Abstract
Upwards of one in ten adults worldwide may be affected by severe periodontitis, making the disease more prevalent than cardiovascular disease. Despite the global scope of periodontal disease, its impact on pain, oral function, and the wellbeing of individuals, and the disproportionate burden of disease and the socio-economic impact on communities, the perception that periodontal disease is a public health problem remains low. Although there have been substantial improvements in our understanding of the etiology of periodontal disease and how we can prevent and control it, these advances have been primarily focused on individual, patient-focused approaches. The prevention of periodontal disease depends on improving currently available individual interventions, and on determining what public health interventions can be effective and sustainable under real-life conditions. Currently, public health approaches for periodontal disease prevention and control are lacking. This review traces the historical strategies for prevention of periodontal disease in an epidemiologic transition context, using a modified model developed for cardiovascular disease, and presents a possible public health approach. Improving periodontal disease prevention and control will need to take into consideration the core activities of a public health approach: assessment, policy development, and assurance.
Introduction
Poor oral health is a global public health concern that is linked to social inequalities and high-risk behaviors such as smoking and unhealthy diet. Many of these high-risk behaviors are considered common or shared risk factors for other chronic, non-communicable diseases, such as diabetes and cardiovascular disease. The burden of poor oral health is high, with substantial social, psychological, and economic impacts that affect individuals, communities and health services. Poor oral health is often characterized as experiencing dental caries, periodontal disease, or complete tooth loss, and recent estimates suggest that as many 3.5 billion people worldwide are affected1. Yet a significant contributor to poor oral health – periodontal disease – has not received the level of attention that has been applied towards dental caries with regards to public health approaches for disease control and prevention.
More than 10% of the adult population worldwide may be affected by severe periodontitis, making it the 11th most prevalent disease globally, more prevalent than cardiovascular disease1,3–5. Within this global rate there are significant disparities by country. Like cardiovascular disease, periodontal disease is a chronic disease marked by an inflammatory process that typically increases in prevalence with age. Unlike cardiovascular disease, the implementation of public health models directed at prevention and control of periodontal disease has been almost non-existent.
The public health approach to preventing disease and promoting health can be organized into three core activities: assessment, policy development, and assurance. Assessment is the systematic examination of health status indicators for a given population6. Although the history of assessment in periodontal disease epidemiology over the past five decades can best be characterized as evolving and improving, implementation of more robust measurement and estimation methodologies has provided a better understanding of the influences, risk factors, and contemporary burden of global periodontitis. However, regarding the other core activities of policy development and assurance (such as evaluating effectiveness), there has been little activity. In a 2005 review article, Peterson and Ogawa articulated a vision for periodontal disease control based on a common risk factor approach and concluded that “countries needed to establish a surveillance system for measuring progress in the control of periodontal disease and promotion of oral health7.” More than a decade later, the public health approach to control and prevent periodontal disease remains mostly an “assessment” activity.
Unlike periodontal disease, the public health approach towards the prevention of cardiovascular disease has seen substantial progress. In 2003, the US Centers for Disease Control and Prevention in collaboration with the American Heart Association and other partners, released a comprehensive public health plan to prevent heart disease and stroke8. Foundational elements of this plan were described in the context of the 3 core functions of public health, where substantial achievements in assessment and policy development were acknowledged, but challenges in assurance were considered where resources were needed to reduce the burden of disease and advance health8. A decade later, the Centers for Disease Control and Prevention and Centers for Medicare and Medicaid Services launched the Million Hearts campaign, spotlighting three major risk reduction areas and optimizing care9. The World Health Organization also launched the Global Hearts Initiative in 201610. Key elements are focusing on improving standardized measurements and methodologies (assessment), developing policies to reduce tobacco use and salt intake, and strengthening assurance activities by building capacity and monitoring implementation.
The development of a comprehensive public health strategy to prevent periodontal disease and improve oral health is still in a nascent stage. For action to reach the scale that has been seen for public health intervention on cardiovascular diseases, awareness of the impact on health associated with periodontal disease must be clearly articulated to frame periodontal disease as a public health problem. To help us better frame the effect of periodontal disease on population health, we can organize the impact of periodontal disease into three general categories:
Local (intra-oral) effects that include gingival bleeding, halitosis, gingival recession, bone loss, tooth displacement, spaces between teeth, tooth mobility, tooth loss, and oral pain.
The impacts on daily living that includes aesthetic and chewing impairment can increase anxiety and feelings of shame and vulnerability. One way to describe these can be expressed as disability adjusted life years, a measure of overall disease burden.
The effect on systemic health likely due to the sharing of common risk factors that affect the oral microbiome or that support systemic inflammation leading to increased associations with cardiovascular diseases (heart disease and stroke), preterm birth/low birthweight, diabetes, chronic obstructive pulmonary disease and pneumonia, pre-eclampsia, chronic kidney disease, delayed conception, and a range of cancers.
Taken together, the cumulative effects of pain, disability, infection, and tooth loss all can lower quality of life and impact well-being. Consequently, this raises an important question in communities with populations disproportionately affected by periodontitis: does periodontal disease qualify as a public health problem?
Periodontal disease qualifies as a public health problem
Public health uses scientific processes to protect and improve the health of people and their communities11. A public health problem can occur based on several conditions, including when the difference between a population’s desired health status and actual health status is substantial, when worsening, or when some groups are more affected by the condition compared to others (disparity). A public health problem can also occur when the burden of the disease (e.g., economic costs or morbidity and mortality) is increasingly affecting individuals and society. A public health problem also conveys the notion of preventability and group action to solve. Essentially, this means there are technically feasible ways to reduce or eliminate the disease that are not limited to individual action but require collective action by people to exhibit the willingness to invest and implement the intervention.
Prevalence and disparity:
Periodontal disease is estimated to be the 11th most prevalent disease globally with an overall prevalence of 7.4% and around 538 million people affected5 (2015). The overall prevalence of periodontitis increases with age, and the incidence rises steeply in adults aged 50–60 years12. It is expected that the burden of periodontitis will continue to increase with the growing ageing population and subsequent increase in tooth retention globally. Periodontitis disproportionately affects the underserved and vulnerable and is a source of social inequity. In the US, 47.2%, or 64.7 million adults, have some form of periodontitis and in adults 65 and older, prevalence increases to 70.1%13,14. According to the Global Burden of Disease Study, periodontal diseases are responsible for 3.5 million years lived with disability3.
Consequences:
Periodontitis is the leading cause of tooth loss in adults worldwide15,16. Individuals with severe periodontal disease are at risk for extensive tooth loss, edentulism, and masticatory dysfunction, thereby affecting their nutrition, quality of life and self‐esteem as well as imposing significant socio‐economic impact. Severe periodontitis, which may result in tooth loss, is found in 5–20% of most adult populations worldwide17–19.
Systemic health link:
Untreated periodontitis is characterized by periodontal pocketing, alveolar bone loss, and inflammation as a result of bacterial challenges to the immune system. Although periodontitis has been shown to be associated with other chronic inflammatory diseases, the exact connections between oral and systemic disease remain complex and obscure. What is clear is that the association with other chronic diseases, such as diabetes, obesity, metabolic syndrome, cardiovascular diseases and stroke – which all have high morbidity, increased mortality, and incur substantial costs to society15,17,20–29 clearly suggests periodontal disease is a public health problem.
Economic burden:
The global cost of lost productivity from severe periodontitis alone has been estimated to be 54 billion USD/year1. The total economic impact of periodontal diseases has been estimated at 440 billion USD (direct and indirect costs) in 201030. In the UK, managing periodontal disease cost the National Health Service over £2.8 billion in 201713,14.
Availability of treatment:
Effective treatments for periodontitis are available and care can be provided early in the natural history of the disease that can eliminate or substantially reduce its negative impact. Prevention of periodontal disease typically consists of patient-performed control of the dental biofilm, professional interventions and control of risk factors. Several strategies have evolved affecting treatment, control, and prevention of periodontal disease based on the concept that primary prevention is the responsibility of the individual and secondary/tertiary prevention is in the domain of the oral health professional, e.g., in-office health education, professionally administered chemotherapeutic applications and advanced cleaning procedures like scaling and root planing.
Evidence from long-term studies indicates that after periodontal therapy, a rate of tooth loss averaging 0.1 tooth/patient/year is observed in subjects participating in professional secondary prevention programs31. This rate is generally compatible with the preservation of the dentition for a lifetime in most subjects. A systematic review reporting tooth survival up to 22 years after periodontal therapy in periodontal specialty practices indicates that tooth loss due to periodontal reasons ranges from 1.5% to 9.8%, while 36 to 89% of individuals receiving treatment do not experience further tooth loss32. Nevertheless, the delivery of appropriate periodontal care to patients requires comprehensive assessments, treatment provided by a highly skilled clinicians, and adequate follow-up care to monitor and maintain periodontal health30,33. This presents several challenges for many health systems globally.
Preventability:
Preventing periodontal disease typically uses an individual approach and focuses on two general areas: (1) promoting oral hygiene activities such as tooth brushing, flossing, and using mouth rinse; and (2) recognizing an individual’s risk factor(s) and recommending intervention for modifiable risks such as smoking. However, population-based strategies for preventing periodontal disease have been limited. The only approach documented to date has been “mass awareness” campaigns34–38 advocating for the importance of oral hygiene and calling for individual behavior modification. In Japan, there is a national promotional effort called the 8020 Campaign.39 This population-based public awareness campaign is focused on encouraging the Japanese people to keep at least 20 teeth by age 80. The program is designed for periodontal disease prevention and is one of few large-scale public health approaches implemented globally.
Perception:
Despite the scope of the prevalence of periodontal disease globally, the impact on pain, oral function, and overall well-being on individuals, and the disproportionate burden of disease and the socio-economic impact on communities, the perception that periodontal disease is a public health problem remains low40–42. This may be reflective of the global perception that oral diseases in general are not serious non-communicable disease problems, or that, unlike dental caries, there are no or limited public health interventions available that can be implemented at the community level.
Individual approach for periodontal disease prevention
The underlying philosophy supporting the individual approach to prevention of periodontal disease draws upon a biological and behavioral model focusing on mechanical biofilm control, reducing putative bacterial load, and eliminating high risk behaviors like smoking. Dental education curricula and research investments globally are focused on this approach. This “biomedical” model has heavily influenced the theoretical understanding of periodontal disease progression and the need for surgical and clinical intervention to secure and maintain periodontal health43. This model also supports the notion that individuals identified as “high-risk” for disease progression should be the focus of therapeutic or preventive interventions. The preventive element in this biomedical approach focuses on supporting changes in hygiene and smoking through chairside advice and counseling.
Consequently, this traditional clinical approach has limited applicability to make any changes in periodontal disease prevalence at the community level. The traditional approach to prevent periodontal disease is costly and can represent a significant economic burden, even in high-income countries43. Overall, it is estimated that the treatment of oral diseases (which includes periodontal disease) is the fourth most expensive condition to treat in developed countries44 and can exceed the total national health care budget for many countries, like Kenya. This approach does not work well for those the disease affects the most – groups affected by health disparities and populations with special needs.
Equally important, the dental workforce in most countries is unable to address the sizeable burden of periodontal disease, especially for secondary or tertiary care. Previous studies/programs have shown mass chairside behavior modification utilizing traditional oral hygiene activities only imparts short-term success as it depends on the compliance of the patient34,35,45,46. Therefore, alternate approaches for public health approaches to prevent, reduce, and control periodontal diseases at the population level.
Epidemiological transition of periodontal diseases and strategies for action
Epidemiology is more than just understanding the distribution and patterns of disease in populations, but is also identifying, monitoring, and promoting activities that prevent or control disease in those populations. The “process” of how patterns of disease changes in concert with changing population dynamics, specifically moving from high mortality communicable diseases to man-made non-communicable disease, is referred to as an epidemiologic transition. To explore historical strategies for prevention of periodontal disease, we described periodontal disease against an epidemiologic transition backdrop using a modified model developed for cardiovascular disease47. Abdel Omran published a seminal paper in 1971 where he hypothesized that epidemiologic transition is an application of epidemiology, inference and interpretation to population dynamics and can be explained by three broad phases that transition from the dominance of infectious disease to the dominance of chronic disease with a focus on “mortality”48. The theory focuses on the complex changes in patterns on the health and disease and their demographic, socio-economic and ecologic-biologic determinants and consequences in various population groups. Although Yusuf and colleagues47 used five phases to explain cardiovascular disease transition, we used three stages which we believe are relevant to periodontal disease that provides an historical overview that captures the “transitional stages” of prevalence and strategies to address periodontal disease (Table 1). Applying these stages to periodontal diseases remains valid as the disease is now considered a chronic disease sharing similar risk factors with cardiovascular diseases and other non-communicable diseases.
Table 1.
Epidemiologic transitional stages of prevalence and strategies of periodontal disease
| Stages | Prevalence | Consequences | Risk factors | Strategy |
|---|---|---|---|---|
| Age of Industrial and Societal Transformation 1900–1960s |
Prevalence of the periodontal disease overshadowed with dental caries dominates oral diseases | Tooth mortality | Focal infection paradigm and part of aging | Extraction of periodontal infected tooth |
| Age of the Receding Pandemics 1960s-1990s |
Prevalence of dental caries recedes and periodontal diseases showed upward increase | Reduction in Tooth mortality |
Individual lifestyle related factors, tobacco, oral hygiene High risk group |
Local factors control, and oral hygiene education gets prominence |
| Age of Degenerative, Lifestyle Diseases and Social Upheaval 2000 onwards |
Periodontal diseases are risk factor for systemic diseases considered along with other chronic diseases |
Systemic consequences Poor quality of life Social and economic consequences |
Interlinking factors with systemic diseases Social factors |
Call for Integrated with chronic disease and action of social determinant factors |
During the first half of the 20th century, dental caries was attributed as the major cause for tooth loss among adults49. The first major index developed to assess the impact of the disease process in populations was for dental caries, introduced by Klein and colleagues in the 1930s50. This index, widely known as the “DMFT” index included missing teeth (the “M” component) because of the high volume of missing teeth as a result of the caries process. Due to the widespread prevalence of dental caries, there was limited understanding of the natural history of periodontal disease and it was generally attributed to the ageing process. The lack of valid population measures (indices) to estimate periodontal diseases made it difficult to understand burden at the community level. Therefore, any evidence of a public health approach to periodontal disease control was essentially non-existent and management of periodontal disease was often therapeutic edentulation among adults51.
In the receding pandemics stage, infectious diseases are being replaced by non-communicable diseases, such as diabetes, cancer, and cardiovascular diseases. Periodontal disease prevalence appears to increase as dental caries control improves, especially in more developed countries. During this period, periodontal epidemiology begins to accelerate with the introduction of a series of indices to measure the distribution of periodontal diseases in populations49. These indices, Russell’s Periodontal Index52 and Ramfjord’s Periodontal Disease Index53, were based on the notion that the natural history of periodontal disease had a linear progression54.
Studies55,56 would soon appear suggesting that poor oral hygiene was the inevitable source of periodontitis and its subsequent linear progression to tooth loss if left untreated. The plaque-is-the-cause of periodontitis concept would quickly become firmly established and as a result, the goal of periodontal therapy was to address the “cause” by achieving optimal plaque control57. It was widely accepted that plaque would cause gingivitis inevitably advancing to periodontitis and that this process would continue to progress in a linear manner49. At one point, it was postulated that poor oral hygiene was attributable to 90% of periodontitis cases58. Understanding the natural progression of periodontal disease would evolve from specific, to nonspecific plaque hypotheses to burst theory where linear progression was no longer favored and to where disease activity for shorter periods of time, followed by longer periods of little or no activity became accepted59,60. As a result, patient focused oral hygiene and plaque control became the principal method for prevention and control of periodontal disease under the direction of skilled professionals during this period.
Efforts to pursue a public health approach for periodontal disease prevention was neglected during this period as dental public health focused on community-based interventions for dental caries, beginning with community water fluoridation. However, an unrelated event in the 1960’s would ultimately have an indirect impact on public health approaches to prevent periodontal disease. In 1964, the first Surgeon General’s Report and Smoking and Health was published61. This report was a watershed moment for efforts directed at smoking cessation to reduce disease and improve heath. Over the years, smoking would be demonstrated to be associated with every major non-communicable disease through successive Surgeon General Reports on tobacco use and health, including periodontal disease. This would culminate in the 2004 Surgeon General’s Report on the health consequences of smoking when the report concluded that “the evidence is sufficient to infer a causal relationship between smoking and periodontitis”62.
Consequently, public health approaches at reducing periodontal disease became focused on a high-risk behavior – tobacco use, but ownership of these efforts were generally more directed by the broader public health community. Tobacco cessation programs emerged, and oral health professionals63 were encouraged to adopt them as part of their primary preventive activities to reduce the potential negative sequelae resulting from severe periodontal disease64,65. Because these activities were patient-focused, tobacco cessation was incorporated into the pre-existing model of using skilled dental professionals to prevent periodontitis through health education and plaque removal. This approach has had a minimal public health effect for several reasons, including limited participation by dental providers, focus on existing smokers, and insufficient incentives for the patient66. More importantly, there is a difference between having a high-risk behavior (e.g., smoking) and being high-risk because of an inherent trait(s) that favors susceptibility. The high-risk behavior is potentially modifiable, whereas having a high-risk trait is non-modifiable.
During the period of receding pandemics, important epidemiological studies also began to show considerable within-group variation in the extent and severity of periodontal disease67–71 that would lead to understanding that even where plaque accumulation is substantial and gingivitis endemic, only a small percentage of individuals are likely to develop severe periodontal disease. In a landmark study, Loe and colleagues reported results from a longitudinal study of a group of Sri Lankan tea workers with no access to dental care54. Even though the majority in this cohort had high levels of plaque, calculus and gingival inflammation, the authors reported that about 8% of the cohort had rapid progression of disease (mean loss of attachment 9 mm), 81% had moderate progression (about 4 mm) and 11% were largely free of periodontitis (less than 1 mm attachment loss by age 3572. Although a comparable study from Norway had much lower prevalence of periodontal disease, similar results were demonstrated suggesting a range of high, moderate and low risk groups72. Consequently, the theory that plaque-is-the-cause of periodontitis was gradually replaced in favor of a disease susceptibility model, implying that for similar levels of poor oral hygiene and gingivitis in a population, groups exist that have varying risk for periodontal disease severity.
In the transition from the receding pandemic period to the age of lifestyle diseases and social upheaval, a seminal study was published that suggested a relationship between poor dental health and acute myocardial infarction20. Over the next decade, numerous studies were published suggesting that oral diseases—mostly periodontal disease—were associated with other diseases characterized by chronic inflammation as an underlying cause4,24,28,29,73–75.
An emerging characteristic of Omran’s third stage of epidemiologic transition – the age of degenerative, lifestyle diseases, and social upheaval – is the recognition that individual behavior and lifestyle modifications are only partially effective at preventing disease. The concept of risk is expanding with the understanding that the social constructs in which people live are also attributable to disease risk and adverse health outcomes. These social constructs, now more commonly referred to as social determinants of health (SDH), are often identified by the varying environments and social settings in which people live76. Advantages in educational attainment, occupational status, income level and accumulated wealth can selectively determine the environment and communities people live in, which in turn can influence disease prevalence77. Similar to many non-communicable diseases, occupational ranking and neighborhood characteristics have been shown to be risk indicators for periodontitis78. As our understanding of SDH improve, so does the way we conceptualize prevention for non-communicable diseases, expanding beyond the traditional interventional framework at the individual level to exploring interventions at the societal level. It is therefore worthwhile to construct upstream polices for managing the macro, social and economic factors that can affect health.
Another important feature of Omran’s third stage, is the rise to prominence of the “degenerative and man-made diseases.” At this time, the linking of systemic diseases with periodontal diseases occurs as potential underlying causes for chronic diseases are explored. As a result, the range of health concerns for which periodontitis has been implicated as a risk indicator has expanded to include cardiovascular diseases (heart disease and stroke), preterm birth/low birthweight, diabetes, chronic obstructive pulmonary disease and pneumonia, pre-eclampsia, chronic kidney disease, delayed conception, and a range of cancers. Tying periodontal disease to other non-communicable diseases and adverse health conditions that cause considerable morbidity and mortality at the population level raises the importance of periodontal disease prevention and the prospect that interventions aimed at reducing periodontal inflammation could impact systemic disease outcomes. However, there are no high-quality studies to date that demonstrate that treating periodontitis reduces some of these adverse health outcomes such as cardiovascular events. Nevertheless, the greater benefit realized with the interconnection of periodontitis and other non-communicable diseases is the recognition of the importance of shared risk factors.
Common risk factor approach for periodontal disease
When searching for population strategies focused specifically on periodontal disease, a review of the literature revealed little information. Among the articles describing or advocating for periodontal disease prevention activities, most were based on theoretical concepts, discussion papers, expert opinions, or consultative workshops. As previously mentioned, a solitary program reported in Japan (the 8020 Campaign) is one of few public health approaches designed for periodontal disease prevention reported in literature.39 There were four studies reporting the evaluation of mass media/awareness programs for oral hygiene and periodontal health.35–38,45 A compendium of our literature search is shown in Table 2. Most of the articles concurred with the supposition that population-focused activities directed at individual behavior modification for enhancing oral hygiene for reduction for periodontal disease is ineffective in achieving constant improvements in periodontal outcomes at the population level4,79–85. Most articles described a public health approach to prevention of periodontal disease as a component of an integrated approach with systemic health, particularly with non-communicable disease prevention activities, focusing on common risk factors. Consequently, thecommon risk factor approach has been conceptualized for the public health approach to prevent periodontal disease. Let the author fill in
Table 2.
Literature summary for public health approach for prevention of periodontal disease
| Source/Articles | Public Health Approach for Periodontal disease prevention |
|---|---|
| Global Periodontal Health: Challenges, priorities and perspectives. FDI- World Oral Health Forum 2017, Proceedings 31 August 2017 Madrid, Spain | Public and professional education as well as reinforced political advocacy for Oral Hygiene behavior-
|
| Prevention of dental Disease: caries and Periodontal disease ann. Rev. Public health. 1981. 2:71–92 |
Frequent Oral prophylaxis approach can be applied on a community basis to children in schools and to adults. |
| Translating science into action – prevention of periodontal disease at patient level. Periodontology 2000, Vol. 60, 2012, 162–172 | Prevention at the patient level must be global and comprehensive. Preventive interventions should be oriented towards influencing patient behavior |
| Common risk factors in the Management of periodontal and associated systemic diseases: The dental setting and Interprofessional Collaboration. J evid base dent pract 2014;14S:4–16 | Risk factor management procedures for Periodontal disease through common risk factor approach. |
| An Evidence-Based Approach to the Prevention of Oral Diseases Med Princ Pract 2003;12(suppl 1):3–11 |
Non-randomized population-wide interventions and evidence on oral health promotion is required for periodontal disease prevention |
| Risk assessment and periodontal prevention in primary care. Periodontology 2000, Vol. 71, 2016, 10–21 |
There is gap in research of extrapolation of the risk factors from a population perspective from an individual perspective for Periodontal risk factors |
| Is It Time to Reassess the Public Health Implications of Periodontal Diseases? A Review of Current Concepts JPHD Vol. 48, No. 4. Fall 1988 | Periodontal disease prevention through Oral Health Promotion |
| Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: A call for global action. J Clin Periodontal. 2017;44:456–462 |
Multiple strategies of oral hygiene awareness program to influence behavior on individual in the community |
| Global burden of oral diseases: emerging concepts, management and interplay with systemic health. Oral Diseases (2016) 22, 609–619 | Integration of oral health (periodontal disease prevention) into general health agenda for optimal health and general well-being. |
| The impact of cigarette smoking on periodontal disease and treatment. Periodontology 2000, Vol. 44, 2007, 178–194 | Periodontal therapy affords multiple opportunities during active and ongoing maintenance therapy for patient education, intervention strategies, and reinforcement of tobacco cessation |
| Translating science into action: periodontal health through public health approaches. Periodontology 2000, Vol. 60, 2012, 173–187 | Important chronic disease entry points for periodontal health are reduction of tobacco use, reduction in consumption of harmful levels of alcohol, a healthy diet and good nutrition and improvement of personal hygiene. Proper sanitation facilities and availability and accessibility of oral hygiene aids are vital to periodontal health’ tackling the social determinants of disease, important behavioral risk factors. |
| Oral health care systems in developing and developed countries Periodontology 2000, Vol. 60, 2012, 98–109 |
Building capacity in oral health care systems, directed towards periodontal disease prevention and primary health care, with special emphasis on meeting the needs of disadvantaged and poor population groups |
| The periodontal disease– systemic health–infectious disease axis in developing countries Periodontology 2000, Vol. 60, 2012, 64–77 |
Integrating periodontal disease and closely related oral diseases into the better funded global prevention programs on malaria, AIDS and nutrition |
| The United States Public Health Service (USPHS) Oral Health Coordinating Committee (OHCC) Oral Health Strategic Framework 2014–2017 |
Enhance national oral health surveillance efforts and developing measures for use in surveillance of periodontal disease for Public health action. |
| Editorial: Periodontal Disease – A Public Health Problem Front. Public Health, 08 January 2016 | Awareness of the periodontal disease problem as a key part of reorienting health services in order to promote periodontal health. |
| Knowledge on periodontal disease before and after a mass media campaign. Swed Dent J (2004) 28:165–71 | Population-based media campaign promoting periodontal knowledge among adults has positive short-term impact only (three months). |
| Factors behind change in knowledge after a mass media campaign targeting periodontitis. Int J Dent Hyg (2006) 4:8–14. | Use of mass media campaign will increase knowledge about periodontitis as a health promotion strategy |
| The effect of a mass-media dental health education campaign. Health Educ Res (1988) 3:243–55. | Oral hygiene behavioral changes have limited outcomes through from other mass media campaigns in health education |
| Assessment of periodontal knowledge following a mass media oral health promotion campaign: a population-based study. BMC Oral Health (2014) 14:31. doi:10.1186/1472–6831-14–31 | Use of mass media periodontal campaign for short term hygiene knowledge improvement among the adult population. |
| Periodontal Health and Disease. A practical guide to reduce the global burden of periodontal disease. FDI 2017 | Use of periodontal health as a priority area for oral health policy and promotion Awareness and literacy national campaign for behavior change |
| Defining a national strategy: The 8020 Campaign in Japan. Campaign, calling for the retention of 20 or more teeth even at the age of 80 started in 2001. Int Dent J. 2001 Jun;51(3 Suppl):200–6. | 8020 Campaign achieved that the percentage of persons achieving 8020 was over 50% - 2016 and improved periodontal outcomes |
| Periodontal health and global public health. Periodontology 2000, Vol. 60, 2012, 7–14 |
|
| Prevention of Periodontal Diseases |
|
| Public Health Perspectives on Surveillance for Periodontal Diseases Periodontal 2007;78:1380–1386. |
monitoring periodontal diseases is invasive, resource-intensive & not feasible. Alternative approaches to periodontal disease surveillance
|
|
Public health aspects of periodontal diseases in Europe. J Clin Periodontal 1991; 18: 362–369 |
|
| Does oral health promotion improve oral hygiene and gingival health? Periodontal 2000. 2005; 37:35–47. |
Short-term reductions in plaque and gingival bleeding at community interventions Public health significance of these changes questionable |
| Strategies and approaches in oral disease prevention Bulletin of the World Health Organization 2005; 83:711–718. | Integration of periodontal disease prevention with Non-Communicable Disease prevention – Common risk factor approach. |
| Strengthening the Prevention of Periodontal Disease: The WHO Approach. J Periodontal 2005; 76:2187–2193. | Integration of periodontal disease prevention with Non-Communicable Disease prevention – Common risk factor approach. |
| The Common Risk Factor Approach – An Integrated Population- and Evidence-Based Approach for Reducing Social Inequalities in Oral Health. Gesundheitswesen 2016; 78: 672–677 | Integration of periodontal disease prevention with Non-Communicable Disease prevention – Common risk factor approach. |
| Sociobehavioral aspects of periodontal disease. Periodontology 2000, Vol. 60, 2012, 54–63 |
|
| Periodontal health through public health – the case for oral health promotion. Periodontology 2000, Vol. 60, 2012, 147–155 | Upstream process through tackling the Marco factors of oral hygiene behavior and Integration of periodontal disease prevention with Non-Communicable Disease prevention – Common risk factor approach. |
| Splash!: a prospective birth cohort study of the impact of environmental, social and family-level influences on child oral health and obesity related risk factors and outcomes. BMC Public Health 2011, 11:505 | This study aims to prospectively examine the impact of drink choices on child obesity risk and oral health status. |
| Reducing pediatric caries and obesity risk in South Asian immigrants: randomized controlled trial of common health/risk factor approach BMC Public Health (2018) 18:680 |
Does a culturally-tailored CR/HFA home-visiting intervention, from child age 6 through 16 months, reduce cariogenic and obesogenic behaviors in SA immigrant families (primary hypothesis)? b) Is this CR/HFA intervention associated with reduced incidence of oral caries and obesity at 18 months of age (secondary hypothesis)? |
| Common risk factor approach to address socioeconomic inequality in the oral health of preschool children – a prospective cohort study BMC Public Health 2014, 14:429 | The aim of the Study of Mothers’ and Infants’ Life Events Affecting Oral Health (SMILE) project is to identify and evaluate the relative importance and timing of critical factors that shape the oral health of young children and then to seek to evaluate those factors in their inter-relationship with socioeconomic influences. |
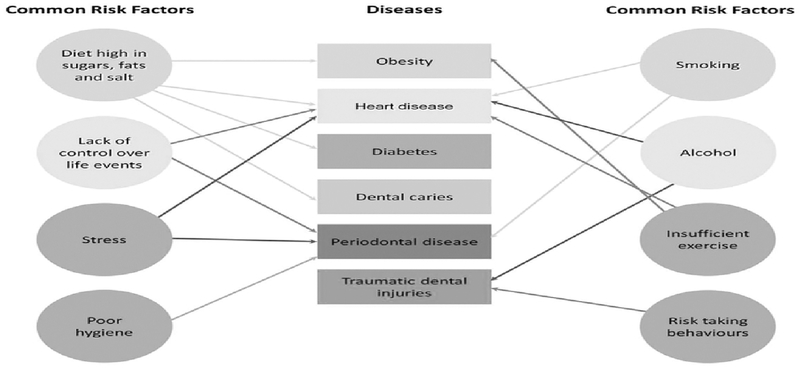
Periodontal disease shares a wide range of risk factors and risk indicators with other conditions (Figure 1). Risk factors that are considered modifiable are often related to lifestyle, e.g. tobacco use and alcohol, diabetes and obesity, dietary factors, (dietary calcium and vitamin D), or stress. Other local factors such as plaque, microbiota, oral hygiene, defective restorations and wearing partial dentures can also constitute risk factors. In addition to individual behaviors and lifestyle risk factors, a range of external factors (SDH) which individuals themselves have little ability to control but must be endured because of the environment and social constructs they live in, affect their oral health. Most of these risk factors, indicators, and SDH are shared with other major non-communicable diseases such as cardiovascular disease, chronic respiratory disease, cancer, and diabetes. Therefore, the common risk factor approach provides the basis for the integration of periodontal disease prevention into public health approaches for non-communicable disease prevention and control activities.
Figure 1.
Common risk factors (Watt 2005)
Smoking and periodontitis as a case study
It’s been well-documented that tobacco use is a major risk factor for periodontitis. It has been estimated that more than half (55.1%) of periodontitis cases in the United States are attributed to smoking,86,87 suggesting that a majority of periodontal disease cases could be preventable through the reduction and cessation of tobacco use. An estimated 14 percent of adults in the U.S., or 34.3 million people, smoked cigarettes in 2017, down from 15.5 percent in 201688. This historic low represents a 67 percent decline from 1965 and highlights how successful public health efforts have been over the past 50 years. Effective population-based tobacco control interventions have included tobacco price increases, high-impact anti-tobacco mass media campaigns, and policy actions implementing comprehensive smoke-free public spaces. These public health approaches have decreased smoking population rates and reduced tobacco-associated diseases and mortality across diverse race/ethnic and socioeconomic groups. Although individual approaches utilized for tobacco cessation activities do work, smoking quit rates remain low (3% to 5%)89 and are resource intensive suggesting that an individual approach would not be as effective as a public health approach, such as implementing effective public policies for health promotion and disease prevention.
Challenges with the common risk factor approach
Even though the common risk factor approach model for periodontal disease prevention is a rational approach as a population-based strategy, it needs to be supported by appropriate evidence to help inform guidelines for implementation. If the foundational knowledge is weak with regards to the expected outcomes, this could lead to nonspecific integration. For instance, quantification of attributable risk for factors that are common between periodontal disease and other major non-communicable diseases are lacking82,90–92. As of this date only smoking’s attributable risk to periodontal disease has been sufficiently quantified, and generally, only in the United States. Without this information for other main risk factors that are modifiable, the opportunities for integration with non-communicable diseases for periodontal disease prevention through common risk factor approach is not as compelling. Quantifying attributable risk that is population-specific for periodontal disease across shared risk factors for the main non-communicable diseases serves three purposes: (1) to provide a benchmark to measure changing patterns of disease; (2) to provide information for policy development; and (3) to ensure the intervention activities at the population level are evaluated appropriately for effectiveness.
Another challenge for the common risk factor approach approach is the limited use of standard reporting guidelines for periodontal disease in epidemiologic studies, which includes the reporting of periodontal disease levels by leading risk factors using common terminology90,91,93. Typically, periodontal disease is monitored by disease characteristics rather than its risk factors or indicators. Achieving success with the integration of periodontal disease prevention into the broader non-communicable disease prevention framework of activities at the global level would be facilitated if surveillance of periodontal disease would follow the WHO’s STEPwise approach to surveillance (STEPS), which is designed as a basic, standardized method for collecting and analyzing data94. The WHO is promoting STEPS for non-communicable disease risk factor surveillance as process to strengthen the common risk factor approach to reduce disease, improve health, and promote wellbeing. Unfortunately, validation of the core STEPS questionnaire with oral health outcomes, including periodontal disease, is not known.
Way forward
In 1951, Marshall-Day raised awareness about the extent of periodontal disease and the deleterious impact on oral health by calling periodontal disease a “serious and universal public health problem.”95 Because there was little epidemiologic data available to support this assertion, he was bringing attention to the need for improvements in periodontal disease assessment. Conversations, workshops, and proceedings followed through 1950s that ushered in several innovations in periodontal assessments over the next six decades. Today, we know much about periodontitis and the impact on oral health and the relationship with overall health. We know how to engage individuals to promote periodontal health and prevent periodontal disease at the individual level, what we don’t know is how to effectively advocate for periodontal disease prevention and control to facilitate oral health at the community level.
Implementing a public health approach to accomplish this will require a multilevel effort focusing on assessment, policy development, and assurance (Box). One important way forward is the recognition of the value that public health informatics could add to a comprehensive strategy in areas of surveillance, prevention, and health promotion. Using the newer tools of data science and technology, public health informatics can help with transforming periodontal disease assessment that better informs decision making. This can facilitate public health policy that supports environments and living spaces that promote periodontal health, encourage oral health literacy leading to individual empowerment, and identify strategies that support integration of periodontal health care into overall health care.
Box. Core Public Health Activities for Periodontal Disease Prevention.
| Assessment |
|
| Policy Development |
|
| Assurance |
|
Summary
Although there have been substantial improvements in our understanding of the etiology of periodontal disease and how we can prevent and control periodontal disease, these advances have been primarily focused on the individual, patient-focused approaches. The prevention of periodontal disease depends not only on improving currently available individual interventions, but in determining what public health interventions can be effective and sustainable under real-life conditions. Population-based effectiveness implies that the benefits of an intervention can reach all those who need it and that the system has the capacity to implement and sustain the intervention. An example of a public health approach that is evolving, showing promise, and gaining international acceptance for non-communicable disease prevention and control is for cardiovascular diseases. Currently, public health approaches for periodontal disease prevention and control are lacking. To remedy this, we should consider the core public health activities of assessment, policy development, and assurance. Part of this effort will require exploring new ways to describe the impact of periodontal disease, to monitor effectiveness of prevention and control activities, and to elevate periodontal disease as an important non-communicable disease public health problem.
Funding Body Agreements & Policy:
This activity was supported by the National Institute of Dental and Craniofacial Research (NIDCR) and the authors were paid a salary by the NIH.
Footnotes
Ethics Statement: The current study was determined exempt from review by the National Institutes of Health Institutional Review Board.
Contributor Information
Chandrashekar Janakiram, Post-Doctoral Fellow in Dental Public Health and Informatics, NIH/National Library of Medicine and the National Institute of Dental and Craniofacial Research.
Bruce A. Dye, Dental Epidemiology Officer and Director of Dental Public Health and Informatics Fellowship Program, NIH/National Institute of Dental and Craniofacial Research.
References
- 1.Marcenes W, Kassebaum NJ, Bernabé E, et al. Global burden of oral conditions in 1990–2010: a systematic analysis. J Dent Res. 2013;92(7):592–597. doi: 10.1177/0022034513490168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dye BA The Global Burden of Oral Disease: Research and Public Health Significance. J Dent Res. 2017;96(4):361–363. doi: 10.1177/0022034517693567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kassebaum NJ, Smith AGC, Bernabé E, et al. Global, Regional, and National Prevalence, Incidence, and Disability-Adjusted Life Years for Oral Conditions for 195 Countries, 1990–2015: A Systematic Analysis for the Global Burden of Diseases, Injuries, and Risk Factors. J Dent Res. 2017;96(4):380–387. doi: 10.1177/0022034517693566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jin LJ, Lamster IB, Greenspan JS, Pitts NB, Scully C, Warnakulasuriya S Global burden of oral diseases: emerging concepts, management and interplay with systemic health. Oral Dis. 2016;22(7):609–619. doi: 10.1111/odi.12428 [DOI] [PubMed] [Google Scholar]
- 5.Vos T, Abajobir AA, Abate KH, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. The Lancet. 2017;390(10100):1211–1259. doi: 10.1016/S0140-6736(17)32154-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Institute of Medicine (US) Committee for the Study of the Future of Public Health. The Future of Public Health. Washington (DC): National Academies Press (US); 1988. http://www.ncbi.nlm.nih.gov/books/NBK218218/. Accessed May 29, 2019. [PubMed] [Google Scholar]
- 7.Petersen PE, Ogawa H Strengthening the Prevention of Periodontal Disease: The WHO Approach. J Periodontol. 2005;76(12):2187–2193. doi: 10.1902/jop.2005.76.12.2187 [DOI] [PubMed] [Google Scholar]
- 8.Labarthe DR, Biggers A, Goff DC, Houston M Translating a plan into action: a Public Health Action Plan to Prevent Heart Disease and Stroke. Am J Prev Med. 2005;29(5 Suppl 1):146–151. doi: 10.1016/j.amepre.2005.07.010 [DOI] [PubMed] [Google Scholar]
- 9.Million Hearts: Meaningful Progress 2012–2016. :14. [Google Scholar]
- 10.WHO | Global Hearts Initiative, working together to promote cardiovascular health. WHO. http://www.who.int/cardiovascular_diseases/global-hearts/en/. Accessed May 29, 2019.
- 11.Rothstein MA Rethinking the Meaning of Public Health. J Law Med Ethics. 2002;30(2):144–149. doi: 10.1111/j.1748-720X.2002.tb00381.x [DOI] [PubMed] [Google Scholar]
- 12.Billings M, Holtfreter B, Papapanou PN, Mitnik GL, Kocher T, Dye BA Age-dependent distribution of periodontitis in two countries: Findings from NHANES 2009 to 2014 and SHIP-TREND 2008 to 2012. J Clin Periodontol. 2018;45 Suppl 20:S130–S148. doi: 10.1111/jcpe.12944 [DOI] [PubMed] [Google Scholar]
- 13.Eke PI, Dye BA, Wei L, Thornton-Evans GO, Genco RJ, CDC Periodontal Disease Surveillance workgroup: James Beck (University of North Carolina, Chapel Hill, USA), Gordon Douglass (Past President, American Academy of Periodontology), Roy Page (University of Washin. Prevalence of periodontitis in adults in the United States: 2009 and 2010. J Dent Res. 2012;91(10):914–920. doi: 10.1177/0022034512457373 [DOI] [PubMed] [Google Scholar]
- 14.Eke PI, Dye BA, Wei L, et al. Update on Prevalence of Periodontitis in Adults in the United States: NHANES 2009 – 2012. J Periodontol. 2015;86(5):611–622. doi: 10.1902/jop.2015.140520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Pablo P, Chapple ILC, Buckley CD, Dietrich T Periodontitis in systemic rheumatic diseases. Nat Rev Rheumatol. 2009;5(4):218–224. doi: 10.1038/nrrheum.2009.28 [DOI] [PubMed] [Google Scholar]
- 16.Benjamin RM Oral Health: The Silent Epidemic. Public Health Rep. 2010;125(2):158–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Albandar JM Epidemiology and risk factors of periodontal diseases. Dent Clin North Am. 2005;49(3):517–532, v-vi. doi: 10.1016/j.cden.2005.03.003 [DOI] [PubMed] [Google Scholar]
- 18.Haynes DR Emerging and future therapies for the treatment of bone loss associated with chronic inflammation. InflammoPharmacology. 2006;14(5):193–197. doi: 10.1007/s10787-006-0006-1 [DOI] [PubMed] [Google Scholar]
- 19.Khalili J Periodontal disease: an overview for medical practitioners. Lik Sprava. 2008;(3–4):10–21. [PubMed] [Google Scholar]
- 20.Association between dental health and acute myocardial infarction. | The BMJ. https://www.bmj.com/content/298/6676/779. Accessed April 2, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Peruzzo DC, Benatti BB, Ambrosano GMB, et al. A systematic review of stress and psychological factors as possible risk factors for periodontal disease. J Periodontol. 2007;78(8):1491–1504. doi: 10.1902/jop.2007.060371 [DOI] [PubMed] [Google Scholar]
- 22.Tezal M, Grossi SG, Ho AW, Genco RJ Alcohol consumption and periodontal disease. The Third National Health and Nutrition Examination Survey. J Clin Periodontol. 2004;31(7):484–488. doi: 10.1111/j.1600-051X.2004.00503.x [DOI] [PubMed] [Google Scholar]
- 23.Guzman S, Karima M, Wang H-Y, Van Dyke TE Association between interleukin-1 genotype and periodontal disease in a diabetic population. J Periodontol. 2003;74(8):1183–1190. doi: 10.1902/jop.2003.74.8.1183 [DOI] [PubMed] [Google Scholar]
- 24.Mealey BL, Oates TW, American Academy of Periodontology. Diabetes mellitus and periodontal diseases. J Periodontol. 2006;77(8):1289–1303. doi: 10.1902/jop.2006.050459 [DOI] [PubMed] [Google Scholar]
- 25.Moynihan P, Petersen PE Diet, nutrition and the prevention of dental diseases. Public Health Nutr. 2004;7(1A):201–226. [DOI] [PubMed] [Google Scholar]
- 26.Borgnakke WS Does Treatment of Periodontal Disease Influence Systemic Disease? Dent Clin North Am. 2015;59(4):885–917. doi: 10.1016/j.cden.2015.06.007 [DOI] [PubMed] [Google Scholar]
- 27.Ryder MI, Nittayananta W, Coogan M, Greenspan D, Greenspan JS Periodontal disease in HIV/AIDS. Periodontol 2000. 2012;60(1):78–97. doi: 10.1111/j.1600-0757.2012.00445.x [DOI] [PubMed] [Google Scholar]
- 28.Periodontitis and systemic diseases: a record of discussions of working group 4 of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. - PubMed - NCBI https://www.ncbi.nlm.nih.gov/pubmed/23627330. Accessed November 18, 2018. [Google Scholar]
- 29.Papapanou PN Systemic effects of periodontitis: lessons learned from research on atherosclerotic vascular disease and adverse pregnancy outcomes. Int Dent J. 2015;65(6):283–291. doi: 10.1111/idj.12185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tonetti MS, Jepsen S, Jin L, Otomo-Corgel J Impact of the global burden of periodontal diseases on health, nutrition and wellbeing of mankind: A call for global action. J Clin Periodontol. 2017;44(5):456–462. doi: 10.1111/jcpe.12732 [DOI] [PubMed] [Google Scholar]
- 31.Trombelli L, Franceschetti G, Farina R Effect of professional mechanical plaque removal performed on a long-term, routine basis in the secondary prevention of periodontitis: a systematic review. J Clin Periodontol. 2015;42 Suppl 16:S221–236. doi: 10.1111/jcpe.12339 [DOI] [PubMed] [Google Scholar]
- 32.Chambrone L, Chambrone D, Lima LA, Chambrone LA Predictors of tooth loss during long-term periodontal maintenance: a systematic review of observational studies. J Clin Periodontol. 2010;37(7):675–684. doi: 10.1111/j.1600-051X.2010.01587.x [DOI] [PubMed] [Google Scholar]
- 33.Ghotane SG, Harrison V, Radcliffe E, Jones E, Gallagher JE Enhanced skills in periodontology: pilot evaluation. BDJ Team. 2017;4(7):17118. doi: 10.1038/bdjteam.2017.118 [DOI] [PubMed] [Google Scholar]
- 34.Zusman SP, Eaton KA, Harris M, Amariei C A pilot project to improve the oral health of orphans and of the elderly in residential care in Constanta, Romania. Community Dent Health. 2015;32(2):89–92. [PubMed] [Google Scholar]
- 35.Gholami M, Pakdaman A, Montazeri A, Jafari A, Virtanen JI Assessment of periodontal knowledge following a mass media oral health promotion campaign: a population-based study. BMC Oral Health. 2014;14:31. doi: 10.1186/1472-6831-14-31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Leous PA, Kazeko LA Approaches to development of programs for prevention and care of periodontal disease at community level. 2006;(3):5. [Google Scholar]
- 37.Mårtensson C, Söderfeldt B, Andersson P, Halling A, Renvert S Factors behind change in knowledge after a mass media campaign targeting periodontitis. Int J Dent Hyg. 2006;4(1):8–14. doi: 10.1111/j.1601-5037.2006.00158.x [DOI] [PubMed] [Google Scholar]
- 38.Kay EJ, Locker D Is dental health education effective? A systematic review of current evidence. Community Dent Oral Epidemiol. 1996;24(4):231–235. [DOI] [PubMed] [Google Scholar]
- 39.Saito H, Kawaguchi Y Halitosis prevention campaign: a report of oral health promotion activities in Japan. Int Dent J. 2002;52 Suppl 3:197–200. [DOI] [PubMed] [Google Scholar]
- 40.Baiju RM, Peter E, Varghese NO, Sivaram R, Streiner DI What makes a tool appropriate to assess patient-reported outcomes of periodontal disease? J Indian Soc Periodontol. 2017;21(2):90. doi: 10.4103/jisp.jisp_144_17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gholami M, Pakdaman A, Virtanen JI Common Perceptions of Periodontal Health and Illness among Adults: A Qualitative Study. International Scholarly Research Notices. doi: 10.5402/2012/671879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Social determinants of health and periodontal disease in Brazilian adults: a cross-sectional study. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3663668/. Accessed April 3, 2019. [DOI] [PMC free article] [PubMed]
- 43.Mariotti A, Hefti AF Defining periodontal health. BMC Oral Health. 2015;15(Suppl 1):S6. doi: 10.1186/1472-6831-15-S1-S6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petersen PE The World Oral Health Report 2003: continuous improvement of oral health in the 21st century--the approach of the WHO Global Oral Health Programme. Community Dent Oral Epidemiol. 2003;31 Suppl 1:3–23. [DOI] [PubMed] [Google Scholar]
- 45.Mårtensson C, Söderfeldt B, Halling A, Renvert S Knowledge on periodontal disease before and after a mass media campaign. Swed Dent J. 2004;28(4):165–171. [PubMed] [Google Scholar]
- 46.Baehni PC Translating science into action--prevention of periodontal disease at patient level. Periodontol 2000. 2012;60(1):162–172. doi: 10.1111/j.1600-0757.2011.00428.x [DOI] [PubMed] [Google Scholar]
- 47.Salim Yusuf, Srinath Reddy, Stephanie Ôunpuu, Sonia Anand Global Burden of Cardiovascular Diseases. Circulation. 2001;104(22):2746–2753. doi: 10.1161/hc4601.099487 [DOI] [PubMed] [Google Scholar]
- 48.Omran AR The Epidemiologic Transition: A Theory of the Epidemiology of Population Change. Milbank Q. 2005;83(4):731–757. doi: 10.1111/j.1468-0009.2005.00398.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Baelum V, López R Periodontal disease epidemiology – learned and unlearned? Periodontol 2000. 2013;62(1):37–58. doi: 10.1111/j.1600-0757.2012.00449.x [DOI] [PubMed] [Google Scholar]
- 50.Henry K, Palmer C Studies on dental caries: V. Familial resemblance in the caries experience of siblings. Public Health Reports. 1938;38:1685–1690. [Google Scholar]
- 51.Black AD Roentgenographic studies of tissues involved in chronic mouth infections. 1918: 38: 924–932. Dent Summ. 1918;38:924–932. [PMC free article] [PubMed] [Google Scholar]
- 52.Russell AL A system of classification and scoring for prevalence surveys of periodontal disease. J Dent Res. 1956;35(3):350–359. doi: 10.1177/00220345560350030401 [DOI] [PubMed] [Google Scholar]
- 53.Ramfjord SP Indices for Prevalence and Incidence of Periodontal Disease. J Periodontol. 1959;30(1):51–59. doi: 10.1902/jop.1959.30.1.51 [DOI] [Google Scholar]
- 54.Löe H, ÅNerud Åg, Boysen H, Smith M The natural history of periodontal disease in man. J Periodontal Res. 1978;13(6):563–572. doi: 10.1111/j.1600-0765.1978.tb00210.x [DOI] [PubMed] [Google Scholar]
- 55.Waerhaug J, Ramfjord S, Kerr D, Ash M Epidemiology of periodontal disease In: Ramfjord SP, Kerr DA, Ash MM eds. World workshop in periodontics. Ann Arbor, MI: University of Michigan, 1966: 179–222. 1966. [Google Scholar]
- 56.Loe H, Theilade E, Jensen SB EXPERIMENTAL GINGIVITIS IN MAN. J Periodontol. 1965;36:177–187. doi: 10.1902/jop.1965.36.3.177 [DOI] [PubMed] [Google Scholar]
- 57.Lindhe J, Nyman S The effect of plaque control and surgical pocket elimination on the establishment and maintenance of periodontal health. A longitudinal study of periodontal therapy in cases of advanced disease. J Clin Periodontol. 1975;2(2):67–79. [DOI] [PubMed] [Google Scholar]
- 58.Rose G Sick Individuals and Sick Populations. Int J Epidemiol. 1985;14(1):32–38. doi: 10.1093/ije/14.1.32 [DOI] [PubMed] [Google Scholar]
- 59.Haffajee A, Socransky S, Goodson J Periodontal disease activity. J Periodontol. 1982;(17):521–522. [DOI] [PubMed] [Google Scholar]
- 60.Goodson JM, Tanner AC, Haffajee AD, Sornberger GC, Socransky SS Patterns of progression and regression of advanced destructive periodontal disease. J Clin Periodontol. 1982;9(6):472–481. [DOI] [PubMed] [Google Scholar]
- 61.50 Years Since the First Surgeon General’s Report on Smoking and Health: A Happy Anniversary? https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3910062/. Accessed April 2, 2019. [DOI] [PMC free article] [PubMed]
- 62.History of the Surgeon General’s Report on Smoking and Health | CDC. https://www.cdc.gov/tobacco/data_statistics/sgr/history/index.htm. Accessed April 2, 2019. [Google Scholar]
- 63.Carr AB, Ebbert J Interventions for tobacco cessation in the dental setting. Cochrane Database Syst Rev. 2012;6:CD005084. doi: 10.1002/14651858.CD005084.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Needleman I, Warnakulasuriya S, Sutherland G, et al. Evaluation of tobacco use cessation (TUC) counselling in the dental office. Oral Health Prev Dent. 2006;4(1):27–47. [PubMed] [Google Scholar]
- 65.Chaffee BW, Couch ET, Ryder MI The tobacco-using periodontal patient: The role of the dental practitioner in tobacco cessation and periodontal diseases management. Periodontol 2000. 2016;71(1):52–64. doi: 10.1111/prd.12120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Solberg LI Incentivising, facilitating, and implementing an office tobacco cessation system. Tob Control. 2000;9(suppl 1):i37–i41. doi: 10.1136/tc.9.suppl_1.i37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Löe H, Anerud A, Boysen H, Smith M The natural history of periodontal disease in man. The rate of periodontal destruction before 40 years of age. J Periodontol. 1978;49(12):607–620. doi: 10.1902/jop.1978.49.12.607 [DOI] [PubMed] [Google Scholar]
- 68.Yoneyama T, Okamoto H, Lindhe J, Socransky SS, Haffajee AD Probing depth, attachment loss and gingival recession. Findings from a clinical examination in Ushiku, Japan. J Clin Periodontol. 1988;15(9):581–591. [DOI] [PubMed] [Google Scholar]
- 69.The prevalence of advanced loss of periodontal attachment in two New Mexico populations - Ismail - 1987 - Journal of Periodontal Research - Wiley Online Library. https://onlinelibrary.wiley.com/doi/abs/10.1111/j.1600-0765.1987.tb01550.x. Accessed April 3, 2019. [DOI] [PubMed] [Google Scholar]
- 70.Hugoson A, Jordan T Frequency distribution of individuals aged 20–70 years according to severity of periodontal disease. Community Dent Oral Epidemiol. 1982;10(4):187–192. [DOI] [PubMed] [Google Scholar]
- 71.Baelum V, Fejerskov O, Manji F Periodontal diseases in adult Kenyans. J Clin Periodontol. 1988;15(7):445–452. [DOI] [PubMed] [Google Scholar]
- 72.Löe H, Anerud A, Boysen H, Morrison E Natural history of periodontal disease in man. Rapid, moderate and no loss of attachment in Sri Lankan laborers 14 to 46 years of age. J Clin Periodontol. 1986;13(5):431–445. [DOI] [PubMed] [Google Scholar]
- 73.Enwonwu CO, Salako N The periodontal disease-systemic health-infectious disease axis in developing countries. Periodontol 2000. 2012;60(1):64–77. doi: 10.1111/j.1600-0757.2012.00447.x [DOI] [PubMed] [Google Scholar]
- 74.Hujoel PP, Drangsholt M, Spiekerman C, DeRouen TA Periodontitis-systemic disease associations in the presence of smoking--causal or coincidental? Periodontol 2000. 2002;30:51–60. [DOI] [PubMed] [Google Scholar]
- 75.Emrich LJ, Shlossman M, Genco RJ Periodontal disease in non-insulin-dependent diabetes mellitus. J Periodontol. 1991;62(2):123–131. doi: 10.1902/jop.1991.62.2.123 [DOI] [PubMed] [Google Scholar]
- 76.Braveman P, Gottlieb L The social determinants of health: it’s time to consider the causes of the causes. Public Health Rep Wash DC 1974. 2014;129 Suppl 2:19–31. doi: 10.1177/00333549141291S206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jürgensen N, Petersen PE, Ogawa H, Matsumoto S Translating science into action: periodontal health through public health approaches. Periodontol 2000. 2012;60(1):173–187. doi: 10.1111/j.1600-0757.2012.00451.x [DOI] [PubMed] [Google Scholar]
- 78.Borrell LN, Crawford ND Socioeconomic position indicators and periodontitis: Examining the evidence. Periodontol 2000. 2012;58(1):69–83. doi: 10.1111/j.1600-0757.2011.00416.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Watt RG, Petersen PE Periodontal health through public health – the case for oral health promotion. Periodontol 2000. 2012;60(1):147–155. doi: 10.1111/j.1600-0757.2011.00426.x [DOI] [PubMed] [Google Scholar]
- 80.Heilmann A, Sheiham A, Watt RG, Jordan RA [The Common Risk Factor Approach - An Integrated Population- and Evidence-Based Approach for Reducing Social Inequalities in Oral Health]. Gesundheitswesen Bundesverb Arzte Offentlichen Gesundheitsdienstes Ger. 2016;78(10):672–677. doi: 10.1055/s-0035-1548933 [DOI] [PubMed] [Google Scholar]
- 81.Sheiham A, Watt RG The common risk factor approach: a rational basis for promoting oral health. Community Dent Oral Epidemiol. 2000;28(6):399–406. [DOI] [PubMed] [Google Scholar]
- 82.WHO | Strategies and approaches in oral disease prevention and health promotion. WHO. https://www.who.int/oral_health/strategies/cont/en/. Accessed November 24, 2018. [PMC free article] [PubMed]
- 83.Petersen PE World Health Organization global policy for improvement of oral health--World Health Assembly 2007. Int Dent J. 2008;58(3):115–121. [DOI] [PubMed] [Google Scholar]
- 84.SIREGARI HE, NDARI R Handling the Periodontal Disease in Community. Iran J Public Health. 2016;45(2):257–259. [PMC free article] [PubMed] [Google Scholar]
- 85.Dumitrescu AL Editorial: Periodontal Disease – A Public Health Problem. Front Public Health. 2016;3. doi: 10.3389/fpubh.2015.00278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Vogtmann E, Graubard B, Loftfield E, et al. Contemporary impact of tobacco use on periodontal disease in the USA. Tob Control. 2017;26(2):237–238. doi: 10.1136/tobaccocontrol-2015-052750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Tomar SL, Asma S Smoking-attributable periodontitis in the United States: findings from NHANES III. National Health and Nutrition Examination Survey. J Periodontol. 2000;71(5):743–751. doi: 10.1902/jop.2000.71.5.743 [DOI] [PubMed] [Google Scholar]
- 88.CDC. Current Cigarette Smoking Among Adults in the United States. Centers for Disease Control and Prevention. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/index.htm. Published February 4, 2019. Accessed May 29, 2019.
- 89.Lancaster T, Stead LF Individual behavioural counselling for smoking cessation. Cochrane Database Syst Rev. 2017;(3). doi: 10.1002/14651858.CD001292.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tomar SL Public Health Perspectives on Surveillance for Periodontal Diseases. J Periodontol. 2007;78 Suppl 7S:1380–1386. doi: 10.1902/jop.2007.060340 [DOI] [PubMed] [Google Scholar]
- 91.WHO | Oral health information systems. WHO. https://www.who.int/oral_health/action/information/surveillance/en/index1.html. Accessed April 3, 2019.
- 92.Chandrashekar J, Farheen T, Joe J, Ramanarayan V Assessment of Common Risk Factors Between Oral Diseases and Non-communicable Diseases in a Hospital-based Population in Kerala, India- A Cross-sectional Study. J Clin Diagn Res. 2019;13(3):16–20. [Google Scholar]
- 93.Holtfreter B, Albandar JM, Dietrich T, et al. Standards for reporting chronic periodontitis prevalence and severity in epidemiologic studies: Proposed standards from the Joint EU/USA Periodontal Epidemiology Working Group. J Clin Periodontol. 2015;42(5):407–412. doi: 10.1111/jcpe.12392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.World Health Organization. WHO Steps Surveillance Manual: The WHO Stepwise Approach to Chronic Disease Risk Factor Surveillance. Geneva: WHO; 2005. [Google Scholar]
- 95.Marshall-Day CD The epidemiology of periodontal disease. J Periodontol. 1951;22(1):13–22; passim. [DOI] [PubMed] [Google Scholar]