Abstract
Ethnopharmacological relevance
Due to the outbreaks such as SARS, bird flu and swine flu, which we frequently encounter in our century, we need fast solutions with no side effects today more than ever. Due to having vast ethnomedical experience and the richest flora (34% endemic) of Europe and the Middle East, Turkey has a high potential for research on this topic. Plants that locals have been using for centuries for the prevention and treatment of influenza can offer effective alternatives to combat this problem. In this context, 224 herbal taxa belonging to 45 families were identified among the selected 81 studies conducted in the seven regions of Turkey. However, only 35 (15.6%) of them were found to be subjected to worldwide in vitro and in vivo research conducted on anti-influenza activity. Quercetin and chlorogenic acid, the effectiveness of which has been proven many times in this context, have been recorded as the most common (7.1%) active ingredients among the other 56 active substances identified.
Aim of the study
This study has been carried out to reveal the inventory of plant species that have been used in flu treatment for centuries in Turkish folk medicine, which could be used in the treatment of flu or flu-like pandemics, such as COVID 19, that humanity has been suffering with, and also compare them with experimental studies in the literature.
Materials and methods
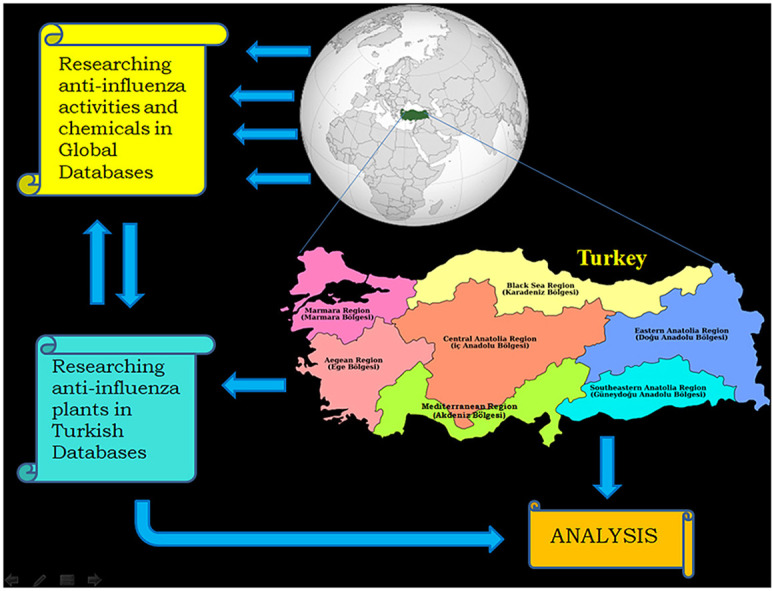
The investigation was conducted in two stages on the subject above by using electronic databases, such as Web of Science, Scopus, ScienceDirect, ProQuest, Medline, Cochrane Library, EBSCO, HighWire Press, PubMed and Google Scholar. The results of both scans are presented in separate tables, together with their regional comparative analysis.
Results
Data obtained on taxa are presented in a table, including anti-influenza mechanism of actions and the active substances. Rosa canina (58.7%) and Mentha x piperita (22.2%) were identified as the most common plants used in Turkey. Also, Sambucus nigra (11.6%), Olea europaea (9.3%), Eucalyptus spp., Melissa officinalis, and Origanum vulgare (7.0%) emerged as the most investigated taxa.
Conclusion
This is the first nationwide ethnomedical screening work conducted on flu treatment with plants in Turkey. Thirty-nine plants have been confirmed in the recent experimental anti-influenza research, which strongly shows that these plants are a rich pharmacological source. Also, with 189 (84.4%) taxa, detections that have not been investigated yet, they are an essential resource for both national and international pharmacological researchers in terms of new natural medicine searches. Considering that the production of antimalarial drugs and their successful use against COVID-19 has begun, this correlation was actually a positive and remarkable piece of data, since there are 15 plants, including Centaurea drabifolia subsp. Phlocosa (an endemic taxon), that were found to be used in the treatment of both flu and malaria.
Keywords: Anti-influenza, Antiviral, Antimalarial, COVID-19, Traditional treatment, Turkey
Graphical abstract
1. Introduction
Plants have always been the primary choice for preventing and treating various diseases faced by human beings, and contain specific or broad-spectrum active compounds for almost any type of disease (Alaoui-Jamali, 2010). People living in Turkey have also benefited from plants in the prevention and treatment of various diseases for centuries. People living in rural areas still have an especially rich medicinal plant repertoire (Ertuğ, 2004). Although herbal cures such as rosehip tea, peppermint-lemon tea and garlic-lemon tea, which are used to prevent and treat flu outbreaks, are well known by the local people, the vast majority of them and their anti-influenza effects have not yet been adequately investigated in vitro by the related industries (Bekut et al., 2018).
In virus classification, influenza viruses are RNA viruses that comprise 4 of the 7 genera of the family Orthomyxoviridae (Kawaoka, 2006), while Human Rhinoviruses (HRVs) are within the genus Enterovirus and the family Picornaviridae (Jacobs et al., 2013). Nevertheless, the flu caused by influenza viruses and the common cold caused by Human Rhinovirus are very similar, although both are types of respiratory virus in terms of disease symptoms (CDC, 2019). In general, it is the most common cause of respiratory viral disease in spring, summer and autumn, while the flu virus is dominant in winter. On the other hand, flu or flu-like viruses are highly contagious and cause serious complications and outbreaks that erupt with a different genetic code each year and even life-threatening pandemics (Jacobs et al., 2013). Nowadays, COVID-19 is one of the most striking examples of a flu-like virus. Due to its fast transmission through direct contact with infected people and contaminated substances or droplets, thousands of patients are dying every day with a fever, cough, and shortness of breath, and, currently, there is no definitive treatment or vaccine, except for some available malaria medicines (Basiri, 2020). There is an urgent need to identify new naturally occurring antiviral molecules, as resistance to anti-influenza drugs appears to be prevalent to an alarming extent (Haidari et al., 2009). Herbal remedies have been used for centuries to treat flu symptoms, and essential oils derived from them have been prescribed as complementary and alternative treatments against influenza (Setzer, 2016). Therefore, to contribute to the treatment of influenza disease and bearing in mind their greater importance, we focused on plants whose successful anti-influenza effects have been tried and trusted by Turkish people for centuries.
Essentially, some antiviral medicines, such as Oseltamivir and Zanamivir, are available for treatment; however, the emergence of drug-resistant strains as a new type of virus is a serious concern (Watanabe and Kawaoka, 2015). In addition, vaccines are only around 50% effective in the elderly, where the highest mortality rates occur (Wang et al., 2006; Rajasekaran et al., 2013), and side effects, such as nausea, vomiting, neuropsychiatric events, abdominal pain, diarrhoea, sinusitis, headache and dizziness, are very common (Grienke et al., 2009). For this reason, natural active ingredients or traditional applications with proven effectiveness are accepted more in the world (Rajasekaran et al., 2013).
Empirical information and bio experiments based on the ethnomedical benefits of plants show that they have the potential to identify new antivirals that can be used against influenza. In particular, the results of research on plant-based antiviral activity and active ingredients against influenza viruses using purified plant chemicals are promising (Grienke et al., 2012). Some of them include determination of the antiviral and cytotoxic effect of quercetin 3-glucoside (Q3G) from Dianthus superbus on influenza virus infection and replication by Nile et al. (2020), revealing the neuraminidase inhibitory effect (on the Influenza Virus replication) of agathisflavone derived from the Anacardium occidentale by De Freitas et al. (2020), and discovering the inhibitory effect of pomegranate (Punica granatum L.) peel extract polymerase activity, RNA replication, and protein expression of the influenza virus by Moradi et al. (2020).
As Velavan and Meyer (2020) stated, the emergence of the COVID-19 flu-like pandemic with high epidemic and mortality rates in early 2020 shows that there is an urgent need for new, effective and various measures against this viral disease. Turkey has the potential for serious research on this topic due to having a very rich (34% of endemic) flora and folkloric experience in plant utilization that has existed for centuries (Güner et al., 2012). Notwithstanding, local research to date, such as detecting Galanthus elwesii and Rheum ribes had a strong antiviral effect against Herpes simplex virus and Sindbis virus among 16 plant influences (Hudson et al., 2000), and investigating the antiviral and cytotoxic effects of the Salvia species (Özçelik et al., 2011) have generally remained at the antiviral level.
In this study, the total list of plant taxa used in Turkish folk medicine against diseases caused by influenza viruses is presented for the first time. It also reveals which of these plants are researched worldwide for anti-influenza activity, along with their active compounds. Taxa that do not have a research record are an important resource for new drug researchers.
2. Materials and methods
2.1. Data collection
This research was conducted in two stages. While, in the first stage, a list of herbs that are used for the treatment of flu in Turkish folk medicine is presented, in the second stage, it was investigated whether there are experimental studies of “anti-influenza” effects of the plants from this list in the world literature. Among these studies those with active compound determination were especially preferred. Various electronic databases, such as Web of Science, Scopus, ScienceDirect, ProQuest, Medline, Cochrane Library, EBSCO, HighWire Press, PubMed and Google Scholar, have been scanned for both studies. In the interest of the plant inventory survey, the national studies conducted in all regions (Fig. 1 ) of Turkey were taken into account. Moreover, to achieve detailed coverage, the database of the Higher Education Council of Turkey National Thesis Center was also included in the research literature. The results of both scans are presented in Table 3, Table 4.
Fig. 1.
Regional map of Turkey.
Table 3.
The list of plant taxa used against influenza in Turkish folk medicine.
| Families | Sc. names | W/C/E | English names | Parts | Preparations | References |
|---|---|---|---|---|---|---|
| Adoxaceae | Sambucus ebulus L. | W | European dwarf elder | Aerial parts | Decoction | Baytop (1999), Tuzlacı and Tolon (2000), Gürbüz et al. (2019) |
| Adoxaceae | Sambucus nigra L. | W | Elderberry, European elder | Leaves, Flowers, Fruits | Infusion | Özhatay et al. (2009), Ugulu et al. (2009), Kalafatçılar and Kalafatçılar (2010), Yeşilada (2012), Karaköse and Karaköse (2017), Ozturk et al. (2017b) |
| Amaranthaceae | Amaranthus retroflexus L. | W | Redroot pigweed, red-rooted pigweed | Leaves | Infusion | Arıtuluk (2010), Polat et al. (2013), Sargin et al. (2013), Yeşilyurt et al. (2017b), Gürbüz et al. (2019), Olgun (2019) |
| Amaranthaceae | Chenopodium album L. | W | Lamb's quarters | Aerial parts | Decoction | Baytop (1999), Şenkardeş (2014), Kılıç (2016) |
| Amaryllidaceae | Allium cepa L. | C | Onion, bulb onion, common onion | Bulbs, Leaves | Eaten raw, Boiling, Juice with some honey | Cansaran and Kaya (2010), Polat et al. (2013), Gökçe (2014), Saraçoğlu (2014), Günbatan et al. (2016), Maranki and Maranki (2016), Paksoy et al. (2016), Uzun and Kaya (2016), Köse (2019), Ekşi et al. (2020) |
| Amaryllidaceae | Allium sativum L. | C | Garlic, onion, shallot, leek, chive, Chinese onion | Leaves, Bulbs, Flowers | Eaten raw or a tablespoon of a tincture prepared with the bulbs, lemon and vinegar is drunk 2–3 times a day | Tuzlacı (2006), Sargin et al. (2013), Gökçe (2014), Şenkardeş (2014), Köse (2019), Ekşi et al. (2020) |
| Anacardiaceae | Rhus coriaria L. | CW | Tanner's sumach, Sicilian sumac | Leaves, Fruits | Infusion, Spice | Tuzlacı and Erol (1999), Tuzlacı and Eryaşar-Aymaz (2001), Akgül et al. (2016) |
| Apiaceae | Cuminum cyminum L. | CW | Cumin | Seeds | Spice | Baytop (1999), Güneş et al. (2018) |
| Apiaceae | Pimpinella anisum L. | CW | Anise, aniseed | Seeds | Infusion after powdering | Genç (2010), Akgül et al. (2016), Ugulu et al. (2009) |
| Apiaceae | Prangos platychlaena Boiss. | E | No English name | Leaves | Infusion after powdering | Tuzlacı and Doğan (2010), Olgun (2019) |
| Asparagaceae | Asparagus acutifolius L. | CW | Wild asparagus | Aerial parts | Infusion | Demirci and Özhatay (2012), Polat et al. (2013), Sargin et al. (2013, 2015a), Demirci-Kayıran (2019), Polat (2019) |
| Berberidaceae | Berberis crataegina DC.a | W | Pipperidge | Roots, Stems | Decoction | Sezik et al. (1992), Arıtuluk (2010) |
| Brassicaceae | Eruca vesicaria (L.) Cav. | CW | Rocket, garden rocket | Leaves | Eaten raw, Salad | Akan and Bakır-Sade (2015), Demirci-Kayıran (2019) |
| Brassicaceae | Erysimum × cheiri (L.) Crantz | CW | Wallflower | Flowers | Infusion | Baytop (1999), Sargin et al. (2013) |
| Brassicaceae | Lepidium sativum L. | CW | Garden cress | Aerial parts | Infusion | Baytop (1999), Ugulu et al. (2009), Gökçe (2014), Bulut and Tuzlacı (2015) |
| Brassicaceae | Raphanus raphanistrum subsp. sativus (L.) Domain | CW | Radish | Tubers | Eaten after mixing with some honey | Sargin et al. (2013), Günbatan et al. (2016), Güneş (2017) |
| Cactaceae | Opuntia ficus-indica (L.) Mill. | CW | Prickly pear, cactus pear, barbary fig | Stems, Fruits | Cataplasm | Baytop (1999), Sargin and Büyükcengiz (2019) |
| Cannabaceae | Celtis tournefortii Lam. | CW | Oriental hackberry | Fruits | Decoction | Polat et al. (2013), Polat (2019), Olgun (2019) |
| Caprifoliaceae | Knautia orientalis L. | W | Oriental widow flower | Flowers | Infusion after drying | Güneş and Özhatay (2011), Güneş (2017) |
| Caprifoliaceae | Morina persica L. | W | Whorl flower | Flowers | Infusion | Şenkardeş (2014), Ozturk et al. (2017a) |
| Compositae | Achillea aleppica DC. | W | Sweet yarrow | Aerial parts | Infusion | Şenkardeş (2014), Kılıç (2019) |
| Compositae | Achillea arabica Kotschy | W | Arabian milfoil | Fruits | Eaten raw, Infusion | Tuzlacı and Erol (1999), Kılıç (2016) |
| Compositae | Achillea cretica L. | W | Cretan milfoil | Flowering branches | Infusion | Bulut et al. (2017b), Yılmaz (2019) |
| Compositae | Achillea millefolium L. | W | Common yarrow | Leaves, Flowers | Infusion | Baytop (1999), Özhatay et al. (2009), Akan and Bakır-Sade (2015) |
| Compositae | Achillea nobilis L. subsp. sipylea (O.Schwarz) Basler | W | Noble yarrow | Aerial parts, Flowers | Infusion | Bulut and Tuzlacı (2015), Sargin et al. (2015a, 2015b), Güner and Selvi (2016), Ozturk et al. (2017b) |
| Compositae | Anthemis cotula L. | W | Dog fennel, stinking chamomile | Aerial parts | Infusion | Güneş and Özhatay (2011), Akgül et al. (2016), Kılıç (2016), Güneş et al. (2018), Polat (2019), Demirci-Kayıran (2019), Kılıç (2019) |
| Compositae | Anthemis fumariifolia Boiss. | E | No English name | Flowers, Flowers | Infusion | Şenkardeş (2014), Kılıç (2016) |
| Compositae | Anthemis haussknechtii Boiss. & Reut. | W | No English name | Aerial parts | Infusion | Akgul et al. (2018), Kılıç (2019) |
| Compositae | Arctium minus (Hill) Bernh. | W | Lesser burdock, little burdock, wild rhubarb | Leaves, Roots | Decoction | Baytop (1999), Günbatan et al. (2016) |
| Compositae | Artemisia absinthium L. | W | Wormwood, grand wormwood, absinthe, absinthium | Flowers, Leaves, Flowering branches, Aerial parts | Infusion after drying | Tuzlacı and Erol (1999), Kılıç (2016) |
| Compositae | Bellis perennis L. | W | Common daisy | Flowers | Infusion | Özçelik et al. (2016), Karaköse and Karaköse (2017), Köse (2019) |
| Compositae | Centaurea drabifolia subsp. floccosa (Boiss.) Wagenitz & Greutera | E | No English name | Flowers | Infusion, Eaten raw by chewing | Ezer and Avcı (2004), Arıtuluk (2010) |
| Compositae | Centaurea iberica Trevir. ex Spreng.a | W | Iberian knapweed, Iberian star-thistle | Leaves | The juice extracted by crushing the leaves is drunk twice a day | Tuzlacı (2006), Çiçek (2019) |
| Compositae | Centaurea jacea L. | W | Brown knapweed | Aerial parts | Infusion | Ergül-Bozkurt and Terzioğlu (2017) |
| Compositae | Centaurea solstitialis L.a | W | Yellow star-thistle, golden starthistle | Aerial parts | Infusion | Tuzlacı and Doğan (2010), Şenkardeş (2014), Bulut and Tuzlacı (2013) |
| Compositae | Cota austriaca (Jacq.) Sch.Bip. | W | Austrian mayweed | Aerial parts | Infusion | Şenkardeş (2014), Kılıç (2019) |
| Compositae | Cota tinctoria (L.) J.Gay | W | Golden marguerite, yellow chamomile | Flowers | Infusion | Ertuğ et al. (2004), Şenkardeş (2014), Bulut and Tuzlacı (2015), Günbatan et al. (2016), Kılıç (2016), Özçelik et al. (2016), Karaköse and Karaköse (2017), Kurt and Karaoğul (2018) |
| Compositae | Crepis vesicaria L. | W | Beaked hawk's-beard | Flowers, Flowers | Infusion | Özhatay et al. (2009) |
| Compositae | Helianthus annuus L. | CW | Common sunflower | Leaves, Flowers, Fruits | Infusion, Decoction, Medicinal bath | Baytop (1999), Cansaran and Kaya (2010), Kalafatçılar and Kalafatçılar (2010), Sargin et al. (2013), Ozturk et al. (2017a) |
| Compositae | Helichrysum arenarium (L.) Moench | W | Dwarf everlast, immortelle | Flowers | Decoction | Tuzlacı and Erol (1999), Akgül et al. (2016), Bağcı et al. (2016), Günbatan et al. (2016) |
| Compositae | Lactuca serriola L. | W | Prickly lettuce | Aerial parts | Infusion | Bulut and Tuzlacı (2013), Şenkardeş (2014) |
| Compositae | Matricaria aurea (Loefl.) Sch.Bip. | W | Golden mayweed | Aerial parts | Infusion | Akgul et al. (2018), Kılıç (2019) |
| Compositae | Matricaria chamomilla L. | W | Chamomile, German chomile | Aerial parts, Flowering branches, Flowers | Infusion | Özer et al. (2005), Özhatay et al. (2009), Kalafatçılar and Kalafatçılar (2010), Sargin et al. (2013, 2015a), Nacakcı and Dutkuner (2015), Akgül et al. (2016), Güneş (2017), İşler (2017), Demirci-Kayıran (2019) |
| Compositae | Pallenis spinosa (L.) Cass. | W | Spiny starwort | Flowering branches, Seeds | Infusion | Ertuğ (2004), Sargin et al. (2015a) |
| Compositae | Silybum marianum (L.) Gaertn. | W | Milk thistle, Marian thistle | Stems, Fruits | Eaten raw after peeling, Infusion | Baytop (1999), Sargin et al. (2015a), Demirci-Kayıran (2019), Kılıç (2019) |
| Compositae | Tanacetum aureum (Lam.) Greuter & al. | W | Golden feverfew | Whole parts | Decoction | Güneş and Özhatay (2011) |
| Compositae | Tanacetum cadmeum (Boiss.) Heywood | E | No English name | Fruits | Eaten raw, Infusion | Tuzlacı and Erol (1999), Kocabas et al. (2017) |
| Compositae | Tanacetum parthenium (L.) Sch.Bip. | W | Feverfew, bachelor buttons | Flowers, Flowers | Infusion | Şenkardeş (2014), Günbatan et al. (2016), Karaköse and Karaköse (2017) |
| Compositae | Tripleurospermum callosum (Boiss. & Heldr.) E.Hossain | E | No English name | Flowers | Infusion | Cansaran and Kaya (2010), Günbatan et al. (2016) |
| Compositae | Tripleurospermum parviflorum (Willd.) Pobed. | W | No English name | Flowers | Infusion | Arıtuluk (2010), Şenkardeş (2014) |
| Compositae | Tussilago farfara L. | W | Coltsfoot | Aerial parts, Flowering branches, Leaves | Infusion | Sargin et al. (2015a), Kılıç (2016), Bulut et al. (2017a) |
| Compositae | Xeranthemum annuum L. | W | Annual everlasting | Aerial parts | Decoction | Özhatay et al. (2009), Tuzlacı and Doğan (2010) |
| Cornaceae | Cornus mas L.a | CW | Cornelian cherry | Fruits | Eaten raw, Decoction, Jam | Koçyiğit and Özhatay (2006), Polat et al. (2013), Köse (2019) |
| Cupressaceae | Juniperus drupacea Labill. | W | Syrian juniper | Fruits, Seeds, Cones | Decoction, Mixture | Ertuğ (2004), Sargin (2015), Kocabaş and Gedik (2016) |
| Cupressaceae | Juniperus oxycedrus L. | W | Cade, cade juniper, prickly juniper | Fruits, Seeds, Leaves, Tars, Cones | Decoction, Infusion | Tuzlacı and Erol (1999), Tuzlacı (2006), Şenkardeş (2014), Nacakcı and Dutkuner (2015), Sargin (2015), Sargin et al. (2015b), Günbatan et al. (2016) |
| Dioscoreaceae | Dioscorea communis (L.) Caddick & Wilkin | W | Black bryony, lady's-seal, black bindweed | Flowering branches, Stems | After boiling, Roasted with onions | Sargin et al. (2013, 2015a), Bulut and Tuzlacı (2015), Gürbüz et al. (2019) |
| Elaeagnaceae | Hippophae rhamnoides subsp. caucasica Rousi | W | Sanddorn, sea bucktorn | Fruits | Infusion, Syrup, jam | Baytop (1999), Şenkardeş (2014) |
| Euphorbiaceae | Euphorbia macroclada Boiss.a | W | No English name | Latex of Stem | Dropped onto a piece of bread, then swallowed. | Şenkardeş (2014), Kılıç (2019) |
| Fagaceae | Quercus ithaburensis subsp. macrolepis (Kotschy) Hedge & Yalt. | W | Valonia oak | Cupula, Seeds | Decoction | Baytop (1999), Sargin et al. (2013, 2015a), Akan and Bakır-Sade (2015) |
| Gentianaceae | Centaurium erythraea Rafn | W | Common centaury, European centaury | Flowering branches | Infusion | Tuzlacı and Eryaşar-Aymaz (2001), Özhatay et al. (2009), Demirci-Kayıran (2019) |
| Hypericaceae | Hypericum perforatum L. | CW | St. John's Wort | Flowering branches, Aerial parts | Infusion | Tuzlacı and Tolon (2000), Tuzlacı and Eryaşar-Aymaz (2001), Tuzlacı (2006), Özhatay et al. (2009), Şenkardeş (2014), Sargin et al. (2015a), Güner and Selvi (2016), Güneş (2017), Kartal and Güneş (2017), Yeşilyurt et al. (2017b), Köse (2019) |
| Iridaceae | Iris caucasica Hoffm. | W | Caucasean Iris | Aerial parts | Infusion | Tuzlacı and Doğan (2010), Polat (2019) |
| Iridaceae | Iris sari Schott ex Baker | E | Tall bearded iris | Flowers | Infusion | Tuzlacı and Doğan (2010), Kılıç (2016) |
| Lamiaceae | Ballota nigra L. | W | Black horehound | Leaves, Aerial parts | Infusion | Özhatay et al. (2009), Arıtuluk (2010) |
| Lamiaceae | Clinopodium acinos (L.) Kuntze | W | Basil-thyme | Aerial parts | Infusion | Özhatay et al. (2009), Kartal and Güneş (2017) |
| Lamiaceae | Clinopodium dolichodontum (P.H.Davis) Bräuchler & Heubl | W | No English name | Aerial parts, Flowering branches | Infusion | Sargin (2015), Ozturk et al, (2017a), Sargin and Büyükcengiz (2019) |
| Lamiaceae | Cyclotrichium origanifolium (Labill.) Manden. & Scheng. | W | Marjoram, leaved calamint | Aerial parts | Infusion, Juice after crashing, Gargle | Sargin (2015), Bağcı et al. (2016), Ozturk et al, (2017b) |
| Lamiaceae | Lavandula angustifolia Mill. | CW | Lavender, true lavender | Leaves | Infusion | Baytop (1999), Bozyel and Merdamert-Bozyel (2020) |
| Lamiaceae | Lavandula pedunculata subsp. cariensis (Boiss.) Upson & S.Andrews | W | Turkish lavender, French lavender | Flowering branches | Infusion | Baytop (1999), Ertuğ (2004), Arıtuluk (2010) |
| Lamiaceae | Lavandula stoechas L. | CW | Spanish lavender, topped lavender | Leaves, Flowering branches | Infusion | Tuzlacı (2006), Bulut and Tuzlacı (2015), Sargin et al. (2015a), Özçelik et al. (2016), Bozyel and Merdamert-Bozyel (2020) |
| Lamiaceae | Marrubium rotundifolium Boiss. | E | Silver edged horehound | Aerial parts | Cataplasm | Arıtuluk (2010), Sargin et al. (2015a), Yeşilyurt et al. (2017a) |
| Lamiaceae | Melissa officinalis L. | CW | Lemon balm | Aerial parts | Infusion | Özhatay et al. (2009), Güneş (2017), Demirci-Kayıran (2019) |
| Lamiaceae | Mentha longifolia (L.) L. | W | Horsemint, Asian mint | Leaves | Infusion | Kilic and Bagci (2013), Gökçe (2014), Sargin et al. (2015a), Günbatan et al. (2016), Özçelik et al. (2016), Yeşilyurt et al. (2017b), Gürbüz et al. (2019) |
| Lamiaceae | Mentha longifolia subsp. typhoides (Briq.) Harley | W | Horse mint | Aerial parts | Eaten raw, Infusion | Güneş and Özhatay (2011), Demirci and Özhatay (2012), Polat et al. (2013), Şenkardeş (2014), Kılıç (2016), Bulut et al. (2017a), Yeşilyurt et al. (2017b), Polat (2019), Kılıç (2019), Çiçek (2019), Olgun (2019) |
| Lamiaceae | Mentha pulegium L. | W | Pennyroyal, pennyrile, squaw mint | Leaves | Infusion | Gökçe (2014), Sargin et al. (2015a), Güner and Selvi (2016), Yeşilyurt et al. (2017b), Akbulut et al. (2019), Köse (2019), Yılmaz (2019) |
| Lamiaceae | Mentha spicata L. | W | Garden mint, spearmint, curly mint, mint, common mint | Aerial parts | Infusion | Tuzlacı and Eryaşar-Aymaz (2001), Tuzlacı (2006), Cakilcioglu et al. (2011), Polat et al. (2013), Tetik et al. (2013), Gökçe (2014), Paksoy et al. (2016), Yeşilyurt et al. (2017b), Güneş (2017), Güneş et al. (2018), Polat (2019), Köse (2019) |
| Lamiaceae | Mentha x piperita L. | CW | Peppermint | Leaves | Infusion with/without lemon juice, Spices | Saraç (2005), Ugulu et al. (2009), Genç (2010), Kalafatçılar and Kalafatçılar (2010), Tetik et al. (2013), Şenkardeş (2014), Sargin et al. (2015a), Sargin and Büyükcengiz (2019), Günbatan et al. (2016), Bulut et al. (2017b), Güneş (2017), Yeşilyurt et al., 2017a, Yeşilyurt et al., 2017b, Bulut et al. (2019), Demirci-Kayıran (2019), Gürbüz et al. (2019), Kılıç (2019) |
| Lamiaceae | Micromeria myrtifolia Boiss. & Hohen. | W | No English name | Aerial parts | Infusion, Spices | Bulut and Tuzlacı (2015), Kocabaş and Gedik (2016), Güzel and Güzelsemme (2018), Çiçek (2019), Sargin and Büyükcengiz (2019) |
| Lamiaceae | Micromeria nervosa (Desf.) Benth. | W | No English name | Aerial parts | Infusion | Ertuğ (2004), Bulut et al. (2017b) |
| Lamiaceae | Ocimum basilicum L. | CW | Basil, great basil | Aerial parts | Infusion | Arıtuluk (2010), Polat et al. (2013), Tetik et al. (2013), Polat (2019) |
| Lamiaceae | Origanum acutidens (Hand.-Mazz.) Ietsw. | E | No English name | Aerial parts | Infusion | Polat (2019) |
| Lamiaceae | Origanum hypericifolium O.Schwarz & P.H.Davis | E | No English name | Aerial parts | Infusion | Bulut et al. (2017a), Yılmaz (2019) |
| Lamiaceae | Origanum majorana L. | W | Sweet marjoram, marjoram | Flowering branches | Infusion | Ertuğ et al. (2004), Bulut and Tuzlacı (2015), Sargin (2015), Sargin et al. (2013, 2015a), Sargin and Büyükcengiz (2019), Demirci-Kayıran (2019) |
| Lamiaceae | Origanum onites L. | W | Pot marjoram, Cretan oregano | Aerial parts | Infusion with/without Sage leaves | Ertuğ (2004), Ertuğ et al. (2004), Tuzlacı (2006), Ugulu et al. (2009), Genç (2010), Kalafatçılar and Kalafatçılar (2010), Sargin et al. (2013, 2015a), Gökçe (2014), Nacakcı and Dutkuner (2015), Akbulut et al. (2019), Yılmaz (2019) |
| Lamiaceae | Origanum saccatum P.H.Davis | E | No English name | Aerial parts, Flowering branches | Infusion | Sargin (2015), Sargin and Büyükcengiz (2019) |
| Lamiaceae | Origanum syriacum subsp. bevanii (Holmes) Greuter & Burdet | W | No English name | Aerial parts, Flowering branches | Infusion | Sargin (2015), Sargin et al. (2015b), Sargin and Büyükcengiz (2019), Guzel and Guzelsemme (2018), Demirci-Kayıran (2019) |
| Lamiaceae | Origanum vulgare L. | W | Ornamental oregano | Aerial parts | Infusion | Ertuğ et al. (2004), Özhatay et al. (2009), Cakilcioglu et al. (2011), Polat et al. (2013), Gökçe (2014), Bulut and Tuzlacı (2015), Çiçek (2019) |
| Lamiaceae | Origanum vulgare subsp. gracile (K.Koch) Ietsw. | W | Russian oregano | Leaves, Flowering branches, Aerial parts | Infusion | Ertuğ et al. (2004), Cakilcioglu et al. (2011), Kilic and Bagci (2013), Ozturk et al, (2017a), Tuzlacı and Doğan (2010), Olgun (2019) |
| Lamiaceae | Origanum vulgare subsp. hirtum (Link) Ietsw. | W | Greek oregano | Aerial parts | Infusion | Ertuğ et al. (2004), Tuzlacı and Eryaşar-Aymaz (2001), Cakilcioglu et al. (2011), Gökçe (2014), Bulut (2016) |
| Lamiaceae | Origanum vulgare subsp. viridulum (Martrin-Donos) Nyman | W | Winter marjoram | Flowering branches | Infusion | Ertuğ et al. (2004), Cakilcioglu et al. (2011), Gökçe (2014), Karaköse and Karaköse (2017) |
| Lamiaceae | Phlomis armeniaca Willd.a | W | No English name | Flowers, Aerial parts | Infusion | Şenkardeş (2014), Dalar et al. (2018), Çiçek (2019), Olgun (2019) |
| Lamiaceae | Prunella vulgaris L. | W | Common selfheal, self-heal | Flowering branches | Infusion | Baytop (1999), Ergül-Bozkurt and Terzioğlu (2017), Karaköse and Karaköse (2017) |
| Lamiaceae | Rosmarinus officinalis L. | CW | Rosemary | Leaves, Stems | Infusion | Ertuğ et al. (2004), Tuzlacı (2006), Yeşilada (2012), Saraçoğlu (2014), Bulut and Tuzlacı (2015), Güner and Selvi (2016), Maranki and Maranki (2016), Kocabas et al. (2017), Kurt and Karaoğul (2018), Akbulut et al. (2019) |
| Lamiaceae | Salvia absconditiflora Greuter & Burdet | E | No English name | Aerial parts, Leaves, Flowers | Cataplasm with dough | Demirci and Özhatay (2012), Sargin (2013), Şenkardeş (2014), Sargin et al. (2015a), Ozturk et al, (2017a), Kılıç (2019) |
| Lamiaceae | Salvia aramiensis Rech.f. | W | Aramenian salve | Leaves | Infusion | Guzel and Guzelsemme (2018) |
| Lamiaceae | Salvia candidissima Vahl | W | Silver sage | Leaves | Infusion | Tuzlacı and Doğan (2010), Olgun (2019) |
| Lamiaceae | Salvia fruticosa Mill. | W | Greek sage, Turkish sage | Aerial parts, Essential oil | Infusion, Lotion | Tanker et al. (1998), Ertuğ (2004), Ertuğ et al. (2004), Tuzlacı (2006), Bulut (2016) |
| Lamiaceae | Salvia multicaulis Vahl | W | Many-stemmed sage | Aerial parts | Infusion, Decoction | Tetik et al. (2013), Çiçek (2019), Olgun (2019) |
| Lamiaceae | Salvia officinalis L. | CW | Culinary sage, golden sage, garden sage | Aerial parts | Infusion | Tanker et al. (1998), Ertuğ et al. (2004), Kalafatçılar and Kalafatçılar (2010), Cakilcioglu et al. (2011), Yeşilada (2012), Akan and Bakır-Sade (2015), Maranki and Maranki (2016), Kurt and Karaoğul (2018), Demirci-Kayıran (2019) |
| Lamiaceae | Salvia palaestina Benth. | W | Palestinian sage | Aerial parts | Infusion | Kocabas et al. (2017), Bulut et al. (2019), Kılıç (2019) |
| Lamiaceae | Salvia sclarea L. | W | Clary sage, clary, clary wort | Flowering branches, Leaves | Infusion | Tuzlacı and Doğan (2010), Demirci-Kayıran (2019) |
| Lamiaceae | Salvia syriaca L | W | Syrian sage | Leaves, Flowers | Infusion | Kilic and Bagci (2013), Şenkardeş (2014) |
| Lamiaceae | Salvia tomentosa Mill. | W | Balsamic sage | Aerial parts | Infusion, Steam compress | Tuzlacı and Erol (1999), Tuzlacı and Eryaşar-Aymaz (2001), Ertuğ et al. (2004), Sargin et al. (2013, 2015a), Güner and Selvi (2016), Özçelik et al. (2016), Bulut et al. (2017a), Guzel and Guzelsemme (2018) |
| Lamiaceae | Salvia verticillata L. | W | Lilac sage | Leaves | Infusion | Köse (2019), Olgun (2019) |
| Lamiaceae | Salvia viridis L. | W | Horminum sage | Leaves, Flowers | Infusion | Paksoy et al. (2016), Güneş (2017) |
| Lamiaceae | Satureja cuneifolia Ten. | W | Apulian savory | Aerial parts | Infusion, Decoction | Ertuğ et al. (2004), Sargin et al. (2013, 2015a), Güneş (2017), Kartal and Güneş (2017) |
| Lamiaceae | Satureja hortensis L. | W | Summer savory | Aerial parts | Infusion | Ertuğ et al. (2004), Cakilcioglu et al. (2011), Kilic and Bagci (2013), Polat et al. (2013), Tetik et al. (2013), Güneş (2017), Çiçek (2019), Olgun (2019) |
| Lamiaceae | Satureja thymbra L. | W | Thyme-leaved savory | Aerial parts, Flowering branches, Essential oil | Infusion, Spice, Lotion | Ertuğ et al. (2004), Nacakcı and Dutkuner (2015), Sargin (2015); Sargin et al. (2015a) |
| Lamiaceae | Satureja wiedemanniana (Avé-Lall.) Velen. | W | No English name | Aerial parts | Infusion | Cansaran and Kaya (2010), Han and Bulut (2015) |
| Lamiaceae | Sideritis arguta Boiss. & Heldr. | E | No English name | Leaves, Flowers | Infusion | Akbulut et al. (2019), Yılmaz (2019) |
| Lamiaceae | Sideritis dichotoma Huter | E | No English name | Aerial parts | Infusion | Cansaran and Kaya (2010) |
| Lamiaceae | Sideritis erythrantha Boiss. & Heldr. | E | No English name | Aerial parts | Infusion, Gargle | Ertuğ et al. (2004), Sargin (2015), Sargin et al. (2015b), Ozturk et al, (2017b) |
| Lamiaceae | Sideritis germanicopolitana Bornm. | E | No English name | Aerial parts | Infusion | Han and Bulut (2015), Günbatan et al. (2016) |
| Lamiaceae | Sideritis huber-morathii Greuter & Burdet | E | No English name | Aerial parts | Infusion | Guzel and Guzelsemme (2018) |
| Lamiaceae | Sideritis leptoclada O.Schwarz & P.H.Davis | E | No English name | Aerial parts | Infusion | Bulut et al. (2017a), Yılmaz (2019) |
| Lamiaceae | Sideritis libanotica Labill. | W | No English name | Leaves, Flowers | Infusion | Arıtuluk (2010), Akbulut et al. (2019) |
| Lamiaceae | Sideritis libanotica subsp. linearis (Benth.) Bornm. | W | No English name | Aerial parts | Infusion | Arıtuluk (2010), Nacakcı and Dutkuner (2015), Demirci and Özhatay (2012) |
| Lamiaceae | Sideritis montana L. | W | Mountain ironwort | Aerial parts | Infusion | Ertuğ et al. (2004), Paksoy et al. (2016), Özhatay et al. (2009) |
| Lamiaceae | Sideritis perfoliata L. | W | No English name | Aerial parts | Infusion | Bulut and Tuzlacı (2015), Kocabaş and Gedik (2016), Bulut et al. (2017a), Ozturk et al, (2017b), Guzel and Guzelsemme (2018) |
| Lamiaceae | Sideritis rubriflora Hub.-Mor. | E | No English name | Aerial parts | Infusion, Gargle | Sargin (2015), Sargin et al. (2015b) |
| Lamiaceae | Sideritis scardica Griseb. | W | Shepherd's tea | Aerial parts | Infusion | Ertuğ et al. (2004), Özhatay et al. (2009), Güneş (2017) |
| Lamiaceae | Sideritis sipylea Boiss. | E | No English name | Aerial parts | Infusion | Ertuğ et al. (2004), Sargin et al. (2013, 2015a) |
| Lamiaceae | Sideritis syriaca subsp. nusairiensis (Post) Hub.-Mor. | E | No English name | Aerial parts | Infusion | Şenkardeş (2014), Kocabaş and Gedik (2016), Guzel and Guzelsemme (2018) |
| Lamiaceae | Sideritis tmolea P. H. Davis | E | No English name | Aerial parts, Flowers | Infusion | Baytop (1999), Ertuğ et al. (2004), Arıtuluk (2010), Sargin et al. (2013, 2015a) |
| Lamiaceae | Sideritis vulcanica Hub.-Mor. | E | No English name | Aerial parts | Infusion | Ertuğ et al. (2004), Polat (2019), Olgun (2019) |
| Lamiaceae | Stachys annua (L.) L. | W | Annual yellow | Aerial parts | Infusion | Şenkardeş (2014), Karaköse and Karaköse (2017) |
| Lamiaceae | Stachys lavandulifolia Vahl | W | Lamb's ear | Aerial parts | Infusion | Polat et al. (2013), Sargin (2015), Sargin and Büyükcengiz (2019), Polat (2019), Olgun (2019) |
| Lamiaceae | Teucrium chamaedrys L. | W | Midget | Aerial parts | Infusion | Tuzlacı (2006), Tuzlacı and Doğan (2010), Kaval et al. (2014) |
| Lamiaceae | Teucrium chamaedrys subsp. sinuatum (Celak.) Rech.f. | W | No English name | Aerial parts | Infusion | Polat et al. (2013), Kaval et al. (2014), Polat (2019) |
| Lamiaceae | Teucrium polium L.a | W | Hulwort, felty germander, mountain germander | Aerial parts | Infusion | Tuzlacı and Erol (1999), Tuzlacı (2006), Koçyiğit and Özhatay (2006), Özhatay et al. (2009), Tuzlacı and Doğan (2010), Cakilcioglu et al. (2011), Kilic and Bagci (2013), Polat et al. (2013), Kaval et al. (2014), Şenkardeş (2014), Günbatan et al. (2016), Dalar et al. (2018), Polat (2019), Bulut et al. (2019), Kılıç (2019), Çiçek (2019), Olgun (2019) |
| Lamiaceae | Thymbra capitata (L.) Cav. | W | Spanish oregano, cone-head thyme | Aerial parts, Flowering branches, Essential oil | Infusion, Lotion, Spice | Ertuğ et al. (2004), Sargin (2015), Sargin et al. (2015a), Yılmaz (2019) |
| Lamiaceae | Thymbra sintenisii Bornm. & Azn. | W | No English name | Aerial parts | Infusion | Ozturk et al, (2017)a, Bulut et al. (2019) |
| Lamiaceae | Thymbra spicata L. | W | Thyme spiked | Aerial parts, Flowers | Infusion, Lotion, Spice | Ertuğ et al. (2004), Tuzlacı (2006), Bulut and Tuzlacı (2013, 2015), Sargin et al. (2013, 2015a; 2015b), Özçelik et al. (2016), Sargin and Büyükcengiz (2019), Akan and Bakır-Sade (2015), Sargin (2015), Güner and Selvi (2016), Kocabaş and Gedik (2016), Kılıç (2019) |
| Lamiaceae | Thymus cilicicus Boiss. & Balansa | W | Cilician thyme | Aerial parts | Infusion | Tuzlacı (2006), Genç (2010), Gökçe (2014), Özçelik et al. (2016), İşler (2017), Guzel and Guzelsemme (2018) |
| Lamiaceae | Thymus haussknechtii Velen. | E | No English name | Leaves | Infusion | Tuzlacı (2006), Genç (2010), Cakilcioglu et al. (2011), Kilic and Bagci (2013), Tetik et al. (2013), Gökçe (2014), Paksoy et al. (2016), İşler (2017) |
| Lamiaceae | Thymus kotschyanus Boiss. & Hohen. | W | No English name | Aerial parts | Infusion | Tuzlacı (2006), Genç (2010), Cakilcioglu et al. (2011), Polat et al. (2013), Kaval et al. (2014), Kocabaş and Gedik (2016), İşler (2017), Kocabas et al. (2017), Polat (2019), Bulut et al. (2019) |
| Lamiaceae | Thymus longicaulis C.Presl | W | Creeping thyme | Flowering branches, Aerial parts | Infusion | Tuzlacı (2006), Genç (2010), Gökçe (2014), Günbatan et al. (2016), İşler (2017), Akbulut et al. (2019), Gürbüz et al. (2019) |
| Lamiaceae | Thymus longicaulis subsp. chaubardii (Rchb.f.) Jalas | W | No English name | Aerial parts | Infusion | Ertuğ et al. (2004), Tuzlacı (2006), Özhatay et al. (2009), Arıtuluk (2010), Genç (2010), Bulut and Tuzlacı (2015), İşler (2017) |
| Lamiaceae | Thymus migricus Klokov & Des.-Shost. | W | No English name | Leaves | Infusion | Ertuğ et al. (2004), Tuzlacı (2006), Genç (2010), Tuzlacı and Doğan (2010), Güneş and Özhatay (2011), İşler (2017) |
| Lamiaceae | Thymus nummularius M.Bieb. | W | No English name | Flowering branches | Infusion | Ertuğ et al. (2004), Tuzlacı (2006), Genç (2010), İşler (2017), Karaköse and Karaköse (2017) |
| Lamiaceae | Thymus praecox subsp. jankae (Celak.) Jalas | W | No English name | Leaves | Infusion | Ertuğ et al. (2004), Tuzlacı (2006), Arıtuluk (2010), Genç (2010), Günbatan et al. (2016), İşler (2017) |
| Lamiaceae | Thymus revolutus Celak. | E | No English name | Aerial parts | Infusion | Tuzlacı (2006), Genç (2010), Kocabas et al. (2017), Sargin and Büyükcengiz (2019) |
| Lamiaceae | Thymus sipyleus Boiss. | W | No English name | Aerial parts | Infusion | Tuzlacı (2006), Cansaran and Kaya (2010), Genç (2010), Gökçe (2014), Şenkardeş (2014), Paksoy et al. (2016), Polat (2019) |
| Lamiaceae | Thymus transcaucasicus Ronniger | W | No English name | Whole parts | Infusion | Tuzlacı (2006), Genç (2010), Güneş and Özhatay (2011), Gökçe (2014), İşler (2017) |
| Lamiaceae | Thymus zygioides Griseb. | W | No English name | Aerial parts, Flowering branches | Infusion | Tuzlacı (2006), Özhatay et al. (2009), Genç (2010), Sargin et al. (2013, 2015a), Gökçe (2014), Bulut and Tuzlacı (2015), İşler (2017) |
| Lamiaceae | Vitex agnus-castus L. | W | Chaste tree, Abraham's balm | Seeds | Decoction, Swallowing | Tuzlacı (2006), Akan and Bakır-Sade (2015), Sargin (2015), Güner and Selvi (2016), Demirci-Kayıran (2019) |
| Lamiaceae | Ziziphora capitata L. | W | No English name | Aerial parts | Infusion | Kilic and Bagci (2013), Kocabas et al. (2017), Kılıç (2019) |
| Lamiaceae | Ziziphora clinopodioides Lam. | W | Blue mint bush | Aerial parts | Infusion | Ertuğ et al. (2004), Tuzlacı and Doğan (2010), Sargin et al. (2013, 2015a) |
| Lamiaceae | Ziziphora taurica M.Bieb. | W | No English name | Aerial parts | Infusion | Baytop (1999), Ertuğ et al. (2004), Sargin et al. (2013, 2015a) |
| Lamiaceae | Ziziphora taurica subsp. cleonioides (Boiss.) P.H.Davis | E | No English name | Aerial parts | Infusion | Ertuğ (2004), Ertuğ et al. (2004), Arıtuluk (2010), Sargin et al. (2013, 2015a) |
| Lamiaceae | Ziziphora tenuior L. | W | No English name | Aerial parts | Infusion | Ertuğ (2004), Sargin et al. (2013), Dalar et al. (2018) |
| Lauraceae | Laurus nobilis L. | CW | Laurel, true laurel, bay, royal bay, sweet bay, Grecian laurel | Leaves, Seeds | Infusion of the leaves with/without quince leaves after drying and pulverizing, Decoction of the seeds | Tuzlacı (2006), Nacakcı and Dutkuner (2015), Kurt and Karaoğul (2018), Gürbüz et al. (2019), Köse (2019) |
| Leguminosae | Ceratonia siliqua L. | CW | Carob, carob tree | Fruits | Eaten raw, Boiling, Molasses | Kurt and Karaoğul (2018), Sargin and Büyükcengiz (2019) |
| Leguminosae | Glycyrrhiza glabra L. | CW | Licorice, liquorice | Leaves, Roots | Infusion after pulverizing | Özer et al. (2005), Saraç (2005), Genç (2010), Sargin et al. (2013, 2015a), Gökçe (2014), Kurt and Karaoğul (2018), Kılıç (2019) |
| Leguminosae | Trifolium repens L. | W | Dutch clover | Aerial parts | Infusion | Cakilcioglu et al. (2011), Kilic and Bagci (2013), Ozturk et al, (2017b) |
| Lythraceae | Punica granatum L. | CW | Pomegranate | Fruits | Eaten raw, Juice | Baytop (1999), Kocabaş and Gedik (2016), Demirci-Kayıran (2019) |
| Malvaceae | Alcea calvertii (Boiss.) Boiss. | W | No English name | Aerial parts | Infusion, Decoction | Akan and Bakır-Sade (2015), Kılıç (2016), Ozturk et al, (2017a) |
| Malvaceae | Alcea excubita Iljin | W | No English name | Flowers, Leaves | Infusion | Tuzlacı and Doğan (2010), Kılıç (2016) |
| Malvaceae | Alcea pallida (Willd.) Waldst. & Kit. | W | Hollyhock, eastern hollyhock | Flowers, Fruits, Aerial parts | Infusion, Decoction | Arıtuluk (2010), Bulut et al. (2017a) |
| Malvaceae | Alcea rosea L. | W | Garden hollyhock, rose mallow | Leaves, Flowers, Roots | Infusion | Şenkardeş (2014), Akgül et al. (2016), Demirci-Kayıran (2019) |
| Malvaceae | Alcea setosa (Boiss.) Alef. | W | Bristly hollyhock | Flowers, Fruits | Infusion | Akgul et al, (2018), Kılıç (2019) |
| Malvaceae | Alcea striata Alef. | W | No English name | Flower, Fruits | Infusion | Akgul et al, (2018), Kılıç (2019) |
| Malvaceae | Althaea officinalis L. | CW | Common marsh | Buds, Flowers | Infusion | Baytop (1999), Genç (2010), Kalafatçılar and Kalafatçılar (2010), Sargin et al. (2015b), Demirci-Kayıran (2019) |
| Malvaceae | Malva neglecta Wallr. | W | Cheeseplant, dwarf mallow | Aerial parts | Infusion, Decoction | Tuzlacı and Erol (1999), Cakilcioglu et al. (2011), Kilic and Bagci (2013), Polat et al. (2013), Tetik et al. (2013), Kaval et al. (2014), Şenkardeş (2014), Dalar et al. (2018), Olgun (2019) |
| Malvaceae | Malva sylvestris (L.) Mill.a | W | Large-flowered mallow, high mallow | Aerial parts | Roasted with rice, radish, onion and butter, Infusion | Tuzlacı and Erol (1999), Özer et al. (2005), Özhatay et al. (2009), Polat et al. (2013), Nacakcı and Dutkuner (2015), Sargin et al. (2015a), Dalar et al. (2018), Demirci-Kayıran (2019), Köse (2019) |
| Malvaceae | Tilia cordata Mill. | W | Bast, small-leaved linden | Leaves, Fruits | Decoction with cinnamon and cloves | Saraç (2005), Kalafatçılar and Kalafatçılar (2010), Gökçe (2014), Şenkardeş (2014), Akgül et al. (2016), Maranki and Maranki (2016), İşler (2017), Yeşilyurt et al. (2017b) |
| Malvaceae | Tilia platyphyllos Scop. | W | Broad-leaved lime | Flowers, Bracts | Infusion | Saraç (2005), Kalafatçılar and Kalafatçılar (2010), Bulut and Tuzlacı (2013), Gökçe (2014), Maranki and Maranki (2016), Bulut et al. (2017b), İşler (2017) |
| Malvaceae | Tilia rubra subsp. caucasica (Rupr.) V.Engl. | W | No English name | Flowers, Leaves, Barks | Infusion, Decoction | Saraç (2005), Tuzlacı (2006), Cansaran and Kaya (2010), Gökçe (2014), Bulut and Tuzlacı (2015), Güner and Selvi (2016), Uzun and Kaya (2016), Maranki and Maranki (2016), İşler (2017), Karaköse and Karaköse (2017), Köse (2019) |
| Malvaceae | Tilia tomentosa Moench | CW | European white lime, silver lime, silver linden | Leaves, Flowers, Fruits, Barks, Bracts, Roots | Infusion, Decoction | Tuzlacı and Tolon (2000), Tuzlacı and Eryaşar-Aymaz (2001), Saraç (2005), Özhatay et al. (2009), Sargin et al. (2013), Gökçe (2014), Bulut and Tuzlacı (2015), Akgül et al. (2016), Maranki and Maranki (2016), Bulut et al. (2017a), İşler (2017), Yeşilyurt et al. (2017a, 2017b), Guzel and Guzelsemme (2018), Kurt and Karaoğul (2018), Gürbüz et al. (2019) |
| Moraceae | Ficus carica L.a | CW | Fig, common fig | Fruits, Leaves | Eaten after drying, Infusion | Sargin et al. (2013, 2015a), Köse (2019) |
| Moraceae | Morus alba L. | CW | White mulberry | Fruits | Syrup | Cakilcioglu et al. (2011), Şenkardeş (2014), Olgun (2019) |
| Myrtaceae | Eucalyptus camaldulensis Dehnh. | CW | Murray red gum, red gum, river red gum, long-beak eucalyptus | Leaves, Essential oils | The 2% infusion is sweetened with honey and drunk 2–3 glasses a day. Medicinal bath, frankincense | Karamanoğlu (1977), Tanker et al. (1998), Baytop (1999), Ertuğ (2004), Saraç (2005), Genç (2010), Kalafatçılar and Kalafatçılar (2010), Ozturk et al. (2017a) |
| Myrtaceae | Eucalyptus globulus Labill. | CW | Blue gum, southern blue gum | Leaves, Essential oils | The 2% infusion is sweetened with honey and drunk 2–3 glasses a day. Medicinal bath, frankincense | Karamanoğlu (1977), Tanker et al. (1998), Baytop (1999), Saraç (2005), Genç (2010), Kalafatçılar and Kalafatçılar (2010), Kurt and Karaoğul (2018) |
| Nitrariaceae | Peganum harmala L. | W | Harmal piganum | Seeds | Infusion | Yeşilyurt et al. (2017a), Bulut et al. (2019), Demirci-Kayıran (2019) |
| Oleaceae | Fraxinus ornus subsp. cilicica (Lingelsh.) Yalt. | E | No English name | Stems, Barks | Infusion | Demirci and Özhatay (2012), Ozturk et al, (2017a) |
| Oleaceae | Olea europaea L.a | CW | Olive, common olive | Fixed oils | Cataplasm with one tablespoon molasses, tarhana and flour | Tuzlacı (2006), Nacakcı and Dutkuner (2015), Sargin et al. (2015a), Köse (2019) |
| Orchidaceae | Dactylorhiza osmanica (Klinge) P.F.Hunt & Summerh. | E | No English name | Tubers | Infusion (with some milk after powdering) | Şenkardeş (2014), Sargin (2015), Sargin and Büyükcengiz (2019) |
| Orchidaceae | Orchis anatolica Boiss. | W | Orchid | Tubers | Infusion, Spice (after powdering) | Baytop (1999), Sargin (2015), Ozturk et al, (2017b) |
| Papaveraceae | Papaver orientale L. | W | Great scarlet poppy | Seeds | Roasted with garlic | Tanker et al. (1998), Baytop (1999), Güneş and Özhatay (2011) |
| Papaveraceae | Papaver rhoeas L. | W | Flanders poppy | Flowers | Infusion | Tanker et al. (1998), Ugulu et al. (2009), Bulut et al. (2017b) |
| Pedaliaceae | Sesamum indicum L. | CW | Sesame, common sesame | Seeds | Crushed and mixed with boiled grape juice, Eaten raw | Baytop (1999), Bağcı et al. (2016), Güneş (2017) |
| Pinaceae | Abies cilicica (Antoine & Kotschy) Carrière | W | Spring grove, cilica fir, hunnewell | Cones, Resins, Buds, Branches | Decoction | Baytop (1999), Ozturk et al. (2017a) |
| Pinaceae | Pinus nigra J.F.Arnold | W | Black pine | Resins, Tars, Essential oils | Decoction, Medicinal bath, frankincense | Tanker et al. (1998), Arıtuluk (2010), Kalafatçılar and Kalafatçılar (2010), Cakilcioglu et al. (2011), Bağcı et al. (2016), Özçelik et al. (2016), Gürbüz et al. (2019) |
| Pinaceae | Pinus sylvestris L. | CW | Redwood, Scots fir | Buds, Resins, Cones, Essential oils | Decoction, Medicinal bath, frankincense | Kalafatçılar and Kalafatçılar (2010), Karaköse and Karaköse (2017), Gürbüz et al. (2019) |
| Plantaginaceae | Plantago major L.a | W | Rat's-tail plantain | Leaves | Infusion | Özhatay et al. (2009), Cakilcioglu et al. (2011), Olgun (2019) |
| Plantaginaceae | Plantago major subsp. intermedia (Gilib.) Lange | W | No English name | Aerial parts, Leaves | Infusion after drying | Arıtuluk (2010), Sargin et al. (2015a), Yeşilyurt et al. (2017a) |
| Polygonaceae | Portulaca oleracea L. | W | Common purslane, fatweed | Aerial parts | Eating raw, Boiling, Roasting | Şenkardeş (2014), Kılıç (2019), Köse (2019), Olgun (2019), Yılmaz (2019) |
| Polygonaceae | Rumex crispus L. | W | Curled dock | Roots | Cataplasm | Tuzlacı (2006), Arıtuluk (2010), Sargin et al. (2015a), Yeşilyurt et al. (2017a) |
| Polygonaceae | Rumex patientia L. | W | Garden patience | Leaves | Decoction | Tuzlacı (2006), Güneş and Özhatay (2011), Ozturk et al, (2017b) |
| Polygonaceae | Rumex scutatus L. | W | Shield dock | Leaves | Infusion | Tuzlacı (2006), Cansaran and Kaya (2010), Ozturk et al, (2017a) |
| Ranunculaceae | Adonis annua L. | W | Annual pheasant's eye | Flowers | Infusion after drying | Baytop (1999), Güneş (2017) |
| Ranunculaceae | Helleborus orientalis Lam. | W | Lenten-rose | Roots | Eaten raw | Tuzlacı and Tolon (2000), Koçyiğit and Özhatay (2006) |
| Ranunculaceae | Nigella arvensis L. | CW | Wild fennel, field fennel flower | Flowers, Seeds | Infusion after drying and crashing | Güneş (2017) |
| Ranunculaceae | Nigella sativa L. | CW | Black cumin | Seeds | Eating raw, Infusion after crashing | Bulut et al. (2017a), Kurt and Karaoğul (2018) |
| Rosaceae | Crataegus monogyna Jacq. | CW | Hawtorn, may | Fruits | Eaten raw, Infusion | Cakilcioglu et al. (2011), Şenkardeş (2014), Sargin et al. (2015a), Olgun (2019) |
| Rosaceae | Crataegus orientalis Pall. ex M.Bieb. | CW | Oriental hawtorn | Fruits | Eaten raw, Infusion | Arıtuluk (2010), Polat et al. (2013), Şenkardeş (2014), Ozturk et al, (2017b) |
| Rosaceae | Crataegus pentagyna Waldst. & Kit. ex Willd. | W | Small-flowered black hawtorn | Flowers | Infusion | Özhatay et al. (2009), Koçyiğit and Özhatay (2006) |
| Rosaceae | Cydonia oblonga Mill | CW | Quince | Leaves, Fruits | Infusion, Cataplasm (with some thyme and tarhana flour), Eaten raw | Cansaran and Kaya (2010), Bulut and Tuzlacı (2013), Polat et al. (2013), Sargin et al. (2013), Şenkardeş (2014), Özçelik et al. (2016), Paksoy et al. (2016), Uzun and Kaya (2016), Güneş (2017), Yeşilyurt et al. (2017a, 2017b), Gürbüz et al. (2019), Köse (2019), Çiçek (2019), Olgun (2019) |
| Rosaceae | Eriobotrya japonica (Thunb.) Lindl. | CW | Loquat | Leaves | Infusion with Cydonia leaves and Tilia flowers | Baytop (1999), Gürbüz et al. (2019) |
| Rosaceae | Malus domestica Borkh. | C | Apple | Fruits | Eaten raw, Juice | Baytop (1999), Olgun (2019) |
| Rosaceae | Mespilus germanica L.a | CW | Medlar, medlar tree | Leaves, Fruits | Infusion Eaten raw | Tuzlacı (2006), Özhatay et al. (2009), Şenkardeş (2014), Köse (2019) |
| Rosaceae | Potentilla speciosa Willd. | W | No English name | Roots | Decoction | Demirci and Özhatay (2012), Ozturk et al, (2017a), Güneş et al. (2018) |
| Rosaceae | Prunus avium (L.) L. | CW | Sweet cherry | Fruits stalks | Paste (from tarhana flour and rye seeds, honey or molasses) | Sargin et al. (2015a), Çiçek (2019), Gürbüz et al. (2019) |
| Rosaceae | Prunus cerasifera Ehrh. | CW | Cherry plum | Fruits | Eaten raw, Infusion, Decoction | Özhatay et al. (2009), Tetik et al. (2013), Çiçek (2019) |
| Rosaceae | Prunus laurocerasus L. | W | Laurel cherry | Leaves | Infusion with Cydonia leaves | Baytop (1999), Bulut (2016), Gürbüz et al. (2019) |
| Rosaceae | Prunus mahaleb L. | CW | Mahaleb cherry | Leaves | Infusion | Baytop (1999), Bulut and Tuzlacı (2013), Bulut et al. (2019) |
| Rosaceae | Prunus spinosa L. | W | Sloe, blackthorn | Fruits | Eaten raw, Decoction | Özhatay et al. (2009), Yeşilyurt et al. (2017b) |
| Rosaceae | Rosa × damascena Herrm. | CW | Rose, damask rose | Fruits | Infusion | Baytop (1999), Ozturk et al, (2017a), Guzel and Guzelsemme (2018) |
| Rosaceae | Rosa × dumalis Bechst. | CW | Glaucous northern dog rose | Fruits, Leaves | Decoction, Infusion | Polat et al. (2013), Polat (2019), Olgun (2019) |
| Rosaceae | Rosa boissieri Cr‚p.a | W | Rose | Leaves, Fruits | Infusion Decoction | Tuzlacı (2006), Olgun (2019) |
| Rosaceae | Rosa canina L.a | CW | Dog rose, briar rose, common briar | Fruits, Leaves, Flowers, Petals, Roots, Stems | Eaten raw, Infusion, Decoction, Jam, Marmalate | Tuzlacı and Erol (1999), Saraç (2005), Koçyiğit and Özhatay (2006), Özhatay et al. (2009), Ugulu et al. (2009), Genç (2010), Kalafatçılar and Kalafatçılar (2010), Tuzlacı and Doğan (2010), Cakilcioglu et al. (2011), Güneş and Özhatay (2011), Demirci and Özhatay (2012), Yeşilada (2012), Bulut and Tuzlacı (2013), Kilic and Bagci (2013), Polat et al. (2013), Sargin et al. (2013, 2015a; 2015b), Tetik et al. (2013), Kaval et al. (2014), Şenkardeş (2014), Bulut and Tuzlacı (2015), Nacakcı and Dutkuner (2015), Akgül et al. (2016), Bağcı et al. (2016), Bulut (2016), Güner and Selvi (2016), Ozturk et al, (2017a), Paksoy et al. (2016), Uzun and Kaya (2016), Bulut et al. (2017a, 2017b), Ergül-Bozkurt and Terzioğlu (2017), Karaköse and Karaköse (2017), Yeşilyurt et al. (2017b), Dalar et al. (2018), Güneş et al. (2018), Guzel and Guzelsemme (2018), Polat (2019), Akbulut et al. (2019), Çiçek (2019), Demirci-Kayıran (2019), Gürbüz et al. (2019), Kılıç (2019), Köse (2019), Sargin and Büyükcengiz (2019) |
| Rosaceae | Rosa hemisphaerica Herrm. | W | Sulphur rose | Fruits | Eaten raw, Decoction | Şenkardeş (2014), Uzun and Kaya (2016) |
| Rosaceae | Rosa xanthina Lindl. | W | Yellow rose | Fruits | Decoction, Jam | Güneş and Özhatay (2011) |
| Rosaceae | Rubus canescens DC. | W | Woolly blackberry | Leaves | Infusion | Özhatay et al. (2009), Kalafatçılar and Kalafatçılar (2010), Polat et al. (2013), Polat (2019), Akbulut et al. (2019) |
| Rosaceae | Rubus sanctus Schreb. | W | Holy bramble | Fruits, Roots, Flowers | Eaten raw or after drying, Decoction, Infusion, Jam, Marmalate | Ertuğ (2004), Kalafatçılar and Kalafatçılar (2010), Şenkardeş (2014), Sargin et al. (2015a), Güneş et al. (2018), Kılıç (2019), Çiçek (2019), Olgun (2019) |
| Rutaceae | Citrus spp. | CW | Oranges, lemons, grapefruits, pomelos, limes | Fruits, Pericarps | Dropped in teas and soups, Juice (sweetened with sugar), Gargle, Eaten fresh, Jam, Marmalade, Hot mush (externally) | Baytop (1999), Ertuğ (2004), Saraç (2005), Genç (2010), Sağıroğlu et al, (2013), Gökçe (2014), Akan and Bakır-Sade (2015), Gürbüz et al. (2019), Köse (2019) |
| Sapindaceae | Aesculus hippocastanum L. | CW | Horse-chestnut, conker tree | Seeds | Peeled, minced, then swallowed | Baytop (1999), Gürbüz et al. (2019), Köse (2019) |
| Scrophulariaceae | Scrophularia chrysantha Jaub. & Spach | W | Figwort | Whole parts | Decoction after drying | Güneş and Özhatay (2011) |
| Solanaceae | Physalis alkekengi L. | W | Bladder cherry | Fruits | Eaten raw, Decoction | Karaköse and Karaköse (2017), Ozturk et al, (2017b) |
| Urticaceae | Urtica dioica L. | W | Stinging nettle, perennial nettle, tall nettle, common nettle | Aerial parts (without flowering) | Infusion | Tuzlacı and Erol (1999), Kilic and Bagci (2013), Polat et al. (2013), Özer et al. (2005), Tetik et al. (2013), Kaval et al. (2014), Şenkardeş (2014), Sargin et al. (2015a), İşler (2017), Ozturk et al, (2017a), Yeşilyurt et al. (2017a, 2017b), Kılıç (2019) |
| Urticaceae | Urtica urens L. | W | Small nettle | Aerial parts | Infusion | Tuzlacı and Erol (1999), Özer et al. (2005), Cakilcioglu et al. (2011), Şenkardeş (2014), İşler (2017), Yeşilyurt et al. (2017b) |
| Violaceae | Viola sieheana W.Becker | W | No English name | Flowers | Infusion | Özhatay et al. (2009), Karaköse and Karaköse (2017) |
| Violaceae | Viola suavis M.Bieb. | W | Russian violet | Aerial parts | Infusion | Ergül-Bozkurt and Terzioğlu (2017) |
| Vitaceae | Vitis vinifera L. | CW | Common grapevine, grapevine, table grape | Fruits, Seeds | Eaten raw or dried, Cataplasm (with tarhana flour), Molasses | Tuzlacı (2006), Polat et al. (2013), Sargin et al. (2013, 2015a), Kılıç (2019), Köse (2019) |
W: Wild plans, C: Cultured plants, WC: Wild and cultured plants, E: Endemic plants.
Boldly highlighted taxa (which are 189 in total and their anti-influenza effects have not been investigated experimentally yet).
The plants that were also identified to be used in the treatment of malaria.
Table 4.
Worldwide anti-influenza activity research results of the taxa detected in the study.
| Plant species | Active compounds identified (and used parts) | Mechanism of action | References |
|---|---|---|---|
| Alcea rosea L. | Not specified (Aerial parts) | Elicits antiviral innate immune responses in serum, bronchoalveolar lavage fluid, small intestinal fluid, and the lungs | Kim et al. (2018) |
| Allium cepa L. | Not specified (Bulbs) | Decreases Hemagglutination Assay (HA) titers and destroys the avian influenza virus subtype H9N2, and the propagation of the virus | Ahmadi et al. (2018) |
| Allium sativum L. | Allicin (Bulbs) | Inhibits viral nucleoprotein synthesis and polymerase activity | Chavan et al. (2016), |
| Crataegus monogyna Jacq. | Chlorogenic acid (Fruits) | Inhibits neuraminidase activity and blocks the release of newly formed virus particles from infected cells | Ding et al. (2017) |
| Cydonia oblonga Mill | Chlorogenic acid, 3-Caffeoylquinic acid (Fruits) | Inhibit influenza viral activity and no effect on hemagglutination inhibition | Hamauzu et al. (2005) |
| Eucalyptus camaldulensis Dehnh. | Not specified (Leaves) | Inhibit virus replication completely | Sadatrasul et al. (2017) |
| Eucalyptus camaldulensis Dehnh. | 1,8-cineole (Leaves, Essential oil) | Increase the production of influenza-specific serum immunoglobulin (Ig) G2a antibodies, stimulate mucosal secretive IgA (s-IgA) responses at the nasal cavity, improve the expression of respiratory tract intraepithelial lymphocytes (IELs) in the upper respiratory tract, and promote dendritic cell (DC) maturation and the expression of co-stimulatory molecules | Li et al. (2017) |
| Eucalyptus camaldulensis Dehnh. | Mentofin (Leaves, Essential oil) | Inactivate Avian Influenza Virus (AIV) | Barbour et al. (2010) |
| Eucalyptus globulus Labill. | 1,8-cineole (Leaves, Essential oil) | Increase the production of influenza-specific serum immunoglobulin (Ig) G2a antibodies, stimulate mucosal secretive IgA (s-IgA) responses at the nasal cavity, improve the expression of respiratory tract intraepithelial lymphocytes (IELs) in the upper respiratory tract, and promote dendritic cell (DC) maturation and the expression of co-stimulatory molecules | Li et al. (2017) |
| Eucalyptus globulus Labill. | Mentofin (Leaves, Essential oil) | Inactivate Avian Influenza Virus (AIV) | Barbour et al. (2010) |
| Eucalyptus globulus Labill. | Citronellol and Eugenol (Leaves, Essential oil) 1,8-Cineole and α-Thujone (Leaves) | Inhibits the hemagglutinin activity, but not the Neuraminidase activity | Vimalanathan and Hudson (2014) |
| Glycyrrhiza glabra L. | 3,4-dihydro-8,8-dimethyl-2H,8H-benzo dipyran-3-ol, Biochanin B, Glabrol, Glabrone, Hispaglabridin B, Licoflavone B, Licorice glycoside B, Licorice glycoside E, Liquiritigenin, Liquiritin, Prunin (Roots) | Inhibit Neuraminidase (NA) activity | Grienke et al. (2014) |
| Hypericum perforatum L. | Hypericin (Flowers) | Inhibits virus-induced cytopathic effect; ie: Lung consolidation and lessening of lung virus titers. | Pu et al. (2009) |
| Hypericum perforatum L. | Isoquercetin (Flowers) | Inhibit the replication of both influenza A and B viruses at the lowest effective concentration | Kim et al. (2010) |
| Hypericum perforatum L. | Chlorogenic acid and Quercetin (Flowers) | Taken together, it was proposed that chlorogenic acid and quercetin could be employed as the effective lead compounds for anti-influenza A H1N1 due to having strong binding abilities with neuraminidase. | Liu et al. (2016) |
| Malus domestica Borkh. | 5-Caffeoylquinic acid (Fruits) | Inhibit influenza viral activity and no effect on hemagglutination inhibition | Hamauzu et al. (2005) |
| Matricaria chamomilla L. | Borneol (Flowers-Essential oil) | Inhibit the replication of the influenza virus A (H1N1) | Sokolova et al. (2017) |
| Melissa officinalis L. | Not specified (Leaves) | Inhibit the HA (hemagglutinin) activity, but not the NA (Neuraminidase) activity | Jalali et al. (2016) |
| Melissa officinalis L. | Not specified (Leaves) | Inhibit replication of AVI through the different virus replication phase, especially throughout the direct interaction with the virus particles | Pourghanbari et al. (2016) |
| Melissa officinalis L. | Tannin (Leaves) | Aqueous extracts of the melissa plant blocked hemadsorption by parainfluenza viruses, but the tannin of this plant has no effect on influenza A and B viruses in hemagglutination and hemadsorption. | Kucera and Herrmann (1967) |
| Mentha x piperita L. | Menthone and Pulegone (Leaves) | Show good antiviral effects in infected mice. | Qi et al. (2012) |
| Mentha x piperita L. | Mentofin (Leaves, Essential oil) | Inactivate Avian Influenza Virus (AIV) | Barbour et al. (2010) |
| Morus alba L. | Cyanidin-3-rutinoside, Rutin, Cyanidin-3-glucoside, Quercetin, Chlorogenic acid (Fruit juice and seeds) | Exhibit 1.3 log inhibition in the pre- and cotreatment of the virus against FL04, a type B virus. Also exhibited significant DPPH radical scavenging and ferric ion-reducing activities in a dose-dependent manner. | Kim and Chung (2018) |
| Nigella sativa L. | Not specified (Seeds) | Enhance immune responsiveness and suppress pathogenicity of influenza viruses in turkeys | Umar et al. (2016) |
| Olea europaea L. | Not specified (Leaves) | Blokes the receptor site of the viruses | Mehmood et al. (2018) |
| Olea europaea L. | Not specified (Leaves) | Shows significant antiviral activity. Olive oil was included in formulations to ameliorate its potential cytotoxic effects. | Vimalanathan and Hudson (2012) |
| Olea europaea L. | Not specified (Fruits) | Both in influenza A/H1N1 and HRV14, replication cycle and progeny virus production were significantly decreased after the treatment with CAPeo (An essential oil combination based on three aromatic plants (Thymbra capitata, Origanum dictamnus and Salvia fruticosa in extra-virgin olive oil) | Tseliou et al. (2019) |
| Origanum vulgare L | β-carotene and Linoleic acid (Aerial parts) | Decrease influenza virus activation by inhibiting the hemagglutination | Mancini et al. (2009) |
| Origanum vulgare L. | Carvacrol (Essential oil) | Shows significant antiviral activity. Olive oil was included in formulations to ameliorate its potential cytotoxic effects. | Vimalanathan and Hudson (2012) |
| Origanum vulgare L. | Not specified (Essential oil) Linalool (Essential oil) Linalool (Essential oil) | Reduce visible cytopathic effects of influenza A/WS/33 virus activity by > 52.8%. | Choi (2018) |
| Papaver rhoeas L. | Kaempferol-3-sophoroside, Kaempferol-3-neohesperidoside, Kaempferol-3-sambubioside, Kaempferol-3-glucoside, Quercetin-3-sophoroside, Luteolin, Chelianthifoline (Pollen) | Display noncompetitive inhibition of H3N2 neuraminidase and reduce the severity of virally induced cytopathic effects | Lee et al. (2016) |
| Peganum harmala L. | Not specified (Seeds) | Inhibit cytopathic effect of influenza virus | Moradi et al. (2017) |
| Pimpinella anisum L. | Not specified (Essential oil) Linalool (Essential oil) Linalool (Essential oil) | Reduce visible cytopathic effects of influenza A/WS/33 virus activity by > 52.8%. | Choi (2018) |
| Portulaca oleracea L. | Not specified (Aerial parts) | Suppress the production of circulating H1N1 and H3N2 and inhibit the binding of virus to cells and decrease the viral load within 10 min to prevent viral infection | Li et al. (2019) |
| Punica granatum L. | Not specified (Seeds) | Inhibit cytopathic effect of influenza virus | Moradi et al. (2017) |
| Punica granatum L. | Ellagic acid, Caffeic acid, Luteolin, and Punicalagin (Fruit juice) | Suppress replication of influenza A virus and inhibit viral RNA replication and agglutination of chicken red blood cells by influenza virus | Haidari et al. (2009) |
| Salvia fruticosa Mill. | Not specified (Aerial parts-Essential oil) | Both in influenza A/H1N1 and HRV14, replication cycle and progeny virus production were significantly decreased after the treatment with CAPeo (An essential oil combination based on three aromatic plants (Thymbra capitata, Origanum dictamnus and Salvia fruticosa in extra-virgin olive oil) | Tseliou et al. (2019) |
| Salvia officinalis L. | Citronellol and Eugenol (Leaves, Essential oil) 1,8-Cineole and α-Thujone (Leaves) | Inhibits the hemagglutinin activity, but not the Neuraminidase activity | Vimalanathan and Hudson (2014) |
| Salvia sclarea L. | Not specified (Essential oil) Linalool (Essential oil) Linalool (Essential oil) | Reduce visible cytopathic effects of influenza A/WS/33 virus activity by > 52.8%. | Choi (2018) |
| Sambucus nigra L. | Not specified (Fruits) | Reduce hemagglutination and inhibit the replication of human influenza viruses | Zakay-Rones et al. (1995) |
| Sambucus nigra L. | Not specified (Fruits) | Reduce visible cytopathic effects and inhibit at an early point in infection, probably by rendering the virus non-infectious | Chen et al. (2014) |
| Sambucus nigra L. | Not specified (Fruits) | Decrease virus titer and inhibit viral protein synthesis or virus particle release. | Shahsavandi et al. (2017) |
| Sambucus nigra L. | Not specified (Fruits) | Suppress viral replication in the bronchoalveolar lavage fluids and increase the level of the IFV-specific neutralizing antibody in the serum | Kinoshita et al. (2012) |
| Sambucus nigra L. | Not specified (Fruits) | Exhibit a specific neuraminidase-inhibiting effect | Krawitz et al. (2011) |
| Silybum marianum (L.) Gaertn. | Silymarin (Seeds) | Reduces cytopathic effect (CPE) and inhibits viral mRNA synthesis with no cytotoxicity | Song and Choi (2011) |
| Thymbra capitata (L.) Cav. | Carvacrol (Essential oil) | Shows significant antiviral activity. Olive oil was included in formulations to ameliorate its potential cytotoxic effects. | Vimalanathan and Hudson (2012) |
| Thymbra capitata (L.) Cav. | Apigenin, Thymol (Aerial parts-Essential oil) | Both in influenza A/H1N1 and HRV14, replication cycle and progeny virus production were significantly decreased after the treatment with CAPeo (An essential oil combination based on three aromatic plants (Thymbra capitata, Origanum dictamnus and Salvia fruticosa in extra-virgin olive oil) | Tseliou et al. (2019) |
| Urtica dioica L. | Lectin (Roots) | Inhibit mannosidases in host cells rendered the progeny viruses more sensitive to the mannose-binding agents and even to the N-acetylglucosamine-binding Urtica dioica agglutinin | Van der Meer et al. (2007) |
| Vitis vinifera L. | Not specified (Fruits) | Exhibit the prevention of the virus infectivity and the antioxidant activities (DPPH scavenging capacity and superoxide anion radical scavenging capacity) | Bekhit et al. (2011) |
| Cota tinctoria (L.) J.Gaya | Not specified (Aerial parts) | No correlation was found between antiviral activity and fatty acid contents of the extracts. | Orhan et al. (2009) |
| Ficus carica L.a | Not specified (Fruits) | The results indicated that the prepared emulsions could elicit a little degree of immunity, but they could not inhibit the anamnestic response and infection. | Najjari et al. (2015) |
| Olea europaea L.a | Not specified (Fruits) | The results indicated that the prepared emulsions could elicit a little degree of immunity, but they could not inhibit the anamnestic response and infection. | Najjari et al. (2015) |
| Origanum acutidens (Hand.-Mazz.) Ietsw.a | Carvacrol (Flowers-Essential oil) | None of the extracts inhibited the reproduction of influenza A/Aichi virus in MDCK cells | Sökmen et al. (2004) |
| Rosmarinus officinalis L.a | Carnosic acid (Aerial parts) | Inhibit both A- and B- type hRSV, while it does not affect the replication of influenza A virus | Shin et al. (2013) |
| Teucrium polium L.a | Not specified (Aerial parts) | No significant effects on influenza virus infectivity | Derakhshan et al. (2015) |
The taxa that have no significant result for virus inactivation.
Only English and Turkish words were used in the search engines. If they exist, their English translations were reviewed for the studies conducted in different languages, such as Chinese, Korean and French. In this context, approximately 700 articles conducted between January 1977 and February 2020 throughout Turkey were excluded since they did not meet the inclusion criteria and a consensus has been provided among the 81 works on the determination of medicinal plants used by local people for centuries. The list of selected plants from these studies is presented in Table 1 .
Table 1.
Eighty one carefully selected works from ethnomedicinal studies conducted in Turkey.
| Selected Studies | Cited Taxa | Citation% | Region | |
|---|---|---|---|---|
| Şenkardeş (2014) | 39 | 17.4 | Central Anatolia | |
| Tuzlacı (2006) | 34 | 15.2 | All Regions | |
| Baytop (1999) | 33 | 14.7 | All Regions | |
| Ertuğ et al. (2004), | 29 | 12.9 | Aegean | |
| Özhatay et al. (2009) | 26 | 11.6 | Marmara | |
| Sargin (2015) | 25 | 11.2 | Mediterranean | |
| Olgun (2019) | 23 | 10.3 | Eastern Anatolia | |
| Polat et al. (2013) | 23 | 10.3 | Eastern Anatolia | |
| Gökçe (2014) | 22 | 9.8 | All Regions | |
| Kılıç (2019) | 22 | 9.8 | Southeastern Anatolia | |
| Genç (2010) | 21 | 9.4 | All Regions | |
| Köse (2019) | 20 | 8.9 | Black sea | |
| Arıtuluk (2010) | 19 | 8.5 | Mediterranean | |
| Sargin et al. (2015a) | 19 | 8.5 | Aegean | |
| Cakilcioglu et al. (2011) | 18 | 8.0 | Eastern Anatolia | |
| Demirci-Kayıran (2019) | 18 | 8.0 | Mediterranean | |
| İşler (2017) | 17 | 7.6 | All Regions | |
| Polat (2019) | 17 | 7.6 | Eastern Anatolia | |
| Gürbüz et al. (2019) | 16 | 7.1 | Black sea | |
| Kalafatçılar and Kalafatçılar (2010) | 16 | 7.1 | All Regions | |
| Bulut and Tuzlacı (2015) | 15 | 6.7 | Marmara | |
| Bulut et al. (2019) | 15 | 6.7 | Southeastern Anatolia | |
| Güneş (2017) | 15 | 6.7 | Marmara | |
| Günbatan et al. (2016) | 14 | 6.3 | Central Anatolia | |
| Çiçek (2019) | 13 | 5.8 | Southeastern Anatolia | |
| Karaköse and Karaköse (2017) | 13 | 5.8 | Black sea | |
| Ozturk et al. (2017)a | 13 | 5.8 | Southeastern Anatolia | |
| Sargin and Büyükcengiz (2019) | 13 | 5.8 | Mediterranean | |
| Tuzlacı and Doğan (2010) | 13 | 5.8 | Eastern Anatolia | |
| Tuzlacı and Erol (1999) | 13 | 5.8 | Mediterranean | |
| Ertuğ (2004) | 11 | 4.9 | Aegean | |
| Güneş and Özhatay (2011) | 11 | 4.9 | Eastern Anatolia | |
| Kılıç (2016) | 11 | 4.9 | Eastern Anatolia | |
| Kilic and Bagci (2013) | 11 | 4.9 | Eastern Anatolia | |
| Guzel and Guzelsemme (2018) | 10 | 4.5 | Mediterranean | |
| Ozturk et al. (2017b) | 10 | 4.5 | Mediterranean | |
| Saraç (2005) | 10 | 4.5 | All Regions | |
| Tetik et al. (2013) | 10 | 4.5 | Eastern Anatolia | |
| Yeşilyurt et al. (2017b) | 10 | 4.5 | Marmara | |
| Akgül et al. (2016) | 9 | 4.0 | Central Anatolia | |
| Bulut et al. (2017a) | 9 | 4.0 | Aegean | |
| Cansaran and Kaya (2010) | 9 | 4.0 | Black sea | |
| Güner and Selvi (2016) | 9 | 4.0 | Marmara | |
| Nacakcı and Dutkuner (2015) | 9 | 4.0 | Mediterranean | |
| Özçelik et al. (2016) | 9 | 4.0 | Mediterranean | |
| Akan and Bakır-Sade (2015) | 8 | 3.6 | Southeastern Anatolia | |
| AkBulut et al. (2019) | 8 | 3.6 | Aegean | |
| Kurt and Karaoğul (2018) | 8 | 3.6 | Black sea | |
| Paksoy et al. (2016) | 8 | 3.6 | Central Anatolia | |
| Sargin et al. (2013) | 8 | 3.6 | Aegean | |
| Yılmaz (2019) | 8 | 3.6 | Aegean | |
| Demirci and Özhatay (2012) | 7 | 3.1 | Southeastern Anatolia | |
| Kaval et al. (2014) | 7 | 3.1 | Eastern Anatolia | |
| Kocabaş and Gedik (2016) | 7 | 3.1 | Mediterranean | |
| Maranki and Maranki (2016) | 7 | 3.1 | All Regions | |
| Tuzlacı and Eryaşar-Aymaz (2001) | 7 | 3.1 | Marmara | |
| Ugulu et al. (2009) | 7 | 3.1 | Aegean | |
| Tanker et al. (1998) | 7 | 3.1 | All Regions | |
| Dalar et al. (2018) | 6 | 2.7 | Eastern Anatolia | |
| Güneş et al. (2018) | 6 | 2.7 | Mediterranean | |
| Kocabas et al. (2017) | 6 | 2.7 | Mediterranean | |
| Bağcı et al. (2016) | 5 | 2.2 | Central Anatolia | |
| Bulut and Tuzlacı (2013) | 5 | 2.2 | Aegean | |
| Bulut et al. (2017b) | 5 | 2.2 | Aegean | |
| Koçyiğit and Özhatay (2006) | 5 | 2.2 | Marmara | |
| Özer et al. (2005) | 5 | 2.2 | All Regions | |
| Sargin et al. (2015b) | 5 | 2.2 | Mediterranean | |
| Uzun and Kaya (2016) | 5 | 2.2 | Central Anatolia | |
| Akgul et al. (2018) | 4 | 1.8 | Southeastern Anatolia | |
| Bulut (2016) | 4 | 1.8 | Marmara | |
| Ergül-Bozkurt and Terzioğlu (2017) | 4 | 1.8 | Black sea | |
| Tuzlacı and Tolon (2000) | 4 | 1.8 | Marmara | |
| Yeşilada (2012) | 4 | 1.8 | All Regions | |
| Yeşilyurt et al. (2017a) | 4 | 1.8 | Black sea | |
| Kartal and Güneş (2017) | 3 | 1.3 | Marmara | |
| Bozyel and Merdamert-Bozyel (2020) | 2 | 0.9 | All Regions | |
| Ekşi et al. (2020) | 2 | 0.9 | All Regions | |
| Han and Bulut (2015) | 2 | 0.9 | Central Anatolia | |
| Karamanoğlu (1977) | 2 | 0.9 | All Regions | |
| Saraçoğlu (2014) | 2 | 0.9 | All Regions | |
| Sağıroğlu et al. (2013) | 1 | 0.4 | Black sea | |
2.2. Data selection
The studies determined to be within the scope of plant screening were reviewed, compared and carefully selected according to the following criteria. Accordingly, a study should:
-
•
be carried out in an area within the borders of Turkey.
-
•
performed on ethnobotanical or ethnopharmacological concept layout.
-
•
include scientific names and local names of the plants used.
In addition, the criterion for choosing the book sources was either the writer having an academic title or the work having been cited. If neither of these were in case, the work was not taken into consideration.
The screening of the resulting plants in the world literature was carried out considering the following criteria. Accordingly, a study should be:
-
•
an experimental (in vitro or in vivo) study, not a review.
-
•
included the scientific name of the plant in its title. In case of writing only the English name of the plant, it is obligatory to include the scientific name in the text.
-
•
carried out under the headings of “anti-flu, anti-influenza or antiviral activities against influenza".
If it contains the active compound(s), it becomes preferable and the mechanism of action is recorded.
2.3. Data arrangement
Table 1 contains the scientific names of plants, their families, local names, English common names, parts used, forms used, and references. The validation of the scientific names of the specified plant taxa was provided by the book Turkey Plant List (Vascular Plants) (Güner et al., 2012), the International Plant Names Index (IPNI: http://www.ipni.org) and the Plant List (http://www.theplantlist.org). English common names of the taxa are placed in the table using the following databases or search engines: EPPO Global Database (https://gd.eppo.int), Plants Database (http://garden.org/plants), USDA PLANTS (https://plants.sc.egov.usda.gov/java), Encyclopedia of Life (https://eol.org), Lebanon Flora (http://www.lebanon-flora.org), Springer Link (https://link.springer.com/article), Flora of Israel Online (http://flora.org.il), Altervista Flora Italiana (http://luirig.altervista.org/flora), and Plants of the World online (http://www.plantsoftheworldonline.org). Taxa for which common English names could not be found have been noted as endemic to Turkey, or containing Irano-Turanian elements.
Finally, the plants were arranged in alphabetical order according to family names. In order to prove the scientific validity of the ethnobotanical data obtained, the research data of the experimental studies regarding the taxa in the list, as found in the world literature, are shown in a separate table (Table 4). In this table, the mechanism of action, active compounds and used parts are also included, in addition to the researched taxa and their references. Great care has been taken to ensure that the findings obtained in these screening studies belong to experimental studies (in vitro or in vivo), not a review.
2.4. Comparative analysis
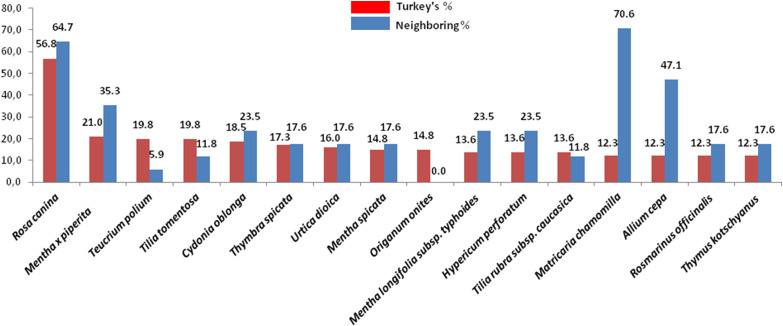
After obtaining the total list of plants with anti-influenza potential in Turkish folk medicine, a comparison was made to determine the similarity percentages in similar studies conducted in neighboring and nearby countries (Table 2 ). To avoid distraction from the subject integrity, not all studies in those countries were included in our comparison. Therefore, only the study with the richest content and the highest percentage of similarity from each country was included in the comparison list. Studies with a similarity percentage >10% were eliminated in the primary elections.
Table 2.
Similarity percentages of neighboring studies (sorted by descending order according to the similarity index).
| Countries | Regions | Total taxa used for influenza | Similar Taxa # | Similarity % | References |
|---|---|---|---|---|---|
| Iraq | Sulaymaniyah (Northern) | 20 | 15 | 75.0 | Ahmed (2016) |
| Bosnia and Herzegovina | Javor Mountain | 15 | 11 | 73.3 | Savić et al. (2019) |
| Cyprus | All | 26 | 19 | 73.1 | Karousou and Deirmentzoglou (2011) |
| Bulgaria | All | 18 | 13 | 72.2 | Kozuharova et al. (2013) |
| Romania | Dobruja (South-Eastern) | 24 | 17 | 70.8 | Pieroni et al. (2014) |
| Kosovo | Southern | 20 | 14 | 70.0 | Mustafa et al. (2015) |
| Croatia | Knin | 18 | 12 | 66.7 | Varga et al. (2019) |
| Georgia | Caucasus | 20 | 13 | 65.0 | Bussmann et al. (2016) |
| Syria | Aleppo | 14 | 9 | 64.3 | Alachkar et al. (2011) |
| Iran | Sirjan in Kerman | 14 | 9 | 64.3 | Nasab and Khosravi (2014) |
| Albania | Alps | 30 | 18 | 60.0 | Mustafa et al. (2012) |
| Greece | Thessaloniki (Northern) | 74 | 44 | 59.5 | Hanlidou et al. (2004) |
| Serbia | South-eastern | 36 | 20 | 55.6 | Jarić et al. (2015) |
| Macedonia | Sharr Mountains | 20 | 9 | 45.0 | Rexhepi et al. (2013) |
| Jordan | Northern Badia | 14 | 6 | 42.9 | Alzweiri et al. (2011) |
| Israel | All | 21 | 8 | 38.1 | Lev and Amar (2000) |
| Montenegro | Prokletije Mountains | 22 | 7 | 31.8 | Menković et al (2011) |
3. Results and discussion
The demand for new antimicrobial agents, especially antivirals, is constantly increasing. This demand arises from the lack of antiviral agents in the market and the emergence of resistant mutants to existing drugs (Vijayan et al., 2004). Throughout our existence, human beings have always been in search of healing from plants in the fight against winter diseases, but clinical studies have to this point been limited. Although the following work is relatively new in Turkey, they are promising for future study: Duman et al. (2018) elicited in vitro antiviral activity of Ribes uva-crispa L and Ribes multiflorum Kit ex Schult, which are naturally grown in Turkey, use the methanol and aqueous extracts of the leaves and fruits; Dogan et al. (2020) revealed anti-RSV effects of Ribes uva-crispa juicy fruit and leaf methanol extracts against the respiratory syncytial virus (RSV) (the cause of a worldwide viral infection), and emphasized their advantages to synthetic drugs; finally, Adem et al, (2020) found that natural polyphenols, such as hesperidin, routine, diosmin and apiin were more effective than nelfinavir in treating COVID-19. The plants (Table 3 ), which have been used by locals in Turkey for centuries for the prevention and treatment of influenza and its adverse effects - from colds to sudden deaths from respiratory failure - need to be investigated in this way. Today, much more research is needed, as outbreaks such as SARS, avian influenza, swine influenza and COVID-19 threaten the existence of human beings every year.
3.1. Regional analysis
Distribution of 81 studies by region was performed as follows: 13 in the Mediterranean (16.0%), 11 in Eastern Anatolia (13.6%), 10 in the Marmara and Aegean region (12.3%), 8 in the Black Sea (12%), 7 in Central and Southeastern Anatolia (11.1%), and 15 general studies across all regions (18.5%). The regional distribution of 921 total citations received was as follows: Mediterranean: 150 (16.3%), Eastern Anatolia: 141 (15.3%), Aegean: 109 (11.8%), Marmara: 98 (10.6%), Central and Southeastern Anatolia: 82 (8.9%), Black Sea: 75 (8.1%), and general studies covering all regions: 184 (20.0%). The reason why the studies conducted in the Mediterranean and Eastern Anatolia regions were highly cited may be due to the fact that there are more plant options, which is the result of having a higher rate of biodiversity and endemism in these regions (Güner et al., 2012) compared to others, that the locals can use in the treatment of influenza. In addition, the topographic structure of the region, and the fact that the region is isolated from city centers in winter conditions (Doğanay and Orhan, 2016) may have been a factor for the people living in these rural areas to choose mostly natural treatment methods.
3.2. Data analysis of ethnomedicinal plants used in flu treatment in Turkey
It has been determined that 224 plants, selected from 81 studies composing of 57 articles, 13 books, seven theses, three chapters and one congress report in total, belonging to 43 families. These plant taxa most commonly belong to the Lamiaceae (88 taxa, 39.3%), Compositae (32 taxa, 14.3%), Rosaceae (21 taxa, 9.4%), Malvaceae (13 taxa, 5.8%), and other families (70 taxa, 31.3%). The most preferred outcome of the Lamiaceae family may be due to the Turkish people's preference for flu treatment, as it is the family that contains the highest dosage of essential oils (Askun et al., 2012). The second family, Compositae, is known as Turkey's most common family (Güner et al., 2012). Infusions prepared from taxa with capitula flower structures such as its representative Chamomile are widely used by local people. Therefore, this was an expected result.
According to studies conducted in different regions of Turkey (Fig. 1), the most common genera are Sideritis (16 taxa, 7.1%), Salvia (12 taxa, 5.4%), Thymus (12 taxa, 5.4%), and Origanum (10 taxa, 4.5%). This finding may indicate that these genus members are more effective in anti-influenza treatment than other genera. In addition, they are the most favored medicinal tea for the locals of Turkey, and even without natural nationwide distribution, it is possible to find these products in almost every public market, herbal and spice shop (Ertuğ, 2004; Dogan, 2012). Some species, such as thyme (Thymus spp.), melissa (Melissa officinalis), lavender (Lavandula angustifolia), cassidony (Lavandula stoechas) and sage (Salvia officinalis), are today being grown in home gardens, balconies or on small farms by rural people for folk medicine use, or for trade and household income (Güneş, 2017; Ekşi et al., 2020). like thyme, melissa, lavender, and sage, Among the identified plants, 145 were wild (64.7%), 49 were wild and cultivated (21.9%), 27 were endemic (12.1%) and 3 (Allium cepa, Allium sativa and Malus domestica) were cultivated (1.3%). These parameters are shown in a column in Table 3; wild taxa as “W", cultivated “C", cultivated & wild “CW” and endemic “E". Most of the plant pieces used are aerial parts (41.1%), flowers/flowering branches/petals (30.8%), leaves (25.0%), fruits (17.4%), seeds/cones (8.5%), roots/bulbs/tubers (6.7%), and other parts (stems, buds, barks, whole parts, resins, tars, cupula, bracts, fruit stalks, essential oils and fixed oils) (14.3%). Those parts were mostly used as infusions (78.6%), decoction/boiling (19.2%), raw eating/swallowing/salad (12.9%), molasses/jam/syrup/juice (7.6%), lotion/drop/cataplasm/vapor compression (6.3%) and other consumption types (roasting, mouthwash, tincture, mixture and pastes) (5.4%) and powdered for spice use (3.1%). The taxa having with the most usage types are Citrus spp (7 types, 3.1%), Rosa canina and Rubus sanctus (5 types, 2.2%) and Vitis vinifera (4 types, 1.8%), while the taxa with the maximum number of consumption parts belong to Rosa canina and Tilia tomentosa (6 parts, 2.7%), and Juniperus oxycedrus (5 parts, 2.2%). Additionally, Rosa canina (with 5 different types of use and 6 different parts) have appeared as the most efficient plants in terms of the total of both part and usage type (Table 3).
3.3. Comparative evaluation of the data with studies of nearby countries
16 taxa, such as Rosa canina (with 46 references and 56.8%) and Mentha x piperita (with 17 references and 21.0%) (Fig. 2 , red color), have been identified as the most frequently cited plants. The reason why these herbs are highly cited may be a reflection of their stronger protective and therapeutic effects against flu; this may be the result of the experience gained in Turkish folk medicine for centuries. We would obviously see this when comparing similar studies between 17 geographically close countries (Fig. 2, blue color). The emergence of the data presented in Table 2 in a similar manner as in Fig. 2 confirms the superior efficacy of these plants, with 76.7% similarity.
Fig. 2.
The most frequently cited plants in Turkey and neighboring countries.
As a matter of fact, similar results were obtained from studies conducted in 17 neighboring countries, comparing with the taxon list presented in the study, including especially Rosa canina (11 countries with 64.7%), Sambucus nigra (8 countrIes with 47.1%) and Mentha x piperita (6 countries with 35.3%). While the similarity was seen mostly in Iraq (75.0%), Bosnia and Herzegovina (73.3%), and Cyprus (73.1%), the least similarity was seen in Montenegro (31.8%) and Israel (38.1%). This may due to the fact that, besides the resemblance of landforms, climate and vegetation, we lived together with the cultures of those countries during the Ottoman period for about 500 years. The reason for the low similarity in Israel and Montenegro may be due to the geographical distance as well as the difference of social-cultural habits, religious rituals, topography and flora (Table 2). It was not very surprising that Matricaria chamomilla emerged as the plant used most in influenza treatment in 12 countries (70.6%) since the spreading area of this plant is very wide and it is very easy for the public to access and use (Fig. 2).
3.4. Comparative analysis with studies in the global literature
Experimental research studies carried out in the world in terms of anti-influenza activities have been determined only for 35 out of 224 taxa (15.6%). Still, among these studies, the active substances were detected for only 18 taxa (8.0%); for the remaining 17 taxa (7.6%), it was observed that they had not been specified (Table 4 ). In Table 4, only “the parts used in research” were given as an idea for these taxa for which active gradients had been “not specified”. It is noteworthy that no investigation has been conducted for 189 (84.4%) taxa yet (they are highlighted in bold in Table 3). Among these 35 taxa, the most common active chemicals are quercetin and chlorogenic acid (7.1%), mentofin (5.4%) and 1,8-cineole (3.6%). The most preferred mechanisms in research are inhibition of viral replication by inhibiting viral nucleoprotein synthesis or polymerase and neuraminidase activity (40.4% out of the 47 mechanisms in total), blocking the receptor site of the viruses by inhibition of neuraminidase, reducing the hemagglutination, or blocking hemadsorption (31.9%), inhibition of the virus-induced cytopathic effect by blocking hemadsorption (21.3%), and stimulating and boosting of the immunity (6.4%). The reason that the six taxa at the end of the list are shown as a line separated from the alphabetical sequence is that there was no significant result for virus inactivation in the experimental studies conducted for them (Table 4).
According to screening results found in the global literature, the most preferred plants in experimental anti-influenza studies are Sambucus nigra (14.3%, out of 35 taxa), Olea europaea (11.4%), followed by Eucalyptus camaldulensis, E. globules, Melissa officinalis and Origanum vulgare (8.6%). The reason for this may be that these plants are easily accessible in nature or from the virtual market environment, and can be obtained for less money. Additionally, eucalyptus trees in Turkey are also known as “malaria trees”, as the infusion prepared from its leaves is used against malaria in traditional medicine (Baytop, 1999; Ertuğ, 2004). Although its effectiveness against COVID-19 has not been fully proven by clinical trials, the widespread use and mass production of chloroquine and similar malaria drugs are permitted in many countries, and positive results continue to be achieved (Millán-Oñate et al., 2020; Touret and de Lamballerie, 2020). This correlation of data has been positive and unexpected because there are fourteen more plants, including Centaurea drabifolia subsp floccosa (an endemic taxon), which have been detected in this study to be used in the treatment of malaria. These fifteen plants are presented in Table 3 by adding the "*" sign to the end of their scientific names.
The percentage of compatibility of the plant parts belonging to these 35 (15.6%) taxa found between the investigation results in the world literature and ethnobotanical results of the study was found to be 92.9%. This result may prove the fact that for centuries, the locals have been equally justified in their preferences of plant usage.
3.5. Comparative evaluation of active compounds
Taxa containing quercetin, which has a typical polyphenol structure with anti-influenza activity, are Hypericum perforatum, Morus alba and Papaver rhoeas (Kim et al., 2010; Liu et al., 2016; Kim and Chung, 2018) (Table 4). It is not accidental that we detected quercetin and chlorogenic acid as the most common active gradients in our screening records, because these compounds are found to be the most effective compounds used in the treatment of influenza. Supporting these findings, Kumar et al. (2003) stated in a study of mice that quercetin (Fig. 3 A) may be useful as a drug to reduce oxidative stress caused by influenza virus infection in the lungs, and to protect them from the toxic effects of free radicals. In another study, Wu et al. (2016) stated that quercetin, which shows inhibitory activity in the early stage of influenza infection, offers a future therapeutic option for developing effective, safe and affordable natural products for the treatment and prophylaxis of influenza virus infections. Moreover, Nile et al. (2020), in an investigation of the antiviral and cytotoxic effects of quercetin 3-glucoside (Q3G) from Dianthus superbus, Q3G (Fig. 3B) found that this substance showed strong antiviral activity against influenza A and B viruses. Therefore, they emphasized that it could be developed and used as a natural anti-influenza drug.
Fig. 3.
The chemical structures of quercetin (A), quercetin 3-glucoside (B) and chlorogenic acid (C).
On the other hand, chlorogenic acid (CHA) is a caffeoylquinic acid constituent (Fig. 3C) found in many vegetables and fruits traditionally used in Turkish folk medicine, such as Cydonia oblonga, Crataegus monogyna, Morus alba, Hypericum perforatum, Eucalyptus globules (Baytop, 1999; Ding et al., 2017; Kim and Chung, 2018). Indeed, many researchers including Ding et al. (2017) and Ren et al. (2019) have pointed out that CHA acts as a neuraminidase blocker to inhibit influenza A virus at both in vitro and in vivo levels, thus they stated that CHA is potentially beneficial in the treatment of influenza.
Among the researches, the taxa containing the most active compounds in terms of anti-influenza activity were Glycyrrhiza glabra (11 chemicals with 31.4% out of the 35), Papaver rhoeas (7; 20.0%), Morus alba (5; 14.3%) and Punica granatum (4; 11.4%) (Table 4). Glycyrrhiza glabra (licorice) is among the oldest and most popular traditional herbal medicines worldwide (Grienke et al., 2014). Also, its roots are one of the most frequently used parts for treating respiratory tract infections in Turkish folk medicine (Baytop, 1999; Ertuğ, 2004). Hence, the roots may have appeared to have the greatest number of active ingredients in the screening. This result overlaps with the findings of Grienke et al. (2014) because they had emphasized that the accumulation of the plant components exhibits 3D similarities to known flu Neuraminidase inhibitors (which are key enzymes in viral replication and the first-line drug target to fight influenza) according to their basis of a shape-focused virtual screening. Therefore, this finding may be pointing out that this plant is more effective and specific than other taxa in terms of anti-influenza activity.
3.6. Ecotic plants
In addition, 9 medicinal exotic herbs were detected to have been traditionally used in the treatment of influenza and sold in herbal and public markets. Zingiber officinale (ginger), Curcuma longa (turmeric), Syzygium aromaticum (cloves), Piper nigrum (black pepper) and Cinnamomum verum (cinnamon) are examples of these plants. Information on which parts, methods, and how often these plants are used in flu treatment is given in Table 5 . The citrus species presented in Table 3 are actually exotic species. For several centuries, they have mainly exhibited a distribution in the Aegean and Mediterranean coasts in Turkey's flora. Citrus limon (lemon), C. sinensis (orange), C. reticulata (tangerine), C. paradisi (grapefruit) and C. x aurantium (citrus) are among these types. Eucalyptus camaldulensis and E. globulus (Eucalyptus trees), another plant that has settled in the flora, are of Australian origin and have been used in forestry, roadside landscaping, drying of the marshes and folk medicine practices, such as combating malaria, since the Ottoman era (Özgün, 2013).
Table 5.
Exotic plants used for influenza treatment in Turkish folk medicine.
| Families | Sc. Names | Local names | English names | Parts | Preparations | Homeland | References |
|---|---|---|---|---|---|---|---|
| Combretaceae | Terminalia chebula Retz. | Kara halile, karahalile | Black myrobalan | Unripe Fruits | Decoction or infusion (after pulverizing) | South Asia | Baytop (1999), Akan and Bakır-Sade (2015) |
| Lauraceae | Cinnamomum verum J.Presl | Tarçın, darçın | Cinnamon, true cinnamon tree | Bark | Decoction or infusion (after pulverizing) with/without cloves | South and Southeast Asia | Baytop (1999), Kocabaş and Gedik (2016), Gürbüz et al. (2019) |
| Lythraceae | Lawsonia inermis L. | Kına, kına otu | Hina, henna tree, mignonette tree, Egyptian privet | Leaves | Infusion of 1% is used in the treatment of lung inflammation. To reduce fever in infants, it is mixed with dried mint, honey and eggs and applied externally to the baby's chest and back. | Northeast Africa | Baytop (1999), Günbatan et al. (2016), Demirci-Kayıran (2019) |
| Myrtaceae | Syzygium aromaticum (L.) Merr. & L.M.Perry | Karanfil | Cloves | Flower buds, Essential oil | Pastille, Infusion, Frankincense | Maluku Islands | Baytop (1999), Sargin et al. (2013) |
| Piperaceae | Piper nigrum L. | Kara biber, karabiber | Black pepper | Unripe Fruits | Infusion prepared with mint (Mentha × piperita) is consumed after the addition of honey. | India | Baytop (1999), Güneş (2017), Gürbüz et al. (2019) |
| Rubiaceae | Cinchona pubescens Vahl | Kınakına, kınakına ağacı | Red cinchona, quina | Bark | 15–30 g of liqueur or wine, containing sulfate salts, is drunk 3 times a day. | Central and South America | Baytop (1999) |
| Zingiberaceae | Zingiber officinale Roscoe | Zencefil | Ginger | Rhizomes | Dried and pulverized rhizomes are used as an infusion or eaten by mixing with honey | South Asia | Baytop (1999), Sargin et al. (2013), Akan and Bakır-Sade (2015), Kocabaş and Gedik (2016), Gürbüz et al. (2019), Demirci-Kayıran (2019) |
| Zingiberaceae | Alpinia officinarum Hance | Havlıcan, havlucan | Lesser galangal | Rhizomes | Decoction or infusion (after pulverizing) | Southeast Asia | Baytop (1999), Sargin et al. (2013), Akan and Bakır-Sade (2015), Kurt and Karaoğul (2018) |
| Zingiberaceae | Curcuma longa L. | Zerdeçal, Hint safranı, safran kökü, sarıboya, zerdeçav | Turmeric | Rhizomes | Decoction or infusion (after pulverizing) with/without lemon and zingiber. Eaten a coffee spoonful with some honey, twice a day | Indian subcontinent and Southeast Asia | Baytop (1999), Akan and Bakır-Sade (2015) |
The point we should especially emphasize here is that, while herbal products to be released for the treatment of influenza are determined by World Health Organisation (WHO) and the European Phytotherapy Scientific Cooperative (ESCOP), and controlled by the Turkish government, these standard practices are not yet available for fresh or dried plant taxa that are traditionally consumed and sold in public markets and herbalist shops in Turkey. Besides, it can never be ignored that medicinal plants are very successful in preventing and treating influenza if used according to the prescriptions specified in their pharmacopoeia. Thus, it is necessary to record traditional-empirical practices with proven trial-and-error methods urgently, to demonstrate their activities and active ingredients in vitro or in vivo studies, and to enlighten the public by adding optimal tariffs to their pharmacopoeia by the relevant official standard institutions.
In our study, it was also determined that 27 endemic plants were used effectively in influenza treatment and collected from nature. The unconscious collection of endemic and endangered species in the red list of the International Association for Nature Conservation (IUCN) should be more carefully monitored using laws, media and educational tools and methods, and the necessary precautions should be urgently taken.
4. Conclusion
Although the first choice for influenza control and reducing the effects of epidemics is a vaccine, it is also known that it is not the fastest and most effective option since modifications in viral proteins require annual adaptation of the influenza vaccine formulation, as noted by Nachbagauer and Palese (2020). Considering the side effects and complications of antiviral medicines, the search for more effective remedies for fast-spreading pandemic influenza strains continues intensively all over the world today.
Due to their easy production, low cost, water-solubility, low toxicity and selective effects, medicinal plants, especially herbal essential oils and antiviral compounds found in their aqueous extracts are the most studied natural ingredients in recent times (Grienke et al., 2009). Therefore, natural products such as traditional herbs show great promise in the development of potentially effective new antiviral drugs. Particularly, recent studies on phytochemicals, such as quercetin, chlorogenic acid, mentofin, and linalool abundantly found in many plants and vegetables, eliminate the efforts and huge costs of finding lots of antiviral vaccines that need to be renewed every year and allow us to be more optimistic about the successful management of the next influenza outbreaks.
Turkey has remarkable potential for serious research on this topic due to having vast ethnomedicinal experience and the richest flora of Europe and the Middle East. This study, conducted in this regard, is the first nationwide ethnomedical screening study conducted on flu treatment with plants in Turkey. In particular, we would like to emphasize that the most common detected genus members, such as Sideritis (16 taxa; 7.1%), Salvia (12; 5.4%), Thymus (12; 5.4%), and Origanum (10; 4.5%) may be more efficient in terms of the anti-influenza targeting than other genera for the interest of the sectors that are researching new natural drug sources.
Through this study, we strongly recommend these 35 (15.6%) plants, which have proved their high anti-influenza activities and inhibition potentials in the experimental studies, to be subject to clinical research and for widespread use in the near future. Also, with 189 (84.4%) taxa detections that have not been investigated yet, it is an important resource for both national and international pharmacological researchers. Clinical research and evaluation studies required for standard compliance for human use, starting especially with the fifteen plant taxa whose use records against both malaria and influenza were presented in this study, can be begun. With a possible mass production of one or more malaria-like drugs, a significant contribution can be provided to the indigenous people living in that region and to the national economy. Therefore, more experimental studies are urgently needed to understand the true value of these plants. Based on the data to be obtained, we believe that the future extension of anti-influenza studies, including plant taxa that are frequently used in Turkish folk medicine, would be a more effective option.
References
- Adem S., Eyupoglu V., Sarfraz I., Rasul A., Ali M. Identification of Potent COVID-19 Main Protease (Mpro) Inhibitors from Natural Polyphenols: An in Silico Strategy Unveils a Hope against CORONA. Preprints. 2020;46(3):1–16. doi: 10.20944/preprints202003.0333.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmadi S., Rajabi Z., Marandi M.V. Evaluation of the antiviral effects of aqueous extracts of red and yellow onions (Allium cepa) against avian influenza virus subtype H9N2. Iran. J. Vet. Sci. Technol. 2018;2(19):23–27. doi: 10.22067/veterinary.v2i10.74060. [DOI] [Google Scholar]
- Ahmed H.M. Ethnopharmacobotanical study on the medicinal plants used by herbalists in Sulaymaniyah Province, Kurdistan, Iraq. J. Ethnobiol. Ethnomed. 2016;12(8):1–17. doi: 10.1186/s13002-016-0081-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akgül G., Yılmaz N., Celep A., Celep F., Çakılcıoğlu U. Ethnobotanical purposes of plants sold by herbalists and folk bazaars in the center of Cappadocica (Nevşehir, Turkey) Indian J. Tradit. Knowl. 2016;15(1):103–108. [Google Scholar]
- Akan H., Bakır-Sade Y. Kâhta (Adıyaman) merkezi ve Narince köyü’nün etnobotanik açıdan araştırılması. Bitlis Eren Univ. J. Sci. Technol. 2015;4(2):219–248. doi: 10.17798/beufen.47724. [DOI] [Google Scholar]
- Akbulut S., Karaköse M., Özkan Z.C. Traditional uses of some wild plants in kale and acıpayam provinces in denizli. Univ. J. For. Fac. 2019;19(1):72–81. doi: 10.17475/kastorman.543529. [DOI] [Google Scholar]
- Akgul A., Akgul A., Senol S.G., Yildirim H., Secmen O., Dogan Y. An ethnobotanical study in Midyat (Turkey), a city on the silk road where cultures meet. J. Ethnobiol. Ethnomed. 2018;14(12):1–18. doi: 10.1186/s13002-017-0201-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alachkar A., Jaddouh A., Elsheikh M.S., Bilia A.R., Vincieri F.F. Traditional medicine in Syria: folk medicine in Aleppo governorate. Natl. Prod. Commun. 2011;6(1):79–84. doi: 10.1177/1934578X1100600119. [DOI] [PubMed] [Google Scholar]
- Alaoui-Jamali M. Springer Science & Business Media; 2010. Alternative and Complementary Therapies for Cancer: Integrative Approaches and Discovery of Conventional Drugs. [Google Scholar]
- Alzweiri M., Al Sarhan A., Mansi K., Hudaib M., Aburjai T. Ethnopharmacological survey of medicinal herbs in Jordan, the Northern Badia region. J. Ethnopharmacol. 2011;137(1):27–35. doi: 10.1016/j.jep.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Arıtuluk Z.C. Hacettepe University, Health Sciences Institute; Ankara: 2010. The Flora and Folk Medicine of Tefenni (Burdur) District. MSc Thesis. [Google Scholar]
- Askun T., Tumen G., Satil F., Modanlioglu S., Yalcin O. In: Underst. Tuberc. - New Approaches Fight. Drug Resist. Cardona P.J., editor. InTech; 2012. Antimycobacterial activity some different Lamiaceae plant extracts containing flavonoids and other phenolic compounds; pp. 309–336. [DOI] [Google Scholar]
- Barbour E.K., Yaghi R.H., Jaber L.S., Shaib H.A., Harakeh S. Safety and antiviral activity of essential oil against avian influenza and Newcastle disease viruses. Intern. J. Appl. Res. Vet. Med. 2010;8(1):60–64. [Google Scholar]
- Basiri M.R. Theory about treatments and morbidity prevention of corona virus disease (Covid-19) J. Pharm. Pharmacol. 2020;8:89–90. doi: 10.17265/2328-2150/2020.03.004. [DOI] [Google Scholar]
- Baytop T. Nobel Tıp Kitabevleri; 1999. Türkiye'de bitkiler ile tedavi: geçmişten Bugüne. İstanbul. [Google Scholar]
- Bağcı Y., Erdoğan Y., Doğu S. Ethnobotanical features of growing plants in Sarıveliler (Karaman) and its environment. Selçuk Univ. J. Sci. Fac. 2016;42(1):84–107. [Google Scholar]
- Bekhit A.E.D.A., Cheng V.J., McConnell M., Zhao J.H., Sedcole R., Harrison R. Antioxidant activities, sensory and anti-influenza activity of grape skin tea infusion. Food Chem. 2011;129(3):837–845. doi: 10.1016/j.foodchem.2011.05.032. [DOI] [PubMed] [Google Scholar]
- Bekut M., Brkić S., Kladar N., Dragović G., Gavarić N., Božin B. Potential of selected Lamiaceae plants in anti (retro) viral therapy. Pharmacol. Res. 2018;133:301–314. doi: 10.1016/j.phrs.2017.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozyel M.E., Merdamert-Bozyel E. Ethnomedicinal uses of genus lavandula (Lamiaceae) in Turkish traditional medicine. Int. J. Acad. Appl. Res. 2020;4(2):5–16. [Google Scholar]
- Bulut G. Medicinal and wild food plants of Marmara island (Balikesir-Turkey) Acta Soc. Bot. Pol. 2016;85(2):3501. doi: 10.5586/asbp.3501. [DOI] [Google Scholar]
- Bulut G., Tuzlacı E. An ethnobotanical study of medicinal plants in Turgutlu (Manisa–Turkey) J. Ethnopharmacol. 2013;149:633–647. doi: 10.1016/j.jep.2013.07.016. [DOI] [PubMed] [Google Scholar]
- Bulut G., Tuzlacı E. An ethnobotanical study of medicinal plants in Bayramiç (Çanakkale-Turkey) Marmara Pharm. J. 2015;19:268–282. doi: 10.12991/mpj.201519392830. [DOI] [Google Scholar]
- Bulut G., Zahid Bozkurt M., Tuzlacı E. The preliminary ethnobotanical study of medicinal plants in Uşak (Turkey) Marmara Pharm. J. 2017;21(2):305–310. doi: 10.12991/marupj.300795. [DOI] [Google Scholar]
- Bulut G., Haznedaroğlu M.Z., Doğan A., Koyu H., Tuzlacı E. An ethnobotanical study of medicinal plants in Acıpayam (Denizli-Turkey) J. Herb. Med. 2017;10:64–81. doi: 10.1016/j.hermed.2017.08.001. [DOI] [Google Scholar]
- Bulut G., Doğan A., Şenkardeş İ., Avci R., Tuzlaci E. The medicinal and wild food plants of Batman city and kozluk district (Batman-Turkey) Agric. Conspectus Sci. 2019;84(1):29–36. [Google Scholar]
- Bussmann R.W., Paniagua Zambrana N.Y., Sikharulidze S., Kikvidze Z., Kikodze D., Tchelidze D., et al. Medicinal and food plants of svaneti and lechkhumi, sakartvelo (Republic of Georgia), caucasus. Med. Aromatic Plants. 2016;5(266) doi: 10.4172/2167-0412.1000266. 2167-0412. [DOI] [Google Scholar]
- Cakilcioglu U., Khatun S., Turkoglu I., Hayta S. Ethnopharmacological survey of medicinal plants in Maden (Elazig-Turkey) J. Ethnopharmacol. 2011;137(1):469–486. doi: 10.1016/j.jep.2011.05.046. [DOI] [PubMed] [Google Scholar]
- Cansaran A., Kaya Ö.F. Contributions of the ethnobotanical investigation carried out in amasya district of Turkey (Amasya-Center, Bağlarüstü, Boğaköy and Vermiş villages; Yassıçal and Ziyaret towns) Biodicon. 2010;3(2):97–116. [Google Scholar]
- CDC Cold versus flu. 2019. https://www.cdc.gov/flu/symptoms/coldflu.htm
- Chavan R.D., Shinde P., Girkar K., Madage R., Chowdhary A. Assessment of anti-influenza activity and hemagglutination inhibition of Plumbago indica and Allium sativum extracts. Pharmacogn. Res. 2016;8(2):105. doi: 10.4103/0974-8490.172562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Zuckerman D.M., Brantley S., Sharpe M., Childress K., Hoiczyk E., Pendleton A.R. Sambucus nigra extracts inhibit infectious bronchitis virus at an early point during replication. BMC Vet. Res. 2014;10(24):1–12. doi: 10.1186/1746-6148-10-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H.J. Chemical constituents of essential oils possessing anti-influenza A/WS/33 virus activity. Osong Publ. Health Res. Perspect. 2018;9(6):348. doi: 10.24171/j.phrp.2018.9.6.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Çiçek İ. Çermik ilçesi ve köylerinin (Diyarbakır) etnobotanik özellikleri. Master Thesis. Bingöl Univ. Sci. Ins. Bingöl. 2019 [Google Scholar]
- Dalar A., Mukemre M., Unal M., Ozgokce F. Traditional medicinal plants of Ağrı Province, Turkey. J. Ethnopharmacol. 2018;226:56–72. doi: 10.1016/j.jep.2018.08.004. [DOI] [PubMed] [Google Scholar]
- De Freitas C.S., Rocha M.E., Sacramento C.Q., Marttorelli A., Ferreira A.C., Rocha N., et al. Agathisflavone, a Biflavonoid from Anacardium occidentale L., inhibits influenza virus neuraminidase. Curr. Top. Med. Chem. 2020;20(2):111–120. doi: 10.2174/1568026620666191219150738. [DOI] [PubMed] [Google Scholar]
- Demirci S., Özhatay N. An ethnobotanical study in Kahramanmaraş (Turkey); wild plants used for medicinal purpose in Andirin. Kahramanmaraş. Turk. J. Pharm. Sci. 2012;9(1):75–92. [Google Scholar]
- Demirci-Kayıran S.D. A research on the present uses of the medicinal plants in De Materia Medica written by Dioscorides in eastern mediterranean region. Lokman Hekim Derg. 2019;9(2):189–202. doi: 10.31020/mutftd.519382. [DOI] [Google Scholar]
- Derakhshan M., Niazmand S., Rezaee R., Derakhshan R. The effects of Teucrium polium L. on human influenza virus. Avicenna J. Phytomed. 2015;5:29–30. [Google Scholar]
- Ding Y., Cao Z., Cao L., Ding G., Wang Z., Xiao W. Antiviral activity of chlorogenic acid against influenza A (H1N1/H3N2) virus and its inhibition of neuraminidase. Sci. Rep. 2017;7:45723. doi: 10.1038/srep45723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dogan Y. Traditionally used wild edible greens in the Aegean Region of Turkey. Acta Soc. Bot. Pol. 2012;81(4):329–342. doi: 10.5586/asbp.2012.037. [DOI] [Google Scholar]
- Dogan H.H., Duman R., Dinç M. Antiviral activity of Ribes uva-crispa L. extracts in vitro. Pak. J. Pharm. Sci. 2020;33(3):1173–1178. doi: 10.36721/PJPS.2020.33.3.REG.1173-1178.1. [DOI] [PubMed] [Google Scholar]
- Doğanay H., Orhan F. Pegem Akademi Yayıncılık; Ankara: 2016. Türkiye Beşeri Coğrafyası. [Google Scholar]
- Duman R., Doğan H.H., Dinç M., Tuncer P. Cytotoxic and antiviral activity of Ribes uva crispa Linn. and Ribes multiflorum Kit. ex Romer and Schultes extracts. Int. J. Pharma Sci. Res. 2018;9(5):1779–1787. doi: 10.13040/IJPSR.0975-8232. [DOI] [Google Scholar]
- Ekşi G., Özkan A.M.G., Koyuncu M. Garlic and onions: an eastern tale. J. Ethnopharmacol. 2020;253:112675. doi: 10.1016/j.jep.2020.112675. [DOI] [PubMed] [Google Scholar]
- Ergül-Bozkurt A., Terzioğlu S. The aromatic-medicinal plant taxa of pure Scots pine stands in sürmene-camburnu (Trabzon) Int. J. Second. Metab. 2017;4(3):517–529. doi: 10.21448/ijsm.377774. [DOI] [Google Scholar]
- Ertuğ F. Wild edible plants of the Bodrum area (muğla, Turkey) Turk. J. Bot. 2004;28:161–174. [Google Scholar]
- Ertuğ F., Tümen G., Çelik A., Dirmenci T. Buldan (Denizli) etnobotanik alan araştırması 2003. TUBA-KED. 2004;2(2):187–218. [Google Scholar]
- Ezer N., Avcı K. Çerkeş (Çankırı) Yöresinde Kullanılan Halk İlaçları. Hacettepe Univ. Eczacı. Fak. Derg. 2004;24(2):66–80. [Google Scholar]
- Genç L. Anadolu University Web-Ofset; Eskişehir: 2010. Tıbbi Ve Aromatik Bitkilerin Kullanım Alanları Ve Etiği. [Google Scholar]
- Gökçe N.P. Doğan Kitap; Istanbul: 2014. Doğanın Mucizesi Şifalı Bitkiler. [Google Scholar]
- Grienke U., Schmidtke M., Kirchmair J., Pfarr K., Wutzler P. Antiviral potential and molecular insight into neuraminidase inhibiting diarylheptanoids from Alpinia katsumadae. J. Med. Chem. 2009;53(2):778–786. doi: 10.1021/jm901440f. [DOI] [PubMed] [Google Scholar]
- Grienke U., Schmidtke M., von Grafenstein S., Kirchmair J., Liedl K.R., Rollinger J.M. Influenza neuraminidase: a druggable target for natural products. Nat. Prod. Rep. 2012;29(1):11–36. doi: 10.1039/c1np00053e. [DOI] [PubMed] [Google Scholar]
- Grienke U., Braun H., Seidel N., Kirchmair J., Richter M., Krumbholz A., et al. Computer-guided approach to access the anti-influenza activity of licorice constituents. J. Nat. Prod. 2014;77(3):563–570. doi: 10.1021/np400817j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günbatan T., Gürbüz İ., Özkan A.M.G. The current status of ethnopharmacobotanical knowledge in Çamlıdere (Ankara, Turkey) Turk. J. Bot. 2016;40(3):241–249. [Google Scholar]
- Güner Ö., Selvi S. Wild medicinal plants sold in Balıkesir/Turkey herbal markets and their using properties. Biodicon. 2016;9(2):96–101. [Google Scholar]
- Güner A., Aslan S., Ekim T., Vural M., Babaç M.T. Nezahat Gökyiğit Botanical Garden and Flora Research Association Publication; Istanbul: 2012. Türkiye Bitkileri Listesi (Damarlı Bitkiler) (S. 47-83. [Google Scholar]
- Güneş F. Medicinal plants used in the Uzunköprü district of Edirne, Turkey. Acta Soc. Bot. Pol. 2017;86(4):1–21. doi: 10.5586/asbp.3565. [DOI] [Google Scholar]
- Güneş F., Özhatay N. An ethnobotanical study from Kars (Eastern) Turkey. Biodicon. 2011;4(1):30–41. [Google Scholar]
- Güneş S., Savran A., Paksoy M.Y., Çakılcıoğlu U. Survey of wild food plants for human consumption in Karaisalı (Adana-Turkey) Indian J. Tradit. Knowl. 2018;17(2):290–298. [Google Scholar]
- Gürbüz İ., Özkan A.M.G., Akaydin G., Salihoğlu E., Günbatan T., Demirci F., Yeşilada E. Folk medicine in düzce province (Turkey) Turk. J. Bot. 2019;43(6):769–784. [Google Scholar]
- Güzel Y., Güzelsemme M. Wild plants used as herbal tea in Antakya and Defne provinces of Hatay. Anadolu J. AARI. 2018;28(1):1–5. [Google Scholar]
- Haidari M., Ali M., Casscells S.W., Madjid M. Pomegranate (Punica granatum) purified polyphenol extract inhibits influenza virus and has a synergistic effect with oseltamivir. Phytomedicine. 2009;16(12):1127–1136. doi: 10.1016/j.phymed.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Hamauzu Y., Yasui H., Inno T., Kume C., Omanyuda M. Phenolic profile, antioxidant property, and anti-influenza viral activity of Chinese quince (Pseudocydonia sinensis Schneid.), quince (Cydonia oblonga Mill.), and apple (Malus domestica Mill.) fruits. J. Agric. Food Chem. 2005;53(4):928–934. doi: 10.1021/jf0494635. [DOI] [PubMed] [Google Scholar]
- Han M.I., Bulut G. The folk–medicinal plants of Kadişehri (Yozgat–Turkey) Acta Soc. Bot. Pol. 2015;84(2):237–248. doi: 10.5586/asbp.2015.021. [DOI] [Google Scholar]
- Hanlidou E., Karousou R., Kleftoyanni V., Kokkini S. The herbal market of Thessaloniki (N Greece) and its relation to the ethnobotanical tradition. J. Ethnopharmacol. 2004;91(2–3):281–299. doi: 10.1016/j.jep.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Hudson J.B., Lee M.K., Sener B., Erdemoglu N. Antiviral activities in extracts of Turkish medicinal plants. Pharm. Biol. 2000;38(3):171–175. doi: 10.1076/1388-0209(200007)3831-SFT171. [DOI] [PubMed] [Google Scholar]
- İşler N. Mustafa Kemal University Press; Hatay: 2017. Genel Tıbbi Bitkiler. [Google Scholar]
- Jacobs S.E., Lamson D.M., St George K., Walsh T.J. Human rhinoviruses. Clin. Microbiol. Rev. 2013;26(1):135–162. doi: 10.1128/CMR.00077-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jalali P., Moattari A., Mohammadi A., Ghazanfari N., Pourghanbari G. Melissa officinalis efficacy against human influenza virus (New H1N1) in comparison with oseltamivir. Asian Pac. J. Trop. Dis. 2016;6(9):714–717. doi: 10.1016/S2222-1808(16)61115-5. [DOI] [Google Scholar]
- Jarić S., Mačukanović-Jocić M., Djurdjević L., Mitrović M., Kostić O., Karadžić B., Pavlović P. An ethnobotanical survey of traditionally used plants on Suva planina mountain (south-eastern Serbia. J. Ethnopharmacol. 2015;175:93–108. doi: 10.1016/j.jep.2015.09.002. [DOI] [PubMed] [Google Scholar]
- Kalafatçılar Ö.A., Kalafatçılar A.İ. Sidas Yayınları; İzmir: 2010. Bitkiler Ve Sağlık. [Google Scholar]
- Karaköse M., Karaköse G.C. Medicinal and aromatic plants of Esenli (giresun) forest planning unit. Int. J. Sec. Metabolite. 2017;4(3):285–305. doi: 10.21448/ijsm.372229. [DOI] [Google Scholar]
- Karamanoğlu K. Ankara University, Faculty of Pharmacy Press; Ankara: 1977. Farmasötik Botanik Ders Kitabı. [Google Scholar]
- Karousou R., Deirmentzoglou S. The herbal market of Cyprus: traditional links and cultural exchanges. J. Ethnopharmacol. 2011;133(1):191–203. doi: 10.1016/j.jep.2010.09.034. [DOI] [PubMed] [Google Scholar]
- Kartal Ç., Güneş F. Medicinal plants used in meriç Town from Turkey. Indian J. Pharm. Educ. 2017;51(3):248–253. doi: 10.5530/ijper.51.3s.23. [DOI] [Google Scholar]
- Kaval I., Behçet L., Cakilcioglu U. Ethnobotanical study on medicinal plants in Geçitli and its surrounding (Hakkari-Turkey) J. Ethnopharmacol. 2014;155(1):171–184. doi: 10.1016/j.jep.2014.05.014. [DOI] [PubMed] [Google Scholar]
- Kawaoka Y. Caister Academic Press; Wymondham: 2006. Influenza Virology: Current Topics. [Google Scholar]
- Kim H., Chung M.S. Antiviral activities of mulberry (Morus alba) juice and seed against influenza viruses. Evid. Base. Complementary Altern. Med. 2018:1–10. doi: 10.1155/2018/2606583. 2606583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y., Narayanan S., Chang K.O. Inhibition of influenza virus replication by plant-derived isoquercetin. Antivir. Res. 2010;88(2):227–235. doi: 10.1016/j.antiviral.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Kilic Ö., Bagci E. An ethnobotanical survey of some medicinal plants in Keban (Elazığ – Turkey) J. Med. Plants Res. 2013;7(23):1675–1684. doi: 10.5897/JMPR2013.4451. [DOI] [Google Scholar]
- Kim M.S., Chathuranga K., Kim H., Lee J.S., Kim C.J. Anti-influenza properties of herbal extract of Althaea rosea in mice. Korean J. Vet. Res. 2018;58(3):153–158. doi: 10.14405/kjvr.2018.58.3.153. [DOI] [Google Scholar]
- Kinoshita E., Hayashi K., Katayama H., Hayashi T., Obata A. Anti-influenza virus effects of elderberry juice and its fractions. Biosci. Biotechnol. Biochem. 2012;76(9):1–6. doi: 10.1271/bbb.120112. 120112. [DOI] [PubMed] [Google Scholar]
- Kocabas Y.Z., Erol A., Aktolun O. Medicinal plants of flora of KSU avsar campus (kahramanmaras) and surrounding areas. Aksaray J. Sci. Eng. 2017;1(2):110–120. doi: 10.29002/asujse.306972. [DOI] [Google Scholar]
- Kocabaş Y.Z., Gedik O. Ethnobotanical researches on plants sold in Kahramanmaraş city center open markets. Iğdır Univ. J. Inst. Sci. Tech. 2016;6(4):41–50. [Google Scholar]
- Köse M. Artvin Çoruh Univ. Sci. Ins. Artvin; 2019. Ethnobotanical Features of Güneysu (Rize) District. Master Thesis. [Google Scholar]
- Kozuharova E., Lebanova H., Getov I., Benbassat N., Napier J. Descriptive study of contemporary status of the traditional knowledge on medicinal plants in Bulgaria. African J. Pharm. Pharmacol. 2013;7(5):185–198. doi: 10.5897/AJPP12.871. [DOI] [Google Scholar]
- Koçyiğit M., Özhatay N. Wild plants used as medicinal purpose in Yalova (northwest Turkey) Turk. J. Pharm. Sci. 2006;3(2):91–103. [Google Scholar]
- Krawitz C., Mraheil M.A., Stein M., Imirzalioglu C., Domann E., Pleschka S., Hain T. Inhibitory activity of a standardized elderberry liquid extract against clinically-relevant human respiratory bacterial pathogens and influenza A and B viruses. BMC Compl. Altrenative M. 2011;11(1):16. doi: 10.1186/1472-6882-11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucera L.S., Herrmann E.C., Jr. Antiviral substances in plants of the mint family (labiatae. I. Tannin of Melissa officinalis. Proc. Soc. Exp. Biol. Med. 1967;124(3):865–869. doi: 10.3181/00379727-124-31872. [DOI] [PubMed] [Google Scholar]
- Kumar P., Sharma S., Khanna M., Raj H.G. Effect of quercetin on lipid peroxidation and changes in lung morphology in experimental influenza virus infection. Int. J. Exp. Pathol. 2003;84(3):127–134. doi: 10.1046/j.1365-2613.2003.00344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurt P., Karaoğul E. Bartın’da Aktarlarda Satılan Tıbbi Aromatik Bitkiler ve Ülkemizdeki Pazar Payları. Bartın Orman Fakültesi Dergisi. 2018;20(1):73–80. doi: 10.24011/barofd.400016. [DOI] [Google Scholar]
- Kılıç Ö. An ethnobotanical survey from Bingol (Turkey) RAJAR. 2016;2(10):685–691. doi: 10.18535/rajar/v2i10.05. [DOI] [Google Scholar]
- Kılıç M. Manisa Celal Bayar Univ. Sci. Ins. Manisa; 2019. An Ethnobotanical Research on Plants Grown in Artuklu (Mardin) Region. PhD Thesis. [Google Scholar]
- Lee I.K., Hwang B.S., Kim D.W., Kim J.Y., Woo E.E., Lee Y.J., et al. Characterization of neuraminidase inhibitors in Korean Papaver rhoeas bee pollen contributing to anti-influenza activities in vitro. Planta Med. 2016;82(6):524–529. doi: 10.1055/s-0041-111631. [DOI] [PubMed] [Google Scholar]
- Lev E., Amar Z. Ethnopharmacological survey of traditional drugs sold in Israel at the end of the 20th century. J. Ethnopharmacol. 2000;72(1–2):191–205. doi: 10.1016/S0378-8741(00)00230-0. [DOI] [PubMed] [Google Scholar]
- Li Y., Xu Y.L., Lai Y.N., Liao S.H., Liu N., Xu P.P. Intranasal co-administration of 1, 8-cineole with influenza vaccine provide cross-protection against influenza virus infection. Phytomedicine. 2017;34:127–135. doi: 10.1016/j.phymed.2017.08.014. [DOI] [PubMed] [Google Scholar]
- Li Y.H., Lai C.Y., Su M.C., Cheng J.C., Chang Y.S. Antiviral activity of Portulaca oleracea L. against influenza A viruses. J. Ethnopharmacol. 2019;241:112013. doi: 10.1016/j.jep.2019.112013. [DOI] [PubMed] [Google Scholar]
- Liu Z., Zhao J., Li W., Shen L., Huang S., Tang J., et al. Computational screen and experimental validation of anti-influenza effects of quercetin and chlorogenic acid from traditional Chinese medicine. Sci. Rep. 2016;6:19095. doi: 10.1038/srep19095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini D.A.P., Mendonça R.M.Z., Pinto J.R., Orico L.D., Rizzo M.A., Stroibel R.C., Mancini-Filho J. Evaluation of compounds from oregano (Origanum vulgare) that inactivate the influenza virus in host animals. Publ. UEPG. 2009;15(1):45–52. doi: 10.5212/publicatio.v15i01.974. [DOI] [Google Scholar]
- Maranki E., Maranki A. Nesil Basım Yayın Gıda Inc; Istanbul: 2016. Kozmik Bilim Işığında Şifalı Bitkiler. [Google Scholar]
- Mehmood M.D., Anwar H., Noreen S., Ameen F., Hassan S., Hussain S. In vivo anti-viral effect of melaleuca alternifolia (tea tree oil) and Olea europaea (olive leaf extract) on vero cell adapted avian influenza virus. Hum. J. 2018;14(1):7–19. [Google Scholar]
- Menković N., Šavikin K., Tasić S., Zdunić G., Stešević D., Milosavljević S., Vincek D. Ethnobotanical study on traditional uses of wild medicinal plants in Prokletije Mountains (Montenegro) J. Ethnopharmacol. 2011;133(1):97–107. doi: 10.1016/j.jep.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Millán-Oñate J., Millan W., Mendoza L.A., Sánchez C.G., Fernandez-Suarez H., Bonilla-Aldana D.K., Rodríguez-Morales A.J. Successful recovery of COVID-19 pneumonia in a patient from Colombia after receiving chloroquine and clarithromycin. Ann. Clin. Microbiol. Antimicrob. 2020;19(16):1–9. doi: 10.1186/s12941-020-00358-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moradi M.T., Karimi A., Lorigooini Z., Pourgheysari B., Alidadi S., Hashemi L. In vitro anti influenza virus activity, antioxidant potential and total phenolic content of twelve Iranian medicinal plants. Marmara Pharm. J. 2017;21(4):843–851. doi: 10.12991/mpj.2017.10. [DOI] [Google Scholar]
- Moradi M.T., Karimi A., Rafieian-kopaei M., Rabiei-Faradonbeh M., Momtaz H. Pomegranate peel extract inhibits internalization and replication of the influenza virus: an in vitro study. Avicenna J. Phytomed. 2020;10(2):143. [PMC free article] [PubMed] [Google Scholar]
- Mustafa B., Hajdari A., Krasniqi F., Hoxha E., Ademi H., Quave C.L., Pieroni A. Medical ethnobotany of the Albanian alps in kosovo. J. Ethnobiol. Ethnomed. 2012;8(6):1–14. doi: 10.1186/1746-4269-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustafa B., Hajdari A., Pieroni A., Pulaj B., Koro X., Quave C.L. A cross-cultural comparison of folk plant uses among Albanians, Bosniaks, Gorani and Turks living in south Kosovo. J. Ethnobiol. Ethnomed. 2015;11(1):39. doi: 10.1186/s13002-015-0023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacakcı F.M., Dutkuner İ. Kumluca (Antalya)’da etnobotanik bir çalışma. Türkiye Ormancılık Dergisi. 2015;19(2):113–119. doi: 10.18182/tjf.421970. [DOI] [Google Scholar]
- Nachbagauer R., Palese P. Is a universal influenza virus vaccine possible? Annu. Rev. Med. 2020;71:315–327. doi: 10.1146/annurev-med-120617-041310. [DOI] [PubMed] [Google Scholar]
- Najjari A.H.A., Rajabi Z., Marandi M.V., Dehghan G. The effect of the hexanic extracts of fig (Ficus carica) and olive (Olea europaea) fruit and nanoparticles of selenium on the immunogenicity of the inactivated avian influenza virus subtype H9N2. Vet. Res. Forum. 2015;6(3):227–231. [PMC free article] [PubMed] [Google Scholar]
- Nasab F.K., Khosravi A.R. Ethnobotanical study of medicinal plants of sirjan in kerman province, Iran. J. Ethnopharmacol. 2014;154(1):190–197. doi: 10.1016/j.jep.2014.04.003. [DOI] [PubMed] [Google Scholar]
- Nile S.H., Kim D.H., Nile A., Park G.S., Gansukh E., Kai G. Probing the effect of quercetin 3-glucoside from Dianthus superbus L against influenza virus infection-In vitro and in silico biochemical and toxicological screening. Food Chem. Toxicol. 2020;135:110985. doi: 10.1016/j.fct.2019.110985. [DOI] [PubMed] [Google Scholar]
- Olgun Ş. Bingöl Univ. Sci. Inst. Bingöl; 2019. Ethnobotanical Characteristics of Arıcak (Elazığ) District. Master Thesis. [Google Scholar]
- Orhan I., Deliorman-Orhan D., Özçelik B. Antiviral activity and cytotoxicity of the lipophilic extracts of various edible plants and their fatty acids. Food Chem. 2009;115(2):701–705. doi: 10.1016/j.foodchem.2009.01.024. [DOI] [Google Scholar]
- Özer Z., Elibüyük l.Ö., Önen H., Elibüyük E.A. In: Toygar K., Toygar N.B., editors. vol. 12. 2005. Sağlıklı bir yaşamdır yabancı otlar: Türk mutfak kültürü üzerine araştırmalar; pp. 113–194. (Türk Halk Kültürünü Araştırma Ve Tanıtma Vakfı). [Google Scholar]
- Özgün C. Osmanlı ağaç kültüründe Yeni Ve Egzotik bir Tür: Okaliptüs. Çağdaş Türkiye Tarihi Araştırmaları Dergisi. 2013;13(26):5–29. [Google Scholar]
- Özhatay N., Akalın E., Genç G.E., Kültür Ş. IV Balkan Botanical Congress; Sofia: 2009. Ethnomedicinal Uses of the Wild Vascular Plants from European Turkey (Turkish Thrace) pp. 613–623. [Google Scholar]
- Özçelik B., Orhan İ.E., Kan Y. Determination of antiviral activity and cytotoxicity of selected sage (Salvia L.) species. J. Pharmacol. Sci. 2011;36:155–160. [Google Scholar]
- Ozcelik H., Cinbilgel I., Muca B., Tavuc I., Koca A., Bebekli O. Sistem Ofset; Ankara: 2016. Burdur Ili Bitki Envanteri (Ekonomik, Nadir Ve Endemik Bitkileri) [Google Scholar]
- Ozturk M., Altay V., Gonenç T.M. In: Drug Discovery from Herbs - Approaches and Applications, Chapter 24, Centre for Science Technology of the Non-aligned and Other Developing Countries. Bhojraj S., et al., editors. DAYA Publishing House; New Delhi: 2017. Herbal from high mountains in the East mediterranean; pp. 327–367. [Google Scholar]
- Ozturk M., Altay V., Gucel S., Altundag E. In: Plant Biodiversity: Monitoring, Assessment and Conservation. Ansari A.A., Gill S.S., editors. CABI; Wallingford: 2017. Chapter 5: plant diversity of the Drylands in Southeast Anatolia-Turkey: role in human health and food security; pp. 83–124. [Google Scholar]
- Paksoy M.Y., Selvi S., Savran A. Ethnopharmacological survey of medicinal plants in Ulukışla (Niğde-Turkey) J. Herb. Med. 2016;6(1):42–48. doi: 10.1016/j.hermed.2015.04.003. [DOI] [Google Scholar]
- Pieroni A., Nedelcheva A., Dogan Y. Local knowledge of medicinal plants and wild food plants among Tatars and Romanians in Dobruja (South-East Romania) Genet. Resour. Crop Evol. 2015;62(4):605–620. doi: 10.1007/s10722-014-0185-3. [DOI] [Google Scholar]
- Polat R. Ethnobotanical study on medicinal plants in Bingöl (City center) (Turkey) J. Herb. Med. 2019;16:100211. doi: 10.1016/j.hermed.2018.01.007. [DOI] [Google Scholar]
- Polat R., Cakilcioglu U., Satıl F. Traditional uses of medicinal plants in Solhan (Bingöl—Turkey) J. Ethnopharmacol. 2013;148(3):951–963. doi: 10.1016/j.jep.2013.05.050. [DOI] [PubMed] [Google Scholar]
- Pourghanbari G., Nili H., Moattari A., Mohammadi A., Iraji A. Antiviral activity of the oseltamivir and Melissa officinalis L. essential oil against avian influenza A virus (H9N2. Virus Dis. 2016;27(2):170–178. doi: 10.1007/s13337-016-0321-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu X.Y., Liang J.P., Wang X.H., Xu T., Hua L.Y., Shang R.F., et al. Anti-influenza A virus effect of Hypericum perforatum. L. extract. Virol. Sin. 2009;24(1):19. doi: 10.1007/s12250-009-2983-x. [DOI] [Google Scholar]
- Qi T., Falong Y., Nan Z., Ting H., Liu Y., Ling G., Xi P., Jinwei L. Study on the effect of anti-influenza virus of the volatile oil of Schizonepetae, menthone and pulegone. Pharmacol. Clin. Chin. Mater. Med. 2012;2 http://en.cnki.com.cn/Article_en/CJFDTotal-ZYYL201202010.htm [Google Scholar]
- Rajasekaran D., Palombo E.A., Yeo T.C., Ley D.L.S., Tu C.L. Identification of traditional medicinal plant extracts with novel anti-influenza activity. PLoS ONE. 2013;8(11) doi: 10.1371/journal.pone.0079293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J., Huang J., Yang B., Lin S., Li J., Liao H., et al. Docking and molecular dynamics: simulation of the inhibition of H5N1 influenza virus (Anhui 2005) neuraminidase (NA) by chlorogenic acid (CHA) Int. J. Clin. Exp. Med. 2019;12(8):9815–9823. [Google Scholar]
- Rexhepi B., Mustafa B., Hajdari A., Rushidi-Rexhepi J., Quave C.L., Pieroni A. Traditional medicinal plant knowledge among Albanians, Macedonians and Gorani in the Sharr Mountains (Republic of Macedonia) Genet. Resour. Crop Evol. 2013;60(7):2055–2080. doi: 10.1007/s10722-013-9974-3. [DOI] [Google Scholar]
- Sadatrasul M.S., Fiezi N., Ghasemian N., Shenagari M., Esmaeili S., Jazaeri E.O., et al. Oil-in-water emulsion formulated with eucalyptus leaves extract inhibit influenza virus binding and replication in vitro. AIMS Microbiol. 2017;3(4):899–907. doi: 10.3934/microbiol.2017.4.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sağıroğlu M., Topuz T., Ceylan K., Turna M. An ethnobotanical survey from Yahyalı (Kayseri) and Tarsus (Mersin) Sakarya Üniversitesi Fen Edebiyat Dergisi. 2013;(2):13–37. [Google Scholar]
- Saraç M.E. Doğan Kitap; Istanbul: 2005. Doğanın Şifalı Eli. [Google Scholar]
- Saraçoğlu İ.A. Gün Ofset; Istanbul: 2014. Tıbbi Bitkiler Ve Bitkisel Sağlık Rehberi. [Google Scholar]
- Sargin S.A. Ethnobotanical survey of medicinal plants in Bozyazı district of Mersin, Turkey. J. Ethnopharmacol. 2015;173:105–126. doi: 10.1016/j.jep.2015.07.009. [DOI] [PubMed] [Google Scholar]
- Sargin S.A., Büyükcengiz M. Plants used in ethnomedicinal practices in Gulnar district of Mersin, Turkey. J. Herb. Med. 2019;15:1–18. doi: 10.1016/j.hermed.2018.06.003. [DOI] [Google Scholar]
- Sargin S.A., Akçicek E., Selvi S. An ethnobotanical study of medicinal plants used by the local people of Alaşehir (Manisa) in Turkey. J. Ethnopharmacol. 2013;150(3):860–874. doi: 10.1016/j.jep.2013.09.040. [DOI] [PubMed] [Google Scholar]
- Sargin S.A., Selvi S., López V. Ethnomedicinal plants of sarigöl district (manisa), Turkey. J. Ethnopharmacol. 2015;171:64–84. doi: 10.1016/j.jep..2015.05.031. [DOI] [PubMed] [Google Scholar]
- Sargin S.A., Selvi S., Büyükcengiz M. Ethnomedicinal plants of aydıncık district of mersin, Turkey. J. Ethnopharmacol. 2015;174:200–216. doi: 10.1016/j.jep.2015.08.008. [DOI] [PubMed] [Google Scholar]
- Savić J., Mačukanović-Jocić M., Jarić S. Medical ethnobotany on the Javor mountain (Bosnia and Herzegovina) Eur. J. Integr. Med. 2019;27:52–64. doi: 10.1016/j.eujim.2019.02.007. [DOI] [Google Scholar]
- Sağıroğlu M., Topuz T., Ceylan K., Turna M. An ethnobotanical survey from Yahyalı (kayseri) and Tarsus (mersin) Sakarya Üniversitesi Fen Edebiyat Dergisi. 2013;2:13–37. [Google Scholar]
- Şenkardeş İ. Marmara Univ. Health Sci. Ins.; Istanbul: 2014. Ethnobotanical Researches in the Southern Districts of Nevşehir (Acıgöl, Derinkuyu, Gülşehir, Nevşehir-Merkez, Ürgüp) PhD Thesis. [Google Scholar]
- Setzer W.N. Essential oils as complementary and alternative medicines for the treatment of influenza. Am. J. Essent. 2016;4(4):16–22. [Google Scholar]
- Sezik E., Zor M., Yesilada E. Traditional medicine in Turkey II. Folk medicine in Kastamonu. Int. J. Pharmacogn. 1992;30(3):233–239. doi: 10.3109/13880209209054005. [DOI] [Google Scholar]
- Shahsavandi S., Ebrahimi M.M., Farahani A.H. Interfering with lipid raft association: a mechanism to control influenza virus infection by Sambucus nigra. Iran. J. Pharm. Res. (IJPR) 2017;16(3):1147. [PMC free article] [PubMed] [Google Scholar]
- Shin H.B., Choi M.S., Ryu B., Lee N.R., Kim H.I., Choi H.E., et al. Antiviral activity of carnosic acid against respiratory syncytial virus. Virol. J. 2013;10(1):303. doi: 10.1186/1743-422X-10-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sökmen M., Serkedjieva J., Daferera D., Gulluce M., Polissiou M., Tepe B., et al. In vitro antioxidant, antimicrobial, and antiviral activities of the essential oil and various extracts from herbal parts and callus cultures of Origanum acutidens. J. Agric. Food Chem. 2004;52(11):3309–3312. doi: 10.1021/jf049859g. [DOI] [PubMed] [Google Scholar]
- Sokolova A.S., Yarovaya O.I., Semenova M.D., Shtro A.A., Orshanskaya I.R., Zarubaev V.V., Salakhutdinov N.F. Synthesis and in vitro study of novel borneol derivatives as potent inhibitors of the influenza A virus. Med. Chem. Comm. 2017;8(5):960–963. doi: 10.1039/C6MD00657D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J.H., Choi H.J. Silymarin efficacy against influenza A virus replication. Phytomedicine. 2011;18(10):832–835. doi: 10.1016/j.phymed.2011.01.026. [DOI] [PubMed] [Google Scholar]
- Tanker N., Koyuncu M., Coşkun M. Ankara University Faculty of Pharmacy Press; Ankara: 1998. Farmasötik Botanik. [Google Scholar]
- Tetik F., Civelek S., Cakilcioglu U. Traditional uses of some medicinal plants in Malatya (Turkey) J. Ethnopharmacol. 2013;146(1):331–346. doi: 10.1016/j.jep.2012.12.054. [DOI] [PubMed] [Google Scholar]
- Touret F., de Lamballerie X. Of chloroquine and COVID-19. Antivir. Res. 2020;177:1–2. doi: 10.1016/j.antiviral.2020.104762. 104762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseliou M., Pirintsos S.A., Lionis C., Castanas E., Sourvinos G. Antiviral effect of an essential oil combination derived from three aromatic plants (Coridothymus capitatus (L.) Rchb. f., Origanum dictamnus L. and Salvia fruticosa Mill.) against viruses causing infections of the upper respiratory tract. J. Herb. Med. 2019;17:100288. doi: 10.1016/j.hermed.2019.100288. [DOI] [Google Scholar]
- Tuzlacı E. Alfa yayınları; Istanbul: 2006. Şifa Niyetine: Türkiye'nin Bitkisel Halk Ilaçları. [Google Scholar]
- Tuzlacı E., Doğan A. Turkish folk medicinal plants, IX: Ovacık (Tunceli) Marmara Pharm. J. 2010;14:136–143. doi: 10.12991/201014449. [DOI] [Google Scholar]
- Tuzlacı E., Erol M.K. Turkish folk medicinal plants. Part II: Eğirdir (Isparta) Fitoterapia. 1999;70(6):593–610. doi: 10.1016/S0367-326X(99)00074-X. [DOI] [Google Scholar]
- Tuzlacı E., Eryaşar-Aymaz P. Turkish folk medicinal plants, part IV: Gönen (Balıkesir) Fitoterapia. 2001;72(4):323–343. doi: 10.1016/S0367-326X(00)00277-X. [DOI] [PubMed] [Google Scholar]
- Tuzlacı E., Tolon E. Turkish folk medicinal plants, part III: Şile (Istanbul) Fitoterapia. 2000;71(6):673–685. doi: 10.1016/S0367-326X(00)00234-3. [DOI] [PubMed] [Google Scholar]
- Ugulu I., Baslar S., Yorek N., Dogan Y. The investigation and quantitative ethnobotanical evaluation of medicinal plants used around Izmir province, Turkey. J. Med. Plants Res. 2009;3(5):345–367. [Google Scholar]
- Umar S., Munir M.T., Subhan S., Azam T., Nisa Q., Khan M.I., et al. Protective and antiviral activities of Nigella sativa against avian influenza (H9N2) in turkeys. J. Saudi Soc. Agric. Sci. 2016 doi: 10.1016/j.jssas.2016.09.004. In press. [DOI] [Google Scholar]
- Uzun M., Kaya A. Ethnobotanical research of medicinal plants in Mihalgazi (Eskişehir), Turkey. Pharm. Biol. 2016;54(12):2922–2932. doi: 10.1080/13880209.2016.1194863. [DOI] [PubMed] [Google Scholar]
- Van der Meer F.J.U.M., de Haan C.A.M., Schuurman N.M.P., Haijema B.J., Verheije M.H., Bosch B.J., et al. The carbohydrate-binding plant lectins and the non-peptidic antibiotic pradimicin A target the glycans of the coronavirus envelope glycoproteins. J. Antimicrob. 2007;60(4):741–749. doi: 10.1093/jac/dkm301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga F., Šolić I., Dujaković M.J., Łuczaj Ł., Grdiša M. The first contribution to the ethnobotany of inland Dalmatia: medicinal and wild food plants of the Knin area, Croatia. Acta Soc. Bot. Pol. 2019;88(2):1–20. doi: 10.5586/asbp.3622. [DOI] [Google Scholar]
- Velavan T.P., Meyer C.G. The COVID‐19 epidemic. Trop. Med. Int. Health. 2020;25(3):278–280. doi: 10.1111/tmi.13383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayan P., Raghu C., Ashok G., Dhanaraj S.A., Suresh B. Antiviral activity of medicinal plants of Nilgiris. Indian J. Med. Res. 2004;120:24–29. [PubMed] [Google Scholar]
- Vimalanathan S., Hudson J. Anti-Influenza virus activities of commercial oregano oils and their carriers. J. Appl. Pharmaceut. Sci. 2012;2(7):214–218. doi: 10.7324/JAPS.2012.2734. [DOI] [Google Scholar]
- Vimalanathan S., Hudson J. Anti-influenza virus activity of essential oils and vapors. Am. J. Essent. 2014;2(1):47–53. [Google Scholar]
- Wang X., Jia W., Zhao A. Anti-influenza agents from plants and traditional Chinese medicine. Phytother Res. 2006;20(5):335–341. doi: 10.1002/ptr.1892. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Kawaoka Y. Influenza virus–host interactomes as a basis for antiviral drug development. Curr. Opin. Virol. 2015;14:71–78. doi: 10.1016/j.coviro.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W., Li R., Li X., He J., Jiang S., Liu S., Yang J. Quercetin as an antiviral agent inhibits influenza A virus (IAV) Entry. Viruses. 2016;8(6):1–18. doi: 10.3390/v8010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeşilada E. Hayykitap; Istanbul: 2012. İyileştiren Bitkiler. [Google Scholar]
- Yeşilyurt E.B., Şimşek I., Akaydin G., Yeşilada E. An ethnobotanical survey in selected districts of the Black Sea region (Turkey) Turk. J. Bot. 2017;41:47–62. doi: 10.3906/bot-1606-12. [DOI] [Google Scholar]
- Yeşilyurt E.B., Şimşek I., Tuncel T., Akaydın G., Yeşilada E. Plants used as folk medicine in some settlements of the Marmara Region. Marmara Pharm. J. 2017;21:132–148. doi: 10.12991/marupj.259891. [DOI] [Google Scholar]
- Yılmaz D. Karadeniz Teknik Univ. Sci. Ins. Trabzon; 2019. Ethnobotanical Features of Datça Peninsula (Muğla) Master Thesis. [Google Scholar]
- Zakay-Rones Z., Varsano N., Zlotnik M., Manor O., Regev L., Schlesinger M., Mumcuoglu M. Inhibition of several strains of influenza virus in vitro and reduction of symptoms by an elderberry extract (Sambucus nigra L.) during an outbreak of influenza B Panama. J. Alternative Compl. Med. 1995;1(4):361–369. doi: 10.1089/acm.1995.1.361. [DOI] [PubMed] [Google Scholar]