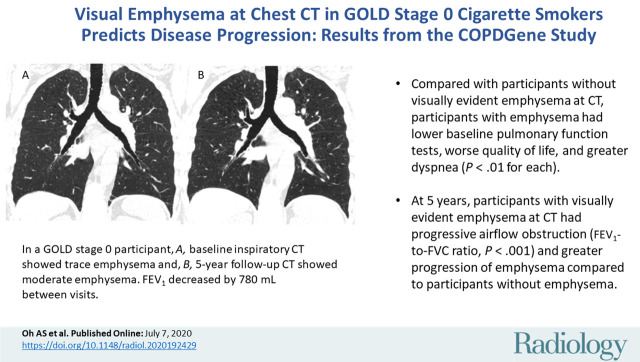
Abstract
Background
The clinical significance of visually evident emphysema on CT images in individuals without spirometric evidence of chronic obstructive pulmonary disease (COPD) by current diagnostic criteria is, to the knowledge of the authors, unknown.
Purpose
To evaluate whether participants with visually evident emphysema at CT were more likely to have progressive disease and increased mortality at 5 years compared with those without visual emphysema.
Materials and Methods
This secondary analysis of the prospective Genetic Epidemiology of COPD study evaluated current or former smokers enrolled between 2008 and 2011 who did not meet current criteria for COPD (defined as Global Initiative for Obstructive Lung Disease stage 0). Statistical analysis was performed by using linear mixed models to estimate mean physiologic, imaging, and clinical outcomes for those with and without visual emphysema. Hazard ratios for mortality were calculated by using Cox regression models with emphysema as the main predictor.
Results
Of the 4095 participants, 48.3% (1979 participants; 1096 men and 883 women; mean age, 57 years ± 8 [standard deviation]) had trace or greater visual emphysema at CT and 51.7% (2116 participants; 1068 men and 1048 women; mean age, 56 years ± 8) had no emphysema at CT. At 5 years, participants with visual emphysema at CT demonstrated progressive airflow obstruction with lower values of ratio of forced expiratory volume in 1 second (FEV1)-to–functional vital capacity (FVC) ratio (−1.7 vs −0.7) and greater progression in quantitative emphysema measured by 15th percentile lung density (−3.3 vs −0.3 HU), adjusted lung density (−3.1 vs −0.2 g/L), and percentage of lung voxels with CT attenuation less than −950 HU (0.17 vs −0.20) than participants without emphysema (P < .001 for each). The rate of quantitative emphysema progression increased with greater grades of emphysema severity within the emphysema group.
Conclusion
The presence of visual emphysema at CT in current and former Global Initiative for Obstructive Lung Disease stage 0 smokers predicted structural and physiologic disease progression.
© RSNA, 2020
Online supplemental material is available for this article.
See also the editorial by Grenier in this issue.
Summary
The presence of visually evident emphysema on CT images in smokers with normal spirometry, labeled as GOLD stage 0, had progressive airflow obstruction and progression of emphysema over 5 years.
Key Results
■ Compared with participants without visually evident emphysema at CT, participants with emphysema had lower baseline pulmonary function tests, worse quality of life, and greater dyspnea (P < .01 for each).
■ At 5 years, participants with visually evident emphysema at CT had progressive airflow obstruction (forced expiratory volume in 1 second–to–forced vital capacity ratio, P < .001) and greater progression in quantitative emphysema measured by adjusted lung density, CT attenuation at the 15th percentile of the lung CT histogram, and percent low attenuation areas less than −950 HU (P < .001 for each) than participants without emphysema.
Introduction
Chronic obstructive pulmonary disease (COPD) is a progressive and debilitating respiratory condition that causes substantial morbidity and mortality worldwide and immense financial burden on the health care system (1,2). Cigarette smoking is the most important risk factor for developing COPD, although only one-third of people who smoke develop COPD during their lifetime (3). The diagnosis is made when a patient with symptoms is confirmed to have physiologic airflow obstruction (ie, postbronchodilator ratio of forced expiratory volume in 1 second [FEV1]-to–functional vital capacity [FVC] ratio less than 0.70) in the absence of an alternative explanation for the symptoms or the airflow obstruction (4). Studies have shown, however, that current and former smokers without spirometric evidence of disease have respiratory symptoms and imaging abnormalities not captured by spirometry measurements, which limits the use of spirometry in early disease detection (5). As a result, smoking-related lung disease remains underdiagnosed in the general population, leading to increased disease burden, comorbidities, and costs (6).
CT can help detect presymptomatic abnormalities in cigarette smokers, before substantial end-organ damage occurs (5). A recent study showed that the visual presence and severity of emphysema at CT is associated with increased mortality risk, independent of the quantitative severity of emphysema (7). However, it remains unclear whether emphysema identified in smokers without spirometric impairment is clinically important. Thus, the purpose of this study was to evaluate differences in progression of physiologic, imaging, and clinical parameters in smokers with and without visual emphysema at CT but without COPD. We chose to use the label Global Initiative for Chronic Obstructive Lung Disease (GOLD) stage 0 for our cohort of nonobstructed smokers, despite that the stage is no longer included in the current GOLD classification system. We hypothesized that participants with visually evident emphysema at CT were more likely to have progressive disease and symptoms and increased mortality at 5 years, compared with those without visual emphysema.
Materials and Methods
This study is a secondary analysis from Genetic Epidemiology of COPD (COPDGene; ClinicalTrials.gov: NCT00608764), a prospective multicenter study focused on the genetic epidemiology of COPD (8). Between 2007 and 2011, 10 192 participants aged 45–80 years with at least a 10-pack-year smoking history were enrolled in this Health Insurance Portability and Accountability Act–compliant study at 21 clinical centers in the United States. Smokers were enrolled on the basis of smoking history and classified by using the GOLD spirometric criteria based on postbronchodilator spirometry. Participants self-identified as non-Hispanic African American or non-Hispanic white ethnicity. Those with respiratory conditions other than asthma and COPD were excluded from the study.
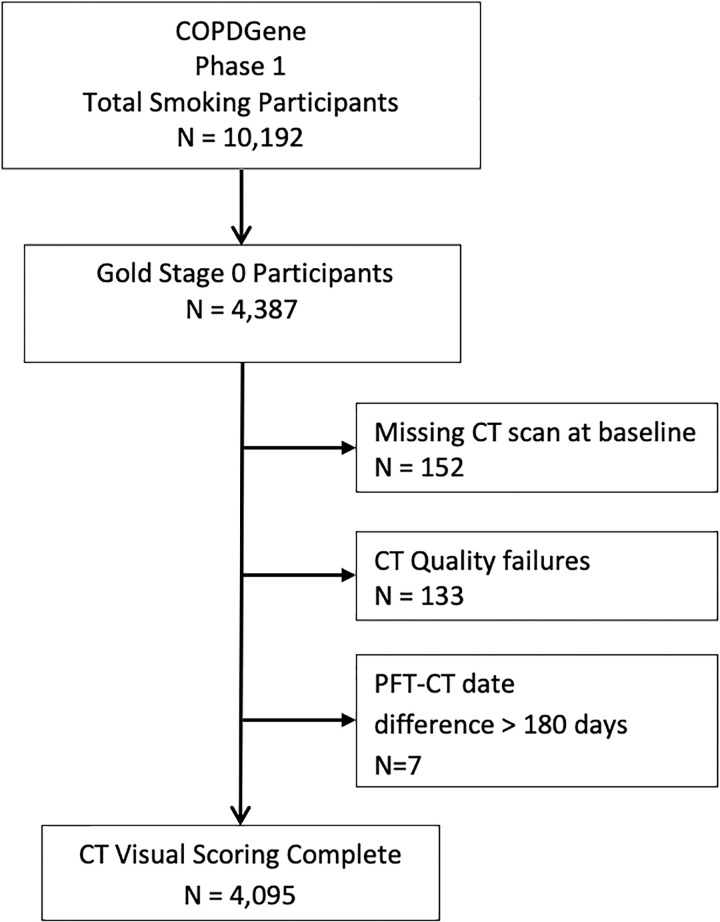
Written informed consent was obtained from all study participants. Our study evaluated the GOLD stage 0 group, defined as current and former smokers with a postbronchodilator ratio of FEV1-to-FVC of at least 0.70 and an FEV1 percentage of at least 80% predicted. Participants with baseline and 5-year follow-up inspiratory CT, visual emphysema scores, and mortality data were included (Fig 1). Clinical and radiologic evidence of lung disease in the GOLD 0 group was previously reported (5). The previous study focused on the GOLD 0 group at baseline, comparing them with never smokers and other COPD groups, whereas we focused solely on the GOLD 0 group and the significance of visually evident emphysema on disease progression at 5-year follow-up.
Figure 1:
Study population consort diagram. COPDGene = Genetic Epidemiology of chronic obstructive pulmonary disease, PFT = pulmonary function testing.
Clinical Evaluation
All participants underwent pre- and postbronchodilator spirometry, evaluation of bronchodilator responsiveness, and 6-minute walk testing by using standard techniques at baseline and 5-year follow-up (8). Standardized questionnaires were used to assess disease-specific impact on quality of life by using the St George Respiratory Questionnaire total score (9), dyspnea by using the modified Medical Research Council dyspnea score cutoff of 2 or greater (10), health-related quality of life by the Medical Outcomes study 36-item short form survey (11), symptoms of chronic bronchitis defined as self-reported cough productive of phlegm for 3 months or more per year for at least 2 consecutive years (12), and history of severe respiratory exacerbations.
Visual Analysis
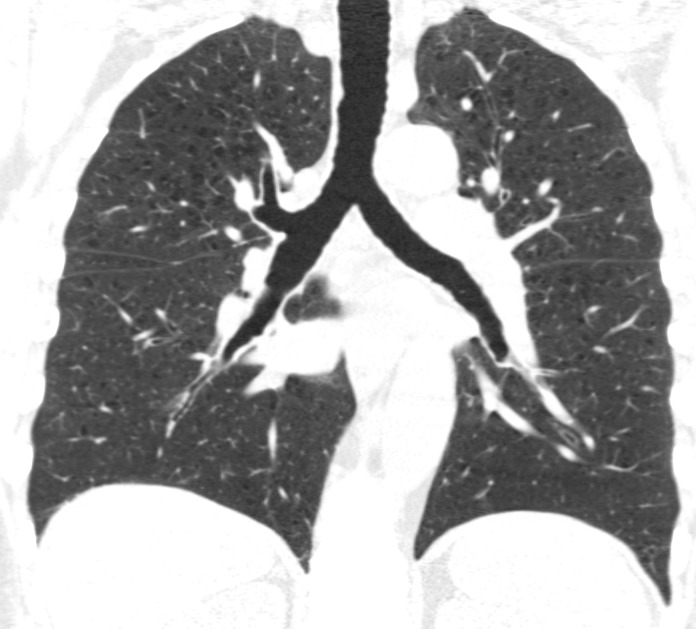
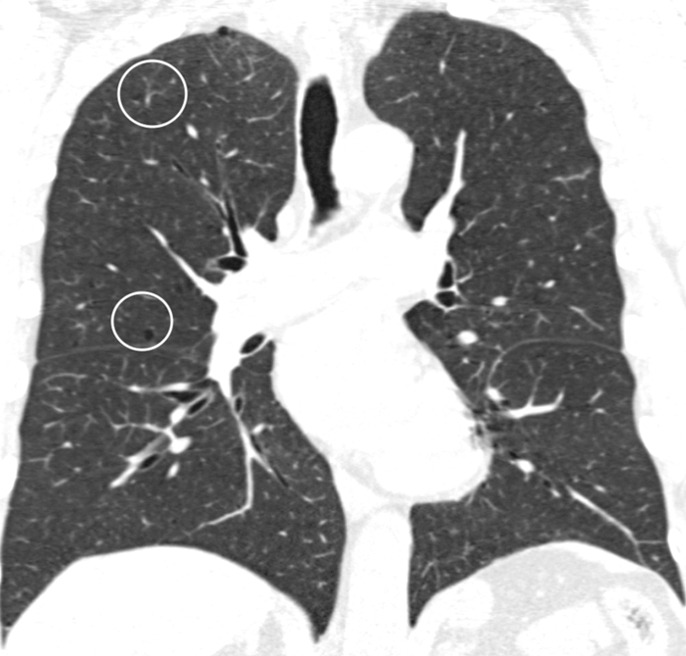
Visual analysis by trained research analysts was based on the Fleischner Society classification system (13) (Fig 2). The details of the methods and analysis were described previously (7).
Figure 2a:
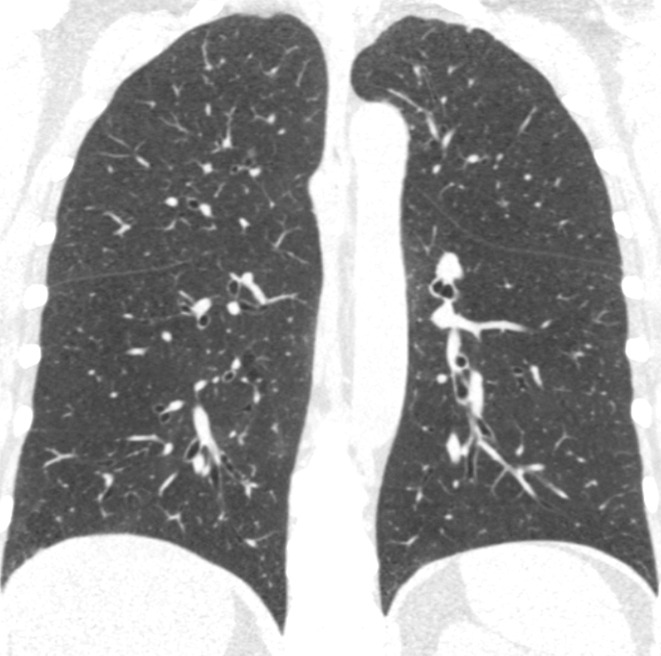
Coronal CT images show progressive grades of parenchymal emphysema on the basis of the Fleischner classification system. (a) Normal CT scan shows no emphysema. (b) Trace centrilobular emphysema (circles). (c) Mild centrilobular emphysema (arrows). (d) Moderate centrilobular emphysema involving more than 5% of the lung zone. (e) Confluent emphysema. (f) Advanced destructive emphysema.
Figure 2b:
Coronal CT images show progressive grades of parenchymal emphysema on the basis of the Fleischner classification system. (a) Normal CT scan shows no emphysema. (b) Trace centrilobular emphysema (circles). (c) Mild centrilobular emphysema (arrows). (d) Moderate centrilobular emphysema involving more than 5% of the lung zone. (e) Confluent emphysema. (f) Advanced destructive emphysema.
Figure 2c:
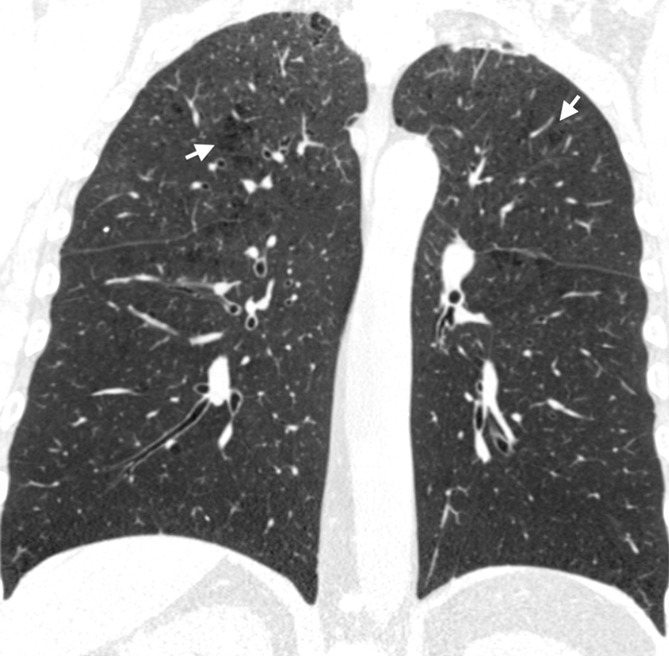
Coronal CT images show progressive grades of parenchymal emphysema on the basis of the Fleischner classification system. (a) Normal CT scan shows no emphysema. (b) Trace centrilobular emphysema (circles). (c) Mild centrilobular emphysema (arrows). (d) Moderate centrilobular emphysema involving more than 5% of the lung zone. (e) Confluent emphysema. (f) Advanced destructive emphysema.
Figure 2d:
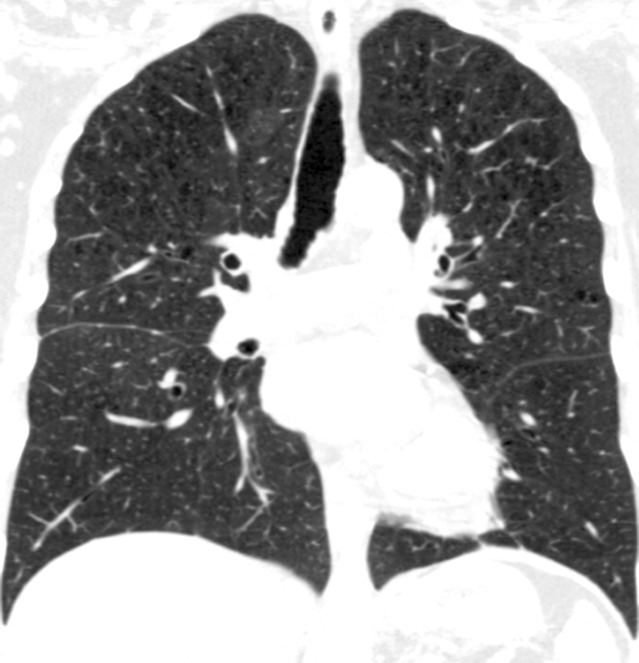
Coronal CT images show progressive grades of parenchymal emphysema on the basis of the Fleischner classification system. (a) Normal CT scan shows no emphysema. (b) Trace centrilobular emphysema (circles). (c) Mild centrilobular emphysema (arrows). (d) Moderate centrilobular emphysema involving more than 5% of the lung zone. (e) Confluent emphysema. (f) Advanced destructive emphysema.
Figure 2e:
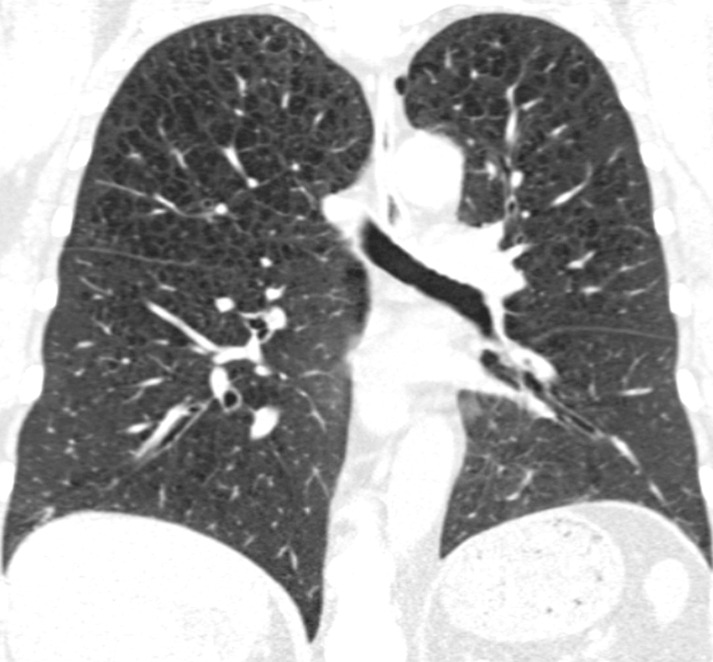
Coronal CT images show progressive grades of parenchymal emphysema on the basis of the Fleischner classification system. (a) Normal CT scan shows no emphysema. (b) Trace centrilobular emphysema (circles). (c) Mild centrilobular emphysema (arrows). (d) Moderate centrilobular emphysema involving more than 5% of the lung zone. (e) Confluent emphysema. (f) Advanced destructive emphysema.
Figure 2f:
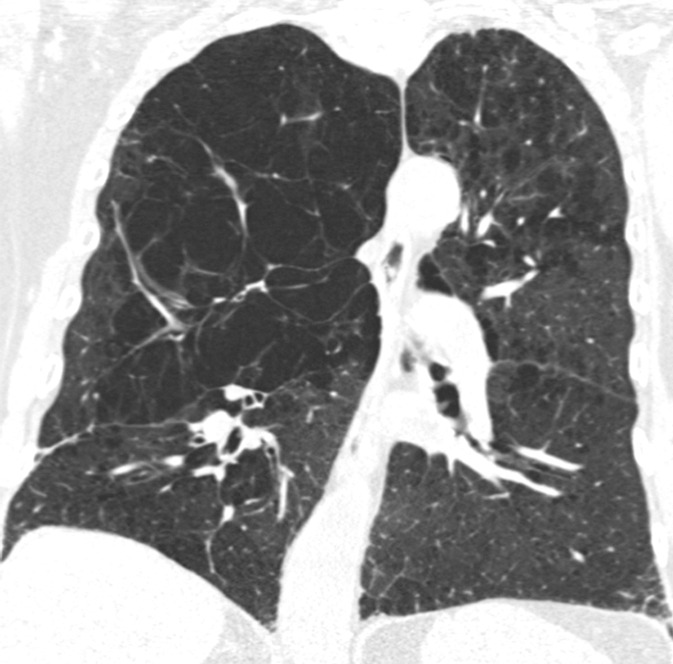
Coronal CT images show progressive grades of parenchymal emphysema on the basis of the Fleischner classification system. (a) Normal CT scan shows no emphysema. (b) Trace centrilobular emphysema (circles). (c) Mild centrilobular emphysema (arrows). (d) Moderate centrilobular emphysema involving more than 5% of the lung zone. (e) Confluent emphysema. (f) Advanced destructive emphysema.
Quantitative CT Analysis
All participants underwent volumetric noncontrast axial inspiratory and expiratory CT by using a standardized protocol (8,14). The scans were reconstructed with a section thickness of 0.625, 0.75, or 0.9 mm depending on the CT manufacturer; corresponding section intervals were 0.625, 0.5, or 0.45 mm, respectively, to achieve near-isotropic voxels (15). Quantitative analysis of emphysema severity and air trapping was performed by using dedicated software programs (3DSlicer, http://www.slicer.org; Pulmonary Workstation 2, Vida Diagnostics, Coralville, Iowa) (15). Emphysema was quantified by using CT attenuation at the 15th percentile of the lung CT histogram (Perc15) (16) and percent low attenuation areas less than −950 HU on inspiratory CT (17,18) as previously described. Volume adjusted lung density (ALD) at the 15th percentile was also used to quantify emphysema by adjusting Perc15 for CT total lung volume. Gas trapping was measured by CT-derived functional residual capacity–to–total lung capacity ratio (19).
Evaluation of Survival Times
Deaths were reported to the central study from the clinical centers largely on the basis of the longitudinal follow-up program. Additional information from the U.S. Social Security Death Index and the COPDGene longitudinal follow-up program was used to determine a survival or censoring time for each participant, taking care to avoid ascertainment bias. Nine sites performed their own Social Security Death Index searches; all others used a centralized search performed by COPDGene staff. The median length of follow-up in this data set was 7.9 years (range, 25 days to 10.5 years).
Statistical Analysis
All analyses were performed by using statistical software (SAS version 9.4; SAS Institute, Cary, NC). A P value of less than .05 was considered to indicate statistical significance. Descriptive statistics of baseline characteristics were calculated and compared between participants with and without visual emphysema or in participants with emphysema between emphysema grades. Participant baseline characteristics were compared by using χ2 tests for categorical data, analysis of variance for normally distributed continuous variables, and Wilcoxon rank sum tests (two groups) or Kruskal-Wallis test (multiple group comparison) for nonnormal continuous variables (6-minute walk distance and number of pack-years smoked, St George Respiratory Questionnaire, and percent low attenuation areas less than −950 HU at inspiratory CT).
Linear mixed models were used to estimate mean physiologic, imaging, and clinical outcomes for those with and without visual emphysema at CT at baseline by including time, visual emphysema status (present, absent), and time multiplied by emphysema status as predictors while adjusting for age, height, weight, sex, ethnicity, and current smoking status. A random intercept was included for study center. For CT outcomes, a fixed term for scanner make and a random intercept for scanner model were also included. For participants, either a random intercept was included, or an unstructured error covariance matrix was used to account for repeated measures during the two visits depending on model convergence issues and goodness-of-fit statistics. Similar models were fit by using levels of emphysema severity (removing those with no presence) in place of presence or absence of visual emphysema severity. Further details of the models are provided in Appendices E1 and E2 (online).
Hazard ratios for mortality were calculated with Cox regression models by using emphysema severity class as the main predictor, with adjustment for age, height, sex, race, and current smoking status. The full model is presented in Appendix E3 (online).
Results
Participant Characteristics
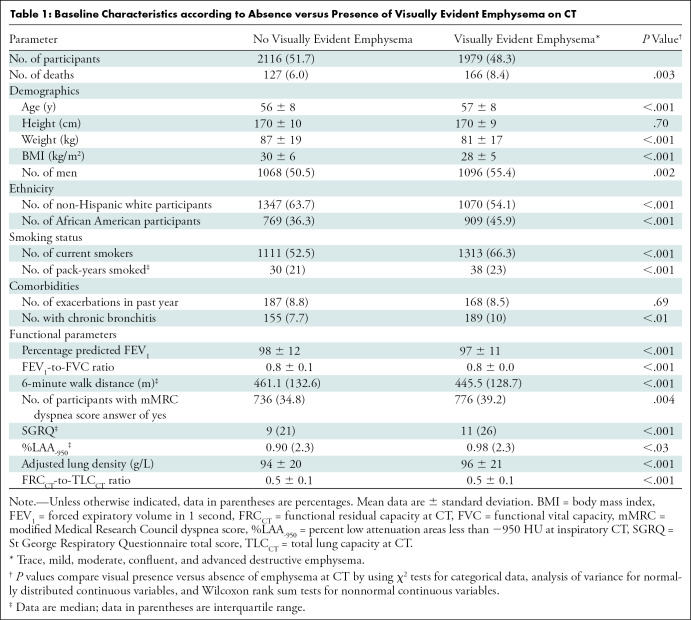
We evaluated 4095 current and former smokers (2164 men and 1931 women) (Table 1). The mean age at enrollment was 57 years ± 8 (standard deviation), with mean ages of 56 years ± 8 for men and 57 years ± 8 for women. No emphysema was found in 2116 of the 4095 participants (51.7%). Trace or greater emphysema was manifest in 1979 of the 4095 participants (48.3%). Compared with participants without emphysema, those with emphysema were older, had a lower body mass index, were more likely to be African American, more likely to be men, had a higher tobacco exposure, and were more likely to be current smokers. The visual presence of emphysema was also associated with a lower baseline FEV1, lower FEV1-to-FVC, shorter 6-minute walk distance, more dyspnea, worse quality of life, and higher prevalence of chronic bronchitis symptoms. Radiologically, participants with emphysema had a higher ALD and higher CT-derived functional residual capacity–to–CT-derived total lung capacity ratio.
Table 1:
Baseline Characteristics according to Absence versus Presence of Visually Evident Emphysema on CT
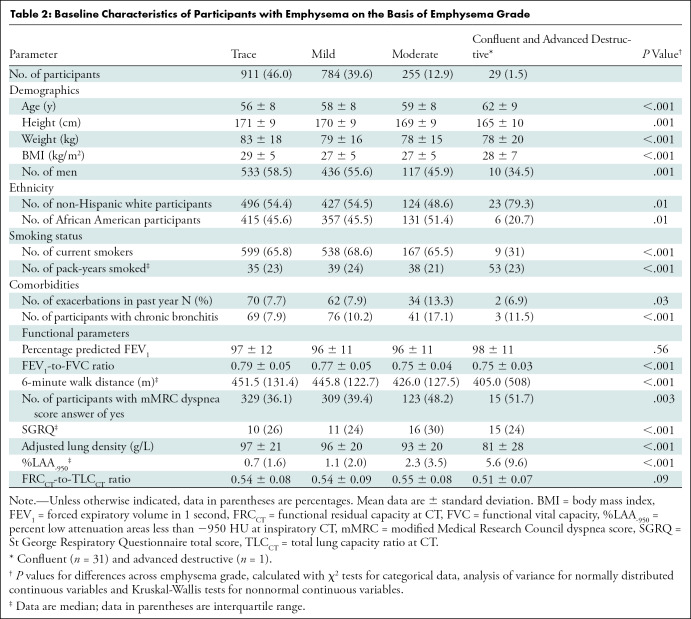
Within the emphysema cohort (Table 2), trace emphysema was present in 911 of the 1979 participants with emphysema (46%), mild in 784 (39.6%), moderate in 255 (12.9%), and confluent or advanced destructive in 29 (1.5%). Participants with greater emphysema severity were more likely to be older, have a lower body mass index, and more likely to be African American with greater tobacco exposure compared with those with less severe emphysema grade. Although emphysema was more common overall in men than in women (1096 men vs 883 women; 55% vs 45%), moderate emphysema was less common in men (117 men vs 138 women of 255 participants with moderate grade emphysema; 46% vs 54%). Further analysis comparing GOLD stage 0 men and women with moderate or greater emphysema did not show a difference between the two groups (P = .26). Additionally, as emphysema grade progressed, participants had lower baseline FEV1-to-FVC, shorter 6-minute walk distance, more dyspnea, worse quality of life, more episodes of chronic bronchitis symptoms, lower ALD, and higher percent low attenuation areas less than −950 HU.
Table 2:
Baseline Characteristics of Participants with Emphysema on the Basis of Emphysema Grade
Multivariable Analysis
At the 5-year follow-up visit, multivariable analyses showed no difference in change in CT-derived functional residual capacity–to–CT-derived total lung capacity ratio (–0.57 vs −0.05, respectively; P = .14), distance walked in 6 minutes (−13.8 vs −16.8 m; P = .54), disease-specific impact on quality of life (St George Respiratory Questionnaire score, 0.22 vs −0.38, P = .33), or general health-related quality of life (Medical Outcomes study 36-item short form survey, −0.87 vs −0.76; P = .80) between the emphysema and no-emphysema groups (Table 3). Participants with visual emphysema did, however, demonstrate progressive airflow obstruction with lower FEV1-to-FVC ratio compared with participants without visual emphysema (−1.7 vs −0.7%; P < .001) and greater progression in quantitative emphysema measured by Perc15 (−3.31 vs −0.30 HU; P < .001), ALD (−3.08 vs −0.22 g/L; P < .001), and natural log percent low attenuation areas less than −950 HU (0.17 vs −0.20; P < .001). An example of participants at GOLD stage 0 without and with emphysema progression and airflow obstruction at baseline and 5 years is shown in Figure 3.
Table 3:
Presence or Absence of Visually Evident Emphysema at Baseline CT in Relationship to Change in CT Parameters, Lung Function, and Clinical Status over 5 years
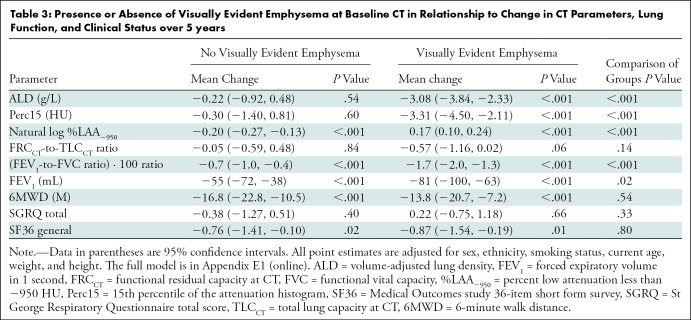
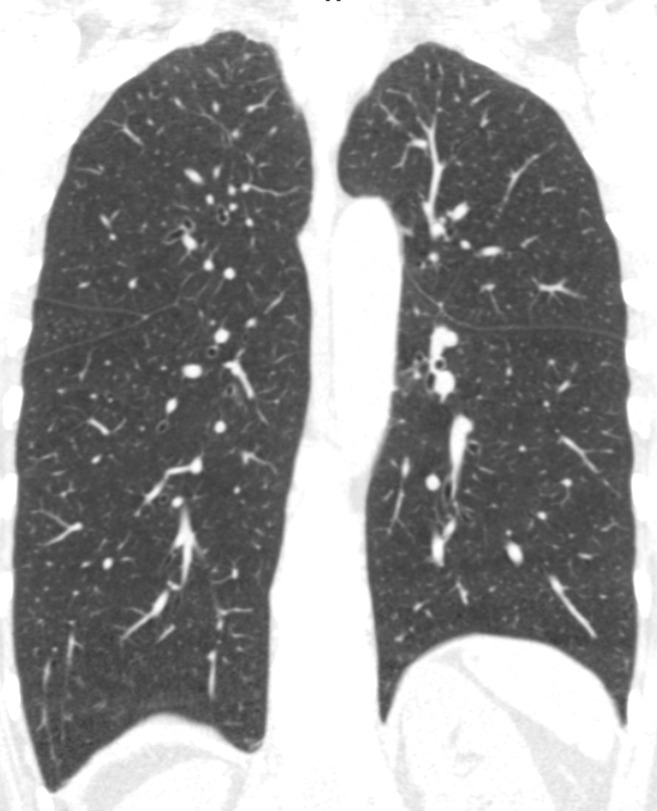
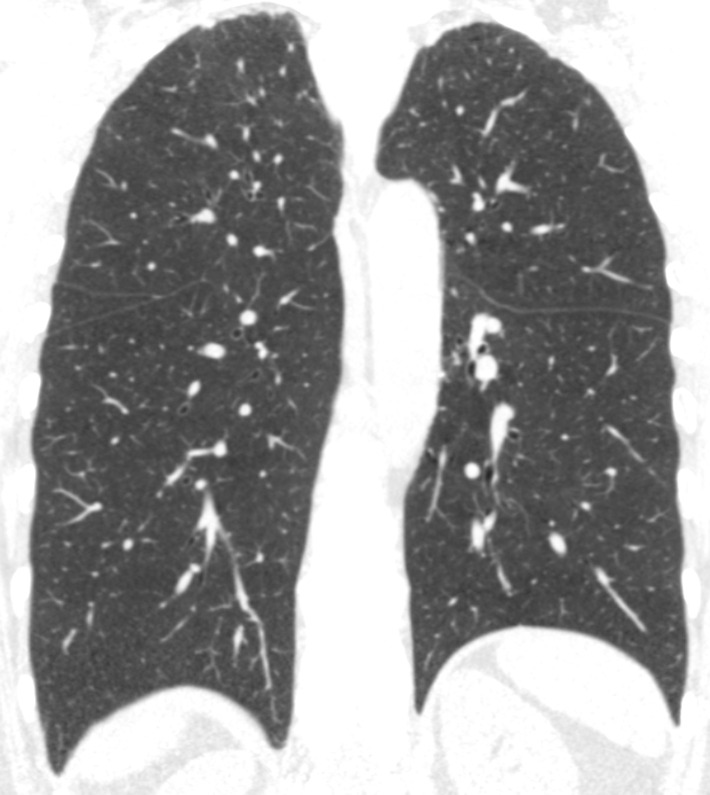
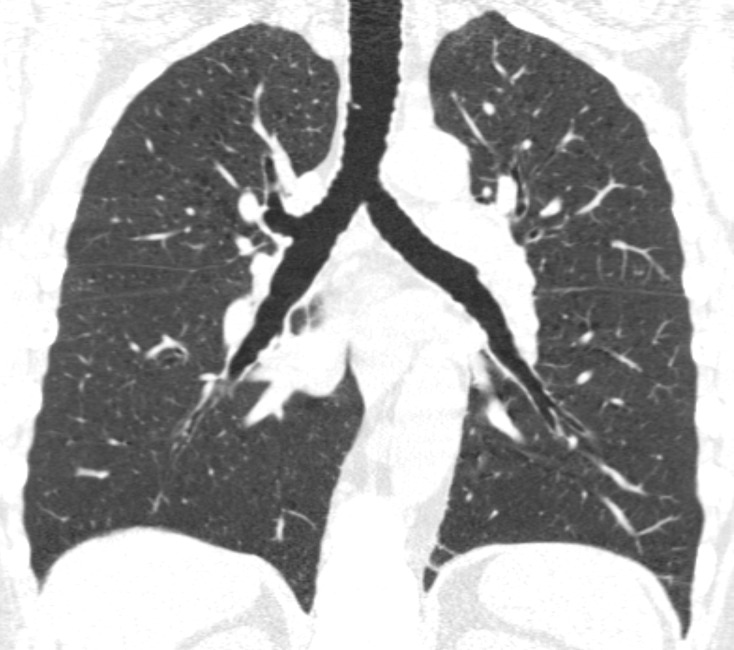
Figure 3a:
Baseline and 5-year follow up inspiratory CT scans in two Global Initiative for Chronic Obstructive Lung Disease stage 0 participants showing no progression and progression. (a) Baseline and (b) 5-year follow-up scans in a 55-year-old female participant without visually evident emphysema and no interval progression at follow-up. Forced expiratory volume in 1 second (FEV1) decreased by 62 mL. (c) Baseline and (d) 5-year follow-up scans in a 49-year-old male participant with trace emphysema at baseline but progression to moderate emphysema at follow-up. FEV1 decreased by 780 mL between visits.
Figure 3b:
Baseline and 5-year follow up inspiratory CT scans in two Global Initiative for Chronic Obstructive Lung Disease stage 0 participants showing no progression and progression. (a) Baseline and (b) 5-year follow-up scans in a 55-year-old female participant without visually evident emphysema and no interval progression at follow-up. Forced expiratory volume in 1 second (FEV1) decreased by 62 mL. (c) Baseline and (d) 5-year follow-up scans in a 49-year-old male participant with trace emphysema at baseline but progression to moderate emphysema at follow-up. FEV1 decreased by 780 mL between visits.
Figure 3c:
Baseline and 5-year follow up inspiratory CT scans in two Global Initiative for Chronic Obstructive Lung Disease stage 0 participants showing no progression and progression. (a) Baseline and (b) 5-year follow-up scans in a 55-year-old female participant without visually evident emphysema and no interval progression at follow-up. Forced expiratory volume in 1 second (FEV1) decreased by 62 mL. (c) Baseline and (d) 5-year follow-up scans in a 49-year-old male participant with trace emphysema at baseline but progression to moderate emphysema at follow-up. FEV1 decreased by 780 mL between visits.
Figure 3d:
Baseline and 5-year follow up inspiratory CT scans in two Global Initiative for Chronic Obstructive Lung Disease stage 0 participants showing no progression and progression. (a) Baseline and (b) 5-year follow-up scans in a 55-year-old female participant without visually evident emphysema and no interval progression at follow-up. Forced expiratory volume in 1 second (FEV1) decreased by 62 mL. (c) Baseline and (d) 5-year follow-up scans in a 49-year-old male participant with trace emphysema at baseline but progression to moderate emphysema at follow-up. FEV1 decreased by 780 mL between visits.
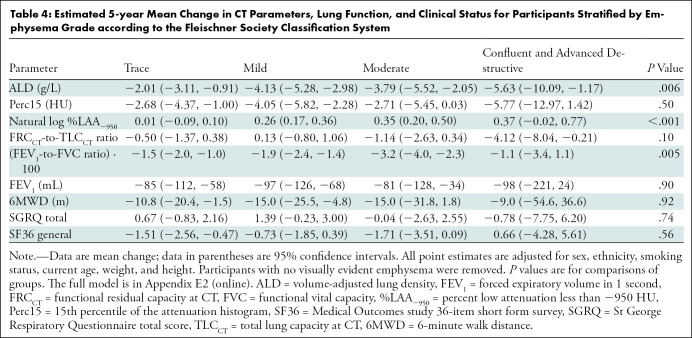
A difference in longitudinal change in ALD, percent low attenuation areas less than −950 HU, and FEV1-to-FVC ratio was present among the groups in the emphysema cohort stratified by emphysema grade (Table 4). Participants with mild or moderate emphysema had greater progression in quantitative emphysema measured by ALD (−4.13 [mild] vs −2.01 g/L [trace]; P = .006) and natural log percent low attenuation areas less than −950 HU (0.26 [mild] or 0.35 [moderate] vs 0.01 [trace]; P < .001) than those with trace emphysema, respectively. The group with moderate emphysema also had a greater decline in FEV1-to-FVC ratio at 5 years compared with the group with trace emphysema (−3.2 vs −1.5%; P = .005).
Table 4:
Estimated 5-year Mean Change in CT Parameters, Lung Function, and Clinical Status for Participants Stratified by Emphysema Grade according to the Fleischner Society Classification System
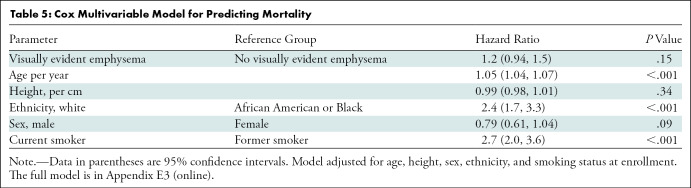
There were 174 deaths in participants with emphysema. The Cox proportional hazards model results are shown in Table 5. The visual emphysema group had greater hazard of death than the group with no emphysema, but it was not statistically significant (hazard ratio, 1.19; 95% confidence interval: 0.94, 1.50; P = .15).
Table 5:
Cox Multivariable Model for Predicting Mortality
Discussion
We demonstrated that Global Initiative for Obstructive Lung Disease (GOLD) stage 0 smokers with visually evident emphysema on baseline CT images had significant and progressive respiratory disease compared with participants without emphysema. The presence of emphysema on CT images was associated with lower baseline pulmonary function tests, shorter 6-minute walk distance, greater dyspnea, and worse quality of life (P < .01 for each). At 5-year follow up, participants with emphysema had increased airflow obstruction (forced expiratory volume in 1 second [FEV1]-to–functional vital capacity [FVC] ratio, P < .001) and greater progression in quantitative emphysema measured by CT attenuation at the 15th percentile of the lung CT histogram, adjusted lung density (ALD), and natural log percent low attenuation areas less than −950 HU than those without emphysema (P < .001 for each). Further stratification of the visually evident emphysema cohort on the basis of the Fleischner Society classification system showed progressive emphysema grade was associated with greater baseline airflow obstruction (FEV1-to-FVC ratio), shorter 6-minute walk distance, more dyspnea, worse quality of life, and greater extent of quantitative emphysema (P < .01 for each). Similarly, at 5-year follow up, participants with more extensive grades of emphysema had a greater degree of airflow obstruction (FEV1-to-FVC ratio, P < .01) and progression in quantitative emphysema (ALD and percent low attenuation areas less than −950 HU, P < .01) than did participants with trace emphysema. Our findings corroborate and extend the results of previous studies (5,20) that established the manifestation of clinical and physiologic disease in the GOLD stage 0 population.
Contrary to what was expected, the visual emphysema cohort had a higher ALD at baseline quantitative imaging (although progressive emphysema grade was associated with declining lung density). This is because participants with emphysema were more likely to be current smokers with higher tobacco exposure and more episodes of chronic bronchitis. Studies have shown that current smokers have denser lungs than never smokers and patients with COPD, which corresponds to measures of local inflammation in the bronchioles and/or airspaces (20–22). These findings, however, did not persist over time. At 5-year follow-up, the emphysema group had a greater decline in lung density and increased airflow obstruction compared with participants without emphysema. This confirms earlier studies of emphysema and airways disease identified on CT scans of ever smokers with normal spirometry and their association with greater rates of lung function decline (23,24). Moreover, we were able to show a gradient of progressive airflow obstruction and quantifiable emphysema with increasing emphysema grade, further validating the Fleischner emphysema classification system as a tool to assess emphysema severity (13). Our findings suggest that the presence of even trace emphysema at baseline CT predicts structural and physiologic progression at 5 years, highlighting the importance of imaging and its complemental role with spirometry in detecting early disease. Additionally, increasing radiologic severity of emphysema at baseline was associated with increased respiratory symptoms, severity of clinical and physiologic impairment, and progression of quantitative emphysema and airflow obstruction. This highlights the importance for the radiologist to routinely record and characterize emphysema at CT (including in patients undergoing lung cancer screening). Given that 67% of the participants with emphysema in our study were current smokers, the identification of emphysema in such individuals might provide an important incentive for smoking cessation.
There was no significant difference in mortality between the two cohorts. In the overall COPDGene population, Lynch et al (7) showed that the visual presence and severity of emphysema was associated with increased mortality risk, though these findings were not found to be significant in the subgroup with trace emphysema. Because almost 50% of our participants with visual emphysema fell into the trace category, the lack of significant mortality difference was not unexpected. Because the rate of progression of COPD is relatively slow, it is conceivable that with a longer follow-up time, the emphysema grade in our studied population could progress and potentially lead to increased mortality risk.
Sato and colleagues have applied the concept of fractal geometry to CT imaging of COPD in mouse and human emphysema studies, demonstrating that the size distribution of clusters of emphysematous regions follow a power law characterized by the fractal dimension, D (25,26). By showing that low attenuation areas on CT images display fractal properties, they determined that D is more sensitive to changes in emphysema progression than percentage low attenuation area (27). By using two Japanese COPD cohorts, they established that a lower D (representing an increase in terminal airspace enlargement) predicts future exacerbations, whereas percent low attenuation volume was associated with a decline in FEV1 and higher 10-year mortality (25). Morphologic assessment of emphysema by using fractal D and percent low attenuation volume in future work may help elucidate different subtypes of emphysema and progression in individuals with GOLD stage 0 who have predominantly trace and mild grades of disease severity.
There were several limitations to our study. The large sample size and number of clinical centers resulted in CT scanner heterogeneity with varying models and manufacturers. Whereas the protocols were designed to minimize this variability, it is possible that this may have led to slight discrepancies in the quantitative imaging measurements. Additionally, whereas COPDGene is more inclusive than many COPD studies, it is not a population-based study, which may impact the generalizability of our findings.
Our study confirmed the limitations in current diagnostic criteria in capturing smoking-related lung injury in GOLD stage 0 participants. As of 2017, there are an estimated 34 million current smokers in the United States (28), of which only half are diagnosed with COPD (29). Many of the remaining 17 million smokers have smoking-related lung disease undiagnosed with the current COPD spirometric criteria (5,30). We have shown that the breadth of symptomatic, structural, and physiologic smoking-related lung disease in these individuals is not adequately captured by the present diagnostic criteria and that participants with emphysema on CT images may require more diligent monitoring for disease progression.
In conclusion, the presence of emphysema in Global Initiative for Obstructive Lung Disease stage 0 participants is an important predictor in determining structural and physiologic progression. Emphysema should be routinely recorded by using an ordinal scale (ie, Fleischner guidelines) or with quantitative CT metrics in radiology reports because CT can play a role in early detection of smoking-related lung disease.
APPENDIX
Study supported by grants from the National Heart, Lung, and Blood Institute (R01HL089897, R01HL089856, U01HL089897, U01HL089856). The COPDGene project is also supported by the COPD Foundation Industry Advisory Board (with contributions from AstraZeneca, Boehringer Ingelheim, Novartis, Pfizer, Siemens, Sunovion, and GlaxoSmithKline).
Disclosures of Conflicts of Interest: A.S.O. disclosed no relevant relationships. M.S. disclosed no relevant relationships. K.P. disclosed no relevant relationships. E.A.R. disclosed no relevant relationships. S.H. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed money paid to authors for board membership from Imidex; disclosed money paid to author for consultancy from Boehringer Ingelheim. Other relationships: disclosed no relevant relationships. J.D.C. disclosed no relevant relationships. D.A.L. Activities related to the present article: disclosed no relevant relationships. Activities not related to the present article: disclosed money to author’s institution for consultancy from Parexel; disclosed money to author’s institution for pending patent regarding systems and methods for classifying severity of COPD. Other relationships: disclosed no relevant relationships.
Abbreviations:
- ALD
- adjusted lung density
- COPD
- chronic obstructive pulmonary disease
- FEV1
- forced expiratory volume in 1 second
- FVC
- forced vital capacity
- COPDGene
- Genetic Epidemiology of COPD
- GOLD
- Global Initiative for Obstructive Lung Disease
- Perc15
- CT attenuation at the 15th percentile of the lung CT histogram
References
- 1.May SM, Li JTC. Burden of chronic obstructive pulmonary disease: healthcare costs and beyond. Allergy Asthma Proc 2015;36(1):4–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Celli BR, MacNee W, ATS/ERS Task Force . Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 2004;23(6):932–946 [Published correction appears in Eur Respir J 2006;27(1):242.]. [DOI] [PubMed] [Google Scholar]
- 3.Vrbica Ž, Labor M, Gudelj I, et al. Early detection of COPD patients in GOLD 0 population: an observational non-interventional cohort study - MARKO study. BMC Pulm Med 2017;17(1):36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Qaseem A, Wilt TJ, Weinberger SE, et al. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Ann Intern Med 2011;155(3):179–191. [DOI] [PubMed] [Google Scholar]
- 5.Regan EA, Lynch DA, Curran-Everett D, et al. Clinical and Radiologic Disease in Smokers With Normal Spirometry. JAMA Intern Med 2015;175(9):1539–1549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larsson KÅ, Janson C, Ställberg B, et al. Extent and impact of late vs early stage COPD diagnosis in the Swedish ARCTIC study. Eur Respir J 2016;48(suppl 60):PA3933. [Google Scholar]
- 7.Lynch DA, Moore CM, Wilson C, et al. CT-based Visual Classification of Emphysema: Association with Mortality in the COPDGene Study. Radiology 2018;288(3):859–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Regan EA, Hokanson JE, Murphy JR, et al. Genetic epidemiology of COPD (COPDGene) study design. COPD 2010;7(1):32–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George’s Respiratory Questionnaire. Am Rev Respir Dis 1992;145(6):1321–1327. [DOI] [PubMed] [Google Scholar]
- 10.Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest 1988;93(3):580–586. [DOI] [PubMed] [Google Scholar]
- 11.Lins L, Carvalho FM. SF-36 total score as a single measure of health-related quality of life: Scoping review. SAGE Open Med 2016;4:2050312116671725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meek PM, Petersen H, Washko GR, et al. Chronic Bronchitis Is Associated With Worse Symptoms and Quality of Life Than Chronic Airflow Obstruction. Chest 2015;148(2):408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lynch DA, Austin JHM, Hogg JC, et al. CT-Definable Subtypes of Chronic Obstructive Pulmonary Disease: A Statement of the Fleischner Society. Radiology 2015;277(1):192–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Han MK, Bartholmai B, Liu LX, et al. Clinical significance of radiologic characterizations in COPD. COPD 2009;6(6):459–467. [DOI] [PubMed] [Google Scholar]
- 15.Schroeder JD, McKenzie AS, Zach JA, et al. Relationships between airflow obstruction and quantitative CT measurements of emphysema, air trapping, and airways in subjects with and without chronic obstructive pulmonary disease. AJR Am J Roentgenol 2013;201(3):W460–W470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Parr DG, Stoel BC, Stolk J, Stockley RA. Validation of computed tomographic lung densitometry for monitoring emphysema in alpha1-antitrypsin deficiency. Thorax 2006;61(6):485–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gevenois PA, de Maertelaer V, De Vuyst P, Zanen J, Yernault JC. Comparison of computed density and macroscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med 1995;152(2):653–657. [DOI] [PubMed] [Google Scholar]
- 18.Lynch DA, Al-Qaisi MA. Quantitative computed tomography in chronic obstructive pulmonary disease. J Thorac Imaging 2013;28(5):284–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hersh CP, Washko GR, Estépar RSJ, et al. Paired inspiratory-expiratory chest CT scans to assess for small airways disease in COPD. Respir Res 2013;14(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Karimi R, Tornling G, Forsslund H, et al. Lung density on high resolution computer tomography (HRCT) reflects degree of inflammation in smokers. Respir Res 2014;15(1):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lederer DJ, Enright PL, Kawut SM, et al. Cigarette smoking is associated with subclinical parenchymal lung disease: the Multi-Ethnic Study of Atherosclerosis (MESA)-lung study. Am J Respir Crit Care Med 2009;180(5):407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Soejima K, Yamaguchi K, Kohda E, et al. Longitudinal follow-up study of smoking-induced lung density changes by high-resolution computed tomography. Am J Respir Crit Care Med 2000;161(4 Pt 1):1264–1273. [DOI] [PubMed] [Google Scholar]
- 23.Remy-Jardin M, Remy J, Boulenguez C, Sobaszek A, Edme JL, Furon D. Morphologic effects of cigarette smoking on airways and pulmonary parenchyma in healthy adult volunteers: CT evaluation and correlation with pulmonary function tests. Radiology 1993;186(1):107–115. [DOI] [PubMed] [Google Scholar]
- 24.Mohamed Hoesein FA, de Hoop B, Zanen P, et al. CT-quantified emphysema in male heavy smokers: association with lung function decline. Thorax 2011;66(9):782–787. [DOI] [PubMed] [Google Scholar]
- 25.Shimizu K, Tanabe N, Tho NV, et al. Per cent low attenuation volume and fractal dimension of low attenuation clusters on CT predict different long-term outcomes in COPD. Thorax 2020;75(2):116–122 [Published correction appears in Thorax 2020 Mar 24:thoraxjnl-2019-213525corr1.]. [DOI] [PubMed] [Google Scholar]
- 26.Sato A, Hirai T, Imura A, et al. Morphological mechanism of the development of pulmonary emphysema in klotho mice. Proc Natl Acad Sci U S A 2007;104(7):2361–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mitsunobu F, Ashida K, Hosaki Y, et al. Complexity of terminal airspace geometry assessed by computed tomography in asthma. Am J Respir Crit Care Med 2003;167(3):411–417. [DOI] [PubMed] [Google Scholar]
- 28.Burden of Cigarette Use in the U.S. Centers for Disease Control and Prevention. https://www.cdc.gov/tobacco/campaign/tips/resources/data/cigarette-smoking-in-united-states.html. Published 2019. Accessed April 21, 2019.
- 29.Chronic Obstructive Pulmonary Disease (COPD). Centers for Disease Control and Prevention. https://www.cdc.gov/copd/index.html. Published 2019. Accessed April 24, 2019. [Google Scholar]
- 30.Woodruff PG, Barr RG, Bleecker E, et al. Clinical Significance of Symptoms in Smokers with Preserved Pulmonary Function. N Engl J Med 2016;374(19):1811–1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.