Abstract
Individuals with diabetes suffering from coronavirus disease 2019 (COVID-19) exhibit increased morbidity and mortality compared with individuals without diabetes. In this Perspective, we critically evaluate and argue that this is due to a dysregulated renin-angiotensin system (RAS). Previously, we have shown that loss of angiotensin-I converting enzyme 2 (ACE2) promotes the ACE/angiotensin-II (Ang-II)/angiotensin type 1 receptor (AT1R) axis, a deleterious arm of RAS, unleashing its detrimental effects in diabetes. As suggested by the recent reports regarding the pathogenesis of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), upon entry into the host, this virus binds to the extracellular domain of ACE2 in nasal, lung, and gut epithelial cells through its spike glycoprotein subunit S1. We put forth the hypothesis that during this process, reduced ACE2 could result in clinical deterioration in COVID-19 patients with diabetes via aggravating Ang-II–dependent pathways and partly driving not only lung but also bone marrow and gastrointestinal pathology. In addition to systemic RAS, the pathophysiological response of the local RAS within the intestinal epithelium involves mechanisms distinct from that of RAS in the lung; however, both lung and gut are impacted by diabetes-induced bone marrow dysfunction. Careful targeting of the systemic and tissue RAS may optimize clinical outcomes in subjects with diabetes infected with SARS-CoV-2.
Introduction
This Perspective focuses on providing an overview of recent studies describing the impact of the coronavirus disease 2019 (COVID-19) pandemic on individuals with diabetes and several possible mechanisms for why individuals with diabetes represent a particularly at-risk population. In December 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was identified as the pathogen responsible for the outbreak that began in Wuhan, China, and rapidly spread throughout China, Europe, and the U.S. Currently, the SARS-CoV-2 virus has infected more than 12 million individuals worldwide, with more than 555,000 COVID-19 cases resulting in death, and the number of individuals becoming infected is increasing. Thus far, SARS-CoV-2 mechanisms of infectivity remain incompletely understood. Some insight, however, has been provided by the previous pandemic of SARS-CoV in 2002, but the brutality of COVID-19 has raised many unanswered questions and the pace of science needs to increase. Here, we put forth the argument that a dysregulated renin-angiotensin system (RAS), typically seen in individuals with diabetes, increases the risk of a poor clinical outcome following COVID-19 infection.
Clinical Burden of COVID-19 in Patients With Diabetes
Conditions associated with increased morbidity and mortality in individuals infected with SARS-CoV-2 are the presence of diabetes, hypertension, cardiovascular disease, and severe obesity (BMI ≥40 kg/m2) (1–3). Considering the high prevalence of hypertension, cardiovascular disease, and obesity in individuals with diabetes, it is difficult to know how diabetes alone directly contributes to the increased risk of adverse outcomes following SARS-CoV-2 infection. Studies indicate that 12–16% of individuals with severe infections have diabetes (1,2). However, a recent meta-analysis of six clinical studies involving 1,687 COVID-19 patients provided evidence that individuals with diabetes exhibited a similar prevalence of being infected with SARS-CoV-2 as the overall population, but presence of diabetes was a critical comorbidity that increased the risk of a poor outcome (4). Certain racial groups such as African Americans and Native Americans are highly prone to developing diabetes and experience disparities in health care making them particularly vulnerable to COVID-19 (5). However, to date there is a paucity of data regarding comorbidities, COVID-19 outcomes, and mechanisms that modulate viral pathogenesis. In this Perspective, we bring attention to specific factors that may complicate COVID-19 in individuals with diabetes including 1) the presence of bone marrow changes (myeloidosis) that predispose those with diabetes to an excessive proinflammatory response (cytokine storm) and contribute to insulin resistance and reduced vascular repair, and worsening function of the heart, kidney, and systemic vasculature as a whole; 2) increased circulating furin levels that could cleave the spike protein and increase infectivity of SARS-CoV-2; 3) dysregulated autophagy that may promote replication and/or reduce viral clearance; and 4) gut dysbiosis that leads to widespread systemic inflammation, increased gut glucose and sodium absorption, and reduced tryptophan and other key amino acid absorption needed for incretin secretion and glucose homeostasis. Central to each of these dysfunctions is the dysregulated RAS, in particular, the global loss of ACE2, which we propose is a unifying mechanism that could lead to the increased risk of morbidity and mortality in individuals with diabetes presenting with COVID-19.
Biochemistry and Physiology of RAS
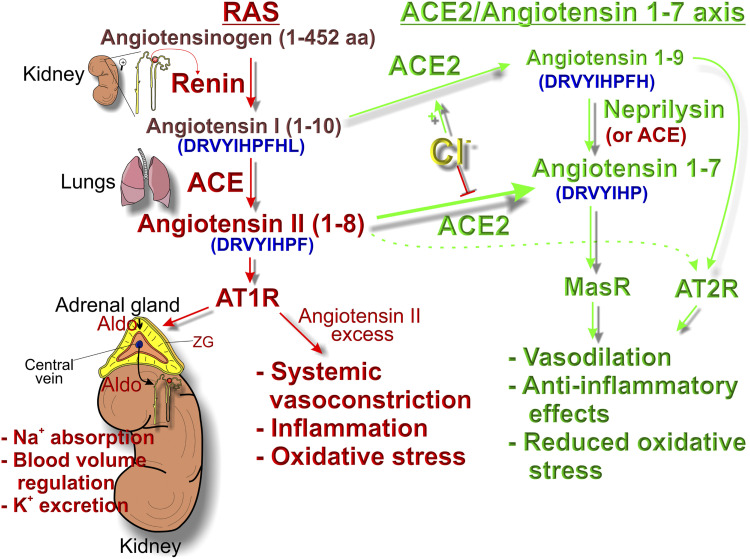
The RAS is a key hormonal circuit tasked in regulating extracellular fluid volume and blood pressure in mammals. If blood pressure falls, the juxtaglomerular cells in the kidneys produce and secrete renin, which cleaves serum angiotensinogen to produce angiotensin I (Ang-I). Then, angiotensin-I converting enzyme (ACE) further converts Ang-I into angiotensin II (Ang-II) in the lungs by removing the C-terminal dipeptide from Ang-I (Fig. 1). Ang-II in turn activates the G-protein–coupled angiotensin type 1 receptor (AT1R) on adrenal zona glomerulosa cells to produce aldosterone, which causes sodium retention, an increase in blood volume, and blood pressure stabilization. Besides its beneficial function in regulating extracellular fluid volume, a dysregulated RAS, as seen in diabetes, could lead to an increase in serum levels of Ang-II that could cause a plethora of potentially harmful effects including vasoconstriction, inflammation, and increased oxidative stress. ACE2 converts Ang-II into Ang-1-7, and Ang-1-7 acts through the Mas receptor (MasR) to oppose the effects of Ang-II. This ability of ACE2 to convert the serum vasopressor Ang-II into the vasodilating Ang-1-7 identifies it as a “negative” regulator of RAS. Notably, COVID-19 patients exhibit increased serum levels of Ang-II (6), which would support less cleavage by ACE2 and thus potentially less ACE2 activity. In addition to systemic RAS, “local” RAS exists within each tissue including a lung RAS, intestinal RAS, and bone marrow RAS. Interestingly, the RAS system in the intestinal mucosa significantly contributes to the regulation of glucose, salt, and water uptake. Emerging COVID-19 gastrointestinal disturbances implicate a central role of intestinal pathophysiology in exacerbation of hyperglycemia and blood pressure in individuals with diabetes infected with SARS-CoV-2.
Figure 1.
The biochemical pathways of the RAS and the beneficial ACE2/Ang-1-7 arm of RAS. The amino acid sequences of hormones are colored in blue and enclosed in parentheses. Renin is produced and secreted in the juxtaglomerular cells of the kidney when plasma NaCl decreases or blood pressure falls. Renin cleaves angiotensinogen to produce Ang-I, which is further converted into Ang-II by ACE in the lungs. Ang-II induces aldosterone secretion from adrenal zona glomerulosa cells, which in turn promotes sodium and water retention in the kidneys, increasing blood pressure. Thus, initial serum Ang-II levels are set by renin. However, the steady-state serum Ang-II level is also markedly affected by the rate of its conversion to Ang-1-7 by ACE2. Therefore, ACE2 activity contributes to regulating the steady-state levels of Ang-II. If we consider an example of a rainwater barrel and assume that renin is the actual rainfall amount, Ang-II is the rainwater, and ACE2 activity is the barrel’s outlet spigot, then the rainfall amount (renin) would always determine the rainwater (Ang-II) inflow rate and level into the barrel. But if we would keep the barrel outlet spigot always open (ACE2 is active) during and after rainstorms, the final level of rainwater would not be as high as in the case if the barrel’s outlet spigot were closed (ACE2 is inactive). Aldo, aldosterone; ZG, zona glomerulosa.
ACE2 Protein: Function, Interaction Between ACE2 and ADAM17, and ACE2 as the Receptor for SARS-CoV and SARS-CoV-2
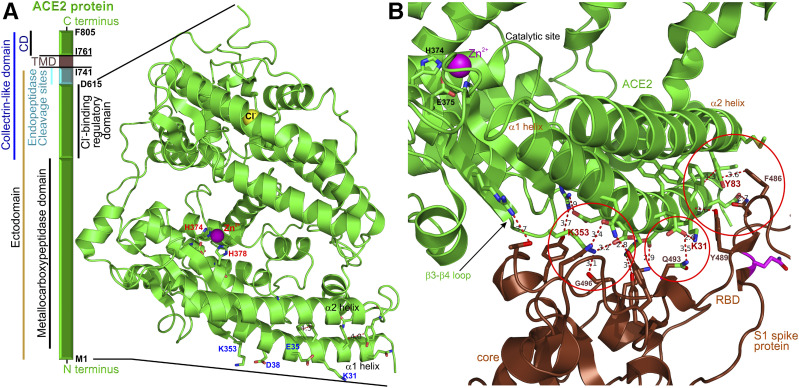
ACE2 was discovered in 2000 (7), just 2–3 years before the first wave of SARS-CoV coronavirus pandemic, when ACE2 was identified as the major SARS-CoV “receptor” on host cells. ACE2 functions as a metallocarboxypeptidase, a plasma membrane–bound proteolytic enzyme (Fig. 2A) that removes a single carboxy-terminal amino acid from specific bioactive oligopeptides, such as Ang-I and Ang-II to form Ang-1-9 and Ang-1-7, respectively (Fig. 1). Unlike ACE, which is a peptidyl dipeptidase removing two carboxy-terminal amino acids from Ang-I and Ang-1-9, ACE2 is not inhibited by typical ACE inhibitors, such as captopril.
Figure 2.
A: Block diagram of the hACE2 protein segments and the structure of its soluble domain, which is shed from the full-length hACE2 after cleavage with endopeptidases, such as ADAM17. The cyan segment depicts the location of the endopeptidase cleavage sites. The amino acids at the borders of the segments are shown in black. The structure depicts the locations of zinc (catalytic) and chloride (regulatory) binding sites. The Cl− binding site, coordinating a single Cl− anion, regulates the efficacy of ACE2 to cleave its substrates (Ang-I and Ang-II). Specifically, ACE2-mediated removal of the terminal leucine from Ang-I is potentiated, whereas the cleavage of the terminal phenylalanine from Ang-II is inhibited in the presence of extracellular chloride anions. The structure of the hACE2 was replotted from pdb ID: 1R42 (50). CD, cytosolic domain; TMD, transmembrane protein. B: The interface of the SARS-CoV-2 virus (brown) and the N-terminal domain of hACE2 (green). The open red circles show the interaction hotspots similar to those identified for the SARS-CoV virus. The catalytic site of hACE2 is visible in the left upper corner. The structure of the hACE2 bound to SARS-CoV-2 receptor binding domain (RBD) was replotted from pdb ID: 6M0J (19).
The ectodomain of ACE2 can be shed into the systemic circulation as a soluble protein, preserving the catalytic activity of ACE2 (soluble ACE2) and its ability to generate in the circulation the vasoprotective peptide, Ang-1-7. Shedding of the ACE2 ectodomain occurs after proteolytic cleavage by plasma membrane–anchored endopeptidases, enzymes capable of breaking nonterminal peptide bonds, such as a disintegrin and metallopeptidase domain 17 protein (ADAM17) (8) or the type II transmembrane serine proteases (TMPRSS2) (9).
Human ACE2 (hACE2) is predominantly expressed in the nasal epithelium, airways, lungs, heart, adipose tissue, kidneys, small intestine, and colon (7,10–12). The high density of hACE2 is found in human nasal epithelium goblet cells, human ciliated cells of the airways, the type 2 alveolar (AT-2) epithelial cells, and bronchial transient secretory cells (10,11). High hACE2 expression in the nasal epithelium is consistent with clinical observations that symptomatic individuals with COVID-19 present with a higher viral load in the nasal cavity compared with the throat (10) and that some COVID-19 patients complain of inability to smell (13). The hACE2-expressing nasal epithelium may provide an “intermediate site” for viral replication before its invasion of the lungs to cause symptomatic COVID-19 and may serve as a sanctuary niche for SARS-CoV-2 survival without spreading to the lungs in human subjects, thus perhaps permitting asymptomatic human-to-human transmission. Notably, the SARS-CoV-2–positive individuals may shed the virus for up to 37 days (3).
The ACE2 protein contains two cofactors: Zn2+ and Cl− ions (Fig. 2A). The zinc binding site, coordinating Zn2+, is critical for the catalytic activity of ACE2 and consists of H374-E375-X-X-H378 in hACE2. The Cl− binding site regulates the efficacy of ACE2 to cleave its substrates (Ang-I and Ang-II) in an extracellular Cl−-dependent manner (7).
The spike proteins of SARS-CoV and SARS-CoV-2 bind the membrane-bound ACE2 to enter the host cells (14–16). The interface of the SARS-CoV-2 receptor binding domain located on the S1 subunit of the spike protein and the N-terminal segments of hACE2 was mapped using cryo-EM and X-ray crystallography (Fig. 2B) (17–19), and the structure data shed light on the underlying mechanisms. There are several virus binding hotspots on the surface of hACE2 that are critical for virus infectivity; these include hotspot 31 (lysine 31), hotspot 353 (lysine 353), and the hydrophobic interaction site (tyrosine Y83) (17). Compared with SARS-CoV, SARS-CoV-2 forms additional hydrogen bonds, dipole-dipole interactions, and salt bridges (19), suggesting stronger interaction. The affinity binding data indicate that the receptor binding domain of SARS-CoV-2 has a greater affinity to hACE2 compared with that of the SARS-CoV virus (17), potentially explaining the enhanced ability of SARS-CoV-2 to quickly spread and infect a great number of hosts. While membrane-bound hACE2 is the major cellular receptor for SARS-CoV-2 binding and internalization, the soluble form of hACE2 is efficient at preventing the coronaviruses attachment to the membrane-bound hACE2 (20).
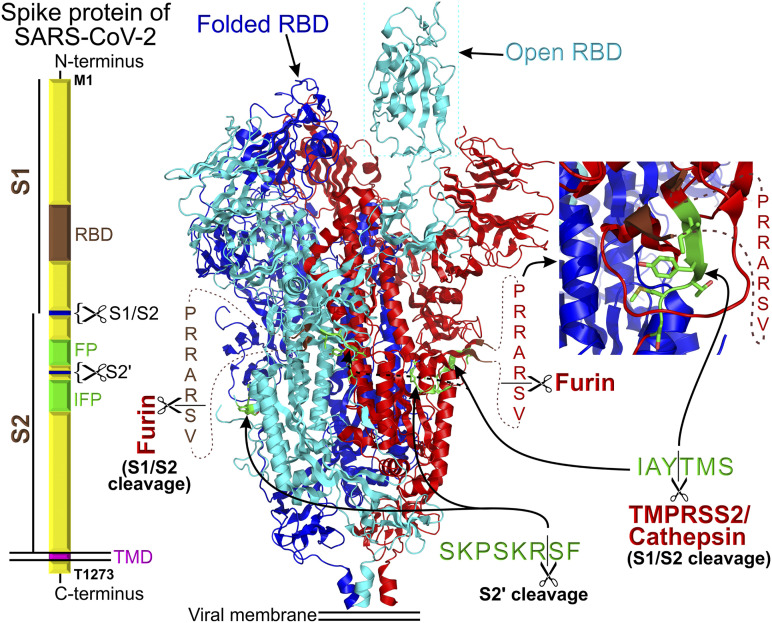
Proteolytic cleavage of the homotrimeric spike protein ectodomains at the S1/S2 subunit junction is critical for entry of coronaviruses into host cells. The ectopeptidase TMPRSS2 and endosomal peptidases cathepsin B/L are the major cellular enzymes that mediate coronavirus “priming” in SARS-CoV-2 (14) by cleaving the spike protein at the S1/S2 cleavage site (IAY↓TMS) (Fig. 3). The SARS-CoV-2 spike protein has an additional canonical furin cleavage site (PRRAR↓SV) located upstream of the conserved IAY↓TMS cleavage site (15,21). This is a unique property of SARS-CoV-2 because the furin site is not present in SARS-CoV (15,21). The presence of a furin-like cleavage site in viral spike-shaped hemagglutinin proteins has been associated with an increased virulence and pathogenicity in avian and human influenza viruses. Consistently, the current pandemic epidemiological data confirm the increased transmissibility and pathogenicity of SARS-CoV-2 as compared with SARS-CoV (22).
Figure 3.
Block diagram of the homotrimeric SARS-CoV-2 spike protein assembly. “RBD” stands for receptor binding domain, “FP” stands for fusion peptide, and the IPF block depicts the location of internal fusion peptide. S1 and S2 are two segments of SARS-CoV-2 ectodomain that can be cleaved with the indicated endopeptidases. The cryo-EM structure (15) of the spike protein is shown in the center of the figure. The proteolytic sites are shown in green. In the structure, the residues preceding and following the furin cleavage site are colored in brown. The inset shows a magnifying view of the TMPRSS2/cathepsin cleavage site. The structure of the homotrimeric SARS-CoV-2 spike protein complex was replotted from pdb ID: 6VYB (15). Each spike protein in the homotrimer is color coded for better identification.
The structure of the SARS-CoV-2 spike protein provides insight on why the addition of the furin cleavage site may increase transmissibility of the virus. According to the structure, the TMPRSS2 cleavage site (IAY↓TMS) is located in a shallow pocket on the lateral surface of the SARS-CoV-2 spike protein (Fig. 3), whereas the short solvent-exposed protein loop harboring the furin-cleavage site (not solved in the structure and shown as the dotted lines in Fig. 3) appears to hang over the TMPRSS2 cleavage site, obstructing access. The newly biosynthesized SARS-CoV-2 viral particles are likely released by budding in a Golgi compartment–dependent manner. Since furin is a Ca2+-dependent endopeptidase, which is present and active only in the Golgi compartment (Fig. 4), the complete cleavage of the furin site is expected in Golgi compartment–processed SARS-CoV-2 spike proteins and is experimentally confirmed (15). Furin-cleaved S1/S2 subunits remain noncovalently bound in the homotrimeric spike protein assembly. It is possible that in the furin precleaved SARS-CoV-2 spike protein, the TMPRSS2 cleavage site is no longer obstructed and is more accessible for TMPRSS2 and/or cathepsins. However, experimental confirmation will be needed for this hypothesis. The SARS-CoV-2 spike protein can be in the closed (folded) or open conformation when the viral receptor binding domain unfolds and extends above the trimeric spike protein structure (Fig. 3). Whether furin cleavage affects the equilibrium between the two spike protein conformations also remains unclear and awaits experimental evidence.
Figure 4.
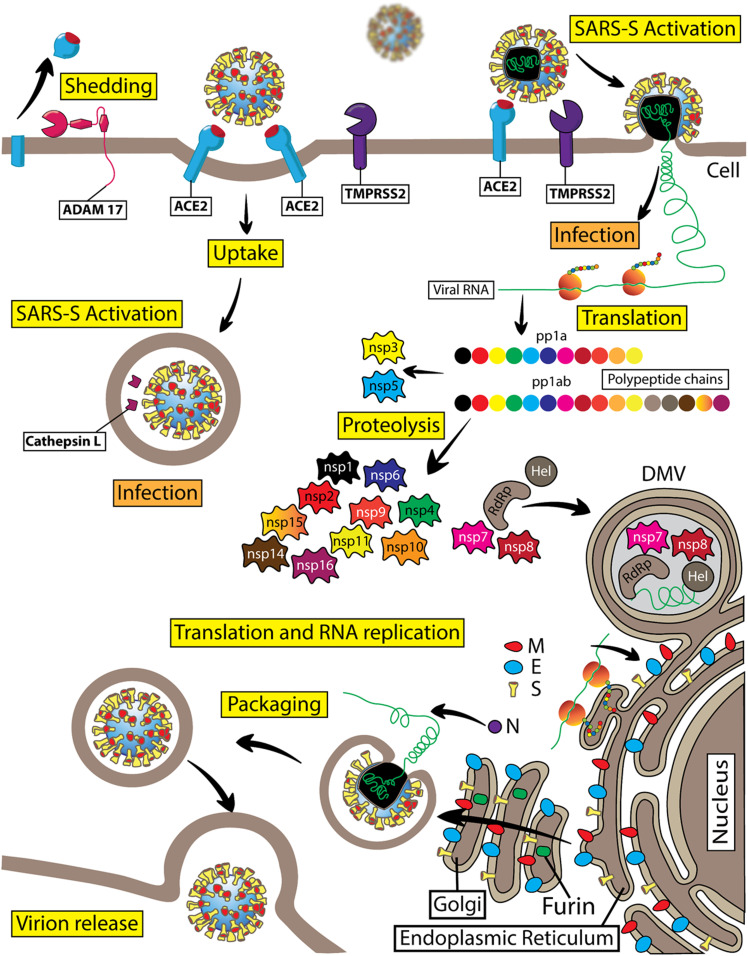
Diagram of the SARS-CoV-2 virus life cycle. SARS-CoV-2 is a member of the Coronaviridae subfamily and belongs to the genus of β-coronaviruses. This is a positive-sense single-stranded RNA virus. SARS-CoV-2 viral RNA serves to code the viral genome and as mRNA for direct protein translation by the host cell ribosomes. Indeed, viral RNA contains a poly-A tail at the 3′ end and a typical mRNA cap structure at the 5′ end. SARS-CoV-2 viral RNA is nonsegmented. Viral RNA genome translation starts with the production of two replicase polyproteins, pp1a and pp1ab, which consist of 11 or 16 covalently linked nonstructural proteins (nsp), respectively. These two large polyproteins are subject to proteolytic cleavage by proteases resulting in the formation of individual nsp1–nsp16. Viral nsp3 functions as a papain-like protease and is important for cleaving the interdomain junctions between nsp1 and nsp4, whereas nsp5 is a chymotrypsin-like protease, which is also named “main protease” because it is responsible for cleaving interdomain junctions between nsp4 and nsp16. Nsp6 can induce small-diameter autophagosome formation in infected cells. Nsp12 (RdRp) is an RNA-dependent RNA polymerase, which is critical for a large-scale replication of viral RNA. Nsp12 requires several cofactors, such as nsp7 and nsp8. The RNA helicase nsp13 (Hel) is important for replication. Nsp14 is a viral N7-methyltransferase ensuring the fidelity of replication. The viral RNA also encodes four structural proteins: the spike protein (S), envelop protein (E), membrane protein (M), and nucleocapsid protein (N). In SARS-CoV-2 virions, viral RNA is enveloped with a membrane that is stabilized by the imbedded structural proteins, including S, E, M, and N proteins. The S or spike protein is a homotrimer that gives the viral particles a characteristic appearance of spiky corona. The S protein is critical for the viral entry into the host cells. The S1 subunit of the SARS-CoV-2 spike protein utilizes the hACE2 protein as its cellular receptor. The TMPRSS2 protein is the key endopeptidase that is important for priming the spike protein of SARS-CoV-2, allowing viral entry into host cells. Cathepsin B/L is an endosomal protease that can substitute TMPRSS2 activity during spike protein priming before viral RNA gains access into the cellular cytosolic compartments. SARS-CoV-2 replication takes place in double membrane vesicles (DMV) that are associated with the specific areas of the rough endoplasmic reticulum or other intracellular membranes, including autophagosomal membranes. The M, E, and N structural proteins together with the S protein are important for formation and stabilization of the SARS-CoV-2 viral particles. Viral structural protein modification takes place in the Golgi compartment before viral particles are ready for budding. Furin is a Ca2+-dependent endopeptidase enriched in the Golgi compartments that precleaves the spike protein at a specific cleavage site in the Golgi compartments, with S1 and S2 subunits remaining noncovalently bound in budding virions. ADAM17 proteolytic activity generates soluble hACE2.
As COVID-19 progresses, SARS-CoV-2 may also involve the lytic release pathway for newly produced viral particles, bypassing the budding process utilizing the furin-containing Golgi compartments (Fig. 4). In such cases, the spike protein of SARS-CoV-2 may remain at least partially uncleaved by intracellular furin. At this stage of COVID-19, extracellular furin may be utilized to complete the cleavage of spike protein’s furin cleavage sites, facilitating the virus spread in the infected host. Notably, circulating levels of furin are elevated in patients with diabetes (23), and patients with diabetes infected with SARS-CoV-2 present with increased mortality (4) and delayed recovery from SARS-CoV-2 infection. Also, individuals with high plasma furin concentration typically have a pronounced dysmetabolic phenotype and elevated risk of diabetes.
RAS Modulates Autophagy
There is increasing evidence that dysregulated autophagy contributes to the pathogenesis of diabetes and its complications. Autophagy is primarily recognized for its essential role in cellular housekeeping and homeostasis through the sequestration and transfer of intracellular components to lysosomes for degradation. However, the endocytic pathway and autophagy are key processes affecting virus infection and replication, including the coronavirus family (24). Viral RNA replication in coronavirus-infected cells occurs in double membrane vesicles that resemble autophagosomes (Fig. 4). Additionally, nonstructural protein 6 (nsp6) of SARS-CoV-2 can generate autophagosomes, and an associated mutation in nsp6 is identified in COVID-19 patients (4,24). Interestingly, inhibition of the canonical autophagy pathway, using in vitro approaches, does not appear to have an effect on SARS-CoV replication, suggesting a noncanonical process. However, a key autophagy protein, LC3, colocalizes with viral replication-transcription complexes, and an S-phase kinase-associated protein 2 (SKP2) reduces autophagy protein Beclin1 levels in coronavirus infections (24,25). In both cases, fusion between autophagosomes and lysosomes is blocked, leading to an accumulation of autophagosomes favoring replication of the virus. Inhibiting SKP2 or enhancing autophagy flux has been shown to reduce the replication of coronaviruses (24,25). RAS can be an important regulator of autophagy. Ang-II activation of angiotensin type 2 receptor (AT2R) attenuates autophagy, whereas Ang-II activation of AT1R induces autophagy through AMPK/mTOR signaling. Ang-1-7 induces autophagy via the cofilin receptor (26). Activation of intestinal RAS promotes Paneth cell autophagy leading to bowel inflammation and arrested release of antimicrobial factors including defensin 5, which inhibits SARS-CoV-2 infection by cloaking ACE2 (27). Given the strong association between the RAS and autophagy, both may serve as therapeutic targets to ablate SARS-CoV-2 infection and replication, and this may further explain the possible beneficial effects of ACE inhibitors/ATR blockers in the treatment of COVID-19, discussed further below.
A Dysregulated RAS May Increase Adverse Outcomes in Individuals Infected With SARS-CoV-2
Several mechanisms may contribute to increased severity of COVID-19 progression in subjects with diabetes. Individuals with diabetes are more vulnerable to most infections and may exhibit decreased viral clearance due to reduced neutrophil chemotaxis, phagocytosis, and intracellular killing of microbes. Under noninfectious conditions, chronic diabetes in both human and rodent models was associated with myeloidosis (7), with monocytes expressing higher levels of proinflammatory cytokines that may, in patients with acute respiratory distress syndrome (ARDS), contribute to cytokine storm.
Once bound to ACE2, SARS-CoV was shown to downregulate cellular expression of ACE2, and the unopposed action of Ang-II was deemed responsible for worsening lung injury (28). Whether this is the case with SARS-CoV-2 is not known. Ang-II receptor blockers, ACE inhibitors, thiazolidinediones, incretin GLP-1 agonists, and statins are typical medications for diabetes that are known to increase ACE2 expression. Lack of evidence regarding the risk or benefit of ACE inhibitors and angiotensin receptor blockers (ARBs) has resulted in the American College of Cardiology, American Heart Association, American Society of Hypertension, and European Heart Association recommendations that patients should continue treatment with their usual antihypertensive therapy (29). However, we would propose that drug-induced increases in ACE2 expression would potentially be beneficial in subjects with diabetes by increasing Ang-1-7 and shifting the RAS axis away from the profibrotic, proinflammatory arm of RAS. Thus, in subjects with diabetes, infection with SARS-CoV-2 would potentially result in additional loss of ACE2 expression in blood vessels and could exacerbate the already compromised vasculature (29).
Implications of COVID-19 Infection on Bone Marrow Dysfunction and Increasing Severity of Diabetic Vascular Complications
The existence of specific RAS systems in organs including the bone marrow has been well established. Local RAS is active in primitive embryonic hematopoiesis (30) and continues to regulate each stage of physiological and pathological blood cell production in the adult via autocrine, paracrine, and intracrine pathways. Local RAS peptides directly regulate myelopoiesis, erythropoiesis, thrombopoiesis, and the development of other cellular lineages (31).
The bone marrow plays a critical role in the pathogenesis of diabetic complications. Individuals with diabetes with vascular complications typically have reduced numbers and migratory function of bone marrow–derived vascular reparative cells, called circulating angiogenic cells (CACs or CD34+ cells). Ang-1-7 improved migration, restored bioavailable nitric oxide, and reduced reactive oxygen species in diabetic CACs. Ang-1-7 gene modification of CACs restored the cells in vivo vasoreparative function (32). A unique set of individuals with diabetes that remained free of microvascular complications, despite >40 years of poor glycemic control, had higher mRNA levels for ACE2 and MasR in their CACs compared with age-, sex-, and glycemia-matched individuals with diabetes with microvascular complications (32). In Akita mice, global loss of ACE2 (ACE2−/y–Akita mice) was associated with a reduction of hematopoietic stem/progenitor cells (HS/PC), a shift of hematopoiesis toward myelopoiesis in bone marrow, and an impairment of HS/PC migration and proliferation. Migratory and proliferative dysfunction of these cells was corrected by exposure to Ang-1-7 (33). These data support that activation of the protective RAS is beneficial for the dysfunctional diabetic bone marrow.
Diabetes-associated bone marrow dysfunction and loss of vascular reparative cells, such as CACs, may contribute to vascular dysfunction in COVID-19 patients that can be manifested as cardiac disease including arrhythmias, viral myocarditis, heart failure, and cardiac arrest (34–36). The impact of global loss of ACE2 in cardiac dysfunction is supported by preclinical studies showing that hearts from Akita mice exhibit marked systolic dysfunction and that ACE2−/y-Akita mice show impaired flow-mediated dilation of the femoral artery in response to ischemia/reperfusion injury, indicative of endothelial dysfunction. In contrast, gain-of-function studies using ACE2 overexpression, via adenoviral gene delivery, in type 1 diabetic rats decreased collagen accumulation and improved left ventricular remodeling and function (7).
The impact of dysregulated RAS is seen in obesity and type 2 diabetes models. Heart failure with preserved ejection fraction (HFpEF) is a proinflammatory state closely linked to obesity-related cardiovascular dysfunction. Loss of ACE2 increases epicardial adipose tissue macrophage polarization to proinflammatory M1-like phenotype and worsens HFpEF in response to diet-induced obesity. Ang-1-7 has potent anti-inflammatory effects in adipose tissue of obese type 2 diabetic mice and protects against diabetic cardiomyopathy and nephropathy. Importantly, Ang-1-7 decreased macrophage M1 polarization and preserved cardiac function in diet-induced obese ACE2 knockout mice (7).
In COVID-19 patients, the prevalence of kidney disease on admission and the development of acute kidney injury during hospitalization is high and associated with in-hospital mortality (37). Patients with diabetes with nephropathy have reduced ACE2. Global loss of ACE2 exacerbates diabetic kidney injury while potentiating Ang-II–mediated cardiorenal fibrosis and oxidative stress in the heart and kidney (7). In Akita mice, recombinant hACE2 (rhACE2) treatment for 4 weeks resulted in decreased glomerular mesangial matrix expansion, which was associated with increased Ang-1-7 levels and lowered Ang-II levels, along with reduced NADPH oxidase activity. The loss of ACE2 via ADAM17 proteolytic cleavage, which is strongly activated in COVID-19 patients, will likely promote further injury to the cardiovascular system and kidneys in patients with diabetes (7,38). Importantly, ACE2 overexpression increases the antihypertensive components of the RAS and pretreatment with rhACE2 prevents Ang-II–induced hypertension in preclinical experimental models. However, these results have yet to be validated in human hypertension.
While the lung is not considered a target tissue for diabetic complications, COVID-19 patients with diabetes experience worse pulmonary disease than those without diabetes. ACE2 knockout mice exhibit ARDS pathology. ARDS triggers multiple pulmonary diseases and is observed in COVID-19 patients. Importantly, ACE deficiency or treatment with AT1R blockers of ACE2−/y mice rescues them from ARDS (38). Taken together, these studies support that in individuals with diabetes with vascular complications, the loss of the protective RAS would serve to intensify SARS-CoV-2–induced pathology.
SARS-CoV-2 Hijacks Gastrointestinal ACE2, Local RAS, and Transporters
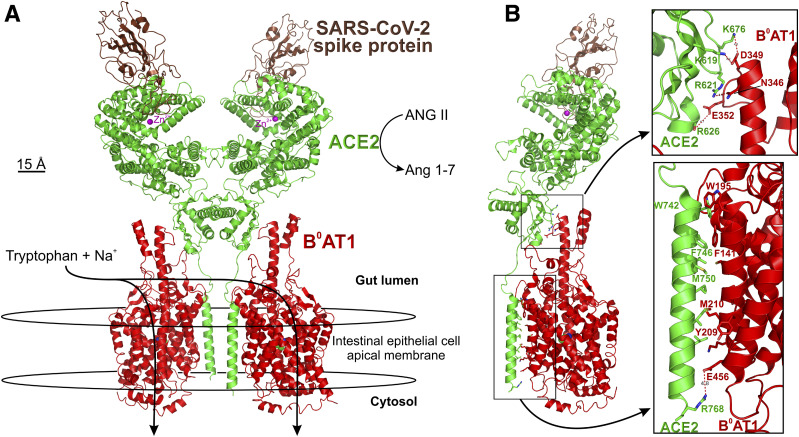
The recent demonstration of SARS-CoV-2 actively infecting human enterocytes and the mounting gastrointestinal symptomology implicate gastrointestinal tract pathophysiology in COVID-19 infection (12,39). The digestive system possesses the body's site of greatest relative expression of ACE2, which in the gut exists as a tetramer with B0AT1 (Fig. 5). While B0AT1 is not expressed in lung pneumocytes, ACE2:B0AT1 complex in the gut acts as a central player in local gut RAS and regulates uptake of glucose, sodium, water, and amino acids (40–42). However, ACE2:B0AT1 complex internalization by SARS-CoV-2 (Fig. 6) destabilizes the gastrointestinal tract’s role in diabetes and blood pressure regulation (Fig. 7).
Figure 5.
Atomic structure of human B0AT1/ACE2 ternary complex bound to spike protein region of SARS-CoV-2. A: The complex comprises a dimer of heterodimers formed by two B0AT1 subunits (red) contacting with two ACE2 subunits (green), with each ACE2 interfacing with a single SARS-CoV-2 spike (brown). The complex was stabilized using Na+ cotransporter B0AT1 transport substrate leucine within the center membrane-spanning domain, known to serve tryptophan, glutamine, and other neutral amino acids in addition to leucine. Intestinal apical membranes express the B0AT1:ACE2 complex, which does not occur in lung pneumocytes (40). B: Side view showing charged moiety interactions in the extracellular region of the gut lumen (top inset) and also hydrophobic interactions of B0AT1 TM3 and TM4 interacting with the single long transmembrane domain of ACE2 within the apical membrane (bottom inset). Data coordinates were obtained from pdb ID: 6M17 (18).
Figure 6.
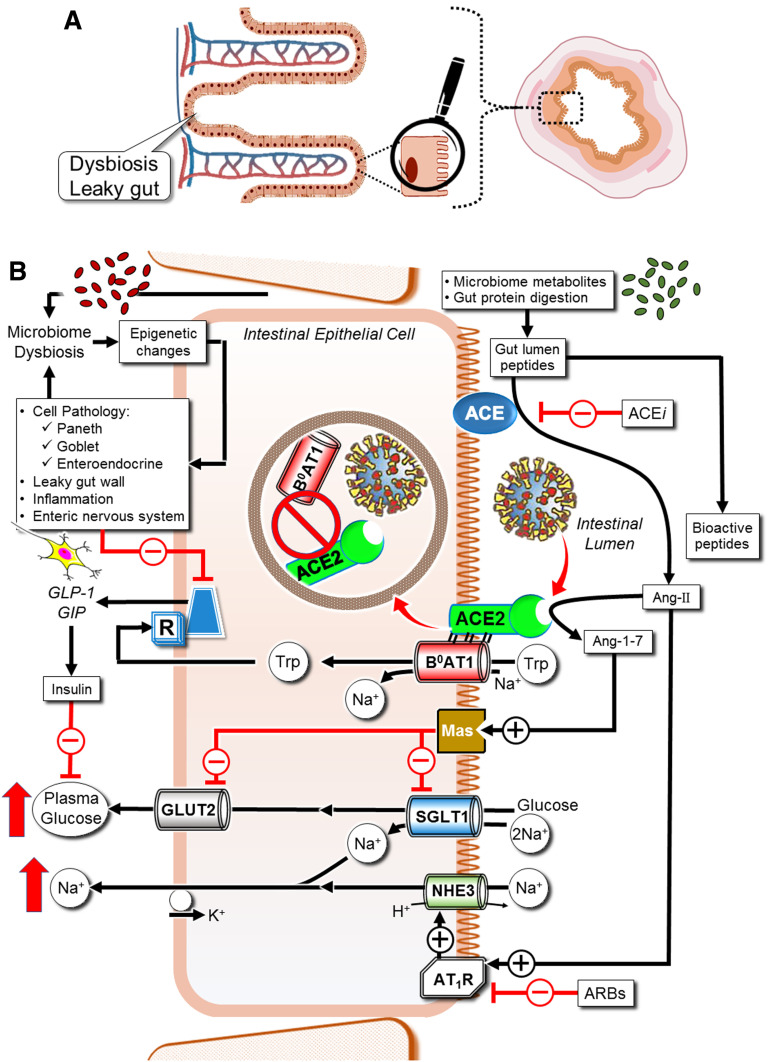
Intestinal epithelial cell RAS and B0AT1 govern glucose, sodium, and inflammation. All RAS components are recapitulated locally in the gut (41). Luminal agonist and antagonist bioactive peptides are derived from interactions of gut digestive enzymes intertwined with microbiome metabolism. Oral ARBs and ACE inhibitor drugs impact gut RAS. Gut RAS governs sodium and glucose uptake via NHE3, SGLT1, and GLUT2. The ACE2:B0AT1 complex dimer of heterodimers (18) serves the Na+-coupled transport of neutral amino acids, including tryptophan. In enteroendocrine L cells, basolateral tryptophan stimulates GLP-1 and GIP secretion. These incretins maintain gut tight junctions, preventing dysbiosis, stimulate pancreatic β-cells, and blunt α-cells, thereby modulating plasma glucose levels. SARS-CoV-2 binding to ACE2 disrupts this homeostasis.
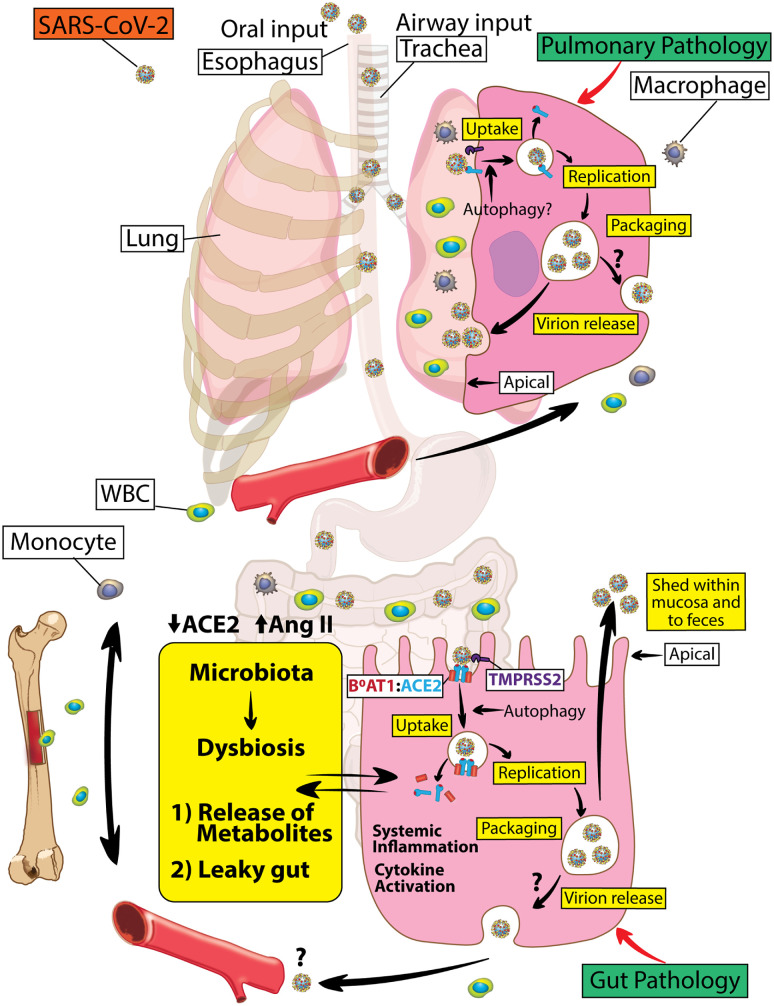
Figure 7.
Dysregulated RAS in lung and gut epithelium of individuals with diabetes with COVID-19. ACE2, a pleiotropic regulator of the RAS, is hijacked as a receptor for SARS-CoV-2 to promote viral infection. Loss of ACE2 indirectly via proteolytic processing, autophagy, and ADAM17-mediated shedding (not shown) partly drives not only lung but also gut disease in individuals with diabetes with COVID-19. SARS-CoV-2 S1 binding to ACE2 initiates internalization of ACE2:B0AT1 complex (gut) or ACE2 (outside of gut). Thus, SARS-CoV-2 by downregulating intestinal ACE2-B0AT1 would promote leaky gut syndrome with elevated plasma bacterial lipopolysaccharides and/or peptidoglycans enhancing systemic inflammation. In the lung, virus internalization also promotes a reduction in ACE2 that results in pulmonary pathology. Careful targeting of the RAS axis will likely optimize clinical outcomes in subjects with diabetes infected with SARS-CoV-2. WBC, white blood cell.
B0AT1 (SLC6A19) is the intestine's primary epithelial apical membrane transporter serving Na+-coupled uptake of neutral amino acids, such as tryptophan. B0AT1 was originally discovered and functionally characterized by Stevens et al. (43), and the transporter was initially named NBB, B, B0, or B(0) in the literature but was subsequently called B0AT1. ACE2 chaperones the trafficking of B0AT1 to form the stabilized dimer of ACE2:B0AT1 (18) in the apical membrane (Fig. 5). Importantly, B0AT1 substrates, notably tryptophan and glutamine, signal downregulation of lymphoid proinflammatory cytokines, promote tight junction formation, activate the release of antimicrobial peptides, and modulate mucosal cell autophagy as defense mechanisms. In the models shown in Figs. 6 and 7, binding of SARS-CoV-2 S1 to ACE2 (18) (Fig. 6) results in downregulating both intestinal ACE2 and B0AT1, with consequences of disrupting sodium and glucose transport, promoting leaky gut syndrome, elevating plasma bacterial lipopolysaccharide, and enhancing inflammation (Figs. 5 and 7).
Intestine, lumen-facing ACE, and ACE2 participate in the food digestion process but are also intertwined in cross talk with gut microbiome metametabolomics of bioactive peptides. Such peptides include a balance of agonists and antagonists of enterocyte apical membrane MasR and AT1R, which are physiologically tasked with regulating uptake of dietary Na+ via NHE3 and glucose absorption via SGLT1 and GLUT2 (Fig. 6).
SARS-CoV-2 Modulation of Insulin and Glucose
Intestinal ACE2:B0AT1 dimer of heterodimers promotes enterocyte Na+-coupled uptake of phenylalanine, glutamine, tryptophan and its microbiome-generated metabolites, and other neutral amino acid agonists of nutrient-sensing receptors. These stimulate release of GLP-1 and GIP into the blood from gut mucosal enteroendocrine L cells (Fig. 6) (44). These incretins circulate to activate pancreatic β-cells, suppress α-cells, and afford brain satiety. SARS-CoV-2 infection of gut mucosa results in endocytosis of apical ACE2, thereby downregulating its activity (45), resulting in gut luminal accumulation of AT1R agonist peptides and disrupting all functions of B0AT1.
Gut–Bone Marrow Connection in Individuals With Diabetes Infected With COVID-19
The dysregulated RAS in the bone marrow with its accompanying myeloidosis promotes chronic inflammation that can contribute to both lung and gut pathology (Fig. 7). An extensive literature supports the concept of communication between the gut and bone marrow. The gut microbiota is a critical extrinsic regulator of hematopoiesis (46), as very low concentrations of microbial antigens set the size of the bone marrow myeloid cell pool, and the size of this pool correlates strongly with the complexity of the intestinal microbiota. In turn, bone marrow cells migrate to the gut and impact gut function via changes in blood flow, gut immunity, and epithelial and endothelial tight junction integrity. Recruitment of bone marrow–derived immune cell to the gut is necessary for host defense and contributes to inflammation resolution and tissue healing. Loss of ACE2 in diabetes results in phylogenetic differences in the gut bacterial community composition with increases in bacteria that have been associated with peptidoglycan generation, which promotes systemic inflammation (47). Overactivation of bone marrow–derived immune cells including proinflammatory monocytes results in secretion of a large number of harmful cytokines into the circulation that promotes insulin resistance. In the patient with diabetes infected with COVID-19, developing pneumonia can be devastating, as preexisting systemic inflammation can rapidly lead to multiple organ failure. Inflammatory cytokine storm is a notable cause of death in critically ill COVID-19 patients and may be driven as much by gut-induced inflammation as lung injury. Thus, imbalance in the bone marrow RAS system (Fig. 7) may represent a central mechanism to not only initiate but also propagate lung and gut injury.
Possible Therapeutics That Modulate RAS
From the perspective of gut enterocyte local RAS, orally delivered ACE inhibitors upregulate expression of both intestinal ACE2 and B0AT1 with their attending nutrient-signaled release of GLP-1, GIP, and mucosal antimicrobial peptides (40) (Fig. 6). In a preclinical colitis model, the ARB irbesartan restored intestinal B0AT1 and ACE2 expression and tryptophan homeostasis with concurrent reduction of intestinal inflammasome activity through an mTOR S6 kinase pathway (48). Irbesartan further shifted the gut microbiota composition toward favorable taxa and away from stress-related dysbiosis (48). Activation of enterocyte AT1R signaled apoptosis with reduced mucosal villus height, while losartan-mediated blockage of gut AT1R resulted in increased mucosal cell proliferation and reduced apoptosis.
Increasing gut ACE2 by engineering probiotic species such as Lactobacillus paracasei (LP) to express this recombinant protein was a strategy used to prevent microvascular complications in diabetic mice. LP expressing the secretable ACE2 fused with the nontoxic subunit B of cholera toxin (which acts as a carrier to facilitate transmucosal transport), showed increased ACE2 activities in serum and tissues, and reduced diabetic complications (49). These results provide proof of concept for feasibility of using engineered probiotic species as a live vector for delivery of decoy hACE2 for possible treatment of enteric COVID-19 infection.
rhACE2 given as intravenous medication may be explored as beneficial to COVID-19 patients with pulmonary complication, as it increases pulmonary blood flow and oxygenation in a pig model of lipopolysaccharide-induced ARDS. Supplementation with ACE2 or inhibition of Ang-II improves outcomes in acute lung injury. A pilot trial demonstrated that rhACE2 is well-tolerated in ARDS patients and showed the anticipated changes in RAS peptides. Taken together, evidence unequivocally supports the concept that ACE2 is critical in pulmonary function and its imbalance in COVID-19 infection contributes to the devastating lung consequences.
An ACE2 activator, diminazene aceturate (DIZE) is a known antiprotozoal drug used in humans, but it has additional benefits including potent anti-inflammatory and antifibrotic activity. DIZE has been used in type 1 diabetes to prevent nephropathy and gastric inflammation. DIZE modulated the RAS by reducing serum Ang-II and the expression of AT1R, but it increased Ang-1-7 (7). DIZE not only increased ACE2 activity but also increased the expression of ACE2 in select cell types where DIZE inhibited the expression of IL-6, IL-8, and MCP-1 at both mRNA and protein levels following stimulation with lipopolysaccharide. Collectively, these results show that DIZE downregulates proinflammatory cytokine production by many distinct cell types and suggest that this drug may provide benefit to COVID-19 patients by reducing pulmonary inflammation and fibrosis, gut inflammation, and cytokine storm.
Conclusion
As the global pandemic unfolds and rapidly spreads, there is an urgent need for basic and clinical studies to address the many unanswered questions posed by COVID-19. This Perspective has directed attention to the disruption of RAS in the lung, gastrointestinal tract, and bone marrow as possible mechanisms of SARS-CoV-2 disease pathogenesis. The dysregulated RAS can potentially impact clinical outcomes in individuals with diabetes resulting in increased morbidity and mortality. ACE2 has emerged as the pleiotropic regulator of the RAS, by metabolizing Ang-II into the beneficial peptide Ang-1-7, while being harmful as the SARS-CoV-2 receptor. Loss of ACE2 indirectly via proteolytic processing, autophagy, and shedding partly could not only drive lung pathology but also gut disease in individuals with diabetes infected with COVID-19. SARS-CoV-2, by downregulating intestinal ACE2-B0AT1, could promote leaky gut syndrome with elevated plasma bacterial lipopolysaccharides and/or peptidoglycans enhancing systemic inflammation. Careful targeting of the RAS axis may represent a strategy for improving clinical outcomes in subjects with diabetes infected with COVID-19.
Article Information
Funding. This study was supported by National Institutes of Health grants R01EY025383, R01EY012601, R01EY028858, and R01EY028037 to M.B.G. A.G.O. was supported in part by R01NS102415.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Footnotes
This article is part of a special article collection available at https://diabetes.diabetesjournals.org/collection/diabetes-and-COVID19-articles.
References
- 1.Guan WJ, Ni ZY, Hu Y, et al.; China Medical Treatment Expert Group for Covid-19 . Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708–1720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhang JJ, Dong X, Cao YY, et al. . Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 2020;75:1730–1741 [DOI] [PubMed] [Google Scholar]
- 3.Zhou F, Yu T, Du R, et al. . Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–1062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fadini GP, Morieri ML, Longato E, Avogaro A. Prevalence and impact of diabetes among people infected with SARS-CoV-2. J Endocrinol Invest 2020;43:867–869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rentsch CT, Kidwai-Khan F, Tate JP, et al. . Covid-19 testing, hospital admission, and intensive care among 2,026,227 United States Veterans aged 54-75 years. 14 April 2020 [preprint]. medRxiv:10.1101/2020.04.09.20059964
- 6.Liu Y, Yang Y, Zhang C, et al. . Clinical and biochemical indexes from 2019-nCoV infected patients linked to viral loads and lung injury. Sci China Life Sci 2020;63:364–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gheblawi M, Wang K, Viveiros A, et al. . Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ Res 2020;126:1456–1474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lambert DW, Yarski M, Warner FJ, et al. . Tumor necrosis factor-alpha convertase (ADAM17) mediates regulated ectodomain shedding of the severe-acute respiratory syndrome-coronavirus (SARS-CoV) receptor, angiotensin-converting enzyme-2 (ACE2). J Biol Chem 2005;280:30113–30119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heurich A, Hofmann-Winkler H, Gierer S, Liepold T, Jahn O, Pöhlmann S. TMPRSS2 and ADAM17 cleave ACE2 differentially and only proteolysis by TMPRSS2 augments entry driven by the severe acute respiratory syndrome coronavirus spike protein. J Virol 2014;88:1293–1307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sungnak W, Huang N, Bécavin C, et al.; HCA Lung Biological Network . SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med 2020;26:681–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lukassen S, Chua RL, Trefzer T, et al. . SARS-CoV-2 receptor ACE2 and TMPRSS2 are primarily expressed in bronchial transient secretory cells. EMBO J 2020;39:e105114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang H, Kang Z, Gong H, et al. . Digestive system is a potential route of COVID-19: an analysis of single-cell coexpression pattern of key proteins in viral entry process. Gut 2020;69:1010–1018 [Google Scholar]
- 13.Butowt R, Bilinska K. SARS-CoV-2: olfaction, brain infection, and the urgent need for clinical samples allowing earlier virus detection. ACS Chem Neurosci 2020;11:1200–1203 [DOI] [PubMed] [Google Scholar]
- 14.Hoffmann M, Kleine-Weber H, Schroeder S, et al. . SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020;181:271–280.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Walls AC, Park YJ, Tortorici MA, Wall A, McGuire AT, Veesler D. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 2020;181:281–292.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Q, Zhang Y, Wu L, et al. . Structural and functional basis of SARS-CoV-2 entry by using human ACE2. Cell 2020;181:894–904.e9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shang J, Ye G, Shi K, et al. . Structural basis of receptor recognition by SARS-CoV-2. Nature 2020;581:221–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yan R, Zhang Y, Li Y, Xia L, Guo Y, Zhou Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020;367:1444–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lan J, Ge J, Yu J, et al. . Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020;581:215–220 [DOI] [PubMed] [Google Scholar]
- 20.Ou X, Liu Y, Lei X, et al. . Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat Commun 2020;11:1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Coutard B, Valle C, de Lamballerie X, Canard B, Seidah NG, Decroly E. The spike glycoprotein of the new coronavirus 2019-nCoV contains a furin-like cleavage site absent in CoV of the same clade. Antiviral Res 2020;176:104742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferretti L, Wymant C, Kendall M, et al. . Quantifying SARS-CoV-2 transmission suggests epidemic control with digital contact tracing. Science 2020;368:eabb6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fernandez C, Rysä J, Almgren P, et al. . Plasma levels of the proprotein convertase furin and incidence of diabetes and mortality. J Intern Med 2018;284:377–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang N, Shen HM. Targeting the endocytic pathway and autophagy process as a novel therapeutic strategy in COVID-19. Int J Biol Sci 2020;16:1724–1731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gassen NC, Niemeyer D, Muth D, et al. . SKP2 attenuates autophagy through Beclin1-ubiquitination and its inhibition reduces MERS-coronavirus infection. Nat Commun 2019;10:5770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Menikdiwela KR, Ramalingam L, Rasha F, et al. . Autophagy in metabolic syndrome: breaking the wheel by targeting the renin-angiotensin system. Cell Death Dis 2020;11:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang C, Wang S, Li D, Wei DQ, Zhao J, Wang J. Human intestinal defensin 5 inhibits SARS-CoV-2 invasion by cloaking ACE2. Gastroenterology. 11 May 2020 [Epub ahead of print]. DOI: 10.1053/j.gastro.2020.05.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kuba K, Imai Y, Rao S, et al. . A crucial role of angiotensin converting enzyme 2 (ACE2) in SARS coronavirus-induced lung injury. Nat Med 2005;11:875–879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Danser AHJ, Epstein M, Batlle D. Renin-angiotensin system blockers and the COVID-19 pandemic: at present there is no evidence to abandon renin-angiotensin system blockers. Hypertension 2020;75:1382–1385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jokubaitis VJ, Sinka L, Driessen R, et al. . Angiotensin-converting enzyme (CD143) marks hematopoietic stem cells in human embryonic, fetal, and adult hematopoietic tissues. Blood 2008;111:4055–4063 [DOI] [PubMed] [Google Scholar]
- 31.Park TS, Zambidis ET. A role for the renin-angiotensin system in hematopoiesis. Haematologica 2009;94:745–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jarajapu YP, Bhatwadekar AD, Caballero S, et al. . Activation of the ACE2/angiotensin-(1-7)/Mas receptor axis enhances the reparative function of dysfunctional diabetic endothelial progenitors. Diabetes 2013;62:1258–1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Duan Y, Beli E, Li Calzi S, et al. . Loss of angiotensin-converting enzyme 2 exacerbates diabetic retinopathy by promoting bone marrow dysfunction. Stem Cells 2018;36:1430–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inciardi RM, Lupi L, Zaccone G, et al. . Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;5:819–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shi S, Qin M, Shen B, et al. . Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol 2020;5:802–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guo T, Fan Y, Chen M, et al. . Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19). JAMA Cardiol 2020;5:811–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cheng Y, Luo R, Wang K, et al. . Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int 2020;97:829–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang K, Gheblawi M, Oudit GY. Angiotensin converting enzyme 2: a double-edged sword. Circulation 2020;142:426–428 [DOI] [PubMed] [Google Scholar]
- 39.Lamers MM, Beumer J, van der Vaart J, et al. . SARS-CoV-2 productively infects human gut enterocytes. Science 2020;369:50–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vuille-dit-Bille RN, Camargo SM, Emmenegger L, et al. . Human intestine luminal ACE2 and amino acid transporter expression increased by ACE-inhibitors. Amino Acids 2015;47:693–705 [DOI] [PubMed] [Google Scholar]
- 41.Garg M, Royce SG, Tikellis C, et al. . Imbalance of the renin-angiotensin system may contribute to inflammation and fibrosis in IBD: a novel therapeutic target? Gut 2020;69:841–851 [DOI] [PubMed] [Google Scholar]
- 42.Bader M, Alenina N, Young D, Santos RAS, Touyz RM. The meaning of Mas. Hypertension 2018;72:1072–1075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stevens BR, Ross HJ, Wright EM. Multiple transport pathways for neutral amino acids in rabbit jejunal brush border vesicles. J Membr Biol 1982;66:213–225 [DOI] [PubMed] [Google Scholar]
- 44.Tolhurst G, Zheng Y, Parker HE, Habib AM, Reimann F, Gribble FM. Glutamine triggers and potentiates glucagon-like peptide-1 secretion by raising cytosolic Ca2+ and cAMP. Endocrinology 2011;152:405–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vaduganathan M, Vardeny O, Michel T, McMurray JJV, Pfeffer MA, Solomon SD. Renin-angiotensin-aldosterone system inhibitors in patients with Covid-19. N Engl J Med 2020;382:1653–1659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Balmer ML, Schürch CM, Saito Y, et al. . Microbiota-derived compounds drive steady-state granulopoiesis via MyD88/TICAM signaling. J Immunol 2014;193:5273–5283 [DOI] [PubMed] [Google Scholar]
- 47.Duan Y, Prasad R, Feng D, et al. . Bone marrow-derived cells restore functional integrity of the gut epithelial and vascular barriers in a model of diabetes and ACE2 deficiency. Circ Res 2019;125:969–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yisireyili M, Uchida Y, Yamamoto K, et al. . Angiotensin receptor blocker irbesartan reduces stress-induced intestinal inflammation via AT1a signaling and ACE2-dependent mechanism in mice. Brain Behav Immun 2018;69:167–179 [DOI] [PubMed] [Google Scholar]
- 49.Verma A, Xu K, Du T, et al. . Expression of human ACE2 in Lactobacillus and beneficial effects in diabetic retinopathy in mice. Mol Ther Methods Clin Dev 2019;14:161–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Towler P, Staker B, Prasad SG, et al. . ACE2 X-ray structures reveal a large hinge-bending motion important for inhibitor binding and catalysis. J Biol Chem 2004;279:17996–18007 [DOI] [PMC free article] [PubMed] [Google Scholar]