Abstract
Background
Acute type A aortic dissection (ATAAD) is a surgical emergency with an operative mortality of up to 30%, a rate that has not changed meaningfully in more than 2 decades. A growing body of research has highlighted several comorbidities and presenting factors in which delay or permanent deferral of surgery may be considered; however, modern comprehensive summative reviews are lacking. The urgency and timing of this review are underscored by significant challenges in resource use posed by the coronavirus disease 2019 (COVID-19) pandemic. This review provides an update on the current understanding of risk assessment, surgical candidacy, and operative timing in patients with ATAAD.
Methods
A literature search was conducted through PubMed and Embase databases to identify relevant studies relating to risk assessment in ATAAD. Articles were selected by group consensus on the basis of quality and relevance.
Results
Several patient factors have been identified that increase risk in ATAAD repair. In particular, frailty, advanced age, previous cardiac surgery, and use of novel anticoagulant medications have been studied. The understanding of malperfusion syndromes has also expanded significantly, including recommendations for surgical delay. Finally, approaches to triage have been significantly influenced by resource limitations related to the ongoing COVID-19 pandemic. Although medical management remains a reasonable option in carefully selected patients at prohibitive risk for open surgery, endovascular therapies for treatment of ATAAD are rapidly evolving.
Conclusions
Early surgical repair remains the preferred treatment for most patients with ATAAD. However, improvements in risk stratification should guide appropriate delay or permanent deferral of surgery in select individuals.
Abbreviations and Acronyms: ATAAD, acute type A aortic dissection; CM, cerebral malperfusion; IRAD, International Registry of Acute Aortic Dissection; MPS, malperfusion syndrome; NOACs, novel or non–vitamin K oral anticoagulant agents; STS, The Society of Thoracic Surgeons
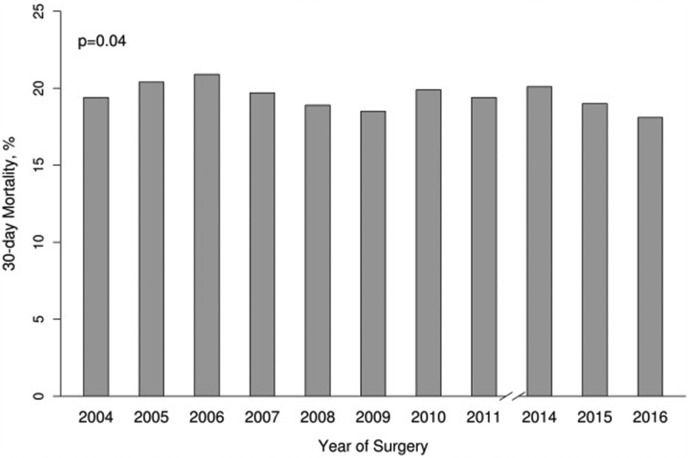
Acute type A aortic dissection (ATAAD) is a surgical emergency, with a mortality rate of up to 90% in patients who do not undergo timely operative intervention.1, 2, 3 Despite significant advances in imaging, perioperative care, and surgical technique, operative mortality rates have remained relatively unchanged, at between 10% and 30%, over the past 2 decades (Figure 1 ).4, 5, 6 Given this relative stagnation in rates of morbidity and mortality associated with ATAAD repair, more recent studies have focused on the identification of factors important for risk stratification in these patients. Furthermore, new options for endovascular management have emerged and are evolving to become efficacious treatment options for carefully selected individuals with ATAAD.7 Finally, in the era of the coronavirus disease 2019 (COVID-19) pandemic, traditional algorithms for the treatment of surgical emergencies must be carefully challenged and reexamined with a focus on minimizing infectious spread, as well as timely and appropriate resource allocation.8 The aims of this review were to provide a commentary on contemporary approaches to the identification of high-risk features in ATAAD and to ascertain when delay, permanent deferral of surgical treatment, or transfer to a specialized center may be considered (Table 1 ).
Figure 1.
The 30-day mortality after repair of acute type A aortic dissection in North America over the era spanning 2004 to 2016 from The Society of Thoracic Surgeons database. (Reproduced from Helder and colleagues,13 with permission from The Society of Thoracic Surgeons.)
Table 1.
Considerations for Potential Deferral of Emergency Surgery or Alternate Therapy in Acute Type A Aortic Dissection
| Risk factors for consideration |
| Frailty and advanced age |
| Visceral and extremity malperfusion and malperfusion syndromes |
| Cerebral malperfusion and major brain injury |
| Previous cardiac surgery and redo sternotomy |
| Preoperative use of novel oral anticoagulants |
| Patient-directed goals of care |
| Refusal of blood products |
| External issues related to resource availability |
| Availability of alternate strategies |
| Applications of current risk prediction tools |
Material and Methods
Articles discussed in this narrative review were identified through a literature search of English language articles in PubMed (1946 to the present) and Embase (1974 to the present), last updated April 25, 2020. The search strategy focused on the identification of articles studying contemporary factors influencing outcomes of ATAAD repair: frailty, age, malperfusion, malperfusion syndrome (MPS), previous cardiac surgery, anticoagulant medications, and risk stratification tools. Additionally, current articles on the ongoing COVID-19 pandemic were identified through additional search of in-press articles in relevant surgical and cardiothoracic surgical journals. We selected the articles on the basis of quality and relevance.
Results
Frailty and Advanced Age
Frailty is a multidimensional syndrome involving loss of reserve across multiple systems that leads to increased vulnerability.9 Although several scoring systems and measurement tools have been developed, assessing frailty in the setting of ATAAD remains a significant challenge given the typical emergency presentation. Consequently, research on the effects of frailty in ATAAD is limited. Nonetheless, frailty assessment in elective proximal aortic surgery has proven useful in the prediction of mortality and discharge disposition.10 , 11 In patients presenting with ATAAD who are at risk for frailty, it remains important to evaluate global functional status, activities of daily living, and comorbid conditions. Several comorbidities, including cerebrovascular disease and severe chronic lung disease, have been shown to be independent predictors of 30-day mortality after ATAAD repair, and these risks are likely exacerbated in the presence of frailty.12 , 13
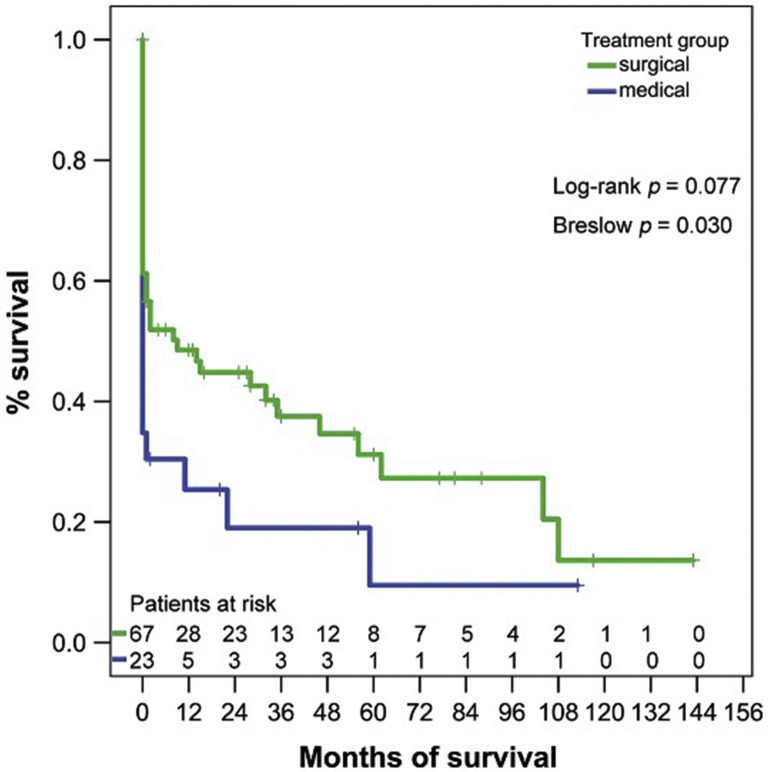
In the absence of validated frailty tools in this patient group, advanced age is often considered a surrogate. Importantly, there is likely to be a significant increase in the number of elderly patients presenting with acute aortic syndromes, in light of the aging population.14 Although many elderly patients do have significant functional limitations, there is no convincing evidence for a “hard” age cutoff with respect to surgery for ATAAD. In fact, several groups have found good short-term outcomes in healthy octogenarians.15 , 16 However, previous work suggested that long-term survival in elderly patients may not be improved compared with patients managed medically (Figure 2 ).17 In elderly individuals with comorbidities or decreased functional status, medical management is a very reasonable course of action. Open and honest communication of both short- and long-term outcomes, along with discussions of goals of care, is very important in this patient group.
Figure 2.
Long-term outcomes of surgical repair vs medical management in octogenarians with acute type A aortic dissection. (Reproduced from Dumfarth and colleagues,17 with permission from the European Association for Cardio-Thoracic Surgery.)
Malperfusion and Malperfusion Syndromes
Malperfusion occurs in 16% to 33% of patients presenting with ATAAD.1 The presence of clinically apparent malperfusion in any organ system at presentation is an ominous sign associated with increased mortality.18 For example, Lawton and colleagues19 demonstrated that patients with malperfusion and severe acidosis had an operative mortality of 92%.
The distinction between malperfusion and MPS is critically important in the optimization of treatment and operative planning. Malperfusion alone has been defined as “inadequate blood flow to the end organs because of dissection related obstruction of the aorta and its branches,” whereas MPS is defined as “tissue necrosis and failure of vital organs (such as viscera or lower extremity) secondary to late-stage malperfusion,” 20 , 21 As such, the diagnosis of MPS requires the presence of both clinical features (eg, abdominal pain, tenderness, oliguria or anuria, motor or sensory neurovascular deficits) and laboratory features (eg, elevated lactate, serum creatinine, liver or pancreatic enzymes, creatinine kinase) indicative of end-organ ischemia.
The presence of MPS indicates active end-organ ischemia and can itself lead to significant exacerbation of the inflammatory cascade, thus further complicating management in these individuals. Mesenteric MPS is of particular concern, with a reported mortality rate of 60% or higher in multiple series. Even with early intervention, the mortality rate in these patients is still up to 42%.22 In light of this finding, many groups have adopted treatment algorithms that delay operative repair of the proximal aorta in the setting of MPS.
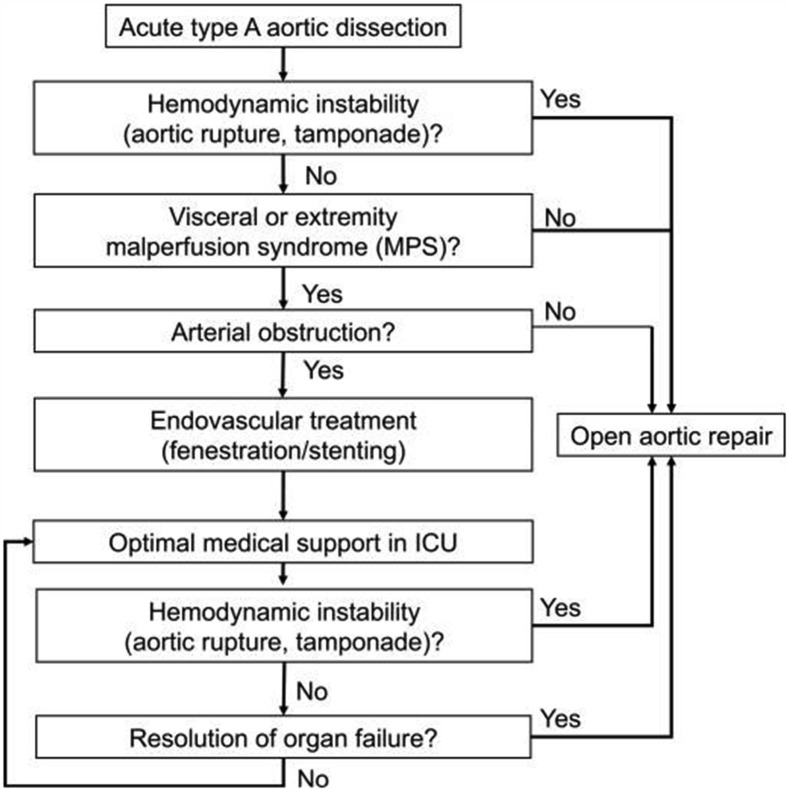
The University of Michigan group was first to describe a novel strategy of operative delay for the treatment of MPS in ATAAD. Their initial landmark study compared the traditional standard of care approach of immediate proximal aortic repair with a cohort managed with initial percutaneous intervention restoring true lumen flow, followed by delayed operative ATAAD repair after resolution of malperfusion injury.20 The mortality rate in the historical cohort treated with immediate aortic repair was 89% compared with only 25% in the group managed with delayed repair after restoring end-organ perfusion (P = .003). In the years since this initial series, these investigators have consistently shown the benefits of this strategy and have demonstrated a 95% success rate in treating malperfused vascular beds percutaneously.23 More current techniques, involving the use of thoracic endovascular aortic repair as the initial step for restoration of flow in MPS, have been successful in comparison with earlier fenestration-based strategies.24 , 25 Additionally, the provisional extension to induce complete attachment (PETTICOAT) technique has also been considered in ATAAD as a means of correcting malperfusion, although this technique requires further study.26 , 27 A standardized algorithm has been developed to summarize the approach to patients without aortic rupture or tamponade (Figure 3 ).28
Figure 3.
Algorithm for managing malperfusion syndromes in patients presenting with acute type A aortic dissection. (ICU, intensive care unit.) (Reproduced from Yang and colleagues,28 with permission from Wolters Kluwer Health, Inc.)
Importantly, the safety of various time periods for surgical delay in these patients has not been definitely shown. However, important patterns can be inferred from previous research on the timing of surgical repair. In 2013, Booher and colleagues29 used the International Registry of Acute Aortic Dissection (IRAD) database to examine a novel classification system for dissection timing. After controlling for delays to initial presentation, these investigators found that longer delays to operative repair were associated with lower follow-up mortality. In this context, their data suggest that patients with MPS who may not initially be surgical candidates but do survive initial MPS management may have favorable surgical outcomes if repair is undertaken in a delayed fashion. In some patients, this may even include delaying repair to the long-term setting in the event that their recovery from MPS is prolonged.
In summary, MPS carries a high risk of morbidity and mortality. Careful workup and patient selection are important in determining optimal procedural approaches to ATAAD repair in these individuals. Operative patients presenting with ATAAD and radiographic concern for malperfusion, but without clear evidence of resultant end-organ dysfunction, are still best treated with immediate ATAAD repair. In patients with MPS who are otherwise operative candidates, delayed repair of the ATAAD is recommended after reversing clinically apparent mesenteric or limb MPS in centers with adequate and timely access to these techniques. Otherwise, in patients with MPS and no evidence of tamponade, transfer to an institution with these capabilities may be the best approach.30
Cerebral Malperfusion
Patients with cerebral malperfusion (CM) represent a unique subset in which treatment decisions are particularly challenging. CM occurs in 7% to 15% of ATAAD cases and is associated with short-term mortality as high as 50% as well as poor long-term survival.31, 32, 33 A study from the IRAD database found that patients with CM who underwent surgery for ATAAD had a higher incidence of postoperative cerebrovascular accidents (17.5% vs 7.2%; P < .001) and in-hospital mortality (25.7% vs 12%; P < .001) than did patients without CM who underwent surgery.34 Hemorrhagic conversion of an ischemic insult during systemic anticoagulation for cardiopulmonary bypass is a significant concern that complicates decisions regarding immediate or delayed operative management.35 , 36
Despite the morbidity of CM in the setting of ATAAD, several studies have demonstrated that early intervention in these individuals can result in improved mortality and significant neurologic recovery.37, 38, 39 In a study by Di Eusanio and colleagues31 using data from IRAD, 84% of patients with stroke and 79% of patients presenting with coma had reversal of brain injury after surgery. Additionally, a recent multicenter study found that 62% of patients with preoperative neurologic deficit had no to moderate postoperative deficits.40 Notably, patient age (odds ratio, 1.041; P = .02) and history of previous stroke (odds ratio, 2.651; P = .03) were predictive of a poor clinical outcome; however, presentation with coma was not. Hemorrhagic conversion occurred in only 7 (5%) patients, and no independent predictors of this complication were identified.40
Patients with ATAAD and CM who undergo surgery should be carefully selected by an experienced multidisciplinary team on the basis of age, frailty, comorbidities, hemodynamic stability, and extent of other MPSs. Preoperative cerebral imaging can aid prognostication because the demonstration of a large infarct or an occluded internal carotid artery may predict a worse neurologic outcome.40 When experienced clinical evaluation otherwise portends a favorable prognosis, early surgery may be performed with reasonable rates of survival and reversal of cerebral ischemia, including patients presenting with coma. Ultimately, this is an evolving realm, and given recent data, including case reports describing advances in percutaneous intervention before surgery, decisions should be considered on a case-by-case basis.40 , 41
Previous Cardiac Surgery
Sternal reentry in patients with previous cardiac surgery poses technical challenges associated with mediastinal adhesions, increased bleeding, and a risk of injury to existing bypass grafts if the earlier operation included coronary bypass grafting.42 Although there were previous thoughts that fibrotic scarring from earlier surgery would provide some protection from tamponade and rupture in ATAAD, this has not consistently proved to be the case in larger database studies.43
Several studies have examined the outcomes of patients with previous cardiac surgery who underwent repair of ATAAD. One such study found no significant difference in 30-day morbidity or mortality in 50 patients who had previous cardiac surgery as compared with patients from the same era without earlier surgery.44 In contrast, in a more recent, much larger study using the Society of Thoracic Surgeons (STS) Adult Cardiac Surgery Database from 2002 to 2017, ATAAD repair in patients with previous cardiac surgery was associated with a greater than 2-fold higher risk of mortality compared with patients without previous cardiac surgery (odds ratio, 2.1; P < .01).45 There was a trend toward decreased operative mortality for patients with previous cardiac surgery who underwent ATAAD repair at high-volume centers (25.7% vs 37.9%; P = .19). In summary, the currently available evidence suggests that patients with ATAAD and a history of previous cardiac surgery require careful deliberation, adequate preoperative planning, and potential consideration for transfer to high-volume centers.
Novel or Non–Vitamin K Oral Anticoagulants
The use of novel or non–vitamin K oral anticoagulants (NOACs), also termed direct oral anticoagulants, has increased dramatically over the past decade.46 Given the absence of reliable reversal agents for several of these medications, they can present significant risks to patients requiring emergency cardiovascular surgery. Studies of their use related to ATAAD are limited to small, single-center experiences or case reports. Although adequate reversal of anticoagulation has been achieved in several cases, outcomes in general remain variable, and high-quality data are lacking.47 In 2018, Hamad and colleagues48 did report 2 successful cases of urgent reversal confirmed by thromboelastometry monitoring in patients taking rivaroxaban and dabigatran, whose procedures were delayed by 60 hours and 40 hours, respectively.
Antidotes for specific NOACs have been approved in recent years. Specifically, idarucizumab has been approved as a reversal agent for dabigatran, and andexanet alfa is approved as a reversal agent for rivaroxaban and apixaban.49 Idarucizumab, a monoclonal antibody fragment, was given before heart transplantation with useful effect in a small case series.50 Andexanet alfa acts as a factor Xa decoy, thus significantly reducing (but not eliminating) anticoagulant activity by binding and sequestering apixaban and rivaroxaban. Its use in the setting of emergency cardiac surgery, particularly with cardiopulmonary bypass, has been described in case reports but requires further investigation.51 Andexanet alfa reverses factor Xa inhibitor levels for approximately 2 to 3 hours, after which levels return to baseline.52
We recommend a multimodal approach in select patients who are taking NOACs and who require emergency surgery for ATAAD. Patient comorbidities and planned extent of operation should be carefully considered, as should other known risk factors for bleeding in proximal aortic repair.53 Depending on institutional capability, initial options include delayed surgical repair until half-life clearance of the agent, global coagulation assay, thromboelastometry assays, or preferably direct measurements of anti–factor Xa levels. Post–cardiopulmonary bypass options to treat coagulopathy include use of the following: antifibrinolytic agents; standard blood products such as platelets, fresh frozen plasma, and cryoprecipitate; recombinant factors such as prothrombin complex concentrate, activated factor VIIa, and human fibrinogen concentrate; and thoughtful administration of anti–factor Xa antidotes.54 , 55
Patient-Centered Decisions
Patient-centered decision making is of utmost importance in ATAAD, given the high-risk nature of this condition. Providers must have open and thoughtful discussion of risks and benefits of all available options, including medical management. Discussions of the use of blood products and overall goals of care are paramount in a patient-centered decision-making process.
Patients Who Refuse Blood Products
In rare cases, patients may wish to avoid transfusion of blood products for personal or religious reasons. Favorable outcomes have been demonstrated in studies of Jehovah’s Witness patients undergoing elective cardiac surgery with appropriate preoperative planning.56 , 57 However, in the setting of ATAAD, which is associated with a high rate of transfusion, the reluctance to receive blood products poses potentially lethal challenges in perioperative management.33 In select patients, delaying surgery to allow clear counseling on the risks of refusing transfusion may be necessary to obtain informed consent and optimize patient outcomes.
Importantly, one must not assume that any individual will uniformly refuse transfusion of any products until properly counseled on the available choices. Furthermore, treatment with purified proteins derived from plasma is acceptable to many patients.58 Options that may be acceptable to patients include treatment with albumin, activated factor VIIa, factor VIII inhibitor bypass activity, prothrombin complex concentrate, and human fibrinogen concentrate.59 Consideration should be given to limiting the scope of the operation with avoidance of prolonged cardiopulmonary bypass times and deep cooling, strict attention to surgical hemostasis, liberal use of recombinant hemostatic factors to facilitate clotting, and vigilant postoperative blood pressure control with early return to the operating room for surgical control of bleeding.60 Finally, initial medical management for stabilization and correction of the acute inflammatory cascade, followed by delayed surgical repair, may be appropriate in some individuals.61
Consideration for Goals of Care
Whether operative or nonoperative management is planned in patients with ATAAD, the patient and their family should be clearly counseled on “best-case and worst-case” scenarios, and clear goals of care discussions should be undertaken. In a recent Veterans Affairs study evaluating 95,204 patients who underwent high-risk surgery, only 770 (0.8%) received a palliative care consultation before surgery.62 Of all the patients who died within 90 days, 29.9% had received a palliative care consultation, and 5.6% received consultation before surgery. Families of these patients reported an overall significant increase in satisfaction with end-of-life care, communication, and support. Although this issue is challenging in the acute care setting, goals of care should always be carefully considered for patients presenting with ATAAD.
Triage Decisions in the Era of COVID-19
In addition to patient-level factors, the ongoing COVID-19 pandemic has highlighted how external forces and hospital resources can influence treatment decisions in patients undergoing complex procedures. As of September 14, 2020, there were more than 29 million confirmed cases worldwide, thus placing an immense strain on health care systems around the globe.63 Additionally, there has been evidence of significant decreases in the number of patients presenting with acute dissection. In New York, El-Hamamsy and colleagues64 reported a 76.5% decrease in expected case volume, thus raising concerns that patients may not be seeking timely care and suggesting the potential for increases in complex delayed presentations.64
In the setting of acute disease such as ATAAD, this pandemic has introduced an additional layer of complexity with regard to operative decision making. In particular, increasingly limited intensive care resources, redeployment of cardiovascular team members, and limitations on inpatient space have reduced the availability of several key elements required to perform resource-intensive ATAAD repair. Formal triage committees are being established in some jurisdictions to aid with challenging decisions regarding scarce resource distribution.65 Recently, the STS COVID-19 Taskforce released a guidance statement that considers 4 levels of tiered case triage on the basis of the impact of COVID-19 on hospital-wide resources.66 In the most extreme fourth tier, treatment of emergency conditions such as ATAAD is still performed according to resource availability; however, treatment in those patients who are considered stable and capable of waiting is deferred until adequate resources can be ensured. In cases of ATAAD in a resource-limited setting, transfer of hemodynamically stable individuals to hospitals with greater immediate capacity may be necessary. In an effort to help surgeons estimate and plan for resource allocation, STS has also launched a Resource Utilization Tool (https://www.sts.org/resources/resource-utilization-tool). This application allows surgeons to estimate operative time, ventilator time, length of stay, and other factors. These systems will be critically important for decisions regarding allocation of limited hospital resources.
Each health system and hospital will face varying pressures throughout this pandemic and will need to make difficult decisions regarding their ability to allocate resources and perform complex procedures.67 Currently, at both Brigham and Women’s Hospital in Boston, Massachusetts and Duke University Medical Center in Durham, North Carolina, patients needing urgent surgery, including ATAAD, are screened for symptoms or exposure to COVID-19, and a rapid (15- to 45-minute) COVID-19 test is sent on admission to the hospital. Barring any systemic resource availability concerns, the patient is taken without delay to the operating room, and the entire perioperative staff is provided with protective personal equipment, including N-95 masks and face shields, until the test results are available, which typically will occur before a skin incision is made. Importantly, there should be no COVID-related delay of transport to the operating room for otherwise operable patients with ATAAD.
Operative decision making must remain fluid in response to the constant changes induced by the current pandemic. In patients with ATAAD and complications such as tamponade or coronary ischemia, emergency repair remains the best available option. In patients with more stable presentations, as outlined earlier, surgeons may consider permanent deferral of surgery or temporary delay until adequate resource planning can be managed. Further, for the patient with ATAAD who tests positive for COVID-19, the decision whether to proceed with emergency surgery adds an additional layer of complexity to medical decision making. Reports of ATAAD repair in patients with COVID-19 are scarce, thereby limiting the delineation of any broad conclusions.68 , 69 However, published outcomes of other cardiothoracic procedures in patients with COVID-19 are bleak and highlight the extremely challenging decision-making process around best practice during this unprecedented pandemic.70
Current Status of Alternate Strategies
Although aortic valve resuspension with supracoronary ascending aorta and hemiarch replacement with circulatory arrest remains the gold standard approach in the majority of patients with ATAAD, other surgical options exist and may be of use in patients who cannot tolerate long, complex procedures. Replacement of the ascending aorta alone, without extension to the hemiarch or total arch, has been studied previously. Although this procedure is associated with higher 30-day mortality,13 it has been used in select individuals in whom there may be advantages to avoiding an open distal repair with circulatory arrest. Furthermore, whereas total arch replacement has been preferred in some high-volume centers for patients with arch vessel involvement, a more conservative approach may suffice in many circumstances.71
In patients who are not surgical candidates, medical management has traditionally been the only alternate strategy available. However, interventional catheter-based options have emerged over the past decade and may be of use in select individuals. Off-label and investigational applications of thoracic endovascular aortic repair in the ascending aorta have been applied to treat ATAAD. Even though this is a higher-risk group of patients, small studies have demonstrated technical success, early mortality rates lower than 15%, and relatively low aorta-related mortality rates in the long-term.72, 73, 74 Much more research and technologic innovation are required before endovascular repair can be considered for widespread use. However, there is promise for these techniques in patients at prohibitive risk for traditional ATAAD repair.
Finally, medical management remains an option for a significant proportion of patients with ATAAD who are not surgical candidates. Recent data from the IRAD database demonstrate that definitive medical management may be a reasonable option in certain high-risk patients, with 30-day survival rates of nearly 40% with medical management alone.75 Predictors of success with medical management in ATAAD have included previous cardiac surgery, normal admission chest roentgenogram, presenting hypertension, non-White race, and most proximal dissection extent limited to the ascending aorta without root involvement (Figure 4 ).75
Figure 4.
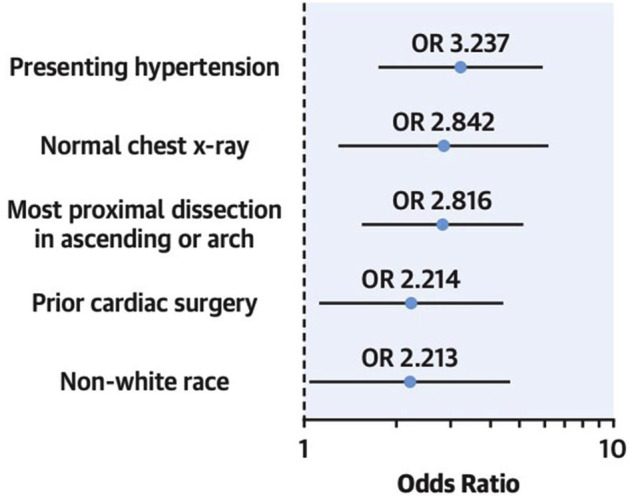
Factors influencing in-hospital survival in medically managed patients with acute type A aortic dissection. (OR, odds ratio.) (Reproduced from Wang and colleagues,75 with permission from Elsevier.)
Risk Stratification Tools
Considering the complex and high-risk presentations associated with ATAAD, risk stratification tools have been developed. The best validated of these tools is the Penn classification, which stratifies patients according to ischemic malperfusion pattern.76 Specifically, this system stratifies risk according to the presence or absence of branch-vessel malperfusion, circulatory collapse, or both. Although the Penn classification has performed well in validation studies, its clinical application remains challenging because of the broad range of presentations in patients with ATAAD. Another clinically based tool, developed from the IRAD database, incorporates preoperative and intraoperative variables to yield a simple risk model with very good utility for predicting death in ATAAD.77
These scoring systems are useful because they can offer rapid estimates of operative mortality; however, they should be used with caution and careful clinical correlation. Specifically, none of the current tools take into account frailty, the presence of other comorbidities, or the complexity of the required repair. A detailed assessment of all factors by an experienced clinical team, along with open and direct conversation with patients and their families, still provides the strongest foundation for decision making in this complex condition.
One important component that is recognized by both scoring systems is the concept of hemodynamic instability or circulatory collapse in the preoperative period. Several studies have found preoperative shock or hemodynamic instability to be associated with significantly higher operative mortality.78 , 79 In the IRAD tool, the presence of hypotension (systolic pressure <100 mm Hg) or shock with or without tamponade (systolic pressure <80 mm Hg) was independently predictive of operative mortality (odds ratio, 3.23).77 Furthermore, patients with cardiac arrest before surgery are at particularly high risk. In a recent study of patients with ATAAD who were undergoing cardiopulmonary resuscitation, Uehara and colleagues80 showed that duration of resuscitation longer than 15 minutes was an extremely strong independent predictor of operative mortality after surgical repair (hazard ratio, 8.27). In the context of these scoring systems, the presence of hemodynamic instability or collapse should be viewed as an ominous sign, with longer durations potentially serving as a relative contraindication to operative repair.
Comment
In conclusion, ATAAD remains a life-threatening condition, with or without surgery. Although our understanding of risk assessment has improved in recent years, clinical decisions remain complex and require a considered, multidisciplinary approach. Optimizing surgical outcomes mandates thoughtful patient selection informed by predicted survival postoperatively, as well as patient-centered discussions of goals of care. Timing of surgery requires a nuanced characterization of the severity and extent of dissection and the potential reversibility of MPSs. Further study in the area of endovascular techniques may improve outcomes in this complex group. In the modern era, with the challenges of resource allocation highlighted by COVID-19, we must hasten our collective understanding and investigation into determining when patients may benefit from surgical delay or permanent deferral of surgery altogether and when transfer to specialized centers may be warranted.
References
- 1.Bonser R.S., Ranasinghe A.M., Loubani M., et al. Evidence, lack of evidence, controversy, and debate in the provision and performance of the surgery of acute type A aortic dissection. J Am Coll Cardiol. 2011;58:2455–2474. doi: 10.1016/j.jacc.2011.06.067. [DOI] [PubMed] [Google Scholar]
- 2.Ehrlich M.P., Ergin M.A., McCullough J.N., et al. Results of immediate surgical treatment of all acute type A dissections. Circulation. 2000;102(suppl 3):III248–III252. doi: 10.1161/01.cir.102.suppl_3.iii-248. [DOI] [PubMed] [Google Scholar]
- 3.Olsson C., Thelin S., Stahle E. Thoracic aortic aneurysm and dissection: increasing prevalence and improved outcomes reported in a nationwide population-based study of more than 14,000 cases from 1987 to 2002. J Vasc Surg. 2007;46:609. doi: 10.1161/CIRCULATIONAHA.106.630400. [DOI] [PubMed] [Google Scholar]
- 4.Kawahito K., Adachi H., Yamaguchi A., Ino T. Preoperative risk factors for hospital mortality in acute type A aortic dissection. Ann Thorac Surg. 2001;71:1239–1243. doi: 10.1016/s0003-4975(00)02654-0. [DOI] [PubMed] [Google Scholar]
- 5.Olsson C. Modifiable risk factors for early mortality in low-risk Penn class Aa acute type A aortic dissection patients -- a descriptive study. Aorta (Stamford) 2017;5:117–123. doi: 10.12945/j.aorta.2017.17.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Trimarchi S., Nienaber C.A., Rampoldi V. Contemporary results of surgery in acute type A aortic dissection: the International Registry of Acute Aortic Dissection Experience. ACC Curr J Rev. 2005;14:45–46. doi: 10.1016/j.jtcvs.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Nienaber C.A., Sakalihasan N., Clough R.E., et al. Thoracic endovascular aortic repair (TEVAR) in proximal (type A) aortic dissection: ready for a broader application? J Thorac Cardiovasc Surg. 2017;153:S3–S11. doi: 10.1016/j.jtcvs.2016.07.078. [DOI] [PubMed] [Google Scholar]
- 8.Nassar A.H., Zern N.K., McIntyre L.K., et al. Emergency restructuring of a general surgery residency program during the coronavirus disease 2019 pandemic: the University of Washington experience. JAMA Surg. 2020;155:624–627. doi: 10.1001/jamasurg.2020.1219. [DOI] [PubMed] [Google Scholar]
- 9.Rockwood K., Song X., MacKnight C., et al. A global clinical measure of fitness and frailty in elderly people. CMAJ. 2005;173:489–495. doi: 10.1503/cmaj.050051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ganapathi A.M., Englum B.R., Hanna J.M., et al. Frailty and risk in proximal aortic surgery. J Thorac Cardiovasc Surg. 2014;147:186–191.e1. doi: 10.1016/j.jtcvs.2013.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Williams J.B., Peterson E.D., Zhao Y., et al. Contemporary results for proximal aortic replacement in North America. J Am Coll Cardiol. 2012;60:1156–1162. doi: 10.1016/j.jacc.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chiappini B., Schepens M., Tan E., et al. Early and late outcomes of acute type A aortic dissection: analysis of risk factors in 487 consecutive patients. Eur Heart J. 2005;26:180–186. doi: 10.1093/eurheartj/ehi024. [DOI] [PubMed] [Google Scholar]
- 13.Helder M.R.K., Schaff H.V., Day C.N., et al. Regional and temporal trends in the outcomes of repairs for acute type A aortic dissections. Ann Thorac Surg. 2020;109:26–33. doi: 10.1016/j.athoracsur.2019.06.058. [DOI] [PubMed] [Google Scholar]
- 14.Ullery B.W., Peterson J.C., Milla F., et al. Cardiac surgery in select nonagenarians: should we or shouldn’t we? Ann Thorac Surg. 2008;85:854–860. doi: 10.1016/j.athoracsur.2007.10.074. [DOI] [PubMed] [Google Scholar]
- 15.Tochii M., Takami Y., Hattori K., et al. Early and late otcomes of surgical repair for Stanford A acute aortic dissection in octogenarians. Circ J. 2016;80:2468–2472. doi: 10.1253/circj.CJ-16-0918. [DOI] [PubMed] [Google Scholar]
- 16.Kawahito K., Adachi H., Yamaguchi A., Ino T. Early and late surgical outcomes of acute type A aortic dissection in patients aged 75 years and older. Ann Thorac Surg. 2000;70:1455–1459. doi: 10.1016/s0003-4975(00)01934-2. [DOI] [PubMed] [Google Scholar]
- 17.Dumfarth J., Peterss S., Luehr M., et al. Acute type A dissection in octogenarians: does emergency surgery impact in-hospital outcome or long-term survival? Eur J Cardiothorac Surg. 2017;51:472–477. doi: 10.1093/ejcts/ezw387. [DOI] [PubMed] [Google Scholar]
- 18.Orihashi K. Malperfusion in acute type A aortic dissection: unsolved problem. Ann Thorac Surg. 2013;95:1570–1576. doi: 10.1016/j.athoracsur.2013.02.025. [DOI] [PubMed] [Google Scholar]
- 19.Lawton J.S., Moon M.R., Liu J., et al. The profound impact of combined severe acidosis and malperfusion on operative mortality in the surgical treatment of type A aortic dissection. J Thorac Cardiovasc Surg. 2018;155:897–904. doi: 10.1016/j.jtcvs.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Deeb G.M., Michael Deeb G., Williams D.M., et al. Surgical delay for acute type A dissection with malperfusion. Ann Thorac Surg. 1997;64:1669–1677. doi: 10.1016/s0003-4975(97)01100-4. [DOI] [PubMed] [Google Scholar]
- 21.Yang B., Norton E.L., Rosati C.M., et al. Managing patients with acute type A aortic dissection and mesenteric malperfusion syndrome: a 20-year experience. J Thorac Cardiovasc Surg. 2018;158:675–687. doi: 10.1016/j.jtcvs.2018.11.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Eusanio M., Trimarchi S., Patel H.J., et al. Clinical presentation, management, and short-term outcome of patients with type A acute dissection complicated by mesenteric malperfusion: observations from the International Registry of Acute Aortic Dissection. J Thorac Cardiovasc Surg. 2013;145:385–390.e1. doi: 10.1016/j.jtcvs.2012.01.042. [DOI] [PubMed] [Google Scholar]
- 23.Deeb G.M., Michael Deeb G., Patel H.J., Williams D.M. Treatment for malperfusion syndrome in acute type A and B aortic dissection: a long-term analysis. J Thorac Cardiovasc Surg. 2010;140:S98–S100. doi: 10.1016/j.jtcvs.2010.07.036. [DOI] [PubMed] [Google Scholar]
- 24.Leshnower B.G., Veeraswamy R.K., Duwayri Y.M., Chen E.P. The TEVAR-first approach to DeBakey I aortic dissection with mesenteric malperfusion. Ann Thorac Surg. 2014;97:693–696. doi: 10.1016/j.athoracsur.2013.06.110. [DOI] [PubMed] [Google Scholar]
- 25.Lombardi J.V., Gleason T.G., Panneton J.M., et al. STABLE II clinical trial on endovascular treatment of acute, complicated type B aortic dissection with a composite device design. J Vasc Surg. 2020;71:1077–1087.e2. doi: 10.1016/j.jvs.2019.06.189. [DOI] [PubMed] [Google Scholar]
- 26.Kotha V.K., Pozeg Z., Herget E., Moon M., Appoo J. Early results of the PETTICOAT technique for the management of acute type A aortic dissection. Aorta (Stamford) 2017;5:124–128. doi: 10.12945/j.aorta.2017.17.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lopes A., Gouveia Melo R., Gomes M.L., et al. Aortic dissection repair using the STABILISE technique associated with arch procedures: report of two cases. EJVES Short Rep. 2019;42:26–30. doi: 10.1016/j.ejvssr.2019.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang B., Rosati C.M., Norton E.L., et al. Endovascular fenestration/stenting first followed by delayed open aortic repair for acute type A aortic dissection with malperfusion syndrome. Circulation. 2018;138:2091–2103. doi: 10.1161/CIRCULATIONAHA.118.036328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Booher A.M., Isselbacher E.M., Nienaber C.A., et al. The IRAD classification system for characterizing survival after aortic dissection. Am J Med. 2013;126:730.e19–730.e24. doi: 10.1016/j.amjmed.2013.01.020. [DOI] [PubMed] [Google Scholar]
- 30.Goldstone A.B., Chiu P., Baiocchi M., et al. Interfacility transfer of Medicare beneficiaries with acute type A aortic dissection and regionalization of care in the United States. Circulation. 2019;140:1239–1250. doi: 10.1161/CIRCULATIONAHA.118.038867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Di Eusanio M., Patel H.J., Nienaber C.A., et al. Patients with type A acute aortic dissection presenting with major brain injury: should we operate on them? J Thorac Cardiovasc Surg. 2013;145:S213–S221.e1. doi: 10.1016/j.jtcvs.2012.11.054. [DOI] [PubMed] [Google Scholar]
- 32.Geirsson A., Szeto W.Y., Pochettino A., et al. Significance of malperfusion syndromes prior to contemporary surgical repair for acute type A dissection: outcomes and need for additional revascularizations. Eur J Cardiothorac Surg. 2007;32:255–262. doi: 10.1016/j.ejcts.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 33.Zindovic I., Gudbjartsson T., Ahlsson A., et al. Malperfusion in acute type A aortic dissection: an update from the Nordic Consortium for Acute Type A Aortic Dissection. J Thorac Cardiovasc Surg. 2019;157:1324–1333.e6. doi: 10.1016/j.jtcvs.2018.10.134. [DOI] [PubMed] [Google Scholar]
- 34.Sultan I., Bianco V., Patel H.J., et al. Surgery for type A aortic dissection in patients with cerebral malperfusion: results from the International Registry of Acute Aortic Dissection. J Thorac Cardiovasc Surg. 2021;161:1713–1720.e1. doi: 10.1016/j.jtcvs.2019.11.003. [DOI] [PubMed] [Google Scholar]
- 35.Tsukube T., Hayashi T., Kawahira T., et al. Neurological outcomes after immediate aortic repair for acute type A aortic dissection complicated by coma. Circulation. 2011;124(11 Suppl):S163–S167. doi: 10.1161/CIRCULATIONAHA.110.011551. [DOI] [PubMed] [Google Scholar]
- 36.Estrera A.L., Garami Z., Miller C.C., et al. Acute type A aortic dissection complicated by stroke: can immediate repair be performed safely? J Thorac Cardiovasc Surg. 2006;132:1404–1408. doi: 10.1016/j.jtcvs.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 37.Conzelmann L.O., Hoffmann I., Blettner M., et al. Analysis of risk factors for neurological dysfunction in patients with acute aortic dissection type A: data from the German Registry for Acute Aortic Dissection type A (GERAADA) Eur J Cardiothorac Surg. 2012;42:557–565. doi: 10.1093/ejcts/ezs025. [DOI] [PubMed] [Google Scholar]
- 38.Estrera A.L., Huynh T.T.T., Porat E.E., Miller C.C., 3rd, Smith J.J., Safi H.J. Is acute type A aortic dissection a true surgical emergency? Semin Vasc Surg. 2002;15:75–82. doi: 10.1053/svas.2002.33093. [DOI] [PubMed] [Google Scholar]
- 39.Leontyev S., Légaré J.-F., Borger M.A., et al. Creation of a scorecard to predict in-hospital death in patients undergoing operations for acute type A aortic dissection. Ann Thorac Surg. 2016;101:1700–1706. doi: 10.1016/j.athoracsur.2015.10.038. [DOI] [PubMed] [Google Scholar]
- 40.Kreibich M., Desai N.D., Bavaria J.E., et al. Preoperative neurological deficit in acute type A aortic dissection. Interact Cardiovasc Thorac Surg. 2020;30:613–619. doi: 10.1093/icvts/ivz311. [DOI] [PubMed] [Google Scholar]
- 41.Heran M.K.S., Balaji N., Cook R.C. Novel percutaneous treatment of cerebral malperfusion before surgery for acute type A dissection. Ann Thorac Surg. 2019;108:e15–e17. doi: 10.1016/j.athoracsur.2018.10.055. [DOI] [PubMed] [Google Scholar]
- 42.Imran Hamid U., Digney R., Soo L., Leung S., Graham A.N.J. Incidence and outcome of re-entry injury in redo cardiac surgery: benefits of preoperative planning. Eur J Cardiothorac Surg. 2015;47:819–823. doi: 10.1093/ejcts/ezu261. [DOI] [PubMed] [Google Scholar]
- 43.Teman N.R., Peterson M.D., Russo M.J., et al. Outcomes of patients presenting with acute type A aortic dissection in the setting of prior cardiac surgery: an analysis from the International Registry of Acute Aortic Dissection. Circulation. 2013;128(11 suppl 1):S180–S185. doi: 10.1161/CIRCULATIONAHA.112.000342. [DOI] [PubMed] [Google Scholar]
- 44.Norton E.L., Rosati C.M., Kim K.M., et al. Is previous cardiac surgery a risk factor for open repair of acute type A aortic dissection? J Thorac Cardiovasc Surg. 2020;160:8–17.e1. doi: 10.1016/j.jtcvs.2019.07.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krebs E.D., Mehaffey J.H., Hawkins R.B., et al. Outcomes after acute type A aortic dissection in patients with prior cardiac surgery. Ann Thorac Surg. 2019;108:708–713. doi: 10.1016/j.athoracsur.2019.02.065. [DOI] [PubMed] [Google Scholar]
- 46.Rodwin B.A., Salami J.A., Spatz E.S., et al. Variation in the use of warfarin and direct oral anticoagulants in atrial fibrillation and associated cost implications. Am J Med. 2019;132:61–70.e1. doi: 10.1016/j.amjmed.2018.09.026. [DOI] [PubMed] [Google Scholar]
- 47.Zimmermann E., Ameer F., Worku B., Avgerinos D. Reversal of direct-acting oral anticoagulants in urgent surgery of the proximal aorta: case series and review of the literature. Curr Pharm Des. 2018;24:4534–4539. doi: 10.2174/1381612825666181226150006. [DOI] [PubMed] [Google Scholar]
- 48.Hamad R., Amr G., Demers P. Delayed surgery in patients with acute type A aortic dissection who are receiving novel oral anticoagulants. J Thorac Cardiovasc Surg. 2018;115:e1–e4. doi: 10.1016/j.jtcvs.2017.08.021. [DOI] [PubMed] [Google Scholar]
- 49.Desai N.R., Cornutt D. Reversal agents for direct oral anticoagulants: considerations for hospital physicians and intensivists. Hosp Pract (1995) 2019;47:113–122. doi: 10.1080/21548331.2019.1643728. [DOI] [PubMed] [Google Scholar]
- 50.Tralhão A., Aguiar C., Ferreira J., et al. Dabigatran reversal with idarucizumab in a patient undergoing heart transplantation: first European report. Thromb J. 2017;15:23. doi: 10.1186/s12959-017-0147-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Flaherty D., Connors J.M., Singh S., Sylvester K.W., Rimsans J., Cornella L. Andexanet alfa for urgent reversal of apixaban before aortic surgery requiring cardiopulmonary bypass. A A Pract. 2019;13:271–273. doi: 10.1213/XAA.0000000000001052. [DOI] [PubMed] [Google Scholar]
- 52.Levy J.H., Douketis J., Weitz J.I. Reversal agents for non-vitamin K antagonist oral anticoagulants. Nat Rev Cardiol. 2018;15:273–281. doi: 10.1038/nrcardio.2017.223. [DOI] [PubMed] [Google Scholar]
- 53.Williams J.B., Phillips-Bute B., Bhattacharya S.D., et al. Predictors of massive transfusion with thoracic aortic procedures involving deep hypothermic circulatory arrest. J Thorac Cardiovasc Surg. 2011;141:1283–1288. doi: 10.1016/j.jtcvs.2010.07.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wagner M.A., Wang H., Benrashid E., et al. Protocolized hemostatic factor use in major thoracic aortic surgery. J Cardiovasc Surg (Torino) 2019;60:633–636. doi: 10.23736/S0021-9509.19.10819-1. [DOI] [PubMed] [Google Scholar]
- 55.Schultz N.H., Lundblad R., Holme P.A. Activated prothrombin complex concentrate to reverse the factor Xa inhibitor (apixaban) effect before emergency surgery: a case series. J Med Case Rep. 2018;12:138. doi: 10.1186/s13256-018-1660-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jassar A.S., Ford P.A., Haber H.L., et al. Cardiac surgery in Jehovah’s Witness patients: ten-year experience. Ann Thorac Surg. 2012;93:19–25. doi: 10.1016/j.athoracsur.2011.06.029. [DOI] [PubMed] [Google Scholar]
- 57.Juraszek A., Kołsut P., Szymański J., et al. Results of open heart surgery in Jehovah’s Witness patients. Single centre experience. Kardiochir Torakochirurgia Pol. 2017;14:164–169. doi: 10.5114/kitp.2017.70529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hughes D.B., Ullery B.W., Barie P.S. The contemporary approach to the care of Jehovah’s witnesses. J Trauma. 2008;65:237–247. doi: 10.1097/TA.0b013e318176cc66. [DOI] [PubMed] [Google Scholar]
- 59.Harris J.E., Varnado S., Herrera E., Salazar E., Colavecchia A.C. Evaluation of postoperative clinical outcomes in Jehovah’s Witness patients who receive prothrombin complex concentrate during cardiac surgery. J Card Surg. 2020;35:801–809. doi: 10.1111/jocs.14463. [DOI] [PubMed] [Google Scholar]
- 60.Pasic M., Ruisz W., Koster A., Hetzer R. Bloodless surgery of acute type A aortic dissection in a Jehovah’s Witness patient. Ann Thorac Surg. 2005;80:1507–1510. doi: 10.1016/j.athoracsur.2004.03.044. [DOI] [PubMed] [Google Scholar]
- 61.DeAnda A., Volman R., Spiess B.D. Repair of type A dissection in a Jehovah’s Witness with prior cardiac operation. Ann Thorac Surg. 2009;87:289–290. doi: 10.1016/j.athoracsur.2008.09.072. [DOI] [PubMed] [Google Scholar]
- 62.Yefimova M., Aslakson R.A., Yang L., et al. Palliative care and end-of-life outcomes following high-risk surgery. JAMA Surg. 2020;155:138–146. doi: 10.1001/jamasurg.2019.5083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.El-Hamamsy I., Brinster D., DeRose J., et al. The COVID-19 pandemic and acute aortic dissections in New York: a matter of public health. J Am Coll Cardiol. 2020;76:227–229. doi: 10.1016/j.jacc.2020.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Truog R.D., Mitchell C., Daley G.Q. The toughest triage — allocating ventilators in a pandemic. N Engl J Med. 2020;382:1973–1975. doi: 10.1056/NEJMp2005689. [DOI] [PubMed] [Google Scholar]
- 66.Haft J.W., Atluri P., Alawadi G., et al. Adult cardiac surgery during the COVID-19 pandemic: a tiered patient triage guidance statement. Ann Thorac Surg. 2020;110:697–700. doi: 10.1016/j.athoracsur.2020.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Emanuel E.J., Persad G., Upshur R., et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020;382:2049–2055. doi: 10.1056/NEJMsb2005114. [DOI] [PubMed] [Google Scholar]
- 68.Fukuhara S., Rosati C.M., El-Dalati S. Acute type A aortic dissection during the COVID-19 outbreak. Ann Thorac Surg. 2020;110:e405–e407. doi: 10.1016/j.athoracsur.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Martens T., Vande Weygaerde Y., Vermassen J., Malfait T. Acute type A aortic dissection complicated by COVID-19 infection. Ann Thorac Surg. 2020;110:e421–e423. doi: 10.1016/j.athoracsur.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang J., Wang A., Kang G., Li D., Hu W. Clinical course of patients infected with SARS-CoV-2 soon after thoracoscopic lung surgery. J Thorac Cardiovasc Surg. 2020;160:e91–e93. doi: 10.1016/j.jtcvs.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Norton E.L., Wu X., Farhat L., et al. Dissection of arch branches alone: an indication for aggressive arch management in type A dissection? Ann Thorac Surg. 2020;109:487–494. doi: 10.1016/j.athoracsur.2019.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Muetterties C.E., Menon R., Wheatley G.H. A systematic review of primary endovascular repair of the ascending aorta. J Vasc Surg. 2018;67:332–342. doi: 10.1016/j.jvs.2017.06.099. [DOI] [PubMed] [Google Scholar]
- 73.Plichta R.P., Hughes G.C. Thoracic endovascular aortic repair for the ascending aorta: experience and pitfalls. J Vis Surg. 2018;4:92. doi: 10.21037/jovs.2018.03.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li Z., Lu Q., Feng R., et al. Outcomes of endovascular repair of ascending aortic dissection in patients unsuitable for direct surgical repair. J Am Coll Cardiol. 2016;68:1944–1954. doi: 10.1016/j.jacc.2016.08.031. [DOI] [PubMed] [Google Scholar]
- 75.Wang A., Montgomery D., Brinster D.R., et al. Predicting in-hospital survival in acute type A aortic dissection medically treated. J Am Coll Cardiol. 2020;75:1360–1361. doi: 10.1016/j.jacc.2020.01.015. [DOI] [PubMed] [Google Scholar]
- 76.Olsson C., Hillebrant C.G., Liska J., Lockowandt U., Eriksson P., Franco-Cereceda A. Mortality in acute type A aortic dissection: validation of the Penn classification. Ann Thorac Surg. 2011;92:1376–1382. doi: 10.1016/j.athoracsur.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 77.Rampoldi V., Trimarchi S., Eagle K.A., et al. Simple risk models to predict surgical mortality in acute type A aortic dissection: the International Registry of Acute Aortic Dissection Score. Ann Thorac Surg. 2007;83:55–61. doi: 10.1016/j.athoracsur.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 78.Long S., Tribble C., Raymond D., Fiser S., et al. Preoperative shock determines outcome for acute type A aortic dissection. Ann Thorac Surg. 2003;75:520–524. doi: 10.1016/s0003-4975(02)04536-8. [DOI] [PubMed] [Google Scholar]
- 79.Zindovic I., Sjogren J., Bjursten H., Danielsson E., Ingemansson R., Nozohoor S. Impact of hemodynamic instability and organ malperfusion in elderly surgical patients treated for acute type A aortic dissection. J Card Surg. 2015;30:822–829. doi: 10.1111/jocs.12633. [DOI] [PubMed] [Google Scholar]
- 80.Uehara K., Matsuda H., Matsuo J., et al. Surgical outcomes of acute type A aortic dissection in patients undergoing cardiopulmonary resuscitation. J Thorac Cardiovasc Surg. 2021;161:1173–1180. doi: 10.1016/j.jtcvs.2019.11.135. [DOI] [PubMed] [Google Scholar]