Abstract
Background
Prostate cancer recurrence is found in up to 40% of men with prior definitive (total prostatectomy or whole-prostate radiation) treatment. Prostate-specific membrane antigen PET agents such as 2-(3-{1-carboxy-5-[(6-[18F]fluoro-pyridine 3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid (18F-DCFPyL) may improve detection of recurrence compared with multiparametric MRI; however, histopathologic validation is lacking.
Purpose
To determine the sensitivity, specificity, and positive predictive value (PPV) of 18F-DCFPyL PET/CT based on histologic analysis and to compare with pelvic multiparametric MRI in men with biochemically recurrent prostate cancer.
Materials and Methods
Men were prospectively recruited after prostatectomy and/or radiation therapy with rising prostate-specific antigen level (median, 2.27 ng/mL; range, 0.2–27.45 ng/mL) and a negative result at conventional imaging (bone scan and/or CT). Participants underwent 18F-DCFPyL PET/CT imaging and 3.0-T pelvic multiparametric MRI. Statistical analysis included Wald and modified χ2 tests.
Results
A total of 323 lesions were visualized in 77 men by using 18F-DCFPyL or multiparametric MRI, with imaging detection concordance of 25% (82 of 323) when including all lesions in the MRI field of view and 53% (52 of 99) when only assessing prostate bed lesions. 18F-DCFPyL depicted more pelvic lymph nodes than did MRI (128 vs 23 nodes). Histologic validation was obtained in 80 locations with sensitivity, specificity, and PPV of 69% (25 of 36; 95% confidence interval [CI]: 51%, 88%), 91% (40 of 44; 95% CI: 74%, 98%), and 86% (25 of 29; 95% CI: 73%, 97%) for 18F-DCFPyL and 69% (24 of 35; 95% CI: 50%, 86%), 74% (31 of 42; 95% CI: 42%, 89%), and 69% (24 of 35; 95% CI: 50%, 88%) for multiparametric MRI (P = .95, P = .14, and P = .07, respectively). In the prostate bed, sensitivity, specificity, and PPV were 57% (13 of 23; 95% CI: 32%, 81%), 86% (18 of 21; 95% CI: 73%, 100%), and 81% (13 of 16; 95% CI: 59%, 100%) for 18F-DCFPyL and 83% (19 of 23; 95% CI: 59%, 100%), 52% (11 of 21; 95% CI: 29%, 74%), and 66% (19 of 29; 95% CI: 44%, 86%) for multiparametric MRI (P = .19, P = .02, and P = .17, respectively). The addition of 18F-DCFPyL to multiparametric MRI improved PPV by 38% overall (P = .02) and by 30% (P = .09) in the prostate bed.
Conclusion
Findings with 2-(3-{1-carboxy-5-[(6-[18F]fluoro-pyridine 3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid (18F-DCFPyL) were histologically validated and demonstrated high specificity and positive predictive value. In the pelvis, 18F-DCFPyL depicted more lymph nodes and improved positive predictive value and specificity when added to multiparametric MRI.
© RSNA, 2020
Online supplemental material is available for this article.
See also the editorial by Zukotynski and Rowe in this issue.
Summary
PET/CT with 2-(3-{1-carboxy-5-[(6-[18F]fluoro-pyridine 3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid demonstrates high specificity and positive predictive value and adds greater value to pelvic multiparametric MRI in correctly recognizing biochemically recurrent prostate cancer.
Key Results
■ In men with histopathologically confirmed recurrent prostate cancer, 2-(3-{1-carboxy-5-[(6-[18F]fluoro-pyridine 3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid (18F-DCFPyL) PET/CT demonstrated a sensitivity, specificity, and positive predictive value (PPV) of 69% (25 of 36), 91% (40 of 44), and 86% (25 of 29).
■ 18F-DCFPyL PET/CT depicted more pelvic lymph nodes than did multiparametric MRI (128 vs 23 nodes).
■ For assessment of local recurrence, the addition of 18F-DCFPyL to multiparametric MRI improved PPV by 38% (P = .02) and improved specificity compared with multiparametric MRI (86% vs 52% respectively; P = .02).
Introduction
With almost 1.3 million new cases in 2018, prostate cancer is the second most common malignancy in men worldwide (1). Fortunately, curative treatment is available for the majority of patients, but after definitive therapy with total prostatectomy and/or whole-prostate radiation, prostate cancer recurrence can occur in up to 40% of treated men (2). Localizing disease recurrence permits more directed treatment of lesions that can slow or stop disease progression (3).
Serum prostate-specific antigen is currently used for surveillance of relapsed prostate cancer and rising levels often trigger a search for sites of disease with imaging (4). PET/CT targeting the prostate-specific membrane antigen (PSMA) is a particularly sensitive research modality for depicting tumors. A variety of PSMA PET agents have been developed, and these can generally be divided into gallium 68 (68Ga)–labeled or fluorine 18 (18F)–labeled probes. Since it was first introduced, 2-(3-{1-carboxy-5-[(6-[18F]fluoro-pyridine 3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid (18F-DCFPyL) has demonstrated high sensitivity with a longer half-life than 68Ga. Therefore, 18F-DCFPyL can be made in centralized commercial radiopharmacies and distributed, whereas 68Ga-labeled agents must be prepared locally (5).
18F-DCFPyL has a high affinity for prostate malignancy and has demonstrated promising early clinical results in identifying metastatic disease in men with high-risk prostate cancer (6). However, histologic validation in the setting of biochemical recurrence is lacking. This is a difficult population from which to obtain histologic specimens because biopsy is not considered standard of care and treatment is often initiated without histologic validation.
Pelvic multiparametric MRI is commonly used to evaluate for local-regional tumor in men with biochemical disease (7–10). A distinct advantage of MRI is that it does not expose patients to ionizing radiation yet provides anatomic information. In recurrent prostate cancer, comparisons of MRI and PSMA PET/CT suggest the two modalities may be complementary (11). Here, we compare the sensitivity, specificity, and positive predictive value (PPV) of 18F-DCFPyL PET/CT and multiparametric MRI by using histologic validation.
Materials and Methods
Study Participants
Between July 2017 and February 2019, men who had undergone radical prostatectomy and/or whole-prostate radiation therapy with rising serum prostate-specific antigen level and no definitive evidence of tumor recurrence at bone scan and/or CT were prospectively recruited for this Health Insurance Portability and Accountability Act–compliant, single institution–approved trial (ClinicalTrials.gov identifier NCT03181867) after written informed consent. (See Appendix E1 [online] for inclusion and exclusion criteria.) Enrolled participants were also analyzed on the detection rate of 18F-DCFPyL as it relates to prostate-specific antigen, which is discussed in a separate article (12). Histopathologic validation results used in the prior article were not the central focus of that study. The current article also differs by addressing the performance of pelvic multiparametric MRI compared with 18F-DCFPyL in this population.
Multiparametric MRI
Participants underwent pelvic multiparametric MRI prior to PET imaging with a 3.0-T scanner (Achieva 3.0T-TX; Phillips Healthcare, Best, the Netherlands). Pelvic multiparametric MRI and 18F-DCFPyL imaging occurred within a mean of 13 days of each other (range, 0–112 days; median interval, 2 days). MRI involved combined use of an endorectal coil (BPX-30; Medrad, Pittsburgh, Pa) filled with 45 mL of a fluorocarbon-based fluid (Fluorinert; 3M, Maplewood, Minn), and the anterior half of a 32-channel cardiac Sense coil (InVivo, Gainesville, Fla). T1-weighted MRI, T2-weighted MRI, diffusion-weighted MRI (including apparent diffusion coefficient map and b = 2000 sec/mm2 diffusion-weighted MRI), and dynamic contrast material–enhanced MRI sequences were performed (Table E1 [online]). Gadoterate meglumine (Dotarem; Guerbet, France) was the contrast agent injected by using the manufacturer’s recommended protocol.
Presence or absence of cancer on T2-weighted, diffusion-weighted, and dynamic contrast-enhanced images was assessed by using a six-grade Likert system for likelihood of recurrence (grade 1, very low; grade 2, low; grade 3, low-moderate; grade 4, moderate; grade 5, moderate-high; grade 6, high likelihood for recurrent prostate cancer). This system has been in use for more than 5 years and modified from the initial three-tier system, which was also created at the National Cancer Institute (13). In this analysis, any finding given a grade was considered positive for detection at MRI. Only lesions within the prostate bed were given a grade. Nodal, soft-tissue, and bone findings suspicious for metastasis were noted on report, but not graded. Images were prospectively read by an experienced genitourinary radiologist (B.T., with more than 12 years of experience) who was unaware of 18F-DCFPyL findings.
18F-DCFPyL PET/CT Imaging
Participants were injected with a mean dose ± standard deviation of 298.96 MBq ± 22.6 (8.08 mCi ± 0.6) 18F-DCFPyL and imaged (head to toe) for a median of 119 minutes (range, 103–140 minutes) after injection with a Discovery MI DR PET/CT (GE Healthcare, Milwaukee, Wis) by using time-of-flight reconstruction, 20-cm axial field of view, and 70-cm bore. Attenuation-corrected images were evaluated with MIM Software (version 6.9.2; MIM Software, Cleveland, Ohio) by two experienced nuclear medicine physicians (L.L. and E.M., each with more than 13 years of experience), who were blinded to MRI findings. By using MIM Software, regions of interest were placed around lesions that were highly suspicious for malignancy based on focal uptake greater than background not due to physiologic activity that fused to a CT abnormality. Standardized uptake values from these regions of interest were not used in this qualitative comparative analysis because of the different modalities. Both physicians read images independently and then discussed findings together to reach consensus. Depending on the location of the lesion, background activity ranged from blood pool, nearby muscle, bone, or lung.
Histopathologic Validation
Histologic samples were obtained from participants by using either transrectal US/MRI fusion (inside the prostate bed) or CT guidance (outside the prostate bed). Specimens were sent to the department of pathology for standard laboratory evaluation per institutional procedure. Results were obtained from official clinical reports. Time from PET imaging to biopsy ranged from 7–106 days (median, 31 days).
Statistical Analysis
Concordance in lesion detection between 18F-DCFPyL PET/CT and multiparametric MRI was estimated by using the proportion of specific agreement defined as the conditional probability of a randomly selected modality depicting a lesion in the same location as the other modality (14). Lesion-level sensitivity, specificity, and PPV were assessed for each modality against pathologic findings. Standard errors and 95% confidence intervals (CIs) of lesion-level estimators were estimated from 2000 bootstrap samples by using random sampling with replacement on the participant level to account for intrapatient correlation arising from multiple lesions. The 2.5th and 97.5th percentiles of 2000 bootstrap samples were taken as the 95% confidence limits. The Wald test based on the bootstrap standard errors was used to test the difference in lesion-level estimators between 18F-DCFPyL and multiparametric MRI. Association between lesion detection and lesion characteristics was tested by using the modified χ2 test (15). Added benefit to PPV of each modality was defined as the difference in PPV between lesions depicted with both imaging modalities and lesions depicted with one imaging modality only. All tests were two sided; P values < .05 were considered to indicate statistical significance. Statistical and graphical analyses were performed by using R software (version 3.2.5; R Foundation for Statistical Computing, Vienna, Austria) (16).
Results
Participant Characteristics
The analysis included 85 men suspected of having prostate cancer recurrence and no evidence of disease at conventional imaging (CT or bone scan) with median serum prostate-specific antigen level of 2.27 ng/mL (range, 0.2–27.45 ng/mL) and median age of 67 years (interquartile range, 60–71 years). Initially, two enrolled participants were excluded because the radiotracer failed quality testing in one participant and MRI was not performed in another. Prostatectomy was the primary treatment in 35 men. Twenty-six men underwent prostate radiation therapy (external beam or brachytherapy), and 24 men underwent combined surgery and radiation after prostatectomy (Fig 1). A history of androgen deprivation therapy was noted in 57 men, with a range of 1.2 years to 9.7 years since the last dose of androgen deprivation therapy and enrollment in the trial (Table 1).
Figure 1:
Participant flowchart. 18F-DCFPyL = 2-(3-{1-carboxy-5-[(6-[18F]fluoro-pyridine 3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid.
Table 1:
Participant Characteristics

Comparison with Histopathologic Analysis
Pathologic verification was obtained in 35 men from 80 locations. Although participants consented to undergo a biopsy, confirmation was not possible in all participants due to anatomic limitations or participant refusal. 18F-DCFPyL exhibited high specificity and PPV (Table 2). Overall sensitivity, specificity, and PPV for MRI were 69% (24 of 35; 95% CI: 50%, 86%), 74% (31 of 42; 95% CI: 42%, 89%), and 69% (24 of 35; 95% CI: 50%, 88%) and those for 18F-DCFPyL were 69% (25 of 36; 95% CI: 51%, 88%), 91% (40 of 44; 95% CI: 74%, 98%), and 86% (25 of 29; 95% CI: 73%, 97%); P = .95, P = .14, and P = .08, respectively (Table 2). There were 26 pelvic lymph node specimens with MRI sensitivity, specificity, and PPV of 57% (four of seven; 95% CI: 25%, 84%), 100% (19 of 19; 95% CI: 100%, 100%), 100% (four of four; 95% CI: 100%, 100%), respectively. Sensitivity, specificity, and PPV for 18F-DCFPyL were 100% (seven of seven; 95% CI: 100%, 100%), 100% (19 of 19; 95% CI: 100%, 100%), and 100% (seven of seven; 95% CI: 100%, 100%), respectively. Besides the small sample size, these results are somewhat biased in favor of MRI because only nodes large enough (and therefore, more detectable) could be biopsied. Tissue correlations were all located within the MRI field of view (prostate bed [44 of 80], pelvic and abdominal lymph nodes [27 of 80], pelvic soft tissue [three of 80], and pelvic bones [three of 80]) except for three specimens (biopsies from two ribs and a clavicle).
Table 2:
Performance of Multiparametric MRI and 18F-DCFPyL
PET- and MRI-concordant lesions were more likely to correlate to pathologic finding. A total of 45 histologic sites were correctly concordant with MRI and 18F-DCFPyL (30 were tumor negative and 15 were tumor positive), while two concordantly positive lesions were tumor negative and two tumor positive lesions were concordantly negative at imaging. There were 28 histologic findings (18 malignant and 10 without tumor) that showed imaging discordance. Analyzed separately and excluding concordant findings, the PPV of 18F-DCFPyL positivity alone was 90% (nine of 10) while that of MRI was 50% (nine of 18). 18F-DCFPyL improved PPV by 38% (P = .02) when combined with MRI for an overall PPV of 88% (15 of 17) (Table 3).
Table 3:
Added Value of Multiparametric MRI and 18F-DCFPyL to PPV
By evaluating just the prostate bed with pathologic validation (44 locations), the sensitivity, specificity, and PPV for multiparametric MRI were 83% (19 of 23; 95% CI: 59%, 100%), 52% (11 of 21; 95% CI: 29%, 74%), and 66% (19 of 29; 95% CI: 44%, 86%) and those for 18F-DCFPyL were 57% (13 of 23; 95% CI: 32%, 81%), 86% (18 of 21; 95% CI: 73%, 100%), and 81% (13 of 16; 95% CI: 59%, 100%), respectively (Table 2). In the prostate bed, as MRI grade increased, there was a tendency toward higher tumor-positive biopsy results, although this relationship was not significant due to the small number in each subgroup (P = .31). In very low or low MRI grades, three proved histologically tumor negative and one was tumor positive. In low-moderate or moderate grades, four proved tumor negative and four were tumor positive. In moderate-high or high grades, three proved tumor negative and 13 were tumor positive.
Within the prostate bed, there were 23 histologically tumor-positive lesions and 21 tumor-negative lesions. At imaging, there were 10 correctly positive concordant lesions, 10 correctly negative concordant lesions, and 21 discordant lesions (Figs 2–4). MRI depicted eight false-positive lesions (which were 18F-DCFPyL negative), whereas 18F-DCFPyL depicted one false-positive lesion (which was MRI negative) that were all histologically diagnosed as normal tissue. In added benefit analysis in the prostate bed, respective PPVs of 75% (three of four) and 53% (nine of 17) resulted for 18F-DCFPyL and MRI independently. 18F-DCFPyL correctly identified three pathologically proven tumors that multiparametric MRI did not depict. Multiparametric MRI correctly depicted nine pathologically proven tumors not seen by using 18F-DCFPyL. The addition of 18F-DCFPyL to MRI improved PPV by 30% (P = .09) while adding MRI to 18F-DCFPyL improved PPV by 8.3% (P = .84) for a combined PPV of 83% (10 of 12) in the prostate bed (Table 3). When combining PPV, both modalities were concordantly positive for pathologically confirmed tumor detection.
Figure 2a:
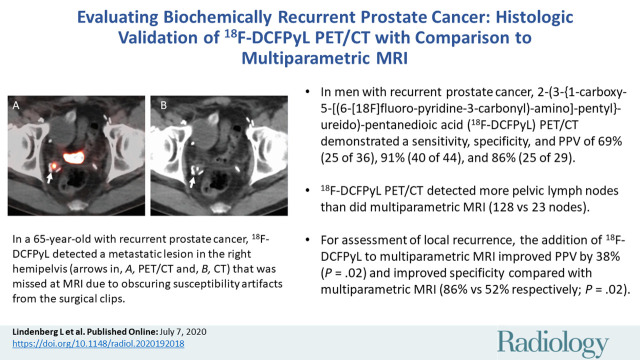
![Images show 2-(3-{1-carboxy-5-[(6-[18F]fluoro-pyridine 3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid (18F-DCFPyL) PET/CT–positive, multiparametric MRI–negative, and biopsy-positive lesion in a 65-year-old man (prostate-specific antigen level of 1.85 ng/mL) with history of prostate cancer and prior radical prostatectomy, now with biochemically recurrent prostate cancer. 18F-DCFPyL depicted pathologically proven metastatic lesion in right hemipelvis (arrow in [a] PET, [b] PET/CT, and [c] CT) that was missed at multiparametric MRI review due to obscuring susceptibility artifacts from surgical clips at (d) T2-weighted MRI, (e) b = 2000 sec/mm2 diffusion-weighted MRI, (f) apparent diffusion coefficient map, and (g) dynamic contrast material–enhanced MRI.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/568c/7457947/df3c62dfd262/radiol.2020192018.fig2a.jpg)
Images show 2-(3-{1-carboxy-5-[(6-[18F]fluoro-pyridine 3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid (18F-DCFPyL) PET/CT–positive, multiparametric MRI–negative, and biopsy-positive lesion in a 65-year-old man (prostate-specific antigen level of 1.85 ng/mL) with history of prostate cancer and prior radical prostatectomy, now with biochemically recurrent prostate cancer. 18F-DCFPyL depicted pathologically proven metastatic lesion in right hemipelvis (arrow in [a] PET, [b] PET/CT, and [c] CT) that was missed at multiparametric MRI review due to obscuring susceptibility artifacts from surgical clips at (d) T2-weighted MRI, (e) b = 2000 sec/mm2 diffusion-weighted MRI, (f) apparent diffusion coefficient map, and (g) dynamic contrast material–enhanced MRI.
Figure 4a:
![Images show 2-(3-(5)-ureido)-pentanedioic acid (18F-DCFPyL) PET/CT–positive, multiparametric MRI–positive, and biopsy-positive lesion in a 62-year-old man (prostate-specific antigen level of 1.48 ng/mL) with history of prostate cancer, treated with external beam radiation therapy, now with biochemically recurrent prostate cancer. Histologically proven lesion in left midbase depicted with 18F-DCFPyL (arrows) ([a] PET and [b] PET/CT) and multiparametric MRI ([c] T2-weighted MRI, [d] b = 2000 sec/mm2 diffusion-weighted MRI, [e] apparent diffusion coefficient map, [f] dynamic contrast material–enhanced MRI) show grade 6 (high likelihood for recurrent prostate cancer).](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/568c/7457947/ce7958da524d/radiol.2020192018.fig4a.jpg)
Images show 2-(3-(5)-ureido)-pentanedioic acid (18F-DCFPyL) PET/CT–positive, multiparametric MRI–positive, and biopsy-positive lesion in a 62-year-old man (prostate-specific antigen level of 1.48 ng/mL) with history of prostate cancer, treated with external beam radiation therapy, now with biochemically recurrent prostate cancer. Histologically proven lesion in left midbase depicted with 18F-DCFPyL (arrows) ([a] PET and [b] PET/CT) and multiparametric MRI ([c] T2-weighted MRI, [d] b = 2000 sec/mm2 diffusion-weighted MRI, [e] apparent diffusion coefficient map, [f] dynamic contrast material–enhanced MRI) show grade 6 (high likelihood for recurrent prostate cancer).
Figure 2b:
![Images show 2-(3-{1-carboxy-5-[(6-[18F]fluoro-pyridine 3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid (18F-DCFPyL) PET/CT–positive, multiparametric MRI–negative, and biopsy-positive lesion in a 65-year-old man (prostate-specific antigen level of 1.85 ng/mL) with history of prostate cancer and prior radical prostatectomy, now with biochemically recurrent prostate cancer. 18F-DCFPyL depicted pathologically proven metastatic lesion in right hemipelvis (arrow in [a] PET, [b] PET/CT, and [c] CT) that was missed at multiparametric MRI review due to obscuring susceptibility artifacts from surgical clips at (d) T2-weighted MRI, (e) b = 2000 sec/mm2 diffusion-weighted MRI, (f) apparent diffusion coefficient map, and (g) dynamic contrast material–enhanced MRI.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/568c/7457947/4a6e88e98d8a/radiol.2020192018.fig2b.jpg)
Images show 2-(3-{1-carboxy-5-[(6-[18F]fluoro-pyridine 3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid (18F-DCFPyL) PET/CT–positive, multiparametric MRI–negative, and biopsy-positive lesion in a 65-year-old man (prostate-specific antigen level of 1.85 ng/mL) with history of prostate cancer and prior radical prostatectomy, now with biochemically recurrent prostate cancer. 18F-DCFPyL depicted pathologically proven metastatic lesion in right hemipelvis (arrow in [a] PET, [b] PET/CT, and [c] CT) that was missed at multiparametric MRI review due to obscuring susceptibility artifacts from surgical clips at (d) T2-weighted MRI, (e) b = 2000 sec/mm2 diffusion-weighted MRI, (f) apparent diffusion coefficient map, and (g) dynamic contrast material–enhanced MRI.
Figure 2c:
![Images show 2-(3-{1-carboxy-5-[(6-[18F]fluoro-pyridine 3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid (18F-DCFPyL) PET/CT–positive, multiparametric MRI–negative, and biopsy-positive lesion in a 65-year-old man (prostate-specific antigen level of 1.85 ng/mL) with history of prostate cancer and prior radical prostatectomy, now with biochemically recurrent prostate cancer. 18F-DCFPyL depicted pathologically proven metastatic lesion in right hemipelvis (arrow in [a] PET, [b] PET/CT, and [c] CT) that was missed at multiparametric MRI review due to obscuring susceptibility artifacts from surgical clips at (d) T2-weighted MRI, (e) b = 2000 sec/mm2 diffusion-weighted MRI, (f) apparent diffusion coefficient map, and (g) dynamic contrast material–enhanced MRI.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/568c/7457947/74b498bd3600/radiol.2020192018.fig2c.jpg)
Images show 2-(3-{1-carboxy-5-[(6-[18F]fluoro-pyridine 3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid (18F-DCFPyL) PET/CT–positive, multiparametric MRI–negative, and biopsy-positive lesion in a 65-year-old man (prostate-specific antigen level of 1.85 ng/mL) with history of prostate cancer and prior radical prostatectomy, now with biochemically recurrent prostate cancer. 18F-DCFPyL depicted pathologically proven metastatic lesion in right hemipelvis (arrow in [a] PET, [b] PET/CT, and [c] CT) that was missed at multiparametric MRI review due to obscuring susceptibility artifacts from surgical clips at (d) T2-weighted MRI, (e) b = 2000 sec/mm2 diffusion-weighted MRI, (f) apparent diffusion coefficient map, and (g) dynamic contrast material–enhanced MRI.
Figure 2d:
![Images show 2-(3-{1-carboxy-5-[(6-[18F]fluoro-pyridine 3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid (18F-DCFPyL) PET/CT–positive, multiparametric MRI–negative, and biopsy-positive lesion in a 65-year-old man (prostate-specific antigen level of 1.85 ng/mL) with history of prostate cancer and prior radical prostatectomy, now with biochemically recurrent prostate cancer. 18F-DCFPyL depicted pathologically proven metastatic lesion in right hemipelvis (arrow in [a] PET, [b] PET/CT, and [c] CT) that was missed at multiparametric MRI review due to obscuring susceptibility artifacts from surgical clips at (d) T2-weighted MRI, (e) b = 2000 sec/mm2 diffusion-weighted MRI, (f) apparent diffusion coefficient map, and (g) dynamic contrast material–enhanced MRI.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/568c/7457947/51eeccf851a7/radiol.2020192018.fig2d.jpg)
Images show 2-(3-{1-carboxy-5-[(6-[18F]fluoro-pyridine 3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid (18F-DCFPyL) PET/CT–positive, multiparametric MRI–negative, and biopsy-positive lesion in a 65-year-old man (prostate-specific antigen level of 1.85 ng/mL) with history of prostate cancer and prior radical prostatectomy, now with biochemically recurrent prostate cancer. 18F-DCFPyL depicted pathologically proven metastatic lesion in right hemipelvis (arrow in [a] PET, [b] PET/CT, and [c] CT) that was missed at multiparametric MRI review due to obscuring susceptibility artifacts from surgical clips at (d) T2-weighted MRI, (e) b = 2000 sec/mm2 diffusion-weighted MRI, (f) apparent diffusion coefficient map, and (g) dynamic contrast material–enhanced MRI.
Figure 2e:
![Images show 2-(3-{1-carboxy-5-[(6-[18F]fluoro-pyridine 3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid (18F-DCFPyL) PET/CT–positive, multiparametric MRI–negative, and biopsy-positive lesion in a 65-year-old man (prostate-specific antigen level of 1.85 ng/mL) with history of prostate cancer and prior radical prostatectomy, now with biochemically recurrent prostate cancer. 18F-DCFPyL depicted pathologically proven metastatic lesion in right hemipelvis (arrow in [a] PET, [b] PET/CT, and [c] CT) that was missed at multiparametric MRI review due to obscuring susceptibility artifacts from surgical clips at (d) T2-weighted MRI, (e) b = 2000 sec/mm2 diffusion-weighted MRI, (f) apparent diffusion coefficient map, and (g) dynamic contrast material–enhanced MRI.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/568c/7457947/5c783205f90d/radiol.2020192018.fig2e.jpg)
Images show 2-(3-{1-carboxy-5-[(6-[18F]fluoro-pyridine 3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid (18F-DCFPyL) PET/CT–positive, multiparametric MRI–negative, and biopsy-positive lesion in a 65-year-old man (prostate-specific antigen level of 1.85 ng/mL) with history of prostate cancer and prior radical prostatectomy, now with biochemically recurrent prostate cancer. 18F-DCFPyL depicted pathologically proven metastatic lesion in right hemipelvis (arrow in [a] PET, [b] PET/CT, and [c] CT) that was missed at multiparametric MRI review due to obscuring susceptibility artifacts from surgical clips at (d) T2-weighted MRI, (e) b = 2000 sec/mm2 diffusion-weighted MRI, (f) apparent diffusion coefficient map, and (g) dynamic contrast material–enhanced MRI.
Figure 2f:
![Images show 2-(3-{1-carboxy-5-[(6-[18F]fluoro-pyridine 3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid (18F-DCFPyL) PET/CT–positive, multiparametric MRI–negative, and biopsy-positive lesion in a 65-year-old man (prostate-specific antigen level of 1.85 ng/mL) with history of prostate cancer and prior radical prostatectomy, now with biochemically recurrent prostate cancer. 18F-DCFPyL depicted pathologically proven metastatic lesion in right hemipelvis (arrow in [a] PET, [b] PET/CT, and [c] CT) that was missed at multiparametric MRI review due to obscuring susceptibility artifacts from surgical clips at (d) T2-weighted MRI, (e) b = 2000 sec/mm2 diffusion-weighted MRI, (f) apparent diffusion coefficient map, and (g) dynamic contrast material–enhanced MRI.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/568c/7457947/bf4146bea914/radiol.2020192018.fig2f.jpg)
Images show 2-(3-{1-carboxy-5-[(6-[18F]fluoro-pyridine 3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid (18F-DCFPyL) PET/CT–positive, multiparametric MRI–negative, and biopsy-positive lesion in a 65-year-old man (prostate-specific antigen level of 1.85 ng/mL) with history of prostate cancer and prior radical prostatectomy, now with biochemically recurrent prostate cancer. 18F-DCFPyL depicted pathologically proven metastatic lesion in right hemipelvis (arrow in [a] PET, [b] PET/CT, and [c] CT) that was missed at multiparametric MRI review due to obscuring susceptibility artifacts from surgical clips at (d) T2-weighted MRI, (e) b = 2000 sec/mm2 diffusion-weighted MRI, (f) apparent diffusion coefficient map, and (g) dynamic contrast material–enhanced MRI.
Figure 2g:
![Images show 2-(3-{1-carboxy-5-[(6-[18F]fluoro-pyridine 3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid (18F-DCFPyL) PET/CT–positive, multiparametric MRI–negative, and biopsy-positive lesion in a 65-year-old man (prostate-specific antigen level of 1.85 ng/mL) with history of prostate cancer and prior radical prostatectomy, now with biochemically recurrent prostate cancer. 18F-DCFPyL depicted pathologically proven metastatic lesion in right hemipelvis (arrow in [a] PET, [b] PET/CT, and [c] CT) that was missed at multiparametric MRI review due to obscuring susceptibility artifacts from surgical clips at (d) T2-weighted MRI, (e) b = 2000 sec/mm2 diffusion-weighted MRI, (f) apparent diffusion coefficient map, and (g) dynamic contrast material–enhanced MRI.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/568c/7457947/be65cdb3a77f/radiol.2020192018.fig2g.jpg)
Images show 2-(3-{1-carboxy-5-[(6-[18F]fluoro-pyridine 3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid (18F-DCFPyL) PET/CT–positive, multiparametric MRI–negative, and biopsy-positive lesion in a 65-year-old man (prostate-specific antigen level of 1.85 ng/mL) with history of prostate cancer and prior radical prostatectomy, now with biochemically recurrent prostate cancer. 18F-DCFPyL depicted pathologically proven metastatic lesion in right hemipelvis (arrow in [a] PET, [b] PET/CT, and [c] CT) that was missed at multiparametric MRI review due to obscuring susceptibility artifacts from surgical clips at (d) T2-weighted MRI, (e) b = 2000 sec/mm2 diffusion-weighted MRI, (f) apparent diffusion coefficient map, and (g) dynamic contrast material–enhanced MRI.
Figure 3a:
![Images show 2-(3-{1-carboxy-5-[(6-[18F]fluoro-pyridine 3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid (18F-DCFPyL) PET/CT–negative, multiparametric MRI–positive, and biopsy-positive lesion in a 67-year-old man (prostate-specific antigen level of 0.42 ng/mL) with history of prostate cancer and prior prostatectomy, now with biochemically recurrent prostate cancer. 18F-DCFPyL images ([a] PET and [b] PET/CT) did not show focal findings. (c) Axial T2-weighted MRI, (d) b = 2000 sec/mm2 diffusion-weighted MRI, (e) apparent diffusion coefficient map, and (f) dynamic contrast material–enhanced MRI show grade 5 (moderate-high likelihood for recurrent prostate cancer) left-sided focal abnormality at prostatectomy bed, which was biopsy proven as recurrent prostate cancer.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/568c/7457947/369c72c02170/radiol.2020192018.fig3a.jpg)
Images show 2-(3-{1-carboxy-5-[(6-[18F]fluoro-pyridine 3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid (18F-DCFPyL) PET/CT–negative, multiparametric MRI–positive, and biopsy-positive lesion in a 67-year-old man (prostate-specific antigen level of 0.42 ng/mL) with history of prostate cancer and prior prostatectomy, now with biochemically recurrent prostate cancer. 18F-DCFPyL images ([a] PET and [b] PET/CT) did not show focal findings. (c) Axial T2-weighted MRI, (d) b = 2000 sec/mm2 diffusion-weighted MRI, (e) apparent diffusion coefficient map, and (f) dynamic contrast material–enhanced MRI show grade 5 (moderate-high likelihood for recurrent prostate cancer) left-sided focal abnormality at prostatectomy bed, which was biopsy proven as recurrent prostate cancer.
Figure 3b:
![Images show 2-(3-{1-carboxy-5-[(6-[18F]fluoro-pyridine 3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid (18F-DCFPyL) PET/CT–negative, multiparametric MRI–positive, and biopsy-positive lesion in a 67-year-old man (prostate-specific antigen level of 0.42 ng/mL) with history of prostate cancer and prior prostatectomy, now with biochemically recurrent prostate cancer. 18F-DCFPyL images ([a] PET and [b] PET/CT) did not show focal findings. (c) Axial T2-weighted MRI, (d) b = 2000 sec/mm2 diffusion-weighted MRI, (e) apparent diffusion coefficient map, and (f) dynamic contrast material–enhanced MRI show grade 5 (moderate-high likelihood for recurrent prostate cancer) left-sided focal abnormality at prostatectomy bed, which was biopsy proven as recurrent prostate cancer.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/568c/7457947/3dfeb2a262a5/radiol.2020192018.fig3b.jpg)
Images show 2-(3-{1-carboxy-5-[(6-[18F]fluoro-pyridine 3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid (18F-DCFPyL) PET/CT–negative, multiparametric MRI–positive, and biopsy-positive lesion in a 67-year-old man (prostate-specific antigen level of 0.42 ng/mL) with history of prostate cancer and prior prostatectomy, now with biochemically recurrent prostate cancer. 18F-DCFPyL images ([a] PET and [b] PET/CT) did not show focal findings. (c) Axial T2-weighted MRI, (d) b = 2000 sec/mm2 diffusion-weighted MRI, (e) apparent diffusion coefficient map, and (f) dynamic contrast material–enhanced MRI show grade 5 (moderate-high likelihood for recurrent prostate cancer) left-sided focal abnormality at prostatectomy bed, which was biopsy proven as recurrent prostate cancer.
Figure 3c:
![Images show 2-(3-{1-carboxy-5-[(6-[18F]fluoro-pyridine 3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid (18F-DCFPyL) PET/CT–negative, multiparametric MRI–positive, and biopsy-positive lesion in a 67-year-old man (prostate-specific antigen level of 0.42 ng/mL) with history of prostate cancer and prior prostatectomy, now with biochemically recurrent prostate cancer. 18F-DCFPyL images ([a] PET and [b] PET/CT) did not show focal findings. (c) Axial T2-weighted MRI, (d) b = 2000 sec/mm2 diffusion-weighted MRI, (e) apparent diffusion coefficient map, and (f) dynamic contrast material–enhanced MRI show grade 5 (moderate-high likelihood for recurrent prostate cancer) left-sided focal abnormality at prostatectomy bed, which was biopsy proven as recurrent prostate cancer.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/568c/7457947/690847c1d70f/radiol.2020192018.fig3c.jpg)
Images show 2-(3-{1-carboxy-5-[(6-[18F]fluoro-pyridine 3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid (18F-DCFPyL) PET/CT–negative, multiparametric MRI–positive, and biopsy-positive lesion in a 67-year-old man (prostate-specific antigen level of 0.42 ng/mL) with history of prostate cancer and prior prostatectomy, now with biochemically recurrent prostate cancer. 18F-DCFPyL images ([a] PET and [b] PET/CT) did not show focal findings. (c) Axial T2-weighted MRI, (d) b = 2000 sec/mm2 diffusion-weighted MRI, (e) apparent diffusion coefficient map, and (f) dynamic contrast material–enhanced MRI show grade 5 (moderate-high likelihood for recurrent prostate cancer) left-sided focal abnormality at prostatectomy bed, which was biopsy proven as recurrent prostate cancer.
Figure 3d:
![Images show 2-(3-{1-carboxy-5-[(6-[18F]fluoro-pyridine 3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid (18F-DCFPyL) PET/CT–negative, multiparametric MRI–positive, and biopsy-positive lesion in a 67-year-old man (prostate-specific antigen level of 0.42 ng/mL) with history of prostate cancer and prior prostatectomy, now with biochemically recurrent prostate cancer. 18F-DCFPyL images ([a] PET and [b] PET/CT) did not show focal findings. (c) Axial T2-weighted MRI, (d) b = 2000 sec/mm2 diffusion-weighted MRI, (e) apparent diffusion coefficient map, and (f) dynamic contrast material–enhanced MRI show grade 5 (moderate-high likelihood for recurrent prostate cancer) left-sided focal abnormality at prostatectomy bed, which was biopsy proven as recurrent prostate cancer.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/568c/7457947/596ff8cee99f/radiol.2020192018.fig3d.jpg)
Images show 2-(3-{1-carboxy-5-[(6-[18F]fluoro-pyridine 3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid (18F-DCFPyL) PET/CT–negative, multiparametric MRI–positive, and biopsy-positive lesion in a 67-year-old man (prostate-specific antigen level of 0.42 ng/mL) with history of prostate cancer and prior prostatectomy, now with biochemically recurrent prostate cancer. 18F-DCFPyL images ([a] PET and [b] PET/CT) did not show focal findings. (c) Axial T2-weighted MRI, (d) b = 2000 sec/mm2 diffusion-weighted MRI, (e) apparent diffusion coefficient map, and (f) dynamic contrast material–enhanced MRI show grade 5 (moderate-high likelihood for recurrent prostate cancer) left-sided focal abnormality at prostatectomy bed, which was biopsy proven as recurrent prostate cancer.
Figure 3e:
![Images show 2-(3-{1-carboxy-5-[(6-[18F]fluoro-pyridine 3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid (18F-DCFPyL) PET/CT–negative, multiparametric MRI–positive, and biopsy-positive lesion in a 67-year-old man (prostate-specific antigen level of 0.42 ng/mL) with history of prostate cancer and prior prostatectomy, now with biochemically recurrent prostate cancer. 18F-DCFPyL images ([a] PET and [b] PET/CT) did not show focal findings. (c) Axial T2-weighted MRI, (d) b = 2000 sec/mm2 diffusion-weighted MRI, (e) apparent diffusion coefficient map, and (f) dynamic contrast material–enhanced MRI show grade 5 (moderate-high likelihood for recurrent prostate cancer) left-sided focal abnormality at prostatectomy bed, which was biopsy proven as recurrent prostate cancer.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/568c/7457947/45910c140a0e/radiol.2020192018.fig3e.jpg)
Images show 2-(3-{1-carboxy-5-[(6-[18F]fluoro-pyridine 3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid (18F-DCFPyL) PET/CT–negative, multiparametric MRI–positive, and biopsy-positive lesion in a 67-year-old man (prostate-specific antigen level of 0.42 ng/mL) with history of prostate cancer and prior prostatectomy, now with biochemically recurrent prostate cancer. 18F-DCFPyL images ([a] PET and [b] PET/CT) did not show focal findings. (c) Axial T2-weighted MRI, (d) b = 2000 sec/mm2 diffusion-weighted MRI, (e) apparent diffusion coefficient map, and (f) dynamic contrast material–enhanced MRI show grade 5 (moderate-high likelihood for recurrent prostate cancer) left-sided focal abnormality at prostatectomy bed, which was biopsy proven as recurrent prostate cancer.
Figure 3f:
![Images show 2-(3-{1-carboxy-5-[(6-[18F]fluoro-pyridine 3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid (18F-DCFPyL) PET/CT–negative, multiparametric MRI–positive, and biopsy-positive lesion in a 67-year-old man (prostate-specific antigen level of 0.42 ng/mL) with history of prostate cancer and prior prostatectomy, now with biochemically recurrent prostate cancer. 18F-DCFPyL images ([a] PET and [b] PET/CT) did not show focal findings. (c) Axial T2-weighted MRI, (d) b = 2000 sec/mm2 diffusion-weighted MRI, (e) apparent diffusion coefficient map, and (f) dynamic contrast material–enhanced MRI show grade 5 (moderate-high likelihood for recurrent prostate cancer) left-sided focal abnormality at prostatectomy bed, which was biopsy proven as recurrent prostate cancer.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/568c/7457947/700e99ad49cd/radiol.2020192018.fig3f.jpg)
Images show 2-(3-{1-carboxy-5-[(6-[18F]fluoro-pyridine 3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid (18F-DCFPyL) PET/CT–negative, multiparametric MRI–positive, and biopsy-positive lesion in a 67-year-old man (prostate-specific antigen level of 0.42 ng/mL) with history of prostate cancer and prior prostatectomy, now with biochemically recurrent prostate cancer. 18F-DCFPyL images ([a] PET and [b] PET/CT) did not show focal findings. (c) Axial T2-weighted MRI, (d) b = 2000 sec/mm2 diffusion-weighted MRI, (e) apparent diffusion coefficient map, and (f) dynamic contrast material–enhanced MRI show grade 5 (moderate-high likelihood for recurrent prostate cancer) left-sided focal abnormality at prostatectomy bed, which was biopsy proven as recurrent prostate cancer.
Figure 4b:
![Images show 2-(3-(5)-ureido)-pentanedioic acid (18F-DCFPyL) PET/CT–positive, multiparametric MRI–positive, and biopsy-positive lesion in a 62-year-old man (prostate-specific antigen level of 1.48 ng/mL) with history of prostate cancer, treated with external beam radiation therapy, now with biochemically recurrent prostate cancer. Histologically proven lesion in left midbase depicted with 18F-DCFPyL (arrows) ([a] PET and [b] PET/CT) and multiparametric MRI ([c] T2-weighted MRI, [d] b = 2000 sec/mm2 diffusion-weighted MRI, [e] apparent diffusion coefficient map, [f] dynamic contrast material–enhanced MRI) show grade 6 (high likelihood for recurrent prostate cancer).](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/568c/7457947/bb948e03e916/radiol.2020192018.fig4b.jpg)
Images show 2-(3-(5)-ureido)-pentanedioic acid (18F-DCFPyL) PET/CT–positive, multiparametric MRI–positive, and biopsy-positive lesion in a 62-year-old man (prostate-specific antigen level of 1.48 ng/mL) with history of prostate cancer, treated with external beam radiation therapy, now with biochemically recurrent prostate cancer. Histologically proven lesion in left midbase depicted with 18F-DCFPyL (arrows) ([a] PET and [b] PET/CT) and multiparametric MRI ([c] T2-weighted MRI, [d] b = 2000 sec/mm2 diffusion-weighted MRI, [e] apparent diffusion coefficient map, [f] dynamic contrast material–enhanced MRI) show grade 6 (high likelihood for recurrent prostate cancer).
Figure 4c:
![Images show 2-(3-(5)-ureido)-pentanedioic acid (18F-DCFPyL) PET/CT–positive, multiparametric MRI–positive, and biopsy-positive lesion in a 62-year-old man (prostate-specific antigen level of 1.48 ng/mL) with history of prostate cancer, treated with external beam radiation therapy, now with biochemically recurrent prostate cancer. Histologically proven lesion in left midbase depicted with 18F-DCFPyL (arrows) ([a] PET and [b] PET/CT) and multiparametric MRI ([c] T2-weighted MRI, [d] b = 2000 sec/mm2 diffusion-weighted MRI, [e] apparent diffusion coefficient map, [f] dynamic contrast material–enhanced MRI) show grade 6 (high likelihood for recurrent prostate cancer).](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/568c/7457947/320a4f5a5f2c/radiol.2020192018.fig4c.jpg)
Images show 2-(3-(5)-ureido)-pentanedioic acid (18F-DCFPyL) PET/CT–positive, multiparametric MRI–positive, and biopsy-positive lesion in a 62-year-old man (prostate-specific antigen level of 1.48 ng/mL) with history of prostate cancer, treated with external beam radiation therapy, now with biochemically recurrent prostate cancer. Histologically proven lesion in left midbase depicted with 18F-DCFPyL (arrows) ([a] PET and [b] PET/CT) and multiparametric MRI ([c] T2-weighted MRI, [d] b = 2000 sec/mm2 diffusion-weighted MRI, [e] apparent diffusion coefficient map, [f] dynamic contrast material–enhanced MRI) show grade 6 (high likelihood for recurrent prostate cancer).
Figure 4d:
![Images show 2-(3-(5)-ureido)-pentanedioic acid (18F-DCFPyL) PET/CT–positive, multiparametric MRI–positive, and biopsy-positive lesion in a 62-year-old man (prostate-specific antigen level of 1.48 ng/mL) with history of prostate cancer, treated with external beam radiation therapy, now with biochemically recurrent prostate cancer. Histologically proven lesion in left midbase depicted with 18F-DCFPyL (arrows) ([a] PET and [b] PET/CT) and multiparametric MRI ([c] T2-weighted MRI, [d] b = 2000 sec/mm2 diffusion-weighted MRI, [e] apparent diffusion coefficient map, [f] dynamic contrast material–enhanced MRI) show grade 6 (high likelihood for recurrent prostate cancer).](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/568c/7457947/4b69fd33e154/radiol.2020192018.fig4d.jpg)
Images show 2-(3-(5)-ureido)-pentanedioic acid (18F-DCFPyL) PET/CT–positive, multiparametric MRI–positive, and biopsy-positive lesion in a 62-year-old man (prostate-specific antigen level of 1.48 ng/mL) with history of prostate cancer, treated with external beam radiation therapy, now with biochemically recurrent prostate cancer. Histologically proven lesion in left midbase depicted with 18F-DCFPyL (arrows) ([a] PET and [b] PET/CT) and multiparametric MRI ([c] T2-weighted MRI, [d] b = 2000 sec/mm2 diffusion-weighted MRI, [e] apparent diffusion coefficient map, [f] dynamic contrast material–enhanced MRI) show grade 6 (high likelihood for recurrent prostate cancer).
Figure 4e:
![Images show 2-(3-(5)-ureido)-pentanedioic acid (18F-DCFPyL) PET/CT–positive, multiparametric MRI–positive, and biopsy-positive lesion in a 62-year-old man (prostate-specific antigen level of 1.48 ng/mL) with history of prostate cancer, treated with external beam radiation therapy, now with biochemically recurrent prostate cancer. Histologically proven lesion in left midbase depicted with 18F-DCFPyL (arrows) ([a] PET and [b] PET/CT) and multiparametric MRI ([c] T2-weighted MRI, [d] b = 2000 sec/mm2 diffusion-weighted MRI, [e] apparent diffusion coefficient map, [f] dynamic contrast material–enhanced MRI) show grade 6 (high likelihood for recurrent prostate cancer).](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/568c/7457947/2c98f78eb378/radiol.2020192018.fig4e.jpg)
Images show 2-(3-(5)-ureido)-pentanedioic acid (18F-DCFPyL) PET/CT–positive, multiparametric MRI–positive, and biopsy-positive lesion in a 62-year-old man (prostate-specific antigen level of 1.48 ng/mL) with history of prostate cancer, treated with external beam radiation therapy, now with biochemically recurrent prostate cancer. Histologically proven lesion in left midbase depicted with 18F-DCFPyL (arrows) ([a] PET and [b] PET/CT) and multiparametric MRI ([c] T2-weighted MRI, [d] b = 2000 sec/mm2 diffusion-weighted MRI, [e] apparent diffusion coefficient map, [f] dynamic contrast material–enhanced MRI) show grade 6 (high likelihood for recurrent prostate cancer).
Figure 4f:
![Images show 2-(3-(5)-ureido)-pentanedioic acid (18F-DCFPyL) PET/CT–positive, multiparametric MRI–positive, and biopsy-positive lesion in a 62-year-old man (prostate-specific antigen level of 1.48 ng/mL) with history of prostate cancer, treated with external beam radiation therapy, now with biochemically recurrent prostate cancer. Histologically proven lesion in left midbase depicted with 18F-DCFPyL (arrows) ([a] PET and [b] PET/CT) and multiparametric MRI ([c] T2-weighted MRI, [d] b = 2000 sec/mm2 diffusion-weighted MRI, [e] apparent diffusion coefficient map, [f] dynamic contrast material–enhanced MRI) show grade 6 (high likelihood for recurrent prostate cancer).](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/568c/7457947/635bd8cb6d85/radiol.2020192018.fig4f.jpg)
Images show 2-(3-(5)-ureido)-pentanedioic acid (18F-DCFPyL) PET/CT–positive, multiparametric MRI–positive, and biopsy-positive lesion in a 62-year-old man (prostate-specific antigen level of 1.48 ng/mL) with history of prostate cancer, treated with external beam radiation therapy, now with biochemically recurrent prostate cancer. Histologically proven lesion in left midbase depicted with 18F-DCFPyL (arrows) ([a] PET and [b] PET/CT) and multiparametric MRI ([c] T2-weighted MRI, [d] b = 2000 sec/mm2 diffusion-weighted MRI, [e] apparent diffusion coefficient map, [f] dynamic contrast material–enhanced MRI) show grade 6 (high likelihood for recurrent prostate cancer).
Comparison of 18F-DCFPyL PET and MRI in All Depicted Lesions
A total of 323 lesions were visualized with either 18F-DCFPyL or MRI in 77 men with 228 visualized only with 18F-DCFPyL, 54 visualized only with MRI, and 41 visualized by using both. Of the 228 lesions only visualized with 18F-DCFPyL, 41 were outside the field of view at MRI and were excluded for the comparative analysis. Lesions were noted in pelvic lymph nodes (42%, 137 of 323), bone metastases (8%, 25 of 323), nonpelvic lymph nodes or soft tissue (27%, 88 of 323), and in the prostate bed (23%, 73 of 323). 18F-DCFPyL depicted more pelvic lymph nodes than did MRI (128 vs 23 nodes). Most lesions visualized at MRI were in the prostate bed (67%, 36 of 54). There were 26 additional lesions that were equivocal or indeterminate after consensus and were excluded from analysis.
For all depicted lesions within the field of view, the concordance rate for 18F-DCFPyL and MRI was 25% (82 of 323) (Table 4). Within the prostate bed, 13 lesions were reported as very low or low grade, 15 were low-moderate or moderate grade, and seven were moderate-high or high grade. One lesion was not graded by using MRI because of an earlier positive result at biopsy. As MRI grade increased, the number of lesions similarly depicted by using 18F-DCFPyL increased as well (P < .001), with a higher concordance rate of 53% (52 of 99) for 26 lesions in the prostate bed. Agreement was seen mainly in the prostate bed while discordance was more likely in pelvic lymph nodes and metastases.
Table 4:
Detected Lesion Concordance for 18F-DCFPyL and MRI
Discussion
Localization of recurrent prostate cancer at its earliest stage is preferred for optimal patient treatment. Multiparametric MRI has already been successfully used in this setting because it provides both anatomic and functional information (17). Prostate-specific membrane antigen (PSMA) PET tracers, however, have been documented to have unprecedented sensitivity for detecting small amounts of residual disease and may have improved specificity since they are based on a ligand-receptor interaction (18). Nevertheless, few publications have validated PSMA PET tracers in the setting of biochemically recurrent prostate cancer. In this study, specificity and positive predictive value (PPV) were high for 2-(3-{1-carboxy-5-[(6-[18F]fluoro-pyridine 3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid (18F-DCFPyL) PET/CT in this population. Sensitivity was lower likely due to inadequate sampling. When comparing 18F-DCFPyL PET/CT to multiparametric MRI, the assessment of local recurrence may be more comparable than the assessment of lymph nodes as there was a selection bias (only larger nodes could be biopsied) in favor of MRI. When comparing the two modalities in the prostate bed, 18F-DCFPyL had higher specificity (86%) than did multiparametric MRI (52%) (P = .02). Notably, 18F-DCFPyL imaging added benefit to multiparametric MRI with a combined PPV of 88% overall.
When comparing all lesions detected (vs only those validated histologically), this study demonstrated MRI concordance of 53% (52 of 99) in the prostate bed but only 25% (82 of 323) agreement when all lesions (particularly lymph nodes) in the field of view were evaluated. 18F-DCFPyL depicted more lymph nodes than did MRI. Highly suspicious MRI lesions were more apt to show concordance with 18F-DCFPyL findings.
When evaluating just the prostate bed, multiparametric MRI identified more lesions than did 18F-DCFPyL (62 vs 37), but many of them proved to be false-positive findings. This result is different than results of studies obtained with gallium 68 (68Ga) PSMA, which depicted significantly more lesions at PET than were detected with multiparametric MRI (19–21). However, multiparametric MRI in our study was more focused on the prostate bed compared with previous studies and included use of a combination of endorectal and surface coils, which provides better spatial resolution. In contrast, nonendorectal coil image acquisition was more commonly used in prior reports.
A strength of our investigation was the use of histopathologic confirmation. Many PSMA PET studies do not include tissue validation especially in the recurrence setting. However, it is important to distinguish between the prostate bed and pelvic nodal recurrence. Prostate bed results for multiparametric MRI revealed sensitivity of 83% (19 of 23) and PPV of 66% (19 of 29), which aligns with a sensitivity and PPV of 88% and 62%, respectively, reported for MRI in previous publications (22). Lawhn-Heath et al (23) studied 68Ga-PSMA-11 with MRI by using a hybrid PET/MR scanner and showed a similar sensitivity (89%) and PPV (91%) but a low specificity (31%), whereas the specificity of 18F-DCFPyL in this study was 91% (40 of 44). This difference may relate to small sample size. Based on the good results with histologic correlation, we believe that the specificity and PPV of 18F-DCFPyL are high and useful features of this agent.
Importantly, we found that 18F-DCFPyL boosted PPV by 38% when added to multiparametric MRI. Other researchers have highlighted the benefits of both modalities in identifying early prostate cancer recurrence, especially in the prostate bed. Metser et al (11) demonstrated that more local lesions were depicted with combined 68Ga PSMA-11 PET/CT and pelvic multiparametric MRI than with either modality alone, but this result was limited to local recurrence, in keeping with our findings. Similarly, Freitag et al (24) found pelvic multiparametric MRI was beneficial in biochemically recurrent prostate cancer evaluations with 68Ga-PSMA-11-PET/CT after prostatectomy. Studies with 68Ga PSMA-11 using a hybrid PET/MRI scanner also point to the advantage of combined modalities over separate scans and that many lesions at MRI would not have been recognized as disease without PSMA avidity (25–27). Although most of these studies lack histologic validation, this data, with its histologic correlation, confirms these findings. Moreover, our analysis indicates that the addition of 18F-DCFPyL to pelvic multiparametric MRI greatly improves PPV.
Our study had several limitations. Our sample of participants and histopathologic findings remains modest. Additionally, directly mapping histologic reports to MRI and PET was not always possible and required some approximation. Biopsies, by their nature, suffer from sampling error and the reported differences in sensitivity undoubtedly underestimates the true detection capabilities of 18F-DCFPyL PET/CT. There is a selection bias favoring the descriptive statistics of MRI in lymph nodes because only larger and easier-to-depict nodes could be targeted for biopsy. This bias is overcome when all lesions are considered and 18F-DCFPyL PET/CT shows its advantage. However, because of the small numbers of histologic confirmation, statistical significance could not be reached in much of the calculations.
In conclusion, 2-(3-{1-carboxy-5-[(6-[18F]fluoro-pyridine 3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid (18F-DCFPyL) PET/CT demonstrates high specificity and positive predictive value for detecting recurrent prostate cancer when validated with biopsy. 18F-DCFPyL greatly improves the performance of MRI for local recurrence. It also had higher detectability than did MRI for pelvic lymph nodes.
APPENDIX
Acknowledgments
Acknowledgments
The authors would like to thank Gary Griffiths, PhD, for his invaluable counsel on the manuscript and Juanita Weaver, BS, and Mirna Martinez for their assistance with this trial.
Supported by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research (ZIA BC 010655). Supported in part by the National Cancer Institute (HHSN261200800001E). The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
Disclosures of Conflicts of Interest: L.L. disclosed no relevant relationships. E.M. disclosed no relevant relationships. B.T. disclosed no relevant relationships. J.H.S. disclosed no relevant relationships. S.E.R. disclosed no relevant relationships. S.A.H. disclosed no relevant relationships. I.L. disclosed no relevant relationships. F.L. disclosed no relevant relationships. A.T. disclosed no relevant relationships. Y.L.M. disclosed no relevant relationships. P.E. disclosed no relevant relationships. D.E.C. disclosed no relevant relationships. W.D. disclosed no relevant relationships. R.M. disclosed no relevant relationships. B.J.W. Activities related to the present article: institution has a cooperative research and development agreement (CRADA) with Philips and the National Institutes of Health (NIH) that has a travel clause, allowing for CRADA-related travel (author is principal investigator). Activities not related to the present article: has patents (planned, pending, or issued) with and receives royalties from Philips, the U.S. government, and NIH. Other relationships: disclosed no relevant relationships. V.K. disclosed no relevant relationships. R.C. disclosed no relevant relationships. E.L. disclosed no relevant relationships. P.P. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: has patents (planned, pending, or issued) with Philips and NIH; receives royalties from Philips. Other relationships: disclosed no relevant relationships. J.F.E. disclosed no relevant relationships. P.L.C. disclosed no relevant relationships.
Abbreviations:
- CI
- confidence interval
- PPV
- positive predictive value
- PSMA
- prostate-specific membrane antigen
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68(6):394–424. [DOI] [PubMed] [Google Scholar]
- 2.Babaian RJ, Troncoso P, Bhadkamkar VA, Johnston DA. Analysis of clinicopathologic factors predicting outcome after radical prostatectomy. Cancer 2001;91(8):1414–1422. [PubMed] [Google Scholar]
- 3.Hoskin P, Sartor O, O’Sullivan JM, et al. Efficacy and safety of radium-223 dichloride in patients with castration-resistant prostate cancer and symptomatic bone metastases, with or without previous docetaxel use: a prespecified subgroup analysis from the randomised, double-blind, phase 3 ALSYMPCA trial. Lancet Oncol 2014;15(12):1397–1406. [DOI] [PubMed] [Google Scholar]
- 4.Szabo Z, Mena E, Rowe SP, et al. Initial Evaluation of [(18)F]DCFPyL for Prostate-Specific Membrane Antigen (PSMA)-Targeted PET Imaging of Prostate Cancer. Mol Imaging Biol 2015;17(4):565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y, Pullambhatla M, Foss CA, et al. 2-(3-{1-Carboxy-5-[(6-[18F]fluoro-pyridine-3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic Acid, [18F]DCFPyL, a PSMA-based PET Imaging Agent for Prostate Cancer. Clin Cancer Res 2011;17(24):7645–7653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gorin MA, Pienta KJ, Siegel BA, et al. A prospective phase 2/3 multi-center study of 18F-DCFPyL PET/CT imaging in patients with prostate cancer; examination of diagnostic accuracy (OSPREY). J Urol 2019;201(Suppl 4):e1100-e. [Google Scholar]
- 7.Gaur S, Turkbey B. Prostate MR Imaging for Posttreatment Evaluation and Recurrence. Radiol Clin North Am 2018;56(2):263–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.An JY, Sidana A, Choyke PL, Wood BJ, Pinto PA, Türkbey IB. Multiparametric Magnetic Resonance Imaging for Active Surveillance of Prostate Cancer. Balkan Med J 2017;34(5):388–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abd-Alazeez M, Ramachandran N, Dikaios N, et al. Multiparametric MRI for detection of radiorecurrent prostate cancer: added value of apparent diffusion coefficient maps and dynamic contrast-enhanced images. Prostate Cancer Prostatic Dis 2015;18(2):128–136. [DOI] [PubMed] [Google Scholar]
- 10.Turkbey B, Choyke PL. Future Perspectives and Challenges of Prostate MR Imaging. Radiol Clin North Am 2018;56(2):327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Metser U, Chua S, Ho B, et al. The Contribution of Multiparametric Pelvic and Whole-Body MRI to Interpretation of 18F-Fluoromethylcholine or 68Ga-HBED-CC PSMA-11 PET/CT in Patients with Biochemical Failure After Radical Prostatectomy. J Nucl Med 2019;60(9):1253–1258. [DOI] [PubMed] [Google Scholar]
- 12.Mena E, Lindenberg ML, Turkbey IB, et al. 18F-DCFPyL PET/CT Imaging in Patients with Biochemical Recurrence Prostate Cancer after Primary Local Therapy. J Nucl Med 2019 Nov 1 [Epub ahead of print]. [Google Scholar]
- 13.Muller BG, Kaushal A, Sankineni S, et al. Multiparametric magnetic resonance imaging-transrectal ultrasound fusion-assisted biopsy for the diagnosis of local recurrence after radical prostatectomy. Urol Oncol 2015;33(10):425.e1–425.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fleiss J. Statistical methods for rates and proportions. 2nd ed. New York, NY: Wiley, 1981. [Google Scholar]
- 15.Shih JH, Fay MP. Pearson’s chi-square test and rank correlation inferences for clustered data. Biometrics 2017;73(3):822–834. [DOI] [PubMed] [Google Scholar]
- 16.R Core Team. R : A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing, 2019. https://www.R-project.org/. [Google Scholar]
- 17.Wu LM, Xu JR, Gu HY, et al. Role of magnetic resonance imaging in the detection of local prostate cancer recurrence after external beam radiotherapy and radical prostatectomy. Clin Oncol (R Coll Radiol) 2013;25(4):252–264. [DOI] [PubMed] [Google Scholar]
- 18.Rauscher I, Maurer T, Beer AJ, et al. Value of 68Ga-PSMA HBED-CC PET for the Assessment of Lymph Node Metastases in Prostate Cancer Patients with Biochemical Recurrence: Comparison with Histopathology After Salvage Lymphadenectomy. J Nucl Med 2016;57(11):1713–1719. [DOI] [PubMed] [Google Scholar]
- 19.Sawicki LM, Kirchner J, Buddensieck C, et al. Prospective comparison of whole-body MRI and 68Ga-PSMA PET/CT for the detection of biochemical recurrence of prostate cancer after radical prostatectomy. Eur J Nucl Med Mol Imaging 2019;46(7):1542–1550. [DOI] [PubMed] [Google Scholar]
- 20.Emmett L, van Leeuwen PJ, Nandurkar R, et al. Treatment Outcomes from 68Ga-PSMA PET/CT-Informed Salvage Radiation Treatment in Men with Rising PSA After Radical Prostatectomy: Prognostic Value of a Negative PSMA PET. J Nucl Med 2017;58(12):1972–1976. [DOI] [PubMed] [Google Scholar]
- 21.Afshar-Oromieh A, Vollnberg B, Alberts I, et al. Comparison of PSMA-ligand PET/CT and multiparametric MRI for the detection of recurrent prostate cancer in the pelvis. Eur J Nucl Med Mol Imaging 2019;46(11):2289–2297. [DOI] [PubMed] [Google Scholar]
- 22.Valle LF, Greer MD, Shih JH, et al. Multiparametric MRI for the detection of local recurrence of prostate cancer in the setting of biochemical recurrence after low dose rate brachytherapy. Diagn Interv Radiol 2018;24(1):46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lawhn-Heath C, Flavell RR, Behr SC, et al. Single-Center Prospective Evaluation of 68Ga-PSMA-11 PET in Biochemical Recurrence of Prostate Cancer. AJR Am J Roentgenol 2019;213(2):266–274. [DOI] [PubMed] [Google Scholar]
- 24.Freitag MT, Radtke JP, Afshar-Oromieh A, et al. Local recurrence of prostate cancer after radical prostatectomy is at risk to be missed in 68Ga-PSMA-11-PET of PET/CT and PET/MRI: comparison with mpMRI integrated in simultaneous PET/MRI. Eur J Nucl Med Mol Imaging 2017;44(5):776–787. [DOI] [PubMed] [Google Scholar]
- 25.Kranzbühler B, Nagel H, Becker AS, et al. Clinical performance of 68Ga-PSMA-11 PET/MRI for the detection of recurrent prostate cancer following radical prostatectomy. Eur J Nucl Med Mol Imaging 2018;45(1):20–30. [DOI] [PubMed] [Google Scholar]
- 26.Grubmüller B, Baltzer P, D’Andrea D, et al. 68Ga-PSMA 11 ligand PET imaging in patients with biochemical recurrence after radical prostatectomy - diagnostic performance and impact on therapeutic decision-making. Eur J Nucl Med Mol Imaging 2018;45(2):235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lake ST, Greene KL, Westphalen AC, et al. Optimal MRI sequences for 68Ga-PSMA-11 PET/MRI in evaluation of biochemically recurrent prostate cancer. EJNMMI Res 2017;7(1):77. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



![Participant flowchart. 18F-DCFPyL = 2-(3-{1-carboxy-5-[(6-[18F]fluoro-pyridine 3-carbonyl)-amino]-pentyl}-ureido)-pentanedioic acid.](https://cdn.ncbi.nlm.nih.gov/pmc/blobs/568c/7457947/bfe63a86ec9c/radiol.2020192018.fig1.jpg)


