Abstract
Recent advances indicate that periodontitis is driven by reciprocally reinforced interactions between a dysbiotic microbiome and dysregulated inflammation. Inflammation is not only a consequence of dysbiosis but, via mediating tissue dysfunction and damage, fuels further growth of selectively dysbiotic communities of bacteria (inflammophiles), thereby generating a self-sustained feed-forward loop that perpetuates the disease. These considerations provide a strong rationale for developing adjunctive host-modulation therapies for the treatment of periodontitis. Such host-modulation approaches aim to inhibit harmful inflammation and promote its resolution or to directly interfere with downstream effectors of connective tissue and bone destruction. This paper reviews diverse strategies to modulate the host periodontal response and discusses their mechanisms of action, perceived safety and potential for clinical application.
Introduction
Chronic periodontitis is clinically characterized by loss of gingival tissue attachment to the tooth, deepening of the gingival crevice (designated ‘periodontal pocket’ in periodontitis), degradation of the periodontal ligament and loss of alveolar bone 1. This destructive process is associated with the presence of subgingival microbial communities and a dense immuno-inflammatory infiltrate in the periodontium that may lead to tooth loss if not appropriately treated. In gingivitis, a reversible form of periodontal disease that does not result in bone loss, the inflammatory process is restricted to the gingival epithelium and the connective tissue without affecting the deeper compartments of the periodontium 1. However, it should be noted that gingivitis is a major risk factor and a necessary pre-requisite for periodontitis and, moreover, can increase the serum levels of inflammatory biomarkers such as C-reactive protein 2–5.
The first systematic model to describe the temporal development of host response events leading to the development of periodontitis dates back in the 1970s when Roy Page and Hubert Schroeder defined four types of histopathologic lesions: the ‘initial’, ‘early’ and ‘established’ lesions exemplifying distinct and sequential stages of gingivitis and the ‘advanced’ lesion featuring bone loss and clinically manifested as periodontitis6. The development of periodontitis correlated with increased complexity of the cellular infiltrate composition from one dominated by neutrophils (initial lesion) to one containing elevated numbers of macrophages and T cells (early lesion) and, additionally, B and plasma cells that predominate in the established and advanced lesions 6. Subsequent discoveries including the characterization of specialized subsets of innate and adaptive leukocytes and the dissection of their crosstalk interactions has offered a more nuanced and mechanistic understanding of periodontal disease pathogenesis with profound implications for treatment 7.
The host immune response to the subgingival tooth-associated biofilm can be potentially protective, thus maintaining balanced host-microbe interactions and a healthy periodontium (‘tissue homeostasis’) 8. Besides the microbiota, additional challenges or potential insults that may contribute to the induction of local immunity include ongoing damage from mastication and perhaps dietary and airborne allergens/particles. In this regard, a predominantly T cell-rich infiltrate and a network of antigen-presenting cells (macrophages, dendritic cells) as well as neutrophils constantly and proactively patrol the periodontium. In contrast, a clinically healthy periodontium contains minimal numbers of B cells and plasma cells and, moreover, the neutrophils present are at lower numbers than in gingivitis or periodontitis. A small population of γδ T cells and of innate lymphoid cells may also be seen in healthy gingiva and are thought to contribute to protective immunity and maintenance of tissue homeostasis 8,9.
However, in individuals with susceptibility to periodontitis, the host response is ineffective, dysregulated, and destructive 10. Whereas the bacteria are required for disease pathogenesis, it is predominantly the host inflammatory response to this microbial challenge that can ultimately inflict damage upon the periodontal tissues 7,11. Resistance or susceptibility to periodontitis appears to be determined by multiple factors that may modify the host response in either a protective or a destructive direction. Such factors include genetic, epigenetic, environmental (such as smoking, stress, and diet), aging, and systemic diseases such as diabetes 12–18 (Figure 1). Observations that gingivitis in certain individuals may remain stable indefinitely, i.e., without necessarily progressing to periodontitis 6, is consistent with the notion that periodontitis requires a susceptible host.
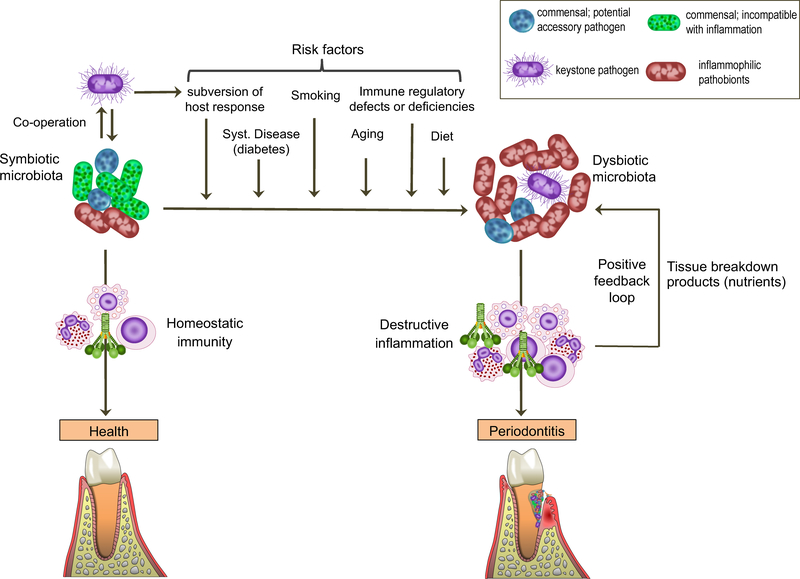
Figure 1. Periodontal disease pathogenesis.
Periodontal health is maintained by homeostatic immunity and is associated with symbiotic microbiota. Periodontitis is associated with a dysbiotic polymicrobial community, in which different members have distinct and synergistic roles that promote destructive inflammation. Keystone pathogens — which are aided by accessory pathogens in terms of nutritional and/or colonization support — initially subvert host immunity leading to the emergence of dysbiotic microbiota, in which commensal-turned pathobionts overactivate the inflammatory response and cause tissue destruction. Inflammation in turn can exacerbate dysbiosis through provision of nutrients for the bacteria (derived from tissue breakdown products; e.g., collagen peptides and heme-containing compounds). Therefore, inflammation and dysbiosis are reciprocally reinforced and generate a positive-feedback loop. This self-sustaining loop may underlie the chronicity of periodontitis, the development of which requires a susceptible host. Risk factors include (but are not limited to) the presence of bacteria that subvert the host response, systemic disease, smoking, aging, high-fat diet, and immune deficiencies. These factors could promote dysbiosis by acting individually or more effectively in combination.
Recent microbiome and mechanistic studies have offered an improved understanding of the nature of host-microbe interactions in periodontitis. Specifically, such studies in humans and animal models have revealed that (i) the periodontitis-associated microbiota is considerably more diverse and complex than previously thought and (ii) the bacteria involved act in disease through polymicrobial synergy and dysbiosis 19–30. In other words, periodontitis is not caused by a single or a select few bacterial species (‘periodontopathogens’) and does not appear to qualify as an infection in the classical sense of the term. Rather, periodontitis is associated with dysbiosis, i.e., an alteration in the abundance or influence of individual species within the polymicrobial community, relative to their abundance or influence in health. Whereas dysbiosis can lead to destructive inflammation, the reverse is also true. In this respect, inflammatory tissue breakdown products (e.g., degraded collagen and heme-containing compounds, sources of amino-acids and iron, respectively) are released into the gingival crevicular fluid. In the gingival crevice/pocket, these inflammatory spoils can be used as nutrients to fuel the selective expansion of a subset of bacterial species (e.g., proteolytic and asaccharolytic pathobionts), thereby exacerbating the microbiota imbalance (dysbiosis) 30–32. In this regard, the addition of serum, hemoglobin, or hemin to an in vitro generated oral multispecies community selectively induces the outgrowth of pathobionts, which interestingly upregulate genes that encode proteases, hemolysins, and molecules involved in hemin acquisition 33. This conversion of the original ‘homeostatic’ community into a ‘dysbiotic’ one can also increase the community’s proinflammatory potential 33. Thus, an initial inflammatory response to subgingival biofilm development (for instance, due to absence of adequate oral hygiene) may select for ‘inflammophilic’ pathobiotic bacteria (incipient dysbiosis), which, upon further growth in a nutritionally favorable inflammatory environment, can further exacerbate inflammation. This feed-forward loop of dysbiosis and inflammation can ultimately cause overt periodontitis in susceptible individuals (Figure 1).
If left untreated, periodontitis can not only lead to tooth loss but may also affect mastication, esthetics and the quality of life 34–36. Almost 50% adults are affected by some form of periodontal disease (ranging from mild to severe) with nearly 10% being afflicted by severe periodontitis 37–39. Although highly variable between different communities, the prevalence of gingivitis is quite high (can reach above 80% in some communities) 40–42. Current standard-of-care therapy in both gingivitis and periodontitis aims to remove the pathogenic microbial biofilm through mechanical debridement (scaling and root planing). However, scaling and root planing is only partially effective for the majority of the periodontitis patients and some individuals (‘refractory periodontitis patients’) do not respond favorably to scaling and root planing 43. Therefore, periodontitis represents a significant health and economic burden 35,44,45. Since tissue damage in periodontitis is mediated primarily by the host inflammatory response, which moreover is exploited by the dysbiotic microbial community for growth and persistence, it can be reasoned that host-response modulation approaches may be promising adjunctive treatments to conventional periodontal therapy. The main objective of this review is to summarize and discuss host-modulation therapies in periodontitis, some of which have already been tested in humans, whereas many more are presently being pursued at a preclinical level. An emphasis will be placed in their mechanisms of action and on their perceived safety and potential for clinical application. The relevance and rationale of the discussed therapeutic interventions can be better understood after a brief outline of the mechanisms of inflammation induction and resolution.
Inflammation and its resolution
Inflammation constitutes an important component of the overall biological response whereby host tissues attempt to cope with various threats, such as invading pathogens, damaged cells, and irritants. Through adaptive changes of the local vasculature and release of various soluble mediators that interact with neutrophils and other cell types, the main functions of inflammation are: (i) elimination of the initial cause of infection or cell injury; (ii) clearance of apoptotic and necrotic cells and debris; and (iii) initiation of tissue repair.
In response to tissue infection, injury or inflammation, neutrophils are the first cells to be recruited from the circulation to the afflicted site. The process of neutrophil extravasation involves a complex cascade of low- and high-affinity adhesive interactions of neutrophils with the endothelium 46–48. Briefly, circulating neutrophils initially engage in transient rolling interactions with the vascular endothelium, mediated by the binding of glycoprotein ligands on neutrophils to their endothelial cell-surface receptors (P- or E-selectin). This rolling-dependent deceleration of neutrophils enables their interaction with chemokines deposited on the luminal surface of endothelial cells 49. Chemokine- and selectin-induced signalling in neutrophils cooperatively induces their β2 integrins to adopt an extended and high-affinity conformation that can effectively bind their counter-receptors on endothelial cells, namely the intercellular adhesion molecules −1 and −2 50,51. The β2 integrins, heterodimeric molecules each containing a distinct CD11 subunit and a common CD18 subunit, are required for firm adhesion of neutrophils onto the endothelium and their subsequent crawling on the endothelial surface, which enables neutrophils to find appropriate sites for extravasation. Firm adhesion and intraluminal crawling are primarily mediated by the β2 integrins LFA-1 (CD11a/CD18) and Mac-1 (CD11b/CD18), respectively 46–48, and genetic deficiency in β2 integrins (owing to CD18 mutations; leukocyte adhesion deficiency) results in few or no neutrophils in peripheral tissues such as the gingiva 52. The function of β2 integrins and hence neutrophil extravasation can be physiologically regulated. A major such mechanism is mediated by a protein secreted by endothelial and other cells, the developmental endothelial locus-1 (Del-1). Del-1 binds LFA-1 and interferes with its adhesive function, thereby downregulating neutrophil recruitment to the periodontium and other peripheral tissues 53,54.
Besides chemokines, other important inflammatory mediators that interact with and activate neutrophils include arachidonic acid derivatives, such as prostaglandins and leukotrienes 55 and complement activation products, such as the anaphylatoxins C3a and C5a 56. The complement system contains some 50 fluid-phase, cell surface-associated, or intracellular proteins that trigger and regulate signaling pathways mediating immune surveillance and homeostasis 56,57. Complement activation is triggered through distinct initiation pathways (classical, lectin or alternative) all of which converge at the third component (C3) leading to enzymatic generation of effector molecules that facilitate the action of antibodies and phagocytes to clear microbial pathogens (via opsonization by C3b), promote recruitment and activation of inflammatory cells (via the C3a and C5a anaphylatoxins) and lyse susceptible pathogens (via the C5b-9 membrane attack complex). However, when complement is dysregulated or overactivated, it can drive or exacerbate the pathogenesis of a number of inflammatory diseases, including periodontitis 58.
Although traditionally associated with acute inflammation, neutrophils are now increasingly appreciated as major players in chronic inflammatory conditions, including atherosclerosis, psoriasis, rheumatoid arthritis, and periodontitis 59–61. In fact, neutrophils are functionally versatile and mediate previously unanticipated functions, including regulation of adaptive immune leukocytes 48,59,62,63. For instance, by releasing the chemokines (C-C motif) ligand 2 (CCL2) and ligand 20 (CCL20), neutrophils can recruit Th17 cells 64. Th17 are CD4+ T helper cells that release interleukin-17 at sites of inflammation and, in periodontitis, represent an osteoclastogenic subset that links T-cell activation to inflammatory bone loss 65. Moreover, neutrophils secrete B-lymphocyte stimulator and a proliferation-inducing ligand (APRIL), two key cytokines that promote the survival, proliferation, and maturation of B lymphocytes into plasma cells 66,67.
The histopathology of periodontitis involves elements of both innate and adaptive immunity. Neutrophils, antigen-presenting cells, and T and B lymphocytes form a sophisticated network of interactions between themselves and with humoral systems, such as complement, to stage immune and inflammatory responses in the periodontium 68–73. It is now well established that complement has functions above and beyond its traditional role of tagging and eliminating microbes. For instance, complement can amplify immune and inflammatory responses by synergizing with Toll-like receptors on innate leukocytes and regulate the activation and differentiation of B cells and T-cell subsets 56,57. In periodontitis, these complex interactions lead to inflammation-induced bone loss, which is largely mediated by a triad of proteins consisting of the receptor activator of nuclear factor-κB ligand (RANKL), its functional receptor RANK, and its decoy receptor osteoprotegerin. RANKL is produced by activated T and B lymphocytes as well as by osteoblasts in the inflamed periodontium 74. The binding of cell-surface or soluble RANKL to RANK on osteoclast precursors triggers osteoclast maturation and activation, although this RANKL/RANK-driven process is antagonized by the decoy receptor osteoprotegerin 7,75.
The ideal outcome of an inflammatory response is its timely termination so that it does not become chronic with potentially adverse effects. Indeed, non-resolving inflammation underlies the pathogenesis of many chronic conditions, including periodontitis 76. The successful resolution of inflammation is an active and well-coordinated process that involves a series of steps. These include down-regulation of proinflammatory mediators and upregulation of regulatory or pro-resolution mediators, termination of neutrophil recruitment, clearance of apoptotic neutrophils by tissue phagocytes (efferocytosis), and initiation of tissue repair 77,78.
A ‘lipid-mediator class switching’ takes place during the resolving phase of inflammation. This temporal switch signifies a transition from an environment rich in pro-inflammatory prostaglandins and leukotrienes to one with high levels of pro-resolving mediators, which include arachidonic acid-derived lipoxins and omega-3 polyunsaturated fatty acid-derived resolvins and protectins 11,79,80. Resolvins are derived from docosahexaenoic acid (D series resolvins) or eicosapentaenoic acid (E series resolvins). When released within the vascular lumen, lipoxins and resolvins can suppress neutrophil transmigration to tissues through several mechanisms, such as modulation of adhesive molecules in both neutrophils and the endothelium. For example, lipoxin A4 blocks leukotriene- or peptidoleukotriene-dependent neutrophil adhesion by down-regulating the expression of P-selectin on endothelial cells and of Mac-1 (CD11b/CD18) on neutrophils 81,82. Additionally, lipoxins and resolvins can inhibit neutrophil recruitment by down-regulating β2 integrin and ICAM-1 expression and upregulating endothelial cell production of nitric oxide, an inhibitor of leukocyte adhesion to vascular endothelium 78,83,84. In addition to restraining neutrophil infiltration, pro-resolving lipid mediators promote neutrophil apoptosis and their phagocytosis by macrophages at the inflamed site 85.
The phagocytic uptake of apoptotic cells is designated efferocytosis and is mediated by a number of endocytic (efferocytic) receptors, including the T-cell immunoglobulin- and mucin-domain-containing molecules −1 and −4, the scavenger receptor CD36, c-Mer tyrosine kinase receptor (MerTK), and the integrins αvβ3, αvβ5 and CD11b/CD18, also known as complement receptor-3. These receptors do not necessarily work independently of each other but actually appear to engage in cooperative interactions 86–88. It should also be noted that not all of these receptors may be expressed by a given phagocyte and the associated clearance mechanisms may operate in a tissue-specific manner.
Efferocytosis is also facilitated by phosphatidylserine exposed on the outer membrane surface of apoptotic cells. This is because phosphatidylserine functions as a major ‘eat-me’ signal and interacts, either directly or indirectly with the help of ‘bridging molecules’, with different types of efferocytic receptors on macrophages 87. An example of the former mechanism involves the efferocytic receptor T-cell immunoglobulin- and mucin-domain-containing molecule-4 which can directly bind phosphatidylserine 89. An example of indirect interaction involves the bridging molecules Del-1 and the related milk fat globule epidermal growth factor-8 (MFG-E8; also designated lactadherin), which promote efferocytosis by each binding to αvβ3 integrin (via an RGD motif on the N-terminal part of the molecules) and to phosphatidylserine (via discoidin-I like domains in the C-terminal part of the molecules) 90–92. Moreover, MerTK can bind phosphatidylserine-bearing apoptotic cells via the opsonin known as growth arrest specific factor-6. Not all efferocytic receptors require phosphatidylserine to function. For instance, Mac-1 (CD11b/CD18), also designated complement receptor-3, on macrophages can mediate the efferocytosis of iC3b-coated apoptotic cells, in other words, this receptor depends on the opsonin iC3b generated as a result of complement activation. Besides their role in apoptotic cell recognition, integrins actively crosstalk with other efferocytic receptor pathways in the context of inflammation resolution. For instance, the T-cell immunoglobulin- and mucin-domain-containing molecule-4 can induce stimulation of Src-family kinases and focal adhesion kinase and integrin activation. In this way, T-cell immunoglobulin- and mucin-domain-containing molecule-4 engages integrins as coreceptors to activate signals required for the engulfment of apoptotic cells 93.
Efferocytosis serves more than a mechanism of waste disposal, that is, to clear apoptotic cells and prevent secondary necrosis and inflammation. Efferocytosis also reprograms the transcriptional profile of the efferocytic macrophage, switching its phenotype to a ‘resolving’ macrophage. Specifically, upon efferocytosis, macrophages are re-programmed to reduce the expression of proinflammatory cytokines (e.g., interleukin-6 and interleukin-23) and increase the expression of regulatory cytokines, such as transforming growth factor-β and interleukin-10 78,80,91,94. Liver X receptors (LXRs) and peroxisome proliferator-activated receptors (PPARs) are ligand-activated transcription factors belonging to the nuclear receptor superfamily and play important roles in efferocytosis and the resolution of inflammation 95,96. LXRs comprise two isoforms (LXRα and LXRβ) and are activated by endogenous oxysterols, i.e., oxidized derivatives of cholesterol. Endogenous ligands of PPARs (existing in distinct isoforms α, β/δ, and γ) include fatty acids and other lipids 97. Engulfment of apoptotic neutrophils triggers LXR signaling in the efferocytic macrophage in response to the sterol lipids of the apoptotic plasma membrane. LXR signaling in turn upregulates the expression of MerTK, thereby enhancing further efferocytosis, and furthermore inhibits proinflammatory gene expression 91,96,98,99. PPARβ/δ signaling is also induced during apoptotic cell uptake, apparently by polyunsaturated fatty acid ligands derived from the apoptotic cell plasma membrane. PPARβ/δ signaling induces the expression of C1q (a complement component that binds phosphatidylserine) and MGF-E8, both of which can promote the phagocytosis of apoptotic cells by macrophages 90,100. Consistent with this, PPARβ/δ-deficient macrophages have impaired capacity to perform efferocytosis and to switch to an anti-inflammatory and pro-resolving phenotype 101. Overall, macrophages exhibit remarkable plasticity, which is crucial for successful resolution of inflammation, hence an important target for host modulation in inflammatory diseases.
Host modulation
Host modulation therapy involves a treatment concept that aims to alter the status or function of the host to treat a disease. In periodontitis, host modulation predominantly refers to efforts to manipulate the immune response in ways that prevent or ameliorate tissue damage. The purpose of some of these approaches is to break a self-sustained vicious cycle that links microbial dysbiosis and destructive inflammation and underlies the chronicity of periodontitis (Figure 1). Conceivably, by controlling the host inflammatory response, it becomes possible to limit the food supply to a microbiota that sustains dysbiosis, thereby creating an environment that can reverse dysbiosis to recover a microbial flora that is compatible with periodontal health. Ideally, effective host-modulation therapy can restore the balance between pro-inflammatory and anti-inflammatory mediators, arrest disease development and promote an environment that is conducive to inflammation resolution and periodontal tissue repair. It would not be feasible to discuss all proposed host response modulation approaches, most of which are at an experimental stage and have not yet been tested in clinical trials. Priority has been given to those where the mechanisms of action are adequately understood (Figure 2).
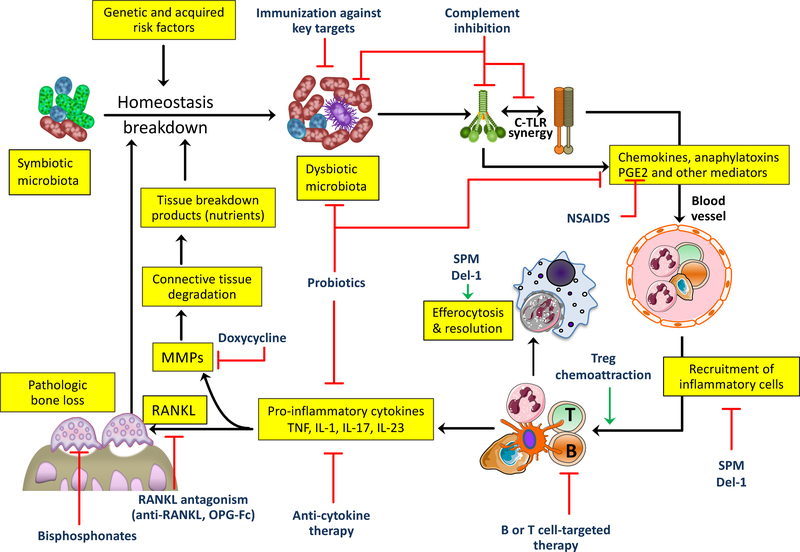
Figure 2. Targets for host-modulation interventions in periodontitis.
Periodontitis arises from the disruption of host-microbe homeostasis in susceptible individuals leading to dysbiosis and destructive inflammation that not only activates osteoclastogenesis and bone loss but also provides nutrients (tissue breakdown products) that enable the dysbiotic microbiota to grow and persist. Shown are important therapeutic targets and potential interventions, most of which are currently at an experimental stage (see text for details). C, complement; Del-1, development endothelial locus-1; NSAIDs, non-steroidal anti-inflammatory drugs; OPG, osteoprotegerin; RANKL, receptor activator of nuclear factor-κB ligand; SPM, specialized pro-resolving mediators; TLR; Toll-like receptor.
Non-steroidal anti-inflammatory drugs
Non-steroidal anti-inflammatory drugs block the cyclooxygenase pathway of arachidonic acid metabolism and have been considered for the treatment of periodontitis 102,103. Non-steroidal anti-inflammatory drugs can be non-selective or selective depending on their ability to block the activity of both enzyme isoforms, cyclooxygenase-1 and −2 or only cyclooxygenase-2, respectively. Cyclooxygenase-1 is constitutively expressed and cyclooxygenase-2 is an inducible isoform expressed in response to inflammation. Cyclooxygenase inhibition blocks the production of prostaglandins, including prostaglandin E2. Prostaglandin E2 is produced by various resident (e.g., fibroblasts) and recruited cell types (e.g., macrophages, and neutrophils) in the periodontium in response to lipopolysaccharide or pro-inflammatory cytokines. Prostaglandin E2 functions as a vasodilator and promotes vascular permeability as well as bone resorption in periodontitis 104,105. Animal model-based studies and clinical trials have shown that non-steroidal anti-inflammatory drugs could inhibit alveolar bone resorption. Although these drugs could improve the clinical outcome of mechanical periodontal treatment, the results decline rapidly after drug withdrawal 102,103,106,107,108. Moreover, current formulations have serious adverse effects that preclude their prolonged use for periodontal therapy. Non-selective non-steroidal anti-inflammatory drugs were associated with gastrointestinal mucosal damage and renal toxicity 109,110. The use of selective cyclooxygenase-2 inhibitors is also problematic as they can induce prothrombotic side-effects 111, which may be attributed to reversal of cyclooxygenase-2–dependent attenuation of expression of tissue factor, a molecule that activates blood clotting 112.
Anti-cytokine therapy
Anti-cytokine treatments involve the use of neutralizing monoclonal antibodies or receptor antagonists to block the action of pro-inflammatory cytokines. Such approaches to inhibit the effects of tumor necrosis factor, interleukin-1, or interlukin-17 resulted in inhibition of periodontitis in preclinical models, thereby also confirming the involvement of these cytokines in inflammatory bone loss 53,113,114. Periodontitis and rheumatoid arthritis share common inflammatory pathways. In this context, a systematic review analyzed the effects of several anti-rheumatic drugs on periodontal inflammation and related biomarkers in rheumatoid patients with periodontitis 115. Such drugs include infliximab (monoclonal antibody to tumor necrosis factor), etanercept (soluble form of tumor necrosis factor receptor) and anakinra (interleukin-1 receptor antagonist). The authors’ conclusion was that there is currently limited evidence to suggest that existing anti-cytokine therapies can reduce periodontal inflammation at least in patients with both periodontitis and rheumatoid arthritis. The insufficient evidence was probably due to the small number of studies performed, which moreover were not specifically designed to address efficacy in periodontitis. Anti-cytokine therapy has potential adverse effects on immunity, which may be less serious for local than for systemic administration. Another potential concern is that specific blockade of a single cytokine may not be very effective if destructive inflammation is driven by a redundant cytokine network 116. This may not be an issue, however, when a specific cytokine pathway is heavily implicated in immune pathology, as is the case with an aggressive form of periodontitis that is associated with leukocyte adhesion deficiency and is driven specifically by the interleukin-23/interleukin-17 axis 52. Indeed, anti-interleukin-23 therapy (through ustekinumab, a monoclonal antibody that blocks the common interleukin-12/interleukin-23 p40 subunit) in a patient with LAD inhibited gingival expression of interleukin-17 and resolved inflammatory lesions without adverse reactions after one year of systemic treatment 117.
Specialized pro-resolution mediators
As discussed in more detail above, specialized pro-resolving lipid mediators, including arachidonic acid-derived lipoxins and omega-3 polyunsaturated fatty acid-derived resolvins and protectins, can mediate inflammation resolution via a variety of mechanisms 78,83,84. A newly dissected pro-resolving mechanism by which resolvins can terminate further neutrophil recruitment involves their ability to upregulate endogenous inhibitors of neutrophil transmigration. Specifically, resolvin D1 was shown to counteract interleukin-17-induced downregulation of the β2 integrin antagonist Del-1, thus potentially contributing to the resurgent expression of Del-1 during the resolution of inflammation 91,118. A number of studies have established the ability of specialized pro-resolving lipid mediators to protect against experimental periodontitis in different animal models, such as rats, rabbits and pigs119–123.
Clinical studies have indicated that dietary supplementation with omega-3 polyunsaturated fatty acids (precursors of resolvins and protectins) may provide a benefit to the treatment of the disease, especially when combined with aspirin 108,124,125. In this regard, aspirin acetylates and changes the enzymatic function of cyclooxygenase-2 triggering the production of E-resolvins from eicosapentaenoic acid and D-resolvins and protectins from docosahexaenoic acid 126. To date, these intervention studies have involved small sample sizes. Larger-scale clinical trials are required to substantiate the initial promising results.
Probiotics
Probiotic microorganisms (probiotics) are being considered as a therapeutic strategy to prevent or treat human inflammatory diseases. The mechanisms of action of probiotics are incompletely understood but appear to modulate both the microbiota and the host response 127,128. Several probiotic preparations were shown to alter the periodontal microbial ecology in a manner that attains a balanced composition compatible with health, although this effect is transient and ceases after the end of the treatment 129. This might be due to the low persistence in the oral cavity of the probiotic strains used, most of which are not oral organisms. Regarding host modulation, probiotics were shown to promote barrier function and the activity of T regulatory cells, and to inhibit pro-inflammatory responses. Several probiotic strains were shown to mitigate experimental periodontitis in animal models. For instance, gastric intubation of Lactobacillus gasseri SBT2055 in mice inhibited Porphyromonas gingivalis-induced gingival inflammation and alveolar bone loss 130. In mice, moreover, topical application of Lactobacillus brevis CD2 was shown to inhibit ligature-induced gingival inflammation and bone loss and reduce the counts of gram-negative bacteria 131. A major mechanism by which L. brevis CD2 exerts anti-inflammatory action is through suppression of nitric oxide synthesis.
In a subsequent human study, L. brevis CD2 or placebo lozenges were applied topically in the mouth (3 times daily for 14 days) and allowed to slowly dissolve. The probiotic had a modest effect in reducing signs of experimental gingivitis in L. brevis CD2-treated volunteers as compared to placebo-treated group 132. A number of clinical studies have used Lactobacillus reuteri; overall, the results from these investigations indicate that this probiotic treatment can be an effective adjunctive treatment to conventional periodontal therapy as they cause significant improvement in clinical indicators of periodontal disease (reviewed in refs. 127,129). Although the combination of probiotic treatment and scaling and root planing is generally more effective than scaling and root planing alone in reducing clinical indices of periodontal inflammation, human studies performed so far involved small sample size and are quite heterogeneous (e.g., use of different probiotic strains, doses, and mode of administration). Therefore, strong conclusions cannot be readily drawn. Indeed, a recent meta-analysis concluded that more clinical studies, especially long-term, are required to strengthen the notion that L. reuteri-based probiotics have clinical efficacy as an adjunct to scaling and root planing 133. Thus, long-term efficacy as well as safety of probiotics need to be established before reliable clinical recommendations could be made 129.
Complement
Early clinical studies have shown considerably higher abundance of complement activation products in the gingival tissue and the gingival crevicular fluid of periodontitis patients than in healthy control individuals 134–140. Consistent with these observations, induction of experimental gingivitis in human volunteers caused progressive complement activation correlating with increased clinical inflammation 141. Conversely, successful periodontal treatment which resolved clinical inflammation inhibited complement activation in the gingival crevicular fluid of the treated patients 142. A cause-and-effect relationship between complement and periodontitis was established in preclinical mouse models using strains deficient in key complement components (C3 or C5aR1), which were found to be protected against experimental periodontitis as compared to wild-type controls 143,144.
The functional significance of C3 in experimental mouse periodontitis and the central location of this molecule in the complement cascade have prompted investigations as to whether C3 inhibition could provide a therapeutic benefit for periodontitis patients. The inhibitor used was Cp40, an improved analog of the compstatin family of C3 inhibitors 145–147. The original compstatin was discovered by screening a phage-displayed peptide library and is a 13-residue, cyclic peptide (I[CVVQDWGHHRC]T-NH2) 148. All members of the compstatin family block the activation of C3 exclusively in humans and non-human primates. Mechanistically, they bind C3 and inhibit its binding to and cleavage by the C3 convertase, thus blocking the generation of downstream effectors regardless of the initiation pathway of complement activation 145,146. Compared to the original version of compstatin, Cp40 has several improved features, including stronger inhibitory action, higher binding affinity for the C3 target and better pharmacokinetic parameters 146. Specifically, Cp40 has subnanomolar affinity for C3 (KD = 0.5 nM) and a plasma half-life (~40 h) that exceeds expectations for most peptidic drugs 146,147,149.
When locally administered by intra-gingival injection in a preventive setting, Cp40 inhibited inflammation and bone loss in young adult non-human primates subjected to ligature-induced periodontitis 143. Cp40 was effective also in a therapeutic setting in multiple subsequent studies. Importantly, in the absence of additional treatments such as scaling and root planing, the drug inhibited pre-existing, naturally occurring periodontitis in aged non-human primates, even when given once every 3 weeks, and its protective effects lasted for at least 6 weeks after drug withdrawal 150,151. In the above-discussed studies 143,150,151, C3 inhibition by Cp40 exerted a strong suppressive effect on interleukin-17, a potent proinflammatory and pro-osteoclastogenic cytokine (Figure 3). In this respect, complement and Toll-like receptors are cooperatively involved in the regulation of interleukin-17 production by both innate and adaptive immune cells, such as Th17 152–157. Although complement has in general complex regulatory effects on interleukin-17 expression, in the periodontium, complement enhances interleukin-17 production in synergy with Toll-like receptor signaling 153. Th17-derived interleukin-17 can mediate bone immunopathology by inducing matrix metalloproteinases and RANKL, thereby contributing to degradation of both connective tissue and the underlying alveolar bone in both human periodontitis and experimental periodontitis in animal models 65,74,158,159(Figure 3). Inhibition of RANKL expression by Cp40 in non-human primate periodontitis 143,150 may in part be attributed to the ability of this C3 inhibitor to suppress interleukin-17 production.
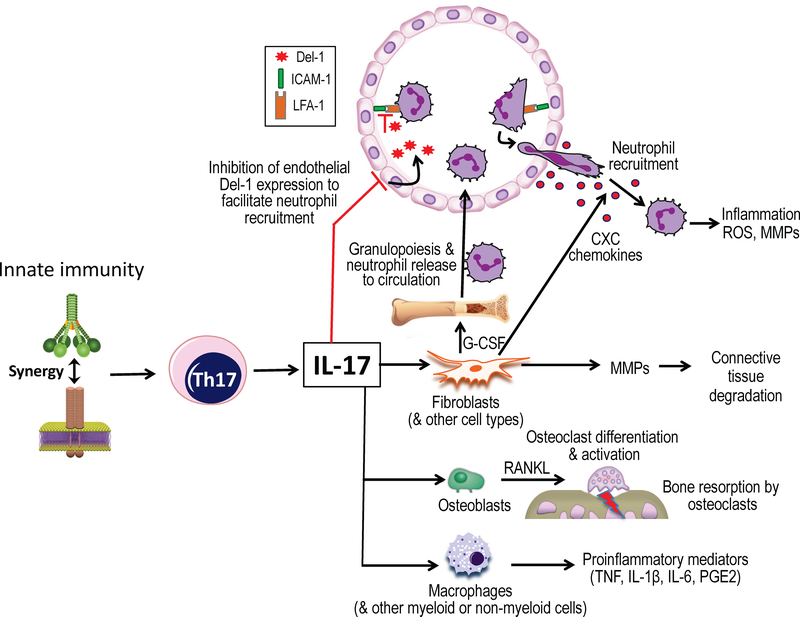
Figure 3: Proinflammatory functions of interleukin-17 with potential for periodontal tissue destruction.
Synergistic complement and Toll-like receptor signaling in antigen-presenting cells enhances interleukin-17 production by adaptive immune cells (Th17). Interleukin-17, in turn, acts predominantly on innate immune and stromal cells to promote inflammatory responses. By upregulating G-CSF, interleukin-17 can orchestrate the production of neutrophils in the bone marrow and their mobilization to the circulation. By inducing CXC chemokines, interleukin-17 can induce the chemotactic recruitment of neutrophils to the periodontium. Additionally, interleukin-17 facilitates neutrophil recruitment by inhibiting negative regulators of the leukocyte adhesion cascade. Specifically, interleukin-17 can inhibit endothelial cell production of Del-1, a homeostatic protein that suppresses neutrophil adhesion and extravasation by blocking the interaction between the LFA-1 integrin on neutrophils and the adhesion molecule ICAM-1 on endothelial cells. Moreover, interleukin-17 activates macrophages and may promote the degradation of both connective tissue and the underlying bone by inducing the production of matrix metalloproteinases and RANKL from stromal cell types.
Cp40 has been licensed by Amyndas Pharmaceuticals and formed the basis for the development of AMY-101, a clinical candidate drug intended for therapeutic intervention in complement-mediated diseases including periodontitis 160,161. AMY-101 has been extensively tested for safety. Monitoring of non-human primates under prolonged (up to 3 months) systemic treatment with AMY-101 revealed no significant differences in hematological, biochemical or immunological parameters in their blood or tissues relative to those of control animals, despite complete inhibition of C3 in the plasma. Furthermore, wounds inflicted in the skin of the AMY-101-treated monkeys did not show signs of infection but in fact showed a trend towards faster wound healing as compared to controls 162. Moreover, AMY-101 was shown to be free of local irritation when intra-gingivally injected in non-human primates 151. More recently, AMY-101 was evaluated in a first-in-human clinical trial in healthy volunteers, in which AMY-101 was shown to be safe and well tolerated 161,163. Overall, the safety and efficacy features of locally administered AMY-101 143,150,151 suggest that this is a promising approach that merits investigation as a host-modulation therapy in human periodontitis.
Homeostatic proteins comprising epidermal growth factor-like and discoidin-like domains
The secreted homologous proteins Del-1 and MFG-E8, which have been mentioned briefly above in the context of neutrophil recruitment and efferocytosis, have an overall identity of about 50% at the amino-acid sequence level and are structurally quite similar: At the N-terminus, both molecules have epidermal growth factor-like repeats (Del-1 has three and MFG-E8 has two such repeats), the second of which contains an integrin-binding RGD motif. At the C-terminus, both Del-1 and MFG-E8 have two discoidin I-like domains which can bind phospholipids and glycosaminoglycans 90,92,164–166. Both proteins were shown to have important homeostatic functions and to inhibit periodontitis in mouse and non-human primate models 53,167–169.
As mentioned earlier, Del-1 can regulate β2 integrin-dependent neutrophil functions, including firm adhesion to the endothelium, thereby suppressing neutrophil recruitment to sites of inflammation 53,54,170 (Figure 3). As a consequence, Del-1-deficient mice display excessive neutrophil infiltration in the gingiva and develop spontaneous periodontal inflammation 53. Moreover, Del-1 is expressed by osteoclasts and regulates their RANKL-induced differentiation as well as restrains their resorptive function 167. More recently, Del-1 was shown to contribute to the resolution of periodontal inflammation 91. In both human and murine periodontitis, inflammation clearance correlated with restoration of Del-1 expression levels after the initial downregulation of Del-1 during initiation of inflammation. Resolution of experimental periodontitis in mice failed in Del-1 deficiency, and Del-1 was functionally connected with resolvins. Indeed, Del-1 was shown to act as a non-redundant downstream effector of inflammation resolution mediated by resolvin D1 and was required for optimal production of resolvin D1 and resolvin E1 91. Therefore, Del-1 is not only a crucial effector of periodontal inflammation resolution but is also positively cross-regulated with resolvins 91,118, likely leading to the generation of a positive-feedback loop that fortifies inflammation resolution. Del-1 was also shown to promote the resolution of acute monosodium urate crystal-induced peritoneal inflammation through its ability to promote effective apoptotic neutrophil clearance (efferocytosis) through an αvβ3 integrin-dependent mechanism that was discussed above 91.
Consistent with its regulatory actions, recombinant Del-1 (fused to the Fc part of IgG; Del-1-Fc) was shown to inhibit periodontal tissue inflammation and bone loss in both mice and non-human NHPs subjected to ligature-induced periodontitis 167. Apparently, Del-1 can block periodontitis by suppressing upstream activities for inflammatory cell recruitment and downstream processes pertaining to osteoclastogenesis, as well as promoting the resolution of inflammation. These findings may potentially translate to human periodontitis since the immune system and periodontal tissue anatomy of monkeys is similar to that of humans; moreover, monkey periodontitis shares important clinical, microbiological, and immunohistological features with the human disease 171.
MFG-E8 expressed by macrophages was shown to promote efferocytosis and hence the resolution of inflammation 90. As noted above, MFG-E8 is structurally similar to Del-1, thus it contains the critical features (RGD motif in the N-terminal part and discoidin-like domains in the C-terminal part) that enhance apoptotic cell phagocytosis. Moreover, MFG-E8 has direct anti-inflammatory activity in macrophages. Specifically, it can bind αvβ3/5-integrins and induce signal transducer and activator of transcription 3 (STAT3)-mediated activation of suppressor of cytokine signaling-3 in macrophages 172. Accordingly, recombinant MFG-E8 inhibits inflammation in animal models of sepsis or cerebral ischemic injury 173,174. More recently, and similar to Del-1, MFG-E8 was shown to suppress the RANKL-induced differentiation of osteoclasts 169. Consistent with its ability to regulate inflammation and osteoclastogenesis, locally administered recombinant mouse MFG-E8 was shown to inhibit periodontal inflammation and bone loss in ligature-induced periodontitis in mice 169. Moreover, recombinant human MFG-E8 inhibited periodontal inflammation and bone loss in non-human primates subjected to ligature-induced periodontitis 168. In humans, the gingival crevicular fluid levels of MFG-E8 are significantly lower in periodontitis as compared to health. However, the gingival crevicular fluid levels of MFG-E8 are significantly elevated in periodontitis patients following non-surgical (scaling and root planing) or surgical periodontal treatment. These findings support the potential utility of MFG-E8 as both a biomarker and therapeutic agent in periodontal disease.
Nuclear metabolic receptor agonists
Nuclear receptors, such as the LXRs and PPARs mentioned above, are ligand-activated transcription factors involved in the expression of genes that regulate cell growth and differentiation, and metabolic homeostasis 175. LXRs and PPARs are constitutively found in the nucleus where they form heterodimers with retinoid x receptors (RXRs) that bind to specific response elements in target genes even in the absence of ligand binding. However, when not occupied by ligands, LXR/RXR and PPAR/RXR heterodimers are transcriptionally inactive as they are complexed with corepressor proteins. In contrast, ligand occupancy of the heterodimers induces conformational changes that release the corepressor proteins and recruit coactivator proteins to the transcriptional complexes leading to target gene expression 175. More recently, LXRs and PPARs were linked to the regulation of inflammation, thus placing them at the intersection of metabolism and immunity 99,175. As alluded to earlier, LXRs and PPARs play important roles in the clearance of apoptotic neutrophils (efferocytosis) by tissue phagocytes 95,96. LXRs are also critical for the ability of Del-1 to induce a pro-resolving macrophage phenotype in the context of efferocytosis 91.
Accumulating evidence suggests that PPAR activation may have protective effects in periodontitis. The activation of PPARδ (also known as PPARβ hence often designated PPARβ/δ) was shown to suppress the ability of P. gingivalis lipopolysaccharide to activate matrix metalloproteinase-2 in human gingival fibroblasts 176. Mechanistically, PPARβ/δ signaling – triggered by the selective agonist GW501516 – inhibited mRNA and protein expression of NADPH oxidase 4 (Nox4), thereby attenuating the production of reactive oxygen species that would in turn induce matrix metalloproteinase-2 activation. Consequently, GW501516-induced activation of PPARβ/δ activation in human gingival fibroblasts blocked P. gingivalis lipopolysaccharide-induced degradation of collagen types I and III. Given the important role of matrix metalloproteinases in periodontitis pathogenesis 177, these findings may provide mechanistic support for an in vivo rat study showing that systemic administration of a selective PPARβ/δ agonist (GW0742) attenuated ligature-induced periodontal inflammation and tissue damage 178. Protective results in ligature-induced periodontitis in rats were obtained also by administering synthetic agonists of PPARα 179 or PPARγ 180.
Targeting adaptive immune cells
B lymphocytes and plasma cells are abundant in the inflammatory infiltrate of advanced chronic periodontitis in humans 6,73,181. The survival, proliferation, and maturation of B lymphocytes depends on two cytokines of the tumor necrosis factor ligand superfamily, namely APRIL and B-lymphocyte stimulator 182,183, which are both upregulated in human periodontitis 184. Antibody-mediated neutralization of APRIL or B-lymphocyte stimulator in mice was shown to diminish the B cell numbers in the gingival tissue and inhibit periodontal bone loss 184. Therapeutic approaches targeting APRIL and/or B-lymphocyte stimulator are under clinical development for other inflammatory (or autoimmune) diseases and a neutralizing monoclonal antibody to B-lymphocyte stimulator (belimumab) has been approved by the FDA for the treatment of systemic lupus erythematosus 185,186. Interestingly, anti-B cell therapy using rituximab (a chimeric monoclonal antibody to CD20) in patients with rheumatoid arthritis resulted in significant reduction of indices that measure clinical periodontal inflammation and tissue destruction 187. Thus, although more studies are required for reliable conclusions, limited evidence suggests the potential of B cell-targeted therapies for the treatment of periodontitis.
CD4+ Foxp3+ regulatory T cells (Tregs) downregulate the induction and proliferation of effector T cells and can be protective in inflammatory and autoimmune conditions 188. In experimental periodontitis in mice, Tregs appear in high numbers after the peak appearance of RANKL-expressing CD4+ T cells 189 and selective Treg depletion exacerbates inflammation and bone loss 70. Tregs therefore mitigate inflammatory tissue damage. However, their protective potential may be compromised in inflamed tissues. For instance, interleukin-23–activated γδ T cells restrain Tregs and shift the balance in favor of effector T helper cells and, moreover, render effector T cells refractory to suppression by Tregs 190. However, local treatment of mice or dogs with a chemoattractant formulation for Tregs, consisting of the chemokine CCL22 encapsulated in degradable polymer, enhanced the recruitment of Tregs and inhibited experimental periodontitis in these models 191.
A recent study has implicated Th17 cells in inflammatory tissue destruction of experimental periodontitis 65. Specific genetic inhibition of Th17 cell differentiation (by knocking out the critical transcription factors Stat3 or RORγt) not only led to the absence of Th17 cells from the gingival tissues but also significantly blocked inflammatory bone loss in a murine model of ligature-induced periodontitis. Human relevance for the importance of Th17 in periodontal disease pathogenesis was established by showing that patients with genetically impaired Th17 cell development (due to STAT3 loss-of-function mutations; autosomal dominant hyper IgE syndrome 192) exhibited significantly decreased periodontal inflammation and bone loss as compared to age- and gender-matched healthy controls and periodontitis patients 65. From a translational perspective, pharmacological inhibition of RORγt using the small-molecule inhibitor GSK805, which inhibits Th17 development and function 193, resulted in significantly decreased periodontal inflammation and bone loss in mice 65. GSK805 preferentially targeted the expansion of Th17 cells without affecting other interleukin-17-secreting cellular sources, such as γδ T cells or innate lymphoid cells that do not appear to contribute to periodontal disease pathogenesis. This study therefore has shown that GSK805 is a selective Th17 inhibitor in periodontitis that can provide protection against this inflammatory disease.
Approaches for direct inhibition of periodontal tissue destruction
A persisting, non-resolving inflammatory response in the periodontal tissue can exert damaging effects through several mechanisms. These include the induction of matrix metalloproteinases, which cause connective tissue degradation 177, and of receptor activator of nuclear factor-κB ligand (RANKL), which stimulates the differentiation and activation of osteoclasts that resorb bone 72,75,194. The destruction of connective and bone tissue can be blocked directly by targeting the effector mechanisms.
Tetracyclines attracted considerable interest when it was discovered that sub-antimicrobial doses of these antibiotics could block the activity of matrix metalloproteinases, which are elevated in inflamed gingiva and can cause collagen breakdown 195. Treatment of periodontitis patients with systemically delivered doxycycline (a potent tetracycline for inhibiting collagenolytic activity) has produced variable results but was generally beneficial as an adjunct to scaling and root planing 196,197. In this respect, treatment with a combination of doxycycline and scaling and root planing could promote clinical attachment gain, reduction in probing depths, and suppression of gingival crevicular fluid levels of certain matrix metalloproteinases, relative to a placebo and scaling and root planing combination. Sub-antimicrobial doxycycline (20 mg, taken twice daily) has received FDA approval and has been marketed as Periostat for the treatment of human periodontitis 196,197.
Bisphosphonates are anti-resorptive drugs that bind the mineral component of bone (hydroxyapatite crystals) and interfere with the action of osteoclasts. One possible mechanism of action of bisphosphonates is promotion of osteoclast apoptosis 198. These drugs have been used for the prevention and treatment of osteoporosis 199. Experiments in animal models of periodontitis showed that systemically administered bisphosphonates could protect against bone loss without affecting inflammation. Similarly, decreased alveolar bone loss and improved mineral density was demonstrated in periodontitis patients administered bisphosphonates (risedronate or alendronate) as an adjunct to scaling and root planing; however, a significant improvement of clinical inflammatory parameters was not consistently observed 200–202. For instance, alendronate did not significantly reduce the gingival index but displayed a trend for improved attachment level. From a safety perspective, osteonecrosis of the jaw is a serious adverse effect observed in a subset of patients receiving bisphosphonates 200,201,202.
Another approach to directly inhibit bone loss is to inhibit the differentiation of osteoclasts by blocking the interaction of RANKL with its receptor RANK on the surface of osteoclast precursors. Proof-of-concept was obtained by showing that the natural inhibitor of RANKL, osteoprotegerin (administered as a fusion protein with the Fc portion of IgG) and anti-RANKL antibody both inhibit periodontal bone loss in rats 203,204. Denosumab is a humanized monoclonal antibody that binds RANKL and prevents its interaction with RANK and has been used for treating osteoporosis 205,206. The efficacy of denosumab has not been specifically tested in periodontitis patients, although it should be noted that osteonecrosis of the jaw has also been reported in patients treated with denosumab 207.
Osteoclasts can also be regulated, directly or indirectly, by secreted frizzled-related proteins (sFRPs) 208,209. For instance, sFRP1 was shown to bind RANKL, thereby preventing its interaction with RANK and hence inhibiting osteoclastogenesis 209. The main targets of sFRPs are, nevertheless, the Wingless/integrase-1 (Wnt) family of proteins. This is because sFRPs are structurally related to the frizzled receptors of Wnt proteins and can act as decoy receptors that antagonize Wnt protein-frizzled receptor interactions 210. In this context, Wnt5a and sFRP5 constitute a typical ligand/antagonist pair 210. The binding of osteoblast-derived Wnt5a to a co-receptor complex involving receptor tyrosine kinase-like orphan receptor-2 and frizzled on osteoclast precursors induces noncanonical Wnt signaling that upregulates RANK expression. This in turn sensitizes the precursors to the action of RANKL, thereby promoting osteoclast differentiation 208. Importantly in this regard, local intrangingival injection of sFRP5 in mice subjected to ligature-induced periodontitis resulted in inhibition of alveolar bone loss, which correlated with decreased numbers of osteoclasts in tissue sections 211. However, given the tight connection between inflammation and osteoclastogenesis, the anti-osteoclastogenic effect of sFRP5 could additionally, or alternatively, be attributed to its anti-inflammatory properties 211–213. Interestingly, there is a reciprocal relationship between sFRP5 and Wnt5a expression in human periodontal health and disease, with Wnt5a dominating in diseased and sFRP5 in healthy tissue 211. In principle, sFRP5 might potentially be both a biomarker and a therapeutic agent in periodontitis.
Vaccination
The first essential condition for immunization against any microbially induced disease is to define the causative agent(s). Thus, the concept of vaccination against periodontitis started to emerge only after specific microorganisms, such as P. gingivalis, Tannerella forsythia, Treponema denticola and Aggregatibacter actinomyctemcomitans were implicated as putative etiologic agents in periodontal diseases in the late 1980s 214. A challenge in vaccine development for periodontitis is that the disease primarily results from collateral tissue damage of the host immune response rather than from direct bacterial action. Therefore, vital to success in developing a periodontitis vaccine is a good understanding of the mechanisms that induce inflammatory tissue damage while generally failing to control subgingival microbial communities. In other words, a vaccine-induced antimicrobial response should not activate a destructive inflammatory response. In this regard, it should be considered that vaccine adjuvants have off-target effects related to induction of trained innate immunity; such effects might confer non-specific heterologous protection against subsequent infections or lead to immune responses that exacerbate inflammatory diseases 215–219. Another challenge for vaccine development is that periodontitis is initiated by synergistic and dysbiotic microbial communities rather than by a few select ‘periodontopathogens’ 19–28.
Despite these considerations, there may be a sufficient rationale for vaccination targeting P. gingivalis. In a mouse model of periodontitis, P. gingivalis acts as a keystone pathogen, i.e., exerts community-wide effects that promote the pathogenicity of the entire biofilm 47. In this context, P. gingivalis subverts the host response in a manner that uncouples inflammation (which is enhanced) from bactericidal activity (which is impaired), thereby leading to the emergence of a dysbiotic microbiota, in which inflammophilic pathobionts aggravate the inflammatory response and cause tissue destruction 23,28,220,221. If P. gingivalis exerts similar effects in human periodontitis, at least in a subset of patients (more below), then it may be a promising therapeutic target.
The first attempts for vaccination against P. gingivalis were performed in rats and utilized whole bacterial cells or broken cell preparations. However, due to the undesirable reactogenicity of these types of vaccines, subsequent attempts in animal models have focused on defined microbial proteins or genetically engineered subunits thereof 214. Such subunit vaccine approaches have so far focused primarily on P. gingivalis virulence factors, particularly its cysteine proteinases (RgpA, RgpB, and Kgp gingipains) hemagglutinin B, as well as its fimbriae 222–226. Immunization with defined subunit immunogens necessitates the use of proper adjuvants more than immunization with whole bacterial cells, as the latter contain intrinsic adjuvant substances (e.g., lipopolysaccharide). Some studies have utilized Freund’s complete or incomplete adjuvant or cholera toxin, while others made use of adjuvants which have been approved for use in humans, such as, aluminum hydroxide (alum) and monophosphoryl lipid A, either alone or supplemented with trehalose dicorynomycolate 214.
Subcutaneous immunization of rats with the hemoglobin-binding domain of P. gingivalis gingipain could induce specific IgG antibodies and moderate protection against alveolar bone loss 227. The same immunogen, when given with Freund’s complete adjuvant, was able to potentiate the antibody responses but failed to confer protection against bone loss 227. This finding may have been due to inflammatory responses induced or primed by the adjuvant, thus interfering with the potential of the immunogen to protect against bone resorption. This study therefore highlights the significance of selecting appropriate adjuvants that can promote specific protective immunity without immunopathological side-effects. In another study, rats were immunized with a mixture of gingipains (RgpA and Kgp) in Freund’s incomplete adjuvant resulting in specific high-titer serum IgG2a antibody responses and protection against P. gingivalis-induced periodontal bone loss 225. Although the control rats were positive for P. gingivalis, this bacterium was undetectable by DNA probe analysis of subgingival dental plaque samples taken from the RgpA/Kgp-immunized animals. Thus, besides possible neutralization of gingipains by antibody, it is possible that P. gingivalis may have been cleared by IgG2a-mediated opsonization and subsequent FcγRII-dependent phagocytosis and killing. Alternatively, specific IgG2a antibodies could inhibit colonization by P. gingivalis. More recently, a chimera vaccine combining key gingipain sequences formulated in alum induced antigen-specific IgG1 antibody and Th2-biased response that protected mice against P. gingivalis-induced periodontitis 228. Although the role of Th2 cells in periodontitis is incompletely understood, this subset does not seem to be involved in periodontitis pathogenesis, in contrast to the Th17 subset 65,69,229. Another approach to immunization against P. gingivalis-induced periodontal disease has exploited Streptococcus gordonii vectors that express cloned segments of the major fimbriae (FimA) of P. gingivalis. Oral immunization of rats with these recombinant bacteria elicited specific salivary IgA and serum IgG antibody responses and protection against subsequent P. gingivalis-induced periodontal bone loss 222.
Vaccination studies in macaque monkeys are particularly valuable as periodontitis in these animals more closely resembles the human diseases than the more convenient and relatively inexpensive rodent models and, importantly, naturally harbor P. gingivalis. However, monkey studies to investigate immunity to periodontal disease have so far had limited success. A few examples are given here. Subcutaneous immunization of monkeys using as immunogen purified P. gingivalis cysteine proteinase in Freund’s incomplete adjuvant elicited specific antibody responses but did not suppress P. gingivalis 230. Moreover, there were no significant differences between immunized and control animals with regard to periodontal bone loss 230. However, in another monkey study, subcutaneous immunization with cysteine proteinase in Syntex Adjuvant Formulation-M (an oil-in-water emulsion stabilized by Tween 80 and pluronic polyoxyethlene/polyoxypropylene block copolymer L121) led to considerably decreased levels of prostaglandin E2 in the gingival crevicular fluid and correlated with significantly reduced bone loss as compared with non-immunized controls 105. This study suggests that suppression of inflammatory mediators by immunization, possibly due to reduced pathogenic challenge as a consequence of antibody-dependent inhibition of colonization or killing, is a potential protective mechanism against periodontal disease. In a similar gingipain-based vaccination study conducted by the same research group, protection against bone loss was associated with decreased counts of P. gingivalis as well as decreased total subgingival bacterial load 231. These findings are consistent with the concept that the presence of P. gingivalis benefits the entire microbial community, as anticipated by the keystone-pathogen hypothesis 47.
Additional studies are needed to better define vaccination strategies and the immune response mechanisms that may be effective in controlling periodontal disease in primates. Specifically, much more has to be done to define adjuvant formulations and immunization routes (mucosal routes are considered safer than systemic ones), in order to develop vaccine candidates that can be effective not only in inducing immune responses to the bacteria, but also in suppressing the disease. Whether a gingipain-based vaccine can have a significant protective impact on periodontal disease in humans remains to be determined. Although P. gingivalis is only one of a plethora of community bacteria implicated in periodontitis, specific immunity to P. gingivalis has been linked to protection against this disease in animal models. This might be explained by the role of P. gingivalis as a keystone pathogen in periodontitis, although it should be borne in mind that P. gingivalis is a risk factor rather than an obligatory factor in periodontal disease pathogenesis. Indeed, the disease depends on a variety of microbial or host factors that could be genetic or acquired (immune deficiencies, immunoregulatory defects smoking, diet, obesity, diabetes and other systemic diseases, aging) and may act alone or in combination to modify the host response and the microbiome in a destructive direction 22,232,233. Thus, although anti-P. gingivalis immunity may be protective in models where the disease is induced by or attributed to this pathogen, such vaccines may be protective only in a subset of human patients in whom periodontitis is primarily driven by the presence of P. gingivalis. In this regard, although P. gingivalis can be detected in periodontal health 234, in many patients, the presence of P. gingivalis in subgingival plaque has been shown to predict imminent disease progression (clinical attachment loss) 235.
Given that some aspects of the adaptive immune response contribute to periodontal tissue destruction and that vaccine adjuvants might cause maladaptive trained immunity, there is currently need for better understanding of the immunoregulatory mechanisms operating in periodontal disease, and for applying this knowledge in designing vaccines including the selection of adjuvants. Thus, besides enhancing specific immunity to target periodontal bacteria, vaccines should also elicit appropriate non-inflammatory modes of immune response that prevent tissue damage and ideally promote inflammation resolution and tissue healing.
Anti-aging approaches
The elderly display increased susceptibility to infectious and inflammatory diseases including periodontal disease 17,236–242. Aging is thought to affect the immuno-inflammatory status and/or the regenerative potential of the periodontal tissue in a manner that increases susceptibility to periodontitis 17,243,244. This ‘age-altered susceptibility’ hypothesis is consistent with findings of aging-related changes in immune and stem cell function that can potentially dysregulate immune responses and impair periodontal tissue repair 17,239,245–252.
The aging of hematopoietic stem cells is a fundamental cause of immune senescence 250,253, which affects both innate and adaptive immunity 237,253,254. In both humans and mice, hematopoietic stem cell function is impaired in old age, including reduced long-term self-renewal capacity, attributable to both hematopoietic stem cell-intrinsic and -extrinsic mechanisms 250,253. Hematopoietic stem cells reside in a specialized microenvironment in the bone marrow, the ‘hematopoietic stem cell niche’, which maintains stem cells in a quiescent state that reduces DNA damage and also supports their survival and self-renewal, and, upon demand, stem cell proliferation and differentiation 250,255–260. Mounting evidence suggests that the hematopoietic stem cell niche is crucial for the regulation of cellular senescence in hematopoietic stem cells 250,255. As a consequence, the physiological aging of endothelial cells, a critical cellular component of the hematopoietic stem cell niche, drives functional aging of young hematopoietic stem cells in ex vivo co-culture experiments 261.
Intriguingly, the homeostatic function of Del-1 is not restricted to peripheral tissues such as the periodontium. Del-1 is also secreted in the bone marrow (by arteriolar endothelial cells, cells of the osteoblastic lineage and by CXCL12-abundant reticular cells) where it serves as a crucial regulator of the hematopoietic stem cell niche 262. Specifically, Del-1 interacts with the αvβ3 integrin on hematopoietic stem cells and promotes their retention, expansion and differentiation toward the myeloid lineage, both under steady-state and stress conditions 262. Thus, the aging-associated deficiency of Del-1 discussed earlier may contribute to defective hematopoiesis in old age. Interestingly, transplanted young endothelial cells rejuvenate the functional activity of hematopoietic stem cells in old mice, thus suggesting that senescence is, at least in part, a reversible process 261. It is tempting to speculate that the hematopoietic stem cell rejuvenation might, in part, be due to expression of normal levels of Del-1 by the transplanted endothelial cells.
Aging-associated alterations in immune function dysregulate the host response in a manner that (i) impairs immunity to pathogens and (ii) promotes non-productive inflammation, the combination of which may increase host susceptibility to a microbe-driven chronic inflammatory disease such as periodontitis 68,239,250,254,263–268. At least in principle, molecular pathways involved in aging and senescence could be targeted therapeutically to rejuvenate stem cells and reverse some of the adverse effects of aging, such as increased susceptibility to inflammatory disorders and reduced responsiveness to vaccination. For instance, mammalian target of rapamycin (mTOR) activity is increased with aging in hematopoietic stem cells and contributes to their senescence; inhibition of mTOR by rapamycin, an FDA-approved drug, restores the capacity of hematopoietic stem cells for self-renewal and hematopoiesis and enables effective vaccination of old mice against a lethal influenza virus infection 269. The improved efficacy of vaccination in old mice after hematopoietic stem cell rejuvenation by rapamycin treatment is not surprising given that aging-related alterations in mature immune cells can be traced back to hematopoietic stem cells 250,253. Importantly, moreover, a recent study showed that administration (via the diet) of rapamycin to old mice reversed aging-associated periodontal bone loss, although the effect on the host inflammatory response was not reported 270.
Aging-related alterations occur also to the niche of the mesenchymal stromal/stem cells in the periodontal ligament and other tissues 251,252,256,258. Specifically, the proliferative and differentiative ability of periodontal ligament-mesenchymal stroma/stem cells is altered with aging in ways that impair tissue homeostasis and repair 251,252,256. Importantly, this periodontal ligament-mesenchymal stroma/stem cell defect is reversible and regulated by the extrinsic microenvironment 252,256. Overall, there is a growing interest in approaches to rejuvenate hematopoietic stem cells or mesenchymal stroma/stem cells (e.g., by targeting stem cell niches) 227,230,247. These emerging therapeutic options may counteract the adverse effects of aging on a number of diseases and perhaps periodontitis, although more data are necessary to determine the feasibility of these approaches 250,253,271.
Conclusions and perspective
Progress in understanding the mechanisms driving periodontal disease pathogenesis has been paralleled with the development of many and diverse approaches to control the disease (Figure 2). Host-modulation therapies involving biologics to target specific components of the immune response may have potential safety issues, including increased risk for infections. However, potential risks and adverse effects are more likely when therapeutics are administered systemically and are prescribed for long-term use, as discussed above for non-steroidal anti-inflammatory drugs. For instance, potential adverse effects on thymocyte and lymph node development by the use of RORγt inhibitors 272,273 are less likely if these inhibitors are administered locally. Being a local inflammatory disease, periodontitis is amenable to local host-modulation treatments. Importantly, local anti-inflammatory therapies, such as complement inhibition, in animal models of periodontitis not only do not predispose to defective immune surveillance in the periodontal tissue but also appear to suppress dysbiotic microbial communities that rely on inflammation for growth and persistence 52,120,121,169,274. In line with this, the bacterial biomass of periodontitis-associated dental plaque increases with increasing clinical inflammation 19. Of course, more reliable information regarding safety can be obtained when candidate drugs enter clinical trials.
At least in principle, the use of endogenous molecules that can both inhibit inflammation and promote its resolution appears to be both safe and effective. For instance, since Del-1 expression is diminished under inflammatory conditions or in old age, restoring its levels by exogenous administration could therapeutically reinstate tissue homeostasis. Therapies utilizing endogenous molecule administration have increased in recent years and endogenous molecule replacement is well tolerated 275,276. The promotion of tissue homeostasis can also be achieved by biologics as long as they are safe and effective. In this regard, although the C3 inhibitor AMY-101 was administered locally in the gingiva of non-human primates, as infrequently as once every 3 weeks and was withdrawn at 6 weeks, the treated animals maintained significantly reduced clinical periodontal/inflammatory indices for at least an additional 6 week-period 151. This long-lasting protective effect was unexpected for a small-molecule inhibitor that was not used throughout the experimental period. One explanation may involve the positive feedback loop between inflammation and dysbiosis 31,32,277 (Figure 1): It is possible that inflammation inhibition by AMY-101 tips the balance towards host-microbe homeostasis, which might be resilient to pathological processes that would tend to re-instate active periodontitis.
With the possible exception of probiotics, most of the aforementioned interventions are intended to be used for treatment rather than prevention of periodontitis. However, at least in principle, most of the above-discussed host-modulation approaches would not necessarily be relevant only in a therapeutic setting; they could also be implemented on a preventive basis, i.e., prior to onset of periodontitis, to high-risk individuals, such as cigarette smokers and diabetic patients 278,279.
The great complexity of pathogenic mechanisms in periodontitis and the involvement of polymicrobial dysbiotic communities, rather than specific pathogens, appear to complicate the development of a periodontitis vaccine. A major challenge is to fine-tune the vaccine-induced host response to maximize protective immunity while minimizing its potentially destructive aspects.
Although our mechanistic understanding of periodontal disease pathogenesis is still incomplete, available knowledge has facilitated promising preclinical studies such as those featured here. Despite the demonstrated potential of rational targeted approaches to inhibit periodontitis, a greater challenge is to clinically evaluate candidate drugs in terms of their risks and benefits, as adjunctive therapies for the treatment of human periodontitis.
Acknowledgements
The authors’ research is supported by U.S. Public Health Service grants from the National Institutes of Health (DE024153, DE024716, DE015254 to GH; DE026152 to GH and TC; and AI068730 and AI030040 to J.D.L), Deutsche Forschungsgemeinschaft (SFB-TR 127 to TC) and the European Commission (FP7-DIREKT 602699 to J.D.L.).
References
- 1.Armitage GC. Periodontal diagnoses and classification of periodontal diseases. Periodontology 2000. 2004;34:9–21. [DOI] [PubMed] [Google Scholar]
- 2.Lang NP, Adler R, Joss A, Nyman S. Absence of bleeding on probing. An indicator of periodontal stability. Journal of clinical periodontology. 1990;17(10):714–721. [DOI] [PubMed] [Google Scholar]
- 3.Schatzle M, Loe H, Lang NP, et al. Clinical course of chronic periodontitis. III. Patterns, variations and risks of attachment loss. Journal of clinical periodontology. 2003;30(10):909–918. [DOI] [PubMed] [Google Scholar]
- 4.Ramseier CA, Anerud A, Dulac M, et al. Natural history of periodontitis: Disease progression and tooth loss over 40 years. Journal of clinical periodontology. 2017;44(12):1182–1191. [DOI] [PubMed] [Google Scholar]
- 5.Pitchika V, Thiering E, Metz I, et al. Gingivitis and lifestyle influences on high-sensitivity C-reactive protein and interleukin 6 in adolescents. Journal of clinical periodontology. 2017;44(4):372–381. [DOI] [PubMed] [Google Scholar]
- 6.Page RC, Schroeder HE. Pathogenesis of inflammatory periodontal disease. A summary of current work. Lab Invest. 1976;34(3):235–249. [PubMed] [Google Scholar]
- 7.Hajishengallis G, Korostoff JM. Revisiting the Page & Schroeder model: the good, the bad and the unknowns in the periodontal host response 40 years later. Periodontology 2000. 2017;75(1):116–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moutsopoulos NM, Konkel JE. Tissue-Specific Immunity at the Oral Mucosal Barrier. Trends in immunology. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dutzan N, Konkel JE, Greenwell-Wild T, Moutsopoulos NM. Characterization of the human immune cell network at the gingival barrier. Mucosal immunology. 2016;9(5):1163–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hajishengallis G Periodontitis: from microbial immune subversion to systemic inflammation. Nature reviews Immunology. 2015;15(1):30–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasturk H, Kantarci A, Van Dyke TE. Paradigm shift in the pharmacological management of periodontal diseases. Frontiers of oral biology. 2012;15:160–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larsson L, Castilho RM, Giannobile WV. Epigenetics and its role in periodontal diseases: a state-of-the-art review. Journal of periodontology. 2015;86(4):556–568. [DOI] [PubMed] [Google Scholar]
- 13.Reynolds MA. Modifiable risk factors in periodontitis: at the intersection of aging and disease. Periodontology 2000. 2014;64(1):7–19. [DOI] [PubMed] [Google Scholar]
- 14.Bagaitkar J, Daep CA, Patel CK, Renaud DE, Demuth DR, Scott DA. Tobacco smoke augments Porphyromonas gingivalis-Streptococcus gordonii biofilm formation. PloS one. 2011;6(11):e27386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Offenbacher S, Divaris K, Barros SP, et al. Genome-wide association study of biologically-informed periodontal complex traits offers novel insights into the genetic basis of periodontal disease. Human molecular genetics. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiao E, Mattos M, Vieira GHA, et al. Diabetes Enhances IL-17 Expression and Alters the Oral Microbiome to Increase Its Pathogenicity. Cell Host Microbe. 2017;22(1):120–128 e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hajishengallis G Aging and its impact on innate immunity and inflammation: Implications for periodontitis. J Oral Biosci. 2014;56:30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Joshi V, Matthews C, Aspiras M, de Jager M, Ward M, Kumar P. Smoking decreases structural and functional resilience in the subgingival ecosystem. Journal of clinical periodontology. 2014;41(11):1037–1047. [DOI] [PubMed] [Google Scholar]
- 19.Abusleme L, Dupuy AK, Dutzan N, et al. The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation. The ISME journal. 2013;7(5):1016–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dewhirst FE, Chen T, Izard J, et al. The human oral microbiome. J Bacteriol. 2010;192(19):5002–5017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duran-Pinedo AE, Chen T, Teles R, et al. Community-wide transcriptome of the oral microbiome in subjects with and without periodontitis. The ISME journal. 2014;8(8):1659–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lamont RJ, Hajishengallis G. Polymicrobial synergy and dysbiosis in inflammatory disease. Trends in molecular medicine. 2015;21(3):172–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hajishengallis G, Liang S, Payne MA, et al. Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe. 2011;10(5):497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dabdoub SM, Ganesan SM, Kumar PS. Comparative metagenomics reveals taxonomically idiosyncratic yet functionally congruent communities in periodontitis. Scientific reports. 2016;6:38993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griffen AL, Beall CJ, Campbell JH, et al. Distinct and complex bacterial profiles in human periodontitis and health revealed by 16S pyrosequencing. The ISME journal. 2012;6(6):1176–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Camelo-Castillo AJ, Mira A, Pico A, et al. Subgingival microbiota in health compared to periodontitis and the influence of smoking. Frontiers in microbiology. 2015;6:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yost S, Duran-Pinedo AE, Teles R, Krishnan K, Frias-Lopez J. Functional signatures of oral dysbiosis during periodontitis progression revealed by microbial metatranscriptome analysis. Genome Med. 2015;7(1):27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lamont RJ, Koo H, Hajishengallis G. The oral microbiota: dynamic communities and host interactions. Nature reviews Microbiology. 2018;16:745–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mira A, Simon-Soro A, Curtis MA. Role of microbial communities in the pathogenesis of periodontal diseases and caries. Journal of clinical periodontology. 2017;44 Suppl 18:S23–S38. [DOI] [PubMed] [Google Scholar]
- 30.Rosier BT, Marsh PD, Mira A. Resilience of the Oral Microbiota in Health: Mechanisms That Prevent Dysbiosis. J Dent Res. 2017:22034517742139. [DOI] [PubMed] [Google Scholar]
- 31.Hajishengallis G The inflammophilic character of the periodontitis-associated microbiota. Molecular oral microbiology. 2014;29:248–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Diaz PI, Hoare A, Hong BY. Subgingival Microbiome Shifts and Community Dynamics in Periodontal Diseases. Journal of the California Dental Association. 2016;44(7):421–435. [PubMed] [Google Scholar]
- 33.Herrero ER, Fernandes S, Verspecht T, et al. Dysbiotic Biofilms Deregulate the Periodontal Inflammatory Response. J Dent Res. 2018:22034517752675. [DOI] [PubMed] [Google Scholar]
- 34.Ferreira MC, Dias-Pereira AC, Branco-de-Almeida LS, Martins CC, Paiva SM. Impact of periodontal disease on quality of life: a systematic review. Journal of periodontal research. 2017;52(4):651–665. [DOI] [PubMed] [Google Scholar]
- 35.Chapple IL. Time to take periodontitis seriously. BMJ. 2014;348:g2645. [DOI] [PubMed] [Google Scholar]
- 36.Brauchle F, Noack M, Reich E. Impact of periodontal disease and periodontal therapy on oral health-related quality of life. Int Dent J. 2013;63(6):306–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eke PI, Dye BA, Wei L, et al. Update on Prevalence of Periodontitis in Adults in the United States: NHANES 2009 to 2012. Journal of periodontology. 2015;86(5):611–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Demmer RT, Papapanou PN. Epidemiologic patterns of chronic and aggressive periodontitis. Periodontology 2000. 2010;53:28–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frencken JE, Sharma P, Stenhouse L, Green D, Laverty D, Dietrich T. Global epidemiology of dental caries and severe periodontitis - a comprehensive review. Journal of clinical periodontology. 2017;44 Suppl 18:S94–S105. [DOI] [PubMed] [Google Scholar]
- 40.Elias-Boneta AR, Encarnacion A, Rivas-Tumanyan S, et al. Prevalence of Gingivitis in a Group of 35- to 70-Year-Olds Residing in Puerto Rico. P R Health Sci J. 2017;36(3):140–145. [PMC free article] [PubMed] [Google Scholar]
- 41.Elias-Boneta AR, Ramirez K, Rivas-Tumanyan S, Murillo M, Toro MJ. Prevalence of gingivitis and calculus in 12-year-old Puerto Ricans: a cross-sectional study. BMC oral health. 2018;18(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Y, Lee S, Hujoel P, et al. Prevalence and severity of gingivitis in American adults. Am J Dent. 2010;23(1):9–13. [PubMed] [Google Scholar]
- 43.Tonetti MS, Chapple IL, Working Group 3 of Seventh European Workshop on P. Biological approaches to the development of novel periodontal therapies--consensus of the Seventh European Workshop on Periodontology. Journal of clinical periodontology. 2011;38 Suppl 11:114–118. [DOI] [PubMed] [Google Scholar]
- 44.Kassebaum NJ, Bernabe E, Dahiya M, Bhandari B, Murray CJ, Marcenes W. Global burden of severe periodontitis in 1990–2010: a systematic review and meta-regression. J Dent Res. 2014;93(11):1045–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Beikler T, Flemmig TF. Oral biofilm-associated diseases: trends and implications for quality of life, systemic health and expenditures. Periodontology 2000. 2011;55(1):87–103. [DOI] [PubMed] [Google Scholar]
- 46.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nature reviews Immunology. 2007;7(9):678–689. [DOI] [PubMed] [Google Scholar]
- 47.Hajishengallis G, Darveau RP, Curtis MA. The keystone-pathogen hypothesis. Nature reviews Microbiology. 2012;10(10):717–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Phillipson M, Kubes P. The neutrophil in vascular inflammation. Nature medicine. 2011;17(11):1381–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marki A, Esko JD, Pries AR, Ley K. Role of the endothelial surface layer in neutrophil recruitment. Journal of leukocyte biology. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mocsai A, Walzog B, Lowell CA. Intracellular signalling during neutrophil recruitment. Cardiovascular research. 2015;107(3):373–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alon R, Dustin ML. Force as a facilitator of integrin conformational changes during leukocyte arrest on blood vessels and antigen-presenting cells. Immunity. 2007;26(1):17–27. [DOI] [PubMed] [Google Scholar]
- 52.Moutsopoulos NM, Konkel J, Sarmadi M, et al. Defective neutrophil recruitment in leukocyte adhesion deficiency type I disease causes local IL-17–driven inflammatory bone loss. Science translational medicine. 2014;6(229):229ra240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Eskan MA, Jotwani R, Abe T, et al. The leukocyte integrin antagonist Del-1 inhibits IL-17-mediated inflammatory bone loss. Nature immunology. 2012;13(5):465–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Choi EY, Chavakis E, Czabanka MA, et al. Del-1, an endogenous leukocyte-endothelial adhesion inhibitor, limits inflammatory cell recruitment. Science. 2008;322(5904):1101–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Capra V, Rovati GE, Mangano P, Buccellati C, Murphy RC, Sala A. Transcellular biosynthesis of eicosanoid lipid mediators. Biochimica et biophysica acta. 2015;1851(4):377–382. [DOI] [PubMed] [Google Scholar]
- 56.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nature immunology. 2010;11(9):785–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hajishengallis G, Reis ES, Mastellos DC, Ricklin D, Lambris JD. Novel mechanisms and functions of complement. Nature immunology. 2017;18(12):1288–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ricklin D, Reis ES, Lambris JD. Complement in disease: a defence system turning offensive. Nat Rev Nephrol. 2016;12(7):383–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Scapini P, Cassatella MA. Social networking of human neutrophils within the immune system. Blood. 2014;124(5):710–719. [DOI] [PubMed] [Google Scholar]
- 60.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nature reviews Immunology. 2013;13(3):159–175. [DOI] [PubMed] [Google Scholar]
- 61.Hajishengallis G, Chavakis T, Hajishengallis E, Lambris JD. Neutrophil homeostasis and inflammation: novel paradigms from studying periodontitis. Journal of leukocyte biology. 2015;98(4):539–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mocsai A Diverse novel functions of neutrophils in immunity, inflammation, and beyond. The Journal of experimental medicine. 2013;210(7):1283–1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Leliefeld PH, Koenderman L, Pillay J. How neutrophils shape adaptive immune responses. Frontiers in immunology. 2015;6:471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pelletier M, Maggi L, Micheletti A, et al. Evidence for a cross-talk between human neutrophils and Th17 cells. Blood. 2009;115:335–343. [DOI] [PubMed] [Google Scholar]
- 65.Dutzan N, Kajikawa T, Abusleme L, et al. A dysbiotic microbiome triggers Th17 cells to mediate oral mucosal immunopathology in mice and humans. Science translational medicine. 2018;10(463):eaat0797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huard B, McKee T, Bosshard C, et al. APRIL secreted by neutrophils binds to heparan sulfate proteoglycans to create plasma cell niches in human mucosa. The Journal of clinical investigation. 2008;118(8):2887–2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Scapini P, Nardelli B, Nadali G, et al. G-CSF-stimulated neutrophils are a prominent source of functional BLyS. The Journal of experimental medicine. 2003;197(3):297–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hajishengallis G Immunomicrobial pathogenesis of periodontitis: keystones, pathobionts, and host response. Trends in immunology. 2014;35(1):3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gaffen SL, Hajishengallis G. A new inflammatory cytokine on the block: re-thinking periodontal disease and the Th1/Th2 paradigm in the context of Th17 cells and IL-17. J Dent Res. 2008;87(9):817–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Garlet GP. Destructive and protective roles of cytokines in periodontitis: A re-appraisal from host defense and tissue destruction viewpoints. J Dent Res. 2010;89(12):1349–1363. [DOI] [PubMed] [Google Scholar]
- 71.Silva N, Abusleme L, Bravo D, et al. Host response mechanisms in periodontal diseases. Journal of applied oral science : revista FOB. 2015;23(3):329–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Taubman MA, Valverde P, Han X, Kawai T. Immune response: the key to bone resorption in periodontal disease. Journal of periodontology. 2005;76(11 Suppl):2033–2041. [DOI] [PubMed] [Google Scholar]
- 73.Berglundh T, Donati M, Zitzmann N. B cells in periodontitis: friends or enemies? Periodontology 2000. 2007;45:51–66. [DOI] [PubMed] [Google Scholar]
- 74.Tsukasaki M, Komatsu N, Nagashima K, et al. Host defense against oral microbiota by bone-damaging T cells. Nature communications. 2018;9(1):701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Belibasakis GN, Bostanci N. The RANKL-OPG system in clinical periodontology. Journal of clinical periodontology. 2012;39(3):239–248. [DOI] [PubMed] [Google Scholar]
- 76.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nature reviews Immunology. 2008;8(5):349–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tabas I, Glass CK. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science. 2013;339(6116):166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kourtzelis I, Mitroulis I, von Renesse J, Hajishengallis G, Chavakis T. From leukocyte recruitment to resolution of inflammation: the cardinal role of integrins. Journal of leukocyte biology. 2017;102:677–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Spite M, Claria J, Serhan CN. Resolvins, specialized proresolving lipid mediators, and their potential roles in metabolic diseases. Cell metabolism. 2014;19(1):21–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ortega-Gomez A, Perretti M, Soehnlein O. Resolution of inflammation: an integrated view. EMBO molecular medicine. 2013;5(5):661–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Papayianni A, Serhan CN, Brady HR. Lipoxin A4 and B4 inhibit leukotriene-stimulated interactions of human neutrophils and endothelial cells. J Immunol. 1996;156(6):2264–2272. [PubMed] [Google Scholar]
- 82.Fiore S, Serhan CN. Lipoxin A4 receptor activation is distinct from that of the formyl peptide receptor in myeloid cells: inhibition of CD11/18 expression by lipoxin A4-lipoxin A4 receptor interaction. Biochemistry. 1995;34(51):16678–16686. [DOI] [PubMed] [Google Scholar]
- 83.Spite M, Serhan CN. Novel lipid mediators promote resolution of acute inflammation: impact of aspirin and statins. Circulation research. 2010;107(10):1170–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Spite M, Norling LV, Summers L, et al. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009;461(7268):1287–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510(7503):92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ravichandran KS, Lorenz U. Engulfment of apoptotic cells: signals for a good meal. Nature reviews Immunology. 2007;7(12):964–974. [DOI] [PubMed] [Google Scholar]
- 87.Ravichandran KS. Beginnings of a good apoptotic meal: the find-me and eat-me signaling pathways. Immunity. 2011;35(4):445–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Erwig LP, Henson PM. Clearance of apoptotic cells by phagocytes. Cell death and differentiation. 2008;15(2):243–250. [DOI] [PubMed] [Google Scholar]
- 89.Foks AC, Engelbertsen D, Kuperwaser F, et al. Blockade of Tim-1 and Tim-4 Enhances Atherosclerosis in Low-Density Lipoprotein Receptor-Deficient Mice. Arteriosclerosis, thrombosis, and vascular biology. 2016;36(3):456–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hanayama R, Tanaka M, Miwa K, Shinohara A, Iwamatsu A, Nagata S. Identification of a factor that links apoptotic cells to phagocytes. Nature. 2002;417(6885):182–187. [DOI] [PubMed] [Google Scholar]
- 91.Kourtzelis I, Li X, Mitroulis I, et al. DEL-1 promotes macrophage efferocytosis and clearance of inflammation. Nature immunology. 2019;20(1):40–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hanayama R, Tanaka M, Miwa K, Nagata S. Expression of developmental endothelial locus-1 in a subset of macrophages for engulfment of apoptotic cells. J Immunol. 2004;172(6):3876–3882. [DOI] [PubMed] [Google Scholar]
- 93.Flannagan RS, Canton J, Furuya W, Glogauer M, Grinstein S. The phosphatidylserine receptor TIM4 utilizes integrins as coreceptors to effect phagocytosis. Molecular biology of the cell. 2014;25(9):1511–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tabas I Macrophage death and defective inflammation resolution in atherosclerosis. Nature reviews Immunology. 2010;10(1):36–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yoon YS, Kim SY, Kim MJ, Lim JH, Cho MS, Kang JL. PPARgamma activation following apoptotic cell instillation promotes resolution of lung inflammation and fibrosis via regulation of efferocytosis and proresolving cytokines. Mucosal immunology. 2015;8(5):1031–1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Alonso-Gonzalez N, Bensinger SJ, Hong C, et al. Apoptotic cells promote their own clearance and immune tolerance through activation of the nuclear receptor LXR. Immunity. 2009;31(2):245–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Li AC, Glass CK. PPAR- and LXR-dependent pathways controlling lipid metabolism and the development of atherosclerosis. J Lipid Res. 2004;45(12):2161–2173. [DOI] [PubMed] [Google Scholar]
- 98.Hong C, Kidani Y, N AG, et al. Coordinate regulation of neutrophil homeostasis by liver X receptors in mice. The Journal of clinical investigation. 2012;122(1):337–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tall AR, Yvan-Charvet L. Cholesterol, inflammation and innate immunity. Nature reviews Immunology. 2015;15(2):104–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Paidassi H, Tacnet-Delorme P, Garlatti V, et al. C1q binds phosphatidylserine and likely acts as a multiligand-bridging molecule in apoptotic cell recognition. J Immunol. 2008;180(4):2329–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chawla A Control of macrophage activation and function by PPARs. Circulation research. 2010;106(10):1559–1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Salvi GE, Lang NP. The effects of non-steroidal anti-inflammatory drugs (selective and non-selective) on the treatment of periodontal diseases. Curr Pharm Des ,. 2005;11(14):1757–1769. [DOI] [PubMed] [Google Scholar]
- 103.Reddy MS, Geurs NC, Gunsolley JC. Periodontal host modulation with antiproteinase, anti-inflammatory, and bone-sparing agents. A systematic review. Annals of periodontology / the American Academy of Periodontology. 2003;8(1):12–37. [DOI] [PubMed] [Google Scholar]
- 104.Heasman PA, Collins JG, Offenbacher S. Changes in crevicular fluid levels of interleukin-1 beta, leukotriene B4, prostaglandin E2, thromboxane B2 and tumour necrosis factor alpha in experimental gingivitis in humans. Journal of periodontal research. 1993;28(4):241–247. [DOI] [PubMed] [Google Scholar]
- 105.Roberts FA, Houston LS, Lukehart SA, Mancl LA, Persson GR, Page RC. Periodontitis vaccine decreases local prostaglandin E2 levels in a primate model. Infection and immunity. 2004;72(2):1166–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bezerra MM, de Lima V, Alencar VB, et al. Selective cyclooxygenase-2 inhibition prevents alveolar bone loss in experimental periodontitis in rats. Journal of periodontology. 2000;71(6):1009–1014. [DOI] [PubMed] [Google Scholar]
- 107.Holzhausen M, Spolidorio DM, Muscara MN, Hebling J, Spolidorio LC. Protective effects of etoricoxib, a selective inhibitor of cyclooxygenase-2, in experimental periodontitis in rats. Journal of periodontal research. 2005;40(3):208–211. [DOI] [PubMed] [Google Scholar]
- 108.Chee B, Park B, Fitzsimmons T, Coates AM, Bartold PM. Omega-3 fatty acids as an adjunct for periodontal therapy-a review. Clin Oral Investig. 2016;20(5):879–894. [DOI] [PubMed] [Google Scholar]
- 109.Lindsley CB, Warady BA. Nonsteroidal antiinflammatory drugs. Renal toxicity. Review of pediatric issues. Clin Pediatr (Phila). 1990;29(1):10–13. [DOI] [PubMed] [Google Scholar]
- 110.Hawkey CJ. Gastroduodenal problems associated with non-steroidal, anti-inflammatory drugs (NSAIDs). Scand J Gastroenterol Suppl. 1993;200:94–95. [DOI] [PubMed] [Google Scholar]
- 111.Warner TD, Mitchell JA. Cyclooxygenases: new forms, new inhibitors, and lessons from the clinic. FASEB J. 2004;18(7):790–804. [DOI] [PubMed] [Google Scholar]
- 112.Ghosh M, Wang H, Ai Y, et al. COX-2 suppresses tissue factor expression via endocannabinoid-directed PPARd activation. J Exp Med. 2007;204(9):2053–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Assuma R, Oates T, Cochran D, Amar S, Graves DT. IL-1 and TNF antagonists inhibit the inflammatory response and bone loss in experimental periodontitis. J Immunol. 1998;160(1):403–409. [PubMed] [Google Scholar]
- 114.Di Paola R, Mazzon E, Muia C, et al. Effects of etanercept, a tumour necrosis factor-alpha antagonist, in an experimental model of periodontitis in rats. British journal of pharmacology. 2007;150(3):286–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Han JY, Reynolds MA. Effect of anti-rheumatic agents on periodontal parameters and biomarkers of inflammation: a systematic review and meta-analysis. Journal of periodontal & implant science. 2012;42(1):3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rogler G, Biedermann L, Scharl M. Anti-Cytokine Strategies beyond Anti-Tumour Necrosis Factor-alpha Therapy: Pathophysiology and Clinical Implications. Dig Dis. 2017;35(1–2):5–12. [DOI] [PubMed] [Google Scholar]
- 117.Moutsopoulos NM, Zerbe CS, Wild T, et al. Interleukin-12 and Interleukin-23 Blockade in Leukocyte Adhesion Deficiency Type 1. The New England journal of medicine. 2017;376(12):1141–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Maekawa T, Hosur K, Abe T, et al. Antagonistic effects of IL-17 and D-resolvins on endothelial Del-1 expression through a GSK-3beta-C/EBPbeta pathway. Nature communications. 2015;6:8272 10.1038/ncomms9272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Van Dyke TE. Pro-resolving mediators in the regulation of periodontal disease. Mol Aspects Med. 2017;58 21–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hasturk H, Kantarci A, Goguet-Surmenian E, et al. Resolvin E1 regulates inflammation at the cellular and tissue level and restores tissue homeostasis in vivo. J Immunol. 2007;179(10):7021–7029. [DOI] [PubMed] [Google Scholar]
- 121.Lee C-T, Teles R, Kantarci A, et al. Resolvin E1 Reverses Experimental Periodontitis and Dysbiosis. The Journal of Immunology. 2016;197:2796–2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Van Dyke TE, Hasturk H, Kantarci A, et al. Proresolving nanomedicines activate bone regeneration in periodontitis. J Dent Res. 2015;94(1):148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bartold MP, Van Dyke TE. Host modulation: controlling the inflammation to control the infection. Periodontology 2000. 2017;75(1):317–329. [DOI] [PubMed] [Google Scholar]
- 124.Naqvi AZ, Hasturk H, Mu L, et al. Docosahexaenoic Acid and Periodontitis in Adults: A Randomized Controlled Trial. J Dent Res. 2014;93(8):767–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.El-Sharkawy H, Aboelsaad N, Eliwa M, et al. Adjunctive treatment of chronic periodontitis with daily dietary supplementation with omega-3 Fatty acids and low-dose aspirin. Journal of periodontology. 2010;81(11):1635–1643. [DOI] [PubMed] [Google Scholar]
- 126.Chen C COX-2’s new role in inflammation. Nature chemical biology. 2010;6(6):401–402. [DOI] [PubMed] [Google Scholar]
- 127.Gatej S, Gully N, Gibson R, Bartold PM. Probiotics and Periodontitis – A Literature Review. Journal of the International Academy of Periodontology. 2017;19(2):42–50. [PubMed] [Google Scholar]
- 128.Laleman I, Teughels W. Probiotics in the dental practice: a review. Quintessence Int. 2015;46(3):255–264. [DOI] [PubMed] [Google Scholar]
- 129.Allaker RP, Stephen AS. Use of Probiotics and Oral Health. Curr Oral Health Rep. 2017;4(4):309–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kobayashi R, Kobayashi T, Sakai F, Hosoya T, Yamamoto M, Kurita-Ochiai T. Oral administration of Lactobacillus gasseri SBT2055 is effective in preventing Porphyromonas gingivalis-accelerated periodontal disease. Scientific reports. 2017;7(1):545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Maekawa T, Hajishengallis G. Topical treatment with probiotic Lactobacillus brevis CD2 inhibits experimental periodontal inflammation and bone loss. Journal of periodontal research. 2014;49:785–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lee JK, Kim SJ, Ko SH, Ouwehand AC, Ma DS. Modulation of the host response by probiotic Lactobacillus brevis CD2 in experimental gingivitis. Oral diseases. 2015;21(6):705–712. [DOI] [PubMed] [Google Scholar]
- 133.Martin-Cabezas R, Davideau JL, Tenenbaum H, Huck O. Clinical efficacy of probiotics as an adjunctive therapy to non-surgical periodontal treatment of chronic periodontitis: a systematic review and meta-analysis. Journal of clinical periodontology. 2016;43(6):520–530. [DOI] [PubMed] [Google Scholar]
- 134.Courts FJ, Boackle RJ, Fudenberg HH, Silverman MS. Detection of functional complement components in gingival crevicular fluid from humans with periodontal diseases. J Dent Res. 1977;56(3):327–331. [DOI] [PubMed] [Google Scholar]
- 135.Attstrom R, Laurel AB, Lahsson U, Sjoholm A. Complement factors in gingival crevice material from healthy and inflamed gingiva in humans. J Periodont Res. 1975;10(1):19–27. [DOI] [PubMed] [Google Scholar]
- 136.Toto PD, Lin L, Gargiulo A. Identification of C3a, IgG, IgM in inflamed human gingiva. J Dent Res. 1978;57(5–6):696. [DOI] [PubMed] [Google Scholar]
- 137.Nikolopoulou-Papaconstantinou AA, Johannessen AC, Kristoffersen T. Deposits of immunoglobulins, complement, and immune complexes in inflamed human gingiva. Acta odontologica Scandinavica. 1987;45(3):187–193. [DOI] [PubMed] [Google Scholar]
- 138.Rautemaa R, Meri S. Protection of gingival epithelium against complement-mediated damage by strong expression of the membrane attack complex inhibitor protectin (CD59). J Dent Res. 1996;75(1):568–574. [DOI] [PubMed] [Google Scholar]
- 139.Schenkein HA, Genco RJ. Gingival fluid and serum in periodontal diseases. II. Evidence for cleavage of complement components C3, C3 proactivator (factor B) and C4 in gingival fluid. Journal of periodontology. 1977;48(12):778–784. [DOI] [PubMed] [Google Scholar]
- 140.Lally ET, McArthur WP, Baehni PC. Biosynthesis of complement components in chronically inflamed gingiva. Journal of periodontal research. 1982;17(3):257–262. [DOI] [PubMed] [Google Scholar]
- 141.Patters MR, Niekrash CE, Lang NP. Assessment of complement cleavage in gingival fluid during experimental gingivitis in man. Journal of clinical periodontology. 1989;16(1):33–37. [DOI] [PubMed] [Google Scholar]
- 142.Niekrash CE, Patters MR. Simultaneous assessment of complement components C3, C4, and B and their cleavage products in human gingival fluid. II. Longitudinal changes during periodontal therapy. Journal of periodontal research. 1985;20(3):268–275. [DOI] [PubMed] [Google Scholar]
- 143.Maekawa T, Abe T, Hajishengallis E, et al. Genetic and intervention studies implicating complement C3 as a major target for the treatment of periodontitis. J Immunol. 2014;192 6020–6027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Liang S, Krauss JL, Domon H, et al. The C5a receptor impairs IL-12-dependent clearance of Porphyromonas gingivalis and is required for induction of periodontal bone loss. J Immunol. 2011;186(2):869–877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ricklin D, Lambris JD. Complement in immune and inflammatory disorders: pathophysiological mechanisms. J Immunol. 2013;190(8):3831–3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mastellos DC, Yancopoulou D, Kokkinos P, et al. Compstatin: a C3-targeted complement inhibitor reaching its prime for bedside intervention. European journal of clinical investigation. 2015;45(4):423–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Qu H, Ricklin D, Bai H, et al. New analogs of the clinical complement inhibitor compstatin with subnanomolar affinity and enhanced pharmacokinetic properties. Immunobiology. 2013;218(4):496–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sahu A, Kay BK, Lambris JD. Inhibition of human complement by a C3-binding peptide isolated from a phage-displayed random peptide library. J Immunol. 1996;157(2):884–891. [PubMed] [Google Scholar]
- 149.Berger N, Alayi TD, Resuello RRG, Tuplano JV, Reis ES, Lambris JD. New Analogs of the Complement C3 Inhibitor Compstatin with Increased Solubility and Improved Pharmacokinetic Profile. Journal of medicinal chemistry. 2018;61(14):6153–6162. [DOI] [PubMed] [Google Scholar]
- 150.Maekawa T, Briones RA, Resuello RR, et al. Inhibition of pre-existing natural periodontitis in non-human primates by a locally administered peptide inhibitor of complement C3. Journal of clinical periodontology. 2016;43:238–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kajikawa T, Briones RA, Resuello RRG, et al. Safety and Efficacy of the Complement Inhibitor AMY-101 in a Natural Model of Periodontitis in Non-human Primates. Molecular Therapy - Methods & Clinical Development. 2017;6:207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Mizutani N, Goshima H, Nabe T, Yoshino S. Complement C3a-induced IL-17 plays a critical role in an IgE-mediated late-phase asthmatic response and airway hyperresponsiveness via neutrophilic inflammation in mice. J Immunol. 2012;188(11):5694–5705. [DOI] [PubMed] [Google Scholar]
- 153.Abe T, Hosur KB, Hajishengallis E, et al. Local complement-targeted intervention in periodontitis: proof-of-concept using a C5a receptor (CD88) antagonist. J Immunol. 2012;189(11):5442–5448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Liu B, Wei L, Meyerle C, et al. Complement component C5a promotes expression of IL-22 and IL-17 from human T cells and its implication in age-related macular degeneration. J Transl Med. 2011;9:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Xu R, Wang R, Han G, et al. Complement C5a regulates IL-17 by affecting the crosstalk between DC and gammadelta T cells in CLP-induced sepsis. European journal of immunology. 2010;40(4):1079–1088. [DOI] [PubMed] [Google Scholar]
- 156.Lajoie S, Lewkowich IP, Suzuki Y, et al. Complement-mediated regulation of the IL-17A axis is a central genetic determinant of the severity of experimental allergic asthma. Nature immunology. 2010;11(10):928–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bosmann M, Sarma JV, Atefi G, Zetoune FS, Ward PA. Evidence for anti-inflammatory effects of C5a on the innate IL-17A/IL-23 axis. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2011;26:1640–1651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Miossec P, Kolls JK. Targeting IL-17 and TH17 cells in chronic inflammation. Nature reviews Drug discovery. 2012;11(10):763–776. [DOI] [PubMed] [Google Scholar]
- 159.Zenobia C, Hajishengallis G. Basic biology and role of interleukin-17 in immunity and inflammation. Periodontology 2000. 2015;69(1):142–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Mastellos DC, Ricklin D, Hajishengallis E, Hajishengallis G, Lambris JD. Complement therapeutics in inflammatory diseases: promising drug candidates for C3-targeted intervention. Molecular oral microbiology. 2016;31(1):3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ricklin D, Mastellos DC, Reis ES, Lambris JD. The renaissance of complement therapeutics. Nat Rev Nephrol. 2018;14(1):26–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Reis ES, Berger N, Wang X, et al. Safety profile after prolonged C3 inhibition. Clin Immunol. 2018;197:96–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Medicine UNLo. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT03316521 2017.
- 164.Hidai C, Kawana M, Kitano H, Kokubun S. Discoidin domain of Del1 protein contributes to its deposition in the extracellular matrix. Cell and tissue research. 2007;330(1):83–95. [DOI] [PubMed] [Google Scholar]
- 165.Chavakis E, Choi EY, Chavakis T. Novel aspects in the regulation of the leukocyte adhesion cascade. Thrombosis and haemostasis. 2009;102(2):191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hidai C, Zupancic T, Penta K, et al. Cloning and characterization of developmental endothelial locus-1: an embryonic endothelial cell protein that binds the alphavbeta3 integrin receptor. Genes & development. 1998;12(1):21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Shin J, Maekawa T, Abe T, et al. DEL-1 restrains osteoclastogenesis and inhibits inflammatory bone loss in nonhuman primates. Science translational medicine. 2015;7(307):307ra155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kajikawa T, Meshikhes F, Maekawa T, et al. MFG-E8 inhibits periodontitis in non-human primates and its gingival crevicular fluid levels can differentiate periodontal health from disease in humans. Journal of clinical periodontology. 2017;44:472–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Abe T, Shin J, Hosur K, Udey MC, Chavakis T, Hajishengallis G. Regulation of osteoclast homeostasis and inflammatory bone loss by MFG-E8. J Immunol. 2014;193(3):1383–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mitroulis I, Kang YY, Gahmberg CG, et al. Developmental endothelial locus-1 attenuates complement-dependent phagocytosis through inhibition of Mac-1-integrin. Thrombosis and haemostasis. 2014;111(5):781–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Page RC, Schroeder HE. Periodontitis in man and other animals- A comparative review. Basel, Switzerland: Karger; 1982. [Google Scholar]
- 172.Aziz M, Jacob A, Matsuda A, Wang P. Review: milk fat globule-EGF factor 8 expression, function and plausible signal transduction in resolving inflammation. Apoptosis. 2011;16(11):1077–1086. [DOI] [PubMed] [Google Scholar]
- 173.Cheyuo C, Jacob A, Wu R, et al. Recombinant human MFG-E8 attenuates cerebral ischemic injury: its role in anti-inflammation and anti-apoptosis. Neuropharmacology. 2012;62(2):890–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Shah KG, Wu R, Jacob A, et al. Recombinant human milk fat globule-EGF factor 8 produces dose-dependent benefits in sepsis. Intensive care medicine. 2012;38(1):128–136. [DOI] [PubMed] [Google Scholar]
- 175.Kidani Y, Bensinger SJ. Liver X receptor and peroxisome proliferator-activated receptor as integrators of lipid homeostasis and immunity. Immunological reviews. 2012;249(1):72–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yoo T, Ham SA, Hwang JS, et al. Peroxisome proliferator-activated receptor delta inhibits Porphyromonas gingivalis lipopolysaccharide-induced activation of matrix metalloproteinase-2 by downregulating NADPH oxidase 4 in human gingival fibroblasts. Molecular oral microbiology. 2016;31(5):398–409. [DOI] [PubMed] [Google Scholar]
- 177.Sorsa T, Tjaderhane L, Konttinen YT, et al. Matrix metalloproteinases: contribution to pathogenesis, diagnosis and treatment of periodontal inflammation. Annals of medicine. 2006;38(5):306–321. [DOI] [PubMed] [Google Scholar]
- 178.Di Paola R, Briguglio F, Paterniti I, et al. Emerging role of PPAR-beta/delta in inflammatory process associated to experimental periodontitis. Mediators of inflammation. 2011;2011:787159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Briguglio E, Di Paola R, Paterniti I, et al. WY-14643, a Potent Peroxisome Proliferator Activator Receptor-alpha PPAR-alpha Agonist Ameliorates the Inflammatory Process Associated to Experimental Periodontitis. PPAR Res. 2010;2010:193019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Di Paola R, Mazzon E, Maiere D, et al. Rosiglitazone reduces the evolution of experimental periodontitis in the rat. J Dent Res. 2006;85(2):156–161. [DOI] [PubMed] [Google Scholar]
- 181.Thorbert-Mros S, Larsson L, Berglundh T. Cellular composition of long-standing gingivitis and periodontitis lesions. Journal of periodontal research. 2015;50:535–543. [DOI] [PubMed] [Google Scholar]
- 182.Mackay F, Schneider P, Rennert P, Browning J. BAFF AND APRIL: a tutorial on B cell survival. Annual review of immunology. 2003;21:231–264. [DOI] [PubMed] [Google Scholar]
- 183.Cancro MP. Signalling crosstalk in B cells: managing worth and need. Nature reviews Immunology. 2009;9(9):657–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Abe T, AlSarhan M, Benakanakere MR, et al. The B Cell-Stimulatory Cytokines BLyS and APRIL Are Elevated in Human Periodontitis and Are Required for B Cell-Dependent Bone Loss in Experimental Murine Periodontitis. J Immunol. 2015;195(4):1427–1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Ding HJ, Gordon C. New biologic therapy for systemic lupus erythematosus. Current opinion in pharmacology. 2013;13(3):405–412. [DOI] [PubMed] [Google Scholar]
- 186.Vincent FB, Morand EF, Schneider P, Mackay F. The BAFF/APRIL system in SLE pathogenesis. Nature reviews Rheumatology. 2014;10(6):365–373. [DOI] [PubMed] [Google Scholar]
- 187.Coat J, Demoersman J, Beuzit S, et al. Anti-B lymphocyte immunotherapy is associated with improvement of periodontal status in subjects with rheumatoid arthritis. Journal of clinical periodontology. 2015;42:817–823. [DOI] [PubMed] [Google Scholar]
- 188.O’Shea JJ, Paul WE. Mechanisms underlying lineage commitment and plasticity of helper CD4+ T cells. Science. 2010;327(5969):1098–1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kobayashi R, Kono T, Bolerjack BA, et al. Induction of IL-10-producing CD4+ T-cells in chronic periodontitis. J Dent Res. 2011;90(5):653–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Petermann F, Rothhammer V, Claussen MC, et al. γδ T cells enhance autoimmunity by restraining regulatory T cell responses via an interleukin-23-dependent mechanism. Immunity. 2010;33(3):351–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Glowacki AJ, Yoshizawa S, Jhunjhunwala S, et al. Prevention of inflammation-mediated bone loss in murine and canine periodontal disease via recruitment of regulatory lymphocytes. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(46):18525–18530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Milner JD, Brenchley JM, Laurence A, et al. Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature. 2008;452(7188):773–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Withers DR, Hepworth MR, Wang X, et al. Transient inhibition of ROR-gammat therapeutically limits intestinal inflammation by reducing TH17 cells and preserving group 3 innate lymphoid cells. Nature medicine. 2016;22(3):319–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Nakashima T, Hayashi M, Takayanagi H. New insights into osteoclastogenic signaling mechanisms. Trends Endocrinol Metab. 2012;23(11):582–590. [DOI] [PubMed] [Google Scholar]
- 195.Golub LM, Goodson JM, Lee HM, Vidal AM, McNamara TF, Ramamurthy NS. Tetracyclines inhibit tissue collagenases. Effects of ingested low-dose and local delivery systems. Journal of periodontology. 1985;56(11 Suppl):93–97. [DOI] [PubMed] [Google Scholar]
- 196.Preshaw PM, Hefti AF, Jepsen S, Etienne D, Walker C, Bradshaw MH. Subantimicrobial dose doxycycline as adjunctive treatment for periodontitis. A review. Journal of clinical periodontology. 2004;31(9):697–707. [DOI] [PubMed] [Google Scholar]
- 197.Caton J, Ryan ME. Clinical studies on the management of periodontal diseases utilizing subantimicrobial dose doxycycline (SDD). Pharmacol Res. 2011;63(2):114–120. [DOI] [PubMed] [Google Scholar]
- 198.Hughes DE, Wright KR, Uy HL, et al. Bisphosphonates promote apoptosis in murine osteoclasts in vitro and in vivo. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 1995;10(10):1478–1487. [DOI] [PubMed] [Google Scholar]
- 199.Baron R, Ferrari S, Russell RG. Denosumab and bisphosphonates: different mechanisms of action and effects. Bone. 2011;48(4):677–692. [DOI] [PubMed] [Google Scholar]
- 200.Salvi GE, Lang NP. Host response modulation in the management of periodontal diseases. Journal of clinical periodontology. 2005;32 Suppl 6:108–129. [DOI] [PubMed] [Google Scholar]
- 201.Kirkwood KL, Cirelli JA, Rogers JE, Giannobile WV. Novel host response therapeutic approaches to treat periodontal diseases. Periodontology 2000. 2007;43:294–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Palomo L, Liu J, Bissada NF. Skeletal bone diseases impact the periodontium: a review of bisphosphonate therapy. Expert Opin Pharmacother. 2007;8(3):309–315. [DOI] [PubMed] [Google Scholar]
- 203.Lin X, Han X, Kawai T, Taubman MA. Antibody to receptor activator of NF-κB ligand ameliorates T cell-mediated periodontal bone resorption. Infection and immunity. 2011;79(2):911–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Han X, Lin X, Yu X, et al. Porphyromonas gingivalis infection-associated periodontal bone resorption is dependent on receptor activator of NF-κB ligand. Infection and immunity. 2013;81(5):1502–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.McClung MR, Lewiecki EM, Cohen SB, et al. Denosumab in postmenopausal women with low bone mineral density. The New England journal of medicine. 2006;354(8):821–831. [DOI] [PubMed] [Google Scholar]
- 206.Pageau SC. Denosumab. MAbs. 2009;1(3):210–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Egloff-Juras C, Gallois A, Salleron J, et al. Denosumab-related osteonecrosis of the jaw: A retrospective study. J Oral Pathol Med. 2017. [DOI] [PubMed] [Google Scholar]
- 208.Maeda K, Kobayashi Y, Udagawa N, et al. Wnt5a-Ror2 signaling between osteoblast-lineage cells and osteoclast precursors enhances osteoclastogenesis. Nature medicine. 2012;18(3):405–412. [DOI] [PubMed] [Google Scholar]
- 209.Hausler KD, Horwood NJ, Chuman Y, et al. Secreted frizzled-related protein-1 inhibits RANKL-dependent osteoclast formation. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2004;19(11):1873–1881. [DOI] [PubMed] [Google Scholar]
- 210.Bovolenta P, Esteve P, Ruiz JM, Cisneros E, Lopez-Rios J. Beyond Wnt inhibition: new functions of secreted Frizzled-related proteins in development and disease. Journal of cell science. 2008;121(Pt 6):737–746. [DOI] [PubMed] [Google Scholar]
- 211.Maekawa T, Kulwattanaporn P, Hosur K, et al. Differential Expression and Roles of Secreted Frizzled-Related Protein 5 and the Wingless Homolog Wnt5a in Periodontitis. J Dent Res. 2017;96(5):571–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ouchi N, Higuchi A, Ohashi K, et al. Sfrp5 is an anti-inflammatory adipokine that modulates metabolic dysfunction in obesity. Science. 2010;329(5990):454–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Nakamura K, Sano S, Fuster JJ, et al. Secreted Frizzled-related Protein 5 Diminishes Cardiac Inflammation and Protects the Heart from Ischemia/Reperfusion Injury. The Journal of biological chemistry. 2016;291(6):2566–2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Hajishengallis G, Russell MW. Molecular approaches to vaccination against oral infections In: Rogers A, ed. Molecular Oral Microbiology. Norfolk, UK: Caister Academic Press; 2008:257–285. [Google Scholar]
- 215.Goodridge HS, Ahmed SS, Curtis N, et al. Harnessing the beneficial heterologous effects of vaccination. Nature reviews Immunology. 2016;16(6):392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Arts RJW, Moorlag S, Novakovic B, et al. BCG Vaccination Protects against Experimental Viral Infection in Humans through the Induction of Cytokines Associated with Trained Immunity. Cell Host Microbe. 2018;23(1):89–100 e105. [DOI] [PubMed] [Google Scholar]
- 217.Netea MG, Joosten LA, Latz E, et al. Trained immunity: A program of innate immune memory in health and disease. Science. 2016;352(6284):aaf1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Mitroulis I, Ruppova K, Wang B, et al. Modulation of Myelopoiesis Progenitors Is an Integral Component of Trained Immunity. Cell. 2018;172(1–2):147–161 e112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Penkov S, Mitroulis I, Hajishengallis G, Chavakis T. Immunometabolic Crosstalk: An Ancestral Principle of Trained Immunity? Trends in immunology. 2019;40(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Maekawa T, Krauss JL, Abe T, et al. Porphyromonas gingivalis manipulates complement and TLR signaling to uncouple bacterial clearance from inflammation and promote dysbiosis. Cell Host Microbe. 2014;15(6):768–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Makkawi H, Hoch S, Burns E, et al. Porphyromonas gingivalis Stimulates TLR2-PI3K Signaling to Escape Immune Clearance and Induce Bone Resorption Independently of MyD88. Frontiers in cellular and infection microbiology. 2017;7(Article no. 359):359 10.3389/fcimb.2017.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Sharma A, Honma K, Evans RT, Hruby DE, Genco RJ. Oral immunization with recombinant Streptococcus gordonii expressing Porphyromonas gingivalis FimA domains. Infection and immunity. 2001;69(5):2928–2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Gibson FC 3rd, Genco CA. Prevention of Porphyromonas gingivalis-induced oral bone loss following immunization with gingipain R1. Infection and immunity. 2001;69(12):7959–7963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.O’Brien-Simpson NM, Pathirana RD, Paolini RA, et al. An immune response directed to proteinase and adhesin functional epitopes protects against Porphyromonas gingivalis-induced periodontal bone loss. J Immunol. 2005;175(6):3980–3989. [DOI] [PubMed] [Google Scholar]
- 225.Rajapakse PS, O’Brien-Simpson NM, Slakeski N, Hoffmann B, Reynolds EC. Immunization with the RgpA-Kgp proteinase-adhesin complexes of Porphyromonas gingivalis protects against periodontal bone loss in the rat periodontitis model. Infection and immunity. 2002;70(5):2480–2486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Katz J, Black KP, Michalek SM. Host responses to recombinant hemagglutinin B of Porphyromonas gingivalis in an experimental rat model. Infection and immunity. 1999;67(9):4352–4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.DeCarlo AA, Huang Y, Collyer CA, Langley DB, Katz J. Feasibility of an HA2 domain-based periodontitis vaccine. Infection and immunity. 2003;71(1):562–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.O’Brien-Simpson NM, Holden JA, Lenzo JC, et al. A therapeutic Porphyromonas gingivalis gingipain vaccine induces neutralising IgG1 antibodies that protect against experimental periodontitis. NPJ Vaccines. 2016;1:16022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Zhao L, Zhou Y, Xu Y, Sun Y, Li L, Chen W. Effect of non-surgical periodontal therapy on the levels of Th17/Th1/Th2 cytokines and their transcription factors in Chinese chronic periodontitis patients. Journal of clinical periodontology. 2011;38(6):509–516. [DOI] [PubMed] [Google Scholar]
- 230.Moritz AJ, Cappelli D, Lantz MS, Holt SC, Ebersole JL. Immunization with Porphyromonas gingivalis cysteine protease: effects on experimental gingivitis and ligature-induced periodontitis in Macaca fascicularis. Journal of periodontology. 1998;69(6):686–697. [DOI] [PubMed] [Google Scholar]
- 231.Page RC, Lantz MS, Darveau R, et al. Immunization of Macaca fascicularis against experimental periodontitis using a vaccine containing cysteine proteases purified from Porphyromonas gingivalis. Oral microbiology and immunology. 2007;22(3):162–168. [DOI] [PubMed] [Google Scholar]
- 232.Laine ML, Crielaard W, Loos BG. Genetic susceptibility to periodontitis. Periodontology 2000. 2012;58(1):37–68. [DOI] [PubMed] [Google Scholar]
- 233.Rhodin K, Divaris K, North KE, et al. Chronic Periodontitis Genome-wide Association Studies: Gene-centric and Gene Set Enrichment Analyses. J Dent Res. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Haffajee AD, Cugini MA, Tanner A, et al. Subgingival microbiota in healthy, well-maintained elder and periodontitis subjects. Journal of clinical periodontology. 1998;25(5):346–353. [DOI] [PubMed] [Google Scholar]
- 235.Reynolds EC, O’Brien-Simpson N, Rowe T, et al. Prospects for treatment of Porphyromonas gingivalis-mediated disease - immune-based therapy. Journal of oral microbiology. 2015;7:29125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.van der Velden U The onset age of periodontal destruction. Journal of clinical periodontology. 1991;18(6):380–383. [DOI] [PubMed] [Google Scholar]
- 237.Miller RA. The aging immune system: primer and prospectus. Science. 1996;273(5271):70–74. [DOI] [PubMed] [Google Scholar]
- 238.Huttner EA, Machado DC, de Oliveira RB, Antunes AG, Hebling E. Effects of human aging on periodontal tissues. Spec Care Dentist. 2009;29(4):149–155. [DOI] [PubMed] [Google Scholar]
- 239.Solana R, Tarazona R, Gayoso I, Lesur O, Dupuis G, Fulop T. Innate immunosenescence: effect of aging on cells and receptors of the innate immune system in humans. Seminars in immunology. 2012;24(5):331–341. [DOI] [PubMed] [Google Scholar]
- 240.Hajishengallis G Too old to fight? Aging and its toll on innate immunity. Molecular oral microbiology. 2010;25(1):25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Dorshkind K, Montecino-Rodriguez E, Signer RA. The ageing immune system: is it ever too old to become young again? Nature reviews Immunology. 2009;9(1):57–62. [DOI] [PubMed] [Google Scholar]
- 242.Willershausen-Zonnchen B, Gleissner C. Periodontal disease in elderly patients. Eur J Med Res. 1998;3(1–2):55–64. [PubMed] [Google Scholar]
- 243.Ebersole JL, Kirakodu S, Novak MJ, et al. Effects of aging in the expression of NOD-like receptors and inflammasome-related genes in oral mucosa. Molecular oral microbiology. 2016;31(1):18–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Gonzalez OA, Nagarajan R, Novak MJ, et al. Immune system transcriptome in gingival tissues of young nonhuman primates. Journal of periodontal research. 2015;doi: 10.1111/jre.12293. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Huang L, Salmon B, Yin X, Helms JA. From restoration to regeneration: periodontal aging and opportunities for therapeutic intervention. Periodontology 2000. 2016;72(1):19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Neves J, Sousa-Victor P, Jasper H. Rejuvenating Strategies for Stem Cell-Based Therapies in Aging. Cell Stem Cell. 2017;20(2):161–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Ebersole JL, Graves CL, Gonzalez OA, et al. Aging, inflammation, immunity and periodontal disease. Periodontology 2000. 2016;72(1):54–75. [DOI] [PubMed] [Google Scholar]
- 248.Shaw AC, Goldstein DR, Montgomery RR. Age-dependent dysregulation of innate immunity. Nature reviews Immunology. 2013;13(12):875–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Naveiras O, Nardi V, Wenzel PL, Hauschka PV, Fahey F, Daley GQ. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature. 2009;460(7252):259–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Geiger H, de Haan G, Florian MC. The ageing haematopoietic stem cell compartment. Nature reviews Immunology. 2013;13(5):376–389. [DOI] [PubMed] [Google Scholar]
- 251.Wu RX, Bi CS, Yu Y, Zhang LL, Chen FM. Age-related decline in the matrix contents and functional properties of human periodontal ligament stem cell sheets. Acta Biomater. 2015;22:70–82. [DOI] [PubMed] [Google Scholar]
- 252.Zheng W, Wang S, Ma D, Tang L, Duan Y, Jin Y. Loss of proliferation and differentiation capacity of aged human periodontal ligament stem cells and rejuvenation by exposure to the young extrinsic environment. Tissue Eng Part A. 2009;15(9):2363–2371. [DOI] [PubMed] [Google Scholar]
- 253.Denkinger MD, Leins H, Schirmbeck R, Florian MC, Geiger H. HSC Aging and Senescent Immune Remodeling. Trends in immunology. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Gomez CR, Nomellini V, Faunce DE, Kovacs EJ. Innate immunity and aging. Exp Gerontol. 2008;43(8):718–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Wagner W, Horn P, Bork S, Ho AD. Aging of hematopoietic stem cells is regulated by the stem cell niche. Exp Gerontol. 2008;43(11):974–980. [DOI] [PubMed] [Google Scholar]
- 256.Sui BD, Hu CH, Zheng CX, Jin Y. Microenvironmental Views on Mesenchymal Stem Cell Differentiation in Aging. J Dent Res. 2016;95(12):1333–1340. [DOI] [PubMed] [Google Scholar]
- 257.Mendelson A, Frenette PS. Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nature medicine. 2014;20(8):833–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Huang GT, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res. 2009;88(9):792–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Nishikawa SI, Osawa M, Yonetani S, Torikai-Nishikawa S, Freter R. Niche required for inducing quiescent stem cells. Cold Spring Harb Symp Quant Biol. 2008;73:67–71. [DOI] [PubMed] [Google Scholar]
- 260.Lynch K, Pei M. Age associated communication between cells and matrix: a potential impact on stem cell-based tissue regeneration strategies. Organogenesis. 2014;10(3):289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Poulos MG, Ramalingam P, Gutkin MC, et al. Endothelial transplantation rejuvenates aged hematopoietic stem cell function. The Journal of clinical investigation. 2017;127(11):4163–4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Mitroulis I, Chen L-S, Singh RP, et al. Secreted protein Del-1 regulates myelopoiesis in the hematopoietic stem cell niche. The Journal of clinical investigation. 2017;127(10):3624–3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Franceschi C, Bonafe M, Valensin S, et al. Inflamm-aging. An evolutionary perspective on immunosenescence. Annals of the New York Academy of Sciences. 2000;908:244–254. [DOI] [PubMed] [Google Scholar]
- 264.Mahbub S, Brubaker AL, Kovacs EJ. Aging of the Innate Immune System: An Update. Curr Immunol Rev. 2011;7(1):104–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Wenisch C, Patruta S, Daxbock F, Krause R, Horl W. Effect of age on human neutrophil function. Journal of leukocyte biology. 2000;67(1):40–45. [DOI] [PubMed] [Google Scholar]
- 266.Butcher S, Chahel H, Lord JM. Ageing and the neutrophil: no appetite for killing? Immunology. 2000;100(4):411–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Butcher SK, Chahal H, Nayak L, et al. Senescence in innate immune responses: reduced neutrophil phagocytic capacity and CD16 expression in elderly humans. Journal of leukocyte biology. 2001;70(6):881–886. [PubMed] [Google Scholar]
- 268.Biasi D, Carletto A, Dell’Agnola C, et al. Neutrophil migration, oxidative metabolism, and adhesion in elderly and young subjects. Inflammation. 1996;20(6):673–681. [DOI] [PubMed] [Google Scholar]
- 269.Chen C, Liu Y, Liu Y, Zheng P. mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells. Science signaling. 2009;2(98):ra75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.An JY, Quarles EK, Mekvanich S, et al. Rapamycin treatment attenuates age-associated periodontitis in mice. Geroscience. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Kim M, Kim C, Choi YS, Kim M, Park C, Suh Y. Age-related alterations in mesenchymal stem cells related to shift in differentiation from osteogenic to adipogenic potential: implication to age-associated bone diseases and defects. Mechanisms of ageing and development. 2012;133(5):215–225. [DOI] [PubMed] [Google Scholar]
- 272.Guo Y, MacIsaac KD, Chen Y, et al. Inhibition of RORgammaT Skews TCRalpha Gene Rearrangement and Limits T Cell Repertoire Diversity. Cell Rep. 2016;17(12):3206–3218. [DOI] [PubMed] [Google Scholar]
- 273.Santori FR, Huang P, van de Pavert SA, et al. Identification of natural RORgamma ligands that regulate the development of lymphoid cells. Cell metabolism. 2015;21(2):286–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Eskan MA, Hajishengallis G, Kinane DF. Differential activation of human gingival epithelial cells and monocytes by Porphyromonas gingivalis fimbriae. Infection and immunity. 2007;75(2):892–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Stoller JK, Aboussouan LS. A review of alpha1-antitrypsin deficiency. American journal of respiratory and critical care medicine. 2012;185(3):246–259. [DOI] [PubMed] [Google Scholar]
- 276.Fieker A, Philpott J, Armand M. Enzyme replacement therapy for pancreatic insufficiency: present and future. Clinical and experimental gastroenterology. 2011;4:55–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Bartold PM, Van Dyke TE. Periodontitis: a host-mediated disruption of microbial homeostasis. Unlearning learned concepts. Periodontology 2000. 2013;62(1):203–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Heitz-Mayfield LJ. Disease progression: identification of high-risk groups and individuals for periodontitis. Journal of clinical periodontology. 2005;32 Suppl 6:196–209. [DOI] [PubMed] [Google Scholar]
- 279.Genco RJ, Genco FD. Common risk factors in the management of periodontal and associated systemic diseases: the dental setting and interprofessional collaboration. The journal of evidence-based dental practice. 2014;14 Suppl:4–16. [DOI] [PubMed] [Google Scholar]