. J Plan Chrom-M TLC. 2012;25:403–408. doi: 10.1556/JPC.25.2012.5.3 [DOI] [Google Scholar]
- 29.Xuan YH, Huang BB, Tian HS, et al. High-glucose inhibits human fibroblast cell migration in wound healing via repression of bFGF-regulating JNK phosphorylation. PLoS One. 2014;9:e108182. doi: 10.1371/journal.pone.0108182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039 [DOI] [PubMed] [Google Scholar]
- 31.Gallucci RM, Sloan DK, Heck JM, Murray AR, O’Dell SJ. Interleukin 6 indirectly induces keratinocyte migration. J Invest Dermatol. 2004;122:764–772. doi: 10.1111/j.0022-202X.2004.22323.x [DOI] [PubMed] [Google Scholar]
- 32.Topçu G. Bioactive triterpenoids from Salvia species. J Nat Prod. 2006;69:482–487. doi: 10.1021/np0600402 [DOI] [PubMed] [Google Scholar]
- 33.Wang X, Morris‐Natschke SL, Lee KH. New developments in the chemistry and biology of the bioactive constituents of Tanshen. Med Res Rev. 2007;27:133–148. doi: 10.1002/med.20077 [DOI] [PubMed] [Google Scholar]
- 34.Lu Y, Foo LY. Polyphenolics of Salvia—a review. Phytochem. 2002;59:117–140. doi: 10.1016/S0031-9422(01)00415-0 [DOI] [PubMed] [Google Scholar]
- 35.Topçu G, Yücer R, Şenol H. Bioactive constituents of anatolian salvia species Salvia Biotechnology. Springer, Cham; 2017:31–132. [Google Scholar]
- 36.Almanza G, Balderrama L, Labbé C, et al. Clerodane diterpenoids and an ursane triterpenoid from Salvia haenkei. Computer-assisted structural elucidation. Tetrahedron. 1997;53:14719–14728. doi: 10.1016/S0040-4020(97)00943-5 [DOI] [Google Scholar]