Abstract
Coronavirus disease 2019 (COVID-19), the infectious disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is an aggressive disease that attacks the respiratory tract and has a higher fatality rate than seasonal influenza. The COVID‐19 pandemic is a global health crisis, and no specific therapy or drug has been formally recommended for use against SARS-CoV-2 infection. In this context, it is a rational strategy to investigate the repurposing of existing drugs to use in the treatment of COVID-19 patients. In the meantime, the medical community is trialing several therapies that target various antiviral and immunomodulating mechanisms to use against the infection. There is no doubt that antiviral and supportive treatments are important in the treatment of COVID-19 patients, but anti-inflammatory therapy also plays a pivotal role in the management COVID-19 patients due to its ability to prevent further injury and organ damage or failure. In this review, we identified drugs that could modulate cytokines levels and play a part in the management of COVID-19. Several drugs that possess an anti-inflammatory profile in others illnesses have been studied in respect of their potential utility in the treatment of the hyperinflammation induced by SAR-COV-2 infection. We highlight a number of antivirals, anti-rheumatic, anti-inflammatory, antineoplastic and antiparasitic drugs that have been found to mitigate cytokine production and consequently attenuate the “cytokine storm” induced by SARS-CoV-2. Reduced hyperinflammation can attenuate multiple organ failure, and even reduce the mortality associated with severe COVID-19. In this context, despite their current unproven clinical efficacy in relation to the current pandemic, the repurposing of drugs with anti-inflammatory activity to use in the treatment of COVID-19 has become a topic of great interest.
Keywords: Cytokine, COVID-19, ACE-2, New drug, IL-6
1. Introduction
The first cases of coronavirus disease 2019 (COVID-19, the disease caused by the SARS-CoV-2 virus) were reported in the city of Wuhan, the capital of Hubei province, China, at the end of 2019 and rapidly spread to other countries. The outbreak was declared a pandemic by the World Health Organization (WHO) on March 11, 2020, raising the level of worldwide monitoring of the disease, and marking the advent of a public health crisis not seen in recent history. There have been a number of significant pandemics in recent human history, such as HIV/AIDS (acquired immunodeficiency syndrome caused by the HIV virus), and the Spanish flu (1918-1919) that infected about 40% of the world’s population and killed about around 50 million people [1], [2]. Although there have been significant advances made in the understanding and treatment of viruses, the current pandemic has still, to a large extent, overwhelmed us.
COVID-19 is an aggressive disease that appears to have a higher fatality rate than seasonal influenza. It has several unusual characteristics, and in the most serious cases attacks the respiratory tract. The death rate is not yet clear, as it depends on the number of undiagnosed and asymptomatic cases [3]. Interestingly, although a considerable number of patients with COVID-19 shown only minor symptoms, like a cold, others die within a few days of being infected. This has been related to the fact that severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) can produce critical pneumonia that is difficult to treat, and may be followed by organ failure, and even death [4]. The treatment of COVID-19 has been based on basic life support, and the mitigation of the effects on the respiratory system caused by acute respiratory distress syndrome (ARDS), which is the leading cause of mortality [5]. Another interesting aspect of COVID-19 is that older patients, and those with comorbidities and immunosuppression from any cause, are more susceptible to the disease [6].
Interestingly, the role that the renin-angiotensin system (RAS), and particularly the ACE2 (Angiotensin-Converting Enzyme 2) receptors, plays in COVID-19 has been a growing area of interest in studies that seek to better understand the disease and find new treatments [7]. SARS-CoV-2 enters cells by attaching itself to a specific protein, the ACE2 receptor, which sits on the cell's surface, so facilitating viral entry into target cells. ACE2 receptors are more abundant in the lungs (one of the reasons why COVID-19 is considered a lung disease) and are also widely expressed in intestinal cells, which could partly explain why some patients have diarrhea [8]. The overexpression of ACE2 enhances the severity of the pulmonary disease caused by coronavirus, but can be attenuated by blocking the RAS pathway [9]. ACE2 overexpression is related to an increase of pro-inflammatory cytokines (PICs) in the lungs of SARS-CoV-2 patients [10], [11]. However, COVID-19 patients with hypertension treated with angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin II type 1 receptor blockers (ARBs) increased CD3 and CD8 T cell counts in peripheral blood, reduced IL-6 levels in peripheral blood, and decreased the peak viral load [7]. Excessively high levels of PICs are associated with worse outcomes in COVID-19 patients [12]. Moreover, a kind of “cytokine storm” appears to be one of the triggers for the condition of some COVID-19 patients to rapidly worsen [5]. It is not yet clear if the use of drugs to manage the levels of cytokines can inhibit COVID-19, but it is certainly an avenue worth exploring.
The use of drugs with distinct molecular mechanisms of action such as hydroxychloroquine (HCQ), chloroquine (CQ), favipiravir, ivermectin, ACEIs, ARBs, and atazanavir, among others, has been proposed. These drugs can be used in association with other drugs to reduce viral loads, or to act as adjuvant drugs to mitigate the severity of COVID-19, particularly in respect of the debilitating respiratory condition that has been one the most dramatic and distressing characteristics of the disease for patients and caregivers. Some of these drugs are strong cytokine modulators, although this is not always their main mechanism of action [13]. However, scientists and researchers across the world have been trying to better understand the disease and its implications, and a great amount has already been discovered. New scientific data being generated at an impressive rate, although for many, the challenges presented by COVID-19 remain insurmountable. In respect of cytokines, there is a growing body of evidence on the role they play in COVID-19, but there are still many gaps in our knowledge, especially regarding the use of drugs in cytokine modulation.
Therefore, this review seeks to explore this gap by performing a review of drugs that could modulate cytokine levels and play some part in the management of severe cases of COVID-19.
1.1. Cytokine dysregulation in COVID-19
Cytokines are water-soluble extracellular polypeptides or glycoproteins, varying between 8 and 30 kDa; they are produced by several types of cells at injury sites, and by cells [5] from immune system through the activation of mitogen-activated protein kinases. Cytokines have been increasingly key in modern medicine as diagnostic, prognostic and therapeutic agents in human disease [14].
The cytokine storm syndrome is defined as a cytokine-mediated systemic inflammatory response induced by several initiating factors, including viruses, resulting in a clinical presentation that could progress to multiple organ failure [15], [16], [17]. The hallmark of cytokine storm syndrome is an uncontrolled activation and amplification of the host immune system, causing the excessive release of a wide range of cytokines, such as, tumor necrosis factor (TNF), interleukin (IL)-1, IL6 and IL-18 [18], [19]. Since the important article published by Mehta et al. (2020) [5], which strongly reinforced the concept of the production of a “cytokine storm” by SARS-CoV-2 infection, many studies have tried to better understand the role of cytokines and the possible clinical repercussions in COVID-19 [20], [21], [22].
The exact role of the “cytokine storm” has not yet been fully elucidated. However, it seems to be related to the severity of ARDS in patients with COVID-19, and it is becoming clear that the establishment of cytokine balance may be an important clinical measure in the treatment of patients. Depending on the amount of cytokine production induced by the immunological activation caused by SARS-CoV-2, three major clinical phenotypes have been identified: mild - characterized by a “drizzle” of cytokines, severe -by a “storm”, and critical – by a “hurricane” of cytokines [23]. Additionally, understanding the underlying mechanism(s) as the disease progresses from mild to critical as a result of immune dysfunction and cytokine dysregulation is crucial to developing an effective treatment [21].
In the pathogenesis of COVID-10, SARS-CoV-2 infection causes a down-regulation of ACE2 protein, which in turn results in excessive production of the vasoconstrictor Ang II. The AngⅡ-AT1R axis activates the nuclear factor κB (NFκB) signaling and the metalloprotease 17 (ADAM17), which stimulates the production of the mature form of epidermal growth factor receptor (EGFR) ligands and TNF-α [24]. The activation of NFκB and STAT3 leads to a hyperinflammatory state [25].
In addition, the viral infection causes the recruitment of macrophages, neutrophils, and natural killer cells which are responsible for producing pro-inflammatory cytokines. These cytokines, IFN, INF-c, TNF-α, IL-1, IL-6, and IL-2 represent the first defense against pathogens. Pathogen-associated molecular patterns (PAMPs) are recognized by pattern-recognition receptors, i.e. toll-like receptors (TLRs) [26]. TLRs mediate the inflammatory signaling cascade activation. TLR activation leads to the production of inflammatory cytokines [27], such IL-6, TNFα, and IL-1β [28]. In the mild phenotype, the adaptive immune cells are then activated and contribute to enhancing the immunological response by directly attacking virus-infected cells or by releasing other proinflammatory cytokines [26]. In most COVID-19 cases, the response is self-limiting, and no tissue damage has been observed.
In severe COVID-19 patients the cytokine storm syndrome may occur. This mechanism causes the release of a great amount of pro-inflammatory cytokines due to the lysis of cells [29]. The activated macrophages contribute by enhancing the massive release of IL-6, IL-1, IL-18, IL-8, granulocyte–macrophage colony-stimulating factor (GMCSF), chemokine CX- C motif ligand (CXCL) 9, and CXCL10 [29], [30]. This excessive cytokine production induced by SARS-CoV-2 infection causes an increase in vascular permeability, resulting in a large amount of blood cells and fluid entering the alveoli, resulting in severe damage to the host cells and respiratory failure [31], [32].
SARS-CoV-2-induced pneumonia is associated with the overexpression of T cells and the rampant production of PICs, mainly IL-6 [33]. COVID-19 pulmonary immunopathology is characterized by several factors, but in respect of the cytokine profile, there is an increase in the levels of IL-1β, IL-2, IL-6, IL-17, IL-8, TNFα and CCL2[34]. IL-6 appears to be key in worsening the pulmonary condition, and in the severity of COVID-19 in the patient [33]. IL-6 has an important role in lung repair responses in viral infections such as SARS-CoV-2, and induces the production of acute phase proteins, including C-reactive protein [35].
The most severe cases have shown high levels of infection-related biomarkers and PICs, and a significant reduction in T cells. Both helper T cells and suppressor T cells in patients with COVID-19 were below normal levels, the lowest level of helper T cells being found in severe patients. Moreover, the percentage of mature naïve helper T cells and memory helper T cells is reduced in patients with SARS. In fact, it is suggested that the disease mainly acts on lymphocytes, especially T lymphocytes, which influence immune modulation and cytokine production [36]. Occasionally, cytokine storm syndrome becomes severe and uncontrolled, showing a clinical phenotype resembling a form of secondary hemophagocytic lymphohistiocytosis/macrophage activation syndrome (HLH/MAS) [37]. In this situation, IL-18 becomes another pivotal mediator of the onset of HLH/MAS [38], with the inflammasome being constantly triggered, releasing an excessive amounts of pro-inflammatory mediators, which leads to multiorgan failure [23]
In this context, an approach that has been highlighted for the treatment of patients with COVID-19 is a reduction in antiviral cytokines, particularly type I interferons (IFNs), which are related to part of the early immune response in viral infections, as they are secreted upon stimulation by pathogen-derived nucleic acids [39]. IFNs activate protective mechanisms against the infection of various viruses to provide harm reduction and elimination of the virus. INF treatment has, therefore, been an approach used for prophylaxis and reduction of viral loads in patients infected with different viruses [40]. Recently, it has been suggested that type III IFNs (IFN-λ) - antiviral cytokines, may be a promising approach to emerging viral infections, such as that produced by SARS-CoV-2. IFN-λ has been reported to be able to inhibit SARS-CoV-1 and MERS-CoV when assessed in in vitro studies [41].
2. Drug repurposing for COVID-19 and affected cytokines
The increasing number of COVID-19 cases has spurred the search for medications to help fight the pandemic. As SARS-CoV-2 is a new virus, rigorous studies to evaluate the effectiveness of existing drugs (through drug repurposing), and to produce a new safe, effective substances are required.
There are currently a variety of classes of drugs being proposed to mitigate the effects of COVID-19. Some of these drugs, such as chloroquine (CQ), and hydroxychloroquine (HCQ), have caused an unjustified frenzy for their indiscriminate use as preventive measures in a number of countries, including Brazil and the USA [42]. Although not the main target of most of the drugs currently used in the treatment of COVID-19, there is growing evidence that the “cytokine storm” may have a significant influence on the development of the disease, especially in critically ill patients [21]. However, there is a lack of information about how these drugs might help to manage important cytokines and contribute to the recovery of patients. Several drugs that have been studied for their potential utility in COVID-19 possess anti-inflammatory profiles in others illness and are being tested against the hyperinflammation induced by SAR-COV-2 infection. Knowledge about the molecular targets of the drugs in SAR-COV-2 infection, and the subsequent effects on immune responses, can help to pave the way for empirical approaches and trials at this stage. Among the drug classes suggested for use in COVID-19 treatment that could modulate the observed inflammatory process are antiviral, anti-rheumatic, anti-inflammatory drug, antineoplastic and antiparasitic drugs (Table 1, Table 2, Table 4 ). All of these classes of drugs have been shown to have favorable effects in COVID-19 patients (see Table 1, Table 2, Table 4).
Table 1.
Antiviral drugs for the management of COVID-19 and cytokines they affected.
| Class | Drug | Main Class | Main mechanism of action | Predominant mechanism to management of COVID-19 | Cytokines | Cytokines study type | References |
|---|---|---|---|---|---|---|---|
| Antiviral drugs | Atazanavir | Antiretroviral drug | Inhibitor of CYP3A and UGT1A1 | Blocks the major protease (Mpro) of SARS-CoV-2 | ↓IL-6 | In vitro | [53] |
| Favipiravir (Avigan) | Antiviral drug | Competitive inhibitor of RNA-dependent RNA polymerase | Interferes with viral replication | ↓ TNF-α |
In vivo In vitro |
[54] | |
| IFN-α2b (interferon) | Antiviral drug Immunomodulatory drug |
Generation of adaptive of immune response Inhibit of DNA replication |
Inhibits the replication of SARS-CoV and MERS-CoV replication |
↑IL-10 ↑IL-2 TNF-α |
In vitro Clinical |
[142], [143], [144], [145] | |
| Lopinavir-ritonavir | Antiviral drug | Proteinase inhib-itorproteinase inhib-itorAntiretroviral proteinase inhibitor | Inhibits the CYP3A‐mediatedmetabolism of LPV, Inhibits 3CLpro by SARS-CoV, affecting viral replication and maturation;Ritonavir inhibits the CYP3A metabolism of Lopinavir, increasing its plasmatic concentration Protease inhibitor |
Lopinavir ↓TNF-α ↓IL-6 Ritonavir ↓ IFN-γ ↓IL-10 |
In vitro | [55] | |
| Remdesivir | Antiviral drug | RNA polymerase inhibitor Nucleoside analogue |
RNA polymerase inhibitor |
↓TNF-α ↓IL-1β ↓IL-6 ↓IL-18 |
In vivo | [60], [146], [147] | |
| Ribavirin | Antiviral drug | Stops viral RNA synthesis | Nucleoside analogue, interferes with the replication of RNA and DNA viruses |
↓TNF-α ↓IL-1β ↓IL-6 ↓IFN-γ |
In vivo | [148] | |
| Umifenovir | Anti-viral drug | Inhibition of membrane fusion of viral envelope and host cell cytoplasmic membrane. | Block the trimerization of spike glycoprotein of SARS-CoV-2 |
↓TNF-α ↓IL-6 ↓IL-8 ↓IL-10 |
In vivo | [149], [150], [56] |
IL: Interleukin; IFNs: Interferons; MERS-CoV: Middle East Respiratory Syndrome Coronavirus; SARS-CoV-2: Severe Acute Respiratory Syndrome coronavirus 2, Tizoxanide: metabolite of nitazoxanide.
Table 2.
Anti-rheumatic and anti-inflammatory drugs for the management of COVID-19 and cytokines they affected.
| Class | Drug | Main Class | Main mechanism of action | Predominant mechanism to management of COVID-19 | Cytokines | Cytokines study type | References |
|---|---|---|---|---|---|---|---|
| Anti-rheumatic drugs | Anakinra | Anti-arthritic agent | Recombinant IL-1Ra antagonist | Interleukin-1 receptor antagonist |
↓TNF-α ↓IL-1β ↓IL-6 |
In vivo |
[67], [151], [69] |
| Baricitinib | Anti-arthritic agent | Potent and selective Janus Kinases (JAK) inhibitor | Interrupts the passage and intracellular assembly of SARS-CoV-2 into the target cells via disruption of AAK1 Block clathrin-mediated endocytosis and thereby inhibit viral infection of cells |
↓TNF-α ↓ IL-4 ↓ IL-6 |
Clinical In vitro |
[152], [153], [116], [154], [117] | |
| Etanercept |
Anti-arthritic agent | Inhibitor of TNFα | TNFα inhibitor |
↓TNF-α ↓IL-1 β ↓IL-6 |
In vivo |
[155], [156], [157], [158], [63] |
|
| Infliximab | Anti-arthritic agent Crohn's disease |
Inhibitor of TNFα | TNFα inhibitor |
↓TNF-α ↓IL-1 ↓IL-6 ↓IL-8 |
Clinical In vivo |
[79], [159] | |
| Tocilizumab |
Anti-arthritic agent | IL-6 receptor inhibitors Monoclonal anti-soluble IL-6 receptor antibody |
Recombinant humanized monoclonal anti-IL6R antibody. It binds both soluble and membrane‐bound IL6R to inhibit IL6‐mediated signaling |
Patients with high IL-6 levels Inhibiting IL-6 signal transduction |
Clinical | [160], [75], [161] | |
|
Anti-inflammatory drugs |
Indomethacin | Nonsteroidal anti-inflammatory drug | Non-selective cyclooxygenase (COX) inhibitor | Inhibition of viral replication and infectious viral particle production Inhibition of viral replication and infectious viral particle production |
↓TNF-α ↓IL-1β ↓IL-6 ↓IL-8 |
In vivo |
[93], [92], [90], [162] |
| Thalidomide |
Phthalimides | Immunomodulatory, anti-inflammatory, and anti-angiogenic agent | Immunomodulatory and anti-inflammatory Immunomodulatory and anti-inflammatory agent |
↓TNF-α ↓IL-1β ↓IL-6 ↑IL-10 ↓IL-17 ↓IFN-γ |
In vivo | [99], [163], [164] | |
| Corticosteroids |
Steroidal anti-inflammatory drug | Suppression of multiple inflammatory genes by linking glucocorticoid recruitment of histone deacetylase 2 for the activated transcription complex. |
Inhibits the NFκB transcription factor. |
↓TNF-α ↓IL-1 ↓IL-2 ↓IL-3 ↓IL-5 ↓IL-6 ↓IL-8 ↑IL-10 ↑IL-12 ↓IL-13 ↓IL-15 ↓IFN-β ↓IFN-λ1 ↓IFN-γ |
In vitro In vivo |
[165], [166], [167] |
AAK1: AP2-associated protein kinase; 1IL: Interleukin; IFNs: Interferons; IL6R: IL-6 receptor; SARS-CoV-2: Severe Acute Respiratory Syndrome coronavirus 2.
Table 4.
Antineoplastic, antibiotic and antiparasitic drugs for the management of COVID-19 and cytokines they affected.
| Class | Drug | Main Class | Main mechanism of action | Predominant mechanism to management of COVID-19 | Cytokines | Cytokines study type | References |
|---|---|---|---|---|---|---|---|
| Antineoplastic drug | Ibrutinib | Antineoplastic drug | Covalent BTK inhibitors | Prevents both B-cell activation and B-cell-mediated signaling (proposal) |
↓TNF-α ↓IL-6 ↓IL-8 ↓IL-10 |
Clinical In vitro |
[122], [123] |
| Ruxolitinib | Antineoplastic and immunomodulating activities | Janus kinase inhibitors | Janus kinase inhibitors |
↓TNF-α ↓IL-6 |
In vitro | [171], [119] | |
| Antibiotic agent | Azithromycin | Antibiotic agent | Binds to the 50S ribosomal subunit, affecting bacterial protein synthesis |
↓TNF-α ↓IL-6 ↓L-8 ↑IL-10 |
In vitro | [172], [173], [174] | |
| Antiparasitic drugs | Chloroquine | Antimalarial drug | Immunosuppression | Alters the pH in the lysosomes Prevents viral fusion and replication. Spike (S)-protein angiotensin converting enzyme 2 (ACE2) blockers |
↓TNF-α ↓IL-6 ↓IL-18 |
In vivo | [175] |
| Hydroxychloroquine | Antimalarial drug Disease-modifying antirheumatic drugs (DMARDs) |
Immunosuppression | Inhibition of cellular receptor of SARS-CoV (ACE2), membrane fusion of the virus in the host, nucleic acid replication, new virus transport, virus release. |
↓TNF-α ↑IL-4 ↓IL-6 ↑IL-10 ↓IL-18 ↓IFN-γ |
Clinical | [137] | |
| Ivermectin | Antiparasitic agent broad spectrum Anti-viral (in vitro) |
Nuclear transport inhibitory activity | Nuclear transport inhibitory activity |
↓TNF--α ↓IL-1ss ↓IL-4 ↓IL-5 ↓IL-6 ↓IL-13 |
In vitro | [126], [132], [131] | |
| Nitazoxanide (anitta) | Antiprotozoal drug | Potentiates interferon alfa and interferon beta production | Affect viral genome synthesis Prevents viral entry and interferes with N-glycosylation |
↓TNF-α ↓IL-1β (Tizoxanide) ↓IL-6 |
In vivo In vitro |
[176], [177], [178] |
|
| Other | Colchicine | Anti-gout | Microtubule inhibitor (colchicine block microtubule polymerization) |
Non-selective inhibitor of NLRP3 inflammasome Anti-inflammatory action |
↓TNF-α ↓IL-1β ↓IL-6 ↓IL-18 |
Clinical In vitro |
[84], [83], [87], [179] |
BTK: Bruton’s tyrosine kinase; IL: Interleukin; IFNs: Interferons; NLRP3: NOD-, LRR- and pyrin domain-containing protein 3; SARS-CoV-2: Severe Acute Respiratory Syndrome coronavirus 2, Tizoxanide: metabolite of nitazoxanide.
2.1. Antiviral drugs
Several recent studies have identified promising antiviral drug candidates that might be effective against SARS-CoV-2 infection by inhibiting some aspects of COVID-19 (Table 1). These include atazanavir[43], favipiravir [44], IFN-α2b (interferon) [45], lopinavir-ritonavir [46], remdesivir [47], ribavirin [48], and umifenovir [49] (Table 1). A number of in silico, preclinical or clinical studies have suggested that these drugs could have favorable clinical outcomes for SARS-CoV-2 patients [50], reducing virus load, and mitigating the “cytokine storm”.
According to Grein et al. (2020) [47], a 10-day course of intravenous administration of remdesivir in patients hospitalized for severe COVID-19 improved clinical symptoms in 68% of the patients. However, in a randomized, double-blind, placebo-controlled, multicenter clinical trial, remdesivir (200 mg on day 1, followed by 100 mg on days 2–10 in single daily infusions) was not associated with statistically significant clinical benefits when administered in severe COVID-19 patients admitted to hospital, despite the authors observing a numerical reduction in the time to clinical improvement. These results require confirmation in larger studies [51].
In a randomized study of a cohort of 240 patients with moderate and severe COVID-19, favipiravir demonstrated better clinical recovery rates at day 7 in moderate illness, but made no difference in severe COVID-19 patients [44]. In another study, umifenovir (200 mg, oral, three times per day, for 7–10 days) administrated in combination with IFN-α2b did not have any beneficial effect in COVID-19 RNA clearance and patient hospitalization when compared to IFN-α2b monotherapy in a cohort, but it did appear to accelerate pneumonia absorption [45]. However, another study suggested that patients with COVID-19 treated with umifenovir had a shorter duration of positive RNA test and an apparent favorable clinical response compared to lopinavir-ritonavir treatment [49].
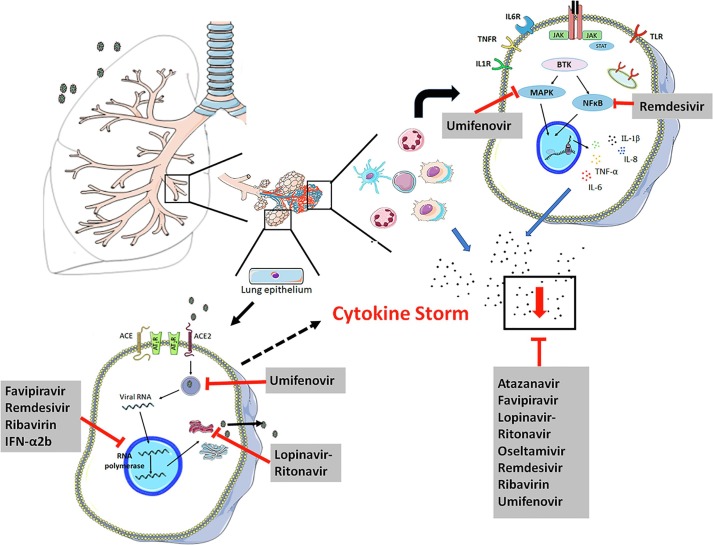
The predominant action mechanism of antiviral drugs in the management of COVID-19 is to interfere with viral replication or inhibit viral fusion in the membrane [52], as can be seen in Table 1 and Fig. 1 . However, this effect, which reduces viral load, is not the only mechanism of action of antiviral drugs. Studies have shown that antiviral drugs, such as atazanavir [53], favipiravir [54], lopinavir-ritonavir [55], remdesivir [51], and umifenovir [56] possess indirect antiviral properties and act as anti-inflammatory molecules, due to their capacity to modify the production of inflammatory mediators [51]. According to some studies [4], the severity of COVID-19 may depend on the magnitude of the “cytokine storm” and, at this stage, drugs that only decrease viremia may not be effective [57]. Thus, antiviral drugs with anti-inflammatory activity could be important in treatment, due to their capacity to minimize the cellular damage caused by the hyperinflammatory process, mitigating the severity of the COVID-19 crisis (see Table 1).
Fig. 1.
Predominant mechanism of antiviral drugs in the management of COVID-19. The entry of SARS-CoV-2 in epithelial/endothelial lung cells, via binding to ACE2, causes apoptotic and necroptotic events that lead to lung injury and the release of large amounts of chemokines, driving the recruitment of immune cells within the lungs. The recruitment of cells promotes the innate immune response by secreting proinflammatory cytokines. A pro-inflammatory feed-forward loop of cytokines acts on innate immune cells and induces exacerbated hyperinflammation (known as the “cytokine storm”), coagulopathy and acute respiratory distress syndrome (ARDS). The current strategy for the management of COVID-19 is based on stopping viral replication and/or attenuating the inflammatory process. Black points represent the pool of cytokines.
As a result of virus recognition, downstream transduction pathways related to inflammatory cascade are activated, including NFκB, IRF3 (IFN regulatory factor-3)[58] and p38MAPK [59], leading to the upregulation of several cytokines and chemokines, and an increase in tissue damage caused by the inflammatory condition. The modulation of this signaling pathway could be crucial for an effective antiviral response against COVID-19. According with Li and Su (2020) [60], the antiviral drug remdesivir inhibits the activation of IRF3 and the NF-κB pathway, decreasing the expression and release of IL-18, IL-6, IL-1β, and TNF-α cytokines. Tanaka et al (2017)[54] showed that favipiravir reduced TNF-α production in macrophage cells medium and BALF from mice lungs, both infected with the influenza virus (H1N1). In addition, the anti-inflammatory effect of umifenovir was determined by Wang et al (2017) [56]. The authors reported that this antiviral drug modulated the production of IL-6, IL-8, IL-10 and TNF-cytokines in vitro and in vivo, after a viral infection. Moreover, umifenovir could treat persistent viral infection by inhibiting the p38MAPK signaling pathway, and modulating the release of cytokines [61]. All these anti-inflammatory activities of antiviral drugs could attenuate the “cytokine storm” caused by the SARS-COV-2 infection, contributing to the recovery of patients. Fig. 1 and Table 1 show the main molecular targets of the antiviral drugs summarized in this article, the effect on viremia, and their anti-inflammatory profiles (see Table 4).
Therefore, antiviral drug therapy may have an important role in the fight against COVID-19 infections due to its capacity to act directly to decrease viral loads, and also to its anti-inflammatory profile that could aid the recovery of COVID-19 patients, contributing to a reduction in the number of severe cases. The antiviral drugs used routinely or suggested for the management of COVID-19 patients are able to mitigate the “cytokine storm” by modulating the production of TNF-α, IL-1β, IL-18, IL-10 and, in particular, IL-6 (Table 1), a cytokine that has been closely correlated with ARDS severity and a worse COVID-19 prognosis. However, no specific antiviral drug has been proven to be effective in the treatment of patients with severe COVID-19. More studies are necessary to confirm the potential useful effects of antiviral therapies.
2.2. Anti-rheumatic drugs
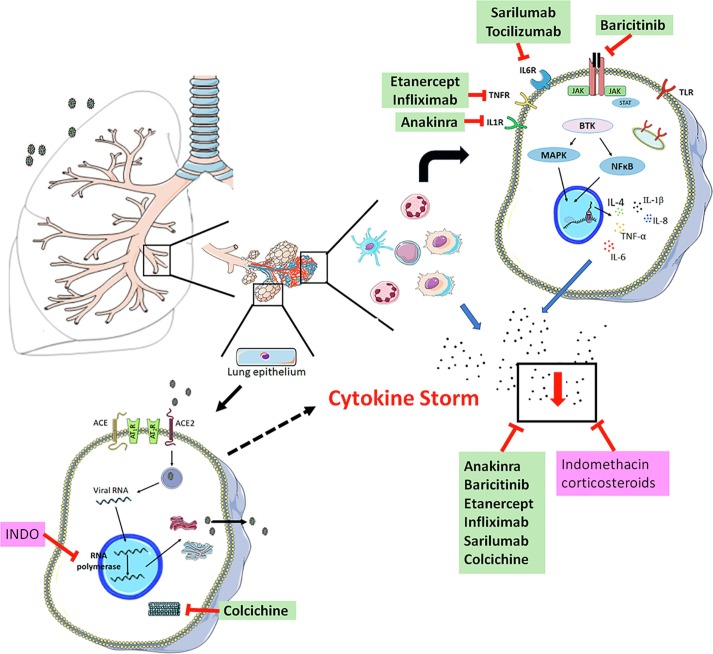
Drugs that have been used in rheumatology are also being studied in relation to SARS-CoV-2 infection [62]. The rationale for the use of these anti-rheumatic and anti-inflammatory drugs is based on the “cytokine storm” that occurs in COVID-19 patients [20], especially in patients with a severe prognosis and T cell depletion [4]. The regulation of this “cytokine storm” is crucial in the treatment of COVID-19 infection. The main mechanisms of action of several anti-rheumatic drugs are based on the inhibition of the inflammatory cascade, blocking TNF-α [63], IL-6 receptors [64] or IL-1Ra [65], as well as are acting as selective Janus kinase (JAK) inhibitors [66] (Fig. 2 ). Table 2 includes details of the anti-rheumatic drugs studied and/or suggested for use in COVID-19 treatment, and the molecular target of each one.
Fig. 2.
Predominant mechanism of anti-rheumatic and anti-inflammatory drugs involved in the management of COVID-19. Black points represent the pool of cytokines. Anti-rheumatic drugs are marked in green; Anti-inflammatory drugs are marked in pink.
According to Dimopoulos et al. (2020) [67], the recombinant IL-1Ra antagonist anakinra may be beneficial for treating severe COVID-19 patients with secondary hemophagocytic lymphohistocytosis (sHLH). Furthermore, Cavalli et al. (2020) [68] reported that in a retrospective cohort study of COVID-19 patients with ARDS treated with high-doses of the drug, it was safe and associated with clinical improvement in 72% of patients. As anakinra acts upstream of the inflammatory signaling pathway [65], its effectiveness in the management of COVID-19 patients could be related to blocking the “cytokine storm”. Studies have shown that IL-1Ra antagonists inhibit TNF-α, IL-6 and IL1β production in animal models [69]. Additionally, a study showed that anakinra treatment protected rat islets in culture against IL-1β, TNFα, and IFNγ stimuli [70], suggesting that this anti-rheumatic drug could have effective tissue protection properties in situations of hyper-inflammation.
Another interesting option is tocilizumab, a recombinant humanized monoclonal anti-IL‐6 receptor antibody. Tocilizumab binds both soluble and membrane‐bound IL‐6R, leading to the inhibition of the IL‐6 signaling pathway [71], and consequent downregulation of JAK-STAT or MAPK/NF-kB-IL-6 signaling cascade activation [72]. Clinical findings suggest that IL-6 is one of the most important cytokines involved in the pathogenesis of COVID-19 [73] and an important target for drugs repurposed for COVID-19 treatment. Tocilizumab has been successfully used in a small series of clinical studies, suggesting that this drug may be a promising medicine to use in the recovery of COVID-19 patients. Preliminary data from a study by Xu et al. (2020) [74] indicated that tocilizumab improved clinical outcomes in severe and critical COVID-19 patients.
According to Capra et al. (2020) [75] tocilizumab appears to increase survival and lead to a favorable clinical course if used early during COVID-19 pneumonia with severe respiratory syndrome. In another study, Luo et al. (2020) treated COVID-19 patients with augmented levels of IL- 6 with the drug, and observed that IL- 6 levels initially increased further, but then reduced in 10 patients. The authors concluded that tocilizumab seems to be an effective treatment option for use in COVID-19 patients with a risk of “cytokine storms” [76]. Thus, tocilizumab could help COVID-19 patients to recover by modulating the inflammatory process, and consequently preventing tissue injury, since IL-6 levels have been closely correlated with ARDS severity and outcome [33]. Moreover, the effects of IL-6 blockade therapy against infection-induced “cytokine storm” have been well documented [77]
Promising results have also been reported in respect of the use of anti-TNF drugs in COVID-19 treatment [78]. Infliximab, a TNFα inhibitor used in the management of Crohn’s disease, was reported to have potential to affect the “cytokine storm” associated with COVID-19. Dolinger et al. (2020) [79], in a case report, reported that a COVID-19 patient with Crohn’s disease treated with infliximab showed improved cytokine profile and reduced clinical symptoms, with normalization of TNF-α, and a reduction in IL-6 and IL-8 levels. The reduction in cytokine concentration induced by infliximab treatment could be associated with downregulation of the p38MAPK pathway [80]. These findings in respect of anti-TNFα drugs need to be cautiously analyzed given the lack of any control group for comparison, and the background treatment with antiviral drugs and corticosteroids.
Additionally, some studies suggest that colchicine, a drug approved to treat autoinflammatory conditions such as gout, could be useful in the management of COVID-19 because of its capability to interfere in the pathogenic mechanisms related to SARS-CoV-2 infection [81]. Montealegre-Gómez et al. (2020) [82] suggested that colchicine could decrease the risk of ARDS in patients with COVID-19 infection through the modulation of TNF-α [83], IL-1β, IL-18 and IL-6 cytokine production [84]. Moreover, colchicine decreases ROS formation and disrupts inflammasome activation, leading to a decrease in inflammatory processes [84], [85]. All these features could contribute to relief of inflammatory symptoms and decrease the risk of organ failure. However, the role of colchicine in the treatment of COVID-19 is controversial in the literature, with some studies raising doubts about its use against SARS-CoV-2 infection [86], [87], as its effects on altering cytosolic pH and suppressing “cytokine storms” are very weak [86] (Table 4).
COVID-19 therapy based on anti-rheumatic and anti-gout drugs has been widely discussed in the scientific literature. The anti-rheumatic drugs suggested for the treatment of COVID-19 patients may be able to mitigate the “cytokine storm” by decreasing the production of TNF-α, IL-1β, IL-6, IL-8, and IL-4, whereas colchicine inhibits TNF-α , IL-1β, IL-18, and IL-6. This class of drugs may block the fulminant response of the immune system, reducing the risk of multiple organ failure and the severity of the disease (Fig. 2). However, additional studies are necessary to prove the efficacy of this therapy in COVID-19 patients and explore the molecular mechanism of this drugs in SARS-CoV-2 infection.
2.3. Anti-inflammatory drugs
Studies have also proposed that nonsteroidal anti-inflammatory drugs are promising candidates for the management of COVID-19. Among them, indomethacin (Table 2), a non-selective cyclooxygenase (COX) inhibitor, has gained attention due to its potent in vitro antiviral profile on human SARS-CoV-1, canine CCoV, and more recently on human SARS-CoV-2 at a low concentration range [88], [89]. The antiviral activity of indomethacin was shown to be associated with blocking viral replication [90]. However, another interesting pharmacological effect of indomethacin that could contribute to COVID-19 patient recovery is its capacity to modulate cytokine production [91], [92], particularly the robust effect this drug has on reducing the release of proinflammatory IL-6 [93]. The ability of indomethacin to attenuate inflammatory processes in combination with its antiviral properties make it a good candidate in the fight against the coronavirus. However, as yet, no clinical study has been undertaken to assess the efficacy of indomethacin as a treatment for COVID-19.
Another family of anti-inflammatory drugs that has been proposed as a treatment for COVID-19 are the corticosteroids (Table 2). Systemic corticosteroids have broad-spectrum actions on the immune system that may mitigate the fulminant systemic inflammatory response that occurs in ARDS, by reducing the “cytokine storm”. However, these use of these drugs in the treatment of severe ARDS and COVID-19 patients is controversial and some studies recommend that their use should be restricted [21], [94]. According to the observational study of Zha et al. (2020) [95], the administration of corticosteroids did not produce any favorable effect in COVID-19 patients, and the study by Lu et al. (2020) reported that the use of high doses of corticosteroids was significantly related with elevated mortality risk [94]. Currently, the use of systemic corticosteroid in COVID-19 is limited to patients with potential lethal complications associated with the “cytokine storm”, and to those patients with elevated serum levels of D-Dimer [96].
Several authors have proposed the use of thalidomide in the treatment of COVID-19 [97]. This drug has an immunomodulatory and anti-inflammatory profile that could help to mitigate the excessive inflammatory mediators release in COVID-19 patients [98], [99]. Thalidomide can block neutrophil chemotaxis at the site of inflammation, inhibit generation of reactive oxygen species (ROS), and modulate the inflammatory process [100]. In addition, it can reduce the production of inflammatory and fibrogenic cytokines in lung tissues [101] However, thalidomide has undesirable side effects, including thrombocytopenia and anemia that indicate it should only be used for short term treatment in patients with the “cytokine storm” [97].
It has been suggested that anti-inflammatory drugs could be used as adjuvant drugs to mitigate the severity of COVID-19 due to their capacity to attenuate the release of inflammatory mediators such as IL-6, TNF-α and IL-1β [92] (Fig. 2). The use of anti-inflammatory drugs in association with other drugs could reduce the debilitating respiratory condition which has been one of the most dramatic and distressing symptoms presented by patients. However, the use of these drugs in COVID-19 should be restricted to certain conditions because of the possible severe side effects.
2.4. Angiotensin-converting-enzyme inhibitors (ACEi) and angiotensin II receptor blockers (ARBs)
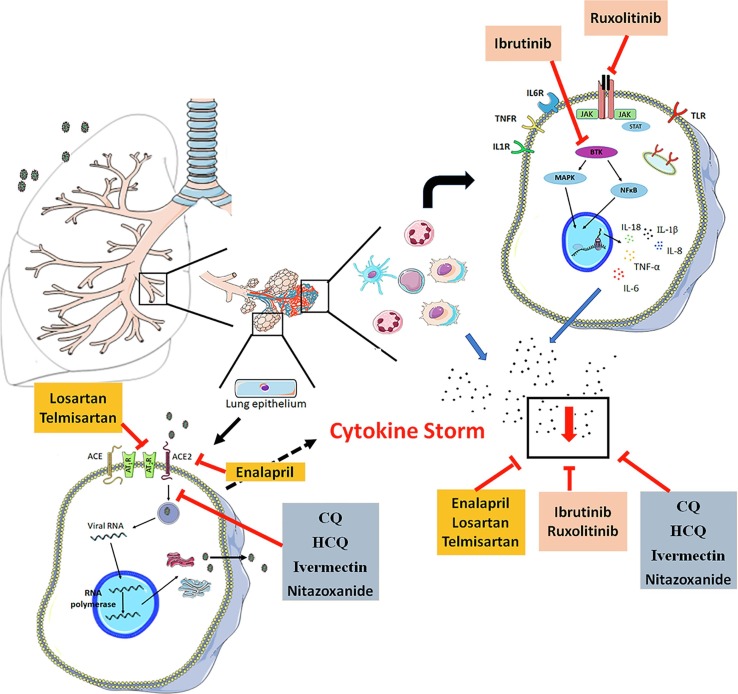
ACE2 and angiotensin II type 1 receptors (AT1-R) play a crucial role in COVID-19 development, and thus targeting them using novel therapeutic strategies may prevent or reduce virus-induced pulmonary, cardiac, and renal injury. Molecular analysis suggests that SARS-CoV-2 use the ACE2 receptor to enter the cell [102], and the overexpression of ACE2 is related to an increase in pro-inflammatory cytokines, and the enhanced pulmonary disease severity caused by coronavirus [9], [10], [11]. In this regard, ACE inhibitor and angiotensin receptor blockers may be a promising therapeutic agent in SARS-CoV-2 infection. The predominant mechanism for the management of COVID-19 used by ACEi and ARBs is a reduction in the interaction between viral protein and ACE2, affecting the internalization of the virus [103]. However, current evidence shows that these molecules could block hypercytokinemia [104] (Fig. 3 ), and thereby act to prevent the progression of the disease.
Fig. 3.
Predominant mechanism of ACEi, ARBs, antineoplastic and anti-parasitic drugs involved in the management of COVID-19. Black points represent the pool of cytokines. ACEi and ARB are marked in orange; Antineoplastic drugs are marked in red; Antineoplastic drugs are marked in red; anti-parasitic drugs are marked in blue.
Interestingly, Monteil et al. (2020) [105] reported that human recombinant soluble ACE2 (hrsACE2), which has already been tested in clinical trials for the treatment of pulmonary hypertension and acute lung injury, may suppress the early stages of SARS-CoV-2 infection in vitro [105], showing that drugs that act on the ACE2 pathway could mitigate the effects of COVID-19. Additionally, Meng et al. (2020) [7] showed that ACEI/ARB therapy improved the clinical outcomes of COVID-19 patients with hypertension, causing a decrease in the viral load by indirectly inhibiting viral replication. The authors hypothesized that ACEI/ARB therapy has an indirect antiviral function by regulating the immune system and blocking inflammatory responses. The ACEi enalapril increased IL-10 and IL-2 levels [104], and downregulated TNF-α, IL-12, IL-6, IL-1β and IL-8 production [106], [107], whereas telmisartan, an AT1R antagonist, decreased TNF-α, IL-6, IL-8 and IL-1β levels [108], [109], confirming the anti-inflammatory profile of ACEI/ARB drugs.
The angiotensin II receptor antagonist losartan, a safe drug with low side effects, is also postulated to exert a protective action against COVID-19 infection [110]. In fact, losartan has been shown to be able to enhance ACE2 expression in vivo [111], preventing the superinfections associated with coronaviruses [110]. In addition, losartan also mitigates COVID-19 symptoms by inhibiting TNF-α, IL-6 and IL-1β production [106], and reducing NFκB and p38MAPK activation [112], [113], thereby attenuating the inflammatory process. This ARB could, therefore, reduce the excessively high levels of PICs that have a harmful role in the outcomes of COVID-19 patients. However, as yet, no clinical study has been undertaken to prove the efficacy of losartan in COVID-19.
Table 3 includes a summary of the main angiotensin-converting-enzyme inhibitors and angiotensin II receptor blockers drugs that have been suggested for use in COVID-19 treatment. ACEi and ARBs therapy could reduce the virus infection and the fulminant inflammatory process caused by the coronavirus, accelerating the recovery of COVID-19 patients, and contributing to the reduction of severe cases. However, the data in the literature about these drugs are controversial, some authors suggest that ARBs increased risk of severity COVID-19 and hospitalization [5], however others authors did not observe risk of worst prognostic and hospitalization [114], [115]. Thus, more studies are required to prove the effectiveness of therapies based on ACEi and ARBs.
Table 3.
ACEi and ARBs drugs for the management of COVID-19 and cytokines they affected.
| Class | Drug | Main Class | Main mechanism of action | Predominant mechanism to management of COVID-19 | Cytokines | Cytokines study type | References |
|---|---|---|---|---|---|---|---|
|
ACEi |
Enalapril |
ACEi | Angiotensin-converting-enzyme inhibitors | Decreases the interaction between viral protein and ACE2, affecting the internalization of the virus |
↓TNF-α, ↓IL-1β ↑IL-2 ↓IL-6 ↓IL-8 ↑IL-10 ↓IL-12 |
In vitro In vivo |
[104], [107], [106] |
|
ARBs |
Losartan | ARBs | AT1R antagonist | Decreases the interaction between viral protein and ACE2 |
↓TNF-α ↓IL-1β ↓IL-6 |
In vitro In vivo |
[106], [168], [169] |
| Telmisartan | ARBs | AT1R antagonist | Decreases the interaction between viral protein and ACE2 |
↓TNF-α ↓IL-1β ↓IL-6 ↓IL-8 ↑IL-10 |
In vitro In vivo |
[108], [109], [170] |
ACE2: Angiotensin-converting enzyme 2; ACEIs: Angiotensin-converting enzyme inhibitors; ARBs: Angiotensin II type 1 receptor blockers; IL: Interleukin; IFNs: Interferons.
2.5. Antineoplastic drugs
Some drugs being considered for use in COVID-19 therapy have been regularly used in leukemias as antineoplastic agents. Indeed, virus-infected cells are pushed to increase the synthesis of nucleic acids, proteins, and lipids, and activate the energetic machinery to complete the replication and stimulated an inflammatory process. The same features are seen in cancer cells, making antineoplastic drugs interesting molecules to test in COVID-19. Among the antineoplastic drugs with immunomodulatory effects, the Janus kinase (JAK) inhibitors and Bruton’s tyrosine kinase (BTK) inhibitors have shown promise in patients with COVID-19 [116] (Fig. 3).
The Janus kinase-signal transducer and activator of transcription (JAK-STAT) signaling pathway in AT1-R, and cytokine receptors are targets for inflammatory mediators that lead to tissue damage in hyperinflammation. Thus, the inhibition of this pathway could suppress the progress of an inflammatory disease, such as COVID-19 [116]. Given the ability of JAKi to block the production of several cytokines, it would be expected that patients with severe COVID-19 and elevated levels of inflammatory cytokines could benefit from treatment with this class of drugs [117]. Table 4 included a description of the JAK and BTK inhibitor drugs that have been already suggested as useful tools for the treatment of SARS-CoV-2 infections.
Ruxolitinib, a Janus-associated kinase (JAK1/2) inhibitor (Fig. 3) approved for the treatment of myeloproliferative neoplasms [118], might be effective against the consequences of the hyperinflammation that occurs in COVID-19 patients. A prospective, multicenter, single-blind, randomized controlled phase II trial involving patients with severe COVID-19 showed that the patients that receive ruxolitinib plus standard of care treatment have a significant improvement in chest condition and a faster recovery from lymphopenia, although no statistical difference was observed in accelerated clinical improvement. The authors of the study suggested that the modulation of cytokine production could be associated with the favorable effects of ruxolitinib on COVID-19 [119]. This is agreement with other studies that show that ruxolitinib inhibits the expression of inflammatory genes by altering the signaling pathway and transcription activators linked to cytokine receptors, such as NFκB, leading to a reduction in cytokine production [120]. The use of the Janus kinase (JAK) inhibitor may be effective in the treatment of COVID-19 patients in whom hyperinflammation has caused a “cytokine storm” and ARDS. However, the expected immunomodulating effect of ruxolitinib must be controlled, as the drug may enhance the risk of opportunistic infections and, in individuals with previous inflammatory conditions, can exacerbate the incidence of thrombocytopenia and anemia [121].
Tyrosine kinase inhibitors, such as Bruton’s tyrosine kinase (BTK) inhibitor, are also being studied for their capacity to interfere with the inflammatory state associated with SARS-CoV-2 infection in severe COVID-19 illness. Preclinical research and case studies have suggested that the BTK inhibitor ibrutinib can provide protection against severe lung injury [122], probably due to its ability to reduce the levels of inflammatory mediators [123]. Treon et al. (2020) and Einhaus et al. (2020) [122], [123] reported that ibrutinib was able to modulate TNF-α, IL-6, IL-8 and IL-10 cytokine levels, and could provide protection against severe lung injury, helping in the recovery of COVID-19 patients.
At this stage, it must be emphasized that current evidence suggests the use of JAK inhibitors and the BTK inhibitor in the treatment of COVID-19. These drugs could block the generation of the “cytokine storm” and, consequently, suppress the fulminant response of the immune system, and multiple organ failure. However, there is concern that JAK inhibitors can suppress the production of several inflammatory cytokines, including TNF-α, IL-6, IL-8, IL-10 (Table 1) [120], [122], and IFN-α, which play an important role in curbing viral activity [124]
2.6. Antiparasitic drugs
Ivermectin is an FDA-approved broad spectrum anti-parasitic agent [125] that has been shown to possess anti-viral activity against several viruses in vitro or in vivo, including SARS-CoV-2 [126], [127], [128], probably by blocking the nuclear trafficking of viral proteins [129]. Caly et al. (2020) [126] reported that ivermectin controlled SARS-CoV-2 replication for up to 48 h in a Vero-h/SLAM cell culture model, showing its potential to reduce SARS-CoV-2 infection. Additionally, ivermectin was also found to have an immunomodulatory profile that changed the function of T-lymphocytes and altered the lymphocyte count [130]. Moreover, this drug was able to decrease the inflammatory process by reducing the production of several cytokines, such as TNF-α, IL-1ss, IL-6, IL-4, IL-13 and IL5 [131], [132]. Thus, ivermectin can inhibit a variety of the inflammatory cytokines which play an important role in the development of the “cytokines storm”, reducing the complications of COVID-19. The evidence in the literature therefore suggests that ivermectin could be useful in the treatment of COVID-19 [133].
Hydroxychloroquine (HCQ) and chloroquine (CQ) are already undergoing clinical trials to assess their use as preventive therapies for COVID-19, with, as yet, contradictory results [134]. Some pre-clinical findings suggest that HCQ/CQ are effective in SARS-CoV-2 infection, with promising preliminary results. HCQ and CQ have been shown to suppress SARS-CoV-2 infection in vitro, with HCQ being more potent than CQ [135]. HCQ has shown an antiviral profile due to its ability to reduce the binding efficiency between ACE2 in host cells and the SARS-CoV-2 spike protein. HCQ has also be reported to be able to inhibit virus fusion with the host cell through the suppression of protease activity in cleaving the coronavirus surface spike proteins [136]. In addition, HCQ also could be effective against SARS-CoV-2 infection by suppressing the “cytokine storm” and consequently reducing disease progression and ARDS development. Other studies have suggested that CQ/HCQ decrease the release of various pro-inflammatory factors such as Il-1, IL-6, IL-18, and TNF-α produced by T cells and B cells, and enhance production of the anti-inflammatory cytokines IL-10 and IL-4 [137], [138]. In addition, the alteration of endosomal pH caused by HCQ/CQ can change signaling in Toll-like receptor (TLR), such as TLR7 and TLR9, inhibiting the activation and production of cytokines [139]. Several clinical studies have described the role of HCQ/CQ treatment, alone or in association with other drugs, in COVID-19 patients, but with weak and inconsistent results [134]. Thus, the effectiveness of these drugs in COVID-19 is not established, with the FDA revoking authorization for the emergency use of hydroxychloroquine and chloroquine to treat COVID-19 in certain hospitalized patients cautions due to the risk of heart rhythm problems. This decision was based on recent results from a large, randomized clinical trial in hospitalized patients [140]. Additional studies are necessary to evaluate the efficacy of these drugs in COVID-19 patients.
Table 4 and Fig. 3 summarize the main anti-parasitic drugs suggested for use in COVID-19 treatment, mainly based on the anti-inflammatory profiles of these drugs. Some evidence suggests that HCQ, CQ, ivermectin and nitazoxanide downregulate pro-inflammatory cytokine levels, attenuating the exacerbated inflammatory process observed in several diseases [131]. These features of anti-parasitic drugs could play a favorable role in the recovery of COVID-19 patients. However, the data from the literature on the use of these drugs is inconclusive and further studies are required to evaluate their efficacy.
3. Conclusion
Therefore, a rational strategy for the treatment of COVID-19 is the repurposing of a number of different types of drugs, including antiviral, anti-rheumatic, anti-inflammatory, antineoplastic, and antiparasitic medications. Interestingly, several drugs proposed for the treatment of COVID-19 have an anti-inflammatory profile, summarized in Table 1, and most are able to reduce IL-6 and TNF-α levels, two cytokines that are important targets for drugs that manage the symptoms of COVID-19. The reduction in PICs is also considered a relevant strategy to improve the clinical conditions of patients with COVID-19, preventing the worsening of the disease.
Faced with the COVID-19 crisis, it is a rational strategy to consider drug repurposing, as has happened with HCQ, an antimalarial drug previously used in the treatment of systemic lupus erythematosus and rheumatoid arthritis, and is now being considered for use as a therapeutic tool for severe cases of COVID-19. As our review shows, other drugs are also being examined, such as ivermectin, an antiparasitic agent commonly used in both humans and animals, which has demonstrated an antiviral profile against a broad range of viruses. It has also been shown to be a potent in vitro inhibitor of SARS-CoV-2 replication [126]. Additionally, the antiviral drug remdesivir, as well as lopinavir-ritonavir, commonly used in the treatment of HIV positive patients, have shown beneficial effects in COVID-19 patients, improving the symptoms or this disease [141]. All these drugs could act directly in virus replication and help to decrease viral load.
Undoubtedly, drugs that directly target SARS-CoV-2 would be effective treatments for COVID-19, however, the reduction of pro-inflammatory cytokines (PICs) could also be crucial for halting the progression of the disease, thereby reducing the number of severe cases and hospitalizations. This hypothesis should not be ignored, because an increase in PICs is associated with a worsened respiratory condition, and with hyperinflammation, widely known as a “cytokine storm,” which can occur when the immune system triggers a runaway response that induces more harm to its own cells than to the virus it is fighting. A strategy that promotes the innate immune system and decreases Th1/Th2 inflammation could help to prevent severe cases of COVID-19 and aid recovery from the disease.
There are several ongoing trials trying to find effective treatments for COVID-19 based on the suppression of the hyperinflammation caused by SARS-CoV-2 infection, which can lead to multiple organ failure and even death in severe cases. These experimental therapies are investigating drugs approved for other inflammatory diseases and include anti-rheumatic and anti-inflammatory agents. As mentioned above, drugs such as tocilizumab and anakinra, which are commonly used to treat arthritis and decrease the inflammatory process, have been applied as a pharmacological tool in COVID-19 patients [67], [75], and have shown promising effects in reducing the complications of COVID-19 and the associated ARDS, due to their capacity to control the inflammatory process. Moreover, some drugs used in COVID-19 patients, such as remdesevir and favipiravir, with proven antiviral activity, act directly on virus replication, and also possess an anti-inflammatory profile, reducing the hyperinflammation caused by the virus. These class of drug have shown favorable effect against SARS-CoV-2, increasing clearance of the virus, and/or reducing cytokine release.
Therefore, repurposed drugs that can attenuate the hyperinflammation induced by SARS-CoV-2 ought to be carefully considered as treatments to combat SARS-CoV-2, due to their capacity to reduce the severity of the disease and improve the prognosis in COVID-19. The reduction of PICs, mainly IL-6, is an important strategy to improve the clinical conditions of patients with COVID-19. Thus, despite their current unproven clinical efficacy, the repurposing of drugs, particularly those with anti-inflammatory activities, has become a significant area of interest in research aimed at finding effective treatments for COVID-19. We sincerely hope that documented drug therapies specifically targeting SARS-CoV-2, or an effective vaccine can be developed to prevent and cure COVID-19 patients as soon as possible.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
This work was supported by grants from FAPITEC-SE, CNPq and CAPES, all agencies from Brazil. The Federal University of Sergipe (UFS) has played an important role in the fight against COVID-19 in Sergipe state (Brazil), and this review is part of EpiSERGIPE project, a wider project to monitor the degree of contamination and the impacts of the new coronavirus in the region. We dedicate this article to all doctors and front-line health workers and other staff who have died or are in the battlefront in the fight against COVID-19.
Author contributions
Luana Heimfarth: conceptualization, data curation, methodological analysis, and writing of the first draft. Mairim Russo Serafini: conceptualization, data curation, methodology, and review & editing manuscript. Paulo Ricardo Saquete Martins-Filho: funding acquisition, research, review & editing manuscript. Jullyana Souza Siqueira Quintans: conceptualization, data curation, methodology, review & editing manuscript, research. Lucindo José Quintans Júnior: funding acquisition, research, and review & editing manuscript.
References
- 1.Kamradt-Scott A. Changing perceptions: of pandemic influenza and public health responses. Am. J. Public Health. 2012;102:90–98. doi: 10.2105/AJPH.2011.300330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yen H.-L., Webster R.G. Pandemic influenza as a current threat. Curr. Top. Microbiol. Immunol. 2009;333:3–24. doi: 10.1007/978-3-540-92165-3_1. [DOI] [PubMed] [Google Scholar]
- 3.Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020;109 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with, novel coronavirus in Wuhan China. Lancet. 2019;395(2020):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. HLH Across Speciality Collaboration, UK, COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395:1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Raoult D., Zumla A., Locatelli F., Ippolito G., Kroemer G. Coronavirus infections: Epidemiological, clinical and immunological features and hypotheses. Cell Stress. 2020;4:66–75. doi: 10.15698/cst2020.04.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meng J., Xiao G., Zhang J., He X., Ou M., Bi J., Yang R., Di W., Wang Z., Li Z., Gao H., Liu L., Zhang G. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg. Microbes Infect. 2020;9:757–760. doi: 10.1080/22221751.2020.1746200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46:586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imai Y., Kuba K., Rao S., Huan Y., Guo F., Guan B., Yang P., Sarao R., Wada T., Leong-Poi H., Crackower M.A., Fukamizu A., Hui C.-C., Hein L., Uhlig S., Slutsky A.S., Jiang C., Penninger J.M. Angiotensin-converting enzyme 2 protects from severe acute lung failure. Nature. 2005;436:112–116. doi: 10.1038/nature03712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He L., Ding Y., Zhang Q., Che X., He Y., Shen H., Wang H., Li Z., Zhao L., Geng J., Deng Y., Yang L., Li J., Cai J., Qiu L., Wen K., Xu X., Jiang S. Expression of elevated levels of pro-inflammatory cytokines in SARS-CoV-infected ACE2+ cells in SARS patients: relation to the acute lung injury and pathogenesis of SARS. J. Pathol. 2006;210:288–297. doi: 10.1002/path.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicholls J.M., Poon L.L.M., Lee K.C., Ng W.F., Lai S.T., Leung C.Y., Chu C.M., Hui P.K., Mak K.L., Lim W., Yan K.W., Chan K.H., Tsang N.C., Guan Y., Yuen K.Y., Peiris J.S.M. Lung pathology of fatal severe acute respiratory syndrome. Lancet. 2003;361:1773–1778. doi: 10.1016/s0140-6736(03)13413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ye R., Liu Z. ACE2 exhibits protective effects against LPS-induced acute lung injury in mice by inhibiting the LPS-TLR4 pathway. Exp. Mol. Pathol. 2020;113 doi: 10.1016/j.yexmp.2019.104350. [DOI] [PubMed] [Google Scholar]
- 13.Li G., De Clercq E. Therapeutic options for the, novel coronavirus (2019-nCoV) Nat. Rev. Drug Discov. 2019;19(2020):149–150. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- 14.Dinarello C.A. Historical insights into cytokines. Eur. J. Immunol. 2007;37(Suppl 1):S34–S45. doi: 10.1002/eji.200737772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Behrens E.M., Koretzky G.A. Review: Cytokine Storm Syndrome: Looking Toward the Precision Medicine Era. Arthritis Rheumatol. (Hoboken, N.J.) 2017;69:1135–1143. doi: 10.1002/art.40071. [DOI] [PubMed] [Google Scholar]
- 16.Chen H., Wang F., Zhang P., Zhang Y., Chen Y., Fan X., Cao X., Liu J., Yang Y., Wang B., Lei B., Gu L., Bai J., Wei L., Zhang R., Zhuang Q., Zhang W., Zhao W., He A. Management of cytokine release syndrome related to CAR-T cell therapy. Front Med. 2019;13:610–617. doi: 10.1007/s11684-019-0714-8. [DOI] [PubMed] [Google Scholar]
- 17.Murthy H., Iqbal M., Chavez J.C., Kharfan-Dabaja M.A. Cytokine Release Syndrome: Current Perspectives. Immunotargets Ther. 2019;8:43–52. doi: 10.2147/ITT.S202015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chousterman B.G., Swirski F.K., Weber G.F. Cytokine storm and sepsis disease pathogenesis. Semin. Immunopathol. 2017;39:517–528. doi: 10.1007/s00281-017-0639-8. [DOI] [PubMed] [Google Scholar]
- 19.Shimabukuro-Vornhagen A., Gödel P., Subklewe M., Stemmler H.J., Schlößer H.A., Schlaak M., Kochanek M., Böll B., von Bergwelt-Baildon M.S. Cytokine release syndrome. J. Immunother. Cancer. 2018;6:56. doi: 10.1186/s40425-018-0343-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., Xie C., Ma K., Shang K., Wang W., Tian D.-S. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020;71:762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarzi-Puttini P., Giorgi V., Sirotti S., Marotto D., Ardizzone S., Rizzardini G., Antinori S., Galli M. COVID-19, cytokines and immunosuppression: what can we learn from severe acute respiratory syndrome? Clin. Exp. Rheumatol. 2020;38:337–342. [PubMed] [Google Scholar]
- 22.Vaninov N. In the eye of the COVID-19 cytokine storm. Nat. Rev. Immunol. 2020;20:277. doi: 10.1038/s41577-020-0305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bindoli S., Felicetti M., Sfriso P., Doria A. The amount of cytokine-release defines different shades of Sars-Cov2 infection. Exp. Biol. Med. (Maywood). 2020;245:970–976. doi: 10.1177/1535370220928964. 1535370220928964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eguchi S., Kawai T., Scalia R., Rizzo V. Understanding Angiotensin II Type 1 Receptor Signaling in Vascular Pathophysiology. Hypertension. 2018;71:804–810. doi: 10.1161/HYPERTENSIONAHA.118.10266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murakami M., Kamimura D., Hirano T. Pleiotropy and Specificity: Insights from the Interleukin 6 Family of Cytokines. Immunity. 2019;50:812–831. doi: 10.1016/j.immuni.2019.03.027. [DOI] [PubMed] [Google Scholar]
- 26.Cooke A., Ferraccioli G.F., Herrmann M., Romani L., Schulze C., Zampieri S., Doria A. Induction and protection of autoimmune rheumatic diseases. The role of infections. Clin. Exp. Rheumatol. 2008;26:S1–S7. [PubMed] [Google Scholar]
- 27.Takeda K., Akira S. TLR signaling pathways. Semin. Immunol. 2004;16:3–9. doi: 10.1016/j.smim.2003.10.003. [DOI] [PubMed] [Google Scholar]
- 28.Hunter C.A., Jones S.A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 2015;16:448–457. doi: 10.1038/ni.3153. [DOI] [PubMed] [Google Scholar]
- 29.Garcia Borrega J., Gödel P., Rüger M.A., Onur Ö.A., Shimabukuro-Vornhagen A., Kochanek M., Böll B. In the Eye of the Storm: Immune-mediated Toxicities Associated With CAR-T Cell Therapy. Hemasphere. 2019;3 doi: 10.1097/HS9.0000000000000191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li X., Geng M., Peng Y., Meng L., Lu S. Molecular immune pathogenesis and diagnosis of COVID-19. J. Pharm Anal. 2020;10:102–108. doi: 10.1016/j.jpha.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang C., Wu Z., Li J.-W., Zhao H., Wang G.-Q. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int. J. Antimicrob. Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leiva-Juárez M.M., Kolls J.K., Evans S.E. Lung epithelial cells: therapeutically inducible effectors of antimicrobial defense. Mucosal. Immunol. 2018;11:21–34. doi: 10.1038/mi.2017.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McGonagle D., Sharif K., O’Regan A., Bridgewood C. The Role of Cytokines including Interleukin-6 in COVID-19 induced Pneumonia and Macrophage Activation Syndrome-Like Disease. Autoimmun Rev. 2020;19 doi: 10.1016/j.autrev.2020.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wan S., Yi Q., Fan S., Lv J., Zhang X., Guo L., Lang C., Xiao Q., Xiao K., Yi Z., Qiang M., Xiang J., Zhang B., Chen Y. Characteristics of lymphocyte subsets and cytokines in peripheral blood of 123 hospitalized patients with 2019 novel coronavirus pneumonia (NCP) MedRxiv. 2020 doi: 10.1101/2020.02.10.20021832. pp. 10.20021832. [DOI] [Google Scholar]
- 35.Wypasek E., Undas A., Sniezek-Maciejewska M., Kapelak B., Plicner D., Stepien E., Sadowski J. The increased plasma C-reactive protein and interleukin-6 levels in patients undergoing coronary artery bypass grafting surgery are associated with the interleukin-6-174G > C gene polymorphism. Ann. Clin. Biochem. 2010;47:343–349. doi: 10.1258/acb.2010.090305. [DOI] [PubMed] [Google Scholar]
- 36.Lin L., Lu L., Cao W., Li T. Hypothesis for potential pathogenesis of SARS-CoV-2 infection-a review of immune changes in patients with viral pneumonia. Emerg Microbes Infect. 2020;9:727–732. doi: 10.1080/22221751.2020.1746199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deane S., Selmi C., Teuber S.S., Gershwin M.E. Macrophage activation syndrome in autoimmune disease. Int. Arch. Allergy Immunol. 2010;153:109–120. doi: 10.1159/000312628. [DOI] [PubMed] [Google Scholar]
- 38.Kaplanski G. Interleukin-18: Biological properties and role in disease pathogenesis. Immunolog. Rev. 2018;281:138–153. doi: 10.1111/imr.12616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kalliolias G.D., Ivashkiv L.B. Overview of the biology of type I interferons. Arthritis Res. Ther. 2010;12(Suppl 1):S1. doi: 10.1186/ar2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin F., Young H.A. Interferons: Success in anti-viral immunotherapy. Cytokine Growth Factor Rev. 2014;25:369–376. doi: 10.1016/j.cytogfr.2014.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prokunina-Olsson L., Alphonse N., Dickenson R.E., Durbin J.E., Glenn J.S., Hartmann R., Kotenko S.V., Lazear H.M., O’Brien T.R., Odendall C., Onabajo O.O., Piontkivska H., Santer D.M., Reich N.C., Wack A., Zanoni I. COVID-19 and emerging viral infections: The case for interferon lambda. J. Exp. Med. 2020;217 doi: 10.1084/jem.20200653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Monteiro W.M., Brito-Sousa J.D., Baía-da-Silva D., de Melo G.C., Siqueira A.M., Val F., Daniel-Ribeiro C.T., Guimarães Lacerda M.V. Driving forces for COVID-19 clinical trials using chloroquine: the need to choose the right research questions and outcomes. Rev. Soc. Bras. Med. Trop. 2020;53 doi: 10.1590/0037-8682-0155-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Beck B.R., Shin B., Choi Y., Park S., Kang K. Predicting commercially available antiviral drugs that may act on the novel coronavirus (SARS-CoV-2) through a drug-target interaction deep learning model. Comput. Struct. Biotechnol. J. 2020;18:784–790. doi: 10.1016/j.csbj.2020.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen C., Zhang Y., Huang J., Yin P., Cheng Z., Wu J., Chen S., Zhang Y., Chen B., Lu M., Luo Y., Ju L., Zhang J., Wang X. Favipiravir versus Arbidol for COVID-19: A Randomized Clinical Trial. MedRxiv. 2020 doi: 10.1101/2020.03.17.20037432. pp. 17.20037432. [DOI] [Google Scholar]
- 45.Xu P., Huang J., Fan Z., Huang W., Qi M., Lin X., Song W. li Yi, Arbidol/IFN-α2b Therapy for Patients With Corona Virus Disease 2019: A Retrospective Multicenter Cohort Study. Microbes Infect. 2020;22:200–205. doi: 10.1016/j.micinf.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Panagopoulos P., Petrakis V., Panopoulou M., Trypsianis G., Penlioglou T., Pnevmatikos I., Papazoglou D. Lopinavir/ritonavir as a third agent in the antiviral regimen for SARS-CoV-2 infection. J Chemother. 2020:1–5. doi: 10.1080/1120009X.2020.1775424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grein J., Ohmagari N., Shin D., Diaz G., Asperges E., Castagna A., Feldt T., Green G., Green M.L., Lescure F.-X., Nicastri E., Oda R., Yo K., Quiros-Roldan E., Studemeister A., Redinski J., Ahmed S., Bernett J., Chelliah D., Chen D., Chihara S., Cohen S.H., Cunningham J., D’Arminio Monforte A., Ismail S., Kato H., Lapadula G., L’Her E., Maeno T., Majumder S., Massari M., Mora-Rillo M., Mutoh Y., Nguyen D., Verweij E., Zoufaly A., Osinusi A.O., DeZure A., Zhao Y., Zhong L., Chokkalingam A., Elboudwarej E., Telep L., Timbs L., Henne I., Sellers S., Cao H., Tan S.K., Winterbourne L., Desai P., Mera R., Gaggar A., Myers R.P., Brainard D.M., Childs R., Flanigan T. Compassionate Use of Remdesivir for Patients with Severe Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hung I.F.-N., Lung K.-C., Tso E.Y.-K., Liu R., Chung T.W.-H., Chu M.-Y., Ng Y.-Y., Lo J., Chan J., Tam A.R., Shum H.-P., Chan V., Wu A.K.-L., Sin K.-M., Leung W.-S., Law W.-L., Lung D.C., Sin S., Yeung P., Yip C.C.-Y., Zhang R.R., Fung A.Y.-F., Yan E.Y.-W., Leung K.-H., Ip J.D., Chu A.W.-H., Chan W.-M., Ng A.C.-K., Lee R., Fung K., Yeung A., Wu T.-C., Chan J.W.-M., Yan W.-W., Chan W.-M., Chan J.F.-W., Lie A.K.-W., Tsang O.T.-Y., Cheng V.C.-C., Que T.-L., Lau C.-S., Chan K.-H., To K.K.-W., Yuen K.-Y. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395:1695–1704. doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu Z., Lu Z., Xu T., Chen C., Yang G., Zha T., Lu J., Xue Y. Arbidol monotherapy is superior to lopinavir/ritonavir in treating COVID-19. J. Infect. 2020;81(1):e21–e23. doi: 10.1016/j.jinf.2020.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bimonte S., Crispo A., Amore A., Celentano E., Cuomo A., Cascella M. Potential Antiviral Drugs for SARS-Cov-2 Treatment: Preclinical Findings and Ongoing Clinical Research. Vivo. 2020;34:1597–1602. doi: 10.21873/invivo.11949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang Y., Zhang D., Du G., Du R., Zhao J., Jin Y., Fu S., Gao L., Cheng Z., Lu Q., Hu Y., Luo G., Wang K., Lu Y., Li H., Wang S., Ruan S., Yang C., Mei C., Wang Y., Ding D., Wu F., Tang X., Ye X., Ye Y., Liu B., Yang J., Yin W., Wang A., Fan G., Zhou F., Liu Z., Gu X., Xu J., Shang L., Zhang Y., Cao L., Guo T., Wan Y., Qin H., Jiang Y., Jaki T., Hayden F.G., Horby P.W., Cao B., Wang C. Remdesivir in adults with severe COVID-19: a randomised, double-blind, placebo-controlled, multicentre trial. Lancet. 2020;395:1569–1578. doi: 10.1016/S0140-6736(20)31022-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gonçalves A., Bertrand J., Ke R., Comets E., de Lamballerie X., Malvy D., Pizzorno A., Terrier O., Calatrava M.R., Mentré F., Smith P., Perelson A.S., Guedj J. Timing of antiviral treatment initiation is critical to reduce SARS-Cov-2 viral load. MedRxiv. 2020 doi: 10.1101/2020.04.04.20047886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fintelman-Rodrigues N., Sacramento C.Q., Lima C.R., da Silva F.S., Ferreira A.C., Mattos M., de Freitas C.S., Soares V.C., Gomes Dias S.da.S., Temerozo J.R., Miranda M., Matos A.R., Bozza F.A., Carels N., Alves C.R., Siqueira M.M., Bozza P.T., Souza T.M.L. Atazanavir inhibits SARS-CoV-2 replication and pro-inflammatory cytokine production. Microbiology. 2020 doi: 10.1101/2020.04.04.020925. [DOI] [Google Scholar]
- 54.Tanaka T., Kamiyama T., Daikoku T., Takahashi K., Nomura N., Kurokawa M., Shiraki K. T-705 (Favipiravir) suppresses tumor necrosis factor α production in response to influenza virus infection: A beneficial feature of T-705 as an anti-influenza drug. Acta Virol. 2017;61:48–55. doi: 10.4149/av_2017_01_48. [DOI] [PubMed] [Google Scholar]
- 55.Fagone P., Mangano K., Quattrocchi C., Cavalli E., Mammana S., Lombardo G.A.G., Pennisi V., Zocca M.-B., He M., Al-Abed Y., Nicoletti F. Effects of NO-Hybridization on the Immunomodulatory Properties of the HIV Protease Inhibitors Lopinavir and Ritonavir. Basic Clin. Pharmacol. Toxicol. 2015;117:306–315. doi: 10.1111/bcpt.12414. [DOI] [PubMed] [Google Scholar]
- 56.Wang Y., Ding Y., Yang C., Li R., Du Q., Hao Y., Li Z., Jiang H., Zhao J., Chen Q., Yang Z., He Z. Inhibition of the infectivity and inflammatory response of influenza virus by Arbidol hydrochloride in vitro and in vivo (mice and ferret) Biomed. Pharmacother. 2017;91:393–401. doi: 10.1016/j.biopha.2017.04.091. [DOI] [PubMed] [Google Scholar]
- 57.Mahevas M., Tran V.-T., Roumier M., Chabrol A., Paule R., Guillaud C., Gallien S., Lepeule R., Szwebel T.-A., Lescure X., Schlemmer F., Matignon M., Khellaf M., Crickx E., Terrier B., Morbieu C., Legendre P., Dang J., Schoindre Y., Pawlotski J.-M., Michel M., Perrodeau E., Carlier N., Roche N., Lastours V.D., Mouthon L., Audureau E., Ravaud P., Godeau B., Costedoat N. No evidence of clinical efficacy of hydroxychloroquine in patients hospitalized for COVID-19 infection with oxygen requirement: results of a study using routinely collected data to emulate a target trial. MedRxiv. 2020 doi: 10.1101/2020.04.10.20060699. pp. 10.20060699. [DOI] [Google Scholar]
- 58.Olejnik J., Hume A.J., Mühlberger E. Toll-like receptor 4 in acute viral infection: Too much of a good thing. PLoS Pathog. 2018;14 doi: 10.1371/journal.ppat.1007390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Grimes J.M., Grimes K.V. p38 MAPK inhibition: A promising therapeutic approach for COVID-19. J Mol Cell Cardiol. 2020;144:63–65. doi: 10.1016/j.yjmcc.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Li Y.-N., Su Y. Remdesivir attenuates high fat diet (HFD)-induced NAFLD by regulating hepatocyte dyslipidemia and inflammation via the suppression of STING. Biochem. Biophys. Res. Commun. 2020;526:381–388. doi: 10.1016/j.bbrc.2020.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang S., Zhi C., Li H., Huang D., Fan Q., Cui J., Liang C. Umifenovir effectively inhibits IL-10 dependent persistent Coxsackie B4 virus infection. Antiviral Res. 2017;141:165–173. doi: 10.1016/j.antiviral.2017.02.018. [DOI] [PubMed] [Google Scholar]
- 62.Zhang W., Zhao Y., Zhang F., Wang Q., Li T., Liu Z., Wang J., Qin Y., Zhang X., Yan X., Zeng X., Zhang S. The use of anti-inflammatory drugs in the treatment of people with severe coronavirus disease 2019 (COVID-19): The Perspectives of clinical immunologists from China. Clin. Immunol. 2020;214 doi: 10.1016/j.clim.2020.108393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Duret P.-M., Sebbag E., Mallick A., Gravier S., Spielmann L., Messer L. Recovery from COVID-19 in a patient with spondyloarthritis treated with TNF-alpha inhibitor etanercept. Ann. Rheum. Dis. 2020;79:1251–1252. doi: 10.1136/annrheumdis-2020-217362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bennardo F., Buffone C., Giudice A. New therapeutic opportunities for COVID-19 patients with Tocilizumab: Possible correlation of interleukin-6 receptor inhibitors with osteonecrosis of the jaws. Oral Oncol. 2020;106 doi: 10.1016/j.oraloncology.2020.104659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Giacomelli R., Sota J., Ruscitti P., Campochiaro C., Colafrancesco S., Dagna L., Iacono D., Iannone F., Lopalco G., Sfriso P., Cantarini L. The treatment of adult-onset Still’s disease with anakinra, a recombinant human IL-1 receptor antagonist: a systematic review of literature. Clin. Exp. Rheumatol. 2020 doi: 10.55563/clinexprheumatol/fsq5vq. [DOI] [PubMed] [Google Scholar]
- 66.Migita K., Izumi Y., Torigoshi T., Satomura K., Izumi M., Nishino Y., Jiuchi Y., Nakamura M., Kozuru H., Nonaka F., Eguchi K., Kawakami A., Motokawa S. Inhibition of Janus kinase/signal transducer and activator of transcription (JAK/STAT) signalling pathway in rheumatoid synovial fibroblasts using small molecule compounds. Clin. Exp. Immunol. 2013;174:356–363. doi: 10.1111/cei.12190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dimopoulos G., de Mast Q., Markou N., Theodorakopoulou M., Komnos A., Mouktaroudi M., Netea M.G., Spyridopoulos T., Verheggen R.J., Hoogerwerf J., Lachana A., van de Veerdonk F.L., Giamarellos-Bourboulis E.J. Favorable anakinra responses in severe COVID-19 patients with secondary hemophagocytic lymphohistiocytosis. Cell Host Microbe. 2020;28:117–123.e1. doi: 10.1016/j.chom.2020.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cavalli G., De Luca G., Campochiaro C., Della-Torre E., Ripa M., Canetti D., Oltolini C., Castiglioni B., Tassan Din C., Boffini N., Tomelleri A., Farina N., Ruggeri A., Rovere-Querini P., Di Lucca G., Martinenghi S., Scotti R., Tresoldi M., Ciceri F., Landoni G., Zangrillo A., Scarpellini P., Dagna L. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: a retrospective cohort study, The Lancet. Rheumatology. 2020;2:e325–e331. doi: 10.1016/S2665-9913(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ozcicek F., Kara A.V., Akbas E.M., Kurt N., Yazici G.N., Cankaya M., Mammadov R., Ozcicek A., Suleyman H. Effects of anakinra on the small intestine mucositis induced by methotrexate in rats. Exp. Anim. 2020;69:144–152. doi: 10.1538/expanim.19-0057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schwarznau A., Hanson M.S., Sperger J.M., Schram B.R., Danobeitia J.S., Greenwood K.K., Vijayan A., Fernandez L.A. IL-1β Receptor Blockade Protects Islets Against Pro-inflammatory Cytokine Induced Necrosis and Apoptosis. J. Cell Physiol. 2009;220:341–347. doi: 10.1002/jcp.21770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chakraborty C., Sharma A.R., Bhattacharya M., Sharma G., Lee S.-S., Agoramoorthy G. COVID-19: Consider IL6 receptor antagonist for the therapy of cytokine storm syndrome in SARS-CoV-2 infected patients. J. Med. Virol. 2020 doi: 10.1002/jmv.26078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saha A., Sharma A.R., Bhattacharya M., Sharma G., Lee S.-S., Chakraborty C., Tocilizumab A Therapeutic Option for the Treatment of Cytokine Storm Syndrome in COVID-19. Arch. Med. Res. 2020 doi: 10.1016/j.arcmed.2020.05.009. S0188440920307827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Smetana K., Brábek J. Role of Interleukin-6 in Lung Complications in Patients With COVID-19: Therapeutic Implications. Vivo. 2020;34:1589–1592. doi: 10.21873/invivo.11947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu X., Han M., Li T., Sun W., Wang D., Fu B., Zhou Y., Zheng X., Yang Y., Li X., Zhang X., Pan A., Wei H. Effective treatment of severe COVID-19 patients with tocilizumab. Proc. Natl. Acad. Sci. U.S.A. 2020;117:10970–10975. doi: 10.1073/pnas.2005615117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Capra R., De Rossi N., Mattioli F., Romanelli G., Scarpazza C., Sormani M.P., Cossi S. Impact of low dose tocilizumab on mortality rate in patients with COVID-19 related pneumonia. Eur. J. Intern. Med. 2020;76:31–35. doi: 10.1016/j.ejim.2020.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Luo P., Liu Y., Qiu L., Liu X., Liu D., Li J. Tocilizumab treatment in COVID-19: A single center experience. J. Med. Virol. 2020;92:814–818. doi: 10.1002/jmv.25801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tanaka T., Narazaki M., Kishimoto T. Immunotherapeutic implications of IL-6 blockade for cytokine storm. Immunotherapy. 2016;8:959–970. doi: 10.2217/imt-2016-0020. [DOI] [PubMed] [Google Scholar]
- 78.Soy M., Keser G., Atagündüz P., Tabak F., Atagündüz I., Kayhan S. Cytokine storm in COVID-19: pathogenesis and overview of anti-inflammatory agents used in treatment. Clin. Rheumatol. 2020;39:2085–2094. doi: 10.1007/s10067-020-05190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dolinger M.T., Person H., Smith R., Jarchin L., Pittman N., Dubinsky M.C., Lai J. Pediatric Crohn’s Disease and Multisystem Inflammatory Syndrome in Children (MIS-C) and COVID-19 Treated with Infliximab. J. Pediatr. Gastroenterol. Nutr. 2020;71:153–155. doi: 10.1097/MPG.0000000000002809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Garfield B.E., Krahl T., Appel S., Cooper S.M., Rincón M. Regulation of p38 MAP kinase in CD4+ lymphocytes by infliximab therapy in patients with rheumatoid arthritis. Clin. Immunol. 2005;116:101–107. doi: 10.1016/j.clim.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 81.Della-Torre E., Della-Torre F., Kusanovic Pcp M., Scotti R., Alvise-Ramirez G., Dagna L., Tresoldi M. Treating COVID-19 with colchicine in community healthcare setting. Clin. Immunol. 2020 doi: 10.1016/j.clim.2020.108490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Montealegre-Gómez G., Garavito E., Gómez-López A., Rojas-Villarraga A., Parra-Medina R. Colchicine, A potential therapeutic tool against COVID-19. Experience of 5 patients. Reumatol Clin. 2020 doi: 10.1016/j.reuma.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Viktorov A.V., Yurkiv V.A. Albendazole and colchicine modulate LPS-induced secretion of inflammatory mediators by liver macrophages. Bull. Exp. Biol. Med. 2011;151:683–685. doi: 10.1007/s10517-011-1415-8. [DOI] [PubMed] [Google Scholar]
- 84.Martínez G.J., Robertson S., Barraclough J., Xia Q., Mallat Z., Bursill C., Celermajer D.S., Patel S. Colchicine Acutely Suppresses Local Cardiac Production of Inflammatory Cytokines in Patients With an Acute Coronary Syndrome. J. Am. Heart Assoc. 2015;4 doi: 10.1161/JAHA.115.002128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Leung Y.Y., Yao Hui L.L., Kraus V.B. Colchicine-Update on mechanisms of action and therapeutic uses. Semin. Arthritis Rheum. 2015;45:341–350. doi: 10.1016/j.semarthrit.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cumhur Cure M., Kucuk A., Cure E. Colchicine may not be effective in COVID-19 infection; it may even be harmful? Clin. Rheumatol. 2020:1–2. doi: 10.1007/s10067-020-05144-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gendelman O., Amital H., Bragazzi N.L., Watad A., Chodick G. Continuous hydroxychloroquine or colchicine therapy does not prevent infection with SARS-CoV-2: Insights from a large healthcare database analysis. Autoimmun. Rev. 2020 doi: 10.1016/j.autrev.2020.102566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Amici C., Di Caro A., Ciucci A., Chiappa L., Castilletti C., Martella V., Decaro N., Buonavoglia C., Capobianchi M.R., Santoro M.G. Indomethacin has a potent antiviral activity against SARS coronavirus. Antivir. Ther. (Lond.) 2006;11:1021–1030. [PubMed] [Google Scholar]
- 89.Xu T., Gao X., Wu Z., Selinger D.W., Zhou Z. Indomethacin has a potent antiviral activity against SARS CoV-2 in vitro and canine coronavirus in vivo. BioRxiv. 2020;2020(04) doi: 10.1101/2020.04.01.017624. pp. 01.017624. [DOI] [Google Scholar]
- 90.Amici C., La Frazia S., Brunelli C., Balsamo M., Angelini M., Santoro M.G. Inhibition of viral protein translation by indomethacin in vesicular stomatitis virus infection: role of eIF2α kinase PKR. Cell. Microbiol. 2015;17:1391–1404. doi: 10.1111/cmi.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gentile L.B., Queiroz-Hazarbassanov N., Massoco C.de.O., Fecchio D. Modulation of Cytokines Production by Indomethacin Acute Dose during the Evolution of Ehrlich Ascites Tumor in Mice. Mediators Inflamm. 2015 doi: 10.1155/2015/924028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Hamad K.M., Sabry M.M., Elgayed S.H., El Shabrawy A.-R., El-Fishawy A.M., Abdel Jaleel G.A. Anti-inflammatory and phytochemical evaluation of Combretum aculeatum Vent growing in Sudan. J. Ethnopharmacol. 2019;242 doi: 10.1016/j.jep.2019.112052. [DOI] [PubMed] [Google Scholar]
- 93.Shacter E., Arzadon G.K., Williams J. Elevation of interleukin-6 in response to a chronic inflammatory stimulus in mice: inhibition by indomethacin. Blood. 1992;80:194–202. [PubMed] [Google Scholar]
- 94.Lu X., Chen T., Wang Y., Wang J., Yan F. Adjuvant corticosteroid therapy for critically ill patients with COVID-19. Crit. Care. 2020;24:241. doi: 10.1186/s13054-020-02964-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zha L., Li S., Pan L., Tefsen B., Li Y., French N., Chen L., Yang G., Villanueva E.V. Corticosteroid treatment of patients with coronavirus disease, COVID-19. Med. J. Aust. 2019;212(2020):416–420. doi: 10.5694/mja2.50577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhou W., Liu Y., Tian D., Wang C., Wang S., Cheng J., Hu M., Fang M., Gao Y. Potential benefits of precise corticosteroids therapy for severe 2019-nCoV pneumonia. Signal Transduct Target Ther. 2020;5:18. doi: 10.1038/s41392-020-0127-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Atrah H.I. Alternative management of Covid-19 infection. Scott Med. J. 2020;65:72–75. doi: 10.1177/0036933020941497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zwingenberger K., Wnendt S. Immunomodulation by thalidomide: systematic review of the literature and of unpublished observations. J. Inflamm. 1995;46:177–211. [PubMed] [Google Scholar]
- 99.Hosseini-Chegeni A., Jazaeri F., Yousefi-Ahmadipour A., Heidari M., Abdollahie A., Dehpour A.R. Thalidomide attenuates the hyporesponsiveness of isolated atria to chronotropic stimulation in BDL rats: The involvement of TNF-α, IL-6 inhibition, and SOCS1 activation. Iran J. Basic Med. Sci. 2019;22:1259–1266. doi: 10.22038/ijbms.2019.32256.7742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Paravar T., Lee D.J. Thalidomide: mechanisms of action. Int. Rev. Immunol. 2008;27:111–135. doi: 10.1080/08830180801911339. [DOI] [PubMed] [Google Scholar]
- 101.Amirshahrokhi K. Anti-inflammatory effect of thalidomide in paraquat-induced pulmonary injury in mice. Int. Immunopharmacol. 2013;17:210–215. doi: 10.1016/j.intimp.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 102.Verdecchia P., Cavallini C., Spanevello A., Angeli F. COVID-19: ACE2centric infective disease? Hypertension. 2020;76:294–299. doi: 10.1161/HYPERTENSIONAHA.120.15353. [DOI] [PubMed] [Google Scholar]
- 103.Albini A., Di Guardo G., Noonan D.M., Lombardo M. The SARS-CoV-2 receptor, ACE-2, is expressed on many different cell types: implications for ACE-inhibitor- and angiotensin II receptor blocker-based cardiovascular therapies. Intern. Emerg. Med. 2020;15:759–766. doi: 10.1007/s11739-020-02364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Albuquerque D., Nihei J., Cardillo F., Singh R. The ACE inhibitors enalapril and captopril modulate cytokine responses in Balb/c and C57Bl/6 normal mice and increase CD4(+)CD103(+)CD25(negative) splenic T cell numbers. Cell. Immunol. 2010;260:92–97. doi: 10.1016/j.cellimm.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 105.Monteil V., Kwon H., Prado P., Hagelkrüys A., Wimmer R.A., Stahl M., Leopoldi A., Garreta E., Hurtado del Pozo C., Prosper F., Romero J.P., Wirnsberger G., Zhang H., Slutsky A.S., Conder R., Montserrat N., Mirazimi A., Penninger J.M. Inhibition of SARS-CoV-2 Infections in Engineered Human Tissues Using Clinical-Grade Soluble Human ACE2. Cell. 2020;181:905–913.e7. doi: 10.1016/j.cell.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Andrzejczak D., Górska D., Czarnecka E. Influence of enalapril, quinapril and losartan on lipopolysaccharide (LPS)-induced serum concentrations of TNF-alpha, IL-1 beta, IL-6 in spontaneously hypertensive rats (SHR) Pharmacol. Rep. 2007;59:437–446. [PubMed] [Google Scholar]
- 107.Lee C., Chun J., Hwang S.W., Kang S.J., Im J.P., Kim J.S. Enalapril inhibits nuclear factor-κB signaling in intestinal epithelial cells and peritoneal macrophages and attenuates experimental colitis in mice. Life Sci. 2014;95:29–39. doi: 10.1016/j.lfs.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 108.Choe S.-H., Choi E.-Y., Hyeon J.-Y., Keum B.R., Choi I.S., Kim S.-J. Telmisartan, an angiotensin II receptor blocker, attenuates Prevotella intermedia lipopolysaccharide-induced production of nitric oxide and interleukin-1β in murine macrophages. Int. Immunopharmacol. 2019;75 doi: 10.1016/j.intimp.2019.105750. [DOI] [PubMed] [Google Scholar]
- 109.Saber S., Basuony M., Eldin A.S. Telmisartan ameliorates dextran sodium sulfate-induced colitis in rats by modulating NF-κB signalling in the context of PPARγ agonistic activity. Arch. Biochem. Biophys. 2019;671:185–195. doi: 10.1016/j.abb.2019.07.014. [DOI] [PubMed] [Google Scholar]
- 110.Gurwitz D. Angiotensin receptor blockers as tentative SARS-CoV-2 therapeutics. Drug Dev Res. 2020;81:537–540. doi: 10.1002/ddr.21656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ishiyama Y., Gallagher P.E., Averill D.B., Tallant E.A., Brosnihan K.B., Ferrario C.M. Upregulation of angiotensin-converting enzyme 2 after myocardial infarction by blockade of angiotensin II receptors. Hypertension. 2004;43:970–976. doi: 10.1161/01.HYP.0000124667.34652.1a. [DOI] [PubMed] [Google Scholar]
- 112.Rukavina Mikusic N.L., Kouyoumdzian N.M., Uceda A., Del Mauro J.S., Pandolfo M., Gironacci M.M., Puyó A.M., Toblli J.E., Fernández B.E., Choi M.R. Losartan prevents the imbalance between renal dopaminergic and renin angiotensin systems induced by fructose overload. l-Dopa/dopamine index as new potential biomarker of renal dysfunction. Metab. Clin. Exp. 2018;85:271–285. doi: 10.1016/j.metabol.2018.04.010. [DOI] [PubMed] [Google Scholar]
- 113.Wang X., Chen X., Huang W., Zhang P., Guo Y., Körner H., Wu H., Wei W. Losartan suppresses the inflammatory response in collagen-induced arthritis by inhibiting the MAPK and NF-κB pathways in B and T cells. Inflammopharmacology. 2019;27:487–502. doi: 10.1007/s10787-018-0545-2. [DOI] [PubMed] [Google Scholar]
- 114.Dworakowska D., Grossman A.B. Renin-angiotensin system inhibitors in management of hypertension during the COVID-19 pandemic. J. Physiol. Pharmacol. 2020;71 doi: 10.26402/jpp.2020.2.01. [DOI] [PubMed] [Google Scholar]
- 115.Morales D.R., Conover M.M., You S.C., Pratt N., Kostka K., Duarte-Salles T., Fernández-Bertolín S., Aragón M., DuVall S.L., Lynch K., Falconer T., van Bochove K., Sung C., Matheny M.E., Lambert C.G., Nyberg F., Alshammari T.M., Williams A.E., Park R.W., Weaver J., Sena A.G., Schuemie M.J., Rijnbeek P.R., Williams R.D., Lane J.C.E., Prats-Uribe A., Zhang L., Areia C., Krumholz H.M., Prieto-Alhambra D., Ryan P.B., Hripcsak G., Suchard M.A. Renin-angiotensin system blockers and susceptibility to COVID-19: a multinational open science cohort study. MedRxiv. 2020 doi: 10.1101/2020.06.11.20125849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Seif F., Aazami H., Khoshmirsafa M., Kamali M., Mohsenzadegan M., Pornour M., Mansouri D. JAK Inhibition as a New Treatment Strategy for Patients with COVID-19. Int. Arch. Allergy Immunol. 2020:1–9. doi: 10.1159/000508247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Spinelli F.R., Conti F., Gadina M. HiJAKing SARS-CoV-2? The potential role of JAK inhibitors in the management of COVID-19. Sci. Immunol. 2020;5:eabc5367. doi: 10.1126/sciimmunol.abc5367. [DOI] [PubMed] [Google Scholar]
- 118.Ajayi S., Becker H., Reinhardt H., Engelhardt M., Zeiser R., von Bubnoff N., Wäsch R. Ruxolitinib. Recent Results Cancer Res. 2018;212:119–132. doi: 10.1007/978-3-319-91439-8_6. [DOI] [PubMed] [Google Scholar]
- 119.Cao Y., Wei J., Zou L., Jiang T., Wang G., Chen L., Huang L., Meng F., Huang L., Wang N., Zhou X., Luo H., Mao Z., Chen X., Xie J., Liu J., Cheng H., Zhao J., Huang G., Wang W., Zhou J. Ruxolitinib in treatment of severe coronavirus disease 2019 (COVID-19): A multicenter, single-blind, randomized controlled trial. J. Allergy Clin. Immunol. 2020;146:137–146.e3. doi: 10.1016/j.jaci.2020.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Li L., He X., Wang X., Sun Y., Wu G., Fang H., Wang C., Luo P., Xia Z. Ruxolitinib protects lipopolysaccharide (LPS)-induced sepsis through inhibition of nitric oxide production in mice. Ann. Transl. Med. 2020;8:546. doi: 10.21037/atm-20-2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Saeed I., McLornan D., Harrison C.N. Managing side effects of JAK inhibitors for myelofibrosis in clinical practice. Expert. Rev. Hematol. 2017;10:617–625. doi: 10.1080/17474086.2017.1337507. [DOI] [PubMed] [Google Scholar]
- 122.Treon S.P., Castillo J.J., Skarbnik A.P., Soumerai J.D., Ghobrial I.M., Guerrera M.L., Meid K., Yang G. The BTK inhibitor ibrutinib may protect against pulmonary injury in COVID-19-infected patients. Blood. 2020;135:1912–1915. doi: 10.1182/blood.2020006288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Einhaus J., Pecher A.-C., Asteriti E., Schmid H., Secker K.-A., Duerr-Stoerzer S., Keppeler H., Klein R., Schneidawind C., Henes J., Schneidawind D. Inhibition of effector B cells by ibrutinib in systemic sclerosis. Arthritis Res. Ther. 2020;22:66. doi: 10.1186/s13075-020-02153-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mantlo E., Bukreyeva N., Maruyama J., Paessler S., Huang C. Antiviral activities of type I interferons to SARS-CoV-2 infection. Antiviral Res. 2020;179 doi: 10.1016/j.antiviral.2020.104811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.González Canga A., Sahagún Prieto A.M., Diez Liébana M.J., Fernández Martínez N., Sierra Vega M.J.J. García Vieitez, The pharmacokinetics and interactions of ivermectin in humans–a mini-review. AAPS J. 2008;10:42–46. doi: 10.1208/s12248-007-9000-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Caly L., Druce J.D., Catton M.G., Jans D.A., Wagstaff K.M. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020;178 doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lv C., Liu W., Wang B., Dang R., Qiu L., Ren J., Yan C., Yang Z., Wang X. Ivermectin inhibits DNA polymerase UL42 of pseudorabies virus entrance into the nucleus and proliferation of the virus in vitro and vivo. Antiviral Res. 2018;159:55–62. doi: 10.1016/j.antiviral.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 128.Tay M.Y.F., Fraser J.E., Chan W.K.K., Moreland N.J., Rathore A.P., Wang C., Vasudevan S.G., Jans D.A. Nuclear localization of dengue virus (DENV) 1–4 non-structural protein 5; protection against all 4 DENV serotypes by the inhibitor Ivermectin. Antiviral Res. 2013;99:301–306. doi: 10.1016/j.antiviral.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 129.Crump A. Ivermectin: enigmatic multifaceted “wonder” drug continues to surprise and exceed expectations. J. Antibiot. 2017;70:495–505. doi: 10.1038/ja.2017.11. [DOI] [PubMed] [Google Scholar]
- 130.Sajid M.S., Iqbal Z., Muhammad G., Iqbal M.U. Immunomodulatory effect of various anti-parasitics: a review. Parasitology. 2006;132:301–313. doi: 10.1017/S0031182005009108. [DOI] [PubMed] [Google Scholar]
- 131.Yan S., Ci X., Chen N., Chen C., Li X., Chu X., Li J., Deng X. Anti-inflammatory effects of ivermectin in mouse model of allergic asthma. Inflamm. Res. 2011;60:589–596. doi: 10.1007/s00011-011-0307-8. [DOI] [PubMed] [Google Scholar]
- 132.Zhang X., Song Y., Ci X., An N., Ju Y., Li H., Wang X., Han C., Cui J., Deng X. Ivermectin inhibits LPS-induced production of inflammatory cytokines and improves LPS-induced survival in mice. Inflamm. Res. 2008;57:524–529. doi: 10.1007/s00011-008-8007-8. [DOI] [PubMed] [Google Scholar]
- 133.Sharun K., Dhama K., Patel S.K., Pathak M., Tiwari R., Singh B.R., Sah R., Bonilla-Aldana D.K., Rodriguez-Morales A.J., Leblebicioglu H. Ivermectin, a new candidate therapeutic against SARS-CoV-2/COVID-19. Ann. Clin. Microbiol. Antimicrob. 2020;19:23. doi: 10.1186/s12941-020-00368-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.PAHO/WHO., COVID-19: Chloroquine and hydroxychloroquine research., (2020). https:iris.paho.org/handle/10665.2/52105 (accessed June 15, 2020).
- 135.Yao X., Ye F., Zhang M., Cui C., Huang B., Niu P., Liu X., Zhao L., Dong E., Song C., Zhan S., Lu R., Li H., Tan W., Liu D. In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Clin. Infect. Dis. 2020;71:732–739. doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vincent M.J., Bergeron E., Benjannet S., Erickson B.R., Rollin P.E., Ksiazek T.G., Seidah N.G., Nichol S.T. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol. J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ghasemnejad-Berenji H., Ghaffari Novin M., Hajshafiha M., Nazarian H., Hashemi S.M., Ilkhanizadeh B., Ghasemnejad T., Sadeghpour S., Ghasemnejad-Berenji M. Immunomodulatory effects of hydroxychloroquine on Th1/Th2 balance in women with repeated implantation failure. Biomed. Pharmacother. 2018;107:1277–1285. doi: 10.1016/j.biopha.2018.08.027. [DOI] [PubMed] [Google Scholar]
- 138.Jang C.-H., Choi J.-H., Byun M.-S., Jue D.-M. Chloroquine inhibits production of TNF-alpha, IL-1beta and IL-6 from lipopolysaccharide-stimulated human monocytes/macrophages by different modes. Rheumatology (Oxford). 2006;45:703–710. doi: 10.1093/rheumatology/kei282. [DOI] [PubMed] [Google Scholar]
- 139.Ewald S.E., Lee B.L., Lau L., Wickliffe K.E., Shi G.-P., Chapman H.A., Barton G.M. The ectodomain of Toll-like receptor 9 is cleaved to generate a functional receptor. Nature. 2008;456:658–662. doi: 10.1038/nature07405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.FDA., FDA cautions against use of hydroxychloroquine or chloroquine for COVID-19 outside of the hospital setting or a clinical trial due to risk of heart rhythm problems., (2020). https://www.fda.gov/drugs/drug-safety-and-availability/.
- 141.Ding J.-G., Li J., Hong L., Yu X.-Q., Ye E.-L., Sun G.-Q., Zhang X.-X., Chen L., Sun Q.-F. Viral kinetics and factors associated with rapid viral clearance during lopinavir/ritonavir-based combination therapy in non-severe COVID-19 patients. Eur. Rev. Med. Pharmacol. Sci. 2020;24:5788–5796. doi: 10.26355/eurrev_202005_21373. [DOI] [PubMed] [Google Scholar]
- 142.Meng Z., Wang T., Li C., Chen X., Li L., Qin X., Li H., Luo J. An experimental trial of recombinant human interferon alpha nasal drops to prevent coronavirus disease 2019 in medical staff in an epidemic area. Infect. Dis. (except HIV/AIDS) 2020 doi: 10.1101/2020.04.11.20061473. [DOI] [Google Scholar]
- 143.Falzarano D., de Wit E., Martellaro C., Callison J., Munster V.J., Feldmann H. Inhibition of novel β coronavirus replication by a combination of interferon-α2b and ribavirin. Sci Rep. 2013;3:1686. doi: 10.1038/srep01686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Curreli S., Romerio F., Secchiero P., Zella D. IFN-alpha2b increases interleukin-10 expression in primary activated human CD8+ T cells. J. Interferon Cytokine Res. 2002;22:1167–1173. doi: 10.1089/10799900260475678. [DOI] [PubMed] [Google Scholar]
- 145.Lagathu C., Eustace B., Prot M., Frantz D., Gu Y., Bastard J.-P., Maachi M., Azoulay S., Briggs M., Caron M., Capeau J. Some HIV antiretrovirals increase oxidative stress and alter chemokine, cytokine or adiponectin production in human adipocytes and macrophages. Antivir. Ther. (Lond.) 2007;12:489–500. [PubMed] [Google Scholar]
- 146.Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Holshue M.L., DeBolt C., Lindquist S., Lofy K.H., Wiesman J., Bruce H., Spitters C., Ericson K., Wilkerson S., Tural A., Diaz G., Cohn A., Fox L., Patel A., Gerber S.I., Kim L., Tong S., Lu X., Lindstrom S., Pallansch M.A., Weldon W.C., Biggs H.M., Uyeki T.M., Pillai S.K. Washington State 2019-nCoV Case Investigation Team, First Case of, Novel Coronavirus in the United States. N. Engl. J. Med. 2019;382(2020):929–936. doi: 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Liao S., Li Y., Lai Y., Liu N., Zhang F., Xu P. Ribavirin attenuates the respiratory immune responses to influenza viral infection in mice. Arch Virol. 2017;162:1661–1669. doi: 10.1007/s00705-017-3291-7. [DOI] [PubMed] [Google Scholar]
- 149.Deng L., Li C., Zeng Q., Liu X., Li X., Zhang H., Hong Z., Xia J. Arbidol combined with LPV/r versus LPV/r alone against Corona Virus Disease 2019: A retrospective cohort study. J. Infect. 2020;81:e1–e5. doi: 10.1016/j.jinf.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Vankadari N. Arbidol: A potential antiviral drug for the treatment of SARS-CoV-2 by blocking the trimerization of viral spike glycoprotein ? Int. J. Antimicrob. Agents. 2020;56:105998. doi: 10.1016/j.ijantimicag.2020.105998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Aouba A., Baldolli A., Geffray L., Verdon R., Bergot E., Martin-Silva N., Justet A. Targeting the inflammatory cascade with anakinra in moderate to severe COVID-19 pneumonia: case series. Ann. Rheum. Dis. 2020 doi: 10.1136/annrheumdis-2020-217706. [DOI] [PubMed] [Google Scholar]
- 152.Yaekura A., Yoshida K., Morii K., Oketani Y., Okumura I., Kaneshiro K., Shibanuma N., Sakai Y., Hashiramoto A. Chronotherapy targeting cytokine secretion attenuates collagen-induced arthritis in mice. Int. Immunopharmacol. 2020;84 doi: 10.1016/j.intimp.2020.106549. [DOI] [PubMed] [Google Scholar]
- 153.Lo Caputo S., Corso G., Clerici M., Santantonio T.A. Baricitinib: a chance to treat COVID-19? J. Med. Virol. 2020 doi: 10.1002/jmv.26033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Richardson P., Griffin I., Tucker C., Smith D., Oechsle O., Phelan A., Stebbing J. Baricitinib as potential treatment for 2019-nCoV acute respiratory disease. Lancet. 2020;395:e30–e31. doi: 10.1016/S0140-6736(20)30304-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Aydın E., Yıldırım Y., Aydın F.Y., Bahadır M.V., Kaplan İ., Kadiroğlu B., Ketani M.A., Yılmaz Z., Kadiroğlu A.K., Yılmaz M.E. Evaluation of the effect of intraperitoneal etanercept administration on oxidative stress and inflammation indicators in the kidney and blood of experimental sepsis-induced rats. Rev. Soc. Bras. Med. Trop. 2020;53 doi: 10.1590/0037-8682-0016-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Lv Y., Jing G., Zhu G., Luo H., Li B., Xie Y., Li C., Wang X. Effects and mechanism of the etanercept on pancreatic encephalopathy. Mol. Med. Rep. 2020;21:2615–2623. doi: 10.3892/mmr.2020.11062. [DOI] [PubMed] [Google Scholar]
- 157.Feldmann M., Maini R.N., Woody J.N., Holgate S.T., Winter G., Rowland M., Richards D., Hussell T. Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed. Lancet. 2020;395:1407–1409. doi: 10.1016/S0140-6736(20)30858-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chen X.Y., Yan B.X., Man X.Y. TNFα inhibitor may be effective for severe COVID-19: learning from toxic epidermal necrolysis. Ther. Adv. Respir. Dis. 2020;14 doi: 10.1177/1753466620926800. 1753466620926800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Savur F., Aydemir O., İlhan N. The effect of infliximab and octreotide on cytokine levels experimental proliferative vitreoretinopathy. Cutan. Ocul. Toxicol. 2020;39:61–66. doi: 10.1080/15569527.2019.1701000. [DOI] [PubMed] [Google Scholar]
- 160.Antwi-Amoabeng D., Kanji Z., Ford B., Beutler B.D., Riddle M.S., Siddiqui F. Clinical Outcomes in COVID-19 Patients Treated with Tocilizumab: An Individual Patient Data Systematic Review. J. Med. Virol. 2020 doi: 10.1002/jmv.26038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Quartuccio L., Sonaglia A., McGonagle D., Fabris M., Peghin M., Pecori D., Monte A.D., Bove T., Curcio F., Bassi F., Vita S.D., Tascini C. Profiling COVID-19 pneumonia progressing into the cytokine storm syndrome: results from a single Italian Centre study on tocilizumab versus standard of care. J. Clin. Virol. 2020 doi: 10.1016/j.jcv.2020.104444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Leik C.E., Walsh S.W. Linoleic acid, but not oleic acid, upregulates production of interleukin-8 by human vascular smooth muscle cells via arachidonic acid metabolites under conditions of oxidative stress. J. Soc. Gynecol. Investig. 2005;12:593–598. doi: 10.1016/j.jsgi.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 163.Chen H., Xu H., Luo L., Qiao L., Wang Y., Xu M., Li Y., Zhu P., Yang B. Thalidomide Prevented and Ameliorated Pathogenesis of Crohn’s Disease in Mice via Regulation of Inflammatory Response and Fibrosis. Front Pharmacol. 2019;10:1486. doi: 10.3389/fphar.2019.01486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Amirshahrokhi K., Khalili A.-R. Thalidomide ameliorates cisplatin-induced nephrotoxicity by inhibiting renal inflammation in an experimental model. Inflammation. 2015;38:476–484. doi: 10.1007/s10753-014-9953-7. [DOI] [PubMed] [Google Scholar]
- 165.Yamaya M., Nishimura H., Deng X., Sugawara M., Watanabe O., Nomura K., Shimotai Y., Momma H., Ichinose M., Kawase T. Inhibitory effects of glycopyrronium, formoterol, and budesonide on coronavirus HCoV-229E replication and cytokine production by primary cultures of human nasal and tracheal epithelial cells. Respir. Investig. 2020;58:155–168. doi: 10.1016/j.resinv.2019.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Neuenschwander H.M., Moreira J.J., Vendruscolo C.P., Fülber J., Seidel S.R.T., Michelacci Y.M., Baccarin R.Y.A. Hyaluronic acid has chondroprotective and joint-preserving effects on LPS-induced synovitis in horses. J. Vet. Sci. 2019;20 doi: 10.4142/jvs.2019.20.e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Torres R.C., Insuela D.B.R., deCarvalho V.F. Mecanismos celulares e moleculares da ação antiinflamatória dos glicocorticóides. Corpus et Scientia. 2012;8:36–51. [Google Scholar]
- 168.Hernández-Fonseca J.P., Durán A., Valero N., Mosquera J. Losartan and enalapril decrease viral absorption and interleukin 1 beta production by macrophages in an experimental dengue virus infection. Arch. Virol. 2015;160:2861–2865. doi: 10.1007/s00705-015-2581-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wong M.H., Chapin O.C., Johnson M.D. LPS-stimulated cytokine production in type I cells is modulated by the renin-angiotensin system. Am. J. Respir. Cell Mol. Biol. 2012;46:641–650. doi: 10.1165/rcmb.2011-0289OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Rothlin R.P., Vetulli H.M., Duarte M., Pelorosso F.G. Telmisartan as tentative angiotensin receptor blocker therapeutic for COVID-19. Drug Dev. Res. 2020 doi: 10.1002/ddr.21679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Li L., He X., Wang X., Sun Y., Wu G., Fang H., Wang C., Luo P., Xia Z. Ruxolitinib protects lipopolysaccharide (LPS)-induced sepsis through inhibition of nitric oxide production in mice. Ann Transl Med. 2020;8:546. doi: 10.21037/atm-20-2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sugiyama K., Shirai R., Mukae H., Ishimoto H., Nagata T., Sakamoto N., Ishii H., Nakayama S., Yanagihara K., Mizuta Y., Kohno S. Differing effects of clarithromycin and azithromycin on cytokine production by murine dendritic cells. Clin. Exp. Immunol. 2007;147:540–546. doi: 10.1111/j.1365-2249.2007.03299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Aghai Z.H., Kode A., Saslow J.G., Nakhla T., Farhath S., Stahl G.E., Eydelman R., Strande L., Leone P., Rahman I. Azithromycin suppresses activation of nuclear factor-kappa B and synthesis of pro-inflammatory cytokines in tracheal aspirate cells from premature infants. Pediatr. Res. 2007;62:483–488. doi: 10.1203/PDR.0b013e318142582d. [DOI] [PubMed] [Google Scholar]
- 174.Ivetić Tkalcević V., Bosnjak B., Hrvacić B., Bosnar M., Marjanović N., Ferencić Z., Situm K., Culić O., Parnham M.J., Eraković V. Anti-inflammatory activity of azithromycin attenuates the effects of lipopolysaccharide administration in mice. Eur. J. Pharmacol. 2006;539:131–138. doi: 10.1016/j.ejphar.2006.03.074. [DOI] [PubMed] [Google Scholar]
- 175.Grassin-Delyle S., Salvator H., Brollo M., Catherinot E., Sage E., Couderc L.-J., Naline E., Devillier P. Chloroquine inhibits the release of inflammatory cytokines by human lung explants. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Hong S.K., Kim H.J., Song C.S., Choi I.S., Lee J.B., Park S.Y. Nitazoxanide suppresses IL-6 production in LPS-stimulated mouse macrophages and TG-injected mice. Int. Immunopharmacol. 2012;13:23–27. doi: 10.1016/j.intimp.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 177.Shou J., Kong X., Wang X., Tang Y., Wang C., Wang M., Zhang L., Liu Y., Fei C., Xue F., Li J., Zhang K. Tizoxanide Inhibits Inflammation in LPS-Activated RAW264.7 Macrophages via the Suppression of NF-κB and MAPK Activation. Inflammation. 2019;42:1336–1349. doi: 10.1007/s10753-019-00994-3. [DOI] [PubMed] [Google Scholar]
- 178.Rossignol J.-F. Nitazoxanide, a new drug candidate for the treatment of Middle East respiratory syndrome coronavirus. J. Infect. Public Health. 2016;9:227–230. doi: 10.1016/j.jiph.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Nasiripour S., Zamani F., Farasatinasab M. Can Colchicine as an Old Anti-Inflammatory Agent Be Effective in COVID-19? J. Clin. Pharmacol. 2020;60:828–829. doi: 10.1002/jcph.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]