Abstract
Type 1 diabetes (T1D) is an autoimmune disease of insulin-producing β-cells. Islet transplantation is a promising treatment for T1D, but long-term graft viability and function remain challenging. Oxidative stress plays a key role in the activation of alloreactive and autoreactive immunity toward the engrafted islets. Therefore, targeting these pathways by encapsulating islets with an antioxidant may delay immune-mediated rejection. Utilizing a layer-by-layer approach, we generated nanothin encapsulation materials containing tannic acid (TA), a polyphenolic compound with redox scavenging and anti-inflammatory effects, and poly(N-vinylpyrrolidone) (PVPON), a biocompatible polymer. We hypothesize that transplantation of PVPON/TA-encapsulated allogeneic C57BL/6 islets into diabetic NOD mice will prolong graft function and elicit localized immunosuppression. In the absence of systemic immunosuppression, diabetic recipients containing PVPON/TA-encapsulated islets maintained euglycemia and delayed graft rejection significantly longer than those receiving nonencapsulated islets. Transplantation of PVPON/TA-encapsulated islets was immunomodulatory because gene expression and flow cytometric analysis revealed significantly decreased immune cell infiltration, synthesis of reactive oxygen species, inflammatory chemokines, cytokines, CD8 T-cell effector responses, and concomitant increases in alternatively activated M2 macrophage and dendritic cell phenotypes. Our results provide evidence that reducing oxidative stress following allotransplantation of PVPON/TA-encapsulated islets can elicit localized immunosuppression and potentially delay graft destruction in future human islet transplantation studies.
Introduction
Autoimmune destruction of insulin-producing β-cells in type 1 diabetes (T1D) results in hyper- and hypoglycemic fluctuations as a result of the lifelong dependence on imperfect exogenous insulin therapies. This lack of proper glucose control has lasting impacts on the life expectancy of patients, increasing their risk for cardiovascular disease, neuropathies, kidney failure, and hypoglycemic unawareness, a feared complication that accounts for 10% of all deaths in patients with T1D (1,2). With the annual incidence rate of T1D increasing at ∼3% each year (3), the search for alternative treatment options has never been more vital.
One therapy that has shown clinical promise in restoring glycemic control and reducing the risk of hypoglycemic events is islet transplantation (4). Islets purified from cadaveric donors are infused into the portal vein of patients with T1D, resulting in >80% of islet transplant recipients becoming insulin independent (5). Systemic immunosuppression is necessary for clinical islet transplantation success but can also reactivate autoimmunity and proinflammatory effector responses involved in graft rejection, contributing to low graft viability and function after 5 years (6).
An innate immune effector molecule that contributes to islet transplantation rejection is reactive oxygen species (ROS) synthesis, which activates redox-dependent pathways involved in cell survival, proliferation, differentiation, and inflammation (7,8). The pancreatic β-cell is sensitive to ROS levels because of reduced levels of antioxidant defenses, such as superoxide dismutase, catalase, and glutathione peroxidase, compared with other tissues, including brain, liver, and kidney, leaving it at risk for redox-driven damage (9–11). During oxidative stress, redox signaling can aid in the activation of immune cells, maturation of effector responses, and enhancement of proinflammatory cytokine and chemokine synthesis (12–14). These signaling cascades can be downregulated in the presence of antioxidants such as catalase, superoxide dismutase, or exogenous free radical scavengers (15–17). Therefore, dampening the generation of ROS at the islet transplant site may suppress proinflammatory immune responses, delay islet graft destruction, and preserve islet function following transplantation.
One strategy to abrogate redox-dependent inflammatory responses without impeding islet function is to encapsulate islets in nanothin (<100 nm in thickness) conformal coatings to elicit localized immunosuppression. Unlike bulk encapsulating materials, nanothin coatings allow a fast exchange of nutrients, oxygen, and glucose-stimulated release of insulin and other endocrine hormones. The multilayer coating includes adsorption of water-soluble polymers on islet surfaces from aqueous solutions in a stepwise manner, leading to conformal polymeric coatings of controllable thickness, composition, and physicochemical properties (18). This approach is exciting because of the possibility of reducing or eliminating the dependence on toxic concentrations of systemic immunosuppressive regimens for islet transplant recipients. We have generated a novel multilayer coating consisting of tannic acid (TA), an immunomodulatory antioxidant rich in phenolic content, and poly(N-vinylpyrrolidone) (PVPON), a hydrophilic and nontoxic polymer, which are layered into a multilayer PVPON/TA coating and held through hydrogen bonds between phenolic groups of TA and carbonyls of PVPON (19) (Fig. 1A). The reliance of this multilayer approach on hydrogen-bonding interactions instead of ionic pairing of polymer layers eliminates the exposure of islets to toxic cationic species and involves noncharged biocompatible macromolecules. Given the sensitivity of β-cells to ROS synthesis, the addition of TA into the encapsulation material may provide additional antioxidant protection from the damaging effects of oxidative stress (9,11,20). We previously demonstrated that PVPON/TA-encapsulated islets maintained in vitro islet function, decreased autoimmune responses, scavenged ROS synthesis without displaying islet toxicity, and restored euglycemia in immunodeficient streptozotocin (STZ)-induced diabetic NOD.Rag mice (19,21,22). However, the ability of PVPON/TA-encapsulated islets to maintain function, delay graft rejection, and provide immunoprotection in mouse models of T1D is not known. Our report demonstrates that islet encapsulation with PVPON/TA layers can elicit localized immunosuppression, prolong islet allograft function, and delay graft rejection by decreasing proinflammatory chemokine synthesis, reducing inflammatory innate immune cell and effector T-cell migration within the islet graft, and concomitantly, enhancing anti-inflammatory M2 macrophage, dendritic cell (DC), and CD8 T-cell responses. The success of PVPON/TA coatings to protect against both autoimmune and alloimmune responses further emphasizes the future human translatability of this novel biotechnology for islet transplantation.
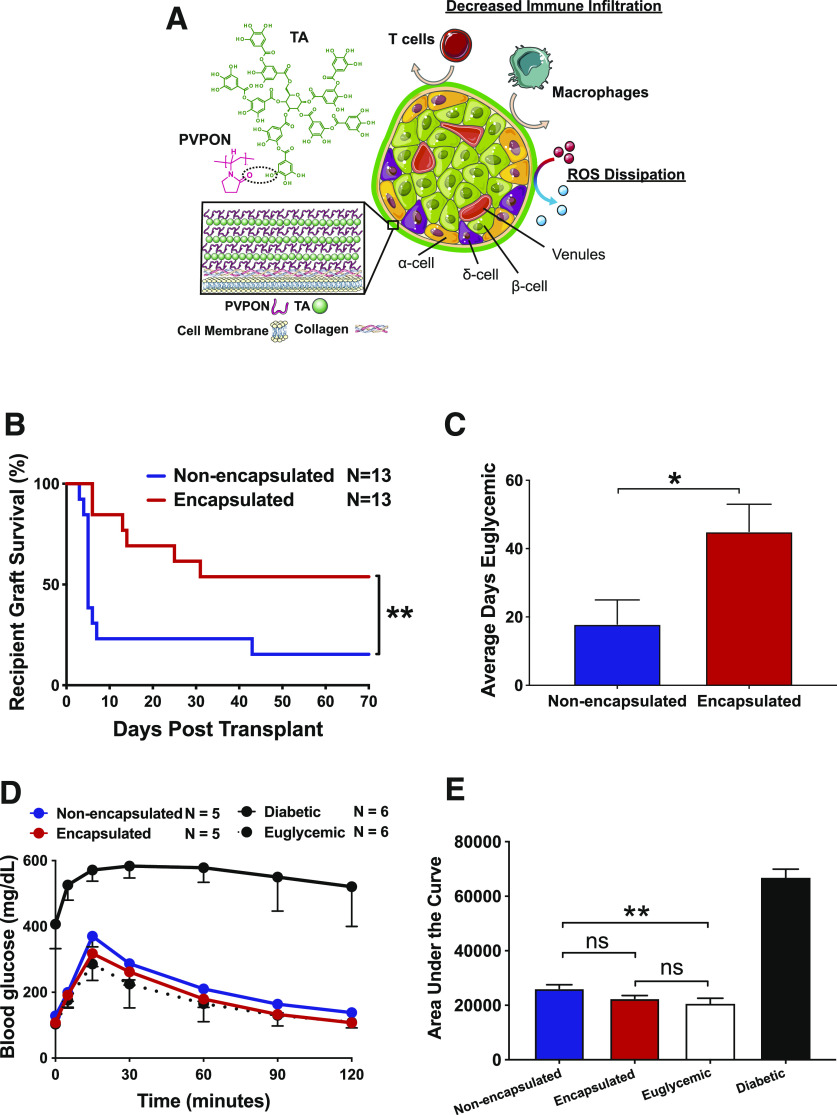
Figure 1.
Transplantation of PVPON/TA-encapsulated islets delays autoimmune-mediated graft failure in diabetic NOD mice. Schematic of PVPON/TA encapsulation of islets and strategy to elicit localized immunosuppression (A). Kaplan-Meier log-rank test of STZ-treated NOD recipients maintaining islet graft function on the basis of blood glucose readings (<300 mg/dL) after transplantation with 250 NOD.scid islets under the kidney capsule with or without PVPON/TA encapsulation (n = 13/group) (B). Student t test of average days recipients remained euglycemic (<300 mg/dL) after transplantation (C). IPGTT with transplanted recipients, diabetic (STZ-treated, nontransplanted) controls, and euglycemic nontransplanted controls at 2 weeks posttransplantation (n = 5–6) (D). Two-way ANOVA with multiple comparisons for area under the curve of the IPGTT assay (E). Data represent four independent experiments. *P < 0.05, **P < 0.01. ns, not significant.
Research Design and Methods
Mice
NOD/ShiLtJ, NOD.scid, and C57BL/6 mice were purchased from The Jackson Laboratory and housed under specific pathogen–free conditions on a 12-h light/dark cycle. Male and female mice between 8 and 12 weeks of age, or 12- to 16-week-old spontaneously diabetic female NOD mice (Supplementary Fig. 1), were used in all experiments in accordance with University of Alabama at Birmingham institutional animal care and use committee–approved mouse protocols.
Islet Isolation and Encapsulation
NOD.scid or C57BL/6 islets were isolated and encapsulated with 3.5 bilayers of hydrogen-bonded PVPON/TA multilayers, with PVPON as the first and the outer layer as described (19,21). PVPON (weight average molecular weight = 1,300,000 g/mol) and TA (molecular weight = 1,700 g/mol) were purchased from Thermo Fisher Scientific.
STZ Induction of Diabetes, Spontaneous Diabetes, and Islet Transplantation
NOD mice were intraperitoneally injected with 175 mg/kg STZ (Sigma-Aldrich) in PBS (pH 7.2) to induce and synchronize diabetes in islet transplant recipients. Diabetes was confirmed after two consecutive blood glucose readings ≥300 mg/dL with a Breeze 2 blood glucose meter (Bayer). Recent-onset diabetic female NOD mice within 1 week of diagnosis from our yearly incidence study were also used as islet transplant recipients to study the protective effects of PVPON/TA-encapsulated NOD.scid islets against autoimmune-mediated rejection. Euglycemia (blood glucose <200 mg/dL) was restored by transplanting 250 nonencapsulated or PVPON/TA-encapsulated NOD.scid or C57BL/6 islets under the kidney capsule as described (23,24). Mice were considered to maintain islet graft function until blood glucose readings ≥300 mg/dL after transplantation. STZ-treated, nontransplanted recipients were used as diabetic controls, and untreated NOD mice were used as euglycemic controls. Mock transplants under the kidney capsule were also performed on STZ-treated mice to distinguish among wound healing, surgery stress responses, and immune-mediated islet graft rejection. An intraperitoneal glucose tolerance test (IPGTT) was performed as we described (25). Kidney nephrectomy of transplanted islets was performed to confirm islet function and restoration of euglycemia in recipients.
Cell Surface and Intracellular Flow Cytometry
Single-cell suspensions of excised kidney islet grafts were made with a Dounce homogenizer, followed by red blood cell lysis, and resuspended at a concentration of 2 × 107 cells/mL. Cells were treated with GolgiPlug (BD Biosciences), Fc receptors were blocked (BD Biosciences), and surface or intracellular flow cytometry was performed according to the manufacturer’s protocol with fluorochrome-conjugated antibodies (Supplementary Table 1A) as described (14). For intracellular staining, cells were fixed and permeabilized (BD Cytofix/Cytoperm) per manufacturer instructions. Cells were collected by Attune NxT Flow Cytometer (Thermo Fisher Scientific) with 500,000 events/sample and analyzed with FlowJo version 10.0.8r1 software.
Quantitative RT-PCR
RNA was isolated from the excised islet graft using TRIzol (Invitrogen) and purified with the RNeasy Kit (QIAGEN). mRNA was converted to cDNA with SuperScript III (Invitrogen) and quantitated using TaqMan gene expression assays (Applied Biosystems) (Supplementary Table 1B) as described (21). Relative gene expression levels were calculated with the 2−ΔΔCt method; Gapdh and Cd8 were used as controls for normalization. Mock transplant samples were used as calibrator controls and set as 1.
Histology and Immunofluorescence
Following islet transplantation, recipients received an intraperitoneal injection of 0.5 g/kg 5,5-dimethyl-1-pyrroline N-oxide (DMPO) (Dojindo Molecular Technologies) every 6 h for a total of three injections (1.5 g/kg total) for immunospin trap detection as described (26). Grafts were excised 24 h (for DMPO tissues) or 40 days after transplantation, flash frozen in optimal cutting temperature compound, and sectioned for hematoxylin-eosin and immunofluorescent staining of free radical-DMPO adducts, islet hormones, and immune cells as described (21) (Supplementary Table 1C for antibody information). Slides were imaged using a Zeiss LSM710 confocal microscope and processed by Zen software (blue edition; Zeiss). Quantitation of DMPO intensity was determined using ImageJ version 1.52a software (National Institutes of Health).
Statistical Analysis
Data were analyzed using GraphPad Prism 8.3. Results were expressed as mean ± SEM. Percentage of euglycemic mice was assessed using the Kaplan-Meier test for cumulative survival, and log-rank test was used to compare survival curves. Differences in days euglycemic was determined using Student t test. Determination of the difference between mean values and SD for each experimental group was assessed using the two-way ANOVA with Tukey multiple comparisons test. In all tests, P < 0.05 was considered significant. All experiments were independently performed at least three times.
Data and Resource Availability
All data generated or analyzed during this study are included in the published article (and its Supplementary Material). The PVPON/TA encapsulation and transplantation protocols for islets generated and analyzed during the current study are available from the corresponding authors upon reasonable request.
Results
Transplantation of PVPON/TA-Encapsulated Islets Delays Autoimmune-Mediated Graft Failure
The induction of oxidative stress is a key innate immune-triggering event that can compromise islet function in both spontaneous T1D and islet transplantation (27). Since PVPON/TA coatings can decrease ROS, proinflammatory cytokines, and chemokines and provide immunoprotection from diabetogenic splenocytes (21,22), we hypothesized that decreasing ROS synthesis by PVPON/TA encapsulation of islets may elicit localized immunosuppression following islet transplantation into autoimmune-prone diabetic NOD mice. NOD mice are a preclinical model for T1D, and islet transplants into these mice can assess the immunoprotective efficacy of PVPON/TA encapsulation to delay both alloimmune and autoimmune responses following transplantation. To facilitate the synchronization of sufficient diabetic recipients for islet transplantation, we treated 8- to 10-week-old male and female NOD mice with STZ to induce diabetes. At this age for islet transplantation in our NOD colony, we can determine the immunoprotective potential of PVPON/TA coatings since insulitis is present and autoreactive T cells exhibit proinflammatory effector responses required for pancreatic β-cell destruction (J.M.B. and H.M.T., unpublished observations). In the absence of systemic immunosuppression, STZ-treated diabetic NOD recipients transplanted with PVPON/TA-encapsulated NOD.scid islets restored euglycemia and exhibited a significant delay in autoimmune-mediated islet graft rejection compared with nonencapsulated controls (Fig. 1B). Comparing the average days transplanted recipients remained euglycemic, we observed a significant increase in graft function with PVPON/TA-encapsulated grafts (Fig. 1C). At 2 weeks posttransplantation, we performed an IPGTT on euglycemic recipients and demonstrated that PVPON/TA-encapsulated islet grafts were glucose-responsive similar to euglycemic controls, while nonencapsulated islet grafts displayed partially impaired glycemic control (Fig. 1D and E). All transplanted mice became hyperglycemic (>300 mg/dL) within 24 h following the removal of the kidney containing the islet graft (data not shown). Not only was immunoprotection of PVPON/TA-encapsulated islets observed in STZ-induced diabetic NOD mice but also transplantation of PVPON/TA-encapsulated NOD.scid islets significantly delayed autoimmune-mediated destruction when transplanted into 12- to 16-week-old recent-onset (<1 week) spontaneously diabetic NOD mice in contrast to nonencapsulated islets (Supplementary Fig. 1). This observation supports our hypothesis that transplantation of PVPON/TA-encapsulated islets can restore euglycemia.
PVPON/TA Encapsulation Promotes an Anti-inflammatory Innate Immune Phenotype
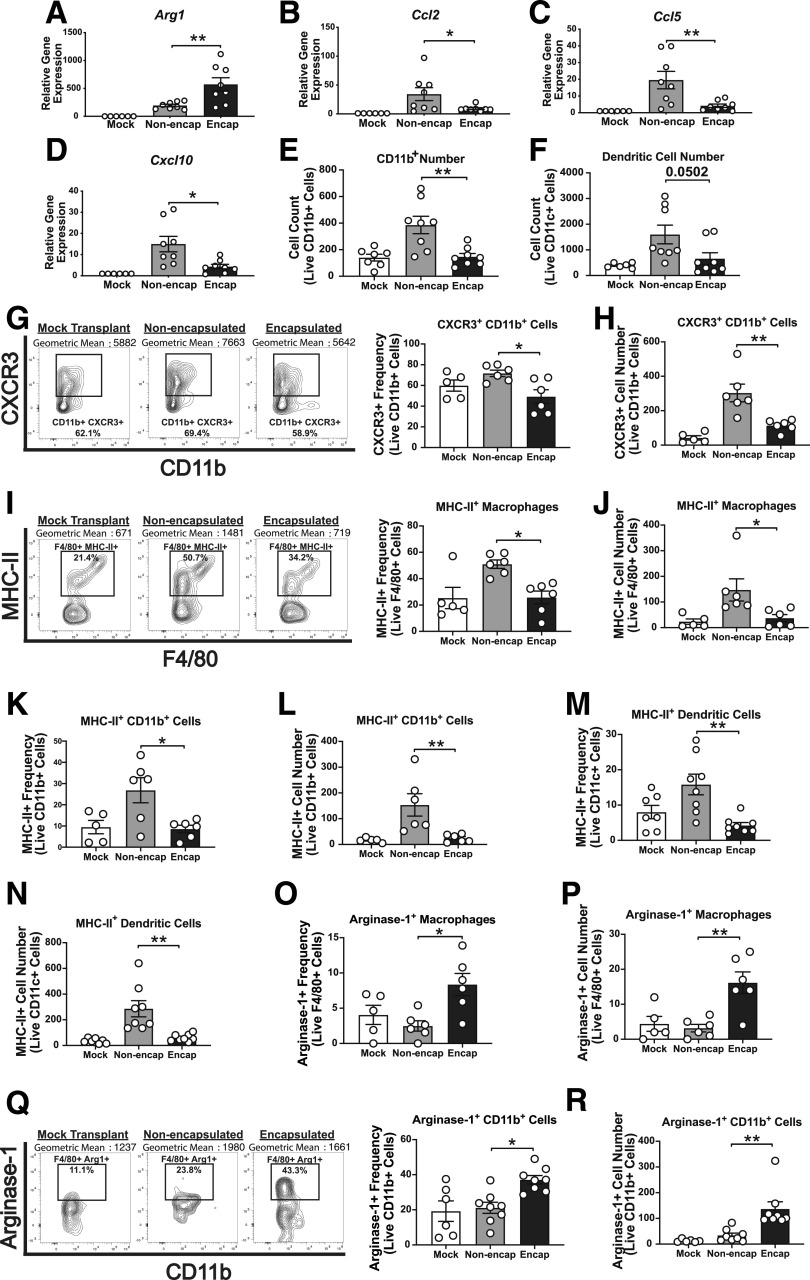
To define whether PVPON/TA encapsulation can elicit localized immunosuppression on innate immune responses during autoimmune-mediated islet transplantation rejection in NOD mice (Fig. 1B), mRNA analysis at 5 days posttransplantation and flow cytometric analysis (gating strategy shown in Supplementary Fig. 2) at 7 days posttransplantation with NOD.scid islets were performed (Figs. 2 and 4–6). We observed a significant increase in the anti-inflammatory gene Arg1 (Fig. 2A) and a significant decrease in the mRNA accumulation of proinflammatory chemokines Ccl2 (Fig. 2B), Ccl5 (Fig. 2C), and Cxcl10 (Fig. 2D) within our encapsulated islet grafts compared with nonencapsulated islets. Confirming the gene expression data, flow cytometry analysis showed significantly reduced numbers of CD11b+ cells (Fig. 2E), CD11c+ DCs (Fig. 2F), and reduced frequencies (Fig. 2G) and cell numbers (Fig. 2H) of CD11b+ cells expressing the chemokine receptor CXCR3+ within encapsulated grafts. We also observed significant reductions in the frequency and cell number of MHC-II+ expression on macrophages (Fig. 2I and J), CD11b+ cells (Fig. 2K and L), and CD11c+ DCs (Fig. 2M and N). Corroborating the decrease in proinflammatory innate immune cells, an increase in the frequencies and cell numbers of arginase-1+ macrophages (Fig. 2O and P) and arginase-1+ CD11b+ cells (Fig. 2Q and R) were present following transplantation of PVPON/TA-encapsulated islets. Our results demonstrate that transplantation of PVPON/TA-encapsulated islets can promote an alternatively activated macrophage phenotype, suppress chemokine synthesis, and reduce MHC-II expression of innate immune cells compared with nonencapsulated islets. The decrease in proinflammatory innate immune responses with PVPON/TA-encapsulated islets can inhibit T-cell migration and contribute to localized immunosuppression because a significant reduction in the numbers of islet graft–infiltrating CD8 T cells was observed (Supplementary Fig. 3A).
Figure 2.
Encapsulated NOD.scid islets skew innate immune responses toward an anti-inflammatory phenotype. NOD.scid islet grafts were excised from STZ-treated NOD mice, and gene expression (day 5) and flow cytometry (day 7) were performed. Graft mRNA was analyzed by quantitative RT-PCR for alternatively activated M2 macrophage marker Arg1 (A) and Ccl2 (B), Ccl5 (C), and Cxcl10 chemokines (D). Flow cytometry analysis of CD11b+ cell number (E), DC number (F), CXCR3+ CD11b+ cell frequency (G), CXCR3+ CD11b+ cell number (H), MHC-II+ macrophage frequency (I), MHC-II+ macrophage number (J), MHC-II+ CD11b+ cell frequency (K), MHC-II+ CD11b+ cell number (L), MHC-II+ DC frequency (M), MHC-II+ CD11c+ cell number (N), arginase-1+ macrophage frequency (O), arginase-1+ macrophage cell number (P), arginase-1+ CD11b+ cell frequency (Q), and arginase-1+ CD11b+ cell number (R) (n = 5–10). Analyzed by two-way ANOVA with multiple comparisons. Data represent three independent experiments. *P < 0.05, **P < 0.01.
Figure 4.
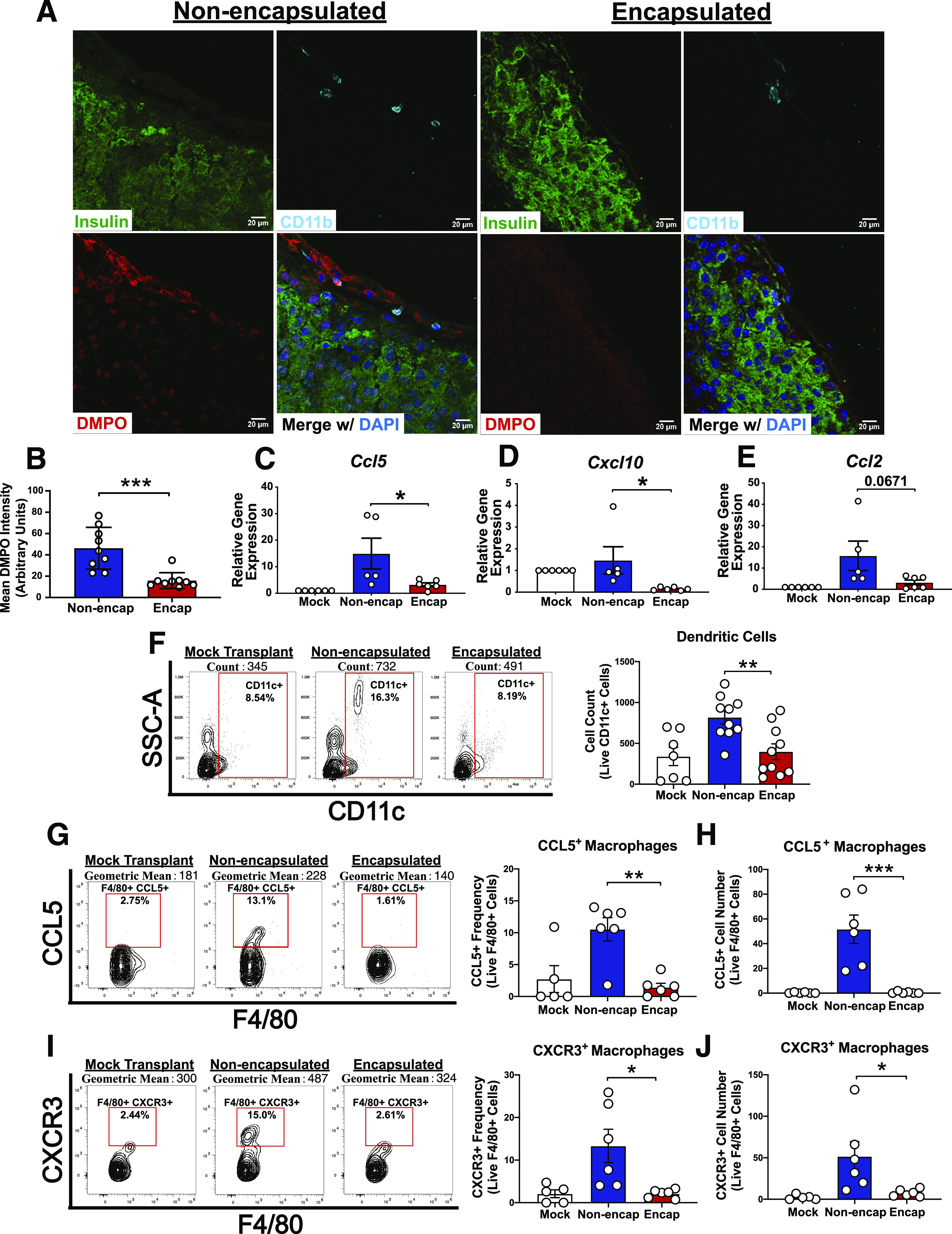
PVPON/TA-encapsulated islet allografts reduce free radical-DMPO adduct formation and chemokine production following allotransplantation. C57BL/6 islet allografts were excised from the kidney capsule 24 h after transplantation into STZ-treated NOD mice and stained for insulin (green), DMPO (red), CD11b (cyan), and DAPI (blue) to detect free radical-DMPO adducts (A). DMPO adduct intensity quantitation analyzed with Student t test (B). C57BL/6 islet allografts were excised from the kidney capsule of STZ-treated NOD mice, and gene expression (day 5) and flow cytometry (day 7) were performed. Graft mRNA was analyzed by quantitative RT-PCR for chemokines Ccl5 (C), Cxcl10 (D), and Ccl2 (E) (n = 5–6). Flow cytometry of islet allograft-infiltrating CD11c+ DC numbers (F), frequency of F4/80+ macrophages expressing CCL5+ (G), cell number of F4/80+ macrophages expressing CCL5+ (H), frequency of F4/80+ macrophages expressing CXCR3+ (I), and cell number of F4/80+ macrophages expressing CXCR3+ (J) (n = 5–10). Gene expression and flow cytometry analyzed by two-way ANOVA with multiple comparisons. Data represent three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001. SSC-A, side scatter area.
Figure 6.
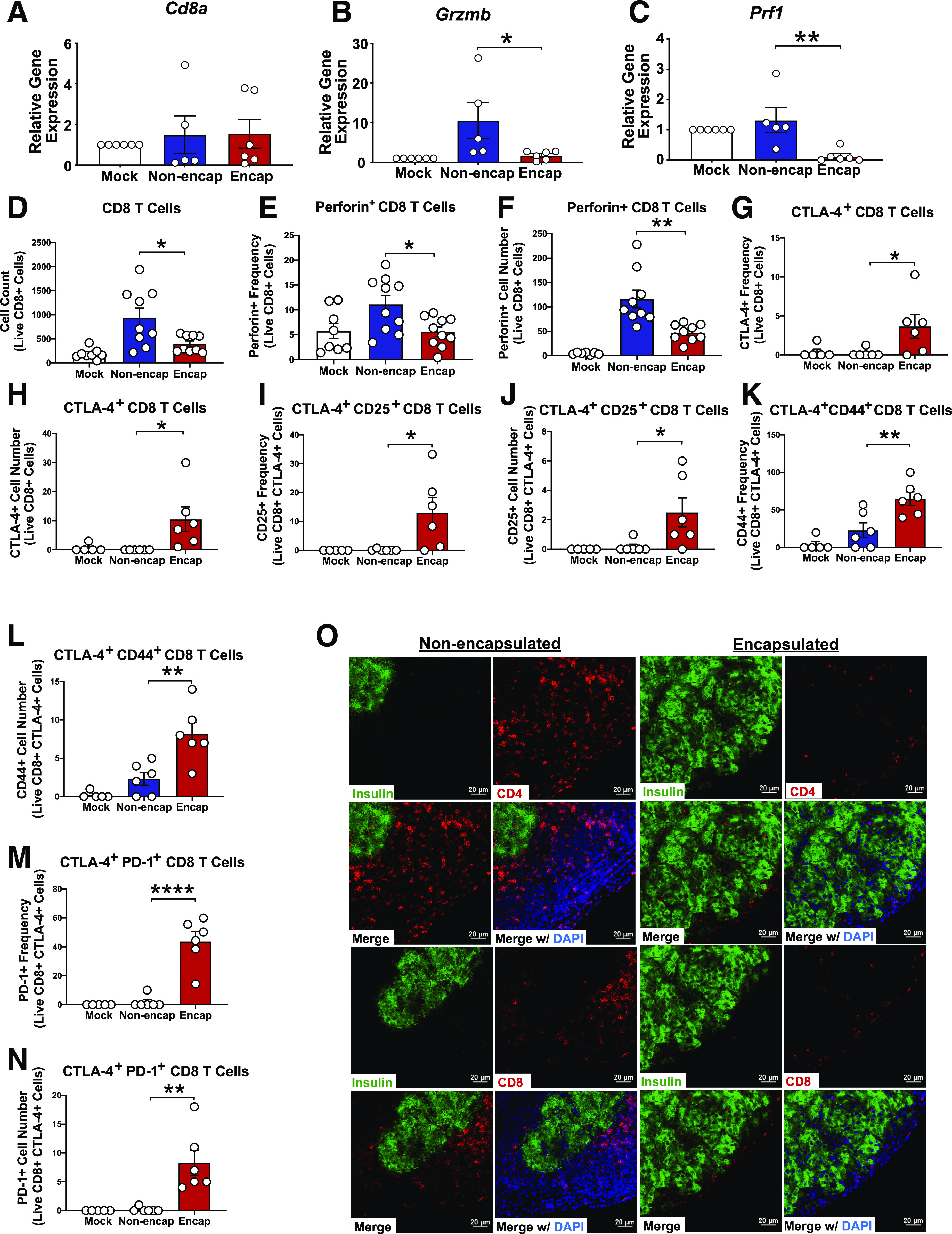
Transplantation of PVPON/TA-encapsulated islets abrogate T-cell infiltration and effector responses. C57BL/6 islet allografts were excised from STZ-treated NOD mice at day 5 posttransplantation for quantitative RT-PCR analysis of T-cell markers Cd8a (A), Grzmb (B), and Prf1 (C) normalized to Gapdh. Flow cytometry at day 7 posttransplantation of islet-infiltrating CD8 T-cell numbers (D), frequency (E) and cell number (F) of perforin+ CD8 T cells, frequency (G) and cell number (H) of CTLA-4+ CD8 T cells, frequency (I) and cell number (J) of CTLA-4+ CD25+ CD8 T cells, frequency (K) and cell number (L) of CTLA-4+ CD44+ CD8 T cells, and frequency (M) and cell number (N) of CTLA-4+ PD-1+ CD8 T cells (n = 5–10). Analyzed by two-way ANOVA with multiple comparisons. Kidney islet allografts were excised 40 days posttransplant, sectioned, and then stained with immunofluorescence to detect CD4 or CD8 T cells (red), insulin (green), or DAPI in nonencapsulated and PVPON/TA-encapsulated islets (O). Data represent three independent experiments. *P < 0.05, **P < 0.01, ****P < 0.0005.
PVPON/TA-Encapsulated Allogeneic Islets Delay Graft Failure in Autoimmune-Prone Mice
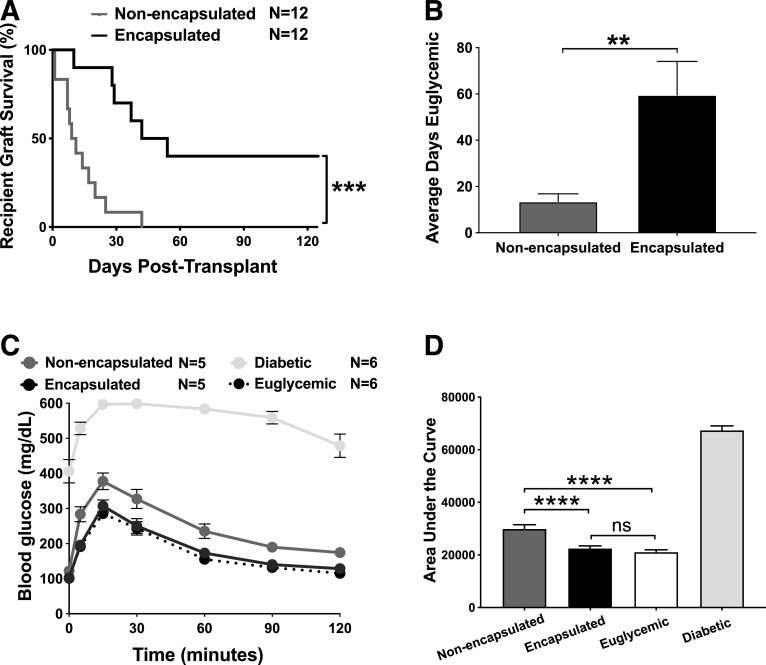
Our results demonstrate that PVPON/TA-encapsulated islets can delay autoimmune-mediated graft destruction (Fig. 1B) and proinflammatory innate immune responses (Fig. 2), but future clinical application in patients with T1D must also elicit localized immunosuppression against alloimmune-mediated inflammatory responses. To determine the clinical efficacy of PVPON/TA encapsulation for islet allotransplants in the absence of immunosuppression, PVPON/TA-encapsulated or nonencapsulated C57BL/6 islets were transplanted into STZ-treated diabetic NOD mice without exogenous immunosuppression. We observed a significant delay in alloimmune-mediated islet graft rejection compared with nonencapsulated controls (Fig. 3A). We also observed a significant increase in the average days PVPON/TA-encapsulated allografts maintained euglycemia compared with nonencapsulated controls (Fig. 3B). At 2 weeks posttransplantation, an IPGTT on euglycemic recipients demonstrated that PVPON/TA-encapsulated islet grafts were glucose responsive similar to euglycemic controls, while nonencapsulated islet grafts displayed partially impaired glycemic control (Fig. 3C and D). These glucose clearance data suggest that PVPON/TA encapsulation has significant beneficial effects on systemic glucose homeostasis and islet function compared with nonencapsulated islet grafts (Fig. 3D). Collectively, our data reveal that conformal coating of islets with PVPON/TA can delay both autoimmune- (Fig. 1B and Supplementary Fig. 1) and alloimmune-mediated rejection (Fig. 3A) of transplanted islets and maintain islet graft function in the absence of systemic immunosuppression.
Figure 3.
Transplantation of PVPON/TA-encapsulated allogeneic islets delays graft failure in diabetic autoimmune-prone mice. Kaplan-Meier log-rank test for percent of STZ-treated NOD recipients maintaining islet allograft function and euglycemia on the basis of blood glucose readings (<300 mg/dL) after transplantation with 250 C57BL/6 islets under the kidney capsule with or without PVPON/TA encapsulation (A) (n = 12). Student t test of average days allograft recipients remained euglycemic (<300 mg/dL) after transplantation (B). IPGTT with allotransplanted recipients, diabetic (STZ-treated, nontransplanted) controls, and euglycemic nontransplanted controls at 2 weeks posttransplantation (C) (n = 5–6). Two-way ANOVA with multiple comparisons for area under the curve of IPGTT assay (D). Data represent four independent experiments. **P < 0.01, ***P < 0.001, ****P < 0.0005. ns, not significant.
Allotransplantation of PVPON/TA-Encapsulated Islets Can Elicit Localized Immunosuppression
To demonstrate that PVPON/TA coatings can decrease in vivo ROS synthesis after allogeneic islet transplantation, we used DMPO, a spin trap that will form free radical adducts detectable by immunofluorescence (28). NOD recipients treated with DMPO and allotransplanted with nonencapsulated or PVPON/TA-encapsulated C57BL/6 islets were euthanized 24 h posttransplantation for the detection of free radical-DMPO adducts at the transplant site. PVPON/TA-encapsulated grafts displayed significantly reduced free radical-DMPO adduct formation compared with nonencapsulated controls that colocalized with CD11b+, insulin+ (Fig. 4A and B), and Ly6G+ cells (data not shown). These results demonstrate that transplantation of PVPON/TA-encapsulated islets can suppress ROS synthesis by islet-infiltrating myeloid cells and insulin-producing β-cells in vivo.
To further determine the immunoprotective effects of PVPON/TA encapsulation on innate immune responses during islet allotransplantation, mRNA analysis displayed a significant decrease in the mRNA accumulation of proinflammatory chemokines Ccl5 (Fig. 4C) and Cxcl10 (Fig. 4D) and a decrease in Ccl2 (Fig. 4E) expression within our encapsulated islet grafts compared with controls at 5 days posttransplantation. Corroborating the reductions in proinflammatory chemokine gene expression, flow cytometry analysis showed significantly reduced CD11c+ DC (Fig. 4F) migration within our PVPON/TA-encapsulated grafts and significant reductions in the frequency and cell number of CCL5+ (Fig. 4G and H) and CXCR3+ (Fig. 4I and J) macrophage populations in allotransplanted PVPON/TA-encapsulated grafts at 7 days posttransplantation. Importantly, these phenotypes observed during alloimmune graft rejection are similar to our results in the autoimmune rejection model (Fig. 2).
Encapsulated grafts also displayed a significant increase in alternatively activated M2 macrophage-associated Arg1 (Fig. 5A), Retnla (Fig. 5B), and Ccl17 (Fig. 5C) mRNA and significantly increased frequencies and cell number of arginase-1+ CD11c+ DCs (Fig. 5D and E), arginase-1+ macrophages (Fig. 5F and G), and CD206+ macrophages (Supplementary Fig. 3J and K) by flow cytometry. While no significant transcriptional changes in macrophage marker F4/80 (Emr1), inducible nitric oxide synthase (Nos2), mannose receptor (Mrc1), or tumor necrosis factor (TNF) (Tnf) were detected (Supplementary Fig. 3B–E), we observed significantly reduced frequencies and cell numbers of TNF+ CD11c+ DCs (Fig. 5H and I), MHC-II+ CD11c+ DCs (Fig. 5J and K), MHC-II+ macrophages (Fig. 5L and M), MHC-II+ CD11b+ cells (Supplementary Fig. 3N and O), TNF+ CD11b+ cells (Supplementary Fig. 3L and M), and CD11b+ cells (Supplementary Fig. 3H and I) with PVPON/TA encapsulation, demonstrating a shift toward an anti-inflammatory environment and induction of localized immunosuppression.
Figure 5.
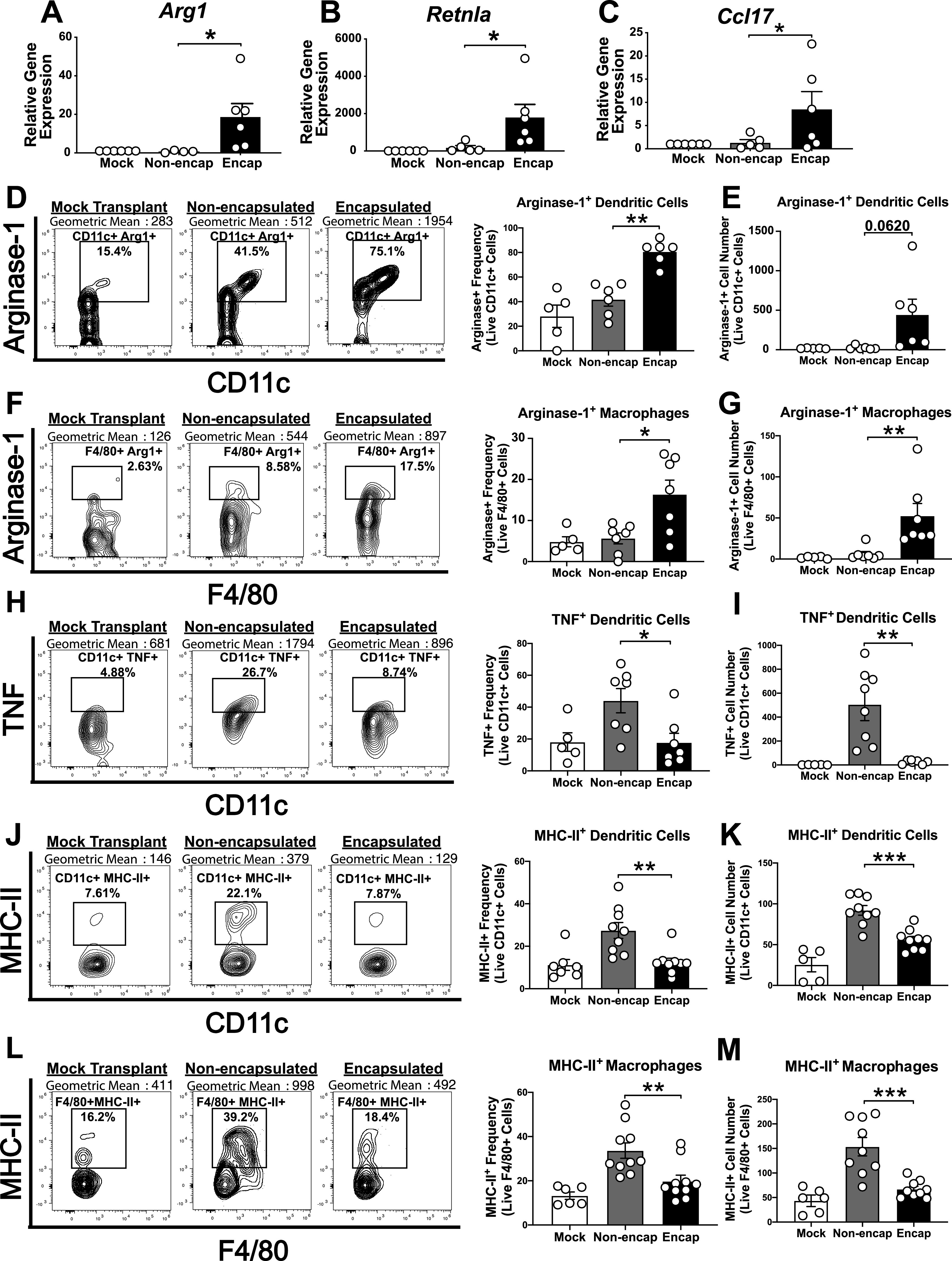
Encapsulated allogeneic islets skew innate immune responses toward anti-inflammatory phenotypes. C57BL/6 islet allografts were excised from STZ-treated NOD mice, and gene expression (day 5) and flow cytometry (day 7) were performed. Graft mRNA was analyzed by quantitative RT-PCR for alternatively activated M2 macrophage markers Arg1 (A), Retnla (B), and Ccl17 (C). Flow cytometry analysis of the frequency and cell number of arginase-1+ CD11c+ DCs (D and E), arginase-1+ F4/80+ macrophages (F and G), TNF+ CD11c+ DCs (H and I), MHC-II+ CD11c+ DCs (J and K), and MHC-II+ F4/80+ macrophages (L and M) (n = 5–10). Analyzed by two-way ANOVA with multiple comparisons. Data represent three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001.
PVPON/TA-Encapsulated Islets Abrogate T-Cell Infiltration and Effector Responses
Since we observed a decrease in innate immune cell infiltration, synthesis of ROS, cytokines, and chemokines, we next wanted to determine whether T-cell infiltration and effector responses were compromised because they directly mediate β-cell destruction during islet transplant rejection (29). Gene expression at 5 days posttransplantation did not show any difference in total Cd8a mRNA accumulation (Fig. 6A), but we observed significant reductions in CD8 T-cell effector Grzmb (Fig. 6B) and Prf1 (Fig. 6C) mRNA within PVPON/TA-encapsulated islet allografts. These differences were maintained when normalized to Cd8a (Supplementary Fig. 3F and G). Flow cytometric analysis at 7 days posttransplantation confirmed that PVPON/TA-encapsulated islets significantly decreased CD8+ T-cell infiltration (Fig. 6D) and perforin+ CD8 T-cell frequencies (Fig. 6E) and cell numbers (Fig. 6F). Additionally, PVPON/TA-encapsulated grafts displayed a significant increase in an anti-inflammatory T-cell response as shown by an increase in CTLA-4+ CD8 T-cell frequency (Fig. 6G) and cell number (Fig. 6H) compared with nonencapsulated grafts. These CTLA-4+ CD8 T cells within our PVPON/TA-encapsulated grafts also displayed significantly enhanced activation profiles, including an increase in the frequency and cell number of CD25 (Fig. 6I and J), CD44 (Fig. 6K and L), and PD-1 (Fig. 6M and N) expression. To further investigate the effects of PVPON/TA encapsulation on adaptive immune cell infiltration, we excised islet allografts at 40 days posttransplantation and performed histology and immunofluorescence staining. PVPON/TA-encapsulated grafts displayed intact islet morphology and insulin expression, while nonencapsulated islet grafts were heavily infiltrated with CD4 and CD8 T cells (Fig. 6O). Our results support the hypothesis that PVPON/TA encapsulation of islets can elicit localized immunosuppression of infiltrating T cells to delay allograft rejection.
Discussion
We demonstrate that encapsulation of islets with PVPON/TA can prolong islet graft function and delay both autoimmune and allogeneic graft rejection in the absence of systemic immunosuppression. Dissipation of ROS synthesis at the site of transplantation shows promise as a potential strategy to elicit localized immunosuppression. Encapsulation with PVPON/TA can protect insulin-secreting β-cells from the damaging effects of oxidative stress and importantly, suppress redox-dependent signals that are necessary to mature and enhance innate and adaptive immune proinflammatory responses involved in islet graft rejection. These data support our hypothesis that decreasing ROS synthesis with PVPON/TA encapsulation of islets can induce localized immunosuppression that is partly due to an increase in alternatively activated M2 macrophages, arginase-1–expressing DCs, and diminished proinflammatory chemokines and cytotoxic CD8 T-cell effector responses.
Using STZ treatment to synchronize hyperglycemia, we transplanted NOD.scid (Fig. 1) or allogeneic C57BL/6 islets (Fig. 3) into STZ-treated diabetic NOD mice. In both autoimmune- and alloimmune-mediated rejection models, we observed significant delays in islet graft failure with PVPON/TA encapsulation. In addition to STZ induction of hyperglycemia, transplantation of PVPON/TA-encapsulated NOD.scid islets into recent-onset spontaneously diabetic female NOD mice was also effective in significantly delaying islet graft failure (Supplementary Fig. 1). These results demonstrate that under different models of alloimmune- and autoimmune-mediated graft rejection, suppressing ROS synthesis with PVPON/TA layers can improve islet graft function and delay immune-mediated rejection.
Our immunospin trap studies are the first to highlight the detection of ROS synthesis following islet allotransplantation by macrophages and β-cells. Importantly, encapsulation of islets with PVPON/TA coatings can significantly dampen free radical-DMPO adduct synthesis at the site of transplantation. Previous studies have shown that abrogating ROS synthesis with antioxidant treatment can decrease the ability of macrophages and DCs to upregulate MHC-II, present antigen, and stimulate T cells (30–32). Therefore, reducing the early generation of ROS following islet transplantation with our PVPON/TA coatings may inhibit the capacity of antigen-presenting cells to properly activate allo- and autoreactive T cells involved in islet transplantation rejection by decreasing the expression of ROS, MHC-II, costimulatory molecules, and proinflammatory cytokines. Our data support this hypothesis, as immunophenotyping of transplanted PVPON/TA-encapsulated islet grafts displayed a reduction in MHC-II expression in macrophage and DC innate immune subsets during both autoimmune- and alloimmune-associated responses (Figs. 2 and 5) as well as reductions in TNF+-expressing DC populations (Fig. 5).
Additionally, suppressing ROS synthesis with PVPON/TA-encapsulated islets not only will protect islets from free radical–mediated damage but also may suppress the synthesis of redox-dependent inflammatory signaling molecules, such as proinflammatory CCL2, CCL5, and CXCL10 (33,34) chemokines, that can further propagate immune-mediated rejection of transplanted islets. These chemokines play an important role in both T1D disease progression (35–37) and islet graft rejection (38). Our results highlight that one mechanism of immunoprotection with PVPON/TA encapsulation of islets could also be due to suppressing chemokine synthesis and prolonging islet graft survival. During both auto- and allogeneic islet graft rejection, PVPON/TA-encapsulated islets significantly reduced levels of proinflammatory CCL2, CCL5, and CXCL10 chemokines, CXCR3 chemokine receptor–positive innate immune cell populations, and islet-infiltrating immune cell subsets at the islet graft site (Figs. 2 and 4).
ROS generation can influence proinflammatory M1 macrophage differentiation (14,39,40), while reductions in ROS synthesis with genetic mouse models or antioxidant treatment can promote an alternatively activated M2 macrophage phenotype (14,41). In both our autoimmune-mediated and our allogeneic islet transplantation models, we demonstrated that PVPON/TA-encapsulated grafts delayed immune-mediated rejection that is partly due to the differentiation of arginase-1 DCs and alternatively activated M2 macrophages expressing arginase-1, Retnla, and Ccl17 mRNA accumulation (Figs. 2 and 5). Considering the important role of proinflammatory M1 macrophages in the initiation of T1D (42) and the immunoprotective role of M2 macrophages (14,43–45), our results provide further evidence that altering the redox balance of immune responses by decreasing ROS with PVPON/TA coatings, antioxidants, or ROS-deficient mouse models can elicit an alternatively activated M2 macrophage phenotype and delay islet graft destruction and autoimmune diabetes (25,34,46–48).
Collectively, these changes in the innate immune response can mitigate efficient adaptive immune responses. From our immunophenotyping studies with auto- and allogeneic islet transplants into diabetic NOD mice, PVPON/TA-encapsulated grafts significantly reduced CD8 T-cell infiltration, most likely as a result of the reductions seen in inflammatory chemokine synthesis. The effector responses of these cells were also affected because PVPON/TA-encapsulated grafts demonstrated reduced Prf1 and Grzmb mRNA accumulation and the frequency of islet-infiltrating perforin+ CD8 T cells by flow cytometry (Fig. 6). While we did not observe any significant changes in either gene expression or flow cytometry with immunosuppressive CD4 regulatory T-cell populations (data not shown), we did identify a significant increase in activated CTLA-4+ CD8 T cells recruited to the site of PVPON/TA-encapsulated islets. Whether this population of CD8 T cells is regulatory and elicits an anti-inflammatory immune response remains to be determined in future studies. Our strategy to decrease ROS synthesis by encapsulating islets with PVPON/TA coatings can mediate localized immunosuppression, inhibit proinflammatory immune responses, and delay islet graft rejection.
Future studies will also investigate how PVPON/TA encapsulation may elicit localized immunosuppression by inhibiting the activation of ROS-dependent signaling pathways involved in maturing innate and adaptive immune effector responses (27,48). These pathways include mitogen-activated protein kinase, Janus kinase/signal transducer and activator of transcription, and nuclear factor-κB that are redox regulated by H2O2 and necessary for optimal immune cell activation and proinflammatory chemokine and cytokine synthesis (15,27). We previously demonstrated that PVPON/TA is effective in decreasing H2O2 synthesis in vitro (21), and with the decrease of free radical-DMPO adducts observed in vivo following transplantation, we postulate that the absence of H2O2 and inability to activate redox-dependent signaling cascades necessary for proinflammatory M1 macrophage polarization (14) may partly explain the observed immunoprotection and localized immunosuppression.
Our approach to inhibit proinflammatory immune responses with PVPON/TA-encapsulated islets without systemic immunosuppression is a significant advancement toward successful islet transplants in humans. PVPON/TA coatings can be modified to increase the number of layers of PVPON and TA for encapsulation, complexed with immune inhibitory receptors, including CTLA-4, PD-L1, and/or anti-inflammatory cytokines (interleukin 10, transforming growth factor-β), to further enhance localized immunosuppression. The use of PVPON/TA coatings is not limited to encapsulation of human islets, as our preliminary studies also demonstrated that PVPON/TA encapsulation does not compromise neonatal porcine islet function (submitted to American Journal of Transplantation) and can also be expanded to include human stem-cell–derived pancreatic β-cells (49,50). As opposed to alginate encapsulation, PVPON/TA capsules do not significantly increase the volume of a human islet allograft, thereby allowing for transplantation into feasible extrahepatic sites, such as the omentum, or subcutaneously. The adaptability of PVPON/TA coatings with various technologies further emphasizes the novelty of this biomaterial to elicit localized immunosuppression and delay islet graft rejection in future translational studies.
Article Information
Acknowledgments. The authors are grateful to Katie Heath and Samuel Blum and Drs. Chad Hunter, Jared Taylor, Joseph Feduska (The University of Alabama at Birmingham), and Lindsey Padgett (La Jolla Institute for Immunology) for critical reading of the manuscript and Dr. Robert Oster (The University of Alabama at Birmingham) for assistance with statistical analyses.
Funding. This work was supported by National Institute of General Medical Sciences Translational and Molecular Sciences grant T32-GM-109780 and Cell, Molecular, and Developmental Biology training grant T32-GM-008111 (J.M.B.); National Science Foundation-Division of Materials Research grant 1608728 (E.K.); National Institute of Diabetes and Digestive and Kidney Diseases R01 award DK-099550 (H.M.T.); and JDRF awards SRA-2016-270-S-B and 2-SRA-2019-692-S-B (H.M.T.).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. J.M.B. designed the research studies, conducted experiments, acquired data, analyzed data, and wrote the manuscript. V.K. and E.K. designed the research studies, conducted experiments, analyzed data, and wrote the manuscript. H.M.T. designed the research studies, analyzed data, and wrote the manuscript. H.M.T. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this article were presented in abstract form at the 78th Scientific Sessions of the American Diabetes Association, Orlando, FL, 22–26 June 2018; the 79th Scientific Sessions of the American Diabetes Association, San Francisco, CA, 7–11 June 2019; and the 80th Scientific Sessions of the American Diabetes Association, 12–16 June 2020.
Footnotes
This article contains supplementary material online at https://doi.org/10.2337/figshare.12497741.
E.K. and H.M.T. share equal seniority.
References
- 1.Steffes MW, Sibley S, Jackson M, Thomas W. Beta-cell function and the development of diabetes-related complications in the diabetes control and complications trial. Diabetes Care 2003;26:832–836 [DOI] [PubMed] [Google Scholar]
- 2.Cryer PE. Severe hypoglycemia predicts mortality in diabetes. Diabetes Care 2012;35:1814–1816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Patterson CC, Gyürüs E, Rosenbauer J, et al. . Trends in childhood type 1 diabetes incidence in Europe during 1989-2008: evidence of non-uniformity over time in rates of increase. Diabetologia 2012;55:2142–2147 [DOI] [PubMed] [Google Scholar]
- 4.Pepper AR, Bruni A, Shapiro AMJ. Clinical islet transplantation: is the future finally now? Curr Opin Organ Transplant 2018;23:428–439 [DOI] [PubMed] [Google Scholar]
- 5.Shapiro AM, Pokrywczynska M, Ricordi C. Clinical pancreatic islet transplantation. Nat Rev Endocrinol 2017;13:268–277 [DOI] [PubMed] [Google Scholar]
- 6.Monti P, Scirpoli M, Maffi P, et al. . Islet transplantation in patients with autoimmune diabetes induces homeostatic cytokines that expand autoreactive memory T cells. J Clin Invest 2008;118:1806–1814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Paiva CN, Bozza MT. Are reactive oxygen species always detrimental to pathogens? Antioxid Redox Signal 2014;20:1000–1037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Trachootham D, Lu W, Ogasawara MA, Nilsa RD, Huang P. Redox regulation of cell survival. Antioxid Redox Signal 2008;10:1343–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lenzen S, Drinkgern J, Tiedge M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic Biol Med 1996;20:463–466 [DOI] [PubMed] [Google Scholar]
- 10.Tiedge M, Lortz S, Drinkgern J, Lenzen S. Relation between antioxidant enzyme gene expression and antioxidative defense status of insulin-producing cells. Diabetes 1997;46:1733–1742 [DOI] [PubMed] [Google Scholar]
- 11.Miki A, Ricordi C, Sakuma Y, et al. . Divergent antioxidant capacity of human islet cell subsets: a potential cause of beta-cell vulnerability in diabetes and islet transplantation. PLoS One 2018;13:e0196570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu C, Whitener RL, Lin A, et al. . Neutrophil cytosolic factor 1 in dendritic cells promotes autoreactive CD8 + T cell activation via cross-presentation in type 1 diabetes. Front Immunol 2019;10:952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Padgett LE, Tse HM. NADPH oxidase-derived superoxide provides a third signal for CD4 T cell effector responses. J Immunol 2016;197:1733–1742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Padgett LE, Burg AR, Lei W, Tse HM. Loss of NADPH oxidase-derived superoxide skews macrophage phenotypes to delay type 1 diabetes. Diabetes 2015;64:937–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flohé L, Brigelius-Flohé R, Saliou C, Traber MG, Packer L. Redox regulation of NF-kappa B activation. Free Radic Biol Med 1997;22:1115–1126 [DOI] [PubMed] [Google Scholar]
- 16.Kyaw M, Yoshizumi M, Tsuchiya K, Kirima K, Tamaki T. Antioxidants inhibit JNK and p38 MAPK activation but not ERK 1/2 activation by angiotensin II in rat aortic smooth muscle cells. Hypertens Res 2001;24:251–261 [DOI] [PubMed] [Google Scholar]
- 17.Kliem C, Merling A, Giaisi M, Köhler R, Krammer PH, Li-Weber M. Curcumin suppresses T cell activation by blocking Ca2+ mobilization and nuclear factor of activated T cells (NFAT) activation. J Biol Chem 2012;287:10200–10209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kozlovskaya V, Zavgorodnya O, Kharlampieva E. Encapsulation and surface engineering of pancreatic islets: advances and challenges. In Biomedicine. Lin C, Ed. London, IntechOpen, 2012, p. 1–32 [Google Scholar]
- 19.Kozlovskaya V, Zavgorodnya O, Chen Y, et al. . Ultrathin polymeric coatings based on hydrogen-bonded polyphenol for protection of pancreatic islet cells. Adv Funct Mater 2012;22:3389–3398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lenzen S. Chemistry and biology of reactive species with special reference to the antioxidative defence status in pancreatic β-cells. Biochim Biophys Acta Gen Subj 2017;1861:1929–1942 [DOI] [PubMed] [Google Scholar]
- 21.Pham-Hua D, Padgett LE, Xue B, et al. . Islet encapsulation with polyphenol coatings decreases pro-inflammatory chemokine synthesis and T cell trafficking. Biomaterials 2017;128:19–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kozlovskaya V, Xue B, Lei W, Padgett LE, Tse HM, Kharlampieva E. Hydrogen-bonded multilayers of tannic acid as mediators of T-cell immunity. Adv Healthc Mater 2015;4:686–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hayward JA, Ellis CE, Seeberger K, et al. . Cotransplantation of mesenchymal stem cells with neonatal porcine islets improve graft function in diabetic mice. Diabetes 2017;66:1312–1321 [DOI] [PubMed] [Google Scholar]
- 24.Szot GL, Koudria P, Bluestone JA. Transplantation of pancreatic islets into the kidney capsule of diabetic mice. J Vis Exp 2007;(9):404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sklavos MM, Bertera S, Tse HM, et al. . Redox modulation protects islets from transplant-related injury. Diabetes 2010;59:1731–1738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khoo NKH, Cantu-Medellin N, St Croix C, Kelley EE. In vivo immuno-spin trapping: imaging the footprints of oxidative stress. Curr Protoc Cytom 2015;74:12.42.1–12.42.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barra JM, Tse HM. Redox-dependent inflammation in islet transplantation rejection. Front Endocrinol (Lausanne) 2018;9:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mason RP. Using anti-5,5-dimethyl-1-pyrroline N-oxide (anti-DMPO) to detect protein radicals in time and space with immuno-spin trapping. Free Radic Biol Med 2004;36:1214–1223 [DOI] [PubMed] [Google Scholar]
- 29.Burrack AL, Martinov T, Fife BT. T cell-mediated beta cell destruction: autoimmunity and alloimmunity in the context of type 1 diabetes. Front Endocrinol (Lausanne) 2017;8:343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tse HM, Milton MJ, Schreiner S, Profozich JL, Trucco M, Piganelli JD. Disruption of innate-mediated proinflammatory cytokine and reactive oxygen species third signal leads to antigen-specific hyporesponsiveness. J Immunol 2007;178:908–917 [DOI] [PubMed] [Google Scholar]
- 31.Harari O, Liao JK. Inhibition of MHC II gene transcription by nitric oxide and antioxidants. Curr Pharm Des 2004;10:893–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burg AR, Tse HM. Redox-sensitive innate immune pathways during macrophage activation in type 1 diabetes. Antioxid Redox Signal 2018;29:1373–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Signal Transduct Target Ther 2017;2:17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Delmastro-Greenwood MM, Tse HM, Piganelli JD. Effects of metalloporphyrins on reducing inflammation and autoimmunity. Antioxid Redox Signal 2014;20:2465–2477 [DOI] [PubMed] [Google Scholar]
- 35.Bradley LM, Asensio VC, Schioetz LK, et al. . Islet-specific Th1, but not Th2, cells secrete multiple chemokines and promote rapid induction of autoimmune diabetes. J Immunol 1999;162:2511–2520 [PubMed] [Google Scholar]
- 36.Morimoto J, Yoneyama H, Shimada A, et al. . CXC chemokine ligand 10 neutralization suppresses the occurrence of diabetes in nonobese diabetic mice through enhanced beta cell proliferation without affecting insulitis. J Immunol 2004;173:7017–7024 [DOI] [PubMed] [Google Scholar]
- 37.Shimada A, Morimoto J, Kodama K, et al. . Elevated serum IP-10 levels observed in type 1 diabetes. Diabetes Care 2001;24:510–515 [DOI] [PubMed] [Google Scholar]
- 38.Yoshimatsu G, Kunnathodi F, Saravanan PB, et al. . Pancreatic β-cell-derived IP-10/CXCL10 isletokine mediates early loss of graft function in islet cell transplantation. Diabetes 2017;66:2857–2867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Griess B, Mir S, Datta K, Teoh-Fitzgerald M. Scavenging reactive oxygen species selectively inhibits M2 macrophage polarization and their pro-tumorigenic function in part, via Stat3 suppression. Free Radic Biol Med 2020;147:48–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rendra E, Riabov V, Mossel DM, Sevastyanova T, Harmsen MC, Kzhyshkowska J. Reactive oxygen species (ROS) in macrophage activation and function in diabetes. Immunobiology 2019;224:242–253 [DOI] [PubMed] [Google Scholar]
- 41.Tan HY, Wang N, Li S, Hong M, Wang X, Feng Y. The reactive oxygen species in macrophage polarization: reflecting its dual role in progression and treatment of human diseases. Oxid Med Cell Longev 2016;2016:2795090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Unanue ER, Wan X. The immunoreactive platform of the pancreatic islets influences the development of autoreactivity. Diabetes 2019;68:1544–1551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jiang K, Weaver JD, Li Y, Chen X, Liang J, Stabler CL. Local release of dexamethasone from macroporous scaffolds accelerates islet transplant engraftment by promotion of anti-inflammatory M2 macrophages. Biomaterials 2017;114:71–81 [DOI] [PubMed] [Google Scholar]
- 44.Husseini M, Wang GS, Patrick C, et al. . Heme oxygenase-1 induction prevents autoimmune diabetes in association with pancreatic recruitment of M2-like macrophages, mesenchymal cells, and fibrocytes. Endocrinology 2015;156:3937–3949 [DOI] [PubMed] [Google Scholar]
- 45.Parsa R, Andresen P, Gillett A, et al. . Adoptive transfer of immunomodulatory M2 macrophages prevents type 1 diabetes in NOD mice. Diabetes 2012;61:2881–2892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tse HM, Thayer TC, Steele C, et al. . NADPH oxidase deficiency regulates Th lineage commitment and modulates autoimmunity. J Immunol 2010;185:5247–5258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tse HM, Milton MJ, Piganelli JD. Mechanistic analysis of the immunomodulatory effects of a catalytic antioxidant on antigen-presenting cells: implication for their use in targeting oxidation-reduction reactions in innate immunity. Free Radic Biol Med 2004;36:233–247 [DOI] [PubMed] [Google Scholar]
- 48.Yarosz EL, Chang CH. The role of reactive oxygen species in regulating T cell-mediated immunity and disease. Immune Netw 2018;18:e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Salama BF, Korbutt GS. Porcine islet xenografts: a clinical source of ß-cell grafts. Curr Diab Rep 2017;17:14. [DOI] [PubMed] [Google Scholar]
- 50.Pagliuca FW, Millman JR, Gürtler M, et al. . Generation of functional human pancreatic β cells in vitro. Cell 2014;159:428–439 [DOI] [PMC free article] [PubMed] [Google Scholar]