Abstract
Non-communicable diseases (NCDs) that involve neurodegenerative disorders and metabolic disease impact over 400 million individuals globally. Interestingly, metabolic disorders, such as diabetes mellitus, are significant risk factors for the development of neurodegenerative diseases. Given that current therapies for these NCDs address symptomatic care, new avenues of discovery are required to offer treatments that affect disease progression. Innovative strategies that fill this void involve the mechanistic target of rapamycin (mTOR) and its associated pathways of mTOR Complex 1 (mTORC1), mTOR Complex 2 (mTORC2), AMP activated protein kinase (AMPK), trophic factors that include erythropoietin (EPO), and the programmed cell death pathways of autophagy and apoptosis. These pathways are intriguing in their potential to provide effective care for metabolic and neurodegenerative disorders. Yet, future work is necessary to fully comprehend the entire breadth of the mTOR pathways that can effectively and safely translate treatments to clinical medicine without the development of unexpected clinical disabilities.
Keywords: Alzheimer’s disease, AMPK, autophagy, apoptosis, diabetes mellitus, dementia, erythropoietin, mTOR, mTORC1, mTORC2
Non-communicable Diseases and Neurodegenerative Disease
With the increasing prevalence of non-communicable diseases (NCDs), it is now estimated that almost seventy percent of the annual deaths that occur each year are the result of NCDs (World Health Organization, 2011, 2017). As a result, over 40 million people die from NCDs each year. Of these individuals, 15 million are between 30 and 69 years old, illustrating that even younger individuals can be impacted by NCDs. For NCDs, greater than 10 percent of the population under 60 years of age is affected in high-income countries (World Health Organization, 2011). In contrast, NCDs affect a much larger proportion of the population in in low and middle-income countries with at least one-third of the population under the age of 60 suffering from NCDs. Interestingly, the rise in NCDs parallels an observed increase in life expectancy of the world’s population (Maiese, 2018d). The age of the global population continues to increase with life expectancy approaching 80 years of age (Maiese, 2014a). In addition, the number of individuals over the age of 65 has doubled during the previous 50 years (Hayutin, 2007). Life expectancy also has been marked by a 1 percent decrease in the age-adjusted death rate from the years 2000 through 2011 (Minino, 2013). It is expected that the number of elderly individuals in large developing countries such as India and China also will increase from five to ten percent over the next several decades (Maiese, 2015f, 2015g). There are a number of reasons that may account for the increased lifespan, but improvements in effective treatments for multiple disorders (Diamanti-Kandarakis et al., 2017; Fann, Ng, Poh, & Arumugam, 2017; Lushchak et al., 2017; Maiese, 2017d; Stefanatos & Sanz, 2018) and broader access to preventive care are believed to have contributed to the increased life span of the world’s population. .
Neurodegenerative disorders form a significant component for NCDs and include more than 600 disease entities that can progressively lead to nervous system dysfunction (Z. Z. Chong, Y. C. Shang, S. Wang, & K. Maiese, 2012; Maiese, 2014b). Both acute and chronic neurodegenerative disorders lead to disability and death for a significant number of individuals worldwide that can approach greater than 30 million individuals worldwide (Borjini et al., 2019; Handa et al., 2019; Maiese, 2019b, 2020).
As a result of improvements in global healthcare and the progressive increase in life span, neurodegenerative disorders are expected to increase in prevalence among the world’s population. For example, the incidence of sporadic cases of Alzheimer’s disease (AD) is expected to significantly increase throughout the globe (Maiese, 2019b; Su et al., 2019). Dementia is now considered to be the 7th leading cause of death. According to the World Health Organization (World Health Organization, 2017), dementia affects all countries throughout the world at a significant financial burden. Cognitive disorders that include AD affect more than 5 million individuals in the United States (US) alone (Filley et al., 2007; Maiese, 2014c). At minimum, 60 percent of dementia cases result from AD (Chong, Li, & Maiese, 2005; Maiese, 2014c, 2015g; Maiese, Chong, Shang, & Wang, 2013a). At least 5 percent of the world’s elderly population suffer from dementia and this represents 50 million individuals. With new cases increasing at a rapid rate, it is estimated by the year 2030, 82 million people are expected to have dementia. If once considers the year 2050, 152 million individuals will suffer from dementia.
Consideration for the financial and service burdens for neurodegenerative disorders are equally staggering. Prior estimates for the cost of neurodegenerative disorders exceeded $700 billion United States dollars (USD) in the United States (US) alone and included dementias, stroke, back pain, epilepsy, trauma to the nervous system, and Parkinson’s disease. Currently, dementia care is considered the most significant cost factor with more than $800 billion USD spent to care for individuals with dementia on an annual basis. These costs approximate 2 percent of the global gross domestic product. By the year 2030, medical and social services could reach in the US to 2 trillion USD annually with the ability to easily overwhelm the system. These projections fail to include the significant financial costs that involve social and adult living care as well as informal and companion care for individual families. In addition, the World Health Organization estimates the need for close to 60 million new health and social care workers (World Health Organization, 2011, 2012, 2017). Other challenges for the care of individuals with neurodegenerative disorders also exist such as when to address the need for these healthcare workers in a timely and implementation of healthcare workers that will occur in efficient manner, since the onset and progression of dementia in individuals is not always well recognized. An additional consideration is that dementia is considered to be under diagnosed throughout the world (Maiese, 2019b, 2019c). Once diagnosis is correctly performed, it can be in the late or end stages of the disease, leaving little time or options for treatment and possibly leading to fragmented care.
Metabolic Disease and Neurodegenerative Disease
Diabetes mellitus (DM) is a significant metabolic disorder of the NCDs that has widespread implications for new treatment options for the global population (Esterline, Vaag, Oscarsson, & Vora, 2018; Klimontov et al., 2019; Maiese, 2016d, 2020). At present, an estimated 460 million individuals currently have DM (Haldar et al., 2015; Jia, Aroor, Martinez-Lemus, & Sowers, 2014; Maiese, 2015a; Maiese, 2015g; Ye & Fu, 2018). At least another 374 million individuals are believed to have metabolic disease and are at risk for developing DM but presently are undiagnosed (Harris & Eastman, 2000; International Diabetes Federation, 2019; Maiese, 2015e; Maiese, Chong, & Shang, 2007). The number of individuals with DM is expected to rise to 700 million individuals by 2045 according to the International Diabetes Federation (International Diabetes Federation, 2019). Obesity and impaired glucose tolerance are closely linked as factors that lead to the development of DM (Barchetta, Cimini, Ciccarelli, Baroni, & Cavallo, 2019; Maiese, 2015a; A. R. Wang et al., 2018). Impaired glucose tolerance and obesity increases the risk of developing DM in young individuals (Maiese, Chong, Shang, & Hou, 2011). Obesity and excess body fat can affect stem cell proliferation, aging, inflammation, oxidative stress injury, and mitochondrial function (Cernea, Tang, Guan, & Yang, 2016; Curjuric et al., 2016; Hill, Solt, & Foster, 2018; Z. Liu, Gan, Zhang, Ren, & Sun, 2018; Maiese, 2016e; Mehta, Rayalam, & Wang, 2018; A. R. Wang et al., 2018).
In regards to the financial considerations for the treatment and care of individuals with DM, almost 80 percent of adults with DM are living in low- and middle-income countries (International Diabetes Federation, 2019). More than $20,000 USD are required to care for each individual with DM per year. The care for patients with DM equals approximately $760 billion USD (International Diabetes Federation, 2019) and consumes more than 17 percent of the Gross Domestic Product in the US as reported by the Centers for Medicare and Medicaid Services (CMS) (Centers for Medicare and Medicaid Services, 2019). With the loss of function and disability that results from DM, an estimated $69 billion USD are consumed from reduced productivity linked to DM.
DM affects all systems of the body including the vascular system and the peripheral and central nervous systems. DM can lead to retinal disease (Maiese, 2015b; Mishra, Duraisamy, & Kowluru, 2018; Ponnalagu, Subramani, Jayadev, Shetty, & Das, 2017), endothelial dysfunction (Arildsen, Andersen, Waagepetersen, Nissen, & Sheykhzade, 2019; S. Ding et al., 2018; Maiese, 2015g, 2018d; Pal, Sonowal, Shukla, Srivastava, & Ramana, 2019), cardiovascular disease (Alexandru, Popov, & Georgescu, 2012; Barchetta et al., 2019; Chiu et al., 2017; S. Ding et al., 2018; Esterline et al., 2018; Maiese, 2016b; Maiese et al., 2007; Maiese, Chong, Shang, & Hou, 2008; Maiese, Li, Chong, & Shang, 2008; Perez-Hernandez et al., 2016; Thackeray et al., 2011), immune function disorders (Kell & Pretorius, 2018; X. Lin & Zhang, 2018; Maiese, 2015c; Maiese, Chong, Shang, & Wang, 2011; Woodhams & Al-Salami, 2017; Y. Zhao et al., 2015), inflammation (Maiese, 2015b; Mishra et al., 2018; Ponnalagu et al., 2017), and injury to the neurovascular unit (Maiese, 2015b; Mishra et al., 2018; Ponnalagu et al., 2017). In the peripheral nervous system, at least 70 percent of individuals with DM can develop some degree of diabetic peripheral neuropathy. DM can lead to both autonomic neuropathy (Albiero et al., 2014) and peripheral nerve disease (Gomes & Negrato, 2014; Gomez-Brouchet et al., 2015). Assessments of the progress of peripheral neuropathies can be challenging, since the disorder is chronic in nature, may be sub-clinical, and prior deficits may go undetected if improved control over glucose homeostasis is initiated. In the central nervous system, DM leads to insulin resistance and dementia that occurs in patients with AD (Caberlotto et al., 2019; Maiese, 2020; Su et al., 2019). DM can affect multiple cellular pathways that lead to the progression of cognitive loss (Maiese, 2015g; Maiese, Chong, Shang, & Hou, 2009; Tang, Ng, Ho, Gyda, & Chan, 2014; Xiang, Mittwede, & Clemmer, 2015; Y. J. Xu, Tappia, Neki, & Dhalla, 2014; Yao, Fujimura, Murayama, Okumura, & Seko, 2017). DM also has been linked to mental illness (Hadamitzky et al., 2018; Ignacio et al., 2016), cerebral vascular injury (Di Rosa & Malaguarnera, 2016; Maiese, 2015e, 2015g; Maiese, Chong, Hou, & Shang, 2008; Tulsulkar, Nada, Slotterbeck, McInerney, & Shah, 2015; F. H. Xiao et al., 2015), impairment of microglial activity (Caberlotto et al., 2019; Maiese, 2020; Su et al., 2019), and can impact stem cell proliferation (Maiese, 2015g; Maiese et al., 2009; Tang et al., 2014; Xiang et al., 2015; Y. J. Xu et al., 2014; Yao et al., 2017).
Innovative Approaches to Target Metabolic Disease and Neurodegenerative Disease
Metabolic disorders, such as DM, pose a significant challenge for the treatment of neurodegenerative disorders. Early diagnosis of DM and quickly focusing on therapeutic targets can offer some degree of protection. Rapid treatment may inhibit the progression of DM (Guo et al., 2019; Klimova & Kristian, 2019; Maiese, 2015a; Maiese, 2018d; Maiese, Chong, Shang, & Wang, 2013b; Othman et al., 2018; Yelumalai, Giribabu, Karim, Omar, & Salleh, 2019). However, tight serum glucose control does not always resolve the complications from DM (Coca, Ismail-Beigi, Haq, Krumholz, & Parikh, 2012; Maiese, Chong, Shang, & Hou, 2011). Use of diet control treatments may be effective to prevent hyperglycemic events, but these strategies have potential risks that can decrease organ mass through processes that involve autophagy (J. H. Lee et al., 2014). In addition to DM, there are other risk factors for neurodegenerative disorders and cognitive loss that include hypertension, low education in early life, and tobacco use (Maiese, 2019b, 2019c). Vascular disease as a result of DM also may lead to dementia (F. Chen et al., 2018; Maiese, 2018a, 2018d; Ong, Wu, Farooqui, & Farooqui, 2018). Current therapies for cognitive loss and AD that may be attributed to metabolic dysregulation are considered to be focused primarily on symptomatic care (Caberlotto et al., 2019; Maiese, 2019c; Su et al., 2019; Sun, Martin, Vanderpoel, & Sumbria, 2019; Ullah, Khan, Shah, Saeed, & Kim, 2019; H. Yu & Blair, 2019). Treatments that are directed to treat AD alone involve the use of cholinesterase inhibitors that may alleviate symptoms, but not disease progression (Ruhal & Dhingra, 2018). Strategies for dementia as a result of vascular disease focus on vascular and metabolic disorders, such as DM (Maiese, 2018b). Novel avenues of discovery are required to address metabolic dysfunction in tandem with neurodegenerative disease, such as cognitive loss. One innovative strategy that offers exciting prospects to fruitfully target both metabolic disease and neurodegenerative disorders involves the mechanistic target of rapamycin (mTOR) and its associated pathways of mTOR Complex 1 (mTORC1), mTOR Complex 2 (mTORC2), and AMP activated protein kinase (AMPK).
The Mechanistic Target of Rapamycin
The mechanistic target of rapamycin (mTOR), a 289-kDa serine/threonine protein kinase, is encoded by a single gene FRAP1 (Z. Z. Chong et al., 2012; Esterline et al., 2018; Jesko, Stepien, Lukiw, & Strosznajder, 2019; Maiese, 2016b) (Figure 1). mTOR also is termed the mammalian target of rapamycin and the FK506-binding protein 12-rapamycin complex-associated protein 1 (Maiese, 2018b, 2018c). The target of rapamycin (TOR) was initially discovered in Saccharomyces cerevisiae with the genes TOR1 and TOR2 (Maiese, Chong, et al., 2013a). Through the use of rapamycin-resistant TOR mutants, TOR1 and TOR2 were found to encode the Tor1 and Tor2 isoforms in yeast (Heitman, Movva, & Hall, 1991). The agent rapamycin is a macrolide antibiotic in Streptomyces hygroscopicus that blocks TOR and mTOR activity (Maiese, 2015e).
Figure 1: Novel Therapeutic Avenues with mTOR.
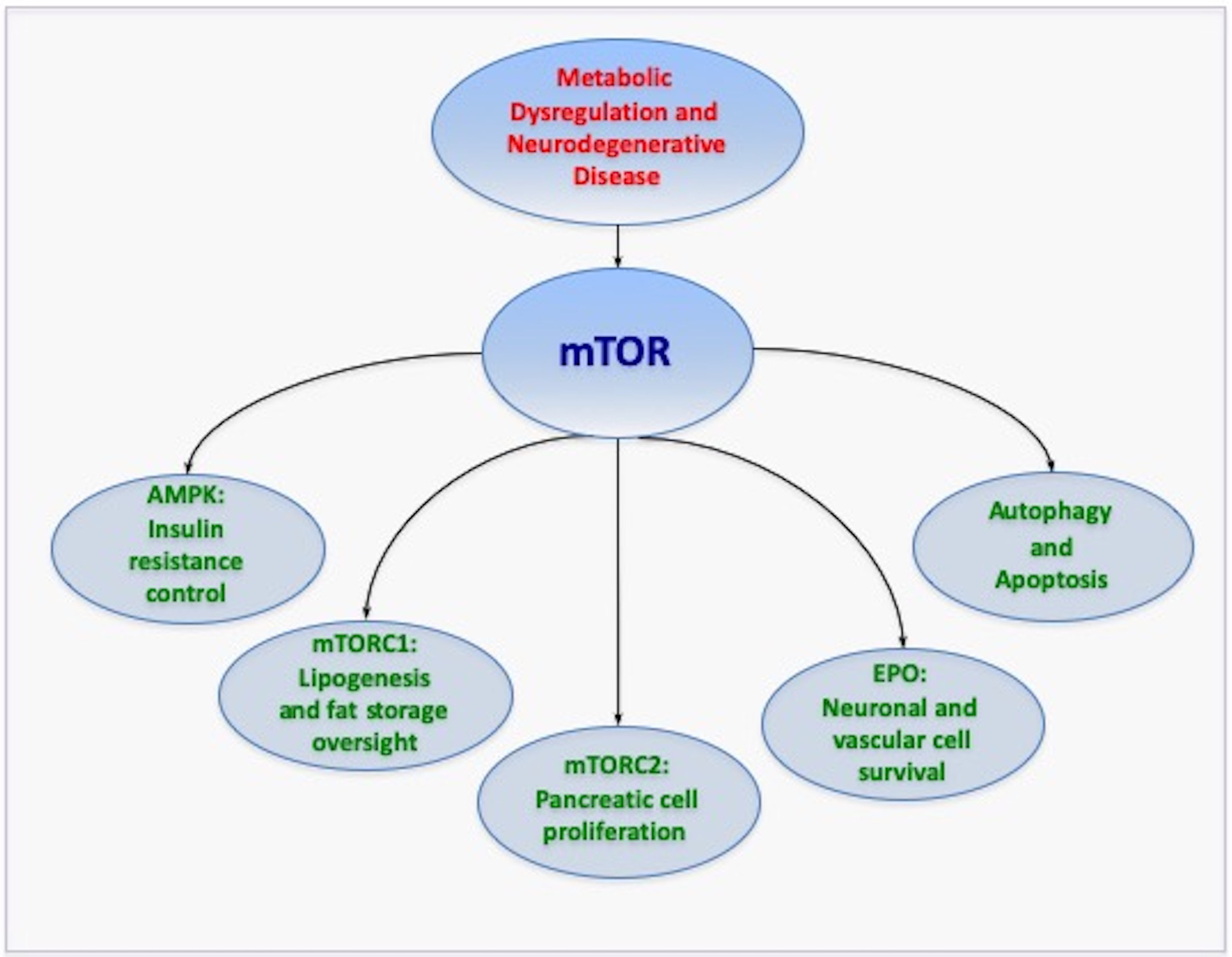
The mechanistic target of rapamycin (mTOR) and its associated pathways of mTOR Complex 1 (mTORC1), mTOR Complex 2 (mTORC2), AMP activated protein kinase (AMPK), and the trophic factor erythropoietin offer novel therapeutic avenues to treat neurodegenerative disorders through metabolic pathways. mTOR and its pathways broadly impact metabolic disease and the nervous system through the oversight of programmed cell death pathways of autophagy and apoptosis. Given that current therapies for metabolic disease and neurodegenerative disorders address only symptomatic care, mTOR and its associated pathways provide strong prospects to treat both the onset and progression of metabolic disease in association with neurodegenerative disorders. Yet, it is clear that comprehensive understanding is required to appreciate the large breadth of mTOR pathways to safely translate treatments to clinical medicine without the onset of unexpected clinical disabilities.
mTOR plays a significant role as the principal component of the protein complexes mTOR Complex 1 (mTORC1) and mTOR Complex 2 (mTORC2) (Hwang & Kim, 2011; Maiese, 2016c; Martinez de Morentin et al., 2014). Rapamycin can prevent mTORC1 activity by binding to immunophilin FK-506-binding protein 12 (FKBP12) that attaches to the FKBP12 -rapamycin-binding domain (FRB) at the carboxy (C) -terminal of mTOR to interfere with the FRB domain of mTORC1 (Maiese, 2014c). The mechanism of how rapamycin blocks mTORC1 activity with the interaction of the domain of FRB is unclear. One consideration may involve allosteric changes on the catalytic domain as well as the inhibition of phosphorylation of protein kinase B (Akt) and p70 ribosomal S6 kinase (p70S6K) (Xue et al., 2009). mTORC1 appears to be more sensitive to inhibition by rapamycin than mTORC2, but chronic administration of rapamycin can inhibit mTORC2 activity as a result of the disruption of the assembly of mTORC2.
mTORC1 and mTORC2 are divided into subcomponents. mTORC1 is composed of Raptor, the proline rich Akt substrate 40 kDa (PRAS40), Deptor (DEP domain-containing mTOR interacting protein), and mammalian lethal with Sec13 protein 8, termed mLST8 (mLST8) (Maiese, Chong, et al., 2013a). mTORC1 can bind to its constituents through the protein Ras homologue enriched in brain (Rheb) that phosphorylates the Raptor residue serine863 and other residues that include serine859, serine855, serine877, serine696, and threonine706 (Foster et al., 2010). The inability to phosphorylate serine863 limits mTORC1 activity, as shown using a site-direct mutation of serine863 (L. Wang, Lawrence, Sturgill, & Harris, 2009). mTOR can control Raptor activity which can be blocked by rapamycin (L. Wang et al., 2009). Deptor, an inhibitor as well, blocks mTORC1 activity by binding to the FAT domain (FKBP12 -rapamycin-associated protein (FRAP), ataxia-telangiectasia (ATM), and the transactivation/transformation domain-associated protein) of mTOR. If the activity of Deptor is decreased, Akt, mTORC1, and mTORC2 activities are increased (Peterson et al., 2009). PRAS40 is known to block mTORC1 activity by preventing the association of p70 ribosomal S6 kinase (p70S6K) and the eukaryotic initiation factor 4E (eIF4E)-binding protein 1 (4EBP1) with Raptor (Maiese, 2014b; Malla, Ashby, et al., 2015). mTORC1 is active once PRAS40 is phosphorylated by Akt. This releases PRAS40 from Raptor to sequester PRAS40 in the cell cytoplasm with the docking protein 14–3-3 (Zhao Zhong Chong, Yan Chen Shang, Shaohui Wang, & Kenneth Maiese, 2012; Fonseca, Smith, Lee, MacKintosh, & Proud, 2007; Shang, Chong, Wang, & Maiese, 2012b; H. Wang et al., 2012; Xiong et al., 2014). mLST8, in contrast to Deptor and PRAS40, promotes mTOR kinase activity. This involves the binding of p70S6K and 4EBP1 to Raptor (D. H. Kim et al., 2003). Interestingly, mLST8 also controls insulin signaling through the transcription factor FoxO3 (Guertin et al., 2006; Maiese, 2015c), is necessary for Akt and protein kinase C-α (PKCα) phosphorylation, and is required for Rictor to associate with mTOR (Guertin et al., 2006).
mTORC1 is associated with metabolic cellular pathways (Esterline et al., 2018; Maiese, 2016d). mTORC1 can stimulate lipogenesis and fat storage (Chakrabarti, English, Shi, Smas, & Kandror, 2010), may increase pancreatic ß-cell mass (Hamada et al., 2009), and can improve glucose homeostasis (Malla, Wang, Chan, Tiwari, & Faridi, 2015). However, in the presence of other cellular pathways and systems such as with silent mating type information regulation 2 homolog 1 (Saccharomyces cerevisiae) (SIRT1), mTORC1 activity may require down-regulation to preserve glucose homeostasis (Y. Li et al., 2011).
mTORC2 has similarities as well as differences to mTORC1. mTORC2 is composed of Rictor, mLST8, Deptor, the mammalian stress-activated protein kinase interacting protein (mSIN1), and the protein observed with Rictor-1 (Protor-1) (Z. Z. Chong et al., 2012; Maiese, 2014b). mTORC2 controls cytoskeleton remodeling through PKCα and cell migration through the Rac guanine nucleotide exchange factors P-Rex1 and P-Rex2 and through Rho signaling (Jacinto et al., 2004). mTORC2 activates protein kinases that includes glucocorticoid induced protein kinase 1 (SGK1), a member of the protein kinase A/protein kinase G/protein kinase C (AGC) family of protein kinases. Protor-1, a Rictor-binding subunit of mTORC2, activates SGK1 (Garcia-Martinez & Alessi, 2008; Pearce, Sommer, Sakamoto, Wullschleger, & Alessi, 2011). The kinase domain of mTOR phosphorylates mSIN1 and prevents lysosomal degradation of this protein. Rictor and mSIN1 also can phosphorylate Akt at serine473 and foster threonine308 phosphorylation by phosphoinositide-dependent kinase 1 (PDK1) to enhance cell survival.
mTORC2 may be necessary to maintain glucose homeostasis (Maiese, 2016d), since loss of this pathway can promote severe hyperglycemia (Treins et al., 2012). Impairment in mTORC2 signaling also leads to oxidative damage and insulin resistance (R. H. Wang et al., 2011). In addition, mTORC2 signaling plays a significant role for the maintenance of pancreatic β-cell proliferation and mass (Gu, Lindner, Kumar, Yuan, & Magnuson, 2011).
AMP activated protein kinase (AMPK): A Negative Modulator of mTOR
The AMP activated protein kinase (AMPK) is closely tied to the mTOR pathway and metabolic disease (Chiu et al., 2017; Du et al., 2015; Jiang et al., 2014; Maiese, 2020) (Figure 1). AMPK can prevent mTORC1 activity through the hamartin (tuberous sclerosis 1)/tuberin (tuberous sclerosis 2) (TSC1/TSC2) complex that inhibits mTORC1 (Maiese, 2017c; Peixoto, de Oliveira, da Rocha Araujo, & Nunes, 2017). Control of the TSC1/TSC2 complex also is overseen though phosphoinositide 3-kinase (PI 3-K), Akt, and its phosphorylation of TSC2. Extracellular signal-regulated kinases (ERKs), protein p90 ribosomal S6 kinase 1 (RSK1), and glycogen synthase kinase −3β (GSK-3β) also can modulate the activity of the TSC1/TSC2 complex. TSC2 functions as a GTPase-activating protein (GAP) that converts G protein Rheb (Rheb-GTP) into the inactive GDP-bound form (Rheb-GDP). Once Rheb-GTP is active, Rheb-GTP associates with Raptor to oversee the binding of 4EBP1 to mTORC1 and increase mTORC1 activity (Sato, Nakashima, Guo, & Tamanoi, 2009). AMPK phosphorylates TSC2 to increase GAP activity to change Rheb-GTP into the inactive Rheb-GDP and to block mTORC1 activity (Inoki, Zhu, & Guan, 2003).
mTOR Pathways and the Intimate Link Between Autophagy and Apoptosis
mTOR and its downstream pathways of AMPK oversee pathways of both autophagy and apoptosis (Cheng et al., 2018; J. Dong, Li, Bai, & Wu, 2019; Dorvash, Farahmandnia, & Tavassoly, 2020; Maiese, 2015d, 2015e, 2016g; Zimmerman, Biggers, & Li, 2018) (Figure 1). This relationship with autophagy and apoptosis even extends to novel signaling systems that involve hydrogen sulfide (Maiese, 2019d; Wu et al., 2018; Q. Xiao, Ying, Xiang, & Zhang, 2018). Programmed cell death includes the pathways of autophagy and apoptosis (Klionsky et al., 2016; Maiese, 2017a; Maiese, Chong, Shang, & Wang, 2012b). Autophagy recycles components of the cytoplasm in cells for tissue remodeling (Preau et al., 2019; Y. Wang & Le, 2019; N. Zhang & Zhao, 2019) and eliminates non-functional organelles (C. Chen et al., 2017; Di Rosa, Distefano, Gagliano, Rusciano, & Malaguarnera, 2016; Klionsky et al., 2016; Maiese, 2015e; Maiese et al., 2012b). Macroautophagy recycles organelles and sequesters cytoplasmic proteins and organelles into autophagosomes. Autophagosomes then combine with lysosomes for degradation and recycling (Maiese, 2014b; White, Datta, & Giordano, 2017). Microautophagy involves lysosomal membranes invagination for the sequestration and digestion of cytoplasmic components (Maiese, 2015f). Chaperone-mediated autophagy (Maiese, 2015c) uses cytosolic chaperones to transport cytoplasmic components across lysosomal membranes (Moors et al., 2017).
Approximately 33 autophagic related genes (Atg) that have been identified in yeast with TOR that can affect multiple disorders including DM (Maiese, 2015e; Weckman et al., 2014). Atg1, Atg13 (also known as Apg13), and Atg17 are associated with Akt and TOR pathways (Klionsky et al., 2016). Either the Atg1 complex in yeast or the UNC-51 like kinase 1 (ULK1) complex in mammals is requires for the induction of autophagy (Kamada et al., 2000; Maiese et al., 2012b). The mammalian homologues of Atg1 are UNC-51 like kinase 1 (ULK1) and ULK2 (Z. Z. Chong et al., 2012). Mammalian Atg13 binds to ULK1, ULK2, and FIP200 (focal adhesion kinase family interacting protein of 200 kDa) to activate ULKs, promote the phosphorylation of FIP200 by ULKs, and lead to autophagy induction (Jung et al., 2009). mTOR activity blocks the onset of autophagy by phosphorylating Atg13 and ULKs to prevent formation of the ULK-Atg13-FIP200 complex (Jung et al., 2009).
Autophagy can be important for clinical aging pathways (Maiese, 2016a, 2017d; Y. Wang & Le, 2019). Studies with Drosophila show that neural aggregate accumulation observed with aging is linked to a reduction in the autophagy pathway. These neural aggregates lead to behavior impairments that can be resolved with the maintenance of autophagy pathways in neurons (Ratliff et al., 2015).
Autophagy is involved in a number of metabolic and neurodegenerative disorders, such as dementia (Crino, 2016; Hsieh, Liu, Lee, Yu, & Wang, 2019; M. Hu et al., 2017; Maiese, 2016a; Maiese, 2017b; Su et al., 2019), AD (Caberlotto et al., 2019; Cheng et al., 2018; Han, Jia, Zhong, & Shang, 2017; Hsieh et al., 2019; L. Li, 2017; Maiese, 2015g, 2017d; Saleem & Biswas, 2017; Su et al., 2019; Z. H. Zhang et al., 2017), Parkinson’s disease (Z. Z. Chong et al., 2012; Jang et al., 2015; Maiese, 2015b; Maiese, Chong, Wang, & Shang, 2013; Moors et al., 2017; Niranjan, Mishra, & Thakur, 2018; Savitt & Jankovic, 2019; Wen et al., 2018), Huntington’s disease (J. H. Lee et al., 2015; Maiese, 2015b, 2018b; Vidal et al., 2012), and DM (Caberlotto et al., 2019; Esterline et al., 2018; Hsieh et al., 2019; Maiese, 2015a; Maiese, 2015a, 2015b; Su et al., 2019; Tong et al., 2018).
In many cases, activation of autophagy with the concurrent inhibition of mTOR can result in neuroprotection in the nervous system (Maiese, 2016c). Autophagy may be particularly important for memory processing in individuals. Tau and Aß neurotoxicity may be reduced with autophagy (Cheng et al., 2018; Morris, Berk, Maes, & Puri, 2019; Z. H. Zhang et al., 2017). Activation of autophagy may be able to reduce Aß levels in the brain as one possible component to limit neurodegenerative decline (Cheng et al., 2018; Maiese, 2017b; Saleem & Biswas, 2017; Su et al., 2019). In regards to pathways associated with mTOR, learning and memory may be preserved with low calorie diets that promote autophagy and limit mTOR activity (W. Dong et al., 2016). Similar results of neuronal protection have also been noted with improved memory, greater insulin signaling, and autophagy activation that can decrease mTOR activity and increase Aß clearance (Han et al., 2017). Other studies find improvements in memory with increased autophagic flux, mTOR inhibition, and resultant tau clearance (Z. H. Zhang et al., 2017).
However, oversight of autophagy is vitally important in a number of scenarios that require mTOR activation for the nervous system. For example, interneuron progenitor growth in the brain relies upon mTOR activity with the inhibition of autophagy (Ka, Smith, & Kim, 2017). In addition, autophagy activation can impair endothelial progenitor cells, lead to mitochondrial oxidative stress, and block new blood vessel formation during elevated glucose exposure (K. A. Kim et al., 2014). During ischemic stroke in rodents, blockade of autophagy may also limit infarct size and rescue cerebral neurons (Yin et al., 2013). Trophic factors that can provide both neuronal and vascular protection in the nervous system, such as erythropoietin (EPO) (Chong, Kang, & Maiese, 2002; Maiese, 2016f; Maiese, Li, & Chong, 2005), can offer protection against neurotoxic by limiting the induction of autophagy and promoting the activation of mTOR (Jang et al., 2015; Maiese, 2017e) (Figure 1). EPO can regulate a number of components of the mTOR pathway, such as PRAS40 and Akt, to promote neuroprotection (Zhao Zhong Chong et al., 2012; H. J. Lee, Koh, Song, Seol, & Park, 2016; Maiese, Chong, Shang, & Wang, 2012a; G. B. Wang, Ni, Zhou, & Zhang, 2014).
In contrast to autophagy, apoptosis has two distinct phases. The early phase involves the loss of plasma membrane phosphatidylserine (PS) asymmetry (Hou, Chong, Shang, & Maiese, 2010; Maiese & Vincent, 2000; Schutters & Reutelingsperger, 2010; Shang, Chong, Hou, & Maiese, 2010; Taveira et al., 2018; Wei et al., 2013; Williams & Dexter, 2014) and a subsequent later phase that leads to genomic DNA degradation (Hou, Wang, Shang, Chong, & Maiese, 2011; S. Kim et al., 2015; S. L. Liu et al., 2017; Lu, Shen, Yang, & Gu, 2016; Maiese, 2012; Taveira et al., 2018). Apoptosis is the result of a cascade activation of nucleases and proteases that involve caspases (Chong et al., 2005; Dai et al., 2017; C. Ding et al., 2017; Gao et al., 2017; Maiese, 2016a; A. Park & Koh, 2019). These processes can influence both the early phase of apoptosis with the loss of plasma membrane PS asymmetry and a later phase that leads to genomic DNA degradation. Loss of membrane PS asymmetry activates inflammatory cells to target, engulf, and remove injured cells (Bailey, Fossum, Fimbel, Montgomery, & Hyde, 2010; Hou et al., 2010; Shang, Chong, Hou, & Maiese, 2009; Wei et al., 2013). Yet, if the engulfment of inflammatory cells can be prevented and not be removed from the nervous system, then functional cells expressing membrane PS residues can be rescued (S. Kim et al., 2015; Maiese, 2015b; Xin et al., 2015; T. Yu et al., 2015). On the flip side, once the destruction of cellular DNA occurs, it is usually not considered to be completely reversible (Maiese, 2015b).
mTOR activation usually can act to prevent apoptotic cell death in the nervous system (Z. Z. Chong et al., 2012; Maiese, 2018c; Wen et al., 2018; Q. Zhou et al., 2016). During periods of mTOR activity, microglia can be protected from oxidative stress (Shang, Chong, Wang, & Maiese, 2011), retinal ganglion cell regeneration occurs (Teotia, Van Hook, Fischer, & Ahmad, 2019), and diabetic peripheral neuropathy can be reduced (J. Dong et al., 2019). This reduction by mTOR of oxidative stress induction has been suggested to be protective in neurodegenerative disorders such as Parkinson’s disease (PD) (C. Zhang et al., 2017). In addition with mTOR activation, Aß toxicity can be prevented (Bellozi et al., 2016; Morris et al., 2019; Shang, Chong, Wang, & Maiese, 2012a; Shang et al., 2012b; Shang, Chong, Wang, & Maiese, 2013; Y. Wang et al., 2015), vascular cell death minimized (Maiese, 2014c; J. A. Park & Lee, 2017), oxidative stress with mitochondrial injury can be blocked (Dai et al., 2019), neuroplasticity can be enhanced (Farmer et al., 2019), neuronal differentiation can be promoted (Huang et al., 2019), neonatal central nervous system hypoxic injury can be averted (Xi, Wang, Long, & Ma, 2018), and reduced stroke volume with decreased apoptotic cell death dependent upon circadian rhythm activity can occur (Beker et al., 2018).
However, it should be noted that apoptosis has an inverse relationship with autophagy (Y. Dong et al., 2019; Maiese, 2017e). Activation of autophagy blocks the activity of mTOR (Dorvash et al., 2020; Maiese, 2019a, 2020). Under some circumstances, cellular protection can occur that ultimately leads to mTOR inhibition. For example, autophagy activation with the blockade of mTOR can prevent injury to dopamine dependent cells (A. Park & Koh, 2019), decrease reactive oxygen species release (Javdan et al., 2018), control neuroprotection with glutamine dependent mechanisms (Y. Zhao et al., 2019), and prevent mitochondrial dysfunction (Dai et al., 2017). In addition, recent work demonstrates that limitations in mTOR activity may reduce markers of senescence and aging in human skin (Chung et al., 2019) and limit aging with extension of cell longevity through AMPK in endothelial cells (H. Zhang et al., 2019). These studies highlight the intimate link between autophagy and apoptosis that center upon mTOR that must be considered for the development of new treatment strategies in the nervous system. The oversight of autophagy and apoptosis require careful modulation since inhibition of mTOR pathways with autophagy activation also can control tumor cell growth (Mu et al., 2019; L. Zhou et al., 2019). Other studies also point to the balance required during embryogenesis for autophagy and apoptosis that can affect body axis formation (G. Xu et al., 2019).
Targeting Metabolic Dysfunction with mTOR Pathways
mTOR has a significant role in cellular metabolic function (Chong & Maiese, 2012; Esterline et al., 2018) (Figure 1). In patients with metabolic syndrome, mTOR activation has been found to be diminished and possibly responsible for insulin resistance and the increased risk of vascular thrombosis (Pasini et al., 2010). Activation of mTOR pathways with p70S6K and 4EBP1 can improve insulin secretion in pancreatic β-cells and increase resistance to β-cell streptozotocin toxicity and obesity in mice (Hamada et al., 2009). Loss of p70S6K activity leads to hypo-insulinemia and glucose intolerance with decreased pancreatic β-cell size (Pende et al., 2000). Activation of mTOR also can protect pancreatic β- cells against cholesterol-induced apoptosis (J. Zhou, Wu, Zheng, Jin, & Li, 2015), lead to enhanced neuronal cell survival in cell models of DM (Y. W. Liu et al., 2015), and lessen glucolipotoxicity (Miao et al., 2013). mTOR activity also appears to be able to modulate insulin signaling in experimental models of AD and maintain astrocyte viability (Crespo et al., 2017), allow for the differentiation of adipocytes (Jung et al., 2015), prevent endothelial cell dysfunction during hyperglycemia (Pal et al., 2019), and preserve glucose homeostasis (Malla, Wang, et al., 2015). In regards to nutrition, mTOR may offer protection as a component of the Mediterranean diet. This diet has been tied to a reduction in Aβ toxicity in astrocytes through enhanced Akt activity by consumption of polyphenol of olives and olive oil that ultimately could prevent the onset or progression of AD (Crespo et al., 2017).
Modulation of the activity of mTOR appears to be a critical point in targeting this pathway. During mTOR inhibition with rapamycin, reduced β-cell function, insulin resistance, and decreased insulin secretion can lead to the progression of DM (Fraenkel et al., 2008). Decreased activity of mTOR has been shown to increase mortality in a mouse model of DM (Sataranatarajan et al., 2016). Without mTOR activity, translocation of glucose transporters to the plasma membrane in skeletal muscle are also affected (Deblon et al., 2012). Interestingly, inhibition of mTOR during DM can sometimes offer protection such as during cerebral ischemia (P. Liu et al., 2016) and be necessary for maintaining a balance between pancreatic β-cell proliferation and cell size (Gu et al., 2011). This suggests that there may be biological feedback pathways with mTOR, such as through AMPK inhibition, to prevent excessive mTOR activity. For example, if mTOR activity is allowed to go unchecked with the down-regulation of AMPK activity during experimental studies, mTOR and p70S6K can lead to glucose intolerance by inhibiting the insulin receptor substrate 1 (IRS-1) (Kang, Chemaly, Hajjar, & Lebeche, 2011).
In addition to potential feedback pathways with mTOR, loss of mTOR activity during metabolic disease may be beneficial through the activation of pathways associated with autophagy. Current studies point to the dysregulation of autophagy as a central pathway that can potentially lead to the progression of cognitive loss with AD and the induction of DM (Caberlotto et al., 2019). At least 33 autophagy-related genes (Atg) that have been identified in yeast and 40 autophagy-related genes involved in the formation of autophagosomes (N. Zhang & Zhao, 2019) that can affect multiple disorders including DM (Maiese, 2015c, 2016d; Weckman et al., 2014). Atg1, Atg13 (also known as Apg13), and Atg17 are associated with the PI 3-K, Akt, and TOR pathways (Klionsky et al., 2016). Earlier studies have shown that autophagy haploinsufficiency with deletion of Atg7 gene in mouse models of obesity promotes increased insulin resistance with elevated lipids and inflammation (Lim et al., 2014). Loss of autophagic proteins Atg7, Atg5, and LC3 can be responsible for diabetic nephropathy (Ma et al., 2016). Autophagy can remove misfolded proteins and eliminate non-functioning mitochondria to maintain β-cell function and prevent the onset of DM (Z. Liu, Stanojevic, Brindamour, & Habener, 2012). Exercise in mice has been shown to promote the induction of autophagy and regulate glucose homeostasis (He et al., 2012). Autophagy can improve insulin sensitivity during high fat diets in mice (Y. Liu et al., 2014) and may be protective to microglia during acute glucose fluctuations (Hsieh et al., 2019).
As a pathway of mTOR, AMPK is vital in regulating cellular metabolism and the pathways of programmed cell death. AMPK has been shown to reduce insulin resistance, since the loss of AMPK results in reduced tolerance to the development of insulin resistance (Y. Liu et al., 2014). AMPK also is involved in the protection of endothelial progenitor cells during periods of hyperglycemia (Chiu et al., 2017). During periods of dietary restriction that may increase lifespan (Balan et al., 2008), AMPK can be one factor to shift to beneficial oxidative metabolism (Moroz et al., 2014). As a result, AMPK can reduce ischemic brain damage in diabetic animal models (P. Liu et al., 2016). In regards to cognition, AMPK activation can improve memory retention in models of AD and DM (Du et al., 2015), may assist with the elimination of ß-amyloid (Aß) in the brain (Zhao, Wang, Wang, & Chen, 2015), facilitate tau clearance (Z. H. Zhang et al., 2017), modulate chronic inflammation in neurodegenerative disorders (Maiese, 2016g, 2017d; Peixoto et al., 2017), and limit Aß neurotoxicity (C. L. Lin et al., 2015).
AMPK functions through both apoptosis and autophagy. During periods of hyperglycemia, AMPK activity may be necessary to increase basal autophagy activity (Klionsky et al., 2016; Maiese, 2015b) and maintain endothelial cell survival (Pal et al., 2019; Weikel, Cacicedo, Ruderman, & Ido, 2016). AMPK also can regulate apoptosis and autophagy during coronary artery disease (Y. Dong et al., 2019), endothelial dysfunction during hyperglycemia (Pal et al., 2019), and oxidative stress cell injury (Shokri Afra et al., 2019; D. Zhao et al., 2019). Anti-senescence activity also can be fostered through mTOR inhibition, AMPK activation, and the acceleration of autophagic flux (H. Zhang et al., 2019).
Present agents used to treat DM such as biguanides and metformin rely upon mTOR and autophagy pathways to restore cellular function. Metformin inhibits mTOR activity, promotes autophagy, and may function at times in an AMPK-independent manner (Kalender et al., 2010). Other reports note that through metformin, AMPK is activated, restores the induction of autophagy, and protects against diabetic cardiac cell apoptosis (He, Zhu, Li, Zou, & Xie, 2013). Metformin also has been shown to limit lipid peroxidation in the brain and spinal cord and decrease caspase activity during toxic insults (Oda, 2017). Such results may be associated with the ability of autophagic pathways to limit oxidative stress under some circumstances (Maiese, 2015a; Zimmerman et al., 2018).
However, it should be recognized that autophagy pathways require a fine balance in activity. In some experimental models of AD, autophagy can be one factor that leads to neuronal cell death (Saleem & Biswas, 2017). Increased activity of autophagy can lead to significant loss of cardiac and liver tissue in diabetic rats during attempts to achieve glycemic control through diet modification (J. H. Lee et al., 2014). During periods of elevated glucose, advanced glycation end products (AGEs), agents that can result in complications during DM, have been shown to lead to the induction of autophagy and vascular smooth muscle proliferation that can result in atherosclerosis (P. Hu, Lai, Lu, Gao, & He, 2012) as well as cardiomyopathy (Y. Lee, Hong, Lee, & Chang, 2012). During elevated glucose exposure, autophagy also can impair endothelial progenitor cells, lead to mitochondrial oxidative stress (Martino et al., 2012), and block angiogenesis (K. A. Kim et al., 2014). Furthermore, chronic inflammatory conditions such as lichen planus have been linked to mTOR inhibition and autophagy activation (L. Wang et al., 2019).
Future Perspectives
More than 40 million people die from NCDs each year (Table 1). Even more concerning to note is that a significant proportion of the 15 million affected by NCDs are between 30 and 69 years old, impacting a significant portion of the growing global population. In addition, NCDs affect a much larger proportion of the population in low and middle-income countries with at least one-third of the population under the age of 60 suffering from NCDs. The rise in NCDs parallels the increase in life expectancy of the world’s population and includes a large component of disease entities that can progressively lead to neurodegenerative disorders. Of these neurodegenerative diseases, dementia is now considered to be the 7th leading cause of death. It is estimated by the year 2030, 82 million people are expected to have dementia and by the year 2050, 152 million individuals will suffer from dementia.
Table 1: Highlights.
The rise in non-communicable diseases (NCDs) parallels an increase in life expectancy of the world’s population leading to a heightened prevalence of metabolic disorders and neurodegenerative diseases, such as cognitive loss.
Metabolic disorders, such as diabetes mellitus, are significant risk factors for the development of neurodegenerative disease.
Given that current therapies for these NCDs address only symptomatic care, the mechanistic target of rapamycin (mTOR) and its associated pathways of mTOR Complex 1 (mTORC1), mTOR Complex 2 (mTORC2), AMP activated protein kinase (AMPK), and the trophic factor erythropoietin offer innovative strategies to treat neurodegenerative disorders through metabolic pathways.
mTOR and its pathways broadly impact metabolic disease and the nervous system through the oversight of programmed cell death pathways of autophagy and apoptosis.
mTOR and its associated pathways offer strong prospects to treat both the onset and progression of metabolic disease that can affect neurodegenerative disorders, but comprehensive understanding is required of the breadth of mTOR pathways to safely translate treatments to clinical medicine without the development of unexpected clinical disabilities.
Intimately tied to neurodegenerative disorders are metabolic disorders, such as DM. DM affects all systems of the body including the neurovascular systems. The number of individuals with DM is expected to rise to 700 million by the year 2045. New work highlights metabolic disorders and DM as significant risk factors that can affect the development of treatment strategies for neurodegenerative disorders. Innovative avenues of investigation are necessary to address metabolic dysfunction in tandem with neurodegenerative disease, especially those disorders that involve cognitive loss and AD.
Currently, treatments for DM and cognitive disease are limited. Tight serum glucose control does not always lead to the resolution of complications from DM (Coca et al., 2012; Maiese, Chong, Shang, & Hou, 2011). In addition, use of diet and body mass control may control hyperglycemic events, but these strategies also can potentially decrease organ mass through processes that involve autophagy (J. H. Lee et al., 2014). In regards to cognitive disease, most available treatments that are directed to treat AD alone involve the use of cholinesterase inhibitors (Ruhal & Dhingra, 2018). Dementia as a result of vascular disease may be treated with therapies that focus on vascular and metabolic disorders, such as DM (Maiese, 2018b). Yet, these treatments for the most part focus upon symptoms and do not alter disease progression. Given these severe limitations for treating neurodegenerative disorders during metabolic dysfunction, it is vital to address novel pathways that can identify the cellular links between metabolic dysfunction and neurodegenerative disorders.
The pathways of mTOR that include mTORC1, mTORC2, AMPK, and trophic factors that include EPO provide exciting opportunities to identify new treatment strategies that can address neurodegenerative disorders that are dependent upon metabolic dysfunction. For example, mTORC1 can stimulate lipogenesis and fat storage (Chakrabarti et al., 2010), may increase pancreatic ß-cell mass (Hamada et al., 2009), and may improve glucose homeostasis (Malla, Wang, et al., 2015). Impairment in mTORC2 signaling results in oxidative damage and insulin resistance (R. H. Wang et al., 2011) and mTORC2 signaling plays an important role for the maintenance of pancreatic β-cell proliferation and mass (Gu et al., 2011). Trophic factors, such as EPO, also can provide cellular protection against neurotoxic insults by promoting the activation of mTOR (Jang et al., 2015; Maiese, 2017e). EPO can regulate a number of components of the mTOR pathway, such as PRAS40 and Akt, to promote neuroprotection (Zhao Zhong Chong et al., 2012; H. J. Lee et al., 2016; Maiese et al., 2012a; G. B. Wang et al., 2014). As a result, EPO may be relevant for clinical disorders such as PD. EPO modulation of mTOR and autophagic pathways may lead to protection of the dopaminergic system (Jang et al., 2015).
These pathways oversee cellular survival and function through the programmed cell death pathways of autophagy and apoptosis. Modulating autophagy and apoptosis, mTOR broadly impacts metabolic disease and the nervous system such as offering protection for pancreatic β- cells (J. Zhou et al., 2015), controlling insulin signaling during AD (Crespo et al., 2017), and preventing endothelial cell dysfunction during hyperglycemia (Pal et al., 2019). Equally important is the function of AMPK as evidenced by its ability to reduce ischemic brain damage in diabetic animal models (P. Liu et al., 2016).
Yet, a number of challenges exist for the development of new treatments for neurodegenerative disorders linked to metabolic disease. Pathways such as mTOR, mTORC1, mTORC2, AMPK, and autophagy require a fine level of control to not only prevent the onset and progression of neurodegenerative disorders, but also to limit the development of undesired or unexpected clinical disabilities. For example, AMPK activation can improve memory retention in models of AD and DM (Du et al., 2015), prevent lipid accumulation and obesity (Maiese, 2015e), and promote neuroprotection (Jiang et al., 2014). Yet, under different cellular environments, inhibition of AMPK activity is required to offer protection of pancreatic islet cells in mice (Guan et al., 2014), limit Aβ toxicity (Shang et al., 2013), and prevent inflammation in the nervous system (Russo et al., 2014). In addition, internal cellular feedback pathways need to be further identified to gain sufficient insight for the careful biological control of mTOR and autophagy in order to successfully translate these pathways to clinical medicine. Unchecked mTOR activity in combination with the down-regulation of AMPK activity can lead to glucose intolerance and progression of neurodegenerative disorders. Current studies have provided an enormous wealth of information for elucidating previously unrecognized contributions of metabolic disease to disorders such as AD. However, given the complexity of the underlying pathways with mTOR and programmed cell death, further comprehensive work is required to properly capture the potential of these pathways to effectively treat metabolic dysfunction and neurodegenerative disease for the growing population that is increasingly affected by these disorders throughout the world.
Acknowledgments:
This research was supported by the following grants to Kenneth Maiese: American Diabetes Association, American Heart Association, NIH NIEHS, NIH NIA, NIH NINDS, and NIH ARRA.
Abbreviations:
- AD
Alzheimer’s disease
- AMPK
AMP activated protein kinase
- Atg
Autophagic related genes
- Deptor
DEP domain-containing mTOR interacting protein
- DM
Diabetes mellitus
- EPO
Erythropoietin
- 4EBP1
Eukaryotic initiation factor 4E (eIF4E)-binding protein 1
- ERKs
Extracellular signal-regulated kinases
- TSC1/TSC2
Hamartin (tuberous sclerosis 1)/tuberin (tuberous sclerosis 2)
- IRS-1
Insulin receptor substrate 1
- mLST8
Mammalian lethal with Sec13 protein 8, termed mLST8
- mTOR
Mechanistic target of rapamycin
- mTORC1
mTOR Complex 1
- mTORC2
mTOR Complex 2
- NCDs
Non-communicable diseases
- p70S6K
p70 ribosomal S6 kinase
- PD
Parkinson’s disease
- PS
Phosphatidylserine
- PDK1
Phosphoinositide-dependent kinase 1
- PRAS40
Proline rich Akt substrate 40 kDa
- Akt
Protein kinase B
- ULK1
UNC-51 like kinase 1
- US
United States
- USD
United States dollars
References
- Albiero M, Poncina N, Tjwa M, Ciciliot S, Menegazzo L, Ceolotto G, … Fadini GP (2014). Diabetes causes bone marrow autonomic neuropathy and impairs stem cell mobilization via dysregulated p66Shc and Sirt1. Diabetes, 63(4), 1353–1365. doi: 10.2337/db13-0894 [DOI] [PubMed] [Google Scholar]
- Alexandru N, Popov D, & Georgescu A (2012). Platelet dysfunction in vascular pathologies and how can it be treated. Thromb Res, 129(2), 116–126. doi: 10.1016/j.thromres.2011.09.026 [DOI] [PubMed] [Google Scholar]
- Arildsen L, Andersen JV, Waagepetersen HS, Nissen JBD, & Sheykhzade M (2019). Hypermetabolism and impaired endothelium-dependent vasodilation in mesenteric arteries of type 2 diabetes mellitus db/db mice. Diab Vasc Dis Res, 16(6), 1479164119865885. doi: 10.1177/1479164119865885 [DOI] [PubMed] [Google Scholar]
- Bailey TJ, Fossum SL, Fimbel SM, Montgomery JE, & Hyde DR (2010). The inhibitor of phagocytosis, O-phospho-L-serine, suppresses Muller glia proliferation and cone cell regeneration in the light-damaged zebrafish retina. Exp Eye Res, 91(5), 601–612. doi: 10.1016/j.exer.2010.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balan V, Miller GS, Kaplun L, Balan K, Chong ZZ, Li F, … Tzivion G (2008). Life span extension and neuronal cell protection by Drosophila nicotinamidase. J Biol Chem, 283(41), 27810–27819. doi:M804681200 [pii] 10.1074/jbc.M804681200 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barchetta I, Cimini FA, Ciccarelli G, Baroni MG, & Cavallo MG (2019). Sick fat: the good and the bad of old and new circulating markers of adipose tissue inflammation. J Endocrinol Invest. doi: 10.1007/s40618-019-01052-3 [DOI] [PubMed] [Google Scholar]
- Beker MC, Caglayan B, Yalcin E, Caglayan AB, Turkseven S, Gurel B, … Kilic E (2018). Time-of-Day Dependent Neuronal Injury After Ischemic Stroke: Implication of Circadian Clock Transcriptional Factor Bmal1 and Survival Kinase AKT. Mol Neurobiol, 55(3), 2565–2576. doi: 10.1007/s12035-017-0524-4 [DOI] [PubMed] [Google Scholar]
- Bellozi PM, Lima IV, Doria JG, Vieira EL, Campos AC, Candelario-Jalil E, … de Oliveira AC (2016). Neuroprotective effects of the anticancer drug NVP-BEZ235 (dactolisib) on amyloid-beta 1–42 induced neurotoxicity and memory impairment. Sci Rep, 6, 25226. doi: 10.1038/srep25226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borjini N, Sivilia S, Giuliani A, Fernandez M, Giardino L, Facchinetti F, & Calza L (2019). Potential biomarkers for neuroinflammation and neurodegeneration at short and long term after neonatal hypoxic-ischemic insult in rat. J Neuroinflammation, 16(1), 194. doi: 10.1186/s12974-019-1595-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caberlotto L, Nguyen TP, Lauria M, Priami C, Rimondini R, Maioli S, … Carboni L (2019). Cross-disease analysis of Alzheimer’s disease and type-2 Diabetes highlights the role of autophagy in the pathophysiology of two highly comorbid diseases. Sci Rep, 9(1), 3965. doi: 10.1038/s41598-019-39828-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Medicare and Medicaid Services. (2019). National Health Expenditure Projections 2018–2027. www.cms.gov.
- Cernea M, Tang W, Guan H, & Yang K (2016). Wisp1 mediates Bmp3-stimulated mesenchymal stem cell proliferation. J Mol Endocrinol, 56(1), 39–46. doi: 10.1530/jme-15-0217 [DOI] [PubMed] [Google Scholar]
- Chakrabarti P, English T, Shi J, Smas CM, & Kandror KV (2010). Mammalian target of rapamycin complex 1 suppresses lipolysis, stimulates lipogenesis, and promotes fat storage. Diabetes, 59(4), 775–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Lu Y, Siu HM, Guan J, Zhu L, Zhang S, … Zhang L (2017). Identification of Novel Vacuolin-1 Analogues as Autophagy Inhibitors by Virtual Drug Screening and Chemical Synthesis. Molecules, 22(6). doi: 10.3390/molecules22060891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen F, Liu Z, Peng W, Gao Z, Ouyang H, Yan T, … Cao Z (2018). Activation of EphA4 induced by EphrinA1 exacerbates disruption of the blood-brain barrier following cerebral ischemia-reperfusion via the Rho/ROCK signaling pathway. Exp Ther Med, 16(3), 2651–2658. doi: 10.3892/etm.2018.6460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J, North BJ, Zhang T, Dai X, Tao K, Guo J, & Wei W (2018). The emerging roles of protein homeostasis-governing pathways in Alzheimer’s disease. Aging Cell, 17(5), e12801. doi: 10.1111/acel.12801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu SC, Chao CY, Chiang EI, Syu JN, Rodriguez RL, & Tang FY (2017). N-3 polyunsaturated fatty acids alleviate high glucose-mediated dysfunction of endothelial progenitor cells and prevent ischemic injuries both in vitro and in vivo. J Nutr Biochem, 42, 172–181. doi: 10.1016/j.jnutbio.2017.01.009 [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Kang JQ, & Maiese K (2002). Erythropoietin is a novel vascular protectant through activation of Akt1 and mitochondrial modulation of cysteine proteases. Circulation, 106(23), 2973–2979. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Li F, & Maiese K (2005). Oxidative stress in the brain: Novel cellular targets that govern survival during neurodegenerative disease. Prog Neurobiol, 75(3), 207–246. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, & Maiese K (2012). Mammalian Target of Rapamycin Signaling in Diabetic Cardiovascular Disease. Cardiovasc Diabetol, 11(1), 45. doi: 10.1186/1475-2840-11-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Shang YC, Wang S, & Maiese K (2012). PRAS40 Is an Integral Regulatory Component of Erythropoietin mTOR Signaling and Cytoprotection. PLoS ONE, 7(9), e45456. doi: 10.1371/journal.pone.0045456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong ZZ, Shang YC, Wang S, & Maiese K (2012). Shedding new light on neurodegenerative diseases through the mammalian target of rapamycin. Prog Neurobiol, 99(2), 128–148. doi: 10.1016/j.pneurobio.2012.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung CL, Lawrence I, Hoffman M, Elgindi D, Nadhan K, Potnis M, … Sell C (2019). Topical rapamycin reduces markers of senescence and aging in human skin: an exploratory, prospective, randomized trial. Geroscience, 41, 6. doi: 10.1007/s11357-019-00113-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coca SG, Ismail-Beigi F, Haq N, Krumholz HM, & Parikh CR (2012). Role of intensive glucose control in development of renal end points in type 2 diabetes mellitus: systematic review and meta-analysis intensive glucose control in type 2 diabetes. Arch Intern Med, 172(10), 761–769. doi: 10.1001/archinternmed.2011.2230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo MC, Tome-Carneiro J, Pintado C, Davalos A, Visioli F, & Burgos-Ramos E (2017). Hydroxytyrosol restores proper insulin signaling in an astrocytic model of Alzheimer’s disease. Biofactors, 43(4). doi: 10.1002/biof.1356 [DOI] [PubMed] [Google Scholar]
- Crino PB (2016). The mTOR signalling cascade: paving new roads to cure neurological disease. Nat Rev Neurol, 12(7), 379–392. doi: 10.1038/nrneurol.2016.81 [DOI] [PubMed] [Google Scholar]
- Curjuric I, Imboden M, Bridevaux PO, Gerbase MW, Haun M, Keidel D, … Probst-Hensch NM (2016). Common SIRT1 variants modify the effect of abdominal adipose tissue on aging-related lung function decline. Age (Dordr), 38(3), 52. doi: 10.1007/s11357-016-9917-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai C, Ciccotosto GD, Cappai R, Wang Y, Tang S, Hoyer D, … Xiao X (2017). Rapamycin confers neuroprotection against colistin-induced oxidative stress, mitochondria dysfunction and apoptosis through the activation of autophagy and mTOR/Akt/CREB signaling pathways. ACS Chem Neurosci, 9(4), 824–837. doi: 10.1021/acschemneuro.7b00323 [DOI] [PubMed] [Google Scholar]
- Dai C, Tang S, Biao X, Xiao X, Chen C, & Li J (2019). Colistin induced peripheral neurotoxicity involves mitochondrial dysfunction and oxidative stress in mice. Mol Biol Rep, 46(2). doi: 10.1007/s11033-019-04646-5 [DOI] [PubMed] [Google Scholar]
- Deblon N, Bourgoin L, Veyrat-Durebex C, Peyrou M, Vinciguerra M, Caillon A, … Foti M (2012). Chronic mTOR inhibition by rapamycin induces muscle insulin resistance despite weight loss in rats. Br J Pharmacol, 165(7), 2325–2340. doi: 10.1111/j.1476-5381.2011.01716.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Rosa M, Distefano G, Gagliano C, Rusciano D, & Malaguarnera L (2016). Autophagy in Diabetic Retinopathy. Curr Neuropharmacol, 14(8), 810–825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Rosa M, & Malaguarnera L (2016). Chitotriosidase: A New Inflammatory Marker in Diabetic Complications. Pathobiology, 83(4), 211–219. doi: 10.1159/000443932 [DOI] [PubMed] [Google Scholar]
- Diamanti-Kandarakis E, Datillo M, Macut D, Duntas LH, Gonos E, Goulis D, … Vryonidou A (2017). Mechanisms in Endocrinology: Aging and Anti-aging Endocrinology: A Combo - Endocrinology Overview. Eur J Endocrinol, 176(6), R283–R308. doi: 10.1530/eje-16-1061 [DOI] [PubMed] [Google Scholar]
- Ding C, Zhang J, Li B, Ding Z, Cheng W, Gao F, … Zhang S (2017). Cornin protects SHSY5Y cells against oxygen and glucose deprivationinduced autophagy through the PI3K/Akt/mTOR pathway. Mol Med Rep, 17(1), 87–92. doi: 10.3892/mmr.2017.7864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S, Zhu Y, Liang Y, Huang H, Xu Y, & Zhong C (2018). Circular RNAs in Vascular Functions and Diseases. Adv Exp Med Biol, 1087, 287–297. doi: 10.1007/978-981-13-1426-1_23 [DOI] [PubMed] [Google Scholar]
- Dong J, Li H, Bai Y, & Wu C (2019). Muscone ameliorates diabetic peripheral neuropathy through activating AKT/mTOR signalling pathway. J Pharm Pharmacol, 71(11), 1706–1713. doi: 10.1111/jphp.13157 [DOI] [PubMed] [Google Scholar]
- Dong W, Wang R, Ma LN, Xu BL, Zhang JS, Zhao ZW, … Zhang X (2016). Influence of age-related learning and memory capacity of mice: different effects of a high and low caloric diet. Aging Clin Exp Res, 28(2), 303–311. doi: 10.1007/s40520-015-0398-0 [DOI] [PubMed] [Google Scholar]
- Dong Y, Chen H, Gao J, Liu Y, Li J, & Wang J (2019). Molecular machinery and interplay of apoptosis and autophagy in coronary heart disease. J Mol Cell Cardiol, 136, 27–41. doi: 10.1016/j.yjmcc.2019.09.001 [DOI] [PubMed] [Google Scholar]
- Dorvash M, Farahmandnia M, & Tavassoly I (2020). A Systems Biology Roadmap to Decode mTOR Control System in Cancer. Interdiscip Sci, 12(1), 1–11. doi: 10.1007/s12539-019-00347-6 [DOI] [PubMed] [Google Scholar]
- Du LL, Chai DM, Zhao LN, Li XH, Zhang FC, Zhang HB, … Zhou XW (2015). AMPK Activation Ameliorates Alzheimer’s Disease-Like Pathology and Spatial Memory Impairment in a Streptozotocin-Induced Alzheimer’s Disease Model in Rats. J Alzheimers Dis, 43(3), 775–784. doi: 10.3233/jad-140564 [DOI] [PubMed] [Google Scholar]
- Esterline RL, Vaag A, Oscarsson J, & Vora J (2018). Mechanisms in Endocrinology: SGLT2 inhibitors; clinical benefits by restoration of normal diurnal metabolism? Eur J Endocrinol. doi: 10.1530/eje-17-0832 [DOI] [PubMed] [Google Scholar]
- Fann DY, Ng GY, Poh L, & Arumugam TV (2017). Positive effects of intermittent fasting in ischemic stroke. Exp Gerontol, 89, 93–102. doi: 10.1016/j.exger.2017.01.014 [DOI] [PubMed] [Google Scholar]
- Farmer K, Abd-Elrahman KS, Derksen A, Rowe EM, Thompson AM, Rudyk CA, … Hayley S (2019). mGluR5 Allosteric Modulation Promotes Neurorecovery in a 6-OHDA-Toxicant Model of Parkinson’s Disease. Mol Neurobiol. doi: 10.1007/s12035-019-01818-z [DOI] [PubMed] [Google Scholar]
- Filley CM, Rollins YD, Anderson CA, Arciniegas DB, Howard KL, Murrell JR, … Ghetti B (2007). The genetics of very early onset Alzheimer disease. Cogn Behav Neurol, 20(3), 149–156. doi: 10.1097/WNN.0b013e318145a8c8 [DOI] [PubMed] [Google Scholar]
- Fonseca BD, Smith EM, Lee VH, MacKintosh C, & Proud CG (2007). PRAS40 is a target for mammalian target of rapamycin complex 1 and is required for signaling downstream of this complex. J Biol Chem, 282(34), 24514–24524. [DOI] [PubMed] [Google Scholar]
- Foster KG, Acosta-Jaquez HA, Romeo Y, Ekim B, Soliman GA, Carriere A, … Fingar DC (2010). Regulation of mTOR complex 1 (mTORC1) by raptor Ser863 and multisite phosphorylation. J Biol Chem, 285(1), 80–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraenkel M, Ketzinel-Gilad M, Ariav Y, Pappo O, Karaca M, Castel J, … Leibowitz G (2008). mTOR inhibition by rapamycin prevents beta-cell adaptation to hyperglycemia and exacerbates the metabolic state in type 2 diabetes. Diabetes, 57(4), 945–957. [DOI] [PubMed] [Google Scholar]
- Gao C, Yu H, Yan C, Zhao W, Liu Y, Zhang D, … Liu N (2017). X-linked inhibitor of apoptosis inhibits apoptosis and preserves the blood-brain barrier after experimental subarachnoid hemorrhage. Sci Rep, 7, 44918. doi: 10.1038/srep44918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Martinez JM, & Alessi DR (2008). mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1). Biochem J, 416(3), 375–385. [DOI] [PubMed] [Google Scholar]
- Gomes MB, & Negrato CA (2014). Alpha-lipoic acid as a pleiotropic compound with potential therapeutic use in diabetes and other chronic diseases. Diabetol Metab Syndr, 6(1), 80. doi: 10.1186/1758-5996-6-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Brouchet A, Blaes N, Mouledous L, Fourcade O, Tack I, Frances B, … Minville V (2015). Beneficial effects of levobupivacaine regional anaesthesia on postoperative opioid induced hyperalgesia in diabetic mice. J Transl Med, 13(1), 208. doi: 10.1186/s12967-015-0575-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Lindner J, Kumar A, Yuan W, & Magnuson MA (2011). Rictor/mTORC2 is essential for maintaining a balance between beta-cell proliferation and cell size. Diabetes, 60(3), 827–837. doi: 10.2337/db10-1194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan FY, Gu J, Li W, Zhang M, Ji Y, Li J, … Hatch GM (2014). Compound K protects pancreatic islet cells against apoptosis through inhibition of the AMPK/JNK pathway in type 2 diabetic mice and in MIN6 beta-cells. Life Sci, 107(1–2), 42–49. doi: 10.1016/j.lfs.2014.04.034 [DOI] [PubMed] [Google Scholar]
- Guertin DA, Stevens DM, Thoreen CC, Burds AA, Kalaany NY, Moffat J, … Sabatini DM (2006). Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCalpha, but not S6K1. Dev Cell, 11(6), 859–871. [DOI] [PubMed] [Google Scholar]
- Guo T, Liu T, Sun Y, Liu X, Xiong R, Li H, … Tian Y (2019). Sonodynamic therapy inhibits palmitate-induced beta cell dysfunction via PINK1/Parkin-dependent mitophagy. Cell Death Dis, 10(6), 457. doi: 10.1038/s41419-019-1695-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadamitzky M, Herring A, Kirchhof J, Bendix I, Haight MJ, Keyvani K, … Schedlowski M (2018). Repeated systemic treatment with rapamycin affects behavior and amygdala protein expression in rats. Int J Neuropsychopharmacol. doi: 10.1093/ijnp/pyy017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haldar SR, Chakrabarty A, Chowdhury S, Haldar A, Sengupta S, & Bhattacharyya M (2015). Oxidative stress-related genes in type 2 diabetes: association analysis and their clinical impact. Biochem Genet, 53(4–6), 93–119. doi: 10.1007/s10528-015-9675-z [DOI] [PubMed] [Google Scholar]
- Hamada S, Hara K, Hamada T, Yasuda H, Moriyama H, Nakayama R, … Yokono K (2009). Upregulation of the mammalian target of rapamycin complex 1 pathway by Ras homolog enriched in brain in pancreatic beta-cells leads to increased beta-cell mass and prevention of hyperglycemia. Diabetes, 58(6), 1321–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han K, Jia N, Zhong Y, & Shang X (2017). S14G-humanin alleviates insulin resistance and increases autophagy in neurons of APP/PS1 transgenic mouse. J Cell Biochem. doi: 10.1002/jcb.26452 [DOI] [PubMed] [Google Scholar]
- Handa JT, Bowes Rickman C, Dick AD, Gorin MB, Miller JW, Toth CA, … Farrer LA (2019). A systems biology approach towards understanding and treating non-neovascular age-related macular degeneration. Nat Commun, 10(1), 3347. doi: 10.1038/s41467-019-11262-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris MI, & Eastman RC (2000). Early detection of undiagnosed diabetes mellitus: a US perspective. Diabetes Metab Res Rev, 16(4), 230–236. [DOI] [PubMed] [Google Scholar]
- Hayutin A (2007). Global demographic shifts create challenges and opportunities. PREA Quarterly, (Fall), 46–53. [Google Scholar]
- He C, Bassik MC, Moresi V, Sun K, Wei Y, Zou Z, … Levine B (2012). Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature, 481(7382), 511–515. doi: 10.1038/nature10758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He C, Zhu H, Li H, Zou MH, & Xie Z (2013). Dissociation of Bcl-2-Beclin1 complex by activated AMPK enhances cardiac autophagy and protects against cardiomyocyte apoptosis in diabetes. Diabetes, 62(4), 1270–1281. doi: 10.2337/db12-0533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heitman J, Movva NR, & Hall MN (1991). Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science, 253(5022), 905–909. [DOI] [PubMed] [Google Scholar]
- Hill JH, Solt C, & Foster MT (2018). Obesity associated disease risk: the role of inherent differences and location of adipose depots. Horm Mol Biol Clin Investig, 33(2). doi: 10.1515/hmbci-2018-0012 [DOI] [PubMed] [Google Scholar]
- Hou J, Chong ZZ, Shang YC, & Maiese K (2010). Early apoptotic vascular signaling is determined by Sirt1 through nuclear shuttling, forkhead trafficking, bad, and mitochondrial caspase activation. Curr Neurovasc Res, 7(2), 95–112. doi: 10.2174/156720210791184899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou J, Wang S, Shang YC, Chong ZZ, & Maiese K (2011). Erythropoietin Employs Cell Longevity Pathways of SIRT1 to Foster Endothelial Vascular Integrity During Oxidant Stress. Curr Neurovasc Res, 8(3), 220–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh CF, Liu CK, Lee CT, Yu LE, & Wang JY (2019). Acute glucose fluctuation impacts microglial activity, leading to inflammatory activation or self-degradation. Sci Rep, 9(1), 840. doi: 10.1038/s41598-018-37215-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu M, Liu Z, Lv P, Wang H, Zhu Y, Qi Q, … Gao L (2017). Nimodipine activates neuroprotective signaling events and inactivates autophages in the VCID rat hippocampus. Neurol Res, 39(10), 904–909. doi: 10.1080/01616412.2017.1356157 [DOI] [PubMed] [Google Scholar]
- Hu P, Lai D, Lu P, Gao J, & He H (2012). ERK and Akt signaling pathways are involved in advanced glycation end product-induced autophagy in rat vascular smooth muscle cells. Int J Mol Med, 29(4), 613–618. doi: 10.3892/ijmm.2012.891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D, Shen S, Cai M, Jin L, Lu J, Xu K, … Feng X (2019). Role of mTOR complex in IGF-1 induced neural differentiation of DPSCs. J Mol Histol. doi: 10.1007/s10735-019-09825-z [DOI] [PubMed] [Google Scholar]
- Hwang SK, & Kim HH (2011). The functions of mTOR in ischemic diseases. BMB Rep, 44(8), 506–511. [DOI] [PubMed] [Google Scholar]
- Ignacio ZM, Reus GZ, Arent CO, Abelaira HM, Pitcher MR, & Quevedo J (2016). New perspectives on the involvement of mTOR in depression as well as in the action of antidepressant drugs. Br J Clin Pharmacol, 82(5), 1280–1290. doi: 10.1111/bcp.12845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoki K, Zhu T, & Guan KL (2003). TSC2 mediates cellular energy response to control cell growth and survival. Cell, 115(5), 577–590. [DOI] [PubMed] [Google Scholar]
- International Diabetes Federation. (2019). Diabetes IDF Diabetes Atlas(9th Edition). [Google Scholar]
- Jacinto E, Loewith R, Schmidt A, Lin S, Ruegg MA, Hall A, & Hall MN (2004). Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol, 6(11), 1122–1128. [DOI] [PubMed] [Google Scholar]
- Jang W, Kim HJ, Li H, Jo KD, Lee MK, & Yang HO (2015). The Neuroprotective Effect of Erythropoietin on Rotenone-Induced Neurotoxicity in SH-SY5Y Cells Through the Induction of Autophagy. Mol Neurobiol, 53(6), 3812–3821. doi: 10.1007/s12035-015-9316-x [DOI] [PubMed] [Google Scholar]
- Javdan N, Ayatollahi SA, Choudhary MI, Al-Hasani S, Kobarfard F, Athar A, & Pazoki-Toroudi H (2018). Capsaicin protects against testicular torsion injury through mTOR-dependent mechanism. Theriogenology, 113, 247–252. doi: 10.1016/j.theriogenology.2018.03.012 [DOI] [PubMed] [Google Scholar]
- Jesko H, Stepien A, Lukiw WJ, & Strosznajder RP (2019). The Cross-Talk Between Sphingolipids and Insulin-Like Growth Factor Signaling: Significance for Aging and Neurodegeneration. Mol Neurobiol, 56(5), 3501–3521. doi: 10.1007/s12035-018-1286-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia G, Aroor AR, Martinez-Lemus LA, & Sowers JR (2014). Invited Review: Over-nutrition, mTOR Signaling and Cardiovascular Diseases. Am J Physiol Regul Integr Comp Physiol, 307(10), R1198–1206. doi: 10.1152/ajpregu.00262.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang T, Yu JT, Zhu XC, Wang HF, Tan MS, Cao L, … Tan L (2014). Acute metformin preconditioning confers neuroprotection against focal cerebral ischaemia by pre-activation of AMPK-dependent autophagy. Br J Pharmacol, 171(13), 3146–3157. doi: 10.1111/bph.12655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung CH, Jun CB, Ro SH, Kim YM, Otto NM, Cao J, … Kim DH (2009). ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol Biol Cell, 20(7), 1992–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung CH, Lee DH, Ahn J, Lee H, Choi WH, Jang YJ, & Ha TY (2015). gamma-Oryzanol Enhances Adipocyte Differentiation and Glucose Uptake. Nutrients, 7(6), 4851–4861. doi: 10.3390/nu7064851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ka M, Smith AL, & Kim WY (2017). MTOR controls genesis and autophagy of GABAergic interneurons during brain development. Autophagy, 0. doi: 10.1080/15548627.2017.1327927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalender A, Selvaraj A, Kim SY, Gulati P, Brule S, Viollet B, … Thomas G (2010). Metformin, independent of AMPK, inhibits mTORC1 in a rag GTPase-dependent manner. Cell Metab, 11(5), 390–401. doi: 10.1016/j.cmet.2010.03.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada Y, Funakoshi T, Shintani T, Nagano K, Ohsumi M, & Ohsumi Y (2000). Tor-mediated induction of autophagy via an Apg1 protein kinase complex. J Cell Biol, 150(6), 1507–1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S, Chemaly ER, Hajjar RJ, & Lebeche D (2011). Resistin promotes cardiac hypertrophy via the AMP-activated protein kinase/mammalian target of rapamycin (AMPK/mTOR) and c-Jun N-terminal kinase/insulin receptor substrate 1 (JNK/IRS1) pathways. J Biol Chem, 286(21), 18465–18473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kell DB, & Pretorius E (2018). No effects without causes: the Iron Dysregulation and Dormant Microbes hypothesis for chronic, inflammatory diseases. Biol Rev Camb Philos Soc. doi: 10.1111/brv.12407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim DH, Sarbassov DD, Ali SM, Latek RR, Guntur KV, Erdjument-Bromage H, … Sabatini DM (2003). GbetaL, a positive regulator of the rapamycin-sensitive pathway required for the nutrient-sensitive interaction between raptor and mTOR. Mol Cell, 11(4), 895–904. [DOI] [PubMed] [Google Scholar]
- Kim KA, Shin YJ, Akram M, Kim ES, Choi KW, Suh H, … Bae ON (2014). High glucose condition induces autophagy in endothelial progenitor cells contributing to angiogenic impairment. Biol Pharm Bull, 37(7), 1248–1252. [DOI] [PubMed] [Google Scholar]
- Kim S, Kang IH, Nam JB, Cho Y, Chung DY, Kim SH, … Shin JW (2015). Ameliorating the Effect of Astragaloside IV on Learning and Memory Deficit after Chronic Cerebral Hypoperfusion in Rats. Molecules, 20(2), 1904–1921. doi: 10.3390/molecules20021904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimontov VV, Bulumbaeva DM, Fazullina ON, Lykov AP, Bgatova NP, Orlov NB, … Rudovich N (2019). Circulating Wnt1-inducible signaling pathway protein-1 (WISP-1/CCN4) is a novel biomarker of adiposity in subjects with type 2 diabetes. J Cell Commun Signal. doi: 10.1007/s12079-019-00536-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimova N, & Kristian T (2019). Multi-targeted Effect of Nicotinamide Mononucleotide on Brain Bioenergetic Metabolism. Neurochem Res. doi: 10.1007/s11064-019-02729-0 [DOI] [PubMed] [Google Scholar]
- Klionsky DJ, Abdelmohsen K, Abe A, Abedin MJ, Abeliovich H, Acevedo Arozena A, … Zughaier SM (2016). Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy, 12(1), 1–222. doi: 10.1080/15548627.2015.1100356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HJ, Koh SH, Song KM, Seol IJ, & Park HK (2016). The Akt/mTOR/p70S6K Pathway Is Involved in the Neuroprotective Effect of Erythropoietin on Hypoxic/Ischemic Brain Injury in a Neonatal Rat Model. Neonatology, 110(2), 93–100. doi: 10.1159/000444360 [DOI] [PubMed] [Google Scholar]
- Lee JH, Lee JH, Jin M, Han SD, Chon GR, Kim IH, … Noh YH (2014). Diet control to achieve euglycemia induces significant loss of heart and liver weight via increased autophagy compared with ad libitum diet in diabetic rats. Exp Mol Med, 46, e111. doi: 10.1038/emm.2014.52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Tecedor L, Chen YH, Monteys AM, Sowada MJ, Thompson LM, & Davidson BL (2015). Reinstating aberrant mTORC1 activity in Huntington’s disease mice improves disease phenotypes. Neuron, 85(2), 303–315. doi: 10.1016/j.neuron.2014.12.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Hong Y, Lee SR, & Chang KT (2012). Autophagy contributes to retardation of cardiac growth in diabetic rats. Lab Anim Res, 28(2), 99–107. doi: 10.5625/lar.2012.28.2.99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L (2017). The Molecular Mechanism of Glucagon-Like Peptide-1 Therapy in Alzheimer’s Disease, Based on a Mechanistic Target of Rapamycin Pathway. CNS Drugs, 31(7), 535–549. doi: 10.1007/s40263-017-0431-2 [DOI] [PubMed] [Google Scholar]
- Li Y, Xu S, Giles A, Nakamura K, Lee JW, Hou X, … Zang M (2011). Hepatic overexpression of SIRT1 in mice attenuates endoplasmic reticulum stress and insulin resistance in the liver. Faseb J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim YM, Lim H, Hur KY, Quan W, Lee HY, Cheon H, … Lee MS (2014). Systemic autophagy insufficiency compromises adaptation to metabolic stress and facilitates progression from obesity to diabetes. Nat Commun, 5, 4934. doi: 10.1038/ncomms5934 [DOI] [PubMed] [Google Scholar]
- Lin CL, Huang WN, Li HH, Huang CN, Hsieh S, Lai C, & Lu FJ (2015). Hydrogen-rich water attenuates amyloid beta-induced cytotoxicity through upregulation of Sirt1-FoxO3a by stimulation of AMP-activated protein kinase in SK-N-MC cells. Chem Biol Interact, 240, 12–21. doi: 10.1016/j.cbi.2015.07.013 [DOI] [PubMed] [Google Scholar]
- Lin X, & Zhang N (2018). Berberine: Pathways to protect neurons. Phytother Res. doi: 10.1002/ptr.6107 [DOI] [PubMed] [Google Scholar]
- Liu P, Yang X, Hei C, Meli Y, Niu J, Sun T, & Li PA (2016). Rapamycin Reduced Ischemic Brain Damage in Diabetic Animals Is Associated with Suppressions of mTOR and ERK1/2 Signaling. Int J Biol Sci, 12(8), 1032–1040. doi: 10.7150/ijbs.15624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu SL, Lin HX, Lin CY, Sun XQ, Ye LP, Qiu F, … Guo L (2017). TIMELESS confers cisplatin resistance in nasopharyngeal carcinoma by activating the Wnt/beta-catenin signaling pathway and promoting the epithelial mesenchymal transition. Cancer Lett, 402, 117–130. doi: 10.1016/j.canlet.2017.05.022 [DOI] [PubMed] [Google Scholar]
- Liu Y, Palanivel R, Rai E, Park M, Gabor TV, Scheid MP, … Sweeney G (2014). Adiponectin stimulates autophagy and reduces oxidative stress to enhance insulin sensitivity during high fat diet feeding in mice. Diabetes, 64(1), 36–48. doi: 10.2337/db14-0267 [DOI] [PubMed] [Google Scholar]
- Liu YW, Zhang L, Li Y, Cheng YQ, Zhu X, Zhang F, & Yin XX (2015). Activation of mTOR signaling mediates the increased expression of AChE in high glucose condition: in vitro and in vivo evidences. Mol Neurobiol. doi: 10.1007/s12035-015-9425-6 [DOI] [PubMed] [Google Scholar]
- Liu Z, Gan L, Zhang T, Ren Q, & Sun C (2018). Melatonin alleviates adipose inflammation through elevating alpha-ketoglutarate and diverting adipose-derived exosomes to macrophages in mice. J Pineal Res, 64(1), 12455. doi: 10.1111/jpi.12455 [DOI] [PubMed] [Google Scholar]
- Liu Z, Stanojevic V, Brindamour LJ, & Habener JF (2012). GLP1-derived nonapeptide GLP1(28–36)amide protects pancreatic beta-cells from glucolipotoxicity. J Endocrinol, 213(2), 143–154. doi: 10.1530/joe-11-0328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y, Shen T, Yang H, & Gu W (2016). Ruthenium Complexes Induce HepG2 Human Hepatocellular Carcinoma Cell Apoptosis and Inhibit Cell Migration and Invasion through Regulation of the Nrf2 Pathway. Int J Mol Sci, 17(5). doi: 10.3390/ijms17050775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lushchak O, Strilbytska O, Piskovatska V, Storey KB, Koliada A, & Vaiserman A (2017). The role of the TOR pathway in mediating the link between nutrition and longevity. Mech Ageing Dev, 164, 127–138. doi: 10.1016/j.mad.2017.03.005 [DOI] [PubMed] [Google Scholar]
- Ma L, Fu R, Duan Z, Lu J, Gao J, Tian L, … Wang L (2016). Sirt1 is essential for resveratrol enhancement of hypoxia-induced autophagy in the type 2 diabetic nephropathy rat. Pathol Res Pract. doi: 10.1016/j.prp.2016.02.001 [DOI] [PubMed] [Google Scholar]
- Maiese K (2012). The Many Facets of Cell Injury: Angiogenesis to Autophagy. Curr Neurovasc Res, 9(2), 1–2. [DOI] [PubMed] [Google Scholar]
- Maiese K (2014a). Cutting through the Complexities of mTOR for the Treatment of Stroke. Curr Neurovasc Res, 11(2), 177–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K (2014b). Driving neural regeneration through the mammalian target of rapamycin. Neural Regen Res, 9(15), 1413–1417. doi: 10.4103/1673-5374.139453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K (2014c). Taking aim at Alzheimer’s disease through the mammalian target of rapamycin. Ann Med, 46(8), 587–596. doi: 10.3109/07853890.2014.941921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K (2015a). Erythropoietin and diabetes mellitus. World J Diabetes, 6(14), 1259–1273. doi: 10.4239/wjd.v6.i14.1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K (2015b). FoxO Proteins in the Nervous System. Anal Cell Pathol (Amst), 2015, 569392. doi: 10.1155/2015/569392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K (2015c). FoxO Transcription Factors and Regenerative Pathways in Diabetes Mellitus. Curr Neurovasc Res, 12(4), 404–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K (2015d). MicroRNAs and SIRT1: A Strategy for Stem Cell Renewal and Clinical Development? J Transl Sci, 1(3), 55–57. [PMC free article] [PubMed] [Google Scholar]
- Maiese K (2015e). mTOR: Driving apoptosis and autophagy for neurocardiac complications of diabetes mellitus. World J Diabetes, 6(2), 217–224. doi: 10.4239/wjd.v6.i2.217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K (2015a). New Insights for Oxidative Stress and Diabetes Mellitus. Oxid Med Cell Longev, 2015(2015:875961). doi: 10.1155/2015/875961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K (2015b). Novel applications of trophic factors, Wnt and WISP for neuronal repair and regeneration in metabolic disease. Neural Regen Res, 10(4), 518–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K (2015f). Programming apoptosis and autophagy with novel approaches for diabetes mellitus. Curr Neurovasc Res, 12(2), 173–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K (2015g). SIRT1 and stem cells: In the forefront with cardiovascular disease, neurodegeneration and cancer. World J Stem Cells, 7(2), 235–242. doi: 10.4252/wjsc.v7.i2.235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K (2016a). The bright side of reactive oxygen species: lifespan extension without cellular demise. J Transl Sci, 2(3), 185–187. doi: 10.15761/jts.1000138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K (2016b). Disease onset and aging in the world of circular RNAs. J Transl Sci, 2(6), 327–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K (2016c). Erythropoietin and mTOR: A “One-Two Punch” for Aging-Related Disorders Accompanied by Enhanced Life Expectancy. Curr Neurovasc Res, 13(4), 329–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K (2016a). Forkhead transcription factors: new considerations for alzheimer’s disease and dementia. J Transl Sci, 2(4), 241–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K (2016b). Molecules to Medicine with mTOR: Translating Critical Pathways into Novel Therapeutic Strategies. Elsevier and Academic Press, ISBN 9780128027332. [Google Scholar]
- Maiese K (2016d). Novel nervous and multi-system regenerative therapeutic strategies for diabetes mellitus with mTOR. Neural Regen Res, 11(3), 372–385. doi: 10.4103/1673-5374.179032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K (2016e). Picking a bone with WISP1 (CCN4): new strategies against degenerative joint disease. J Transl Sci, 1(3), 83–85. [PMC free article] [PubMed] [Google Scholar]
- Maiese K (2016f). Regeneration in the nervous system with erythropoietin. Front Biosci (Landmark Ed), 21, 561–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K (2016g). Targeting molecules to medicine with mTOR, autophagy and neurodegenerative disorders. Br J Clin Pharmacol, 82(5), 1245–1266. doi: 10.1111/bcp.12804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K (2017a). Autophagy to the Rescue. Curr Neurovasc Res, 14(3), 199. doi: 10.2174/1567202614666170724160119 [DOI] [PubMed] [Google Scholar]
- Maiese K (2017b). Forkhead Transcription Factors: Formulating a FOXO Target for Cognitive Loss. Curr Neurovasc Res, 14(4), 415–420. doi: 10.2174/1567202614666171116102911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K (2017c). Harnessing the Power of SIRT1 and Non-coding RNAs in Vascular Disease. Curr Neurovasc Res, 14(1), 82–88. doi: 10.2174/1567202613666161129112822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K (2017d). Moving to the Rhythm with Clock (Circadian) Genes, Autophagy, mTOR, and SIRT1 in Degenerative Disease and Cancer. Curr Neurovasc Res, 14(3), 299–304. doi: 10.2174/1567202614666170718092010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K (2017e). Warming Up to New Possibilities with the Capsaicin Receptor TRPV1: mTOR, AMPK, and Erythropoietin. Curr Neurovasc Res, 14(2), 184–189. doi: 10.2174/1567202614666170313105337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K (2018a). Dampening the Progression of Dementia. Curr Neurovasc Res, 15(2). doi: 10.2174/1567202615666180510165839 [DOI] [PubMed] [Google Scholar]
- Maiese K (2018b). The mechanistic target of rapamycin (mTOR) and the silent mating-type information regulation 2 homolog 1 (SIRT1): oversight for neurodegenerative disorders. Biochem Soc Trans, 46(2), 351–360. doi: 10.1042/bst20170121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K (2018c). Novel Treatment Strategies for the Nervous System: Circadian Clock Genes, Non-coding RNAs, and Forkhead Transcription Factors. Curr Neurovasc Res, 15(1), 81–91. doi: 10.2174/1567202615666180319151244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K (2018d). Sirtuins: Developing Innovative Treatments for Aged-Related Memory Loss and Alzheimer’s Disease. Curr Neurovasc Res, 15(4). doi: 10.2174/1567202616666181128120003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K (2019a). Dissecting the Biological Effects of Isoflurane through the Mechanistic Target of Rapamycin (mTOR) and microRNAs (miRNAs). Curr Neurovasc Res, 16(5). doi: 10.2174/1567202616999191024151901 [DOI] [PubMed] [Google Scholar]
- Maiese K (2019b). Impacting dementia and cognitive loss with innovative strategies: mechanistic target of rapamycin, clock genes, circular non-coding ribonucleic acids, and Rho/Rock. Neural Regen Res, 14(5), 773–774. doi: 10.4103/1673-5374.249224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K (2019c). MicroRNAs for the Treatment of Dementia and Alzheimer’s Disease. Curr Neurovasc Res, 16(1), 1–2. doi: 10.2174/1567202616666190208094159 [DOI] [PubMed] [Google Scholar]
- Maiese K (2019d). Releasing the Genie in the Bottle: Molecular Signaling with Hydrogen Sulfide. Curr Neurovasc Res, 16(2). doi: 10.2174/1567202616999190430124021 [DOI] [PubMed] [Google Scholar]
- Maiese K (2020). Cognitive impairment with diabetes mellitus and metabolic disease: innovative insights with the mechanistic target of rapamycin and circadian clock gene pathways. Expert Rev Clin Pharmacol, 13(1), 23–34. doi: 10.1080/17512433.2020.1698288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ, Hou J, & Shang YC (2008). Erythropoietin and oxidative stress. Curr Neurovasc Res, 5(2), 125–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ, & Shang YC (2007). Mechanistic insights into diabetes mellitus and oxidative stress. Curr Med Chem, 14(16), 1729–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ, Shang YC, & Hou J (2008). Rogue proliferation versus restorative protection: where do we draw the line for Wnt and forkhead signaling? Expert Opin Ther Targets, 12(7), 905–916. doi: 10.1517/14728222.12.7.905 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ, Shang YC, & Hou J (2009). FoxO proteins: cunning concepts and considerations for the cardiovascular system. Clin Sci (Lond), 116(3), 191–203. doi:CS20080113 [pii] 10.1042/CS20080113 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ, Shang YC, & Hou J (2011). Novel Avenues of Drug Discovery and Biomarkers for Diabetes Mellitus. J Clin Pharmacol, 51(2), 128–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ, Shang YC, & Wang S (2011). Translating cell survival and cell longevity into treatment strategies with SIRT1. Rom J Morphol Embryol, 52(4), 1173–1185. [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ, Shang YC, & Wang S (2012a). Erythropoietin: new directions for the nervous system. Int J Mol Sci, 13(9), 11102–11129. doi: 10.3390/ijms130911102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ, Shang YC, & Wang S (2012b). Targeting disease through novel pathways of apoptosis and autophagy. Expert Opin Ther Targets, 16(12), 1203–1214. doi: 10.1517/14728222.2012.719499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ, Shang YC, & Wang S (2013a). mTOR: on target for novel therapeutic strategies in the nervous system. Trends Mol Med, 19(1), 51–60. doi: 10.1016/j.molmed.2012.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ, Shang YC, & Wang S (2013b). Novel directions for diabetes mellitus drug discovery. Expert Opin Drug Discov, 8(1), 35–48. doi: 10.1517/17460441.2013.736485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Chong ZZ, Wang S, & Shang YC (2013). Oxidant Stress and Signal Transduction in the Nervous System with the PI 3-K, Akt, and mTOR Cascade. Int J Mol Sci, 13(11), 13830–13866. doi: 10.3390/ijms131113830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Li F, & Chong ZZ (2005). New avenues of exploration for erythropoietin. Jama, 293(1), 90–95. doi:293/1/90 [pii] 10.1001/jama.293.1.90 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, Li F, Chong ZZ, & Shang YC (2008). The Wnt signaling pathway: Aging gracefully as a protectionist? Pharmacol Ther, 118(1), 58–81. doi:S0163–7258(08)00017-X [pii] 10.1016/j.pharmthera.2008.01.004 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maiese K, & Vincent AM (2000). Membrane asymmetry and DNA degradation: functionally distinct determinants of neuronal programmed cell death. J Neurosci Res, 59(4), 568–580. [DOI] [PubMed] [Google Scholar]
- Malla R, Ashby CR Jr., Narayanan NK, Narayanan B, Faridi JS, & Tiwari AK (2015). Proline-rich AKT substrate of 40-kDa (PRAS40) in the pathophysiology of cancer. Biochem Biophys Res Commun, 463(3), 161–166. doi: 10.1016/j.bbrc.2015.05.041 [DOI] [PubMed] [Google Scholar]
- Malla R, Wang Y, Chan WK, Tiwari AK, & Faridi JS (2015). Genetic ablation of PRAS40 improves glucose homeostasis via linking the AKT and mTOR pathways. Biochem Pharmacol. doi: 10.1016/j.bcp.2015.04.016 [DOI] [PubMed] [Google Scholar]
- Martinez de Morentin PB, Martinez-Sanchez N, Roa J, Ferno J, Nogueiras R, Tena-Sempere M, … Lopez M (2014). Hypothalamic mTOR: the rookie energy sensor. Curr Mol Med, 14(1), 3–21. [DOI] [PubMed] [Google Scholar]
- Martino L, Masini M, Novelli M, Beffy P, Bugliani M, Marselli L, … De Tata V (2012). Palmitate activates autophagy in INS-1E beta-cells and in isolated rat and human pancreatic islets. PLoS ONE, 7(5), e36188. doi: 10.1371/journal.pone.0036188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta J, Rayalam S, & Wang X (2018). Cytoprotective Effects of Natural Compounds against Oxidative Stress. Antioxidants (Basel), 7(10). doi: 10.3390/antiox7100147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao XY, Gu ZY, Liu P, Hu Y, Li L, Gong YP, … Li CL (2013). The human glucagon-like peptide-1 analogue liraglutide regulates pancreatic beta-cell proliferation and apoptosis via an AMPK/mTOR/P70S6K signaling pathway. Peptides, 39, 71–79. doi: 10.1016/j.peptides.2012.10.006 [DOI] [PubMed] [Google Scholar]
- Minino AM (2013). Death in the United States, 2011. NCHS Data Brief(115), 1–8. [PubMed] [Google Scholar]
- Mishra M, Duraisamy AJ, & Kowluru RA (2018). Sirt1- A Guardian of the Development of Diabetic Retinopathy. Diabetes. doi: 10.2337/db17-0996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moors TE, Hoozemans JJ, Ingrassia A, Beccari T, Parnetti L, Chartier-Harlin MC, & van de Berg WD (2017). Therapeutic potential of autophagy-enhancing agents in Parkinson’s disease. Mol Neurodegener, 12(1), 11. doi: 10.1186/s13024-017-0154-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moroz N, Carmona JJ, Anderson E, Hart AC, Sinclair DA, & Blackwell TK (2014). Dietary restriction involves NAD -dependent mechanisms and a shift toward oxidative metabolism. Aging Cell, 13(6), 1075–1085. doi: 10.1111/acel.12273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris G, Berk M, Maes M, & Puri BK (2019). Could Alzheimer’s Disease Originate in the Periphery and If So How So? Mol Neurobiol, 56(1), 406–434. doi: 10.1007/s12035-018-1092-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu N, Lei Y, Wang Y, Wang Y, Duan Q, Ma G, … Su L (2019). Inhibition of SIRT1/2 upregulates HSPA5 acetylation and induces pro-survival autophagy via ATF4-DDIT4-mTORC1 axis in human lung cancer cells. Apoptosis. doi: 10.1007/s10495-019-01559-3 [DOI] [PubMed] [Google Scholar]
- Niranjan R, Mishra KP, & Thakur AK (2018). Inhibition of Cyclooxygenase-2 (COX-2) Initiates Autophagy and Potentiates MPTP-Induced Autophagic Cell Death of Human Neuroblastoma Cells, SH-SY5Y: an Inside in the Pathology of Parkinson’s Disease. Mol Neurobiol. doi: 10.1007/s12035-018-0950-y [DOI] [PubMed] [Google Scholar]
- Oda SS (2017). Metformin Protects against Experimental Acrylamide Neuropathy in Rats. Drug Dev Res, 78(7). doi: 10.1002/ddr.21400 [DOI] [PubMed] [Google Scholar]
- Ong WY, Wu YJ, Farooqui T, & Farooqui AA (2018). Qi Fu Yin-a Ming Dynasty Prescription for the Treatment of Dementia. Mol Neurobiol. doi: 10.1007/s12035-018-0908-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Othman MAM, Rajab E, AlMubarak A, AlNaisar M, Bahzad N, & Kamal A (2018). Erythropoietin Protects Against Cognitive Impairment and Hippocampal Neurodegeneration in Diabetic Mice. Behav Sci (Basel), 9(1). doi: 10.3390/bs9010004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal PB, Sonowal H, Shukla K, Srivastava SK, & Ramana KV (2019). Aldose reductase regulates hyperglycemia-induced HUVEC death via SIRT1/AMPK-alpha1/mTOR pathway. J Mol Endocrinol. doi: 10.1530/jme-19-0080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park A, & Koh HC (2019). NF-kappaB/mTOR-mediated autophagy can regulate diquat-induced apoptosis. Arch Toxicol. doi: 10.1007/s00204-019-02424-7 [DOI] [PubMed] [Google Scholar]
- Park JA, & Lee CH (2017). Temporal changes in mammalian target of rapamycin (mTOR) and phosphorylated-mTOR expressions in the hippocampal CA1 region of rat with vascular dementia. J Vet Sci, 18(1), 11–16. doi: 10.4142/jvs.2017.18.1.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasini E, Flati V, Paiardi S, Rizzoni D, Porteri E, Aquilani R, … Agabiti-Rosei E (2010). Intracellular molecular effects of insulin resistance in patients with metabolic syndrome. Cardiovasc Diabetol, 9, 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce LR, Sommer EM, Sakamoto K, Wullschleger S, & Alessi DR (2011). Protor-1 is required for efficient mTORC2-mediated activation of SGK1 in the kidney. Biochem J, 436(1), 169–179. [DOI] [PubMed] [Google Scholar]
- Peixoto CA, de Oliveira WH, da Rocha Araujo SM, & Nunes AKS (2017). AMPK activation: Role in the signaling pathways of neuroinflammation and neurodegeneration. Exp Neurol, 298(PtA), 31–41. doi: 10.1016/j.expneurol.2017.08.013 [DOI] [PubMed] [Google Scholar]
- Pende M, Kozma SC, Jaquet M, Oorschot V, Burcelin R, Le Marchand-Brustel Y, … Thomas G (2000). Hypoinsulinaemia, glucose intolerance and diminished beta-cell size in S6K1-deficient mice. Nature, 408(6815), 994–997. [DOI] [PubMed] [Google Scholar]
- Perez-Hernandez N, Vargas-Alarcon G, Posadas-Sanchez R, Martinez-Rodriguez N, Tovilla-Zarate CA, Rodriguez-Cortes AA, … Rodriguez-Perez JM (2016). PHACTR1 Gene Polymorphism Is Associated with Increased Risk of Developing Premature Coronary Artery Disease in Mexican Population. Int J Environ Res Public Health, 13(8). doi: 10.3390/ijerph13080803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson TR, Laplante M, Thoreen CC, Sancak Y, Kang SA, Kuehl WM, … Sabatini DM (2009). DEPTOR is an mTOR inhibitor frequently overexpressed in multiple myeloma cells and required for their survival. Cell, 137(5), 873–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnalagu M, Subramani M, Jayadev C, Shetty R, & Das D (2017). Retinal pigment epithelium-secretome: A diabetic retinopathy perspective. Cytokine, 95, 126–135. doi: 10.1016/j.cyto.2017.02.013 [DOI] [PubMed] [Google Scholar]
- Preau S, Ambler M, Sigurta A, Kleyman A, Dyson A, Hill NE, … Singer M (2019). Protein recycling and limb muscle recovery after critical illness in slow- and fast-twitch limb muscle. Am J Physiol Regul Integr Comp Physiol. doi: 10.1152/ajpregu.00221.2018 [DOI] [PubMed] [Google Scholar]
- Ratliff EP, Mauntz RE, Kotzebue RW, Gonzalez A, Achal M, Barekat A, … Finley KD (2015). Aging and Autophagic Function Influences the Progressive Decline of Adult Drosophila Behaviors. PLoS ONE, 10(7), e0132768. doi: 10.1371/journal.pone.0132768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhal P, & Dhingra D (2018). Inosine improves cognitive function and decreases aging-induced oxidative stress and neuroinflammation in aged female rats. Inflammopharmacology, 26(5), 1317–1329. doi: 10.1007/s10787-018-0476-y [DOI] [PubMed] [Google Scholar]
- Russo E, Andreozzi F, Iuliano R, Dattilo V, Procopio T, Fiume G, … De Sarro G (2014). Early molecular and behavioral response to lipopolysaccharide in the WAG/Rij rat model of absence epilepsy and depressive-like behavior, involves interplay between AMPK, AKT/mTOR pathways and neuroinflammatory cytokine release. Brain Behav Immun, 42, 157–168. doi: 10.1016/j.bbi.2014.06.016 [DOI] [PubMed] [Google Scholar]
- Saleem S, & Biswas SC (2017). Tribbles Pseudokinase 3 Induces Both Apoptosis and Autophagy in Amyloid-beta-induced Neuronal Death. J Biol Chem, 292(7), 2571–2585. doi: 10.1074/jbc.M116.744730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sataranatarajan K, Ikeno Y, Bokov A, Feliers D, Yalamanchili H, Lee HJ, … Kasinath BS (2016). Rapamycin Increases Mortality in db/db Mice, a Mouse Model of Type 2 Diabetes. J Gerontol A Biol Sci Med Sci, 71(7), 850–857. doi: 10.1093/gerona/glv170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato T, Nakashima A, Guo L, & Tamanoi F (2009). Specific activation of mTORC1 by Rheb G-protein in vitro involves enhanced recruitment of its substrate protein. J Biol Chem, 284(19), 12783–12791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savitt D, & Jankovic J (2019). Targeting alpha-Synuclein in Parkinson’s Disease: Progress Towards the Development of Disease-Modifying Therapeutics. Drugs. doi: 10.1007/s40265-019-01104-1 [DOI] [PubMed] [Google Scholar]
- Schutters K, & Reutelingsperger C (2010). Phosphatidylserine targeting for diagnosis and treatment of human diseases. Apoptosis, 15(9), 1072–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang YC, Chong ZZ, Hou J, & Maiese K (2009). FoxO3a governs early microglial proliferation and employs mitochondrial depolarization with caspase 3, 8, and 9 cleavage during oxidant induced apoptosis. Curr Neurovasc Res, 6(4), 223–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang YC, Chong ZZ, Hou J, & Maiese K (2010). Wnt1, FoxO3a, and NF-kappaB oversee microglial integrity and activation during oxidant stress. Cell Signal, 22(9), 1317–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang YC, Chong ZZ, Wang S, & Maiese K (2011). Erythropoietin and Wnt1 Govern Pathways of mTOR, Apaf-1, and XIAP in Inflammatory Microglia. Curr Neurovasc Res, 8(4), 270–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang YC, Chong ZZ, Wang S, & Maiese K (2012a). Prevention of beta-amyloid degeneration of microglia by erythropoietin depends on Wnt1, the PI 3-K/mTOR pathway, Bad, and Bcl-xL. Aging (Albany NY), 4(3), 187–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang YC, Chong ZZ, Wang S, & Maiese K (2012b). WNT1 Inducible Signaling Pathway Protein 1 (WISP1) Targets PRAS40 to Govern beta-Amyloid Apoptotic Injury of Microglia. Curr Neurovasc Res, 9(4), 239–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang YC, Chong ZZ, Wang S, & Maiese K (2013). Tuberous sclerosis protein 2 (TSC2) modulates CCN4 cytoprotection during apoptotic amyloid toxicity in microglia. Curr Neurovasc Res, 10(1), 29–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shokri Afra H, Zangooei M, Meshkani R, Ghahremani MH, Ilbeigi D, Khedri A, … Nourbakhsh M (2019). Hesperetin is a potent bioactivator that activates SIRT1-AMPK signaling pathway in HepG2 cells. J Physiol Biochem. doi: 10.1007/s13105-019-00678-4 [DOI] [PubMed] [Google Scholar]
- Stefanatos R, & Sanz A (2018). The role of mitochondrial ROS in the aging brain. FEBS Lett, 592(5), 743–758. doi: 10.1002/1873-3468.12902 [DOI] [PubMed] [Google Scholar]
- Su M, Naderi K, Samson N, Youssef I, Fulop L, Bozso Z, … Davis S (2019). Mechanisms Associated with Type 2 Diabetes as a Risk Factor for Alzheimer-Related Pathology. Mol Neurobiol. doi: 10.1007/s12035-019-1475-8 [DOI] [PubMed] [Google Scholar]
- Sun J, Martin JM, Vanderpoel V, & Sumbria RK (2019). The Promises and Challenges of Erythropoietin for Treatment of Alzheimer’s Disease. Neuromolecular Med. doi: 10.1007/s12017-019-08524-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang PC, Ng YF, Ho S, Gyda M, & Chan SW (2014). Resveratrol and cardiovascular health--promising therapeutic or hopeless illusion? Pharmacol Res, 90, 88–115. doi: 10.1016/j.phrs.2014.08.001 [DOI] [PubMed] [Google Scholar]
- Taveira GB, Mello EO, Souza SB, Monteiro RM, Ramos AC, Carvalho AO, … Gomes VM (2018). Programmed cell death in yeast by thionin-like peptide from Capsicum annuum fruits involving activation of capases and extracelullar H(+) flux. Biosci Rep. doi: 10.1042/bsr20180119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teotia P, Van Hook MJ, Fischer D, & Ahmad I (2019). Human retinal ganglion cell axon regeneration by recapitulating developmental mechanisms: effects of recruitment of the mTOR pathway. Development, 146(13). doi: 10.1242/dev.178012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thackeray JT, Radziuk J, Harper ME, Suuronen EJ, Ascah KJ, Beanlands RS, & Dasilva JN (2011). Sympathetic nervous dysregulation in the absence of systolic left ventricular dysfunction in a rat model of insulin resistance with hyperglycemia. Cardiovasc Diabetol, 10, 75. doi: 10.1186/1475-2840-10-75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong J, Lai Y, Yao YA, Wang XJ, Shi YS, Hou HJ, … Liu XB (2018). Qiliqiangxin Rescues Mouse Cardiac Function by Regulating AGTR1/TRPV1-Mediated Autophagy in STZ-Induced Diabetes Mellitus. Cell Physiol Biochem, 47(4), 1365–1376. doi: 10.1159/000490822 [DOI] [PubMed] [Google Scholar]
- Treins C, Alliouachene S, Hassouna R, Xie Y, Birnbaum MJ, & Pende M (2012). The combined deletion of S6K1 and Akt2 deteriorates glycaemic control in high fat diet. Mol Cell Biol. doi: 10.1128/mcb.00514-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulsulkar J, Nada SE, Slotterbeck BD, McInerney MF, & Shah ZA (2015). Obesity and hyperglycemia lead to impaired post-ischemic recovery after permanent ischemia in mice. Obesity (Silver Spring), 24(2), 417–423. doi: 10.1002/oby.21388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullah R, Khan M, Shah SA, Saeed K, & Kim MO (2019). Natural Antioxidant Anthocyanins-A Hidden Therapeutic Candidate in Metabolic Disorders with Major Focus in Neurodegeneration. Nutrients, 11(6). doi: 10.3390/nu11061195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal RL, Figueroa A, Court FA, Thielen P, Molina C, Wirth C, … Hetz C (2012). Targeting the UPR transcription factor XBP1 protects against Huntington’s disease through the regulation of FoxO1 and autophagy. Hum Mol Genet, 21(10), 2245–2262. doi: 10.1093/hmg/dds040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang AR, Yan XQ, Zhang C, Du CQ, Long WJ, Zhan D, … Luo XP (2018). Characterization of Wnt1-inducible Signaling Pathway Protein-1 in Obese Children and Adolescents. Curr Med Sci, 38(5), 868–874. doi: 10.1007/s11596-018-1955-5 [DOI] [PubMed] [Google Scholar]
- Wang GB, Ni YL, Zhou XP, & Zhang WF (2014). The AKT/mTOR pathway mediates neuronal protective effects of erythropoietin in sepsis. Mol Cell Biochem, 385(1–2), 125–132. doi: 10.1007/s11010-013-1821-5 [DOI] [PubMed] [Google Scholar]
- Wang H, Zhang Q, Wen Q, Zheng Y, Philip L, Jiang H, … Zheng W (2012). Proline-rich Akt substrate of 40kDa (PRAS40): a novel downstream target of PI3k/Akt signaling pathway. Cell Signal, 24(1), 17–24. doi: 10.1016/j.cellsig.2011.08.010 [DOI] [PubMed] [Google Scholar]
- Wang L, Lawrence JC Jr., Sturgill TW, & Harris TE (2009). Mammalian target of rapamycin complex 1 (mTORC1) activity is associated with phosphorylation of raptor by mTOR. J Biol Chem, 284(22), 14693–14697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Wu W, Chen J, Li Y, Xu M, & Cai Y (2019). miR122 and miR199 synergistically promote autophagy in oral lichen planus by targeting the Akt/mTOR pathway. Int J Mol Med. doi: 10.3892/ijmm.2019.4068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RH, Kim HS, Xiao C, Xu X, Gavrilova O, & Deng CX (2011). Hepatic Sirt1 deficiency in mice impairs mTorc2/Akt signaling and results in hyperglycemia, oxidative damage, and insulin resistance. J Clin Invest, 121(11), 4477–4490. doi: 10.1172/jci46243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, & Le WD (2019). Autophagy and Ubiquitin-Proteasome System. Adv Exp Med Biol, 1206, 527–550. doi: 10.1007/978-981-15-0602-4_25 [DOI] [PubMed] [Google Scholar]
- Wang Y, Wang YX, Liu T, Law PY, Loh HH, Qiu Y, & Chen HZ (2015). mu-Opioid receptor attenuates Abeta oligomers-induced neurotoxicity through mTOR signaling. CNS Neurosci Ther, 21(1), 8–14. doi: 10.1111/cns.12316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weckman A, Di Ieva A, Rotondo F, Syro LV, Ortiz LD, Kovacs K, & Cusimano MD (2014). Autophagy in the endocrine glands. J Mol Endocrinol, 52(2), R151–163. doi: 10.1530/jme-13-0241 [DOI] [PubMed] [Google Scholar]
- Wei L, Sun C, Lei M, Li G, Yi L, Luo F, … Xu P (2013). Activation of Wnt/beta-catenin Pathway by Exogenous Wnt1 Protects SH-SY5Y Cells Against 6-Hydroxydopamine Toxicity. J Mol Neurosci, 49(1), 105–115. doi: 10.1007/s12031-012-9900-8 [DOI] [PubMed] [Google Scholar]
- Weikel KA, Cacicedo JM, Ruderman NB, & Ido Y (2016). Knockdown of GSK3beta Increases Basal Autophagy and AMPK Signaling in Nutrient-laden Human Aortic Endothelial Cells. Biosci Rep, 36(5), pii e00382. doi: 10.1042/bsr20160174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen Z, Zhang J, Tang P, Tu N, Wang K, & Wu G (2018). Overexpression of miR185 inhibits autophagy and apoptosis of dopaminergic neurons by regulating the AMPK/mTOR signaling pathway in Parkinson’s disease. Mol Med Rep, 17(1), 131–137. doi: 10.3892/mmr.2017.7897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White CR, Datta G, & Giordano S (2017). High-Density Lipoprotein Regulation of Mitochondrial Function. Adv Exp Med Biol, 982, 407–429. doi: 10.1007/978-3-319-55330-6_22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CJ, & Dexter DT (2014). Neuroprotective and symptomatic effects of targeting group III mGlu receptors in neurodegenerative disease. J Neurochem, 129(1), 4–20. doi: 10.1111/jnc.12608 [DOI] [PubMed] [Google Scholar]
- Woodhams L, & Al-Salami H (2017). The roles of bile acids and applications of microencapsulation technology in treating Type 1 diabetes mellitus. Ther Deliv, 8(6), 401–409. doi: 10.4155/tde-2017-0010 [DOI] [PubMed] [Google Scholar]
- World Health Organization. (2011). Description of the global burden of NCDs, their risk factors and determinants. Global status report on noncommunicable diseases 2010(April), 1–176. [Google Scholar]
- World Health Organization. (2012). Dementia: A public health priority. Geneva: World Health Organization, 1–4. [Google Scholar]
- World Health Organization. (2017). Global action plan on the public health response to dementia 2017–2025. 1–44. [Google Scholar]
- Wu D, Wang H, Teng T, Duan S, Ji A, & Li Y (2018). Hydrogen sulfide and autophagy: A double edged sword. Pharmacol Res. doi: 10.1016/j.phrs.2018.03.002 [DOI] [PubMed] [Google Scholar]
- Xi JS, Wang YF, Long XX, & Ma Y (2018). Mangiferin Potentiates Neuroprotection by Isoflurane in Neonatal Hypoxic Brain Injury by Reducing Oxidative Stress and Activation of Phosphatidylinositol-3-Kinase/Akt/Mammalian Target of Rapamycin (PI3K/Akt/mTOR) Signaling. Med Sci Monit, 24, 7459–7468. doi: 10.12659/msm.908142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang L, Mittwede PN, & Clemmer JS (2015). Glucose Homeostasis and Cardiovascular Alterations in Diabetes. Compr Physiol, 5(4), 1815–1839. doi: 10.1002/cphy.c150001 [DOI] [PubMed] [Google Scholar]
- Xiao FH, He YH, Li QG, Wu H, Luo LH, & Kong QP (2015). A genome-wide scan reveals important roles of DNA methylation in human longevity by regulating age-related disease genes. PLoS ONE, 10(3), e0120388. doi: 10.1371/journal.pone.0120388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Q, Ying J, Xiang L, & Zhang C (2018). The biologic effect of hydrogen sulfide and its function in various diseases. Medicine (Baltimore), 97(44), e13065. doi: 10.1097/md.0000000000013065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin YJ, Yuan B, Yu B, Wang YQ, Wu JJ, Zhou WH, & Qiu Z (2015). Tet1-mediated DNA demethylation regulates neuronal cell death induced by oxidative stress. Sci Rep, 5, 7645. doi: 10.1038/srep07645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong X, Xie R, Zhang H, Gu L, Xie W, Cheng M, … Zhao H (2014). PRAS40 plays a pivotal role in protecting against stroke by linking the Akt and mTOR pathways. Neurobiol Dis, 66, 43–52. doi: 10.1016/j.nbd.2014.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu G, Shen H, Nibona E, Wu K, Ke X, Al Hafiz MA, … Zhao H (2019). Fundc1 is necessary for proper body axis formation during embryogenesis in zebrafish. Sci Rep, 9(1), 18910. doi: 10.1038/s41598-019-55415-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu YJ, Tappia PS, Neki NS, & Dhalla NS (2014). Prevention of diabetes-induced cardiovascular complications upon treatment with antioxidants. Heart Fail Rev, 19(1), 113–121. doi: 10.1007/s10741-013-9379-6 [DOI] [PubMed] [Google Scholar]
- Xue Q, Nagy JA, Manseau EJ, Phung TL, Dvorak HF, & Benjamin LE (2009). Rapamycin inhibition of the Akt/mTOR pathway blocks select stages of VEGF-A164-driven angiogenesis, in part by blocking S6Kinase. Arterioscler Thromb Vasc Biol, 29(8), 1172–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao T, Fujimura T, Murayama K, Okumura K, & Seko Y (2017). Oxidative Stress-Responsive Apoptosis Inducing Protein (ORAIP) Plays a Critical Role in High Glucose-Induced Apoptosis in Rat Cardiac Myocytes and Murine Pancreatic beta-Cells. Cells, 6(4). doi: 10.3390/cells6040035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye Q, & Fu JF (2018). Paediatric type 2 diabetes in China-Pandemic, progression, and potential solutions. Pediatr Diabetes, 19(1), 27–35. doi: 10.1111/pedi.12517 [DOI] [PubMed] [Google Scholar]
- Yelumalai S, Giribabu N, Karim K, Omar SZ, & Salleh NB (2019). In vivo administration of quercetin ameliorates sperm oxidative stress, inflammation, preserves sperm morphology and functions in streptozotocin-nicotinamide induced adult male diabetic rats. Arch Med Sci, 15(1), 240–249. doi: 10.5114/aoms.2018.81038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin B, Liang H, Chen Y, Chu K, Huang L, Fang L, … Luo B (2013). EGB1212 post-treatment ameliorates hippocampal CA1 neuronal death and memory impairment induced by transient global cerebral ischemia/reperfusion. Am J Chin Med, 41(6), 1329–1341. doi: 10.1142/s0192415x13500894 [DOI] [PubMed] [Google Scholar]
- Yu H, & Blair RH (2019). Integration of probabilistic regulatory networks into constraint-based models of metabolism with applications to Alzheimer’s disease. BMC Bioinformatics, 20(1), 386. doi: 10.1186/s12859-019-2872-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T, Li L, Chen T, Liu Z, Liu H, & Li Z (2015). Erythropoietin attenuates advanced glycation endproducts-induced toxicity of schwann cells in vitro. Neurochem Res, 40(4), 698–712. doi: 10.1007/s11064-015-1516-2 [DOI] [PubMed] [Google Scholar]
- Zhang C, Li C, Chen S, Li Z, Ma L, Jia X, … He C (2017). Hormetic effect of panaxatriol saponins confers neuroprotection in PC12 cells and zebrafish through PI3K/AKT/mTOR and AMPK/SIRT1/FOXO3 pathways. Sci Rep, 7, 41082. doi: 10.1038/srep41082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Yang X, Pang X, Zhao Z, Yu H, & Zhou H (2019). Genistein protects against ox-LDL-induced senescence through enhancing SIRT1/LKB1/AMPK-mediated autophagy flux in HUVECs. Mol Cell Biochem, 455(1). doi: 10.1007/s11010-018-3476-8 [DOI] [PubMed] [Google Scholar]
- Zhang N, & Zhao Y (2019). Other Molecular Mechanisms Regulating Autophagy. Adv Exp Med Biol, 1206, 261–271. doi: 10.1007/978-981-15-0602-4_13 [DOI] [PubMed] [Google Scholar]
- Zhang ZH, Wu QY, Zheng R, Chen C, Chen Y, Liu Q, … Song GL (2017). Selenomethionine mitigates cognitive decline by targeting both tau hyperphosphorylation and autophagic clearance in an Alzheimer’s disease mouse model. J Neurosci, 37(9), 2449–2462. doi: 10.1523/jneurosci.3229-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D, Sun X, Lv S, Sun M, Guo H, Zhai Y, … Wang X (2019). Salidroside attenuates oxidized lowdensity lipoproteininduced endothelial cell injury via promotion of the AMPK/SIRT1 pathway. Int J Mol Med. doi: 10.3892/ijmm.2019.4153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Wang ZC, Wang KF, & Chen XY (2015). Abeta peptide secretion is reduced by Radix Polygalaeinduced autophagy via activation of the AMPK/mTOR pathway. Mol Med Rep, 12(2), 2771–2776. doi: 10.3892/mmr.2015.3781 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Scott NA, Fynch S, Elkerbout L, Wong WW, Mason KD, … Thomas HE (2015). Autoreactive T cells induce necrosis and not BCL-2-regulated or death receptor-mediated apoptosis or RIPK3-dependent necroptosis of transplanted islets in a mouse model of type 1 diabetes. Diabetologia, 58(1), 140–148. doi: 10.1007/s00125-014-3407-5 [DOI] [PubMed] [Google Scholar]
- Zhao Y, Wang Q, Wang Y, Li J, Lu G, & Liu Z (2019). Glutamine protects against oxidative stress injury through inhibiting the activation of PI3K/Akt signaling pathway in parkinsonian cell model. Environ Health Prev Med, 24(1), 4. doi: 10.1186/s12199-018-0757-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Wu J, Zheng F, Jin M, & Li H (2015). Glucagon-like peptide-1 analog-mediated protection against cholesterol-induced apoptosis via mammalian target of rapamycin activation in pancreatic betaTC-6 cells −1mTORbetaTC-6. J Diabetes, 7(2), 231–239. doi: 10.1111/1753-0407.12177 [DOI] [PubMed] [Google Scholar]
- Zhou L, Gao W, Wang K, Huang Z, Zhang L, Zhang Z, … Huang C (2019). Brefeldin A inhibits colorectal cancer growth by triggering Bip/Akt-regulated autophagy. Faseb J, fj201801983R. doi: 10.1096/fj.201801983R [DOI] [PubMed] [Google Scholar]
- Zhou Q, Chen B, Wang X, Wu L, Yang Y, Cheng X, … Cao P (2016). Sulforaphane protects against rotenone-induced neurotoxicity in vivo: Involvement of the mTOR, Nrf2, and autophagy pathways. Sci Rep, 6, 32206. doi: 10.1038/srep32206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman MA, Biggers CD, & Li PA (2018). Rapamycin treatment increases hippocampal cell viability in an mTOR-independent manner during exposure to hypoxia mimetic, cobalt chloride. BMC Neurosci, 19(1), 82. doi: 10.1186/s12868-018-0482-4 [DOI] [PMC free article] [PubMed] [Google Scholar]


