Abstract
In the midst of the COVID-19 pandemic, we herein report the case of an elderly female with multiple comorbidities coming with typical symptoms of the viral infection in addition to the unusual presentation of bradycardia due to complete heart block requiring pacemaker placement.
This may be a rare complication of the disease but one has to keep a high index of suspicion since this virus has an ability to affect multiple organ systems with many ways yet to be uncovered.
<Learning objective: 1. COVID-19 might affect the conduction system of the heart as part of its disease process. 2. Future studies are needed to address the mechanism of which COVID-19 affects the conduction system to improve the recognition of this phenomenon. 3. It is pertinent to recognize any metabolic and pharmacological risk factors related to conduction block in patients with COVID-19, and to provide close monitoring to high-risk patients in order to recognize this rare complication and treat it early on.>
Keywords: COVID-19, Complete heart block, Bradycardia
Introduction
The novel coronavirus (COVID-19) has been associated with arrhythmias and cardiomyopathy in some reports. We report a case of new complete heart block in the setting of COVID-19 infection.
Case report
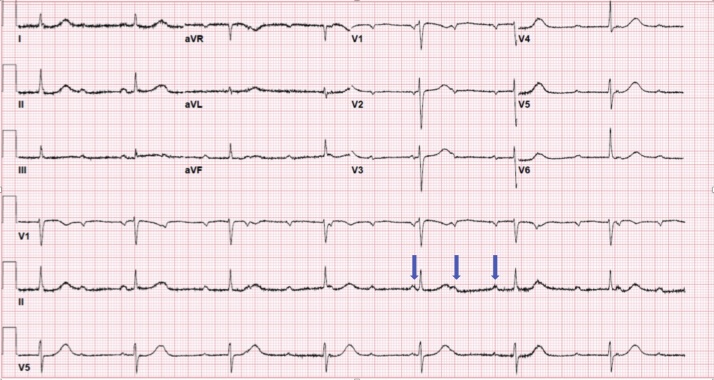
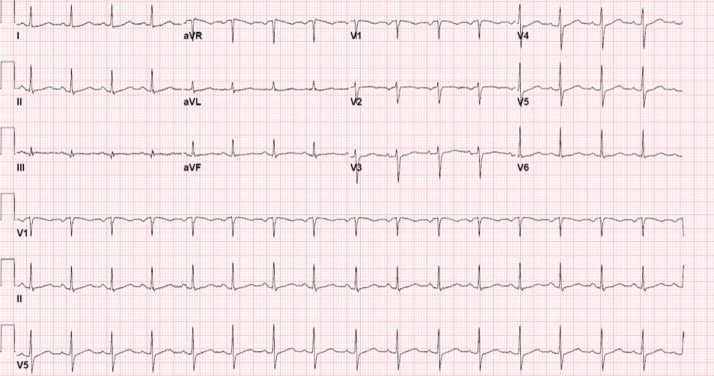
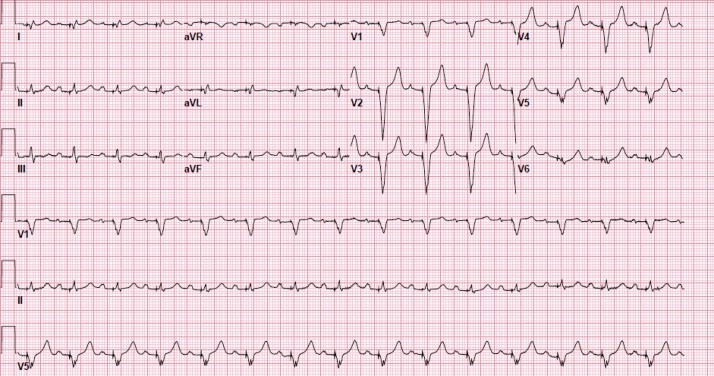
A 71-year-old female patient, who resides in a nursing home, with past medical history of Parkinson’s disease, tardive dyskinesia, type II diabetes mellitus, and bipolar disorder presented to our hospital with non-productive cough, shortness of breath on minimal exertion, and orthopnea for three days prior to her admission to the hospital. She also reported loss of smell and taste sensation for the past month. She was found to have new onset slow heart rate during routine vital signs check at the nursing home that was not noticed previously one day prior to her admission to the hospital. She never experienced similar symptoms in the past. Her medications included iloperidone, valbenazine, baclofen, glyburide, metformin, and sitagliptin. On presentation, her vital signs showed a blood pressure of 144/50 mmHg, heart rate of 42 beats per minute (BPM), respiratory rate of 16 per minute, oxygen saturation of 97% on room air, and temperature of 36.7 °C. Physical examination revealed intact mental status, normal jugular venous pressure, clear lung sounds, normal S1 and S2 heart sounds with regular bradycardia, soft abdomen, and no lower extremities swelling. Her electrocardiogram (ECG) showed complete heart block with junctional escape rhythm at a ventricular rate of 42 BPM and a dissociated atrial rate of 100 BPM (Fig. 1). Differential diagnosis of the complete heart block at that point included myocardial infarction, electrolyte abnormality, or infectious etiology. Chest X-ray showed clear chest. Laboratory results were significant for leukocytosis (white blood cell count 18.3 K/μL), anemia (10.4 g/dL), hyponatremia (Na 123 mmol/L), and elevated inflammatory markers with C-reactive protein of 109 mg/L and fibrinogen of 606 mg/dL (reference range <5.1 mg/L and 212–516 mg/dL, respectively). Otherwise, her venous blood gas, thyroid function, high-sensitivity troponin-I, brain natriuretic protein, and electrolytes were within normal ranges. COVID-19 nasal swab reverse transcription polymerase chain reaction (RT-PCR) tested positive for the infection. She denied using any atrioventricular blocking agents or having previous heart problems. A review of her home medications did not uncover any adverse negative dromotropic effect. Transthoracic echocardiography showed normal biventricular wall motion, left ventricular ejection fraction of 70%, thickened mitral valve leaflets, and dilated left atrium. During her first hospital day, the patient remained hemodynamically stable with a heart rate in the 40–45 BPM range on telemetry, complete heart block, and junctional escape rhythm. Reviewing her ECG from one year previously showed normal sinus rhythm (Fig. 2). The patient underwent a dual chamber pacemaker implantation on the following day. Her symptoms of shortness of breath and orthopnea resolved after placing the dual chamber pace maker but her non-productive cough persisted. She spent 8 days in the hospital, where she remained asymptomatic except for having a cough, until a repeat RT-PCR for COVID-19 tested negative for the virus before she was discharged back to the nursing home without posing the risk of infecting other residents. Her inflammatory markers started to trend toward normal limits at the time of discharge. The patient was seen in the clinic one month after discharge where she denied any symptoms and her cough and loss of smell sensation had recovered. Her device interrogation revealed ventricular pacing at 100% of the time and her repeat ECG showed atrial-sensed and ventricular-paced rhythm (Fig. 3).
Fig. 1.
Complete heart block with junctional escape ventricular rhythm at a rate of 42 BPM and a dissociated atrial rate at 100 BPM (blue arrows).
Fig. 2.
Previous electrocardiogram for the same patient before COVID-19 infection demonstrating normal sinus rhythm at a rate of 98 BPM.
Fig. 3.
Follow-up electrocardiogram after one month of discharge demonstrating atrial-sensed and ventricular-paced rhythm at 90 BPM.
Discussion
The COVID-19 virus has caused a widespread pandemic infecting millions of people. There is evidence that the virus targets angiotensin-converting enzyme 2 (ACE-2) receptors found in type 1 and type 2 pneumocytes in the respiratory tract [1]. Cardiac involvement has been reported with varying incidence as one of the manifestations that confer a worse prognosis [2]. It has been reported that there was a correlation between paradoxical prolongation or lack of shortening of the PR interval with increasing heart rate and higher mortality and intubation rate in COVID-19 patients, suggesting a worse prognosis when conduction abnormalities are present [3]. Cardiac arrhythmias have been reported in association with cardiac injury [2], [4], [5]. In a large physician survey predominantly involving electrophysiologists, sinus bradycardia and complete heart block were the most observed bradyarrhythmia in hospitalized patients [6]. The exact mechanism as to how COVID-19 causes cardiac injury remains unclear. A systemic inflammatory response with a cytokine surge as seen in our patient has been suggested to play a role in this injury [7]. Troponin elevation and contractility dysfunction suggest myocardial inflammation and tissue damage [8]. Hypercoagulable states and plaque destabilization causing coronary events, and demand ischemia in the setting of severe hypoxia related to respiratory disease are other suggested mechanisms for the COVID-19 related cardiac injury [8]. There have only been few reported cases of complete heart block [4], [5]. It is difficult to determine if cardiac arrhythmias that are reported in patient cohorts are related to myocarditis, electrolyte abnormalities, or medication side effects as many of the medications administered to treat the infection are associated with arrhythmia [6]. This patient presented with complete heart block and was not given any therapeutics for COVID-19 prior to her hospitalization suggesting a different etiology causing her heart block. Injury to the conduction system can be related to direct virus invasion. However, this seems unlikely given no clear evidence of the viral RNA presence on cardiac biopsies [7]. While this patient had risk factors for coronary artery disease, including diabetes mellitus and her advanced age which can contribute to conduction abnormalities, there was a low index of suspicion for a coronary artery ischemic event to have been the main culprit of her complete heart block. This was based on the lack of chest pain, negative cardiac biomarkers, normal ventricular wall motion on echocardiogram, and the lack of ischemic changes on her ECG. The timing of the complete heart block happening after one month of her first reported COVID-19 symptom makes the coincidental occurrence of the complete heart block and the COVID-19 infection less likely, and raises the suspicion that both diseases are related to each other. A cardiac biopsy from a 50-year-old male patient infected with COVID-19 revealed interstitial mononuclear inflammatory cell infiltrate suggesting an inflammatory process in myocytes [9]. This process is typical of myocarditis which has been associated in the past with conduction abnormalities [10]. The patient’s elevated inflammatory markers suggest an inflammatory response possibly causing myocarditis. This patient however presents a diagnostic challenge given the fact that her cardiac enzymes were normal. Her echocardiogram did not show any evidence of cardiac dysfunction. During her one-month follow-up, the complete heart block did not recover despite her recovery form COVID-19 infection and the associated inflammatory state of the viral infection. She will need more long-term follow-up to determine if her conduction system will recover at some point in the future. It would be interesting to look into the viral effects of COVID-19 that are selective to myocardial conductive tissue in the future to determine the pathophysiology of this patient’s disease process and whether the conduction system damage is permanent after the clearance of the virus and the resolution of the inflammation.
Conclusion
The novel COVID-19 virus infection has been expanding its clinical spectrum and has affected the cardiovascular system in different patterns. This rare case of new onset complete heart block in the setting of COVID-19 infection adds more knowledge to the association between this virus and the cardiovascular system. It is pertinent to closely address any metabolic and pharmacological risk factors related to conduction block in patients infected with COVID-19, and to monitor patients who have other risk factors for conduction block, whenever possible, in cardiac monitored units in order to recognize this rare complication and to reverse it early on.
Conflict of interest
The authors confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Acknowledgement
The patient is warmly thanked for permitting the publication of his case.
References
- 1.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C. Pathological findings of COVID-19 associated with acute respiratory distress syndrome [published correction appears in Lancet Respir Med. 2020 Feb 25] Lancet Respir Med. 2020;8(4):420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pavri B.B., Kloo J., Farzad D., Riley J.M. Behavior of the PR interval with increasing heart rate in patients with COVID-19. Heart Rhythm. 2020 doi: 10.1016/j.hrthm.2020.06.009. S1547-5271(20)30551-30558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azarkish M., Laleh Far V., Eslami M., Mollazadeh R. Transient complete heart block in a patient with critical COVID-19. Eur Heart J. 2020;41:2131. doi: 10.1093/eurheartj/ehaa307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El Assaad I., Hood-Pishchany M.I., Kheir J., Mistry K., Dixit A., Halyabar O. Complete heart block, severe ventricular dysfunction and myocardial inflammation in a child with COVID-19 infection. JACC Case Rep. 2020 doi: 10.1016/j.jaccas.2020.05.030. PII:S2666-0849(20)30486-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gopinathannair R., Merchant F.M., Lakkireddy D.R., Etheridge S.P., Feigofsky S., Han J.K. COVID-19 and cardiac arrhythmias: a global perspective on arrhythmia characteristics and management strategies. J Interv Card Electrophysiol. 2020:1–8. doi: 10.1007/s10840-020-00789-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basu-Ray I., Nk Almaddah, Adeboye A., Soos M.P. Cardiac manifestations of coronavirus (COVID-19) StatPearls. 2020;(April) [PubMed] [Google Scholar]
- 8.Babapoor-Farrokhran S., Gill D., Walker J., Rasekhi R.T., Bozorgnia B., Amanullah A. Myocardial injury and COVID-19: possible mechanisms. Life Sci. 2020;253 doi: 10.1016/j.lfs.2020.117723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xu Z., Shi L., Wang Y., Zhang J., Huang L., Zhang C. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420–422. doi: 10.1016/S2213-2600(20)30076-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tse G., Yeo J.M., Chan Y.W., Lai E.T.H., Yan B.P. What is the arrhythmic substrate in viral myocarditis? Insights from clinical and animal studies. Front Physiol. 2016;7:308. doi: 10.3389/fphys.2016.00308. [DOI] [PMC free article] [PubMed] [Google Scholar]