Graphical abstract
Keywords: Clean water and sanitation, Green and sustainable remediation, High-performance photocatalyst, Advanced materials, Reaction mechanisms for photodegradation
Abstract
Antibiotics are widely present in the environment due to their extensive and long-term use in modern medicine. The presence and dispersal of these compounds in the environment lead to the dissemination of antibiotic residues, thereby seriously threatening human and ecosystem health. Thus, the effective management of antibiotic residues in water and the practical applications of the management methods are long-term matters of contention among academics. Particularly, photocatalysis has attracted extensive interest as it enables the treatment of antibiotic residues in an eco-friendly manner. Considerable progress has been achieved in the implementation of photocatalytic treatment of antibiotic residues in the past few years. Therefore, this review provides a comprehensive overview of the recent developments on this important topic. This review primarily focuses on the application of photocatalysis as a promising solution for the efficient decomposition of antibiotic residues in water. Particular emphasis was laid on improvement and modification strategies, such as augmented light harvesting, improved charge separation, and strengthened interface interaction, all of which enable the design of powerful photocatalysts to enhance the photocatalytic removal of antibiotics.
1. Introduction
In the last few decades, antibiotics have been broadly utilized not only for human therapies, such as curing infectious diseases like COVID-19, transplants, chemotherapy, and surgical interventions, but also for therapeutic and non-therapeutic purposes in aquaculture and animal husbandry and for enhancing crop production [1], [2]. However, the use and overuse as well as the delayed metabolism of antibiotics have led to the inevitable discharge of their residues into aquatic environments, resulting in refractory pollution sources [3]. Conventional wastewater treatment plants (WWTPs) cannot efficiently remove antibiotic residues [4], [5]. This leads to their distribution into ecological systems [6], [7] and eventually into the human body through the food chain or drinking water [8], [9], [10]. Moreover, the long residence times of antibiotic residues in aquatic environments, even at low concentrations, may lead to the propagation of bacteria with antibiotic resistance and even multiple drug resistance, which may cause life-threatening infections [3], [11], [12]. Therefore, developing an effective approach to degrade or remove antibiotic residues from aquatic environments is crucial.
Different methods have been developed to treat antibiotic residues in water and wastewater before their final release into the environment. Table 1 summarizes the current main approaches, including traditional techniques and newly developed methods.
Table 1.
Methods for antibiotic degradation or removal in water.
| Treatment | Materials | Antibiotic | Operating conditions | Disadvantages | Ref. |
|---|---|---|---|---|---|
| Adsorption processes | Anionic surfactant sodium dodecyl sulfate (SDS) | Amoxicillin Ampicillin |
Contact time: 40 min Agitation speed: 350 rpm Temperature: 40 °C pH: 4 |
Low removal capacities; difficult separation; secondary environmental pollution; unsatisfactory recycling ability |
[13] |
| KOH-modified biochar | Norfloxacin Sulfamerazine Oxytetracycline |
Temperature: 15–35 °C pH 3–9 |
[14] | ||
| Graphene oxide/cellulose nanofibril hybrid aerogel | Doxycycline Chlortetracycline Oxytetracycline Tetracycline |
Temperature: 25 °C | [15] | ||
| Pyrogenic carbonaceous materials | Ciprofloxacin | Contact time: 72 h Temperature: 25 °C pH 7.5 and 9.5 |
[16] | ||
| Clean and dried Kigelia pinnata modified with ionic liquid | Ibuprofen Ketoprofen Ampicillin Diclofenac |
pH 2.5 or 5 | [17] | ||
| Manure-derived biochars | Lincomycin | pH 6 or 10 | [18] | ||
| Lanthanum modified diatomite | Tetracycline antibiotics | Contact time: 24 h pH 3–10 |
[19] | ||
| Adsorption processes | Grape stalk | Ofloxacin Chrysoidine |
pH 4, 7, and 9 | Low removal capacities; difficult separation; secondary environmental pollution; unsatisfactory recycling capacity |
[20] |
| Cleaned and dried Pachydictyon coriaceum and Sargassum hemiphyllum | Tetracycline | Temperature: 15–35 °C pH 3–9 Salinity: 0–100 mM NaCl |
[21] | ||
| Spent mushroom substrate | Sulfamethyldiazine Sulfamethazine Sulfathiazole Sulfamethoxazole |
Temperature: 15 °C pH 3–11 |
[22] | ||
| Coagulation /flocculation /sedimentation |
Amino-acid-modified-chitosan flocculants | Norfloxacin Sulfadiazine Tylosin |
Temperature: 25 °C pH 6, 7, 8 |
Antibiotics cannot be completely removed and secondary pollution occurs readily | [23] |
| Ozonation | Ozone | Amoxicillin Doxycycline Ciprofloxacin Sulfadiazine |
Neutral pH | Demands high equipment and energy costs | [24] |
| Ozone/zero-valent iron | Flumequine | Contact time: 1h Initial pH 2.5 Fe (0) dosage: 60 g/L Ozone flow rate: 0.25 L/min Temperature: 30 °C |
[25] | ||
| Ozonation | Medium-high frequency ultrasound and ozone | Amoxicillin | Medium-high ultrasonic frequency waves: 575, 861, 1141 kHz pH 7, 10 |
Demands high equipment and energy costs | [26] |
| Ozone | Flumequine | Contact time: 6 min Temperature: 25 °C pH 3, 5, 7, 9, 11 |
[27] | ||
| Ozone | Ofloxacin | Temperature: 25 °C pH: 2, 7, 12 |
[28] | ||
| Multistage ozone and biological treatment system | Amoxicillin | Temperature: 25 °C pH: 10 |
Complex; high operating costs; continuous use is impractical | [29] | |
| Chemical coagulation and microfiltration | Ibuprofen Ephedrine Propranolol |
Different doses of ZnO nanoparticles: 0.5, 0.7, 1.0, 1.3, 1.5, 1.7 g/L pH 7, 9 |
[30] | ||
| Electric coagulation and photo-electro-Fenton process | Metronidazole | pH: 1, 3, 5, 7, 9 Metronidazole concentration: 50 mg/L Voltage: 5–30 V H2O2: 0–0.02 mol/L Temperature: ~20 °C UV at 230 nm Number of UV lamps: 1–4 |
[31] | ||
| Combined processes | A membrane bioreactor (MBR) integrated with solar Fenton oxidation | Sulfamethoxazole Erythromycin Clarithromycin |
H2O2: 20–100 mg/L pH: 2.8 [Fe2+]: 5 mg/L |
Complex; high operating costs; impracticability in continuous use | [32] |
| Integrated adsorption-membrane filtration process | Norfloxacin Ofloxacin |
pH: 7 Temperature: 25 °C UV at 276 nm and 293 nm pressure range: 34.47–172.36 kPa |
[33] | ||
| Ultraviolet, chlorination, ozone disinfection | Antibiotic resistance genes | Chlorine concentrations: 2–32 mg/L Ozone concentration: 2–10 mg/L UV (mJ/cm2): 10–160 |
[34] | ||
| Adsorptive magnetic ion exchange resin | Sulfamethoxazole Tetracycline Amoxicillin |
Contact time: 30 min Temperature: 25 °C MIEX resin dosage: 5 mL/LAntibiotic: 1000 μg/L |
[30] | ||
| Nanofiltration and chlorination | Sulfanilamide Sulfadiazine Sulfamethoxazole Sulfadimethoxine |
Membrane effective area: 40.92 cm2 Operating pressure: 5 bar Temperature: 25 °C Flow rate: 120–150 mL/min Initial pH: 5.6, 7.2, 10 Initial concentration: 2.0 × 10−5 M |
[35] |
These current methods can be loosely classified into three types according to the treatment mechanisms or principles involved: physical removal, biological treatment, and chemical degradation. As shown in Table 1, physical methods such as adsorption, sedimentation, flocculation, and filtration only separate the antibiotic residues from the water and generate problematic products such as brine and contaminated adsorbents. Alternatively, biological approaches have recently emerged, and most antibiotic residues in the environment can be removed through this route [36], [37], [38]. However, the artificial introduction of active organisms into aquatic environments may disrupt the ecological balance of their biomes, which may cause irreversible ecosystem damage. Moreover, biological processes suffer from some key disadvantages such as being time-consuming and often unreliable. Therefore, the chemical degradation of antibiotics has gained strong interests.
Different chemical approaches such as ozonation, chlorination, and Fenton’s oxidation have been developed for the treatment of antibiotic residues in water, as shown in Table 1. Unfortunately, complete mineralization is difficult to achieve or may otherwise be prohibitively lengthy. In some cases, these methods may kill non-target organisms due to their low selectivity, which causes unintended damages [39], [40]. Additionally, this method incurs high capital and high operating costs. Combinations of physical and chemical degradation processes can significantly reduce the toxicity of treated effluents during the removal of antibiotic residues from water. Nevertheless, these methods are complex and costly [41].
Alternatively, photocatalysis has broad application prospects for environmental remediation due to its unique advantages, including (1) easily-attainable reaction conditions (i.e., near ambient temperature and mostly ambient pressure), the use of oxygen in the air to produce a powerful oxidant, and the use of solar radiation as an energy source; (2) potentially complete decomposition of organic pollutants into innocuous inorganic molecules such as carbon dioxide and water; (3) strong redox ability, low cost, no adsorption saturation, and long durability. Therefore, photocatalysis has increasingly garnered worldwide interest and has been broadly implemented in novel energy extraction and environmental control strategies.
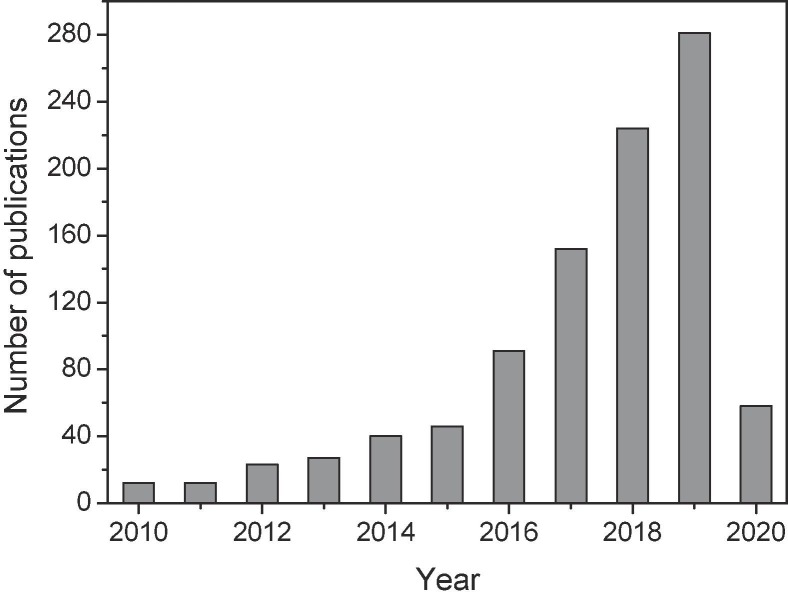
Photocatalysis is an advanced oxidation process that has previously been applied in the treatment of antibiotic residues. As shown in Fig. 1 , less than 40 studies had been published on the photocatalytic treatment of antibiotic residues, prior to 2014. However, a sharply increasing number of publications on this subject have been released thereafter. Substantial progress has been made in recent years; however, photocatalysis still suffers from some key imperfections, including insufficient visible light utilization, rapid annihilation of photogenerated carriers, and incomplete mineralization, all of which strongly restrict its commercial application. A thorough overview concerning the fundamentals, improvement, and modification of strategies and challenges of the photocatalytic treatment of antibiotic residues was yet to be compiled, and therefore, these topics have been comprehensively addressed herein.
Fig. 1.
Number of search results of recent publications addressing the photocatalytic treatment of antibiotic residues using “photocatalytic” and “antibiotic treatment” as keywords (collected from the Web of Science Core Database: March 9, 2020).
This article comprehensively reviews the latest advances in the photocatalytic treatment of antibiotic residues in water. Specifically, the fundamentals of photocatalysis will be introduced. Different strategies will then be summarized and classified, followed by a discussion on the improvement and modification strategies to enhance the photocatalytic degradation of antibiotics. Finally, this review will conclude with a summary of major challenges and key perspectives.
2. Basic principles of photodegradation
When a semiconductor is exposed to irradiation with energy beyond its optical band gap, electrons are excited and shifted from its valence band (VB) towards the conduction band (CB), producing an equal number of positively charged holes in the VB. When the potential of VB vs NHE is more positive than or , and the potential of CB vs NHE is more negative than−0.33 V vs NHE), the semiconductor will be able to generate and . Thereafter, the photoinduced electrons and holes separate and migrate to the surface of the semiconductor, and redox reactions will occur at the reactive site on the semiconductor surface (Fig. 2 ) [42], [43].
Fig. 2.
Schematic representation of the semiconductor photocatalysis process [44]
The reaction mechanisms of semiconductor photocatalysis are typically expressed by the following equations [45], [46]:
Semiconductor Light Energy (λ) Semiconductor ( ) (1)
OH () (2)
OH () (3)
( − 0.33 V vs NHE) (4)
Pollutant Active species Degradation Products (5)
Through these chemical processes, solar energy can be directly converted and effectively utilized. For instance, Zhao et al. [47] successfully prepared ZnSe quantum dot (QD)/g-C3N4 composites with remarkable photocatalytic properties and utilized them for the photocatalytic degradation of ceftriaxone sodium. Based on the results, OH and h+ were found to be the main active materials. The potential reaction mechanisms are described below.
ZnSe QDs/g-C3N4 hv h+ e−
h+ H2O H+ •OH
e− 1/2O2 H+ •OH
h+ •OH Ceftriaxone sodium CO2 H2O other small molecules
However, limited optical utilization and the quick annihilation of photoexcited electron-hole pairs diminish the effects of photocatalytic activity. Photocatalysts are able to overcome these deficits, provided that they meet the following criteria: (1) appropriate spectral absorption range, (2) proper band energy structure for sufficient separation and transport of electron–hole pairs, and (3) adequate active sites for adsorption or reaction [48], [49], [50]. It is essential to satisfy all three of the aforementioned prerequisites to improve photocatalytic efficiency, and abundant efforts have been made to systematically design photocatalysts and optimize photocatalytic dynamics.
3. Strategies for photocatalytic activity improvement
The photocatalytic treatment of antibiotic residues in aquatic environments has recently become the focus of much attention, and diverse strategies have been proposed to improve the photocatalytic efficiency (Scheme 1 ). Examples of such reported strategies are summarized in Table 2 .
Scheme 1.
Strategies for photocatalytic efficiency improvement.
Table 2.
Summary of previous studies using different strategies to improve photocatalytic degradation of antibiotics.
| Strategies | Catalysts and concentration | Light source | Antibiotic and concentration | Degradation efficiency | Ref. |
|---|---|---|---|---|---|
| Vacancies | BiOCl with abundant oxygen vacancies 0.5 g/L |
300-W Xe lamp (λ > 420 nm) |
Tetracycline hydrochloride 10 mg/L |
Approximately 87% optimum within 2 h | [51] |
| Vacancies | Oxygen vacancy-rich mesoporous ZrO2 1 g/L |
300-W Xe lamp (λ > 420 nm) |
Tetracycline hydrochloride 40 mg/L |
Approximately 80% optimum within 150 h | [52] |
| Vacancies | BiOBr microspheres with oxygen vacancies 1 g/L |
10-W LED lamp (0.4 mW‧cm−2) | Tetracycline (TC) 20 mg/L |
Approximately 94% optimum within 90 min | [53] |
| Vacancies | ZnWO4-x nanorods with oxygen vacancy 0.2 g/L |
Hg lamp 300-W UV or 300-W Xe lamp (UV–Vis-NIR) | Tetracycline 20 mg/L |
Approximately 91% optimum within 90 min | [54] |
| Vacancies | Bi2MoO6 with oxygen vacancy 0.4 g/L |
300-W Xe lamp (λ > 400 nm) |
Ciprofloxacin 20 mg/L |
Approximately 55% optimum within 120 min | [55] |
| Doping | Carbon-doped g-C3N4 0.5 g/L |
Sunlight | Tetracycline 20 mg/L |
Approximately 90% optimum within 90 min | [56] |
| Doping | P-O co-doped g-C3N4 1 g/L |
350-W Xe lamp (λ>420 nm) | Enrofloxacin10 mg/L | Approximately 90% within 80 min | [57] |
| Doping | I and K co-doped g-C3N4 2 g/L |
300-W Xe lamp (λ>420 nm) | Sulfamethoxazole 10 mg/L |
Approximately 99% optimum within 45 min | [58] |
| Doping | Bi3+/g-C3N4 0.2 g/L |
300-W Xe lamp (λ>420 nm) | Tetracycline 10 mg/L |
Approximately 94% optimum within 30 min | [59] |
| Doping | Cr3+/SrTiO3 1 g/L |
250-W Xe lamp (λ>420 nm) | Tetracycline 10 mg/L |
Approximately 70% optimum within 60 min | [60] |
| Doping | Fe2+/Fe3+ immobilized on TiO2/fly-ash cenospheres 2 g/L |
150-W tungsten halogen lamp (λ>420 nm) | Ciprofloxacin 10 mg/L |
Approximately 80% optimum within 60 min | [61] |
| Doping | Ce3+ doped Bi2O3 0.6 g/L |
300-W lamp (visible light) | Tetracycline 20 mg/L |
Approximately 89% optimum within 180 min | [62] |
| Doping | Ti3+/N co-doped TiO2/diatomite granule 5 g/L |
150-W Xenon lamp with a UV light filter | Tetracycline 10 mg/L |
92% optimum within 150 min | [63] |
| Quantum dots | CQDs modified Bi2MoO6 1 g/L |
300-W Xe lamp (λ>400 nm) | Ciprofloxacin 20 mg/L |
88% optimum within 2h | [64] |
| Quantum dots | CQDs/BiOBr microspheres 1 g/L |
Visible light irradiation | Ciprofloxacin 1 g/L |
Approximately 65% optimum within 180 min | [65] |
| Quantum dots | TiO2/C-dots 0.1 g/L-0.5 g/L |
Average intensity sunlight irradiation (72 klx) | Levofloxacin 10 mg/L |
Approximately 99% optimum within 90 min | [66] |
| Quantum dots | ZnSe QDs/g-C3N4 Not provided |
300-W Xe lamp (λ>400 nm) | Ceftriaxone sodium Concentration not provided |
Approximately 80% optimum within 120 min | [47] |
| Quantum dots | Ag2O/TiO2 quantum dots 0.25 g/L |
400-W halogen bulb (similar to sunlight) | Levofloxacin 10 mg/L |
Approximately 81% optimum within 90 min | [67] |
| Quantum dots | CQDs/BiOI 0.5 g/L |
300-W Xe lamp (λ>400 nm) | Tetracycline 20 mg/L |
Approximately 70% optimum within 120 min | [68] |
| Quantum dots | CQDs/BiOBr 0.3 g/L |
300-W Xe lamp (λ>400 nm) | Tetracycline 20 mg/L |
Approximately 60% optimum within 120 min | [69] |
| Quantum dots | MoS2 modified Zn-AgIn5S8 quantum dots 0.1 g/L |
250-W Xe lamp (λ>420 nm) | Tetracycline 10 mg/L |
Approximately 74% optimum within 4 min | [70] |
| Quantum dots | 3D ZnS-RGO nanospheres 1 g/L |
300-W Hg vapor lamp | Norfloxacin 20 mg/L |
92% optimum within 4h | [71] |
| Phase junction | Porous core–shell homojunction 0.5 g/L |
UV lamp (254 nm, 90 W, Philips) | Tetracycline hydrochloride 50 mg/L |
Approximately 81% within 300 min | [72] |
| Facet junction | CaCu3Ti4O12 Co-exposed (0 0 1) and (1 1 1) facets 0.4 g/L |
300-W Xe lamp visible light |
Tetracycline 0.24 g/L |
Approximately 99% within 50 min | [73] |
| Facet junction | AgBr tetradecahedrons with co-exposed (1 0 0) and (1 1 1) facets 1 g/L |
500-W halogen tungsten lamp (λ>420 nm) | Sulfadiazine 20 mg/L |
Approximately 90% optimum within 90 min | [74] |
| Schottky heterojunction | Ag/Ag2MoO4 0.4 g/L |
500-W Xe lamp (λ>420 nm) | Ciprofloxacin 20 mg/L |
Approximately 99% optimum within 60 min | [75] |
| Schottky heterojunction | Ag/TiO2 (hollow nanosphere) 0.5 g/L |
125-W high-pressure Hg lamp, (λ>435.8 nm) | Metronidazole 15 mg/L |
Approximately 95% optimum within 120 min | [76] |
| Schottky heterojunction | Bi/BiOBr (nano-flowers) 0.8 g/L |
300-W Xe lamp (λ>420 nm) | Tetracycline hydrochloride Ciprofloxacin and Doxycycline 1 g/L |
Approximately 100% optimum within 30 min | [77] |
| Schottky heterojunction | BiOCl-Ag (2D) 1.0 g/L |
200-W Xe arc lamp (λ<420 nm) | Sulfonamides 10 mg/L |
Approximately 80% optimum within 5h | [78] |
| Schottky heterojunction | Ag/Bi3O4Cl 0.5 g/L |
250-W Xe lamp (λ>420 nm) | Tetracycline 10 mg/L |
Approximately 94% optimum within 120 min | [79] |
| Schottky heterojunction | Ag/CCN 0.5 g/L |
300-W Xe lamp (λ>420 nm) | Tetracycline 10 mg/L |
75% optimum within 15 min | [80] |
| Schottky heterojunction | Pt/g-C3N4 0.5 g/L |
300-W Xe lamp (λ>400 nm) | Tetracycline hydrochloride 20 mg/L |
Approximately 84% optimum within 40 min | [81] |
| Schottky heterojunction | Bi (Spheres)/g-C3N4 0.5 g/L |
300-W Xe lamp (λ>420 nm) | Amoxicillin 100 mg/L |
Approximately 5% optimum within 4 h | [82] |
| Schottky heterojunction | W-doped BaTiO3 0.2 g/L |
Visible light irradiation | Tetracycline 20 mg/L |
Approximately 80% optimum within 3h | [83] |
| Schottky heterojunction | Fe, Co, Ni, Fe-Co-, and Fe-Ni-doped ZnO 0.6 g/L |
300-W Xe lamp (λ=365 nm) | Oxytetracycline 20 mg/L |
Approximately 87% optimum within 2h | [84] |
| Schottky heterojunction | Pt/Bi/TiO2 1 g/L |
300-W halogen-tungsten lamp (λ>420 nm) | Amoxicillin 10 mg/L |
Approximately 87% optimum within 2h | [85] |
| Schottky heterojunction | Au/Pt/g-C3N4 1 g/L |
500-W Xe lamp (λ>400 nm) | Tetracycline hydrochloride 20 g/L |
Approximately 90% optimum within 3h | [86] |
| Schottky heterojunction | 0D Bi nanodots/2D Bi3NbO7 nanosheets 0.5 g/L |
300-W Xe lamp (λ>400 nm) | Ciprofloxacin 10 mg/L |
Approximately 86% optimum within 120 min | [87] |
| Type Ⅱ heterojunction | AgI/BiVO4 0.3 g/L |
300-W Xe lamp (λ>420 nm) | Tetracycline (TC) 20 mg/L |
Approximately 94% optimum within 1h | [88] |
| Type Ⅱ heterojunction | 3D porous CdS/TiO2 1 g/L |
300-W Xe lamp (λ>420 nm) | Tetracycline and oxytetracycline (OTC) 40 mg/L |
Approximately TC: 67% and OTC: 81% optimums within 50 min | [89] |
| Type Ⅱ heterojunction | MgFe2O4/MoS2 | Radiation intensity: 47 mW/cm2 | Tetracycline 10 mg/L |
Approximately 92% optimum within 120 min | [90] |
| Type Ⅱ heterojunction | ZnWO4-CdS 1 g/L |
300-W Xe lamp (λ>420 nm) | Ciprofloxacin 15 mg/L |
Approximately 90% optimum within 1h | [91] |
| Type Ⅱ heterojunction | ZnO@ZnS nanorod 3×3 cm chip |
500-W Xe lamp | Tetracycline 10 mg/mL |
Approximately 80% within 140 min | [92] |
| Type Ⅱ heterojunction | MoS2/PbBiO2I 0.3 g/L |
300-W Xe lamp (λ>400 nm) | Ciprofloxacin 10 mg/L |
Approximately 80% optimum within 6h | [93] |
| Type Ⅱ heterojunction | Bi2SiO5/Bi12SiO20 1 g/L |
100-W high-pressure Hg lamp | Tetracycline 10 mg/L |
Approximately 79% optimum within 30 min | [94] |
| Type Ⅱ heterojunction | SrTiO3/Fe2O3 1 g/L |
250-W Xe lamp (λ>420 nm) | Tetracycline 10 mg/L |
Approximately 83% optimum within 140 min | [95] |
| Type Ⅱ heterojunction | p-C3N4/f-BiOBr 1 g/L |
250-W Xe lamp (λ>400 nm) | Tetracycline 30 mg/L |
Approximately 94% optimum within 300 min | [96] |
| Type Ⅱ heterojunction | Bi2O7Sn2-Bi7O9I3 1 g/L |
Halogen lamp as simulated solar light | Tetracycline 35 mg/L |
80% optimum within 90 min | [97] |
| Type Ⅱ heterojunction | NiFe2O4/Bi2O3 1 g/L |
150-W xenon lamp (λ>420 nm) | Tetracycline 10 mg/L |
Approximately 91% optimum within 90 min | [98] |
| Type Ⅱ heterojunction | BiVO4/rGO 0.9 g/L |
250-W Xe lamp (λ>420 nm) | Tetracycline 25 mg/L |
99% optimum within 90 min | [99] |
| Type Ⅱ heterojunction | SrTiO3 nanocube coated CdS microsphere 1 g/L |
250-W Xe lamp (λ>400 nm) | Ciprofloxacin 10 mg/L |
Approximately 94% optimum within 120 min | [100] |
| Type Ⅱ heterojunction | g-C3N4/BiPO4 0.3 g/L |
250-W high-pressure Hg lamp | Ciprofloxacin 10 mg/L |
Approximately 97% optimum within 120 min | [101] |
| Type Ⅱ heterojunction | g-C3N4/Ag3PO4 0.5 g/L |
300-W Xe lamp (λ>400 nm) | Ciprofloxacin Concentration not provided |
Approximately 67% optimum within 15 min | [102] |
| Type Ⅱ heterojunction | In2S3/NaTaO3 0.5 g/L |
300-W Xe lamp | Tetracycline hydrochloride 10 mg/L |
Approximately 80% optimum within 180 min | [103] |
| Type Ⅱ heterojunction | Polyaniline/Bi4O5Br2 0.4 g/L |
Visible light (λ>420 nm) |
Ciprofloxacin 10 mg/L Tetracycline 20 mg/L |
CIP: 99% optimum within 50 min, TC: approximately 86% optimum within 240 min | [104] |
| Type Ⅱ heterojunction | CdS nanoparticles/porous carbon polyhedrons 1 g/L |
300-W Xe lamp (λ>420 nm) | Cephalexin 20 mg/L |
Approximately 90% optimum within 90 min | [105] |
| Type Ⅱ heterojunction | Microsphere-like In2S3/InVO4 0.5 g/L |
300-W Xe lamp (λ>420 nm) | Tetracycline 10 mg/L |
Approximately 71% optimum within 60 min. | [106] |
| Type Ⅱ heterojunction | g-C3N4/Bi4O5Br2 0.5 g/L |
300-W Xe arc lamp | Ciprofloxacin 10 mg/L |
Approximately 67% optimum within 150 min | [107] |
| Type Ⅱ heterojunction | Bi2WO6/g-C3N4 1 g/L |
300-W Xe lamp UV light | Ceftriaxone sodium 10 mg/L |
Approximately 94% optimum within 120 min | [108] |
| Type Ⅱ heterojunction | Flower-root shaped Bi2O3/Bi2MoO6 0.4 g/L |
500-W Xe lamp (λ>420 nm) | Tetracycline 10 mg/L |
Approximately 70% optimum within 190 min | [109] |
| Type Ⅱ heterojunction | Covalent triazine framework modified BiOBr nanoflake 0.2 g/L |
500-W Xe lamp | Tetracycline 10 mg/L Ciprofloxacin 10 mg/L |
Approximately TC: 90% and CIP: 60% optimums within 50 min | [110] |
| Type Ⅱ heterojunction | mpg-C3N4 and Bi2WO6 nest-like structure 0.5 g/L |
300-W Xe lamp (λ>420 nm) | Tetracycline hydrochloride 50 mg/L |
Approximately 75% optimum within 120 min | [111] |
| Type Ⅱ heterojunction | TiO2 nanoparticle/SnNb2O6 nanosheet heterojunctions 1 g/L |
500-W tungsten lamp | Tetracycline hydrochloride 35 mg/L |
Approximately 76% optimum within 240 min | [112] |
| Type Ⅱ heterojunction | Bi4Ti3O12/BiOCl (2D/0D) composite 0.67 g/L |
300-W Xe lamp | Tetracycline hydrochloride 20 mg/L |
Approximately 84% optimum within 150 min | [113] |
| Type Ⅱ heterojunction | 2D-2D g-C3N4/Bi4O5Br2 0.1 g/L |
300-W Xe lamp (λ>400 nm) | Ciprofloxacin 10 mg/L |
Approximately 50% optimum within 30 min | [114] |
| Type Ⅱ heterojunction | 2D/2D Bi4Ti3O12/I-BiOCl 0.375 g/L |
350-W Xe arc lamp (λ>420 nm) | Ciprofloxacin 10 mg/L Tetracycline hydrochloride 10 mg/L |
Approximately 90% optimum within 120 min | [115] |
| Type Ⅱ heterojunction | Carbon-doped carbon nitride/Bi12O17Cl2 1 g/L |
300-W Xe lamp (λ>420 nm) | Tetracycline 20 mg/L |
94% optimum within 60 min | [116] |
| Type Ⅱ heterojunction | CuBi2O4/CuO 1 g/L |
Visible light (λ>400 nm) |
Metronidazole 50 mg/L |
36% optimum within 120 min | [46] |
| p-n heterojunction | p-n type BiOCl/titanium phosphate nanoplates 0.4 g/L |
300-W Xe lamp | Ciprofloxacin 5 mg/L |
Approximately 100% within 5 min | [117] |
| p-n heterojunction | p-n type CoO/g-C3N4 0.5 g/L |
300-W Xe lamp (λ>420 nm) | Tetracycline 10 mg/L |
Approximately 90% within 60 min | [118] |
| p-n heterojunction | p-n type Cu2O/SrTiO3 1 g/L |
150-W Xe lamp (λ>420 nm) | Tetracycline 10 mg/L |
Approximately 79% within 100 min | [119] |
| p-n heterojunction | p-n type flower-like BiOCl/BiOCOOH p-n 1 g/L |
300-W Xe lamp simulated sunlight | Tetracycline 20 mg/L |
Approximately 80% within 60 min | [120] |
| p-n heterojunction | p-n type Ag2O/g-C3N4 1 g/L |
500-W Xe lamp (λ>400 nm) | Tetracycline hydrochloride 20 mg/L |
Approximately 94% within 3h | [121] |
| p-n heterojunction | p-n type Co3O4-C3N4 0.5 g/L |
Sunlight | Tetracycline 48 mg/L |
Approximately 97% within 180 min | [122] |
| p-n heterojunction | p-n type 3D flower-like BiOBr/Bi2SiO5 1 g/L |
300-W Xe lamp (λ>420 nm) | Tetracycline 20 mg/L |
91% within 120 min | [123] |
| p-n heterojunction | n-p type SnO2 nanoparticles/BiOI 1 g/L |
300-W Xe lamp (λ>420 nm) | Oxytetracycline hydrochloride 10 mg/L |
Approximately 94% optimum within 90 min | [124] |
| p-n heterojunction | p-n type N-graphene QDs-BiOI/MnNb2O6 0.5 g/L |
250-W Xe lamp (λ>420 nm) | Tetracycline 10 mg/L |
Approximately 87% optimum within 60 min | [125] |
| p-n heterojunction | p-n type Fe3O4 quantum dots modified BiOCl/BiVO4 0.5 g/L |
300-W Xe lamp (λ>420 nm) | Sulfamethoxazole (SMX, 5 mg/L), TC (20 mg/L), norfloxacin (NOR, 10 mg/L), and CIP (10 mg/L) | SMX: 91% within 90 min, TC: 87% within 30 min, NOR: 89% within 60 min, CIP: 87% within 90 min | [126] |
| Double heterojunction | CDs/MoS2/TiO2 nanobelt 0.5 g/L |
250-W Xe lamp (λ>420 nm) | Tetracycline 10 mg/L |
Approximately 82% within 3h | [127] |
| Double heterojunction | (g-C3N4)-ZnO/halloysite nanotubes (HNTs) 1 g/L |
350-W Xe arc lamp | Tetracycline 20 mg/L |
Approximately 87% optimum within 60 min | [128] |
| Double heterojunction | Ultrathin g-C3N4 nanosheets coupled with amorphous Cu doped FeOOH nanoclusters as 2D/0D heterogeneous catalysts 0.2 g/L |
500-W Xe lamp (λ>420 nm) | Tetracycline 10 mg/L |
Approximately 90% within 40 min | [129] |
| Double heterojunction | 2D/2D/2D CoAl-LDH/g-C3N4/RGO ternary heterojunction 0.25 g/L |
300-W halogen lamp visible-light irradiation | Tetracycline 20 mg/L |
Approximately 100% optimum within 60 min | [130] |
| Double heterojunction | Ag-AgVO3/g-C3N4 0.2 g/L |
300-W Xe lamp (λ>410 nm) | Tetracycline 30 mg/L |
Approximately 84% optimum within 120 min | [131] |
| Double heterojunction | ZnFe2O4/Ag/Ag3VO4 1 g/L |
Visible-light irradiation | Tetracycline 10 mg/L |
Approximately 60% within 10 min | [132] |
| Double heterojunction | NiS and MoS2 nanosheet co-modified g-C3N4 ternary heterostructure 1 g/L |
250-W metal halide lamp (λ>400 nm) | Tetracycline 10 mg/L Ciprofloxacin 10 mg/L |
Approximately CIP: 71% and TC: 96% optimums, within 120 min | [133] |
| Double heterojunction | AgCl/Ag3PO4/ g-C3N4 1 mg/L |
Visible-light irradiation (λ>400 nm) | Sulfamethoxazole 50 mg/L |
Approximately 100% optimum within 90 min | [134] |
| Double heterojunction | 3D Ag3PO4/TiO2@MoS2 0.5 g/L |
800-W Xe arc lamp | OTC 5 mg/L ENR 5 mg/L |
Approximately OTC: 75%, ENR: 92% within 10 min | [135] |
| Double heterojunction | Bi2O3/BiOCl supported on graphene sand (BO/BOC/GSC) composite (BO/BOC/CT) 0.5 g/L |
Solar light intensity (35 × 103 ± 1000 lx) | Oxytetracycline 46 mg/L Ampicillin 37 mg/L |
BO/BOC/GSC: approximately 90% for AMP and OTC BO/BOC/CT: approximately 80% for AMP and OTC |
[136] |
| Double heterojunction | Core-shell structured Fe3O4@SiO2@CdS 0.2 g/L |
1000-W tungsten-halide lamp (Philips) (λ>420 nm) | Tetracycline 100 mg/L |
Approximately 80% optimum within 21 min | [137] |
| Double heterojunction | RGO-CdS/ZnS 0.5 g/L |
300-W Xe lamp (λ>420 nm) | Tetracycline 15 mg/L |
Approximately 90% optimum in 60 min | [138] |
| Double heterojunction | TiO2/Bi2WO6/carbon fibers 3 g/L |
300-W Xe lamp (λ>400 nm) | Tetracycline hydrochloride 10 mg/L |
Approximately 95% optimum within 60 min | [139] |
| Z-scheme heterojunction | Z-scheme beta-Bi2O3@g-C3N4 core/shell nanocomposite 0.5 g/L |
250-W Xe lamp (λ>420 nm) | Tetracycline 10 mg/L |
Approximately 80% optimum within 50 min | [140] |
| Z-scheme heterojunction | Z-scheme WO3-g-C3N4 0.5 g/L |
300-W Xe arc lamp (1.5 AM solar simulator) | Sulfamethoxazole 10 mg/L |
Approximately 92% optimum within 4h | [141] |
| Z-scheme heterojunction | Z-scheme AgI nanoparticle-sensitized Bi5O7I microspheres 0.5 g/L |
300-W Xe lamp (λ>400 nm) |
TC (20 mg/L), DTC (10 mg/L), OTC (10 mg/L), or CIP (10 mg/L) | Approximately TC: 95%, DTC: 90%, OTC: 80% and CIP: 90% optimums within 40 min | [142] |
| Z-scheme heterojunction | Z-scheme CdTe/TiO2 0.8 g/L |
400-W halogen lamp (λ>400 nm) | Tetracycline hydrochloride 20 mg/L |
Approximately 78% optimum within 30 min | [143] |
| Z-scheme heterojunction | Type II AgI/CuBi2O4 Z-scheme AgBr/CuBi2O4 0.5 g/L |
300-W Xe lamp (λ>420 nm) |
Tetracycline 10 mg/L |
Approximately Type II catalyst: 80% and Z-scheme catalyst: 90% optimums within 30 min | [144] |
| Z-scheme heterojunction | Z-scheme mesoporous Sn3O4 nanoclusters/g-C3N4 nanosheets 0.5 g/L |
500-W Xe lamp (λ>420 nm) |
Tetracycline hydrochloride 10 mg/L |
Approximately 72% optimum within 120 min | [145] |
| Z-scheme heterojunction | Z-scheme Bi3TaO7 QDs/g-C3N4 nanosheets (NSs) 0.5 g/L |
LED lamp (λ=420 nm, 86 W) |
CIP and CPX 10 mg/L |
Approximately CIP: 91%, CPX: 77% CPX within 120 min | [146] |
| Z-scheme heterojunction | Z-scheme WO3 nanosheet/K+Ca2Nb3O10− ultrathin nanosheet 1 g/L |
250-W xenon lamp as simulated sunlight (no filters). | Tetracycline hydrochloride 35 mg/L |
Approximately 86% optimum within 120 min | [147] |
| Z-scheme heterojunction | Z-scheme AgI/BiOBr 0.5 g/L |
300-W Xe lamp (λ>420 nm) | Ciprofloxacin 10 mg/L |
Approximately 91% optimum within 1h | [148] |
| Z-scheme heterojunction | Z-scheme Ag3PO4/g-C3N4 0.05 g/L |
300-W Xe lamp (λ>400 nm) | Sulfamethoxazole 1 mg/L |
Approximately 99% optimum within 90 min | [149] |
| Z-scheme heterojunction | Z-scheme CdS-Au-BiVO4 (0 1 0) 0.5 g/L |
300-W Xe lamp (λ>420 nm) | Tetracycline 10 mg/L |
91% optimum within 90 min | [150] |
| Z-scheme heterojunction | Z-scheme BiVO4/Ag/Cu2O 0.4 g/L |
300-W Xe lamp (λ>420 nm) | Tetracycline 20 mg/L |
Approximately 91% optimum within 90 min | [151] |
| Z-scheme heterojunction | Z-scheme ZnFe2O4/Ag/PEDOT 0.2 g/L |
250-W xenon lamp (1.8×105 lx) | Tetracycline 20 mg/L |
Approximately 72% optimum within 120 min | [152] |
| Z-scheme heterojunction | Organic-inorganic Z-scheme PANI/Ag/Ag2MoO4 ~ 0.2 g/L |
40-W UV tube (Phillips) | Ciprofloxacin 3 mg/L |
Approximately 100% optimum within 40 min | [153] |
| Z-scheme heterojunction | Z-scheme (0 0 1) BiOCl-Au-CdS 1 g/L |
300-W Xe lamp (AM 1.5) | Sulfadiazine 20 mg/L |
Approximately 91% optimum within 4h | [154] |
| Z-scheme heterojunction | Z-scheme iodine vacancy-rich BiOI/Ag@AgI 0.3 g/L |
300-W Xe lamp (λ>420 nm) | Tetracycline 20 mg/L |
Approximately 86% optimum within 60 min | [155] |
| Z-scheme heterojunction | Z-scheme Ag2CO3/Ag/WO3 0.5 g/L |
300-W Xe lamp (λ>420 nm) | CIP and TC 10 mg/L |
Approximately CIP: 84% and TC: 81% optimums within 90 min | [156] |
| Z-scheme heterojunction | Z-scheme AgI/Ag/Bi3TaO7 0.5 g/L |
300-W Xe lamp (visible light) | Sulfamethoxazole 5 mg/L |
Approximately 98% optimum within 100 min | [157] |
| Z-scheme heterojunction | Z-scheme MIL-53(Fe)/Ag/g-C3N4 80 mg/L |
Visible light | Clioquinol 10 mg/L |
95% optimum within 100 min | [158] |
| Z-scheme heterojunction | Z-scheme CeVO4/3D RGO aerogel/BiVO4 0.5 g/L |
500-W Xe lamp | Tetracycline 20 mg/L |
Approximately 100% optimum within 60 min | [159] |
| Z-scheme heterojunction | TCPP/rGO/Bi2WO6 0.3 g/L |
300-W Xe lamp (λ>420 nm) | Tetracycline 15 mg/L |
Approximately 84% optimum within 60 min | [160] |
| Z-scheme heterojunction | Z-scheme Ag3PO4/Bi2S3/Bi2O3 1 g/L |
300-W Xe lamp (λ>420 nm) |
Sulfamethazine (SAZ) and cloxacillin (CLX) 10 mg/L |
Approximately SAZ: 99% and CLX: 90% optimums within 90 min | [161] |
| Z-scheme heterojunction | RGO-Ag2O/TiO2 0.4 g/L |
350-W Hg lamp (λless than356 nm), 300-W Xe arc lamp (visible light), 300-W infrared lamp, and 156-W APOLLO solar simulator | Tetracycline 10 mg/L |
100% and approximately 100% optimums within 60 min, approximately 90% optimums within 120 min | [162] |
| Z-scheme heterojunction | Z-scheme g-C3N4/Ag2CO3/graphene oxide 0.8 g/L |
300-W Xe lamp (λ>420 nm) |
Tetracycline 20 mg/L |
Approximately 82% optimum within 60 min | [163] |
| Z-scheme heterojunction | Z-scheme nitrogen-doped graphene QDs-BiVO4/g-C3N4 0.5 g/L |
250-W Xe lamp (λ>420 nm) |
Tetracycline 10 mg/L |
Approximately 91% optimum within 30 min | [164] |
| Z-scheme heterojunction | Z-scheme graphitic carbon nitride (CN) and reduced graphene oxide (rGO) with AP 1 g/L |
Both intense sunlight and weak indoor light irradiation | Norfloxacin 10 mg/L |
Approximately 100% optimum within 30 min and 85% optimum within 2h | [165] |
| Z-scheme heterojunction | Z-scheme WO3/Fe3O4/g-C3N4 1 g/L |
300-W Xe lamp (λ>400 nm) |
Tetracycline 20 mg/L |
89% optimum within 120 min | [166] |
| Z-scheme heterojunction | Z-scheme nitrogen-doped hollow mesoporous carbon spheres (N-HMCs) modified g-C3N4/Bi2O3 1 g/L |
300-W Xe lamp (λ>420 nm) |
Tetracycline hydrochloride (TCH) and ciprofloxacin hydrochloride (CFH) 10 mg/L |
Approximately 90% and 80% optimum within 60 min | [167] |
| Exposing active facets | Bi2O2(OH)(NO3) nanosheets with (0 0 1) active exposing facets 1 g/L |
UV light irradiation (300-W Hg lamp) |
Tetracycline hydrochloride 10 mg/L |
Approximately 98% optimum within 25 min | [168] |
| Exposing active facets | Ultrathin Bi2O2(OH)xCl2-x solid solution with exposed (0 0 1) facets 0.5 g/L |
Visible light (420-nm LED) |
Ciprofloxacin 20 mg/L |
Approximately 90% optimum within 150 min | [169] |
| Exposing active facets | Nanosheet BiVO4 with oxygen vacancies and exposed (0 0 1) facets 1 g/L |
500-W Xe lamp without optical filters to simulate the sunlight | Oxytetracycline 20 mg/L |
Approximately 96% optimum within 2h | [170] |
| Exposing active facets | Various well-defined Bi2WO6 crystals ~ 0.67 g/L |
300-W Xe lamp (λ>420 nm) |
Ciprofloxacin 10 mg/L |
Approximately 70% optimum within 5h | [171] |
| Exposing active facets | Doped BiOCl nanoplates ~ 0.67 g/L |
300-W Xe lamp (λ>420 nm) | Tetracycline hydrochloride 30 mg/L |
Approximately 90% optimum within 100 min | [172] |
| Exposing active facets | (0 0 1) Ag@NC-TiO2 square nanosheets 1 g/L |
350-W Xe arc lamp (λ>420 nm) | Ciprofloxacin 10 mg/L |
Approximately 97% optimum within 150 min | [173] |
| Exposing active facets | TiO2@g-C3N4 core–shell quantum 1 g/L |
Xe lamp irradiation. | Tetracycline 20 mg/L |
Approximately 100% optimum within less than 10 min | [174] |
| Porous materials | Intercalate structure g-C3N4@ATP 1 g/L |
300-W Xe lamp (λ>420 nm) | Tetracycline 20 mg/L |
Approximately 90% optimum within 2h | [175] |
| Porous materials | 3D hierarchical mesoporous BiOI Optimum 1.5 or 0.68 g/L |
1000-W tungsten halogen lamp (λ>420 nm) |
Tetracycline hydrochloride Optimum 2.1 or 2 mg/L |
Approximately 100% optimum within 37.5 or 101.5 min | [176] |
| Porous materials | BiOI hollow microspheres 1 g/L |
300-W Xe lamp (λ>400 nm) | Tetracycline 20 mg/L |
80% optimum within 120 min | [177] |
| Porous materials | Ultra-thin Bi2MoO6 nanosheets 0.5 g/L-1.5 g/L |
Sunlight | Ofloxacin 10 mg/L |
Approximately 71% optimum within 90 min | [178] |
| Tailoring morphology | Rod-like SrV2O6 2–9 g/L |
500-W tungsten lamp (λ>400 nm) | Metronidazole 20 mg/L |
98% optimum within 60 min | [179] |
| Tailoring morphology | TiO2 nanobelts | Simulative solar light | Amikacin 5 mg/L |
Approximately 70% optimum within 150 min | [180] |
| Tailoring morphology | ZnO Nanotubes 0.035 g/L |
300-W Xe lamp (AM1.5 filter (1000 Wm−2)) | Ciprofloxacin 6.6 mg/L |
Approximately 12% optimum within 2h | [181] |
| Tailoring morphology | Bi5FeTi3O15 0.4 g/L |
300-W Xe lamp (λ>420 nm) |
Tetracycline hydrochloride 4.8 mg/L |
Approximately 99% optimum within 1h | [182] |
| Tailoring morphology | Navel-like Bi2WO6 hierarchical microspheres | UV light irradiation (λ=365 nm) | Norfloxacin (Concentration not provided) |
Approximately 67% optimum within 8h | [183] |
| Tailoring morphology | spearhead-like g-C3N4 1 g/L |
Xe lamp (35 W, 6000 K) |
Tetracycline 20 mg/L |
Approximately 70% optimum within 180 min | [184] |
| 3D aerogel | g-C3N4@CA/B-PET 5 g/L |
Artificial solar light (125 mW/cm2) |
Sulfaquinoxaline sodium 10 mg/L |
Approximately 100% optimum within 60 min | [185] |
| 3D aerogel | BiVO4/3D RGO aerogel/CeVO4 0.5 g/L |
500-W Xe lamp (visible light) |
Tetracycline 20 mg/L |
Approximately 90% optimum within 120 min | [159] |
| 3D aerogel | 3D MoS2 nanosheets/graphene aerogel 0.8 mg/L |
300-W Xe lamp (λ>420 nm) |
Tetracycline hydrochloride 60 mg/L |
Approximately 10% optimum within 75 min | [186] |
3.1. Strategies to increase light harvesting
3.1.1. Defect engineering to increase light harvesting
Defects play two distinct roles in the photocatalytic process: (1) they act as trapping sites for electrons or holes. Depending on the nature of the defects (deep trap or shallow trap), it may improve the diffusion length of charge carriers (shallow trap) or promote the charge recombination (deep trap) thus reducing the photocatalytic efficiency; (2) they may extend the light absorption by modulating the electronic structure of the photocatalyst (e.g. creation of midgap impurities level), thereby influencing the photocatalytic activity. However, elaborative designs such as vacancy construction and elemental doping are necessary to take full advantage of the improvements and avoid or minimize the disadvantages [187], [188], [189], [190].
3.1.1.1. Vacancies for the improvement of light absorption
Vacancies are types of point defects that not only manipulate the composition of catalysts without introducing impurities but also allow for the customization of suitable band structures by modulating their types and concentrations. The concentration of the vacancies should be controlled within a delicately balanced range. Excessive vacancies would provide too many recombination centers for photoinduced electrons and holes, whereas insufficient vacancies would fail to achieve the desired photocatalytic performance. Vacancies may be categorized into three types: 1) anion vacancies (such as sulfur [191], [192], [193], [194], [195], halogen [196], [197], [198], [199], nitrogen [200], [201], [202] and oxygen vacancies (OV) [203], [204], [205], [206], [207]), 2) cationic vacancies (such as bismuth [208], [209], [210], carbon [211] and titanium vacancies [212], [213], [214], [215]), and 3) mixed vacancies, which combine anion and cation vacancies [216], [217]. For example, mesoporous ZrO2 containing abundant oxygen vacancies was successfully synthesized by Zhang et al. [52] via a template-less solvothermal approach, following calcination. The absorption edge of the obtained sample was extended into the visible range, and the modified surface characteristics promoted adsorption ability towards organic compounds, leading to an enhancement of the visible-light-driven photodegradation of organic contaminants. Similarly, brown ZnWO4-x nanorods containing oxygen vacancies exhibited a broadened photo-absorption capacity from the ultraviolet (UV) to the near-infrared (NIR) region, whereas conventional white ZnWO4 nanorods without OV exhibited a UV absorption onset at 365 nm [54].
Fig. 3 shows the existence of an OV-mid-gap state and three possibilities for electron excitation: (i) UV-driven direct excitation from the VB towards the CB, (ii) visible-light-induced jump from VB to OV defect states, and (iii) near-infrared-light-driven transition from the OV-derived defect state to the CB [54]. The induced defect, or the inter-band energy level, enables electron jumps with less energetic illumination, thereby improving the light harvest capacity of ZnWO4-x nanorods with abundant OV [54]. Moreover, Liu et al. [218] reported that anion vacancy could be produced in a TiO2 lattice when Ti3+ was present. Excess energy levels below the CB would be induced in the presence of these anion vacancies [219], [220], [221], [222]. Overlapping of these levels with CB occurs by increasing the concentration of these vacancies, resulting in a decreased bandgap of TiO2. Therefore, the visible-light-driven transition can take place from intrinsic VB to Ti3+ states. This behavior can extend the optical response to an onset wavelength of longer than 400 nm [223].
Fig. 3.
Schematic of the OV-induced photocatalytic process on ZnWO4-x[54]
3.1.1.2. Heteroatoms or ion doping for light response improvement
The introduction of heteroatoms or ions into the photocatalyst, in the form of dopant, is an effective light response management method. Non-metal dopants (e.g., C [224], [225], B [226], [227], [228], [229], P [57], [230], S [231], [232], N [233], [234], [235], [236], and halogen [237], [238]) and metallic dopants (e.g. Fe3+ [239], [240], Ce3+ [62], Cr3+ [241], W6+ [242], Ti4+ [243], Cu2+ [241], Co2+ [244], [245] and Er3+ [246]) were most widely studied in semiconductor photocatalysts, especially metal oxides. Generally, a method can either introduce an impurity energy level lower than the CB minimum or higher than the VB maximum to narrow down the bandgap, enhancing light absorption.
Recently, N-doped TiO2 photocatalysts with tunable doping contents were prepared by calcining sol–gel TiO2 powder in the presence of NH3 flow at 450–800 °C [247]. The light absorption capacity of samples prepared at a temperature region of 450–600 °C increased gradually with temperature, and their colors varied from pale yellow to emerald green as a result of the production of N 2p (localized states) beyond the VB of TiO2 and the generation of OV (Fig. 4 (a)). When NH3 treatment reached T > 600 °C, anatase TiO2 was transformed into rutile, leading to a remarkable reduction in its Brunauer-Emmett-Teller (BET) area and the production of a TiN layer on the TiO2 surface. The materials treated at 700 and 800 °C exhibited a conspicuous response to a wide range of spectra spanning from the visible to the NIR range, which originated from the production of OV and Ti3+ defects. Similarly, Wang et al. [248] synthesized lantern-shaped g-C3N4 with a Eu3+ dopant and found that a doping level was developed below the CB upon the introduction of Eu3+, resulting in a narrower bandgap and lowering the electron position compared to that of raw CN. This improved both the optical light response and photocatalytic performance.
Fig. 4.
(a) UV–vis diffuse reflectance spectra of a) 1) raw TiO2 (5 0 0), and N-TiO2 (T) samples prepared at 2) 450, 3) 500, 4) 550, 5) 600, 6) 700 and 7) 800 °C [247], (b) diffuse reflection spectra (DRS) curves of undoped and doped TDHG [63], Inset: Photographs of TDHG, N-TDHG, b -TDHG and b/N-TDHG photocatalysts.
In some cases, co-doping (e.g., the addition of two non-metals [57], [231], two metal ions [246], or a metal ion paired with a non-metal ion [63]) has been used to avoid imbalances of charges during aliovalent doping [187]. For example, b/N-TDHG (black Ti3+/N co-doped TiO2/diatomite hybrid granule) could be obtained from a sol–gel process [63]. The light absorption of b/N-TDHG (Fig. 4(b)) exhibited a more pronounced redshift than other TDHG counterparts. Particularly, it had the strongest visible-light response among all obtained photocatalysts, which resulted from the synergistic action of co-doped N and Ti3+.
3.1.2. Addition of light sensitizers to enhance light absorption
The addition of photosensitizers is another simple method of enhancing the photoactivity of wide bandgap semiconductors in visible light. In recent years, a variety of QDs (e.g., ZnSe QDs [47], carbon QDs (CQDs) [64], [65], [66], [68], [69], [249], [250], [251], [252], [253], graphene [254], CdS [255], [256], Fe3O4 [257], g-C3N4 [258], CsPbBr3 [259] and MoS2 [260]) have been combined into semiconductors to enhance light absorption. Among them, CQDs have become the most widely used QDs to upgrade wide-bandgap semiconductors owing to their low cost, non-toxicity, and high stability [250], [261]. For example, up-converting CQDs were immobilized on the surface of La2Ti2O7 (LTO) nanosheets to promote visible-light-induced photocatalysis [252]. The pristine (i.e., unmodified) LTO nanosheets had no optical response from 400 to 800 nm (Fig. 5 (a)). In contrast, all CQD/La2Ti2O7 (C-LTO) composites exhibited distinct responses in the above range, and the response could be modulated accordingly by changing the amount of CQD.
Fig. 5.
(a) Light absorption curves [252] and (b) photocatalytic mechanisms of CQD/TNT photocatalyst [253]
Zhao et al. [253] successfully synthesized TiO2 nanotube (CQD/TNTs) composites with an outstanding photocatalytic performance. These CQD co-catalysts had remarkable up-converting photoluminescence (PL) features. Longwave infrared rays (LWIR, >600 nm) absorption could be converted into optical light of shorter than 600 nm, enabling the production of IR-induced electrons and holes on TNTs (Fig. 5(b)). Additionally, the introduction of CQDs can accelerate the harvesting of photoexcited electrons and prolong the lifetime of photoinduced carriers.
3.2. Interfacial engineering strategies for carrier migration improvement
The construction of a junction interface is a typical approach to facilitate photocatalysis by modulating the separation/migration of the photoinduced electrons and holes at the interface. Therefore, in-depth studies have been conducted to design photocatalyst junction interfaces to monitor carrier migration capabilities. According to their phase composition, junction interfaces can be divided into homogenous junction interfaces and heterojunction interfaces.
3.2.1. Homogenous junction interfaces
Homogenous junction interfaces are built by identical compounds in the absence of additional components and have been the focus of significant interest. The construction of homogenous junction interfaces is primarily based on the established phase junction or facet junction, creating an effective migration pathway for the photoinduced charges.
3.2.2. Phase junction
Most crystals (e.g., TiO2 [262], [263], [264], [265], [266], [267], [268], [269], [270], CdS [271], Bi2O3 [272], MoS2 [273] and ZnIn2S4 [274]) have many different natural or artificial phases. The construction of the phase junction is a significant approach to improve the photocatalytic performance through accelerating the migration of electrons and holes. Many studies have been conducted on the design of photocatalyst phase junctions to boost carrier migration characteristics and capabilities [275], [276]. Jia et al. [277] studied the theoretical basis of the phase changes and interfacial characteristics of Cu2ZnSnS4 with a hetero-phase junction. A type-II band structure could be formed at the hetero-phase junction of (1 0 1), (1 1 0), and (1 0 0) facets, thereby facilitating the photoelectric capacity. However, type-I heterojunctions may be aligned at the phase junction of (1 1 2) and (0 0 1) facets, hindering the generation of charges and improving optoelectronic properties owing to a high potential barrier. Similarly, according to the band engineering design theory, a bonding-region-width-controlled phase junction surrounded by cubic CdS and a hexagonal counterpart was designed by Ai et al. [271]. The electron–hole separation was optimized at an appropriate bonding region width (0.76 nm), which exhibited the highest photocatalytic efficiency (45% quantum efficiency) among all the examined samples, in addition to good photocorrosion resistance.
Artificial phases caused by vacancies or self-doping have been used to construct a phase junction to control the separation of electrons and holes. Recently, Cao et al. [201] constructed a g-C3N4 p-n homojunction through introducing nitrogen vacancies (NVs). The differences in Mott-Schottky plots between bulk g-C3N4 (CN) and PN-2 indicated that the conductivity characteristics of the homojunction were consistent with a p-n model (Fig. 6 (a) and (b)). Additionally, the PN-2 homojunction exhibited a distinct improvement in its photocatalytic performance to treat Rhodamine B (RhB), compared to CN, which was attributed to the efficient separation of the photoexcited charges at the PN-2 homojunction (Fig. 6 (c) and (d)). Chade Lv et al. [278] synthesized Bi5+-BVO (Bi5+-self-doped Bi4V2O11) nanotubes and formed p-n homojunctions by implementing the oxygen vacancy strategy. Density functional theory (DFT) simulations and laboratory findings reveal that Bi5+ auto-doping reduces the bandgap of Bi4V2O11 (BVO), thereby enhancing light capture. Furthermore, Bi5+ auto-doping confers both n- and p-type semiconductor characteristics to BVO, which enables the construction of p-n homojunctions to delay the quick annihilation of electrons and holes.
Fig. 6.
Mott-Schottky curves on (a) CN and (b) PN-2; (c and d) schematic of carrier migration at the p-n homojunction [201]
3.2.3. Facet engineering
Previous studies have reported that, unlike isolated surfaces, the coexistence of multiple crystal planes in single particles could be recognized as interactive surfaces with synergistic effects [279], [280]. This means that redox reactions occur locally at the separate planes, resulting in spatial charge separation in semiconductors. For example, multi-morphologic silver bromide (AgBr) crystals containing distinct exposed planes were prepared by synchronously injecting silver nitrate and potassium bromide precursors [74]. The shape and exposed planes of the target sample could be easily modulated by varying the density of Br− ions, which can significantly reduce the surface barrier of (1 0 0) and (1 1 1) planes and affect their growth rates. C-AgBr ((1 0 0) facet exposed cubes), T-AgBr ((1 0 0) and (1 1 1) facet exposed tetradecahedrons), and O-AgBr ((1 1 1) facet exposed octahedrons) could be obtained at specific Br− concentrations of 100, 100.5, and 101 mM. The obtained T-AgBr exhibited improved photocatalytic performance for the degradation of methyl orange (MO) and sulfadiazine (SD) owing to the formed facet heterojunction structures between the (1 1 1) and (1 0 0) facets. When irradiated with optical light, the electrons and holes are separately transported to the (1 0 0) and (1 1 1) facets. Therefore, electrons and holes could not merely be efficiently separated, but back-reaction was effectively prevented owing to the isolation of active redox sites.
3.2.4. Heterojunction interfaces
The formation of homojunctions is considerably challenging owing to its complexity and high cost. Therefore, the creation of heterojunction interfaces has been proposed as an alternative to improve charge separation efficiency and has been widely investigated in the past decades [281]. Typically, heterojunctions are divided into five categories: (1) Schottky heterojunctions, (2) type I heterojunctions, (3) type II heterojunctions, (4) p-n heterojunctions, and (5) Z-scheme heterojunctions.
3.2.5. Schottky heterojunctions
At the semiconductor–metal interface, photoinduced electrons may typically flow from the former to the latter to match their Fermi energies and form a Schottky barrier. The formed Schottky junction can efficiently capture the electron, which promotes electron-hole separation. To further comprehend the mechanisms by which the Schottky junction improves photocatalysis, He et al. [282] prepared a series of Schottky-junction Ag-loaded carbon nitride fibers (Ag/CNFs) through loading NaBH4-reduced Ag on preformed CNFs. The Schottky-type Ag/CNFs exhibited outstanding photodegradation performance for tetracycline (TC) under visible irradiation. The enhanced photodegradation activity resulted from the synergistic effect between surface plasmon resonance (SPR) on silver nanoparticles (Ag NPs) and the efficient isolation of the photoinduced carriers at the constructed Schottky heterojunction. Moreover, on account of the SPR absorption of specific metals, the light response could even be extended to the entire sunlight range. Therefore, in addition to promoting charge separation, semiconductors and plasmonic metals could also jointly improve light harvesting.
Jiang and co-workers synthesized a novel Ag/Bi3O4Cl photocatalyst with different amounts of Ag via a facile photo-deposition process [79]. The TC degradation on 1.0 wt% Ag/Bi3O4Cl was drastically more pronounced, compared to pristine Bi3O4Cl, and 94.2% TC could be degraded in 120 min (Fig. 7 (a)). The improved photocatalytic properties resulted from the synergism of the photoinduced electrons on Bi3O4Cl and SPR from Ag NPs, which improved visible-light harvesting and facilitated the isolation of photoinduced electrons and holes (Fig. 7(b)).
Fig. 7.
(a) Photodegradation of TC by the as-synthesized plasmonic Ag/Bi3O4Cl under visible irradiation, (b) possible photocatalytic mechanisms on the Ag/Bi3O4Cl samples [79], (c) photocatalytic kinetics of prepared g-C3N4, Pt/g-C3N4, Au/g-C3N4, and Au/Pt/g-C3N4 nanocomposites [86] (d) schematic of the g-C3N4 photoinduced charge transport [284]
In other studies, bimetallic-supported semiconductor catalysts (Pt/Au/TiO2 [283], [284], Au/Ag/TiO2 [285], Pd-Cu/TiO2 [286] and Ag-Cu/TiO2 [287]) exhibited super activity and high selectivity, which cannot be found in single metals owing to the synergistic effect of bimetals. For example, Xue et al. [86] prepared plasmonic Au/Pt/g-C3N4 via a simple calcination-photo-deposition process. The prepared heterostructure photocatalyst exhibited an optimized photodegradation performance for antibiotic tetracycline hydrochloride (TC-HCl) treatment, and the visible-light-driven degradation was 3.4 times higher than that of pristine g-C3N4 (Fig. 7(c)). This enhanced photodegradation performance was attributed to the synergism between the SPR absorption on Au and the electron-trap effect of Pt NPs, which facilitated the light-harvesting capability and isolation of photoinduced charges on g-C3N4, thereby jointly boosting the photocatalytic properties. Similarly, TiO2 nanotube arrays (Au-Pt/TNTAs) with small quantities of Au-Pt were successfully prepared. Notably, these structures exhibited remarkably enhanced visible-light harvesting and carrier separation capacity. These enhancements in photodegradation activity derived from the synergism from interfacial Schottky junctions and the synergistic effect between ternary Au, Pt, and TNTAs (Fig. 7(d)) [284].
3.2.6. Type I or II heterojunctions
Compared to single semiconductors, the formation of type I semiconductor heterojunctions can promote photocatalytic efficiency. However, electrons and holes are enriched on the same semiconductor, which cannot effectively restrain the recombination of photoinduced electrons and holes. This makes type I semiconductor heterojunctions questionable prospects for photocatalytic property improvement [288], [289]. To date, large numbers of traditional type II heterojunctions have been intensively investigated, some of which have rendered drastically optimized photocatalysis performances. For example, compared to pristine MgFe2O4, newly synthesized MgFe2O4/MoS2 heterojunctions can accelerate electron–hole dissociation, albeit without extending the light-harvesting scope [90]. The as-synthesized MgFe2O4/MoS2 sample delivered a prominent photoelectrochemical performance in TC treatment and H2 evolution owing to its unique bandgap structure. In another study, a novel 2D-2D thin-layered g-C3N4/Bi4O5Br2 type II heterojunction was synthesized by Ji’s group [114] through a simple ionic-liquid-assisted solvothermal process (Fig. 8 ). This 2D g-C3N4 component had a layered microstructure, providing enough potential to successfully build intense interface interactions with exfoliated Bi4O5Br2 nanosheets. Implementing the 2D layered g-C3N4 component provided several clear advantages, including an enlarged specific surface area (SBET), broadened optical light harvesting range, optimized electron–hole separation, and accelerated transport of the interfacial electrons and holes, all of which enhanced the photocatalytic performance of the Bi4O5Br2 system within a given light range.
Fig. 8.

Preparation process for g-C3N4/Bi4O5Br2 nanocomposites [114]
3.2.7. p-n heterojunction
Schottky and type II heterojunctions may successfully split electron–hole pairs. However, it is still necessary to restrain the superfast electron–hole annihilation at the interface. Therefore, p-n heterojunctions have been implemented and widely used to manage photocatalytic properties. For instance, Kang et al. [118] constructed a high-performance CoO/g-C3N4 p-n junction through a simple solvothermal process. The prepared p-n junction exhibited excellent visible-light-induced photocatalytic properties and consistency for TC treatment. These excellent photocatalytic properties were attributed to the production of an interfacial electric field around the junction, which effectively prevented the annihilation of photo-induced charges. Furthermore, a SnO2/BiOI n-p heterojunction was successfully achieved by Wen et al. [124] by depositing SnO2 NPs onto the outer surface of BiOI nanosheets. The obtained n-p heterojunction exhibited a strong visible-light-driven photocatalysis capacity and durability for the degradation of MO and oxytetracycline HCl. This excellent photocatalytic activity resulted from the constructed p-n heterojunction, which significantly limited the annihilation of electrons and holes.
Similarly, a novel three-dimensional (3D) layered BiOBr/Bi2SiO5 p-n heterostructure was successfully synthesized to efficiently degrade TC [123]. The prepared BiOBr/Bi2SiO5 p-n heterostructure exhibited an optimized visible-light-driven photocatalytic degradation of TC, which was 3.6-fold higher than that of BiOBr. Moreover, this high TC photodegradation performance remained constant for 5 cycles. This optimized photodegradation property of the BiOBr/Bi2SiO5 heterostructure was ascribed to the following factors: (1) the introduced BiOBr facilitated light harvesting within a broad optical range, and (2) the formed p-n heterojunction restrained the annihilation of photoinduced electrons and holes. These features prominently promoted the generation of ·OH, ·O2 –, and H+, leading to a promoted photocatalytic activity.
3.2.8. Z-scheme heterojunction
The development of Z-scheme photocatalysis was inspired by photosynthetic systems in nature and has attracted tremendous interests since the first report of a conventional Z-scheme photosystem in 1979 (Fig. 9 (a)) [290], [291]. Depending on the charge transport route involved, Z-scheme photosystems are classified as conventional, all-solid-state, and direct, which are distinguished by the specific transport medium used to facilitate the charge transport of reversible redox ion pairs, electronic conductors, and direct interfacial contacts, respectively [291], [292], [293], [294], [295]. For example, Ma et al. prepared a Z-scheme WO3/K+Ca2Nb3O10 − binary 2D-2D heterojunction photocatalyst from a facile hydrothermal co-assembly method at ambient conditions [147]. The as-prepared WO3/K+Ca2Nb3O10 − photocatalyst exhibited a remarkable improvement in the photodegradation of TC-HCl under simulated sunlight irradiation, compared to pristine WO3 and K+Ca2Nb3O10 −. 20%-WO3/K+Ca2Nb3O10 − exhibited the maximum activity, which was 5.1 and 2 times higher than those of WO3 and K+Ca2Nb3O10 −, respectively. These enhanced properties and sustainability were attributed to the tightly-coupled heterointerfaces, increased SBET area, optimized optical-capturing capability, and improved carrier dynamics of the semiconductor in the Z-scheme procedure.
Fig. 9.
(a) Roadmap of Z-scheme photocatalytic system evolution [293]; (b) suggested photoinduced charge transport on g-C3N4(60)/TNTAs [296]
Yang et al. [155] designed a new Z-scheme heterostructure Ag@AgI/VI-BOI (iodine-vacancy-rich BiOI), whereby Ag@AgI NPs were loaded locally on the outer surface of defect-rich BiOI nanosheets. This Z-scheme heterostructure possessed a remarkable visible-light-induced photocatalysis capacity for TC treatment, which was significantly higher than those of pristine BiOI, VI-BOI, and Ag@AgI. The enhanced photocatalytic properties of this Z-scheme heterostructure originated from the synergistic action between VI (iodine vacancies), interface junctions between AgI and VI-BOI, and Ag0. A direct visible-light-induced solid-state C3N4 NS/TNTAs (g-C3N4 nanosheet/TiO2 nanotube array) Z-scheme heterostructure (Fig. 9(b)) was successfully fabricated in-situ by adding preformed g-C3N4 nanosheets into the anodizing bath solution [296]. Enhanced photocatalytic performances and good stability were observed on nanosheet-supplemented photocatalysts, and g-C3N4(60)/TNTAs exhibited an optimum activity for the degradation of rhodamine B (RhB) and colorless TC-HCl. The optimized photodegradation performance was derived from an enhanced light harvesting capacity, a suppressed carrier recombination, and an extended carrier lifetime.
3.2.9. Double heterojunction
In addition to heterojunctions, double heterojunctions such as QDs Schottky heterojunctions combined with type II, Z-scheme heterojunction [127], [130], [131], [132], type I combined with Z-scheme heterojunctions, and double Z-scheme heterojunctions have been applied to treat antibiotics (e.g., tetracycline hydrochloride, ciprofloxacin, oxytetracycline, ciprofloxacin, etc.) in water [135]. Tang et al. [297] prepared ternary Ag/CuNb2O6/CuFe2O4 heterostructures. The prepared multicomponent heterostructures exhibited stronger visible-light-induced photodegradation properties towards methylene blue (MB) and the pesticide imidacloprid than their single (CuNb2O6) and binary (Ag/CuNb2O6 or CuNb2O6/CuFe2O4) counterparts. The excellent photodegradation performance of the obtained ternary heterostructure was attributed to an enhanced visible-light harvesting capacity and rapid charge recombination restraint.
Jo et al. [130] developed a novel ternary 2D/2D/2D-configured LDH/CN/RGO heterostructure (CoAl-layered double hydroxide/g-C3N4/reduced graphene oxide) from a simple one-step hydrothermal synthesis. The prepared LDH/CN/RGO heterostructure exhibited a significantly superior visible-light-induced photodegradation activity for the treatment of Congo red (CR) and TC in water. Particularly, the LDH/CN/RGO heterostructure containing RGO (1 wt%) and LDH (15 wt%) featured optimum photodegradation properties in all the synthesized photocatalysts. The significantly enhanced photodegradation efficiency and outstanding consistency of the heterostructure LDH/CN/RGO were primarily attributed to the intense interface interaction between the CN, LDH, and RGO components (Fig. 10 ). This strong interfacial effect was further facilitated by the specifically arranged 2D/2D/2D microstructure, which ultimately accelerated the charge transport at the interface and efficiently separated photoinduced electrons and holes.
Fig. 10.
Photocatalytic degradation mechanisms of CR and TC by a ternary LDH/CN/RGO heterostructure [130]
3.3. Strategies to manage surface reactions
Although charges can be effectively separated, recombination in the process of electron transport remains problematic when the charges cannot be consumed promptly due to low activity or limited active sites on the semiconductor surface. Therefore, strategies have been proposed to promote intrinsic active sites on photocatalysts and the adsorption of pollutants.
3.3.1. Selective exposure of high energy facets
Typically, the redox reactions that occur on the outer surface or interface of semiconductors are highly susceptible to the exposed facets. Based on crystal anisotropy data, it is widely known that different crystal facets have different atomic arrangements, which result in different physical and chemical properties, including anisotropic surface electronic structures, tunable surface energy, and diverse molecule absorption abilities and reactivities [279].
Hao et al. [168] synthesized Bi2O2(OH)(NO3) nanosheets (BON-NS) with a predominant exposure of the reactive (0 0 1) plane through a wet-chemical process using sodium dodecylbenzene sulfonate (SDBS) as a template. They found that the BON-NS samples consisted of loosely stacked nanosheets, which provided an exceptionally high specific surface area, as well as more efficient charge separation. This feature was likely derived from the dominant exposure of reactive (0 0 1) facet and the [Bi2O2(OH)]+ stacking layers down the c axis, which induces the orientation of an auto-built electric field. This allows the developed BON-NS to have an elevated capability for carrier separation and migration, leading to significantly superior UV-light photocatalysis performance for the treatment of antibiotics (tetracycline hydrochloride) than their bulk counterparts [168]. Additionally, hexagonal WO3 (h-WO3) was synthesized by employing sodium sulfate and ammonium sulfate as end-capping reagents through diverse hydrothermal methods [298]. Particularly, h-WO3 NSs with exposed (0 0 2) planes exhibited the highest visible-light photodegradation performance owing to their large BET surface and interior electron–hole separation on high-energy (0 0 2) reactive planes.
3.3.2. Preparation of 2D porous materials
Porous materials have numerous notable advantages, including a high-density active core that facilitates photocatalytic reactions, high light absorption rates derived from the reflection and scattering of incident light within the pores, and high specific surface areas that improve the adsorption of contaminants and accelerate surface reactions.
Two-dimensional (2D) materials, including black phosphorus (BP), transition metal dichalcogenides (TMDs), metal oxides, metal carbides, metal nitrides, hexagonal boron nitride (h-BN), g-C3N4, and layered double hydroxides (LDH), have attracted substantial interest in the field of photocatalysis [299], [300], [301]. When performing as photocatalysts, the high BET surface and abundant surface reactive sites of 2D candidates may result in enhanced catalytic activity. To further improve photocatalytic properties, 2D porous materials with a more accessible BET surface and additional reactive sites are developed to completely expose the active sites to the contaminants and participate in the oxidation–reduction process, which degrades the contaminants and produces H2/O2.[302] Unfortunately, 2D porous materials, such as MoSe2 [303], [304], MoS2 [305], [306], [307], and g-C3N4 [308], [309], [310], [311], [312], [313], [314], [315], [316], [317], [318], [319], [320], [321], [322] are mostly used in photocatalytic hydrogen evolution or photodegradation of organic dyes and are rarely applied for the degradation of antibiotics. Nonetheless, 2D porous materials could be promising candidates for the photocatalytic treatment of antibiotics.
Kang et al. [311] prepared few-layer g-C3N4 NSs with foamy pores by ultrafast liquid-N2-frozen exfoliation of its bulk counterpart within 10 s. The foamed porous super-thin g-C3N4 NSs exhibited interesting advances such as well-crystallized characteristics, a narrowed energy band, an additional unveiled edge, a slightly-augmented BET surface (135.6 m2/g) with an increased mesopore, and an enhanced charge transport capability. Unlike massive g-C3N4, the super-thin porous g-C3N4 exhibited a remarkably promoted capability to generate reactive oxygen species (ROS) and a 4-fold improvement in performance for visible-light-excited RhB decomposition. This primarily resulted from an increase in reactive sites and a shortened route for charge transport. These results suggest that this technology could be further developed to generate high-performance super-thin g-C3N4 NSs for contaminant treatment.
Template-free 2D porous super-thin g-C3N4 NSs with doped oxygen atoms were synthesized by She et al. [316]. The photodegradation performance (60.8%) of MO and the mean H2 production speed (~189.3 μmol·h−1) were nearly 71 and 5.2 times higher than those in the bulk phase, respectively. This enhanced photocatalysis derived from unique features such as an enriched adsorbable/reactive site, a reinforced oxidation–reduction capability and enhanced charge migration.
3.3.3. Preparing 3D aerogel materials
Aerogel photocatalysts with 3D continuous network structures can provide ultra-large accessible surface areas, rich porosities, and a large number of photocatalytic active sites, which can result in high absorption capacity and enhanced photocatalytic performance. Moreover, 3D aerogel materials can solve the problem of dispersion and recovery of photocatalytic materials in practical applications [186]. Liu et al. [159] recently fabricated ternary CeVO4/3D RGO (reduced graphene oxide) aerogel/BiVO4 photocatalysts (Fig. 11 ) and used them for visible-light-induced O2 generation and TC removal. The synthesized aerogel composites displayed a well-defined 3D hierarchical microstructure built by the two vanadates and RGO. The optimum heterostructure exhibited a significantly facilitated visible-light-excited H2O oxidation capacity as well as TC degradation. This optimized photocatalysis capacity resulted from the large BET surface and ternary Z-scheme heterojunction of the aerogel. These features enabled the 3D RGO aerogel to connect to the other semiconductors and act as distinct dual pathways to further enhance the charge transport.
Fig. 11.
Synthesis of BiVO4/RGO/CeVO4 heterostructures [159]
A freestanding MoS2 nanosheets/graphene aerogel (3D MoS2 NS/GA) heterostructure was recently created through a simple hydrothermal process [186]. The obtained 3D MoS2 NS/GA exhibited an excellent visible-light photodegradation efficiency for TC-HCl disposal with superior stability and reusability. Chen et al. [185] prepared powerful cellulose aerogel/blended polyester fibers (3D g-C3N4@CA/B-PET) via a simple process by supporting g-C3N4 NSs on B-PET reinforced cellulose aerogel (CA). The prepared g-C3N4@CA/B-PET photocatalyst exhibited a superior photocatalytic performance to treat sulfaquinoxaline sodium and Cr (VI), compared to pristine g-C3N4. Moreover, heterostructure g-C3N4@CA/B-PET aerogel can be easily recycled and reused and is remarkably resistant to light and water currents.
4. Summary and outlook
The hazards of antibiotics and their treatment methods were briefly discussed herein, followed by a discussion on the current state of photocatalytic degradation of antibiotics in water. Photocatalysis plays an important role in treating antibiotic residues in water due to its superb features. However, efforts have to be made to further improve the efficiency for the wide application of this technology. The strategies for the improvement of degradation efficiency are the following:
-
(1)
increasing light-harvesting capacity via defect engineering
-
(2)
enhancing charge separation via interface engineering
-
(3)
accelerating surface reaction.
These strategies are promising based on previous studies. However, for further practical applications, many challenges remain to be addressed:
-
a)
It is difficult to identify the exact occurrence, formation, concentration, and types of defects (especially for vacancies) using current characterization techniques. This produces barriers for understanding the relationship between the structure and performance, which limits the further development of these technologies.
-
b)
The construction of traditional heterojunctions via interface engineering has been intensively studied. Nonetheless, only a few previous studies have focused on homojunctions and double heterojunctions, given that current preparation methods are complex, time-consuming, non-eco-friendly, and theoretically lacking.
-
c)
The BET surface and the number of active sites of a catalyst are key factors for accelerating surface reactions. In addition to increasing the specific surface area by controlling catalyst morphology, the selective exposure of highly active high-specific-surface-area crystal faces has recently become a popular research topic. However, most of these materials are used for hydrogen production, with few instances of antibiotic treatment applications.
Despite these challenges, this review provides valuable information on improvement and modification strategies for the design of high-performance photocatalysts to treat antibiotic residues in water. Besides, it is difficult to treat the antibiotic residues in practice by a sole technology. Innovating a complex system comprising photocatalysis and photoelectrocatalysis or electrocatalysis could be a promising alternative for the efficient removal of antibiotic residues in water. This review provides new insights into the design of high-efficiency photocatalysts for the degradation of antibiotic residues, thereby furthering the development of photocatalysis for water treatment and other fields.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the Zhejiang Provincial Natural Science Foundation of China (No. LY17E020008), the Technology Foundation for Selected Overseas Chinese Scholars of Zhejiang Province, and Fundamental Research Funds for the Provincial Universities of Zhejiang (2020YW53). This work was also carried out with the support of the Cooperative Research Program for Agriculture Science and Technology Development (Project No. PJ014758), the Rural Development Administration in the Republic of Korea.
References
- 1.Kim K.-R., Owens G., Kwon S.-I., So K.-H., Lee D.-B., Ok Y.S. Occurrence and environmental fate of veterinary antibiotics in the terrestrial environment. Water Air Soil Pollut. 2011;214:163–174. doi: 10.1007/s11270-010-0412-2. [DOI] [Google Scholar]
- 2.Rodriguez-Mozaz S., Chamorro S., Marti E., Huerta B., Gros M., Sanchez-Melsio A., Borrego C.M., Barcelo D., Balcazar J.L. Occurrence of antibiotics and antibiotic resistance genes in hospital and urban wastewaters and their impact on the receiving river. Water Res. 2015;69:234–242. doi: 10.1016/j.watres.2014.11.021. [DOI] [PubMed] [Google Scholar]
- 3.Kerrigan J.F., Sandberg K.D., Engstrom D.R., LaPara T.M., Arnold W.A. Small and large-scale distribution of four classes of antibiotics in sediment: association with metals and antibiotic resistance genes. Environ. Sci. Processes Impacts. 2018;20:1167–1179. doi: 10.1039/c8em00190a. [DOI] [PubMed] [Google Scholar]
- 4.Dinh Q.T., Moreau-Guigon E., Labadie P., Alliot F., Teil M.J., Blanchard M., Chevreuil M. Occurrence of antibiotics in rural catchments. Chemosphere. 2017;168:483–490. doi: 10.1016/j.chemosphere.2016.10.106. [DOI] [PubMed] [Google Scholar]
- 5.Karthikeyan K.G., Meyer M.T. Occurrence of antibiotics in wastewater treatment facilities in Wisconsin, USA. Sci. Total Environ. 2006;361:196–207. doi: 10.1016/j.scitotenv.2005.06.030. [DOI] [PubMed] [Google Scholar]
- 6.Siedlewicz G., Bialk-Bielinska A., Borecka M., Winogradow A., Stepnowski P., Pazdro K. Presence, concentrations and risk assessment of selected antibiotic residues in sediments and near-bottom waters collected from the Polish coastal zone in the southern Baltic Sea-Summary of 3 years of studies. Mar. Pollut. Bull. 2018;129:787–801. doi: 10.1016/j.marpolbul.2017.10.075. [DOI] [PubMed] [Google Scholar]
- 7.Dong D., Zhang L., Liu S., Guo Z., Hua X. Antibiotics in water and sediments from Liao River in Jilin Province, China: occurrence, distribution, and risk assessment. Environ. Earth Sci. 2016;75:1202. doi: 10.1007/s12665-016-6008-4. [DOI] [Google Scholar]
- 8.Awad Y.M., Kim S.-C., Abd El-Azeem S.A.M., Kim K.-H., Kim K.-R., Kim K., Jeon C., Lee S.S., Ok Y.S. Veterinary antibiotics contamination in water, sediment, and soil near a swine manure composting facility. Environ. Earth Sci. 2013;71:1433–1440. doi: 10.1007/s12665-013-2548-z. [DOI] [Google Scholar]
- 9.Rajapaksha A.U., Vithanage M., Lim J.E., Ahmed M.B., Zhang M., Lee S.S., Ok Y.S. Invasive plant-derived biochar inhibits sulfamethazine uptake by lettuce in soil. Chemosphere. 2014;111:500–504. doi: 10.1016/j.chemosphere.2014.04.040. [DOI] [PubMed] [Google Scholar]
- 10.Vithanage M., Rajapaksha A.U., Tang X., Thiele-Bruhn S., Kim K.H., Lee S.-E., Ok Y.S. Sorption and transport of sulfamethazine in agricultural soils amended with invasive-plant-derived biochar. J. Environ. Manage. 2014;141:95–103. doi: 10.1016/j.jenvman.2014.02.030. [DOI] [PubMed] [Google Scholar]
- 11.McConnell M.M., Truelstrup Hansen L., Jamieson R.C., Neudorf K.D., Yost C.K., Tong A. Removal of antibiotic resistance genes in two tertiary level municipal wastewater treatment plants. Sci. Total Environ. 2018;643:292–300. doi: 10.1016/j.scitotenv.2018.06.212. [DOI] [PubMed] [Google Scholar]
- 12.Jimenez-Tototzintle M., Ferreira I.J., da Silva Duque S., Guimaraes Barrocas P.R., Saggioro E.M. Removal of contaminants of emerging concern (CECs) and antibiotic resistant bacteria in urban wastewater using UVA/TiO2/H2O2 photocatalysis. Chemosphere. 2018;210:449–457. doi: 10.1016/j.chemosphere.2018.07.036. [DOI] [PubMed] [Google Scholar]
- 13.Boukhelkhal A., Benkortbi O., Hamadache M. Use of an anionic surfactant for the sorption of a binary mixture of antibiotics from aqueous solutions. Environ. Technol. 2018;40:3328–3336. doi: 10.1080/09593330.2018.1472301. [DOI] [PubMed] [Google Scholar]
- 14.Luo J., Li X., Ge C., Müller K., Yu H., Huang P., Li J., Tsang D.C.W., Bolan N.S., Rinklebe J., Wang H. Sorption of norfloxacin, sulfamerazine and oxytetracycline by KOH-modified biochar under single and ternary systems. Bioresour. Technol. 2018;263:385–392. doi: 10.1016/j.biortech.2018.05.022. [DOI] [PubMed] [Google Scholar]
- 15.Wang J., Yao Q., Sheng C., Jin C., Sun Q. One-Step Preparation of Graphene Oxide/Cellulose Nanofibril Hybrid Aerogel for Adsorptive Removal of Four Kinds of Antibiotics. Journal of Nanomaterials. 2017;2017:1–10. doi: 10.1155/2017/5150613. [DOI] [Google Scholar]
- 16.Zhao Q., Zhang S., Zhang X., Lei L., Ma W., Ma C., Song L., Chen J., Pan B., Xing B. Cation-Pi Interaction: A Key Force for Sorption of Fluoroquinolone Antibiotics on Pyrogenic Carbonaceous Materials. Environ. Sci. Technol. 2017;51:13659–13667. doi: 10.1021/acs.est.7b02317. [DOI] [PubMed] [Google Scholar]
- 17.Lawal I.A., Moodley B. Sorption mechansim of pharmaceuticals from aqueous medium on ionic liquid modified biomass. J. Chem. Technol. Biotechnol. 2017;92:808–818. doi: 10.1002/jctb.5063. [DOI] [Google Scholar]
- 18.Liu C.H., Chuang Y.H., Li H., Teppen B.J., Boyd S.A., Gonzalez J.M., Johnston C.T., Lehmann J., Zhang W. Sorption of Lincomycin by Manure-Derived Biochars from Water. J. Environ. Qual. 2016;45:519–527. doi: 10.2134/jeq2015.06.0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li G., Feng Y., Zhu W., Zhang X. Enhanced adsorptive performance of tetracycline antibiotics on lanthanum modified diatomite. Korean J. Chem. Eng. 2015;32:2109–2115. doi: 10.1007/s11814-015-0058-2. [DOI] [Google Scholar]
- 20.Nurchi V.M., Crespo-Alonso M., Pilo M.I., Spano N., Sanna G., Toniolo R. Sorption of ofloxacin and chrysoidine by grape stalk. A representative case of biomass removal of emerging pollutants from wastewater. Arabian J. Chem. 2019;12:1141–1147. doi: 10.1016/j.arabjc.2015.01.006. [DOI] [Google Scholar]
- 21.Li W.C., Wong M.H. A comparative study on tetracycline sorption by Pachydictyon coriaceum and Sargassum hemiphyllum. Int. J. Environ. Sci. Technol. 2014;12:2731–2740. doi: 10.1007/s13762-014-0690-0. [DOI] [Google Scholar]
- 22.Zhou A., Zhang Y., Li R., Su X., Zhang L. Adsorptive removal of sulfa antibiotics from water using spent mushroom substrate, an agricultural waste. Desalin. Water Treat. 2016;57:388. doi: 10.1080/19443994.2014.979239. [DOI] [Google Scholar]
- 23.Jia S., Yang Z., Ren K., Tian Z., Dong C., Ma R., Yu G., Yang W. Removal of antibiotics from water in the coexistence of suspended particles and natural organic matters using amino-acid-modified-chitosan flocculants: A combined experimental and theoretical study. J. Hazard. Mater. 2016;317:593–601. doi: 10.1016/j.jhazmat.2016.06.024. [DOI] [PubMed] [Google Scholar]
- 24.Alsager O.A., Alnajrani M.N., Abuelizz H.A., Aldaghmani I.A. Removal of antibiotics from water and waste milk by ozonation: kinetics, byproducts, and antimicrobial activity. Ecotoxicol. Environ. Saf. 2018;158:114–122. doi: 10.1016/j.ecoenv.2018.04.024. [DOI] [PubMed] [Google Scholar]
- 25.Ji Y., Pan Z., Yuan D., Lai B. Advanced Treatment of the Antibiotic Production Wastewater by Ozone/Zero-Valent Iron Process. CLEAN - Soil, Air, Water. 2018;46:1700666. doi: 10.1002/clen.201700666. [DOI] [Google Scholar]
- 26.Kidak R., Dogan S. Medium-high frequency ultrasound and ozone based advanced oxidation for amoxicillin removal in water. Ultrason. Sonochem. 2018;40:131–139. doi: 10.1016/j.ultsonch.2017.01.033. [DOI] [PubMed] [Google Scholar]
- 27.Feng M., Yan L., Zhang X., Sun P., Yang S., Wang L., Wang Z. Fast removal of the antibiotic flumequine from aqueous solution by ozonation: Influencing factors, reaction pathways, and toxicity evaluation. Sci. Total Environ. 2016;541:167–175. doi: 10.1016/j.scitotenv.2015.09.048. [DOI] [PubMed] [Google Scholar]
- 28.Tay K.S., Madehi N. Ozonation of ofloxacin in water: by-products, degradation pathway and ecotoxicity assessment. Sci. Total Environ. 2015;520:23–31. doi: 10.1016/j.scitotenv.2015.03.033. [DOI] [PubMed] [Google Scholar]
- 29.Marcelino R.B.P., Leao M.M.D., Lago R.M., Amorim C.C. Multistage ozone and biological treatment system for real wastewater containing antibiotics. J. Environ. Manage. 2017;195:110–116. doi: 10.1016/j.jenvman.2016.04.041. [DOI] [PubMed] [Google Scholar]
- 30.Hassan S.S.M., Abdel-Shafy H.I., Mansour M.S.M. Removal of pharmaceutical compounds from urine via chemical coagulation by green synthesized ZnO-nanoparticles followed by microfiltration for safe reuse. Arabian J. Chem. 2016;12:4074–4083. doi: 10.1016/j.arabjc.2016.04.009. [DOI] [Google Scholar]
- 31.Kamarehie B., Ahmadi F., Hafezi F., Abbariki A., Heydari R., Karami M.A. Experimental data of electric coagulation and photo-electro-phenton process efficiency in the removal of metronidazole antibiotic from aqueous solution. Data in Brief. 2018;18:96–101. doi: 10.1016/j.dib.2018.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karaolia P., Michael-Kordatou I., Hapeshi E., Alexander J., Schwartz T., Fatta-Kassinos D. Investigation of the potential of a Membrane BioReactor followed by solar Fenton oxidation to remove antibiotic-related microcontaminants. Chem. Eng. J. 2017;310:491–502. doi: 10.1016/j.cej.2016.04.113. [DOI] [Google Scholar]
- 33.Sharma V., Vinoth Kumar R., Pakshirajan K., Pugazhenthi G. Integrated adsorption-membrane filtration process for antibiotic removal from aqueous solution. Powder Technol. 2017;321:259–269. doi: 10.1016/j.powtec.2017.08.040. [DOI] [Google Scholar]
- 34.Zheng J., Su C., Zhou J., Xu L., Qian Y., Chen H. Effects and mechanisms of ultraviolet, chlorination, and ozone disinfection on antibiotic resistance genes in secondary effluents of municipal wastewater treatment plants. Chem. Eng. J. 2017;317:309–316. doi: 10.1016/j.cej.2017.02.076. [DOI] [Google Scholar]
- 35.Ramli M.R., Sulaiman N.M., Mohd M.A., Rabuni M.F. Performance of chlorination process during nanofiltration of sulfonamide antibiotic. Water Sci. Technol. 2015;72:1611–1620. doi: 10.2166/wst.2015.367. [DOI] [PubMed] [Google Scholar]
- 36.Akyon B., McLaughlin M., Hernandez F., Blotevogel J., Bibby K. Characterization and biological removal of organic compounds from hydraulic fracturing produced water. Environ. Sci. Processes Impacts. 2019;21:279–290. doi: 10.1039/c8em00354h. [DOI] [PubMed] [Google Scholar]
- 37.Cetecioglu Z., Atasoy M. Biodegradation and inhibitory effects of antibiotics on biological wastewater treatment systems. Toxicity and Biodegradation Testing. 2018:29–55. [Google Scholar]
- 38.Zhang J., Lin H., Ma J., Sun W., Yang Y., Zhang X. Compost-bulking agents reduce the reservoir of antibiotics and antibiotic resistance genes in manures by modifying bacterial microbiota. Sci. Total Environ. 2019;649:396–404. doi: 10.1016/j.scitotenv.2018.08.212. [DOI] [PubMed] [Google Scholar]
- 39.de Souza Santos L.V., Meireles A.M., Lange L.C. Degradation of antibiotics norfloxacin by Fenton, UV and UV/H2O2. J. Environ. Manage. 2015;154:8–12. doi: 10.1016/j.jenvman.2015.02.021. [DOI] [PubMed] [Google Scholar]
- 40.Yahya M.S., Oturan N., El Kacemi K., El Karbane M., Aravindakumar C., Oturan M.A. Oxidative degradation study on antimicrobial agent ciprofloxacin by electro-Fenton process: kinetics and oxidation products. Chemosphere. 2014;117:447–454. doi: 10.1016/j.chemosphere.2014.08.016. [DOI] [PubMed] [Google Scholar]
- 41.Homem V., Santos L. Degradation and removal methods of antibiotics from aqueous matrices-a review. J. Environ. Manage. 2011;92:2304–2347. doi: 10.1016/j.jenvman.2011.05.023. [DOI] [PubMed] [Google Scholar]
- 42.Chang S., Yang X., Sang Y., Liu H. Highly Efficient Photocatalysts and Continuous-Flow Photocatalytic Reactors for Degradation of Organic Pollutants in Wastewater. Chem Asian J. 2016;11:2352–2371. doi: 10.1002/asia.201600363. [DOI] [PubMed] [Google Scholar]
- 43.Gaya U.I., Abdullah A.H. Heterogeneous photocatalytic degradation of organic contaminants over titanium dioxide: a review of fundamentals, progress and problems. J. Photochem. Photobiol., C. 2008;9 [Google Scholar]
- 44.Colmenares J.C., Luque R. Heterogeneous photocatalytic nanomaterials: prospects and challenges in selective transformations of biomass-derived compounds. Chem Soc Rev. 2014;43:765–778. doi: 10.1039/c3cs60262a. [DOI] [PubMed] [Google Scholar]
- 45.Elmolla E.S., Chaudhuri M. Photocatalytic degradation of amoxicillin, ampicillin and cloxacillin antibiotics in aqueous solution using UV/TiO2 and UV/H2O2/TiO2 photocatalysis. Desalination. 2010;252:46–52. doi: 10.1016/j.desal.2009.11.003. [DOI] [Google Scholar]
- 46.Nogueira A.C., Gomes L.E., Ferencz J.A.P., Rodrigues J.E.F.S., Gonçalves R.V., Wender H. Improved Visible Light Photoactivity of CuBi2O4/CuO Heterojunctions for Photodegradation of Methylene Blue and Metronidazole. The Journal of Physical Chemistry C. 2019;123:25680–25690. doi: 10.1021/acs.jpcc.9b06907. [DOI] [Google Scholar]
- 47.Zhao Y., Wang Y., Shi H., Liu E., Fan J., Hu X. Enhanced photocatalytic activity of ZnSe QDs/g-C3N4 composite for Ceftriaxone sodium degradation under visible light. Mater. Lett. 2018;231:150–153. doi: 10.1016/j.matlet.2018.08.034. [DOI] [Google Scholar]
- 48.Saravanan R., Gracia F., Stephen A. Basic Principles, Mechanism, and Challenges of Photocatalysis. Nanocomposites for Visible Light-induced Photocatalysis. 2017:19–40. [Google Scholar]
- 49.Tu W., Zhou Y., Zou Z. Versatile Graphene-Promoting Photocatalytic Performance of Semiconductors: Basic Principles, Synthesis, Solar Energy Conversion, and Environmental Applications. Adv. Funct. Mater. 2013;23:4996–5008. doi: 10.1002/adfm.201203547. [DOI] [Google Scholar]
- 50.Tan H.L., Abdi F.F., Ng Y.H. Heterogeneous photocatalysts: an overview of classic and modern approaches for optical, electronic, and charge dynamics evaluation. Chem Soc Rev. 2019;48:1255–1271. doi: 10.1039/c8cs00882e. [DOI] [PubMed] [Google Scholar]
- 51.Sun J., Xu H., Li D., Zou Z., Wu Q., Liu G., Yang J., Sun L., Xia D. Ultrasound-assisted synthesis of a feathery-shaped BiOCl with abundant oxygen vacancies and efficient visible-light photoactivity. New J. Chem. 2018;42:19571–19577. doi: 10.1039/c8nj04165b. [DOI] [Google Scholar]
- 52.Zhang J., Gao Y., Jia X., Wang J., Chen Z., Xu Y. Oxygen vacancy-rich mesoporous ZrO2 with remarkably enhanced visible-light photocatalytic performance. Sol. Energy Mater. Sol. Cells. 2018;182:113–120. doi: 10.1016/j.solmat.2018.03.023. [DOI] [Google Scholar]
- 53.Lyu J., Hu Z., Li Z., Ge M. Removal of tetracycline by BiOBr microspheres with oxygen vacancies: Combination of adsorption and photocatalysis. J. Phys. Chem. Solids. 2019;129:61–70. doi: 10.1016/j.jpcs.2018.12.041. [DOI] [Google Scholar]
- 54.Osotsi M.I., Macharia D.K., Zhu B., Wang Z., Shen X., Liu Z., Zhang L., Chen Z. Synthesis of ZnWO4−x nanorods with oxygen vacancy for efficient photocatalytic degradation of tetracycline. Progress in Natural Science: Materials International. 2018;28:408–415. doi: 10.1016/j.pnsc.2018.01.007. [DOI] [Google Scholar]
- 55.Xu X., Ding X., Yang X., Wang P., Li S., Lu Z., Chen H. Oxygen vacancy boosted photocatalytic decomposition of ciprofloxacin over Bi2MoO6: Oxygen vacancy engineering, biotoxicity evaluation and mechanism study. J. Hazard. Mater. 2019;364:691–699. doi: 10.1016/j.jhazmat.2018.10.063. [DOI] [PubMed] [Google Scholar]
- 56.Panneri S., Ganguly P., Mohan M., Nair B.N., Mohamed A.A.P., Warrier K.G., Hareesh U. Photoregenerable, bifunctional granules of carbon-doped g-C3N4 as adsorptive photocatalyst for the efficient removal of tetracycline antibiotic. ACS Sustainable Chem. Eng. 2017;5:1610–1618. doi: 10.1021/acssuschemeng.6b02383. [DOI] [Google Scholar]
- 57.Huang J., Li D., Li R., Zhang Q., Chen T., Liu H., Liu Y., Lv W., Liu G. An efficient metal-free phosphorus and oxygen co-doped g-C3N4 photocatalyst with enhanced visible light photocatalytic activity for the degradation of fluoroquinolone antibiotics. Chem. Eng. J. 2019;374:242–253. doi: 10.1016/j.cej.2019.05.175. [DOI] [Google Scholar]
- 58.Paragas L.K.B., de Luna M.D.G., Doong R.A. Rapid removal of sulfamethoxazole from simulated water matrix by visible-light responsive iodine and potassium co-doped graphitic carbon nitride photocatalysts. Chemosphere. 2018;210:1099–1107. doi: 10.1016/j.chemosphere.2018.07.109. [DOI] [PubMed] [Google Scholar]
- 59.Wang M., Jin C., Li Z., You M., Zhang Y., Zhu T. The effects of bismuth (III) doping and ultrathin nanosheets construction on the photocatalytic performance of graphitic carbon nitride for antibiotic degradation. J. Colloid Interface Sci. 2019;533:513–525. doi: 10.1016/j.jcis.2018.08.113. [DOI] [PubMed] [Google Scholar]
- 60.Cai F., Tang Y., Chen F., Yan Y., Shi W. Enhanced visible-light-driven photocatalytic degradation of tetracycline by Cr3+ doping SrTiO3 cubic nanoparticles. RSC Adv. 2015;5:21290–21296. doi: 10.1039/C4RA13821J. [DOI] [Google Scholar]
- 61.Huo P., Lu Z., Wang H., Pan J., Li H., Wu X., Huang W., Yan Y. Enhanced photodegradation of antibiotics solution under visible light with Fe2+/Fe3+ immobilized on TiO2/fly-ash cenospheres by using ions imprinting technology. Chem. Eng. J. 2011;172:615–622. doi: 10.1016/j.cej.2011.06.003. [DOI] [Google Scholar]
- 62.Zhang W., Gao S., Chen D. Preparation of Ce3+ doped Bi2O3 hollow needle-shape with enhanced visible-light photocatalytic activity. J. Rare Earths. 2019;37:726–731. doi: 10.1016/j.jre.2018.12.007. [DOI] [Google Scholar]
- 63.Chen Y., Wu Q., Liu L., Wang J., Song Y. The fabrication of self-floating Ti3+/N co-doped TiO2/diatomite granule catalyst with enhanced photocatalytic performance under visible light irradiation. Appl. Surf. Sci. 2019;467–468:514–525. doi: 10.1016/j.apsusc.2018.10.146. [DOI] [Google Scholar]
- 64.Di J., Xia J., Ji M., Li H., Xu H., Li H., Chen R. The synergistic role of carbon quantum dots for the improved photocatalytic performance of Bi2MoO6. Nanoscale. 2015;7:11433–11443. doi: 10.1039/C5NR01350J. [DOI] [PubMed] [Google Scholar]
- 65.Liang Z., Yang J., Zhou C., Mo Q., Zhang Y. Carbon quantum dots modified BiOBr microspheres with enhanced visible light photocatalytic performance. Inorg. Chem. Commun. 2018;90:97–100. doi: 10.1016/j.inoche.2018.02.013. [DOI] [Google Scholar]
- 66.Sharma S., Umar A., Mehta S.K., Ibhadon A.O., Kansal S.K. Solar light driven photocatalytic degradation of levofloxacin using TiO2/carbon-dot nanocomposites. New J. Chem. 2018;42:7445–7456. doi: 10.1039/C7NJ05118B. [DOI] [Google Scholar]
- 67.Kaur A., Salunke D.B., Umar A., Mehta S.K., Sinha A., Kansal S.K. Visible light driven photocatalytic degradation of fluoroquinolone levofloxacin drug using Ag2O/TiO2 quantum dots: a mechanistic study and degradation pathway. New J. Chem. 2017;41:12079–12090. doi: 10.1039/C7NJ02053H. [DOI] [Google Scholar]
- 68.Di J., Xia J., Ji M., Wang B., Yin S., Xu H., Chen Z., Li H. Carbon quantum dots induced ultrasmall BiOI nanosheets with assembled hollow structures for broad spectrum photocatalytic activity and mechanism insight. Langmuir. 2016;32:2075–2084. doi: 10.1021/acs.langmuir.5b04308. [DOI] [PubMed] [Google Scholar]
- 69.Ji M., Zhang Z., Xia J., Di J., Liu Y., Chen R., Yin S., Zhang S., Li H. Enhanced photocatalytic performance of carbon quantum dots/BiOBr composite and mechanism investigation. Chin. Chem. Lett. 2018;29:805–810. doi: 10.1016/j.cclet.2018.05.002. [DOI] [Google Scholar]
- 70.Gong G., Liu Y., Mao B., Wang B., Tan L., Li D., Liu Y., Shi W. Mechanism study on the photocatalytic efficiency enhancement of MoS2 modified Zn-AgIn5S8 quantum dots. RSC Adv. 2016;6:99023–99033. doi: 10.1039/c6ra19949f. [DOI] [Google Scholar]
- 71.Bai J., Li Y., Jin P., Wang J., Liu L. Facile preparation 3D ZnS nanospheres-reduced graphene oxide composites for enhanced photodegradation of norfloxacin. J. Alloy. Compd. 2017;729:809–815. doi: 10.1016/j.jallcom.2017.07.057. [DOI] [Google Scholar]
- 72.Lyu J., Shao J., Wang Y., Qiu Y., Li J., Li T., Peng Y., Liu F. Construction of a porous core-shell homojunction for the photocatalytic degradation of antibiotics. Chem. Eng. J. 2019;358:614–620. doi: 10.1016/j.cej.2018.10.085. [DOI] [Google Scholar]
- 73.Hailili R., Wang Z.-Q., Gong X.-Q., Wang C. Octahedral-shaped perovskite CaCu3Ti4O12 with dual defects and coexposed (001), (111) facets for visible-light photocatalysis. Appl. Catal. B. 2019;254:86–97. doi: 10.1016/j.apcatb.2019.03.086. [DOI] [Google Scholar]
- 74.Bao S., Wang Z., Gong X., Zeng C., Wu Q., Tian B., Zhang J. AgBr tetradecahedrons with co-exposed 100 and 111 facets: simple fabrication and enhancing spatial charge separation using facet heterojunctions. J. Mater. Chem. A. 2016;4:18570–18577. doi: 10.1039/c6ta06594e. [DOI] [Google Scholar]
- 75.Li J., Liu F., Li Y. Fabrication of an Ag/Ag2MoO4 plasmonic photocatalyst with enhanced photocatalytic performance for the degradation of ciprofloxacin. New J. Chem. 2018;42:12054–12061. doi: 10.1039/c8nj02327a. [DOI] [Google Scholar]
- 76.Boxi S.S., Paria S. Visible Light Induced Enhanced Photocatalytic Degradation of Organic Pollutants in Aqueous Media Using Ag Doped Hollow TiO2 Nanospheres. RSC Adv. 2015;5:37657–37668. doi: 10.1039/C5RA03421C. [DOI] [Google Scholar]
- 77.Cao F., Wang J., Wang Y., Zhou J., Fan W. An in-situ Bi-decorated BiOBr Photocatalyst for Synchronously Treating Multiple Antibiotics in Water. Nanoscale Advances. 2019;1:1124. doi: 10.1039/c8na00197a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhu W., Zhong L., Yan Z., Yan X. Deposition of Silver nanoparticles onto two dimensional BiOCl nanodiscs for enhanced visible light photocatalytic and biocidal activities. RSC Adv. 2016;6:64911–64920. doi: 10.1039/c6ra09964e. [DOI] [Google Scholar]
- 79.Jiang E., Liu X., Che H., Liu C., Dong H., Che G. Visible-light-driven Ag/Bi3O4Cl nanocomposite photocatalyst with enhanced photocatalytic activity for degradation of tetracycline. RSC Adv. 2018;8:37200–37207. doi: 10.1039/C8RA07482H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tian C., Xu T., Sha L., Yan Q., Wu Y. Cellulose Nanofibrils Anchored Ag on Graphitic Carbon Nitride for Efficient Photocatalysis under Visible Light. Environ. Sci. Nano. 2018;5:1–44. doi: 10.1039/C8EN00570B. [DOI] [Google Scholar]
- 81.Zhao L., Song W., Chao O., Hao W., Zeng D., Xie C. Enhanced visible-light photocatalytic performance of highly-dispersed Pt/g-C3N4 nanocomposites by one-step solvothermal treatment. RSC Adv. 2017;7:33552–33557. doi: 10.1039/c7ra04931e. [DOI] [Google Scholar]
- 82.Wei Z., Liu J., Fang W., Xu M., Qin Z., Jiang Z., Shangguan W. Photocatalytic hydrogen evolution with simultaneous antibiotic wastewater degradation via the visible-light-responsive bismuth spheres-g-C3N4 nanohybrid: Waste to energy insight. Chem. Eng. J. 2019;358:944–954. doi: 10.1016/j.cej.2018.10.096. [DOI] [Google Scholar]
- 83.Demircivi P., Simsek E.B. Visible-light-enhanced photoactivity of perovskite-type W-doped BaTiO3 photocatalyst for photodegradation of tetracycline. J. Alloy. Compd. 2019;774:795–802. doi: 10.1016/j.jallcom.2018.09.354. [DOI] [Google Scholar]
- 84.Guo J., Li Y., Hu D., Liu H. Preparation of transition-metal-doped ZnO nanophotocatalysts and their performance on photocatalytic degradation of antibiotic wastewater. Desalin. Water Treat. 2014:1–8. doi: 10.1080/19443994.2014.961171. [DOI] [Google Scholar]
- 85.Salimi M., Behbahani M., Sobhi H.R., Gholami M., Jafari A.J., Kalantary R.R., Farzadkia M., Esrafili A. A new nano-photocatalyst based on Pt and Bi co-doped TiO2 for efficient visible-light photo degradation of amoxicillin. New J. Chem. 2019;43:1562–1568. doi: 10.1039/c8nj05020a. [DOI] [Google Scholar]
- 86.Xue J., Ma S., Zhou Y., Zhang Z., He M. Facile photochemical synthesis of Au/Pt/g-C3N4 with plasmon-enhanced photocatalytic activity for antibiotic degradation. ACS Appl. Mater. Interfaces. 2015;7:9630–9637. doi: 10.1021/acsami.5b01212. [DOI] [PubMed] [Google Scholar]
- 87.Wang K., Li Y., Zhang G., Li J., Wu X. 0D Bi nanodots/2D Bi3NbO7 nanosheets heterojunctions for efficient visible light photocatalytic degradation of antibiotics: Enhanced molecular oxygen activation and mechanism insight. Appl. Catal. B. 2019;240:39–49. doi: 10.1016/j.apcatb.2018.08.063. [DOI] [Google Scholar]
- 88.Chen F., Yang Q., Sun J., Yao F., Wang S., Wang Y., Wang X., Li X., Niu C., Wang D. Enhanced photocatalytic degradation of tetracycline by AgI/BiVO4 heterojunction under visible-light irradiation: mineralization efficiency and mechanism. ACS Appl. Mater. Interfaces. 2016;8:32887–32900. doi: 10.1021/acsami.6b12278. [DOI] [PubMed] [Google Scholar]
- 89.Du Y.-B., Zhang L., Ruan M., Niu C.-G., Wen X.-J., Liang C., Zhang X.-G., Zeng G.-M. Template-free synthesis of three-dimensional porous CdS/TiO2 with high stability and excellent visible photocatalytic activity. Mater. Chem. Phys. 2018;212:69–77. doi: 10.1016/j.matchemphys.2018.03.033. [DOI] [Google Scholar]
- 90.Fan W., Li M., Bai H., Xu D., Chen C., Li C., Ge Y., Shi W. Fabrication of MgFe2O4/MoS2 heterostructure nanowires for photoelectrochemical catalysis. Langmuir. 2016;32:1629–1636. doi: 10.1021/acs.langmuir.5b03887. [DOI] [PubMed] [Google Scholar]
- 91.Huo P., Tang Y., Zhou M., Li J., Ye Z., Ma C., Yu L., Yan Y. Fabrication of ZnWO4-CdS heterostructure photocatalysts for visible light induced degradation of ciprofloxacin antibiotics. J. Ind. Eng. Chem. 2016;37:340–346. doi: 10.1016/j.jiec.2016.03.043. [DOI] [Google Scholar]
- 92.Ji B., Zhang J., Zhang C., Li N., Zhao T., Chen F., Hu L., Zhang S., Wang Z. Vertically aligned ZnO@ ZnS nanorod chip with improved photocatalytic activity for antibiotics degradation. ACS Applied Nano Materials. 2018;1:793–799. doi: 10.1021/acsanm.7b00242. [DOI] [Google Scholar]
- 93.Li M., Yin S., Wu T., Di J., Ji M., Wang B., Chen Y., Xia J., Li H. Controlled preparation of MoS2/PbBiO2I hybrid microspheres with enhanced visible-light photocatalytic behaviour. J. Colloid Interface Sci. 2018;517:278–287. doi: 10.1016/j.jcis.2018.01.096. [DOI] [PubMed] [Google Scholar]
- 94.Li W., Wen Z., Tian S., Shan L., Xiong Y. Citric acid-assisted hydrothermal synthesis of a self-modified Bi2SiO5/Bi12SiO20 heterojunction for efficient photocatalytic degradation of aqueous pollutants. Catal. Sci. Technol. 2018;8:1051–1061. doi: 10.1039/x0xx00000x. [DOI] [Google Scholar]
- 95.Liu C., Wu G., Chen J., Huang K., Shi W. Fabrication of a visible-light-driven photocatalyst and degradation of tetracycline based on the photoinduced interfacial charge transfer of SrTiO3/Fe2O3 nanowires. New J. Chem. 2016;40:5198–5208. doi: 10.1039/c5nj03167b. [DOI] [Google Scholar]
- 96.Ma Z., Deng L., Fan G., He Y. Hydrothermal synthesis of p-C3N4/f-BiOBr composites with highly efficient degradation of methylene blue and tetracycline. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019;214:103–110. doi: 10.1016/j.saa.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 97.Motlagh H.F., Haghighi M., Shabani M. Sono-solvothermal fabrication of ball-flowerlike Bi2O7Sn2-Bi7O9I3 nanophotocatalyst with efficient solar-light-driven activity for degradation of antibiotic tetracycline. Sol. Energy. 2019;180:25–38. [Google Scholar]
- 98.Ren A., Liu C., Hong Y., Shi W., Lin S., Li P. Enhanced visible-light-driven photocatalytic activity for antibiotic degradation using magnetic NiFe2O4/Bi2O3 heterostructures. Chem. Eng. J. 2014;258:301–308. doi: 10.1016/j.cej.2014.07.071. [DOI] [Google Scholar]
- 99.Soltani T., Tayyebi A., Lee B.-K. Photolysis and photocatalysis of tetracycline by sonochemically heterojunctioned BiVO4/reduced graphene oxide under visible-light irradiation. J. Environ. Manage. 2019;232:713–721. doi: 10.1016/j.jenvman.2018.11.133. [DOI] [PubMed] [Google Scholar]
- 100.Wu G., Xiao L., Gu W., Shi W., Jiang D., Liu C. Fabrication and excellent visible-light-driven photodegradation activity for antibiotics of SrTiO3 nanocube coated CdS microsphere heterojunctions. RSC Adv. 2016;6:19878–19886. doi: 10.1039/c5ra21651f. [DOI] [Google Scholar]
- 101.Xia J., Zhao J., Chen J., Di J., Ji M., Xu L., Chen Z., Li H. Facile fabrication of g-C3N4/BiPO4 hybrid materials via a reactable ionic liquid for the photocatalytic degradation of antibiotic ciprofloxacin. J. Photochem. Photobiol., A. 2017;339:59–66. doi: 10.1016/j.jphotochem.2017.02.010. [DOI] [Google Scholar]
- 102.Xu H., Zhao H., Song Y., Yan W., Xu Y., Li H., Huang L., Yin S., Li Y., Zhang Q. g-C3N4/Ag3PO4 composites with synergistic effect for increased photocatalytic activity under the visible light irradiation. Mater. Sci. Semicond. Process. 2015;39:726–734. doi: 10.1016/j.mssp.2015.04.013. [DOI] [Google Scholar]
- 103.Xu J., Luo B., Gu W., Jian Y., Wu F., Tang Y., Shen H. Fabrication of In2S3/NaTaO3 composites for enhancing the photocatalytic activity toward the degradation of tetracycline. New J. Chem. 2018;42:5052–5058. doi: 10.1039/C7NJ05123A. [DOI] [Google Scholar]
- 104.Xu Y., Ma Y., Ji H., Huang S., Xie M., Zhao Y., Xu H., Li H. Enhanced long-wavelength light utilization with polyaniline/bismuth-rich bismuth oxyhalide composite towards photocatalytic degradation of antibiotics. J. Colloid Interface Sci. 2019;537:101–111. doi: 10.1016/j.jcis.2018.10.109. [DOI] [PubMed] [Google Scholar]
- 105.Yang C., Cheng J., Chen Y., Hu Y. CdS nanoparticles immobilized on porous carbon polyhedrons derived from a metal-organic framework with enhanced visible light photocatalytic activity for antibiotic degradation. Appl. Surf. Sci. 2017;420:252–259. doi: 10.1016/j.apsusc.2017.05.102. [DOI] [Google Scholar]
- 106.Yuan X., Jiang L., Liang J., Pan Y., Zhang J., Wang H., Leng L., Wu Z., Guan R., Zeng G. In-situ synthesis of 3D microsphere-like In2S3/InVO4 heterojunction with efficient photocatalytic activity for tetracycline degradation under visible light irradiation. Chem. Eng. J. 2019;356:371–381. doi: 10.1016/j.cej.2018.09.079. [DOI] [Google Scholar]
- 107.Zhao J., Ji M., Di J., Ge Y., Zhang P., Xia J., Li H. Synthesis of g-C3N4/Bi4O5Br 2 via reactable ionic liquid and its cooperation effect for the enhanced photocatalytic behavior towards ciprofloxacin degradation. J. Photochem. Photobiol., A. 2017;347:168–176. [Google Scholar]
- 108.Zhao Y., Liang X., Wang Y., Shi H., Liu E., Fan J., Hu X. Degradation and removal of Ceftriaxone sodium in aquatic environment with Bi2WO6/g-C3N4 photocatalyst. J. Colloid Interface Sci. 2018;523:7–17. doi: 10.1016/j.jcis.2018.03.078. [DOI] [PubMed] [Google Scholar]
- 109.Zhen H., Khan M.A., Xia M., Lei W., Wang F. Controllable synthesis of flower-root shaped Bi2O3/Bi2MoO6 heterostructures as an efficient photocatalyst under visible light irradiation. J. Photochem. Photobiol., A. 2019;372:78–88. doi: 10.1016/j.jphotochem.2018.11.021. [DOI] [Google Scholar]
- 110.Zhu S.-R., Qi Q., Fang Y., Zhao W.-N., Wu M.-K., Han L. Covalent triazine framework modified BiOBr nanoflake with enhanced photocatalytic activity for antibiotic removal. Cryst. Growth Des. 2017;18:883–891. doi: 10.1021/acs.cgd.7b01367. [DOI] [Google Scholar]
- 111.Zhu X., Liu J., Zhao Z., Yan J., Xu Y., Song Y., Ji H., Xu H., Li H. Hydrothermal synthesis of mpg-C3N4 and Bi2WO6 nest-like structure nanohybrids with enhanced visible light photocatalytic activities. RSC Adv. 2017;7:38682–38690. doi: 10.1039/c7ra06681c. [DOI] [Google Scholar]
- 112.Jin Y., Jiang D., Li D., Chen M. Construction of ultrafine TiO2 nanoparticle and SnNb2O6 nanosheet 0D/2D heterojunctions with abundant interfaces and significantly improved photocatalytic activity. Catal. Sci. Technol. 2017;7:2308–2317. doi: 10.1039/c7cy00366h. [DOI] [Google Scholar]
- 113.Liu W., Dai Z., Liu Y., Zhu A., Zhong D., Wang J., Pan J. Intimate contacted two-dimensional/zero-dimensional composite of bismuth titanate nanosheets supported ultrafine bismuth oxychloride nanoparticles for enhanced antibiotic residue degradation. J. Colloid Interface Sci. 2018;529:23–33. doi: 10.1016/j.jcis.2018.05.112. [DOI] [PubMed] [Google Scholar]
- 114.Ji M., Di J., Ge Y., Xia J., Li H. 2D–2D stacking of graphene-like g-C3N4/Ultrathin Bi4O5Br 2 with matched energy band structure towards antibiotic removal. Appl. Surf. Sci. 2017;413:372–380. doi: 10.1016/j.apsusc.2017.03.287. [DOI] [Google Scholar]
- 115.Qian K., Xia L., Jiang Z., Wei W., Chen L., Xie J. In situ chemical transformation synthesis of Bi4Ti3O12/I–BiOCl 2D/2D heterojunction systems for water pollution treatment and hydrogen production. Catal. Sci. Technol. 2017;7:3863–3875. doi: 10.1039/C7CY01162H. [DOI] [Google Scholar]
- 116.Zhou C., Lai C., Xu P., Zeng G., Huang D., Li Z., Zhang C., Cheng M., Hu L., Wan J. Rational design of carbon-doped carbon nitride/Bi12O17Cl2 composites: a promising candidate photocatalyst for boosting visible-light-driven photocatalytic degradation of tetracycline. ACS Sustainable Chem. Eng. 2018;6:6941–6949. doi: 10.1021/acssuschemeng.8b00782. [DOI] [Google Scholar]
- 117.Ao Y., Bao J., Wang P., Wang C., Hou J. Bismuth oxychloride modified titanium phosphate nanoplates: a new p-n type heterostructured photocatalyst with high activity for the degradation of different kinds of organic pollutants. J. Colloid Interface Sci. 2016;476:71–78. doi: 10.1016/j.jcis.2016.05.021. [DOI] [PubMed] [Google Scholar]
- 118.Guo F., Shi W., Wang H., Han M., Li H., Huang H., Liu Y., Kang Z. Facile fabrication of a CoO/g-C3N4 p-n heterojunction with enhanced photocatalytic activity and stability for tetracycline degradation under visible light. Catal. Sci. Technol. 2017;7:3325–3331. doi: 10.1039/c7cy00960g. [DOI] [Google Scholar]
- 119.Liu C., Li P., Wu G., Luo B., Lin S., Ren A., Shi W. Enhanced photoelectrochemical and photocatalytic activity by Cu2O/SrTiO3 p–n heterojunction via a facile deposition–precipitation technique. RSC Adv. 2015;5:33938–33945. doi: 10.1039/c5ra03086b. [DOI] [Google Scholar]
- 120.Li S., Chen J., Liu Y., Xu K., Liu J. In situ anion exchange strategy to construct flower-like BiOCl/BiOCOOH pn heterojunctions for efficiently photocatalytic removal of aqueous toxic pollutants under solar irradiation. J. Alloy. Compd. 2019;781:582–588. doi: 10.1016/j.jallcom.2018.12.114. [DOI] [Google Scholar]
- 121.Ma S., Xue J., Zhou Y., Zhang Z. Enhanced visible-light photocatalytic activity of Ag2O/g-C3N4 p–n heterojunctions synthesized via a photochemical route for degradation of tetracycline hydrochloride. RSC Adv. 2015;5:40000–40006. doi: 10.1039/c5ra04075b. [DOI] [Google Scholar]
- 122.Suyana P., Ganguly P., Nair B.N., Mohamed A.P., Warrier K., Hareesh U. Co3O4-C3N4 p-n nano-heterojunctions for the simultaneous degradation of a mixture of pollutants under solar irradiation. Environ. Sci. Nano. 2017;4:212–221. doi: 10.1039/c6en00410e. [DOI] [Google Scholar]
- 123.Wang J., Zhang G., Li J., Wang K. Novel three-dimensional flowerlike BiOBr/Bi2SiO5 p-n heterostructured nanocomposite for degradation of tetracycline: Enhanced visible light photocatalytic activity and mechanism. ACS Sustainable Chem. Eng. 2018;6:14221–14229. doi: 10.1021/acssuschemeng.8b02869. [DOI] [Google Scholar]
- 124.Wen X.-J., Niu C.-G., Zhang L., Zeng G.-M. Fabrication of SnO2 nanopaticles/BiOI n-p heterostructure for wider spectrum visible-light photocatalytic degradation of antibiotic oxytetracycline hydrochloride. ACS Sustainable Chem. Eng. 2017;5:5134–5147. doi: 10.1021/acssuschemeng.7b00501. [DOI] [Google Scholar]
- 125.Yan M., Hua Y., Zhu F., Gu W., Jiang J., Shen H., Shi W. Fabrication of nitrogen doped graphene quantum dots-BiOI/MnNb2O6 p-n junction photocatalysts with enhanced visible light efficiency in photocatalytic degradation of antibiotics. Appl. Catal. B. 2017;202:518–527. doi: 10.1016/j.apcatb.2016.09.039. [DOI] [Google Scholar]
- 126.Jiang R., Wu D., Lu G., Yan Z., Liu J., Zhou R., Nkoom M. Fabrication of Fe3O4 quantum dots modified BiOCl/BiVO4 pn heterojunction to enhance photocatalytic activity for removing broad-spectrum antibiotics under visible light. J. Taiwan Inst. Chem. Eng. 2019;96:681–690. doi: 10.1016/j.jtice.2019.01.010. [DOI] [Google Scholar]
- 127.Liu C., Chen J., Che H., Huang K., Charpentier P.A., Xu W.Z., Shi W., Dong H. Construction and enhanced photocatalytic activities of a hydrogenated TiO2 nanobelt coated with CDs/MoS2 nanosheets. RSC Adv. 2017;7:8429–8442. doi: 10.1039/c6ra28479e. [DOI] [Google Scholar]
- 128.Li J., Zhou M., Ye Z., Wang H., Ma C., Huo P., Yan Y. Enhanced photocatalytic activity of g-C3N4-ZnO/HNT composite heterostructure photocatalysts for degradation of tetracycline under visible light irradiation. RSC Adv. 2015;5:91177–91189. doi: 10.1039/C5RA17360D. [DOI] [Google Scholar]
- 129.Zhang S., Gao H., Huang Y., Wang X., Hayat T., Li J., Xu X., Wang X. Ultrathin g-C3N4 nanosheets coupled with amorphous Cu-doped FeOOH nanoclusters as 2D/0D heterogeneous catalysts for water remediation. Environ. Sci. Nano. 2018;5:1179–1190. doi: 10.1039/C8EN00124C. [DOI] [Google Scholar]
- 130.Jo W.-K., Tonda S. Novel CoAl-LDH/g-C3N4/RGO ternary heterojunction with notable 2D/2D/2D configuration for highly efficient visible-light-induced photocatalytic elimination of dye and antibiotic pollutants. J. Hazard. Mater. 2019;368:778–787. doi: 10.1016/j.jhazmat.2019.01.114. [DOI] [PubMed] [Google Scholar]
- 131.Chen D., Li B., Pu Q., Chen X., Wen G., Li Z. Preparation of Ag-AgVO3/g-C3N4 composite photo-catalyst and degradation characteristics of antibiotics. J. Hazard. Mater. 2019;373:303–312. doi: 10.1016/j.jhazmat.2019.03.090. [DOI] [PubMed] [Google Scholar]
- 132.Jing L., Xu Y., Qin C., Liu J., Huang S., He M., Xu H., Li H. Visible-light-driven ZnFe2O4/Ag/Ag3VO4 photocatalysts with enhanced photocatalytic activity under visible light irradiation. Mater. Res. Bull. 2017;95:607–615. doi: 10.1016/j.materresbull.2017.06.003. [DOI] [Google Scholar]
- 133.Lu X., Wang Y., Zhang X., Xu G., Wang D., Lv J., Zheng Z., Wu Y. NiS and MoS2 nanosheet co-modified graphitic C3N4 ternary heterostructure for high efficient visible light photodegradation of antibiotic. J. Hazard. Mater. 2018;341:10–19. doi: 10.1016/j.jhazmat.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 134.Zhou L., Zhang W., Chen L., Deng H., Wan J. A novel ternary visible-light-driven photocatalyst AgCl/Ag3PO4/g-C3N4: Synthesis, characterization, photocatalytic activity for antibiotic degradation and mechanism analysis. Catal. Commun. 2017;100:191–195. doi: 10.1016/j.catcom.2017.06.049. [DOI] [Google Scholar]
- 135.Shao N., Wang J., Wang D., Corvini P. Preparation of three-dimensional Ag3PO4/TiO2@ MoS2 for enhanced visible-light photocatalytic activity and anti-photocorrosion. Appl. Catal. B. 2017;203:964–978. doi: 10.1016/j.apcatb.2016.11.008. [DOI] [Google Scholar]
- 136.Priya B., Raizada P., Singh N., Thakur P., Singh P. Adsorptional photocatalytic mineralization of oxytetracycline and ampicillin antibiotics using Bi2O3/BiOCl supported on graphene sand composite and chitosan. J. Colloid Interface Sci. 2016;479:271–283. doi: 10.1016/j.jcis.2016.06.067. [DOI] [PubMed] [Google Scholar]
- 137.Shi W., Lu D., Wang L., Teng F., Zhang J. Core-shell structured Fe3O4@ SiO2@ CdS nanoparticles with enhanced visible-light photocatalytic activities. RSC Adv. 2015;5:106038–106043. doi: 10.1039/C5RA22295H. [DOI] [Google Scholar]
- 138.Tang Y., Liu X., Ma C., Zhou M., Huo P., Yu L., Pan J., Shi W., Yan Y. Enhanced photocatalytic degradation of tetracycline antibiotics by reduced graphene oxide-CdS/ZnS heterostructure photocatalysts. New J. Chem. 2015;39:5150–5160. doi: 10.1039/c5nj00681c. [DOI] [Google Scholar]
- 139.Xu P., Shen X., Luo L., Shi Z., Liu Z., Chen Z., Zhu M., Zhang L. Preparation of TiO2/Bi2WO6 nanostructured heterojunctions on carbon fibers as a weaveable visible-light photocatalyst/photoelectrode. Environ. Sci. Nano. 2018;5:327–337. doi: 10.1039/C7EN00822H. [DOI] [Google Scholar]
- 140.Hong Y., Li C., Yin B., Li D., Zhang Z., Mao B., Fan W., Gu W., Shi W. Promoting visible-light-induced photocatalytic degradation of tetracycline by an efficient and stable beta-Bi2O3@ g-C3N4 core/shell nanocomposite. Chem. Eng. J. 2018;338:137–146. doi: 10.1016/j.cej.2017.12.108. [DOI] [Google Scholar]
- 141.Zhu W., Sun F., Goei R., Zhou Y. Construction of WO3-g-C3N4 composites as efficient photocatalysts for pharmaceutical degradation under visible light. Catal. Sci. Technol. 2017;7:2591–2600. doi: 10.1039/x0xx00000x. [DOI] [Google Scholar]
- 142.Chen F., Yang Q., Yao F., Wang S., Sun J., An H., Yi K., Wang Y., Zhou Y., Wang L. Visible-light photocatalytic degradation of multiple antibiotics by AgI nanoparticle-sensitized Bi5O7I microspheres: Enhanced interfacial charge transfer based on Z-scheme heterojunctions. J. Catal. 2017;352:160–170. doi: 10.1016/j.jcat.2017.04.032. [DOI] [Google Scholar]
- 143.Gong Y., Wu Y., Xu Y., Li L., Li C., Liu X., Niu L. All-solid-state Z-scheme CdTe/TiO2 heterostructure photocatalysts with enhanced visible-light photocatalytic degradation of antibiotic waste water. Chem. Eng. J. 2018;350:257–267. doi: 10.1016/j.cej.2018.05.186. [DOI] [Google Scholar]
- 144.Guo F., Shi W., Wang H., Han M., Guan W., Huang H., Liu Y., Kang Z. Study on highly enhanced photocatalytic tetracycline degradation of type Ⅱ AgI/CuBi2O4 and Z-scheme AgBr/CuBi2O4 heterojunction photocatalysts. J. Hazard. Mater. 2018;349:111–118. doi: 10.1016/j.jhazmat.2018.01.042. [DOI] [PubMed] [Google Scholar]
- 145.Li C., Yu S., Dong H., Liu C., Wu H., Che H., Chen G. Z-scheme mesoporous photocatalyst constructed by modification of Sn3O4 nanoclusters on g-C3N4 nanosheets with improved photocatalytic performance and mechanism insight. Appl. Catal. B. 2018;238:284–293. doi: 10.1016/j.apcatb.2018.07.049. [DOI] [Google Scholar]
- 146.Wang K., Zhang G., Li J., Li Y., Wu X. 0D/2D Z-scheme heterojunctions of bismuth tantalate quantum dots/ultrathin g-C3N4 nanosheets for highly efficient visible light photocatalytic degradation of antibiotics. ACS Appl. Mater. Interfaces. 2017;9:43704–43715. doi: 10.1021/acsami.7b14275. [DOI] [PubMed] [Google Scholar]
- 147.Ma X., Jiang D., Xiao P., Jin Y., Meng S., Chen M. 2D/2D heterojunctions of WO3 nanosheet/K+ Ca2Nb3O10−ultrathin nanosheet with improved charge separation efficiency for significantly boosting photocatalysis. Catal. Sci. Technol. 2017;7:3481–3491. doi: 10.1039/x0xx00000x. [DOI] [Google Scholar]
- 148.Yu H., Huang B., Wang H., Yuan X., Jiang L., Wu Z., Zhang J., Zeng G. Facile construction of novel direct solid-state Z-scheme AgI/BiOBr photocatalysts for highly effective removal of ciprofloxacin under visible light exposure: Mineralization efficiency and mechanisms. J. Colloid Interface Sci. 2018;522:82–94. doi: 10.1016/j.jcis.2018.03.056. [DOI] [PubMed] [Google Scholar]
- 149.Zhou L., Zhang W., Chen L., Deng H. Z-scheme mechanism of photogenerated carriers for hybrid photocatalyst Ag3PO4/g-C3N4 in degradation of sulfamethoxazole. J. Colloid Interface Sci. 2017;487:410–417. doi: 10.1016/j.jcis.2016.10.068. [DOI] [PubMed] [Google Scholar]
- 150.Bao S., Wu Q., Chang S., Tian B., Zhang J. Z-scheme CdS-Au-BiVO4 with enhanced photocatalytic activity for organic contaminant decomposition. Catal. Sci. Technol. 2017;7:124–132. doi: 10.1039/C6CY01980C. [DOI] [Google Scholar]
- 151.Deng Y., Tang L., Zeng G., Feng C., Dong H., Wang J., Feng H., Liu Y., Zhou Y., Pang Y. Plasmonic resonance excited dual Z-scheme BiVO4/Ag/Cu2O nanocomposite: synthesis and mechanism for enhanced photocatalytic performance in recalcitrant antibiotic degradation. Environ. Sci. Nano. 2017;4:1494–1511. doi: 10.1039/c7en00237h. [DOI] [Google Scholar]
- 152.Lu Z., Yu Z., Dong J., Song M., Yang L., Liu X., Ma Z., Hang S., Yan Y., Huo P. Facile microwave synthesis of a Z-scheme imprinted ZnFe2O4/Ag/PEDOT with the specific recognition ability towards improving photocatalytic activity and selectivity for tetracycline. Chem. Eng. J. 2018;337:228–241. doi: 10.1016/j.cej.2017.12.115. [DOI] [Google Scholar]
- 153.Mondal P., Satra J., Ghorui U.K., Saha N., Srivastava D.N., Adhikary B. Facile Fabrication of Novel Hetero-Structured Organic-Inorganic High-Performance Nanocatalyst: A Smart System for Enhanced Catalytic Activity toward Ciprofloxacin Degradation and Oxygen Reduction. ACS Applied Nano Materials. 2018;1:6015–6026. doi: 10.1021/acsanm.8b00937. [DOI] [Google Scholar]
- 154.Li Q., Guan Z., Wu D., Zhao X., Bao S., Tian B., Zhang J. Z-scheme BiOCl-Au-CdS heterostructure with enhanced sunlight-driven photocatalytic activity in degrading water dyes and antibiotics. ACS Sustainable Chem. Eng. 2017;5:6958–6968. doi: 10.1021/acssuschemeng.7b01157. [DOI] [Google Scholar]
- 155.Yang Y., Zeng Z., Zhang C., Huang D., Zeng G., Xiao R., Lai C., Zhou C., Guo H., Xue W. Construction of iodine vacancy-rich BiOI/Ag@AgI Z-scheme heterojunction photocatalysts for visible-light-driven tetracycline degradation: transformation pathways and mechanism insight. Chem. Eng. J. 2018;349:808–821. doi: 10.1016/j.cej.2018.05.093. [DOI] [Google Scholar]
- 156.Yuan X., Jiang L., Chen X., Leng L., Wang H., Wu Z., Xiong T., Liang J., Zeng G. Highly efficient visible-light-induced photoactivity of Z-scheme Ag2CO3/Ag/WO3 photocatalysts for organic pollutant degradation. Environ. Sci. Nano. 2017;4:2175–2185. doi: 10.1039/C7EN00713B. [DOI] [Google Scholar]
- 157.Ren M., Chen J., Wang P., Hou J., Qian J., Wang C., Ao Y. Construction of silver iodide/silver/Bismuth Tantalate Z-scheme photocatalyst for effective visible light degradation of organic pollutants. J. Colloid Interface Sci. 2018;532:190–200. doi: 10.1016/j.jcis.2018.07.141. [DOI] [PubMed] [Google Scholar]
- 158.Patra S.K., Rahut S., Basu J.K. Enhanced Z-scheme photocatalytic activity of a π-conjugated heterojunction: MIL-53 (Fe)/Ag/g-C3N4. New J. Chem. 2018;42:18598–18607. doi: 10.1039/c8nj04080j. [DOI] [Google Scholar]
- 159.Liu Q., Shen J., Yang X., Zhang T., Tang H. 3D reduced graphene oxide aerogel-mediated Z-scheme photocatalytic system for highly efficient solar-driven water oxidation and removal of antibiotics. Appl. Catal. B. 2018;232:562–573. doi: 10.1016/j.apcatb.2018.03.100. [DOI] [Google Scholar]
- 160.Hu K., Chen C., Zhu Y., Zeng G., Huang B., Chen W., Liu S., Lei C., Li B., Yang Y. Ternary Z-scheme heterojunction of Bi2WO6 with reduced graphene oxide (rGO) and meso-tetra (4-carboxyphenyl) porphyrin (TCPP) for enhanced visible-light photocatalysis. J. Colloid Interface Sci. 2019;540:115–125. doi: 10.1016/j.jcis.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 161.Shao B., Liu X., Liu Z., Zeng G., Liang Q., Liang C., Cheng Y., Zhang W., Liu Y., Gong S. A novel double Z-scheme photocatalyst Ag3PO4/Bi2S3/Bi2O3 with enhanced visible-light photocatalytic performance for antibiotic degradation. Chem. Eng. J. 2019;6:16426–16436. doi: 10.1016/j.cej.2019.03.013. [DOI] [Google Scholar]
- 162.Hu X., Liu X., Tian J., Li Y., Cui H. Towards full-spectrum (UV, visible, and near-infrared) photocatalysis: achieving an all-solid-state Z-scheme between Ag2O and TiO2 using reduced graphene oxide as the electron mediator. Catal. Sci. Technol. 2017;7:4193–4205. doi: 10.1039/C7CY01349C. [DOI] [Google Scholar]
- 163.Liu H.-Y., Liang C., Niu C.-G., Huang D.-W., Du Y.-B., Guo H., Zhang L., Yang Y.-Y., Zeng G.-M. Facile assembly of g-C3N4/Ag2CO3/graphene oxide with a novel dual Z-scheme system for enhanced photocatalytic pollutant degradation. Appl. Surf. Sci. 2019;475:421–434. doi: 10.1016/j.apsusc.2019.01.018. [DOI] [Google Scholar]
- 164.Yan M., Zhu F., Gu W., Sun L., Shi W., Hua Y. Construction of nitrogen-doped graphene quantum dots-BiVO4/g-C3N4 Z-scheme photocatalyst and enhanced photocatalytic degradation of antibiotics under visible light. RSC Adv. 2016;6:61162–61174. doi: 10.1039/c6ra07589d. [DOI] [Google Scholar]
- 165.Wang J., Chen H., Tang L., Zeng G., Liu Y., Yan M., Deng Y., Feng H., Yu J., Wang L. Antibiotic removal from water: A highly efficient silver phosphate-based Z-scheme photocatalytic system under natural solar light. Sci. Total Environ. 2018;639:1462–1470. doi: 10.1016/j.scitotenv.2018.05.258. [DOI] [PubMed] [Google Scholar]
- 166.Pan Z., Ma W., Wang L. Construction of a magnetic Z-scheme photocatalyst with enhanced oxidation/reduction abilities and recyclability for the degradation of tetracycline. RSC Adv. 2016;6:114374–114382. doi: 10.1039/c6ra24096h. [DOI] [Google Scholar]
- 167.Shao B., Liu Z., Zeng G., Wu Z., Liu Y., Cheng M., Chen M., Liu Y., Zhang W., Feng H. Nitrogen-Doped Hollow Mesoporous Carbon Spheres Modified g-C3N4/Bi2O3 Direct Dual Semiconductor Photocatalytic System with Enhanced Antibiotics Degradation under Visible Light. ACS Sustainable Chem. Eng. 2018;6:16424–16436. doi: 10.1021/acssuschemeng.8b03480. [DOI] [Google Scholar]
- 168.Hao L., Huang H., Guo Y., Zhang Y. Multifunctional Bi2O2(OH)(NO3) nanosheets with 001 active exposing facets: efficient photocatalysis, dye-sensitization, and piezoelectric-catalysis. ACS Sustainable Chem. Eng. 2018;6:1848–1862. doi: 10.1021/acssuschemeng.7b03223. [DOI] [Google Scholar]
- 169.Wu X., Li K., Li Y., Zhang G. Motivating visible light photocatalytic activity of ultrathin Bi2O2(OH)xCl2−x solid solution with exposed 001 facets by the co-effect of oxygen vacancy and OH replacement. Nanoscale. 2018;10:15294–15302. doi: 10.1039/C8NR04469D. [DOI] [PubMed] [Google Scholar]
- 170.Xu J., Bian Z., Xin X., Chen A., Wang H. Size dependence of nanosheet BiVO4 with oxygen vacancies and exposed 0 0 1 facets on the photodegradation of oxytetracycline. Chem. Eng. J. 2018;337:684–696. doi: 10.1016/j.cej.2017.12.133. [DOI] [Google Scholar]
- 171.Ma Y., Liu Q., Wang Q., Qu D., Shi J. Insight into the origin of photoreactivity of various well-defined Bi2WO6 crystals: exposed heterojunction-like surface and oxygen defects. RSC Adv. 2016;6:18916–18923. doi: 10.1039/c5ra27295e. [DOI] [Google Scholar]
- 172.Liu W., Shang Y., Zhu A., Tan P., Liu Y., Qiao L., Chu D., Xiong X., Pan J. Enhanced performance of doped BiOCl nanoplates for photocatalysis: understanding from doping insight into improved spatial carrier separation. J. Mater. Chem. A. 2017;5:12542–12549. doi: 10.1039/C7TA02724A. [DOI] [Google Scholar]
- 173.Jiang Z., Lv X., Jiang D., Xie J., Mao D. Natural leaves-assisted synthesis of nitrogen-doped, carbon-rich nanodots-sensitized, Ag-loaded anatase TiO2 square nanosheets with dominant 001 facets and their enhanced catalytic applications. J. Mater. Chem. A. 2013;1:14963–14972. doi: 10.1039/c3ta13248j. [DOI] [Google Scholar]
- 174.Wang W., Fang J., Shao S., Lai M., Lu C. Compact and uniform TiO2@ g-C3N4 core-shell quantum heterojunction for photocatalytic degradation of tetracycline antibiotics. Appl. Catal. B. 2017;217:57–64. doi: 10.1016/j.apcatb.2017.05.037. [DOI] [Google Scholar]
- 175.Zhu Z., Fan W., Liu Z., Dong H., Yan Y., Huo P. Construction of an attapulgite intercalated mesoporous g-C3N4 with enhanced photocatalytic activity for antibiotic degradation. J. Photochem. Photobiol., A. 2018;359:102–110. doi: 10.1016/j.jphotochem.2018.04.003. [DOI] [Google Scholar]
- 176.Dehghan A., Dehghani M.H., Nabizadeh R., Ramezanian N., Alimohammadi M., Najafpoor A.A. Adsorption and visible-light photocatalytic degradation of tetracycline hydrochloride from aqueous solutions using 3D hierarchical mesoporous BiOI: synthesis and characterization, process optimization, adsorption and degradation modeling. Chem. Eng. Res. Des. 2018;129:217–230. doi: 10.1016/j.cherd.2017.11.003. [DOI] [Google Scholar]
- 177.Di J., Xia J., Ge Y., Xu L., Xu H., He M., Zhang Q., Li H. Reactable ionic liquid-assisted rapid synthesis of BiOI hollow microspheres at room temperature with enhanced photocatalytic activity. J. Mater. Chem. A. 2014;2:15864–15874. doi: 10.1039/c4ta02400a. [DOI] [Google Scholar]
- 178.Gupta G., Umar A., Kaur A., Sood S., Dhir A., Kansal S. Solar light driven photocatalytic degradation of Ofloxacin based on ultra-thin bismuth molybdenum oxide nanosheets. Mater. Res. Bull. 2018;99:359–366. doi: 10.1016/j.materresbull.2017.11.033. [DOI] [Google Scholar]
- 179.Karthik R., Kumar J.V., Chen S.-M., Kumar P.S., Selvam V., Muthuraj V. A selective electrochemical sensor for caffeic acid and photocatalyst for metronidazole drug pollutant - A dual role by rod-like SrV2O6. Sci. Rep. 2017;7:7254. doi: 10.1038/s41598-017-07423-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Chen Q., Liu H., Xin Y., Cheng X. TiO2 nanobelts-Effect of calcination temperature on optical, photoelectrochemical and photocatalytic properties. Electrochim. Acta. 2013;111:284–291. doi: 10.1016/j.electacta.2013.08.049. [DOI] [Google Scholar]
- 181.Bojer C., Schöbel J., Martin T., Ertl M., Schmalz H., Breu J. Clinical wastewater treatment: Photochemical removal of an anionic antibiotic (ciprofloxacin) by mesostructured high aspect ratio ZnO nanotubes. Appl. Catal. B. 2017;204:561–565. doi: 10.1016/j.apcatb.2016.12.003. [DOI] [Google Scholar]
- 182.Hailili R., Wang Z.-Q., Xu M., Wang Y., Gong X.-Q., Xu T., Wang C. Layered nanostructured ferroelectric perovskite Bi5FeTi3O15 for visible light photodegradation of antibiotics. J. Mater. Chem. A. 2017;5:21275–21290. doi: 10.1039/c7ta06618j. [DOI] [Google Scholar]
- 183.Chen Q., Mao Y., Bing N., Zou Y., Zhu L. Preparation and optical properties of three-dimensional navel-like Bi2WO6 hierarchical microspheres. Chin. Chem. Lett. 2018;30:783–786. doi: 10.1016/j.cclet.2018.10.036. [DOI] [Google Scholar]
- 184.Hernández-Uresti D., Vázquez A., Obregón S., Ruíz-Gómez M. Novel g-C3N4 photocatalytic coatings with spearhead-like morphology prepared by an electrophoretic deposition route. Mater. Lett. 2017;200:59–62. doi: 10.1016/j.matlet.2017.04.097. [DOI] [Google Scholar]
- 185.Chen S., Lu W., Han J., Zhong H., Xu T., Wang G., Chen W. Robust three-dimensional g-C3N4@cellulose aerogel enhanced by cross-linked polyester fibers for simultaneous removal of hexavalent chromium and antibiotics. Chem. Eng. J. 2019;359:119–129. doi: 10.1016/j.cej.2018.11.110. [DOI] [Google Scholar]
- 186.Yang X., Chen Z., Fang J., Yang Q., Zhao W., Qian X., Zhou D., Liu C., Chen M. Freestanding 3D MoS2 nanosheets/graphene aerogel heterostructure as a recyclable photocatalyst for efficiently degrading antibiotic residues. Mater. Lett. 2019;252:5–7. doi: 10.1016/j.matlet.2019.05.084. [DOI] [Google Scholar]
- 187.Bai S., Zhang N., Gao C., Xiong Y. Defect engineering in photocatalytic materials. Nano Energy. 2018;53:296–336. doi: 10.1016/j.nanoen.2018.08.058. [DOI] [Google Scholar]
- 188.Zhang N., Gao C., Xiong Y. Defect engineering: a versatile tool for tuning the activation of key molecules in photocatalytic reactions. Journal of Energy Chemistry. 2019;37:43–57. doi: 10.1016/j.jechem.2018.09.010. [DOI] [Google Scholar]
- 189.Chen D., Zou Y., Wang S. Surface chemical-functionalization of ultrathin two-dimensional nanomaterials for electrocatalysis. Mater. Today Energy. 2019;12:250–268. doi: 10.1016/j.mtener.2019.01.006. [DOI] [Google Scholar]
- 190.Tan H.L., Amal R., Ng Y.H. Alternative strategies in improving the photocatalytic and photoelectrochemical activities of visible light-driven BiVO4: a review. J. Mater. Chem. A. 2017;5:16498–16521. doi: 10.1039/c7ta04441k. [DOI] [Google Scholar]
- 191.Du C., Zhang Q., Lin Z., Yan B., Xia C., Yang G. Half-unit-cell ZnIn2S4 monolayer with sulfur vacancies for photocatalytic hydrogen evolution. Appl. Catal. B. 2019;248:193–201. doi: 10.1016/j.apcatb.2019.02.027. [DOI] [Google Scholar]
- 192.Han Y., Zhou J., Dong J. Electronic and magnetic properties of MoS2 nanoribbons with sulfur line vacancy defects. Appl. Surf. Sci. 2015;346:470–476. doi: 10.1016/j.apsusc.2015.02.016. [DOI] [Google Scholar]
- 193.Li G., Fu C., Wu J., Rao J., Liou S.-C., Xu X., Shao B., Liu K., Liu E., Kumar N., Liu X., Fahlman M., Gooth J., Auffermann G., Sun Y., Felser C., Zhang B. Synergistically creating sulfur vacancies in semimetal-supported amorphous MoS2 for efficient hydrogen evolution. Appl. Catal. B. 2019;254:1–6. doi: 10.1016/j.apcatb.2019.04.080. [DOI] [Google Scholar]
- 194.Li X., Cheng Y., Wu Q., Xu J., Wang Y. Synergistic effect of the rearranged sulfur vacancies and sulfur interstitials for 13-fold enhanced photocatalytic H2 production over defective Zn2In2S5 nanosheets. Appl. Catal. B. 2019;240:270–276. doi: 10.1016/j.apcatb.2018.09.008. [DOI] [Google Scholar]
- 195.Shang H., Wang T., Zhang W. Sulfur vacancy formation at different MoS2 edges during hydrodesulfurization process: A DFT study. Chem. Eng. Sci. 2019;195:208–217. doi: 10.1016/j.ces.2018.11.049. [DOI] [Google Scholar]
- 196.Eliyahu I., Horowitz Y.S., Oster L., Mardor I., Druzhyna S., Biderman S. Kinetic modeling of Fluorine vacancy/F center creation in LiF:Mg, Ti including vacancy-interstitial recombination: Evaluating the factors leading to the lack of supralinearity in the optical absorption F center concentration dose response. Nucl. Instrum. Methods Phys. Res., Sect. B. 2015;343:15–25. doi: 10.1016/j.nimb.2014.11.017. [DOI] [Google Scholar]
- 197.Wang Q., Liu Z., Liu D., Liu G., Yang M., Cui F., Wang W. Ultrathin two-dimensional BiOBrxI1-x solid solution with rich oxygen vacancies for enhanced visible-light-driven photoactivity in environmental remediation. Appl. Catal. B. 2018;236:222–232. doi: 10.1016/j.apcatb.2018.05.029. [DOI] [Google Scholar]
- 198.Anzaldo Olivares B., Moreno O.P., Téllez G.H., Rosas E.R., Bustamante F.J.M., Castro Sánchez M.E., Sharma P., Mendoza A., Pérez R.G. Green emission band induced by crystal defects in halogenated (-Br, -Cl, -F) chiral imines with a benzo[b]thiophene-based moiety. Opt. Mater. 2019;94:337–347. doi: 10.1016/j.optmat.2019.06.007. [DOI] [Google Scholar]
- 199.Yang Y., Zeng Z., Chen Z., Huang D., Zeng G., Rong X., Cui L., Zhou C., Hai G., Xue W. Construction of iodine vacancy-rich BiOI/Ag@AgI Z-scheme heterojunction photocatalysts for visible-light-driven tetracycline degradation: transformation pathways and mechanism insight. Chem. Eng. J. 2018;349:808–821. doi: 10.1016/j.cej.2018.05.093. [DOI] [Google Scholar]
- 200.Cao J., Nie W., Huang L., Ding Y., Lv K., Tang H. Photocatalytic activation of sulfite by nitrogen vacancy modified graphitic carbon nitride for efficient degradation of carbamazepine. Appl. Catal. B. 2019;241:18–27. doi: 10.1016/j.apcatb.2018.09.007. [DOI] [Google Scholar]
- 201.Cao J., Pan C., Ding Y., Li W., Lv K., Tang H. Constructing nitrogen vacancy introduced g-C3N4 p-n homojunction for enhanced photocatalytic activity. J. Environ. Chem. Eng. 2019;7 doi: 10.1016/j.jece.2019.102984. [DOI] [Google Scholar]
- 202.Liang L., Shi L., Wang F., Yao L., Zhang Y., Qi W. Synthesis and photo-catalytic activity of porous g-C3N4: Promotion effect of nitrogen vacancy in H2 evolution and pollutant degradation reactions. Int. J. Hydrogen Energy. 2019;44:16315–16326. doi: 10.1016/j.ijhydene.2019.05.001. [DOI] [Google Scholar]
- 203.Ji M., Chen R., Di J., Liu Y., Li K., Chen Z., Xia J., Li H. Oxygen vacancies modulated Bi-rich bismuth oxyiodide microspheres with tunable valence band position to boost the photocatalytic activity. J. Colloid Interface Sci. 2019;533:612–620. doi: 10.1016/j.jcis.2018.08.097. [DOI] [PubMed] [Google Scholar]
- 204.Liu J., Xie F., Li R., Li T., Jia Z., Wang Y., Wang Y., Zhang X., Fan C. TiO2-x/Ag3PO4 photocatalyst: Oxygen vacancy dependent visible light photocatalytic performance and BPA degradative pathway. Mater. Sci. Semicond. Process. 2019;97:1–10. doi: 10.1016/j.mssp.2019.03.002. [DOI] [Google Scholar]
- 205.Qi W., Zhang F., An X., Liu H., Qu J. Oxygen vacancy modulation of {010}-dominated TiO2 for enhanced photodegradation of Sulfamethoxazole. Catal. Commun. 2019;118:35–38. doi: 10.1016/j.catcom.2018.09.014. [DOI] [Google Scholar]
- 206.Song Z., Dong X., Fang J., Xiong C., Wang N., Tang X. Improved photocatalytic degradation of perfluorooctanoic acid on oxygen vacancies-tunable bismuth oxychloride nanosheets prepared by a facile hydrolysis. J. Hazard. Mater. 2019;377:371–380. doi: 10.1016/j.jhazmat.2019.05.084. [DOI] [PubMed] [Google Scholar]
- 207.Singh M., Jampaiah D., Kandjani A.E., Sabri Y.M., Della Gaspera E., Reineck P., Judd M., Langley J., CoxJ. N. van Embden, Oxygen-deficient photostable Cu2O for enhanced visible light photocatalytic activity. Nanoscale. 2018;10:6039–6050. doi: 10.1039/C7NR08388B. [DOI] [PubMed] [Google Scholar]
- 208.Bai Y., Ye L., Chen T., Wang L., Shi X., Zhang X., Chen D. Facet-Dependent Photocatalytic N2 Fixation of Bismuth-Rich Bi5O7I Nanosheets. ACS Appl. Mater. Interfaces. 2016;8:27661–27668. doi: 10.1021/acsami.6b08129. [DOI] [PubMed] [Google Scholar]
- 209.Di J., Chen C., Zhu C., Ji M., Xia J., Yan C., Hao W., Li S., Li H., Liu Z. Bismuth vacancy mediated single unit cell Bi2WO6 nanosheets for boosting photocatalytic oxygen evolution. Appl. Catal. B. 2018;238:119–125. doi: 10.1016/j.apcatb.2018.06.066. [DOI] [Google Scholar]
- 210.Jin X., Ye L., Xie H., Chen G. Bismuth-rich bismuth oxyhalides for environmental and energy photocatalysis. Coord. Chem. Rev. 2017;349:84–101. doi: 10.1016/j.ccr.2017.08.010. [DOI] [Google Scholar]
- 211.Cao S., Fan B., Feng Y., Chen H., Jiang F., Wang X. Sulfur-doped g-C3N4 nanosheets with carbon vacancies: General synthesis and improved activity for simulated solar-light photocatalytic nitrogen fixation. Chem. Eng. J. 2018;353:147–156. doi: 10.1016/j.cej.2018.07.116. [DOI] [Google Scholar]
- 212.Pan Y., Li Y.Q., Zheng Q.H., Xu Y. Point defect of titanium sesquioxide Ti2O3 as the application of next generation Li-ion batteries. J. Alloy. Compd. 2019;786:621–626. doi: 10.1016/j.jallcom.2019.02.054. [DOI] [Google Scholar]
- 213.Wang T., Liu L., Ge G., Liu M., Zhou W., Chang K., Yang F., Wang D., Ye J. Two-dimensional titanium oxide nanosheets rich in titanium vacancies as an efficient cocatalyst for photocatalytic water oxidation. J. Catal. 2018;367:296–305. doi: 10.1016/j.jcat.2018.09.026. [DOI] [Google Scholar]
- 214.Zuo F., Wang L., Wu T., Zhang Z., Borchardt D., Feng P. Self-doped Ti3+ enhanced photocatalyst for hydrogen production under visible light. J. Am. Chem. Soc. 2010;132:11856–11857. doi: 10.1002/chin.201049012. [DOI] [PubMed] [Google Scholar]
- 215.Chen X., Liu L., Peter Y.Y., Mao S.S. Increasing solar absorption for photocatalysis with black hydrogenated titanium dioxide nanocrystals. Science. 2011;331:746–750. doi: 10.1126/science.1198640. [DOI] [PubMed] [Google Scholar]
- 216.Nowotny M.K., Sheppard L.R., Bak T., Nowotny J. Defect chemistry of titanium dioxide. Application of defect engineering in processing of TiO2-based photocatalysts. The Journal of Physical Chemistry C. 2008;112:5275–5300. doi: 10.1002/chin.200831228. [DOI] [Google Scholar]
- 217.Ren L., Zhou W., Sun B., Li H., Qiao P., Xu Y., Wu J., Lin K., Fu H. Defects-engineering of magnetic γ-Fe2O3 ultrathin nanosheets/mesoporous black TiO2 hollow sphere heterojunctions for efficient charge separation and the solar-driven photocatalytic mechanism of tetracycline degradation. Appl. Catal. B. 2019;240:319–328. doi: 10.1016/j.apcatb.2018.08.033. [DOI] [Google Scholar]
- 218.Liu X., Xu H., Grabstanowicz L.R., Gao S., Lou Z., WangB. huang, Y. Dai, T. Xu, W. Ti3+ self-doped TiO2−x anatase nanoparticles via oxidation of TiH2 in H2O2. Catal. Today. 2014;225:80–89. doi: 10.1016/j.cattod.2013.08.025. [DOI] [Google Scholar]
- 219.Wen M., Zhang S., Dai W., Li G., Zhang D. In situ synthesis of Ti3+ self-doped mesoporous TiO2 as a durable photocatalyst for environmental remediation. Chin. J. Catal. 2015;36:2095–2102. doi: 10.1016/s1872-2067(15)60992-5. [DOI] [Google Scholar]
- 220.Xing M., Fang W., Nasir M., Ma Y., Zhang J., Anpo M. Self-doped Ti3+-enhanced TiO2 nanoparticles with a high-performance photocatalysis. J. Catal. 2013;297:236–243. doi: 10.1016/j.jcat.2012.10.014. [DOI] [Google Scholar]
- 221.Wang X., Li Y., Liu X., Gao S., Huang B., Dai Y. Preparation of Ti3+ self-doped TiO2 nanoparticles and their visible light photocatalytic activity. Chin. J. Catal. 2015;36:389–399. doi: 10.1016/s1872-2067(14)60234-5. [DOI] [Google Scholar]
- 222.Wang J., Yang P., Huang B. Self-doped TiO2−x nanowires with enhanced photocatalytic activity: Facile synthesis and effects of the Ti3+ Appl. Surf. Sci. 2015;356:391–398. doi: 10.1016/j.apsusc.2015.08.029. [DOI] [Google Scholar]
- 223.Deng X., Zhang H., Guo R., Ma Q., Cui Y., Cheng X., Xie M., Cheng Q. Effect of Ti3+ on enhancing photocatalytic and photoelectrochemical properties of TiO2 nanorods/nanosheets photoelectrode. Sep. Purif. Technol. 2018;192:329–339. doi: 10.1016/j.seppur.2017.10.029. [DOI] [Google Scholar]
- 224.Mohamed M.A., Zain M.F.M., Jeffery Minggu L., Kassim M.B., Jaafar J., Saidina Amin N.A., Mastuli M.S., Wu H., Wong R.J., Ng Y.H. Bio-inspired hierarchical hetero-architectures of in-situ C-doped g-C3N4 grafted on C, N co-doped ZnO micro-flowers with booming solar photocatalytic activity. J. Ind. Eng. Chem. 2019;77:393–407. doi: 10.1016/j.jiec.2019.05.003. [DOI] [Google Scholar]
- 225.Fakhri A., Khakpour R. Synthesis and characterization of carbon or/and boron-doped CdS nanoparticles and investigation of optical and photoluminescence properties. J. Lumin. 2015;160:233–237. doi: 10.1016/j.jlumin.2014.12.019. [DOI] [Google Scholar]
- 226.Gu Q., Liu J., Gao Z., Xue C. Homogenous boron-doping in self-sensitized carbon nitride for enhanced visible-light photocatalytic activity. Chemistry-An Asian Journal. 2016;11:3169–3173. doi: 10.1002/asia.201601201. [DOI] [PubMed] [Google Scholar]
- 227.Cavalcante R.P., Dantas R.F., Wender H., Bayarri B., González O., Giménez J., Esplugas S., Machulek A. Photocatalytic treatment of metoprolol with B-doped TiO2: Effect of water matrix, toxicological evaluation and identification of intermediates. Appl. Catal. B. 2015;176–177:173–182. doi: 10.1016/j.apcatb.2015.04.007. [DOI] [Google Scholar]
- 228.Giannakas A.E., Antonopoulou M., Daikopoulos C., Deligiannakis Y., Konstantinou I. Characterization and catalytic performance of B-doped, B-N co-doped and B–N–F tri-doped TiO2 towards simultaneous Cr(VI) reduction and benzoic acid oxidation. Appl. Catal. B. 2016;184:44–54. doi: 10.1016/j.apcatb.2015.11.009. [DOI] [Google Scholar]
- 229.Zheng J., Liu Z., Liu X., Yan X., Li D., Chu W. Facile hydrothermal synthesis and characteristics of B-doped TiO2 hybrid hollow microspheres with higher photo-catalytic activity. J. Alloy. Compd. 2011;509:3771–3776. doi: 10.1016/j.jallcom.2010.12.152. [DOI] [Google Scholar]
- 230.Liu Z., Sun K., Wei M., Ma Z. Phosphorus-doped cerium vanadate nanorods with enhanced photocatalytic activity. J. Colloid Interface Sci. 2018;531:618–627. doi: 10.1016/j.jcis.2018.07.077. [DOI] [PubMed] [Google Scholar]
- 231.Farhadian N., Akbarzadeh R., Pirsaheb M., Jen T.C., Fakhri Y., Asadi A. Chitosan modified N, S-doped TiO2 and N, S-doped ZnO for visible light photocatalytic degradation of tetracycline. Int. J. Biol. Macromol. 2019;132:360–373. doi: 10.1016/j.ijbiomac.2019.03.217. [DOI] [PubMed] [Google Scholar]
- 232.Jia Y., Wu C., Lee B.W., Liu C., Kang S., Lee T., Park Y.C., Yoo R., Lee W. Magnetically separable sulfur-doped SnFe2O4/graphene nanohybrids for effective photocatalytic purification of wastewater under visible light. J. Hazard. Mater. 2017;338:447–457. doi: 10.1016/j.jhazmat.2017.05.057. [DOI] [PubMed] [Google Scholar]
- 233.Huang H., Song Y., Li N., Chen D., Xu Q., Li H., He J., Lu J. One-step in-situ preparation of N-doped TiO2@C derived from Ti3C2 MXene for enhanced visible-light driven photodegradation. Appl. Catal. B. 2019;251:154–161. doi: 10.1016/j.apcatb.2019.03.066. [DOI] [Google Scholar]
- 234.Huang W.C., Ting J.-M. Novel nitrogen-doped anatase TiO2 mesoporous bead photocatalysts for enhanced visible light response. Ceram. Int. 2017;43:9992–9997. doi: 10.1016/j.ceramint.2017.05.012. [DOI] [Google Scholar]
- 235.Suwannaruang T., Kidkhunthod P., Chanlek N., Soontaranon S., Wantala K. High anatase purity of nitrogen-doped TiO2 nanorice particles for the photocatalytic treatment activity of pharmaceutical wastewater. Appl. Surf. Sci. 2019;478:1–14. doi: 10.1016/j.apsusc.2019.01.158. [DOI] [Google Scholar]
- 236.Vaiano V., Sacco O., Sannino D., Ciambelli P. Photocatalytic removal of spiramycin from wastewater under visible light with N-doped TiO2 photocatalysts. Chem. Eng. J. 2015;261:3–8. doi: 10.1016/j.cej.2014.02.071. [DOI] [Google Scholar]
- 237.Dumrongrojthanath P., Phuruangrat A., Thongtem S., Thongtem T. Hydrothermal preparation of visible-light-driven Br-doped Bi2WO6 photocatalyst. Mater. Lett. 2017;209:501–504. doi: 10.1016/j.matlet.2017.08.089. [DOI] [Google Scholar]
- 238.Guo M., He H., Cao J., Lin H., Chen S. Novel I-doped Bi12O17Cl2 photocatalysts with enhanced photocatalytic activity for contaminants removal. Mater. Res. Bull. 2019;112:205–212. doi: 10.1016/j.materresbull.2018.12.023. [DOI] [Google Scholar]
- 239.Sekhar H., Rao D.N. Spectroscopic studies on Fe3+ doped CdS nanopowders prepared by simple coprecipitation method. J. Alloy. Compd. 2012;517:103–110. doi: 10.1016/j.jallcom.2011.12.039. [DOI] [Google Scholar]
- 240.Yan X., Xue C., Yang B., Yang G. Novel three-dimensionally ordered macroporous Fe3+-doped TiO2 photocatalysts for H2 production and degradation applications. Appl. Surf. Sci. 2017;394:248–257. doi: 10.1016/j.apsusc.2016.10.077. [DOI] [Google Scholar]
- 241.Dutta D.P., Raval P. Effect of transition metal ion (Cr3+, Mn2+ and Cu2+) doping on the photocatalytic properties of ZnWO4 nanoparticles. J. Photochem. Photobiol., A. 2018;357:193–200. doi: 10.1016/j.jphotochem.2018.02.026. [DOI] [Google Scholar]
- 242.Zou Y., Gong Y., Lin B., Mellott N.P. Photodegradation of methylene blue in the visible spectrum: An efficient W6+ ion doped anatase titania photocatalyst via a solvothermal method. Vacuum. 2016;126:63–69. doi: 10.1016/j.vacuum.2016.01.018. [DOI] [Google Scholar]
- 243.Wang J., Sun Y., Wu C., Cui Z., Rao P. Enhancing photocatalytic activity of Bi2MoO6 via surface co-doping with Ni2+ and Ti4+ ions. J. Phys. Chem. Solids. 2019;129:209–216. doi: 10.1016/j.jpcs.2019.01.014. [DOI] [Google Scholar]
- 244.Gaurav A., Beura R., Kumar J.S., Thangadurai P. Study on the effect of copper ion doping in zinc oxide nanomaterials for photocatalytic applications. Mater. Chem. Phys. 2019;230:162–171. doi: 10.1016/j.matchemphys.2019.03.056. [DOI] [Google Scholar]
- 245.Li F., Li H., Guan L.-X., Yao M.-M. Nanocrystalline Co2+/F− codoped TiO2–SiO2 composite films for environmental applications. Chem. Eng. J. 2014;252:1–10. doi: 10.1016/j.cej.2014.04.107. [DOI] [Google Scholar]
- 246.Liu Y., Zhu G., Gao J., Zhu R., Hojamberdiev M., Wang C., Wei X., Liu P. A novel synergy of Er3+/Fe3+ co-doped porous Bi5O7I microspheres with enhanced photocatalytic activity under visible-light irradiation. Appl. Catal. B. 2017;205:421–432. doi: 10.1016/j.apcatb.2016.12.061. [DOI] [Google Scholar]
- 247.Petala A., Tsikritzis D., Kollia M., Ladas S., Kennou S., Kondarides D.I. Synthesis and characterization of N-doped TiO2 photocatalysts with tunable response to solar radiation. Appl. Surf. Sci. 2014;305:281–291. doi: 10.1016/j.apsusc.2014.03.062. [DOI] [Google Scholar]
- 248.Wang M., Guo P., Zhang Y., Lv C., Liu T., Chai T., Xie Y., Wang Y., Zhu T. Synthesis of hollow lantern-like Eu(III)-doped g-C3N4 with enhanced visible light photocatalytic perfomance for organic degradation. J. Hazard. Mater. 2018;349:224–233. doi: 10.1016/j.jhazmat.2018.01.058. [DOI] [PubMed] [Google Scholar]
- 249.Que Q., Xing Y., He Z., Yang Y., Yin X., Que W. Bi2O3/Carbon quantum dots heterostructured photocatalysts with enhanced photocatalytic activity. Mater. Lett. 2017;209:220–223. doi: 10.1016/j.matlet.2017.07.115. [DOI] [Google Scholar]
- 250.Sharma S., Dutta V., Singh P., Raizada P., Rahmani-Sani A., Hosseini-Bandegharaei A., Thakur V.K. Carbon quantum dot supported semiconductor photocatalysts for efficient degradation of organic pollutants in water: A review. J. Cleaner Prod. 2019;228:755–769. doi: 10.1016/j.jclepro.2019.04.292. [DOI] [Google Scholar]
- 251.Zhang J., Kuang M., Wang J., Liu R., Xie S., Ji Z. Fabrication of carbon quantum dots/TiO2/Fe2O3 composites and enhancement of photocatalytic activity under visible light. Chem. Phys. Lett. 2019;730:391–398. doi: 10.1016/j.cplett.2019.06.011. [DOI] [Google Scholar]
- 252.Zhang Z., Wu L., Wang P., Zhang Y., Wan S., Guo X., Jin W., Zhang J. Carbon quantum dots modified La2Ti2O7 nanosheets for visible light photocatalysis. Mater. Lett. 2018;230:72–75. doi: 10.1016/j.matlet.2018.07.086. [DOI] [Google Scholar]
- 253.Zhao F., Rong Y., Wan J., Hu Z., Peng Z., Wang B. High photocatalytic performance of carbon quantum dots/TNTs composites for enhanced photogenerated charges separation under visible light. Catal. Today. 2018;315:162–170. doi: 10.1016/j.cattod.2018.02.019. [DOI] [Google Scholar]
- 254.Yuan A., Lei H., Xi F., Liu J., Qin L., Chen Z., Dong X. Graphene quantum dots decorated graphitic carbon nitride nanorods for photocatalytic removal of antibiotics. J Colloid Interface Sci. 2019;548:56–65. doi: 10.1016/j.jcis.2019.04.027. [DOI] [PubMed] [Google Scholar]
- 255.Aziz M.I., Mughal F., Naeem H.M., Zeb A., Tahir M.A., Basit M.A. Evolution of photovoltaic and photocatalytic activity in anatase-TiO2 under visible light via simplistic deposition of CdS and PbS quantum-dots. Mater. Chem. Phys. 2019;229:508–513. doi: 10.1016/j.matchemphys.2019.03.042. [DOI] [Google Scholar]
- 256.Zhu Y., Wang Y., Chen Z., Qin L., Yang L., Zhu L., Tang P., Gao T., Huang Y., Sha Z., Tang G. Visible light induced photocatalysis on CdS quantum dots decorated TiO2 nanotube arrays. Appl. Catal. A: Gen. 2015;498:159–166. doi: 10.1016/j.apcata.2015.03.035. [DOI] [Google Scholar]
- 257.Jiang R., Wu D., Lu G., Yan Z., Liu J., Zhou R., Nkoom M. Fabrication of Fe3O4 quantum dots modified BiOCl/BiVO4 p-n heterojunction to enhance photocatalytic activity for removing broad-spectrum antibiotics under visible light. J. Taiwan Inst. Chem. Eng. 2019;96:681–690. doi: 10.1016/j.jtice.2019.01.010. [DOI] [Google Scholar]
- 258.Lin X., Liu C., Wang J., Yang S., Shi J., Hong Y. Graphitic carbon nitride quantum dots and nitrogen-doped carbon quantum dots co-decorated with BiVO4 microspheres: A ternary heterostructure photocatalyst for water purification. Sep. Purif. Technol. 2019;226:117–127. doi: 10.1016/j.seppur.2019.05.093. [DOI] [Google Scholar]
- 259.Qian X., Chen Z., Yang X., Zhao W., Liu C., Sun T., Zhou D., Yang Q., Wei G., Fan M. Perovskite cesium lead bromide quantum dots: a new efficient photocatalyst for degrading antibiotic residues in organic system. J. Clean. Prod. 2019;249 doi: 10.1016/j.jclepro.2019.119335. [DOI] [Google Scholar]
- 260.Liu Y., Li C.F., Li X.Y., Yu W.B., Dong W.D., Zhao H., Hu Z.Y., Deng Z., Wang C., Wu S.J., Chen H., Liu J., Wang Z., Chen L.H., Li Y., Su B.L. Molybdenum disulfide quantum dots directing zinc indium sulfide heterostructures for enhanced visible light hydrogen production. J. Colloid Interface Sci. 2019;551:111–118. doi: 10.1016/j.jcis.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 261.Lu K.-Q., Quan Q., Zhang N., Xu Y.-J. Multifarious roles of carbon quantum dots in heterogeneous photocatalysis. Journal of Energy Chemistry. 2016;25:927–935. doi: 10.1016/j.jechem.2016.09.015. [DOI] [Google Scholar]
- 262.Liu J., Huo J., Zhang M., Dong X. Branched TiO2 nanorod arrays owning the surface anatase/rutile junctions for dye sensitized solar cells. Thin Solid Films. 2017;623:25–30. doi: 10.1016/j.tsf.2016.12.042. [DOI] [Google Scholar]
- 263.Liu J., Yu X., Liu Q., Liu R., Shang X., Zhang S., Li W., Zheng W., Zhang G., Cao H., Gu Z. Surface-phase junctions of branched TiO2 nanorod arrays for efficient photoelectrochemical water splitting. Appl. Catal. B. 2014;158–159:296–300. doi: 10.1016/j.apcatb.2014.04.032. [DOI] [Google Scholar]
- 264.Pang L.-X., Wang X.-Y., Tang X.-D. Enhanced photocatalytic performance of porous TiO2 nanobelts with phase junctions. Solid State Sci. 2015;39:29–33. doi: 10.1016/j.solidstatesciences.2014.11.004. [DOI] [Google Scholar]
- 265.Qiu Y., Ouyang F. Fabrication of TiO2 hierarchical architecture assembled by nanowires with anatase/TiO2(B) phase-junctions for efficient photocatalytic hydrogen production. Appl. Surf. Sci. 2017;403:691–698. doi: 10.1016/j.apsusc.2017.01.255. [DOI] [Google Scholar]
- 266.Wang X., Shen S., Feng Z., Li C. Time-resolved photoluminescence of anatase/rutile TiO2 phase junction revealing charge separation dynamics. Chin. J. Catal. 2016;37:2059–2068. doi: 10.1016/s1872-2067(16)62574-3. [DOI] [Google Scholar]
- 267.Zhou Y., Chen C., Wang N., Li Y., Ding H. Stable Ti3+ Self-Doped Anatase-Rutile Mixed TiO2 with Enhanced Visible Light Utilization and Durability. The Journal of Physical Chemistry C. 2016;120:6116–6124. doi: 10.1021/acs.jpcc.6b00655. [DOI] [Google Scholar]
- 268.Zhou Y.-D., Zhao Z.-Y. Interfacial structure and properties of TiO2 phase junction studied by DFT calculations. Appl. Surf. Sci. 2019;485:8–21. doi: 10.1016/j.apsusc.2019.04.193. [DOI] [Google Scholar]
- 269.Liu R., Li H., Duan L., Shen H., Zhang Q., Zhao X. Influences of annealing atmosphere on phase transition temperature, optical properties and photocatalytic activities of TiO2 phase-junction microspheres. J. Alloy. Compd. 2019;789:1015–1021. doi: 10.1016/j.jallcom.2019.02.198. [DOI] [Google Scholar]
- 270.Byrne C., Moran L., Hermosilla D., Merayo N., Blanco Á., Rhatigan S., Hinder S., Ganguly P., Nolan M., Pillai S.C. Effect of Cu doping on the anatase-to-rutile phase transition in TiO2 photocatalysts: theory and experiments. Appl. Catal. B. 2019;246:266–276. doi: 10.1016/j.apcatb.2019.01.058. [DOI] [Google Scholar]
- 271.Ai Z., Zhao G., Zhong Y., Shao Y., Huang B., Wu Y., Hao X. Phase junction CdS: high efficient and stable photocatalyst for hydrogen generation. Appl. Catal. B. 2018;221:179–186. doi: 10.1016/j.apcatb.2017.09.002. [DOI] [Google Scholar]
- 272.Shi Y., Luo L., Zhang Y., Chen Y., Wang S., Li L., Long Y., Jiang F. Synthesis and characterization of α/β-Bi2O3 with enhanced photocatalytic activity for 17α-ethynylestradiol. Ceram. Int. 2017;43:7627–7635. doi: 10.1016/j.ceramint.2017.03.057. [DOI] [Google Scholar]
- 273.Zhao W., Liu X., Yang X., Liu C., Qian X., Sun T., Chang W., Zhang J., Chen Z. Synthesis of Novel 1T/2H-MoS2 from MoO3 Nanowires with Enhanced Photocatalytic Performance. Nanomaterials. 2020;10:1124. doi: 10.3390/nano10061124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Chen Y., He J., Li J., Mao M., Yan Z., Wang W., Wang J. Hydrilla derived ZnIn2S4 photocatalyst with hexagonal-cubic phase junctions: A bio-inspired approach for H2 evolution. Catal. Commun. 2016;87:1–5. doi: 10.1016/j.catcom.2016.08.031. [DOI] [Google Scholar]
- 275.Ma Y., Wang X., Li C. Charge separation promoted by phase junctions in photocatalysts. Chin. J. Catal. 2015;36:1519–1527. doi: 10.1016/s1872-2067(15)60874-9. [DOI] [Google Scholar]
- 276.Yang K., Li X., Yu C., Zeng D., Chen F., Zhang K., Huang W., Ji H. Review on heterophase/homophase junctions for efficient photocatalysis: The case of phase transition construction. Chin. J. Catal. 2019;40:796–818. doi: 10.1016/s1872-2067(19)63290-0. [DOI] [Google Scholar]
- 277.Jia Z.-J., Zhao Z.-Y. Properties of phase transition and interfaces of Cu2ZnSnS4 with hetero-phase junctions. Appl. Surf. Sci. 2019;481:1044–1052. doi: 10.1016/j.apsusc.2019.03.201. [DOI] [Google Scholar]
- 278.Lv C., Chen G., Zhou X., Zhang C., Wang Z., Zhao B., Li D. Oxygen-Induced Bi5+-Self-Doped Bi4V2O11 with a p-n Homojunction Toward Promoting the Photocatalytic Performance. ACS Appl Mater Interfaces. 2017;9:23748–23755. doi: 10.1021/acsami.7b05302. [DOI] [PubMed] [Google Scholar]
- 279.Pan J., Liu G. Facet control of photocatalysts for water splitting. Semiconductors for Photocatalysis. 2017:349–391. [Google Scholar]
- 280.Tan H.L., Wen X., Amal R., Ng Y.H. BiVO4 010 and 110 relative exposure extent: governing factor of surface charge population and photocatalytic activity. J. Phys. Chem. Lett. 2016;7:1400–1405. doi: 10.1021/acs.jpclett.6b00428. [DOI] [PubMed] [Google Scholar]
- 281.Wu H., Tan H.L., Toe C.Y., Scott J., Wang L., Amal R., Ng Y.H. Photocatalytic and photoelectrochemical systems: similarities and differences. Adv. Mater. 2020;32:1904717. doi: 10.1002/adma.201904717. [DOI] [PubMed] [Google Scholar]
- 282.He F., Wang S., Zhao H., Wang Y., Zhang J., Yan Q., Dong P., Tai Z., Chen L., Wang Y., Zhao C. Construction of Schottky-type Ag-loaded fiber-like carbon nitride photocatalysts for tetracycline elimination and hydrogen evolution. Appl. Surf. Sci. 2019;485:70–80. doi: 10.1016/j.apsusc.2019.04.164. [DOI] [Google Scholar]
- 283.Gołąbiewska A., Lisowski W., Jarek M., Nowaczyk G., Michalska M., Jurga S., Zaleska-Medynska A. The effect of metals content on the photocatalytic activity of TiO2 modified by Pt/Au bimetallic nanoparticles prepared by sol-gel method. Molecular Catalysis. 2017;442:154–163. doi: 10.1016/j.mcat.2017.09.004. [DOI] [Google Scholar]
- 284.Yang X., Chen Z., Zhou D., Zhao W., Qian X., Yang Q., Sun T., Shen C. Ultra-low Au-Pt Co-decorated TiO2 nanotube arrays: construction and its improved visible-light-induced photocatalytic properties. Sol. Energy Mater. Sol. Cells. 2019;201 doi: 10.1016/j.solmat.2019.110065. [DOI] [Google Scholar]
- 285.Li W., Li B., Meng M., Cui Y., Wu Y., Zhang Y., Dong H., Feng Y. Bimetallic Au/Ag decorated TiO2 nanocomposite membrane for enhanced photocatalytic degradation of tetracycline and bactericidal efficiency. Appl. Surf. Sci. 2019;487:1008–1017. doi: 10.1016/j.apsusc.2019.05.162. [DOI] [Google Scholar]
- 286.Lisowski P., Colmenares J.C., Łomot D., Chernyayeva O., Lisovytskiy D. Preparation by sonophotodeposition method of bimetallic photocatalysts Pd-Cu/TiO2 for sustainable gaseous selective oxidation of methanol to methyl formate. J. Mol. Catal. A: Chem. 2016;411:247–256. doi: 10.1016/j.molcata.2015.10.031. [DOI] [PubMed] [Google Scholar]
- 287.Reddy K.L., Kumar S., Kumar A., Krishnan V. Wide spectrum photocatalytic activity in lanthanide-doped upconversion nanophosphors coated with porous TiO2 and Ag-Cu bimetallic nanoparticles. J. Hazard. Mater. 2019;367:694–705. doi: 10.1016/j.jhazmat.2019.01.004. [DOI] [PubMed] [Google Scholar]
- 288.Huang H., Liu C., Ou H., Ma T., Zhang Y. Self-sacrifice transformation for fabrication of type-I and type-II heterojunctions in hierarchical BixOyIz/g-C3N4 for efficient visible-light photocatalysis. Appl. Surf. Sci. 2019;470:1101–1110. doi: 10.1016/j.apsusc.2018.11.193. [DOI] [Google Scholar]
- 289.Zhu Y., Wan T., Wen X., Chu D., Jiang Y. Tunable Type I and II heterojunction of CoOx nanoparticles confined in g-C3N4 nanotubes for photocatalytic hydrogen production. Appl. Catal. B. 2019;244:814–822. doi: 10.1016/j.apcatb.2018.12.015. [DOI] [Google Scholar]
- 290.Bard A.J. Photoelectrochemistry and heterogeneous photo-catalysis at semiconductors. Journal of Photochemistry. 1979;10:59–75. doi: 10.1016/0047-2670(79)80037-4. [DOI] [Google Scholar]
- 291.Li H., Tu W., Zhou Y., Zou Z. Z-Scheme Photocatalytic Systems for Promoting Photocatalytic Performance: Recent Progress and Future Challenges. Adv. Sci. 2016;3:1500389. doi: 10.1002/advs.201500389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Zhou P., Yu J., Jaroniec M. All-solid-state Z-scheme photocatalytic systems. Adv. Mater. 2014;26:4920–4935. doi: 10.1002/adma.201400288. [DOI] [PubMed] [Google Scholar]
- 293.Low J., Jiang C., Cheng B., Wageh S., Al-Ghamdi A.A., Yu J. A review of direct Z-scheme photocatalysts. Small Methods. 2017;1:1700080. doi: 10.1002/smtd.201700080. [DOI] [Google Scholar]
- 294.Low J., Yu J., Jaroniec M., Wageh S., Al-Ghamdi A.A. Heterojunction photocatalysts. Adv. Mater. 2017;29:1601694. doi: 10.1002/adma.201601694. [DOI] [PubMed] [Google Scholar]
- 295.Zhou D., Yu B., Chen Q., Shi H., Zhang Y., Li D., Yang X., Zhao W., Liu C., Wei G., Chen Z. Improved visible light photocatalytic activity on Z-scheme g-C3N4 decorated TiO2 nanotube arrays by a simple impregnation method. Mater. Res. Bull. 2020;124 doi: 10.1016/j.materresbull.2019.110757. [DOI] [Google Scholar]
- 296.Zhou D., Chen Z., Yang Q., Shen C., Tang G., Zhao S., Zhang J., Chen D., Wei Q., Dong X. Facile construction of g-C3N4 nanosheets/TiO2 nanotube arrays as Z-scheme photocatalyst with enhanced visible-light performance. ChemCatChem. 2016;8:3064–3073. doi: 10.1002/cctc.201600828. [DOI] [Google Scholar]
- 297.Tang Y., Zhang D., Li Y., Huang B., Li H., Pu X., Geng Y. Fabrication of magnetically recoverable Ag/CuNb2O6/CuFe2O4 ternary heterojunction composite for highly efficient photocatalytic degradation of pollutants. Sep. Purif. Technol. 2019;220:78–88. doi: 10.1016/j.seppur.2019.03.049. [DOI] [Google Scholar]
- 298.Wang X., Fan H., Ren P. Effects of exposed facets on photocatalytic properties of WO3. Adv. Powder Technol. 2017;28:2549–2555. doi: 10.1016/j.apt.2017.07.005. [DOI] [Google Scholar]
- 299.Peng B., Ang P.K., Loh K.P. Two-dimensional dichalcogenides for light-harvesting applications. Nano Today. 2015;10:128–137. doi: 10.1016/j.nantod.2015.01.007. [DOI] [Google Scholar]
- 300.Li Y., Gao C., Long R., Xiong Y. Photocatalyst design based on two-dimensional materials. Mater. Today Chem. 2019;11:197–216. doi: 10.1016/j.mtchem.2018.11.002. [DOI] [Google Scholar]
- 301.Tan C., Cao X., Wu X.J., He Q., Yang J., Zhang X., Chen J., Zhao W., Han S., Nam G.H., Sindoro M., Zhang H. Recent Advances in Ultrathin Two-Dimensional Nanomaterials. Chem. Rev. 2017;117:6225–6331. doi: 10.1021/acs.chemrev.6b00558. [DOI] [PubMed] [Google Scholar]
- 302.Yang K., Li J., Zhou L., Zhang T., Fu L. Synthetic strategies of two-dimensional porous materials towards highly effective catalysts. FlatChem. 2019;15 doi: 10.1016/j.flatc.2019.100109. [DOI] [Google Scholar]
- 303.Chia X., Pumera M. Inverse Opal-like Porous MoSex Films for Hydrogen Evolution Catalysis: Overpotential-Pore Size Dependence. ACS Appl. Mater. Interfaces. 2018;10:4937–4945. doi: 10.1021/acsami.7b17800. [DOI] [PubMed] [Google Scholar]
- 304.Lei Z., Xu S., Wu P. Ultra-thin and porous MoSe2 nanosheets: facile preparation and enhanced electrocatalytic activity towards the hydrogen evolution reaction. PCCP. 2016;18:70–74. doi: 10.1039/c5cp06483j. [DOI] [PubMed] [Google Scholar]
- 305.Li Y., Yin K., Wang L., Lu X., Zhang Y., Liu Y., Yan D., Song Y., Luo S. Engineering MoS2 nanomesh with holes and lattice defects for highly active hydrogen evolution reaction. Appl. Catal. B. 2018;239:537–544. doi: 10.1016/j.apcatb.2018.05.080. [DOI] [Google Scholar]
- 306.Wang L., Li X., Zhang J., Liu H., Jiang W., Zhao H. One-pot synthesis of holey MoS2 nanostructures as efficient electrocatalysts for hydrogen evolution. Appl. Surf. Sci. 2017;396:1719–1725. doi: 10.1016/j.apsusc.2016.11.235. [DOI] [Google Scholar]
- 307.Wang S., Li H., Sawada H., Allen C.S., Kirkland A.I., Grossman J.C., Warner J.H. Atomic structure and formation mechanism of sub-nanometer pores in 2D monolayer MoS2. Nanoscale. 2017;9:6417–6426. doi: 10.1039/c7nr01127j. [DOI] [PubMed] [Google Scholar]
- 308.Ge J., Zhang L., Xu J., Liu Y., Jiang D., Du P. Nitrogen photofixation on holey g-C3N4 nanosheets with carbon vacancies under visible-light irradiation. Chin. Chem. Lett. 2019;31:792–796. doi: 10.1016/j.cclet.2019.05.030. [DOI] [Google Scholar]
- 309.Guo S., Zhu Y., Yan Y., Min Y., Fan J., Xu Q. Holey structured graphitic carbon nitride thin sheets with edge oxygen doping via photo-Fenton reaction with enhanced photocatalytic activity. Appl. Catal. B. 2016;185:315–321. doi: 10.1016/j.apcatb.2015.11.030. [DOI] [Google Scholar]
- 310.Jiang Y., Sun Z., Tang C., Zhou Y., Zeng L., Huang L. Enhancement of photocatalytic hydrogen evolution activity of porous oxygen doped g-C3N4 with nitrogen defects induced by changing electron transition. Appl. Catal. B. 2019;240:30–38. doi: 10.1016/j.apcatb.2018.08.059. [DOI] [Google Scholar]
- 311.Kang S., Zhang L., Yin C., Li Y., Cui L., Wang Y. Fast flash frozen synthesis of holey few-layer g-C3N4 with high enhancement of photocatalytic reactive oxygen species evolution under visible light irradiation. Appl. Catal. B. 2017;211:266–274. doi: 10.1016/j.apcatb.2017.04.050. [DOI] [Google Scholar]
- 312.Li Y., Jin R., Xing Y., Li J., Song S., Liu X., Li M., Jin R. Macroscopic Foam-Like Holey Ultrathin g-C3N4 Nanosheets for Drastic Improvement of Visible-Light Photocatalytic Activity. Adv. Energy Mater. 2016;6:1601273. doi: 10.1002/aenm.201601273. [DOI] [Google Scholar]
- 313.Li Y., Ruan Z., He Y., Li J., Li K., Jiang Y., Xu X., Yuan Y., Lin K. In situ fabrication of hierarchically porous g-C3N4 and understanding on its enhanced photocatalytic activity based on energy absorption. Appl. Catal. B. 2018;236:64–75. doi: 10.1016/j.apcatb.2018.04.082. [DOI] [Google Scholar]
- 314.Liang Q., Li Z., Huang Z.-H., Kang F., Yang Q.-H. Holey Graphitic Carbon Nitride Nanosheets with Carbon Vacancies for Highly Improved Photocatalytic Hydrogen Production. Adv. Funct. Mater. 2015;25:6885–6892. doi: 10.1002/adfm.201503221. [DOI] [Google Scholar]
- 315.Liu Q., Wang X., Yang Q., Zhang Z., Fang X. A novel route combined precursor-hydrothermal pretreatment with microwave heating for preparing holey g-C3N4 nanosheets with high crystalline quality and extended visible light absorption. Appl. Catal. B. 2018;225:22–29. doi: 10.1016/j.apcatb.2017.11.044. [DOI] [Google Scholar]
- 316.She X., Liu L., Ji H., Mo Z., Li Y., Huang L., Du D., Xu H., Li H. Template-free synthesis of 2D porous ultrathin nonmetal-doped g-C3N4 nanosheets with highly efficient photocatalytic H2 evolution from water under visible light. Appl. Catal. B. 2016;187:144–153. doi: 10.1016/j.apcatb.2015.12.046. [DOI] [Google Scholar]
- 317.Shi L., Chang K., Zhang H., Hai X., Yang L., Wang T., Ye J. Drastic Enhancement of Photocatalytic Activities over Phosphoric Acid Protonated Porous g-C3N4 Nanosheets under Visible Light. Small. 2016;12:4431–4439. doi: 10.1002/smll.201601668. [DOI] [PubMed] [Google Scholar]
- 318.Song T., Zhang P., Wang T., Ali A., Zeng H. Alkali-assisted fabrication of holey carbon nitride nanosheet with tunable conjugated system for efficient visible-light-driven water splitting. Appl. Catal. B. 2018;224:877–885. doi: 10.1016/j.apcatb.2017.11.039. [DOI] [Google Scholar]
- 319.Song T., Zhang P., Wang T., Ali A., Zeng H. Vopor-polymerization strategy to carbon-rich holey few-layer carbon nitride nanosheets with large domain size for superior photocatalytic hydrogen evolution. Appl. Surf. Sci. 2019;464:195–204. doi: 10.1016/j.apsusc.2018.09.062. [DOI] [Google Scholar]
- 320.Zhang D., Guo Y., Zhao Z. Porous defect-modified graphitic carbon nitride via a facile one-step approach with significantly enhanced photocatalytic hydrogen evolution under visible light irradiation. Appl. Catal. B. 2018;226:1–9. doi: 10.1016/j.apcatb.2017.12.044. [DOI] [Google Scholar]
- 321.Zhang J.-W., Gong S., Mahmood N., Pan L., Zhang X., Zou J.-J. Oxygen-doped nanoporous carbon nitride via water-based homogeneous supramolecular assembly for photocatalytic hydrogen evolution. Appl. Catal. B. 2018;221:9–16. doi: 10.1016/j.apcatb.2017.09.003. [DOI] [Google Scholar]
- 322.Zhao S., Zhang Y., Zhou Y., Wang Y., Qiu K., Zhang C., Fang J., Sheng X. Facile one-step synthesis of hollow mesoporous g-C3N4 spheres with ultrathin nanosheets for photoredox water splitting. Carbon. 2018;126:247–256. doi: 10.1016/j.carbon.2017.10.033. [DOI] [Google Scholar]